Author: Gupta VC. Tuohy M.G. Kubiсek K.P. Saddler D. Xu F.
Tags: bioenergy
ISBN: 978-0-444-59561-4
Year: 2014
Text
BIOENERGY
RESEARCH:
ADVANCES AND
APPLICATIONS
Edited by
VIJAI K. GUPTA, MARIA G. TUOHY, CHRISTIAN P. KUBICEK,
JACK SADDLER, FENG XU
AMSTERDAM • BOSTON • HEIDELBERG • LONDON • NEW YORK • OXFORD
PARIS • SAN DIEGO • SAN FRANCISCO • SYDNEY • TOKYO
Elsevier
225, Wyman Street, Waltham, MA 02451, USA
The Boulevard, Langford Lane, Kidlington, Oxford OX5 1GB, UK
Radarweg 29, PO Box 211, 1000 AE Amsterdam, The Netherlands
Copyright Ó 2014 Elsevier B.V. All rights reserved.
No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means
electronic, mechanical, photocopying, recording or otherwise without the prior written permission of the publisher
Permissions may be sought directly from Elsevier’s Science & Technology Rights Department in Oxford, UK: phone (+44) (0) 1865
843830; fax (+44) (0) 1865 853333; email: permissions@elsevier.com. Alternatively you can submit your request online by
visiting the Elsevier web site at http://elsevier.com/locate/permissions, and selecting Obtaining permission to use Elsevier
material
Notice
No responsibility is assumed by the publisher for any injury and/or damage to persons or property as a matter of products
liability, negligence or otherwise, or from any use or operation of any methods, products, instructions or ideas contained in the
material herein. Because of rapid advances in the medical sciences, in particular, independent verification of diagnoses and drug
dosages should be made
British Library Cataloguing in Publication Data
A catalogue record for this book is available from the British Library
Library of Congress Cataloging-in-Publication Data
A catalog record for this book is available from the Library of Congress
ISBN: 978-0-444-59561-4
For information on all Elsevier publications
visit our web site at store.elsevier.com
Printed and bound in Great Britain
14 15 16 17 10 9 8 7 6 5 4 3 2 1
Preface
The finite nature of fossil fuels and the emission of
greenhouse gases as result of the consumption, these
resources provide the impetus to seek alternative sources of clean energy, which can be produced in a sustainable manner. This important quest underpins the
essential requirement for research and development on
various types of bioenergy. Bioethanol production has
been the focus of considerable research in the context
of liquid fuels for transportation. The use of starchbased (first-generation) agricultural products as
substrates as bioethanol feedstocks is possible but raises
some concerns because of potential competition with
food production. Although numerous investigations
on bioenergy have been performed over the past
decades to clarify the potential of, and to develop
processes for the use of agricultural crops and biomass
as feedstock for fuel and energy, the recent period has
seen a renewed intensity of research on biomass to bioenergy conversion technologies and processes, with the
aim of developing economical and sustainable solutions
at commercial scale. To support economic sustainability,
biorefinery systems have been implemented to convert
renewable materials, such as wood or agricultural crops,
into additional valuable products such as platform and
feedstock chemicals, and pharma compounds. It is
envisaged that the biorefinery concept should enable
a transition from the traditional fossil fuel-based platforms for production of commodity products to more
environmentally favorable and sustainable bio-based
processes. For researchers and industrialists alike, the
biorefinery approach brings both significant scientific
and technical challenges and much opportunity for technological innovation.
Second-generation bioenergy uses the lignocellulose
present in woody biomass, forestry residue, agricultural residues, food wastes, agricultural wastes and
animal wastes. Agricultural residues include the straw
from wheat and rice, sugar cane bagasse, stem and
roots from food crops, the top ends of trees like eucalyptus not used in paper manufacture, and fast developing tall grasses (e.g. Miscanthus spp., coastal
grasses, etc.). A detailed understanding of the composition of the lignocellulosic waste is essential to
develop and optimize mechanistic models for its
conversion. Inclusion of pretreatment processes to aid
the integration of waste streams into the raw materials
for ethanol plants in such models is essential to
increase both fuel (ethanol)/bioenergy yields, recover
valuable coproducts and biorefinery feedstocks, as
well as to reduce process costs. Hydrolysis of lignocellulosic materials is the first step for either digestion to
biogas (methane) or fermentation to ethanol. Hydrolysis using enzymes (generally derived from microbial
sources) is the preferred option as enzymes can be
used to selectively convert carbohydrate-rich biopolymers in biomass to fermentable sugars, without formation of by-products that inhibit downstream bioenergy
and biorefinery conversion processes. However,
pretreatment of the lignocellulose to reduce its recalcitrance to enzymatic and microbial conversion is essential. Pretreatment by physical, chemical or biological
means is an essential process for ethanol production
from lignocellulosic materials. Pretreatment also
enhances the biodegradability of the wastes for ethanol
and biogas production and increases accessibility of
the enzymes to the biopolymers present in the
biomass/waste feedstocks. Research is necessary to
improve process efficiencies in the areas of pretreatment and bioconversion, and to explore new technologies for conversion of lignocellulose to bioenergy.
Similarly, the major challenge for microalgal biodiesel
production is the high cost of producing microalgal
biomass, and the current significant environmental,
safety and sustainability concerns surrounding the
recovery and extraction of lipid fractions used for biodiesel production. In this sector, the key issues to be
solved are the costs for harvesting the algae, protection
of the high-oil microalgae from the contamination by
other algae, and the development of environmentally
and operationally more benign extraction processes.
Another important issue for both lignocellulosic
ethanol and microalgal biodiesel processes involves
the development of technologies for the utilization of
coproducts and residues formed through primary
bioconversion processes which should increase overall
process economics. Utilization of each fraction in
biomass agricultural wastes provides an effective way
to minimize environmental pollution, address food
security problems and improve agricultural waste
management approaches.
ix
x
PREFACE
This book focuses on current innovative methods and
technological developments which are aimed at overcoming the bottlenecks in biofuel and bioenergy
processes. Reviews of the potential of lignocellulosics
for the production of (bio)chemicals are also included.
Chapters on biorefining routes resulting in a product
with higher market value than ethanol have been
included. It is envisaged that once such approaches
have reached viable commercial scale, global dependence on petroleum for a host of products used in
day-to-day applications will be reduced, and a more
sustainable global bioeconomy will result.
Editors
Foreword
Our present industrial civilization relies on the
consumption of enormous amounts of energy and
much of today’s economic wealth is based on a petroleum-based economy. Petroleum not only is used as
energy in transport but also is the starting material of
many other products of our daily life including such
diverse products as plastics, pharmaceuticals, solvents,
fertilizers, pesticides and clothing up to the tarmac,
which we use for the transport of these products.
However, our continued reliance on fossil fuels will
make it impossible to reduce greenhouse gas emissions
to stop environmental problems such as global warming. Without decisive actions, the global usage of energy
and energy-related emissions of carbon dioxide is predicted to double by 2050. Although there is an active
debate when the demand for oil will exceed its supply
(Peak Oil), it is clear that our present economic system
will need a major shift to develop effective alternatives
including a more sustainable economy. This sustainable
development will be based on renewable energy and
biomass sources as well as more efficient ways to use
these.
Traditionally, biomass has been used to produce food,
feed and wood fiber. But biomass can also provide
energy in the form of (bio)fuels and it can be used as
a source of feedstock chemicals replacing the petroleum-based products. The development of such a biobased economy is occurring already at a relatively rapid
pace and some of its products are already on the market
including first-generation biofuels. The commercial
viability of this approach will depend largely on the
availability of cost-competitive technologies capable of
converting (waste) biomass within a holistic concept of
a biorefinery to biofuels and other bio-based products.
Biorefiningdthe sustainable processing of biomass
into food/feed ingredients, chemicals, materials and
bioenergydaims to use the available biomass resources
as efficient as possible. At the moment, a wide range of
biomass conversion technologies are under development to improve efficiencies, lower costs along the
whole supply chain and improve the environmental
performance. But there is also a need for further technological innovation leading to more efficient and cleaner
conversion of a more diverse range of feedstocks. These
include not only existing lignocellulosic waste residues
from forestry, agriculture and urban communities but
also the generation of new feedstocks from energy crops
or microalgae. A first wave of cellulosic biofuels demonstration plants is now reaching completion producing
transportation fuels from agro-, forestry and process
residues. To make the overall process more market
competitive, these plants co-produce added-value biobased products thereby supplying processes that are
less energy or chemically intensive compared to their
petroleum-based counterparts.
Increasing deployment of biomass will include also
other challenges for our society including an increasing
competition for land, questions of biodiversity and soil
quality or the availability of water resources. But
biomass will be an important part of the future energy
mix thereby contributing to a low CO2 future. Excluding
biomass from the energy mix would significantly
increase the cost of decarbonizing our energy system.
This book has been initiated to describe the current
stage of knowledge on bioenergy research from various
perspectives, thereby outlining also those areas where
further progress is needed.
Dr. Bernhard Seiboth
Professor, Head of Molecular Biotechnology, Vienna
University of Technology, Vienna, Austria
xi
List of Contributors
Bruno C. Aita Department of Chemical Engineering, Federal
University of Santa Maria, Santa Maria, Brazil
Y. Allahverdiyeva Department of Biochemistry, University
of Turku, Turku, Finland
Samuel Amartey Division of Biology, Imperial College of
Science, Technology and Medicine, South Kensington,
London, UK
M. Anusree Biotechnology Division, National Institute for
Interdisciplinary Science and Technology (NIIST), CSIR,
Trivandrum, Kerala, India
E.M. Aro Department of Biochemistry, University of Turku,
Turku, Finland
Rama Raju Baadhe Department of Biotechnology, National
Institute of Technology, Warangal, Andhra Pradesh, India
Mikhail Balakshin
Prussia, PA, USA
Renmatix, R&D Department, King of
Ciarán John Forde AER BIO, National Institute for Bioprocessing Research & Training (NIBRT), Blackrock, Co.
Dublin, Ireland
Michael P. Garver Department of Paper and Bioprocess
Engineering, College of Environmental Science and
Forestry, State University of New York, Syracuse, NY, USA
Juliana M. Gasparotto Department of Chemical Engineering, Federal University of Santa Maria, Santa Maria,
Brazil
Maria Gavrilescu Department of Environmental Engineering and Management, Gheorghe Asachi Technical
University of Iasi, Iasi, Romania; Academy of Romanian
Scientists, Bucharest, Romania
Nishant Gopalan Biotechnology Division, National Institute
for Interdisciplinary Science and Technology (NIIST), CSIR,
Trivandrum, Kerala, India
Alex Berlin Novozymes, Protein Chemistry Department,
Davis, CA, USA
Vipin Gopinath Biotechnology Division, National Institute
for Interdisciplinary Science and Technology (NIIST),
CSIR, Trivandrum, Kerala, India
Susan Boland AER BIO, National Institute for Bioprocessing
Research & Training (NIBRT), Blackrock, Co. Dublin,
Ireland
Richard J.A. Gosselink Food and Biobased Research, Wageningen UR, Wageningen, The Netherlands
John Bosco Carrigan AER BIO, National Institute for Bioprocessing Research & Training (NIBRT), Blackrock, Co.
Dublin, Ireland
Maria Aparecida F. Cesar-Oliveira Research Center in
Applied Chemistry, Department of Chemistry, Federal
University of Paraná, Curitiba, Paraná, Brazil
Daniel P. Chielle Department of Chemical Engineering,
Federal University of Santa Maria, Santa Maria, Brazil
Rhykka Connelly UT Algae Science and Technology
Facility, University of Texas at Austin, Austin, TX, USA
Claudiney S. Cordeiro Research Center in Applied Chemistry, Department of Chemistry, Federal University of
Paraná, Curitiba, Paraná, Brazil
Ed de Jong
Netherlands
Avantium
Chemicals,
Amsterdam,
The
Kiran S. Dhar Biotechnology Division, National Institute for
Interdisciplinary Science and Technology (NIIST), CSIR,
Trivandrum, Kerala, India
Hanshu Ding Department of Protein Chemistry, Novozymes
Inc., Davis, California, USA
Thaddeus Chukwuemeka Ezeji The Ohio State University,
Department of Animal Sciences and Ohio State Agricultural
Research and Development Center (OARDC), Wooster, OH,
USA
Tingyue Gu Department of Chemical and Biomolecular
Engineering, Ohio University, Athens, OH, USA
Vijai K. Gupta Molecular Glycobiotechnology Group,
Department of Biochemistry, School of Natural Sciences,
National University of Ireland Galway, Galway, Ireland
Patrick C. Hallenbeck Département de Microbiologie et
Immunologie, Université de Montréal, Montréal, Québec,
Canada
Daniel J. Hassett Department of Molecular Genetics,
Biochemistry and Microbiology, University of Cincinnati,
College of Medicine, Cincinnati, OH, USA
Alan Hernon AER BIO, National Institute for Bioprocessing
Research & Training (NIBRT), Blackrock, Co. Dublin, Ireland
Charles Hyland Department of Civil & Environmental
Engineering, The University of Auckland, Auckland, New
Zealand
Tao Jin Key Laboratory of Pollution Processes and Environmental Criteria (Ministry of Education), College of Environmental Science and Engineering, Nankai University,
Tianjin, China
Vasiliki Kachrimanidou Department of Food Science and
Human Nutrition, Agricultural University of Athens,
Athens, Greece
Rodrigo Klaic Department of Chemical Engineering,
Federal University of Santa Maria, Santa Maria, Brazil
xiii
xiv
LIST OF CONTRIBUTORS
Nikolaos Kopsahelis Department of Food Science and
Human Nutrition, Agricultural University of Athens,
Athens, Greece
Shirley Nakagaki Research Center in Applied Chemistry,
Department of Chemistry, Federal University of Paraná,
Curitiba, Paraná, Brazil
S.N. Kosourov Department of Biochemistry, University of
Turku, Turku, Finland
K.
Apostolis A. Koutinas Department of Food Science and
Human Nutrition, Agricultural University of Athens,
Athens, Greece
Christian P. Kubicek Research Area Biotechnology and
Microbiology, Institute of Chemical Engineering, TU Wien,
Gumpendorferstrasse Wien, Austria
Jyothi Kumaran Human Health Therapeutics, National
Research Council Canada, Ottawa, ON, Canada; School of
Environmental Sciences, University of Guelph, Guelph,
ON, Canada
Gustavo B. Leite Département de Microbiologie et
Immunologie, Université de Montréal, Montréal, Québec,
Canada
Madhavan Nampoothiri Biotechnology Division,
National Institute for Interdisciplinary Science and Technology (NIIST), CSIR, Trivandrum, Kerala, India
W.J. Oosterkamp
Netherlands
Oosterkamp Oosterbeek Octooien, The
Anthonia
O’Donovan Molecular
Glycobiotechnology
Group, Department of Biochemistry, School of Natural
Sciences, National University of Ireland Galway, Galway,
Ireland
Irmene Ortı́z Departamento de Procesos y Tecnologı́a, Universidad Autónoma Metropolitana - Cuajimalpa, México
D.F., México
Ravichandra Potumarthi Department of Chemical Engineering, Monash University, Clayton, Victoria, Australia
Wensheng Qin Department of Biology, Lakehead University, ON, Canada
Xiangling Li Aquatic and Crop Resource Development,
National Research Council Canada, Ottawa, ON, Canada;
College of Chinese Medicine, Guangzhou University of
Chinese Medicine, Guangzhou, China
Rodolfo Quintero Departamento de Procesos y Tecnologı́a,
Universidad Autónoma Metropolitana - Cuajimalpa,
México D.F., México
Shijie Liu Department of Paper and Bioprocess Engineering, College of Environmental Science and Forestry,
State University of New York, Syracuse, NY, USA
Nasib Qureshi United States Department of Agriculture,
National Center for Agricultural Utilization Research,
ARS, Bioenergy Research, Peoria, IL, USA
Fan Lu College of Bioengineering, Hubei University of
Technology, Wuhan, Hubei Province, China
Luiz P. Ramos Research Center in Applied Chemistry,
Department of Chemistry, Federal University of Paraná,
Curitiba, Paraná, Brazil
Miranda Maki Department of Biology, Lakehead University,
ON, Canada
Nirupama Mallick Agricultural and Food Engineering
Department, Indian Institute of Technology, Kharagpur,
West Bengal, India
Shovon Mandal Section of Ecology, Behavior and Evolution,
University of California, San Diego, CA, USA
Marcio A. Mazutti Department of Chemical Engineering,
Federal University of Santa Maria, Santa Maria, Brazil
Mark P. McHenry School of Engineering and Information
Technology, Murdoch University, Perth, Western Australia,
Australia
Marie Meaney AER BIO, National Institute for Bioprocessing Research & Training (NIBRT), Blackrock, Co.
Dublin, Ireland
Naveen Kumar Mekala Department of Biotechnology,
National Institute of Technology, Warangal, Andhra
Pradesh, India
Clive Mills AER BIO, National Institute for Bioprocessing
Research & Training (NIBRT), Blackrock, Co. Dublin,
Ireland
Jéssica M. Moscon Department of Chemical Engineering,
Federal University of Santa Maria, Santa Maria, Brazil
Adrian Muller Research Institute of Organic Farming FiBL,
Zurich, Switzerland; Institute for Environmental Decisions,
Swiss Federal Institutes of Technology (ETH), Zurich,
Switzerland
Gabrielly V. Ribeiro Department of Chemical Engineering,
Federal University of Santa Maria, Santa Maria, Brazil
Paulo R.S. Salbego Department of Chemical Engineering,
Federal University of Santa Maria, Santa Maria, Brazil
Ajit K. Sarmah Department of Civil & Environmental Engineering, The University of Auckland, Auckland, New
Zealand
Gauri Dutt Sharma
garh, India
Bilaspur University, Bilaspur, Chattis-
Dong Shen Tong Research Group for Advanced Materials &
Sustainable Catalysis (AMSC), Breeding Base of State
Key Laboratory of Green Chemistry Synthesis Technology,
College of Chemical Engineering and Materials Science,
Zhejiang University of Technology, Hangzhou, Zhejiang,
China
Fabiane M. Stringhini Department of Chemical Engineering,
Federal University of Santa Maria, Santa Maria, Brazil
Maria G. Tuohy Molecular Glycobiotechnology Group,
Department of Biochemistry, School of Natural
Sciences, National University of Ireland Galway, Galway,
Ireland
Victor Ujor The Ohio State University, Department of
Animal Sciences and Ohio State Agricultural Research
and Development Center (OARDC), Wooster, OH, USA
Luiz J. Visioli Department of Chemical Engineering, Federal
University of Santa Maria, Santa Maria, Brazil
LIST OF CONTRIBUTORS
Hongyu Wang Key Laboratory of Pollution Processes and
Environmental Criteria (Ministry of Education), College of
Environmental Science and Engineering, Nankai University, Tianjin, China
Colin Webb School of Chemical Engineering and Analytical
Science, University of Manchester, Manchester, England,
United Kingdom
Lin Mei Wu Research Group for Advanced Materials &
Sustainable Catalysis (AMSC), Breeding Base of State Key
Laboratory of Green Chemistry Synthesis Technology,
College of Chemical Engineering and Materials Science,
Zhejiang University of Technology, Hangzhou, Zhejiang,
China
Fernando Wypych Research Center in Applied Chemistry,
Department of Chemistry, Federal University of Paraná,
Curitiba, Paraná, Brazil
Feng Xu Department of Protein Chemistry, Novozymes Inc.,
Davis, California, USA
Trent Chunzhong Yang Aquatic and Crop Resource Development, National Research Council Canada, Ottawa, ON,
Canada
Jie Yang Key Laboratory of Pollution Processes and Environmental Criteria (Ministry of Education), College of
xv
Environmental Science and Engineering, Nankai University, Tianjin, China
Yanbin Yin Department of Biological Sciences, Northern
Illinois University, DeKalb, IL, USA
Wei Hua Yu Research Group for Advanced Materials &
Sustainable Catalysis (AMSC), Breeding Base of State Key
Laboratory of Green Chemistry Synthesis Technology,
College of Chemical Engineering and Materials Science,
Zhejiang University of Technology, Hangzhou, Zhejiang,
China
Chun Hui Zhou Research Group for Advanced Materials &
Sustainable Catalysis (AMSC), Breeding Base of State Key
Laboratory of Green Chemistry Synthesis Technology,
College of Chemical Engineering and Materials Science,
Zhejiang University of Technology, Hangzhou, Zhejiang,
China; The Institute for Agriculture and the Environment,
University of Southern Queensland, Queensland,
Australia
Minghua Zhou Key Laboratory of Pollution Processes and
Environmental Criteria (Ministry of Education), College of
Environmental Science and Engineering, Nankai University, Tianjin, China
C H A P T E R
1
Current Bioenergy Researches: Strengths
and Future Challenges
Naveen Kumar Mekala 1, Ravichandra Potumarthi 2,*,
Rama Raju Baadhe 1, Vijai K. Gupta 3
1
Department of Biotechnology, National Institute of Technology, Warangal, Andhra Pradesh, India,
Department of Chemical Engineering, Monash University, Clayton, Victoria, Australia, 3Molecular Glycobiotechnology
Group, Department of Biochemistry, School of Natural Sciences, National University of Ireland Galway, Galway, Ireland
*Corresponding author email: ravichandra.potumarthi@monash.edu; pravichandra@gmail.com
2
O U T L I N E
Introduction
Different Forms of Bioenergy
1
3
Biopellets
3
Bioethanol
Feedstock for Bioethanol
Pretreatment of Lignocelluloses
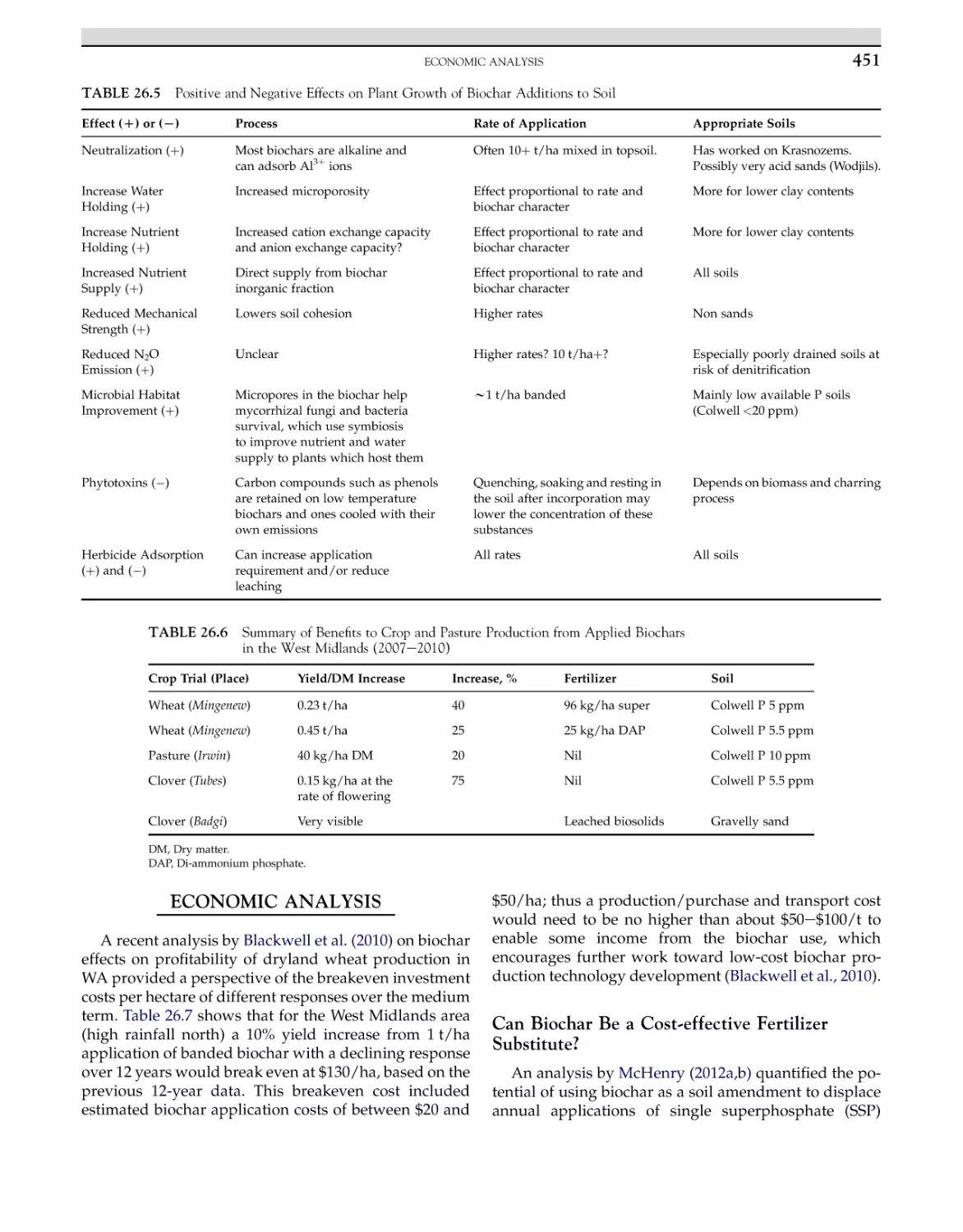
Biological Pretreatment
Physical Pretreatment
Chemical
Pretreatment
Bioethanol Fermentation
Molecular Biology Trends in Bioethanol
Production Development
Bioreactors in Ethanol
Production
Immobilization of Cells for Ethanol
Production
3
3
4
5
6
6
7
8
8
9
10
10
11
12
12
13
Biogas
Biogas Feedstock
Household Digesters for Biogas
Fixed Dome Digesters
Floating Drum Digesters
Social and Environmental Aspects of Biogas Digesters
14
15
15
15
16
17
Conclusion
17
References
18
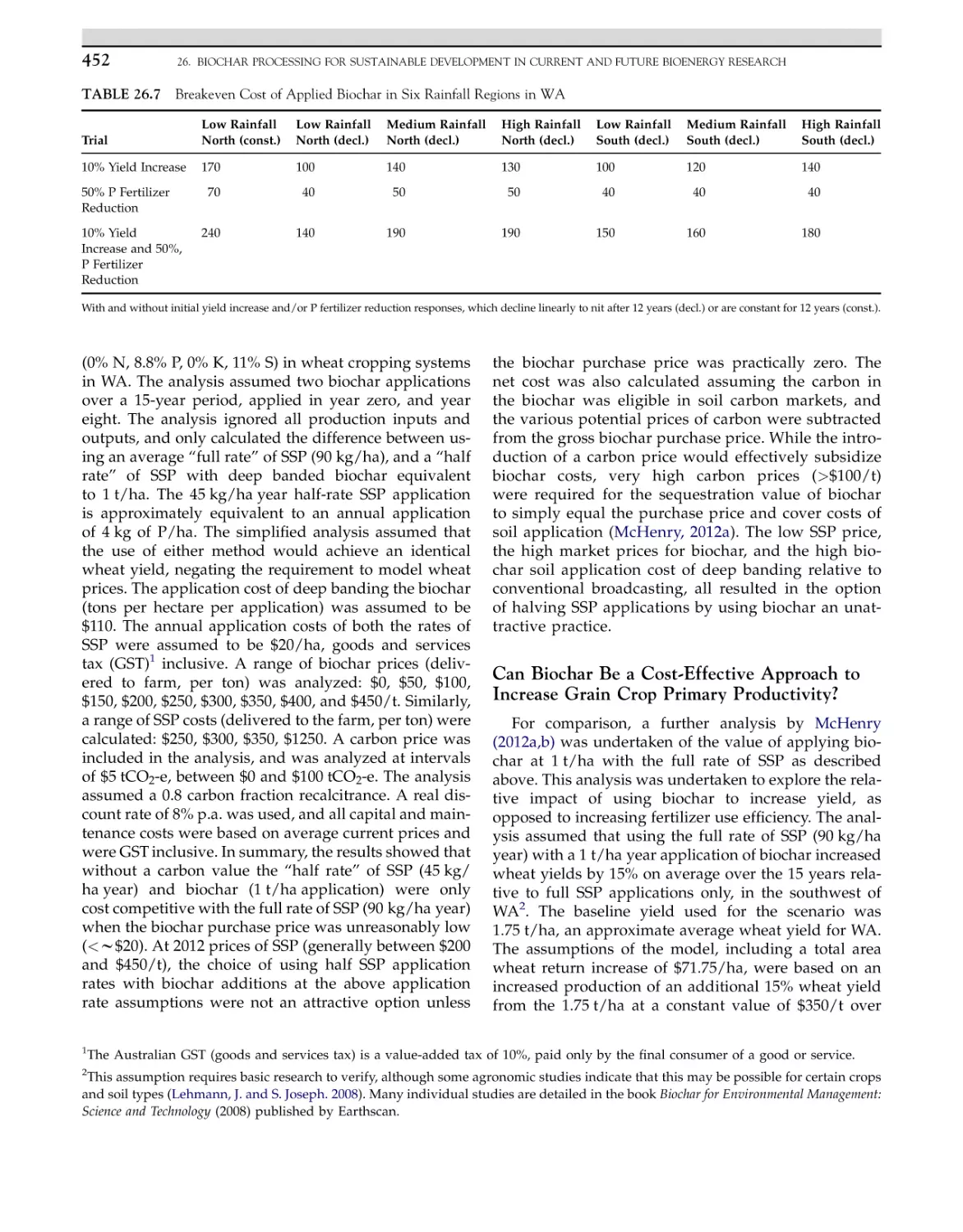
9
INTRODUCTION
rate of consumption, crude oil reserves, natural gas
and liquid fuels were expected to last for around 60
and 120 years, respectively (British Petroleum Statistical Review, 2011). An additional challenge with fossil
fuel consumption is emission of greenhouse gases
(GHGs). In 2010, an average of 450 g of CO2 was
emitted by production of 1 kWh of electricity from the
coal (Figure 1.1) (International Energy Agency Statistics, 2012). It is also clear that coal’s share of the global
Modern world is facing two vital challenges, energy
crisis and environmental pollution. Energy is a key
component for all sectors of modern economy and
plays an elementary role in improving the quality of
life (US DOE, 2010). In current situations, approximately 80% of world energy supplies rely on rapidly
exhausting nonrenewable fossil fuels. At the current
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00001-2
Biodiesel
Feedstocks for Biodiesel
Biodiesel from Pure Vegetable Oil
Biodiesel from Animal Fat Wastes
Other Waste Cooking Oils
Algae as a Biodiesel Source
Bioreactors for Biodiesel Production
1
Copyright Ó 2014 Elsevier B.V. All rights reserved.
2
1. CURRENT BIOENERGY RESEARCHES: STRENGTHS AND FUTURE CHALLENGES
Energy production
Level : World
Legend
Mtoe :
[ 2 347.03 ; 2 347.03 ]
[ 1 993.36 ; 2 347.03 ]
[ 1 605.20 ; 1 993.36 ]
[ 1 253.92 ; 1 605.20 ]
[ 1 160.87 ; 1 253.92 ]
[ 1 066.08 ; 1 160.87 ]
[ 727.64 ; 1 066.08 ]
[ 63.86 ; 727.64 ]
FIGURE 1.1 Global energy production chart signifies the growing demand for energy. Source: IEA, 2012. (For color version of this figure, the
reader is referred to the online version of this book.)
energy continues to rise, and by 2017 coal will come
close to surpassing oil as the world’s top energy source.
China and India lead the growth in coal consumption
over the next 5 years. Research says China will surpass
the rest of the world in coal demand during the outlook
period, while India will become the largest seaborne
coal importer and second largest consumer, surpassing
the United States (IEA, 2012).
Growing global energy needs, release of environmental pollutants from fossil fuels and national security
have finally tuned the attention in clean liquid fuel as a
suitable alternative source of energy. The alternative bioenergy sources, not only cut the dependence on oil trade
and reduce the doubts caused by the fluctuations in oil
price, but also secure reductions in environmental pollution due to their high oxygen content (Huang et al., 2008;
Boer et al., 2000).
In this context, the availability of bioenergy in its two
main appearances, wood and agro energy can offer
cleaner energy services to meet basic energy requirements. This century could see a remarkable switchover
from fossil fuel-based energy to bioenergy-based economy, with agriculture and forestry as the main sources
of feedstock for biofuels such as wood pellets, fuelwood, charcoal, bioethanol, and biodiesel (Agarwal,
2007). Moreover, energy crops can be part of highly
specialized and various agricultural production chains
and biorefineries, where a variety of bioproducts could
be obtained besides bioenergy, which are important for
their economic competitiveness (United Nations Environment Program, 2006).
The exploitation of currently unused by-products
and growing energy crops can address other existing
environmental concerns. Perennial energy crops and
plantations are generally characterized by higher biodiversity compared with conventional annual crops. By
providing more continuous soil cover, they reduce the
impact of rainfall and sediment transport, thereby preventing soil erosion. The introduction of annual energy
crops into crop systems allows for diversification and
expansion of crop rotations, replacing less favorable
monocropping systems (Kheshgi et al., 1996). Deforested, degraded and marginal land can be rehabilitated
with bioenergy plantations, thus helping to combat
desertification and hopefully reducing market and geosocial pressures on high-quality arable land.
Biofuels can be obtained in bulk when they are derived
from agricultural crops, crop residues and processing
wastes from agroindustries, forests, etc. Despite this
immense potential, existing biofuel policies have been
very costly; they produce slight reductions in fossil fuel
use and increase, rather than decrease, in GHG emissions
(Wuebbles and Jain, 2001). However, recent volatility and
rise in international fossil fuel prices, make biomass
increasingly competitive as energy feedstock.
Current bioenergy research around the globe
should direct us toward reduced production cost, higher
energy conversion efficiency and greater costeffectiveness of biofuels. After all we are aware of a fact
“use of biomass as a potentially large source of energy in
the 21st century will have a significant impact in rural,
agricultural and forestry development” (UNEP, 2006).
3
BIOETHANOL
Different Forms of Bioenergy
Organic matter holding bioenergy sources in side is
known as biomass. We can utilize this biomass in
many different ways, through something as simple as
burning wood for heat, or as complex as growing genetically modified microbes to produce cellulosic ethanol
(Adler et al., 2009). Since nearly entire bioenergy can
be traced back to energy from sunlight, bioenergy has
the key advantage of being a renewable energy source.
Here, in this chapter we will discuss various forms of
bioenergy and their application in detail.
BIOPELLETS
Today, wood pellets are an imperative and wellaccepted fuel in lots of different countries and the
according markets are likely to rise even further in future.
For these reasons, it is feared that the inadequate availability of cheap wood as a feedstock for pellets will limit
this market increase (Marina et al., 2011; Larsson et al.,
2008). As alternative, autumn leaves from urban areas,
as a seasonal available waste material, are the possible
substitutes for or additives to wood. In lot of Western
countries, wood pellets become a more and more significant fuel for the use in small furnaces for household
buildings or in industries as a climate-neutral alternative
to crude oil or natural gas (Verma et al., 2012; Nielsen
et al., 2009). This pelletized biomass has a number of
advantages like tolerance against microbial degradation,
high transport and storage density of bioenergy, and the
process of pelletization is quite simpler (Figure 1.2).
BIOETHANOL
Bioethanol is the most common biofuel worldwide.
It is produced by simple fermentation of sugars derived
from wheat, corn, sugar beets, sugarcane, molasses and
any sugar or starch sources that alcoholic beverages can
be made from (Cara et al., 2008). Bioethanol can be used
in petrol engines as a substitute for gasoline. Bioconversion of lignocellulosics into fermentable sugars is a biorefining area in which enormous research labors have
been invested, as it is a prerequisite for the subsequent
bioethanol production (Broder et al., 1992). Although
extensive research has been carried out to meet the
potential challenges of bioenergy generation, there is
no self-sufficient process or technology available today
to convert the lignocellulosic biomass to bioethanol
(Tu et al., 2007).
Use of bioethanol-blended fossil fuel for automobiles
can significantly cut the petroleum use and exhaust
GHG emission. Bioethanol can be produced from
different kinds of raw materials and these raw materials
are classified into three categories of agricultural raw
materials: simple sugars, starch and lignocelluloses
(Mustafa and Havva, 2009). Bioethanol from sugarcane,
under proper conditions, is essentially a clean fuel and
has several advantages over petroleum-derived gasoline
in reducing GHG emissions and improving air quality in
metropolitan cities. Conversion technologies for producing bioethanol from cellulosic biomass resources such as
forest materials, agricultural residues and urban wastes
are under development and have not yet been established commercially (Demirbas, 2008).
Feedstock for Bioethanol
Across the globe, there is a rising need to find out new
and cheap carbohydrate sources for bioethanol production (Mohanty et al., 2009). Presently, a serious focus is
on biofuels made from renewable energy crops such as
sugarcane, corn, wheat, soybeans, etc. In a given production line, the comparison of the biomass includes several
issues: (1) cultivation practices, (2) chemical composition of the biomass, (3) use of resources, (4) emission
of GHGs, (5) availability of land and land use practices,
(6) soil erosion, (7) energy balance, (8) price of the
(b)
(a)
Species 1
Species 2
...
Drying
Milling
Conditioning
Species n
Pelletizing
Leaf mixture
Analyzing
Leaf pellets
FIGURE 1.2 (a) Experimental flow sheet for pelletization of leaves; (b) leaf pellets. (For color version of this figure, the reader is referred to
the online version of this book.)
4
1. CURRENT BIOENERGY RESEARCHES: STRENGTHS AND FUTURE CHALLENGES
FIGURE 1.3 Major lignocellulosic feedstock
explored for bioethanol production. Source:
Taherzadeh and Karimi, 2007.
biomass, (9) contribution to biodiversity and landscape
value losses, (10) direct economic value of the feedstock,
(11) water requirements and water availability, (12) creation or maintain of employment, and (13) logistic cost
(transport and storage of the biomass) (Gnansounou
et al., 2005).
Bioethanol feedstock can be divided into three major
groups: (1) sugar-based feedstock (e.g. sugarcane, beet
sugar, sorghum and fruits), (2) starchy feedstock (e.g.
corn, sweet potato, rice, potatoes, cassava, wheat and
barley), and (3) lignocellulosic feedstock (e.g. wood,
straw, grasses, and corncob). In short term, production
of bioethanol as a fuel is almost entirely dependent on
starch and sugars from existing food crops (Smith,
2008; Potumarthi et al., 2012). The negative part in producing bioethanol from starch and sugar is that the feedstock tends to be costly and demanded by other
applications as well (Enguidanos et al., 2002). Lignocellulosic biomass is envisaged to provide a major portion
of the raw materials for bioethanol production in the
long term due to its low cost and high availability
(Gnansounou et al., 2005).
Up to 2003, about 60% of global bioethanol was obtained from sugarcane and 40% from all other crops
(Dufey, 2006). Brazil utilizes sugarcane for bioethanol
production, while the United States and other western
countries mainly use starch from corn, wheat and barley
(Linde et al., 2008). Brazil is the largest producer of sugarcane with about 672,157,000 tons of global production
followed by India, second largest producer with
285,029,000 ton production (Food & Agricultural Organization of United Nations, 2013).
Bioethanol production in Brazil is less expensive than
in the United States from corn or in Europe from sugar
beet, because of lower labor costs, shorter processing
time, lower transport costs, and other input costs. After
sugarcane, starch is the high-yield feedstock for bioethanol production, but pretreatment is necessary to
produce bioethanol by fermentation (Pongsawatmanit
et al., 2007). Starch is a homopolymer consisting monomers of D-glucose and for bioethanol production it is
necessary to break down this carbohydrate for obtaining
glucose syrup, which can be further transformed into
bioethanol by yeasts. Starch-based feedstock are the
most utilized for bioethanol production in America
and Europe.
Biomass from agricultural waste (wheat straw, sugarcane bagasse, etc.), wood, and energy crops are attractive materials for bioethanol production since it is the
most abundant reproducible assets on earth (Figure 1.3).
The existing biomass from crops could produce up to
442 American billion liters per year of bioethanol
(Bohlmann, 2006). Thus, the total possible bioethanol
production from crop residues and wasted crops is
491 American billion liters per year, about 16 times
higher than the existing world bioethanol production.
Advantages of biofuels are as follows: (1) biofuels are
easily available from common biomass sources, (2) biofuels have a considerable environmentally friendly potential, and (3) they are biodegradable and contribute
to sustainability (Balat, 2007; Mekala et al., 2008).
Although lignocellulosic biomass is the best alternative
source, initial pretreatment is a must to attain simple
sugars for simultaneous ethanol fermentation.
Pretreatment of Lignocelluloses
Woody materials including bark, wood, and mixture
of other residues from the forest contain cellulose, hemicelluloses, lignin and small amount of other biomass
5
BIOETHANOL
of these lignocelluloses separates the sugars and lignin
and disrupts the crystalline portion of the biopolymers
(Hu et al., 2008). Different pretreatment methods have
been explored for achieving the optimistic situations
with different biomass.
In general, pretreatment methods can be divided into
biological pretreatment, physical pretreatment, and
chemical pretreatment according to the pretreatment
procedure. Some pretreatments combine two or more
of broadly explored methodologies. Table 1.1 recaps
some of the broadly explored pretreatment methods as
per the classification (Sun and Cheng, 2002).
FIGURE 1.4 Chemical composition of lignocellulosic biomass (SW,
soft wood; HW, hard wood).
contents (Figure 1.4). Cellulose is the major component
in plant biomass and it is made of anhydroglucopyranose or glucose residues, which can be converted to
glucose and act as major source of hexoses in woody
feedstock (Alvira et al., 2010). Due to the hydrogen
bonds in it, cellulose is a highly crystalline structure
and it is difficult to hydrolyze. Unlike cellulose, hemicelluloses are heteropolymers composed of both fivecarbon sugars and six-carbon sugars, including glucose,
mannose, arabinose, xylose and others (Bochek, 2003).
Due to its amorphous structure, hemicellulose is easily
breakable by dilute acid or base. Lignin is the third major part in wood and comprises the glue that guards
woody biomass from pathogens. Lignin mainly consists
of phenolic units and with available technology we
cannot use lignin as a source of bioethanol. Pretreatment
Biological Pretreatment
Most pretreatment technologies require selected and
expensive amenities or equipment that have high power
requirements, depending on the process. In particular,
physical and chemical processes require rich energy for
biomass conversion, whereas, biological treatment via
microorganisms is a safe and environmentally friendly
method and is increasingly being promoted as a process
that does not require high energy, even for lignin
removal from a lignocellulosic biomass (Okano et al.,
2005; Potumarthi et al., 2013; Ravichandra et al., 2013).
Phanerochaete chrysosporium is one among the best
microbial models to study the lignin degradation by
white rot fungi. Fungi breaks down lignin anaerobically through a family of extracellular enzymes collectively termed as lignases (Howard et al., 2003). Two
families of lignolytic enzymes are generally considered to play vital role in the enzymatic degradation:
peroxidases (lignin peroxidase) and phenol oxidase
TABLE 1.1 Pretreatment Methods of Lignocellulosic Feedstock
Energy
Pretreatment
Source
Means
Effect
Biological pretreatment
Microorganisms
Actinomysis, fungi
Removes lignin and reduces the degree
of polymerization (DP) of celluloses
Physical pretreatment
Comminution
Ball milling, compression milling, colloidal
milling
Decreases the particle size, crystallinity
and DP of cellulose
Steam explosion
High-pressure steam
Partially hydrolyze cellulose and
hemicelluloses
Ultrasonic radiation
Electron beam, gamma rays, microwave
Increases the surface area and softens the
lignin
Acid
Hydrochloric acid, hydrofluoric acid, nitric
acid, sulfuric acid, peracetic acid, etc.
Alkaline
Sodium hydroxide, sodium carbonate,
ammonia, ammonium sulfate, lime, etc.
Decreases in crystallinity and DP of
cellulose; partial or complete
degradation of hemicellulose;
delignification
Gases
Chlorine dioxide, nitrogen dioxide
Cellulose solvents
DMSO, cadoxen, CMCS
Chemical pretreatment
Source: Moiser et al., 2005.
6
1. CURRENT BIOENERGY RESEARCHES: STRENGTHS AND FUTURE CHALLENGES
(Malherbe and Cloete, 2003). Other enzymes are not
fully explored including glucose oxidase, methanol oxidase, glyoxal oxidase, and oxidoreductase (Eriksson,
2000). Another best example was Trichoderma reesei,
which is a mesophilic cellulolytic fungus isolated in
the mid-1950s. By the mid-1970s, an impressive collection of more than 14,000 cellulolytic fungi were isolated
against cellulose and other insoluble fibers (Coyne et al.,
2013). Trichoderma reesei, although a good producer of
hemi and cellulolytic enzymes, is unable to degrade
lignin (Mekala et al., 2008; Gupta et al., 2013).
Actinomycetes are also best tested for their task in
lignin biodegradation. Lignolytic enzymes like peroxidases, ligninase and manganese peroxidase were
discovered in P. chrysosporium (Saritha et al., 2012).
Based on this, P. chrysosporium was taken up for biological delignification of wood and paddy straw in ethanol
production. But, the extent of delignification was inadequate to expose a significant portion of cellulose for
enzymatic hydrolysis. Thus, from the reports available,
it is evident that white rot fungi and actinomycete can
be used jointly to remove lignin from lignocellulosic
substrates, and further studies are required to shorten
the incubation time and to optimize the delignification
process.
Physical Pretreatment
MECHANICAL COMMINUTION
The objective is to cut the particle size and crystallinity of lignocellulosic biomass in order to increase
the surface area and reduce the degree of polymerization. Methods like chipping, grinding and milling can
be used to improve the further enzymatic hydrolysis.
However, this process is not economically feasible
due to the high energy requirement (Tassinari et al.,
1980). During comminution, vibratory ball milling is
found to be more efficient in breaking down the cellulose molecules of spruce and aspen chips and
improving the digestibility of the biomass than ordinary ball milling (Sun and Cheng, 2002). The power
requirement of mechanical comminution of agricultural
materials depends on the final particle size and the
waste biomass characteristics.
STEAM EXPLOSION
It is a hydrothermal pretreatment in which the lignocellulose is subjected to pressurized water vapors for
few seconds to several minutes, and then suddenly depressurized. In this process, combination with the partial
hydrolysis of hemicelluloses and solubilization, the
lignin is redistributed and removed up to certain level
from the material (Pan et al., 2005). Although this technique is cost-effective, it generates toxic by-products
and the hemicelluloses degradation is partial (Saritha
et al., 2012).
ULTRASONIC PRETREATMENT
This technique is extensively used for the treatment of
sludge from wastewater treatment plants. An experiment on carboxyl methyl cellulose with irradiation
increased the rate of enzymatic hydrolysis up to 200%
approximately (Imai et al., 2004). The mechanism of action in ultrasonic treatment remains unknown. One
guess is that, the hydrogen bonds in the crystalline cellulose structures were broken due to irradiation energy,
whose energy is higher than the hydrogen bond energy
(Bochek, 2003).
EXTRUSION
This process disrupts the crystal structure of lignocellulose and increases the accessibility of carbohydrates to
enzyme. In this case, materials are subjected to heating,
mixing and shearing resulting in physical and chemical
modifications in biomass structure (Karunanithy et al.,
2008). However, the process is novel and not widely
applied.
Chemical Pretreatment
ACID HYDROLYSIS
During acid hydrolysis, concentrated acids like HCl
and H2SO4 have been used to pretreat lignocellulosic
biomass. Although acids are influential agents for cellulose hydrolysis, intense acids are poisonous, corrosive,
and require chemical reactors that are resistant to corrosion. In addition, concentrated acid must be removed after hydrolysis of celluloses into simple sugars, which
simultaneously enter into alcoholic fermentation
(Potumarthi et al., 2013; Ravichandra et al., 2013). Hydrolysis using dilute acid has been industriously developed
for pretreatment of lignocellulosic biomass (O’Donovan
et al., 2013). The dilute sulfuric acid pretreatment can
attain high reaction rates and drastically improve cellulose hydrolysis. Dilute acids at lower temperatures,
saccharification suffered from low yields because of
sugar decomposition (Chen et al., 2009). High temperatures, with dilute acids are favorable for cellulose hydrolysis. In recent times, dilute acid hydrolysis processes use
less harsh environment and achieve high xylan to xylose
conversion rates. Achieving high xylan to xylose conversion yields is required to achieve favorable economics,
because xylan is the third most promising carbohydrate
in many lignocellulosic feedstocks (Sun and Cheng,
2002). Primarily two types of dilute acid pretreatment
processes
are
well
studied:
high-temperature
(T > 160 C), continuous flow process for low solids
loading (5e15% (weight of biomass/weight of reaction
mixture)) (Converse et al., 1989), and low-temperature
(T < 160 C), batch process for high solids loading
(10e40%) (Esteghlalian et al., 1997). Although dilute
acid hydrolysis can significantly improve the cellulose
7
BIOETHANOL
breakdown, overall cost is typically higher when
compared with few other physicochemical pretreatment
processes such as steam explosion.
ALKALINE HYDROLYSIS
Usually alkaline hydrolysis was carried out at low
temperature and pressure and it may be completed
even at ambient conditions. The only drawback of this
process is time; it might be hours or even days to complete the hydrolysis. During lime pretreatment, some calcium is tainted into nonrecoverable salts or included in
the biomass (Chang et al., 2001). Other alkaline pretreatment methods include calcium, potassium, sodium and
ammonium hydroxides as reactants based on biomass
category. Among these reactants, sodium hydroxide receives the most attention followed by lime, due to its
advantage of being low cost and secure to use, as well
as it is easily recoverable from water as insoluble
CaCO3 by reaction with CO2. Further delignification of
feedstocks can be enhanced by supplying surplus air/
oxygen (Hu et al., 2008). We can compare alkaline pretreatment of feedstocks to Kraft pulping, where lignin
was removed efficiently, thus improving the reactivity
of polysaccharides. Alkaline hydrolysis also effectively
removes acetyl groups and uronic substitutions from
hemicellulose; thus, the surface of hemicellulose becomes
more accessible to the hydrolytic enzymes.
AMMONIA HYDROLYSIS
Ammonia has abundant desirable characteristics as a
pretreatment reagent. It is a valuable swelling reagent
for lignocellulosic biomass, and ammonia has high
selectivity for reactions with lignin over those with carbohydrates (Kim et al., 2003). It is one of the most extensively used commodity chemicals with about one-fourth
the cost of sulfuric acid on molar basis. Its high volatility
makes it easy to recover and recycle. It is a nonpolluting
and noncorrosive chemical. One of the known reactions
of aqueous ammonia with lignin is cleavage of ether
(CeOeC) bonds in lignin as well as ester bonds in the
ligninecarbohydrate complex (Lewin and Roldan,
1971). This above reaction indicates that ammonia pretreatment selectively cuts the lignin content in biomass.
Lignin is believed to be a major hindrance in enzymatic
hydrolysis and there are several advantages by
removing lignin early in the conversion process before
it faces the biological treatment.
OZONOLYSIS
Ozone is a leading oxidant that demonstrates high
delignification efficiency. This ozonolysis is done at
room temperature and at normal pressure. In this case
we do not locate any inhibitory by-products, which
affect the simultaneous fermentation steps (Saritha
et al., 2012). An important drawback is ozone requirement in large quantities, which can make the process
economically unapproachable (Sun and Cheng, 2002).
Bioethanol Fermentation
Once the lignocelluloses were hydrolyzed into simple
sugars, they have to be fermented to ethanol. The hydrolyzate now contains various hexoses and pentoses, mainly
glucose and xylose, depending on the substrate and the
pretreatment method applied. Currently, fermentation
of simple sugars is mostly done using yeast cultures
(Saccharomyces cerevisiae), because of its well-known characteristics, toughness and high ethanol yield. However, S.
cerevisiae can only ferment hexoses and not the pentoses.
The pentose sugars can be fermented in an additional
step by another microorganism or by S. cerevisiae itself
through genetic engineering approaches, so that it is
able to ferment pentoses as well (Van Zyl et al., 2007).
List of most popular yeast strains used for ethanol fermentation are mentioned in Table 1.2. Besides a high yield, an
important aspect with fermentation is alcohol tolerance in
the fermenting organisms. A strategy to defeat this crisis
is to have a system where the ethanol is recovered at regular intervals to keep the alcohol concentrations under
control. Another problem is inhibitory compounds that
TABLE 1.2 Yeast Species That Produce Ethanol as the Main Fermentation Product
Strain/Species
Temperature
( C)
pH
Carbon Source/Concentration
(g/l)
Incubation
Time (h)
Ethanol Concentration
Produced (g/l)
27817- S. cerevisiae
30
5.5
Glucose/(50e200)
18e94
91.8
L-041- S. cerevisiae
30e35
e
Sucrose/(100)
24
50
ATCC 24860-S. cerevisiae
30
4.5
Molasses/(1.6e5.0)
24
18.5
Bakers’ yeastdS. cerevisiae
28
5.0
Sucrose/(220)
96
96.71
CMI237- S. cerevisiae
30
4.5
Sugar/(160)
30
70
27774- Kluyveromyces fragilis
30
5.5
Glucose/(20e120)
18e94
48.6
Source: Lin and Tanaka, 2006.
8
1. CURRENT BIOENERGY RESEARCHES: STRENGTHS AND FUTURE CHALLENGES
TABLE 1.3
Comparison between Biodiesel and Petroleum Diesel
Advantages
(1)
(2)
(3)
(4)
(5)
(6)
(7)
Domestically produced from nonpetroleum, renewable resources
Can be used in most diesel engines, especially in recent ones
Less air pollutants (other than nitrogen oxides)
Less greenhouse gas emissions (e.g. B20 reduces CO2 by 15%)
Biodegradable
Nontoxic
Safer to handle
are produced during the pretreatment. As mentioned
above they can be reduced by an additional detoxification
step, but this is an expensive operation (Van Maris et al.,
2006).
Molecular Biology Trends in Bioethanol
Production Development
In the last few years technologies breakthrough has
compelled us for an alternative feedstock due to considerable shortage in agricultural land. In this sense, advances in metabolic pathway engineering/genetic
engineering have led to the development of microbes
skilled enough to convert biomass into ethanol (Das
Neves et al., 2007). Generally, such development depends on expansion of the substrate range and inclusion
of other biomass sources like arabinose or xylose in
strains that cannot ferment sugars other than glucose.
Examples of such microorganisms include genetically
modified Escherichia coli, Saccharomyces sp., and Zymomonas mobilis, etc. (Davis et al., 2006).
In cellulosic ethanol industry, aside from Pichia stipitis,
natural xylose fermenting yeast, more efforts are being
taken in obtaining recombinant bacterial and yeast strains
that are able to ferment pentose sugars, such as arabinose
and xylose. Figure 1.5 is one among the best examples
depicting recombination process in microbes, where the
tail end in E. coli and Klebsiella oxytoca or the front end of
S. cerevisiae and Z. mobilis can be recombined for improved
production of ethanol (Hagerdal et al., 2006).
Disadvantages
(1)
(2)
(3)
(4)
(5)
(6)
Use of blends above B5 not yet approved by many auto makers
Lower fuel economy and power (10% lower for B100, 2% for B20)
Currently more expensive
B100 generally not suitable for use in low temperatures
Concerns about B100’s impact on engine durability
Slight increase in nitrogen oxide emissions possible in some
circumstances
Moreover, genetic engineering of plants is another
promising area, which most likely plays a key role in biofuel industry. The latest hybrid varieties have helped us
considerably in improving starch yield from energy
crops. For example, 25 kg of corn contains about 15 kg
of starch. In the near future, that same 25 kg may contain
as much as 17 kg of starch through hybrid corn. This
would result in a gain of nearly $2 million in annual income by processing the same amount of corn in a
120 million liter per year ethanol production (DOE, 2007).
Bioreactors in Ethanol Production
A major commitment in cost-effective lignocellulosic
bioethanol production is to employ reactor systems
yielding the maximal cellulose conversion with the minimal enzyme. As a result, one of the most vital parameters for the fabrication and operation of bioreactors for
lignocellulosic conversion is the efficient use of the enzymes to gain high specific rates of cellulose conversion
(yield of glucose attained/amount of enzymes). The
maximization of the product concentration, i.e. the
amount of glucose obtained per liquid volume, is also
a significant parameter as well as the optimization of
the volumetric productivity.
When hydrolysis is carried out with biomass comprised
of high cellulose levels, the product concentration will
drive up. For this reason, few researchers are attempting
the enzymatic biomass conversion with high biomass
loads (Jorgensen et al., 2007). The most imperative
FIGURE 1.5 Strains that can be metabolically
engineered for ethanol production. Source: Hagerdal
et al., 2006. (For color version of this figure, the reader
is referred to the online version of this book.)
9
BIODIESEL
difficulty in high biomass loads is related to the viscosity of
reaction mixture, which also influences the rheology of the
mixture. In particular, mixing and mass transfer limitations and presumably increased inhibition by intermediates come into play. A variety of fed-batch strategies have
been adopted with the scope of supplying the substrate
without reaching excessive viscosities and unproductive
enzyme binding to the substrate (Rudolf et al., 2005).
General criteria in bioreactor design and in the choice
of the operating conditions could be use of bioreactors or
reaction regimes that allow a rapid decrease in the
glucose concentration; running of the reactions at low
to medium substrate concentrations in order to maintain
higher conversion rates and thus obtain higher volumetric output of the reactor (Andric et al., 2010).
The combination of the bioreactor with a separation
unit has obtained prospective results with product
inhibited or equilibrium limited enzyme-mediated conversions, because it potentially removes the products as
they are accumulated (Gan et al., 2002). In this regard,
membrane bioreactors could be a feasible process
configuration. Unlike the Solid State Fermentation
(SSF) approach in which the glucose consumption is carried out by the microbes simultaneously accessible in
the hydrolyzate, the use of membrane bioreactors would
finish the same function without any compromise in the
reaction parameters. A membrane bioreactor (Figure 1.6)
is a multitasking reactor that combines the reaction with
a separation, namely, in this case the product was taken
away by membrane separation, as one integrated unit
(in situ removal) or alternatively in two or more separate
units. The membrane bioreactors used for this separation processes are mainly ultra- and nanofiltration types
(Pinelo et al., 2009). However, the use of this technology
is restricted by the accumulation of unreacted lignocellulosics in large level and/or continuous processing
(Andric et al., 2010). Already in the past, few scientists
enhanced the efficiency of the continuous stirred tank
bioreactor by incorporating membrane separation technologies during the reactor design.
Recently, an advanced reactor system was intended
that removes the reducing sugars during the enzymatic
hydrolysis of cellulose through a system consisting in a
tubular reactor, in which the substrate was retained
with a porous filter at the bottom and buffer entered at
the top through a distributor (Yang et al., 2006). This hollow fiber ultrafiltration module with polysulfone membrane enabled the permeation and the separation of the
sugars. To keep the volume constant in the tubular reactor,
the entire buffer was recycled back from the ultrafiltration
membrane and the makeup buffer was continuously supplied from the reservoir. In some applications an additional microfiltration unit has exceptionally been used to
retain the unconverted lignin-rich solid fraction due to
the presence of firmly bound enzymes or has been
employed to remove the unconverted substrate from the
reactor. These setups result in slightly complex process
layouts for the hydrolysis (Knutsen and Davis, 2004).
It is obvious that the optimization of the reactor designs
will allow overcoming both the rheological and inhibition
limit of the bioconversion and maximizing the enzymatic
conversion. Therefore, the reactor design becomes more
relevant for large-scale processing of cellulosic biomass.
Immobilization of Cells for Ethanol Production
For bioreactor application, immobilization of cells is a
technique that has proved augmented ethanol productivity, operation stability and easier downstream processing, compared to processes using suspended cells (Das
Neves et al., 2007). However, the specific advantages of
immobilized cells depend on the nature of cells, reactor
design and nature of the process. Entrapment of cells in
natural polymers by ionic gelation (alginate) or by thermal precipitation (carrageenan and agar) is a method
commonly used for cell immobilization (Ogbonna et al.,
1991). Immobilization by passive adhesion to surfaces
has great potential for industrial application since the
immobilization method is relatively simple. The use of
cheap carriers ensures that this method can be exploited
with minimal increase in the overall production cost.
Thus, one limiting factor of this technology is that it can
only be adapted for practical industrial production if
the expected increase in bioethanol productivity can
overcome the increase in the production costs (cost of
the carrier and immobilization) (Ogbonna et al., 1996).
BIODIESEL
FIGURE 1.6 Schematic of membrane bioreactor integrated with
membrane distillation (MD) process for alcohol distillation. Source:
Gryta, 2012. (For color version of this figure, the reader is referred to
the online version of this book.)
Biodiesel is a form of diesel fuel manufactured from
vegetable oils, animal fats, or recycled restaurant
greases. It is safe, biodegradable, and produces less air
10
1. CURRENT BIOENERGY RESEARCHES: STRENGTHS AND FUTURE CHALLENGES
pollutants than petroleum-based diesel. Biodiesel can be
used in its pure form (B100) or blended with petroleum
diesel. Common blends include B2 (2% biodiesel), B5,
and B20.
Biodiesel is an ideal biofuel contender that eventually could replace petroleum based diesel. Currently,
biodiesel production is still too costly to be
commercialized. Due to the static cost associated with
oil extraction and biodiesel processing and the
variability in biomass production, future cost-saving
efforts for biodiesel production should focus on the
production of oil-rich feedstocks like microalgae,
nonedible oils, etc.
As discussed above, biodiesel is costlier than conventional diesel fuel, although it is rarely quoted as being
competitive, as it will be if existing fluctuations in feedstocks/product prices are favorable. Using the distribution of these prices over the last 20 years, less than 5% of
costebenefit analyses based on fixed prices over the
project life will show a positive result in producing biodiesel. If the feedstocks/product prices are varied each
year, as will be the case in reality, biodiesel production
will always be more expensive than conventional diesel
(Duncan, 2003).
Feedstocks for Biodiesel
Biodiesel can be made from any oil/lipid source; the
major components of these sources are triacylglycerol
molecules. In general, biodiesel feedstocks can be categorized into three groups: pure vegetable oils, animal
fats, and waste cooking oils.
TABLE 1.4
Biodiesel from Pure Vegetable Oil
The first group is pure oils derived from various
crops and plants such as soybean, canola (rapeseed),
corn, cottonseed, flax, sunflower, peanut, and palm.
These are the most widely used feedstocks by commercial biodiesel producers. The oil composition from vegetable crops is pure; this cuts down on preprocessing
steps and makes for a more consistent quality of biodiesel product. However, there is an obvious disadvantage for vegetable oils as biodiesel feedstocks: wide scale
production of crops for biodiesel feedstocks can cause
an increase in worldwide food and commodity prices.
Such a “food vs fuels” debate has reached national attention when using vegetable oils for biodiesel production.
Alternative feedstocks usually arise out of necessity
from regions of the world where the above materials
are not locally available or as part of a concerted attempt
to reduce reliance on imported petroleum.
JATROPHA CURCAS (JATROPHA)
The nonedible oil from Jatropha curcas (Jatropha) has
recently attracted extensive attention as a feedstock for
biodiesel production in India and other climatically parallel regions of the world (Kumartiwari et al., 2007;
Kalbande et al., 2008). The Jatropha tree is a perennial
shrub belonging to the Euphorbiaceae family whose
seeds contain up to 30 wt% oil. This plant can be found
in tropical and subtropical regions such as Africa, Indian
subcontinent, Central America, and other countries of
Asia. Since Jatropha oil contains a relatively elevated
percentage of saturated fatty acids (Table 1.4), the corresponding methyl esters display relatively poor low
Biodiesel Production from Feedstocks High in Free Fatty Acids
Feedstock
FFA (wt%)
Pretreatment
Catalyst for Transesterification
Yield (wt%)
References
Pongamia pinnata
Up to 20
H2SO4
KOH
97
Naik et al. (2008)
Jatropha curcas
14/<1
H2SO4
KOH
99þ
Kumartiwari et al. (2007)
Madhuca indica
20
None
Pseudomonas cepacia
96þ**
Kumari et al. (2007)
Nicotiana tabacum
35/<2
H2SO4
KOH
91
Veljkovic et al. (2006)
Calophyllum inophyllum
22/<2
H2SO4
KOH
85
Sahoo et al. (2007)
Zanthoxylum
bungeanum
45.5/1.16*
None
H2SO4
98
Zhang and Jiang (2008)
Brown grease
40/<1
Diarylammonium
catalysts
NaOCH3
98þ**
Ngo et al. (2008)
Waste cooking oil
7.25/<1*
H2SO4
NaOH
90**
Meng et al. (2008)
Waste fryer grease
5.6
H2SO4
KOH
90þ
Issariyakul et al. (2007)
Sorghum bug oil
10.5
None
H2SO4
77e94
Mariod et al. (2006)
* Acid value (mg KOH/g) was given instead of FFA.
** Conversion to esters (wt%) is provided instead of yield.
BIODIESEL
temperature operability, as evidenced by pour point
(PP) value of 2 C (Kumartiwari et al., 2007).
PONGAMIA PINNATA (KARANJA)
Another nonedible biomass originated in India is
Pongamia pinnata (Karanja), which is a medium-sized deciduous plant that grows fast in damp and subtropical
environments and matures in 5e7 years to tender fruit
that contains two kidney-shaped kernels (Mohibbeazam
et al., 2005). The oil content of Karanja kernels ranges between 25 wt% and 40 wt% (Karmee et al., 2005;
Mohibbeazam et al., 2005). The primary fatty acid found
in Karanja oil is oleic acid (45e70 wt%), followed by
palmitic, linoleic, and stearic acids (Karmee et al., 2005;
Naik et al., 2008). The low-temperature operability of
the parallel methyl esters from karanja is superior to
that of jatropha oil methyl esters as a result of the fairly
high percentage of oleic acid in karanja oil, as evidenced
by cloud point (CP) and PP values of 2 C and 6 C,
respectively (Srivastava and Verma, 2008).
MADHUCA INDICA (MAHUA)
Madhuca indica, commonly known as “Mahua”, is a
tropical plant found frequently in the central and northern plains and forests of India. It belongs to the family
Sapotaceae and grows rapidly up to 20 m in height, possesses evergreen or semievergreen foliage, and is well
adapted to dry environments (Ghadge and Raheman,
2006; Kumari et al., 2007). The fruit is nonedible,
obtained from the tree in 4e7 years and contains one to
two kidney-shaped kernels (Mohibbeazam et al., 2005).
The oil content of dried Mahua seeds is about 50 wt%.
Mahua oil is characterized by free fatty acid (FFA) content of around 20 wt% and a comparatively high percentage of saturated fatty acids such as stearic (14.0 wt%)
and palmitic (17.8 wt%) acids (Ghadge and Raheman,
11
2006). The remaining fatty acids are mostly spread
among unsaturated components such as linoleic
(17.9 wt%) and oleic (46.3 wt%) acids (Singh and Singh,
1991). The relatively high percentage of saturated fatty
acids (35.8 wt%) found in Mahua oil results in relatively
poor low-temperature properties of the parallel methyl
esters, as evidenced by PP value of 6 C (Ghadge and
Raheman, 2006).
NICOTIANA TABACUM (TOBACCO)
Nicotiana tabacum, commonly referred as tobacco, is a
commercial shrub with pink flowers and green capsules
containing abundant small seeds grown in a large number of countries around the world. The foliage of the
plant is the commercial product and used in the preparation of cigarettes and other tobacco-containing products. The oil content of the seeds, a by-product from
tobacco, ranges from 36 wt% to 41 wt% (Usta, 2005).
This tobacco seed oil contains more than 17 wt% FFAs
(Veljkovic et al., 2006) and is high in linoleic acid
(69.5 wt%), along with oleic (14.5 wt%) and palmitic
(11.0 wt%) acid in significant amounts. Due to high linoleic acid content of tobacco seed oil, the corresponding
methyl esters display relatively low kinematic viscosity
(3.5 mm2/s) in comparison to most other biodiesel fuels
(Usta, 2005).
Biodiesel from Animal Fat Wastes
The feedstock issues are very critical, which affect the
economic potential of biodiesel production, since feedstock accounts around 75% of the biodiesel total cost
(Figure 1.7). Recently, alternative lipid residues such as
waste frying oil and nonedible animal fats have also
received substantial attention from the biofuel sector.
To take benefit of these low-cost and low-quality
resources, a suitable act would be to reuse residues in
FIGURE 1.7 Biodiesel production cost summary sheet. Source: Pruszko, 2007. (For color version of this figure, the reader is referred to the
online version of this book.)
12
1. CURRENT BIOENERGY RESEARCHES: STRENGTHS AND FUTURE CHALLENGES
order to integrate sustainable energy supply and waste
management in food processing facilities. Animal fats
are typically considered as waste by-products and less
expensive than commodity vegetable oils, which make
them attractive as feedstock for biodiesel production.
These animal wastes are collected from chicken, cow,
pork lard, and other animals such as fish and insects.
BEEF TALLOW AND CHICKEN FAT
Animal fats like beef tallow and chicken fat are byproducts from the meat industry and stand for cheap
feedstock for biodiesel production. The key fatty acids
found in beef tallow were oleic (47.2 wt%), palmitic
(23.8 wt%), and stearic (12.7 wt%) acids. The prime fatty
acids contained in chicken fat include oleic (40.9 wt%),
palmitic (20.9 wt%), and linoleic (20.5 wt%) acids (Wyatt
et al., 2005). Due to very low concentration of polyunsaturated fatty acid in beef tallow, the corresponding
methyl esters illustrate excellent oxidative stability, as
evidenced by an oil stability index (OSI) value of 69 h
at 110 C. In addition, other physical properties of beef
tallow methyl esters include kinematic viscosity
(40 C) of 5.0 mm2/s, a flash point (FP) of 150 C, and
CP, PP and cold filter plugging point (CFPP) values of
11, 13, and 8 C respectively (Moser, 2009). In chicken
fat, due to high polyunsaturated fatty acid content, the
corresponding methyl esters display poor oxidative stability, as evidenced by an OSI value of 3.5 h at 110 C.
Burning the B20 blends of beef tallow and chicken fat
methyl esters results in NOx exhaust emissions of only
2.4% versus 6.2% of B20 blend of soybean methyl esters
(SME) (Wyatt et al., 2005).
PORK LARD
Pork lard is a by-product of the food industry and
symbolizes a low-cost feedstock for biodiesel production. The main fatty acids in pork lard includes stearic
(121 wt%), linoleic (127 wt%), oleic (44.7 wt%), and palmitic (26.4 wt%) acids (Jeong et al., 2009). Due to high
saturated fatty acid content in pork lard, the corresponding methyl esters exhibit quite high CFPP value of 8 C
and a relatively low iodine value (IV) of 72, along with
a typical kinematic viscosity (40 C) of 4.2 mm2/s.
Another study determined that pork lard methyl esters
have a kinematic viscosity (40 C) of 4.8 mm2/s, FP of
160 C, OSI value of 18.4 h at 110 C, and CP, PP, and
CFPP values of 11, 13, and 8 C, respectively (Wyatt
et al., 2005). Furthermore, combustion of B20 blends of
pork lard methyl esters results in NOx exhaust emissions of only 3.0% versus 6.2% for a B20 blend of SME.
Other Waste Cooking Oils
Waste oils may include a variety of low-worth materials such as used cooking or frying oils, acid oils, tall
oil, vegetable oil soapstocks, and other waste materials.
Waste oils are usually characterized by relatively high
FFA and water contents and potentially contain a variety
of solid materials that must be removed by filtration
prior to conversion to biodiesel (Moser, 2009). In the
case of used cooking or frying oils, hydrogenation to increase the useful cooking lifetime of the oil may result in
the introduction of relatively high-melting trans constituents, which influence the physical properties of the
resulting biodiesel. Used frying or cooking oil is mainly
acquired from restaurants and may cost between free to
50% less expensive than commodity vegetable oils,
depending on the source and the availability (Predojevic, 2008). The physical properties of methyl esters prepared from used cooking or frying oils include
kinematic viscosities (40 C) of 4.23 (Meng et al., 2008),
4.79, and 4.89 mm2/s; FP of 171 C; cetane number of
55, IV of 125, CFPP values of 1 and 6 C (Cetinkaya
and Karaosmanoglu, 2004), CP values of 9 and 3 C,
and PP values of 3 and 0 C (Phan and Phan, 2008).
The disparities in the physical property data among
the various studies may be a result of feedstock origin
or due to differences in product purity.
Algae as a Biodiesel Source
Algae can also be used to produce energy in a number
of ways. One of the most competent ways is through
exploitation of the algal oils to produce biodiesel. Algal
biomass contains three major components: carbohydrates, proteins, and lipids/natural oils (Dunahay
et al., 1996). Because the natural oil made by microalgae
is in the form of triacylglycerol molecule, which is the
right kind of oil for producing biodiesel, microalgae
are the exclusive focus in the algae to biofuel arena.
Actual biodiesel yield per hectare is about 80% of the
yield of the parent crop oil given in Table 1.5.
In view of Table 1.5, microalgae emerged to be the
only source of biodiesel that has the potential to
completely replace petroleum diesel. Unlike other oil
crops, microalgae grow extremely rapidly and many
are exceedingly rich in oil. Microalgae commonly double their biomass within 24 h. Biomass doubling times
during exponential growth are commonly as short as
3e4 h. Oil content in microalgae can exceed 70% by
weight of dry biomass (Metting, 1996; Spolaore et al.,
2006). Oil levels up to 50% are quite common. Oil productivity, the mass of oil produced per unit volume of
the microalgal broth per day, depends on the algal
growth rate and the oil content of the biomass. Microalgae with high oil productivities are desired for producing biodiesel.
CHEMICAL TRANSESTERIFICATION PROCESS
FOR BIODIESEL PRODUCTION
The source oil used in making biodiesel consists of triglycerides (Figure 1.7), in which three fatty acid
BIODIESEL
TABLE 1.5 Comparison between Few Biodiesel Sources
Crop
Oil Yield (l/hectare)***
Land Required (M he)
Corn
172
1540
Soybean
446
594
Jatropha
1892
140
Coconut
2698
99
Oil palm
5950
45
Microalgae*
136,900
2
Microalgae**
58,700
4.5
* About 70% oil (by wt) in biomass.
** About 30% oil (by wt) in biomass.
***
For meeting 50% of all transport fuel needs of the United States.
molecules are esterified with a molecule of glycerol. In
biodiesel production, triglycerides are reacted with
methanol in a reaction known as transesterification or
alcoholysis. Transesterification produces methyl esters
of fatty acids that are biodiesel and glycerol (Figure 1.7).
The reaction occurs stepwise: triglycerides are first converted to diglycerides, then to monoglycerides and
finally to glycerol.
At equilibrium, transesterification needs 3 mol of
alcohol for every mole of triglyceride to produce 1 mol
of glycerol and 3 mol of methyl esters (Figure 1.8). Industrial processes use 6 mol of methanol for each mole
of triglyceride (Fukuda et al., 2001). This large excess
of methanol ensures that the reaction is driven in the direction of methyl esters, i.e. toward biodiesel. Yield of
methyl esters exceeds 98% on a weight basis.
Transesterification is catalyzed by acids and alkalis
(Fukuda et al., 2001). Alkali-catalyzed transesterification
is about 4000 times quicker than the acid-catalyzed reaction. Thus, alkalis such as sodium and potassium hydroxide are frequently used as commercial catalysts at
a concentration of about 1% by weight of oil. Alkoxides
such as sodium methoxide (CH3ONa) act like better catalysts than sodium hydroxide and are being increasingly used. Use of lipases offers significant advantages,
but it is currently not feasible because of the relatively
high cost of the catalyst (Chisti, 2007). Alkali-catalyzed
transesterification is carried out at about 60 C under
one atmospheric pressure, as methanol boils off at
65 C at atmospheric pressure. Under these conditions,
reaction takes about 90 min to complete (Meher et al.,
FIGURE 1.8 Transesterification of oil to biodiesel.
13
2006). A higher temperature can be used in combination
with higher pressure, but the process becomes expensive. During reaction, methanol and oil do not mix;
hence, the reaction mixture shows two liquid phases.
Other alcohols can be used, but methanol is the least
expensive. To stop yield loss due to saponification reactions (soap formation), the oil and alcohol must be dry
and the oil should have a least of FFAs. Biodiesel is
recovered by repeated washing with water to remove
glycerol and methanol.
ENZYMATIC TRANSESTERIFICATION PROCESS
FOR BIODIESEL PRODUCTION
Lipases (triacylglycerol hydrolase, EC 3.1.1.3.) are enzymes that catalyze the breakdown of carboxylic ester
link in the triacylglycerol molecule to form FFAs, diand monoglycerides and glycerol. Although their purpose is to catalyze hydrolysis of ester links, they can
also catalyze the esterification, the conception of this
link between alcohol hydroxyl groups and carboxyl
groups of carboxylic acids. Therefore, they can catalyze
hydrolysis, alcoholysis, esterification and transesterification and they have a wide spectrum of biotechnological applications (Kirk et al., 2002). Lipases are also
highly specific as regio, chemo and enantioselective catalysts. Thanks to protein engineering, it is possible to
enhance catalytic potential of lipases and “tailor” them
to exact application and process situation, enabling
further expansion of their industrial applications (B
van Beilen and Li, 2002). Among lipases from animal,
plant and microbial origins, the most commonly used
are microbial lipases. They have abundant advantages
over lipases from animal and plant sources. Using microbes it is possible to achieve a higher yield of enzymes,
and to genetically control the strain in obtaining a lowcost lipase with preferred properties for the conversion
of fats and oils into biodiesel. In addition, the enzymatic
yield is independent of potential seasonal variations and
it is possible to achieve rapid growth of microbes in lowcost media (Gupta et al., 2004).
Bioreactors for Biodiesel Production
Microalgae are unicellular microscopic organisms,
like simple plants with no leaves and roots that grow
through photosynthesis process. They capture carbon
dioxide during photosynthesis and convert it into feedstock that can be used as food, fertilizer, a source of medicine and biodiesel (Chojnacka and Marquez-Rochaet,
2004).
Growing algae in open pond system raise several concerns such as impossibility to control growth settings
and contamination threats. Algal cells in open ponds
are exposed to the environment, light deficiency, subject
to risk of contamination, and heterogenous medium
14
1. CURRENT BIOENERGY RESEARCHES: STRENGTHS AND FUTURE CHALLENGES
Exhaust gas
Culture medium
Harvest
Airlift system
Air
CO2
Samples
Solar
receiver
FIGURE 1.9 Schematic of tubular photobioreactor with airlift
system. Source: Molina et al., 2001.
depending upon the mixing mechanism, the shape of
the ponds and the depth of the pond (Chojnacka and
Marquez-Rochaet, 2004). On the other hand, closed
ponds (photobioreactors) mitigate fluid culture contamination, and enhance full control over algal growth parameters such as homogenous culture, pH, light
penetration, and carbon dioxide input. They would
use less space with high algal biomass yield. However,
they are costly to build and maintain (Mulumba and
Farag, 2012).
Design of tubular photobioreactor (TPBR) for algal cell
growth was depicted in Figure 1.9. It has a main tank connected to two spiral tubes set in sequence. Both spiral
parts were clear polyvinyl chloride (PVC) tubes of 100
external diameter and 3/400 internal diameter. The capacity of both spiral parts was 3.4 gallons. The main tank
served as a feeding point of medium to the PVC tubes
with a maximum capacity of 5 gallons (Chisti, 2007). Culture medium was pumped into the tubings at a fixed flow
rate. These tubes provided an area of 20 ft2 exposed to the
fluorescence light. Air compressor supplies air to the
TABLE 1.6
system for aeration and to serve as a source of carbon dioxide (CO2). The air flow rate was set in the ranges of
190e210 gallons/h. In TPBR, selected algal strain was
cultured using fresh medium with no modification. Algal
growth and pH were measured over a period of time
varying between 12 days and 14 days. A sample was
taken every 2 days to quantify the turbidity using a spectrophotometer at 682 nm and cell counts were performed
using a microscope. The pH of culture was measured using pH test strips.
The selected algal strain shows the typical growth
curve of other microbes, which include lag, exponential
or log, stationary and lytic phases. The length of each
phase depends on light penetration, nutrients concentration, mixing mechanism, and the solubility of oxygen
in medium. After reaching a stationary or lysis phase,
algal culture was harvested by centrifugation followed
by lyophilization to produce dry algal feedstock. Crude
lipid from dried algal biomass was extracted using
either modified Folch method (Cooksey et al., 1987) or
Soxhlet extractor (Mulumba, 2010; Chojnacka and
Marquez-Rochaet, 2004). In both methods, polar and
nonpolar solvents such as methanol and chloroform/
hexane were used (Table 1.6). The combination of polar
and nonpolar solvents enhances the extraction of both
polar and nonpolar lipid.
BIOGAS
Biogas is obtained by anaerobic digestion (AD) of
organic materials, which occurs inside the anaerobic biodigester. Chemical composition of this biogas depends
on several parameters, such as type of digester
employed, the kind of organic material and the constancy of the feeding process of the biodigester. The
most significant biogas components are methane
(CH4), carbon dioxide (CO2) and sulfuric components
(H2S). The composition of biogas is a crucial parameter,
Biodiesel Production with Various Lipases
Lipase
Source
Acyl Acceptor
Solvent
Yield (%)
References
Candida antarctica B
Waste cooking palm oil
Methanol
tert-butanol
79.1
Halim et al. (2009)
Thermomyces lanuginosus
Soybean oil
Ethanol
n-hexane/solvent free
70e100
Rodrigues et al. (2010)
Pseudomonas fluorescens,
Candida rugosa
Jatropha seed oil
Ethanol
Solvent free
98
Shah and Gupta (2007)
Rhizomucor miehei,
Penicillium cyclopium
Soybean oil
Methanol
Solvent free
68e95
Guan et al. 2010
Candida antarctica
Sunflower oil
Methyl acetate
Solvent free
>95
Ognjanovic et al. (2009)
Thermomyces lanuginosus
Rapeseed oil
Methanol
Solvent free
95
Li et al. (2006)
Candida antarctica
Jatropha seed oil, karanja oil
Ethyl acetate
Solvent free
>90
Modi et al. (2007)
15
BIOGAS
because it allows identifying the suitable purification
system, which aims to remove sulfuric gases and reduce
the water volume, contributing to recover the combustion fuel conditions (Boe et al., 2007). Other important
data collected from biogas analysis is referent to the
low heat value, that combined to the efficiency and
biogas consumption is important to estimate the electric
generation potential. However, biogas production is
much variable because it depends on several parameters, such as the kind of organic material (Liu et al.,
2004). Biogas production involves three steps: fermentation, which includes hydrolysis and acid genesis,
acetone genesis and methane genesis. In the fermentation process, during the hydrolysis the organic material
is converted into smaller molecules and this material is
transformed in soluble acids by acidogenese. Next step
is acetanogenese process, transforming the products obtained in the first step into acetic acid, hydrogen and carbon dioxide. The last step is referent to metanogenese
process, producing methane gas through anaerobic bacteria (Figure 1.10) (Seadi et al., 2008; Boe et al., 2007).
Biogas Feedstock
Awide range of biomass types can be used as substrates
for the production of biogas by AD. The most common
biomass categories used in biogas production are listed
below and in Table 1.7. Animal manure and slurry, agricultural residues and by-products, digestible organic wastes
from food and agro industries, organic fraction of municipal waste and from catering, and sewage sludge, etc. are
best study sources for biogas production.
Recently, various novel feed stocks has been tested
and introduced for biogas synthesis in many countries,
the dedicated energy crops (DECs), crops grown specifically for energy and biogas production. DECs can be
herbaceous (grass, maize, and raps) and also woody
crops (willow, poplar, and oak), although the woody
crops need particular delignification treatment before
AD.
In AD, substrates can be classified according to the
following criteria: methane yield, origin, dry matter
(DM) content, etc. Table 1.7 gives a summary on the
Carbohydrate
Sugar
Household Digesters for Biogas
It is difficult to accept one particular type of digester
for household biogas production. The design of the
digesters is diversified based on the availability of substrate, geographical location, and climatic conditions.
For example, a digester designed in mountainous regions has less gas volume in order to avoid gas loss.
For tropical countries, it is recommended to have digesters underground due to the geothermal energy
(Bin, 1989). Of all the different digesters developed, the
fixed dome model developed in China and the floating
drum model developed in India sustained to perform
well until today (Rajendran et al., 2012). Recently, plug
flow digesters are gaining attention due to its portability
and easy operation.
Fixed Dome Digesters
The fixed dome digesters (Figure 1.11) is also called
“hydraulic” or “Chinese” digesters and it is the most
frequent model developed and used in China for biogas
production (Rajendran et al., 2012). In this case, digester
is filled through the inlet pipe until the level reaches the
base level of the expansion chamber. Biogas that is produced is accumulated at the upper part of the digester
called storage part. The difference in the levels between
the slurry inside the digester and the expansion chamber
develops pressure inside due to accumulation of biogas.
This accumulated biogas requires space and presses the
substrate apart and enters into the expansion chamber.
The slurry flows back into the digester straight away after
Carbon acids,
alcohols
Fats
Fatty acids
Proteins
Amino acids
Hydrolysis
characteristics of some digestible feedstocks. Substrates
with DM content less than 20% are used for what is
called wet digestion (wet fermentation), which includes
animal slurries and manure as well as various wet
organic wastes from food industries. When the DM content is as high as 35%, it is called dry digestion (dry
fermentation), and it is typical for energy crops and silages. The choice of types and amounts of feedstock
for the AD substrate mixture depends on their DM content as well as the content of sugars, lipids and proteins.
Acidogenesis
Hydrogen
carbon dioxide
ammonia
Acetic acid
hydrogen
carbon dioxide
Acetogenesis
Methane
carbon dioxide
Methanogenesis
FIGURE 1.10 Biochemical process in anaerobic digester. (For color version of this figure, the reader is referred to the online version of
this book.)
16
TABLE 1.7
1. CURRENT BIOENERGY RESEARCHES: STRENGTHS AND FUTURE CHALLENGES
Characteristics of Some Digestible Feedstocks
Type of
Feedstock
Organic
Content
C:N
Ratio
DM
(%)
VS% of
DM
Biogas Yield
(m3/kg VS*)
Unwanted Physical
Impurities
Cattle slurry
Carbohydrate,
protein, lipid
6e20
5e12
80
0.20e0.30
Bristles, soil, water,
straw, wood
Poultry slurry
Carbohydrate,
protein, lipid
3e10
10e30
80
0.35e0.60
Grit, sand, feathers
Stomach/intestine
content
Carbohydrate,
protein, lipid
3e5
15
80
0.40e0.68
Animal tissues
Concentrated whey
75e80% lactose
20e25% protein
e
20e25
90
0.80e0.95
Transportation
impurities
Flotation sludge
65e70% proteins
30e35% lipids
e
e
e
e
Animal tissues
Straw
Carbohydrates,
lipids
80e100
70e90
80e90
0.15e0.35
Sand, grit
Food remains
Carbohydrate,
protein, lipid
e
10
80
0.50e0.60
Bones, plastic
* VS - volatile solids
Source: Seadi et al., 2008.
Floating Drum Digesters
gas is released (Adeoti et al., 2000). Fixed dome digesters
are usually built underground and the size of the digester
depends on the place, number of households, and the
amount of substrate available every day. Generally size
of these digesters normally varies between 5 m3 and
150 m3 in various parts of Asia (Tomar, 1994). Instead of
having a digester for each home, a large-volume digester
is used to produce biogas for 10 to 20 homes, and is called
community-type biogas digesters. In countries where
houses are clustered as in Africa, these types of biogas
digesters are more viable (Adeoti et al., 2000).
The floating drum digester was first time constructed
by Khadi and Village Industries Commission and this
model was developed in 1962 (Figure 1.12). Although
the model is old, it is one of the most extensively used
designs for household purposes in India. This design
includes a movable inverted drum placed on a wellshaped digester. The inverted steel drum acts as a storage tank, which can move up and down depending on
the quantity of accumulated biogas at the top of the
digester. The weight of this inverted drum applies the
Outlet for
bio-gas
Slurry of cattle
dung and water
S
Mixing
tank
Slab cover
Dome
M
V
Gas valve
Slab cover
D
Ground
level
Overflow
tank
F
Bio-gas
I
Inlet
chamber
Spent slurry
Dung and water
mixture
T
O
Outlet
chamber
Underground
digester tank
FIGURE 1.11 Schematic sketch of fixed dome digester. Source: GMI, India, 2013. (For color version of this figure, the reader is referred to the
online version of this book.)
17
CONCLUSION
Gas control valve
Gas stove
FIGURE 1.12 Floating drum digester.
Source: Working of biogas plants Working of biogas
plants, 2013, www.tutorvista.com. (For color
version of this figure, the reader is referred to
the online version of this book.)
Gas holder
Mixing tank
Over flow tank
Inlet pipe
Outlet pipe
Digester tank
Inlet tank
Partition wall
pressure needed for biogas flow through the pipeline
(Singh and Sooch, 2004).
Floating drum digesters manufacture biogas at a stable pressure with variable volume. In floating drum
reactor, by position of the drum, the amount of biogas
accumulated under the drum is easily noticeable. However, the floating drum needs to be coated with paint at
regular intervals to avoid rusting. Additionally, fibrous
materials in biomass will block the movement of the
digester. Hence, their accumulation must be avoided if
possible (Adeoti et al., 2000). In Thailand, the floating
dome has been customized with two cement jars on
each side of the floating drum. The average size of these
digesters is around 1.2 m3 (Gosling, 1982). For small and
medium-size farms the size varies from around 5
to 15 m3. Singh and Singh (1991) compared 14
different biogas plants with a floating drum model and
optimized the various parameters for maximum biogas
production.
Social and Environmental Aspects of Biogas
Digesters
Change in the global climate is a major threat that the
world is facing today. The nonrenewable energy consumption in the past has led to global warming that
needs to be addressed (Bilen et al., 2008). The household
digesters could reduce the pressure on the environment
by dropping deforestation and GHG emissions followed
by loss of cultivable land, and soil erosion (Gautam et al.,
2009). Biogas production in rural areas can partly reduce
global warming (Pei-dong et al., 2007). By using biogas
in rural households economical, environmental, and social benefits were achieved (Yang et al., 2011). Even
though both carbon dioxide and methane are major contributors to the greenhouse effect, the global warming
effect of methane is 21 times greater than that of carbon
dioxide (Dhingra et al., 2011). However, houses equipped with biogas systems exhibit leakage of gases in the
biogas systems. Fortunately, the households with biogas
plants have 48% less emissions compared to households
without biogas systems (Pathak et al., 2009). It is worth
talking about 10% of households, which had methane
leakage (Yang et al., 2011). Research has already shown
that by replacing firewood and coal with biogas, the
emission of CO2 and SO2 would be reduced by 4193
thousand tons, and 62.0 thousand tons, respectively
(Pei-dong et al., 2007).
CONCLUSION
We conclude that by accelerating research in areas of
bioenergy, we can make significant contributions to
sustainable development and use of feedstock. We
must realize that by maximizing biomass conversion efficiency, we can minimize raw material requirements,
while at the same time the financial position of various
market sectors (e.g. energy, agriculture, and forestry)
are strengthened. There is an international agreement
on the fact that the feedstock accessibility is inadequate
so that the raw materials should be used as competently as possible, i.e. expansion of multipurpose industries (biorefineries) that can utilize variable
biomass sources as raw materials for bioenergy production. The main constraint in making this biorefinery a
successful path is bringing the stakeholders together,
who normally operate in different market sectors (e.g.
energy, agriculture and forestry, fuel transportation,
etc.). Above all, the government should make policies
to help overcome the threshold by dropping production costs in the form of feedstock in tariffs, feedstock
18
1. CURRENT BIOENERGY RESEARCHES: STRENGTHS AND FUTURE CHALLENGES
in premiums, tax exemptions, etc. These encouragements can be targeted at different parts of the supply
chain like feedstock producers, energy producers, and
distributors.
References
Adeoti, O., Ilori, M.O., Oyebisi, T.O., Adekoya, L.O., 2000. Engineering
design and economic evaluation of a family-sized biogas project in
Nigeria. Technovation 20, 103e108.
Adler, P.R., Sanderson, M.A., Weimer, P.J., Vogel, K.P., 2009. Plant
species composition and biofuel yields of conservation grasslands.
Ecol. Appl. 19, 2202e2209.
Agarwal, A.K., 2007. Biofuels (alcohols and biodiesel) applications as
fuels for internal combustion engines. Prog. Energy Combust. Sci.
33, 233e271.
Alvira, P., Tomas, P.E., Ballesteros, M., Negro, M.J., 2010. Pretreatment
technologies for an efficient bioethanol production process based
on enzymatic hydrolysis: a review. Bioresour. Technol. 101,
4851e4861.
Andric, P., Meyer, A.S., Jensen, P.A., Johansen, K.D., 2010. Reactor
design for minimizing product inhibition during enzymatic
lignocellulose hydrolysis II. Quantification of inhibition and
suitability of membrane reactors. Biotechnol. Adv. 28 (3),
407e425.
Balat, M., 2007. An overview of biofuels and policies in the European
Union countries. Energy Sources Part B 2, 167e181.
Bilen, K., Ozyurt, O., Bakırc, K., Karsl, S., Erdogan, S., Yılmaz, M.,
Comakl, O., 2008. Energy production, consumption, and environmental pollution for sustainable development: a case study in
Turkey. Renewable Sustainable Energy Rev. 12, 1529e1561.
Bin, C., 1989. The current status of agricultural geothermal utilization
in China. Biomass 20, 69e76.
Bochek, A.M., 2003. Effect of hydrogen bonding on cellulose solubility
in aqueous and nonaqueous solvents. Russ. J. Appl. Chem. 76 (11),
1711e1719.
Boe, K., Steyer, J.P., Angelidaki, I. 2007. Monitoring and Control of the
Biogas Process Based on Propionate Concentration Using Online
VFA Measurement. Presented at 11th IWA World Congress on
Anaerobic Digestion, 23e27 September 2007, Brisbane, Australia.
Boer, G.J., Flato, G., Reader, M.C., Ramsden, D., 2000. A transient
climate change simulation with greenhouse gas and aerosol forcing: experimental design and comparison with the instrumental
record for the 20th century. Clim. Dyn. 16, 405e425.
Bohlmann, G.M., 2006. Process economic considerations for production of ethanol from biomass feedstocks. Ind. Biotechnol. 2, 14e20.
British Petroleum Statistical Review, 2011. BP statistical review of
world energy. Available at. http://www.bp.com/en/global/
corporate/about-bp/statistical-review-of-world-energy-2013/
statistical-review-1951-2011.html (Accessed on 10.03.2013).
Broder, J.D., Barrier, J.W., Lightsey, G.R., 1992. Conversion of cotton
trash and other residues to liquid fuel from renewable resources.
In: Cundiff, J.S. (Ed.), Proceedings of an Alternative Energy Conference. American Society of Agricultural Engineers, St. Joseph,
MI, pp. 189e200.
Cara, C., Ruiz, E., Mercedes, B., Paloma, M., Ma, J.N., Eulogio, C.,
2008. Production of fuel ethanol from steam-explosion pretreated
olive tree pruning. Fuel 87 (6), 692e700.
Cetinkaya, M., Karaosmanoglu, F., 2004. Optimization of basecatalyzed transesterification reaction of used cooking oil. Energy
Fuel 18, 1888e1895.
Chang, V.S., Kaar, W.E., Burr, B., Holtzapple, M.T., 2001. Simultaneous
saccharification and fermentation of lime treated biomass. Biotechnol. Lett. 23 (6), 1327e1333.
Chen, M., Zhao, J., Xia, L., 2009. Comparison of four different chemical
pretreatments of corn stover for enhancing enzymatic digestibility.
Biomass Bioenergy 33, 1381e1385.
Chisti, Y., 2007. Biodiesel from microalgae. Biotechnol. Adv. 25,
294e306.
Chojnacka, K., Marquez-Rocha, F.J., 2004. Kinetic and stoichiometric
relationships of the energy and carbon metabolism in the culture of
microalgae. Biotechnology 3, 21e34.
Converse, A.O., Kwarteng, I.K., Grethlein, H.E., Ooshima, H., 1989.
Kinetics of thermochemical pretreatment of lignocellulosic materials. Appl. Biochem. Biotechnol. 20 (21), 63e78.
Cooksey, K.E., Guckert, J.B., Williams, S.A., Callis, P.R., 1987.
Fluorometric determination of the neutral lipid content of
microalgal cells using nile red. J. Microbiol. Methods 6,
333e345.
Coyne, J., Gupta, V.K., O’Donovan, A., Tuohy, M.G., 2013. The role of
fungal enzymes in global biofuel production technologies. In:
Gupta, V.K., Tuohy, M.G. (Eds.), Biofuel Technologies. Springer
Science Publishers, USA, pp. 121e143.
Das Neves, M.A., Kimura, T., Shimizu, N., Nakajima, M., 2007.
State of the art and future trends of bioethanol production. In:
da Silva, J.A.T. (Ed.), Dynamic Biochemical Process Biotech
Molecular Biology. Global Science Books Ltd, Middlesex, UK,
pp. 1e14.
Davis, L., Rogers, P., Pearce, J., Peiris, P., 2006. Evaluation of
Zymomonas-based ethanol production from a hydrolysed waste
starch stream. Biomass Bioenergy 30, 809e814.
Demirbas, A., 2008. The importance of bioethanol and biodiesel from
biomass. Energy Sources, Part B 3, 177e185.
Department of Energy, 2007. Office of Energy Efficiency and Renewable Energy. Washington DC, US. Available at: http://www.doe.
gov. (accessed 14.03.2013).
Dhingra, R., Christensen, E.R., Liu, Y., Zhong, B., Wu, C.F., Yost, M.G.,
Remais, J.V., 2011. Greenhouse gas emission reductions from domestic anaerobic digesters linked with sustainable sanitation in
rural China. Environ. Sci. Technol. 45, 2345e2352.
Dufey, A., 2006. Biofuels Production, Trade and Sustainable Development: Emerging Issues. Sustainable Markets Discussion, Paper
No. 2. International Institute for Environment and Development,
London.
Dunahay, T.G., Jarvis, E.E., Dais, S.S., Roessler, P.G., 1996. Manipulation of microalgal lipid production using genetic engineering.
Appl. Biochem. Biotechnol. 57, 223e231.
Duncan, J. 2003. Costs of biodiesel production. Prepared for: Energy
Efficiency and Conservation Authority: 1e26.
Enguidanos, M., Soria, A., Kavalov, B., Jensen, P., 2002. Techno-Economic Analysis of Bioalcohol Production in the EU: A Short
Summary for Decision-Makers. Report EUR 20280 EN. IPTS/JRC,
Sevilla.
Eriksson, K.E.L., 2000. Lignocellulose, lignin, ligninases. In:
Schaechter, M. (Ed.), Encyclopedia of Microbiology. Academic
Press, San Diego, pp. 39e48.
Esteghlalian, A., Hashimoto, A.G., Fenske, J.J., Penner, M.H., 1997.
Modeling and optimization of the dilute-sulfuric-acid pretreatment of corn stover, poplar and switchgrass. Bioresour. Technol.
59, 129e136.
FAO, United Nations, 2013. Food and Agricultural Organization of
United Nations: Economic and Social Department: The Statistical
Division. Available at: http://faostat.fao.org/. (accessed
15.03.2013).
Fukuda, H., Kondo, A., Noda, H., 2001. Biodiesel fuel production by
transesterification of oils. J. Biosci. Bioeng. 92, 405e416.
Gan, Q., Allen, S.J., Taylor, G., 2002. Design and operation of an integrated membrane reactor for enzymatic cellulose hydrolysis.
Biochem. Eng. J. 12 (3), 223e229.
REFERENCES
Gautam, R., Baral, S., Herat, S., 2009. Biogas as a sustainable energy
source in Nepal: present status and future challenges. Renewable
Sustainable Energy Rev. 13, 248e252.
Ghadge, S.V., Raheman, H., 2006. Process optimization for biodiesel
production from mahua (Madhuca indica L.) oil using response
surface methodology. Bioresour. Technol. 97, 379e384.
GMI, India, 2013. Global Methane Initiative, India. Available at:
http://www.methanetomarketsindia.com/1/agri.htm. (accessed
20.03.2013).
Gnansounou, E., Bedniaguine, D., Dauriat, A. 2005. Promoting bioethanol production through clean development mechanism: findings and lessons learnt from ASIATIC project. In Proceedings of
the 7th IAEE European Energy Conference, 28e30 August 2005.
Bergen, Norway.
Gosling, D., 1982. Biogas for Thailand’s rural development: transferring the technology. Biomass 2, 309e316.
Gryta, M., 2012. Desalination of industrial effluents using integrated
membrane processes. In: Ning, R.Y. (Ed.), Advancing Desalination.
InTech, Rijeka, Croatia, pp. 37e56.
Guan, F., Peng, P., Wang, G., Yin, T., Peng, Q., Huang, J., Guan, G.,
Li, Y., 2010. Combination of two lipases more efficiently catalyzes
methanolysis of soybean oil for biodiesel production in aqueous
medium. Process Biochem. 45 (10), 1667e1682.
Gupta, R., Gupta, N., Rathi, P., 2004. Bacterial lipases: an overview of
production, purification and biochemical properties. Appl.
Microbiol. Biotechnol. 64 (6), 763e781.
Gupta, V.K., Tuohy, M.G., Sharma, G.D., 2013. Biotechnology of Trichoderma: an overview. In: Gupta, V.K., Tuohy, M.G., Sharma, G.D.,
Gaur, S. (Eds.), Applications of Microbial Genes in Enzyme Technology. Nova Science Publishers, USA, pp. 375e393.
Hagerdal, B.H., Grauslund, G.M., Liden, G., Zacchi, G., 2006. Bioethanoldthe fuel of tomorrow from the residues of today. Trends
Biotechnol. 24, 549e556.
Halim, S.F.A., Kamaruddin, A.H., Fernando, W.J.N., 2009. Continuous
biosynthesis of biodiesel from waste cooking palm oil in a packed
bed reactor: optimization using response surface methodology
(RSM) and mass transfer studies. Bioresour. Technol. 100 (2),
710e716.
Howard, R.L., Abotsi, E., Jansen van Rensberg, E.L., Howard, S., 2003.
Lignocellulose biotechnology: issues of bioconversion and enzyme
production. Afr. J. Biotechnol. 2, 602e619.
Hu, G., Heitmann, J.A., Rojas, O.J., 2008. Feedstock pretreatment
strategies. Bioresources 3 (1), 270e294.
Huang, H.J., Ramaswamy, S., Tschirner, U.W., Ramarao, B.V., 2008. A
review of separation technologies in current and future biorefineries. Sep. Purif. Technol. 62, 1e21.
Imai, M., Ikari, K., Suzuki, I., 2004. High-performance hydrolysis of
cellulose using mixed cellulose species and ultrasonication pretreatment. Biochem. Eng. J. 17 (2), 79e83.
International Energy Agency Statistics, 2012. CO2 Emissions from the
Consumption of Coal. Available at. http://www.eia.gov/cfapps/
ipdbproject/IEDIndex3.cfm?tid¼1&pid¼1&aid¼8 (Accessed on
10.03.2013).
Issariyakul, T., Kulkarmi, M.G., Dalai, A.K., Bakhshi, N.N., 2007.
Production of biodiesel from waste fryer grease using mixed
methanol/ethanol system. Fuel Process. Technol. 88, 429e436.
Jeong, G.W., Yang, H.S., Park, D.H., 2009. Optimization of transesterification of animal fat ester using response surface methodology. Bioresour. Technol. 100, 25e30.
Jorgensen, H., Vibe-Pedersen, J., Larsen, J., Felby, C., 2007. Liquefaction of lignocellulose at high solids concentrations. Biotechnol.
Bioeng. 96 (5), 862e870.
Kalbande, S.R., More, G.R., Nadre, R.G., 2008. Biodiesel production
from non-edible oils of jatropha and karanj for utilization in electrical generator. Bioenergy Res. 2, 170e178.
19
Karmee, S.K., Chadha, A., 2005. Preparation of biodiesel from crude
oil of Pongamia pinnata. Bioresour. Technol. 96 (13), 1425e1429.
Karunanithy, C., Muthukumarappan, K., Julson, J.L. 2008. Influence of
High Shear Bioreactor Parameters on Carbohydrate Release from
Different Biomasses. American Society of Agricultural and Biological Engineers Annual International Meeting, 29 Junee2 July,
2008, Providence, Rhode Island.
Kheshgi, H.S., Jain, A.K., Wuebbles, D.J., 1996. Accounting for the
missing carbon sink with the CO2 fertilization effect. Clim. Change
33, 31e62.
Kim, T.H., Kim, J.S., Sunwoo, C., Lee, Y.Y., 2003. Pretreatment of corn
stover by aqueous ammonia. Bioresour. Technol. 90, 39e47.
Kirk, O., Borchert, T.V., Fuglsang, C.C., 2002. Industrial enzyme
application. Curr. Opin. Biotechnol. 13 (4), 345e351.
Knutsen, J.S., Davis, R.H., 2004. Cellulase retention and sugar removal
by membrane ultrafiltration during lignocellulosic biomass hydrolysis. Appl. Biochem. Biotechnol. 113e116, 585e599.
Kumari, V., Shah, S., Gupta, M.N., 2007. Preparation of biodiesel by
lipase-catalyzed transesterification of high free fatty acid containing oil from Madhuca indica. Energy Fuel 21, 368e372.
Kumartiwari, A.K., Kumar, A., Raheman, H., 2007. Biodiesel production from jatropha oil (Jatropha curcas) with high free fatty
acids: an optimized process. Biomass Bioenergy 31, 569e575.
Larsson, S.H., Thyrel, M., Geladi, P., Lestander, T.A., 2008. High
quality biofuel pellet production from pre-compacted low density
raw materials. Bioresour. Technol. 99, 7176e7182.
Lewin, M., Roldan, L.G., 1971. The effect of liquid anhydrous
ammonia in the structure and morphology of cotton cellulose.
J. Polym. Sci. Part C 36, 213e229.
Li, L., Du, W., Liu, D., Wang, L., Li, Z., 2006. Lipase-catalyzed transesterification of rapeseed oils for biodiesel production with a novel
organic solvent as the reaction medium. J. Mol. Catal. B: Enzym. 43
(1e4), 58e62.
Lin, Y., Tanaka, S., 2006. Ethanol fermentation from biomass resources:
current state and prospects. Appl. Microbiol. Biotechnol. 69, 627e642.
Linde, M., Galbe, M., Zacchi, G., 2008. Bioethanol production from
non-starch carbohydrate residues in process streams from a drymill ethanol plant. Bioresour. Technol. 99, 6505e6511.
Liu, J., Olsson, G., Mattiasson, B., 2004. Control of an anaerobic reactor
towards maximum biogas production. Water Sci. Technol. 50 (11),
189e198.
Malherbe, S., Cloete, T.E., 2003. Lignocellulosic biodegradation: fundamentals and applications: a review. Environ. Sci. Biotechnol. 1,
105e114.
Marina, S., Verena, E.M.S., Martin, K., 2011. Pelletizing of autumn
leavesdpossibilities and limits. Biomass Convers. Biorefin. 1,
173e187.
Mariod, A., Klupsch, S., Hussein, H., Ondruschka, B., 2006. Synthesis
of alkyl esters from three unconventional Sudanese oils for their
use as biodiesel. Energy Fuel 20, 2249e2252.
Meher, L.C., Vidya-Sagar, D., Naik, S.N., 2006. Technical aspects of
biodiesel production by transesterificationda review. Renewable
Sustainable Energy Rev. 10, 248e268.
Mekala, N.K., Singhania, R.R., Sukumaran, R.K., Pandey, A., 2008.
Cellulase production under solid-state fermentation by Trichoderma
reesei RUT C30: statistical optimization of process parameters.
Appl. Biochem. Biotechnol. 151, 122e131.
Meng, X., Chen, G., Wang, Y., 2008. Biodiesel production from waste
cooking oil via alkali catalyst and its engine test. Fuel Process.
Technol. 89, 851e857.
Metting, F.B., 1996. Biodiversity and application of microalgae. J. Ind.
Microbiol. 17, 477e489.
Modi, M.K., Reddy, J.R.C., Rao, B.V.S.K., Prasad, R.B.N., 2007. Lipasemediated conversion of vegetable oils into biodiesel using ethyl
acetate as acyl acceptor. Bioresour. Technol. 98 (6), 1260e1264.
20
1. CURRENT BIOENERGY RESEARCHES: STRENGTHS AND FUTURE CHALLENGES
Mohanty, S.M., Behera, S., Swain, M.R., Ray, R.C., 2009. Bioethanol
production from mahula (Madhuca latifolia L.) flowers by solidstate fermentation. Appl. Energy 86, 640e644.
Mohibbeazam, M.M., Waris, A., Nahar, N.M., 2005. Prospects and
potential of fatty acid methyl esters of some non-traditional seed
oils for use as biodiesel in India. Biomass Bioenergy 29, 293e302.
Molina, E., Fernandez, J., Acien, F.G., Chisti, Y., 2001. Tubular photobioreactor design for algal cultures. J. Biotechnol. 92, 113e131.
Moser, B.R., 2009. Biodiesel production, properties, and feedstocks.
In Vitro Cell. Dev. Biol.: Plant 45, 229e266.
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y.Y., Holtzapple, M.,
2005. Features of promising technologies for pretreatment of
lignocellulosic biomass. Bioresour. Technol. 96 (6), 673e686.
Mulumba, N. 2010. Production of Biodiesel from Microalgae, M.S.
Thesis, Chemical Engineering Dept., University of New Hampshire, Durham, NH, USA.
Mulumba, N., Farag, I.H., 2012. Tubular photobioreactor for microalgae biodiesel production. Int. J. Eng. Sci. Technol. 4 (2),
703e709.
Mustafa, B., Havva, B., 2009. Recent trends in global production and
utilization of bio-ethanol fuel. Appl. Energy 86, 2273e2282.
Naik, M., Meher, L.C., Naik, S.N., Das, L.M., 2008. Production of
biodiesel from high free fatty acid karanja (Pongamia pinnata) oil.
Biomass Bioenergy 32, 354e357.
Ngo, H.L., Zafiropoulos, N.A., Foglia, T.A., Samulski, E.T., Lin, W.,
2008. Efficient two-step synthesis of biodiesel from greases. Energy
Fuel 22, 626e634.
Nielsen, N.P.K., Gardner, D.J., Poulsen, T., Felby, C., 2009. Importance
of temperature, moisture content, and species for the conversion
process of wood into fuel pellets. Wood Fiber Sci. 41, 414e425.
Ogbonna, J.C., Matsumura, M., Kataoka, H., 1991. Effective oxygenation of immobilized cells through reduction in bead diameters: a
review. Process Biochem. 26, 109e121.
Ogbonna, J.C., Tomiyama, S., Tanaka, H., 1996. Development of a
method for immobilization of non-flocculating cells in loofa (Luffa
cylindrica) sponge. Process Biochem. 31, 737e744.
Ognjanovic, N., Bezbradica, D., Knezevic-Jugovic, Z., 2009. Enzymatic
conversion of sunflower oil to biodiesel in a solvent-free system:
process optimization and immobilized system stability. Bioresour.
Technol. 100 (21), 5146e5154.
Okano, K., Kitagaw, M., Sasaki, Y., Watanabe, T., 2005. Conversion of
Japanese red cedar (Cryptomeria japonica) into a feed for ruminants
by white-rot basidiomycetes. Anim. Feed Sci. Technol. 120 (3),
235e243.
O’Donovan, A., Gupta, V.K., Tuohy, M.G., 2013. Recent updates in
acid pretreatments and SEM analysis of acid pretreated grass
biomass. In: Gupta, V.K., Tuohy, M.G. (Eds.), Biofuel Technologies; Recent Developments. Springer Science Publishers, USA,
pp. 97e118.
Pan, X., Xie, D., Gilkes, N., Gregg, D.J., Saddler, J.N., 2005. Strategies to
enhance the enzymatic hydrolysis of pretreated softwood with
high residual lignin content. Appl. Biochem. Biotechnol. A 124,
1069e1079.
Pathak, H., Jain, N., Bhatia, A., Mohanty, S., Gupta, N., 2009. Global
warming mitigation potential of biogas plants in India. Environ.
Monit. Assess. 157, 407e418.
Pei-dong, Z., Guomei, J., Gang, W., 2007. Contribution to emission
reduction of CO2 and SO2 by household biogas construction in
rural China. Renewable Sustainable Energy Rev. 11, 1903e1912.
Phan, A.N., Phan, T.M., 2008. Biodiesel production from waste cooking oils. Fuel 87, 3490e3496.
Pinelo, M., Jonsson, G., Meyer, A.S., 2009. Review: membrane technology for purification of enzymatically produced oligosaccharides: molecular features affecting performance. Sep. Purif.
Technol. 70 (1), 1e11.
Pongsawatmanit, R., Temsiripong, T., Suwonsichon, T., 2007.
Thermal and rheological properties of tapioca starch and
xyloglucan mixtures in the presence of sucrose. Food Res. Int.
40, 239e248.
Potumarthi, R., Baadhe, R.R., Jetty, A., 2012. Mixing of acid and base
pretreated corncobs for improved production of reducing sugars
and reduction in water use during neutralization. Bioresour.
Technol. 119, 99e104.
Potumarthi, R., Baadhe, R.R., Jetty, A., 2013. Simultaneous pretreatment and saccharification of rice husk by Phanerochaete chrysosporium for improved production of reducing sugars. Bioresour.
Technol. 128, 113e117.
Ravichandra, R., Baadhe, R.R., Bhattacharya, S., 2013. Fermentable
sugars from lignocellulosic biomass: technical challenges. In:
Gupta, V.K., Tuohy, M. (Eds.), Biofuel Technologies: Recent
Developments. Springer, Germany, pp. 3e27.
Predojevic, Z.J., 2008. The production of biodiesel from waste frying
oils: a comparison of different purification steps. Fuel 87,
3522e3528.
Pruszko, R. 2007. Alternative feedstocks and biodiesel production.
Presented at Practical Biodiesel Blueprint Conference (23e24 Jan,
2007), Kuala Lumpur, Malaysia.
Rajendran, K., Aslanzadeh, S., Taherzadeh, M.J., 2012. Household
biogas digestersda review. Energies 5, 2911e2942.
Rodrigues, R.C., Pessela, B.C.C., Volpato, G., Fernandez-Lafuente, R.,
Guisan, J.M., Ayub, M.A.Z., 2010. Two step ethanolysis: a simple
and efficient way to improve the enzymatic biodiesel synthesis
catalyzed by an immobilized-stabilized lipase from Thermomyces
lanuginosus. Process Biochem. 45 (8), 1268e1273.
Rudolf, A., Alkasrawi, M., Zacchi, G., Liden, G., 2005. A comparison
between batch and fedbatch simultaneous saccharification and
fermentation of steam pretreated spruce. Enzyme Microb. Technol.
37 (2), 195e204.
Sahoo, P.K., Das, L.M., Babu, M.K.G., Naik, S.N., 2007. Biodiesel
development from high acid value polanga seed oil and performance evaluation in a CI engine. Fuel 86, 448e454.
Saritha, M., Arora, A., Lata, 2012. Biological pretreatment of lignocellulosic substrates for enhanced delignification and enzymatic
digestibility. Indian J. Microbiol. 52 (2), 122e130.
Seadi, T.A., Rutz, D., Prassl, H., Kottner, M., 2008. Biogas Handbook.
University of Southern Denmark, Esbjerg, Denmark.
Shah, S., Gupta, M.N., 2007. Lipase catalyzed preparation of biodiesel
from Jatropha oil in a solvent free system. Process Biochem. 42 (2),
409e414.
Singh, A., Singh, I.S., 1991. Chemical evaluation of mahua (Madhuca
indica (M. longifolia)) seeds. Food Chem. 40, 221e228.
Singh, K.J., Sooch, S.S., 2004. Comparative study of economics of
different models of family size biogas plants for state of Punjab,
India. Energy Convers. Manage. 45, 1329e1341.
Smith, A.M., 2008. Prospects for increasing starch and sucrose yields
for bioethanol production. Plant J. 54, 546e558.
Spolaore, P., Joannis-Cassan, C., Duran, E., Isambert, A., 2006.
Commercial applications of microalgae. J. Biosci. Bioeng. 101,
87e96.
Srivastava, P.K., Verma, M., 2008. Methyl ester of karanja oil as an
alternative renewable source energy. Fuel 87, 1673e1677.
Sun, Y., Cheng, J., 2002. Hydrolysis of lignocellulosic materials for
ethanol production: a review. Bioresour. Technol. 83 (1), 1e11.
Taherzadeh, M.J., Karimi, K., 2007. Acid-base hydrolysis process for
ethanol from lignocellulosic material: a review. Bioresour. Technol.
2 (3), 472e499.
Tassinari, T., Macy, C., Spano, L., Ryu, D.D.Y., 1980. Energy requirements and process design considerations in compression
milling pretreatment of cellulosic wastes for enzymatic hydrolysis.
Biotechnol. Bioeng. 22, 1689e1705.
REFERENCES
Tomar, S.S., 1994. Status of biogas plant in India. Renewable Energy 5,
829e831.
Tu, M.B., Chandra, R.P., Saddler, J., 2007. Evaluating the distribution
of cellulases and the recycling of free cellulases during the hydrolysis of lignocellulosic substrates. Biotechnol. Prog. 23,
398e406.
United Nations Environment Program, 2006. Introducing the International Bioenergy Platform (IBEP). Available at: http://www.
unep.org/training/programmes/Instructor%20Version/Part_2/
Activities/Innovations_and_Technology/Energy/Supplemental/
Bioenergy.pdf. (accessed 11.03.2013).
US DOE, 2010. U.S. Department of Energy’s Bioenergy Research
Centers: An Overview of the Science, DOE/SC-0127. Office of
Biological and Environmental Research within the DOE Office of
Science. Available at: genomicscience.energy.gov/centers/
brcbrochure.pdf. (accessed 15.03.2013).
Usta, N., 2005. Use of tobacco seed oil methyl ester in a turbocharged
indirect injection diesel engine. Biomass Bioenergy 28, 77e86.
van Beilen, J.B., Li, Z., 2002. Enzyme technology: an overview. Curr.
Opin. Biotechnol. 13 (4), 338e344.
Van Maris, A.J.A., Abbott, D.A., Bellissimi, E., Brink, J.V.D., 2006.
Alcoholic fermentation of carbon sources in biomass hydrolysates
by Saccharomyces cerevisiae: current status. Anton. Leeuw. Int. J. G.
90 (4), 391e418.
Van-Zyl, W.H., Lynd, L.R., Haan, R.D., McBride, J.E., 2007. Consolidated bioprocessing for bioethanol production using Saccharomyces
cerevisiae. Biofuels 108, 205e235.
21
Veljkovic, V.B., Lakicevic, S.H., Stamenkovic, Z.B., Todorovic, O.S.,
Lazic, M.L., 2006. Biodiesel production from tobacco (Nicotiana
tabacum) seed oil with a high content of free fatty acids. Fuel 85,
2671e2675.
Verma, V.K., Bram, S., Delattin, F., Laha, P., Vandendael, O.,
Hubin, A., 2012. Agropellets for domestic heating boilers: standard laboratory and real life performance. Appl. Energy 90 (1),
17e23.
Working of biogas plants. 2013. Tutorvista.com. Available at: http://
www.tutorvista.com/content/physics/physics-ii/fission-andfusion/biogas-plants.php#construction-of-the-floating-gas-holdertype-plant. (accessed 21.03.2013).
Wuebbles, D.J., Jain, A.K., 2001. Concerns about climate change and
the role of fossil fuel use. Fuel Process. Technol. 71, 99e119.
Wyatt, V.T., Hess, M.A., Dunn, R.O., Foglia, T.A., Haas, M.J.,
Marmer, W.M., 2005. Fuel properties and nitrogen oxide emission
levels of biodiesel produced from animal fats. J. Am. Oil Chem.
Soc. 82, 585e591.
Yang, S., Ding, W., Chen, H., 2006. Enzymatic hydrolysis of rice straw
in a tubular reactor coupled with UF membrane. Process. Biochem.
41 (3), 721e725.
Yang, J., Chen, W., Chen, B., 2011. Impacts of biogas projects on agroecosystem in rural areasda case study of Gongcheng. Front. Earth
Sci. 5, 1e6.
Zhang, J., Jiang, L. 2008. Acid-catalyzed esterification of Zanthoxylum
bungeanum seed oil with high free fatty acids for biodiesel production. Bioresource. Tech.
C H A P T E R
2
Bioenergy Research: An Overview on
Technological Developments and Bioresources
Vijai K. Gupta 1,*, Ravichandra Potumarthi 2, Anthonia O’Donovan 1,
Christian P. Kubicek 3, Gauri Dutt Sharma 4, Maria G. Tuohy 1,*
1
Molecular Glycobiotechnology Group, Department of Biochemistry, School of Natural Sciences, National
University of Ireland Galway, Galway, Ireland, 2Department of Chemical Engineering, Monash University,
Clayton, Victoria, Australia, 3Research Area Biotechnology and Microbiology, Institute of Chemical Engineering,
TU Wien, Gumpendorferstrasse Wien, Austria, 4Bilaspur University, Bilaspur, Chattisgarh, India
*Corresponding author email: vijai.gupta@nuigalway.ie, maria.tuohy@nuigalway.ie
O U T L I N E
Introduction
23
Current Bioenergy Practices
25
Main Biofuel Technologies and Current Processes
26
Technological Routes for Bioenergy Production
Biomass Pretreatment
Hydrolysis
Fermentation
Combined Pretreatment, Hydrolysis and
Fermentation Strategies
Advanced Biomass-to-Biofuels
Development Platform
28
28
29
29
29
Bioenergy Resources and Biofuels Development
Program
Sustainability
Bioenergy Feedstocks and Dedicated
Biofuel Crops
Lignocellulosic Feedstocks
Dedicated Bioenergy Crops
Feedstocks for Biodiesel
36
Conclusions
41
References
41
37
37
38
39
30
INTRODUCTION
nontoxic; biofuel spillages present far less risk than
fossil fuel spillages (Hahn-Hagerdal et al., 2006).
Bioenergy is a term broadly used to describe gaseous,
liquid or solid energy products that, for the most part,
are derived from biological raw materials (biomass). In
the 1990s bioethanol was a promising technological
option to reduce transportation sector greenhouse gas
(GHG) emissions (Lynd, 1996); most of this ethanol
was derived from so-called first-generation, starchand sucrose-rich feedstocks. Bioethanol is readily
made from starchy seeds, tubers, or roots of plants
such as maize (Zea mays), barley (Hordeum vulgare),
Fossil fuels such as petrol, diesel or crude oil, are
nonrenewable sources of fuel and are not natural
resources in many countries making these nations dependent on fossil fuel-rich countries at enormous expense.
The rising cost and simultaneous depletion of fossil fuels,
in addition to political instability in key countries,
means the competitiveness of biomass-derived energy
has increased considerably. Additionally bioenergy
sources, including biofuels, pose a reduced threat to
the environment because they are biodegradable and
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00002-4
33
23
Copyright Ó 2014 Elsevier B.V. All rights reserved.
24
2. BIOENERGY RESEARCH: AN OVERVIEW ON TECHNOLOGICAL DEVELOPMENTS AND BIORESOURCES
wheat (Triticum aestivum), rice (Oryza sativa), potato
(Solanum tuberosum), sweet potato (Ipomea batatas),
cassava (Manihot esculenta), Jerusalem artichoke (Helianthus tuberosus), etc. and from the sugar-rich stems
and roots of sugarcane (Saccharum officinarum), sweet
sorghum (Sorghum vulgare), and sugar beet (Beta vulgaris). Indeed, the basic technology for making ethanol
from such crops is centuries old (Lemus and Parrish,
2009). Currently bioethanol is produced commercially
by fermentation of sugars derived from corn, sugar
cane and sugar beet. It is expected that, in light of the
increase in global population and the “food versus
fuel” debate, there will be limits to the supply of these
feedstocks for biofuel production in the near future ultimately making first-generation biofuels an unsustainable approach to meet future energy needs (O’Donovan
et al., 2013; Groom et al., 2008; Simpson et al., 2008).
Perennial herbaceous energy crops make good feedstocks because they do not require annual reseeding
once established, need fewer energy inputs (such as fertilizers and pesticides) than annual cropland, and can be
grown on marginal lands (Dien et al., 2005). They also
have environmental benefits that include reduced soil
erosion, enhanced carbon sequestration, and conservation of wildlife habitats (Lemus and Lai, 2005). The
major herbaceous energy crops that have been selected
for bioethanol production in the United States are switch
grass (Panicum virgatum), Miscanthus (Miscanthus spp.
Anderss.), canary grass (Phalaris arundinacea), giant
reed (Arundo donax L.), and alfalfa (Medicago sativa L.).
They are considered to have energetic, economic, and
environmental advantages over food crops for ethanol
production. While these dedicated energy crops contain
substantial amounts of holocellulose (cellulose and
hemicelluloses) in their cell walls, their feedstock quality
for livestock makes them less attractive options for fuel
ethanol and bioenergy generation (Hill et al., 2006).
It is essential to approach renewable energy (REN)
production through the application of complementary
technologies. The recent “food versus fuel” debate has
motivated the development of technologies to utilize
nonfood crops as well as food wastes and agriprocessing wastes, in biomass to bioenergy strategies;
therefore, lignocellulosic residues and other nonfood
plant biomass types are considered attractive alternative
feedstocks.
Large efforts are being made worldwide in order to
develop technologies that generate clean, sustainable
energy sources from nonfood biomass feedstocks that
could substitute fossil fuels (Ragauskas et al., 2006;
Levin et al., 2006). At present, in the United States,
biomass provides about 40 times as much energy as
photovoltaics (Banerjee, 2011) and represents 78% of
the total REN generated worldwide (International
Energy Agency (IEA), 2010). Biofuels are the only viable
energy source for the foreseeable future and can
provide sustainable development in a manner that will
address socioeconomic and environmental concerns
(Demirbas, 2005).
Bionergy derived from second-generation feedstocks,
i.e. lignocellulosic materials is now the prime target for
commercial biofuel production (O’Donovan et al.,
2013; Demain, 2009). Lignocellulosic biomass is an abundant, domestic, renewable feedstock source rich in complex carbohydrates, which can be converted to liquid
transportation fuel and other chemicals by strategies
involving carbohydrate degradation and subsequent
fermentation. More than 70% of lignocellulosic biomass
is made up of the complex biopolymers, cellulose, hemicellulose, and lignin. The organization of these structural polymers in the plant cell wall makes such
feedstocks highly recalcitrant to bioconversion and difficult to use as a raw material in ethanol production
compared with starch (O’Donovan et al., 2013;
Abramson et al., 2010; Somerville et al., 2010). However,
lignocellulosic biomass in the form of wood and agricultural residues is virtually inexhaustible (Sarkar et al.,
2012; Zhang et al., 2007; Zhang and Lynd, 2006; Lynd
et al., 2002; Kuhad et al., 1997). Agricultural residuals
or by-products are annually renewable, abundantly
available and account for more than 180 million tons
of biomass per year (Kapdan and Kargi, 2006). The
most abundant lignocellulose agricultural residues are
corncobs, corn stover, wheat, rice, barley straw, sorghum
stalks, coconut husks, sugarcane bagasse, switchgrass,
pineapple and banana leaves (Demain et al., 2005; Kim
and Dale, 2004). Cereal crops, pulse crops and harvestable palm oil biomass are also being produced in large
amounts worldwide annually (Rajaram and Verma,
1990). In addition, wood and paper industries generate
huge amounts of residual lignocellulosic biomass. Along
with agricultural and forestry wastes and residues,
locally available nonfood plant biomass and municipal
solid wastes are potential candidates to meet demands
for biofuel and bioenergy production, since additional
costs for cultivation and harvesting are not involved. It
is likely that the diversity of raw materials will support
the decentralization of fuel production with geopolitical,
economic, and social benefits (Wyman, 2007), thus
bringing further socioeconomic benefits.
The concept of replacing fossil fuels with alternative
biobased energy sources and fuels has been markedly
enhanced by the realization that plant biomass also
has the potential to provide a wide range of feedstock
(bio)chemicals that can yield high-value commodity
products and offset bioenergy production costs in
lignocellulose-based biorefinery approaches (Zhu and
Zhuang, 2012; Cherubini, 2010; FitzPatrick et al., 2010;
Percival Zhang, 2008; Taylor, 2008; Kamm and Kamm,
2004). Bioenergy production processes (e.g. anaerobic
CURRENT BIOENERGY PRACTICES
digestion and thermochemical treatments) can also
generate organic wastes that still have significant market
potential, for example, as organic fertilizers and biochars, which are most important for soil enrichment.
The developments in biorefining have underpinned
several recent and promising advances in bioenergy
(Aden et al., 2002).
Bioethanol, biobutanol and biomethane are promising future bioenergy and biofuel sources. Biomethane
is produced most frequently through anaerobic digestion, in which biomass is converted by consortia of
bacteria via hydrolysis, fermentation, acetogenesis and
methanogenesis reaction steps to methane and smaller
amount of other gases (Keating et al., 2012; Liew et al.,
2012; McHugh et al., 2003; Mata-Alvarez et al., 2000).
Liquid fuels that are being produced from biomass are
typically of higher quality and burn more cleanly than
petroleum-based diesel and jet fuels. Biofuels also
reduce the release of volatile organic compounds, as
the addition of ethanol to gasoline oxygenates the fuel
mixture causing it to burn more completely. Biodiesel
is another important biofuel usually produced from
oleaginous crops, such as rapeseed, soybean, sunflower,
palm and from microalgae through a chemical transesterification process of their oils with shortchain alcohols,
mainly methanol (Antolı́n et al., 2002). Thus, a shift to
biofuels for current fuel needs would reduce energy
dependency on oil imports and could boost rural development, providing farmers and crop producers with an
additional source of income.
CURRENT BIOENERGY PRACTICES
Biofuels account for the major proportion of
bioenergy production worldwide, with most of the fuels
being derived through biochemical processes. For this
reason, this review will focus in the main on current practices used in the production of the main biofuels. The
major producers of bioethanol are Brazil and the United
States, both of which account for about 89% of world production (World Development Report, 2008; Lichts, 2010),
while the European Union is the world’s largest producer
of biodiesel (OECD-FAO, 2009). The United States has
been the world’s largest producer of ethanol fuel since
2005 and the world’s largest exporter since 2010. In
2011, the United States produced 52.6 billion liters (13.9
billion US liquid gallons) of ethanol, while Brazil produced 21.1 billion liters (5.57 billion US liquid gallons),
representing 24.9% of the world’s total ethanol used as
fuel (Renewable Fuels Association, 2012).
Fuel ethanol production is considerably more modest
in the European Union, where France, Germany and
Spain are the largest producers of bioethanol producing
950, 581 and 346 million liters, respectively, in 2008
25
(European Bioethanol Fuel Association, 2009). Countries
such as Poland, Hungary and Slovakia have also
increased their bioethanol output producing 200, 150
and 94 million liters of bioethanol, respectively
(European Bioethanol Fuel Association, 2009). Sweden
is the leading country in Europe in terms of the use of
ethanol as fuel, the impetus for which is driven by
government policy. Although most of the ethanol is
imported, Swedish gas stations are required by an act
of parliament to offer at least one alternative fuel.
Furthermore, reductions in biofuel prices to the
consumer have also encouraged biofuel consumption.
Government incentives for biofuel replacement of
gasoline are now being implemented in other countries
worldwide, motivated by ever-increasing oil costs,
depleting fossil fuel resources, GHG emission targets
and the need for greater diversification to support agricultural and rural development (Mussato et al., 2010).
