Tags: military affairs engineering design handbook
Year: 1971
Similar
Text
ttCNMNUT
*NCF 7К-Ш
ENGINEERING DESIGN
HANDBOOK
EXPLOSIVES SERIES
PROPERTIES OF EXPLOSIVES
OF MILITARY INTEREST
I.S. ARMY MATERIEL С0ММАЮ JANUARY 1971
HEADQUARTERS
UNITED STATES ARMY MATERIEL COMMAND
WASHIhCON, D. C. 70315
AMC PAMPHLE. 29 ’anuary 19’1
No. 706-177*
ENGINEERING DESIGN HANDBOOK
PROPERTIES OF EXPLOSIVES OF MILITARY INTEREST
?&•.
РЕКРАСЖ........................................................................ v
ШВПАП0К ABD SYMBOLS.............................................................Vil
ШКШХПОМ......................................................................... 1
JMatol, 80/20................................................................... 12
Aantol, 60/40................................................................. 14
Aantol, 50/50................................................................... 16
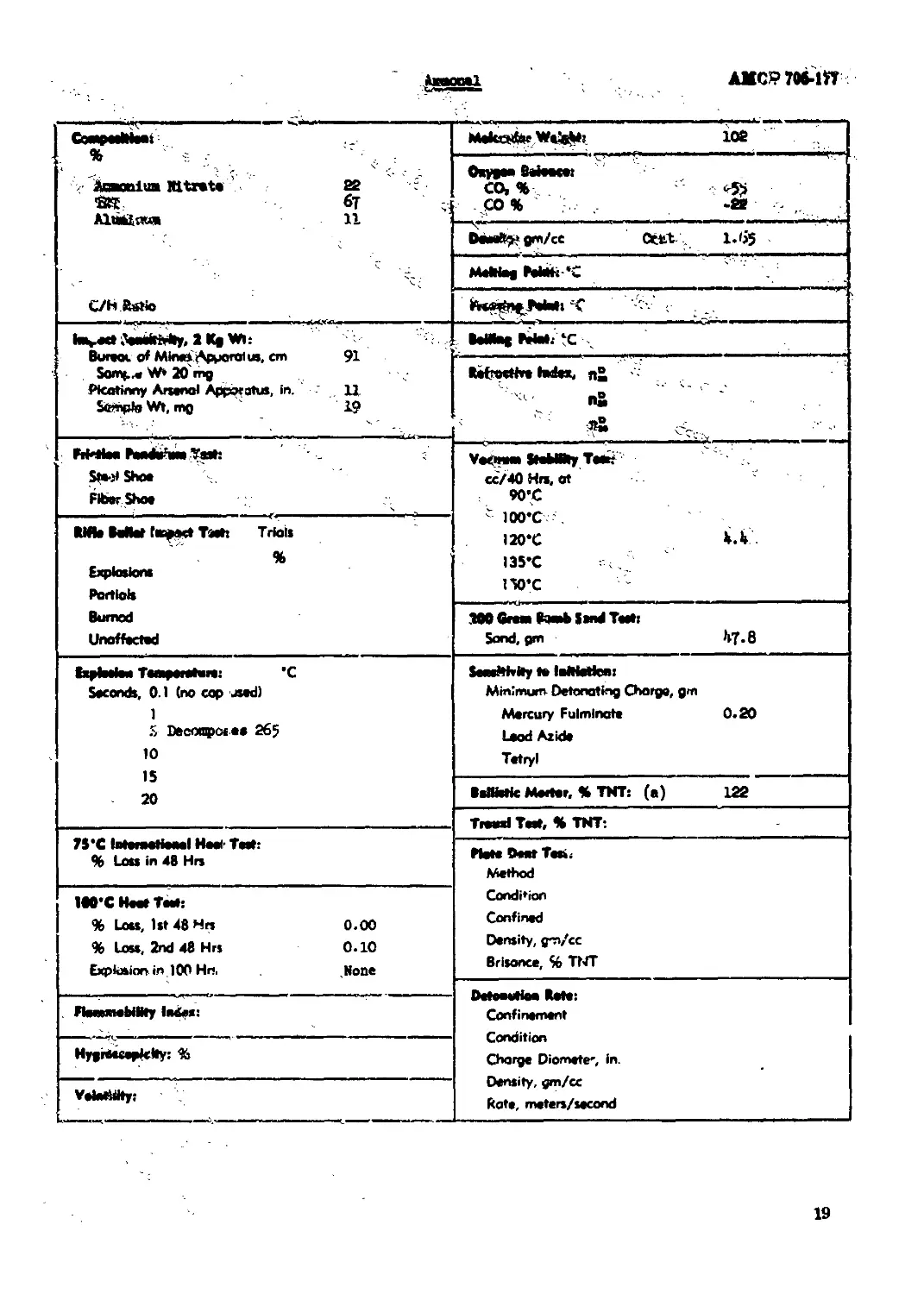
BmmmsI......................................................................... 19
Anaoolu* Nitrate............................................................. 21
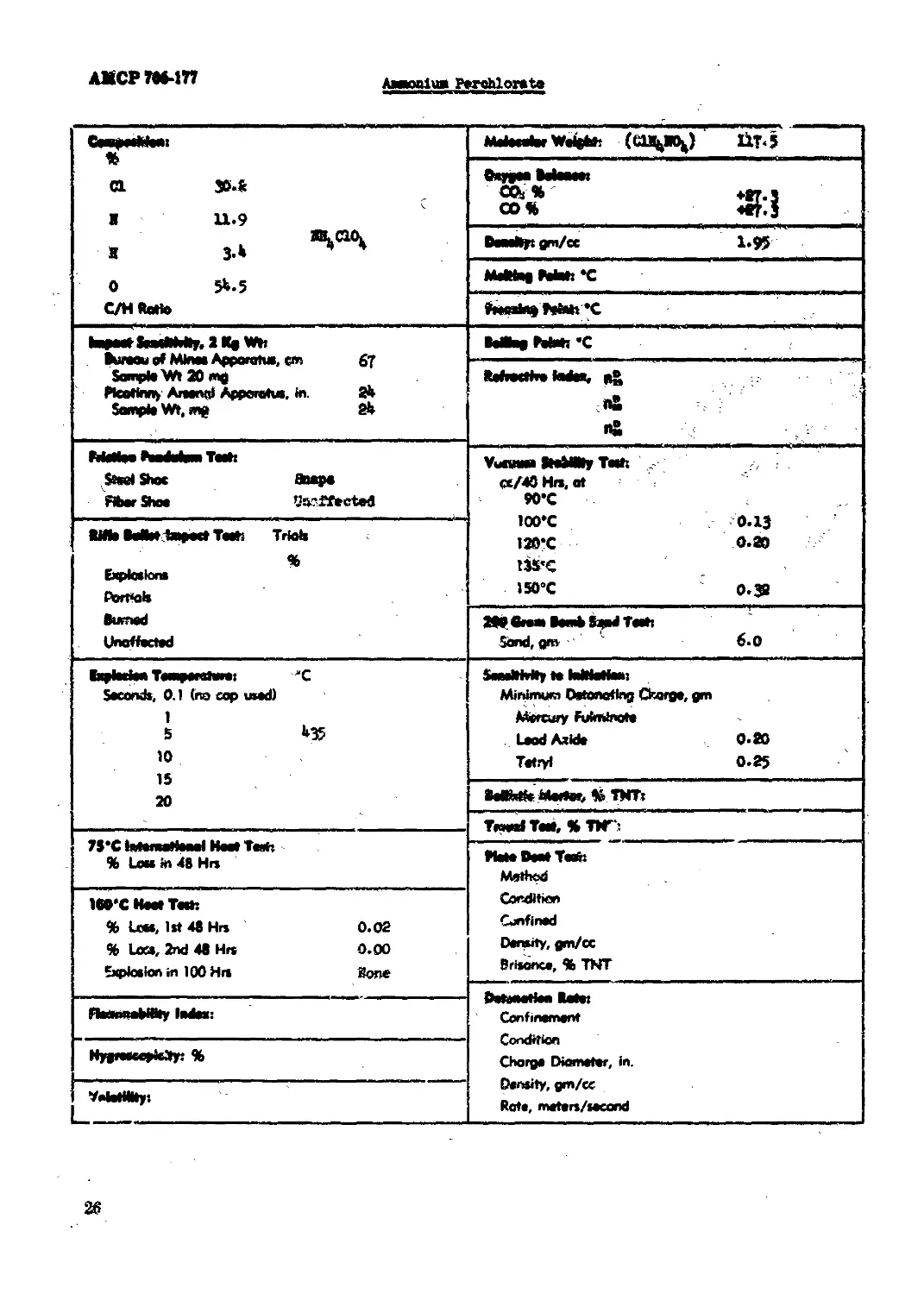
Jmaonfu* Perchlorate.......................................................... 26
Ananxiliss Picj a te—See; Explosive D
Baratol......................................................................... 29
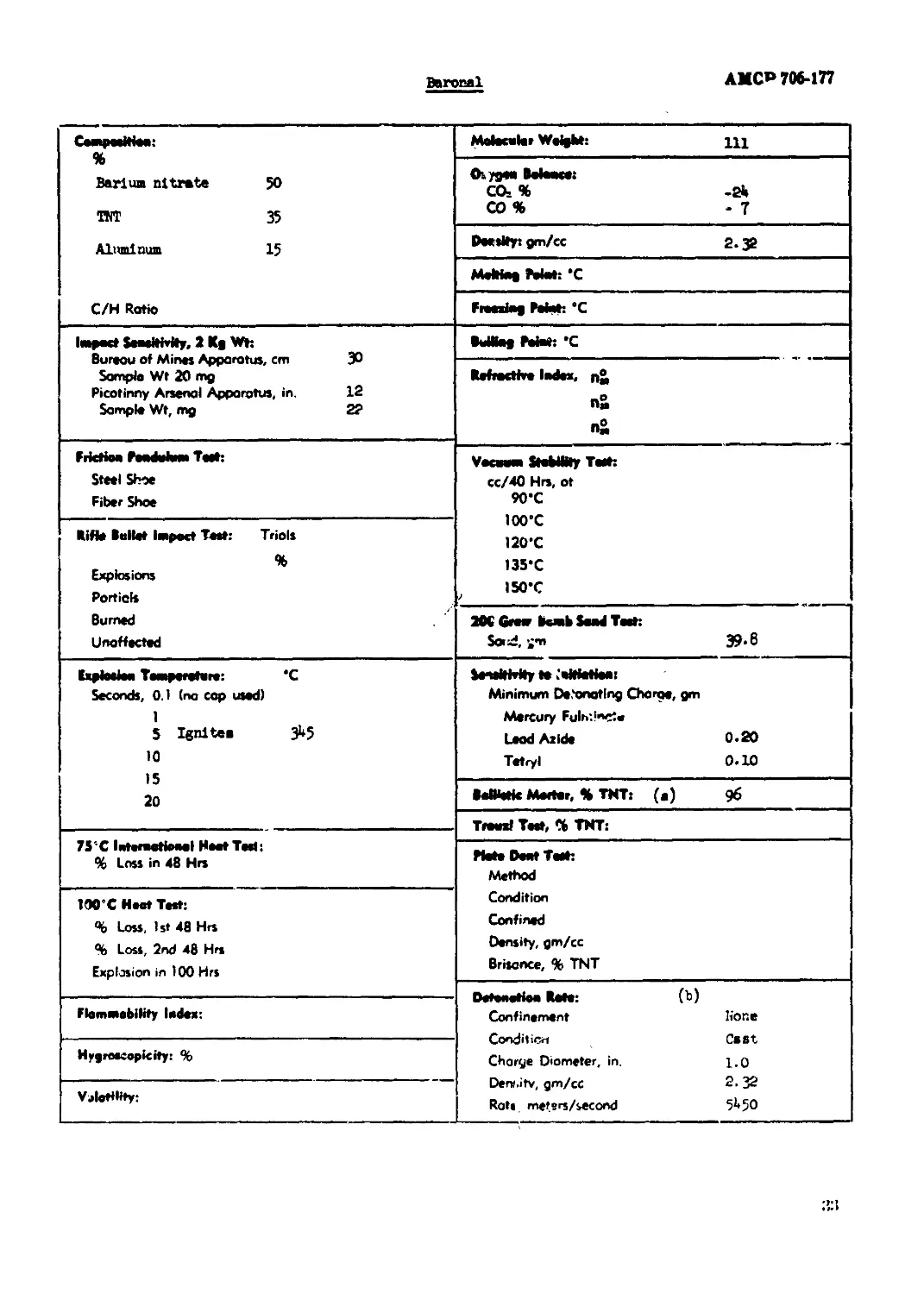
Baronal........................................................................ 33
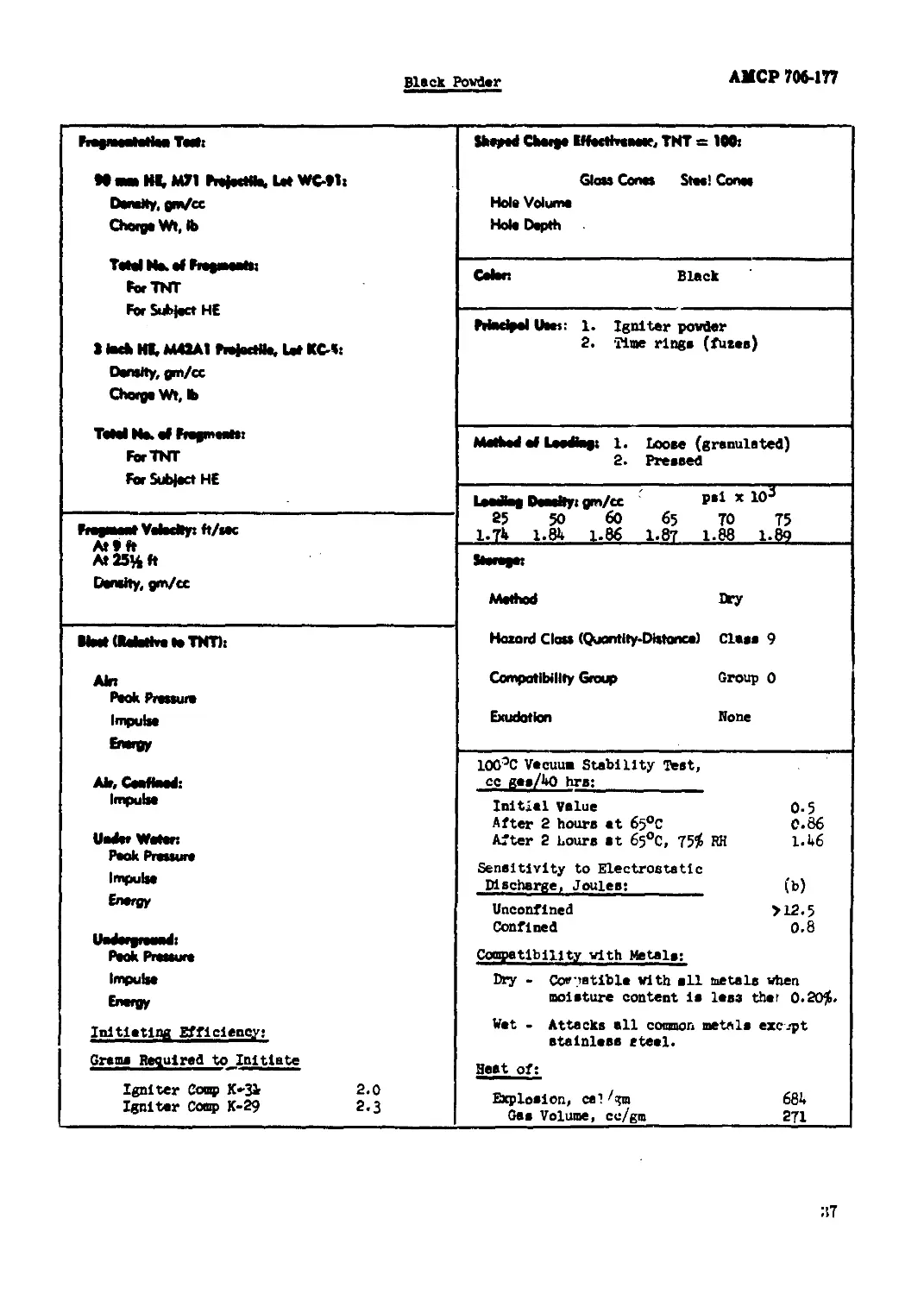
Black Powder.................................................................... 35
1,2,4-Butanetrlol Trinltrete (BTTN) Liquid.................................... 40
Conpooltlon A-3............................................................... 43
Conpooltlo- В................................................................... 46
Cocpoeltlon B, Desensitised..................................................... 51
Conposltion C................................................................. 53
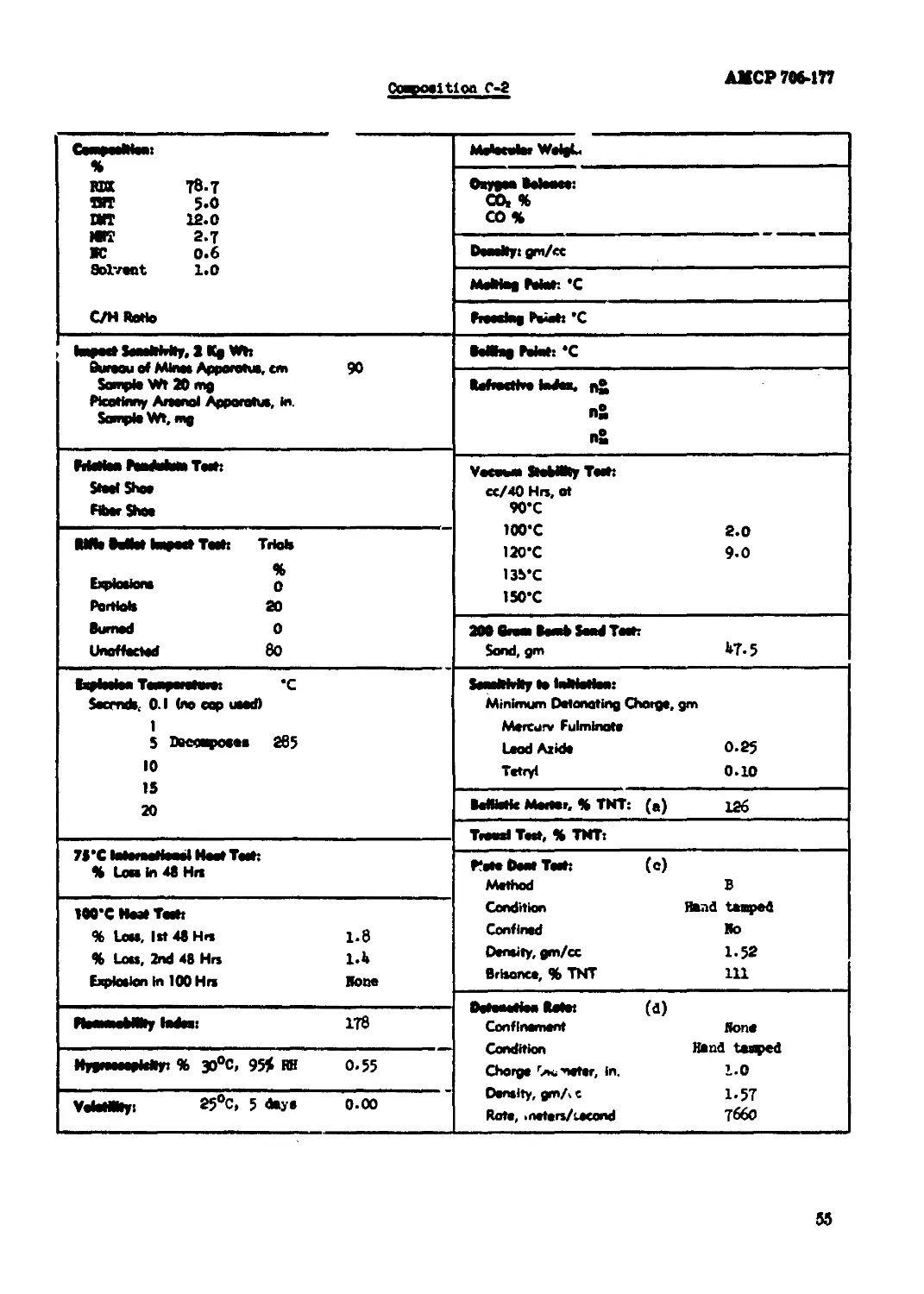
Composition C-2.............................................................. 55
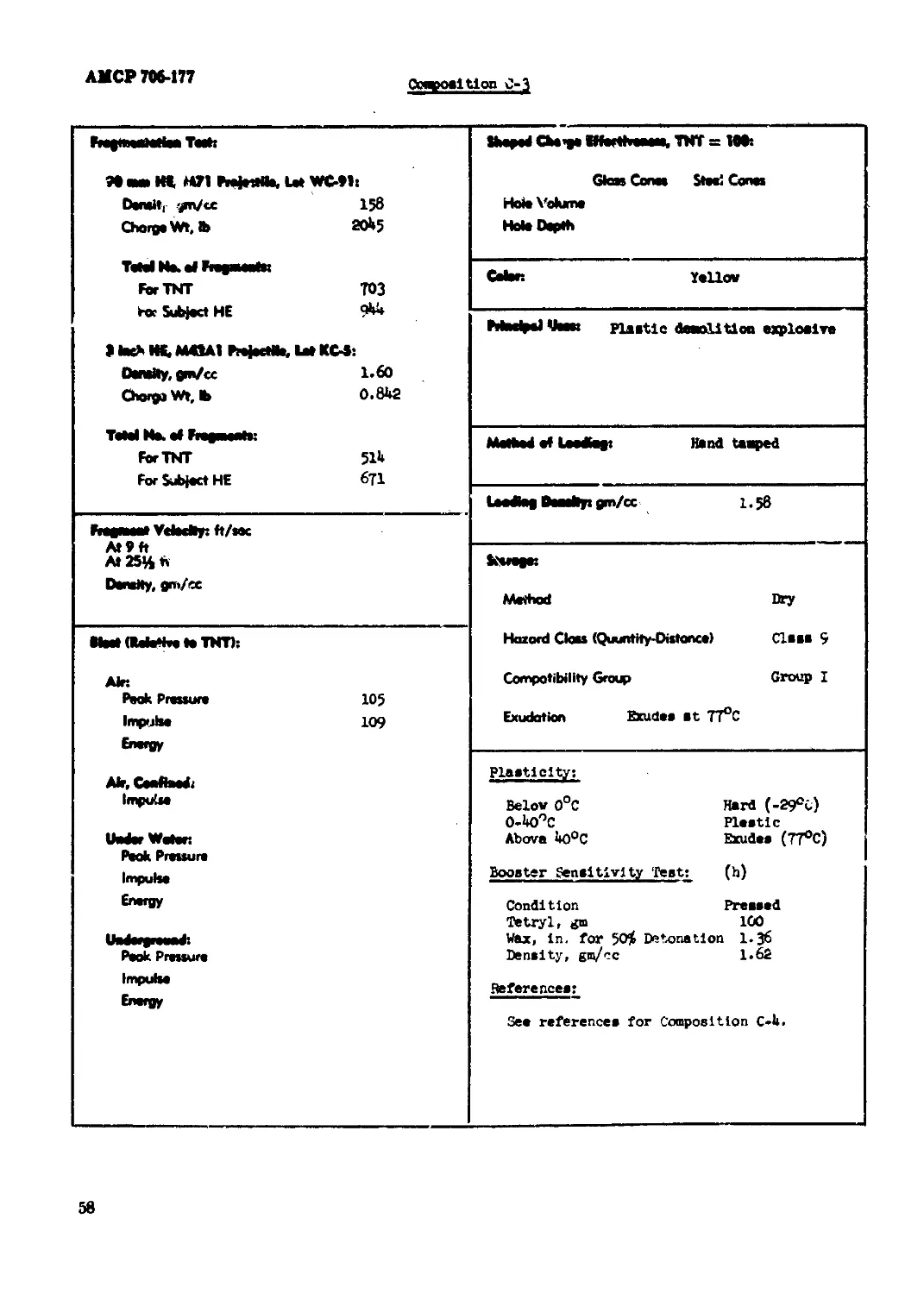
Composition C-3................................................................. 57
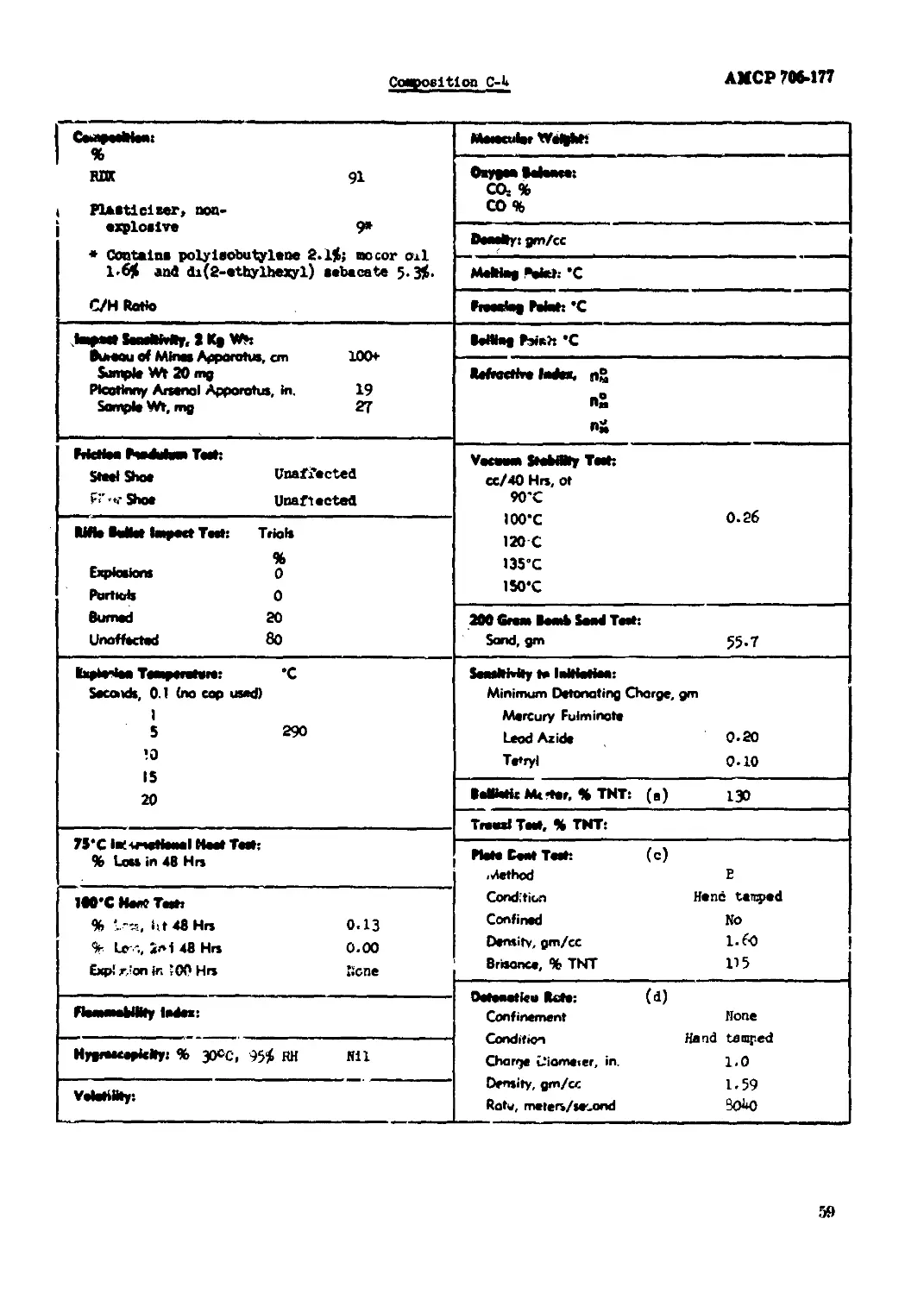
Conposltlon C-4................................................................. 59
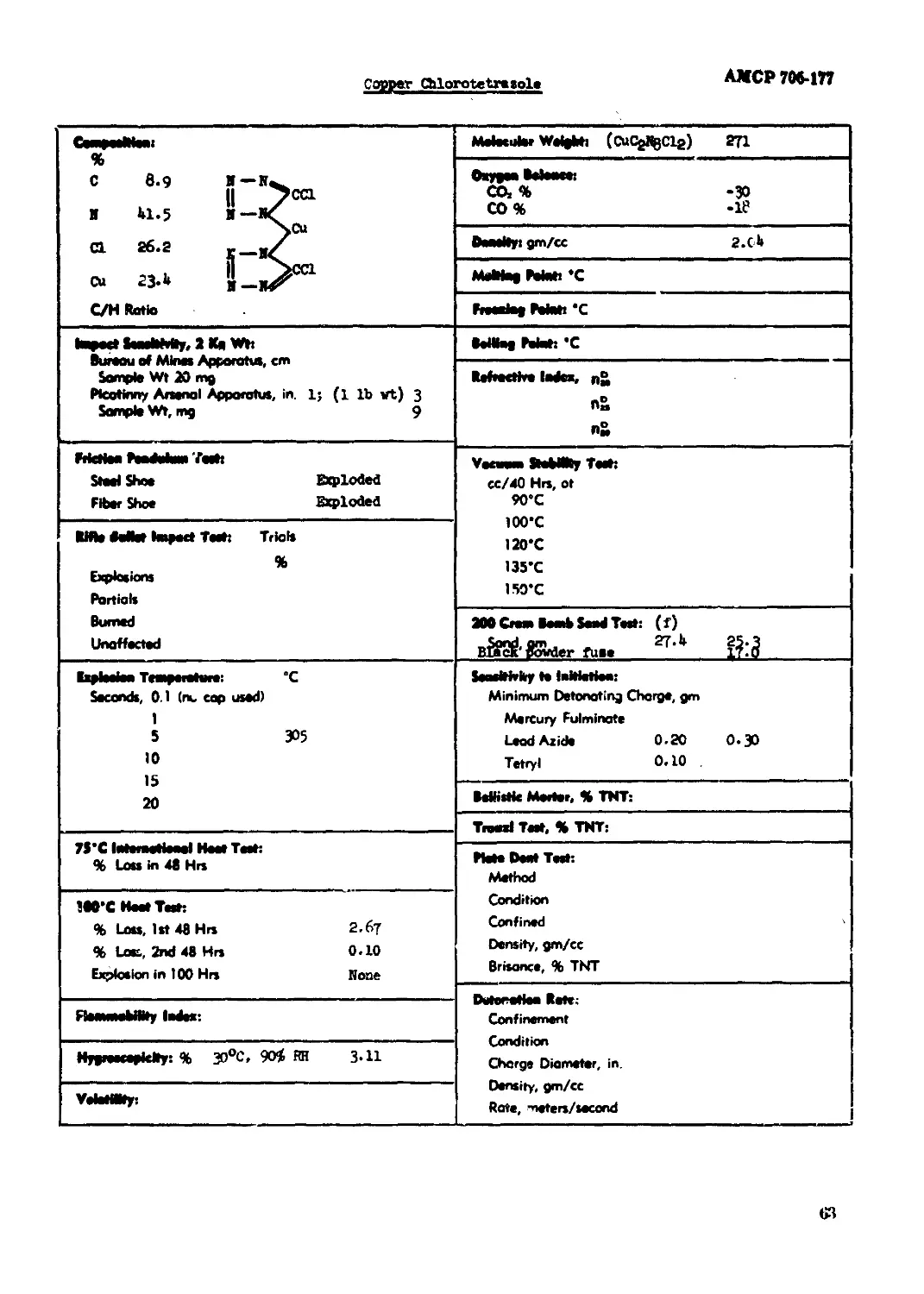
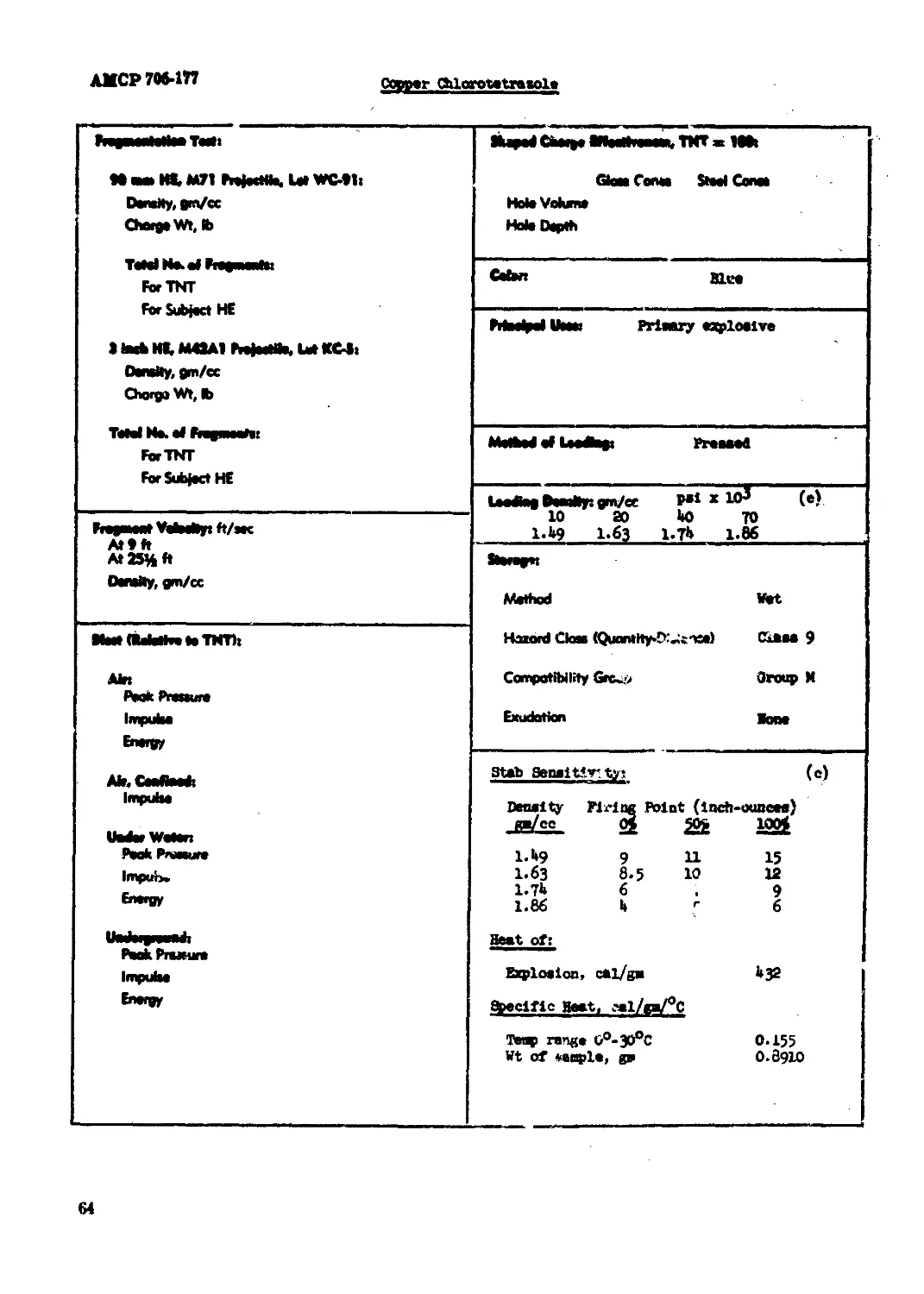
Copper Chlorotetrazole........................................................ 63
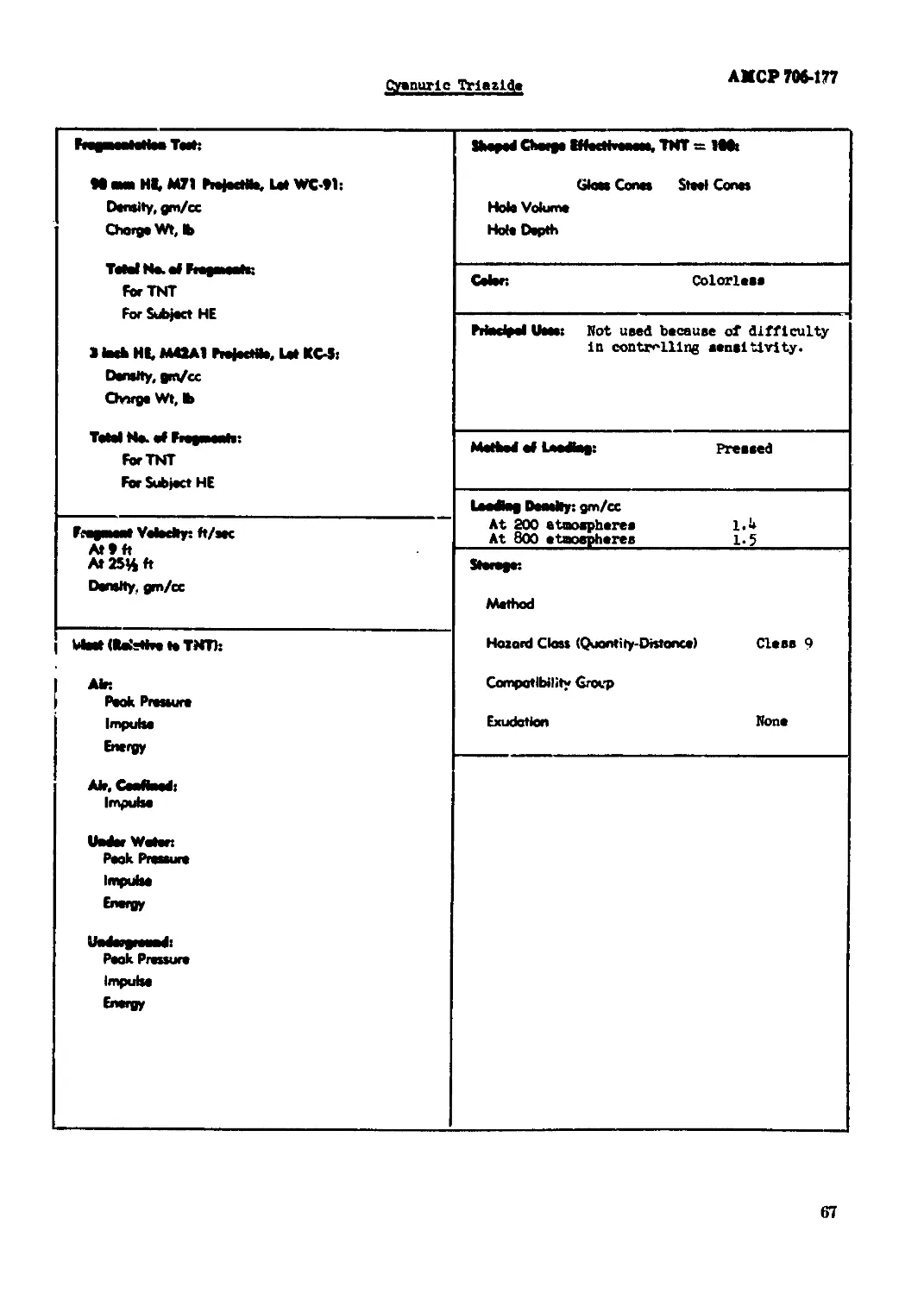
Cyanuric TriazIde............................................................... 66
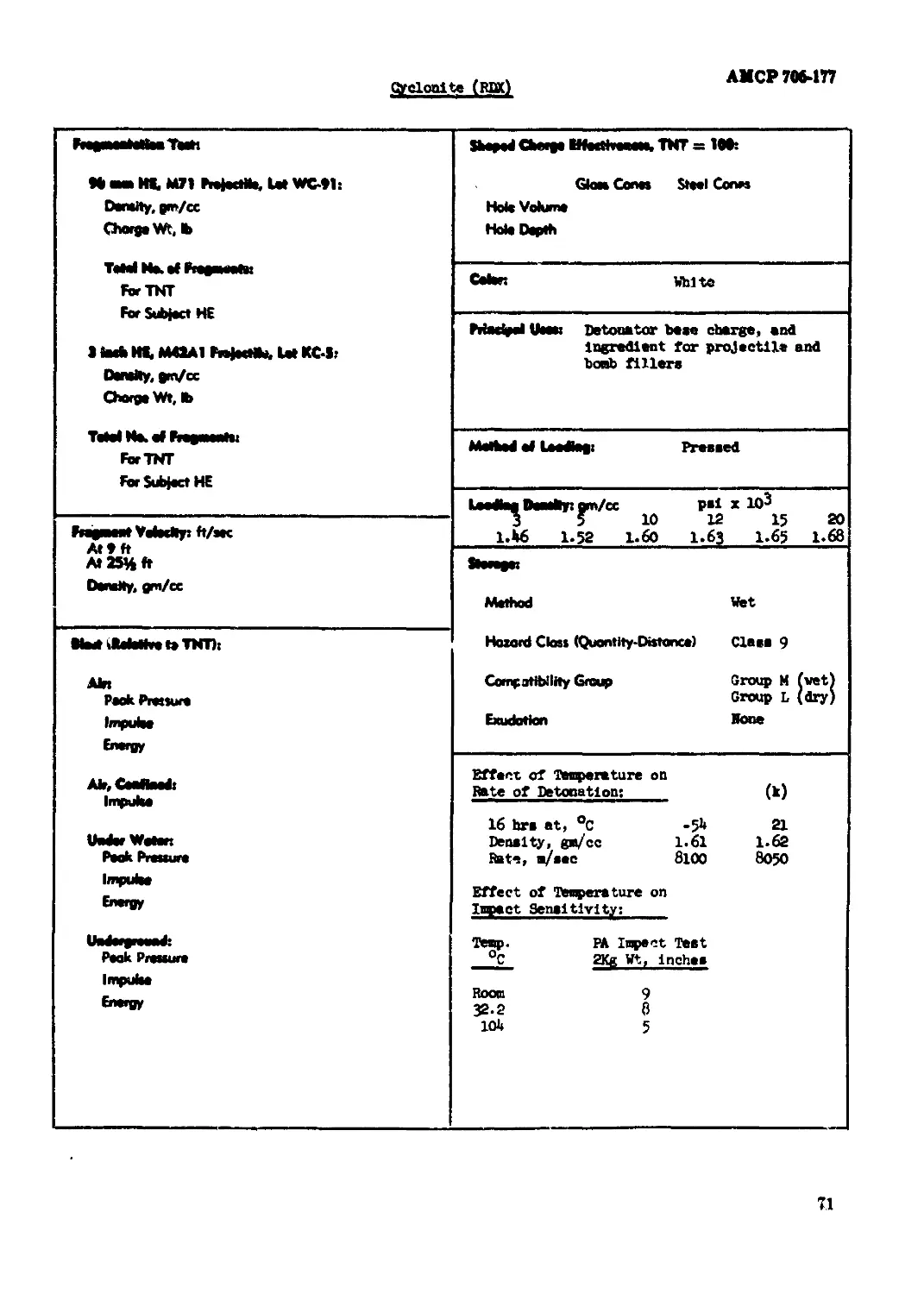
Cyclonite (BOX)................................................................. 69
Cyclotol, 75/25............................................................... 76
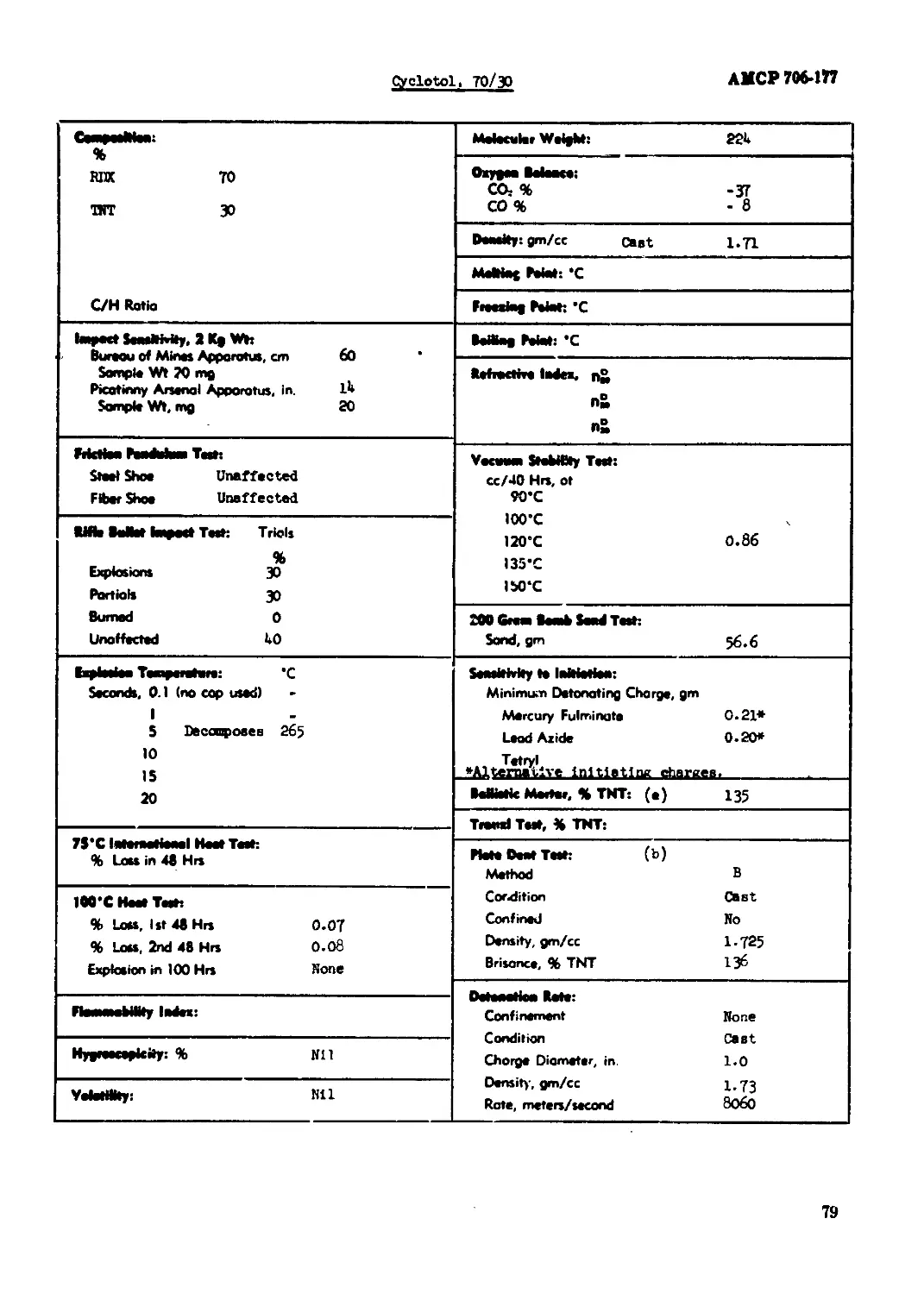
Cyclotol, 70/30................................................................. 79
Cyclotol, 65/35................................................................. 81
Cyclotol, 60/40................................................................. 83
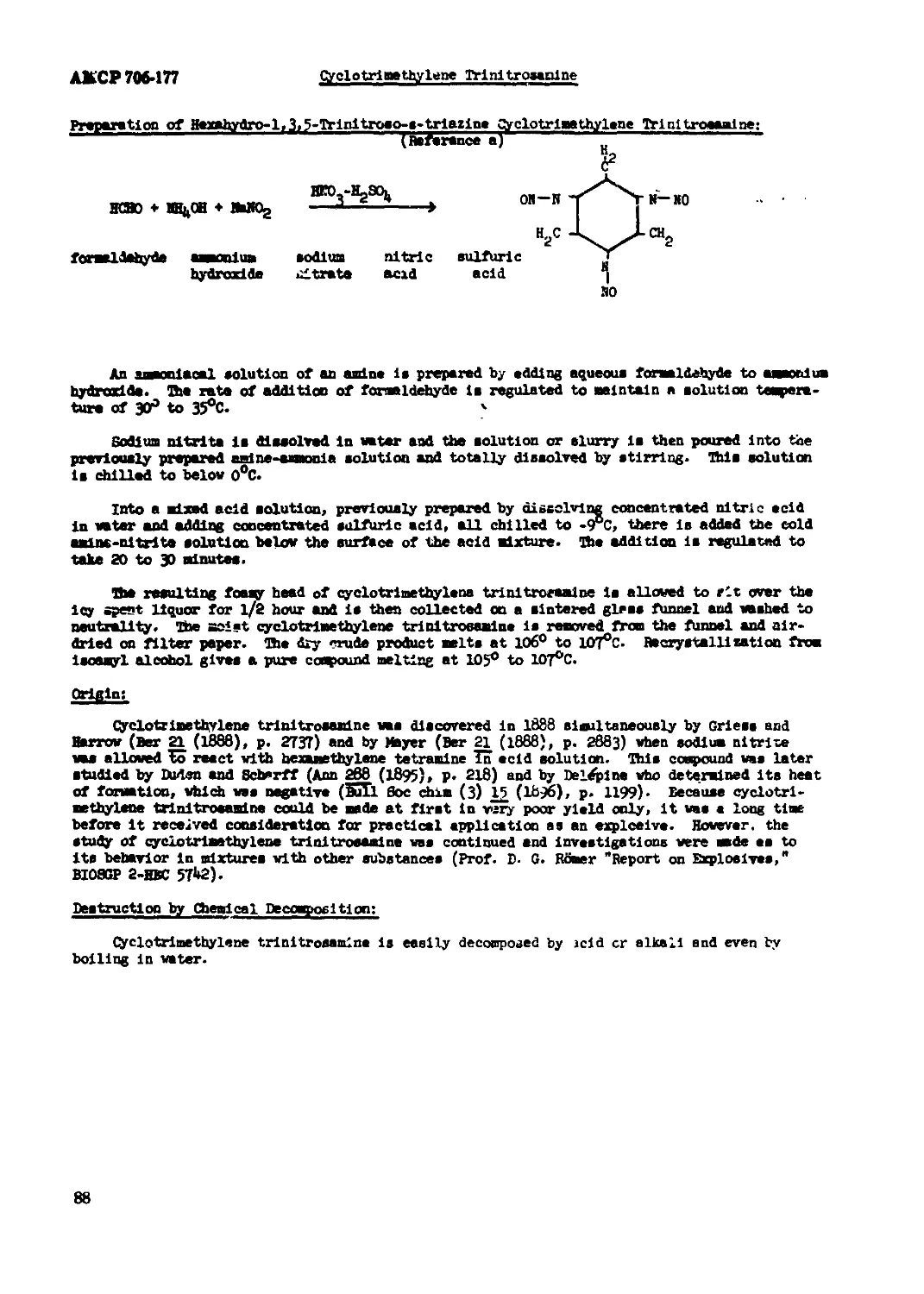
Cyclotrinethylene Trinitrosanine................................................ 86
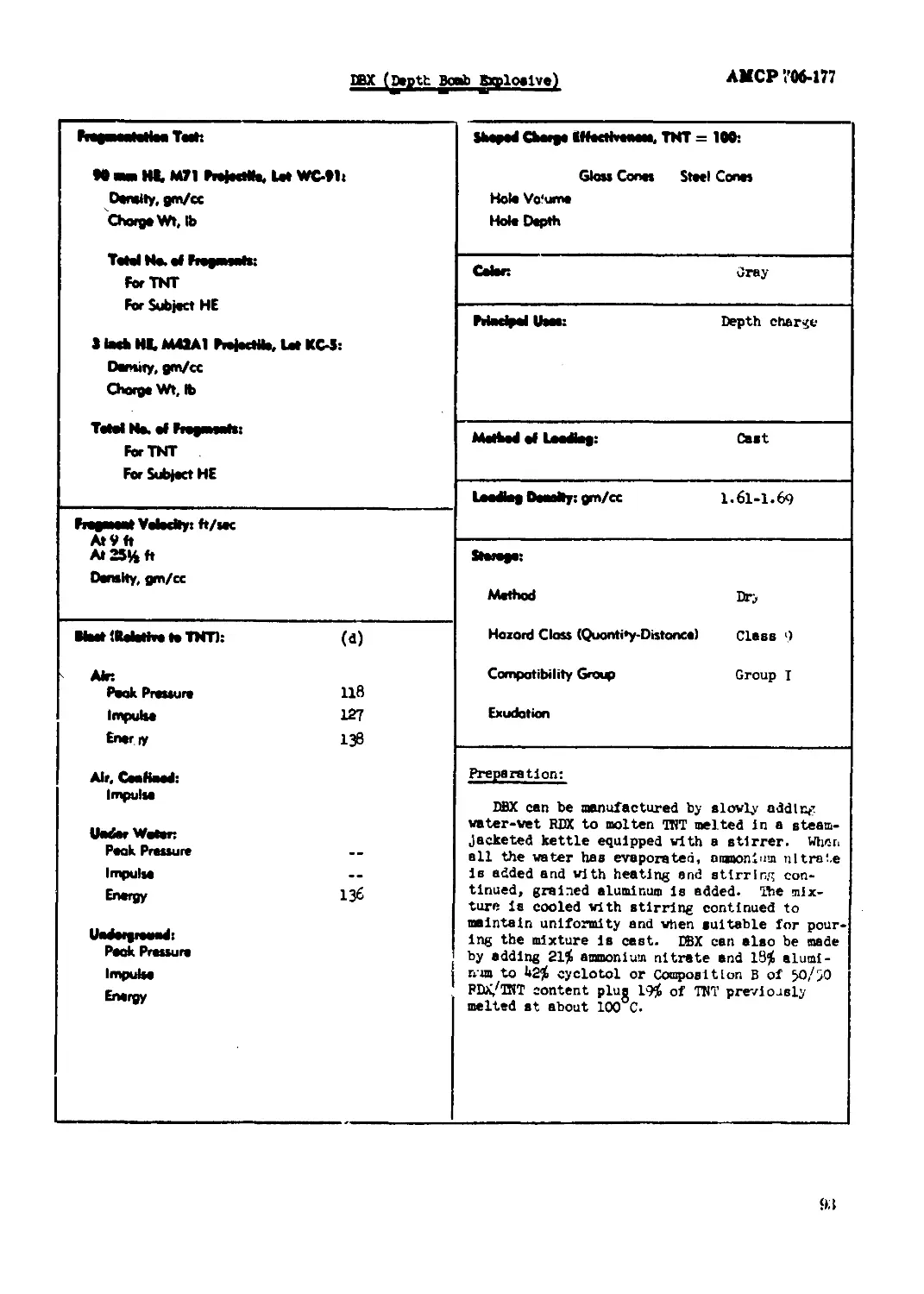
ЭВХ (gepth Bpnh Explosive).......................................................91
1,3~01аа1по~2,4,6-Тг1п1сгоЬспгепе (DATNB)....................................... 95
Mazodlnltiaphenol............................................................... 99
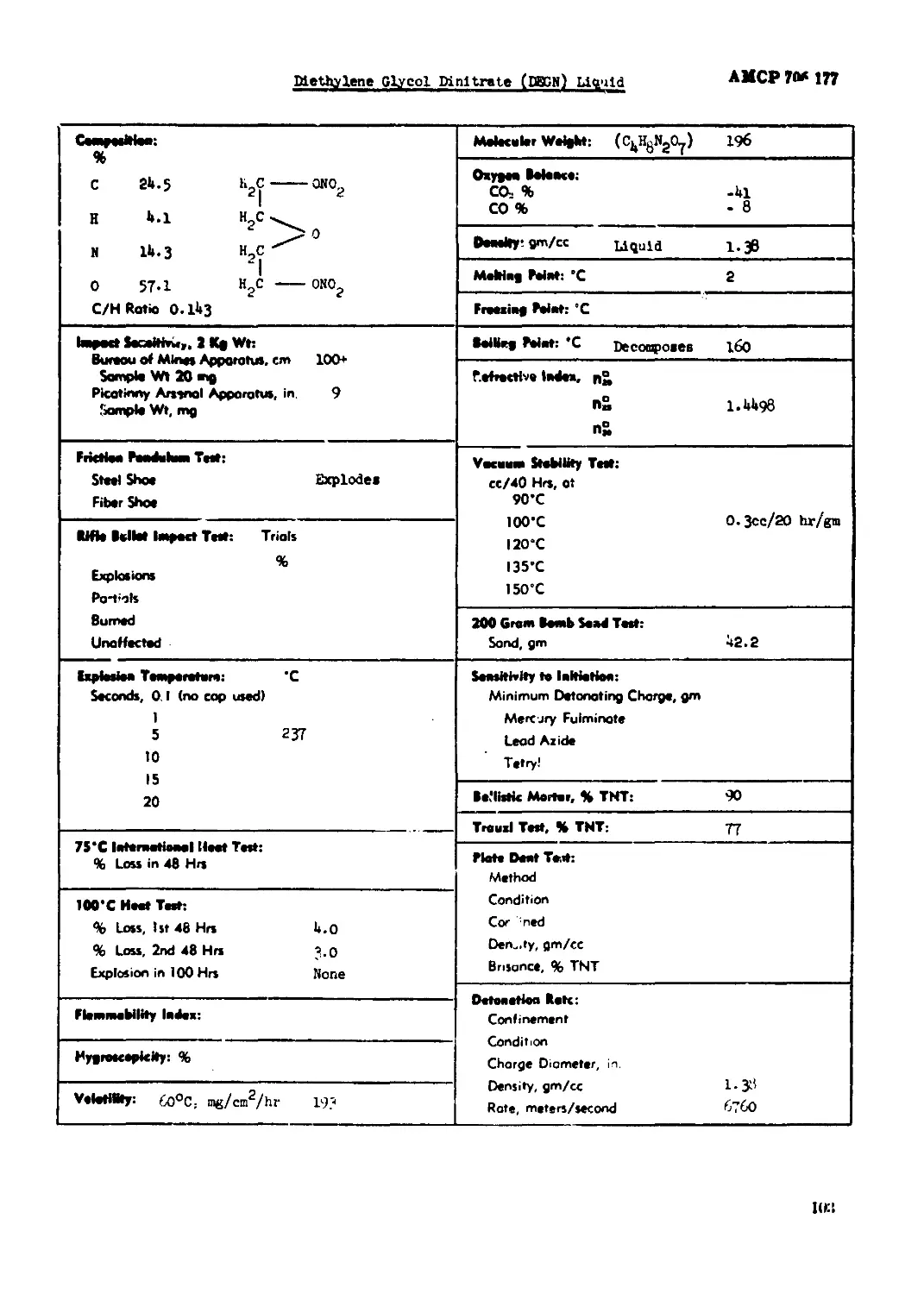
Methylene Glycol Mnltrate (DECK) Liquid.........................................103
Bis(2,2-Dlnttropropyl) Punarate (DNPF)..........................................107
BlsIl^-MnitropL'opyl) Succinate (DNPS)..........................................110
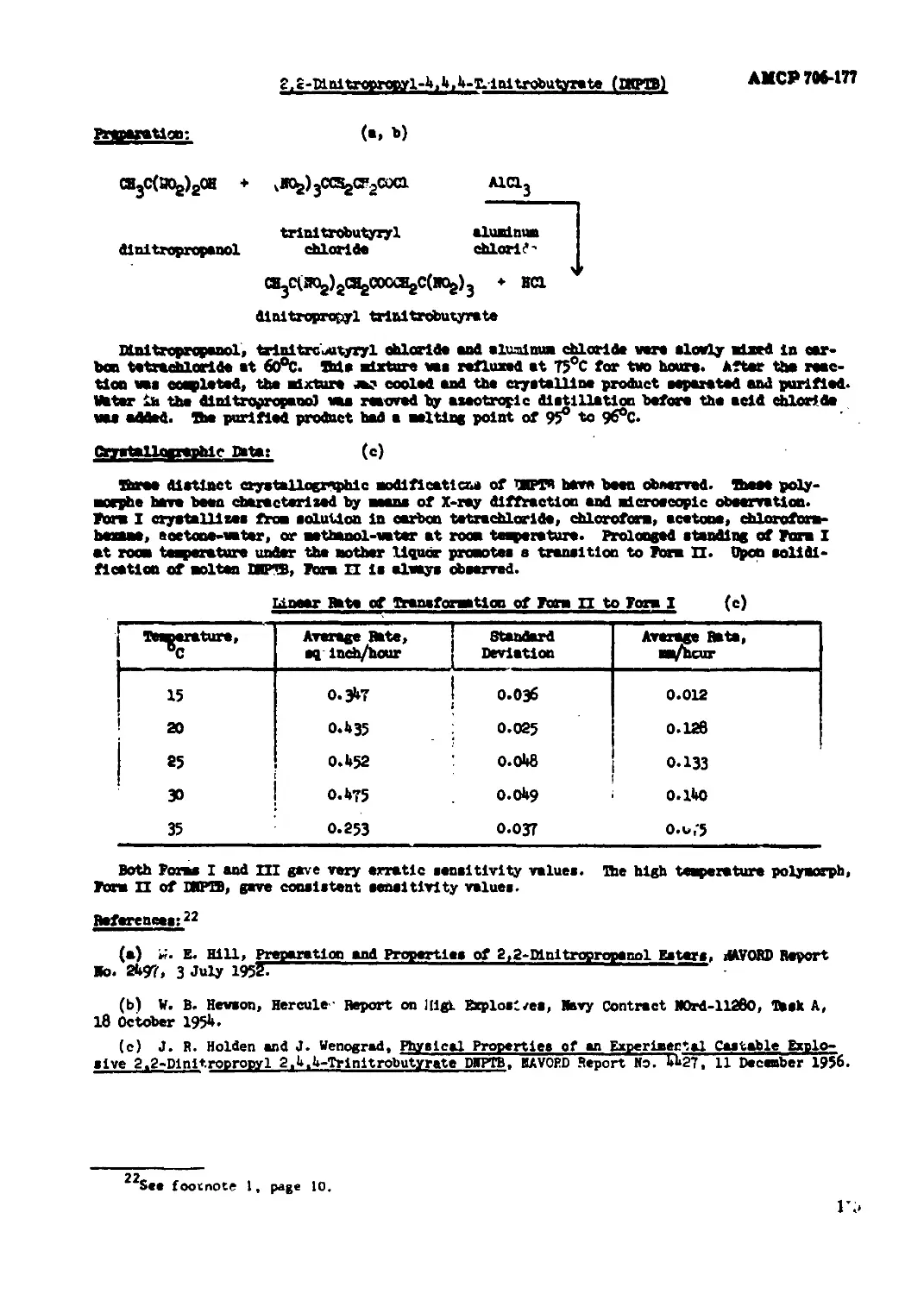
2,2-Dlnltropropyl-4,4.4-Trlnirrobjtyrate (DHPTB)................................113
*This paephlef supersedes ЛИС? 706-177, 2? March 1967, Including Change 1,
20 December 1967.
АМСР 706-177
TABLE OF CONTENTS (cont'r)
Pays
2,4- Dinitrotoluene (DNT)..............................................11C
Dipentecrythrltol Hexdaltrate (PPEHN).............................................1 c
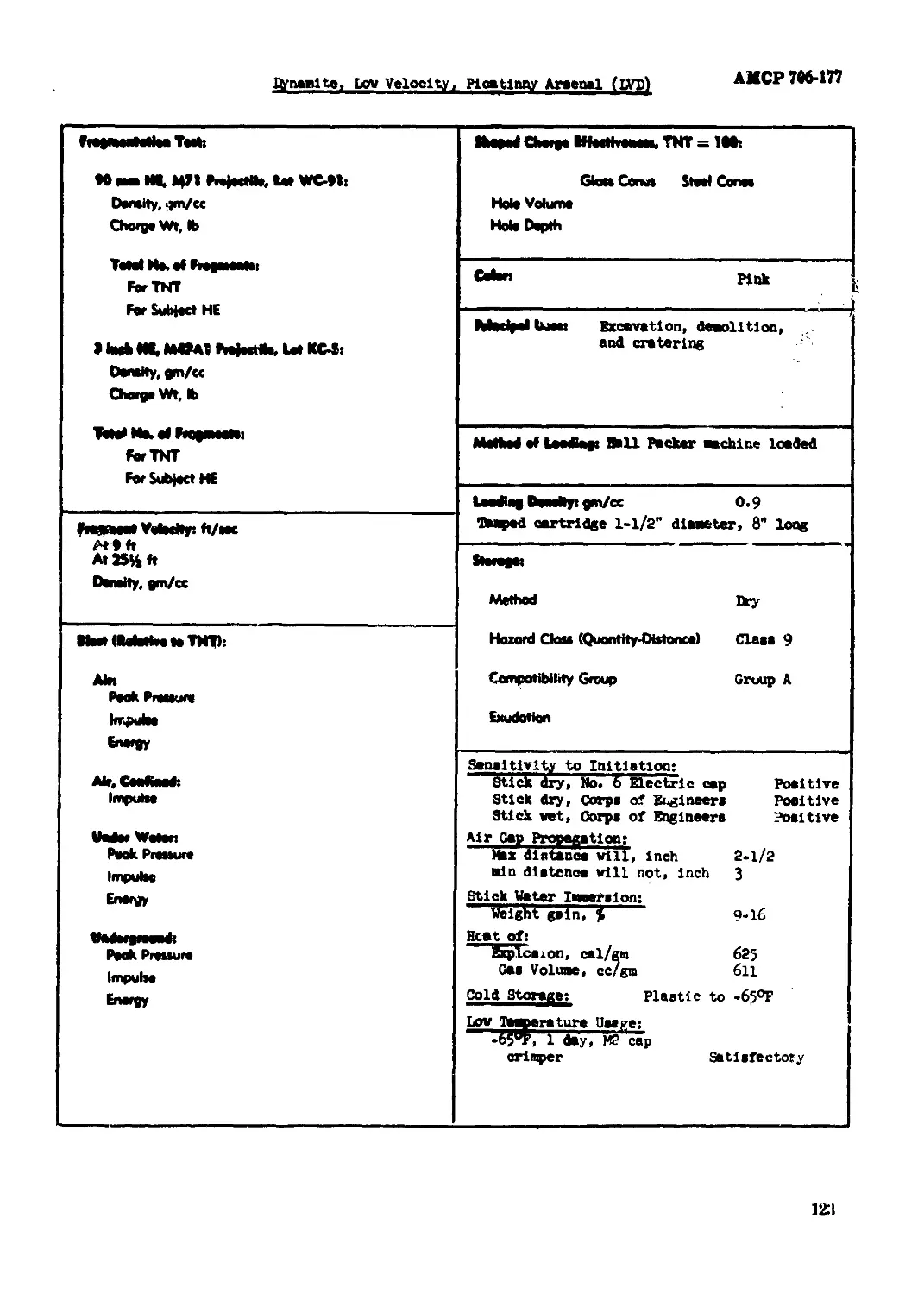
Dynaaite, Lus Velocity, Plcatlnny Aisenal (LVD)...................................
Dynaaite, Medlua Velocity, Hercules (MV!>)...................................... 125
EC Blank bra......................................................................128
EBU--See: Haleltc
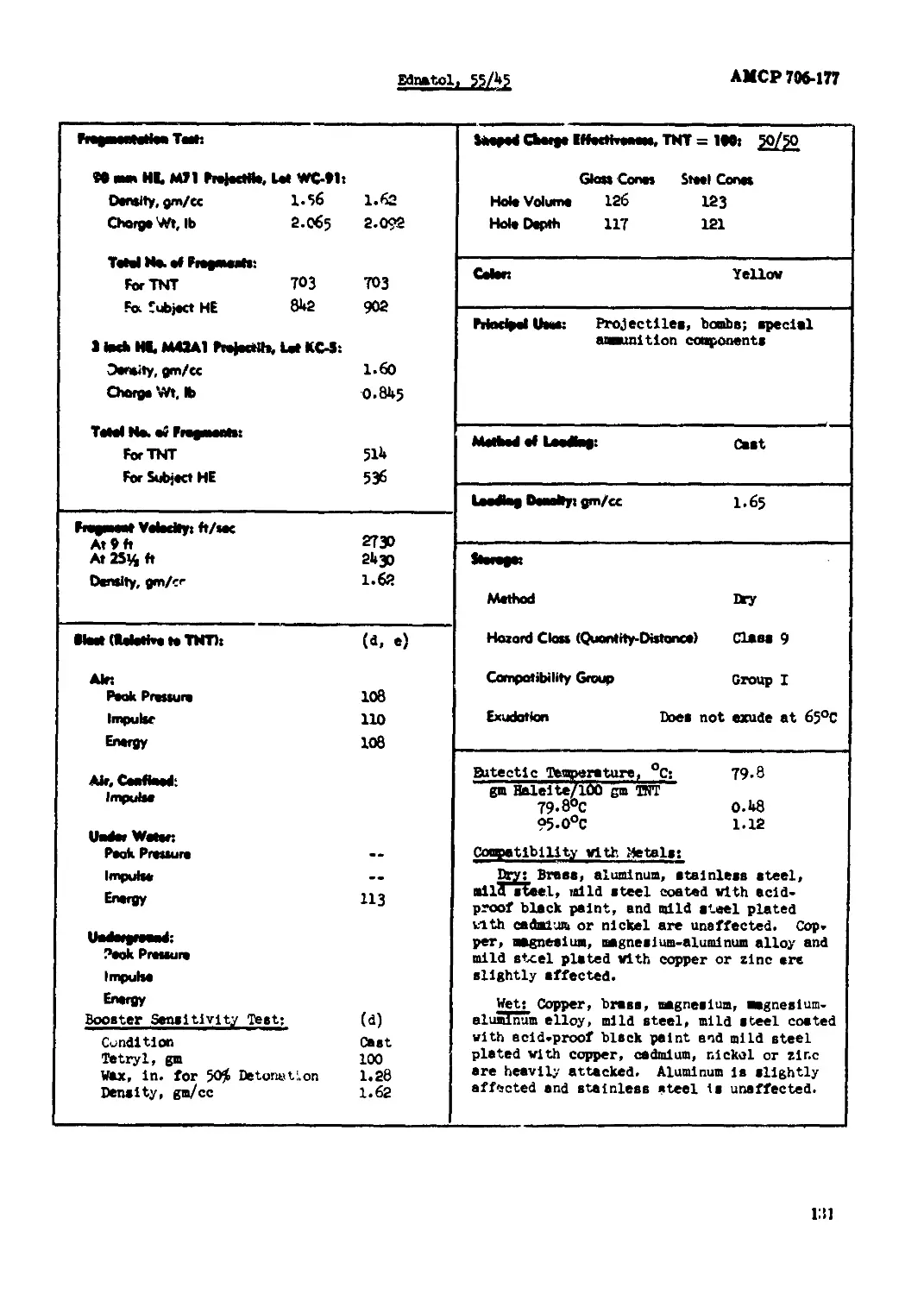
Ednaul. 55/45....u............................................................ 120
Ethylene Glycol Di-Trinitrobutyrite (GiNB)........................................133
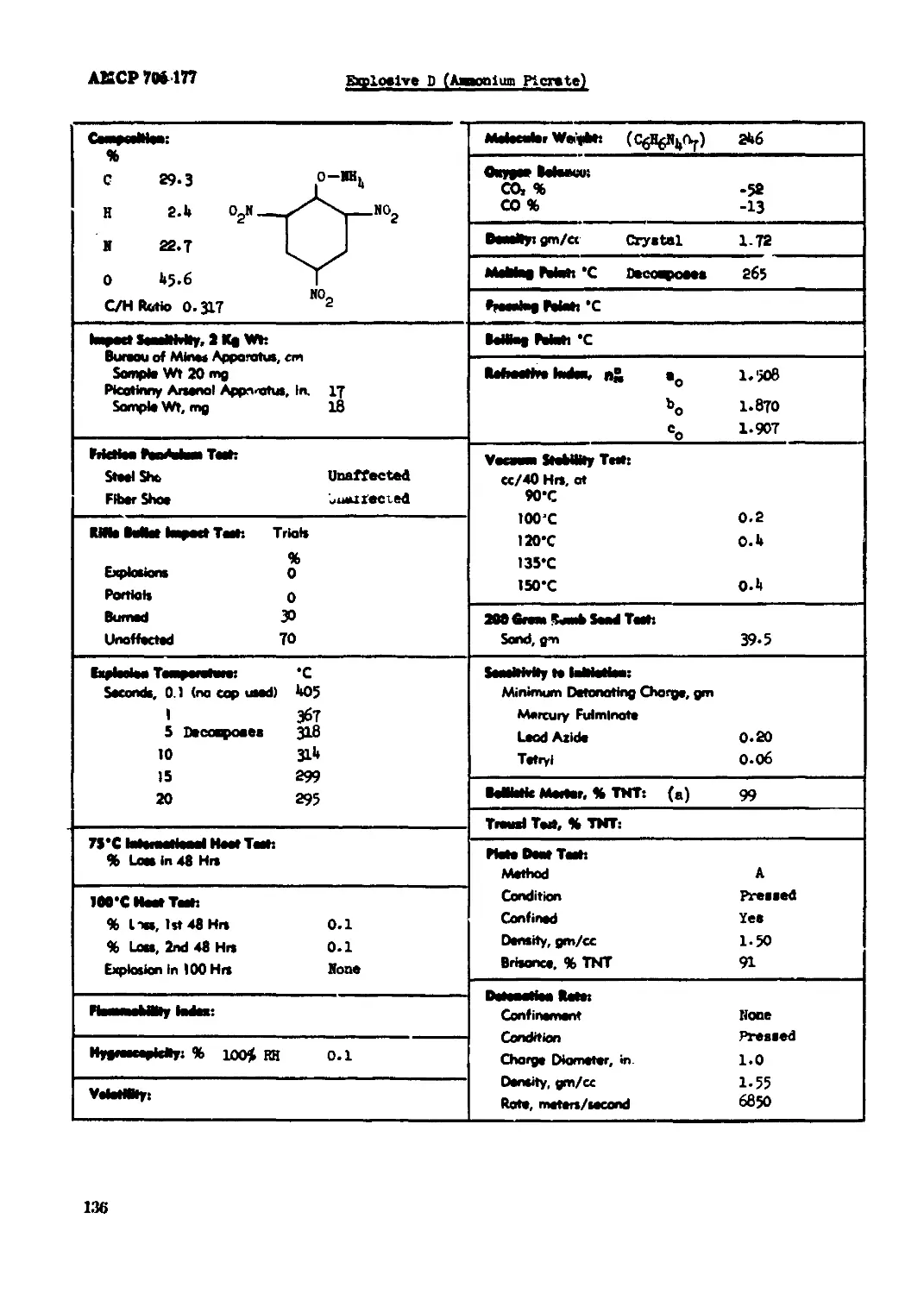
Explosive D (Aaaonlun Picrate)........ ......................................... 136
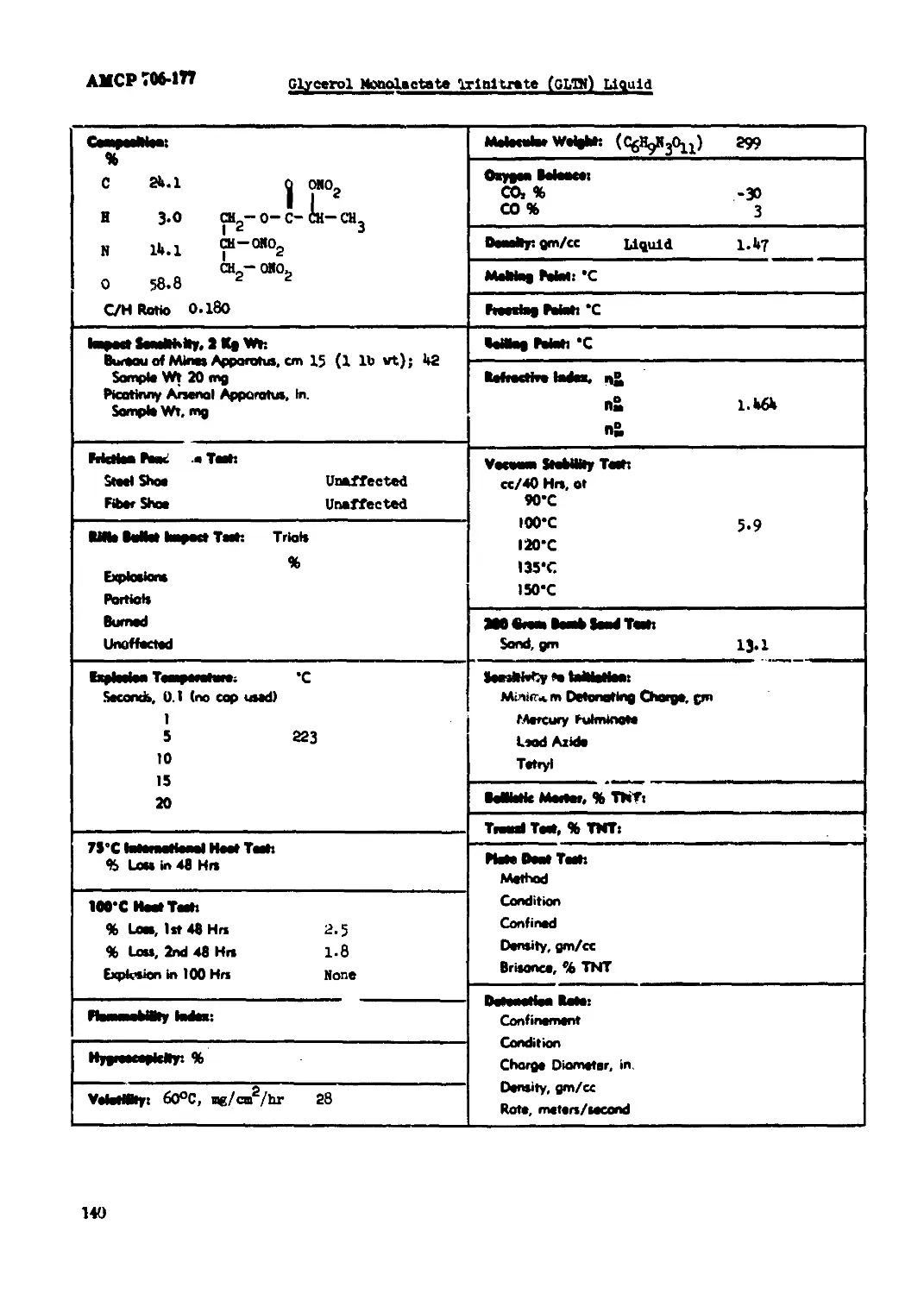
Glycerol Monolactate Trinitrate (GLTN) Liquid.....................................140
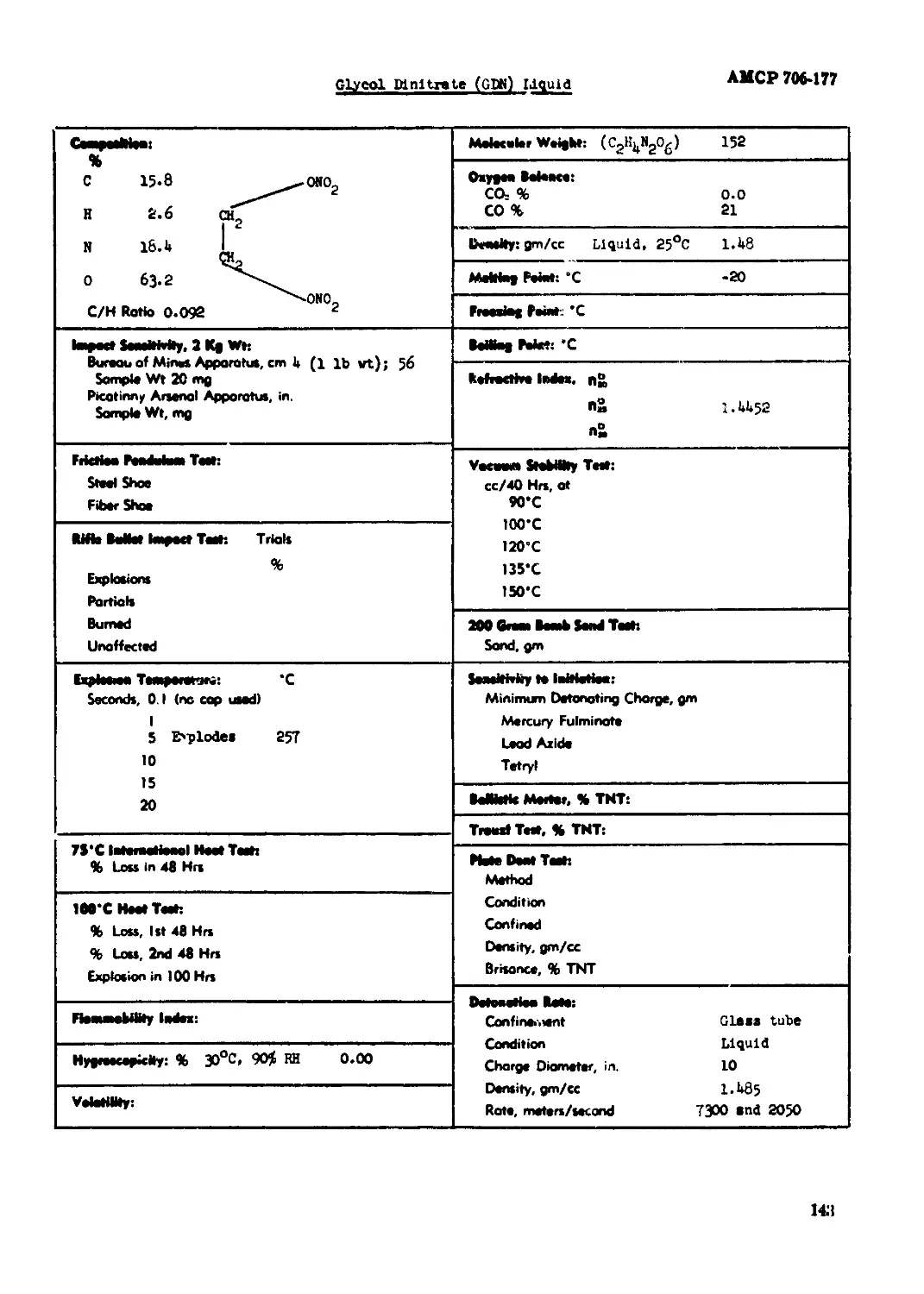
Glycol Dinitrate (РЖ) Liquid......................................................143
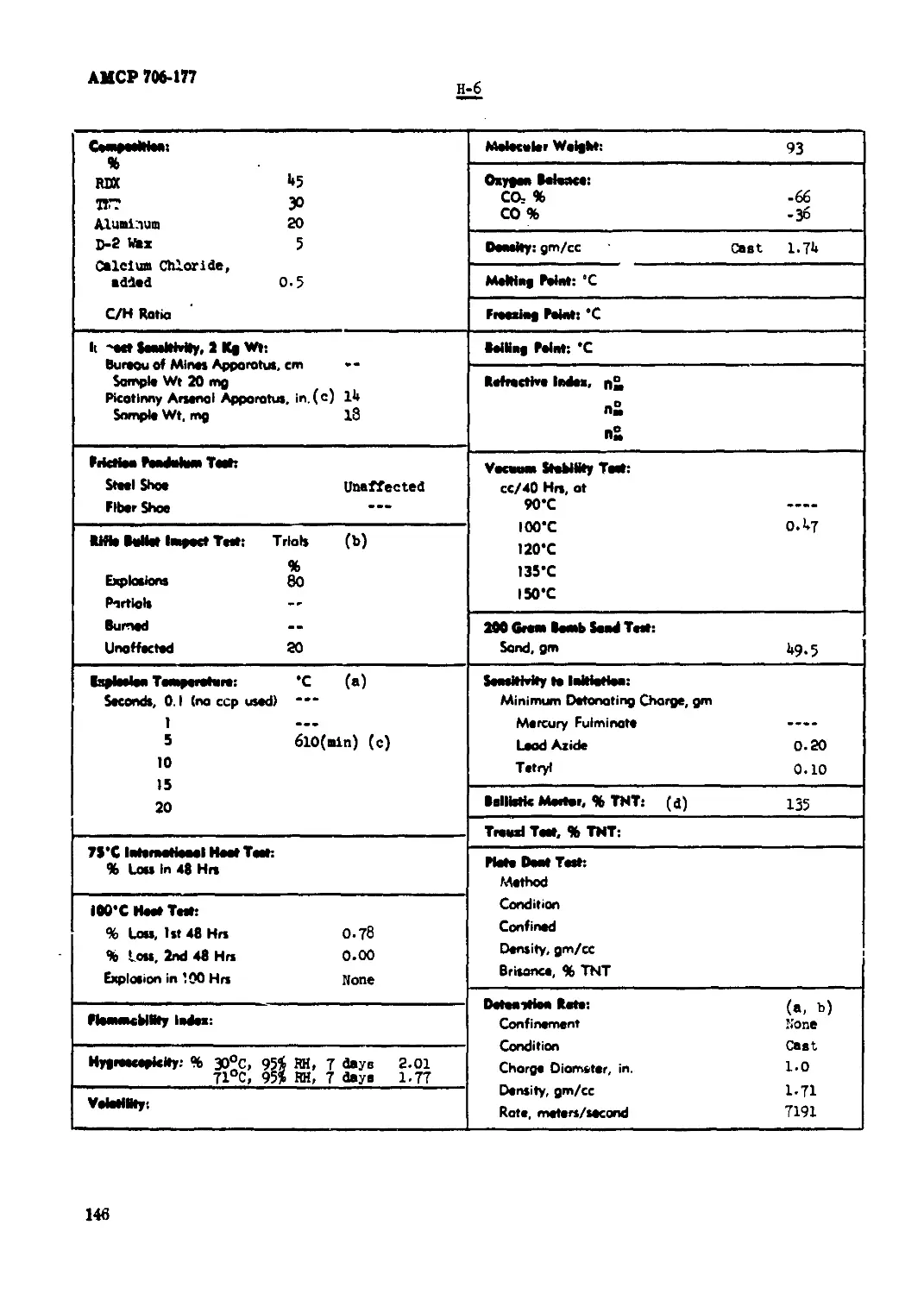
P-6.............................................................................. 146
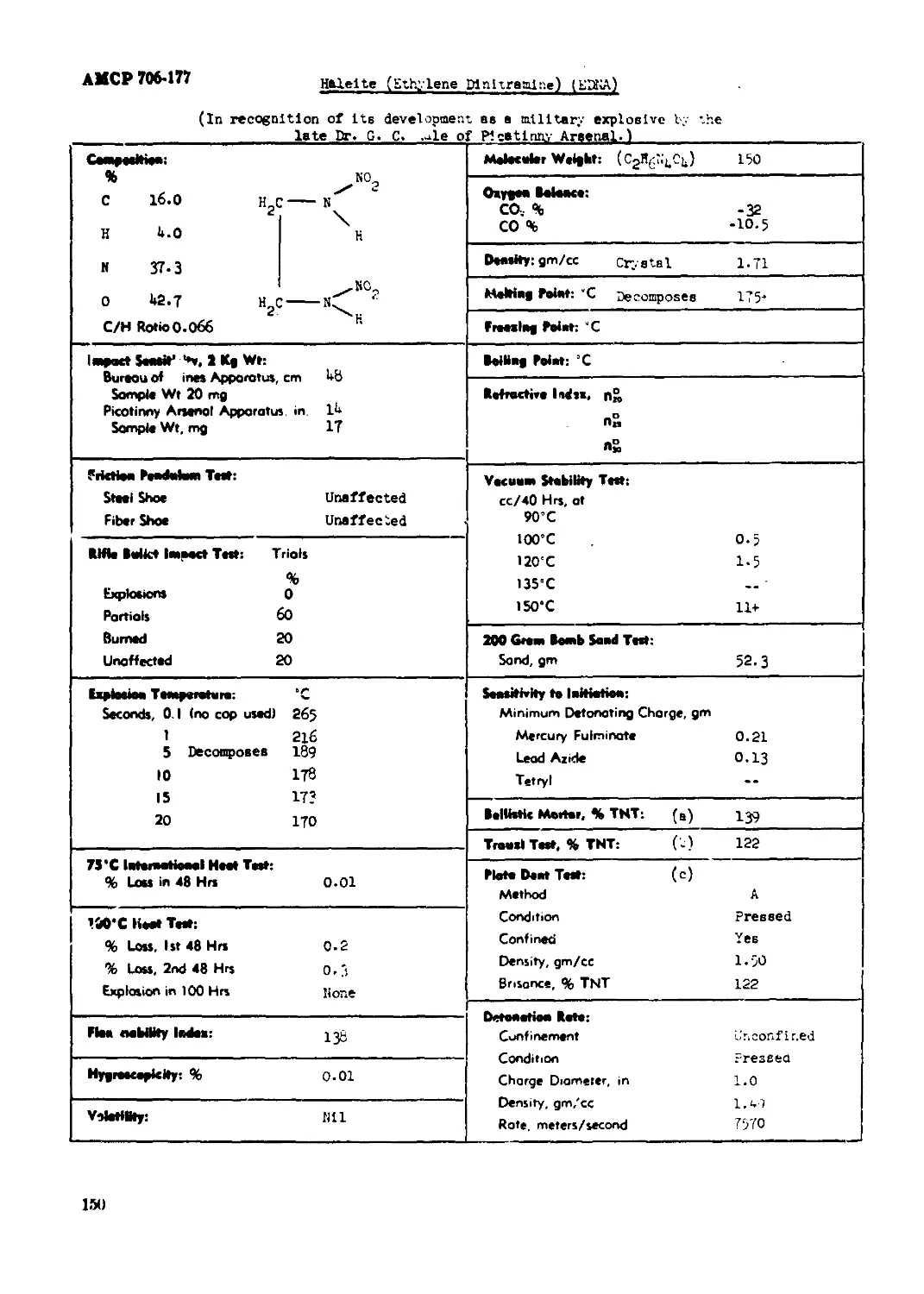
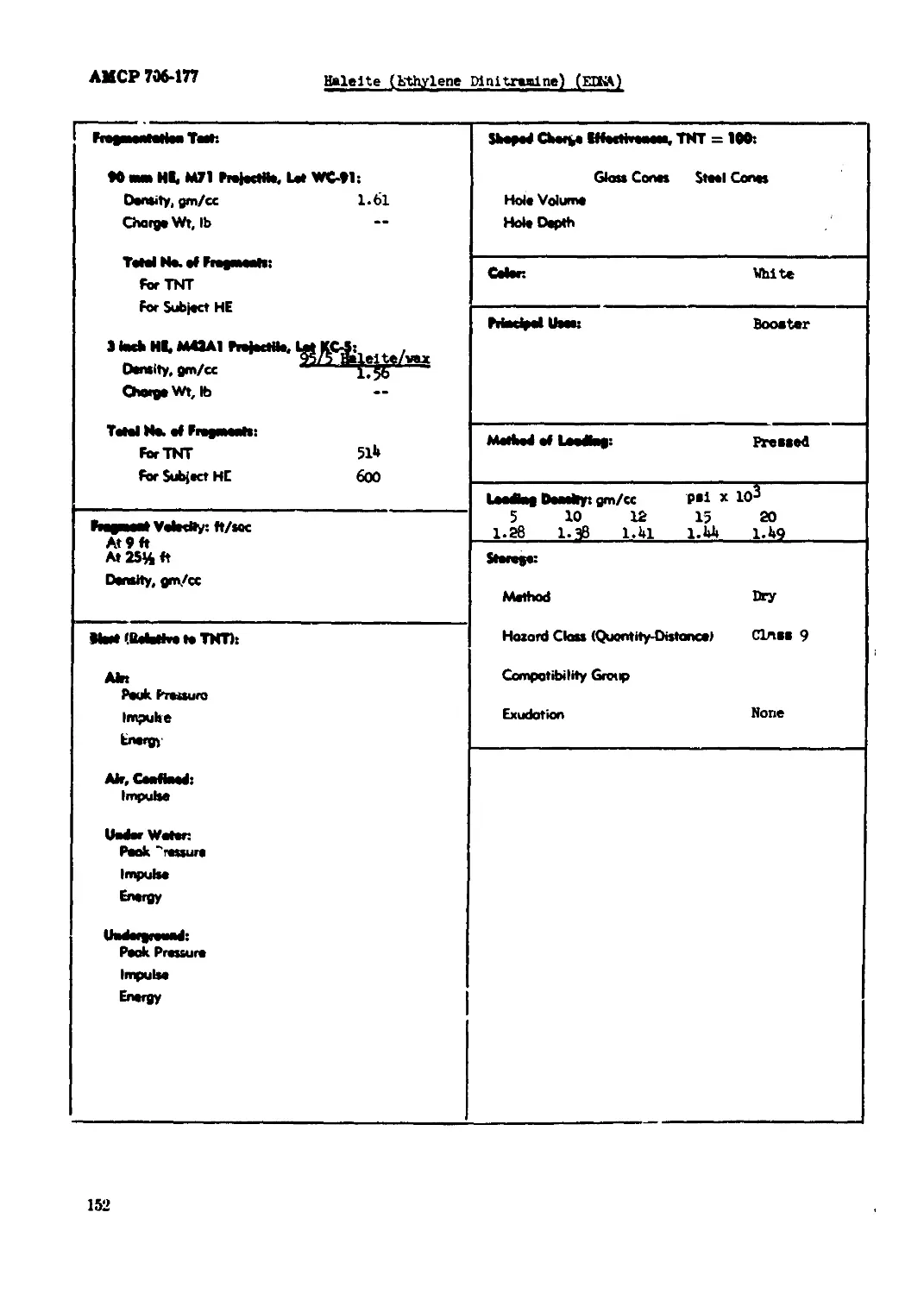
Halelte (Ethylene Dinittaalne) (EDNA).............................................150
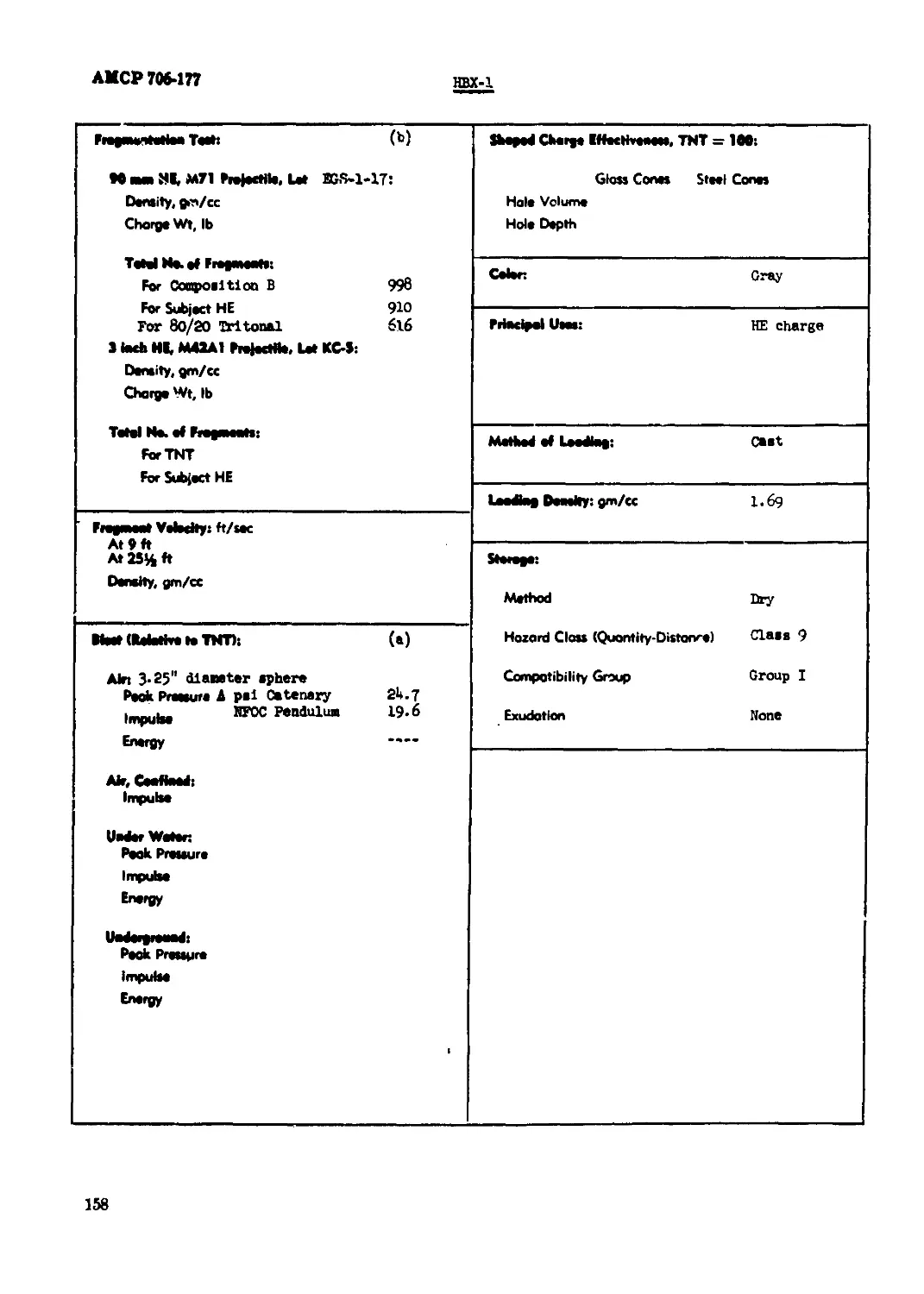
HBX-1......................................................................... 156
HEX- .............................................................................159
HEX-24............................................................................164
HEX-48........................................................................... 166
2,4,6,2',4‘-6'-Hexanltro-oxanlilde (HNO)....................................... .170
beta-IMX........................................................................ 173
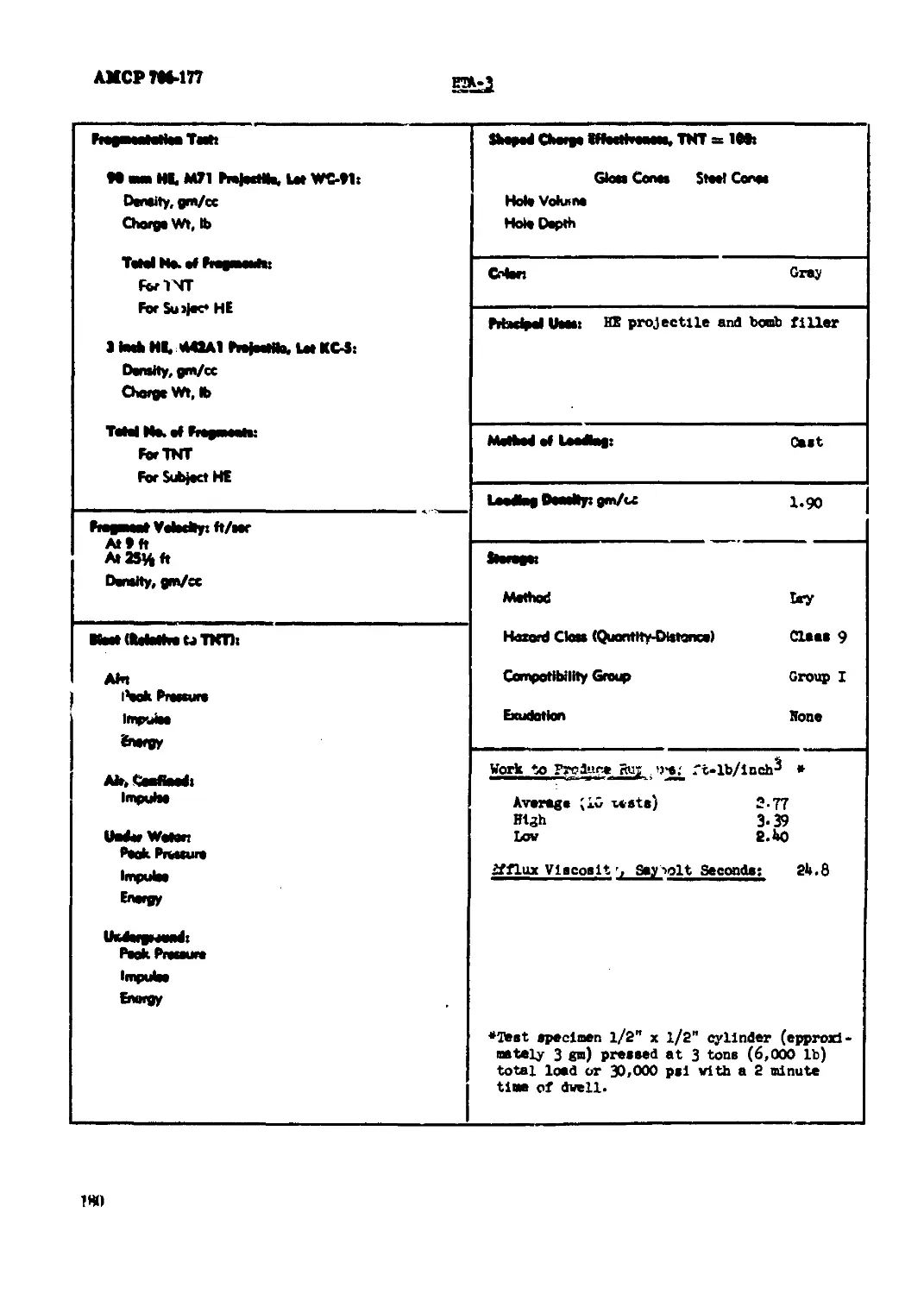
HTA-3.............................................................................178
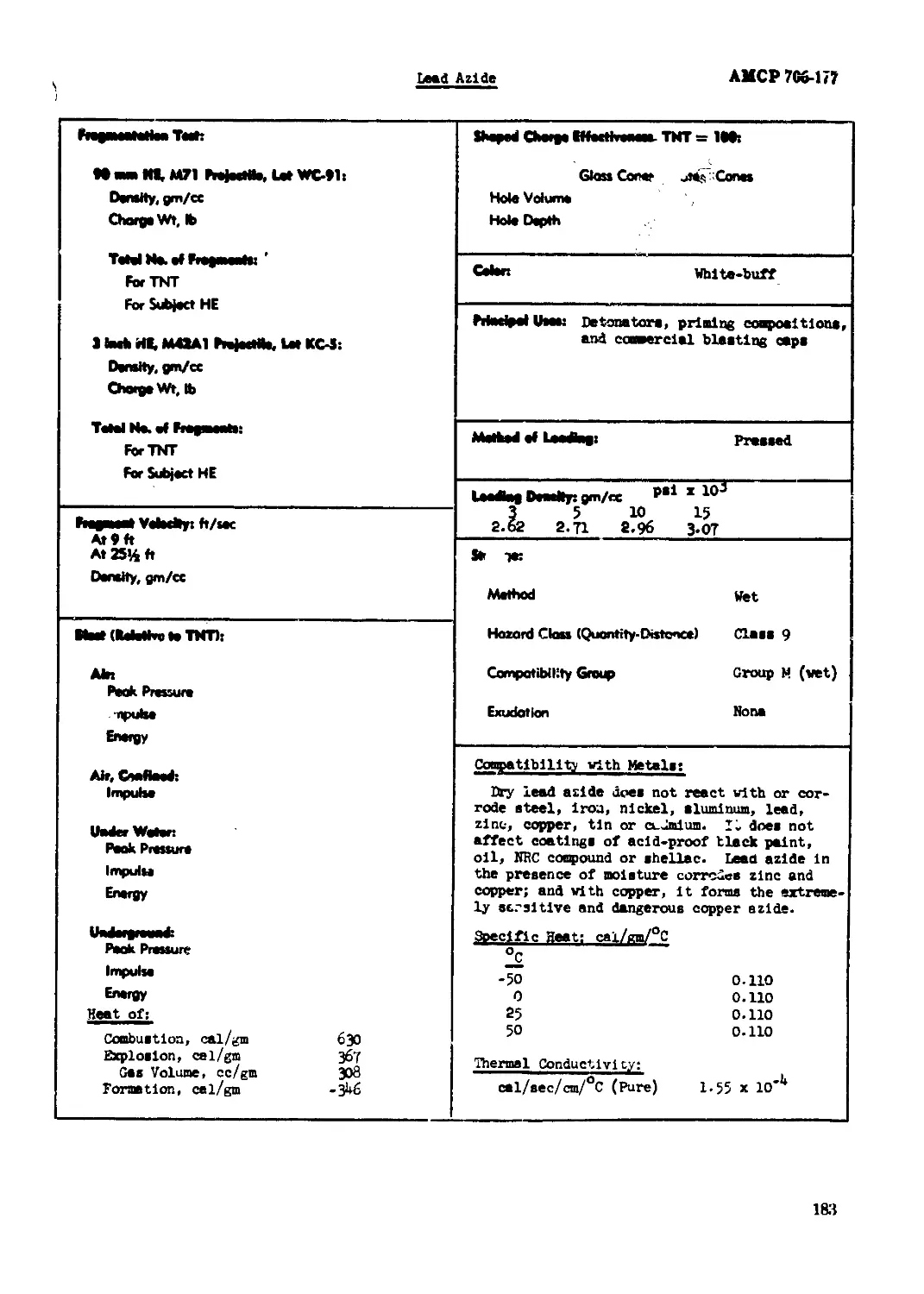
Lead Aside............................................................ -.........182
Lead 2,4-Dinitroresorclnate (LUiF.)...............................................187
Lead 4,6-Dlnltroresocrlnol Basic (LDNR Basic).....................................190
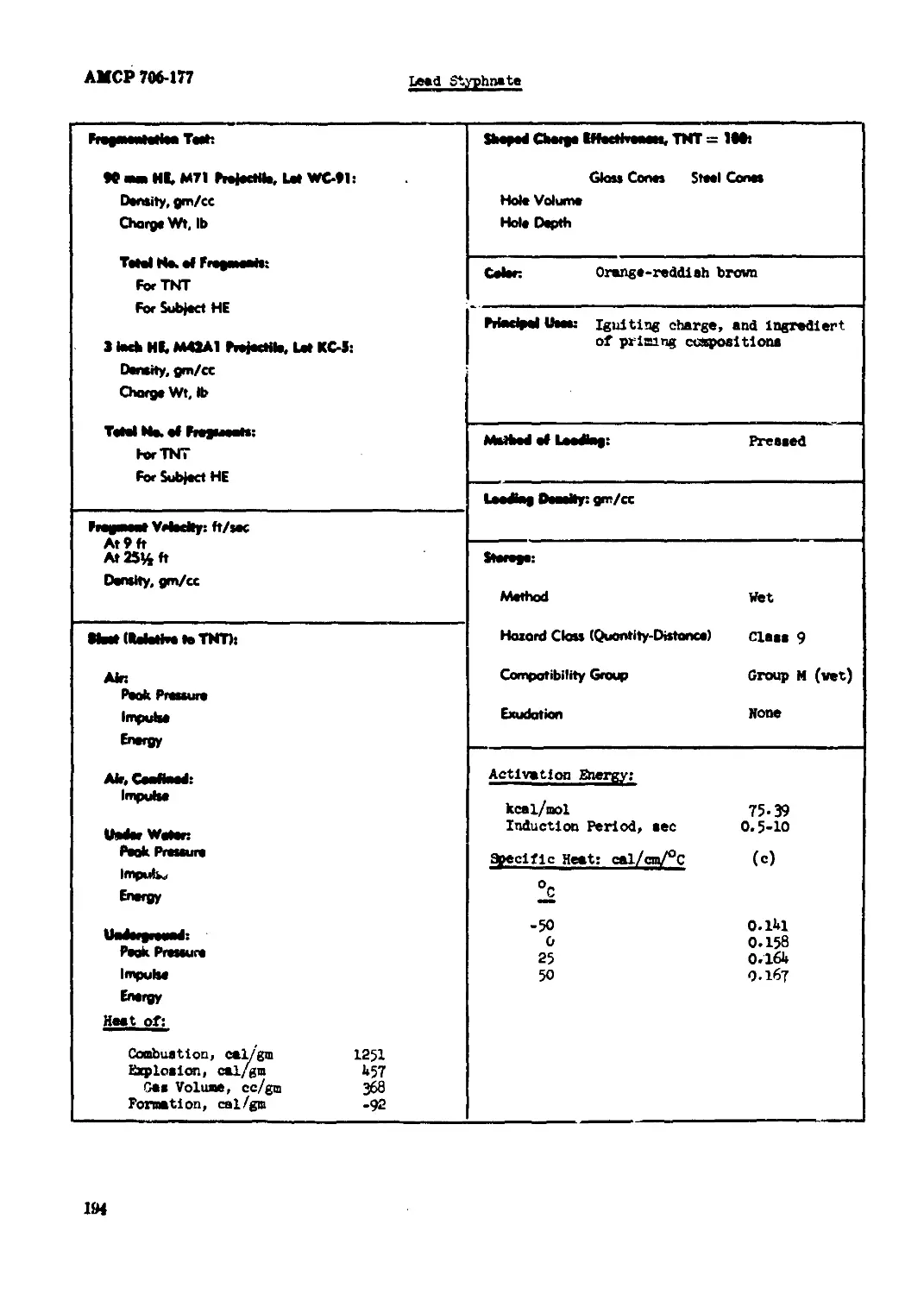
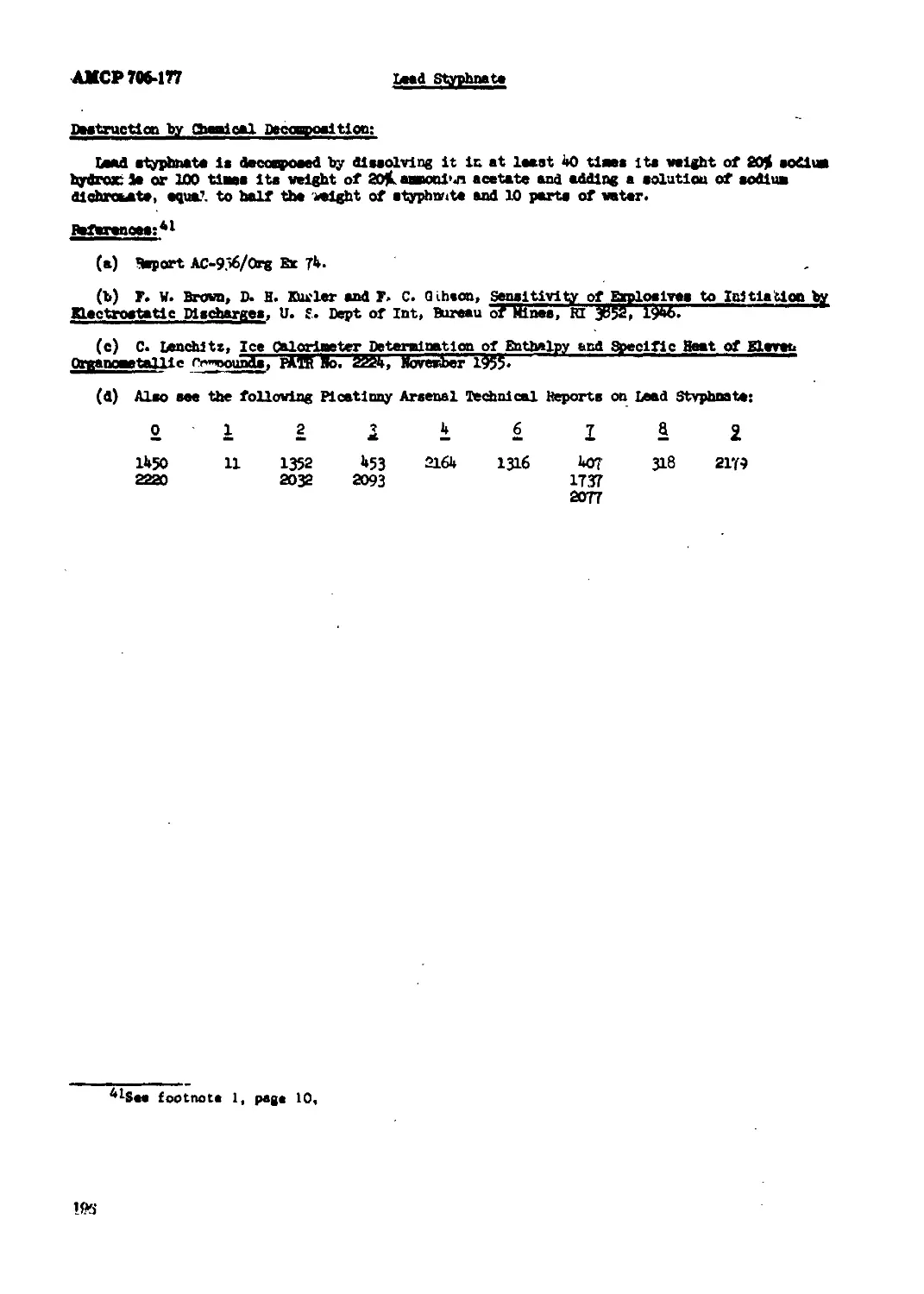
Lead Styphnate.................................................................. 193
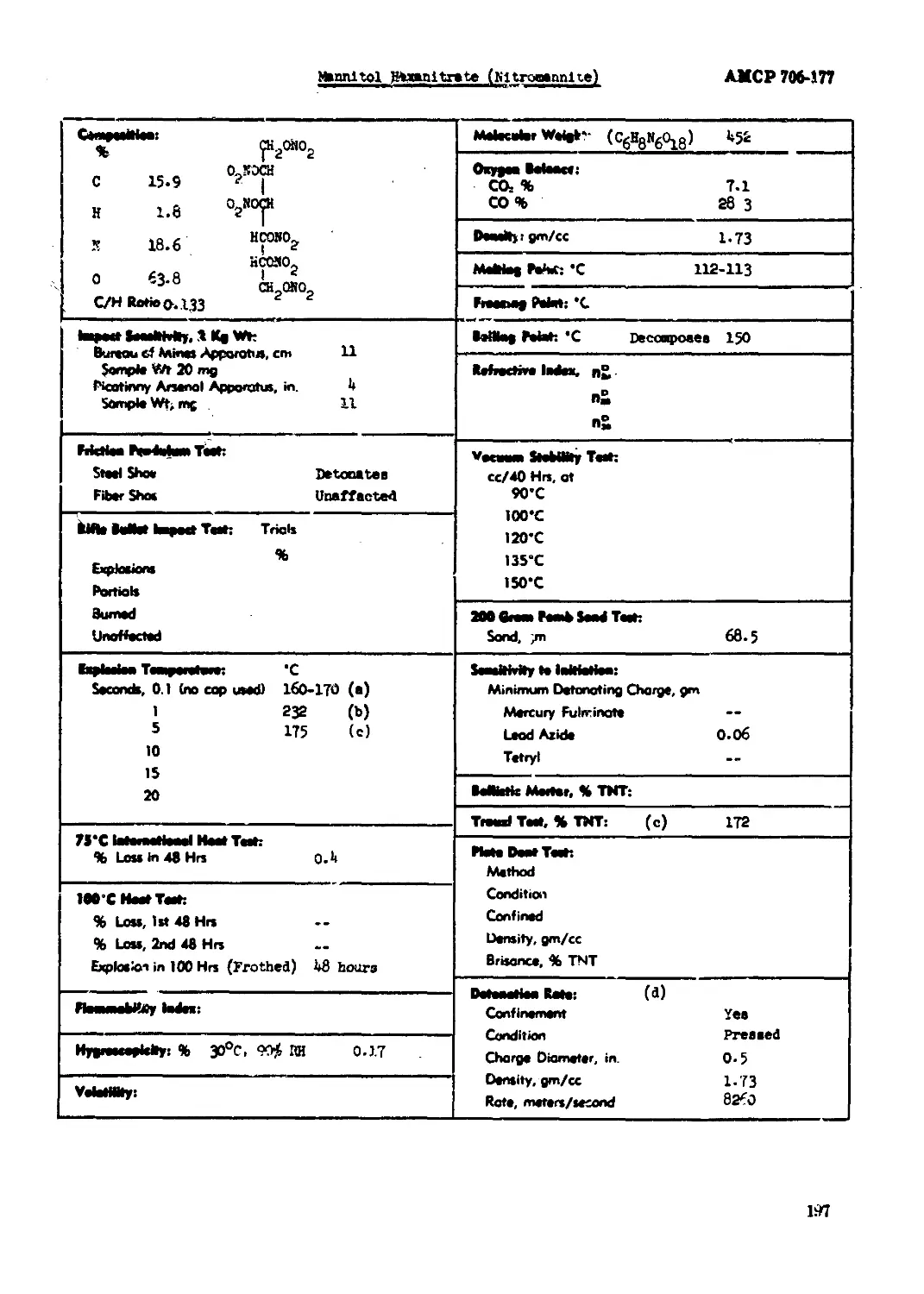