The major feedstock for bioethanol in Brazil is sugarcane including bagasse, while corn grain/maize is the
main feedstock used for bioethanol production in
the United States. As mentioned earlier, bioethanol can
be produced from any sugar or starch crop in firstgeneration processes, but other potential resources for
bioethanol include sugar beet, cassava, maize, oil
palm, rapeseed, soybean, corn stover, grass, leaves, agricrop residues and various locally available nonfood
plant biomass like Jatropha, Miscanthus, willow, hemp
and switchgrass. Table 2.1 summarizes the major
crops/biomass currently (ranked in order of importance) in use for biofuel and bioenergy production in
different countries. Shapouri (1995), Shapouri et al.
(2002) concluded that the energy content of bioethanol
was higher than the energy required to produce it,
although other researchers would argue as to the
economic viability of bioethanol in the absence of an
accompanying high-value biorefinery process.
Production of ethanol from lignocellulosic biomass is a
complex process where the biomass often requires pretreatment to render the holocellulose more accessible to
a mixture of enzymes, which are utilized to saccharify
or hyrolyze the complex polysaccharides to fermentable
sugars. Pretreatment processes can be expensive, toxic
and corrosive and may require a subsequent costly detoxification step (Agbor et al., 2011; Zhang and Lynd, 2004;
Sun and Cheng, 2002). In addition, preparation of
fermentable sugars and the inhibitory effect of lignin
and carbohydrate-derived compounds, formed during
pretreatment of the lignocelluloses, are the major bottlenecks in bioconversion processes (Viikari et al., 2007).
However, since biomass energy is derived from renewable resources, its production can still be advantageous
if proper management technologies are utilized in
biomass harvesting, pretreatment and processing, and if
biomass feedstocks are produced sustainably. Plant
26
TABLE 2.1
2. BIOENERGY RESEARCH: AN OVERVIEW ON TECHNOLOGICAL DEVELOPMENTS AND BIORESOURCES
Major Crops Used for the Production of Biofuels
Crop
Countries
Cassava
Nigeria/Brazil/Thailand/Indonesia
Corn Stover
United States/Latin America
Hemp
Ireland/United Kingdom/United States/other
European countries/China
Jatropha
India/China/other Asian countries/Africa
Maize
United States/China/Brazil/Mexico
Miscanthus
Ireland/United Kingdom/other European countries
Oil Palm
Malaysia/Indonesia/Nigeria/Thailand
Rapeseed
China/Canada/India/Germany
Soybean
United States /Brazil/Argentina/China
Sugar Beet
France/United States/Germany/Russia
Sugarcane
Brazil/India/China/Thailand
Switch Grass
Ireland/United Kingdom/United States/other
European countries/China
Willow
Ireland/United Kingdom/other European countries
Source: Müller et al., 2008; De Fraiture and Berndes 2009.
biomass to energy or chemicals can be economical only if
all of the components in the biomass are converted into
fuel, chemicals or other value-added components in a
true biorefinery approach (FitzPatrick et al., 2010;
Cherubini, 2010; Percival Zhang, 2008; Kamm and
Kamm, 2004).
MAIN BIOFUEL TECHNOLOGIES AND
CURRENT PROCESSES
Biofuels, biogas and syngas are energy carriers that
store the energy derived from biomass. A wide range
of biomass sources can be used to produce bioenergy
in a variety of forms (see Figure 2.1). As mentioned,
food, fiber and wood process residues from the industrial sector; energy crops, short rotation crops and agricultural wastes from the agriculture sector; and
residues from the forestry sector are being used to produce biofuels, generate electricity, heat, combined heat
and power, and other forms of bioenergy (Department
of Energy (DOE), 2011; Chu and Majumdar, 2012).
Figure 2.1 provides an overview of the main biological
and chemical technology platforms that are currently
in use for the production of different types of bioenergy,
including biofuels.
Liquid biofuels produced from agricultural crops, e.g.
cereals, maize, sugarcane, sugar beet and sweet sorghum and from vegetable oil, as well as biogas, are
referred to as first-generation biofuels, while those produced from lignocellulosic biomass and nonfood crops
are referred to as second-generation biofuels (Yuan
et al., 2008). Biofuels produced from algae are termed
third-generation biofuels (Brennan and Owende, 2010;
see Table 2.2).
The many sources of biomass used for energy purposes are often scattered across large and diverse
geographical areas. Even today, most energy derived
from biomass originates from by-products of food,
fodder and fiber production. For instance, the main byproducts of forest processing industries are used to
produce fuels wood and charcoal, and black liquor
(a by-product of pulp mills) is a major fuel source for
bioelectricity generation in countries such as Brazil,
Canada, Finland, Sweden and the United States. A
considerable amount of heat and power is derived from
recovered and/or recycled woody biomass and
increasing amounts of energy are being recovered from
biomass derived from cropland (straw and cotton stalks)
and forest land (wood chips and pellets). In sugar- and
FIGURE 2.1 Main technology platforms
for bioenergy production (including biofuels) and by-/coproducts from various
feedstocks. Source: Department of Energy
(DOE), 2011; Chu and Majumdar, 2012.
(For color version of this figure, the reader is
referred to the online version of this book.)
27
MAIN BIOFUEL TECHNOLOGIES AND CURRENT PROCESSES
TABLE 2.2 Bioenergy Outputs, Feedstocks Utilized and By-/Coproducts
Bioenergy Category
Feedstock(s) Used
Bioenergy Outputs
Solid Biofuels
Woody material, dried manure
Used as dried biomass for energy
1. Rapeseed oil, sunflower oil and other
1. As transport fuel
2. For generation of electricity
By-/Coproducts
FIRST GENERATION
Plant Oils (Vegetable Oil)
plant oil, waste vegetable oil
2. Rapeseed oil, palm oil, Jatropha and
other plant oil
Biodiesel
Bioethanol
Biogas
Oil cake as animal feed
and heat in decentralized
combined heat and power (CHP)
stations
1.
2.
3.
4.
Europe: rapeseed, sunflower, soya
United States: soya, sunflower,
Canada: soya, rapeseed, canola
South and Central America: soya,
palm, Jatropha, castor
5. Africa: palm, soya, sunflower, Jatropha
6. Asia: palm, soya, rapeseed, sunflower, Jatropha
Transesterification of oils and
fats to provide fatty acid methyl
ester and to use transport fuel
1.
2.
3.
4.
5.
Europe: cereals, sugar beets
United States: corn
Canada: maize, cereals
Brazil: sugarcane
South and Central America:
sugarcane, cassava
6. Africa: sugarcane, maize
7. Asia: sugarcane, cassava
Fermentation (sugar), hydrolysis
and fermentation (starch) used in
transport fuel
Energy crops (viz. maize, Miscanthus,
wood from short rotation and multiple
cropping system), agriculture residues
(waste leaves, twigs, plant material),
biodegradable waste material including
sewage from any source
Fermentation of biomass used in
decentralized system for energy
requirement or through supply into
gas pipeline (as purified biomethane)
1. For generation of electricity and heat in
1. Oil cakes as animal feed
2. Glycerin
3. Oil cakes as residue for
energy recovery
1. Maize and cereals yield
animal feedstocks
2. Sugarcane bagasse used
for energy recovery
Residues used as fertilizer
(soil conditioners and
nutrient recycling)
CHP-based power stations
2. For transport fuel: either 100% biogas
fuel or blending with
natural gas used as fuel
Solid Biofuels
Wood, grass cuttings, switchgrass,
perennial rye grass, grass press cake,
grains, straw, charcoal, domestic
refuse and dried manure
1. Densification of biomass by
carbonization (charcoal)
2. Residuals and waste for
generation of electricity and
heat (e.g. industrial waste in CHP)
Residues used as fertilizer
(soil conditioners and
nutrient recycling)
SECOND GENERATION
Bioethanol
Lignocellulosic biomass like straw, stalks
of wheat, corn stover and wood, bioenergy
crops, e.g. Miscanthus, willow, witchgrass
(Panicum virgatum L.), reed canary grass
(Phalaris arundinacea L.), and alfalfa
(Medicago sativa L.), agave, Jatropha
Biodiesel and Range of
Lignocellulosic biomass like straw,
Biofuels such as
wood and secondary raw materials
Biohydrogen, Biomethane;
2,5-Dimethylfuran,
Dimethyl ether, Mixed
Alcohols
Breakdown of lignocellulosic
biomass in many steps via
hydrolysis to fermentation to
produce bioethanol
(Both biochemical and thermal
platforms are being used as
technological innovation)
1. Residues used as fertilizer
(soil conditioners and
nutrient recycling)
2. Range of biochemicals
Gasification of low-moisture biomass
provides syngas (mix of CO, CO2, H2,
CH4, and hydrocarbons) from which
liquid fuels and other chemicals are
being derived
Various feedstocks for
chemical industry to
produce range of
biochemical and plastics
Bioreactors for ethanol,
transesterification and pyrolysis for
biodiesel
Liquid biofuels processing
Biopolymers, high-protein
animal feedstocks,
agricultural fertilizers
THIRD-GENERATION BIOFUELS
Biodiesel, Aviation Fuels, Macroalgae, microalgae
Bioethanol, Biobutanol,
Biohydrogen, Bio-Oils from
Algae
Source: Farine et al., 2011; Stefan et al., 2009.
28
2. BIOENERGY RESEARCH: AN OVERVIEW ON TECHNOLOGICAL DEVELOPMENTS AND BIORESOURCES
coffee-producing countries, bagasse and coffee husks are
used for direct combustion to produce heat energy and
steam (http://www.thebioenergysite.com/articles/172/
biofuels-and-agriculture). In terms of bioenergy, however, the big growth area in recent years has been in the production of liquid biofuels for transport using agricultural
crops as feedstocks. The bulk of the biofuel output is in
the form of bioethanol (sugar crops or starchy crops),
or biodiesel (oil crops, e.g. oilseed rape and Jatropha).
Table 2.2 provides an overview of some of the main feedstocks used for target bioenergy outputs under first-, second- and third-generation biofuel production strategies.
TECHNOLOGICAL ROUTES FOR
BIOENERGY PRODUCTION
Suitable conversion technologies are needed in order
to effectively breakdown or deconstruct biomass into
simple sugars, carbohydrate derivatives or bio-oils that
are more easily converted into fuels in combination
with downstream conversion technologies to subsequently upgrade these intermediates into bioenergy,
biofuels and value-added bioproducts.
At present, the bioconversion of lignocellulose is
carried out in four major steps viz. pretreatment, hydrolysis, fermentation and separation/purification to
recover bioenergy/biofuels and residues (more recently,
the recovery of coproducts has becoming increasingly
important). The pretreatment of lignocellulose materials
is considered a key step in bioenergy production and
indeed in biorefining as it accelerates the hydrolysis procedure, by enhancing cellulose accessibility and
increasing pore size, which, in theory, leads to higher
sugar yields for fermentation. An ideal pretreatment
should remove lignin and thus reduce the crystallinity
of cellulose (Lynd et al., 2002), increase porosity and
accessibility of the cellulose (and hemicellulose) to
enzymatic hydrolysis, release/generate low levels of
inhibitory compounds, be low cost and have low energy
requirements. The overall result should be a reduction in
the recalcitrance of lignocellulose and an increase in
accessibility to enzymes.
In general, accessibility of cellulose is achieved
through the removal of lignin and hemicellulose
polymers through various pretreatment methods, which
can be defined as chemical, physical or biological
(O’Donovan et al., 2013; Dashtban et al., 2009;
Taherzadeh and Karimi, 2008; Ong, 2004; Howard
et al., 2003).
Biomass Pretreatment
Pretreatments vary from hot-water extraction and
steam pretreatments (often with an oxidant or other
chemical) to weak and strong acid and alkali pretreatments (Sun and Cheng, 2002). Physical pretreatments
include mechanical communition, milling and ultrasound methods (Agbor et al., 2011; Balat, 2011), as well
as irradiation.
Chemical pretreatment methods include ammonia fiber explosion (AFEX), organosolv treatment and the
addition of either acid or alkali (Isroi et al., 2011; Ong,
2004; Dashtban et al., 2009). The use of acid as a catalyst,
normally H2SO4, targets the hemicellulose to dissolve
with lignin and cellulose remaining as solids, whereas
the addition of alkali, normally NaOH, mainly targets
lignin, leaving mainly cellulose as a solid with hemicelluloses (Dashtban et al., 2009; Ong, 2004). Physicochemical pretreatments combine a mild chemical treatment
with high pressure and temperature and include
methods ranging from uncatalyzed solvolysis (hydrothermolysis) to steam explosion with chemical additives
such as carbon dioxide or sulfur dioxide, AFEX and
“popping” techniques (Mosier et al., 2005; Pan et al.,
2005; Wi et al., 2011). Recent developments include
pretreatments based on alkali soaking (NaOH) coupled
with extrusion (Karunanithy and Muthukumarappan,
2011). Steam explosion consists of steaming the lignocellulose at high pressure followed by either a rapid or
slow reduction in pressure to dissolve the hemicelluloses into solution and allow the cellulose and lignin
to remain as solids (Ong, 2004; Dashtban et al., 2009).
SO2 or CO2 can be used as catalysts, although SO2 can
be highly toxic to downstream fermentation microorganisms (Ong, 2004).
Although physical and chemical pretreatments can
effectively reduce the recalcitrance of lignocellulosic
compounds within a shorter time frame, they result in
many environmental and cost concerns for industries.
They require high-energy input alongside highpressure reactors and can produce toxic compounds
and wastewaters (Isroi et al., 2011).
Biological pretreatment methods include the use of
microorganisms in order to delignify the lignocellulose
material (Ravichandra et al. 2013; Dashtban et al.,
2009). The enzymes produced by the microorganisms
selectively disrupt the fibril and lignin structures of
the plant cell wall and provide the advantages of lower
energy demands, minimal waste production and
reduced effects on the environment (Isroi et al., 2011;
Dashtban et al., 2009). Microbial delignification is a
gentle and effective approach to remove up to 31.59%
lignin from biomass such as corn stover (Wan and Li,
2010) but results in a low rate of downstream hydrolysis.
Pretreatment times required for direct microbiological
methods are lengthy, being typically from 18 to 35 d.
Nonetheless, enzymatic delignification is an alternative
option and different “-omics” technologies are likely to
yield new enzymatic delignification systems from
TECHNOLOGICAL ROUTES FOR BIOENERGY PRODUCTION
different white rot and brown rot fungi (Martinez et al.,
2009).
The method chosen for pretreatment is dependent
upon the lignocellulosic material and the hydrolysis to
be carried out afterward. If the hydrolysis step is accompanied by microbial enzymes, which are optimized at a
lower pH (4e6), an acidic pretreatment is preferred as
the first step in the bioconversion process (Dashtban
et al., 2009).
Hydrolysis
Hydrolysis is the process by which the lignocellulose
polymers are reduced (saccharified) to yield fermentable
sugars (hexoses and pentoses) (Harris and DeBolt, 2010).
There are two methods of hydrolysis used within the
bioenergy and biorefining processes, namely, acid hydrolysis and enzymatic hydrolysis (Potumarthi et al.,
2013, 2012; Dashtban et al., 2009; Ong, 2004).
Acid hydrolysis is the older method of the two and
has been implemented on an industrial scale since
World War I. In this particular process, dilute or concentrated acid, normally H2SO4 as it is cheapest, is used to
hydrolyze the cellulose with the reaction temperatures
dependent upon the molarity; dilute acids require
higher temperatures (above 200 C) while concentrated
acids require lower temperatures. The acid hydrolysis
approaches are less attractive due to low yields with
dilute acid and the recovery and environmental factors
involved with use of concentrated acids (Ong, 2004).
In enzymatic hydrolysis, the lignocellulose is broken
down into the corresponding monomeric sugars by specific enzymes produced from bacteria or fungi ( Coyne
et al., 2013; Gupta et al., 2013; Ong, 2004; Dashtban
et al., 2009). This approach is more complex, expensive
and time consuming, in comparison to the acid hydrolysis approach, but has the advantage of little or no byproducts to dispose of at the end of the biorefining
process (Ong, 2004) and it can be used for more selective
fractionation in a biorefinery context (Menon and Rao,
2012). Pretreatment of lignocelluloses with acid or alkali
partially removes the lignin and hemicellulose but also
substantially disrupts the fibrillar structure of biomass.
Therefore, acid or alkali pretreated lignocellulosic
biomass can be saccharified enzymatically to produce
fermentable sugars. This results in faster hydrolysis
rates and higher glucan enzymatic digestibility. A
common belief is that lignin removal in particular
promotes faster and more efficient enzymatic cellulose
hydrolysis (Zhu et al., 2008).
Fermentation
Fermentation, the third step of bioconversion, converts the hydrolysates, mainly glucose, xylose, arabinose
29
and mannose to bioethanol using microorganisms. In
addition to bioethanol, fermentation can be used to
generate other useful end products, e.g. biobutanol, fatty
acids, lactic acid, bioplastics or other biochemicals. The
hydrolysates are often detoxified before fermentation
due to the production of inhibitory compounds, such
as phenolics and furan derivatives, in the pretreatment
and hydrolysis steps (Dashtban et al., 2009; Ong, 2004).
Saccharomyces cerevisiae is the most commonly used
microorganism as it has a high fermentation rate and
the application of recombinant DNA techniques has
enabled the bioengineering of strains, capable of
converting arabinose and xylose, as well as glucose, to
bioethanol (Dashtban et al., 2009). This allows utilization
of a larger amount of the hydrolysates, thus giving a
higher percentage yield of bioethanol.
Combined Pretreatment, Hydrolysis and
Fermentation Strategies
Different combinations of the first three bioconversion
steps have been investigated in order to reduce
production costs, increase end-product yield and reduce
time required for bioconversion. Sequential hydrolysis
and fermentation provides the opportunity of optimizing
each process separately, although it can result in the use
of large amounts of enzymes such as b-glucosidase to
overcome end-product inhibition during the hydrolysis
making this a costly process (Blanch, 2012; Dashtban
et al., 2009). Simultaneous saccharification and fermentation (SSF) combines both steps into one reaction, which in
theory allows direct fermentation of hydrolysates into
bioethanol with a reduction in enzyme costs. However,
involved both reactions and end-product yields can be
compromised in SSF (Dashtban et al., 2009; Ong, 2004).
Another method termed consolidated bioprocessing can
be used to combine all three steps into one with the use
of one or many microorganisms (Hasunuma et al., 2013;
Matano et al., 2013; Amore and Faraco, 2012; Blanch,
2012; Hasunuma and Kondo, 2012; Girio et al., 2010;
Dashtban et al., 2009). This particular process possesses
the potential to reduce bioethanol production costs to
competitive fuel levels. Although significant advances
have been made with regard to CBP (Hansunuma et al.,
2013; Hyeon et al., 2013; Matano et al., 2013; Olson
et al., 2012), more research into the microbial cell factories, enzymes and physicochemical and catalytic conditions (pH, temperature, and synergies) is required (Olson
et al., 2012; Menon and Rao, 2012; Van Dyck and
Pletschke, 2012).
However, key technologies are available to convert a
variety of biomass into electricity, gas, or different liquid
fuels (Table 2.3). These technologies use various types
of feedstocks, and are produced in different ways
(Farine et al., 2011).
30
TABLE 2.3
2. BIOENERGY RESEARCH: AN OVERVIEW ON TECHNOLOGICAL DEVELOPMENTS AND BIORESOURCES
Biomass to Bioenergy Routes for Important
Feedstocks
Conversion
Route
Energy
Product
Type
Starch (Wheat, Sorghum,
Barley, Oat and Triticale Grain)
Fermentation
Ethanol
Sucrose (C-Molasses and
Sugarcane Sugar)
Fermentation
Ethanol
Oil (Canola, Animal Tallow,
Waste Oil Mixture, Algae,
Pongamia Seed)
Transesterification Biodiesel
Lignocellulose (Stubble from Annual
Crops, Bagasse, Sugarcane (Whole
Plant), Products and Residues from
Native Forest, and Hardwood and
Softwood Plantations, Wood Waste
Mixture and Coppice Eucalyptus)
Enzymatic
fermentation
Ethanol
Lignocellulose (Stubble from Annual
Crops, Bagasse, Sugar Cane (Whole
Plant), Products and Residues from
Native Forest, and Hardwood and
Softwood Plantations, Wood Waste
Mixture and Coppice Eucalyptus)
Combustion
Electricity
Feedstock/Biomass
Source: Farine et al., 2011.
Advanced Biomass-to-Biofuels
Development Platform
The lignocellulosic substrates include woody substrates such as hardwood (birch, aspen, etc.) and softwood (spruce, pine, etc.), agri residues (wheat straw,
sugarcane bagasse, corn stover, etc.), dedicated energy
crops (switchgrass, willow, hemp, Miscanthus, etc.),
weedy materials (Eichhornia crassipes, Lantana camara,
etc.), and municipal solid waste (food and kitchen waste,
etc.). The diversity of raw materials will allow the decentralization of fuel production with geopolitical, economic, and social benefits (Van Dyck and Pletschke,
2012; Wyman, 2007). Despite the success achieved in
the laboratory, there are limitations to success with
lignocellulosic substrates on a commercial scale (Chandel and Singh, 2011) as each source of biomass brings
a unique technological challenge.
The advanced biomass-to-biofuels development
platform has multiple goals, including the use of new
enzymes to take full advantage of available carbohydrates, the development of new lines of bioenergy
crops with increased fermentation productivity
(Carpita, 2012; Abramson et al., 2010), the development
of new uses for coproducts, and the reduction of processing and energy costs. Lignocelluloses have three
main components: cellulose, hemicelluloses, and lignin.
Cellulose is the most abundant organic polymer on the
earth. It is a homopolymer of sugars containing six
carbon atoms linked together in a chain that constitutes
the largest proportion of the plant cell wall. Hemicelluloses are heteropolysaccharides consisting of short
branched chains of hexoses, e.g. mannose units in
mannans and pentoses such as xylose units in xylans
(Chandel et al.,. 2010; Girio et al., 2010; Kuhad
et al., 1997).
Table 2.4 summarizes the basic cell wall composition
of some important lignocellulosic biomass used in bioenergy generation. In general, hardwoods contain
18e25% lignin, 45e55% cellulose, and 24e40% hemicelluloses, while softwoods contain 25e35% lignin,
45e50% cellulose, and 5e35% hemicelluloses. Grasses
normally contain 10e30% lignin, 25e40% cellulose,
and 25e50% hemicelluloses (Balat, 2011; Sanchez, 2009;
Howard et al., 2003; Malherbe and Cloete, 2003; Betts
et al., 1991). Agri-biomass commonly comprises about
40% cellulose, 25% hemicellulose and 18% lignin. The
structure and components of the cell walls of weeds
are significantly different from those of most plant
species, which may influence digestibility during the
bioconversion process to bioethanol (Van Dyck and
Pletschke, 2012; Chandel and Singh, 2011; Sarkar et al.,
2009).
The hydrolytic breakdown of cellulose in nature
involves the use of enzymes including cellobiohydrolases, endoglucanases and b-glucosidases produced by
microbes or other biological agents, alone or in combination (Turner et al., 2010; Kuhad et al., 1997). More recent
studies have shown that additional oxidoreductase
enzymes (glycosyl hydrolase family 61 polysaccharide
monooxygenases and cellobiose dehydrogenase) are
essential components in a complete cellulose-degrading
enzyme system (Horn et al., 2012; Kittl et al., 2012; Langston et al., 2011). The sugar chains of cellulose can be hydrolyzed to produce glucose and cellooligosaccharides,
most of which can be fermented using ordinary baker’s
yeast. To attain economic feasibility a high ethanol
yield is a necessity. Producing monomer sugars from cellulose and hemicellulose at high yields is far more
difficult than deriving sugars from sugar- or starchcontaining crops, e.g. sugarcane or maize (Van Dyck
and Pletschke, 2012; Tuohy et al., 1994). Therefore,
although the cost of lignocellulosic biomass is far
lower than that of sugar and starch crops, the cost of
obtaining sugars from such materials for fermentation
into bioethanol has historically been far too high to
attract industrial interest. For this reason, it is crucial to
solve the problems involved in the conversion of
lignocellulosic biomass to sugar and further to ethanol
(Agbor et al., 2011; Galbe and Zacchi, 2002).
The heterogeneity in feedstock and the influence
of different process conditions on microorganisms
and enzymes makes the biomass-to-ethanol process
31
TECHNOLOGICAL ROUTES FOR BIOENERGY PRODUCTION
TABLE 2.4 Cell Wall Compositions (%) of Different Lignocellulosic Sources
Biomass Type
Cellulose
Hemicellulose
Lignin
References
Birch
40.0
23.0
21.0
Olsson and Hahn-Hägerdal, 1996
Willow
37.0
23.0
21.0
Olsson and Hahn-Hägerdal, 1996
Aspen
51.0
29
16
Olsson and Hahn-Hägerdal, 1996
Spruce
43
26
29
Olsson and Hahn-Hägerdal, 1996
Pine
44e46.4
8.8e26
29.4
Wayman and Parekh, 1990; Olsson and
Hahn-Hägerdal, 1996
Hemlocks
47.5
22.0
28.5
Wayman and Parekh, 1990
HARD WOOD
SOFT WOOD
AGRICULTURAL FEEDSTOCKS/RESIDUE
Sugarcane Bagasse
33
30
29
Neureiter et al., 2002
Sorghum Bagasse
44.4
35.5
3.9
Dogaris et al., 2009
Wheat Straw
37e38.2
21.2e29
15e23.4
Wiselogel et al., 1996; Lee et al., 2007a
Corn Stover
37.5e26
22.4e29
17.6e19
Zhu et al., 2008; Lee et al., 2007a
Rice Straw
33.0
26.0
7.0
Severe and ZoBell, 2012
Barley Straw
43.3
29.6
7.7
Severe and ZoBell, 2012
Oat Straw
41.0
16.0
11.0
Mussatto and Teixeira, 2010; Severe and
ZoBell, 2012
Sunflower
34.06e42.1
5.18e29.7
7.72e13.4
Mussatto and Teixeira, 2010; Tutt and Olt, 2011
Silage
39.27
25.96
9.02
Tutt and Olt, 2011
Jerusalem Artichoke
20.95e25.99
4.50e5.48
5.05e5.70
Tutt and Olt, 2011
Reed
49.40
31.50
8.74
Tutt and Olt, 2011
Coffee Grounds
8.6
37.6
NA
Mussatto et al., 2011
Rye Straw
37.6
30.5
19.0
Mussatto and Teixeira, 2010
Soya Stalks
34.5
24.8
19.8
Mussatto and Teixeira, 2010
Leaves (Mixed Biomass)
15e20
80e85
0
Sun and Cheng, 2002; Harmsen et al., 2010
Nut Shells
25e30
25e30
30e40
Sun and Cheng, 2002; Harmsen et al., 2010
Orchard Grass
52.3
42.9
6.6
Jung and Vogel, 1986
Smooth Bromegrass
49.8
41.9
7.6
Jung and Vogel, 1986
Indiangrass
49.8
43.1
6.7
Jung and Vogel, 1986
Big Bluestem
47.6
47.4
4.5
Jung and Vogel, 1986
Ensiled Grass
37.85
27.33
9.65
Tutt and Olt, 2011
Coastal Bermuda grass
25
35.7
6.4
Sun and Cheng, 2002; Harmsen et al., 2010
Grasses (Mixed Biomass)
25e40
35e50
10e30
Sun and Cheng, 2002; Harmsen et al., 2010
Switchgrass (Perennial Grass)
31.0e37
20.4e29
17.6e19
Wiselogel et al., 1996; Lee et al., 2007b;
Tutt and Olt, 2011
Miscanthus
40e42
18e30.15
7e25
Sørensen et al., 2008; Tutt and Olt, 2011
Alfalfa
33
18
8
Sreenath et al., 2001
ENERGY CROPS
(Continued )
32
TABLE 2.4
2. BIOENERGY RESEARCH: AN OVERVIEW ON TECHNOLOGICAL DEVELOPMENTS AND BIORESOURCES
Cell Wall Compositions (%) of Different Lignocellulosic Sourcesdcont’d
Biomass Type
Cellulose
Hemicellulose
Lignin
References
Hemp
53.86
10.60
8.76
Tutt and Olt, 2011
Jatropha
34
10
12
Singh et al., 2008, Abreu, 2009; Jingura et al.,
2010; Yamamura et al., 2012
Algae
7.1
16.3
1.52
Ververis et al., 2007
Saccharum spontaneum
45.10
22.75
24.38
Chandel et al., 2009
Lantana camara
45.1
17.0
27.25
Pasha et al., 2007
Prosopis juliflora
45.5
20.38
24.65
Gupta et al., 2009
Eichhornia crassipes
18.2
48.7
3.50
Kumar et al., 2009
Crofton Weed Stem
37.6
22.4
16.4
Zhao et al., 2007
C. odorata (Siam Weed)
41.0
17.3
20.7
Zhao et al., 2010
Processed Paper/Black Paper
47
25
12
Ackerson et al., 1991
Waste Papers from Chemical
Pulps
60e70
10e20
5e10
Sun and Cheng, 2002; Harmsen et al., 2010
Newspaper
40e61
25e40
18e30
Ackerson et al., 1991; Sun and Cheng, 2002
Brown Bin Waste/Food Waste
42.51e49.53
0.73e7.41
10.9e14.33
Komilis and Ham, 2003; Lamborn, 2009
Sorted Refuse
60
20
20
Sun and Cheng, 2002; Harmsen et al., 2010
Primary Wastewater Solids
8e15
NA
24e29
Sun and Cheng, 2002; Harmsen et al., 2010
Solid Cattle Manure
1.6e4.7
1.4e3.3
2.7e5.7
Sun and Cheng, 2002; Harmsen et al., 2010
Poultry Waste
11
16
4
FAO, 1980
Spent Mushroom Compost
38
19
25
Jordan et al., 2008
Swine Waste
6.0
28
NA
Sun and Cheng, 2002; Harmsen et al., 2010
Dried Distilled Grains with
Solubles (DDGS)
16e22
8.2e15
0e3.1
Blaschek and Ezej, 2007; Kim et al., 2008;
Pasangulapati et al., 2012,
Eastern Red Cedar
40.3
8.5
35.9
Pasangulapati et al., 2012
Poplar
39.8
14.8
29.1
Blaschek and Ezej, 2007
WEEDS
SOLID WASTE
FOREST RESIDUE
NA, data not available.
complex. Ethanol can be produced from lignocellulosic
materials in various ways. The main difference between
the process alternatives is the hydrolysis steps, which as
mentioned previously, can be performed by dilute acid,
concentrated acid or enzymatically. Some of the process
steps are more or less the same, independent of the hydrolysis method used. For example, enzyme production
will be omitted in an acid hydrolysis process; likewise,
the recovery of acid is not necessary in an enzyme hydrolysis process (Galbe and Zacchi, 2002).
To achieve lower production costs, the sustainable
supply of cheap raw materials is a necessity. It is also
essential to ensure that all components of the biomass
are utilized and resulting by-products and wastes are
used in a biorefinery system. When lignocellulosic raw
materials are used, the main by-product is lignin, which
can be used as an ash-free solid fuel for production of
heat and/or electricity, for which there are no foreseeable market limits. However, in addition, lignin can be
used for a range of additional high-value products that
have the potential to enhance overall process economics
significantly (Azadi et al., 2013; Lange et al., 2013;
Doherty et al., 2011; Collinson and Thielemans, 2010).
Accordingly, it will only be possible to produce large
amounts of low-cost ethanol if lignocellulosic feedstocks
such as fast-growing trees, grass, aquatic plants,
waste products (including agricultural and forestry
residues) and municipal and industrial waste are used
BIOENERGY RESOURCES AND BIOFUELS DEVELOPMENT PROGRAM
(Van Dyck and Pletschke, 2012; Wheals et al., 1999). The
potential of using lignocellulosic biomass for energy
production is even more apparent when one realizes
that it is the most abundant renewable organic component in the biosphere (Claassen et al. 1999). Currently
enzyme hydrolysis has high yields (70e85%) of bioconversion, and improvements are still possible (85e95%)
(Van Dyck and Pletschke, 2012; Sills and Gossett, 2011;
Redding et al., 2010; Hu and Wen, 2008).
BIOENERGY RESOURCES AND BIOFUELS
DEVELOPMENT PROGRAM
Current bioenergy resources consist of residues from
forestry and agriculture, various organic waste streams
and dedicated biomass production from pasture land,
wood plantations and sugar cane (Figure 2.2). At present, the main biomass feedstocks for electricity and
heat generation are forestry and agricultural residues
and municipal waste in cogeneration and cofiring power
plants. In the longer term, lignocellulosic crops could
provide bioenergy resources for second-generation biofuels, which are considered more sustainable, provide
land use opportunities and will reduce the competition
with food crops (http://www.ga.gov.au/image_cache/
GA16706.pdf).
Major feedstock sources for future biofuel production
are likely to be high biomass producing plant species
such as poplar, pine, switchgrass, sorghum maize,
Miscanthus, hemp, Jatropha, willow and cassava. With
33
growing interest in the utilization of plant biomass for
the production of ethanol and other biofuels, the use
of plant species as biofuel feedstocks has become a focal
point in research. Due to concerns about diverting grain
and seed from human food and livestock feed to biofuel
feedstock production, emphasis has shifted to the use of
lignocellulose-derived biofuel production, and research
is now directed at improving not only lignocellulosic
yield but also quality traits in these species (Banerjee,
2011; Mueller et al., 2011; Tyner, 2010).
A long-term opportunity exists to produce fuels from
nonedible lignocellulosic biomass from plants (Heather
and Somerville, 2012). Sugarcane, energy cane, elephant
grass, switchgrass, and Miscanthus have intrinsically
higher light, water and nitrogen use efficiency and are
fast-growing biomass/crops for bioenergy work program. Work on perennial grasses such as switchgrass
(Panicum spp.), prairie cordgrass (Spartina spp.), big
bluestem (Andropogon spp.), little bluestem (Schizachyrium spp.) and others could produce significant biomass
in a variety of biomass throughout the northern plains
and southeastern grasslands in the United States
(Gonzalez-Hernandez et al., 2009). Woody biomass can
be harvested sustainably for lumber and paper and
may, therefore, provide biofuel feedstock for some regions (Malmsheimer et al., 2011). Table 2.5 summarizes
the countrywise contribution of current biofuel yield
from different feedstocks.
As mentioned previously, biomass energy can come
from numerous sources and produce several types of
fuels. Ethanol is typically produced from biomass
FIGURE 2.2
Share of biomass sources in the world.
Source: IEA, 2009. (For color version of this figure, the reader
is referred to the online version of this book.)
34
TABLE 2.5
2. BIOENERGY RESEARCH: AN OVERVIEW ON TECHNOLOGICAL DEVELOPMENTS AND BIORESOURCES
Countrywise Contribution of Current Biofuel Yield from Their Available Feedstocks
Crop Yield
Conversion
Efficiency
Total Biofuel
Yield
Crop
Global/Country
Wise Estimates
Biofuel Types
(Tons/ha)
(l/ton)
(l/ha)
Sugar Beet
Global
Bioethanol
46.0
110
5060
SugarCane
Global
Bioethanol
65.0
70
4550
Cassava
Global
Bioethanol
12.0
180
2070
Maize
Global
Bioethanol
4.9
400
1960
Rice
Global
Bioethanol
4.2
430
1806
Wheat
Global
Bioethanol
2.8
340
952
Sorghum
Global
Bioethanol
1.3
380
494
Sugar Cane
Brazil
Bioethanol
73.5
74.5
5476
Sugar Cane
India
Bioethanol
60.7
74.5
4522
Oil Palm
Malaysia
Biodiesel
20.6
230
4736
Oil Palm
Indonesia
Biodiesel
17.8
230
4092
Maize
United States
Bioethanol
9.4
399
3751
Maize
China
Bioethanol
5.0
399
1995
Cassava
Brazil
Bioethanol
13.6
137
1863
Cassava
Nigeria
Bioethanol
10.8
137
1480
Soybean
United States
Biodiesel
2.7
205
552
Soybean
Brazil
Biodiesel
2.4
205
491
Jatropha
India
Biodiesel
2.0
340
680
Sources: Rajagoapl and Zilberman, 2007, Naylor et al., 2007, FAO, 2008.
high in carbohydrates (sugar, starch and cellulose) during a fermentation process. Recent developments in
fermentation processes now allow almost any plant
type to be used to produce ethanol. The most promising natural oils, such as rapeseed oil, have been
used to produce biodiesel, which performs much like
petroleum-derived diesel fuel. Apart from agricultural,
forestry and other by-products, the main source of
lignocellulosic biomass for second-generation biofuels
is likely to be from “dedicated biomass feedstocks”,
such as certain perennial grass and forest tree species.
Genomics, genetic modifications and other biotechnologies are all being investigated as tools to produce
plants with desirable characteristics for secondgeneration biofuel production, for example, plants
that produce less lignin (a compound that cannot be
fermented into liquid biofuel), plants that produce enzymes themselves for cellulose and/or lignin degradation, or plants that produce increased cellulose or
overall biomass yields. Grass, leaves, agri crops, agricrop residues and currently available nonfood plant
biomass are the dominant source of lignocellulosic
materials (Carpita, 2012; Ambavaram et al., 2011;
Abramson et al., 2010; Davison et al., 2006; Nguyen
et al., 1999, 2000).
Bioenergy resources used in current biofuels development programs, potential future resources and the
related bioenergy outputs are summarized in Table 2.6.
Bioenergy resources are difficult to estimate due to their
multiple and competing uses. Production statistics exist
for current commodities such as grain, sugar, pulp wood
and saw logs; however, these commodities are currently
largely committed to food, animal feed and materials
markets. Potential feedstocks for the future include
modified strains of existing crops, new tree crops
and algae. There are many factors to be taken into account for each bioenergy resource, such as moisture content, resource location and distribution, and type of
conversion process that is most suitable. Different sources of biomass require very different production
systems and therefore a variety of sustainability issues
can arise. These range from very positive benefits (e.g.
use of waste material, or growing woody biomass on
degraded agricultural land) through to large-scale
diversion of high-input agricultural food crops for biofuels (O’Connell et al., 2009).
TABLE 2.6 Potential Resources and the Bioenergy Outputs
Biomass Groups
Current Resources
Agriculture- Related
Wastes and
By-Products
Livestock wastes:
• Manure
• Abattoir wastes solids
By-products:
• Wheat starch
• Used cooking oil
Electricity and
heat generation
Transport biofuel
production
Crop and food residues from harvesting and
processing:
• Large scale: rice husks, cotton ginning, and
cereal straw
• Small scale: maize cobs, coconut husks and
nut shells
• Crop stubble: The residue remaining after
the harvest of grain crops such as wheat,
barley and lupins
• Grasses (various varieties including wild
sorghum, kangaroo grass, tall fescue,
perennial ryegrass)
Electricity and
heat generation
Sugar Cane
Bagasse (the stem residue remaining
after the crushing to remove sugarrich juice from sugar cane), fibrous
residues of sugar cane milling
process sugar and C-molasses
Electricity and
heat generation
Transport biofuel
production
Trash, leaves and tops
from harvesting
Electricity and
heat generation
Energy Crops
High yield, short rotation crops
grown specifically:
• Sugar and starch crops
• Oil-bearing cropsdsunflower,
canola, juncea and soya beans
• Palm oil
• Jatropha (plant that produces
seeds containing inedible oil
content of 30e40% seed weight)
Transport biofuel
production
Woody crops, genetically modified (GM)
crops, tree crops, coppice (short rotation tree
species, e.g. eucalyptus, poplar), woody weeds
(e.g. camphor, laurel), new oilseed (Pongamia,
camelina, and cotton seed), sugar (agave)
crops, algae (micro and macro), and
Halophytes (salt water and coastal/desert
plant varieties, e.g. salicornia, marsh grasses,
mangroves)
Electricity and
heat generation
Transport biofuel
production
Forest and Forest
Residues
Wood from plantation forests
Electricity and
heat generation
Wood from plantation forests, native forestry
operations, bark, sawdust, pulpwood (wood
used for processing into paper and related
products) and harvest residues
Electricity and
heat generation
Transport biofuel
production
Wood-Related
Waste
Saw mill residues:
• Wood chips and saw dust
Pulp mill residues:
• Black liquor and wet wastes
Electricity and
heat generation
Commercial and industrial waste, food-related
wastes, garden organics, palettes, furniture,
paper and cardboard material and urban
timber
Electricity and
heat generation
Meat and livestock by-product
Electricity and
heat generation
Electricity and
heat generation
Landfill Gas
Methane emitted from landfills
mainly municipal solid wastes and
industrial wastes
Electricity and
heat generation
Sewage Gas
Methane emitted from the solid
organic components of sewage
Electricity and
heat generation
Tallow
Bioenergy Type
Transport biofuel
production
35
Source: Sustainable Aviation Fuel Road Map 2011; Batten and O’Connell 2007; IEA, 2006.
Future Resources
BIOENERGY RESOURCES AND BIOFUELS DEVELOPMENT PROGRAM
Urban Solid
Waste
Bioenergy Type
36
2. BIOENERGY RESEARCH: AN OVERVIEW ON TECHNOLOGICAL DEVELOPMENTS AND BIORESOURCES
SUSTAINABILITY
Consideration of the sustainability of biomass to bioenergy programs based on utilizing lignocellulosic feedstocks is both timely and important in terms of the
current plans for commercial valorization of this sector
(Third International Conference on Lignocellulosic
Ethanol;
http://www.biofuelstp.eu/events/3rd-icleapril-2013.pdf). Sustainability of second-generation bioenergy is also been driven and supported by European
and International directives and certification programs,
including the Renewable Energy Directive 2009/28/EC
(EU-RED), International Sustainability and Carbon Certification programs and standards, the Roundtable on
Sustainable Biofuels and the Global Bioenergy Partnership (Scarlat and Dallemand, 2011). The sustainability
of biomass to bioenergy programs has been a subject
of great interest in Sweden, Canada and the western
United States as well as in some Asian countries
(Nguyen et al., 1999, 2000; Wu et al., 1999). The ecological and sustainable potential of biomass sources for
fuel production is estimated to reach 130 TWh/year in
Sweden by around 2020 (Parrika, 1997). Issues such as
land use, environmental impact, logistics and resource
management must be considered in terms of feedstock
production. In addition, the sustainability of the bioconversion process(es) and downstream outputs, and the
ability to meet REN and GHG emission targets must
be carefully evaluated. High on the priority list of
most national governments is the need to support rural
development and sustain the local and national economies. Consequently, biomass to bioenergy programs
need to be subjected to detailed life cycle analysis
(LCA), where all of the aforementioned considerations
are evaluated. LCA can also help derisk biomass to bioenergy processes (Buonocore et al., 2012). The use of
conventional crops for energy use can also be expanded,
with careful consideration of land availability and food
demand. For sustainable bioenergy development lignocellulosic crops (both herbaceous and woody) could be
produced on marginal, degraded and surplus agricultural lands and, in theory, could provide the bulk of
the biomass resource in the medium term along with
aquatic biomass (algae) as a significant contribution in
the longer term (Richardson, 2008). However, significant
progress needs to be made to scale-up algal production
and processing in an economic manner to make algal
biomass to bioenergy a commercially viable option.
First-generation biofuels face both social and environmental challenges, largely because they use food crops
that could lead to food price increases and possibly indirect land use change (ILUC). Nonfood biomass, e.g.
lignocellulosic feedstocks such as organic wastes,
forestry residues, high-yielding woody or grass energy
crops and algae have the potential to provide possible
solution to this problem, if developed and managed in
a sustainable manner. The use of these feedstocks for
second-generation biofuel production would significantly decrease the potential pressure on land use,
improve GHG emission reductions when compared to
some first-generation biofuels, and result in lower environmental and social risks (Bauen et al., 2009 IEA
Report).
The environmental impacts of conventional crop production have been researched in far greater detail than
those of lignocellulosic crop production. Technically,
the potential supply of energy from lignocellulosic
biomass depends largely on the amount of land that is
available for growing energy crops. In parallel, the
need to meet the growing worldwide demand for
food, protect biodiversity, manage soil and water reserves sustainably and fulfill additional socioeconomic
objectives must be addressed. Bioenergy crop production can have positive impacts, for example, it can help
to improve the soil structure and fertility of degraded
lands. However, conversion of areas with sparse vegetation to high-yielding lignocellulosic plantations or ILUC
may lead to substantial reductions in ground water
recharge and water supply, which may lead to deteriorating conditions in water-scarce areas (Upham et al.,
2011; Cabral et al., 2010; Smeets and Faaij, 2010). The
cultivation of short rotation biomass crops may lead to
nutrient removal or depletion (van den Broek et al.,
2000), and important habitats may be lost through both
land conversion and intensification (Pedroli et al.,
2012). Aesthetic considerations also need to be considered in terms of the impact of cultivating and harvesting
short rotation bioenergy crops (Hardcastle, 2006). Sound
agricultural methods exist that can achieve major increases in feedstock productivity in neutral or positive
environmental conditions in order to provide a continuous supply of energy crops/biomass waste, which
can support the important role of bioenergy chains
in socioeconomic development (Figure 2.3; Dornburg
et al., 2008). The issue of biomass logistics is also a factor
that needs careful consideration in terms of feedstock
supply, processing technology selection, sitting of commercial production facilities and overall sustainability
(Stephen et al., 2010).
Recent studies have shown the potential of recycled
wastewater for biomass production in an integrated natural water treatment approach (Fedler and Duan, 2011),
which suggests that through innovative and careful
consideration of environmental impacts solutions can
be found that have multiple potential benefits. It has
been suggested that the application of strict sustainability criteria, standards and a requirement for certification
(Scarlat and Dallemand, 2011; Schubert and Blasch, 2010;
van Dam et al., 2010) of feedstocks, land use and
SUSTAINABILITY
37
FIGURE 2.3 Sustainability of bioenergy crop supply chains and environmental effects. Source: Dornburg et al., 2008. (For color version of this
figure, the reader is referred to the online version of this book.)
bioenergy programs globally could both alleviate
concerns and provide a more harmonized framework
globally for sustainable development of secondgeneration bioenergy (Cornelissen et al., 2012; Van
Stappen et al., 2011).
Bioenergy Feedstocks and Dedicated
Biofuel Crops
There are two principal sources of biomass-based
REN for second-generation bioenergy and biofuels: (1)
wastes and residues from agriculture and forestry and
(2) dedicated bioenergy crops. Wastes such as wood
and agricultural residues, municipal wastes, and
poultry litter are typically less expensive to supply to
end point users, and are likely to play an important
role in early development of commercial-scale REN supplies. However, analyses of future demand for REN
indicates that these wastes may be capable of supplying
only 14e30% of the total potential production of cellulosic ethanol and only approximately 18e60% of the
production potential that could be derived from producing energy crops on currently idle or potentially
available agricultural lands (Robert and Abbott, 2012;
Brown, 2009; Lynd et al., 1991). Thus dedicated energy
crops will be required to meet the demands of a growing
REN market. Such crops, grown in the vicinity of the end
point industrial user and specifically for the conversion
process being used, offer important advantages of more
systematic control of fuel quality, supply, and price
stability than wastes derived from dispersed sources,
which will be subject to alternative competitive end
point uses and associated price fluctuations. The potential feedstocks for second-generation biofuel production
considered in this study are biomass from crop residues,
other nonfood energy crops, wood/forestry residues,
Miscanthus, willow, hemp, Jatropha, switchgrasses
and algae (Bauen et al., 2009).
Lignocellulosic Feedstocks
The major components of lignocellulosic feedstocks
are cellulose and hemicellulose that can be converted
to sugars through a series of thermochemical and biological processes and eventually fermented to bioethanol, other solvent biofuel or biogas. Therefore,
lignocellulosic feedstocks are mainly categorized as
agricultural residues (e.g. crop residues and sugarcane
bagasse), forest residues, herbaceous and woody energy
38
2. BIOENERGY RESEARCH: AN OVERVIEW ON TECHNOLOGICAL DEVELOPMENTS AND BIORESOURCES
crops. There are three principal technological end points
for bioenergy crops: (1) conversion to liquid fuels, (2)
combustion (alone or in combination with fossil fuels)
to produce heat, steam, or electricity, and (3) gasification
to simpler gaseous products that can have various uses.
Agricultural residues can differ significantly in their
chemical composition, which can lead to different bioenergy and biofuel yields per unit feedstock (Carriquiry
et al., 2010). Commonly available agricultural residues
for bioenergy production are barley straw, corn stover,
rice straw, sorghum straw, wheat straw and sugarcane
bagasse and having available carbohydrate contents of
70%, 58.3%, 49.3%, 61%, 54% and 67.2%, respectively.
If the available carbohydrate of these feedstocks is fully
converted to bioenergy, they can potentially yield
approximately 367, 503, 392, 199, 1413 and 3133 liters
biofuel/hectare, respectively (Carriquiry et al., 2010;
US-DOE, 2008a, b; Kim and Dale, 2004). Forest residues
include logging residues produced from harvest operations, fuel wood extracted from forestlands, and primary and secondary wood-processing mill residues
(Perlack et al., 2005). Some important forest residues
include hardwoods (black locust and hybrid poplar),
softwoods (eucalyptus and pine) and switchgrass,
which comprise approximately 57.15e66.45% carbohydrate, depending on the residue (Menon and Rao,
2012; van Dyck and Pletschke, 2012; Alves et al., 2010;
Carriquiry et al., 2010; US-DOE, 2008a, b; Merino and
Cherry, 2007; Hamelinck et al., 2005 Howard et al.,
2003). However, several factors restrict the potential
use of forest residues for biofuel production (Perlack
et al., 2005). The first factor is the economic cost of
transportation as limited accessibility largely increases
the operational costs of logging/collection activities.
Another factor is a potential reduction in recoverability
of harvest areas due to environmental considerations
(Richardson, 2008). Therefore a shift in research efforts
to dedicated biofuel crops is taking place in order to
have a continuous supply of feedstock for secondgeneration bioenergy requirements.
Dedicated Bioenergy Crops
From the industrial perspective, both fuel cost and
quality relative to corresponding fossil fuels are essential considerations (Menon and Rao, 2012; Graham
et al., 1995). Dedicated bioenergy crops can be broadly
categorized into grassy (herbaceous or forage) and
woody (tree) crops. Perennial forage crop species are a
promising feedstock source for second-generation biofuels. Switchgrass (P. virgatum L.) is frequently
mentioned because of its relatively low water and nutrition input and costs, positive environmental impact and
adaptability to low-quality land (Keshwani and Cheng,
2009). Switchgrass has a wide and natural distribution
from Central America to Southern Canada. Switchgrass
as an energy crop is classified as a lignocellulosic crop
because the cell walls primarily are digested to form
sugars, which can subsequently be fermented to produce liquid fuels. Switchgrass research has been conducted cooperatively by the US Department of
Agriculture and the University of Nebraska since the
mid-1930s, with a primary focus on bioenergy since
1990 at several institutions. Progress has been made in
switchgrass breeding and genetics, molecular genetics,
fertility management, production economics and energetics, harvest and storage management, ecosystem services and ethanol yield. Other perennial forage crops
such as alfalfa (M. sativa L.), reed canary grass (P. arundinacea L.), napier grass (Pennisetum purpureum Schumach.), and Bermuda grass (Cynodon spp.) also have
potential as dedicated bioenergy crops (Carriquiry
et al., 2010).
The rationale for developing purpose-grown lignocellulosic crops for energy is that less intensive production
techniques and poorer quality land can be used for these
crops, thereby avoiding competition with food production on better quality land. A potential limitation of
some forms of bioenergy is that biochemical composition, energy content, and contamination with alkali
metals can limit their usefulness for certain industrial
applications (Miles et al., 1993). Analysis of the energy
content, the levels of alkali and ash and combustion
properties of switchgrass indicates that this biomass is
a versatile feedstock that is well suited for use in combustion, gasification, and liquid fuel production systems
(Alonso et al., 2010; McKendry, 2002; McLaughlin
et al., 1996). A complete field-validated biomass production system has been developed for the Midwest and
Central Plains of the United States. Even with favorable
economic and sustainability results from field trials,
switchgrass for bioenergy has not been adopted on a
large or commercial scale as yet. This is likely to be
due to a number of factors, including the need for efficient conversion technologies, farmers’ reluctance to
plant switchgrass without a viable bioenergy market
and reluctance to build commercial biorefineries
without a viable long-term feedstock supply already in
place. Production, economic, net energy and sustainability research completed to date fully supports the use
of switchgrass as a biomass energy crop (Mitchell
et al., 2012).
Another potential biofuel crop is Miscanthus sp.,
which has been used for forage and thatching in Japan
for thousands of years, and managed through burning
and grazing in vast prairies similar to those managed
by Native American tribes in the central United States
(Stewart et al., 2009). Miscanthus is a grass native to
Asia and is a compelling herbaceous biomass feedstock
for Europe (Lewandowski et al., 2003), in part because of
its cold tolerance and low nitrogen requirements.
SUSTAINABILITY
A drawback to the use of this species is that it takes 2e3
years to start full production as it must be established
and propagated by rhizome cuttings. Other major limitations are (1) limited availability of genotype, (2) losses
over winter, and (3) high costs in establishing the crop
(Carriquiry et al., 2010; Lewandowski et al., 2003). Giant
Miscanthus has been studied in the European Union and
is used commercially in some member states for
bedding, heat and electricity generation (Jones and
Walsh, 2001). Most production currently occurs in
England with some production also in Spain, Italy,
Hungary, France, and Germany. Recently, a renewed interest in this native species has occurred in China and
Japan and multiple research and commercialization projects have commenced. In the United States, research on
the use of Miscanthus began at the University of Illinois
at Urbana-Champaign in 2001 (Pyter et al., 2007) and has
expanded rapidly to other US universities. Giant Miscanthus has been proposed for use in the United States
in combined heat and power generation, as a supplement or on its own (Khanna et al., 2008; Heaton et al.,
2008). It is also a leading candidate feedstock for cellulosic ethanol (Department of Energy (DOE), 2006).
Although it is widely touted for cellulosic ethanol, giant
Miscanthus has traits that are likely to make it better
suited for thermochemical conversion processes over
biological fermentation, at least using existing technology (Williams and Douglas, 2011).
Reed canary grass is commonly used for hay and
forage. It is well adapted to temperate agroeconomic regions and to weathered soils (Carlson et al., 1996). Reed
canary grass can be slow to establish and can become an
invasive species in native wetland (Merigliano and
Lesica, 1998). Alfalfa is a forage crop that can be used
to both supply biomass feedstock and high-quality animal feed (Delong et al., 1995). Several other subtropical
and tropical grasses have been explored as potential
biomass feedstocks in the United States, including
Bermuda grass (Boateng et al., 2007), napier grass
(Schank et al., 1993), eastern gamagrass and prairie cordgrass (Carriquiry et al., 2010; Boe and Lee, 2007; Springer
and Dewald, 2004). Dedicated fast-growing woody
energy crops with potential include fast-growing tree
species. Important attributes include the relatively high
yield potential, wide geographical distribution and relatively low levels of nutrient and manpower input needed
when compared to annual crops, as well as their versatility as a source of solid and liquid energy has also
been highlighted (Smeets et al., 2007). Poplar (Populus
spp.), willow (Salix spp.), and eucalyptus are among
the species most frequently mentioned in this latter category. Biofuels produced from short rotation coppice species like willow could help reduce dependence on fossil
fuels. To maximize yields per hectare, light interception
and utilization of the plant canopy need to be optimized
39
(Cunniff and Cerasuolo, 2011; Carriquiry et al., 2010).
Poplar and willow have been grown successfully using
municipal waste-derived fertilizers and irrigated with
municipal or industrial wastewater, thereby decreasing
two waste streams yet achieving nutrient and water inputs needed for high yields (Powlson et al., 2005).
Pressure to increase the use of woody biomass for
bioenergy and biofuel production could lead to conversion of forests to plantations with short rotation tree
species, e.g. poplar (Populus spp.) and willow (Salix
spp.) (Karp et al.,2011; Carriquiry et al., 2010; Zalesny
et al., 2009). Cellulosic ethanol is derived from grasses,
agricrop and wood residues and fast-growing trees
(such as poplar or willow) and typically yields >10
times more energy than is needed to produce the fuel
(Carriquiry et al., 2010); Powlson et al., 2005. However,
with the case of crop and forest residues, the logistics of
feedstocks obtained from dedicated energy crops is still
a challenging issue to be resolved as these feedstocks
are bulky and difficult to transport.
Feedstocks for Biodiesel
Jatropha (Jatropha curcas) is an oilseed species that has
generated the most excitement in recent years in terms
of its potential as a feedstock for biodiesel production.
It is a multipurpose bush or low-growing tree, native
to tropical America that can be used as a hedge, to
reclaim land and as a commercial crop (Carriquiry
et al., 2010; Azam et al., 2005; Openshaw, 2000). Jatropha
is now grown in many tropical and subtropical regions
within Asia and Africa. The oil derived from Jatropha
has been shown to yield a biodiesel that meets European
and US quality standards (Pandey et al., 2012; Akbar
et al., 2009; Azam et al., 2005). Jatropha is known as a
diesel fuel plant; the seed can yield a substantial quantity of oil that can be converted to biodiesel without
prior refining (Carriquiry et al., 2010; Becker and
Makkar, 2009). This plant is currently underutilized
but could help in meeting the challenges of global biofuel demand (37 billion gallon) by 2016. Jatropha can
be grown in semiarid conditions and/or marginal soils
without large investment inputs (Jongschaap et al.,
2007). While nonedible and toxic to humans and some
animals (toxic substances include toxalbumin curcin,
phorbol, saponins, trypsin inhibitor and a toxic lectin;
Rakshit et al., 2013; Pimentel et al., 2012; Carels, 2009),
its oil can be burnt directly or processed into biodiesel,
which makes it an especially attractive biofuel crop in
remote rural areas (Akbar et al., 2009; Jongschaap
et al., 2007). The interest in Jatropha has been fueled
by very optimistic claims of a concurrent capability to
producing high oil yields and recovering wasteland
(Achten et al., 2008). However, to date, critical questions
remain regarding its ability to be economically viable
40
2. BIOENERGY RESEARCH: AN OVERVIEW ON TECHNOLOGICAL DEVELOPMENTS AND BIORESOURCES
when grown in poor environmental conditions. Attainment of consistently high yields has only been achieved
with relatively high levels of nutrient inputs and on
good soils (International Energy Agency (IEA) Bioenergy, 2008). Nonetheless, the possibility of cultivating
energy crops such as J. curcas L. has the potential to
enable some smallholder farmers, producers and processors to improve their economic and social conditions,
and support rural development. In addition to growing
on degraded and marginal lands, this crop has special
appeal, in that it grows under drought conditions and
animals do not graze on it (Pandey et al., 2012; Carriquiry et al., 2010).
Another important biodiesel feedstock are microalgae,
which comprise a diverse group of aquatic photosynthetic microorganisms that grow rapidly and have the
capability to yield large quantities of lipids adequate for
biodiesel production (Ahmad et al., 2011; Amaro et al.,
2011; Singh et al., 2011; Carriquiry et al., 2010; Mata
et al., 2010; Li et al., 2008; Chisti, 2007; World Watch Institute (WWI), 2007). Algae were initially investigated as a
potential source of fuel during the gas scare of the 1970s
(Li et al., 2008). The National Renewable Energy Laboratory (NREL) started its algae feedstock studies in the
late 1970s, but its research program was discontinued in
1996. Recent renewed interest has led the NREL to restart
its research into the bioenergy/biodiesel potential of
algae (Donovan and Stowe, 2009). The potential for algae
to provide biomass for biodiesel production is now
widely accepted. Furthermore, algae are recognized
among the most efficient raw material for this purpose,
and some studies (Carriquiry et al., 2010; Chisti, 2007)
assert microalgae represent the “only source of biodiesel
that has the potential to completely displace fossil diesel”.
One of the main advantages is the ability of microalgae to
produce large amounts of biomass per unit of land. In
addition, microalgae can be grown in saline water, coastal
seawater, freshwater and on nonarable land, hence
reducing the competition for land with conventional agriculture (Khan et al., 2009), and creating economic opportunities in arid or salinity affected regions (Carriquiry
et al., 2010; Schenk et al., 2008)
Cultivation of microalgae, which is considered one of
the major bottlenecks to commercial development, is
being done mainly on open ponds, on closed bioreactors, and in hybrid systems (Brennan and Owende,
2010; Mata et al., 2010; Ugwu et al., 2008). While conventional open ponds are old systems for biomass production and account for the majority of microalgae
cultivated today, closed bioreactors that achieve higher
biomass productivity are being developed (Khan et al.,
2009; Schenk et al., 2008). Open ponds are often
perceived to be less expensive than bioreactors, as
they require less capital and are cheaper to operate
(Carriquiry et al., 2010; Khan et al., 2009). However,
open ponds are more susceptible to contamination
from unwanted species (Schenk et al., 2008), suffer
from high water losses due to evaporation and reduced
process control and reproducibility. Algal biomass
production systems can be adapted to various levels of
operational and technological skills; some microalgae
yield chemically useful fatty acid profiles and an unsaponifiable fraction, which supports biodiesel production
with high oxidation stability (Natrah et al., 2007;
Minowa et al., 1995; Dote et al., 1994; Milne et al.,
1990). In a biorefinery context, the lipid profiles of microalgae can also provide a valuable source of omega-3
fatty acids, such as docosahexaenoic acid and eicosapentaenoic acid (Yen et al., 2013; Doughman et al., 2007).
Some important microalgal species are listed in Table
2.7 with their corresponding oil content. The physical
and fuel properties of biodiesel from algal oil are comparable, in general, to those of fuel diesel (Amin, 2009;
Rana and Spada, 2007; Miao and Wu, 2006).
TABLE 2.7 Oil Content of Some Algae
Species
Oils (% Dry Matter
of Lipid)
FRESHWATER MICROALGAE
Scenedesmus obliquus
11e55
Scenedesmus dimorphus
6e40
Chlorella vulgaris
14e56
C. emersonii
25e63
C. protothecoides
23/55
C. sorokiana
22
C. minutissima
57
Spirulina maxima
4e9
MARINE MICROALGAE
Crypthecodinium cohnii
20e51.1
Dunaliella bioculata
8
D. salina
14e20
D. tertiolecta
16.7e71
Dunaniella sp.
17.5e67
Nannochloris sp.
20e56
Nannochloropsis sp.
12e53
Neochloris oleoabundans
29e65
Phaeodactylum tricornutum
18e57
Pyremnesium parvum
22e38
Skeletonema costatum
13.5e51.3
Tetraselmis suecica
8.5e23
Sources: Mata et al., 2010; Bruton et al., 2009; Gouveia and Oliveira 2009.
REFERENCES
The use of microalgae could be a suitable alternative
in the future, if improved high-rate production systems
are available at scale, because these algae are one of the
most efficient biological producers of oils on the planet
and are a versatile biomass source (Demirbas, 2011;
Mata et al., 2010; Macedo, 2007; Campbell, 1997). In
fact, microalgae with a lower oil content (w30% of the
dry biomass) could yield 58,700 L oil/hectare per year
or 51,927 kg biodiesel/hectare per year. In comparison,
Jatropha (J. curcas L.), with an oil content of 28% (dry
weight), can yield 741 L oil/hectare or a biodiesel productivity of 656 kg biodiesel/hectare per year (Mata
et al., 2010). On average, the biodiesel production yield
from microalgae can be 10e20 times higher than the
yield obtained from oleaginous seeds and/or vegetable
oils (Mata et al., 2010; Gouveia and Oliveira, 2009; Chisti,
2007; Tickell, 2000). Therefore, in the future microalgae
may become one of the Earth’s most important renewable fuel feedstocks for an number of reasons: their
higher photosynthetic efficiency, biomass productivities,
faster growth rates (in comparison with terrestrial
plants), higher CO2 fixation and O2 production rates,
and ability to grow in liquid medium, in variable
climates and in ponds on nonarable land including
marginal areas unsuitable for agricultural purposes
(e.g. desert and seashore lands). Microalgae can also
grow in nonpotable water or even in systems to
combine waste treatment and biomass production
(Zeng et al., 2012). They also use far less water than
traditional crops and do not displace food crops;
their production is not seasonal and biomass can be
harvested daily (Chisti, 2007, 2008; Spolaore et al.,
2006; Campbell, 1997).
CONCLUSIONS
In summary, achieving the feedstock yields to meet
bioenergy requirements will generally require lignocellulosic crops rather than food crops. Pretreatment is
likely to be required, and could be conducted close to
the site of harvesting, as the pretreated biomass would
be reduced in bulk, and thus cheaper to transport.
The ideal pretreatment should be low cost, yield minimum levels of inhibitory compounds, result in a
minimum loss of the main polysaccharides and enable
maximum recovery of different fractions from the
biomass. Pretreated biomass is also more amenable to
downstream enzymatic bioconversion. There are major
challenges ahead to reduce bioenergy production
costs, many of which can provide significant opportunities for fundamental research and innovation in
science and engineering. Bioenergy production, especially from second- and third- generation feedstocks,
can yield many socioeconomic benefits. Selection of
41
the appropriate feedstocks in combination with positive
sustainable agronomic and resource management approaches will reduce global dependency on fossil fuels.
However, well-integrated and well-conceived strategies
are required so that bioenergy can maintain the environment, support biodiversity, conserve water resources,
lead to a reduction in emissions and enable rural
development. Lignocellulosic biomass has several
advantages over conventional sugar- and starch-based
raw materials and has been projected to be one of the
main sources of bioenergy and biofuels in the near
future. With the application of existing technologies
and future advances, biomass to bioenergy can provide
a significant positive alternative in the energy and
biofuel sector.
Acknowledgments
The authors are grateful for research funding from Enterprise Ireland
and the Industrial Development Authority, through the Technology
Centre for Biorefining and Bioenergy (TCBB), as part of the Competence Centre program under the National Development Plan
2007e2013. The support of Mr B. Bonsall, Technology Leader (TCBB),
and Prof. V. O’Flaherty, Chair of Microbiology, School of Natural Sciences, & Deputy Director of the Ryan Institute for Environmental,
Marine and Energy Research at NUI Galway, Ireland, is gratefully
acknowledged.
References
Abramson, M., Shoseyov, O., Shani, Z., 2010. Plant cell wall reconstruction toward improved lignocellulosic production and processability. Plant. Sci. 178, 61e72.
Abreu, F., 2009. Alternative By-Products from Jatropha. http://www.
ifad.org/events/Jatropha/harvest/F_Abreu.ppt.
Achten, W.M.J., Verchot, L., Franken, Y.J., Mathijs, E., Singh, V.P.,
Aerts, R., Muys, B., 2008. Jatropha bio-diesel production and use.
Biomass Bioenergy 32, 1063e1084.
Ackerson, M.D., Clausen, E.C., Gaddy, J.L., 1991. Production of
ethanol from MSW via concentrated acid hydrolysis of the lignocellulosic fraction. Energy Biomass Wastes 15, 725e743.
Aden, M., Ruth, K., Ibsen, J., et al., 2002. Lignocellulosic Biomass to
Ethanol Process Design and Economics Utilizing Co-Current
Dilute Acid Pre-hydrolysis and Enzymatic Hydrolysis for Corn
Stover (Report No. NREL/TP-510e32438). National Renewable
Energy Laboratory, USA.
Agbor, V.B., Cicek, N., Sparling, R., Berlin, A., Levin, D.B., 2011.
Biomass pretreatment: fundamentals towards application. Biotechnol. Adv. 29, 675e685.
Ahmad, A.L., Yasin, N.H.M., Derek, C.J.C., Lim, J.K., 2011. Microalgae
as a sustainable energy source for biodiesel production: review.
Renewable Sustainable Energy Rev. 15, 584e593.
Akbar, E., Yaakub, Z., Kamarudin, S.K., Ismail, M., Salimon, J., 2009.
Characteristics and composition of Jatropha curcas oil seed from
Malaysia and its potential as biodiesel feedstock. Eur. J. Sci. Res. 29,
396e403.
Alonso, D.M., Bond, J.Q., Dumesic, J.A., 2010. Catalytic conversion of
biomass to biofuels. Green Chem. 12, 1493e1513.
Alves, F.F., Bose, S.K., Francis, R.C., Colodette, J.L., Iakovlev, M.,
Heiningen, A.V., 2010. Carbohydrate composition of eucalyptus,
bagasse and bamboo by a combination of methods. Carbohydr.
Polym. 82, 1097e1101.
42
2. BIOENERGY RESEARCH: AN OVERVIEW ON TECHNOLOGICAL DEVELOPMENTS AND BIORESOURCES
Amaro, H.M., Guedes, A.C., Malcata, F.X., 2011. Advances and
perspectives in using microalgae to produce biodiesel. Appl. Energy 88, 3402e3410.
Ambravaram, M.M.R., Krishnan, A., Trijatmiko, K.R., Pereira, A., 2011.
Coordinated activation of cellulose and repression of lignin
biosynthetic pathways in rice. Plant. Physiol. 155, 916e931.
Amin, S., 2009. Review on biofuel oil and gas production processes from
microalgae. Energy Conversion and Management 50, 1834e1840.
Amore, A., Faraco, V., 2012. Potential of fungi as category I consolidated BioProcessing organisms for cellulosic ethanol production.
Renewable Sustainable Energy Rev. 16, 3286e3301.
Antolı́n, G., Tinaut, F.V., Briceño, Y., Castaño, V., Pérez, C.,
Ramı́rez, A.I., 2002. Optimisation of biodiesel production by
sunflower oil transesterification. Bioresour. Technol. 83, 111e114.
Azadi, P., Inderwildi, O.R., Farnood, R., King, D.A., 2013. Liquid fuels,
hydrogen and chemicals from lignin: a critical review. Renewable
Sustainable Energy Rev. 21, 506e523.
Azam, M.M., Waris, A., Nahar, N.M., 2005. Prospects and potential of
fatty acid methyl ester of some nontraditional seed oils for use as
biodiesel. Ind. Biomass Bioenergy 29, 293e302.
Balat, M., 2011. Production of bioethanol from lignocellulosic materials via the biochemical pathway: a review. Energy Convers.
Manage. 52, 858e875.
Banerjee, A., 2011. Food, feed, fuel: transforming the competition for
grains. Dev. Change 42, 529e557.
Batten, D., O’Connell, D., 2007. Biofuels in Australia: Some Economic
and Policy Considerations. RIRDC Publication No07/177. Rural
Industries Research and Development Corporation and CSIRO,
Canberra.
Bauen, A., Gerndes, G., Junginger, M., Londo, M., Vuille, F., Ball, R.,
Bole, T., Chudziak, C., Faaij, A., Mozafferian, H., 2009. Bioenergy e
A Sustainable and Reliable Energy Source: A Review of Status and
Prospects. IEA BIOENERGY. ExCo: 2009:06.
Becker, K., Makkar, H.P.S., 2009. Jatropha curcas: a potential source for
tomorrow’s oil and biodiesel. Lipid Toxicol. 20, 104e107.
Betts, W.B., Dart, R.K., Ball, A.S., Pedlar, S.L., 1991. Biosynthesis and
structure of lignocellulose. In: Betts, W.B. (Ed.), Biodegradation: Natural and Synthetic Materials. Springer, Berlin, Germany, pp. 139e155.
Blanch, H., 2012. Bioprocessing for biofuels. Curr. Opin. Biotechnol.
23, 390e395.
Blaschek, H.P., Ezej, T.C., 2007. Science of alternative feedstocks. In:
Corn-Based Ethanol in Illinois and the US: A Report from the
Department of Agricultural and Consumer Economics. University
of Illinois, pp. 112e128.
Boateng, A., Anderson, W., Phillips, J., 2007. Bermuda grass for biofuels: effect of two genotypes on pyrolysis product yield. Energy
Fuels 21, 1183e1187.
Boe, A., Lee, D., 2007. Genetic variation for biomass production in
prairie cordgrass and switchgrass. Crop. Sci. 47, 929e934.
Brennan, L., Owende, P., 2010. Biofuels from microalgae e a review of
technologies for production, processing and extraction of biofuels
and co-products. Renewable Sustainable Energy Rev. 14, 557e577.
Brown, J.N., 2009. Development of a Lab-Scale Auger Reactor for
Biomass Fast Pyrolysis and Process Optimization Using Response
Surface Methodology. Graduate Theses and Dissertations. Paper
10996, Iowa State University, USA.
Bruton, T., Lyons, H., Lerat, Y., Stanley, M., BoRasmussen, M., 2009. A
Review of the Potential of Marine Algae as a Source of Biofuel in
Ireland. Sustainable Energy, Ireland.
Buonocore, E., Franzese, P.P., Ulgiati, S., 2012. Assessing the environmental performance and sustainability of bioenergy production in
Sweden: a life cycle assessment perspective. Energy 37, 69e78.
Cabral, O.M.R., Rocha, H.R., Gash, J.H.C., LIgo, M.A.V., Freitas, H.C.,
Tatsch, J.D., 2010. The energy and water balance of a Eucalyptus
plantation in southeast Brazil. J. Hydrol. 388, 208e216.
Campbell, C.J., 1997. The Coming Oil Crisis. Multi-science Publishing
Company and petroconsultants S.A, Essex, England.
Carels, N., 2009. Jatropha curcas: a review. Adv. Bot. Res. 50, 39e86.
Carlson, I.T., Oram, R.N., Surprenant, J., 1996. Reed canary grass and
other Phalaris species. In: Moser, L.E., Buxton, D.R., Casler, M.D.
(Eds.), Cool-Season Forage Grasses. Am. Soc. Agron, Madison, WI,
pp. 569e604.
Carpita, N., 2012. Progress in the biological synthesis of the plant cell
wall: new ideas for improving biomass for bioenergy. Curr. Opin.
Biotechnol. 23, 330e337.
Carriquiry, Miguel A., Xiaodong, Du, Timilsina, Govinda R., 2010.
Second-Generation Biofuels: Economics and Policies. Policy
Research Working Paper 5406. http://econ.worldbank.org.
Chandel, Anuj K., Singh, Om V., 2011. Weedy lignocellulosic feedstock
and microbial metabolic engineering: advancing the generation of
‘Biofuel’. Appl. Microbiol. Biotechnol. 89, 1289e1303.
Chandel, A.K., Narasu, M.L., Chandrasekhar, G., Manikeyam, A.,
Rao, L.V., 2009. Use of Saccharum spontaneum (wild sugarcane) as
biomaterial for cell immobilization and modulated ethanol production by thermotolerant Saccharomyces cerevisiae VS3. Bioresour.
Technol. 100, 2404e2410.
Chandel, A.K., Singh, O.V., Rao, L.V., 2010. Biotechnological applications of hemicellulosic derived sugars: state-of-the-art. In:
Singh, O.V., Harvey, S.P. (Eds.), Sustainable Biotechnology:
Renewable Resources and New Perspectives. Springer,
Netherland, pp. 63e81.
Cherubini, F., 2010. The biorefinery concept: using biomass instead of
oil for producing energy and chemicals. Energy Convers. Manage.
51, 1412e1421.
Chisti, Y., 2007. Biodiesel from microalgae. Biotechnol. Adv. 25,
294e306.
Chisti, Y., 2008. Biodiesel from microalgae beats bioethanol. Trends.
Biotechnol. 26, 126e131.
Chu, S., Majumdar, A., 2012. Opportunities and challenges for a
sustainable energy future. Nature 488, 294e303.
Claassen, P.A.M., Sijtsma, L., Stams, A.J.M., De Vries, S.S.,
Weusthuis, R.A., 1999. Utilisation of biomass for the supply of
energy carriers. Appl. Microbiol. Biotechnol 52, 741e755.
Collinson, S.R., Theilemans, W., 2010. The catalytic oxidation of
biomass to new materials focusing on starch, cellulose and lignin.
Coord. Chem. Rev. 254, 1854e1870.
Cornelissen, S., Koper, M., Deng, Y.Y., 2012. The role of bioenergy in a
fully sustainable global energy system. Biomass Bioenergy 41,
21e33.
Coyne, J., Gupta, V.K., O’Donovan, A., Tuohy, M.G., 2013. The role of
fungal enzymes in global biofuel production technologies. In:
Gupta, V.K., Tuohy, M.G. (Eds.), Biofuel Technologies. Springer
Science Publishers, USA, pp. 121e143.
Cunniff, J., Cerasuolo, M., 2011. Lighting the way to willow biomass
production. J. Sci. Food Agric. 91, 1733e1736.
Dashtban, M., Schraft, H., Qin, W., 2009. Fungal bioconversion of
lignocellulosic residues; opportunities & perspectives. Int. J. Biol.
Sci. 5, 578e595.
Davison, B., Drescher, S., Tuskan, G., Davis, M., Ngheim, N., 2006.
Variation of S/G ratio and lignin content in a Populus family influences the release of xylose by dilute acid hydrolysis. Appl.
Biochem. Biotechnol 130, 427e435.
De Fraiture, C., Berndes, G., 2009. Biofuels and water. In:
Howarth, R.W., Bringezu, S. (Eds.), Biofuels: Environmental
Consequences and Interactions with Changing Land Use. Proceedings of the Scientific Committee on Problems of the Environment (SCOPE) International Biofuels Project Rapid Assessment,
22e25 September 2008, Gummersbach Germany. Cornell University, Ithaca NY, USA, pp. 139e153. http://cip.cornell.edu/
biofuels/.
REFERENCES
Delong, M.M., Swanberg, D.R., Oelke, E.A., Hanson, C., Onischak, M.,
Schmid, M.R., Wiant, B.C., August, 1995. Sustainable biomass energy production and rural economic development using alfalfa as
a feedstock. In: Klass, D.L. (Ed.), August, 1995. Second Biomass
Conf. of the Americas: Energy, Environment, Agriculture, and
Industry, vol. 21e24, pp. 1582e1591. Portland, OR, USA.
Demain, A.L., Newcomb, M., Wu, D.J.H., 2005. Cellulase, clostridia,
and ethanol microbiology. Mol. Biol. Rev. 69, 124e154.
Demain, A.L., 2009. Biosolutions to the energy problem. J.Ind.Microbiol. Biotechnol 36, 319e332.
Demirbas, M.F., 2011. Biofuels from algae for sustainable development. Appl. Energy 88, 3473e3480.
Demirbas, A., 2005. Bioethanol from cellulosic materials: a renewable
motor fuel from biomass. Energy Sources 27, 327e337.
Department of Energy (DOE), 2011. In: Quadrennial Technology
Review Report. Technology Assessments, vol. 2. US Department of
Energy. http://energy.gov/articles/department-energy-releasesinaugural-quadrennial-technology-review-report.
Department of Energy (DOE), 2006. Breaking the Biological Barriers to
Cellulosic Ethanol. A Joint Research Agenda (DOE/SC-0095). U.S.
Department of Energy, Rockville, Maryland.
Dien, B.S., Iten, L.B., Skory, C.D., 2005. Converting herbaceous energy
crops to bioethanol; a review with emphasis on pretreatment
processes. In: Hou, C.T. (Ed.), Handbook of Industrial Biocatalysis,.
Taylor and Francis, Boca Raton, FL, pp. 1e11. (Chapter 23).
Dogaris, I., Vakontios, G., Kalogeris, E., Mamma, D., Kekos, D., 2009.
Induction of cellulases and hemicellulases from Neurospora crassa
under solid-state cultivation for bioconversion of sorghum bagasse
into ethanol. Ind. Crops. Prod. 29, 404e411.
Doherty, W.O.S., Mousavioun, P., Fellows, C.M., 2011. Value-adding to
cellulosic ethanol: lignin polymers. Ind. Crops Prod. 33, 259e276.
Donovan, J., Stowe, N., 2009. Is the Future of Biofuels in Algae?
RenewableEnergyWorld.com
06/12/2009.
http://www.
renewableenergyworld.com/rea/news/article/2009/06/is-thefuture-ofbiofuels-in-algae.
Dornburg, V., Faaij, A., Langeveld, H., van de Ven, G., Wester, F., van
Keulen, H., van Diepen, K., Ros, J., van Vuuren, D., van den
Born, G.J., van Oorschot, M., Smout, F., Aiking, H., Londo, M.,
Mozaffarian, H., Smekens, K., Meeusen, M., Banse, M., Lysen, E.,
van Egmond, S., 2008. Biomass Assessment: Assessment of Global
Biomass Potentials and Their Links to Food, Water, Biodiversity,
Energy Demand and Economy. Report 500102 012.
Dote, Y., Sawayama, S., Inoue, S., Minowa, T., Yokoyama, S., 1994.
Recovery of liquid fuel from hydrocarbon-rich microalgae by
thermo-chemical liquefaction. Fuel 73, 1855e1857.
Doughman, S.D., Krupanidhi, S., Sanjeevi, C.B., 2007. Omega-3 fatty
acids for nutrition and medicine: considering microalgae oil as a
vegetarian source of EPA and DHA. Curr. Diabetes Rev. 3, 198e203.
European Bioethanol Fuel Association, November, 2009. Production
Data.
FAO, 1980. Feed from Animal Wastes: State of Knowledge. http://
www.fao.org/docrep/004/X6518E/X6518E00.htm#TOC.
FAO, 2008. The State of Food and Agriculture 2008. Biofuels: Prospects, Risks and Opportunities, Rome.
Farine, D., O’Connell, D., Raison, J., May, B., O’Connor, M.,
Crawford, D., Alexander Herr - Herry, Herr, Taylor, J., Jovanovic, T.,
Campbell, P., Dunlop, M., Rodriguez, L., Poole, M., Braid, A.,
Kriticos, D., 2011. An assessment of biomass for bioelectricity and
biofuel, and for greenhouse gas emission reduction in Australia.
GCB Bioenergy 4, 148e175.
Fedler, C.B., Duan, R., 2011. Biomass production for bioenergy using
recycled wastewater in a natural waste treatment system. Resour.,
Conserv. Recycl. 55, 793e800.
FitzPatrick, M., Champagne, P., Cunningham, M.F., Whitney, R.A.,
2010. A biorefinery processing perspective: treatment of
43
lignocellulosic materials for the production of value-added products. Bioresour. Technol. 101, 8915e8922. ftp://ftp.fao.org/
docrep/fao/011/i0100e/i0100e02.pdf.
Galbe, M., Zacchi, G., 2002. A review of the production of ethanol
from softwood. Appl. Microbiol. Biotechnol. 59, 618e628.
Girio, F.M., Fonseca, C., Caravalheiro, F., Duarte, L.C., Marques, A.,
Bogel-Lukasik, R., 2010. Hemicelluloses for fuel ethanol: a review.
Bioresour. Technol. 101, 4775e4800.
Gonzalez-Hernandez, J.L., Sarath, G., Stein, J.M., Owens, V.,
Gedye, K., Boe, A., 2009. A multiple species approach to biomass
production from native herbaceous perennial feedstocks. In Vitro
Cell. Dev. Biol.: Plant 45, 267e281.
Gouveia, L., Oliveira, A.C., 2009. Microalgae as a raw material for
biofuels production. J. Ind. Microbiol. Biotechnol. 36, 269e274.
Graham, R.L., Lichtenberg, E., Roningen, V.O., Shapouri, H.,
Walsh, M., 1995. The Economics of Biomass Production in the
United States. Proc. Second Biomass of the Americas Conference,
Portland, OR.
Groom, M.J., Gray, E.M., Townsend, P.A., 2008. Biofuels and biodiversity: principles for creating better policies for biofuel production. Conserv. Biol. 22, 602e609.
Gupta, R., Sharma, K.K., Kuhad, R.C., 2009. Separate hydrolysis and
fermentation (SHF) of Prosopis juliflora, a woody substrate, form the
production of cellulosic ethanol by Saccharomyces cerevisiae and
Pichia stipitis NCIM 3498. Bioresour. Technol. 100, 1214e1220.
Gupta, V.K., Tuohy, M.G., Sharma, G.D., 2013. Biotechnology of
Trichoderma: an overview. In: Gupta, V.K., Tuohy, M.G.,
Sharma, G.D., Gaur, S. (Eds.), Applications of Microbial Genes in
Enzyme Technology. Nova Science Publishers, USA, pp. 375e393.
Hahn-Hagerdal, B., Galbe, M., Gorwa-Grauslund, M., Liden, G.,
Zacchi, G., 2006. Bio-ethanol the fuel of tomorrow from the
residues of today. Trends. Biotechnol. 24, 549e556.
Hamelinck, C., Hooijdonk, G., Faaij, A., 2005. Ethanol from lignocellulosic biomass: techno-economic performance in short-, middleand long-term. Biomass Bioenergy 28, 384e410.
Hardcastle, P.D., 2006. A Review of the Potential Impacts of Short
Rotation Forestry. Forestry Commission, Farnham, UK.
Harmsen, P.F.H., Huijgen, W.J.J., Bermúdez López, L.M.,
Bakker, R.R.C., 2010. Literature Review of Physical and Chemical
Pretreatment Processes for Lignocellulosic Biomass. BioSynergy
project. Food & Biobased Research Centre, Wageningen University,
The Netherlands.
Harris, D., DeBolt, S., 2010. Synthesis, regulation and utilization of
lignocellulosic biomass. Plant Biotechnol. J. 8, 244e262.
Hasunuma, T., Kondo, A., 2012. Development of yeast cell factories for
consolidated bioprocessing of lignocellulose to bioethanol through
cell surface engineering. Biotechnol. Adv. 30, 1207e1218.
Hasunuma, T., Okazaki, F., Okai, N., Hora, K.Y., Ishii, K., Kondo, A.,
2013. A review of enzymes and microbes for lignocellulosic biorefinery and the possibility of their application to consolidated
bioprocessing. Bioresour. Technol. 135, 513e522.
Heather, Y., Somerville, C., 2012. Development of feedstocks for
cellulosic biofuels. F1000 Biology Reports. 4 (10).
Heaton, E.A., Flavell, R.B., Mascia, P.N., Thomas, S.R., Dohleman, F.G.,
Long, S.P., 2008. Herbaceous energy crop development: recent
progress and future prospects. Curr. Opin. Biotechnol. 19, 202e209.
Hill, J., Nelson, E., Tilman, D., Polasky, S., Tiffany, D., 2006. Environmental, economic, and energetic costs and benefits of biodiesel and
ethanol biofuels. Proc. Nat. Acad. Sci. USA 103, 11206e11210.
Horn, S., Vaaje-Kolstad, G., Westereng, B., Eijsink, V., 2012. Novel
enzymes for the degradation of cellulose. Biotechnol. Biofuels 5, 45.
Howard, R., Abotsi, E., Jansen van Rensburg, E., Howard, S., 2003.
Lignocellulose biotechnology: issues of bioconversion and enzyme
production. Afr. J. Biotechnol. 2, 602e619.
Http://www.ga.gov.au/image_cache/GA16706.pdf
44
2. BIOENERGY RESEARCH: AN OVERVIEW ON TECHNOLOGICAL DEVELOPMENTS AND BIORESOURCES
Http://www.thebioenergysite.com/articles/172/biofuels-andagriculture
Hu, Z., Wen, Z., 2008. Enhancing enzymatic digestibility of switchgrass by microwave-assisted alkali pretreatment. Biochem. Eng. J.
38, 369e378.
Hyeon, J.E., Jeon, S.D., Han, S.O., in press. Cellulosome-based, Clostridium-derived multi-functional enzyme complexes for advanced
biotechnology tool development: Advances and applications.
Biotechnol. Adv. (Available online: 3.04.13.).
IEA, 2006. Energy Technology Perspectives e Scenario’s and Strategies to 2050. OECD/IEA, Paris, France.
IEA, 2009. World Energy Balances 2009. OECD/IEA, Paris.
International Energy Agency (IEA) Bioenergy, November, 2008. From
First- to Second-Generation Biofuel Technologies: An Overview of
Current Industry and RD&D Activities. IEA.
International Energy Agency (IEA), 2010. Energy Technology
Perspectives: Scenarios and Strategies to 2050. OECD/IEA, Paris, 706.
Isroi, Millati, R., Syamsiah, S., Niklasson, C., Nur Cahyanto, M.,
Lundquist, K., Taherzadeh, M., 2011. Biological pretreatments of
lignocelluloses with white-rot fungi and its applications: a review.
BioResources 6, 5224e5259.
Jingura, R.M., Musademba, D., Matengaifa, R., 2010. An evaluation of
utility of Jatropha curcas L. as a source of multiple energy carriers.
Int. J. Eng. Sci. Technol. 2, 115e122.
Jones, M.B., Walsh, M., 2001. Miscanthus for Energy and Fibre. James
and James Ltd., London.
Jongschaap, R.E.E., Corre, W.J., Bindraban, P.S., Brandenburg, W.A.,
2007. Claims and facts on Jatropha curcas L. Report 158. Plant Res.
Int. (Wageningen).
Jordan, S.N., Mullen, G.J., Murphy, M.C., 2008. Composition variability of spent mushroom compost in Ireland. Bioresour. Technol.
99, 411e418.
Jung, H.G., Vogel, K.P., 1986. Influence of lignin on digestibility of
forage cell wall material. J. Anim. Sci. 62, 1703e1712.
Kamm, B., Kamm, M., 2004. Principles of biorefineries. Appl. Microbiol. Biotechnol. 64, 137e145.
Kapdan, L.K., Kargi, F., 2006. Biohydrogen production from waste
materials. Enzyme Microb. Technol. 38, 569e582.
Karp, A., Hanley, S.J., Trybush, S.O., Macalpine, W., Pei, M., Shield, I.,
2011. Genetic improvement of willow for bioenergy and biofuels.
J. Integr. Plant Biol. 53, 151e165.
Karunanithy, C., Muthukumarappan, K., 2011. Optimization of alkali
soaking and extrusion of prairie cord grass for maximum sugar
recovery by enzymatic hydrolysis. Biochem. Eng. J. 564, 71e82.
Keating, C., Cysneiros, D., Mahony, T., O’Flaherty, V., 2012. The hydrolysis and biogas production of complex cellulosic substrates
using three anaerobic biomass sources. Water. Sci. Technol. 67,
293e298.
Keshwani, D., Cheng, J., 2009. Switchgrass for bioethanol and other
value-added applications: a review. Bioresour. Technol. 100,
1515e1523.
Khan, S.A., Rashmi, Hussain, M.Z., Prasad, S., Banerjee, U.C., 2009.
Prospects of biodiesel production from microalagae in India.
Renewable Sustainable Energy Rev. 13, 2361e2372.
Khanna, M., Dhungana, B., Clifton-Brown, J., 2008. Costs of producing
miscanthus and switchgrass for bioenergy in Illinois. Biomass
Bioenergy 32, 482e493.
Kim, E., Martinez Amezcua, J.C., Utterback, P.L., Parsons, C.M., 2008.
Phosphorus bioavailability, true metabolizable energy, and amino
acid digestibility of high protein corn distillers dried grains and
dehydrated corn germ. Poult. Sci. 87, 700e705.
Kim, S., Dale, E.B., 2004. Global potential bioethanol production from
wasted crops and crop residues. Biomass Bioenergy 26, 361e375.
Kittl, R., Kracher, D., Burgstaller, D., Haltrich, D., Ludwig, R., 2012.
Production of four Neurospora crassa lytic polysaccharide
monooxygenases in Pichia pastoris monitored by a fluorimetric
assay. Biotechnol. Biofuels 5, 79.
Komilis, D.P., Ham, R.K., 2003. The effect of lignin and sugars to the
aerobic composting of solid waste. Waste Manage. 23, 419e423.
Kuhad, R.C., Singh, A., Eriksson, K.E.L., 1997. Microorganisms and
enzymes involved in the degradation of plant fiber cell walls. In:
Eriksson, K.E.L. (Ed.), 1997. Advances in Biochemical Engineering
Biotechnology, vol. 57, pp. 46e125.
Kumar, A., Singh, L.K., Ghosh, S., 2009. Bioconversion of lignocellulosic fraction of water-hyacinth (Eichhornia crassipes) hemicellulose
acid hydrolysate to ethanol by Pichia stipitis. Bioresour. Technol.
100, 3293e3297.
Lamborn, J., 2009. Characterisation of Municipal Solid Waste
Composition into Model Inputs. Third Workshop on the Hydroe
PhysicoeMechanical Properties of Wastes (HPM3), Braunschweig,
Germany.
Lange, H., Decina, S., Crestini, C., in press. Oxidative upgrade of
lignin e recent routes reviewed. Eur. Polym. J.
Langston, J.A., Shaghasi, T., Abbate, E., Xu, F., Vlasenko, E.,
Sweeney, M.D., 2011. Oxidoreductive cellulose depolymerization
by the enzymes cellobiose dehydrogenase and glycoside hydrolase
61. Appl. Environ. Microbiol. 77, 7007e7015.
Lee, D.K., Owens, V.N., Boe, A., Jeranyama, P., 2007a. Composition of
Herbaceous Biomass Feedstocks. A report by Plant Science
Department, South Dakota State University. http://agbiopubs.
sdstate.edu/articles/SGINC1-07.pdf.
Lee, D.K., Owens, V.N., Doolittle, J.J., 2007b. Switchgrass and soil
carbon sequestration response to ammonium nitrate, manure, and
harvest frequency on conservation reserve program land. Agron. J.
99, 462e468.
Lemus, R., Parrish, D.J., 2009. Herbaceous crops with potential for biofuel
production in the USA. CAB Reviews: Perspectives in Agriculture,
Veterinary Science, Nutrition and Natural Resources 4 (no. 057).
Lemus, R., Lai, R., 2005. Bioenergy crops and carbon sequestration.
Crit. Rev. Plant Sci. 24, 1e21.
Levin, D.B., Islam, R., Cicek, N., Sparling, R., 2006. Hydrogen production by Clostridium thermocellum 27405 from cellulosic biomass
substrates. Int. J. Hydrogen Energy 31, 1496e1503.
Lewandowski, I., Scurlock, J., Lindvall, E., Christou, M., 2003. The
development and current status of perennial rhizomatous grasses as
energy crops in the US and Europe. Biomass Bioenergy 25, 335e361.
Li, Y., Horsman, M., Wu, N., Lan, C.Q., Dubois-Calero, N., 2008.
Biofuels from microalgae. Biotechnol. Prog. 24, 815e820.
Lichts, F.O., 2010. Industry Statistics: 2010 World Fuel Ethanol
Production. Renewable Fuels Association.
Liew, L.N., Shi, J., Li, Y., 2012. Methane production from solid-state
anaerobic digestin of lignocellulosic biomass. Biomass Bioenergy
46, 125e132.
Lynd, L.L., Cushman, J.H., Nichols, R.J., Wyman, C.F., 1991. Fuel
ethanol from cellulosic biomass. Science 231, 1318e1323.
Lynd, L.R., 1996. Overview and evaluation of fuel ethanol from
cellulosic biomass: technology, economics, the environment, and
policy. Annu. Rev. Energy. Environ. 21, 403e465.
Lynd, L.R., Weimer, P.J., van Zyl, W.H., Pretorius, I.S., 2002. Microbial
cellulose utilization: fundamentals and biotechnology. Microbiol.
Mol. Biol. Rev. 66, 506e577.
Macedo, I.C., 2007. Etanol de Cana de Acucar no Brasil. Presentation
at the Seminar on Technologies for Future Ethanol Production in
Brazil. Instituto Tecnologia Promon, Sao Paulo, Brazil.
Malherbe, S., Cloete, T.E., 2003. Lignocellulose biodegradation:
fundamentals and applications. Environ. Sci. Bioechnol. 1, 105e114.
Malmsheimer, R.W., Bowyer, J.L., Fried, J.S., Gee, E., Izlar, R.L.,
Miner, R.A., Munn, I.A., Oneil, E., Stewart, W.C., 2011. Stewart:
managing forests because carbon matters: integrating energy,
products, and land management policy. J. For. 109, S7eS48.
REFERENCES
Martinez, A.T., Ruiz-Duenas, F.J., Martinez, M.J., del Rio, J.C.,
Gutierrez, A., 2009. Enzymatic delignification of plant cell walls:
from nature to mill. Curr. Opin. Biotechnol. 20, 348e357.
Mata, T.M., Martins, A.A., Caetano, N.S., 2010. Microalgae for
biodiesel production and other applications: a review. Renewable
Sustainable Energy Rev. 14, 217e232.
Mata-Alvarez, J., Mace, S., Llabres, P., 2000. Anaerobic digestion of
organic solid wastes. An overview of research achievements and
perspectives. Bioresour. Technol. 74, 3e16.
Matano, Y., Hasunuma, T., Kondo, A., 2013. Cell recycle batch
fermentation of high-solid lignocellulose using a recombinant
cellulose-displaying yeast strain for high yield ethanol production
in consolidated bioprocessing. Bioresour. Technol. 135, 403e409.
McHugh, S., Carton, M., Mahony, T., O’Flaherty, V., 2003. Methanogenic population structure in a variety of anaerobic bioreactors.
FEMS. Microbiol. Lett. 219, 297e304.
McKendry, Peter, 2002. Energy production from biomass (part 3):
gasification technologies. Bioresour. Technol. 83, 55e63.
McLaughlin, S.B., Samson, R., Bransby, D., Weislogel, A., Sept. 1996.
Evaluating Physical, Chemical, and Energetic Properties of Perennial Grasses as Biofuels. Proc. Bioenergy 96, Nashville, TN, pp. 1e8.
Menon, V., Rao, M., 2012. Trends in bioconversion of lignocellulose:
biofuels, platform chemicals & biorefinery concept. Prog. Energy
Combust. Sci. 38, 522e550.
Merigliano, M.F., Lesica, P., 1998. The native status of reed canary
grass (Phalaris arundinacea L.) in the inland Northwest USA. Nat.
Areas J. 18, 223e230.
Merino, S.T., Cherry, J., 2007. Progress and challenges in enzyme
development for biomass utilization. Adv. Biochem. Eng.
Biotechnol. 108, 95e120.
Miao, X., Wu, Q., 2006. Biodiesel production from heterotrophic
microalgal oil. Bioresour. Technol. 97, 841e846.
Miles, T.R., et al., 1993. Alkali Slagging Problems in Biomass Fuels.
Proc. First Biomass Conference of the Americas, Burlington, VT,
pp. 406e421.
Milne, T.A., Evans, R.J., Nagle, N., 1990. Catalytic conversion of
microalgae and vegetable oils to premium gasoline, with shape
selective zeolites. Biomass 21, 219e232.
Minowa, T., Yokoyama, S.Y., Kishimoto, M., Okakurat, T., 1995. Oil
production from algal cells of Dunaliella tertiolecta by direct thermochemical liquefaction. Fuel 74, 1735e1738.
Mitchell, Rob, Vogel Kenneth, P., Uden, Daniel R., 2012. The feasibility
of switchgrass for biofuel production. Biofuels 3, 47e59.
Mosier, N., Wyman, C.E., Dale, B.D., Elander, R.T., Lee, Y.Y.,
Holtzapple, M., Ladisch, M.R., 2005. Features of promising
technologies for pretreatment of lignocelluolsic biomass. Bioresour.
Technol. 96, 673e686.
Mueller, S.A., Anderson, J.E., Wallington, T.J., 2011. Impact of biofuel
production and other supply and demand factors on food price
increases in 2008. Biomass Bioenergy 35, 1623e1632.
Müller, A., Schmidhuber, J., Hoogeveen, J., Steduto, P., 2008. Some
insights in the effect of growing bio energy demand on global food
security and natural resources. Water Policy 10, 83e94.
Mussatto, S.I., Teixeira, J.A., 2010. Lignocellulose as raw material in
fermentation processes. In: Méndez-Vilas, A. (Ed.), Current
Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology, Microbiology Series, vol. 2.
FORMATEX.
Mussato, S.I., Dragone, G., Guimaraes, P.M.R., Silva, J.P.A., Carneiro, L.M.,
Roberto, I.C., Vicente, A., Domingues, L., Teixeira, J.A., 2010. Technological trends, global market, and challenges of bio-ethanol production. Biotechnology Advances 28, 817e830.
Mussatto, S.I., Carneiro, L.M., Silva, J.P.A., Roberto, I.C., Teixeira, J.A.,
2011. A study on chemical constituents and sugars extraction from
spent coffee grounds. Carbohydr. Polym. 83, 368e374.
45
Natrah, F., Yosoff, F.M., Shariff, M., Abas, F., Mariana, N.S., 2007.
Screening of Malaysian indigenous microalgae for antioxidant
properties and nutritional value. J. Appl. Phycol. 19, 711e718.
Naylor, R., Liska, A.J., Burke, M.B., Falcon, W.P., Gaskell, J.C.,
Rozelle, S.D., Cassman, K.G., 2007. The ripple effect: biofuels, food
security, and the environment. Environment 49, 31e43.
Neureiter, M., Danner, H., Thomasser, C., Saidi, B., Braun, R., 2002.
Dilute acid hydrolysis of sugarcane bagasse at varying conditions.
Appl. Biochem. Biotechnol. 98, 49e58.
Nguyen, Q.A., Tucker, M.P., Keller, F.A., Eddy, F.P., 2000. Two-stage
dilute acid pretreatment of softwoods. Appl. Biochem. Biotechnol.
84e86, 561e576.
Nguyen, Q.A., Tucker, M.P., Keller, F.A., Beaty, D.A., Connors, K.M.,
Eddy, F.P., 1999. Dilute acid hydrolysis of softwoods. Appl. Biochem. Biotechnol. 77e79, 133e142.
O’Connell, D., Braid, A., Raison, J., Handberg, K., Cowie, A.,
Rodriguez, L., George, B., November 2009. Sustainable Production
of Bioenergy; a Review of Global Bioenergy Sustainability Frameworks and Assessment Systems. RIRDC Publication No.09/167.
Rural Industries Research and Development Corporation and
CSIRO, Canberra.
O’Donovan, A., Gupta, V.K., Tuohy, M.G., 2013. Recent updates in acid
pretreatments and SEM analysis of acid pretreated grass biomass.
In: Gupta, V.K., Tuohy, M.G. (Eds.), Biofuel Technologies; Recent
Developments. Springer Science Publishers, USA, pp. 97e118.
OECD/FAO, 2009. Agricultural Outlook, 2009e2018.
Olson, D.G., McBride, J.E., Shaw, J., Lynd, L.R., 2012. Recent progress
in consolidated bioprocessing. Curr. Opin. Biotechnol. 23,
396e405.
Olsson, L., Hahn-Hägerdal, B., 1996. Fermentation of lignocellulosic
hydrolysates for ethanol production. Enzyme Microb. Technol. 18,
312e331.
Ong, L.K., 2004. Conversion of lignocellulosic biomass to fuel ethanol a brief review. Planter 80, 517e524.
Openshaw, K., 2000. A review of Jatropha curcas: an oil plant of unfulfilled promise. Biomass Bioenergy 19, 1e15.
Pandey, V.M., Singh, K., Singh, J.S., Kumar, A., Singh, B., Singh, R.P.,
2012. Jatropha curcas: a potential biofuel plant for sustainable
environmental development. Renewable Sustainable Energy Rev.
16, 2870e2883.
Pan, X., Xie, D., Gilkes, N., Gregg, D.J., Saddler, J.N., 2005. Strategies to
enhance the enzymatic hydrolysis of pretreated softwood with
high residual lignin content. Appl. Biochem. Biotechnol. (part A)
124, 1069e1079.
Parrika, M., 1997. Biosimse a Method for the Estimation of Woody
Biomass for Fuel in Sweden (Ph.D. thesis). Department of ForestryIndustry-Market Studies, Swedish University of Agricultural
Sciences, Uppsala, Sweden.
Pasangulapati, V., Ramachandriya, K.D., Kumar, A., Wilkins, M.R.,
Jones, C.L., Huhnke, R.L., 2012. Effects of cellulose, hemicellulose
and lignin on thermochemical conversion characteristics of the
selected biomass. Bioresour. Technol. 114, 663e669.
Pasha, C., Valli, N., Rao, L.V., 2007. Lantana camara for fuel ethanol
production using thermotolerant yeast. Lett. Appl. Microbiol. 44,
666e672.
Pedroli, B., Elbersen, B., Frederiksen, P., Grandin, U., Heikkilä, R., Krogh,
P.H., Izakraovicová, Z., Johansen, A., Meiresonne, L., Spijker, J., 2012.
Is energy cropping in Europe compatible with biodiversity? Opportunities and threats to biodiversity from land-based production of
biomass for bioenergy purposes. Biomass Bioenergy.
Percival Zhang, Y.-H., 2008. Reviving the carbohydrate economy via
multi-product lignocellulose biorefineries. J. Ind. Microbiol. Biotechnol. 35, 367e375.
Perlack, R., Wright, L., Turhollow, A., Graham, R., Stokes, B.,
Erbach, D., April, 2005. Biomass as Feedstock for a Bioenergy and
46
2. BIOENERGY RESEARCH: AN OVERVIEW ON TECHNOLOGICAL DEVELOPMENTS AND BIORESOURCES
Bioproducts Industry: The Technical Feasibility of a Billion-Ton
Annual Supply. Department of Energy/GO-102005e2135.
Pimentel, L.A., Riet-Correa, B., Dantas, A.F., Medeiros, R.M.T., RietCorrea, F., 2012. Poisoning by Jatropha ribifolia in goats. Toxicon 59,
587e591.
Potumarthi, R., Baadhe, R.R., Jetty, A., 2012. Mixing of acid- and basepretreated corn cobs for improved production of reducing sugars
and reduction in water use during neutralization. Bioresour.
Technol. 119, 99e104.
Potumarthi, R., Baadhe, R.R., Nayak, P., Jetty, A., 2013. One step biological pretreatment of rice husk by Phanerochaete chrysosporium for
the production of reducing sugars. Bioresour. Technol. 128, 113e117.
Powlson, D.S., Richie, A.B., Shield, I., 2005. Biofuels and other approaches for decreasing fossil fuel emissions from agriculture.
Ann. Appl. Biol. 146, 193e201.
Pyter, R., Voigt, T.B., Heaton, E.A., Dohleman, F.G., Long, S.P., 2007.
Giant miscanthus: biomass crop for Illinois. In: Janick, J.,
Whipkey, A. (Eds.), Issues in New Crops and New Uses. ASHS
Press, Alexandria, VA, pp. 39e42.
Ragauskas, A.J., Williams, C.K., Davison, B.H., Britovsek, G.,
Cairney, J., Eckert, C.A., Frederick, W.J.J.R., Hallett, J.P., Leak, D.J.,
Liotta, C.L., 2006. The path forward for biofuels and biomaterials.
Science 311, 484e489.
Rajagopal, D., Zilberman, D., 2007. Review of Environmental, Economic and Policy Aspects of Biofuels. Development Research
Group, The World Bank. Policy Research Working Paper WPS4341.
Rajaram, S., Verma, A., 1990. Production and characterization of
xylanase from Bacillus thermoalkalophilus growth on agricultural
wastes. Appl. Microbiol. Biotechnol. 34, 141e144.
Rakshit K., Devappa, J.S., Roach, H.P.S., Makkar, K.B., in press.
Occular and dermal toxicity of Jatropha curcas phorbal esters.
Ecotoxicol. Environ. Saf.
Rana, R., Spada, V., 2007. Biodiesel production from ocean biomass. In:
Proceedings of the Fifteenth European conference and exhibition.
Berlin.
Ravichandra, P., Rama, R.B., Sankar, B., 2013. Fermentable sugars from
lignocellulosic biomass: technical challenges. In: Gupta, V.K.,
Tuohy, M.G. (Eds.), Biofuel Technologies: Recent Developments.
Springer Verlag, Germany, pp. 3e27.
Redding, A.P., Wang, Z., Keshwani, D.R., Cheng, J.J., 2011. High
temperature dilute acid pretreatment of coastal Bermuda grass for
enzymatic hydrolysis. Bioresour. Technol. 102, 1415e1424.
Renewable Fuels Association, 2012. Accelerating Industry
Innovationd2012, Ethanol Industry Outlook. Renewable Fuels
Association, pp. 3e23.
Richardson, J., 2008. Production of biomass for energy from sustainable forestry systems: Canada and Europe. Short Rotation Crops
International Conference.
Robert, F., Abbott, Z., 2012. Energy Crop Opportunities in the Western
Upper Peninsula of Michigan. Michigan Technological University,
USA.
Sanchez, C., 2009. Lignocellulosic residues: biodegradation and
bioconversion by fungi. Biotechnol. Adv. 27, 185e194.
Sarkar, P., Bosneaga, E., Auer, M., 2009. Plant cell walls throughout
evolution: towards a molecular understanding of their design
principles. J. Exp. Bot. 60, 3615e3635.
Sarkar, N., Ghosh, S.K., Bannerjee, S., Aikat, K., 2012. Bioethanol
production from agricultural wastes. An overview. Renewable
Energy 37, 19e27.
Scarlat, N., Dallemand, J.-F., 2011. Recent developments of biofuels/
bioenergy sustainability certification: a global overview. Energy
Policy 39, 1630e1646.
Schank, S., Chenowyth, D., Turick, C., Mendoza, P., 1993. Napier grass
genotypes and plant parts for biomass energy. Biomass Bioenergy
4, 1e7.
Schenk, P., Thomas-Hall, S.R., Stephens, E., Marx, U.C.,
Mussgnug, J.H., Posten, O.Kruse, C., Hankamer, B., 2008. Second
generation biofuels: high-efficiency microalgae for biodiesel production. Bioenergy Res. 1, 20e43.
Schubert, R., Blasch, J., 2010. Sustainability standards for bioenergy e
a means to reduce climate change risks? Energy Policy 38,
2797e2805.
Severe, J., ZoBell, D.R., 2012. Technical Aspects for the Utilization of
Small Grain Straws as Feed Energy Sources for Ruminants:
Emphasis on Beef Cattle. AG/BeefCattle/2012e03. Department of
Extension and Agriculture, Utah State University, USA.
http://extension.usu.edu/files/publications/publication/AG_
BeefCattle_2012-03.pdf.
Shapouri, H., Duield, J.A., Grabosk, M.S., 1995. Estimating the Net
Energy Balance of Corn Ethanol. Agricultural Economic Report
721. US Department of Agriculture, USA, Washington, DC.
Shapouri, H., Duield, J.A., Wang, M., 2002. The Energy Balance of
Corn Ethanol: An Update. Agricultural Economic Report 813. US
Department of Agriculture, USA, Washington, DC.
Sills, D.L., Gossett, J.M., 2011. Assessment of commercial hemicellulases for saccharification of alkaline pretreated biomass. Bioresour. Technol. 102, 1389e1398.
Simpson, T.W., Sharpley, A.N., Howarth, R.W., Paerl, H.W.,
Mankin, K.R., 2008. The new gold rush: fueling ethanol production
while protecting water quality. Environ. Qual. 37, 318e324.
Singh, A., Nigam, P.S., Murphy, J.D., 2011. Mechanism and challenges in
commercialization of algal biofuels. Bioresour. Technol. 102, 26e34.
Singh, R.N., et al., 2008. SPRERI experience on holistic approach to
utilize all parts of Jatropha curcas fruit for energy. Renewable
Energy 33, 1868e1873.
Smeets, E.M.W., Faaij, A.P.C., 2010. The impact of sustainability
criteria on the costs and potentials of bioenergy production e
applied for case studies in Brazil and Ukraine. Biomass Bioenergy
34, 319e333.
Smeets, E.M.W., Faaij, A., Lewandowski, I.M., Turkenburg, W.C., 2007.
A bottom up assessment and review of global bio-energy potentials for 2050. Prog. Energy Combust. Sci. 33, 56e106.
Somerville, C., Youngs, H., Taylor, C., Davis, S.C., Long, S.P., 2010.
Feedstocks for lignocellulosic biofuels. Science 329, 790e792.
Sørensen, A., Teller, P.J., Hilstrøm, T., Ahring, B.K., 2008. Hydrolysis of
Miscanthus for bioethanol production using dilute acid presoaking
combined with wet explosion pre-treatment and enzymatic treatment. Bioresour. Technol. 99, 6602e6607.
Spolaore, P., Joannis-Cassan, C., Duran, E., Isambert, A., 2006. Commercial applications of microalgaedreview. J. Biosci. Bioeng. 101,
87e96.
Springer, T., Dewald, C., 2004. Eastern gamagrass and other Tripsacum
species. In: Moser, L., Burson, B., Sollenberger, L. (Eds.), Warmseason (C4) Grasses. American Society of Agronomy, Madison, WI.
Sreenath, H.K., Koegel, R.G., Moldes, A.B., Jeffries, T.W., Straub, R.J.,
2001. Ethanol production from alfalfa fiber fractions by saccharification and fermentation. Process Biochem. 36, 1199e1204.
Stefan, B., Helmut, S., O’Brien, M., Kauppi, L., Howarth, R.W.,
McNeely, J., 2009. Towards Sustainable Production and Use of
Resources: Accessing Biofuels. International Panel for Sustainable
Resource Management. United Nations Environment Programme
(UNEP). www.unep.org.
Stephen, J.D., Mabee, W.E., Saddler, J.N., 2010. Biomass logistics as a
determinant of second-generation biofuel facility scale, location
and technology selection. Biofuels, Bioproducts Biorefin. 4,
503e518.
Stewart, R.J., Toma, Y., Fernandez, F.G., Nishiwaki, A., Yamada, T.,
Bollero, G.A., 2009. The ecology and agronomy of Miscanthus
sinensis, a species important to bioenergy crop development, in its
native range in Japan: a review. GCB Bioenergy 1, 126e153.
REFERENCES
Sun, Y., Cheng, J., 2002. Hydrolysis of lignocellulosic materials for
ethanol production: a review. Bioresour. Technol. 83, 1e11.
Sustainable Aviation Fuel Road Map, 2011. Towards Establishing a
Sustainable Aviation Fuels Industry in Australia and New
Zealand. CSIRO Energy Transformed Flagship, PO Box 330,
Newcastle, NSW 2300 Australia.
Taherzadeh, M.J., Karimi, K., 2008. Pretreatment of lignocellulosic
wastes to improve ethanol and biogas production: a review. Int. J.
Mol. Sci. 9, 1621e1651.
Taylor, G., 2008. Biofuels and the biorefinery concept. Energy Policy
36, 4406e4409.
Tickell, J., 2000. From the Fryer to the Fuel Tank. The complete guide
to using vegetable oil as an alternative fuel. Tallahasseee, USA.
Third International Conference on Lignocellulosic Ethanol. 2013.
http://www.biofuelstp.eu/events/3rd-icleapril-2013.pdf
Tuohy, M.G., Laffey, C.D., Coughlan, M.P., 1994. Characterisation of
the individual components of the xylanolytic enzyme system of
Talaromyces emersonii. Bioresour. Technol. 50, 37e42.
Turner, K.M., Ayyachamy, M., Brebion, J., Gupta, V.K., Tuohy, M.G.,
2010. Fungal thermozymes: a new route for the synthesis of
functional prebiotics. In: Gupta, V.K., Tuohy, M.G., Gaur, R.K.
(Eds.), Fungal Biochemistry and Biotechnology. Lambert Academic
Publishing, Germany.
Tutt, M., Olt, J., 2011. Suitability of various plant species for bioethanol
production. Agron. Res. Biosyst. Eng. 1, 261e267.
Tyner, W.E., 2010. The integration of energy and agricultural markets.
Agric. Econ. 41, 193e201.
Ugwu, C.U., Aoyagi, H., Uchiyama, H., 2008. Photobioreactors for
mass cultivation of algae. Bioresour. Technol. 99, 4021e4028.
Upham, P., Riesch, H., Tomei, J., Thornley, P., 2011. The sustainability
of forestry biomass supply for EU bioenergy: a post-normal
approach to environmental risk and uncertainty. Environ. Sci.
Policy 14, 510e518.
US-DOE, 2008a. Biomass Program. Department of Energy, The Office
of Energy Efficiency and Renewable Energy, Washington, DC.
http://www1.eere.energy.gov/biomass/biofuels_initiative.html.
US-DOE, 2008b. Theoretical Ethanol Yield Calculator. http://www1.
eere.energy. gov/biomass/ethanol_yield_calculator.html.
Van Dam, J., Junginger, M., Faaij, A.P.C., 2010. From the global efforts
on certification of bioenergy towards an integrated approach based
on sustainable land use planning. Renewable Sustainable Energy
Rev. 14, 2445e2472.
Van den Broek, R., van den Burg, T., van Wijk, A., Turkenburg, W.,
2000. Electricity generation from eucalyptus and bagasse by sugar
mills in Nicaragua: a comparison with fuel oil electricity generation on the basis of costs, macro-economic impacts and environmental emissions. Biomass Bioenergy 19, 311e335.
Van Dyck, J.S., Pletschke, B.I., 2012. A review of lignocellulose
bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes e factors affecting enzymes, conversion
and synergy. Biotechnol. Adv. 30, 1458e1480.
Van Stappen, F., Brose, I., Schenkel, Y., 2011. Direct and indirect land
use change issues in European sustainability initiatives: state-ofthe-art, open issues and future developments. Biomass Bioenergy
35, 4824e4834.
Ververis, C., Georghiou, K., Danielidis, D., Hatzinikolaou, D.G., Santas, P.,
Santas, R., Corleti, V., 2007. Cellulose, hemicelluloses, lignin and ash
content of some organic materials and their suitability for use as paper
pulp supplements. Bioresour. Technol. 98, 296e301.
Viikari, L., Alapuranen, M., Puranen, T., Vehmaanperä, J., SiikaAho, M., 2007. Thermostable enzymes in lignocellulose hydrolysis.
Adv. Biochem. Eng. Biotechnol. 108, 121e145.
Wan, C., Li, Y., 2010. Microbial pretreatment of corn stover with
Ceriporiopsis subvermispora for enzymatic hydrolysis and ethanol
production. Bioresour. Technol. 101, 6398e6403.
47
Wayman, M., Parekh, S.R., 1990. Biotechnology of Biomass Conversion; Fuel and Chemicals from Renewable Resources. Open
University Press, Milton Keynes, 181e232.
Wheals, A.E., Basso, L.C., Alves, D.M.G., Amorim, H.V., 1999. Fuel
ethanol after 25 years. Trends Biotechnol. 17, 482e487.
Wi, S.G., Chung, B.Y., Lee, Y.G., Yang, D.J., Bae, H.-J., 2011. Enhanced
enzymatic hydrolysis of rapeseed straw by popping pretreatment
for bioethanol production. Bioresour. Technol. 102, 5788e5793.
Williams, M.J., Douglas, J., 2011. Planting and managing giant miscanthus as a biomass energy crop. USDA plant materials program,
Technical Note No. 4.
Wiselogel, A., Tyson, S., Johnson, D., 1996. Biomass feedstock
resources and composition. In: Wyman, C.E. (Ed.), Handbook on
Bioethanol: Production and Utilization. Taylor & Francis,
Washington, DC, pp. 105e118.
World Development Report, 2008. Biofuels: The Promise and the
Risks. The World Bank, 70e71.
World Watch Institute (WWI), 2007. In: Hunt, S., Sterling, V.A. (Eds.),
Biofuels for Transport: Global Potential and Implications for Sustainable Energy and Agriculture.
Wu, M.M., Chang, K., Gregg, D.J., Boussaid, A., Beatson, R.P.,
Saddler, J.N., 1999. Optimization of steam explosion to enhance
hemicellulose recovery and enzymatic hydrolysis of cellulose in
softwoods. Appl. Biochem. Biotechnol. 77 (79), 47e54. www.
ieabioenergy.com/DownLoad.aspx?DocId¼6494.
Wyman, C.E., 2007. What is (and is not) vital to advancing cellulosic
ethanol. Trends Biotechnol. 25, 153e157.
Yamamura, M., Akashi, K., Yokota, A., Hattori, T., Suzuki, S.,
Shibata, D., Umezawa, T., 2012. Characterization of Jatropha curcas
lignins. Plant Biotechnol. 29, 179e183.
Yen, H.-W., Hu, I.-C., Chen, C.-Y., Ho, S.-H., Lee, D.-J., Chang J-, S.,
2013. Microalgae-based biorefinery e from biofuels to natural
products. Bioresour. Technol. 135, 166e174.
Yuan, J.S., Tiller, K.H., Al-Ahmad, H., Stewart, N.R., Stewart, C.N.,
2008. Plants to power: bioenergy to fuel the future. Trends Plant
Sci. 13, 421e429.
Zalesny Jr., R.S., Wiese, A.H., Bauer, E.O., Riemenschneider, D.E., 2009.
Ex situ growth and biomass of Populus bioenergy crops irrigated and
fertilized with landfill leachate. Biomass Bioenergy 33, 62e69.
Zeng, X., Danquah, M., Zheng, C., Potumarthi, R., Xiao, D.C., Lu, Y.,
2012. NaCS-PDMDAAC immobilized autotrophic cultivation of
Chlorella sp. for wastewater nitrogen and phosphate removal.
Chem. Eng. J. 187, 185e192.
Zhang, Y.H.P., Ding, S.-Y., Mielenz, J.R., Cui, J.-B., Elander, R.T.,
Laser, M., Himmel, M.E., McMillan, J.R., Lynd, L.R., 2007.
Fractionating recalcitrant lignocellulose at modest reaction conditions. Biotechnol. Bioeng. 97, 214e223.
Zhang, Y.H.P., Lynd, L.R., 2004. Toward an aggregated understanding
of enzymatic hydrolysis of cellulose: non-complexed cellulase
systems. Biotechnol. Bioeng. 88, 797e824.
Zhang, Y.H.P., Lynd, L.R., 2006. A functionally based model for hydrolysis of cellulose by fungal cellulose. Biotechnol. Bioeng 94,
888e898.
Zhao, X., Zhang, L., Liu, D., 2010. Pretreatment of Siam weed stem
by several chemical methods for increasing the enzymatic
digestibility. Biotechnol. J. 5, 493e504.
Zhao, X., Zhang, L., Liu, D., 2007. Comparative study on chemical
pretreatment methods for improving enzymatic digestibility of
crofton weed stem. Bioresour. Technol. 99, 3729e3736.
Zhu, J.Y., Zhuang, X.S., 2012. Conceptual net energy output for biofuel
production from lignocellulosic biomass through biorefining. Prog.
Energy Combust. Sci. 38, 583e598.
Zhu, L., O’Dwyer, J.P., Chang, V.S., Granda, C.B., Holtzapple, M.T.,
2008. Structural features affecting biomass enzymatic digestibility.
Bioresour. Technol. 99, 3817e3828.
C H A P T E R
3
Use of Agroindustrial Residues
for Bioethanol Production
Luiz J. Visioli, Fabiane M. Stringhini, Paulo R.S. Salbego, Daniel P. Chielle,
Gabrielly V. Ribeiro, Juliana M. Gasparotto, Bruno C. Aita, Rodrigo Klaic,
Jéssica M. Moscon, Marcio A. Mazutti*
Department of Chemical Engineering, Federal University of Santa Maria, Santa Maria, Brazil
*Corresponding author email: mazutti@ufsm.br
O U T L I N E
Introduction
49
Raw Material
Sugar-Containing Residues
Starch-Containing Residues
Cellulose-Containing Residues
50
51
51
52
Sugar Production and Fermentation
52
Separate Hydrolysis and Fermentation
Simultaneous Saccharification and Fermentation
Concluding Remarks
55
References
55
INTRODUCTION
toward the use of fuels derived from plant feedstock
(Ferreira-Leitão et al., 2010).
Agroindustrial and forestry residues, which are byproducts of key industrial and economical activities,
stand out as potential raw materials for the production
of renewable fuels, chemicals and energy (FerreiraLeitão et al., 2010). Biofuels can also be derived from
fishery products or municipal wastes, also including
by-products and wastes originated from agroindustry,
food industry and food services (Nigam and Singh,
2011). The key advantage of the utilization of renewable
sources for the production of biofuels is the utilization of
natural bioresources (that are geographically more
evenly distributed than fossil fuels) and the produced
bioenergy provides independence and security of energy supply (Nigam and Singh, 2011). The use of agricultural residue and waste substrates as raw materials is
advantageous as their availability is not hindered by a
requirement for arable land for the production of food
The last years have verified a pronounced demand
for fossil fuels worldwide due to increase in industrialization and motorization (Agrawal et al., 2007). Nowadays, fossil fuels represent around 80% of all primary
energy consumed in the world, where 58% is employed
in the transport sector (Escobar et al., 2009). The estimates show that the global energy demand is projected
to grow by more than 50% by 2025, with much of this increase in demand emerging from several rapidly developing nations. Clearly, increasing demand for finite
petroleum resources cannot be a satisfactory policy for
the long term (Ragauskas et al., 2006).
Biofuels are a renewable energy source produced
from natural (plant) materials, which can be used as a
substitute for petroleum fuels (Demirbas, 2011). The
global demand for liquid biofuels more than tripled in
last decade, indisputably showing the increasing trend
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00003-6
52
55
49
Copyright Ó 2014 Elsevier B.V. All rights reserved.
50
3. USE OF AGROINDUSTRIAL RESIDUES FOR BIOETHANOL PRODUCTION
and feed. Reusing agricultural waste products is one
goal of environmental sustainability and has become
an option to add value to producers (Manique et al.,
2012). In addition, waste utilization prevents its accumulation, which is of great environmental concern due to its
potential for contamination of rivers and underground
water (Ferreira-Leitão et al., 2010).
The most well-known first-generation biofuel is
ethanol (Nigam and Singh, 2011), which is currently being produced from sugarcane or corn and will often be
referred to as bioethanol (Demirbas, 2011). Ethanol has
long been considered as a suitable alternative to fossil
fuels either as a sole fuel in cars with dedicated engines
or as an additive in fuel blends with no engine modification requirement when mixed up to 30%. Today, bioethanol is the most dominant biofuel and its global
production showed an upward trend over the last
25 years. Worldwide production capacity in 2006
was about 49 109 liters per year, and total output in
2015 is forecast to reach over 115 109 liters (Talebnia
et al., 2010).
Feedstock containing significant amounts of sugar, or
materials that can be converted into sugars, such as
starch or cellulose, can also be used in the production
of ethanol (Nigam and Singh, 2011). The production of
ethanol from cellulosic feedstock has a growing interest
worldwide. Cellulosic biomass is an abundant renewable resource on earth and includes various agricultural
residues. Some of these agricultural residues such as
straw, corn husk, and sugarcane residue represent an
abundant, inexpensive, and readily available source of
renewable lignocellulosic biomass. At the present time,
this readily available biomass is considered as a waste
and is disposed of through agricultural burning after
harvest (Dawson and Boopathy, 2007).
Agricultural residues are produced in large quantities
throughout the world. Approximately, 1 kg of residue is
produced for each kilogram of grain harvested. These
residues are renewable resources that could be used to
produce ethanol and many other value-added products
(Dawson and Boopathy, 2007). Among these residues,
ethanol can be produced from biomass feedstocks such
as sucrose-containing feedstocks (e.g. sugar beet, sweet
sorghum, and sugarcane), starchy materials (e.g. wheat,
corn, barley, cassava, and rice), and lignocellulosic
biomass (e.g. wood, straw, grasses, and various crop residues). These biomass feedstocks can reduce about 50%
of the price of the ethanol produced, depending on the
type of the biomass used (Hong and Yoon, 2011).
Lignocellulosic waste materials obtained from energy
crops, wood and agricultural residues represent the
most abundant global source of renewable biomass.
Among the agricultural residues, wheat straw is the
largest biomass feedstock in Europe and the second
largest in the world after rice straw. About 21% of the
world’s food depends on the wheat crop and its global
production needs to be increased to satisfy the growing
demand of human consumption; therefore, wheat straw
would serve as a great potential feedstock for production of ethanol in the twenty-first century (Talebnia
et al., 2010).
The use of lignocellulosic energy crops, and particularly
low-cost biomass residues, offers excellent perspectives
for large-scale application of ethanol in transportation
fuels. These materials will increase the ethanol production
capacity and reduce production cost to a competitive level.
Bioethanol from these materials provides a highly costeffective option for CO2 emission reduction in the transportation sector (Patle and Lal, 2007).
The utilization of lignocellulosic biomass has been
closely associated with a new technological concept,
so-called biorefinery, which emerges as key to the
significant expansion of the desired production of
ethanol. Fermentative processes stand out, where
microbial metabolism is used for the transformation
of simple raw materials in products with high aggregate value. Experts believe that the biorefineries are
likely to be a key industry of the twenty-first century,
even responsible for a new industrial revolution,
because of the importance of the technologies they
employ and their effects on the actual industrial model
(Santos et al., 2010).
Regarding crop residues that have proper application
in energy supply, the energetic generation cost for useful
energy is a matter for consideration. Studies done so far
suggest that, when transport distances are similar, the
most efficient energetic use of lignocellulosic materials
such as agricultural residues is the application for the
generation of electricity. Applied in this way, crop residues are most efficient in replacing fossil fuels, much
more so than when crop residues are converted to
ethanol for use in cars. However, when road transport
distances to power-generating plants are very large, it
may be that energetic uses that require a much lower
input of transport fuel become energetically more attractive (Reijnders, 2008).
Based on these aspects, the main objective of this
work is to present an overview about bioethanol
production from agroindustrial residues, which were
based on low-priced feedstocks such as crop residues,
municipal/industrial solid waste, and food residues.
For this purpose, papers and overviews since 2006
were reported.
RAW MATERIAL
Bioethanol is usually produced out of organic-based
matter with high contents of sugar fermentation by
enzymes produced from yeast. The yeast converts
51
RAW MATERIAL
six-carbon sugars (mainly glucose) to ethanol, because
starch is much easier than cellulose to convert to glucose
(Nigam and Singh, 2011). Bioethanol is produced similarly to other alcohols such as spirits using natural products like wheat, maize and sugar beet. Hence, the
suitable raw materials required for bioethanol production could be any of those materials that contain considerable amounts of carbohydrates to provide fermentable
sugars for bioconversion into bioethanol. Then an optimized microbial fermentation process can be used for
the bioconversion of sugars released from carbohydrates
into ethanol (Nigam and Singh, 2011).
Agricultural waste materials are inexpensively found
outside the human food chain in large amounts and can
be obtained throughout the year. These agricultural biomasses are the potential feedstocks for bioethanol production, including the cellulosic biomass, as well as
starchy waste agricultural materials, and they provide
low-cost and uniquely sustainable resources, improvement on energy security, development of the economy,
as well as cleaning the environment and atmosphere
by the disposing of problematic solid wastes and getting
wealth out of wastes. Synthetically, 7% ethanol can be
made from petroleum resources and 93% ethanol
through fermentation process using microorganisms to
convert biomass materials into ethanol (Kahn et al.,
2011).
Sugar-Containing Residues
Raw materials (containing saccharides) include sweet
sorghum, sugar beet, banana, mango, watermelon, sugarcane and other fruits; these are examples of sugar
feedstock. The wastes of these sugar-containing sources
can be fermented by using different microorganisms of
interest. However, the use of these materials for
bioethanol production is highly expensive and humans
use them as food (Kahn et al., 2011).
The first research about bioethanol showed in
Table 3.1 reports the production of biofuel using residues of fruits and vegetables. These are an important
source of sugar for ethylic fermentation because the processing of fruits have a great potential to generate residues that can be used. The authors report the use of
enzymes in the process; this is necessary because these
residues have a lot of fiber that can be hydrolyzed (Patle
and Lal, 2007). The use of wastes is the main aspect of
two other studies (Gouvea et al., 2009; Ge et al., 2011).
Gouvea et al. (2009) reported the use of a residue very
abundant in Brazil, that is, the coffee husk. As well as
in the case of fruit and vegetable residues the floating
seaweed wastes have other kinds of carbohydrates that
were hydrolyzed by enzymes (Wu et al., 2011). Ge
et al. (2011) reported the use of a principal sugar source
for ethylic fermentation, the stalk juice of sugarcane.
This juice has great quantity of glucose in its composition and has a low cost. More studies about sugarcane
juice were omitted since the main subject of this chapter
is agroindustrial residues.
Starch-Containing Residues
Starch materials are also potential resources for the bioethanol production. Starch molecules are polysaccharides
made up of long chains of glucose units covalently linked.
Before the fermentation process, the starchy materials are
broken into simple glucose molecules after which the simple sugar units are easily fermented by the microbes.
Examples of starchy materials used for bioethanol production include cereal grains, potato, sweet potato, beans, cassava, maize, wheat and cereal grains. As these materials
are also too expensive and included in the human food
TABLE 3.1 Sugar Raw Material
Raw Material
Sugar Production
Operation
Ethanol Yield
References
Fruit and vegetable
residues (apple, carrot,
mango, orange, pineapple,
sapota and tomato)
Acid and enzymatic
hydrolysis was used
previously to the
fermentation to release the
sugar from residues
Separated hydrolysis and
fermentation (SHF)
Apple: yield of 79.8%
Carrot: yield of 70%
Mango: yield of 82.8%
Orange: yield of 91.3%
Pineapple: yield of 75.4%
Sapota: yield of 97.7%
Tomato: yield of 72.4%
Patle and Lal (2007)
Coffee husks
Not applicable
Not applicable
Ethanol production was
84.9 2.9 g/kg dry basis
Gouvea et al. (2009)
Floating seaweed wastes
Acid pretreatment
(H2SO4), enzymatic
hydrolysis fermentation
Separated hydrolysis and
fermentation (SHF)
The maximum yield of
glucose reached 277.5
mg/g (FSW) that was
80.8% fermented to ethanol
Ge et al. (2011)
Sugarcane stalk juice
Not applicable
Not applicable
The theoretical yield was
88%
Wu et al. (2011)
52
TABLE 3.2
3. USE OF AGROINDUSTRIAL RESIDUES FOR BIOETHANOL PRODUCTION
Starch Raw Material
Raw Material
Sugar Production
Operation
Ethanol Yield
References
Food residues
The hydrolysis was
made by enzymes
Simultaneous saccharification
and fermentation (SSF)
The ethanol concentrations
were 19, 35, and 39 g/dm3
when the concentrations of
food residue were 50, 100, and
120 g/dm3
Hong and Yoon (2011)
Raw corn starch
Enzymatic
saccharification
Simultaneous saccharification
and fermentation (SSF)
The largest production of
ethanol was 85 g/kg (raw
material)
Moukamnerd et al. (2010)
Potato starch
residue
Hydrolysis acid (HCl
and H2SO4)
Separated hydrolysis and
fermentation (SHF)
The maximum yield of ethanol
(5.52 g/l)
Hashem and Darwish
(2010)
chain, wastes are collected from places where they are
crushed into flour or from industries where they are
used for various products (Kahn et al., 2011).
Table 3.2 reports the use of starchy residues in ethanol
production. As starch is an important component of the
food chain, it is expected that the wastes of the processing be more used for ethylic fermentation (Moukamnerd
et al., 2010). Another important point that can be
observed is the predominant use of simultaneous
saccharification and fermentation (SSF) in the use of
starch for energy generation and in both cases hydrolysis was achieved by enzymes (Hong and Yoon, 2011;
Moukamnerd et al., 2010). Hashem and Darwish (2010)
reported the use of potato starch residue stream produced during chips manufacture and the authors have
used separate hydrolysis and fermentation (SHF) and
a very low acid hydrolysis of the starch to reduce the
cost associated with this necessary treatment.
Cellulose-Containing Residues
Another way to produce bioethanol is using cellulosic
materials. Examples of cellulosic materials are bagasse,
straw, paper, cardboard, wood and materials of plant
cellulosic fibers such hemp, giant reed, eucalyptus tree
and Miscanthus. Cellulosic resources are immensely
widespread and found abundantly everywhere. These
cellulosic materials have the potential to be used for the
production of bioethanol since they are not commonly
used in the human food chain and exist in large amounts.
Moreover, these materials are inexpensive as compared to
the sugar and starchy feedstocks and preferably used for
bioethanol production. Cellulosic materials are called
lignocelluloses because they are composed of lignin,
cellulose and hemicelluloses (Kahn et al., 2011).
The cellulosic residue more reported in Table 3.3 for
ethanol production is sugarcane bagasse (Dawson and
Boopathy, 2007; Santos et al., 2012; Wu et al., 2011;
Buaban et al., 2010). This can be explained because of
the great use of its juice for sugar ethylic fermentation
and the residue is generated just in the alcohol manufacture (Santos et al., 2012). Furthermore, the quantity of
this waste available is very great and its direct combustion is not the best economical way to use this resource
(Wu et al., 2011). The hydrolysis process more employed
for saccharification of the bagasse is enzymatic. This is
because of the inefficiency of acid hydrolysis in a very
complex matrix. Moreover many studies are trying
SSF, which can reduce one step of the process.
The agricultural wastes (apart from sugarcane) are
studied much for the ethanol production. This is because
they are present around the world (comes from diverse
agricultural crops). Thus these residues can mean the
energetic independence of many countries. Rice plant
(Kitamoto et al., 2011) and Lycoris radiata Herbert (Liu
et al., 2012) are examples of how much singular are the
wastes that the researches are using to produce biofuels.
Similarly to sugarcane, Talebnia et al. (2010) have tested
SSF and SHF of wheat straw, and obtained good results
in the two cases. In Table 3.3 it is possible to see that
pretreatment (physical or chemical) is usually necessary,
and this is a difficulty in this production.
SUGAR PRODUCTION AND
FERMENTATION
Separate Hydrolysis and Fermentation
SHF uses different stages for enzyme production, cellulose hydrolysis and fermentation of glucose. In this
process, the hydrolysis of cellulose occurs before the
fermentation of glucose, which is carried out in different
reactors. In this case, the temperature of hydrolysis and
fermentation can be optimized individually (Hamelinck
et al., 2005). For this reason, this process is preferably
used for ethanol production from lignocellulosic material, as can be seen in Table 3.3, since the optimal temperature for acid or enzymatic hydrolysis is different from
that of fermentation.
TABLE 3.3 Cellulosic Raw Material
Sugar Production
Operation
Ethanol Yield
References
Postharvest sugarcane residue
Alkaline pretreatment (H2O2) to
remove lignin and acid hydrolysis
(H2SO4)
Separated hydrolysis and
fermentation (SHF)
Ethanol production of
335.67 mg/l after 12 days of
fermentation
Dawson and Boopathy
(2007)
Agricultural residues and hay
(wheat, barley, and triticale
straw and barley, triticale,
pearl millet, and sweet
sorghum hay)
Chemical pretreatment (NaOH or
H2SO4) and enzymatic hydrolysis
Separated hydrolysis and
fermentation (SHF)
Production between 52.00%
and 65.82% of the theoretical
ethanol yield
Chen et al. (2007)
Cotton stalk, triticale hay,
barley, triticale, and wheat
straw
Enzymatic hydrolysis of
lignocelluloses
Separated hydrolysis and
fermentation (SHF)
Ethanol yields range between
0.21 and 0.28 (g/g reducing
sugars)
Chen et al. (2007)
Wheat straw
The several kinds of pretreatment
and hydrolysis are found in the
reference
Simultaneous saccharification and
fermentation (SSF)
The largest ethanol yield
reported by the authors was
81%
Talebnia et al. (2010)
Wheat straw
The several kinds of pretreatment
and hydrolysis are found in the
reference
Separated hydrolysis and
fermentation (SHF)
Ethanol yield ranging from
65% to 99% of theoretical value
Talebnia et al. (2010)
Production canola residue
Acid (H2SO4) and alkali (NaOH)
pretreatment, followed by
enzymatic hydrolysis
Separated hydrolysis and
fermentation (SHF)
The best yield obtained in the
fermentation was 45% for
ethanol production or around
95 l per dry ton of raw material
George et al. (2010)
Corncob residues
Chemical pretreatment (acid or
alkali) and enzymatic hydrolysis
Simultaneous saccharification and
fermentation (SSF)
The yield range was between
25.2% and 27.1% of theoretical
value to ethanol production
Liu et al. (2010)
Floating seaweed wastes
(FSWs)
Acid pretreatment (H2SO4),
enzymatic hydrolysis
fermentation
Separated hydrolysis and
fermentation (SHF)
The maximum yield of glucose
reached 277.5 mg/g FSW that
was 80.8% fermented to
ethanol
Ge et al. (2011)
Sugarcane bagasse
Pretreated by steam explosion at
200 C and delignification with
NaOH, and enzymatic hydrolysis
Simultaneous saccharification and
fermentation (SSF)
Ethanol concentration was
higher than 25 g/l
Santos et al. (2012)
SUGAR PRODUCTION AND FERMENTATION
Raw Material
(Continued)
53
54
TABLE 3.3
Cellulosic Raw Materialdcont’d
Sugar Production
Operation
Ethanol Yield
References
Rice plants
Physical and chemical
pretreatment and enzymatic
saccharification
Simultaneous saccharification and
fermentation (SSF)
Ethanol in fresh matter
(169 g/kg dry matter) was
produced
Kitamoto et al. (2011)
Lycoris radiata Herbert
(Amarylllidaceae) residues
The residue was hydrolyzed by
enzymes before the fermentation
Separated hydrolysis and
fermentation (SHF)
Not determined
Liu et al. (2012)
Sugarcane bagasse
Enzymatic hydrolysis
Separated hydrolysis and
fermentation (SHF)
The theoretical yield was 88%
Wu et al. (2011)
Soybeans hulls
Enzymatic hydrolysis without
pretreatment
Simultaneous saccharification and
fermentation (SSF)
Ethanol concentrations of
25e30 g/l were obtained
Mielenz et al. (2009)
Sugarcane bagasse
Mechanical pretreatment by ball
milling, with enzymatic
hydrolysis
Separated hydrolysis and
fermentation (SHF)
Yield of 56.9% of theoretical
production
Buaban et al. (2010)
Sugarcane bagasse
Mechanical pretreatment by ball
milling, with enzymatic
hydrolysis
Simultaneous saccharification and
fermentation (SSF)
Yield of 52.9% of the
theoretical production
Buaban et al. (2010)
Corn stover
Enzymatic hydrolysis
Simultaneous saccharification and
fermentation (SSF)
The yield was between 69%
and 98% of the theoretical
ethanol production
Yoo et al. (2012)
SHF, separated hydrolysis and fermentation; SSF, simultaneous saccharification and fermentation.
3. USE OF AGROINDUSTRIAL RESIDUES FOR BIOETHANOL PRODUCTION
Raw Material
55
REFERENCES
The main characteristic of SHF is that the technique
allows a high number of steps. Thereby the hydrolysis
can be carried out with better efficiency than in SSF.
Another important aspect to the use of this one is the
unnecessity of development or trials of new microorganism or enzymes that are able to produce ethanol
or sugar at different medium of that when usually
fermentation happens (George et al., 2010). Tables 3.2
and 3.3 show that the SHF is the predominant technique used for saccharification of any source of
carbohydrates.
Simultaneous Saccharification
and Fermentation
Research in ethanol has been targeted for the development of second-generation technology, including the
strategy of SSF process, which combines in a single
unit the cellulose enzymatic hydrolysis and the ethanol
fermentation (Santos et al., 2010). In the SSF process,
glucose released by cellulase action is directly converted
to ethanol by the fermenting microorganisms, which alleviates problems caused by the end product.
The consumption of glucose and the presence of
ethanol in the culture medium would reduce the risk
of undesired contamination by glucose-dependent organisms. Recently, consolidated bioprocessing, which
combines enzyme production, saccharification and
fermentation in a single step, has gained recognition as
a potential bioethanol production system, because the
costs of capital investment, substance and other raw materials, and utilities associated with enzyme production
can be avoided using microorganisms with the capability for efficient cellulose hydrolysis and ethanol production (Hasunuma and Kondo, 2012).
Recently, there are many reports that SSF is superior
to the traditional saccharification and subsequent
fermentation in the ethanol production because the
SSF process can improve ethanol yields by removing
end-product inhibition of saccharification process and
decrease the enzyme loading. Moreover, SSF requires a
single fermenter for the entire process and eliminates
the need for separating reactors for saccharification
and fermentation leading to reduce the investment
cost (Boonsawang et al., 2012).
Difference between SHF and SSF is in an incipient step
of their development. It is possible to note that a significant
number of studies reported in Tables 3.2 and 3.3 are making a comparison between the two techniques, which
shows that the SSF researches are trying to develop an efficient process to substitute the SHF method. On the other
hand the starchy raw materials have a great use in SSF
fermentation; this can be explained by the simplicity of
this substrate compared to cellulosic (the efficiency of
enzymatic hydrolysis is better) and the conditions
of operation can be milder, facilitating the adaptation
of a fermentation microorganism.
CONCLUDING REMARKS
As can be seen from the tables above, there is a
growing interest in ethanol production from agroindustrial residues of a variety of sources including grains,
straws, stalks and husks such as cotton, barley, triticale,
wheat, coffee, rice, canola, sugarcane and other fruits
and vegetables. In terms of volume, lignocellulosic
material is the predominant raw material for secondgeneration ethanol. However, the production costs
associated with the use of lignocellulosic ethanol is
high, making it necessary to develop an efficient process
for hydrolysis and fermentation, where the use of simultaneous saccharification and hydrolysis is seen a promising technology, but there is also the necessity to
genetically modify a microorganism to grow at high
temperatures or obtain an enzyme to carry out the hydrolysis at normal fermentation temperature. Low-cost
biomass residues offer excellent perspective for largescale application of ethanol.
Acknowledgments
The authors thank CAPES for the scholarships and SCIT-RS and CNPq
for the financial support of this work.
References
Agrawal, R., Singh, N.R., Ribeiro, F.H., Delgass, W.N., 2007. Sustainable fuel for the transportation sector. Proc. Natl. Acad. Sci. 104,
4828e4833.
Boonsawang, P., Subkaree, Y., Srinorakutara, T., 2012. Ethanol production from palm pressed fiber by prehydrolysis prior to simultaneous saccharification and fermentation (SSF). Biomass
Bioenergy 40, 127e132.
Buaban, B., Inoue, H., Yano, S., Tanapongpipat, S., Ruanglek, V.,
Champreda, V., Pichyangkura, R., Rengpipat, S., Eurwilaichitr, L.,
2010. Bioethanol production from ball milled bagasse using an
on-site produced fungal enzyme cocktail and xylose-fermenting
Pichia stipitis. J. Biosci. Bioeng. 110, 18e25.
Chen, Y., Sharma-Shivappa, R.R., Chen, C., 2007. Ensiling agricultural
residues for bioethanol production. Appl. Biochem. Biotechnol.
143, 80e92.
Dawson, L., Boopathy, R., 2007. Use of post-harvest sugarcane residue
for ethanol production. Bioresour. Technol. 98, 1695e1699.
Demirbas, A., 2011. Competitive liquid biofuels from biomass. Appl.
Energy 88, 17e28.
Escobar, J.C., Lora, E.S., Venturini, O.J., Yanez, E.r E., Castillo, E.F.,
Almazan, O., 2009. Biofuels: environment, technology and food
security. Renewable Sustainable Energy Rev. 13, 1275e1287.
Ferreira-Leitão, V., Gottschalk, L.M.F., Ferrara, M.A., Nepomuceno, A.L.,
Molinari, H.B.C., Bon, E.P.S., 2010. Biomass residues in Brazil: availability and potential uses. Waste Biomass Valorization 1, 65e76.
56
3. USE OF AGROINDUSTRIAL RESIDUES FOR BIOETHANOL PRODUCTION
Ge, L., Wang, P., Mou, H., 2011. Study on saccharification techniques
of seaweed wastes for the transformation of ethanol. Renewable
Energy 36 (84), 89.
George, N., Yang, Y., Wang, Z., Sharma-Shivappa, R., Tungate, K.,
2010. Suitability of canola residue for cellulosic ethanol production.
Energy Fuels 24, 4454e4458.
Gouvea, B.M., Torres, C., Franca, A.S., Oliveira, L.S., Oliveira, E.S., 2009.
Feasibility of ethanol production from coffee husks. Biotechnol. Lett.
31, 1315e1319.
Hamelinck, C.N., van Hooijdonk, G., Faaij, A.P., 2005. Ethanol from
lignocellulosic biomass: techno-economic performance in short,
middle, and long term. Biomass Bioenergy 28, 384e410.
Hashem, M., Darwish, S.M.I., 2010. Production of bioethanol and
associated by-products from potato starch residue stream by
Saccharomyces cerevisiae. Biomass Bioenergy 34, 953e959.
Hasunuma, T., Kondo, A., 2012. Consolidated bioprocessing and
simultaneous saccharification and fermentation of lignocellulose to
ethanol with thermotolerant yeast strains. Process Biochem. 47,
1287e1294.
Hong, Y.S., Yoon, H.H., 2011. Ethanol production from food residues.
Biomass Bioenergy 35, 3271e3275.
Kahn, R.A., Kahn, A.N., Ahmed, M., Kahn, M.R., Shah, M.S.,
Azam, N., Sadullah, F., Dian, F., Ullah, S., Kahn, N., 2011. Bioethanol sources in Pakistan: a renewable energy resource. Afr. J.
Biotechnol. 10, 19850e19854.
Kitamoto, H.K., Horita, M., Cai, Y., Shinozaki, Y., Sakaki, K., 2011.
Silage produces biofuel for local consumption. Biotechnol. Biofuels
4, 46.
Liu, K., Lin, X., Yue, J., Li, X., Fang, X., Zhu, M., Lin, J., Qu, Y.,
Xiao, L., 2010. High concentration ethanol production from
corncob residues by fed-batch strategy. Bioresour. Technol. 101,
4952e4958.
Liu, S., Ding, Z.G., Zhang, L., Gu, Z., Wang, X., Sun, X., Shi, G., 2012.
Ethanol production from Lycoris radiata Herbert (Amarylllidaceae)
residues as a new resource. Biomass Bioenergy 37, 237e242.
Manique, M.C., Faccini, C.S., Onorevoli, B., Benvenutti, E.V., 2012. Rice
husk ash as an adsorbent for purifying biodiesel from waste frying
oil. Fuel 92, 56e61.
Mielenz, J.R., Bardsley, J.S., Wyman, C.E., 2009. Fermentation of soybean hulls to ethanol while preserving protein value. Bioresour.
Technol. 100, 3532e3539.
Moukamnerd, C., Kino-oka, M., Sugiyama, M., Kaneko, Y.,
Boonchird, C., Harashima, S., Noda, H., Ninomiya, K., Shioya, S.,
Katakura, Y., 2010. Ethanol production from biomass by repetitive
solid-state fed-batch fermentation with continuous recovery of
ethanol. Appl. Microbiol. Biotechnol. 88, 87e94.
Nigam, P.S., Singh, A., 2011. Production of liquid biofuels from
renewable resources. Prog. Energy Combust. Sci. 37, 52e68.
Patle, S., Lal, B., 2007. Ethanol production from hydrolysed agricultural wastes using mixed culture of Zymomonas mobilis and Candida
tropicalis. Biotechnol. Lett. 29, 1839e1843.
Ragauskas, A.J., Williams, C.K., Davison, B.H., Britovsek, G., Cairney, J.,
Eckert, C.A., Frederick Jr, W.J., Hallett, J.P., Leak, D.J., Liotta, C.L.,
Mielenz, J.R., Murphy, R., Templer, R., Tschaplinski, T., 2006. The
path forward for biofuels and biomaterials. Science 311, 484.
Reijnders, L., 2008. Ethanol production from crop residues and soil
organic carbon. Resour., Conserv. Recycl. 52, 653e658.
Santos, D.S., Camelo, A.C., Rodrigues, K.C.P., Carlos, L.C., Pereira, N.,
2010. Ethanol production from sugarcane bagasse by Zymomonas
mobilis using simultaneous saccharification and fermentation (SSF)
process. Appl. Biochem. Biotechnol. 161, 93e105.
Santos, J.R.A., Lucena, M.S., Gusmão, N.B., Gouveia, E.R., 2012.
Optimization of ethanol production by Saccharomyces cerevisiae
UFPEDA 1238 in simultaneous saccharification and fermentation
of delignified sugarcane bagasse. Ind. Crops Prod. 36, 584e588.
Talebnia, F., Karakashev, D., Angelidaki, I., 2010. Production of bioethanol from wheat straw: an overview on pretreatment, hydrolysis and fermentation. Bioresour. Technol. 101, 4744e4753.
Wu, L., Li, Y., Arakane, M., Ike, M., Wada, M., Terajima, Y.,
Ishikawa, S., Tokuyasu, K., 2011. Efficient conversion of sugarcane
stalks into ethanol employing low temperature alkali pretreatment
method. Bioresour. Technol. 102, 11183e11188.
Yoo, C.G., Kuo, M., Kim, T.H., 2012. Ethanol and furfural production
from corn stover using a hybrid fractionation process with zinc
chloride and simultaneous saccharification and fermentation (SSF).
Process Biochem. 47, 319e326.
C H A P T E R
4
Recent Advancements in Pretreatment
Technologies of Biomass to Produce Bioenergy
Irmene Ortı´z*, Rodolfo Quintero
Departamento de Procesos y Tecnologı́a, Universidad Autónoma Metropolitana - Cuajimalpa, México D.F., México
*Corresponding author email: irmene@correo.cua.uam.mx
O U T L I N E
Lignocelullosic Biomass
57
Pretreatment of Lignocelullosic Biomass
for Biofuels Production
Trends in Pretreatments
Other Pretreatments
62
62
58
Pretreatment Modeling
65
58
58
59
60
Environmental and Economical Aspects
65
Concluding Remarks
66
References
66
Types of Pretreatments
Biological Pretreatments
Physical Pretreatments
Chemical Pretreatments
Physicochemical
Pretreatments
61
LIGNOCELULLOSIC BIOMASS
resulting in the aggregation of chains into elementary
crystalline fibrils of 36 cellulose chains, while hemicelluloses are complex branched heterogeneous polysaccharides composed of monomeric residues: D-glucose,
D-galactose, D-mannose, D-xylose, L-arabinose, D-glucuronic acid and 4-O-methyl-D-glucuronic acid; and lignin
is a complex amorphous network formed by polymerization of phenyl propane units and constitutes the
most abundant nonpolysaccharide fraction in lignocellulose (Jørgensen et al., 2007; Lewis et al., 2005).
Biofuels produced from native lignocellulose are
known as second-generation biofuels. In this process
the cellulose is converted into glucose, which is easily
fermented to ethanol, while the hemicellulosic fraction
is converted into monomeric sugars (mainly pentoses),
a fermentation that is considerably harder to accomplish
(Dias et al., 2011). The physicochemical and structural
compositions of native lignocellulose are, however,
recalcitrant to direct enzymatic hydrolysis of cellulose
(Mosier et al., 2005). Therefore, a pretreatment step is
Lignocellulosic biomass is composed primarily of cellulose, hemicelluloses (mainly xylan), lignin and smaller
amounts of other compounds. Typically, the composition of lignocellulosic biomass by weight is 40e50% cellulose, 20e40% hemicellulose, 10e30% lignin and other
components such as minerals, oils, soluble sugars, pectins, proteins, and ashes (Jørgensen et al., 2007; Lewis
et al., 2005; Wyman et al., 2005).
Cellulose, hemicelluloses and lignin are present in
varying amounts in the different parts of the plant and
they are intimately associated to form the structural
framework of the plant cell wall; also, the content of
the different sugars of the hemicelluloses varies significantly between different plants depending on plant species, age and growth conditions (Jørgensen et al., 2007).
Cellulose is the most abundant constituent of the
plant cell wall; its linear structure enables the formation
of both intra- and intermolecular hydrogen bonds
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00004-8
57
Copyright Ó 2014 Elsevier B.V. All rights reserved.
58
4. RECENT ADVANCEMENTS IN PRETREATMENT TECHNOLOGIES OF BIOMASS TO PRODUCE BIOENERGY
invariably required to render the cellulose amenable to
enzymatic hydrolysis (Zheng et al., 2009).
The total estimated availability of usable biomass in the
world is about 2 billion dry tons per year (Lewis et al.,
2005). Therefore, the enormous potential of secondgeneration fuels and the increasing interest toward developing effective, low-cost and environmentally friendly
pretreatments for breaking down the close association of
the structures of the biomass.
by-products, e.g. lignin; (9) to be cost-effective by operating in reactors of moderate size, minimizing the heat
and power requirements, chemicals and capital equipment; and (10) be scalable to industrial size (Alvira
et al., 2010; Brodeur et al., 2011; Jørgensen et al., 2007;
Yang and Wyman, 2008).
This chapter reviews the advances in the most studied
pretreatments and those recently proposed in scheme of
the biochemical biorefinery, including kinetics, mechanistic and economical models proposed for describing
some of these pretreatment processes.
PRETREATMENT OF LIGNOCELULLOSIC
BIOMASS FOR BIOFUELS PRODUCTION
TYPES OF PRETREATMENTS
One of the most promising emerging biorefinery platforms is the biochemical path that focuses on fermentation of sugars extracted from lignocellulosic feedstocks
(Carvalheiro et al., 2008). This technology involves three
basic steps: (1) conversion of biomass to sugar or other
fermentation-feedstock, (2) bioconversion of these
biomass intermediates using biocatalysts, and (3) process products to yield added value chemicals, fuelgrade ethanol and other fuels, heat and/or electricity
(Carvalheiro et al., 2008).
The first step involves a pretreatment process the goal
of which is to alter or remove structural and compositional impediments to hydrolysis in order to improve
the rate of enzyme hydrolysis and increase yields
of fermentable sugars from cellulose or hemicellulose
(Mosier et al., 2005). The effectiveness of enzymatic
hydrolysis of pretreated lignocellulosic biomass can be
significantly enhanced if lignin and its derivatives are
removed or effectively modified before adding enzymes
because lignin and its derivatives interfere with the path
for cellulases action and they are also toxic to microorganisms, slowing down enzymatic hydrolysis (Qing
et al., 2010; Yang and Wyman, 2008).
The ideal pretreatment process produces a disrupted,
hydrated substrate that is easily hydrolyzed but avoids
the formation of sugar degradation products and
fermentation inhibitors (Agbor et al., 2011). Furthermore, there is an overall consensus on the several technical, operational and economical characteristics that a
successful pretreatment should accomplish, including
(1) maximize the production of highly digestible solids
that enhances sugar yields during enzyme hydrolysis;
(2) avoid the degradation of sugars including those
derived from hemicellulose; (3) not require the addition
of toxic compounds or minimize their use; (4) fermentation compatibility, minimizing the formation of inhibitors for the enzymes or microorganisms in the
subsequent steps; (5) effectiveness at low moisture content; (6) broad applicability for multiple crops, sites ages
and harvesting times; (7) not required size reduction of
biomass; (8) maximize the production of other valuable
The pretreatment methods cause physical and/or
chemical changes in the lignocellulosic biomass; thus,
pretreatment technologies are usually classified into
physical, chemical, physicochemical, and biological.
For the purposes of classification, steam and water
are excluded from being considered chemical agents
for pretreatment since extraneous chemicals are not
added to the biomass (Mosier et al., 2005). This chapter
focuses on chemical, physical and physicochemical
pretreatments but a brief description of biological
treatments are include in order to contrast and
compare them.
Biological Pretreatments
Biological pretreatments employ microorganisms
mainly brown, white and soft-rot fungi, which degrade
lignin, hemicellulose and cellulose in small proportion
(Alvira et al., 2010). Recently, this approach has received
renewed attention as biological pretreatments have
several advantages over conventional physical/chemical pretreatment methods, such as they are considered
as environmentally friendly, low capital cost, low
energy, no chemicals requirement, and mild environmental conditions (Saritha and Lata, 2011). However,
the main drawbacks to develop biological methods are
the low hydrolysis rate obtained in most biological
materials and the relatively long time of the pretreatment compared to physical/chemical methods. Consequently, more space and longer processes are required,
which increase the operating costs (Alvira et al., 2010;
Saritha and Lata, 2011).
The white-rot fungi are able to decompose all wood
fractions, including lignin because they produce various
enzymes involved in lignin degradation such as lignin
peroxidase, laccase, manganese peroxidase, versatile
peroxidase, and H2O2-forming enzymes such as glyoxal
oxidase and aryl alcohol oxidase. White-rot fungi also
produce cellulases, xylanases and other hemicellulases
that are required in the hydrolysis. Almost all
TYPES OF PRETREATMENTS
white-rot fungi produce manganese peroxidase and laccase, but only some of them produce lignin peroxidase
(Isroi et al., 2011).
Several white-rot fungi such as Phanerochaete chrysosporium, Ceriporia lacerata, Cyathus stercolerus, Ceriporiopsis subvermispora, Pycnoporus cinnarbarinus, Pleurotus
ostreaus, Dichomitus squalens, Coriolus versicolor, Trichoderma reesei, Aspergillus terreus, Aspergillus awamori, Bjerkandera adusta, Phlebia tremellosus, Fusarium proliferatum,
and Pleurotus florida have been examined on different
lignocellulosic biomass (Alvira et al., 2010; Cui et al.,
2012; Isroi et al., 2011; Kuhar et al., 2008; Pinto et al.,
2012; Saritha and Lata, 2011; Wan and Li, 2011). Recently,
some bacterial laccases have also been characterized
from Azospirillum lipoferum and Bacillus subtilis (Saritha
and Lata, 2011). However, they face three major challenges associated with lignin structure: (1) the lignin
polymer is large; therefore, ligninolytic systems must
be extracellular, (2) lignin structure comprises interunit
carbonecarbon and ether bonds; therefore, the degradation mechanism must be oxidative rather than hydrolytic, and (3) lignin polymer is stereoirregular, therefore the
ligninolytic agents must be much less specific than
degradative enzymes (Isroi et al., 2011).
As mentioned before, one of the most main drawbacks of biological pretreatments is the time of the pretreatment; reported time of treatment is between 7 and
60 days (Giles et al., 2011; Mahalaxmi et al., 2010; Wan
and Li, 2011). After 18 days of pretreatment, C. subvermispora effectively delignified corn stover, switchgrass,
and hardwood with glucose yields during enzymatic
hydrolysis that reached 56.50%, 37.15%, and 24.21%,
respectively (Wan and Li, 2011). Also, glucose yield
after 21 days of pretreatment with Poria subvermispora
and Irpex lacteus reached 69% and 66% of cellulose
available in the wheat straw, respectively, with an
ethanol yield of 62% in both cases (Salvachua et al.,
2011).
Biological pretreatment has also been used before pyrolysis of biomass to produce fuel. The biological pretreatment of corn stover can optimize the thermal
decomposition, decrease the reaction temperature and
reduce the gas contamination (SOx), making the
biomass pyrolysis more efficient and environmentally
friendly. Biological pretreatment can decrease the activation energy and reacting temperature of the hemicellulose and cellulose pyrolysis (up to 36 C), shorten the
temperature range of the active pyrolysis (up to 14 C),
and increase the thermal decomposition rate (Isroi
et al., 2011; Yang et al., 2011).
A cost-competitive biological pretreatment of lignocellulose requires continuous studying and testing more microorganisms for their ability to delignify the plant
material quickly and efficiently (Saritha and Lata, 2011).
Also, integrated methods, such as, cotreatment with
59
organic solvents, diluted acids, supercritical CO2 and
ionic liquids (ILs); mutation breeding and crossbreeding
of fungal mycelia to obtain engineering strains; and integration of fungal pretreatment with simultaneous
saccharification and fermentation to produce biofuels
and value-added products should be studied (Tian
et al., 2012).
Physical Pretreatments
Physical methods involve breakdown of biomass size
by coarse size reduction, chipping, shredding, grinding,
and milling in order to increase the available specific
surface area and reduce the degree of polymerization,
enhancing the digestibility of lignocellulosic biomass
(Agbor et al., 2011; Brodeur et al., 2011). However, it
has been shown that further reduction of biomass particle size below 0.4 mm has little effect on the rates and
yields of biomass hydrolysis (Agbor et al., 2011).
Chipping reduces heat and mass transfer limitations;
grinding and milling are more effective at reducing the
particle size and cellulose crystallinity than chipping,
probably as result of the shear forces generated during
milling. Vibratory ball milling has been used with
more effective results in reducing cellulose crystallinity
than ordinary ball milling. Also, disk milling, which produces fibers, has been reported as more efficient in
enhancing cellulose hydrolysis than hammer milling,
which produces finer bundles (Agbor et al., 2011). Stirring ball milling also could significantly damage the
structure of biomass, resulting in the variation of surface
morphology, the increase in amorphous region ratio and
hydrogen bond energy, and the decrease in crystallinity
and crystalline size (Liao et al., 2011).
The energy requirements for physical pretreatments
are dependent on the biomass characteristics, final particle size and reduction in crystallinity, for example,
hardwoods require more energy input than agricultural
residues (Agbor et al., 2011). Taking into account the
high-energy requirement of milling on an industrial
scale and the rise in energy demands, this method is
not economically feasible and likely will not be used in
a full-scale process (Agbor et al., 2011; Saritha and
Lata, 2011). In most cases where the only option available for pretreatment is physical, the required energy
is higher than the theoretical energy content available
in the biomass (Brodeur et al., 2011).
The pretreated biomasses by physical methods are
subjected to heating, mixing and shearing resulting in
physical and chemical modifications (Karunanithy and
Muthukumarappan, 2011; Lamsal et al., 2010; Saritha
and Lata, 2011). Also, Agbor et al., in 2011, suggested
that the materials can be milled after chemical pretreatment with significantly reduction of (1) milling energy
consumption, (2) reduce cost of solid liquid separation
60
4. RECENT ADVANCEMENTS IN PRETREATMENT TECHNOLOGIES OF BIOMASS TO PRODUCE BIOENERGY
because the pretreated chips can be easily separated,
(3) eliminate energy-intensive mixing of pretreatment
slurries, (4) liquid to solid ratio and (5) did not result
in the production of fermentation inhibitors.
Another physical method is extrusion that disrupts
the lignocellulose structure and increases the accessibility of carbohydrates to enzyme attack. This method
has reported the improvement on sugar recovery up to
63.5% (Karunanithy and Muthukumarappan, 2011).
Other physical pretreatments involve the use of gamma
rays that cleave the b-1,4 glycosidic bonds, thus giving a
larger surface area and lower crystallinity. This method
will undoubtedly be very expensive on a large scale
with huge environmental and safety concerns (Agbor
et al., 2011). Proton beam irradiation has also been tested
reporting a glucose conversion of 68% of the theoretical
maximum at 72 h (Kim et al., 2011b).
The effect of microwave and microwave-chemical pretreatments on densification characteristics and physical
quality of pellets has also been investigated showing
that microwave pretreatment was significantly able to
disintegrate the lignocellulosic structure of wheat and
barley straw grinds (Kashaninejad and Tabil, 2011). These
pretreatments also have been tested on barley husks,
sweet sorghum bagasse, bamboo, coconut husk and garden biomass (Choudhary et al., 2012; Ding et al., 2012;
Gabhane et al., 2011; Jackowiak et al., 2011; JankerObermeier et al., 2012; Roos et al., 2009; Wu et al., 2012).
However, some restrictions on energy consumption
must be accomplished to obtain a positive energy balance
with these pretreatments. Ultrasounds and ultrasoundassisted alkaline pretreatment also has been reported
(Sun et al., 2002; Velmurugan and Muthukumar, 2012b).
On the other hand, some continuous system includes a
wet disk milling of rice straw (Hideno et al., 2012) and
pulsed electric field of wood chip and switchgrass
(Kumar et al., 2011).
Chemical Pretreatments
In general, chemical pretreatments show a high degree
of selectivity for the biomass component they degrade;
they also involve relatively harsh reaction conditions,
which may not be ideal in a biorefinery scheme due
to the possible production of toxic substances and their
possible effects on downstream biological processing
(FitzPatrick et al., 2010). Degradation of lignin has
been observed in most chemical pretreatments, and
particularly in dilute-acid and lime pretreatments
(Samuel et al., 2011).
Acid treatments solubilize the hemicellulose, and by
this, make the cellulose more accessible. The main reaction that occurs during acid pretreatment is the hydrolysis of hemicellulose, especially xylan as glucomannan is
relatively acid stable. The condensation and precipitation
of solubilized lignin components is an unwanted reaction, as it decreases digestibility (Hendriks and Zeeman,
2009). Dilute-acid pretreatment is considered as one of
the promising pretreatment methods despite its highenergy (steam or electricity) requirements and/or
corrosion-resistant high-pressure reactors, and extensive
washing, which increases the cost (Isroi et al., 2011). On
the other hand, pretreatments with strong acids for the
ethanol production is not an attractive option, because
there is a risk of formation of inhibiting compounds
(Hendriks and Zeeman, 2009). Other weak organic acids
such as lactic acid and phosphoric acid have been also
investigated (Monauari et al., 2011).
During alkaline pretreatment the first reactions taking
place are solvation and saponification. This causes a
swollen state of the biomass and makes it more accessible
for enzymes and bacteria. At “strong” alkali concentrations, dissolution, “peeling” of end groups, alkaline hydrolysis, degradation and decomposition of dissolved
polysaccharides can take place. Alkali extraction can
also cause solubilization, redistribution and condensation
of lignin and modifications in the crystalline state of the
cellulose (Hendriks and Zeeman, 2009).
ILs are generally defined as salts that melt at or below
100 C, providing liquids exclusively composed of ions.
Simple inorganic salts (e.g. NaCl) melt at very high temperatures (803 C), rendering unfeasible their routine
use as solvents for organic chemical processing (Tadesse
and Luque, 2011). ILs have been termed green solvents
due to their negligible vapor pressure (Patel and Lee,
2012). The application of ILs to biomass valorization
and pretreatment recently started to attract a great deal
of attention because they are capable of disrupting the
hydrogen bonds between different polysaccharide
chains, thus decreasing the compactness of cellulose
and making the carbohydrate fraction more susceptible
to hydrolysis (Tadesse and Luque, 2011). Additionally,
the recovering and the recycling of ILs has been proposed for decreasing the cost of the pretreatment process
(Tadesse and Luque, 2011). However, cost and energyintensive recycling of the solvents are major constraints
preventing ILs from commercial viability (Fu and
Mazza, 2011). Another drawback of ILs is the fact that
cellulases are inactivated even at low concentrations of
ILs (Wang et al., 2011a). The ILs pretreatment has been
tested using coadjuvant metal or acid catalysts to obtain
higher conversion and/or yields of intermediates
(Tadesse and Luque, 2011).
Organosolv pretreatment is the process to extract
lignin from lignocellulosic feedstocks with organic solvents or their aqueous solutions. This process is similar
to that used in industrial paper-making processes but
the degree of delignification for pretreatment is not
demanded to be as high as that of pulping. Generally,
organosolv processes are conducted at high temperatures
TYPES OF PRETREATMENTS
(100e250 C) using low boiling point solvents (methanol
and ethanol), high boiling point alcohols (ethylene glycol,
glycerol, tetrahydrofurfuryl alcohol) and other classes of
organic compounds including ethers, ketones, phenols,
organic acids, and dimethyl sulfoxide (Agbor et al.,
2011). This pretreatment removes extensive lignin and
nearly complete hemicellulose, enhancing the enzymatic
digestibility as a consequence of the increase in accessible
surface area and pore volume (Agbor et al., 2011; Zhao
et al., 2009). The organosolv pretreatment is more expensive at present than the leading pretreatment processes;
however, organosolv can provide some valuable byproducts that might lead it to be a promising pretreatment for biorefining lignocellulosic feedstock in the
future (Zhao et al., 2009).
The advantages of organosolv pretreatment includes,
organic solvents are always easy to recover by distillation and recycled for pretreatment; the chemical recovery in organosolv pulping processes can isolate lignin
as a solid material and carbohydrates as syrup, both of
which show promise as chemical feedstocks. However,
there are inherent drawbacks to the organosolv pretreatment, such as air and water pollution, the pretreated
solids always need to be washed with organic solvent
before water washing in order to avoid the reprecipitation of dissolved lignin, which leads to cumbersome
washing arrangements (Zhao et al., 2009).
Physicochemical Pretreatments
The objective of steam pretreatment, steam explosion or liquid hot water, is to solubilize the hemicellulose to make the cellulose better accessible for
enzymatic hydrolysis and to avoid the formation of
inhibitors (Hendriks and Zeeman, 2009). During steam
pretreatment parts of the hemicellulose hydrolyze and
form acids, which could catalyze the further hydrolysis
of the hemicellulose. To avoid the formation of inhibitors, the pH should be kept between 4 and 7 during
the pretreatment (Hendriks and Zeeman, 2009). The
aqueous fractionation of native lignocellulosic materials with hot, compressed water (also known as hydrothermal processing or autohydrolysis) has been
proposed as a fractionation method for biorefineries,
as it enables the simultaneous removal of watersoluble extractives and the solubilization of hemicelluloses, yielding a solid phase enriched in lignin and
cellulose (Gullón et al., 2012). Liquid hot water has
the major advantage that solubilized hemicellulose
and lignin products are present in lower concentrations, when compared to steam pretreatment, due to
higher water input. These lower concentrations reduce
the risk on degradation products like furfural and the
condensation and precipitation of lignin compounds
(Hendriks and Zeeman, 2009).
61
Wet oxidation is another oxidative pretreatment
method, which uses oxygen as oxidation agent. The soluble sugars produced during wet-oxidation pretreatment are mainly polymers opposite to the monomers
produced during steaming or acid hydrolysis as pretreatment. Phenolic monomers are no end products
during wet oxidation but are further degraded to carboxylic acids (Hendriks and Zeeman, 2009; Martin
and Thomsen, 2007).
Carbon dioxide pretreatment is conducted with highpressure carbon dioxide at high temperatures of up to
200 C with duration of several minutes. Explosive
steam pretreatment with high-pressure carbon dioxide
causes the liquid to be acidic and this acid hydrolyses
especially the hemicellulose. Carbon dioxide is also
applied as supercritical carbon dioxide (35 C, 73 bars)
for depolymerization of the sugars present in biomass,
increasing the glucose yield probably caused by increase
in pore size (Hendriks and Zeeman, 2009). This method
is considered as a “green” pretreatment because it does
not require neutralization or pH adjustment prior to
enzymatic hydrolysis (King et al., 2012).
Ammonia fiber explosion (AFEX), ammonia recycled
percolation (ARP) and soaking aqueous ammonia (SAA)
are alkaline pretreatment methods that use liquid
ammonia to pretreat biomass. The difference between
AFEX and ARP processes is that the first is carried out
in liquid ammonia and the second one in an aqueous
ammonia solution.
AFEX is a physicochemical pretreatment process in
which lignocellulosic biomass is exposed to liquid
ammonia at high temperature and pressure for a period
of time, and then the pressure is suddenly reduced
(Kumar et al., 2009). The AFEX pretreatment simultaneously reduces lignin content and removes some hemicellulose while decrystallizing cellulose and it has the
advantage of ammonia being recyclable due to its high
volatility (Yang and Wyman, 2008). AFEX has been
shown to complete conversion of cellulose to fermentable sugars but removes or loses little lignin or hemicellulose. In a typical AFEX process, the dosage of liquid
ammonia is 1e2 kg of ammonia/kg of dry biomass,
the temperature is 90 C, and the residence time is
30 min (Kumar et al., 2009). However, AFEX pretreatment at 40 C and longer residence times, up to 8 h,
has also been proposed with comparable yields of sugar
and ethanol (Bals et al., 2012).
AFEX treatment is a batch process while continuous
processing in an extruder is an approach called FIBEX
(fiber extrusion) that significantly reduces both the
time required for treatment and the ammonia levels
required with similar hydrolysis results to those for
AFEX (Yang and Wyman, 2008).
ARP is another process based on ammonia, which
recycles aqueous ammonia solution (5e15 wt%) through
62
4. RECENT ADVANCEMENTS IN PRETREATMENT TECHNOLOGIES OF BIOMASS TO PRODUCE BIOENERGY
a reactor packed with biomass at elevated temperatures
(80e180 C). Ammonia in aqueous solution and at high
temperature breaks down lignin via the ammoniolysis
reaction but has virtually no effect on carbohydrates
(Geddes et al., 2011). A major challenge for ARP is to
reduce liquid loadings to keep energy costs low (Yang
and Wyman, 2008). SAA is a modified version of
AFEX but it uses moderate temperatures (25e60 C) to
reduce the liquid amount during pretreatment. At
ambient temperatures the duration could be up to
10e60 days while at higher temperatures (150e190 C)
the duration of pretreatment is reduced to minutes
(Agbor et al., 2011). The cost of ammonia, and especially
of ammonia recovery, drives the cost of the ammoniarelated pretreatments (Kumar et al., 2009).
TRENDS IN PRETREATMENTS
A search made in the database Current Contents ConnectÒ for key words “lignocellulosic biomass” and “pretreatment” resulted in a total of 1217 articles published
from 2000 to 2012. From the total, 91.2% corresponded
to research papers and 8.8% to review papers
(Figure 4.1). The number of published papers has
increased exponentially from 2007 to 2012 as shown in
Figure 4.1, indicating the relevance that the topic has
gained in the recent years. Among the reviews documents, 37.2% corresponded to reviews directly related
to pretreatments, pointing out the importance of this
step in the concept of biorefinery. The remaining documents corresponded to reviews on the general topic of
biofuels from lignocellulosic biomass.
FIGURE 4.2 Classification of published papers from 2000 to 2012
in the topic of pretreatments of lignocellulosic biomass. Source: With
data of Current Contents ConnectÒ.
For the research papers, the search was refined to
select only papers that focus on the pretreatment processes obtaining a total of 692 papers and around 54%
of these papers were published in 2012. The research
papers related to pretreatment were classified into nine
categories as shown in Figure 4.2. From this analysis
we can conclude that alkaline, acid, thermal and IL pretreatments are the most reported, the comparison and
combination of them is also widely reported. However,
this variety of studied pretreatments indicates that there
is not a prevalent pretreatment suggesting that further
investigation on the topic is required.
Other Pretreatments
FIGURE 4.1 Published papers from 2000 to 2012 in the topic
) Original papers and (
) review
lignocellulosic biomass. (
papers. Source: With data of Current Contents ConnectÒ.
The pretreatments described above such as steam
explosion, liquid hot water, dilute acid, lime, and
ammonia pretreatments are the most studied methods
because they have potential as cost-effective pretreatments (Kazi et al., 2010; Mosier et al., 2005; Piccolo and
Bezzo, 2009; Tao et al., 2011; Wyman et al., 2005). Other
alternatives such as biological, ultrasonication, microwave, organosolvs, ILs, and combinatorial methods are
also essayed; however, they are either low effective,
long-time treatment or too expensive, and further investigation and improvements have to be reached before
they can be competitive. In this section multiple
or combinatorial pretreatments and other alternative
pretreatments will be discussed.
Biological pretreatments must decrease the time
of the process in order to be competitive in an industrial
concept of biorefinery; to reach this objective its combination with chemicals and/or physical methods
TRENDS IN PRETREATMENTS
has been proposed by several studies. For example, the
combination of a biological pretreatment by I. lacteus
or P. subvermispora with a mild alkali pretreatment
improved significantly ethanol production without the
production of inhibitor compounds for downstream processes (Salvachua et al., 2011; Zhong et al., 2011). Other
two-step pretreatment proposed consisted in a mild
physical or chemical step (ultrasonic and H2O2) and a
subsequent biological treatment by P. ostreatus, increasing
significantly the lignin degradation compared to those of
one-step pretreatments (Yu et al., 2009); also, pretreatment by white-rot fungi has been combined with organosolv pretreatment in an ethanol production process from
beech wood chips (Salvachua et al., 2011); the combination of biological and mild acid pretreatment was
reported as a promising method to improve enzymatic
hydrolysis and ethanol production from water hyacinth
with low lignin content (Ma et al., 2010). Another combination of biological pretreatment with thermal processing
for wheat straw consisted in a first phase of biodegradation by P. chrysosporium (10 days) and a thermal decomposition using pyrolysis (Zeng et al., 2011). Also, a
combination of fungal treatment with liquid hot water
treatment was conducted to enhance the enzymatic
hydrolysis of Populus tomentosa (Wang et al., 2012).
Sugarcane bagasse is one of the most promising
biomass considered in biorefineries; thus, several
studies have proposed combined pretreatments. The
ultrahigh-pressure explosion combined with alkaline
treatment (0.5% NaOH) at 125 C for 120 min significantly decreased the particle size and disrupted the
microstructure, with a significant delignification and
increased enzymatic digestibility of sugarcane bagasse
(Chen et al., 2010). A combined treatment with dilute
sulfuric acid and microwave heating up to 190 C for
5 min has also been studied. This treatment resulted in
an increment of the specific surface area of bagasse,
almost complete removal of hemicellulose and significant reduction of the crystalline structure of cellulose
(Chen et al., 2011), while microwaveealkali treatment
at 450 W for 5 min resulted in almost 90% of lignin
removal from the bagasse (Binod et al., 2012). Also,
bagasse has been subjected to sono-assisted alkaline pretreatment (Velmurugan and Muthukumar, 2012a). Acid,
alkaline or sequential acid/alkaline solutions have been
tested to conversion into bio-oil in a pyrolysis process at
low-temperature conversion under He or O2/He atmospheres at 350e450 C (Cunha et al., 2011). A twostage process for delignification of sugarcane bagasse
uses alkali and peracetic acid combination (Teixeira
et al., 2000; Zhao et al., 2011b).
Same strategies (acidic/alkaline) have been proposed
for corn stover. For example, a two-stage process
consists of use of 0.07 wt% sulfuric acid at 170 C,
2.5 ml/min for 30 min and ARP (15 wt% ammonia)
63
at the same temperature, 5.0 ml/min for 60 min. In the
first stage hemicellulose was recovered while in the
following stage lignin was recovered. This treatment
brought about enzymatic digestibility of 90% using 60
filter paper units/g glucan cellulase enzyme loadings
(Kim, 2011). Another combined treatment proposed for
corn stover is SAA (15 wt% ammonia) with solution containing also 20 wt% ethanol at 60 C for 24 h preserving
the hemicellulose in solid form (Kim et al., 2009). Also,
the use of NaOH (0.3 N) and a step of particle size
homogenization has reported a significant enhancement
of enzymatic hydrolysis (Li et al., 2004). The synergistic
effect of preimpregnation by sulfuric acid (3 wt%) and
steam explosion (190 C) has been investigated; after
48 h of digestion the yield of glucose was 93% of the
theoretical (Zimbardi et al., 2007).
Sequential stages of autohydrolysis and ethanole
water mixtures were used to pretreat olive tree trimmings
recovering up to 42% of the polysaccharides contained in
the raw material (Requejo et al., 2011). Also, this process
has been tested with uncatalyzed ethanolewater solutions of Eucalyptus globulus wood (Romani et al., 2011).
Mixtures of ethanol/water/acetic acid in an autoclave
have been also used (Teramoto et al., 2008). This combined
process causes the solubilization of hemicelluloses and
lignin, leaving solids enriched in cellulose. A treatment
of ethanosolv catalyzed with FeCl3 (0.1 M) at 170 C for
72 h has been proposed for barley straw allowing enzymatic digestibility of 89%. This treatment had a particularly strong effect on enzymatic digestibility and
cellulose recovery (Kim et al., 2010).
Another pretreatment at pH 1 (hydrochloric acid) and
subsequently at pH 13 (sodium hydroxide) released 69%
and 95% of the theoretical maximal amounts of glucose
and xylose, respectively, from the straw and removal of
68% of the lignin (Pedersen et al., 2010). The opposite
sequence alkaline stage (ammonia) followed by acidic
stage (dilute sulfuric acid by percolation) has also been
used to treated rice straw (Kim et al., 2011a).
Microwave-based heating (190 C) was used to pretreat switchgrass presoaked in alkali solutions (0.1 g/g)
resulting in release of 90% of maximum potential sugars.
This value was significantly higher than the one obtained
with conventional heat and it was attributed to the
disruption of recalcitrant structures under microwave
heating (Hu and Wen, 2008).
Significant disintegration of lignocellulosic structure of wheat, barley straw grinds, switchgrass and
coastal bermuda grass has been reported with the
microwaveechemical (NaOH or Ca(OH)2) pretreatments (Kashaninejad and Tabil, 2011; Keshwani and
Cheng, 2010). Also, microwave-assisted pretreatment
of woody biomass with ammonium molybdate activated by H2O2 has also been proposed resulting in a
selective delignifying system (Verma et al., 2011).
64
4. RECENT ADVANCEMENTS IN PRETREATMENT TECHNOLOGIES OF BIOMASS TO PRODUCE BIOENERGY
For hydrogen production from Miscanthus by Thermotoga elfii, high delignification values were obtained
by the combination of mechanical (one-step extrusion)
and chemical pretreatments (NaOH at 70 C) resulting
in a 33% conversion into monosaccharides of the initial
biomass after enzymatic hydrolysis (de Vrije et al.,
2002).
A two-stage pretreatment method was proposed and
tested for deconstruction of Miscanthus; first, biomass is
pretreated at 50 C, 1.0e4.0% alkaline peroxide solutions
to remove up to 64% of hemicellulose and 64% of lignin.
The remaining solids were subjected to a second pretreatment at 121 C with electrolyzed water (Wang
et al., 2010).
On the other hand, application of a dehydration process to the mechanochemical pretreatment process of the
bioethanol production system has been proposed for
energy saving and cost reduction. However, the dehydration process has problems with the loss of sugars
eluted in the liquid phase during the hydrothermal process (Yanagida et al., 2011).
Combination of hot compressed water (hydrothermal
treatment) and mechanochemical milling, including a
dewatering step for Eucalyptus and rice straw, has been
proposed for ethanol production (Fujimoto et al., 2008;
Hideno et al., 2012). Torrefaction is a mild thermal pretreatment (T < 300 C) that improves biomass milling
and storage properties (Chen et al., 2012; Fisher et al.,
2012). This treatment has gained attention in recent
years and some biomasses that have been treated
include oil palm fiber and eucalyptus, Norwegian birch,
spruce, Miscanthus and white oak sawdust; residues
from coffee grain, sugarcane, sawdust and rice husk
bagasse (Chen et al., 2012; Lu et al., 2012; Medic et al.,
2012; Protasio et al., 2012; Srinivasan et al., 2012; Tapasvi
et al., 2012; Tumuluru et al., 2012). Wet torrefaction (hot
compressed water 200e260 C) and dry (nitrogen,
250e300 C) has been tested with Loblolly pine with
mass yield of solid product ranging between 57% and
89%, and energy densification to 108e136% of the original feedstock (Yan et al., 2009).
Extrusion has also been used in combination with
alkali (1.70%, w/v NaOH) soaking for pretreatment of
prairie cord grass at a barrel temperature of 114 C,
122 rpm screw speed resulted in an 82% of sugar recovery after enzymatic hydrolysis (Karunanithy and Muthukumarappan, 2011). An alkali-combined extrusion
pretreatment of corn stover obtained glucose and xylose
sugar yields of 86.8% and 50.5%, respectively. The conditions used were alkali loading of 0.04 g/g dry biomass, a
screw speed of 80 rpm, residence time for extrusion is
27 min, temperature of 140 C and washed with water
(Zhang et al., 2012b). Also, glucose conversion of 95%
was reported from soybean hulls using a thermomechanical extrusion pretreatment (screw speed 350 rpm, 80 C
and in-barrel moisture content 40% wt) (Yoo et al., 2011).
A study of high-temperature (110e130 C), concentratedacid (5e30 wt.%) hydrolysis kinetics was undertaken for
pretreated pine in a corotating twin-screw extruder
reactor, obtaining more than 50% of the theoretical
glucose in roughly 25 min (Miller and Hester, 2007).
A successive pretreatment of ball-milled bamboo consisted in ultrasound treatment in ethanol solution at
20 C from 0 up to 50 min. After that the samples were
dissolved with 7% NaOH/12% urea solutions at 12 C,
followed by successive extractions with dioxane, ethanol,
and dimethyl sulfoxide (Li et al., 2010). Other treatments,
such as SAA and proton beam irradiation, have been
tested with rice straw and approximately 90% of the
theoretical glucose conversion was obtained at 12 h
(Kim et al., 2011b). Microwave pretreatment also has
been combined with alkali to pretreat cashew apple
bagasse founding that alkali exerted influence on glucose
formation (Rodrigues et al., 2011).
A pretreatment method using ammonia and ILs
reported a synergy effect for rice straw, achieving 82%
of the cellulose recovery and 97% of the enzymatic
glucose conversion with recycling of the ILs (Nguyen
et al., 2010). Pretreatment of wheat straw with combined
sulfuric acid (0e3%, w/v) and Tween-20 (concentration,
0e1%) was evaluated with modification of lignin surface
(Qi et al., 2010). Other surfactants, such as, Tween-80,
dodecylbenzene sulfonic acid, and polyethylene glycol
4000, have also been used combined with diluted acid
to treat corn stover and bagasse (Qing et al., 2010;
Sindhu et al., 2012).
Other pretreatments include technology used in kraft
pulp mills for the efficient conversion of lignocellulosic
biomass into ethanol (Gonzalez et al., 2011). Sulfite pretreatment to overcome recalcitrance of lignocellulose
consists of sulfite treatment of wood chips under acidic
conditions followed by mechanical size reduction using
disk refining (Li et al., 2012; Zhang et al., 2012a). Pretreatment of corn stalk with sulfite (7%) at a temperature of
180 C for 30 min was successfully performed (Liu
et al., 2011; Zhu et al., 2009). Silage preparation is a
well-known procedure for preserving plant material;
the effects of Fe(NO3)3 pretreatment conditions on sugar
yields were investigated for corn stover silage. Ensiling
techniques, with and without supplemental enzymes,
also have been reported as a cost-effective pretreatment
(Chen et al., 2007; Sun et al., 2011; Thomsen et al.,
2008). Also, FeSO4 (0.1 mol/L at 180 C for 20 min) was
investigated as a catalyst for the pretreatment of corn stover, observing significantly increased hemicellulose
degradation in aqueous solutions with high xylose
recovery and low cellulose removal (Zhao et al., 2011a).
Lignocellulose pretreatment featuring modest reaction
conditions (50 C and atmospheric pressure) was demonstrated to fractionate lignocellulose to amorphous
ENVIRONMENTAL AND ECONOMICAL ASPECTS
cellulose, hemicellulose, lignin, and acetic acid by using a
nonvolatile cellulose solvent (concentrated phosphoric
acid), a highly volatile organic solvent (acetone) and
water (Zhang et al., 2007).
PRETREATMENT MODELING
For a rational design of pretreatment processes is
required experimental investigation of physical changes
and chemical reactions that occur during pretreatment;
however, due to the wide range of pretreatments and
biomass available for biorefinery, the development of
effective and mechanistic models can provide a large
amount of information to optimize operational conditions. Furthermore, several key criteria regarding technical, economical, and environmental considerations
should be critically analyzed when adapting these technologies for the nascent biorefinery industry (Sousa
et al., 2009). In this section some models that particularly
focus on pretreatments in the scheme of a biorefinery
plant are discussed.
The most commonly developed models for the pretreatment are kinetic models with assumptions of a
first-order dependence of reaction rate on biomass components and an Arrhenius-type correlation between rate
constant and temperature (Wang et al., 2011b). In view of
the heterogeneous nature of the reactions involved in the
pretreatment, the uses of severity factor, artificial neural
network, and fuzzy inference systems, represent alternative approaches for predicting the behavior of the systems (Wang et al., 2011b).
A multiscale model of hydrothermal pretreatment
methods, including microscale, mesoscale and macroscale, was used to elucidate the mechanisms involved
in the breakage of hemicellulose of wood (Hosseini
and Shah, 2009).
A model that simulates a biorefinery plant integrating
first- and second-generation ethanol production process
from sugarcane, surplus bagasse and trash included a
selected pretreatment method followed, or not, by a
delignification step. The simulation indicated that the
best results were obtained for steam explosion pretreatment at high solids loading and hydrolysis time between
24 and 48 h (Dias et al., 2011). Also, a mathematical
model for a countercurrent shrinking-bed reactor for
pretreatment/hydrolysis of hardwood cellulose predicts
that dilute sulfuric acid (0.08 wt%) and with optimal
adjustment of other operating parameters resulted in
80e90% yield with 2e4 wt% product concentration.
This model also indicates that acid concentration and
temperatures acutely affect the reactor performance
in cellulose hydrolysis. In contrast, hemicellulose
hydrolysis is less sensitive to acid concentration and
temperature allowing broader latitude in operating
65
conditions (Lee et al., 2000). Also, methods of optimization have been used to acid-catalyzed pretreatment process showing that the sulfuric acid concentration plays
the major role during the pretreatment of areca nut
husk (57% contribution) followed by the duration of
operation (24.98% contribution) and solid loading
(14.3% contribution) (Sasmal et al., 2011). Other authors
have reported that for alkali pretreatment of cereal crop
residues, the temperature had the greatest impact on
sugar release, followed by alkali concentration and treatment time (Vancov and McIntosh, 2011). A statistical
optimization method proposed variables such as temperature, sulfuric acid concentration and reaction time
to release xylose from sugarcane bagasse as a useful
means of trading off the combined effects of these three
variables on total xylose recovery yields (Um and Bae,
2011). Other works considered also the reduction of
the acid concentrations and reaction times to optimize
the pretreatment process. This kinetic model predicted
optimum conditions to pretreatment of corn stover of
150 C, 0.6% HNO3 and 1 min of reaction time for
maximal xylose, glucose and arabinose yields and minimal yield of acetic acid and furfural (Zhang et al., 2011).
To deeply understand the factors that affect the conversion of lignocellulosic biomass to fermentable sugars,
experimental results should be bridged with process
simulations (Wang et al., 2011b).
ENVIRONMENTAL AND
ECONOMICAL ASPECTS
A renewable biofuel economy is projected as a
pathway to decrease dependence on fossil fuels as well
as to reduce greenhouse gas (GHG) emissions. Ethanol
produced on large scale from lignocellulosic raw materials is considered the most potential next-generation
automotive fuel. However, the environmental impact
of biorefinery processes must be assessed. The GHG
emissions have been evaluated in some processes of
ethanol production for many authors, reporting that
ethanol emissions could be 20e90% lower than those
from fossil fuels, depending on the scheme of production and the biomass used. Some works have proposed
the life cycle assessment model to evaluate the environmental implications of the production of ethanol founding that compared to conventional gasoline, life cycle
GHG emissions are lower for ethanol blends, specifically
up to 145% lower for E85 mixture (85% ethanol and 15%
gasoline v/v) derived from Ethiopian mustard, associated with the low intensive energy and high biomass
yield of this crop (Gonzalez-Garcia et al., 2010).
Production of fuel ethanol from lignocellulosic feedstock has been modeled for design optimization through
mass and energy balances in terms of ethanol yield and
66
4. RECENT ADVANCEMENTS IN PRETREATMENT TECHNOLOGIES OF BIOMASS TO PRODUCE BIOENERGY
power generation as well as from a financial point of view
in order to identify critical parameters of the processes
productivity and profitability (Piccolo and Bezzo, 2009).
Technoeconomic analysis will play a key role in
process development and targeting of technical and
economic barriers for these new fuels and feedstocks
(Aden and Foust, 2009). Some of these economic analyses indicate that about 18e20% of the total projected
cost for biological production of cellulosic ethanol can
be attributed to pretreatment; then, reducing ethanol
cost requires optimizing pretreatment strategies and
conditions to the most economical possible and to
accelerate commercial applications (Banerjee et al.,
2010; Yang and Wyman, 2008).
Most of the current process design and economic
results are described for dilute-acid pretreatment followed by enzymatic hydrolysis and fermentation. The
projection made in 2007 of some models for ethanol
costs in 2012 at commercial scale of corn stover conversion process was $0.35 per liter (Aden and Foust, 2009).
However, commercial plants from lignocellulosic materials are still under development. The variation in estimated ethanol production cost is considerable, ranging
from about 0.13 to 0.81 US$ per liter ethanol. This can
be explained to a large extent by actual process differences and variations in the assumptions underlying
the technoeconomic evaluations (Galbe et al., 2007).
Other studies indicate that dilute-acid pretreatment
process has the lowest product value compared to hot
water and AFEX pretreatments for three downstream
process variations (pervaporation, separate five-carbon
and six-carbon sugars fermentation, and onsite enzyme
production) (Kazi et al., 2010).
The technical and economic challenges for softwood
to ethanol processes with SO2-catalyzed steam explosion
and ethanol organosolv pretreatments have been
analyzed concluding that organosolv pretreatment has
the advantage of high-value coproduct from lignin
(Mabee et al., 2006).
CONCLUDING REMARKS
The technical feasibility of extracting sugars from
lignocellulosic biomass applying a pretreatment process
and the subsequent steps of enzymatic hydrolysis and
fermentation to obtain bioenergy and other products
has been established for the past decades. However,
the economical feasibility of this process is still under
development. The biochemical and chemical paths of
biorefineries are not economically competitive with
first-generation biofuels. The steam explosion, liquid
hot water, dilute acid, lime, and ammonia pretreatments
are the most studied methods because they have
potential as cost-effective pretreatments. Furthermore,
other alternatives such as biological, ultrasonication,
microwave, organosolvs, and IL pretreatments require
further investigation and improvements have to
be reached before they can be competitive. The several
options proposed in pretreatments indicate that there
is not a prevalent technology and it is probable that
each biorefinery plant should have to select the best
option according to their feedstock and target products.
Further research must be performed in order to evaluate
if one pretreatment or combined stages of pretreatment
are economical and environmentally feasible prior to
enzymatic hydrolysis step. Also, consolidated process
of pretreatment, hydrolysis or even including fermentation process, should be evaluated from both economical
and environmental points of view.
References
Aden, A., Foust, T., 2009. Technoeconomic analysis of the dilute sulfuric acid and enzymatic hydrolysis process for the conversion of
corn stover to ethanol. Cellulose 16, 535e545.
Agbor, V.B., Cicek, N., Sparling, R., Berlin, A., Levin, D.B., 2011.
Biomass pretreatment: fundamentals toward application. Biotechnol. Adv. 29, 675e685.
Alvira, P., Tomas-Pejo, E., Ballesteros, M., Negro, M.J., 2010. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour. Technol.
101, 4851e4861.
Bals, B.D., Teymouri, F., Campbell, T., Jin, M., Dale, B.E., 2012. Low
temperature and long residence time AFEX pretreatment of corn
stover. BioEnergy Res. 5, 372e379.
Banerjee, S., Mudliar, S., Sen, R., Giri, B., Satpute, D., Chakrabarti, T.,
Pandey, R.A., 2010. Commercializing lignocellulosic bioethanol:
technology bottlenecks and possible remedies. Biofuels, Bioprod.
Biorefin. 4, 77e93.
Binod, P., Satyanagalakshmi, K., Sindhu, R., Janu, K.U.,
Sukumaran, R.K., Pandey, A., 2012. Short duration microwave
assisted pretreatment enhances the enzymatic saccharification and
fermentable sugar yield from sugarcane bagasse. Renewable
Energy 37, 109e116.
Brodeur, G., Yau, E., Badal, K., Collier, J., Ramachandran, K.B.,
Ramakrishnan, S., 2011. Chemical and physicochemical pretreatment of lignocellulosic biomass: a review. Enzyme Res. 2011, 1e17.
Carvalheiro, F., Duarte, L.C., Gı́rio, F.M., 2008. Hemicellulose
biorefineries: a review on biomass pretreatments. J. Sci. Ind. Res.
67, 849e864.
Chen, D., Guo, Y., Huang, R.B., Lu, Q., Huang, J., 2010. Pretreatment
by ultra-high pressure explosion with homogenizer facilitates
cellulase digestion of sugarcane bagasses. Bioresour. Technol. 101,
5592e5600.
Chen, W.-H., Lu, K.-M., Tsai, C.-M., 2012. An experimental analysis on
property and structure variations of agricultural wastes undergoing torrefaction. Appl. Energy 100, 318e325.
Chen, W.H., Tu, Y.J., Sheen, H.K., 2011. Disruption of sugarcane
bagasse lignocellulosic structure by means of dilute sulfuric acid
pretreatment with microwave-assisted heating. Appl. Energy 88,
2726e2734.
Chen, Y., Sharma-Shivappa, R.R., Chen, C., 2007. Ensiling agricultural
residues for bioethanol production. Appl. Biochem. Biotechnol.
143, 80e92.
REFERENCES
Choudhary, R., Umagiliyage, A.L., Liang, Y., Siddaramu, T.,
Haddock, J., Markevicius, G., 2012. Microwave pretreatment for
enzymatic saccharification of sweet sorghum bagasse. Biomass
Bioenergy 39, 218e226.
Cui, Z., Wan, C., Shi, J., Sykes, R.W., Li, Y., 2012. Enzymatic
digestibility of corn stover fractions in response to fungal pretreatment. Ind. Eng. Chem. Res. 51, 7153e7159.
Cunha, J.A., Pereira, M.M., Valente, L.M.M., de la Piscina, P.R.,
Homs, N., Santos, M.R.L., 2011. Waste biomass to liquids: low
temperature conversion of sugarcane bagasse to bio-oil. The effect
of combined hydrolysis treatments. Biomass Bioenergy 35,
2106e2116.
de Vrije, T., de Haas, G.G., Tan, G.B., Keijsers, E.R.P., Claassen, P.A.M.,
2002. Pretreatment of Miscanthus for hydrogen production by
Thermotoga elfii. Int. J. Hydrogen Energy 27, 1381e1390.
Dias, M.O.S., Cunha, M.P., Maciel, R., Bonomi, A., Jesus, C.D.F.,
Rossell, C.E.V., 2011. Simulation of integrated first and second
generation bioethanol production from sugarcane: comparison
between different biomass pretreatment methods. J. Ind. Microbiol.
Biotechnol. 38, 955e966.
Ding, T.Y., Hii, S.L., Ong, L.G.A., 2012. Comparison of pretreatment
strategies for conversion of coconut husk fiber to fermentable
sugars. Bioresources 7, 1540e1547.
Fisher, E.M., Dupont, C., Darvell, L.I., Commandre, J.M., Saddawi, A.,
Jones, J.M., Grateau, M., Nocquet, T., Salvador, S., 2012. Combustion and gasification characteristics of chars from raw and torrefied
biomass. Bioresour. Technol. 119, 157e165.
FitzPatrick, M., Champagne, P., Cunningham, M.F., Whitney, R.A.,
2010. A biorefinery processing perspective: treatment of lignocellulosic materials for the production of value-added products.
Bioresour. Technol. 101, 8915e8922.
Fu, D.B., Mazza, G., 2011. Aqueous ionic liquid pretreatment of straw.
Bioresour. Technol. 102, 7008e7011.
Fujimoto, S., Inoue, H., Yano, S., Sakaki, T., Minowa, T., Endo, T.,
Sawayama, S., Sakanishi, K., 2008. Bioethanol production from
lignocellulosic biomass requiring no sulfuric acid: mechanochemical pretreatment and enzymic saccharification. J. Jpn. Pet. Inst. 51,
264e273.
Gabhane, J., William, S., Vaidya, A.N., Mahapatra, K., Chakrabarti, T.,
2011. Influence of heating source on the efficacy of lignocellulosic
pretreatment - a cellulosic ethanol perspective. Biomass Bioenergy
35, 96e102.
Galbe, M., Sassner, P., Wingren, A., Zacchi, G., 2007. Process engineering economics of bioethanol production. Biofuels 108, 303e327.
Geddes, C.C., Nieves, I.U., Ingram, L.O., 2011. Advances in ethanol
production. Curr. Opin. Biotechnol. 22, 312e319.
Giles, R.L., Galloway, E.R., Elliott, G.D., Parrow, M.W., 2011. Two-stage
fungal biopulping for improved enzymatic hydrolysis of wood.
Bioresour. Technol. 102, 8011e8016.
Gonzalez, R., Treasure, T., Phillips, R., Jameel, H., Saloni, D., 2011.
Economics of cellulosic ethanol production: green liquor pretreatment for softwood and hardwood, greenfield and repurpose scenarios. Bioresources 6, 2551e2567.
Gonzalez-Garcia, S., Moreira, M.T., Feijoo, G., 2010. Comparative
environmental performance of lignocellulosic ethanol from
different feedstocks. Renewable Sustainable Energy Rev. 14,
2077e2085.
Gullón, P., Romani, A., Vila, C., Garrote, G., Parajo, J.C., 2012. Potential
of hydrothermal treatments in lignocellulose biorefineries. Biofuels, Bioprod. Biorefin. 6, 219e232.
Hendriks, A., Zeeman, G., 2009. Pretreatments to enhance the
digestibility of lignocellulosic biomass. Bioresour. Technol. 100,
10e18.
Hideno, A., Inoue, H., Yanagida, T., Tsukahara, K., Endo, T.,
Sawayama, S., 2012. Combination of hot compressed water
67
treatment and wet disk milling for high sugar recovery yield in
enzymatic hydrolysis of rice straw. Bioresour. Technol. 104,
743e748.
Hosseini, S.A., Shah, N., 2009. Multiscale modelling of biomass pretreatment for biofuels production. Chem. Eng. Res. Des. 87,
1251e1260.
Hu, Z.H., Wen, Z.Y., 2008. Enhancing enzymatic digestibility of
switchgrass by microwave-assisted alkali pretreatment. Biochem.
Eng. J. 38, 369e378.
Isroi Millati, R., Syamsiah, S., Niklasson, C., Cahyanto, M.N.,
Lundquist, K., Taherzadeh, M.J., 2011. Biological pretreatment of
lignocelluloses with white-rot fungi and its applications: a Review.
Bioresources 6, 5224e5259.
Jackowiak, D., Bassard, D., Pauss, A., Ribeiro, T., 2011. Optimisation of
a microwave pretreatment of wheat straw for methane production.
Bioresour. Technol. 102, 6750e6756.
Janker-Obermeier, I., Sieber, V., Faulstich, M., Schieder, D., 2012. Solubilization of hemicellulose and lignin from wheat straw through
microwave-assisted alkali treatment. Ind. Crops Prod. 39, 198e203.
Jørgensen, H., Kristensen, J.B., Felby, C., 2007. Enzymatic conversion
of lignocellulose into fermentable sugars: challenges and opportunities. Biofuels, Bioprod. Biorefin. 1, 119e134.
Karunanithy, C., Muthukumarappan, K., 2011. Optimization of alkali
soaking and extrusion pretreatment of prairie cord grass for
maximum sugar recovery by enzymatic hydrolysis. Biochem. Eng.
J. 54, 71e82.
Kashaninejad, M., Tabil, L.G., 2011. Effect of microwave-chemical pretreatment on compression characteristics of biomass grinds.
Biosyst. Eng. 108, 36e45.
Kazi, F.K., Fortman, J.A., Anex, R.P., Hsu, D.D., Aden, A., Dutta, A.,
Kothandaraman, G., 2010. Techno-economic comparison of process
technologies for biochemical ethanol production from corn stover.
Fuel 89, S20eS28.
Keshwani, D.R., Cheng, J.J., 2010. Microwave-based alkali pretreatment of switchgrass and coastal Bermuda grass for bioethanol
production. Biotechnol. Prog. 26, 644e652.
Kim, J.W., Kim, K.S., Lee, J.S., Park, S.M., Cho, H.Y., Park, J.C.,
Kim, J.S., 2011a. Two-stage pretreatment of rice straw using
aqueous ammonia and dilute acid. Bioresour. Technol. 102,
8992e8999.
Kim, S.B., Kim, J.S., Lee, J.H., Kang, S.W., Park, C., Kim, S.W., 2011b.
Pretreatment of rice straw by proton beam irradiation for efficient
enzyme digestibility. Appl. Biochem. Biotechnol. 164, 1183e1191.
Kim, T.H., 2011. Sequential hydrolysis of hemicellulose and lignin in
lignocellulosic biomass by two-stage percolation process using
dilute sulfuric acid and ammonium hydroxide. Korean J. Chem.
Eng. 28, 2156e2162.
Kim, T.H., Nghiem, N.P., Hicks, K.B., 2009. Pretreatment and fractionation of corn stover by soaking in ethanol and aqueous
ammonia. Appl. Biochem. Biotechnol. 153, 171e179.
Kim, Y., Yu, A., Han, M., Choi, G.W., Chung, B., 2010. Ethanosolv
pretreatment of barley straw with iron(III) chloride for enzymatic
saccharification. J. Chem. Technol. Biotechnol. 85, 1494e1498.
King, J.W., Srinivas, K., Guevara, O., Lu, Y.-W., Zhang, D., Wang, Y.-J.,
2012. Reactive high pressure carbonated water pretreatment prior
to enzymatic saccharification of biomass substrates. J. Supercrit.
Fluids 66, 221e231.
Kuhar, S., Nair, L.M., Kuhad, R.C., 2008. Pretreatment of lignocellulosic material with fungi capable of higher lignin degradation and
lower carbohydrate degradation improves substrate acid hydrolysis and the eventual conversion to ethanol. Can. J. Microbiol. 54,
305e313.
Kumar, P., Barrett, D.M., Delwiche, M.J., Stroeve, P., 2009. Methods for
pretreatment of lignocellulosic biomass for efficient hydrolysis and
biofuel production. Ind. Eng. Chem. Res. 48, 3713e3729.
68
4. RECENT ADVANCEMENTS IN PRETREATMENT TECHNOLOGIES OF BIOMASS TO PRODUCE BIOENERGY
Kumar, P., Barrett, D.M., Delwiche, M.J., Stroeve, P., 2011. Pulsed
electric field pretreatment of switchgrass and wood chip species
for biofuel production. Ind. Eng. Chem. Res. 50, 10996e11001.
Lamsal, B., Yoo, J., Brijwani, K., Alavi, S., 2010. Extrusion as a thermomechanical pre-treatment for lignocellulosic ethanol. Biomass
Bioenergy 34, 1703e1710.
Lee, Y.Y., Wu, Z.W., Torget, R.W., 2000. Modeling of countercurrent
shrinking-bed reactor in dilute-acid total-hydrolysis of lignocellulosic biomass. Bioresour. Technol. 71, 29e39.
Lewis, R.S., Datar, R.P., Huhnke, R.L., 2005. Biomass to ethanol.
In: Encyclopedia of Chemical Processing, Vol. 1, pp. 142e151.
Li, M.F., Fan, Y.M., Xu, F., Sun, R.C., Zhang, X.L., 2010. Cold sodium
hydroxide/urea based pretreatment of bamboo for bioethanol
production: characterization of the cellulose rich fraction. Ind.
Crops Prod. 32, 551e559.
Li, X., Luo, X., Li, K., Zhu, J.Y., Fougere, J.D., Clarke, K., 2012. Effects of
SPORL and dilute acid pretreatment on substrate morphology, cell
physical and chemical wall structures, and subsequent enzymatic
hydrolysis of lodgepole pine. Appl. Biochem. Biotechnol. 168,
1556e1567.
Li, Y., Ruan, R., Chen, P.L., Liu, Z., Pan, X., Lin, X., Liu, Y., Mok, C.K.,
Yang, T., 2004. Enzymatic hydrolysis of corn stover pretreated by
combined dilute alkaline treatment and homogenization. Trans.
ASAE 47, 821e825.
Liao, Z.D., Huang, Z.Q., Hu, H.Y., Zhang, Y.J., Tan, Y.F., 2011. Microscopic structure and properties changes of cassava stillage residue
pretreated by mechanical activation. Bioresour. Technol. 102,
7953e7958.
Liu, Y., Wang, G.S., Xu, J.L., Zhang, Y., Liu, C.F., Yuan, Z.H., 2011.
Effect of sulfite pretreatment to overcome the recalcitrance of
lignin (SPORL) on enzymatic saccharification of corn stalk.
Bioresources 6, 5001e5011.
Lu, K.-M., Lee, W.-J., Chen, W.-H., Liu, S.-H., Lin, T.-C., 2012. Torrefaction and low temperature carbonization of oil palm fiber and
eucalyptus in nitrogen and air atmospheres. Bioresour. Technol.
123, 98e105.
Ma, F.Y., Yang, N., Xu, C.Y., Yu, H.B., Wu, J.G., Zhang, X.Y., 2010.
Combination of biological pretreatment with mild acid pretreatment for enzymatic hydrolysis and ethanol production from water
hyacinth. Bioresour. Technol. 101, 9600e9604.
Mabee, W.E., Gregg, D.J., Arato, C., Berlin, A., Bura, R., Gilkes, N.,
Mirochnik, O., Pan, X.J., Pye, E.K., Saddler, J.N., 2006. Updates on
softwood-to-ethanol process development. Appl. Biochem.
Biotechnol. 129-132, 55e70.
Mahalaxmi, S., Jackson, C.R., Williford, C., Burandt, C.L., 2010. Estimation of treatment time for microbial preprocessing of biomass.
Appl. Biochem. Biotechnol. 162, 1414e1422.
Martin, C., Thomsen, A.B., 2007. Wet oxidation pretreatment of
lignocellulosic residues of sugarcane, rice, cassava and peanuts for
ethanol production. J. Chem. Technol. Biotechnol. 82, 174e181.
Medic, D., Darr, M., Shah, A., Potter, B., Zimmerman, J., 2012. Effects
of torrefaction process parameters on biomass feedstock upgrading. Fuel 91, 147e154.
Miller, S., Hester, R., 2007. Concentrated acid conversion of pine
sawdust to sugars. Part II: high-temperature batch reactor kinetics
of pretreated pine sawdust. Chem. Eng. Commun. 194, 103e116.
Monauari, S., Galbe, M., Zacchi, G., 2011. Influence of impregnation
with lactic acid on sugar yields from steam pretreatment of sugarcane bagasse and spruce, for bioethanol production. Biomass
Bioenergy 35, 3115e3122.
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y.Y., Holtzapple, M.,
Ladisch, M., 2005. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 96, 673e686.
Nguyen, T.A.D., Kim, K.R., Han, S.J., Cho, H.Y., Kim, J.W., Park, S.M.,
Park, J.C., Sim, S.J., 2010. Pretreatment of rice straw with ammonia
and ionic liquid for lignocellulose conversion to fermentable
sugars. Bioresour. Technol. 101, 7432e7438.
Patel, D.D., Lee, J.-M., 2012. Applications of ionic liquids. Chem. Rec.
12, 329e355.
Pedersen, M., Vikso-Nielsen, A., Meyer, A.S., 2010. Monosaccharide
yields and lignin removal from wheat straw in response to catalyst
type and pH during mild thermal pretreatment. Process Biochem.
2010, 1181e1186.
Piccolo, C., Bezzo, F., 2009. A techno-economic comparison between
two technologies for bioethanol production from lignocellulose.
Biomass Bioenergy 33, 478e491.
Pinto, P.A., Dias, A.A., Fraga, I., Marques, G., Rodrigues, M.A.M.,
Colaco, J., Sampaio, A., Bezerra, R.M.F., 2012. Influence of ligninolytic enzymes on straw saccharification during fungal pretreatment. Bioresour. Technol. 111, 261e267.
Protasio, T.d.P., Bufalino, L., Mendes, R.F., Ribeiro, M.X., Trugilho, P.F.,
Leite, E.R.d.S., 2012. Torrefaction and carbonization of briquettes
made with residues from coffee grain. Rev. Bras. Eng. Agric.
Ambient 16, 1252e1258.
Qi, B.K., Chen, X.R., Wan, Y.H., 2010. Pretreatment of wheat straw by
nonionic surfactant-assisted dilute acid for enhancing enzymatic hydrolysis and ethanol production. Bioresour. Technol. 101, 4875e4883.
Qing, Q., Yang, B., Wyman, C.E., 2010. Impact of surfactants on pretreatment of corn stover. Bioresour. Technol. 101, 5941e5951.
Requejo, A., Peleteiro, S., Rodriguez, A., Garrote, G., Parajo, J.C., 2011.
Second-generation bioethanol from residual woody biomass.
Energy Fuels 25, 4803e4810.
Rodrigues, T.H.S., Rocha, M.V.P., de Macedo, G.R., Goncalves, L.R.B.,
2011. Ethanol production from cashew apple bagasse: improvement of enzymatic hydrolysis by microwave-assisted alkali pretreatment. Appl. Biochem. Biotechnol. 164, 929e943.
Romani, A., Garrote, G., Lopez, F., Parajo, J.C., 2011. Eucalyptus globulus wood fractionation by autohydrolysis and organosolv
delignification. Bioresour. Technol. 102, 5896e5904.
Roos, A.A., Persson, T., Krawczyk, H., Zacchi, G., Stalbrand, H., 2009.
Extraction of water-soluble hemicelluloses from barley husks.
Bioresour. Technol. 100, 763e769.
Salvachua, D., Prieto, A., Lopez-Abelairas, M., Lu-Chau, T.,
Martinez, A.T., Martinez, M.J., 2011. Fungal pretreatment: an
alternative in second-generation ethanol from wheat straw.
Bioresour. Technol. 102, 7500e7506.
Samuel, R., Foston, M., Jaing, N., Cao, S.L., Allison, L., Studer, M.,
Wyman, C., Ragauskas, A.J., 2011. HSQC (heteronuclear single
quantum coherence) (13)C-(1)H correlation spectra of whole
biomass in perdeuterated pyridinium chloride-DMSO system: an
effective tool for evaluating pretreatment. Fuel 90, 2836e2842.
Saritha, M., Lata, A.A., 2011. Biological pretreatment of lignocellulosic
substrates for enhanced delignification and enzymatic digestibility.
Indian J. Microbiol.
Sasmal, S., Goud, V.V., Mohanty, K., 2011. Optimisation of the acid
catalysed pretreatment of areca nut husk fibre using the Taguchi
design method. Biosyst. Eng. 110, 465e472.
Sindhu, R., Kuttiraja, M., Binod, P., Preeti, V.E., Sandhya, S.V., Vani, S.,
Sukumaran, R.K., Pandey, A., 2012. Surfactant-assisted acid pretreatment of sugarcane tops for bioethanol production. Appl.
Biochem. Biotechnol. 167, 1513e1526.
Sousa, L.D., Chundawat, S.P.S., Balan, V., Dale, B.E., 2009. ’Cradleto-grave’ assessment of existing lignocellulose pretreatment technologies. Curr. Opin. Biotechnol. 20, 339e347.
Srinivasan, V., Adhikari, S., Chattanathan, S.A., Park, S., 2012. Catalytic pyrolysis of torrefied biomass for hydrocarbons production.
Energy Fuels 26, 7347e7353.
Sun, R.C., Sun, X.F., Ma, X.H., 2002. Effect of ultrasound on the
structural and physiochemical properties of organosolv soluble
hemicelluloses from wheat straw. Ultrason. Sonochem. 9, 95e101.
REFERENCES
Sun, Y.S., Lu, X.B., Zhang, R., Wang, X.Y., Zhang, S.T., 2011. Pretreatment of corn stover silage with Fe(NO(3))(3) for fermentable sugar
production. Appl. Biochem. Biotechnol. 164, 918e928.
Tadesse, H., Luque, R., 2011. Advances on biomass pretreatment using
ionic liquids: an overview. Energy Environ. Sci. 4, 3913e3929.
Tao, L., Aden, A., Elander, R.T., Pallapolu, V.R., Lee, Y.Y., Garlock, R.J.,
Balan, V., Dale, B.E., Kim, Y., Mosier, N.S., Ladisch, M.R., Falls, M.,
Holtzapple, M.T., Sierra, R., Shi, J., Ebrik, M.A., Redmond, T.,
Yang, B., Wyman, C.E., Hames, B., Thomas, S., Warner, R.E., 2011.
Process and technoeconomic analysis of leading pretreatment
technologies for lignocellulosic ethanol production using switchgrass. Bioresour. Technol. 102, 11105e11114.
Tapasvi, D., Khalil, R., Skreiberg, O., Khanh-Quang, T., Gronli, M.,
2012. Torrefaction of Norwegian birch and spruce: an experimental
study using macro-TGA. Energy Fuels 26, 5232e5240.
Teixeira, L.C., Linden, J.C., Schroeder, H.A., 2000. Simultaneous
saccharification and cofermentation of peracetic acid-pretreated
biomass. Appl. Biochem. Biotechnol. 84-6, 111e127.
Teramoto, Y., Tanaka, N., Lee, S.H., Endo, T., 2008. Pretreatment of
eucalyptus wood chips for enzymatic saccharification using combined sulfuric acid-free ethanol cooking and ball milling.
Biotechnol. Bioeng. 99, 75e85.
Thomsen, M.H., Holm-Nielsen, J.B., Oleskowicz-Popiel, P.,
Thomsen, A.B., 2008. Pretreatment of whole-crop harvested, ensiled
maize for ethanol production. Appl. Biochem. Biotechnol. 148, 23e33.
Tian, X.-f., Fang, Z., Guo, F., 2012. Impact and prospective of fungal
pre-treatment of lignocellulosic biomass for enzymatic hydrolysis.
Biofuels, Bioprod. Biorefin. 6, 335e350.
Tumuluru, J.S., Boardman, R.D., Wright, C.T., Hess, J.R., 2012. Some
chemical compositional changes in Miscanthus and white oak
sawdust samples during torrefaction. Energies 5, 3928e3947.
Um, B.H., Bae, S.H., 2011. Statistical methodology for optimizing the dilute
acid hydrolysis of sugarcane bagasse. Korean J. Chem. Eng. 28,
1172e1176.
Vancov, T., McIntosh, S., 2011. Effects of dilute acid pretreatment
on enzyme saccharification of wheat stubble. J. Chem. Technol.
Biotechnol. 86, 818e825.
Velmurugan, R., Muthukumar, K., 2012a. Sono-assisted enzymatic
saccharification of sugarcane bagasse for bioethanol production.
Biochem. Eng. J. 63, 1e9.
Velmurugan, R., Muthukumar, K., 2012b. Ultrasound-assisted alkaline
pretreatment of sugarcane bagasse for fermentable sugar production: optimization through response surface methodology. Bioresour. Technol. 112, 293e299.
Verma, P., Watanabe, T., Honda, Y., 2011. Microwave-assisted pretreatment of woody biomass with ammonium molybdate activated
by H2O2. Bioresour. Technol. 102, 3941e3945.
Wan, C., Li, Y., 2011. Effectiveness of microbial pretreatment by
Ceriporiopsis subvermispora on different biomass feedstocks.
Bioresour. Technol. 102, 7507e7512.
Wang, B., Wang, X.J., Feng, H., 2010. Deconstructing recalcitrant
Miscanthus with alkaline peroxide and electrolyzed water. Bioresour. Technol. 101, 752e760.
Wang, W., Yuan, T., Wang, K., Cui, B., Dai, Y., 2012. Combination of
biological pretreatment with liquid hot water pretreatment to
enhance enzymatic hydrolysis of Populus tomentosa. Bioresour.
Technol. 107, 282e286.
Wang, Y., Radosevich, M., Hayes, D., Labbe, N., 2011a. Compatible
ionic liquid-cellulases system for hydrolysis of lignocellulosic
biomass. Biotechnol. Bioeng. 108, 1042e1048.
Wang, Z.Y., Xu, J.L., Cheng, J.J., 2011b. Modeling biochemical conversion of lignocellulosic materials for sugar production: a review.
Bioresources 6, 5282e5306.
69
Wu, Y., Zhang, C., Liu, Y., Fu, Z., Dai, B., Yin, D., 2012. Biomass char
sulfonic acids (BC-SO3H)-catalyzed hydrolysis of bamboo under
microwave irradiation. Bioresources 7, 5950e5959.
Wyman, C.E., Dale, B., Richard, T.E., Holtzapple, M., Ladisch, M.,
Lee, Y.Y., 2005. Coordinated development of leading biomass
pretreatment technologies. Bioresour. Technol. 96, 1959e1966.
Yan, W., Acharjee, T.C., Coronella, C.J., Vasquez, V.R., 2009. Thermal
pretreatment of lignocellulosic biomass. Environ. Prog. Sustainable
Energy 28, 435e440.
Yanagida, T., Fujimoto, S., Bespyatko, L., Tsukahara, K., Hideno, A.,
Sawayama, S., Minowa, T., 2011. Introduction of dehydration
process into mechanochemical pretreatment for bioethanol production. J. Jpn. Pet. Inst. 54, 215e221.
Yang, B., Wyman, C.E., 2008. Pretreatment: the key to unlocking lowcost cellulosic ethanol. Biofuels, Bioprod. Biorefin. 2, 26e40.
Yang, X.W., Ma, F.Y., Yu, H.B., Zhang, X.Y., Chen, S.L., 2011. Effects of
biopretreatment of corn stover with white-rot fungus on lowtemperature pyrolysis products. Bioresour. Technol. 102,
3498e3503.
Yoo, J., Alavi, S., Vadlani, P., Amanor-Boadu, V., 2011. Thermo-mechanical extrusion pretreatment for conversion of soybean hulls to
fermentable sugars. Bioresour. Technol. 102, 7583e7590.
Yu, J., Zhang, J.B., He, J., Liu, Z.D., Yu, Z.N., 2009. Combinations of
mild physical or chemical pretreatment with biological pretreatment for enzymatic hydrolysis of rice hull. Bioresour. Technol. 100,
903e908.
Zeng, J.J., Singh, D., Chen, S.L., 2011. Thermal decomposition kinetics
of wheat straw treated by Phanerochaete chrysosporium. Int. Biodeterior. Biodegrad. 65, 410e414.
Zhang, C., Zhu, J.Y., Gleisner, R., Sessions, J., 2012a. Fractionation of
forest residues of Douglas-fir for fermentable sugar production by
SPORL pretreatment. Bioenergy Res. 5, 978e988.
Zhang, R., Lu, X.B., Sun, Y.S., Wang, X.Y., Zhang, S.T., 2011. Modeling
and optimization of dilute nitric acid hydrolysis on corn stover.
J. Chem. Technol. Biotechnol. 86, 306e314.
Zhang, S., Keshwani, D.R., Xu, Y., Hanna, M.A., 2012b. Alkali combined extrusion pretreatment of corn stover to enhance enzyme
saccharification. Ind. Crops Prod. 37, 352e357.
Zhang, Y.H.P., Ding, S.Y., Mielenz, J.R., Cui, J.B., Elander, R.T.,
Laser, M., Himmel, M.E., McMillan, J.R., Lynd, L.R., 2007. Fractionating recalcitrant lignocellulose at modest reaction conditions.
Biotechnol. Bioeng. 97, 214e223.
Zhao, J., Zhang, H.M., Zheng, R.P., Lin, Z.X., Huang, H., 2011a. The
enhancement of pretreatment and enzymatic hydrolysis of corn
stover by FeSO(4) pretreatment. Biochem. Eng. J. 56, 158e164.
Zhao, X.B., Cheng, K.K., Liu, D.H., 2009. Organosolv pretreatment of
lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol.
Biotechnol. 82, 815e827.
Zhao, X.B., Wu, R.C., Liu, D.H., 2011b. Production of pulp, ethanol
and lignin from sugarcane bagasse by alkali-peracetic acid
delignification. Biomass Bioenergy 35, 2874e2882.
Zheng, Y., Pan, Z., Zhang, R., 2009. Overview of biomass pretreatment
for cellulosic ethanol production. Int. J. Agric. Biol. Eng. 2, 51e58.
Zhong, W.X., Yu, H.B., Song, L.L., Zhang, X.Y., 2011. Combined pretreatment with white-rot fungus and alkali at near roomtemperature for improving saccharification of corn stalks. Bioresources 6, 3440e3451.
Zhu, J.Y., Pan, X.J., Wang, G.S., Gleisner, R., 2009. Sulfite pretreatment
(SPORL) for robust enzymatic saccharification of spruce and red
pine. Bioresour. Technol. 100, 2411e2418.
Zimbardi, F., Viola, E., Nanna, F., Larocca, E., Cardinale, M.,
Barisano, D., 2007. Acid impregnation and steam explosion of corn
stover in batch processes. Ind. Crops Prod. 26, 195e206.
C H A P T E R
5
Biofuels and Bioproducts Produced through
Microbial Conversion of Biomass
Trent Chunzhong Yang 1, Jyothi Kumaran 2,3, Samuel Amartey 4,
Miranda Maki 5, Xiangling Li 1,6, Fan Lu 7, Wensheng Qin 5,*
1
Aquatic and Crop Resource Development, National Research Council Canada, Ottawa, ON, Canada, 2Human Health
Therapeutics, National Research Council Canada, Ottawa, ON, Canada, 3School of Environmental Sciences, University
of Guelph, Guelph, ON, Canada, 4Division of Biology, Imperial College of Science, Technology and Medicine, South
Kensington, London, UK, 5Department of Biology, Lakehead University, ON, Canada,
6
College of Chinese Medicine, Guangzhou University of Chinese Medicine, Guangzhou, China,
7
College of Bioengineering, Hubei University of Technology, Wuhan, Hubei Province, China
*Corresponding author email: wqin@Lakeheadu.ca
O U T L I N E
Lignocellulosic Biomass and its Pretreatment
Nonbiological Pretreatment
Physical Pretreatments
Chemical Pretreatments
Physicochemical Pretreatments
Biological Pretreatment with Microorganisms
Potential Advantages over Nonbiological
Pretreatment
Biological Degradation of Lignin
Commonly used Microorganisms for Biological
Pretreatment
Natural Microorganisms and Practical Applications
in Bioconversion
Application of White-Rot Fungus in Treatment
of Different Biomasses
White-Rot Fungus Pretreatment of Biomass
for Animal Feed
White-Rot Fungus Pretreatment in Biological
Pulping
White-Rot Fungus Pretreatment of Biomass
for Biofiber
Brown-Rot Fungi
Soft-Rot Fungi
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00005-X
72
72
72
72
72
73
73
73
73
74
74
75
75
75
75
76
Bacteria
Genetically Modified Microorganisms for Biomass
Conversion
Rational Engineering
Metabolic Engineering of Microbial Pathways
for Enhanced Bioproduct Production
Strategies of Using Microbial Pretreatment
to Enhance Sugar Release for Biofuel and
Bioproduct Production
Application of Microbial Pretreatment for Biogas
Production
Application of Microbial Pretreatment for Biomass
Conversion
Strategies for Microorganism Application
in Biomass
Commonly Used Microorganisms in Biomass
Conversion and Some Application Examples
Other Bioproducts Produced by Microbial Conversion
of Biomass: Introduction
References
77
77
77
78
79
80
81
81
82
84
87
71
Copyright Ó 2014 Elsevier B.V. All rights reserved.
72
5. BIOFUELS AND BIOPRODUCTS PRODUCED THROUGH MICROBIAL CONVERSION OF BIOMASS
LIGNOCELLULOSIC BIOMASS AND ITS
PRETREATMENT
Lignocellulose is the primary building block of plant
cell walls and is composed mainly of cellulose, hemicelluloses, lignin and small quantities of pectin, proteins,
extractives and ash. The cellulose, hemicelluloses and
lignin are present in varying amounts in the different
parts of the plant and are intimately associated to form
the complex structural framework of the plant cell
wall where cellulose and hemicellulose are bound
together with lignin and other components to form a
tight matrix. The composition of lignocellulose depends
on plant species as well as growth conditions and age.
Lignocellulose biomass is a renewable, sustainable,
abundant and cheap resource for producing renewable
biofuels and bioproducts. However, their conversion
into fermentable sugar before fermentation is a major
hurdle due to its complex structure and recalcitrant
nature. While hydrolysis of cellulose and hemicellulose
yields fermentable sugars, they are not easily accessible
due to the crystalline structure of cellulose and interference by the phenyl-propanoid polymer, lignin.
Bioconversion of carbohydrates from lignocellulosic
feedstocks into fermentable sugars is a key challenge
in the biorefinery process. Efficient, cost-effective and
environmentally benign pretreatment and hydrolysis
methods are required. The primary purpose of pretreatment is to change the architecture of the cell wall by
delignification and disrupting the cellulose structure
and making the lignocellulosic biomass accessible and
reactive to allow high rates and yields on enzymatic
hydrolysis. Pretreatment has been considered as one of
the most expensive processing steps in biomass to
fermentable sugar conversion (Mosier et al., 2005).
This article focuses mainly on biological conversion
of biomass with microorganisms. However, nonbiological pretreatments, as well as the most frequently studied and applied procedures, will also be discussed.
Nonbiological Pretreatment
A variety of nonbiological pretreatment methods have
been extensively reviewed. These include physical,
chemical, physicochemical and other combinations of
procedures (Alvira et al., 2010; Chandra et al., 2007; da
Costa Sousa et al., 2009; Hendriks and Zeeman, 2009;
Sun and Cheng, 2002; Taherzadeh and Karimi, 2008).
Based on their effects on biomass structure, pretreatments can be divided into different categories: those
that increase enzyme accessibility to crystalline cellulose
by decreasing the fiber’s degree of polymerization or by
facilitating hemicellulose and/or lignin removal to create
pores in the cellulose fibrils. Since hemicellulose and
lignin are the two main protective coats surrounding
cellulose, they have to be removed or altered in order to
achieve fast enzymatic hydrolysis of the biomass. However, to obtain high sugar yield for both hexoses and pentoses, an ideal pretreatment procedure should efficiently
remove or modify lignin and also hydrolyze hemicellulose, but not degrade these hemicellulose sugars (Ohgren
et al., 2007). Some of the most widely investigated procedures are briefly described.
Physical Pretreatments
These include mechanical methods to chip, grind and
mill the biomass to reduce particle size and, potentially,
the crystallinity and degree of polymerization of lignocellulose in order to maximize the downstream enzyme
hydrolysis process (Tassinari et al., 1980). Recently, a
novel extrusion method was developed where the
biomass materials are subjected to heating, mixing and
shearing to cause both physical and chemical modifications to the material in order to increase cellulose accessibility (Karunanithy and Muthukumarappan, 2010a,b;
Karunanithy et al., 2012).
Chemical Pretreatments
These are mainly alkali and acid pretreatments.
Alkali pretreatments increase cellulose digestibility by
enhancing lignin solubilization and decreasing cellulose
crystallinity. This method is more effective on agricultural biomass than on wood material (Kumar et al.,
2009; Playne, 1984). Acid pretreatment, mostly diluted
acid pretreatments, increase cellulose accessibility
mainly by solubilizing hemicellulose. It can be used as
either a pretreatment or a direct hydrolysis process but
leads to toxic degradation products that inhibit downstream fermentation (Alvira et al., 2010). On the contrary, ozonolysis uses the powerful oxidant ozone to
delignify lignocellulosic materials at room temperature
and does not form inhibitory compounds, yet it is
economically unviable due to large amounts of ozone
consumed (Sun and Cheng, 2002). On the other hand,
organosolv process can efficiently remove lignin and
result in minimal cellulose loss. This is a promising process if economic solvents are available at commercial
scales (Wood and Saddler, 1988; Zhao et al., 2009).
Physicochemical Pretreatments
Steam explosion is the most studied and commonly
used physicochemical method and extensively reviewed
(Hsu, 1996; McMillan, 1994; Saddler et al., 1993). During
this hydrothermal procedure, biomass is subjected to
pressurized steam for a short time and then suddenly
depressurized. The process leads to hemicellulose
degradation and lignin transformation and as a result,
increases pore volumes in the pretreated biomass, leading to enhanced enzymatic accessibility (Grous et al.,
1986). It is recognized as one of the most cost-effective
COMMONLY USED MICROORGANISMS FOR BIOLOGICAL PRETREATMENT
processes for hardwoods and agricultural residues,
but less effective for softwoods (Sun and Cheng, 2002).
Another disadvantage is the production of inhibitory
compounds. Addition of diluted acids can decrease pretreatment time and temperature thus reducing the production of inhibitory compounds and also enhancing
softwood pretreatment efficiency (Ballesteros et al.,
2006; Duff and Murray, 1996; Jørgensen, 2007; Kumar
et al., 2009; Stenberg et al., 1998). As a relatively energy
and environmentally friendly procedure, steam explosion had been scaled up and used in pilot-scale production at Iogen (Canada) and is to be used in many of the
planned commercial size facilities worldwide.
Other physicochemical methods explored include
ammonia fiber explosion (AFEX) (Alizadeh et al., 2005;
Teymouri et al., 2004, 2005), carbon dioxide explosion
(Zheng et al., 1995, 1998), liquid hot water (LHW) pretreatment (Kim et al., 2009; Mosier et al., 2005), ultrasound pretreatment (Gonzalez-Fernandez et al., 2012;
Sasmal et al., 2012), and microwave pretreatment
(Azuma et al., 1984; Ma et al., 2009; Ooshima et al., 1984).
For practical application, different pretreatment
methods have to be tested for each specific biomass to
determine the best procedure that is compatible with
the downstream hydrolytic enzyme cocktail. For
example, in a recent report describing switchgrass hydrolysis, different pretreatment methods were tested
including ammonia fiber expansion (AFEX), dilute
acid (DA), LHW, lime, lime þ ball milling, soaking in
aqueous ammonia, and sulfur dioxide (SO2). It was
demonstrated that lime þ ball milling lead to the highest
overall sugar yield (98.3%) from pretreated biomass
with xylanase addition (Falls et al., 2011).
Biological Pretreatment with Microorganisms
Potential Advantages over Nonbiological
Pretreatment
Microbial pretreatment by solid state cultivation
(SSC) has the potential to be a low-cost, environmentally
friendly alternative to chemical approaches. Existing
nonbiological pretreatment methods as described above
have largely been developed on the basis of physicochemical technologies such as steam explosion, microwave radiation, ionizing radiation, dilute acid, alkali,
and oxidation or various combinations of these methodologies (Mosier et al., 2005). Most of these methods
require expensive, complicated, high-pressure and
corrosion-resistant equipment and may consume large
amounts of energy and water. Furthermore, chemical
pretreatments can be detrimental to subsequent enzymatic hydrolysis and microbial fermentation in addition
to producing acidic or alkaline waste water, which
requires predisposal treatment to ensure environmental
safety (Keller et al., 2003). Due to its low energy and
73
material costs, mild reaction conditions with simple
equipment, and environmental benefits, microbial/biological pretreatment has received increased attention
as an alternative to physicochemical or thermochemical
pretreatments (Kumar and Wyman, 2009; Rabinovich
et al., 2004; Sanchez, 2009; Saritha et al., 2012a; Shi
et al., 2008; Sun and Cheng, 2002; Zeng et al., 2011).
Biological Degradation of Lignin
Lignin is a complex, heterogeneous phenylpropanoid
polymer that is linked to both hemicelluloses and cellulose to form an impenetrable physical and chemical
barrier for biodegradative systems (Sanchez, 2009;
Blanchette, 1991). Unless lignin is modified or removed,
hydrolytic enzymes cannot penetrate and effectively
degrade woody substrates. In addition to producing
the extracellular polysaccharide degradative enzymes,
such as cellulases, xylanases, and mannanases, saprophytic fungi have a unique oxidative and extracellular
lignolytic system called Fenton’s reagents to degrade
lignin and open phenyl rings (Green and Highley,
1997; Jensen et al., 2001; Arantes et al., 2012; Contreras
et al., 2007; Irbe et al., 2011; Kramer et al., 2004; Ray
et al., 2010; Suzuki et al., 2006; Yanase et al., 2010b). In
addition to cellulase and hemicellulases, lignolytic
enzymes have also been detected in some strains. Particularly, species among the Basidiomycotina fungi that
cause white rots of wood may simultaneously degrade
lignin and cell wall carbohydrates (Sanchez, 2009).
Furthermore, a small number of the white-rot fungi preferentially degrade lignin leading to little to no loss of
cellulose (Blanchette, 1991). For practical applications,
these species that can selectively remove lignin without
extensive cellulose degradation are of special interest.
The most widely studied white-rot fungus, Phanerochaete
chrysosporium, can significantly degrade lignin and
simultaneously degrade a small fraction of cellulose
and hemicellulose, whereas others such as Ceriporiopsis
subvermispora tend to remove lignin in advance of cellulose and hemicellulose (Blanchette et al., 1992; Hatakka,
1994; Sanchez, 2009).
COMMONLY USED MICROORGANISMS
FOR BIOLOGICAL PRETREATMENT
Microbial pretreatment makes use of microorganisms
and their enzyme systems to breakdown lignin and/or
hemicellulose present in lignocellulosic biomass. So
far, the isolated and identified lignocellulolytic microorganisms mainly include fungi and a few bacterial
strains. Fungi including brown-, white-, and soft-rot
fungi are the predominant organisms responsible for
lignocellulose degradation, and among the fungi, the
Basidiomycetes that cause both white and brown rots
74
5. BIOFUELS AND BIOPRODUCTS PRODUCED THROUGH MICROBIAL CONVERSION OF BIOMASS
are the most rapid degraders (Bennet et al., 2002;
Loguercio-Leite et al., 2008; Rabinovich et al., 2004;
Sanchez, 2009; ten Have and Teunissen, 2001). Several
Basidiomycetes such as P. chrysosporium, C. subvermispora,
Phlebia subserialis, Pleurotus ostreatus, and Irpex
lacteus have been shown to efficiently degrade lignin
in different lignocellulosic materials (Hatakka and
Usi-Rauva, 1983; Keller et al., 2003; Sawada et al., 1995;
Taniguchi et al., 2005; Zeng et al., 2011).
Natural Microorganisms and Practical
Applications in Bioconversion
Application of White-Rot Fungus in Treatment
of Different Biomasses
CORN STOVER
When corn stover is pretreated with C. subvermispora
for downstream bioethanol production, lignin is selectively degraded up to 31.59% with a limited cellulose
loss of less than 6% during an 18-day pretreatment.
Longer pretreatment time was found to increase lignin
removal, resulting in correspondingly higher glucose
yields from enzymatic hydrolysis. The highest overall
ethanol yield of 57.80% was obtained with 35-daypretreated corn stover (Wan and Li, 2010).
In a later study, the effectiveness of C. subvermispora
pretreatment on different types of feedstocks, including
corn stover, wheat straw, soybean straw, switchgrass,
and hardwood was tested. After an 18-day pretreatment, corn stover, switchgrass, and hardwood were
effectively delignified, leading to a two- to threefold increase in glucose yield over those of the untreated raw
materials. In contrast, wheat straw and soybean straw
did not show glucose yield increase after undergoing
the same pretreatment, suggesting the importance of using a specific strain for pretreatment of specific biomass
(Wan and Li, 2011).
Pretreatments of corn stover with the white-rot fungus
I. lacteus CD2 also resulted in significant lignin degradation with limited cellulose loss (Zeng et al., 2011). Pretreatment of corn stover with Cyathus stercoreus led to a
three- to fivefold improvement in enzymatic cellulose
digestibility (Keller et al., 2003). Pretreatment of corn stover with a newly isolated white-rot fungus, Trametes
hirsuta yj9, led to selective lignin degradation up to
71.49% and a significant increase in enzymatic digestibility of 73.99% after a 42-day pretreatment (Sun et al., 2011).
Pretreatment of corn stover fractions (leaves, cobs,
and stalks) with the white-rot fungus C. subvermispora
showed that the leaves were the least recalcitrant to
fungal pretreatment with a 45% lignin degradation as
well as higher carbohydrate degradation after 30 days
of pretreatment. However, corn cobs produced the highest sugar yield after fungal pretreatment (Cui et al., 2012).
SOFTWOOD
The effect of pretreatment on the softwood Pinus densiflora by three white-rot fungi, Ceriporia lacerata, Stereum
hirsutum, and Polyporus brumalis, has been investigated.
Among the three white-rot fungi tested, S. hirsutum
selectively degraded the lignin rather than the holocellulose component. Consistently, extracellular enzymes
from S. hirsutum showed higher activity of ligninase
and lower activity of cellulase than those from the other
white-rot fungi. In addition, the available pore size and
surface area in the pretreated wood were increased,
possibly due to degradation of lignin and a small
portion of hemicellulose by the secreted enzymes. Sugar
yield of the S. hirsutum pretreated wood also greatly
increased compared to a nonpretreated sample, indicating S. hirsutum might be a potentially effective fungus
for use in biological pretreatment of woody biomass
(Lee et al., 2007).
COTTON STALKS
Conditions for pretreatment of cotton stalks using
P. chrysosporium by SSC have also been explored. While
substrate moisture content significantly affects lignin
degradation, supplementation with modified salts did
not affect the reaction process. Over a period of 14
days, SSCat 75% moisture content without salts resulted
in 27.6% lignin degradation, 71.1% solids recovery and
41.6% availability of carbohydrates, suggesting that microbial pretreatment by SSC has the potential to be a
low-cost, environmentally friendly alternative to chemical approaches (Shi et al., 2008).
RICE STRAW
Fungal pretreatment of rice straw for improved enzymatic saccharification has been reported. Yamagishi
et al. (2011) tested 17 C. stercoreus isolates for their ability
to treat rice straw for improved enzymatic hydrolysis.
A negative correlation was found between cellulase
and xylanase activity in these isolates and enzymatic
saccharification yields in the pretreated straw. A 25-day
pretreatment with the strain C. stercoreus TY-2 led to a
more than fivefold increase in enzymatic saccharification
yield compared to untreated control samples, suggesting
this isolate has the potential for biological pretreatment
of rice straw under conditions of low energy input. A
15-day pretreatment of rice straw with P. chrysosporium
in an optimized media resulted in a treated biomass
with an enzymatic digestibility of 64.9% of the theoretical
maximum glucose yield. When the fungal-pretreated rice
straw was used as a substrate in simultaneous saccharification and fermentation (SSF), a 9.49 g/l ethanol concentration, 58.2% of the theoretical maximum production
yield, and 0.40 g/l/h productivity were achieved after
24 h and a 62.7% of the theoretical maximum ethanol
yield was expected after 96 h (Bak et al., 2009).
COMMONLY USED MICROORGANISMS FOR BIOLOGICAL PRETREATMENT
When rice straw was pretreated with the wood-rot fungus, Dichomitus squalens, for 15 days, an enzymatic digestibility of 58.1% of theoretical glucose yield was reached for
the treated biomass. When the pretreated rice straw was
used as a substrate for ethanol production in SSF, the
ethanol production yield and productivity were 54.2%
of the theoretical maximum and 0.39 g/l/h, respectively,
after 24 h (Bak et al., 2009). Taniguchia et al. (Taniguchi
et al., 2005) reported the effect on rice straw composition
and susceptibility to enzymatic hydrolysis after pretreatment with four white-rot fungi (P. chrysosporium, Trametes
versicolor, C. subvermispora, and P. ostreatus). Among the
four strains, P. ostreatus selectively degraded the lignin
fraction of rice straw rather than the cellulose component. A 60-day pretreatment of rice straw with P. ostreatus
led to a total weight loss of 25% and 41% lignin degradation, but only a 17% loss of cellulose and a 48% loss of
hemicellulose. A 48-h enzymatic hydrolysis lead to
52% holocellulose and 44% cellulose solubilization in
the pretreated rice straw corresponding to a net sugar
yield of 33% from holocellulose and 32% from cellulose.
PADDY STRAW
A recent report of a study on the pretreatment of
paddy straw with the white-rot fungus T. hirsuta (Microbial Type Culture Collection) MTCC 136 showed high
ligninase and low cellulase activities. It showed that
within 10 days of solid state fermentation, the carbohydrate content was enhanced by 11.1% and a much higher
yield of sugars was obtained after enzymatic hydrolysis.
Saccharification efficiency of the biologically pretreated
paddy straw with the commercial enzyme AcceleraseÒ 1500 reached 52.69% within 72 h suggesting the
delignification potential of T. hirsuta for pretreatment
of lignocellulosic substrate and facilitating efficient
enzymatic digestibility of cellulose (Saritha et al., 2012b).
White-Rot Fungus Pretreatment of Biomass
for Animal Feed
Pretreatment of lignocellulosic biomass with the
white-rot fungi increases biodegradability and leads to
high-quality ruminant feed. For example, white-rot
fungi-treated cedar wood shows significant improvement for rumen digestibility (Okano et al., 2005).
When high-lignin forages such as grass, oat straw and
alfalfa stems were treated with various white-rot fungi,
substantial improvements in digestibilities have also
been obtained (Akin et al., 1995, 1993; Jung et al., 1992).
White-Rot Fungus Pretreatment in Biological
Pulping
White-rot fungi have also been used in biological pulping (biopulping) to reduce the utilization of chemicals
in the pulping industry and decrease the environmental
hazard caused by the traditional pulping process (Singh
75
et al., 2010). Biopulping process removes not only lignin
and hemicellulose but also some of the wood extractives.
It can also improve paper quality and significantly reduce
the electrical energy and cooking time required for pulping wood chips (Ali and Sreekrishnan, 2001; Hunt et al.,
2004; Singh et al., 2010). When C. subvermispora was
used for biopulping of agricultural residues including
rice, wheat and barley straw samples, the tensile strength
and burst factor of hand sheets produced from the biopulping process improved significantly compared to
the chemical process (Yaghoubi et al., 2008). Blanchette
et al. (Blanchette et al., 1992) evaluated the potential
application in biopulping of 19 strains of P. chrysosporium
and 9 strains of C. subvermispora. For the P. chrysosporium
isolates, only a few strains preferentially removed large
amounts of lignin from wood while the majority of the
isolates removed all cell wall components nonselectively.
In contrast, all nine isolates of C. subvermispora led to
moderate weight losses and preferential degradation of
lignin in aspen, birch and loblolly pine wood.
White-Rot Fungus Pretreatment of Biomass
for Biofiber
Microbial pretreatment can also improve the feature of
the fiber in biomass for biocomposite production. For
example, corn stalk pretreated with the white-rot fungus
Trametes hirsuta has been used to produce fiberboard by
hot pressing without adhesive. The corn stalk-based
fiberboard made of the pretreated biomass has an increase of 3.40- and 8.87-fold in moduli of rupture and
elasticity, respectively, over the fiberboard made from
untreated corn stalk. Further analyses showed that the
increase in the mechanical properties of the fiberboard
resulted from the pretreated biomass possessing more
than twice the number of hydroxyl groups, an 18%
higher crystallinity, and twice the polysaccharide content
of untreated corn stalk (Wu et al., 2011).
Brown-Rot Fungi
Brown-rot fungi are Basidiomycete fungi that, unlike
white-rot fungi, selectively modify and then completely
hydrolyze lignocellulose polysaccharides, typically
without secreting an exoacting glucanase and without
removing lignin (Schilling et al., 2009; Tewalt and Schilling, 2010). The wood decay resulting from the action of
brown-rot fungi leads to an increased volume of pores in
the wood cell wall and decreased degree of polymerization of holocellulose along with a dramatic weight loss
(Flournoy et al., 1991). Depolymerization of holocellulose occurs rapidly during the early decay process leading to an extensive degradation of holocellulose in wood
(Blanchette, 1995; Irbe et al., 2011; Kumar et al., 2009) and
as high as 75% wood strength loss even when only 1%
weight loss has occurred (Green and Highley, 1997;
Richards, 1954; Wilcox, 1978).
76
5. BIOFUELS AND BIOPRODUCTS PRODUCED THROUGH MICROBIAL CONVERSION OF BIOMASS
The exact mechanism for brown-rot decay is still unclear. For the selective removal of polysaccharides, a
two-step procedure has been proposed: a nonenzymatic
radical-based modification of the wood cell wall through
small molecules, followed by secretion of enzymes to
catalyze the breakdown of polysaccharides into their
sugar monomers (Green and Highley, 1997; Tewalt and
Schilling, 2010). However, cellulose and hemicellulose
removal by brown-rot fungi does not open up cell walls
to facilitate enzyme penetration (Flournoy et al., 1991).
Primarily because enzymes are too large to penetrate
the decayed wood, attack by cellulolytic enzymes may
only be limited to a localized, superficial area (Baldrian
and Valaskova, 2008; Flournoy et al., 1991). It has been
proposed that Fenton’s reagents and not enzymes are
responsible for rapid wood decomposition early in
brown-rot decay (Green and Highley, 1997; Jensen
et al., 2001; Ray et al., 2010). Other study results also
support that hydroxyl radicals (HO_) generated through
Fenton chemistry (H2O2eFe(II)) initiate lignocellulose
breakdown (Arantes et al., 2012; Contreras et al., 2007;
Hammel et al., 2002; Kaneko et al., 2005; Kramer et al.,
2004; Suzuki et al., 2006). Consequently, this suggests
that reactive oxygen species play an important role
in the early stages of wood degradation by brown-rot
fungi (Irbe et al., 2011). In brown-rot wood decay, hemicellulose is removed considerably faster than cellulose
(Curling et al., 2002; Highley, 1987; Monrroy et al.,
2011). Consistently, the total secretome hemicellulase
expression and activity for brown-rot fungi peak prior
to cellulase activity (Lyr, 1960; Martinez et al., 2009).
Hemicellulose is embedded in cellulose microfibrils
and its prior removal may facilitate cellulose degradation and removal (Green and Highley, 1997). Continual
degradation of holocellulose by brown-rot fungi leads
to gradually increased weight loss but the percent crystallinity in decayed wood increases apparently at an
early stage, peaks between 2 and 4 weeks and then decreases implying structural changes of cellulose chains
during fungal attack (Howell et al., 2009). Towards the
end of brown-rot decay, nearly 100% of carbohydrates
can be removed; however, most of the lignin remains
(Eriksson et al., 1990). Only a small fraction of the lignin
is oxidized, demethylated and depolymerized, often
leading to lignin-derived volatile components (Ewen
et al., 2004; Irbe et al., 2011; Schilling et al., 2012).
Recently, the potential application of brown-rot fungi
for the pretreatment of biomass to increase downstream
enzymatic hydrolysis has been explored. When spruce
and pine woods were treated with one of two brownrot fungi, Gloeophyllum trabeum or Fomitopsis pinicola,
saccharification efficiency was increased significantly
even though total sugar yield was low, probably due
to low enzyme loading (Schilling et al., 2009). In another
effort, G. trabeum-treated pine wood block only led to a
maximum 22% glucose release even though 60 FPU Celluclast was loaded, suggesting brown-rot fungus G. trabeum modification of pine wood may not be sufficient to
increase cellulose accessibility (Tewalt and Schilling,
2010). Similarly, when the brown-rot fungi G. trabeum
and Laetoporeus sulphureus were used for the pretreatment of the wood Pinus radiate and Eucalyptus globules,
the highest glucose yield was 14% after 8 weeks of
biodegradation (Monrroy et al., 2011). On the other
hand, when G. trabeum was used to pretreat different
biomass including aspen, spruce, or corn stover, sugar
yield was significantly increased up to threefold. In the
best case, a 2-week pretreatment of aspen by G. trabeum
led to a 72% cellulose-to-glucose yield corresponding to
51% yield relative to original glucan. For corn stover, a
weak colonization with minor degradation by another
tested brown-rot fungus, Postia placenta, resulted in
more than a twofold increase in sugar yield (Schilling
et al., 2012). Similar to wood biomass, when corn stover
is pretreated with the brown-rot fungus Fomitopsis sp.
IMER2, the amorphous regions of the cellulose are preferentially degraded in contrast to the significant lignin
degradation by the white-rot fungus I. lacteus CD2
(Zeng et al., 2011). In another successful case, simple pretreatment of Scots pine (Pinus sylvestris) with the brown
rot fungus Coniophora puteana for 15 days permitted recovery of greater than 70% of the glucose present in
the biomass, with a total wood mass loss of 9%, suggesting great potential for use of this specific group of fungi
in lignocellulosic biomass pretreatment (Ray et al., 2010).
Brown-rot fungi therefore hold significant potential for
practical application in biological pretreatment.
Soft-Rot Fungi
Even though the process of wood decay by many
common white- and rot fungi has been well characterized, other types of decay caused by soft-rot fungi or
bacteria are still not well understood (Blanchette et al.,
2002, 2004). Soft rot is caused by fungi taxonomically
classified in the phylum Ascomycota, including related
asexual taxa. The term soft rot is used because it was first
identified from soft, decayed wood surfaces in contact
with excessive moisture (Findlay, 1984). Soft rot can
also occur in dry environments (Blanchette, 2000) and
seems to predominate in extreme environments such
as excessively wet or dry sites, where white- and
brown-rot fungi growth is inhibited, and in substrates
that do not favor the growth and development of other
types of fungi (Blanchette, 1995; Blanchette et al.,
2004). Soft-rot fungi attack the lignocellulose matrix in
wood by formation of cavities (type I) or cell wall
erosion (type II). Cellulases and hemicellulases, but not
ligninases, are involved in soft-rot attack leading to
extensive loss of the carbohydrate polymers; high
amounts of lignin remain even in advanced stages of
COMMONLY USED MICROORGANISMS FOR BIOLOGICAL PRETREATMENT
soft rot (Blanchette, 1995; Eriksson et al., 1990; Nilsson et
al, 1989). The most studied and applied soft-rot fungus,
Trichoderma reesei, and its mutants, are mainly used for
large-scale commercial production of cellulases and
hemicellulases (Durand et al., 1988; Esterbauer et al.,
1991; Tomme et al., 1988).
Bacteria
Bacteria degrade plant cell walls through three main
morphological forms: tunneling, erosion, and cavitation
(Blanchette, 1995; Daniel et al., 1987; Singh and Butcher,
1991, 1985; Singh et al., 1990). An early study has
confirmed that the Gram-positive filamentous bacterium Streptomyces viridosporus degrades softwood lignin
into low molecular weight fragments (Crawford et al.,
1982). Furthermore, enzymes similar to the fungal system such as peroxidases, ligninases and manganese peroxidases have been implicated in bacterial biomass
delignification (Glenn and Gold, 1983; Kirk et al.,
1986). Interestingly, some bacteria can attack high
lignin-containing hard wood that is considered durable
and resistant to fungal decay (Nilsson et al., 1992; Singh
and Butcher, 1991). However, compared to fungi, bacteria are not as efficient for lignocellulosic biomass pretreatment, as shown by a recent work comparing eight
microorganisms including fungi and bacteria, for pretreatment of sugarcane waste (Singh et al., 2008).
Genetically Modified Microorganisms
for Biomass Conversion
Since the 1990s, bacteria, fungi and yeasts have been
genetically engineered for the industrial production of
biofuels and bioproducts. More conventionally, the
improvement of microorganisms for biomass conversion
has been done using classical chemical mutagenesis, a
random approach followed by the screening and selection of a desired trait. Nevertheless, with advancements
in molecular biology and biotechnology approaches,
the improvement of microorganisms via rational engineering of proteins and metabolic engineering of pathways has become more prevalent (Strohl, 2001). This is
due to the economic needs of the industry, which
demands the development of strains that produce greater
yields and a different variety of products. Specifically, in
the bioconversion of biomass, researchers face challenges
related to the substrate such as appropriate enzymes for
conversion and microorganisms that produce them,
fermentation of nonglucose sugars (i.e. xylose), and
“consolidated bioprocessing”, where the production of
enzymes for biomass conversion (i.e. cellulose production), hydrolysis or modification of the biomass (i.e.
cellulose hydrolysis), and fermentation of solubilized
carbohydrates occur in a single step (Lynd et al., 1999).
Therefore, prior to engineering microorganisms for
77
biomass conversion it is important to select host organisms with desired characteristics; with emphasis on
strains that can utilize low-cost substrates, have high
product yield, competitive fitness, and are more robust
to environmental stresses (Lynd et al., 1999). Once a
good host has been selected based on targeted physiological characteristics and functionalities, one can identify
the additionally desirable characteristic that will then
be engineered into the host, whether targeting proteins
such as enzymes through rational engineering or changing the metabolism and/or metabolic flux through metabolic engineering (Zhang et al., 2009).
Rational Engineering
Generally speaking, rational engineering refers to
planned biochemical changes to a protein through the
use of protein sequence and structure information, which
in theory corresponds to a physiological or functional
change in the proteins behavior. The engineered changes
are usually predicted using computational biology and
protein sequence data. However, there is limited structural information available for enzymes, for example,
in structureefunction relationshipdso predictions on
behavioral changes after rational engineering still remain
in a trial-like state (Maki et al., 2009). Nonetheless, with
increasing knowledge of biomass substrates and a
rigorous test of our knowledge about enzyme interactions with plant-based biomass, rational engineering
can be a valuable tool in the economical production of
biofuels and value-added by-products.
Briefly, rational design of proteins can be summed up
in three simple steps: (1) a suitable enzyme is chosen
based on desired characteristics, (2) using computational
biology or a high resolution crystallographic structure,
the amino acid sites to be changed are identified, and
(3) mutants produced from rationally engineered proteins are characterized (Percival Zhang et al., 2006).
Moreover, rational modifications to enzymes often
include amino acids substitutions using site-directed
mutagenesis, which can be used to increase the stability
of enzymes (i.e. thermostability), substrate specificity,
cofactor specificity, and the elucidation of enzymatic
mechanisms (Bornscheuer and Pohl, 2001). In the field
of biomass conversion to biofuels and bioproducts, the
use of rational design has pioneering examples as outlined here.
For the most part, there are numerous reviews that
summarize studies that revealed the mechanism of
cellulase and other biomass-converting genes through
the use of site-directed mutagenesis (Schulein, 2000;
Wilson, 2004; Wither, 2001). On the contrary, very few
researchers have reported increasing cellulase and other
biomass-converting activities or enhancing properties
through site-directed mutagenesis. However, Baker
et al. were able to improve the activity of endoglucanase
78
5. BIOFUELS AND BIOPRODUCTS PRODUCED THROUGH MICROBIAL CONVERSION OF BIOMASS
Cel5A of Acidothermus celluloyticus toward microcrystalline cellulose by 20% (Baker et al., 2005). This was
accomplished utilizing a high-resolution crystallographic structure (Sakon et al., 1996) to determine a series of mutations designed to alter the active cleft
through a change in chemistry of the product-leaving
side. As a result, structural information allowed endproduct inhibition to be alleviated by a substitution of
a nonaromatic residue at site 245; a Y245G mutant
increased the KI of cellobiose by 15-fold.
In a similar study, site-directed mutagenesis was used
to improve the catalytic activity of endo/exocellulase
Cel9A in Thermobifida fusca by 40% with soluble and
amorphous cellulose, such as carboxymethyl cellulose
(CMC) and swollen cellulose. Through the use of computer modeling, the conserved phenylalanine residue
F476 (one of three residues) was found at the end of the
carbohydrate binding module and appeared to play an
important role in the initial binding of the cellulase to
substrate. Also, computer modeling was used to predict
that a new hydrogen bond could be created as a result of
mutating the conserved phenylalanine residue F476 to a
tyrosine. Therefore, the observed increase in catalytic activity of mutant F476Y is thought to be attributed to better
binding properties, which are key for placing the soluble
and amorphous cellulose chains in the carbohydrate
binding domain (Escovar-Kousen et al., 2004).
Rational engineering of enzymes can also be used to
improve characteristics such as thermostability and
alkalinity in addition to specific activity. The roles of
highly conserved residues (Asp 60, Tyr 35 and Glu
141), near the catalytic site, were investigated in the pHdependent activity of xylanase XYL1p from Scytalidium
acidophilum using site-directed mutagenesis. In doing
so, three single mutants, D60N, Y35W and E141A,
were created and the activities of three combined xylanase mutants DN/YW, DN/EA and YW/EA were evaluated at different pHs and temperatures. An increased
pH optimum of 0.5e1.5 pH units and lower specific
activities were observed in all the mutants except one.
Mutant E141A exhibited a 50% increase in specific activity at pH 4.0 and had an overall higher catalytic efficiency than wild-type enzyme (Al Balaa et al., 2009).
This work presents some important knowledge in acidophilic adaptation and, at the same time, is a prime
example of how rational engineering can lead to the
development of enzymes more suitable for the bioconversion industry environment, with competitive catalytic efficiency maintained.
Finally, the possibility of using rational engineering to
improve the pH optimum and catalytic efficiency of laccase enzymes, involved in the oxidation of lignin, has
been increasing as several researchers explore important
residues conserved in laccases from fungi (Rogers et al.,
2009). In one compelling example, researchers replaced
an Asp residue in position 206 with an Asn residue in
a laccase from T. versicolor, using site-directed mutagenesis. Upon expression of mutants in the yeast Yarrowia
lipolytica, it was noted that catalytic activity was significantly affected as the pH optimum was raised by 1.4 pH
units (Madzak et al., 2006), highlighting the interaction between the reducing substrate and the binding
pocket of laccase. This study, like those discussed previously, pave the way for future development of efficient
biomass-converting enzymes.
Metabolic Engineering of Microbial Pathways
for Enhanced Bioproduct Production
Contrary to rational engineering, partial and/or additional metabolic pathways of microorganisms can be
engineered to enhance bioproduct production. The
term “metabolic engineering” was first coined by Bailey
and was described as a vast variety of manipulations
and experimental procedures to improve the productivity of a desired metabolite by an organism (Bailey, 1991).