Mannitol Hexanitrate (Nltroaannlte)...............................................197
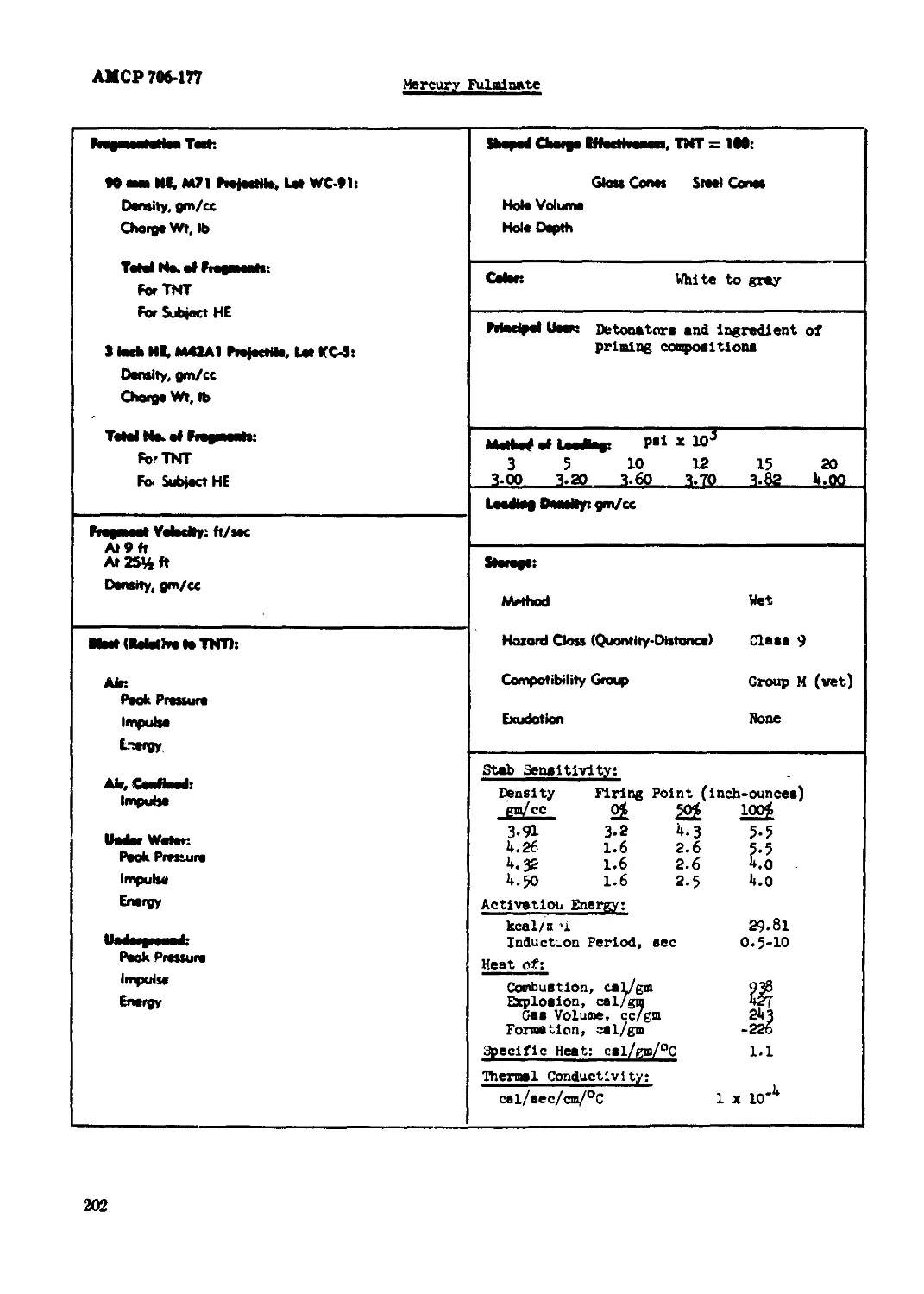
Mercury Fulainate.................................................................201
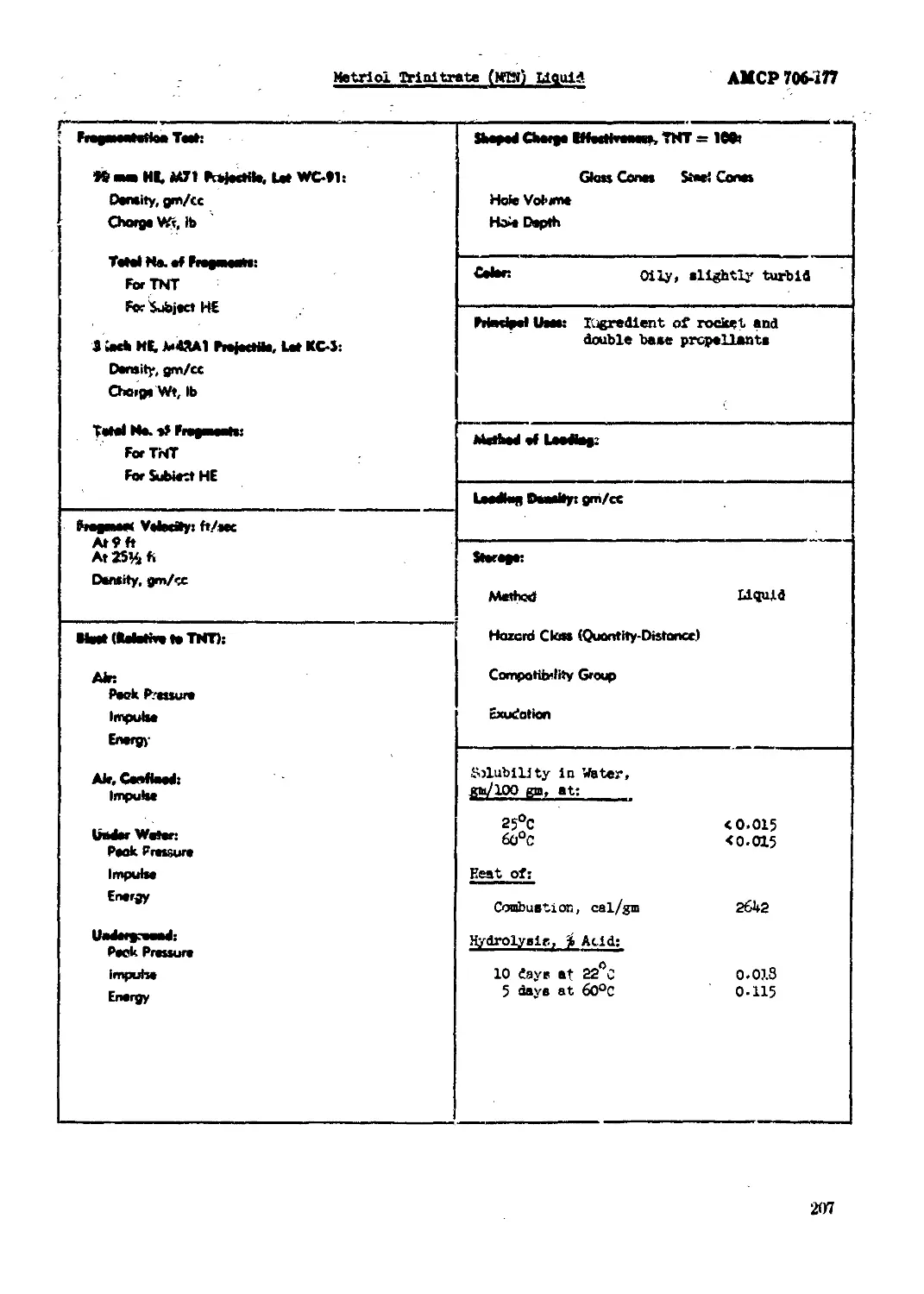
Metrlol Trinitrate (MTN) Liquid (or Trlnethylolethane Trinitrate).................206
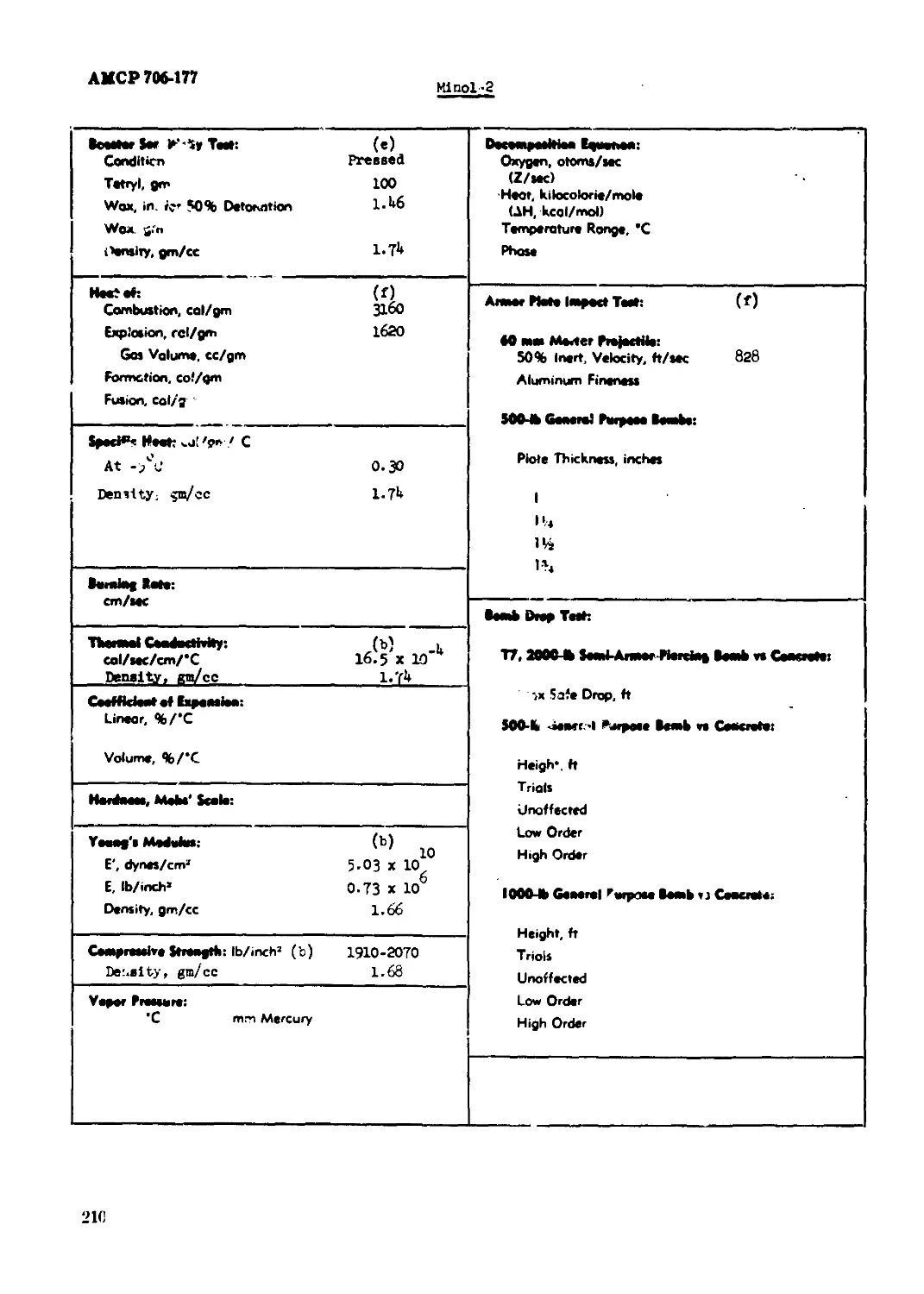
Minol-2...........................................................................209
MOX-1............................................................................ 213
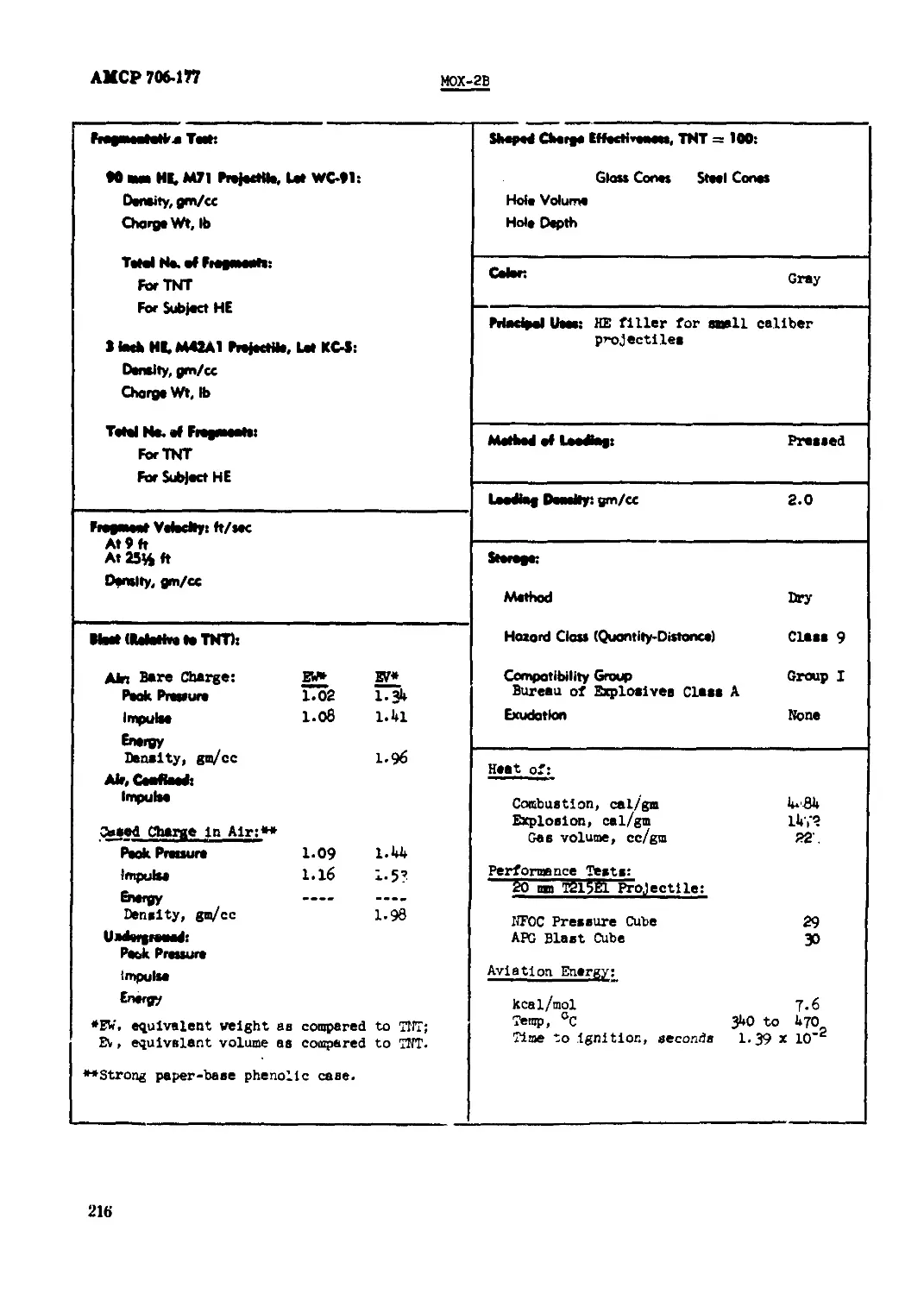
MOX-2B............................................................................215
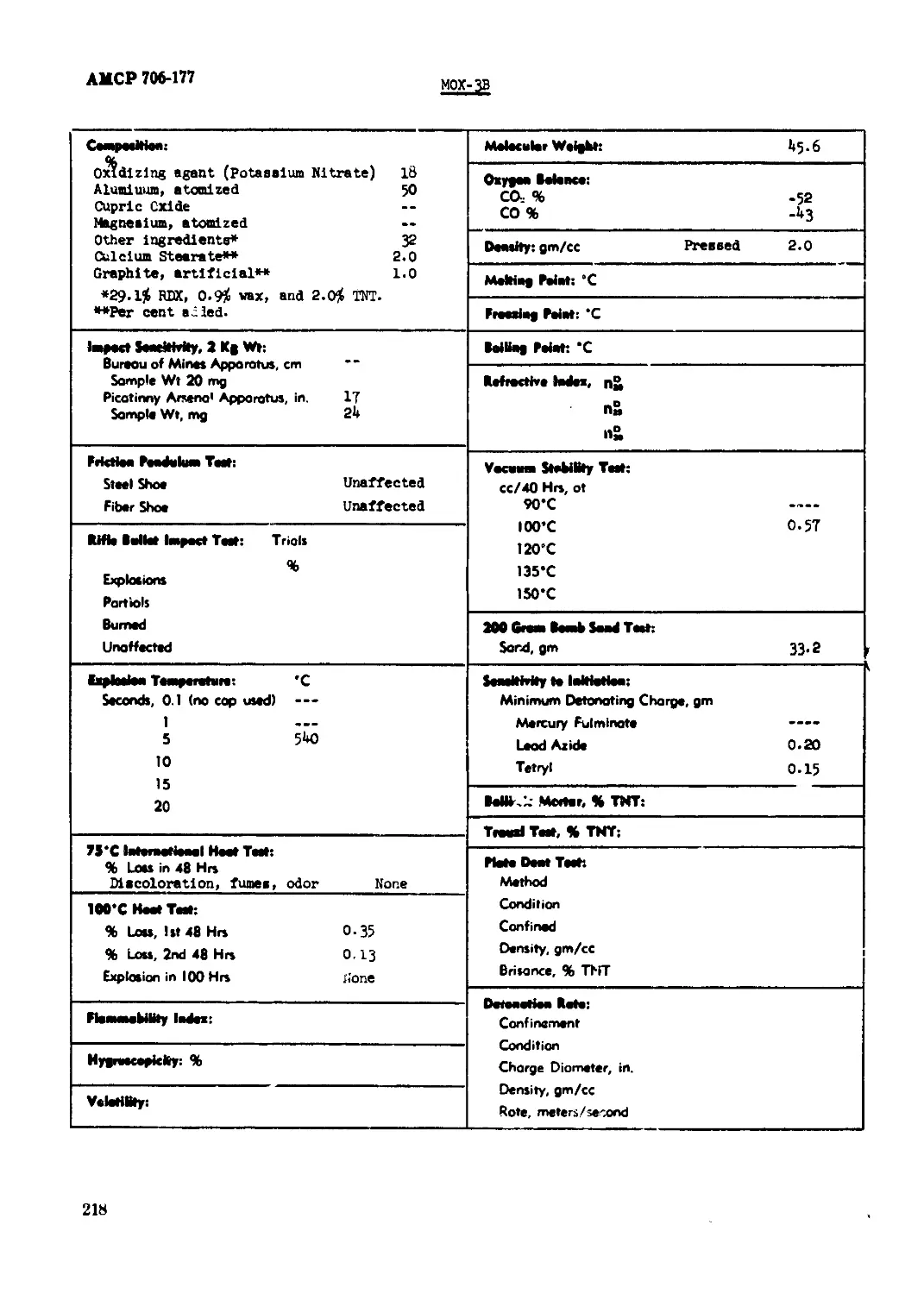
MOX-3B........................................................................ 218
MOX-4B... ...................................................................... 220
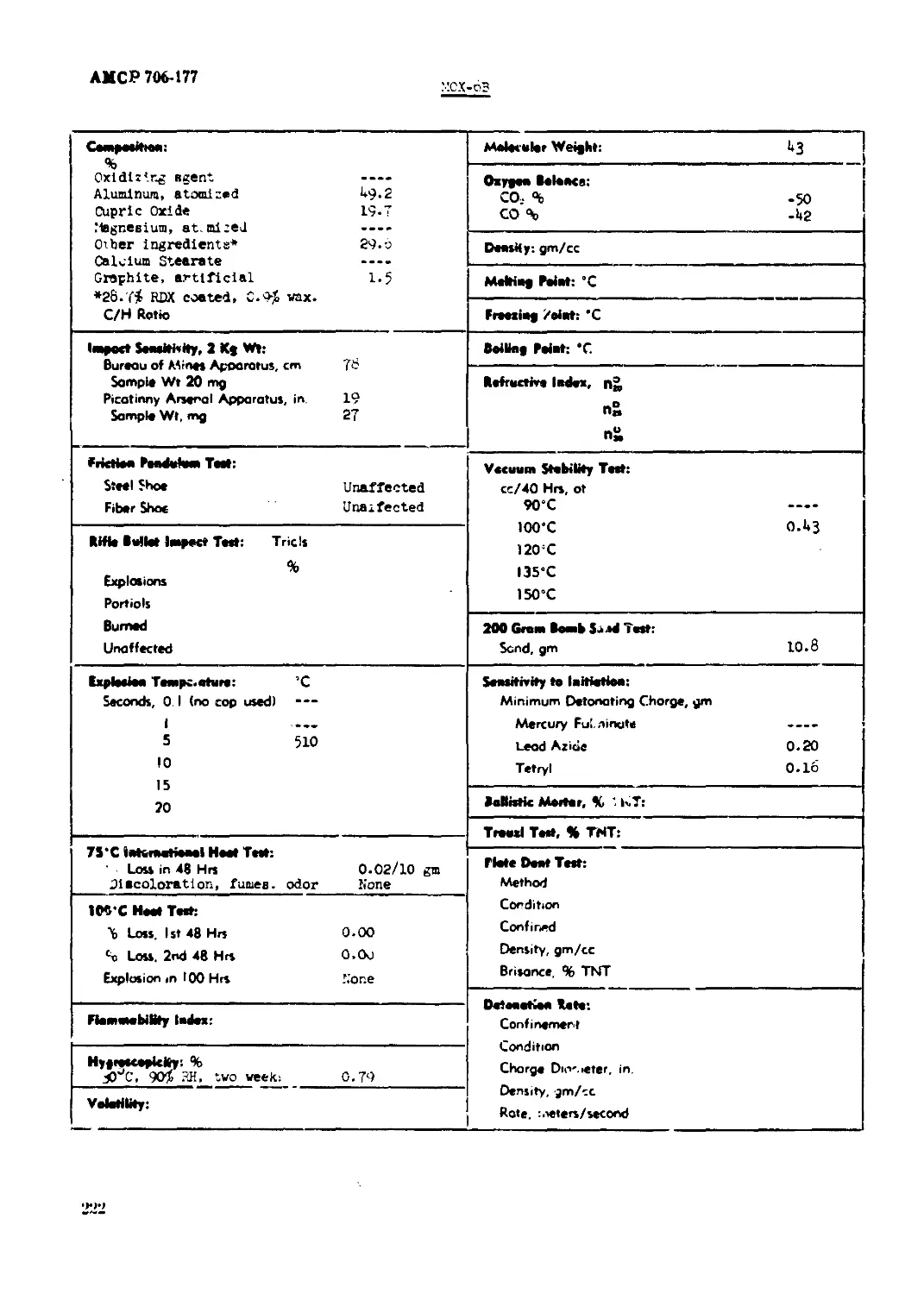
MOX-6B......................................"................................... ..222
Nitrocellulose. 12.61 N (NC).................................................... 226
Nitrocellulose, 13.45’ N (NC).....................................................227
Nitrocellulose, 14.141 N (NC).....................................................228
ii
ЛКСР 706-177
TABLE OF CONTENTS (cont'd)
ftw
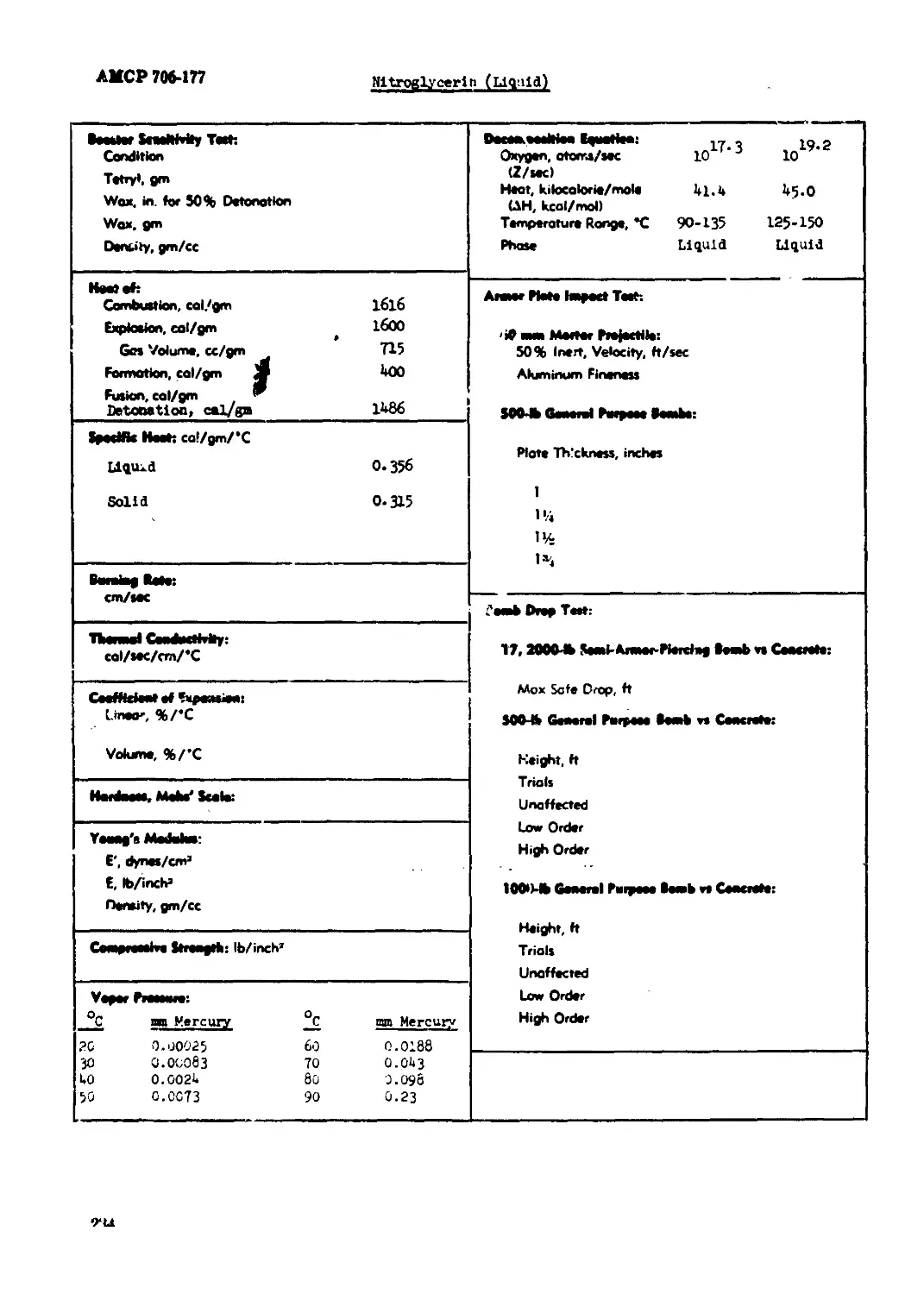
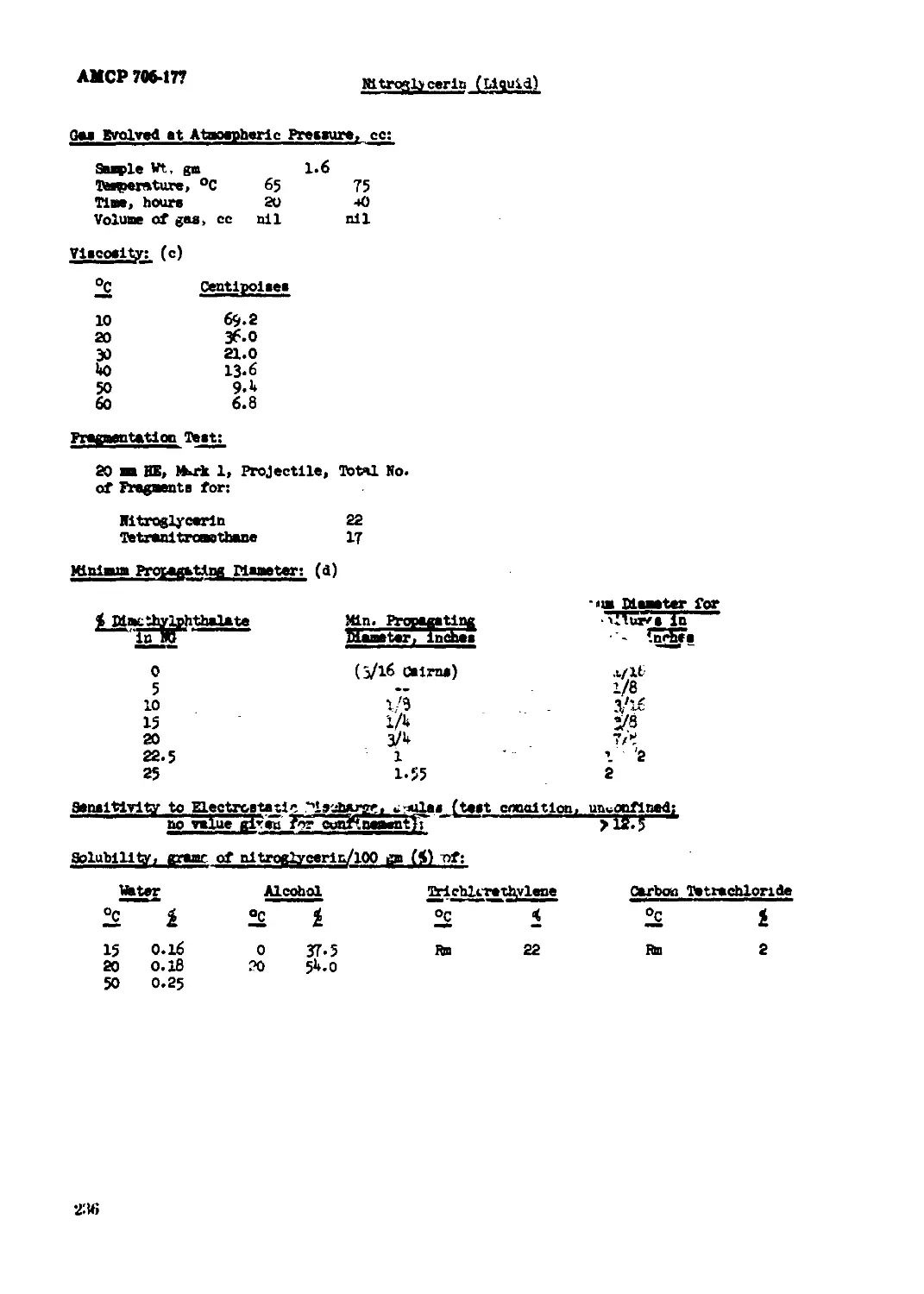
Pltroglycerin (Liquid).............................................................233
Ritroguanidine.................................................................... .. 239
Hitroiaobutylglycervl Trinitrate i NIBTN) Liquid)................................243
KitroaathMie—Stt: PLX Liquid
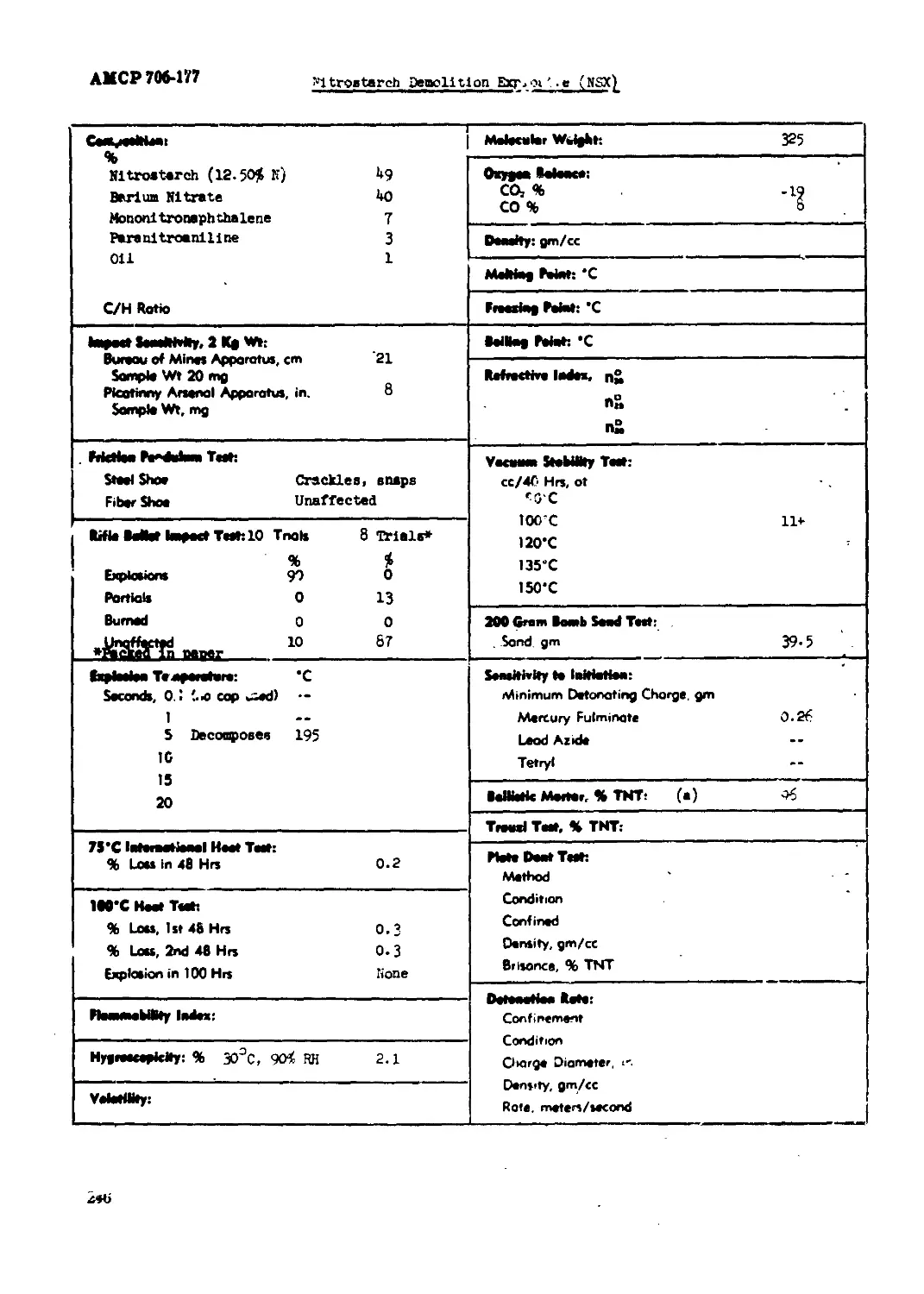
Nitroetnrch Oaaolltlcn S>iloalve (JSX)...........................................246
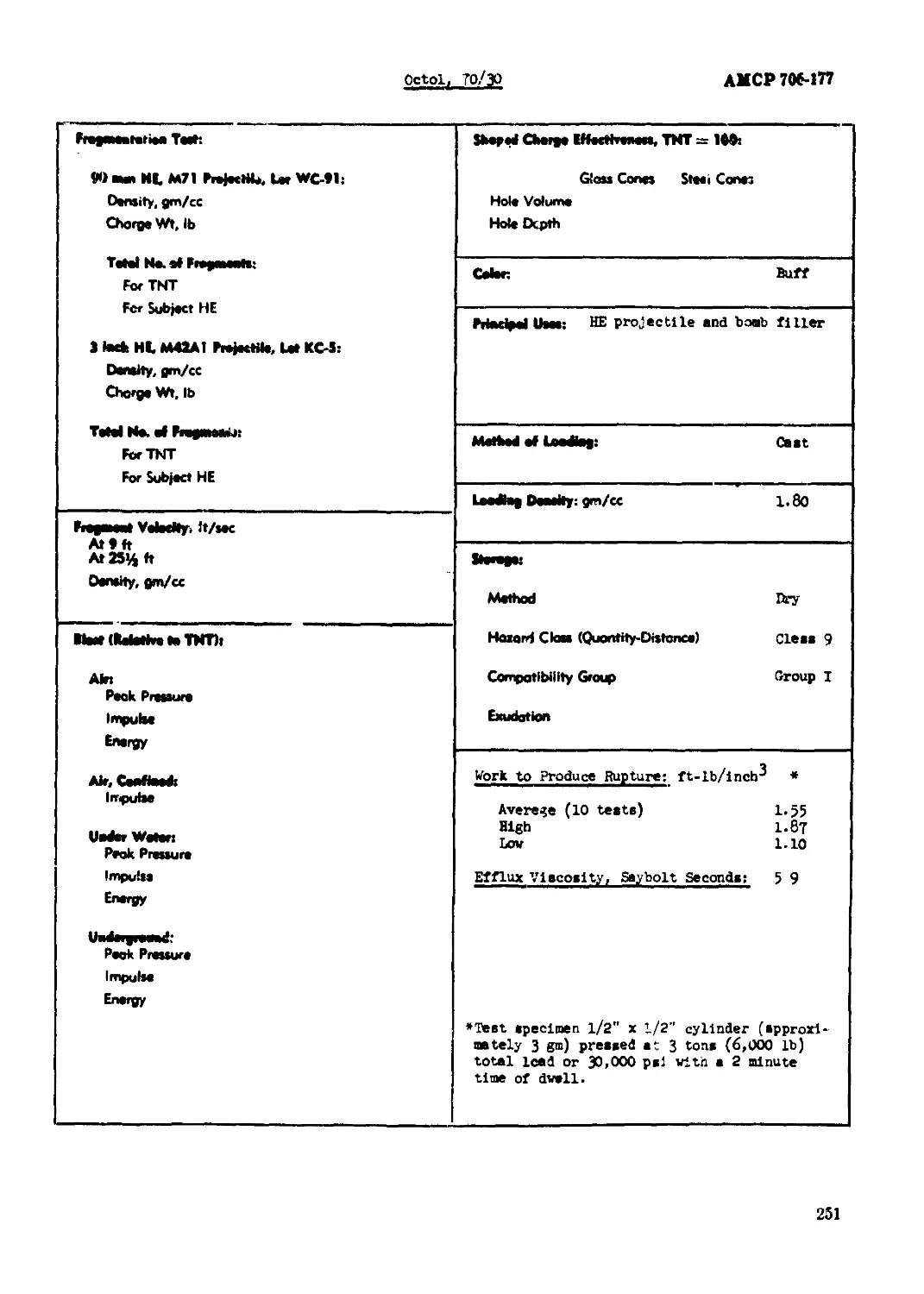
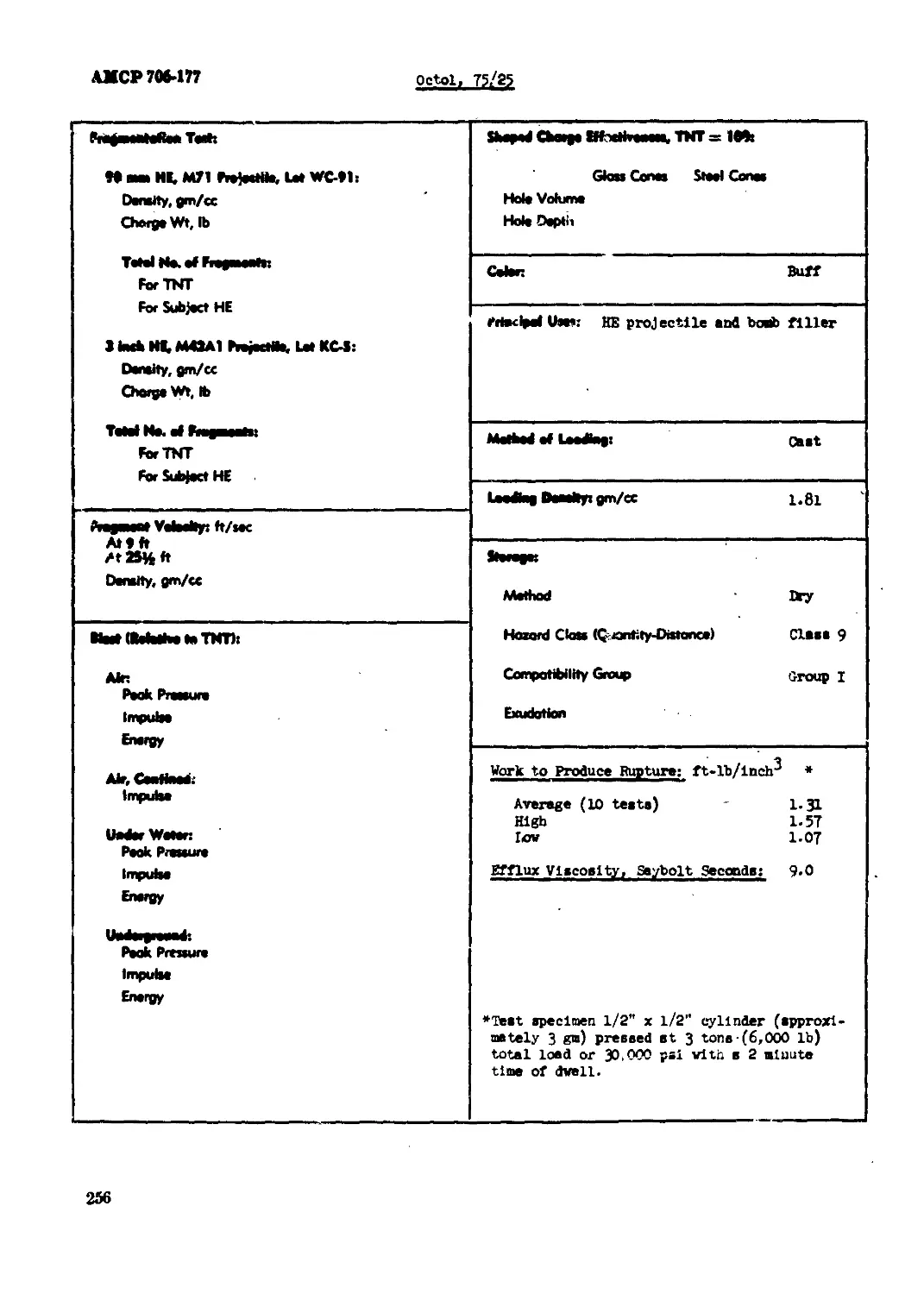
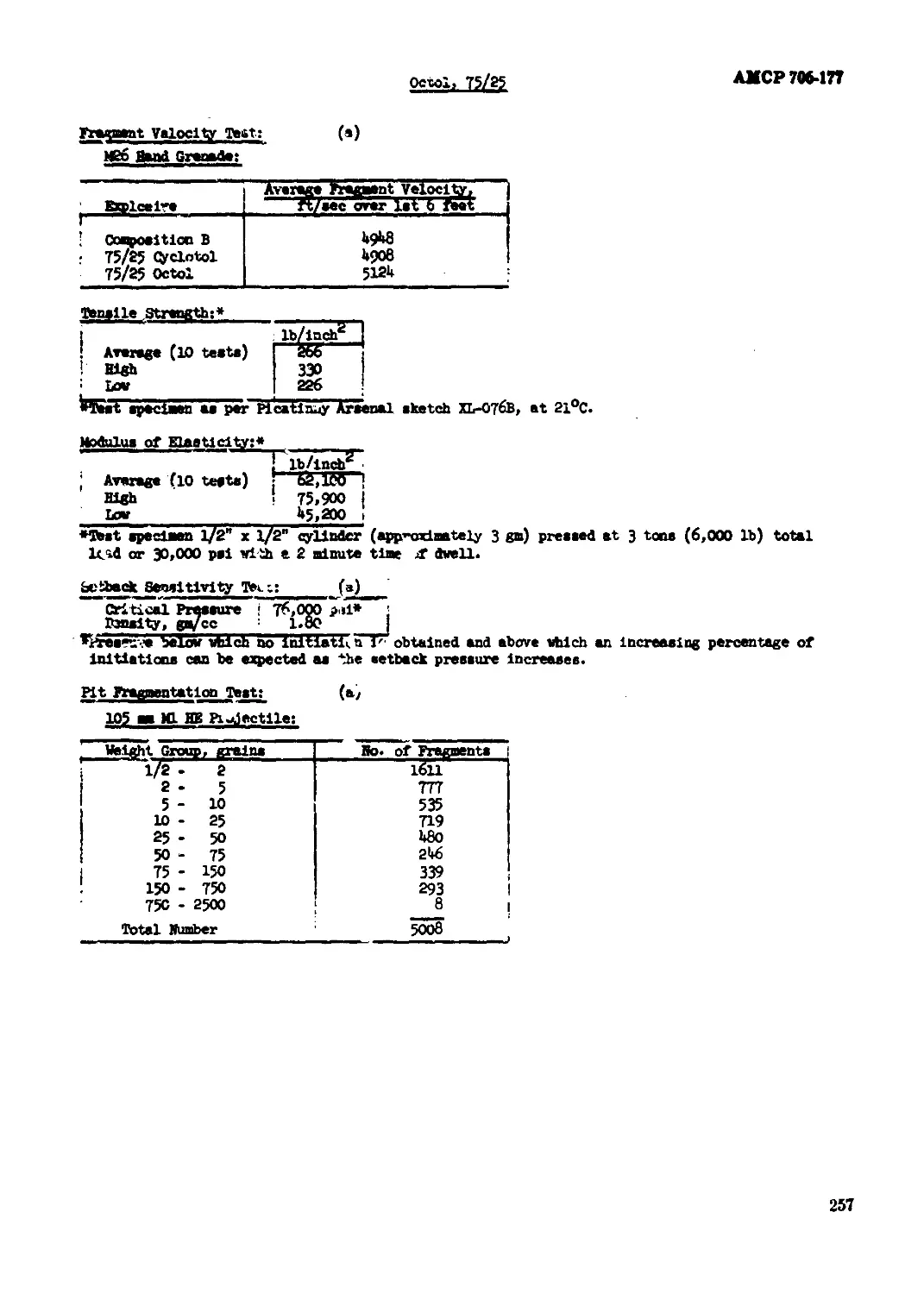
Octol, 70/30............л..........................................................249
Octol, 75/25...................................................................... 254
PB-RDX.............................................................................25Э
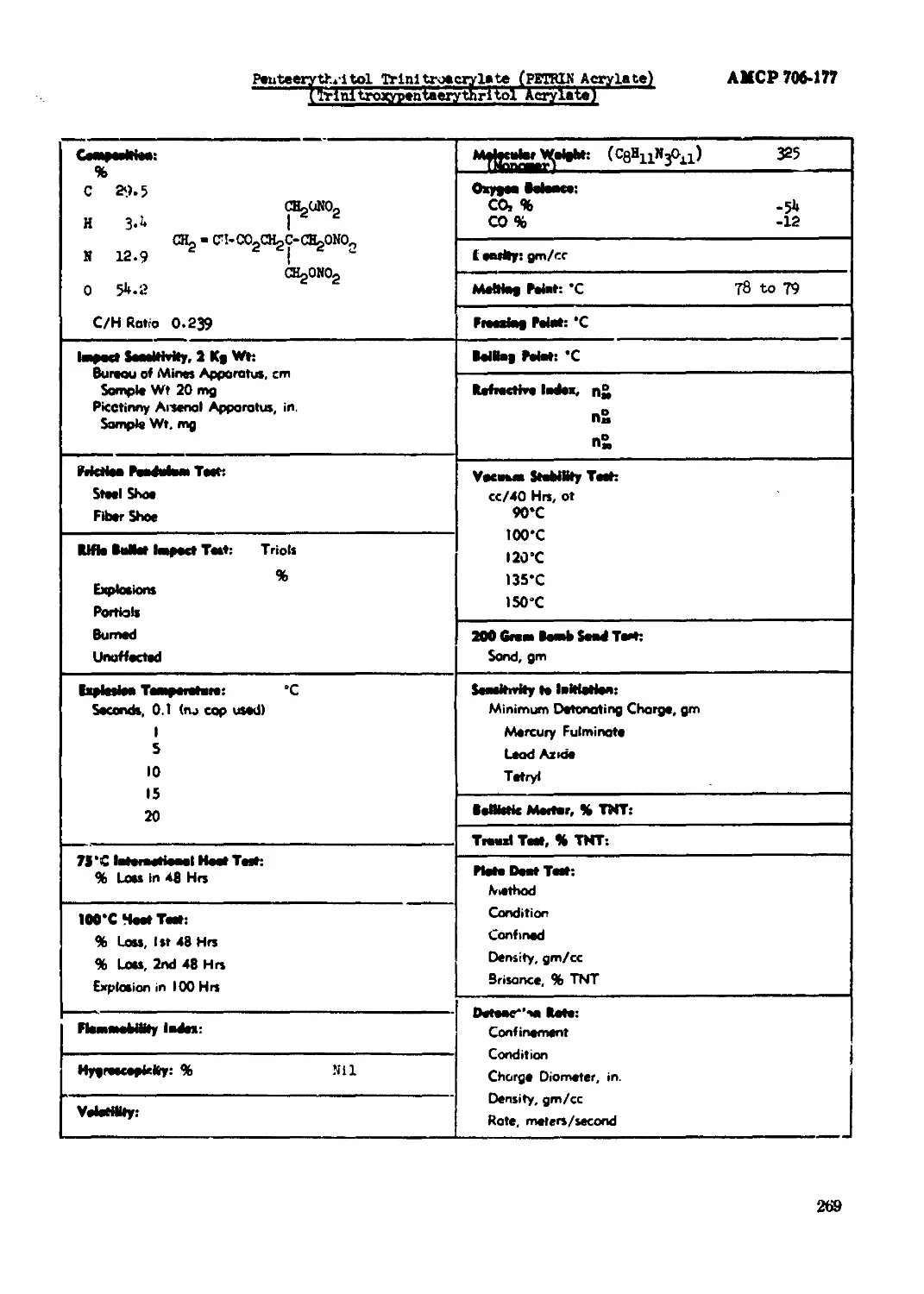
Pantaerythrltol Trinitrate (PETRIN)................................................265
Partaerythrltox Trinitroacryla'.e (.PETRIN Acrylate) (Trlnitroxypentaerythrl tol
Acrylate)...................................................................269
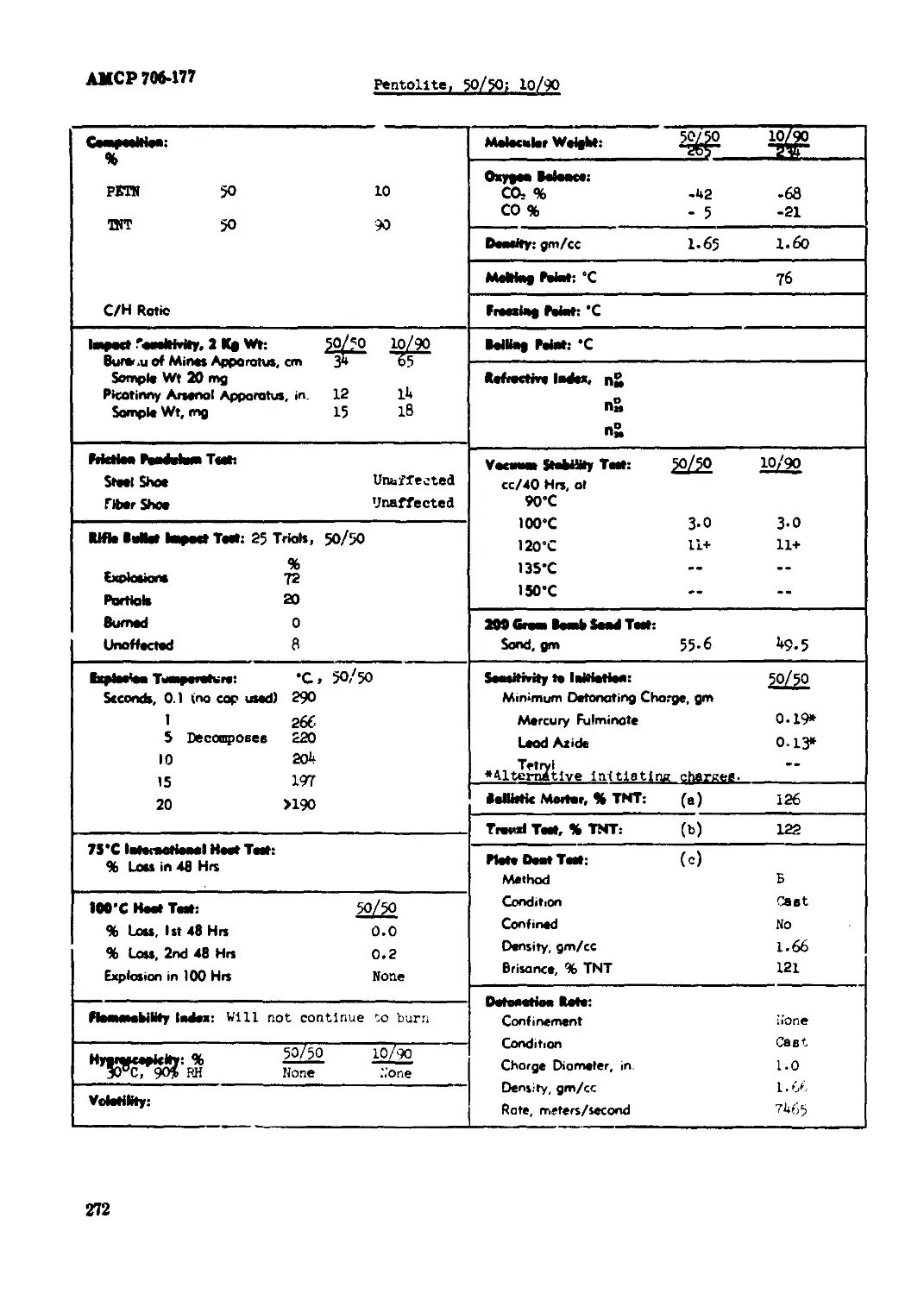
Pentolite, 50/50; 10/90......................................................... 272
PETR (Pantaerythcitol Tetraritrate)................................................276
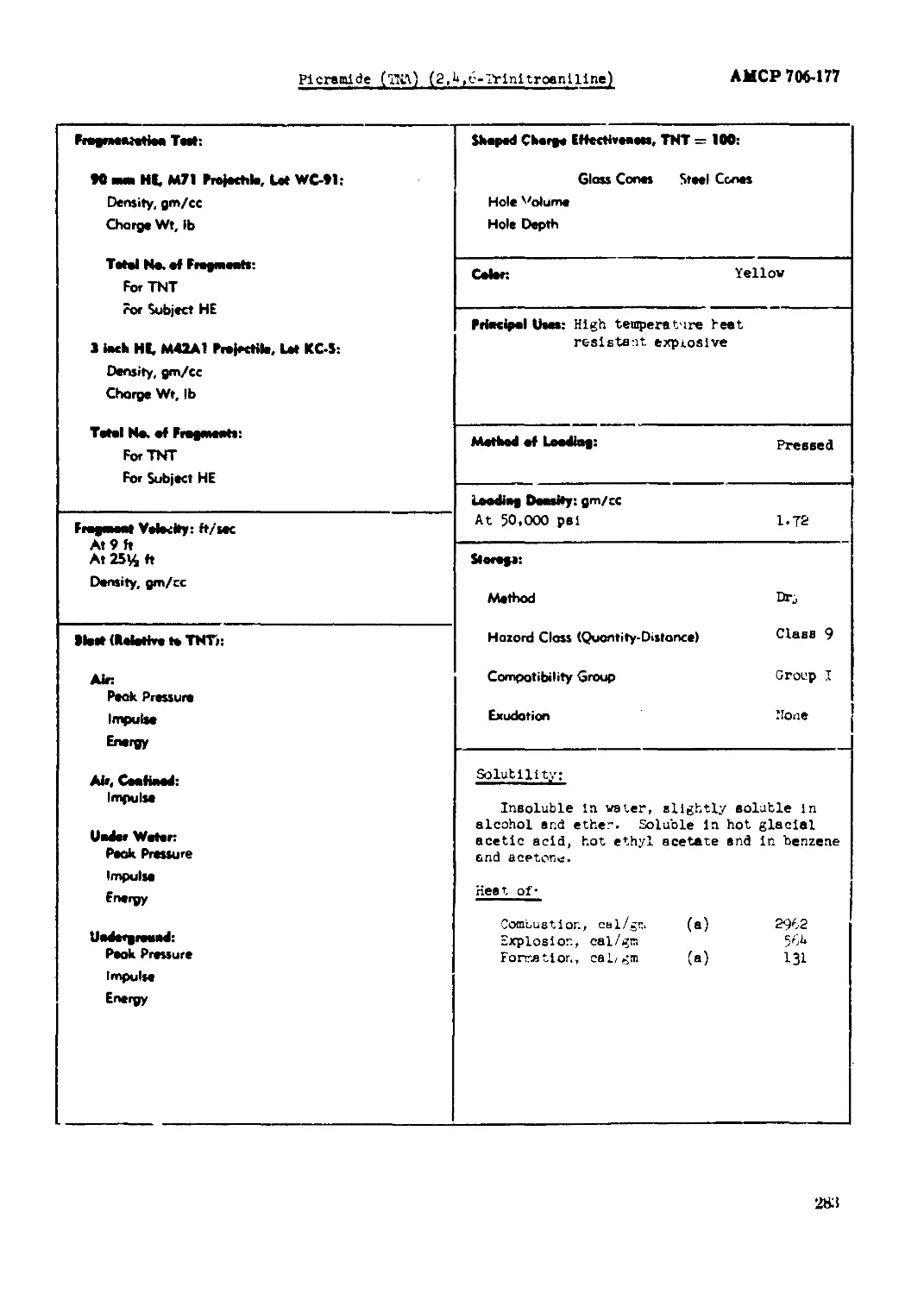
Picnaide (TEA) (2,4,6-Trinitroanlllne)........................................... 282
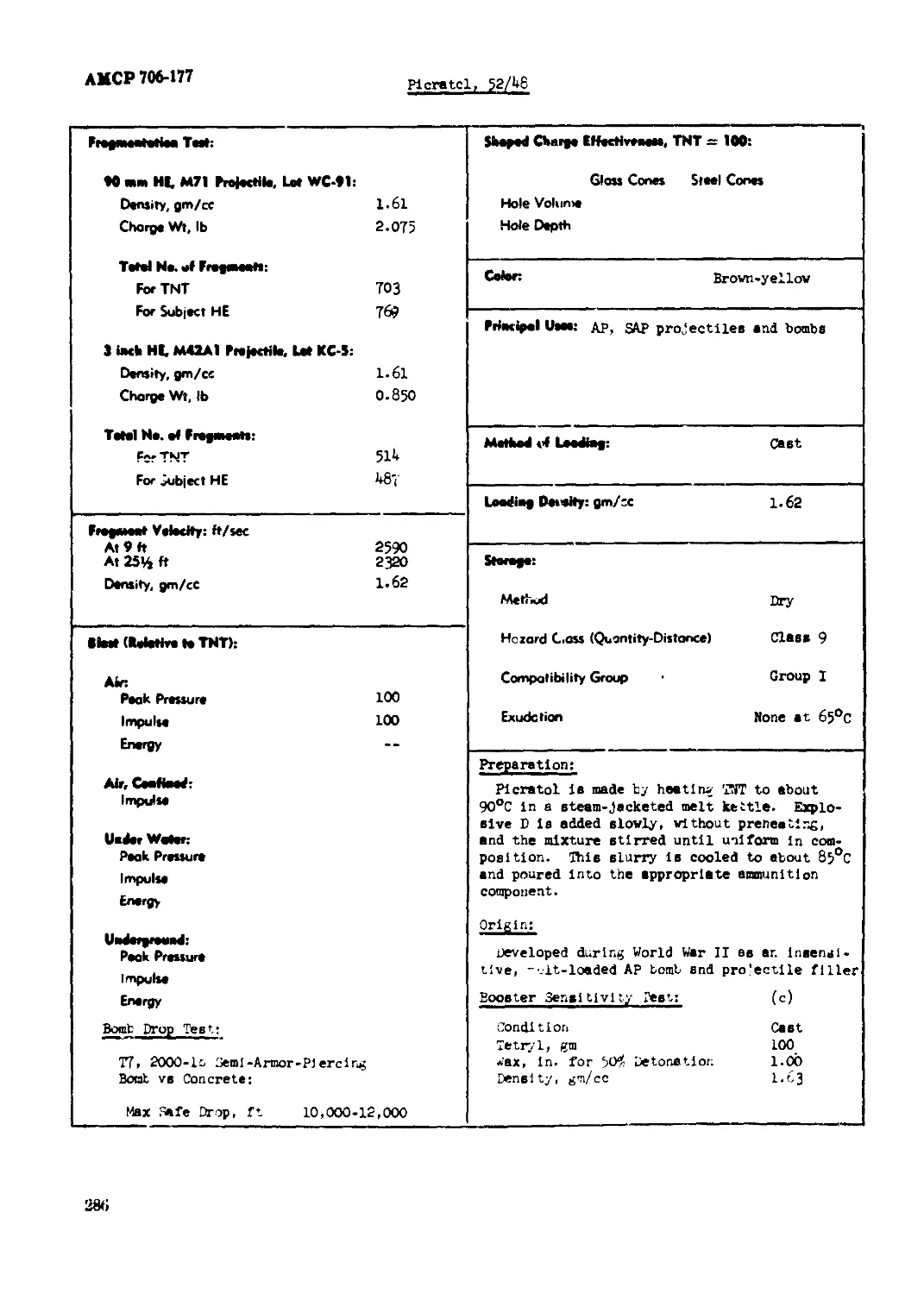
Picratol, 52/48................................................................ 285
Picric Acid....................................................................... 288
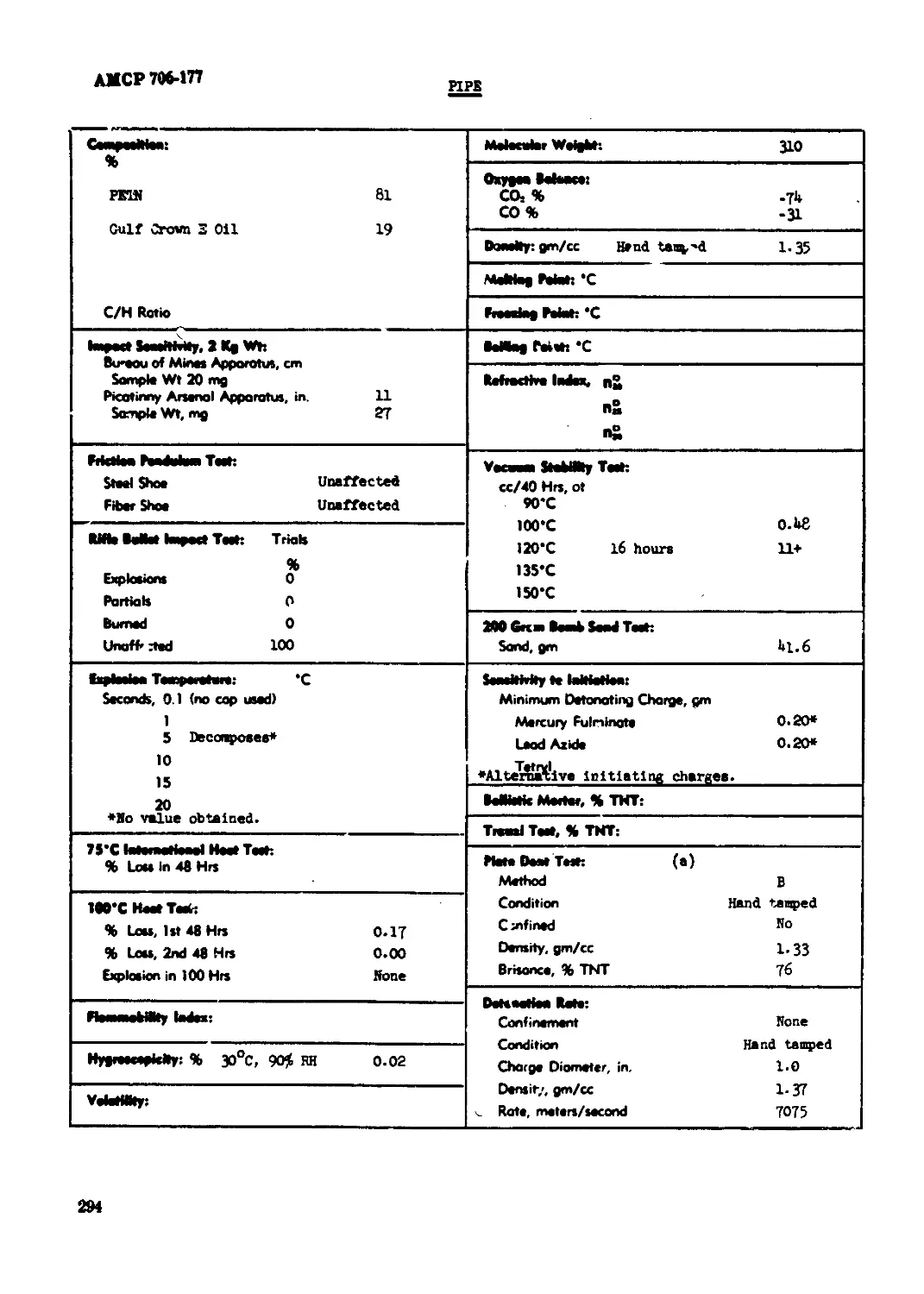
P’»F...............................................................................294
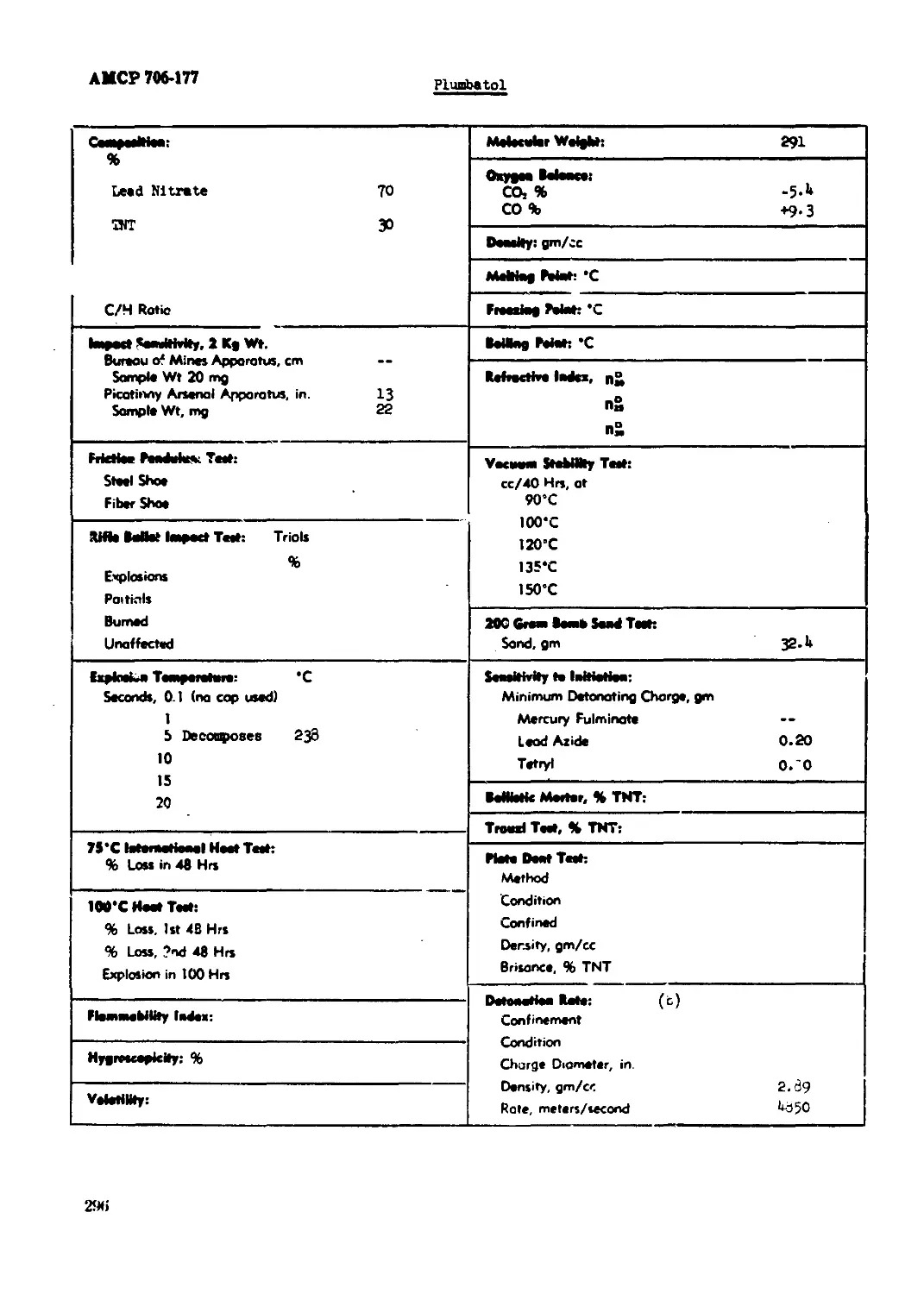
Pluri>atol..,,.x...................................................................296
PLX (Litui43....;..................................................................298
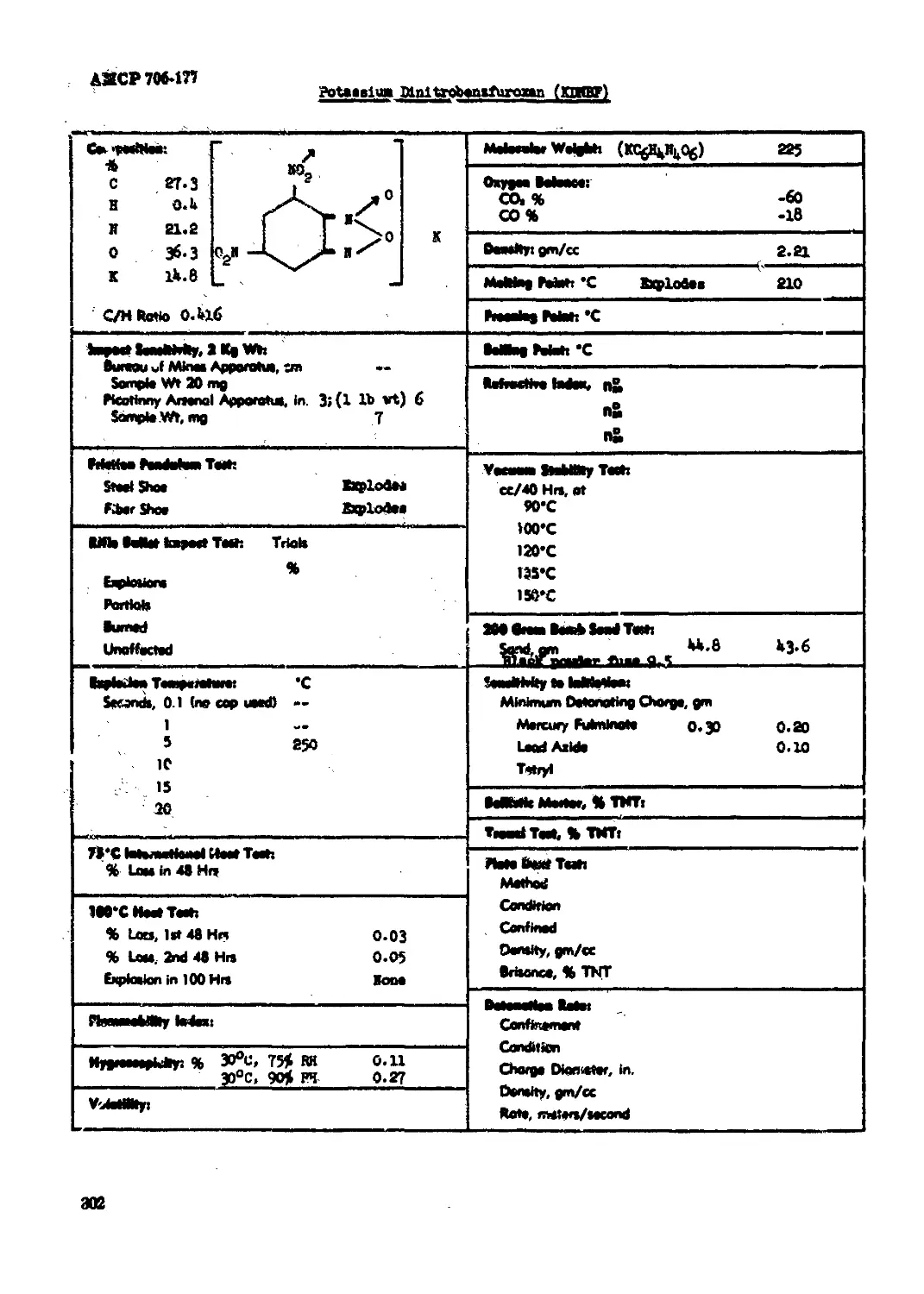
Potaaaiua Di».lirob«.zfuroxan (KDNBF).............................................302
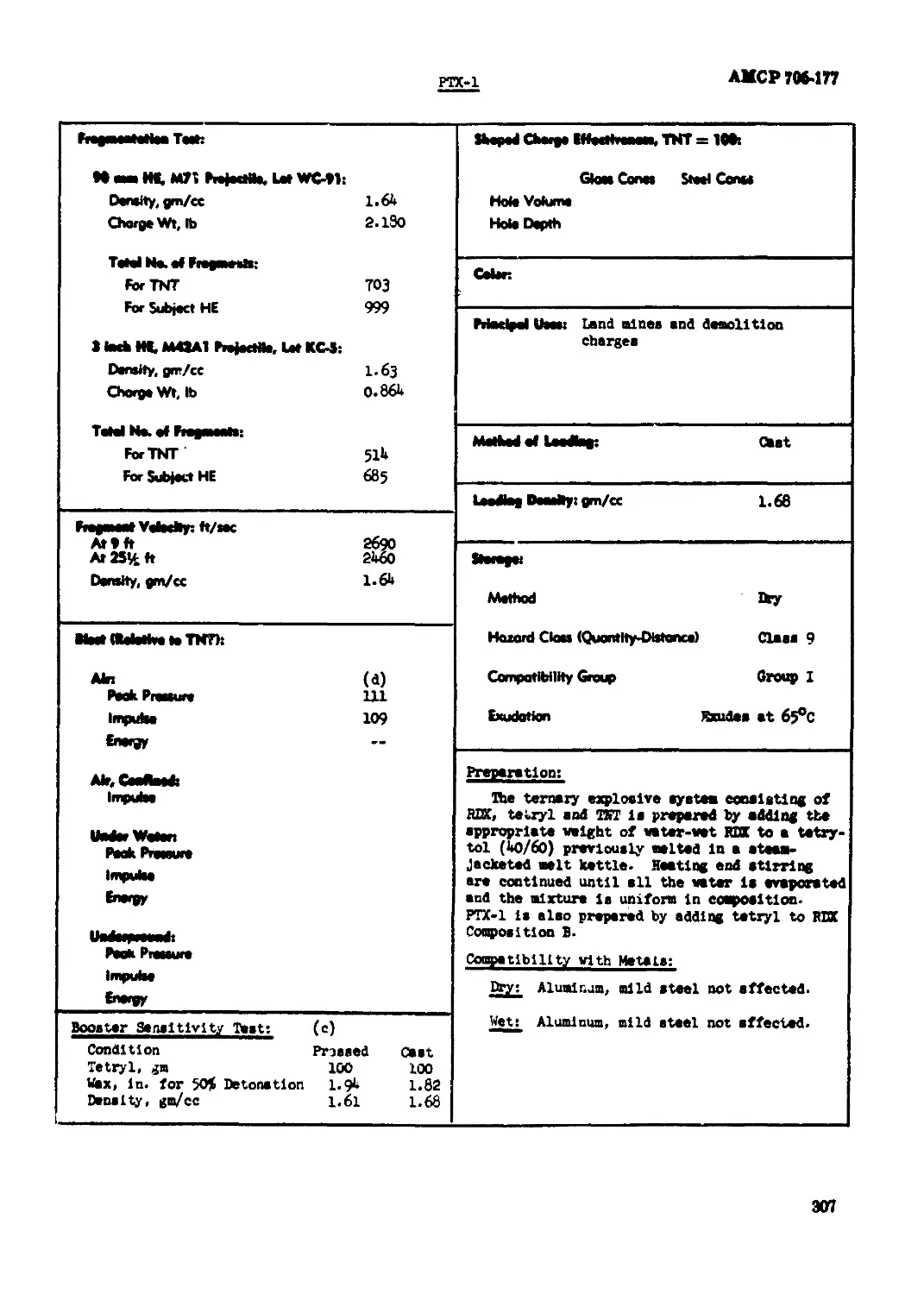
PTX-1............................................................................ 306
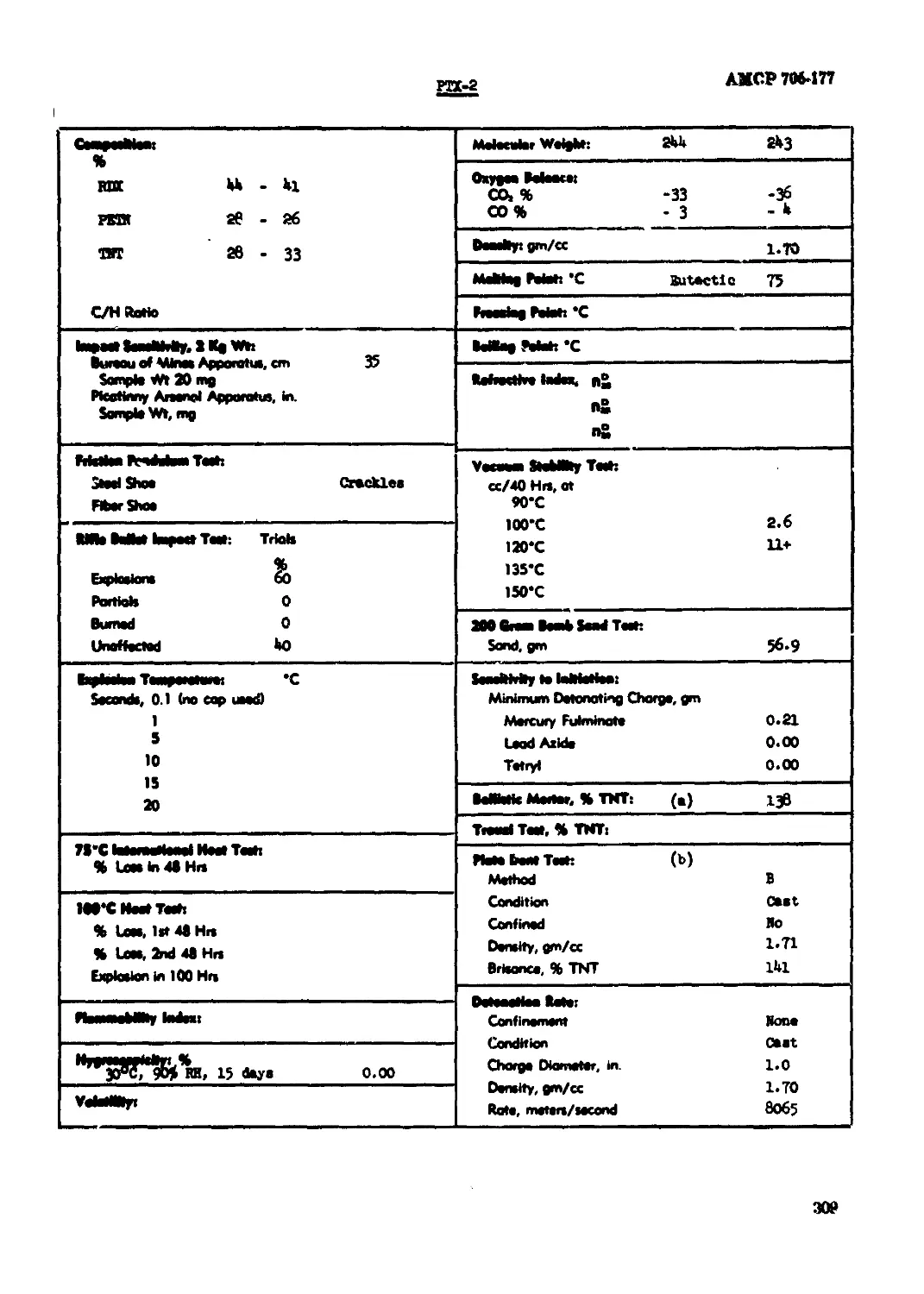
PTX-2..............................................................................309
PVA-4..............................................................................312
PVR (Polyvinyl Nltr-te)............................................................315
RDX—o’ea; Cyclonite- Coapoaltiona A-3: В; C-2; C-3; .'-4
RIPE.............................................................................. 318
Silver Azide..................................................................... 320
Теtrecent...................................................................... 324
Tetranitrocarbezole (TRC)......................................................... 327
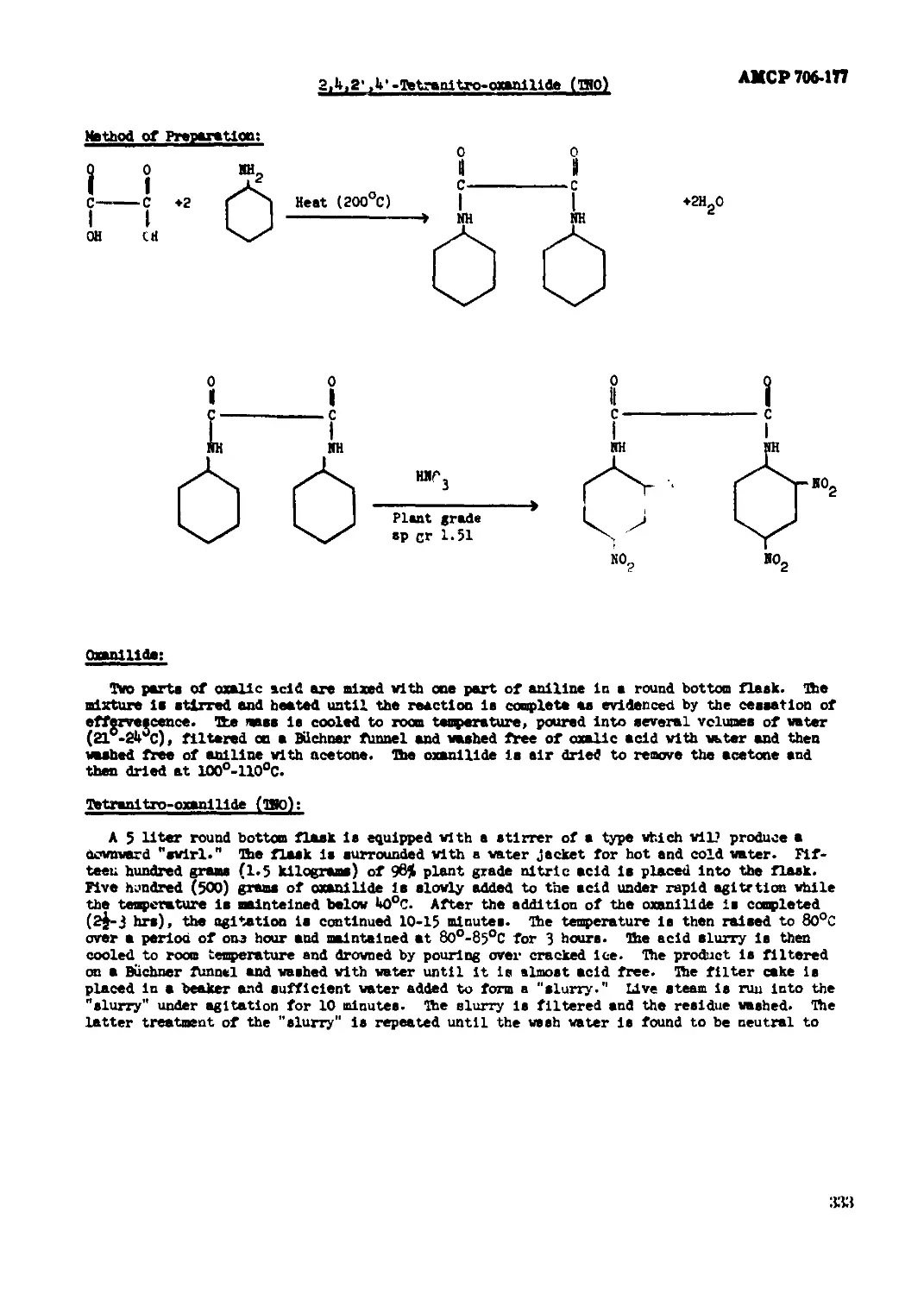
2,4,2',4'-Tetranitro-oxanlllde (TRO)...............................................331
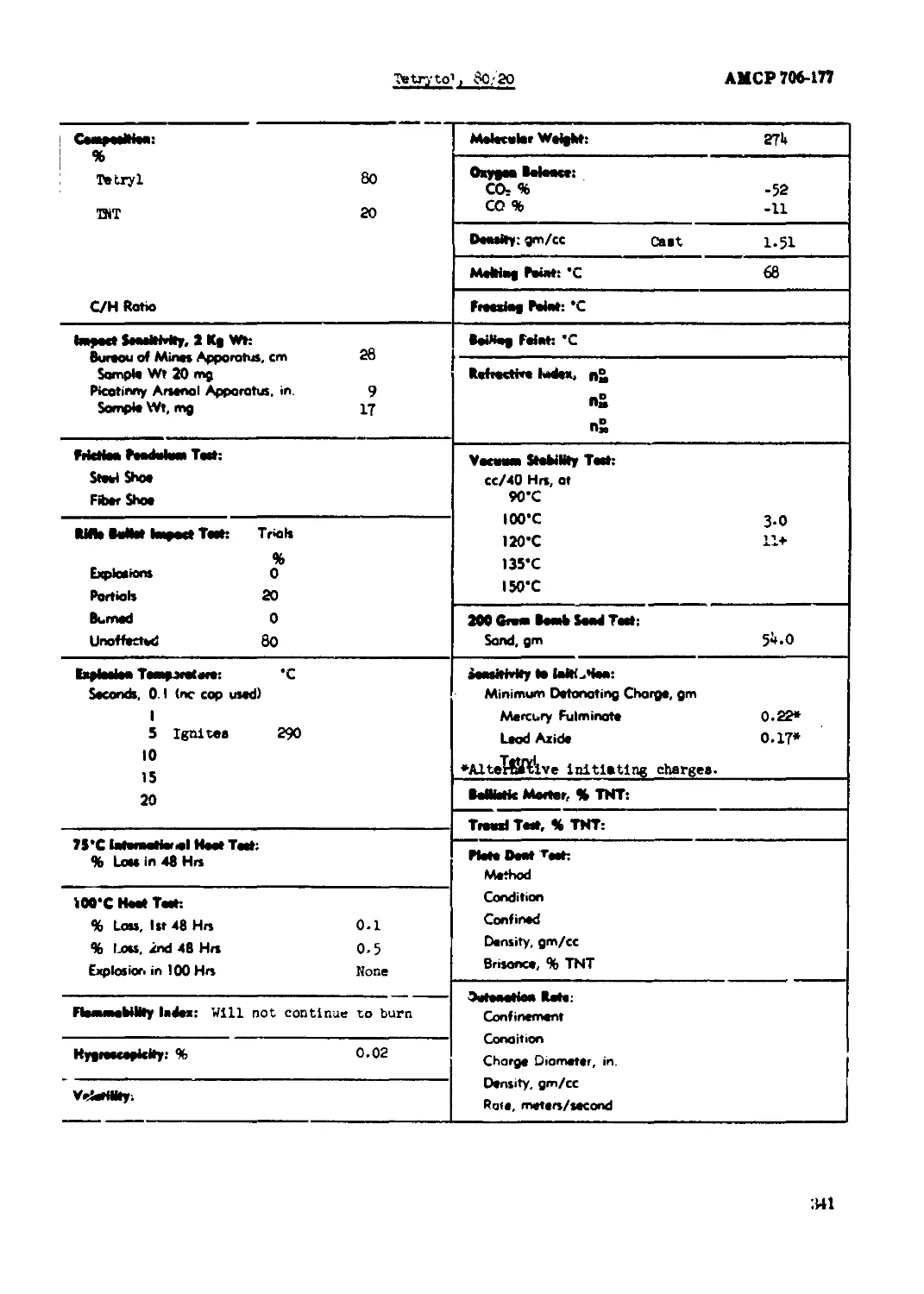
Tetryl......................................................................... 335
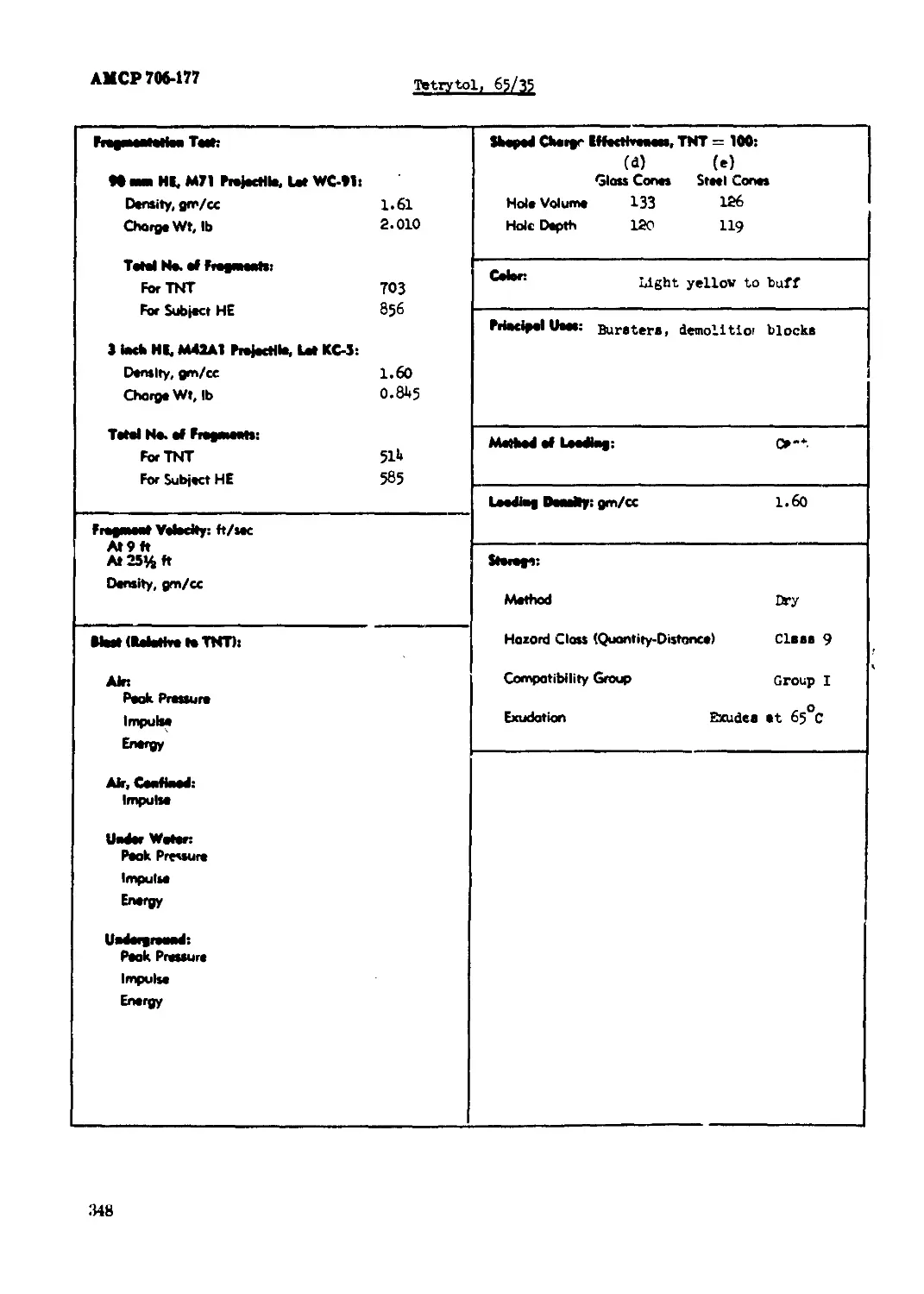
Tetrytol, 80/20.............................................................. 341
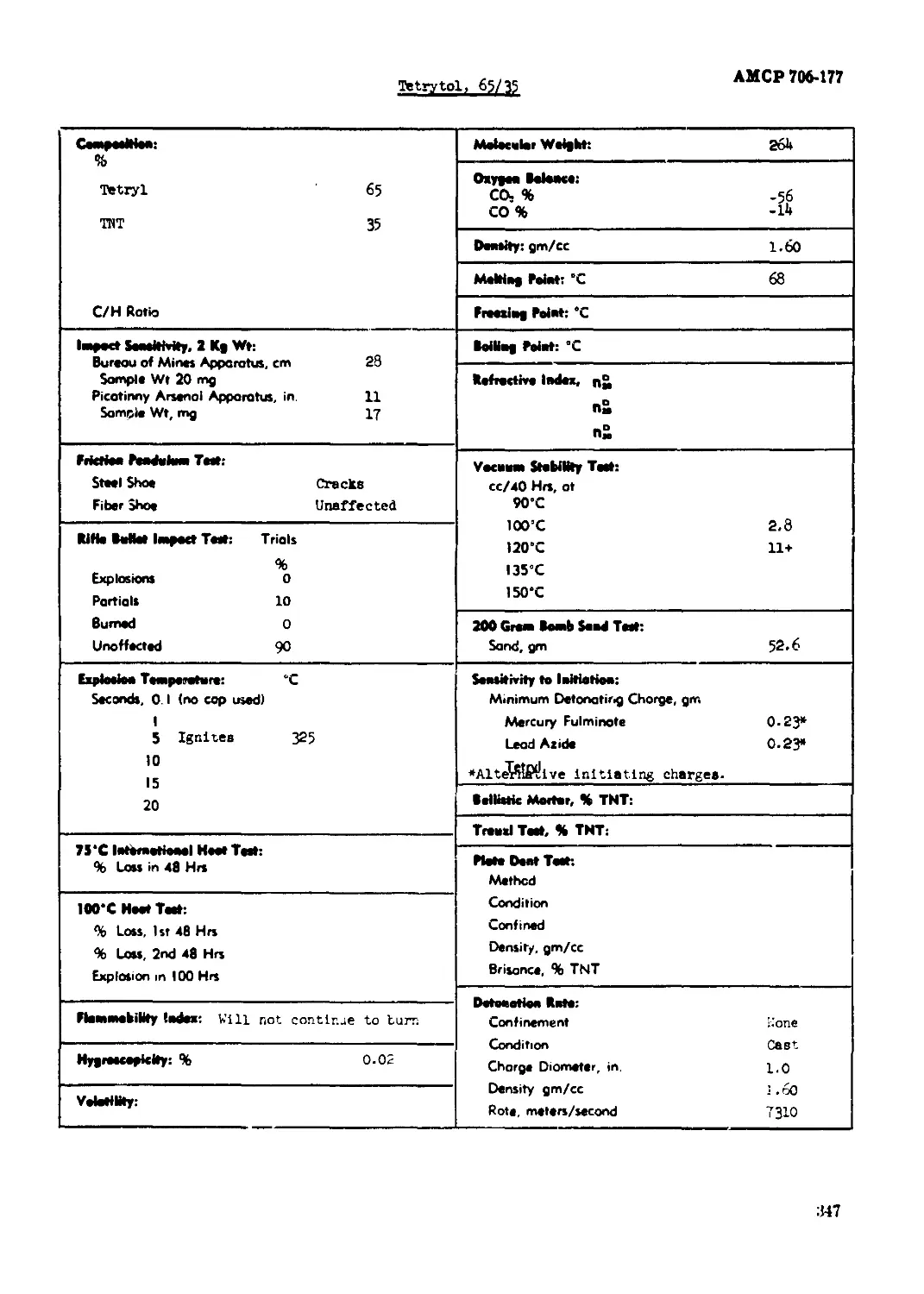
Tetrytol, :.V25................................................................ 343
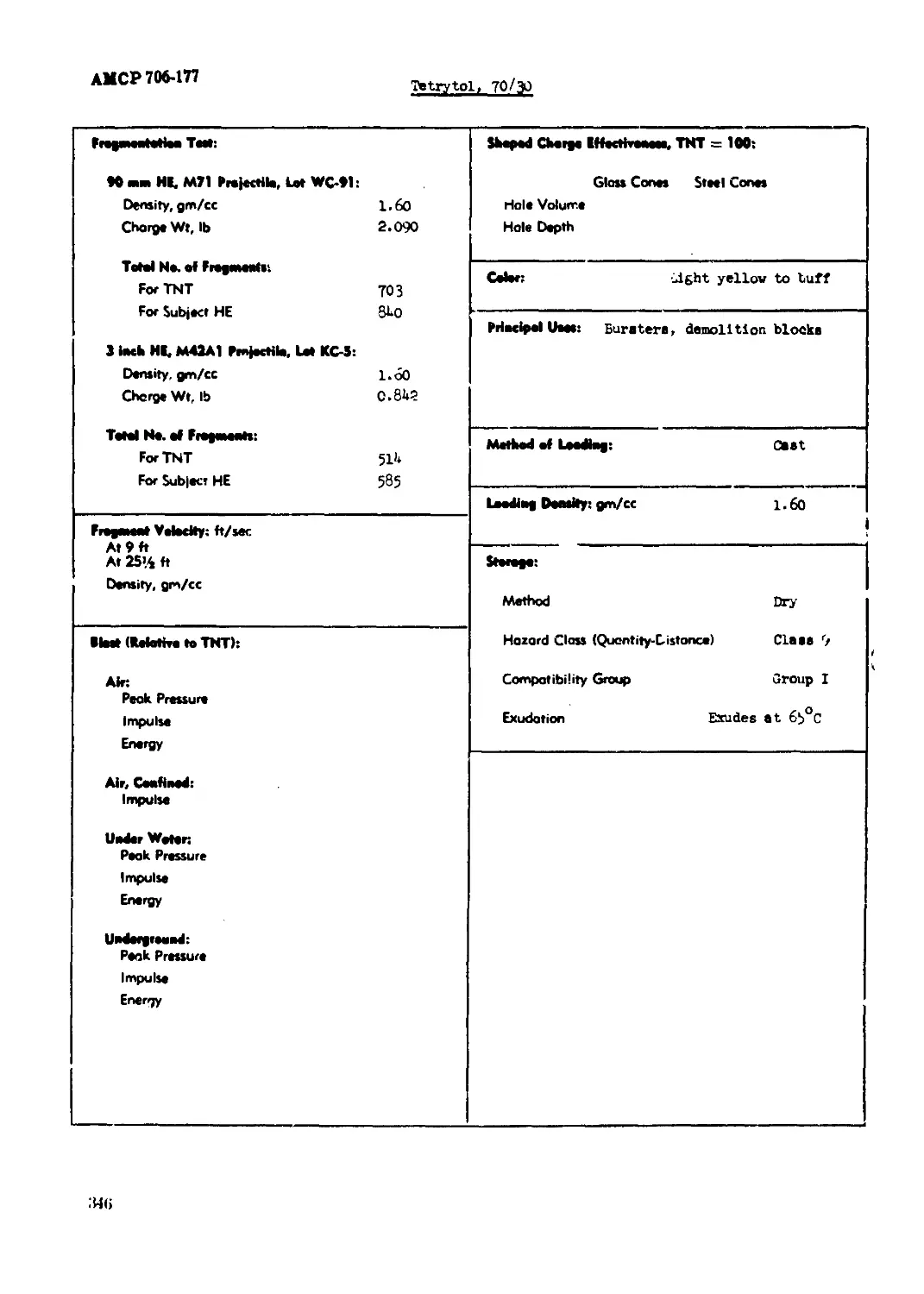
Tetrytol, 70/30............................................................... 345
Tetrytol, 65/35................................................................... 34?
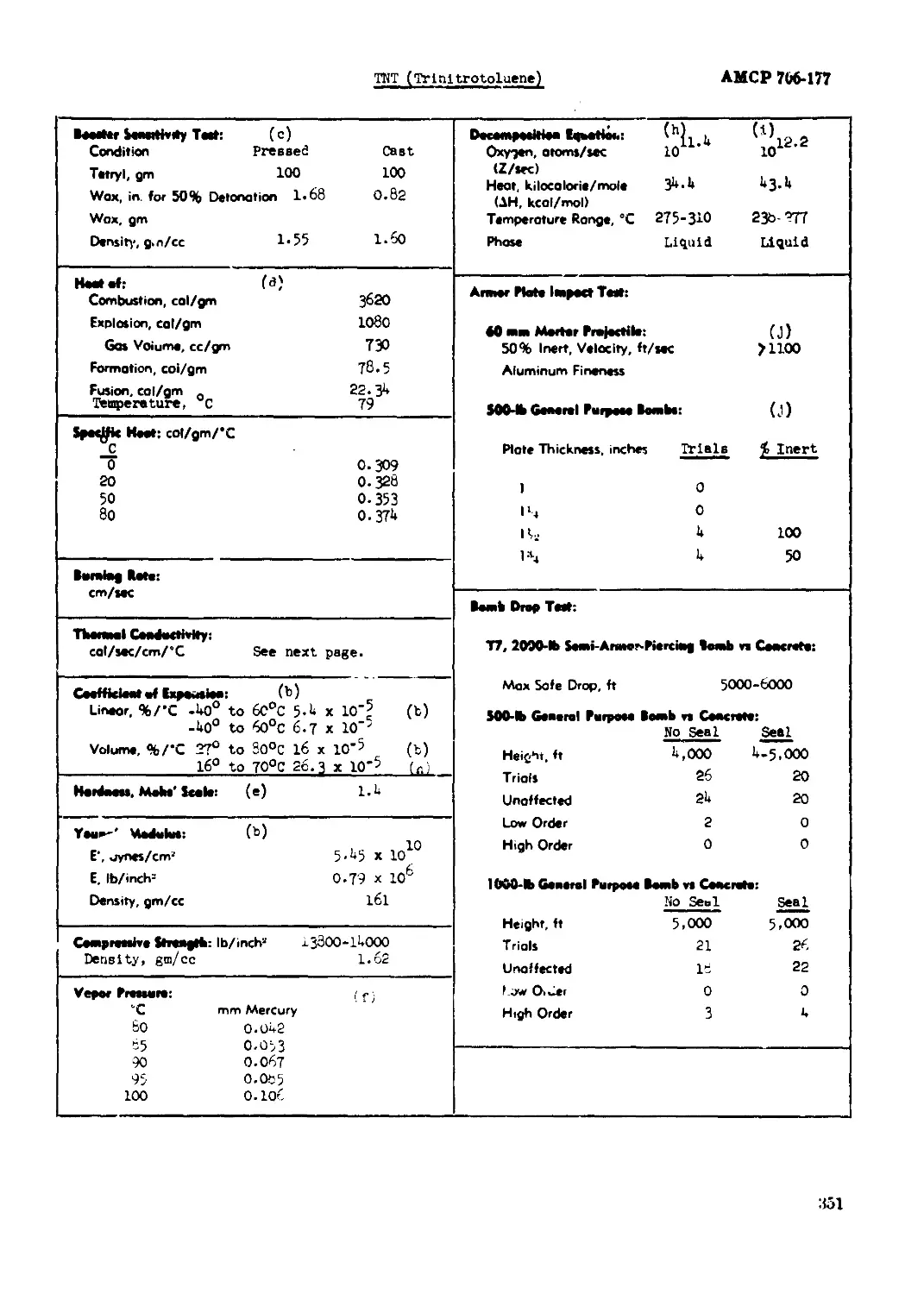
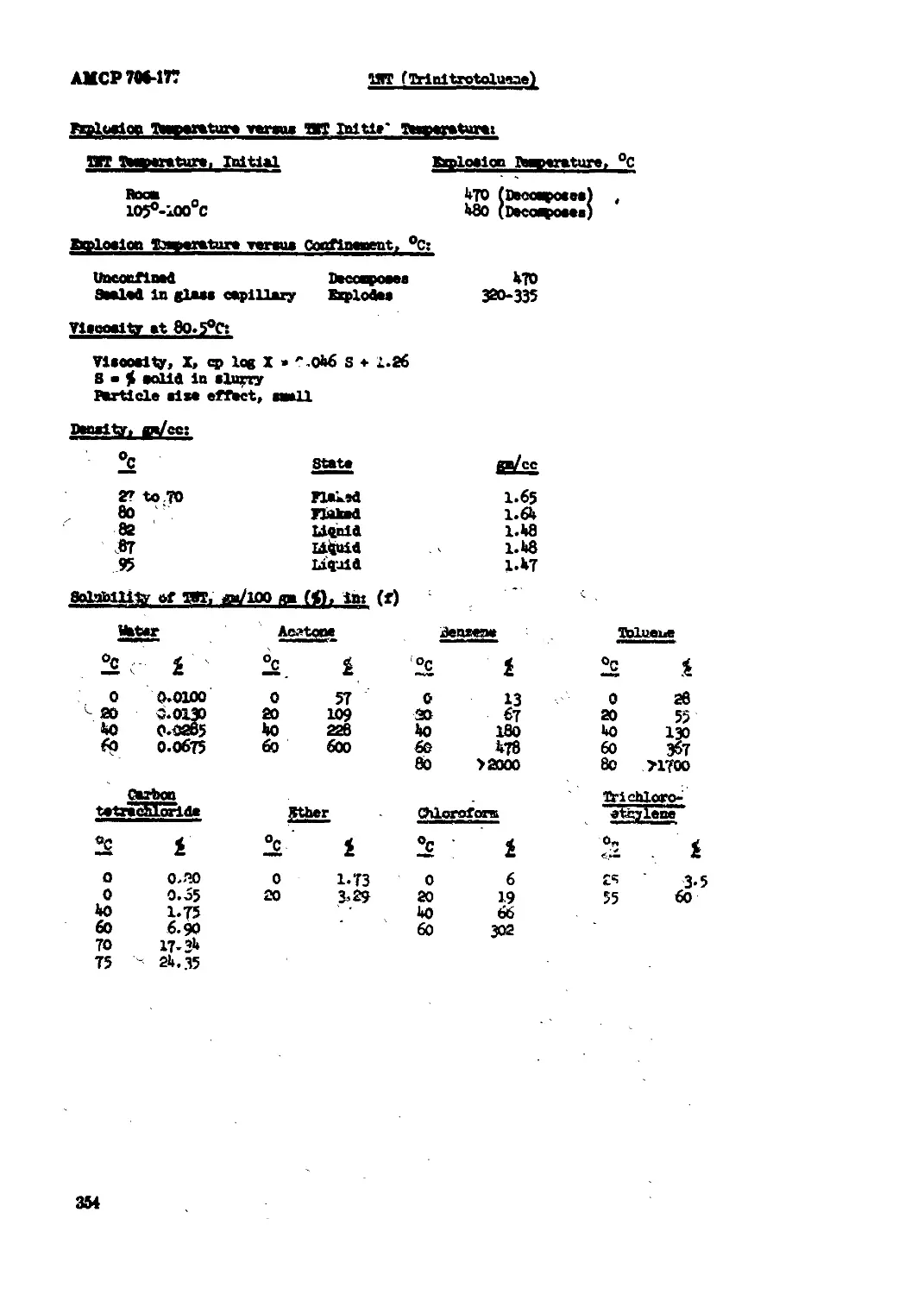
ТЭТ (Trinitrotoluene)............................................................ 350
111
АМСРТОМП
T8BL8 ЭГ CONTKMTS (con.'d)
Й82.
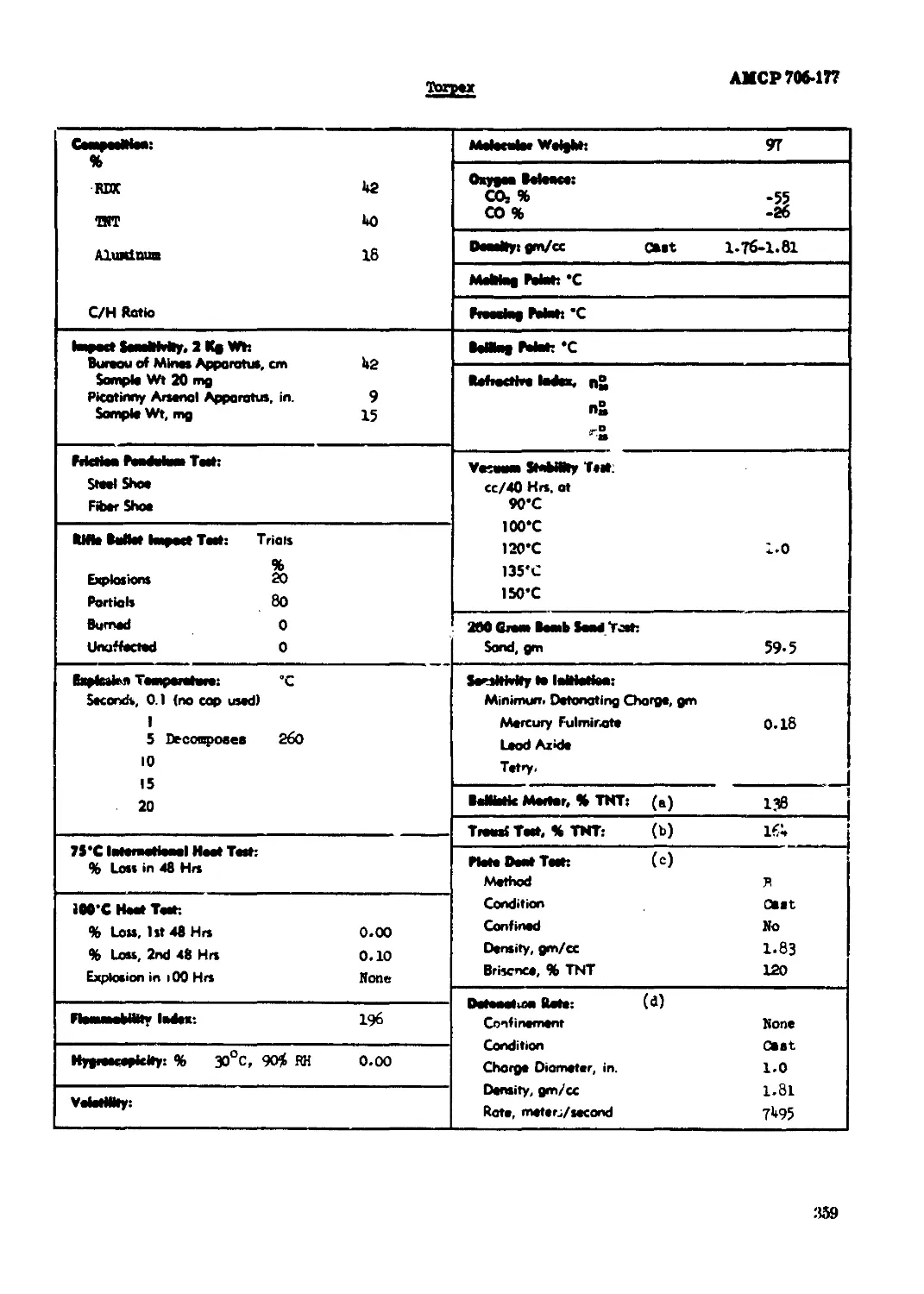
forpas......................................................................... 359
lt3t3-TriaKiuo»2,4t6-Triaitrobaiu«M (TAIKB)................................. .3u*
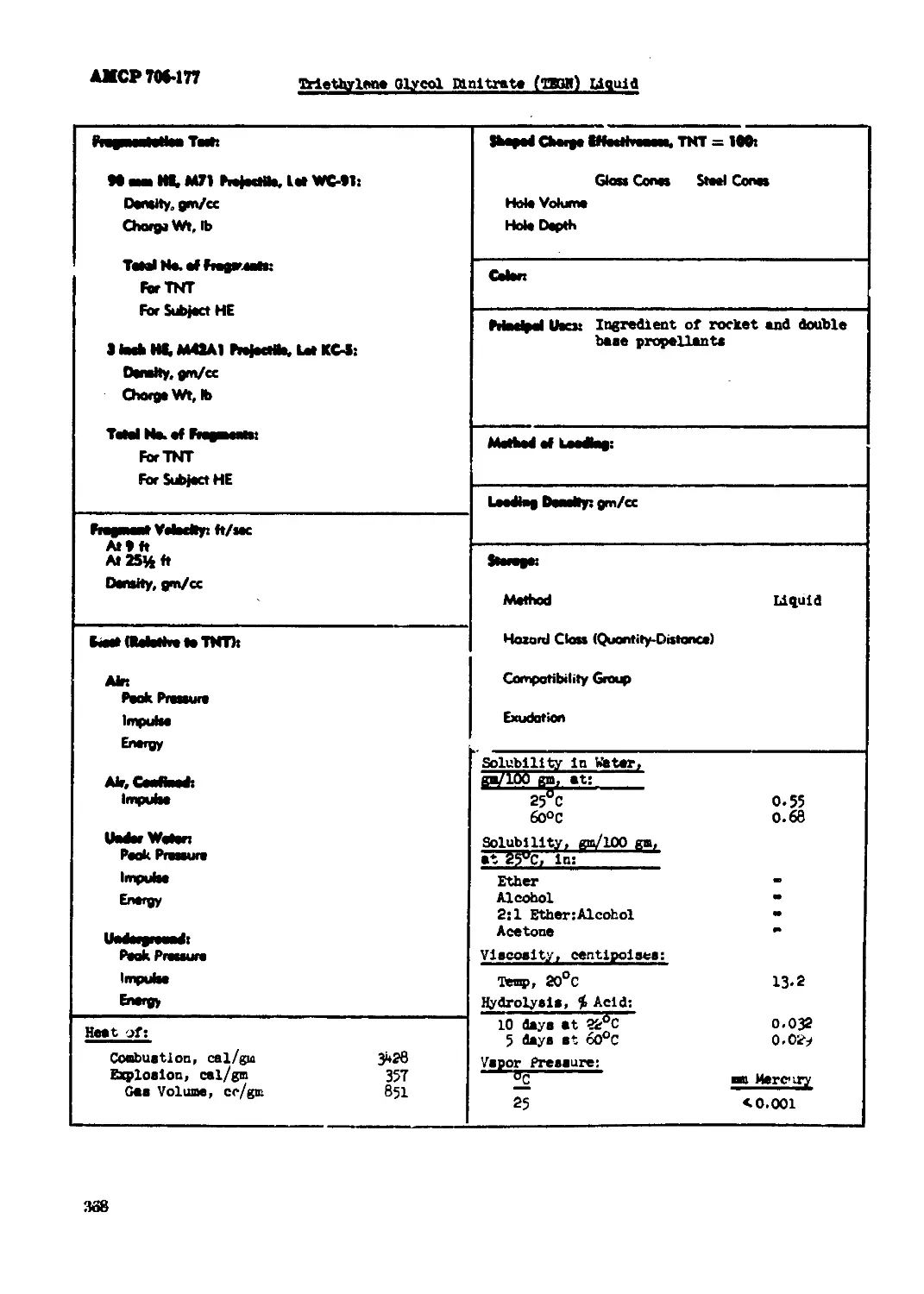
Triethylana Glycol binitrata (¥SGM) Liquid.................................. 367
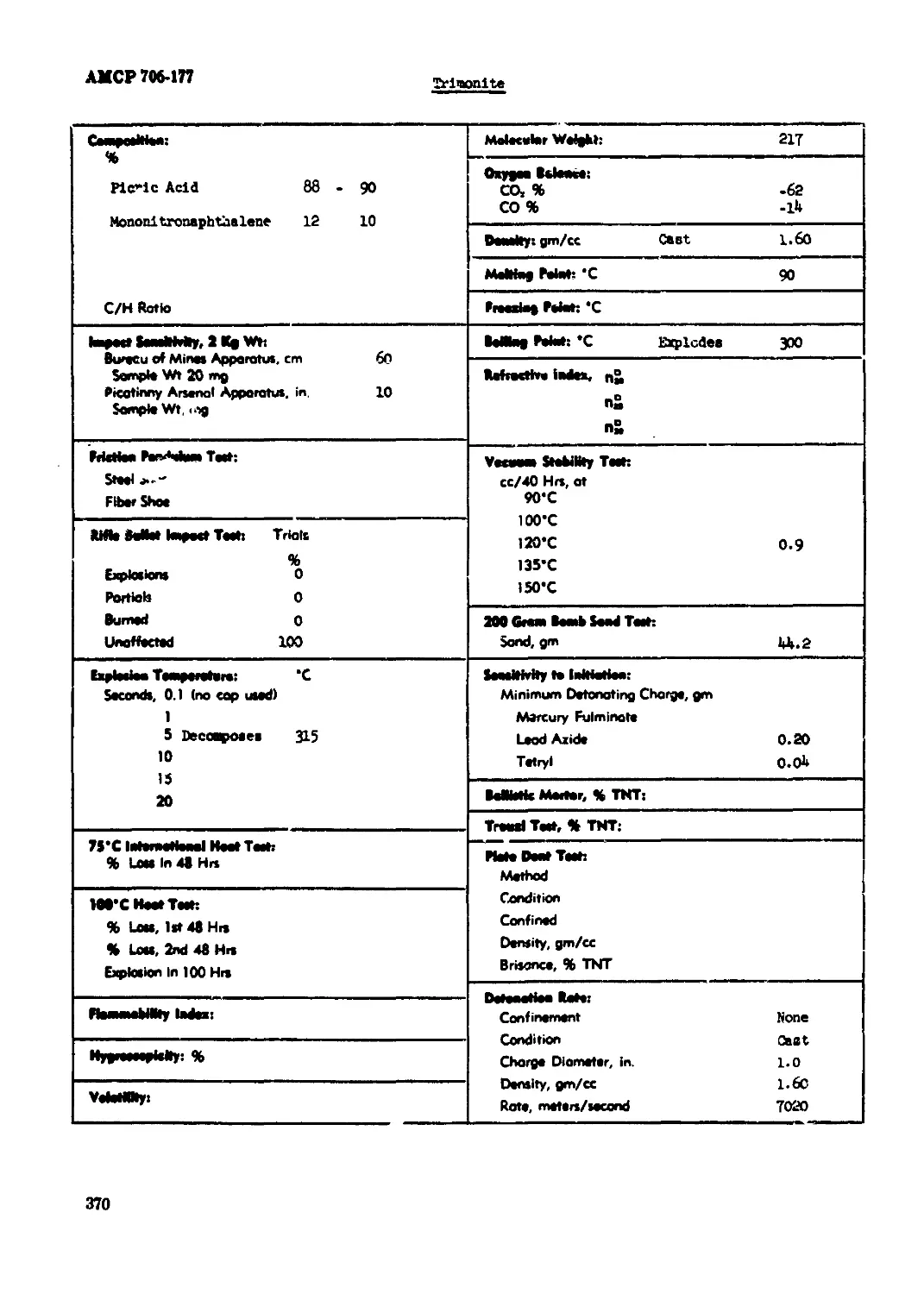
Trlacuita..................................................................... 370
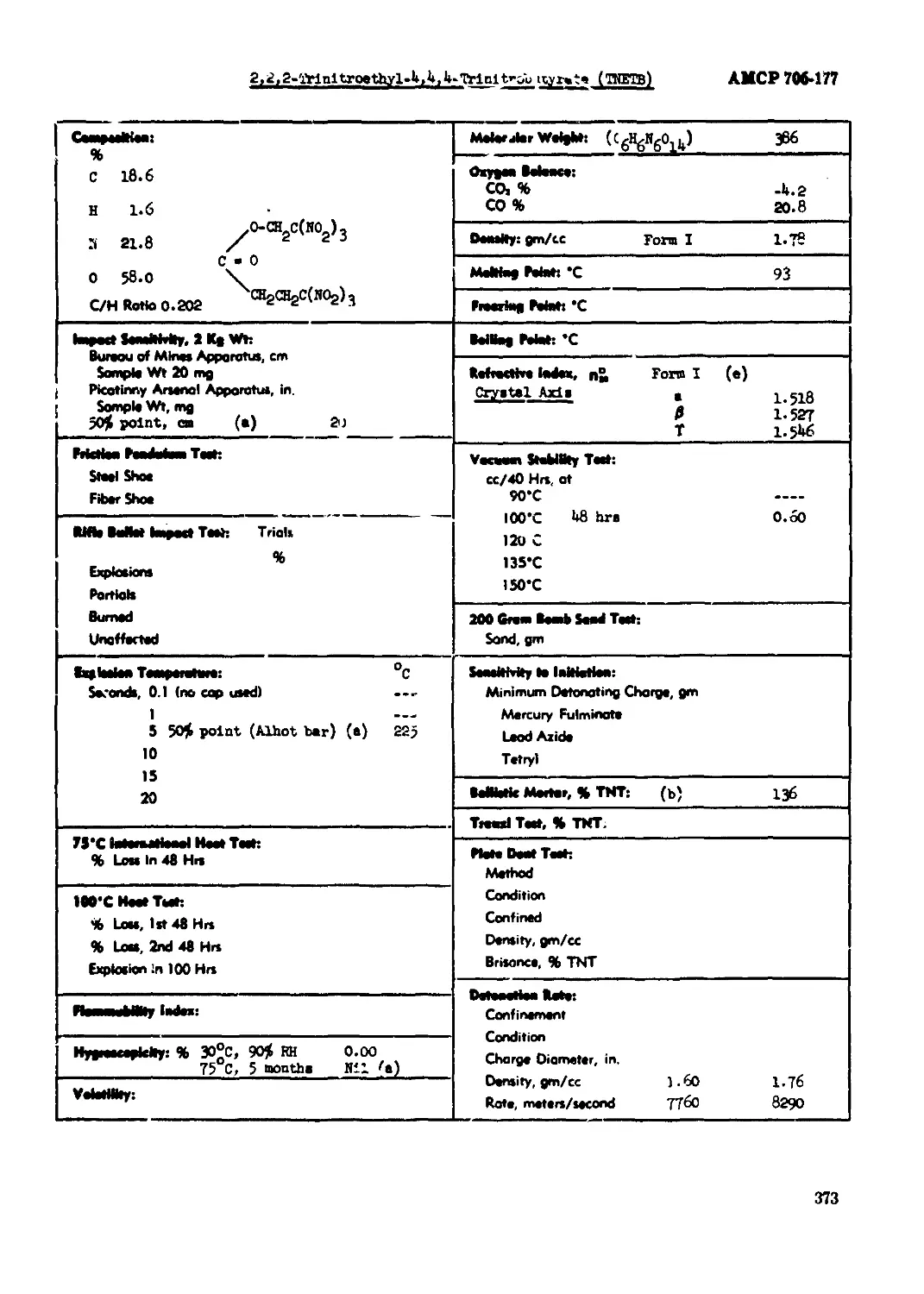
212,2-Trlnitroathyl~«> 4,4-Ttlnitrobutyrate (TKKTB) ............................373
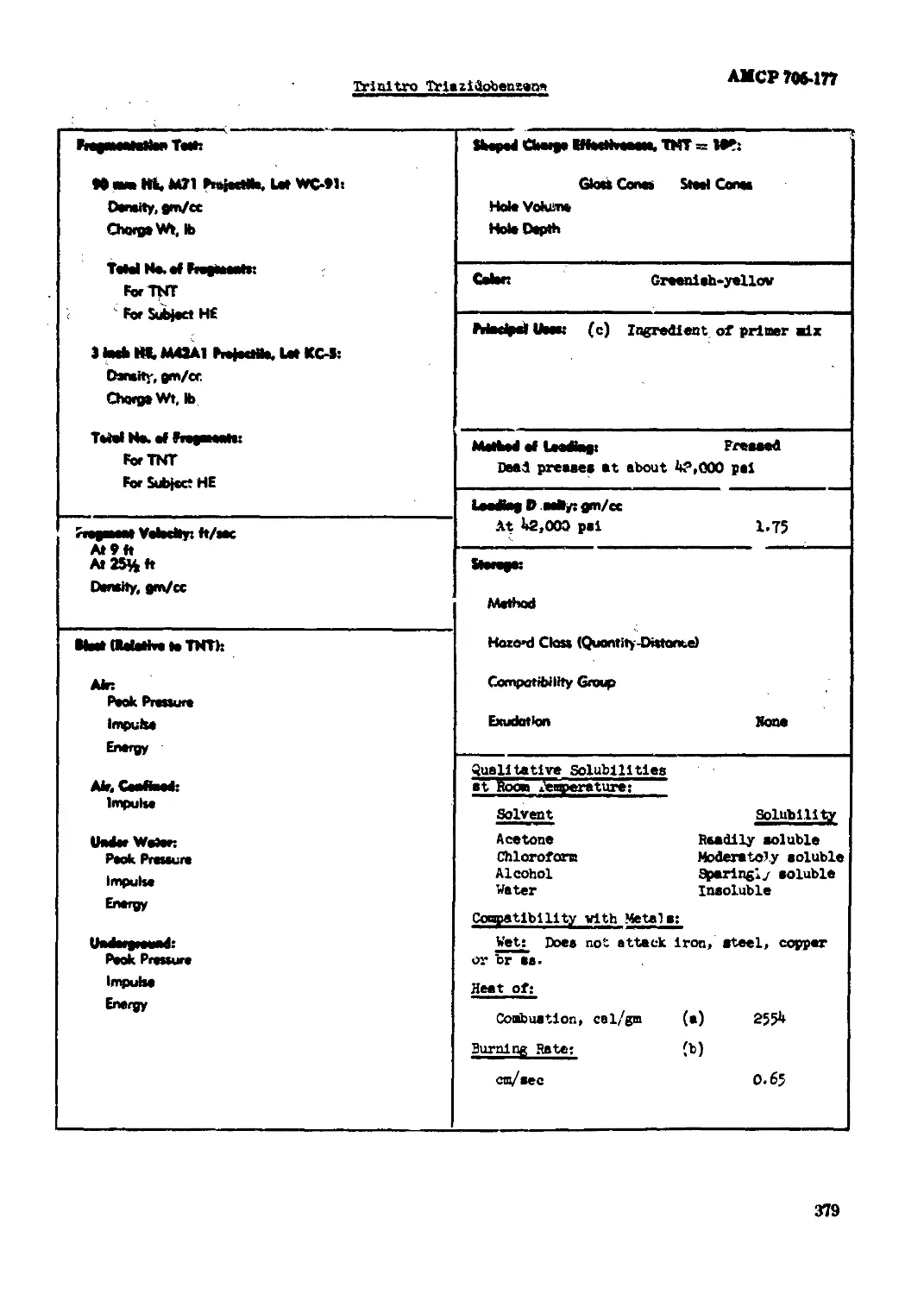
Trinitro Triasodibanaara.................................................... 370
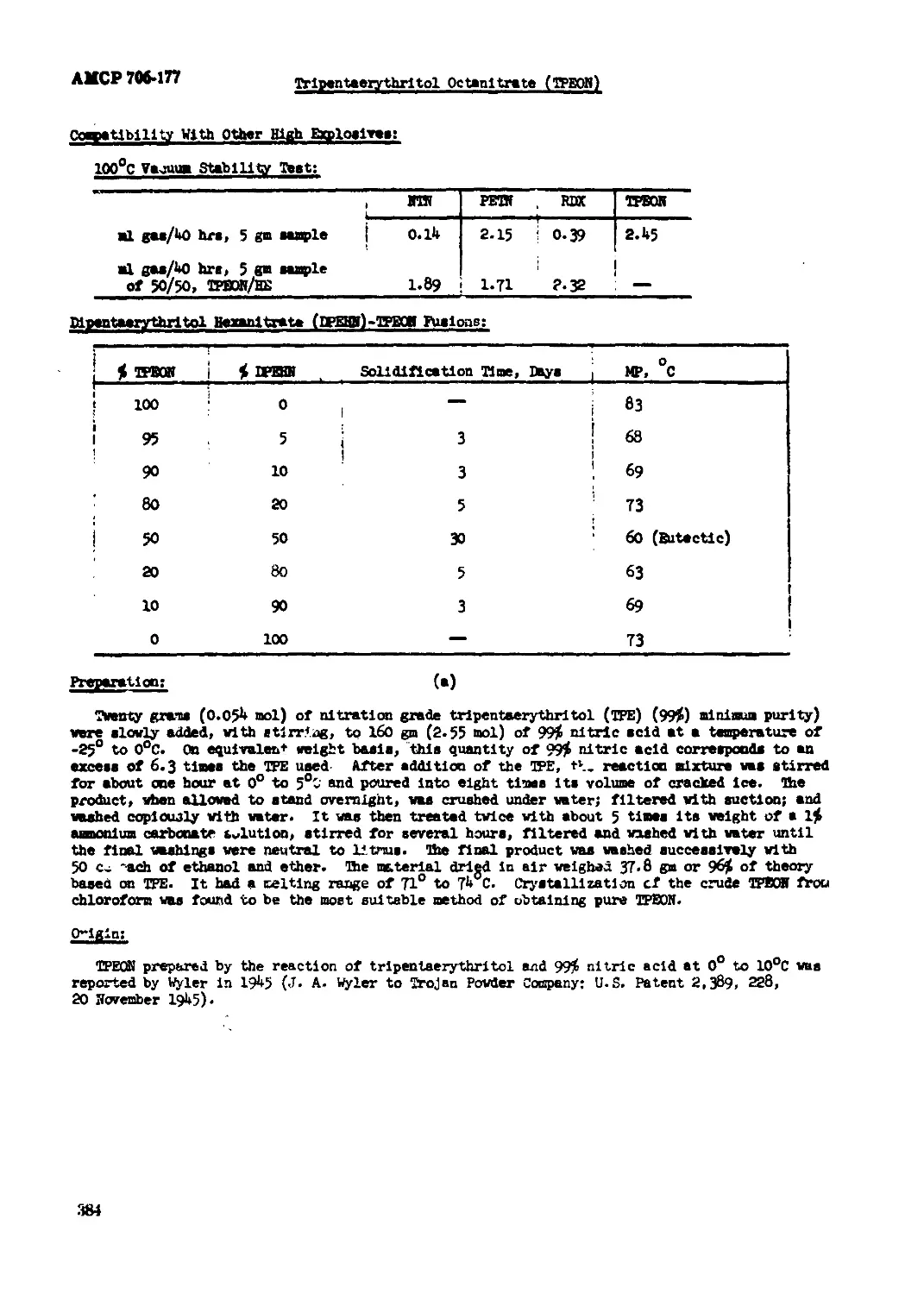
Tripuneaarychritol Octaaitrata (TPMH).................................... .....381
Tritonal, 00/20.................................................................ЗГ6
Taltax Ko. 448................................................................ .391
1v
АМСР 706-177
PREFACE
The* engineering Design Handbott Serlis of the Any Materiel Coaanand 1s a coordinated
series of nandixeks containing bail: infonaation and fundamental data useful In the design
and development of Any materiel ant systems. The handbooks are authoritative reference
<jok$ of practical Information and quantitative facts helpful in the design and development
of Anqy materiel so that It will meet th*, tectickl and technical needs of the Armed Forces.
AMCP 7(*>-177, Properties of Explosive.' of Military Intsrsst, is one of a series on
Explosives. One hundred an< ten explosive compounds or mixtures are listed herein, alpha-
betically, with their properties, Including composition variation. Thes* explosives were
selected because of their current or probab.' application to military use.
The tabulated data reflect the results cf tests, and were irst compiled for publica-
tion at Plcatlnny Arsenal, Dover, New Jersey, by N- R- ’’’nmlinson, Jr. These data were
later revised by Oliver E. Sheffield, also of Plcatlnny Arsenal. fc* the Engineer! 'g Hand-
book Office of Duke University, prime co tractor to the Arap Nauirl Ccamand.
The Handbooks are readily available to all elements cf AMC, including personnel and
contractors having a need and/or requirement. The Anqy Materiel Coamend pcilcy fs to re-
lease these Engineering Design Handbooks to ether DOO activities and their contractors
and to other Government agencies In accordance with current Anqy Regulation 70-31, dated
9 September 1966. Procedures for acquiring these Handbooks follow:
a. Activities within AMC and other DOO agencies order direct on an official
form from:
CoawMndlnv Officer
Letterkeniyy Anqy Depot, ATTN: AMXLE-ATD
Chambersburg, Pennsylvania 17201
b. Contractors who a.vo Department of Defense contracts should submit their
requests through their contracting officer proper Justification to the address
listed In par. a.
c. Government agencies other than DU1 having need for the Handbooks may submit
their requests directly to the address listed in par. a or to:
Comnding General
U. £. Anqy Materiel Command
ATTN: AMCAN-ABS
Washington, D. C. 20315
d. Industries not having Government contracts (this Includes colleges and
Universities) must forward their requests to:
Cowanding General
U. S. Anqy Materiel Command
ATTN: AMCRD-TV
Washington, 0. C. 20315
e. All foreign requests must be submitted through the Washington, D.
Embassy to:
Assistant Chief of Staff for Intelligence
Foreign Liaison Office
Department of the Anqy
Washington, 0. C. 20310
All requests, other than those originating within OOD, must be accompanied by a
valid justification.
Comments and suggestions on this handbook are welcomed and should be addressed
to Anqy Research Office-Durham, Box CM, Duke Station, Durham, North Carolina 27706.
AMCP70G-177
ABBREVIATIONS AND SYMBOLS
-V
AC
ACS
AISI
Ann
Ann chin phys
AP
APG
atz.
Ball
Bar
BIOS GP2-HEC
BM
Bull Soc c<iln
CA
calc
Chan Ket Eng
Chin at Ind
Coup rand
c₽
CR
dec
AH
DRP
E
E
Gazz chin ital
CP
HE
HEAT
Ind Eng Cham
J Am Chem Soc
J Chem Ind
J Chen Soc
J Frank Inst
J Ind Explo-
sives Soc
J praxt Chea
LA
Land-Bornst
H
M t
Heш poudr
8
approximately. ihi* eymool is cued before numbers.
Advisory Council on Scientific Research and Develop-
ment, Great Britain.
American Chemical Society.
American Iron rnd Stael Institute.
Liebig’s Annalen der Chemie.
Annales de chlaie at de physique.
armor-piercing.
Aberdeen Proving Ground.
etmosphern; atmospheric pressure.
Beilatein Organische Chenie, 4th Edition.
Berichte der Deutschen Chemiachen Gesellschaft.
British Intelligence Overseas Service or Objective
Subcommittee, Group 2, Halstead Exploiting Center.
Bureau of Mines, United States Department of Interior.
Bulletin de la societe' chlmique de France.
Chemical Abstracts.
calculated.
Chemical and Metallurgical Engineering.
Chinle et Industrie.
Compton rendus hebdonadaires des reances de
I’Academie des Sciences (Parlp).
ceutipolce.
l.omptes rendus hebdonadaires des saances de
I’academle des Sciences (Paris).
decomposes.
difference in heat (l.e., heat evolved) by decomposition.
Deutsches Reichspatent.
modulus of elasticity or "Young's modulus"; longitudinal
stress/change in length; (force/area)/(alongation/
length); expr-ssed in lb/inc\ .
same as Z, but expressed in dynes/cm?.
Gazzetta C’.ilmlca Italiana.
general purpose.
high explosive.
high explosive antitank.
Industrial & Engineering Chemistry.
Journal of the American Chemical Society
The Journal of the Society of Chemical Industry (London).
Journal of the Chemical Society (London).
Journal of the Franklin Institute.
Journal of the Industrial Explosives Society (Japan).
Journal fur praktische Chemie,
lead az'de
Lardolt-Bornsteln Phys1ka1ish-Chemische Tabellen,
5th Edition (Berlin).
molar.
Monatshefte fur Chenie (Wein).
Memorial dea poudres et aalp^tres (Paris).
milligram.
>11
АМСР 706477
ABBREVIATIONS AND SYMBOLS (cont'd)
In
1
m/a
MW
NAVOED
NC
HDRC
NFOC
NG
HOL
HOTS
HRC
OB
OCM
OSRD
PA
PATH
Phil Tran*
Fogg Ann
Proc Boy Soc
Rec trev chin '
RH
RI
SAB
SAP
eol
Spec
aid dev
TH
TM/TO
Trans Farad Soc
vac stab
Z angew Chen
Z anorg Cher*
Z ges Schleis-
SprengstofI*
Z/ssc
minimum,
milliliter,
meterc per secund,
molecular weight.
Bureau of Ordnance (V. S. ’’vy)
nitrocellulose.
Index of refraction, wit1" D band of sodium es light
source, at twenty degrees centigrade.
Rational Defense Reeesrch Committee.
Rational Fireworks Ordnance Corporetlon.
nitroglycerin.
U. S- Naval Ordnance Laboratory, White Oak, Silver
Spring, Maryland.
U. S. Naval Ordnance Teat Station, China Lake, Cellf
Rational Research Council,
oxygen balance.
Ordnance Committee Minutes.
Office of Scientific Research end Development
Plcatlnny Araenal.
Picatlnay Arsenal Technical Report.
Philosophical Transactions of the Royal Society of
London.
Poggendorf's Annalen der Physlk.
Proceedings of the Royal Society of London.
Recuall des traveux chlmiquea des Pays-Bas.
relative humidity.
Report of Investigation.
Society of Automotive Engineers,
aeml-armor-plercing.
solution.
Specifications,
standard deviation.
Technical Manual, Department of the Агву,
joint publication, as a TM and as a Department of th
Air Force Technical Order.
Transactions of the Faraday Society
vacuum stability.
Zeltschrift fur angewandte Chemie.
Zeltschrift fur anorganlache und allgemeine Chemie.
Zeltschrift fur das gesemte Schless und Sprengstoff-
wessen (Muncben).
atoms of oxygr ’ per second.
Will
АМСРШ177
RROF^RTIES OF EXPLOSIVES OF MILITARY INTEREST
INTRODUCTION
1. ятыдмиу A RgQKT ar SnOAKDARD ДЯГВ. Mo effort «ы «лйе tc cover au the existing
literature, litter open or 'classified security inforaation, on ечу explosive, tether, the
mln resouzu* has teen retorts frcsi facilities using. standard c*? vell-knovn test procedures.
g, ОИйХЖ» Oompilation of date resulting in this handbook was undertaken by Plcatlnny Arsenal
perteOMiabo desired to provide a manual tabulating the characteristics of explosives, based
on tests, with regard to current, and possible future, interest. Ле first resulting Plcatlnny
Areere'l publication was dated go June 19*»9. Revision 1, PA Technical Report Bo. 1T*>O, dated
Aprli. WS, with revisions, provides the data used herein.
j. flOCHU Tabulated date of teste on one hundred and ten explosive comptAinds ar mixtures
Include sensitivity to Metlon, impact, heat; performance characteristics or effbctivunees
in weapons; physical and chenical properties; and method of preparatloc, synthesis or manufhc-
tore, with comments on I .storiesI origin, and supplementary references.
... МИИВСТ ИОВАДОИЗ ЛИР SOURCES. Ле references, as to sources of date or for more details
in Methods of testing, have been listed, when available, at the e.'d of each section devoted to
a given explosive coopound, explosive mixture, or explosive Ingres tent. Where no reference is
given, it can be assured that these date represent typical values dbv.lned by standard proce-
dures. When available any reference should be consulted for aore details in interpreting test
date.
Also there are listed Plcatlnny Arsenal Technical Reports which contain <lditlcnal informa-
tion on the particular exnlosive. These report numbers are given In ascending order, in columns
corresponding co their teixinal digits, and in accordance with the "Uniterm Iidex" prepared for
Plcatlnny Arsenal by DocusentatLon Incorporated under Contract DAI-S^-OS^-SOl-ORD-CP)-^ (1955).
5. ЯИЛЖДОН Cf TOMS AMD METHODS Of ШЗДВВ. Data ьге tabulated herein on three form-type
pager, in the folfwlng sequence of headings. Many of these terms are self-explanatory.
a. First tabular page.
(1) Marne of the explosive in each Instance.
(2) "Composition."
(3) ’'Inpact Sensitivity: 2 Kg Wt."
(a) Iqpect sensitivity test for solids, (a)*
А sample (approxfrecely 0.02 gram) of explosive is subjected to the action of a falling
weight, usually 2 kilograms. A 20-mllligram sample of explosive Is always used in the Bureau
of Mines (BM) apparatus when testing solid explosives. The weight of sample used In the Pica-
tinry Arsenal (PA) apparatus is Indicated in each case. The Impact test value is the minimum
•Reference publications (a through q), applying to thic introduction. are listed at the end of
the introduction.
1
AMCF’M-ГП
height at, which st’ least ana of 10 t-tEola resul+tib fen^sai-en. For the BN apparatus, the unit
of height is the centimeter; for the PA apparatus» it is -the inch. In ths fbrmr, .the explo-
sive is held between two flat, parallel. hardened (C 63 x 2) utecl surfaces; in the latter case,
it is placed In the dapraesioti °i" a small eteel die-cup, capped by a thin brass cover, in the
center of which is placed a tlotted-vented-cyllndrical steel plug, slotted side deem. In the
M apparatus, the ijqact inpulse is transmitted to the sample by the upper flat surface, in the
PA, by the vented pltg. The main difference* between the two tests are that the PA tact (1)
involves greater confinement, (2) distributes the translational iamulse over a smaller area
(due to the inclined sides of the die-cup cavity), end (3) involves a frictional component
'against the inclined sides).
The teat value obtained with the PA apparatus depends, to a narked degree, on the sanpl*'
density. This value indicates the hasard to be expected on subjecting the particular sample
to an impact blow, but is of value in assessing a material's inherent sensitivity only If the
apparent density (charge weight) is recorded along with the inpact test value. The values tabu-
lated herein were obtained ол notarial screened between $0 and 100 mesh, U. S. Standard Screens
where nlngle component explosives are involved, and through 50 mesh for the mixtures.
(b) Impact sensitivity test for liquids, (b)
The И Impact Test for liquids Is run in the sane way as foi solids. The dis-cup Is filled
and the top of the liquid meniscus adjusted to coincide with the plane of the top rin of the
die-cup. To date, this visual observation has been found adequate to assure that the liquid
does not wet the die-cup ria after the brass cap has been set in place. Thus fhr the repro-
duclbllltyof data dbtelnnd in this way indicate that variations in «ample else obtained are
not significant.
In the case of the BN apparatus, the procedure that was described for solids is used with
the following variations:
1. Tan weight of explosive tested is 0.<J07-ga.
2. A disc of desiccated filter paper (Whstear. Ro. 1) 9.5-mlllineter disaster.- is laid on
eadT’drop, on the anvil, and then the plunger is lowered on the sample absorbed in the filter
paper.
(b) "Friction Pendulum Test.” (c)
A 7.0-gm sample of explosive, 50-100 mesh, is exposed to the action of a steel, or fiber,
shoe swinging as a pendulum at the end of a long steel rod. The betevior of the sawpie is
described qualitatively to indicate its reaction to this experience, l.e-, the most energetic
reaction io explosion, and in decreasing order of severity of reactio n snaps, cracks, and
unaffected.
(5) "Rifle Bullet Impact Test.' (d)
Approximately 0.5-pound of explosive is leaded in the same manner as it is losded for actual
use: that is, cast, pressed, or liquid in a 3-inch pipe nipple (2-inch inside diameter, 1/16-
inch wsdl) closed on each end by a cap. Hie loaded item, in the standard test, contains a small
edr space which can, if desired, be filled by inserting a wax pltvy* The loaded item la sub-
jected to the inpact of a caliber .30 bullet fired perpendicularly to the long axis of the pipe
nipple, from a distance of 90 feet.
2
АМСР 706-177
(6) "Explosion Tesperature. ’ (a)
A 0.02-gm sample (0.01-gm Id th* case of Initiator») of explosive, Loose loaded in a №>. 8
bleating cap, la литЗ for a short period In <1 Wood в metal bath. -S«e temperature determined
la that which produces srnloolon, ignition or dace position o? the sample in 5 second?, and the
behavior of the sasqile is Indicated by "Explode»" or "Ignites” or "Decompose»" placed beside the
value. Where values were available for tinea other than 5 seconds, these have been included,
for 0.1-second values, no cap was used- but the explosive was placed directly on Wood1» metal
bath, itasedlately after cleaning, The valuo 0.1 second Is estimated, not determined, and repre-
sents an Interval regarded as Instantaneous to the observer's eye. Dashes Indicate no action-
(7) "75°C International Beat Test." (a)
A 10-gm sample is heated, for 48 hours at 75°C. The sample a‘ter this exposure is observed
for signs of decospositlon or volatility.
(8) "100°C Heat Test." (a)
A 0.6-gm sample it heated for two 48-hour periods at 100°C. It is also noted whether expo-
sure at 100°C for 100 hours results in explosion.
(9) "Flamatllity Index." (h)
The measure of the likelihood that ‘. bare cher^e will catch fire when exposed to flames 1»
the Index of flaxnabillty. The test is made by bringing an oaqdiydrogan flame to bear on the
explosive. The naxlnum time of exposure which gives no Ignition In 10 trials and the minimum
exposure which gives ignition in each of 10 trials are determined. The index of flamsablllty
is 100 divided by the mean of the two times in seconds. The most flamaable substances have high
indices, e.g., 250.
(10) "Hygroscopicity."
A 5- to 10-gm sample Is exposed for hygroscopicity under the etated conditions, until equili-
brium 1» attained, or In cases vhere either the rate is extremely low, or very large amounts of
water are picked up, for the stated time. The sample, If solid, is prepared by sieving through
a JO and on a 100 mesh screen.
(11) "Volatility.”
A ,1-gm stable Is exposed for volatility under the stated conditions. Ihe sample if solid
is prepared by sieving through a 50 and on a 100 mesh sieve.
(12) "Molecular Weight."
The molecular weight (MW) of a mixture can be calculated from the equation
MW of mixture • . 100
_5_+ _L+ _£_ + JL
mw^ mvg mvj mwn
where a, b, c and & sre the weight p-.:rcents of the compcnents, and mvi, ш»2, “*3 and mvc their
corresponding molecular weights.
3
Aitcpm-in
(13) "Oxygen Balance.”
Пи «W* balance (СВ) 1* cal. lated from the esplrioal fontul* of a ooxpound in percentage
at «КПШ required for complete oouveraion of oarwoe to carton dioxide (or oartcn aoooxlda) and
hyteegen to water. Vhan aetal la present the reactions are asaniaed to occur in the following
' dan
Metal + 0 ------- Metal Oxide
C + HgO 11 CO +
00g + Hg ------fr CO + HgO
200 * Qg ------> 2C0a
Procedure flor calculating uxygea balance is to de tend ne the nunber of gnaatoaa of «organ which
are омам or deficient for 100 grans of a co^ound. This nuaber aul .'plied by the atonic «sight
of oxygen gives
the oaQrpen balance: 1600 (21 + - Z)
4 nolacular weight of ooapound oxygen balance to COg and IgO, vt.ere X • atoaa of ^rton.
X atom of hydrogen, Z • atosH of oxygen. The oxygen balance of a stature la equal to the
oust of the percent coa^oaltlyn ties» the oxygen balance for each coagxxient.
the oarton/hydrogen (c/g) ratio is calculated aa follower
(lb) "Density."
(1$) "Melting Point."
(16) "РГеехХод Print."
(1?) "filing Point."
(18) "Refractive index.”
(19) ’Vacuua Stability Heat.” (a)
A 5.0-gn easgle (1.0 в* far initiators), after having been carefully dried is heated for
bO hours, in vacuo at the desired tenperature.
(20) "200 Oran Bcab 3und Test."
(a) SSnd test for solids, (a)
A O-b-ga sample of explosive, pressed at 3000 pounds per squsure inch into a So. 6 cap, is
initiated by lead aside, or mercury fUlainate (or, if necessary, ty lead aside und tetryl), in
a sand test bosh containing 200 gn of "on 30 nesh" Ottawa sand. The amount of aside, or of
tetryl, that oust be used, to insure that the sear Is .rushes the Msytini». ™»t. weight of sand,
is designated aa its sensitivity to Initiation and ths net weight of sand crushed, finer than
4
АМСР 706-177
30 mesh, 1* tarmod the —ad beet ytlue. The net weight of слой crushed le obtained by гЛ-
tzacting fT— the tctai*lhe amount —uahed by the Initiator idten ehot alone.
(b) Send test for liquid-’. (b)
Ihe —nd test for liquid! la nad. in accordance with the procedure given for solids except
that the following procedure for loaning the teat aaaplea la eubstituted:
Cut the doted end from a Ko. 6 bleating cep and load one end of the resulting cylinder
with 0.20 ga of lead aside and 0.25 gm of tetryl, uaing a pressure of 3000 pal for coneoil-
dating each charge. With a pin, prick the powder train in one end of a piece of miner’a black
powder fU— 8 or 9 indict long. Crimp to the pricked end a loaded cylinder, taking cere that
the end of the fu— la held firmly against the charge in the cep. crisp near the mouth of the
cep ao aa to avoid squeezing the charge. Transfer a weighed portion of O.bOO ga of the teat
explosive to an almnum cap, taking precautions when the explosive la liquid to insert +ne
aa—le in such a manner that aa little aa r—Bible adheres to the aide valla of the cap, ~id
sben a solid material la being tented use material fine enough to paaa through a Ko. 100 U. S.
Standard Sieve. Die caps used shall be of the following dimensions: length 2.00 Inches, in-
ternal diameter 0.2^8-inch, —11 thickness 0.025-lnch. Press solid explosives, after Insertion
into the aluminum cap, by means of hand pressure to an apparent density of approximately 1.2 ga
per cubic centimeter. Dili was done by exerting band pressure on a wooden plunger until the
plunger had entered the cap to a depth of 3>93 centimeten. Folloving are the dimensions of
the Interior of the cap: height 5-00 cm, ar— of cross section 0.312 square centimeters. In-
sert the cylinder containing the fuse and explosive charge of tetryl and lead aside into the
aimnua cap containing the test explosive for the determination of —nd crushed.
(21) "Sensitivity to Initiation."
This is sensitivity to initiation aa described under the preceding heading. The minion,
detonating charge, ingress, required to detonate the explosive sample, la given.
(22) "Ballistic Mortar, J> ТЭТ." (e)
The amount of sample uuder test which la necessary to raise the heavy ballistic mortar to
the same height to which It t s raised by IO gm of trinitrotoluene (ТЭТ) is determined. The
sample la th— rated, on a proportionate basis, as having a certain ТЭТ valie, i.e., as being
a certain percent aa effective aa ТЭТ In this respect. The formula is
ТЭТ value - —IP , ,,, x 100.
sa—>le weight
The ballistic mortar consists of a long cospound supporting rod, at the end of which la sup-
ported a heavy short-nosed mortar. The mortar contains a chamber about 6 inches in di see rer
and 1 foot long. A projectile occupies about 7 Inches of the rhaaher and the sample to be
tested occupies a small portion of the remainder of the chamber. When the sample la detonated,
the projectile 1j driven into a sand bank, and the mortar swings through — angle which la
carted on peper by a pencil attached to the mortar. The angle thus indicates the height to
which the pendulum is rtlaed by the explosion, and thia latter represents the energy measured
by this test procedure.
(23) "Trauzl Test, $ ТЭТ." (d)
A sample of the explosive to be tested (of the order of 10 gm) Is exploded in a cavity, or
borehole, 25-nm In diameter and 125-mn deep, In a lead block 200-am in diameter and 200-nm in
height. The borehole 1^ made centrally in the up; ~ face of —ch block, which Is cast in e mold
from desilverized 1—d of the best quality. Although these tests have been sole ' nder a variety
5
АМСР 706-17?
of condition», where possible th* data have been taken from or related to those of Reference f
(Nkoum). Here a No 8 blasting cap was ueed for Initiation of thf sample contained in glass.
The weight of saz^le used им adjusted to give, with the initiator, a tot*.1, expansion of 250 to
JOO cc, since within this range expansion and sample weight were linearly related under the con-
ditions '* Taoun’s test. Thus expansions for equivalent weights were readily calculated, and
the tes; alue expressed in percent of the expansion of an equivalent weight of TNT,
(2b) "Plate Dent Test." (d)
Two methode were used for plate dent testa.
(a) Method A - Ine charge is contained in a copper tube, having an internal diameter
of 3/l»-lnch and 1/16-1 nch wall. This loaded tube Is placed vertically on a square piece of
cold-rolled steel plate, 5/8-inch thick; U-inch and З-1/U-inch square plate gave the sane re-
sults. The steel plate *a in a horizontal position and rests in turn on a short length of
heavy steel tubing 1-1/2 inches ID and 3 inches 0D. The charge rests on the centei of the
plate md the centers of the charge, plate, and supporting tube are in the same line. A 20-gm
charge of the explosive under test is ooostered by a 5-6® pellet of tetryl, in turn initiated
by a No- 8 detonator.
(b) Mathod В - A 1-5/8-lnch diameter, 5-inch long uncased charge la fired on a
l-j/U-lnch thick, 5-square Inch cold-rolled steel plate, with one or more similar plates us
backing. The charge is Initiated with a Ko. 8 detonator and two 1-5/8-inch diemeter, JO-gm
tetryl boosters.
Plate dent test value, or relative brisance = Saagle Dent Depth x
Dent Depth for ШТ at 1.Ы ga/ac.
(25) "^location Rate." (g)
The detonation rates reported in the tables contained herein were determined principally by
using the rotating drum camere, under the conditions stated, e.g., usually charges 1 inch in
diameter, 20 inches long, wrapped in cellulose acetate sheet, and initiated by a system designed
to produce high order stable detonation at the maximum rate under the particular ejndltions. A
tj-nical initiating system for this consisted of four tetryl pellets 0.995 inch In diameter, 0.75
inch long, pressed to 1.50 gm/cc, with a Corps of Engineers special blasting cap placed in a
ce .tral hole In the end pellet.
b. Second tabular page.
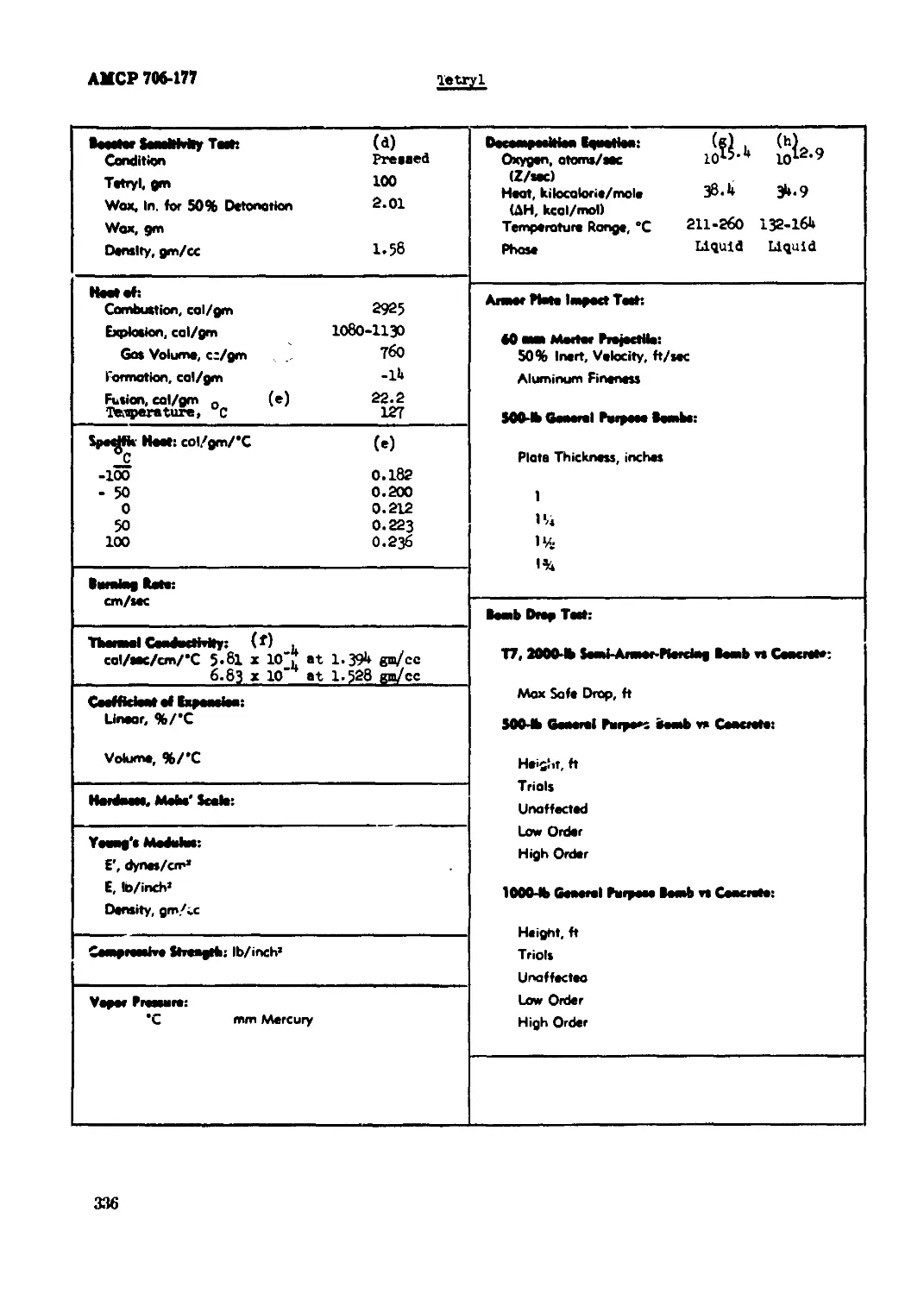
(1) "Booster Sensitivity Test." (p)
The booster sensitivity test procedure is a scaled up modification of thu Bruqeton method
(unconilned charge). The source of the shock consists of twe tetryl pellets, each 1.57 inches
diameter by 1.60 inches high, of approximately 100 gm total weight. The initial shock is de-
graded through wax spacers of cast Acrewax B, 1*5/6 inches diameter. The test charges are 1-5/8
inches diameter by 5 inches long. The value given is the thickness of wax in inches at the 50$
detonation point. The weight of tetryl pellet noted is the minimum which will produce detona-
tion with the spacer indicated.
(2) "Heat of (calorimetric tests). (i)
Heats of combustion and explosion are generally determined on samples weighing of the order
of 1 to 2 gm, In standard calorimeter bombs such as the Parr or Emerson, approximately UOO cc
(for low loading density), or the Boas, approximately 1»5 cc (for high loading density). For
6
АМСР 706-177
heats of combustion the ицЦ« is burned under ebout 1*0 atmospheres of oxygen; flor heats of
explosion, nitrogen, or cm atmosphere of air la used*
(3) "Specific Beat."
(1*) "Burning Rate."
(5) "Themed. Conductivity"
(6) "Coefficient of Expansion. ”
(7) "mrdneaa, Nohs' Scale."
(8) "Toung'a Modulus."
(9) "Coapresslve Suer4? th."
(10) "Vapor Pressure."
(11) "Decomposition Bjuatlon."
(12) "Armor Plate Impact Test." (j)
(a) 6o-sn Mortar Projectile.
A modified бО-sn, M*<M2, mortar projectile is loaded with the explosive to be tested, drilled
to the proper depth (about 1/2 inch), and a flat-based steel plug screwed into the projectile to
give a smooth dose-fit between the plug base and the charge. The pert of the plug outside the
projectile is rounded off In the for* of a spherical section. The loaded projectile with fins
attached Is fired fro* a five foot length of 2-3/8 Inches ID x 3-3/8 Inches OD Shelby steel tub-
Э. The igniter Jid propelling charge, consisting of an igniter for a 2. Эб-lnch rocket (basoo-
, 5 gm of 4F blck pu«uer, and a quantity of shotgun propellant sufficient to give the desired
velocity (read from a calibration chart) are conveniently loaded into the "gun" through в simple
breech plug. The velocities are Measured electronically, and the reaction, inert or affected,
Is determined by observation (e.g., whether or not flash occurs on impact). Within the range of
flight stability of the projectile, 200-1100 ft/sec, the 50^6 point is located.
(b) 500-lb General purpose Bombs-
(13) "Bomb Drop Test."
Bomb drops are made using bombs assembled in the conventional manner, as for service usage,
but containing either ineit or sloe bated fuzes. The target is usually reinforced concrete.
c. Third tabular page.
(1) "fragmentation Test." (1)
The weight of each empty projectile and weight of water displaced by the explosive charge is
determined, and from this the specific gravity of the charge is calculated. All 3-1nch and
90-nm nrojectiles are initiated by M20 Booster pellets, and those used with 3-inch HE. M*»2A1,
Dot КС-5 and 90-na> HE, МП, Dot WC-91 projectiles are controlled In weight and height aa follows:
22.50 ± 0.10 gm, and 0.1*80 to O.U85 inch.
АМСР7М4П
The projectile asssshled with fur», actuated by a Blasting Chp, Эреclal, Type П (Spec ,'<9-
20) pieced directly on a lead of соефегеЫе dlsmetev and booster, are placed la heme con-
structed of half-inch pine. The 90-sm projectile» are fragmented in boxes 21 x 10-1/2 x 10*1/2
inches and the 3-inch projectiles in bans 15 x 9 x 9 inches outside dii.rulcns. The box with
projectile is placed on about b feet of sand in • sieel fregmentation tub, the detonator wires
•re connected, sad the box cowered with approximately b feet ноте of ssud. The projectile is
fired and the sand run onto a gyrating b-mesh screen on which the fragments are recovered.
(2) "fragment Velocity."
Charges 10-1/8 Inche» long and 2 laches in diameter, containing a booster cavity, filled by
a T2-gm tetryl pellet (1*3/8 inches diameter, 2 inches long, average density 1,59b) are fired
in a nodal projectile of ЭмХЬу aeenleaa tubing, 2 Inchon ID, 3 inches 0D, SAS 1020 steel, with
a welded-on cold rolled steel base. The projectile is so fired in a cheater, connected to a
corridor containing velocity stations, that a desired wedge of projectile casing fregmenta can
be observed. The fragment velocities are determined by shadow photograph»), using flash bulbs,
and rotating drum cameras, each behind three slits. The drum cameras have a writing speed of
30 asters per second.
(3) "Blast (Relative V ТЭТ)."
The blast pressures and irvulses given were determined alooet exclusively with tourmaline
gages, and the usual necessary specialised electrical circuits, shielded cc-axial cables,
oscillographs, • \c- In general, the data represent results of tests with large cased chargee.
(b) "amped Charge Effectivenees, THT « 100." (k, m)
Unccnflned chargee 2 inches in diameter and 6 Inches long, boostered by a 10-gn pressed
tetryl pellet, set in a 20-sm pellet (truncated cone) of cast 6o/bo cyclotol, are shot against
3-inch homogeneous armor plate at a 1*3/16 inches standoff. The cores used are noinirdal Pyre,
glass funnels, sealed off at the start of the stem, 2 inches in diameter, 0.110 to 0.125 inch
well thickaeee,
Unoouflned charge» 1.63 inches in diameter and 6 inches long «re tested at a standoff of
I.63 Inches against stacks of b x b x 1 inch mild steel plates. M9A1 steel cones are used.
Results are averages of b trials.
(5) "Color."
(6) "Principal Uses."
(7) "Method of Loading."
(8) "Loading Density."
(9) "Storage."
Ammunition and bulk explosives in storage represent varying degrees of hazard and compati-
bility. This has led to their being divided into a nuober cf hazard classes and compatibility
groups as indicated in subparagraphs (b) and (c) below.
(a) Jfcthod: Wet or dry.
(b) Hazard Class (Quantity-DlLtance).
8
АМСРПЫП
й sunt tian and bulk explosives ar* divided into quantity-distance claaiei, daac 1 through
12, according to the daaage expected if they explode or ignite (Kefarence: Amy Materiel Oosumim*.
Regulation, ANCR >45-100, ARC Safety Manual, chapter 17). All standard exploaivea in bulk are
ineluded In four of theoe daaeea: riaan 6, 2A, 9, and 12 (W 9-1910/TO UA-1-ЭМ*
(c) Corjetibllity Group.
Ekploaivea and uaunition are grouped for compatibility with respect to the folloving ftctors:
1. Effects of explosion of the item.
2. Rate of deterioration.
3. 9e~Xxtivity to initiation.
it. Type of packlt^.
5. Effects of fire involving the itaa.
6. Quantity of explosive per unit.
(d) Exudation.
d. Miscellaneous entries.
Where available and appropriate, the foilwing or related data are given, in space at
the bottom of the third fora, or on plain pages.
(1) Solubility.
(2) Methods of aanuftcture.
(3) Historical inforsatlon.
(1) Bulk cc^roMibility nodulus. (4)
Ihe direct experlnsntal naasursnant of the dynamic bulk modulus of a solid is difficult, and
few such nsasursmenta have been nade. One apparatus has been developed at the iieval Ordnance
laboratory and is described in detail in Reference 4. Bulk modulus (its reciprocal is the com-
pressibility) is refined aa the ratio of stress to strain when the stress is a pressure applied
equally on all surlkoes of the aaaplo and the strain is the insulting cftange in volume per unit
volume.
• (5) Hydrolysis testa. (0)
The 2uO-hour hydrolysis tost is conducted aa foliose: A 5*ga aaaple of the dry nitrocellu-
lose is weighed accurately in a tare-weighed 250-cc Pyrex flask hr.vlng a ground glass connection
for a fyrtx condenser. Then 100 cc of distilled enter is added to the nitrocellulose in the
flask and the flask fitted to the condenser. The flask is placed in a steen bath in which the
water is kept ЬоШэд constantly by aeons of electric hotplates. At the end of 240 hours the
amount of solid developed by the hydrolysis of the nitrocellulose is neasured by an electromatic
pH nethod.
(6) Sensitivity to initiatlu. by electrostatic discharge, (n)
9
АМСРТОв-177
Die аацйеа are tested under two amounts of confinement, designated as unccnflned and con*
lined. In the unccnfined test, а ммфХе of spproxiBately 0.05 8“ is dumped Into a «hallow de-
pression in a «tael block and flattened out with e spatula. in the confined tests (partly
oonflrwd)- the eac^le of approximately 0.05 gm la Introduced into soft-glass tube (-*T» IIx
18 sss long) which fits over a natal peg. The volume of the «разе around the charge at zero gap
la *» 0.15 cc; at a gap of 0.6 an, It 0.4 cc. In addition to providing noderate confine-
ment, thia ajetem also minimizes dispersion of the sample by the teat eperk, and reduces the
affect of ив tert al bei. - repelled from the needle point by electrostatic field effect.
When a teat is to be nada, the needle point electrode is screwed up until the gap between
electrodes is greater than the critical gap discharge at the test voltage. The sample is then
placed in position, the high-voltage terminal of the charged condenser la switched to the point
electrode by means of a mercury switch, end the electrode Is screwed down until discharge occurs.
The spark energy (in Joules), for zero probability of ignition, is determined.
(7) Destruction by chemical decaq*ositlon.
Burning is the preferred method of destroying explosives. Initiating type explosives (in
Quantity) are usually destroyed by detonrtlon with demolition blocks. Destruction of explo-
sives by chemical decosg-osltion can be effectively used where small laboratory Quantities are
involved. Procedures given are standard for only lead azide, mercury fulminate and nitrogly-
cerin.
(8) other intonation.
(9) References.
6. Н8ПНВКЖЗ СМДР PT РПЯОШСЯКЖ.1
a. W. H. Rinkwnbach and A. J. dear. Standard Laboratory Procedures for Sensitivity,
Brisance, and Stability of Bzplosives, BAlb ’о. 14ol, 18 №rdh 1944, Revised 2b February 1950.
b. W. fl. Tomlinson, Jr. and A. J. dear, Development of Standard Testa — Application of
thelwact and Sand Testa to the Study of Bltroglycertu and Other Liquid ^plosives, BATRl(o.
173B, 13 June 1949.
c. J. H. Mdvor, Friction Pendulum, PA Testing Manual 7-1, 8 May 1950.
d. Departments of ths Any and the Air Force Joint Technical Manual and Technical Order,
Ш 9-1910/tc llA-l-3b, Military Explosives, April 1955.
a. J. H. Mdvor, wanistlc Mortar Test, PA Testing Manual 7-2, 8 May 1950.
f. Ph. Mourn, Z gee Schiese-Sorengetoffw, pp. 181, 229, 267 (27 June 1932).
g. 0. J. MieUer, Equipment for the Study of the Detonation Process, PATH No. 1465,
4 July 19И.
h. NEBC Interim Report, Preparation and Testing of Explosives, Nos. PT-19 snd PT-20,
February-April 1944.
1. Unnie E. Newman, PA Chemical Laboratory Report Nos. 127815 and 134476, 11 January 1951.
J. Report АС-2963/Org Expl 179.
For Information regarding source of references, inquiries should be made to the Commander,
U.S. Army Research Office--Durham, ATTN: CRDARD-EH, Box CM, Duke Station, Durham, North Carolina
27706.
10
ЛМСР706-1П
к. Baatern Dabcaatory, du Pont, Xmreatiaation of Ovlty Effect, Section III, Variation at
ОкгШг Effect vl th Ooapoaltion, MERC Contract V-bVfe-OTUX-LTtj,
1- j. И. Kclror, Pmpeentaticn Teat Irocetoea, RA Tseti^ Manual 5-1, 2A Auguet 1950.
a. Maatam laboratory, du Pont, Inveetigatlon of Cavity Effect, Final Report, 18 Septaaber
19^3, ШКС Contract V.5T2-0BD-5723*
n. p. W. Brown, D. H. Khalcr, and F. C. Gibaon, senaitivlty of Kxploeivea to Initiation
by ELectroatatic Maehargua, U. S. Department of Interior, Bureau of nnea, R. I. yt&, 1^6,
o. D. D. Sager, Study of Add Adeorptloc and Rydrolyaie of Oelluloee Bitrate and Oelluloae
Sulphate, BAIR Jfo. 1?4 12 JanuaryTJS.
p. L. C. SOdth and E. H. Ryater, Huraical Teatirg of Exploairea, Part Ш, Ittacellaneoua
Senaltlvity Teata, perforaance Jeata, CiffiD Report Яо. Hfkb, zf tieceaber 1^45.
Q. c* S. Sandler, An Acouatic Technique for Maaauring the Effective Broadc KUiNpdulna
of KLaatidty and Aaeodated boon factor of Rubber and raaatioa, MVORD Report lb. 1^й. 1 Зёр-
tMber 19$Ь.
W. S. Craaer, Bulk Cotdreealbllity Data on Severe! Bploairea, MAVORD Deport Mo. ^3SO,
15 Saptaaber 1956.
11
amcpw-177
Anetol, 80/gp
MoImvIw Wk^htt
Atteonlua Kltrete 80 ttr ao Ому^мь BoImmoi co» % *1 CO % +11
Sentityi gm/cc (Mat 1.46
MoMsf Mott *C
C/H Ratto •WW1 w
Import SoMbMty» 1 Kg Wti Bureau of Mines Apparatus, cm 90 Sample Wt 20 mg PleoNnny Arsenol Apparatus, tn. 15 Sampto Wt, mg 17 Ob^^o WWoWalJ гвип w
4? *4? ai i
пш1<|) ^явясяя1 lew* Stool Shoo Unaffected fiber Shoo Unaffected gl^Mmy cc/40 Hn, at 90"C 100*C 0.И I2O*C 0.95 !35X J5O*C 6-8
Mo BaBrt Import Toth 5 Trioh % Eaptaetons 0 Partial» 0
Burned 0 Unaffected 100 M8 «rem Bomb Seed Tertt Sand, gm 35.5
Raptedoe Teeteeralere: *C Second», 0.1 (no cap toed) 1 5 Оесстфооее 260 10 Вй (явМвМойз Minimum Detonating Charge, gm Mercury Fulminate Load Azide 0.20 Totryi 0.07
20 BoMrtic Mono*, % TNT: (в) IJO
Treed Tort, % TXT: (b) 123
7J'C tatomotieeef Meet Tertt % Lorn in 48 Hr» 0.06
teeto Doot Tort: Method
1M*C Hoot Tert: % Lost, l«t 48 Hr» 0.03 % Loot, 2nd 48 Hr» 0.05 Exptoeton in 100 Hr» Bone Condition Confined Dontity, gm/cc Brisance, % TNT
НмвямЫВ^ 1в4вк* Confinement Nor» Bone
Ышмадд*1ркм* 3D°C. «X RH. 2 dove 61 Charge Dtomev»', In. 1.0 1.0 Density, gm/ce 1.46 1.50 Rote, meteri/Mcond 4500 5100
УеЫМу: Я11
13
Amatol, 8o/ao
AMCP 706-177
^^F^t^l^ll^llt^leele^t ^^^Oltt TO м M, МП MmNK let WC41t Density, gm/cc Charge Wt,M ТМЫ Ne.ef PiegnMaOoi For TNT For Subject HE 11мЬ M, MOA1 PiefcoMa. let KC4i Density, gm/cc Chorge Vlft, fc Total No. of FmgmoaMt For TNT For Subject HE Glatt Conoo Stool Cento Ной Volume Ной Depth
Cohn Buff-yellow
Maelpel Uteot Boabo, HB projectileo
MoNMI of Itofcg: Qtot
Leeftag Doatlft: gm/<x l*b6
Fregawat Тойону: ft/oac (f) At 2 ft 1900 At 25% ft 1750 Density, gm/cc
Method Dry Hazord Cbm (Quonttty-Diiionca) Claoo 9 CompotMHty Group Group I Exudation Doee not exude at 65°C
Moot Пайейей TNT): Ain Peak Pressure Impulse Bwgy AiCf СмЯмА Impulse IMorWatan Амк Pressure bapulte Energy PookPrsosum Impulse Bnaiyy
Booeter Beneitlvity Toot: (e) Condition Proceed Tetryl, ga 100 Hex, In. for 50^ Detonation O.83 Denolty, g^cc I.65 Heat of: (d, e) Ccribuatlon, cal/gn 100Й» Eiploeloa, cel/gn l>90* Gao Volune, cc/ga 930* «Calculated Iron co^oeltlon of mixture.
13
АМСР 706-177
Altai, брДо
CmvmMm: АмюШив Nitrate 60 ШТ Uo С/Н Ratio Molecular Weight: lot>
Oxygen Balonce: CO, % -18 CO % +2
Density: gm/cc Cast 1.60
n • V
Freezing Feint: 'C
hnpeet SenehMty, 2 Kg Wh Bureau of Mina Apparatus, cm 95 Sample Wt 20 mg Picatinny Arsenal Apparatus, In. 16 Sample Wt, mg 17 ВеШад Feint: *C
Refrectire Index, n» n£ n£
« -« - в *- rnWWIi rwWSVWIII 1ЯП 3VCV1 злое Fiber Shoe Vacwae StablHly Test. cc/40 Hrs, at 90*C 100‘C 120*C 135’C 150‘C
Rifle Baflet Inspect Tee»: Trials % Explosions Partials Burned Unaffected
200 Dram Bomb Send Test: Sand, gm U1.5
Expleeien Tempereiwie: 'C Seconds, 0.1 (no cap used) 1 5 Decomposes 270 10 15 20 Seneithtty to InMioHon: Minimum Detonating Charge, gm Mercury Fulminate Lead Azide 0.20 Tetryl 0.06
BeNietic Metter, % TNT: (e) 128
TreuzI Teet, % TNT:
75’C Interactional Heat Tee»: % Loes in 48 Hrs
Ftate Dent Test ; Method Condition Confined Density, gm/cc Brisance, % TNT
100'C Hoe* Toot: % Loss, 1st 48 Hrs % Loes, 2nd 48 Hrs Explosion in 100 Hrs
Dotenetiea Kate: Confinemen. Гопе Condition Cast Charge Diameter, In. 1.0 Density, gm/cc 1-50 Rate, meters/second 5760
Hygnoeeplclly: %
VeloMky: Nil
14
Amatol, 6o/4p
AMCP 706-177
И м Ht, МЛ Projectile, и» WC-»1
Density, gm/cc 1.49
ChorgeWt, lb 1.971
Tefal No. of Fregmeats:
For TNT 703
For Subject HE 583
3 tach HI, M42A1 Projectile, Lot KM:
Density, gm/cc 1.57
Chorge Wt, lb 0.827
ЬАл лА Bee•
For TNT 514
For Subject HE 4o8
Fregosos* Vatedty: ft/sec
At ? ft
At 25ЦН
Density, gm/cc
Sita* (Rotative ta TNT):
Ain
Peak Pressure 95
Impulse 85
Energy 84
Air, Cemftaed:
Impulse
Under Wotan
Peek Pressure
Impute*
Energy
Ui^w^vMMdt
Peak Pressure
Impulse
Energy
Staged Cksrge KHeetfteeess, TNT = = 10fr.
Glass Cones Steel Cones
Hole Volume Hole Depth
Color-. Buff-yellow
Prtadpel Uses: Bombs, HE projectiles
Mittal of Leedtag: Oset
Leedtag Pieilty: gm/cc loO
Method Dry
Hazard Class (Quantity-Distance) Claes 9
Compatibility Group Group I
Exudation Does not exude at 65°C
Heat of; (d, a )
Combustion, cal/gm 1658»
Explosion, cal/ga 633*
Gee Volume, cc/gm 380»
•Calculated from composition of mixture.
амср mm
g/У.
Омфяййяш ML Mslliahr Weight: 118
W Aaaoniva ai*raU 50 ЯТ 50 OefBM fchmtt CO, % .87 CO % .3
Basdtyi gm/cc Cast 1.5<
MeRtap Raiott *C
C/H Ratio Fraccing Mott *C
la^oat 8миВМу, 1 Kt Wh Rureou of Mini» Apparatus, cv 95 Sample Wt 20 mg Hcalinny Artanol Apporatus, in. 16 Sample Wt, mg •Л1
Rofmctiae ladar, n° l*a Па
NoNao Raodahm Taatt Steel Shoe Itaeff acted RberShoe UnrTfacted Vocoom SteHRty Taatt cc/40 Hrs, at WC 100’C e c.2 120*C 1.0 135*C 150*C
*Ma RoRf Repeat Taatt Triols % Expkaione о Partials 0
Burned 0 Unaffected 100 200 Rmm Beads Saad Taatt Sand, gm 42.5
RbgdMloe ^Tampoeetiae: C Seconds, 0.1 (no cep used) 1 5 Юоеоа^оам 265 10 Minimum Detonating Charge, gm Mercury Fulminota LeadAxide 0-» Tetryl °'°5
20 BeKeNc Metter, % TNT: (e) 124
Tread Test, % TWT:
TS*C tatenaotieaei Meet Taatt % Lea in 46 Hrs Hale Beat Taatt Method В
1»’C Meet Taatt % Leas, 1st 48 Hts % uaa, 2nd 48 Hri Explosion in 100 Hrs Condition Cast Confined Bo Density, gm/cc 1*55 Brisonca, % TNT 52
АмшиЫМу Confinement None Note
% Nil Charge Diameter, in. 1.0 1*0 Density, gm/cc 1.55 1-55 Rote, meters/wcond 64JD 6230
VohMRyt
16
АМСРТИ-177
?М(ВМММММ1 Ttttt 1 Ibeped Choige EHeetboacea, TNT =
М Ml Kt, МЛ РмйаЫЬ. Let WC-»I Density, gm/cc CSorgsWt, b i 1.55 2.053 GIms Cores Steal Ной Volume 53 Hole Depth 69 Cones («)
TeSel Ke. el htjMSte For TNT For Subnet HE 3 task ME, MttAl MxNh, IM KC4i Density, gm/cc ChovWtb 703 Cslsn Buff-ye)low
1*5* 0.819 Pitaslpel Uses: Boaba, HK projectiles
Total He- of FregsLsats: For TNT For SubHet HE 51* 385 Mo4n4 ef laedtag: cast 4.
LoeNag Ооаайу: gm/cc 1.59
тп^МЯК т9ШЯЯу1 ТТ/9К At» ft At 25^ ft Density, gm/cc
Method Jky
Nest (ВаМЬе ta TNT): Hozord doss (QuonNftUMstance) Class 9
Ain Peok Prainira Impulse Energy 9f 87 Compatibility Group Exudation Does not exude st Group I 65°C
Ate Impulse Under Wotan talk Pressure Impulse Energy fteok Pressure Impulse Бюгду 93 10* 10* 10* Booster Sensitivity Test: (s) Condition cast Tetryl, gr- 100 Wax, in. for 5OJ Detonation 0.60 Density, ga/cc 1.55 Heat of: (d. e) Combustion, cal/gm 1990 Expiceion, cal/g’" 703* Gee Voluas, cc/ga 855* «Calculated fron composition of mixture. Specific Heat: cal/gm/°C (i) ¥еч>, ЙУ» to 5УГ О.3В3 Bomb йгор Tea*: T7, 2000-lb Seai-ArsKir-Piercing Boab vs Concrete:
Max Safe Drop, ft *000-5000
17
АКСР 706-177
Amatol» 60/20, бО/кр. jO/'jO
Compatibility with Medals:
Dry - Metals unaffected are bine, Iron, tin, brtua, brass t'.i plated, brass MRO coated,
brain shellac coated, nickel aluminum, steel, steel plated rth nickel, zinc or tin, stain-
less steel, Parke?1ted ateal, and ateel coated vith acid-proof black paint. Metals «lightly
affected are copper, bronze, lead and copper plated steel.
Preparation:
In preparing amatols the proper granulation of a.Tonlum nitrate ii required If tue crxirum
density of the cast anatol is desired. Ihe asmxxrt.u nitrate should be dried so ae to contain
not more than 0.25^ moisture. It should be heated to about 90°C before being added to the
appropriate weight of molten ВГГ contained in a melting vessel equipped with an agitator. Con-
tlkje mixing to Insure uniformity end load by pouring into «bill or bomba.
Origin;
Developed by the britisb during world War I in order to conserve ТЯТ.
References?2
(a) L- C. Smith and 2. H. prater, Physical Teatizu of Explosives. tert П1, Miscellaneous
Sensitivity Testa, Performance Jests, OSSD Report 574o, 27 December 19^5-
(b) Report ЛС-17/Ptaya 2x 1.
(c) D< P. McDougall, Methods of Physical Testing, OSD Report Bo. 803, Ц Auguot 1942.
L- C. Smith ind K. G. tyster, Physical testing of Explosives, Part III - Miscellaneous
Sensitivity testa; Performance Jests, 0S№ Report tto.5746, 27 December 19^5-
(i) Joesittee of Dtv 2 and 8, НЖС, Report on HEX and Tritonal, OSES Report Bo. $4o6,
31 July 1945.
(e) Philip C. Keenan and Dorothy Pipes, Table of Military High Explosives, Second Revision,
SAVORD Report Ko. 87-46, 26 July 19U.
(f) R. W. Drake, Fragment Velocity and Panel Penetration of Several Explosives in Simulated
Shells, OSRD Report Mo. 562^, 2 January 1946. ..
(g> Eastern Laboratory, du Pont, Investigation of Cavity Effect, Final Report, 18 September
1943, НИС Contract W-672-ORD-5723.
(h) Also see the following Plcatlnny Arsenal technical Reports on Amatols:
0 1 2 1 it 5 6 7 8 2
240 681 132 743 364 6$ 266 1207 548 549
350 731 182 1173 694 425 556 1457 638 799
630 901 1302 1373 734 695 666 1797 838 929
950 1051 1352 1323 874 715 986 1827 1098 1129
1300 1311 1372 11*93 1344 735 1376 2167 1148 1219
1530 11*51 1552 1763 1145 1446 1388 1369
1651 1225 1636 1568 1559
1345 1796 1838
1455
18Й5
(i) та 9-1910/ТО 11A-1-34, Military Explosives, April 1955.
^See footnote 1, page 10.
IS
АимооаХ
AMCP 706-1УТ
—. 1 " —- --— * - :~' < : taeniimMtnt» 22 ®t бТ :. Ахмадом ii 4 ” • C/HRsJio Mekarfar WsieMs 102
Oxypea Befeace: CO. % -5> CO % -22
D*aeft>*gm/ce Ottt 1.65
Mefeiap Pefcfe'C
ta^ct Д«*ЙМ1у> 1 Kfl Wt: Bureoc of Mines Apuoral us, cm 91 Somj..« W* 20 mg Picotinny Arsenal Apparatus, in. XX Sample Wt, mo 19
Refrocthr* todex, n° n£
awH^W^WIW ,-гЖт Sfe-ji She* Fiber Shoe Veciraas StebBRy T**»f cc/40 Hrs, at 90’C ' 100’C 120’C Vb 135’C 150’C
RM* Mlat teepect Tash Trials % Explosions Partials Burned Unaffected
M0 Great Ikaab Send Teets Sand, pm ^7.8
txpfesiw T«*p*r*tem: ’C Seconds, 0.1 (no cap used) 1 5 Decrxnpct ** 26$ 10 15 20 SeaeMrity te lellietfea: Minimum Detonating Charge, gm Mercury Fulminate 0.20 Lead Azide Tetryl
BeWetic Merter, % TNT: (a) 122
Traaxl Test, % TNT:
75 *C 1вкпмНм*1 Heeir Tat: % Loss in 48 Hrs Piet* De*t Ten, Method Condition Confined Density, gm/cc Brisance, % Tt4T
1M*C Hte T*eh % Loss, 1st 48 Hrs 0.00 % Loss, 2nd 48 Hrs 0.10 Explosion in 100 Hr<, .None
Deteaatfe* Rote: Confinement Condition Charge Diomete', in. Density, gm/cc Rote, meters/second
. ЛмятЫЯеу Indies:
НудгМеерДОу: %
VeWJdty: .. ,
19
AMCP TW-177
АмюпаХ
ЕмфаавОаНаа T*ah Moped Ctetga МаеКмамм, TNT = 1 Wt
M wan Ш, Mil Ь*)мМ*. U* WC-tlt DamNy, gm/cc ChorpeWt.b Gloat Cone» Steel Сопи Hole Volume Hole Depth
Total Na. efFtagamate For TNT For Subject HE Cttet Mattel Ukatt Projectile filler
Э Ы| к M4SA1 МмМа, и» KC4t
Оману, gm/cc 1.65
Charge Wt, Ь
Tidal Na. of Fragamele: For TNT 655 For Subject HE 550 Malted of Leodtag: (Mat leodbg OaaeHyt gm/cc 1.65
Кодаки* Vate»y: ft/eec At» ft At2»%ft Density, gm/cc z Iteage: Method Dry
Meet (tehlivo to TNT): Hotonf Ctee (QvontlhMMtance) Claaa 9
Ain Rook Рммиге Impute Епечу CcmpotibUiiy Group Exudotion
ЛЬ» CmA*W: knpuhft Origin: Castable mixture developed in United States during World War !•
Under Water: Hook Pressure Impute En rgy References: (a) W. R. Tomlinson, Jr., Physical and Ex- ploalve Properties of Military Explosive», PATH Ko. 1372, 29 ktereaber 19<»3>
Peek Pressure Impute Energy (b) Also see the following Picatinny Ar- senal Technical Report» on AaMonele: 1108, 1286, 1292, 1308 and 1783.
Preparation:
Procedure sane же described under Лае tele,
except aluainua la edded to the eamonlum nTT
trete-ПГТ aoXten Mixture under agitation u..-
tll uniforalty in coapceition la obtained.
Loading in accomrlished by pouring into the
anoroorlate prolbctlle.______________________
20
Awwilw» Mtrete
АМСР7М-1П
--inmni I 35 В 5 JBk», 0 60 C/H Ratio Msi.idsr Weight: (Ць 80
«зднаМиом CO % +20
•eodlrt gm/cc Crystal 1.T3
MeMng Mott *C 170
Mooing Meh ’C
Imped ймМйу. 2 Kg Wit Bureau of Mino» Apparatus, cm 100t Sample Wt 20 mg Plcatinny Arsenal Apporotu», In. 31 Sample Wt, mg 17 wnj rwBBBve M
Bafca dfee hist, £
»-_e.- TfMlWB IVWl Stool Shoo Unaffected Aber Shoe Unaffected Vacuum BtobMBy Test: cc/40 Hrs, ot 90‘C ioo*c 0.3 120*C O.J 135*C 150*C 0.3
KMo МЫ ta^oot Todt Trial» % ЕфЫоп» 0 Partials 0 Burned 0 Unaffected 100
2M«nm Bead Send Test: Sand, gm Ml
Kxpfssiea Tompossduse: C Seconds, 0.1 (no cap used) 1 5 Ignites 465 10 15 20 Minimum Detonating Charge, gm Mercury Fulminate Lead Aside 0.20 Tetryl 0*25
BaMdic Alerter, % TNT: (s) 56
Tread Tert, % TNT:
7S*C lolereerieeel Heat Tod: (a) % Loss in 48 Hrs 0.0
Hole Beat Tertt Method Condition Confined Density, gm/cc Brisance, % TNT
180'C Heat Test: % Loss, 1st 48 Hrs 0.?L % Loss, 2nd 48 Hrs 0.13 Explosion in 100 Hrs Bone
DotoeoHee Rote: (b) Confinement None Strong Condition Solid Liquid Charge Diameter, in. 1.25 4.5 Density, gm/cc O.9 1.4 Rate, meters/second 1000 2500
ЛввммЫШу Index*
NrgmsMpfcBy: % Brtwme ЭО°С, 90jC RH Extreme
VdrtMly: Decomposes et 210°C
21
Axcpm-m
A—oalua Mitreto
СмчМоп Tetryl gm Wok, In. for 30* Detonation Density, gm/cc ОеееацреМеа ЦмНеаг (f) , R (h) e Oxygen, otome/eec 10iJ,° lo“’3 (Z/see) Heat, kilocotorie/moh *0.5 38.3 (ДН. ксЫ/moi) Temperature Range, *C 2*3-861 817-867 Phase Ucuid
Neat oh CcmbuMion, col/gm 3*6 Expiodon, cal/gm 3*6 Gm Volume, cc/gm 9Й0 Formoticn, cal/gm 1096 Fusion, сЫ/рп 18.83 Anaer Note hepect Test: 50* Inert, Velocity, ft/eec Aluminum Fineness МФ4Ь Шммм1 PwptM ВвмЬвз Plate Thickness, inches 1 1* 1* 1*
Speallb Nooh cai/gm/’C (•) °C °C 150 0.109 0 0.397 «100 0.330 50 0Л14 -50 0.3» Ю0 0.426
ВвмЬф Sflist cm/sec
iemb Drop Tash 17,3000-* ЗеткЛлпепЛогс1од leaA vs CeeereSo: AAax Safe Drop, ft SOCMb Oseirsl Perpsii Beak vs Coecistst Height, ft Trials Unaffected low Order High Order IOGO-Ь Geaorel Perpeee Bomb v. Concrete: Height, ft Trials Unaffected Low Order High Order
eol/soc/cm/X 8.9-3.9 x 10"^
СмМШм* W ЬфмвСмц Linear, */*C Volume, */*C
Yooog's Modolas: E', dynes/cm* E, b/lnch* Density, gm/cc
fe^M^Le Ik/irx-k* *M888^W8BWw 9VW88^aRe 1О/ «Tsfi
Vapor Ргомого: (g) *C mm Mercury 188 3-25 205 7.*5 216 II.55 223 15.80 t8T
22
Anaonlum nitrate
AMCP7W-177
M mat Ж, MFI MmNK u» WCM; Penalty, gm/cc ChorgeWl, k> Tktal Na. «1 Progmoata For TNT For Subject HE 2 Meh NCMOA1 NtcNh, Lat KC4t Penalty, gm/cc ChwgaWt, k> Total Mu of Ftogouata: For TNT For Subject HE *epod Cteago eHoetkeeoa, TNT = Wt GtacConee Steel Cones Hole Volume OQ>0 ВЛрчм
Celon Colorleev
Prtetpd Iteet Explosive ingredient of mixtures used In bcebe or large caliber projectilea
Method of laetegi Preaeed or cent depending on conceit Ion of mixture
Leedhg deao*yt gm/cc Variable
Fragmaet Vslstbp ft/sec At 9 ft At 25% ft Penalty, gm/cc
Method Dry Hoiord Clou (Quantity-Pittance) С1ам 12 Compatibility Group Group D Exudottai Hone
MaatOhbMrateTNDt Ain Peak Pressure Impute Energy СмИв*4х Impute Uadar Wotan Peak Praaaure Impute Energy Underground: Peak Preaaure Impute Energy
Effect of Teapereture on Inpact 8enaitivity (Chenlcally pure grade); (b) Deep. РЛ Itgpect Teet °C 2 Kg Wt, inchee 25 31 75 28 100 27 150 27 175 12 Compatibility with Matala; (a) In the preeence of moisture, annoniun nitrate reacte with copper, iron eteel, brass, lead end cadmium. Entropy: (g) cal/mol at 25°C 36.O
23
АМСР 796-177
A—lue nitrate
Solubility of wecat— nitrate, юга— in 100 era— (1) of; (a)
Ifcter Alcohol A—tic Add Mltrlc Acid
20 l$e Ao 5 27-0 0.39 15 73.0 21.7
AO 897 60 T.5 80.9 5.8 30 106 20.8
60 Aa 78 10.5 101.0 20.7 75 SOI 31.6
80 580 120.0 125
100 871
Pre—ration;
A——1— nitrate ie prepared by the neutrallxati— of an aqueoua aolution of a sonic with
nitric acid and evaporation of the solnticn. Ihe product which la very pure la dried In a
graining kettle.
Origin;
First prepared by Glauber in 1659 end first used aa an explosive ingredient in 186? when a
Svadlsh patent was granted to Chlaaon and Borrbln for a co—oaite dynamite.
Destruction by Chemi—1 Deco—osltion;
A—turn nitrate la deccmpoaed by strong slkalias vitb the liberation of a—onia, and by
sulfuric add with the for—tlon of a—maium sulfate and nitric add.
Hafer—a;3
(a) Depart—nta of the Any and the Air Force IM 9-19Ю/Т0 lia-1-ЗА, Military Explosives,
April 1955.
(b) P. F. Macy, T. D. Dudderar, E. F. Rease and L. И. Er? keen, investigation of Sensitivity
of Fertiliser grade As—irfum Bitrate to Explosion, BAIR Mo. 1658, 11 July 19A7«
(c) D. P. ZfecDougall, Methods of Physical Testing, OSRD Report Bo. 803, 11 August I9A2.
(d) L. C. Sdtb and 8. G. Ryater, Physical Testing of Explosives, Part III - Miscellaneous
Sensitivity Testa; Performance Testa, dan) Report Mo. 5746, 27 December 19**5*
(a) Inter—tiooal Critical Tables, McGraw-Hill Book Co., N. Y-, Land-Bornet.
G. D. Clift and В. T. Federoff, A Manual for Explosives laboratories, Vol. II, Lefax
Society, Inc., Philadelphia, 19A3.
(f) R. J. Finkelstein and G. Gamov, Theory of the Detonation Process, NAVORD Report Mo. 90-
A6, 20 April 19A7.
(g) George Felck, The Dissociation Pressure and Free Bnergy of Formation of Aamonlim Bi-
trate, Arthur D. Little, Inc., J Aa Chee Soc, |6, 5b^B-60 (19y>).
(h) M. A. Cook end M. Taylor Abegg,*Isothermal Decomposition of Explosives? University of
Utah, Tnd rng than. June 1956, pp. 1090 to 1095-
•’See footnote 1, page 10
24
/iiwonlw itratc
AMCPTM*177
(1) Alao sea tbe fbllovlnc PlMUmy Aiwenel №ehnlcel Reports on Awonitm mtrata;
, 0 1 e 2 * 2 6 I 8 2
a*o «81 18г 1*3 3» 59« 907 5*8 799
so Т» 1302 1323 9» 11*5 666 HIT «38 13«9
1051 iia 1T83 109* 1225 676 19*7 938 1*09
1290 12*1 2183 121* 1*55 9*6 2167 1008
1780 uai 123* 1«55 1106 1038
1391 139* 1675 1696
1*31 1725
25
АМСР7М-1П
/nntua Perchlorata
<L Metocatoe Weights (CU^M^) 11T-5
a ».s Ж 11.9 BdMNBt Stf Ж?
J.» Beaeiipt gm/cc 1«95
0 5b.5 Melttag Petat: *C
C/H Ratto rVMWny vW« V
hugest SnedlMly, 1 Kg Wh . Bureau of Mine* Apparatus, cm 67 Sample Wt 20 mg Fkottony Areonal Apparatus, in. 2b Sample Wt, mg 2b Baling Potato *C
I ft ft ft
Pitaitae Heduhaa Teeta Stool Shoe Snaps Aber Shoe teffteted Vueaaa ItoMWr Taeft . CC/40 Hri, at КГС 100*C 0.13 120*C 0.80 135’C ISO’C 0.^
Rifle Mtotimpoct Teets Trial* % Explotions ttorttek
Burned Unaffected 280 вина Bomb S^nd Teeh Sand, grn ' 6.0
(epleefeft ТеяфеЮм**! "C Seconds, 0.1 (no cop used) 1 S b35 10 1C *•___tablln Minimum Detonating Charge, gm Mercury Fufminote LsodAxide 0-80 Tetryl 0.25
20 BelMto Mortor, bb TWTr
-- TmedTato, » ТРГ:
7S*C latemotfeael Hoot Teett % Lots in 48 Hr»
Plate Dent Тей: Method
WC Hoot Тей! % Loos, let 48 Hr» 0.02 % La», 2nd 48 Hrs 0.00 Explosion m 100 Hr* Bone Condition Confined Density, gm/cc Brisance, % TNT
jpg- e toe» 0_ j aWCWwBV^mVVy BIBSVBe 9M0MtlM RMBt Confinement
NvMeceMcetvs % rsyjsww*r^wtt|t /w Charge Diameter, in. Density, gm/cc Rate, meters/socand
VetotMity:
АяюаНмд Perchlorate
I - ,
NegnMdMtet Теск M ай Mb МЛ l^wNK Ш WC41i Dsnsay. gm/cc i? " ChorgsW»,»» . ;.\. /м^вммйк For TNT ЕогЭДос'НЕ > ЧЙМ, MM1 h«Hfe U» K€4k Jwrty, •**/& ;• ' . TteMMm af faMfe Um TNT C ; for SubNet HE Steped Charge МееНееми, TNT = IWt Gloae Cones Steel Cones raw vomww Hole Depth . c
Ccte: Colarleee
hleNpel Umm, Zxploelve ingredient of Mixtures .tied in pyroto«hnlee and aa projectile filler
Method el Medhg: Proceed or cant depending on ccripoeltion of mixture
leedlng Deeehy: gm/cc ; Variable
ч^Я^^ШвШК П/ м^йс At 9 ft .’- : At2Sfcft Density, gm/cc
«WWJVe Method Dry Hozoed Clast (Quantity-2.»t..^e) H.ese 9 Compatibility Group Exudafic Bone
МмЮЫШееиТНТ): ' AM ЯкАРпашге Instate <. ^*ЧУ Atof ' кгф&е . ItafaWtBc StehPrmurs Impute. Energy Ur4w^mm4j Pooh Pressure Impute Energy
Solubility da Water дц/ЮО co saturated eolation; O^C 12 2J°C 2C 60°C 39 100°C 38 Prepetition; 'Bte- perchlorates ere prepared by the action of the add on a suitable base; by the ther- mal decornoeition of certain chlorates; end by the electrolysis of chlorates (see origin). Heat of; Fortaetloii, cal/gm 665
27
AMO7W-1H
Annul иш Pvrohlorate
Orsini (в)
g. Mlteeherliob fleet ргфагеб, in 1S32, crystals of ажое1ия perchlorate free hariun
porthleeate and мвмАав Mlftrte (Рои Aan Sg, 300). T. Schloslng treated a hot notation of
eetiM perchlorate with eoamolua chloride, and on cooling, crystals of vwilua parohlorate
мм obtaiaod (Ооцр rood, Jt. 18б9, ДОГц). U. Alviei treated a nixture of 76 porta at aa-
vnlt* nitMto with £13 porta at sodlua perchlorate, and obtained crop of anell crystals of
емааАав narahloeato thick were purified by recrystfilllest-’.on frou hot voter (Сети Patent,
103,993, ?Ь?8). A> hiolatl idxed uagneeiua or celciue perchlorate with MMtHun chloride e&C
wyrtela of аамйХио perchlorate depoaited f*oa the solution of very soluble aagneeiun or oal-
iloa chloride (Oomn Patent, 112, 682, 1899).
BafMancaat*
(a) V. B. tfllneon, Jr., Fhyeleal ^nd fttploelve Propertlee of Military teplorlvee, Pa2R
Яо. 1372, 29 Bomber 19M-
(b) T. b> Devil, the Cheoirtry of Powder and gxploeivei, John Wiley end Sone, Inc., Uev
Tort, igki. >
(с) 3. V. Mellor, A Cooprehenaive amtlee on Inorganic and iheoretical Omietry, Vol. II
LooBarams, Zfewen and Co. , London, 19^2, P- 39b.
(d) Alao see the following Picetinny Arsenal Technical Reports cm Armonivii Perchlorate:
0 «» 1 c 1 £ 2 6 2
ICO 521 843 354 1095 1726 1049
1783 604 1725 1969
8^4 2205
ее footnote 1, pege 10.
28
Beretol
ЛМСР 706-177
Compoeltioa: % Barium nitrate 67 TNT 33 C/H Rotio Molecular Weight: 125
Oxygen Mance: CO, % -3 co % +13
Density: gm/cc Cast 2.55
Melting Point: °C
Frvexing Point: *C
Impoct Sensitivity, 2 Kg Wt: Bureau of Mines Apparatus, cm 35 Sample Wt 20 mg Picatinny Arsenal Apparatus, in. 11 Sample Wt, mg 2t Boiling Point: 'C
Refractive Index, ni nS n£
Friction Pendulum Test: Steel Shoe Fiber Shoe Vacuum Stability Toot: cc/40 Hrs, at 90*C 100’C I2O°C 135 °C I5O“C
Rifle Bullet Impact Tost: Trials % Explosions Portiols Burned Unaffected
200 Gram Bomb Sand Toot: Sand, gm 26.8
Explosion Temperature: °C Secc xh, 0 1 (no cap used) 1 5 Ignites 385 10 15 20 Sensitivity to Initiation: Minimum Detonating Charge, gm Mercury Fulminate lead Azide 0.20 Tetryl 0.10
Ballistic Matter, % TNT:
Trouxl Test, % TNT : Plate Den Test: (e) 7327 Method В Conditior Ject. Confined No Density, gm/cc 2-52 Brisance, % TNT 61
75 C tirtemotioMol Hoot Test: % Loss in 48 Hrs
100 C Heat Ти*: % Loss, 1st 48 Hrs % Loss, 2nd 40 Hrs explosion in 100 Hrs
DetMeilM Rett*; Confinement Condition Charge Diameter, in. Density gm/cc Rate, meters/seccnd
Flammability Index:
Hygroscopicity: % 30°C, 90Й Mi 0.00
Volatility:
АМСР 706-177
Bexttwl
Beeetwr ТйеУуВу Teat:
. Condition . ORSt .
Tetryl, gm 10c
Wax, m. for 90% Detonation 0.32
Wax, gm'
Denelty, grhztc . S-S5
'KNMtoft
Combustion, eal/gm
ExpiaMon, tai/gm
Gas Volume, d;/gm
Serftwtlon, eol/gm
Reson, cci/gm 75/25 Bar* to 1 2-6Г (d)
ItaeeetpMifae ЕфюМю:
Ox/реп, etsrse/sec
iZ/»*c)
Heat, ktloealorte/moHi
(AH, kcal/mol)
Twntperakae Range.- *C
FbaM '
Апи* Hfctw hegec* Teat:
M еые Mertur Л^'тлЙв)
$0% inert. Vetocity, ft/sec
Aluminum Fineness.
SpecNk Heett col/gm/’C («) 75/25 Beretol •' v ' ' - • . X Ptoto 'thicktfw, inches
°C. J wato
-75 0-152 . 75 0.260 - . . 5
0 0,14 25 0.180 50 0.229 85 .«• ' 100 0.213 0.a»l О.ЭП: ' L . - 1%
Rtwetag Rate: cm/fifc <
_;.Э>вя«е1 CeodesKvfcy: ccf/tec/cm/’C
Linear, %/'С
Max Safe Drop, ft
SMhfe SiMoref fergeae Bomb ч Cewcittei
Г
Volume, %ГС
КбгЗеем, Make* Seek;
E', dynes/ctm
’E, tt>/inch*
Deve.it/, gm/cc
Height, ft
- Trio1»
Jnaffpcied
Low Order
High Order
10ЭСМьвлмы«1 forpwc* )«mb n Concrete:
Height, ft
Ceetproechra-fM'iglb: lb/inch1
Yager bewere:
‘ ’C .
mm Mercury
Unof)ec'«* ,
l.ow Order
High О«йг
iil)
Beretol
Амсртм-m
Twtt M ям» M. MJ1 taMk Let WC-»1: Density, gm/cc Charge Wt. b For TNT For Subject HE 9 inch HE, M42A1 PsejecMa, Let KC-St Density, gm/cc Charge Wt, b ^Л Кш^кп^Ьа IVWI ^^Ve We Пч^ЯВИае» For TNT For Subject HE Shaped Charge EHesttreoees, TNT = 1B0: Gloss Cones Steel Cones Hole Volume Hole Depth
Cofer:
Medpei Usees Bos* filler
Merited of I sodfag: cast
Leedhj D xn*y: gm/cc 2.55
FregnMet Vsiesby: ft/sec At» ft At 25% ft Density, gm/cc
IIWN^» Method Dry Hazard Class (Quantity-Distance) Claes 9 Compatibility Group Group I Exudation
Msst (BsleHvo to TNT): Abt Peek Pleasure Impulse Energy Ab, Confined: Impulse Under Wafer: Peek Pressure Impulse Energy Peak Pressure Impulse Energy
Preparation: The appropriate vel&ht of barium nltnte heeted to about 90°C la added to molton TNT contained in a melting vessel equipped with an agitator. Continue mixing until 'inffnrm, and load by pouring at the lowest practical temperature. Origin: Paratol, an explosive conta'nlng berlum nitrate and TNT, the proportions varied to suit the lequlred purposes, vaa developed during World War I.
31
АМСР 706-177
Baratol
References;5
(a) D. P. KcDougall, Methods of Phy ileal Testing, OSRD Report Bo. 803, 11 August 1942.
(b' L. C. Saith and E. G. tyeter, Rnralcal Ihsting of ExploiIves, Pert HI - Miscellaneous
Sensitivity Teste; Performance Testa, 6S№ Itepori Ho. 5746, 27 December 1945 •
(c) Also see the following Pleatinny Arsenal Technical Reports on Baretol:
0 2 6 8
2010 1783 2226 2138
2160 2233
(d) C. Lenchiti, W. Beach and R. Vallcky, Enthalpy Change», Heat of Fusion and Specific
Beat of Basic Expletives, РАШ Ko. 2504, January 1959.
^See footnote 1, page 10.
H2
Ba ro cal
АМС» 706-177
ГдаиммШм1 % Barium nitrate 50 TNT 35 Aluminum 15 C/H Ratio Molecular Weight: щ
Osygen Boloace: CO- % -2a CO % -7
Doeslty: gm/cc 2.32
Melttag Mat: *C
Freezing Mart: “C
Impact Sensitivity, 2 Kg Wt: Bureou of Minas Apparatus, cm 30 Sample Wt 20 mg Picotirmy Arsenal Apparatus, in. 12 Sample Wt, mg 22 1M-- gz* e No
Refractive Index, n® n« П&
Friction Pendulum Test: Steel Shoe Fiber Shoe Vacuum SfobHity Test: cc/40 Hrs, ot 90*C lOO'C 120'C 135’C I5O"C / 2K Grew bomb Saad Tost: SorJ.i-n 39-6
Rifle Bullet Impact Test: Triols % Explosions Porticls Burned Unaffected
Explosion Temperature: *C Seconds, 0.1 (no cop used) 1 5 Ignlten 3**5 10 15 20 < t»e__ta_ '«Jgl^laar W lwWWIWW* Minimum Detonating Charge, gm Mercury Fulr>'Jnnt« Leod Azide 0.20 Tetryl 0.10
BeMstic Mester, % TNT: (a) 96
Trawxl Tost, % TNT:
7S’’C International Heat Test: % Loss in 48 Hrs
Plate Dent Test: Method Condition Confined Density, gm/cc Brisance, % TNT
100’C Heat Test: % Loss, 1st 48 Hrs % Loss, 2nd 48 Hrs Explosion in 100 Hrs
Detonation Rote: (b) Confinement hone Condition Cast Charge Diameter, in. i.q Derr ,i tv, gm/cc 2- 32 Rats mefsrs/second 5^50
Flammobility Index:
Hygroscopicity: %
Volatility:
АМСР 706-177
Beronal
PregmoateHea Teat: М ям на. МП FioiosNh, IM WC-91: Density, gm/cc Charge Wt, b IWwi ^^Ve vsWJMVR*** For TNT For Subject, HE I lack HI, M42A1 FiejocNh, IM KC-S : Density, gm/cc Charge Wt, b a^Mt ^^*e ОТ VVV^HWOTve For TNT For Subject HE * /к М^Мкмам ТЫТ tftfte w40OT^^ 1 VW 1 ** O^^Me Gloss Cones Steel Cones Hate Volume Hate Depth
Cohn
Prtadpel Uses: Bomb filler
Method M Loedlag: Cast
Leadbg Dsaetty: gm/cc 232
FragmeM Vs 1st by; ft/sec At9ft At 25% ft Density, gm/cc
Storage: Method Dry Hazard Class (Quontity-Distcnce) Class 9 Compatibility Group Group I Exudotion
Meet (Ralativo te TND: AM Peak Pressure Impulse Energy Ab, Ceaflaed: Impulse Uadar Water: Peak Pressure Impulse Energy Peak Pressure Impulse Energy
Preparation: Procedure same as described under Bnratol except aluminum is added to the barium ni- trate-fflT molten mixture under agitation until uniformity in comparison is obtained. Booster Sensitivity Test: Condition Cast Tetryl, gm 100 №x, in. for 50^ Detonation 0-86 Density, gm/cc 2.32 Heat of: Corab'.stlon, cal/gm 2099 Explosion, cel/gm 1135 Gas Volume, ce/gu klO
:м
ЛМСР 706477
Beronal
References;6
(а) L. C. Skdth and E. Q. Ryster, Physical Testing of Explosives, Part III - Rjecellsneoua
Sensitivity Teetsi Perforaance Teets, Ьбкй hepori 1Йо. 57*6» ST Baoakbe* iw?
(b) Q. ... Masterly, Д» Mte of Detonation of Various Explosive Ccaaou.ie, OSRD Report Ro.
1219, 22 Nbraaiy 19*3-
N. n. Bunrita, Де mte of Detonation of Vartans ои-™м» and Mixtures. OSRD Report
о. 5&1, 15 January 19*6.
(e) D. P. MacDougall, Methods of Physical Tevtlne, OSRD Report Ko. 803, И August 19^2-
(d) Arthur D. Little Report, Study of Pure Explosive Ccapounds, Part III, Correlation of
CHUceltlon of Mixture rlth Jerforeemce, Contwwt Ко. 1А-19-ое0^0ВВ-1й, 1 Nay 1950.
(e) 8. J. Lovell, Propagation of Detonation in Long and Narrov Ooluans of Explosives, PAIR
10. 2138, ЛЪхиагу \----------------------------------------- ----------
6See footnote 1, page 10.
35
АМСР 700-177
Black Power
% Potaeeiua nitrate Th.0 Sulfur 10.4 Charcoal 15-6 C/H Ratio Molecular Weight: 84
Oxygen Balance: CO, % .22 CO % -2
Density: S /cc Variable
*C
PfWKM| uVtWao 4o
hngect Sensitivity, 2 Kg Wt: Bureau of Mlrvu Apparatus, cm 32 Sample Wt 20 mg Pleotinny Arsenal Apparatus, in. 16 Sample Wt, mg 16 BeMng Feint: *C
Reftoctive Index, n« n£ n£
YnM« HwsW^ 1 Steel SSnape fiber Shoe Unaffected WCWwNBI ^wwlBty a Wo cc/40 Hrs, at 90'C 100‘C 0.5 120*C 0.9 I35*C ISO'C
Rifle Ballot Impact Test: Triols % Explosions Portiois Burned Unaffected
200 Grom Bams Sand Tost: Sand, gm 8
Explosion Temperature: *C Seconds, 0.1 (no cap used) 510 1 490 5 Ignites 42? Ю 356 15 20 Sensitivity io Initiation: Minimum Detonating Charge, gm Mercury Fulminate I od Aside Зепа^йтге to igniting fuse
Ballistic Mortar, % TNT: 50
TreuxI Tost, % TNT: (a) 10
75*C Inlefnatienel Hoot Test: % Lou in 48 Hrs 0.31
Ffato Dent Test: Method Condition Confined Density, gm/cc Brisance. % TNT
IM*C Heat Test: % Lest, 1st 48 Hrs % Lou, 2nd 48 Hrs Explosion In 100 Hrs
Detooetloo Roto: Confinement Condition Charge Diometer, in. Density, gm/cc 1-6 Rate, meters/second 400
Flammability Index:
„ , ЯЬ^С, 7555 ян 0.75 Hygroosopteity: % 25OC, 90* RH 1,91 30°C, 90* RH 2.51
Volatility:
36
Black Powder
AMCP 706-177
ТмФ( Shaped Charge Iffectbenorr, TNT = 100:
МмМ,МЛ Piejestib. Let WG01: Density, gm/cc Chorge Wt, lb Gloss Cone* Steel Cone* Hole Volume Ной Depth
Total He. ef Fregaeentt: For TNT For Subject HE 1 inch HI, M42A1 Projectile, let КСЛ: Density, gm/cc Chorge Wt, b Color: Black
Principal User: 1. Igniter powder 2. Time rings (fuzes)
Total He. of Fragmoetat For TNT For Subject HE Matbed of Loading: 1. Loose (granulated) 2. Pressed
Leading Density: gm/cc ' P*1 * loJ
Fregeseet Velocity: ft/aec At» ft At25Hft Density, gm/cc 25 50 60 65 70 75 1.7b 1.84 1.86 1.87 1.88 1.89
Method Cry
Flanl (ReMre ta THT): Hazard Cloaa (Quontity-Distanco) Class 9
Ain Peek Pressure Impute Energy Compatibility Group Group 0 Exudation None
Air, Confined: 100°C Vecuua Stability Test, cc ges/bO hrs:
Impute Under Woter: Peak Pressure Impute Initial value 0-5 After 2 hours at 65°C 0.86 After 2 Lours st 65°C, 75$ RH 1.46 Sensitivity to Electrostatic Discharge, Joules: (b)
Energy Peak Pressure Impute Energy Initinting Efficiency: Grams Required to Initiate Igniter Comp K-31 2.0 Igniter Coop K-29 2.3 Unconfined >12.5 Confined 0.8 Compatibility with Metals; Dry - Cov'?atible with all metals when moisture content is less the: 0.20$. Wet - Attacks all common metals exc.-pt stainless eteel. Heat of; Explosion, cel684 Ges Volume, ce/gm 271
37
АМСР 706-177
Black Powder
Preparation:
Willav or alder charcoal, flour of sulphur and 2-j£ of water are placed in a tumbling barrel
and aind for a abort period (about 1/2 hour). The mixture is transferred to a "wheel sdll” and
crystalline potassium nitrate containing moisture is added and the mixture is in> orporated
for several hours. During the incorporation period the mixture is kept deep (2-JjC moisture) by
ed-diug water at interval». The mill cake la then pressed at 6000 psi between aluminum plates.
The cres'ied cakes are broken up between rubber or wood rolls. The material is screened and tee
various particle sisea are separated as desired. The screened material is then transferred to
canvas trays and dried in hot air ovens at 6o°C. If it is desired to glaze the black powder,
the material before drying is polished by rotation in a tumbling barrel to give it a smooth
surface, it is next screened to remove the dust. The smooth particles are then placed in a
wooden barrel and rotated with graphite. The material is again screened to remove the excess
graphite, and dried. Material finer than JbO U. S. Sieve is not graph!ted.
WARNING
The batches of black powder must be of sufficient size to cover the bed of the "wheel mill."
If the wheels run off on the bare bed, explosions usually result.
Origin;
The exact date of the discovery of black powder is unknown. Historians attribute its dis-
covery to the Chinese, Hindus or Arabs. The Greeks used it during the 7th Century. Marcus
Graecus in the 9th Century and Boger Bacon in the 13th Century described coaposltions similar
to the present powder. Beginning with the 16th Century, the ccnposition of black powder con-
taining potassium nitrate, charcoal and sulfur has remained unchanged with respect to the pro-
portionality (75/15/10) of the ingredients.
Destruction by Chemical Decomposition:
Black powder can be desensitized by leaching with water to dissolve the potassium nitrate.
The washings must be disposed of separately because the residue of sulfur and charcoal is com-
bustible but not explosive.
Reference.»:7
(e) Hi. Haoum, nitroglycerine and Hitroglycerine Explosives, Baltimore, 1926.
(b) F. W. Brown, D. H. Kusier and F. C. Gibson, Sensitivity of Explosivasto Initiation by
Electrostatic Diecharges, U. S. Department of the Interior, Bureau of Mines fef jbjb, 1946.
(c) Also see the following Plcatlnny Araenal Technical Reports on Black Powder:
^See footnote 1, page 10.
:lx
АМСР 706-177
Bl»c> Povdar
0 1 2 1 6 2 6 I 8 2
250 91 222 163 356 65 56 367 188 379
710 671 272 ЗбЗ 656 615 176 607 318 819
850 661 322 653 566 565 356 637 628 839
1010 901 672 863 556 605 686 567 558 869
1650 1111 692 IO63 576 1165 766 757 598 859
1261 582 U53 596 1275 1256 867 6о8 899
1651 7б2 1263 656 1815 1316 1097 618 1259
1561 872 1333 666 1885 1536 1737 698 1309
1711 1022 16?3 1523 776 1905 1576 1797 838 1339
19U 1622 866 1915 1586 1807 898 1369
1951 1712 I663 1116 1966 1827 1068 1589
2051 1802 1813 П56 1388 1739
1912 1863 1266 1526 186?
1973 1506 1778 1889
1808
1838
1928
2178
АМСР 706-177
l,2,U-Butanetriol Trinity It (BTTN) Liquid
% C 19-9 H„C-0N0o M 2.9 2| 2 Mehculsr Weight: (С^КЛ^) 241
Oxygon Beieaco: CO,. % -17 co % 10
N 17-5 / Density: gm/cc LlQu 14 1.52
Hlzwv*Wo 0 59-7 \ * HpC-ONOg C/H Rotio 0,13 FWnt* ”C
Freesiag Feiat: °C
lipect SenoitMty, 2 Kg Wt: Buroou of Mines Apporotus, cm 58 Sample Wt 20 mg Picotirmy Arsenal Apparatus, in. «1 Sample Wt, mg Bailing Feint: °C
Reflective lodes, 1.473b nS.
Friction Fonduhnn Teet: Steel Shoe Fiber Shoe Vecaem Stability Tost: cc/40 Hrs, ot 90 °C loo'C 2.33 120 °C 135“C 1ЫГС
Rifle Ballet Imps Л Тем: Trials % Explosions portiols
Burrvjd Unaffected 200 Grain Bomb Send Tost: Sand, gm 1»8.6
Ixpiesioa Temperature. "C Seconds, 0.1 (no cap used) 1 5 Decomposes 230 10 Sensitivlry to Inltietien: Minimum Detonating Charge, gm Mercury Fulminate Lead Azide 0.20 Tetry! 0.10
20 Ballistic Meteor, Si TNT:
Tceoxl Tost, % TNT:
7S C loternationel fleet Test: % Loss in 48 Hrs
Flats Dent Toot: Method
100 C float Tost: % Loss, 1 st 48 Hrs 1.5 % Loss, 2nd 4b drs 1*2 Explosion in 100 Hrs None Condition Confined Density, gm/cc Brisance, % TNT
Detonation Roto: Confinement Condition Charge Diameter, in Density, gm/cc Rate, meters/second
Flammability Index:
Hygroscopicity: % (a) 100°T, 957 RH, 2L hrs 0.1b
»» 8 ▼•mtwy: o 60°C, mg/cr.yhr 4C
1,2A-BatHnetrioJ 'iYl'.il tin11- (BL'LTi) ,'Jq.ld
AMCP 706-177
re^jan^i^steti^t^s ^Fest . ♦0 лм HE. M71 Prejectiio, Let WC-91: Density, gm/cc Charge Wt, lb Total No. of Fregments: rar TNT For Subject HE 3 lech HE. M42A1 Projectile, Let K£ S- Density, gm/cc Charge Wt, lb Total No. M Frogmeoto: For TNT For Subject HE $hop«d Charge EHecHveaou, TNT - 100: Gloss Cones Steel Cones Hole Volume Hole Depth
Color: Yellow oil
Principal Uses: Explosive plasticizer for nItrocell :lose
Method of Lending:
Lending Density: gm/cc L • 5£
Fragment Velocity: ft/sec At 9 ft At 25^ ft Density, gm/cc
Stor«9«: Method
Btoot to TNT):
Ain
°eak Pressure
'mpulse
Energy
Air, Confined:
Impulse
Under Water:
Peak Pressure
Impulse
Energy
Uadeeg round:
Peak Pressure
impulse
Energy
Hee . of :
Cor.: jt'.iO:., -ul/ •::.
=:?7 , CG]/.--
z Vd.-t, c- -
ft)
:)
Hazard Class (Quantity-Distance)
Compatibility Group
Exudation
lol.c’ it. Ic ’Лгег, £/100 et: (e)
HG C 0.0’
FT-C 0.15
Solucill*, of Wa ter I, (8)
sn/100 gra: 0.0E
SoltAli:*-, ..—./LOO
6- 25°C, Ir:
Ether .,1
Alcohol <v
£:L i^.h^rrAlcv.ol
r C2*.0fZ-
АМСР 706-177
1,2,1-Butanctriol Trinitrate (ВЯЖ) Liquid
Pregaratlon (labotetcxy Procedure):
lb a cooled Mixture of 73-8 ga of 1005b nitric add, 16.2 gas of 106.2jt aulfUrlc add and
60.0 go of 96.15! sulfuric add, 30 gas of the original (or redistilled) 1,2Д-butanetriol was
added dropvise with agitation for a period of thirty minutes. Hie teapei-ature of the reaction
alxture was kept at 0°-5°C. When the agitation was completed stirring was continued for one
and one-half hours. The alxture was po-ired into ice water, and the resulting oil suspension
was extracted with three 100 nilliliter portions of ether. The ccablned ether extracts ware
washed with water, then with a scdiua bicarbonate solution and finally with water. The neu-
tralised extract was dried with anhydrous calcium chloride and then the ether was evaporated.
The yellow oil was dried in a vacwa desiccator over anhydrous calri.ua chloride until ths Mate-
rial was brought to constant weight.
Origin;
1,2,4-butanetri.ol was firat synthesised by Ihgner and Ginsberg in 1891» by oxidising allyl
carbinol with potaasiua perouganate under nild conditions (Вег 27, 2b37). Recently the U. S.
Rubber laboratory, under the direction of P. mvney, devised a new synthesis carried out with
allyl acetate and foraaldehyde to give 1,2,1-butane triacetate which was readily hydrolysed to
butanetriol (U. S. Rubber COopeny Quarterly Report, May 19W). Working with pure l,2,h-butane-
triol prepared by an l^roved technique of the Wagner Bethod, the U. 8. Ravel laboratory in
19U8 nitrated the butanetriol on a laboratory and a pilot plant scale (Reference a).
References;8
(a) J. A. Gallaghan, F. facri, J. Bednarlk, and F. McCoD.ua, Tue Synthesis ofl,2,4-Butane-
triol and the Evaluation of Its Trin?.trate, U. S. Naval Powder Factoiy Tecimlcai Report ^o. 19,
------------------------------------------
(b) Also see ths following Plcatlnny Arsens. Technical Reports on Butaiaetriol Trinitrate:
1755 and 1786.
^ee cootnote 1, page 10.
42
Cospoeltlon A-3
AMCP 706-177
OL Mehcokr Weight: 227
rax 91 «u 9 Osygoa Bdoaee: CO, % CO % -W ’23
Density: gm/cc 12,000 psi 1.65
Mefeiag Feint: 'C
C/H Ratio Froeda^ Mat: 'C
Intact SeoeitMty, 2 Kg Wt: Bureau of Mine* Apporotui, cm Sample Wt 20 mg Picotinny Arsenal Apparatus, In. Sample Wt, mg Boiling Mot: ‘C
16 17 RofrocHee Index, n£ nS
M A--IS vavCHVIB fWBB^^PsOBBB 1 w• Steel Shoe Fiber Shoe Unaffected Unaffected Vncrsm SixblHt; Tost: cc/40 Hrs, ot 90*C 100*C 120*C 135*C 150-C 0-3 0.6
RKSe BaMet Impact Test: Trials % Explosion» 0 Partials 0
Burned 0 Unaffected 100 200 Grom Bomh Sand Test: Sand, gm 51.5
Seconds, 0.1 (no cap used) 1 5 Decompose! 10 15 •c 250 Ssesitivify to Initietien: Minimum Detonating Charge, gm Mercury Fulminate 0.22* Load Azide 0.25» * AlJsrSatlve Initiating charges
20 Ballistic Mortar, % TNT: (•) 135
Traer1 Test, % TNT:
75'C laMnteHeaol Hoot Test: % Loss In 48 Hrs
Plato Dent Т» t: Method (b) В В
100‘C Heat Tash % Loss, 1st 48 Hrs % Loss, 2nd 48 Hrs Explosion in 100 Hrs 0.15 0.15 Hone Condition Confined Density, gm/cc Brisonce, % TNT Preased Xo 1.61 126 Preened No 1.20 75
Dotonatiea Rato: Confinement Condition Charge Diameter, in. Density, gm/cc Rate, meters/second (c)
FlemmeMiity Index: 195 None Pressed 1.0
Hygroscopicity: % зо°с, 9O> RH O.C
Volatility: 50°C, 15 da; в 1.0’ 1-59 8100
43
АМСР 706-177
Composition А-3
FvOfMMrtvHMI 90 мт HE. M71 FrvjecHla. Lot WC-91: Density, gm/cc 1 - 62 Charge Wt, lb 2.102 Total No. of Fragment*: For TNT 703 ForSui.ectHE 11J8 3 inch НК, M42A1 PraiecHle, Lot KC-S: Density, gm/cc 1 • 64 Charge Wt, lb 0.861 Total No. of Frogmortft: For TNT 514 For Subject HE 710 Shope*. Charge Iffoctivenoe*, TNT = IM: Glass Cones Steel Cones Hole Volume Hole Depth
Соки: White-buff
Principal Uses: HE, SAP, AP projectiles; Shaped Charges
Method of Loading: Pressed
Loading Density: gm/cc Pel x 3 I? 1.4т 1.65
Fragment Velocity: ft/sec At 9 ft 2800 At 254 ft 2530 Density, gm/cc 1.61
Storage; Method Dry 1 lozard Class (Quantity-Distance) Class 9 Campatibilil,' Group Group I E> .Jotton J not exude at 65°C when waxes □citing sharply at or above 75°C are used.
list* (Relative to TNT): Air: Peak Pressure Impulse Energy Air, Confined: Impulse Under Water: Peak Pressure Impulse Energy Undergroeed: Peak Pressure Impulse Energy
Preparation: A water slurry of KDX 1* heated to 100°C with agitation. Wax and a wetting agent are added and the mixture, under agitation, la cooled below the melting point of the wax. The wax coated RDX is collected on s filter and al»- dried et T5°C- Effect of Temperature on Fate of Detonation: (e) 16 hrs at, °C -54 21 Density, gm/cc 1.51 1-51 Rate, ш/sec 7бОО 7б?0 Booster Sensltivlty Test: (d) Condition Pressed Tetryl, gm LOO Wax, ir.. for Detonation 1.70 Deneity, gm/cc 1.62 Heat of: -•bustIon2 cal/gm 1210
44
AMCP 706-177
Composition A-3
Compatibility with Metals:
Dry - Aluminum, stainless steel, mild steel, mild ateel coated ’dth acid-proof black paint
and mild steel plated with nickel or zinc are unaffected. Copper, magnesium, magneslum-alumlnua
alloy, brass and mild steel plated with cadmiur. or coppti are slightly affected.
Wet - Stainless ateel la unaffected. Copper, alvjnlnum, magnesium, brasr, mild steel, mild
steel coated wit., acid-proof black paint and mild fteel plated with copper, cadmium, nickel or
zinc are slightly affected.
Origin:
Developed by the British during World War II as RDX and beeswax. Subsequent changes in the
United States replaced beeswax with synthetic wax-?, changed the granulation of RDX and improved
the method of manufacture.
Destruction by Chemical Decomposition;
RDX Composition A-3 (RDX/wax, 91/9) is decomposed by adding It slowly to 2$ times its weight
of boiling 5% sodium hydroxide. Boiling of the solution Is continued for one-half hour.
References:9
(a) L. C. Smith and E. G. faster, Physical Testing of Explosives, Part III - Miscellaneous
Sensitivity Tests; Performance Tests, OSRD Report No. 57^6, 27 December 19^5-
(b) D. P. MacDougall, Methods of Physical Testing, OSRD Report No. 803, 11 August 19^2.
(c) G. H. Messerly, The Rate of Detonation of Various Explosive Compounds, OSRD Report No.
1219, 22 February I9U3.
M. D. Hurwitz, The Rate of Detonation of Various Compounds and Mixtures, OSRD Report
No. 5611, 15 January 19^8-
(d) L. C- Smith and S. R. Vaiton, A Consideration of RDX/Wax Mixtures as a Substitute for
Tetryl In Boosters, NOL Memo 1O,3O3« dated 15 June 19^9-
(e) W. F. McGarry and T. W. Stevens, Detonation Rat .3 of the More Important Military Explo-
sives at Several Different Temperatures, R&TR No, 2303-"November 1956.
(f) Also see the following Picatinny Arsenal Technical Reports on RDX Composition A-3:
0 1 2 1 1 5 6 7 8 9
1380 1**51 IU92 1U93 142U 1325 1556 16o7 1ЗЗ0 1639
1910 1761 2112 1611f 1585 1936 1737 1388 2179
163b 1595 li’97 17^3
215*+ 171; 183З
1635
2235
^See footnote 1, page 1C.
АМСР 706-177
C<wo»itlon В
СвмрмМс*! % мк 6о тнт >ю УШХ, added 1 C/H Ratio Meieceier Weight: 22L
Oxygen leleeee; CO, % -43 CO % 10
Densify: gm/cc fleet I.65
MeMng Met: *C (1) ?S-80
Pressing Met: *C
Mngoet SeaeMvity, 2 Kg Wit Bureau of Mines Apparatus, cm 75 Sample Wt 20 mg Picatimry Arsenal Apparatus, in. Sample Wt, mg 19 Beflieg Matt *C
Refractive Index, n“ nS
vWCSV^MI rgH^^^WI В VS*o Steel Shoe Unaffected Fiber Shoe Unaffected Vacuum StobMty Test: cc/40 Hrs, ot 90*C IGO’C 0.7 120*C 0.9 I35*C 150*C 11+
Rifle Beflet hnpoct Test: Triols % Explosions 3 Partials 13 Burned ** Unaffected 80
200 Great Oemb Saad Test: Send, gm 5^*0
bpteeiea Tomperatora: *C Seconds, 0.1 (no cap used) 526 1 368 5 Decaopoees 278 Ю 255 15 »25u 20 »25O + t»t- Г- - Л- 1иИ1в*1ал> era^^WlvWy eV IllWraiWllr Minimum Detonating Charge, gm Mercury Fulminate 0.22* Lead Azide 0.20* * AjftsPnatlve Initietluc сЬагяев
Ballistic Mortar, % TNT: ') 133
Trauxi Tost, % 1NT: (b) 130
75*C iMenwHenel Hoot Test: % Loss in 48 Hrs
Plots Dent Test: (c) Method В Condition Cast Confined No Density, gm/cc ' . 71 Brisance, % TNT 132
100’C fleet Tert: % Loss, 1st 48 Hrs 0.2 % Loss, 2nd 48 Hrs 0.2 Explosion in 100 Hrs None
DetoMtien Rote: Confinement None Condition Cast Charge Diameter, in 1.0 Density, gm/cc 1.68 Rote, meters/second 7840
ГНяммЫНгу Index: 177
Hygraecopteity: % 30°C, 90# RH 0.02
VeleHBty:
46
Cc^poaitica В
АМСР 706-177
Baaatar SeutMvDy Тем. (d) Condi tt-п Cast Tetryl, gm 100 Wax, in. for 50% Detonation l.bO Wax, gm Density, gm/rc 1.6$ Oxygen, otoma/sac (Z/sec) Hoot, kilocolorie/mola (AH, kcol/mol) Temperature Range, *C °hase
Нее» at; (a) Combustion, csl/gm 2790 Explosion, col/gm 12b0 Gas Volume, cc/gm Fo-motioo, col/gm Fusion, col/gm (1) 6.0 У«сЖс Hoot: col/gm/’C (' ) °C Cc -75 0.235 75 0.376 0 0.220 85 O.35U 25 0.2J1 90 0.3Ы 50 0.305 100 0.312 Armor Heir Isspoct Test: (e) M smi Mortar Hojaatlie: 50% Inert, Velocity, ft/sec 209 Aluminum fineness SOtMb Gene-el Purpose Bombs: Plote Thickness, inches Brials Inert 1 It 100 l',4 6 50 liA 2 0 Hi 0
BansMg Xetv:
cm/sec Bomb Drop Tost:
TbatHMl Caadotthiiy: ccil/sec/cm/'C T7, 2000-lt Soml-Armor-Herciag Boasb vs Coacmls:
CoaHldan* of fwpaoeloa: llnoor, %/‘C Volume, %/’C Max Safs Drop, ft SOO-Jb Geaerel Purpose Bomb vs Coecrote: Ko Seel Seel Height, ft UOOO bCO Triol, 65 39 Uno'>«cted 58 Эб
Herdaoee, Mobs' Seals:
E', dynes/cm’ E, Ib/inch* Density, gm/cc Low Order 2 2 High Order 5 1 1000-lb Geaerel Purpose Bomb vs Concrete: Height, ft Triols Unaffected
Compressive Strength: lb/inch’ (b) 1610-2580 Density, ga/cc 1.68
Vapor Praasere: "C mm Mercury Low ( 1er High Order
41
ЛМСР’06-177
Composition В
Fregmeetotien Test: 90 arm HE. M71 Projectile, Lot WC-91: Shaped Charge Effectiveness, TNT = 100:
(g) Glass Cone: Hole Volume 178 Hole Depth 12? (h) Steel Cone: 162 1Ь8
Density, gm/cc Chorge Wt, lb 1.65 2.18?
Tc -el No. of Fragmeotc: Color:
Far TNT For Subject HE 703 996 Yellow-brown
Principal Ums: Fragmentation bombs, HE
3 Inch HE. M42A1 Projectile, Lot KC-S: 1.67 projectiles, grenades, shaped charges
Density. gm/cc
Charge Wt, lb 0.C.-2
Total No. of Fragments: Method of Lending:
For TNT 51U
For Subject HE 701
Loading Density: gm/cc 1.68
Fragarant Velocity: ft/sec At 9 ft
29U0
At 25'. ft 2680 Storage:
Density, gm/cc 1.68 Method Dry
Bloat (Releftee to TNT): (1) Hozord Class (Quantity-Distance) Class 9
Ain Compatibility Group Group I
Peak Pressure 110
Impulse 110 Exudation Very slight when stored st 71°C
Energy 116
Air, Confined: Origin;
Impulse 75 RDX Composition В was developed by the
British between World War I and World War II.
Under Water: It vac standardized by the United States
Peak Pressure 110 eerly in World War II.
Impulse 108 Effect of Temperature on
Energy 121 Rate of Detonation: _ (i)
U ode rf roved; Peak Pressure 16 hrs at, °C 21
10“ Density, gm/cc Rate, m/sec 1.^9 1.69 7720 7660
Impulse Bulk Modulus at Room (:)
Energy Temperature (25°-3O°C)
tracer re.llus cubed 107 Wax In Como В 12 3
> nes/em^ x 10‘10 5.10 3.5-, 2.3b
Densit.;, e’m/cc Viscosity, poises: 1. :'2 1.70
lemp, •; 3° ~ 3-1
2. ?
4h
AMCP 706-177
Composition В
Compatibility with Metals;
Dry - Magnesium, aluminum, magneslum-alumlnuir alley, mild steel, utainlees steel, mild steel
coated with acid-proof black paint and mild steel plated with zinc or nickel are unaffected.
Copper, brass and mild Steel plated with copper C” cadmium are slightly affected.
Wet - Aluminum and stainless steel are unaffected. Copper, brass, mild steel, mild steel
coated with acid-proof black paint end mild steel plated with cadmiu ;, copper, nickel or zine
are slightly affected. Magnesium and magnesium-aluminum alloy are more heavily affected.
Preparation:
Water vet REX is added slowly with stirring to molten T”’" melted in a steam-jacketed kettle
at a temperature of 100°C. Some water is poured off and heating and stirring are continued un-
til all moisture is evaporated. Wax is then added and when thoroughly mined, the composition
ie cooled to a satisfactory pouring temperature. It is cast directly Into ammunition components
or in the form of chips vhen Composition В ie to be stored.
Destruction by Chemical Decomposition:
RDX Composition В is decomposed in 12 parts by weight of technical grade acetone heated to
15°C. While this is stirred vigorously, there is added 12 parts of a solution, heated to 70°C,
of 1 part sodium sulfide (NagS'^gO) in 1 parts water. Ihe sulfide solution is added slowly so
that the temperature of the acetone solution does not rise above 6O°C. After addition _ com-
plete, stirring Is continued for one-half hour.
References:10
(a) L. C. Smith and E. G. Eyster, Physical Testing of Explosives, Part III - Miscellaneous
Senslti.^ty Tests; Performance Tests, OJRD Report Ko. 57^6, 27 December 19^5-
(b) Philip C. Keenan and Dorothy Pipes, Table of Military High Explosives,- Second Revision,
NAVORD Report Ko. 87-^6, 26 July 19U6.
(c) D. P. MacDougall, Methods of Physical Testing, OSRD Report No. 803, 11 August 1912.
(d) L. C. Smith and S. R. Walton, A Consideration of RDX/Wax Mixtures as a Substitute for
Tetryl in Boosters, KOL Memo 10,303, 15 Juns 1919-
(e) "jmmittee of Divisions 2 and 8, NDRC, Report on and IM tonal, t'RD Report No. 5^06,
31 July 1915.
(f) W. R. Tomlinson, Jr., Blast Effects of Bomb Explosives, PA Tech Div Lecture, 9 April
1913. --------------------------------------------------------
(g) Eastern Laboratory, du Pont, Investigation of Cavity Effect, Sec III, Variation of Cavi-
ty Effect with Explosive Composition, NDRC Contract W672-ORD-5723.
(h) Eastern Laboratory du Pont, Investigation of Cavity Effect, Final Report. E Lab du Pont,
contract W-672-0RD-5723, 13 September 19^3-
(1) W. F. McGarry and T. W. Stevens, Detonation Rates of the More Important Military Explo-
sives at Several Different Temperatures, PATR No. 238 3, November, 195^.
**’See footnote I, page 10.
49
АМСР 7064 77
Cow>o«ition В
(J > W. & Craner, Bulk Cwwibillty Data on Several High Rxploalvea, KAvORD Report No.
к380, 15 ЭецлйЬег 195Г
(kJ Alto see the following Plcatlnny Arsenal Technical Reports on RDX Composition B:
0 1 2 2 к 2 6 I 8 2
1360 1211 lkO2 1313 122k 1325 lk66 1207 1338 1339
1530 1451 lk82 11133 lk2k lk35 lk?6 Ik 37 1388 1379
2100 2131 1592 ieo3 19kk 1585 1556 lk57 lk38 IU69
2160 2190 2151 1903 2053 2063 2103 2233 200k 210k 1595 1865 1885 2055 2125 2155 2175 2235 1756 1956 225' l'r37 1797 2007 2ik7 lk50 1688 1728 1828 1838 1978 2008 2138 2168 1819 'OlO
(X) C. Lenchits,
of Basic Buploaives,
V. Beach and R. Vai icky, Enthalpy Changes, Heat of Fusion end Specific Heat
PATR No. 250b, January 1959-
50
Ccegosltlon В, Desaneltlzed
ЛМСР 706-177
СеечмЖоп; I* II** RDX 60 55-2 TNT W 40.0 Wtx, added, (Stanoilnd or Arlstowex, 1650/ 5 1700F) , Vinylseal (MA28-1U), added 2 Vistanex (B120) 1.2 Albccer Wax 3.6 C/H Ratio M^slarWaigM: It ±1 See 0vr.1n.it t.e See (Упор П
Osygen Belence: CO- % See Cyclonite See Соцр В CO See Cyclonite See Coeqp В
Density: gin/rr Cast I.65 I.65
Making Feint: *C
Pressing Feint: 'C
hnpoct Sensitivity. 1M Wt: 1^ Bureau of Mine* Apparatus, cm 95 Sample Wt 20 mg Picotinny Arsenal Apparatus, in. 1U 13 Sample Wt, mg 17 16 Befllng Feint: *C
Reflective lodes, nb
Frictioa Fendehmi Tost: Stwl Shoe Unaffected Fiber Shoe Unaffected Vocuem Stability Tost: I* II*« cc/40 Hrs, at 90-C 100’C 120‘C 0.99 0.92 135’C 150*C 11+ 11+
Rifle Reflet Isapect Test: Triols % I* II** Explosions 0 0 Portiols 0 0 I Burned 5 0 Unaffected 95 100
«rani Bomb Send Tost: I* II*« Sand, gm 52.7 55,0
bpleoion Ta sretere: ’С I* II** Seconds, 0.1 (no cop used) 1 5 Decomposes 260 270 10 15 20 Sensitivity to hMotieo: I* II*« Minimum Detonating Chorge, gm Mercury Fulminate Load Azide 0.22 0-26 Tetryl
Ballistic Matter, % TNT:
Trooxl Test, % TNT:
75'C InteraotioMl fleet Test: % Lou in 48 Hrs
Plate Dent Tost: Method Condition Confined Density, gm/cc Brisance, % TNT
lOv C fleet Test: I* IT** °C Loss, 1st 48 Hrs 0.05 0.12 % Lots, 2nd 48 Hrs 0.19 0.18 Explosion in 100 Hrs None None
Detonation Ha's: Confinement Condition Chorge Diameter, in. Density, gm/cc Rate, meters/second
FlenMnoNlity Index:
Hygroscopicity: % 30°C, 90< RH 0.00 o.oc
VeMfllty; Nil Nil
♦Desensitized Сош: designated I, uses emulsified wax.
♦•Dusens'tlzed Comp 3, designated II, uses _ »ted RDX-
51
AMCP 706-177
Composition В, Desensitised
Fragmentation Test: Shaped Charge Effectiveness, TNT 100:
90 mm HI, МП Prajectito, Lot WC-91: Density, gm/cc Charge Wt, lb Gloss - Hole Volume Hole Depth ones Steel Cones
Total No. of Fragment»: Гэг TNT Color: Yellow-brown
ror ouoiecr nt 3 inch HE, M42A1 Projectile, Lot.KC-3: Density, gm/cc ТП65 Charge Wt, lb 0.87 II<Ht 0 86 Principal Use»: Boris
Total No. of Fragment»: For TNT For Subject HE 51b 609 659 Method of Loading: Cast
Loading Density: gm/cc 1.65
Fragment Velocity: ft/sec At 9 ft 25Ц ft Density, gm/cc
Storage: Method Dry
Obit (Relative to TNT): Hozord Class (Quantity-Distance) Class 9
Air: Peak Pressure Impulse Energy Air, Confined: Impulse Under Water: Peak Pressure Compatibility Group Exudation Croup I
Viscosity, poises: I* II»*
Temp, 83°C 95°3 References; 3- 5 3-1 2.6 2.7
Impulse Energy Underground: Peak Pressure Impulse Energy (a) See the following Plcatlnny Arsenal Technical Reports on RDX Composition B, Desensitized: 1 1 2 2 21>1 1313 1Л35 1756 2053 I0C5
♦Desenciti zed Comp B, deci Kiulsi f iwnx. * *Deser.3i t i zed л,опр i!, dec: coated -.DX. ated II, uses uses
АМСР 706-177
Coop^eitlon С
% ROC 88.3 Pl.eticizer, non» explosive 11.7* *Nonexplosive oily plasticizer containing 0.6)1 lecithin. C/H Ratio Meleceler Weight:
Oxygen Bateece: CO, % CO %
Density: gm/cc
Freezing Feint: 'C
Impact SsorMvMr, 2 tg Wh Витки of Mines Appcroti e, cm 100 Sompie Wt 20 mg Picotinny Arsenal Aoparatus, in. Sample Wt, mg ВеШпд Point; *C
Refractive Index, n» n£ П»
*-* -»* 1 вва> Steel Shoe Fiber Shoe Wt Mh* fmaocr Test: Trials % Explosions 0 Partials 0 Burned 0 Unaffected 100 Veewam Stability Test: cc/40 Hrs, at 90'C loo-c 0.3 I2CC 0.7 135-C 150‘C
200 (ararn Bomb Saad Test: Sand, gtn L6.5
Ksphrlea Temperatine: *C Swonds, 0.1 (no cap used: 1 5 Decoeposes 2Э5 10 15 20 Sensitivity to Initiation: Minimum Detonating Charge, gm Mercury Fulminate Load Aside 0.25 Tetryl 0.11
BaUetic Mortar. % TNT: (a) 120
Tread Teat. % TN*,:
7S’C kHoraaHeaef Heer Test: % Lass in 48 Hrs
Plate Dent Tost: Method A Condition Hand Tamped Confined Yes Density, gm/cc I.58 Brisoncn. % TNT 112
tOO'C Heer Tech % Loss, 1st 48 Hrs C.OL % Lou, 2nd 4P Hrs 0.00 explosion in 100 Hrs Nene
Doteeetiea Reto: Confinement Condition Charge Diometer, in Density, gm/cc Rote, .neters/recond
FlwMwbHtoy lurfwt
Hygraetspklty: % 30°C, 95i RH 0.25
VirstMty: 25°C, 5 days 0.00
АМСРТМ4П
Oocposltlon с
W ее HI, МЛ Pmriri^ let WC-Л: Density, gm/cc Charge Wt, b Tetri No. e* FtepsMata For TXT For Subject HE НееЫЦМОА! PnJeoMo, LMKC-I: Density, gm/cc Charge Wt, ft Tetri No. of Pregeseatst For TNT For Subject HE Shaped Charge MeeHveeass, TXT = IM: (f) (8) Gloss Canes Steel Cone? Hrie Volume 113 Ilk Hole Depth 101 lx
Ceiort White
PrieeMri Usesr Plastic denolltloo ех?1оо1те •
Method of Loedhgi Bond teaped
Leedtag Oeathyt gm/cc 1.^9
Fmgmenf Vrieri^n ft/sec At» ft At 25% ft Density, gm/cc
Method Dry Harard Class IQuantlty-OiManco) Class 9 Compatibility Group Group I Exudation grades abase UO°C
Nsst Mriethe N TXT): Ain Peak Pressure Impuhe Energy Ah* CoedBods Imputes Under Weten Peak Pressure Impulse Energy Peak Pressure Impulse Energy
Plasticity; Belov 0°C Brittle (0°C) 0-kO°C Plastic Above UO°C Exudes (kO°C) references: Boa references for Composition C-k.
54
АМСР 706-177
СгжюеШоа С-2
M^kbBbv WbIqCm
гак 78.7 ВГГ 5.0 ШТ 12.0 МТ 2.7 КС О.б Solvent 1.0 co %
Density: gm/cc
JAoIHb^ FMbF* *C
С/Н Ratio heeeiag Peieti 'C
hnpeat SenakMty, 2 К« Wh 0^ e
Sample Wt 20 mg Picotinny Artanol Apporotus, in. Sample Wt, mg eg og of
PftaNMi Тей* Steel Shoe Pri— *0 HDtC УМ Veceem SteOMy Teat: cc/40 Hrs, ot wc lOO’C 2.0 !20"C 9.0 I3S*C 1SO*C
RMh Mtat hapact Taatt Trial» % ЬфЫопа 0 Partial» 20
Burned 0 Unaffected 80 200 вяиа Beodt Seed Test: Sand, gm iiT-5
Socmdi. 0.1 (no cop used) 1 5 Deeoapoees 285 10 it 15 • 0— MHOWryWy OWwelWe^ROo Minimum Detonating Charge, gm Mercurv Fulminate Lead Aside 0.25 Tetryl 0.10
20 BelUette Metter, % TNT: (a) 126
Treud Tost, % TNT:
71’C IntomaHeeol Hoot Teat: % Loe» in 48 Hr»
P*ete Doot Test: (a) Method В
100*C Neat Tech % Lou, let 48 Hrs 1.8 % Lou, 2nd 48 Hr» 1Л Expiceion in 100 Hrs Bone Condition Hand tamped Confined Mo Density, gm/cc 1.52 Brisonce, % TNT 111
ReaaaeMBy lades: 178 МЮВОТВ0В ВВЯО1 —TO Confinement Bone
HygreoeepMy: % 30°C, 95jC RH 0.55 Charge rm.'neter. In. 1.0 Density, gmA e 1.57 Rate, .neters/iocond 766O
VotaMty: 25°C, 5 daye 0.00
55
АМСР 706-177
Composition C-g
Frogmumtatie* Tost: Shaped Charge EHectlveooee, TNT = IOC:
M м HL M71 Projectile, Let WC-91: Glass Cores Steel Ccnes
Density, g-n/cc Hale Volume
Charge Wt, lb Hole Depth
Total No. of Freguxnts: For TNT For Subject HE C-*5 White
Principal Uses: Plastic demolition explosive
1 tach HL M42A1 Projectile, Lot KC-5:
Density, gm/cc Charge Wt, lb
Total No. of FrogoMMls: For TNT For Subject HE Method of Loedtag: Hand tailed
Lookup Density: gm/cc 1.57
FrogoMot Velocity: ft/sec At 9 ft
At 25% ft
Density, gm/cc Method Dry
hot (Motive to TNT)- Hozcrd Class (Quontity-Distonce) Cleos 9
Air Compotlbility Group Group I
Peak Pressure Impulse Energy Exudation Volatilizes above 52°C
Air, Comflaed: Plasticity:
Impulse Under Water Peak Pressure Belcv 0°C Plastic (-30°C) O-J*O°C Plastic
above J*O°C Hard (52°C)»
i.-.pulse fnergy •Due to vol! tall zat J on of plasticizer.
References;
Undcry round: Peak Pressure See references for Composition C-U.