More specifically, examples of metabolic engineering
can include increased productivity and/or yield,
improvement of substrate uptake, widening the scope
of substrate range for an organism, modification of
metabolic flux, and elimination of unnecessary or
competing metabolic pathways (Stephanopoulos, 1999).
Metabolic engineering, similar to rational engineering, requires the selection of a good host/microorganism as a candidate for the production of biofuels
and/or bioproducts from biomass. This could include
engineering desired pathways into well-studied host
microorganisms such as Escherichia coli and Saccharomyces cerevisiae; these microorganisms have been used
for industrial-scale production for several years. However, some experts suggest that engineering desired
pathways into microorganisms that already possess
industrial properties may be more successful. This is
due to the potential for metabolic burden to the cell;
new metabolic pathways require amino acids, redox
cofactors, and energy for synthesis and function of its
enzymes (Lee et al., 2008a).
Furthermore, metabolic engineering poses several
general challenges for researchers including the development of recombinant DNA technologies for selected
host microorganisms, development of quantitative tools,
methods to understand flux modification in complex
biological systems, and the development of quantitative
techniques to determine changes in fluxes or metabolite
concentrations (Cameron and Tong, 1993). A few successful examples of metabolic engineering to improve
general host and select host microorganisms metabolism
for the digestion and conversion of biomass are outlined
below.
Recently, the development of genome-scale modeling
permits the prediction of how new metabolic pathways
STRATEGIES OF USING MICROBIAL PRETREATMENT TO ENHANCE SUGAR RELEASE FOR BIOFUEL AND BIOPRODUCT PRODUCTION
may impact growth and product production using metabolic models. These models result in a more rational
approach to metabolic engineering (Patil et al., 2004).
Moreover, stoichiometric models can be defined by
established equations through the use of metabolic
flux analysis (MFA); this is established by measuring exchange fluxes experimentally (Lee et al., 2008b). For
example, the native metabolism of E. coli under different
growth conditions (Kayser et al., 2005) and during recombinant protein production (Ozkan et al., 2005) has
been determined using MFA. For efficient application
in biofuel and bioproduct production, genome-scale
models should be developed with constraints to optimize flux in desired pathways, while balancing important cofactors and energy metabolites (Lee et al., 2008b).
Host microorganisms such as E. coli and S. cerevisae
have been improved time and again for the fermentation
of sugars to ethanol. In particular, due to the broad range
of carbohydrates metabolized by E. coli, it has been a potential candidate for the expression of ethanologenic
pathways in some studies. For example, a portable
cassette called the production of ethanol operon (PET
operon) was used to genetically engineer the homoethanologenic pathway from Zymomonas mobilis into E. coli,
which included the pyruvate decarboxylase and alcohol
dehydrogenase B genes. Using the PET system, these
genes were integrated into the chromosome of E.coli at
the pfl locus. Meanwhile the fumarate reductase (frd)
gene was deleted to eliminate succinate production,
therefore preventing carbon loss. These metabolic
changes resulted in the recombinant strain KO11, which
produced ethanol yields as high as 95% in complex medium (Jarboe et al., 2007; Ohta et al., 1991). However,
host strains such as E.coli may encounter metabolic burdens and are often not naturally adapted to the toxicity
of end products like ethanol. Thus, there have also been
some attempts to metabolically engineer known
biomass-converting bacteria or fungal strains.
Typically, bacteria produce more desirable end products through facultative and anaerobic digestion, as is
the case for bacteria belonging to the class Clostridia.
Much of the metabolic engineering in these species focuses on product formation, which may include the
elimination of undesirable products such as in the case
of an engineering project conducted on Clostridium
acetobutylicumda well-known ethanogenic strain studied often for the production of butanol. In brief, the acetoacetate decarboxylase gene (adc) was disrupted in the
hyperbutanol-producing strain C. acetobutylicum EA
2018 using TargeTron technology (Sigma Aldrich) (Jiang
et al., 2009). TargeTron is a group II intron developed for
rapid and site-specific gene disruption in prokaryotes.
The disruption of adc led to an increase in butanol ratio
from 70% to 80.05%, with a simultaneous reduction in
acetone of 0.21 g/l (Jiang et al., 2009).
79
In contrast, one can implement metabolic engineering
to improve native metabolism in microorganisms by
engineering entirely novel pathways for desired product
formation, which is more practically done in hosts able
to hydrolyze biomass, such as the example with Clostridium cellulolyticum. Recently, Higashide et al. demonstrated the production of isobutanol from crystalline
cellulose in C. cellulolyticum (Higashide et al., 2011).
In this study, the development of valine biosynthesis
pathway required the expression of five genes, alsS,
ilvC, ilvD, kivD, and ahdA, to convert pyruvate into isobutanol. Consequently, only the expression and function
of kivD (2-keto-acid decarboxylase) and alsS (alphaacetolactate synthase) were confirmed; nonetheless
modified C. cellulolyticum produced up to 660 mg/l of
isobutanol over a 7- to 9-day growth period (Higashide
et al., 2011).
These examples of engineering and modeling to
improve the metabolic capabilities of strains helped
lay the foundation for future development of biomassconverting microorganisms. Combined with the ability
to rationally design enzymes with greater stability
and/or increased specific activity the modification of
microorganisms in industrial production of biofuels
and bioproducts looks promising.
STRATEGIES OF USING MICROBIAL
PRETREATMENT TO ENHANCE SUGAR
RELEASE FOR BIOFUEL AND
BIOPRODUCT PRODUCTION
The advantages of biological pretreatment include
minimum facility cost, low energy requirement and
mild environmental conditions. However, for practical
application, there are two major disadvantages associated with this process. First, fungi growth consumes holocellulose as an energy source leading to significant
carbohydrate loss; second, most biological pretreatments are long processes due to slow microbial growth
and delignification reaction rates. Since lignin breakdown in the biomass would lead to enzyme access to cellulose and hemicellulose, selective lignin degradation by
white-rot fungi hold some promise for real application
in biomass pretreatment if the procedure can be cut
shorter and sugar consumption can be controlled to an
insignificantly low level. However, not even white-rot
fungi can use lignin as a sole carbon and energy source;
fungi growth inevitably results in carbohydrate loss
(Fan et al., 2012; Sanchez, 2009). Strategies taken to
shorten biological pretreatment time and decrease carbohydrate consumption include (1) selection for naturally occurring white-rot fungi that preferentially
attack lignin (Ander Eriksson, 1977; Kirk and Moore,
1972; Lee et al., 2007; Muller and Trosch, 1986; Salvachua
80
5. BIOFUELS AND BIOPRODUCTS PRODUCED THROUGH MICROBIAL CONVERSION OF BIOMASS
et al., 2011), (2) selection of cellulase-deficient mutants
(Akin et al., 1993; Eriksson et al., 1980; Ruel et al.,
1981), or (3) repression of cellulase and hemicellulase
expression (Yang et al., 1980). As an example of strain selection, among 22 screened Basidiomycetes, mostly the
white-rot fungi Pleurotus sp. “florida” preferentially attacks lignin in wheat straw to increase cellulose accessibility. After 90 days pretreatment with Pleurotus sp.
“florida”, the resulting biomass can release the same
amount of glucose as Avicel, the lignin-free cellulose
(Muller and Trosch, 1986). However, pretreatment using
this strain is still time consuming.
Furthermore, there are many limitations to the strategies for strain improvement. First, carbohydrate consumption is needed for microbial growth; therefore,
strains can only be selected for increased delignification
and decreased sugar loss and not for minimal sugar loss.
In addition, decreasing the secretion of carbohydrate hydrolysis enzymes would lower the reaction rate and lead
to even longer pretreatment time. Genetic modification
of white-rot fungi to improve the required features
may help resolve some of the drawbacks, but the technical process is quite challenging (Fan et al., 2012).
Another way to improve the biological pretreatment
process is through optimization of nutrients, temperature, and preprocessing time to reach a balance between
maximum sugar release and minimum sugar loss
within the shortest possible time. Based on the enzymatic
activity profile obtained in a 28-day pretreatment analysis, switchgrass is pretreated with P. chrysosporium for
7 days. The pretreatment of switchgrass led to higher
glucan, xylan, and total sugar yields than the unpretreated sample, suggesting enzyme profile assays may
be utilized for initial estimation of pretreatment time in order to enhance sugar yields and reduce sugar loss (Mahalaxmi et al., 2010). By monitoring compositional changes
during biological pretreatment, a 15-day pretreatment
time was selected for the pretreatment of the woody biomasses Prosopis juliflora and Lantana camara with the
white-rot fungus Pycnoporus cinnabarimus (Gupta et al.,
2011). This 15-day pretreatment resulted in a relatively
small weight loss in the pretreated feedstocks with
decreased lignin and increased holocellulose contents.
Enzymatic hydrolysis of the pretreated biomass led to
sugar releases of 389 and 402 mg per gram of dried solid.
Alternatively, as a compromise, preliminary microbial
pretreatment of biomass can be used in combination
with downstream thermochemical, chemical or other pretreatment. This procedure would reduce, for example,
the amount of acid needed combined with lower temperature and shorter time, thus reducing energy and chemical costs. In addition, there would be less biomass
degradation and inhibitor production compared to conventional thermochemical pretreatment. Preliminary
tests showed that after corn stover pretreatment with
P. chrysosporium, the shear forces needed to obtain the
same shear rates of 3.2e7 rev/s were reduced 10- to
100-fold, respectively. The digestibility of C. stercoreuspretreated corn stover showed a three- to fivefold
improvement in enzymatic cellulose digestibility (Keller
et al., 2003). Sawada et al. reported that combination of
fungal pretreatment with less severe steam explosion
maximizes enzymatic saccharification of beech wood
meal (Sawada et al., 1995). Compared to steam explosion
alone, combined pretreatments improve saccharification
by 20e100% of the polysaccharide in the wood. However,
17% of the holocellulose was degraded during fungal
pretreatment, and there was an unspecified holocellulose
loss during steam explosion at optimum 215 C for
6.5 min (Sawada et al., 1995). Pretreatment of wheat straw
with P. juliflora followed by acid hydrolysis led to a reduction in acid load and an increase in sugar release as well as
ethanol yield (Kuhar et al., 2008).
Interestingly, a recent study showed that by simply
changing the pretreatment sequence, i.e. when the
wood Pimus radiata biomass was treated first with steam
explosion followed by fungi pretreatment, a 10-fold
increase in glucose yield was achieved after enzymatic
hydrolysis (Vaidya and Singh, 2012). A combination of
selected fungal pretreatment with a mild alkali treatment of wheat straw led to a maximum of 69% glucose
yield and an ethanol yield of 62% with no inhibitor formation during the pretreatment (Salvachua et al., 2011).
Also, a combination of the white-rot fungus Lenzites
betulina C5617 pretreatment with LHW treatment
enhanced the enzymatic hydrolysis of the poplar wood
Populus tomentosa led to the highest hemicellulose
removal of 92.33%, which was almost two times higher
than that of LHW treatment alone and a 2.66-fold
increase in glucose yield (Wang et al., 2012).
Application of Microbial Pretreatment
for Biogas Production
A promising application for microbial pretreatment
of lignocellulosic materials is for increasing biogas yield
in the anaerobic fermentation process. Anaerobic digestion of organic waste and residues not only provides a
good solution for the sustainable processing and treatment of large amounts of biomaterials, but also leads
to value-added renewable energy production. Natural
lignocellulosic materials can only be converted to biogas
at a very low efficiency due to their resistance to anaerobic
digestion. The low biogas conversion rate results from the
resistance to enzymatic attack by the biomass due to the
tight association of lignin, cellulose, and hemicellulose.
Under anaerobic conditions, cellulose and hemicellulose
can be degraded during biogas production but not lignin
(Fernandes et al., 2009). Pretreatment procedures to
increase the accessibility of holocellulose are necessary
STRATEGIES OF USING MICROBIAL PRETREATMENT TO ENHANCE SUGAR RELEASE FOR BIOFUEL AND BIOPRODUCT PRODUCTION
to increase biogas production. Different pretreatment
methods, including physical and chemical pretreatments,
effectively enhance anaerobic digestion, but these procedures have disadvantages as described beforehand. A
microbial pretreatment followed by another step of biological process seems very promising and close to practical application as shown by some following examples.
Pretreatment of wheat straw with Pleurotus sp. "florida" doubles both cellulase digestibility of the treated
biomass and the resulting biogas yield, compared with
untreated wheat straw (Muller and Trosch, 1986). Pretreatment of softwood in the presence of wheat bran
with the white-rot fungus C. subvermispora, which can
effectively degrade the lignin component, enhanced
methane fermentation of softwood to 35% of the theoretical yield, based on holocellulose content of the biomass.
In contrast, pretreatment with Pleurocybella porrigens,
which has a lower ability to decompose lignin, led to
no significant changes (Amirta et al., 2006).
Application of a lignocellulose degrading composite
microbial system with high xylanase activity (XDC-2),
instead of a pure culture of microorganisms for biomass
pretreatment has also been tested. XDC-2 is composed of
26 different clones from three phyla: Clostridiales, Proteobacteria, and Bacteriodetes. However, these degrade
mainly carbohydrate but not lignin. After a 5-day
pretreatment with XDC-2, corn stalk was efficiently
degraded by nearly 45%, and the cellulose and hemicellulose contents were decreased by 22.7% and 74.1%,
respectively. Biodegradability of the pretreated biomass
is improved resulting from changes in chemical structure due to decreased holocellulose content. Compared
with untreated corn stalks, total biogas production and
methane yield were increased by 68.3% and 87.9%,
respectively, and the technical digestion time (T80) was
shortened by 35.7% (Yuan et al., 2011).
Effectiveness of biological pretreatments in enhancing
corn straw biogas production has also been reported with
complex microbial agents including yeast (S. cerevisiae,
Coccidioides immitis, and Hansenula anomala), cellulolytic
bacteria (Bacillus licheniformis, Pseudomonas sp., Bacillus
subtilis, and Pleurotus florida), and the lactic acid bacteria
Lactobacillus deiliehii. A 15-day pretreatment of corn straw
at ambient temperature led to reduced contents of total
lignin, cellulose, and hemicellulose, and increased content of hot-water extractives. Anaerobic digestion of the
pretreated material resulted in 33.07% more biogas yield,
75.57% more methane yield, and 34.6% shorter technical
digestion time compared with the untreated sample
(Zhong et al., 2011).
In conclusion, under proper conditions, microbial/
biological pretreatment can be an effective method for
improving biodegradability and enhancing downstream
biological conversion efficiency of biomass into bioenergy and other value-added bioproducts.
81
Application of Microbial Pretreatment
for Biomass Conversion
Strategies for Microorganism Application
in Biomass
Most naturally occurring microorganisms cannot utilize untreated lignocellulose efficiently for the production of biofuel or bioproducts due to the inaccessibility
of the carbohydrate polymers, even though many of
them secrete a variety of hydrolytic enzymes. For efficient utilization, biomass must first be pretreated to
open up the cell wall and then hydrolyzed by acidic or
enzymatic processes to fermentable sugar monomers.
In addition to monomeric sugars, the pretreatment and
acidic hydrolysis processes may also produce low molecular weight organic acids like acetic acid, furfural,
hydroxymethylfurfural and various lignin-degradation
products that are potent inhibitors of microbial metabolism (Larsson et al., 1999; Palmqvist and HahnHägerdal, 2000).
For an economically viable manufacturing process
from lignocellulosic biomass, both hexose and pentose
sugars produced during hydrolysis of both cellulose
and hemicelluloses need to be utilized efficiently. In the
course of cellulosic biomass conversion into biofuels
and bioproducts, four biologically mediated processes
are involved: (1) saccharolytic enzyme production, (2)
enzymatic hydrolysis of biomass, (3) fermentation of hexose sugars, and (4) fermentation of pentose sugars (Lynd
et al., 2005, 2002). For an industrially viable process, each
of the four steps must be rapid and efficient. As suggested
by a recent calculation, an economically competitive
fermentation process for industrial application needs to
approach an anaerobic yield of w95% of the theoretical
yield, produce around 100 g/l of end product with a productivity of more than 2 g/l/h (Sheridan, 2009).
DIFFERENT PROCESSES OF MICROORGANISMMEDIATED BIOMASS CONVERSION
For enzymatic hydrolysis and fermentation, different
strategies have been explored including separate
hydrolysis and fermentation (SHF), SSF nonisothermal
simultaneous saccharification and fermentation (NSSF),
simultaneous saccharification and cofermentation
(SSCF), or consolidated bioprocessing (CBP) (Lynd
et al., 2002; Taherzadeh and Karimi, 2007). Each process
has advantages and disadvantages.
For SHF, the main advantage is the possibility to
separately optimize hydrolysis and fermentation steps
and the main drawback is the inhibition of cellulase activity by the released sugars, mainly cellobiose and glucose
(Taherzadeh and Karimi, 2007). SSF, different from SHF,
combines the enzymatic hydrolysis and fermentation
in one step, thus minimizing the product inhibition of
cellulase enzymes as the released sugars are immediately
82
5. BIOFUELS AND BIOPRODUCTS PRODUCED THROUGH MICROBIAL CONVERSION OF BIOMASS
consumed by the microorganism. In addition, cellulase
production and fermentation of hemicellulose hydrolysis
products occur in two additional, discrete process steps.
This process has many advantages over SHF such as
increased ethanol yield, decreased enzyme loading,
decreased contamination, and lower capital cost. The disadvantages are differences between optimum temperatures for enzyme hydrolysis and fermentation and
inhibition of cellulase by the produced ethanol (Lynd
et al., 2002; Olofsson et al., 2008).
To solve the issue of temperature difference, the NSSF
process was proposed (Wu and Lee, 1998) in which
saccharification and fermentation occur simultaneously
but in two separate reactors, each operated at its own
optimum temperature. Compared to SSF, NSSF
increased ethanol yield and productivity with a reduced
overall enzyme loading of 30e40%. The disadvantage is
increased capital cost for extra equipment.
In SSCF, enzymatic biomass hydrolysis and fermentation of both cellulose and hemicellulose hydrolysis
products all occur in a single bioreactor with a single
microorganism (Teixeira et al., 2000). It is considered
an improved process compared to SSF, which requires
two bioreactors with two different microorganisms
and two different biomass production setups (Hamelinck et al., 2005; McMillan, 1997; McMillan et al.,
1999). However, SSCF usually requires a metabolically
engineered microorganism that can robustly coferment
both glucose and xylose (Teixeira et al., 2000) without
synthesis of side products. For example, when a naturally occurring strain, Lactobacillus pentosus (American
Type Culture Collection, ATCC 8041), was used in an
SSCF process using pretreated corn stover as substrate
and the commercial cellulase Spezyme-CP for hydrolysis, the maximum yield of lactic acid was >90% of the
theoretical maximum on the basis of all available
fermentable sugars. However, acetic acid was also produced through a different metabolic pathway that assimilates pentoses (xylose and arabinose). Another
drawback of the process is the difficulty in improving
lactic acid concentration due to end-product inhibition
of the nonengineered strain (Zhu et al., 2007).
All the above-mentioned processes require a separate
enzyme production step or an external supply of enzymes for biomass hydrolysis. In CBP, enzyme production, biomass hydrolysis, and fermentation of pentoses
and hexoses are accomplished in a single reactor by
mono- or cocultures of microorganisms (Lynd et al.,
2002). The obvious advantages of CBP are decreased capital costs and no extra cost for enzyme production or purchasing (Hamelinck et al., 2005; Lynd et al., 2005).
However, since naturally occurring microorganisms
cannot simultaneously synthesize enough of the necessary saccharolytic enzymes and convert released sugars
into the desired end products, the CBP configuration
requires the development of engineered microorganisms
(Hasunuma and Kondo, 2012a; Xu et al., 2009). Such
“superbugs” need to not only secrete high titer, robust enzymes, but also efficiently produce ethanol and other bioproducts at high yields under harsh environments
containing toxic compounds. CBP is gaining increasing
recognition as a potential breakthrough for low-cost
biomass processing (Hasunuma and Kondo, 2012a; van
Zyl et al., 2007). The company Mascoma Corporation
claims to have successfully engineered microorganisms
for industrial CBP application (http://www.mascoma.
com/).
Commonly Used Microorganisms in Biomass
Conversion and Some Application Examples
A large number of microorganisms are capable of
degrading plant cell walls including bacteria and fungi.
With few exceptions, two distinct cellulolytic strategies
have been adapted by the aerobic and anaerobic groups.
While aerobic bacteria and fungi produce numerous
individual, extracellular enzymes with many of them
acting in synergy for effective hydrolysis, anaerobic
bacteria and fungi possess a unique extracellular multienzyme complex, termed the cellulosome, that can
efficiently hydrolyze crystalline cellulose (Bayer et al.,
2004, 1998; Doi and Kosugi, 2004; Fontes and Gilbert,
2010; Lamed et al., 1983; Lynd et al., 2002; Schwarz,
2001; Shoham et al., 1999; Steenbakkers et al., 2003).
Metabolic utilization of the monomeric sugars from
hydrolyzed biomass leads to the natural production of
biofuels and bioproducts, mostly as side products by
different microorganisms. For ethanol fermentation of
lignocellulosic biomass, most frequently considered
microorganisms include the bacteria E. coli, Z. mobilis
and Clostridium phytofermentans; themophilic bacteria
such as Clostridium thermocellum; yeasts such as S. cerevisiaeand Pichia stipitis; and filamentous fungi (Amore and
Faraco, 2012; Hahn-Hagerdal et al., 2007; Weber et al.,
2010; Xu et al., 2009).
Like ethanol, the majority of other potential biofuels
and bioproducts are naturally produced by various microorganisms as side products. The viability of a
fermentation process for industrial application depends
on its cost-competitiveness. As listed in Table 5.1, most
microorganisms cannot use polymeric carbohydrates
directly as fermentation substrates; therefore, biomass
has to be broken down into monomeric sugars to be
used as fermentation substrates. For an economically
viable manufacturing process of biofuels from lignocellulosic biomass, pentose utilization is essential.
Therefore, an optimal microorganism should be able
to simultaneously ferment both hexose and pentose
sugars and give rise to high productivities and yields.
In addition, it should have high tolerance to fermentation inhibitors and end products and resist microbial
STRATEGIES OF USING MICROBIAL PRETREATMENT TO ENHANCE SUGAR RELEASE FOR BIOFUEL AND BIOPRODUCT PRODUCTION
83
TABLE 5.1 Typical Features of Representative Microorganisms for Biofuel Production
Strain
Pros
Cons
References
E. coli
Pentose utilization
Not resistant to environmental
stress, low ethanol and butanol
tolerance
(Jeffries, 1983; Knoshaug and
Zhang, 2009; Shin et al., 2010;
Trinh and Srienc, 2009; Yomano
et al., 1998, 2008)
Z. mobilis
High ethanol yield and productivity;
high ethanol tolerance
Cannot metabolize pentose sugars
(Rogers et al., 1982; Weber et al.,
2010)
Clostridium phytofermentans
(ethanol),
Clostridium acetobutylicum
(butanol)
Saccarify cellulose and hemicellulose,
ferment hexose and pentose sugars
Slow growth rate, low
productivity, sensitive to
bacteriophage infection
(Jones et al., 2000; Lee et al.,
2008a,b; Maki et al., 2009; Warnick
et al., 2002)
S. cerevisiae
High robustness, highly resistant to
toxic inhibitors and end products
Cannot naturally ferment pentose
sugars
(Olofsson et al., 2008; Yanase et al.,
2010a,b)
P. stipitis
Naturally ferment xylose
Lower sugar consumption rate
than S. cerevisiae; sequential
fermentation of glucose and
xylose
(Agbogbo and Coward-Kelly,
2008; Jeffries, 1983; Jeffries et al.,
2007; Parekh and Wayman, 1986)
Kluyveromyces marxianus
Thermotolerance allowing higher
fermentation temperature, optimum
SSF process at lower enzyme loading,
lower operation cost, potential
application in CBP
Poor xylose fermentation,
undesirable side product
(Babiker et al., 2010; Banat et al.,
1992; Hasunuma and Kondo,
2012a,b; Yanase et al., 2010a,b)
Clostridium thermocellum
Thermophilic anaerobe that grows
fast on crystalline cellulose, both
cellulolytic and ethanologenic,
hydrolyze homocellulose and
directly ferment hexose sugars to
ethanol and organic acids, no need
for external enzyme addition
No pentose fermentation,
branched fermentation pathways
lead to acetate and lactate byproducts, low ethanol production
efficiency, low ethanol tolerance
(Demain et al., 2005; Lynd et al.,
2005; Ng et al., 1981; Raman et al.,
2011; Roberts et al., 2010; Zhang
and Lynd, 2005)
T. reesei
Hyper producer of cellulolytic
enzymes, extensive knowledge and
tools for genetic manipulation and
practical application
Extensive efforts needed for strain
development, low ethanol yield
and productivity, low ethanol
tolerance
(Amore and Faraco, 2012; Xu
et al., 2009)
contamination, e.g. bacteriophage infections (Weber
et al., 2010).
No naturally occurring microorganism has all the
required features. Promising means to develop a
microorganism for sustainable bioethanol/bioproduct
production include breeding technologies, genetic engineering and the search for undiscovered species
(Weber et al., 2010). For production of a particular
product from a specific biomass, native organisms
can be selected from a group of different species of microbes based on their fermentation performance, such
as substrate utilization efficiency, inhibitor resistance,
and productivity (Rumbold et al., 2010, 2009). The
yeast S. cerevisiae is by far the most widely used organism in the existing fermentation industry. To improve
its application in bioethanol fermentation from
biomass, targeted evolution strategy has been applied
to obtain inhibitor-tolerant S. cerevisiae that can resist
individual or multiple inhibitors (Ding et al., 2012;
Heer and Sauer, 2008; Liu, 2006). When adaptation
and selection processes were applied to the parental
fungus Rhizopus oryzae, a new strain was obtained
that exhibited significantly improved efficiency of substrate utilization and enhanced production of L-(þ)lactic acid from corncob hydrolysate. The final product
concentration, yield, and volumetric productivity more
than doubled compared with its parental strain (Bai
et al., 2008).
Applications of thermotolerant mesophilic microorganisms in the fermentation process have considerable
potential for cost-effective ethanol and other bioproduct
production. The thermotolerant yeast Kluyveromyces
marxianus grows well at temperatures as high as
45e52 C and can efficiently ferment ethanol at temperatures of between 38 and 45 C. A 5 C increase in the
fermentation temperature can greatly decrease fuel
84
5. BIOFUELS AND BIOPRODUCTS PRODUCED THROUGH MICROBIAL CONVERSION OF BIOMASS
ethanol production costs (Babiker et al., 2010). Results
from solid state fermentation of sweet sorghum stalk
to ethanol with the thermotolerant yeast strain Issatchenkia orientalis IPE 100A showed great potential for its
practical application in large-scale, deep-bed solid state
fermentation (Kwon et al., 2011).
The thermotolerant Bacillus coagulans strain 36D1 can
ferment both hexoses and pentoses from enzymatically
hydrolyzed biomass at 50e55 C and pH 5.0 producing
L (þ)-lactic acid as the primary fermentation product.
Since such conditions are closer to the optimum fungal
enzyme functioning requirements, the amount of
enzyme required for cellulose conversion is significantly reduced in comparison with yeast or lactic acid
bacteria currently used by the industry as microbial
biocatalysts. In addition, both biomass conversion
efficiency and product yield are greatly increased with
a dramatically decreased fermentation time, thus
reducing the cost of both the process and final product
(Ou et al., 2009).
The anaerobic mesophilic bacterium C. phytofermentans (ATCC 700,394) is a promising native microorganism for biomass conversion since its genome
encodes the highest number of enzymes for degradation
of lignocellulosic material among sequenced Clostridial
genomes (Warnick et al., 2002; Weber et al., 2010). It secretes noncomplex, individual enzymes to hydrolyze
both cellulose and hemicelluloses to both hexose and
pentose sugars, which are mostly directly consumed,
producing ethanol and acetate as the major products
(Warnick et al., 2002; Weber et al., 2010). When used in
the CBP process with pretreated corn stover as substrate, at optimal conditions with low solid loading
(0.5% w/w), C. phytofermentans hydrolyzed 76% of
glucan and 88.6% of xylan in 10 days. These values
reach 87% and 102% of those obtained by SSCF process
using commercial enzymes and S. cerevisiae 424A with
an ethanol titer of 2.8 g/l corresponding to 71.8% of
that yielded by SSCF (3.9 g/l) (Jin et al., 2011a). However, using a similar process with high solid loading (4%
w/w), the side product acetate became a major product
(Jin et al., 2012).
Even though C. thermocellum seems a good candidate
for ethanol fermentation from cellulosic biomass, there
are a few disadvantages as listed in Table 5.1. Despite
its ability to degrade lignocellulosic waste to both hexose and pentose sugars, it can only utilize hexose sugars
from cellulose and not the pentose sugars derived from
hemicellulose (Lynd et al., 2002; Taylor et al., 2009). This
drawback could be solved by the use of mixed cultures
for the degradation and fermentation of all sugars
derived from lignocellulosic materials. For example,
the anaerobic thermophile Thermoanaerobacterium saccharolyticum, which can ferment xylan and almost all
soluble biomass sugars, would be a good candidate for
coculture with C. thermocellum. A twofold reduction of
the bioethanol production cost from lignocellulose could
be achieved when using thermophilic anaerobic mixed
cultures (Demain et al., 2005; Lynd et al., 2002). Since
there is currently no perfect CBP microbe that can
degrade lignocellulosic biomass efficiently and at the
same time utilize all the sugars released from biomass
to produce mostly ethanol, coculture or community/
mixed fermentation may be a suitable option (Barnard
et al., 2010; Demain, 2009; Jin et al., 2011a). Chen
reviewed 35 coculture systems for ethanol production
by cofermentation of glucose and xylose and concluded
that even though still in its infancy, this strategy is promising as it can increase ethanol yield and productivity,
shorten fermentation time, and reduce process costs
(Chen, 2011).
FUTURE PERSPECTIVES
For a particular product made from lignocellulosic
biomass fermentation, it will be difficult to predict
which particular microorganism should be finally
used in commercial production. For different processes,
it is possible that different species may be required. For
bioethanol production, S. cerevisiae has some advantages since it is already widely used in large-scale,
first-generation bioethanol production with wellestablished processes and technology. An ideal biomass
sugar fermentation process needs to reach high product
yield by fermenting all biomass sugars including
glucose, xylose, arabinose, mannose, and galactose
with an optimal microorganism that is resistant to toxic
materials/chemicals in biomass hydrolysates such as
acids, phenolics, salts, and sugar oligomers. In addition, the microorganism should be robust, resistant to
contamination and environmental stresses, with minimal metabolic by-product production. To achieve these
goals, metabolic engineering, or extensive physiological reprogramming of the producing organisms may
provide solutions.
Other Bioproducts Produced by Microbial
Conversion of Biomass: Introduction
The use of microorganisms in conversion processes to
produce usable material from biomass sources has been
ongoing for several decades. Most of the reports in the
literature discuss the development of bioprocesses that
are involved in the production of simple sugars, which
are then used to produce bioethanol or related compounds for use as biofuels. However, there are new
trends emerging for the use of biomass conversion by
microbes, as shown in Table 5.2. Biomass conversion
processes may eventually be implemented to produce
a much greater array of useful bioproducts, in addition
to biofuels.
STRATEGIES OF USING MICROBIAL PRETREATMENT TO ENHANCE SUGAR RELEASE FOR BIOFUEL AND BIOPRODUCT PRODUCTION
85
TABLE 5.2 List of Bioproducts Produced by Different Microorganisms
Bioproduct
Organism
Conversion
References
Biofuel
Clostridium thermosaccharolyticum
Xylose to ethanol
(Mistry and Cooney,
1989)
Engineered Escherichia coli
Cell wall sugars to biofuel
(Doran-Peterson
et al., 2008)
Lactobacillus buchneri NRRL B-30929
Xylose and glucose to ethanol and
chemicals
(Liu et al., 2009)
Saccharomyces cerevisiae
Heptanal to heptanol
(Verma et al., 2010)
Saccharomyces cerevisiae AM12
Spent shiitake mushroom medium
(using Meicelase) into ethanol
(Asada et al., 2011)
Pichia stiptis NCIM3498 and Saccharomyces
cerevisiae-VS3
Hemicellulosic hydrolysate to ethanol
(Chandel et al., 2011)
Methanosarcinales and Methanomicrobiales
Coal to methane
(Wawrik et al., 2012)
Saccharomyces cerevisiae daughter strains
Pretreated pine to ethanol
(Hawkins and
Doran-Peterson, 2011)
Trichoderma reesei xylanase
Wheat biomass to bioethanol
(Juodeikiene
et al., 2012)
Saccharomyces cerevisiae
Lignocellulose-derived sugars to
ethanol
(Madhavan et al., 2012)
Clostridium saccharoperbutylacetonicum
n-butyrate to n-butanol
(Richter et al., 2012)
Burkholderia sp. C20
Microalgal oil to biodiesel
(Tran et al., 2012)
Cyathus stercoreus and Ceriporiopsis
subversmispora
Grass stem pretreatment
(Akin et al., 1995)
Ceriporia lacerata, Stereum hirsutum, and
Polyporus brumalis
Softwood pretreatment
(Lee et al., 2007)
Ceriporiopsis subvermispora
Corn stover pretreatment for
enzymatic hydrolysis and ethanol
production
(Wan and Li, 2010)
Trametes versicolor
Canola straw pretreatment for biofuel
production
(Canam et al., 2011)
Pleurotus ostreatus
Wood degradation
(Piskur et al., 2011)
Irpex lacteus
Straw saccharification
(Pinto et al., 2012)
Tramete hirsuta
Paddy straw pretreatment for
improved enzymatic saccharification
(Saritha et al., 2012b)
Phanerochaete chrysosporium
Pretreatment of cornstalk to enhance
enzymatic saccharification and
hydrogen production
(Zhao et al., 2012)
Aureobasidium pullulans (yeastlike mold
strain)
Glucose to gluconic acid
(Anastassiadis
et al., 2003)
Enterobacter aerogenes 230S
L-Psicose
(Rao et al., 2008)
Debaryomyces hansenii
D-xylose
and sugarcane bagasse
hemicellulose to xylitol
(Prakash et al., 2011)
Agromyces sp. C42 and Stenotrophomonas
sp. A10b (from yellow mealworm gut)
Lignocellulose to reducing sugars
(Qi et al., 2011)
Ustilago maydis
Fungal lignocellulosic biomass to
glucose and other sugars
(Couturier et al., 2012)
Pretreated/delignified
biomass
Simple sugars
to L-tagatose
(Continued)
86
TABLE 5.2
5. BIOFUELS AND BIOPRODUCTS PRODUCED THROUGH MICROBIAL CONVERSION OF BIOMASS
List of Bioproducts Produced by Different Microorganismsdcont’d
Bioproduct
Lipids
Organic chemicals
Other
Organism
Conversion
References
Debaryomyces hansenii NRRL Y-7426
Distilled grape marc hemicellulosic
hydrolysates to xylitol
(Salgado et al., 2012)
Candida athensensis SB18
D-xylose and horticultural waste
hemicellulosic hydrolysate to xylitol
(Zhang et al., 2012a)
Acidotermus celluloyticus endoglucanase
Cellulose to glucose
(Zhang et al., 2012b)
Cellulolytic fungus of Aspergillus oryzae A-4
Wheat straw to lipid
(Lin et al., 2010)
Engineered Escherichia coli
Simple sugars to fatty esters, fatty
alcohols and waxes
(Steen et al., 2010)
Ustilago maydis
Crude glycerol to glycolipids
(Liu et al., 2011)
Cryptococcus curvatus
Crude glycerol to oleic acid, palmitic
acid, stearic acid and linoleic acid
(Thiru et al., 2011)
Trichosporon coremiiforme
Organic acids and residual sugars
(following butanol fermentation) to oil
(Chen et al., 2012a)
Trichosporon cutaneum
Corncob acid hydrolysate to oil
(Chen et al., 2012b)
Lipomyces starkeyi
Cellobiose and xylose into intracellular
lipids
(Gong et al., 2012)
Rhodococcus opacus DSM 1069
and PD630
Lignin model compounds to
triglycerides
(Kosa and Ragauskas,
2012)
Clostridium lentocellum SG6
Cellulose to acetic acid
(Tammali et al., 2003)
Saccharomyces uvarum SW-58
Ethyl 4,4,4-trifluoroacetoacetate to
ethyl (R)-4,4,4-trifluoro3-hydroxybutanoate [(R)-2]
(He et al., 2007)
Engineered E. coli
Glucose to glucuronic and glucaric acid
(Moon et al., 2009)
Phanerochaete chrysosporium
Rice straw biodelignification in the
presence of dirhamnolipid
biosurfactant
(Liang et al., 2010)
Schizophyllum commune
Cinnamic acid derivatives to phenols
(Nimura et al., 2010)
Aspergillus parasiticus speare BGB
Glycyrrhizinic acid in liquorice to
18-beta glycyrrhetinic acid
(Wang et al., 2010)
Gliocladium spp. and E. coli
Cellulosic biomass to hydrocarbons
(Ahamed and
Ahring, 2011)
Actinobacillus succinogenes
Sugarcane bagasse hemicellulose
hydrolysate to succinic acid
(Borges and Pereira,
2011)
Engineered Thermobifida fusca
Untreated lignocellulosic biomass to
1-propanol
(Deng and Fong, 2011)
Plasticicumulans acidivorans/Thauera
selenatis mixed culture
Lactate, lactate/acetate mix to poly3-hydroxy butyrate
(Jiang et al., 2011)
Klebsiella pneumoniae
Glycerol and xylose cofermentation to
1,3-propanediol
(Jin et al., 2011b)
Clostridium ragsdalei
Acetone to isopropanol
(Ramachandriya
et al., 2011)
Pseudonocardia carboxydivorans
Compactin to pravastatin
(Lin et al., 2011)
Ganoderma sp. rckk02
Wheat straw to nutritive ruminant feed
(Shrivastava et al., 2012)
Brevundimonas sp. SGJ
L-Tyrosine
(Surwase et al., 2012)
to
L-dihydroxyphenylalanine
Lactobacillus brevis TCCCC13007
Monosodium glutamate to gammaaminobutyric acid
(Zhang et al., 2012c)
REFERENCES
References
Agbogbo, F.K., Coward-Kelly, G., 2008. Cellulosic ethanol production
using the naturally occurring xylose-fermenting yeast, Pichia stipitis. Biotechnol. Lett. 30, 1515e1524.
Ahamed, A., Ahring, B.K., 2011. Production of hydrocarbon compounds by endophytic fungi Gliocladium species grown on cellulose. Bioresour. Technol. 102, 9718e9722.
Akin, D.E., Rigsby, L.L., Sethuraman, A., Morrison, W.H.,
Gamble, G.R., Eriksson, E.K., 1995. Alterations in structure,
chemistry, and biodegradability of grass lignocellulose treated
with the white rot fungi Ceriporiopsis subvermispora and Cyathus
stercoreus. Appl. Environ. Microbiol. 61, 1591e1598.
Akin, D.E., Sethuraman, A., Morrison, W.H., Martin, S.A.,
Eriksson, E.K., 1993. Microbial delignification with white rot fungi
improves forage digestibility. Appl. Environ. Microbiol. 59,
4274e4282.
Al Balaa, B., Brijs, K., Gebruers, K., Vandenhaute, J., Wouters, J.,
Housen, I., 2009. Xylanase XYL1p from Scytalidium acidophilum:
site-directed mutagenesis and acidophilic adaptation. Bioresour.
Technol. 100, 6465e6471.
Ali, M., Sreekrishnan, T.R., 2001. Aquatic toxicity from pulp and paper
mill effluents: a review. Adv. Environ. Res. 5, 175e196.
Alizadeh, H., Teymouri, F., Gilbert, T.I., Dale, B.E., 2005. Pretreatment
of switchgrass by ammonia fiber explosion (AFEX). Appl. Biochem. Biotechnol. 121-124, 1133e1141.
Alvira, P., Tomas-Pejo, E., Ballesteros, M., Negro, M.J., 2010. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour. Technol.
101, 4851e4861.
Amirta, R., Tanabe, T., Watanabe, T., Honda, Y., Kuwahara, M., 2006.
Methane fermentation of Japanese cedar wood pretreated with a
white rot fungus, Ceriporiopsis subvermispora. J. Biotechnol. 123, 71e77.
Amore, A., Faraco, V., 2012. Potential of fungi as category I consolidated bioprocessing organisms for cellulosic ethanol production.
Renewable Sustainable Energy Rev. 1, 3286e3301.
Anastassiadis, S., Aivasidis, A., Wandrey, C., 2003. Continuous gluconic acid production by isolated yeast-like mould strains of
Aureobasidium pullulans. Appl. Microbiol. Biotechnol. 61, 110e117.
Ander, P., Eriksson, K.E., 1977. Selective degradation of wood components by white-rot fungi. Physiol. Plant 41, 239e248.
Arantes, V., Jellison, J., Goodell, B., 2012. Peculiarities of brown-rot
fungi and biochemical Fenton reaction with regard to their potential as a model for bioprocessing biomass. Appl. Microbiol.
Biotechnol. 94, 323e338.
Asada, C., Asakawa, A., Sasaki, C., Nakamura, Y., 2011. Characterization of the steam-exploded spent shiitake mushroom medium
and its efficient conversion to ethanol. Bioresour. Technol. 102,
10052e10056.
Azuma, J.I., Tanaka, F., Koshijima, T., 1984. Enhancement of enzymatic
susceptibility of lignocellulosic wastes by microwave irradiation.
J. Ferment. Technol. 62, 377e384.
Babiker, M.A., Banat, A., Hoshida, H., Ano, A., Nonklang, S.,
Akada, R., 2010. High-temperature fermentation: how can processes for ethanol production at high temperatures become superior to the traditional process using mesophilic yeast? Appl.
Microbiol. Biotechnol. 85, 861e867.
Bai, D.M., Li, S.Z., Liu, Z.L., Cui, Z.F., 2008. Enhanced L-(þ)-lactic acid
production by an adapted strain of Rhizopus oryzae using corncob
hydrolysate. Appl. Biochem. Biotechnol. 144, 79e85.
Bailey, J.E., 1991. Toward a science of metabolic engineering. Science
252, 1668e1675.
Bak, J.S., Ko, J.K., Choi, I.G., Park, Y.C., Seo, J.H., Kim, K.H., 2009.
Fungal pretreatment of lignocellulose by Phanerochaete chrysosporium to produce ethanol from rice straw. Biotechnol. Bioeng.
104, 471e482.
87
Baker, J.O., McCarley, J.R., Lovett, R., Yu, C.H., Adney, W.S.,
Rignall, T.R., Vinzant, T.B., Decker, S.R., Sakon, J., Himmel, M.E.,
2005. Catalytically enhanced endocellulase Cel5A from Acidothermus cellulolyticus. Appl. Biochem. Biotechnol. 121-124, 129e148.
Baldrian, P., Valaskova, V., 2008. Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol. Rev. 32, 501e521.
Ballesteros, I., Negro, M.J., Oliva, J.M., Cabanas, A., Manzanares, P.,
Ballesteros, M., 2006. Ethanol production from steam-explosion
pretreated wheat straw. Appl. Biochem. Biotechnol. 129-132,
496e508.
Banat, I.M., Nigam, P., Marchant, R., 1992. Isolation of thermotolerant,
fermentative yeasts growing at 52 C and producing ethanol at
45 C and 50 C. World J. Microbiol. Biotechnol. 8, 259e263.
Barnard, D., Casanueva, A., Tuffin, M., Cowan, D., 2010. Extremophiles in biofuel synthesis. Environ. Technol. 31, 871e888.
Bayer, E.A., Belaich, J.P., Shoham, Y., Lamed, R., 2004. The cellulosomes: multienzyme machines for degradation of plant cell wall
polysaccharides. Annu. Rev. Microbiol. 58, 521e554.
Bayer, E.A., Shimon, L.J., Shoham, Y., Lamed, R., 1998. Cellulosomes structure and ultrastructure. J. Struct. Biol. 124, 221e234.
Bennet, J.W., Wunch, K.G., Faison, B.D., 2002. Use of fungi in
biodegradation. In: Hurst, C.J. (Ed.), Manual of Environmental
Microbiology. ASM Press, Washington, D.C.
Blanchette, R.A., 1991. Delignification by wood-decay fungi. Annu.
Rev. Phytopathol. 29, 381e398.
Blanchette, R.A., 1995. Degradation of the lignocellulose complex in
wood. Can. J. Bot. 73 (suppl. 1), 999e1010.
Blanchette, R.A., 2000. A review of microbial deterioration found in
archaeological wood from different environments. Int. Biodeterior.
Biodegrad. 46, 189e204.
Blanchette, R.A., Burnes, T.A., Erdmans, M.M., Akhtar, M., 1992.
Evaluating isolates of Phanerochaete chrysosporium and Ceriporiopsis
subvermispora for use in biological pulping processes. Holzforschung 46, 109e115.
Blanchette, R.A., Held, B.W., Farrell, R.L., 2002. Defibration of wood in
the expedition huts of Antarctica: an unusual deterioration process
occurring in the polar environment. Polar Rec. 38, 313e322.
Blanchette, R.A., Held, B.W., Jurgens, J.A., McNew, D.L.,
Harrington, T.C., Duncan, S.M., Farrell, R.L., 2004. Wooddestroying soft rot fungi in the historic expedition huts of
Antarctica. Appl. Environ. Microbiol. 70, 1328e1335.
Borges, E.R., Pereira Jr., N., 2011. Succinic acid production from sugarcane bagasse hemicellulose hydrolysate by Actinobacillus succinogenes. J. Ind. Microbiol. Biotechnol. 38, 1001e1011.
Bornscheuer, U.T., Pohl, M., 2001. Improved biocatalysts by directed
evolution and rational protein design. Curr. Opin. Chem. Biol. 5,
137e143.
Cameron, D.C., Tong, I.T., 1993. Cellular and metabolic engineering.
An overview. Appl. Biochem. Biotechnol. 38, 105e140.
Canam, T., Town, J.R., Tsang, A., McAllister, T.A., Dumonceaux, T.J.,
2011. Biological pretreatment with a cellobiose dehydrogenasedeficient strain of Trametes versicolor enhances the biofuel potential of canola straw. Bioresour. Technol. 102, 10020e10027.
Chandel, A.K., Singh, O.V., Narasu, M.L., Rao, L.V., 2011. Bioconversion of Saccharum spontaneum (wild sugarcane) hemicellulosic hydrolysate into ethanol by mono and co-cultures of Pichia stipitis
NCIM3498 and thermotolerant Saccharomyces cerevisiae-VS(3). New
Biotechnol. 28, 593e599.
Chandra, R.P., Bura, R., Mabee, W.E., Berlin, A., Pan, X., Saddler, J.N.,
2007. Substrate pretreatment: the key to effective enzymatic hydrolysis of lignocellulosics? Adv. Biochem. Eng. Biotechnol. 108,
67e93.
Chen, X.F., Huang, C., Xiong, L., Chen, X.D., Chen, Y., Ma, L.L., 2012a.
Oil production on wastewaters after butanol fermentation by
oleaginous yeast Trichosporon coremiiforme. Bioresour. Technol. 118,
594e597.
88
5. BIOFUELS AND BIOPRODUCTS PRODUCED THROUGH MICROBIAL CONVERSION OF BIOMASS
Chen, X.F., Huang, C., Xiong, L., Chen, X.D., Ma, L.L., 2012b. Microbial oil production from corncob acid hydrolysate by Trichosporon
cutaneum. Biotechnol. Lett. 34, 1025e1028.
Chen, Y., 2011. Development and application of co-culture for ethanol
production by co-fermentation of glucose and xylose: a systematic
review. J. Ind. Microbiol. Biotechnol. 38, 581e597.
Contreras, D., Rodriguez, J., Freer, J., Schwederski, B., Kaim, W., 2007.
Enhanced hydroxyl radical production by dihydroxybenzenedriven Fenton reactions: implications for wood biodegradation.
J. Biol. Inorg. Chem. 12, 1055e1061.
Couturier, M., Navarro, D., Olive, C., Chevret, D., Haon, M., Favel, A.,
Lesage-Meessen, L., Henrissat, B., Coutinho, P.M., Berrin, J.G.,
2012. Post-genomic analyses of fungal lignocellulosic biomass
degradation reveal the unexpected potential of the plant pathogen
Ustilago maydis. BMC Genomics 13, 57.
Crawford, D.L., Barder, M.J., Pometto III, A.L., Crawford, R.L., 1982.
Chemistry of softwood lignin degradation by Streptomyces viridosporus. Arch. Microbiol. 131, 140e145.
Cui, Z.F., Wan, C.X., Sykes, R., Shi, J., Li, Y.B., 2012. Enzymatic
digestibility of corn stover fractions in response to fungal pretreatment. Ind. Eng. Chem. 51, 7153e7159.
Curling, S.F., Clausen, C.A., Winandy, J.E., 2002. Relationships between
mechanical properties, weight loss, and chemical composition of
wood during incipient brown-rot decay. For. Prod. J. 52, 34e39.
da Costa Sousa, L., Chundawat, S.P., Balan, V., Dale, B.E., 2009.
’Cradle-to-grave’ assessment of existing lignocellulose pretreatment technologies. Curr. Opin. Biotechnol. 20, 339e347.
Daniel, G., Nilsson, T., Singh, A.P., 1987. Degradation of lignocellulosics by unique tunnel-forming bacteria. Can. J. Microbiol. 33,
943e948.
Demain, A.L., 2009. Biosolutions to the energy problem. J. Ind.
Microbiol. Biotechnol. 36, 319e332.
Demain, A.L., Newcomb, M., Wu, J.H., 2005. Cellulase, clostridia, and
ethanol. Microbiol. Mol. Biol. Rev. 69, 124e154.
Deng, Y., Fong, S.S., 2011. Metabolic engineering of Thermobifida fusca
for direct aerobic bioconversion of untreated lignocellulosic
biomass to 1-propanol. Metab. Eng. 13, 570e577.
Ding, M.-Z., Wang, X., Yang, Y., Yuan, Y.-J., 2012. Comparative metabolic profiling of parental and inhibitors-tolerant yeasts during
lignocellulosic ethanol fermentation. Metabolomics 8, 232e243.
Doi, R.H., Kosugi, A., 2004. Cellulosomes: plant-cell-wall-degrading
enzyme complexes. Nat. Rev. Microbiol. 2, 541e551.
Doran-Peterson, J., Cook, D.M., Brandon, S.K., 2008. Microbial conversion of sugars from plant biomass to lactic acid or ethanol. Plant
J. 54, 582e592.
Duff, S.J.B., Murray, W.D., 1996. Bioconversion of forest products industry waste cellulosics to fuel ethanol: a review. Bioresour.
Technol. 55, 1e33.
Durand, H., Tiraby, G., 1988. Genetic improvement of Trichoderma
reesei for large scale cellulase production. Enzyme Microb. Technol.
10, 341e346.
Eriksson, K.E., Grunewald, A., Nilsson, T., Vallander, L., 1980. A
scanning electron microscopy study of the growth and attack on
wood by three white-rot fungi and their cellulaseless mutants.
Holzforschung 34, 207e213.
Eriksson, K.E.L., Blanchette, R.A., Ander, P., 1990. Microbial and
Enzymatic Degradation of Wood and Wood Components.
Springer-Verlag, Berlin, Germany.
Escovar-Kousen, J.M., Wilson, D., Irwin, D., 2004. Integration of
computer modeling and initial studies of site-directed mutagenesis
to improve cellulase activity on Cel9A from Thermobifida fusca.
Appl. Biochem. Biotechnol. 113-116, 287e297.
Esterbauer, H., Steiner, W., Labudova, I., Hermann, A., Hayn, M., 1991.
Production of Trichoderma cellulase in laboratory and pilot scale.
Bioresour. Technol. 36, 51e65.
Ewen, R.J., Jones, P.R., Ratcliffe, N.M., Spencer-Phillips, P.T., 2004.
Identification by gas chromatography-mass spectrometry of the
volatile organic compounds emitted from the wood-rotting fungi
Serpula lacrymans and Coniophora puteana, and from Pinus sylvestris
timber. Mycol. Res. 108, 806e814.
Falls, M., Shi, J., Ebrik, M.A., Redmond, T., Yang, B., Wyman, C.E.,
Garlock, R., Balan, V., Dale, B.E., Pallapolu, V.R., et al., 2011.
Investigation of enzyme formulation on pretreated switchgrass.
Bioresour. Technol. 102, 11072e11079.
Fan, Z., Wu, W., Hildebrand, A., Kasuga, T., Zhang, R., Xiong, X., 2012.
A novel biochemical route for fuels and chemicals production from
cellulosic biomass. PLoS One 7, e31693.
Fernandes, T.V., Bos, G.J., Zeeman, G., Sanders, J.P., van Lier, J.B., 2009.
Effects of thermo-chemical pre-treatment on anaerobic biodegradability and hydrolysis of lignocellulosic biomass. Bioresour.
Technol. 100, 2575e2579.
Findlay, W.P.K., 1984. Soft rot of timberda review. J. Indian Acad.
Wood Sci. 15, 1e11.
Flournoy, D.S., Kirk, T.K., Highley, T.L., 1991. Wood decay by brownrot fungi: changes in pore structure and cell wall volume. Holzforschung 45, 383e388.
Fontes, C.M., Gilbert, H.J., 2010. Cellulosomes: highly efficient nanomachines designed to deconstruct plant cell wall complex carbohydrates. Annu. Rev. Biochem. 79, 655e681.
Glenn, J.K., Gold, M.H., 1983. Decolorization of several polymeric
dyes by the lignin-degrading basidiomycete Phanerochaete chrysosporium. Appl. Environ. Microbiol. 45, 1741e1747.
Gong, Z., Wang, Q., Shen, H., Hu, C., Jin, G., Zhao, Z.K., 2012.
Co-fermentation of cellobiose and xylose by Lipomyces starkeyi for
lipid production. Bioresour. Technol. 117, 20e24.
Gonzalez-Fernandez, C., Sialve, B., Bernet, N., Steyer, J.P., 2012. Comparison of ultrasound and thermal pretreatment of Scenedesmus
biomass on methane production. Bioresour. Technol. 110, 610e616.
Green, F., Highley, T.L., 1997. Mechanism of brown-rot decay: paradigm or paradox. Int. Biodeterior. Biodegrad. 39, 113e124.
Grous, W.R., Convers, A.O., Grethlein, H.E., 1986. Effect of steam
explosion pretreatment on pore size and enzymatic hydrolysis of
poplar. Enzyme Microb. Technol. 8, 274e280.
Gupta, R., Mehta, G., Khasa, Y.P., Kuhad, R.C., 2011. Fungal delignification of lignocellulosic biomass improves the saccharification of
cellulosics. Biodegradation 22, 797e804.
Hahn-Hagerdal, B., Karhumaa, K., Fonseca, C., Spencer-Martins, I.,
Gorwa-Grauslund, M.F., 2007. Towards industrial pentosefermenting yeast strains. Appl. Microbiol. Biotechnol. 74, 937e953.
Hamelinck, C.N., Van Hooijdonk, G., Faaij, A.P.C., 2005. Ethanol from
lignocellulosic biomass: techno-economic performance in short-,
middle- and long-term. Biomass Bioenergy 28, 384e410.
Hammel, K.E., Kapich, A.N., Jensen, K.A., Ryan, Z.C., 2002. Reactive
oxygen species as agents of wood decay by fungi. Enzyme Microb.
Tech. 30, 445e453.
Hasunuma, T., Kondo, A., 2012a. Consolidated bioprocessing and
simultaneous saccharification and fermentation of lignocellulose to
ethanol with thermotolerant yeast strains. Process Biochem. 47,
1287e1294.
Hasunuma, T., Kondo, A., 2012b. Development of yeast cell factories
for consolidated bioprocessing of lignocellulose to bioethanol
through cell surface engineering. Biotechnol. Adv. 30, 1207e1218.
Hatakka, A., 1994. Lignin-modifying enzymes from selected white-rot
fungi - production and role in lignin degradation. FEMS Microbiol.
Rev. 13, 125e135.
Hatakka, A.I., Usi-Rauva, A.K., 1983. Degradation of 14C-labelled
poplar wood lignin by selected white-rot fungi. Eur. J. Appl.
Microbiol. Biotechnol. 17, 235e242.
Hawkins, G.M., Doran-Peterson, J., 2011. A strain of Saccharomyces
cerevisiae evolved for fermentation of lignocellulosic biomass
REFERENCES
displays improved growth and fermentative ability in high solids
concentrations and in the presence of inhibitory compounds. Biotechnol. Biofuels 4, 49.
He, J., Mao, X., Sun, Z., Zheng, P., Ni, Y., Xu, Y., 2007. Microbial
synthesis of ethyl (R)-4,4,4-trifluoro-3-hydroxybutanoate by
asymmetric reduction of ethyl 4,4,4-trifluoroacetoacetate in an
aqueous-organic solvent biphasic system. Biotechnol. J. 2, 260e265.
Heer, D., Sauer, U., 2008. Identification of furfural as a key toxin in
lignocellulosic hydrolysates and evolution of a tolerant yeast
strain. Microb. Biotechnol. 1, 497e506.
Hendriks, A.T., Zeeman, G., 2009. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 100,
10e18.
Higashide, W., Li, Y., Yang, Y., Liao, J.C., 2011. Metabolic engineering
of Clostridium cellulolyticum for production of isobutanol from
cellulose. Appl. Environ. Microbiol. 77, 2727e2733.
Highley, T., 1987. Changes in chemical components of hardwood and
softwood by brown-rot fungi. Mater. Org. 21, 39e45.
Howell, C., Steenkjær Hastrup, A.C., Goodell, B., Jellison, J., 2009.
Temporal changes in wood crystalline cellulose during degradation by brown rot fungi. Int. Biodeterior. Biodegrad. 63, 414e419.
Hsu, T.-A., 1996. Pretreatment of biomass. In: Wyman, C.E. (Ed.),
Handbook on Bioethanol, Production and Utilization. Taylor and
Francis, USA.
Hunt, C., Kenealy, W., Horn, E., Houtman, C., 2004. A biopulping
mechanism: creation of acid groups on fiber. Holzforschung 58,
434e439.
Irbe, I., Andersone, I., Andersons, B., Noldt, G., Dizhbite, T.,
Kurnosova, N., Nuopponen, M., Stewart, D., 2011. Characterisation
of the initial degradation stage of Scots pine (Pinus sylvestris L.)
sapwood after attack by brown-rot fungus Coniophora puteana.
Biodegradation 22, 719e728.
Jarboe, L.R., Grabar, T.B., Yomano, L.P., Shanmugan, K.T.,
Ingram, L.O., 2007. Development of ethanologenic bacteria. Adv.
Biochem. Eng. Biotechnol. 108, 237e261.
Jeffries, T.W., 1983. Utilization of xylose by bacteria, yeasts, and fungi.
Adv. Biochem. Eng. Biotechnol. 27, 1e32.
Jeffries, T.W., Grigoriev, I.V., Grimwood, J., Laplaza, J.M., Aerts, A.,
Salamov, A., Schmutz, J., Lindquist, E., Dehal, P., Shapiro, H., et al.,
2007. Genome sequence of the lignocellulose-bioconverting and
xylose-fermenting yeast Pichia stipitis. Nat. Biotechnol. 25,
319e326.
Jensen Jr., K.A., Houtman, C.J., Ryan, Z.C., Hammel, K.E., 2001.
Pathways for extracellular Fenton chemistry in the brown rot
basidiomycete Gloeophyllum trabeum. Appl. Environ. Microbiol. 67,
2705e2711.
Jiang, Y., Marang, L., Kleerebezem, R., Muyzer, G., van
Loosdrecht, M.C., 2011. Polyhydroxybutyrate production from
lactate using a mixed microbial culture. Biotechnol. Bioeng. 108,
2022e2035.
Jiang, Y., Xu, C., Dong, F., Yang, Y., Jiang, W., Yang, S., 2009. Disruption
of the acetoacetate decarboxylase gene in solvent-producing Clostridium acetobutylicum increases the butanol ratio. Metab. Eng. 11,
284e291.
Jin, M., Balan, V., Gunawan, C., Dale, B.E., 2011a. Consolidated bioprocessing (CBP) performance of Clostridium phytofermentans on
AFEX-treated corn stover for ethanol production. Biotechnol. Bioeng. 108, 1290e1297.
Jin, P., Li, S., Lu, S.G., Zhu, J.G., Huang, H., 2011b. Improved
1,3-propanediol production with hemicellulosic hydrolysates (corn
straw) as cosubstrate: impact of degradation products on Klebsiella
pneumoniae growth and 1,3-propanediol fermentation. Bioresour.
Technol. 102, 1815e1821.
Jin, M., Gunawan, C., Balan, V., Dale, B.E., 2012. Consolidated bioprocessing (CBP) of AFEX-pretreated corn stover for ethanol
89
production using Clostridium phytofermentans at a high solids
loading. Biotechnol. Bioeng. 109, 1929e1936.
Jones, D.T., Shirley, M., Wu, X., Keis, S., 2000. Bacteriophage infections
in the industrial acetone butanol (AB) fermentation process. J. Mol.
Microbiol. Biotechnol. 2, 21e26.
Jørgensen, H., Kristensen, J.B., Felby, C., 2007. Enzymatic conversion
of lignocellulose into fermentable sugars: challenges and opportunities. Biofuels Bioprod. Bioref 1, 119e134.
Jung, H.G., Valdez, F.R., Abad, A.R., Blanchette, R.A., Hatfield, R.D.,
1992. Effect of white rot basidiomycetes on chemical composition
and in vitro digestibility of oat straw and alfalfa stems. J. Anim.
Sci. 70, 1928e1935.
Juodeikiene, G., Basinskiene, L., Vidmantiene, D., Makaravicius, T.,
Bartkiene, E., 2012. Benefits of beta-xylanase for wheat biomass
conversion to bioethanol. J. Sci. Food Agric. 92, 84e91.
Kaneko, S., Yoshitake, K., Itakura, S., Tanaka, H., Enoki, A., 2005.
Relationship between production of hydroxyl radicals and degradation of wood, crystalline cellulose, and a lignin-related compound or accumulation of oxalic acid in cultures of brown-rot
fungi. J. Wood Sci. 51, 262e269.
Karunanithy, C., Muthukumarappan, K., 2010a. Effect of extruder
parameters and moisture content of switchgrass, prairie cord grass
on sugar recovery from enzymatic hydrolysis. Appl. Biochem.
Biotechnol. 162, 1785e1803.
Karunanithy, C., Muthukumarappan, K., 2010b. Influence of extruder
temperature and screw speed on pretreatment of corn stover while
varying enzymes and their ratios. Appl. Biochem. Biotechnol. 162,
264e279.
Karunanithy, C., Muthukumarappan, K., Gibbons, W.R., 2012. Extrusion pretreatment of pine wood chips. Appl. Biochem. Biotechnol.
167, 81e99.
Kayser, A., Weber, J., Hecht, V., Rinas, U., 2005. Metabolic flux analysis
of Escherichia coli in glucose-limited continuous culture. I. Growthrate-dependent metabolic efficiency at steady state. Microbiology
151, 693e706.
Keller, F.A., Hamilton, J.E., Nguyen, Q.A., 2003. Microbial pretreatment
of biomass: potential for reducing severity of thermochemical
biomass pretreatment. Appl. Biochem. Biotechnol. 105e108, 27e41.
Kim, Y., Hendrickson, R., Mosier, N.S., Ladisch, M.R., 2009. Liquid hot
water pretreatment of cellulosic biomass. Methods Mol. Biol. 581,
93e102.
Kirk, T., Tien, M., Johnrud, S.C., Eriksson, K.E., 1986. Lignin-degrading activity of Phanerochaete chrysosporium Burds: comparison of
cellulase-negative and other strains. Enzyme Microb. Technol. 8,
75e80.
Kirk, T.K., Moore, W.E., 1972. Removing lignin from wood with whiterot fungi and digestibility from resulting wood. Wood Fiber 4,
72e79.
Knoshaug, E.P., Zhang, M., 2009. Butanol tolerance in a selection of
microorganisms. Appl. Biochem. Biotechnol. 153, 13e20.
Kosa, M., Ragauskas, A.J., 2012. Bioconversion of lignin model compounds with oleaginous Rhodococci. Appl. Microbiol. Biotechnol.
93, 891e900.
Kramer, C., Kreisel, G., Fahr, K., Kassbohrer, J., Schlosser, D., 2004.
Degradation of 2-fluorophenol by the brown-rot fungus Gloeophyllum striatum: evidence for the involvement of extracellular
Fenton chemistry. Appl. Microbiol. Biotechnol. 64, 387e395.
Kuhar, S., Nair, L.M., Kuhad, R.C., 2008. Pretreatment of lignocellulosic material with fungi capable of higher lignin degradation and
lower carbohydrate degradation improves substrate acid hydrolysis and the eventual conversion to ethanol. Can. J. Microbiol. 54,
305e313.
Kumar, P., Barrett, D.M., Delwiche, M.J., Stroeve, P., 2009. Methods for
pretreatment of lignocellulosic biomass for efficient hydrolysis and
biofuel production. Ind. Eng. Chem. Res. 48, 3713e3729.
90
5. BIOFUELS AND BIOPRODUCTS PRODUCED THROUGH MICROBIAL CONVERSION OF BIOMASS
Kumar, R., Wyman, C.E., 2009. Effects of cellulase and xylanase
enzymes on the deconstruction of solids from pretreatment of
poplar by leading technologies. Biotechnol. Prog. 25, 302e314.
Kwon, Y.J., Wang, F., Liu, C.Z., 2011. Deep-bed solid state fermentation
of sweet sorghum stalk to ethanol by thermotolerant Issatchenkia
orientalis IPE 100. Bioresour. Technol. 102, 11262e11265.
Lamed, R., Setter, E., Bayer, E.A., 1983. Characterization of a cellulosebinding, cellulase-containing complex in Clostridium thermocellum.
J. Bacteriol. 156, 828e836.
Larsson, S., Reimann, A., Nilvebrant, N.-O., Jönsson, L.J., 1999.
Comparison of different methods for the detoxification of lignocellulose hydrolyzates of spruce. Appl. Biochem. Biotechnol. 77,
91e103.
Lee, J.W., Gwak, K.S., Park, J.Y., Park, M.J., Choi, D.H., Kwon, M.,
Choi, I.G., 2007. Biological pretreatment of softwood Pinus densiflora by three white rot fungi. J. Microbiol. 45, 485e491.
Lee, S.K., Chou, H., Ham, T.S., Lee, T.S., Keasling, J.D., 2008a. Metabolic engineering of microorganisms for biofuels production: from
bugs to synthetic biology to fuels. Curr. Opin. Biotechnol. 19,
556e563.
Lee, S.Y., Park, J.H., Jang, S.H., Nielsen, L.K., Kim, J., Jung, K.S., 2008b.
Fermentative butanol production by Clostridia. Biotechnol. Bioeng.
101, 209e228.
Liang, Y.S., Yuan, X.Z., Zeng, G.M., Hu, C.L., Zhong, H., Huang, D.L.,
Tang, L., Zhao, J.J., 2010. Biodelignification of rice straw by Phanerochaete chrysosporium in the presence of dirhamnolipid.
Biodegradation 21, 615e624.
Lin, C.L., Tang, Y.L., Lin, S.M., 2011. Efficient bioconversion of compactin to pravastatin by the quinoline-degrading microorganism
Pseudonocardia
carboxydivorans
isolated from
petroleumcontaminated soil. Bioresour. Technol. 102, 10187e10193.
Lin, H., Cheng, W., Ding, H.T., Chen, X.J., Zhou, Q.F., Zhao, Y.H., 2010.
Direct microbial conversion of wheat straw into lipid by a cellulolytic fungus of Aspergillus oryzae A-4 in solid-state fermentation.
Bioresour. Technol. 101, 7556e7562.
Liu, S., Bischoff, K.M., Hughes, S.R., Leathers, T.D., Price, N.P.,
Qureshi, N., Rich, J.O., 2009. Conversion of biomass hydrolysates
and other substrates to ethanol and other chemicals by Lactobacillus
buchneri. Lett. Appl. Microbiol. 48, 337e342.
Liu, Y., Koh, C.M., Ji, L., 2011. Bioconversion of crude glycerol to
glycolipids in Ustilago maydis. Bioresour. Technol. 102, 3927e3933.
Liu, Z.L., 2006. Genomic adaptation of ethanologenic yeast to biomass
conversion inhibitors. Appl. Microbiol. Biotechnol. 73, 27e36.
Loguercio-Leite, C., Michels, J., Baltazar, J.M., 2008. New records of
lignocellulolytic basidiomycetes (Fungi): Parque Estadual da Serra
do Tabuleiro (P.E.S.T.), Santa Catarina, Brazil. Biotemas 21, 7e14.
Lynd, L., Wyman, C.E., Gerngross, T.U., 1999. Biocommodity engineering. Biotechnol. Prog. 15, 777e793.
Lynd, L.R., van Zyl, W.H., McBride, J.E., Laser, M., 2005. Consolidated
bioprocessing of cellulosic biomass: an update. Curr. Opin. Biotechnol. 16, 577e583.
Lynd, L.R., Weimer, P.J., van Zyl, W.H., Pretorius, I.S., 2002. Microbial
cellulose utilization: fundamentals and biotechnology. Microbiol.
Mol. Biol. Rev. 66, 506e577.
Lyr, H., 1960. Formation of ectoenzymes by wood-rotting and woodinhabiting fungi on different culture media. Part V. A complex
medium as carbon source. Arch. Microbiol. 35, 258e278.
Ma, H., Liu, W.W., Chen, X., Wu, Y.J., Yu, Z.L., 2009. Enhanced
enzymatic saccharification of rice straw by microwave pretreatment. Bioresour. Technol. 100, 1279e1284.
Madhavan, A., Srivastava, A., Kondo, A., Bisaria, V.S., 2012. Bioconversion of lignocellulose-derived sugars to ethanol by engineered
Saccharomyces cerevisiae. Crit. Rev. Biotechnol. 32, 22e48.
Madzak, C., Mimmi, M.C., Caminade, E., Brault, A., Baumberger, S.,
Briozzo, P., Mougin, C., Jolivalt, C., 2006. Shifting the optimal pH
of activity for a laccase from the fungus Trametes versicolor by
structure-based mutagenesis. Protein Eng. Des. Sel. 19, 77e84.
Mahalaxmi, S., Jackson, C., Williford, C., Burandt, C.L., 2010. Estimation of treatment time for microbial preprocessing of biomass.
Appl. Biochem. Biotechnol. 162, 1414e1422.
Maki, M., Leung, K.T., Qin, W., 2009. The prospects of cellulaseproducing bacteria for the bioconversion of lignocellulosic
biomass. Int. J. Biol. Sci. 5, 500e516.
Martinez, D., Challacombe, J., Morgenstern, I., Hibbett, D.,
Schmoll, M., Kubicek, C.P., Ferreira, P., Ruiz-Duenas, F.J.,
Martinez, A.T., Kersten, P., et al., 2009. Genome, transcriptome, and
secretome analysis of wood decay fungus Postia placenta supports
unique mechanisms of lignocellulose conversion. Proc. Natl. Acad.
Sci. USA 106, 1954e1959.
McMillan, J.D., 1994. Pretreatment of lignocellulosic biomass. In:
Himmel, J.O.B.M.E., Overend, R.P. (Eds.), Enzymatic Conversion
of Biomass for Fuels Production. ACS, Washington, USA,
pp. 292e324.
McMillan, J.D., 1997. Bioethanol production: status and prospects.
Renew. Energ 10, 295e302.
McMillan, J.D., Newman, M.M., Templeton, D.W., Mohagheghi, A.,
Hatakka, 1999. Simultaneous saccharification and cofermentation
of dilute-acid pretreated yellow poplar hardwood to ethanol using
xylose-fermenting Zymomonas mobilis. Appl. Biochem. Biotechnol.
77-79, 649e665.
Mistry, F.R., Cooney, C.L., 1989. Production of ethanol by Clostridium
thermosaccharolyticum: II. A quantitative model describing product
distributions. Biotechnol. Bioeng. 34, 1305e1320.
Monrroy, M., Ortega, I., Ramirez, M., Baeza, J., Freer, J., 2011. Structural change in wood by brown rot fungi and effect on enzymatic
hydrolysis. Enzyme Microb. Technol. 49, 472e477.
Moon, T.S., Yoon, S.H., Lanza, A.M., Roy-Mayhew, J.D., Prather, K.L.,
2009. Production of glucaric acid from a synthetic pathway in recombinant Escherichia coli. Appl. Environ. Microbiol. 75, 589e595.
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y.Y., Holtzapple, M.,
Ladisch, M., 2005. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 96,
673e686.
Muller, H.W., Trosch, W., 1986. Screening of white-rot fungi for biological pretreatment of wheat straw for biogas production. Appl.
Microbiol. Biotechnol. 24, 180e185.
Ng, T.K., Ben-Bassat, A., Zeikus, J.G., 1981. Ethanol production by
thermophilic bacteria: fermentation of cellulosic substrates by
cocultures of Clostridium thermocellum and Clostridium thermohydrosulfuricum. Appl. Environ. Microbiol. 41, 1337e1343.
Nilsson, T., Daniel, G.F., Kirk, T.K., Obst, J.R., 1989. Chemistry and
microscopy of wood decay by some higher ascomycetes. Holzforschung 43, 11e18.
Nilsson, T., Singh, A.P., Daniel, G.F., 1992. Ultrastructure of the attack
of Eusideroxylon zwageri wood by tunnelling bacteria. Holzforschung 46, 361e367.
Nimura, Y., Tsujiyama, S., Ueno, M., 2010. Bioconversion of cinnamic
acid derivatives by Schizophyllum commune. J. Gen. Appl. Microbiol. 56, 381e387.
Ohgren, K., Bura, R., Saddler, J., Zacchi, G., 2007. Effect of hemicellulose and lignin removal on enzymatic hydrolysis of steam pretreated corn stover. Bioresour. Technol. 98, 2503e2510.
Ohta, K., Beall, D.S., Mejia, J.P., Shanmugam, K.T., Ingram, L.O., 1991.
Genetic improvement of Escherichia coli for ethanol production:
chromosomal integration of Zymomonas mobilis genes encoding
pyruvate decarboxylase and alcohol dehydrogenase II. Appl.
Environ. Microbiol. 57, 893e900.
Okano, K., Kitagawa, M., Sasaki, Y., Watanabe, T., 2005. Conversion of
Japanese red cedar (Cryptomeria japonica) into a feed for ruminants
by white-rot basidiomycetes. Anim. Feed Sci. Technol. 120, 235e243.
REFERENCES
Olofsson, K., Bertilsson, M., Liden, G., 2008. A short review on SSF - an
interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol. Biofuels 1, 7.
Ooshima, H., Aso, K., Harano, Y., Yamamoto., T., 1984. Microwave
treatment of cellulosic materials for their enzymatic-hydrolysis.
Biotechnol. Lett. 6, 289e294.
Ou, M.S., Mohammed, N., Ingram, L.O., Shanmugam, K.T., 2009.
Thermophilic Bacillus coagulans requires less cellulases for simultaneous saccharification and fermentation of cellulose to products
than mesophilic microbial biocatalysts. Appl. Biochem. Biotechnol.
155, 379e385.
Ozkan, P., Sariyar, B., Utkur, F.O., Akman, U., Hortacsu, A., 2005.
Metabolic flux analysis of recombinant protein overproduction in
Escherichia coli. Biochem. Eng. J. 22, 167e195.
Palmqvist, E., Hahn-Hägerdal, B., 2000. Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition.
Bioresour. Technol. 74, 25e33.
Parekh, S., Wayman, M., 1986. Fermentation of cellobiose and wood
sugars to ethanol by Candida shehatae and Pichia stipitis. Biotechnol.
Lett. 8, 597e600.
Patil, K.R., Akesson, M., Nielsen, J., 2004. Use of genome-scale microbial
models for metabolic engineering. Curr. Opin. Biotechnol. 15, 64e69.
Percival Zhang, Y.H., Himmel, M.E., Mielenz, J.R., 2006. Outlook for
cellulase improvement: screening and selection strategies. Biotechnol. Adv. 24, 452e481.
Pinto, P.A., Dias, A.A., Fraga, I., Marques, G., Rodrigues, M.A.,
Colaco, J., Sampaio, A., Bezerra, R.M., 2012. Influence of ligninolytic enzymes on straw saccharification during fungal pretreatment. Bioresour. Technol. 111, 261e267.
Piskur, B., Bajc, M., Robek, R., Humar, M., Sinjur, I., Kadunc, A.,
Oven, P., Rep, G., Al Sayegh Petkovsek, S., Kraigher, H., et al., 2011.
Influence of Pleurotus ostreatus inoculation on wood degradation
and fungal colonization. Bioresour. Technol. 102, 10611e10617.
Playne, M.J., 1984. Increased digestibility of bagasses by pretreatment
with alkalis and steam explosion. Biotechnol. Bioeng. 26, 426e433.
Prakash, G., Varma, A.J., Prabhune, A., Shouche, Y., Rao, M., 2011.
Microbial production of xylitol from D-xylose and sugarcane
bagasse hemicellulose using newly isolated thermotolerant yeast
Debaryomyces hansenii. Bioresour. Technol. 102, 3304e3308.
Qi, W., Chen, C.L., Wang, J.Y., 2011. Reducing sugar-producing bacteria
from guts of Tenebrio molitor Linnaeus (yellow mealworm) for
lignocellulosic waste minimization. Microbes Environ. 26, 354e359.
Rabinovich, M.L., Bolobova, A.V., Vasil’chenko, L.G., 2004. Fungal
decomposition of natural aromatic structures and xenobiotics: a
review. Appl. Biochem. Microbiol. 40, 1e17.
Ramachandriya, K.D., Wilkins, M.R., Delorme, M.J., Zhu, X.,
Kundiyana, D.K., Atiyeh, H.K., Huhnke, R.L., 2011. Reduction of
acetone to isopropanol using producer gas fermenting microbes.
Biotechnol. Bioeng. 108, 2330e2338.
Raman, B., McKeown, C.K., Rodriguez, M., Brown Jr., S.D.,
Mielenz, J.R., 2011. Transcriptomic analysis of Clostridium thermocellum ATCC 27405 cellulose fermentation. BMC Microbiol. 11, 134.
Rao, D., Gullapalli, P., Yoshihara, A., Jenkinson, S.F., Morimoto, K.,
Takata, G., Akimitsu, K., Tajima, S., Fleet, G.W., Izumori, K., 2008.
Direct production of L-tagatose from L-psicose by Enterobacter aerogenes 230S. J. Biosci. Bioeng. 106, 473e480.
Ray, M.J., Leak, D.J., Spanu, P.D., Murphy, R.J., 2010. Brown rot fungal
early stage decay mechanism as a biological pretreatment for softwood
biomass in biofuel production. Biomass Bioenergy 34, 1257e1262.
Richards, D.B., 1954. Physical changes in decaying wood. J. For. 52,
260e265.
Richter, H., Qureshi, N., Heger, S., Dien, B., Cotta, M.A., Angenent, L.T.,
2012. Prolonged conversion of n-butyrate to n-butanol with Clostridium saccharoperbutylacetonicum in a two-stage continuous culture
with in-situ product removal. Biotechnol. Bioeng. 109, 913e921.
91
Roberts, S.B., Gowen, C.M., Brooks, J.P., Fong, S.S., 2010. Genome-scale
metabolic analysis of Clostridium thermocellum for bioethanol production. BMC Syst. Biol. 4, 31.
Rogers, C.J., Blanford, C.F., Giddens, S.R., Skamnioti, P.,
Armstrong, F.A., Gurr, S.J., 2009. Designer laccases: a vogue for
high-potential fungal enzymes? Trends Biotechnol. 28, 63e72.
Rogers, P.L., Lee, K.J., Skotnicki, M.L., Tribe, D.E., 1982. Ethanol production by Zymomonas mobilis. Adv. Biochem. Eng. 23, 37e84.
Ruel, K., Barnoud, F., Eriksson, K.E., 1981. Micromorphological and
ultrastructural aspects of spruce wood degradation by wild-type
Sporotrichum pulverulentum and its cellulase-less mutant Ce144.
Holzforschung 35, 157e171.
Rumbold, K., van Buijsen, H.J., Gray, V.M., van Groenestijn, J.W.,
Overkamp, K.M., Slomp, R.S., van der Werf, M.J., Punt, P.J., 2010.
Microbial renewable feedstock utilization: a substrate-oriented
approach. Bioeng. Bugs 1, 359e366.
Rumbold, K., van Buijsen, H.J., Overkamp, K.M., van
Groenestijn, J.W., Punt, P.J., van der Werf, M.J., 2009. Microbial
production host selection for converting second-generation feedstocks into bioproducts. Microb. Cell Fact 8, 64.
Saddler, J.N., Ramos, L.P., Breuil, C., 1993. Steam pretreatment of
lignocellulosic residues. In: Saddler, J.N. (Ed.), Bioconversion of
Forest and Agricultural Plant Wastes. C.A.B. International, Wallingford, UK, pp. 73e92.
Sakon, J., Adney, W.S., Himmel, M.E., Thomas, S.R., Karplus, P.A.,
1996. Crystal structure of thermostable family 5 endocellulase E1
from Acidothermus cellulolyticus in complex with cellotetraose.
Biochem. 35, 10648e10660.
Salgado, J.M., Rodriguez, N., Cortes, S., Dominguez, J.M., 2012. Effect
of nutrient supplementation of crude or detoxified concentrated
distilled grape marc hemicellulosic hydrolysates on the xylitol
production by Debaryomyces hansenii. Prep. Biochem. Biotechnol.
42, 1e14.
Salvachua, D., Prieto, A., Lopez-Abelairas, M., Lu-Chau, T.,
Martinez, A.T., Martinez, M.J., 2011. Fungal pretreatment: an
alternative in second-generation ethanol from wheat straw. Bioresour. Technol. 102, 7500e7506.
Sanchez, C., 2009. Lignocellulosic residues: biodegradation and
bioconversion by fungi. Biotechnol. Adv. 27, 185e194.
Saritha, M., Arora, A., Lata, 2012a. Biological pretreatment of lignocellulosic substrates for enhanced delignification and enzymatic
digestibility. Indian J. Microbiol. 52, 122e130.
Saritha, M., Arora, A., Nain, L., 2012b. Pretreatment of paddy straw
with Trametes hirsuta for improved enzymatic saccharification.
Bioresour. Technol. 104, 459e465.
Sasmal, S., Goud, V.V., Mohanty, K., 2012. Ultrasound assisted lime
pretreatment of lignocellulosic biomass toward bioethanol production. Energy Fuels 26, 3777e3784.
Sawada, T., Nakamura, Y., Kobayashi, F., Kuwahara, M., Watanabe, T.,
1995. Effects of fungal pretreatment and steam explosion pretreatment on enzymatic saccharification of plant biomass. Biotechnol. Bioeng. 48, 719e724.
Schilling, J.S., Ai, J., Blanchette, R.A., Duncan, S.M., Filley, T.R.,
Tschirner, U.W., 2012. Lignocellulose modifications by brown rot
fungi and their effects, as pretreatments, on cellulolysis. Bioresour.
Technol. 116, 147e154.
Schilling, J.S., Tewalt, J.P., Duncan, S.M., 2009. Synergy between pretreatment lignocellulose modifications and saccharification efficiency in two brown rot fungal systems. Appl. Microbiol.
Biotechnol. 84, 465e475.
Schulein, M., 2000. Protein engineering of cellulases. Biochem. Biophys. Acta. 1543, 239e252.
Schwarz, W.H., 2001. The cellulosome and cellulose degradation by
anaerobic bacteria. Appl. Microbiol. Biotechnol. 56, 634e649.
Sheridan, C., 2009. Making green. Nat. Biotechnol. 27, 1074e1076.
92
5. BIOFUELS AND BIOPRODUCTS PRODUCED THROUGH MICROBIAL CONVERSION OF BIOMASS
Shi, J., Chinn, M.S., Sharma-Shivappa, R., 2008. Microbial pretreatment of cotton stalks by solid state cultivation of Phanerochaete
chrysosporium for bioethanol production. Bioresour. Technol. 99,
556e6564.
Shin, H.D., McClendon, S., Vo, T., Chen, R.R., 2010. Escherichia coli
binary culture engineered for direct fermentation of hemicellulose
to a biofuel. Appl. Environ. Microbiol. 76, 8150e8159.
Shoham, Y., Lamed, R., Bayer, E.A., 1999. The cellulosome concept as
an efficient microbial strategy for the degradation of insoluble
polysaccharides. Trends Microbiol. 7, 275e281.
Shrivastava, B., Nandal, P., Sharma, A., Jain, K.K., Khasa, Y.P.,
Das, T.K., Mani, V., Kewalramani, N.J., Kundu, S.S., Kuhad, R.C.,
2012. Solid state bioconversion of wheat straw into digestible and
nutritive ruminant feed by Ganoderma sp. rckk02. Bioresour.
Technol. 107, 347e351.
Singh, A.P., Butcher, J.A., 1991. Bacterial degradation of wood cell
walls: a review of degradation patterns. J. Inst. Wood Sci. 12,
143e157.
Singh, A.P., Butcher, S.A., 1985. Degradation of CCA-treated Pinus
radiata posts by erosion bacteria. J. Inst. Wood Sci. 10, 140e144.
Singh, A.P., Nilsson, T., Daniel, G.F., 1990. Bacterial attack of Pinus
sylvestris wood under near-anaerobic conditions. J. Inst. Wood Sci.
11, 237e249.
Singh, P., Sulaiman, O., Hashim, R., Rupani, P.F., Peng, L.C., 2010.
Biopulping of lignocellulosic material using different fungal species: a review. Rev. Environ. Sci. Biotechnol. 9, 141e151.
Singh, P., Suman, A., Tiwari, P., Arya, N., Gaur, A., Shrivastava, A.K.,
2008. Biological pretreatment of sugarcane trash for its conversion
to fermentable sugars. World J. Microbiol. Biotechnol. 24, 667e673.
Steen, E.J., Kang, Y., Bokinsky, G., Hu, Z., Schirmer, A., McClure, A.,
Del Cardayre, S.B., Keasling, J.D., 2010. Microbial production of
fatty-acid-derived fuels and chemicals from plant biomass. Nature
463, 559e562.
Steenbakkers, P.J., Harhangi, H.R., Bosscher, M.W., van der
Hooft, M.M., Keltjens, J.T., van der Drift, C., Vogels, G.D., op den
Camp, H.J., 2003. Beta-Glucosidase in cellulosome of the anaerobic
fungus Piromyces sp. strain E2 is a family 3 glycoside hydrolase.
Biochem. J. 370, 963e970.
Stenberg, K., Tengborg, C., Galbe, M., Zacchi, G., 1998. Optimisation of
steam pretreatment of SO2-impregnated mixed softwoods for
ethanol production. J. Chem. Technol. Biotechnol. 71, 299e308.
Stephanopoulos, G., 1999. Metabolic fluxes and metabolic engineering.
Metab. Eng. 1, 1e11.
Strohl, W.R., 2001. Biochemical engineering of natural product
biosynthesis pathways. Metab. Eng. 3, 4e14.
Sun, F.-h., Jiang, L., Yue-xiang, Y., Zhi-ying, Y., Liu, X.-F., 2011. Effect of
biological pretreatment with Trametes hirsuta yj9 on enzymatic
hydrolysis of corn stover. Int. Biodeterior. Biodegrad. 65, 931e938.
Sun, Y., Cheng, J., 2002. Hydrolysis of lignocellulosic materials for
ethanol production: a review. Bioresour. Technol. 83, 1e11.
Surwase, S.N., Patil, S.A., Jadhav, S.B., Jadhav, J.P., 2012. Optimization
of L-DOPA production by Brevundimonas sp. SGJ using response
surface methodology. Microb. Biotechnol. 5, 731e737.
Suzuki, M.R., Hunt, C.G., Houtman, C.J., Dalebroux, Z.D.,
Hammel, K.E., 2006. Fungal hydroquinones contribute to brown
rot of wood. Environ. Microbiol. 8, 2214e2223.
Taherzadeh, M.J., Karimi, K., 2007. Enzymatic-based hydrolysis processes for ethanol from lignocellulosic materials: a review. Bioresources 2, 707e738.
Taherzadeh, M.J., Karimi, K., 2008. Pretreatment of lignocellulosic
wastes to improve ethanol and biogas production: a review. Int.
J. Mol. Sci. 9, 1621e1651.
Tammali, R., Seenayya, G., Reddy, G., 2003. Fermentation of cellulose
to acetic acid by Clostridium lentocellum SG6: induction of
sporulation and effect of buffering agent on acetic acid production.
Lett. Appl. Microbiol. 37, 304e308.
Taniguchi, M., Suzuki, H., Watanabe, D., Sakai, K., Hoshino, K.,
Tanaka, T., 2005. Evaluation of pretreatment with Pleurotus ostreatus for enzymatic hydrolysis of rice straw. J. Biosci. Bioeng. 100,
637e643.
Tassinari, T., Macy, C., Spano, L., 1980. Energy requirements and
process design considerations in compression-milling pretreatment of cellulosic wastes for enzymatic hydrolysis. Biotechnol.
Bioeng. 22, 1689e1705.
Taylor, M.P., Eley, K.L., Martin, S., Tuffin, M.I., Burton, S.G.,
Cowan, D.A., 2009. Thermophilic ethanologenesis: future prospects for second-generation bioethanol production. Trends Biotechnol. 27, 398e405.
Teixeira, L.C., Linden, J.C., Schroeder, H.A., 2000. Simultaneous
saccharification and cofermentation of peracetic acid-pretreated
biomass. Appl. Biochem. Biotechnol. 84-86, 111e127.
ten Have, R., Teunissen, P.J., 2001. Oxidative mechanisms involved in
lignin degradation by white-rot fungi. Chem. Rev. 101, 3397e3413.
Tewalt, J., Schilling, J., 2010. Assessment of saccharification efficacy in
the cellulase system of the brown rot fungus Gloeophyllum trabeum.
Appl. Microbiol. Biotechnol. 86, 1785e1793.
Teymouri, F., Laureano-Perez, L., Alizadeh, H., Dale, B.E., 2004.
Ammonia fiber explosion treatment of corn stover. Appl. Biochem.
Biotechnol. 113-116, 951e963.
Teymouri, F., Laureano-Perez, L., Alizadeh, H., Dale, B.E., 2005.
Optimization of the ammonia fiber explosion (AFEX) treatment
parameters for enzymatic hydrolysis of corn stover. Bioresour.
Technol. 96, 2014e2018.
Thiru, M., Sankh, S., Rangaswamy, V., 2011. Process for biodiesel
production from Cryptococcus curvatus. Bioresour. Technol. 102,
10436e10440.
Tomme, P., Van Tilbeurgh, H., Pettersson, G., Van Damme, J.,
Vandekerckhove, J., Knowles, J., Teeri, T., Claeyssens, M., 1988.
Studies of the cellulolytic system of Trichoderma reesei QM 9414.
Analysis of domain function in two cellobiohydrolases by limited
proteolysis. Eur. J. Biochem. 170, 575e581.
Tran, D.T., Yeh, K.L., Chen, C.L., Chang, J.S., 2012. Enzymatic transesterification of microalgal oil from Chlorella vulgaris ESP-31 for
biodiesel synthesis using immobilized Burkholderia lipase. Bioresour. Technol. 108, 119e127.
Trinh, C.T., Srienc, F., 2009. Metabolic engineering of Escherichia coli for
efficient conversion of glycerol to ethanol. Appl. Environ. Microbiol. 75, 6696e6705.
Vaidya, A., Singh, T., 2012. Pre-treatment of Pinus radiata substrates by
basidiomycetes fungi to enhance enzymatic hydrolysis. Biotechnol.
Lett. 34, 1263e1267.
van Zyl, W.H., den Haan, R., McBride, J.E., 2007. Consolidated bioprocessing for bioethanol production using Saccharomyces cerevisiae. Adv. Biochem. Eng. Biotechnol. 108, 205e235.
Verma, S., Ray, A.K., De, B.K., 2010. Bioconversion of heptanal to
heptanol by Saccharomyces cerevisiae. Yeast 27, 269e275.
Wan, C., Li, Y., 2010. Microbial pretreatment of corn stover with Ceriporiopsis subvermispora for enzymatic hydrolysis and ethanol
production. Bioresour. Technol. 101, 6398e6403.
Wan, C., Li, Y., 2011. Effectiveness of microbial pretreatment by Ceriporiopsis subvermispora on different biomass feedstocks. Bioresour.
Technol. 102, 7507e7512.
Wang, J., Sun, Q., Gao, P., Wang, J.F., Xu, C., Sun, Q.L., 2010. Bioconversion of glycyrrhizinic acid in liquorice into 18-beta-glycyrrhetinic acid by Aspergillus parasiticus speare BGB. Prikl. Biokhim.
Mikrobiol. 46, 462e466.
Wang, W., Yuan, T., Wang, K., Cui, B., Dai, Y., 2012. Combination of
biological pretreatment with liquid hot water pretreatment to
REFERENCES
enhance enzymatic hydrolysis of Populus tomentosa. Bioresour.
Technol. 107, 282e286.
Warnick, T.A., Methe, B.A., Leschine, S.B., 2002. Clostridium phytofermentans sp. nov., a cellulolytic mesophile from forest soil. Int.
J. Syst. Evol. Microbiol. 52, 1155e1160.
Wawrik, B., Mendivelso, M., Parisi, V.A., Suflita, J.M., Davidova, I.A.,
Marks, C.R., Van Nostrand, J.D., Liang, Y., Zhou, J., Huizinga, B.J.,
et al., 2012. Field and laboratory studies on the bioconversion of
coal to methane in the San Juan Basin. FEMS Microbiol. Ecol. 81,
26e42.
Weber, C., Farwick, A., Benisch, F., Brat, D., Dietz, H., Subtil, T.,
Boles, E., 2010. Trends and challenges in the microbial production
of lignocellulosic bioalcohol fuels. Appl. Microbiol. Biotechnol. 87,
1303e1315.
Wilcox, W.W., 1978. Review of literature on the effects of early stages
of decay on wood strength. Wood Fiber 9, 252e257.
Wilson, D.B., 2004. Studies of Thermobifida fusca plant cell wall
degrading enzymes. Chem. Rec. 4, 72e82.
Wither, S.G., 2001. Mechanism of glycosyl transferase and hydrolases.
Carbohydr. Polym. 44, 325e337.
Wood, T.M., Saddler, J.N., 1988. Increasing the availability of cellulose
in biomass materials. Methods Enzymol. 160, 3e11.
Wu, J., Zhang, X., Wan, J., Ma, F., Tang, Y., 2011. Production of fiberboard using corn stalk pretreated with white-rot fungus Trametes
hirsute by hot pressing without adhesive. Bioresour. Technol. 102,
11258e11261.
Wu, Z., Lee, Y.Y., 1998. Nonisothermal simultaneous saccharification
and fermentation for direct conversion of lignocellulosic biomass
to ethanol. Appl. Biochem. Biotechnol. 7072, 479e492.
Xu, Q., Singh, A., Himmel, M.E., 2009. Perspectives and new directions for the production of bioethanol using consolidated bioprocessing of lignocellulose. Curr. Opin. Biotechnol. 20, 364e371.
Yaghoubi, K., Pazouki, M., Shojaosadati, S.A., 2008. Variable optimization for biopulping of agricultural residues by Ceriporiopsis subvermispora. Bioresour. Technol. 99, 4321e4328.
Yamagishi, K., Kimura, T., Watanabe, T., 2011. Treatment of rice straw
with selected Cyathus stercoreus strains to improve enzymatic
saccharification. Bioresour. Technol. 102, 6937e6943.
Yanase, S., Hasunuma, T., Yamada, R., Tanaka, T., Ogino, C.,
Fukuda, H., Kondo, A., 2010a. Direct ethanol production from
cellulosic materials at high temperature using the thermotolerant
yeast Kluyveromyces marxianus displaying cellulolytic enzymes.
Appl. Microbiol. Biotechnol. 88, 381e388.
Yanase, S., Yamada, R., Kaneko, S., Noda, H., Hasunuma, T.,
Tanaka, T., Ogino, C., Fukuda, H., Kondo, A., 2010b. Ethanol
production from cellulosic materials using cellulase-expressing
yeast. Biotechnol. J. 5, 449e455.
Yang, H.H., Effland, M.J., Kirk, T.K., 1980. Factors influencing fungal
degradation of lignin in a representative lignocellulosic, thermomechanical pulp. Biotechnol. Bioeng. 22, 5e77.
93
Yomano, L.P., York, S.W., Ingram, L.O., 1998. Isolation and characterization of ethanol-tolerant mutants of Escherichia coli KO11 for fuel
ethanol production. J. Ind. Microbiol. Biotechnol. 20, 132e138.
Yomano, L.P., York, S.W., Zhou, S., Shanmugam, K.T., Ingram, L.O.,
2008. Re-engineering Escherichia coli for ethanol production. Biotechnol. Lett. 30, 2097e2103.
Yuan, X., Li, P., Wang, H., Wang, X., Cheng, X., Cui, Z., 2011.
Enhancing the anaerobic digestion of corn stalks using composite
microbial pretreatment. J. Microbiol. Biotechnol. 21, 746e752.
Zeng, Y., Yang, X., Yu, H., Zhang, X., Ma, F., 2011. Comparative studies
on thermochemical characterization of corn stover pretreated by
white-rot and brown-rot fungi. J. Agric. Food Chem. 59,
9965e9971.
Zhang, J., Geng, A., Yao, C., Lu, Y., Li, Q., 2012a. Xylitol production
from D-xylose and horticultural waste hemicellulosic hydrolysate
by a new isolate of Candida athensensis SB18. Bioresour. Technol.
105, 134e141.
Zhang, Q., Zhang, W., Lin, C., Xu, X., Shen, Z., 2012b. Expression of an
Acidothermus cellulolyticus endoglucanase in transgenic rice seeds.
Protein Expression Purif. 82, 279e283.
Zhang, Y., Song, L., Gao, Q., Yu, S.M., Li, L., Gao, N.F., 2012c. The twostep biotransformation of monosodium glutamate to GABA by
Lactobacillus brevis growing and resting cells. Appl. Microbiol.
Biotechnol. 94, 1619e1627.
Zhang, Y., Zhu, Y., Zhu, Y., Li, Y., 2009. The importance of engineering
physiological functionality into microbes. Trends Biotechnol. 12,
664e672.
Zhang, Y.H., Lynd, L.R., 2005. Cellulose utilization by Clostridium
thermocellum: bioenergetics and hydrolysis product assimilation.
Proc. Natl. Acad. Sci. USA 102, 7321e7325.
Zhao, L., Cao, G.L., Wang, A.J., Ren, H.Y., Dong, D., Liu, Z.N.,
Guan, X.Y., Xu, C.J., Ren, N.Q., 2012. Fungal pretreatment of
cornstalk with Phanerochaete chrysosporium for enhancing enzymatic saccharification and hydrogen production. Bioresour. Technol. 114, 365e369.
Zhao, X., Cheng, K., Liu, D., 2009. Organosolv pretreatment of
lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol.
Biotechnol. 82, 815e827.
Zheng, Y., Lin, H.-M., Wen, J., Cao, N., Yu, X., Tsao, G.T., 1995. Supercritical carbon dioxide explosion as a pretreatment for cellulose
hydrolysis. Biotechnol. Lett. 17, 845e850.
Zheng, Y., Lin, H., Tsao, G.T., 1998. Pretreatment for cellulose hydrolysis by carbon dioxide explosion. Biotechnol. Prog. 14, 890e896.
Zhong, W., Zhang, Z., Luo, Y., Sun, S., Qiao, W., Xiao, M., 2011. Effect
of biological pretreatments in enhancing corn straw biogas production. Bioresour. Technol. 102, 11177e11182.
Zhu, Y., Lee, Y.Y., Elander, R.T., 2007. Conversion of aqueous
ammonia-treated corn stover to lactic acid by simultaneous
saccharification and cofermentation. Appl. Biochem. Biotechnol.
137-140, 721e738.
C H A P T E R
6
Databases for Bioenergy-Related Enzymes
Yanbin Yin
Department of Biological Sciences, Northern Illinois University, DeKalb, IL, USA
email: yyin@niu.edu
O U T L I N E
Plant Biomass
95
Bioenergy-Related Enzymes and Regulation
96
Databases and Web Servers
CAZy Database
CAT and dbCAN
FOLy Database
98
98
101
102
Purdue Cell Wall Genomics and UC-Riverside Cell
Wall Navigator Databases
Plant Coexpression Network Databases: PlaNet and
ATTED
102
Future Perspectives
103
References
103
PLANT BIOMASS
Note that unlike celluloses, hemicelluloses and
pectins both refer to a collection of complex polysaccharides mostly with side chains. Hemicelluloses contain
four major groups: xyloglucans, mannans, xylans and
mixed-linkage glucans, while pectins contain three major groups: galacturonans, rhamnogalacturonan I and
rhamnogalaturonan II. Each of the groups of hemicelluloses and pectins do not refer to a single type of polysaccharides; they often refer to polysaccharides with the
same backbone structure (sugars and linkages) while
with different side chains. Due to this reason, all
these biopolymers are cross-linked and interwoven
(Somerville et al., 2004; Himmel et al., 2007) to form
very complex and heterogeneous cell wall structures.
Particularly celluloses are wrapped by hemicelluloses
and buried in a lignin network and not accessible to
enzymes so that the degradation efficiency is very low
if no costly chemical pretreatment is applied.
Although celluloses are simple polymer of glucose
linked by beta-1,4,-glucosidic bond, the complexity of
chemical compositions of hemicelluloses and pectins is
remarkably high (Somerville et al., 2004). The reasons
are as follows: (1) there are 14 different monosaccharaides (sugar units) found in hemicelluloses and pectins
(Pauly and Keegstra, 2008b); (2) the possible glycosidic
The major components of plant biomass are
carbohydrate-rich cell walls, composed of different biopolymers such as polysaccharides and lignins as well
as some minor wall structural glycoproteins (Somerville
et al., 2004). Biomass used for biofuel production is primarily derived from secondary cell walls. For example,
wood cells from poplar trees contain a thin layer of primary cell walls and multiple layers of much thicker and
tougher secondary cell walls. All plant cells of different
tissues have primary cell walls while only in developed
cells (stopped growing) secondary cell walls appear
(Cosgrove, 2005). The chemical compositions in primary
and secondary cell walls differ significantly (Mohnen
et al., 2008). The primary cell wall contains no lignins
and the polysaccharides include celluloses, hemicelluloses (primarily xyloglucans and mannans in dicots
and xylans in monocots) and pectins. However, in the
secondary cell walls, there are higher percentage of celluloses, different hemicellulosic polysaccharides (primarily xylans in both dicots and monocots) and lignins. For
example, wood secondary cell walls contain 35e50%
celluloses, 25e30% hemicelluloses (mostly xylans) and
15e30% lignins (Himmel et al., 2007).
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00006-1
102
95
Copyright Ó 2014 Elsevier B.V. All rights reserved.
96
6. DATABASES FOR BIOENERGY-RELATED ENZYMES
linkages formed between two sugars are extremely
diverse as theoretically they can be connected between
any hydroxyl group of two sugars and (3) sugars in
the polysaccharides can be further modified by, e.g.
methylation, acetylation or esterification.
Lignins, however, are complex heterogeneous
phenolic polymers and chemically very distinct from
polysaccharides. They are formed by three major
monomers: hydroxyphenyl (H), guaiacyl (G) and
syringyl (S) units, which are derived from coumaryl
alcohol, coniferyl alcohol and sinapyl alcohol, respectively (Boerjan et al., 2003). All the biopolymers
in plant cell walls are cross-linked and interwoven
(Somerville et al., 2004; Himmel et al., 2007) to form
very complex and heterogeneous structures, which is
believed to enable cell walls recalcitrant to enzymatic
degradation.
Besides, cell wall compositions and structures also
vary from tissue to tissue. The reason is because the
cell wall biosynthetic enzymes are differentially regulated and expressed in different tissues. Furthermore,
different plants, especially those of distant evolutionary
clades, have very distinct cell wall biopolymer compositions. For example, grasses generally have significantly
higher percentage of xylans than trees (Pauly and
Keegstra, 2008a).
For biofuel production, polysaccharides especially
celluloses are favored as their degradation releases
fermentable sugars. Lignins, however, are phenolic
polymers and chemically distinct from polysaccharides,
not giving rise to sugars and meant to be removed in the
biofuel production. In order to develop transgenic
plants with modified cell wall compositions (i.e. higher
cellulose and lower lignin content), we need a better understanding of how plant cell wall polysaccharides and
lignins are synthesized.
BIOENERGY-RELATED ENZYMES
AND REGULATION
Because of the overwhelming complexity of cell
wall polymers in terms of their chemical compositions,
linkages and structures, plant biomass formation and
microbial degradation involve a surprisingly large number of genes in plant and microbes, respectively. For
example, presumably every different glycosidic bond in
the polysaccharides will be formed using a different
enzyme. It is estimated that w10% of genes in Arabidopsis
genome are involved in cell wall synthesis and modification (Yong et al., 2005), which account for w2000
genes encoding enzymes for sugar and lignin precursor
synthesis, polysaccharide and lignin synthesis and
modification, lignin-polysaccharide cross-linking, transcription factors (TFs)and signaling proteins, etc.
The most important enzymes are clearly those
involved in polysaccharide synthesis and lignin synthesis. To form polysaccharides, glycosyltransferases (GTs)
take the activated sugar donors, nucleoside diphosphate
sugars (NDP-sugars), as the substrates to build glycosidic bonds between two sugars. Except for celluloses,
other cell wall polysaccharides are mostly synthesized
in Golgi apparatus, where GTs, NDP-sugar biosynthetic
enzymes and sugar transporters are located and work
together. Glycoside hydrolases (GHs), on the other
hand, are used to break glycosidic bonds through hydrolysis reactions to release sugars from polysaccharides. In
plants this is often used to modify existing polysaccharides, e.g. when plant cells are growing, while in microbes GHs are the most critical enzymes degrading
plant biomass. Clearly not all GH and GT enzymes are
involved in cell wall polysaccharide metabolism, as
many of them are involved in metabolism of storage
polysaccharides, glycoproteins, glycolipids and other
glycol-conjugates that are not relevant to plant cell walls.
All GT and GH enzymes are categorized by the CAZy
(Carbohydrate Active enZyme) database (CAZyDB)
(Cantarel et al., 2009), which provides a general classification scheme for all carbohydrate active enzymes
(CAZymes) and is widely accepted by the carbohydrate
research community. So far there are a limited number of
enzymes biochemically or genetically characterized to
be involved in plant cell wall synthesis or modification,
many of which belong to some large GH and GT families. For example, the GT2 family is known to include
cellulose synthases and some hemicellulose backbone
synthases (Lerouxel et al., 2006), such as mannan synthases (Dhugga et al., 2004; Liepman et al., 2005), putative xyloglucan synthases (Cocuron et al., 2007), and
mixed linkage glucan synthases (Burton et al., 2006).
With respect to the synthesis of xylan, the most abundant hemicellulose, proteins of GT43, GT47 and GT8
are likely to be involved (Zhong et al., 2005; Brown
et al., 2007; Lee et al., 2007; Pena et al., 2007; Persson
et al., 2007; York and O’Neill, 2008; Brown et al., 2009;
Wu et al., 2009).
Some of these known cell wall-related CAZyme families are included in Purdue’s Cell Wall genomics database (Yong et al., 2005) and UC-Riverside’s (UCR) Cell
Wall Navigator database (Girke et al., 2004), and more
families are discussed in the literature or to be characterized in terms of their roles in biomass-related polysaccharide formation and degradation. For example, a
few recent papers (Scheller and Ulvskov, 2010; Driouich
et al., 2012) and a book (Ulvskov, 2011) updated our
knowledge about the GT family members involved in
cell wall synthesis: GT2, 8, 31, 34, 37, 43, 47, 61, 64, 75,
77, while there must be more GT families not included
and to be identified as cell wall related (CWR), e.g.
GT92 (Liwanag et al., 2012).
BIOENERGY-RELATED ENZYMES AND REGULATION
Lignins are complex heterogeneous polymers with
lots of aromatic rings. The monolignol synthesis
pathway that starts from phenylalanine to synthesize
G, S and H units has been relatively well known, with
about 10 gene families characterized encoding most of
the enzymes in the pathway (Humphreys and Chapple,
2002; Boerjan et al., 2003; Vanholme et al., 2008; Xu et al.,
2009; Zhong and Ye, 2009; Li and Chapple, 2010; Weng
and Chapple, 2010; Carpita, 2012). All these lignin
synthesis-related enzymes have been extensively
reviewed in the literature and are included in Purdue’s
Cell Wall genomics database. Transporting the units to
the outside of the cell and assembling them into lignin
polymers are less understood but some candidate transporters and two major enzyme families, peroxidase and
laccase, are suggested (McCaig et al., 2005; Liu et al.,
2011; Zhang et al., 2011; Alejandro et al., 2012; Carpita,
2012; Handford et al., 2012; Sibout and Hofte, 2012).
As with all other metabolic pathways, biomass formation and degradation are also under strict regulation.
However, compared to enzymatic activities, regulatory
mechanism is even more difficult to elucidate. In plants,
only a handful of TFs are known to regulate cell wall
synthesis. The most studied process is the regulation
of lignin biosynthesis (Zhong and Ye, 2009; Zhong
et al., 2010; Zhao and Dixon, 2011; Wang and Dixon,
2012). TF families NAC, WRKY, and MYB among a
few others have been shown to directly or indirectly control the monolignol synthesis. Some of the TF family
members are global regulators that regulate the entire
secondary cell wall synthesis, including the synthesis
of celluloses and xylans, suggesting that the different
biopolymers in biomass are not synthesized independently but in a coordinated way. On the other hand,
genetically modifying the regulation of cell wall biosynthesis represents a very promising way to improve the
desired traits of bioenergy crops. For example, Wang
et al. showed that a mutation found in a WRKY TF could
rewire the regulatory network of secondary cell wall
synthesis and improve 50% of the biomass production
in Arabidopsis (Wang et al., 2010). Similarly, micro ribonucleic acids (miRNAs) are also excellent targets for
controlling the regulation of cell wall synthesis (Fu
et al., 2012), which is less discussed in the literature.
Clearly looking for novel transcription regulators, either
TFs and miRNAs, and further building the regulatory
network of cell wall synthesis is the ultimate goal for
the elucidation of the mechanism of biomass formation.
Recently a few plant journals published special issues
on plant cell wall researches: Plant Physiology (McCann
and Rose, 2010), Current Opinion in Plant Biology
(Pauly and Keegstra, 2008b), Frontiers in Plant Science
(Debolt and Estevez, 2012) and Molecular Plant (has a
cell wall biology category). Particularly, a number of
review articles published in these special issues and a
97
few book chapters (Table 6.1) gave overviews of latest
progress in a specific area of cell wall research and are
very useful for pointing to the original research papers
reporting the characterization of specific CWR genes.
In terms of degradation, cell wall polysaccharides are
degraded by microbial GHs and other CAZymes that
are defined and categorized in CAZyDB. Lignins are
mostly degraded by microbes too particularly by certain
fungi (Dashtban et al., 2009). Enzymes involved in the
degradation include fungal laccases and peroxidases,
which are categorized in the FOLy (fungal oxidative
enzymes) database (Levasseur et al., 2008). Note that
these two families are not restricted to fungi. Instead
they both belong to large protein families having many
homologs in various organisms such as plants, animals
and bacteria, bearing slightly different biochemical
activities (Welinder, 1992). As mentioned above, these
enzyme families are also used for lignin polymerization
TABLE 6.1 Selected Publications for CWR Genes
Category
Publications
Lignins
(Humphreys and Chapple, 2002; Boerjan et al.,
2003; Vanholme et al., 2008; Xu et al., 2009; Zhong
and Ye, 2009; Li and Chapple, 2010; Weng and
Chapple, 2010; Carpita, 2012)
Celluloses
(Somerville, 2006; Joshi and Mansfield, 2007; Gu
and Somerville, 2010; Endler and Persson, 2011;
Lei et al., 2012)
Xylans
(York and O’Neill, 2008; Carpita, 2012; Doering
et al., 2012)
Hemicelluloses
(Sandhu et al., 2009; Scheller and Ulvskov, 2010;
Carpita, 2012; Driouich et al., 2012)
Pectins
(Mohnen, 2008; Harholt et al., 2010; Driouich
et al., 2012)
NDP-sugars
(Reiter and; Vanzin, 2001; Seifert, 2004; Reiter,
2008; Bar-Peled and O’Neill, 2011)
TFs*
(Zhong and Ye, 2009; Zhong et al., 2010; Zhao and
Dixon, 2011; Carpita, 2012; Wang and Dixon,
2012)
Transporters
(Liu et al., 2011; Zhang et al., 2011; Alejandro
et al., 2012; Carpita, 2012; Handford et al., 2012;
Sibout and Hofte, 2012)
miRNAs
(Sun; Sun et al.; Fu et al., 2012)
GTs*
(Yokoyama and Nishitani, 2004; Scheller and
Ulvskov, 2010; Driouich et al., 2012; Harholt et al.,
2012)
Other CAZymes
(Yokoyama and Nishitani, 2004; Minic, 2008)
DUF* families
(Carpita, 2012; Hansen et al., 2012; Harholt et al.,
2012)
Bioinformatics
(Egelund et al., 2004; Yong et al., 2005; Penning
et al., 2009; Michel et al., 2010)
* TF, transcription factor; GT, glycosyltranferase; DUF, domain of undefined function.
98
6. DATABASES FOR BIOENERGY-RELATED ENZYMES
in plants. There is also increasing evidence to show that
such enzymes are also used for lignin degradation in
bacteria (Claus, 2003; Li et al., 2009; Bugg et al., 2011a,
2011b).
Notably many cell wall biosynthesis-related gene
families are also rooted in bacteria (Royo et al., 2000;
Nobles and Brown, 2004; Emiliani et al., 2009; Yin
et al., 2009; Weng and Chapple, 2010; Yin et al., 2010,
2011; Popper et al., 2011). In other words, although carbohydrate and lignin-rich plant cell walls are almost
unique to plants, the biosynthetic machinery has
evolved from ancient gene families that were already
present in early prokaryotes. On the degradation side,
microbes are responsible for breaking down biomass,
while plants also contain homologs of many microbial
degrading enzymes such GHs and peroxidases. Obviously plants also inherited these enzymes for different
purposes: modify existing polysaccharide or complete
the lignification process.
Similarly, less is known about the regulation of
enzymes for the polysaccharide and lignin degradation
in microbes than the enzymes themselves. As opposed
to plants, microbes involved in biomass degradation
are more taxonomically distributed spanning from
eukaryotic fungi (e.g. Neurospora crassa) to prokaryotic
bacteria (Clostridium thermocellum). As a result, the regulation systems in these divergent organisms are often
not very conserved, e.g. many of the TFs found in fungi
are not present in bacteria and vice versa. Furthermore,
there are numerous model microbes used for bioenergy
research and the transcription regulators regulating
cellulases, hemicellulases or ligninases are highly
dispersed in the literature, e.g. (Aro et al., 2005; Portnoy
et al., 2011; Coradetti et al., 2012; Sun et al., 2012). All
these make the curation and annotation of the regulators
and targeting cis elements to be very difficult. Recently,
global gene expression data (e.g. microarray) and other
omics data have been generated to help study the regulation of biomass degradation (Nataf et al., 2010; Raman
et al., 2011; Riederer et al., 2011; Yang et al., 2012), which
represents the future trend of understanding biofuel
production at the systems biology level. Similar to regulators for cell wall synthesis, there is a lack of web-based
bioinformatics databases to include the regulatory genes
for bioenergy-related degradation enzymes.
DATABASES AND WEB SERVERS
Our knowledge about cell wall biosynthesis and
biodegradation has steadily increased in the past
decades, although there is still a long way to go to fully
understand these extremely complex processes. So far
our knowledge is at best fragmented and characterized
genes are often from various organisms and dispersed
in the literature. Therefore, dedicated, centralized
and frequently updated databases of bioenergy-related
genes are crucial to guide the development of transgenic
biofuel crops and the annotation of newly sequenced
metagenomes/genomes to look for novel enzymes.
Like other biology research areas, bioenergy research
has also been benefiting from bioinformatics. Table 6.2
provides a list of bioinformatics databases and webbased resources developed specifically for bioenergyrelated enzymes as well as some general bioinformatics
web resources that are valuable for bioenergy research.
Here we offer a summary of a few selected resources
that are particularly useful.
CAZy Database
For bioenergy research, CAZymes are obviously the
most important enzymes. The CAZyDB team started
to classify and annotate CAZyme proteins from GenBank, UniProt and PDB to protein families since the
early 1990s. It is the original database that defined
over 300 CAZyme protein families throughout the past
20 years and the most comprehensive database
providing high-quality manual annotation by extracting
associated knowledge from the literature (Cantarel et al.,
2009). Its Web site regularly updates every few weeks,
mainly by assigning new proteins in public databases
to existing CAZyme families by sequence similarity
search or creating new families if there are new biochemically characterized CAZyme proteins (supported
by published papers) that do not belong to existing
CAZyme families. Sometimes the functional annotation
information (e.g. known activities) for some families is
also updated if relevant literature came out.
Currently the database comprises five classes of protein families: in addition to GHs and GTs, there are three
other classes, carbohydrate esterases (CEs), polysaccharide lyases (PLs) and carbohydrate binding modules
(CBMs). As aforementioned, GTs are used for building
polysaccharides or glycolconjugates, while GH for
breaking them. CE and PL are also used for breaking carbohydrate molecules while using different mechanisms
or cutting different chemical bonds. CBMs, as indicated
by names, are structural modules used for recognizing
and binding different sugars. Currently the five classes
contain 94 GT families, 131 GH families, 16 CE families,
22 PL families and 64 CBM families. Each class also has
an unclassified family, meaning proteins are annotated
to belong to a certain class but are not able to be assigned
to any existing families in that class. Each family is
named with the class name followed by a sequential
number, e.g. GT2. Note such name does not indicate
any biochemical activity of each family. The reason is
that these families are defined solely based on sequence
similarity: there are many cases that one family contains
99
DATABASES AND WEB SERVERS
TABLE 6.2 Bioenergy-Related Databases
General
Plant biomass
formation
Database
Description
References
CAZyDB
General carbohydrate active enzyme database; a classification
scheme with five classes (GH, GT, CE, PL and CBM) and over 300
families; supported by the biochemical literature; links to proteins in
GenBank, UniProt and structures in PDB; subfamilies for selected
families; Enzyme commission annotation and biochemically curated
function annotation
(Cantarel et al., 2009)
CAT
CAZyme analysis toolkit; allow CAZyme annotation of user
submitted data using BLAST (basic local alignment search tool) and
Pfam-based search
(Park et al., 2010)
dbCAN
Web server for automated CAZyme annotation; allow submission of
predicted protein sequences from newly sequenced genomes and
metagenomes and return a table and graphical diagram to show the
matched CAZyme domains; users could also download HMMs
representing all CAZyme domains to perform annotation locally by
running HMMER3 (hmmer.org)
(Yin et al., 2012)
Survey of databases
for cell wall
synthesis
A paper reviewed nine public databases
(Cao et al., 2010)
XTH World
Xyloglucosyl transferase gene nomenclature; gene structure and
literature in Arabidopsis, rice and tomato
MAIZEWALL
Cell wall gene catalogue, expression data and literature in maize
(Guillaumie et al., 2007)
coreCarb
PlaNet family tool; Arabidopsis CAZyme proteins; sequence,
expression, regulon (coexpressed genes), phylogeny data
(Mutwil et al., 2009)
GolgiP
Web server for prediction of Golgi localized proteins
(Chou et al., 2010)
Purdue cell wall
genomics
General classification scheme for plant cell wall synthesis; with
mutants and spectrotype information; also including lignin
synthesis and modification and NDP-sugar synthesis genes and
signaling proteins etc.; phylogeny for gene families in Arabidopsis,
rice, maize and sorghum; literature
(Yong et al., 2005)
UC-Riverside cell
wall navigator
Similar classification scheme for plant cell wall synthesis proteins to
Purdue cell wall database but not including lignin-related and
signaling proteins; including sequence, literature and microarray
expression data; primarily for Arabidopsis and rice, but also generally
from UniProt
(Girke et al., 2004)
Stanford cellulose
Designed for the CesA (cellulose synthase) superfamily and
homologs; deprecated web site
(Richmond and
Somerville, 2000)
Rice GT
Part of the rice phylogenomics database; rice GT protein phylogeny,
sequence, expression, mutants, ortholog, BLAST
(Cao et al., 2008)
Csl families
Web site supplemental to (Yin et al., 2009); protein sequences,
alignments and phylogeny of the CesA superfamily in fully
sequenced plant and algal genomes
(Yin et al., 2009)
pDAWG
CAZymes in fully sequenced plant and algal genomes based on
search against CAZyDB; phylogeny, predicted subcellular
localization and proteineprotein interaction data; BLAST
(Mao et al., 2009)
PPI for cell wall
Proteineprotein interaction graphs for cell wall-related proteins in
Arabidopsis
(Zhou et al., 2010a)
PlaNet
General coexpression database for seven plant organisms and
comparison among them; highest reciprocal rank based
coexpression and clustering using Heuristic Cluster Chiseling
Algorithm (HCCA, Mutwil et al.); queried gene-centered display of
coexpression network
(Mutwil et al., 2011)
(Continued)
100
6. DATABASES FOR BIOENERGY-RELATED ENZYMES
TABLE 6.2 Bioenergy-Related Databasesdcont’d
Biomass
degradation
Misc
Database
Description
References
Cell wall
coexpression
database
Biclustering coexpression analysis of cell wall-related genes from
Purdue cell wall genomics database; coexpression modules and
graphs generated using Cytoscape (Shannon et al., 2003); cisregulatory elements identified in promoter regions of genes of a
same module
(Wang et al., 2012)
ATTED
General coexpression database and predicted cis-regulatory
elements for Arabidopsis and rice; mutual rank based; also identified
conserved coexpression links and referred to proteineprotein
interaction data
(Obayashi et al., 2009)
GAS db
Glycosyl hydrolase AnnotationS (GAS) database; GH data identified
from UniProt and JGI metagenomes based on CAZyDB and Pfam
search; featured with the graphical domain diagrams and
comparison between two selected bacteria
(Zhou et al., 2010b)
FOLyDB
Fungal enzymes for lignin degradation; 10 families of lignin oxidases
and auxiliary enzymes; proteins from GenBank, UniProt and PDB
(Levasseur et al., 2008)
PeroxiBase
General peroxidase database including peroxidases (EC 1.11.1.x)
from over 1000 organisms; lignin-related peroxidases are a subset of
the database
(Fawal et al., 2012)
LccED
General laccase database and their homologs in the multicopper
oxidase superfamily
(Sirim et al., 2011)
Biofuel feedstock
genomic
resource (BFGR)
Database of 54 plant organisms with sequenced genomes or
significant amount of EST (expressed sequence tag) data; integrated
data including expression, ortholog and paralog, pathway
prediction, and functional information
(Childs et al., 2012)
BESC-KB
Knowledgebase for the Bioenergy Science Center of DOE; a web
portal to a number computational tools and databases dedicated for
bioenergy research and developed within the center
(Syed et al., 2012)
Pathway-genome
database of poplar
Populus trichocarpa metabolic pathways generated automatically
through the Pathway Tool; currently the NDP-sugar biosynthetic
pathways were manually curated by experts
(Nag et al., 2012)
JGI IMG/M
Joint Genome Institute’s integrated microbial genomes and
metagenomes web site
(Markowitz et al., 2012)
Phytozome
JGI’s plant genome web site; currently most sequenced plant
genomes are available in this web site
(Goodstein et al., 2012)
proteins characterized with different biochemical activities. Recent efforts from the CAZyDB team suggest
that further classification of family into subfamilies
could be useful as subfamily may contain proteins
with the same activity (Stam et al., 2006; Lombard
et al., 2010; Aspeborg et al., 2012).
CAZyDB’s annotation also evolved in the past 20
years. Among the 327 CAZyme families as of December
2012, there are 10 depreciated families; they were
created during the life course of CAZyDB but later
were deleted since they were shown not related to carbohydrate metabolism or due to some other reasons. However, to keep the existing nomenclature system
unchanged, these family names remain in the system
but indicated to be deleted on the Web pages for these
families. Other examples include CE10 family, whose
Web page was not updated since 2002 because after
the family was created it was shown that most CE10
family members do not take carbohydrates as substrate;
CBM33 was thought to be a carbohydrate active binding
module but later shown likely to be an oxidase family.
For a decade, CAZyDB provides an HTML page for
each family to list member proteins and associated functional information. In recent updates, CAZyDB added a
Web page for each genome, providing a list of GenBank
protein accession numbers of that genome together with
the CAZyme family assignment for each protein, which
is termed “CAZyome” of an organism. So far, there are
almost 2400 genomes spanning from eukaryotes to
prokaryotes and viruses annotated in CAZyDB. It is
said that such genome-scale annotation of CAZyme
proteins is done semiautomatically (Coutinho and
DATABASES AND WEB SERVERS
Henrissat, 2011). A backend automated domain modulebased search is performed first and then manual curation
will be conducted to remove false positives or include
false negatives. Obviously this process is rather accurate
and of high quality but time consuming because it is
done manually and requires expert knowledge. Indeed
such genome annotation can only be done by the collaboration with the CAZyDB team, which is often invisible
to and out of the control of the users, e.g. people who
sequenced the genome. Over the past 10 years, the
CAZyDB team has done expert CAZyme annotation
for dozens of genome sequencing projects that led to a
lot of collaborative genome annotation papers.
CAT and dbCAN
With more and more bioenergy-related genomes of
plants and microbes as well as environmental metagenomes sequenced, there is an urgent need for automated
CAZyme annotation. Although such annotation will not
reach a quality as accurate as the expert annotation from
CAZyDB, it is expected to be much faster and users can
control the annotation at their will. Moreover, nowadays
all newly sequenced genomes are relying on generic
protein domain/family databases such as Pfam (Finn
et al., 2006), InterPro (Hunter et al., 2009), and conserved
domain database (CDD, Marchler-Bauer et al., 2009) for
automatic genome annotation. Clearly annotation from
these databases is often too general and too far from
the exact function; the precisely actual function still
needs to be determined by experimental approaches.
However, most genome annotators are still interested
in such genome-scale annotation, as it can give them a
quick summary about what the genome encodes, how
large the gene families are and how that compares to
other genomes.
In fact, even CAZyDB’s manual annotation (assign
proteins to existing CAZyme families) on newly
sequenced genomes is unlikely to be 100% correct.
Considering every new genome contains a high percentage of proteins that are not experimentally studied, the
manual curation is still largely based upon additional
bioinformatics analysis such as BLAST search against
public protein sequence databases (e.g. UniProt (Bairoch
et al., 2005)) and domain databases (e.g. Pfam) and
inspection of top matches.
With these in mind, automated CAZyme annotation is
still very useful, e.g. particularly for a quick and general
overview of how many CAZymes and what CAZymes a
newly sequenced genome has. Using the annotated
CAZyme proteins and classification scheme in CAZyDB
as the foundation, two bioinformatics efforts have
been published since 2010, both supporting automated
CAZyme annotation, given a protein sequence dataset
predicted from a genome/metagenome. The CAZyme
101
Analysis Toolkit (CAT) (Park et al., 2010) allows a BLAST
search against CAZyme proteins annotated by CAZyDB
and also a Pfam domain-based search. The simple BLAST
search suffers from the inability to accurately annotate
the prevalent multidomain CAZyme proteins. The
Pfam domain-based search can solve this problem.
These Pfam domains are either given by CAZyDB in
the CAZyme family Web pages or identified to correspond to CAZyme family using an association rule built
by CAT. However, there are only 142 (46%) of over 300
CAZyme families linked to Pfam domains by CAZyDB.
In fact, many of the Pfam domains were originally
created after CAZyDB.
We recently developed dbCAN (Yin et al., 2012) to
define a signature domain model for all CAZyme families. Aside from the 142 CAZyme families annotated
with a Pfam domain, we managed to associate other
CAZyme families to functional domains in a broader
and general protein domain database CDD . This way,
we were able to find a CDD domain for 248 CAZyme
families. For the remaining families, we performed a
literature curation by reading relevant biochemical
papers that are linked to these families by CAZyDB. In
the end, we extracted the domain regions in all the member proteins annotated in CAZyDB and built a multiple
sequence alignment (MSA) for each of the CAZyme family. These MSAs were further processed and represented
by hidden Markov models (HMMs), statistical models
widely used in the bioinformatics field to represent protein sequence alignments, e.g. by Pfam.
As of June 2012, dbCAN has 320 HMMs representing
317 CAZyme families and three cellulosome modules.
We provide all these HMMs freely to the public so that
they can run domain-based tool hmmscan of the
HMMER 3.0 package (hmmer.org) to annotate their
genomes/metagenomes in a local computer, exactly the
way that people perform Pfam, InterPro or CDD annotation. To help users who do not know how to run hmmscan
on a Linux PC, we offer the web server (http://csbl.bmb.
uga.edu/dbCAN/annotate.php) so that people can submit their sequences for annotation on the web. The 320
CAZyme family-specific HMMs are our key contribution
to the carbohydrate research community and ideally
should be included in the general protein domain/family
database such as Pfam in the future.
In addition to the Web server, dbCAN also provides a
database where precomputed CAZyme homologs in a
number of protein databases are showed on the Web.
Particularly, starting from the 320 dbCAN HMMs, we
scanned public metagenome datasets such as NCBIenv-nr, CAMERA (Seshadri et al., 2007), JGI metagenomes
(Markowitz et al., 2012), human gut metagenomes
(Meta-HIT) (Qin et al., 2010) and cow rumen metagenomes (Hess et al., 2011) as well as plant (Goodstein
et al., 2012), bacterial and fungal genomes. Tests on
102
6. DATABASES FOR BIOENERGY-RELATED ENZYMES
Arabidopsis thaliana (plant) and C. thermocellum (bacteria)
using CAZyDB as the positive set suggest that the automated CAZyme annotation achieved a fairly good accuracy (A. thaliana: sensitivity ¼ 96.3%, precision ¼ 78.8%
and average ¼ 87.6%; C. thermocellum: sensitivity ¼ 99.3%
and precision ¼ 89.4%). Particularly the sensitivity is over
95% for both organisms, meaning dbCAN annotation
tends not to lose true CAZyme proteins.
category to support their classification. Proteins of the
two databases are primarily from model organisms
such as Arabidopsis and rice. However, it is easy to use
the sequences as query to search for their homologs and
even orthologs in other plants. The protein accessions
and classification complied by the two databases serve
as an excellent overview of the current achievement
made by the entire plant cell wall community in terms
of our latest understanding of cell wall synthesis.
FOLy Database
Inspired by CAZyDB, Levasseur et al. developed a
new database named FOLy, for the classification of ligninases in fungi (Levasseur et al., 2008), as these enzymes
are critical for breaking down lignins in the biomass but
are not included in CAZyDB. Similar to CAZyDB,
FOLyDB started from biochemically characterized proteins or structures to recruit homologs from GenBank,
UniProt and PDB databases. Based on sequence similarity, three lignin oxidase families and seven lignin degrading auxiliary enzyme families were created, each
containing biochemically characterized proteins together
with their sequence homologs. Similarly, FOLyDB is
featured with expert manual curation of continuingly
published literature to include more characterized proteins in order to create new families and populate the
database. Like CAZyDB, it is not designed for automated
genome annotation but BLAST and Pfam domain-based
search against annotated proteins in FOLyDB has been
widely used to annotate newly sequenced genomes for
ligninases.
Purdue Cell Wall Genomics and UC-Riverside
Cell Wall Navigator Databases
Unlike the above general protein family databases,
Purdue’s Cell Wall Genomics (Yong et al., 2005) and
UCR’s Cell Wall Navigator (Girke et al., 2004) databases
are specifically designed for plant cell wall biosynthesis.
As opposed to sequence similarity-based classification,
both databases categorize proteins based on their physiological roles in cell wall synthesis. In UCR’s database,
there are five categories: monosaccharide synthesis,
polysaccharide synthesis, reassembly, structural proteins
and glycoprotein synthesis, basically all centered on carbohydrate molecules in cell walls. However, Purdue’s
database is even broader with six categories: pathways
for substrate generation, polysaccharide synthases,
secretion and targeting pathways, assembly/architecture and growth, differentiation and secondary wall
formation and signaling and response pathways. Particularly, Purdue’s database also includes lignin synthesis
and polymerization proteins as well as signaling proteins
involved in cell wall synthesis. Both databases also provide references and the literature associated with each
Plant Coexpression Network Databases: PlaNet
and ATTED
As we mentioned earlier, regulation of biomass formation and degradation is also extremely important.
Studying the regulation has been benefited a lot from
coexpression analysis of microarray data and recently
on high-throughput RNA sequencing data. There are
numerous tools, Web based or stand alone, allowing
for coexpression analysis with user-submitted genes as
query or by general browsing. Many online tools even
offer prebuilt coexpression networks, with nodes in the
network graphs representing genes and edges representing coexpression relationships. These coexpression
networks are very informative and insightful, in terms
of suggesting candidate genes involving in the same
metabolic pathways or potential regulators of genes of
interest to the users (Ruprecht and Persson, 2012).
Although there are many such tools available, in plant
cell wall field PlaNet (Mutwil et al., 2011) family tools
(coreCarb (Mutwil et al., 2009), AraNet, GeneCat) stand
out as they were developed by researchers of the cell
wall field, have a Web-based interface and have been
shown to be effective in suggesting new genes for cell
wall synthesis (Mutwil et al., 2009; Ruprecht et al., 2011).
PlaNet placed query genes in network graphs of three
levels: (1) the coexpressed node vicinity network, containing the query gene and genes coexpressed two steps
away and the links among them; (2) a larger coexpression cluster containing the query gene and genes coexpressed, which resulted from running a heuristic
clustering algorithm; and (3) the largest meta-network
with nodes now representing all coexpression clusters
instead of individual genes. PlaNet also has a module
called NetworkComparer, allowing a comparative analysis of gene expression networks across seven plant organisms. Such comparative coexpression analysis has
recently become very popular as it can help deal with
missing data in a single species, reduce false positives
identified as coexpressed in a single species, and enable
to study the conservation of coexpression network from
an evolution perspective (Movahedi et al., 2012).
An earlier tool ATTED-II (Obayashi et al., 2009) is also
well known, which was developed by plant biologists
since 2003 as a database for Arabidopsis tissue-specific
REFERENCES
(ATTED) expression. Compared to PlaNet, ATTED-II
provides richer annotation and a lot of useful links to
external resources in the query gene browse page. It
also has a nicer network graph with less genes (top
certain amount of genes for better visualization) and
gene function information (biologically meaningful
gene names) and labeled TFs. Besides, ATTED-II also
predicted cis-regulatory elements in the upstream regions of coexpression genes. However, ATTED-II
currently includes only two plants: Arabidopsis and
rice, and the gene locus page is only available for
Arabidopsis.
FUTURE PERSPECTIVES
As a perspective for the future development of
bioenergy-related databases, we ask: what do we
need from newly developed databases? Nucleic Acids
Research publishes a prestigious annual Database Special Issue since 20 years ago. Most databases published
there for a particular class of proteins such as plant
TFs (Guo et al., 2008), peroxidases (Fawal et al., 2012),
transporters (Saier et al., 2006) and peptidases (Rawlings
et al., 2012), all provide the following data or functionalities: (1) a general classification of targeted protein
families, manually collected references, a list of characterized proteins curated from the literature and/or predicted member proteins; (2) secondary data derived
from further in-depth bioinformatics analysis, such
as computer-based functional annotation (e.g. Gene
Ontology or protein domain annotation), sequence
alignment, phylogenetic trees, predicted protein structures etc.; (3) simple Web-based BLAST search against
the sequence database and text search using keywords;
(4) long-term maintenance to update regularly with
new data; and (5) plenty of documentation such as
help, FAQ or tutorial pages. These could be considered
as criteria for a good protein family database.
Although the plant biomass formation-related databases listed in Table 6.2 are all very useful, none of
them have sufficiently integrated various functional
omics data. Biologists working on one model plant often
want to take advantage of these data to study their interested genes, e.g. investigate fully sequenced plant genomes to look for orthologs, or transcriptome data
(microarray and RNA-seq) for expression profiles or
look for coexpressed genes and go to the upstream regions for candidate cis-regulatory motifs; all these analyses have to be done using individual bioinformatics
tools or servers, which often requires expert knowledge
to run or to interpret the results. In addition, many of the
databases are outdated and none of them have included
all CWR genes. For example, Purdue’s database is an
excellent resource, but it does not include many of the
103
newly characterized CWR genes such as TF family
NAC, WRKY, MYB members shown to control lignin
synthesis; many of the newly characterized CAZyme
families such as GT43, 61, 75, transporters for NDPsugars and monolignols; miRNAs; DUF (domain of unknown function) families etc. It includes neither much
annotation data nor any search functionalities.
Therefore, the future plant CWR gene databases
should aim to include all experimentally characterized
CWR genes from any organisms, associated sequences
and functional descriptions collected from the published
literature, e.g. those listed in Table 6.1. Such characterized gene list could be highly useful for annotating
sequenced bioenergy plants such as switchgrass, poplar,
maize, sorghum and Eucalyptus grandis. The CWR gene
repertories for these organisms will be highly valuable
for the bioenergy research community as people are
trying to select candidate CWR genes to knock down
or overexpress for developing transgenic plants in these
model organisms. Gene families for CWR genes and
other extensive secondary bioinformatics data should
also be included in the databases, particularly phylogeny (used to identify orthologs from homologs), predicted cis-regulatory element, conserved coexpression
network modules of known CWR genes, expression
profiling, coexpressed gene list including noncoding
RNAs, genomic location, gene neighborhood, epigenomics, proteineprotein interactions, structures, subcellular locations, single-nucleotide polymorphism, indels,
etc. Similar databases should also be developed for plant
CAZymes and include the above bioinformatics-derived
data types. The reason is that CAZyDB now only
covered 2 (A. thaliana and Oryza sativa) of over 40
sequenced plant and green algal genomes, not to
mention there are more incomplete genomes and transcriptomes (ESTs and RNA-seq data).
References
Alejandro, S., Lee, Y., Tohge, T., Sudre, D., Osorio, S., Park, J., Bovet, L.,
Lee, Y., Geldner, N., Fernie, A.R., Martinoia, E., 2012. AtABCG29
is a monolignol transporter involved in lignin biosynthesis. Curr.
Biol. 22, 1207e1212.
Aro, N., Pakula, T., Penttila, M., 2005. Transcriptional regulation of
plant cell wall degradation by filamentous fungi. FEMS Microbiol.
Rev. 29, 719e739.
Aspeborg, H., Coutinho, P.M., Wang, Y., Brumer 3rd, H., Henrissat, B.,
2012. Evolution, substrate specificity and subfamily classification
of glycoside hydrolase family 5 (GH5). BMC Evol. Biol. 12, 186.
Bairoch, A., Apweiler, R., Wu, C.H., Barker, W.C., Boeckmann, B.,
Ferro, S., Gasteiger, E., Huang, H., Lopez, R., Magrane, M.,
Martin, M.J., Natale, D.A., O’Donovan, C., Redaschi, N., Yeh, L.S.,
2005. The universal protein resource (UniProt). Nucleic Acids Res.
33, D154eD159.
Bar-Peled, M., O’Neill, M.A., 2011. Plant nucleotide sugar formation,
interconversion, and salvage by sugar recycling. Annu. Rev. Plant
Biol.
104
6. DATABASES FOR BIOENERGY-RELATED ENZYMES
Boerjan, W., Ralph, J., Baucher, M., 2003. Lignin biosynthesis. Annu.
Rev. Plant Biol. 54, 519e546.
Brown, D.M., Goubet, F., Wong, V.W., Goodacre, R., Stephens, E.,
Dupree, P., Turner, S.R., 2007. Comparison of five xylan synthesis
mutants reveals new insight into the mechanisms of xylan synthesis. Plant J. 52, 1154e1168.
Brown, D.M., Zhang, Z., Stephens, E., Dupree, P., Turner, S.R., 2009.
Characterization of IRX10 and IRX10-like reveals an essential role
in glucuronoxylan biosynthesis in Arabidopsis. Plant J. 57, 732e746.
Bugg, T.D., Ahmad, M., Hardiman, E.M., Rahmanpour, R., 2011a.
Pathways for degradation of lignin in bacteria and fungi. Nat.
Prod. Rep. 28, 1883e1896.
Bugg, T.D., Ahmad, M., Hardiman, E.M., Singh, R., 2011b. The
emerging role for bacteria in lignin degradation and bio-product
formation. Curr. Opin. Biotechnol. 22, 394e400.
Burton, R.A., Wilson, S.M., Hrmova, M., Harvey, A.J., Shirley, N.J.,
Stone, B.A., Newbigin, E.J., Bacic, A., Fincher, G.B., 2006. Cellulose
synthase-like CslF genes mediate the synthesis of cell wall (1,3;1,4)beta-D-glucans. Science 311, 1940e1942.
Cantarel, B.L., Coutinho, P.M., Rancurel, C., Bernard, T., Lombard, V.,
Henrissat, B., 2009. The carbohydrate-active enZymes database
(CAZy): an expert resource for glycogenomics. Nucleic Acids Res.
37, D233eD238.
Cao, P.J., Bartley, L.E., Jung, K.H., Ronald, P.C., 2008. Construction of a
rice glycosyltransferase phylogenomic database and identification
of rice-diverged glycosyltransferases. Mol. Plant 1, 858e877.
Cao, P.J., Jung, K.H., Ronald, P.C., 2010. A survey of databases for
analysis of plant cell wall-related enzymes. BioEnergy Res. 3,
108e114.
Carpita, N.C., 2012. Progress in the biological synthesis of the plant
cell wall: new ideas for improving biomass for bioenergy. Curr.
Opin. Biotechnol. 23, 330e337.
Childs, K.L., Konganti, K., Buell, C.R., 2012. The biofuel feedstock
genomics resource: a web-based portal and database to enable
functional genomics of plant biofuel feedstock species. Database
(Oxford) bar061.
Chou, W.C., Yin, Y., Xu, Y., 2010. GolgiP: prediction of golgi-resident
proteins in plants. Bioinformatics 26, 2464e2465.
Claus, H., 2003. Laccases and their occurrence in prokaryotes. Arch.
Microbiol. 179, 145e150.
Cocuron, J.C., Lerouxel, O., Drakakaki, G., Alonso, A.P.,
Liepman, A.H., Keegstra, K., Raikhel, N., Wilkerson, C.G., 2007. A
gene from the cellulose synthase-like C family encodes a beta-1,4
glucan synthase. Proc. Natl. Acad. Sci. USA 104, 8550e8555.
Coradetti, S.T., Craig, J.P., Xiong, Y., Shock, T., Tian, C., Glass, N.L.,
2012. Conserved and essential transcription factors for cellulase
gene expression in ascomycete fungi. Proc. Natl. Acad. Sci. USA
109, 7397e7402.
Cosgrove, D.J., 2005. Growth of the plant cell wall. Nat. Rev. Mol. Cell
Biol. 6, 850e861.
Coutinho, P.M., Henrissat, B., 2011. Annotating carbohydrate-active
enzymes in plant genomes: present challenges. In: Ulvskov, P.
(Ed.), Annual Plant Reviews: Plant Polysaccharides, Biosynthesis
and Bioengineering. Wiley-Blackwell, Oxford, UK, pp. 93e107.
Dashtban, M., Schraft, H., Qin, W., 2009. Fungal bioconversion of
lignocellulosic residues; opportunities & perspectives. Int. J. Biol.
Sci. 5, 578e595.
Debolt, S., Estevez, J.M., 2012. Current challenges in plant cell walls:
editorial overview. Front. Plant Sci. 3, 232.
Dhugga, K.S., Barreiro, R., Whitten, B., Stecca, K., Hazebroek, J.,
Randhawa, G.S., Dolan, M., Kinney, A.J., Tomes, D., Nichols, S.,
Anderson, P., 2004. Guar seed beta-mannan synthase is a member
of the cellulose synthase super gene family. Science 303, 363e366.
Doering, A., Lathe, R., Persson, S., 2012. An update on xylan synthesis.
Mol. Plant 5, 769e771.
Driouich, A., Follet-Gueye, M.L., Bernard, S., Kousar, S., Chevalier, L.,
Vicre-Gibouin, M., Lerouxel, O., 2012. Golgi-mediated synthesis
and secretion of matrix polysaccharides of the primary cell wall of
higher plants. Front. Plant Sci. 3, 79.
Egelund, J., Skjot, M., Geshi, N., Ulvskov, P., Petersen, B.L., 2004.
A complementary bioinformatics approach to identify potential
plant cell wall glycosyltransferase-encoding genes. Plant Physiol.
136, 2609e2620.
Emiliani, G., Fondi, M., Fani, R., Gribaldo, S., 2009. A horizontal gene
transfer at the origin of phenylpropanoid metabolism: a key
adaptation of plants to land. Biol. Direct 4.
Endler, A., Persson, S., 2011. Cellulose synthases and synthesis in
Arabidopsis. Mol. Plant 4, 199e211.
Fawal, N., Li, Q., Savelli, B., Brette, M., Passaia, G., Fabre, M.,
Mathe, C., Dunand, C., 2012. PeroxiBase: a database for large-scale
evolutionary analysis of peroxidases. Nucleic Acids Res.
Finn, R.D., Mistry, J., Schuster-Bockler, B., Griffiths-Jones, S.,
Hollich, V., Lassmann, T., Moxon, S., Marshall, M., Khanna, A.,
Durbin, R., Eddy, S.R., Sonnhammer, E.L., Bateman, A., 2006. Pfam:
clans, web tools and services. Nucleic Acids Res. 34, D247eD251.
Fu, C., Sunkar, R., Zhou, C., Shen, H., Zhang, J.Y., Matts, J., Wolf, J.,
Mann, D.G., Stewart Jr., C.N., Tang, Y., Wang, Z.Y., 2012. Overexpression of miR156 in switchgrass (Panicum virgatum L.) results
in various morphological alterations and leads to improved
biomass production. Plant Biotechnol. J. 10, 443e452.
Girke, T., Lauricha, J., Tran, H., Keegstra, K., Raikhel, N., 2004. The cell
wall navigator database. A systems-based approach to organismunrestricted mining of protein families involved in cell wall
metabolism. Plant Physiol. 136, 3003e3008.
Goodstein, D.M., Shu, S., Howson, R., Neupane, R., Hayes, R.D.,
Fazo, J., Mitros, T., Dirks, W., Hellsten, U., Putnam, N.,
Rokhsar, D.S., 2012. Phytozome: a comparative platform for green
plant genomics. Nucleic Acids Res. 40, D1178eD1186.
Gu, Y., Somerville, C., 2010. Cellulose synthase interacting protein: a
new factor in cellulose synthesis. Plant Signaling Behav. 5,
1571e1574.
Guillaumie, S., San-Clemente, H., Deswarte, C., Martinez, Y.,
Lapierre, C., Murigneux, A., Barriere, Y., Pichon, M., Goffner, D.,
2007. MAIZEWALL. Database and developmental gene expression
profiling of cell wall biosynthesis and assembly in maize. Plant
Physiol. 143, 339e363.
Guo, A.Y., Chen, X., Gao, G., Zhang, H., Zhu, Q.H., Liu, X.C., Zhong, Y.F.,
Gu, X., He, K., Luo, J., 2008. PlantTFDB: a comprehensive plant
transcription factor database. Nucleic Acids Res. 36, D966eD969.
Handford, M., Rodriguez-Furlan, C., Marchant, L., Segura, M.,
Gomez, D., Alvarez-Buylla, E., Xiong, G.Y., Pauly, M., Orellana, A.,
2012. Arabidopsis thaliana AtUTr7 encodes a golgi-localized
UDP-glucose/UDP-galactose transporter that affects lateral root
emergence. Mol. Plant 5, 1263e1280.
Hansen, S.F., Harholt, J., Oikawa, A., Scheller, H.V., 2012. Plant glycosyltransferases beyond CAZy: a perspective on DUF families.
Front. Plant Sci. 3, 59.
Harholt, J., Sorensen, I., Fangel, J., Roberts, A., Willats, W.G.,
Scheller, H.V., Petersen, B.L., Banks, J.A., Ulvskov, P., 2012. The
glycosyltransferase repertoire of the spikemoss Selaginella moellendorffii and a comparative study of its cell wall. PLoS One 7,
e35846.
Harholt, J., Suttangkakul, A., Vibe Scheller, H., 2010. Biosynthesis of
pectin. Plant Physiol. 153, 384e395.
Hess, M., Sczyrba, A., Egan, R., Kim, T.W., Chokhawala, H.,
Schroth, G., Luo, S., Clark, D.S., Chen, F., Zhang, T., Mackie, R.I.,
Pennacchio, L.A., Tringe, S.G., Visel, A., Woyke, T., Wang, Z.,
Rubin, E.M., 2011. Metagenomic discovery of biomass-degrading
genes and genomes from cow rumen. Science 331, 463e467.
REFERENCES
Himmel, M.E., Ding, S.Y., Johnson, D.K., Adney, W.S., Nimlos, M.R.,
Brady, J.W., Foust, T.D., 2007. Biomass recalcitrance: engineering
plants and enzymes for biofuels production. Science 315, 804e807.
Humphreys, J.M., Chapple, C., 2002. Rewriting the lignin roadmap.
Curr. Opin. Plant Biol. 5, 224e229.
Hunter, S., Apweiler, R., Attwood, T.K., Bairoch, A., Bateman, A.,
Binns, D., Bork, P., Das, U., Daugherty, L., Duquenne, L.,
Finn, R.D., Gough, J., Haft, D., Hulo, N., Kahn, D., Kelly, E.,
Laugraud, A., Letunic, I., Lonsdale, D., Lopez, R., Madera, M.,
Maslen, J., McAnulla, C., McDowall, J., Mistry, J., Mitchell, A.,
Mulder, N., Natale, D., Orengo, C., Quinn, A.F., Selengut, J.D.,
Sigrist, C.J., Thimma, M., Thomas, P.D., Valentin, F., Wilson, D.,
Wu, C.H., Yeats, C., 2009. InterPro: the integrative protein signature database. Nucleic Acids Res. 37, D211eD215.
Joshi, C.P., Mansfield, S.D., 2007. The cellulose paradoxesimple molecule, complex biosynthesis. Curr. Opin. Plant Biol. 10, 220e226.
Lee, C., O’Neill, M.A., Tsumuraya, Y., Darvill, A.G., Ye, Z.H., 2007. The
irregular xylem9 mutant is deficient in xylan xylosyltransferase
activity. Plant Cell Physiol. 48, 1624e1634.
Lei, L., Li, S., Gu, Y., 2012. Cellulose synthase complexes: composition
and regulation. Front. Plant Sci. 3, 75.
Lerouxel, O., Cavalier, D.M., Liepman, A.H., Keegstra, K., 2006.
Biosynthesis of plant cell wall polysaccharides - a complex process.
Curr. Opin. Plant Biol. 9, 621e630.
Levasseur, A., Piumi, F., Coutinho, P.M., Rancurel, C., Asther, M.,
Delattre, M., Henrissat, B., Pontarotti, P., Asther, M., Record, E.,
2008. FOLy: an integrated database for the classification and
functional annotation of fungal oxidoreductases potentially
involved in the degradation of lignin and related aromatic compounds. Fungal Genet. Biol. 45, 638e645.
Li, J., Yuan, H., Yang, J., 2009. Bacteria and lignin degradation. Front.
Biol. China 4, 29e38.
Li, X., Chapple, C., 2010. Understanding lignification: challenges
beyond monolignol biosynthesis. Plant Physiol. 154, 449e452.
Liepman, A.H., Wilkerson, C.G., Keegstra, K., 2005. Expression of
cellulose synthase-like (Csl) genes in insect cells reveals that CslA
family members encode mannan synthases. Proc. Natl. Acad. Sci.
USA 102, 2221e2226.
Liu, C.J., Miao, Y.C., Zhang, K.W., 2011. Sequestration and transport of
lignin monomeric precursors. Molecules 16, 710e727.
Liwanag, A.J., Ebert, B., Verhertbruggen, Y., Rennie, E.A.,
Rautengarten, C., Oikawa, A., Andersen, M.C., Clausen, M.H.,
Scheller, H.V., 2012. Pectin biosynthesis: GALS1 in Arabidopsis
thaliana is a beta-1,4-galactan beta-1,4-galactosyltransferase. Plant
Cell.
Lombard, V., Bernard, T., Rancurel, C., Brumer, H., Coutinho, P.M.,
Henrissat, B., 2010. A hierarchical classification of polysaccharide
lyases for glycogenomics. Biochem. J. 432, 437e444.
Mao, F.L., Yin, Y.B., Zhou, F.F., Chou, W.C., Zhou, C., Chen, H.L.,
Xu, Y., 2009. pDAWG: an integrated database for plant cell wall
genes. BioEnergy Res. 2, 209e216.
Marchler-Bauer, A., Anderson, J.B., Chitsaz, F., Derbyshire, M.K.,
DeWeese-Scott, C., Fong, J.H., Geer, L.Y., Geer, R.C.,
Gonzales, N.R., Gwadz, M., He, S., Hurwitz, D.I., Jackson, J.D.,
Ke, Z., Lanczycki, C.J., Liebert, C.A., Liu, C., Lu, F., Lu, S.,
Marchler, G.H., Mullokandov, M., Song, J.S., Tasneem, A.,
Thanki, N., Yamashita, R.A., Zhang, D., Zhang, N., Bryant, S.H.,
2009. CDD: specific functional annotation with the conserved
domain database. Nucleic Acids Res. 37, D205eD210.
Markowitz, V.M., Chen, I.M., Chu, K., Szeto, E., Palaniappan, K.,
Grechkin, Y., Ratner, A., Jacob, B., Pati, A., Huntemann, M.,
Liolios, K., Pagani, I., Anderson, I., Mavromatis, K., Ivanova, N.N.,
Kyrpides, N.C., 2012. IMG/M: the integrated metagenome data
management and comparative analysis system. Nucleic Acids Res.
40, D123eD129.
105
McCaig, B.C., Meagher, R.B., Dean, J.F., 2005. Gene structure and
molecular analysis of the laccase-like multicopper oxidase (LMCO)
gene family in Arabidopsis thaliana. Planta 221, 619e636.
McCann, M., Rose, J., 2010. Blueprints for building plant cell walls.
Plant Physiol. 153, 365.
Michel, G., Tonon, T., Scornet, D., Cock, J.M., Kloareg, B., 2010. The cell
wall polysaccharide metabolism of the brown alga Ectocarpus siliculosus. Insights into the evolution of extracellular matrix polysaccharides in Eukaryotes. New Phytol. 188, 82e97.
Minic, Z., 2008. Physiological roles of plant glycoside hydrolases.
Planta 227, 723e740.
Mohnen, D., 2008. Pectin structure and biosynthesis. Curr. Opin. Plant
Biol. 11, 266e277.
Mohnen, D., Bar-Peled, M., Somerville, C.R., 2008. Cell wall polysaccharide synthesis. In: Himmel, M.E. (Ed.), Biomass Recalcitrance: Deconstructing the Plant Cell Wall for Bioenergy. Blackwell
Publishing, pp. 94e159.
Movahedi, S., Van Bel, M., Heyndrickx, K.S., Vandepoele, K., 2012.
Comparative co-expression analysis in plant biology. Plant Cell
Environ. 35, 1787e1798.
Mutwil, M., Klie, S., Tohge, T., Giorgi, F.M., Wilkins, O., Campbell, M.M.,
Fernie, A.R., Usadel, B., Nikoloski, Z., Persson, S., 2011. PlaNet:
combined sequence and expression comparisons across plant networks derived from seven species. Plant Cell 23, 895e910.
Mutwil, M., Ruprecht, C., Giorgi, F.M., Bringmann, M., Usadel, B.,
Persson, S., 2009. Transcriptional wiring of cell wall-related genes
in Arabidopsis. Mol. Plant 2, 1015e1024.
Mutwil, M., Usadel, B., Schutte, M., Loraine, A., Ebenhoh, O., Persson,
S. Assembly of an interactive correlation network for the Arabidopsis genome using a novel heuristic clustering algorithm. Plant
Physiol. 152, 29e43.
Nag, A., Karpinets, T.V., Chang, C.H., Bar-Peled, M., 2012. Enhancing
a pathway-genome database (PGDB) to capture subcellular localization of metabolites and enzymes: the nucleotide-sugar biosynthetic pathways of Populus trichocarpa. Database (Oxford). bas013.
Nataf, Y., Bahari, L., Kahel-Raifer, H., Borovok, I., Lamed, R.,
Bayer, E.A., Sonenshein, A.L., Shoham, Y., 2010. Clostridium thermocellum cellulosomal genes are regulated by extracytoplasmic
polysaccharides via alternative sigma factors. Proc. Natl. Acad. Sci.
USA 107, 18646e18651.
Nobles, D.R., Brown, R.M., 2004. The pivotal role of cyanobacteria in
the evolution of cellulose synthases and cellulose synthase-like
proteins. Cellulose 11, 437e448.
Obayashi, T., Hayashi, S., Saeki, M., Ohta, H., Kinoshita, K., 2009.
ATTED-II provides coexpressed gene networks for Arabidopsis.
Nucleic Acids Res. 37, D987eD991.
Park, B.H., Karpinets, T.V., Syed, M.H., Leuze, M.R., Uberbacher, E.C.,
2010. CAZymes analysis toolkit (CAT): web service for searching
and analyzing carbohydrate-active enzymes in a newly sequenced
organism using CAZy database. Glycobiology 20, 1574e1584.
Pauly, M., Keegstra, K., 2008a. Cell-wall carbohydrates and their
modification as a resource for biofuels. Plant J. 54, 559e568.
Pauly, M., Keegstra, K., 2008b. Physiology and metabolism ’Tear down
this wall’. Curr. Opin. Plant Biol. 11, 233e235.
Pena, M.J., Zhong, R., Zhou, G.K., Richardson, E.A., O’Neill, M.A.,
Darvill, A.G., York, W.S., Ye, Z.H., 2007. Arabidopsis irregular
xylem8 and irregular xylem9: implications for the complexity of
glucuronoxylan biosynthesis. Plant Cell 19, 549e563.
Penning, B.W., Hunter 3rd, C.T., Tayengwa, R., Eveland, A.L.,
Dugard, C.K., Olek, A.T., Vermerris, W., Koch, K.E., McCarty, D.R.,
Davis, M.F., Thomas, S.R., McCann, M.C., Carpita, N.C., 2009.
Genetic resources for maize cell wall biology. Plant Physiol. 151,
1703e1728.
Persson, S., Caffall, K.H., Freshour, G., Hilley, M.T., Bauer, S.,
Poindexter, P., Hahn, M.G., Mohnen, D., Somerville, C., 2007. The
106
6. DATABASES FOR BIOENERGY-RELATED ENZYMES
Arabidopsis irregular xylem8 mutant is deficient in glucuronoxylan
and homogalacturonan, which are essential for secondary cell wall
integrity. Plant Cell 19, 237e255.
Popper, Z., Michel, G., Herve, C., Domozych, D.S., Willats, W.G.,
Tuohy, M.G., Kloareg, B., Stengel, D.B., 2011. Evolution and
diversity of plant cell walls: from algae to flowering plants. Annu.
Rev. Plant Biol. 62, 567e590.
Portnoy, T., Margeot, A., Seidl-Seiboth, V., Le Crom, S., Ben
Chaabane, F., Linke, R., Seiboth, B., Kubicek, C.P., 2011. Differential
regulation of the cellulase transcription factors XYR1, ACE2, and
ACE1 in Trichoderma reesei strains producing high and low levels of
cellulase. Eukaryotic Cell 10, 262e271.
Qin, J., Li, R., Raes, J., Arumugam, M., Burgdorf, K.S., Manichanh, C.,
Nielsen, T., Pons, N., Levenez, F., Yamada, T., Mende, D.R., Li, J.,
Xu, J., Li, S., Li, D., Cao, J., Wang, B., Liang, H., Zheng, H., Xie, Y.,
Tap, J., Lepage, P., Bertalan, M., Batto, J.M., Hansen, T., Le Paslier, D.,
Linneberg, A., Nielsen, H.B., Pelletier, E., Renault, P., SicheritzPonten, T., Turner, K., Zhu, H., Yu, C., Jian, M., Zhou, Y., Li, Y.,
Zhang, X., Qin, N., Yang, H., Wang, J., Brunak, S., Dore, J., Guarner, F.,
Kristiansen, K., Pedersen, O., Parkhill, J., Weissenbach, J., Bork, P.,
Ehrlich, S.D., 2010. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59e65.
Raman, B., McKeown, C.K., Rodriguez Jr., M., Brown, S.D.,
Mielenz, J.R., 2011. Transcriptomic analysis of Clostridium thermocellum ATCC 27405 cellulose fermentation. BMC Microbiol. 11, 134.
Rawlings, N.D., Barrett, A.J., Bateman, A., 2012. MEROPS: the database of proteolytic enzymes, their substrates and inhibitors.
Nucleic Acids Res. 40, D343eD350.
Reiter, W.D., 2008. Biochemical genetics of nucleotide sugar interconversion reactions. Curr. Opin. Plant Biol. 11, 236e243.
Reiter, W.D., Vanzin, G.F., 2001. Molecular genetics of nucleotide sugar
interconversion pathways in plants. Plant Mol. Biol. 47, 95e113.
Richmond, T.A., Somerville, C.R., 2000. The cellulose synthase
superfamily. Plant Physiol. 124, 495e498.
Riederer, A., Takasuka, T.E., Makino, S., Stevenson, D.M.,
Bukhman, Y.V., Elsen, N.L., Fox, B.G., 2011. Global gene expression
patterns in Clostridium thermocellum as determined by microarray
analysis of chemostat cultures on cellulose or cellobiose. Appl.
Environ. Microbiol. 77, 1243e1253.
Royo, J., Gimez, E., Hueros, G., 2000. CMP-KDO synthetase: a plant
gene borrowed from gram-negative eubacteria. Trends Genet. 16,
432e433.
Ruprecht, C., Mutwil, M., Saxe, F., Eder, M., Nikoloski, Z., Persson, S.,
2011. Large-scale co-expression approach to dissect secondary cell
wall formation across plant species. Front. Plant Sci. 2, 23.
Ruprecht, C., Persson, S., 2012. Co-expression of cell-wall related
genes: new tools and insights. Front. Plant Sci. 3, 83.
Saier Jr., M.H., Tran, C.V., Barabote, R.D., 2006. TCDB: the transporter
classification database for membrane transport protein analyses
and information. Nucleic Acids Res. 34, D181eD186.
Sandhu, A.P., Randhawa, G.S., Dhugga, K.S., 2009. Plant cell wall
matrix polysaccharide biosynthesis. Mol. Plant 2, 840e850.
Scheller, H.V., Ulvskov, P., 2010. Hemicelluloses. Annu. Rev. Plant Biol.
61, 263e289.
Seifert, G.J., 2004. Nucleotide sugar interconversions and cell wall
biosynthesis: how to bring the inside to the outside. Curr. Opin.
Plant Biol. 7, 277e284.
Seshadri, R., Kravitz, S.A., Smarr, L., Gilna, P., Frazier, M., 2007. CAMERA: a community resource for metagenomics. PLoS Biol. 5, e75.
Shannon, P., Markiel, A., Ozier, O., Baliga, N.S., Wang, J.T.,
Ramage, D., Amin, N., Schwikowski, B., Ideker, T., 2003. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498e2504.
Sibout, R., Hofte, H., 2012. Plant cell biology: the ABC of monolignol
transport. Curr. Biol. 22, R533eR535.
Sirim, D., Wagner, F., Wang, L., Schmid, R.D., Pleiss, J., 2011. The laccase
engineering database: a classification and analysis system for laccases and related multicopper oxidases. Database (Oxford) bar006.
Somerville, C., 2006. Cellulose synthesis in higher plants. Annu. Rev.
Cell Dev. Biol. 22, 53e78.
Somerville, C., Bauer, S., Brininstool, G., Facette, M., Hamann, T.,
Milne, J., Osborne, E., Paredez, A., Persson, S., Raab, T.,
Vorwerk, S., Youngs, H., 2004. Toward a systems approach to understanding plant cell walls. Science 306, 2206e2211.
Stam, M.R., Danchin, E.G., Rancurel, C., Coutinho, P.M., Henrissat, B.,
2006. Dividing the large glycoside hydrolase family 13 into subfamilies: towards improved functional annotations of alphaamylase-related proteins. Protein Eng. Des. Sel 19, 555e562.
Sun, G. MicroRNAs and their diverse functions in plants. Plant Mol.
Biol. 80, 17e36.
Sun, J., Tian, C., Diamond, S., Glass, N.L., 2012. Deciphering transcriptional regulatory mechanisms associated with hemicellulose
degradation in Neurospora crassa. Eukaryotic Cell 11, 482e493.
Sun, Y.H., Shi, R., Zhang, X.H., Chiang, V.L., Sederoff, R.R. MicroRNAs in trees. Plant Mol. Biol. 80, 37e53.
Syed, M.H., Karpinets, T.V., Parang, M., Leuze, M.R., Park, B.H.,
Hyatt, D., Brown, S.D., Moulton, S., Galloway, M.D.,
Uberbacher, E.C., 2012. BESC knowledgebase public portal. Bioinformatics 28, 750e751.
Ulvskov, P., 2011. In: Ulvskov, P. (Ed.), Annual Plant Reviews: Plant
Polysaccharides, Biosynthesis and Bioengineering. Wiley-Blackwell, Oxford, UK.
Vanholme, R., Morreel, K., Ralph, J., Boerjan, W., 2008. Lignin engineering. Curr. Opin. Plant Biol. 11, 278e285.
Wang, H., Avci, U., Nakashima, J., Hahn, M.G., Chen, F., Dixon, R.A.,
2010. Mutation of WRKY transcription factors initiates pith secondary wall formation and increases stem biomass in dicotyledonous plants. Proc. Natl. Acad. Sci. USA 107, 22338e22343.
Wang, H.Z., Dixon, R.A., 2012. On-off switches for secondary cell wall
biosynthesis. Mol. Plant 5, 297e303.
Wang, S., Yin, Y., Ma, Q., Tang, X., Hao, D., Xu, Y., 2012. Genome-scale
identification of cell-wall related genes in Arabidopsis based on coexpression network analysis. BMC Plant Biol. 12, 138.
Welinder, K.G., 1992. Superfamily of plant, fungal and bacterial peroxidases. Curr. Opin. Struct. Biol. 2, 388e393.
Weng, J.K., Chapple, C., 2010. The origin and evolution of lignin
biosynthesis. New Phytol. 187, 273e285.
Wu, A.M., Rihouey, C., Seveno, M., Hornblad, E., Singh, S.K.,
Matsunaga, T., Ishii, T., Lerouge, P., Marchant, A., 2009. The Arabidopsis IRX10 and IRX10-LIKE glycosyltransferases are critical for
glucuronoxylan biosynthesis during secondary cell wall formation.
Plant J. 57, 718e731.
Xu, Z., Zhang, D., Hu, J., Zhou, X., Ye, X., Reichel, K.L., Stewart, N.R.,
Syrenne, R.D., Yang, X., Gao, P., Shi, W., Doeppke, C., Sykes, R.W.,
Burris, J.N., Bozell, J.J., Cheng, M.Z., Hayes, D.G., Labbe, N.,
Davis, M., Stewart Jr., C.N., Yuan, J.S., 2009. Comparative genome
analysis of lignin biosynthesis gene families across the plant
kingdom. BMC Bioinform. 10 (Suppl. 11), S3.
Yang, S., Giannone, R.J., Dice, L., Yang, Z.K., Engle, N.L.,
Tschaplinski, T.J., Hettich, R.L., Brown, S.D., 2012. Clostridium
thermocellum ATCC27405 transcriptomic, metabolomic and proteomic profiles after ethanol stress. BMC Genomics 13, 336.
Yin, Y., Chen, H., Hahn, M.G., Mohnen, D., Xu, Y., 2010. Evolution and
function of the plant cell wall synthesis-related glycosyltransferase
family 8. Plant Physiol. 153, 1729e1746.
Yin, Y., Huang, J., Gu, X., Bar-Peled, M., Xu, Y., 2011. Evolution of
plant nucleotide-sugar interconversion enzymes. PLoS One 6,
e27995.
Yin, Y., Huang, J., Xu, Y., 2009. The cellulose synthase superfamily in
fully sequenced plants and algae. BMC Plant Biol. 9, 99.
REFERENCES
Yin, Y.B., Mao, X.Z., Yang, J.C., Chen, X., Mao, F.L., Xu, Y., 2012.
dbCAN: a web resource for automated carbohydrate-active
enzyme annotation. Nucleic Acids Res. 40, W445eW451.
Yokoyama, R., Nishitani, K., 2004. Genomic basis for cell-wall diversity in plants. A comparative approach to gene families in rice
and Arabidopsis. Plant Cell Physiol. 45, 1111e1121.
Yong, W., Link, B., O’Malley, R., Tewari, J., Hunter, C.T., Lu, C.A.,
Li, X., Bleecker, A.B., Koch, K.E., McCann, M.C., McCarty, D.R.,
Patterson, S.E., Reiter, W.D., Staiger, C., Thomas, S.R.,
Vermerris, W., Carpita, N.C., 2005. Genomics of plant cell wall
biogenesis. Planta 221, 747e751.
York, W.S., O’Neill, M.A., 2008. Biochemical control of xylan biosynthesis - which end is up? Curr. Opin. Plant Biol. 11, 258e265.
Zhang, B., Liu, X., Qian, Q., Liu, L., Dong, G., Xiong, G., Zeng, D.,
Zhou, Y., 2011. Golgi nucleotide sugar transporter modulates
cell wall biosynthesis and plant growth in rice. Proc. Natl. Acad.
Sci. USA 108, 5110e5115.
107
Zhao, Q., Dixon, R.A., 2011. Transcriptional networks for lignin biosyn-x
thesis: more complex than we thought? Trends Plant Sci. 16, 227e233.
Zhong, R., Lee, C., Ye, Z.H., 2010. Evolutionary conservation of the
transcriptional network regulating secondary cell wall biosynthesis. Trends Plant Sci. 15, 625e632.
Zhong, R., Pena, M.J., Zhou, G.K., Nairn, C.J., Wood-Jones, A.,
Richardson, E.A., Morrison 3rd, W.H., Darvill, A.G., York, W.S.,
Ye, Z.H., 2005. Arabidopsis fragile fiber8, which encodes a putative
glucuronyltransferase, is essential for normal secondary wall
synthesis. Plant Cell 17, 3390e3408.
Zhong, R., Ye, Z.H., 2009. Transcriptional regulation of lignin
biosynthesis. Plant Signaling Behav. 4, 1028e1034.
Zhou, C., Yin, Y., Dam, P., Xu, Y., 2010a. Identification of novel proteins
involved in plant cell-wall synthesis based on protein-protein
interaction data. J. Proteome Res. 9, 5025e5037.
Zhou, F., Chen, H., Xu, Y., 2010b. GASdb: a large-scale and comparative exploration database of glycosyl hydrolysis systems. BMC
Microbiol. 10, 69.
C H A P T E R
7
Isobutanol Production from Bioenergy Crops
Thaddeus Chukwuemeka Ezeji 1,*, Nasib Qureshi 2, Victor Ujor 1
1
The Ohio State University, Department of Animal Sciences and Ohio State Agricultural Research
and Development Center (OARDC), Wooster, OH, USA, 2United States Department of Agriculture,a
National Center for Agricultural Utilization Research, ARS, Bioenergy Research, Peoria, IL, USA
*Corresponding author email: ezeji.1@osu.edu
O U T L I N E
Background/Introduction
109
Keto Acid Pathways for Higher Alcohol
Production
Feasibility of Using Bioenergy Crops as Sustainable
114
Feedstocks for Isobutanol Production
110
Biochemistry of Isobutanol Fermentation
112
Technologies That have been Developed for
Simultaneous Butanol Fermentation and Recovery 115
Metabolic Engineering of Microorganisms for
Isobutanol Production
113
BACKGROUND/INTRODUCTION
Isobutanol (Inte‘rnational Union of Pure and Applied
Chemistry nomenclature: 2-methylpropan-1-ol) is a
branched four-carbon alcohol [(CH3)2CHCH2OH], with
a boiling point of 108 C, a melting point of 108 C,
and a relative density of 0.806 at 15 C (Budavari,
1996). It is also known as isobutyl alcohol or 2-methyl1-propanol. Isobutanol has a vapor pressure of
10.43 mm Hg or 13.9 hPa at 25 C (Daubert and Danner,
1985) and a water solubility of 85.0 g/l at 25 C (Valvani
et al., 1981). These properties reveal that isobutanol is
lighter than, and also soluble in water. While isobutanol
is produced industrially via carbonylation (incorporation of carbon monoxide into organic/inorganic compounds) of propylene, isobutanol can be produced
biologically via fermentation of glucose with a potential
to use lignocellulosic biomass. Isobutanol is naturally
Conclusion and Future Perspective
116
References
116
produced in low amounts by Saccharomyces cerevisiae as
a degradation product of valine. The first report of biological production of isobutanol was by Dickinson et al.
(1998) who demonstrated that S. cerevisiae was able to
produce isobutanol using 13C-labeled valine as substrate.
It was hypothesized that the product of valine transamination, a-ketoisovalerate, had four potential routes to
isobutanol,
which
include
(1)
catalysis
of
a-ketoisovalerate by branched-chain a-keto acid dehydrogenase to produce isobutyryl-CoA and subsequently
isobutanol; (2) catalysis of a-ketoisovalerate to isobutanol
by pyruvate decarboxylase (PDC); (3) reduction of
a-ketoisovalerate to a-hydroxyisovalerate by a-ketoisovalerate reductase; and (4) use of the PDC-like enzyme
encoded by YDL080c to produce isobutanol. Given the
fact that riddance of branched-chain a-keto acid dehydrogenase activity in an lpd1 disruption mutant did not prevent the formation of isobutanol, S. cerevisiae cell
a
Mention of trade names or commercial products in this article is solely for the purpose of providing scientific information and does not
imply recommendation or endorsement by the United States Department of Agriculture. USDA is an equal opportunity provider and
employer.
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00007-3
109
Copyright Ó 2014 Elsevier B.V. All rights reserved.
110
7. ISOBUTANOL PRODUCTION FROM BIOENERGY CROPS
homogenates could not convert a-hydroxyisovalerate to
isobutanol, and a strain with a disrupted PDC-like
gene, YDL080c, produced wild-type levels of isobutanol,
hence, routes 1, 3, and 4 were eliminated in S. cerevisiae.
Notably, elimination of PDC activity in a pdc1 pdc5
pdc6 triple mutant abolished isobutanol production
thus, buttressing the notion that this route is the right
channel to isobutanol biosynthesis.
As a feedstock chemical, isobutanol is used for the
production of isobutyl acetate, which is subsequently
used for the production of lacquers. It also finds use as
a direct solvent, production of amino resins, isobutyl
amines, and acrylate and methyl acrylate esters. The
largest use for isobutanol is for the production of zinc
dialkyldithiophosphates, an additive for lube oils,
greases and hydraulic fluids, in which it functions as
an antiwear and antioxidant additive. The second most
significant applications of isobutanol are in the production of isobutyl acetate and as a solvent, primarily for
surface coatings and adhesives (Bizziari et al., 2002).
Recent advances in liquid biofuel technology
(Mariano et al., 2011, 2012; Atsumi et al., 2008), depletion
of petroleum reserves, global population growth, environmental and energy security concerns, have revived
research efforts aimed at producing environmentally
friendly liquid fuel chemicals. Indeed, global population
is projected to reach 8.92 American billion by 2050 and
world energy use may increase 53% by 2035. Consequently, there is an exigent need to source for or develop
new fuels to fill potential shortfalls and, possibly replace
our fast depleting petroleum reserves. Between 1980 and
2010, efforts have been focused on engineering microorganisms to make production of ethanol from biomass
more efficient for use as a biofuel. Compared to ethanol,
longer chain alcohols (e.g. n-propanol, n-butanol and
isobutanol) have greater energy content, lower vapor
pressure, and lower hygroscopicity, which make them
superior alternatives to ethanol as a biofuel (Ladisch,
1991; Ezeji et al., 2005).
Isobutanol has the potential to substitute gasoline or
serve as a gasoline supplement and can be produced
from domestically abundant biomass sources including
lignocellulosic biomass. Lignocellulosic biomass, which
may contain xylan, arabinan, galactan, glucuronic, acetic, ferulic, and coumaric acids, is the most abundant
renewable resource on the planet (Koukiekolo et al.,
2005) and has great potential as a substrate for isobutanol production (Higashide et al., 2011). Substrate cost
has long been recognized to have significant influence
on biofuel price and has been identified as a major factor
affecting economic viability of n-butanol production by
fermentation (Qureshi and Blaschek, 2000). Production
of isobutanol from low-cost lignocellulosic biomass
which does not compete with food crops may be critical
for cost-effective fermentative production of isobutanol.
Whereas majority of producing microorganisms
including S. cerevisiae use glucose as preferred substrate
for growth and alcohol production, recent advances in
genetic engineering have made it possible to metabolically engineer these microorganisms to expand their
substrate range to include pentose sugar components
of lignocellulosic biomass hydrolysates such as xylose
and arabinose, as in the case of ethanologenic microorganisms such as Escherichia coli (Dien et al., 1999, 2000),
Zymomonas mobilis (Zhang et al., 1995; Deanda et al.,
1996), and S. cerevisiae (Jin et al., 2005; Wisselink et al.,
2007; Garcia Sanchez et al., 2010). This chapter describes
the biochemistry of isobutanol production from
biomass, latest developments in isobutanol production
technology, and efforts directed toward development
of more efficient and cost-effective processes for isobutanol production from biomass.
KETO ACID PATHWAYS FOR HIGHER
ALCOHOL PRODUCTION
Keto acids are organic acids with ketone functional
group on the second carbon, typically known as a-carbon,
and are present in microorganisms as intermediate products of amino acids production pathways, and degradation of biosynthesized amino acids to alcohols is what
is commonly known as Ehrlich pathway (Figure 7.1).
Different strains of S. cerevisiae and yeasts belonging to
genera such as Endomycopsis, Candida, and Hansenula are
known to produce higher alcohols via keto acid pathways
(Singh and Kunkee, 1976; Cronk et al., 1979).
In addition to ethanol and CO2 production,
S. cerevisiae produces a variety of relatively lowmolecular weight flavor compounds such as alcohols,
diacetyl, esters, organic acids, organic sulfides, and
carbonyl compounds during fermentation (Ter Schure
Amino acid
Transamination: e.g. 2-ketoglutaric acid
L -glutamic
acid
α-Keto acid (e.g. L -glutamic acid )
Decarboxylation: removal of CO2
Aldehyde (e.g. isobutyraldehyde)
NAD(P)H
Dehydrogenase
NAD(P)+
Alcohol (e.g. isobutanol)
FIGURE 7.1 Simplified biochemistry of branched chain higher
alcohol production from amino acids.
111
KETO ACID PATHWAYS FOR HIGHER ALCOHOL PRODUCTION
TABLE 7.1 Intermediate Products from Potential Amino Acid Degradation (Ehrlich Pathway)
Amino acid
a-Keto Acid
Fusel Aldehyde
Fusel Alcohol
Fusel Acid
Isoleucine:
HO2CCH(NH2)
CH(CH3)CH2CH3
a-Ketomethylvalerate
Methylvaleraldehyde
Amyl alcohol
Methylvaleric acid
Leucine:
HO2CCH(NH2)
CH2CH(CH3)2
a-Ketoisocaproate
Isoamylaldehyde
Isoamyl alcohol
Isovaleric acid
Methionine:
HO2CCH(NH2)
CH2CH2SCH3
a-Keto-g-(methylthio)
butyrate
Methional
Methionol
3-(Methylthio)
propionic acid
Phenylalanine:
C6H5CH2CH(NH2)
COOH
Phenylpyruvate
2-Phenylacetaldehyde
2-Phenylethanol
2-Phenylacetate
Tryptophan:
C11H12N2O2
3-Indole pyruvate
3-Indole acetaldehyde
Tryptophol
2-(Indole-3-yl) acetic
acid
Tyrosine: C9H11NO3
p-Hydroxyphenylpyruvate
p-Hydroxyphenylacetaldehyde
p-Hydroxyphenylethanol
p-Hydroxyphenylacetic acid
Valine: HO2CCH(NH2)
CH(CH3)2
a-Ketoisovalerate
Isobutyraldehyde or
isovaleraldehyde or
isobutanal
Isobutanol
Isobutyric acid
et al., 1998; Hazelwood et al., 2008). These compounds
are formed via the Ehrlich pathway involving branchedchain amino acids such as isoleucine, leucine, methionine, phenylalanine, tryptophan, tyrosine, and valine,
and biocatalysts such as transaminase, decarboxylase,
and alcohol dehydrogenase (ADH) (Figure 7.1, Table 7.1
Ryan and Kohlhaw, 1974). This pathway is prevalent
in yeast and is especially active when yeast is cultivated
in growth medium whose carbon source is solely amino
acids. Notably, catabolism of isoleucine, leucine, methionine, phenylalanine, tryptophan, tyrosine, and valine
by S. cerevisiae via Ehrlich pathway generates
2-methylbutanol (active amyl alcohol), 3-methylbutanol
(isoamyl alcohol), methionol, 2-phenylethanol, tryptophol, p-hydroxyphenyl ethanol, and isobutanol, respectively (Figure 7.1; Table 7.1). These relatively long-chain
alcohols are often referred to as fusel oils or fusel alcohols.
During ethanolic fermentation by S. cerevisiae, small quantities of these alcohols (fusel oil) are produced (Singh and
Kunkee, 1976). Whereas this mixture of alcohols may
contribute flavor and body to wines, it can produce an
off-flavor in wines when the acceptable concentration
threshold is exceeded. Indeed, the catabolism of amino
acids in S. cerevisiae and its regulation has been studied
extensively (Ter Schure et al., 1998; Hazelwood et al.,
2008; Dickinson et al., 1998, 2000).
By transamination of amino acids to a-keto acids
followed by decarboxylation of a-keto acids to aldehydes,
these aldehydes can undergo reduction reaction to
produce alcohols (Figure 7.1; Table 7.1). Scientists are
exploiting this biosynthetic pathway to take advantage
of the amino acid biosynthesis capability of producing
microorganisms such as E. coli and S. cerevisiae to produce fusel alcohol, of which isobutanol appears to be
the most attractive. In particular, the specificity of decarboxylases has been suggested to be an important factor
influencing the composition of fusel alcohols (Harrison
and Collins, 1968; Suomalainen and Keranen, 1967).
Furthermore, the amount of fusel alcohols produced by
different yeasts and specific ADH activities with the corresponding alcohols as substrates was found to be
related as well (Singh and Kunkee, 1976). In recent years,
metabolic engineering strategy using heterologous hosts
such as E. coli and Clostridium cellulolyticum to produce
higher alcohols from glucose and cellulose, respectively,
is under investigation (Atsumi et al., 2008; Higashide
et al., 2011). Depending on the source, 2-ketoacid decarboxylase (KDC, encoded by the kivd gene) and ADH
(encoded by the adh2 gene), which play critical roles in
fusel oil production, may have broad substrate specificities toward the catalysis of 2-ketoacids and generation
of isobutanol (Figures 7.1 and 7.2). When these two
genes, kivd from Lactococcus lactis and adh2 from S. cerevisiae, were cloned and overexpressed in E. coli, approximately six long-chain alcohols including 1-propanol,
1-butanol, isobutanol, 2-methyl-1-butanol, 3-methyl1-butanol, and 2-phenylethanol were produced (Atsumi
et al., 2008). This strategy exploits the presence of a
highly active amino acid biosynthetic pathway in the
host microorganism, keto acid pathway, and the ability
112
7. ISOBUTANOL PRODUCTION FROM BIOENERGY CROPS
Glucose
Acetyl-CoA
PDA1
Pyruvate
2-acetolactate
ILV2
NAD(P)H
2-acetolactate
ILV5
NAD(P)+
NAD(P)H
ILV5
2,3-dihydroxy-isovalerate
NAD(P)+
Mitochondria
ILV2
Pyruvate
FIGURE 7.2 Schematic diagram depicting pathways
leading to valine and isobutanol biosynthesis in S. cerevisiae.
Genes encoding enzymes that catalyze each step are indicated and are as follows: ADH2 (alcohol dehydrogenase),
Bat1 and Bat2 (branched chain amino acid aminotransferases), ILV2 (acetolactate synthase), ILV3 (dihydroxyacid
dehydratase), ILV5 (acetohydroxyacid reductoisomerase),
Kdc/kivd/Pdc, 2-ketoacid decarboxylase (pyruvate decarboxylase); and PDA (pyruvate dehydrogenase complex).
(For color version of this figure, the reader is referred to the
online version of this book.)
ILV3
2,3-dihydroxy-isovalerate
2-ketoisovalerate
ILV3
2-ketoisovalerate
PDC6, 5, 1 (kivd)
L-glutamate
BAT1
2-oxoglutarate
L -Valine
Isobutyraldehyde
ADH2
NAD(P)H
Isobutanol
L-glutamate
BAT2
2-oxoglutarate
NAD(P)+
2-ketoisovalerate
PDC6, 5, 1 (kivd)
Isobutyraldehyde
ADH2
Cytosol
NAD(P)H
NAD(P)+
Isobutanol
of the host to reroute its 2-ketoacid intermediates for
alcohol synthesis (Atsumi et al., 2008). The amount of
individual alcohol produced is compared with the level
of its corresponding ketoacid. For example, when alsS
from Bacillus subtilis and ilvCD from E. coli were overexpressed in E. coli, the resulting strain accumulated
remarkable amounts of 2-ketoisovalerate (KIV) in the
fermentation broth. Furthermore, when kivd and adh2
were co-expressed in this recombinant strain, approximately 22 g/l isobutanol was produced over the course
of 112 h of fermentation (Atsumi et al., 2008). Notably,
AlsS of B. subtilis, kivd from L. lactis, adh2 from S. cerevisiae, and YqhD (nicotinamide adenine dinucleotide phosphate (NADPH)-dependent ADH) from E. coli have high
affinity for pyruvate and 2-ketoacids, 2-ketoacids, isobutyraldehyde, and isobutyraldehyde, respectively.
BIOCHEMISTRY OF ISOBUTANOL
FERMENTATION
In the presence of excess sugar, yeast, especially S. cerevisiae, has a strong tendency to undergo alcoholic fermentation, even when oxygen is available in excess (Van Diken
and Scheffers, 1986). In the biochemical pathway for
carbohydrate metabolism in most yeasts, two modes of
disaccharide metabolism exist. While extracellular hydrolysis of sucrose to glucose and fructose followed by transport of these monosaccharides into the cell is the most
common method for sucrose metabolism in yeast, transport of disaccharides by protonesugar symport followed
by intracellular hydrolysis occurs in maltose and lactose
metabolism (Weusthuis et al., 1994). However, hydrolysis
of sucrose can occur either intracellularly or extracellularly in S. cerevisiae (Santos et al., 1982), followed by the
phosphorylation of glucose to glucose-6-phosphate,
which is subsequently catabolized to pyruvate via the
EmbdeneMeyerhofeParnas pathway (Figure 7.2).
Although most of the synthesized pyruvate is decarboxylated to acetaldehyde (ethanal) by PDC followed by the
reduction of acetaldehyde to ethanol by ADH, a small
proportion of the pyruvate is converted to fusel alcohols
such as isobutanol (Figures 7.1 and 7.2; Table 7.1).
KIV is an important precursor for valine biosynthesis,
which is also shared by isobutanol production. KIV
biosynthesis is initiated by the condensation of two
pyruvate molecules to 2-acetolactate, which is catalyzed
by acetolactate synthase (ILV2 þ ILV6; Figure 7.2).
Notably, ILV6 is the regulatory subunit of acetolactate
synthase and an enhancer of ILV2 catalytic activity
METABOLIC ENGINEERING OF MICROORGANISMS FOR ISOBUTANOL PRODUCTION
(Chen et al., 2011). The 2-acetolactate is reduced to
2,3-dihydroxyisovalerate via catalysis by acetohydroxyacid reductoisomerase (ILV5), the precursor for KIV
biosynthesis (Figure 7.2; Velasco et al., 1993). Thus, KIV is
produced through catalysis of 2,3-dihydroxyisovalerate
by dihydroxyacid dehydratase (ILV3). Further, the bidirectional conversion between KIV and valine is catalyzed by
aminotransferases (Bat1 and Bat2). While aminotransferase
Bat1 is present in the mitochondrial matrix of S. cerevisiae,
aminotransferase Bat2 is present in the cytosol (Kispal
et al., 1996; Chen et al., 2011). Next, KIV is decarboxylated
by PDC, a KDC, to isobutyraldehyde and subsequently,
reduced to isobutanol by ADHs (Figure 7.2).
METABOLIC ENGINEERING
OF MICROORGANISMS FOR
ISOBUTANOL PRODUCTION
In an effort to metabolically engineer microorganisms
for efficient isobutanol production, various researchers
sought to understand isobutanol production at both
the molecular and protein levels. A few years before
2013, a number of studies directed at finding molecular
and biochemical bases for isobutanol synthesis have
been conducted (Table 7.2). Using a prokaryote (E. coli)
as host, Atsumi et al. (2008) demonstrated that KIV
can serve as a precursor for efficient isobutanol production from glucose in a biosynthetic pathway consisting
of 2-KDC from L. lactis and ADH2 from S. cerevisiae
with broad-range substrate specificity in combination
with the expression of the alsS gene (encoding AHAS
(acetohydroxy acid synthase)) from B. subtilis and the
ilvCD gene from E. coli. A follow-up study on the role of
different ADHs on isobutanol production with E. coli
showed that chromosomally encoded YqhD is the major
isobutyraldehyde-converting enzyme, and ADH2 from
S. cerevisiae contributes only to a minor extent to isobutanol
production in E. coli (Atsumi et al., 2010). This supposition
was made after yqhD gene was deleted from the genome
of E. coli; the generated recombinant E. coli strain (yqhD
deficient) accumulated isobutyraldehyde during fermentation and experienced 80% reduction in isobutanol
production. Using an a eukaryote (S. cerevisiae) and working on the assumption that inefficient production of isobutanol from glucose may be due to limited supply of KIV, a
precursor for the valine biosynthesis pathway (Figure 7.2),
Lee et al. (2012) added exogenous KIV (0.5 g/l) into the
growth medium. As expected, supplementation of the
medium with KIV improved the production of isobutanol,
which suggests that the endogenous pathway for producing KIV in S. cerevisiae (and potentially, other producing
microorganisms) is the limiting step in the isobutanol production pathway. Consequently, this finding made KIV
biosynthesis a rational target for metabolic engineering
113
toward designing more robust isobutanol-producing
strains.
Development of hyper-isobutanol-producing strains
has typically followed one of the three approaches: (1)
identification of rate-limiting steps in Ehrlich/2-ketoacid
biosynthetic pathways and overexpression of KIV biosynthetic genes; (2) improvement of heterologous expression
of enzymes of the isobutanol pathway by codon optimization; and (3) removal of feedback inhibition and deletion of
other competitive pathways, especially competition for
pyruvate. Pursuant to the first strategy, Lee et al. (2012)
screened and identified a 2-KDC exhibiting a relatively
higher activity on KIV through in vitro activity assays of
KDC using crude extracts of transformants overexpressing KDCs from various microorganisms. The highest
KDC activity with KIV was observed from the transformant expressing kivd from L. lactis subsp. lactis KACC13877.
Subsequently, Chen et al. (2011) evaluated the effect of
overexpressing the genes, ILV2, ILV3, ILV5, ILV6, and
BAT2, involved in valine metabolism, in different combinations in S. cerevisiae, on isobutanol production.
Following cultivation of the ILV2, ILV3, and ILV5 overexpressing strain (ILV235_XCY561) and the reference strain
(CEN.PK113-5D) in mineral glucose medium supplemented with uracil in fermentors under anaerobic conditions, the recombinant strain ILV235_XCY561 produced
0.97 0.14 mg isobutanol/g glucose, which was sixfold
higher than the control strain (Chen et al., 2011), hence
attesting to the fact that overexpression of the genes
ILV2, ILV3, and ILV5 may have led to a higher concentration of KIV, which resulted in higher isobutanol production. In parallel, Atsumi et al. (2009) engineered a
cyanobacterium, Synechococcus elongatus, by expressing a
KDC gene (kivd) from L. lactis in this cyanobacterium
using an expression cassette under the control of the isopropyl-b-D-thiogalactoside-inducible promoter Ptrc and
integration into neutral site I (Bustos and Golden, 1992)
by homologous recombination (Golden et al., 1987), and
strain SA578 was generated. To improve flux toward
KIV, alsS gene from B. subtilis and the ilvC and ilvD genes
from E. coli were integrated into neutral site II (Andersson
et al., 2000) in the genome of strain SA578 to generate strain
SA590, which produced high levels of isobutyraldehyde
upon cultivation in a Roux culture bottle at 30 C (Atsumi
et al., 2009, Figure 7.2). Given the fact that isobutyraldehyde can easily undergo a reduction reaction to produce
isobutanol, Atsumi et al. (2009) evaluated feasibility of
using ADHs (ADH2 from S. cerevisiae, YqhD from E. coli,
and AdhA from L. lactis) and Kivd from L. lactis (strain
SA590) to achieve this reduction reaction. These genes
(ADH2, YqhD, and AdhA) were integrated downstream
of kivd individually, hence, strains SA413, SA561 and
SA562 were generated, respectively. Whereas YqhD, an
NADPH-dependent enzyme, was the most active one in
S. elongatus, AdhA and ADH2 were nicotinamide adenine
114
7. ISOBUTANOL PRODUCTION FROM BIOENERGY CROPS
TABLE 7.2 Isobutanol Levels Produced by Genetically Modified Microorganisms during Fermentation
Species
Isobutanol
Titer (g/l)
Modified or Overexpressed
Genes
Escherichia coli
21.2
Carbon Source
References
Random mutagenesis and
selection
Hexose and pentose*
Smith and Liao (2011)
Clostridium cellulolyticum
0.66
alsS, ilvC, ilvD, kivd, yqhD
Cellulose
Higashide et al. (2011)
Saccharomyces cerevisiae
0.00136
xyla, xks1, tal1, aro10, adh2, ilv2,
ilv5, ilv3
Hexose and pentose
Brat and Boles (2013)
Saccharomyces cerevisiae
0.143
kdc (kivd), adh (adh6)
Hexose and pentose*
Kondo et al. (2012)
Saccharomyces cerevisiae
0.151
kivd, Ilv2, ilv3, ilv5
Hexose and pentose*
Lee et al. (2012)
Corynebacterium glutamicum
2.2
alsS, ilvC, ilvD, kivd
Hexose and pentose*
Smith et al. (2010)
Corynebacterium glutamicum
2.6
alsS, ilvC, ilvD, kivd, adhA
Hexose and pentose*
Smith et al. (2010)
Synechococcus elongatus 7942
0.018
kivd, yqhD
Carbon dioxide
Atsumi et al. (2009)
Synechococcus elongatus 7942
0.45
alsS, ilvC, kivd, yqhD
Carbon dioxide
Atsumi et al. (2009)
Synechocystis sp. strain PCC 6803
0.114
kivd, adhA
Carbon dioxide and hexose
Varman et al. (2013)
* Recombinant strains capable of using pentose sugars as carbon source are available.
Abbreviations: adhA, alcohol dehydrogenase from Lactococcus lactis; aro10, phenylpyruvate pyruvate decarboxylase; alsS, acetolactate synthase from Bacillus subtilis;
ilv2, acetolactate synthase; ilv3 (ilvD), dihydroxyacid dehydratase; ilv5 (ilvC), acetohydroxyacid reductoisomerase; Kdc/kivd/Pdc, 2-ketoacid decarboxylase
(pyruvate decarboxylase); yqhD, alcohol dehydrogenase/aldehyde reductase; xyla, xylose isomerase from Clostridium phytofermentans; xks1, xylulokinase; tal1,
transaldolase.
dinucleotide-dependent, and generated strain SA579,
which produced 450 mg/l isobutanol.
The second strategy derives from differences in
codon bias among different microorganisms, based on
their individual transfer RNA content and requisite
expression levels of specific proteins in each microorganism (Ikemura, 1985; Percudani et al., 1997). Given
the fact that codons at the beginning of an open reading
frame play a critical role in protein expression (Vervoort
et al., 2000), codon bias influences heterologous expression of foreign proteins to a great extent. For instance,
heterologously expressed protein levels in E. coli
(Atsumi et al., 2010) and S. cerevisiae (Brat and Boles,
2013) were improved by codon optimization, especially
at the 50 end of the coding sequence.
To realize the full potential of heterologously overexpressed genes in producing microorganisms with respect
to efficient isobutanol production, six genes including
adhE (ADH), ldhA (lactate dehydrogenase), frd (fumarate
reductase), fnr (encodes redox-sensing transcription
regulator, which partakes in the regulation of lactate
synthesis), pta (phosphate acetyltransferase), and pflB
(pyruvate formate lyase) that are involved in byproduct
formation in E. coli were deleted following overexpression of AlsS (B. subtilis), IlvC (E. coli), and IlvD (E. coli)
(Atsumi et al., 2008). These deletions may have increased
the level of pyruvate available for the valine biosynthesis
pathway. As a consequence, the isobutanol strain
(JCL260/pSA55/pSA69) produced more than 22 g/l in
112 h (Atsumi et al., 2008). In a similar study, Kondo
et al. (2012) overexpressed 2-KDC and ADH in S. cerevisiae to enhance the endogenous activity of the Ehrlich
pathway followed by overexpression of Ilv2, which catalyzes the first step in the valine synthetic pathway and
deletion of the PDC1 gene encoding a major PDC with
the intent of reducing ethanol flux via pyruvate. As a
result, S. cerevisiae YTD306 was generated. Upon cultivation of S. cerevisiae YTD306 along with modification of
culture conditions, a 13-fold increase in isobutanol titer
was produced (from 11 mg/l to 143 mg/l) when
compared with the control (Table 7.2, Kondo et al., 2012).
The strategy described here, in which amino acid biosynthetic and 2-ketoacid degradation pathways were
exploited for isobutanol production, represents a new
paradigm for biofuel production. Indeed, this paradigm
employs non-CoA-mediated chemistry and uses only
pyruvate as a precursor, unlike ethanol and butanol
production by native alcohols producing microorganisms that are CoA-dependent (Ezeji et al., 2010; Atsumi
et al., 2008).
FEASIBILITY OF USING BIOENERGY
CROPS AS SUSTAINABLE FEEDSTOCKS
FOR ISOBUTANOL PRODUCTION
Perennial grasses (lignocellulosic biomass) such as
switchgrass (Panicum virgatum), Miscanthus, and Napier
grass (Pennisetum purpureum) have been gaining attention recently for use in biofuel production because of
TECHNOLOGIES THAT HAVE BEEN DEVELOPED FOR SIMULTANEOUS BUTANOL FERMENTATION AND RECOVERY
their low energy requirement for production in the US
and Europe, and high biomass yield (Khanna et al.,
2008). Miscanthus species most often used in biomass
research is the sterile hybrid Miscanthus x giganteus, a
hardy and fast growing C4 grass that is cultivated via
rhizomes (Lewandowski et al., 2000). Yields per acre
vary depending on where the crop is grown. The typical
yield is 4e10 tons/acre, but yields have been known to
reach 16 tons/acre in southern Europe (Lewandowski
et al., 2000). Recent research on M. x giganteus and
switchgrass at the University of Illinois has produced
an average yield of 12 tons/acre and a maximum of
24.7 tons/acre for M. x giganteus and approximately
5 tons/acre for switchgrass (Heaton et al., 2008). The
harvestable biomass of Miscanthus is 190% greater than
that of corn and could produce 742 more gallons of
ethanol per acre (Heaton et al., 2008) or 600 more gallons
of butanol per acre. Napier grass, which belongs to sugarcane family and a native to Africa, is now found in
most tropical and subtropical regions of the world (Pennisetum purpureum, 2013). It has a high moisture content
of 70e80% and reaches maturation following 8 months
of plantation/rationing. Approximately two-thirds of
Napier grass biomass (dry weight) is composed of
sugars: glucan (38.43%), xylan (20.20%), galactan
(2.02%), arabinan (2.73%) and mannan (0.23%), and
lignin accounted for 20.93% of the lignocellulosic material, with ash (7.75%) and extractives (1.76%) comprising
the remaining fraction (Takara and Khanal 2011). Napier
grass has a rapid and dense growth, which have attracted
the attention of researchers as a potentially ideal source
for lignocellulosic biomass. Napier grass is capable of
producing 42 dry tons/acre/year, approximately double
the biomass yields of sugarcane and switchgrass
(McLaughlin and Kszos, 2005; Takara and Khanal, 2011).
Nonetheless, one of the key steps in the lignocellulosic biomass-to-fermentable sugars conversion is pretreatment. The goal of pretreatment is to disrupt the
biomass structure and disentangle ligninecarbohydrate
complex such that enzymatic hydrolysis of the carbohydrate fraction of the lignocellulosic biomass-to-simple
sugars can be achieved more rapidly and with greater
yield (Mosier et al., 2005; Ezeji and Blaschek, 2010).
Economic analysis of the current pretreatment methods
has shown that the relatively high costs of biofuel
(ethanol) production from lignocellulosic biomass arise
mainly from costs associated with three factors: (a) harsh
pretreatment conditions (high temperature, high pressure, use of acids or bases, long residence time, and so
on, allowing for inhibitor formation); (b) overuse
of expensive enzymes; and (c) recovery of end products
(low ethanol concentration in beer; Eggeman and Elander, 2005; Ezeji and Blaschek, 2010). Technologies that
lead to improvement in any of these areas will help to
make isobutanol production using energy crops as
115
feedstock more cost-effective. Moreover, energy crops
can be genetically modified to improve biomass yield
(per acre per year) without the risk of compromising
grain yield or quality along with reducing their recalcitrance to efficient deconstruction to monomeric sugars.
While producing microorganisms have not been
shown to directly utilize lignocellulosic biomass as a carbon source for isobutanol production, Higashide et al.
(2011) recently demonstrated the first production of isobutanol from crystalline cellulose using C. cellulolyticum.
This breakthrough was accomplished after a couple of
attempts. First, the activities of the first three enzymes
in the isobutanol production pathway were examined
by transforming plasmids expressing alsS or alsS ilvCD
into C. cellulolyticum and no C. cellulolyticum alsS or
alsS ilvCD transformants were obtained. Realizing that
alsS and alsS ilvCD transformants could not be obtained,
a second attempt wherein genes encoding B. subtilis
a-acetolactate synthase, E. coli acetohydroxyacid isomeroreductase, E. coli dihydroxyacid dehydratase, L. lactis
KDC, and E. coli and L. lactis ADHs (alsS, ilvCD, kivd,
and adhA, complete isobutanol production pathway
genes) were expressed in C. cellulolyticum (Higashide
et al., 2011). Despite a mutation in alsS, the alsS ilvCD
kivd adhA strain produced 140 and 420 mg/l isobutanol
from cellobiose and cellulose, respectively. When plasmids expressing kivd yqhD alsS ilvCD, in which alsS
was the third gene in the operon, was constructed and
transformed into C. cellulolyticum, 364 and 660 mg/l
isobutanol were produced from cellobiose and cellulose,
respectively (Table 7.2). Given the fact that isobutanol
production technology has been changing at a rapid
pace, this accomplishment in which cellulose is used
as a carbon source is significant because it opens the
frontier for utilizing lignocellulosic biomass such as
energy crops for isobutanol production.
TECHNOLOGIES THAT HAVE BEEN
DEVELOPED FOR SIMULTANEOUS
BUTANOL FERMENTATION AND
RECOVERY
Production of isobutanol by fermentation is looking
attractive owing to two main reasons: (1) the higher
tolerance of producing microorganisms to isobutanol,
usually 36.9e51.9 g/l as compared to n-butanol (called
butanol in the later sections of this chapter), which is
20e30 g/l in selected hyper-producing strains and (2)
having a lower boiling point (108 C vs 118 C) than
butanol, which comparatively may be economical to
recover. The above titer values for the isobutanol are
without simultaneous product recovery (Baez et al.,
2011). However, in this report, it was mentioned that
in situ recovery by gas stripping improves isobutanol
116
7. ISOBUTANOL PRODUCTION FROM BIOENERGY CROPS
production. To the authors’ knowledge, this is the only
report where isobutanol fermentation and recovery
were performed simultaneously.
Fermentative production of isobutanol or butanol
can be economically achieved in two ways: (1) by
developing the high-tolerant or high-producer strain,
which also offers some benefits during the recovery
process, and (2) using energy-efficient process engineering techniques to simultaneously remove the toxic
product. Interestingly, the first approach has been
reported for isobutanol fermentation (Baez et al.,
2011) with great success. For butanol producing strains,
numerous attempts have been made to improve performance; however, success has been limited, with
maximum titer stagnating around 21 g/l (total acetone
butanol ethanol (ABE) 32.6 g/l) (Chen and Blaschek,
1999). Nevertheless, butanol has drawn significant
amount of attention from the process engineering point
of view. One of the main focuses has been the development of integrated process technologies where fermentation and simultaneous product recovery have been
integrated. The reader is directed to a couple of following
reports, where much higher production of ABE than in a
batch system has been achieved. Employing such integrated systems (gas stripping and perstraction), cumulative ABE production from 461 g/l to 698 g/l (Ezeji et al.,
2013; Jeon and Lee, 1989) has been achieved compared to
21 g/l butanol or 51.9 g/l isobutanol. It should also
be noted that several simultaneous product recovery
systems such as adsorption, liquideliquid extraction,
pervaporation, ionic liquid extraction, and reverse
osmosis have been investigated (Qureshi et al., 2013).
Also, other advances have been made for butanol production from agricultural residues such as wheat straw
(Qureshi et al., 2007; Qureshi et al., 2008a), barley straw
(Qureshi et al., 2010a), corn stover (Parekh et al., 1988;
Qureshi et al., 2010b), switchgrass (Qureshi et al.,
2010b), and distillers dry grains and soluble (Ezeji and
Blaschek, 2008).
CONCLUSION AND
FUTURE PERSPECTIVE
Given the higher microbial tolerance of isobutanol
and its greater volatility in comparison to butanol, it is
likely that simultaneous product recovery using gas
stripping, perstraction, and/or pervaporation would
achieve even higher production levels than reported
for butanol, thus benefiting economics of isobutanol
production process. To make this biofuel even more
attractive, recent advances in fermentation of lignocellulosic biomass such as separate hydrolysis of lignocellulosic biomass, combined with fermentation, and
product recovery separate hydrolysis, fermentation,
and recovery (SHFR), and simultaneous saccharification,
fermentation, and recovery (SSFR) should be applied
(Qureshi et al., 2013). In a process where wheat straw
was used to produce butanol by SSFR, 192 g/l ABE was
produced from 430 g/l of lignocellulosic sugars (Qureshi
et al., 2008b). At this stage, engineering producing microorganisms by applying similar method already reported
by Higashide et al. (2011) to utilize pentose sugars such
as arabinose and xylose as substrates for the production
of isobutanol is looking promising.
Owing to the fact that butanol producing cultures
have the potential to be tolerant to isobutanol, it is
reasonable to express isobutanol-producing genes in solventogenic Clostridium species. Such an undertaking
would have two advantages: (1) ability of the developed
strain to utilize pentose sugars that accounts for about
30e40% of carbohydrates present in lignocellulosic
biomass and (2) the developed strain may produce
higher titers of isobutanol than yeast or E. coli.
References
Andersson, C.R., Tsinoremas, N.F., Shelton, J., Lebedeva, N.V.,
Yarrow, J., Min, H., Golden, S.S., 2000. Application of bioluminescence to the study of circadian rhythms in cyanobacteria.
Methods Enzymol. 305, 527e542.
Atsumi, S., Hanai, T., Liao, J.C., 2008. Non-fermentative pathways for
synthesis of branched-chain higher alcohols as biofuels. Nature
451, 86e89.
Atsumi, S., Higashide, W., Liao, J.C., 2009. Direct photosynthetic
recycling of carbon dioxide to isobutyraldehyde. Nat. Biotechnol.
27, 1177e1180.
Atsumi, S., Wu, T.-Y., Eckl, E.-M., Hawkins, S.D., Buelter, T.,
Liao, J.C., 2010. Engineering the isobutanol biosynthetic pathway
in Escherichia coli by comparison of three aldehyde reductase/
alcohol dehydrogenase genes. Appl. Microbiol. Biotechnol. 85,
651e657.
Baez, A., Cho, K.M., Liao, J.C., 2011. High-flux isobutanol production
using engineered Escherichia coli: a bioreactor study with in situ
product removal. Appl. Microbiol. Biotechnol. 90, 1681e1690.
Brat, D., Boles, E., 2013. Isobutanol production from D-xylose by
recombinant Saccharomyces cerevisiae. FEMS Yeast Res. 13, 241e244.
Budavari, S., 1996. The Merck Index. An Encyclopedia of chemicals,
Drugs, and Biologicals. Merck Research Laboratories, Division of
Merck & Co., Inc, Whitehouse Station, NJ.
Bustos, S.A., Golden, S.S., 1992. Light-regulated expression of the
psbD gene family in Synechococcus sp. strain PCC 7942: evidence
for the role of duplicated psbD genes in cyanobacteria. Mol. Gen.
Genet. 232, 221e230.
Bizziari, S.N., Gubler, R., Kishi, A., 2002. CEH Marketing Research
Report for Plasticizer Alcohols.
Chen, C.-K., Blaschek, H.P., 1999. Acetate enhances solvent production
and prevents degeneration in Clostridium beijerinckii BA101. Appl.
Microbiol. Biotechnol. 52, 170e173.
Chen, X., Nielsen, K.F., Borodina, I., Kielland-Brandt, M.C.,
Karhumaa, K., 2011. Increased isobutanol production in Saccharomyces cerevisiae by overexpression of genes in valine metabolism.
Biotechnol. Biofuels 4, 21.
Cronk, T.C., Mattick, L.R., Steinkraus, K.H., Hackler, L.R., 1979. Production of higher alcohols during Indonesian tape ketan fermentation. Appl. Environ. Microbiol. 37, 892e896.
REFERENCES
Daubert, T.E., Danner, R.P., 1985. Data Compilation Tables of Properties of Pure Compounds. 1985. Design Institute for Physical
Property Data, American Institute of Chemical Engineers.
Deanda, K., Zhang, M., Eddy, C., Picataggio, S., 1996. Development of
an arabinose-fermenting Zymomonas mobilis strain by metabolic
pathway engineering. Appl. Environ. Microbiol. 62, 4465e4470.
Dickinson, J.R., Harrison, S.J., Hewlins, M.J., 1998. An investigation of
the metabolism of valine to isobutyl alcohol in Saccharomyces cerevisiae. J. Biol. Chem. 273, 25751e25756.
Dickinson, J.R., Harrison, S.J., Dickinson, J.A., Hewlins, M.J., 2000. An
investigation of the metabolism of isoleucine to active amyl alcohol
in Saccharomyces cerevisiae. J. Biol. Chem. 275, 10937e10942.
Dien, B.S., Iten, L.B., Bothast, R.J., 1999. Conversion of corn fiber to
ethanol by recombinant E. coli strain FBR3. J. Ind. Microbiol. Biotechnol. 22, 575e581.
Dien, B.S., Nichols, N.N., OBryan, P.J., Bothast, R.J., 2000. Development of new ethanologenic Escherichia coli strains for fermentation
of lignocellulosic biomass. Appl. Biochem. Biotechnol. 84, 181e196.
Eggeman, T., Elander, R.T., 2005. Process and economic analysis of
pretreatment technologies. Bioresour. Technol. 96, 2019e2025.
Ezeji, T.C., Blaschek, H.P., 2008. Fermentation of dried distillers’ grains
and soluble (DDGS) hydrolysates to solvents and value-added products by solventogenic clostridia. Bioresour. Technol. 99, 5232e5242.
Ezeji, T.C., Blaschek, H.P., 2010. Butanol production from lignocellulosic biomass. In: Blaschek, H.P., Ezeji, T.C., Scheffran, J. (Eds.),
Biofuels from Agricultural Wastes and Byproducts. WileyBlackwell, Ames, IA, pp. 19e37.
Ezeji, T.C., Karcher, P.M., Qureshi, N., Blaschek, H.P., 2005. Improving
performance of a gas stripping-based recovery system to remove
butanol from Clostridium beijerinckii fermentation. Bioprocess
Biosyst. Eng. 27, 207e214.
Ezeji, T., Milne, C., Price, N.D., Blaschek, H.P., 2010. Achievements
and perspectives to overcome the poor solvent resistance in
acetone and butanol-producing microorganisms. Appl. Microbiol.
Biotechnol. 85, 1697e1712.
Ezeji, T.C., Qureshi, N., Blaschek, H.P., 2013. Microbial production of a
biofuel (acetone-butanol-ethanol) in a continuous bioreactor:
impact of bleed and simultaneous product removal. Bioprocess
Biosyst. Eng. 36, 109e116.
Garcia Sanchez, R., Karhumaa, K., Fonseca, C., Sànchez Nogué, V.,
Almeida, J.R.M., Larsson, C.U., Bengtsson, O., Bettiga, M., HahnHägerdal, B., Gorwa-Grauslund, M.F., 2010. Improved xylose and
arabinose utilization by an industrial recombinant Saccharomyces
cerevisiae strain using evolutionary engineering. Biotechnol. Biofuels 3, 13.
Golden, S.S., Brusslan, J., Haselkorn, R., 1987. Genetic engineering of
the cyanobacterial chromosome. Methods Enzymol. 153, 215e231.
Harrison, G.A.F., Collins, E., 1968. Gas-chromatographic determination of individual a-keto acids in beer. Proc. Am. Soc. Brew. Chem.
1968, 101e105.
Hazelwood, L.A., Daran, J.M., van Maris, A.J., Pronk, J.T.,
Dickinson, J.R., 2008. The Ehrlich pathway for fusel alcohol
production: a century of research on Saccharomyces cerevisiae
metabolism. Appl. Environ. Microbiol. 74, 2259e2266.
Heaton, E.A., Dohleman, F.G., Long, S.P., 2008. Meeting US biofuel
goals with less land: the potential of Miscanthus. Global Change
Bio. 14, 2000e2014.
Higashide, W., Li, Y., Yang, Y., Liao, J.C., 2011. Metabolic engineering
of Clostridium cellulolyticum for production of isobutanol from
cellulose. Appl. Environ. Microbiol. 77, 2727e2733.
Ikemura, T., 1985. Codon usage and tRNA content in unicellular and
multicellular organisms. Mol. Biol. Evol. 2, 13e14.
Jeon, Y.J., Lee, Y.Y., 1989. In situ product separation in butanol
fermentation by membrane-assisted extraction. Enzyme Microb.
Technol. 11, 575e582.
117
Jin, Y.-S., Alper, H., Yang, Y.-T., Stephanopoulos, G., 2005. Improvement of xylose uptake and ethanol production in recombinant
Saccharomyces cerevisiae through an inverse metabolic engineering
approach. Appl. Environ. Microbiol. 71, 8249e8256.
Khanna, M., Dhungana, B., Clifton-Brown, J., 2008. Costs of producing
Miscanthus and switchgrass for bioenergy in Illinois. Biomass
Bioenergy 32, 82e493.
Kispal, G., Steiner, H., Court, D.A., Rolinski, B., Lill, R., 1996. Mitochondrial and cytosolic branched-chain amino acid transaminases
from yeast, homologs of the myc oncogene-regulated Eca39 protein. J. Biol. Chem. 271, 24458e24464.
Kondo, T., Tezuka, H., Ishii, J., Matsuda, F., Ogino, C., Kondo, A., 2012.
Genetic engineering to enhance the Ehrlich pathway and alter
carbon flux for increased isobutanol production from glucose by
Saccharomyces cerevisiae. J. Biotechnol. 159, 32e37.
Koukiekolo, R., Cho, H.-Y., Kosugi, A., Inui, M., Yukawa, H., Doi, R.Y.,
2005. Degradation of corn fiber by Clostridium cellulovorans cellulases and hemicellulases and contribution of scaffolding protein
CbpA. Appl. Environ. Microbiol. 71, 3504e3511.
Ladisch, M.R., 1991. Fermentation-derived butanol and scenarios for
its uses in energy-related applications. Enzyme Microb. Technol.
13, 280e283.
Lee, W.-H., Seo, S.-O., Bae, Y.-H., Nan, H., Jin, Y.-S., Seo, J.-H., 2012.
Isobutanol production in engineered Saccharomyces cerevisiae by
overexpression of 2-ketoisovalerate decarboxylase and valine
biosynthetic enzymes. Bioprocess Biosyst. Eng. 35, 1467e1475.
Lewandowski, I., Clifton-Brown, J.C., Scurlock, J.M.O., Huisman, W.,
2000. Miscanthus: European experience with a novel energy crop.
Biomass Bioenergy 19, 209e227.
Mariano, A.P., Qureshi, N., Filho, R.M., Ezeji, T.C., 2011. Bioproduction
of butanol in bioreactors: new insights from simultaneous in situ
butanol recovery to eliminate product toxicity. Biotechnol. Bioeng.
108, 1757e1765.
Mariano, A.P., Qureshi, N., Filho, R.M., Ezeji, T.C., 2012. Assessment of
in situ butanol recovery by vacuum during acetone butanol ethanol
(ABE) fermentation. J. Chem. Technol. Biotechnol. 87, 334e340.
McLaughlin, S.B., Kszos, L.A., 2005. Development of switchgrass
(Panicum virgatum) as a bioenergy feedstock in the United States.
Biomass Bioenergy 28, 515e535.
Mosier, N., Wyman, C., Dale, B.E., Elander, R., Lee, Y.Y.,
Holtzapple, M., Ladisch, M., 2005. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour.
Technol. 96, 673e686.
Parekh, S.R., Parekh, R.S., Wayman, M., 1988. Ethanol and butanol
production by fermentation of enzymatically saccharified
SO2-prehydrolysed lignocellulosics. Enzyme Microb. Technol.
10, 660e668.
Pennisetum purpureum, 2013. Pacific Island Ecosystems at Risk (PIER).
Available at: <http://www.hear.org/pier/species/pennisetum_
purpureum.htm> (accessed May, 2013).
Percudani, R., Pavesi, A., Ottonello, S., 1997. Transfer RNA gene
redundancy and translational selection in Saccharomyces cerevisiae.
J. Mol. Biol. 268, 322e330.
Qureshi, N., Blaschek, H.P., 2000. Economics of butanol fermentation
using hyper-butanol producing Clostridium beijerinckii BA101.
Trans IChemE 78, Part C, 139e144.
Qureshi, N., Saha, B.C., Cotta, M.A., 2007. Butanol production from
wheat straw hydrolysate using Clostridium beijerinckii. Bioprocess
Biosyst. Eng. 30, 419e427.
Qureshi, N., Saha, B.C., Hector, R.E., Hughes, S.R., Cotta, M.A., 2008a.
Butanol production from wheat straw by simultaneous saccharification and fermentation using Clostridium beijerinckii: part
Idbatch fermentation. Biomass Bioenergy 32, 168e175.
Qureshi, N., Saha, B.C., Cotta, M.A., 2008b. Butanol production from
wheat straw by simultaneous saccharification and fermentation
118
7. ISOBUTANOL PRODUCTION FROM BIOENERGY CROPS
using Clostridium beijerinckii: part IIdfed-batch fermentation.
Biomass Bioenergy 32, 785e791.
Qureshi, N., Saha, B.C., Dien, B., Hector, R., Cotta, M.A., 2010a. Production of butanol (a biofuel) from agricultural residues: part Iduse
of barley straw hydrolysate. Biomass Bioenergy 34, 559e565.
Qureshi, N., Saha, B.C., Hector, R.E., Dien, B., Hughes, S.R., Liu, S., Iten, L.,
Bowman, M.J., Sarath, G., Cotta, M.A., 2010b. Production of butanol (a
biofuel) from agricultural residues: part IIduse of corn stover and
switchgrass hydrolysates. Biomass Bioenergy 34, 566e571.
Qureshi, N., Liu, S., Ezeji, T.C., 2013. Cellulosic butanol production
from agricultural biomass and residues: recent advances in technology. In: Lee, J. (Ed.), Advanced Biofuels and Bioproducts.
Springer Science þ Business Media, New York, pp. 247e265.
Ryan, E.D., Kohlhaw, G.B., 1974. Subcellular localization of isoleucinevaline biosynthetic enzymes in yeast. J. Bacteriol. 120, 631e637.
Santos, E.,L., Rodriguez, M., Elorza, V., Sentandreu, R., 1982. Uptake
of sucrose by Saccharomyces cerevisiae. Arch. Biochem. Biophys. 216,
652e660.
Singh, R., Kunkee, R., 1976. Alcohol dehydrogenase activities of wine
yeasts in relation to higher alcohol formation. Appl. Environ.
Microbiol. 32, 666e670.
Smith, K.M., Liao, J.C., 2011. An evolutionary strategy for isobutanol
production strain development in Escherichia coli. Metab. Eng. 13,
674e681.
Smith, K.M., Cho, K.-M., Liao, J.C., 2010. Engineering Corynebacterium
glutamicum for isobutanol production. Appl. Microbiol. Biotechnol.
87, 1045e1055.
Suomalainen, H., Keranen, A.J.A., 1967. Keto acids formed by Baker’s
yeast. J. Inst. Brew. 73, 477e484.
Takara, D., Khanal, S.K., 2011. Green processing of tropical banagrass
into biofuel and biobased products: an innovative biorefinery
approach. Bioresour. Technol. 102, 1587e1592.
Ter Schure, E.G., Flikweert, M.T., van Dijken, J.P., Pronk, J.T.,
Verrips, C.T., 1998. Pyruvate decarboxylase catalyzes decarboxylation of branched-chain 2-oxo acids but is not essential for fusel
alcohol production by Saccharomyces cerevisiae. Appl. Environ.
Microbiol. 64, 1303e1307.
Valvani, S.C., Yalkowsky, S.H., Rosemand, T.J., 1981. Solubility and
Partitioning. IV. Aqueous Solubility and Octanol-Water Partition
Coefficients of Liquid Non-electrolytes. J. Pharm. Sci. 70, 502e507.
Van Diken, J.P., Scheffers, W.A., 1986. Redox balances in the metabolism of sugars by yeasts. FEMS Microbiol. Rev. 32, 199e224.
Varman, A.M., Xiao, Y., Pakrasi, H.B., Tang, Y.J., 2013. Metabolic
engineering of Synechocystis sp. strain PCC 6803 for isobutanol
production. Appl. Environ. Microbiol. 79, 908e914.
Velasco, J.A., Cansado, J., Pena, M.C., Kawakami, T., Laborda, J.,
Notario, V., 1993. Cloning of the dihydroxyacid dehydrataseencoding gene (ILV3) from Saccharomyces cerevisiae. Gene 137,
179e185.
Vervoort, E.B., van Ravestein, A., van Peij, N.N.M.E., Heikoop, J.C.,
van Haastert, P.J.M., Verheijden, G.F., Linskens, M.H.K., 2000.
Optimizing heterologous expression in Dictyostelium: importance
of 5’ codon adaptation. Nucleic Acids Res. 28, 2069e2074.
Weusthuis, R.A., Pronk, J.T., van den Broek, P.J.A., van Dijken, J.P.,
1994. Chemostat cultivation as a tool for studies on sugar transport
in yeasts. Microbiol. Rev. 58, 616e630.
Wisselink, H.W., Toirkens, M.J., del Rosario Franco Berriel, M.,
Winkler, A.A., van Dijken, J.P., Pronk, J.T., van Maris, A.J.A., 2007.
Engineering of Saccharomyces cerevisiae for efficient anaerobic
alcoholic fermentation of L-arabinose. Appl. Environ. Microbiol.
73, 4881e4891.
Zhang, M., Eddy, C., Deanda, K., Finkestein, M., Picataggio, S., 1995.
Metabolic engineering of a pentose metabolism pathway in ethanologenic Zymomonas mobilis. Science 267, 240e243.
C H A P T E R
8
Lipase-Catalyzed Biodiesel Production:
Technical Challenges
Rama Raju Baadhe 1, Ravichandra Potumarthi 2,*, Vijai K. Gupta 3
1
Department of Biotechnology, National Institute of Technology, Warangal, Andhra Pradesh, India,
2
Department of Chemical Engineering, Monash University, Clayton, Victoria, Australia,
3
Molecular Glycobiotechnology Group, Department of Biochemistry, School of Natural Sciences,
National University of Ireland Galway, Galway, Ireland
*Corresponding author email: ravichandra.potumarthi@monash.edu; pravichandra@gmail.com
O U T L I N E
Introduction
119
Chemistry of Biodiesel
120
Transesterification
120
Disadvantages of Chemical Transesterification
120
Advantages of Using Lipases in
Biodiesel Production
121
Historical Background of Lipase
121
Animal Oils/Fats
Waste Oils/Fats
Algae Oils
123
123
124
Choice of Enzyme
124
Molar Ratio (Alcohol/Oil)
124
Temperature
124
Water Content
126
Acyl Acceptors
126
Lipase-Catalyzed Transesterification Done
in Two Approaches
121
Solvents
126
Advantages of Immobilized Lipase
122
Reactor System
126
Technical Challenges
123
Conclusions
127
Feedstock
Vegetable Oils
123
123
References
127
INTRODUCTION
World’s commercial primary energy needs are mostly
supplied through fossil fuels and accounts about 87% of
total energy source (OPEC, 2011, 2012). Primary energy
demand by 2035 increases to 54% and still fossil fuels contributes 82% of the global total by 2035 (OPEC, 2012). All
fossil-fuel sources are finite and if the crude oil consumption continued at current usage rates, it will last
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00008-5
only for 54.2 years (British Petroleum Statistical Review,
2012). Projected demand for oil reaches 110 mb/day by
2035. Among the fossil fuel, diesel fuels have an essential function in the industrial, transportation and agricultural sectors in developing countries. Gradual
depletion of crude oil and emission of greenhouse gases
in to the environment triggers the alarm for suitable
alternative fuels for use in diesel engines (Ganesan
et al., 2009). Biodiesel is one of the attractive and
119
Copyright Ó 2014 Elsevier B.V. All rights reserved.
120
8. LIPASE-CATALYZED BIODIESEL PRODUCTION: TECHNICAL CHALLENGES
alternative fuels along with bioethanol. Biodiesel or fatty
acid methyl esters (FAMEs) are mono-alkyl esters of
long-chain fatty acids, derived from transesterification
of triglycerides (plant or animal or algal origin). It can
be used directly in its pure form or as a blend with conventional diesel fuel in diesel engines (Ma and Hanna,
1999). This fuel is biodegradable and nontoxic and has
low emission profiles when compared to petroleum
diesel (Krawczyk, 1996). But the cost of biodiesel, however, is the main obstacle to commercialize the product.
There are four primary ways of making biodiesel:
direct use and blending (Ma and Hanna, 1999), micro
emulsions (Schwab et al., 1987), thermal cracking (pyrolysis) (Sonntag, 1979) and transesterification. However,
the first three have some limitations and drawbacks in
case of physiochemical properties of biodiesel (Schwab
et al., 1987). Transesterification is the well-known method
and involves conversion of oils or fat to FAMEs or fatty
acid ethyl esters in the presence of a catalyst such as
acid, base or lipase (Bisen et al., 2010). The conventional
method for producing biodiesel involves acid and base
catalysts to form fatty acid alkyl esters. Processing
expenses and environmental concerns associated with
biodiesel production and difficulties connected with
by-products recovery have led to the search for alternative production methods and alternative sources (Bisen
et al., 2010). Enzyme-mediated transesterification can
be a moderate alternative to produce biodiesel in its
pure form, which also makes its separation easy against
the by-product (glycerol). But still due to the cost of
enzyme, commercialization of biodiesel has not come to
reality. Though there are many attempts made for biodiesel production through enzyme-mediated method
(Ranganathan et al., 2008; Sanchez and Vasudevan,
2006; Lai et al., 2005; Noureddini et al., 2005; De Oliveira
et al., 2004; Xu et al., 2004; Sha et al., 2003; Belafi-Bako
et al., 2002; Iso et al., 2001; Fukuda et al., 2001; Freedman
et al., 1984), profitable commercial production was not
achieved for industrial utilization. Recombinant DNA
and protein engineering technologies improved the
quantities and catalytic efficiency of lipase (Akoh et al.,
2007). There are several technical challenges that need
to be addressed to make biodiesel production profitable.
Some of them associated with enzyme transesterification
process. In this chapter, some of the technical challenges
3
CH2–O–CO–R
2 H–C–O–CO–R′
1
CH2–O–CO–R′′
1,2,3,-O-tri-acyl -glycerol
R′, Rand R′′ are saturated or
unsaturated chains
FIGURE 8.1 Chemical structure of triacylglycerol. Source: Bisen
et al. (2010).
involved in the lipase-catalyzed biodiesel production
were discussed.
CHEMISTRY OF BIODIESEL
Chemically biodiesel is defined as mono-alkyl
(methyl or ethyl) esters of triacylglycerol. All vegetable
oils, algal lipids and animal fats (triacylglycerol/triglyceride molecules) consist of a three-carbon chain forms
the glycerol backbone, which consists of three long fatty
acid chains (Figure 8.1). Amounts of each fatty acid present in molecules determine the properties of triacylglycerol (Knothe, 2001).
TRANSESTERIFICATION
In chemical transesterification process, fatty acid reacts with any alcohol and forms mono-alkyl ester (biodiesel) in the presence of a catalyst (acid, base and
enzyme). General reaction scheme of biodiesel production is shown in Figure 8.2. The reaction has two inputs:
triacylglycerol and the alcoholdcommonly ethanol or
methanol is used (Meher et al., 2004).
DISADVANTAGES OF CHEMICAL
TRANSESTERIFICATION
• Requires high reaction temperatures.
• Soap formation: in the base-catalyzed
transesterification process, free fatty acid (FFAs) level
of feedstock should be less, otherwise it will result in
too much soaps formation.
• Recovery of by-product: purification of glycerol is
very difficult.
FIGURE 8.2 The scheme of transesterification reaction: (R ¼ CH3) alcohol is methanol.
LIPASE-CATALYZED TRANSESTERIFICATION DONE IN TWO APPROACHES
• Pretreatment step needed: FFAs level of the
feedstocks should not exceed 3 wt%, beyond which it
has to undergo pretreatment steps before
transesterification (Leung et al., 2010).
• Yield of methyl esters: yields of the methyl esters are
lower compared to enzymatic transesterification.
• Purification of methyl esters: purification of methyl
esters requires repeated washing which increases
process operational cost.
• Less active: since alkali catalysts (NaOH and KOH)
are inexpensive, they are preferred but activity is less
(Demirbas, 2008).
• Energy consumption: alkali-catalyzed
transesterification needed large energy consumption
during downstream biodiesel refining process
(Madras and Kolluru, 2004).
• Corrosion: when H2SO4 is used as catalyst, it leads to
corrosion of the reactor and huge wastewater
generated during neutralization of mineral acid
(Atadashi et al., 2013).
• Use of homogenous catalysts makes biodiesel
product separation difficulty and recovery of catalyst
cumbersome (Atadashi et al., 2013).
• Acid-catalyzed transesterification reaction needs
higher alcohol-to-oil molar ratios (Atadashi et al., 2013).
• In base-catalyzed transesterification reaction, large
amount of catalyst is needed.
Difficulties arise during chemical catalysis can be
overcome by enzyme-mediated (biocatalysts) transesterification and they are becoming increasingly important
in biodiesel preparation due to their ability to beat chemical catalysts. Lipases (E.C.3.1.1.3) are widely considered
as biocatalysts to catalyze transesterification and esterification reactions.
ADVANTAGES OF USING LIPASES IN
BIODIESEL PRODUCTION
Advantages of using lipases in biodiesel production
are the following:
• Ability to work under different media environments
includes biphasic system and monophasic system
(aqueous and nonaqueous) (Mittelbach, 1990; Linko
et al., 1994; Mukesh et al., 1994).
• They can be produced in bulk and are sturdy and
adaptable enzymes.
• Separation is not necessary if transesterification
process with lipase carried out in a packed-bed
reactor.
• Proficiencies like short-chain, alcohol-tolerant and
higher thermo stability of lipase make it very
appropriate for use in biodiesel production (Ghaly
et al., 2010).
121
• Thermal stability of lipases makes it possible to run
the transesterification process at elevated
temperatures which allows (1) increased solubility of
lipids and other hydrophobic substrates in water;
(2) higher diffusion rates; (3) decreased substrate
viscosities; (4) increased reactant solubilities;
(5) faster reaction rates; and (6) reduced risk of
microbial contamination.
HISTORICAL BACKGROUND OF LIPASE
Over 300 years ago, triglycerides hydrolyzing
enzymes have been studied well. Nearly 70 years ago,
lipase’s catalysis ability and synthesis ability have
been known. In 1856, Claude Bernard first revealed
that pancreatic juice contains an enzyme (lipase) that
hydrolyzed the insoluble oil droplets and converted
them in to soluble products. In 1901 activity of microbial
lipases has been observed in Bacillus prodigiosus, Bacillus
pyocyaneus and Bacillus fluorescens. Serratia marcescens,
Pseudomonas aeruginosa and Pseudomonas fluorescens
are the best-studied lipase-producing bacteria (Fariha
et al., 2006).
Lipase-catalyzed biodiesel production was reported
first by Mittelbach (1990). Depending upon the specificity, lipases are divided into three groups: (1)
1,3-specific, (2) fatty acid-specific, and (3) nonspecific.
Among these three, 1,3-specific lipases discharge fatty
acids from positions 1 and 3 of a glyceride and hydrolyze ester bonds in these positions (Antczak et al.,
2009; Ribeiro et al., 2011).
LIPASE-CATALYZED
TRANSESTERIFICATION DONE IN TWO
APPROACHES
1. Extracellular/free lipase lipases (i.e. recovered and
purified from the cultivation broth): For industrial
level production of extracellular lipases, bacteria,
yeast and fungi are preferred. Lipases from different
sources are able to catalyze the same reaction
(Table 8.1). Bacterial and fungal lipases are mostly
used in biodiesel production and recently,
Streptomyces sp. was proved as an effective lipase
producing microbe and the enzyme produced was
appropriate for biodiesel production (Cho et al.,
2012). Use of free or extracellular enzymes was
limited due to their price. Cost of the enzyme was
increased due to their specific separation and
purification techniques. Extracellular lipases are
soluble enzymes and they are dispersed in the
solution and can move freely during the catalytic
reaction, thus difficult to handle and reuse (Iso et al.,
2001). One auspicious approach to overcome this
122
8. LIPASE-CATALYZED BIODIESEL PRODUCTION: TECHNICAL CHALLENGES
TABLE 8.1
Different Microbial Sources for Lipases Used in
Biodiesel Production
Lipase Producing
Microorganisms
References
Pseudomonas fluorescens
Iso et al. (2001)
Pseudomonas cepacia
Noureddini et al. (2005)
Candida antarctica
Nelson et al. (1996)
Rhizopus delemar
Nelson et al. (1996)
Rhizopus oryzae
Ghamgui et al. (2004)
Mucor miehei
Nelson et al. (1996)
Geotrichum candidum
Nelson et al. (1996)
Candida rugosa
Shimada et al. (2002);
Ma et al. (2002);
Chowdary and Prapulla (2002)
Rhizomucor miehei
Soumanou and Bornscheuer (2003a,b)
Thermomyces lanuginosa
Iso et al. (2001);
Xu et al. (2003);
Soumanou and Bornscheuer (2003a,b);
Du et al. (2003)
Aspergillus niger
Haas et al. (2002)
Pseudomonas cepacia
Noureddini et al. (2005)
Chromobacterium viscosum
Yahya et al. (1998)
Photobacterium lipolyticum
Yahya et al. (1998)
Streptomyces sp.
Yahya et al. (1998);
Cho et al. (2012)
TABLE 2
difficulty is to immobilize the enzyme in a way that
can be separated and reused later by using simple
separation methods like centrifugation and filtration
(Iso et al., 2001; Cao, 2005).
2. Intracellular/immobilized lipases; i.e. lipases
remain either inside or attached to the cell wall.
In this case, enzyme is immobilized (naturally)
directly or together with the whole cell
(intracellular). This strategy eliminates downstream
operations and promises the recycling of enzymes.
Alternatively lipases can be immobilized
synthetically by different mechanisms.
Immobilization restricts movement of the enzyme
and constrains its location to an inert support
or a carrier (Cao, 2005). Various methods
like adsorption, covalent bonding, entrapment,
and cross-linking are available for enzyme
immobilization. The choice of method and support
material is a protuberant factor for obtaining an
efficient lipase (Table 8.2) (Sevil et al., 2012).
ADVANTAGES OF IMMOBILIZED LIPASE
1. Enzyme becomes more stable.
2. Immobilization of enzyme increases surface area of
biocatalyst.
3. Option of regeneration and reuse of the immobilized
lipase.
4. Protection from solvent inhibition.
Comparison of Different Immobilization Methods
Characteristics
Entrapment
Covalent Bonding
Cross-Linking
Adsorption
Protection from microbes
Yes
No
Possible
No
Immobilized process
Difficult
Difficult
Difficult
Easy
Interaction
Strong
Strong
Strong
Weak
Nature of immobilization
Irreversible
Irreversible
Irreversible
Reversible
Recovery of lipase activity
High
Low
Moderate
Low
Enzyme leakage
Yes
No
No
Yes
Regeneration of
immobilized lipase
Impossible
Impossible
Impossible
Possible
Immobilized cost
Low
High
Moderate
Low
Immobilization efficiency
depend on
Nature of the polymer
Surface -CHO groups
of support and surface amino
acid residues of the enzyme
Interaction between
enzyme and the carrier
pH, temperature
and ionic strength.
Enzyme stability
High
High
High
Low
Active site
Not effected
Effected
Effected
Not effected
Mass transfer limitation
Yes
No
No
No
FEEDSTOCK
5. Separation of product and enzyme is easier.
6. Avoids contamination of enzyme or whole cell.
7. Rigid external support expected to increase optimal
temperature, thereby fasten reaction rate.
TECHNICAL CHALLENGES
Yield of biodiesel through lipase catalysis is effected
by (1) feedstock quality, (2) choice of enzyme (extracellular or intracellular), (3) molar ratio (alcohol/oil), (4)
temperature, (5) water content, (6) acyl acceptors, (7) solvent and (8) reactor system.
FEEDSTOCK
One of the main barriers for commercialization of biodiesel production is choice and availability of feedstock,
which comprise nearly 80% of the overall biodiesel production cost. Diverse kinds of feedstocks are available
such as edible and nonedible vegetable oil, animal fats,
waste oil, microbial oil and microalgae oil and they
can be used for enzyme-catalyzed transesterification
(Sevil et al., 2012).
Vegetable Oils
Vegetable oils are well-known for their high heat content and they are alternative fuels for diesel engines. High
viscosity restricts their consumption directly in diesel engines, which leads to many problems (Koh and Ghazi,
2011; Singh and Singh, 2010). Most widely used edible
vegetable oils in enzymatic transesterification are soybean (Wenlei and Ning, 2010; Du et al., 2003), sunflower
(Karout and Pierre, 2009), palm (Talukder et al., 2011;
Matassoli et al., 2009), corn (Mata et al., 2012), cottonseed
(Chattopadhyay et al., 2011), canola (Jang et al., 2012) and
olive (Sanchez and Vasudevan, 2006). Higher quality of
edible oil is good feedstock to produce biodiesel by enzymatic transesterification. However, major concern is the
economic viability of biodiesel since refined vegetable
oils are expensive. Also, use of high-value edible vegetable oil as biodiesel feedstocks has caused food crisis.
Furthermore, percentage of oil and yield per hectare are
effective parameters in selecting potential renewable
feedstock for biodiesel production (Nielsen et al., 2008).
Hence, in order to make biodiesel production more
economical, low-cost and nonedible oils need to be
preferred. Babassu (Orbignya martiana), Jatropha curcas
(Linnaeus), neem (Azadirachta indica), polanga (Calophyllum inophyllum), karanja (Pongamia pinnata), rubber seed
tree (Hevea brasiliensis), mahua (Madhuca indica and
Madhuca longifolia), tobacco (Nicotiana tabacum), etc. are
most widely used nonedible oil sources for biodiesel
123
production. Biodiesel produced from these nonedible
oils meets key specifications of biodiesel as per the standard organization requirements (Mohibbe et al., 2005).
All these low-cost feedstocks contain large amount of
FFA which leads to undesirable soap formation during
traditional base-catalyzed transesterification. However,
high free-acid content is not a problem in enzyme
transesterification.
Animal Oils/Fats
Animal oils differ from vegetable oil in their fatty acid
composition. Vegetable oils have high content of unsaturated fatty acids (mainly oleic and linoleic acid), while
animal fat has higher proportion of saturated fatty acids.
Commonly used animal fats for biodiesel production via
enzymatic route contains lard (Jike et al., 2007), lamb
meet, beef tallow, chicken fat and animal fat mix (Vivian
Feddern et al., 2011). Waste animal fats from animal processing industries and slaughter houses are also a good
source for animal fats; however it is a decent alternative
instead of their direct dispose in to environment. Their
favorable features like noncorrosive nature, high cetane
number, and renewable nature makes them a good
source for biodiesel production. But their relatively
high FFA (5e30%) and water content led to soap formation in chemical transesterification process and their
saturated fats prone them to oxidation and crystallization at high temperatures (Huynh et al., 2011). Removal
of contaminants is another problem from animal fats,
which generally contain phospholipids, or gums, and
cause insoluble precipitates when they come into contact with water. Gums are removed by adding water
and citric or phosphoric acid to the animal fats followed
by centrifugal separation of precipitates. Phospholipids
get separated with glycerin during processing, or by water washing/ion exchange separation. Removal of sulfur
contents is also a serious issue. Beef tallow and some
chicken fat contain around 100 ppm of sulfur. Vacuum
distillation is the only reliable technique for reducing
the sulfur level to permissible levels (15 ppm) (Farm
energy, 2012).
Waste Oils/Fats
Used edible oils generally recycled as animal feed or
used as a raw material for lubricant and paints and the
rest discharged into the environment (Watanabe et al.,
2001). To eliminate environment and human health risk
caused by waste oils/fats (Chen et al., 2006) and to lower
biodiesel production cost, usage of waste oils/fats for
biodiesel production is recommended (Watanabe et al.,
2001). Waste cooking oil, animal fats, yellow grease,
brown grease and waste from vegetable oil refining
industries are major sources of waste oil for biodiesel
124
8. LIPASE-CATALYZED BIODIESEL PRODUCTION: TECHNICAL CHALLENGES
production (Huynh et al., 2011). Waste oils are rich in high
percentage of FFA and high water content, so lipasemediated transesterification is a promising method for
production of biodiesel with high yields (Huynh et al.,
2011). Novozym 435 is capable of converting used olive
oils (Sanchez and Vasudevan, 2006). Novozym 435 is
capable of converting used olive oils in to biodiesel
(Sanchez and Vasudevan 2006).
Algae Oils
Usage of algae oil for biodiesel production has
considerable interest because of their availability costs
compared to edible oils and animal fats. High photosynthetic rate, rapid growth rate, and high productivity
make algae a good renewable source for oil/fats. High
lipid content (20e40%), tolerance to water, and smaller
land usage up to 132 times less compared to terrestrial
oil crops make them more prominent choice for oil
source (Karatay and Donmez, 2011). Usage of algae oil
could reduce the food scarcity problem caused by bioenergy crops (Mata et al., 2010). However, technological
development is needed to improve the microalgae oil
extraction processes.
CHOICE OF ENZYME
Lipases from bacteria and fungi are the most
commonly used for transesterification. In general at reaction temperatures 30e50 C, the best enzymes will
show conversions above 90%. Catalytic reaction time,
alcohol type and enzyme condition (free enzyme or
immobilized) are also the crucial parameters for selecting enzyme (Table 8.3). Immobilized Pseudomonas cepacia
lipases converts the jatropha oil into FAME in 8 h with
ethanol; for the same free enzyme, it took 90 h for transesterifying soybean oil with methanol.
MOLAR RATIO (ALCOHOL/OIL)
According to the stoichiometric ratio of transesterification, it requires 3 mol of alcohol and 1 mol of triglyceride to yield 3 mol of fatty acid alkyl esters and 1 mol of
glycerol. Since it is a reversible reaction, traditional
transesterification process biodiesel yield always
improved due to excess amount of alcohol over fatty
acids in triglycerides (Sevil et al., 2012). But for
enzyme-catalyzed transesterification, methanol exhibits
negative effects on enzyme activity thus decrease the
production yield. High molar ratio of alcohol to triglycerides increases the glycerol solubility and effects the
separation of glycerol. Glycerol in solution, drives the
equilibrium back to the left, lowering the yield of alkyl
esters. Apart from this, highly hydrophilic nature of
alcohol eliminates water layer essential for the enzymes
and inactivates them. Gradual mixing of alcohol is a
potential approach for molar ratio optimization in
solvent-free systems. Most of the studies show molar
ratio 9:1, which seems to be the most appropriate. Addition of cosolvents, such as n-hexane and tetrahydrofuran,
slightly discharges this problem and increases the reaction rates, but a more complicated process should apply
for separation of biodiesel (Fan, 2012; Meher et al., 2006).
A two-step reaction system was reported to avoid the
inactivation of lipase by addition of excess amounts of
methanol (Watanabe et al., 2007). Moreno-Pirajan done
experiments on different molar ratios of methanol and
ethanol and his studies with various alcohol types and
palm oil with 10.4 M ratios by using Candida rugosa
lipase yielded 85 mol% of methyl esters (Moreno-Pirajan
and Giraldo, 2011). Zaidi et al. 2002 reported inhibition
coefficient of alcohol increased from 0.034 mol/l to
0.42 mol/l, when number of carbon atoms increased
from 1 (methanol) to 18 (oleyl alcohol), respectively.
Dizge and Keskinler (2008) conducted experiments at
different molar ratios of methanol to canola oil and
proved that up to certain molar ratio (1:6), there is an increase in ester production; upon increasing molar ratio,
it has shown the negative effect by decreasing the formation of esters due to enzyme inactivation. Finally
amount of alcohol needed varies suggestively depending on the origin of the lipase and triglycerides. Optimization of molar ratio is a big technical challenge and
there is much scope in this area for designing the molar
ratios for different types of lipids and enzyme (Sevil
et al., 2012).
TEMPERATURE
Transesterification can occur at different temperatures varying from 25 C to 60 C depending on the oil
used and many studies reported the effect of temperature on transesterification which influences reaction
rate and yield of esters. Generally high temperatures increase the ester yields (Freedman et al., 1984). However,
increased temperature beyond optimum point promotes
the denaturation and higher thermal deactivation of
enzyme, thus decreases catalytic activity (Sevil et al.,
2012). In batch process, optimal temperature range was
40e50 C whereas in repeated batch process, it lost its
activity at 40 C (Kose et al., 2002). Various research
groups worked to find out the effect of temperature on
biodiesel production with immobilized enzymes. Iso
et al.’s (2001) studies on the transesterification reaction
using free and immobilized lipase produced by P. fluorescens at a temperature range from 40 C to 70 C
revealed that conversion rate was the highest at 60 C,
TABLE 8.3
Different Lipase-Catalyzed Biodiesel Production Process Conditions
Oil/Fat
Alcohol
Yield (%)
Pseudomonas fluorescens
Soybean oil
Methanol
90
35
Kaieda et al. (2001)
Immobilized Candida antarctica lipase
B (Novozym 435)
Jatropha oil
Ethyl acetate
91.3
e
Modi et al. (2007)
Candida antarctica lipase
Cottonseed oil
Methanol
97
e
Royon et al. (2007)
Candida antarctica
Tallow
Methanol
74
e
Lee et al. (2002)
Immobilized Mucor miehei lipase
(Lipozyme IM-20)
Mowrah, mango,
kernel, sal
C4eC18:1 alcohols
86.8e99.2
e
De et al. (1999)
Candida antarctica lipase
(lipase SP-435)
Sunflower
Secondary alcohols
61.2e83.8
e
Mittelbach, (1990)
Rhizopus miehei lipase
(Lipozyme IM-60)
Methanol
19.4
e
Rhizopus miehei lipase
(Lipozyme IM-60)
Ethanol
65.5
e
Pseudomonas fluorescens lipase
Methanol
3
e
References
Candida rugosa
Waste ABE
Methanol, ethanol, 1-propanol,
3,1-butanol, iso-butanol, isoamylalcohol, and n-octanol
e
e
Noureddini et al. (2005)
Immobilized Candida sp. 99e125
Salad
Methanol
e
40
Nie et al. (2006)
Novozym 435, Lipozyme TL IM and
Lipozyme RM IM
Soybean
Ethanol
e
25
Hernandez-Martin and
Otero (2008)
Candida sp. 99e125
Waste cooking
Methanol
e
40e50
Chen et al. (2009)
Novozym 435
Rapeseed
Methanol
e
40
Jeong and Park (2008)
Novozym 435
Tung and palm
Methanol and ethanol
e
55
Wang et al. (2011a,b)
Novozym 435
Cottonseed
Dimethyl carbonate as organic
solvent
e
50
Su et al. (2007)
Novozym 435
Canalo
Methanol
e
38
Chang et al. (2005)
Novozym 435
Olive
Methanol
e
40
Sanchez and Vasudevan
(2006)
Novozym 435
Soybean
T-amyl
e
40
Zheng et al. (2009)
Novozym 435
Sunflower
Methanol
e
45
Ognjanovic et al. (2009)
Novozym 435
Stillingia
Methanol
e
40
Liu et al. (2009)
TEMPERATURE
Lipase
Optimum
Temperature
( C)
125
126
8. LIPASE-CATALYZED BIODIESEL PRODUCTION: TECHNICAL CHALLENGES
whereas free lipase activity highly decreased at 70 C
and immobilized enzyme activity remained active.
This work reveals the fact that increase in thermal stability of enzyme is due to immobilization enzyme and rigid
external carrier provides temperature resistance for
lipase molecule. The studies about the effect of temperature for lipase transesterification are shown in Table 8.3.
WATER CONTENT
Water content plays a key role in enzymatic transesterification as it is vital to sustain the threedimensional conformations of enzyme catalytic site.
Presence of an oilewater interface creates a favorable
environment for the conformation of active site (AlZuhair et al., 2006, 2003). Water interacts with the
enzyme hydrophilic groups located on surface, and
changes the conformation of hydrogen bond interactions inside enzyme, leading to transformation of lipase
active (Gao et al., 2006). Generally lipase activity increases with increase in water content up to 15% (w/w
of oils). Beyond 15%, the conversion rate decreased
slightly. But 20% of water content also efficiently catalyzed alcoholysis using lipases from Rhizopus delemar
and Rhizomucor miehei (Tweddell et al., 1998). About
5% of initial water content was suggested as optimum
for biodiesel production from jatropha oil using various
lipases (Shah and Gupta, 2007). Thus, the optimum level
of initial water (moisture) is based on the type of biocatalyst and reaction conditions.
ACYL ACCEPTORS
Generally transesterification reactions are conducted
using straight- and branched-chain alcohols. Because
of abundant availability and low cost, methanol is the
widely used short-chain alcohol acyl acceptor for biodiesel production (Fan, 2012). The negative effect of
methanol on enzyme activity alleviates by stepwise
addition of alcohols. Ethanol, n-butanol and i-butanol,
n-amylalcohol and i-amylalcohol, and n-propanol were
also used during transesterification. But increase in C
number of the alcohols has not significantly influenced
fatty acid ester contents and shown the negative effect
(Soumanou and Bornscheuer, 2003a,b). Also, it is generally believed that primary alcohols are more suitable
than secondary alcohols and alcohols with less than
eight carbon atoms can be used under the conditions
that gave the highest conversion of the oils to FAME.
Methyl acetate had no negative effect on enzymatic activity. No changes were detected in lipase activity even
after being continuously used for 100 batches (Sulaiman,
2007). Recently, ethyl acetate, methyl acetate, butyl
acetate, vinyl acetate and dimethyl carbonate (DMC)
are considered as novel acyl acceptors. The work
revealed by Er-Zheng et al. (2007) proved that DMC
gives two- to threefold higher conversion than those of
conventional acyl acceptors (methanol and methyl acetate) and is also ecofriendly, neutral, odorless, cheap,
noncorrosive, nontoxic, and exhibits good solvent
properties.
SOLVENTS
In the above section (molar ratio) discussed that excess
amount of alcohols increases FAME yield. In order to increase the solubility of alcohol (not the enzyme), solvents
are used and they alleviate negative effect of methanol on
the catalyst and precede the transesterification. Enzyme
should be insoluble in solvent; otherwise, it will not be
active (Kanerva et al., 1990; Antczak et al., 2009). Various
hydrophilic and hydrophobic organic solvents such as
cyclohexane, n-hexane, tert-butanol, petroleum ether,
isooctane and 1,4-dioxane are mainly studied organic solvents in enzymatic biodiesel production. If organic solvent is used as medium, overall alcohol is added at the
beginning of the reaction. In solvent-free reaction medium, alcohol is added stepwise to prevent enzyme activity with high alcohol concentration (Sevil et al., 2012).
REACTOR SYSTEM
Development of enzymatic biodiesel production at
commercial scale is dependent on the reactor systems.
Various reactors, including batch reactors, packed-bed
reactors and supercritical reactors, are studied for biodiesel production. Most of the studies have done on
batch reactors and packed-bed reactors. Batch reactors
are simple to use in the laboratory. But shear stress
caused by stirrer would disrupt the enzyme life (Tan
et al., 2010). Batch operation is a laborious process and
is not suitable for automation (Chen et al., 2010).
Packed-bed reactors are continuous and are a good alternative for batch reactors to lower the shear stresses
(long-term enzyme stability) and to make the process
economical (Wang et al., 2010). In addition, this system
offers high bed volume and is simple to scale up
(Hama et al., 2011). Because of its continuous mode,
stepwise addition of alcohol is possible in order to
reduce the inactivation of the enzyme caused by excess
alcohol. Lipase inhibition due to the cloggage by glycerol accumulation inside the reactor is a major challenge
(Xu et al., 2012). This can be resolved using more than
one column in the reactor. Yoshida et al. (2012) developed a reactor in which a reactant solution is pumped
through a column containing immobilized recombinant
REFERENCES
Aspergillus oryzae and the effluent from the column is
recycled into the same column with a stepwise addition
of methanol. This reactor system gave better lipase activity up to five cycles with 96.1% FAME content.
Wang et al. (2011b) developed a four-packed-bed
reactor in order to provide longer residence time to the
reaction mixture in the reactor and to lower lipase inhibition by product accumulation. A single-packed-bed
reactor and the four-packed-bed reactor were used to
produce biodiesel by using refined soybean oil with
P. cepacia lipase. Over 88% conversion rate and great stability were achieved with the four-packed-bed reactor
compared to single-packed-bed reactor (Wang et al.,
2010). This process improved the reaction efficiency
and additionally, the cost of biodiesel production can
be reduced by effective recycling of enzyme (Fjerbaek
et al., 2009).
Supercritical reactors are also investigated for enzymatic biodiesel production. D. Oliveira and J.V. Oliveira (2001) produced biodiesel from palm kernel oil
in the presence of Novozym 435 and Lipozyme IM in
supercritical carbon dioxide. Lipozyme IM showed better conversion (77.5%). But the problem is high pressure (beyond 200 bar) used in this process. Study by
Taher et al. (2011) has given only 49.2% conversion
rate with lamb-meat fat in supercritical carbon dioxide
by Novozym 435. Supercritical reactors could not be
commercialized due to low conversion rate and high
cost of the system. Subsequently, a technically
improved packed-bed reactor system with high transesterification efficiency is a good alternative for industrial scale-up of enzymatic biodiesel production in an
economic way.
CONCLUSIONS
Energy crisis and environmental concerns raised the
necessity for the new biofuels. Biodiesel is a clean alternative to fossil fuel. A green approach for biodiesel production through enzymatic biodiesel production has
gained a lot of attention due to the drawbacks of chemical methods. Promising enzymatic processes are established for biodiesel production. The main obstacle for
the industrialization of enzymatic process would be
overall cost of production. Production cost could be
reduced by increasing the productivity or by increasing
the catalytic efficiency of lipases. Immobilization and genetic engineering methods appear to be an attractive way
to obtain more active, stable, and reusable lipases in
different reaction systems. Operational parameters like
water content, temperature, solvent, acyl acceptors, and
so on plays key role in transesterification process. Along
with all these technical operational conditions, novel
bioreactor designing has also promising challenges
127
in order to make biodiesel a great potential commercial
fuel in future.
References
Akoh, C.C., Chang, S.W., Lee, G.C., Shaw, J.F., 2007. Enzymatic
approach to biodiesel production. J. Agric. Food Chem. 55,
8995e9005.
Al-Zuhair, S., Jayaraman, K.V., Krishnan, S., Chan, W.H., 2006. The
effect of fatty acid concentration and water content on the production of biodiesel by lipase. Biochem. Eng. J. 30, 212e217.
Al-Zuhair, S., Hasan, M., Ramachandran, K.B., 2003. Kinetic hydrolysis of palm oil using lipase. Process Biochem. 38, 1155e1163.
Antczak, M.S., Kubiak, A., Antczak, T., Bielecki, S., 2009. Enzymatic
biodiesel synthesisdkey factors affecting efficiency of the process.
Renewable Energy 34, 1185e1194.
Atadashi, I.M., Aroua, M.K., Abdul Aziz, A.R., Sulaiman, N.M.N.,
2013. The effects of catalysts in biodiesel production: a review.
J. Ind. Eng. Chem. 19, 14e26.
Belafi-Bako, K., Kovacs, F., Gubicza, L., Hancsok, J., 2002. Enzymatic
biodiesel production from sunflower oil by Candida antarctica
lipase in a solvent-free system. Biocatal. Biotransform. 20, 437e439.
Bisen, P.S., Sanodiya, B.S., Thakur, G.S., Bhagel, R.K., Prasad, G.B.K.S.,
2010. Biodiesel production with special emphasis on lipase catalysed transesterification. Biotechnol. Lett. 32, 1019e1030.
British Petroleum Statistical Review of World Energy, 2012. (Accessed
on 22 August, 2012) available on http://www.bp.com/
sectionbodycopy.do?categoryId¼7500&contentId¼7068481.
Cao, L., 2005. Immobilised enzymes: science or art? Curr. Opin. Chem.
Bio. 9, 217e226.
Chang, H.M., Liao, H.F., Lee, C.C., Shieh, C.J., 2005. Optimised synthesis of lipase-catalyzed biodiesel by Novozym 435. J. Chem.
Technol. Biotechnol. 80, 307e312.
Chattopadhyay, S., Karemore, A., Das, S., Deysarkar, A., Sen, R., 2011.
Biocatalytic production of biodiesel from cottonseed oil: standardization of process parameters and comparison of fuel characteristics. Appl. Energy 88, 1251e1256.
Chen, G., Ying, M., Li, W., 2006. Enzymatic conversion of waste
cooking oils in to alternative fuel-biodiesel. Appl. Biochem. Biotechnol. 129e132, 911e921.
Chen, Y.H., Huang, Y.H., Lin, R.H., Shang, N.C., 2010. A continuousflow biodiesel production process using a rotating packed bed.
Bioresour. Technol. 101, 668e673.
Chen, Y., Xiao, B., Chang, J., Fu, Y., Lv, P., Wang, X., 2009. Synthesis of
biodiesel from waste cooking oil using immobilized lipase in fixed
bed reactor. Energy Convers. Manage. 50, 668e673.
Cho, S.S., Park, D.J., Simkhada, J.R., Hong, J.H., Sohng, J.K., Lee, O.H.,
Yoo, J.C., 2012. A neutral lipase applicable in biodiesel production
from a newly isolated Streptomyces sp. CS326. Bioprocess Biosyst.
Eng. 35, 227e234.
Chowdary, G.V., Prapulla, S.G., 2002. The influence of water activity
on the lipase catalyzed synthesis of butyl butyrate by transesterification. Process Biochem. 38, 393e397.
De Oliveira, D., Di Luccio, M., Faccio, C., Rosa, C.D., Bender, J.P., Lipke, N.,
Menoncin, S., Amroginski, C., De Oliveira, J.V., 2004. Optimization of
enzymatic production of biodiesel from castor oil in organic solvent
medium. Appl. Biochem. Biotechnol. 113e116, 771e780.
De, B.K., Bhattacharyya, D.K., Bandhu, C., 1999. Enzymatic synthesis of
fatty alcohol esters by alcoholysis. J. Am. Oil Chem. Soc. 76, 451e453.
Demirbas, A., 2008. A Realistic Fuel for Alternative Diesel Engines.
Human press, Springer, Verlag, London. p. 208.
Dizge, N., Keskinler, B., 2008. Enzymatic production of biodiesel from
canola oil using immobilized lipase. Biomass Bioenergy 32,
1274e1278.
128
8. LIPASE-CATALYZED BIODIESEL PRODUCTION: TECHNICAL CHALLENGES
Du, W., Xu, Y., Liu, D., 2003. Lipase-catalysed transesterification of
soya bean oil for biodiesel production during continuous batch
operation. Biotechnol. Appl. Biochem. 38, 103e106.
Er-Zheng, S., Zhang, M.J., Zhang, J.G., Gao, J.F., Wei, D.Z., 2007.
Lipase-catalyzed irreversible transesterification of vegetable oils
for fatty acid methyl esters production with dimethyl carbonate as
the acyl acceptor. Biochem. Eng. J. 36, 167e173.
Fan, X., 2012. Enzymatic biodiesel productiondthe way of the future.
Lipid Technol. 24, 31e32.
Fariha, H., Shah, A.A., Hameed, A., 2006. Industrial applications of
microbial lipases. Enzyme Microb. Technol. 39, 235e251.
Farm energy, 2012. Animal fats for biodiesel production. (Accessed on
20 February, 2013) available on http://www.extension.org/pages/
30256/animal-fats-for-biodiesel-production.
Fjerbaek, L., Christensen, K.V., Norddahl, B., 2009. A review of the
current state of biodiesel production using enzymatic transesterification. Biotechnol. Bioeng. 102, 1298e1315.
Freedman, B., Pryde, E.H., Mounts, T.L., 1984. Variables affecting the
yields of fatty esters from transesterified vegetable oils. J. Am. Oil
Chem. Soc. 61, 1638e1643.
Fukuda, H., Kondo, A., Noda, H., 2001. Biodiesel fuel production by
transesterification of oils. J. Biosci. Bioeng. 92, 405e416.
Ganesan, D., Rajendran, A., Thangavelu, V., 2009. An overview on the
recent advances in the transesterification of vegetable oil for biodiesel production using chemical and biocatalyst. Rev. Environ.
Sci. Biotechnol. 8, 367e394.
Gao, Y., Tan, W.T., Nie, K.L., Wang, F., 2006. Immobilization of lipase
on macroporous resin and its application in synthesis of biodiesel
in low aqueous media. Chin. J. Biotechnol. 22, 114e118.
Ghaly, A.E., Dave, D., Brooks, M.S., Budge, S., 2010. Production of
biodiesel by enzymatic transesterification: review. Am. J. Biochem.