Impulse Energy
1
AX СР 706-177
Соеров iti on С-3
СееарееШеа: Mehceter Weight:
Те гак Tetryl srr 77 3 b Oxyp.a FeterHe: CC, *. co %
ШТ мкт 10 5 Deaeity: gm/cc
ИС 1 >
C/HRotto Freeeiag Met: ’C
1яфМ* >—2 Kg Wt: Витки of Minot Apporotus, Sample Wt 20 mg Pkotinny Arsenal Apporotu Sample Wt, mg loot lb 33 BeMag Mat: *C
CFW », ?n. Refractive lade*, ng i£ ng
FiKV^RI ТгаВЮТМИе IWXne Steel Shoe Fiber Shoe Unaffected Unaffected Vocaaai StebMy Teet: cc/40 Hr», ot 90*C 100*C 120T 1355' 150'C 1.21 11+
RMe BmNet hupect Teet: Trial* % Explosion* 0 Partial* 40
Burned Unaffected 0 60 2f 7 Stoat Beoib Vad Teet: Sond, gm 53-1
fapieeioa Teetperatere: Second*, 0.1 (no cap used) 1 5 Decomposes 10 IS 20 •c 280 МННПтНу W Minimum Detonating Chorge, gm Mercury Fulminate Lead Azide Tetryl 0.20 0.08
BaMetic Mortar, % TNT: (•) 126
Travel Test, % TNT: (b) 117
75’C leferaatieael Heel Teet: % La** in 48 Hr*
Mete Dent Teet: Method (c) В
IBO'C Hoot Teet: % Lou, 1st 48 Hr* % Lou, 2nd 48 Hn Explosion In 100 Hr* 3.20 I.63 Kone Condition Confined Density, gm/cc Briionce, % TNT Hand tamped No 1-57 118
Detaootiea Rate: Confinement (a) Hand
Нвмм*ЫИ1/ 1я4м: None tamped 1.0 1.60 7625
HygraocepkBy: % ЭО°С, 95$ RH 2.b Chorge Diameter, in. Density, gm/cc Rote, meters/teccsd
VelelMty: 25°C, 5 days 1.15
57
AMCP 706-177
Composition С-3
Shaped Che<ge illsrlhseMS, TNT = IN:
9» мм M, МП PrefcttHe, Let WC-91: Densit, jm/cc 158 Charge Wt, lb 2O*»5 Glees Cones Steel Cones Hole Volume Hole Depth
Total Na. of Fregawats: For TNT 703 лк». Cater. Yellow
roc SUOjOCt Ht r** > lech Hi, M«U1 Projectile, lot KC-5: Density, gm/cc 1.60 Charge Wt, b 0.842 Prteeips) Usae: Plastic desolltlon explosive
I^mwV ^^Ve 4^ «WJMWNVVo For TNT For Subject HE 514 671 Method of Leadbgt Hand taaped
Loadteg Deaekyt gm/cc 1.58
4* /ma* Td^SIVyi It/а^Ъ
At 9 ft At25fth Density, gm/cc Mefhod Dry
Most (UieHeo to TNT): Hazard Class (Quuntity-Distonce) Cisse 9
Air. Peak Pressure Impulse Energy 105 109 Compatibility Group Group I Exudation Exudes st 77°C
Air, Coefaod: Impulse Under Water: Peak Pressure Plasticity; Belov 0°C Hard (-29°C) 0-40°C Plastic Above 4o°C Exudes (77°C) Booster Sensitivity 'Test: (h)
Energy Vi^^HjsWWWi Peak Pressure Impuhe Energy Condition Pressed Tetryl, gm 100 Wax, in. for 50^ Detonation I.36 Density, gm/cc 1.62 Beforences: See references for Composition C-4.
58
СоеровItlon С-1
АМСР 706477
CerapeeMon: * ЮЖ t Плене! вег, поп- Mmecufer WefeRft
91 Oeygea Roieaeo: COs % CO %
I explosive у» * Contain! polyiaobutylene по cor oil 1-6)1 and di(2-ethylhexyl) lab*cate 5.3)6. Deaeity: gm/cc
Mefeiog e*teh *C
C/H Ratio нееемр FeMrs
»*п*ГеВу, 1 K| W»: taeou of Mine* Apparatus, cm Sample Wt 20 mg Picotinny Arsenol Apparatus, in. Sample Wt, mg ВеШад Fsfeh *C
19 27 Refractive ledex, ng n£
FfictfM FwAAmi Teet* Steel Shoe ₽"-«• Shoe Unaffected Unaftacted fe» — cc/40 Hrs, ot 90'C 100*C 120 C 135°C 150*C 0.26
RM* Bsdlet impact Test: Trials % Explosions 0 Partials 0
Burned Unaffected 20 80 200 Gram Bomb Seed Test: Sand, gm 55-7
txpi*"t*e Temperetere: Seconds, 0.1 (no cap used) 1 5 ’0 15 20 •c 290 • Ы V. *»* «*__ «VBBmWBvBay wW lOBBVWn^BMe Minimum Detonating Charge, gm Mercury Fulminate Lead Azide Te»ryl 0-20 0.10
Ba№*HcM<fter, % TNT: (a) 130
Trend Teet, % TNT:
75*C lafwwHoMl Meet Test: % Los* in 48 Hrs
Mete Cent Test: -Aethod (c) E
100’C Her* Test: % я, i 11 48 Hrs % Lp'., ini 48 Hrs Exp! rjon in iOO Hrs 0.13 0.00 I.'cne Cond.Tkrfi Confined Density, gm/cc Brisance, % TNT Heno tanped No 1.60 | П5
Ootoaetleu Rote: Confinement (d) Hand
ЯеампеМЙу lodes: None tanped 1.0
Hygroscopicity: % ЗО^С, 95$ RH Hll CondifKH Charge Riometer, in. Density, gm/cc Rotw, meters/second
VefeHHty: 1.59 3010
59
АМСР 706-177
exposition С-к
^vw^MMRWHWi *V^ N «и» HI. МЛ Projsctite. Lot WC-»1: Density, gm/cc Charge Wt, k> rol Ne. of Fragments: For TNT For Sublet HE 1 inch M. M42A1 Project*, Lot KC*S: Density, gm/cc Charge Wt, b Total Na, of Fragments: For TNT For Subject HE Sbeped Charge Iffotthsesoa. TNT = >09: (jkm Cones Steel Cores Hole Voiijme Ho>« Depth
Celer: Light brow:
Principal Utas: Plastic demolition explosive
Method of Leedk.p: Hand tamped
loodhg Density: gm/cc 1.60
Fiegnsol Vetedty: ft/tec At 9 ft At 25ЦН Density, gm/cc
Metfr d Dry Hazard Class (Quantity-Distance) Cisse 9 Compatibility Group Group I Exudation None at 77°C
Bteot (Ratetbe to TNT): Air Peak Pressure Impulse Energy Ah’. Cenfinod: Impulse Under Water: Peak Pressure Impulse Energy Peak Pressure Impulse Energy
Effect of Tempereture on (1) Rate of Detonation: 16 hrs et, °C -5k 21 Density, gm/cc 1.J6 1>35 Rate, m/sec 7020 TOkO Plasticity: Belov 0°C Plastic (<7°C) O-kO°C Fleet!c Above kO°C Fleatlc (T. °C)
60
AMCP 706*177
Compoaltiona С, С-2, С-3» C-U
Preparetl on;
In manufacturing Cu^oeition C-3, the aised plasticlxlng agent la heated in a melting kettle
at 1OO°C. Wter-wet RIX la added and heating and stlxring are continued until all the eater ie
evaporated. Thia mixture la then cooled and hand preened into demolition blocks or special item
amnni tian.
Cosgroaitlon C-l* la prepared by hand kneading and rolling, or in a Schrader Bowl mixer, RIX
of Mt micron alze or leas with the polyisobutylene-plaetlcizer previously made up in ether, the
thoroughly blended explosive is dried in air at 6O°C and loosely packed by hand tamping to ite
maximum density.
Origin:
Developed by the British during World War II au a plastic explosive which could be band
shaped. It was standardized in the United States during World Her II and subsequent development
led to mixtures designated C-2, C-3 and C-b.
Destruction by Chemical Decoapoeition:
Composition C-3 is decomposed by adding it slowly tc ь solution composed of 1 1/b parts
sodium hydroxide, 11 parte '«ter, and b parts 95$ alcohol, bested to 5O°C. After addition of
Composition C-3 la complete, the solution is heated to 8O°C and mintained at this temperature
for 15 minutes.
References;П
(a) Committee of Div 2 and 8, !TT.v. Report on HdX and Trxtonal, OSRD No. 5bo6, 31 July 19b5.
(b) Philip C. Keenan and Dorothy Pipes, Table of Military High Explosives, Second Revision,
NAVORD Report Nd. 87-b6, 26 July 19b6.
(c) D. P. MacDougall, Methods of Physical Testing, OSRD Report No. 803, 11 August 19b2.
L. C. Staith end E. G. Eyster, Physical Testing of Explosives, Part III - Miscellaneous
Sensitivity Testa; Performance Testa, OSRD Report No. 571*6, 27 December 191*5.
(d) G. H. Messerly, The Rate of Detonation of Various Explosive Compounds, 01®D Report No.
1219, 22 February 19b3-
M. D. Hurwitz, Ihe Rate of Detonation of Various Compounds and Mixtures, OSRD Report No.
5611, 15 January 19b6.
w" R" UomlinBon, Jr., Blast Effects of Bomb Explosives, PA Tech Div Lect'ire, 9 April
(f) Eastern Laboratory, du Pont, Investigation of Cavity Effect, Sec ITT, Variation of Cavi-
ty Effect vith Explosive Composition,~BpRC Coi.tract Wb72-ORI>>?23.
(g) Eastern Laboratory, du Pont, Investigation of Cavity Effect, Final Report, 18 September
191*3, NDPC Contract W-672-ORD-5723.
(h) L. C. Smit end S. R. Walton, A Consideration of RDX/Wax Mixtures as a Substitute for
In try! in Boosters, NOL Memo 10,303, 15 June 191*9.
^See footnote 1, page 10.
61
АМСР 706-177
Coipooitions С, С-2» С-3» C-k
(1) V» Ж. McGarry art T. W. Stevens, Detonation Bates of the Mare Iwxartant Rill try Xxplo-
stves at Several ftnporetures, ВМЯ So. '’3B3, Soveaber 19%.
(j ) Also ме the following Plcatlnny Arsenal Technical Reports on ВПК Composition С:
0 1 2 к 2 6 I 8 2
£&£ 1260 1293 1518 1838
о» c-e 1611 1293 1ПЗ 21% 1595 1416 1к16 1797 1518 1518
°M 9^ 1695 1885 1556 1766 1766 1907 2038 1828 1958 1819
62
Copper Chlorotetresole
АМСР 706-177
Meleeuler Weigh»: (CuCjNgClg) 871
% С 8.9 N—N^ и У™ И L1.5 и—жГ Oxygen Beteece: CO- % -30 co % -le
\Си О. 26.2 г—м< MmByt gm/cc 2.(1»
Си 23-к И—Я^001
С/Н Rotio 0^ nvHMj гяввт 4>
impest SeneitMty, 1 К. Wh Bureau of Mln»» Apparatus, cm Sample Wt 20 mg Pleotinny Arsenal Apparatus, in. 1; (1 lb vt) 3 Sample Wt, mg 9 BeHIng Me»: °C
Refractive Index, n° Па nb
McNea Peedoleni Tee»: Steel Shoe Exploded Fiber Shoe Exploded Veceaae StabMBy Tee»: cc/40 Hrs, ot 90°C 100°C 120°C I35’C ISPC
RMe Mie» hnpect Tee»: Triole % Eclosions Partials
Burned Unaffected 200 Crem Bemb Send Test: (f) B^eSrder fue. ^.3
Ixpheiee Trnrpeietuie: °C Seconds, 0.1 (n. cop used) 1 5 305 10 SeneBMty te InMetien: Minimum Detonating Charge, gm Mercury Fulminate Lead Azide 0.20 0.30 Tetryl 0.10
20 BeHistic Merter, % TNT:
Treed Tee», % TNT:
7S°C iMenwtieeel Hee» Tee»: % Loss in 48 Hrs
Ptete Dent Test: Method
100’C Meet Test: % Loss, 1st 48 Hrs 2.6? % Loss, 2nd 48 Hrs 0.10 Explosion in 100 Hrs None Condition Confined ' Density, gm/cc Brisance, % TNT
BnSe. Confinement Condition Charge Diameter, in. Density, gm/cc Rate, meters/second
НмммЬЛЙу IWts*
HygraccepicJty: % 30°C, 90£ RH 3-11
TVHIhrji
63
АМСР 706-177
Copper Chlorotetraiole
.1 1 "111 111 Shaped Charge МиНгоои*, TNT a Nti
M ша HE, МЛ rraiMtih, let WC-»1: Density, gm/cc Charge Wt, K> Gloss Const Steel Cents Hole Volume Hole Depth
Total Мм «1 Fragments: For TNT For SubHet HE 1 МНЕ,MOA1 hsMk LotKCJi Density, gm/cc Chorge Wt, lb Cebn Mee
Prtoripel Usok Prlsary explosive
Total No. of FrogmeeN: For TNT For SubHet HE Method of Lee** Proceed
Loodkrg Deeri* gm/cc Vе1 x W3 (e)
Fregateet Wbel* ft/sec Ad 9 ft At25Hft Density, gm/cc 10 20 UO l.M ЬбЗ 1.7*1 70 1.86
Staroget Method Wet
Most (Molise taTNTh Haxord Class (Quantlty.£K«;cxe) CiBae 9
Abt Peak Pressure Impulse Energy Compatibility Grc»;> Exudation Group M Hone
Impulse Under Wotan Poofc Pressure Import. Energy Stab aensitf.v tyt (a) Density Firing Point (inch>ouncee) jb'cc. 2 1& 1Л9 9 11 15 1.63 8.5 10 12 1.7L 6 , 9 1.66 U r 6
РеокРгшемге Impulse Heat of: Explosion, eal/ga 432
Energy Specific Heat, ral/ga/°C
Те* range G°-30°C Wt of *мф!е, gv O.i55 0.3910
64
AMCP 706-177
Copper Chlorotetxeiole
Fropaxation: (a)
five змш of 5-anlnotetrazole are dissolved in a nixture at 200 al of eater and TO al of
concentrated Hd. Pnot^h kerosene or nujol (which gives a «lightly cleaner product) is added
to provide в ley» of oil epproodaetely 1/h" thick on the surface. with only Moderate stirring
and external cooling to 10°-15°C> a aolutljn of 5 i{reea of sodiun nitrite in 70 cc of water la
added rapidly by naana of a burette extending below the oil layer. lenediately after thia addi-
tion, a solution at r ga at cupric chloride In a alnluwa anount of water la added all at once,
and stirring la continued for about 1 hour. The r*. -? ion Mixture la allowed to stand for a few
Minutes till the bright blue copper ealt ceparetaa. Ле oil is reMOred by decantation and any
be reuoed. Ihe ealt ie filtered; vaahed vith water alcohol, and fccher; and dried - giving e
yield of 6 grans or 7M-
НОВО
N+-C1-
♦2H20
I—I N—N
I \ci , Cum I \-Cl-,
R vll J UlrW _ I L T Rg
LZ
Cu
— I
^CCl
✓
—N
Origin:
Ihe copper salt of 5-cNtarotetrazole was first described in 1929 by R. Stolle (vith
E. Schick, F. Henke-Stark and L. Krause) who prepared the ccagxxind by reaction of the diazo-
nium chloride of 5-aninotetrazole vith copper chloride (Her feA, 1123).
References:12
(a) H. J. Gaughrac and J. V. F. Khuftaan, Synthesis and Properties of Halotetrazoie Salts,
PAIR Bo. 2136, February 1955.
(b) A. M. Anzalone, J. E. Abel and A. C. Forsyth, Characteristics of Explosive Substances.
for Application In AMMinition, EAIR Bo. 2179, May 1955-
(с) A. C. Forsyth, Pfc, 3. Krasner end R. J. Gaughran, Development of Optimum Explosive
Trains. An Investigation Concerning Stab Sensitivity versus Loading Density of Some Initiating
!^хДЬ7ВДГко. aU,. Rt,r^ry ------4---------- ----------------------
12See footnote 1, page 10.
65
АМСР 700-177
Qranurlc Trlazlde
% C IT.6 Nj И 82.4 /^\ If N rl Лнз C/H Rotio Melecttier Weight: (C3N12) 204
^Soygea Baletsce, CO, % -4T.1 CO % -23.5
Jeasity: gm/cc Crystal 1.5b
Making Potato *C 94
ггают| rvNws u
taipoct Sensitivity, 2 Kg Wto Bureau of Mint* Apporotus, cm 1 kg vt T Sample Wt 20 mg Pleotinny Arsenal Apparatus, in. Sample Wt, mg BeMtag Potato *C
Refractive Index, n° nS n£
FiWl^RI аМИ^^ИОМО • •o Steel Shoe Fiber Shoe Vacuum Stability Tost: cc/40 Hrs, ot 90‘C 100’C 120-C 1?5*C 150‘C
Rifle Mht Impact Tests Trials % Explosions Partials Burned Unaffected
200 6rum Booth Sand Tost: Sand, gm 32.2
bptaiM Тм^мКн: ’C Seconds, 0.1 (no cap used) 252 1 5 10 15 20 Sensitivity to InMatien: Minimum Detonating Gxirge, gm Mercury Fulminate Lead Aside 0.20 Tetryl 0.10
Ballistic Mortar, % TNT:
Tread Tost, % TNT:
75*C Intemational fleet Test: % Loss in 48 Hrs
Plata Dent Tech Method Condition Confined Density, gm/cc Brisance, % TNT
100‘C fleet Test; % Loss, 1st 48 Hrs % Lou, 2nd 48 Hrs Explosion in 100 Hrs
Dotonetien Reto: Confinement Condition Charge Diameter, in. 0-3 Density, gm/cc 1.15 Rate, meters/second 5550-5600
НмммЬМЙу 1и4вх:
QL. rvy^i^vc^^vwy* TV
ValetMty: Decomposes above 100°C !
66
AMCP 706-177
Cyanuric Triazide
M ома HI. M71 Piejsctlle, Let WC-»1: Density, gm/cc Chorge Wt. k> Мж 1 W FV^^pHiVMVWe For TNT For Subject HE 1 task HI. MOA1 PiojectMe, Let KC-Si Density, gm/cc Chorge Wt, ta Y^^M Ma *- . * ImY^ n^^pMiVMW • For TNT For Subject HE j Шаалкмма TMY — IM. WWIJi Koo^YVwV^RBYVVe e W •“ g^^Pe doss Cones Steel Cones Holo Volume Hole Depth
Color: Colorlees
Piin rip el Usee: Not used because of difficulty in controlling sensitivity.
Meth» I of Loedtag: Pressed
Landtag Density: gm/cc At 200 atnospheres 1Л At 800 etnoepheres 1-5
Fragment Velocity: h/мс At» ft At25ftft Density, gm/cc
Stove^^o Method Hozord Close (Quontily-Distonce) Class 9 Compatibility Group Exudation None
। West (Idstive fe TNT): | Ab: j Peok Pressure Impulse Energy Ab. Cleftali: Impulse Under Wotan Peak Pressure Impulse Energy ВВа^^м^^аоп^^Ло Peak Pressure Impulse Energy
67
Cyanuric Triazide
AMCP 705-177
Prtparaticn:
S|y th* reaction at cyanuric chloride vith an aqueous solution at sodium aside
:-ci
H--C
z\
ЗМаИ, —»
3KaCl
Recrystallisation should ba avoided aa it leads to vary large crystals which explode when
broken.
Origin:
Cyanuric Triaside was prepared la iBVf by Cahours from chlorine and nethyl cyanate.
Later Jane* improved the process (JCS gl, 266 (1887) and in 1921 E. Ott patented the prepara-
tion from cyanuric chloride and sodium aside (Baf b) Thylor and Rinkeribech prepared cyanuric
triaside in a pure state and determined ita properties (Ref c).
initiating Efficiency:
Reported to be more efficient than lead aside, capable of initiating Explosive D.
Solubility;
Insoluble in water; readily soluble in hot ethanol, acetone, benzene, and ether.
Heat of:
Fonatlon, cal/ga -1090 to -H38
References:13
(a) A. H. Blatt, Cowilatlon of Data on Organic Explosives, OSRD Report Bo. 2014,
29 February 1944.
(b) Ott and Choe, Bar ^4, 179 (1921).
(c) IWylor and Rickenbach, Bureau of Minas, И 2513 (1923).
mylar end Rinkenbach, J Frank Inst 204, 369 (1927).
l%a* footnote 1, page 10.
Qrelonite* (RDX)
А И СР 706-177
СмеаНи! Motecrrier Weight: (C^NgOg) 222
те Z X C 16.3 OgN-N N—ML H 2.7 Hol CHp > Oeygea Balance: CO, % CO % -22 0.0
H 37.8 \/ Dsaeity: gm/cc Crystal 1.82
0 ъз.2 Ж>2 MoMag Mat: *C 204
C/HRotio 0.095 Кмап1*а
Impact SeasMrMy. T Kg Wlh Buroou of Mines Apparatus, cm Sample Wt 20 mg Picotinrry Arsenal Apparatus, in. Sample Wt, mg & ВеШад Mat: *C
8 18 Refractors Index, nJ l£ nb
ГПКП*Я ГР^ИЯЯ * Wsl Steel Shoe Explodes Fiber Shoe Unaffected Vacuum StebMRy Test: cc/40 Hrs, ot 90"C 100X 120*C 135*C 150’C 0.7 0.9 2.5
Rifle Ballot Impest Test: Trials % Explosions 100 Partials 0
Burned 0 Unaffected 0 200 firom Booth Saad Ted Sand, gm 60.2
lepiooloa Tessporeture: *C Seconds, 0.1 (no cap used) >>05 1 316 5 Оесофоеее 260 10 240 15 235 20 • _—-Sa^R^^ Minimum Detonating Charge, gm Mercury Fulminate Load Azide * Alternltive Initiating chargee, 0.19» 0.05*
BoMriic Metter, % TNT: (•) 150
Trees! Tost. % TNT: (b) 157
9**Л Uteam^laMf *-* * —*- • 9 w ЮТ^тЯ^Р^ИВРВ uv^^te Ifmi % Loss in 48 Hrs
0.03 Plate Doot Test: Method (ch A
100‘C Hoot Test: % Loss, 1st 48 Hrs % Loes, 2nd 48 Hrs Explosion In 100 Hrs 0 04 0.00 Mono Condition Confined Density, gm/cc Prisance, % TNT Pressed Yes 1.50 135
HemmebMty lades: (d) 278 Confinement None Pressed 1.0 I.65 3180
Hygroscopicity: % 25°C, 100$ RH 0.02 Charge Diameter, in. Density, gm/cc Rate, meters/socond
VofaHBty: Nil
*Neme given by Clarence J. Beln of Pice tinny Arsenal. Germans cell It Hexogen; Italians call
it Ofc; British, RDX.
лмсртм-m
Cyclonite (RDX)
vWH^^r вИоНТгЖу I BWi Condition Tetryl, gm Wax, kt. for 30% Detonation Wok, gm Density, gm/cc DeeompooMon Bguetioa. (1) ,0 c Oxygen, otoms/soc IO10*’ (Z/soc) Heat, kilocalorie/mde bf. 5 (AH, kcol/mol) Temperature Range, *C 213-299 Phase Liquid
Xsotefi Combustion, col/gm 2285 Explosion, col/gm 12BC Gas Volume, cc/gm 90S Formation, col/gm »$S Solution, cnl/nol (28-55% BS^) 7.169* •Aamming cyclonite uniwoleculnl- Armor Hate Impost Test: Wmm WIWTWr 50% Inert, Velocity, ft/sec Aluminum Fineness
Specific host: col/gm/*C °C °C 20 0.298 100 О.Ьоб bO O.33I 120 O.b2? 60 0.360 Ibo O.bb6 80 0.38b Plate Thickness, inches 1 1’Zs 1% 1%
cm/soc
Bomb Drop Test:
Thermal CeeducNvIly; (h) . cal/soc/cm/*C 1.263 6.91 x 10*? Density, gn/cc 1-533 6.96 x 10~* 17,2M04b Semi Armor Herring Bomb vs Concrete:
Linear, %/*C Volume, %/*C Max Safe Drop, ft S0%* Geesrel Purpose Bomb vs Generate: Height, ft Trials Unaffected
Young's Modulus: E', dynos/cm* E, b/inch* Density, gm/cc Low Order High Order lOOCMb General Purpose Bomb vs Generate: Height, ft Trials Unaffected
CaortWoesive Strength: tb/ineh’
Vaer.wl тенге: *C mm Mercury Low Order High Order
70
АМСР 706-177
Cyclonite (RDX)
WmM, Mil Pujsctih, Lot WC-61: Density, gm/cc Charge Wt,b Tefal Ha. ef Fragaseata: For TNT For Subject HE 1 Saab M, MOA1 F»|ec«U Let KC-Si Density, gm/cc ChorgeWt, R> Tefal He. of Fragments: For TNT For Subject HE Shaped Charge SHectiveeass, TNT = 106: GIom Cones Steel Cones Hole Volume Hole Depth
Color: White
Priadpai Utas: Detonator beae charge, and Ingredient for projectile and bomb fillers
Method af Loedtog: Pressed
Loading Daaehy: gm/cc P«1 x 10^ 3 5 10 12 15 20 1.46 1.52 1.60 I.63 1.65 1.68
Ffagnmet Vatadty: ft/sec At» ft At25Hft Density, gm/cc
4-^ S tux dvwmoci "* Hozord Class (tyantity-Distonce) Class 9 Compatibility Group Group M (vet) Group L (dry) Exudation Hone
Май «KaisHvo t* TNT): Abt Pack Pressure Impulse Energy Ahr, Confined: Impulse Under Wotan Peak Pressure Impulse Energy Peak Pressure Impulse Energy
Effect of Temperature on Rate of Detonation; (k) 16 hrs at, °C -51» 21 Denalty, gn/cc 1.61 1.62 Rate, a/sec 8100 8050 Effect of Temperature on Inject Sensitivity; Temp. Bk Impact Test °C 2Kg Wt, Inches Room 9 32.2 8 104 5
71
АМСР 706-177
Сус Ionita (ШК)
Solubility of Qyclonita; gm/100 ст of the following aubctancea; (j)
Water Alcohol Acetone Henzene Tolueno
Од 30 0.005 “o’ оТоБЬ “o’ -A i A 20 0.05 “S’ A15
50 0.025 20 0.105 20 7.3 ho 0-09 20 0.02
70 O.CT* h0 0.2h0 ho 11.5 60 0.20 hO 0.05
90 0.19 60 0.579 60 18. 80 O.hl 60 0.13
wo O.ae Ethyl acetate 78 1.195 Carbon tetrachloride Methanol Bthar 80 0.30 100 0.65 ЯТ
i- A Од 50 0.005 Од 0 ол4 _£ 10 0.05 S’ A
9h 18. 60 C.007 20 0.23 20 0.056 85 5-0
70 0.009 hO O.hf 30 0.076 90 5-55
lecaevl Methyl 60 1.1 Althosrethyl 95 6-2 100 7.0 105 7-9 Trichloro-
•oetate acetate Chlorobenzene ethylene
Og_ 0 0.02 •22- 4- 20 275 _°C < 20 0.15 ± 20 0.33 20 oTSo
20 0.03 30 3-3 30 0.16 30 O.hh 30 0.22
ho 0.065 ho h.l hO 0.19 hO O.56 ho 0.2h
60 0.22 50 5-6 50 0.25 50 0.7h 50 0.26
80 0.5h 100 1.35 Tetra- 1^^>~ laobutanol Chloroform Meaityloxlde
S’ 4g- Jk ЭВ 0.18 Од 20 0.0 Од g 20 0.01 °C I ~SF 3З2
Cyclo- Mitro- Mitro- tyclo- 97 12.2
brazene ethene pentanone Acetonitrile
& °S_ Л. 25 1.5 S A 4g.A ar 11.5 °C % 2S”fl
97 25 97 12.h 93 19 90 37 82 33
Methyl ethyl ketone
72
Cyclonite (RM)
AMCP 706-177
Solubility of Cyclonite, Holeton Lot B-2-5 in Various Solvent»:
Boiling Heated Cryatalline Fora
Solvent Potntj Grade or Source4 28°C
Acetone 56 CP 8.2 I6.5 at 60°C hexagonal-thick
Qrclohextnone 155.6 CP 13.0 24.0 at 93°C cubic (naaalve fora)
Rttronethane 100.8 1-5 12.4 at 9T°C platen
Acetonitrile 81.6 Mi acet Chea. Co. 11.3 33.4 at 93°C platen
1-litroprcpane c 126.5 H Preet 1.4 10.6 at 93°C short needlee
P-Rltropropanr - 120 BC Praui 2.3 11.6 at 93°C abort needlee
2,4-Pentanedione 140.5 Carbide Ь Carbon 2.9 18.3 at 93°C flat prisns
NethyUodbutylketone 115.8 2.4 9-6 at 93°C long priane
n-Propylacetate 101.6 К Sad label 1.5 6.0 at 93°C long priane, все» cubic
n-Botylformte 105.6 К Red Label 1.4 4.6 at 9S°C long priane
Ethyl acetate 77-1 Baker*в T 2.0 6.1 at boil. hexagonal platea
n-Rrapylpropiooate 121 К Red label 0.8 1.6 at 93°C abort priane, всем cubic
Butyleoetate 126.5 К Technicel 1.1 4.0 at 93°C long priana
Netbylethy Iketone 79.6 5-6 13*9 at boil. coerce platea
litroethane 114.2 К Red label 3.6 19-5 at 93°C platea
laopropylacetate 88-90 CP 1.1 3.2 at boil. long prions
Mealtyloxide 128 Bi Red label 4.8 14-5 at 93°C platea
n-Anylaeetate 146 CP 1.0 2.1 at 93°C priane
Ctnathylcarbonate 88-91 К Red label 1.4 6.6 at boil. platea
Dtethylcarbonate 125-126.5 Bi Red label 0.7 3.2 at 93°C priane
laoHgylacetate 132 CP 1.2 3-6 at 93°C priane
Etbylpropionate 96-100 К Red label 3.0 10.7 at 93°C fairly thick hex platea
Methyl-n-butyret. IOI.5-IO3.5 Ж Red Label 1.2 4.9 at 93°C needlea
Cydcpentanone 130.6 BC Red label 11.5 39.0 at 93-5°C hexagonal platea
Acrylonitrile 77.3 Qyananid Co. 4.0 16.4 at boil. flat platea
Methylcellosolveacstate 144.5 Carbide & Carbon 1.6 8.8 at 93°C naaalve hexagons and priane
* EX, EMtnan Kodak; Preet, practical.
73
АМСР 706-177
Cyclonite (RDX)
Frmparaticn:
(Sumaary Technical Report of tbm NDRC, Div 8, Vol 1)
zs°2
/и
сн2 XfH2
(CHj, M. ♦ Uno, + 2HH.N0. + 6(СН,С0)о 0 II
г 6 “ з * з 3 * O.H-N N-NO.
2 \ .X 2
“2
Ammonium nitrate and acetic anhydride are placed in a flask and, while the mixture is stirred
at 75°C, the following three liquids are introduced concurrently and proportionately: acetic an-
hydride, concentrated nitric add, and a solution of hexaadne in glacial acetic add. the final
mixture is held for a short tine at 75°C, diluted with water to 30^ acetic add, and aineered to
hydrolyse unstable reaction by-products, which are a mixture of various nitrated and acetylated
derivatives of hexadna fragments. After simmering, the slurry is cooled and the precipitated
cyclonite removed by filtration. The yield is 78)1 of the theoretical amount (2 moles) of cyclo-
nite melting at 199°C- By dissolving the aneonlum nitrate in the nitric add, a continuous pro-
cess, based on 3 liquids, Is possible.
The product Is recrystallized from acetone, or Cyclohexanone, to (a) remove acidity, (b)
control particle else and (c) to produce stable The preparative procedure described
above, the Bachmann or Combination process, yields cyclonite containing 3-84 НИХ.
Origin:
First prepared by Benning in 1899 (German Patent 104,280) and later by von Hertz (U. S.
Patent 1, 402,693) In 1922 who recognized its value as an explosive. Kot used on a large scale
In explosive aamunltion until World War II.
Destruction by Chemical Decomposition;
Cyclonite (RDX) is decomposed by adding it slowly to 25 times its weight oZ boiling 5jl sodium
hydroxide. Boiling should be continued for one-half hour.
References:14
(а) Г< C. Smith and E. G. Eyeter, Physical Testing of Explosives, Part III - Miscellaneous
Sensitivity Tests; Performance IBata, OSRD Report No. 5746, 27 December 1945.
(b) Ph. Naoum, Z. кеа Schiess &>rengstoffw, pp. 181, 229, 267 (27 June 1932).
(c) D. P. MacDougall, Methods ot Physical Testing, OSRD Report No. 803, 11 August 1942.
(d) Philip C. Keenan and Dorothy Pipes, Table of Military High Explosives, Second Revision,
NVVORD Report No. 87-46, 26 July 1946.
^See footnote 1, page 10.
74
АМСР 706-177
Cyclonite (ВМС)
(•) Arsaaant Research Department (Woolvich), Solubility of RMC In Mitrlc Add (ARD Kxpl Rpt
322/1*3 SeptaAer 19**3)-
(f) Report AC-2587.
(g) International Critical Tables
land. Bomat.
В. T. Fedoroff at al, A fanual for Bxploslves laboratories, Lefax Society Inc, Phila-
delphia, 19U3-6.
(h) S. Hutchinson, Pw Therral Pansitiveneaa of Explosives. Hie Thermal Conductivity of
toloslvo Keter1*'1»- AC 2bol, Rret Report, August 1^42.
(1) R. J. Finkelstein and Q. Gaaov, Theory of the Detonation Process, KAVORD Report Ko. 90-
1*6, 20 April 19*7.
(j) International Critical Tables.
(k) W. F. HcOorry and T. W. Stevens, Detonation fates of the More Iwcrtant Military Explo-
sives at Several Different Tesperatures, вкЛ Ko. 2383, Ifcveabe; 1956.
(1) Also see the following Picatinny Arsenal Technical Reports on Cyclonite:
0 1 2 2 1* 2 6 1 8 2
1170 1211 582 863 1181* 65 1236 857 11*38 709
1290 121*1 13k2 1193 11*11* 1175 1316 1207 11*58 1379
1360 1311 1352 1293 11*51* 1185 11*16 11*27 11*98 11*29
lt50 11*21 1372 11*33 1611* 11*35 11*1*6 11*37 1578 11'1*9
17б0 11*81 11*02 11*83 I63U 11*1*5 11*66 1517 1838 11*69
1980 1561 11*52 1503 2024 1715 11*76 1617 1958 1709
2100 1611 11*92 1693 2151* 1855 *516 1687 1958 1909
1651 171*1 1751 1761 2131 2151 1532 2062 2112 1713 1793 1923 2201* 1885 1915 1935 2095 2125 2205 1556 1756 1766 1796 1836 1936 1956 2016 2056 2176 1737 171*7 1787 1797 1957 211*7 222? 2008 2028 2178 2198 2059 2179
75
АМСР 700-177
Cyclotol, 75/25
* *1T ’ НИХ 75 ПИ 25 С/Н Rotio Msfeceter Weight: 22b
Oaygea Baiaoco: CO, % -35 CO % -6
Density: gm/cc Oast 1.71
MeMag Pokt: *C
Impact SeneltMty, 2 Kg Wlh Bureou of Mines Apparatus, cm Sample Wt 20 mg Pleatinny Arsenal Apparatus, in. Sample Wt, mg BMieg Point: *C
Refractive laden, n£ n£ n»
— •-*» - rHWsJB FWW^I*I l*№ S'eel Shoe Unaffected fiber Shoe Unaffected Vecaam SfoMty Teet: cc/40 Hrs, at 90-C 100'C 0.23 120*C 0.1Ч 1Э5*С 150*C
ШЯе BaOat Impact Teet: Trials % Explosions 30 Partials Эаокеа Uo Burned 0 Unaffected 30
200 Oram Bomb Saad Taett Sand, gm
Espleeioa TaatpereSuee: C Seconds, 0 1 (no <_np used) 1 5 10 15 20 SeaoMdty to MMfea: Minimum Detonating Charge, gm Mercury Fulminate Load Aside Tetryl
Вi Bl stir Matter, % TNT:
Tread Teet, % TNT:
75‘C lntimetis»el Hoot Test: % Loss in 48 Hrs
Ptete Dent Test: Method Condition Confined Density, gm/cc Brisance, % 1 NT
100‘C Hoot Teet: % Loss, 1st 48 Hrs % Loss, 2nd 48 Hrs Explosion in 100 Hrs
Detonation Rate: Confinement None Hone Condition Cast Cast Charge Diameter, in. 1.0 1.0 Density, gm/cc 1.70 1-71 Rote, meters/second 8035 793^
ПесптЫШу Index:
AZ oVy^^^^W^N*8*ye TO
VelotMty:
76
Cyclotol, 75/2$
AMCP 706-177
. - yjonomon Tetryl, gm Wax, in. for 50% Detonation Wax, gm DeraHy, gm/cc .Oxygen, atoms/sec * (Z/soc) Hoot, kilocolorie/mole UH, kcol/mol) Temperature Range, *C Pham
Neat aft Combustion, col/gm 2625* ЕфЫсп, col/gm 1225* Gm Volume, cc/gm 662 Formation, col/gm Rolan, col/gm (h) 5*0 •Calculated fraa ccapoeltlaa of mixture. Armor Hole Impact Test: M awn Metter Projectile: 50% Inert, Velocity, ft/мс Aluminum Fineness Pia*e Thickness, inches 1 1% >4
SpocNk Heat: cal/gm/*C (h> 2c -75 0.220 75 0-352 0 0.225 85 0.325 25 0.25b 90 0.332 50 0.296 100 0.351
cm/iec
Bomb Deep Tact: T7, SMMb SeaM-Anner-Mercing Bomb vs Concrete: Max Safe Drop, ft 50Mb General Pmpoee Bomb vs Ceetitle: Height, ft Trials Unaffected Low Order High Order 100O-* General hrrpeee Bomb vs Creuoto: Height, ft Trials Unaffected Low Orde: High Order
Thermal CiafxtlMl). col/sec/cm/*C
Linear, %/*C Volume, %/"C
НмАмвВу Meiw* ScbIb*
E', dynes/cm’ E, tb/fneh* Density, gm/cc
Compreeoive Strength: Ib/inch*
Vapor Praeeera: *C mm Mercury
77
АХСР7М-177
Cyclotol, 75/25
H MI HI, МЛ Рч|ес*И«, IM WC-01: Density, gm/cc 1.72 Charge Wt, lb 2.22 Tefei Me. of Fragments: For TNT 703 For Subject HE 151U 1 Inch HI, MOA1 hefccHta. let KC-5: Density, gm/cc Charge Wt, lb For TNT For Subject HE Shaped Cbmge tHocttveaess, TNT = IM: Glass Cones Steel Cones Hole Volume Hole Depth
СЛг Yellow-buff
Princlpel Uses: 9taped charge bomb «specially fragmentation; HE projectiles; grenades
Method of laodtog: Cast
Loodtog Density: gm/cc 1.71
tfm^mm^^raa A* гя^мн тютну* !</••* At 9 ft At 25Ц ft Density, gm/cc
Method Dry Hazard Class IQuontity-Detonce) Class 9 Compatibility Group Group I Erudation
Meet WslMHiN TNT): 'd) Ain РеокРгА-е 111 Impume 126 Energy Air, CsaWaed: Impulse Under Water: Peak Pressure Impulse Energy Peek Pressure ImpuU: Energy
Preparation; See Coeposltlon В Origin; Developed by the British between World Wars I and II and standardized in the United States early in World War Ц. Black Modulus et Room Temperature (25°-30°C): lynte/cm^ x 10-W 3.09 Density, gm/cc 1.7k Absolute Viscosity, poises;* Temp, 85UC 210*» 90°C Efflux Viscosity, Ssybolt Seconds:
Temp, 85°C 9-1U * Compositions using Spec Graae Type A, Cisse A RDX. * * Composition prepsred using RDX of optimum particle size.
78
Cyclotol, 70/30
АМСР 706-177
Molecular Weight: 22b |
гак то тит зо Oxygen Вокам: CO, % -37 CO % - 8
Density: gm/cc Cast I.71
*C
С/Н Ratio Freezing Poke: 'C
Impact Sensitivity, 2 Kg Wt: Bureau of Minot Apparatus, cm 60 Sample Wt 70 mg Picatinny Arsenal Apparatus, in. lb Sample Wt. mg 20 *C
Refractive Index, n“ nb n*
» * . -*- » ГПС*МЯ ГТОЮТтоТО ЮТ*! Steel Shoe Unaffected Fiber Shoe Unaffected C^mKlMDne Tande ▼ Ouawin 1 wm 0 cc/40 Hrs, ot 90'C 100’C 120’C 0.86 135"C 150*C
Rifle Mht Impact Tost: Trials % Explosions 30 Partials 30
Burned 0 Unaffected bO 200 Grom Bomb Send Tost: Send, gm 56.6
Explosion Temperature: *C Seconds. 0.1 (no cop used) 1 5 Decaapoees 265 10 15 20 JwWmtvWy TO IINValn^VOOe Minimum Detonating Charge, gm Mercury Fulminate 0-21* Load Azide 0.20* Tetryl •Alternative Initiating charges.
BaiUefic Mortar, % TNT: (e) 135
Trartzl Test, % TNT:
7S*C iMotaatioMl Hoot Test: % Lots in 48 Hrs
Plate Dent Test: (b) Method В
100'C Hoot Test: % Lou, 1st 48 Hrs 0.07 % Lou, 2nd 48 Hrs 0.08 Explosion in 100 Hrs None Condition Cast Confined No Density, gm/cc 1.725 Brisonce, % TNT 136
Dotoeotien Rote: Confinement None Condition Cast Chorge Diameter, in. 1.0 Density, gm/cc 1-73 Rote, meters/second 9o60
Flammability Index:
Hygroscopicity: % Nil
VektMty: Nil
79
AMCP 706-177
Cyclotol, 70/30
FiwgnmaJaHoa Test: Shopod Charge HfoeNveaeaa, TNT = 100:
W waa Hi, M71 Projectile, La* WC-91: Density, gm/cc 1-71 Charge Wt, fb 2.213 Gloss Cones Steel Cones (e) Hole Volume Hole Depth 130
Total No. of FregoMati: For TNT For Subject HE » tach HI, MttAI Рм)есМе, Density, gm/cc Charge Wt, It 703 Colon Yellow-buff
LotKC-5: 1.72 0.923 Prtadpol Ums; Shaped charge boobs; especially fragmentation HE projectiles, grenades
0 ^^Ve V* rw^^pHiVMVtae For TNT For Subject HE 51U 826 Methed of Loodtag: Cast
Loadtag Dsaoity: gm/cc 1.71
FregaMot Till city: ft/ис At 9 ft At25ftft Density, gm/cc
Method Dry
Blast (Kotattie to TNT): (a) Hasord Class (Quantity-Distance) dess 9
Ain Peak Prauure Impuhe Energy 110 120 Compatibility Group Group I Exudation
Air, CsaHrsd; Impuhe Under Wotan Peek Pressure Impuhe Energy Peek Pressure Impuhe Energy Preparation; See Composition В Origin; Developed by the British between World Wars 1 end TI and standar’ieed in the United States early in World War *1. Absolute Viscosity, poises:* Temp, 85°C 90°C 53-2 Efflux Viscosity, Saybolt Seconds: Ten?’. 85°C 5 Heat of; *» Combustion, cal/gm 2685 Explosion, cal/gm 1213 Ges Volume, cc/gm 851»
* Composition using Spec Grade Type A, Class A RDX. ** Calculated fr< oosition of mixture.
80
Cyclotol, 65/35
АМСР 706-177
См*мМи: гак ШТ 35 22t
Oxypea Baieoee: CO- % CO % .hO - Q
Deneityi gm/cc cast 1.71
MeMag Paint: *C
С/Н Ratio » ..e же. pf» vWfl Ve
impeet SeaeMvfty, 2 Kg Wh Вилюй of Minot Aoparatus. cm Sample Wt 20 mg Picatinny Arsenal Apparatus, in. Sample Wt. mg Beflinf Point: *C
ж * a. В—— D МПЖСПтр Hi^VMp l>M nb n£
Steel Shoe Fiber Shoe Unaffected Unaffected cc/40 Hrs, ot 90*C 100‘C 120’C 135’C 150‘C
Rifle Met impeet Test: Trials % Explosions Partials
Burned Unaffected 200 Grero li—b Seed Taatt Sand, gm 55-h
ЬрЫоа Те apoeotere: bC Seconds, 0.1 (no cap used) I 5 Deconpoees 270 10 IS 20 » - Л- | — 3VH*WWWy TO 1ЯИчЯ^Я« Minimum Detonating Charge, gm Mercury Fulminate Load Azide Tetryl
BoMeflc Metter, % TNT: (e> 13h
True! Test, % TNT:
7S*C Intern etieeal fleet Test: % Loss in 48 Hrs
Plete Dent Test: Method
10ГС fleet Test: % Lass, 1st 48 Hrs % Loss, 2nd 48 Hrs Explosion in 100 Hrs Condition Confined Density, gm/cc Brisance, % TNT
Р^М^М^мВ^ЯВ flWtu, Confinement
Р1еаммЫЙу Index: None CBbt 1.0 1.72 7^';
Nygrescopiclty: % Nil Condition Charge Diameter, in. Density, gm/cc Rate, meters/second
▼ VBSVHwye Nil
KI
АМСР 706-177
Cyclotol, 65/35
FragaraatoHee Test: Shaped Charge EHecthreaetb TNT = 100:
М к М, W1 Рм|мМв, 1м Density, gm/cc Chorge Wt, b WC-*1: 1.71 2.253 GIom Canes Steel Cones (•) Hole Volume Hole Depth 1Э0
For TNT For Subject HE 8 tach Nt, MOA1 riciccNh, Le Density, gm/cc Chorge Wt, b 703 G**** Yellow-buff
tKC-S: 1.71 0.922 Prleilpel Uses: Shaped charge taebo; especially fragmentation HE projectiles, grenades
Tseei Ho. of Fragments: For TNT For Sublet HE 5U 769 Mollwtf W LmAsq s Cast
Leedtag Daaahyt gm/cc 1-71
Fragmeat VeheKy: ft/sac At 9 ft At 25% ft Density, gm/cc
Method Dry
MratOtaMratoTHTh Hoxord Class (Quantity-Oistonca) Claes 9
Abt Peak Proieure Impute Energy Compatibility Group Exudation Group I
Alr.CeaNaadt Impute Under Water: Peak Pressure Preparation: See Composition В Origin: Developed by the British :etveec World Wars I end II end standardized in the United States early in World War II. Eutectic Temperature, °C: 79
impuw Energy Peak Pressure Impulse Energy Heat of: » Combustion, cal/gm 2755 Explosion, cal/gm 1205 Ges Volume, cc/gm 8U5 * Calculated from composition of mixture. gm REX/100 gm ПГГ 79°C U.16 95°C 5.85 Absolute Viscosity, poises:* Temp, 85°C 30.2 90°C 26.0 * Composition using Spec Grade Type A, Cless A RDX.
82
Cyclotol, 6o/bQ
AMCP 706-177
RDX 60 1ST bO C/H Ratio Molecular Weight: 22b
Osygea 0*leas*: CO- % .b3 CO % 10
Deadly: gm/cc caet 1.68
MoMsf PefaH eC
to a B.l-» g^ FIWaMf rw» V
Imrm* SeadtMty, 2 Kg Wh Bureau of Mino* Apporotus, cm 75 Sample Wt 20 mg Picrtinny Arsenal Apporotus, in. lb Sampi* Wt, mg 19 ~ ЬЛаДа 0^
io io io
^Irietio^s ^^o^s^^o^tass ^f**tt Siad Shot Unaffected Fiber Shot Unaffected Vacaaan StebMy Toot: cc/40 Hr», ot 90'C 100"С 120"C 0.29 135’C 150‘C
Ute Mht hapact Test; Trial* % Expiation* 5 Perticih 55 Burned 25 Unaffected 15
200 Gram leant lead Teth Sand, gm 5b. 6
ОарЫеа Twagereterii "C Second*, 0.1 (no cop used) 1 5 Decavoeee 280 10 15 20 • UL.1»— «_ lelhlaO^^r vMMvwwwy W IHtVW^RO* Minimum Detonating Charge, gm Mercury Fulminate 0.22» Load Azide 0.20» •Alternative inltlatlna charaee.
OaNiaHc Mertar, % TNT: (a) I.33
Traazl Test, % TNT:
75'C leiereeHeael Hoot Teet: % Lott in 48 Hr*
Plato Doot Teat: (b) Method В Condition Caet Confined No Density, gm/cc 1 • 72 Brisonce, % TNT 132
100*C Hoot Teet: % Lou, 1st 48 Hr* % Lou, 2nd 48 Hr* Expiation in 100 Hr*
Botoaetiea Rote: Confinement None Condition Cast Charge Diameter, in. 1.0 Density, gm/cc 1.72 Rote, meters/second 7900
НудяесорМу: % Nil
VetetiOty: Nil
83
АМСР 706*177
Cyclotol, 60/U0
Frogaseatetioai Test: СЬмм TfetT vMMpWI WWwr^ KnVMirvMItf 1^1 *Wl
90 bmi HI, M71 PrafsaMe, Let WC-91: Glass Cones Steel Canes (e)
Density, gm/cc 1.65 Hate Volume 178 162
Charge Wt, k> 2.187 Hole Depth 125 1W
Cebn Yellow-buff
For TNT 703
For Subject HE 998
Pits tip el Ueeet Shaped charge tomb;
3 Ы1 HI, M42A1 PmjactMa, Lat KC-S: especially fragmentation HE projectiles, grenades
Density, gm/cc 1.67
Charge Wt, № 0.882
0 «^We "OVgOO^RUVe Method of Laedteg: Cast
For TNT 51A
For Subject HE 701
Leadtag Density: gm/cc 1.68
FregaMat VebcKy: ft/sec (c)
At 9 ft 2965
At 25 V> ft 2800
Density, gm/cc *>
Method Dry
Most (Misties ie TNT): (a) Hozord Class (Qiantity-Distonce) Claes 9
Air. Compatibility Group Group I
Peak Pressure 101»
Impulse 116 Exudation
Energy ••
Air, CeeWaed; Preparation; See Composition В
Impulse Origin; Developed by the British between
Worl? Wars I and II end standardized in
Under Water: the United States early in World War IT.
Peak Pressure
Impulse Bulk Modulus st Room
Energy Temperature (25°-30°C):
Dynes/cra2 x 10'10 k.lk
Уй4мд*ошв^! Density, gm/cc 1.72
Peak Pretture
Impulse Absolute Viscosity, poises;*
Energy Temp, 85°C 12.3
iiest of: « 90°C
Sanitation, caL/gm 2820
Explosion, cal/gm 1195 * Compositions using Spec Crsde Type A,
Cat Volaxe, ec/gm 8l<5 Class A RDX.
Compress tve Strength: lb/i nch2
1.1*0 .-n'cc 5200-3000
' Cole;.1эi.eci from eon-position of mlxtur-.-.
Cyclotol, 75/25, 70/30, 65/35
АМСР706-1П
2SSSS22SL15
(a) !>. C. Smith and E. 0. Ryster, Ihyaioal Jesting of Explosives, Part Щ - Miecellaneoue
Sensitivity Testa; Performance Teets, 0€KD Report Mo. 57 Ab, 27 Dectaibar 1945.
(b) D. P. McDougall, Methods of Physical Testing, OSRD Report Mo. 803, 11 August 1942.
(a) R. W. Drake, Fragment Velocity and Panel Penetration of Several Explosives in Simu-
lated Shells, OSRD Report Mo. 2 January i$4b.
(d) V. Phlllpchuk, Free Air Bleat Evaluation of ЫЯ-ИТ-А1, ROC-UTT, and MT-Metal Sy stems,
Motional Northern teary Report, B-Kj4, Aprtl 19$6. ----------------------- ------
(e) Eastern laboratory, du Pont, Investigation of cavity Effect. Section III, Variation of
Cavity Effect with Ccsexmltlon, HUtC Contract U-6lT2-ORl>-5723.
(f) W. S. Cremer, Bulk Oomnressibillty Data on Several High Explosives, NAVORD Retx>rt No.
4300, 15 September 1956*
(g) Also see the following Picatinny Arsenal Technical Reports on Cyclotols:
0 1 2 2 4 2 6 I 8 2
1290 1651 1482 1483 1824 1435 1476 1427 1396 1469
1530 1741 1793 19° 3 1834 1944 2004 1585 1756 1796 I876 1507 1747 1488 1838 1509 1709
(h) C. Lenchjtz, W. Beach and R. Valicky, Enthalpy Changes, Heat of Fusion end Specific
Heat of Baaic Explosives, PATR No. 2504, January 1959-
TSs
ее footnote 1, page 10.
85
лмсртм-m
Cydotrlsmthylene TTlnltrooemlne
СетрееШоа: H % I2 Motecutar Weight: (CjHgN^Oj) IjL
C 20.6 C - _ 0-H-N_rZZX^ N-N—0 n p || CO, % -55 CO % >28
x ад.з н c_l Lch 2 2 Density: gm/cc
0 27.6 J Making Mot: *C 105 to 107
C/H Ratio 0.12 J PfMsfaf *C
Impest IsseltM» 1 Kg Wh Bureau of Mino* Apparatus, cm Sample Wt 20 mg Pteotinny Arsenal Apparatus, in. 15 to 22 Sample Wt, mg 17 to 20 BeHing Feint: *C
Refractive ladex, nb П» l£
Mctiea Poodniem Test: Steel Shoe Unaffected Fiber Shoe Unaffected Vocnem StabMy Test: (c) cc/40 Hrs, at 90’C 0.20 100‘C 9.19 3-71* •Average value of 5 8» people twice recrystal- lized from ieoenyl alcohol.
Rifle Mht Impost Test: Trials % Explosions Partials
Burned | » •• «- -S UnOrtWCTvo 200 6ram Bomb Sood Test: Send, gm 59-2 5h.l
Ixplssimi Temperature: C Seconds, 0.1 (no cop used) 1 5 220 10 15 •VKIIVWIVy W IWWtmli Minimum Detonating Charge, gm Mercury Fulminate 0.200** Lead Azide 0.100** ♦•Al'Sffitftlve initiating charges.
20 Ballistic Mortar, % TNT: 130
Treed Tost, % TNT:
75 *C letenratioeel Hoot Test: % Loss in 48 Hrs
Piets Doot Test: Method
100*C Hoot Test: % Loss, 1st 48 Hrs 8.79 % Loss, 2nd 4B Hrs 2.98 Explosion in 100 Hrs None Condition Confined Density, gm/cc Brisance, % TNT
Dotonotion Rote: 'b) Confinement None Condition Cast Charge Diometer, in. 1.2
FieemrebiOty lodes:
Hygroscopicity: % 30°C, 90$ RH 0.02
TWWrlltyr Density, gm/cc 1.L2 Rote, meters/second 7000 to 7300
Mi
Cyclotrioethylene Trlnltrotiainlne
АМСР 706477
J fU« _ MNpW WyO 1П 1 — 100:
96 ana NL M71 Prajectih. 1st WC-91: Density, gm/cc Charge Wt, b Gloss Cone» Steel Cone* Hole Volume Hoh Depth
Tetei He. ef Fragarants: For TNT For Subject HE 1 tach HL M42A1 hijidlh. Lar KC*S: Density, gm/cc Charge Wt, b Cohn Yellow
Prlailgsl the»: Ingredient of pm Jac tile filler
Tefal No. ef Fragments: For TNT For Subject HE Molted ef lesdfagt Pressed ar cut with added neltlng point depressant*
Loodhg Density: gm/cc See below
Fragmewt Vshsityt ft/sec At 9 ft At 25% ft Density, gm/cc
Method Dry
Most (Metho tsTNT): Hozord Closs (Qiorttity-Distance) Class 9
Ate Peak Pressure Impute Energy Compatibility Group Exudation Group M Hone
Aiv Impute Density at Ve-ious Pressures: lb/lnchg ga/cc
Under Water: Prok Pressure Impute Energy Peek Pressure Impute Energy 2,k20 M30 9,650 Ik,500 24,200 33,800 42,500 Heat of; Combustion, cal/gm Explosion, cal/gm Formation, u'>i/gm 1.10 1.23 1-37 1.44 1-53 1.57 1.59 3158 8?6 -914
87
АМСР 706-177
Cyclotrinethylene Trinitrosanine
Preparation of Haxahydro-1,3.5-Trinitroso-s-triazin» qyclotrlwthylene Trinitrosanine;
(Reference a)
t?
«зав + ЯВ40Н ♦ mnoj -----> °"~B ’'~K0
H2C -LJ-ch2
formaldehyde ammonlua sodium nitric sulfuric
hydroxide ultrate acid acid *|
SO
An aiMoniacal solution of an anine is prepared by adding aqueous fownldehyde to aciriua
hydroxide. Die rate of addition of formaldehyde is regulated to Maintain a solution tempera-
ture of 30° to 35°C. '
Sodlua nltrits is dissolved in eater and the solution or slurry is then poured into the
previously prepared erine-aaaonia solution and totally dissolved by stirring. This solution
is chilled to below 0°C.
Into a nixed acid solution, previously prepared by dissolving concentrated nitric ecld
in wter and addir® concentrated sulfuric acid, all chilled to -<rC, there is added the cold
anlns-nltrite solution belov the surface of the acid Mixture. The addition is regulated to
take 20 to 30 ainutee.
The resulting foeay head of cyclotrimethylena trinitrosanine la allowed to fit over the
icy spent liquor for 1/2 hour and is then collected on a sintered glees funnel and waked to
neutrality, toe aci»t cyclotrinethylene trinitrosamino is reeoved from the funnel and air-
dried on filter paper. The dry crude product Belts at 106° to Ю7°С. Reerystallication from
isoamyl alcohol gives a pure compound melting at 105° to 107°C.
Origin:
Cyclotrinethylene trlnitrosanine wa discovered in 1888 aiaultaneoualy by Griess and
Barrow (Ber 21 (1888), p. 2737) and by Mayer (Ber 21 (1888), p. 2883) when sodlua nitrite
was allowed To react with hexamethylene tetramine Tn ecld solution. This compound was later
studied by Duden and Scharff (Ann 268 (1895), P- 218) and by De lupine who determined its heat
of formation, which was negative (Bull Soc chin (3) 15 (1896), p. 1199). Because cyclotri-
nethylene trinitrosanine could be made at first in v^ry poor yield only, it was a long time
before It received consideration for practical application as an explcelv». However, the
study of cyclotrinethylene trialtroeaadne was continued and investigations were made ea to
its behavior In mixtures with other substances (Prof. D. G. Romer "Report on Explosives,"
BI09GP 2-HBC 57^2).
Destruction by Chemical Decosgosltlon;
Cyclotrlmethylene trinitrosamlna is easily decomposed by acid cr alkali and even by
boiling in water.
88
QyclotrlMthylene Trlnitroeaadne
AMCP 706-177
High Ifcagereture Decomposition, 0.02 ga it. 10 <tl Teat Tube; (b)
Imnersed 10 minute» in bath heated »t 5°/minute
Teup. C
(1) Melting begins | 105
Decooposltlon begins , 150
Xitrous gas 160
Entire decomposition 170
(2) Sose bubbles no
Very slow decoaposltion 150
Decomposes in 2 minutes 200
Deco^oses in 40 seconds 250
Immediate decomposition Э00
L04? №ra Stability: (to)
Cyclotrlmethy'.ene Irlnitrosamine loosely packed in covered wooden boxes for six yeere at
ambient temperaUre and protected free the sun:
1. Exploar e shoved no color change.
2. Melting point decreased froa 104.5° to 104°C.
3. Coefficient of "Utilisation Prectique" decreased froa 125-5 to 1’3-5-
4. An "bel Tbat et 110°C gave no color to iodine starch paper In 15 minutes.
Fusion Tests, Mixtures of Cyclotrimethylene Trlnltroaamlne and TUT: (b)
Cyclotri methylene Trlnitiosamine, > Malting Point, C
10 74
20 68
30 62
40 55
42 55 (Eutectic)
50 61
60 69
70 95 77 95
Eutectic Composition With ЮТ: (b)
42^ Cyclotriorthylene Trlnl troeamine
58< THT
Rate of Detonation, meters/second
7,000
89
АМСР 706-177 Суclotrimethvlenv - 1 nitrosamino
Reaction of Qyclotrinathylene Trial troaaaine With Other Materiels: (b)
1. Iron pooler Slight reaction
2. Copper powder Slight reaction
3. Aluminum powder Slight reaction
A. 2 parta picric add + 1 pert R-Salt e. Violent decomposition after 2 hours at 10°C b. Violent deco^oeition after 10 to 15 minutes at 10C°C
5. 2 parts nltr^lycerln + 1 part R-Salt Ro evidence of deco^oeltiot after 5 days at 90°C
1
Detonation I».te: (b)
Coni' oeaent Paper cartridge
C.edition reseed
Charge Dlsmeter, In. 1.18
Rate, maters/second Density, gm/cc
5180 0.85
5760 1.00
6600 1.20
7330 l.Uo
7600 1.50
7800 1.57
References:lt
(a) Arthur D. Little, Inc. Progress Report No. 106, Fundamental Development of High Explo-
sives, April 1955, Contract Ho. DAI-19-O2O-5Ol-ORD<P)-33.
(b) Louie Me'dard and Meurice Du tour, "Etude Dee Prcprietes De La Cj do tri methylene
Trinltroaamine," ИЙа poudr, 37, 192^ (195M-
(с) H. A. Bronner and J. V. R. Kaufman, "Synthesis and Properties of R-Salt," PATR in
preparation 1959-
(d) Also see the following Pica tinny Arsenal Technical Reports on Cyclotri, ethylene
Trinitrosamlne: 1171*, 2179*
l^See footnote 1, puge 10.
ПВХ (Depth ВовЬ Kxplojive)
АМСР 706-177
*Т-ГТ-“Т-« Aeonlua Bitrate 21 ШЖ 21 ПТ iiO Alusdnun 18 C/H Rotio Molecular Weigh*: 83 Oxygon Balance: CO, % -46 CO % -26 -
Density: gm/cc Coot 1.68
Making Patot: *C
»
impact SeaoRMfy, 2 Kg Wt: Bureau of Mino* Apparatus, cm 35 Sample Wt 20 mg Pleotinny Arsenal Apparatus, in. 13 Sample Wt, mg 14 ВеШпд Mat: "C Refrectiro lode*, ng ng ng
WI^^Ww^NBO в w* Stool Shoo Fiber Shoe hJ _o-*oe^ Tto^e ▼ VC^^BBBB e e cc/40 Hrs, at 90"C 100’C 120‘C 6.15 135‘C 150‘C
ШЯе Вове* Impact Tost: Trials % Explosions Portia Is Burned Unaffected
200 Gram Bomb Sand Test: Sand, gm 58.5
Eoptootoe Temperature: C Seconds, 0.1 (no cap used) 1 5 Ignite* U00 10 IS 20 S<insitMty to Initiation: Minimum Detonating Charge, grr Mercury Fulminate Load Azide 0.20 Tetryl 0.10
Ballistic Mortar, % TNT: (e) 146
Ttosad Test, % TNT:
75*C Internotioael Hoot Tost: % Los* In 48 Hrs
Plato Dent Test: (b) Method В Condition (Met Confined No Density, gm/cc l.?6 Brisance, % TNT 102
100‘C Hoot Tart: % Loss, 1st 48 Hrs % Loss, 2nd 48 Hrs Explosion in 100 Hrs
DeteMfoft Reto: (c) Confinement None Condition Ceet Charge Diameter, in. 1.6 Density, gm/cc 1-65 Rate, meters/second 6600
НмммЫМу ledex:
Hygroscopicity: %
VoIrtiNty:
91
лмср тм-m
EBX (Depth Scab Explosive)
^^ееефде Jeenftfeft^^ —_*«^»— w<jnQn?Qri Tetryl, gm Wax, in. for 50% Detonation Wax, gm <•> (Met 100 1-35 Oxygen, atoms/soc (Z/sec) Heat, kilocalorie/mole (AH, kcol/mol) Temperature Range, °C
Density, gm/cc 1.76 Phase
Neat eft Combustion, col/gm (d) Armor Pteta Impact Test:
Explosion, col/gm Got Volume, cc/gm 1700 M пип Mortar Projectile: 50% Inert, Velocity, ft/sec
Formation, col/gm Aluminum Fineness
Fusion, col/gm SOO-» Senerwi Purpose Bombs:
Specific Heat: col/gm/ -5°C, density 1.75 gn/cc (d) 0.25 Piote Thickness, inches
1 14
IH
Ha
Burning Rote: cm/soc
Bomb Drop Test:
cal/sec/cm/*C Density 1.75 gm/cc 13-2 x 10A T7, 2000-» Semi Armor Piercing Bomb м Concrete:
Coefficient of Fcpanalea: Linear, %/«C -73°-75°C lt.5 x 10' Max Safe Drop, ft SOO-» Qonerel Pwpeoe Bomb м Concrete:
Volume. %/'C Height ft Trials Unaffected
Hardness, Mobs' Scale:
h^edofaS* E', dynes/cm” (d) ЮЛ X 1010 Lew Order High Order
E, fo/lnch* Density, gm/cc 1.51 x 10° 1.72 10004 Clonorul Purpeee Bomb м Concrete: Haight, ft Trials
Compnooire Strength: ib/inch’ (а) 3210-3380
Density 1.78 gm/cc Unaffected
Vapor Preaeure: "C mm Mercury Low Order High Order
92
UPC (Dtpth Bomb Explosive)
AMCP 706-177
^FegoMmleiiea TmI: Mm Hl МЛ PseJeeMa, U* WC-»1: Density, gm/cc Chorge Wt, lb ВWww V4V* vv^^pHVWW*» For TNT For Subject HE 3 lash HL M43A1 Pnjoctifs, let KC-5: Density, gm/cc Chorge Wt, lb Total Na. ef Ftegmeats: For TNT For Subject HE Shaped Charge IHecthreaom, TNT ~ 100: Gloss Cones Steel Cones Hole Volume Hole Depth
Cater: Gray
Principal Usee: Depth charge
Method ef leading; Cast
Landing Density: gm/cc 1.61-1.69
Frogmeat Velocity: ft/iec AtVft At2$Kft Density, gm/cc
Method Dr^ Hazard Class (Quanti*y-Distanee) Class 9 Compatibility Group Group I Exudation
Meet IRehtho to TNT): (d) Ain Peak Pressure 116 Impulse 127 bier jy 138 Air, Cea Kami: Impulse Under Water: Peak Pressure Impulse Energy 136 Uadergroaad: Peak Pressure Impulse Energy
Preparation: DBX can be manufactured by slovly adding water-vet RDX to molten TUT melted in a steam- jacketed kettle equipped with a stirrer. When all the water has evaporated, ammonium nitrate is added and with heating end stirring con- tinued, grained aluminum is added, fte mix- ture is cooled with stirring continued to maintain uniformity and when suitable for pour- ing the mixture is cast. DBX can also be made by adding 21$ ammonium nitrate and 18$ alumi- num to 1*2$ cyclotol or Composition В of J0/>0 FDX/UiT content plus 19$ of TNT previously melted st about 100°C.
93
АМСР 706-177
ИХ (Depth Bomb Eg>lo«lve)
Origin:
ИХ пае developed and ured by the United State» and Great Britain during World Whr II.
Reference»:17
(a) L. C. Smith and E. G. filter, Physical Testing of Explosive», Part HI - Miscellaneous
Sensitivity Teata; Performnce Teat», 0®D Report No.57Ab, 2? December 1945^
(b) D. P. MacDougall, Kathode of Phyilcal Teatlng, OSRD Report No. 803, 11 August 1942.
(c) G. H. Msaaerly, The Rate of Detonation of Varloui Exploiive Compound», OSRD Report No.
1219, 22 February 1943.
M. D. Hundt», The Bate of Detonation of Various Coupounda and Mixture», OSRD Report
No. 5611, 15 January 1355!-----------------------------------------------------
(d) Philip C. Keenan and Dorothy Pipea, T»ble of Military High Exploaivea, Second Reviaion,
NAVORD Report No. 87-46, 26 July 1946.
(e) L. C. Stadth and S. R. Whiten, A Oonaideratlon of RUX/WAx Mixture» aw a Subatitute for
Tetryl in Booatera, NOL Meno 10,303, 15 June 1949.
(f) Also see the follaving Plcatlnny Arsenal Technical Reports on DBX: 1585 and 1635.
l7S»e footnote I, page 10.
94
1,3-01X8100-2,1*,6-Trinitrobensene (DMHB)
AMCP 706-177
* ин С 29.6 l" ON H 2.1 'i | Melectdor Weight: (CfcHjNjOg) 21*3
- N02 Oxygen Beienee: CO, % CO %
И 26.8 ПТ ~ NH2 Density: gm/cc Crystal 1.83
И0_ 0 39-5 2 Melting Feint: *C (a) 290
C/H Ratio 0.38o Preening Feint: *C
hugest SeMiHrity, 2 Kg Wt: Bureau of Minn Apparatus, cm Sample Wt 20 mg Pkatinny Arsenol Apparatus, In. Sample Wt, mg BoWng Paint: *C
18 9 Refractive Index, n£ n£ nb
Steel Shoe Fiber Shoe Vecuum Stability Test: cc/40 Hrs, ot 90'C 100’C 120*C
Rifle Buflst hnpoct Teet: Trials
% Explosions Partials 135’C 150’C
Burned Unaffected 200 Grant Bomb Send Test: Sand, gm 46.6
Expheiea Temperature: *C Seconds, 0.1 (no cap used) pBWRlTWy eV ВШеЮТЮТОе Minimum Detonating Chorge, gm
1 5 Mercury Fulminate Lead Azide 0.20
10 15 Tetryl 0.10
20 Ballistic Mortar, % TNT: 100
Treezl Test, % TNT:
78’C latoraofleeel fleet Test: % Loss In 48 Hrs
Plate Dent Test: Method
IM'C fleet Test: % Loss, 1st 48 Hrs % Loss, 2nd 48 Hrs Explosion in 100 Hrs 0.00 0.L None Condition Confined Density, gm/cc Brisonce, % TNT
Detonation Rato: Confinement
НмммЫНГу Index: None Pressed 0.5 1.35 7500
Condition Charge Diameter, in. Density, gm/cc Rate, meters/socond
Hygroscopicity: %
Volatility:
95
AMCP 796-177
1 3-Diamlno-2,li,6-Tri;.ttrobenzene (DMWB)
i^MWrWWH 9 VW* M ем Hl МП Projectile, Let WC-91: Density, gm/cc Charge Wt, lb Total He. «1 Fragments: For TNT For Subject HE 3 inch HI, M42A1 Projectile, Lot KC-S: Density, gm/cc Charge Wt, lb Total No. of Fregareata: For TNT For Subject HE iheped Charge IHectiveaoM, TNT = IM: Gloss Cone* Steel Cones Hole Volume Hole Depth
Colen Yellow
Principal Uses:
Method of Loading: Pressed
Loading Density: gm/cc At 50,000 pel 1.65
Fragment Velocity: ft/sec At 9 ft At 25'/г ft Density, gm/cc
(Аша**, MWrVJWr Method Dry Hazard Class (Quantity-Distance) Compatibility Group Exudation None
float (Motive to TNT): Ain Peak Pressure Impulse Energy Air, Coafiaod: Impulse Under Weter: Peek Pressure Impulse Energy Underground: Peok Pressure Impulse Energy
Cook-Off Temperature: °C 320 Time, minutes 8 Heat of: Explosion, cal/gm 2876
АМСР 706-177
l,3-Diamlno-2,4,6-Trlnltrobenzene (РАТКИ)
Preparation;
Fifty grams (50 go) of dry styphnic ecld was added to 200 gm of anhydrous pyridine with
stirring. Иге resultinr slurry was stirred for an additional 30 minutes. The yellow product,
dlpyridinlum styphnate, was collected by filtration and washed with approximate 100 milli-
liters of diethyl ether. Hie product was dried over phosphorus (v) oxide, at room tempera-
ture, for 5 hours. Yield of 77 gm (94^), melting point 168° to J.TO°C (literature malting
point 173 C).
To 50 milliliters of phosphorus oxytrichloride, 29-8 gm of the dlpyridinlum styphnate were
added iu small portions, with stirring. The reaction mixture was then warmed on a steam bath
for 15 minutes. This solution was quenched in 500 gm of ice water. Ihe light yellow precipi-
tate vas separated by filtration and washed with water until the washing was neutral to lit-
mus- Yield of 1, 3-dlchloro-2,4,6-trinitrobenzene 20.4 gm (98$). MP 130° to 131°C (literature
MP 126°c).
A suspension of 3 gm of l,3-dichloro-2,4;6-trinitrobenzene in 9 milliliters of absolute
methanol was prepared. This slurry was cooled to 0°C, and dry ammonia was bubbled into the
stirred suspension. After 20 minutes the reaction mixture was allowed to warm to room tem-
perature, filtered by suction and washed wi 'h methanol and ether until a negative Beilstein
test for chloride ion was obtained or. the washings. Yield of 1,3-diamino-2,4,6-trinitrobenzene
2.5 gm (77%)» MP 288° to 290°C (literature MP 285°C).
Origin;
DAUfB, also called 2,4,6-trinitro-l,3-diamj.no7benzol or 2,4,6-trinitro-phenylenediamine-
(1,3), was first obtained by Noelting and Collin in 1884 (Ber 17, 260) and also by Barr in
1888 (Ber 21, 1546) from 2,4,6-trinltrorasorcin dimethylether Tn contact with snmonlacel
alcohol for"several days. J. J. Blanksma obtained the same pro.iuct in 1902 by reacting
either 2-chloro-2,4,6-trlnitroanlsole or 3-chloro-2,4,6-trinltrophenetol with ammoniacal
alcohol (Rec trav chim 21, 324) and from 2,4,6-trinitroresorcln inethylethyl ether with emtno-
nlacal alcohol (Bee trav chim 27, 56 (1908)).
Melsenheimer and Patzig in 1906 prepared DAHIB in the form of yellow needles, MP 280°C
from 1,3,5-trinitrobenzene ydroxylamlne and sodium methylste in methyl alcohol (Ber 39, 254o).
The product was slightly soluble in glacial acetic acid but poorly soluble in other solvents.
It decomposed into NHo and 2,4,6-trinitroresorcln when boiled with dilute NaOH or KOH (Beil 13,
60). 3
Korner and Contardi prepared DAINB by the reaction of either 2,4-dlchloro-l,3,5-trlnltro-
benzene or 2,4-dlbromo-l,3,5-trinitrobenzene with annoniacal alcohol st room temperature or
better by heating to 100°C (Atti R. Accad Lincel (5), 171, 473 (1908)); (5) 18 I, 101 (1909)).
A method of preparation by prolonged reaction of N-nltro-N-methyl-2,3,4,6-tetranitroanillne
with a saturated aanonls solution was reported In 1913 by van Romburgh and Scheers (Akad
Amsterdam Versl 22, 297).
C. F- Van Ulin obtained DAHIB melting at 301°C by reacting a concentrated aqueous aramonis
solution with N-nltro-N,N,N-trimethyl-2,4,6-trlnltrophenylenedlamine-(l, 3) or with N-nltro-
N-ir.ethyl-N-phenyl-2,4,6-trlnitrophenylenediamine-(l,3) (Rec trav chim 38, 89-IOO (1919)).
later Van luin and Van Lenr.ep reacted concentrated aqueous ammonia with 2,4,6-trinltro-3-
aminoenisole or 2,4,6-trinitro-3-aminophenetol to obtain DATK3 melting at 237° to 288°C (Rec
truv chim 39, 147-77 (1920)). In 1927 Lorang prepared the same compound by boiling 2,4,6-
tri-itro-1,3”bls ( -nitroethyl ureldo) benzene with water or by heating it with ammoniacal
alcohol in a tube at 100°C (Rec trav chim 46, 619) (Bell 1 17, 7 II 33).
АМСР7М4П
1.3-Djaaino-2.t,6-Srinitrobenaeoe (РМЯВ)
A recent report deacrtbee toe preparation of DfcBlB in two atepa froa ccamrcially avail-
able etartinc an tart ala. Hret e-uitroanillne we nitrated vith ДОО^-ПОе acid Mixture to
tetranitroaniline. She crude tetranitroaniline we converted by netbandlc awonla to
diaatnotrinltro-benxene in a high degree of purity. A converaion of 100 parte of a-nitroani-
line into 110 parte of 0АТП wa obtained by thia Method, which can eaaily be carried out on
a cauaarcial acala.
98
Diazodinitrophenol
АМСР706-1П
fl ft! Mofocolor Weight: (C^H^tOj) 210
* I • C 3^.3 [ 5 H 0.9 Г > or. r I CO, % .61 CO % -15
Density: gm/cc Crystal 1.63
0 38.1 ° Melting Feint: *C 157
C/H Ratio 1.056 Freexing Point: *C
hnpnct SsaskMty, 1 Kg Wt: Bureau of Mines Apparatus, cm Sample Wt 20 mg Pkatirmy Arsenal Apparatus, in. 4; (1 lb vt) 7 Sample Wt, mg 15 Boiling Point: *C
RwttWWtVw BW^^VMp nft nft
Friction PendahMi Teet: Steel Shoe Detonates Fiber Shoe Detonates Уосшик SMbMty Test: cc/40 Hrs, ot 90‘C 100’C 7-6 120‘C I35’C ISO’C
Rifle • let Impoct Teet: Trials % Explosions Partials
Burned Unaffected МО Cron» Booth Sood Test: ЙйЛклМег fuse l»5.i
Expheien Teamperetere: ‘C Seconds, 0.1 (no cap used) 1 200 5 195 10 180 |C Minimum Detonating Chorge, gm Mercury Fulminate Lecd Azide 0.20 Tetryl 0.10
20 BeNieHc Moetar, % TNT: (a) 97
Trend Teat, % TNT:
75‘C IntotnoHenel Meet Test: % Loss in 48 Hrs
Piste Dent Toot: Method
100’C Hoot Test: % Loss, 1st 48 Hrs 2-10 % Loss, 2nd 48 Hrs 2.20 Explosion in 100 Hrs None Condition Confined Density, gm/cc Brisance, % TNT
Detonation Rate: Confinement
ЛееммЫКу Index:
Hygroscopicity: % 30°C, 90< RH 0.04 Chorge Diameter, in. Density, gm/cc 0.9 1.5 1.6 Rote, meters/second ^L00 6600 6900
VoMRty: 50°C, 30 months Unaffected
•Until it ii established which picramic acid (melting point 169°C) isomer is involved (Ref: J
Chem Soc, 2082, August 19^*9). ~
99
AMCP' 6-177
Diazodini trophenol
90 чиа HE, M7I Projectile, Lot WC-91: Density, gm/cc Charge Wt, lb Total Na. of Fragments: For TNT For Subject HE 3 tach HE, M42A1 Projectile, Lot KC-5: Density, gm/cc Chorga Wt, lb Tata, No. of Fragments: For TNT For Subject HE Shaped Charge IffocthreaeM, TNT = 100: Glass Cones Steel Cones Hole Volume ' Hole Depth
Color: Yellow needles
Principal Usee: Percussion caps
Method of Leading; Pressed
Loading Oeaafty: gm/cc Apparent 0.27 At 3000 psi 1.14
Fragment Vetocity: ft/sec At 9 ft At 254 ft Density, gm/cc
Storage: Method Under water Hazard Class (Quontity-Dislanco) Class 9 Compatibility Group Exudation None
Maat dtototive teTNT): Air Peak Pressure Impulse Energy Air, Caaftaod: Impulse Under Water: Peak Pressure Impulse Energy Uadergrooad: Peak Pressure Impulse Energy
Solubility: Soluble in nitroglycerin, nitrobenzene, aniline, pyridine, concentrated hydrochloric acid, and in most common organic solvents. Hea - of; Combustion, cel/gm 3243 Explosion, cal/gm 820 Gas Volume, cc/gm 86^ Sensitivity to Electrostatic Discharge, Joules; (fc) 0.012
100
АМСР 706-177
Dlazodinltro) ;ol
Solubility: gm/100 aj of the follaving substKices; (c)
.Solubility at 50°C
Solvent
Ethyl acetate
Methanol
Ethanol
Ethylenedichloride
Carbon tetrachloride
Chloroform
Benzene
Toluene
Petroli us ether
Ethyl ether
Carbon dliulfll
1
2.45
1-25
2.1*3
0.79
trace
0.11
0.23
0.15
Insoluble (at 20°C)
0.08 (30°C)
trace (30°C)
Preparation: (Chemistry 01* Powder and Explos.veB, Davie)
Ten gm >f picramic acid is sr^pendeG in 120 cc of % hydrochloric acid, and under efficient
agitation at about 0°C. 3-6 gm eodi л nitrite in 10 cc water ie dumped into the suspension.
Stirring is continued for 20 minutes, the product filtered off rnd washed thoroughly with ice
water. Ihe dark brown product, if dissolved in acetone and precipitated in water, turns bril-
liant yellow.
Origin:
Discovered by Griess in 1858 (Annalen 106, 123; 113, 205 (18o0) and studied extensively by
L. V. Clark (ind Eng diem 25, 6cj <19339- lieveloped for commercial use In 1928. Ibis com-
pound was patented in the (mited States by Professor William M. Dene.
Destruction by Chemical Decomposition:
Dlazodinitrophenol is decomposed ... adding the water-wet material to 100 times its weight
of 10$ eodi m hydroxide. Nitrogen gas la evolved.
Reference; 18
(a) F.illip C. Keenan and Dorothy '’1--B. Table of Military High Explosives, Second Revision,
WORD Report No. 87-46, 26 Jul;- 1946.
(b) F. W. „row.., D. K. Kusler and Г. C. Gibson, Sensitivity of Explosives to Initiation by
footnote page 10.
101
AMCP 706-177
Diazodlni trophenol
Electrostatic Discharges, U. S. Dept of Int, Bureau of Minas, RI 3852, 1946.
(c) L. V. Clark, "Diaxodinlt'-ophenol, A Detonating ESrloslve," Ind Eng Chen 25, 663
(1933).
Seidell, Solubilities of Inorganic and Organic Compounds, Van Nostmnd and Co., Я. Y.
(d) Also see the following Picatinny Arsenal Technical Reports on Diazodinltrophenol:
0 2 4 2 7 8 2
150 1352 3b 355 8i7 318 2179
610 214 1830
2120
102
Diethylene Glycol Dinitrate (DBGN) Liquid
AMCP 7<* 177
% c 2U.5 H2C 0N02 h u.i нс,— J> 0 N 1U.3 H,C Molecular Weight: (C^HgN^) 196
Oxygen Beieace: CO, % CO % -41 - 8
Density- gm/cc Liquid 1-30
0 57-1 H2C ono2 C/H Ratio 0.11*3 Melting Paint: *C 2
Freezing Paint: 'C
Impact SeooitivM,, 2 Kg Wt: Bureau of Minn Apparatus, cm 100+ Sample Wt 20 mg Picatinny Arsenal Apparatus, in. 9 Sample Wt, mg ВЫНяу Feiirt: *C Бесошроеев 160
f.efmctive Index, n» Пш n£> I.U98
Friction Peedohan Test: Steel Shoe Explodes Fiber Shoe Vacuum Stability Tost: cc/40 Hrs, ot 90’C 100’C I2O°C I35’C 150°C 0.3cc/20 hr/gni
Rifle Ballot Impact Test: Trials % Explosions Pa't’ols
Burned Unaffected 200 Gram Bomb Sand Test: Sand, gm 42.2
Ixpleeien Tomporefam: *C Seconds, 0.1 (no cap used) 1 5 237 10 15 Sensitivity to Initiation: Minimum Detonating Charge, gm Mercury Fulminate Lead Azide Tetryl
20 Be'listic Mortar, % TNT: 90
Trauzl Test, % TNT: 77
7S*C Interactional fleet Test: % Loss in 48 Hrs
Plate Dent Te.rt: Method
100’C fleet Test: % Loss, 1st 48 Hrs L.O % Loss, 2nd 48 Hrs 3.0 Explosion in 100 Hrs None Condition Cor ned Den^.ty, gm/cc Brisance, % TNT
DeteneHoa Rate: Confinement Condition Charge Diameter, in.
FlemmebiHty Index:
Hygroscopicity: % i.y, 6760
VeletiHty: 60°C; mg/cm2/hr 19? Density, gm/cc Rote, meters/second
kk:
АМСР 706-177
Methylene Glycol Dlnltrete (DEGN) Liquid
Booster Sensitivity Test:
Condition
Tetryl, gm
Wax, in. for 50% Detonation
Wax, gm
Density, gm/cc
Decompositicfi Igooffon:
Oxygen, atoms/sec
(Z/sec)
Heat, kilocolorie/moie
UH, kcal/mol)
Temperature Range, °C
Phase
Meal of:
Combustion, cal/gm 2792
Explosion, cal/gm 841
Gas Volume, cc/gm 796
Formcrion, cal/gm 2020
Fusion, cal/gm
Specific Heat: col/gn.. C
Armor Plate Impact Tost:
60 mm Mortar Projectile:
50% Inert, Velocity, ft/sec
Aluminum Fineness
500-fo Gaaoral Purpose Bomba:
Plate Thickness, inches
1
1’4
I’.i
l\
Bunsing Bate:
cm/sec
Thorsnol Conductivity:
col/sec/cm/°C
Coefficient of Expansion:
linear, %/'C
Volume, %/‘C
Hardness, Mohs' Scale:
Young's Modulus:
E', dynes/cm2
E, lb'inch2
Density, gm/cc
Compressive Strength: lb/mch-
Vepor Pressure:
C mm Mercury
20 0.003’
•л 0.130
Bomb Drop Tost:
T7, 2000-lb Stmi-Armor-t forcing Bomb vs Concrete:
Max Safe Drop, ft
500-lb General Purpoct Bomb vs Concrete:
Height, ft
TrK
Unofrs.red
Low Order
High Order
1000-lb General Purpose Bomb vs Concrete:
Height, fr
Trials
Unaffected
Low Order
High Order
KM
Methylene Glycol Dlnltrate (PBGN) Liquid
AMCP 706-177
WmiHI. M71 Projectile, Let WC-91: Density, gm/cc Charge Wt, lb Totol He. ef Fregaisnti; For TNT For Subject HE 3 tech HI. M42A1 Projectile, Lot KC-S: Density, gm/cc Charge Wt, lb Tefal He. ef Fragments: For TNT For Subject HE Shaped Charge ЕНесНееме*, TNT z. 100: Gluis Com: Steel Cones Hole Vc 'jme Hole Depth
Cohc Colorless
PriBcip*! Utm: Propellant :ompositlons
Method of Loading:
Landing Density: gm/cc
Frogaianf Velocity: ft/sec At 9 ft At 25 Vi ft Density, gm/cc
Storage: Method Liquid Hazard Class (Quantity-Distance) Class 9 Compatibility Group Exudation
Blest (Relative to TNT): Ain Peak Pressure Impulse Energy Air, Confieod: Impulse Under Water: Peck Pressure Impulse Energy Underground: Peck Pressure Impulse Energy
Preparation: D2GN can be prepared with approxl- netely 55$ yield by eddlng diethyleneglycol to mixed acid (50$ ИН%' 45$ HgSO^, and 5$ HgO). The temperature "'is kept at 30°C or lower. The separated LEON is purified Ъ, washing with successive portions of water, dilute sodium carbonate solution and water until neutral. Hydrolysis. $ Acid; 10 days at 22UC 0.003 5 days at oO°C 0.003 Solubility In Water, gm/100 ym, »l:
25UC ' d.4o 60°C 0.60 Solubility, em/100 gm, at 2>°C, 1:.:
Viscosity, centipoises; Temp, 20°C d.l
Ether ?0 Alcohol 00 2:1 Ether:Alcohol 00 Acetone 00
АМСР 706-177
Di ethylene Glycol Dinitrate (Ю) Liquid
Origin:
Flrat prepared and studied by Wn. H. Rlnkenbach in 1927 (Ind Eng Chen 19 > 925 (1927) end
later by Rlnkenbach and H. A. Aeronson (Ind Eng Chen 23, 160 (1931)) both of Picatinny Arsenal.
Tsed in propellant coapoaitiona by the Gemane durlngworld War II.
leitruotioc by Chemical Deconpoaition:
ШШ la decoapoaed by adding it slowly to 10 tinea its weight of 18$ aodiun sulfide
(BegS'SdigO). Seat la liberated by this reaction but thia ie not haza? ’ous if stirring la
aa Intel nod during the addition of ШЯ and continued until solution la complete.
References:19
,5ee the following Pi за tinny Arsenal Technical Reports on DEGN:
0 1 2 2 4 6 I 2
50 231 72 673 1*94 346 487 279
180 551 602 1443 1624 1516 1427 579
620 1391 1262 1616 1487 1439
1490 1421 1392 1786 1817
1990
та--------
See footnote 1, page 10.
106
BiB(2,2-Dinltropropyl) Fuaarate (IHPF)
AMCP 706-177
% C 31-6 aiCO2CH2c(NO2)2CH3 К 11*.7 CHC02CH2C(N02)2CH3 0 50.5 C/H Rotio Molecular Weight: (с10Н1г\с12) ^0
Oxygee Balance: CO. % -59 CO % -17
Density: gm/cc Crystal 1.60
Paint: °C Form^ jg
Framing Point: *C
Inspect SoneMvity, 2 Kg Wt: Bureau of Mines Apparatus, cm 100* Sample Wt 20 mg Pleotinny Arsenal Apparatus, in. 18 Sample Wt, mg 18 Bolling Point: °C
RHrecCWe 1в4«ж, nJ Пи
Fifctioa Pondulmn Test: Steel Shoe Unaffected Fiber Shoe Unaffected Vacuum Stability Test: cc/40 Hrs, ot 90°C —- iDO’C 0.66 120°C 135’C 0.91 150*C
title Belief Inspect Test: Triols % Explosions Portiols । Burned ' Unaffected
200 Gram Bomb Sand Test: Sand, gm
Baploeion Temperature: °C Seconds, 0.1 (na cop used) — 1 1* Snokee 250 10 15 20 7S C Intematioaol Meet Test: % Loss in 48 Hrs Sensitivity to Initiation: Minimum Detonating Charge, gm Mercury Fulminate Lead Azide Tetryl
Ballistic Matter, % TNT:
Trouzl Test, % TNT:
Pinto Dent Tost: Method Condition Confined Density, gm, cc Brisance, % TNT
l00*C Heat Test: % Loss, 1st 48 Hrs % Lass, 2nd 48 Hrs Ertplosion m 100 Hrs
DotcnnHen Rate: Confinement Condition Charge Diometer, in. Density, gm/cc 1.1*9 ate, meters/second /OJO
Flemmability lodes:
Hygraocepicity: %
Volatility:
Ш7
СР 706-177
В1в(£,£-Р1пКгоргору1) Fumarate (DNPF)
Frogmentation Teet: Shaped Charge EffectivenoM, TNT = 100:
M mm HE, МП PrejocHlo, Lot WC-01: Glass Cones Steel Cones
Density, gm/cc Hole Volume
Charge Wt, lb Hole Depth
Total No. of Frogmonte: For TNT For Subject HE Color: White
Principal Ums:
3 loch HE, M42A1 Projectile, Lot KC-3:
Density, gm/cc Charge V/t, lb
Total Ne. of Fragments: For TNT Far Subject HE Method of Loading: Cast
Loading Density: gm/cc 1.90
Frogmeet Velocity: ft/sec At 9 ft At 25V4 К
<n - T 0VVvV|V'
Deni'ty, gm/cc Method Dry
Bknt I Relative to TNT): Hazard Clots (Quantity-Distance)
A.r: Compatibility Grc p
Peak Pressure Impulse Energy Exudation Hone
Air, Confined: Impulse Under Water: Peak Pressure Heat of: Combustion, cal/gm Detonation, cel/gm Viscosity, poises: 3070 (calculated) 7»7 (calculated)
Impulse Energy & .etnp, 'Xj.90C iOC.5 C 0.5-*. O.U39
Undorgreend: Peak Pressure Impulse Liquid Density, gm/cc:
Гегар, •Xi.9°C 10*',. ‘j°c 1. У.2 1 • 379
Energy Or is 1
ri'.hee i zea ir. 199? 1? !•!. E. ill 11 *f
U, iisva! 6rdnei.ce labors'.ot . WhI it- Oek.
.’ter, Intid.
Kin
Bis(2,2-Dlnltropropyl) Fumarate (DNPF)
AMCP 706-177
Preparation;
(a, b)
hc-coci hc-co2ch2c(no2)2ch3
|| + гоцсС'ЮрксНрОН aici, |
HC-COCI -------HC-C02CH2C(N02)2CH3
3.3 mol 7.3 mol 1.6 mol 33$ yield
fumaryl chloride 2,2-dlnitropropanol aluminum bis(dinltropropyl] fumarate
chloride
Di nitropropanol was nixed with chloroform (1320 milliliters) and the mixture heated to
boil'.ng. Ihe distillate was collected in a water separator. At first the distillate was
cloudy and this was dried with calcium chloride before being returned to the system. When
no more water was collected in the water separator, the mixture was cooled to room tempera-
ture and the separator removed. Fumaryl chloride was introduced, followed by the aluminum
chloride which was added in four equal portions. Air was blown into the flask for a minute
to effect mixing, and the reaction sustained itself without the addition of heat for one hour.
Steam was gradually introduced so that the reflux temperature was reached 2-1/2 hours after
the beginning of the reaction. After 3 hours of reflux, the hot liquid was poured into a
bucket. As cooling took place the slurry was vigo .sly agitated until It finally set up at
room temperature. This material was broken up and mixed with dllu'e ice cold 1IC1. The solid
product was collected on a sintered funnel, washed with water and irith he*auc. The crude
material was recrystallized from methanol to give a product melting at d6°C (urcorrected),
but after storage for several days the melt'ng point was 89°C.
References;20
(e) M. E. Hill. Preparation and Properties of 2,2-Dinltropropam 1 Esteys, NAVCRD Report
No. 21?7, 3 July 1951th
(b) D. L. Kouba and H. D. McNeil, Jr., Hercules Report on High Explosives, '-vy Contract
NOrd-11230, Task A, 26 May 1951*-
109
Bla(2,2-Dinitropropyl) Succinate (Ot?S)
АМСР 706-177
fimptaltltn. C 31.1* H 3-7 0 50.2 CH2C02CH2C(N02)2CH3 C/H Ratio 0.250 Meiecoler Weight: (C^.i^N^O^) 332
Oxygen Balaace: co, % -63 CO % -21
Density: gm/cc Crystal 1.51
MeMng Point: *C 86
Framing Point: *C
fmpsrt SeosMvity, 1 Kg Wt: Bureau of Mine» Apparatus cm Sample Wt 20 mg Pleotinny Arsenal Apparatus, in. Sample Wt, mg Bailing Point: *C
Refractive Index, nJ Пи nb
FVWVWOB vWWO^WO I VMv3 Steel Shoe Fiber Shoe K* СЛа^ИХДпо Ta^r YKVv* ЮТ» cc/40 Hrs, at 90-C —— ,00’C 0.10 120‘C 135*C 150*C
Rifle BaHot ''npoct Test: Trial» % Explosion* Partial» Burned Unaffected
200 Orem Bamh Seed Test; Sand, gm
Ixptaien Temperature: *C Seconds, 0.1 (no cap mod) — 1 5 >l»00 10 15 20 SoesitMty to InMetioa: Minimum Detonating Charge, gm Mercury Fulminate Lead Azide Tetryl
Ballistic Metter, % TNT:
Trenxl Teat, % TNT:
7S’C InterneHonol Hoot Test: % Lots in 48 Hrs
Plato Dent Test: Method Condition Confined Density, gm/cc Brisance, % TNT
100‘C Heet Test: % Lots, 1st 48 Hrs % Loss, 2nd 48 Hrs Explosion in 100 Hrs
Confinement Condition Charge Diameter, in. Density, gm/cc Rate, metors/second
Memmebillty lades:
Hygroscopicity: %
VolaHBty:
110
Bis(S,S-Dinltropropyl) Succinate (IMPS)
AMCP 706-177
Fragmentation Test: M м KI, M71 Projectile, Let WC-P1: Density, gm/cc Charge Wt, lb For TNT For Subject HE 1 inch HI, M42A1 Pmjirtih, Let KC-S: Density, gm/cc Chorge Wt, № ЬЛ* la^Maaatai For TNT For Subject HE Sbeped Charge IHocihraaees, TNT = IBOt Glass Cones StMl Cones Hole Volume Hol* Depth
Calar: White
Priaeipd Uses:
Method ef leering: Cast
Laoriag OeaeBy: gm/cc
Frogamet Velsrilyt ft/ioc At 9 ft At 23% ft Density, gm/cc
Storage: Method Ery Hazard Class (Quantity-Distance) Compatibility Group Exudation None
Im* (Bcietiee Ю TNT): Ain Peak Pressure Impulse Energy Air, Canfiaad: Impulse Under Water: Psok Pressure Impulse Energy UaBevgeeooBt Peek Pressure Impulse Energy
Origin: Synthesized in 1953 by M. E. Hill of the U.S. Naval Ordnance laboratory, White Oak, Maryland.
111
AMCP 706-177
В1й(2,2-И.п1 tropropyl) Succinate (DNPS)
Preparation:
гсНзССк^гСЯг0*4
,COC1
CH^CoCx
AlClj
jJigCOOO^CC no2) 2ch3
CHgCOOCHgCfNOgJ^j
2HC1
di ni tropropanol
succinyl
chloride
aluminum
chloride
bis(2,2-dinitropropyl) succinate
A methylene chloride solution of dinitropropanol (0*02 mol 'n 15 milliliters) was mixed
with 0.01 mol of succinyl chloride. To this solution 0.003 mcl of crushed anhydrous aluminum
chloride was added. It was necessary to cool the reaction vessel due to the vigorousness of
the reaction. After 25 minutes at room temperature the reaction solution was refluxed 1-1/2
hours. Fine needle-like crystals formed upon cooling and adding hexane. Ihe crystals were
slurried in dilute hydrochloric acid and on recrystallization from methanol gave a 935> yield
of INPS (melting point 850 to 85.6°C).
References:21
(a) M. E. Hill, Synthesis of Hew High Explosives, NAVORD Report No. 2965, 1 April 1=»53-
2^S*_- foo:nr>(e ! , png.- 10.
112
2,8-Plnltropropyl-4,4,4-Trinltrcjutyrete (ГНРТВ)
AMCP 706-177
% C 23-6 H 8.5 ^хОСИгС(’»Э2)2СН3 M 19-T C““0 Melecata Weigh: 355
Oxygen Belence: CO, % .89 CO % +2.3
Density: gm/cc Cryetel ’.68
0 54.2 ^CHaCHgCtMOj) MeMnn »C Form 1 11 ГОГШ 11 95 • Fora III 59
C/HRotio » . e Л. g^ i^MWi ta
Ippoet fanefcMty, 1 Kg Wh ШвЖв^ VMeft *C
Sample Wt 20 mg Pfcotinn* Anenol Apparatus, tat. Sample Wt, mg f jo 8a jo
**—а — ЭЮТ1 ЭПОВ RtarShoe ^MwiniOpy BWBVo cc/40 Hn, at 90*C 100'C 0.5 I2O*C 135*C 150*C
Me Batat tappet T«t: Trial* % Expiation* tartiak
Burned UacsHoctad 208 Orem Bomb Send Teet: Sand, gm
Seconds, 0.1 (no cap wed) — 1 5 300 10 15 •BWwWWy W ^^ТО^ЯВМПе Minimum Detonating Chorge, gm Mercury Fulminate Lead Az Ide Tetryl
20 Beilietic Mortar, % TNT:
Truori Toot, % TNT:
7J‘C taemaltawl Heat Tach % Lorn In 48 Hr*
Fleto Dent Toot: Method
ЮГС Neat Tetit % Loss, let 48 Hr* % Loes, 2nd 48 Hr» Expiation In 100 Hrs Condition Confined Density, gm/cc Brisance, % TNT
BWBVe Confinement Condition Charge Diameter, in. Density, gm/cc 1.6? Rate, meters/second ?600
VtoiMnoMttty Iodo**
ИмммввШкн % ^e^V p * Л4
VetatMty:
113
АМСР7М4П
2,2-Dlnltropropyl-U,U,U-Trinltrobutyrete (ЕКРта)
М ям Ж, МЛ ЫН'* ut wc-*l: Density, gm/cc ChorgeWt. lb TaNl No. •* Pmgawafa: For TNT For Subnet HE 3bob ME. MOAl haiecHb, Ie» KC-5: Density, gm/cc Oov Wt, lb ТЬМИа, «1 *M*mmk For TXT For Subnet HE Glow Cones Stool Conet Hob Volume MoUm ***— OOW UW0VT1
Coion White
MnfeolUtM:
Method of Leedhy: Cost
Loodbg Deooity: gm/cc 1.67
Fmgmsal УМмкг Ft/sec At 9 Ft At ИЦА Density, gm/cc
Method Dry Hoiord Clow (Quontrty-Diitonco) Compatibility Group Exudation None
Nsst (Astaiioe b TNT): Ain book Prweuro №auhe Energy Ак.Саайм* Impuhi UMhtWMn Fonk Pressure Impulse Energy ВЬмйг PaaituM Impulse Energy
Beet of; (c) „ , . - ' Solvent Trenoltlon, cel/gn CCli, IMF I Fill 6.8 l».8 II > I -16.6 -82.0 Heat of Solution, 30°C; ДН Solution, eel/ga Material CCIl IMF Form III 89.5 8.1 Form I 35.6 12.8 Form II 19-1 -9.I Origin, Synthesized in 1952 by M. E. Kill of the U.S. Naval OrCnanco Laboratory, White Oak, Maryland.
114
2,c-ianltropropyl-l*,l*,l*-T.inltrdbutyrete (ШРТВ)
АМСР 706-177
Preparation;
(•> Ь)
CBjCfuo^gOH + кю5)3сс5га’2сос1 aici3
trinltrbbutyryl alusdnun
dlnitropropanol chloride chlor!f-
CS3C(RO^)2C^COOCSgC(H(^)3 * HO.
dinltropropyl trlbltrobutyrate
Dfnitroprcpanol, trlnitrc*xityryl chloride and eluainun chloride were «lowly nixed in car-
bon tetrachloride et 60°C. Thia mixture was refluxed et 75°C for two houra. After the reac-
tion was completed, the nixture лег cooled and the crystalline product sapareted and purified.
Miter Is* the dlnltropropanoj vac removed by auotropic distillation before the odd chloride
mi added* the purified product had a uniting point of 95° to 9б°С*
Cryatellcugephie Dita: (c)
three distinct crystallographic eodiflcattcns of ШРТН have been observed. These poly-
orphe have been characterised by naans of X-ray diffraction and nlcrooeaplc observation.
Pom I crystallizes free solution in carbon tetrachloride, chloroforn, acetone, chlorofOrn-
besane, aoctone-nater, or ethanol-nater at roon temperature. Prolonged standing of Para I
at roon te^wrature under the mother liquor pronotes в transition to Torn П. Upon solidi-
fication of seitan ШРТВ, Torn П is always observed.
Linear Hate of Transfomaticn of Porn П to Fore I (c)
TC^erature, Average Bate, sq inch/hour Standard Deviation Average Rata, иц/hcur
15 O.JU? о.оэб 0.012
2° 0.1*35 0.025 0.126
85 0.U52 0.01*8 0.133
30 0.1*75 0.01*9 0.11*0
35 0.253 0.037 0.u,'5
Both Porno I and HI gave very erratic sensitivity values. The high teapereture polynorph,
Pom П of EHPTO, gave consistent sensitivity values.
References;22
(а) и. E. Hill, Preparation and Properties of 2,2-Dinltropropanol Eaters, AVORD Report
Ho. 21*97, 3 July 195?^
(b) W. B. Hewson, Hercule- Report on lllgl Explosives, Hsvy Contract HOrd-1126O, tbsk A,
18 October 1951».
(c) J. R. Holden and J. Wenograd, Physical Properties of an Experimental Castable Explo-
sive 2.2-Dinitropropyl 2.1*.1*-Trinitrobutyrate DHPTB. 8AV0ED Report Но. 1*1>27. 11 December 1956.
22
See footnote 1, page 10.
АМСР 706-177
2, U-И nitro toluene
CainpociHee: % CE3 C U6.3 1 H 3-3 Г T Metacetar Weight: (CjH^NgO^) 1.82
Oxygen Betaace: CO- % -lib CO % - 53
к 15-U Density: gm/cc 1.521
0 35-0 NJ Mohtag Mat: *C 71
2 C/HRotto 0.579 е^ЯКЯЯф rWv»
hugest loMbirity, 1 Ke Wtt Bureou • Mines Apparatus, cm sonrb wtaomg Pfeatinny Arsenal Apparatus, in. Sample Wt, mg Befltag Mat: *C Decoapoees 300
Refractire Index, ц» fa л° п»
FnCnMi «Я0ЯЯ IKW* Steel Shue Unaffected Fiber Shoe Unaffected RfOp— ▼VCWMB Jtflflflwy |W cc/40 Hrs, at 90’C 100‘C 120’C 0-0U 135*C 150*C
Rifle Refltt hapnct Test: T rich. % Explosions C Pcrtiols 0
Burned 0 Unoffected IDO 200 Orem Bomb Saad T< - Sand, gm 19,3
е Wfl^^WW«w« Seconds, 0.1 (no cop used) 1 5 Decomposes 310 10 * ** а- Сж» -»»..— vVIWovevOVy W HRWe^mwVHe Minimum Detonating Charge, gm Mercury Fulminate Lead Azide 0.20 Tetryl 0.25
20 BeOhtic Mortar, % TNT: (e) 71
Trend Teel, % TNT: (ъ) 6U
71 ‘C tateraotieael fleet Tech % Loes in 48 Hr»
Ptalo Dent Tert: Method
1M*C fleet Teet: % Loes. 1st 48 Hrs % Loss, 2nd 48 Hrs Explosion in 100 Hrs Condition Confined Density, gm/cc Brisance, % TNT
n*«*obMty Index: Confinement Condition Charge Diameter, in. Density, gm/cc Rate, meters/socond
flygreesepiclty: % 25°c, 100$ RH 0.00
VeleiiBty:
llfi
2,1»-'dtd trot oluene (PIT) AMCP 7*6*177
АМСР706-1П
2,4-DLnitrotoluene (ШТ)
Preparation;
8м пн?»
Solubility! ra/100 ga of the folloving substances:
25 o.i*:
» 0.29
45 0.49
55 О.ТГ
60 1.03
nitroglycerin water
°C J 2£ 1
20 30 22 0.027
50 0.037
100 0.251»
Solubility at 15°C> in:
1 sare* i
65.076 'эВеСВ (absolute) 3-039
2Л31 Khtr (absolute) 7-422
60.644 Acetone 81.9)1
45.U7O Ethyl acetate 57*929
5.014 0^2 2.306
1.916 Pyridine 76.810
Origin;
Occur! ae 754 of the product! obtained on the nitration of toluene, the reaaining 254 being
ainly 2,6-ПГГ and other iscaers of ШТ. Alio occurs as an iapurity In crude WT obtained by
standard aanuiecturing process, oaed In explosive Mixtures at least since 1931.
References:23
(a) L* C. arith and E. 0. Xyster, physical lasting of Explosives, Part III - Miscellaneous
Ssnsltlvity Testa: Pexfoxaance Tests, OSRD Report bo. 5746, Iff December 1945*
(b) A. H. Blatt, OoiBllatioc of Bate on Organic Explosives, OSRD Report йи. 2011», 29 Febru-
ary 1944.
(c) Report AC-2661.
(d) Also see the folio» .ng Plcatlnny Arsenal Technical Reports on П4Т:
0 1 2 1 4 1 6 1 8 2
810 1351 72 43 394 1615 186 97 768 69
1830 1501 372 5S 804 2125 1556 817 938 149
1651 922 1044 1816 837 1538 249
1781 1142 673 1084 1896 279
1821 1672 1023 1094 779
2031 1692 1663 1164 1749
2221 1743 1324
2013 1464
1521»
1671»
1754
2094
23Ses footnote 1, page 10.
118
Dipentaerythritol Hexanltrate (DPSOi)
AMCP 706-177
% c 21.7 и 2.9 N 15.2 0И0., f)HO„ 0 60,2 ch. ch 1 - 1 2 Ohb3CH2<j -CH, - c - сн2-ссн2о«ог C/H Ratio 0.15b °"^2 ^2 Nits tote. Weight: (C^H^W 551»
BdMcto CO, % -26 co % > 3
Beodly: gm/cc Crystal 1.63
Meting Peiatt *C 73-7
fceseing Point: *c
hwpoN f trail hr Ity, 1 Kg Wt: Bureau of Mine» Apparatus cm 14 Samp*» Wt 20 mg Picatinny Arsenal Apparatus, in. k Sample Wt, mg 10 fc_t_д. g^ MM| rtIWi к
ВеВяеНге lades, n“ ПЙ n»
flMee ВеаМша Teet: Steel Shoe Explodes Fiber Shoe Unaffected Vsara Stability Tost: cc/40 Hrs, ot 90*C 100‘C 3-7 120*C lie 135*C 150*C
BMt Mht teepoet Tsat: Trials % Explosions POrtfok Burned Unaffected
BBBBroni Bomb Sand Tost: Sand, gm 57. h
Exptoofea Tarapeeeteee: *C Seconds, 0.1 (no cap used) 1 300 5 Explodes 255 10 15 20 р^М1П*>*У SW Minimum Detonating Charge, gm Mercury Fulminate Load Aside Tetryl
BeNieHc Matter, % TNT: (B) 1U2
Travel Teet, % TNT: (Ъ) 128
П *€ teSeeeeliee».' Neat Tee»: % Loss In 4B Hn
Plate Doot Toot: Method Condition Confined Density, gm/cc Brisance, % TNT
lOO’C Moot Toot: % Loss, 1st 48 Hrs 0.11 % Loss, 2nd 4В Hrs 0.10 Explosion in 100 Hrs None
Detoootiea Bote: (c) Confinement copper tube Condition Preseed Charge Diameter, In. 0.39 Density, gm/cc 1, Rate, meters/socond 7bl0
— A-tBPe— A-A
Hygroscopicity: % 0.03
VehMtey:
11»
АМСР 706-177
М penta erythritol Hexanitrate (РРШН)
VW^pOWHVWVWIO 0 ••*• M MM HI, MFI Prejsrtilt, U* WC-91: Density, gm/cc Charge Wt, lb Tetol He. of FroguMata For TNT For Subject HE 1 Uch HI, MttAl PniecHh, Let KC4: Density, gm/cc Charge Wt, lb Tefal Ho. of РмдмеаН: For TNT For Subject HE Shopod Charge MfecHreoess, TNT = 1#C: Glass Cones Steel Cones Hole Volume Hole Depth
Cefar. White
Priadpol Usesi Ingredient of priming coapoaitlona
Method of Landtag: Pressed
Leedtag Deashyt gm/cc At 3000 to «Ю0О psi l.$9
Aa FVVJMWw ▼•WCWyX «T/jec At 9 ft At25Hft Density, gm/cc
Method Dry Hazorc* Class (Qunntity-Dtstonco) Class 9 Compatibility Group -udotion
tat (ReMeo to TNTh Abt Peak Pressure Impulse Energy Air, СеаКлеА Impuhe Under WaSen Peak ProMuro Impulse Energy Peak Pressure Impulse Energy
Preparation: (Chemistry of Powder and Explosives, Davis) afHO-CHg^C Dehydrate a (HO-CBg)3C-O-C(CB2-ce)3 'XJ » (о2ио-сн2)3с-о-с(снг-сио2)3 Dipentaerythrftol Hexsnltrate Is procured In the pure stati (melting point 72°C) by fractional crystillization of crude РИВ frost moist acetone. Origin: Formed as an inpurity in the prepa- ration of РПЯ. Properties first described by W. Frederick end W. Br&n in 1930 (Berlchte 63, 2861 (1930); Z. gee Sehless- Sprengsto/lv 2J, 73-6, 125-7, 156-8 (1932)> Heat of: Combustion, cal/gm 2260
120
Bipentaarythritol Hexanitrate (ДЛИН)
АМСР 706-177
BsfVreneee :24
(а) L. C. arith and B. Q. Xyster, Barsioal Tasting of Explosives, Part III - Miscellaneous
Osnsitivity Tests} Perfmaance Tests, OffiD Report Во. 57*6, <T beceear
(b) A. Stettbacher, Pie Schloss und a>rengstoffe, Leipsix, p. 363.
(с) T. I*. Mria, P>a Cbcalitnr of Pcwdar and Kxploaivea, John Wilay and Эопа, Inc., Bov
York (1!*3) »• 218-283.
(d) ft. Livingston, Characteristics of bmi wives НИХ and ИИ, PfcTR Во. 15&-, 6 Heptaaber
1945<
24See footnote 1, page 10.
121
АМСР 706-177
Hynamlte, Lov Velocity» Plea tinny Arsenal (LVD)
CoaopaoMea: 99.5/0.5 RDX/1-М dye» 17-5 % ПГГ 6?. 8 TTlpentaerythritoJ 8.6 68/32 Vlatac no l/DOS binders** h.l Ccllulooe acetate, 1Я-1 2.0 *RDX, doss E; 1-MA la 96$ pure 1-aethylaalno- anthraquinone. **Viatac № 1 ia lov MW polybutene; DOS is dioctylsetacate. C/H Ratio
^Fsygea ^Jebe^tee. CO, % CO %
Boaoffyt gm/cc Loading 0.9
MoBtag Fatah °C
FseoJag Fatah °C
IM^KV Л Ута» Buraou tf Mines Apparatus, cm Sample Wt 20 mg Picotinny Arsenal Apparatus, In. 22 Sample Wt, mg 19 BoNtag Fatal: °C
Rrhoeffra tadea, n° n£ n»
Mctfea Poadutam Toot: Steel Shoe Unaffected Fiber Shoe Unaffected Уе'дииа кабЯКу Teat: cc/40 Hrs, at 90°C 100°С I2O°C 0.90 I35°C I5O°C
Me Ballot kapoct Testi Trials % Eaplooiora Partials Burned Unaffected
MB Orata Bomb Saad Test Sand, gm 1ф. 5
ЬрМеа Temperature: °C Seconds, 0.1 (no cap used) 1 5 Ignites 480 10 15 20 tameMUBs» 6а ‘ Hr*1 OTBBWWBVy IWHWsWffr Minimum Detonating Chorge, gm Mercury Fulminate Load Azide 0.20 Tetryl 0.15
BeWsffi Berber, % . KT: gg
Treesl Toot, л Г ,T:
7J°C tataraeMoael Meet Toot: % Loss In 48 Hrs
Ftate Boat Teat. Method Conditio^ Confined Density, gm/cc Brisance, % TNT
100‘C Hoot Tom. % Lost, 1st 48 Hrs % Loss, 2nd 48 Hrs Explosion in 100 Hrs
Botcaetioo tote: Confinement None Condition Hand tamped Chorge Diometer, in. 1-25 Density, gm/cc 0.0 Rote, meten/second J1)?; or 1Л^00 ft/вес
НееммЫЙу lades:
Hygtiitsplsity: % 0.31 71°C. 95$ RH. 80 dove Satisfactory
VeleHBey:
122
Ifrnaiilto, Low Velocity, Picatinny Arsenal (ЦП))
AMCP 706-177
NtagU Chetge ВНоеНеоам, TNT = 1И:
•0 «ев Na. Ц71 ProjocHta. tn» WC-TI: Density, gm/cc ChorgeWt, lb Gloss Conus Steel Сопи Hole Volume Hole Depth
Total He. el fwgsMMet For TNT For Subject HE Cohn pink NodpollMee: Excavation, demolition,
> 1ей Nt, M4TA1 Prefect*, Ut KGS: and cratering
Density, gm/cc
Charge Wt, lb
Totof Ma, of Ftogmoete: For TNT For Subject HE Method of Leedieg: Bell Pucker machine loaded Loading Density: gm/cc 0.9
ftaanwat Vehdtyi ft/sec M t ft At 25ft ft Dimity, gm/cc Dashed cartridge 1-1/2” diameter, 8" 1оод Method Dry
Meet WNillnte TNT): Hazard Clou (Quantity-Distonce) Class 9
Ain Peek Pressure Impulse Energy Compatibility Group Group A Exudation
Alv Impulse Sensitivity to Initiation; Stick dry, No. 6 Electric cep Positive Stick dry, Corps of Engineers Positive Stick vet, Corps of Engineers Positive
Under Wotan Peak Pressure Impulse Energy Air Gap Propagation; Mas distance will, inch 2-1/2 min dlstenae will not, inch 3 Stick Water Issnersion; Weight gain, % 9-16
Peak Pressure Impulse Energy Hot of: kxplcsion, cal/gm 625 Gas Volume, cc/gm 611 Cold Storage: Plastic to -65°F Low Temperature Usage; -65°r, 1 day, М2 cap crimper Satisfectoty
12:1
АМСР 7*477
Ifrnanlte> Low Velocity, Plcatlnny Arsenal (pro)
Preparation;
Tc date thia dynes! to baa bean prepared on a laboratory scale, the details of which are
clasalfled. It has been ehcam, however, to be sachlne loadable on a Hall packing aacMne*
Origin:
obel invented the original dynaaite In 1866 and gave the naae dynaaite to slxtures of
nitroglycerin and kieselguhr. П» strength of a dynaaite war Indicated by the percentage
of № in the alxture. Later oxidants and casbustibles were substituted for the kieselguhr,
and aasonlos nitrate nA/аг nitrostarch replaced the BG, bringing into existence new types
of dynaaltes. World *r П ailitary operations required special dasolltlon and crate ;ng
explosives free free the objectionable characteristics of Ж? and aany "dynaaite substitutes"
were developed for q^^dfio applications, The subject low velocity dynaaite waa developed in
1956 by Plcatlnny Arsenal (Ref a).
BefOroncee;
(a) H. V. Tcigt, Dwelowwnt of Low-Velocity Military Explosives Equivalent to Coanerdal
Mites, PA Technical hwSrt Йаг~сь'1ЭДС-------------------' ----------- ------------------------
(b) Also see the foilowirg Plcatlnny Arsenal Technical Reports on lynasites:
0 1 2 4 1 6 I 8 2
12б0 1360 1720 1760 1381 1611 782 153!' 864 1464 1285 1416 1436 1506 2056 507 957 848 1826 1819
2*See footnote 1, page 10.
124
Dynamite, Mediun Velocity, Hercules (МУР)
АМСР 706-177
^rwnhhm ЛИ 75 Wf 15 Starch 5 SU Ho. 10 Oil к Viatanex oil gel* 1 *80/15/5, ОН Во. Ю weight oil/Vistanex B- ICOXC/Navy П2 aax, C/H Ratio Molccvlw
Osrygea Boieace: ca% .51 co %
Density: gm/cc Loading 1.1
МаШад Point: "C
* o^ rwBIBrJ v>
invest 8миМа«у, 1 Kg Wt: Buraou of Mine Apporotua, cm >100 Sample Wt 20 mg 18 Picotinny Arsenal Apparatus, tot. 25 Sample Wt, mg Nitroglycerin Equivalent, J, 60
al al at c c c
PtMaa Rcndohaa Teats Steal Shoe Cracklee Fiber Shoe Unaffected Vmbmb StaMMty Teet* cc/40 Hrs, ot 90’C 100’C 0.80 120*C 0-9*> 135’C I5O*C
RMe BuBat Inspect Teat: Triofs % Essploaione C Portiob 0 Burned 10 Unaffected 90
2M Gram Bomb Seed Teet: Sand, gm 52.6
8арвве1ео ITaeape^etsaee. Cf Seconds, 0.1 (no cop used) 1 5 10 15 20 vGMBVBBWv^^^ WV В BwB^R^^^B* Minimum Detonating Charge, gm Marcury Fulminate Lead Azide q,20 Tetryl 0.10
Inilirtic Mertsr, % TNT: ]^2
Treeri Teat, % TNT:
T^^Ae 9 9 mt ^BB^^^^^B^^^^^^MBB^^B В % Lota tot 48 Hrs
Plate Dasti Teat: Method Condition Confined Density, gm/cc Brier, xa, % TNT
MB'C Moot Teat: % Lett. 1st 48 Hrs 0.62 % Um, 2nd 48 Hrs 0.12 Explosion tot 100 Hrs None
Uataiietiaa Rote: f enfinement None Condition Machine tamped Charge Diameter, in. 1.50 Density, gm/cc 1, i Rote, meters/second бООО-ббОО; or 20,000 ft/вес
Ib4*s*
71°C, 95# BH, 30 days Satiefactory
ValJlilj:
125
AMCP T06-177 ч Dynemlta, Msdlum Velocity, Hercules (WD)
FiegamMlsB Tash Sheped Charge Effectiveness, TXT = 1«h
N «мв M, МЛ h^MHto, Ut WC-»1: Density, gm/cc Charge Wt, lb Glass Cones Steel Cones Hole Volume Hob Depth
For TNT For Subject HE 1 inab Nt, MOA1 РмЦсМе, IM KC-S: Density, gm/cc ChorgeWt, lb Cohn Buff
Mwipol Ums: Excavation, demolition. and entering
For TNT For Subject HE Method of Leedhg: Hall Packer Bachina leaded
leodhg DeaeNyt gm/cc 1.1
hspM Yataity: **/sec At 9 ft At2SVift Density, Qn/cc Cartridge 1-1/2" diameter, 8" long
Method Dry
*tat (RahHvela TNT): Hazard Class (Quantity-Distance) Class 9
Ain took Pressure Impulse Bmw Compatibility Group Exudation Group A
Alp* СовЛввйк Impulse UederWelor: Peak Pressure Impulse Bmw Peak Pressure Impulse Enorw Sensitivity to Initiation; Stick dry, Ho. b Electric cep Positive Stick dry. Corps of Engineers Positive Stick wt, Corps of Engineers > 50^ Positive Air Gap Propagation; Max distance will, inch 1 Mln distance vlll not, Inch 2-1/2 Quarry Performance; 1* tons rock/ton explosive Stick Water TurnersIon: Velght gain, f, 25-27 Heat of; Explosion, cal/gm 935 Ges Volume, cc/gm 9^5 Cold Storage; Plastic to -70°F Low Tswerature Usage:
-6^4^, 1 day, Mz cap crimper Satisfactory
126
Dynaaite, Medium Velocity, Hercule» (МУР)
AMCP7N-177
Preparation;
Manufactured on atandard dynaaite Una and packaged on a Hall packing aadilne. Patella of
handling materiala and technique» of manufacture are claaaifled.
Origin:
Military force» frequently require excetritiou, demolition, and cratering operatlonc for
which atandard high exploaive» are unauitnble. Ooanercial bleating aiploaivea, except black
powder, are called dyneaita» although they nay contain no nitroglycerin. The cub Jact dynaaite
aub»tivute ana developed in 1952 by the Hercule» Povdar Ccapeny (Ref a).
Beferencea:26
(a) V. R. Baldwin, Jr., Bleating Exploeivea (Pynamite Subatitute), Hercule» Povdar Coapany
Vocmal Progreaa Report, RI 2006, 15 Auguat 1952, Any Contract РА-^б-ОЭ^ОВР-ИО.
(b) и* V. Voigt, Development of Low-Velocity Military Bploeivea Equivalent to Ooanarrlal
Dynaaitee, BA Technical Report Bo. 2374, March 1957.
26Sea footnote 1, page 10.