Biotechnol. 6, 54e76.
Ghamgui, H., Karra-Chaa bouni, M., Gargouri, Y., 2004. 1-Butyl oleate
synthesis by immobilized lipase from Rhizopus oryzae: a comparative study between n-hexane and solvent free system. Enzyme
Microb. Technol. 35, 335e363.
Haas, M.J., Piazza, G.J., Foglia, T.A., 2002. Enzymatic approaches to
the production of biodiesel fuels. In: Kuo, T.M., Gardner, H.W.
(Eds.), Lipid Biotechnology. Marcel Dekker Inc., New York,
pp. 587e598.
Hama, S., Tamalampudi, S., Yoshida, A., Tamadani, N., Kuratani, N.,
Nodaa, H., Fukuda, H., Kondo, A., 2011. Enzymatic packed-bed
reactor integrated with glycerol-separating system for solventfree production of biodiesel fuel. Biochem. Eng. J. 55, 66e71.
Hernandez-Martin, E., Otero, C., 2008. Different enzyme requirements
for the synthesis of biodiesel: Novozym 435 and Lipozyme TL IM.
Bioresour. Technol. 99, 277e286.
Huynh, L.H., Kasim, N.S., Ju, Y.H., 2011. Biodiesel production from waste
oils. In: Pandey, A.K., Larroche, C., Ricke, S.C., Dussap, C.G.,
Gnansounou, E. (Eds.), Biofuels: Alternative Feedstocks and Conversion Processes. Academic press, Elsevier Inc, USA, pp. 375e392.
Iso, M., Chen, B., Eguchi, M., Kudo, T., Shrestha, S., 2001. Production
of biodiesel fuel from triglycerides and alcohol using immobilized
lipase. J. Mol. Catal. B: Enzym. 16, 53e58.
Jang, M.G., Kim, D.K., Park, S.C., Lee, J.S., Kim, S.W., 2012. Biodiesel
production from crude canola oil by two-step enzymatic processes.
Renewable Energy 42, 99e104.
Jeong, G.T., Park, D.H., 2008. Lipase-catalyzed transesterification of
rapeseed oil for biodiesel production with tert-butanol. Appl.
Biochem. Biotechnol. 148, 131e139.
Jike, L., Nie, K., Xie, F., Wang, F., Tan, T., 2007. Enzymatic synthesis of
fatty acid methyl esters from lard with immobilized Candida sp.
99e125. Process Biochem. 42, 1367e1370.
Kaieda, M., Samukawa, T., Kondo, A., Fukuda, H., 2001. Effect of
methanol and water contents on production of biodiesel fuel from
plant oil catalyzed by various lipases in a solvent-free system.
J. Biosci. Bioeng. 91, 12e15.
Kanerva, L.T., Vihanto, J., Halme, M.H., Loponen, J.M., Euranto, E.K.,
1990. Solvent effects in lipase-catalysed transesterification
reactions. Acta Chem. Scand. 44, 1032e1035.
Karatay, S.E., Donmez, G., 2011. Microbial oil production from thermophile
cyanobacteria for biodiesel production. Appl. Energy 88, 3632e3635.
Karout, A., Pierre, A.C., 2009. Partial transesterification of sunflower
oil with ethanol by a silica fiber reinforced aerogel encapsulated
lipase. J. Sol-Gel Sci. Technol. 52, 276e286.
Knothe, G., 2001. Historical perspectives on vegetable oil-based diesel
fuels. Inform 12, 1103e1107.
Koh, M.Y., Ghazi, T.I.M., 2011. A review of biodiesel production from
Jatropha curcas L. Oil. Renewable Sustainable Energy Rev. 15,
2240e2251.
Kose, O., Tooter, M., Aksoy, H.A., 2002. Immobilized Candida
Antarctica lipase catalyzed alcoholysis of cotton seed oil in a
solvent-free medium. Bioresour. Technol. 83, 125e129.
Krawczyk, T., 1996. Biodieseldalternative fuel makes inroads but
hurdles remain. Inform 7, 801e829.
Lai, C.C., Zullaikah, S., Vali, S.R., Ju, Y.H., 2005. Lipase-catalyzed
production of biodiesel from rice bran oil. J. Chem. Technol. Biotechnol. 80, 331e337.
Lee, K.T., Foglia, T.A., Chang, K.S., 2002. Production of alkyl ester as
biodiesel from fractionated lard and restaurant grease. J. Am. Oil
Chem. Soc. 79, 191e195.
Leung, D.Y.C., Wu, X., Leung, M.K.H., 2010. A review on biodiesel
production using catalyzed transesterification. Appl. Energy 87,
1083e1095.
Linko, Y.Y., Lamsa, M., Huhtala, A., Linko, P., 1994. Lipase catalysed
transesterification of rapeseed oil and 2-ethyl-1-hexanol. J. Am. Oil
Chem. Soc. 71, 1411e1414.
Liu, Y., Xin, H., Yan, Y.J., 2009. Physicochemical properties of stillingia
oil: feasibility for biodiesel production by enzyme transesterification. Ind. Crops Prod. 30, 431e436.
Ma, L., Persson, M., Adlercreutz, P., 2002. Water activity dependence
of lipase catalysis in organic media explains successful transesterification reactions. Enzyme Microb. Technol. 31, 1024e1029.
Ma, F., Hanna, F.A., 1999. Biodiesel production: a review. Bioresour.
Technol. 70, 1e15.
Madras, G., Kolluru, C.R., 2004. Synthesis of biodiesel in supercritical
fluids. Fuel 83, 2029e2033.
Mata, T.M., Martins, A.A., Caetano, S.N., 2010. Microalgae for biodiesel production and other applications: a review. Renewable
Sustainable Energy Rev. 14, 217e232.
Mata, T.M., Sousa, I.R.B.G., Vieira, S.S., Caetano, N.S., 2012. Biodiesel
production from corn oil via enzymatic catalysis with ethanol.
Energy Fuels 26, 3034e3041.
Matassoli, A.L.F., Corrêa, I.N.S., Portilho, M.F., Veloso, C.O.,
Langone, M.A.P., 2009. Enzymatic synthesis of biodiesel via alcoholysis of palm oil. Appl. Biochem. Biotechnol. 155, 347e355.
Meher, L.C., Sagar, D.V., Naik, S.N., 2006. Technical aspects of biodiesel production by transesterificationda review. Renewable
Sustainable Energy Rev. 10, 248e268.
Mittelbach, M., 1990. Lipase catalyzed alcoholysis of sunflower oil.
J. Am. Oil Chem. Soc. 67, 168e170.
Modi, M.K., Reddy, J.R.C., Rao, B.V.S.K., Prasad, R.B.N., 2007. Lipasecatalyzed mediated conversion of vegetable oils into biodiesel
using ethyl acetate as acyl. Bioresour. Technol. 98, 1260e1264.
Mohibbe, A.M., Amtul, W., Nahar, N.M., 2005. Prospects and potential
of fatty acid methyl esters of some non-traditional seed oils for use
as biodiesel in India. Biomass Bioenergy 29, 293e302.
Moreno-Pirajan, J.C., Giraldo, L., 2011. Study of immobilized Candida
rugosa lipase for biodiesel fuel production from palm oil by flow
micro calorimetry. Arab. J. Chem. 4, 55e62.
REFERENCES
Mukesh, D., Banerji, A.A., Bevinakatti, H.S., 1994. A note on transesterifications of vegetable oils catalysed by lipase in a packed
tubular reactor. Indian Chem. Eng., Sect. A 36, 193e196.
Nelson, L.A., Foglia, T.A., Marmer, W.N., 1996. Lipase-catalysed production of biodiesel. J. Am. Oil Chem. Soc. 73, 1191e1195.
Nie, K., Xie, F., Wang, F., Tan, T., 2006. Lipase catalyzed methanolysis
to produce biodiesel: optimization of the biodiesel production.
J. Mol. Catal. B: Enzym. 43, 142e147.
Nielsen, P.M., Brask, J., Fjerbaek, L., 2008. Enzymatic biodiesel production: technical and economical considerations. Eur. J. Lipid Sci.
Technol. 110, 692e700.
Noureddini, H., Gao, X., Philkana, R.S., 2005. Immobilized Pseudomonas cepacia lipase for biodiesel fuel production from soybean oil.
Bioresour. Technol. 96, 769e777.
Ognjanovic, N., Bezbradica, D., Knezevic-Jugovic, Z., 2009. Enzymatic
conversion of sunflower oil to biodiesel in a solvent-free system:
process optimization and the immobilized system stability. Bioresour. Technol. 100, 5146e5154.
Oliveira, D., Oliveira, J.V., 2001. Enzymatic alcoholysis of palm kernel oil
in n-hexane and SC CO2. J. Supercrit. Fluids 19 (2), 141e148.
OPEC: Organization of the Petroleum Exporting Countries, 2011.
World Oil Outlook. available on. http://www.opec.org/opec_
web/static_files_project/media/downloads/publications/WOO_
2011.pdf (accessed on 21.12.2012).
OPEC: Organization of the Petroleum Exporting Countries, 2012.
World Oil Outlook. available on. http://www.opec.org/opec_
web/static_files_project/media/downloads/publications/
WOO2012.pdf (accessed on 12.07.2012).
Ranganathan, S.V., Narasimhan, S.L., Muthukumar, K., 2008. An
overview of enzymatic production of biodiesel. Bioresour. Technol
99, 3975e3981.
Ribeiro, B.D., De Castro, A.M., Coelho, M.A.Z., Freire, D.M.G., 2011.
Production and use of lipases in bioenergy: a review from the
feedstocks to biodiesel production. Enzyme Res. 2011, 1e16.
Royon, D., Daz, M., Ellenrieder, G., Locatelli, S., 2007. Enzymatic
production of biodiesel from cottonseed oil using n-butanol as a
solvent. Bioresour. Technol. 98, 648e653.
Sanchez, F., Vasudevan, P.T., 2006. Enzyme catalyzed production of
biodiesel from olive oil. Appl. Biochem. Biotechnol. 135, 1e14.
Schwab, A.W., Bagby, M.O., Freedman, B., 1987. Preparation and
properties of diesel fuels from vegetable oils. Fuel 66, 1372e1378.
Sevil, Y., Terzio
glu, P., Özçimen, D., 2012. Lipase applications in biodiesel production. In: Fang, Z. (Ed.), BiodieseldFeedstocks, Production and Applications. InTech, Rijeka, Croatia, pp. 210e250.
Sha, S., Sharma, S., Gupta, M.N., 2003. Mini review: enzymatic
transesterification for biodiesel production. Indian J. Biochem.
Biophys. 40, 392e399.
Shah, S., Gupta, M.N., 2007. Lipase catalyzed preparation of biodiesel
from jatropha oil in a solvent free system. Process Biochem. 42,
409e414.
Shimada, Y., Watanabe, Y., Sugihara, A., Tominaga, Y., 2002. Enzymatic alcoholysis for biodiesel fuel production and application of
the reaction to oil processing. J. Mol. Catal. B: Enzym. 17, 133e142.
Singh, S.P., Singh, D., 2010. Biodiesel production through the use of
different sources and characterization of oils and their esters as the
substitute of biodiesel: a review. Renewable Sustainable Energy
Rev. 12, 200e216.
Sonntag, N.O.V., 1979. Reactions of fats and fatty acids. In: Swern, D.,
Bailey, A.E. (Eds.), Industrial Oil and Fat Products, fourth ed.
Wiley, New York, USA, p. 99.
Soumanou, M.M., Bornscheuer, U.T., 2003a. Improvement in lipasecatalyzed synthesis of fatty acid methyl esters from sunflower
oil. Enzyme Microb. Technol. 33, 97e103.
Soumanou, M.M., Bornscheuer, U.T., 2003b. Lipase-catalyzed alcoholysis of vegetable oils. Eur. J. Lipid Sci. Technol. 105, 656e660.
129
Su, E.Z., Zhang, M.J., Zhang, J.G., Gao, J.F., Wei, D.Z., 2007. Lipasecatalyzed irreversible transesterification of vegetable oils for fatty
acid methyl esters production with dimethyl carbonate as the acyl
acceptor. Biochem. Eng. J. 36, 167e173.
Sulaiman, A.Z., 2007. Production of biodiesel: possibilities and challenges. Biofuels, Bioprod. Biorefin. 1, 57e66.
Taher, H., Al-Zuhair, S., AlMarzouqui, A., Hashim, I., 2011. Extracted
fat from lamb meat by supercritical CO2 as feedstock for biodiesel
production. Biochem. Eng. J. 55, 23e31.
Talukder, M.M.R., Das, P., Fang, T.S., Wu, J.C., 2011. Enhanced enzymatic transesterification of palm oil to biodiesel. Biochemical. Eng.
J. 55, 119e122.
Tan, T., Lu, J., Nie, K., Deng, L., Wang, F., 2010. Biodiesel production
with immobilized lipase: a review. Biotechnol. Adv. 28, 628e634.
Tweddell, R.J., Kermasha, S., Combes, D., Marty, A., 1998. Esterification, interesterification activities of lipase from Rhizopus niveus and
Mucor miehei in three different types of organic media: a comparative study. Enzyme Microb. Technol. 22, 439e445.
Vivian, F., Junior, A.C., De Pra, M.C., de Abreu, P.G., dos Santos
Filho, J.I., Higarashi, M.M., Sulenta, M., Coldebella, A., 2011. Animal fat wastes for biodiesel production. In: Stoytcheva, M.,
Gisela, M. (Eds.), BiodieseldFeedstocks and Processing Technologies. InTech, Rijeka, Croatia, pp. 45e70.
Wang, Y.N., Chen, M.H., Ko, C.H., Lu, P.J., Chern, J.M., Wu, C.H.,
Chang, F.C., 2011a. Lipase Catalyzed Transesterification of Tung
and Palm Oil for Biodiesel. World Renewable Energy Congress;
Sweden, 8e13 May.
Wang, X., Liu, X., Zhao, C., Ding, Y., Xu, P., 2011b. Biodiesel production in packed-bed reactors using lipaseenanoparticle biocomposite. Bioresour. Technol. 102, 6352e6355.
Watanabe, Y., Pinsirodom, P., Nagao, T., Asao, Y., Kobayashi, T.,
Nishida, Y., Takagi, Y., Shimada, Y., 2007. Conversion of acid oil byproduced in vegetable oil refining to biodiesel fuel by immobilized
Candida antarctica lipase. J. Mol. Catal. B: Enzym. 44, 99e105.
Watanabe, Y., Shimada, Y., Sugihara, A., Tominaga, Y., 2001. Enzymatic conversion of waste edible oil to biodiesel fuel in a fixed-bed
bioreactor. J9830. J. Am. Oil Chem. Soc. 78, 703e707.
Wenlei, X., Ning, M., 2010. Enzymatic transesterification of soybean oil
by using immobilized lipase on magnetic nano-particles. Biomass
Bioenergy 34, 890e896.
Xu, Y., Nordblad, M., Woodley, J.M., 2012. A two-stage enzymatic
ethanol-based biodiesel production in a packed bed reactor.
J. Biotechnol. 162, 407e414.
Xu, Y., Du, W., Liu, D., Zeng, J., 2003. A novel enzymatic route for
biodiesel production from renewable oils in a solvent-free medium. Biotechnol. Lett. 25, 1239e1241.
Xu, Y.Y., Du, W., Zeng, J., Liu, D.H., 2004. Conversion of soybean oil to
biodiesel fuel using Lipozyme TL 1M in a solvent-free medium.
Biocatal. Biotransform. 22, 45e48.
Yahya, A.R.M., Anderson, W.A., Moo-Young, M., 1998. Ester synthesis
in lipase catalysed reactions. Enzyme Microb. Technol. 23, 438e450.
Yoshida, A., Hama, S., Tamadani, N., Fukuda, H., Kondo, A., 2012.
Improved performance of a packed-bed reactor for biodiesel production through whole-cell biocatalysis employing a high-lipaseexpression system. Biochem. Eng. J. 63, 76e80.
Zaidi, A., Gainer, J.L., Carta, G., Mrani, A., Kadiri, T., Belarbi, Y.,
Mir, A., 2002. Esterification of fatty acids using nylon-immobilized
lipase in n-hexane: kinetic parameters and chain-length effects.
J. Biotechnol. 93, 209e216.
Zhang, B., Weng, Y., Xu, H., Mao, Z., 2012. Enzyme immobilization for
biodiesel production. Appl. Microbiol. Biotechnol. 93, 61e70.
Zheng, Y., Quan, J., Ning, X., Zhu, L.M., Jiang, B., He, Z.Y., 2009.
Lipase-catalyzed transesterification of soybean oil for biodiesel
production in tert-amyl alcohol. World J. Microbiol. Biotechnol.
25, 41e46.
C H A P T E R
9
Bioelectrochemistry of Microbial Fuel Cells
and their Potential Applications in Bioenergy
Minghua Zhou 1,*, Jie Yang 1, Hongyu Wang 1, Tao Jin 1,
Daniel J. Hassett 2, Tingyue Gu 3,*
1
Key Laboratory of Pollution Processes and Environmental Criteria (Ministry of Education), College of Environmental
Science and Engineering, Nankai University, Tianjin, China, 2Department of Molecular Genetics,
Biochemistry and Microbiology, University of Cincinnati, College of Medicine, Cincinnati, OH, USA,
3
Department of Chemical and Biomolecular Engineering, Ohio University, Athens, OH, USA
*Corresponding author email: gu@ohio.edu;zhoumh@nankai.edu.cn
O U T L I N E
Introduction
132
Bioelectrochemistry of MFC
Electrode Reactions in MFC
Anode Reaction
Cathode Reaction
Electron Transfer Methods
DET for Anodic Biofilms
MET for Anodic Biofilms
Electrogens in Biofilms for MFCs
Biocathodes
Electron Transfer for Biocathodes
DET for Biocathodes
MET for Biocathodes
132
132
132
133
133
134
134
135
136
137
137
137
Biofilm Electrochemistry for Enhanced MFC
Performance: A Molecular Biology Perspective
Bacterial Metabolism: How to Power MFCs through
Respiratory/Anaerobic Fluxes
Mediator-Less Factors Affecting MFC Performance
TFP (or “Nanowires”): Geobacter and Shewanella
Species as Model Organisms
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00009-7
139
139
139
Cytochromes (Cell-Bound)
Brief Synopsis of the S. oneidensis MR-1
Bioelectrochemical Machinery in Reverse:
Potential Role in the Biosynthesis of Biofuels
in MFCs
Mediators for Accelerated Electron Transfer
in Biofilms
Flavins
Phenazines
140
142
142
142
143
MFCs for Wastewater Treatment with Concomitant
Electricity Production
143
MFC Reactor Designs
143
Substrates Used in MFCs
145
Simple Biodegradable Organics
145
Wastewater Types
146
Lignocellulosic Biomass
146
Summary and Perspectives
147
References
147
139
131
Copyright Ó 2014 Elsevier B.V. All rights reserved.
132
9. BIOELECTROCHEMISTRY OF MICROBIAL FUEL CELLS AND THEIR POTENTIAL APPLICATIONS IN BIOENERGY
INTRODUCTION
Currently, the energy sources utilized in our society
are mainly fossil fuels such as oil, natural gas and coal
(Makarieva et al., 2008). However, their supplies are
limited and nonrenewable (Logan, 2009). When fossil
fuels are combusted, their carbon, sulfur and nitrogen
contents are converted into carbon oxides, sulfur oxides
and nitric oxides, respectively, resulting in greenhouse
gas emission and environmental pollution (e.g. acid
rain). With dwindling oil reserves, global warming signs
and worsening air pollution in many countries, more
efforts are devoted to the use of renewable energy
such as solar, wind and bioenergy. Bioenergy is a sustainable alternative to fossil fuels as part of an integrated
energy solution to alleviate the worldwide energy crisis
and environmental pollution problems (Srikanth and
Venkata, 2012).
Recently, microbial fuel cells (MFCs) have been intensively investigated in many academic labs as a potential
technology for bioenergy production from organic carbon sources such as wastewater, sludge and some lignocellulosic biomass (Allen and Bennetto, 1993; Lovley,
2006b; Rhoads et al., 2005). In a typical MFC, the
microbes forming the anodic biofilm oxidize the substrates (organic material) by anaerobic respiration
(Bond and Lovley, 2003; Logan et al., 2006) and release
electrons (e) and protons (Hþ) (Srikanth and Venkata,
2012). The electrons are transferred to the anode and
then reach the cathode via an external circuit. Simultaneously, protons in the solution travel through a proton
exchange membrane (PEM) and reach the cathode
where electrons are used to reduce oxygen. In this
fashion, electricity is generated by converting the energy
stored in the chemical bonds in the organic matter (or
feedstock) provided to each system (Choi et al., 2003a;
Gil et al., 2003; Huang et al., 2011b; Moon et al., 2006;
Osman et al., 2010). Thus, MFCs produce bioelectricity
directly instead of a biofuel in the process of degrading
organic matter in the wastewater (Chaudhuri and Lovley, 2003; Oh and Logan, 2005; Park and Zeikus, 2000).
Bioelectricity production by an MFC was first reported
by Petter (1911). Not much research was done on MFCs
until 1980s when mediators were found to improve
MFC power density greatly. However, externally supplied
mediators such as methyl viologen, neutral red, and thionine are not sustainable. They are expensive and toxic,
limiting their uses to academic research (Du et al., 2007).
In recent years, some microorganisms such as Shewanella
putrefaciens (Kim et al., 2002), Rhodoferax ferrireducens
(Chaudhuri and Lovley, 2003), and Geobacteraceae sulfurreducens (Bond and Lovley, 2003) have been found to transfer electrons from the cytoplasm where metabolic
respiration occurs to an external electrode surface (anode),
resulting in the development of mediator-less MFCs.
Intensive research efforts from 1990 to 2010 have
improved MFC power densities by several orders of
magnitude to up to several watts per square meter
(anode area) under optimal laboratory conditions.
Recently, Tong et al. (2012) compared the power densities
between MFCs and conventional fuel cells and found
that MFCs were still behind by three orders of magnitude. It is unrealistic to expect MFCs to catch up with
chemical fuel cells because the latter uses pure hydrogen,
ethanol or other high-energy-density fuels rather than
wastes. However, it is still necessary to improve MFC
power densities much further to make the MFC power
generation practically useful. Major breakthroughs are
needed in biofilm engineering, materials for electrodes
and reactor configuration to achieve far better bioelectrochemical performance and to lower the currently
rather high costs in MFC construction, maintenance
and operation (Zhou et al., 2012). This chapter addresses
various bioelectrochemical issues in MFC operation for
the improvement of MFC performance.
BIOELECTROCHEMISTRY OF MFC
Electrode Reactions in MFC
A typical MFC reactor contains an anodic chamber, a
cathodic chamber and a PEM partitioning the two chambers. Figure 9.1 shows a dual-chamber MFC.
Anode Reaction
The microbes in the anodic chamber oxidize substrates such as glucose, acetate and some refractory
organics. For example, glucose is oxidized as follows
to generate electrons, protons and carbon dioxide
(Pham et al., 2006):
C6 H12 O6 þ 6H2 O/6CO2 þ 24Hþ þ 24e
(9.1)
Resistance
e
e–
–
PEM
Anode
Cathode
H 2O
CO2
e–
e–
Substrate
H+
O2
H+
Air
FIGURE 9.1 Schematic diagram of a microbial fuel cell. (For color
version of this figure, the reader is referred to the online version of this
book.)
BIOELECTROCHEMISTRY OF MFC
Because electrons cannot “swim” in an aqueous solution, the oxidation reaction must occur in a biofilm that is
capable of transferring electrons to the anode. In the
absence of a suitable oxidant in the anodic chamber to
absorb the electrons, electrons will be transferred to the
anode by the biofilm (Zhao et al., 2009). The electrons
reach the cathode via an external circuit linking the
anode and the cathode, where they are used to reduce
an oxidant such as oxygen (Figure 9.1). A load is placed
on the external circuit to harvest the electricity. To maintain electroneutrality, protons must carry an equal
amount of positive charges from the anodic chamber to
the cathodic chamber usually through a PEM. Inefficient
proton migration will result in accumulation of protons
that causes acidity in the anodic chamber (Xu et al., 2012).
In the anodic chamber, anaerobic conditions are very
important to guarantee the substrate oxidation by the
microbes through anaerobic respiration (Liu et al.,
2005b; Logan et al., 2006). Oxygen leaked into the anodic
chamber from outside air or through diffusion from the
cathodic chamber (Figure 9.1) would reduce Coulombic
efficiency of the MFC by directly oxidizing the organic
matter in the anodic chamber. In this case, energy
will be released as low-grade heat instead of electricity.
A PEM plays an important role of preventing oxygen
diffusion from the cathodic chamber to the anodic chamber (Li et al., 2011), while allowing positive charges to go
through it via a proton exchange process. If nonoxygen
oxidants such as sulfate and nitrate are present in sufficient quantities in the anodic chamber feed stream, the
biofilm on the anode must not be able to catalyze their
reduction because it would divert the electrons released
from oxidation of organic matters for the local reduction
of sulfate or nitrate. A buffer solution that usually contains NH4Cl, NaH2PO4, Na2HPO4, KCl, and so on is
often used to enhance the proton transfer in laboratory
MFC investigations (Liu et al., 2011). The presence of a
buffer solution increases the conductivity, thus reducing
internal resistance of the MFC (Liu et al., 2005a).
Cathode Reaction
The cathode reaction has a major impact on MFC
performance. The electrons coming from the anode via
the external circuit, the protons coming from the anodic
chamber via the PEM and the electron acceptors (e.g. O2)
will react with the help of catalysts on the cathode
(Pham et al., 2006):
24Hþ þ 24e þ 6O2 / 12H2 O
(9.2)
Reactions (9.1) and (9.2) form a thermodynamically
favorable redox reaction, that is, the aerobic oxidation
of glucose. However, a thermodynamically favorable
reaction may not proceed at an appreciable rate if the
kinetics is too slow. In an MFC, anode and cathode reactions almost always require catalysis. For the anode
133
reaction, a biofilm is required to catalyze organic carbon
oxidation and electron transfer. For the cathode, oxygen
reduction rate is very slow without catalysis. The
cathodic reaction efficiency depends on the concentration and type of electron acceptors, proton concentration, electrode structure and its catalytic ability (Zhou
et al., 2012).
In order to improve electricity generation, a good catalytic cathode is crucial since the catalysts can reduce the
activation energy and thus greatly increase the reaction
rate. Currently, for oxygen reduction, platinum (Pt)
appears to be most effective. However, it is extremely
expensive and, thus, unrealistic for most practical applications even when only Pt coating is used. Some alternative catalysts have been explored such as MnOx,
CoTMPP, PbO2, iron(II) phthalocyanine (FePc) and
recently the biocathode (Roche and Scott, 2008; Zhou
et al., 2011).
Oxygen is the most popular acceptor because of its
high standard potential (0.818 mV), low cost and environmental “friendliness”. However, the rate of oxygen
reduction is very low on the cathode surface, resulting
in a high overpotential, which is one of the most important limiting factors in MFCs (Gil et al., 2003). Potassium
ferricyanide (K3[Fe(CN)6]) can overcome this handicap
(Logan et al., 2006; Nevin et al., 2008; Park and Zeikus,
2003). However, the regeneration of K3[Fe(CN)6]is a
problem because it usually is not sufficiently oxidized
by oxygen. It needs to be replenished periodically
(Franks and Nevin, 2010). In addition, K3[Fe(CN)6] can
diffuse into the anodic chamber through the PEM,
thereby influencing the desired anaerobic conditions of
anodic chamber (Logan et al., 2006). Potassium permanganate is also used as an acceptor, and the power density
was reported to be higher than that with K3[Fe(CN)6] and
oxygen (You et al., 2006). In practice, wastewater streams
are low-grade energy sources that are pale in comparison
to pure fuels such as hydrogen or ethanol as a fuel. This
inherently means that a large volume of water must be
treated to harvest a sufficient amount of electricity. This
makes all externally added soluble catalysts impractical,
limiting them to academic investigations.
To overcome the requirement for catalysis by oxygen
oxidation on the cathode, biocathodes have been explored
(Biocathodes Section). Various biofilms have been tested
on cathodes to biocatalyze oxygen or a nonoxygen
oxidant such as nitrate and perchlorate (Shea et al.,
2008; Srikanth et al., 2012; Zhang and Angelidaki, 2012).
Electron Transfer Methods
How the electrons released by organic carbon oxidation in the bacterial anaerobic cytoplasm are transferred
by the biofilm to the anode surface is an important factor
in MFC performance (Neto et al., 2010). Major advances
134
9. BIOELECTROCHEMISTRY OF MICROBIAL FUEL CELLS AND THEIR POTENTIAL APPLICATIONS IN BIOENERGY
have been made between 2000 and 2010 in understanding the electron transfer mechanisms by electrogens.
There are two primary mechanisms: one is the direct
electron transfer (DET) and the other, mediated electron
transfer (MET).
DET for Anodic Biofilms
DET occurs via a direct physical contact between the
microbial cell wall and the anode surface, or via a pilus
that links the two. Gene expression studies (Holmes
et al., 2008) and electrochemical analysis (Busalmen
et al., 2008) have demonstrated that there are active sites
for cytochrome proteins on the outer cell surface (Franks
and Nevin, 2010; Zhou et al., 2012). When the microbes
contact the anode surface, the cytochromes can transfer
the electrons from the inside of the microbial cell wall to
the outer cell wall and then to the anode surface (Rinaldi
et al., 2008) (Figure 9.2(a)). Shewanella putrefaciens (Kim
et al., 2002), R. ferrireducens (Chaudhuri and Lovley,
2003) and G. sulfurreducens (Bond and Lovley, 2003)
use such cytochromes to achieve electron transfer. One
major disadvantage for this mechanism is that only a
monolayer of sessile cells in a biofilm can transfer electrons to the anode surface. This explains why the power
and current densities of MFCs relying on this kind of
DET are lower, sometimes by several orders of magnitude, than that of MFCs with MET (Schröder, 2007)
because MET can utilize more than one monolayer.
Recently, some researchers have observed that some
microbial strains (such as Shewanella oneidensis and
G. sulfurreducens; Logan and Regan, 2006; Torres et al.,
2010) can produce pili (conductive nanowires) to form
physical conductive connections between the cell wall
and the anode surface while the microbial cell wall is
at a short distance from the anode (Reguera et al.,
2005; Rinaldi et al., 2008). An extensive pilus network
would allow several layers of sessile cells to donate electrons to the anode, thus multiplying the MFC power
output. Summers et al. found a whole cell aggregate
(a)
Substrate
H+
A
n
o
d
e
(b)
H+
Substrate
e–
CO2
e–
H+
Cytochrome
CO2
Conductive pili or
filament
FIGURE 9.2 Different DET methods: (a) direct cell wall-electrode
contact (b) conductive pili or filament linkage. (For color version of
this figure, the reader is referred to the online version of this book.)
consisting of G. sulfurreducens and Geobacter metallireducens is conductive when the coculture was grown on
ethanol. The c-type cytochrome OmcS of G. sulfurreducens was suspected to play a key role in accepting electrons from G. metallireducens (Summers et al., 2010).
This overcomes the inability of G. sulfurreducens to use
H2 for interspecies electron transfer. The ability of interspecies electron exchange suggests that a nonelectrogenic species in a synergistic biofilm consortium may
contribute to electricity generation as long as its electrons can be taken up by an electrogenic species through
interspecies electron transfer. In addition to H2, other
molecules such as formate may act as an electron shuttle
for biofilm communities (Morita et al., 2011).
An exciting new discovery by Pfeffer et al. (2012)
provides new hope for greatly enhancing electron transfer in microorganisms. They found that some filamentous bacteria in marine sediments are capable of
transferring electrons over centimeter-long distances
via conductive filaments that are 200 nM or wider in
diameter. This distance is far greater than the much
thinner pili could achieve. This indicates that potentially
many more layers of sessile cells could be networked via
the conductive filaments than pili could achieve.
Figure 9.2 is a schematic illustration of DET via direct
cell walleelectrode contact and via a pili or conductive
filament linkage.
MET for Anodic Biofilms
MET THROUGH EXOGENOUS REDOX MEDIATORS
Some microbes such as Escherichia coli, Pseudomonas sp.,
Proteus and Bacillus (Lovley, 2006a) cannot directly transfer electrons to the anode and must rely on mediators
(Lovley, 2006a). When the oxidized mediators reach
the surface of the microbes, they penetrate the cell membrane of the microbes, and they are reduced by electrons.
The reduced mediators pass through the cell membrane
again and reach the anode surface where then they are
reoxidized (losing the electrons). In this fashion, electrons
are transferred to the anode while the oxidized mediators enter the microbes again, thereby continuing the
redox cycle (Figure 9.3(a)) (Neto et al., 2010; Rabaey
et al., 2005b).
Properties of good exogenous mediators should
be the ability to (a) cross cell membranes with ease;
(b) receive electrons from electron donors without
interfering with other metabolic processes; (c) deliver
electrons inside the cytoplasm for oxidation reactions
and regenerate at rapid rates; (d) have good solubility
and stability in both oxidized and reduced forms;
(e) have no cytotoxicity; and (f) not be consumed by
microbes in the biofilm as a nutrient (Bao and Wu,
2004). These mediators include thionine, neutral red,
2-hydroxy-1,4-naphthoquinone, phenazines, quinines,
BIOELECTROCHEMISTRY OF MFC
(a)
CO2
Med red
(b)
A
n
o
d
e
e–
Substrate
H2
CO2
Substrate
135
anodic chamber, thus improving the MFC performance
(Osman et al., 2010). However, in continuous flow
MFCs for wastewater treatment, the secondary metabolites can be insufficient due to diluted concentrations as
a result of flow (Lee et al., 2003; Rabaey et al., 2005c),
thus resulting in the decline of the performance after
the flow starts (Lovley, 2006a; Osman et al., 2010).
MET THROUGH PRIMARY METABOLITES
H+
Med ox
e–
H+
Electrocatalyst
FIGURE 9.3 The mechanism of MET: (a) exogenous or secondary
metabolites and (b) primary metabolites. (For color version of this
figure, the reader is referred to the online version of this book.)
Fe(III) ethylenediaminetetraacetic acid, methylene blue,
phenothiazines, phenoxazines and others (Choi et al.,
2003b; Lovley, 2006a; McKinlay and Zeikus, 2004;
Newman and Kolter, 2000; Osman et al., 2010; Park and
Zeikus, 2000). However, these mediators are unsuitable
for practical applications because they are costly and
most of them are toxic and recalcitrant, harmful to the
environment (Erable et al., 2010a; Lovley, 2006a).
MET THROUGH THE SECONDARY METABOLITES
Researchers have found that some microbes can transfer electrons without DET in the absence of exogenous
redox mediators. These microbes such as S. putrefaciens,
S. oneidensis, G. sulfurreducens, Pseudomonas aeruginosa,
and Clostridium butyricum can produce their own mediators (Angenent et al., 2004; Erable et al., 2010a; Fitzgerald
et al., 2012; Newman and Kolter, 2000; Rabaey et al.,
2005a). The presence of these microbes in the mixed
cultures enhances electron transfer. These mediators
mainly include phenazine derivatives like pyocyanine
and 2-amino-3-carboxy-1,4-naphthoquinone (Osman
et al., 2010).
In practical applications, the secondary metabolites
(endogenous redox mediators) may be very important
to MFCs because they can transfer the electron without
the exogenous redox mediators (Schröder, 2007). The
mechanism of electron transfer by the secondary metabolites is similar to that of the exogenous electrochemical
redox mediators (Figure 9.3(a)). The secondary metabolites can be reused, and one metabolite molecule can
transfer thousands of electrons (Schröder, 2007). So a
small amount of the secondary metabolites can singlehandedly enhance the rate of electron transfer and
thus increasing power density and improve the MFC
performance without introducing costly exogenous
mediators.
In batch-mode operations, these microbes are very
suitable because the mediators will accumulate in the
The other endogenous redox mediators are primary
metabolites. Some microbes can produce fermentation
products such as hydrogen (H2), hydrogen sulfide
(H2S), alcohols and ammonia (Erable et al., 2010a).
When these primary metabolites reach the surface of
the anode, they are oxidized, and the released electrons
will be further transferred to the anode surface.
There are two types of anaerobic metabolism that can
produce primary redox metabolites: one is anaerobic
respiration, and the other is fermentation. Some microbes
such as Proteus vulgaris, E. coli, P. aeruginosa and Desulfovibrio desulfuricans can produce sulfide which may
serve as the mediator to transfer electrons (Bullen et al.,
2006; Schröder, 2007):
þ
Cytoplasm : SO2
4 þ 9H þ 8e /HS þ 4H2 O (9.3)
þ
Anode : HS þ 4H2 O/SO2
4 þ 9H þ 8e
(9.4)
This process relies on sulfate reducing bacteria (SRB)
that cannot metabolize carbohydrates. A fermentation
process can produce small organic acids and alcohols
that can be used in anaerobic respiration (Schröder,
2007). Many SRB degrade the substrates incompletely
and this lowers the MFC power output. Electrode
poisoning by sulfide due to its easy absorption on the
electrode surface is also a major drawback (Reimers
et al., 2006; Ryckelynck et al., 2005).
Fermentation also produces primary metabolites
such as hydrogen, ethanol and formate. They can be
oxidized directly by electrolysis on an anode such as
platinum or tungsten carbide (Rosenbaum et al., 2006).
For example, through electrocatalysis, the molecular
hydrogen near and on an anode surface would be
oxidized to Hþ, accompanying the electron transfer
(Figure 9.3(b)). Molecular hydrogen is known to be
used as an electron carrier used by hydrogenasepositive microbes such as some SRB in microbiologically
influenced corrosion (Gu, 2012). Thus, it contributes to
power generation.
Electrogens in Biofilms for MFCs
The microbial species in a biofilm covering an anode
are important because they determine the mode of electron transfer and the mechanism of electricity generation as well as what forms of organic material can be
136
9. BIOELECTROCHEMISTRY OF MICROBIAL FUEL CELLS AND THEIR POTENTIAL APPLICATIONS IN BIOENERGY
utilized in the feed stream. Theoretically, a myriad of
microorganisms may be useful for MFCs, but most of
them have no direct electrochemical activity and thus
cannot transfer electrons directly from the cytoplasm
to the anode, i.e. they are not electrogenic. However,
many microorganisms with the addition of a soluble
redox mediator can act as electron transfer intermediates
to transfer electrons. Table 9.1 shows the microbial species and the electron transfer mechanism in the anodic
chamber that can perform such processes.
In MFCs, mixed cultures usually possess higher electron transfer efficiency than the pure culture because its
specificity to the microbe is very strong and its growth
rate is relatively slow (Hassan et al., 2012). Mixed cultures are often found to perform better than pure stains.
This is because a synergistic biofilm consortium contains
various syntrophic species, with each organism contributing specific roles. A consortium can adapt to substrate
variations in wastewater and harsh environmental conditions because generally a biofilm consortium is far
more robust metabolically than a pure-culture biofilm.
The consortium is able to self-select the most efficient
electron transfer mechanism if several are available.
Biocathodes
Biocathodes use biofilms as catalysts to improve the
cathode reaction, avoiding using precious metal catalysts. Another unique advantage of biocathodes is that
oxidants other than oxygen can be used, including sulfate, nitrate, carbon dioxide, Hþ, Fe(III), Cr(VI), U(VI),
Mn(IV), tetrachloroethene, fumarate, perchlorate, and
trichloroethene (Huang et al., 2011c). In addition, the
sustainability of MFC may be improved with the elimination of problems such as sulfur poisoning of Pt and
the requirement for electron mediators in the cathodic
chamber (He and Angenent, 2006).
There are two types of biocathodes: aerobic and anaerobic. Aerobic biocathodes reduce oxygen (electron
acceptor). The biofilm on the cathode surface can catalyze
the oxidation of transition metal compounds, such as
Fe(II) and Mn (II), releasing the electrons to oxygen.
MFCs with aerobic biocathodes can produce higher power
density than that of anaerobic biocathodes (Srikanth and
Venkata, 2012).
The use of a biocathode also means that an MFC can
potentially be used to treat an additional wastewater
stream in the cathodic chamber. It may be a wastewater
stream containing sulfate or nitrate that can come from
agricultural runoff (Srikanth and Venkata, 2012). However, the accumulation of microbial metabolites in the
cathode chamber can inhibit microbial activities. In
addition, metabolites which act as electron donors for
bacteria can also compete against the cathode, and therefore reduce the MFC performance (Hamid et al., 2008).
TABLE 9.1 The Microbial Species in the Anodic Chamber
Microbe
Electron Transfer
References
Escherichia coli K12
MET
Erable et al. (2010a)
Clostridium beijerinckii
MET
Erable et al. (2010a)
Clostridium butyricum
MET
Erable et al. (2010a)
Proteus vulgaris
MET
Kim et al. (2000a,b);
Thurston et al. (1985)
Shewanella putrefaciens
MET/DET
Kim et al. (1999, 2002)
Geothrix fermentans
MET
Bond and Lovley (2005)
Pseudomonas aeruginosa
MET
Rabaey et al. (2005a)
Shewanella oneidensis
MET/DET
Biffinger et al. (2008,
2007); Hou et al. (2009);
Manohar et al. (2008);
Qian et al. (2009);
Ringeisen et al. (2006)
Desulfuromonas
acetoxidans
DET
Bond et al. (2002)
Geobacter sulfurreducens
DET
Holmes et al. (2004)
Geobacter metallireducens
DET
Holmes et al. (2004);
Min et al. (2005)
Rhodoferax ferrireducens
DET
Holmes et al. (2004)
Desulfobulbus
propionicus
DET
Holmes et al. (2004)
Aeromonas hydrophila
MET
Pham et al. (2003)
Clostridium butyricum
DET
Niessen et al. (2004)
Hansenula anomala
DET
Prasad et al. (2007)
Rhodopseudomonas
palustris
MET
Xing et al. (2008); Zhou
et al. (2012)
Enterococcus faecium
MET
Rabaey et al. (2005a)
Desulfovibrio
desulfuricans
DET
Cooney et al. (1996)
Erwinia dissolvens
MET
Vega and Fernandez
(1987)
Escherichia coli
MET/DET
McKinlay and Zeikus
(2004); Schröder et al.
(2003)
Desulfovibrio vulgaris
MET
Tsujimura et al. (2001)
Shewanella putrefaciens
IR-1
DET
Schröder (2007)
Shewanella putrefaciens
MR-1
DET
Schröder (2007)
Shewanella putrefaciens
SR-1
DET
Schröder (2007)
Aeromonas hydrophila
PA 3
DET
Schröder (2007)
Clostridium sp. EG 3
DET
Schröder (2007)
BIOELECTROCHEMISTRY OF MFC
137
Furthermore, Zhou et al. (2013) indicated that the
voltage output for the combined redox reaction
involving Eqn (9.3) and the oxidation of an organic carbon such as acetate may be too small for MFC after subtracting various overpotentials.
systems, the mixed culture biocathodes also can transfer
electrons via DET. When nitrate, carbon dioxide or trichloroethene is used as the electron acceptor, the DET
in the mixed culture biocathode improves the power
generation (Aulenta et al., 2010; Cao et al., 2009; Clauwaert et al., 2007a).
Electron Transfer for Biocathodes
MET for Biocathodes
There are numerous investigations on the mechanisms of electron transfer to the anode by the microbes,
while the reports about electron transfer to a biocathode
are rather limited (Lovley, 2008). The two electron transfer directions are opposite. The biocathode is an electron
donor while the anode is an electron acceptor. Despite
this difference, biocathodes use the same electron transfer mechanisms, DET and MET (Rosenbaum et al., 2011),
because biofilm electron transfer can be bidirectional.
MET THROUGH EXOGENOUS REDOX MEDIATORS
DET for Biocathodes
Similar to DET for anodes, DET for biocathodes also
requires physical contact of the microbial cell wall
with the electrode surface. At the site of direct contact,
the electrons directly transfer to the outer cell
membrane-bound redox macromolecules (such as
c-type cytochromes) from the electrode (Figure 9.4(a);
Huang et al., 2011c). However, this kind of DET can
only utilize a monolayer of sessile cells on the cathode,
thus limiting the biocathode performance. With an increase in biofilm thickness, the power generation
decreased due to mass transfer resistance to oxidant
diffusion from the bulk fluid to the cathode surface
(Behera et al., 2010). Geobacter species and mixed
cultures that use nitrate, fumarate, tetrachloroethene,
O2, CO2, U(VI)/U(IV), and so on as an electron acceptor
generally transfer the electrons via DET (Table 9.2). On
biocathodes, most of the microbes are found to be
Gram negative although some Gram-positive microbes
exhibit the DET mechanism in cyclic voltammetry
(Huang et al., 2011c). Compared with the pure culture
(a)
Oxidized
acceptor
C
a
t
h
o
d
e
(b)
Oxidized
acceptor
Med red
e–
Reduced
acceptor
Med ox
Reduced
acceptor
FIGURE 9.4 The mechanism of electron transfer in biocathode: (a)
DET and (b) MET. (For color version of this figure, the reader is
referred to the online version of this book.)
Similar to bioanodes, the same exogenous mediators
including neutral red, methyl viologen and the anthraquinone-2,6-disulfonate can be used for biocathodes
(Hatch and Finneran, 2008; Park and Zeikus, 1999; Steinbusch et al., 2010) to enhance MFC performance significantly. When mediators are added into the cathode
chamber, they are reduced by the electrons donated by
the cathode. The reduced mediators reach the microbial
cell wall and then transfer the electrons through the
wall while the mediators are oxidized. Subsequently,
the oxidized mediators diffuse back to the cathodic surface for reuse. This cyclic process is illustrated in
Figure 9.4(b). Usually one mediator molecule can accomplish thousands of cycles. These mediators are relatively
short-lived and costly, making their use unsustainable.
Just like their use for bioanodes, these exogenous mediators are used only in laboratory investigations of MFC
mechanisms for academic purposes. Pili can also be
used by microbes to transfer extracellular electrons to
the cytoplasm (Zhou et al., 2013).
In manganese-oxidizing bacteria, manganese (IV)
plays an important role in the electron transfer. This
mechanism is similar to the exogenous mediator MnO2
on the biocathode surface. It is first reduced to MnOOH
by the electrons donated from the cathode and then
Mn2þ is released. Finally, with the help of manganeseoxidizing bacteria, Mn2þ was oxidized by dissolved oxygen to regenerate MnO2 (Nguyen et al., 2007). The
power density can be improved by two orders of magnitude, compared with the abiotic cathode (Rhoads et al.,
2005), making it attractive for potential practical
applications.
MET THROUGH SELF-EXCRETED REDOX MEDIATORS
Apart from exogenous redox mediators, some
microbes can excrete metabolites that are redox active.
For example, Pseudomonas spp. can produce phenazines
(Venkataraman et al., 2010) and S. oneidensis can produce
flavins (Marsili et al., 2008). These mediators can be used
by biocathodes indirectly. In the presence of these mediators, the rate of electron transfer is enhanced. These
mediators are more easily utilized by other microbes
than their producers (Rosenbaum et al., 2011). Therefore,
in biocathodes, the self-excreted mediators play an
important role in a synergistic biofilm consortium covering a cathode. Their mechanism of electron transfer is
138
9. BIOELECTROCHEMISTRY OF MICROBIAL FUEL CELLS AND THEIR POTENTIAL APPLICATIONS IN BIOENERGY
TABLE 9.2 The Microbial Species in the Biocathode Chamber
Species
Electron Transfer
Oxidant/End Product
References
Burkholderia cepacia
DET
O2/H2O
Cournet et al. (2010)
Brevundimonas diminuta
DET
O2/H2O
Branhamella catarrhalis
DET
O2/H2O
Bacillus subtilis
DET
O2/H2O
Acinetobacter sp.
DET
O2/H2O
Shigella flexneri
DET
O2/H2O
Escherichia coli
DET
O2/H2O
Enterobacter cloacae
DET
O2/H2O
Pseudomonas aeruginosa
DET
O2/H2O
Pseudomonas fluorescens
DET
O2/H2O
Kingella denitrificans
DET
O2/H2O
Staphylococcus carnosus
DET
O2/H2O
Kingella kingae
DET
O2/H2O
Micrococcus luteus
DET
O2/H2O
Geobacter sulfurreducens
DET
Fumarate/succinate
Dumas et al. (2008)
Mixed culture
DET
O2/H2O
Aldrovandi et al. (2009)
Leptothrix discophora SP-6
MET
O2/H2O
Nguyen et al. (2007)
Hydrogenophilic methanogenic culture
DET
CO2/CH4
Hþ/H2
Villano et al. (2010)
Mixed culture
DET
CO2/CH2O*
Cao et al. (2009)
Geobacter sulfurreducens
DET
U(VI)/U(IV)
Gregory and Lovley (2005)
Shewanella putrefaciens
MET
O2/H2O
Freguia et al. (2010)
Acinetobacter calcoaceticus
MET
O2/H2O
Mixed culture
DET
O2/H2O
Rabaey et al. (2008)
Mixed culture
DET
O2/H2O
Erable et al. (2010b)
Mixed culture
MET
O2/H2O
Clauwaert et al. (2007b)
DET
ClO
4 /Cl
Shea et al. (2008)
MET
ClO
4 /Cl
Thrash et al. (2007)
Azospira suillum
MET
ClO
4 /Cl
Mixed culture
DET
Cr(VI)/Cr(III)
Huang et al. (2011a); Tandukar et al. (2009)
Mixed culture
DET
TCE/cis-DCE
Aulenta et al. (2010)
Mixed culture
Dechloromonas agitata
Desulfovibrio vulgaris
MET
þ
H /H2
þ
Lojou et al. (2002)
DET
H /H2
Jeremiasse et al. (2010)
DET
NO
3 /N2
Clauwaert et al. (2007a)
Mixed culture
DET
NO
3 /N2
Lefebvre et al. (2008)
Mixed culture
MET
Acetate/ethanol
Steinbusch et al. (2010)
Mixed culture
Mixed culture
* CH2O represents the approximate formula of biomass.
BIOFILM ELECTROCHEMISTRY FOR ENHANCED MFC PERFORMANCE: A MOLECULAR BIOLOGY PERSPECTIVE
similar to that used by exogenous redox mediators. Table
9.2 shows some reported microbial species for
biocathodes.
BIOFILM ELECTROCHEMISTRY
FOR ENHANCED MFC PERFORMANCE:
A MOLECULAR BIOLOGY PERSPECTIVE
Mechanisms by which bacteria generate power in
MFCs have been intensively investigated in recent years.
How might we make “super-bug” electrogens using the
power of genetic manipulation? There are many factors
that we may ponder. The MFC literature is rife with biological, biochemical and biophysical aspects of highly
electrogenic bacteria including members of the genera
Geobacter, Shewanella, and Rhodoferax species, and many
other Gram-positive and -negative electrogenic bacteria
(Huang et al., 2012; Guo et al., 2012). Many of these organisms can exist and/or thrive in a myriad of different
niches, including those involving significant variations
in temperature, pH, osmolarity, pollutants, biocides,
and metabolizable/nonmetabolizable carbon sources.
As such, many studies involving MFCs house single species bacteria, bacterial “consortia,” media or feedstock,
anode/cathode materials, MFC design and design material, flow rates, Coulombic efficiency and other MFC parameters that can impact power density measurements.
Bacterial Metabolism: How to Power MFCs
through Respiratory/Anaerobic Fluxes
Many bacteria are metabolically versatile organisms,
and can utilize nearly every carbon-containing compound produced in nature. As stated earlier, they are
capable of aerobic and/or anaerobic respiration as well
as fermentation. Aerobic respiration requires molecular
oxygen (O2) while the latter can use alternative electron
2
acceptors including but are not limited to NO
3 , SO4 ,
3þ
Fe , dimethyl sulfoxide (DMSO), trimethylamine
N-oxide (TMAO), and CO2. A member of the tricarboxylic acid cycle (TCA) cycle, fumarate, can also be used.
Interestingly, forms of electron acceptors including
oxides of iron and manganese (Shi et al., 2007), vanadium, selenium, tellurium and toxic metals including
chromium, arsenic and cobalt can also be used by some
organisms. Thus, depending on the organism(s), the
goal of genetically unmodified bacteria is to couple
oxidation of organic matter and reduction of terminal
electron acceptor (in most cases, the anode of the MFC).
However, in MFCs, the electron acceptor in most cases,
with the exception of biocathodes, and bioanode/biocathode MFCs, is the anodic surface. This can occur in
what are commonly termed mediator-dependent or
mediator-less MFCs (discussed below). Many facultative
139
and obligatory anaerobic bacteria can undergo an anaerobic process known as fermentation that does not require
functional cytochromes, respiratory chains and produces
far less energy in the form of adenosine triphosphate
(ATP) than, for example, glucose respiration in E. coli.
Mediator-Less Factors Affecting MFC
Performance
Many studies have been conducted in the past
w7 years to either isolate superior unknown electrogens
or improve the electrogenic properties of existing organisms possessing such capacity. The power of various molecular genetics tools (mutations, deletions, gene
transfer, overexpression, etc.) is the central force underlying the discovery of such strains. However, surfacelocalized factors such as bacterial type IV pili (TFP)
represent a major mediator-less protein that contributes
significantly to some of the more extensively studied
electrogenic bacteria.
TFP (or “Nanowires”): Geobacter and Shewanella
Species as Model Organisms
TFP have been found to be critical for the transfer of
electrons generated metabolically to metal oxides
(e.g. iron oxides; Reguera et al., 2005) that represent
just one component of an MFC anode. These are the
extendable (fully extended outside the cell, followed
by retraction, degradation, and new pilus synthesis)
proteinaceous appendages (for fundamental structures
of three TFP of P. aeruginosa, N. gonorrhoeae and V. cholerae, see Figure 9.5(aec) from Craig et al., 2004). Pili
are often essential for optimal biofilm formation in
many bacterial genera (Zechner et al., 2012), a requirement for mediator-less current on the MFC electrode(s)
surface. Geobacter members are capable of reducing
oxide of either insoluble iron (Fe3þ) or manganese (Mn4þ)
that are directly coupled with organic carbon oxidation.
The pilus extends from many bacteria to bind to and
retract from surfaces for biofilm formation and dispersion in some bacteria (O’Toole and Kolter, 1998) and is
capable of a “grappling hook” retraction mechanism,
followed by degradation, new pilus synthesis and
extension, followed again by the complete retractione
degradationesynthesiseextension loop. The pili of electrogenic G. sulfurreducens have been termed “nanowires”
due to their highly conductive properties (Reguera et al.,
2005) that appear to differ markedly from similar members of the same genus (e.g. G. metallireducens). These
“nanowires” have also been described in S. oneidensis
(Gorby et al., 2006) and are likely in many other bacteria.
The electrogenic importance of the pilus was proven
in a deletion mutant strain of G. sulfurreducens pilA that
generated nearly 10-fold less the power density than
that of wild-type, pilusþ bacteria (Reguera et al., 2006).
140
9. BIOELECTROCHEMISTRY OF MICROBIAL FUEL CELLS AND THEIR POTENTIAL APPLICATIONS IN BIOENERGY
FIGURE 9.5 Superimposition of the
ribbon structures of (a) P. aeruginosa PAK
pilin, (b) N. gonorrhoeae pilin and (c)
V. cholerae TcpA. Details of the superimposition parameters are based upon the
color of each pilin where PAK pilin is in
blue, gonococcal pilin is in white, and
TcpA is in red. Source: Figure from Craig
et al. (2004) with permission from Nature
Publishing Group. (For interpretation of
the references to color in this figure
legend, the reader is referred to the online
version of this book.)
These results were independent of anode composition,
whether it is inexpensive, highly reproducible conductive graphite, or gold, an expensive yet sometimes problematic (reproducibility issues) anodic material (Richter
et al., 2008). A longer isoform of PilA is critical for
optimal power generation than a shorter PilA (Richter
et al., 2012). Many genes, including those involved in
flagella and pilus biosynthesis in G. sulfurreducens (and
even bacterial human opportunistic pathogens, Proteus
mirabilis and P. aeruginosa (Totten et al., 1990; Zhao
et al., 1999)) are controlled by the nitrogen sigma factor,
RpoN (Leang et al., 2009), as identified by microarray
analyses. Thus, predictably strains lacking RpoN are
not electrogenic when compared to wild-type bacteria.
Yi et al. (2009) demonstrated indirect evidence that
T4P in KN400 strain of G. sulfurreducens not only formed
more robust biofilms but also provided superior power
generation (KN400 current (7.6 A/m2) and power
(3.9 W/m2); wild-type DL1-(1.4 A/m2 and 0.5 W/m2)).
An excellent review by Lovley et al. (2011) has shed light
on the unique processes involved in G. sulfurreducens
metabolism and how such unique metabolic properties lends to its reputation as a highly electrogenic
organism.
Gorby et al. (2006) have shown that S. oneidensis MR-1
produce conductive pili in response to a reduction in or
a lack of a terminal electron acceptor. Those researchers
linked electron carrier proteins (c-type decaheme cytochromes MtrC and OmcA, see below) as well as mutations in the type II secretion pathway, where there are
often periplasm protein modifications (e.g. disulfide
bond formation) within Gram-negative bacteria. Thus,
despite possessing pili, bacteria lacking specific cytochromes possessed reduced electrogenic properties.
Yi et al. (2009) isolated a mutant of G. sulfurreducens
DL1 (KN400 strain) that was more effective in current
production than wild-type bacteria. The paradoxical
results were manifested with KN400 forming thinner
biofilms, increased current production, great nanowire
production, flagellum production, far less outersurface c-type cytochromes and, above all, lower MFC
internal resistance. Recently, however, an artificial
matrix termed a conductive artificial biofilm (CAB)
was developed that allows for adherence and nearly
11-fold increased conductive properties of Shewanella
biofilm bacteria (Yu et al., 2011).
Cytochromes (Cell-Bound)
Redox properties of some bacterial cytochromes
(either membrane-bound or soluble cytochromes (e.g.
cytochrome c) electron carriers) have been connected
with the conductive properties of pili (described above).
Typically, these are critical for normal respiratory
functions in both prokaryotic and eukaryotic cells.
In recent years, electrogenesis by metal-oxidizing
Shewanella and Geobacter species as described above are
facilitated by the production of pili and flagella, yet cytochromes have also emerged as one of the major drivers
of the electrogenic process. This is due, in part, to the organisms harboring such compounds transport and
cellular localization of these redox-active cytochromes
to the surface or near-surface of the aforementioned organisms (Figure 9.6). Thus, the surface (e.g. an iron oxide
(Fe3þ) anode) has to be readily accessible to component(s) of the respiratory pathway of such organisms
for optimal electrogenesis to occur. Using S. oneidensis
as a model organism for examining the role of cytochromes in the electrogenic process, there are at least
BIOFILM ELECTROCHEMISTRY FOR ENHANCED MFC PERFORMANCE: A MOLECULAR BIOLOGY PERSPECTIVE
mtrD
(a)
mtrE
mtrF
omcA
mtrC
mtrA
mtrB
mtrC
mtrA
mtrB
mtrC
mtrA
141
S. oneidensis MR-1
A. hydrophila
cymA mtrD
Fe(III) reducers
mtrE
mtrF
undA
mtrG
orfA omcA
mtrB
F. balearica
mtrK2
mtrK3
mtrK1 cymA1
mtrC
mtrA
mtrB
mtrJ
mtrI
orfC cymA 2orfB mtrH
R. ferrireducens
mtoD mtoA
(b)
mtoB
mtoC
D. aromatica RCB
mtoC mtoD mtoA
mtoB
G. capsiferriformans ES-2
Fe(II) oxidizers
mtoD mtoA
mtoB
cymA
pioB
pioC
S. lithotrophicus ES-1
pioA
R. palustris TIE-1
FIGURE 9.6 (a) Genetic organization of iron-reducing vs (b) iron-oxidizing bacteria. Note close proximity of the Mtr (metal reducing) loci in
the iron-reducing and Mto (metal oxidizing) cluster organisms. Source: Figure from Shi et al. (2012a) under STM Permission Guidelines. (For color
version of this figure, the reader is referred to the online version of this book.)
10 gene products involved in iron reduction that are critical for some features of electrogenesis in this organism,
an event that has been studied by many research groups
for more than two decades (Arnold et al., 1990). Conveniently, most of the genes (especially mtr genes)
involved in the process of iron oxide reduction and electrogenesis in MFCs are located in close proximity on the
S. oneidensis genome (Figure 9.6). Figure 9.6 lists the organisms that are also iron-oxidizing bacteria for
Fe(III) oxide
Chelator
Fe(II)
Chelator–Fe(III)
OmcA
MtrC
OmcA
Flavinsox
FlavinsRE
MtrB
OM
MtrA
PS
e–
CymA
IM
quinol
quinone
quinol
quinone
comparative purposes. Of the loci involved in metal
reduction, these include mtrDEF, outer membrane
cytochrome (omcA), followed by the mtrCAB genes.
Figure 9.7 is a simplified recent schematic diagram of
the mechanism of precisely how this process functions
in S. oneidensis, elegantly described by Shi et al.
(2012b). Prior to this exhaustive process of mechanistic
functionality, the first genes found to be required for
iron and manganese oxide reduction were performed
FIGURE 9.7 Model of the decaheme reduction of
insoluble iron by S. oneidensis OmcA (outer membrane
cytochrome A), MtrABC (redox-active metal reducing
proteins) incorporating the influence of inner membrane menaquinone pool as well as CymA (tetraheme
cytochrome). Source: Figure from Shi et al. (2012b);
courtesy of Frontiers Editorial Office in Switzerland. (For
color version of this figure, the reader is referred to the
online version of this book.)
142
9. BIOELECTROCHEMISTRY OF MICROBIAL FUEL CELLS AND THEIR POTENTIAL APPLICATIONS IN BIOENERGY
in S. putrefaciens using transposon mutagenesis in 1998
(Beliaev and Saffarini, 1998). MtrB was found to be an
outer membrane cytochrome while the upstream locus,
mtrA, encodes a periplasmic decaheme cytochrome.
MtrC of both S. putrefaciens and S. oneidensis is also an
outer membrane cytochrome with apparent terminal
iron reductase activity (Beliaev et al., 2001; Hartshorne
et al., 2007). Shi et al. (2006) demonstrated that OcmA,
yet another decaheme cytochrome, binds under acidic
conditions to MtrC and, in fact, these form a highaffinity protein complex with one another. MtrF, MtrD
and MtrE appear to be homologs of MtrCAB, yet one
set cannot replace the other functionally, although
some components can coaggregate. The outlier is that
the mtrFDE loci appear to be highly expressed in biofilms and the Mtr system, in general, is required for
optimal biofilm formation (Coursolle et al., 2010),
similar to aggregation of bacteria on a conductive surface such as an iron oxide anode in MFCs.
In summary, the order of electron flow for optimal
electrogenesis of S. oneidensis is the following: cytoplasmic membrane-bound menaquinone, periplasmic
tetraheme CymA with electron flowing through the
b-barrel of MtrB to the decaheme cytochromes MtrA/
F and finally to two other decaheme proteins, MtrC
and OmcA (Figure 9.7). The final destination for electrons prior to reduction of iron oxides is MtrC. Thus,
again, it is intuitive that the genes encoding those
proteins involved in iron reduction are localized in
the following order on the S. oneidensis genome,
mtrDEFeomcAemtrCAB, respectively. Similar to the
discovered mechanism of proton pumping in the
F1F0-ATP synthase using bacteriorhodopsin in membrane vesicles, scientists have proven that the protein
complex of MtrCAB conducts electron when embedded
within membrane vesicles (Hartshorne et al., 2009). In
2010, Tai et al. (2010) assessed potential networks of
transcriptional regulation between chemotactic and
electron transport properties and found that previously
unknown roles of genes including cheA (a chemotaxis
gene), mgtE-1 (an Mg2þ transport gene) and SO4572
(a triheme cytochrome gene). More recently, Leang
et al. (2010) showed that the redox-cytochrome OmcS
of G. sulfurreducens actually binds to the conductive
pili, thereby contributing to their electrogenic properties. However, using a whole-cell cyclic voltammetric
analysis of various mutant strains including (DmtrC/
DomcA), transmembrane pili (DpilM-Q, DmshH-Q, and
DpilM-Q/DmshH-Q) and flagella (Dflg), Carmona et al.
(2011) demonstrated that even with such mutations
in place, often there are “by-pass” mechanisms of
electron transfer, still allowing for some level of electrogenesis using cyclic voltammetric techniques. A synopsis of these results is shown in schematic form in
Figure 9.8.
Brief Synopsis of the S. oneidensis MR-1
Bioelectrochemical Machinery in Reverse:
Potential Role in the Biosynthesis of Biofuels
in MFCs
The multiple proteins and other factors involved in
bacterial electrogenesis in MFCs are complex. A process termed electron flow reversal, or, better put, electron
diversion, is critical for a nonelectrogenic process for
the purpose of generating single or multiple compounds of value. Ross et al. (2011) have helped simplifying many features of this process in their 2011
publication. Obviously, the goal of scientists working
with electrogenic bacteria is to maximize their power
density while wasting the energy harness in the carbon
skeletons they consume for sustenance. From the above
information collectively, it appears that TFP and cytochromes involved in the Mtr respiratory pathway facilitate the transfer of direct current in the form of
electrons to one or more electrodes. Figure 9.9 helps
simplifying what is currently understood of these systems and other drivers that will be discussed below.
This process is also clearly dependent upon the carbon
sources (or feedstock in more complex, multisubstrate
systems). In that study, multiple isogenic mutants
were created that (1) lacked the periplasmic fumarate
reductase (FccA) and thus could not reduce fumarate
using electrons derived from electrodes, a process
adversely affected by nearly 90% by (2) deletion of
mtrB, or worse, (3) the periplasmic cytochrome, MtrA,
and prevention of menaquinone biosynthesis.
Mediators for Accelerated Electron Transfer
in Biofilms
Flavins
In addition to the mediator-less form of electrogenesis
discussed above (TFP and soluble/insoluble cytochromes), S. oneidensis is an electrogenic bacterium that
can also secrete extracellular, soluble, and redox-active
mediators. Mediators can accept electrons in the anaerobic extracellular milieu or directly from the bacterial
cell surface, and, due to their lower redox potential
(Eo0 in V), they donate electrons directly to the anodic
surface. One group of the S. oneidensis mediators is flavins. In addition to pili and cytochromes, S. oneidensis
produces extracellular flavins that contribute to the electrogenic process and can actually be reduced, in part, via
the Mtr/Omc system. Such flavins include riboflavin
(vitamin B2) (Figure 9.10), flavin (isoalloxazine from
which flavins are derived), and flavin mononuclotide
(FMN). Thus, cells that are unbound to the metal oxide
surface are still capable of reducing it, although the
reduction process also requires reduced members of
MtrC and OmcA (Figure 9.7).
143
MFCS FOR WASTEWATER TREATMENT WITH CONCOMITANT ELECTRICITY PRODUCTION
Flavinox
e–
OmcA
(a)
Anode
Anode
FlavinRed
e–
OmcA
MtrC
Flavinox
Anode
MtrA
e–
e–
MET-1
CymA
FlavinRed
Anode
e–
MtrC
OM
MtrB
MtrB
e–
MtrA
MtrA
Periplasm
e–
e–
DET-1
OM
MtrB
MtrB
MET-1
CymA
MtrA
Periplasm
DET-1
IM
IM
Wild-type, ΔpilM-Q, ΔmshH-Q,
ΔpilM-Q/ ΔmshH-Q and Δflg
ΔmtrC/ΔomcA
IM
e–
DET-2
Wild-type,
ΔmtrC/ΔomcA
ΔmshH-Q and Δflg
(c)
FlavinRed
e–
Anode
e–
MET-2
e–
OM
OM
e–
MET-3
DET-2
IM
IM
msh-type pilus
MET-2
pil-type pilus
OM
e–
Flavinox
FlavinRed
e–
pil-type pilus
Flavinox
Anode
(b)
Anode
e–
DET-3
msh-type pilus
Anode
Wild-type,
ΔmtrC/ΔomcA
ΔpilM-Q and Δflg
ΔpilM-Q and
ΔpilM-Q/ ΔmshH-Q
e–
MET-3
OM
e–
DET-3
IM
ΔmshH-Q and
ΔpilM-Q/ ΔmshH-Q
(d)
Anode
Anode
Anode
Thick biofilm
Wild-type, ΔmtrC/ΔomcA
and ΔpilM-Q
Δflg
ΔmshH-Q and
ΔpilM-Q/ ΔmshH-Q
Poor biofilm
formation
FIGURE 9.8 DET and MET electron transfer pathways utilized by S. oneidensis and selected mutant strains. (a) Electron transfer via the
cytochrome pool. Transmembrane pilus electron transfer via (b) pil-type pilus and via (c) msh-type pilus, and (d) biofilm formation behaviour.
OM: Outer membrane and IM: Inner membrane. Source: Figure from Carmona et al. (2011) with permission from Elsevier
Phenazines
Phenazines are tricyclic, redox-active compounds that
are produced by a number of species of the genus Pseudomonas. Pseudomonas aeruginosa, depending upon the
mutations acquired in a specific microniche, can produce,
or in the case of mutations within negative regulators
or modulators such as RpoS, actually overproduce
redox-active 1-hydroxyphenazine, pyorubrin, or pyocyanin (Figure 9.11). The process of phenazine biosynthesis in these organisms was highlighted by Mavrodi
et al. (2001) where the entire pathway is based upon
anthranilate synthesis and genes beginning with the
acronym phn (for phenazine). A classic demonstration
of the electrogenic contribution of P. aeruginosa phenazines to the electrogenic properties of this organism
was shown using the power of classical bacterial genetics.
Using a mutant approach, Rabaey et al. (2005a) showed
that pyocyanin, pyorubrin, and 1-hydroxyphenazine
could act as excellent mediators in MFCs. Bacteria that
could not produce these mediators possessed reduced
electrogenic properties relative to those genetically incapable of producing them, or organisms whose media
were amended with known quantities of each phenazine.
Given the known electrochemical potential of each of
the aforementioned mediators, it is not surprising that
power density was greatest in MFCs containing pyocyanin > pyorubrin (aeruginosin A; Rabaey et al., 2005a) >
1-hydroxyphenazine. Supportive of these results were
those of Luo et al. (2009b) in the isolation of strain RE7.
MFCS FOR WASTEWATER TREATMENT
WITH CONCOMITANT ELECTRICITY
PRODUCTION
MFC Reactor Designs
There are many different types of MFC bioreactors.
They include single-chamber, dual-chamber, multichamber, membrane-less, multianode, multicathode
and so on. Many MFC reactors were discussed by Du
144
9. BIOELECTROCHEMISTRY OF MICROBIAL FUEL CELLS AND THEIR POTENTIAL APPLICATIONS IN BIOENERGY
(a)
Cytoplasmic
membrane
Periplasmic
space
Outer
membrane
Extracellular
space
e–
MtrA
MtrC
soluble
mediator
Electrode
direct
MtrB
CymA
MQ MQH2
CymA
FccA
fumarate succinate
(b)
CymA
–300 mV
MtrA:MtrC
FccA
Potential window of electrode
linked fumarate reduction
V vs SHE
–200 mV
–100 mV
0 mV
fum/suc
+100 mV
FIGURE 9.9 (a) Mechanism of electron reversal (or inward electron flux) in S. oneidensis and the gene products involved in this process.
(b) Fumarate reduction in S. oneidensis: windows of redox and midpoint (deep-red lines) potentials for each electron carrier. Source: Figure from
Ross et al. (2011) with permission from Public Library of Science. (For interpretation of the references to color in this figure legend, the reader is
referred to the online version of this book.)
et al. (2007). More recently, Zhou et al. (2013) reviewed
some new MFC reactors and their combinations,
including MFCs operated as microbial electrolysis cells
(MECs) to produce bio-products such as hydrogen and
methane. It should be pointed out that improvement
in MFC reactor design must consider cost and maintenance. Complicated designs are not only costly but
also prone to biofouling, causing maintenance and sustainability problems. A simplistic tubular MFC reactor
with convective axial flow was proposed (Zhou et al.,
2013). To reduce cost and fouling, no membrane was
used. To prevent oxygen back-diffusion into the anodic
region, a substantial flow rate from the anode to the
cathode is required. This means that the biofilm has to
be highly efficient in the digestion of organic matter in
wastewater streams. This type of design will become
attractive only when robust “super-bug” biofilms are
successfully engineered.
MFCS FOR WASTEWATER TREATMENT WITH CONCOMITANT ELECTRICITY PRODUCTION
145
FIGURE 9.10
(a). The riboflavin (vitamin B2) biosynthetic genes of S. oneidensis. (b). Structures of riboflavin, isoalloxazine, and flavin
mononucleotide. Source: Figure courtesy of Dr Jeff Gralnick of University of Minnesota. (For color version of this figure, the reader is referred to the
online version of this book.)
the metabolic pathways to utilize high-grade organic
carbons such as cellulose, hemicellulose, various hexoses and phenylpropane moieties (components of
lignin). Most of the electrogenic microbes capable of
DET feed only on low-grade organic carbons such as
VFAs and alcohols. Only a few organisms such as
R. ferrireducens (Chaudhuri and Lovley, 2003; Schröder,
2007) utilize glucose, while Geobacter and Shewanella
strains cannot (Lovley, 2006a). This limits MFC
power output because high-grade organic carbons are
unutilized.
Simple Biodegradable Organics
FIGURE 9.11
Microcentrifuge tubes containing chloroformextractable pyocyanin (blue bottom layer and the “merlot” colored)
and water-soluble pyorubrin layer (top). The tube on the left is
derived from a lasI rhlI mutant that is incapable of quorum sensing
and, as such, is incapable of producing pigments, while that on the
right is from rpoS mutant bacteria that overproduce both pyocyanin
and pyorubrin. Source: Suh et al. (1999). (For interpretation of the
references to color in this figure legend, the reader is referred to the
online version of this book.)
Substrates Used in MFCs
In MFCs, the substrates greatly impact their performances such as power density and Coulombic
efficiency (Pant et al., 2010). The substrates range
from the simple volatile fatty acids (VFAs) to complex
compounds such as lignocellulosic biomass. Anaerobes
evolved when the earth’s atmosphere was still anaerobic long before aerobes evolved. Many of them lack
Acetate and glucose are two common substrates in
laboratory studies. Compared to the recalcitrant
substrates, they are far more readily utilized by
microbes for energy generation. Thus, they are usually
used as the carbon source for microbes used in
MFCs. Acetate has an advantage that at normal temperatures, it is not a good nutrient for fermentation and
methanogenesis. In contrast, glucose is a fermentable
sugar that can be consumed by the processes of fermentation and methanogenesis (Pant et al., 2010). Thus, the
Coulombic efficiency of acetate is usually higher than
glucose. However, glucose can be used to promote the
microbial diversity of a biofilm consortium. When
glucose was used as the substrate, a maximum power
density of 216 mW m2 was achieved (Rabaey et al.,
2003), while it reached 506 mW m2 for acetate (Liu
et al., 2005b). Some other simple substrates such as butyrate have also been used as the substrate in MFCs.
146
9. BIOELECTROCHEMISTRY OF MICROBIAL FUEL CELLS AND THEIR POTENTIAL APPLICATIONS IN BIOENERGY
Wastewater Types
Various wastewaters have been tested as substrates for
MFCs because they contain many different kinds of
organic carbon molecules. They are attractive for use in
MFCs because the organic carbons are otherwise wasted.
As shown in Table 9.3, the output power density is
dependent on the wastewater quality (high COD values)
and the MFC reactor structure. For example, a maximum
power density of 528 mW m2 for brewery wastewater
was obtained (Feng et al., 2008), while an average power
density of 72 mW m2 was achieved for domestic wastewater (Sharma and Kundu, 2010). Some biorefractory
wastewaters such as dye, leachates and pharmaceutical
wastewater have also been tested for MFC power generation. A landfill leachate containing heavy metals, dissolved organic matters and other matters achieved a
maximum power density of 1.38 mW m2 (Greenman
et al., 2009). A maximum power density of 9.1 W m3
was achieved when using phenol as the sole carbon
source. While glucose was added as a supplement, the
maximum power density increased to 28.3 W m3 (Luo
et al., 2009a). In addition, some refractory compounds
such as pyridine, quinoline and indole were also used
as substrates for MFCs (Hu et al., 2011).
Lignocellulosic Biomass
Lignocellulosic biomass includes corn stover, straw,
wheat stover, algae and others. The primary components
in lignocellulosic biomass are cellulose, hemicellulose
and lignin. Compositions differ for different types of
biomass. Lignocellulosic biomass is considered unfermentable because most microbes cannot degrade it
TABLE 9.3 An Updated List of Substrates Used in MFCs
Substrates
Reactor Style
Pmax (mW mL2)
References
Glucose
Dual chamber
283
Rahimnejad et al. (2011)
Cheese whey
Dual chamber
42
Stamatelatou et al. (2011)
Food waste
Single chamber
207.2**
Kannaiah and Venkata (2011)
Palm oil mill effluent with acetate
Dual chamber
622
Jong et al. (2011)
Dairy wastewater
Single chamber
5.7*
Ayyaru and Dharmalingam (2011)
Leachates
Single chamber
20.9
Va’zquez-Larios et al. (2011)
Composite food waste
Single chamber
107.89
Goud et al. (2011)
Pharmaceutical wastewater
Single chamber
177.36
Velvizhi and Venkata (2011)
Azo dye
Single-chamber
e
Sun et al. (2011)
Human feces wastewater
Dual chamber
70.8
Du et al. (2011)
Synthetic penicillin wastewater with
glucose
Single chamber
101.2*
Wen et al. (2011)
Paper wastewater
Single chamber
125***
Velasquez et al. (2011)
Dairy wastewater
Single chamber
25***
Brewery and bakery wastewaters
Single chamber
10***
Distillery wastewater
Single chamber
245.34
Mohanakrishna et al. (2012)
Sewage sludge
Tubular MFC
73
Yuan et al. (2012)
Primary clarifier effluent
Single chamber
13
Ishii et al. (2012)
Alcohol distillery wastewater
Dual chamber
1000
Ha et al. (2012)
Agriculture wastewater
Single chamber
13
Nimje et al. (2012)
Domestic wastewater
Single chamber
42
Paper wastewater
Single chamber
8
Food/dairy wastewater
Single chamber
15
Bad wine
Dual chamber
3.82*
3
* In W m .
** Calculated from power and current densities.
***
In mA m2.
Rengasamy and Berchmans (2012)
REFERENCES
without pretreatment and lignin is optimally degraded
under aerobic conditions via several dioxygenasetype enzymes, although some anaerobic bacteria can
degrade it, albeit slowly. Pretreatment methods include
mechanical, hydrothermal, biological, chemical, ammonia or supercritical CO2 explosion and ionic liquid
extraction (Gu, 2013). An MFC using corn stover after
steam-explosion pretreatment as the substrate achieved
a maximum power density of 861 mW m2 (Zuo et al.,
2006). MFCs fed with Chlorella vulgaris and Ulva lactuca
powders achieved maximum power densities of
0.98 W m2 (277 W m3) and 0.76 W m2 (215 W m3),
respectively (Velasquez-Orta et al., 2009).
Cellulose is relative easy to utilize by MFCs compared
with lignocellulosic biomass. A maximum power density of 272 mW m2 was achieved using carboxymethyl
cellulose as substrate in an MFC (Rezaei et al., 2009).
This means that it is possible to utilize the tissue paper
(cellulose) in municipal wastewater as substrate.
Table 9.3 shows the list of substrates used for MFCs
studied until 2013.
SUMMARY AND PERSPECTIVES
This chapter discusses the operating principles of
MFCs and various aspects in bioelectrochemistry in
MFC research. Although tremendous advances have
been made around 2013 in academic MFC research
including a much better understanding of biofilm electrochemistry and better reactor designs, major technological
hurdles remain for practical MFC applications beyond
powering sensor devices. It is unreasonable to expect
MFCs to reach power densities on par with those from
chemical fuel cells because MFCs are powered by lowenergy-density fuels such as dilute organic matter in
wastewaters. However, it is still necessary to increase
MFC power generation to what would be considered a
useful level (e.g. to offset part of the energy input in
wastewater treatment), much higher than what has
been achieved.
Various approaches have been attempted to increase
MFC performance including improved reactor designs,
electrode and membrane materials, feedstock selection
and modification, introduction of exogenous mediators,
and utilization of secreted endogenous mediators.
Unfortunately, many of the improvements come with
inherent cost increases with little hope for practical
applications. Some MFC researchers have come to
realize that a breakthrough in biofilm engineering
should be explored. Recent discoveries such as interspecies electron transfer, conductive cell aggregates and
long-distance conductive filaments provide new hope
for means to engineer robust “super-bug” biofilms
with greatly enhanced electron transfer capacity and a
147
voracious appetite for complex organic matter digestion.
The dawn of a new era for MFC research might be
in sight and the synergistic involvement of biochemical
and environmental engineers, microbiologists and molecular biologists may soon bear fruit in this exciting
field of practical research.
References
Aldrovandi, A., Marsili, E., Stante, L., Paganin, P., Tabacchioni, S.,
Giordano, A., 2009. Sustainable power production in a membraneless and mediator-less synthetic wastewater microbial fuel cell.
Bioresour. Technol. 100, 3252e3260.
Allen, R.M., Bennetto, H.P., 1993. Microbial fuel-cells electricity production from carbohydrates. Appl. Biochem. Biotech. 39/40, 27e40.
Angenent, L.T., Karim, K., Dahhan, M.H.A., Wrenn, B.A., Rosa, D.E.,
2004. Production of bioenergy and biochemicals from industrial
and agricultural wastewater. Trends Biotechnol. 22, 477e485.
Arnold, R.G., Hoffmann, M.R., Dichristina, T.J., Picardal, F.W., 1990.
Regulation of dissimilatory Fe(III) reduction activity in Shewanella
putrefaciens. Appl. Environ. Microbiol. 56, 2811e2817.
Aulenta, F., Reale, P., Canosa, A., Rossetti, S., Panero, S., Majone, M.,
2010. Characterization of an electro-active biocathode capable of
dechlorinating trichloroethene and cis-dichloroethene to ethene.
Biosens. Bioelectron. 25, 1796e1802.
Ayyaru, S., Dharmalingam, S., 2011. Development of MFC using
sulphonated polyether ether ketone (SPEEK) membrane for electricity generation from waste water. Bioresour. Technol. 102,
11167e11171.
Bao, Y., Wu, X., 2004. Progress in research for biofuel cell. Electrochemistry 10, 1e8.
Behera, M., Jana, P.S., Ghangrekar, M.M., 2010. Performance evaluation of low cost microbial fuel cell fabricated using earthen pot
with biotic and abiotic cathode. Bioresour. Technol. 101, 1183e1189.
Beliaev, A.S., Saffarini, D.A., 1998. Shewanella putrefaciens mtrB encodes
an outer membrane protein required for Fe(III) and Mn(IV)
reduction. J. Bacteriol. 180, 6292e6297.
Beliaev, A.S., Saffarini, D.A., McLaughlin, J.L., Hunnicutt, D., 2001. MtrC,
an outer membrane decahaem c cytochrome required for metal
reduction in Shewanella putrefaciens MR-1. Mol. Microbiol. 39, 722e730.
Biffinger, J.C., Pietron, J., Ray, R., 2007. A biofilm enhanced miniature
microbial fuel cell using Shewanella oneidensis DSP10 and oxygen
reduction cathodes. Biosens. Bioelectron. 22, 1672e1679.
Biffinger, J.C., Pietron, J., Bretschger, O., 2008. The influence of acidity
on microbial fuel cell containing Shewanella oneidensis. Biosens.
Bioelectron. 24, 906e911.
Bond, D.R., Lovley, D.R., 2003. Electricity production by Geobacter
sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 69,
1548e1555.
Bond, D.R., Lovley, D.R., 2005. Evidence for involvement of an electron shuttle in electricity generation by Geothrix fermentans. Appl.
Environ. Microbiol. 71, 2186e2189.
Bond, D.R., E.Holmes, D., tender, L.M., Lovely, D.R., 2002. Electrodereducing microorganisms that harvest energy from marine sediments. Science 295, 483e485.
Bullen, R.A., Arnot, T.C., Lakeman, J.B., Walsh, F.C., 2006. Biofuel cells
and their development. Biosens. Bioelectron. 21, 2015e2045.
Busalmen, J.P., Nunez, A.E., Berna, A., Feliu, J.M., 2008. C-type cytochromes wire electricity-producing bacteria to electrodes. Angew.
Chem. Int. Edit. 47, 4874e4877.
Cao, X., Huang, X., Liang, P., Boon, N., Fan, M., Zhang, L., Zhang, X.,
2009. A completely anoxic microbial fuel cell using a photobiocathode for cathodic carbon dioxide reduction. Energy Environ.
Sci. 2, 498.
148
9. BIOELECTROCHEMISTRY OF MICROBIAL FUEL CELLS AND THEIR POTENTIAL APPLICATIONS IN BIOENERGY
Carmona, M.A.A., Harnisch, F., Fitzgerald, L.A., Biffinger, J.C.,
Ringeisen, B.R., Schröder, U., 2011. Cyclic voltammetric analysis of
the electron transfer of Shewanella oneidensis MR-1 and nanofilament
and cytochrome knock-out mutants. Bioelectrochemistry 81, 74e80.
Chaudhuri, S.K., Lovley, D.R., 2003. Electricity generation by direct
oxidation of glucose in mediatorless microbial fuel cells. Nat.
Biotechnol. 21, 1229e1232.
Choi, Y., Jung, E., Kim, S., Jung, S., 2003a. Membrane fluidity sensoring microbial fuel cell. Bioelectrochemistry 59, 121e127.
Choi, Y., Kim, N., Kim, S., Jung, S., 2003b. Dynamic behaviors of redox
mediators within the hydrophobic layers as an important factor for
effective microbial fuel cell operation. B. Korean Chem. Soc. 24,
437e440.
Clauwaert, P., Rabaey, K., Aelterman, P., Schamphelaire, L.D.,
Pham, H.T., Boeckx, P., Boon, N., Verstraete, W., 2007a. Biological
denitrification in microbial fuel cells. Environ. Sci. Technol. 41,
3354e3360.
Clauwaert, P., Ha, D.V.D., Boon, N., Verbeken, K., Verhaege, M.,
Rabaey, K., Verstraete, W., 2007b. Open air biocathode enables
effective electricity generation with microbial fuel cells. Environ.
Sci. Technol. 41, 7564e7569.
Cooney, M.J., Roschi, E., Marison, I.W., Comninellis, C., Stockar, U.v.,
1996. Physiologic studies with the sulfate-reducing bacterium
Desulfovibrio desulfuricans evaluation for use in a biofuel cell.
Enzyme Microb. Tech. 18, 358e365.
Cournet, A., Délia, M.L., Bergel, A., Roques, C., Bergé, M., 2010.
Electrochemical reduction of oxygen catalyzed by a wide range of
bacteria including gram-positive. Electrochem. Commun. 12,
505e508.
Coursolle, D., Baron, D.B., Bond, D.R., Gralnick, J.A., 2010. The Mtr
respiratory pathway is essential for reducing flavins and electrodes
in Shewanella oneidensis. J. Bacteriol. 192, 467e474.
Craig, L., Pique, M.E., Tainer, J.A., 2004. Type IV pilus structure and
bacterial pathogenicity. Nat. Rev. Microbiol. 2, 363e378.
Du, Z., Li, H., Gu, T., 2007. A state of the art review on microbial fuel
cells: a promising technology for wastewater treatment and bioenergy. Biotechnol. Adv. 25, 464e482.
Du, F., Li, Z., Yang, S., Xie, B., Liu, H., 2011. Electricity generation
directly using human feces wastewater for life support system.
Acta Astronaut. 68, 1537e1547.
Dumas, C., Basseguy, R., Bergel, A., 2008. Microbial electrocatalysis
with Geobacter sulfurreducens biofilm on stainless steel cathodes.
Electrochim. Acta 53, 2494e2500.
Erable, B., Duteanu, N.M., Ghangrekar, M.M., Dumas, C., Scott, K.,
2010a. Application of electro-active biofilms. Biofouling 26, 57e71.
Erable, B., Vandecandelaere, I., Faimali, M., Delia, M.L., Etcheverry, L.,
Vandamme, P., Bergel, A., 2010b. Marine aerobic biofilm as biocathode catalyst. Bioelectrochemistry 78, 51e56.
Feng, Y., Wang, X., Logan, B.E., Lee, H., 2008. Brewery wastewater
treatment using air-cathode microbial fuel cells. Appl. Microbiol.
Biotechnol. 78, 873e880.
Fitzgerald, L.A., Petersen, E.R., Ray, R.I., Little, B.J., Cooper, C.J.,
Howard, E.C., Ringeisen, B.R., Biffinger, J.C., 2012. Shewanella oneidensis MR-1 Msh pilin proteins are involved in extracellular electron
transfer in microbial fuel cells. Process Biochem. 47, 170e174.
Franks, A.E., Nevin, K.P., 2010. Microbial fuel cells, a current review.
Energies 3, 899e919.
Freguia, S., Tsujimura, S., Kano, K., 2010. Electron transfer pathways
in microbial oxygen biocathodes. Electrochim. Acta 55, 813e818.
Gil, G.C., Chang, I.S., Kim, B.H., Kim, M., Jang, J.K., Park, H.S.,
Kim, H.J., 2003. Operational parameters affecting the performance
of a mediator-less microbial fuel cell. Biosens. Bioelectron. 18,
327e334.
Gorby, Y.A., Yanina, S., McLean, J.S., Rosso, K.M., Moyles, D.,
Dohnalkova, A., Beveridge, T.J., Chang, I.S., Kim, B.H., Kim, K.S.,
Culley, D.E., Reed, S.B., Romine, M.F., Saffarini, D.A., Hill, E.A.,
Shi, L., Elias, D.A., Kennedy, D.W., Pinchuk, G., Watanabe, K.,
Ishii, S., Logan, B., Nealson, K.H., Fredrickson, J.K., 2006. Electrically conductive bacterial nanowires produced by Shewanella
oneidensis strain MR-1 and other microorganisms. Proc. Natl. Acad.
Sci. USA 103, 11358e11363.
Goud, R.K., Babu, P.S., Mohan, S.V., 2011. Canteen based composite
food waste as potential anodic fuel for bioelectricity generation in
single chambered microbial fuel cell (MFC): bio-electrochemical
evaluation under increasing substrate loading condition. Int.
J. Hydrogen Energy 36, 6210e6218.
Greenman, J., Gálvez, A., Giusti, L., Ieropoulos, I., 2009. Electricity
from landfill leachate using microbial fuel cells: comparison with a
biological aerated filter. Enzyme Microb. Tech. 44, 112e119.
Gregory, K.B., Lovley, D.R., 2005. Remediation and recovery of uranium from contaminated subsurface environments with electrodes. Environ. Sci. Technol. 39, 8943e8947.
Gu, T., 2012. New understandings of biocorrosion mechanisms and
their classifications. J. Chem. Technol. Biotechnol. 4, 3e6.
Gu, T., 2013. Green Biomass Pretreatment for Biofuels Production.
Springer, Berlin-New York.
Guo, K., Hassett, D.J., Gu, T., 2012. Microbial fuel cells: electricity
generation from organic wastes by microbes. Advances in microbial fuel cells for potential energy production from organic feed
streams. In: Arora, R. (Ed.), Microbial Biotechnology: Energy and
Environment. CAB International, Oxon, United Kingdom, ISBN
978-1845939564. (Chapter 12).
Ha, P.T., Lee, T.K., Rittmann, B.E., Park, J., Chang, I.S., 2012. Treatment of
alcohol distillery wastewater using a bacteroidetes-dominant thermophilic microbial fuel cell. Environ. Sci. Technol. 46, 3022e3030.
Hamid, R.Y., Carver, S.M., Christy, A.D., Tuovinen, O.H., 2008.
Cathodic limitations in microbial fuel cells: an overview. J. Power
Sources 180, 683e694.
Hartshorne, R.S., Jepson, B.N., Clarke, T.A., Field, S.J., Fredrickson, J.,
Zachara, J., Shi, L., Butt, J.N., Richardson, D.J., 2007. Characterization of Shewanella oneidensis MtrC: a cell-surface decaheme
cytochrome involved in respiratory electron transport to extracellular electron acceptors. J. Biol. Inorg. Chem. 12, 1083e1094.
Hartshorne, R.S., Reardon, C.L., Ross, D., Nuester, J., Clarke, T.A.,
Gates, A.J., Mills, P.C., Fredrickson, J.K., Zachara, J.M., Shi, L.,
Beliaev, A.S., Marshall, M.J., Tien, M., Brantley, S., Butt, J.N.,
Richardson, D.J., 2009. Characterization of an electron conduit
between bacteria and the extracellular environment. Proc. Natl.
Acad. Sci. USA 106, 22169e22174.
Hassan, S.H.A., Kim, Y.S., Oh, S.E., 2012. Power generation from cellulose using mixed and pure cultures of cellulose-degrading bacteria in a microbial fuel cell. Enzyme Microb. Technol. 51, 269e273.
Hatch, J.L., Finneran, K.T., 2008. Influence of reduced electron
shuttling compounds on biological H2 production in the
fermentative pure culture Clostridium beijerinckii. Curr. Microbiol.
56, 268e273.
He, Z., Angenent, L.T., 2006. Application of bacterial biocathodes in
microbial fuel cells. J. Electroanal. Chem. 18, 2009e2015.
Holmes, D.E., Bond, D.R., O’Neil, R.A., Reimers, C.E., Tender, L.R.,
Lovley, D.R., 2004. Microbial communities associated with electrodes harvesting electricity from a variety of aquatic sediments.
Microb. Ecol. 48, 178e190.
Holmes, D.E., Mester, T., O’Neil, R.A., Perpetua, L.A.,
Larrahondo, M.J., Glaven, R., Sharma, M.L., Ward, J.E., Nevin, K.P.,
Lovley, D.R., 2008. Genes for two multicopper proteins required
for Fe(III) oxide reduction in Geobacter sulfurreducens have different
expression patterns both in the subsurface and on energyharvesting electrodes. Microbiology 154, 1422e1435.
Hou, H., Li, L., Cho, Y., 2009. Microfabricated microbial fuel cell arrays
reveal electrochemically active microbes. PLoS One 4, 6570.
REFERENCES
Hu, W.J., Niu, C.G., Wang, Y., Zeng, G.M., Wu, Z., 2011. Nitrogenous
heterocyclic compounds degradation in the microbial fuel cells.
Process Saf. Environ. Prot. 89, 133e140.
Huang, L., Chai, X., Cheng, S., Chen, G., 2011a. Evaluation of carbonbased materials in tubular biocathode microbial fuel cells in terms
of hexavalent chromium reduction and electricity generation.
Chem. Eng. J. 166, 652e661.
Huang, L., Cheng, S., Chen, G., 2011b. Bioelectrochemical systems for
efficient recalcitrant wastes treatment. J. Chem. Technol. Biotechnol. 86, 481e491.
Huang, L., Regan, J.M., Quan, X., 2011c. Electron transfer mechanisms,
new applications, and performance of biocathode microbial fuel
cells. Bioresour. Technol. 102, 316e323.
Huang, L., Cheng, S., Hassett, D.J., Gu, T., 2012. Wastewater treatment
with concomitant bioenergy production using microbial fuel cells.
In: Sharma, S.K., Sanghi, R. (Eds.), Water Treatment and Pollution
Prevention: Advances in Research. Springer-Verlag, Berlin-New
York, pp. 405e452. (Chapter 18).
Ishii, S.i., Suzuki, S., Norden-Krichmar, T.M., Nealson, K.H.,
Sekiguchi, Y., Gorby, Y.A., Bretschger, O., 2012. Functionally stable and phylogenetically diverse microbial enrichments from
microbial fuel cells during wastewater treatment. PLoS One 7,
1e12.
Jeremiasse, A.W., Hamelers, H.V., Buisman, C.J., 2010. Microbial
electrolysis cell with a microbial biocathode. Bioelectrochemistry
78, 39e43.
Jong, B.C., Liew, P.W.Y., Juri, M.L., Kim, B.H., Mohd Dzomir, A.Z.,
Leo, K.W., Awang, M.R., 2011. Performance and microbial
diversity of palm oil mill effluent microbial fuel cell. Lett. Appl.
Microbiol. 53, 660e667.
Kannaiah, G.R., Venkata, M.S., 2011. Pre-fermentation of waste as a
strategy to enhance the performance of single chambered microbial fuel cell (MFC). Int. J. Hydrogen Energy 36, 13753e13762.
Kim, B.H., Ikeda, T., Park, H.S., Kim, H.J., Hyun, M.S., Kano, K.,
Takagi, K., Tatsumi, H., 1999. Electrochemical activity of an Fe(III)reducing bacterium, Shewanella putrefaciens IR-1, in the presence of
alternative electron acceptors. Biotechnol. Technol. 13, 475e478.
Kim, N., Choi, Y., Jung, S., 2000a. Development of microbial fuel cells
using Proteus vulgaris. B. Korean Chem. Soc. 21, 44e48.
Kim, N., Choi, Y., Jung, S., 2000b. Effect of initial carbon sources on the
performance of microbial fuel cells containing Proteus vulgaris.
Biotechnol. Bioeng. 70, 109e114.
Kim, H.J., Park, H.S., Hyun, M.S., Chang, I.S., Kim, M., Kima, B.H., 2002.
A mediator-less microbial fuel cell using a metal reducing bacterium, Shewanella putrefaciens. Enzyme Microb. Technol. 30, 145e152.
Leang, C., Krushkal, J., Ueki, T., Puljic, M., Sun, J., Juarez, K.,
Nunez, C., Reguera, G., DiDonato, R., Postier, B., Adkins, R.M.,
Lovley, D.R., 2009. Genome-wide analysis of the RpoN regulon in
Geobacter sulfurreducens. BMC Genomics 10, 331.
Leang, C., Qian, X., Mester, T., Lovley, D.R., 2010. Alignment of the
c-type cytochrome OmcS along pili of Geobacter sulfurreducens.
Appl. Environ. Microbiol. 76, 4080e4084.
Lee, J., Phung, N.T., Chang, I.S., Kim, B.H., Sung, H.C., 2003. Use of
acetate for enrichment of electrochemically active microorganisms
and their 16S rDNA analyses. FEMS Microbiol. Lett. 223, 185e191.
Lefebvre, O., Mamun, A.A., Ng, H.Y., 2008. A microbial fuel cell
equipped with a biocathode for organic removal and denitrification. Water Sci. Technol. 58, 881e885.
Li, W.W., Sheng, G.P., Liu, X.W., Yu, H.Q., 2011. Recent advances in the
separators for microbial fuel cells. Bioresour. Technol. 102,
244e252.
Liu, H., Cheng, S., Logan, B.E., 2005a. Power generation in fedbatch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environ. Sci. Technol. 39,
5488e5493.
149
Liu, H., Cheng, S., Logan, B.E., 2005b. Production of electricity from
acetate or butyrate using a single-chamber microbial fuel Cell.
Environ. Sci. Technol. 39, 658e662.
Liu, G., Yates, M.D., Cheng, S., Call, D.F., Sun, D., Logan, B.E., 2011.
Examination of microbial fuel cell start-up times with domestic
wastewater and additional amendments. Bioresour. Technol. 102,
7301e7306.
Logan, B.E., 2009. Exoelectrogenic bacteria that power microbial fuel
cells. Nat. Rev. Microbiol. 7, 375e381.
Logan, B.E., Regan, J.M., 2006. Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol. 14, 512e518.
Logan, B.E., Hamelers, B., Rozendal, R., Schröder, U., Keller, J.,
Freguia, S., Aelterman, P., Verstraete, W., Rabaey, K., 2006. Microbial fuel cells: methodology and technology. Environ. Sci. Technol.
40, 5181e5192.
Lojou, E., Durand, M.C., Dolla, A., Bianco, P., 2002. Hydrogenase
activity control at Desulfovibrio vulgaris cell. J. Electroanal. Chem.
14, 913e922.
Lovley, D.R., 2006a. Bug juice: harvesting electricity with microorganisms. Nat. Rev. Microbiol. 4, 497e508.
Lovley, D.R., 2006b. Microbial fuel cells: novel microbial physiologies
and engineering approaches. Curr. Opin. Biotechnol. 17, 327e332.
Lovley, D.R., 2008. The microbe electric: conversion of organic matter
to electricity. Curr. Opin. Biotechnol. 19, 564e571.
Lovley, D.R., Ueki, T., Zhang, T., Malvankar, N.S., Shrestha, P.M.,
Flanagan, K.A., Aklujkar, M., Butler, J.E., Giloteaux, L.,
Rotaru, A.E., Holmes, D.E., Franks, A.E., Orellana, R., Risso, C.,
Nevin, K.P., 2011. Geobacter: the microbe electric’s physiology,
ecology, and practical applications. Adv. Microb. Physiol. 59,
1e100.
Luo, H., Liu, G., Zhang, R., Jin, S., 2009a. Phenol degradation in
microbial fuel cells. Chem. Eng. J. 147, 259e264.
Luo, H.P., Liu, G.l., Zhang, R.D., Cao, L.X., 2009b. Isolation and
characterization of electrochemical active bacterial Pseudomonas
aeruginosa strain RE7. Huan Jing Ke Xue 30, 2118e2123.
Makarieva, A.M., Gorshkov, V.G., Li, B.L., 2008. Energy budget of the
biosphere and civilization: rethinking environmental security
of global renewable and non-renewable resources. Ecol. Complex.
5, 281e288.
Manohar, A.K., Bretschger, O., Nealson, K.H., 2008. The use of
electrochemical impedance spectroscopy (EIS) in the evaluation of
the electrochemical properties of a microbial fuel cell.
Bioelectrochemistry 72, 149e154.
Marsili, E., Baron, D.B., Shikhare, I.D., Coursolle, D., Gralnick, J.A.,
Bond, D.R., 2008. Shewanella secretes flavins that mediate extracellular electron transfer. Microbiology 105, 3968e3973.
Mavrodi, D.V., Bonsall, R.F., Delaney, S.M., Soule, M.J., Phillips, G.,
Thomashow, L.S., 2001. Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J. Bacteriol. 183, 6454e6465.
McKinlay, J.B., Zeikus, J.G., 2004. Extracellular iron reduction is
mediated in part by neutral red and hydrogenase in Escherichia coli.
Appl. Environ. Microbiol. 70, 3467e3474.
Min, B., Cheng, S., Logan, B., 2005. Electricity generation using
membrane and salt bridge microbial fuel cells. Water Res. 39,
1675e1686.
Mohanakrishna, G., Mohan, S.K., Mohan, S.V., 2012. Carbon based
nanotubes and nanopowder as impregnated electrode structures
for enhanced power generation: evaluation with real field wastewater. Appl. Energy 95, 31e37.
Moon, H., Chang, I.S., Kim, B.H., 2006. Continuous electricity production from artificial wastewater using a mediator-less microbial
fuel cell. Bioresour. Technol. 97, 621e627.
Morita, M., Malvankar, N.S., Franks, A.E., Summers, Z.M.,
Giloteaux, L., Rotaru, A.E., Rotaru, C., Lovley, D.R., 2011. Potential
150
9. BIOELECTROCHEMISTRY OF MICROBIAL FUEL CELLS AND THEIR POTENTIAL APPLICATIONS IN BIOENERGY
for direct interspecies electron transfer in methanogenic wastewater digester aggregates. MBio. 2, e00159e11.
Neto, S.A., Forti, J.C., Andrade, A.R., 2010. An overview of enzymatic
biofuel cells. Electrocatalysis 1, 87e94.
Nevin, K.P., Richter, H., Covalla, S.F., Johnson, J.P., Woodard, T.L.,
Orloff, A.L., Jia, H., Zhang, M., Lovley, D.R., 2008. Power output
and columbic efficiencies from biofilms of Geobacter sulfurreducens
comparable to mixed community microbial fuel cells. Environ.
Microbiol. 10, 2505e2514.
Newman, D.K., Kolter, R., 2000. A role for excreted quinones in
extracellular electron transfer. Nature 450, 94e97.
Nguyen, T.A., Lu, Y., Yang, X., Shi, X., 2007. Carbon and steel surfaces
modified by Leptothrix discophora SP-6: characterization and
implications. Environ. Sci. Technol. 41, 7987e7996.
Niessen, J., Schröder, U., Scholz, F., 2004. Exploiting complex carbohydrates for microbial electricity generation-a bacterial fuel cell
operating on starch. Electrochem. Commun. 6, 955e958.
Nimje, V.R., Chen, C.Y., Chen, H.R., Chen, C.C., Huang, Y.M.,
Tseng, M.J., Cheng, K.C., Chang, Y.F., 2012. Comparative
bioelectricity production from various wastewaters in microbial
fuel cells using mixed cultures and a pure strain of Shewanella
oneidensis. Bioresour. Technol. 104, 315e323.
O’Toole, G.A., Kolter, R., 1998. Flagellar and twitching motility are
necessary for Pseudomonas aeruginosa biofilm development. Mol.
Microbiol. 30, 295e304.
Oh, S.E., Logan, B.E., 2005. Hydrogen and electricity production from
a food processing wastewater using fermentation and microbial
fuel cell technologies. Water Res. 39, 4673e4682.
Osman, M.H., Shah, A.A., Walsh, F.C., 2010. Recent progress and
continuing challenges in bio-fuel cells. Part II: microbial. Biosens.
Bioelectron. 26, 953e963.
Pant, D., Van Bogaert, G., Diels, L., Vanbroekhoven, K., 2010. A review
of the substrates used in microbial fuel cells (MFCs) for sustainable
energy production. Bioresour. Technol. 101, 1533e1543.
Park, D.H., Zeikus, J.G., 1999. Utilization of electrically reduced
neutral red by Actinobacillus succinogenes: physiological function of
neutral red in membrane driven fumarate reduction and energy
conservation. J. Bacteriol. 181, 2403e2410.
Park, D.H., Zeikus, J.G., 2000. Electricity generation in microbial fuel
cells using neutral red as an electronophore. Appl. Environ.
Microbiol. 66, 1292e1297.
Park, D.H., Zeikus, J.G., 2003. Improved fuel cell and electrode
designs for producing electricity from microbial degradation.
Biotechnol. Bioeng. 81, 348e355.
Petter, M.C., 1911. Electrical effects accompanying the decomposition
of organic compounds. Proc. R Soc. Lond. B Biol. Sci. 84, 260e276.
Pfeffer, C., Larsen, S., Song, J., Dong, M., Besenbacher, F., Meyer, R.L.,
Kjeldsen, K.U., Schreiber, L., Gorby, Y.A., El-Naggar, M.Y.,
Leung, K.M., Schramm, A., Risgaard-Petersen, N., Nielsen, L.P.,
2012. Filamentous bacteria transport electrons over centimetre
distances. Nature 491, 218e221.
Pham, C.A., Jung, S.J., Phung, N.T., Lee, J., Chang, I.S., Kim, B.H.,
Yi, H., Chun, J., 2003. A novel electrochemically active and Fe(III)reducing bacterium phylogenetically related to Aeromonas hydrophila, isolated from a microbial fuel cell. FEMS Microbiol. Lett. 223,
129e134.
Pham, T.H., Rabaey, K., Aelterman, P., Clauwaert, P., De
Schamphelaire, L., Boon, N., Verstraete, W., 2006. Microbial fuel
cells in relation to conventional anaerobic digestion technology.
Eng. Life Sci. 6, 285e292.
Prasad, D., Arun, S., Murugesan, M., Padmanaban, S.,
Satyanarayanan, R.S., Berchmans, S., Yegnaraman, V., 2007. Direct
electron transfer with yeast cells and construction of a mediatorless
microbial fuel cell. Biosens. Bioelectron. 22, 2604e2610.
Qian, F., Baum, M., Gu, Q., Morse, D.E., 2009. A 1.5 mL microbial fuel
cell for on-chip bioelectricity generation. Lab Chip 9, 3076e3081.
Rabaey, K., Lissens, G., Siciliano, S.D., Verstraete, W., 2003. A microbial
fuel cell capable of converting glucose to electricity at high rate and
efficiency. Biotechnol. Lett. 25, 1531e1535.
Rabaey, K., Boon, N., Hofte, M., Verstraete, W., 2005a. Microbial
phenazine production enhances electron transfer in biofuel cells.
Environ. Sci. Technol. 39, 3401e3408.
Rabaey, K., Lissens, G., Verstraete, W., 2005b. Microbial fuel cells
performances and perspectives. In: Biofuels for Fuel Cells:
Renewable Energy from Biomass Fermentation. IWA Publishing,
London.
Rabaey, K., Ossieur, W., Verhaege, M., Verstraete, W., 2005c. Continuous microbial fuel cells convert carbohydrates to electricity. Water
Sci. Technol. 52, 515e523.
Rabaey, K., Read, S.T., Clauwaert, P., Freguia, S., Bond, P.L.,
Blackall, L.L., Keller, J., 2008. Cathodic oxygen reduction catalyzed
by bacteria in microbial fuel cells. ISME J. 2, 519e527.
Rahimnejad, M., Ghoreyshi, A.A., Najafpour, G., Jafary, T., 2011.
Power generation from organic substrate in batch and continuous flow microbial fuel cell operations. Appl. Energy 88,
3999e4004.
Reguera, G., McCarthy, K.D., Mehta, T., Nicoll, J.S., Tuominen, M.T.,
Lovley, D.R., 2005. Extracellular electron transfer via microbial
nanowires. Nature 435, 1098e1101.
Reguera, G., Nevin, K.P., Nicoll, J.S., Covalla, S.F., Woodard, T.L.,
Lovley, D.R., 2006. Biofilm and nanowire production leads to
increased current in Geobacter sulfurreducens fuel cells. Appl.
Environ. Microbiol. 72, 7345e7348.
Reimers, C.E., Girguis, P., Stecher, H.A., Tender, L.M., Ryckekynck, N.,
Whaling, P., 2006. Microbial fuel cell energy from an ocean cold
seep. Geobiology 4, 123e136.
Rengasamy, K., Berchmans, S., 2012. Simultaneous degradation of bad
wine and electricity generation with the aid of the coexisting biocatalysts Acetobacter aceti and Gluconobacter roseus. Bioresour.
Technol. 104, 388e393.
Rezaei, F., Richard, T.L., Logan, B.E., 2009. Analysis of chitin particle
size on maximum power generation, power longevity, and
Coulombic efficiency in solidesubstrate microbial fuel cells.
J. Power Sources 192, 304e309.
Rhoads, A., Beyenal, H., Lewandowski, Z., 2005. Microbial fuel cell
using anaerobic respiration as an anodic reaction and biomineralized manganese as a cathodic reactant. Environ. Sci.
Technol. 39, 4666e4671.
Richter, H., McCarthy, K., Nevin, K.P., Johnson, J.P., Rotello, V.M.,
Lovley, D.R., 2008. Electricity generation by Geobacter sulfurreducens
attached to gold electrodes. Langmuir 24, 4376e4379.
Richter, L.V., Sandler, S.J., Weis, R.M., 2012. Two isoforms of Geobacter
sulfurreducens PilA have distinct roles in pilus biogenesis, cytochrome localization, extracellular electron transfer, and biofilm
formation. J. Bacteriol. 194, 2551e2563.
Rinaldi, A., Mecheri, B., Garavaglia, V., Licoccia, S., Nardo, P.D.,
Traversa, E., 2008. Engineering materials and biology to boost performance of microbial fuel cells: a critical review. Energy Environ.
Sci. 1, 417.
Ringeisen, B.R., Henderson, E., Wu, P.K., Pietron, J., Ray, R., Little, B.,
Biffinger, J.C., Jones-Meehan, J.M., 2006. High power density from
a miniature microbial fuel cell using Shewanella oneidensis DSP10.
Environ. Sci. Technol. 40, 2629e2634.
Roche, I., Scott, K., 2008. Carbon-supported manganese oxide nanoparticles as electrocatalysts for oxygen reduction reaction (orr) in
neutral solution. J. Appl. Electrochem. 39, 197e204.
Rosenbaum, M., Zhao, F., Schröder, U., Scholz, F., 2006. Interfacing
electrocatalysis and biocatalysis with tungsten carbide: a high-
REFERENCES
performance, noble-metal-free microbial fuel cell. Angew. Chem.
Int. Edit. 118, 6810e6813.
Rosenbaum, M., Aulenta, F., Villano, M., Angenent, L.T., 2011. Cathodes as electron donors for microbial metabolism: which extracellular electron transfer mechanisms are involved? Bioresour.
Technol. 102, 324e333.
Ross, D.E., Flynn, J.M., Baron, D.B., Gralnick, J.A., Bond, D.R., 2011.
Towards electrosynthesis in Shewanella: energetics of reversing the
mtr pathway for reductive metabolism. PLoS One 6, 16649.
Ryckelynck, N., Stecher, H.A., Reimers, C.E., 2005. Understanding
the anodic mechanism of a seafloor fuel cell: Interactions between geochemistry and microbial activity. Biogeochemistry 76,
113e139.
Schröder, U., 2007. Anodic electron transfer mechanisms in microbial
fuel cells and their energy efficiency. Phys. Chem. Chem. Phys. 9,
2619e2629.
Schröder, U., Niessen, J., Scholz, F., 2003. A generation of microbial
fuel cells with current outputs boosted by more than one order of
magnitude. Angew. Chem. Int. Edit. 42, 2880e2883.
Sharma, V., Kundu, P.P., 2010. Biocatalysts in microbial fuel cells.
Enzyme Microb. Tech. 47, 179e188.
Shea, C., Clauwaert, P., Verstraete, W., 2008. Adapting a denitrifying
biocathode for perchlorate. Water Sci. Technol. 58, 1941e1946.
Shi, L., Chen, B., Wang, Z., Elias, D.A., Mayer, M.U., Gorby, Y.A.,
Ni, S., Lower, B.H., Kennedy, D.W., Wunschel, D.S., Mottaz, H.M.,
Marshall, M.J., Hill, E.A., Beliaev, A.S., Zachara, J.M.,
Fredrickson, J.K., Squier, T.C., 2006. Isolation of a high-affinity
functional protein complex between OmcA and MtrC: two outer
membrane decaheme c-type cytochromes of Shewanella oneidensis
MR-1. J. Bacteriol. 188, 4705e4714.
Shi, L., Squier, T.C., Zachara, J.M., Fredrickson, J.K., 2007. Respiration
of metal (hydr)oxides by Shewanella and Geobacter: a key role for
multihaem c-type cytochromes. Mol. Microbiol. 65, 12e20.
Shi, L., Rosso, K.M., Zachara, J.M., Fredrickson, J.K., 2012a. Mtr
extracellular electron-transfer pathways in Fe (III)-reducing or Fe
(II)-oxidizing bacteria: a genomic perspective. Biochem. Soc. Trans.
40, 1261e1267.
Shi, L., Rosso, K.M., Clarke, T.A., Richardson, D.J., Zachara, J.M.,
Fredrickson, J.K., 2012b. Molecular underpinnings of Fe(III) oxide
reduction by Shewanella oneidensis MR-1. Front. Microbiol. 3, 50.
Srikanth, S., Reddy, M.V., Mohan, S.V., 2012. Microaerophilic
microenvironment at biocathode enhances electrogenesis with
simultaneous synthesis of polyhydroxyalkanoates (PHA) in bioelectrochemical system (BES). Bioresour. Technol. 125, 291e299.
Srikanth, S., Venkata, M.S., 2012. Change in electrogenic activity of the
microbial fuel cell (MFC) with the function of biocathode microenvironment as terminal electron accepting condition: influence on
overpotentials and bio-electro kinetics. Bioresour. Technol. 119C,
241e251.
Stamatelatou, K., Antonopoulou, G., Tremouli, A., Lyberatos, G., 2011.
Production of gaseous biofuels and electricity from cheese whey.
Ind. Eng. Chem. Res. 50, 639e644.
Steinbusch, K.J., Hamelers, H.V., Schaap, J.D., Kampman, C.,
Buisman, C.J., 2010. Bioelectrochemical ethanol production
through mediated acetate reduction by mixed cultures. Environ.
Sci. Technol. 44, 513e517.
Suh, S.J., Silo-Suh, L., Woods, D.E., Hassett, D.J., West, S.E.,
Ohman, D.E., 1999. Effect of rpoS mutation on the stress response
and expression of virulence factors in Pseudomonas aeruginosa.
J. Bacteriol. 181, 3890e3897.
Summers, Z.M., Fogarty, H.E., Leang, C., Franks, A.E.,
Malvankar, N.S., Lovley, D.R., 2010. Direct exchange of electrons
within aggregates of an evolved syntrophic coculture of anaerobic
bacteria. Science 330, 1413e1415.
151
Sun, J., Hu, Y., Hou, B., 2011. Electrochemical characterization of the
bioanode during simultaneous azo dye decolorization and
bioelectricity generation in an air-cathode single chambered microbial fuel cell. Electrochim. Acta 56, 6874e6879.
Tai, S.K., Wu, G., Yuan, S., Li, K.C., 2010. Genome-wide expression
links the electron transfer pathway of Shewanella oneidensis to
chemotaxis. BMC Genomics 11, 319.
Tandukar, M., Huber, S.J., Onodera, T., Pavlostathis, S.G., 2009. Biological chromium(VI) reduction in the cathode of a microbial fuel
cell. Environ. Sci. Technol. 43, 8159e8165.
Thrash, J.C., Trump, J.I., Weber, K.A., Miller, E., Achenbach, L.A.,
Coates, J.D., 2007. Electrochemical stimulation of microbial
perchlorate reduction. Environ. Sci. Technol. 41, 1740e1746.
Thurston, C.F., Bennetto, H.P., Delaney, G.M., 1985. Glucose metabolism in a microbial fuel cell stoichiometry of product formation
in a thionine-mediated Proteus vulgaris fuel cell and its relation to
coulombic yields. J. Gen. Appl. Microbiol. 131, 1393e1404.
Tong, M., Du, Z., Gu, T., 2012. Converting low-grade biomass to
produce energy using bio-fuel cells. In: Zhou, Y. (Ed.), Eco- and
Renewable Energy Materials. Springer, Berlin-New York, ISBN
978-3-642-33496-2, pp. 73e97.
Torres, C.I., Marcus, A.K., Lee, H.S., Parameswaran, P., Brown, K.R.,
Rittmann, B.E., 2010. A kinetic perspective on extracellular electron
transfer by anode-respiring bacteria. FEMS Microbiol. Rev. 34,
3e17.
Totten, P.A., Lara, J.C., Lory, S., 1990. The rpoN gene product of
Pseudomonas aeruginosa is required for expression of diverse genes,
including the flagellin gene. J. Bacteriol. 172, 389e396.
Tsujimura, S., Fujita, M., Tatsumi, H., Kano, K., Ikeda, T., 2001. Bioelectrocatalysis-based dihydrogen/dioxygen fuel cell operating at
physiological pH. Phys. Chem. Chem. Phys. 3, 1331e1335.
Va’zquez-Larios, A.L., Solorza-Feria, O., Va’zquez-Huerta, G., EsparzaGarcı’a, F., Rinderknecht-Seijas, N., Poggi-Varaldo, H.c.M., 2011.
Effects of architectural changes and inoculum type on internal
resistance of a microbial fuel cell designed for the treatment of
leachates from the dark hydrogenogenic fermentation of organic
solid wastes. Int. J. Hydrogen Energy 36, 6199e6209.
Vega, C.A., Fernandez, I., 1987. Mediating effect of ferric chelate
compounds in microbial fuel-cells with Lactobacillus plantarum,
Streptococcus lactis, and Erwinia dissolvens. Bioelectrochemistry 17,
217e222.
Velasquez, O.S.B., Head, I.M., Curtis, T.P., Scott, K., 2011. Factors
affecting current production in microbial fuel cells using different
industrial wastewaters. Bioresour. Technol. 102, 5105e5112.
Velasquez-Orta, S.B., Curtis, T.P., Logan, B.E., 2009. Energy from algae
using microbial fuel cells. Biotechnol. Bioeng. 103, 1068e1076.
Velvizhi, G., Venkata, M.S., 2011. Biocatalyst behavior under selfinduced electrogenic microenvironment in comparison with
anaerobic treatment: evaluation with pharmaceutical wastewater
for multi-pollutant removal. Bioresour. Technol. 102, 10784e10793.
Venkataraman, A., Rosenbaum, M., Arends, J.B.A., Halitschke, R.,
Angenent, L.T., 2010. Quorum sensing regulates electric current
generation of Pseudomonas aeruginosa PA14 in bioelectrochemical
systems. Electrochem. Commun. 12, 459e462.
Villano, M., Aulenta, F., Ciucci, C., Ferri, T., Giuliano, A., Majone, M.,
2010. Bioelectrochemical reduction of CO2 to CH4 via direct and
indirect extracellular electron transfer by a hydrogenophilic
methanogenic culture. Bioresour. Technol. 101, 3085e3090.
Wen, Q., Kong, F., Zheng, H., Cao, D., Ren, Y., Yin, J., 2011.
Electricity generation from synthetic penicillin wastewater in
an air-cathode single chamber microbial fuel cell. Chem. Eng. J.
168, 572e576.
Xing, D.F., Zuo, Y., Cheng, S., 2008. Electricity generation by Rhodopseudomonas palustris DX-1. Environ. Sci. Technol. 42, 4146e4151.
152
9. BIOELECTROCHEMISTRY OF MICROBIAL FUEL CELLS AND THEIR POTENTIAL APPLICATIONS IN BIOENERGY
Xu, J., Sheng, G.P., Luo, H.W., Li, W.W., Wang, L.F., Yu, H.Q.,
2012. Fouling of proton exchange membrane (PEM) deteriorates
the performance of microbial fuel cell. Water Res. 46,
1817e1824.
Yi, H., Nevin, K.P., Kim, B.C., Franks, A.E., Klimes, A., Tender, L.M.,
Lovley, D.R., 2009. Selection of a variant of Geobacter sulfurreducens
with enhanced capacity for current production in microbial fuel
cells. Biosens. Bioelectron. 24, 3498e3503.
You, S., Zhao, Q., Zhang, J., Jiang, J., Zhao, S., 2006. A microbial fuel
cell using permanganate as the cathodic electron acceptor. J. Power
Sources 162, 1409e1415.
Yu, Y.Y., Chen, H.L., Yong, Y.C., Kim, D.H., Song, H., 2011. Conductive
artificial biofilm dramatically enhances bioelectricity production in
Shewanella-inoculated microbial fuel cells. Chem. Commun. 47,
12825e12827.
Yuan, Y., Chen, Q., Zhou, S., Zhuang, L., Hu, P., 2012. Improved
electricity production from sewage sludge under alkaline conditions in an insert-type air-cathode microbial fuel cell. J. Chem.
Technol. Biotechnol. 87, 80e86.
Zechner, E.L., Lang, S., Schildbach, J.F., 2012. Assembly and mechanisms of bacterial type IV secretion machines. Philos. Trans. R. Soc.
Lond. B Biol. Sci. 367, 1073e1087.
Zhang, Y., Angelidaki, I., 2012. Bioelectrode-based approach for
enhancing nitrate and nitrite removal and electricity generation
from eutrophic lakes. Water Res. 46, 6445e6453.
Zhao, H., Li, X., Johnson, D.E., Mobley, H.L., 1999. Identification of
protease and rpoN-associated genes of uropathogenic Proteus mirabilis by negative selection in a mouse model of ascending urinary
tract infection. Microbiology 145 (Pt 1), 185e195.
Zhao, F., Slade, R.C., Varcoe, J.R., 2009. Techniques for the study and
development of microbial fuel cells: an electrochemical perspective. Chem. Soc. Rev. 38, 1926e1939.
Zhou, M., Chi, M., Luo, J., He, H., Jin, T., 2011. An overview of electrode
materials in microbial fuel cells. J. Power Sources 196, 4427e4435.
Zhou, M., Jin, T., Wu, Z., Chi, M., Gu, T., 2012. Microbial fuel cells for
bioenergy and bioproducts. In: Gopalakrishnan, K., Leeuwen, J.v.,
Brown, R. (Eds.), Sustainable Bioenergy and Bioproducts.
Springer-Verlag, Berlin-New York, pp. 131e172.
Zhou, M., Wang, H., Hassett, D.J., Gu, T., 2013. Recent advances in
microbial fuel cells (MFCs) and microbial electrolysis cells (MECs)
for wastewater treatment, bioenergy and bioproducts. J. Chem.
Technol. Biotechnol. 88, 508e518.
Zuo, Y., Maness, P.C., Logan, B.E., 2006. Electricity production from
steam-exploded corn stover biomass. Energy Fuel 20, 1716e1721.
C H A P T E R
10
Second-Generation Biofuel from
High-Efficiency Algal-Derived Biocrude
Rhykka Connelly
UT Algae Science and Technology Facility, University of Texas at Austin, Austin, TX, USA
email: r.connelly@cem.utexas.edu
O U T L I N E
Introduction
153
Biodiesel
158
Microalgal Biofuel History
154
Production of Biodiesel from Microalgae
159
Microalgae Biomass/Biofuel
ProductiondCultivation
Comparison of Biodiesel to Petrodiesel
160
155
Bioethanol
161
Phototrophic Microalgae
155
Bioethanol Production Process
161
Heterotrophic Microalgae
155
Biomethane
164
Nutrients
156
Biohydrogen
165
Contamination
156
Biocrude
166
Mixing
156
Properties of Subcritical Water
166
Culture Techniques
156
Hydrothermal Catalytic Liquefaction
167
Open-Pond Culture
157
HTL Summary and Outlook
167
Photobioreactors
157
Conclusions
167
Processing Microalgal Biomass for Biofuels
158
References
168
Microalgal Biomass to Biofuels
158
INTRODUCTION
First-generation, or conventional, biofuels are derived
from sugars, starches, or vegetable oils from traditional
agricultural crops and waste oils. Given firstgeneration biofuels’ impact on agricultural crop
demand and prices, alternative feedstocks have been
sought out. Microalgae have since been identified
as a viable second-generation biofuels feedstock
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00010-3
(Figure 10.1). The advantages of using microalgae for
biofuel production in comparison with other available
feedstocks have been extensively reported.
There are an estimated 100,000 microalgae species,
each with specific properties that allow them to exist
in nearly every environment on Earth, including arid climates that do not sustain most agricultural crops. Therefore, microalgal production systems need not displace
other traditional land-based crops intended for human
153
Copyright Ó 2014 Elsevier B.V. All rights reserved.
154
10. SECOND-GENERATION BIOFUEL FROM HIGH-EFFICIENCY ALGAL-DERIVED BIOCRUDE
FIGURE 10.1 The progression from first- to second-generation biofuels. (For color version of this figure, the reader is referred to the online
version of this book.)
or livestock consumption, which in turn greatly reduces
the impact to the food distribution chain. Further, microalgae may be harvested multiple times a year, which
greatly increases yearly production yields. The cultivation of microalgae for biofuels production can also be
coupled with other beneficial production schemes to
improve net income and positively address environmental concerns. Some possibilities currently being
investigated include the following:
strategies, microalgae intended for biofuel production
can potentially revolutionize a large number of biotechnology areas concurrently, including pharmaceuticals,
cosmetics, nutrition and food additives, aquaculture,
and pollution prevention.
3
• Reclamation of nutrients such as NHþ
4 , NO3 , PO4 ,
and others from wastewater, which reduces costs
associated with cultivating the algae and treating
wastewater (Zhu, 2013; Batten, 2013).
• Utilization of waste CO2 from industrial flue gases,
which reduces greenhouse gas emissions while
producing biofuel (González-López, 2012).
• Cultivation and extraction of value-added
metabolites within microalgae intended for biofuel
production. In this scenario, the value-added
metabolite is extracted prior to, or during, the biofuel
production stream. Commercially relevant products
include a large range of fine chemicals and bulk
products, such as polyunsaturated omega fatty acids,
antioxidants, high-value bioactive compounds,
natural dyes, sugars, and proteins (Mimouni, 2012;
Skjånes et al., 2013).
• After oil and target metabolite extraction, the
processed algal biomass can be used as a nutrient-rich
livestock feed, or used as sustainable organic fertilizer
due to its high N:P ratio (Mulbry, 2005; Stamey, 2012).
Beginning in the 1950s, Golueke et al. (1957) conducted early work on the anaerobic digestion of microalgal
biomass for the production of methane fuel. The energy
crisis in 1973 prompted the formation of The National
Renewable Energy Laboratory (NREL) under the Jimmy
Carter Administration. From 1978 to 1996, NREL conducted the most authoritative study to date on the development of biofuels from algae (Sheehan et al., 1998). The
study concluded that under controlled conditions, algae
are capable of producing 40 times the amount of oil for
biodiesel per unit area of land when compared to terrestrial oilseed crops such as soy and canola, and that the
use of wastewater as a nutrient source for algae propagation was the most practical approach for near-term
production of algal biodiesel (Sheehan et al., 1998;
Oswald, 2003). Despite the promise of cost-effective
fuel production from microalgae, interest in renewable
energy quickly waned as the energy crisis subsided
and fuel prices fell. The recent world-wide escalation
in oil prices has renewed interest in microalgae as a biofuels feedstock.
Since the original NREL study, other groups also have
conducted analyses of full-scale algae-to-biodiesel production (Benemann et al., 1982; Weissman and Goebel,
Because of this variety of value-added biological derivatives, coupled with environmental sustaining
MICROALGAL BIOFUEL HISTORY
HETEROTROPHIC MICROALGAE
1987; Beal, 2012a). Although these and other studies have
indicated a great potential for profitable biofuel from
microalgae, they also highlighted the need for system improvements, in both cultivation management and processing schemes to improve yields and reduce costs in
order to be competitive with fossil fuels. For example,
even when robust algae growth was achieved, inefficient
processing techniques such as biomass centrifugation
and drying followed by solvent extraction made recovery
of biofuels cost-prohibitive. To overcome this barrier,
changes to the system have been introduced, including
processing techniques that eliminate the need for expensive dewatering regimens such as centrifugation and drying of the harvested biomass prior to oil extraction with
solvents. One suggested path forward is a solventless
wet stream process whereby microalgae are concentrated
using pH-driven flocculation using inexpensive lime, followed by rupturing of the cells by pulsed electric field,
and ultimate recovery of released lipids by cross-flow
filtration. When coupled with waste streams for CO2
and nutrients, this process has a positive return on investment (Beal, 2012b). Another suggested path forward toward practical biofuel extraction from microalgae is the
use of hydrothermal liquefaction (HTL) processing.
This method eliminates the need for solvents to break
open algae cells, instead relying on heat and pressure to
remove the water from the biomass. An ancillary benefit
of the HTL method is that in addition to lipids, other
organic metabolites such as carbohydrates, proteins,
and nucleic acids can likewise be converted to biocrude
during the HTL process. Thus, a cultivation strategy
needs only to focus on the production of biomass rather
than inducing the accumulation of lipids at the expense
of cellular proliferation. Ultimately, cultivation and processing strategies should be firmly supported by realtime analysis of fuel precursors such as lipids that can
be converted to biodiesel, carbohydrates that can be converted to bioethanol, and the organic biomass that can be
converted to biocrude. Detailed analytical feedback is
necessary to optimize growth conditions to maximize
specific biofuel precursors.
MICROALGAE BIOMASS/BIOFUEL
PRODUCTIONdCULTIVATION
The intended final biofuel product defines successful
microalgae cultivation. If biodiesel is the final product,
algal strains should be selected and cultured to produce
maximal saturated fatty acids. If biocrude is the desired
product, high organic content, or a simple abundance of
biomass, is required. Whatever the target product, successful cultivation requires specific environmental conditions to drive the production of specific fuel precursors.
Major parameters that influence biomass production
155
include adequate light (wavelength and intensity), temperature, CO2 concentration, nutrient composition,
salinity, contaminants, and mixing conditions.