127
АМСР 706-177
EC Blank Fire
Смфмййв Molecular Weight: Approximately 503
Nitrocellulose, 13-256 N 80 BariuB Nitrate 8 Potaaalua Nitrate 8 Oxygm Маасе. CO % -25
Starch 3 Dlphenylandne 0.75 Aurlne 0.25 Density: gm/cc
Making Point: "C
C/H Ratio
Impact Sonettivily, 2 Kg Wh Bureau of Minas Apporatus, cm 19 Sample Wt 20 mg Picatinny Arsenal Apporatus, in. Sample Wt, mg 20 BeWag Point: 'C
Refractive Index, nJ ni r.i
• • -* ^---а_-а . rnOnW rWWWBI IMBos Steel Shoe Snape Fiber Shoe cc/40 Hrs, at 90‘C 100‘C I2O*C 135‘C 150‘C
RMb BnMat Impact Teat: Trials % Explosions Partials
Burned Unaffected 200 Gram Bomb Sand Tost: Sand, gm 16.8
Ь^йЫм ТмфмоНи*: 9C Seconds, 0.1 (no cop used) 1 5 Decomposes 200 10 >K Sensitivity to Initiation: Minimum Detonating Charge, gm Mercury Fulminate 0.22 Lead Azide Tetryl
20 BaMstic Mortar, % TNT:
Travel Test, % TNT:
79‘C latamstiaeal Heat Test: % Lou In 49 Hrs 1.8
Piets Dent Toot: Method
100'C Heat Teat: % Lou, 1st 48 Hrs 2.0 % Lou, 2nd 48 Hrs 0.2 Explosion in 100 Hrs None Condition Confined Density, gm/cc Brisance, % TNT
Detonation Rate: Confinement Condition Charge Diameter, in. Density, gm/cc Rate, meters/second 1
Hygroscopicity: % ypCt m g.2
"BHVHIVf «
128
EC Blank Иге
АМСР 706-177
Fvegawalatism Teats •0 мае HI. МЛ Pnjirtili. Let WC-91: Density, gm/cc Chorge Wt, lb For TNT Foe Subject HE > tach H8, M42A1 PrajecMs. Let KC-1: Daneity, gm/cc Chorge Wt, lb TsSsiNe, ef Ямдама*к For TNT For Subject HE Shaped Charge EffeeHeeoese, TUT = ItO: Gloss Сопы Steel Cones Hole Volume Hole Depth
Cator:
Pitadpel Uses: Grenades; caliber • 30 blank Motbo'' '4 Laodtag: Loose
Lnadtag PreeWyi gm/cc o.ltO
Frogeumt Votacby: ft/ssc At 9 ft At 25% ft Daneity, gm/cc
Method wet Hazard Class (Quantity-Distance) Class 0 Compatibility Group Group J Exudation
Meet ttrlMbete TNT): Abt Peak Pressure Impube Energy Airf См41м4г Impube Under Wotan Peek Pressure Impube Energy Peak Pressure Impube Enerav
Preparation; EC Blank Fire 1b a partially collolded propellant manufactured by a pro- cess using either acetone and ethanol or a mixture of butyl acetate and benzene to gelatinize only a part of the nitrocellu- lose. The process is controlled co that the product passes through a No. 12 sieve and is retained on a No. 50 sieve. Origin; Invented in 1882 as bulk sporting (smoke- less) powder by W. F. Reid and D. Johnson at the Explosive Совфапу (whence the name "FC") In England (British Patent 619). 120°C Heat Test; u. Salmon Pink i.50 Red Fumes ЗЭО+ Explodes 300*
References:2 4 a) See the following Picatlnny Arsenal Technical Reports on EC Blank Fire: 091, 901, 372 , 512 , 822 , 233, 1373 , 851*, 65, 667, 817, 69, 579 and 1399-
'See footnote 1, page 1C.
129
AMCP 706-177
Ednatol, 55A?
(W Meleceler Weight: 178
Halelte (Ethylene Dinltranine) 55 TNT 1*5 Oxygen lelaaca: CO: % .51 CO % -17
Densify: gm/cc Cast 1.62
Melting Pahth *C Eutectic 80
C/H Rot . ^^0004^6^ ^^еФЯФе
aspect Sensitivity, 1 Kg Wt: Bureou of Mines Apporotus, cm 95 Sample Wt 20 mg Picatinny Arsenal Apparatus, in. Sample Wt, mg 20 BeMiog BeHft "C
ot oS oS c c c
Гггаа^П vwH^^NW iwmas Steel Shoe Unaffected Fiber Shoe Unaffected Vecaam StebMty test: cc/40 Hn., at 90'C 100'C 1.0 120"C 11* I35*C 150’C
Mia BoMat Impact Test: Tols % Explosions 0 Partials 0
Burned 7 Unaffected 93 200 Seem Bomb Sood Teat: Sand, gm 1*9.1»
Expteeiea Taaipeeelvee: » C Seconds, 0.1 (no cap used? 1*35 1 21*8 5 Decomposes 190 10 183 >5 176 20 168 wWVBVTvDVy vw WBBOwBV^B^NBe Minimum Detonating Charge, gm Mercury Fulminate 0.22» Lead Azide 0.26» •Alternative initiating charge».
BoMeNc Mertw, % TNT: (a) 119
•Ccripoaltlon Helelte/TNT, 60/l*0. trawl Teat, % TNT: (b) 120
75‘C leteieatiaael Heei Test: % loss in 48 Hrs
Pfete Deaf feat: 52/W Method В
100*C Heat Teat: % Loes, 1st 48 Hrs 0.2 % Lou, 2nd 48 Hrs o.l Explosion in 100 Hrs None Condition Cast Confined No Density, gm/cc 1.62 Brisance, % TNT 112
Confinement None
HeamaeMBty lodes: Will not continue to bum
Hypraeaaalclty: % None Condition Cost Charge Diameter, in. 1.0 Density, gm/cc 1-63 Rate, meters/second 73**O
Te^^mW^^y J
130
Ed na to 1, 55A5
АМСР 706-177
ФО ям* Hl« Mil FvMecHb, Ut WC-01: Density, gm/cc 1-56 1.62 Charge Wt, lb 2.065 2.092 Total Ma. of Pragmesfi: For TNT 703 703 Fa Subject HE 8U2 902 1 loch HI. M42A1 Pw|eeHh, Let KC-S: □entity, gm/cc 1-60 Charge Wt, lb O.8U5 Total Na. of FragsMOH: For TNT 514 For Subject HE 536 Steeped Charge INecHveaeso, TNT = 1Ю: 50/50 Gloss Cones Steel Cones Hole Volume 126 123 Ной Depth 117 121
Cohn Yellow
Principal Um: Projectiles, bombs; special ammunition components
Method of Loodhg: Cast
Uedfag Oeastey: gm/cc 1,65
Ffogeteet Veiedty: ft/sec Mh 2730 At 25Ц ft 21*30 Density, gm/er 1.62
Method Ety Hazard Class (Quantity-Distance) Class 9 Compatibility Group Group I Exudation Does not exude at 65°C
Mast (Rehthre fa TNT): (d, e) Air: Peak Pressure 108 Impulse 110 Energy 108 Air, Caafhad; Impulse Under Water: Peak Pressure Impute* Energy 113 Peak Pressure Impulse Energy Booster Sensitivity Test; (d) Condition Cast Tetryl, gm 100 Wax, In. for 50% Detonstl-on 1.28 Density, gm/cc 1.62
Butectic Temperature, °C; 79-8 gm Halelte/ldo gm ТЯГ 79.8°C 0.1*8 95-O°C 1.12 Compatibility vith Metals: Dry: Brass, aluminum, stainless steel, mild steel, mild steel coated with acid- proof black paint, and mild steel plated vith cadmium or nickel are unaffected. Cop- per, magnesium, magnesium-alumlnum alloy and mild steel plated vith copper or zinc ere slightly affected. Wet; Copper, brass, magnesium, magnesium- aluminum elloy, mild steel, mild steel coated vith acid-proof black paint and mild steel plated vith copper, cadmium, nickel or zinc are heavily attacked. Aluminum is slightly affected and stainless steel is unaffected.
131
АМСР 706-177
Ednatol, 55/45
Preparation:
Wet Halelte 1* added «lowly to molton TUT heated at about 100°C in a steen Jacketed eelting
kettle equipped with a stirrer. Heating and stirring are continued until all moisture la
evaporated. Loading la done by pouring the mixture cooled to 85°C.
Origin:
Mixture» of Halelte (ЕЛА) and ТЯГ, deaignated Ednatol; were developed at Picatlnny Arsenal
Juat prior to World War II.
References;-8
(a) L. C. Saith and E. G. Ryeter, Phy a leal Te»tlng of Explosives, Part III - Miscellaneous
Sensitivity Tests; Performance Te»ta, OSRD Report Ho. 5^46, 27 December 1^4^.
(b) Philip C. Keenan and Dorothy C. Pipe», Table of Military High Explo»lve«, Second Revi-
sion, KAVORD Report Ho. 87-46, 26 July 1946.
(c) D. P. McDougall, Methods of Physical Те»ting, OSRD Report Ho. 803, 11 August 1942.
(d) L. C. Smith and S. R. Welton, A Consideration of RDX/Wax Mixture» a» a Substitute for
Tetryl in Booster», HOL Memo 10,303, 15 June 1$49.
v* Tomlinson, Jr., Blast Effects of Bomb Explosive», PA Tech Div Lecture, 9 April
(f) Eastern laboratory, du Pont, Investigation of cavity Effect, Sec III, Variation of
Cavity Effect with Cocpoeltion, ВИС Contract W-672-0RD-5?ft.
(g) Eastern Laboratory, du Pont, Investigation of Cavity Effect, Final Report, 18 Septem-
ber 1943, НПГС Contract W-672-0RD-5727
(h) Also see the following Picatlnny Arsenal Technical Reports on Ednatol:
0 1 2 1 4 2 6 1 8 2
1290 1291 1162 1193 1294 1325 1796 1457 1198 1279
1400 1451 1372 1363 1434 1395 1477 1388 1469
1420 1651 1482 1493 1885 1737 1838
1530 1797
28S.S footnote 1, page 10,
132
Ethylene Glycol Di-Trlnltrobutyrete (GTHB)
AMCP 706-177
CTHMinirt c 25.6 И 2.6 Mehtaier Weight: (C^Hj^KgOj^) i»68
Oxygen Baleace: CO, % CO % o'#
^HgCOgCHgCHgCfiiO^) Density: gm/cc Crystal I.63
0 %? Making Mnt: *C .96
C/H Ratio 0.235 Fleecing Point: *C
Inspect 1емМ«йу, 1 Kg Wtt Tiffing Flint: *C
е^ШЯ^Шеп wt II1 Igag^aes w• Sample Wt 20 mg Picatinny Arsenal Apparatus, in. Sample Wt, mg Kefiecthre lades, ni nl t£
e-»e_ _ ^г^^^Б^^^^ЯВ В ЯЯ*е Steel Shoe Fiber Shoe Vecaaee Stability Teet: cc/40 Hrs, ot 90*C 100’C 120*C 135*C 150*C
ШНе BaNet Impact Teet: Trlan % Expiations Partials
Burned Unaffected 200 Cram Bomb Send Teet: Sand, gm
ЯЯрВЯЯ^ИВ • WW^WWWeWt Seconds, 0.1 (no cop used) —- 1 5 50jt point 230 10 15 4» -» a*-- e t.e .e _ . ПЯВВЯВдВТЯу ew BflBaWIBVWe Minimum Detonating Charge, gm Mercury Fulminate Lead Azide Tetryl
20 Ballietic Mortar, % TNT:
Treusl Test, % TNT:
79*C letemetionel Meet Teet: % Low tn 48 Hrs
Ptatn Dent Test: Method
100*C Heat Teet: % Lou, 1st 48 Hrs % Lou, 2nd 48 Hrs Explosion In 100 Hrs Condition Confined Density, gm/cc Brisance, % TNT
ЯеомиЫМу lades: 9В«ПЯв«МЯ™КОВПе Confinement
kLeaaMouplaihr* QL иОПбпЮП Charge Diametnr, in. Density, gm/cc Rate, meters/second 1.63 73UO
»*- a.^eae^.
133
АХО» 706-177
Ethylene Glycol Dl-Trinitrobutyrete (gTNB)
ЕюдамаСаМеа Tash M ома HI, MFI Prelect#*, Ut WC-»1: Density, gm/cc Charge Wt, lb Total No. ef FtagsMata: For TNT For Subject HE ) bnb HI, M42A1 PrejecM*, Lae KC-St Dantity, gm/cc Onego Wt, lb Total No. of FfagoMOtai For TNT For Subject HE Shaped Charge Iffectbeae**, TNT = IN: Qon Cone* Steel Cone* Holo Volume Holo Depth
Cxlxtt
Pile dp *1 Uses: Costing medium for HE compounds
Method ef Leadtag: Cast
Leodtag Dooaity: gm/cc 1.60
Ftaganmt Vdodtyt ft/eec At» ft At 25% ft DansHy, gm/cc
Steroge: Method Dry Hazard Clast (Quantny-Distance) Compatibility Group Exudation None
Moat (Marita taTMDi Ain Peak Promurn Impube Energy Air, Ceafiaed: Impube Under Wotan Peak Pleasure Impube Energy Peak PiWMuro Impube Energy
preparation; (a) By the addition of nitroform to ethylene glycol diacrylate. As the method of prepa- ration often leads to products difficult to purity, a preparation from ethylene glycol and pure trialtrobutyric add la In process. Origin: First synthesized In 1951 by the U.S. Rubber Соераny, Research and Development General Laboratories, Passaic, Nev Jersey. Viscosity, poises: Tenp, 98.9° C 0.2U6 106.5°C 0.193 Llguld Density, gm/cc: Tenp, 98.9°C I.U67 106.5°C 1Л59
ГИ
AMCP 706-177
Ethylene Glycol M-T^lnitrobutyrate (GOTB)
Reference»»2?
(a) U. S. Rubber Соирапу Progress Report Ko. 1U, Navy Contract NOrd-10129, 1 Tebruary 1951
to 1 May 1951.
(b) U. S. Naval Ordnance Laboratory, Silver Spring, Maryland, Letter free Dr. 0. H. John-
son to Coonnding Officer, Plcatlnny Araenal, 8 April 1955 (OREBB l*T1.86/M»-3, Registry No.
39815); and NOL Letter free» Or. D. V. Slckaan to Cotenanding Officer, Plcatlnny Arsensd,
29 Noveaber 1955 (ORTBB l»71.86/159-1} Serial No. 3289**).
2?See footnote I, page 10.
13d
АКСР7М1П
Explosive D (Aanonium Picrate)
Melecahr Wei'gbtt (CgHgNj,<^) 246
c 29.3 |0-BHlt H 2.4 O2N N02 Osygee Вейми: CO, % .52 CO % -13
N 22. T 1 \ Oeeeltyi gm/cc Crystal 1.72
0 45.6 I Meting Mott *C Decadence 265
N0~ C/H Rotio 0.317 2 В—8^*
ImpactSaaeMMty, 2Kg W* Bureau of Mines Apparatus, cm Sample Wt 20 mg Pleotinny Arsenal Appri'Utus, In. 17 Sample Wt, mg 18 BoiSag Matt *C
leMbkdo, nJ oo l.'5O8 ь0 1.870 % 1‘907
Frktiea Pin «also Test: Steel She Unaffected Fiber Shoe Luaireeted Veceam Stability Test: cc/40 Hrs, at 90*C 100C 0.2 120*C 0.4 135*C 150‘C 0.4
KIHo BaNot Impeet Tech Trials % Explosions 0 Partials 0
Burned 30 Unaffected 70 208 Gram Seed Tent: Sand, gm 39-5
Eapheiee Tsf sreten1 *C Seconds, 0.1 (no cap used) 405 1 367 5 Decoepoees 31B 10 314 IS PQQ *OHSBWTwy ra VIHnVoVBBBe Minimum Detonating Charge, gm Mercury Fulminate Lead Aside 0.20 Tetryl 0.06
20 295 BeiHstir Mester, % TNT: (a) 99
Trmal Tert, % TNT:
TCtA 9 9 mt В % Lok in 48 Hrs
Plate Boat Teat: Method A
IBO’C Hoot Test-. % Itk, 1st 48 Hrs 0.1 % Loss, 2nd 48 Hrs 0.1 Explosion In 100 Hrs Kone Condition Pressed Confined Yes Density, gm/cc 1.50 Brisance. % TNT 91
Confinement None
HygreecogteMy: % юо^ RH 0.1 COnOniCrV > 1 VBBVU Charge Diameter, in. 1.0 Density, gm/cc 1.55 Rate, meters/second 6850
VeMBfyt
136
Explosive D (A—onlua Picrate)
AMCP 706-177
W mm M. M71 Pmteate. Ut WC-H:
Density, gm/cc Charge Wt, lb 1.50 1.9k
BW^VV ^w^MBBWBBR
For TNT 703
For Subject HE 6k9
1 teh M. MOA1 Prate*. Loe KC-S :
Density, gm/cc 1-55
Charge Wt, R> 0.82
B^^BV V* nVJMvMK
Far TNT 51k
Far Subject HE 508
At 9 ft
At2Sftft
Density, gm/cc
Steel Сейм
BtetOteettetoTNTh
Abt
F так Pressure
Impute
Energy
impute
Under Weton
Peak Pressure
Impute
Peak Praeauro
Impute
Holt Volume
Koto Depth
Cote: Yellov-cnnge
Ptedpel Item AP frc^-ctile* and botes
Hotbed of Leading: Pressed
Leedhra Denote: gm/cc psi x 103
- 3 5 10 12 15 20
1.33 l.kl l.k? 1.1»9 1.51 1-53
Method Dry
Hazard Clou (<>nntity-Distance) Close 9
Compatibility Group Group I
txudation Hone et 65°C
Sensitivity to Electrostatic
Diecharge, Joules: (a)
through 100 Mesh:
Confined 6.0
Unconfined 0.02?
Booster Sensitivity tWr.v: (e)
Condition Pressed
Tetryl, ga 100
Wax, in. for 504 Detonation 1.27
Density, gs/cc 1.5k
Beat of;
Combustion, cal/gm 2890
Explosion, cal/gm 800
Formation, cal/gm 395
137
АМСР 706-iW
Explosive D (AsMonium Picrate)
Preparation;
Explosive D la sanufhotured by suspending picric add In hot vatar and neutralizing it vith
gaseous or liquid aaaonla. As the picrate Is formed, It gees Into solution; on cooling, it
precipitates* An excess of авакШа leads to formation of the red form of anaKnlum picrate.
This should be avoided. The sepaisted crystals are washed vith cold voter and dried.
Effect of Storage on Sand Test Values;
Minimum
Mercury Sand
Storage. Fulminate Crushed
Years fgn) Trtgl (gaj
0 0.06 23
3-5 50 0.25 23
2 * Honal 0.03 23
1» » Е0ШМ1 0.04 23
2 *» 50 0.24 - 23
* After 3-5 years at 50°C.
*♦ After 3*5 years at 50°C and 2 years at nagazlna temperature.
Solubility: gm/100 gm «), of: (e)
Hater Alcohol Ethyl Acetate
1 2£ £ fc 1
20 1.1 0 0.515 0 0.290
100 75 10 0.690 10 . 0.300
30 1.050 30 0.380
50 1.890 50 0Л50
80 3.620 80 O.56O
Origin:
First piepared by Marchand in 1841 and used by Brugere In admixture vlth potassium nitrate
as a propellant in 1869. Used aa a high explosive after 1900.
Destruction by Chemical Dsccspoeitlon;
Explosive D (assKniua picrate) is deccapoaed by diasolving in 30 times Its velght of a
solution made from 1 part of sodium sulfide (BegS'^gO) in 6 parte of water.
References:
(e) L. C. Smith and E. G. Eye ter, Physical Testing,of Exploeivau, Part- III - Miscellaneous
Sensitivity Tests; Perfomance Tests, Offib Report Но. 5746, 27 Dececber 1945.
SOSee footnote 1, page 10.
138
Stploaive D (Awonlua Picrate)
AMcpmtn
(b) D- P. McDougall, Method! of Physical Та»ting, OSRD Report Ko. 803, 11 August 1942.
(c) U C. SBlth rod S. R, Wteltoo, A Consideration of РДХ/Мх Mixture» aa a Substitute for
Tetryl in Boosters, .OL Neeo 10,303, 1$ June
(d) V. Brown, D. H. Builer and F. C. Gibson, Sensitivity of Explosives to Initiation
by Electrostatic Diecharges, U. S. Dept of Int, Bureau of Mines, Bi 3o52,
(e) Various sources in the open literature.
(f) Also see the following Picatlnny Arsenal Technical Reports on Explosive D:
0 1 2 2 4 2 .6 I 8 2
3U0 14Ы 132 8^3 69*t 65 266 1737 328 1729
870 ,6 1 582 70b 425 556 1797 836 1759
13&? 1172 87<* 1585 796 183B
1352 123b 1655 986
1372 172k 1725 1466
lit 92 1885 1796
1895
11Ю
АМСР 706-177
Glycerol Monolactate Irinitrate (GLOi) Liquid
СТПМ1ТГ11-. c 24.1 о оно. 1 1 H 3.0 CH- 0- C- CH- CH, Mateeater Weight: (CgH^OjJ 299
co, % co % -30 3
1 < N 14.1 CT-0M02 J Density: gm/cc Liquid 1Л?
CH - OHO- 0 58.8 22 MalNag Petal: *C
C/H Rotio 0.180 F(WmB| rWWi
Repast SeaeMtity, 1 Kg Wt: Bweou of Mine* ^porotus, cm 15 (1 lb wt); 42 Sample Wt 20 mg Pleotinny Anenal Apparatus, In. Sample Wt. mg oWONVe Vp
jo go /о 1.464
Mclisa Pent Tash Steel Shoe Fiber Shoe Unaffected Unaffected ц m.-ltee-. ЧР- wwwMWBey 8W1 cc/40 Hrs, ot 90’C 100’C I2O*C I35*C I5O*C 5-9
RM* Better Impeet Taet: Trial* % Explosion* Partial*
Burned Unaffected NO Orem Веой toad Totat Sand, gm IM
Second*, 0.1 (no cap ussd) 1 5 223 10 15 20 vWB0VeV4«y ‘"W MOHONRO^MOI Minim», m Detonating Charge, gm tAercury Fulminate Lead Azide Tetryl
ВеШаНс Merter, % THT:
Trauxl Test, % TNT:
9 9 W * * % Los* in 48 Hr*
Plate Beat Teat: Method
100'C Heat Test: % Loe*, 1st 48 Hr* % Lou, 2nd 48 Hr* Explosion in 100 Hrs 2.5 1.8 None Condition Confined Density, gm/cc Brisonce, % TNT
FlMMMsMSly ledee* Confinement
QC Condition Charge Diameter, in.
VetaMky: 60°C, ag/cm2/hr 28 Density, gm/cc Rote, meters/second
140
Glycerol Monolaetate Trinitrate I.GLTW) Liquid
AMCP 706-177
FregaseePstiee Teat: NeeM, W1 hejertNe, Let WC-»1: Density, gm/cc ChorgeWt, k> Tetel Na. et Fragaaanto: For TNI For Subject HE 1 lack HI. М4ЭА1 NljulWk. Let KC-S: Density, gm/cc Chorga Wt, lb IWw ^^Ba гжВИИЮТКМа For TNT Far Subject HE Gloss Cenas Steel Cones • Hole Volume Hole Depth
Cebr:
Principal Ueas: Gelatinlzer for nitrocellulose
Merited of Lending:
Leadteg Doaeky: gm/cc
Fragment Velocity: ft/sec At 9 ft At25H<t Density, gm/cc
Method Liquid Hazard Class (Quantity-Distance) Claes 9 Compatibility Group Exudation
Blast (leietbe to TNT): Air: Peak Pressure Impulse Energy Air, Cenflned: Impulse Under Water: Peek Pressure Impulse Energy Peek Pressure Impulse Energy
Hydrolysis, jt Add: 10 days at 22°C 0.021 '5 days at 60°C 0.01b Solubility in Water, gm/100 gm, at; 25°C <0.01 60° C <0.015 Solubility, gm/100 gm, at 25°C, in; Ether > 2:1 Ether:Alcohol - Acetone Heat of; Combustion, cal/gm 2hO7
141
АМСР 706-Ш
Glycerol Monolactate Trinitrate (МДМ) Liquid
Preparation:
Glycerol monolactate (GML) '.в prepared by heating a glycerol lactic add mixture containing
Ц exceaa lactic acid at 116°C for 112 hours with dry air bubbling through the liquid. The
product which contains 0.67^ free add is carefully nixed with 6 parts of 4о/бО HBOo/HgSOt
aaintained at 2O°C, stirred for 1 hour, cooled to 5°C, and poured on ice. It is extracted
with ether, water-washed, adjusted to pH 7 by shaking with a sodium bicarbonate solution, and
again water-washed tn.—e tines, it is then dried with calcium chloride, filtered and freed
of ether by bubbling vi'h eir until min'.ael loss in weight is obtained. The product has a
nltrate-nltrcgen conUat of 13.43^ (theoretical 14.1^ N). Another batch, prepared from 1ML
obtained from glycerol-lactic acid containing 6.% excess glycerol, bad a nitrate-nitrtgeu
content of 14.yjf, corresponding to a mixture containing 5-5^ nitroglycerin. It it not con-
sidered practicable to prepare the pure GLTK.
Origin:
The preparation of a nitrated ester of lactic acid and glycerol, by nitrating a glyceryl
lactate with nitric and sulfuric acids, for use in explosives, was reported in 1931 by Charles
Stine and Charles Burke (U. S. Patent 1,792,515)*
П>е preparation of glycerol monolactate by heating glycerol with equimolar proportions of
a lactic acid ester of an alcohol boiling below 100°C (ethyl lactate) ms patented by Richie
H. Locke in 1936 (British Patent 456,525 end U. S. Patent 2,087,980).
Reference;31
(a) P. F. Macy and A. A. Saffitz, Explosive Plasticizers for nitrocellulose, rAIR Ho.
1616, 22 July 1946.
31See footnote 1, page 10,
142
Glycol Иnitrate (GDN) liquid
AMCP 706*177
Molecular Weight: (СДК^) 152
C 15.8 ^^,oso2 H 2.6 CH2 Oxygee Balance: CO: % 0.0 CO % 21
N 18.4 1 Density: gm/cc Liquid, 25°C 1.48
o 63.2 Making Point: °C -20
^^0N0_ C/H Rotio 0.092 2 Fraesieg Point *C
Impact Sensitivity, 3 Kg Wt: Bureau of Mines Apporotus, cm 4 (1 lb vt); 56 Sample Wt 20 mg Picotirmy Arsenal Apparatus, in. Sample Wt, mg BeWng Patat: *C
kehnctive Index, n£ П» 1.4452 nJ
Steel Shoe Fiber Shoe wVWWWwy leWi cc/40 Hrs, at 90*C 100°C 120’C I35*C 150‘C
Riffs BoNct Impact Tech Trials % Explosions Partials
Burned Unaffected 200 Gram Bomb Sand Toot: Sand, gm
Кж^ймм* ТмфмвИива °C Seconds, 0.1 (no cap used) 1 5 Explodes 257 10 Sensitivity to Iniffetioa: Minimum Detonating Charge, gm Mercury Fulminate Load Azide Tetryl
20 I sillstir Mortar, % TNT:
Troxel Test, % TNT:
7S'C Interaaiioecl Heat Tom: % Loss in 48 Hrs
Plate Dent Toot: Method
100’C Hoot Teat: % Loss, 1st 48 Hrs % Loss, 2nd 48 Hrs Explosion in 100 Hrs Condition Confined Density, gm/cc Brisance, % TNT
FlenunebUity Index: Confiner.ient Glace tube Condition Liquid Charge Diameter, in. 10 Density, gm/cc 1.485 Rato, meters/secand 7300 and 2050
Hygroscopicity: % 30°C, 90$ RH 0.00
Volatility:
14И
AMCP 706*177
Glycol Di nitrate (GEK) Liquid
g бон M мм HE. МУ1 Pra|ecMe. Lat WC-T1: Density, gm/cc ChorgeWt, tb For TNT For Subject HE 1 loch Ht. M42A1 Рге|ес*Не. Let KC-S: Density, gm/cc ChorgeWt, tb e Wwl V^Ae V* rlW^I^Ml» For TNT For Subject HE Shaped Charge IKocHveaesa, TNT = 100: Gloss Cones Steel Cones Hole Volume Hole Depth
Color: Yellow
Priadpal Uses: Ingredient of nonfreezing dynamite
Leedtog DeaeKy: gm/cc
Fragment Yehilty ft/sec At 9 ft At 254 ft Density, gm/cc
Method Liquid Hazard Class (Quantity-Distance) dees 9 Compatibility Group Exudation
Elect UUietire to TNT): Ah: Peck Pressure Impulse Energy Ah. CsaHasd: Impulse Under Water; Peak Pressure Impulse Energy Peek Pressure Impulse Energy
Solubility in 1000 cc Whter; Temp, °C Grama 15 6.2 20 6.8 50 9-2 Viscosity, centipoises; Temp, 20°C 1.2 Vanor Pressure; °C nn Mercury 0 0.0011 20 0.038 10 0.26 6o 1.3 80 5.9 100 22.0 Heat of; Combustion, cal/gm 1761 Formation, cal/gm (b) 366
1+4
Glycol Dinitrate (GDN) Liquid
Alt CP 706-177
Preparation;
Glycol dinitrate (ethylene glycol dinitrate, dinltroglycol, nitroglycol, dinitrodimethyl-
eneglycol) may he prepared by nitration of ethylene glycol, HOCH2CH2OH, vith a mixed nitric
acid in the same apparatus that ie used for the preparation of nitroglycerin, lhe glycol is
prepared by synthesis from ethylene, and ethylene chlorohydrin:
ch2 - ch2
H0C1
-------> HOCH2CH2C1
H °
—-------> HOCH„CH OH
NaHCO3
Origin:
Henry vas the first to prepare and identify glycol dinitrate (Ber J, 529 (1870) and Ann
chim phys [4 ] 27, 243 (1872) but Kekuld had previously nitrated ethylene and obtained an un-
stable oil vhicn he supposed to he glycol nitrate-nltrate. No Immediate practical use vas
uade of glycol dinitrate because glycol Itself vas relatively rare and expensive at the time.
It wi 1904 before a patent vas granted covering the use of GIV as an explosive (DRP 179,789).
but it vas seven years later before its actual use as an explosive vas recorded ( M4m poudr 16
(1911) p. 214). П>е principal physical properties of GDK vere determined or recorded by Rinx-
enbach (Ref b).
References:
(а) И». Naoum, Nitroglycerin and Nitroglycerin Explosives, translation, E. M. Synsnes, The
Williams and Wilkins Company, Baltimore (19»), p. 224.
(b) WB>. H. Rinkenbach, "The Properties of Glycol Dinitrate," Ind Eng Chem 18, 1195 (1926).
(c) WB>. H. Rinkenbach, "Glycol Dinitrate in Eynamlt'.- Manufacture," Chem Met Eng, 34, 296
(1927).
(d) WB>. H. Rinkenbach, Application of the Vacuum Stability Test to Nitroglycerin end Nitro-
glycerin Explosives, PATR 162*, 27 August 1946.
32See footnote I, page 10.
145
АМСР 706-177
н-6
% RDX *»5 7ГТ 30 Aluninum 20 D-2 Wax 5 Calcium Chloride, added 0.5 C/H Rotio Molecolor Weight: 93
Oxygen Balance: CO: % -66 CO % -36
Density: gm/cc Cast 1.74
Molting Point: °C
Freezing Point: °C
It "eat Sensitivity, 2 Kg Wt: Buraou of Mines Apparatus, cm -- Sample Wt 20 mg Pleotinny Arsenal Apporatus, in. (c) 14 Sample Wt, mg 18 Boiling Point: *C
Refractive Index, ni ni nS
ГПСПМ V яп Steel Shoe Unaffected Fiber Shoe Vacuum Stability Test: cc/40 Hrs, at 90°C ---- 100‘C 0Л7 120°C 135°C I5O*C
Rifle Ballet Impest Test: Trials (b) % Explosions 80 Partials Burned Unaffected 20
200 Gram Bomb Seed Test: Sand, gm 49.5
IspMee Temperature: *C (a) Seconds, 0.1 (no cep used) — 1 5 610(nin) (c) 10 15 20 Sensitivity to Initietiea: Minimum Detonating Charge, gm Mercury Fulminate ---- Lead Azide 0.20 Tetryl 0.10
Ballistic Mortar, % TNT: (d) 135
Treuxi Toot, % TNT:
7S‘C Intomatiooel Meet Toot: % Lou In 48 Hrs
Plate Doot Test: Method Condition Confined Density, gm/cc Brisance, % TNT
100*С Hoot Test: % Lou, 1st 48 Hrs 0.78 % Lou, 2nd 48 Hrs 0.00 Explosion in ',00 Hrs None
DetenMioa Rate: (в| ь) Confinement None Condition Cast Charge Diameter, in. 1*0 Density, gm/cc 1-71 Rate, meters/socand 7191
FlommcWHty Index:
Hygroscopicity: % зо°с, 95$ ЙН, 7 days 2.01 71°C, 95$ RH, 7 days 1,77
VeMBty:
146
Н-6
АМСР 706-177
Booster («мШтНу Test:
Condition Oxygen, otoms/soc
Tetryl, gm (Z/sec) Hoot, kilocolorie/molo
'Vox, in, (л 50% Detonation (AH, kcol/mol)
Wax, gm Temperature Rango, *C
Density, gm/cc Phase
Meet of: Combustion, col/gm 3972 Armor Mote Impost Test:
Explosion, col/gm 923 M mm Mortar Projectile:
Gas Volume, cc/gm 733 50% inert. Velocity, ft/sec
Formation, cal/gm Fusion, cal/gm T8°C (b) 10.25 Aluminum Fineness 9004b General Potpoee Bombe:
Specific Heat: c.„ „m/'C (b)
30°C 0.269 Plate Thickness, inches
50°C 0.268 1 1’4
IMr
1%
Burning Rote:
cm/sec Bomb Drop Teet:
Tbermol Coodoctivity: cal/sec/cm/’C 35°C (b) _3 1.10 X 10 T7, 20004b Somi-Armor-Plercing Bomb vs Concrete:
Coefficient ef Exponsioa: Max Safe Drop, ft
Linear, dZ/ inch 0°C 14) x 10"!* 9004b General Purpose Oemb vs Concrete:
35°C 83 x ЮТ
70°C 13л X lo Height, ft Trials
Hardnoei. Mobe' Seek: Unaffected
Low Order
Young'» Modulus: High Order
E', dynes/cm’ 9.0 x 10
E, lb/inchа L.30 x lu5 1000-0 General Purpose Bomb vs Concrete:
Density, gm/cc 1.71 Height, ft
Compressive Strength: lb/i.tcha See be;cv Trials
Unaffected
Vapor Pressure: Low Order
’C mm Mercury 2 Compressive Strength: It/inch IO83 High Order
Density, gm/cc 1.71
Ultimate aefDrmation, % 1-32
147
AMCP 706*177
Frogasoatetloa Tost: (ъ) П — Ш, М71 PrejocMe, Let EGS-1-17: Density, gm/cc Chorge Wt, lb hl^ VVW ^^Ve rw^NOIKli For Composition В 998 For Subject HE 711* For 30/20 Tritonal 616 3 tach HI, МЛ2А1 Projectile, Lit KC-S: Density, gm/cc ChorgeWt, lb Total Ke. ef Fregaseals: For TNT For Subject HE Shaped Charge Effectiveness, TNT = 100: Glass Cones Steel rones Hole Volume Hole Depth
Color: Gray
Principal Usee: HE charge
Method of Leodtag: Oast
Lending Density: gm/cc 1.71
Fragnteat Velocity: ft/sec At 9 ft At 25% ft Density, gm/cc
Storage: Method Dry Hazard Class (Quantity-Distance) Class 9 Compatibility Group Group I Exudation None
Mort (Rointivo to TNT): (a) Air: 3-25'1 diameter sphere Peek Pressure A psi Catenary 25.к Impulse NFOC Pendulum 19-8 Energy — - Air, Ceeftaed: Impulse Under Water: Peak Pressure Impulse Energy Peak Pressure Impulse Energy
1
148
АМСР 706-177
Effect of Altitude, Charge Diameter and Degree of Confinement on Det .nation Velocity*
(Deference e)
i SxDloelve Siaulated Altitude, One-Inch Column TWO-Jnch Column !
Confined Unconfined n/a Confined I Unconfined
'Teet m/a n/e m/e
: TUT, Ground 6820 6720 6670 5270
' density, ga/cc 1-59 * 30,000 6660 6930(2) 6610 6760(1*)
• 60,000 6800 - 6520 61*00(1»)
90,000 6810 6720 6550 6610(1)
Average 6798 6790 6588 6260
B-6, Ground 7190 7360 73**0 6870
density, ga/cc I.69 30,000 7300(2) 71*30 7360 7980
60,000 7280 71*90 7550 7010
90,000 7300(3) 7270 7500 7000
Average 7268 7385 7*»30 7215
♦Confined charge in 1/1»" steel tube, AlSI 1015 seamlees, 1" diameter 18" long, and 2" diameter 7" long. All means were determined from sets of five values unless otherwise indicated by ( ). A 26 gn tetryl booster was used to initiate each charge.
Average Fragment Velocities at Various Altitude»* (e)
Explosive Charge Diameter, Inches Siaulated Altitude, Feet
Ground m/e 30,000 n/e 60,000 m/e 90,000 m/e
BIT, 1 291*0 1 2991 3119 2868 1
density, gm/cc 1.51 2 3623 — j 1*191 1 4 5077 1*980 -
: H-6, j 1 31:61 3UO5 3^67 S563
2 1*6O3 1»726 1*998 5288
♦Outside diameter 2.5V; inside •‘tameter 2.01*"; length 7".
References;
See HBX-1; HBX-3 reference list.
149
АМСР 706-177
Halelte (Ethylene Dinitramine) (ЕЖА)
(In recognition of its development as a military explosive by the
late Dr. G. C. .ale of Plcatlnny Arsenal.)
* ^no2 C 16.0 H2C N Molecoler Weight: (CgHgl.'^O^) 150
Oxygen Balance: CO.. % -32 CO % -10-5
H L.O N 37-3 0 U2.7 H„C H
Density: gm/cc Crystal 1.71
Melting Feint: "C Decomposes 1754
C ^*4 Ц C/H Rotio0.066 Froeslng Feint: C
Impact Seasif '♦v. 2 Kg Wt: Boiling Feint: ’C
Sample Wt 20 mg Refractive ladsx, n°
Pkotinny Arsenol Apparatus, in. 1« _D
Sample Wt, mg 17 & oft E G
Friction Foodalnm Test: Vacuum Stability Test:
Steel Shoe Unaffected cc/40 Hrs, at
Fiber Shoe Unaffected 90’C
100 C 0. p
Rifle Baikt Impact Test: Trials I2O"C 1-5
% 135=C
Explosions 0
Partials So 150-C 11+
Burned 20 200 Grom Bomb Send Test:
Unaffected ю Sand, gm 52.3
Expiation Tomperetare: 6/» Sensitivity to Initietioe:
Seconds, 0.1 (no cop used) 265 Minimum Detonating Charge, gm
1 216 Mercury Fulminate 0.21
5 Decomposes 189 Lead Azide 0.13
10 178 Tetryl
|C 173
20 170 Ballistic Mortar, % THT: (a) 139
— — — Troasl Test, % TNT: ( t) 122
7S°C Intemationol Hoot Test:
% Loss in 48 Hrs 0.01 Plata Dent Test: (c)
Method A
WC Hoot Test: Condition Pressed
% Loss, 1st 48 Hrs 0.2 Confined Yes
% Loss, 2nd 48 Hrs 0.3 Density, gm/cc 1.50
Explosion in 100 Hrs Hone Brisance, % TNT 122
1 Detonation Rote:
Flea nobility Index: 138 Confinement L'nconfir.ed
— 1 —III—I. - Condition Pressed
Hygroscopicity: % 0.01 Charge Diameter, in 1.0
Density, gm/cc 1. +1
VolotiBty: Mil Rote, meters/second 7570
151)
Haleite (Ethylene Dlnlt.-^-ie) (EDNA)
AMCP 706-177
Booster SeMkh'ty Teet: (d) Condition Pressed Tetryl, gm 100 Wax, in. for 50% Detonation 2.03 Wax, gm Density, gm/« 1.42 Decompositten Kquatioa: (e) „ (e) (f) Oxygen, otoms/soc 1СГ '° K> ’1 IO11*1 (Z/sec) Heat, kiiocalorie/mole 30*5 37-3 30«8 (AH, kcal/mcl) Temperature Range, CC 184-251* -- 144-164 ' Phase Liquid Solid Solid
Hset of: Combustion, cal/gm 21*77 Explosion, col/gm 1276 Gas Volume, cc/gm 906 Formation, cal •'em 134 Fusion, cal/grr Armor Mote Impact Test: M mm Metter Projectile: 50% Inert, Velocity, ft/sec Aluminum Fin*ness S004b General Purpose Bombs: Plate Thickness, inches 1 14 ; IV,.
Specific Hoot: col/gm/‘C
Bandog Kate: cm/sec
Bomb Drop Tost: T7, 20064b Semi-Armor-Piercing Bomb vs Concrete: Max Safe Drop, ft S00 lb General Purpose Bomb vs Concrete: Height, ft Trials Unaffected Low Order High Order 1000-lb General Purpose Bomb VS Concrete: Height, ft Trials Unaffected Low Order High Order
Tbotmol Conductivity: col/sec/cm/'C
Coefficient of Expansion: Linear, %/*C Volume, %/”C
Herd»», Mobs' Scale:
Young's Modulus: E', dynes/cm“ E. Ib/inch1 Density, gm/cc
Compressive Strength: Ib/inch-1
VtpM PrttMre; ’C mm Mercury
151
AMCP 706-177
Haleite (Ethylene Dinitramine) (ЕШЧ)
1 riv^NmWwII !• •0 MM HI, Mil Pra|ecMe, Let WC-91: Density, gm/cc 1.61 ChorgeWt, lb Total No. of Fragments: For TNT For Subject HE 3 W HI. М4ЭА1 FMMHH. Density, gm/cc "l.Jb Chorge Wt, lb Total No. of Fragments: For TNT 51*» For Subject HE 600 Shaped Choree KHocthrenoee, TNT = 100: Glass Cones Steel Cones Hole Volume Hole Depth
Color-. White
Principal Usee: Booster
Method of Loodtag: Preened
Loedtag Doneby: gm/cc P«i x 10^ 5 10 12 15 20 1.28 1.38 l.bl 1.Ы» 1Л9
Fragment Velocity: ft/soc At 9 ft At25ftft Density, gm/cc
Storage: Method Dry Hazard Class (Qjontity-Distance) ClASS 9 Compatibility Grotgr Exudation None
NMOUMoHvetaTHT): Abt Peak Pressure Impuhe Energy Ab, Confined: Impulse Under Water: Rook "ressure Impulse Energy (fadofyMMds Peak Pressure Impulse Energy
152
Halelte (Ethylene Dinitramine) (ЕЖА)
АМСР 706-177
Compatibility with Me tali:
Pty - Copper, brass, aluminum, mild ateel, stainless steel, mild steel coated with acid-
proof'black paint, and mild steel plated with copper nickel, cadmium or zinc are unaffected.
Magnesium and magneslum-aluminum alloy are «lightly affected.
Wet - Copper, braes, mild steel coated with acid-proof black paint, and mild ateel plated
vlth capper, cadmium, nickel or zinc are heavily corroded. Aluminum Is slightly affected and
stainless steel Is unaffected.
Impact Sensitivities of Various Crystal Habits;
Bureau of Mines Isoect Test, 2 Kg Wt;
Habit cm
1st plate 2nd plate Bi-pynmld Bracydome Sphenoid 55 55 71 66 h6
Solubility; gm/100 gm (10 of;
Water Al coho?.
°C J °C J
20 0.25 20 1.00
h0 0.75 ho 2.h6
60 2.13 60 5.2?
80 6.38 78 10.h
100 >20
Preparation:
(Summary Technical Report of the KIRC, Div 8, Vol 1)
CH2O + НСЯ —» KO CH2C3
(98% yield)
HO CH2CH ♦ ЯН3 -* НН2СН2СЯ ♦ H20
(82% yield)
ин2сн2сн + 2H2 -» H2N ch2ch2bh2
(88% yield)
CH2—BH2
CH2--HH2
C02->
CO + H20
153
АМСР 706-177
Halelte (Ethylene Dinitramine) (ЕША)
CH„— NH — NO_
| 2 * CO
CH2— NH—N02
The raw materials used in this process are cheap and available; the first three reactions
proceed smoothly, rapidly and In good yield (70$ overall), and only the third requires high
pressures. The reaction of ethylenediamine vith carbon dioxide at about 220°C and 820 atmos-
pheres has been worked out and is more satisfactory for the preparation of ethyleneurea than
the use of chlorethyl carbonate or urea and better than the reaction of acetic anhydride and
ethylenediamine to yield N,N'-dlacetyl-ethylenediamine which can be treated in a way similar
to the above to yield Halelte.
Ethyleneurea is very easily nitrated, with strong nitric acid (98$), nt ordinary temperature,
and in a very short time, and the dlnitroetbyleneurea produced appears ... ..jdrolyze, yielding
Halelte, Immediately after solution In water at 95°C. Doth the nitration and hydrcly-ts are
practically quantitative.
Origin:
First described in 1877 by Franchimont and KLotbie (Rec trav chim 7, 1" and 244) but it
was 1935 before its value as an explosive was recognized. Standardized during World War II
as a military explosive.
Destruction by Chemical Decomioeition:
Halelte is decomposed by addition to hot, dilute sulfuric acid. Nitrous oxide, acetalde-
hyde and rthyxene glycol are evolved. Halelte is also decomposed by addition to 5 times its
weight of 20^ sodium hydroxide.
References:33
(a) L. C. Smith and E. G. Eyster, Physical Testing of Explosives, Part III - Miscellaneous
Sensitivity Tests; Performance Tests, OSRD Report No. 574b, 27 December 1945.
(t) Report АС-2983/Org Ex 179-
(c) D. P. MacDougall, Methods of Physical Testing, OSRD Report No. 803, 11 August 1942.
(d) L. C. Smith and S. R. Walton, A Consideration of RDX/Wax Mixtures as a Substitute for
Tetryl in Boosters, NOL Memo 10,303, 15 June 1949.
(e) R. J. Finkelstein and G. Ganiev, Theory of the Detonation Process, NAVORD Report !io.
90-46, 20 April 1947.
(f) M. A. Cook and M. Taylor Abbag, "Isothermal Decomposition of Explosives." University
of Utah, Ind Eng Chem (June 1956) pp. 1090-1095-
33,.ee footnot- -, page 10.
154
Halelte (Ethylene Dinitramine) (ЕГНА)
AMCP 706*177
(ь) Also see the following Picatlnny Arsenal Technical Repores on Halelte:
0 1 2 2 1 2 6 7 8 2
1200 1231 1162 1113 111 1255 786 897 1198 1279
1290 1151 1232 1193 1291 1325 1796 1737 1288 1319
1360 1651 1252 1923 1131 1395 1797 1378 1379
1380 1352 1885 1937 1388 1169
1100 1372 1838 1189
1600 2179
155
AMCP 706-177
НВХ-1
% RDX *Ю TUT 38 Aluminum 17 D-2 Wax 5 Calcium Chloride, added O.J C/H Rotio Mehcoisr Weight: 102
Oxygen Baleace; CO, % .68 CO % -35
Density: gm/cc де j.72
MeMag Print: *C
Pressing Point: *C
* • * if— BBOpWo p • Bureau of Mines ilpporotus, cm Sample Wt 20 mg Picatinny Arsenal Apparatus, In. 16 Sample Wt, mg 21 Bolling Point: *C
Refractive Index, nJ Пи n°
Friction Pendnioni Teet: (b) Steel Shoe Unaffected Fiber Shoe — Vocosm StobiRty Test: (a, b) cc/40 Hrs, at 90"C —— 100’C 0Л7 120-C 0.98 I35’C —— !50*C 11+
Bitie Ballet Impact Test: Trials (b) % Explosions 73 Partials Burned Unaffected 28
200 Grom Bomb Send Test: Sand, gm 48.1
Expiesiea Tempesotote: *C (a) Seconds, 0.1 (no cap used) — 1 5 WO 10 15 20 <►-- _e-.e e. s r.l _ MMHSBWTwf WW BIBWBVnVOOe Minimum Detonating Charge, gm Mercury Fulminate .... Lead Azide 0.20 Tetryl 0.10
Ballistic Mester, % TNT: (d) 133
Trees! Test, % TNT:
7S*C Intesnath. at Teat: % Loss In 48 t _ •
Plate Dent Test: Method Condition Confined Density, gm/cc Brisonce, % TNT
100’C Meat Test: (b) % Loss, ht 48 Hrs 0.058 % Loes. 2nd 48 Hrs 0.00 Explosion in 100 Hrs none
Detonation Rate: (s, b) Confinement None Condition Cast Chorga Diameter, in. 1.0 Density, gm/cc I.69 Rote, meters/sscond 7221*
Flammability Index:
Hygroscopicity: % 30°C, 9% RH, 7 days 2.98 7-°C, 95t RH, 7 days 1.13
v V^^VBSWy •
156
НВХ-1
АМСР 706-177
двивт1тИу В Mve (c) 0*сфм^м1Н*11 ЦмНм:
Condition Cast Oxygen, atoms/sec
Tetryl, gm 100 1-25 (Z/soc) Hoot, kilocalorie/mole
Wax, in. for 50% Detonotion (AH, kca mol)
Wqx, 9m Temperature Range, °C
Density, gm/cc 1-73 Phase
Hosp of: Combustion, cal/gm (b) 3862 Armor Mete Impact Tost:
Explosion, cal/gm 919 M mm Mortar Projectile:
Gas Valumo, cc/gm 50% Inert, Velocity, ft/sec
Formation, cal/gm 758 Aluminum Fineness
Fusion, cal/gm Т8°С 9-25 *M-lb General Purpose Bombs:
Specific Hoof: col/gm/°C (b)
30°r 0.249 Plate Thickness, inches
50°c 0.264 1 Hi
|i/,.
l*i
Btwoiag Rato:
cm/soc Bomb Drop Test:
Thermol Conductivity: (b) , T7, 20004b Semi Armor-Piercing Bomb vs Concrete:
cal/soc/cm/*C 35°C 0.97 x 1O'J
Coefficient of Expaasisc: (b) Max Safe Drop, ft
Linear, ДХЛпсЬ 0°C 35°C 46 x lO'j* 95 x 10" 5004b General Purpose Bomb vs Concrete:
70°C 159 x 10*4 Height, ft
T rials
Hardness, Mohs' Scale: Unaffected
Young's Modulus: Low Order
(b)
E', dynos/cm’ 9 High Order
10.3 X 10
E, lb/inch’ 1.49 x 10", 1000-lb General Purpose Bomb vs Concrete:
Density, gm/cc 1.69 Height, ft
Compressive Strength: Ib/inch1 See below Triols
Unaffected
Vapor Pressure: *C mm Mercury Compressive Strength: lt/lnch2 Density, gm/cc (b) 1303 1.69 Low Order High Order
Ultimate deformation, % 1.3s
157
АМСР 706-177
НВХ-1
FragssoataHon Тай: (ь) 90 mm Ml. М71 Projectile, Let EGS-1-17: Density, o>'r>/cc Charge Wt, lb Total Me. «f Frogmeats: For Composition В 996 For Subject HE 910 For 80/20 Tritonal 616 3 tach HI, M42A1 FielecMe, let KC-J: Density, gm/cc Charge Wt, lb Total No. of Fregmeats: For TNT For Subject HE Sbopod Charge IffecHvenees, TNT = 100: Gloss Cones Steel Cones Hole Volume Hole Depth
Color: Cray
Principal Uses: HE charge
Method el Loading: Cast
Loading Deeoity: gm/cc 1.69
Fragment Velocity: ft/sec At 9 ft At 25% ft Density, gm/cc
Method Dry Hazard Class (Quantity-Distance) Class 9 Compatibility Group Group I Exudation None
Meet (Relative to TNT): (a) Ain 3*25" dlaaeter sphere Pock Pressure A pel Catenary 21».7 Impulse KFOC 19.6 Energy Air, Coeftaed: Impulse Under Water: Peek Pressure Impulse Energy Underground: Peek Pressure Impulse Energy 1
158
нвх-з
АМСР 706-177
СмпрмЖм: % Molecular Weight: 61»
RDX 31 TNT 29 Oxygon Beience: CO, % .75 CO % .1*9
D-2 Wax 5 Density: gm/cc Cent 1.8U
Calcium Chloride, added 0. 5 Moiling Point: C
C/H Ratio Freexlng Feint: °C
Impact Sensitivity, 2 Kg Wt: Bureau of Mines Apparatus, cm Sample Wt 20 mg Picatinny Arsenal Apparatus, in. 15 Sample Wr, mg 23 Boiling Point: °C
Refrecthrs Index, nJ Пд П&
Friction Pendulum Test: Steel Shoe Unaffected Fiber S’ ’e --- • Vacuum Stability Toot: (a, b) cc/40 Hrs, ot 90°C .... 100°С 0.U5 120°C 135°C I5O*C
Rifle Ballet Imprxt Test: Trials (b) % Explosions 78 Partials
Burned Unaffected 22 200 Gram Bomb Send Tost: (b) Sand, gm LU.9
tiplesion Temperature: °C (a) Seconds, 0.1 (no cop used) —- 1 5 500 10 15 20 la » Sa.t_«r •МЮТПТЯу fw BVwVWnJ Minimum Detonating Charge, gm Mercury Fulminate Lead Azide 0.20 Tetryl 0.10
BoKisflc Mortar, % TNT: (d) 111
Trenal Test, % TNT:
7S°C Intometianel Heat Test: % Loss in 48 Hrs
Piste Dent Test: Method
10VC Heat Tost: (b) % Loss. 1st 48 Hrs 0-70 % Loss, 2nd 48 Hrs 0.00 Explosion in 100 Hrs None Condition Confined Density, gm/cc Brisonce, % TNT
Oeteaetioa Rote: (a, b) Confinement None Condition Cast Chorge Diameter, in. 1.0
Flammability Index:
Hyg.nscopicity: % 30°C, 95* RH. 7 days 2.01 (b) 71°C, 95^ RH. 7 days 0.31
Volatility: Density, gm/cc 1.81 Rote, meters/second 6917 |
159
АМСР 706-177
нвх-з
Beeeter Sensitivity Test: Condition Tetryl, gm Wox, in. for 50% Detonotion Wox, gm Density, gm/cc Oxygen, ctoms/soc (Z/sec) Hoot, kilocolorie/mole (AH, kcol/moi) Temperature Range, °C Phase
Heat of: Combustion, cal/gm Explosion, cal/gm Gas Velum*, cc/gm Formation, cal/gm Fusion, col/gm (b) 4495 877 491 9-30 Armor Plots Impact Tost: M mm Mortar Profoctile: 50% Inert, Velocity, ft/sec Aluminum Fineness SOO-lb General Purpose Bembs:
Specific Hunt: ccl/gm/'C 30°C 0.254 Plots Thickness, inches
50rc 0.254 1 14 I'A
Berning Rote:
cm/scc Bomb Drop Tost:
o %^peovc*en^^r• col/soc/cm/'C 35°C 0>) -3 1.70 x 10 3 T7, 2000-b Somi-Armor-Piercing Bomb vs Cowcrafw:
Coefficient of Expo mien: Linear, A i /1 nch 0°C 35°C 70°C (bj 40 x lo'^ 8’ x 10*4 130 x 10" Max Safe Drop, ft SOO-й General Purpose Bomb vs Concrats: Height, ft Trials Unaffected
Hardness, Mobs' Scale:
Young's Modulus: E', dynes/cm’ E, Ib/inch’ Density, gm/cc (b) 11.5 x 109 1.67 X 105 1.81 Low Order High Order 1000-lb General Purpose Bomb VS Concrete: Height, ft Trials Unaffected
Compressive Strength: Ib/inch’ See below
Vapor Pressure: 'C mm Mercury L_-Uffiresslye. strength: Ib/inch^ 1/10 Low Order High Order
Dens.ty, .-n/cc Ultimate deformation, ,6 1.61 1.37
АМС?7Си-177
M MM Nt, МУ1 Projectile, La* BGS-1-17: Demity, gm/cc Chorga Wt, lb Tetel Na. of Frogman**: For Oospoeltlon В 993 Frr Subject HE Wo F"- 8o/2O Tritonal 616 1 tach Ht, M43AI Projoctita, La* KC-S: Damity, gm/cc Charge Wt, lb Total Na. of Frogaaeah: FcrTNT For Subject HE Shaped Charge Iffoctiveaea*. TNT = 100: Gio** Cone* Steel Corm Hole Volume Ной Depth
Color: Gre?
Principal Uae*: HE charge
Method of Leadtag: Caat
Loadtag Deealty: gm/cc 1-81
Fragment Vetadty: ft/мс At 9 ft At 254 ft Damity, gm/cc
Method Dry Hazard 2km (Quantity-Distance) Claae 9 Compatibility Group Group I Exudation Hone
Bleat (Rotative te TNT): (a) Ain 3-25" diameter ephere Rook Ргемиге Д pal Catenary 25.3 Impuhe KFOC Pendulum 20.6 Cnergy Air, Confined: Imp-jho Under Water: Peek Preuure Impulse Energy Peak Ртамиго Impuhe Energy
161
АМСР 7С6-177
НВХ-1; НВХ-3
ЧЪе Stability of НВХ Coapoaitions Made With and
Without beiiccanti and Containing Added Moisture *
Explosive Coaposit ion Moisture, 1 i Acidity, 100wC Vac Stab Test Hygroscopicity, % $5% ta
cc gae Hours
30°C 71°C
I • : Standard HBX-1 0.73 0.011 0.47 40 +2.98 +1.13
+0.2% moisture 0.68 4o
+0.4% moiature 0.62 40
+0.6% mole cure t I 0.50 40
HBX-1 without CaCL, 0.00 0.029 0.36 40 -0.06 -O.25
+0.2% moiature 0.25 40
+0.4% moiature 0.23 40
+0.6% moiature 0.27 40
HBX-1 with silica gel 0.06 0.031 0.73 40 +0.08 +0.0U
Standard HBX-3 0.54 0.012 0.45 40 +2.01 +0.31
+0.2% moiature 0.47 40
+0.4% moiature 0.43 40
+0.6% moiature 0.41 40
HBX-3 without СаСЪ, 0.02 0.049 0.46 40 -0.06 -0.29
+0.2% moisture 0.26 40
+0.4% moiature 0.26 40
+0.6% moiature 0.20 40
HBX-3 vith silica gel 0.04 0.100 0.45 40 +0.09 +0.05
Standard 3-6 0.71 0.017 0.47 40 +2.01 +1-77
+d.2% moiature 0.88 40
+0.4% moisture O.63 40
+0.6% moisture 0.65 40
H-6 without CaCl„ 0.03 0.082 о.ьо 40 -0.06 -0.25
+0.2% moisture 0.10 40
+0.4% moisture j.25 40
+0.6% moisture 0.23 40
H-6 with silica gel 0.05 0.028 0.43 40 +0.09 +0.06
* All samnlee were ground to 20/100 meah size, 7 daya before teata. Silica gel used wee
Fisher 'ientific Cwnpany, Lot 541492, through 100 meah U. S. Standard Sieve.
I<i2
AMCP 706-177
HBX-l; HBX-3
Preparation:
HBX explosive mixtures are prepared by melting TNT in a stearn-Jaeketed melt kettle equipped
with a mechanical stirrer. Water-wet ! JX is added slowly with stirring and heating until all
the water ie evaporated. Aluminum is added, and the composition is stirred until uniform.
D-2 wax and calcium chloride are then added. The desensitizer wax, also known as Composition
D-2, consists of ОДС paraffin and other waxes, 1U^ nitrocellulose and 2$ lecithin. The mixture
is cooled from approximately 95° to 100°C to a temperature considered suitable for casting
(the lowest practicable pour temperature). HBX can also be made by adding the calculated
amount of TNT to Composition В to outain the desired proportion of RDX/TNT. The appropriate
weights of the other ingredients are added to complete the mixture.
Origin:
Developed during World War II, as relatively insensitive mixtures, by adding 5^ desensiti-
zer to Toroex II, for high blast explosive applications.
References: 34
(а) о. E. Sheffield, Blast Properties of Explosives Containing Aluminum or Other ;>tal
Additives, PATR No. 2353, November 1956.
(b) S. D. Stein, G. J. Horvat end о. E. Sheffield, Some Properties and Characteristics
of HBX-l, HBX-3 and H-б, PATR No. 2U31, June 1957-
(c) L. C. Smitn and S. R. Walton, A Consideration of RDX/WAx Mixtures as a Substitute for
Tetryl in Boosters, NOL Memo. 10,303, 15 June 19*»9-
(d) S. R. Whlton, Reno^t on the Program to Develop an Improved HBX-Tvpe Explosive, HAVORD
Report No. 1502, 26 July 19$0.
(e) A. W. O'Brien, Jr., C. W. Plunmer, R. P. Woodburn and V. Philipchuk, Detonation
Velocity Determinations and Fragment Velocity Letermlnatlons of Varied Explosive ^sterna
and Conditions, national Northern Corporation Final Summary Report NNC-F-13, February 1956
(tontract lAl-19-O20-5Ol-ORD-(P)-59).
xi') Also see the following Picatinny Arsenal Technical Renerts on HBX Explosives: 1756,
2138, 2169.
footnote I, page 10.
1G3
АМСР 706-177
HEX-24
CeetpeBiMo** % Potassium Perchlorate 32 (17 microns) Aluminum, atomized 48 (20 microns) RDX (througn 325 meeh) 16 Asphaltum (through 100 mean) 4 C/H Ratio Molecular Weight: 47.6
Oxygee Beieoce: CO: % -42 CO % -34
к?9
Molting Feint: °C
Froesing Feint: °C
Impact Sensitivity, 2 Kg Wt: Bureau of Mines Apparatus, cm Sample Wt 20 mg Picatinny Arsenal Apparatus, in. 16 Sample Wt, mg 24 Bolling Feint: °C
Refractive Index. nJ Пи nS
*-* вот^ИМИИ 0• Steel Shoe Detonates Fiber Shoe UnaJi'cted Rifle Ballot Impoct Test: Trials % Explosions Portials Burned Unaffected Vacuum StabiBty Test: cc/40 Hrs, ot 90’C —- ioo°c 1.25 I2O°C I35°C 150°C
200 Grom Bomb Sand Test: Sand, gm 12.5
Explosion Temperature: °C Seconds, 0.1 v " a used) — 1 5 520 10 IS 20 SensFiaity to Inrtiaeioa: Minin... Detorat ng Charge, gm Mercury Fulminate Lead Azide 0-20 Tetryl 0.25
Ballistic Mortar, % THT:
Treexl Test, % THT:
7S*C International Meet Test: * Lou in 46 Hrs
Fiet*. Dent Tost: Method Condition Confined Density, gm/cc Brisance, % TNT
100 °C Hoot Test: % Lou, ‘.st 48 Hrs 0.13 % Lou, 2nd 46 Hrs 0.00 Explosion in 100 Hrs rone
Dttoaorion Rett; Confinement Condition Charge Diameter, in. Density, gm/cc Rote, meters/second
Flammability lodes:
Hygroscopicity: % :ione
VotatiU/;
164
HEX-21*
АМСР 706-177
Frogase^fetie^t ^Test: Им HE, M71 ЬфсНЕ*. Let WC-91: Density, gm/cc Charge Wt, la Total He. ef Fregmentt: For TNT For Subject HE 3 inch HE, M42A1 FrejecHto, let XC-S: density, gm/cc Charge Wt, lb Tetei Ne. of Frngmnnts: For TNT For Subject HE Shaped Charge Itfectiveaesa, TNT = 100: Glass Cones Steel Cones Hole Volume Hole Depth
Color: Gray
Principal Uses: HE filler for small caliber projectiles
Method ef Loodhg: Pressed
Leading Density: gm/cc Pressed at 20,000 psi 2.1
FragsMOt Velocity: ft/sec At 9 ft At 25% ft Density, gm/cc
(Далаал, Method Dry Hazard Class (Quantity-Distance) Compatibility Group Exudation None
Moot (Relative to TNT): Air: Peak Pressure Impulse Energy Air, Confined: Impulse Under Water: Peak Pressure Impulse Energy Uedsrgreand: Peak Pressure Impulse Enarav
S-etlc Tests: 20 дп T215E1 Projectile; pA Peak Pressure, psi 55 NFOC 20" Blast Cube U* APG 2U” Blest Cube U* Static Tests; 20 mm M97 Projectile; HEX-24 Tritonal Toгрех Foxboro psi 1$ 12.U 13.0 Catenary psi 1*6 --— —- Ikiration, mlcrosec 533 ---- .... APG 21*" Blast Cube 36 21* 32 Heat of: Combustion, cal/gm 1*197 Explosion, cal/gm 1856 Gas volume, cc/gm 159
Flame Temperature, °K 2552 Activation Energy, kcal 20.1» Temp, °C 1*50 to 570 Specific reaction rate, к 1.61» x 10'5
165
AMCP 706-177
HEX-UI
% Potaeslum Perchlorate 32 (17 ml crone) Aluminum, flaked (1 micron) U8 RDX (through 325 mesh) 16 Aephaltum (through 100 mesh) h C/H i\?tio Molecular Weight: 47. L
Oxygen Balance: CO. % -L2 CO % -з1*
Melting Feint: °C
Framing Point: °C
Impact Sensitivity, 2 Kg Wt: Buroov of Mines Apporotus, cm Sample Wt 20 mg Picotinny Arsenal Apparatus, in. Sample Wt, mg Boiling Point: °C
Refractive Index, n° П»
Friction Pendohim Test: Steel Shoe Partially detonates Fiber Shoe Unaffected Vacuum Stability Test: cc/40 Hrs, at 90°C —- 100’C 1.52 120C I35°C I5O°C
Rifle Bullet Impact Test: Trials % Explosions Partials Burned Unaffected
200 Gram Bomb Sand Teet: Sand, gm 23-7
Explosion Temperature: C Seconds, 0.1 (no cap used) 1 5 51*5 10 IS 20 Sensitivity to IniriaHoa: Minimum Detonating Charge, gm Mercury Fulminate ---- Lead Azide 0*20 Tetryl 0.25
Ballistic Mortar, % TNT:
Trauxl Teat, % TNT:
7S°C lotereetioMl Hect Test: % Loss in 48 Hrs
Plate Dent Test: Method Condition Confined Density, gm/cc Bnsonce, % TNT
100°C Heat Test: % Loss, 1st 48 Hrs % Loss, 2nd 48 Hrs Explosion in 100 H rs
Detonation Rate: Confinement Condition Charge Diameter, in Density, gm/cc Rote, meters/second
Flammability Index:
Hygroscopicity: %
VoietiliJy:
lliti
НЕл-W
AMCP 706-177
Fragmentation T*«t: 90 mm HE, M71 Projectile, Let WC-91: Density, gm/cc Charge Wt, lb Total No. of Fragment,: For TNT For Subject HE 3 inch HE, M42A1 Projectile, Lot KC-5: Density, gm/cc Chorge Wt, lb Total No. of Fragments: For TNT For Subj^.t HE Shaped Charge Fftactivenaos, TNT = 100: Glass Cones Steel Cones Hol* Volume Hole Depth
Color Grny
Principol Uses: HE filler for snail caliber projectiles
Method of Loodtag: Pressed
Loading Density: gm/cc Pressed at 20,000 1.62
Fragment Velocity: ft/sec At 9 ft At 25>4 ft Density, gm/cc
Storage: Method Dry Hazard Class (Quantity-Distance) Compatibility Group Exudation None
Blast (Rotative to TNT): Alt: Peak Pressure Impulse Energy Air, Confined: Impulse Under Water: Peak Pressure Impulse Energy Underground: Peak Pressure Impulse Energy ? lame lempera^ . T, 1 7 & • 1 0;, ; : er , й-Ю I 25»” .e:np. •• ,0 •,<> •• ‘0 ‘pec i ? •- геб'Г i >:, re •к -x. x P
Static Tests: 20 mm T215E1 Projectile: PA Peak Pressure, psi 77 ГРОС 2C" Blest Cube k5 APG 2k" Blast Cube 12 Stetlc Tests: 20 шт M97 Projectile: HEX-kr. Tin Tetryl Fos'coro psi 17-3 2.8 3- 5 Catenary psi кз 2з 2Й duration, ndcrosec 517 5^0 530 APT 2k" Blast Cute 29 10 uea-. ji: ;om. *sMot.* cal **1г-) Lxpl js : . cal t-y. 1'35 ,ae ’,'gL .me, '-c 200
167
АМСР 706-177
НЕХ-48
Cook-Off Tests; (с)
20 мд Т215Е1 HEX-48 Loaded Project Пев with Ifre-Ccsted RDX Top-Off
Projectile No. Cut-Off Temp. °C Cook-Off
1 2 3 4 170 150 155 150 to 175 Yes (198) No Yes (190) No
national northern Projectile Load:
j M0X-2B (no top-off) ( I M0X-2B (Tetryl top-off) | j M0X-2B (97/3» RDX/wax top-off) j MOX-2 (no top-off) 195 | 150 ' 175 ; 175 '
Fragment Penetration Tests; (c)
1 1 Avg. No. of Penetrations per
j 1 Round in Zone 65°-130°
j Projectile Filler Altitude, Feet Г" j 0.020" 0.040" 0.051"
j T215E1 1 1 HEX-48 Ground 60,000 35^ 676 264 282 ; 380
T282EL MDX-2B Ground 60,000 634 807 290 367 235 250
EX8 Mod 0 M0X-2B Ground 60,000 476 672 268 264 224 256
lhe fragment penetration teat records numbers of complete penetrations of aluminum panels
of various thicknesses at 2.5 feet from the static detonation. The total penetrations recorded
on the 24ST-3 aluminum panels occurred vith the projectile nose always pointed toward C° and
the base toward 180°.
The test data indicate that on the thicker panel*, 0.o4o" and 0.051," the HEX-48 loaded
T215E1 projectile produced mora comple e fragment penetrrtions at ground and altitude than
M0X-2B loaded T282E1 and EX8 Mod 0 projectiles.
168
АМСР 706-177
HEX-21*; HEX-48
Preparation;
П>е HEX compositions were prepared by blending the appropriate weight of the dry ingredients
in a Patterson-Kelly twin-shell blender for at least 30 minutes.
..n alternate procedure for 100 to 200 gram batches used a "Cradle-Roll" mixing device.
Bds device consisted of a half-barrel type container constructed of wood and lined with an
electrical conductive material. A plastic roll was allowed to move over the Ingredients by
remote control action of the container. The roll action prevented caking of the mix but had
no adverse effect on the particle size of the ingradients, lhe period of time required to
obtained a uniform and intimate mixture was approximately fifteen minute».
Origin:
Hie development of "slow-burning" explosive mixtures which would produce Increased blast
effects in enclosed or nearly enclosed spaces directed attention to their use for possible
military application. In 1950 Plcatlnny Arsenal developed a high capacity filler for 20mm
projectiles consisting of 85/10/5 RDX/alumlnum/deeeneltizer which was more powerful than stan-
dard tetryl filler. However, In comparison vith MOX type explosives, there was little doubt
as to th superior performance of the MOX sdxture. HEX (higu ?”ergy e^losive) mixtures were
developed at Plcatlnny Arsenal In 1953 (Ref a) as superior hlgK bx»t compositions suitable
for use In small caliber projectlies.
References; 33
(a) 0. E. Sheffield and E. J. Murray, Development of Explosives"' Metallized Explosives—
High Blast Fillers for Shell Caliber Shell, Plcatlnny Arsen 1 Memorandum Report No. MR-49,
21 December 1ф$3.
(b) 0. E. Sheffield, Properties of MOX-Туре Explosive Mixtures, PAIR No. 2205, October
1-955-
(c) National Northern Corporation, Le> er from Dr. С. M. Saffer, Jr., to Commanding
Officer, Plcatlnny Arsenal, 12 June 195’i
^See footnote 1, pege 10.
169
АМСР 706-177
2,4,i.,2' ,L' .о' -Неха::1 tro-oxat:i II de (: v! )
С от position: C 33-0 J c 1 i H 1.2 NH NH N 21.9 C’’’TZ| ' 0 1*3-9 X/' X.X C/H Ratio 0.797 NO, No, Molecular Weight:
Oxygen lelence: CO; % -53-4 CO % - v>.4
Density: gm/cc
Melting Point: C Decomposes 302
Freexing Point: C
Impact Sensitivity, 2 Kg Wt: Bureau of Mines Apparatus, cm Sample Wt 20 mg Picotinny Arsenal Apparatus, in 15 Sample Wt, mg 12 Boiling Point: ’C
Refractive Index, nJS nM
Friction Pendulum Test: Steel Shoe Unaffected Fiber Shoe U..affected Vacuum Stability Tost: cc/40 Hrs, at 90 °C loO'C o.Lo 120 C 135-C 150 C
Rifle Ballet Impact Test: Trials % Explosions Partials Burned Unaffected
200 Grom Bomb Send Toil: Sand, gm 52.1
Explosion Temperature: °C Seconds, 0 1 (no cop used) 5 334 10 15 20 Sensitivity to Initiation: Minimum Detonating Charge, gm Mercury Fulminate Lead Azide 0- 30 Tetryl 0.25
Bollittic Mortar, % TNT:
Trouxl Test, % TMT:
75‘C Interactional Heat Tost: % Loss in 48 Hrs
Plate Dent Test: Method Condition Confined Density, gm/cc Bnsance, % TNT
10CC Hoot Test: % Loss, 1st 48 Hrs 0.07 % Loss, 2nd 48 Hrs 0.05 Explosion in 100 Hrs None
Detonation Rote: Confinement Condition Charge Diametet, in Density, gm/cc Rate, meiers/second
Flammability Index:
Hygroscopicity: % ^°C, XT1, id: 0. . i
Volatility:
170
2,U,b,2'A',o’-Hexanltro-oxenilide (HNo)
AMCP 706-177
Fragmentation Tet 90 mm HI, M71 Projectile, Lot WC-9I: Density, gm/cc Chorge Wt, lb Total No. of Fragment!: For TNT For Subject HE 3 inch HE, M4J> 1 Projectile, Lot KC-5: Density, gr, 'zz Cho< -e Wt, b Total No. of Fmamento: For TNT For Subject HE Shaped Cherge Effectiveness, TNT = 100: Glass Cones Steel Cones Hole Volume Hole Depth
Color: Almost white
Principal Urn: Igniter powder; pyrotechnic compositions
Method of Loading: Pressed snd extruded
Loading Density: gm/cc
Fragment Velocity: ft/sec At 9 ft At 25>4 ft Density, gm/cc
Storage: Method Dry Hazard Class (Quantity-Distance) Class j Compatibility Group Exudation None
Blatt (Relative to TNT): Air: Peak Pressure Impulse Energy Air, Confined: Impulse Under Water: Peak Pressure Impulse Energy Underground: Peak Pressure Impulse Energy
171
AMCP 706-177
2,4,б,2' ,4’ ,G' -Hexanitro-oxsoiilide (HNO)
Solubility In the following substances:
Solvent
Nitrobenzene Whiter Alcohol (Ethyl) Acetone Benzene Butyl acetate Carbon tetrachloride Dimethylformamide Ether (Ethyl) Acetic Acid Nitric Acid Crystalline form <3 gm in 100 cc, at 23°C - 5 gm in 100 cc, at 210°C 0.10 gm in 100 cc, at 100°C Insoluble Insoluble Insoluble Insoluble Insoluble Very soluble Insoluble Insoluble Soluble Long rectangular glistening plates from nitrobenzene
Preparation:
To prepare hexanltro-oxanilide, first prepare tetranitro-oxanllide as described herein
under the entry ”2,4,2' ,4'-Tetranitro-oxanllide (ПЮ)."
A 1.5 liter round bottom flask is equipped with a stirrer of the type which causes a down-
ward swirl, ihe flask Is Jacketed for hot and cold water. 187 grams of nitric acid of speci-
fic gravity 1.49 (comaercial grade) is placed into the flask and 100 grams of sulphuric acid
is added to the nitric acid under agitation. T:.e mixed acid Is cooled to 10°C. 29.2 grama of
tetranitro-oxanllide Is slowly added to the mixed acid under rapid agitation maintaining the
temperature at 8°-10°C. After the addition of the MG is completed (approximately 25 minutes)
the temperature is raised to 85°C over a period of 2 hours and held at 85°-9O°C for one hour.
Ihe hexanltro-oxanilide (HNO) "slurry" is filtered on a Juchrer funnel and purified as ex-
plained under "Tetranitro-oxanllide."
Origin;
A. 0. Perkin In 1892 obtained hexanltro-oxanilide directly by heating to boiling a solution
of tetranitro-oxanllide in a mixture of sulfuric and nitric acids. He also prepared the same
compound from oxanillde by the action of a boiling mixture ~>t fuming nitric and sulfuric acids
(J Chem Soc. 61, 462 (1892)).
References: 36
(a) L. Gowen and R. Dwlggens, Case Cun Ignition Studies, NA.VOFLD Report No. 2321, 13 June
1952.
(b) D. Dubrow and J. Kristai, Substitution of Tetranitro Oxanillde and Hexanitro Oxanillde
for Tetranitro Carbazole, PA Pyrotechnic Research laboratory ftepoi-t 54-TF1-S3, 20 December 19$U.
(c) S. Livingston, Preparation o; Tetianitro Carbazole PA Cr.eulcsl Reb-utcu Laboratcr
Report 136,330, 11 April 1651.
(d) S. Livingston, Development of Improved Ignition Type Powders, PATR No. 226/, July 19 6.
36s ее footnote I, page 10.
172
beta-НИХ (e)
АМСР 706-177
Самрмтея: tt. JL 2 Mohetfr' Weight: (^HgNgOg) 296
с 16.2 o.x-n ^‘и-мо, 2 1 f 3 И 2.7 H2(J СН„ Oxygon Btleeca: CO. % .21.6 CO % 0.0
ь 5Г.9 о2к-»^^-»-"ог Density: gm/cc Crystal 1.90
О из-2 ®2 MetHag 9ЛЛ^С 2»₽1и^ “thod £□ Keffer Micro Hot Sta« 2ВО
C/H Robo 0.095 Freeaieg Feint: *C
hapaet SeaMefcy. Я Kg Wt: Buraou of Mines Apparatus, ст 32 Sompta Wt 20 mg Pitotinny Arsenal Apporotus, in. 9 Sample Wt, mg 23 BoMag Pelat: *C
RefcecHve Index, nb Пп гё
McHm FmMm Tut Steel Shoa Explodea Fiber Shoe Unaffected Vecaam StabOky Teat: cc/40 n>; ot WC 100’C 0.3г I2O’C 0Л5 I35*C I5O"C 0.62
*Ma Mht hnpoct Teat: Trials % Explosions Partials
Burned Unaffected 200 баеаа BooA Sand Teat: Sand, gm 60Л
kpirrien ТсаарогеПм: *C Seconds, 0.1 (no cop used) 38O 1 5 327 10 306 IS W ЮТаДВВ^^МВе Minimum Detonating Charge, gm Mercury Fulminate Load Azide 0.30 Tetryl
20 BoMeNe Mortar, % THT: 150
Trensl Tert, % TNT: 14$
% Loan in 48 Hrs
Hole Doot Tost: Method
100*C Moot Teat: % Loes, 1st 48 hrs 0.05 % Loss, 2nd 48 Hrs 0.03 Explosion in 100 Hrs Kone Condition Confined Density, gm/cc Brito..;., % TNT
Detonation Rota. Confinm^w
ГмаомЫСОу ledea:
NygpMcepieftyt % 30°C, 9# RH (c) 0.00 Charge Diameter, in. Density, gm/cc l.SV Rate, meters/second 912U
173
АМСР 706-177
beta-НЮС
ВеееЬЙИОМИЬЬгО^ Teets vonumv. Tetryl, gm Wax. tot. for 504 DManotlon Wax, gm Density, gm/cc Peeempeiltiee IgoeNeat (el. Oxygen, otome/iec. IO1’1' (Z/eec) Heat, kilocolorie/mol* 52.7 (AH, keol/mal) Temperature Range, *C 271-31*» Pho** Liquid
Meet eft Combustion, col/gm 2Jb.' Expiation, cal/gm (e) 1356 Go* Volume, cc/gm Formation, col/gm (e) -60.5 Futian, col/gm Anna* Plata карам Test: 60 moi Metter Рга|ес1й*: 50% Inert, Velocity, ft/tec Aluminum Finan*** MOUbGeaetol Гогрове Boeebet Plate Thlckne**, inches 1 14
Specific Heet: cal/gm/‘C Recryctellized (g) 2£ -T5 0.153 85 0.288 0 0.228 90 0.290 25 0.2b3 100 0.295 50 0.266 125 0-307 75 0.282 150 0.315
Botaieg Koto: crn/tec
BeaA Orap Teet: T7, 2000-& Semi Areier Hei*feg Betnb n Ceetietei Max Sat* Drop, ft 9004b General Parpeee Веой «« Ceecmte: Height, ft Trial* Unaffected Low Order High Order '»wCi-й Geaerat Parpeee Beeeb va Cea crate: Height, ft Trial» Unaffeae.i Low Order High Order
Tkeraiel Cendocthrfcy: col/rac/cm/’C
Linear, %/*C Volume, %/*C 1
HttfeM, Make' Scotei (e) 2.3
Yaeep's MoMw: E', dyns»/cma E, Ib/inch' D»f,j'»y. gm/cc
CfWtwebe Streegtk: Ib/irtch*
Veper Preeeurat *C mm Mercury
17
beta-HMX
AMCP 706-177
NO,
Preparation: (b)
CH,— N— CHO
I 2 I 2
0_N — N N — NO
2 I . L
NO2
Two men are required to regulate the addition of reagents and control the tenperatvjr* dur-
ing the initial stage addition; one arn can complete the procedure. A 1-11tar 5-necked flask
la used, the center neck for an efficient stirrer, one aide neck for a therncmter, and the
other песка for burrettes and a gaa outlet (to water trap). The flask la placed In a pan vith
steam and cold water inlets, for temperature control.
Five cc of acetic anhydride and 250 cc glacial acetic add are poured into the flack and
the temperature brought to **5 ♦ 1°C, and held there for the duration of the entire reaction.
The reagents (a solution of 33-6 gm hexamine in 55 gm of glacial acetic acid, 100 cc of ace-
tic anhydride ard **0 cc of a aolutlon of **2.3/57.7-assscml urn nitrete/960 nitric add) are then
added simultaneously, continuously and equivalently over a 25-minute period. The reaction
mixture la aged 15 minutea.
The aeoood atage reagents (6o cc of **2.3/57.7, ammonium nitrate/960 nitric add and 150 cc
acetic anhydride) are then added simultaneously, continuously and equivalently over a 25-min-
ute period. The mixture la aged 65 minutes, poured into 1.5 liter of water and simmered on a
bath for 12 hours. Cool, filter and dry the RDX-HMX precipitate (yield 730 НИХ).
The КПХ la destroyed, leaving HMX, as follows: 1025 gm of the crude product are placed in
a solution of 15 gm sodium tetraborate decahydrate in 5 liters of water, heated to boiling
vith agitation, and 5 Я ЯаСН added at the rate of 3 cc/min. When about 730 cc have been added
the рЯ increases sharply from a little over 8.7 to over 9.7 which corresponds to complete
destruction of the 4D1. Filter th< HMX free the hot mixture; yield 612 gm, вф 279.5°-2бО.5°С.
Recrystallization from nitromethane yields material belting at 261°-282°C.
Origin;
Wes discovered as an impurity (by-product) in the nitration or hexamethylene-tetrumins to
form REX. It is now manufactured directly bythe procass described above and has valuable use
in exj,-l<y_ve systems.
Removal of RDX from HKX-°PX Mixtures and Recovery of a RDX-HMX Mixture (This procedure appears
suitable for use vith ndxtu-es containing 800 or more НИХ);
175
АМСР 706-177
beta-HMX
Procedure;
500 grama of HMT containing 12.25$ RDX are plant d in a 1500 cc beaker, 500 co of acetone
la added and the Blurry la agitated for eeveral minutes at room teqerature. Before co^leta
settling, the j^DX-HMX-acctonu solution is decanted.
To the residual HMX-RDX, another 500 cc of acetone is added. The slurry is heated ou the
steeabath and while tolllag, agitated for several nlnutes. The boiling RDX-HMX-acctone solu-
tion is decanted. The residual НИХ is now washed with cold aceto* >e 11 to a funnel. This HMX
is now taken up in 95$ alcohol, filtered and dried. Yield 353-9 gm or 70.7®$.
All the acetone extracts are combined and evaporated to dryness. Yield 137-5 В» or 26.%.
Yield Balance;
Pure HMX Obtained - 353-9 gm
Total RDX-HMX mixture recovered -
1ЭТ-5 SB
Sables taken during process -
2.1» gn
boss during process
70-78$
26.5O5C
O.A8$
2.2A$
Total
100.00$
Various satples were analysed for RXD content;
1. Crude BCC
2. After first acetone washing
3. After second acetone washing
A. After third acetone washing
RDX-Btt sample recovered
12.25$ RDX
6.0$ RDX
2.0$ RHC
O.Oi F4
5b. 5$ RDX
Prepsuration of ' ine Particle-site HMX by the Aspirator jfethod;
1. Diesel'e 1100 gm HMX in AAOO cc of dimethyl sulfoxide.
2. Piiter the HMX solution,
3. Connect a clean aspirator to the water line.
A. Place a 55 gallon clean drum under the aspirator.
5. Pseten a polyethylene tubing, long enough to reach easily to the bottom of the ШХ-
dismthyl sulfoxide eontzlner, to the side Intake of v^a aspirator.
6. fasten to the boctorn of tha aspirator another polyethy. ene tube long enough to reach
to the bottom of the 55 gallon drum.
7. Open the water ftuicet and then place the polyethylene tube in the HMX container.
3. ’.’bite aLlij- fine HMX separates out in the drum. Total duration of run is approximately
7 ‘xiautes.
9. After all the HMX solution la sucked out of the container, the • .ter is turned off.
10. The materiel ic filtered and water washed.
11. If dry HMX is required, the material < n be alcohol and ether washed.
A more efficient method to recover the RDX-HMX mixture;
1. Filter the combined hot acetone extracts.
2. Pour while agitating the filtered extracts into at least A times its volume of water.
3> Filter and dry, etc.
176
beta-Bg
АМСР темп
Oulor:
White
Storage;
Method ixy
Retard (Яма (Quauf ty-Dlatance) (Дам 9
Co^wtlbility Group Group 1 (dry)
Group M (wt)
Exudation Hana
Refarencea;37
(i) 0. E. Sheffield, E. J. Mxrrey, A. L. Roaen and B. W. Khnouae, Properties of Bg, PA
ОинХза! Research Laboratory Report Bo. 52-0:1.-23, T April 1952.
(b) W. E. Bantaann, The Preparation of Bg, OSRD Report Ifo. 1961» 3 Rcveabar 19*3-
(c) S. Livingaton, Character! a tica of Eaploalvea Bg ind ДШН, PAIR Bo. 1561, 6 September
19*5.
(d) R. J. Hnkelatein and G. Gaanv, iheory of the Datenation Proceaa, MAVORD Report Bo.
9O-*6, 20 April 19*7.
(a) 0. H. Johnsen, Bg aa a Military Explneive, KAVORD Report Во. АЗП, 1 October 1956.
(f) Alao nee the following Plcatlnny Areenal Technical Report! on Bg:
15*12
17*1 2103 2016 1737 1709
2059
(g) C. Len<a, W. Beach and R< Vallcky, Enthalpy Changea, Heat of Fuaion and Specific
Heat of Beale ExploaiTee, PAIR Ho. 250*, January 1959.
footnote 1, page 10.
177
AMCP 706-177
НЧА-З
% нмх 1 ткт VB^BWo 91
49 29 Oxygon Вашем: COr % co % -5’ -27 -
Alusinua 22 Density: gm/cc Cast 1.90
Making Paint: *C
C/H Rotio Fraoakig Point: *C
Impact ТемНгку, 1 Kg Wt: Bt-reou of Miras Ap^cratus, cm Sample Wt 20 mg Picatinny Arsenol Apparatus, In. Sample Wt, mg ВеМпеММ: *C
17 25 RmI«cHv« iMboMp «1® fit# n£
ГгКПЯ омВВввВВИв |*м< Steel Shoe Fiber Shoe Unaffected Unaffected ▼Omw\*odB ^WEHty • еОво cc/-’0 Hrs, о» 90’C ’OO’C 120‘C 135‘C 150’C
RNte Mht Impact Tact: lOTriols, $ 3/16" Steel Explosions X) Portial» 1/8" Al 50 0.37
Burned 10 Unaffected 0 50 2M Яrem Bomb Seed Tea: Sand, gm 61.3
Seconds, 0.1 (no cap used) 1 5 Паме erratically 10 '5 20 °C 370 » Km -*»• *» AVKMWvBVy oTa inHwe^Rli Minirr um Detonating Charge, gm M< rcury Fulminate Load Aside Tetryl 0-30
BoiBstic Mortar, % TNT: 120
Tteool Tost, % TNT:
71 ‘C hrtomotirssl Heat Teat: % Loss In 48 Hrs
Piets Dent Toot: Method
100‘C Neat Teat- % Loss, 1st 48 Hrs % Loes, 2nd 48 Hrs Explosion in 100 Hrs Condition Confined Density, gm/cc Brisance, % TNT
ЛеемевЫЙу Index: D« MMlhNb Betas Confinement None Test 1.0
Hygroscopicity: % Condition Charge Diameter, in. Density, gm/cc Rote, meters/socond
VotatiBty: 1.9C 7866
178
нтж-з
АМСР 706-177
^^МгЧ^М ИИИИТЯу lOMt
Condition
Tetryl, gm
Wax, In. for 50% Detonation
Wax, gm
Density, gm/cc
Oxygen, atoms/soc
(Z/ssc)
Meat, k-locnlorio/mole
UH, k»;/mol)
Temperature Range, *C
Phase
Hoot of:
Combustion, col/gm З687
Expic Ion, col/gm 1190
Got Volume, cc/gm - 660
Formation, col/gm ----
Fusion, col/gm
Specific Hit: col/gm/ ‘C
32э to 7UOC 0.21*5
Burning tto:
cm/see
imiwi Ъмвсшлпяу:
col/tec/cm/’C
Linear, %/‘C
Volume, %/*C
*J~L *----*—-
^VV^MBWf ^RVBBD еЮТМое
E', dynes/cm*
E, Ib/inch*
Density, gm/cc
Compressive Strueth t lb/inchu 22б0
;<-e below
Voper Piotwre:
'C mm Mercury
Coopressiva Strength; lb/inch’
Average (i0 tests j
High
Low
2260
2530
1910
Ultimate Deformation; $
Average (10 tests) 2.61
High 3-22
_________________________________________ Low__________________________________________2.52
Test specimen 1/2" x 1/2" cylinder (epproxlmetely 3 gm) pressed at i tons (6,000 lb) total
load or 30.000 pel with a 2 minute tin* of dwel*.
Armor Hte Impact Test:
16 mm Metter PrafecHH:
50% Inert, Velocity, ft/sjc
Aluminum Fineness
)ФФ*1Ь вяяям! Pwpses
Plate Thickness, inches
1
14
H4
Hi
Bomb Drop Test:
T7, 3000-k Send Armor Piercing Bomb vs Concrete:
Max Safe Drop, ft
SINMb Sonoml Purpeco Bomb vs Concrete:
Height, ft
T rials
Unaffected
Low Order
High Order
10004 Gene.ei Purpose Bomb vs Generate:
Height, ft
Trials
Unaffected
Low Order
High Order
179
АМСР тлп
ета-з
М мм НЕ. МЛ ЬфсНМ, Lee WC-P1: Density, gm/cc Charge Wt, lb Shaped Charge EftaeNeeaess, TNT = IMt Glass Cones Steel Canes Hale Vohsne Hole Depth
ml В Wwl о^^^НЯ^МЮТмф Far TNT ForSutja^HE 1 ieeh HI. <M2A1 PtojaaMo. Lar KC-3: Density, gm/cc Charge Wt, lb Cotoet Gray
Prbdpal Uses: HE projectile and bomb finer
Tefal Na. ef Frageteaio: For TNT Far Subject HE Method of Laadtag: Oast
Leedhg Doaefty: gm/cc 1-90
Fragmeat Veiecby: ft/ser At» ft At 25ft ft Density, gm/cc
Method bry
Meet (leMee u TNDi Hazard Class (Quantity-Distance) Claes 9
AM | iSak Pressure Impulse Energy Compatibility Group Exudation Group I None
Ah, Cesfiead: Impulse Uodar Water: Peak Pressure Work to Frclure Hu- u-e; .'t-lb/inch^
Average ;1G casts) 2-T High 3-39 Lav 2.UC Afflux VlecoeitSey iolt Seconds: 211.8
Energy
Peak Pressure Impulse Energy
♦Test specimen 1/2" x 1/2" cylinder (epproxi* metely 3 gm) pressed at 3 tons (6,000 lb) total load or 30,000 psi vith a 2 minute time of dwell.
’НО
AMCP 7Г1П
Modulua of Blaeticity: •
Ib/inch4
Average 89,200
High 97,1*00
Low : 76,300
* ttat aped мп 1/2" x 1/i” cylinder (approximately 3 8й) preased at 3 toon (6,000 lb)
total load or j),000 pal with a 2 ninuta tine of dwell.
Setback Senaitivity Teat: (a)
Critical Preaaure 119,000 pal »
Density, gm/cc 1-92
* Preaaure below which no initiation la obtained and above which an increasing
percentage of initlationa can be expected aa the aetback preaaure increeaea.
Preparation:
Procedure aiailar to that used for Torpex.
References:38
(a) let IndoraoMut frost Chief, ftploeives Development Section, to Chief, Bxploaives
Research section, Piea.tinny Arsenal, dated 12 Nay 1953. Subject: "Properties of Octols
and HTA-3."
(b) R. Brom and R. Vellcky, Heat Capacity of HTA-3, Picatinny Arsenal General laboratory
Report So. >в-Е1-5О9, 5 May 1958.
3®See footnote 1, page 10.
181
AMCP 706-177
Lead Azide
СвмрмШм Meieceier Weight: (PbNg) 291
N 28.8 N—N- N-po— Ox>geo Meece: C\% .5.5 CV% -5.5
Pb 71.2 Deneih: gm/cc ^ys^1 , л ^80 w Dextrinated 4.38
MeNag t.Mnt: *C Decoeposes
C/H Ratio Freezing Mett *C
hnpect Ssaelthlty, 2 Kg Wt: Pure Dextrine ted Bureau of Mines Apporotus, cm 10 17 Sample Wt 20 mg Picatinny Arsenal Apporotus, in. 3 5 Sample Wt, mg 30 28 ВеШад Feint: *C
Refractive ledex.. it»» n£ n£
ж , ee^Be^W*» 1W• Steel Shoe Explodes Fiber Shoe Explodes Vacant* Stability Tact: Dextrine ted cc/40 Hrs, at 90*C 100’C 1.0 ’?0’C 0.07 13b v. Г-VC
Rifle Bsdiet hnpocf Teet: Trials % Explosions Portion
Bumod U reflected 200 Greer Bernb Saad Teat: ^flSk^povder fuse 19.< !
Bsylesiea Texspetetere: *C Seconds, 0.1 (no cop used) 39o ’ 35o 5 Explcaes 3W> 10 335 15 335 20 335 SaasMviiy to InMfoHea: Minimum Detonoting Charge, gm Mercury Fulminate Lead Azide Tetryl
Brflietic Merfer, % TNT-
Treed Test, % TNT: (a) 39
7S*C.tatetaaHeaol Hee* Teet: % Lou in 48 Hi
Plata Dent Teat: Method
100‘C Hast Teet: % Loe», 1st 48 Hrs 0. 34 % Loss, 2nd 48 Hrs 0.05 Explosion in 100 Hrs None Condition Confined Density, gm/cc Brisonce, % TNT
Detonation Rote: Pure Lead Azide Confinement
ЯаоимЫЭсу Index:
IhgieoteMibL % Dextrineted Not. Dextrine ted 30^C , 90£ RH 0.8 0.03 Charge Diometer, In. Density, gm/cc 2.0 3.0 4.0 Rate, meters/second 4070 4630 5180
VefatiNty:
182
Lead Azide
АМСР 766-177
Fragsseateliea Teet* W мм Ml, MT1 Prajestde, Let WC-91: Density, gm/cc Charge Wt, lb Total Na. of Fragmeata ' For TNT For Subject HE 1 taeb ЙЕ, M42A1 hoje Hilt. Let KC-S: Density, gm/cc Chorge Wt, lb T^^mI bl* *1 IVawU ^^Ve ^ta For TNT For Subject HE — a o*. Ш*а|1ммам TMT — wNWgw Vnwmalv^WVSM* 1 Rl Gloss Conor jtey Cones Hole Volume Hole Depth
Cohn White-buff
Principe! Uses: De tone tore, priming compositions, and commercial blasting caps
Method ef Loadtag: Pressed
Leedtag Deadly: gm/cc pil x 103 3 5 Ю 15 2.62 2.71 2.96 3.07
Fragmeat Vetacby: ft/sec At 9 ft At 25% ft Density, gm/cc
Str тс Method Wet Hazard Clots (Quantity-Distance) Class 9 Compatibility Group Group H (vet) Exudation Nona
Meet (Reiethv to TNT): Abt Peak Pressure npulse Energy Ab, Cnafioed: Impulse Under Wotan Peak Pressure Impulse Energy Peak Pressure Impulse Energy Heat of; Combustion, cal/gm 630 Explosion, cel/gm 36'f Gas Volume, cc/gm 308 Formation, cal/gm -3^6
Coa^atibility with Metals: Ury lead azide does not react with or cor- rode ateel, iron, nickel, aluminum, lead, zinc, copper, tin or cudmlum. Il does not affect coatings of acid-proof black paint, oil, NRC coepound or shellac. Lead azide in the presence of moisture corrodes zinc and copper; and with copper, it forms the extreme- ly sensitive and dangerous copper azide. Specific Heat: cal/gm/°C 2c -50 0.110 0 0.110 25 0.110 50 0.110 Thermal Conductivity: cal/sec/cm/°C (Pure) 1.55 x 10*1*
183
АМСР 706-177
load Aside
CosjetibiHty with Mstale:
Пгу:' Steel, iron, niohel, iluninun, lead, sine, copper, tin, stainless steel, braes and
broue were unaffected by six yean* contact vith dry lead aside at aabient tes^wature and
50°C* NMzel, rhrone-nf ckel and Inconel vere unaffected under the eane conditions' in two and
one-half years.
Wrt; Copper and sine are rapidly attacked by aolst lead azide, while aluainun is not
attacked in 24 hours. Monel, chrcm-nickel and Inconel are not attacked by lend aside (|£
Moisture) after 2? «onthc* exposure at aabient te^erature and 5O°C, and J-l nagnesiun-aluni-
ra alley is very slightly corroded.
Lead Aside
Lead Aside Lead Aside plus dbj
Lead Aside plus' plus rayl Meo»
Denols Jested 2» HEter 20» Hater hoi It
friction Pendultai Best:
(All IA daztrlnated)
Shoe fiber fiber Steel fiber Steel fiber
№>. of Irialo 1 10 12 13 1» 1
SVloaions 1 0 0 0 1 1
0 2 0 2 0
Unaffected о 10 10 1C 1 0
J»et Sensitivity, 2 Ks wt:
(All IA dextrlnated)
Pl Apparatus, inches 1» 9 9 1»
Activation fcergy: (c)
Koel/e^s 23. Th
Induction Period, seconds 0.5-10
Initiating Ifflclen,-v, Grans Required to Give Complete Initiations of:
Dextrlnated Azine (gn)
ИТ 0.25
Bstryl 0.10
RUX 0.05
BB 0.02
Sensitivity to Static Discharge, Joules (Pure Lead Azide) (b) 0.0070
184
Lead Aside
АМСР 706-177
Comatiblllty of Dextrin»ted Lead Azide with Black Powder:
100°C Vacuum Stability Test, cc/ko 'nr:
Smile Wt (gm) Material cc
1.0 1.0 2.0 ' Lead Azide 0.50 Black Powder 0.3B 50/50,Lead Azlde/Black Powder 1-26
Solubility of Pure Lead Aside; gn/100 at of Water:
i
20 0.05
Preparation of Lead Aside (Dextrin»ted): (du Pont procedure)
2 Ba — Я* № И + rb (Ж>3)2~’ И>(Я3)2 + 2 BaBOj
Lead nitrate solution: Thia 5» prepared by dlsnolvlng 164 lbs lead nitrate and 8.25 IK
dextrine la deionized water, the solution allowed to eettla, an* sodium hydroxide added to
bring the solution to a pH >if 5.4. iba final concentration of the solution la then adjusted
t*, 7.Ц lead nitrate, 0.3756 dextrine by addition of deionized water.
Ute lead «aide is precipitated at a aolutlon taiventure of 160°F, using 60 parte lead
nitrate and 50 parte aodlun etide aolutlon. She latter la added to the forner in 23 ndnutea,
under agitation (no baffles are used in the precipitation таем!), the nlxture coded to room
te^psrztu- in 12 ndnutes, and allowed to settle 10 nlnutes. The nether liquor is decanted
and the remaining slurry saihed before packing.
Od^n^
Pint prepend In 1891 by T. Curtiuj (Ber 24, 3345-6) by adding lead acetate to a solution
of aodlun or modus azide. T. Hyronimus (rireach Patent 364,792) should be credited witn the
first attest in 1907 to use load aside with sone success in the explosive industry. Its com-
mercial mnufhetun started in Europe before World War II and In the United States since 1931
as Military or commercial grade "dextrinated" lead azide.
Destruction by Caemlcal Decomoeition;
Land azide can be decoavooed by
(;) nlxizg with at least five time its '-eight of a 106 solution of sodium hydroxide
and allowing the mixture to stand for 16 hours. Decant the supernatant solution of sodium
azide and drain into the soil.
(2) dissolving in a 106 solution of amouiun acetate and adding a 106 solution of
•odium or potassium bichromate until no акте lead chromate Is precipitated.
(3) wetting with 500 times ite weight of water, slowly addlt« 12 times its weight
of 256 sodium nitrite, stirring, and then adding 14 times ite weight of 366 nitric or glacial
acetic add A red color produced by the addition of ferric chloride solution indicates Lead
Azide is still present.
185
AJKCP 706-177
Lead Azide
(4) dlaaolving in $0 time its weight of 157t eerie aenonium nitrate. n>e azJ<H ie
deccag>v«ed with the evolution of nitrogen. '
References: 39
(a) И». Naoua, Z gee Schieas Sprengatoffv, 181, 229, 267 (27 June 1932).
(b) F. W. Brown, D. H. Kualer and F. C. Gibe:», Sensitivity of Ezploeiyea to Initiation
by Electrostatic Diachargea, U. S. Dept of Int, Bureau of Minea, HI 3852, 19^*6-
(с) C. bench!tz, Ice Calorimeter Determination of Enthalpy and Specific Heat of Eleven
Organcawtallic Ooapounda, PACT ^22h, Bovember I'-55.
(d) Also «ее the follovlig Picatiruy Araejal Technical Reports on Lead Azide:
0 1 2 2 4 2 6 I 8 2
550 561 832 393 r,k 255 326 567 628 609
580 861 852 1393 784 525 856 637 708 ns
600 1451 882 1493 824 1325 866 657 748 749
760 1651 932 2093 944 1485 1316 707 788 .769
IU50 1132 2133 2161» 1486 1737 838 849
1152 2201» 1556 2227 1388 999
1352 1528 2179
1372 18 38
2198
J^See footnote I, page 10.
186
Load 2,4-Djnitroresnrcinate (LEHR)
АМСР 706-177
ftngtttHsn, C 17-8 Г I ° H 0.5 N 6.9 < — ЯО2 0 23.Т Pb рь 51.1 kJ— 0 L »o2 J C/H Rrtio 0.549 . Molecule'Weight: (PbCgHgHgOg) 405
Oxygon Baleace: co= % -32 CO % - 8
Density: gm/cc crystal 3,2
Framing Point: *C
Impact Sonoitivby, 2 Xg Wt: Bureau of Mines Apparatus, от 1 Л 30 Sample Wt 20 mg Pleotinny Arsenal Apparatus, in. Sample Wt, mg 20 BeMtag Point: *C
Refractive Index, n° Па n£
Steel Shoe Fiber Shoe Vacuum Stability Tmt: cc/40 Hrs, at 90"C 100*C 120°C (73 minutes) Explodes 135’C 150’C
RNh BaRot Impact Tmt: Trials % Explosions Partials Burned Unaffected
200 Gram Bomb Seed Tosh ^G&fpowder fuse 20
(xphoioo Temperature: ’C Seconds, 0.1 (no cap used) 1 5 Explodes 265 10 15 20 m о» 0- e—. »— v~v - «CKUvovWy Iw 1МЮТМ. Minimum Detonating Charge, gm Mercury Fulminate Lead Azide Tetryl
BeBiMte Mortar, % TNT:
Trarnl Test, % TNT;
7ГС Intornoticaal Hoot Test: % Loss in 48 Hrs
Plate Done Met.ic Condition Confined Density, gnr /cc । Brisance.. % TNT
100'C Hoot Tmt: % Loss, ’st 48 Hrs 0.20 % Lou, 2nd 48 Hrs J.02 Explosion in 100 Hrs None
Dotenetion Rato: Confinement Condition Charge Diameter, in. Density, gm/cc Rate, meters/second
ЛмммЫШу 1м4вж:
Hygroscopicity: % jo°c, 90^ RH 0.73
Vefoeffity:
187
амср темп
Load 8,l-£tlnltroreeorclnete (ЫЖВ)
Preg^aoaOotloa Toot: 9* яма M, МП Prajoetiio. Let WC-P1: Density, gm/cc ChorgeWt, lb TaM No. *f FtogMata For TNT For Subject HE 1taeh НА M42A1 PiojortAe. Let KC4: Density, gm/cc ChorgeWt, lb Total No. gf FmgMota For TNT For Subject HE SbepodCbor?e IHoetheooM. TNT = 1И: Gloss Cone* Steel Cone* Hob Volume Hob Depth
Color: Red or yellow
Priadpol Umu Electric detonator*
Method of Landtag: Proceed
Leodtag Deoafcy: gm/cc
At» ft At 25% ft Density, g.<i/cc
Mtthod Wet Hoxord Class (Quantity-Distance) Cloe* 9 Compatibility Group Exudation Hone
Mart (laiatfvo ta TNT): Abt Peak Premure Impube Energy Air, Confined: Impuhe Under Wotan Peok Pressure Impube Energy Uedeegroead t Peak Pressure Impube Energy
Initiating Efficiency; 0.1 gm LMR does not initiate tetryl proceed at 3000 pci. Heat of; Explocion, cal/gm 270
188
Lead 2,4-pinltroresordnate (ЦЖВ)
АМСР 706-177
Prepaxatlon;
To • «elution of 5 grana of purifier ninitroreaorcin and 2.65 grana of anhydroua aodlun
' te in 500 cc of boiling water la а ’Л «lowly a aolutlon of 10 grana of lead nitrate
uaMolved in 60 cc of boiling water. Им reaction nlxuire la constantly atirred during the
addition of the lead «alt and for about an hour attemrd while the aolutlon la allowed to
coox to rocn tenperature. 'Tie precipitate la filtered and waahed thoroughly flrat with water
and then with alcohol ar ether. It la dried in a atean oven.
Origin:
iД-dinltroreaorcin wan deacxibed in the 1881 edition of BeHeteln (Beil VII, 88?). The
aane coa^ound wee deacxibed in mare detail by WeaelBky, Benedlkt and Bffbl in 1882 (M II, 323).
The land aalt of 2,4-dlnitrore«arcinol appeara to have been prejared between World War I and
World War П by treating reaordnol with nltroua add and oxidizing the reaultlng dinltroao-
rmaordnol to dlnltrareaorcirul. lead nitrate aolutlon waa then added to a aolutlon of the
2,4-dlnltrore«arclnol to which aodlun carbonate had been added to fora the soluble aodlun ealx
(J. D. Popper, ML® Ko. 480, March 193*). The 1ЛИ exiats In two forne differing In phyalcal
charactadatlea but poaaeaalng alnilar explosive properties. These forma are red and orange
in color (K. 8. Warren, PATR 1448, SepteaAer 1944).
..fex w*»:40
(a) See the following Picatlnny Arsenal Technical Reports on read 2,4>dnitroreeordnate:
0 2 4 8 2
480 453 1004 1328 859
5Й0 1448 1079
^Se* footnote 1, page 10.
18»
АМСР 706-177
Lead 4,6-Dlni.troreBorcinol Basic (UWR Basic)
GT-H*‘ 0_ C 11.2 1 ; o.6 v Moiecnier Weight: (Pb^H^NgOg) 646
Pb —OH Qxygea Baience: CO. % CO % 1 1 v.8
0 19.8 Pb 6k.1 L 0 — Pt — OH Density: gm/cc
N0_ Making feint: *C 213
2 C/H Ratio 0.177 Preening feint: *C
* GaaWUhr 4 If a Wfe MHMnVwji Л WJ WT» Bureau of Mines Apparatus, cm 1 Sample Wt 20 mg Ококппу Arsenal Apparatus, in. Sample Wt, mg kg vt 60 20 BsKng feint: *C
Beftective Ыаа, п» nS П»
e_»e__ te_OP--.- П1м1К1 гЧВнЮТЮТ I VBT3 Steel Shoe Fiber Shat Vecoam Stahkky Test: cc/40 Hrs, at WC lOO’C 120*C ’35‘C 150-C
ШЯе BoNet impeet Tech Trials % Explosions Partials
Burned Unaffected 200 Gram Bomb Send Teat: %HW*Bavder fuse 15
^ix^rieei^xn l^^reB^reeedsrr^i. Seconds, 0.1 (no cap used) 1 5 Explodes 295 10 15 20 • |т1В^й1ата MHSBTWwy nW рИИо^М^МВ» Minimum Detonating Charge, gm Mercury Fulminate Lead Azide Tetryl
BsBlsHr Metter, % ПГТ-.
Tranzi Test, % TNT:
75'C tetetnaHeoel Hoot Tmt: % Loss In 48 Hrs
Plate Dent Toot: Method
100*C Hoot Tee»: % Loss, 1st 48 Hrs % Loss, 2nd 48 Hrs Explosion in 100 Hrs o.L 0.0 None Condition Confined Density, gm/cc Brisance, % TNT
Confinement Condition Charge DiometC'. in
Indn*
Hygroscopicity: %
▼WWWmy» Density, gm/cc Rate, metors/second
190
Lead U,6-Dlnitrorenorcinol Basic (1ДНВ Basic)
AMCP 706-177
M ем* НЕ, МЛ PmjacMo. UM WC-»1: Density, gm/cc ChorgeWt, tb Total Na. a* Fragment*: For TNT For Subje^ HE 1 iach Ht, M42A1 PmjeMile. IM KC-S: Density, gm/cc Charge Wt, lb BVKVK VV *W^pi^MK*o For TNT For Subject HE Shaped Charge Iffectboneee. TNT = 1И: GkmCone* Steel Cone* Hole Volume Hole Depth
Color Red or yellow
Principal Ueea: Electric detenatore
Matted of Leadhg: Preeeed
Leadhg Deaaby: gm/cc
Fragment Veh thy; ft/toc At» ft At 254 ft Density, gm/cc
Method Wet Hozord Clou (Quontity-Drttonca) Claoo 9 Compatibility Group Exudation Косе
Meet (ReleMrete TNT): Abt Rook Ргемиге Impube Energy Ab, Confined: Impuhe Under Wetan Rook Ргемиге Impuhe Energy Peek Ргемиге Impuhe Energy
Initiating Efficiency; O.U gm ЫЖН Basic does not initiate tetryl pressed at 3000 psi.
191
АМСР 706-177
Lead 4,6-PinitroreBorcinol Bail с (ЫЖВ Basic)
Preparation:
Pb(CH3COO)2
O-Pb-OH;
O-Pb—OH
N02
(a) One hundred grams of pure resorcin is fused in a porcelain casserole and 1 mediate Im-
paired on a glass plate. After cooling, the cake le ground In c mortar to pace a U. S. Stan-
dard Bo. 6 meah screen. Four hundred grama of 98 percent nitric acid in a one pint capacity
Deear Jar la stirred mechanically while carbon dioxide snow ie added in email piece». When
the teaverature falls to -20®C, 40 grama of the granulated resorcin ie added In small quanti-
ties. Simultaneous addition of solid carbon dioxide aa required prevents a rise of tempera-
ture of more than 5 degrees throughout the entire experiment. Five minutes after the last
portion of resorcin is introduced, the mixture is further cooled to minus 5O°C, and finally
drowned vith vigorous stirring In fl.e times its volume of cracked ice. in water. This mix-
ture is allowed to stand for one ho*ir and the product then filtered, washed, and partially
dried, weight 43.6 grams. The crude 4,6-Шл is purified by first dissolving the product In
an equecus 5 percent sodium hydrox de solution (17-4 grams of sodium hydroxide in cc of
water). The resulting solution if then neutralized by gradually adding it to a boiling solu-
tion of 21.4 grams of 96 percent sulphuric add in 150 cc of water. The resulting precipitate
of 4,6-ПШ Is filtered hot on a suction filter and air-dried. Yield, 27.5 grams (37*8 percent
of the theoretical).
(b) Five hundredths (0.05) mole (18-96 grams) of load acetate is dissolved in 67 cc of
warm water, into which is gradually stirred 0.10 mole (4.0 grams) of sodium hydroxide dis-
solved in 67 cc of water. Stirring is continued for five mini tea. After settling, the white
lead hydroxide is washed by decantation three times with 100 cc portions of distilled water,
and used isnediately for the next operation.
(с) A O.O278 mole (5-56 grams) quantity of the 4,6-ШВ prepared under (a) above, is dis-
persed in 270 cc of water by vigorously beating vith a motor stirrer. After heating this
dispersion to 90°C, the 0.05 mole of lead hydroxide prepared above in slurry form is intro-
duced in snail portions. Agitation is continued for three hours at 90°C. The bMic lead
4,6-IHR is washed cuca by decantation, and again on the filter with alcohol. After drying
overnight in a desiccator charged with calcium chloride, the product weighs 15.6 grams.
Origin;
Both the 2,4- and 4,6-dinitroresorcin were described in some detail by Waselsky, Benedikt
and Hubl in 1882 (M II, 323)* T^pke prepared the 4,6-dinitiore>,orcin in 1883 by iiydrolyzing
the nitration product of resorcin dlecetate (Ber 16, 551). А ’логе direct and economical
method of preparation suitable for production scale manufacture was developed during World
war II by the British (Ministry of Supply Pouch Item W-154-21S, "Mrnufacture of 4,6-Ddnitro-
resorcln and Lead 4,6-Dinltroresorcinste"). This procedure consisted of preparing 4,6-
dlnltroresorcinol by direct nitration of granulated resorcin and allowing the product in slurry
to react with sn excess of lead hydroxide at 90°C. This basic salt can be prepared in two
forms: (1) a micro-crystalline, yellow, lov-dsnsity form and (2) a denser, brick-red form.
Both products have the same chemical composition and the same sensitivity to impact (PATH
144S, September 1944).
IffJ
AMCP 706-177
Lead Styphnate
% Г 0 C 15-1» H 0.6 0„N -f у- no„ N 9.0 * 1 | г PbH-0 0 Э0-8 L J— 0 г Pb Ui.2 1 И02 C/H Ratio O.32OL- J Meiocaier Weight: (pbCgH^Og) U68
Oxygen Balance: CO, % -19 CO % 2
Density: gm/cc Crystal 3.02
Melting Point: ’C Explodes 260-310
Fieesiag Point: *C
* * * If — Ц«- ЯИ^Ям «^ЯОвОаоТяуд Л ^Tc* Bureau of Mine* Apparatus, cm 17 Sample Wt 20 mg Picatinny Arsenal Apparatus, in. 3» (8 oz vt) 8 Samp1* Wt, mg 22 BeiSag Point: *C
Refractive Index, ng ng ng
гпспоя rwB«weei 1 w* Stool She* De too* tee Fiber Shoe Deton*tee Vecanm Stability Tort: cc/40 Hrs, at 90’C 100’C O.U • 20’C 0.3 I35’C 150'C
RWb BeBet Impact Teet: Trial* . % Exptos'cn* Partial* Burned Unaffected
200 Grein Bomb Send Tost: B^*<& SJmnier fuse ?1.1
Kxplesiea Temperateie: 'C Second*, 0.1 (no cop used) 1 5 Explodes 262 10 276 15 272 2C 267 Sensitivity to Initietioo: Minimum Detonating Charge, gm Mercury Fulminate Treco* Lead Azide Trace* * <.0Й'Йп, alternative
Ballistic Mortar, 4 TNT:
Troexl Toot, % TNT: (a) UO
75 ‘C International Meet Test: % Lou in 48 Hrs
Piofo Dent Test: Method Condition Confined Density, gm/cc Brisonce, % TNT
WC Hee* Tost: % Lou, 1st 48 Hrs О.38 % Lou, 2nd 48 Hu 0.73 Explosion in 100 Hrs None
Dotonotiea Reto: Confinemen’ Condition Chorge Chur.eter Density, gm/cc 2.9 Rote, meters/second 5200
ЛеммЫК* Index:
Hygrascopicity: % 25°C, 100$ RH 0.05 30°C, 90$ RH 0.02
Volatility:
193
АМСР 706-177
Lead Styphnste
99 ома HU M71 PrejecHlo, Lot WC-91: Density, gm/cc Charge Wt, lb Shaped Charge IHactivoaoss, TNT = IN: Glass Cones Steel Cones Hole Volume Hole Depth
Т«Ш Ho. of ггедемаН: For TNT For Subject HE 3 tach HU М42Л1 Projectile, Lot KC-S: Density, gm/cc Charge Wt, lb Color: Orange-reddish brawn
Prlacipai Uses: igniting charge, and ingredient of prising compositions
Total No. ef FrejssoaH: For TNT For Subject HE ef Pressed
Leodiag Doosity: gm/cc
Pregawat Vdecfty: ft/soc At 9 ft At 25^4 ft Density, gm/cc
Method Wet
Moot (Kdotivo to TNT): Air: Peak Pressure Impulse Energy Hazard Clou (Quontity-Distonce) Compatibility Group Exudation Class 9 Group M (vet) Kone
Air Ceaftaod: Impulse Ueder Wafer: Peak Pressure Impute Energy Peek Pressure Impulse Energy Heat of; Combustion, cal/gm Explosion, cal/gm Gas Volume, cc/gm Formation, cal/gm -251 1*57 368 -92 Activation Energy; kcal/mol Induction Period, sec Specific Heat; cal/cm/°C 2c -50 0 25 50 75-39 0.5-10 (c) 0.141 0.158 0.164 O.167
ISM
Lead Styphnata
АМСР 706-177
Preparation;
♦рь(сн3соо)2
PbHgo ♦ гсн^сооа
Dissolve 14.4 /ф lead nitrate and 1 cc of acattc n-cld in 320 cc distilled eater. Dis-
solve 4 gm 2,4,6-trinltroresorcinol and 1.73 8“ sodlun ceruonate in 80 cc di«tilled eater.
Add the lw€d acetate solution to the trinitroreaorclnol solution, under agitation, keeping
the temperature at 7O°-75°C and continue stirring for 3 hours at this teq^erature. Cool to
20°C in 5 hours. Evaporate the solution to 1/3 its volume, cool, filter and eash the product
well with eater (to neutrality).
Sensitivity to Static Discharge, joules; (b) 0.0009
Loss In Weigh; at 1O5°C: 1°
3 hours 0.02
6 hours 0.23
9 hours 0.23
Effect of Storage for 2 Months at 30°C, on;
Explosion Tbqerature Test Value Nil
Sand Test Value Я11
Sensitivity to Initiation Mil
Solubility, gm/100 gm (<) in;
Glycol Diacetate
°C jt
20-25 0.1
Origin:
First described in 1914 by von Hurtz and found to be a relatively poor initiator by Wall-
baum in comparison to other primary explosives. (Z ges Schless Sprengstoffw J4, 126, 161, 197
(1939)) Moisak shoved that lead styphnste could be used as an insulating (cover) material
for lest azide providing protection from mechanical and chemical Influences and, at the same
time, increasing the detonating ability of the total charge (Transactions of Butlerov Inst
Chem Tech Kasan (Russia) 2, 81-5 (1935)-
195
AMCP 706-177
Lead Styphnate
Destruction by Chemical Decaapoeition:
Land styphnate is deccepoaed by dissolving it in at least 40 times ita wight of 20* sodium
hydrac ie or IDO times ita wight of 20*, аамоп1>л acetate and adding a solution of sodium
dlchroaate, equal to half the wight of styphnnte and 10 parts of wter.
References:*1
(a) Report AC-9j6/0rg Be 74.
(Ъ) T. W. Brown, D. H. Hurler and F. C. Giheon, Sensitivity of Exploelvea to Initiation by
Electrostatic Dischargee, U. £. Dept of Int, Bureau of Mines, RI 3B52, 19^6.
(с) C. Lenchltz, Ice Calorimeter Determination of Enthalpy and Specific Heat of Eleven
Organometallic CcwoounS, PACT Mo. 2224, Boverber 1955»
(d) Also see the following Picatinny Arsenal Technical Reports on Lead Styphnate:
0 1 2 ? 4 6 1 a 2
1450 2220 11 1352 2032 *53 2093 2164 1316 hen 1737 2077 318 2179
4tS« footnote 1, page 10,
Mannitol gtaanltrate (Kitroaannlte)
AMCP 706-177
% С 15.9 °2S0|H H 1.8 °2ИОР К 18.6 НГ°2 HCGSO* 0 63.8 * ‘ ° '’•° сн„ояо„ C/H Rofio 0.133 г г Metocoter Weig»' (CgHgB^g) 452
Oxygen Belaec*: CCL % 7.1 CO % 28 3
Deneffy: gm/cc I.73
Meffing Pohc *C 112-113
Fieeneg Met: *C
» -- » *» *- « К- fwmnvwjj л ^*тт Buraou ef Mine Apparatus, cm 11 Sample Wt 20 mg Pieotinny Arsenal Apparatus, in. 4 Sample Wt, me 11 BeMag Feint: *C Decomposes 150
Refractive Index, n£ n« n»
ПЖПЯ1 ^^ВВ^^ИВНВ ЮТ*» Steal Shoa Detonates Fiber Shot Unaffected ЮТе cc/40 Hrs, at 90'C I00*C 120”C 135"C 150*C
Riffe Buffet Impest Teat: Trials % Explosions Portia is Burned Unaffected
200 6ren> Pemb Send Test: Sond, /n 68.5
Seconds, 0.1 (no cap used) 160-170 (a) 1 232 (b) 5 175 (c) 10 15 20 vVHMWvgvy W IwWwI^Rir Minimum Detonating Charge, gm Mercury Fulminate Lead Azide 0.06 Tetryl
OeOiette Merter, % TNT:
Trees! Teet, % TNT: (c) 172
75‘C 1а1еигаНмга1 Heat Teat: % Loss In 48 Hrs 0.4
Plete Dent Test: Method Condition Confined Density, gm/cc Brisance, % TNT
100’C Meet Teet: % Loss, 1st 48 Hrs % Loss, 2nd 48 Hrs Explosion in 100 Hrs (Frothed) 48 hours
Petenntien Rote: (d) Confinement Yes Condition Pressed Charge Diameter, in. 0.5 Density, gm/cc 1.73 Rate, meters/second 82^0
Wygreecepicity: % 30°C, 90# I<H 0.17
Т*ЮТЯну>
197
АМСР 706-177
Mannitol Hexa nitrate (Nitromannite}
Ftagmc Mates TMh Maa Hl,M71 Plate**. La* WC-P1: Density, gm/cc Chorge Wt, lb Total Na. of РгадомаН: Far TNT For Subject HE 1 iacb HL М42Л1 Prejirtile, La* KC-S: Density, gm/cc Chorge Wt, >b Tatai No. ef Pra^ueeN: For TNT For Subject HE Sbepud Charge EHocteeaees, TNT = IK: '.tea Cone* Steel Cone» Hole Volume Hole Depth
Color:
Prladpei Uses: Secondary charge In detonator* (ref 1), and in blasting caps designed to be initiated by a fuse (ref j) i
Method ef Leediag: Pressed
Leedhg Deaaky: gm/cc
Fragaaaa* Vatodty: ft/ис At9ft At25feft Daraity, gm/cc
Method Dry Hazard Ctes (Quantity-Distance) dess 9 Compatibility Group Exudation None
-Май (RaiaHve to TNT): Ab: Peak Pressure Impute Energy », Ceaflaed: Impute Peak Pressure Impute Energy Peek Pressure Impute Energy
65.5°C KI Test; Minutes 6 Heat of; (e, f, g) Combustion, cal/gm ISIS 152_> Explosio.., cal/gm 1390 ibsb 1U68 1520 Farmation, csl/cz 337 3U5 366
198
ttannitol Hexanitratо (nitrrwannlte)
AMCP 706-177
Solubility; ____
a. Insoluble in water.
b. Slightly soluble in cold alcohol (2.9 gm at 13°C).
c. Slightly aoluble in ether (4 gm at 9°C).
4. Very aoluble in hot alcohol.
Preparation; (Laboratory Method) (k)
a. Cool to belov O°C, 5C gm of 96$-10O$ nitric acid placed in a 300 milliliter Erlenmeyer
Pyrex flask provided with a thermometer and inneraed in an Ice-aalt mixture.
b. Introduce in email portions, 10 gm of d-nmnnitol, while swirling the 'leak to break up
any lumps of mannite which might form. Keep tha temperature below 0°C.
c. After solution is canplate, add 100 gm of concentrated sulfuric acid from a dropping
funnel, swirling the flask in an ice-salt mixture to keep the temperature below 0°C.
d. Filter the resulting porridge-like slurry through a filter paper previously hardened
by treatment with mixed acid.
e. Rinse the precipitate directly on the filter with water followed by dilute aqueous
sodium carbonate and finally with water. (The resulting crude mannitol hexanitrate gives
lb. 2$ H aa vLterndned by the nitrometer.)
f. Dissolve the crude mannitol hexanitrate in boiling alcohol and filter through a water-
heated funnel.
g. Bring the filtra-e to boiling and gradually add hot water until the appearance of the
first turbidity.
h. Cool in an ice-salt bath, separate and dry the crystals. (Yield should be about 23
gm of material, melting .t H2°-113°C and having 18.58$ Я, the nitrogen being determined by
t.u nitrometer. Theoretical yKld would be 24.8 gm.)
Origin;
Mannitol hexanitrate was 'discovered in 1847 by As car. io Sobrero who recommended it as a sub-
stitute for mercury fulminate in percussion caps (Cotjp rend. 1847. 121). It ia the hexanltrlc
ester of d-rannitol which Ir. widely distributed nature, particularly in .!• slant Frax'.nus
omus. N. Sokoloff, s Russian chemist investigated the explosive properties of end recom-
mended in 1878 a method of prentiacion. Mannitol hexanitrate was thoroughly studied by Tlerthe-
lot, Sarran and Vieille. Lusonte, Menard, Strecker, Tlchanowich (Ph. Kaoum, Nitroglycerin and
Nitroglycerin Expletives, Baltimore, 1928, pp. 156, 217-250), and particularly by J . H. Wigner
(Ber 36, 79A (1903))• More recent data have been reviewed by Guastalla and Racciu ("Modern
Explores," Industrie Chimica 8, 1093-1102 (1933)).
References;4*2
(a) G. C. Hale, Abstract of Available Information on the Preparation and Explosive Proper-
ties of Hexanitrcmannlte, PA Special Report По. 238, 30 July 1925.
A2See footnote 1, page 1C.
19!)
АМСР 706-177
Mannitol Haxanitrate (Mtmaannite)
(b) C. A. Taylor and W™ H. Rinkenbach, "Sentitiveneaa of Detonating СоцкмпДа to frictional
B^act, Bgpact, and Heat," J. Frank Inst 204, 369-76 (1927)*
(c) Hi. Baoua, Z gee Schlett - Storengatoffv (Munich), pp. 181, 229, 26? (27 June 1932).
(d) H. Khat, Z anew Chea, £, /4 (1923)*
(e) A. Schmidt, Z gee Schlett - Sprengetoffv 29, 262, (1934).
landolt and Bernstein, E IH, p. 2914.
(f) A. Narahall, btplotlvea, Ihelr ttoufacture, Properties. Jetta, and History, Vol Ш,
London (1932) p. 39* №. Maoua.Hitroglycerin and Hiiroglycerin Exploalvet, Baltlaore, (1928),
pp. 156, 247-250.
(g) A. Scheldt, Z get Schlett - Sprengetoffv 2£, 2б2 (1934) G. Fleury, L. Bria tend and
P. Ihoete, "Structure and Stability of MJ trie Eater a," Coap rend 224, 1016-18 (1947).
W. R. Joalinacn, Jr., Fundamental Properties of High Ехр1се1тев. BSraodynaalc Relatione for
Ute in the Eatiaation of Explosive Properties, PATH Mo. 1651. 22 April 1947.
(h) JMrren and VieUe, мАп poudr 2, 161 (1884-1889).
(1) k. von Hurts, U. S. Patent 1,878,652 (20 September 1932).
(j) L. A. Burrova, U. S. Patent 2,427,899 (23 Septeaher 1947).
(к) В. T. Fedoroff, Handbook of Exploalvt and Belated Itama, Picatlnny Arsenal (unpub-
Uahed).
(1) 0. E. Sheffield, Literature Survey on Mannitol Heaanitrate, Bl Chealcal RMeartii labo-
ratory Report Во. 52-M.-16, 23 January 1952.
(a) Also tee the following Picatlnny Arsenal Technical Rriporta on Mannitol Heaanitrate:
2 4 £ 6
1352 24 85 6
64
20G
Mercury Fulalnate
АМСР 706-177
% с 8Л К 9-8 Hrlsretor Weight» (HgCgl^Oj) 285
о —H —c He Oeleece* co, % co % -17 .<=.5
0 п.2 0 —N— C DeaeRy: gm/cc Crystal 1».1»3
Be 70.6 Melting Feint: V Decos^oees
С/Н Ratto
taapaet SeaeMvRy, 2 Kg Wh Bureau of Minos Apporatus, cm 5; (1 kg vt) 35 Sample Wt 20 mg Pleotinny Arsenal Apporatus, in. 2; (1 lb vt) 1» Sample Wt, mg 30 BeNfeg Point: *C
RehocNee lades. n« ПЙ n&
5*eel Shoe Fiber Shoe Explodes Explodes Vocanm SlebiBty Tost: cc/40 Hrs, ot 90*C 100’C 120*C 135*C 150*C
ВШ- R » »-~- Lxptosiont Partials Trials % Explodes
Burned Unaffected 200 (Мем ВешО ScmO Teeh _ fuie 24.1»
Seconds, 0.1 (no cop used) 263 ' 239 - Explodes 210 Ifr-*' 199 ic tali e. ur »ж- |—v BMBIlrvWy W ВЮТ^^^ИВе Minimum Detonating Chorge, gm Mercury Fulminate Leod Azide Tetryl
20 190 BeWstie Metier, % TNT:
Trausi Test, % TNT: (a) 51
7S C leSoreeHoael Hee* Tosh % Loss in 48 Hrs 0.18 Plato Dent Test: Method
100‘C Hee* Tosh Exploded In 16 hours % Loss, 1st 48 Hrs % Loes, 2nd 48 Hrs Explosion in 100 Hrs Condition Confined Density, gm/cc Brisonco, % TNT
Detseetise Role: Confinement
НммюМКу ledex: Pressed 3.0 1».O 4250 5000
Hygroscopicity: % 30°C, 90^ RH 0.02 Charge Diameter, :л. Density, gm/cc 2.0 Rote, meters/second 3500
▼ВЮТЯВеу *
201
AMCP 706-177
Mercury Fulminate
90 аыа Ml, M71 PraHctite. U» WC-91: Dimity, gm/cc ChorgeWt, lb bk _£ For TNT For Subject HE 1 tack HI. M42A1 ProjecHte. Lot KC-S: Density, gm/cc Chorga Wt, lb For TNT Fo< Subject HE Г*- -Я Лкемд Ммбкамм TMT 1AA* WWWf^^ vv^MVVW^MIg V^B OW* Glass Cones Steel Cones Hole Volume Hole Depth
Cohr: White to grey
Ptbdpei User: Detonators and ingredient of priming compositions
Method of Leedbg: P*1 x ЮЛ 3 5 10 12 15 20 3-00 3.20 3.60 3.70 3.02 4.00
Leading Doaoity: gm/cc
Ftegaroat Vebdly: ft/sec At 9 ft At 25% ft Density, gm/cc
Method Wet Hazard Class (Quantity-Distance) Class 9 Compatibility Group Group M (vet) Exudation None
Bbat (Kebthra Io TND: Ur. Peak Pressure Impulse Ltergy Air, Ceafbed: Impulse Uador Water. Peak Pressure Impube Energy Peak Pressure Impulse Energy
Stab Senaitivity: Density Firing Point (inch-ounces) gm/cc Of, 50# 100# 3-91 3-2 4.3 5-5 4.26 1.6 2.6 5.5 4.32 1.6 2.6 4.0 4.50 1.6 2.5 4.0 Activation Energy: kcal/п ’i 29.81 Induction Period, sec 0.5*10 Heat of; Combustion, cal/gm 938 Explosion, cal/gm 427 Gas Volume, cc/gm 243 Formation, cal/gm -226 Specific Heat: cal/gm/°C 1.1 Thermal Conductivity; cal/sec/cm/°C 1 x IO"1*
202
Mercury Fulminate
АМСР 706-177
Initiating Efficiency; Grams Required to Give Coeplete Initiation of:
Fulminate, gm
ТЯТ Tetryl RDC PET* 0.Й5 o.a? 0.19 0.17
Compatibility with Metals:
tty; Reacts rapidly vith aluminum and magnealum. Reacts slovly vith copper, zinc, brass
and Nonce. Iron and steel are not affected.
Wet; Reacts inoedlately vith aluainun and Magnesium. Reacts rapidly vith copper, zinc,
brass and bronze. Iron end steel are not affected.
Sensitivity to Static Discharge, Joulea; (b)
0.025
The Effect, of Storage at 50°C (tty) on the Purity of Mercury Fulminate
Months Recrystallized Lots Uncry stall!zed Lots
SsM£ 2Z2 2S 221 2& 5o!>. 6-7/31 505.3-5/n
0 99.75 99.77 99-79 99.79 98.86
4 98.7
6 99-45 99.54 99-47 95-95 98.7
8 97.4
9 94.95
10 94.9
12 98.74 99.56 97.49 99.06 90.65
13 98.26 98.79
14 98.22
15 97.52 99-30 99.30 98.19 83*76
16 97-00 99.01 97-75
17 95.70 98.66 96.69
18 94.81 98.58 98.46 95.90 79-99
23 74.52
26 63.80
Chemistry:
Mercuric fulminate readily decomposes in the presence of aqueous solutions, chlorides, car-
bonate and many other materials. ttie to the presence of small amounts of mercury, iomed by
exposure to light or elevated temperatures, it readily foms amalgams vith copper, brass and
bronze, thus components containing these metals must be protectively coated If used vith ful-
minate.
Solubility, Grams of Mercury Fulminate In 100 Grams of Water
°C £
12 0.07
49 0.18
203
АМСР 706-177
Mercury Fulminate
Preparation:
(Chemistry of Povdcr and Explosives, Davie)
CH3-CH2-OH —* CH3—CHO —» CH2-CH0 jjH-CHO
NO N —OH
н ]°2 |°2
o <----------CH <------------ C-COOH «-----CH—COOH
. I I и
’ —ОН К N—OH
c OH
I------» Нв(СЖС)2
Five gm mercury is dissolved in 25 cc df nitric add (sp gr 1.42) without agitation, and
this solution poured into 50 cc of 900 ethyl alcohol, resulting in a vigorous reaction,
attended by evolution of white fumes and subsequent appearance of fulminate crystals. Red
fumes then appear aa precipitation of the product accelerates, and then white fumes again are
evolved as the reaction moderates. After about 20 minutes the reaction la over: water is
added, and the crystals are repeatedly washed, by decantation, with water to remove all acidi-
ty. The product la purified, rendered white, by solution in strong aanonium hydroxide, fol-
lowed by repredpltetlon with 300 acetic acid.
Origin:
Mercury fulminate was first prepared by John K. von Lowenstern (1630-1703) and in 1800 its
preparation and properties were first described in detail by Edward Howard in a paper presented
to the Royal Society of London (EhilTrans, 204 (1800). It was 186? before the compound was
used as an initiating agent, when Alfred Nobel invented the bleating cap and used mercury ful-
minate to detonate nitroglycerin (British Patent 1345 (1867)).
Destruction by Chemical Deccsposltlcn:
Mercury fulminate is decomposed by adding it, while stirring, to at least 10 times its
weight of 200 sodium thiosulfate. Some poisonous cyanogen gas may be evolved.
References:43
(a) Ph. Hacum - Z ges Schies^-Sprengstoffv (Munich), pp. 181, 229, 267 (27 June 1932).
(b) F. V. Brown, D. H. KUsler, and F. C. Gibson, Sensitivity of Explosives to Initiation by
Electrostatic Dlschargas, U. S. Dept of Int, Bureau of Mines, fu 3852, l£t6.
43Sea footnote 1, page 10.
204
Mercury Fulminate
AMCP 706-Щ
(c) Alao see the following Pl cat tinny Areenal Technical Report» on Mercury Fulminate:
0 2 1 4 1 6 I 8 1
250 Э01 132 23 144 65 266 277 28 199
480 381 4 52 203 294 105 366 297 78 609
510 561 522 393 53b 255 556 407 278 749
550 1651 582 433 624 285 566 537 318 849
610 782 833 694 365 866 567 788 999
6bo 882 U83 784 415 986 637 1838 1079
760 932 1393 874 425 1316 857 1389
1220 1192 2093 1104 1325 1486 1737 2179
1450 1352 1365 1556
1375 2146
1722
2032
275
AMCP 706-177 Metriol Tri nitrite (MTW) Liquid (or Trime*hylolethane Trinitrate)
% C 23.5 02«0—CH2 H 3-5 л Molecular Weight: 'C^HgN^O^) 255
Oxyge leleets: CO-. % CO % -35 - 3
02N0—CH2 И 16.6 У - C — CH j Deaeity: gm/cc Liquid 1.1»7
o„no—сн9 0 56.1» 2 2 Mehiag Point: ’C -3
C/H Ratio 0.150 Froeaiag Point: *C
Impact Sermitivity, 2 Kg Wt: Buraou of Mine* Apparatus, cm V; (1 lb wt) U Sample Wt 20 mg Picatinny Arsenal Apparatus, in. Sample Wt, mg 20 BeUbg Paint: *C
Refractive Index, nL n£ П» 1.1»752
Длд!*. m— л. a. -- 0^W» Steel Shoe Fiber Shoe Explode* Vacuum Stability Tost: cc/40 Hrs, at 90’C 100"C cc/ga 1.9 120'C 135’C 150’C
ШЯе Betiet Impact Teat: Triols % Expiation* Partial*
Burned Unaffected 200 Gram limb Sand Teat: Sond, gm ^3-7
Expleeiea Temperature: ’C Seco.ds, 0.1 (no cop used) 1 5 Ignite* 235 10 15 20 Seetitivity fa laitietiaa: Minimum Detonating Charge, gm Mercury Fulminate Lead Azide Tetryl
BaNietic Mortar, % TNT: (a) 136
Trauxl Teet, % TNT: (b) 1UO
75 *C latere etiaeel Meet Test: % Los* in 48 Hrs
Plate Doot Teat: Method
100'C Hee* Test: % Low, 1st 48 Hrs % Um, 2nd 48 Hrs Expiation n 100 Hrs 2-5 1.8 None Condition Confined Density, gm/cc Brisonce, % TNT
FleoMnability Index: Detmratiaw-Bata: Confinement Condition Charge Diameter, in. Density, gm/cc Rote, meters/second
Hygroscopicity: % 30°C, 9O£ RH 0.07
Volatility: 60°C, mg/er.^/hr 21»
206
Me trio! Trinitrate (MTU) Liquid
AM CP 706-177
r~— ' FiegnwalwHea Test: Shaped Charge IHoeHeonaee, TNT = IMt
W яма HI. Ю1 МйсНк Lot WC-M: Density, gm/cc Chorge Wr, № Gass Cones Steel Cones Hole Vol' ime Ной Depth
Total No. of FrogownN: For TNT «•••« Oily, slightly turbid
For Subject HE 3 Mi HE, M4M1 PreiecHte, Lee KC-3: Density, gm/cc Charge Wt, lb
PiMpel Uses: Ingredient of rocket end double base propellants
Total No. tf FraguMoH: For TNT ForSubiettHE Method ef Leading:
Loedieg Density: gm/cc
hijai* VeteoTy: ft/sec At 9 ft At 25% ft Density, gm/cc
Storage: Method Liquid
Meet (Motive to TNT): Hazard Ckes (Quantity-Distance)
Ain Peck Pressure Impulse Energy Compatibility Group exudation
Air. Confined: Impulse Solubility in Water, gm/100 gm, at:
Under Water: Peak Pressure Impulse 25°C 60°C Eeat of; <0.015 <0.015
Energy Combustion, cal/gm 2642
Peck Pressure impulse Energy Hydrolysis, jb Acid; 10 days at 22°C 5 days at 60°C 0.018 0-115
207
АМСР 706-177
Metriol Tri nit,-ate (MOT) Liquid
Preparation:
Metriol (trimethylolmethylmethane) is obtained by the following procedure, baaed on work
by Hosaeuc (Annalen 276, 76 (1893):
Into a $ liter round bottom flask l.a weighed 2700 gms of water. To thia are added 267 got
of formaldehyde and 60 gms of propionaldehyde. The mixture la stirred for a few seconds.
TO the mixture la added 150 gms of calcium oxide previously slaked with бСО gms of water.
The mixture la heated In boiling water for four hours, and then allowed to cool spontaneously
overnight. After filtering off the insoluble calcium hydroxide, the solution la heated and
treated with a saturated aqueous solution of oxalic acid to precipitate all the calcium. Ths
precipitated calcium oxalate la filtered off, and the pale-yellow filtrate concentrated aa
ouch aa possible on the steam bath to a thick lemon-yellow syrup. After dissolving in abso-
lute alcohol, the solution is filtered and concentrated in the steam beth to about twice the
voirjm. of the concentrated syrup. The aolutlon is then chilled in a cold box to hasten cry-
stallization. After allowing it to warm up to just above 0°C, the mixture is filtered. The
resulting product Is not sufficiently pure and is recrystallized from absolute alcohol. OTe
melting point of the product (40. 3 gm) is then about 19t>°C (Hosaeus gives 199°C).
Me tricl is nitrated by carefully mixing it with 3*5 parts of 65/35 HNOo/HoSO^ maintained
at 20°C, stirring for 30 minutes, cooling to 5°C, and pouring the reaction mixture on Ice. It
is extracted with ether, water-washed, and adjusted to pH 7 by shaking with 3 sodium bicar-
bonate solution and again water-washed three times. It is then dried with calcium chloride,
filtered, and freed 01* ether by bubbling with dry air until minimal ret a of loss In weight is
attained. TL- yield is 88$ of the theoretical. The product has a nitrate-nltrogen contact
of 16.35$ (calc listed: 16.47$). Its refractive index at 25°C ie 1.4752.
Origin:
MOT, according to Italian sources, va« first prepared and patented by Bombrlnl-Parodi-
Delfino Cospany of Italy unucr the name "metriolo." A German Patent of 1927 also describes
the preparation and gives some properties. This compound was known in Trance before World
War II under the name of "Hitropentaglycerin" and Burbot and Thomas determined its beet of
combustion (Ref b).
References:
(a) A. H. Blatt, Compilation of Data on Organic Explosives, OSRD Report Ho. 2014,
29 February 1944.
(b) E. Bvrlot and M. Thomas, Mem poudr 29, 262 (1939).
(c) Also zee the following Picatlnny Arsenal Technical Reportn on Metriol Trinitrate:
1616 and 1817.
‘‘‘‘Set footnote I, page 10.
206
Mlnol-2
AMCP 706-177
Comptsifioa: OL Meleceler Weight: 71
TO Anuonluvi Nitrate 40 тат bO Aluminum 20 Oxygen Bnleaco: CO- % CO % 1 8
Density: gm/cc 1.62-1.68
Melting feiet' "C
C/H Rotio Freesing Point: *C
Impost SeneMvIty, 2 Kg Wt: Bureau of Mine* Apparatus, err Sample Wt 20 mg Picatinny Arsenal Apporatut, in. Sample Wt, mg Bailing Point: *C
13 17 Refractive Index, n“ n£ i»£
FlMVBWO 1ВВП Steel Shoe Fiber Shoe Vecanm Stability Teat: cc/40 Hrs, ot 90°C 100‘C 120’C 135’C 150’C
Rifle Ballet Impact .it: Tnil* % Expiations Partials 2.1
Burned Unaffected 200 Grew Bomb Send Tost: Send, gm
Тмярвгаймве 'С Seconds, 0.1 (no cap used) 1 S Ignites U35 10 15 2f » ImldiebLmt fWHVITWy W imWsWHi Minimum Detonoting Charge, gm Mercury Fulminate Lead / side Tetryl
BeUistic Mortar, % TNT: (a) 14 3
Trend Test, % TNT: (b) 165
78’C lateiaetisaol Heet Test: % Lou in 4B Hrs
Plots Dent Test: (c) Method В
100’C Hoot Teet: % Lou, 1st 48 Hrs % Lou, 2nd 48 Hrs Explosion in 100 Hrs Condition Confined Density, gm/cc Brisance, % TNT Pressed No 1.73 66
100 и^ВОЯВВ^^МВ ftBVBe Confinement Condition Chorga Diameter, in. Density, gm/cc r .e, meters/second Kone Cast L.6
ее Az • TO
ValetWty: Lorjj 5820
2(Л»
АМСР 706-177
Minoi-г
Bomtov Sot Txt: (e) Condition Pressed Tetryl, gm 100 Wax, in. re* 50% Detonation 1.U6 Wax J.'n i tensity, gm/cc l*7b Oxygen, otoms/sec (Z/sec) -Heat, kilocolorie/mole (AH, kcal/mol) Temperature Range, *C Phase
Heel of: (f) Combustion, cal/gm 3160 Explosion, rcl/gm 1620 Gas Volume, cc/gm Formation. col/gm Fusion, Cal/5 Armor Plate Impact Test: (f) M mm Merfer Projectile: 50% Inert, Velocity, ft/sec 828 Aluminum Fineness SM-* GonereJ Purpose limbi: Piole Thickness, inches 1 H* 1%
Sped*** Heat: «.ut'pn ' C At -Д’ 0.30 Density; gm/cc 1.7b
Burning Kale: cm/sec
limb Drop Test: 17, 200C-lb Somi-Armor Pierciag Bomb vs Cinrrsts: ix Safe Drop, ft SOO-lb -somc.'I Purpose Bomb vs Concrete: Heigh*, ft T rials Unaffected Low Order High Order lOCNMb GeiMrel FwpoM tomb yj Семга**; Height, ft Triols Unaffected Low Order High Order
Theimul Conductivity: (b) _i col/sec/cm/’C 16.5 x 10 ” Density, gm/cc l.'fb
Coefficient of ExpaMien: Linear, %/‘C Volume, %/"C
Hardness, Mohs' Scale:
Taung's Modulus: (b) E', dynes/cm3 5-03 x 10 E, Ib/inch3 0.73 x 106 Density, gm/cc 1.66
Compressive Strength: Ib/inch3 (b) 1910-2070 Density, gm/cc 1.68
Veper Praeenra: •C mm Mercury
210
mnoi-2
АМСР 706-177
FregaseatMlea Teet:
Км WI, МП ЬфеМе, Let WC-91:
Density, gm/cc
Charge Wt, lb
Tetd Mo. ef Frsgiasats:
For TNT
For Subject HE
3 tech HE. M42A1 NejocHte. Let KC-S:
□entity, gm/cc
Chorge 'Vt, lb
Ы* *4
^PT vv^P^^Vv^N^vvc
For TNT
For Subject HE
Sboped Otetge IffectteeaeM, TNT = i&t
Glass Cones Steel Cones
Hole Volume
Hole Depth
Cater: Gray
Principal Uses: Boob в and depth charged
hAetbed el Loediog: Cast
Leediag Doecity: gm/cc 1.62-1.68
Fregmiet Velocity: ft/sec
At 9 ft
At 254 »t
Density, gm/cc
Mett 'ЬкНчъТМП:
Air:
Peek Pressure 115
Impulse 116
Energy 133
Air, Coafteod: Impulse 90
Under Water: Pack Pressure 108
Impulse 126
Energy 110
Underground: Peek Pressure 131
Impulse 139
Energy 14?
Method Dry
Hazard Class (Quantity-Distance) Class 9
Compatibility Group Group I
Exudation
Preparation:
Mlnol is a castable mixture consisting of
40 percent HIT, 10 percent annonlum nitrate,
end 20 percent powdered aluminum and there-
fore can be prepared by adding the dry in-
gredients to molten W at 903C under agita-
tion. Mlnol also can be prepared by adding
25 parts of aluminum to 100 parts of 50/50
amatol previously prepared.
211
:*мср/о«-П7
mnoi-2
Origin;
Minols are British ternary explosive* developed during World War II. There are three for-
mulations:
Composition, jt; Minol-1 Minol-2 Minol-3
ТЯТ 18 10 12
Aancnium Nitrate 12 10 38
Aluminum 10 20 20
References:
(a) L. C. Smith and E. G. Eyster, Physical Testing of Explosives, Part LU - Miscellaneous
Sensitivity Tests; Performance Tests, OSRD Report Ko. 5tw>, 2? December $•
(b) Philip C. Keenan and Dorothy Pipes, Ihble of Military High Explos'ves, Second Revision,
WORD Report Ko. 87-16, 26 July 19*^6.
(c) D. P. MacDougall, Methods of Physical Testing, OSRD Report No. 803, 11 August 1912.
(d) G. H. Messerly, The Bate of Detonation of Various Explosive Compounds, OSRD Report No.
1219, 22 February 19^3-
M. D. Hurwitz, The Bate of Detonation of Various Compounds and Mixtures, OSRD Report
No. 5611, 15 January 19>Яь
(e) L. C. Smith and S. F Walton, A Consideration of KDX/Wax Mixtures as a Substitute for
Tetryl in Boosters, NOL Memo 10,303, 15 June
(f) Coemittee of Div 2 and 8, NDRC, Report on HBX and Tritonal, OSRD No. 5I06, 31 July 19^5*
(g) W. R. Tomlinson, Jr., Blast Effects of Bomb Explosives, PA Technical Div Lecture,
9 April 1Л8.
(h) Also see the following Picatinny Arsenal Technical Reports on Minol-2: 1585 and 1635-
^See footnote I, page 10.
212
МОХ-1
АМСР 706-177
Г--»пГ1-. Oxidizing agent (Ammonium Perchlorate) Aluminum, atomized Cupric Oxide Magnesium, atomized Other ingredient (Tetryl) Calcium Stearate Graphite, artificial C/H Rotio Metacotar Weight: LO.6
35.0 26.2 Oxygen Botaoce: CO, % CO % 1 8
26.2 9.7 1.9 1.0 Density: gm/cc Pressed 2.0
Melting Point: °C
Freezing Feint: *C
taipact ScaritMty, 2 Kg Wt: Bureau of Mines Apparatus, cm Sample Wt 20 mg Picatinny Arsenal Apparatus, in. Sample Wt, mg Boiling Feint: *C
13 22 1«4«Жр Па» Пп п»
mCoQw vwOBW^^^^O 1 Wee Steel Sloe Fiber Shoe De tonetea Unaffected Vecmnn Stability Teat: cc/40 Hrs, ot 90'C 100’C 120 °C 135*C 150*C 0.4?
Rifle Bniht Impact Teet: Trials % Explosions Partials
Burned Unaffected 200 Gram Bomb Send Teet: Sand, gm 10.6
RspMen Tompeeotete: *C Seconds, 0.1 (no cap used) — 1 5 285 10 15 20 Sensitivity te Inltietien: Minimum Detonating Charge, gm Mercury Fulminate Lead Azide Tetryl 0.20 0.25
BalNetic Mortar, % TNT:
Trend Teet, % TNT:
75*C IMernatisnal Heel Tezt: % Loss in 48 Hrs Diecoloration, fuues, odor
None Plate Dent Test: Method
100'C Hoot Test: % Loes, 1st 48 Hrs % Loes, 2nd 48 Hrs Explosion in 100 Hrs 0.10 0.01 None Condition Confined Density, gm/cc Brisance, % TNT
Flammability tadex: ROTwe Confinement Condition Chorge Diameter, in. Density, gm/cc Rote, meten/second
Hygroocepicky: %
Volatility:
213
АМСР 706-177
МОХ-1
Fregnioatetiea Teeh Shaped Charge Iffocttvenem, TNT = 160:
90 ем» НЕ. МП hejectHe, U» WC-91: Density, gm/cc Charge Wt, lb y' Glass Cones Steel Cones Hole Volume Hole Depth
Total Ns, ef Ire|n»ti: For TNT For Subject HE 3 tach HE. M42A1 Projectile, Lot KC-S: Density, gm/cc Chorge Wt, lb Color: Gray powder mixture
Principal Um»: small caliber antiaircraft projectiles
Total Ns, of Fragment»: For TNT For Subject HE Method of Leading: Pressed
Leading Фомку: gm/cc
Fragment Velocity: ft/sec At 9 ft At 25% ft Density, gm/cc At 30.000 pel 2.0
Storage: Method Dry
Mast (Relative to TNT): Hozord Closs (Quantity-Distance) Class 9
Air: Peek Pressure Impulse Energy Compatibility Group Group I Bureau ef Explosives Classification Class A Exudation
Air, Confined: Impulse Under Water: Peak Pressure Impulse Energy Underground: Peak Pressure Impulse Energy Heet of: Combustion, cal/gm Explosion, cal/gm Gas volume, cc/.yi Performance Tests: 20 am T21$E1 Projectile; IIFOC Pressure Cube APG Blest Cute Activation Energy: kcal/mol Temp, C Time to ignition, seconds эос 1.78 1*087 2087 212 35 1*0 12.5 to 38p x 10"4
214
М0Х-2В
AMCP 706-177
Семроокюа; % Oxidizing agent (Aoeonium Perchlorate) Altainua, atoilzed Cupric Oxide (tegneiiua, atoaized Other Ingredients* Calciua Stearate Graphite, artificial *5.8,1 КЗ and 3.9JI MT cootedyg Mohceler V-t’ht: 42
35-0 52Л Oxygon Balance: CO, % co % -4?
Density: gm/cc Pressed 2.0
1.9 1.0 Making Mat: ‘C
В 1 Ж - * . » 9 S' orWBNB^ vVliwo
hapect Sonoi^vity, 2 Kg Wh Bureau of M’nes Apparatus, cm Sample Wt 20 mg Picatinny Arsenal Apparatus, in. Sample Wt, mg Bailing Point: "C
12 24 Refractive Index, n* n£ n£
"В^БЖЮТ •1 VSe a Steel Shoe Fiber Shoe Unaffected Unaffected Vacua» Stability Toot: cc/40 Hrs, ot 90*C 100’C I2O"C 135’C 150"C 0.21
Rifle Ballet Impact Teet: Trials % Explosion:> Partials
Bur ied Unaffected 200 Gram Booth Saad Test: Sand, gm 11.5
txploeien Trmpcratora: 'C Seconds, 0.1 (n cop used) — 1 5 375 10 15 20 Sensitivity to Initiation: Minimum Detonating Charge, gm Mercury Fulminate Load Azide Tetryl 0.20 0.20
BaNiotic Mortar, % TNT:
Trarul Toot, % TNT:
П C Intemotionol Hoot Test: % Loes in 48 Hrs Diocoloration. fUaeo, odor
None Plate Dent Toot: Method
100‘C Hoot Tool: % Loes, 1st 48 Hrs % Loss, 2nd 48 Hrs Explosion in 100 Hrs 0.27 0.12 None Condition Confined Density, gm/cc Brisance, % TNI'
- Detonation Rote: Confinement Condition Charge Diometer, in
Flammability Index:
Hygroscopicity: %
Volatility: Density, gm/cc Rats, moters/soconc'
215
АМСР 706-1Т7
М0Х-2В
Shaped Charge Effectiveness, TNT = 100:
•0 мм HI, МУ1 ProjecMIs, LM WC-91: Gloss Cones Steel Cones
Dantity, gm/cc Hole Volume
Charge Wt, lb Hole Depth
Tefal No. ef FMgaMOte: For TNT Cote: Gray
For Subject HE
Principal Uses: HE filler for stell caliber
1 loch HE, M42A1 ProjocHte, Let KC4: project!lea
Density, gm/cc
Chorge Wt, lb
Total Me. ef Fragments: Method ef Lending: Pressed
For TNT
For Subject HE
Loading Density: gm/cc 2.0
Fregawet Veledty: ft/мс
At 9 ft
At 2514 ft Stwegt:
Deralty, gm/cc
Method Dry
Meet (Motive to TNT): Hazard Class (Quantity-Distance) Close 9
Ain Bare Charge: EW* EV* Compatibility Group Group I
Pook Pressure 1.02 1.34 Bureau of Explosives Class A
Impute 1.08 1.41 Exudation None
Energy
Density, gm/cc 1.96
Heat of:
Air, Confined:
Impute Combustion, cal/gm 4.-84
Explosion, cal/gm 14','?
Cased Charge in Air:** Gas volume, cc/gm 22
Pook Pretsure 1.09 1.44
Impute 1.16 1-5? Performance Tests:
BNtey So min T21^E1 Projectile:
Denaity, gm/cc I.98 IiTOC Pressure Cube 29
Uadorgretrnd: APG Blast Cube 30
Peek Pressure
impute Aviation Energy:
Energy Itcal/raol 7.6
*EW, equivalent weight as compared to TNT; Temp, °C 340 to 470o
Ev, equivalent volume aa compered to TNT. Time to Ignition, seconds 1.39 x 10’2
♦•Strong paper-base phenolic case.
216
М0Х-2В
АМСР 70< >-177
Effect of Altitude, Charge Diameter and Degree of Confinement on Detonation Velocity»
(Reference g)
Simulated Altitude, Peet One-Inch Column Confined Unconfined Two-Inch Column Confined Unconfined
m/s m/a n/B П/В
Ground 30,000 60,000 90,000 Average Charge would not j prorogate detonation, i 4730 *♦530(3) 4430 4290 Charge would not propa- gate detona- tion.
41*95
«Confined charge in 1/4" steel tube, AISI 1015 seamleca, 1" diameter 18" long, and
2" diameter 7" long. All means were determined from sets of five value» unless
otherwise Indicated by ( ). A 26 gm tetryl booster was used to initiate each charg:.
Average Fragment Velocity at Various Altitudes» (g)
Explosive J Charge Diameter, I Inches । - Slbiuleted Altitude, Feet
Ground 30,000 bb,oob ,
m/s j /• m/s i Vs 1
MDX-2B, 1 1 2012 I *H* ** *» 1
density, gm/cc 207 2 3514 3351 3247 ! **
«Outside diameter 2.54"; inside diameter 2.01*"; length 7".
••Charge would not propagate detonation.
217
AMCP 706-177
мох-зв
Competition z Oxidizing agent (potassium Nitrate) 18 Alumiuum, atomized JO Cupric Oxide Magnesium, atomized Other ingredients* 32 Calcium Stearate»* 2.0 Graphite, artificial** 1.0 *29-15l RDX, 0.9$ wx, and 2.0$ TNT. **Per cent ailed. Molecular Weight: 45.6
Oxygon Balance: CO-.- % .52 CO % -43
Density: gm/cc Pressed 2.0
Making Point: °C
Freezing Point: °C
Impact Seaekivity, 2 Kg Wt: Bureau of Mines Apparatus, cm Sample Wt 20 mg Picatinny Arseno1 Apparatus, in. 17 Sample Wt, mg 24 Boiling Feint: °C
Refractive Index, n° Пм П»
Friction Pendulum Test: Steel Shoe Unaffected Fiber Shoe Unaffected Vacuum Stability Toot: cc/40 Hrs, ot 90°C -— IOO°C 0.57 120°C 135’C 150°C
Rifle Ballet Impact Test: Trials % Explosions Partials Burned Unaffected
200 Great Bomb Saad Test: Sand, gm 33.2
Fsplnsiea Temperature: 'C Seconds, 0.1 (no cap used) —- 1 5 540 10 15 20 Sonokhrity to Initietiea: Minimum Detonating Charge, gm Mercury Fulminate - Load Azide 0.20 Tetryl 0.15 Ballb.L- Mortar, % TNT:
Treed Teet, % TNT:
75°C International Hoot Toot: % Loss in 48 Hrs Discoloration, fumes, odor None
Plata Dent Toot: Method Condition Confined Density, gm/cc Brisance, % TNT
100’C Heat Toot: % Loss, 1st 48 Hrs 0.35 % Lou, 2nd 48 Hrs 0.13 Explosion in 100 Hrs None
Detonation Roto: Confinement Condition Charge Diometer, in. Density, gm/cc Rote, meters/se-xmd
Flammability Index:
Hygroscopicity: %
Volatility:
218
мох-зв
АМСР 706-177
Shaped Сйф Effectiveness, TNT - 100:
90 mm HE. M71 Projectile, Let WC-01:
Density, gm/cc
Charge Wt, lb
Gloss Cones Steel Cones
Hole Volume
Hole Depth
al Kemtama^o* В vWVe VT • For TNT For Subject HE 3 lack HE, M42A1 Projectile, Lot KC4: Density, gm/cc Charge Wt, lb Total No. of Fragments: For TNT For Subject HE
Color: Gray powder mixture
Principal Uses: Small caliber antiaircraft projectiles
Method ef Lending: Pressed
Lending Density: gm/cc At 30,000 psi <v2.0
Pre gm ret Velocity: ft/sec At 9 ft
At 254 ft
Density, gm/cc
Method
Dry
Blest (Raletive to TNT):
Hozord Clou (Quantity-Distance)
Claes 9
Peek Pressure
Impulse
Energy
Compatibility Group Group 1
Bureau of Explosives Class A
Ab, Confined:
Impulse
Heat of:
Under Water:
Peak Pressure
Impulse
Energy
Pook Pressure
Impulse
Energy
Combustion, cal/gm
Explosion, cal/gm
Gas volume, cc/gm
Performance Tests:
20 mm T215EL Projectile:
NFOC Pressure Cube
APG Blast Cube
Activation Energy:
kcal/mgl
Temp, C
Time to ignition,
seconds
*♦331
900
232
37
52
not Included
erratic ig-
under condl-
Values
due to
nition
tlons of test.
219
АМСР 706-177
MOX-1*B
Crnyiiltlsn. Oxidizing agent (Barium Nitrate) 18 Aluminum, atomized 50 Cupric Oxide Magnesium, atomized Other i ngredients* 32 Calcium Stearate»* 2.0 Graphite, artificial** 1.0 *29.15l RDX, 0.951 wz, and 2.0* 1ЯТ. **Per cent added. Molecular Welpm : Mj
Oxygen Balonco: CO, % ~53 CO % -^3
Daneity: ф ,'cc Pressed 2.0
Making Pein”: ’C Freesir? Point: °C
Impact Seaaitivity, 2 Kg Wt: Buraou of Mine* Apparatus, cm 78 Sample Wt 20 mg Picatinny Arsenal Apparatus, in. 18 Sample Wt, mg 26 Boding PaW: °C
Refractive Index, n» Пт n“
Friction Pendulum Vest: Steel Shoe Sparks Fiber Shoe Unaffected V OCUwIR MowWBRTtjF 1 a cc/40 Hrs, ot 90°C IOO°C J.67 I2O°C 135’C 150°C
Rifle BoNet Impact Teat: Trials % Explosions Partials Bur.ted Unaffected
200 Gram Bomb Sand Teet: Send, gm 33.6
tephiiee Temperature: °C Seconds, 0.1 (no cap used) --- 5 610 10 15 20 Sensitivity to Initietien: Minimum Detonating Charge, gm Mercury Fulminate Load Azide 0.20 Tetryl 0.15
BeWetic Mortar, % TNT:
Treuzi Teet, % TNT-
75‘C International Meet Teet: % 1 '.4 и 41 Hrs Discoloration, furaes, odor None
Plato Dent Teet: Method Condition Confined Density, gm/cc Brisance, % TNT
100°C Heat Tee»: % Lou,' st 48 Hrs 0.22 % Lou, 2nd 48 Hrs 0.12 Explosion in 100 Hrs None
^^еФе^ВеФбФЯВ ^^фффе Confinement Condition Chorge Diameter, in. Density, gm/cc Rate, meters/second
Flammability .adex:
Hygroscopicity: %
Volatility:
220
МСХ-ЬВ
AMCP 706-177
| Fragmentation Tost: 90 ома HL МП he|octi . Lot WC-91: Density, gm/cc Charge Wt, lb Total No. of Fn^taeafs: Fur TNT For Subject HE 3 lack HL M42A1 Projei * Ut KC-S: Density, gm/cc Charge Wt, lb Total No. of FiogaMata: For TNT For Sub,ret HE Shaped Charge effectiveness, TNT = 100: Gloss Cones Steel Cones Hole Volume Hole Depth
Colon Gray powder mixture
Priacipei Use»: Small caliber entlaircraft projectties
McAod of Veoavsp: p.-essed
Loodiag Density; gm/cc
FragaMat Velocity: ft/sec At 9 ft At25H ft Density, gm/cc At 30,000 psi 0
Method 2r;, Hozord Close'Quantity-Distance) Clscs e Cenpct'bility G—up Group I Bureau of Explosives Class A
Met. (Koiotivo fa TNT): Ain Peak Pressure Impulse Energy Air, Ceafiaad: Impulse Uador Woion Peak Pressure Impulse Energy Underground: Peak Pressure impulse Energy
Heat of: Cocu-uotlon, cal/z.:; 1*392 Explosion cr.l/gm 709 Gas volute, cc/gm 208 Performance Tests: 20 an T21$E1 Projectile: KFOC Pressure Cube APG Blest Cube ^3 Aviation Energy кcal/mol Values not included Temp, °C due to erratic Ignl- Time to ignition, tlor. under conditions seconds of test.
221
АМСР 706-177
% Oxldlx'.r^ egent —- Aluminum, atomized 49.2 Cupric Oxide 19.7 .‘tagneaium, at. mi zed Other ingredients* 29.6 Calvlum Stearate —- Molecular Weight: 43 !
Osygen Boleace: CO.. % -50 CO % .42
DeasHy: gm/cc
Graphite, artificial 1.J *28. (% RDX coated, wax. C/H Ratio Making Point: °C
Freezing /oint: °C
Impact Sonsitioity, 2 Kj Wt: Bureau of AAines Apparatus, cm 7o Sample Wt 20 mg Picatinny Arsenal Apparatus, in 19 Sample Wt, mg 27 Boiling Point: °C
Refractive ladex, n» Па П»
Friction Psndslem Test: Steel Shoe Unaffected Fiber Shoe Unaifected Vacuum Stability Test: cc/40 Hrs, ot 90°C —- 100°C 0.43 )20=C I35°C 150’C
Rifle Bullet Impeet Test: Trie Is % Explosions Portiols
Burned Unaffected 200 Grom Bomb Test: Send, gm 10 > 8
Explosion Tempe.ata re: ’C Seconds, 0.1 (no cop used) — 1 ... 5 510 10 15 20 Sensitivity to Initiation: Minimum Detonating Chorge, gm Mercury Fu.ninate .... Lead Azide 0.20 Tetryl 0.16
Ballistic Mortar, % ' 1- 7:
Treuzl Tost, % TNT:
7S‘C intamatioaol Heat Test: Loss in 48 Hrs 0.02/10 gm Oilcoloratlon, fumes, odor None
Plate Dent Test: Method
IN C Heat Test: 4> Loss, 1st 48 Hrs 0.00 4 Loss. 2nd 48 Hrs O.CXj Explosion ,n 100 Hrs None Condition Confined Density, gm/cc Brisance. % TNT
Deteaettai life: Confinement Condition Chorge Din*. ieter, in. Density, gm/cc Rote. :.Aet«fs/second
Flammobility lades:
Hygroscopicity: % jO’-'C. 90^t ЙН, two week: 0.79
Volatility:
мох-бв
АМСР 706-177
Y^a*. *9 mm НС, МЛ Projectile, Ut WC-91: Density, gm/cc Charge Wt, lb Total No. of Frogmoats: For TNT For Subject HE 2 tach HI. M42A1 Projectile, Let KC-S: Density, gm/cc Charge Wt, lb Total No. of Fragments: For TNT For Subject HE Shaped Charge EHecthreoeu, TNT = 100: Glass Cones Steel Cone* Hole Volume Hole Depth
Color: Gray powder mixture
Frtacipal Usa: Smell caliber antiaircraft projectiles
Method of Leading: Pressed
Loading Daneity: gm/cc At '«0,000 psi ~2.0
Fragment Velocity: ft/sec At 9 ft At 254 ft Density, gm/cc
Storage: Method Dry Hazard Class (Quantity-Distance) Class 9 Compatibility Group Grc P 1 Bureau of Explosives Class A
taC (Kotatr.eHTNT): lir. Peak Pressure Impulse Energy Air, Confined: Impulse Under Water: Peak Pressure Impulse Energy Undergraend: Peak Pressure Impulse Energy
Heat of: Combustion, cal/gm 1*293 Explosion, cal/gm 750 Ibb volume, cc/gm 204 Activetion Energy: kcsl/mgl Values not included emp, C due to erratic igr.l- ."!me to Ignition, tlon under conditions seconds of test. 1
AMCP 706-177
MOX-1; MOX-2B; МОХ-ЗЬ; МОХ-bB; МОХ-бВ
Preparation:
We various ingredients used in the preparation of MOX explosives are coated separately as
follows:
Di chromated Atomized Aluminum - Seventy-five grams of chemically pure grade sodium dichro-
ma te is dissolved in 1300 ml Hi 11terв of water at 100°C under mechanical agitation. Six hun-
dred grams of the atomized aluminum powder is added gradually (2 to 3 minutes) and stirring
is continued for half an hour. Ihe dichromated metal is filtered, washed with water (13 to 20
times) until the washings show only a slight cloudiness with silver nitrate, the water-vet
product is then dried in an oven at 30°C. Ihe dried material is hand-rolled to reduce any
conglomerates, and blended before use-
Wax-Coated RDX - Eighteen grams of molten Be Square Special Wax (manufacturer - 100° to
185° Fahrenheit grade amber) is added to 582 grams of finely divided RDX (water precipitated
from acetone solution) in a water slurry under mechanical agitation. The temperature of the
vex-RDX slurry is maintained above the melting point of the wax (about y0°C). The stirring
is continued for half an hour. After cooling to 5O°C, the wax-coated RDX is recovered by
filtration in a Buchner funnel and dried tn air. The RDX thus coated and presumed to be 3^
waxed RDX or a 97/3 RDX/wax mixture is hend-rollsd to crush any conglomerates formed, and
blended by hand before use.
ИТ-Coated Barium Nitrate - Thirty grams of HIT in alcohol solution is added to 270 grams
of barium nitrate in an alcvhol slurry under agitation. Tha temperature of the TNT-barium
nitrate mixture is maintained ut 80°C and stirring is continued until most of the alcohol is
evaporated. The coated material ie spread in a thin layer on a tray to dry in air overnight.
He barium nitrate thus coated with lOft TNT is reduced to an intimate mixture by hand-rolling
ard blending before use.
TNT-Coated Potassium Nitrate - The ИТ-coated potassium nitrate is prepared by the same
procedure as is used for coating barium nitrate.
TIDX/TNT-Coated Anmcnium Perchlorate - The ammonium per chic ue is coated by dissolving the
appropriate weights of RDX and ИТ in hot > 'tobol. After add.. tha ammonium perchlorate,
the ’’ rry is stirred until most of the solvent, .s evaporated. The treated ammonium perchlo-
rate spread, on a tray to dry overnight. Agglomerates formed during the process are crushed
by band-rolling end blending ths mixture before use.
HT-Coated RDX - Sixty grams of rolten TNT are added to s water slurry of 5**0 gram.' of
finely divided RDX Iwater precipitated from acetone solution) under mechanical agitation.
We temperature of the TNT-RDX elurry is maintained at about 90°C and stirring is continued
for half an hour. Alter cooling to about 50°C. the ИТ-coeted RDX is recovered by filtration.
The RDX thus treated, and presumed to be lOJt coated or a 90/10 RDX/TNT mixture, ie further
blended by hand after rolling to crush any aggregates formed during the process.
'Ле MOX explosive mixtures are prepared by blending the appropriate weigh's ol the dry
Ingredients ir. a Patterson-Kelly twin-shell blender for at lerst 30 minutes.
Origin:
MOX tyne explosive m.ix’-res were developed beginning in 1950 by National Northern, techni-
cal division of the National Fireworks Ordnance Corporation, West Hano’.er. Masraohusetts.
,‘Ю.<-1; М0Х-2В; МОХ-ЗВ; МОХ-1В; МОХ-бЗ
АМСР 706-177
References:^1
(а) А. 0. Mire-chi and А. Т. Wilscn. Development of МОХ Explosives for Improved 20 стп
Amunition, Navy Contract NOrd-10975, Teak 1, National Fireworks Ordnance Corporation; First
Yearly Summary, August 1950 to August 1951.
(Ъ) A. T. Wilson, Development of MOX Explosives: Various Oxidants in MOX, First Progress
Report NFOC-6, Navy Contract N0rd-123B2, National Fireworks Ordnance Corporation, December 1952.
(c) A. 0. Mirarcbi, Properties of Explosives: Theory of the MOX Explosion, First Progress
Report NF0C-10, Navy Contract NOrd-11393, National Fireworks Ordnance Corporation, December
1952.
(d) A. 0. Mimrchi, Properties of Explosives. MOX ^ L^aives in Various Atmospheres,
Firat Progress Report NFOC-9, Navy Contract NOrd-11393, National Fireworks Ordnance Corpora-
tion, 1952.
(e) A. T. Wilson, Development of MOX Explosives: Composition Variations, First Progress
Report NFOC-7, Navy Contract N0rd-li3bi, National Fireworks Ordnance Corporation, 1952.
(f) A. T. Wilson, Development of MOX Explosives: Various Oxidants in MOX, Second Progress
Report NFOC-14, Navy Contract NOrd-136M, national Fireworks Ordnance Corporation, October
1953-
(g) A. W. O'Brien, Jr., C. W. Plummer, R. P. Woodburn and V. Philipchuk, Detonation
Velocity Determinations and Fragment Velocity Determinations ox Varied Explosive Systems
and Conditions, National Northern Corporation Fins! Seminary 'eport NNC-F-13, February 1958
(Contract B3T19-O2O-5Ol-ORD-(P)-58).
(h) P. Z. Kalanski, Air Blast Evaluation cf M0X-2B Cased and Bare Charges, NAVORD Report
No. 3755, 5 April 1956.
(i) Also see the following Picatlnny Arsenal Technical Reports on MOX Explosives: 1935,
1969, 2201. 2205-
^See footnote 1 , p.
АМСР 706-177
Nitrocellulose, .g.6jb (NC)
Composition: Г / % ’но С 26.U6 ! . Н н 2>Й М Пн Г'Х N 12.60 X Н Н 0 >6.16 с-к J< X-ONOp 1 >< х L к _ n Molecolor Weight: (272.39)n
Oxygen Faience: CO.. % -35 CO % 0.6
Density: gm/cc
Malting Feint: *C
С/Н Ratio 0.23 Froexing Point: *C
Impact Sensitivity, 2 Kg Wt: Bureau nf Mines Apparatus, cm 8 Sample Wt 20 mg Picatinny Arsenal Apparatus, in. 3 Sample Wt, mg 5 Boiling Point: "C
Refractive Index, n» П?. n°
Fricciea Pendulum Toot: Steel Shoe E'ter Shoe Vacuum Stability Teet: cc/40 Hrs, ot 90“C 0.17 IC0"C 1.0 I2O°C 16 hours 11.+ 135'C 150‘C 200 Gram Bomb Sand Tost: Sand, gm 4>.0
Rifle Ballet Impact Toot: Trials % Explosions Portiols Burned Unaffected
Explode* Temperature: °C Seconds, 0.1 (no cop used) 1 5 Decomposes 170 10 15 20 Sensitivity to Initiatioa: Minimum Detonating Charge, gm Mercury Fulminate Lead Aside 0.10 Tetryl
Ballistic Mortar, % TNT:
Tratal Toot, % TNT:
7S*C InHmoHofMl Нм* Test: Lou in 48 Hrs
Plate Doot Toot: Method Condition Confined Density, gm/cc Brisance, % TNT
100'C Heat Teel: % Loss, 1st 48 Hrs % Loss, 2nd 48 Hrs Explosion in 100 Hrs
Detonation Roto: Confinement Condition Chorge Diomeler, in. Density, gm/cc Rote, meters/second
Flammability Index:
Hygroscopicity: % 30°C, 9C$ RH 3
Volatility: 60°C, n^/cn/v/hr 0.0
226
Nitrocellulose, 13.45j6 N (iic)
AMCP 706-1’7
% c 25.29 н 2.52 N 13-1*5 0 58.74 X-0N02 C/H Ratio 0.23 1 1 tv* 0 X—p / \ - ж ж X XX ! __i u Molocvler Weight: <r86-34)n
Oxygen Boknce CO- % -29 CO % 4.7
Density: gm/cc
МаШад Mat: °C Deconrpoeee
Freezing Point: °C
Impact Sensitivit,, 2 Kg Wt: Bureau of Mines Apparatus, cm 9 Sample Wt 20 mg Picatinny Arsenal Apparatus, in. 3 Semple Wt, mg 5 Boiling Point: *C
Fefracthre Index, ng ng ng
Friction Pondahim Test: Steel Shoe Fiber Shoe Vacaam . ' *4ty Toot: cc/40 H.s, at 50°C O’. 42 100*C 1.5 I2O°C 11.+ 135°C 150°C
Rifle Ballet Impact Tost: Triols % Explosions Portiols Burned Unaffected
200 Gram Boma Send Toot: Sand, gm 49.0
Explosion Temporaturo: °C Seconds, 0 1 (no cap used) 1 5 230 10 IS 2G Seasitivkty to Inttintkn: Minimum Detonating Choree, pm Mercury Fuhnin. ,• Lead Azide 0.10 Tetryl
Ballistic Mottos, % TNT: 125
Tread Tert, % TNT:
7S°C latoraetieaol Hoot Toot: % Loss in 48 Hrs
Plate tent Tort: Method Condition Confined Density, gm/cc Brisance, % TNT
1U0°C Hoot Test: % Loss, 1st 48 Irs 0. 3 % Loss, 2nd 48 Hrs 0,0 Explosion m 100 Hrs None
Ж —_ —nJ — _ B-*-, V^Tert^nrtrt "vTw к .onfinement Condition Chorge Diameter, in Density, gm/cc 1,20 Rate, melers/second 7300
flommohilrrz Index:
Hygrascopkity: % 30°C, 90/> ~ 2
Volatility: 6O°C, og/ca^/nr 0.0
227
AMCP 706-177
Nitrocellulose. ib.lbjt N (lie)
% f . C 2b. 25 H 2.3T И lb.lb 0 59-2U x-ono2 C/H Ratio 0.23 ‘1 C h 1 2I~SU Г"х x 1 H ° / \ A x J n Molecular Weight: (297• 15)n
Oxygen Balance: CO; % -2b О % 8
Density: gm/cc 1.65-1.70
Melting Point: °C Decomposes
Freezing Point: °C
Impact Sensitivity, 2 Kg Wt: Bureau of Mines Apparatus, cm 8 Sample Wt 20 mg Picatinny Arsenal Apparo’js, in. 3 Sample Wt, mg 5 Boiling Point: °C
Refractive Index, nJ nJ nJ
Friction PendahMi Teet: Steel Shoe Fiber She: Vocones Stability Tost: cc/40 Hrs, at 90”C l.b6 !00°C lb hours 11.+ l;T°C 16 hours Ц.+ 135 C I5O°C
Rifle Ballet Impoct Teet: Triols % Explosions Partials Burned Unaffected
20Э Crees Bomb Sand Toot: Sand, gm 52. 3
txpiosioa Temperature: ’C Seconds, 0.1 (nc cop used) 1 10 15 20 Sensitivity to Initiation: Minimum Detonating Charge, gm Mercury Fulmmo’e Lead Azide C.10 Tetryl
Ballistic Mortar, % TN F:
Trauzl Test, % TNT:
7S*C Intornatioaal Heat Teet: % Loss in 48 Hrs
Plate Dent Toot-. Method Condition Confined Density, fm/cc Brisance, % TNT
100 C Heat Teet: % Loss. 1st 48 Hrs % Loss, 2nd 43 Hra Explosion in 100 Hrs
Detonation Rote: Confinement Condition Charge Diameter, in Density, gm/cc Rate, meters/second
Flammability Index:
Hygroscopicity: % 30°C, 90S KH 1
Volatility: 60°C, mg/cm^/hr 0.0
228
nitrocellulose (NC)
АМСР 706-177
90 mm HI, M71 Projectile, Lot WC-91: Density, gm/cc Charge Wt, lb Total Na. ef FrogtaoeH: For TNT For Subject HE 3 inch HL M42A1 Projectile, Let KC4: Density, gm/cc Chorge Wt, lb TTet^tl ^ir^t^jasoate. For TNT For Subject HE Shaped Charge FWoctivenees, TNT = 100: Glass Cones Steel Cones Holo Volume Hole Depth
Color: Volte
Principal Usee: Pyroxylin (12jb N). blasting explosives; pyrocellulose (12.бО$ Л), smokeless powder; guncotton (13-35$ N minimum), propellants
Method ri hooding:
Lending Deaeity: gm/cc
Fragment Velocity: ft/sec At9ft At25ttf< Density, gm/cc
SttfVQQo Method Vet (8% to 3#> wa.nr) Hoiord Clou (Quontity-Distonce) Class 12 Compatibility Group Group M (wet) Exudation None
Meet (Kolotive to TNT): Ain Peak Pressure Impulse Energy Ab, Coafloci' Impulse Under Water: Peak Pressure Impulse Energy Underground: Peak Pressure Impulse Energy
Heat of; Combustion, cal/jm PU09* 2313»* 2226*** Explosion, cal/gm 855* 965** 1058*** Gas Volume, cc/gm 919* 883»* 853*** Fornatlon, cal/gm 617* 561«* 513*** * 12.6# N * * 13-^556 N * ** 1U.1U$ N Vapor Pressure: °C стп Mercury 25 0.00 60 0.00
229
АМСР 706*177
Nitrocellulose (NC)
Solubility in Water, gm/100 gm, at: 1£.6)6 h 13. b5* N 14.0» N
25°C Insoluble insoluble Insoluble
6o°c Insoluble Insoluble Insoluble
Solubility, gm/100 gm, 25°C, in:
Ether Insoluble Insoluble Insoluble
Alcohol Very slight- ly soluble Practlcslly insoluble Insoluble
2: l-Ether:Alcohol Soluble Slightly soluble (6)6-11)6) Practically insoluble (1 - 1)
Acetone Soluble Soluble Soluble
2b>Hour Hydrolysis Test, » Nitric Aci- 1.22 1.03
Preparation of NitrocelluloBe from Cotton Linters:
(laboratory Procedure)
Nitration: Second cut cotton linters, previously dried to a moisture content of 1е«:я than
0.5^, are nitrated by timers ion in mixed acid under the following conditions:
Ratio of Mixed Acid to cotton 55 to 1
Composition of Mixed Acid (approximate)
a. for 12.6)6 N: HgSO^ 63.5)6, HNOj 21)6, H2O 15-5)6
b. for 13. Ц N: H2SO4 68)6, HN03 22)6, H20 10.0)6
Temperature of acid at the start 3b°C
Time of nitration 2b minutes
During the nitration period the mixture is turned over occasionally to keep the acid homo-
geneous. The mixture is then filtered on a Buchner funnel with suction for about three min-
utes and then drowned rapidly with strong hand stirring in at least 50 volumes of cold vtter.
After the nitrocellulose has settled, most ol the water is decanted and fresh water added.
The nitrocell lose-water mixture is boiled and the acidity adjusted to 0.25^ to 0-50)6 as ligSOj.
The sour boil is continued for at least 2b hours for pyrocellulose and at least bO hours for
gun-cotton. Additional boiling with changes of water are made in accordance with the govern-
ing specification (jAN-N-2bb).
Pulping: The nitrocellulose Is then pulped in a laboratory Holland-type ptper beater.
Enough sodium carbonate is added to keep the reaction faintly alkaline to phenolphthalein.
Pulping is continued to the desired degree of fineness.
Poaching: After washing the nitrocellulose from the beater, the mixture is filtered and
the product boiled for b hours with fresh water while stirring mechanically. From time to
time a little cc-dlvia carbonate solution is added to maintain the mixture faintly 1 Lkaline I?
phenolphthalein. The water is decanted and the boiling continued. According to the specifi-
cation, the total boiling treatment with poachi. Is as follows:
230
NitrocelluloBe (NC)
АМСР 706-177
U houra boiling with or without sodium carbonate
2 hours boiling without sodium carbonate
1 hour boiling without sodium carbonate
1 hour boiling without sodium carbonate.
Each boil is followed by settling and change of water.
Washing; The nitrocellulose is then washed by mechanical agitation with water. A minimum
of two washes are given. If a sample taken after ths water washes gives a minimum test of
35 minutes in the 65.5°C Heat Test and 30 minutes in the 13^-5°С Heat Test, the nitrocellulose
is satisfactorily stabilized. Otherwise additional washes should be g±ven.
Origin;
Cellulose occurs in nature. It is wood fiber, cell wall and the structural material of all
plants. Cotton fiber is pure, cellulose. Nitrocellulose was discovered about 184? by C. F.
Schonbein at Basel .d R. Bcttger at Frankfort-on-the-Main independently of each other when
cotton vas nitrated. T. J. Pelouze had nitrated paper earlier (1838) and was probably the
first to prepare nitrocellulose.
Pyroxylin or collodion, which is soluble in a mixture of ether and ethanol, contains from
8$ to 12% nitrogen. It is used in the manufacture of celluloid and in composite blasting
explosives.
Pyrocellulose, a type of nitrocellulose of 12.6% nitrogen content, con?;. ’y soluble i.i 2
mixture of parts ether and one part ethanol, was developed by Mendeleev ( ,1-1895). This
material, when colloided, formed the first smoke 1е^з powder for military use in the ’united
States (1898).
Guncotton for military purposes t у contains a minimum of lj.35% nitrogen. It is only
slightly soluble in ether-ethanol, but completely soluble in acetone. Principal use is in
flashless powders and as flame carriers. I1*. 14$ N nitrocellulose represents a theoretical
limit.
In the manufacture ~f propellants, there is used a mixture of pyrocellulose and guncotton
(b’’"deri nitrocellulose) of 15*15% to 13-25% nitrogen content.
restruct!on by Chemical Decomposition;
Nitrocellulose is decomposed by adding it, with stirring, tc 5 times its weight of 10$
sodium hydroxide heated t; ?0°C. Stirring is continued for 15 minutes after all the nitro
cellulose bee bee.; added.
References;47
(a) See the followin' Picatinr.; Arsenal Technical Reports on Nitrocellulose:
1, page 10.
AMCP 706-177
Nitrocellulose (NC)
0
10
1 2 2 4 2 6 1 8 2
41 72 13 4 125 86 167 8 19
101 332 33 24 475 576 327 198 29
2?] 402 43 114 485 586 407 208 69
351 422 133 174 495 796 717 278 169
551 542 233 194 555 916 787 388 279
831 572 253 334 705 1016 987 408 499
851 652 273 374 965 1026 1187 588 659
971 662 6=3 394 1065 1066 1197 718 669
1031 752 673 724 Г.25 1206 1267 758 709
.1041 802 68ч 804 1135 1256 1297 778 739
1071 952 773 894 1205 1276 1327 808 779
1151 1012 793 1024 1265 1306 1407 838 809
1201 1032 963 1054 1275 1316 1427 8r8 909
1221 1142 1023 1074 1#5 1516 1447 1058 1119
1231 1242 12. 1 1084 1375 .’55* 1487 1228 1159
1331 1262 1273 1174 1745 1616 1587 1238 1249
1351 1362 1443 1274 1755 1.786 1637 1248 1309
1391 1392 1653 1304 1845 2056 1717 1348 1329
1401 1642 1753 1314 1905 1817 1398 1349
1421 1812 1813 1384 1915 1827 1478 1399
1501 1852 186° 1394 1955 1847 1528 1439
1541 1912 18' 3 1454 2107 1638 1449
1681 1992 1973 1674 2137 1678 1619
1691 2022 1754 1838 1799
1731 2102 1814 1898 1809
1781 1824 1918 1869
1811 2144 2098 2119
1831 2206 2189
1841
1851
1931
1961
199i
2071
2101
2181
2201
232
ititroglycerlr. (Lliuid)
АМСР 706-177
Metocufer Weight: (с^ЦЯ,0д) 227
ъ с 15-9 н с—оно. 2| 2 Н 2.2 НС — <И02 И 18.5 н2^—оио2 о 63.U C/H Rotic 0.Ю9 Bsleecw* CO, % 3.5 CO % 2U. 5
25°c, Liquid 1.59; Daaslty.gm/cc г
— *C Labile form 2.2 j reran к. fom .13,2
Fnsslrg Potato 'C
СмлШмЬы 9 If м 4tfBe MipW* «ОТВСЖеТмур * ВВЦ WTT Buraou of Мпг» Apparatus, an 1> Sample ?*. 20 mg Picotinny Arwrol Apparatus, In. 1 lb wt 1 Sample Wt, mg BeMag Potato 'С Decompose* 11»5
BrfsecNse Udes, nb 1.1»732 ni 1.1»T13 n£
Steal She* Explodes Fiber Shoe Vaaaaai StabMy Test: cc/40 Hrs, at 90*C се/да^б hr* 1. 6 100'C cc/ge/16 hr* 11+ 120'C I35*C 150*C
RNh BaMst Impact Tlssto Trials % Explosions 100 Partials 0
Burned 0 Unaffected 0 NO Штата Barah Saud Test: Sand, gm Liquid aathod 51.5
Ixpteste"» ТмерогеНие: *C Seconds, 0.1 bw cap used) 1 5 Explode* 222 10 Minimum Detonating Charge, gm Mercury Fjlminet* lead Atid* Tetryl
20 BaOMe Metter, « TNT: (a) 140
Ttaata T«*t.«T*rri (b) 181
75' I tatereetieaU Heat Test: 9 Loss In 48 Hrs
Ptete D«H Test: Method
IBB'CHeutTesto % Loss, 1st 48 H« 3*6 % Lou, 2nd 48 Hrs 3>5 Exp osion In 160 Hrs None Condition Confined Density, gn/cc Brisance, % TNT
ОеГмеНм Bate: Confinement Sl**« J'eel Condition Liquid Liquid Charge Diameter, in. 0.39 1,25 Density, gm/cc 1.6 l.o Rote, meters/seennd 1600-1906 7700
НеташЬИку lades:
Hygrercepfeky: % 30°C, 9C# RH 0.06
VetafMy: 60°C, вг?/сш2/Ьг 0.11
zn
АМСР 706-177
nitroglycerin (Liquid)
- »- - *——t»t--t^— •Wwiwl’Wy 1 Condition Tetryl, gm Wax. in. for 50% Detonation Wax. gm DenUty, gm/cc Oxygen, otoms/sec 10 * 10 (Z/tec) Hoot, kilocalorie/mole 41.4 45.0 (AH, kcal/mol) Temperature Range, *C 90-135 125-150 Phase Liquid Liquid
Heat of: Combustion, cal/gm 1616 Expiation, col/gm , 1600 Gai Volume, cc/gm 715 Formation, cal/gm JV ^00 Fusion, cal/gm " Detonation, cal/в» l1^ Armor Plate hnpoct Tech <iP mm Metter Projectile: 50% Inert, Velocity, ft/sec Aluminum Fineness 5Ф0-* General Parpeoe Bembe: Mote Th.’cknass, inches 1 1'4 IM:
Spodflc Heth cal/gm/*C Liquid 0.356 Solid 0-315
cm/sec
CoaA Drop Test: 17,200Ф* Semi-Armer-Pierciep Bomb vs Coeemte: ЛЛох Safe Drop, ft SOO-lb General Perpoea bomb vs Caacrete: Height, ft Trials Unaffected Low Order High Order 1000-lb General Pwpeea Bomb vs Concrete: Height, ft Trials Unaffected Low Order High Order
col/sec/rm/’C
CoeMdont of TxpeMien: Linoo-, %/’C Volume, %/’C
E', dynes/cm1 E, Ib/inch3 Density, gm/cc
Compraeelve Strength: Ib/inch1
Vopor Prosoma: °C an Mercury °C m Mercury 2C 0.00025 60 0.0186 30 О.ООО83 70 0.043 UO 0.0021 80 0.098 50 O.OC73 90 0.23
•XU
Nitroglycerin (Liquid)
AMCP 706-177
90 nun HI, M71 PiejecMe, Let WC-91: Detu.*y, gm/cc Charge Wt, lb Tefal No. of Fragssaets: For TNT For Subject HE, 3 inch HI, M42A1 Projectile, Let KC-3: Density, gm/cc Charge Wt, lb Total No. of fregowatt: For TNT For Subject HE Shaped Charge IffosttooMM, TNT s llbt Gloss Cones Steel Cones Hole Volume Hole Depth Color: Colcrless
Principal Usee: Propellant ingredient, demoli- tion explosive ingredient, grenade burster Ingredient
МоИмй W LmAaq ‘
Loading Density: gm/cc
Fragment Velocity: ft/sec At 9 ft At 25 Ц ft Docsity, gm/cc
Method With acetone or other desensitizer, generally not stored Hazard Class (Quantity-Distance) Class 9 Compatibility Group Exirdatian
toot (lototfre to TNT): Air: Peek Pressure Impulse Energy Air. Cer^ned: Impuhe Under Water: Peek Pressure Impulse Energy Peek Pressure Impulse Energy
Heat of Transition, cal/gm:
Transition: Liquid —• labile 5.2 labile —» stable 28.0 Liquid —» stable 33-2 Hydrolysis, % Acid; 10 days at 22°C <0.002 5 days at 60°C 0.005 82.1°C KI Test; Minutes 10+
235
AMCP 706-177
Nitroglycerin (Liquid)
0— Evolved at Atmospheric Pressure, cc;
Эач>1е Wt, gm 1.6
TMverature, °C 65 75
Time, hours 20 -tO
Volume of gas, cc nil nil
Viscosity: (c) Centipoises
10 20 30 Uo 50 6o 69.2 36.0 21.0 13.6 9Л 6.8
Fragmentation Teat:
20 m HE, Жгк 1, Projectlie, Total No.
of Fregsenta for:
Nitroglycerin 22
Tetranitroawthane 17
Minimum Propagating Diameter: (d)
<im Diameter for
It Pliacthj^lphthalate Mln. Propagating Blur'S in
Tilameter, Inches inches
0 (3/16 Cairns) Л/16
5 1/6
10 1/9 3/16
15 1Д 3/8
20 3Д 7/1?
22.5 1 '2
25 1-55 2
Senaltlvlty to Electro ata tic ^'auharge, uvulas (teet condition, unvonflned;
no value given Tv? conf-neaentlT " " >li>5
Solubility, gramc of nltroglycerin/lOO дп «) t>f;
Water Alcohol TricM-frethylene Carbon Tetrachloride
i °C 1 °C 4 °C 2
15 0.16 0 ЭТ-5 Rm 22 Rm 2
20 0.18 20 51.0
50 O.25
2:16
Nitroglyceri 1 (Liquid)
АМСР 706-177
Carbon Disulfide gm/ICO gn (*), at 25°C in
°C J Ether 2:1, Ether: Alcohol >100
Ambient 1 Acetone ”
Soluble in all Proportions in:
Methanol
Acetone
Ether
Ethyl acetate
Asyl acetate
Methyl nitrate
Ethyl nitrate
Nitroglycol
Tetrani trodlglyceri ne
Acetic acid
Benzene
Toluene
Solubility in NO, of:
Alcohol IWT
°C J °C J
О ЗЛ 20 ’5
20
50
Phenol
Pyridine
Xylene
Nitrobenzene
p-Nitrotolusne
Liquid DKT
Chloroform
Ethyl chloride
Ethyl bromide
Tetrachloroethylene
DI chloroethylene
Trimethyleneglycol Ш.nitrate
TNT Water
20 30 25 0.06
ch„— он
I 2
CH ---OH
I
CH2---CH
+ 3HNO3
№2-0N02
ch —oso„
I 2
CH2—0N02
+ 3H20
Glycerine it usually nitrated at 25°C, or below, by adding it very slowly to a well agitated
mixture of nitric and sulfuric adds, e.g., **0/59.5/0.5, nitric acid/sulfurlc add/water, us-
ing an acid/glycerine ratio of approximately 6. Agitation of the rearcion mixture is accom-
plished by use of compressed air. A rapid temperature rise, or appearance of red fumes, auto-
matically requires dumping of the charge, Immediately, into a drowning vessel filled with
water. After all the glycerine has bean added to the nitrator, agitation and cooling are con-
tinued until the temperature drops to about 15°C, and the charge is then run into a separator
where the NG rises to the top, and is run off to the neutralizer- The nitroglycerin is
washed first with water, then with sodium carbonate, and finally with water. The resultant
NG when washed with water, produces washings which do not color phenolphthalein, and itself
is neutral to litmus paper.
237
АМСР 736-177
Nitroglycerin (Liquid)
Origin:
Nitroglycerin was first prepared in 1846 or 184т by Ascanio Sobrexo, an Italian chemist
(Mam Acad Torino (2) 10, 195 (184т)). For several years after this discovery, nitroglycerin
attracted llttxe interest as oa explosive until Alfred Nobel in 1864 patented improvements in
its manufacture and method of initiation (British Patent 1813)• Nobel gave the name dynamite
to mixtures of nitroglycerin and ton-explosive absorbents, such as charcoal, siliceous earth
or Kieselguhr (British Patent 1345 (186?)) later developments led to gelatine dynamites,
ammonia dynamites, and so colled straight dynamites, The first propellents using nitrogly-
cerin vere called Ballistite (Nobel, British Patent 1471 (1888)) and Cordite (/bel and Dever,
British Patents 5614 and 11,664 (1889)).
Destruction by Chemical Decogwaition;
Nitroglycerin Is decomposed by adding it slowly to 10 times its weight of 18$ sodium sul-
fide (NagS-'JHg'1). ’ieat is liberated by this roection; but this is not hazardous if stirring
is maintained during the addition of nitroglycerin and continued until solution is complete.
References: 48
(a) A. H. Blatt, Coepilation of late on Organic Explosives, OSRD Report No. 2014, 29 Feb-
ruary 1°44.
(b) Ph. ifaoum, 2. gee Schiess-Sprangstoffw, pp. 181, 229, 267 (27 June 1932).
(c) Inndolt - Bernstein.. ttysitelisch-Chemische Tabellen, 5th Ed. (1923) •
International Critical Tables.
В. T. fedoroff et al, A Manual for Explosive Laboratories, Vol T-IV, lafax Society,
Inc., Philadelphia, 1943, 1946.
(d) H. A. Strecker, Initiation, Propagation and Luminosity Studies of Liquid Explosives,
OSRD Report No. 5609, 3 leoember 194$.
(e) Also see the following Picatlnny Arsenal Tec hnical Reports on Nitroglycerin:
1 2 1 4 ч X. 6 7 8 2
620 511 652 233 454 1155 1206 817 768 69
660 551 672 343 494 1235 1456 837 1348 249
8oo 701 792 673 1024 1955 1196 1197 1398 579
1020 891 922 903 1074 2015 1556 129т 1738 709
1150 911 1142 1023 1084 1616 1637 1918 1349
1210 1410 1620 1680 1031 1041 1151 1191 1221 1611 1651 1691 1731 1781 1851 1931 2021 2181 2201 1232 1362 1542 1662 16% 1742 1752 1992 1443 1643 1663 1863 1993 1454 1524 1624 1674 1754 1786 1816 1896 2056 1817 1847 2096 1359 2119
48See footnote 1, page 10,
238
Hitroguanidlne
AMCP ZVe-177
& Molecular Weight: (CfyfyOg) 104
c 11.5 nh2 H 3-9 НИ— c Osygoa OeSaace: co, % -31 CO % -15.4
N 53-8 'Y* Deaelty: gm/c Crystal 1.72
NO,. 0 30.8 2 M0M41 Niat: *C 238
C/H Rotio 0.038 ГОгЖг
hapoct Seaoitivity. 2 Kg Wt: Bureau of Minos Apparatus, an 47 Simple Wt 20 mg Picatinny Arsenal Apparatus, in. 26 Sample Wt, mg 7 a_wt_- lulah. *f* Ж^ЯВоее
Refractive lodes, n» n& n°
Т^Д, f a\ ГтЩН rWWwHI eWOTa \~J Steel Shoe Unaffected Fibe.' Shoe Unaffected T^h^wHi 4vw*vv<y • bw; cc/40 Hrs, at 90*C ioo’c ; "-3T 120'C 0-44 135*C 150*C
Rifle Met tagaet Teet: 5 Trials (e) % Explosions 0 Partials 0
Burned 0 Unaffected 100 200 Grom BeaA Scad Toot: Sand, gm 3^.0
Iwpliiln Tomperetere: °C Seconds, 0.1 (no cap used) 1 5 Тэееошровев 275 10 15 Saaaitivity to laMotioa: Minimum Ootonoting Charge, gm Mercury Fulminate Lead Azide 0.20 Tetryl 0.10
20 BoOtatic Mortar, % TNT: (а) ю4
. Travsl Toot. % TNT: (b) 101
79’C latemotioeel Hoot Teet: % Lou in 4» Hrs 0.04
Hate Deot Test: (c) Method A
ICO'C Hoot Test: % L-su, 1st 48 Hrs 0.18 % ’ла, 2nd 48 Hrs C.09 Explosion in 100 Hrs None Condition Pressed Confined Ho Density, gm/cc 1.50 Brisance, % TNT 95
DetanetiM Rata: (e) Confinement Condition Charge Diameter, in.
71еяммЫШу Index:
Hygroscopicity: % 30°C, 90^ RH .Nine
Volatility: None Density, f m/cc 1-55 Rote, meters/second 7^50
239
Mltroguanidlne
AMCP /иб-177
Frogsseatetioa Test. W нмп HE. МП Projectile. Let WC-91: Deteity, gm/cc Charge Wt, lb Yetel No. ef FregeseeH: For TNI For Subject HE 3 tech HE. M42A1 Projectile. Let KC4: Density, gm/cc ChorgeWt, lb Tefal No. of Fregnients: For TNT For Subject HE Shaped Charge Effectiveness. TNT = 100: Gloss Cones Steel Cones Hole Volume Hole Depth
Celon Colorless
Principal Uses: Propellant composition ingredient, bursting chorga ingredient
MMhed eff Leodtef:
Loading Density; gm/cc At 3300 psi 0.95
FrogoMat Votedty: ft/sec At» ft At 25Ц ft Domity, gm/cc
Storage. Method Dry Hazard Class (Quantity-Distance) Cisse 9 Compatibility Group Croup I Exudation
Moot (Motive to TNT): Alt: Peek Pressure Impulse Energy Air, Csnfieeft Impulse Under Woton ’oak Pressure Impulse Energy Underground: Peak Pressure Impulse Energy
Solubility, gm/100 gm ($), in; °c„ Water 25 0.44 100 9.1 < 1.0 H Potassium Hydroxide 25 l.i Sulfuric Acid 0 3-*** 25 8.0* * gm/100 cc solution Booster Sensitivity Test: (d) Condition Pressed Tetryl, gm 100 Wax, in. for 50$ Detonation 0.6Г Density, gm/cc 1.41 Heat of; Combustion, cal/gm 1995 Explosion, cal/gm 751 Gas Volume, cc/gm 1077 Formation, cal/gm 227
240
Nitroguanidine
АМСР 706-177
Preparation;
(Chemistry of Powder and fxplosirei, Davis)
V.
N02 — uh
C — NH
HHO,
Dehydration j
C *»KH
V
Four hand ed gee of dry guanidine nitrate is added in email portions to JOO cc concentrated
sulfuric acid at 10°C, or below. As soon as all crystals have disappeared the milky solution
is poured into 3 liters of ice-water, and allowed to stand until crystallization is complete.
The product is filtered, rinsed with water, and recrystallized from about 4 liters of boiling
water, yield about 90^.
Origin;
Xltroguanidine was first prepared in 1877 by Jousselin, but it was 1900 before it found
use in propellant ««positions. During World War I, nitroguanidine was used by the Gereons
as an Ingredient of bursting charge explosives.
Destruction by Chemical Decospositlon;
Xltroguanidine is decoEposed by dissolving in 15 times its weight of kyfi sulfuric acid at
roots temperature and warming the solution until gas is evolved Hee ting is continued for one-
half hour.
Hefansccss;49
(a) I>. C. Smith and E. G. Foster, Physical Testing of Explosives, Pa-1 III - Miscellaneous
Sensitivity lasts; Performance Tests, OSRD i’eport Ko. 571*6, 27 December 1945.
(o) Canadian Report, CE-12, 1 May-15 August 191*1.
(c) D. P. MacDougall, Methods of Physical Testing, OSRD Report No. flOJ, 11 August 1942.
(d) L. C. Smith and S. R. Walton, A Consideration of RDX/Wax Mixtures as a Substitute for
Tetryl in Boosters, HOL Memo 10,303, 15"June 1949.
(a) Departments of the Агву and the Air Force TH 9-19Ю/ТО 11A-1-34, Military Explosives,
April 19».
ла
Sea footnote I, page 10,
241
АМСР 704-177
Mltroguenidlne
G?) Also ем t he following Picatinny Araenal Technical Reports on Ritroguanldlne:
i 1 2 2 6 1 8 2
1U90 1391 2181 2201 1282 1392 2142 1183 1423 2193 1336 907 2177 758 1439 1749
242
Nitroisdbutylelycerol Trinitrate (М1ВТЯ) Llguid
AMCP7M-177
' Ceerperitiev Metorafar Weight: (С^ОДО^ ) 286
ъ c 16.8 0g«0-CH2 H 2.1 Oxyg** Bataeeo: CO, % o.o CO % 22
OgWO^CHg 11 C KUg N 19.6 Piailtyi gm/cc 20°C 1.64
. OJO-CH< 0 61.5 2 2 Bml^t i
C/H Rotiu 0.126 Framing Nat: *C .39
Impact Seoaietvity, 2 Kf Wt: Bureau of Mina* Apparatus, cm 25 Sample Wt 20 mg Picotinny Arsenal Apparatus, In. Sample Wt, mg rwrai
Refractive Index, n£ n> 1.1*896 i£ 1.1*871*
Frictiao FwMue Test: Steel Shoe Fiber Shoe 0 Ta^t cc/40 Hrs, ot 90’C 100 °C 120°C 135°C 150°C
Rifle Reflet Impart Test: Trials % Explosions Partials
Burned Unaffected 200 Стам Всей Read Teet:
Captation Tomperetare: °C Seconds, 01 (no cap used) 1 5 Ignites 185 10 a мДйЬЬм Minimum Detorrating Chorge, gm Mercury Fubnlnote Lend Azide Te:r0
13 20 Bafltaic Mortar, % TNT*
Trawl Teet, % TNTt
79°C iMarnetioaol fleet Teet: % Loes in 48 Hrs
Flett Dorrt Teat: Method
100’C Meet Test: % Lass, 1st 48 Hrs % loss, 2nd 48 hrs Explosion in 100 Hrs Condition Confined Density, gm/cc Brisonce, % TNT
DateooMea Rate: Confinement Gloea (1 gm well) Condition Liguid Chorge Diameter, In. 0*39 Density, gm/cc 1.64 Rate, metors/socond 7860
— i—.4 IWVvXi
Об nyjlWRe^WWyr
VetoMityt p 25°c, ng/ca /21* hre 0.127 x 10"’
243
АМСР 706-177
Nitroleobutylalyeerol Trinitrate (КНУД?) liquid
«е хм к мл PNiMtn*, ut wc-*it Oamity, gm/cc Cxage Wt, b Total Не. Ргадвиейк For TNT Fo. Subject HE 1laab HE, M42A1 NMmMo, Lrt KC4: Density, gm/cc Charge Wt, № Total No. of Fmgteaote: For TNT For Sublet HE Shaped Charge Hterttvearte, TNT - 100i Stat Come Steal Conor Hole Volume Hole Depth
Cebn Yello» oil
Mariyil UteK Gelatin!xlng agent for nitrooelluloae
MetteE ef LaeMagt
Leortag Daedty: gm/cc
FragoMot Vetatey: ft/iec At 9 ft At 254 ft Dimity, gm/cc
Method Liquid Hozord Ctate K^nntity-Dirtonc») Compatibility Group Exudation
Mart (EoteHre ta TNT): Ab! Prok Protiure Impute Energy Air, CiaWitfc Impute Under Water; Peek Pranure Impute Energy ШЮТ0^|ПЯВЯ* Ptek Pressure Impute Energy
Solubility; Soluble In netayl and ethyl alconole, ace- tone, ether, ethjlenedichloride, chloroform and benzene. Ineoluble In 'fltter carbos;.dleulp'iide, 1 and petroie-A® ether. | Toxicity; Slight, decidedly Ъ-чь J-—, nitroglycerin. Gelatinising Action: .
Slight or. nitrocellulose. . 62.2°C KI Test; Mir .tea 2
244
Nltrolsobutylglycarol Trinitrate (NIBUt) Liquid
AMCP 706-177
Preparetl on;
A total of 675 gm 31% formalin is added to 150 gm nitromethane containing 2 gm potassium
с“гЪл--**« homd-hydrate. n>a *•—* 200 gm formalin ie added slowly, keeping the teverature
below 30°C> and then the heat of reaction is allowed to raise the tes^ereture to 60°C, and
the mixture then heated two hours at 90°C. The reaction mixture Is then concentrated at re-
Aiced - res' 're and diluted, and this process repeated several times to remove formaldehyde.
After the *lnal concentration the cooled mixture is filtered and the crystalline product recry-
stallised froa alcohol and then sever* 1 times from ether and dried.
The nitrated product is then obtained by nitrating 50 gm nltrolsdbutylglycerol vith 300 gm
mixed acid (бо/ЗВ/2, sulfuric add/nitric acid/wter) below 15°C for 1.5 hours.
Origin:
This explosive (also called SrlmethyloInitronethane Trinitrate, Nitrolsobutanetrlol Trini-
trate, Nltrolsobutylglycerln Trinitrate and incorrectly but widely used Bitroisobutylglycarol
trinitrate) was first described in 1912 by Hofwlmer (Z ges Schiess - aprengstoffw 7, 43
(1912). Bofvlsur prepared the compound by the condensation of 3 moles of formaldehyde vith
1 mole of nitromethane in the presence of pctassium bicarbonate, the subsequent nitration of
the prohict. The explosive can now be produced from coike, air, and natural gas.
References:so
(a) E* A. Aaronaon, Study of Explosive. Derived iron Nitroparaffins, PAT® Ho. 1125,
24 October 1941.
(b) M. Aubry, Nfe poudr, 2£, 197-204 (1932*33); CA 2£, 4o83 (1933)-
(c) A. Stettbecher, HltTvceUuloae £, 159-62, 181-4, 203-6 (1934); CA 2g, 1250 (1935)-
(d) V. de C. Crater, U.S. Patent 2,112,749 (March 1938); CA J2, 3964 (1938).
(e) E. J. Hlbehman, E. H. Pierson, and H. B. Haas, Ind Eng them 32, 427-9 (1940); CA 34,
3235 (1940).
(f) A. Stettbecher, Z ges Schiess Sprengstoffv 62-4 (1942); CA j8, 255 (1944).
^®See footnote 1, page 10.
24
АМСР 706-177
l-’l trostarch Demolition Bagso> ’ -e (NSX£
СепмооМеа: Molecular Waight: 325
4b Nitrostarch (12.50^ K) **9 Barium Nitrate 40 Mononltronephthalene 7 C(X % -19 CO % 0
Paranitroeniline 3 Oil 1 Density: gm/cc
Making Met: 'C
C/H Rotio Freezing Faint: *C
tapeet SoaoRMty, 2 Kg Wt: Buraou of Mine* Apparatus, cm 21 Sample Wt 20 mg Picotinny Arsenal Apparatus, in. 8 Sample Wt, mg Bailing Point: *C
Refractive Index, n£ П» n£
. Friction Rondnlem Test: Steel Shoe Crackles, snaps Fiber Shoe Unaffected Vacaaa* Stability Tost: cc/46 Hrs, ot '0‘C 100’C 11* 120‘C 135“C 150-C
Rifle Mot hnpnct Test: 10 Tnols 8 Trials* % I Explosions 90 0 Partials 0 13
Burned 0 0 -Unaffected 10 67 •naked In never 200 Gram Bomb Sand Test: . Sand gm 39- 5
(xphoien Teaporotere: "C Seconds, 0.1 С л cop uted) — 1 5 Decomposes 195 10 it Sensitivity to Initiation: Minimum Detonating Charge, gm Mercury Fulminate 0.26 Lead Azide Tetryl
20 ellietic Mortar, % TNT: (a) 96
Treat! Tost, % TNT:
75’C latoractfenel Meet Tost: % Loss In 48 Hrs 0.2
Plate Doot Test: Method
100‘C Heat Toot: % Lass, 1st 48 Hrs 0.3 % Loss, 2nd 48 Hrs 0.3 Expiation in 100 Hrs None Condition Confined Density, gm/cc Brisance, % TNT
WtvIWwII MW* Confinement Condition Charge Diameter, Density, gm/cc Rate, meters/second
PlemmebiRty Index:
Hygroscopicity: % 30°C, 90^ RH 2.1
Nitrostarch Demolition Explosive (WSX)
amcp w-m
•0 ими HL M71 Projectile, Lot WC-01: Density, gm/cc Chorge Wt, lb Tetol No. ef Frogmen!»: For TNT For Subject HE 3 inch HL M43A1 Projectile, Let KC-5: Density, gm/cc Chorge Wt, lb Total No. of Frogeroata: For TNT For Subject HE Shaped Charge EHoctleoaoss, TNT = 100: Gloss Cones Steel Cones Hole Volume Hole Depth
Color:
Priadpol Meat: Demolition, bursting charges, and priming coog>oaiticne
Method of Loading: Band temped
Loading Density: gm/cc Apparent 0*92
Fragment Vtiocity: ft/sec At9ft At25Hft Density, gm/cc
Method Dry Hazard Class (Quantity-Distance) Class 9 Compatibility Group Group I Exudation Bone
Nett (ЫоНео to TNT): Air: Peek Pressure Impulse Energy Air, Caofinod: Impulse Under Water: Peak Pressure Impulse Energy Underground: Peak Pressure Impulse Energy
120°C Heat Teat:
Minutes Salmon Pink jd Red Fumes 255 Explodes 256
247
AMCP 706-177
Nitrostarch reisolition Explosive (USX)
Preparation: (b)
The nitration of starch proceeds vith the formation of hexanitro starch according to the
folloving equation:
2C^H1005 * 6НВО3-* +
Tapioca starch is ccnsidured the best for nitration purposes, although other starches give
iairly stable products* The starch, pretreated to remove oils, tats k."d water soluble im-
purities, is dried and screened* Feeding of the dried starch into stalnloea steel nitre tore
containing nixed acid (62]C-63jt ВГО3 and ЗТ6-386 HgSOiJ is done elovly vith constant agitation
of the Mixture. ihe heat evolved Bist be controlled by cooling coils. Ihe nitrated starch
is separated fton the spent add, washed vith a large amount of water and centrifuged. Fine!
dryiz^ is on trays heated to 35°-4o°C vith air. This product is so sensitive even a static
discharge night cause explosion.
Hitrostarch demolition explosives contain a high percentage of nitrootarch, an oxidising
agent, Mineral oil, a stabiliser and/or other ingredients.
Origin:
Kitrostarch was first prepared in 1833 by Bran cannot, vho called it xyloidlne (Ann chia
phys [23 52, 290 (1833))* T. J. Palouse studied xyloidlne further and reported its explosive
properties (Coapt rend 7, 713 (1838). It found military use in the United States during
World Шгв I and II as blasting explosives and as an ingredient of bursting charges and pris-
ing ccaposltions.
References;
(a) W. R. Tomlinson, Jr., Ihysical and Explosive Properties of Military Explosives, PAU?
Ho. 1372, 29 Bovesfcer 1943-
(b) G. D. Clift and В. T. Fedoroff, A Manual for Explosives laboratories, Vol I, Lefhx
Society, Ihe., Philadelphia (1942).
(c) Also see the following Picatinny Arsenal Technical Reports on Kitrostarch Explosives:
1 2 4 J 8 2
1611 782 1031 Ш7 838 1269
2032 848
^'see footnote 1, page 10.
248
АМСР 706-177
Octol, ТО/30
% нмх то ОТТ 30 С/Н Ratio Meiocsler Weight: 265
Oxygen Beteoce: co, % -38 CO % -7.5
Deaeity: gm/cc Coat 1.80
Mohing Meh *C
Freaafag Pbioh *C
hngeet SaaaMvHy, 1 Kg Wk Bureau of Minot Apparatus, cm Sample Wt 20 mg ₽ cotirmy Arterial Apparatus, in. 18 Sample Wt, mg 26 o^^^^^ao w
Reflective lades, nJ n» ni
fhwwBR rwmisM iiw» Steel Shoe Unaffected Fiber Shoe Unaffected ▼V*^W ЮТ* cc/40 Hrs, ot 90*C lOO’C 120‘C 0.3T 135*C 150*C
Mia BoKst hepecf Toth Triols % Explosions Portiols Burned Unoffoeted
200 Great Booth Saad Test: Sond, gm Exploratory 58.4
Seconds, 0.1 (no cop used) — 1 5 Huas erratically 335 10 IS 20 A* Minimum Detonating Charge, gm Mercury Fulminate ---- ^ood Aside 0,30 Tetryl
BeliNic Moder, % TMT: 115
Tieeri Teet, % TNT:
П C fototMtioeol Heat Teat: % Lot* in 48 Hrs
Plate Dent Teet: Method Condition Confined Density, gm/cc Brisance, % TNT
180"C Hoot Tosh % Lost, 1st 48 Hrs % Loss, 2nd 48 Hrs Explosion m 100 Hrs
Detonation Roto: Confinement None Condition Cast Chorge Diameter, in 1.0 Density, gm/cc 1-80 Rote, meters/socond 83T7
Л/
VoMBty:
249
АМСР 706-177
Осlol, 70/30
HWWW WWWleWy 1 Wv« Condition Tetryl, gm Wox. in. io» 50% Detonotion Wax, gm Dantity, gm/cc DtcoiMpoeiHQii Кфю11м№ Oxygen, otomt/toc (2/sec) Heat, kilocolorie/mole (AH, kcol/mol) Temperature Range, °C Phase
Heotot: Combustion, cal/gm 2722 Expiation, cai/gm Ю71, Gas Volume, cc/gm 84? Formation, cal/gm Fusion, cal/gm Armor Plate Impact Test: M mm Mortar Projectile: 50% Inert, Velocity, ft/se>. Aluminum Fineness S00-G General Parpose Bombs: Plate Thickness, inches 1 |i/_. 1%
Specific Hoot: c ’ /gm/’C
• ---l^a I^Ai cm/soc
Bomb Drop Test: T7, 2000-№ Semi-Агтоь Piercing Bomb vs Concrete: Max Safe Drop, ft SOCMb General Parpose Bomb vs Concrete: Height, ft T rials Unaffected Low Order High Order 1000-lb Generel Ригрме Bomb vi Ceecrete: Height, ft Triols Unaffected Low Order High Order
1 A^MS^Ri*ewTl^y e cal/sec/cm/'C
Cceffi cleet of Ixpeneiea: Linear, %/‘C Volume, %/‘C
M_U^k.1 Vai., a^^Ba^^^^WVp ^W^Ve
E', dynes/cm* E, ib/inch’ Density, gm/cc
Compressive Strength: Ib/inch’ 1310 See below
Yegor Pressers: 'C mm Mercury Compressive Strength: Ib/inch^ * Average (10 tests) 1510 High 17L0 Low 1330
Ultimate Deformation: J
Average (10 tests) 2.26 High 2.58 Low 1-97
•Test specimen 1/2” x 1/2' cylinder (approximately 3 »m) pressed a •_ 3 tans ('..ООО Ih) total
load or 30»000 psi w'th a 2 minute time of dwell.
250
Octol, ТО/ЗО
AMCP 706-1П
FragMOntatiea Tosh Shaped Chorga EffecHvenoes, TNT — IM:
W мм Mt, M71 PrejecHU, Ur WC-Fl: Density, gm/cc Chorge Wt, lb Gass Cones Steei Cone; Hole Volume Hole Depth
Ыл Вм^м^йа< В игеМ» For TNT For Subject HE 3 tach HI, M42A1 Projectile, let KC-S: Density, gm/cc Charge Wt, lb Buff
Principal Uses: HE projectile end boob filler
Total He. of FregoMMij: For TNT For Subject HE МвМм4 Loo^tB^e Cast
Landtag Deneity: gm/cc 1.80
Fragment Vrhrlty, Jt/sec At* ft At2SH ft Density, gm/cc
Method Dry
bat (fatothre la TWDi Hasarri Class (Quontity-Drstonce) Ciess 9
Ain Peak Ргемиге Impuhe Energy Compatibility Group Exudation Group I
Air, Confined: Impuhe Under Wotan Peak Pressure Impuhe Work to Produce Rupture: ft-lb/incb^ *
Averege (10 tests) High Low Efflux Viscosity, Ssybolt Seconds: 1.55 1.87 1.10 5 9
Energy
Underground: Peak Pressure Impulse Energy •Test specimen 1/2" x 1/2" cylinder (approxi- mately 3 gm) pressed st 3 tons (6,000 lb) total lead or 30,000 psi with s 2 minute time of dwell.
251
АМСР 706-177
Octol, ТО/ЗО
Effect of Altitude Charge Ddsmeter and Degree of Confinement on Detonation Velocity*
(Reference b)
| Explcaive Simulated Altitude, One-Inch Column Two-Inch Column
Confined Unconfined Confined Unconfined
Feet m/s m/e m/s m/s
! 70/30, RuX/lST; ! density, gi^cc 1.62 Ground 7900 8100 7660 8030 7800
30,000 8020 8120 7900(4)
i t 60,000 8040 8140 8010 7950
90,000 8060 7980 8010 7710
' Average I 8005 8085 7895 7873
70/30, НШ/ОТТ; density, ga/cc 1.61 Ground 7060 7900(4) 7870 J 7640(4)
30,000 8050 8060 7930 i 7710
60,000 8020 7930 7890 7650
90,000 7950 8000 7940 ' 7650
Average 7995 7973 7908 7663
•70/30 Octol confined charge in 1/4" steel tube, AISI 1015 seamless, 1” diameter 18” long,
and 2" diameter 7" long. All means were determined from sets cf five values unless other-
wise indicated by ( ). A 26 gm tetry booster was used to initiate each charge.
Average Fragment Velocities at Various Altitudes* (g)
Explosive Charge Diameter, Inches 1 Simulated Altitude, Peet
! Ground 30,000 60,000 > 90,000
j m/s 1 =V'« m/s m/s
70/30, rdx/mt 1 j 3415 3672 3666 3685
2 4647 i 5192 5236 6011
70/30, нш/мт 1 3366 3680 4014 3617
2 l*70'j 5464 6089 6111
•Outside diameter 2.54"; inside diameter 2.04"; length 7".
252
Octol, 70/30
AMCP 706-177
Tsnsile Strength:*
Ib/lnch*
Average (8 tests) 169
High 204
Lov 128
♦Teat speeimn as per Picatinny Arsenal sketch XL-O76B, at 21°C,
todulus of
lb/incha
Average (10 tests) ?3,2W
High 79,300
Lav 63,OC
Test specimen 1/2"
load or 30,000 psi
vith a 2 minute time of dwell,
3 gn) preened at 3 tons (6,000 lh) total
Setback flensitiT ity
Test:
Critical Pressure 92,000 psi*
Deisity, fjb/cc 1.72
•Pressure below which no initiation is obtained and above which an increasing percentage of
initiations can be expected as the setbeck pressure increases.
Pit Fregmentation Test:
105 t Ml HE Projectile;
Wight Group, grains Ro. of Fragment»
1/2 - 2 1297
2 - 5 665
5 - Ю 497
10 - 25 661
25 - 50 4?_
50 - 75 247
75 - 150 322
150 - 750 295
750 - 2500 12
1 Total Humber
4i67
253
АМСР 706-177
Octol^
См*мМм> ВМС ит Molecular Weight: 276
75 25 Oxygen Belence: CO, % CO % -?вз
Density: gm/cc Car: 1.81
Making Point: °C
C/HRotio rVWBolOJ FWUts
hvact SaaiOMty, 2 Kg Wt: Bureau of Minos Apparatus, cm Sample Wt 20 mg Picotinr.y Arsenal Apparatus, in. Sample Wt, mg •eifag Petah °C
17 25 Refractive Index, n& n£ n»
PficHsu Teet* Steel Shoe Fiber Shoe Unaffected Unaffected Yacuam ItabMly Test: cc/40 Hrs, ot 90*C 100°C I2O°C 135°C 150°C
2Же Mht Impact Teet: lOTriols $ 3/16" Steal Explosions 70 Partials 1/8" Al 70 0-39
Burned Unaffected 30 30 200 Gram Booth Send Tech Send, gm Exploratory 62.1
Seconds, 0.1 (no cop used) 1 S Planes erratically 10 55 20 °C 350 * ». _ One. »s plniMiTWy Iw 1*8«ЮТВ90В{ Minimum Detonating Charge, gm Mercury Fulminate Lead Aside Tetryl 0.30
Ballistic Mortar, % TNT: 116
Transi Tost, % TNT:
7I*C latoraoHeoet Meet Test: % Loss in 48 Hrs
Hate Doot Teat: Method
100°C Hoot Tosh % Loss, 1st 48 Hrs % 2nd 48 Hrs Explosion in 100 Hrs Condition Confined Density, gm/cc Brisonce, % TNT
FtaorasebiOty lodes: Confinement None Cast 1.0 1.81 86L3
ny^ww^wwyj tO Condition Charge Diameter, in. Density, gm/cc Rote, meters/socond
VetatMyi
2M
Octol, 75/25
AMCP 706-177
w^MWvwy о Condition Tetryl, got Wox, in. for 50% Detonation Wox, got Density, gm/cc Oxygon, otoma/soc (Z/sec) Hoot, kllocolorie/mole (AH, kcol/mol) Temperature Ronge, *C Phase
Meet eh Combustion, col/gm 26?6 Explosion, col/gm 1131 Ges Volume, cc/gm 830 Formation, col/gm Fusion, col/gm 29.4* «Calculated for 76-9)1 HMX, 23.16 HIT. Armor Mote Import Teet: 40 asm Mortar MejocHlet 50% Inert. Velocity, ft/sec Aluminum Fineness Plate Thickness, inches 1 1% 1% 1%
Specific Heat: col/gm/'C ** -79 C 0.200 -50° to +80°C 0.240 33° to 74°C 0-245 90° to 150cC 0.323 ♦♦Determined for 76.96 HMX, 23.16 ™T.
Berning Rote: cm/sec
Bomb Drop Teat: T7,2QM-lb Semi Armer Raraleg Boasb vs Ciasrsta: Max Sofe Drop, ft SOCMb Oeaerel Parpeoe Bomb vs Concrete: Height, ft Trials Unaffected Low Order High Order 10004b Osnrrel Parpeoe Bomb vs Caatrete: Height, ft Trials Unaffected Low Order High Order
Thermal Connectivity: coi/sec/em/’C
Coefficient of Ixpeesion: Linear, %/*C Volume, %/*C
Mote* Scete*
E', dynes/cm* E, Ib/inch* Density, gm/cc
Compressive Strength: Ib/inch1 13^0 See below
Vapor Pressure: *C mm Mercury p Compressive Strength; Ib/inch ♦«♦ Average (10 teats) 1340 High I56O Low 1040
Ultimate Deformation: 6 Average (10 testa) 2.43 High 2.69 Low 2.04
♦♦«Test apecimen 1/2" x 1/2" cylinder (approximately 3 6°) preseed at 3 ton» (6,000 lb) total
load or 30,000 psi with a 2 minute time of dwell.
255
АМСР 706*177
Octol, 75/25
PW^p^RHWVVWVl • Ммо M MM MI. M71 PreJetfKs, La» WC-»1: Density, gm/cc Chorge Wt, lb For TNT For Subject HE 1 lash Kt, M43A1 PrejKtfc. La» KC4: Density, gm/cc Chorge Wt, lb Total No. ef Fragmaaii: For TNT For Subject HE Shaped Ctesge Hbatireeeea, TNT s 1И: Gloss Cones Steel Cones Hole Volume Hole Depth
Cote: Buff
iMadgei Uses: HE projectile and boob filler
Method ef Leodiag: cast
Leedtag Deaaity: gm/cc 1.81
tagnreat Vateily: ft/sec Attft Л'ЭД* Danatty, gm/cc
Method №y Hazard Class (Q'jontity-Distance) Class 9 Compatibility Group Group I Exudation
Meet (Rotate to TNT»: Air: Peak Pleasure Impulse Energy Air, Cantina».' Impute Under Water: Peek Pressure Impute Energy Peak Pressure Impute Energy
Work to Produce Rupture; ft-lb/lnch^ * Average (10 teats) 1-31 High 1.57 low 1.07 Efflux Viscosity, Saybolt Seconds; 9.0 *Test specimen 1/2" x 1/2" cylinder (approxi- mately 3 8") pressed st 3 tons (6,000 lb) total load or 30.000 pal with a 2 mluute time of dwell.
256
Octol, 75/25
АМСР706-1П
Fragment Velocity Teat:
неб gand grenade:
(’)
EQlceire Average Fragment Velocity, Я/sec ovglsiTra
1 Co^osition В 75/25 Cyclotol 75/25 Octol 1*9**8 1*908 512U
Ib/inch*
! Average (10 tests) 2Ы>" *
I High 330
Lov 226
aa per
ketch XL-O76B, at 21°C.
Modulus of ELaatlclty:*
I Ib/inch^
J Average (10 testa) • 62,ICO |
High > 75,900
Low_____________________ 1*5,200 i
♦ftit apedmen 1/2" x 1/2" cylinder (approximately 3 gm) pressed at 3 tons (6,000 lb) total
Icid or 30,000 psi with e 2 minute time jT dwell.
Setback Sensitivity Tate: (a)
Critical Pressure j 76,000 pai« ;
Banslty, ga/cc 1.80 I
Trtrea—i-.e below which no initiati(.n 1' obtained and above which an increasing percentage of
initiations can be expected as the setback pressure increases.
Pit Fragmentation Test:
105 — Ю. HE Piujectile;
Weight Group, grains Bo. of Fragments
1/2 - 2 1611
2 - 5 777
5 - 10 535
10 - 25 719
25 - 50 1*80
50 - 75 21*6
75-150 339
150-750 293
750 - 2500 8
Total Number 5OOS
257
AMCP 706-177
Octol, 70/30; Ootol, 75/25
Preparation:
Whter-vet HMX 11 added slowly to molten ЛСГ In a steam-Jacketed kettle at a tea^errture of
100°C. Ihe mixture is heated and stirred until all. moisture id evaporated, ihe composition
is cooled to a aatiaActoiy pouring temperature and cast directly into aaounition coavonenta
or prepared in the form of chips to ha stored for later use*
References;52
(a) 1st Indorsement from Chief, Explosives Development Section, to Chief, Explosives
Research Section, Picatinny Arsenal, dated 12 №y 1958* Subject: "Properties of Octols anC
HTA-3."
(b) A. W. O'Brien, Jr., C. ft. Plumer, R. P. Woodburn and V. Ihilipchuk, Detonation Velod-
ty Determinations and Fragment Velocity Determinations of Verted Explosive Systems and Condi-
tions, hitiooal northern Corporation renal Isumary Report MMC-F-13, February 1958 (Contract
BCTT9-O2O-501-0RD- (p) 58).
^2See footnote 1, page 10.
258
PB-RDX
АМСР 706-177
СмфмМм: % RDX 90 Polystyrene (unmodified) 8.5 Dioctylphthalate 1.5 C/H Ratio Molecolor Weight: 2U5
Oxygon Science: CO, % -62 CO % -18
tt-i-ftr П-/" Unpresaed 0.81 Tfellet areneed at 40.000 nal 1.62
MiMag Point: *C
Freezing Point: ’C
Impost Ssnsitirity, I Ke Wt: Unpressed Bureau of Mines Apparatus, cm 28 Sample Wt 20 mg Pkotinny Arsenal Apparatus, in. 15 Sample Wt, mg 20 BeMag Point: *C
Beftocthe Index, n“ П» n£
voewVv^RB о^МО^^В^^ЯОО В« Steel Shoe Unaffected Fiber Shoe Unaffected W^^cno^M Cb^baltbw T^^e cc/40 Hrs, at 90‘C —— 100'C 120’C 0.41 135‘C 150*C
Rifle BoNet Impeet Teel: 10 Trials » % Explosions 10 Partials 90 Burned 0 Unaffected 0
200 всем Bomb Sand Tost: Send, gm
ЬрМеа Tompwatoio: *C Seconds, 0 1 (no cap used) — 1 5 Smokes 275 10 15 20 m *ALrtBu . •MWWfxWy ™ aOBvw^^W^Mio Minimum Detonating Charge, gm Mercury Fulminate Load Azide Tetryl
BeOietic Metier, % TNT:
Trewd Tost, % TNT:
7!*C IntomaHenel Heat Teel: % Lou in 48 ! !.-»
Plate boat Test: Method Condition Confined Density, gm/cc Brisance, % TNT
100‘C Meet Teet: % Lou, 1st 48 Hrs 0.00 % Lou, 2nd 48 Hrs 0.00 Explosion in 100 Hrs None
DetoMtHi Bote: Confina.nent Condition Charge Diameter, in. Density, gm/cc Rate, meters/second
Flammability Index:
Hygroscopicity: %
* Test procedure described in PATR No. 2247, Mny 1956.
259
АМСР 706-177
PB-RDX
^^^ВЯ^Яа v^MCWVvwy ЮТ» *- — vonoiTion Tetryl, gm Wox, in. for 50% Detonation Wax, gm Density, gm/cc Oxygen, otoms/soc (Z/sec) Hoot, kiloeolorie/molo (AH, kcol/mol) Temperature Range, °C Phase
Neat of: Combustion, col/gm 3027 Explosion, cal/gm 983 Gas Volume, cc/gm Formation, col/gm Fusion, col/gm Armor Plate Impart Teat: M mm Mertor Projectile: 50% Inert, Velocity, ft/sec Aluminum Fineness Виолево Booths* Plote Thickness, inches 1 1'4 I’/i Hr
Ipacffis Heat: col/gm/°C
>emi»i Rate: cm/sec
Bomb Drop Tort: T7,20064b Somi-Annor-PiorcJag Bomb vs Concrete: Max Safe Drop, ft 500-ib General Purpose Bemb *s Concrete: Height, ft Triols Unaffected Low Order High Order 1000-lb General Purpat» Bomb *t Concrete: Height, ft Triols Unaffected Low Order High Order
CMBBecthrity» col/MC/cm/’C
Cot^ficioet ef Expansion: Linear, %/°C Volume, %/°C
Yeoeg'i Medatae: See below E', dynes/cm* E, Ib/inch1 Density, gm/cc
Ctxiprtnlvt Strength: Ib/inch’ 2403 2149 Percent 8.9 13.1
Vapor Ргамага: °C mm Mercury Young's Modulus: • (a) Temperature 2 Ambient 95°C E, lb/ii ch (svg of 5 ) 39,953 ЗМГй Density, gm/cc 1.60 1.57
•Pellet» (Lot OAC-596-55) O.75O Inch diameter by 0.750 Inch Long, pressed at 30.000 pst with
30-second dwell.
260
PB-RDX
AMCP 706-177
W яма Nt. M71 Projectile, Let WC-01: Density, gm/cc ChorgeWt, lb ^ЦРЛ vWV* a^•• For TNT For Sublet HE 1 lack ME. M42A1 Projectile. La» KC-S: Density, gm/cc Chorga Wt. lb Tatal Na. of Fragmeais: For TNT For Subject HE Shaped Charge MfoeHveaoea. TNT = 1*0t Gloss Cones Steel Cones Hole Volume Hole Depth
Cofer: White
Priacipai Usee: High neehanical strength explosive
Mediad of Leedfap: Pressed
Leading Oeaeky: gm/cc Pre,,e<i’ p,i x 0 10 20 30 1.10 l.k9 1-59 1-62
Frogaroet Vehoity: ft/sec At 9 ft At 25Ц ft Density, gm/cc
Method Dry Hozord Close (Quontpy-Distance) Class 9 Compatibility Group Group I Exudation None
Moat (ЫмЬе la TNT): Ain Peak Pressure Impulse Energy Air. Conflnadt Impulse Under Water: Peak Pressure Impulse Ene<gy Uadorgreaad: Peak Pressure Impulse Energy
Rockwell Hardness, "R" Scale: (e) 1/2 inch diameter Penetrator, 60 Kg Load; Pellet Specific No.* Gravity Hardness 1 1.62k 8k 2 1.623 90 3 1.611 8k к 1.600 80 5 1-590 75 6 1.571 73 7 1.5k8 62 8 1.52k k9 ♦Pellets (Lot HOL-F-93) were 1-1/2 inches in diameter and ЗА inch high.
261
.’'СР 706-177
PB-RDX
Sensitivity of PB-RDX and 96/2 RDX/Stearic Acid
rex-.ets* to Initiation by Type II Special Blastlng*‘caps (a)
Gap (Distance From Base of Cap to Pellet). Inches
Pellets ( 6.25?’ 0.300 d.tfd 0.400 o.W d.Jdd 0.Т5У
PB-RDX with Pellet Density 1.55 gm/cc
№. of Trials 8 5 6 2 1 1
Average Dep uh of Plate Indentation, inches ** 0.082 o. 090 0.087 0.080 0.080
No. of Frilures 0 1 3 4 1 1 I
PB-RDX vith Pellet Density 1.60 gm/cc
No. of Trials 3 8 9 4 3 5 2
Average Depth of Plate Indentation, inches •* 0.090 0.089 0.087 O.v,- 0.087 0.075
No. of Failures 0 0 2 2 3 2
98/2 RDX/Stearic Add With Pellet Density I.63 gm/cc
No. of Trials 5 3 c 5 5 5
Average Depth of Plate Indentation, inches »* 0.109 0.096 0.095 0.092 0.097 0.087
No. ef Failures 0 1 0 3 4 4 5
• Pellets 0.92 Inch diameter, 0.375 Inch height.
** Mild steel plate 5" x 5" x 1".
Performance of PB-RDX as Booster: (b, d)
Ten 2.75 Inch HEAT Ml Rocket Heads were unaffected in performance by storage at 71°C for
38 days. Thus, PB-RDX vas not desensitized by contact with INT-bearlng explosives. Tetryl,
similarly used, becomes desensitized vhen stored in bursting chargee at elevated temperatures.
In addition, 108 modified МЭ07А1 57 nm> projectiles were firad for performance against
armor. Each round contained a PB-RDX booster pellet. There vas no evidence in these firings
that the projectiles were inadequately boostered.
262
АМСР 706-177
PB-RDX
Preparation:
The purchase description sheet for polystyrene-bonded RDX (X-RA-PD-1088., 25 October 1956)
requires that the PB-RDX shall be a mixture of RDX. coated and surrounded by a homogeneous
mixture of polystyrene and dioctylpnthalate. The specified percentage of RDX shall consist
of a mixture of 75$ ТЛ* B, Class A RDX and 25$ Type B, Class E RDX. The granulation of the
unpressed composition shall be as follove:
Through U. S. Standard Si eve No. Minimum $ 1 MBjcinuiD %
6 100 __
12 60
20 -- 2
35 -• 0
Two methods have been reported for the preparation of PB-RDX (Reference: Los Alamos Scien-
tific laboratory, Contract W-74O5-Eng 36 with U.S. Atomic Energy Comiesion, Report No. IA-
1448). The earlier method employed a Baker-Perkins type mixer to blend the components. This
procedure gave a product with good pressing characteristics. However, the molding composition
was nonuniform in granulation and tended to be dusty. The slurry method of PB-RDX preparation
gave a product which was uniform, free-flowing and dustless. In addition, PB-RDX granulated
by the slurry method exhibited satisfactory drying, handling and pressing characteristics.
The final procedure incorporating the better features found from ths study of such variables
as solvents, solvent/plastic ratios, lacquer addition and temnerature, agitation, RDX particle
size distribution, dispersants and rosin additive, was aa folxovs (Reference c):
Forty-two and five-tentl.s grams (42.5 S®) of polystyrene and 8 cc dioctylphthalate were
dissolved In 200 cc toluene in a lacquer dissolver. Steam was Introduced into the jacket
until the temperature reached 65°C. The lacquer was agitated constantly until it was ready
to be added to the granulator. This lacquer contained a 1:4 ratio of plastic-plasticizer to
toluane.
Four hundred and fifty grems (450 gm) of RDX and 4500 grams of HgO (ratio 1:10) were added
to the grenulator. The agitator was set for 400 rpm and the temperature was raised to 75°C
by introducing steam into the jacket. Die temperature differential between the lacquer solu-
tion and the RDX/water slurry was 5° to 10°C.
Die lacquer solution was poured through the emerging funnel into the grenulator. As soon
as the lacquer was added, a solution of gelatin in water was added, end the mixture was agi-
tated until the lacquer was well dispersed in the RDX slurry (approximately 5 minutes). Granu-
lation took place at this point. Steam was introduced again into the jacket to distill the
solvent until the temperature reached 98°C. Cooling water was then run into the Jacket to
cool the batch to 4o°C. Die coated material from the granulator was collected on a Buchner
funnel and dried in a tray at 70°C for 24 hours. Temperatures below 70°C did not furnish
enough heat, but a temperature of SO°C produced stickiness and caking of PB-RDX.
Origin;
An explosive consisting of RDX coated with polystyrene plasticized with dioetyphthalate
was Initially developed in 1952 for the Atomic Energy СоыЛвв1оп by Lob Alamos Scientific
Laboratory of the University of California (contract W-7405-Eng 36 with U. S. Atomic Energy
263
AMCP 706-177
ra-RCT
Co—iaaicn, Report Ко* IA-1M8). She аресШс ftnulaticn of 90/0.5/1*5 Rnc/polyatyrene/
dioctylphthalate мм aubeequently atandardlaed by Loe Alaaoa. This exploeive, originally
deeignated PBX, baa boon redaaHnatad П-ИК. П» detailed re^uire—nta for tbe preeent
polyatyrene>bonded !UK(n>RIK) are (Ivan In purehaae deacripticn Х-ЙА-РО-1066, 25 October 1956.
Refarenceaiи
(a) B. j. Zlotucha, T. w. Stevena and С. I. Jaoobaou, Cbaracterietica of Polyatyrene-
Bcndad RPX(PB-KIX), PATR Bo. 2^97, April 1958.
(b) A. J. PMcaaio, The Suitability of a Kara FHX Booater Pallet in the 2.75 Inch Ml ДАТ
Rocket Bead, HWR Bo. 22^1, Kovaabar 19!>$*
(c) J. L. Venaillicn and H. C. Dubberly, plaatlc-Bonded ИД, Ita Preparation by the Slurry
Method, Bolaton Dafbnae Corporation, Control Bo. 20-T-lh Sarlea A (t*d 1ОЯ), *> rfuah 1953-
(d) C. J. Eichinger, Report on Cartridge ПАТ 57 ай M3PTA1 (Mod) id th Modified Coppar
Linar, Aberdeen Proring Ground, Develop—nt and Proof aervicea, Firat Report on PC Project
ЯЗЗЗД, October 1957.
ee footnote 1, page 10.