PHOTOTROPHIC MICROALGAE
Phototrophic microalgae use carbon dioxide (carbon
source), sunlight (energy source), and nutrients to proliferate. Two properties of light energy are important for
algal growth and metabolism: quality of the light spectrum and quantity of the light photons. As phototrophs,
light-harvesting pigments (chlorophyll and carotenoids)
absorb light at specific wavelengths to drive the photosynthetic process. Light absorption, however, is hindered both by light scattering through increasing
depths of the culture medium and by mutual shading
as the culture increases in density. Antenna structures
of microalgae are excessively efficient at harvesting light
energy, absorbing all the photons that hit them even
though only a fraction of those photons are used for
photosynthesis. This deprives nearby algae from
absorbing photons and consequently leads to low productivity. Aggressive mixing of the culture mitigates
some of these effects, but cannot completely overcome
the light penetration limitations inherent in a photosynthetic system.
HETEROTROPHIC MICROALGAE
Several wild-type and genetically modified species of
microalgae have been reported capable of growing phototrophically, heterotrophically or both (mixotrophically). Unlike phototrophic algae that require light
energy, heterotrophic algae have no such requirement.
Instead, these algae utilize organic carbons supplied in
the media to drive cellular proliferation and lipid accumulation. Without the limitations imposed by inefficient
light harvesting due to mutual shading and light scattering in the medium, the densities of heterotrophic cultures can far exceed the densities achieved in
phototrophic systems. Increased densities can translate
to higher biofuel precursor yields. For example, when
Chlorella protothecoides was grown heterotrophically using an organic carbon source, oil accumulation far
exceeded that seen in corresponding autotrophic cells
(Miao and Wu, 2004). Hence, heterotrophic production
has several advantages over phototrophic systems
including increased densities that eliminate the need
for dewatering, and increased process control that facilitates the maintenance and rapid growth of monocultures and the creation of a consistent product. The
primary limitation for commercial-scale heterotrophic
production of biofuel oils in microalgae is the cost of
156
10. SECOND-GENERATION BIOFUEL FROM HIGH-EFFICIENCY ALGAL-DERIVED BIOCRUDE
the organic carbon source. Sugars such as glucose and
acetate have been utilized as the primary carbon source
at the bench scale, but become cost-prohibitive at production scale. It is therefore unsurprising that increased
efforts to identify microalgal species that can thrive on
waste sugars, such as bagasse or cellulosic waste, are
underway.
NUTRIENTS
To maximize biomass production and the accumulation of fuel precursors, algal cultures must be supplied
with various concentrations of macronutrients, vitamins, and trace elements depending on species requirements. While there are limited reports on optimal levels
of nutrients required for mass algal cultures, it is generally accepted that required macronutrients are nitrogen
and phosphorus (Brzezinski, 1985; Harrison and Berges,
2005). Trace elements such as cobalt, copper, molybdenum, zinc, and nickel are likewise necessary, and in
some species are considered to be effective in hydrogen
production (Ramachandran and Mitsui, 1984). There appears to be no consensus on the optimal ratios for these
nutrients, even for specific species grown successively in
the same system. Therefore, nutrients are often added in
excess to avoid nutrient limitations (Richmond, 1999;
Sanchez et al., 1999; Acien Fernandez et al., 2001).
One strategy to reduce costs associated with adding
excess nutrients involves culturing microalgae in
reclaimed water or wastewater blends. The use of algae
to absorb nutrients in the wastewater processing stream
has been widely employed by water treatment facilities
(Megharaj et al., 1992; Tredici et al., 1992; Nurdogan and
Oswald, 1995; Kaya and Picard, 1995; Craggs et al.,
1995). The green microalga Scenedesmus obliquus has
demonstrated vitality in urban wastewaters, registering
growth rates similar to those reported for a complete synthetic medium. This freshwater alga tolerates a wide range
of temperature and pH, making it versatile for water purification (Kessler, 1991). Similar findings for other algal species continue to emerge, along with the energy return on
investment analyses that confirm the utility of coupling
scaled algal (EROI) production with nutrient reclamation
from waste streams, resulting in decreased costs for both
algal growth and water treatment (Beal, 2012b).
CONTAMINATION
Another barrier to the large-scale production of algae
biofuels is the maintenance of axenic or nearly axenic cultures. In particular, cultivation systems that are open to
the environment (e.g. open ponds) are easily susceptible
to contamination by unwanted species if extreme care is
not taken. A new open pond is typically inoculated
with the desired strain of microalgae with the hope that
the algae will aggressively proliferate and dominate the
pond flora. Over time, it is likely that undesired species
will be introduced, which may graze on the algae or
outcompete the inoculated species and lead to severely
reduced yields. Once a competitor has taken residence
in a pond, it is extremely difficult to eradicate (Schenk
et al., 2008). It is therefore crucial to aggressively monitor
cultures to identify and eradicate contaminates as soon as
possible. A number of strategies have been employed to
minimize culture contaminations. Cultivating algal
extremophiles that tolerate and outcompete invasive species in particular environments (e.g. pH and salinity) facilitates open-pond production. High bicarbonate
concentrations allow Spirulina to be grown in open ponds
with few invasive algae, and high-saline environments
allow marine algae like Dunaliella salina to be grown in
“relative pure cultures” (Anderson, 2005). Another popular strategy involves shortening the longevity of the culture; cultures are scaled and harvested before major
contamination can occur (Benemann, 2008). Cultivation
of microalgae in closed photobioreactors (PBRs) offers
another level of protection against predators. Occasionally, cultures can be treated with antibiotics and antifungals to eliminate bacteria and fungi, but this practice
can lead to microbial resistance and render the treatment
ineffective. Predator ciliates can be treated with dioctyl
sulfosuccinate, which is used to eliminate ciliates in the
udders of milking cows (Abou Akkada, 1968) with minimal harm done to the algae.
MIXING
At high algae concentrations, a thin top layer of cells
absorbs nearly all lightdthis phenomenon can be
avoided by proper mixing. Mixing must sufficiently
keep algae cells in suspension, aid distribution of CO2
and O2, and provide uniform exposure of light to all
cells. Mixing also decreases the boundary layer around
cells, which facilitates increased uptake of metabolic
products (Molina Grima et al., 1999).
CULTURE TECHNIQUES
The choice of cultivation systems is an important
aspect that significantly affects the efficiency and costeffectiveness of a microalgal biofuel production process
(Lee, 2001; Pulz, 2001; Carvalho et al., 2006). A wide variation exists among the microalgal cultivation systems
for the production of biomass. Raceways, PBRs, and fermenters, which are the three most widely used microalgae culture systems, will be discussed below.
157
PHOTOBIOREACTORS
OPEN-POND CULTURE
Large-scale cultivation of microalgae in outdoor
open-pond systems is well documented (Benemann
and Oswald, 1996; Borowitzka, 2005). Open ponds
most closely resemble the natural milieu of microalgae.
Indeed, ponds can be natural bodies of water, excavated
ditches that are unlined or lined with impermeable materials, or they can be constructed above ground with
walls (Figure 10.2). Despite a certain variability in shape,
the most common technical design for open-pond systems is raceway cultivators driven by paddle wheels
and usually operating at water depths of 15e20 cm
(Figure 10.1). At these water depths, biomass concentrations of up to 1000 mg/l and productivities of
60e100 mg/(l/day), i.e. 10e25 g/(m2/day) are possible.
Similar in design are the circular ponds commonly seen
in Asia and the Ukraine (Becker, 2007). Such circular
ponds usually have the provision of a centrally located
rotating arm (similar to those used in wastewater treatment) for mixing and may have productivities ranging
between 8.5 and 21 g/m2 day (Benemann and Oswald,
1996). On the other hand, thin-layer, inclined ponds
consist of slightly inclined shallow trays and may
achieve productivities up to 31 g/m2 day (Doucha and
Livansky, 2006). Because these ponds are open to the
environment, they are most suitable for algal species
that can tolerate extreme environmental conditions
(salinity, pH, nutrient loads, etc.) to the exclusion of
invasive species. Such algal species include fast growers
such as Chlorella, Spirulina, and Dunaliella, which thrive
in highly alkaline or saline environments (Chisti, 2007).
Limitations to successful scale-up of microalgae in
open-pond systems include contamination, evaporation,
limited species suitability, low-volumetric productivities, and the need for large land area.
PHOTOBIOREACTORS
The problems associated with open systems have
encouraged the development of closed system PBRs.
PBRs can be located indoors under supplemental illumination or outdoors utilizing natural sunlight. Various
types of PBRs have been designed depending on
growers’ needs; these include tubular PBRs, vertical
bubble columns and airlift reactors, combined bubble
column and inclined tubular reactors, helical PBRs,
and flat-plate PBRs (Tredici and Zittelli, 1998; Sanchez
et al., 1999; Berzin, 2005; Ugwu et al., 2005) (Figure 10.3).
Closed PBRs allow for tighter regulation and control of
nearly all the biotechnologically important parameters
FIGURE 10.2 (a) Open-pond production systems at Seambiotic in TelAviv, Israel and (b) Cyantotech in Kona, Hawaii. (For color version of
this figure, the reader is referred to the online version of this book.)
FIGURE 10.3 (a) Horizontal photobioreactors used in the biomass production
plant in Klötze, Saxony-Anhalt (ÓBioprodukte
Prof. Steinberg GmbH) and (b) vertical photobioreactors used at the University of Texas.
(For color version of this figure, the reader is
referred to the online version of this book.)
158
10. SECOND-GENERATION BIOFUEL FROM HIGH-EFFICIENCY ALGAL-DERIVED BIOCRUDE
and confer the following fundamental benefits: a
reduced contamination risk, reduced CO2 losses, reproducible cultivation conditions, controllable hydrodynamics, and temperature (Pulz, 1992). However,
widespread implementation has been hampered by the
high capital costs associated with PBRs.
PROCESSING MICROALGAL
BIOMASS FOR BIOFUELS
There are several methods to process microalgae into
biofuel products. Figure 10.4 shows some of the more
common approaches to (1) harvest/dewater microalgae,
(2) release fuel precursors by compromising the integrity
of the algae, followed by (3) conversion of fuel precursors to biofuel products.
Many algal species can be preconcentrated by simply
allowing unmixed cells to settle by gravity. Additional
concentration can be achieved by flocculation, centrifugation, microfiltration, and drying. Freshly clarified media can be recycled back to the growth environment,
although there are limited data regarding the number
of times growth media can be recycled. Concentrated
wet algal cells may be subsequently compromised by
passage through a pulsed electric field, mechanical
bead-milling, sonication, or enzymatic degradation
(Beal, 2012a). Solvent extraction of the biomass generally requires that the biomass is dried as an initial
step. Once fuel precursors are exposed, they may be
converted as fuel products by specific fuel conversion
approaches. These methods are discussed in detail
below.
FIGURE 10.4
MICROALGAL BIOMASS TO BIOFUELS
Microalgae can provide several different types of
renewable biofuels, and numerous options exist for the
conversion of components of microalgal biomass to biofuel. These include methane produced by anaerobic
digestion of the algal biomass (Spolaore et al., 2006); biodiesel derived from microalgal oil (Roessler et al., 1990;
Sawayama et al., 1995; Dunahay et al., 1996; Sheehan
et al., 1998; Banerjee et al., 2002; Gavrilescu and Chisti,
2005); biohydrogen (Ghirardi et al., 2000; Akkerman
et al., 2002; Melis, 2002; Fedorov et al., 2005; Kapdan
and Kargi, 2006); and biocrude derived from organics
comprising microalgae. An important distinction to
note is whether extracted compounds, whole biomass,
or both will be converted to biofuel. Microalgae that
contain high-value bioproducts (e.g. carotenoids,
sulfated polysaccharides, and phycobilliproteins) may
undergo a two-phase extraction scheme where the
value-added product is fractionated from the biofuel
production stream prior to conversion of lipids to biodiesel and carbohydrates to bioethanol. Alternatively,
the remaining organic fraction of the biomass can be
converted to biocrude by HTL.
BIODIESEL
Biodiesel is derived from fatty acyl lipids from plant
and animal sources. Table 10.1 shows the average oil
yield per hectare from various crops. Using the average
oil yield per acre, the footprint needed to meet 50% of
the U.S. transport fuel needs is calculated. For example,
Microalgal biomass-to-biofuel processing pathway choices. (For color version of this figure, the reader is referred to the online
version of this book.)
159
PRODUCTION OF BIODIESEL FROM MICROALGAE
TABLE 10.1
Comparison of Biodiesel Feedstocks: Oil Yields vs Land Area Necessary to Meet 50% of Current
Transportation Fuel Demand
Crop
Oil yield
(l/ha)
Land Area
Needed (M ha)
Percentage of Existing
US Agricultural Area
Corn
172
1540
846
Soybean
446
594
326
Canola
1190
223
122
Jatropha
1892
140
77
Coconut
2689
99
54
Oil palm
5950
45
24
Microalgae (30% oil by wt)
58,700
4.5
2.5
Microalgae (70% oil by wt)
136,900
2
1.1
Source: Christi, 2007.
the high-yielding crop oil palm requires a 45 Mha cropping area, or 24% of the existing agricultural footprint in
the US to meet only 50% of the current transport fuel
needs.
Given the large agricultural footprint required, it is
clear that land-based oilseed crops cannot realistically
satisfy current demand. Lipid-rich microalgae, however,
hold more promise as a sustainable feedstock that
can significantly contribute toward demand. Under
controlled conditions, the footprint required to produce
an order of magnitude higher oil yields requires an order of magnitude smaller cropping area compared to
oil palm, assuming an oil content of 30% in the microalgae. A caveat to these numbers is that the microalgal oil
yields given in Table 10.1 are based on experimentally
demonstrated biomass productivity in PBRs. Demonstrated biodiesel yields on a larger scale have been
much smaller. The large-scale cultivation of lipid-rich
microalgae remains a significant challenge in the algae
biofuel industry and thus still under intense
investigation.
PRODUCTION OF BIODIESEL FROM
MICROALGAE
Biodiesel is derived from plant and animal lipids.
Lipids are subdivided in two main classes based on their
chemical characteristics: polar and nonpolar (neutral)
lipids. Neutral lipids include the tri- and diglycerides,
waxes, and isoprenoid-type lipids. Monoglycerides
divide neutral lipids from polar lipids. Polar lipids
include phospholipids (e.g. phosphatidylinositol and
phosphatidylethanolamine), free fatty acids, and glycerol. Desirable feedstocks for biodiesel production are
composed of a higher proportion of saturated fatty
acyl neutral, rather than polar lipids. Compared to animal fats and other seed-based oils, many microalgal species have been reported to contain a relatively greater
proportion of polar lipids to neutral lipids (triglycerides)
and the predominance of long-chain polyunsaturated
fatty acids (greater than C18). However, several species
of microalgae have been shown to produce various
lipids, hydrocarbons, and other complex oils suitable
for biodiesel production (Banerjee et al., 2002; Guschina
and Harwood, 2006). To accurately predict yields from
microalgae, it is critical to understand the lipid composition of the feedstock. The fluorescence probe Nile Red is
often used to monitor neutral lipid composition within
microalgae. However, Nile Red cannot provide information regarding carbon chain length or saturation of fatty
acids. Gas chromatography is often utilized for the identification of specific fatty acids and the separation, identification and quantification of specific lipid classes by
High-performance liquid chromatographyeevaporative
light scattering detection (HPLC-ELSD) has recently
been described (Jones et al., 2012). An informed realtime understanding of the lipid composition of the culture may lead to better cultivation practices, which can
drive the accumulation of desirable lipids and ultimately
higher biodiesel yields.
The oil to biodiesel conversion process is termed
transesterification (Figure 10.5). During transesterification, an alcohol (e.g. methanol and ethanol) is reacted
with vegetable oil (fatty acid) in the presence of catalyst.
Catalysts include alkalis (e.g. KOH and NaOH) or acids
(e.g. H2SO4) to produce fatty acid methyl esters (FAME)
or fatty acid ethyl esters and glycerol. Generally, methanol is preferred for transesterification because it is
less expensive than ethanol. Transesterification requires
3 mol of alcohol for every 1 mol of triglyceride to produce 1 mol of glycerol and 3 mol of methyl esters. This
160
10. SECOND-GENERATION BIOFUEL FROM HIGH-EFFICIENCY ALGAL-DERIVED BIOCRUDE
FIGURE 10.5 The transesterification of
triglyceride to 3 mol each of fatty acid
methyl esters and glycerol.
reaction is reversible in nature and eventually arrives
at equilibrium (Fukuda et al., 2001). The produced biodiesel is immiscible and thus easily separated from glycerol by phase partitioning the biodiesel in a nonpolar
solvent such as hexane or heptane. The solvent is
later recovered by distillation. Transesterification is an
inexpensive way of transforming the large, branched
molecular structure of the vegetable oils into smaller,
straight-chain molecules of the type required in regular
diesel combustion engines.
Using microalgae as a feedstock, biodiesel can be produced from extracted algal oils or by direct conversion
of the biomass. The production of biodiesel from
extracted microalgal oil proceeds as described above.
For direct conversion of the biomass to biodiesel, the
microalgae are first concentrated to a paste-like consistency. The cells are then incubated in methanol or
ethanol in the presence of a strong acid or base at an
elevated temperature. In this process, fatty acids derived
from not only triglycerides but also diglycerides and
free fatty acids are transesterified to biodiesel. The
remaining residue contains starch and proteins, which
can further be processed into ethanol, animal feed, or
used as a feedstock in an anaerobic fermenter.
TABLE 10.2
COMPARISON OF BIODIESEL
TO PETRODIESEL
Biodiesel is a proven fuel. The conversion of vegetable oil to biodiesel was first described as early as
1853 by Patrick Duffy, many years before the first diesel
engine became functional (Duffy, 1853). Rudolf Diesel’s
engine was built several years later, running for the first
time on August 10, 1893 using nothing but peanut oil
feedstock. In a 1912 speech, Diesel said, “the use of vegetable oils for engine fuels may seem insignificant today
but such oils may become, in the course of time, as
important as petroleum and the coal-tar products of
the present time.”
Fossil fuel-derived petrodiesel is produced from the
fractional distillation of fossil fuel crude oil. It contains
w75% saturated hydrocarbons and 25% aromatic hydrocarbons (including naphthalenes and alkylbenzenes). Compared to petrodiesel, biodiesel molecules
are comprised almost entirely FAME saturated, or
monosaturated, hydrocarbons and w5% aromatic compounds. Table 10.2 shows a comparison between the
properties of biodiesel to petrodiesel. Biodiesel has a
higher lubricity and thus better lubricating properties
Fuel Properties of Biodiesel and Petrodiesel
Property
Biodiesel
Petrodiesel
Production process
Chemical reaction
Reaction þ fractionation
Cetane number
51e62
44e49
Oxygen
10e12% free oxygen
Very low
Aromatics
5%
18e22%
Sulfur
None
0.05%
Flash point
300e400 F
125 F
Lubricity
Much greater than diesel. Comparable to oil
lubricants
Low-sulphur fuel has low lubricity factor
Biodegradability
Biodegrades readily
Poor biodegradability
Toxicity
Essentially nontoxic
Highly toxic
BIOETHANOL PRODUCTION PROCESS
than fossil diesel, which reduces wear on fuel systems
and engine components. Biodiesel likewise has higher
cetane ratings than today’s lower sulfur diesel fuels.
The cetane number is a measure of a fuel’s ignition
delay, or the time period between the start of injection
and the first identifiable pressure increase during combustion of the fuel; the higher the cetane number the
more easily the fuel will combust. Therefore higher cetane biodiesel should cause an engine to run more
smoothly and quietly. Biodiesel’s higher flash point
makes biofuel vehicles much safer in accidents than
those powered by petrodiesel or gasoline. Biodiesel is
biodegradable and nontoxic and also contains little to
no sulfur, which makes it a much cleaner burning fuel
compared to petrodiesel (Hai et al., 2000; Anderson
et al., 2002; Hoekema et al., 2002; Choi et al., 2003; Grima
et al., 2003; Zijffers et al., 2008; Brindley et al., 2011).
Biodiesel has higher oxygen content than petrodiesel, which can also reduce pollution emissions. However, this benefit is offset by the fact that biodiesel is
more likely to oxidize (react with oxygen), producing
contaminants (gumming/sludge) that will plug fuel
filters, leave deposits on injectors and cause injector
pump problems. Further, continuous oxidization leads
to the fuel becoming more acidic, which in turn causes
corrosion on the components in the injection system. It
will also dissolve fossil-diesel sludge built up over
time and send it through fuel lines, plugging fuel filters. Biodiesel cloud or gel point is higher than
pump diesel, meaning that it tends to gel at low temperatures more readily which can lead to poor cold
starting. Clearly, there are both benefits and drawbacks for using biodiesel in today’s automobile
engines.
BIOETHANOL
First-generation bioethanol is usually produced by
alcoholic fermentation of starch (e.g. corn and wheat)
or sugar (e.g. sugarcane, sugar beet and sweet sorghum).
Second-generation bioethanol feedstocks include lignocellulosic grasses, woody biomass, and algae. Bioethanol
is an already well-established fuel in Brazil and the USA
(Goldemberg, 2007). Owing to mandates enacted by the
Brazilian government in 1976, all light-duty fleet vehicles are required to operate using a blend of gasoline
and bioethanol fluctuating between 10% and 25%, or
E10eE25. In 2003, the Brazilian car manufacturing industry introduced flexible-fuel vehicles that can run on
any proportion of gasoline (E20eE25 blend) and hydrous ethanol (E100) (Horta Nogueira, 2004). Sales
reached an impressive 92.3% share of all new cars and
light-vehicle sales for 2009, and overall bioethanol production reached 5.5 billion U.S. liquid gallons.
161
Although the vast majority of bioethanol is produced
by fermentation of corn glucose in the United States or
sugarcane sucrose in Brazil (Rosillo-Calle and Cortez,
1998), bioethanol can be derived from any material
that contains sugars, including microalgae. Unlike
land-based food crops, the production of bioethanol
from microalgae does not divert agricultural foods
away from grocer’s shelves. This is especially true for
corn and corn products, which serve as base ingredients
of many processed foods. Further, microalgae can be
cultivated in areas nonsuitable for traditional agricultural crops and can be harvested many times a year.
Therefore, in the U.S., microalgae are generally thought
to be the only practical alternative to current bioethanol
crops such as corn and soybean (Chisti, 2007; Hu et al.,
2008; Singh and Gu, 2010).
Matsumoto et al. (2003) screened several strains of
marine microalgae with high-carbohydrate content and
identified a total of 76 strains with a carbohydrate content ranging from 33% to 53% . It has been estimated
that approximately 46e140 kl of ethanol/ha year can
be produced from microalgae (Mussatto, 2010). This
yield is several orders of magnitude higher than yields
obtained from other bioethanol feedstocks (Table 10.3).
BIOETHANOL PRODUCTION PROCESS
Monomeric sugars can be converted to ethanol
directly, while starches and cellulose first must be hydrolyzed to fermentable sugars either enzymatically or
chemically (Bashir and Lee, 1994). Like most biofuels
processes, bioethanol production from microalgae begins with the concentration of algae. The algae are
then further dried and ground to a powder. In the next
step of the process, the algae mass is hydrolyzed and
Saccharomyces cerevisiae yeast is added to the biomass
to begin the fermentation process. The resulting fermented mash contains about 11e15% ethanol by volume
as well as the nonfermentable solids from algae and
yeast cells. Ethanol is then distilled off the mash at
w96% strength. Despite widespread knowledge of this
fermentation process, the details of the conversion process of algal celluloses-to-bioethanol are only partially
understood. Celluloses comprise a large fraction of algal
cells walls. These molecules are tightly packed and
enzymatic access is often limited without a pretreatment
step (Figure 10.6).
Many authors have reported that it is essential to
introduce a pretreatment stage to release and convert
the complex carbohydrates entrapped in the cell wall
into simple sugars necessary for yeast fermentation. Cellulose can be made more accessible by the addition of an
acid (Figure 10.7). Arantes and Saddler (2010) have suggested a model where prior to hydrolysis of cellulose to
162
TABLE 10.3
10. SECOND-GENERATION BIOFUEL FROM HIGH-EFFICIENCY ALGAL-DERIVED BIOCRUDE
Comparison of Bioethanol Feedstocks
Feedstock
Productivity
(dry mg/ha year)
%Fermentable
Carbohydrate
Corn
7*
80{{
Switchgrass
{{
3.6e15*
Woody biomass
70e85
xx
15{{
5.6
1.05
2.8e11.5
0.4e1.8
7e18.7
4e7.7
12
{{
10e22
Lignin Productivity
(dry mg/ha year)
{{
76.4
x
%Lignin
Fermentable
Carbohydrate Productivity
(dry mg/ha year)
{
{{
25e35
{
Chlorella sp.
127.8e262.8
33.4
0
42.7e87.8
0
Tetraselmis suecia
38*e139.4**
11e47*
0*
4.2e65.5
0
Arthrospira sp.
27e70*
15e50*
0*
4.1e35
0
* Dismukes et al., 2008.
x
Ragauskas et al., 2006.
{
Kristensen, 1990.
** Zittelli et al., 1999.
xx
Chisti, 2007.
{{
Sanchez et al., 1999.
FIGURE 10.6
The microalgal bioethanol production process. (For color version of this figure, the reader is referred to the online version of
this book.)
FIGURE 10.7
Acid-driven hydrolysis of cellulose.
BIOETHANOL PRODUCTION PROCESS
163
FIGURE 10.8 Theoretical breakdown of cellulose into monomeric units of glucose. Source: Arantes and Saddler (2010). (For color version of
this figure, the reader is referred to the online version of this book.)
monomeric units, cellulases must adsorb onto the surface
of the insoluble cellulose (Figure 10.8). The action of the
cellulases serves to loosen tightly packed fibrous cellulosic
networks and create additional access to cellulose chains
buried within the fibrils. Then the synergistic action of
exo- and endoglucanases cleave accessible molecules to
form soluble cello-oligosaccharides, or oligomers of 6
sugar units. These oligosaccharides are quickly hydrolyzed to primarily cellobiose, or two glucose molecules
linked by a b (1/4) bond. Cellobiose hydrolyzation to
glucose monomers is usually completed by the extraneous
addition of b-glucosidase.
Once glucose monomers have been rendered, bioethanol from microalgal biomass can be produced
through two distinct pathways: direct dark fermentation
or yeast fermentation of saccharified biomass. Whereas
direct dark fermentation yields are typically much
lower, the yeast fermentation process is a very wellestablished, relatively high-yield, low-energy-intensive
process. Because microalgae can be harvested multiple
times a year, some species have been shown to
theoretically yield an order of magnitude more bioethanol compared to a land-based crop such as corn
(Table 10.3). Further, using microalgae as a raw material
is strongly advantageous as algae sugars may be derived
from multiple sourcesdfrom intracellular starches and
from the cellulosic cell wall. Nevertheless, to achieve
higher yields, it is still necessary to screen for high
starch-producing algal strains coupled with identifying
mechanisms and culture conditions for inducing
maximal accumulation of intracellular starches.
In comparison to terrestrial feedstocks that contain
lignin, certain species of microalgae and cyanobacteria
have high potentiality for bioethanol production due
to their high productivity rates, high biomass fermentable carbohydrate content, and lack of lignin. Lignin is
a recalcitrant substance (i.e. not easily degraded) present
in the cell walls of terrestrial biomass that cannot be converted to bioethanoldits processing is a major impediment for bioethanol production (Ragauskas et al.,
2006). Microalgae’s potential can be highlighted by the
fact that 75% of algal complex carbohydrates can be
164
10. SECOND-GENERATION BIOFUEL FROM HIGH-EFFICIENCY ALGAL-DERIVED BIOCRUDE
hydrolyzed into a fermentable hexose monomer, and the
fermentation yield of bioethanol is w80% of the theoretical optimal value (Huntley and Redalje, 2007). Harun
et al. (2009) have shown that the blue-green Chlorococum
sp. produces a maximum bioethanol concentration of
3.83 g/l obtained from 10 g/l samples that are preextracted for lipids versus those that remain as dried intact
cells. This indicates that microalgae can be used for the
production of both lipid-based biofuels and ethanol biofuels from the same biomass as a means to increase their
overall economic value (Jones and Mayfield, 2012). The
microalgae Chlorella vulgaris and Porphyridium sp.,
particularly, have been considered as promising feedstocks for bioethanol production because they can accumulate up to 37% and 54% (dry weight) of starch,
respectively. The potential for simple, low-cost methods
of bioethanol production from microalgae and cyanobacteria are real. The next phase of biofuel research
should develop improved methodologies to increase
intracellular ethanol production efficiencies.
BIOMETHANE
Biomethane (CH4) production from microalgal
biomass is of interest because the efficiency of algal
biomass production per hectare is estimated to be
5e30 times greater than that of the terrestrial crop plants
(Sheehan et al., 1998). Golueke and Oswald (1959) published one of the first feasibility studies using microalgae
for CH4 production and concluded that the process was
feasible (Golueke and Oswald, 1959). There are two
well-established methods of CH4 production: (1) harvest
of an algal polyculture from a wastewater treatment
pond, or (2) axenic growth of specific algae at a bench
scale (Asinari Di San Marzano et al., 1982; Yen and
Brune, 2007). The digestion process is described in
Figure 10.9. It begins with bacterial hydrolysis of the
algal biomass. Organic polymers, such as lipids, carbohydrates, and proteins, are first broken down to soluble
derivatives, which are further fractionated into carbon
dioxide, hydrogen, ammonia, and organic acids by
acidogenic bacteria. Acetogenic bacteria then convert
these resulting organic acids into acetic acid, along
with additional ammonia, hydrogen, and carbon dioxide. Finally, methanogens convert these products to
methane and carbon dioxide. Regardless of operating
conditions and species, the proportion of methane in
the biogas produced for the majority of studies falls in
the range 69e75%. Anaerobic digestion is an effective
process for biological oxygen demand removal, but it
is not effective for nutrient removal. Thus, there is a
need for further treatment of effluent from anaerobic digesters before it can be discharged into the environment.
The nutrient-rich digestate also produced can be used as
fertilizer. This process of converting microalgae to CH4
is dependent on several key metrics, namely (1) pH,
(2) retention time, (3) mixing, (4) composition of the
biomass and (5) composition of the surrounding milieu.
One of the most important factors influencing CH4
biogas production from algal biomass has been reported
to be pH. At high pH, due to high alkalinity from NH3
release, the gas production will shift toward CH4. The
oxidation state of the biomass also affects biogas quality,
which in turn drives the proportion of methane released
(Sialve et al., 2009). Due to lowered content of sulfated
amino acids, the microalgal biomass digestion releases
a lower amount of hydrogen sulfide than do other types
of organic substrates (Becker, 1988). The composition of
the microalgal feedstock also affects biomethane yields.
The relatively high lipid, starch, and protein contents
and the absence of lignin make microalgae an ideal
candidate for efficient biomethane production via
fermentation in biogas plants. Theoretically, higher
FIGURE 10.9 Anaerobic digestion process of microalgae. (For color version of this figure, the reader is referred to the online version of this
book.)
165
BIOHYDROGEN
Hydrogen is seen as one of the most promising fuels
for the future owing to the fact that it is renewable and
liberates large amounts of energy per unit weight
without evolving CO2 when combusted. Biohydrogen
production has several advantages over hydrogen production by photoelectrochemical or thermochemical
processes. For example, whereas electrochemical
hydrogen production requires the use of solar batteries
with high energy requirements to split water and form
the hydrogen product, biohydrogen production by
photosynthetic microorganisms only requires simple
PBRs with low energy requirements. A select group of
green algae (including Chlamydomonas reinhardtii) and
cyanobacteria offer an alternative route to renewable
H2 production (Levin et al., 2004; Sakurai and Masukawa,
2007). Cyanobacteria are able to diverge the electrons
emerging from the two primary reactions of oxygenic
photosynthesis directly into the production of H2,
making them attractive for the production of renewable
H2 from solar energy and water.
Cyanobacteria utilize two enzymatic pathways for H2
production, either nitrogenases or bidirectional hydrogenases (Angermayr et al., 2009). Nitrogenases require ATP,
whereas bidirectional hydrogenases do not require ATP
for H2 production, hence making them more efficient
and favorable for H2 production with a much higher
turnover. The fundamental aspects of cyanobacterial hydrogenases, and their more applied potential use as
future producers of renewable H2 from sun and water,
are receiving increased international attention. At the
same time, significant progress is being made in the understanding of the molecular regulation of the genes
encoding both the enzymes and the accessory proteins
H2 O/2Hþ þ 2e þ 1 2 O2
2Hþ þ 2e /H2
H2 Combustion
H2 þ 1 2 O2 /H2 O þ 285:8 kJ=mol
=
BIOHYDROGEN
needed for the correct assembly of an active hydrogenase.
With the increasing interest of both scientific and public
communities in clean and renewable energy sources,
and consequent funding opportunities, rapid progress
will likely be made in the fundamental understanding
of the regulation of cyanobacterial hydrogenases at both
genetic and proteomic levels. Bandyopadhyay et al.
(2010) have described Cyanothece sp. ATCC 51142, a unicellular, diazotrophic cyanobacterium with capacity to
generate high levels of hydrogen under aerobic conditions. Wild-type Cyanothece sp. 51142 can produce
hydrogen at rates as high as 465 mmol/mg of chlorophyll/h in the presence of glycerol. Authors also report
that hydrogen production in this strain is mediated by
an efficient nitrogenase system, which can be manipulated to convert solar energy into hydrogen at rates that
are several fold higher, compared to other previously
described wild-type hydrogen-producing photosynthetic
microbes. These strains have evolved the ability to use solar energy to produce H2 from water (Esquı́vel, 2011;
Levin et al., 1961). The theoretical conversion efficiency
from light to H2 is calculated to be as high as w10%
(Levin et al., 1961).
Photosystem II (PSII) drives the first stage of the process (Figure 10.10), by splitting H2O into protons (H2),
electrons (e), and O2.
H2 Production
=
cellular lipid contents will result in higher methane
yields. Thus lipid-rich microalgae make attractive substrates for anaerobic digestion, as they have a higher
gas production potential when compared to carbohydrates and proteins (Li et al., 2002; Cirne et al., 2007).
The hydraulic and solid retention time is another key
metric in the anaerobic process. The hydraulic and solid
retention time is a measure of the average length of the
time that a soluble compound remains in a constructed
bioreactor. Retention times should be sufficiently high
to allow active bacterial populations (e.g. methanogens)
to remain in the reactor yet not limit hydrolysis, which is
considered to be the rate-limiting step in the overall conversion of complex substrates to methane. Moreover,
optimal loading rates and hydraulic retention times
must be enhanced to ensure efficient conversion of
organic matter, and will depend on algal substrate
composition and accessibility.
Normally, the photosynthetic light reactions and the Calvin cycle produce carbohydrates that fuel mitochondrial
respiration and cell growth. Under anaerobic conditions,
however, mitochondrial oxidative phosphorylation is
largely inhibited, which leads some organisms (e.g. Chlamydomonas reinhardtii) to reroute the energy stored in
FIGURE 10.10
Biohydrogen production by microalgal respiration.
(For color version of this figure, the reader is referred to the online
version of this book.)
166
10. SECOND-GENERATION BIOFUEL FROM HIGH-EFFICIENCY ALGAL-DERIVED BIOCRUDE
carbohydrates to a chloroplast hydrogenase (HydA),
likely using an NAD(P)HPQ e transfer mechanism,
to facilitate ATP production via photophosphorylation.
Thus, hydrogenase reacts with Hþ (from the medium)
and e (from reduced ferredoxin) to produce H2 gas
that is subsequently excreted from the cell. The combustion of the recovered H2 yields only heat and H2O and
thus is a model green technology.
Several renewable energy laboratories have
concluded that production efficiencies must be
improved from 0.2% photon to H2 conversion
efficiency at 20 W/m2 illumination to w7e10% at
230 W/m2 illumination (day light) to make the process
economically viable. Through extensive preliminary
work, the efficiency of this process has been enhanced
to w1.0% from light to H2 and 2% to biomass. The H2
gas produced in such mutants has a purity of
w90e95% and typical yields are 500 ml H2 for a 1 l
culture (10 days; 110 W illumination). Without further
purification, the H2 gas can used to power a
small-scale fuel cell car.
In addition to work with Chlamydomonas, a large
number of unicellular, filamentous, freshwater, and marine cyanobacterial species have been reported to produce large quantities of biohydrogen. Among other
species, Anabaena azollae, Anabaena cylindrica, Anabaena
variabilis, Arthrospira (Spirulina) platensis, Cyanothece,
Gloeocapsa alpicola, and Nostoc muscorum have been reported to produce high levels of hydrogen gas (Jeffries
et al., 1978; Aoyama et al., 1997; Antal and Lindblad,
2005). In particular, Anabaena sp. is reported to produce
relatively large quantities of biohydrogen. Among these
species, nitrogen-starved A. cylindrica cells produce the
highest concentration of biohydrogen (30 ml H2/l/h)
(Margheri et al., 1990).
These cyanobacterial strains use two sets of enzymes
to generate hydrogen gas. The first enzyme is nitrogenase, and it is found in the heterocysts of filamentous
cyanobacteria when grown under nitrogen-limiting conditions. Hydrogen is produced as a by-product of fixation of nitrogen into ammonia. The reaction consumes
16 ATP for fixation of 1 mol of N2, and results in formation of 1 mol of H2. The other hydrogen-metabolizing or
hydrogen-producing enzymes in cyanobacteria are hydrogenases, which occur as two distinct types in
different cyanobacterial species. The first type is uptake
hydrogenase (encoded by hupSL), which has the ability
to oxidize hydrogen via oxyhydrogenation or the Knallgas reaction. The other type of hydrogenase is reversible
or bidirectional hydrogenase (encoded by hoxFUYH),
and it is capable of uptake and production of hydrogen
(Schmitz et al., 1995; Tamagnini et al., 2002). Hydrogen is
an important fuel source and is widely applied in fuel
cells, coal liquefaction, upgrading of heavy oils, and
several other operations. Hydrogen can be produced
biologically by various means, including the steam
reformation of bio-oils, dark- and photofermentation of
organic materials, and photolysis of water catalyzed by
special microalgal species (Kapdan and Kargi, 2006;
Ran et al., 2006; Wang et al., 2008).
BIOCRUDE
In addition to direct combustion, there is growing
attention to conversion of biomass into liquid energy
carriers. Applying more traditional biofuel production
processes (e.g. lipid extraction followed by transesterification, fast pyrolysis and gasification) to algal biomass
requires that the algae be dried prior to use. Unless access to waste heat is available, the energy required to
first concentrate the biomass to a paste followed by complete drying far exceeds to energy value of the produced
biocrude. An alternative production pathway called hydrothermal liquefaction (HTL) bypasses the drying step
and converts the algal biomass into a hydrocarbonbased biocrude fuel in the aqueous phase. A simple
comparison of the enthalpies of liquid water at 350 C
and water vapor at 50 C (i.e. drying the biomass) indicates that processing in liquid water saves 921 kJ/kg.
PROPERTIES OF SUBCRITICAL WATER
In HTL, water is an important reactant and catalyst,
and thus the biomass can be directly converted without
an energy-consuming drying step, as in the case of pyrolysis (Bridgwater, 2004). As hot compressed liquid water approaches its thermodynamic critical point
(Tc ¼ 373.95 C, Pc ¼ 22.064 MPa), its dielectric constant
decreases due to a decrease in hydrogen bonding between water molecules (Figure 10.11). At these conditions, water is still in a liquid state, and has a range of
exotic properties very different from liquid water at
room temperature. Among them is increased solubility
of hydrophobic organic compounds, such as free fatty
acids (Holliday et al., 1997). Subcritical water can also
sustain acid and base ions simultaneously and promotes
radical-driven chemistry. These properties make subcritical water an excellent medium for fast, homogeneous
and efficient conversions of algal organics to biocrude.
But this technology is not without challengesdthe solubility of some salts in the reacting medium decreases
significantly leading to excess precipitate in the system.
Salts present in the HTL process are typically subdivided into two categories: Type I and Type II. Type 1
salts, such as NaCl, still exhibit relatively high solubility
at subcritical conditions. Type 2 salts such as Na2SO4, on
the other hand, have very limited solubility at these conditions (Hodes, 2004). If Type II salts are present in the
167
CONCLUSIONS
FIGURE 10.11
The critical point of water. (For color
version of this figure, the reader is referred to the online
version of this book.)
reaction medium, the decreased solubility can lead to
what’s known as “shock precipitate” which can adsorb
onto the walls of processing equipment causing fouling
and eventually blockage. Technologies designed to
remove or reduce salts from the production stream are
currently being evaluated (Marrone, 2004).
processing of microalgae, heterogeneous catalysts may
provide a more attractive option than homogeneous catalysts because heterogeneous catalysts can be more easily
separated from the reaction products. Further, the yields
of HTL biocrude using heterogeneous catalyst have
been reported to be as high as 71% (Zhang et al., 2013).
HYDROTHERMAL CATALYTIC
LIQUEFACTION
HTL SUMMARY AND OUTLOOK
The principal role of HTL is to fractionate organic
macromolecules into simpler molecular units that can
then be further upgraded to produce specific liquid
fuels. The HTL environment promotes the hydrolytic
cleavage of ester linkages in lipids, peptide linkages in
proteins, and glycosidic ether linkages in carbohydrates.
The speed and efficiency of these cleavage reactions can
be improved by the addition of catalysts to the reaction
medium. Catalysts are generally classified as homogeneous and heterogeneous. In chemistry, homogeneous
catalysis is a sequence of reactions that occur when a
catalyst is codissolved in the same phase as the reactants. The most reported homogeneous catalyst for
HTL processing of microalgae is Na2CO3 (Tekin, 2013;
Zhang et al., 2013). While it has been reported that the
addition of Na2CO3 to the HTL process increases the
overall biocrude yield from microalgae, others have reported that Na2CO3 negatively impacts yields derived
from lipids or proteins, but improves yields of precursors derived from carbohydrates (Biller et al. 2011).
The effects of other homogeneous catalysts (e.g. KOH,
HCOOH, and CH3COOH) on HTL of microalgae have
been examined and ordered according to effectiveness
Na2CO3 > CH3COOH > KOH > HCOOH. For HTL
Though only a limited amount of work has been done
to date, it is clear that hydrothermal catalytic conversion
of algae can produce hydrocarbons for liquid biofuels.
Thus, there is tremendous potential for this field and
the outlook is bright. The majority of the work to date
on producing liquid fuels from hydrothermal conversion of aquatic biomass has focused on homogeneous
catalysis by metal salts or alkali. More recent studies,
however, are beginning to examine heterogeneous catalysts due to advantages in separation and selectivity of
the catalyst. More work is needed to identify better heterogeneous catalysts for these applications. In particular,
the development of nonprecious metal-based catalysts
would provide a major advance.
CONCLUSIONS
Microalgae are a promising source of clean, renewable biofuel. Not only can it be grown and produced
on a large scale, it can be grown in virtually every part
of the world including locations that are considered to
be otherwise unsuitable to agricultural production and
thus lie dormant. However, several challenges remain
to its full execution: (1) the successful production of
168
10. SECOND-GENERATION BIOFUEL FROM HIGH-EFFICIENCY ALGAL-DERIVED BIOCRUDE
feedstock on a large scale; (2) the development of processing methods that are cost-effective and leave intact
the desired molecular end-products; and (3) a richer understanding of microalgal chemistry and product accumulation during both growth and processing phases.
Whether employing open ponds or PBRs for biomass
production, cultures must be carefully monitored to
maintain the desired composition of the culture. Factors
such as nutrient loads, mixing and light source, and contaminants all drive the production of biomass and thus
biofuel precursors. There is a growing trend toward processing microalgae directly from the aqueous stream,
eliminating costly drying steps and conserving water.
As such, HTL is an emerging process that converts
biomass to biocrude in hot, compressed water, thereby
eliminating the need for drying or organic solvents.
Further, all organic components serve as the feedstock
for the HTL process rather than discreet components,
such as lipids for biodiesel or ethanol for bioethanol.
Biohydrogen is another provocative fuel derived from
microalgae. Whatever the feedstock and biofuel process,
additional improvements to each of the technologies are
required to make the production of renewable fuels from
microalgae cost-effective. These improvements can only
result from systems using real-time analytical feedback
to inform growth and processing and from innovations
derived from a multidisciplinary approach.
References
S&T, January, 2003. The Addition of Ethanol from Wheat to GH
Senius. S & T Consultants, Delta, BC.
Abou Akkada, A.R., Bartley, E.E., Berube, R., Fina, L.R., Meyer, R.M.,
Henricks, D., Julius, F., 1968. Simple method to remove completely
ciliate protozoa of adult ruminants. Appl. Environ. Microbiol. 16
(10), 1475e1477.
Acien Fernandez, F.G., Fernandez Sevilla, J.M., Sanchez Perez, J.A.,
Molina Grima, E., Chisti, Y., 2001. Airlift-driven external-loop
tubular photobioreactors for outdoor production of microalgae:
assessment of design and performance. Chem. Eng. Sci. 56,
2721e2732.
Akkerman, I., Janssen, M., Rocha, J., Wijffels, R.H., 2002. Photobiological hydrogen production: photochemical efficiency and bioreactor design. Int. J. Hydrogen Energy 27, 1195e1208.
Anderson, R., 2005. Algal Culturing Techniques, first ed. Elsevier,
Burlington, MA.
Anderson, G.A., Kommareddy, A., Schipull, M.A., 2002. Photobioreactor Design. Paper No. MBSK02e216: An ASAE/CSAE
Meeting Presentation, Canada.
Angermayr, S.A., Hellingwerf, K.J., Lindblad, P., de Mattos, M.J., 2009.
Energy biotechnology with cyanobacteria. Curr Opin Biotechnol.
20, 257e263.
Antal, T.K., Lindblad, P., 2005. Production of H2 by sulphur-deprived
cells of the unicellular cyanobacteria Gloeocapsa alpicola and Synechocystis sp. PCC 6803 during dark incubation with methane or at
various extracellular pH. J. App Microbiol. 98, 114e120.
Aoyama, K., Uemura, I., Miyake, J., Asada, Y., 1997. Fermentative
metabolism to produce hydrogen gas and organic compounds in a
cyanobacterium, Spirulina platensis. J. Ferment. Bioeng. 83, 17e20.
Arantes, V., Saddler, J.N., 2010. Access to cellulose limits the efficiency
of enzymatic hydrolysis: the role of amorphogenesis. Biotechnol.
for biofuels 3, 1e11.
Asinari Di San Marzano, C.M., Legros, A., Naveau, H.P., Nyns, E.J.,
1982. Biomethanation of the marine algae Tetraselmis. Int. J.
Sustainable Energy 1, 263e272.
Bandyopadhyay, S.S., Navid, M.H., Ghosh, T., Schnitzler, P., Ray, B.,
2011. Structural features and in vitro antiviral activities of sulfated
polysaccharides from Sphacelaria indica. Phytochemistry 72,
276e283.
Banerjee, A., Sharma, R., Chisti, Y., Banerjee, U.C., 2002. Botryococcus
braunii: A renewable source of hydrocarbons and other chemicals.
Crit. Rev. Biotechnol. 22, 245e279.
Bashir, S., Lee, S., 1994. Fuel ethanol production from agricultural
lignocellulose feedstocks e a review. Fuel Science Technol. Int’l 12,
1427e1473.
Batten, D., Beer, T., Freischmidt, G., Grant, T., Liffman, K., Paterson, D.,
Priestley, T., Rye, L., Threlfall, G., 2013. Using wastewater and highrate algal ponds for nutrient removal and the production of bioenergy and biofuels. Water Sci. Technol. 67 (4).
Beal, C.M., Hebner, R.E., Romanovicz, D.K., Connelly, R.L., 2012a.
Progression of lipid profile and cell structure in a research-scale
production pathway for algal biocrude. Renewable Energy 50,
86e93.
Beal, C.M., Stillwell, A.S., King, C.W., Cohen, S., Berberoglu, H.,
Bhattarai, R.P., Connelly, R.L., Webber, M.E., Hebner, R.E.,
2012b. Energy return on investment for algal biofuel production coupled with wastewater treatment. Water Environ. Res. 84
(9), 692e710.
Becker, E.W., 1988. Microalgae for human and animal consumption.
In: Borowitzka, M.A., Borowitzka, L.J. (Eds.), Microalgal Biotechnology. Cambridge University Press, Oxford, pp. 222e249.
Becker, E.W., 2007. Micro-algae as a source of protein. Biotechnol. Adv.
25, 207e210.
Benemann, J.R., 2008. Open Ponds and Closed Photobioreactors e
Comparative Economics (Slide Presentation). Paper presented at
the Fifth Annual World Congress on Industrial Biotechnology &
Bioprocessing, April 27e30, Chicago, Illinois.
Benemann, J.R., Oswald, W.J., 1996. Systems and Economic Analysis
of Microalgae Ponds for Conversion of Carbon Dioxide to Biomass
(Final Report: Grant No. DE-FG22-93PC93204). Pittsburgh Energy
Technology Center, Pittsburgh, PA (U.S. Department of Energy).
Benemann, J.R., Goebel, R.P., Weissman, J.C., Augenstein, D.C., 1982.
Microalgae as a Source of Liquid Fuels (Final Technical Report,
Contract Deacos 81 ER 30014). U.S. Department of Energy.
Berzin, I., Nov. 24, 2005. Photobioreactor and Process for Biomass
Production and Mitigation of Pollutants in Flue Gases. United
States Patent Application. Pub no. US2005/0260553 A1, USA,
Publication Date.
Biller, P., Riley, R., Ross, A.B., 2011. Catalytic hydrothermal processing
of microalgae: decomposition and upgrading of lipids. Bioresour
Technol. 102, 4841e4848.
Borowitzka, M.A., 2005. Culturing microalgae in outdoor ponds. In:
Anderson, R.A. (Ed.), Algal Culturing Techniques. Elsevier, Burlington, MA, pp. 205e218.
Bridgwater, A.V., Maniatis, K., 2004. The production of biofuels by the
thermochemical processing of biomass. In: Archer, M.D., Barber, J.
(Eds.), Molecular to Global Photosynthesis. IC Press, London,
pp. 521e612.
Brindley, C., Fernandez, F.G.A., Fernandez-Sevilla, J.M., 2011. Analysis
of light regime in continuous light distributions in photobioreactors. Bioresour. Technol. 102, 3138e3148.
Brzezinski, M.A., 1985. The SieCeN ratio of marine diatoms: interspecific variability and the effect of some environmental variables.
J. Phycol. 21, 34e357.
REFERENCES
Carvalho, A.P., Meireles, L.A., Malcata, F.X., 2006. Microalgal reactors:
A review of enclosed system designs and performances. Biotechnol. Prog. 22, 1490e1506.
Chisti, Y., 2007. Biodiesel from microalgae. Biotechnol. Adv. 25,
294e306.
Choi, S.L., Suh, I.S., Lee, C.G., 2003. Lumostatic operation of bubble
column photobioreactors for Haematococcus pluvialis cultures using
a specific light uptake rate as a control parameter. Enzyme Microb.
Technol. 33, 403e409.
Cirne, D.G., Paloumet, X., Bjornsson, L., Alves, M.M., Mattiasson, B.,
2007. Anaerobic digestion of lipid-rich wastedeffects of lipid
concentration. Renewable Energy 32, 965e975.
Craggs, R.J., Smith, V.J., McAuley, P.J., 1995. Wastewater nutrient
removal by marine micro-algae cultured under ambient conditions
in mini-ponds. Wat. Sci. Technol. 31, 151e160.
Dismukes, G.C.D., Carrieri, N., Bennette, G., Ananyey, M., 2008.
Aquatic phototrophs: efficient alternatives to land-based crops for
biofuels. Curr. Opin. Biotechnol. 19, 235e240.
Doucha, J., Livansky, K., 2006. Productivity, CO2/O2 exchange and
hydraulics in outdoor open high density microalgal (Chlorella sp.)
photobioreactors operated in a middle and southern European
climate. J. Appl. Phycol. 18, 811e826.
Duffy, P., 1853. XXV. On the constitution of stearine. Q. J. Chem. Soc.
London 5, 303.
Dunahay, T.G., Jarvis, E.E., Dais, S.S., Roessler, P.G., 1996. Manipulation of microalgal lipid production using genetic engineering.
Appl. Biochem. Biotechnol. 57, 223e231.
Esquı́vel, M.G., Amaro, H.M., Pinto, T.S., Fevereiro, P.S., Malcata, F.X.,
2011. Efficient H2 production via Chlamydomonas reinhardtii. Trends
Biotechnol. 29 (12), 595e600.
Fedorov, A.S., Kosourov, S., Ghirardi, M.L., Seibert, M., 2005.
Continuous H2 photoproduction by Chlamydomonas reinhardtii
using a novel two-stage, sulfate-limited chemostat system. Appl
Biochem Biotechnol. 121e124, 403e412.
Fukuda, H., Kondo, A., Noda, H., 2001. Biodiesel fuel production by
transesterification of oils. J. Biosci. Bioeng. 92, 405e416.
Gavrilescu, M., Chisti, Y., 2005. Biotechnologyda sustainable alternative for chemical industry. Biotechnol. Adv. 23, 471e499.
Ghirardi, M.L., Zhang, J.P., Lee, J.W., Flynn, T., Seiber, M.,
Greenbaum, E., 2000. Microalgae: a green source of renewable H2.
Trends Biotechnol. 18, 506e511.
Goldemberg, J., 2007. Ethanol for a sustainable energy future. Science
315, 808e810.
Golueke, C.G., Oswald, W.J., 1959. Biological conversion of light
energy to the chemical energy of methane. Appl. Microbiol. 7,
219e227.
Golueke, C.G., Oswald, W.J., Gotaas, H.B., 1957. Anaerobic digestion
of algae. Appl. Microbiol. 5, 47e55.
González-López, C.V., Acién Fernández, F.G., Fernández-Sevilla, J.M.,
Sánchez Fernández, J.F., Molina Grima, E., 2012. Development of a
process for efficient use of CO2 from flue gases in the production
of photosynthetic microorganisms. Biotechnol. Bioeng. 109 (7),
1637e1650.
Grima, E.M., Belarbia, E.H., Fernandez, F.G.A., Medina, A.R.,
Chisti, Y., 2003. Recovery of microalgal biomass and metabolites:
process options and economics. Biotechnol. Adv. 20, 491e515.
Guschina, I.A., Harwood, J.L., 2006. Lipids and lipid metabolism in
eukaryotic algae. Prog. Lipid Res. 45, 160e186.
Hai, T., Ahlers, H., Gorenflo, V., Steinbuchel, A., 2000. Axenic cultivation of anoxygenic phototrophic bacteria, cyanobacteria and
microalgae in a new closed tubular glass photobioreactor. Appl.
Microbiol. Biotechnol. 53, 383e389.
Harrison, P., Berges, J., 2005. Marine culture media. In: Andersen, R.A.
(Ed.), Algal Culturing Techniques. Elsevier, Burlington, MA,
pp. 21e33.
169
Harun, R., Danquah, M.K., Forde, G.M., 2009. Microalgal biomass as a
fermentation feedstock for bioethanol production. J. Chem. Technol. Biotechnol. 85, 199e203.
Hodes, M., Marrone, P.A., Hong, G.T., Smith, K.A., Tester, J.W., 2004.
Salt precipitation and scale control in supercritical water oxidation part A: fundamentals and research. J. Supercrit Fluids 29,
265e288.
Hoekema, S., Bijmans, M., Janssen, M., Tramper, J., Wijffles, R.H.,
2002. A pneumatically agitated flat-panel photobioreactor with
gas re-circulation: anaerobic photoheterotrophic cultivation of a
purple non-sulfur bacterium. Int. J. Hydrogen Energy 27,
1331e1338.
Holliday, R.L., King, J.W., List, G.R., 1997. Hydrolysis of vegetable oils
in sub- and supercritical water. Ind. Eng. Chem. Res. 36, 932e935.
Horta Nogueira, L., 2004. Perspectivas de un Programa de Biocombustibles en America Central: Proyecto uso Sustentable de
Hidrocarburos. Comision Economica para America Latina y el
Caribe (CEPAL).
Hu, Q., Sommerfeld, M., Jarvis, E., Ghirardi, M., Posewitz, M.,
Seibert, M., Darzins, A., 2008. Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J.
54, 621e639.
Huntley, M., Redalje, D., 2007. CO2 mitigation and renewable oil from
photosynthetic microbes: A new appraisal. Mitigation Adapt.
Strategy Global Change 12, 573e608.
Jeffries, T.W., Timourian, H., Ward, R.L., 1978. Hydrogen production
by Anabaena cylindrica: effects of varying ammonium and ferric
ions, pH and light. Appl. Environ. Microbiol. 35, 704e710.
Jones, J., Manning, S., Montoya, M., Keller, K., Poenie, M., 2012.
Extraction of algal lipids and their analysis by HPLC and mass
spectrometry. J. Am. Oil Chem. Soc. 89 (8), 1371e1381.
Jones, C.S., Mayfield, S.P., 2012. Algae biofuels: versatility for the
future of bioenergy. Curr Opin Biotechnol. 23, 346e351.
Kapdan, I.K., Kargi, F., 2006. Bio-hydrogen production from waste
materials. Enzym. Microb. Technol. 38, 569e582.
Kaya, V.M., Picard, G., 1995. The viability of Scenedesmus bicellularis
cells immobilized on alginate screens following nutrient starvation
in air at 100% relative humidity. Biotechnol. Bioeng. 46, 459e464.
Kessler, E., 1991. Scenedesmus: problems of a highly variable genus of
green algae. Botanica Acta 104, 169e171.
Kristensen, E., 1990. Characterization of biogenic organic matter by
stepwise thermogravimetry (STG). Biogeochemistry 9, 135e159.
Lee, Y.K., 2001. Microalgal mass culture systems and methods: their
limitations and potential. J. Appl. Phycol. 13, 307e315.
Levin, G.V., Clendenning, J.R., Gibor, A., Bogar, F.D., 1961. Harvesting
of algae by froth flotation. Appl. Microbiol. 10, 169e175.
Levin, D.B., Lawrence, P., Murry, L., 2004. Biohydrogen production:
prospects and limitations to practical application. Int. J. Hydrogen
Energy 29, 173e185.
Li, Y.Y., Sasaki, H., Yamashita, K., Seki, K., Kamigochi, I., 2002. Highrate methane fermentation of lipid-rich food wastes by a highsolids co-digestion process. Water Sci. Technol. 45, 143e150.
Margheri, M.C., Tredici, M.R., Allotta, G., Vagnoli, L., 1990. Heterotrophic metabolism and regulation of uptake hydrogenase activity
in symbiotic cyanobacteria. In: Polsinelli, M., Materassi, R.,
Vincenzini, M. (Eds.), Developments in Plant and Soil Sciences-Bio
Nitrogen Fixation. Kluwer Academic Publications, Dordrecht,
pp. 481e486.
Marrone, P.A., Hodes, M., Smith, K.A., Tester, J.W., 2004. Salt precipitation and scale control in supercritical water oxidation part B:
Commercial/full-scale applications. J. Supercrit. Fluids 29,
289e312.
Matsumoto, M., Hiroko, Y., Nobukazu, S., Hiroshi, O., Tadashi, M.,
2003. Saccharification of marine microalgae using marine bacteria
for ethanol production. Appl. Biochem. Biotechnol. 105, 247e254.
170
10. SECOND-GENERATION BIOFUEL FROM HIGH-EFFICIENCY ALGAL-DERIVED BIOCRUDE
Megharaj, M., Peardon, H.W., Venkateswarlu, K., 1992. Removal of
nitrogen and phosphorus by immobilized cells Chlorella vulgaris
and Scenedesmus bijugatus isolated from soil. Enz. Microb. Technol. 14, 656e658.
Melis, A., 2002. Green alga hydrogen production: Progress, challenges
and prospects. Int. J. Hydrogen Energy 27, 1217e1228.
Miao, X.L., Wu, Q.Y., 2004. High yield bio-oil production from fast
pyrolysis by metabolic controlling of Chlorella protothecoides.
J. Biotechnol. 110, 85e93.
Mimouni, V., Ulmann, L., Pasquet, V., Mathieu, M., Picot, L.,
Bougaran, G., Cadoret, J.P., Morant-Manceau, A., Schoefs, B., 2012.
The potential of microalgae for the production of bioactive molecules of pharmaceutical interest. Curr. Pharm. Biotechnol. 13 (15),
2733e2750.
Molina Grima, E., Acien Fernandez, F.G., Garcia Camacho, F.,
Chisti, Y., 1999. Photobioreactors: light regime, mass transfer, and
scale-up. J. Biotechnol. 70, 231e248.
Mulbry, W., Westhead,.EK., Pizarro, C., Sikora, L., 2005. Recycling of
manure nutrients: use of algal biomass from dairy manure treatment
as a slow release fertilizer. Bioresour. Technol. 96 (4), 451e458.
Mussatto, S.I., Dragone, G., Guimarães, P., Silva, J.P., Carneiro, L.M.,
Roberto, I.C., Vicente, A., Domingues, L., Teixeira, J.A., 2010.
Technological trends, global market, and challenges of bio-ethanol
production. Biotechnol. Adv. 28, 817e830.
Nurdogan, Y., Oswald, W.J., 1995. Enhanced nutrient removal in high
rate ponds. Water Sci. Technol. 31, 33e43.
Oswald, W.J., 2003. My sixty years in applied algology. J. Appl. Phycol. 15, 99e106.
Pulz, O., 1992. Cultivation Techniques for Microalgae in Open and
Closed Ponds. Proceedings of the First European workshop on
microalgal biotechnology. Potsdam, p. 61.
Pulz, O., 2001. Photobioreactors: Production systems for phototrophic
microorganisms. Appl. Microbiol. Biotechnol. 57, 287e293.
Ragauskas, A.J., Williams, C.K., Davison, B.H., Britovsek, G., Cairney, J.,
Eckert, C.A., Frederick Jr., W.J., Hallett, J.P., Leak, D.J., Liotta, C.L.,
Mielenz, J.R., Murphy, R., Templer, R., Tschaplinski, T., 2006. The
path forward for biofuels and biomaterials. Science 311, 484e489.
Ramachandran, S., Mitsui, A., 1984. Recycling of hydrogen production
system using an immobilized blue green algae Oscillatoria sp.
Miami BG7, solar energy and sea water. In: Abstracts of the VII
International Biotechnology Symposium, pp. 183e184.
Ran, J.-H., Wei, X.-X., Wang, X.-Q., 2006. Molecular phylogeny and
biogeography of Picea (Pinaceae): implications for phylogeographical studies using cytoplasmic haplotypes. Mol. Phylogenet.
Evol. 41, 405e419.
Richmond, A., 1999. Physiological principles and modes of cultivation
in mass production of photoautotrophic microalgae. In: Cohen, Z.
(Ed.), Chemicals from Microalgae. Taylor & Francis, London, UK,
pp. 353e386.
Roessler, P.G., 1990. Environmental control of glycerolipid metabolism
in microalgae: commercial implications and future research directions. J. Phycol. 26, 393e399.
Rosillo-Calle, F., Cortez, L., 1998. Towards proalcohol II: a review of the
Brazilian bioethanol programme. Biomass Bioenergy 14, 115e124.
Sakurai, H., Masukawa, H., 2007. Promoting R & D in photobiological
hydrogen production utilizing mariculture-raised cyanobacteria.
Mar. Biotechnol 9, 128e145.
Sanchez, E.J., Novotny, L., Xie, X.S., 1999. Near-field fluorescence
microscopy based on two-photon excitation with metal tips. Phys.
Rev. Lett. 82, 4014e4017.
Sawayama, S., Inoue, S., Yokoyama, S., 1995. Phylogenetic position of
Botryococcus braunii (Chlorophyceae) based on small subunit ribosomal RNA sequence data. J. Phycol. 31, 419e420.
Schenk, P., Thomas-Hall, S., Stephens, E., Marx, U., Mussgnug, J.,
Posten, C., Kruse, O., Hankamer, B., 2008. Second generation
biofuels: high-efficiency microalgae for biodiesel production. Bioenergy Res. 1, 20e43.
Schmitz, O., Boison, G., Hilscher, R., Hundeshagen, B., Zimmer, W.,
Lottspeich, F., Bothe, H., 1995. Molecular biological analysis of a
bidirectional hydrogenase from cyanobacteria. Eur. J. Biochem.
233, 266e276.
Sheehan, J., Dunahay, T., Benemann, J., Roessler, P., 1998. A Look Back
at the U.S. Department of Energy’s Aquatic Species Program e
Biodiesel from Algae. National Renewable Energy Laboratory.
NREL/TP-580e24190.
Sialve, B., Bernet, N., Bernard, O., 2009. Anaerobic digestion of
microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnol. Adv. 27, 409e416.
Singh, J., Gu, S., 2010. Commercialization potential of microalgae for
biofuels production. Renewable and Sustainable Energy Reviews
14, 2596e2610.
Skjånes, K., Rebours, C., Lindblad, P., 2013. Potential for green
microalgae to produce hydrogen, pharmaceuticals and other highvalue products in a combined process. Crit. Rev. Biotechnol. 33 (2),
172e215.
Spolaore, P., Joannis-Cassan, C., Duran, E., Isambert, A., 2006. Commercial applications of microalgae. J. Biosci. Bioeng. 101, 87e96.
Stamey, J.A., Shepherd, D.M., de Veth, M.J., Corl,., B.A., 2012. Use of
algae or algal oil rich in n-3 fatty acids as a feed supplement for
dairy cattle. J. Dairy Sci. 95 (9), 5269e5275.
Tamagnini, P., Axelsson, R., Lindberg, P., Oxelfelt, F., Wunschiers, R.,
Lindblad, P., 2002. Hydrogenases and hydrogen metabolism of
cyanobacteria. Microbiol. Mol. Biol. Rev. 66, 1e20.
Tekin, K., Karagoz, S., 2013. Non-catalytic and catalytic hydrothermal
liquefaction of biomass. Res. Chem. Intermed. 39 (2), 485e498.
Tredici, M.R., Margheri, M.C., Zittelli, G.C., Biagiolini, S., Capolino, E.,
Natali, M., 1992. Nitrogen and phosphorus reclamation from
municipal wastewater through an artificial food-chain system.
Bioresource Technol. 42, 247e253.
Tredici, M.R., Zitelli, G.C., 1998. Efficiency of sunlight utilization:
tubular versus flat photobioreactors. Biotechnol. Bioeng. 587,
187e197.
Ugwu, C.U., Ogbonna, J.C., Tanaka, H., 2005. Characterization of light
utilization and biomass yields of Chlorella sorokiniana in inclined
outdoor tubular photobioreactors equipped with static mixers.
Process Biochem. 40, 3406e3411.
Wang, B., Li, Y., Wu, N., Lan, C.Q., 2008. CO2 bio-mitigation using
microalgae. Appl. Microbiol. Biotechnol. 79, 707e718.
Weissman, J.C., Goebel, R.P., 1987. Design and Analysis of Microalgal
Open Pond Systems for the Purpose of Producing Fuels. SERI/
STR-231-2840. Solar Energy Research Institute, Golden, Colorado.
Yen, H.W., Brune, D.E., 2007. Anaerobic co-digestion of algal sludge
and waste paper to produce methane. Bioresour. Technol. 98,
130e134.
Zhang, J., Chen, W.T., Zhang, P., Luo, Z., Zhang, Y., 2013. Hydrothermal liquefaction of Chlorella pyrenoidosa in sub- and supercritical ethanol with heterogeneous catalysts. Bioresour Technol.
133, 389e397.
Zhu, L., Wang, Z., Shu, Q., Takala, J., Hiltunen, E., Feng, P., Yuan, Z.,
2013. Nutrient removal and biodiesel production by integration of
freshwater algae cultivation with piggery wastewater treatment.
Water Res. 47 (13), 4294e4302.
Zijffers, J.W.F., Salim, S., Janssen, M., Tramper, J., Wijffels, R.H., 2008.
Capturing sunlight into a photobioreactor: ray tracing simulations
of the propagation of light from capture to distribution into the
reactor. Chem. Eng. J. 145, 316e327.
Zittelli, C.G., Lavista, F., Bastianini, A., Rodolfi, L., Vincenzini, M.,
Tredici, M.R., 1999. Production of eicosapentaenoic acid by Nannochloropsis sp. cultures in outdoor tubular photobioreactors. J
Biotechnol. 70, 299e312.
C H A P T E R
11
Microalgae: The Tiny Microbes
with a Big Impact
Shovon Mandal 1, Nirupama Mallick 2,*
1
Section of Ecology, Behavior and Evolution, University of California, San Diego, CA, USA,
Agricultural and Food Engineering Department, Indian Institute of Technology, Kharagpur,
West Bengal, India
*Corresponding author email: nm@agfe.iitkgp.ernet.in
2
O U T L I N E
Fatty Acid Methyl Esters and Fuel
Properties
Renewable Energy
171
Petroleum Fuel Scenario in India
172
Biodiesel
172
Microalgae: Viable Feedstocks for Biodiesel
173
Waste Utilization for Biodiesel Production: A Case
Study with Scenedesmus obliquus in a
Recirculatory Aquaculture System
179
Selection of Potent Strains
173
Concluding Remarks
181
Genetic Engineering Approach
175
References
181
Microalgal Biodiesel Production
177
RENEWABLE ENERGY
Energy is an important currency for human society.
The world population growth and rapid economic progresses are expected to result in considerable increase
in the demand for energy. In the reference scenario,
the International Energy Agency has projected an
increase in energy need by 55%, between 2005 and
2030, at an average annual rate of 1.8% (IEA, 2007).
Driven by such increasing demand, and the dwindling
fuel production, the cost of petroleum fuel has gone up
sky high in recent times, which can jeopardize the economic progresses of a nation. Despite the fuel crisis,
increasing concentrations of CO2 and other heattrapping greenhouse gases (GHGs) in the atmosphere,
primarily due to the combustion of fossil fuels, is
clearly the prime reason for rapid warming of the
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00011-5
179
planet (Shay, 1993). The use of renewable energy is
largely motivated from the standpoint of global energy
crisis and environmental issues. Renewable energy is a
form of energy that is produced from natural sources
like sunlight, wind, hydropower, geothermal and
biomass, which can be naturally replenished. Currently,
renewable energy supplies only w18% of the world’s
energy consumption (Kumar et al., 2010). Most of these
renewable energy sources (hydropower, wind, solar
and geothermal) target the electricity market, while
the majority of world energy consumption (about
two-thirds) is derived from liquid fuels (Campbell,
2008; Hankamer et al., 2007). This has stimulated recent
interest to explore alternative sources for petroleumbased fuels and much of the attention has been focused
on biomass-derived liquid fuels or biofuels (Haag, 2007;
Schneider, 2006).
171
Copyright Ó 2014 Elsevier B.V. All rights reserved.
172
11. MICROALGAE: THE TINY MICROBES WITH A BIG IMPACT
PETROLEUM FUEL SCENARIO IN INDIA
India ranks seventh as the world’s energy producer
accounting for about 2.5% of the world’s total annual
energy production, and world’s fifth largest energy
consumer with about 3.5% of the global primary energy
demand (IEA, 2007; Planning Commission, Govt. of
India, 2007). Despite being among the largest energy
producer, India is a net importer of energy, largely
due to huge imbalance between energy consumption
and production. About 30% of India’s total primary
energy need is being met by petroleum oil, of which
76% is imported. India’s transportation fuel requirements are unique in the world. India consumes almost
five times more diesel fuel than gasoline, whereas all
other countries in the world use more gasoline than
diesel fuel (Khan et al., 2009). Thus, search for alternatives to diesel fuel is of special importance in India.
Bioalcohols are unsuitable substitutes for diesel
engines, because of their low cetane numbers (CNs) along
with poor energy content per unit biomass (Bhattacharyya
and Reddy, 1994; Rao and Gopalkrishnan, 1991). Therefore, biodiesel is the only option to fulfill the requirements in future.
BIODIESEL
Biodiesel is chemically monoalkyl esters of longchain fatty acids derived from vegetable oils or animal
fats. The history of using vegetable oil as an alternative
fuel dates back to 1900, when Rudolph Diesel used peanut oil as fuel in the World Exhibition in Paris. It was
found that vegetable oils, in general, have acceptable
CNs and calorific values comparable with the conventional diesel. However, the major problem with the
direct use of vegetable oils as fuel of compression ignition engine is their high viscosity, which interferes with
the fuel injection and atomization contributing to
incomplete combustion, nozzle clogging, injector
coking, severe engine deposits, ring sticking and gum
formation leading to engine failure (Knothe, 2005;
Meher et al., 2006; Singh and Rastogi, 2009). Therefore,
vegetable oils need to be modified to bring their
combustion-related properties closer to those of diesel
fuel. One possible method to overcome the problem
of high viscosity of vegetable oils is their chemical
modification to esters, what is nowadays called as
“biodiesel”.
Biodiesel has emerged as the most suitable alternative to petroleum diesel fuel owing to its ecofriendly
characteristics and renewability (Krawczyk, 1996). It
burns in conventional diesel engines with or without
any modifications while reducing pollution (100%
less sulfur dioxide, 37% less unburned hydrocarbons,
46% less carbon monoxide, and 84% less particulate
matter) in comparison to the conventional diesel fuel
(McMillen et al., 2005). The basic feedstocks for the
production of first-generation biodiesel were mainly
edible vegetable oils like soybean, rapeseed, sunflower
and safflower. The use of first-generation biodiesel has
generated a lot of controversy, mainly due to their
impact on global food markets and food security for
diverting food away from the human food chain. The
second-generation biodiesel was produced by using
nonedible oil sources like used frying oil, grease, tallow,
lard, karanja, jatropha and mahua oils (Alcantara
et al., 2000; Francis and Becker 2002; Canakci and
Gerpen, 1999; Dorado et al., 2002; Ghadge and
Raheman, 2006; Mittelbach, 1990). Nevertheless, the
cost of biodiesel production is still a major obstacle
for large-scale commercial exploitation, mainly due to
the high feed cost of vegetable oils (Lang et al., 2001).
Moreover, the first- as well as the second-generation
biodiesel based on terrestrial plants initiate land
clearing and potentially compete with net food production (Chisti, 2008; Marsh, 2009). The focus of researchers
has now been shifted to the next generation biodiesel.
The third-generation biodiesel is both promising and
different; it is based on simple microscopic organisms
that live in water and grow hydroponically, i.e.
microalgae.
The possibilities of biodiesel production from edible
oil resources in India is almost impossible, as primary
need is to first meet the demand of edible oil that is being
imported. India accounts for 9.3% of the world’s total oil
seed production and contributes as the fourth largest
edible oil producing country. Even then, about 46% of
edible oil is imported for catering the domestic needs
(Jain and Sharma, 2010). So the nonedible oil resources
like Jatropha, pongamia, mahua, etc. seem to be the
only possibility for biodiesel production in the country.
The Government of India has duly realized the importance of biodiesel and introduced a nationwide program
under the National Biodiesel Mission in 2003 with the
aim of achieving a target of meeting 13.4 Mt of biodiesel
(@ 20% blending) from Jatropha curcas by 2012, and to
achieve the target about 27 billion of planting materials
are required to be planted over 11.2 million hectares of
land (Planning Commission, Govt. of India, 2003). At
the current rate of consumption, if all petroleumderived transport fuel is to be replaced with biodiesel
from Jatropha oil, Jatropha would need to be grown
over an area of 384 million hectares, which is more
than 100% of the geographic area of India (Khan et al.,
2009). Therefore, India must find additional, reliable,
cost-effective and sustainable feedstock for biodiesel
production. In this context, biodiesel from microalgae
seems to be a suitable substitute for diesel fuel in the
long run.
SELECTION OF POTENT STRAINS
MICROALGAE: VIABLE FEEDSTOCKS
FOR BIODIESEL
Microalgae are a diverse group of photosynthetic
organisms whose systematics is based on the kinds
and combinations of photosynthetic pigments present
in different species. They can grow in diverse environmental conditions, and are able to produce a wide
range of chemical products with applications in feed,
food, nutritional, cosmetic and pharmaceutical industries. These are primitive organisms with a simple
cellular structure and a large surface to volume body
ratio, which gives them the ability to take up a large
amount of nutrients. While the mechanism of photosynthesis in microalgae is similar to that of higher
plants, they have the ability to capture solar energy
with an efficiency of 10e50 times higher than that of
terrestrial plants (Li et al., 2008). Moreover, because
the cells grow in aqueous suspension, they have
more efficient access to water, CO2 and other nutrients. For these reasons, microalgae are capable of producing more amount of oil per unit area of land in
comparison to that of all other known oil-producing
crops (Chisti, 2007; Haag, 2007). The per hectare yield
of microalgal oil has been projected to be
58,700e136,900 l/year depending upon the oil content
of algae, which is about 10e20 times higher than the
best oil producing crop, i.e. palm (5950 l/ha year,
Chisti, 2007). The most acclaimed energy crop, i.e.
Jatropha has been estimated to produce only 1892 l/ha
year. More importantly, due to being aquatic in nature,
algae do not compete for arable land for their cultivation; they can be grown in freshwater or saline, and
salt concentrations up to twice that of seawater can
be used effectively for few species (Aresta et al.,
2005; Brown and Zeiler, 1993). The utilization of
wastewaters that are rich in nitrogen and phosphorus
may bring about remarkable advantages by providing
N and P nutrients for growing microalgae, while
removing N and P from the wastewaters (Mallick,
2002). This implies that algae need not compete with
other users for freshwater (Campbell, 2008). On top
of these advantages, microalgae grow even better
when fed with extra carbon dioxide, the main GHG.
If so, these tiny organisms can fix CO2 from power stations and other industrial plants, thereby cleaning up
the greenhouse problem. Each ton of algae produced
consumes about 1.8 ton of CO2 (Chisti, 2007). Thus,
the integrated efforts to cleanup industrial flue gas
with microalgal culture by combining it with wastewater treatment will significantly enhance the environmental and economical benefits of the technology
for biodiesel production by minimizing the additional
cost of nutrients and saving the precious freshwater
resources.
173
SELECTION OF POTENT STRAINS
Realizing the oil-yielding potentialities with much
faster growth rate and efficient CO2 fixation, microalgae
appear to be the best option as a renewable source of
biodiesel that has the potentiality to completely replace
the petroleum diesel fuel. However, the lipid content
in the selected microalga/strain is required to be high;
otherwise the economic performance would be hard to
achieve.
Each species of microalga produces different ratios of
lipids, carbohydrates and proteins. Nevertheless, these
tiny organisms have the ability to manipulate their
metabolism by simple manipulations of the chemical
composition of the culture medium (Behrens and Kyle,
1996); thus, high lipid productivity can be achieved.
Physiological stresses such as nutrient limitation/deficiency, salt stress and high light intensity have been
employed for directing metabolic fluxes to lipid biosynthesis of microalgae. Many reports are available, where
attempts have been made to raise the lipid pool of
various microalgal species. Table 11.1 summarizes those
studies.
Exceptionally, an oil content of 86% of dry cell weight
(dcw) was reported in the brown resting state colonies of
Botryococcus braunii, while the green active state colonies
were found to account for 17% only (Brown et al., 1969).
However, the major obstacle in focusing B. braunii as an
industrial organism for biodiesel production is its poor
growth rate (Dayananda et al., 2007). Nitrogen limitation/deficiency has been found to raise the lipid content
of a number of microalgal species profoundly. For
instance, Piorreck and Pohl (1984) reported an increased
lipid pool from 12% to 53% (dcw) in Chlorella vulgaris
under nitrogen-limited condition. Unlike the green algae,
the blue-green algae viz. Anacystis nidulans and Oscillatoria rubescens contained the same quantities of lipid at
different nitrogen concentrations. It was observed by
Illman et al. (2000) that four species of Chlorella (Chlorella
emersonii, Chlorella minutissima, C. vulgaris and Chlorella
pyrenoidosa) could accumulate lipid up to 63, 57, 40 and
23% (dcw), respectively, in low N-medium. These values
in control vessels were, respectively, 29%, 31%, 18% and
11% in the above order. In the same year, Takagi et al.
(2000) observed an increase in intracellular lipid pool
up to 51% (dcw) against 31% control in 3% CO2-purged
cultures of Nannochloris sp. UTEX LB1999 grown in
continuous low nitrate (0.9 mM)-fed medium. Chlorella
protothecoides also showed a rise in lipid pool from 15%
to 55% (dcw), when grown heterotrophically with
glucose (1%) under reduced nitrogen concentration
(Miao and Wu, 2004). Similarly, C. protothecoides depicted
a lipid pool of 55% (dcw) when grown heterotrophically
with corn powder hydrolysate under nitrogen limitation
(Xu et al., 2006).
174
11. MICROALGAE: THE TINY MICROBES WITH A BIG IMPACT
TABLE 11.1
A List of Studies on Increased Lipid Accumulation in Microalgae under Various Specific Conditions
Microalga
Growth Condition
Lipid Content as
Percent of Dry
Cell Weight
Botryococcus braunii
Brown resting state
86 (17*)
Brown et al. (1969)
Chlorella vulgaris
Nitrogen limitation
53 (12*)
Piorreck and Pohl (1984)
Chlorella emersonii
Nitrogen limitation
63 (29*)
Illman et al. (2000)
Chlorella minutissima
57 (31*)
Chlorella vulgaris
40 (18*)
Chlorella pyrenoidosa
23 (11*)
References
Nannochloris sp. UTEX
LB1999
Nitrogen limitation
51 (31*)
Takagi et al. (2000)
Chlorella protothecoides
Heterotrophy with 0.1%
glucose under nitrogen
limitation
55 (15*)
Miao and Wu (2004)
Heterotrophy with corn
powder hydrolysate under
nitrogen limitation
55 (15*)
Xu et al. (2006)
Dunaliella sp.
1 M NaCl
71 (64*)
Takagi et al. (2006)
Chlorella sp.
Heterotrophy with 1%
sucrose
33 (15*)
Rattanapoltee et al. (2008)
Scenedesmus obliquus
Nitrogen and phosphorus
limitations in presence of
thiosulphate
58 (13*)
Mandal and Mallick (2009)
Neochloris oleoabundans
Nitrogen deficiency
56 (29*)
Gouveia and Oliveira (2009)
Nannochloropsis oculata
NCTU-3
2% CO2
50 (31*)
Chiu et al. (2009)
Nannochloropsis
sp. F&M-M24
Nitrogen deficiency
60 (31*)
Rodolfi et al. (2009)
Phosphorus deficiency
50 (31*)
Nannochloropsis oculata
Nitrogen limitation
15 (8*)
Chlorella vulgaris
Converti et al. (2009)
16 (6*)
Choricystis minor
Nitrogen and phosphorus
deficiencies
60 (27*)
Sobczuk and Chisti (2010)
Haematococcus pluvialis
High light intensity
35 (15*)
Damiani et al. (2010)
High light intensity under
nitrogen deficiency
33 (15*)
Chlorella protothecoides
Heterotrophy with sweet
sorghum hydrolysate
under nitrogen limitation
50 (15*)
Gao et al. (2010)
Chlorella zofingiensis
Nitrogen limitation
55 (27*)
Feng et al. (2011)a
Isochrysis
zhangjiangensis
High nitrogen (0.9%)
supplementation
53 (41*)
Feng et al. (2011)b
Dunaliella tertiolecta
Nitrogen deficiency
26 (12*)
Jiang et al. (2012)
Thalassiosira pseudonana
Chlorella vulgaris
* Lipid content of control culture.
20 (13*)
Nitrogen, phosphorus and
iron limitations
57 (8*)
Mallick et al. (2012)
GENETIC ENGINEERING APPROACH
Gao et al. (2010) used sweet sorghum hydrolysate
instead of corn powder for C. protothecoides culture,
and lipid yield of 50% (dcw) was recorded. Nitrogen
limitation/starvation also enhanced the lipid content
in Neochloris oleoabundans, Nannochloropsis oculata,
C. vulgaris, Chlorella zofingiensis, Dunaliella tertiolecta
and Thalassiosira pseudonana (Converti et al., 2009; Feng
et al., 2011a; Gouveia and Oliveira, 2009; Jiang et al.,
2012). However, the marine microalga Isochrysis zhangjiangensis was found to accumulate lipid under high
nitrate concentration, rather than limitation or depletion
(Feng et al., 2011b).
Limitation of phosphate was also found to enhance
lipid accumulation in Ankistrodesmus falcutus and Monodus subterraneus (Kilham et al., 1997; Khozin-Goldberg
and Cohen, 2006). Rodolfi et al. (2009) screened 30
microalgal strains for lipid production, among which
the marine genus Nannochloropsis sp. F&M-M24
emerged as the best candidate for oil production (50%
under phosphorus deficiency against 31% control).
Sobczuk and Chisti (2010) observed an increase in intracellular lipid content up to 60% (dcw) against 27%
control in Choricystis minor under simultaneous nitrate
and phosphate deficiencies. In Scenedemus obliquus, lipid
accumulation up to 58% (dcw) was recorded when
subjected to simultaneous nitrate and phosphate limitations in presence of sodium thiosulphate (against 13%
under control condition, Mandal and Mallick, 2009).
Simultaneous nitrate, phosphate and iron limitations
have also been reported to stimulate lipid accumulation
in a microalga C. vulgaris (57% against 8% control,
Mallick et al., 2012).
In addition to nutrient limitations/deficiencies, other
stress conditions may also enhance lipid accumulation
in microalgae. Takagi et al. (2006) studied the effect of
NaCl on accumulation of lipids and triacylglycerides
in the marine microalga Dunaliella sp. Increase in initial
NaCl concentration from 0.5 M (seawater) to 1.0 M
resulted in a higher intracellular lipid accumulation
(71% dcw). Damiani et al. (2010) studied the effects of
continuous high light intensity (300 mmol photons/
m2 s) on lipid accumulation in Haematococcus pluvialis
grown under nitrogen-sufficient and nitrogen-deprived
conditions. A lipid yield of 33e35% was recorded under
the high light intensity as compared to 15% yield in control cultures. Nitrogen deprivation was, however, not
found to raise the lipid content of H. pluvialis cultures.
Nutrient limitations/deficiencies or physiological
stresses required for accumulation of lipids in microalgal cells is associated with reduced cell division
(Ratledge, 2002). The overall lipid productivity is therefore compromised due to the low biomass productivity.
For instance, Scragg et al. (2002) studied the energy
recovery from C. vulgaris and C. emersonii grown in complete Watanabe medium and also in low nitrogen
175
medium. The results showed that the low nitrogen
medium, although induced higher lipid accumulation
in both the test algae with high calorific values, the
overall energy recovery was lower in comparison to
Watanabe’s medium. A commonly suggested counter
measure is to use a two-stage cultivation strategy, dedicating the first stage for cell growth/division in nutrient
sufficient medium, and the second stage for lipid accumulation under nutrient starvation or other physiological stresses. To get maximal biomass and lipid yield,
CO2 can also be utilized. Chiu et al. (2009) reported an
increased accumulation of lipid (from 31% to 50%
dcw) in the stationary phase cultures of N. oculata
NCTU-3 grown under 2% CO2 aeration.
GENETIC ENGINEERING APPROACH
High oil-yielding transgenic microalgae could be a
promising source for biodiesel production. However,
the biotechnological processes based on transgenic
microalgae are still in infancy. In manipulation of genetically modified algae for high oil content, acetyl-CoA
carboxylase (ACCase) was first isolated from the diatom
Cyclotella cryptica by Roessler (1990), and then successfully transformed into the diatoms C. cryptica and
Navicula saprophila (Dunahay et al., 1995, 1996; Sheehan
et al., 1998). A plasmid was constructed that contained
acc1 gene driven by the cauliflower mosaic virus 35S
ribosomal gene promoter (CaMV35S) and the selectable
marker nptII from Escherichia coli. Introduction of
plasmids into the diatoms was mediated by microprojectile bombardment. The acc1 was overexpressed with
the enzyme activity enhanced by threefold. These experiments demonstrated that ACCase could be transformed
efficiently into microalgae, although no significant
increase in lipid accumulation was observed in the
transgenic diatoms (Dunahay et al., 1995, 1996).
Recently, diacylglycerol acyltransferases (DGATs)
homologous genes have been identified in the genome
of Chlamydomonas reinhardtii and were overexpressed
in the same microalga (Russa et al., 2012). This resulted
in an enhanced mRNA expression level of DGAT genes,
but did not boost the intracellular triacylglycerol (TAG)
synthesis. Thus, till date, there is no success story with
respect to lipid overproduction in microalgae using the
genetic engineering approach.
Extensive studies have also been carried out on
enhancement of lipid production using genetic engineering approaches in different bacterial and plant
species, which may provide valuable background for
future studies with microalgae. Some of these studies
are summarized in Table 11.2. The cytosolic ACCase
from Arabidopsis sp. was overexpressed in Brassica napus
(rapeseed) plastids. The fatty acid content of the
176
TABLE 11.2
11. MICROALGAE: THE TINY MICROBES WITH A BIG IMPACT
A List on Trials to Enhance Lipid Biosynthesis in Transgenic Organisms
Gene (Enzyme)
Source Species
Receiver Species
Result
References
acc1 (ACCase)
Cyclotella cryptica
Cyclotella cryptica
3-fold rise in ACCase activity,
no change in lipid content
Dunahay et al.
(1995, 1996)
Navicula saprophila
3-fold rise in ACCase activity,
no change in lipid content
acc1 (ACCase)
Arabidopsis sp.
Brassica napus
2-fold rise in plastid ACCase,
6% rise in fatty acid content
Roesler et al. (1997)
LPAT
Saccharomyces cerevisiae
Brassica napus
6-fold rise in oil content
Zou et al. (1997)
accA, accB, accC, accD
(ACCase)
E. coli
E. coli
6-fold rise in fatty acid synthesis
Davis et al. (2000)
are1 and are2 (DGAT)
Arabidopsis thaliana
Saccharomyces
cerevisiae
9-fold rise in TAG content
Bouvier-Nave et al.
(2000)
DGAT
Arabidopsis sp.
Arabidopsis sp.
70% rise in lipid content
Jako et al. (2001)
acc1 (ACCase)
Arabidopsis sp.
Solanum tuberosum
5-fold rise in TAG content
Klaus et al. (2004)
acs (ACS)
E. coil
E. coli
9-fold rise in ACS activity
Lin et al. (2006)
malEMt and malEMc
(malic enzyme, ME)
Mortierella alpina and
Mucor circinelloides
M. circinelloides
2.5-fold rise in lipid accumulation
Zhang et al. (2007)
fadD, ACCase,
thioesterase (TE)
E. coil
E. coli
20-fold rise in fatty acid synthesis
Lu et al. (2008)
wri1
Brassica napus
Arabidopsis thaliana
40% rise in oil content
Liu et al. (2010)
Acyl-ACP thioesterase
Diploknema butyracea,
Ricinus communis and
Jatropha curcas
E. coli
0.2e2.0 g/l free fatty acid yield
Zhang et al. (2011)
DGAT
Chlamydomonas reinhardtii
C. reinhardtii
29-Fold rise in mRNA level,
no change in TAG
Russa et al. (2012)
Source: Modified from Courchesne et al. (2009)
recombinant was 6% higher than that of the control
(Roesler et al., 1997). In prokaryotes like E. coli, overexpression of four ACCase subunits resulted in sixfold
rise in the rate of fatty acid synthesis (Davis et al.,
2000), confirming that the ACCase-catalyzed committing
step was indeed the rate-limiting step for fatty acid
biosynthesis in this strain. Nevertheless, Klaus et al.
(2004) achieved an increase in fatty acid synthesis and
a more than fivefold rise in the amount of TAG in Solanum tuberosum (potato) by overexpressing the ACCase
from Arabidopsis in the amyloplasts of potato tubers.
Transformation of rape seed with a putative sn-2-acyltransferase gene from Saccharomyces cerevisiae was carried out by Zou et al. (1997), leading to overexpression
of seed lysophosphatidate acid acyl-transferase (LPAT)
activity. This enzyme is involved in TAG formation
and its overexpression led to profound rise in oil content
from 8% to 48% on seed dry weight basis. However,
it was cautioned that the steady state level of diacylglycerol could be perturbed by an increase in LPAT
activity in the developing seeds. Transformations of
S. cerevisiae with the Arabidopsis DGAT were performed
by Bouvier-Nave et al. (2000). About 600-fold rise in
DGAT activity in the transformed S. cerevisiae was
observed, which led to a ninefold increase in TAG
accumulation. DGAT gene has also been overexpressed
in the plant Arabidopsis and it was shown that the oil
content was enhanced in correlation with the DGAT
activity (Jako et al., 2001). All these results suggest that
the reaction catalyzed by ACCase, LPAT and DGAT
are important rate-limiting steps in lipid biosynthesis.
A few enzymes that are not directly involved in lipid
metabolism have also been demonstrated to influence
the rate of lipid accumulation. For instance, it was
observed by Lin et al. (2006) that by overexpressing
the acs gene in E. coli, the acetyl-CoA synthase activity
was increased by ninefold, leading to a significant
increase in the assimilation of acetate from the medium,
which can contribute to lipid biosynthesis. The genes
coding for malic enzyme from Mucor circinelloides (malEMt) and from Mortierella alpina (malEMc), respectively,
were overexpressed in M. circinelloides which led to a
2.5-fold increase in lipid accumulation (Zhang et al.,
2007). Lu et al. (2008) reported a 20-fold enhancement
MICROALGAL BIODIESEL PRODUCTION
of fatty acid productivity of E. coli by combining four
targeted genotypic changes: deletion of the fadD gene
encoding the first enzyme in fatty acid degradation,
overexpression of the genes encoding the endogenous
ACCase, and overexpression of both an endogenous
thioesterase (TE) as well as a heterologous plant TE.
Overexpression of wri1 gene from B. napus in transgenic
Arabidopsis thaliana resulted into 40% increased seed oil
content (Liu et al., 2010). Zhang et al. (2011) studied
the effects of the overexpression of different acyl-ACP
TE genes from Diploknema butyracea, Ricinus communis
and J. curcas on free fatty acid contents of E. coli. The
strain carrying the acyl-ACP TE gene from D. butyracea
produced approximately 0.2 g/l of free fatty acid while
the strains carrying acyl-ACP TE genes from R. communis and J. curcas produced the free fatty acid at a high
level of more than 2.0 g/l.
MICROALGAL BIODIESEL PRODUCTION
Microalgal biodiesel production is relatively new and
not very well explored. Some reports are available,
where attempts have been made to produce biodiesel
from algae (Table 11.3). Miao and Wu (2006) reported
that lipid extracted from the heterotrophically grown
microalga, C. protothecoides, transformed into biodiesel
with a yield of 63% under 1:1 weight ratio of H2SO4 to
oil, and 56:1 molar ratio of methanol to oil at 30 C for
a reaction time of 4 h. Xu et al. (2006) characterized the
biodiesel obtained from the C. protothecoides oil by
acid-catalyzed transesterification. The most abundant
fatty acid methyl ester (FAME) in C. prothecoides biodiesel was methyl oleate (61% of total FAME) followed
by methyl linoleate (17%) and methyl palmitate (13%).
Subsequently, Li et al. (2007) showed that it was feasible
to grow C. protothecoides in a commercial-scale bioreactor. Using 75% immobilized lipase, these researchers
claimed w98% conversion could be obtained in 12 h
when the reaction condition with respect to solvent
type, water content and pH were optimized. Hossain
and Salleh (2008) studied biodiesel production from
Oedogonium and Spirogyra species using NaOH as catalyst. Algal oil and biodiesel production was higher in
Oedogonium sp. than in Spirogyra sp. Umdu et al. (2009)
studied the effects of Al2O3 supported CaO and MgO
catalysts in the transesterification of lipid of N. oculata.
These researchers found that pure CaO and MgO were
not active, and CaO-Al2O3 catalyst showed the highest
activity. Biodiesel yield was increased up to 98%
from 23% under CaO-Al2O3 catalyzed reaction when
methanol: lipid ratio was increased from 6:1 to 30:1.
Lipid extracted from N. oleoabundans was found to
have an adequate fatty acid profile and iodine value
according to the biodiesel specifications of European
177
Standards (EN, Gouveia et al. 2009). Converti et al.
(2009) analyzed the FAMEs in biodiesel produced from
N. oculata and C. vulgaris. The most abundant composition was methyl palmitate, which was 62% and 66%,
respectively, in N. oculata and C. vulgaris biodiesel.
However, the concentration of linolenic acid (18%) in
N. oculata could not meet the requirement of European
legislation for biodiesel. Johnson and Wen (2009)
prepared biodiesel from the microalga Schizochytrium
limacinum by direct transesterification of algal biomass.
Parameters such as free glycerol, total glycerol, acid
number, soap content, corrosiveness to copper, flash
point and viscosity met the American Society for Testing
and Materials (ASTM) and European standards, while
the water and sediment content, as well as the sulfur
content did not pass the standards. Damiani et al.
(2010) studied biodiesel production from H. pluvialis
using potassium hydroxide as the catalyst. The major
constituent of H. pluvialis biodiesel was palmitic acid followed by linoleic, oleic and linolenic acid methyl esters.
The iodine value was within the limit established by
European standards. Chinnasamy et al. (2010) produced
biodiesel by a two-step transesterification process
(acid-catalyzed followed by base-catalyzed) from a consortium of 15 native algae cultivated in carpet industry
wastewater. Algal methyl esters were predominated by
linolenic, linoleic, palmitic and oleic acids. The biodiesel
was found to contain 0.0155% and 0.0001% bound and
free glycerin, respectively, and met the ASTM and European standard specifications.
Patil et al. (2012) optimized the direct conversion of
wet Nannochlopsis sp. biomass to biodiesel under supercritical methanol treatment, without using any catalyst.
In the supercritical state, at high pressure and temperature, the methanol molecules enabled simultaneous
extraction and transesterification of lipids in wet
algal biomass. The abundant FAME in Nannochlopsis
sp. biodiesel was methyl oleate (37%) followed by
methyl palmitolate (32%) and methyl palmitate (8%).
Velasquez-Orta et al. (2012) compared in situ transesterification of C. vulgaris with acid as well as alkaline catalysts, in which the oil extraction step was eliminated.
FAME yield reached a maximum of 77.6% after
45 min using a catalyst (NaOH) ratio of 0.15:1 and solvent ratio of 600:1 at 60 C under constant stirring
rate of 380 rpm. However, with sulfuric acid as catalyst
FAME yield reached up to 96.9% with catalyst : oil
ratio of 0.35:1 for a reaction time of 20 h. Recently,
Mallick et al. (2012) characterized the biodiesel obtained
from the C. vulgaris oil by acid-catalyzed transesterification. The fuel properties (density, viscosity, acid value,
iodine value, calorific value, cetane index, ash and
water contents) of C. vulgaris biodiesel are comparable
with the international (ASTM and EN) and Indian
standards (IS).
TABLE 11.3
Attempts on Biodiesel Production from Microalgae
Major Ester
Physical Property
References
Chlorella protothecoides
H2SO4-catalyzed (63%)
NC
Density: 0.86 kg/l, viscosity: 5.2 cSt, flash
point: 115 C, acid value: 0.37 mg KOH/g,
heating value: 41 MJ/kg
Miao and Wu (2006)
H2SO4-catalyzed (63%)
Methyl oleate: 61%, methyl linoleate:
17%, methyl palmitate: 13%
Density: 0.86 kg/ l, viscosity: 5.2 cSt, flash
point: 115 C, solidifying point: 12 C, acid
value: 0.37 mg KOH/g
Xu et al. (2006)
Lipase-catalyzed (98%)
Methyl oleate: 65%, methyl linoleate:
18%, methyl palmitate: 10%
NC
Li et al. (2007)
Oedogonium sp.
NaOH-catalyzed (95%)
NC
NC
Hossain and Salleh (2008)
Spirogyra sp.
NaOH-catalyzed (93%)
Nannochloropsis oculata
Heterogeneous catalyst (Al2O3supported CaO & MgO) (98%)
NC
NC
Umdu et al. (2009)
Neochloris oleoabundans
BF3-catalyzed (NR)
Methyl oleate: 38%, methyl palmitate:
17%, methyl stearate: 14%, methyl
linolenate: 8%
Iodine value: 72 g I2/100 g
Gouveia et al. (2009)
Nannochloropsis oculata
Acid-catalyzed (NR)
Methyl palmitate: 62%, methyl
linolenate: 18%, methyl linoleate: 12%,
methyl oleate: 6%
NC
Converti et al. (2009)
Chlorella vulgaris
Methyl palmitate: 66%, methyl
linolenate: 12%, methyl linoleate: 11%,
methyl oleate: 7%
Schizochytrium limacinum
H2SO4-catalyzed (66%)
Methyl palmitate: 57%, methyl
ester of C22: 6:30%
Viscosity: 3.87 cSt, flash point: 204 C,
moisture content: 0.11%, acid value: 0.11 mg
KOH/g, total glycerin: 0.097%, free glycerin:
0.003%,
Johnson and Wen (2009)
Haematococcus pluvialis
KOH-catalyzed (NR)
Methyl palmitate: 23%, methyl
linoleate: 20%, methyl oleate: 19%,
methyl linolenate: 16%
Iodine value: 111 g I2/100 g
Damiani et al. (2010)
A consortium of 15
native microalgae
Acid-catalyzed followed
by base-catalyzed (64%)
Methyl linolenate: 28%, methyl
linoleate: 20%, methyl palmitate:
16%, methyl oleate: 12%
Bound glycerin: 0.0155%, free glycerin:
0.0001%
Chinnasamy et al. (2010)
Nannochloropsis sp.
Supercritical methanol
Methyl oleate: 37%, methyl palmitoleate:
23%, methyl palmitate: 8%
NC
Patil et al. (2012)
Chlorella vulgaris
Alkaline in situ (78%)
Methyl linolenate: 22%, methyl oleate:
21%, methyl stearate: 11%
NC
Velasquez-Orta et al. (2012)
Chlorella vulgaris
HCl-catalyzed (NR)
Methyl palmitate: 62%, methyl oleate:
20%, methyl linoleate: 10%
Density: 0.88 kg/l, viscosity: 4.5 cSt, calorific
value: 38.4 MJ/kg, iodine value: 56.2 g I2/
100 g, acid value: 0.6 mg KOH/g, cetane
index: 54.7, ash content: 0.01%, water
content: 0.03%
Mallick et al. (2012)
NR, not reported; NC, not characterized.
11. MICROALGAE: THE TINY MICROBES WITH A BIG IMPACT
Name of the Alga
178
Properties of Biodiesel
Transesterification Process
with % Conversion
WASTE UTILIZATION FOR BIODIESEL PRODUCTION: A CASE STUDY WITH SCENEDESMUS OBLIQUUS
FATTY ACID METHYL ESTERS AND
FUEL PROPERTIES
As stated before, biodiesel is the best substitute for petrodiesel due to its fuel properties, which are very close to
those of diesel. Diesel is a mixture of C15 to C18 hydrocarbons obtained from crude oil in the distillation range of
250e350 C. It contains only carbon and hydrogen atoms,
which are arranged in straight or branched chain structures, as well as aromatic configurations. Diesel may
contain both saturated and unsaturated hydrocarbons.
Biodiesel rather has a different chemical structure than
the conventional diesel fuel. It is monoalkyl esters of
long-chain fatty acids derived from various types of vegetable oils. The fatty acids are of C12 to C24, with over 90%
of them being between C16 and C18.
Fuel properties of biodiesel that are influenced by the
fatty acid profile and, in turn, by the structural features
of various fatty acid esters is CN, which ultimately affects the exhaust emission, heat of combustion, cold
flow, oxidative stability, viscosity, and lubricity. Structural features of a fatty acid ester molecule that influence
the physical and fuel properties are chain length and
degree of unsaturation (Knothe, 2005). Since biodiesel
is produced in quite differently scaled plants from vegetable oils of varying origin and quality, it is necessary to
install a standardization of fuel quality to guarantee
engine performance without any difficulties.
CN is widely used as diesel fuel quality parameter
related to the ignition delay time and combustion quality. The higher the CN is the better are the ignition properties (Meher et al., 2006). High CNs ensure good cold
start properties and minimize the formation of white
smoke. The longer the fatty acid carbon chains and the
more saturated the molecules are, the higher are the
CNs (Bajpai and Tyagi, 2006). According to Knothe
and Dunn. (2003), high CNs are observed for esters of
saturated fatty acids such as palmitic and stearic acids.
The oxidation stability decreased with increase in the
contents of polyunsaturated FAMEs (Ramos et al., 2009).
The limitation of unsaturated fatty acids is also necessary due to the fact that heating of higher unsaturated
fatty acids results in polymerization of glycerides. This
can lead to the formation of heavy deposits in the machines (Mittelbach, 1996).
One of the major problems associated with the use of
biodiesel is its poor cold flow property, indicated by relatively high cloud point and pour point. Saturated fatty
acids have significantly higher melting points and
crystallize even at room temperature. Thus biodiesel
produced from the sources with high amounts of saturated fats would show higher cloud points and pour
points. Viscosity also increases with the increasing
degree of saturation and chain length (Knothe, 2005).
Unsaturated fatty acids exhibit better lubricity than
179
saturated ones (Kenesey and Ecker, 2003). Heat of combustion increases with the chain length and decreases
with unsaturation (Goering et al., 1982). The increase
in heat content results from a gross increase in number
of carbon and hydrogen as well as increase in the ratio
of these elements relative to oxygen. Therefore no single
fatty acid could fulfill every fuel properties. Rather, a
very good compromise can be reached by considering
a fuel rich in the monounsaturated fatty acids, such as
oleate or palmitoleate, and low in both saturated and
polyunsaturated fatty acids (Durrett et al., 2008).
WASTE UTILIZATION FOR BIODIESEL
PRODUCTION: A CASE STUDY
WITH SCENEDESMUS OBLIQUUS
IN A RECIRCULATORY
AQUACULTURE SYSTEM
Nowadays, waste disposal is a worldwide problem. In
agricultural countries like India, waste discharges from
agriculture, agrobased industries and city sewages are
the main sources of water pollution. Conventional wastewater treatment systems do not seem to be the definitive
solution to pollution and eutrophication problems. The
major drawbacks are cost and lack of nutrient recycling
(Eisenberg et al., 1981). Secondary sewage treatment
plants are specifically designed to control the quantity of
organic compounds in wastewaters. Other pollutants
including nitrogen and phosphorus are only slightly
affected by this type of treatment (Gates and Borchardt,
1964). Owing to the ability to use nitrogen and phosphorus
for growth, algae can successfully be cultivated in such
type of wastewaters (Mallick, 2002). This has been evolved
from the early work of Oswald (Oswald et al., 1953) using
microalgae in tertiary treatment of municipal wastewaters. The widely used microalgae cultures for nutrient
removal are Chlorella (González et al., 1997; Lee and Lee,
2001), Scenedesmus (Martinez et al., 1999, 2000) and Spirulina (Olguı́n et al., 2003). Nutrient removal efficiency of
Nannochloris sp. (Jimenez-Perez et al., 2004), B. brauinii
(An et al., 2003), and Phormidium sp. (Dumas et al., 1998;
Laliberte et al., 1997) has also been investigated. One of
the well-known algae-based bioprocesses for wastewater
treatment is high-rate algal ponds (Cromar et al., 1996;
Deviller et al., 2004). Recently, corrugated raceways
(Craggs et al., 1997; Olguı́n et al., 2003), triangular
photobioreactors (Dumas et al., 1998), and tubular
photobioreactors (Briassoulis et al., 2010; Molina et al.,
2000) have been developed for nutrient removal.
Among agroindustries, a large quantity of wastewater is generated from intensified aquaculture practices. The main source of potentially polluting waste in
fish culture is feed derived, mainly unconsumed and
undigested feed and fish excreta. Discharging these
180
11. MICROALGAE: THE TINY MICROBES WITH A BIG IMPACT
effluents directly into water resources causes eutrophication of the receiving waters. Qian et al. (1996) reported
the collapse of a prawn industry in China due to
outbreak of pathogenic bacteria caused by high nutrient
load. A few studies have shown the efficiency of algae
biofilters in removing nitrogen from fish effluents
(Cohen and Neori, 1991; Jimenez del Rio et al., 1996;
Schuenhoff et al., 2003). These works are based on the
use of seaweeds of the genera Ulva and Gracilaria to treat
effluent water from aquaculture.
Recently, we intend to explore an integrated approach
to produce biodiesel with simultaneous waste recycling
by a green microalga S. obliquus with three types of
wastes, viz. poultry litter (PL), fish pond discharge
(FPD), and municipal secondary settling tank discharge.
Our initial trial under laboratory batch culture conditions (Mandal and Mallick, 2011) encouraged us to
conduct a small-scale field experiment in a recirculatory
aquaculture system (RAS) using FPD and PL with the
same microalga (Mandal and Mallick, 2012). Figure 11.1
presents a schematic diagram of RAS, developed at
Agricultural and Food Engineering Department, Indian
Institute of Technology Kharagpur, West Bengal, India.
The effluent from a fish pond was pumped into a settling
tank for removal of large solids. After 24 h, the supernatant was siphoned to an inclined plate settler for
removal of fine solids. To have a clear picture of the
inclined plate settler readers are requested to refer
Sarkar et al. (2007). The effluent was then entered into
fiber-reinforced plastic tanks (length 125 cm, breadth
60 cm, depth 45 cm) for culturing the test microalga.
FPD has a very high load of solid particles in suspension, which contributes to increase in turbidity. Experiments carried on with sedimented and nonsedimented
FPD showed that the nutrient removal efficiency of S.
obliquus was higher in the sedimented one. Further experiments with sedimented FPD demonstrated that
biomass and lipid yield was maximum at 15 cm culture
depth with stirring. In seasonal variation study, the
maximum algal biomass and lipid productivity was
recorded during summer when sunshine hour was relatively large. During the summer season, when S. obliquus
cultures pregrown in FPD supplemented with 5 g PL/l
were transferred to the optimized conditions to maximize the lipid accumulation (to have details on optimized condition readers are requested to refer Mandal
and Mallick, 2009), lipid yield was raised by more than
sixfold (up to 780 mg/l, Mandal and Mallick, 2012). During rainy and winter seasons, comparable lipid yield
was recorded by providing artificial lights for few hours.
Thus an areal lipid productivity of 14,000 l/ha year
(approximately) has been projected assuming 11 cultivation cycles per year, leaving the rest of the period for
cleaning and maintenance of the system (Mandal and
FP : fish pond
CP : centrifugal pump
ST : settling tank
IPS : inclined plate settler
ACT : algae culture tank
RWT : remediated water tank
ST
RWT
FIGURE 11.1
Diagrammatic representation of recirculatory aquaculture system (RAS).
REFERENCES
Mallick, 2012). Nevertheless, this value is w8 times
higher than that of Jatropha, one of the most acclaimed
energy crops (Khan et al., 2009).
181
a project (Studies on Microalgal Triacylglycerols as a Source of Biodiesel)
to continue research efforts in this exciting and imminent field.
References
CONCLUDING REMARKS
Chisti (2007) envisioned a lipid productivity of
58,700e136,900 l/ha year, considering lipid content of
30e70% of dry biomass. However, to attain this,
increasing the volumetric and areal production rates
should be the focus (Grobbelaar, 2012). Grobbelaar
(2009) projected the upper limits of biomass productivity
of about 200 g (dcw)/m2 day. Six decades of worldwide
research on outdoor mass cultivation of microalgae, however, have demonstrated only a diminutive fraction of
this, where the highest value being recorded was 30 g
(dcw)/m2 day (Lee, 2001). As opined by Grobbelaar
(2012), with the available strains, an average long-term
rate close to 50 g (dcw)/m2 day could be attainable by
optimizing various conditions, such as culture depth,
mixing, nutrients and CO2 supply, temperature and light,
and controlling predators, pathogens and alien microalgal invasion in open raceways. This equates to an annual
productivity of about 150 tons of algal biomass per hectare. Thus, maximum lipid productivity would vary
between 50,000 and 84,000 l/ha year, considering lipid
content of 30e50% of dry biomass.
For the last few years, there has been a worldwide
impetus to achieve commercial-scale production of biodiesel from microalgae. In October 28, 2010, US Naval
base in Norfolk, Virginia, completed a successful test
by running a 15 m gunboat with 50:50 mix of algaebased fuel and diesel. However, the cost of this mix
was $112 for liter. In March 2012, the US Navy put
another milestone by sailing a fleet ship w1200 miles
on “Soladiesel”, an algae-based fuel blend. Recently, researchers at Brookhaven National Laboratory, USA,
have announced the development of a new process
that could significantly lower the cost. Nevertheless,
cost of producing microalgal biodiesel can be reduced
further by using a biorefinery-based production strategy, like a petroleum refinery, where each and every
component is used to produce valuable products. There
is much to be researched in this exciting and upcoming
field, and the only thing we can say for certain is that the
best method/technology of biofuel production will survive and rise above the others. It is our job as researchers to find this, and be proud to be a part of this
endeavor.
Acknowledgments
Nirupama Mallick is thankful to NFBSFARA, Indian Council of Agricultural Research, New Delhi, India, for financial support in the form of
Alcantara, R., Amores, J., Canoira, L., Fidalgo, E., Franco, M.J.,
Navarro, A., 2000. Catalytic production of biodiesel from soybean
oil, used frying oil and tallow. Biomass Bioenergy 18, 515e527.
An, J.Y., Sim, S.J., Lee, J.S., Kim, B.K., 2003. Hydrocarbon production
from secondarily treated piggery wastewater by the green algae
Botryococcus braunii. J. Appl. Phycol. 15, 185e191.
Aresta, M., Dibenedetto, A., Carone, M., Colonna, T., Fagale, C., 2005.
Production of biodiesel from macroalgae by supercritical CO2
extraction and thermochemical liquifaction. Environ. Chem. Lett.
3, 136e139.
Bajpai, D., Tyagi, V.K., 2006. Biodiesel: source, production, composition, properties and its benefits. J. Oleo Sci. 55, 487e502.
Behrens, P.W., Kyle, D.J., 1996. Microalgae as a source of fatty acids.
J. Food Lipids 3, 259e272.
Bhattacharyya, S., Reddy, C.S., 1994. Vegetable oils as fuel for internal
combustion engine: a review. J. Agric. Eng. Res. 57, 157e166.
Bouvier-Nave, P., Benveniste, P., Oelkers, P., Sturley, S.L., Schaller, H.,
2000. Expression in yeast and tobacco of plant cDNAs encoding
acyl CoA: diacylglycerol acyltransferase. Eur. J. Biochem. 267,
85e96.
Briassoulis, D., Panagakis, P., Chionidis, M., Tzenos, D., Lalos, A.,
Tsinos, C., Berberidis, K., Jacobsen, A., 2010. An experimental
helical-tubular photobioreactor for continuous production of
Nannochloropsis sp. Bioresour. Technol. 101, 6768e6777.
Brown, A.C., Knights, B.A., Conway, E., 1969. Hydrocarbon content
and its relationship to physiological state in the green alga
Botryococcus braunii. Phytochemicals 8, 543e547.
Brown, L., Zeiler, K.G., 1993. Aquatic biomass and carbon dioxide
trapping. Energy Convers. Manage. 34, 1005e1013.
Campbell, M.N., 2008. Biodiesel: algae as a renewable source for
liquid fuel. Guelph Eng. J. 1, 1916-1107.
Canakci, M., Gerpan, J.V., 1999. Biodiesel production via acid catalysis.
Trans. ASAE 42, 1203e1210.
Chinnasamy, S., Bhatnagar, A., Hunt, R.W., Das, D.C., 2010. Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour. Technol. 101, 3097e3105.
Chisti, Y., 2008. Biodiesel from microalgae beats bioethanol. Trends
Biotechnol. 26, 126e131.
Chisti, Y., 2007. Biodiesel from microalgae. Biotechnol. Adv. 25,
294e306.
Chiu, S.Y., Kao, C.Y., Tsai, M.T., Ong, S.C., Chen, C.H., Lin, C.S., 2009.
Lipid accumulation and CO2 utilization of Nannochloropsis oculata
in response to CO2 aeration. Bioresour. Technol. 100, 833e838.
Cohen, I., Neori, A., 1991. Ulva lactuca biofilters for marine fishpond
effluents: 1. ammonia uptake kinetics and nitrogen content. Bot.
Mar. 34, 475e482.
Converti, A., Casazza, A.A., Ortiz, E.Y., Perego, P., Del Borghi, M.,
2009. Effect of temperature and nitrogen concentration on the
growth and lipid content of Nannochloropsis oculata and Chlorella
vulgaris for biodiesel production. Chem. Eng. Process 48,
1146e1151.
Courchesne, N.M.D., Parisien, A., Wang, B., Lan, C.Q., 2009.
Enhancement of lipid production using biochemical, genetic and
transcription factor engineering approaches. J. Biotechnol. 141,
31e41.
Craggs, R.J., McAuley, P.J., Smith, V.J., 1997. Wastewater nutrient
removal by marine microalgae grown on corrugated raceway.
Water Res. 31, 1701e1707.
Cromar, N.J., Fallowfield, H.J., Martin, N.J., 1996. Influence of environmental parameters on biomass production and nutrient
182
11. MICROALGAE: THE TINY MICROBES WITH A BIG IMPACT
removal in a high rate algal pond operated by continuous culture.
Water Sci. Technol. 34, 133e140.
Damiani, M.C., Cecilia, A.P., Diana, C., Patricia, I.L., 2010. Lipid
analysis in Haematococcus pluvialis to assess its potential use as a
biodiesel feedstock. Bioresour. Technol. 101, 3801e3807.
Davis, M.S., Solbiati, J., Cronan, J.E., 2000. Overproduction of acetylCoA carboxylase activity increases the rate of fatty acid biosynthesis in Escherichia coli. J. Biol. Chem. 275, 28593e28598.
Dayananda, C., Sarada, R., Usha Rani, M., Shamala, T.R.,
Ravishankar, G.A., 2007. Autotrophic cultivation of Botryococcus
braunii for the production of hydrocarbons and exopolysaccharides
in various media. Biomass Bioenergy 31, 87e93.
Deviller, G., Aliaume, C., Nava, M.A.F., Casellas, C., Blancheton, J.P.,
2004. High-rate algal pond treatment for water reuse in an integrated marine fish recirculating system: effect on water quality and
sea bass growth. Aquaculture 235, 331e344.
Dorado, M.P., Ballesteros, E., Almeida, J.A., Schellert, C.,
Lohrlein, H.P., Krause, R., 2002. An alkali-catalyzed transesterification process for high free fatty acid waste oils. Trans.
ASAE 45, 525e529.
Dumas, A., Laliberté, G., Lessard, P., de la Noüe, J., 1998. Biotreatment
of fish farm effluents using the cyanobacterium Phormidium
bohneri. Aqua. Eng. 17, 57e68.
Dunahay, T.G., Jarvis, E.E., Roessler, P.G., 1995. Genetic transformation
of the diatoms Cyclotella cryptica and Navicula saprophila. J. Phycol.
31, 1004e1012.
Dunahay, T.G., Jarvis, E.E., Dais, S.S., Roessler, P.G., 1996. Manipulation of microalgal lipid production using genetic engineering.
Appl. Biochem. Biotechnol. 57, 223e231.
Durrett, T., Benning, C., Ohlrogge, J., 2008. Plant triacylglycerols as
feedstocks for the production of biofuels. Plant J. 54, 593e607.
Eisenberg, M., Koopman, B., Benemann, J.R., Oswald, W.L., 1981.
Algal bioflocculation and energy conservation in microalgal
sewage ponds. Biotechnol. Bioeng. Symp. 11, 429e448.
Feng, D., Chen, Z., Xue, S., Zhang, W., 2011b. Increased lipid
production of the marine oleaginous microalgae Isochrysis zhangjiangensis (Chrysophyta) by nitrogen supplement. Bioresour.
Technol. 102, 6710e6716.
Feng, P., Deng, Z., Hu, Z., Fan, L., 2011a. Lipid accumulation and
growth of Chlorella zofingiensis in flat plate photobioreactors
outdoors. Bioresour. Technol. 102, 10577e10584.
Francis, G., Becker, K., 2002. Biodiesel from Jatropha plantations on
degraded land. University of Hohenheim, Stuttgart, Germany p 9.
http://www.youmanitas.nl/pdf/Bio-diesel.pdf.
Gao, C., Zhai, Y., Ding, Y., Wu, Q., 2010. Application of sweet sorghum
for biodiesel production by heterotrophic microalga Chlorella
protothecoides. Appl. Energy 87, 756e761.
Gates, W.E., Borchardt, J.A., 1964. Nitrogen and phosphorus extraction
from domestic waste water treatment plant effluents by controlled
algal culture. Res. J. Water Pollut. Control Fed. 36, 443e462.
Ghadge, S.V., Raheman, H., 2006. Process optimization for biodiesel
production from mahua (Madhuca indica) oil using response
surface methodology. Bioresour. Technol. 97, 379e384.
Goering, C.E., Schwab, A.W., Daugherty, M.J., Pryde, E.H.,
Heakin, A.J., 1982. Fuel properties of eleven vegetable oils. Trans.
ASAE 25, 1472e1477.
González, L.E., Cañizaresb, R.O., Baenaa, S., 1997. Efficiency of
ammonia and phosphorus removal from a colombian agroindustrial wastewater by the microalgae Chlorella vulgaris and
Scenedesmus dimorphus. Bioresour. Technol. 60, 259e262.
Gouveia, L., Oliveira, A.C., 2009. Microalgae as a raw material for
biofuels production. J. Ind. Microbiol. Biotechnol. 36, 269e274.
Gouveia, L., Marques, A.E., da Silva, T.L., Reis, A., 2009. Neochloris
oleoabundans UTEX #1185: a suitable renewable lipid source for
biofuel production. J. Ind. Microbiol. Biotechnol. 36, 821e826.
Grobbelaar, J.U., 2009. Upper limits of photosynthetic productivity
and problems of scaling. J. Appl. Phycol. 21, 519e522.
Grobbelaar, J.U., 2012. Microalgae mass culture: the constraints of
scaling-up. J. Appl. Phycol. 24, 315e318.
Haag, A.L., 2007. Algae bloom again. Nature 447, 520e521.
Hankamer, B., Lehr, F., Rupprecht, J., Mussgnug, J.H., Posten, C.,
Kruse, O., 2007. Photosynthetic biomass and H2 production by
green algae: from bioengineering to bioreactor scale-up. Physiol.
Plant. 131, 10e21.
Hossain, A.B.M.S., Salleh, A., 2008. Biodiesel fuel production from
algae as renewable energy. Am. J. Biochem. Biotechnol. 4, 250e254.
Illman, A.M., Scragg, A.H., Shales, S.W., 2000. Increase in Chlorella
strains calorific values when grown in low nitrogen medium.
Enzyme Microb. Technol. 27, 631e635.
International Energy Agency, 2007. World Energy Outlook. Executive
Summary, China and India insight. Paris, France, pp.14.
Jain, S., Sharma, M.P., 2010. Prospects of biodiesel from Jatropha in
India: a review. Renewable Sustainable Energy Rev. 14, 763e771.
Jako, C., Kumar, A., Wei, Y., Zou, J., Barton, D.L., Giblin, E.M.,
Covello, P.S., Taylor, D.C., 2001. Seed-specific over-expression of an
Arabidopsis cDNA encoding a diacylglycerol acyltransferase
enhances seed oil content and seed weight. Plant Physiol. 126,
861e874.
Jiang, Y., Yoshida, T., Quigg, A., 2012. Photosynthetic performance,
lipid production and biomass composition in response to nitrogen
limitation in marine microalgae. Plant Physiol. Biochem. 54, 70e77.
Jimenez del Rio, M., Ramazanov, Z., Garcia-Reina, G., 1996. Ulva rigida
(Ulvales, Chlorophyta) tank culture as biofilters for dissolved
inorganic nitrogen from fishpond effluents. Hydrobiologia 326,
61e66.
Jimenez-Perez, M.V., Sanches-Castillo, P., Romera, O., FernandezMoreno, D., Perez-Martinez, C., 2004. Growth and nutrient
removal in free and immobilized planktonic green algae isolated
from pig manure. Enzyme Microb. Technol. 34, 392e398.
Johnson, M.B., Wen, Z., 2009. Production of biodiesel fuel from the
microalga Schizochytrium limacinum by direct transesterification of
algal biomass. Energy Fuels 23, 5179e5183.
Kenesey, E., Ecker, A., 2003. Oxygen bond to improve the lubricity of
fuel. Tribol. Schmierungstech. 50, 21e26.
Khan, S.A., Rashmi, M.Z., Hussain, S., Prasad, Banerjee, U.C., 2009.
Prospects of biodiesel production from microalgae in India.
Renewable Sustainable Energy Rev. 13, 2361e2372.
Khozin-Goldberg, I., Cohen, Z., 2006. The effect of phosphate starvation
on the lipid and fatty acid composition of the fresh water eustigmatophyte Monodus subterraneus. Phytochemicals 67, 696e701.
Kilham, S.S., Kreeger, D.A., Gulden, C.E., Lynn, S.G., 1997. Effects
of nutrients limitation on biochemical constituents of Ankistrodesmus falcatus. Freshwater Biol. 38, 591e596.
Klaus, D., Ohlrogge, J.B., Neuhaus, H.E., Dormann, P., 2004. Increased
fatty acid production in potato by engineering of acetyl-CoA
carboxylase. Planta 219, 389e396.
Knothe, G., 2005. Dependence of biodiesel fuel properties on the structure
of fatty acid alkyl esters. Fuel Process. Technol. 86, 1059e1070.
Knothe, G., Dunn, R.O., 2003. Oxidative stability of biodiesel/jet fuel
blends by oil stability index (OSI) analysis. J. Am. Oil Chem. Soc.
80, 1047e1048.
Krawczyk, T., 1996. Biodiesel alternative fuel makes inroads but
hurdles remain. Inform 7, 801e829.
Kumar, A., Kumar, K., Kaushik, N., Sharma, S., Mishra, S., 2010.
Renewable energy in India: current status and future potentials.
Renewable Sustainable Energy Rev. 14, 2434e2442.
Laliberte, G., Lessard, P., de la Noüe, J., Sylvestre, S., 1997. Effect of
phosphorus addition on nutrient removal from wastewater with
the cyanobacterium Phormidium bohneri. Bioresour. Technol. 59,
227e233.
REFERENCES
Lang, X., Dalai, A.K., Bakhshi, N.N., Reany, M.J., Hertz, P.B., 2001.
Preparation and characterization of biodiesel from various bio-oils.
Bioresour. Technol. 80, 53e62.
Lee, Y.-K., 2001. Microalgal mass culture systems and methods: their
limitation and potential. J. Appl. Phycol. 13, 307e315.
Lee, K., Lee, C.G., 2001. Effect of light/dark cycles on wastewater
treatments by microalgae. Biotechnol. Bioprocess Eng. 6, 194e199.
Li, X., Xu, H., Wu, Q., 2007. Large-scale biodiesel production from
microalga Chlorella protothecoides through heterotrophic cultivation
in bioreactors. Biotechnol. Bioeng. 98, 764e771.
Li, Y., Horsman, M., Wu, N., Lan, C.Q., Dubois-Calero, N., 2008.
Biofuels from microalgae. Biotechnol. Prog. 24, 815e820.
Lin, H., Castro, N.M., Bennett, G.N., San, S.Y., 2006. Acetyl-CoA
synthetase overexpression in Escherichia coli demonstrates more
efficient acetate assimilation and lower acetate accumulation: a
potential tool in metabolic engineering. Appl. Microbiol.
Biotechnol. 71, 870e874.
Liu, J., Hua, W., Zhan, G., Wei, F., Wang, X., Liu, G., Wang, H., 2010.
Increasing seed mass and oil content in transgenic Arabidopsis by
the overexpression of wri1-like gene from Brassica napus. Plant
Physiol. Biochem. 48, 9e15.
Lu, X., Vora, H., Khosla, S., 2008. Over production of free fatty acids in
E. coli: implications for biodiesel production. Metab. Eng. 10,
333e339.
Mallick, N., 2002. Biotechnological potential of immobilized algae for
wastewater N, P and metal removal: a review. BioMetals 15,
377e390.
Mallick, N., Mandal, S., Singh, A.K., Bishai, M., Dash, A., 2012. Green
microalga Chlorella vulgaris as a potential feedstock for biodiesel.
J. Chem. Technol. Biotechnol. 87, 137e145.
Mandal, S., Mallick, N., 2009. Microalga Scenedesmus obliquus as a
potential source for biodiesel production. Appl. Microbiol.
Biotechnol. 84, 281e291.
Mandal, S., Mallick, N., 2011. Waste utilization and biodiesel production by the green microalga Scenedesmus obliquus. Appl. Environ. Microbiol. 77, 374e377.
Mandal, S., Mallick, N., 2012. Biodiesel production by the green
microalga Scenedesmus obliquus in a recirculatory aquaculture
system. Appl. Environ. Microbiol. 78, 5929e5934.
Marsh, G., 2009. Small wonders: biomass from algae. Renewable
Energy Focus 9, 74e78.
Martinez, M.E., Castillo, J.M., El Yousfi, F., 1999. Photoautotrophic
consumption of phosphorus by Scenedesmus obliquus in a continuous
culture. Influence of light intensity. Process Biochem. 34, 811e818.
Martinez, M.E., Sanchez, S., Jimenez, J.M., El Yousfi, F., Munoz, L.,
2000. Nitrogen and phosphorus removal from urban wastewater
by the microalga Scenedesmus obliquus. Bioresour. Technol. 73,
263e272.
McMillen, S., Shaw, P., Jolly, N., Goulding, B., Finkle, V., 2005.
Biodiesel: Fuel for Thought, Fuel for Connecticut’s Future.
Connecticut Center for Economic Analysis Report. University of
Connecticut, pp. 56.
Meher, L.C., Vidya Sagar, D., Naik, S.N., 2006. Technical aspects of
biodiesel production by transesterification - a review. Renewable
Sustainable Energy Rev. 10, 248e268.
Miao, X., Wu, Q., 2004. High yield bio-oil production from fast
pyrolysis by metabolic controlling of Chlorella protothecoides.
J. Biotechnol. 110, 85e93.
Miao, X., Wu, Q., 2006. Biodiesel production from heterotrophic
microalgal oil. Bioresour. Technol. 97, 841e846.
Mittelbach, M., 1990. Lipase-catalyzed alcoholysis of sunflower oil.
J. Am. Oil Chem. Soc. 67, 168e170.
Mittelbach, M., 1996. Diesel fuel derived from vegetable oils, VI:
specifications and quality control of biodiesel. Bioresour. Technol.
27, 435e437.
183
Molina, E., Fernández, A.F.G., Camacho, G.F., Rubio, C.F., Chisti, Y.,
2000. Scale-up of tubular photobioreactors. J. Appl. Phycol. 12,
355e368.
Olguı́n, E.J., Galicia, S., Mercado, G., Perez, T., 2003. Annual productivity of Spirulina (Arthrospira) and nutrient removal in a pig
wastewater recycle process under tropical conditions. J. Appl.
Phycol. 15, 249e257.
Oswald, W.J., Gotaas, H.B., Ludwig, H.F., Lynch, W., 1953. Algae
symbiosis in oxidation ponds: III. phototosynthetic oxygenation.
Sewage Ind. Wastes 25, 692e704.
Patil, P.D., Gude, V.G., Mannarswamy, A., Deng, S., Cooke, P.,
Munson-McGee, S., Rhodes, I., Lammers, P., Nirmalakhandan, N.,
2012. Optimization of direct conversion of wet algae to biodiesel
under supercritical methanol conditions. Bioresour. Technol. 101,
118e122.
Piorreck, M., Pohl, P., 1984. Formation of biomass, total protein,
chlorophylls, lipids and fatty acids in green and blue-green algae
during one growth phase. Phytochemicals 23, 217e223.
Planning Commission, 2003. Report of the Committee on Development of Biofuel. Government of India, New Delhi, India, pp. 140.
Planning Commission, 2007. Eleventh Five Year Plan 2007e2012, Energy Sector. Government of India, New Delhi, India, pp. 65.