264
Ponta erythritol Trinitrate (РИММ)
АМСР 706-177
Metecoiw Weight: 271
W С 22.1 В 3.3 Г2^ Оцфмв Betaoce* co, % co % -87 3
HOCHg—С —CHgOHOg Density: gm/cc 1-5^
сн_о1ю9 0 59.1 * MeMng Point: *C 26 to 28
С/Н Rotio 0.1U1 Mh^^a^Aa ПВ*К1В| rWWi V
hnpeet 8оааШеОу, 1 Kg Wh Buraou of Mine* Apporatus, cm Sample Wt 20 mg Picatinny Arsenal Apparatus, In. 5 to 10 Sample Wt, mg 38 BaMng Point: *f It m Hg Deeoapooea 130
Refractive Cades, n£ n£ n&
vоОП Steel Shoe Fiber Shoe Vocraras StobMy Teeh cc/40 Hrs, ot 90‘C lOCX 120’C 135*C 150’C 2.54 to 5.69
RMo BaNet Impost Test: Trials % Eolations Partials
Burned unarrecrva 200 Gram Borah load Tosh Sond, gm
(яр1мйя ТифМвНм: *C Seconds, 0.1 (no cap used) 1 S 10 С-мЫ—Цы n^Xal^A^mo ^VOOVOVwvWy W VONBO^w^MBo Minimum Detonoting Chorgo, gm Mercury Fulminate Lead Azide Tetryl
20 Bolistic M wter % TNT:
Trawd Tost, % TNT:
75’C lotaseoHeaol Hoot Toth % Loss in 48 Hrs
Piste Dent Tosh Method
100’C Heat Tosh % Loss, 1st 48 Hrs % Loss, 2nd 48 Hrs Explosion in 100 Hrs Condition Confined Density, gm/cc Brisance, % TNT
Confinement
Р1мммЬМ1у tamest
uoneiTKx* Charge Diameter, in. Density, gm/cc Rote, meters/socond
Volatility:
265
AzCCP 706-177
Pentaerythritol irinitrate (PETRIN)
Fsegaseatetiea Teat* •0 asm HE, M71 FrajecHle, let WC-91: Density, gm/cc Charge Wt, lb Tefal No. ef FrwgaseeH: For TNT For Subject HE 3 tech HE. M42A1 hajactils, Lot KC-S: Density, gm/cc Chorge Wt, lb Ыж .—Ж 0^We W* ev^^HiWlHVo For TNT For Subject HE Shaped Charge EHoctiveoou, TNT = 100: Gloss Cones Steel Cones Hole Volume Hole Depth
Сект: ^5^
Friadpel Usee: Explosive, propellent or igniter ingredient
Method ti Laediag:
Laedtag Density: gm/cc
Frogmens Velocity: ft/sec At 9 ft At2Sft ft Density, gm/cc
Storage: Method Dry Hazard Class (Quantity-Distance) Compatibility Group Exudation None
Meet (OeleHoe to TNT): Ain Peak Pressure Impulse Energy Alt, Cos.laed: Impube Umdes Weton РкЧ pressure Impulse Energy Peak Pressure Impulse Energy Absolute Viscosity, poises: Temp, 17°C 14.8 23°C 4.3 28 C 3.0 38°c 1.2 1
PETRIN esters are listed in reference (b) and most of these esters have been shown to have explosive properties. An Infrared spectrophotometric procedure was developed for the determination of the acetone con+ent of PETRIN (ref c). A 2.5 gm setnple of PETRIN is dissolved in chloroform end the volume increased to 25 milliliters in a volumetric flask. The acetone content of the PETRIN solution ie determined by Its Infra- red absorption at 5.82^* in a 0-5 mm cell- A double beam method is used with a reference cell containing chloroform end acetone-free PETRIN. The quantity of the letter must be carefully adjusted to give a good balance be- tween the test sample and reference cells for the stror.z PETRIN peak at 6.02^< maximum. Heat of; Explosion, cal/vm 1204
266
Pentacrythrltol Trinitrate (PETRIN)
AMCP 706-177
Preparation:
CCC^OH)^ + 3HNOj ----------------> OHCH^CHgNO^ + 3^0
pentaerythritol nitric sulfuric pentaerythritol water
add acid trinitrate
MW 136 MW 63 MW 98 MW 271 MW 18
The earlier' procedure uaed for the manufacture of PETRIN waa that developed at Alleghany
Ballistics Laboratory. In this process* called the "A process," 80$ HNO:; and the solid pen-
taerythritol were charged to the reactor and 80$ HgSOj^ was added slowly at a rate to permit
control of temperature at 0° to 5°C. Ibis mixture was held for a 2-1/2-t.our reaction period,
then drowned in water and filtered to give a cake containing both the tri- end tetra-nitrates
of pentaerythritol. The cake was dissolved in acetone and neutralized In solution with am-
moniu carbonate, after which the PE® - .s precipitated by the addition of water. After fil-
tration, the PETRIN was recovered from the filtrate by stripping off the solvent under vacuum.
Yields by this process averaged about to$.
An Improved process, called the "B process,” used the same primary reaction procedure but
a different work-up procedure. After tha reaction holding period, water was addpd to dilute
the mixed acid and the batch was extracted in situ with methylene chloride. Iba organic
layer was separated, neutralized with aqueous sodium bicarbonate, and stripped of methylene
chloride under vacuum to yield the product directly. Yields by this process were about $0$
and quality of the product was much improved over that of the "A procass."
The "C process," currently in use, involves essentially the simultaneous synthesis and
extraction of PETRIN from the reaction mixture. Methylene chloride approximately equal to
tlit toual weight of the other components Is added to the reaction mixture before the sulfuric
acid. After s ь-ltpble time following the addition of sulfuric acid, the solvent is removed
and v.i- seed by fresh solvent one or more times. The combined extracts are neutralized and
concetcrate;. Because of their Initially relatively large volume, PETR —be r*—.oved by
flltratlo,. from the concentrated PETRIN solution before the flnel solvent la stripped. Yields
by this process have been 60$ to 65$.
Origin;
The nitration products of pentaerythritol or itc derivative containing not more than three
NO» groups were patented for use as explosives, propellants or ignition materials in 1936
(German Patents 638Д32 and 638Л33; CA 31, 1212 (1937)).
A process in which pentaerythritol monoacetate was converted to penteerythritol trinitrate
monoacetate, which was then saponified under carefully controlled conditions to PETBIN, was
repoited in 195b (N. S. Marans, D. E. Elrlck and R. F. Preckel, J Am Chem Soc 7o, 1301*).
’’ETRIN was also prepared by the nitretion of penteerythritol with a mixture of~So$ HNOa and
60$ HoSOl in 1955 (A. I- Camp, N. S. Mbrans, D. E. Elrick and R. F. Preckal, J Am Chetb Soc
77, 751).
267
АМСР70С-1П
Pentaerythritol Trinitrat* (ИМИ)
ReferanceeP*
(a) Rota and Вши Совпаду, Redstone Arsenal Diriiica, Proceea for the Ifcnuftctnre «?
Pentaerythritol Brlnltrete Monoacrylate and Petrin Acrylate bropelliuita, 12
(b) Ж* Berio», R. H. Barth and J. Ж» Snow, Me Pentaerythritoll, ACS Monograph Bo. 136,
p. 65, Reinhold Publishing Corporation, Bev York, 1ЙЙ.
(c) R. H. Pierson, An Infrared apectrophotcnetrlc Method for Deterainaticc of Acetone
OW^nt^^ Pentaex^ttoitol^nltraie^b.8. Meval Ordnance ^ait Station ВепоЯ teas IbTTT
^Чее footnote 1, page 10.
2вв
AMCP 706-177
Penteerythrltel Trialtraecrylate (РВПШ< Acrylate)
llrinltroxypeniaerytnrlloi Acrylatej
% c 29.5 CHgONOg CHg - CI-COgOHgC-CHgONO,, CJ^ONOj, (Wu) 325
Oxygen Boleace: CO, % co % -54 -12
f entity: gm/cc
Matting Point: °C 78 to 79
C/H Ratio О.239 Pressing Point: °C
Imped Sensitivity, 2 Kg Wh Buraou of Mines Apparatus, cm Sample Wt 20 mg Picctinny Aisenol Apparatus, in. Sample Wt. mg BeiHng Point: °C
Refractive Index, njj n£ nS,
*» *-*i*— Steel Shoe Fiber Shoe Vacates Stability Test: cc/40 Hrs, ot 90°C 100°C 120 °C )35°C 150°C
Rifle Ballet Impact Test: Triols % Explosions Portiols
Burned Unaffected 200 Grom Всей Send Teet: Sand, gm
bpMea Tsmperofare: °C Seconds, 0.1 (nj cop used) 1 5 10 Sensitivity to InMotion: Minimum Detonating Charge, gm Mercury Fulminate Lead Azide Tetryl
20 Bsflistic Mortar, % TNT:
Treoxl Test, % TNT:
7S*C International fleet Test: % Loes In 48 Hrs
Plata Dent Test: Aiethod
100°C fleet Teet: % Loss, 1st 48 Hrs % Loes, 2nd 48 Hrs Explosion in 100 Hrs Condition Confined Density, gm/cc Brisance, % TNT
Dotoae*’mi Rote: Confinement
Flammability lades:
Hygroscopicity: % Nil Charge Diometer, in. Density, gm/cc Rote, meters/second
Volatility:
269
Pentaerythritol Trlnltroacrylate (PETRIN Acrylate)
ЛМС9ПНП
FI^JIHWRwnWi * V*Vo Shaped Charge ЕНесНеммее, TNT -- 100:
90 м HE. МП PrejecHb, Lot WC-91: Gloss Cones Steel Cones
Density, gm/cc Charge Wt, lb Hole Volume Hole Depth
Total No. of Frogmiett: Color: White
For TNT For Subject HE Principal Usee: Ingredient of composite
3 tach HE. M42A1 Prejoctile, Let KC-S: rocket propellants -
Density, gm/cc
Charge Wt, lb
Total Ne. of Fraga ret r: Method of Ltidtag:
For TNT For Subject HE Loodtog Doasity: gm/cc
Fraga rat Vetadta: ft/sec At 9 ft At 25 >4 ft Density, gm/cc Method Dry at temperatures below melting point
В Scot (Relative to TXT): Hozord Class (Quantity-Distance)
Air: Compatibility Group
Pook Pressure Impulse Energy Exudation None
Air, Coe.".; ed: Impulse , Heat of: Combustion, Л/ца 2923
Under Water Peak Pressure lm<ulse Energy Explosion, cal/gm 791
UndergfMWrfe
Peak Ргемиге
Impulse
Energy
270
Pentaerythrl л .rinitrobcrylate (PETRIN Acrylate)
AMCP 706-177
Preparation: (a)
носнгс(сн2Мо3)3 * CHg • CHCOci «• c6h5«(ch3)2 -----
pentaerythritol acrylyl dimethyl
trinitrate (PETRIN) chloride aniline
MW 2?1 MW 90-5 MW 121
(tyNOCHgJjCCHgOCCH - dig + C6H5H(CH3)2HC1 <------------
pentaerythritol trinitrate mono- dimethylanine
acrylate (PETRIN acrylate) hydrochloride
MW 325
The original synthesis for PETRIN acrylate employed trifluoroacetic anhydride and glacial
acrylic acid ss the acrylation agent for PETRIN. These two me..erlaIs were charged to a re-
action vessel and the initial reacticn was controlled by the slov addition of PETRIN at a
temperature of 10° to 15°C. Following a period of one hour, the batch was drowned in water,
precipitating the FuIRIN acrylate. This solid was separated by filtration, dissolved in chlo-
roform, and neutralized in solution with sodium bicarbonate. The product was then crystal-
lized during a period of 16 hours at 0°C and dried under vacuum to remove traces of solvent.
The yield for this process was about 60$.
A significant improvement in yield (to about 74)1) and purity (approximately 9ЭД was
realized by the substitution of methanol for chloroform and crystallization of the product
from the solution without neutralization, residual acid being removed by washing the filter
cake with water.
Because of the high coat and hygroscopic nature of trifluoroacetic anhydride, a new pro-
cess, based on dimethylaniline '.nd acrylyl chloride, was considered. This process is cur-
rently under development in tne Rohm and Haas Chemical Processing facilities and is not con-
sidered optimum. Yields averaged and product purities averaged 93-5$.
PETRIN Acrylate Propellants;
PETRIN acrylate could be used as a monopropellant because it has a specific impulse of
214 lb-eec/lb and a burning rate of 0.2 in/вес. The addition of an oxidizer increases both
the impulse and burning rate.
A composition which presently appears most promising is as follows:
PETRIN acrylate ( > 97jl purity), % Triathylene glycol ‘rinitrate, % Glycol diacrylate, % Ammonium perchlorate, Hydroquinone, $ Composition NM 34?3 (binder) 11.8 (plasticizer) 2-9 (croaslinker) 51.0 (oxidizer) 0.014 (polymerization inhibitor)
Measured specific impulse 238 lb-eec/lb, at density of 1.3.
Reference:5'
(a) Rohm and Haas Company, Redstone Arsenal Division, Process for the Manufacture of
Pentaerythritol Tetranitrate Monoecrylate and Petrin Acrylste Propellants, 12 March 1956-
5^See footnote 1, page 10.
271
АМСР 706-177
Pentolite, 50/50; Ю/90
Molecular Weight: '5^^
PSTN 50 10 ТОТ 50 90 Oxygen Balance: ca % .42 -68 CO % .5 -21
Density: gm/cc 1.65 1 • 60
Molting Point: °C 76
C/H Rotio Froexing Feint: *C
Impact TomitMty, 2 Kg Wt: 50/50 10/90 Buror.uof Mines Apporatus, cm 3* 65 Somple Wt 20 mg Pleotinny Arsenal Apparatus, in. 12 14 Sample Wt, mg 15 18 Bailing Point: ‘C
Refractive Index, n& П» n°
pvmv^w i **>o Steel Shoe Unaffected Fiber Shoe Unaffected Vacuum Stability Toot: 50/50 10/90 cc/40 Hrs, at 90”C 100‘C 3-0 3-0 120’C 11+ 11+ 135‘C 150‘C
Rifle Ballet hnpaet Teel: 25 Trials, 50/50 % Explosions 72 Partials 20
Burned 0 Unaffected 8 209 Gram Bomb Sand Test: Sand, gm 55-6 49.5
sptoelea Temperature: *C, 50/50 Seconds, 0.1 (no cap used) 290 1 266 5 Decomposes 220 10 204 15 197 20 >190 Sensitivity to leitiotien: 50/50 Minimum Detonating Charge, gm Mercury Fulminate 0.19* Load Azide 0.13* MlternXtlve initiatine charcee.
Ballistic Mortar, % TNT: (a) 126
Trovzl Test, % TNT: (b) 122
75‘C International Heel Teel: % Loss in 48 Hrs
Piet* Dm* Teat: (c) Method Б
100‘C Heat Teel: 50/50 % Loss, 1st 48 Hrs 0.0 % Loss, 2nd 48 Hrs 0.2 Explosion in 100 Hrs None Condition Cast Confined No Density, gm/cc 1.66 Brisance, % TNT 121
D**omHm Rote: Confinement Попе Condition Cast Chorge Diometer, in. 1.0 Density, gm/cc 1.66 Rote, meters/second 7465
PhaMMbility Index: Will not continue '.0 burn
Hygroscopicity: % ^2122 1212° 30°C, 90% RH None None
Volatility:
272
Pentolite, 50/ЬО: Ю/'чО
AMCP 706-177
Booster Sensitivity Teet: (d) 50/SO Condition Pressed Cast Tetryl, gm 100 100 Wox, in. for 50% Detonation 2.36 2.08 Wax, gm Density, gm/cc 1.60 I.65 Oecompeeitioa Eguotloa: Oxygen, atoms/soc (Z Чес) Heat, kilocolorie/male 11H, kcoi/mol) Temperature Range, °C Phase
Hout Combustion, col/gm Explosion, cal/gm 1220 Gas Volume, cc/gm Formation, col/gm Fusion, cal/gm Armor Plate Impact Toot: 50/50 60 mm Mortar Projectile: 50% Inert, Velocity, ft/sec 170 Aluminum Fineness 300-lb General Purpose Bombs: Plate Thickness, inches 1 14 lit l-ъ
Specific Heat: col/gm/“C
Burning Rate: cm/sec
Bomb Drop Toot: T7, 2MX>-№ Semi-Armor-Piorcing Bomb и Concrete: Max Safe Drop, ft SOO-lb General Purpose Bomb vs Concrete: Height, ft Trials Unorfected Low Order High Order 1000-lb General Purpose Bomb n Concrete: Height, ft Trials Unaffected Low Order High Order
Thermal Conductivity: col/sec/cm/’C
Coefficient of Expansion: Linear, %/°C Volume, %/”C
Hardness, Mohs' Scale:
Young's Modules: E', dynes/cm3 E, Ib/inch3 Density, gm/cc
Compressive Strength: Ib/inch* 2000-2200 Dens’ ‘.j, gm/cc 1.6c
Vapor Pressure: X mm Mercury
273
АМСР 706-177
Pentolite, 50/50; 10/90
М ж HU МП Projectile. Lot WC-91: ^0 Shaped Charge Iffecthrooeee, TNT = IN: 50/50 10/90 50/50 25/75 Gloss Coneeff) Steel Cones (g)
Density, gm/cc 1.65 Hole Volume 157 105 149 119
Chorge Wt, lb 2.147 Hole Depth 116 116 131 119
Tetel No. ef Frogeseats: For TNT For Subj< ct HE 3 loch HU M42A1 Projectile, Let KC-S: 703 963 Color: Yellow-vhite
Principal Usee: Shaped charges, bursting charges, demolition blocks
Density, f.m/cc 1.65
Charge Wt, lb 0.672
Tetel No. of FrogoMMs: For TNT For Subject HE 514 650 Method of Leading: Cast
Loading Density: gm/cc 50/50 10/90
Fragment Velocity: ft/sec At 9 ft At2Sftft 2810 2580 1.65 1.60
Density, gm/cc 1.66 Method Dry
Meet (Rotative to TNT): (•) Haxard Class (Quantity-Distance) Class 9
Air: Peak Pressure 105 Compatibility Group Group I
Impulse 107 Exudation
Energy ..
Compatibility with Metals:
Air, Ceoftoed: Impulse Under Water: Peak Pressure Impulse Dry; Copper, brass, aluminum, magnesium, megaesium-aluminum alloy, mild steel coated with acid-proof blsck paint, end mild steel plated vlth copper, cadmium or nickel are not affected. Zinc plated steel is only slightly affected.
Enjrgy Underground: Peak Pressure Impulse We*.: Stainless steel, aluminum and mild steeTTor ted with acid-proof black paint are not sift ted. Copper, brass, magnesium, mag- nee lum-slumlnum slloy, mild steal and mild steel plated with copper, cadmium, zinc or nickel are slightly effected.
Energy Effect of Temperature on (h)
Eutectic Temperature, °C: 76 Rate of Detonation: , 16 hrs at, °C -54 21 Density, gm/cc I.67 1.66 Hate, m/sec 7470 7440
gm PEUi/100 gm TNT 7б°С 95°C 13-0 26.3
274
Pentolite, 50/50; ю/90
AMCP 706-177
Preparation:
Pentolite ie manufactured by either the «lurry method or coprecipitation of PEIN and ТЭТ*
m the slurry method PEIN, in water, ie «timed and heated above 80°C* ТЭТ 1« added and when
molten, it coate the particle» of PEIN* The elurry is cooled vith rapid «timing and the «вра-
га ted granules are collected on a filter and dried below 75°C.
In ^precipitation, PEIN and ТЭТ are dissolved separately in acetone. The ecluticne are
mimed and the explosives are precipitated «imultaneouel; by pouring the mixed solution into
cold water under vigorous agitation. Tha precipitated solid is collected on a filter and
dried in air*
Origin;
Standardized during World War II, with the 50-50 PETN/INT mixture being the more important
for bursting charges and booster-surround charges.
References:56
(a) L. C* Stalth and E. G* Eyster, Physical Testing of Explosives, Part III - Miscellaneous
Sensitivity Tests; Performance Tests, OSRD Report ho. 5746, 27 December 1945.
(b) Philip C. Keenan and Dorothy Pipes, Table of Military High Explosives, Second Revision,
WORD Report No. 87-46, 26 July 1946.
(c) D. P* MacDougall, Methode of Physical Testing, OSRD Report No. 803, 11 August 1942.
(d) L. C. Smith and 5. R. Walton, A Consideration of RDX/Wax Mixtures as a Substitute for
Tetryl in Boosters, NOL Memo 10,303, 15 June 1949. —————————
^^e) W. R. Tbmlinson, Jr., Blast Effects of Bomb Explosives, PA Tech Div Lecture, 9 April
(f) Eastern Laboratory, du Pont, Investig. on of Cavity Effect. Sec IH, Variation of
Cavity Effect vith Btplo«lve Composition, JCTC Contract W672-0RD-5723.
(g) Eastern Laboratory, du Pont, invactigation of Cavity Effect, Final Report, Contract
W-672-0RD-5723, E. lab, du Pont, 18 September 1943-
(h) W. F. McGarry and T. W. Stevens, Detonation Rates of the More Important Military Explo-
sives at Several Different Temperatures, PATR ho. 2383» November 19§6.
(1) Also see the following Picatinny Arsenal Technical Report on Pentolite:
0 1 2 2 4 5 6 7 8
1360 1291 1212 1133 1284 1325 1436 1477 1388
1420 1451 1262 1193 2004 1466 1677 1598
1570 1651 1374 1213 1796 1737 1668
1363 I838
56gee f octnote 1, page 10.
275
АМСР 706-177
РЬТН (Pentaerythritol Tetranitrate)
Ceesposltion: % C 19.0 °H°2 H 2.5 |K2 N 17.7 02»0-CH2-C-CH2-0N02 0 60.8 J“2 C/H Rctio 0-13** 0S02 Molecular Weight: (CjHgN^O^) 316
Oxygoa Helence: co, % -io CO % 15
Densify: gm/cc CryeUl I.77
MeltHg Point: °C 141
Freezing Point: °C
Impeet Sensitivity, 2 Kg Wt: Buraou of Mines Apparatus, cm 17 Somple Wt 20 mg Picatinny Arsenal Apparatus, in. 6 Sample Wt, mg 16 Boiling Point: °C
Refractive Index, n» П» П»
BricHen Pendulum Test: Steel Shoe Crackles FiStr Shoe Unaffected Vacuum Stability Toot: cc/40 Hrs, al 90°C 100°C 0.5 120°C 11+ I35°C 150’C
Riffe Ballet Impact Test: 5 Triols * % Explosions 100 Portiols 0 Burned 0 Unaffected 0 *4.ЯМ. mnl.tnrn tn «*тр1»я
200 Gram Bomb Sand Toot: Sand, gm 62.7
Expleeiea Temperature: °C Seconds, 0.1 (no cop used) 272 1 244 5 Deccsuposet 225 10 211 15 20 Sensitivity to Initiation: Minimum Detonating Charge, gm Mercury Fulminate 0.17* Load Azide 0.03* •Alternative initiating charaes.
Ballistic Mortar, % TNT: (a) 145
Troozl Toot, % TNT: (b) 173
75 °C lotoraatioaol Heat Toot: % Loss in 48 Hrs 0.02
Plata bent Teet: (c) Method A Condition Pressed Confined Yes Density, gm/cc 1.50 Brisonce, % TNT 129
100cC Heat Tee»: % Loss, 1st 48 Hrs 0.1 % Loss, 2nd 48 Hrs 0.0 Explosion in 100 Hrs Kone
Detonation Rato: Confinement None Condition Preaped Chorge Dnmeter, in. 1.00 Density, gm/cc 1.70 Rote, meters/second 8300
Flammability lade.: Will not continue tn turn
Hygroscopicity: % 30°C, 90/ PJI 0.0
Volatility: 0.0 1
АМСР 706-177
PETW (Penteerythritol Tetranitrate)
Booster Seneitivity Teet: (с) Condition Pressed Tetryl, gm 5 Wox, in. for 50% Detonotlon Wax, gm 3 Density, gm/cc 1.6 Decomposition Ignntion: (') „ (•) (J) Oxygen, atoms/sec lO1^'0 IO20'° 10Z’‘l (Z/soc) Heat, kilocalorie/mole 1*7-0 50.9 52.3 (AH, kcal/mol) Temperature Ronge, °C 161-233 108-120 137-157 Phase Liquid Solid At melt- ing paint
Meet of: Combustion, col/gm I960 Explosion, col/gm 138? Gas Volume, cc/gm 790 Formation, col/gm 383 Fusion, col/gm Алпог Plate Impact Test: 60 mm Mester Proloctile: 50% Inert, Velocity, ft/soc Aluminum Fineness 5OO-lb General Purpose Bombs: Plate Thickness, inches 1 P/4 >4 1%
Specific Hoot: col/gm/°C (d) Room Temperature 0.26
Burning Bote: cm/sec
Bomb Drop Toot: T7, 2MXMb Somi-Armor-Piercing Bomb vs Concrete: Max Safe Drop, ft 500-lb General Purpose Bomb vs Concrete: Height, ft Trials Unaffected Low Order High Order 1000-lb General Purpose Bomb vs Concrete: Height, ft Triols Unallotted Low Order High Order
Thermal Conductivity: col/sec/cm/’C
Coefficient of Expansion: Linear, %/°C Volume, %/°C
Hardness, Mohs' Scale: 1.9
Young's Modalos: E', dynes/cm1 E, Ib/inch1 Density, gm/cc
Compressive Strength; Ib/inch1
Vapur Pressure: *C mm Mercury
AMCP 706-177
Pgm (Pentaerythritol Tetranitrate)
Shaped Charge Effectiveness, TNT = 100:
M пип HI. МП PieiecMe, La» WC-91: Glos» Cones Steel Cones
Density gm/cc Hole Volume
ChorgeWt, lb Hole Depth
Total No. e* Fragments: For TNT For Subject HE в-*5 White
Principal Uses:
3 tach HE, M42A1 hoiectita, Let KC-S: Claes A - Detonating fuse end boosters
Density, gm/cc Charge Wt, lb Class В • Priming compositions
Ma *4 * *•-- 1 ^ге^Я W vV^^pH^MWVa For TNT For Subject HE .‘.4Aod of Loading:
Leading Osnilty: gm/cc P*- *
Fragment Velocity: ft/sec At 9 ft At 254 f* J 5 10 20 30 40 1.37 1.58 l.(SU 1.71 1.73 1-71*
Storage:
Density, gm/cc Me*hod Wet
Meet (Motive to TNT): Hazard Clou (Quantity-Distance) Claes 9
Air: Comoatibility Group Group M (vet)
Peak Pressure Impulse Energy Exudation None
Air. Confined: Impulse Bulk Modulus at Room (1) Temperature (25^-ЭОис):
Under Water: Dynes/cm2 x 10”10 L.60 Density, gm/cc 1.77
Peak Pressure Impulse Energy
Underground:
Peak Pressure Impulse Energy
278
PETS (Penteerythritol Tetranitrate)
АМСР 706-177
Cosgatibility vith Metals:
Пгу; Copper, brass, aluminum, magnesium, megnesium-eluminum alloy, stainleas ateel, mild
steel, mild ateel coated with acid-proof black paint and mild ateel plated with copper, cad-
mium, nickel ar zinc are not affected.
Wet; Stainless steel is unaffected and aluminum only vary slightly so after prolonged
storage- Copper, brass, magnesium, megnesium-sluminum alloy, mild steel, mild steel coated
vith acid-proof black paint and mild ateel plated with cadmium, copper, nickel ar zinc are
affected.
Sensitivity of PETS to electrostetic discharge, Joules; Through 100 Mesh: (g)
Unconfined 0.06
Confined 0.21
Solubility, grama of РЕШ per 100 grams (%) of; (h)
TH chlorethylene
~ or Alcohol Acetone Benzene Toluene
°C 1 °C 4 4 °C 4
0 0.070 0 14.37 0 0.150 0 0.150
20 0.195 20 24.95 20 0.450 20 0.430
40 0.415 40 30.56 40 1.160 40 0.620
60 1.205 60 42.68 80 7.900 60 2.490
80 5.850
100 15-920
112 30.900
/ -Ethozy-ethyl-
Methyl acetate Ether acetate Chlorobenzene
£c 4 °C 4 °C 4 °C 4
20 13 0 0.200 20 1.5 20 0.35
30 17 20 0.340 30 4.1 30 2.8
40 22 34.7 0.450 40 7.6 40 6.1
50 31 50 11.2 50 9.2
60 14.2 60 12.2
Carbon
Ethylenedi chloride Methanol Tetrachloroethane tetrachloride
£c 4 4 °C 4 °C 4
10 0.9 20 0.46 20 0.18 20 0.096
30 1.5 40 1.15 30 0,27 30 0.108
50 2.6 60 2.6 4o 0.40 40 0.118
50 0.58 50 0.121
279
АМСР 706-177
РЕШ (Pentar?y‘aritol Те trail1 trete)
laopropanol
Isobutancl
Chloroform
TNT
°C 1 °C 1 °C £ 0., L t
15 0.02 20 C 2? 20 0.09 80 19-3
20 0.01' 30 0.31 85 25.0
30 0.15 40 0.39 90 32.1
4o 0.36 50 0.52 95 39-5
50 0.46 1’00 48.6
about 13$ PEHN 105 58.2
Eutetic of the system PSTN- TNT lu 110 70.0
and 87% TNT at 76"c. 115 87-J
120 115
125 161
Preparation:
(Nitroglycerin and Nitroglyceidn Explosives, Naoum)
8HCHO + CHjCHO + 0a(0H)2 ~* SCCaigOH)^ Oa(HCOO)2 CfCHgOK)), + 1»HNO,—♦ c(aigONO2)4 + ^HjjO
1/ In this preparation 1940 gm of formaldehyde and 600 gm of ncetellehj le are dieeolved
In 90 liters of vater containing 1600 gm suspended slaked line. The reaction la complete In
about 3 weeks if agitated several tinea a day. The solution lu filtered, the calcium formate
precipitated with oxalic add, filtered off, and ths vater removed under reduced preaauie.
On cooling the mother liquor about 1200 gm crude pentaery.-thritol, melting point 235°-24o°C
are obtained. Purification la ret.dily effected by atlrr'ig vith n little alcohol, filtering
and recrystallization from vater.
2. T_. 400 cc of strong vhlte nltrl< eld, era added 100 gm of pentaerythritol (through
50 meah), at 5°C or belov, under good ab. itlon. Z<ftsr addltior is complete stirring, at
5°C, is continued for 15 minutes. The mixture it drowned in 3 liters of ice-water, filtered,
the product washed free of add vith vater and then digested 1 hoc- in 1 liter of hot 0.b£
sodium carbonate solution. The product is filtered, and recrystallized from acetone.
Origin;
РИЯ vas known аз an explosive in 1494 when it was proposed as an add!' 'on to smokeless
powdem to raise their flammability and ease of combustion (German Patent 81.664 (1894).
Modern methods of preparation are described by Vignon «nd Gerin (Compt rend ijV 590 (1901)
and Oatman Patent 265,025 (1912) end A. Stettbscher (Z ges Schless - Sprangat ffw 11, 112,
162 (1916) and 24, 259 (1929))- PEIN vas not used on в practical basis until after World War Z.
Destruction by Cheui-al Decomposition;
PETN is d .otapor by din-solving in 8 times its weight of technical grade acetone and burn-
ing the solution 1 a shallow container. If preferred, v-rm the acetone solution to 40°C,
stir and add 7 parts by vt/ight, to each part of PETTI, of a solution of 1 part sodium sulfide
(Na2S-9HgO) in 2 parts water heated to 80°C. Hie aqueous solution should be added at such a
rate that the acetone solution does not boil. After mixing is complete continue stirring for
one-half hour.
280
PEUt (pentaerythritol "’etranltrate)
AMCP 706-177
References:5'
(a) L. C. Smith and E. G. Eyster, Physical Testing of Explosives, Part III - Miscellaneous
Sensitivity Teste; Performance Tests- OSRD Report No. 5746, 27 December 1945-
(b; Ph. Naova, Z ges Schiess Sprengstoffw, pp. 151, 229, £6/ (27 June 1932).
(c) D. P- MacDougall, Methods of Physical Testing, OSRD Report No. 803, 11 August 1942.
(d) International Critical Tables.
(e) M- A. Cook and M. T. Abegg, "Isothermal Decomposition of Explosives,” University of
Utah, Ind & Eng Them, (June 1956), pp. 1090-1095.
(ft A. J. B. Robertson, "The Thermal Decomposition of Pencaerythrltol Tetranitrate, Nitro-
glycerin. Etnyuenediamine DLnitrate and Ammonium Nitrate," J Chem Ind 67. 221 (1948).
(g) F. W. Broun, D. H. Kusler and F. C. Gibson, Sensitivity of Explosives to Initiation
by Electrostatic Discharges, U.S. Dept of Int, Bureau of Mines, RI 3852, 1946.
(h) Various sources in the open literature.
(i) W. S. Cramer, Bulk Compressibility IX *a on Several High Explosives, NAVORD Report No.
4380, 15 September 195^3
(J) Also «ее the following Picatinny Arsenal Technical Reports on PETN:
0 1 2 i 4 2 6 7 8 2
760 1041 772 343 904 1305 1246 407 318 1379
1170 1311 922 863 1274 1325 1276 527 833 1429
1260 1381 1182 IO63 1284 1-45 1316 857 12^8 1489
1290 1451 1192 1133 1414 1705 1376 1247 1318 1556
1300 1561 1212 1253 1885 1446 1517 1388 2179
1320 1611 1262 1343 2125 1456 1617 1568
1360 1651 I 342 1493 1466 1737 3 598
1380 1352 1533 1556 '-797 183C
1390 1372 1796 2178
1430 lb >2
1450
1570
281
Picrandde (THA) (2,4,6-Trinltroanillne)
АМСР 706-177
% С 31-5 МН2 н 1-3 су яео N 24.5 0 42.2 N02 С/Н Rot io 0.500 Molecolor Weight: (CgH^N^Og) 228
Oxygen 8do*ce: CO: % -56 CO % -14
Density: gm/cc Crystal l.?6
Melting Feint: 'C 189 to 190
Freezing Point: 'C
Impact Sensitivtty, 2 Kg Wt: Bureou of Mine* Apporotus, cm Somple Wt 20 mg Picotinny Arsenal Aopcrotus. >n. 23 Sample Wt, mg 20 loiMna Point- *C Decomposes before boiling w point
Riffrwcflv* Index, n£ П» nS
Friction Pendohim Teat: Ste*' Shoe Fiber She* Vecaam Stability Test: :c/40 Hrs, at 90”C —— l00"C 0.9 120’C 135C 150’C
Rifle Pellet Impact Teet: Trial* % Explosions Portia's Burned Unaffected
2tX> Gram Bomb Send Test: Send, gm 48. X
Expleeioa Temperature: °C Seconds, 0.1 (no cap used) 1 5 10 15 20 Sensitivity to Initiation: Minimum Detonating Chorge, gm Mercury Fulminote L«ad Azide 0- 30 Tetryl
Ballistic Mortar, % TNT: ]_00
Treasl Test, % TNT: 107
7S‘C Internotionel Hoot Teet: % Loss in 48 Hrs
Plate Dent Test- Method Condition Confirmed Density, gm/cc Brisance, % TNT
100 C Heat Teet: % Loss, i st 48 Hrs % Loss, 2nd 48 Hrs Explosion in 100 Hrs
Detoft«He« Rote: Confinement None Condition Pressed Charge Diometer, in. 0.5 Density, gm/cc 1.72 «otc. meters/second 7300
Flammability Index:
Hygroscopicity: %
Volatility:
2Н2
Plcramlde (1ИЛ) (2,4,6-Trini troanlline)
AMCP 706-177
Fragmouetioa Toot: M м HI, M71 Projectile, Lot WC-9I: Density, gm/cc Charge Wt, ib Total No. of Fregmeats: For TNT For Subject HE 3 inch HI. M42A1 Projectile, Lot KC-S: Density, gm/cc Charge Wt, Ib Total Ne. ef Fregmeats: For TNT For Subject HE Shaped Charge EHectiveaoss, TNT — 100: Gloss Cones Steel Cones Hole Volume Hole Depth
Color: Yellow
Principal Uses: High temperat?ire beat resistant explosive
Method of Loading: Pressed
loading Density: gm/cc At 50,000 pal 1.7&
Fragment Velocity: ft/sec At 9 ft At 25% ft Density, gm/cc
Storage: Method Dry Hozord Class (Quontity-Distonce) Class 9 Compatibility Group Group I Exudation Hone
Bleat (Rotative to TNT»: Ain Peak Pressure Impulse Energy Air, Coafiaod: Impulse Uader Woton Peak Pressure Impulse Energy Underground: Peak Pressure Impulse Energy
Solubility; Insoluble In water, slightly soluble In alcohol and ether. Soluble In hot glacial acetic acid, hot ethyl acetate end in benzene and acetone. Heat of- Combustion, cal/gm (a) 2962 Explosion, cal/gm 564 Formation, cal/gm (a) 131
2K1
АМСР 706-177
Picramide (HiA) (2,1*,6-'IYinitroan;Line)
Preparation:
Five grams of picryl chloride were dissolved in 180 milliliters of absolute methanol.
The solution vas then satur»^ed vith anhydrous, gaseous ammonia. The time required vas
approximately 30 minutes. The amino derivative precipitated in 7S$ yield (3-6 gm) melting
at 190°C (literature MP 189°C).
Origin:
Picramide (2,4 6-trinttroanillne) vas first prepared in 1854 'ey Pisani who treated picryl
chloride vith am .onium carbonate (CR 39, 853) • The use of picramide, as a brisant explosive,
vas patended by Chemlsche Febrile GriesHeim 26 May 1894 (German Patent 84,628). Meisenhelraer
and Patzlg refuted trinitrobenzene with hydroxy laciine in cold alcohol solution to obtain
picramide (Ber 39, 2534 (1906)). Witt and Witte obtained the compound by nitrating a solu-
tion of aniline in glacial acetic acid or concentrated HgSO;. at about 5°C vith concentrated
HNO3 (Ber 41, ^091 (1908)). Holleman gives details of the prep atlon from p-nitroenlline
and ггош acetanilide (Rec tra/ chim 49, 112 (1930)).
Reference:58
(a) William H. Rinkenbach, "The Heals of Combustion and Formation of Aromatic Nitro
Compounds," J Am Chem Зое 52, 116 (1930).
58See footnote 1, png .0.
284
?icratol, 52/45
АМСР 706-177
Composition: % Explosive D 52 ITT 4; Molecular Weight: < %.•
Oxygen Balance: CO. % CO % -19
Density: gm/cc Cast 1.62
Melting Point: C
C/H Rotio Freexing Point: C
Impact Sensitivity, 2 Kg Wt: Bureau of Mines Apparatus, cm 100+ Sample Wt 20 mg Picotinny Arsenol Appa.—^., in 17 Sample Wt, mg 19 Boiling Point: C
Refractive Index, n» n,° nJ,
Frictioa i*endulum Test: Steel Shoe Unaffected Fiber Shoe Unaffected Vacuum Stability Test: cc/40 Hrs, -. •>o C 100 C 0.3’’ 120 С 0.C& 135 C 150 C 0.7
Rifle Ballet Impact Test: Triols % Explosions 0 Portiols 0
Burned 1*0 Unaffected 60 200 Gram Bomb Saad Teet: Send, Qm l»^o0
Explosion Temperature: C Seconds, 0 1 (no cop used) 4% 1 354 5 Decomposes 2b; 10 2'4 15 2’0 20 255 SeaeHMty to InftiaHW Mtn mum Detonating 'J.oige Qm Me чигу Fulminate Leoo Ajide 0.20 Tetryl (>,0'
Beilistic Mortar, 4 TNT: > n) ,.x>
Trauel Test, 4 TNT:
75’C Intemetienel Heat Test: % Loss in 48 Hrs 0-0
Plato Dent "sst: 1 Method
100’C Heat Tost: % Loss, 1st 48 Hrs 0.0 % Loss, 2nd 46 Hrs 0.0, Explosion in ICO Hrs "ore Condemn *e' Confined Density, gm/cc i .' ' Bn&unce. TNT
DetanetHMi Rate: z ) Confinement <- Condition 4L' i i Charge Diameter, in . '
Flammability Index.
Hygrescepicity: % jO°C, Юк лН 0.02
Volatility: Density, gm/cc , * | Rare, metert/yecond 1 |
АМСР 706-177
Picratcl, 52/48
•0 mm HI. M71 Proiectifa, Lot WC-tl: Density, gm/cc 1.61 Chorge Wt, lb 2.075 Tefal Me. of FrogoMeft: For TNT 703 For Subject HE 769 3 lack НЕ, M42A1 Projectile, Lot KC-S: Density, gm/cc 1.61 Chorge Wt, lb 0.850 Tefal Na. of Fragments: F~TNT 514 For Subject HE 48'f Shaped Charge EffocHveaoM, TNT = 100: Gloss Cones Steel Cones Hole Volume Hole Depth
Color: Brown-yellow
Principal Uses: др, SAP projectiles and bombs
Method «4 Lending: Cast
Loading Density: gm/cc 1.62
Fragment Velocity: ft/sec At 9 ft 2590 At 25Ц ft 2320 Density, gm/cc 1.62
Method Dry Hazard Gass (Quantity-Distance) Class 9 Compatibility Group Group I Exudation None at 65°C
het (Relative to TNT): Air. Peak Pressure 100 Impulse 100 Energy Air, Confined: Impulse Under Water: Peak Pressure Impulse Energy Underground: Peak Pressure Impulse Energy Bomb Drop Test: 17, 2000-ln Seml-Armor-Piercing Boat ve Concrete: Max Safe Drop, ft 10,000-12,000
Preparation: Picratol is made by heating BiT to about 90°C in a steam-jacketed melt kettle. Explo- sive D is added slowly, without preheating, and the mixture stirred until uniform in com- position. This slurry is cooled to about 85°C and poured into the appropriate ammunition component. Origin; Developed during World War II as an insensi- tive, --it-loeded AP bomb end projectile filler Booster Sensitivity Peet: (c)
Condition Cast Tetryl, gm 100 Wax, in. for 50^ Detonation 1.00 Density, gm/cc I.63
286
Picratol, 52/Ufl
AMCP 706-177
Reference»; 59
(a) 1- C. Smith and E. G. Eyater, Physical Testing of Explosives, Part III - Miscellaneous
Sensitivity Tests; Performance Tests, OSRD Report Ho. 2? December JQ45.
(b) D. P. MacDougall, Methods of Physical ^testing, OSRD Report No. 803. 11 August 191*2.
(c) L. C. Smith and S. R. Welton, A Consideration of RDX/Wax Mixtures as a Substitute for
Tetryl in Boosters, NOL Memo 10,303, 15 June 1949.
(d) R. W. Drake, Fragment Velocity and Panel Penetration of Several Explosives in Simu-
lated Shells, OSRD Report Уо. 5^2^, 2 January 1946.
(e) Also see the following Picatinny Arse: <•'.! Technical Reports on Picratol:
0 2 6 7 8 2
l<»70 1885 1466 1737 1838 1729
1796 1797
1956
^4See footnote I, page 10.
287
AMCP 706-177
Picric Acid
Composition: % OH c 31,5 1 H 1.3 °2N“| J Molecular Weight: (Cj-.H,'. 3°7:’ 229
— NO., Oxygen Balance: CO- % CO % -'•5 -3-5
N 18-3 Lx" Density: gm/cc Cry s ta 1 1.76
0 U8-9 ' Z z NO, C/H Rotio 0.656 г Molting Point: °C 122
Freezing Point: ’C
Impact Sensitivity, 2 Kg Wt: Bureou of Mines Apparatus, cm Sample Wt 20 mg Picatinny Arsenal Apparatus, in. Sample Wt, r.ig Й5 Boiling Point: °C
13 17 Refractive Index, П» n°
Friction Pendtrium Test: Steel Shoe Fiber Shoe Vacuum Stability Tost: cc/40 H >, at r C lOO’C 120’C I35’C 150’C 0.2 O.5
Rifle Ballet Impact Teet: Trials % Explosions 0 Partiols 60
Burned 10 Unaffected 0 200 Gram Bomb Send Tost: Sand, gm 48.5
Explosion Temperature: "C Seconds, 0.1 (no cop used) 1 5 Decomposes 320 10 15 20 Sensitivity to Initiation: Minimum Detonating Chorga, gm Mercury Fulminate Lead Azide Tetryl *Alternative initiating charges. 0.26* 0.21*
Ballistic Mortar, % TNT: (•) 112
| reaxl Tost, % TNT: (1) 101
7S‘C International Hoot Teet: % Loss in 48 Hrs
0 or Plate Dent Test: Method (c) A
100C Heat Teat: % Loss, 1st 48 Hrs % Loss, 2nd 48 Hrs Explosion m 100 Hrs 0.03 0.09 I.'one Condition Confined Density, gm/cc Brisance, % TNT T3 О W L-A Ы o • о £
Detonation Roto: Confinement Condition Charge 0 imeter, in. Density, gn /cc Role, meters/second
Flammability Indan: Uncor, П r.ed
Hygroscopicity: % J0OQt ;<J: 0.01 ' .0 1.25
Volatility: "Ъ0
2MH
Pieri >-• Acid
АМСР 706-177
Itattw Sonsitivity Toot: (c) Condition Pressed Cast Tetryl, gm 10 5 Wox, in for 50% Detonotion Wax, gm 2 0 Density, gm/cc l.e 1. Г Decomposition Eq notion: Oxygen, atoms/sec (Z.'sec) Heat, kilocolorie/mole (AH, kcol/mol) Temperature Range. C Phase
Hoot of: Combustion, col/gm 2c72 Explosion, col/gm 1000 Gas Volume, cc/gm h7~ Formation, col/gm 2Lo Fusion, cal/gm (-) 20.1 Temperature, 122 Armor Plate Impact Test: 60 mm Mortar Projectile: 50% inert. Velocity, ft/sec Aluminum Fineness 500-lb General Purpose Bombs: Plate Thickness, inches 1 14 1'2 1*1
Specific Hoot: col/gm/’C (e) °C ~ 0.23b 30 0.258 60 0.2S2 90 0.31C 120 0 337
Burning Roto: cm/sec
Bomb Drop Tost: Г7, 2000-lb Semi-Armor Piercing Bomb vs Concrete: Max Safe Drop, ft 500-lb General Purpose Bomb vs Concrete: Height, ft Trials Unaffected Low Order High Order e 1000-lb General Purpose Bomb vt Concrete; Height, ft Trials Unaffected Low Order High Order
Thermal Conductivity: (f) co!/sec/cm/'C 6.21 x 10 Density, gm/cc ..Лоб
Coofficioot of Expansion: Linear, %/’C Volume, %/'C
Hardness, Mohs' Scale: 2 • 1
Young's Modulus: E', dynes/cm2 E, Ib/inch2 Density, gm/cc
Comprossive Strength: Ib/inch2
Vapor Pressure: ’ C mm Mercury 1 25 2 2J> >0
АМСР 706-177
Picric Acid
РгадммМНм Teet: «• ям HI. МП Prajoetile. U» WC-P1: Density, gm/cc Charge Wt, lb Total He. ef Fragments: For TNT For Subject HE 3 inch HE. M42A1 Projectile, Let KC-3: Density, gm/cc Charge Wt, lb Total No. ef FrogmeoH: For TNT For Subject HE Shaped Charge Effectfroame, TNT = 100: Glass Cones Steel Canes Hole Volume Hole Depth
Cobrt Yellow
Principal Uses: Formerly projectile filler, now explceive admixture; and for the manufac ire of Explosive D
Method of Lending: Pressed
Leading Denoity: gm/cc PBi x Ю3 3 5 10 12 15 ao l.lio 1.50 1.57 1-59 1.01 1.6b
Fragment Vaiedty: ft/sec Ar? ft At 254 ft Density, gm/cc
ЗФмр*0Фо Method Dry Huxord Class (Quantity-Distance) Class 9 Compatibility Group Group I Exudation None
Meet (Alioth. fe TNT): Air Peak Pressure Impulse Energy Air, Coafiaod: Impulse Under Water: Peak Pressure Impulse Energy 11 ц^аяаемк^ • Peak Pressure Impulse Energy
290
AMCP 706-177
Pi eric Acid
solubility: gram per 100 gram i£) of: (g)
Wter Alcohol Benzene Toluene Ether
i 1 L 1 °C f 2£ 1
0 0.85 0 4.1 0 ~2 20 ~13 20 ~3
20 1.1’ 20 6.9 20 9.6 60 -30 3h-7 3-96
40 1.88 40 Ik 0 ho 27.5
6o 2.98 60 59
80 4.53
100 7.1
rar bon
Chlorofora Ethyl seetato teTrc chloride Pyridine Acetone
2£ 1 1 2c i 1 2£ 1
20 ~2 20 42 20 -/0.07 10 24 20 125
60 -6 3° 50 60 ~0.4 30 37.5 30 137
40 58 50 48 ho 16h
50 69 50 208
Mothanol laopropyl alcohol Propanol-1 Carbon dieulfide
°C f cC ha Ie? t*. £c 1
0 14 10 6.4 0 2.4 20 0.12
20 19 30 9-8 20 3-3 30 0.16
40 31 50 15.5 hO 5-h
50 41 50 7.4
Preparation: (Sumary Hop. rt of Н1ЖС, Div 8, Vol I)
СбНб + нй(но3)2 — — » CgHjBgeOj + hko3 (1)
+ HgOj I *• CgHjBO + Bg(NO3)2 (2)
СбН5Я0 + 2B0 - — * j6®5®2^°3 (3a)
C^jH^NgWO^ + HgO — » сбн5он + r2 + hho3 (3b)
CgHjOH + HRO3 . 52г > OgHCgH^OH + HgC ) (3c)
CgH-NO M»o3 OJiCgH.OH oxidation and rearrangement 4 (h)
OgNCgCH + НЯ03 . > (OgMjgCgH^H + HgO (5)
(OgMjgCgHjOH + HWO? №>2 ¥ (O-JS^C^OH + HgO (6)
291
АМСР 706-177
Picric Ac*d
The two variables of greatest *raportance in this process are nitric acid concentration and
the effe"t.lve concentration of benzene (i.e., benzene dissolved in the oxynitration solution).
The optimal, concentration of nitric acid is in the range 10.4 to 11.6 molar (or the equivalent
of 50$ co 5r$ by weight for pure acid). The acid concentration greatly influences the over
all rate -f reaction, below 10.4 molar th< rate falls off rapidly, while above 10.4 molar the
rates i-i both the oxynitration reaction anu various side reactions, such as direct nitration,
increase rapidly. The range mentioned above seems, in general, to give the lowest proportion
of neutral nitro-compounds to nitro-phenols . ith, ut the ваше time, an adequate rste of ext ni-
tration. The oxynitration solution must be fortified frequently, or, preferably, continuously
with nitric acid. Strengths of nitric acid between 95$ and 96$ are best, due to the smaller
increase in reaction volume than if weaker acid were used. The une of absolute nitric acid
requires that Its direct contact with liquid benzene be avoided.
The effective concentration of benzene 1. -obably toe most critical variable affecting
the proportion of neutral nitro-coinpounds to 11; t-iphenols and amount- of colored l.y-produc-s.
Saturation of the oxyuitratlon solution v ‘Л be; tune в undeslrab.' a,.d thus in batch processes
slow benzene addition is preferable to tl.c ddltion cf it in one portion; In continue, s pro-
cesses where an excess of benzene is useu .he rat» of agitation is important.
The contentration of mercuric nitrate catalyst does not appear to be a critical factor
?’' r _ xaij.2.'- wi.de range. Concentrations of 0.3, to 0-5 mole of mercuric nitrate per liter
of oxynltratiu. solution have been found to give satisfactory results In most cases.
A continuous pj 'Cess, known as the continuous lo.’.ution process, works on the following
cycle. The oxy nitre ton solution is saturated dth benzene by vigorous agitation with excess
benzene at r'om temperature, the saturated solution is separated from excess benzene and cir-
culated thro gh a heated coil; it Is then cooled to room temperature ' ..a agitat. i again, with
benzene, which extrac . the orgsnlc product and resaturatos the oxynl-ration solution. In
evaluating th’.; process, the rate of formation of dinltrophenol per liter of reacting solution
in the coll *” deterr.V.ed; 7G gm of dinltropheno1 per liter per hour Is repr«ser tatlve perfor-
mance. Hie dlniuenol is, of course, nitrated to picric acid.
Origin:
Picric Acid was first prepared in 1771 by Woulff wuc found the reaction of nitric acid
and indigo yielded a dye. Hausizsm Isolated Pieri A- 1 In 1774 and st-d ed It further
(Journal de physique ^2, 165 (173b)). The prep таt’c- was studied by many chemists but in
1841 Laurent established Its iden.lty (Ann chin yhys 1Л, 211 (1841)). It was .fed us a
yellow dye unttl Hirpln. in 1л5, proposed I'ic»l . 1 as a burst! ц. charge lur high exp1 jive
shell (French .’atent 1'.<,512). Ihe British adopted . leric Acid as a military explosive in
uocki under toe часе of lyddite and other nations soon began to иве It es the first melt-
loaded high explosive. Mixtures of other explosives end Picric Acid were developed until it
was gradually repla-.ei ty 777 about 1900. 7-:day P11 1c Acid is used for the manufacture of
Explosive L.
Leftructioi. '.y 7r.en.lca. ;<c0E.P05it;й:.^
Picric Acid .a decomposed dissolving 2y times i-s weight of a solution nde from
1 part sodium hydroxide and i-1 parts sod::::. sulfide 1’ha^.T IHgC) ir. 200 parts 01' water. Some
hydrogen sulfide and aononiv are evolve'-.
292
Picric Л
АМСР 706-177
Refwnncea:
(а) I» С. £Mith and Е. G. Hyster, Physical Testing of Pmloslves, Part III - MircellaneouB
Sensitivity Kata; Perforaanc» Testa, OSRD Report Bo. 5746, 2? December 19*»5-
(b) Hi. Haoua, Z gee Schiess-Sprengstoffv, pp. 181, 229, 26? ^27 June 1932).
(c) D. P. MacDougall, Methods of Fnyaical Tearing, OSRD Report No. 803, 11 August 1942.
(d) G. H. Mssserly, Ke Rate of Detonation of Various Explosive Compounds, OSRD Report
Bo. 1219, 22 February 194T
M. D. Hurwitz, The Rate or Detonation of Various Compounds and Mixtures, OSRD Report
Ho. 5611, 15 January 1946.
(e) International Critical Tables.
(f) E. Hutchinson, The Thermal Sensitiveness of Explosives. The Thermal Conductivity
Esploelve Materials, AC Report No. 2661, First Report. August 1942.
(g) Values taken from various sources in the open literature.
(h) Also see' the following Picatinny Arsenal Technical Reports on Picric Acid:
1 2 1 4 2 6 I 8 2
1651 132 1303 694 65 266 1347 1118 IS 9
582 764 425 556 1557
1172 874 1585 926
1352 976
1372 986
1446
1556
*^Saa footnote 1, pegs 10.
293
АМСР 706-177
ИРЕ
СмрмШм. РЕМ 81 Gulf Orow 3 Oil 19 C/H Rotio Motecater Weight: 310
Oxygen leisare: CO, % -TL CO % -31
Ooaeity: gm/cc Bend tanp-d 1-35
>a-A»s ж- t-ж- vVMBdo Че
vWIHee чр
Impact leaeltiilQ, 2 Kg Wt: Bu-eou of Minot Apparatus, an Sample Wt 20 mg Picatinny Arsenol Apparatus, in. 11 Sample Wt, mg 27 oNag Pm st: *C
Refractive Index, nS lȣ
Stool Shoo Unaffected Fiber Shoe Unaffected Vocamn Stebiflty Tost: cc/40 Hrs, ot 90'C 100’C 0.1*8 120*C 16 hours 11+ 135’C ISO'C
Rifle Reflet hnpact Teet: Trials % Explosions 0 Portiols 0 Burned 0 Unaffr :ted 100
2M Grcm Bomb Saad Test: Sand, gm L1.6
(xpiesiea Temporetera: *C Seconds, 0.1 (no cop used) 1 5 Deconposes* 10 15 20 *Eo value obtained. W »»« »- A- t i«r »r NMWlvWy W МтЮТЯЯ, Minimum Detonating Charge, gm Mercury Fulminate 0.20* Lead Azide 0.20* •Alternative initiating chargee.
MHetic Metter, % TXT:
TiwbI Teet, % TNT:
7S*C latemetfeeel Meet Teet: % Loss in 48 Hrs
Plato Boat Test: (a) Method В Condition Hand tanped C »if ined Density, gm/cc 1. 33 Brisance, % TNT 76
100'C Heat Test: % Loss, 1st 48 Hrs 0.17 % Loss, 2nd 48 Hrs 0.00 Explosion in 100 Hrs None
DeHaetiea Rote: Confinement None Condition Hand tamped Charge Diometcr, in. 1.0 Density, gm/cc 1.37 v Rote, meters/sacond 7075
ч а еасж R_ j FWUMflvNRy
Hygroscopicity: % 30°C, 90jt RH 0.02
Voietidty:
294
PIPE
AMCP 706-177
FrogmaaMNea Test: Shaped Charge ЕНосНгамаа, TNT = 100:
Им И1, M71 ProJectHe, Lot WC-91: Density, gm/cc Chorgr Wt, Ib 1.33 1.723 Gloss Cones Steel Cones Hole Volume Hole Depth
TeToi Na. a* Fragments: For TNT For Subject HE 1 inch HI. MOA1 Projectile, Lot KC-S: Density, gm/cc ChorgeWt, Ib 703 519 1.39 0.735 Cohn
Principal Uses: Plastic denolltion explosive
Total No. of Fregmaeta; For TNT For Subject HE 51b 1*28 Masked of Leedhe-t Band tamped
Loading Density: gm/cc 1.35
Fregmoat Vebdty: ft/sec At 9 ft At 25ft ft Density, gm/cc
Method Dry
Mast (Rotative to TNT): Hozord Close (Quontity-Distonce) Class 9
Ain Peek Pressure Impulse Energy Compatibility Group Group I Exudation
Air, Confined: Impulse Under Wotor: 4. Pook Pressure Origin:
PIPE, a mechanical mixture of PEIS and Gulf Crown E Oil, was developed in the United States during World War II. References:bl
Impulse Energy (a) L- C- Smith end E. G- Eyater, Physical Testing of Explosives, Part III-Mlacellaneoua
Sensitivity Tests; Performance Teats, OSRt Re-
Underground: Peek Procure Impulse port He. 27 December l^ij. (b) S. Livingston, Properties of Explosives
RIPE, PIPE and PEP-3, Picatinny Arsenal Technl-
Energy Preparation: PIPE is manufactured by simple mechanical mixing of PETN in oil. cal Report 1517, 2U April 19*»5-
^See footnote I , page 10.
295
АМСР 706-177
Plumbatol
Campeekioa: Mdecahr Weight: 291
Lead Nitrate xNT 70 Я0 Oxygea Balance: CO. % CO % -5.U +9-3
Density: gm/cc
Making Point: ‘C
C/H Rotio Freexing Feint: *C
Impact Mnskivity, 2 Kg Wt. Bureau o' Mines Apparatus, cm Sample Wt 20 mg Picotinny Arsenal Apparatus, in. Sample Wt, mg BeiBng Point: *C
13 22 Refractive Index, n“ n£ n°
mSVIW c vv*« Steel Shoe Fiber Shoe Vacuum Stability Test: cc/40 Hrs, ot 90°C 100*C 120"C 135*C 150"C
Rifle Boflot Impact Teet: Triols % Explosions Poitkils
Burned Unaffected 200 Gram Barna Send Test: Send, gm 32-U
ExptaeUn Tamperafara: *C Seconds, 0.1 (no cop used) 1 5 Decomposes 23З 10 15 Sensitivity tn Initiation: Minimum Detonoting Chorge, gm Mercury Fulminate Lead Azide Tetryl 0.20 0.0
20 BeNietic Mortar. % TNT:
Tread Tost, % TNT:
7S*C latoraotioaol Heat Test: % Loss in 48 Hrs
Plate Dent Test: Method
1WC Hoot Teet: % Loss. 1st 46 Hrs % Loss, ?nd 48 Hrs Explosion in 100 Hrs Condition Confined Density, gm/cc Brisance, % TNT
Detonation Rate: (c) Confinement Condition Charge Diameter, in. Density, gm/cc Rate, meters/second
Flommobility Index:
Hygroscopicity: % 2.89 1*850
Volatility:
2W>
Plumbatol
АМСР 706-177
Пив ШЛО! tajactih. Let WC-91: Density, gm/cc Chorge Wt, lb кА* лЛ IHPH OYV» nW^eWBO* ForTNT For jubjecr HF 3 lack HI, M4IA1 he|sstMs. Let KC-5: Danatty, gm/cc Chorge Wt, lb ForTNT For Subject HE Shaped Chorge IHecHvoaese, TNT = 100: Gloss Cones Steel Cones (a) Hole Volume Ilk Hole Depth 10 3
Cebn Light yellow
Friar ip si Usr«
Mattel a* Loedtag: cast
Leodiag Deaaity: gm/cc
Fregawat Velocity: ft/sec At 9 ft At 25% ft Danatty, gm/cc
Method Dry Hazard Class (Quantity-Distance) Claes 9 Compatibility Group Group I Exudation
Meat (Ratetfee to TNT): Ain Rack Pressure Impute Energy Air, Caaftaai; Impulae Uader Wotan Peak Pressure Impute Energy Uedorgroaad: Pook Pressure Impute Energy Preparation: Plumbato1 la manufactured by simple mechanical mixing of lead nitrate in molten TUT.
Origin; An explosive containing 70$ lead nitrate and 30$ TNT has neen used in Belgium under the name of "Marcarlte." References:62 (a) Eastern laboratory, du Pont, Inveatl- gatlon of Cavity Effect, Sec III, Variation of
Cavity irffect with Explosive Corposltlon, NDRC
Contract W-6T2-ORD-5T23. (b) Л^грев Dictionary of Applied Chem- istry, Fourth Edition, Vol TV, tongmana. Gre n and Company, London - Nev lork - Toronto, p. 46k.
62Sc« footnote i, page 10.
297
AMCP 706-177
PLX (Liquid)
% * Moloculer Weight: 2|Z<i
Mltioeetbene 100 95 Sthylr.nedlaeine -- 5 •The Bixture 95/5 Nitranethane/Kthylenediamlne la designated PLX (for Picatlrny Liquid Explo- sive). See note under Storage. " Oxygen Beleece: CDs % -39 -h8 CO % -13 -21
Pensky: gm/cc 1.1L 1.12
Making Feint: *C .29
C/H Ratio В—0^^ rs^WNl| w
Inspect SenoMvfcy, 2 Kg Wt: 100 95/5 Bureau of Mines Apporotu», cm 100* 100* Sample Wt 20 mg Picatinny Arsenal Apparatus, in. Sample Wt, mg 20 20 BoMhg Feint: *C 101
3 3 3 BO BO BO
0M^Me Steel Shoe Unaffected Fiber Shoe Unaffected Vactrnm Stability Test: cc/40 Hrs, at 90*C 100’C 120’C 135*C 150*C
Me Beget lamest Tash 10 Trials 5 Trials % < Explosion» 0 i Partials 0 0
Burned 0 □ Unaffected 100 100 к 200 Grans Bomb Send Test: 100 9^ Sand, gm 8.1 50.6
Ixptseien Tsa^eraterf *C °C Seconds, 0.1 100 95/5 1 5 430 iiJO 10 15 SenoMrity to InCHstien: Minirmxn Detonoting Charge, gm Mercury Fulminate Lead Aside Tetryl
20 leMetic Mester, % TNT: 131»
Trees! Teet, % PA 127
-» * A*—> <P—< % Loes in 48 Hrs
Flute Sent Test: Method
100’C Neat Test: % Loes, 1st 48 Hrs % Loss, 2nd 48 Hrs Explosion in 100 Hrs Condition Confined Density, gm/cc brisance, % TNT
Confinement Glass Glass Condition Liquid Liquid Charge Diameter, in. 1.25 0-9L Density, gm/cc 1.1L 1.12 пЮТЙИ.6210 6165
Hygroscopicity: %
Volatility:
298
PUC (Liquid)
АМСР 706-177
Baaater SteiRhOy Teat: Ki trone thana -as*-»— ЧЛП0ПЮП Tetryl, gm Wax, in. for 50% Dafonction Wax, gm Daneity, gm/cc DeeoMpeaMen Sgaatiaa: (d) Mltrootethane Oxygen, otoma/sec 10х*"° (2/joc) Hoot, ktlocolorie/mole $6*6 (AH, kcol/mol) Temperature Rcnge, *C ЗВО-кЗО Phase Gaeeoua
Heat aft (a) Combustion, col/gm 2830 Explosion, col/gm Gaa Volume, cc/gm Formation, cal/gm -3U8 Fusion, col/gm Vapor! sati or, cal/gn 1U9 Amnf Kit* йнрмФ Twt? мм 50% kwrt, Velocity, ft/мс Aluminum Ппенмв Э0Ф4Ь Фмвм! Рмрм* ВммЬв! Plate Thickness, inches 1 14 1% 14
Spodflc Hoots col/gm/'C (b) C 0.4209 - O.OOO76t + 0.0000061t2 p for 15 C to 70°C
Boraiag Rate: cm/sec
Beete Dreg Teat: 77, MOD* leaii Anasr Harriag Beate os Generate: Max Safe Drop, ft S004b fisarrel hwpeee BeaA os Concrete: Height, ft Trials Unaffected Low Order High Order 10004b General Pnrpeoe BeaA vs Concrete: Height, ft Trials Unoffoctea Low Order High Order
I WMVWBWB wMW^^WWvWyS col/soc/cm/"C
CMNidratwf Ьрммомк Linear, %/*C Volume, %/*C
ИмИмов» MbW Seek*
Yeung's Modubre: E', dynes/cm> E, Ib/inch* Density, gm/cc
Ceateraaohe Sheagib: Ib/inch*
Vapor Ргемага- (c) ‘C mm Mercury 70 258 85 444
299
АМСР 706-177
PLX (Liquid)
N мм HI, МЛ PtofeclKo, U» WC-ll: Density, gm/cc Chorge Wt, lb Total No. of Рмдмийк ForTNT For Subject HE Э Meh HI, M42AT Pnjirtih, Let KC4: Density, gm/cr. Charge W:, It Tot*; No. of Fsugasoafs: ForTNT For Subject HE Shaped Cbergo If let II tenets, TNT = 100: Glass Cones Steel Cones Hole Volume Hole Depth
Cofer: Light yellow
Pnacipol Usee: Minefield clearing
Method ef Loadfeg: Pumping
Loodtag Density: gm/cc 122 22/^ 1.1k 1.12
Fragment Velocity: ft/tec At 9 ft At 25% ft Density, gm/cc
Storage: Method Components stored separately; mixed only when reedy to use Hazard Class (Quontity-Distonco) Compatibility Group Exudation
Moot (Relative to TNT): Abt Peak Pressure Impulse Energy Air, Г Seed: Impurse Under Water: Pook Pressure Impulse Energy Peak Pressure Impulse Energy 1
Minimum Propagating 100 95/5 Thickness, tn: d.$ 0.063 Viscosity, centipoises: t«) Tern;, 10°C O.7MJ 25°C 0.625 kO°C 0.533 Compatibility with Metals; Stainless steel, mild steel sad duriron not effected; corrodes nrass.
:юо
рц: (Liquid)
AMCP 706-177
Origin:
Nitrcmthene bar been known tine* 1872 (Kolbw, J pralrt (2) (1872), but was
available only ae a laboratory product until it appeared ы «к industrial chemical in 1940.
A nuaber of patents have been issued for nitroMt&ane prodawsi ae a by-product of the titra-
tion of propene (U. 8. Patent 1,967*667 (1934); Srltieh Patent. t 3,707 (1937): and Canadian
Patent 371,007 (1938).
Uie development of nitaroeethane liquid explosives vw based ca intonation that nltro-
ethane ie sensitised to initiation and propagation of deb-onatioo by the addition of various
seines, this study Made at Picatinny Arsenal in 19*5 indtoatod tint mixtures of nitromethane
with 55& of ethylenediamine, n-butyl-amine, or morpholine ebrwod considerable promise for ap-
plication in nine-field clearance (L. H. Eriksen and J. V'. ttoven. MT?. No. 1565, 17 September
19*>5)-
References^3
(a) E. E. Holcomb and C. F. Dorsey, "IfcerEXxaymslc Properties of Nitroparafflna,'* Ind
Engl Chen 41, 2788 (1949).
(b) J. W. Williams, "A Study of the Myslcel Properties of Sltrcmethane," J Ав Cbem Soc
£[, 2644 (1925).
(c) L. Medard, "Explosive Properties of Nitromethane," Мяа poudr 33, 125 (1951)-
(d) T. L. Cottrell, T. E. Graham and T. J. rield, "The Thermal Decomposition of Nitro-
ne thanes," Transactions of the Faraday Soclet.. 47, 58U (1951).
(e) F. Bellinger, H. B. Friedman, W. H. Bauer, V' w> Bastes and W. С. Э111, "Chemical
Propellents: Stability of Mononitrcns'ixiMe," Ind Engr Chen 40, 1320 (1948).
(f) Also see the following Picatinny Arsenal Technical Reports on Nitrorethar.fc:
0 1 ; 2 2' 6 1 8 2
1660 1681 2113 1565 - 2016 1747 1708 1619
1831
G^See footnote
ptfge 10.
301
gotMeiu» Dlnltroben*furoxen (КДИУ)
АНСР 706477
« С 27-3 H 0.1» N 21.2 Miliwlor Weight: (KC^N^Og) 225
B02 -0 * b\L XXQXXvVo CO, % co % >60 •18
0 эб-з P2’\y ^0 к Deadly: gm/cc 2.21
К U.8 4 -J M0M4 Mo»t *C Bxplotee 210
C/H Ratio 0Л16 Bvsoeim *C
tappet SeneBMty. 1 Kg Wfc BeMeg Molt *C
SompkWt20mg Picotinny Artenal Apporatu*. in. 3» (I lb *t) 6 SampleWT, mg 7 wWVWWW В8ВОТВЦ tit* n& П»
elWTWi 1 Steel Shoe Fiber Shot iltt! KxplO&M Sxplodo* "KVM ЖММВку ТЯП CC/40 Hr», Ol 90-C 100’C 120’C T»’C 1K»C
Uh Ballet impeet Tut: Trial* % ЕфЬМопе Portiota
Burned UlwTWCVM MB Knn Bemb Send Tert: Ц-6 1*3-6
laptoUea T«np«mlara: *C Sewnd», 0.1 (no cop ueed) — 1 5 250 10 Minimum Detonating Chorge, gm Mercury Fulminate 0.30 Uad Azide Tetryl 0.20 0.10
20 BeKMic Motto», % THT:
TraoelTeet, % THT:
7>C нНлнпша Moot Teeh % Ln* in 48 Hr* Moto BiHtf Tat: Method
Ж‘СЫТг* % Lots, lot 48 Hr* % Law. 2nd 48 Hr* Expiation in 100 Hr* 0.03 0.05 lone vonornon . Confined Daneity, gm/cc Briunce, % TNT
НвоммМВу total: Confinement
30°C, 75* RH J0°C, 90* PH 0.11 0.27 vonwtion Chorge Diameter, in. Deneity, gm/cc Rate, meun/tocond
V'jtatMtjn
Э02
Petaaolu» Dlnitrdbensfuroxan (ДЖВР)
АМСР70МП
i Oessmr SeasMsOy Tosh Condition Tetryl, gm Wox, In. for 30% Catenation Wax, gm Comity, gm/ec Oxygen, etorm/sec <Z/sec) Hoot 1и1осо1оНв/яюИ (AH, kcol/mol) Tomperoturs Kongs, % Phase
Neat eft Combustion, col/gm 2209 Explosion, col/gm 725 Gm Volume, cc/gm 60U Formation, cal/gm Fusion, col/gm Atoms Halo Impeet Tostt ee^e ^ЛевВее 50% Inert, Velocity, ft/sec A. la molm ^om Оа^^мое ^awvwnuni rwwnio* Plate TNckneas, Inches 1 1% 1% 1%
OpooMe Heats eol/gm/’C (b) °C -50 0.217 0 0.E17 25 0-217 t . 50 0.217
Oosalag lotos cm/soc
tanb Deep Tostt Мех Sole Crop, ft Height, ft Triols unoTTecsvo Lew Order High Order 10004b fleaeral Petpoae Bomb «о ГгапеОо; Height, ft Triols vnoTvecwo Low Order High Order
сЫ/soe/cm/'C
CoofHstoor of Ixpoaslea: Unoar, %/*C Volume, %/*C
Мейе* Scsle*
Ysong't Medefas: E', dynes/cm* E, Ib/inch* Comity, gm/cc
**- *’—*- ^НетИуПОе *Vp егоъа*
heiwi! X mm Mercury
303
АЫСР 706-1П
Potassium Dlnltrobcctfuroxai: (KDKBF)
FregmeeteHoa Teeh N м Ht, MFI PioieeMa, la» WC-91: Density, gm/cc Chorge Wt, lb Total Me. of FregnunH: For ThC Fo« Subject HE Э lash Ht, M42A1 FrejocbU, U» KC-5: □entity, gm/cc Chorge Wt, lb Total Ke. of Fregmeots: ForTNT For Sublet HE Shaped Charge If teeth on roe. TNT = IM: Gloss Cones Steel Cones Hole Volume Hole Depth
Colon Orange to brown
Principal Uses: Primary exploeive
Method of leodiag: Pressed
Leodiag Deaeity: gm/cc Pel x 10 20 JO 40 80 1.63 l.T 2-81 1.86 1.98
Fregewet Velocity: ft/sec At 9 ft At 25% ft Density, gm/cc
Stwefe: Method Vet Hazard Clos. (Quontity-Distonce) Class 9 Compatibility Group Group M (vei) Exudation
Meet Utebtfveto TNT): Air. Pool Pressure Impulse Energy Air, CoaHmed: Impulse Under Water: Peek Pressure . Impulse Energy Undergrennd: Peek Pressure Impulse Energy
Solubility in wet gm/100 gm solvent, st;
30°C 0-245 Stab Sensitivity; Density Firing Point (inch-ounces) gm,'cc Oft 5Oft 1005 1.63 73 И 84 1.77 66 75 S3 l.cl 42 48 64 1.86 12 15 18 1.93 11 17 21 l.Ofi 7 11 14 A ? 11 vat Ion E.-.ergy;
kcai/moi Induction-Perl'-'!, see 0.5-10
:hm
Potaadun Djnitrobenzfuroxan (KDfBF)
AMCP 706-177
ftwparatlon of Potaadua 9dt of h,6-dnitrobentfuroxan: (a)
Benxfuroxan, aade by the reaction of ortho-dtrcaniline alkaline aodiun hypochlorite,
ш diarolved in 6 parte of 96jl sulfuric add tad nitrated at _?°-2O°C with a U to 1 aulfuric-
dtric add datura. The ealt was prepared by neutralization of the l,6-dinltrdbenrfurox*n
with potaadua bicarbonate followed by recrys tall! tation froa hot water. The product feme
in mbH golden orange platea which explode at 2L0°C.
Origin:
The potaadwa salt of 1,6-dinitrcbenzfUroxan vaa first prepared in 1899 by von P. IToet
(Ann Jgfc, 56 (1899))*
Beferences:64
(a) B. J. Gaughran, J. P. Picard and J. V. R. Khufban, "Contribution to the Cbedstry of
nemfliirnim Derivatives," J Aa Ома Soc j6, 2233 (1954*
(b) C- Denchits, Toe Oalariaater Deterdnation of Bhtbalpy and Spedflc Heat of Eleven
(IraanMetallic Ccwounda, feMB fe. ЙЙЙЬ» Boveder 1955.
(e) Also aee the following Picatinny Araenal Technical Beporta on Potaaaiun Dinltro-
bauftaraan:
£ 2
2122 2093
6 2
21U 2179
^See footnote 1, page 10.
306
АМСР 706-17?
РТХ-1
MJecrter Waight: 252
Oswv Iolanta;
RDX 30 -*5
Tetryl 50 ЭЯ - 9
THT 20 Density: gm/cc 1.68
r Mte T Met: ’C Eutectic 67
C/HRotto sWIWi 4»
Impact SoaoHMy, 2 Kg Wt: eMog faint: "C
Sampte Wt 20 mg Refrecttee lades, n£
Picatinny Arsenal Apparatus, in.
Sampte Wt, mg n«
П»
Vdsddlvoi Tut* Vocwam SlsbMty Teat:
Stem Shoa cc/40 Hrs, at
Aber Shoa 90*C 3-0
100‘C
RMeMtet Impact Teat: Trioh 120"C
% Explosions 20 135’C
Rortiak 20 150‘C -
•timed 0 200 6mm lamb Stead Tec
Unaffected 60 Sand, gm 54.8
M testate vKKVIaWWy VHIV^^V^Mie
Second», 0.1 (no cop used) Minimum Detonating Charge; gm
1 Mercury Fulminate 0.23»
S Lead Aside 0.22»
10 IK «Alterative initiating ohanrea.
20 MNtefc Meteor, % TNT: (a) 132
Tread Tote, % TNT:
* Low In 48 Hrs Note Doot Toot: (b)
Method В
НГС Note Teter Condition Coat
% Ute, let 40 Hr» Confined No
% Ute, 2nd 40 Ha Density, gm/cc 1.68
Evteoion in 100 Hrs Brisance, % TNT 127
Detonation Rate:
ЙМммЫЙ1у lodM! Confinement None.
Condition
HygieeiMlilryi % 3rC, 90l> RH> 15 days 0.00 Charge Diameter, in. 1.0
Density, gm/cc 1.64
Votes*?: Rate, meters/tecond 7655
306
РТХ-1
AMcpyofr-m
N MM HI. МЛ hejecMe, La» WC-tl: □entity, gm/cc 1.64 ChorgeWt, Ib 2.180 Teiol No. of РмдмШк For TNT 703 For Subject HE 999 1 tach Ht, M42A1 Projectile, Let KC-S: Density, gm/cc I.63 Charge Wt, Ib 0.864 I wVWB ВМШа For TNT 514 For Subject HE 685 Shaped Charge IHesHveoaea, TNT = IBM GIom Cones Steel Сопы Hole Volume Hole Depth
Catar:
Principal Usm: Lend mines and demolition charges
Method of leodfag; Chet
Leedtag Deaahy: gm/cc 1.68
Модема» Vetachy: ft/sec A»» ft 2690 At 254 ft 2460 Density, gm/cc 1.64
Method Dry Hoxord Clou (Quontlty-Otstonca) Claes 9 Compatibility Group Group I Exudation Exudes at 65°C
Meet flteMeo to TNT): Ain (a) Peak Pressure 111 Impulse 109 Energy Alt* СмАмбх Impute* Under Weesn Peek Pressure Impuhe Energy Peak Pressure Impuhe Energy
Preparation;
Ihe ternary explosive system consisting of RDX, tetryl end TXT 1s prepared by adding the appropriate weight of water-wet REX to a tetry- tol (40/60) previously melted in a steam- jacketed melt kettle. Heating end stirring are continued until all the water is evaporated and the mixture is uniform in cosposltion. PTX-1 is also prepared by adding tetryl to RDX Composition B. Compatibility with Metals:
Dry; Aluminum, mild steel not affected. Wet; Aluminum, mild steel not effected.
Booster Sensitivity Teet; (c) Condition Pressed Cost Tetryl, gm 100 100 Wax, in. for 50£ Detonation 1.94 1,32 Density, gm/cc 1.61 1.68
307
ЛХСР 706-177
PTX-1
Origin;
Die possibility of employing ternary mixtures to obtain explosives haring greater power
and higher brisance than binary mixtures was suggested by the analysis of Russian 76 am,
armor piercing high exploeive rounds (R*Dl Mo. 13U, 17 July 19^3). The Risslan type ternary
explosives, based on the composition and laboratory studies of such Mixtures, were indicated
to be effective pressed fillers. In conducting s preliminary study of csstable ternary explo-
sive Mixtures suggested by the Russian fillers, a mixture consisting of ftHi/teEryl/TMT, desig-
nated PTX-1 was developed which had exploeive and physical properties offering considerable
advantage for military applications (RADI Во. 13^0, 27 October 19*13; end 1379, 11 January
19*A).
A PTX-3 ccapoaition, prepared by the addition of Halelte to *0/60 tetrytol, else offered
prosdse but United to applications where the charge would not be required to withstand stor-
age at 6j°C without exudation.
References:65
(a) L. C. Smith and E. G. Ryster, fhysical Testing of Explosives, Part Щ - Miscellaneoua
Sensitivity Testa; Performance Tests, OSRD Report Bo. 57*^>, 27 December 19*5.
(b) D. P. MacDougall, Methods at Physical Testing, OSRD Report Ko. 003, 11 Augur .. 19*2.
(c) L. C. Smith and S. R. Walton, A Consideration at RIg/wax Mixtures as a Substitute for
Tetryl in Boosters, MOL Memo 10,303, 15 June 19*9-
(d) V. R. Tomlinson, Jr., Blast Effects of Bomb Explosives, Rd Tech Div Lecture,
9 April 19W.
(e) Also see the following Picatinny Arsenal Technics^. Reports on PTX-1:
0 8 1 6 1 2
1530 11Ю2 1623 1H66 1>»37 1379
1506 1U29
1*69
6^Ses footnote 1, pngs 10.
308
РД-2
АМСР 706-177
CmvmNm: RDX Mt . 1ц FKDt 28 - яб ОТТ 28-33 C/H Ratto Metecater Weight: 2U 2^3
Oxygee Oelearst CO» % -33 -36 CO % - з - *»
Benally: gm/cc x.TO
MaBteg Mat: *C Butectic 75
a b^ ГЮТВоЯЦ twIWi ч»
IrapMtfaneBMy, 1 Kg Wlh Buraou of Mterae Apparatus, cm 35 Sampte Wt 20 mg PiMtteny Anonol Apparatus, in. Sampte Wt, mg fc. sao— WBMBQ TWBBin w
Refractive Max, ni n£ n£
HWllVH ^^^ММЯНВ ЮТ* Steal Shao Cracklee гютг эгия Vaearan SteMBy Tact: cc/40 Hrs, at 90"C 100’C 2.6 120*C H+ 135*C 150*C
RMteOoBot hugest Ta«: Trials Exptooiora 6o Partials 0 Burned 0 Unaffected ho
200 Brara Borah Sand Test: Sand, gm 56.9
Oeplsoloo Tesnporetrav: C Seconds, 0.1 (no cap used) 1 5 10 IS 20 m—1м1в1М^Ае «аввявппву w ooobbbm^ws Minimum Detonating Chorge, gm Mercury Fulminate 0.21 Lead Azido 0.00 Tetryl 0.00
DaBhHc Mester,«TNT: (•) 136
Trauol Toot, % TNT:
7S*C Mset Testi % Loes In 40 Hn
Ptata boat Test: (b) Method В Condition Coot Confined Ro Density, gm/cc 1’71 Brisance, % TNT 1Ы
IN'C Neat Toth % Ute, 1st 40 Hrs « Lora, 2nd 40 Hrs Explosion in 100 Hn
BoteaoHea Bote: Confinement None Condition Coot Chorge Diameter, in. 1.0 Density, gm/cc 1.70 Rate, meters/socond 8065
ЯшвяшММу In4nt
- ^4 ” 30o<5, 9W RH, 15 «toys 0.00
VateMByt
Э№
AMCP 706-177
PTX-2
^^^BgasoaSaaloa ^fosls п мм НА МП PiojecMe, LM WC-91: Density, gm/cc 1.68 ChorgeWt,» 2.226 Total He. of fugiMi'hi For TNT 703 For Subject HE 1128 3 lack НА MOA1 PropMto U»KC4s Density, gm/cc 1.70 ChorgeWt, Ib 0.897 TsBel Nou of For TNT 51b For Subject HE 7$O ЯмрИ Charge Necttoeeee, TNT = 160: Gloss Cones Steel Cones Hole Volume ~ 130 Hole Depth
feeler:
htaslgslltota Shaped chargee Fragmentation charges
Method of Lsodtag: Chat
Leedtag ttaaohy: gm/cc 1.70
Frogawat Vatadty: ft/мс At 9 ft 3020 At 25ЦЙ 2850 Density, gm/cc I.70
Msthod Ury Hczord Class (Quantity-Distance) Claes 9 Compatibility Group Group I Exude <ian Hone et 65°C
Etot (Malto to ТИП AM (d) Peak Pressure 113 Imputo 113 Energy *• Air, Ceatosd: Imputo POok Pressure Imputo Energy Peek Frescura hnputo Energy
Preparation: / The ternary explosive system consisting of RDX, РИЯ and TUT is prepared by adding the appropriate weight of water-wet RDX to a pen- tolite (ЭО/70) previously eeltod in e atoaa- Jвeketed melt kettle. Heating and atirrlng are continued until ill the water la evaporated and the mixture is uni fora in composItion. PTX-2 is also prepared by adding water-wet РЕП to RDX Composition 3. Coeetlblllty vith Metals:
Dry: Aluminum, mild steel not affected. Wet: Aluadnum not affected.
Booster Sensitivity Test: (c)
Condition Pressed Cost Tetryl, ga 100 100 Hex, In. for 506 Detonation 1.87 2.32 Density, ga/cc 1.70 1.61
310
m-г
АМСР 70МП
Origin!
9м possibility of eag>loylng ternary nixturua to obtain explosives baring greeter power
and higher brisance than binary Mixtures aha auggeeted by the analysis of Russian 76 an, amor-
piercin} high exploeive rounds (RAR во. 13H, IT July 1943) • 9м Russian type ternary explo-
sives, baaed on tte ccsvoaition and laboratory studies of ouch nlxturea, were indicated to be
effective nressed fl Ilera. In conducting a preliainexy study of castsble ternary exploeive
ndxtyrea suggested by tte Ruaaian fl Here, a nlxture eonaiating of Rld^ita(/9n, deeignated
PTX-2 tea developed which ted exploeive and physical properties offering conalderable advan-
tage for sdlltery applications (MIR Mo. 1)60, October 19*3; end 1379, 11 January 19**).
A PTX-* coupositicn, prepared by the addition of Halelte to 30/70 Pentolite, also offered
proedse but tecauae of border-line stability in accelerated stability tea to, РП-* oust be
proven by long tens storage to be acceptable for uae in atandard a—iinl tion.
RefUrencest**
(a) L, C. Beith and E. 0. Ryster, Physical Tenting of Explosives, Part in - Miscellaneous
Sensitivity Htetet Perfoiwanoe Haeta,
(b) D. P« MacDougall, Methods of Physical Heating, OBRD Report Bo. 803, 11 August 19*2-
(1) L> C. Bal th and 8, R, Walton, A Consideration of RDt/Wax Mixtures as a Substitute flor
Tetryl in Boosters, MOL Meao 10,303, 1JJ June 19*9-
?^d) V. R. Honlinaon, Jr,, Blast Effects of Boob Exploeivwe, FA Tech Div Lecture, 9 April
(e) Also see tte following Picatinny Arsenal Technical Reports on PTX-2:
023*2662
1530 1*62 1*83 1*1* 1**5 1*66 1836 1379
1623 1*29
1*69
“dee footnote I, page 10.
311
лж.срмьлтг
P7A-U
См^мМвв Milandir Weight: 217
нт 90 Polyvinyl Acetate 8 Oapgea Meaeet CO, % -37 CO % -10
DihutylphthaLate 2 DeaaKfi gm/cc Pressed 1.60
°C 9g
C/HRrtio
ЬцмМ lisillUty, 2 Kg Wt: Buraou of Mines Apparatus, an 39 Sample Wt 20 mg Picotinny Anenol Apparatus, in. 9 Sample Wt, mg 13 BeMsf VtolRtt *C
Refrastwe laden, n° ni ni
Fiielioo Twts Steel Shoe Crackles Rhor Shoe Unaffected уК^ЯМ рЮТИиу ЮТ* cc/40 Hrs, ot 90*C 100’C 0.15 120’C 0.88 I35’C 150’C 11+
BMe Mot Ьцм* Test: 5 Tric k * % Explosions 20 Portide 0
Burned 60 *f№%92fs at -46°C - Unaffected 200 Grom Bomb Send Test: Sand, gm 58.5
^^s^tlseie^a ^Ps^^t^l^ieotoe^i: Seconds, 0.1 (no cop used) — 1 330 5 Оесоацюеее 375 10 265 IS 20 ее we_ s- Utel^U-, pmWwrvwy W nHlIBnSiOo Minimum Detonating Charge, gm Mercury Fulminate Lead Azide 0.22 Tetryl
IsBletit hterter, % THT:
Trani Teet, % TNT:
7S*C leteraeHoeel Hoot Teat: % Lou in 48 Hrs
Plate Doot Test: Method
100*C Heat Teat: % Lou, 1st 48 Hrs 0.10 % Lou, 2nd 48 Hra 0.06 Explosion in 100 Hrs Hone Condition Confined Density, grn/cc Brisance, % TNT
Confinement Hone
Hygresiiylrltyi % 30°C, 90£ RH 0.20 Charge Diamater, in. 1.0 Density, gm/cc 1.60 Roto, meters/second 7910
VetoMfcy: 55°C, vacuo, 6 hrs 0.03
312
АМСР706-1П
N м M, МЛ МмМ* 1*» WC41t DtmMy, gm/cc Chorga W»,lb For TNT hr Subject HE 1 MM, М4Ы1 МмЫЬ, be* K4: OaraRy, gm/cc Chorga Wt.lb TM Ne. of РядемМаз For TNT For Subject Ht * n_ - VMV » gMfWV inWIIvVNWII, 1 П a a^^H Glace Cena* Steel Сом* Hole Volume Кой Depth
Coiert White
Prieeipoi Umk Deaolitioo chargee
MclbeA of Laedtag: Preaaed or extruded
Uedtag Daaaky*. gm/cc I.6o
Рядам* Vabakyt ft/мс At 9 ft At23Hft OonaHy, gm/cc
Method Dry Hazard CloM (Quontity-Dtetanca) Claoo 9 Compatibility Group Group I Exudation gone at 71°C
Waat (Relative to TNTh Ain ReokPrwwe Impuhe &*gy AW Impuhe Uader Wotan Рмк Ргемиге Impube &*gy (Ыидмамк Pack Ргамым IttpuiM &*gy
Ploaticity; -kO°C Cracked 25°C 0.3
313
АМСР 706-177
FVA-J*
Preparation;
bploelve IVA-4, a seed-plastic composition of Canadian origin, consists of 900 RDX, dfl
polyvinyl acetate and 20 dlbutylphthalata (1ЙР). Thia femulation wee developed by ЙГ. Suthur-
land of Sbevlnigan Cbeodcals, Ltd. In evaluating various types of polyvinyl acetate соивег-
clally available in the United Staten, a type obtained free Union Carbide and Carbon, under
the Industrie! naead or designation *AXMT *» the aovt proadaiog coating for RDX in the pro*
portions M«/lVA(ATAT)/lBP 9ty6/2.
A practical eethod of preparing this covooition vaa by the addition of e aolution of the
coating agent to an aqueous ROC slurry. Based on the quality of the product end the pellet
densities obtained, a procedure of adding an acetone aolution of IVA a- IBP to a hot eater
Blurry of RDX, under agitation, vas adopted as atandard.
Мйгеу?67
(a) See the following Picatinny Arsenal Technical Reports on IVA-4: 1532 and 1бэ4.
footnote I, page 10.
314
РУМ (Polyvinyl Mitrate)
АМСР 706-177
*--rnTi-i с ат и 3.U | (BgC-ai-ceog)n М 15.6 • о 5* C/H Ratio 0,203 Meteeuter Weight: (c8H3Ba3)n (89)n
Ощ^м ОЫмсФх CC. % A5 CO % .9
Вошку: gm/cc
Making Noh *C (Soft Pb) 50
м^мИемвы^ U.860 Buraou of Mita Apparatus cm — Sampte M* 20 mg Picotinny Artenol Apporaii|(, In. Sampte W|,mp
f |b fa fa
о— Vratan •VraHM VWV Steal Shot Crackle* RberSho* Unaffected Veceem Stebflky Trate cc/40 Hn, ot 90’C 100’C 16 hour% 11+ 120’C 16 boure ll* 13S’C 150’C
UbMOtetaTM; Trial» % Explosions rVMWQV Burned aa—»_ л UnGTWCWQ
2BB Stem Beta Sand Tate: Sand, pm k9-9
Repteteea Teeiperata*: *C Second», 0.) (no cop umd) 1 S 265 10 IS 20 SoBoMvIty to (яШяНвА* Minimum Detonating Charge, gm Mitfojtnf Fubninote Lead Ax ide Tetryl
BakMte Metta. % TNT:
Trata Tate,« TNT:
99 w ИЯНЯИ1М1 SfOW ••w* % Loot In 41 Hr*
Plate Boot Toeh аа-*о- - -* IVWIVIUO Condition Confined Oeneity, gm/cc Brieanco, % TNT
IBB’C Neat Tetei % Lora, IttOBKn 1.9 % Ute, 2nd 4« Ha 2.1 Expteaion in 100 Ha Bone
VaOTVomWeeWeW Catation Ok ge Diameter, In. DoneHy, gm/cc Rate, metore/eooond
НшшмЫВу |в4рвх
Hyp % 30°C, 90* RH 0.62
VihNBlji
3U
амсрнс-го
Ю (Polyvinyl Mtwto)
9» MM IM, МП MwNK lot wwi: Density, gm/oc Charge Wt, to Tatoi N*> «1 Тмдаиаап ForTNT For Subnet HE > tach ML MOA1 Рм|ааМК Lot KC4t Corally, gm/cc Chops Wt, b Total Mat of FragMsatat ForTNT For Sublet HE Shogad Chapa hHanlinata TNT ж NM (аПмСопм SiaaiConM HoteVoiuma Hob Depth
Cai»
Laadtag IMaAyt gm/cc
Марам» WhaMjift/aac At 9 It At 254 Л DamHy, gm/cc
ЛЛ -.A- _ -a fvwmoQ Hazoid Oom tQuonttty-DiaMnce) ^aa^Mb^^SUlaa yJff^KKVMf ЧЖНф Enidotion
MMMahNwtoTWDt ' Ain Wt Maaawa tagulta bw AMCsaMas* (хфиПа Ыа Wotan АккАммиго Iwpultt EMW AmAMsmum 1м|ш1м в*рг
65.$°C P T«at: Minutes 6o 13h-5°C Boat Teat: Minutes Salnoo Pink Йв RedPumss 25 Kxplodes JOO* g^tO-Hour gydrolyala Test; t ШЮ3 j.OT Heat of; Coadjustloo, cal/go 29^0 Explosion, cal/go 900 Gm Voluas, cc/jo 838
316
рта (Polyvinyl Hitrate) AMCP 706477
Preparation;
Polyvinyl alcohol io nixed vith acetic anhydride. Th« mixture la cooled to -5°C and the
nitric add la added slowly while the пом la being atirr d- The temperature la controlled
by the rate of add addition so that when ull the add haj been added the temperature does
not ria* above SO°C.
Vhen the nitration la complete, the mixture ie drowned by allowing a fine atreon 1 the
syrupy liquid to fleer from the nitrator and dx'intimately with a large stream of water. This
causes the product to precipitate in a fine state.
Пе finely divided precipitate la purified by boiling in frequent changes of water.
Origin:
Пе first preparation of polyvinyl nitrate waa reported in 1929 by solution of polyvinyl
alcohol in concentrated sulfuric add and treatment with nitrating add at a temperature not
over 50°C. (demon Patent 537,303). Inter patehta issued relative to polyvinyl nitrate in-
cluded П. 8. Patent 2,Hj8,U87 (1938) and demon Patent 737,199 (19^3) .
317
АМСР706-1П
RIPE
CoespotiNen: % ШЖ 65 Gulf Crown Е Oil 15 C/H Ratio Metosotar Weight: 230
Oxygen Beleece: CO» % . -70 CO %. -35
Dsesky: gm/cc Hand taoped I.37
^M^BBBIB^ s>URi \e
Frosting Beta; *C
। speet SontiMty, 1 Kg Wh Buroou of Mines Apporotus, cm 53 Sociple Wt 20 mg Picatinny Areanol Apparatus, in. 13 Sample Wt, mg 25 te_»ne T ^^BBBBBe \s
go go io
Stool Shao Unaffected Rbor Shag Unaffected Vecaaot StehiWy Tool: cc/40 Hrs, at 90*C 100’C 0-3*t 120*C 0.56 135*C x 150*C
Mb Baliti taped Tost: Trials % Captations 0 Partiata 0 Burned 0 Unaffected 100
280 Oram too* Send Test: Sand, gm *Ю.1
fapfssioi Tot psootwo: ’C Seconds, 0.1 (no cap used) 1 5 Decomposes; no value obtained 10 15 20 at ws а. ж_ e r»r же Вм^е^^га^ВСВе Minimum Detonating Charge, gm Mercury Fulminate Lead Aside 0.20 Tetryl
SaBtatic Mortar, % TNT: (a) 118
Tranti Teet, % TNT:
* 9 w 1 Wee % Loos in 43 Hrs
Plate Deaf Toot: (ъ) Method В Condition Hand tanped Confined No Density, gm/cc . 1>37 Briionia, % TNT 85
1M*C Hoot Testi % Loss, 1st 4iHw 0.63 •. .4 Loss, 2nd 48 Hrs -01 -ixplotion in 100 Hrs None
ftetOe Confinement Nora: Condition. Hand tanped Chorge Diameter, in. 1.0 Density, gm/cc 1. 37 Rote, meters/second 7390
ЙррямрМр % 30°C, 90$ RH 0-04
VtitiMty*
31F
F-ГРЕ
АМСР 706-177
FvegeideMHee Teet?
«• man HI, МУ1 MHctfe.Let WC-tl: V Density, gm/cc 1.3S
Cha.-5s W lb 1.^6
Tatel H*. ef РгедемМ*.'7
For TNT 703 -
For SubJirtHE _ J fMhKVtadW Рго)мМе, Let KC-S: 592
Density, gm/ec ? 1.U2
Chorge Wt, lb . , . . 0.756
• x" ? '
TotalH».efFr*gmeaj«( -
For TNT 51b
For Subject ЙЕ \ 501
--
Рядами* VetacMyt ft/sec AtPft At 25% ft 2650 2370
Density, gm/cc 1.395
Btast (RstaHveteTNT): Abt Peek Prostum Impube bergy Ab, Ceebtaed: Impube Under Wotan Peek Pressure Impulse Energy Ua^MMabta4t Peok Pressure Impulw Energy Preps ration; RIPE Is manufactured t. simple mechsnlс»I
mixing of RDX In oil.
Hol* Volume -
Hdrtfepih
Whit*
Plastic demolition exploaive
Landtag DsasMy: gm/cc
Method
Hand tamped
1.37
ГГу
Hozord Class (Quontity-Dbtonce)
Compatibility Group
Kone at 85°C in 30
Esudorion Kone at 95°C in W
Exudes at 105°C in
Origin:
Clean 9
30
Group I
hrs
hr*
k8 hrs
RIPE, a a*chan1cel mixture of RDX and Gulf
Crown E Oil, we* developed in the United States
during World war II,
R*ferenceaih"
(a) U- C. Smith and E, G. Xyster, Physical
Testing ttf Explosives, Part HI - Miscellaneous
Sensitivity Tests; Performance Tania, dfltb Be-~
port No.'^t. 5?'b»cemler"T545'.-------
(t) D- P. MscDougsll, Methods of Physical
Testing, OSRD R*port No. 86}, it August
(c) Also see the following Picatlnny A.-aenal
Technical Reports on RIPE: 1713, 1695 and 1517<
hHSee footnote 1, pagt- iо.
319
АМСР 706-177
Silver Azide
Melecelar Weight: (AgN^) 150
N 28.0 Ag 72.0 Oxygen lalaata: CO, % -5 CO % -5
Ag-N-«"« Dasilty: gm/cc Crystal 5.1
JuUMsg Meh ’C.. (a) 251
C/H Ratio _ . _ , . silver hd nitrogen. Steering Meh C
hnpeel SeeaMrOy, 2 Kg Wh BriOeg Meh *C
e^Ota ^Я^че a IB IMV J Iga^^ga no a will w Sample Wt 20 mg Picotinny Arsenal Apparatus, in. 3 Sample Wt, mg 18 ft ft ft
Mttiea Pte delta Tash PA Stall Apparatus Steal Shoe Detonates Fiber Shoe Detonates vKWMR МЮТЮТу 1 cc/40 Hi*, at WC 100*C 120‘C 135’C 150‘C
RMe BeKst hnpect Teat: Trial* % Explotion* ta ^t-t- ГОГТЮ»
Burned Unaffected 200 Gram Bea* Seed Teah ЙаЬУооийег fuse^ 18. Q ___
ExpUsiee Ttaparaleie. *C Second*, 0.1 (no cap uced) 310 1 5 Explodes 290 10 15 1— gGHHItvWy МНП^м^ИВ* Minimum Detonating Charge, gm AAercury Fulminate Lead Azide Tttryl
20 BeHiaHc Mortar, % TNT:
TroexiTeat, % Hg(ONC)2 (c) 88
7S'C letimstlaaal Hoot Teat: % Loa* In 48 Hr*
Plote Doot Teah Method
WC Hoot Teat: % Loaa, lit 48 Hr* % Loaa, 2nd 48 Hr* Explotion in 100 Hr* Condition Confined Density, gm/ce Brisance, % TNT
DeBMMtWfl Retos Confinement Condition Charge Diameter, in.
HsoMMbdity Index:
Hygroscopicity: % (b) 25°C, 100'S RH 0.04
Volatility: 75°C, 2L hrs 0.00 Density, gm/cc Rote, meters/tecond
320
Silver Azide
AMCP 706477
РмфяиМвИоо Shaped Charge IHoaHvorssa, TNT = 166t
N ама HI, M71 PsejMtMa, Ut WC31: Density, gm/cc ChorgeWt, Ib Gloss Cones Steel Cones Hole Volume Hole Depth
Total Ne. of Fregassats: For TNT For Subject HE S bob M, MttAI ProjosSMs, Let KC-S: DoraKy, gm/cc ChorgeWt, Ib Celon Whl te to grey
Maslpel Uses: Initiator
ToM Ha. of Fregamata: For TNT For Subject HI Mothe! el Uedhgt Pressey'
X. Loodhsg Osaaftyt gm/cc Variable
Frepaeat Vaieoftyt ft/sec
At У ft At 25% ft Density, gm/cc Method Wet
Meet (BeioHve ta TNTh Hozord Class (Quantity-Distance) С1ам 9
Ain Peak Pressure Impulse &tergy Compatibility Group Exudation (uOUp M None
А1г,СмЯм£ Initiating Efficiency:
Impulse Uader Wotan Peak Pressure Graae Required to Give Complete Initiation of TNT Solubility in 100 gm Solvent (c) 0.02-0.05
Impulse &tergy UWw^vmnMIs Peak Pressure Impulse Energy Explosive Power; (f) at Room Temperature:
Solvent Water (b) Ansaonium hydroxide Nitric acid Ether (b) Ett_. 1 alcohol, 95^ Acetone Unaffected by water and COg. Grams 0.006 Soluble Decomposes 0.017 0.006 0.015 (a)
Kilogram meters 192,000 Heat of:
$ Mercury Fulminate 1.097 Exploalon, cal/gm ic. a) Formation, cal/gm (e) 1*52 67.8
321
АМСР 706*177
Silver Azide
Preparation;
ММ3 + AgJK>3~* AgN3 I + KaHO3
Prepare the following aqueous aolutiooe:
•> 5^ НЫ<з> sodiua azide, 50 ее
b. 256 AglO^, silver nitrate, 25 ее
The silver nitrate aolution la placed in a 200 ее conductive rubber beaker equipped vith a
hard wood stirrer operated by an sir notor. Thu sodlua azide solution Is plaeed in a separa-
tory funnel fastened in e ring stand above the beaker containing the Oliver nitrate. A long
cord (10 ft) is fastened to the stopcock of the separatory funnel so that the funnel can be
ez^tled by reacts control. The silver nitrate solution is ow stirred very tepidly and the
sodlua azide la slowly ten into the nitrate solution. Stirring is continued for 5 minutes.
The content^ of the beaker are filtered through folded filter paper and vashed free of sodlua
azide and silver nitrate vith distilled water.
Silver azide should be stored under water in a conductive rubber container. Thia prepara-
tion will yield approximately 7 grans.
The preparation should be conducted under a hood and behind a barricade. The product ob-
tained hy the above procedure has a very fine particle size, alnoet colloidal. Very fine sil-
ver azide ie safer to handle and is just as efficient and stable as the largo, coarse crystal-
line mterial (Ref b). When a thin flln of fine silver azide is precipitated on Mercury fhl-
ninate, tetryl, etc., these substances are as efficient weight for weight as pure silver azide
(Ref g). White silver azide is less effected by light than aercury or lead azide (Ref h).
ions colorless crystals which explode on breaking are obtained fmn ammonium hydroxide.
Origin!
Silver azide was first prepared in 1890-1 by T. Curtius (Ber 2J, 3032; Ber 24, 3344-5) by
passing hydxezoic add (HM-) into neutral silver nitrate solution. Thylor and~Kinkenbach pre-
pared pure "eollodial" aggfegatee and showed its sensitivity depends upon its particle size
(Any Ordnance 5, 824 (1925). Silver azide was found in a detonator of foreign aeunltion for
the first tine in 1945 (Ref 1).
References
(a) A. R. Hitch, "Thermal Decomposition of Certain Inorganic IM nitrides," J An Chen Soc
40, 1195 (1918).
(b) C. A. Taylor and Wn. H. Rinkenbach, "Silver Azide: An Initiator of Detonation," Army
Ordnance, vol 5, p. 824 (1925)*
(с) E. De V. S. Colver, High Explosives, London and Rev York, p. 527.
(d) A. Stettbacher, Spreng u. Schlesstoffe, Reacher, Zurich, p. 97 (1948).
(e) A. Marshall, Explosives, 2nd Ed, Vol II, p. 767, London.
(f) A. Stettbacher, Z gee Schiess-Sprengstoffw 10, pp. 193-214 (1915).
69s«« footnote 1, page 10.
322
311тет Aside
АМСР 706-177
(в) Т> Blechta, Chi» et Ind Special Io. 921-5 (June 1933); C. A. 26, (46.
(h) L. Wohler and W. Krupko, Berlohte W, 20^7-2050 (1913).
(1) F. G. Harerlak, graainatloo of 120/k5 Ж Hg Shell, Italian (MOM-M6U) , BA® Mo. 1515,
10 April 19*»5-
323
АМСР 706-177
Tetracene
CmvmNm. С 12.8 NH мн Н 4.3 • А , , с-кн-кн-к» н-с К 74.4 1 \ „ КН- нн-нн-ко О 8.5 2 C/H Ratio 0.068 Molecular Weight: (CgHgNloO) 188
^hrygea Boleace. CO, % .60 CO % .43
Density: gm/cc At 3000 pel 1.05
Melting Bolat: ’C Explodes 140-160
Pieeeieg Peksh *C
bnpoct SiMiNvky, 1 Kg Wh Bureau of Mines Apparatus, cm 7 Sample Wt 20 mg Picotinny Arsenal Apparatus, in. 2; (8 az vt) 8 Sample Wt, mg
Reflective Index, n» n£ l£
MsHoa Poedninm Test; Steel Shoe Fiber Shoe Vicmm StoMSly Tests cc/40 Hrs, ot WC 100’C 120’C 135’C 150’C
Rifle BaNet Impest Test: Trials % Estonians rUrTtOI* Burned Unaffected
MO Gram Bemb Seed Test: Sn&^cvder fuse 4,0
lepiseiee Teaiperotuse. C Seconds, 0.1 (no cop used) 1 5 160 10 15 20 • ImM^Uar eMBwVTWy BBBOVBVVe^MBe Minimum Detonating Chorge, gm Mercury Fulminate 0.40 Load Azide Tetryl
BoHietic Mortar, % THT:
Trmal Teet, % TNT: (e) 61
75’C letsmetieael Meet Test: % Lass in 48 Hrs 0.5
Plots Doot Test: Method Condition Confined Density, gm/cc Brisance, % TNT
100’C Heat Tech % Loss, 1st 48 Hrs 23.2 % Loss, 2nd 48 Hrs 3. 4 Explosion in 100 Hrs None
Confinement Condition Chorge biometer, in. Density, gm/cc Rote, meters/socond
ЛоемпоЬМИу lodes:
Hygroscopicity: % 30°C, 90$ RH 0.77
xo a -«ч- ▼ФЮТвМу*
324
Tetracene
AMCP 706-177
РмфяиМвИоо Tm9s Shaped Charge IHsdhssf, TNT = 100:
W sass HI, M71 PrajesMa, Let WC-91: Density, gm/cc Chorga Wt, Ib Glat* Cones Steel Cones Hole Volume Hob Depth
Total He. af Ftagmoab: For TNT For Subject HE 1 ieth HI. M42A1 PmjecMe, Let KC-J: Density, gm/cc ChorgeWt, Ib Color: pale yellov
Pitadpel Ihoet Priming compositions and detonators
Total No. af FragmoaH: For TNT For Subject HE Method of Leedbg: Pressed
Leedbg Bsaslfri gm/cc
Fi nf—t ValecHy: ft/sec At 9 ft At 2ЭД ft Density, gm/cc At ЭООО pel 1.05
rVWWiOQ Vet
loot (Melbeta TNT): Hazard Class (Quantity-Distance) Class 9
Abt Peek P.essure Impuhe Energy Compatibility Group Exudation Group M
Air, Caafieed: Impube Uader Water: Peek Pressure Impube Solubility:
Practically insoluble in water, alcohol, acetone, ether, benzene, carbontetrachloride or ethylenedichloride. Sensitivity to Electrostatic Discharge, Joules: (b)
btergy Peek Pressure Impulse Energy Unconfined Confined Heat of; Explosion, cal/gm Gas Volume, cc/gm Initiating Efficiency: 0.010 0.012 658 1190
Tetracene is not efficient in initiating high explosives.
325
АМСР 706-177
Tetracene
Preparation;
(Rinkenbach and Burton, Лицу Ordnance 12, 120 (1931))-
Tetracene ie prepared by dissolving 5 вяв of emlnoguanidlne dinitrate in 30 cc of voter,
cooling to 0°C and nixing vith a solution of 2.5 gms of sodium nitrate in 15 cc of water. The
temperature is maintained at about 10°C and C.5 gm of acetic acid ia added. The tetracene
separates out and is washed with water, alcohol and ether. It is then dried.
Tetracene may also be prepared by placing aminoguanidine sulphate and sodium nitrite in a
large beaker and adding water her ed to J0°C. The heat of reaction causes the mixture to boll;
after standing for two or three hours the separated tetracene is filtered off, washed thoroughly
and dried.
Origin:
Tetracene was first prepared in 1910 by Hofflnan and Roth (Ber 4j, 682) who also studied its
chMdcal reactions and determined its structure (Hoffman et al, Ber 43, 1067, 1866 (1910); Ber
44, 2496 (1911); and Ann 380, 131 (19H)). W. H. Rinkenbach and 0. Si-ton made an extensive
sTudy of tetracene and described Its manufacture and explosive properties (Army Ordnance 12,
120 (1931)).
Destruction by Chemical Decomposition:
Tetracene is decomposed by adding it to boiling water and continuing boiling for some tine
to insure complete decomposition.
References;70
(a) D. P. MacDougall, Methods of Physical Testing, OSRD Report No. 803, 11 August 1942.
L. C. Smith and E. G. Eyeter, Physical Testing of Explosives. Partlll - Miscellaneous
Sensitivity Tests; Performance Tests, OSRD Report No. 57^, 27 December 1945-
(b) F. V. Brown, D. H. Kusler and F. C. Glbsoq, Sensitivity of Explosiyesto initiation
by KLectroatatic Discharges, U. S. Dept of Int, Bureau of Mines, RI 3852, 19*‘6<
(c) Also see the following Plcatlnny Arsenal Technical Reports on Tetracene:
0 1 2 4 7 8 2
1450 11 453 1104 407 318 859
2164 2179
7^See footnote I, page 10.
328
Tetranltrocarbazole (ТИС)
АМСР 706-177
Molecular Weight: (C^^BjOg) 3*»7
* O_N Н NO с *:.6 Н 1.4 Г Т 1 1 Oxygen Balance: CO- % -85 CO % .30
°2N "к J О И°2 В 20.0 Density: gm/cc
0 37.0 C/H Ratio 1.032 Melting Point: °C Pure 1,3,6,8-lsomer 296
Fro wing Point: *C
Impact Sensitivity, 2 Kg Wt: Buraou of Mint» Apparatus, cm 100+ Sampte Wt 20 mg Pkatinny Arsenal Apparatus, in. 18 Sampte Wt, mg 14 Boiling Paint: *C
Refractive Index, n° l£
Friction Pendulum Test: Steel Shoe Unaffected Fiber Shoe Unaffected Vacuum StebiBty Test: cc/40 Hrs, ot 90'C 100°C 0.2 120*C 0.2 I35*C 150’C
Bifte Bultet Impost Teet: Triols % Explosions Partials
Burned Unaffected 200 Gram Bomb Saud Tost: Sand, gm 41.3
Ixpteetee Teeverehwe: *C Secono, 0.1 (no cap used) 3 IX composes 1*70 10 » tnS--te_- e -t O^wBurTWy W iHWu^ravOBB» Minimum Detonating Charge, gm Mercury Fulminate Lead Azide 0.20 Tetryl 0.25
20 Ballistic Mortar, % TNT:
Trend Test, % TNT:
75’C letenMmoee: Heat Test: % Loss in 48 Hrs
Plate Dant Toot: Method
WO'C Heat Test: % Lou, 1st 48 Hrs 0.15 | % Los*, 2nd 48 Hrs 0.05 Explosion in 100 Hrs Bone Condition Confined Density, gm/cc Brisance, % TNT
1 e^oA. Confinement Condition Charge Diameter, in. Density, gm/cc Rote, meters/sacond
<к^1вмямЫ1йу
Hygroscopicity: % 30°C, 90$ RH 0.01
Volatility:
327
АМСР 706-177
TetrenitrocarbecoJe (Pic)
Ftogmoaesttae Test: Shaped Chorge Effeetiveaesa, TNT: = 1W>
N мм М, МП МмМ«, Lm Wfr«lt Density, gm/cc Chorge Wt, lb Glc* Cones Steel Cones Hole Volume LJjJ* Retell пою uopvn
ToBol MOb OJ FfOJBHOeBJ ForTNT For Subject HE S tach HA M43A1 Piijsctita, Let KC4: Density, gm/cc Chorge Wt, tb Celon Light yellow
Principal Uses: Coqponent of igniter end pyrotechnic co^osltione
Total No. of МдммМг ForTNT For Subject HE Moihorf off ЬмЛкф Pressed
Leading Denrify: gm/cc
FrogMoat Vetaefty: ft/sec At» ft At2S%ft Density, gm/cc
Method Dry
Meat Utatalbe to TNT): Hazard Class (Quantity-Distance) Class 9
Ain Pooh Pressure Impute Energy Compatibility Grog: Exudation
Abp СмЛмА Impute Under Wetan Peak Pressure Impute Ьмгду Peak Press'jre Impute Energy Solubility in Water, gm/100 go Щ, st;
95°C Qualitative Solubilities; Solvent Nitrobenzene Acetone Benzene Chloroforo Carbontetrachlorlde Ether Ether, pet-roleun 0.10 Solubility Very soluble Soluble Insoluble Insoluble Insoluble Insoluble Insoluble
328
Tetranitrocarbazole (ДС)
AMCP 706-177
Preparation;
Sulfonation; Fifty-elx gms of carbazole is dissolved in 320 gms of HgSOv (966, specific
gravity 1.81). ihe solution is agitated during the addition of the carbazole and the tempera-
ture maintained at 25°-33°C. After the addition of the carbazole is completed, the agitation
is continued and solution completed by raising the tesperature to 80°-85°C and maintaining
this temperature for one hour. Де sulphate is now cooled to 20°C.
Witration; ihe sulfonate solution is slowly added to 168 gms of HHOq (Plant grade specific
gravity 1.525 at 15°C) salntalning the temperature at 30° to 50°C. (Tina required - 1 hour 25
minutes). Ihe te^erature is then gradually raised to 70° to 75°C and nndntalned for one hour
after which the temperature is raised to 85° to 90°C and held for one hour, then lowered to
room t04>exature before drowning.
Drowning; The nitration mixture is drowned by pouring it into 2 to 3 volumes of ice and
water.
Filtering; The separated light yellov product Is filtered on s Buchner Funnel end washed
with water twice to remove most of the acid.
Purification; The ДС is placed In hot water (95° to 100’c) and boiled for five to ten
minutes with rapid agitation, allowed to settle then filtered and washed once. This proce-
dure Is repeated twice, making a total of three ''boilings.” The final wash is acid free.
Drying: The TWC is spread in a thin layer and dried at 100° to 110°C for four hours.
Yield; 73-3*.
Melting Point of ДС as prepared: 28o°C (compares to 296°C for pure 1,3,6,8-lsooer in pre-
ceding 8ata).
Origin;
The preparation of Те tranl trocarbar. ole (BiC) was first reported in 1880 by C. Graebe (Ann
202, 26 (1880» who nitrated carbazole with 9b£ nitric acid. Similar procedures were followed
by R. Escalee (Ber 37 , 359t> (190b))and P. Zierch (Ber 12, 3000 i9O9U However, G. L. Ciami-
cian and P. P. Silber observed the formation of four isomeric ТИС в when acetyl carbazole van
treated with fuming nitric acid (Gazz chim ital 12, 272 1882)1 In 1912 and 1913 patents were
Issued to the dyestuff manufacturer, Casella and Company, covering the preparation of polynl-
trocarbazoles (German Patent 268,173 and French Patent 164,538). The Casella process of
:й>9
AMCP 706-177
Tetranitrocarbazole (INC)
preparing polynltrocarbazoles by dissolving carbazole In sulfuric acid and treating the solu-
tion of sulfonic adds vith strong nitrating agents is essentially the process used today in
the United States. The crude product, thus prepared, contains principally 1,3,6,8-TNC (W.
Boreche and B. G. B. Scholten Ber 50, 596 (1917) and about 10^ of the 1,2,6,8-ПГС isomer
(D. B. Murphy et al J Am Chem Soc 7%*, 1*289 (1953)- TNC was used in explosives by the Germans
during World Whr II.
References;71
(a) D. B. Mirphy, F. R. Schvartr, J. P. Picard-and J. V. R. Kauftaan, "Identification of
Isomers Formed in the Nitration of Carbazole,'' J Am Chem Soc, 75, 1*289-1(291 (1953).
(b) S. Livingston, Preparation of Tetrad trocsrbazole, PA Chemical Research laboratory
Report No. 136,330, 11 April 1951.
(c) D. Br. Murphy et al, Long Range Basic Technical Research Leading to the Development of
Improved Ignition Type Powders - The Chemistry of Tetranitrocarbazole, pA Memorandum Report
No. 22, 2 September 1952-
(d) S. Livingston, Development of Improved Ignition Type Powders, PATH No. 2267, July I956.
(e) Also see the following Picatinny Arsenal Technical Reports on Tetranitrocarbazole:
0 2 7
2180 1802 1973 V.81* 161*7
1937
7^ee footnote 1, per/. 1C.
330
2,U,2’ ,4’ -Tetrar.itro-oxanillde (TWO)
AMCP 706-177
<v 0 0 Molecular Weight: (С^НдЯдО^) U20
* II c l»0.0 c H 1.9 f f Oxygen Boleace: CO- % -84 CO % -31
Я 20.0 Г I Г 1 2 Density: gm/cc
0 38,1 Melting Point: °C Decoapoaea 313
C/H Ratio 0.735 «°2 N^2 Freezing Point: °C
Inject Smteitivity, 2 Kg Wt: Buraou of Mina* Apparatus, cm Sample Wt 20 mg Picotinny Arsenal Apparatus, in. 30 Sample Wt, mg 11 Boiling Point: °C
Refractive Index, ria n.° nS,
nCTW® 1 w^a * Steel Shoe Unaffected Fiber Shoe Unaffected Vacuum Stability Test: cc/40 Hrs, at 90*C 100°C 120’C 0.11 135*C 150°C
Rifle •Het Impact Teat: Triols % Explosions Partials
Burned Unaffected 200 Gram Bomb Send Test: Sand, gm 1& 3
Explesioa Temperature: °C Seconds, 0.1 (no cop used) 1 5 392 10 1C Sensitivity to Initiation: Minimum Detonating Chorge, gm Mercury Fulminate Lead Azide 0.20 Tetryl 0.25
1 9 20 Ballistic Mortar, % THT:
Trauzl Tost, % TNT:
7S*C iateraoMaaai Hoot Test: % Loss in 48 Hrs
Plete Deel Teet: Method
100*C Hoot Tost: % Loss, 1st 48 Hr* 0.07 % Loss, 2nd 48 Hrs 0.00 Explosion in 100 Hrs None Condition Confined Density, gm/cc Brisance, % TNT
Detonation Rote: Confinement Condition Charge Diometer, in. Density, gm/cc Rote, meters/second
Flammability Index:
Hygroscopicity: % 30°C, 90% HH Trace
Volatility:
331
АМСР 706-177
2,U,c',lt,-Tetxmnitro-oxanlllde (СТО)
AmgMSOtoNsm Test: Shape! Charge IHnlhseMS, TNT = IM:
М ама HI, М71 Nojectita. Lot WC-T1: C jnsity, gm/cc Charge Wt, ib Glass Cones Steel Cones Hole Volume Hole Depth
Total No. of FrogoMafz: ForTNT For Subject HE > tech HI, M42A1 PmjscMs, Lot KC-9: Density, gm/cc Charge Wt, Ib Cohn Light yellow
Prtadpel Usm: Component of block powder type end pyrotechnic соц>оа1Ь1опа
1 VW w ^W^M^BWIVa ForTNT For Sub. oct HE Method ef Loadtap: Pressed end extruded compositions
Loadtap Deeeily: gm/cc
Fregmaat Vetacfty: ft/sec At 9 ft At 254 ft Density, gm/cc
Method Dry
Btast(tabtiveteTNT): Hazard Class (Qiantity-Distance) Claes 9
Ain Peak Pressure Impute biergy Compatibility Group Exudation
Mt, Caeftaod: Impute Under Wafer: Peak Pressure Impute Energy Peak Pressure Impute biergy Solubility, gm/100 cc Solvent, in:
water Nitrobenzene Qualitative Solubilities: Solvent Ethyl alcohol Benzene Butyl acetate Oarbon tetra chloride Ethyl ether Acetic ecld Nitric acid Caustic potash Dimethyl formamide V 1 100 <0.10 150 >15 Solubility Insoluble Insoluble Insoluble Insoluble Insoluble Soluble Soluble Soluble Very soluble
:«2
2Л,2',У-Tetranitro-oxanllide (СТО)
AMCP 706-177
Method of Preparation;
♦2H20
Oxanllide:
Two parte of oxalic add are nixed with one part of aniline in a round bottom Паек. The
mixture ie stirred and heated until the reaction ie complete aa evidenced by the cessation of
effervescence. The паев ie cooled to roan temperature, poured into several vcluoee of water
(21°-2U°c), filtered on a Bilchner funnel and washed free of oxalic add vith water and then
washed free of aniline vith acetone. The oxanllide is air dried to remove the acetone and
then dried at 100°-110°C.
Tetranitro-oxanllide (MO):
A 5 liter round bottom flask ie equipped vith a stirrer of a type vhich vll? produce a
downward "svirl." The flask is surrounded with a water Jacket for hot and cold water. Fif-
teen hundred gratae (1.5 kilograms) of 96% plant grade nitric acid is placed into the flask.
Five hundred (500) grams of oxanllide is slowly added to the acid under rapid agitrtion while
the temperature is mainteined below kO°C. After the addition of the oxanllide is completed
(2^-j hrs), the agitation is continued 10-15 minutes. The temperature is then raised to 8O°C
ever a period of опз hour and maintained at 8o°-85°C for 3 hours. The acid slurry is then
cooled to room temperature and drowned by pouring ever cracked ice. The product is filtered
on a Buchner funnel and washed vith water until it is almost acid free. The filter cake is
placed in a beaker and sufficient water added to fora a "slurry." Live steam is run into the
"slurry" under agitation for 10 minutes. The slurry is filtered and the residue washed. The
latter treatment of the "slurry" is repeated until the wash water is found to be neutral to
АМСР 706-177
2,U,2',U'.T»tranitro-oxanllide (TWOj
litmus paper- The TNO la washed with alcohol, then acetone, air dried and finally dried at
100°-110°C.
Yield - 90^ to 97-5^ of theoretical.
Origin;
A. G. Perkin in 1892 obtained tetranitro-oxanilide directly by heating a solution of finely
powdered oxanilide in nitric acid. He also obtained the earn compound by the action of a
cooled mixture of nitric and eulfUric acids on oxanilide and precipitating the product by
pouring the solution into water (j Chem Soc 61, 460 (1892).
References;72
(a) S. Livingston, Development of Improved Ignition Type Powders, PAIR Ho. 226?, July 1956
(b) D. Dubrow and J, Kristal, Substitution of Tetranitro Oxanilide and Hexanitro Oxanilide
f^Tetranitro Carbazole, PA Pyrotechnic Research laboratory Report 5^-TF 1-88, 2d becember
^See footnote 1, page 10.
334
АМСР 706-177
Tetryl
СмчмеЖм Molecular Weight: (CjHjNjOg) 287
С 29-3 н 1-7 02К HjC—N — "°2 YN02 Oxypea Beleace: CO- % CO % -7*7 4 - 8
N 2U.1* Density: gm/cc Crystal 1-73
0 1*4.6 M02 Motting Point: *C 130
C/H Ratio 0.1*20 Fraosinp Point: *C
Impact Sensitivity, 2 Kg Wt: Bi-roou of Mines Apparatus, Sample Wt 20 mg Picotinny Arsenal Apparatu Sample Wt, mg 26 BeMag Point: *C
S, in. 8 18 Refractive Index, n° ni nS,
Friction Pondulam Teet: Steel Shoe Fiber Shoe Crackles Unaffected Vacuum Stability Test: cc/40 Hr«. at 90-C 100’C 120’C 135*C 150’C 0.3 1.0 11+
Rifle Ballet Impact Test: Trials % Explosions 13 Partials 54
Burned Unaffected 10 23 200 Gram Bomb Sand Test: Sand, gm 54.2
Fwplesiee Temperature: Seconds, 0.1 (no cap used) 1 5 Ignites 10 ]5 •c 340 31b 257 238 94A Sensitivity to InMetion: Minimum Detonating Charge, gm Mercury Fulminate Lead Azide »Alt«TiJ^lve initiating chargee. 0.20» 0.10*
20 231* Ballistic Mortar, % TNT: (a) 130
Travel Tost, % TNT: (b) 125
75°C International fleet Teet: % Loss in 48 Hrs
0.01 Plate Doot Test: (c) Method A В
1W: Heat Teet: % toss, 1st 48 Hrs % Less, 2nd 48 Hrs Explosion in ICO Hrs 0.1 0.0 None Condition Pressed Pressed Confined Yes No Density, gm/cc 1-50 1.59 1<3б Brisance, % TNT 116 115 96
Detonation Reto: Confinement Condition Chorge Diometer, in.
Flammability Index: 241* None Pressed 1.0
Hygroscopicity: % on°C; go^ jqj 0.01*
Volatility: 25°C 0.00 Density, gm/cc Rote, meters/second 1-71 7650
335
AMCP 706-177
tetryl
ID^WVW ЛИ^яТтНу 1 VOv* Condition Tetryl, gm Wox, In. for 50% Detonation Wox, gm Density, gm/cc (a) Preseed 100 2.01 1.58 Decomposition Iquetien: (g) (h) Oxygen, atoms/мс 1015'4 IO12'9 (Z/soc) Moot, kilocolorie/mole 38.U JU.9 (AH, kcal/mol) Temperature Range, °C 211-260 132-16U Phase Liquid Liquid
Hoot of: Combustion, cal/gm Explosion, cal/gm Gas Volume, c:/gm Formation, cal/gm Fusion, cal/gm _ (e) Temperature, C 2925 1080-1130 ТбО -11» 22.2 127 Aimer Mate Impact Test: M asm Matter Projectile: 50% Inert, Velocity, ft/sec Aluminum Fineness SOO-W General Perpose Bombs:
Spo^fk Heat: col/gm/°C -100 - 50 0 50 100 (e) 0.182 0.200 0.212 0.223 O.236 Plots Thickness, inches 1 V,4 1Ц.
Berning Mie:
cm/sec Bomb Drop Test:
Thermal Ceedesllill>: ( ?) , col/soc/cm/’C 5-81 x 10"? 6.63 x 10 at 1.391* gm/cc at 1.528 gm/cc T7, 2000-» Ssml Armor Horcing Bomb vs Concrete:
СмНШм! ef KK^Mstoss Linear, %/°C Max Safe Drop, ft SOO-» General Purport bomb V» Concrete:
Volume, %/°C Height, ft Trials Unaffected
Hardness, Mobs' Scale:
MbedvhM* E', dynes/cm’ E, Ib/inch’ Density, gm/ic Low Order High Order 1000-» General Perpose Bomb vs Concrete: Height, ft Triols Unaffocteo
Compressive Strength; Ib/inch’
Vapor Pressure: ’C mm Mercury Low Order High Order
336
АМСР 706-177
^ir^i^ioa^iae^iti^i^s ITest *
99 она Hl. М71 Pia|octih, Lal WC-91:
Density, gm/cc I.58
Charge Wt, Ib 2.052
Totol Na. ef Frepmaets:
ForTNT 703
For Subject HE 864
3 inch Ht, M42A1 Projectile, Let KC-S:
Density, gm/cc 1.62
Chorge Wt. lb 0.848
Total Na. ef FrogaauM:
ForTNT 514
• For Subject HE 605
Shaped Charge tffecthreaees, TNT = 100:
Gloss Cones Hole Volume Hole Depth Steel Cones
Cefor: Light yellov
Principal Uses: Boosters; ingredient of explo-
sive mixtures, detonators, and
blasting caps
Method ef Leodiag: Pressed
Leodiag Density: gm/cc See belov
Fn|a>i»t Vebdty: ft/sec
At 9 ft
At 2514 ft
Density, gm/cc
Bloat (Mathre to TNT):
Ahn
Peek Pressure
Impulse
Energy
Air, Caafiaad:
Impulse
Under Water:
Peak Pressure
Impulse
Energy
Uadetgrooad:
Peak Pressure
Impulse
Energy
Method Dr-
Hazard Class (Qiantity-Distance) Class 9
Compatibility Group Group L
Exudoti'in Does 1 not < exude at 65°C
Loading Density: gm/cc
Cast 1.62 Pressed psi X 103
0 3 5 10 12 15 20
0-9 1-40 1.47 1-57 1.60 1.63 l-6r
30
1.71
Effect of Temperature on (j)
Rate of Detonation:
]6 hra at, °C -54 21
Density, gm/cc 1.52 1-53
Rate, m/aec 7150 7170
АМСР 706-177
Tetryl
Preparation;
(Manufacture of Tetryl by Dlnitromonomethylanillae Process, Wannamaker Chemical Cc ., Inc.)
C/-H(NO)C1 + CH.SH, + NaOH — СЛМНО '+ NaCl ♦ H,0
©322 32 0322 3 2
C6H3(NO2)2-NH-CH3 ♦ 2HNO3x^
O2N_jX^\^ N02
I J ♦ 2H20
no2
To a solution of 202.5 gm dinitrochlorbenzene in 200 cc benzene, at 75°C with good agita-
tion, in 15 to 20 minutes, add 112 gm of 30% aqueous monomethylsmine- Then add 129 gm of 31%
aqueous sodium hydroxide, in 15 to 20 minutes, at such a rate as to cause refluxing; continue
agitation for 3 hours at 70°C. The mixture is concentrated to a liquid temperature of 101°-
102°C, cooled, filtered and the precipitate washed with distilled watei until the washings
give no test with silver nitrate, dried at 60°C (melting point 167.2°c)
The dlnitrcoethylanlline is nitrated to tetryl by solution of it in &8% sulfuric add (197
gm nitroanlline/1190 gm sulfuric) at 25°C, followed by addition of nitric add. The process
is carried out so that the water content remains at 16%. Solution (per 197 gm nltroanlline)
requires $ to 10 minutes, nitration, by addition of the aulfuric add solution to nitric acid,
about 1 hour at 30°C, plus U8 minutes at 50° to 55°C st the end. The mixture is then cooled
to 20°C and filtered. The tetryl is dumped into 1 liter water, washed 2 or 3 times with 200
cc cola water, and then stirred 10 to 15 minutes at 50°C with 500 cc water, filtered warm aH
then washed with water until the washings are neutral to metlr-1 orange. The tetryl dried to
constant weight at 70°C weighs about 270 gm.
Tetryl filtered from an acid containing 87% sulfuric acid (or more) -13% water, at l»0oC
(or over) may fire in 30 minutes to 1 hour and 30 minutes, if not drowned in water. A safe
nitration procedure, even on plant scale involves:
1. The concentration of sulfuric in the opent acid is maintained at a lov level (approx
80/1.6/18.2 sulfurlc/nitrlc/water)•
2. Nitration maximum temperature is 50°C.
3. The slurry is cooled to 35°C before filtration.
U. Filtration time prior to drowning, is minimized (15 minutes maximum).
The crude tetryl produced is recrystallized to remove impurit : and occluJed acid and to
control its granulation.
338
AMCP 706-177
Sensitivity of tetryl electrostatic diecharge, Joules; through 100 mesh; (1)
Unccnflned 0.007
Confined 4.4
Solubility of tetryl, grana In 100 grana (£) of;
Water Carbon tetra chlor: de Ether 95^ Alcohol
2c i °C J
0 0.0050 0 0.007 0 0.188 0 0.320
20 0.0075 20 0.015 10 0.330 10 0.425
40 0.0110 40 0.058 20 0.418 20 О.563
80 0.0810 60 0.154 30 0.493 30 0.76
100 0.184 50 1.72
75 5-33
Chloroforrs Carbon disulfide Ethylene dl chloride Acetone
°C °C i 2s i 2c i
0 0.28 0 0.009 25 4-5 20 75
20 0.39 10 0.015 75 45 30 95
40 1.20 20 0.021 40 * 116
60 2.65 30 0.030 50 138
Tri chloroethylene Ethyl ace^1 ’te Benzene Toluene
°C 1 °c 1 °C
0 0.07 20 ~40 20 7.8 20 8.5
20 0.12 30 10.0
40 0.26 40 12.5
60 0.67 50 16.0
80 1.50
86 I.76
20
30
40
50
3-3
4.4
5-4
6.0
TOT
°C <
80 82
100 149
120 645
Origin;
Tetryl was first described In 1879 by Michler and Meyer (Ber 12, 1792), van Rotnburgh and
Martens studied Its properties and proved Its structure (Rec trav chlr. 2, 108 (1883); 6, 215
(1887); and Ber 19, 2126 (1886)). Tetryl was not used as an explosive until World War-I.
339
АМСР 706-177
Tetryl
Destruction by Chemical Decomposition:
Tetryl is decomposed by dissolving in 12 times its weight of a solution prepared from 1
part by weight of sodium sulfite (NagSOy THgO) in 4 parts vote?. The sulfite solution may
be heated to 8o°C to facilitate decomposition of the Tetryl.
References:73
(a) L. C. Smith and E. G. Eyater, Physical Testing of Explosives, Pert III - Miscellaneous
Sensitivity Tests; Performance Tests, OSRD Report No. 57^6, 27 December 1945-
(b) Ph Naoum, Z gee Schiess-—Sprengstoffw, pp. 181, 229, 267 (27 June 1932)
(c) D. P. MacDougall, Methods of Physical Testing, OSRD Report No. 803, H August 1942.
(d) L. C. Smith and S. R. Walton, A Consideration of RDX/WSx Mixtures as e Substitute for
Tetryl in Boosters, NOL Memo 10,303, 15 June 1949.
(e) C. A. Taylor and Wm. H. Rinkenbach, "The Solubility of Trinitro-PhenyImethy1-N1tram!ne
(Tetryl) in Organic Solvents," J Am Chem Stc Ug, (1923) P- 10b.
(f) E. Hutchinson, The The-ral Sensitiveness of Explosives. The Thermal Conductivity of
"xplosive Materials, AC Й061, First Report, August 1942.
(g) R. J. Finkelstein snd G. Gamov, Theory of the Detonation Process, NAVORD Report No.
90-46, 20 April 1947.
(h) M. A. Cook and M. T. Abegg, "Isothermal Decomposition of Explosives," University of
Utah, Ind Eng Chem 1090-1095 (June 1956).
(i) J. W. Brown, D. H. Easier and F. C. Gibson, Sensitivity of Explosivesto Initiation
by Electrostatic Discharges, u. S. Dept of Int, Bureau of Mines, RI 385^, 1946.
(j) W. F. McGarry and T. W. Stevens, Detonation Rates of t',e More Important Military
Explosives at Several Different Temperatures, PAIR ко. &3B3, November 19*6.
(k) Also see the following Picatinny Arseni 1 Technical Reports on Tetryl:
0 1 *2 2 4 2 6 7 4/ 9
30 11 132 453 84 65 266 117 28 129
600 361 582 493 144 195 •556 197 438 179
no 381 832 623 294 425 786 637 628 319
810 621 882 £33 314 525 986 707 708 609
1180 861 1192 863 694 565 1086 807 788 709
1290 1041 1352 1113 774 625 1126 837 838 849
1350 1131 1372 1373 784 635 1316 857 1418 999
1360 1261 1402 2053 874 845 1376 1C4? 1788 1029
1400 133-2 1452 2163 904 925 1416 1137 1828 1209
1450 1500 1510 1677 1431 1471 1611 1651 1592 2233 1134 116’ 1234 1264 2C2U 2201 1145 1285 1405 15&“ 1885 1935 2105 2125 1446 1466 1556 1636 1956 1287 1337 1367 1437 1737 1797 1937 1838 1379 1429 1489 1819 1969
2205
3See *ootnote ), oage 10.
:14<»
Те try to1, 6c,-2O
AMCP 706-177
СеаарееЖеа: * Tetryl 8о тат го C/H Ratio Molecular Weight: 2Tb
Oxygea Balance: CO- % -52 CO % -11
Density: gm/cc Cast 1.51
Melting Paint: *C 68
Freexing Point: *C
Impact SeweMvity, 2 Kg Wt: Bureau of Minas Apparatus, cm 26 Sample Wt 20 mg Picatinny Arsenal Apparatus, in. 9 Sample Wt, mg 17 Bailing Feint: *C
Refractive Index, n» nS n?,
А*—T^rnAa Steel Shoe Fiber Shoe Vacuum Stability Test: cc/40 Hrs, at 90’C 100’C 3.0 120’C 11+ 135’C I5O*C
ШЯе BaNot Impact Test: Trials % Explosions 0 Partials 20 Burned 0 Unaffected 80
200 Grom Bomb Send Test: Sand, gm 54.0
Ixpfaiisn TeaapzreCere: 'C Seconds, 0.1 (nc cap used) 1 5 Ignites 290 10 15 20 SeniltMty te InM^San: Minimum Detonating Charge, gm Mercury Fulminate 0.22* Lead Azide 0.17* ♦AltelfiPt'ive initiating chargee.
Ballistic Mortar, % TNT:
Treed Test. % TNT:
7S*C Intemetieriel Hoof Test: % Loss in 48 Hrs
Plate Dent Tast: Method Condition Confined Density, gm/cc Brisance, % TNT Dutonetion Rato: Confinement Conoition Charge Diometer, in. Density, gm/cc Rate, meters/second
100‘C Heat Test: % Loss, 1st 48 Hrs 0.1 % IjOss, 2nd 48 Hrs 0.5 Explosion in 100 Hrs None
Flammability Index: Will not continue to burn
Hygroscopicity: % 0.02
VoJaHlity;
;н1
АМСР 706-177
Tetrytol, 8o/2O
Shaped Charge lifocHvoMV, TNT = 100:
M пмп HI. M7' PtelecHte, Lot WC-91: Gloss Cones Steel Cones
Density, gm/cc Che go Wt, lb Hole Volume Hole Depth
Total No. of Fragments: Colon Light у allow to buff
For TNT For Subject HE Principal Utee: Bursters, demolition blocks
3 tech HI. M42A1 PraHctite. Let KC-S:
Density, gm/cc
Chorge Wt, lb
Tstel No. of Fragments: For TNT For Subject HE s*-«s - -A J 1 m—ЛЗ W tes^^^^^Va^M 0 loading Daneity: gm/cc
Fragment Votecity: h/sec
At 9 ft At 2ЭД ft Density, gm/cc $t*f*0t* Method Dry
test (kotethre to TNT): Hazard Class (Quantity-Distance) Class 9
Ain Compatibility Group Grotrp I
Peak Pressure Impulse Energy Exudation Exudes st 65°C
Air, Confinod:
Impulse
Under Water:
Peak Pressure
Impulse
Energy
Underground:
Peak Pressure
Impulse
Energy
:142
Te.rytol, 75/2$
АМСР 706-177
CeeaperiHea: at Molecular Weight: 270
Tetryl 1NT 75 25 Oxygea Balance: CO, % CO % -5* -12
Density: gm/cc Cast 1-59
Melting Point: °C 68
C/H Rotio Freezing Point: *C
bapeet Sensitivity, 2 Kg Wt: Bureau of Mines Apparatus, cm Sample Wt 20 mg Picatinny Arsenol Apparatus, in. Sample Wt, mg Boiling Point: °C
10 17 Refractive Index, nJ, n.° nJ,
FricHoa Pendulum Teet: Steel Shoe Fiber Shoe Crackb Unaffected Vocmtm Stability Toot: cc/40 Hrs, ot 90°C 100°C 120 °C 135°C I5O’C 3-0 11+
Rifle Bullet Impact Teet: Triols % Explosions 0 Portiols 30
dur.sd 0 Unof /acted 70 200 Gram Bomb Saad Teet Sand, nm 53-7
lx, iesiea Teeeperetare: “C : (- ends, 0 1 (no cop used) 1 5 Ignites 310 10 15 20 Sensitivity to Initiatiea: Minimum Detonating Chorge, gm Mercury Fulminate Lead Azide ♦Alternative initiating charges. 0.23» 0.19*
BellistK Mortar, % TNT: (a) 122
Truu.l Test, % TNT:
7S'C IwtevaaHeaal Heat Teet: % Lou in 48 Hrs
Plate Deaf Teet: Method (b) 3 В
100-C Heat Teet; % Loss, 1st 48 Hrs % Loss, 2nd 48 Hrs Explosion m 100 Hrs Condition Confined Density. gm/cc Brisance, % TNT Cast No l.tt 11" Cast Yer 1.62 lli*
Fhmmebility ledei: Will not continue to burn Detvnottea Bate: Confinement Condition Charge Diameter, in Density, gm/cc Rate, meten/second None Cas’. 1.0
Hygroscopicity: % 0.03
Volatility: 1.60 7385
АМСР 706-177
Tetrytol, 75/25
Prigssutitiia Teet: Shaped Charge Iffectbrenoes, TNT = 100:
90 емп HI. M71 Ptejectila, Let WC-91: Density, gm/cc 1.39 Chorge Wt, <b 2.101 Gloss Cones Steel Cones (d) Hole Volume 127 Hole Depth 120
Total Ne. ef Frepmeets: For TNT 703 For Subject HE 657 Э tech HI. M42A1 Projectile, let KC-S: Density, gm/cc 1.60 Chorge Wt, lb O.8U5 Calen Light yellow to buff
Principal Ueee: Bursters, demolition blocks
Tetel Na. ef Fragaeeats: For TNT 511* For Subject HE 591 ef Leedlwj s Oast
Leading tteaeHy: gm/cc 1.59
FregaMat Velocity: ft/sec At 9 ft At 25ty ft Density, gm/cc
St*f*0te Method Dry
Blaet (Motive U TNT): Ain Peek Pressure Impulse Energy Hoxord Class (Quontity-Distonca) Compatibility Group Exudation Class 9 r Group I Exudes at 65°C
Alt, Confined: Impulse Under Water: Peek Pressure Eutectic Temperature, °C: 67-5
gr Tetryl''lOO gm TNT 67.5°C Booster Sensitivity Test: 51-82 (c)
Impulse Energy UedergrewH: Peak Pressure Impulse Energy Condition Tetryl, gm Wax, in. for 50$ Detonation Density, gm/cc Cast 100 1.65 1.66
344
Tetrytol, 7О/ЗО
AMCP 706-177
% Tetryl 70 TNT 30 C/H Rotio Melecelar Weight: 266
Oxygea Beleace; CO- % -55 CO % -13
Deasity: gm/cc Cast 1.60
Melting Point: *C 68
Fieeslng Point: *C
Inject Sensitivity, 2 Kg Wt: Bureau of Mines Apparatus, cm 28 Sample Wt 20 mg Picotinny Arsenal Apparatus, in. 11 Sample Wt, mg 18 oiling Point: *C
Hefrective Index, nJ Пи nj>
Frictiea Peadehim Teet; Steel Shoe Unaffected Fiber Shoe Unaffected Veceern Stability Test: cc/40 Hrs, ot 90‘C 100’C 3-2 I2O’C 11+ 135‘C 150-C
Hifle leilet Ir :pect Test: Triols % Exp'osions 0 Partials 55 Burned 0 Unaffected 1*5
200 Great Bomb Saad Teet: Sand, gm 53" 2
Ixplesiea Temperature: 'C Seconds, 0.1 (no cop used) 1*16 1 387 5 Ignites 320 10 302 15 289 20 275 Ssasitivity to laiHetiea: Minimum Detonating Charge, gm Mercury Fulminate 0.23* Lead Azide 0.22* •AlteniSl'lve initiating charge*.
Ballistic Matter, % TNT: (a) 120
Tranxl Test, % TNT:
7S*C InteraaHeaal Meet Test: % Loss in 48 Hrs
Plate Dent Test: (b) Method В Condition Cast Confined Yea Density, gm/cc 1.60 Brisance, % TNT 117
100*C Heat Test: % Loss, 1st 48 Hrs 0.1 % Loss, 2nd 48 Hrs 0.1 Explosion in 100 Hrs None
DateaaHaa Rate: Confinement None Condition Coat Charge DinrnetSr, in. 1.0 Density, gm/cc 1.60 Rote, meters/second 73'*O
FlammebUity Index: Will not continue to burn
Hygroscopicity: % 0.02
Volatility:
:цг>
АМСР 706-177
Tetrytol, 70/30
Fragmentation Teet: М « HI. М71 Projectile, Let WC-91: Density, gm/cc 1.60 Charge Wt, lb 2.090 Total No. of Fragment»; For TNT 703 For Subject HE 81*0 3 inch HI. M43A1 Projectile, Lof KC-5: Density, gm/cc l.oO Chorge Wt, lb 0.81*2 Tetel Ne. ef Fragments: For TNT 511* For Subject HE 5З5 Shaped Charge Iffectfvenom, TNT = 100: Glass Cones Steel Cones Hole Volume Hole Depth
Color: Jght yellow to tuff
Principal Uses: Bursters, demolition blocks
Method of Leading: Cast
Leading Density: gm/cc 1.60
Fragment Velocity: ft/sec At 9 ft At 25>4 ft Density, gm/cc
Storage: Method Dry Hazard Class (Quantity-Distance) Class 9 Compatibility Group Croup I Exudation Exudes at 65°C
lest (Relative to TNT): Air: Peak Pressure Impulse Energy Air, Confined: Impulse Under Water: Peak Pressure Impulse Energy Underground: Peak Pressure Impulse Energy
;м<>
AMCP 706-177
Tetrytol, 65/35
% Tetryl 65 TNT 35 C/H Rotio Molecular Weight: 264
Oxygea Balance: CO, % -56 CO % -I1*
Density: gm/cc 1.60
Melting Point: °C 68
Freezing Point: °C
Impact Sonsifivity, 2 Kg Wt: Bureou of Mines Apparatus, cm 23 Sample Wt 20 mg Picatinny Arsenoi Apparatus, in. 11 Sample Wt, mg 17 Boiling Point: °C
Refractive Index, n, ni n°
Frictieo Penduhmi Test: Steel Shoe Cracks Fiber Shoe Unaffected Vacuum Stability Teet: cc/40 Hrs, ot 90’C 100"C 2,8 120°C 11+ I35°C 150°C
Rifle Bullet Inspect Teet: Trials % Explosions 0 Partials 10 Burned 0 Unaffected 90
200 Grom Bomb Sand Teet: Sand, gm 52.6
Explosion Tomporetare: °C Seconds, 0.1 (no cap used) 1 5 Ignites 325 10 15 20 Sensitivity to Initiation: Minimum Detonatir,g Chorge, gm Mercury Fulminate 0-23* Lead Azide 0.23* *Alt^i*t!K!ive initiating chargee.
Ballistic Mortar, % TNT:
Trend Test, % TNT:
7S‘C Intbrnetioaol Meet Teet: % Loss in 48 Hrs
Piste Dent Test-. Method Condition Confined Density, gm/cc Brisance, % TNT
100-C fleet Teet: % Loss, 1st 48 Hrs % Loss, 2nd 48 Hrs Explosion m 100 Hrs
Detanotiea Rato: Confinement Kone Condition Cast Chorge Diometer, in. 1.0 Density gm/cc j. 60 Rote, meters/second 7310
FlMmebiHty ledex: Will not continue to turn
Hygroscopicity: % 0.02
Volefflity:
347
АМСР 706-177
Tetrytol, 65/35
М nun HI, М71 Projectile, Lot WC-91: Density, gm/cc 1.61 Charge Wt, lb 2-010 Tetel No. ef Fregmenti: ForTNT T03 For Subject HE 856 3 Inch HI. M42A1 PtojocHh, Let KC-5: Density, gm/cc 1.60 Chorge Wt, Ib 0.845 Tetel No. ef FtogmeMe: ForTNT 51*» For Subject HE 585 Shaped Chorge IffectiveaoM, TNT = 100: (d) (e) Glass Cones Steel Cones Hole Volume 133 126 Hole Depth 120 119
Light yellow to buff
Friecipel Uses: Bursters, demolltlor blocks
MafrhaJ af LmJIm* П»-*
Loading Density: gm/cc 1.60
Fragment Velocity: ft/sec At 9 ft At 25% ft Density, gm/cc
Storegs: Method Dry Hazard Class (Quantity-Distance) Cisse 9 Compatibility Group Group 1 Exudation Exudes et 65°C
Blest (Motive to TNT): Ain Peak Pressure Impulse Energy Air, Confined: Impulse Under Weton Peak Pressure Impulse Energy Undetgraond: Peak Pressure Impulse Energy
348
АМСР 706-177
Tetrytol, 80/20, 75/25, 70/30, €5/33
Compatibility with Metale:
Dry: Copper, brass, aluminum, magnesium, stainless ateel, mild steel, mild steel coated
with add proof black paint and mild ateel plated vith copper, cadmium, zinc or nickel are
unaffected. Magneslum-aluminum alloy la slightly affected.
Wet; Stainless steel and mild steel coated vith acid-proof black paint are unaffected.
Copper, brass, aluminum, magnesium, magneslum-aluminum alloy, mild steel and mild steel plated
vith cadmium, copper, zinc or nickel are slightly affected.
Preparation:
Tetrytols a-e manufactured by heating UiT in a melting kettle, equipped with a stirrer,
until all the '£NT Is melted. The necessary amount oftetryl is added and heating and stirring
are continued. The temperature is allowed to drop from 100°C until the mixture is of maximum
viscosity suitable for pouring. Part of the tetryl dissolves iu THT forming a eutectic mix-
ture which contains 55 percent tetryl. This mixture freezes at 67.5°C.
Origin;
Tetrytols were developed during World War II. Hie 70/30 tetryl/lNT casteble mixture is
the most important in military applications.
References:
(s) L. C. Smith ar- E. G. Jester, Physical Testing of Explosives, Part III, Miscellaneous
Sensitivity Tests, Performance Tests, OSRD Report No. 5*716, 27 December 1915-
(b) D. P. MacDougall, Methods of Physical Testing, OSRD Report No. 803, 11 August 191-2.
(c) L. C- Smith and S. R. Walton, A Consideration of RDX/wax Mixtures as a Substitute for
Tetryl in Boosters, N0L Memo 10,303, 15 June 1969.
(d) Eastern laboratory, du Pont, Investigation of Cavity Effect, Sec III, Variation of
Cavity Effect with Explosive Cocposltion, NDRC Contract w67^-ORD-5723.
(e) Eastern Laboratory, du Pont, Investigation of Cavity Effect, Final Report, Eastern
Lab, du Pont, 18 September 1913, NDRC Contract W-672-0RD->723.
(f) Also see the following Picatlnny Arterial Technical Reports on Tetrytol
0 1 2 2 2 6 7 8 9
1260 1291 1372 1193 1285 1376 1177 1158 1379
1360 1311 1213 1325 1136 1737 1388
1120 1151 13б3 1885 1166 1797 1838
1500 1651 1193 2125 1506
1530 1951
I^See footnote I, page 10.
349
AMCP 706-177
TNT (Trinitrotoluene)
Сам peeitioa! Molecular Weight: (CyH^N^Og) 227
C 37-0 H 2.2 0 N — NO Oxygea Belonce; CO: % CO % -71» -25
2 N 13.5 Density: gm/cc Crystal 1.65
0 U2-3 Melting Point: °C 91
C/H Ratio о.5**9 X02 Freexlng Point; °C
Impact Sensitivity, 2 Kg Wt: Bureau of Mines Apparatus, 'ample Wt 20 mg Picatinny Arsenal Apparatus Sample Wt, mg 95-100 1U-15 17 Boiling Point: °C
, in. Refractive Index, nJ, a 8 T 1-51*30 1.671*2 1.717
Frictien Pendulum Teet: Steel Shoe F>ber Shoe Unaffected Unaffected Vacuum Stability Teet: cc/40 Hrs, ot 90 “C IOO°C I2O°C I35°C I5O°C
Rifle Ballet Impact Teet: Triols % Explosions 1* Partials 0 0.10 0.23 0.1*1* 0.65
Burned Unaffected СГч О 200 Gram Bomb Send Teet: Sand, gm 1*8.0 ^'5
Explosion Temperature: Seconds, 0.1 (no cop used) 1 5 Decomposes 10 15 20 •с 570 520 £75 1*65 Sensitivity to Initiation: Minimum Detonating Charge, gm Mercury Fulminate Lead Azide ♦Alternative initiating charges. 0.21** 0.27*
Ballistic Mortar, % TNT: Std 100
Trauzl Tam. « TNT: Std«?.00
7S*C IntametioMl Heat Teet: % Loss in 48 Hrs
0.01 Plate Beat Test: (a) Method A A в
1WC Heat Teet: % Loss, 1 st 48 Hrs % Loss, 2nd 48 Hrs Explosion in 100 Hrs 0.2 0.2 None Condition Cast Pi seed Confined Yes Yes Density, gm/cc 1.61 1.50 Brisance, % TNT 100 100 Cast No 1.61 100
Detoaetioa Rate: Confinement Unconfined Condition Pressed Chorge Diometer, in. 1.0 Density, gm/cc 1. 56 Rote, meters/second 6825
FlemmeMHty Index: (Ъ) 100 Ur.confi ne< Cast 1.0
Hygroscopicity: % 30°C, 90$ RH 0.03
Veietility: 30°C Nil 1.56 661*0
изо
TNT (Trinitrotoluene)
АМСР 706-177
Booster Seemtivity Test: (c) Condition Pressed Cast Tetryl, gm 100 100 Wax, in. for 50% Detonation 1*68 0.82 Wox. gm Density, g.n/cc 1-55 1.60 Decompesitioa Cquetior.: 12 2 Oxygen, otoms/sec 10 10х (Z/sec) Heat, kilocalorie/mole 3b. b Ьз.Ь (AH, kcol/mol) Temperature Range, °C 275-ЗЮ 23b-277 Phase Liquid Liquid
Heat of: (d) Combustion, cal/gm 3^20 Explosion, cal/gm 1080 Gas Voiume, cc/gm 730 Formation, col/gm 78.5 Fusion, col/gm а 22. 3b Temperature, “c 79 Armor Plale Impact Test: M mm Mortar Projectile: ( J ) 50% Inert, Velocity, ft/sec >1100 Aluminum Fineness 500-k Generel Purpose Bombs: ( J ) Plate Thickness, inches Trials $ Inert 1 0 1*4 0 11,2 b 100 b 50
Specie Heel: col/gm/'C C o 0.309 20 0.328 50 0.353 80 0.37b
Burning Rote: cm/sec
Bomb Drop Test: T7, 2090-lb Somi-Armer-Piercing Bomb vs Concrete: Max Safe Drop, ft 5000-6000 500-lb General Purpose Bomb vs Concrete: No Seal Seal Height, ft 1,000 1-51000 Triol» 26 20 Unoffected 21 20 Low Order 2 0 High Order 0 0 ItKrO-lb General Purpose Bomb vs Concrete: No Seal Seal Height, ft 5,000 5,000 Trials 21 26 Unaffected 16 22 • ow 0>Ler 0 0 High Order 3 b
Thermal Conductivity: col/sec/cm/’C See next page. Coefficient of txpewsiea: (b) Lineor, %/'C -b0° to 6C°C 5-b x 10*5 (b) -b0° to 60°C 6.7 x 10’' Volume, %/'C 27° to SO°C 16 x 10’5 (b) 16° to 70°C 26.3 x 10*5 (n)
Hordnoss, Mobs'Scole: (e) l.b
You»'" Modulus: (b) E’, uynes/cm2 5-^5 x 10 E, Ib/inch3 0.79 x 106 Density, gm/cc 161
Compressive Strength: Ib/inch3 1330O-1L00O Density, gm/cc 1.62
Vepor Pressure: ( r . ‘C mm Mercury 80 0.042 65 0.053 90 0.067 95 0.065 100 0.106
:J51
АМСР 706-177
TNT (Trinitrotoluene)
Fie,msiHetias Test: I Skeftd Cberya IHoctbreaess. TNT = 100:
*0 MM HI. M71 Projectile, Let WC-01: G»c_? Cones Steel Cones
pensity, gm/cc 1.60 Hi '• Volume 10l 100
tS«r-e Wt, lb 2.10A Hole Depth ИХ» 100
Tetel Ne. of Ftogmonta: For TNT Гог Subject HE 703 Color: Light yellow
763
Priodpot User. C-P bombs, ?E projectile#,
3 lech H£, M42A1 Ptojoctile, Let KC-S: 1.60 demolition chergis, depth charges, grenades, propellent compositions
Density; gm/cc
Charge Wt, Ib o.au
Tetel Nje, ef Fregetttte: Method ef Leading: 1- Cast
ForTNT For Subject HE 5b- jlk 2. Pressed
Leodiag Density: gm/cc See belov
Freqmeet Vetodty: ft/sec At 9 ft W 2600
Ap2S%ft 2У0
•"density, gm/cc 1 ' 1.58 Method Dry
Vtast (Mothe to TNTh Hazard Class i Quantity-Distance) Cisse 9
Air.- Compatibility Group Group I
Peak Pressure 100
Impulse 100 Exudation None st 65°C
Energy 100
Air, Ceefieec. Impulse Loading Density; gm/cc
100 1. Cost 1.58-1.59 2. Pressed psi x 10
Under Water: 3 5 10 15 20 30 50
Peak Pressure 100 1.35 l.W 1.1*5 1-52 1.55 1-59 1.6
Impulse 100 Thermal Cor.ductlvl ty;
Energy 100 csl/sec/cm/°C
UMorgretmd: Peak Pressure 1U0 Density 1.19 gm/cc (&} 5-28 x 10 !* 1.51 gm/cc (g) 7.12 x 10’7 1.54 gm/cc (b) 5.6 x 10*”
Impure IOC 1.67 gm/cc (g) 12.21 x IO*4
Energy lOv Viscosit;., polees;
Tenu . 85°C 0.139 0.095
Bulk 4cd.; .8 et ?oom Temperst re (25o-30°C): Dynes,-’em'’ x 10" *-u Pensl -y, £m/;n (n) 2.92
ИИ (Prinitrotoluene)
AMCP ТвМП
Effect of Sispemture зс Mte of Detonation: (1)
TSepernture of Charge, °C *5b 21 60 Sours at TM^araturc 16 16 24 Density, gp/cc 1-63 1.62 1.6» 60 72 1.64
Fite, mtere/second 6700 6820 6770 6510 Эимй*1У1ч> t; Electrostatic Diacharge, Joules; through 100 Utah: Ubeonflnsl 0.06 Confined 4Л Pepact Bssaitivltr versus TOay gature: Picatliry Arsenal АтрртЛов, 2 eg wt, inches: °C inchoe Ao 17 Boca U 80 7 90 3 105-120 2 (5 expl in 20 trials) ZOeet Sensitivity versus Loading Jbthod; Large Tnpact Apparatus, Inches: -
Pressed at 1.60 gp/cc 70 Oast at i-60 gs/c-c j6 Bifl» Pallet frjact aene'-tivltr versus Bey&rrtare, Oonflwent; Boost itandera Iron Bost: Hospers turu 105° to 110°C
So Air фме Male 10 explosions 1 vary low order Air Эросе Trials 10 Explosions 0 tin cr Oartboard Bcriba; With or Without Air Space male 10 r losicns 0 10 J 10 0 10 0
353
АМСР 706-177
MIT fTrlnltrotolueae)
BmltMoa versus МИ Inltlt' Tww*t>mi
ИТ ftwoireture, Initial Kaploelon ftiiarature, °C
Roca 470 (Deocnpoaes) t
105°-100 C HSO (DecoRXJses)
Explosion Twerature versus Confto—ent, °C:
Uncoefined Decceposes 470
3ealed in glass capillary Explodes 320-335
Viscoaltar at 8q.5°C:
Viscosity, X, cp log X » '*-046 S * 1.26
8 £ solid in slurry
Particle alи effect, snail
Density, ga/cc;
°C State gn/cc
2? to 70 Flaked 1.65
80 Flibd 1.64
82 Liftnld 1.48
&Г Liquid 1.48
95 Liquid 1.47
Solubility of ТЮ, at/100 яа (Я, tot (f) c
Hater Acatona jenreae Iblueue
c- i 2 4 °C •an* 4
0 0.0100 0 57 0 13 0 26
20 0*0130 20 109 30 67 20 55
40 0.0285 40 228 4o 180 40 130
60 0.0675 6o 600 6c 478 60 367
so >2000 80 >1700
carbon toichloro-
tetrachloride Ether Chloroform ethylene
!S 1 °C aee* 1 2£ 1 «4 4
0 0.Я0 0 1.73 0 6 3-5
0 0.5 5 20 3.29 20 19 55 60
40 1-75 40 66
6o 6.90 60 302
70 17- ’4
75 24.35
354
АМСР 706-177
ИТ (Trinitrotoluene)
ftnrtdina Methyl acetate di chi arid»
2c < ft J J
20 Iho 20 73 20 3h
hO 9® hO 135 hO 123
GO 6ho 50 280 60 hGO
TO 1250
• 5
*9
96
tetrechloro- leorrapyl
ethene Aniline alcohol Ethenol
i os. i 2c i i 1
20 IB Ю 6.1 20 0.76 0 0.62
hO 5C 30 U.5 hO 1.96 20 1.25
50 M0 50 29 50 2.95 hO 2.8$
70 Th 60 8.h
80 130 70 15
Xsobutyl alcohol Carton disulfide Chlordbenaene
°c I 2c 1 2£ i
0 0.20 0 O.lh 20 35
20 0.61 20 O.hh 3, 51
hO l.hl hO l.h hO 79
50 2.55 50 116
Prer^rutioe.
(AC 7258, 7259, 7260 - litretian Kinetics)
(CheiidetCT at Powder and Exploeive». Davie)
,02
In elder proeeeses trinitrotoluene (ПТ) vas «lowly «uid Laboriouily nitrated ir. throe
stages using successively stronger add». Today, however, a «ingle stage nitration ie pos-
sible, in a short tine (leas than one hour) producing TUT at a coat of a little leea than
6//I0. Tn England, a tvs stage continuous prvсеве vas developed during World Wbr II; in the
firat counter cuirent sta^e, to' as van nitrated to the none stage nononitrоtoluene (МВТ);
in tiie second rtsga, also count current, MKB vas nitrated to HIT.
355
AMCP 706-177 TUT (Trilltrotoluene^
It «i the British work, on the kinetics of nitration of toluene to TUT, that first pointed
out the basic importance to nitration processes of the nltroxyl ion (N0g+), on the one hand,
and the role of the blsulfbte ion (НЭО4-) and unionised sulfuric acid on the other. These
concepts were successful 1^ explaining the maximin in nitration rate occurring et a sulfuric
acid content of 92#. This work, for instance, leads to the following equation for the rate of
forestion of ТЯТ from DHT:
l.CSF) - К (ЯОо*) [к* (HSOfc-) + К" (HpSOjtH (ШТ)
Tnree Stage Process; Toluene (100 gm) is nlorated to the mono derivative by slowly adding
a mixture of 2^4 gm sulfuric acid (sp gr 1.84) and 14? gm nitric acid (sp gr 1.42) to it at
30°-4o°C, with good agitation. Acid addition requires 1-1.5 hour, and stirring et 30°-40°C
is continued 30 minutes longer. The mixture is cooled and the lover layer of spent ecid
drawn off*
Half the crude none is dissolved in 109 gm sulfuric add (sp gr 1.84) with cooling, the
solution heated to 50°C and a mixture of 54.5 gm nitric add (sp gr I.50) and 54.5 gm sul-
furic acid (bp gr 1.84) added, under agitation, at such a rate that the temperature is main-
tained between 90° and 100°C. Acid addition requires 1 hour, and stirring at 90°-100°C is
coati ued 2 acre hours.
While the dlnltratlcn mixture is still at 90°C, 145 gm fuming sulfuric acid (oleum con-
taining 15f free S0^) is added slowly. A mixed add of 92*$ gm each nitric acid (sp gr 1.50)
and 15t oleum is sltvly added, under good agitation at 100°-11 ' over 1^-2 hours. The mix-
ture is stirred at 100 -115°C for 2 mor» hours, cooled, filtereo, and the THT cake broken up
and washed with water. The ТЯТ is washed 3-4 times with hot water (85°-95°C) with good agi-
tation. The predict can be purified either by recrystallization from alcohol or by washing
it with 5 times its weight of 5* sodium bisulfite solution at 90°C for J hour with vigorous
stirring, washing with hot water until the washings are colorless, end cooling slowly with
stirring to granulate the product.
Origin:
TRT was first prepared in 1863 by Wllbrand (Ann 1Д8, 1(8), later by fcciIsteln and KUhlberg
(Bar 2/ 202 (1870) end also Tlanann (Ber 217 418?6), eac'i using different methods of start-
ing materials. It was nearly 30 years later when Haussrmem undertook its manufacture on an
industrial scale (Z angew pen, 1891, p. 508; J Chem Ind, 1891, p. 1028). After 1901 TBT
began to be used extensively aa a military explosive anf Germany became the flrat nation to
adopt it aa a standard shell filler (1902-1904). Curing World War I ell the mejcr powers of
the world were using ТЯТ, with the quantity used limited only by the available supply of
toluene. Prior to World War II the development of synthetic toluene from petroleum mode
available In the United States, an almost unlimited su;yly of this raw material. Because of
the general suitability of 1ST for melt-loading and its extensive use in binary and ternary
explosive mixtures, ТЙТ is considered the mo.jt inportant military explosive known today.
Destruction by Chemical Decongiosltlon:
TUT ic decomposed by sddirg it slowly, while stirring, to 30 times its weight of s solution
prepared by dissolving 1 part of sodium sulfide (HegS^HgO) in 6 parts of water.
References;7$
(a) D. P. MacDougall, Methods of Physical Testing, OSRD Report No. 803, 11 August 1942.
7^See footnote 1, page 10.
356
АМСР 706-177
HIT (Trinitrotoluene)
(Ъ) Philip С. Keenan and Dorothy Pipe a, №ble of Mi 11 try High Explosives, SJcond Revi-
sion, MAVORD Report No. 87-46, 26 July 1946.
(c) L. c. S*ith and 8. a. Wlton, A Consideration of RDl/Wax Mixtures as a Substitute for
Tetryl In Boosters, HOL Memo 1O,303j 15 June 1949»
(d) L. C. Stith *nd E. H. Eyater, Physical Testing of Ex.'loeivea, Partin, Miscellaneous
SMurftlvity Taste, Pt,^fo"nance lasts, OSRD Report No.5746, 27 December 1945.
(e) Report AC-2587.
(f) International Critical Tables and various other sources in the open literature.
(g) K. Hutchinson, The Thermal Sensitiveness of Explosives. The Therm] Conductivity of
Exploeive Materials, AC-2&0I, First Report, August 1942.
(h) A. J. B. Robertson, Trane Farad Society, 44,- 977 (1948).
(1) M. A. Cook end M. T. Abegg,"Isotharml Decoepoaition of Explosives,й University of
Utah, Ind Eng Oo (June 1956), pp. 1090-1095-
(J) Comittee of Div 2 and 8, MRC, Report on HKL and Tritonal, OSRD No. 5406, 31 July
19*»5.
(к) H. W. Brake, yragswnt Velocity and Panel Penetration of Several Explosives in 31ыи-
lated Shells, OSRD Report Bo. 5622, 2 January 1946-
(1) W. I. McGarry and T. W. Stevens, Detonatlgn Rates of the More lyrtant Military
Explosives at Several Different Taaperaturea, BATS Ko. 2333, November 1956.
() W. 8. eraser, ^ilk Ccapresslblllty Data on Several High Explosives, WORD Report Ko.
4380, 15 September 19?6'
(n) Kantrov, Journal of Chemical Indust у (Russia) 6, 1929, pp. 1686-1688.
(0) Also see the following picatinny Arsenal Technical Reports on HT:
0 1 2 2 4 r 6 7 8 2
10 291 132 43 364 €5 86 41 118 99
30 551 582 83 694 195 266 87 288 249
24o 731 782 133 874 425 556 507 638 269
350 861 892 273 904 555 666 527 738 319
630 891 972 513 1094 695 956 597 768 389
760 901 1072 643 1104 735 986 707 838 499
810 971 1182 673 1124 805 1046 807 1088 7Q9
1120 1041 U.92 743 1224 975 1146 817 1098 739
u4o 1121 1272 853 1284 1145 1276 337 1128 779
1170 1311 1292 863 1294 1155 J 376 1107 1143 799
1260 1391 1342 Ю63 1304 1225 1446 1147 1158 889
1270 1431 1352 1123 1314 1285 1466 1217 1188 929
1360 1451 1372 1133 1344 1305 U76 1247 1198 939
14co 1491 1402 1193 1414 1Ы5 1556 1307 1228 1099
1460 1651 1452 1243 1444 1395 1636 1417 1258 1109
1500 1821 1472 1323 1454 1425 1756 1427 1308 1129
357
АМСР 706-177
ТИТ ( Tried U-otolue;^)
0 2 2 U 2 6 I 8 9
1530 11*92 1373 152<* 11*35 1956 11*37 1318 1139
15ЙО 1562 11*93 151*1* 1М*5 2216 11*57 133В 1179
1550 1582 1553 1561* 11*95 11*97 1388 1199
1730 1712 1633 16С’+ 1515 1537 11*18 1259
2010 2100 2160 1862 1693 1823 2063 2163 1671* 1751* 1921* 2061* 2211* 1535 1585 1.605 1635 1665 1865 1965 1715 1885 2125 2175 151*7 1557 1577 1597 1677 1737 1797 1827 181*7 2007 211*7 2167 11*28 1578 1618 1688 1726 1828 183В 1858 2008 2138 2168 1289 1339 1369 1379 11*19 11*29 1U69 11*89 1529 151*9 1629 1689 1709
1729
I7U9
1809
1819
1879
19*»9
2159
2179
358
АМСР 706-177
Тогрех
СамрмШм. RDX 12 ПГТ **О АХшйпив 16 C/H Rotto
Oxygen Beteece: CO, % .55 CO % -26
Density: gm/cc Cast 1.76-1.81
^M^MBWBH r^^tei wr
Лйяф* °C
Impact SoasitMty. 2 Kg Wlh Buraou of Minas Apparatus, cm k2 Sampte Wt 20 mg Picotinny Arsenal Apparatus, in. 9 Sample Wt, mg 15 BeMtag Point: °C
e ’o ?o ft
• Ж—ЧР— vFvCnWBB F^R^^WB^MBB В VvFe Steel Shoe Fiber Shoe *F л^Л1 ▼V7WBBB «VWVBIBFy 9 vw. cc/40 Hrs. at 90’C 100’C 120’C 1.0 135'C 150’C
Rifle Ballot Impact Tost: Trials % Explosions 20 Partials 80 Burned 0 Unaffected 0
200 Grom Bomb Sand Yost: Sand, gm 59.5
Explcairn Temperature: °C Seconds, 0.1 (no cap used) 1 5 Decoapoeee 2б0 10 x v o.o a»_. e^s»s - же — BHBb^^mWOWo Minimum Detonating Chorge, gm Mercury Fulminate 0.18 Leod Az*de Tetry.
ЫШс МмКг, % TNT: (в) 13g
Trani Test, % TNT: (b) 161
7S*C Intetnattenal Hoot Test: % Loss in 48 Hrs
Plate Dent Test: (c) Method Я Condition Coot Confined Ko Density, gm/cc 1.83 Briscnce, % TNT 120
100’C Heat Test: % Lou, 1st 48 Hrs 0.00 % Loss, 2nd 48 Hrs 0.10 Explosion in lOO Hrs None
Detoaetum Kate: (d) Confinement None Condition Cost Chorge Diameter, in. 1.0 Density, gm/cc 1.81 Rote, meterj/second 7**95
FtemamMitv Index: 196
Hygroscopicity: % 30°C, 90$ RH 0.00
▼ ^MeFBBBFy •
:й9
AMCP 706-177
Torpex
Bssobbf SeoeiHvJly Condition (c) Pressed Cssc
Tetryl, gm 10 5
Wax, in. for 50% Detonation
Wax, gm 2 0
Density, gm/cc 1.6L 1.81
Meet oft Combustion, cal/gm (•) зт'ю
Explosion, cal/gm Gas Volume, cc/gm Formation, cal/gm Fusion, ca!/gm 1800
Specific Meet: cal/gm/*C At -5°C (b) 0.22
Density, gm/cc 1.82
At 15°C 0.2U
Busalag Rate: cm/sec
1 ММИМв* WVHVM*VtvWy« col/sec/cm/'C Density, ga/cc (b) 9-7 x 10'1* 1.82
Oxygen, otems/sec (Z/sec) Hoot, kJlocoloHe/moie (AH, kcol/mel) Temperature Ronge, *C Phase
Алеет Plate Inspect Test:
M mas Mostar PseieaMe: (•)
50% Inert, Velocity, ft/sec 185
Aluminum Fineness
Plote Thickness, inches
1
1’4
14
1%
T7,2000-lb Sewti-Arsaer-Pierctaf iamb vs Cesser ' s:
Lineor, %/*C -73 <» 75°C M x 10‘5 (b)
Volume, %/’C
KarAk i* Mdn Scala:
E', dynes/cm*
E, Ib/inch3
Density, gm/cc
(b) 10
9-53 x 10*~
I.38 x 10°
Max Soft Drop, ft
5004b вамга! Puspeso Bomb vs Cesscrete:
Height, ft
Triols
Unoffected
Low Order
High Order
100Q-* (aeaerel Purpose Is mb vs < esscsata:
Density, gm/cc
2100-2300
C
mm Mercury
Height, ft
Triols
Unoffected
Low Order
High Order
:йо
Torpex
AMCP 706-177
Fregeeeafotien Teet: 00 мм HI. M71 Projectile, La* WC-tl: Density, gm/cc ChorgeWt, Ib 1.75 2.316 Shaped Charge IffecHvoMos, TNT = 100: У/У>-?ДЗ-? Glass Corm Steel Corm Hole Volume 150 lb$ Hole Depth 127 131
Total Nv. ef Fregnreats: For TNT For Subject HE 3 Inch HI. M42A1 Projectile, Let KC-5: Density, gm/cc ChorgeWt, Ib 703 891 1.79 0.9b0 Cohn Grey
Principal Uses: Depth chargee, bombe
Tetd No. ef FragawetK For TNT For Subject HE 51b 647 Method of Lending: Cost
Lending Donahy: gm/cc 1.76-1.81
Ргмумм» Velocity: ft/soe At 9 ft At 23Ц ft Density, gm/cc
2960 2660 Method Dry
Meet (BeiaHvw to TNT): (e) t.'uord Cioss 'Quantity-Distance) C14SS 9
Ain Peek Pressure Impulse Energy 122 125 lb6 Compntlb lity Group exudation Group I
Air, СееЯмб: Impuhe Effect of Temperature on
116 ХвхЖ'.-t SeneltiYity: Тещ. FA Ibpect Test
Under Weter: Peek Pressure Impuhe Energy 116 127 153 vb 2 Kg Wt, locbee 25 15 32 7 10b 8
Viecoeity, poises:
Peak Pressure Impuhe Energy Temp, 83°C 95°C b-5 2.3
361
АМСР 706-177
Torpex
Preparation:
Torpex la manufactured by heating TNT to approrlnat* ly 100°C in a steam-jacketed kettle
equipped with a stirrer. Hiter wet RDX Is added alowly to the molten THT, while mixing end
heating, until all the water is evaporated. Aluminum is added end the mixture ie stirred
until unifora. The mixture ie cooled, vith continued к birring, until it is suitable for
pouring* Torpex can also be aad: by adding the calculated amount of THT to Composition В
to maintain the desired proportion of RDX/TNT, heating and stirriM, and adding 18 percent
of aluminum to complete the mixture.
Origin;
Tjrpex, a castable high explosive, was developed in England during World War II for use
as a filler in warheads, mines and depth bombs. Several varletlone in the composition of
torpex h'.ve been evaluated but the following are those used in ' rvlce munitions:
Torpex 2 Torpex 2
unwaxed waxed
(•) (b) (c)
RDX, % 42 41.6 41.4
TNT, $ 40 39.7 39.5
Aluminum, $ 18 18.0 17.9
Wax, Ц 0.7 0.7
Calcium chloride, <f> 0.5
(a) Made from Composition B-2 or 6o/4o cyclotol.
(b) Made by the addition of aluminum to Composition B.
(c) Made by the addition of calcium chloride to Torpex 2.
Wax has the undesirable effect of (1) tending tc coagulate the aluminum, thus giving a less
homogeneous and more viscous product, (2) lowering the density of the cast explosive from
1.72*1.75 to I.06-I.7O for waxed torpex, and (3) lowering the compressive strength from ffOO
psi to 1970 psi for waxed torper. However, wax is used in service torpex for reasons of
safety, since there is evidence that its presence lowers the sensitivity of the expletive to
iopact as measured by laboratory drop tests and bullet sensitivity tests of small charges
(Bureau of Ord Res Memo Rpt No. 2b, January 19U5).
References:76
(s) Committee of Div 2 and 8, NDRC, Repor. on HBX and Tritonal, OSRD No. 5406, 31 July
19^5.
(b) Philip C. Keenan and Dorothy C. Pipes, Table of Military High Explosives, Second Revi-
sion, WORD Report No. 87-46, 26 July 1946.
(c) D. P. MacDougall, Methods of Physical Testing, OSRD Report No. 803, 11 August 1942.
L. C. Staith and E. H. Eyster, Physical Testing of Explosives, Part III, Miscellaneous
Sensitivity Tests, Performance Teats, OSRD Report No. 57^6, 27 December 19^*5.
76Se« footnote 1, page 10.
362
АМСР 706*177
223Й?.
(d) G. В. Messer ly, The Rate of Detonation of Vari out Explosive Cotnounds, OJRD Report
Bo. 1219, 22 February 195У~
M. D. Hurvlxx, The Rate of Detonation of Various Cospounds and Mixture», OSRD Report
BO. 56U, 15 January 19*Л.——————
(e) ’. ,Г' Tomllnaon, Jr., Bleat Effbcta oi Boab Explosives, PA Tech Div Lecture, 9 April
19*G. - - ---------------------- ---------
(f) Eastern Laboratory du Pont, Investigation of Cavity Effect, Sec III, Variation of
Oevlty Effect with Explosive Coaposition, ВИС Contract Wb72-6M>.'fl2j.
(b) Also see the following Plcatlnny Arsenal Technical Reports on Torpex:
0 1 2 A 2 6 I P
1530 1651 1292 2353 1585 1635 1885 1796 1797 1638
2355
363
АМСР 706-177
1.3i5-Trlamino-2,L,6-THnltrobensene (ТММВ)
Meteexlar Weightt (CgKgHgOg) 258
* „ NH9 С 27-9 . 2 н 2.з VY|“N°£ Oxvgen Bateaeel CO» % -56 CO % -19
Я 32.6 Н2Я -4.J-BH2 Caaoky: gm/cc Crystal I.93
0 37-? К02 MoMag Points *C 333 (b, e) 360 (a)
C/H Ratio 0.302 ммкт •Г* I^WWe \e
Import SamMMty, 2 Kg Wh Bureos of Minas Apporatus, cm Semple Wt 20 r-.g Picatinny Arsenal Apparatus, in. 11 Sample Wt, mg 7 OeMteg Mott *C
jo go jo
Friction PeeMom Tosh Steel Shoe Fiber Shoe Vetoed Stability Tash cc/40 Hr. at 90'C 100’C (a, b) O.36 120*C 135’C I50-C
Aifte Belief Impact Toot: Trials % Explosions Portiols
Burned Unaffected 200 Greet Bomb Seed Toots Sand, gm U2.9
Fxpleoisa Tempemhtre: *C Seconds 0.1 (no cap used! 1 5 10 MMiiMVWwy obowwv^^oi Minimum Detonating Charge, gm Mercury Fulminate ---- Lead Azide 0.30 Tetryl — --
20 FHltatt* Mertar, % TNT:
TtaasITeot,* FNT:
7S*C Intenmtionel Heat Toot: % Loss in 48 Hrs
Plate Dent Toot: Method
11ГС Hoot Toot: % Loss, 1st 48 Hrs 0.00 % Loss, 2nd 48 Hrs 0.00 Explosion in 100 Hrs Bone Condition Confined Density, gm/cc Brisance, % TNT
Detcoetlea Kate: 2- 'inoment None
FtenMneNOfy Index:
Hygscsee^Jelty: % C’torge Diameter, in. 0.5 Density, gm/cc 1.60 Rote, metem/second '7500
Vm^AlMAn..
364
l,3,5*Trtealno-2,k,6-T mltfbbenxene (UMWB)
лхсрж-т
M Mi Ml МП МмМК I* *M>| Омйу,*к/сс ОшреМК,» TMei Mb. of Ffopamatu For TNT ForSubMdHE »1мЬМ «МШ MwMk Ut KC4t Dmty, gm/cc Cheeps Wt,b For TNT For SubHct HE (taped Ctaspe fffisthsseii. TNT = IWt GIom Cones Stool Cones Hob Volume Ч -1- -*- now u^tn
Crtmt Yollow
Nm^I UtaM
Mollwi of teeftopt Preoood
loedtap Ooortyt gm/cc At 50,000 pal 1.80
FofMot VeWte 1t/tK At 9 ft At 25% ft Омйу, pin/cc
Method Iky Horad Ctet (Оиопгйу-ОШопм) CompoHbiUty Group O* >_»> CXUQQnon
MM fthtebe leTNTh Ain АмкРшмо Impute Energy AW, СмЯмй Impute IWoWtta ftggll PpkMMV &ИГ8У Pock Pressure Impute Fnorgy
Detonation Velocity; (a, b. c) Penalty, gn/cc Metera/aec 1.290 5360 1.3**5 5626 1.675 6550 . 1.675 6575 1.082 7035 1.635 7220 Heat of; Explosion, cal/gm 2831
365
АЖСРОДП
l,3,5~Trianitio-2,k,6-Trlnitrcbenxene (йЯВ)
Prewatioa: (а)
Absolute alcohol (200 milliliter») ш saturated vith eaennla and than 'i.5 gw (0.088 wol)
of l,3>5-tribroeo-2,k,6-trinitrobenx«», prepared according tc Hill (НОТОЮ Report Bo. 3709,
2 Nbruaxy 1953), w« added. The flask wi stoppered and alloved to stand et rocw tespexeture
ter a day. addlticnal awwonia was bubbled into the nixture, which was then basted under reflux
for thirty ilnutes, filtered hot, and the insoluble prcduct collected on a Buchir * funnel.
The product wi washed with water, alcohol, and dried. The k.7 gw of notarial recovered Me
recrystalliMd frcw nitrobenssne.
A dieadvantage of the above wetted was that it could not be used for the prepaxwtion of
large quantities of ТАИВ. Since it did not seen fbariblw to develop a net netted of prepara-
tion, an investigatiau was node of the reported aadnetlon reactions (see Origin below). An
attempt sue aade (Ref f) to find a wodlfication which vmld produce high yields of a pure pro-
duct. The process which evolved free this study nay be suenarisad as follows (Ref J): 1,3,5-
trichlorobensene was nitrated "in one step** to l,3,5-wichlcro-2,k,6-trinltrbbensene in 891t
yield. The crude nitration product was aadnated in bensene with swami a gas to ЗУЛЯ®, in
yields of at least 95lt>
Origin:
ТМП wi prepared for the first tine in 1888 by C. L. Jackson and J. F. Wing, who f md
the coupound Insoluble in alcatel, ether, chloroform, benzene, and g’ adal acetic add; and
soluble in nitrobenzene and aniline (Aner Chew Journal W, 282 (1688'). B. Flhraehein and
B. L. Heines prepared TATEB trow benzene tree pentanltroanillne by gradually adding it to 100
aqueous awotrla (J (hew Soc, Pt 2,3tAj (1928)). After bailing, an orange-yellow pgwdar £>*-
lag above JOO°C was obtained. ТМг product corresponded to that described by Jac4»on and wins-
These authors, as wall as Palner (Aaer Chew Journal Ik, 378 (189*.,), attested to reduce ТАЯВ
to texa-awinbtwnsene Either decomposition occurred*ar a hydrochloric» <•’ penta-awinobenxene
was formed. Harsch- « and Holmes succeeded in reducing ПЯВ vith pnvLylt.-lraxine by heating
then together up to 200°C (J Ctew Soc, Pt 1,33k (1929)) (Beil 1^, JOI ы.Д ЖП, 1k?).
References;77
(a) F. lay lor, Jr., Synthesis Of Же High Explosives II, Derivatives of 1,3,5-Trlbromo-
2,k,6-THnltrobenzene, RAvCttD Report Ro. WO5, IMovember 1956.
(b) Ih D. Haavton, Snell Scale Dstcoction velocity Measurewents ftow May 1951 to May 195k,
WORD Report ro. 3731, June 195*b ' =
(с) E. M. Fisher end E. A. Christian, Explosion Effects Dots Sheets, KAVORD Report Ro.
2986, Ik June 1955-
77--------
See footnote I, page 10.
368
Olycol Plnltrete (ДЮМ) Mquld с AMCP 706-177
.ocoikl Mekcaler Weight: (C^Kj^KgOg* 2h0
С 89.9 HgC^ й 5-^ Osy^es BcfaftCV! CO, % .89 CO % -27
Н 11.7 1 K-^gm/cc |$C l.g
н_сх 0 53-0 /0 MolHeg Met: *C
C/H Rotio 0.177 H2C'4‘CHgOBOg rVMSMiJ FMW! 4»
1н*м1 ХмакМу, 1 Kg Wtt Bureau of Minot ^poeetus, cm 100* Somple Wt 20 mg Picatinny Arsenal Apparatus, in. 1*3 Sample Wt, mg IaIIUa lul^o *Л ^wHwe Ke
Refractive Index, n£ 1Л51Ю n£ n£
PgjgllQQ Twtt Stool Shoo Unaffected Fiber Shoe Unaffected Vecanrai StebiBly Teat: cc/40 Hn, at 90’C 100*C 0.1*5 120*C 8 hours 0.8 135’C 150*C
RMe BeKet hugest Teat: Triols % Expiosicns PwrTvQVl
Burned unovTvcrw 2006reai Boeab Send Teet: Sand, gm 1U.7
Seconds, 0.1 (no cop used) 1 5 223 10 *wm*wy * вявиям^^н* Minimum Detonating Chorge, gm Mercury Fulminate Lead Axide Tetryl
20 BeMoHc Metter, % TNT:
Treed Teat, % TNT:
79*C IntonraHeuei Hoot Teat: % Loaa in 48 Hit
Floto boat Toot: Method
lore Neat Tea»: % Loaa, 1st 48 Hrs 1.8 % Loaa, 2nd 48 Hu 1.6 Explosion in 100 Hn Hone Condition Confined Density, gm/cc Brisance, % TNT
ЯвмямЫВ!^ fades* Confinement Shelby steel Condition Liquid Chorge Diameter, in. 1.25 Density, gm/cc 1«33 Rote, meten/socund Fells
QL vVyS"^*^*^***** *
VdMMtr 60°C, ae/ca^/hr LO
367
АМСР 706-177
THethylene Glycol Dlnitrate (TB3M) Liquid
Nm M.M7I ProjecMs, I* WC-»1: Density, gm/cc Chorga Wt, lb Total Na. af hagriHi For TNT For Subject HE 1 task HE. M42A1 Projectile. Lot KC-S: Density, gm/cc Chorge Wt, lb Total No. of Fragments: For TNT For Subject HE Sheped Charge EHoetiveneoe, TNT = IM: Gloss Cones Steel Cones Hob Volume Hole Depth
Color:
Principal Uses: Ingredient of rocket end double base propellants
ef Urih|:
Lending Density: gm/cc
Aa /ала оЯ^НЯИЕ ▼ МОСКу! о% J Ж At» ft At 25ft ft Density, gm/cc
Method Liquid Hazard Class (Quantity-Distance) Compatibility Group Exudation
beet (Rotative *e TNT): Ata Peak Pressure Impube Energy Air.CseftasE: Impulse Under Wotan Peak Pressure Impube Energy Peak Pressure Impube Energy
Solubility in Water, gm/100 gm, at: 25°C 0.55 60°C 0.68 Solubility, gm/100 gm, at 25bC, in; Ether Alcohol “ 2:1 Ether: Alcohol » Acetone *• Viscosity, centipoises: Temp, 20°C 13-2 Hydrolysis, Add: 10 days at 22°C 0.032 5 days st 6o°C 0.02^ Vapor Pressure; °C i Mercury 25 <0.001
Heet of; Combustion, cal/gm 3428 Explosion, cal/gm 357 Gas Volume, cc/gm 851
368
Triethylene Glycol Dibitrate (ЯЮК) Liquid
AMCP 706-177
Origin:
Lourenco prepared triethylene glycol In 1863 by heating glycol vith ethylene bromide in a
sealed tube at U5°-12C°C (Ann (3) 67, 275) • Later in the ваше year Wurtt prepared tri ethy-
lene glycol by heating ethylene oodlEs vith glycc' at 100°C. By action of nitric acrd triethy-
lene glycol was oxi di ted to (HgOOC-CEp^O-O^Ja (.3) 69, 331, 351).
3» Germans and Italian* were the first to prepare and use ffiJN during World War II aa an
Ingredient of rocket and propellant powders. The cosnerclal production of TEGN in quantity
la still difficult and its ure u a plasticizer for nitrocellulose is being replaced by other
liquid nitrates.
Preparation:
friethylena glycol la purified by fractional distillation under vacuum in an 18-inch VI-
geaux fractioning colunm. The assembly as a «hole la equivalent to 4.5 theoretical plates.
The distillation la conducted using a 5 to 1 reflux ratio, at a pot teqperature of approxi-
mately 18O°C, and a take-off teaperature of approximetely 120°C.
The puriiled triethyleneglycol (TEG) la nitrated by carefully stirring it int' 2-5 parts
of 65/30/5 nitric acid/sulphuric add/water maintained at 0 t 5°C- The rate of cooling la
sufficient that 300 gm of TEG cau be added within 40 minutes. The mixture is stirred and
held it 0+ 5°C, for 30 additional nlnutes. It la then drowned by pouring ont, a large quan-
tity of xce and extracted three times vith ether. The combined extract ir water-washed to a
pH of about 4, shaken vith an excess of sodium bicarbonate solution, and further washed vith
1* sodium bicarbonate solution until the washings are colorless. The ethersal solution is
water-washed until it has the same pH value as distilled water It is carefulseparated
from excess water, treated vith chemically pure calcium chloride to remove ,‘issolved water,
and filtered. The ether is removed by bubbling vith dry air until в minima; rate of loss in
weight is attained. The yield is 1.3U gm per gm TEG (84* of theoretical) and the nitrogen
content of different batches range from 11.60 to 11.69* by the nitrometer method (calculated
11.67*).
References:78
(t) See the following Picatlnny Arsenal Technical Reports on THGN:
2 1 6 I 8
1953 1745 1786 1767 1638
2193 2056 1817
78See footnote I, puge 10.
369
AMCP 706-177
Trigonite
Molecular Weigbl: 217
TV Plc’-ic Acid 88-90 Mononltronapbthalene 12 10 Oxygea Bsleaee: CO, % -62 CO % -1U
Density: gm/cc Cast 1.60
Matting Paint: *C 90
C/H Rotio Frosting Point: *C
hagert SeaaitMy, 1 Kg Wh Bureau of Mina* Apparatus, cm 60 SompleWtJOmg Picatinny Arsenol Apparatus, in. 10 Sample Wt, .ng BeMag Paint: *C Explodes 300
Refrectiva index, n" n£ i£
Prietiea РепАйма Test: Steel j..- Fiber Shoe Vicaem Stability Teat: cc/40 Hrs, at 90'C 100’C 120*C 0.9 135*C 150’C
RM* BuNat hnpecf Test: Triols % Explosions 0 Portioh 0
Burned 0 Unaffected 100 200 Grom Bomb lead Teat: Bond, gm LA. 2
Faple*i*e ToMpefetara: *C Seconds, 0.1 (no cop used) 1 5 Deccapoaee 315 10 И-—i«r .tx.. ХКНПТну VV Minimum Detonating Charge, gm Mercury Fulminate Lead Azide 0.20 Tetryl O.OA
20 OaMatic Mortar, % TNT:
Treat! Teat, * TNT:
7S*C 1а*агмНем1 Hoot Test: % LOH In 48 Hrs
Plate Dent Teet: Mathod
10ГС Meet Teat: % Loss, 1st 40 Hr* % Loh, 2nd 48 Hr* Expiation in 100 Hr* Condition Confined Density, gm/cc Bri*onca, % TNT
Defeaatieo Rett: Confinement None
НеяммЫМу Index:
Chorge Diameter, in. 1.0 Density, gm/cc 1.6C Rote, meters/second 7020
VeteHMyi
370
Trimonlte
АМСР 706-177
N мм HI. МП РмИсЖа. Let WC-91: Density, gm/cc Chorge W:, Ib ForTNT For Subject HE Э lack Hl. M41A1 PwhcHh, Lrt KC-S: Density, gm/cc Charge Wt, Ib IWW« HWr W ForTNT For Subject HE Shaped Chorda Iffostivenoss, TNT = 100; Glass Cones Steel Canes Hole Volume Hole Depth
Cote:
Principal Uses: THT substitute in projectiles end bcatbe
Molted ef Leodiag: Cast
Leodiag Deadly: gm/cc 1- 60
Fregasoat Velocity: ft/soe At 9 ft At2SHft Density, gm/cc
Method Dry Hazard Class (Quantity-Distance) Class 9 Compatibility Group Group I Exudation Exudes et 50°C
Blast (Motive to TNT): Ain Peak Pressure Impulse Energy Air, Confined: Impute Under WMen Peak Pressure Impute Energy ^^4o Peak Pressure Impute Energy
Preparation:
Picric acid and alpba-mononitronapbthalena ere salted together in an aluminum or tin steam Jacketed melt kettle equipped with a stirrer. Although picric add alone requires a high tem- perature for its melt loading (120°C), the mixture forms a eutectic melting at *9°C. Cere must he taken to prevent the formation of dan- gerous metallic picrates. Trlmonits la of interest as an emergency substitute for ПГГ.
371
АМСР 706-177
Trlmoalte
Origin:
Trlmonite, a castable mixture of picric add/mononitronapbthalene vas developed by the
British during World War II as an improvement over tridite which la a mixture of 80/20 picric
add/dlnitrophenol. Both mixtures are suitable for melt-loading below 100°C and therefore
represent en improvement over melt-loading picric acid alone (melting point 122°C). However,
tridite is slightly inferior to picric add as an explosive end dlnitrophenol is object!en-
able because of its toxicity. Trimonite is also slightly inferior to picric acid and ТЯТ as
an explosive. Because of the lov eutectic temperature of the picric add-mononitronaphthalene
mixture (U9°C), Tridite exudes when stored at elevated temperatures. It does not possess the
dlsadvantsges of picric add (corrosive action on metals, ease of decomposition, etc.) and is
a comparatively inexpensive substitute for ПП.
References:
(a) See the following Picatlnny Arsenal Technical Reports on Trimonite:
2 2 6 8
1352 1325 926 1098
1372 976 1836
ЧП--------
' See footnote 1, page *0.
.'Г72
2,2,2-'rrj.nltroethyl*l*,^,U-Trinlt.‘c,u tty rate (IWETB)
AMCP 706*177
% C 16.6 H 1.6 O-CH С(ЯО ), 3 21.8 / 2 2 3 C - 0 0 58.0 \ с/h Rotio 0.202 снгснгссяог). Meier jler Weight: (CgHgHgO^) ?66
Oxygea Beleece: CO, % Л.2 CO % 20.8
Doneity: gm/cc Form I l.?S
Molting Point: *C 93
m a B—1_ж. rVVVFNIf FMRv»
hnpeet Sensitivity, 2 Kg Wt: Bureou of Mino* Apporotus, cm Sompis Wt 20 mg Pkotinny Arsenol Apparatus, in. Sample Wt, mg 50jC point, cm (a) 2) Belling Feint: *C
Refractive Index, nJ Form I (e) Crystal Axle , 1.518 fi 1.527 T I.5U6
Stool Shoo Fiber Shoo Vacwn Stability Teat: cc/40 Hrs, at 90’C —— 100 °C U8 hr* O.oO 120 C 135'C 150*C
RMo BrnNot hmpoct Tooh Trials % Explosion* Partial* Burned Unaffected
200 Grant Boaab Send Teat: Sond, gm
ВхуЫм ToMfMfehwo: °C Seconds, 0.1 (no cap used) — 1 --- 5 50^ point (Alhot bar) (a) 225 10 15 20 > i»* r. a_«а*_ лЛ^.— ИНеН|Тну IMWIVnVW* Minimum Detonating Charge, gm Mercury Fulminate Lead Azido Tetryl
Ballistic Mortar, % TNT: (b) 136
Trent Teat, % TNT.
7S"C intoraatissel Moot Teat: % Loa* In 48 Hr*
Plate Dent Teet: Method Condition Confined Density, gm/cc Brisonce, % TNT
WC Hoot Tub % Lot*. 1st 48 Hr* % Los*. 2nd 48 Hr* Explosion in 100 Hrs
Detonetian Rote: Confinement Condition Chorge Diameter, in. Density, gm/cc ].6O 1.76 Rote, meters/second 7760 8290
ЯмвяшЫШу
Hygroscopicity: % 30°C, 90jt RH 0.00 75°C, 5 months Hil ^a)
▼ ^MBVnBvy *
373
АМСР 706-177
2,?,2-Trlnltroethyl-L,L,4-lYlnUrobutyrete (TNETB)
* - _ CoaoMluU» BWmut I v Condition Tetryl, gm Wax, in. for 50% Detonation Wax, gm Doroity, gm/cc Oxygen, atoms/sec U.U x 102^ (Z/sec) Heat, kilocalorie/mole U3. U (AH, kcal/mol) Temperature Range, °C Phase Liquid
Heat of: Combustion, col/gm 1685 Explosion, cal/gm Gas Volume, cc/gm Formation, eol/gm 307 Fusion, col/gm Subline+'.ou, cnl/gm (e. t) 80L Armor Plate Impeet Tact: M mm Mortar Projectile: 50е inert. Velocity, ft/sec Aluminum Fineness 5004b General Purpose Bomba: Plote Thickness, inches 1 14 H/2 1%
Specific 'teat: cal/gm/'C
Buroiog koto: cni/sec
Bomb Drop Teat: T7, 2000-lb Semi-Armor-Piercing Bomb vt Concrete: Max Safe Drop, ft 500-lb General Purpose Bomb vs Concrete: Height, ft Trials Unaffected Low Order High Order 1000-к General Purpose Bomb vs Concrete: Height, ft Trials Unaffected Low Order High Order
Thermal Conductivity: col/sec/cm/'C
Coefficient ef Expansion: Linear, %/*C Volume, %/‘C
Herdami, Mobs' Scale:
£', dynes/cm* E, Ib/inch’ Density, gm/cc
Compraecive strength: ib/inch3
Vapor Fracture: (e) *C mm Mercury 65 3-3 x Юд 75 1.3 x 10 35 b.2 x 10* 100 2.3 x 10'£ 120 1.1 x 10‘2
374
2,2,2-Trlnltroethyl-4,4,4-Trlnltrobutyrate (TNETB)
АМСР 706-177
rraMR^I-i^S^Hi * Wi 90 пып HI. МУ1 Projectile, lot WC-»:: identity, gm/cc Chorge Wt, lb Total No. af Fragments: For TNT For Subject HE 3 tach HI. M43A1 Projectile, Let KC-S: Density, gm/cc Charge Wt, ib Tefal No. of Fragmeets: For TNT For Subject HE Shaped Charge Effectivenett, TNT = IN: Glatt Cones Steel Cones Hole Volume Hole Depth
Colee: Colorless
Mncipel Uses:
Leading Density: gm/cc Form I 1.783 Form П 1.677 Liquid, 99°C. 1.551
Fragment Velocity: ft/sec At 9 ft At 25% ft Density, gm/cc
Storage: Method Wet Hoznrd Class (Qiantity-Distonce) Compatibility Group Exudation
Iasi (Relative to H-6i: Sphere Cylinder (h) Air: l-lb Charge: EW* EV* iiW» EV* Peak Pressure 0.91 0.64 0.81 0-75 Impulse 0.73 O.67 0.74 O.69 Energy Air, Confined: Impulse Under Water: Peak Pressure Impulse Energy Underground: Peak Pressure Impulse Energy *EW, equivalent weight of H-6 feh. a unit weight of test mixture for equal performance at. the same test distance; EV, equivalent volume of H-б for a unit volume of test mixture for equal performance at the same test distance.
Bruceton Safety Test Results; (g) Mean and standard deviation of lengths of 0.300 diameter cylinder across which initia- tion Is possible for 50$ certainty: TNT 0.391 ♦ 0.C40 RDX Comp В O.381 + 0.042 TT3TB 0.920 + 0.059 Absolute Viscosity, poises; (e) Temp, 98.9°C 0.173 106.5°C O.138
375
АМСР7М-177
2,2,2-Irinitroe<hyl-b>b,b-Irinitrobutyrate (ПИИЭ)
Solubility (Boom Тиюегеturc); (a)
Solvent Solubility
j Utter * i Insoluble
j tuBarane Insoluble
’ Carbon tetrachloride 1 Insoluble ;
! Ethanol 5 gm/ХЭО gm solvent ;
Chloroform 5 gm/WO gm solvent 1
| Beuene 10 gm/100 gm solvent
3ttrceethan*i Very soluble
Glacial acetic acid Very soluble
Ethyl acetate Very soluble
ИИВ Ferms Butectics With thf Following Cowpounds: (a)
SW«iemmsmmmammeemMrawmm»»emsmm«een»WMSsmme
I BBBS (bis(trlnitroethyl) succinate)
| ВВП (bis(tri*ltroethyl) nitramine)
| TSB (trinitrobenzene)
; Compound A (C^HgM^O- formed by
condensation of1,'1-dinitroethane)
Trinitroethyl trinitrobenzoate (27jt)
Cryetallctfraphlc Data:
(•)
Three polymorphic czyatalline forme have been observed. Low tamperatuie Fora I goes through
a solid-solid transition at 89°C giving Fora II. Form II has a melting poi... of 92.5° to 93^C.
On cooling, Fora II does not transform reversibly to Fora I when 69°C is reached. However,
Fora II will transform to Fora I nt room temperature, usually taking a few hours to do so.
Fora III was observed, vllch appeared to be stable over a vary narrow temperature range on
the order of 0.2° to 0.3°C near 92.5°C.
Preparation: (d)
(»о2)3сся2анрсос1 + (HOgJgCHgOH ^эоц
trinlcrobutyryl chloride trinltroethanol sulfuric
add
(во2)3сся2сн2соос^с(яо2)3 + HC1 *-------------
2,2,2-trinitroethyl-l,h,b-trinltro- hydrochloric
butyrate acid
laboratory experiments indicate that the present slow step involving overnight treatment
of b,h,h-trlnitrobutyryl chloride with 2,2,2-trinltroethanol and aluminum chloride can be
replaced by a fast and simple esterification in sulfuric acid. Using 100>’ sulfuric acid un-
fortified НрЗОц, the ester can be prepared in yields of 95^ to In 24 hours st 25°C, in
5 hours at 50°C, or lu 3 hours at 65°C. Abort 65°C the reaction time is less, but the yield
fall- off and a less pure product is obtained. The crude white crystalline product on recryt-
tallization from dilute methanol gives a material melting at 92° to 93°C.
37b
2,2,2-'Brinitroe1hylA,b,U-Trinitrobutyrete (ЗИД)
АМСР7М-177
Oriaint (e)
IBB belongs to a kv cIsm of aiplodvM characterlMd by tridtromthyl troupe,
-C(B0b)v She «haedstry cf thia claa» of оафоивДа ш studied in Gerweny by Dre. Schenck
end (ш Teenleihair*’. who diecovered in 19^2-19^3 *t trldtroaethaae or dtrcftae, HCfBOfe)»
wm the eouroe of aw axplodve terlvutivM. Dr. Sctvnck prepared the stable solid alcohol?
2,2,2-trldtroethanol, froe nitrofom and forwaldehyde. Dr. Schlaaalschaidt reacted nitrofora
with tinea turn ted organic cowoupda, such м acrylic add, and predieted in X9*»3 that the eater
ofA,h,k-tridtrobutyric add with trinitroethenol would be an interes tJ»»c asploalve.
Ш 19^7 the U.S. Navy began a prograa to esplore these coupe* ’>• Ute initial task of in-
vestigatiug the ohaedstzy at trinitroathanol wm undertaken by v л Hercules Powder Coweny
(Bevy Contract BOrd-9925). Ihe U.S. Rubber Coapany studied the cheat в try of nitrefora (Bevy
Contract BOrd-10,129)• Attar preparation at the first laboratory aaaplM of JSKIB, consider-
able intarMt wm aroused, m early 1950 the Naugatuck Chedcel Division of U.8. Rubber Com-
pany wm assigned to prepare 100 pounds of SBIB. Ihe Bureau of Ordnance in July 1953 reined
the protection to 800 pounds with the aMistance of the Hercules Powder Соцрепу in aug^ ing
the protection at Brugatuck (BBvy Contract BOrd-11,280). ШЯВ ie a high oxygen contei
uplodve.
RefWrenoesi80
fa) J. N. Roaen, Properties of Trinitroethyl Trinltrobutyrete ШИВ, KAVOR? Report Хэ.
1758, 17 Deceaber 1952a-
(b) Bureau of Nines Report Bo. 3107, Part IX, Ballistic Mortar Tests on Irtnitrouthyl
Tridtrobutyrate, 5 April 1950.
(e) b. D. ВафЬсп and G. flradebe, Evaluation of 2,2,2-Trit 'troethyl-h.hA-Trinitrobu*,, -ata
<C a Constituent of CMtable Explosives, ХАУонЬ Report No- 261 . 30 September 1952.
(d) U.S. Rubber Сафапу Quarterly Progress Report No. 23, °ynth»ds of Bev Propellan—
and Baplodves, Navy Contracts NQrd-10-129 and -12,663, 19 August 1?53-
(e) N. X. Rill, 0. H. Johnson, J. M. Rosen, D. V. Sicknan end F. Thylor, Jr., Preparation
and PropertlM of В0ЯВ, a Bev CMtable High Btploeive, KAVORD Report Ko. 3685, 27 January 1955.
(f) M. E. Hill, RynthMis of Bev Hid» Baplosives, KAVORD Report No. 2965 1 April 1953-
(g) Jacob Ravitt, A Sensitivity Test for CMtable Mould Explosives, Including Results
for fleas Nev 1Шег1а1аГИШ) bcori bo. “-------- ---------
(h) R. V. Gipson, SoMitiTity of Explosives, IX; Selected Physico-Chenical Data of .ten
Pure High Explosives, NAVORD Report No. 6130, IB June 1958.
8®See footnote I, page 10.
377
АМСР 706-177
Trinitro Trtazitobenteue
Ceapmrtien; MehetderWeiglrt: (С606Я12) ЗЭб
?°2 c ta.k ->1/ Jl 50.0 "з I | B3 SRC • °?S \^~S02 Oxygen Wevn CO, % -29 CO % 0.0
iMnelty: gm/cc Crystal 1.81
Q 2v«t c ^у**^ *- К . Matting Mat: "C Deconpoaee 131
3 • C/HRoHo vBBOKNtf WPvS 4» ......^ .
hapect SenritMy, 1 Kg Wfc Bureau of Mines Apparatus, cm (•) & 25 Soovto Wt 20 mg Pkatirmy Anenol Apparatus, in. Sample Wt, mg Fifai; *C
3 3 3 go Bo go
а * -« _ —»- Ca*a1 Скм 3*wl эпов Fiber Shoe VnctHMW Stability Tort: cc/40 Hr*, ot 90"C 100’C 120’C I35’C 150’C
Uh BaBrt hnpoct Tert: Trial* % Expiation* Partial*
Burned Unaffected 200 Gram Bomb Send Tert: Sand, gm
||рЫй Temporatnre: ’C ' (e.). Second*, 0.1 (no cap used) 1 5 150 10 15 20 • •• <ТГ*жа-»е-_ •WwWWy |ИЯ^Я^И8е Minimum Detonating Charge, gm Mercury Fulminate Lead Azide Tetryl
ВоЯМс Moder, % TNT:
Trernri Tert, % pm: 90
V(*^ 4Pm^&« в У V veW* 1 We % La** in 48'Hr* Hole Dent Toth Method
100’C Neat Tert: % Loss, 1st 48 Hr* % La**, 2nd 48 Hr* Expiation in 100 Hr* Condition Confined Density, gm/cc Britance, % TNT
Deteaatioa Beta: Confinement Condition Charge Diameter, in. Density, gm/cc Rote, meters/second
РСмкяиЫ№у 1й4*ж;
Hygreecepicify: % 30°C, 90^ № 0.00
VeteHUty:
378
Trinitro Triazidobentenn
AMCP 706-177
Я ем Hl. МЛ Prajectih. L* WC-91: Density, gm/cc Chorg» Wt, Ib bia ^Л IWWI rWt wt FVW^BBMRBs ForTNT For Subject HE 3 ЬиЫМ. M42A1 Projectile, Let KC-3: Density, gm/cc Charge Wt, Ib ЧРХДеьВ al 1VW V^V* Vm vl^^WWsr ForTNT For Subject HE Shaped Charge Effeetiveaess. TNT = l»f; Gloss Cones Steel Cones Hole Volume Hole Depth
Color: Greenish-yellav
Priadpal Usee: (c) Ingredient of printer mix
Method ef leading) Pressed Deed presses at about 42,000 pel
Leadteg D веку: gm/cc At 42,000 pel 1.75
rvegmaat Velocity: ft/sec At 9 ft At 25% ft Density, gm/cc
Method Hazonl Class (Quantity-Distance) Compatibility Group Exudation None
Meet (Relative to TNT): Ain Peak Pressure Impulse Energy Air, Confined: Impulse Under Water: Peok Pressure Impulse Energy UWif^VMNMle Peak Pressure Impulse Energy
Qualitative Solubilities et Room .'empereture: Solvent Solubility Acetone Readily soluble Chloroform Moderately soluble Alcohol Sparingly soluble Water Insoluble Compatibility vith Metals: Wet; Does not attack iron, steel, copper or or sa. Heet of; Combustion, cal/gm (a) 2554 Burning Rate; (b) cm/sec 0.65
379
Aniline 1» chlorinated tc fore tri chia semi line. The aaino group is eliminated by the :
diazo reaction. The resulting i ya-tri chlorobenzene la nitrated. Thia nitration is carried
cut by dissolving the material la w» 32# oleum, adding strong nitric add, and heating to
lbO°-15O°C until no trinltro trichlcrobenzone(me?.ting taint 18T°C) precipitates (Bef f)-
The chlorine groups are then replaced by azo groups. This is acccepllahed by adding ar ace-
tone solution at the trinitro trlchlorobenzeoe, or better, and po-dered substance alone, to
an actively stirred aolutlon Gt sodium eside in alcdbol. The precipitated trinitro triazida-
benzese ia collected on e filter, washed Vith alcohol, water and dried. It way be purified
by dissolving in chlaioinrm, allowing the oclutfcn to cool, and collecting the greenish yellow
crystals (welting point 131°C with decovpoaitioa).
Origin:
This initiating explosive was first prepared in 1923 by Turek who also perfected ita manu-
facture.
Hefcrtncea:81
(a) S. Self, Tecta of Explosive Conpounda Submitted by Arthur D. Little, Inc., BATR 1750,
2b October I$b9.
(h) A. x. Belyaeva and A. E. Belyaeva CR a.s. USSR 52, 503-505 (19^6) Chemical Abatracte
bl, b310.
A. E. Belyaeva and A. F. Belyaeva, Doklady Akad Sauk. USSR 56, b91-b?b (19b?).
(c) French Patent 893,9**!, lb November 19bb (Chemical Abstracts bj, 83Tb).
(d) A. D. Ioffe, "Thermal Decomposition and Explosion of Azides," Proc. Roy Soc A208,
188-199 (1951).
(e.) T. L. Davis, The Chemistry of Fcvder and Explosives, John Wiley and Sons, Inc.,
New York (19b3), p. b3?.
(f) 0. Turek, Chim et Ind 26, ,781 (1931); German Patent b98,050; British Patent 298,981.
B^See footnote 1, page 10.
380
TMpenta erythritol Octant treta (TPECW)
AMCP 706-177
timtliiTtjinii Metecater Weight: ( С15Н2^к802б) 7ЭЙ
C Й4.6 5 3.3 c;; м15.3 0 C56-S - с/ниЪ^®20*^ г4" а.г« Oxygea Baleeae: CO, % CO % JAP! • CM •
Deaeifr: gm/cc Crystal 1.58
*C 82 to 84
ra e— 1^*^- g^>
!>|Ц|И1>»*У,1М Wfc . Bureau of Minep Apporatue, cm Sompte Wt 20 mg Picatinny Ammai Apparatus, in. 9 Sompte Wt,i*y 24 te-eoe Bto^^e V^NII^p
3 3 3 go go go
_. a..* ор_-ж. , ПМНОП гШвЯИВ l*CT* Steel Shoa Unaffected Rbar Shot Unaffected Yoeaam SteHfty Teet: cc/40 Hr*, at 90’C 100’C Pure 120*C Specially purified 135*C 150*C 2.4S 1.94
Mte BaGat hugest Toot: Trial* % Ецйо*>оп* ’ Pottiate
Burned Unaffected 200 Gram Bemb Saad Teat: Send, gm 58.9
lepteetea Temgotstura: C Second*, 0.1 (nc cap used) — 1 5 225 10 15 te ur 1. a e-e ^e vOMMBVavluj иИрЮТаОЮе Minimum Detonating Charge, gm Mercury Fulminate Lead Azide Tetryl 0.30
20 BaGtette Mortar, % TNT:
Traate Teat, % TNT:
7J’C tateraatieaal Hoot Teat: % Lota in 48 Hrs
Plate Dent Teet: Method
100’C Moat Teet: % Lot», lit 48 Hr* 1.15 % Loss, 2nd 48 Hr* 0.75 Explosion ы 100 Hr* None Condition Confined Density, gm/cc Brisance, % TNT 4
Deraaetten Rate: Confinement
НмммЫВ1у 1в4ежх None Preeaed 0-5 I.56 7650
f . * e- fu Chorgo Diometer, in.
VeteNBty: Density, gm/cc Rote, meters/second
АМСР 706-177
Trlpentaerythrltol Octanitrate (ТРКЖ)
Condition * Tetryl, 0"» Wax, In. for 50% Detonation Wax, gm Density, gm/cc Oxygen, alomi/soe (Z/mc) Heat, kiloeolorie/mole 23.1 (AH, kcal/mol) Temperature Range, *C 215 to 250 Phase Liquid
Им* oft Combutticn, col/gm 2632 Explosion, cal/gm 1085 Gas Volume, ee/gm 7б2 Formation, cal/gm Futian, cat/gm Armor Plate Impost Test: ГЛ ' ^^Чр 50% Inart, Velocity, ft/soe Aluminum Fineness Омяйм! Plate Thickness, inches 1 14 H4
Ipeetth Hoot: cal/gm/*C Specific lopuloe; Ib-eec/lb (calculated) 2<tO
Bearing Roles cm/tec
limb Drop Test: 77,20004b torn! Armor Piercing Bomb «s Concrete: Max Safe Drop, ft 5004b Geeeral Porpeeo Bomb ее Coocrete: Height, ft Trials Unaffected Low Order High Order 10004b General Purpose Bomb ss Concrete: Height, ft Trials Unaffected Low Order High Order
Thermal Condostivity: col/tec/cm/*C
CetfRciM* ef Ьрвмйя: Linear, %/*C Volume, %/*C
E‘, dynes/cm* E, Ib/inch* Density, gm/cc
Cemprasehro Strength: Ib/inch’
Vepor Praeeeri: ‘C mm Mercury
382
Tripentaerythritol Octanitr:v (TPBON)
AMCP 706-177
FrigmietiMie Tash Shaped Charge ОМееНелаеее, TNT s 100:
N «им НО, МП hejecMe, let WC-01; Density, gm/cc Chorge Wt, lb Glass Conee Steel Cone* Hob Volume Hole Depth
For TNT For Subject HE 3 inch НЕ. M42A1 PtnjscMa, Let KC-5: Dantity, gm/cc Chorge Wt. lb Color White
Pi In sb si Uwn High explosive end ее possible plasticiser for nitroeellnlose
о Bowe гчв» mjBBWims For TNT For Subject HE Method ef Loading: Cast or pressed
Leedtag Daeellyt gm/cc Pressed at 60,000 pal 1.565
Fregment Velocity: ft/sec
At 9 ft AtJ5%ft Density, gm/cc Method Dry
bet (ReMve ie TNT): Hazard Class (Quantity-Distance)
Air Peak Pressure Impube Energy Compatibility Gloup Exudation Bone
CsefhMdt Impube Hygroecopicity, Gain or Lose in Wt, It;
Time, Hra < RH at 30°C
Under Water 1*0 70 QO
Peak Pressure Impube Energy UWw^vmnMIo Peak Pressure Impuhv Energy 2k -0.008 -to.01 -Ю.Ф U8 -0.02 -0.01 «0.02 144 -0.0k -0.03 -0.02 192 -0.0k -0.02 216 -0.00k -o.Ol +0.03 Solubility: Solvent Solubility Water Insoluble Alcohol Soluble Chloroform Soluble Acetone, hot Very soluble Benzene, hot Very soluble
383
АМСР 706-177
Trlpentaerythrltol Octanitrate (TPEOR)
Ccwatibility With Other High Explosivee:
100°C Vacuum Stability Tbst:
ГИ? PETR RDX TPEON
al gaa/ltO hrs, 5 gm sample 0.1U 2.15 i 0.39 2.45
al gas/йО hrs, 5 gm sample of 50/50, tpbot/he 1.89 1-71 i 2-32
Dlpentaarythritol Hexanitrata (UJEHH)-HiE0H Fusions;
j % TPEON % IPffiN Solidification Time, Daye MP, °C
I 100 0 I 83
! 95 5 1 3 68
90 10 3 69
80 20 5 73
i 50 50 30 60 (Eutectic)
20 80 5 63
10 90 3 69
0 100 — 73
Preparation: (a)
Twenty grata (0.05^ mol) of nitration grade trlpentaerythrltol (TPE) (99#) minimum purity)
were slowly added, with stirring, to 160 ga (2-55 mol) of 99# nitric add at a temperature of
-25° to 0°C. On equivalent weight baaia, thia quantity of 99# nitric add corresponds to an
excess of 6.3 tinea the TPE used After addition of the TPE, t1.. reaction mixture vas stirred
for about one hour at 0° to 5°1 and poured into eight times its volume of cracked ice. The
product, when allowed to stand overnight, was crushed under vater; filtered vith suction; and
washed copiously with vater. It was then treated twice with about 5 time its Wight of a 1#
amcnlum carbonate solution, stirred for several hours, filtered and washed with vater until
the final washings were neutral to litmus. The final product was washed successively with
50 c- -ach of ethanol and ether. The material dried in air weighed 37.6 gm or 96# of theory
based on TPE. It had a xeltlng range of 71° to 7* C. Crystallization cf the crude ТРЕСЖ frou
chloroform was found to be the most suitable method of obtaining pure TPEON.
Origin;
TPEOK prepared by the reaction of trlpentaerythrltol and 99# nitric acid at 0° to 10°C vas
reported by Wyler in 19^5 (J. A. Wyler to Trojan Povder Company: U.S. Patent 2,389, 228,
20 November 19^5).
Tripentaerythritol Octanitrate (MBON)
AMCP 706-17?
Befarencaet*3
(•) J. J. laNonte, И. J. Jackaon, 8. Uvlncaton, L. B. SLlbexoan and X. N. Jonee, The
Preparation and XKOloaiYe Prooertleeof Tripantaerythritol Octanitrate, PATH No. 81*90,”1558.
(b) K. Saaba, J. Yaaaahlte and 8. Tanaka, "Pentaerythritol Mtranitrete," J Ind KxploalTes
Soc (Japan) 1£, 868-9 (19541 <* & ПЯвЗ (1955).
(a) 8. D. Braver and H. Hankin, The Stability of MM and Pantollta, OSRD Report No. ihlh.
(d) X. Berlov, R. H. Barth and J. X. Snov, Tha Pantaexythritola, ACS Nonosreph NO. 1Эб,
Reinhold Publlehlng Corporation, Nev lark, 1958.
gZsae footnote 1, page 10.
385
АМСР 706-177
Tritonal, 80/20
Matesater Weight: 81
пгт 8о Alualnua 20 CO, % -77 CO % -30
Deaaky: gm/cc Oast 1.72
MoRiag fate»: *C
C/H Ratio а W^^^uB^B^
hapect SaaaMvky, 2 Kg Wt: Bureau of Mines Apporotus, cm 05 Sampte Wt 20 mg Picotinny Arsenal Apparatus, in. 13 Sompte Wt, mg 16 еЖад Paint: *C
Rohoctiae ladcx, n£ i£ nJ,
• vWVWIO vW^WBMOOO 0вв», Steel Shot Unaffected Fiber Shot Unaffected Vacaem StobWty Teat: cc/40 hrs, ot W'C 100’C 0.1 120*C 0.2 135’C 150X 0.8
Rifte Mlat Impact Taah Triois % Explosions 60 Portioli 0
Burned 0 Unoffoctod LO 200 Grom Bomb Send Teat: Send, gm
fapteatee Taa^aeetara, *C Second», 0.1 (no cop uaad) 610 1 520 5 Daccnpoaea kfO 10 W5 15 Minimum Detonating Charge, gm Mercury Fulminate Lead Azide 0.20 Tetryl 0.10
20 BaKtette Mortar, % TNT: (e) 12*»
Titatei Tost, % TNT: (b) 125
75‘C lateraatiaaal Heat Tear: % Lorn in 48 Hn
Plata Dent Teat: (c) Method В
100’C Мае» Taah % Loa», 1st 48 Hrs % Lou, 2nd 48 Hrs Explosion in 100 Hrs Condition Oaat Confined No Density, gm/cc i.. 75 Brisance, % TNT 93
Docaaottea Reto: Confinement None None Condition Cast Pressed Charge Diameter, in. 1.0 1.0
РЬомвеЫйу lades: 100
Hygeeecepiclty: % JO°C, 90% RH 0.00
VetaHHiy: Density, gm/cc 1.71 1.72 Rote, meters/socond 6L75 67OO
386
Tritonal, 80/20
АМСР 706-177
Booster 6маЫ*Му Test: ( d) CondHon Cost Tetryl, gm 100 Won, in. for СЭ% Detonation 0.5в Wox, gm Density. gm/cc 1«75 Oxygen, otoms/soe (Z/soe) Hoot, kllocolorie/mole UH, kcol/mol) Temperature Range, *C Photo
Hooteft (c) Combustion, col/gm ЬЬвО Explosion, cal/gm 1770 Got Volume, cc/gm Formation, cal/gm Fusion, cal/gm Amor Note Impact Test: (e) ЛЛ >«__»- m _e^_».am__ W ИИИ MVnW TaVjMVnVe 5C % Inert, Velocity, ft/sec 509 >1100 Aluminum Fineness 100 12 Plate Thickness, inches Trials f Inert 1 0 1'4 *6 100 6 33 1% 0
Ipedfie Hoot; cal/gm/*C (b) At -5°C 0.23 Density, gm/cc 1.7b At 20°C 0.31
Berelag Bote: ст/мс
Bomb Drop Test: (e) T7,20004b Eeml Anas i-Pierciag Bomb vs Concrete: Max Safe Drop, ft S004b General Perpoeo Bomb vs Csecrete: Seel Height, ft 1736b 5735b Trinh 3)» l*i Unaffected 32 1** Low Order 0 0 High Order 2 0 10004b General Perpoeo Bomb vs Coacrelei Seal Height, ft Trials 2b Unaffected 23 Low Order 0 High Order 1
Tberami CeedecHvity: . col/soc/cm/’C (b) 11 x 10* Density, gm/cc 1.73
CMffldMtW fapMiiM: Linear, %/*C Volume, %/*C
Mete* ScMe*
Tseng's MeMee: (b) E', dynes/cm* 6.6? x 10^ E, Ib/inch* 0.97 x 106 Density, gm/cc 1-72
Compressive Strength: Ib/inch* (b) 23**0 Density, gm/cc 1.75
Vepor Fremerei *C mm Mercury
.'«7
АМСР7М-177
Tritonal, 80/20
^Ч^Я1^Я|Н l«VT N мм M, WI Pre|aaNh, La» WC»h Dsrctty. gm/cc 1*T1 ChorgeWt,* 2.272 TeSei Ne. ef FrogmanM: For TNT ГОЗ For Subnet HE 616 I lash HE. МСЫ1 ProfecNta. La» KC-E: Density, gm/cc 1.73 Chorga Wt. Ib 0.91b Total Na. of Рг«дмеа1к For TNT 51*» For Sublet HE W5 Glow Cones Steel Cones Hob Volume HoCatfctrih
Cahn Grey
Pile sly al Uaew GP boats
МеМмЕ of LeoNagt Cast
Leading BoatKyt tfm/x 1.65-1*72
Недоме» Vatecfty: ft/sec At 9 ft 2b60 At2$Hft 2380 Density, gm/cc 1*72
Method Ury Hazard Class (QuantityOistonce) dess 9 Compatibility Gruun Group I Exudation
Moat (laMsote TNT): (f) Abt Peak Ргамига 110 Impube 115 Energy 119 Aif CeofhM^t Impube ЦО UNaTste. Pooh Ргемиге 105 Impulse 118 Energy l?-9 Peak Pressure 117 Impube 127 Energy 1#
Preparation; Tritonal is prepared by adding ТЭТ and alualnua aenarately to a stoaa-Jenkoted nelt kettle equipped vith a stirrer. Beating nf the kettle and nixing of the ingrediente are continued until all the THT la melted. When the viacoaity of the rixture ia conaldared satisfactory (about 85°C), the tritonal la poured into projectiles or honba the aaae aa тэт.
388
АМСР 706-177
Tritonal, 80/20
Origin;
Ite Addition of aluminum to increase the power of explosive» was proposed by Xacales in
1899 end patented by Roth in 1900 (German Patent 172,327)* Some recent studies, directed
towards establishment of the optlmua aaount of aluainua in the TRT/Aluainua system, have shown
that (1) the blast effect increases to a maximum when the aluainua content is 306 (Ref g);
the brisance, as aeasured by the Sand Teat, peaaea through a atxlaua st about 1?6 aluainua
(Ref h); in Fragaentation Testa, no aaxiaua 1s observed, additions of aluainua causing a de-
crease in efficiency over the entire range free Об to 706 aluminum (Ref 1); and (It) the rate
of detonation of east charges is continuously decrease! by additioc* of aluainua up to M)6
(Ref j). Per all practice! purposes -t is concluded that the addition of 186 to 206 aluainua
to TOT iagtrores ite performance to a aaxiaua* this conclusion is in agreement with that of
British worker* who measured performance of eluainlzed TOT-aixturee based on extensive Lead
Block Test data (Ref k).
Tritonal, consisting of 806 TOT and 206 aluainua, van developed and standardised in the
United States during World War 17 for uae in bombs.
References;8!
(e) L. C. Sai th and В. H. tyster, Physical Testing of Explosives, Partin, Miscellaneous
Sensitivity Tests,- Performance Tests, OSRD Report Bo* 574b, ZT Decesmer 19^5*
(b) Ibllip C. Keenan and Dorothy Pipes, Table of Military High Explosives, Second Revi-
sion, HAVORD Report Ro. 87-U6, 26 July 19**6.
(c) D. P. MacDougall, Methods of Physical Testing, OSRD Report Ko. 803, H August 19^2*
(d) L. C. Sai th and B. R. Walton, A Consideration of RDf/Wax Mixtures as a Substitute for
Tetryl in Boosters, MOL Memo 10,303, 15 June 19*9*
(e) Committee of Div 2 and 8, HCRC, Report on HBX and Tritonal, OSRD to. 5>»Об, 31 July 1945*
^f) W. R. Tomlinson, jr., Blast Effects of Bomb Explosives, BA Tech Div Lecture, 9 April
(g) W. B* Kennedy, R* F. Arentxen and C. W. Tait, Survey of the Performance of ДТ/А1 on
the Basis of Air-Blast Pressure and Impulse, OSRD Report Ho. !649, Division 2, Monthly Report
Ko. MB-6, g Janua'r>1^5.------------ -------
(h) W. R. Ttelinson, Jr., Develop Bev Hich Explosive Filler for AP Shot, BAIR Ko. 1290,
First Progress Report, 19 May 19**3*
(i) W. R. Tomlinson, Jr., Develop Rev High Explosive Filler for AP Shot, PAIR Bo. IJBO,
Second Progress Report, 12 January 1§M.
(j) L* S. Wise, Effect of Aluminum on the Kate of Detonation of TOT, PAIR Ho. 1550,
26 July 19*»5*
(k) Armament Research Dept, The Effect of Aluminum on the Pcver of Explosives, British
Report AC-6U37, May 19W» (Explosives Report
^Te' footnote 1, page 10.
38Э
АМСР 706-177
Tritonal, 8o/gp
(1) Also see the following Plcatlnny Arsenal Technlcel Reports on Tritonal:
0 £ A £ 6 J 6
1530 1693 ЙЙ 1635 1956 1737 2138
15Ю 2353 2127
2010
390
Veltex Mo. U8*
AMCP 706-177
% HMX 70.0 Nitrocellulose (13-15% Я) 15-0 Nitroglycerin 10.7 2-8itrodipbenylainine 1.3 Triacetln 3-0 C/H Rotio Metocohr Weight: 261
Oxygea Belerce: CO, % -26 CO % -0.5
Density: gm/cc Pressed 1.72
Melting Point: *C
Freesiag Point: *C
Caiwct Sensitivity, 2 Kg Wt: bureau of Mines Apparatus, cm Sample Wt 20 mg Picotirmy Arsenal Apparatus, in. Sample Wt, mg Boiling Point: *C
Reflective Index, nJ l£ n?,
Friction Pendoleni Teet: Steel Shoe Unaffected Fiber Shoe Unaffected Vecwnm Stability Teet: cc/40 Hrs, ot 90’C .... 100’C 1.29 120*C 29 hours i > 135*C 150"C
Rifle Bullet Impact Teet: Triols % Explosions Portiols Burned Unoffectod
200 Grem Oeseb Send Test: Send, t>m 66.4
Explosion Tempereture: ‘C Seconds, 0.1 (uc cop used) 1 5 10 15 20 *-- *<!! flan * *“* -* М%Вя1гну TO НЮТО^мМООе Minimum Detonating Charge, gm Mercury Fulminate — - Lead Azide 0.30 Tetryl ----
OaMsHc Metter, % TNT:
Trend Teet, % TNT:
7S°C lntenMtieael fleet Teet: % Loss in <e Hrs
Plate Dent Teet: Method Condition Confined Density, gm/cc Brisance, % TNT
93 C fleet Test: % Loss. 1st 48 Hrs 0.28 % Loss, 2nd 48 Hrs 1-12 Explosion in 100 Hrs None
Detonation Reto: Confinement Condition Chorge Diameter, in. Density, gm/cc Rote, meters/second (calculated) 3500
Flemmebility Index:
Hygroscopicity: %
Volatility:
♦See footnote relieving pagt.
391
АМСР 706-177
Veltex МО. U8*
vonomon Tetryl, gm Wox, in. for 50% Detonation Wox. gm Density, gm/cc Oxygen, otoms/soe (Z/ssc) Hoot, kilocolorie/mole UH, kcal/mol) Temperature Range, *C Phase
Nest oft Combustion, cal/gm 2359 Explosion, col/gm 1226 Gas Volume, cc/gm Formation, cal/gm Fusion, cal/gm Armor Holo Impact Test: 66 ssm Mortar Fssfsst6st 50% Inert, Velocity, ft/sec Aluminum Fineness Plate Thickness, inches 1 14
Coaoresslon st Rupture; % 8.26 WOrk to Produce Rupture; ft-lb/lnch3 9-62
0имЬф BfltWs cm/soe
Bomb Drop Tert: T7, 200Mb Semi Amur Horsing Bomb vs Centrete. Max Safe Drop, ft SfNMb General Pntpeee Bomb vs Concrete: Height, ft Trials Unaffected Law Order High Order 100Mb General Pnrpeee Bomb vs Concrete: Height, ft Trials Unaffected Law Order High Order
1И7ЯЛ WMSMVWlvyt cal/soe/cm/’C
CetfUctarteff fapMiiMs Linear, %/’C Volume, %/*C
Meta* Scsfle*
E', dynss/cm* 0.2k x 10^° E, Ib/inch’ C 35 x Ю5 Density, gm/cc
- CiKi»nuhi Strength: Ib/inch’ 2720
Vapor Prossere: *C mm Mercury •Meme assigned by Dr. Mar- M. Jones, formerly of PA; based on original development by James H. Veltman.
392
Veltex No. U8
АМСР 706-177
N м Ш, M71 ProjMtib, Let WC-F1: Dantity, gm/cc Charge Wt, £ For TNT For Subject HE 1 inch HI, M42A1 ProjMtib. Lot KC-S: Dantity, gm/cc Charge Wt, № Total He. ef FragxMc*: For TNT For Subject HE Shaped Charge EHaaHoooess, TNT = 100: Glass Cones Steel Cones Hob Volume Hob Depth
Color: Orange
Principal Uses: High enchant cal strength schlnehle explosive
Method of Loedhsg: Pressed
Loading Dansby: gm/cc At 6,700 pal 1.72
Fiagaeeat Vdeckyt ft/sec At 9 ft At 25% ft Dantity, gm/cc
Method Dry Hazard Class (Qjantity-Distance) Compatibility Group Exudation None Machinability Excellent
Blest (RatoHeo to TNT): Air: Peak Pressure Impulse Energy Air, Confined: Impube Under Water: Peak Pressure Impulse Energy Peak Pressure Impulse Energy
393
АМСР 706-177
Veltex No. Щ
Preparation;
The preparation of this class of explosive compositions is illustrated by ti.e metnod used
far Veltex Ho. 4U8: Place 675 cc of water in a slurry kettle equipped vith an aglt’t —. Add
5*85 gm of 2-nitrodiphenylamine and agitate for several minutes to obtain dispersion. Then
add 93«7 gm of water-wet nitrocellulose (dry weight 67-5 gm) in small portions, р-ise the
temperature to WJ°C and maintain this temperature, but continue the agitation. A mixture of
48.2 gm of nitroglycerin and 13>5 gm of triacetin Is added over a 5-mlnuto period, with the
mixing continuing for an additional 10 minutes at 48°C. The 1NX (350 gm) ic ;<-lded over a
5-minute period with agitation continued for 30 minutes at 48°C The slurry 13 cooled to
roon temperature and filtered. The filter cake is dried to a moicture content between 6% and
126* The incorporation of this mix is completed ’ rolling 50 gm portions at a temperature
of approximately 90°C* The finished coll d Is then prehested on s heat table st бо’с. In-
crements of 25 gm each are pressed at 67a, psi for four minutes at 71°C. A cylinder is then
built up by pressing together four 25 S-- increments for a dwell time of 15 minutes.
Pidgin;
Veltex is the name given to a series of closely related nitrocellulose compositions pre-
pared in 1957 at Picatinny Arsenal by the solventless process used for propellants. These
compositions all contain a high percentage of solid high explosive. They were investigated
to determinate the suitability of the Holtex type exploeive developed by Hispano Suiza of
Switzerland, France and Spain, but for which the composition was not reported (Ref s). Com-
positions similar to Veltex No. W*8 and containing 606 to 806 HMX, with either nitroglycerin
or triethyleneglycol dinitrete as colloiding agent for nitrocellulose, have also been prepared-
In general these compositions shoved lover heat stability than that of conventional high ex-
plosive compositions.
Reference;84
(a) U.S. Air Intelligence Information Report IR-269-55, Holtex--Hispano Sulza Exploeive,
4 May 1955-
^^See footnote I, page 10.
fa I., h. J HNM f.VJ РИ1М1М, OHi( r. I'-? I <> 4J<1 .WA’
394
AMCP 706-177
(AMCRD-TV)
FOR THE COMMANDER:
OFFICIAL:
Lt. R. HORNE
HColorel, GS
Chief, HQ Admin Mgt Ofc
CHARLES T. HORNER, JR.
Major General, USA
Chief of Staff
DISTRIBUTION:
Special
ENGINEERING DESIGN HANDBOOKS
Listed below ere the Handbooks which have been published or ere currently under preparation. Handbooks with publication
dates prior to I August 1962 were published as ?0-*er1es Ordnance Corps Pamphlets. AMC Circular 310-38, 19 July I М3,
^designated those publications as 706-serles лис Paaphlots (e.g., DROP 20-138 was redesignated AMCP TQi-138). A'l new.
reprinted» or revised Handbooks are being published os 7O6*sp*ies AMC Paaphlets.
no*
»tP 706-
11 tie
100 •Design Guidance for Producib! Ht*
104 «Value Engineering
106 Elements of Arms went Engineering, «art One»
Sources of Energy
107 Elements of Armament Engineering» Part Two.
Bail(sties
106 Elements of Armament Engineering. Part Three,
weapon System and Components
109 •Taolet of the Cumulative Binomial Probabilities
110 Experimental Statistics. Section 1. Bas<r Con-
cepts and Analysis of Hr**ure«iont DaU
111 Experimental Statistics. Section 2» Analysis c-
Enumeratlve and Classificatory Dau
112 Expcrl«ufiul Statistics. Section 3» Planning
and Analysis of Comparative Experiments
113 Experimental Statistics. S*ctlor 4. Special
Topics
114 Experimental Statistics. Sectiun Tables
IIS Envlronmenui Series, Part One, Basic Environ-
mental Concepts
116 ‘Environmental Serins, Part Two, Basic Environ-
menui Factors
120 ‘Criteria for Environmental Control of Nobile
Systems
>21 «‘Packaging and Pack Engineering
123 ‘Hydraulic Fluids
125 Electrical wire and Cable
127 ‘Infrared NPItary Systems. Part One
126(5) ‘Infrared Military Systems. Part U)
130 Design for Air ’ransport «nd A. droj of
Nauricl
133 ‘Moir,*’neb111ty trg’-“eri»g Tnoory and Practice
134 Mr rtaln«»4Mty Guide f^r Dtb-gn
135 ln4»ntions. Patent», «nd belated Matters
136 Ser -wecMnlsm», Section 1. Theory
137 Servomechanisms, Section 2, Measurement and
Signal Converters
136 Servomechanisms, Section 3, Amplification
139 Servomechan'.sms. Section 4. Power Elements ang
System Design
14o Trajectories. Differential fleets, and Data
for Projectiles
145 ‘Dynamics of a Tracking Gimbal System
150 Interior Ballistics of Guns
160(5) Elements of Terminal Ballistics. Part One. oil
Mecnanisms and Vulnerability (и 1
I6I(S| Elements of Terminal Ballistics. Part Two.
Collection and Anal-s’s of Data Concerning
Targets (u)
I62(SAD) Elements of Terminal Salhsbcs, Part Throe.
Application to Missile and Spaxe Targets 'u;
165 Llouid-F11 led Projectile Design
170(C| “Armor and Its Application (U)
175 Solid Propellants, Part One
4(C) Sol id Propellants, Part Two (lO
7 Procerties of Explosives of MiliUry Interest
176(C) ^Properties of Exp'OVvOk of Military Interest,
Section 2 (U) (REPLACED -177)
179 Explosive Trains
180 ‘Principles of Explosive Behav'ir
IBS Military Pyrotechnics. Part One. Theory and
Application
164 Military Pyro*echmcs. Part Two. Safety,
procedures and Glossary
|y; Military Pyrotechnics, part Three, Properties
of Materials Used m .yrotectmic Compositions
188 ‘Military Pyrotechnics, Pert Four. Design fff
Ammunition 1 r Pyrotechnic Effects
189 Mibury Pyrotechnics, Fart Five. Bibliography
190 ‘Army weapon Sys tec Analysis
igi «System Analysis and Ust-Effectiveness
195 ‘Development Guide for Reliability, Part One.
Introduction, Background, and Planning for
Army Materiel Reauiroments
196 ‘Development &Hde for Reliability. Part r«o.
Design for Reliability
«97 ‘Development Guide for Reliability, Part Three,
Reliability prediction
138 «Development Guida for Reliability, Part Four.
#eflability Measurement
199 ‘Development Guide for Reliability. Part five,
Contracting for Reliability
200 *D»velopment Guide for Reliability, Pa-- 5^,
Mathematical Appendix and Glossary
201 ‘Rotorcraft Engineering. Part jne. PreHm^'y
Design
202 •Rotorcraft Engineering, Part Two. Detail
Design
203 ‘Rotorcraft Engineering. Part three. Qualifica-
tion Assurance
205 ‘Timing Systems and Components
210 Fuzes
211(C) Fuzes. Proximity, Electrical, Par* One (U)
2I2<S) Fuzes, "’reitmity, Electrical. Part Two (U)
2I3(S) Fuaes, Proximity. Electrical, Part Three (U)
214(S) fu«s. Proximity, Electrical, Part Four (U)
2154/ Fuirs. Proximity, Electrical, Part Five (U)
23$ ‘Hardening Weapon Systems Against RF Energy
239(5) ‘Small Arms Ammunition fu;
2*C(S) Grenaues (u)
24|(s/ «Lend Mines (’J,
•'*2 Design for Control Of Projectile Flight
Characteristics (REPLACES -2*6)
244 Aew^nltion, ^action I, Artillery Aaumnitlon*-
Generai, with ’able of Contents, Glossary,
and Index for Senes
245(C) АмшпШоп, Section 2, Design for Terminal
Effects (U)
24b ♦AawMiit’cn, Section 3, Design for Control of
Flight Characteristics (REPLACED 8* -242)
24 7 Amnuni t’on, Section 4, Design for Projection
228 •Ammnlti<->, Section 5, Inspection Aspects of
Artillery Ambition design
249 АмшлШог, Section 6, Manufacture of Metallic
Components o* Artillery Ammunition
250 Gun$--General
251 Muzzle Devices
252 Gun Tubes
255 Spectral Characteristics of Mu;yle Has"
2bQ Automatic weapons
270 Propellant Actuated Devices
2B0 Design of AerodywicaDy Stabilized Fr-e
Rockets
28l(SR0) Weapon System Ef»ect’veness (u)
282 ♦Propulsion and P’c^el’anrs (REPLACED 8т *28$)
*8 J Aerodynamics
284(C) Trajectories -’ul
285 E’emants of Aircraft and Missile Propulsion
REPLACES -2И?'
286 Structures
2904: Hrh*ads--Gene'al '>;
‘91 3urface-lb-Atr Missiles, Part One, System
IcUgrtt’On
292 uce-to-Air iles. Part Two, weapon
Control
29J Surface-to-Air missiles. Part Three. CompuUrs
294,$) Surfpre-to-Air Missiles, Part Four, Missile
Armameri t ' у ’
295'$) Surface-to-4'r M titles. ₽art «i«, Co-nter
«measures
296 Surface-to-Air miss lies, ®tirt Structure:
and Рсл<ц Sources
2*»7;$) Sur*ace-U-Air hiss-les. ₽*r| Sever, Sample
Proci** I о)
)/’ firr Cor.t*bl 5yste«H--General
J29 F-*e Luntrol Сотри*mg Systems
y\i Compensating
JJSlSRC) ‘Det’(P E -1 nears’ fU-dear Effects Manual,
volume I, Munitions and weapon S.s* 'O’;
336(SRD; ‘Dt-iigr. Engineer 4wtlear Effects Manual,
tpium» II, EUcTHjric }»s!ems and i ogiiTicai
Systems (U)
337'SRD) *Des> Ш Engineers hud«ar Effects Ha«ua’
•olume HI, huclear Environment ;u)
3Jet SRC' ‘Desid* Engined’ s' hue Tear (< ectt Manual.
Уа1цм* |y, Nudeer i*4<ts 'u:
i4u Carriages a*>d Mounts--Genera‘
34i Cradles
342 Recoil Systems
J43 To' Cer/»oges
344 te. о* Carrtages
345 touli U'dtors
346 Elevating >tocnanis*s
34J Traversing Me<h*n;
360 wheeled Ampniciani
35f. Autoaet've Atimuti!/
356 Automotive ksponsiivs
35/ AyUeetive Bodies an»-
•UNDER PREPAKATlOH--not jval’abie
♦OBSDLETE-.Qut о» stock
••REVISION UNDtt PREPARATION