Qian, P., Wu, C.Y., Wu, M., Xie, Y.K., 1996. Integrated cultivation of the
red alga Kappaphycus alvarezii and the pearl oyster Pinctada
martensi. Aquaculture 147, 21e35.
Ramos, M.J., Fernández, C.M., Casas, A., Rodrı́guez, L., Pérez, Á.,
2009. Influence of fatty acid composition of raw materials on
biodiesel properties. Bioresour. Technol. 100, 261e268.
Rao, P.P., Gopalkrishnan, K.V., 1991. Vegetable oils and their methyl
esters as fuels diesel engines. Indian J. Technol. 29, 292e297.
Ratledge, C., 2002. Regulation of lipid accumulation in oleaginous
micro-organisms. Biochem. Soc. Trans. 30, 1047e1050.
Rattanapoltee,
P.,
Chulalaksananukul,
W.,
James,
A.E.,
Kaewkannetra, P., 2008. Comparison of autotrophic and heterotrophic cultivations of microalgae as a raw material for biodiesel
production. J. Biotechnol. 136, S412.
Rodolfi, L., Zittelli, G., Bassi, N., Padovani, G., Bonini, N., Bonini, G.,
Tredici, M.R., 2009. Microalgae for oil: strain selection, induction of
lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 102, 100e112.
Roesler, K., Shintani, D., Savage, L., Boddupalli, S., Ohlrogge, J., 1997.
Targeting of the Arabidopsis homomeric acetyl-coenzyme A
carboxylase to plastids of rapeseeds. Plant Physiol. 113, 75e81.
Roessler, P.G., 1990. Purification and characterization of acetyl-CoA
carboxylase from the diatom Cyclotella cryptica. Plant Physiol. 92,
73e78.
Russa, L.M., Bogen, C., Uhmeyer, A., Doebbe, A., Filippone, E.,
Kruse, O., Mussgnug, J.H., 2012. Functional analysis of three type2 DGAT homologue genes for triacylglycerol production in the
green microalga Chlamydomonas reinhardtii. J. Biotechnol. 162,
13e20.
Sarkar, S., Kamilya, D., Mal, B.C., 2007. Effect of geometric and process
variables on the performance of inclined plate settlers in treating
aquacultural waste. Water Res. 41, 993e1000.
Schneider, D., 2006. Grow your own? Would the widespread adoption
of biomass-derived transportation fuels really help the environment. Am. Sci. 94, 408e409.
Schuenhoff, A., Shpigel, M., Lupatsch, I., Ashkenazi, A., Msuya, F.E.,
Neori, A., 2003. A semi-recirculating, integrated system for the
culture of fish and seaweed. Aquaculture 221, 167e181.
Scragg, A.H., Illman, A.M., Carden, A., Shales, S.W., 2002. Growth of
microalgae with increased calorific values in a tubular bioreactor.
Biomass Bioenergy 23, 67e73.
Shay, E., 1993. Diesel fuel from vegetable oils: status and opportunities. Biomass Bioenergy 4, 227e242.
184
11. MICROALGAE: THE TINY MICROBES WITH A BIG IMPACT
Sheehan, J., Dunahay, T., Benemann, J., Roessler, P., 1998. A Look Back
at the U.S. Department of Energy’s Aquatic Species Program Biodiesel from Algae. National Renewable Energy Laboratory
Report. U.S. Department of Energy, pp. 323.
Singh, I., Rastogi, V., 2009. Performance analysis of a modified 4-stroke
engine using biodiesel fuel for irrigation purpose. Int. J. Environ.
Sci. 4, 229e242.
Sobczuk, T.M., Chisti, Y., 2010. Potential fuel oils from the microalga
Choricystis minor. J. Chem. Technol. Biotechnol. 85, 100e108.
Takagi, M., Karseno, Yoshida, T., 2006. Effect of salt concentration on
intracellular accumulation of lipids and triacylglyceride in marine
microalgae Dunaliella cells. J. Biosci. Bioeng. 101, 223e226.
Takagi, M., Watanabe, K., Yamaberi, K., Yoshida, T., 2000. Limited
feeding of potassium nitrate for intracellular lipid and triglyceride
accumulation of Nannochloris sp. UTEX LB1999. Appl. Microbiol.
Biotechnol. 54, 112e117.
Umdu, E.S., Tuncer, M., Seker, E., 2009. Transesterification of
Nannochloropsis oculata microalga’s lipid to biodiesel on Al2O3
supported CaO and MgO catalysts. Bioresour. Technol. 100,
2828e2831.
Velasquez-Orta, S.B., Lee, J.G.M., Harvey, A., 2012. Alkaline in situ
transesterification of Chlorella vulgaris. Fuel 94, 544e550.
Xu, H., Miao, X., Wu, Q., 2006. High quality biodiesel production from
a microalgae Chlorella protothecoides by heterotrophic growth in
fermenters. J. Biotechnol. 126, 499e507.
Zhang, Y., Adams, I.P., Ratledge, C., 2007. Malic enzyme: the controlling activity for lipid production? Overexpression of malic
enzyme in Mucor circinelloides leads to a 2.5-fold increase in lipid
accumulation. Microbiology 153, 2013e2025.
Zhang, Z., Li, M., Agarwal, A., San, K.-Y., 2011. Efficient free fatty acid
production in Escherichia coli using plant acyl-ACP thioesterases.
Metab. Eng. 13, 713e722.
Zou, J., Katavic, V., Giblin, E.M., Barton, D.L., MacKenzie, S.L.,
Keller, W.A., Hu, X., Taylor, D.C., 1997. Modification of seed
oil content and acyl composition in the Brassicaceae by expression
of a yeast sn-2 acyltransferase gene. Plant Cell 9, 909e923.
C H A P T E R
12
Biobased Fats (Lipids) and Oils from Biomass
as a Source of Bioenergy
Ciarán John Forde, Marie Meaney, John Bosco Carrigan, Clive Mills,
Susan Boland, Alan Hernon*
AER BIO, National Institute for Bioprocessing Research & Training (NIBRT), Blackrock, Co. Dublin, Ireland
*Corresponding author email: alan.hernon@aer-bio.com
O U T L I N E
Introduction
185
Sources of Biolipids
Plant-Derived Biolipids
Edible Lipids
Nonedible Lipids
Waste Edible oil
Animal-Derived Biolipids
Microalgae and Other Oleaginous MicroorganismsDerived Biolipids
186
186
186
187
187
188
Supply and Projected/Purrent Volume
190
Energy Balance
192
Processing of Biolipids and Properties of
Biolipid-Derived Biofuels
Extraction
Steam Distillation
Maceration (Solvent Extraction)
Enzymatic Hydrolytic Maceration
Expression (Cold Pressing)
189
193
193
193
193
193
194
INTRODUCTION
Biolipids have been an important source of energy
since prehistoric times. While the term “biofuel” is
now often synonymously used with “biodiesel”, the first
biofuels used were wood or other plant materials, which
were burnt to provide heat, light, protection from
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00012-7
Hot Continuous Extraction (Soxhlet)
Countercurrent Extraction
Ultrasound Extraction (Sonication)
Supercritical Fluid Extraction
194
194
194
194
Properties of Pure Plant Oil
Degumming
Alkaline Neutralization
Winterization
Bleaching
Transesterification
195
195
195
195
196
196
Properties of Biodiesel
196
Biomass to Liquid Fuels (Bio-oil)
Gasification
Cleaning Process
Synthesis
197
197
197
197
Conclusion
198
References
198
predators and for cooking. The earliest lamps recorded
were made using plant material that was soaked with
animal fat, such as lard. Later lamps, which used oils,
were introduced in the eighteenth century, with early
lamp fuels being oils from fish, whale and a variety of
nut and other plant sources. Whale oil was much sought
after for a lamp fuel as it produced a cleaner flame with
185
Copyright Ó 2014 Elsevier B.V. All rights reserved.
186
12. BIOBASED FATS (LIPIDS) AND OILS FROM BIOMASS AS A SOURCE OF BIOENERGY
less odor and smoke. Another source of light was
candles, which were made from tallow and other oils
rendered from animal waste. These fuels are known as
primary biofuels, fuels that are used without any significant processing in contrast to secondary biofuels where
significant processing is required before the raw products can be used as fuels. As they were discovered,
coal, gas and petroleum products (kerosene in particular) slowly replaced tallow and other animal-based
fuels. Similarly, the use of biolipids as transport fuel is
not novel; in fact, in 1900 when Rudolf Diesel showcased
his internal combustion engine at The Exposition
Universelle in Paris it was fuelled by peanut oil (Stauffer
and Byron, 2007). However, advancements in the use of
petroleum as fuel at the turn of the century resulted in
the selection of this abundant, cheap and efficient hydrocarbon as the fuel of choice for transport. It was not until
the oil crisis of 1973 when oil became expensive and the
security of supply became paramount that biolipids
were investigated again; however, this interest was short
lived as the supply of crude oil from the Organization of
Arab Petroleum Exporting counties was restored in
1974. Now over 100 years after Diesel’s invention we
are almost completely dependent on this finite, expensive and polluting hydrocarbon (petroleum) as a transport fuel. Consequently, the use of petroleum-based
products has resulted in a significant number of environmental issues including global warming via the
greenhouse gas (GHG) effect. Also, in an era when it is
generally accepted that we have reached peak oil
production and it is projected that the demand for transport fuel will increase globally by 39% in the next
10 years interest in the use of biolipids as fuel has
reached new heights. Recent years have seen significant
research, investment and advances in sustainable energy technologies such as solar, wind, geothermal, tidal
and hydroelectrical. It should be noted, however, that
these energy sources, along with nuclear power, relate
to the generation of electricity. Currently electricity
only accounts for about 33% of the world energy market,
whereas liquid fuels account for the remaining 67% of
global energy consumption. These figures, along with
the finite nature of crude oil stocks, illustrate the need
to drastically increase the production of sustainable
liquid fuels (Schenk et al., 2008). Alternative liquid fuel
sources are continually being sought (Bereczky, 2012;
Singh and Singh, 2010) and while the obvious solution
is to revert to the use of vegetable oil used in 1900, there
are several problems with that approach. Most notably is
the need to use arable land to feed the world’s exponentially growing population. Land use for the production
of liquid biofuels has become a hotly debated topic since
2007 when a combination of poor harvests and allocation of vast quantities of land for the production of
biofuel (mostly corn ethanol) resulted in a spike in world
food prices (Tenenbaum, 2008). The ease in supply of
food to the world market in 2007/2008 acted at an indicator to what will happen in the future as the world’s
population increases beyond 8 billion people and we
struggle to meet the nutritional needs of humankind. It
will simply be impossible to grow enough terrestrial
crops to meet the worlds nutritional and energy needs.
It is therefore necessary to explore the use of biolipids
from all sources including lipids from plant, animal
and microalgae sources. Recovering lipids from waste
products like recovered vegetable oil and beef tallow
will also have a role to play in meeting our insatiable
demand for energy. Therefore, it is important to
judicially select biolipids that require the minimum
land usage (maximizing ton of oil per hectare) and lipids
with good fuel properties, as discussed below. In addition, the energy consumed in growing and recovering
the biolipid is also an important consideration when
selecting a biomass for the production of biofuel.
SOURCES OF BIOLIPIDS
Biolipids can be derived from plant, animal, oleaginous microorganisms and algal sources. The composition of biolipids derived from each of these sources
differs greatly and has varying degrees of suitability to
the biofuel production industry. The major lipids
produced from each of these sources are listed below
and the degree of suitability to the production of biofuel
production is discussed.
Plant-Derived Biolipids
In 2007, 95% of world biodiesel was produced via
edible plant oils, which were supplied by the agricultural industry, with the vast majority supplied by
rapeseed oil, 84% (Food and Agriculture Organization,
2008). Overall, plant lipids are divided into three major
categories: edible, nonedible and waste vegetable oils
described below.
Edible Lipids
The main edible oils used for biofuel production are
rapeseed, palm and soy bean oils. Edible oils have the
disadvantage of competing directly with food production. The use of edible oils for the production of biodiesel
competes directly with the use of land for the production of food and without proper planning results in
reduced food production (Gui et al., 2008). However,
the productivity from edible oils is high in terms of oil
yield and the quality of the resulting biofuel. The oil
yield from palm is the highest of the commonly grown
edible oil crops at 5 tons per hectare while rapeseed
SOURCES OF BIOLIPIDS
produces 1 ton per hectare and soy bean 0.52 tons per
hectare. A high lipid yield is vital for the economical
production of biofuel from these plants. Although the
productivity from palm oil is particularly high its use
as a biofuel is limited as it is the world’s most commonly
used edible lipid and thus competition for the oil
between the food and biofuel industry would result in
an increase in the price of this oil (Lam et al., 2009). In
terms of the suitability for biofuel, palm oil has a high
degree of saturation and thus is not the most suitable
for biofuel production with the resulting fuel having
poor cold flow properties. However, the cold flow properties of a lipid can be altered by the use of cold filtration
(Kerschbaum et al., 2008) or alternatively the use of alcohols such as ethanol, isopropanol or isobutanol, which
results in the production of fatty alkyl esters with lower
freezing points and therefore improved cold flow properties (Dunn, 2009). There are also some environmental
and ecological concerns surrounding palm oil production, with the clearing of rain forests to make way for
palm plantations. The plantation costs of edible oil crops
are relatively low with the exception of palm oil, which
has a higher cost; however, this is offset by the high oil
yield from the crop. The overall estimated energy
balance of rapeseed and soybean is similar at 3.7 and
3.4, respectively, while palm oil is significantly higher
at 9.6 due to the high yields (Food and Agriculture
Organization, 2008). Currently rapeseed oil is the most
commonly used plant oil used in biodiesel production
because it makes an excellent biofuel with excellent
cold flow properties. The main disadvantage of using
rapeseed oil is the growth of rapes is difficult and unsustainable as it must be part of a one in five rotation due to
the large quantity of nutrients required for the growth of
the organism and the buildup of pathogens and disease
in the environment targeting rapeseed if grown
annually.
Nonedible Lipids
Nonedible oils that may be used in biofuel production
include Jatropha, Pongamia, jojoba, linseed and cotton
seed oil. Nonedible oils are not suitable for human
consumption due to the presence of toxic compounds
in the oils, for example, curicin present in Jatropha oils
is a toxic lectin. Biofuels from nonedible lipids have
many advantages over the edible alternative including
the ability of these organisms to grow in harsh nutrientand moisture-limiting conditions and the reduction in
carbon emissions. Nonedible oils are generally more
cost-effective as they do not have applications in food
production and thus are lower value oils, containing
low sulfur concentrations and low aromatic compound
concentrations and the lipids produced are biodegradable (No, 2011). A disadvantage of using nonedible oils
187
is the large amounts of free fatty acids (FFAs) that cannot
be converted into biodiesel using an alkaline catalyst
(Demirbas et al., 2011).
Jatropha is one of the most widely used nonedible oils
due to the high potential yield of 0.5e12 tons per hectare
per year; the yield is highly effected by the conditions in
which it is produced, and the ability of the organism to
grow in harsh environmental conditions of low water
availability and low nutrient content (Francis et al.,
2005). The oil produced by Jatropha has good cold flow
properties due to the composition of the oil. The Jatropha
plant is a small tree and produces seeds with high lipid
content. In addition to the drought resistance within the
plant it is also pest tolerant and unpalatable to animals
and grows rapidly with a lifetime of 30 years; each of
these factors makes it a suitable choice for the production of biofuels. The ability of the plant to grow in harsh
conditions led to Jatropha being considered a revolutionary plant that could provide the solution for the production of large volume of lipids without competing with
the food industry. However, when grown in marginal
lands studies revealed that the number of seeds produced by the plant was quite low and although the
tree is capable of growing in low nutrient conditions,
the lipid production is low (Pandey et al., 2012). Therefore, the economic returns of Jatropha grown on marginal
lands is low; however, growing the crop in developing
areas with poor land may be a viable method of production of oil on a small scale. The energy balance from the
crop is also low if only the seeds are used for the production of biofuel; however, the value is increased if all
components, for example, the husks are also utilized
(Prueksakorn and Gheewala, 2006).
Waste Edible oil
Waste edible oil (WEO) is the waste product of cooking or frying foods. The disposal of WEO is difficult and
thus the use of WEO as a biofuel would both alleviate
the problem of disposal in addition to providing a
renewable source of biodiesel. WEO has a high volume
of FFAs, 0.5e15% in comparison with the 0.5% content
of refined virgin vegetable oil, which cannot be converted to biodiesel using an alkaline catalyst as the
FFAs undergo a saponification reaction with the catalyst
thus reducing efficiency and yield (Knothe et al., 2005).
The problem may be overcome by using a supercritical
methanol transesterification for the transesterification
process rather than an alkaline catalyst (Kusdiana and
Saka, 2004). The volume of WEOs available is quite
high with approximately 1 million tons produced in
Europe each year while 10 million tons are produced
annually in the United States (Gui et al., 2008). WEO is
available two to three times cheaper than virgin
vegetable oils (Phan and Phan, 2008) and the high
188
12. BIOBASED FATS (LIPIDS) AND OILS FROM BIOMASS AS A SOURCE OF BIOENERGY
volume of WEO available means it a viable method for
biodiesel production. WEO has a higher estimated
energy balance than rapeseed and soybean of 5.8;
however, the value is lower than that of palm oil at 9.5
(Food and Agriculture Organization, 2008).
Animal-Derived Biolipids
As outlined above many biological sources can be
used for the generation of biofuels (Demirbas et al.,
2011; Vasudevan et al., 2005); however, one source of
biomass for the production of biodiesel that is often
overlooked is the waste fat from animals (e.g.
(Ali et al., 2012; Duku et al., 2011; Feddern, 2011;
Panneerselvam et al., 2011; Wisniewski Jr et al., 2010)).
Generally three broad categories of waste animal fats
are describeddtallow and related raw fats from processing industries, yellow grease from waste cooking oil
used to cook, for example, chicken, and brown grease
that is obtained from traps used to prevent waste fats
and oils being released into the environment. Animal
fats can be sourced as room temperature solids or
semisolids from a variety of animals and include tallow
and suet (cattle and mutton), lard (pigs), schmaltz
(poultry especially chicken and goose), duck, fish oil
and dairy products (milk, butter) (Jayasinghe and
Hawboldt, 2011; Kerihuel et al., 2005; Mrad et al., 2012;
Panneerselvam et al., 2011; Wisniewski Jr et al., 2010).
It is also possible to reclaim waste animal fats from
wastewater (Awad et al., 2012a). Many of the properties
of animal fats used put to specific uses have been known
for a long time (Andés, 1898; Shahidi and Zhong, 2005).
A significant percentage of waste animal fat can be converted to biodiesel using similar techniques to those
used for plant oils, the main process being transesterification, described later (Prosková et al., 2009). The
triglycerides in animal fats are saturated, compared to
unsaturated plant triglycerides, and this has some implications when used as biodiesel. In particular the cloud
point, the temperature at which the oils solidify, is
higher for animal fats. However, when used as additives
to other sources of diesel, for example, 5% or 20%
biodiesel (B5 or B20 blends), the high cloud point does
not affect the blend overall.
Production of biodiesel from waste animal fats has
been shown using a variety of methods including a
novel, integrated method in which fat from lamb meat
is continuously extracted by supercritical CO2 followed
by enzymatic production of biodiesel (Schenk et al.,
2008). Feedstocks containing high levels of FFAs require
an additional preproduction step to convert the FFAs
into esters, which can subsequently be converted into
biodiesel. Waste sources that contain high levels of
FFAs require a separate step (acid catalyzed pretreatment) before the base catalyzed reactions can be used
to provide maximal yields of biodiesel (Canakci and
Van Gerpen, 2001; Knothe et al., 2005; Popescu and
Ionel, 2011). Multistep processes using waste restaurant
oil and animal (pig) fat containing high levels of FFAs
can achieve high yields of biodiesel of up to 80% by
volume on a small scale (Math et al., 2010). Other high
FFA content oils, including used cooking oils, rendered
animal fat and some inedible plant oils (Mathiyazhagan
et al., 2011) can be processed in a similar fashion (Bakir
and Fadhil, 2011).
The feasibility and sustainability of using waste
animal fats as feedstocks for biofuel production has
been the subject of many studies in many areas, for
example, general studies (Demirbas, 2009; Nigam and
Singh, 2011), Australia (Puri et al., 2012), Ghana (Duku
et al., 2011), the United States (Groschen, 2002), Brazil
(Aranda et al., 2009), Ireland (Thamsiriroj and Murphy,
2010) and Hungary (Lakó et al., 2008). In addition, the
use of animal fats from waste tissue may also have environmental benefits, such as being considered as a waste
management process and as a fuel source that does not
compete with food resources (e.g. soybean), the food
versus fuel debate. Table 12.1 shows typical values
reported for triglycerides in several animal fats in comparison to values for soy, a commonly used plantderived feedstock. In all cases, waste animal fats contain
high levels of the fatty acids that are capable of being
converted to methyl esters by transesterification
reactions to produce usable biodiesel. From a sustainability point of view an estimate of the total annual US
production of animal fats as compared to plantderived oils is shown in Table 12.2.
Vegetable oils tend to be produced for human consumption, whereas animal fats form part of a wide
group of animal by-products that are rendered into
many products that may be used in part for human consumption (e.g. production of gelatin). All animal byproducts, including fats, are coded and classified
(Alakangas et al., 2011) according to their intended use
and animal fats not intended for human consumption
are controlled in the European Union by Regulation
(EC) No 1069/2009 and related legislation. Similarly,
TABLE 12.1
Percentages of Fatty Acids in Animal Fats
Fatty Acid
Beef Tallow Pork Lard Chicken Fat Whale Soy
Myristic 14:0
1.4e6.3
0.5e2.5
1
Palmitic 16:0
20e37
20e32
25
Palmitoleic 16:1 0.7e8.8
Stearic 18:0
1.7e5
4e8
e
7e12 w10
8
7e18 e
1e3
6e40
5e24
6
Oleic 18:1
26e50
35e62
41
Linoleic 18:2
0.5e5
3e16
18
w5
28e32 w20
1e2
w50
SOURCES OF BIOLIPIDS
TABLE 12.2
Total Annual Production of US Fats and Oils
Vegetable Oil Production (billion pounds per year)
Canola
1.04
Corn
2.49
Cottonseed
0.617
Soybean
Sunflower
Total Vegetable Oil
19.61
0.731
24.49
Animal Fats (billion pounds per year)
Edible Tallow
1.859
Inedible Tallow
3.299
Lard & Grease
1.63
Yellow Grease
1.40
Poultry Fat
1.42
Total Animal Fat
9.61
Source: U.S. Department of Agriculture, 2010; U.S. Census Bureau, 2010.
the storage of animal fats for use as fuels also needs to be
addressed. The storage of raw animal fat under unsuitable conditions can lead to oxidation and other undesirable chemical and microbial processes that can affect the
quality of the final biodiesel product. The stability of the
final biodiesel:diesel blend can also be affected by longterm storage under unsuitable conditions, and additives
such as antioxidants might be added to improve stability
(Geller et al., 2008; Jain and Sharma, 2010).
With the advent of Bovine spongiform encephalopathy
(BSE) and more specifically Transmissible spongiform
encephalopathies (TSE), there is a greater need to monitor
human health issues when using waste animal fats for the
production of biofuel, at all stages of the production process. The rendering industry recognizes that safe product
(fats) can only be supplied if certain standards are adhered
to (Woodgate and Van Der Veen, 2004). The raw materials
could well have microbial contamination including pathogenic bacteria and possibly prion material (Baribeau
et al., 2005; Brown et al., 2007; Bruederle et al., 2008; Greene
et al., 2007). There is also concern that prions will survive
the rendering process itself (Bruederle et al., 2008). These
concerns have in part led to the publication of guidelines
for the safe handling and use of biodiesels (National
Renewable Energy Laboratory, 2009).
Many trials of waste animal fat biodiesel-powered
engines have been published (Darunde Dhiraj and
Deshmukh Mangesh, 2012; Kleinová et al., 2011;
Panneerselvam et al., 2011; Varuvel et al., 2012). One trial
using public transport buses (Proc, 2006) showed that
the biodiesel does not have any harmful effects on
the engines at B5 and B20 mixes and also shows
189
environmental benefit by way of reduced exhaust
pollutants. However, there are other potential health
and environmental issues in using animal fats as a feedstock for biodiesel production (Greene et al., 2007) and
the production of safe biodiesel is in part dependent
on a safe feedstock (Woodgate and Van Der Veen,
2004). Finally, the processes involved (e.g. rendering,
cleanup, transesterification, etc.) in the production of
biodiesel will generate waste that also needs to be
assessed (Ellis, 2007).
Microalgae and Other Oleaginous
Microorganisms-Derived Biolipids
Microalgae are a heterogeneous group of organisms
consisting of both prokaryotes such as cyanobacteria
and eukaryotes such as diatoms (Bacillariophyta),
dinoflagelates (Dinophyta), green algae (Chlorophyta),
yellow-green algae (Xanthophyta), and red algae
(Rhodophyta) (Brennan and Owende, 2010; Hu et al.,
2008). Similarly, other oleaginous microorganisms are
defined as microorganisms with lipid content in excess
of 20%. The number of bacteria that produce lipids that
could be used for biodiesel production is very small. As
a result, bacteria are mainly used for special lipid production such as Docosahexaenoic acid (DHA). Many yeasts
and fungi also produce high quantities of lipid. Yeasts
with high lipid content include Candida curvata (58%),
Cryptococcus albidus (65%), Lipomyces strakeyi (64%) and
Rhodotorula glutinous (72%). Oleaginous fungi include
Aspergillus oryzae (57%), Mortierella isabellina (86%), Humicola lanuginose (75%) and Mortierella vinacea (66%) (Meng
et al., 2009). In terms of microalgae, species are generally
unicellular organisms but there are also a number of simple multicellular organisms that occur as colonial or filamentous groups of cells. Microalgae are capable of
autotrophic, heterotrophic and mixotrophic growth.
Microalgae populate a wide variety of ecological niches
due to a wide range of tolerance for various growth conditions such as availability of nutrients, salinity, pH and
temperature (Brennan and Owende, 2010; Gong and
Jiang, 2011; Schenk et al., 2008). Currently, microalgae
contribute very little biolipid to the overall bioenergy
market as full-scale commercialization has yet to be
realized. Despite this fact, microalgae remain the feedstock with the greatest potential for supplying future
demand for bioenergy in the form of liquid fuels. The
idea of using microalgae as a source of biolipids for biofuel is not a new one, however. For example, the Aquatic
Species Program was launched in 1978 by what is now
known as the National Renewable Energy Laboratory
(NREL) with its main focus being, “the production of biodiesel from high lipid-content algae grown in ponds, utilising waste CO2from coal fired power plants” (Sheehan
et al., 1998). Over 3000 microalgae strains were initially
190
12. BIOBASED FATS (LIPIDS) AND OILS FROM BIOMASS AS A SOURCE OF BIOENERGY
collected, 300 of which were eventually identified as oil
rich. When the program was officially closed in 1998
the conclusions were that no “fundamental engineering
and economic issues” were identified that would hamper
the feasibility of large-scale microalgae culture. The authors noted, however, that total biomass and algal lipids
produced were still below “theoretical potential, and the
requirements for economic viability” (Sheehan et al.,
1998). The economic viability was, of course, based on a
time when oil prices in the United States were among
their all-time lowest at less than $20 per barrel (adjusted
for inflation). Today the average oil price is approximately $100 per barrel and this, along with increased
pressure to reduce GHG emissions as well as significant
technical advances, has made microalgae-derived biofuels even more relevant to meet current bioenergy
demands.
SUPPLY AND PROJECTED/PURRENT
VOLUME
Growing microalgae for biolipid production usually
involves a lag phase of growth followed by a stationary
phase induced by some sort of “stress” This “stress”,
often nitrogen depletion, induces a switch in the metabolism of the microalgae, which encourages the production of storage lipids in the form of triacylglycerides
(TAGs) rather than cell division (Meng et al., 2009;
Widjaja et al., 2009). Currently microalgae can be grown
at industrial scale autotrophically in open raceway
ponds (Sapphire Energy, 2013) or closed photobioreactor
(PBR) systems (Solix BioSystems, 2013). In addition,
many microalgae species have the ability to grow
heterotrophically, in closed fermenters, given a suitable
carbon source (Solazyme Inc., 2013). Open culture systems, such as race way ponds, are significantly lower
cost in terms of capital expenditure. They require greater
land area than closed systems and are more prone to
contamination by invasive species. Water loss due to
evaporation can also be a significant problem when
compared to closed systems (Chisti, 2007; Pulz, 2001;
Sheehan et al., 1998). Closed systems, on the other
hand, such as PBRs or fermenters are by their nature
closed and thus less likely to be contaminated. Nutrient
concentration can be more easily controlled and water
loss through evaporation is negligible. However, some
have argued that loss of cooling water, used to control
temperature, negates any savings made from using a
closed culture system. The tighter control over culture
conditions facilitated by a closed culture system, along
with more sterile cultures, results in PBRs producing
much greater levels of microalgae biomass, when
compared to raceway ponds. However, the increased
production capability must be offset against the much
larger capital cost involved in commissioning and maintaining a closed culture system (Carvalho et al., 2006;
Pulz, 2001; Ugwu et al., 2008). Hybrid systems have
also been proposed whereby a closed system is used
for the log phase production of biomass and the nutrient
depleted lag phase is allowed to occur in large raceway
ponds. It is hoped that the relatively concentrated inoculation of the raceway ponds will not allow any invasive
species to become established (Greenwell et al., 2010;
Huntley and Redalje, 2007; Rodolfi et al., 2008).
Microalgae present significant potential as a
source of biolipids for bioenergy over more traditional
sources of biolipids such as palm, soya or Jatropha for a
number of reasons. Firstly, the oil content of microalgae
as a percentage of the dry weight, shown in Table 12.3,
is generally in the range of 20e70%, although levels
above 40% are rarely observed (Borowitzka, 1988).
Similarly, the potential yield of biolipids and derived
biodiesel from microalgae per area far outweighs that
of any current oilseed crop. For example, one of the
best available studies of large-scale algae cultivation
produced 0.1 g/l day or 20e23 g dry weight/m2 day.
A conservative lipid content of 30% could therefore
yield 24,000 l biodiesel/ha year (Moheimani and
Borowitzka, 2006; Schenk et al., 2008). This compares
extremely favorably with both Jatropha (1892 l
biodiesel/ha year) and oil palm (5950 l biodiesel/ha
year) (Schenk et al., 2008).
The high potential yield of biodiesel from microalgaederived biolipids is due to a number of factors including
the growth rate of microalgae (Scott et al., 2010) all year
round production capability (Schenk et al., 2008) and the
higher photon conversion efficiency compared to terrestrial plants (Melis, 2009). Unlike algae-derived biofuels,
first-generation biofuels directly competed with food
crops for arable land sparking the “Food vs Fuel” debate
(Gui et al., 2008). Although second-generation fuel crops
such as Jatropha can grow on marginal land (Francis
et al., 2005), microalgae are capable of growing on
nonarable land ensuring competition for land with
food crops is significantly reduced. Similarly, in terms
of other resource demands, 1 kg of algae biomass
requires 1.83 kg of CO2 to grow (Chisti, 2007) and
much research has investigated the potential of industrial flue gases as a source of this CO2 (Bilanovic et al.,
2009). This possibility of both sequestering excess CO2
from flue gases that would otherwise be released into
the atmosphere, while also increasing the growth rate
of microalgae to be used for bioenergy, offers both
environmental and economic advantages (Pires et al.,
2012; Yun et al., 1997). More recently, the apparent
“peak phosphorus” problem has been identified
whereby phosphorus will become a limiting resource
in agriculture. As a result, the potential industrial scale
culture of microalgae, which requires a phosphorus
SUPPLY AND PROJECTED/PURRENT VOLUME
TABLE 12.3
191
Lipid Content and Biomass Productivity of Biofuel Relevant Algae Species
Algae Species
Lipid Content
(% Dry Weight)
Biomass Productivity
(g/l day)
Botryococcus braunii
25e75
Chlorella protothecoides
15e58
Chlorella emersonii
63
(Gouveia and Oliveira, 2009)
Chlorella minutissima
57
(Gouveia and Oliveira, 2009)
Chlorella protothecoides
55
(Gouveia and Oliveira, 2009)
Chlorella sorokiana
22
(Gouveia and Oliveira, 2009)
Chlorella sorokiniana
19e22
Chlorella sp.
28e32
(Chisti, 2007)
Chlorella vulgaris
56
(Gouveia and Oliveira, 2009)
Chlorococcum sp.
19
Crypthecodinium cohnii
20
(Chisti, 2007)
Cylindrotheca sp.
16e37
(Chisti, 2007)
Dunaliella bioculata
8
(Gouveia and Oliveira, 2009)
Dunaliella primolecta
23
(Chisti, 2007)
Dunaliella salina
6e25
(Gong and Jiang, 2011; Gouveia and
Oliveira, 2009)
Ellipsoidion sp.
27
Isochrysis sp.
25e33
(Chisti, 2007)
Monallanthus salina
20
(Chisti, 2007)
Nannochloris sp.
20e35
0.038e0.061
(Chisti, 2007)
Nannochloropsis oculata
22e30
0.084e0.142
(Gong and Jiang, 2011)
Nannochloropsis sp.
31e68
Neochloris oleoabundans
29e65
Nitzschia sp.
45e47
Pavlova lutheri
36
0.05
(Gong and Jiang, 2011)
Pavlova salina
31
0.049
(Gong and Jiang, 2011)
Phaeodactylum tricornutum
18e57
0.045
(Chisti, 2007; Gong and Jiang, 2011)
Scenedesmus dimorphus
16e40
(Gouveia and Oliveira, 2009)
Scenedesmus obliquus
35e55
(Gouveia and Oliveira, 2009)
Scenedesmus sp.
20e21
Schizochytrium sp.
50e77
(Chisti, 2007)
Spirulina maxima
4e9
(Gouveia and Oliveira, 2009)
Tetraselmis sueica
15e23
(Chisti, 2007)
References
(Chisti, 2007)
1.214
(Gong and Jiang, 2011)
0.045
(Gong and Jiang, 2011)
0.054
(Gong and Jiang, 2011)
0.047
(Gong and Jiang, 2011)
(Chisti, 2007; Gong and Jiang, 2011)
0.090e0.134
(Chisti, 2007; Gong and Jiang, 2011;
Gouveia and Oliveira, 2009)
(Chisti, 2007)
0.041e0.054
and nitrogen source for growth, would also be affected
(Cordell et al., 2009). Both phosphorus and nitrogen
are available in plentiful supply within waste water
streams (Sawayama et al., 1995; Yun et al., 1997).
(Gong and Jiang, 2011)
Commercial harvesting of algae blooms from wastewater has already been demonstrated in New Zealand
(Aquaflow, 2013) and the use of wastewater streams as
a nutrient source in large-scale cultivation of microalgae
192
12. BIOBASED FATS (LIPIDS) AND OILS FROM BIOMASS AS A SOURCE OF BIOENERGY
has been well studied and implemented. Similarly, in
terms of water usage, microalgae cultivation, particularly in closed cultivation systems, demonstrates significant water savings when compared to traditional
biofuel crops. Many microalgae species are also capable
of growing in brackish water most notably Dunaliella
salina (Weldy and Huesemann, 2007).
ENERGY BALANCE
Any credible source of bioenergy should not only be
economically viable but also environmentally sustainable. The economic and environmental impacts of any
source of bioenergy, including biolipids from microalgae, will usually be measured in terms of energy return
on energy investment (EROI) and/or GHG emissions.
These economic and environmental impacts of biofuels
and microalgae biofuels in general have been hotly
debated in recent years. A number of life cycle analyses
(LCAs) have been undertaken with seemingly conflicting results (Benemann et al., 2012; Liu et al., 2011;
Resurreccion et al., 2012; Sun et al., 2011). Similar
disparities arose in the case of second-generation biofuels such as corn ethanol before the introduction of
the Energy and Resources Group (ERG) Bioenergy
Meta-Model (Farrell et al., 2006). The results of reported
LCA analyses are hindered by the lack of fully integrated commercial-scale microalgae to bioenergy systems from which to obtain accurate measurements.
Estimates are based on projections from laboratoryand pilot-scale tests, as well as some commercial data.
Despite these facts an overall meta-analysis concluded
that algae-based biodiesel would result in energy consumption and GHG emissions on par with terrestrial alternatives (Liu et al., 2011). In this study the authors
consider a microalga-based bioenergy system whereby
CO2 and nitrogen for microalgae cultivation are recycled
from waste streams and the microalgae coproducts are
used for further bioenergy production in the form of
methane. This concept of an integrated “biorefinery”
has been proposed previously (Borowitzka, 1995, 1999;
Chisti, 2007; Martı́n and Grossmann, 2012). As alluded
above, the “biorefinery” concept envisages the main inputs into the cultivation process such as carbon, nitrogen and phosphorus being supplied through various
waste streams. Similarly, the microalgae product resulting from cultivation could be fully “refined” into a number of outputs including biolipids for bioenergy,
biolipids for nutraceutical applications, proteins for animal feeds, sugars for bioethanol production, etc. At present, where fully commercial scale cultivation of
microalgae and conversion to fuel alone is still not
economically feasible, the “biorefinery” concept appears
to offer the best short to medium term path to scale-up.
In addition to the potential economic and environmental advantages of using microalgae-derived biolipids, the properties of the resulting biodiesel product
are also worth considering. As detailed later in this
chapter, biodiesel is produced by transesterification of
the biolipids from an appropriate feedstock. Much like
the plant- and animal-based biolipids discussed previously, the profile of the microalgae-derived biolipids
that undergo transesterification will ultimately determine the quality of the biodiesel product. This profile
will include the level of polyunsaturated fatty acids
(PUFAs), the level of FFAs and the level of TAGs.
Although the lipid profile of microalgae varies among
species and even among the same species under
different conditions of growth, approximately 80% of
the lipid content of microalgae, in general, will be
made up of storage lipids in the form of TAGs. TAGs
are made up of three fatty acid chains, usually with a
chain length of C14 to C22 for microalgae-derived biolipids, joined to glycerol through three ester bonds (Scott
et al., 2010). These TAGs can be easily transesterified in
the presence of methanol, as described later in the chapter, to fatty acid methyl esters (FAMEs), which make up
biodiesel. The presence of FFAs, however, results in the
formation of soaps during transesterification in the presence of a base catalyst such as NaOH. This increases the
downstream processing required to produce a finished
biodiesel product. Similarly, the presence of PUFAs in
biolipids derived from some microalgae species can
cause tar formation resulting from fatty acid chains
cross-linking (Burton et al., 2009). A high PUFA content
could also mean that a biodiesel product would not pass
European standards for biodiesel (EN14214), which demand the content of FAMEs with four or more double
bonds to be below 1% mol (Knothe et al., 2005). Other
properties that have been considered with regard to
other feedstocks mentioned in this chapter include the
cloud point, the cetane number and the oxidation stability of the biodiesel fuel. It has been suggested that biodiesel from microalgae oils may face significant
performance problems regarding cold flow and oxidative stability in particular (Knothe, 2011); however,
exceptions to this observation may apply to some microalgae such as Trichosporon capitatum. Also, in a recent
study, biodiesel derived from the microalgae Chaetoceros
gracilis was found to generate similar torque and power
to soy-derived biodiesel. In terms of emissions, the
C. gracilis-derived biodiesel also produced less CO,
NOx and hydrocarbons than petroleum diesel (Wahlen
et al., 2012).
It is clear that the potential for algae to supply a sustainable source of biolipid for transportation fuel and
other forms of bioenergy is not in doubt. However, there
remain technical, economic and environmental challenges to be overcome. In a recent report by the National
PROCESSING OF BIOLIPIDS AND PROPERTIES OF BIOLIPID-DERIVED BIOFUELS
Research Council in the United States entitled, “Sustainable development of algal biofuels” a number of sustainability concerns were highlighted. These included EROI;
GHG emissions and resource usage such as land, water,
nitrogen, phosphorus, and carbon dioxide (National
Research Council, 2012). None of these concerns,
however, were considered a “definitive barrier to sustainable development of algal biofuels”. This is because
a number of strategies have already been implemented
to tackle these challenges. As mentioned previously
the use of wastewater streams can drastically reduce
resource usage and GHG emissions as well as greatly increase EROI. Current projects, at industrial scale, such as
Sapphire Energy’s “Green Crude Farm” (Sapphire
Energy, 2013) aim to have a capacity of 1 million gallons
per year of finished biofuel product. It is predicted that
this will result in a 60e70% reduction in GHG emissions
compared to traditional fossil crude oil, which, if
achieved, will make the potential of microalgaederived biofuel a very definite reality.
PROCESSING OF BIOLIPIDS AND
PROPERTIES OF BIOLIPID-DERIVED
BIOFUELS
Independent of the biomass source, biolipids can be
used in various ways as a source of bioenergy. There
are a number of basic steps involved in processing biolipids to biofuel. These can include some or all of lipid
extraction, degumming, neutralization, winterization,
bleaching and transesterification. The sources of
biomass and how they are produced have been
described previously in this chapter and the first processing step will usually involve efficient extraction of
the biolipid from the biomass. Following extraction,
some biolipids can be used in their pure form as pure
plant oils (PPOs). Other biolipids are further processed,
usually into biodiesel. Here the extraction step is
followed by purification and stabilization of the biolipid
and the conversion to biodiesel. The various steps
involved in processing biolipids are described below,
beginning with extraction, along with the fuel properties
of both PPO and biodiesel.
Extraction
Extraction is a process consisting of the separation of
a specific substance from a complex matrix. In the
context of extraction lipids from biomass, the purpose
is to use standardized extraction procedures to isolate
the biomolecules of interest, i.e. lipids, concurrent to
rejection of the remaining inert biomass. This is most
commonly achieved by using a selective solvent known
as menstruum (Handa, 2008), or by solventless physical
193
extraction means. The resultant lipid may be ready for
use in the form of fluid extracts, it may be further processed into a variety of biofuel and nutraceutical products, or it may be fractionated to isolate individual
chemical entities or a combination of the above as
proposed by the “biorefinery” concept discussed previously. The most common biolipid extraction procedures
are summarized below.
Steam Distillation
Steam distillation is a process that is commonly
applied to the extraction of essential oils (Gutierrez
et al., 2009). Plant material is placed into a still where
pressurized steam penetrates the plant material causing
internal lipid vacuoles to rupture. Upon exposure to the
surrounding environment, the lipid evaporates to form a
mixture of easily separable vapors (essential oil and
water). The vapors condense and the distillate (separated into two immiscible layers) is collected in a graduated tube connected to a condenser. The aqueous phase
is recirculated into the flask, while the volatile oil is
collected separately. The main disadvantage associated
with steam distillation is that thermolabile components
risk being degraded (Sarker et al., 2005). A combination
of solvents and steam distillation is often used to
improve the final product of a biodiesel production
process.
Maceration (Solvent Extraction)
Maceration is used for creating extracts and resins in
a simple yet well-established procedure. Whole or
coarsely powdered biomass is placed in intimate contact
with a suitable extractant in a closed vessel. The mixture
is allowed to stand at room temperature for a defined
period of time, typically at least 3 days, with frequent
agitation (using mechanical shakers or mixers) to ensure
homogeneity (Sarker et al., 2005). The organic phase is
separated from the solids by either filtration, decantation or in some cases centrifugation and the remaining
solid material is pressed to ensure efficient solvent
recovery. The recovered liquid phases are combined
and clarified for further processing. This process can
be repeated several times to achieve maximum lipid
recovery. The main disadvantage associated with maceration is that the process can be quite onerous, potentially
taking from a few days up to several weeks (Takahashi
et al., 2001).
Enzymatic Hydrolytic Maceration
Certain plant materials require enzymatic maceration
prior to lipid release as their volatile components
are glycosidically bound. Enzymes can be either
194
12. BIOBASED FATS (LIPIDS) AND OILS FROM BIOMASS AS A SOURCE OF BIOENERGY
endogenous or exogenous to the biomass. For example,
methyl salicylate (wintergreen oil) is an organic ester
that is naturally produced by many species of plants.
The plant leaves contain the precursor gaultherin and
the enzyme primeverosidase; when the leaves are
macerated in warm water, the endogenous enzyme
acts on the gaultherin and liberates free methyl salicylate
and primeverose (Handa, 2008). In the case of the exogenous addition of enzymes, recent advances in the field
of algal lipids have demonstrated the addition of complex mixtures of enzymes to selectively degrade cell
walls in a cascade of hydrolytic reactions. Released
lipids are isolated and collected for further processing
(Liang et al., 2012).
Expression (Cold Pressing)
Expression or cold pressing is commonly used in the
production of essential and food oils. The term expression refers to any physical process in which the essential
oil glands in the biomass are crushed or broken to
release the oil. The resulting oilewater emulsion is typically separated by centrifugation. Traditionally, cold
pressing was conducted by hand; however, for largescale commercialization, this is impractical. Thus, with
the advancement of industrialization, a number of
machines have been designed to achieve the same
results on commercial scale. It is important to note that
oils extracted using this method have a relatively short
shelf life (Martı́nez et al., 2008).
Hot Continuous Extraction (Soxhlet)
In this method, finely ground biomass is placed in a
porous bag or “thimble” made of strong cellulose, which
is placed in the extraction chamber of a Soxhlet apparatus. The menstruum is heated, and the condensed
extractant drips into the thimble containing the biomass,
ensuring intimate continuous contact with the biomass.
When the level of liquid in the extraction chamber
reaches overflow, the liquid contents siphon into the
heating chamber. This process is continuous and is
carried out until complete extraction is achieved
(Morrison and Coventry, 2006). The advantage of this
method is that large amounts of lipid can be extracted
with a much smaller quantity of solvent.
Countercurrent Extraction
Counter-current extraction is a process whereby wet
raw material is pulverized using toothed disc disintegrators to produce slurry in a semicontinuous stream.
As the pulverization of the biomass is in aqueous media,
the heat generated during comminution is counterbalanced by the slurry water, preserving thermolabile
compounds. The slurry stream is moved in one direction
within the cylindrical extractor where it comes
into discreet contact with a suitable menstruum
(Vishwakarma, 2010). Complete extraction is possible
when the quantities of solvent and material and their
respective flow rates are optimized. The quantity of
solvent required is generally minimal and as the process
is most often conducted at room temperature, the threat
to thermodegradation of volatile compounds is negated
(Handa, 2008).
Ultrasound Extraction (Sonication)
The use of sonication is an emerging technology
that is gaining widespread industrial acceptance due
to recent advances in the scalability of the technology
(Awad et al., 2012b; Dolatowski et al., 2007). In the
context of lipid extraction from biomass, ultrasound
technology is used to increase the permeability of
biomass cell walls by generating cavitation events.
These events are created by the use of high frequencies
(20e2000 kHz) to generate a microbubble in solution;
the intensity of the waves leads to the eventual
collapse of the bubble generating extreme localized
pressure and temperature events in close proximity
to the biomass. These cavitation events assist in the
rupturing of the cell walls to release the intercellular
constituents into the surrounding environment. Once
the biomolecules of interest are released from the
biomass they can be recovered using conventional
techniques. One disadvantage of using ultrasonics in
the occurrence of sonolysis, i.e. the occasional but
deleterious effect that when high power (typically
greater than 20 kHz) is applied in aqueous media it
can lead to the formation of free radicals and hydrogen
peroxide. These are generated at the interfacial double
layer established during cavitations, which subsequently diffuse into solution (O’Donnell et al., 2010).
Supercritical Fluid Extraction
Another technology in the extraction space is supercritical fluid extraction (SFE) whereby a solvent is
subjected to temperature and pressure conditions to
adjust the properties to those intermediate to a gas
and liquid in a dedicated reactor setup. This in turn
effects the solubilization of solutes in a matrix
(Wenclawiak, 1992). The main supercritical solvent
employed is carbon dioxide. Carbon dioxide (critical
conditions: T ¼ 30.9 C and P ¼ 73.8 bar) is cheap, environmentally friendly and has generally recognized as
safe status from the US Food and Drug Administration. Supercritical CO2 (SC-CO2) is also attractive
because of its high diffusivity combined with its
easily tunable solvent strength (Herrero et al., 2010).
PROPERTIES OF PURE PLANT OIL
However, due to its chemical nature, it possesses
several polarity limitations. As mentioned previously,
solvent polarity is particularly important when
extracting polar solutes and when strong matrix interactions are present. To augment the process, organic
solvents are commonly added to the carbon dioxide
extracting fluid to alleviate the polarity limitations
(Handa, 2008). CO2 is gaseous at room temperature
and pressure, which makes recovery very simple and
provides solvent-free products, i.e. once the liquid
depressurizes, the CO2 returns to a gaseous state,
and only the extracted products remain. SFE using
CO2 can be operated at low temperatures, which
allows the extraction and integrity preservation of
thermolabile compounds (Mendiola et al., 2007).
PROPERTIES OF PURE PLANT OIL
Following extraction from biomass, biolipids can be
used as pure oil (generally plant) or can be converted
to biodiesel by a process known as transesterification,
described later. However, the use of PPO as a fuel requires the modification of diesel engines unlike biodiesel, which, particularly when blended with
petroleum diesel, can be used in unmodified diesel
engines. These engine modifications are needed as
PPO is more than 10 times as viscous as biodiesel. As
a result, it has a tendency to gum up in cold weather,
which can be somewhat overcome by blending with
traditional fossil diesel. Nevertheless, it has some
advantages: with a flash point of over 300 C, storage
and transport are simplified. According to the VwVwS
(Verwaltungsvorschrift wassergefährdende Stoffe),
which is the national German regulation on water
hazard classification, PPO is not designated as even a
hazard to water given that it is biodegradable. In an
unmodified engine, poor atomization of the fuel will
lead to coking of the injectors and accumulation of
soot deposits. Modification is designed to preheat fuel
or involves installation of a two-tank system. In the
latter, the engine is started with diesel and only changes
to PPO when the operating temperature has been
reached. It must switch back to diesel before being
turned off, to flush out the remainder of the PPO in
order to ready the engine for the next operation. Other
options exist, such as the specialist engine developed
by Ludwig Elsbett in the 1970s. The fuel emissions of
PPO are also much lower in sulfur emissions when
compared to the fossil equivalent. For a detailed overview see (Russo et al., 2012). After extraction, if the
biolipid is not to be used as PPO, or other pure oil, it
needs to be further processed into a more useable biofuel, usually biodiesel. Here the biolipid goes through
a series of processing steps beginning with degumming.
195
Degumming
Following extraction and regardless of the process
described above, the end product will generally be a rather
impure biolipid that contains undesirable contents such as
FFAs, tocopherols, waxes and possibly phosphatides. The
latter, if not removed before storage, will produce a thick
gum over time. Gums are formed when the biolipid
absorbs water, which causes some of the phosphatides
(such as phosphocholine) to become hydrated and thereby
lipid insoluble. Accordingly, hydrating the gums and
removing the hydrated gums from the oil before storage
can prevent the formation of a gum deposit. This treatment is called water degumming and involves the addition of water at 60e90 C before the phase is separated.
An optimum temperature is sought, as it must not be so
high as to increase the solubility of phosphatides in oil.
A temperature that is too low will increase the viscosity,
making phase separation more difficult. It is never applied
to fruit oils like olive oil and palm oil, since these oils have
already had considerable water contact during their production. The removal of nonhydratable phosphatides
(such as phosphatidic acid) requires the addition of an
acid, usually citric or phosphoric, which will form a sludge
that can be easily removed (Dijkstra and Van Opstal, 1989).
This addition of acid is proportional to the amount of
phosphorous already contained in the sample. In addition, this acid also reduces any iron salts and decreases
chlorophyll contamination. Enzymatic degumming focuses on the use of lipases, which convert nonhydratable
lipids to more hydratable forms. Although the process
has been tried at a larger scale for 20 years, it has not
made the advancement toward widespread use (Dijkstra,
2010; Yang et al., 2008).
Alkaline Neutralization
As mentioned previously, the presence of FFAs in the
biolipid is detrimental to oil quality and function,
including biodiesel production. Removal typically involves the reaction of these FFAs with an alkaline solution. In the edible oil industry, usually only caustic
soda is used for this reaction, but potassium hydroxide
is also used by some producers. The acidity of the FFA
comes from the Hþ of the carboxyl group. This Hþ of
the functional group of the stearic acid reacts with the
OH group of the caustic soda (NaOH) to produce
soap and water. In addition to the removal of FFAs, other
undesirable nonglyceride materials are also removed in
this fashion such as phenol, oxidized fatty compounds,
heavy metals and phospholipids.
Winterization
Most biolipids do not need dewaxing, as they contain
little or no waxes. Only biolipids of higher melting
196
12. BIOBASED FATS (LIPIDS) AND OILS FROM BIOMASS AS A SOURCE OF BIOENERGY
temperatures, such as sunflower oil and rice bran oil,
give a hazy appearance during winter season due to
precipitation of dissolved waxes. Hence, they require
being dewaxed. This is carried out by chilling the oil
to 10e15 C, followed by filtration of precipitated solids.
The oil thus treated has a sparkling appearance, even in
winter temperatures.
Bleaching
Oil bleaching, which is performed in order to prepare
a sufficiently light-colored product of enhanced appearance and improved stability, is usually achieved by
treating the crude or refined oil with powdered
absorbent. These absorbents usually contain a calcium
montmorillonite (fuller’s earth) or natural hydrated
aluminum silicate (bentonite). Adsorption of color
bodies, trace metals and oxidation products, as well as
residual soaps and phospholipids remaining after
washing neutralized oils takes place, if possible. Acidactivated clays are the major adsorbent used, although
active carbons and synthetic silicas are also applied
industrially with more specific goals. Thus, active
carbons are used specifically to eliminate polycyclic
aromatic hydrocarbons from some oils, especially fish
oils and pomace oils, while synthetic silicas are quite
efficient in adsorbing secondary oxidation products,
phospholipids and soaps (León-Camacho et al., 2003).
There are a number of good sources of material with
more detailed descriptions of each process found online
at the Lipid Library (Hardwood and Weselake, 2013), in
“Proceedings of the World Conference on Oilseed
Technology and Utilization” (Applewhite, 1993) and
finally in, Edible Oil Processing (Hamm and Hamilton,
2000).
Transesterification
Despite being energetically favorable, the direct use
of plant or other biolipids in fuel engines is problematic
as described earlier. Briefly, due to high viscosity (over
10 times higher than diesel fuel) and low volatility,
they do not burn efficiently and can form deposits in
the fuel injector of diesel engines. Furthermore, acrolein
(a highly toxic substance) is formed through thermal
decomposition of glycerol. Different ways have been
considered to reduce the high viscosity of plant and
other biolipids, but the principal method is to engage
in chemical transesterification to produce biodiesel,
which could be used in the common diesel engine
with minor modification.
As mentioned previously, biolipids consist primarily
of triglycerides, which are three hydrocarbon chains
connected by glycerol. The bonds are hydrolyzed to
allow the formation of FFAs, which are mixed and
reacted with methanol or ethanol to form methyl (or
ethyl) fatty acid esters. The use of methanol (methanolysis) is widespread and considered advantageous, as it
is cheaper than ethanol (although in Brazil, ethanol 90
is plentiful) and has less azeotrophic qualities (Encinar
et al., 2007). The same reaction using ethanol is more
complicated as it requires a water-free alcohol, as well
as a biolipid with low water content, in order to obtain
good glycerol separation. Methanolysis can happen by
heating 80e90% methanol with a small amount of catalyst. The received biodiesel after methanolysis is FAME
and with ethanol to form fatty acid ethyl ester. The use of
ethanolysis reaction using bioethanol has been
discussed as being possibly more environmentally
favorable as it would involve the use of a nonfossil
fuel. Apart from this, ethanol is less toxic and slightly
increases the cetane number of the biofuel. Although
transesterification can proceed in the absence of
catalysts, the reaction proceeds much too slowly to be
economically viable and thus typically requires an acidic
or alkaline catalysis. Among the most commonly used
alkaline catalysts in the biodiesel industry are potassium
hydroxide (KOH) and sodium hydroxide (NaOH)
flakes, which are inexpensive, easy to handle and can
be transported and stored easily. For this reason, they
are preferred by smaller producers. Alkyl oxide solutions of sodium methoxide (NaOCH3) or potassium
methoxide (KOCH3) in methanol, which are now
commercially available, are the preferred catalysts for
large continuous-flow production processes.
In the transesterification process, the effective species
of catalysis is the methoxide radicals (CH3O) and the
activity of a catalyst depends upon the amount of methoxide radicals (Komers et al., 2001a,b). For sodium or
potassium hydroxide, the methoxide ion is prepared in
situ by reacting methanol with hydroxide, a reaction
that will also produce water that remains in the system.
Hydrolysis of triglycerides and alkyl esters may occur
due to the presence of this water, which further leads
to the formation of FFAs and thus to a soap. Saponification may also occur if a strong base, e.g. NaOH or KOH,
is present in the system by reacting with esters and
triglycerides directly. All these problems can be avoided
completely if sodium and potassium methoxide
solutions, which can be prepared water-free, are applied
(Singh et al., 2006).
PROPERTIES OF BIODIESEL
Untreated biodiesel blend stocks, generated by transesterification, generally exhibit poor oxidation stability,
which can result in long-term storage problems.
Biodiesel has many similar fuel economies to fossil
diesel. Although it has about 10% less energy content
BIOMASS TO LIQUID FUELS (BIO-OIL)
per volume, its cetane number and lubricating effect are
higher, which is advantageous (Rutz and Janssen, 2007).
The higher oxygen content leads to better combustion
and fewer pollutants, particularly sulfur oxides. Biodiesel is produced in a pure form (100% biodiesel blend
stock, referred to as “B100” or “neat biodiesel”) and is
typically blended with petroleum-based diesel fuel.
Such biodiesel blends are designated as BXX, where
XX represents the percentage by volume of pure
biodiesel contained in the blend (e.g. “B5” or “B20”).
According to a “Technical Statement on the Use of
Biodiesel in Compression Ignition Engines” released in
2009 by the Truck and Engine Manufactures Association, neat biodiesel and higher percentage biodiesel
blends can cause a variety of engine performance
problems. These include fuel filter plugging, injector
coking, piston ring sticking and breaking, elastomer
seal swelling and hardening/cracking, and severe
engine lubricant degradation and dilution. The report
goes on to state that when converting from petroleumbased diesel to a biodiesel blend, residual fuel system
deposits may accumulate in fuel filters due to the high
solvency of the fuel. Thus, more frequent filter service
may be required until the fuel system deposits are stabilized. More information on biofuel handling can be
found in "Biodiesel Handling and Use Guide: Fourth edition
(Revised)" published by NREL (National Renewable
Energy Laboratory, 2009).
BIOMASS TO LIQUID FUELS (BIO-OIL)
While the focus of this chapter has been on biolipids it
is important to note that any biomass can be converted to
“bio-oil” via a high-temperature process known as pyrolÒ
ysis. This “bio-oil” also known as Synfuel or Sunfuel is
currently only produced on a small scale and it very
much belongs to the second-generation biofuels, as it is
a way of generating fuel from a range of biomass
including straw, wood or other materials high in lignin,
which are difficult to convert to bioethanol. The potential
for mass production remains enormous. The production
of this biomass to liquid or BtL fuel can vary in
complexity and can vary depending on the individual
needs, but it essentially comprises the following steps.
Gasification
Gasification is a form of incomplete combustion in
which a fuel is burnt in an oxygen-deficient atmosphere.
An energy-rich gas, consisting principally of methane,
CO and hydrogen, is formed but heat release is minimized. Thus an energy-rich fuel (biomass) is converted
into an energy-rich gas. There are differing processes
for gasification. For example, a description of the
197
Ò
Carbo-V process first developed by Chloren Industries
but now owned by Linde Engineering GmbH was outlined in the Biofuel Technology Handbook (Rutz and
Janssen, 2007). This involves low-temperature gasification, where low-temperature pyrolysis with air or oxygen at 400e500 C allows the continuous production of
a gas containing both tars (volatile component) and
char (carbon solids). This is followed by a hightemperature gasification, where the gas is further
oxidized (again hypostoichiometrically) in a combustion
chamber. The third part involves blowing the pulverized
char into the hot gasification medium. Pulverized char
and gasification medium react endothermically in the
gasification reactor and are converted into a raw synthesis gas. Other gasification processes can be found, such
as the recently developed BioliqÒ , which was formed
by Lurgi AG (Frankfurt Germany) with Karlsruhe Institute of Technology (Karlsruhe Germeny).
Cleaning Process
After gasification, it is usual to have many impurities
and thus cleaning remains one of the most important
and most technical challenges. Remaining tars tend to
be refractory and difficult to remove by thermal or physical processes. Generally, the impurities in biosyngas produced from the gasifier can be grouped into three types:
(1) organic impurities, such as tars, benzene, toluene, and
xylenes; (2) inorganic impurities, such as O2, NH3, HCN,
H2S, Carbonyl sulfide (COS), and HCl; and (3) other impurities, such as soot and dust. Both thermal cracking,
which involves the addition of steam and oxygen at
200e1000 C, and catalytic cracking at lower temperatures is possible, as is low-temperature scrubbing with
an oil-based medium may all encompass the process. A
multicontaminant syngas treatment process created by
Southern Research Institute, Birmingham, Alabama,
USA, uses a candle filter, which can be catalytic, closely
coupled with the gasifier. A variety of sorbents is injected
into the gasifier or between the gasifier and filter to
remove various contaminants (e.g. alkali metals, sulfur
species, and halides) both by reaction in the gas phase
and on the filter cake. Catalysts may be incorporated
into the candle filter or the filter may be coated with a
catalyst to crack tar and ammonia depending on the operating temperature of the candle filter. An outline of the
process can be seen in Figure 12.1.
Synthesis
Two methods are available for this production step,
but the Fischer-Tropsch (FT) synthesis is the most
widely known. It was developed at the Kaiser-Wilhelm
Institute for Research on Coal (Mühlheim/Ruhr) in
1925. In Germany, coal to liquid fuels have been
198
12. BIOBASED FATS (LIPIDS) AND OILS FROM BIOMASS AS A SOURCE OF BIOENERGY
FIGURE 12.1 Biobased fats (lipids)
and oils from biomass as a source of
bioenergy. Integration of a catalytic filter
into a gasifier for combined particle
separation and tar removal from
biomass gasification gas. Source: Courtesy of Southern Research Institute, Birmingham, Alabama, USA. (For color
version of this figure, the reader is
referred to the online version of this
book.)
produced with the help of FT synthesis since 1938.
During the process, CO and H2, with the aid of a
catalyst, will form hydrocarbons. A variety of catalysts
exist, but the most common are usually transition metals
such as cobalt. In the case of biomass, however, an iron
catalyst is often favored (Hu et al., 2012).
The other process is the methanol-to-gasolineÒ
method, in which the syngas is first transformed into
methanol as an intermediate state. In a following step,
fuels can be obtained from this compound. Finally, after
separating the produced liquid hydrocarbons into
heavy, medium and light fractions, these hydrocarbons
are refined and blended to achieve the desired fuel
properties.
CONCLUSION
The search for a sustainable supply of fuel that does
not contribute to global warming has consumed environmental scientists for decades. While it is unlikely
there is a “silver bullet” solution to the pending energy
crisis the use of biolipids has enormous potential to
meet a large proportion of the global transport fuel
requirements. Similarly, no Single lipid source is produced in sufficient quantities to impact on the world’s
fuel supplies; therefore, a combination of all biolipids
outlined above will be required if biolipids are to be a
realistic alternative to petroleum-based fuels. While
plant-derived biolipids currently dominate the liquid
bioenergy markets, microalgae remain the most promising source of biolipids in the future. The limited land
usage requirement and efficient carbon fixing capabilities of microalgae make them the ideal choice as a
source of biolipids; however, there are a number of
stumbling blocks to be overcome before algal biofuels
are a commercial reality. These include the challenge of
growing algae at industrial scale to meet the increasing
demand for liquid transport fuel, the energy input
involved in harvesting and dewatering algae and finally
the cost and environmental impact of efficiently extracting biolipids from algae. These challenges are far from
insurmountable, however, and each challenge is being
tackled by numerous academic institutions and increasingly, by large, multinational energy, food and industrial
chemical companies. This concerted effort with regard
to algae biofuels, coupled with the more established
plant- and animal-based biofuel industries can supply
a significant portion of the world’s energy needs in the
future.
References
Alakangas, E., Nikolaisen, L., Sikkema, R., Junginger, M., 2011. Combined nomenclatures (CN Codes) for biomass fuels. Available
from: www.vtt.fi/inf/julkaisut/muut/2011/D2-4-EUBIONETIII_
CN_code_report.pdf (accessed 15.02.13.).
Ali, A., Ahmad, F., Farhan, M., Ahmad, M., 2012. Biodiesel production
from residual animal fat using various catalysts. Pak. J. Sci. 64,
282e286.
Andés, L.E., 1898. Animal Fats and Oils: Their Practical Production, Purification and Uses for a Great Variety of Purposes,
Their Properties, Falsification and Examination; a Handbook
for Manufacturers of Oil-and Fat-products, Soap and
Candle Makers, Agriculturists, Tanners, Etc. Scott, Greenwood,
London, 240.
REFERENCES
Applewhite, T., 1993. Proceedings of the World Conference on Oilseed
Technology and Utilization. Amer Oil Chemists Society, USA.
pp. 507.
Aquaflow, 2013. Blenheim municipal wastewater. Available from:
www.aquaflowgroup.com/projects/blenheim-municipalwastewater (accessed 10.01.13.).
Aranda, D.A.G., da Silva, C.C.C.M., Detoni, C., 2009. Current processes in Brazilian biodiesel production. Int. Rev. Chem. Eng. 1,
603e608.
Awad, S., Paraschiv, M., Varuvel, E.G., Tazerout, M., 2012a. Optimization of biodiesel production from animal fat residue in wastewater using response surface methodology. Bioresour. Technol.
129, 315e320.
Awad, T., Moharram, H., Shaltout, O., Asker, D., Youssef, M., 2012b.
Applications of ultrasound in analysis, processing and quality
control of food: a review. Food Res. Int. 48, 410e427.
Bakir, E.T., Fadhil, A.B., 2011. Production of biodiesel from chicken
frying oil. Pak. J. Anal. Environ. Chem. 12, 95e101.
Baribeau, A.M., R. Bradley, P. Brown, J.J. Goodwin, U. Kihm, E. Lotero,
D. O’Connor, M. Schuppers and D. Taylor. 2005. Biodiesel from
specified risk material tallow: an appraisal of TSE risks and
their reduction. Available from: www.iea-amf.vtt.fi/pdf/
annex30_vol1.pdf (accessed 15.02.13.).
Benemann, J., Woertz, I., Lundquist, T., 2012. Life cycle assessment
for microalgae oil production. Disrupt. Sci. Technol. 1, 68e78.
Bereczky, Á., 2012. Alternative fuels and technologies for compression
ignition internal combustion engines. J. KONES Powertrain
Transp. 19, 43e51.
Bilanovic, D., Andargatchew, A., Kroeger, T., Shelef, G., 2009. Freshwater and marine microalgae sequestering of CO2 at different C
and N concentrations e response surface methodology analysis.
Energy Convers. Manage. 50, 262e267.
Borowitzka, M.A., 1988. Fats, oils and hydrocarbons. In:
Borowitzka, L.J., Borowitzka, M.A. (Eds.), Micro-Algal Biotechnology. Cambridge University Press, Cambridge, pp. 257e287.
Borowitzka, M.A., 1995. Microalgae as sources of pharmaceuticals and
other biologically active compounds. J. Appl. Phycol. 7, 3e15.
Borowitzka, M.A., 1999. Commercial production of microalgae: ponds,
tanks, and fermenters. Prog. Ind. Microbiol. 35, 313e321.
Brennan, L., Owende, P., 2010. Biofuels from microalgae - a review of
technologies for production, processing, and extractions of biofuels
and co-products. Renewable Sustainable Energy Rev. 14, 557e577.
Brown, R.S., Keller, B.O., Oleschuk, R.D., 2007. Detection of prion
proteins and TSE infectivity in the rendering and biodiesel
manufacture processes. Available from: www.iea-amf.vtt.fi/pdf/
annex30_vol2.pdf (accessed 15.02.13.).
Bruederle, C.E., Hnasko, R.M., Kraemer, T., Garcia, R.A., Haas, M.J.,
Marmer, W.N., Carter, J.M., 2008. Prion infected meat-and-bone
meal is still infectious after biodiesel production. PloS One. 3, 1e8.
Burton, T., Lyons, H., Lerat, Y., Stanley, M., Rasmussen, M.B., 2009. A
review of the potential of marine algae as a source of biofuel in
Ireland. Available from: www.seai.ie/Publications/Renewables_
Publications_/Bioenergy/Algaereport.pdf (accessed 21.02.13.).
Canakci, M., Van Gerpen, J., 2001. Biodiesel production from oils and
fats with high free fatty acids. Trans. ASME. 44, 1429e1436.
Carvalho, A.P., Meireles, L.A., Malcata, F.X., 2006. Microalgal reactors:
a review of enclosed system designs and performances. Biotechnol.
Prog. 22, 1490e1506.
Chisti, Y., 2007. Biodiesel from microalgae. Biotechnol. Adv. 25, 294e
306.
Cordell, D., Drangert, J.O., White, S., 2009. The story of phosphorus:
global food security and food for thought. Global Environ. Change
19, 292e305.
Darunde Dhiraj, S., Deshmukh Mangesh, M., 2012. Biodiesel
production from animal fats and its impact on the diesel engine
199
with ethanol-diesel blends: a review. Int. J. Emerging Technol. Adv.
Eng. 2, 179e185.
Demirbas, A., 2009. Political, economic and environmental impacts of
biofuels: a review. Appl. Energy. 86, S108eS117.
Demirbas, M.F., Balat, M., Balat, H., 2011. Biowastes-to-biofuels.
Energy Convers. Manage. 52, 1815e1828.
Dijkstra, A.J., 2010. Enzymatic degumming. Eur. J. Lipid Sci. Technol.
112, 1178e1189.
Dijkstra, A.J., Van Opstal, M., 1989. The total degumming process.
J. Am. Oil Chem. Soc. 66, 1002e1009.
Dolatowski, Z.J., Stadnik, J., Stasiak, D., 2007. Applications of ultrasound in food technology. Acta Sci. Pol. Technol. Aliment. 6, 89e
99.
Duku, M.H., Gu, S., Hagan, E.B., 2011. A comprehensive review of
biomass resources and biofuels potential in Ghana. Renewable
Sustainable Energy Rev. 15, 404e415.
Dunn, R.O., 2009. Cold-flow properties of soybean oil fatty acid
monoalkyl ester admixtures. Energy Fuels 23, 4082e4091.
Ellis, M., 2007. Air Quality and Biodiesel Production. Nebraska
Department of Environmental Quality. Available from: www.
deq.state.ne.us/Publica.nsf/c4afc76e4e077e11862568770059b73f/
9bf9daf0963eb8728625729f00575cbd/$FILE/06-272.pdf (accessed
14.02.13.).
Encinar, J., González, J., Rodrı́guez-Reinares, A., 2007. Ethanolysis of
used frying oil. Biodiesel preparation and characterization. Fuel
Process. Technol. 88, 513e522.
Farrell, A.E., Plevin, R.J., Turner, B.T., Jones, A.D., O’Hare, M.,
Kammen, D.M., 2006. Ethanol can contribute to energy and environmental goals. Science 311, 506e508.
Feddern, V.J., De Praá, A.C., de Abreu, M.C., dos Santos Filho, P.G.,
Higarashi, J.I., Sulenta, M.M., Coldebella, A., 2011. Animal fat
wastes for biodiesel production. In: Montero, M.S.A.G. (Ed.),
Biodiesel - Feedstock and Processing Technologies. InTech,
pp. 45e70.
Food and Agriculture Organization, 2008. The state of food and
agriculture 2008 biofuels: prospects, risks and opportunities.
Available from: www.fao.org/docrep/011/i0100e/i0100e00.htm
(accessed 14.02.13.).
Francis, G., Edinger, R., Becker, K., 2005. A concept for simultaneous wasteland reclamation, fuel production, and socioeconomic development in degraded areas in India: need,
potential and perspectives of Jatropha plantations. Nat. Resour.
Forum 29, 12e24.
Geller, D.P., Adams, T.T., Goodrum, J.W., Pendergrass, J., 2008. Storage
stability of poultry fat and diesel fuel mixtures: specific gravity and
viscosity. Fuel 87, 92e102.
Gong, Y., Jiang, M., 2011. Biodiesel production with microalgae as
feedstock: from strains to biodiesel. Biotechnol. Lett. 33, 1269e
1284.
Gouveia, L., Oliveira, A.C., 2009. Microalgae as a raw material for
biofuels production. J. Ind. Microbiol. Biotechnol. 36, 269e274.
Greene, A.K., Dawson, P.L., Nixon, D., Atkins, J.R., Pearl, G.G., 2007.
Safety of animal fats for biodiesel production: a critical review of
the Literature. Available from: www.iea-amf.vtt.fi/pdf/annex30_
vol3.pdf (accessed 15.02.13.).
Greenwell, H., Laurens, L., Shields, R., Lovitt, R., Flynn, K., 2010.
Placing microalgae on the biofuels priority list: a review of the
technological challenges. J. R. Soc., Interface 7, 703e726.
Groschen, R.. Overview of the Feasibility of Biodiesel from Waste/
Recycled Greases and Animal Fats. Minnesota Department of Agriculture. Available from: www.mda.state.mn.us/news/publications/
renewable/wastefatsfeasability.pdf (accessed 08.02.13.).
Gui, M.M., Lee, K.T., Bhatia, S., 2008. Feasibility of edible oil vs nonedible oil vs waste edible oil as biodiesel feedstock. Energy 33,
1646e1653.
200
12. BIOBASED FATS (LIPIDS) AND OILS FROM BIOMASS AS A SOURCE OF BIOENERGY
Gutierrez, J., Barry-Ryan, C., Bourke, P., 2009. Antimicrobial activity of
plant essential oils using food model media: efficacy, synergistic
potential and interactions with food components. Food Microbiol.
26, 142e150.
Hamm, W., Hamilton, R.R.J., 2000. Edible Oil Processing. Sheffield
Academic Press, Sheffield. p. 281.
Handa, S.S., 2008. An overview of extraction techniques for medicinal
and aromatic plants. In: Handa, S.S., Khanuja, S.P.S., Longo, G.,
Rakesh, D.D. (Eds.), Extraction Technologies for Medicinal and
Aromatic Plants. International Centre for Science and High
Technology (ICS), Trieste, pp. 21e52.
Hardwood, J.L., Weselake, R.J., 2013. AOCS lipid library. Available
from: www.lipidlibrary.com (accessed 10.01.13.).
Herrero, M., Mendiola, J.A., Cifuentes, A., Ibáñez, E., 2010. Supercritical fluid extraction: recent advances and applications.
J. Chromatogr. A 1217, 2495e2511.
Hu, Q., Sommerfeld, M., Jarvis, E., Ghirardi, M., Posewitz, M.,
Seibert, M., Darzins, A., 2008. Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J.
54, 621e639.
Hu, J., Yu, F., Lu, Y., 2012. Application of FischereTropsch synthesis in
biomass to liquid conversion. Catalysts 2, 303e326.
Huntley, M.E., Redalje, D.G., 2007. CO2 mitigation and renewable oil
from photosynthetic microbes: a new appraisal. Mitigation Adapt.
Strategies Global Change 12, 573e608.
Jain, S., Sharma, M., 2010. Stability of biodiesel and its blends: a
review. Renewable Sustainable Energy Rev. 14, 667e678.
Jayasinghe, P., Hawboldt, K., 2011. A review of bio-oils from waste
biomass: focus on fish processing waste. Renewable Sustainable
Energy Rev. 16, 798e821.
Kerihuel, A., Senthil Kumar, M., Bellettre, J., Tazerout, M., 2005. Use of
animal fats as CI engine fuel by making stable emulsions with
water and methanol. Fuel 84, 1713e1716.
Kerschbaum, S., Rinke, G., Schubert, K., 2008. Winterization of biodiesel by micro process engineering. Fuel 87, 2590e2597.
Kleinová, A., Vailing, I., Lábaj, J., Mikulec, J., Cvengros, J., 2011.
Vegetable oils and animal fats as alternative fuels for diesel engines
with dual fuel operation. Fuel Process. Technol. 92, 1980e1986.
Knothe, G., 2011. A technical evaluation of biodiesel from vegetable
oils vs algae. Will algae-derived biodiesel perform? Green Chem.
13, 3048e3065.
Knothe, G., Van Gerpen, J.H., Krahl, 2005. The Biodiesel Handbook,
vol. 1. AOCS press, Champaign, IL. USA. p. 304.
Komers, K., Machek, J., Stloukal, R., 2001a. Biodiesel from rapeseed
oil, methanol and KOH. 2. Composition of solution of KOH in
methanol as reaction partner of oil. Eur. J. Lipid Sci. Technol. 103,
359e362.
Komers, K., Stloukal, R., Machek, J., Skopal, F., 2001b. Biodiesel from
rapeseed oil, methanol and KOH. 3. Analysis of composition of
actual reaction mixture. Eur. J. Lipid Sci. Technol. 103, 363e371.
Kusdiana, D., Saka, S., 2004. Effects of water on biodiesel fuel production by supercritical methanol treatment. Bioresour. Technol.
91, 289e295.
Lakó, J., Hancsók, J., Yuzhakova, T., Marton, G., Utasi, A., Rédey, Á,
Popa, V., 2008. Biomassea source of chemicals and energy for
sustainable development. Environ. Eng. Manage. J. 7, 499e509.
Lam, M.K., Tan, K.T., Lee, K.T., Mohamed, R., 2009. Malaysian palm
oil: surviving the food versus fuel dispute for a sustainable future.
Renewable Sustainable Energy Rev. 13, 1456e1464.
León-Camacho, M., Viera-Alcaide, I., Ruiz-Méndez, M., 2003. Elimination of polycyclic aromatic hydrocarbons by bleaching of olive
pomace oil. Eur. J. Lipid Sci. Technol. 105, 9e16.
Liang, K., Zhang, Q., Cong, W., 2012. Enzyme-assisted aqueous
extraction of lipid from microalgae. J. Agric. Food Chem. 60,
11771e11776.
Liu, X., Clarens, A.F., Colosi, L.M., 2011. Algae biodiesel has potential
despite inconclusive results to date. Bioresour. Technol. 104, 803e
806.
Martı́n, M., Grossmann, I.E., 2012. On the systematic synthesis of
sustainable biorefineries. Ind. Eng. Chem. Res. 52, 3044e3064.
Martı́nez, M.L., Mattea, M.A., Maestri, D.M., 2008. Pressing and supercritical carbon dioxide extraction of walnut oil. J. Food Eng. 88,
399e404.
Math, M., Kumar, S.P., Chetty, S.V., 2010. Optimization of biodiesel
production from oils and fats with high free fatty acids. Indian J.
Sci. Technol. 3, 318e321.
Mathiyazhagan, M., Ganapathi, A., Jaganath, B., Renganayaki, N.,
Sasireka, N., 2011. Production of biodiesel from non-edible plant
oils having high FFA content. Int. J. Chem. Environ. Eng. 2, 119e
122.
Melis, A., 2009. Solar energy conversion efficiencies in photosynthesis:
minimizing the chlorophyll antennae to maximize efficiency. Plant
Sci. 177, 272e280.
Mendiola, J.A., Herrero, M., Cifuentes, A., Ibañez, E., 2007. Use of
compressed fluids for sample preparation: food applications.
J. Chromatogr., A 1152, 234e246.
Meng, X., Yang, J., Xu, X., Zhang, L., Nie, Q., Xian, M., 2009. Biodiesel
production from oleaginous microorganisms. Renewable Energy
34, 1e5.
Moheimani, N.R., Borowitzka, M.A., 2006. The long-term culture of
the coccolithophore Pleurochrysis carterae (Haptophyta) in outdoor
raceway ponds. J. Appl. Phycol. 18, 703e712.
Morrison, W., Coventry, A., 2006. Extraction of lipids from cereal
starches with hot aqueous alcohols. Starch-Stärke. 37, 83e87.
Mrad, N., Varuvel, E.G., Tazerout, M., Aloui, F., 2012. Effects of biofuel
from fish oil industrial residueediesel blends in diesel engine.
Energy 44, 955e963.
National Renewable Energy Laboratory, 2009. Biodiesel Handling and
Use Guide. Revised, Fourth ed. National Renewable Energy
Laboratory, Washington, D.C., USA.
National Research Council, 2012. Sustainable Development of Algal
Biofuels in the United States. The National Academies Press,
Washington, D.C. USA. p. 232.
Nigam, P.S., Singh, A., 2011. Production of liquid biofuels from
renewable resources. Prog. Energy Combust. Sci. 37, 52e68.
No, S.Y., 2011. Inedible vegetable oils and their derivatives for alternative diesel fuels in CI engines: a review. Renewable Sustainable
Energy Rev. 15, 131e149.
O’Donnell, C., Tiwari, B., Bourke, P., Cullen, P., 2010. Effect of ultrasonic processing on food enzymes of industrial importance. Trends
Food Sci. Technol. 21, 358e367.
Pandey, V.C., Singh, K., Singh, J.S., Kumar, A., Singh, B., Singh, R.P.,
2012. Jatropha curcas: a potential biofuel plant for sustainable
environmental development. Renewable Sustainable Energy Rev.
16, 2870e2883.
Panneerselvam, S.I., Parthiban, R., Miranda, L.R., 2011. Poultry fatda
cheap and viable source for biodiesel production. Proc. Int. Conf.
Environ. Sci. Technol. (ICEST 2011) 6, 371e374.
Phan, A.N., Phan, T.M., 2008. Biodiesel production from waste cooking oils. Fuel 87, 3490e3496.
Pires, J.C.M., Alvim-Ferraz, M.C.M., Martins, F.G., Simões, M., 2012.
Carbon dioxide capture from flue gases using microalgae:
engineering aspects and biorefinery concept. Renewable Sustainable Energy Rev. 16, 3043e3053.
Popescu, F., Ionel, I., 2011. Waste animal fats with high FFA as a
renewable energy source for biodiesel production - concept,
experimental production and impact evaluation on air quality. In:
Manzanera., M. (Ed.), Alternative Fuel. Intech, pp. 93e108.
Proc, K.B., Barnitt, R., Hayes, R.R., Ratcliff, M., McCormick, R.L., 2006.
100,000-mile evaluation of transit buses operated on biodiesel
REFERENCES
blends (B20). In: Paper presented at Society of Automotive Engineers Powertrain and Fluid Systems, Toronto, Canada.
Prosková, A., Kopicová, Z., Kucera, J., Skarková,
L., 2009. Acid
catalysed transesterification of animal waste fat. Res. Agric. Eng.
55, 24e28.
Prueksakorn, K., Gheewala, T.E., 2006. Energy and greenhouse gas
implications of biodiesel production from Jatropha curcas L. In:
Paper presented at Proceedings of the Second Joint International
Conference on Sustainable Energy and Environment, Bangkok,
Thailand.
Pulz, O., 2001. Photobioreactors: production systems for phototrophic
microorganisms. Appl. Microbiol. Biotechnol. 57, 287e293.
Puri, M., Abraham, R.E., Barrow, C.J., 2012. Biofuel production:
prospects, challenges and feedstock in Australia. Renewable
Sustainable Energy Rev. 16, 6022e6031.
Resurreccion, E.P., Colosi, L.M., White, M.A., Clarens, A.F., 2012.
Comparison of algae cultivation methods for bioenergy production
using a combined life cycle assessment and life cycle costing
approach. Bioresour. Technol. 126, 298e306.
Rodolfi, L., Chini Zittelli, G., Bassi, N., Padovani, G., Biondi, N.,
Bonini, G., Tredici, M.R., 2008. Microalgae for oil: strain selection,
induction of lipid synthesis and outdoor mass cultivation in a lowcost photobioreactor. Biotechnol. Bioeng. 102, 100e112.
Russo, D., Dassisti, M., Lawlor, V., Olabi, A., 2012. State of the art of
biofuels from pure plant oil. Renewable Sustainable Energy Rev.
16, 4056e4070.
Rutz, D., Janssen, R., 2007. Biofuel Technology Handbook, vol. 1. WIP
Renewable Energies, 149.
Sapphire Energy, 2013. Green crude farm. Available from: www.
sapphireenergy.com/locations/green-crude-farm.html (accessed
10.01.13.).
Sarker, S.D., Latif, Z., Gray, A.I., 2005. Natural Products Isolation,
second ed. Springer, New York, USA. p. 515.
Sawayama, S., Inoue, S., Dote, Y., Yokoyama, S.Y., 1995. CO2 fixation
and oil production through microalga. Energy Convers. Manage.
36, 729e731.
Schenk, P.M., Thomas-Hall, S.R., Stephens, E., Marx, U.C.,
Mussgnug, J.H., Posten, C., Kruse, O., Hankamer, B., 2008. Second
generation biofuels: high-efficiency microalgae for biodiesel production. BioEnergy Res. 1, 20e43.
Scott, S.A., Davey, M.P., Dennis, J.S., Horst, I., Howe, C.J.,
Lea-Smith, D.J., Smith, A.G., 2010. Biodiesel from algae: challenges
and prospects. Curr. Opin. Biotechnol. 21, 277e286.
Shahidi, F., Zhong, Y., 2005. Marine mammal oils. In: Shahidi, F. (Ed.),
Bailey’s Industrial Oil and Fat Products. John Wiley & Sons, Inc.,
USA. pp. 259e278.
Sheehan, J., Dunahay, T., Benemann, J., Roessler, P., 1998. A Look Back
at the US Department of Energy’s Aquatic Species Program:
Biodiesel from Algae. National Renewable Energy Laboratory.
Available from: www.nrel.gov/biomass/pdfs/24190.pdf (accessed
10.01.13.).
Singh, S., Singh, D., 2010. Biodiesel production through the use of
different sources and characterization of oils and their esters as the
substitute of diesel: a review. Renewable Sustainable Energy Rev.
14, 200e216.
Singh, A., He, B., Thompson, J., Van Gerpen, J., 2006. Process optimization of biodiesel production using alkaline catalysts. Appl.
Eng. Agric. 22, 597e600.
201
Solazyme Inc. 2013. Biotechnology that creates renewable oils from
microalgae. Available from: http://solazyme.com/technology
(accessed 10.01.13.)
Solix BioSystems, 2013. Available from: www.solixbiofuels.com
(accessed 10.01.13.).
Stauffer, E., Byron, D., 2007. Alternative fuels in fire debris analysis:
biodiesel basics. J. Forensic Sci. 52, 371e379.
Sun, A., Davis, R., Starbuck, M., Ben-Amotz, A., Pate, R., Pienkos, P.T.,
2011. Comparative cost analysis of algal oil production for biofuels.
Energy 36, 5169e5179.
Takahashi, H., Hirata, S., Minami, H., Fukuyama, Y., 2001. Triterpene
and flavanone glycoside from Rhododendron simsii. Phytochemistry
56, 875e879.
Tenenbaum, D.J., 2008. Food vs fuel: diversion of crops could cause
more hunger. Environ. Health Perspect. 116, A254eA257.
Thamsiriroj, T., Murphy, J., 2010. How much of the target for biofuels
can be met by biodiesel generated from residues in Ireland? Fuel.
89, 3579e3589.
Ugwu, C., Aoyagi, H., Uchiyama, H., 2008. Photobioreactors for mass
cultivation of algae. Bioresour. Technol. 99, 4021e4028.
U.S. Census Bureau, 2010. Current industrial reports - M311K - fats
and oils: production, consumption, and stocks. Available from:
http://www.census.gov/cir/www/311/m311k.html
(accessed
10.01.13.).
U.S. Department of Agriculture, 2010. Oil Crops Yearbook. Available
from: usda.mannlib.cornell.edu/MannUsda/viewDocumentInfo.
do?documentID¼1290 (accessed 10.01.13.).
Varuvel, E.G., Mrad, N., Tazerout, M., Aloui, F., 2012. Experimental
analysis of biofuel as an alternative fuel for diesel engines. Appl.
Energy. 94, 224e231.
Vasudevan, P., Sharma, S., Kumar, A., 2005. Liquid fuel from biomass:
an overview. J. Sci. Ind. Res. 64, 822e831.
Vishwakarma, S., 2010. Countercurrent extraction of 2, 3-Butanediol.
Int. J. Chem. Eng. Appl. 1, 147e150.
Wahlen, B.D., Morgan, M.R., McCurdy, A.T., Willis, R.M.,
Morgan, M.D., Dye, D.J., Bugbee, B., Wood, B.D., Seefeldt, L.C.,
2012. Biodiesel from microalgae, yeast, and bacteria: engine performance and exhaust emissions. Energy Fuels 27, 220e228.
Weldy, C.S., Huesemann, M.H., 2007. Lipid production by Dunaliella
salina in batch culture: effects of nitrogen limitation and light
intensity. J. Undergrad. Chem. Res. 7, 115e122.
Wenclawiak, B., 1992. Analysis with Supercritical Fluids: Extraction
and Chromatography. Springer-Verlag, Munich, Germany.
Widjaja, A., Chien, C.C., Ju, Y.H., 2009. Study of increasing lipid
production from fresh water microalgae Chlorella vulgaris.
J. Taiwan Inst. Chem. Eng. 40, 13e20.
Wisniewski Jr, A., Wiggers, V.R., Simionatto, E.L., Meier, H.F.,
Barros, A.A.C., Madureira, L.A.S., 2010. Biofuels from waste fish
oil pyrolysis: chemical composition. Fuel 89, 563e568.
Woodgate, S., Van Der Veen, J., 2004. The role of fat processing and
rendering in the European Union animal production industry.
Biotechnol. Agron. Soc. Environ. 8, 283e294.
Yang, B., Zhou, R., Yang, J.G., Wang, Y.H., Wang, W.F., 2008. Insight
into the enzymatic degumming process of soybean oil. J. Am. Oil
Chem. Soc. 85, 421e425.
Yun, Y.S., Lee, S.B., Park, J.M., Lee, C.I., Yang, J.W., 1997. Carbon dioxide fixation by algal cultivation using wastewater nutrients.
J. Chem. Technol. Biotechnol. 69, 451e455.
C H A P T E R
13
Use of Volatile Solids from
Biomass for Energy Production
W.J. Oosterkamp
Oosterkamp Oosterbeek Octooien, The Netherlands
email: willemjan@oosterkamp.org
O U T L I N E
Introduction
204
Biodegradability
204
Addition of Macro- and
Micronutrients
204
Digestion Systems
Family-Size Biogas Plant
Wet Digesters
Scum Layer Digester
Solid Biomass Digester
211
211
211
211
212
Addition of Microbes
205
Increase in Solids Content in Wet Digesters
212
Addition of Enzymes
206
Loading and Unloading of Digesters
212
Pretreatments
Biological Pretreatment with
Enzymes
Chemical Pretreatment
Hot Water Treatment
Mechanical Pretreatment
207
Treatment of Digestate in Wet Digesters
212
Use of Methane
213
Chemical Conversion of Volatile Solids
Combustion
Gasification
213
213
213
Longer Retention Times
207
Energy Crops
207
Thermal Conversion of Volatile Solids
Slow Pyrolysis
Flash Pyrolysis
214
214
214
Food Processing Residues
Rice Husks
Bagasse
Coffee Husks and Mucilage
207
207
207
208
Crop Residues
209
Discussion
Maximum Methane Yield
Nutrient Recycling
Soil Fertility
Digesters
214
214
214
214
214
Spent Bedding
209
Conclusions
214
Kitchen and Garden Waste
209
References
215
Aquatic Weeds
209
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00013-9
207
207
207
207
203
Copyright Ó 2014 Elsevier B.V. All rights reserved.
204
13. USE OF VOLATILE SOLIDS FROM BIOMASS FOR ENERGY PRODUCTION
INTRODUCTION
All-renewable energy resources are required to
reduce dependency on fossil fuels from politically unstable regions. Biomass is one such renewable energy
resource. Farm and food processing residues are
preferred but, where economic, energy plants can be
used.
Biomass as such cannot replace fossil fuels. Such
materials have to be converted into gas, liquid or electricity. Biological volatilizing (anaerobic digestion) converts organic by-products and residues into methane
and carbon dioxide, an energy source that can be used
for cooking, the production of electricity and as transportation fuel.
In Asia there are over 10 million family-size anaerobic
digestion plants utilizing manure and some straw. The
biogas is used for cooking. There are significant health
advantages in using biogas, compared to the local alternative of the burning of cattle manure, leaves and wood
inside the houses.
There are a few thousand centralized biogas plants in
Europe that use manure with a whole range of easily
digestible residues. Other biogas plants in Europe use
sludge from wastewater cleanup plants. They convert
the biogas into electricity and heat. Carbon dioxide is
removed from the biogas in a number of recent plants;
the gas is compressed and injected into the natural gas
grid.
The digestate, after the production of biogas, should
be used as an organic fertilizer. This will recycle the
macro elements nitrogen, potassium, phosphorus and
carbon to the soil. Recycling of carbon is essential for
high soil productivity and will reverse the trend of
lowering of crop yields (Hossain, 2001).
The energy content of the animal residues (mostly
manure) produced worldwide is equivalent to an
average power of 50e150 W per person (9e25 EJ/a).
The energy content of crop residues (mostly straw) is
also 50e150 W per person (Hoogwijk et al., 2003).
Worldwide energy consumption is 2.5 kW per person
(500 EJ/a). Oil production worldwide is 1 kW per person (80 million barrels a day). Biogas from straw and
manure can replace about 10e30% of the world oil production. This substitution can be doubled by the use of
forest residues.
only part of it can be depolymerized into soluble components. Anaerobic digestion is a complex process that
is slow compared to chemical processes. Chynoweth
et al. (1987) have published on the processes involved
in the anaerobic digestion of biomass. Hydrolytic bacteria break down the cellulose and hemicellulose into
organic acids and neutral compounds. Hydrogen producing bacteria convert the acids into hydrogen.
Homoacetogenic bacteria convert hydrogen into acetic
acid. Methanogenic bacteria convert acetic acid into
methane. A by-product in these conversions is carbon
dioxide.
Anaerobic biodegradation potential assay is performed by mixing the material with digestate from an
operating digester or by mixing the material with a
defined nutrient medium according to Owen et al.
(1979). The methane produced is measured at different
times.
Chandler et al. (1980) made a correlation based on 15
different lingocellulosic materials.
yCH4 ¼ a ðb c li Þ
(13.1)
where
yCH4 is the methane yield in l/kg volatile solids (VS)
a ¼ 440 l/kg is the conversion between methane yield
and VS reduction (Jerger et al., 1982).
b ¼ 0.83 fitted constant.
c ¼ 2.8 fitted constant.
li is lignin fraction of the VS
This correlation gives a standard deviation of
80 l/kg VS for straws and woody biomass (Table 13.1).
A different correlation was developed for straws and
woody biomass.
(13.2)
y CH4 ¼ a ð1 li Þ 1 edt
d ¼ f * (1 g * li) is exponential factor.
f ¼ 0.025 fitted constant.
g ¼ 3 fitted constant.
This correlation assumes that biodegradation can be
described as a first-order process. Shielding of cellulose
and hemicellulose by lignin is reflected in the exponential factor. This shielding eventually breaks down. This
correlation performs better with a standard deviation
of 32 l/kg VS.
BIODEGRADABILITY
ADDITION OF MACRO- AND
MICRONUTRIENTS
Agricultural waste materials like straw and the solid
fraction of manure are lignocellulosic materials. These
materials are strong, flexible and protected against
decay. They consist of cellulose, hemicellulose and
lignin. Lignin cannot be converted into biogas, and
Solubilization of lignocellulosic materials is inhibited
by free fatty acids produced during hydrolyzation and
subsequent acidogenesis. Increasing the number of
methanogenic bacteria reduces the concentration of
free fatty acids. Macronutrients nitrogen and phosphate
205
ADDITION OF MICROBES
TABLE 13.1
Substrate
Biodegradability
Lignin (%)
Test Period
(days)
Methane
Yield (l/kg)
Calculated
Methane Yield
Chandler
et al., 1980
Maize I
8
70
313
298
286
Amon et al., 2003
Maize II
6
70
326
315
312
Amon et al., 2003
Maize III
4
70
287
332
338
Amon et al., 2003
Rice Straw
10
40
240
200
260
Somayai et al., 1994
Barley
13
100
300
299
221
Moeller et al., 2006
Wheat Straw
17
100
270
258
169
Moeller et al., 2006
Maize straw
16
70
200
221
182
Mo et al., 2011
Willow
14
139
289
328
208
Lehtomaeki 2006
Oat Straw
21
150
320
261
117
Lehtomaeki 2006
Rapeseed Straw
20
154
240
277
130
Lehtomaeki 2006
Cotton Wood
25
120
140
174
65
Jerger et al., 1982
Hybrid Poplar
26
120
130
157
52
Jerger et al., 1982
Sycamore
26
120
190
157
52
Jerger et al., 1982
Black Alder
28
120
70
121
26
Jerger et al., 1982
Lignin data for rice straw, barley straw, wheat straw and maize straw are from Godin et al. (2010). The others are from the authors of the biodegradability tests. Godin
et al. give for oats straw a lignin value of 0.14.
and the micronutrients S, Ca, Mg, Fe, Ni, Co, Mo, Zn,
Mn, Se and Cu (Demirel et al.., 2011) are required for
the multiplication of methanogenic bacteria. Scherer
et al. (1983) determined the chemical composition of a
number of methanogenic bacteria (Table 13.2). Scherer
(2011) advises also on the minimum concentration
of ions in a digester. The basal medium of GuengoerDemirci et al. (2004) and the recommendations of Speece
(1987) are similar. Lebuhn et al. (2010) formulated a special cocktail. They commented that for maize silage Co
should be added at 0.1 mg/kg VS and sodium at
30 mg/kg VS. Speece (1987) has a recommendation for
Se. Se was not limiting in the tests by Lebuhn et al.
(2010).
Straws, husks, bagasse and woody biomass are generally deficient in macro- and micronutrients. The same
holds for cattle manure in Asia as cattle feed mostly on
rice straw. Manures in Europe and North America
have an excess of nitrogen.
Jerger et al., 1982 found a 60% increase in methane
yield in half the time in batch-fed anaerobic potential
assays with extra-micronutrients (Table 13.3). They
also added NH4CL and KH2PO4 to reduce the C/N ratio
to 15 and the C/P ratio to 75. Similar results have been
obtained by Komatsu et al. (2007). They obtained a
methane yield of 280 l/kg VS with sewage sludge and
rice straw at a hydraulic retention time of 20 days in a
continuously operating digester at 36 C. Somayaji
et al. (1994) had 240 l/kg VS in 40 days for rice straw.
The addition of micronutrients has an effect of 10e70%
on the methane production.
Sewage water cleanup sludges are a source of macro
and micronutrients. Average primary sewage sludge has
the right concentration for Ni and Mo. Co is an order of
magnitude too low. In some sludges the concentrations
of Fe, Co and Ni are too low (Speece, 1988). Concentrations for Ca, Fe, Zn, Mn and Cu are an order of magnitude too high for its use in agriculture (Wolf et al., 2005).
Optimum nutrient conditions are cost-effective.
Industrial fertilizers should be used, lacking organic
sources of nitrogen and phosphate. For each kilogram
of dry lignocellulosic biomass a maximum of 40 g of
urea and 20 g of phosphate are required. Human urine
is a good source of nitrogen and phosphate. Human
feces are also good but require storage for more than
100 days in order to prevent the spread of illnesses. Ecosan toilets (Terefe and Edström, 1999) separate urine and
feces, so that urine can be directly used and feces stored
for the required period.
ADDITION OF MICROBES
Op den Camp et al. (1991) describe an acidogenic
reactor with rumen-derived bacteria. A hydraulic
retention time of 12 h and a solids retention time of
72 h resulted in a methane yield of 440 l/kg VS for cellulose and 120 l/kg VS for barley and rye straw. The
206
13. USE OF VOLATILE SOLIDS FROM BIOMASS FOR ENERGY PRODUCTION
TABLE 13.2
Nutrients
Methanogenic
Bacteria
Scherer et al.,
1983 (g/kg)
Speece, 1987
(mg/l)
Lebuhn et al.,
2010 (mg/l)
Scherer (2011)
Ions (mg/l)
Sewage Sludge
Basal Medium
Guengor-Demirci
and Demirer, 2004
(mg/l)
Loeffen et al.
Kelly et al., 2005
1984 (g/kg) (g/kg)
N
100
800
33
80
P
20
19
23
40
K
25
130
3
S
10
40
16
Ca
3
15
39
Mg
3
4
4
Fe
2
10
0.300
11
11
Ni
0.120
0.1
0.160
2
0.12
0.080
Co
0.065
5
0.220
0.5
2.4
0.004
Mo
0.040
0.1
0.115
0.5
0.26
0.030
Zn
0.340
0.001
0.24
1.740
Mn
0.015
0.260
0.14
0.260
Cu
0.085
0.19
0.850
Se
0.1
0.010
0.05
1.5
0.6
0.01
Author
Scherer et al., 1983
Concentrations for methanogenic bacteria
Guengor-Demirci
et al., 2004
Basal medium
Kelly et al., 1984
Sewage sludge; median values of 200 sludge samples in the United States. Values vary by an order of magnitude from
sample to sample
Loeffen et al. 2005
Sewage sludge data from the Netherlands 2002
TABLE 13.3
Effect of Micronutrients on Wood Substrates
Methane Yield (l/kg VS)
Woody Biomass
Without Micro
Elements
120 days
With Micro
Elements 60 days
Cotton Wood
140
200
Hybrid Poplar
130
270
Sycamore
190
220
Black Alder
70
135
methane was produced in a second reactor separated
from the first by a filter with 0.03 mm pore size. The
liquid without the free fatty acids was recycled to the
first reactor. Soluble lignin products (humic acids)
inhibited further degradation of the straws. The
German company Ares Technology is performing tests
at pilot plant scale. Typical conversion yields are
around 50% (Strecker, 2012).
Weiss et al. (2009) isolated and multiplied hemicellulytic bacteria. These were immobilized on trace metal
activated zeolite. Digestion of second-stage sludge from
a biogas plant gave a methane yield of 215 l/kg VS after
34 days (35 C) and 150 l/kg VS for the control.
ADDITION OF ENZYMES
Some European companies (Telschow, 2006; Chollet,
2011) advertise the application of special enzyme combinations in biogas digesters. A 30% faster digestion or a
10% higher biogas yield is reported.
Water cleanup secondary sludge is a source of
enzymes. The secondary sludge consists mainly of bacteria and the intracellular liquid of these bacteria contains lyses enzymes.
207
FOOD PROCESSING RESIDUES
PRETREATMENTS
Biological Pretreatment with Enzymes
Shredded straws, bagasse and husks are seasonal
products and need to be stored before being used as substrate in a digester. Storage with silage can be used to
improve the biodigestability of the substrate. Methane
yield for maize silage increased from 290 l/kg VS to
330 l/kg VS using the enzyme mixture Microfern
(Bossuwe, 2011).
Methane yield increased from 145 l/kg VS (fresh
reed) to 200 l/kg VS (reed silage prepared with the
enzyme mixture Methaplus; Helbig, 2009). Komatsu
et al. (2007) report an increase in methane yield from
280 l/kg VS to 310 l/kg VS for rice straw soaked in a
solution of an unspecified enzyme codigested with
sewage sludge.
Chemical Pretreatment
Lime (calcium hydroxide) is a relatively cheap chemical and calcium improves the fertility of the soil. In its
production about 0.8 kWh/kg high-temperature thermal energy is used. Gunnerson et al.; (1987) advise to
compost straw with lime, water and dung. In this
method a fraction of VS is lost. Raju et al., 2010 demonstrated an increase of 60% in biogas production using a
pretreatment at 0.015 kg Ca(OH)2 per kilogram VS.
The pretreatment with 1.5% CaOH is equivalent to
an increase in retention time from 32 to 100 days
(Moeller et al., 2006). Klopfenstein (1978) found for
hemicellulose and cellulose an increase of 80% and
20%, respectively, for sodium hydroxide using corncobs as substrate. The yield increase was only 25% using calcium hydroxide both for hemicellulose and
cellulose.
Pretreatment with a minimum amount of dilute
acids at 50e100 C dissolves the hemicellulose and
leaves a solid residue that is highly porous (Tsao,
1987). German biogas tanks have an acid pretreatment
(Sauter, 2012). Lebuhn et al. (2010) report technical difficulties with the acid pretreatment and no increase in
methane yield.
Schober et al. (2006) and Busch et al. (2006) describe
an aerated percolation reactor followed by a methanogenese reactor. They report shorter retention times for
kitchen and garden waste and maize silage compared
to wet systems.
Hot Water Treatment
Raju et al. (2010) obtained a 40% increase in methane
yield using a 15 min pretreatment of wheat and rapeseed straw at 75 C.
Mechanical Pretreatment
Jerger et al., 1983 found an increase in the methane
yield from 270 l/kg VS for particles of hybrid poplar
<8 mm to 310 l/kg VS for particles <0.8 mm. The duration of the tests was 90 days. Slotyuk (Oechsner, 2012)
found an increase from 230 l/kg VS for 10 mm wheat
straw particles to 300 l/kg VS for 1 mm particles. The
duration of the tests was 35 days (Table 13.4).
LONGER RETENTION TIMES
Doubling of the retention times increases the gas
yield with 30e50% (Table 13.5). It is unfortunate that
the tests were not done at optimum nutrient concentrations. Calculated yields for shorter retention times using
Eqns (1) and (2) are compared with measured yields in
Table 13.6. The standard deviation between measurement and calculation is 35 l/kg VS (similar to the correlation for longer retention times) (Table 13.7).
ENERGY CROPS
About 7% of the land used for agriculture in Germany
is planted with maize destined for methane production.
There are a number of other energy crops with higher
production costs (Boese, 2010). Some of these crops
have a higher methane yield per hectare (Table 13.8).
The humus content of the soils will decrease when
only maize is planted as crop (Willms et al., 2009).
FOOD PROCESSING RESIDUES
Rice Husks
The production of rice husks is about 100 million
tons per year. Only a fraction of it is used as animal
bedding or as fuel for energy production. In Asia briquettes are produced from rice husks. These are expensive to produce, due to the silicon content of the husks.
Hill et al. (1981) obtained 110 l/kg VS at a retention
time of 17 days. Pretreatment with 8% NaOH gave a
methane yield of 200 l/kg VS (Vevekanandan et al.,
2011).
Bagasse
Bagasse is the pressed stalks from sugarcane. It is
washed to remove nearly all the sugar in the stalks
and leaves the factory at 50% humidity with 5% sugar
remaining. World production is 140 million tons (dry
weight). Most of the bagasse is used as fuel in the sugar
208
13. USE OF VOLATILE SOLIDS FROM BIOMASS FOR ENERGY PRODUCTION
TABLE 13.4
Effect of Nutrients
Author
Process
Temperature
Test
Duration
Control
Methane
(l/kg VS)
Test Methane
(l/kg VS)
RICE STRAW
Bardiya et al., 1999
25 ppm Fe
Batch
Ambient
40 days
63
120
Bardiya et al., 1999
Single dose Ni
Batch
Ambient
40 days
63
110
20 mM FeSO4
Continuously fed
37 C
16 days
128
180
CATTLE MANURE
Preeti Rao et al., 1993
Guengoer-Demirci
et al., 2004
Basal medium
Batch
35 C
90 days
260
290
Lar et al., 2010
Ca 1.5 g/l
Batch
35 C
Lar et al., 2010
Fe 0.4 g/l
Lehtomaeki, 2006
Ali et al., 2010
25% Jatropha press cake
Haque et al., 2006
160
185
50 days
190
225
20 days
150
215
95
115
35 C
Batch
30% barley straw
50 days
35 C
Continuously fed
27 C
Batch
2% dry matter urine
Batch
28e35 C
40 days
195
260
10% w/w Glauconite
Continuously fed
55 C
15 days
67
90
165 days
250
300
280
380
270
370
SWINE MANURE
Hansen et al.,1999
35 C
Ahn et al., 2006
3% CaCl
Batch
Moeller et al., 2006
30% wheat straw
Continuously fed
0.2 mg/l Se
Continuously fed
FOOD WASTE
Zhang et al., 2010
TABLE 13.5
35 C
Effect of Retention Time on Methane Yield (l/kg VS)
Author
Material
Process
Temperature
15
(days)
30
(days)
Hansen et al., 1999
Swine manure
Continuously fed
55 C
67
180
Shyam 2001
Cattle manure
Continuously fed
Moeller et al., 2006
Wheat Straw
Torres-Castillo et al., 1995
Barley straw
Torres-Castillo et al., 1995
Lehtomaeki 2006
Lehtomaeki 2006
Lehtomaeki 2006
50
(days)
100
(days)
22 C
100
160
35 C
190
270
Batch leachate
recycling
35 C
145
195
Barley straw
Batch leachate
recycling
25 C
Oats straw
Batch
35 C
Rapeseed straw
Willow
Batch
Batch
factory and some is made into paper and fiberboard.
The factories have an excess of bagasse and this is
stored in the open air. The stacks produce methane
and open fires are common giving off soot and
polluting the air. Bagasse has a low biodegradability
of 120 l/kg VS (Table 13.8).
160
140
240
300
145
165
200
155
35 C
35 C
200
(days)
240
270
Coffee Husks and Mucilage
The production of coffee husks and mucilage is
5 million tons per year. They are dumped near the factories causing methane emissions and degrading the
environment. They have a high lignin content of 21%
209
AQUATIC WEEDS
TABLE 13.6
Measured and Calculated Methane Yield
for Shorter Retention Times
Methane
(l/kg VS)
Lignin days
Yield Calculated
Wheat
Straw
17
50
190
165
Moeller et al., 2006
Barley
Straw
13
50
145
205
Torres-Castillo
et al., 1995
Oats Straw 14
50
240
195
Lehtomaeki 2006
Oats Straw 14
30
140
195
Lehtomaeki 2006
Rapeseed
Straw
20
50
165
140
Lehtomaeki 2006
Rapeseed
Straw
20
30
145
91
Lehtomaeki 2006
Willow
14
30
155
135
Lehtomaeki 2006
(Zoca et al., 2012). Padua Ferreira et al. (2011) gives a
value of 28%. Biodigestability is 70 l/kg (Frederiks,
2012). Methane capture can be economic using a cheap
biodigester and long retention times. The coffee factories
can use the methane for electricity production. Coffee
farmers can install small digesters and use the methane
for cooking.
TABLE 13.7
Methane Yield, Costs and Humus Gain
for Various Energy Crops
Methane
Yield (m3/ Methane Costs
ha)
(V/m3)
Humus Gain
(kg/ha a)
Reye (Grains)
2400
0.39
550
Meadows
2900
0.40
Reye
(Whole Plants)
3200
0.34
Grass
3400
0.42
800
Maize Silage
4300
0.30
950
Reye
(Whole Plants)
and Grass
(Intermediate
Crop)
4800
0.35
Barley
(Whole Plants)
and Sorghum
(Intermediate
Crop)
4800
0.39
Energy Beets
4800
0.42
Rye (Green)
and Maize
5200
0.38
CROP RESIDUES
The residue from grain crops amounts to nearly
3000 million tons per year (Table 13.8). The most
important are maize, wheat and rice. A fraction of
this is used as animal fodder or animal bedding. Animal fodder turns into manure and bedding becomes a
bedding manure mixture. The fraction that is left in the
fields can be used for anaerobic digestion. During
threshing the straws are deposited in the fields in
swaths and can be picked up after a few days. Losses
are between 20% and 50%. The costs for collecting
the residues are high. Schmaltschinski (2008) estimates
75 V per ton of shredded straw on the truck at the field
in Germany. Mo et al. (2011) report a value of 50 V in
Poland. Baled straw sells for 140 V in the Netherlands.
Substrate costs are 0.30 V/m3 methane for shredded
straw and 0.50 V/m3 for baled straw at 100 days retention times (Table 13.8).
SPENT BEDDING
Straw used as animal bedding can be collected and
digested. It is then an organic fertilizer. The costs for
this are mainly transport costs. The use of spent bedding
means that all straw can contribute to methane production. Barsega et al. (1994) digested cattle bedding from
wheat straw.
Spent bedding of rice husks from piggery housings
gave less than 20% reduction in VS (Tait et al., 2009).
Weathering in a pig shed improved the biodegradability.
Bonilla et al. (1985) successfully digested spent poultry
litter of rice chaff.
Spent wheat bedding from ducks gave a methane
yield of 310 l/kg VS (Buisonje, 2009).
KITCHEN AND GARDEN WASTE
Around 100 kg/person kitchen and garden waste are
separately collected in Germany (Kern et al., 2008). VS
are around 40%. Extrapolation to the world population
would give 150 million tons of VS per year.
AQUATIC WEEDS
860
Sewage from most urban areas in tropical countries is
discharged untreated in rivers and lakes. This leads to
eutrofication. Fast growing aquatic weeds use the nutrients and form thick mats hindering fishing and navigation. Lake Victoria is a prime example. Biological control
of the waterweeds is seen as one method to reduce the
problems, but does not address the root cause of
210
TABLE 13.8
Substrate Availability
Availability
(109 kg/a)
Rice Husks
100
Uses
Fuel, animal
bedding
Pretreatment
4% NaOH
Test Duration
(days)
Temperature ( C)
CH4 Yield
(l/kg VS)
Author
17
35
140
Hills et al., 1981
40
Ambient
200
Vevekanandan et al., 2011
Joseph et al., 2009
Bagasse
200
Fuel, paper, board
60
35
120
Coffee Husks
10
Fuel
45
30
80
Frederiks, 2012
Wheat Straw
800
Fuel, animal
bedding
1.5% CaOH
32
37
260
Raju et al., 2010
None
32
37
170
Raju et al., 2010
None
100
37
270
Moeller et al., 2006
100
37
300
Moeller et al., 2006
Ambient
240
Somayaji et al., 1994
200
Mo et al., 2011
35
240
Lehtomaeki 2006
Barley Straw
200
Animal bedding
Rice Straw
700
Animal fodder
40
Maize Straw
1400
Animal fodder
70
Rapeseed Straw
150
Animal bedding
150
Poultry Litter/Rice
Straw
52
Ambient
230
Bonilla et al., 1985
Duck Litter/Wheat
Straw
50
30
310
Buisonje 2009
Cattle Bedding/Wheat
Straw
27
35
270
Barsega et al., 1994
250
Vandevivere et al., 2003
240
Vaidyanathan et al., 1985
Kitchen/Garden Waste
150
Compost
Aquatic Weeds
100
Compost
90
30
13. USE OF VOLATILE SOLIDS FROM BIOMASS FOR ENERGY PRODUCTION
Material
211
DIGESTION SYSTEMS
TABLE 13.9
Methane Yield and Test Conditions Water Hyacinth
Test
Duration (days)
Methane
Yield (l/kg VS)
125
190
Digested water hyacinth
90
240
Swine manure
60
60
10
Fresh rumen residue
65
250
20e30
10
Fresh rumen residue
95
200
25e35
20
30
20
Author
T ( C)
Wolverton, 1979
25
Vaidyanathan et al., 1985
29
30
29
Moorhead et al, 1993
35
5
13
Almoustapha et al., 2008
30e40
Almoustapha et al., 2008
Ofoefule et al., 2009
VS (Kg/m3)
C/N ratio
Seed
17
18
eutrofication. Controlling of one type of waterweed will
give others the opportunity to become a pest. There are a
number of instances where aquatic weeds are used as an
organic fertilizer (Jandl, 2010), but this practice is not
widespread.
Aquatic weeds are digested in Luzira prison Kampala,
Uganda, (Lindsay et al., 2000) and at the Songhai agricultural training centre in Porto Novo Benin (Jandl, 2010).
Harvesting of aquatic weeds is expensive.
Antunuassi et al. (2002) calculate a cost of 15,000 V/ha
or 0.15 V/kg VS. Veitch (2007) suggest significant cost reductions using outboard motor-powered launches with a
rake and a land-based backhoe.
Anaerobic digestion of whole plants is not common.
There are a number of tests with chopped (10e60 mm)
water hyacinth (Table 13.9). Gas yields are high for the
experiments of Wolverton et al. (1979), Vaidyanathan
et al. (1985), and Almoustapha et al. (2008). Swine
manure is not well suited as a seed for water hyacinth
digestion as it has little biogas bacteria and this explains
the low yield of Moorhead et al. (1993). Duration of the
tests by Ofoefule et al. (2009) is too low.
Moorhead et al., 1993 have done tests with ground
(1.6 mm) water hyacinth and chopped water hyacinth
(12.6 mm), resulting in a 15% lower gas yield for the
chopped water hyacinth. The results for the digestion
of whole plants will be also lower than for ground water
hyacinth.
DIGESTION SYSTEMS
Family-Size Biogas Plant
Straws and most other biomass have a tendency to
float on the water and form a scum layer. Tests in India
have shown that these types of materials will generate
biogas when they have been in contact with digester
fluid for at least three days. This can be achieved by
filling the digester with a quantity of wet compost. The
compost will float. New material is added from the
bottom of the digester. This material will push the
compost upward but will stay under the digester liquid,
as the compost being wet is relatively heavy. The resulting digested material forms a thick mat. The digested
material can be removed once per year after opening
the plant (Oosterkamp, 2003).
A plant suitable to convert maize stalks and other
biomass into biogas is of the water jacket floating
drum plant type. The water jacket reduces the emission
of methane. In fixed dome plants 10% of the methane
production is lost. The main modification is the use of
a large 0.3 m diameter inlet pipe. Through this pipe
the waste biomass can be pushed down into the digester
proper. The floating drum can be removed and digested
material taken out from the top.
Wet Digesters
Most digester systems in Europe are manure based
(wet) and/or use maize silage for their feed. Some of
these wet systems add about 10% straws (VS). Two or
more sequentially linked digesters give about 15% more
gas yield than a single continuously stirred digester
(Angelidaki et al., 2005). The wet systems have the disadvantage that solids (10e15%) need to be kept in suspension by an impeller. With a high percentage of grasses
and straw scum layers are formed that need to be
removed mechanically after opening the digester.
Scum Layer Digester
Sauter (2012) circumvents this problem by spraying
digestate on top of the scum layer using water canons.
The patent of Rossow (2011) claims that the scum layer
can be 5 m in thickness. It is to be expected that digested
particles with a high lignin content will be washed
down, sink to the bottom and can be collected there
using a pump. The input material is loaded into the
digester through an input shaft with its exit below
the lower level of the scum layer in the digester. It will
be acidified for a few days in the first step of the
212
13. USE OF VOLATILE SOLIDS FROM BIOMASS FOR ENERGY PRODUCTION
digestion process. The acids will be transformed into
methane and carbon dioxide in the other parts of the
digester. Schoenberg and Linke (2012) tested a 45 l
scum layer digester with whole plants (Silphium perfoliatum) as substrate. A loading rate of 8 kg/(m3d) was
possible with a methane yield of 215 l/kg VS.
Solid Biomass Digester
VandeVivere et al. (2003) gave an overview of anaerobic digesters for solid biomass. These “dry” (20e40%
solids) systems are used for the digestion of kitchen
and garden waste and the mechanically sorted fraction
of municipal waste, but can be used for most types of
solid farm and food processing by-products.
The Kompogas (VandeVivere et al., 2003) system
consists of a cylindrical plug flow reactor in which
the fermenting wastes are mixed and moved by paddles. Temperature is 55 C and retention time 15 days.
Capacity is limited to 10,000 tons/a due to the
maximum dimensions of the axis with paddles. Capacity of these systems in Europe and Qatar is more than
1 million tons/a. Methane yields are 200e300 l/kg VS
(VandeVivere et al., 2003).
There are some batch systems with leachate recirculation. Methane yield is up to 40% less due to channeling
of the leachate in the substrate (Vandevivere). Mussoline
et al., 2012 obtained 175 l/kg VS for rice straw and
swine manure in a digestion period of 1 year. Temperatures ranged from 15 to 35 C. The cell with 6500 m3
capacity was filled with cylindrical bales. Packing
density was 100 kg/m3.
Storage of straw for 6 months is expensive (25 V/ton).
In Western Europe storage cum digester tanks can be
used. The idea is to fill these tanks in the period Julye
October with shredded straw and a fraction of old
digestate together with macro- and micronutrients.
Mussoline (2012) demonstrated this concept during
one year with rice straw bales and swine manure. Daily
power generation was directly correlated to the digester
temperature.
German tanks with concrete tops are 120 V/m3 and
with foil tops 70 V/m3. At a packing density of 10%
this amounts to an investment of 700e1200 V/ton straw
stored and digested.
INCREASE IN SOLIDS CONTENT
IN WET DIGESTERS
A reduction in digestion plant size can be obtained by
increasing the solids content. The amount of work in
transporting and spreading the digestate is also reduced.
Ong et al. (2000) obtained an increase in gas yield
compared to a continuously fed and stirred reactor in
a continuously fed nonstirred reactor with the outlet in
the middle. It seems that some solids removal from the
bottom has to take place, as accumulation of inert solids
will reduce the effective volume of the reactor.
Shyam (2001) demonstrated that cow manure can be
digested at 18% total solids. The method increases the
gas yield with 40% using practically the same equipment as before.
LOADING AND UNLOADING
OF DIGESTERS
Manure-based (wet) digesters use pumps for loading.
Straw is mixed with digestate and pumped in the
digester. An alternative is using an auger and pushing
the straw to below the liquid level in the digester. Heavy
solids are removed from the bottom of the digester using
a rotating rake and pump. Straw disintegrates during
digestion and is pumped off together with the digested
manure.
In the scum layer plant straw can be loaded with front
loaders using an inlet shaft with an outlet to below the
liquid level. Shredded straw can also be blown into the
inlet shaft. Solids are removed in the same way as in
wet digesters. The remaining digestate is pumped out.
Plug flow dry digesters are loaded and unloaded with
augers.
Leachate recirculation digesters are operated in batch
mode. The digesters are opened and the digestate is
unloaded with front loaders. Capacity of these digesters
is relatively low due to the loading procedure.
TREATMENT OF DIGESTATE
IN WET DIGESTERS
A screw press or a centrifuge can separate the digestate into a solid and a liquid fraction. Recycling of the
solid fraction will increase the solid content of the
digesters further. The recycled solid fraction will yield
between 30 and 100 l/kg VS depending on the pH and
protein content (Balsari et al., 2010).
Disposal of the liquid fraction into a sewer or into surface waters requires the removal of phosphate and nitrogen. Phosphate can be separated by the addition of
magnesium and precipitation of magnesium ammonium
phosphate (struvite). The production of ammonium
requires about 30 MJ/kg. The ammonium can be recovered by adding extra phosphate and magnesium to the
effluent.
Tuerker and Celen (2007) give a cost for chemicals of
7.7 $/kg N removed (price level of 2001) for magnesium
chloride and phosphoric acid and sodium hydroxide.
About a third of the cost is for the sodium hydroxide
CHEMICAL CONVERSION OF VOLATILE SOLIDS
necessary for the adjustment of pH. Struvite is not
a conventional fertilizer and the price is quite speculative, but it should be at least the value of the phosphate
2.9 $/kg N.
Dvorak et al., 2011 heat the liquid fraction of digestate
up to 70 C and use aeration for the removal of CO2. This
results in an increase in the pH to nearly 10 and a shift of
the ammonia ions to gaseous ammonia. The diet of dairy
cows is rich in calcium. Consequently the phosphate in
the digestate is in the form of insoluble calcium phosphate particles. CO2 bubbles keep these particles in suspension. Elimination of the CO2 results in settling of the
particles in a quiescent tank. The system removes 70% of
ammonia and 80% of phosphate. End products are a solution (35%) of ammonium sulfate and phosphatecontaining solids.
Karakashev et al. (2005) came to the conclusion that
microfiltration is unsuited for treatment of digested
pig manure due to membrane clogging. They developed
a method at laboratory scale to clean the supernatant
after decanting-centrifuging. It involves a up-flow
anaerobic sludge blanket reactor, precipitation of
magnesium-ammonia-phosphate (struvite) by adding
magnesium oxide, partial aeration and ammonium
removal by anaerobic ammonia-oxidizing bacteria.
Phosphate and nitrogen can also be concentrated by
removing water from the liquid fraction. Waste heat
(50e70 C) from electricity generation removes only
fraction of the water in a single pass. Up to three passes
are possible.
Distillation with vapor recompression has been tried
(Melse et al. 2005). The electric energy consumption
was about 0.3 MJe/kg water removed. Technical and
economical reasons led to abandonment of the process.
The Biorek (Preez et al., 2005) process uses a two-step
filtration and reverse osmosis process to increase the
solids content. One project in the Netherlands was
stopped due to operational difficulties.
The 20 MWe biogas plant in Penkum, Germany, uses
a decanter followed by a swinging screen for the
removal of solids and evaporation and reverse osmosis
for the removal of salts (Herbes, 2010).
USE OF METHANE
At present most methane from anaerobic digestion is
used for cooking and in gas or dual fuel engines (diesel
engines where part of the fuel is substituted by biogas)
to generate electricity with an overall efficiency of
30e50%. In Western Europe part of the excess heat is
used in residences and factories. In a few instances
fuel cells are used to generate electricity and hightemperature process heat. It is, however, better to
remove the carbon dioxide and to inject the gas into
213
the natural gas grid. It can then be used to generate electricity in 60% efficient combined cycle plants
In Europe and in several states of the United States
there are requirements to gradually introduce biomassderived fuels in the transport sector. Approximately
5 million cars currently run on compressed natural gas
and could run on compressed methane from anaerobic
digestion.
An alternative is to liquefy the gas and use the liquefied gas as biofuel in vehicles. This is done in Snurrevarden (Norway) and Gasum (Finland).
Anaerobic digestion and the use of compressed
methane is more energy efficient than the hydrolysis
of cellulose and hemicellulose to sugars and conversion
of these sugars into alcohol. This alcohol has to be
distilled in order for it to be used as a transportation
fuel.
In areas where there is no natural gas infrastructure,
methane in high-pressure bottles can replace bottled liquefied petroleum gas (LPG or propane). Energy densities of 20% of that of LPG bottles can be reached at a
pressure of 4 MPa using bottles filled with activated
carbon.
CHEMICAL CONVERSION OF
VOLATILE SOLIDS
Combustion
It is estimated that around 3 billion people worldwide
rely on wood, stubble, dung and leaves for cooking fuel.
Burning biomass fuels on open fires and in inefficient
stoves releases many harmful pollutants. These pollutants result in excess respiratory illnesses and death in
women and children. Known as a “silent killer”, over
1.6 million children die annually throughout the developing world from the consequences of exposure to
biomass fuel smoke (Edelstein et al., 2008). Improved
stoves reduce the fuel consumption and indoor pollution by 50% (Ravindranath et al., 1997; Halim, 2008).
Co-combustion of solid biomass and coal is reviewed
by Cremers (2009).
Combustionesteam cycle. This combustion of solid
biomass and the use of a steam cycle is not very energy
efficient (32%, Yang et al., 2006). The maximum temperature is limited as potassium and calcium together with
silicon form at high temperatures glasslike deposits on
the furnace walls. Corrosion problems occur in strawbased furnaces at 500 C (Hansen et al., 2000).
Gasification
Gasifying cook stoves are described by Field (2012).
A high-pressure liquid ash gasifyer has an efficiency
214
13. USE OF VOLATILE SOLIDS FROM BIOMASS FOR ENERGY PRODUCTION
of 50%. These are large installations with capacities of
over 200 MWe. At present only 15% biomass is cogasified with coal (Drift, 2008).
THERMAL CONVERSION OF
VOLATILE SOLIDS
Slow Pyrolysis
Only a few percent of chlorine and potassium are carried in the pyrolysis gas and bio-oil at a pyrolysis temperature of less than 700 C (Jensen et al., 1999). About 40%
of the energy is at a temperature of 700 C in the gas fraction. Fifty percent of the energy remains in the biochar in
slow pyrolysis at 400 C. Total efficiency is 20% (Halwachs, 2010).
Flash Pyrolysis
In flash pyrolysis the production of liquid bio-oil is
maximized using high temperatures and high heating
rates. The liquid fraction of fast pyrolysis products contains a high concentration of potassium and calcium and
its use in diesel engines and gas turbines is not prudent.
DISCUSSION
Maximum Methane Yield
Jerger et al. (1982) demonstrated that a correct ratio of
macronutrients and minimum values of micronutrients
offer a way to increase the methane yield at low costs.
Tests on the biodegradability should be made with
optimal concentrations of macronutrients and sufficient
micronutrients. Some companies offer the service to test
for these conditions in biogas plants. It would be wise
for Indian cattle manure plants to formulate packages
with the required macro- and micronutrients.
Komatsu et al. (2007) showed that primary sewage
sludge is an excellent medium of both macro and micronutrients. Secondary sludge not only has methanogenic
bacteria but also contains enzymes that break down
straws, bagasse and husks. They used 65% VS sewage
sludge and demonstrated that high concentrations of
micronutrients are not harmful. The codigestion of lignocellulosic materials with sewage sludge is attractive. The
proteins in the sludge are difficult to digest. Codigestion
reduces the VS more than digestion the sludge and lignocellulosic materials separately (Rashed et al., 2008).
Longer retention times will increase the methane
yield further given optimal nutrient conditions. This
requires more or larger digester tanks. The tanks form
only 20% of the total investment in a biogas installation
and retention time can be increased fivefold when the
methane yield is doubled for the same investment per
cubic meter methane. The size for concrete tanks is
limited to around 3000 m3 and multiple tanks are normally required.
Much work has been done on mechanical, chemical
or biological pretreatment of straws, bagasse and husks.
These are effective giving up to 50% more methane. No
comparison has been made with longer retention times
in terms of investments and operational costs. Enzyme
addition when making silage and boiling water addition
during loading of straw and husks are the next most
cost-effective treatments. This involves no extra
handling of the substrates.
Nutrient Recycling
Nutrient recycling back to the soil is possible with
anaerobic digestion. Most of the nitrogen and phosphate
is in the liquid fraction of the digestate, when the digestate is separated in a solid and liquid fraction. The liquid
fraction could be spread at nearby fields. Lignocellulosic
biomass should be codigested with sewage sludge,
where nutrient recycling is not economic.
Soil Fertility
Soil fertility is enhanced by humus. Humus is in turn
produced by the degradation of lignin. The lignin
remains in the digestate in anaerobic digestion and can
be used as “fertilizer”. A similar effect is obtained by
the biochar from the pyrolysis of lignocellulosic biomass
(terra preta). No comparison has been made on investment costs and operational costs between pyrolysis
and anaerobic digestion.
Digesters
Solid biomasses need to be digested at optimum
nutrient conditions and long retention times for
maximum methane yields. The wet scum layer digester
is one option. The maximum solids concentration in the
systems without giving operational difficulties has not
been established.
Batch systems with leachate recycling are also an
option. Substrate handling is minimized. The disadvantage is their small size and the danger of explosions
during opening of the digesters.
CONCLUSIONS
Anaerobic digestion of food processing and crop residues can contribute to reduce the dependency on fossil
fuels. Energy recovery in methane is 70% with digestion
periods between 100 and 150 days, depending on the
REFERENCES
lignin content of the substrates and temperature of the
digestion. Digestion times can be reduced with optimum concentrations of macro and micronutrients.
Food processing residues are a cheaper substrate for
anaerobic digestion than crop residues, as collection
has already been paid for. Systems using an auger or
paddles to transport the substrate inside the digester
tank have a significant higher methane yield than batch
systems with leachate recycling. Their investment warrants only their use for kitchen and garden wastes, for
which a gate fee is paid. Losses in batch systems may
be reduced by longer retention times. Storage cum
digester tanks with leachate recycling will reduce substrate handling to a minimum.
References
Ahn, J.H., Do, T.H., Kim, S.D., Hwang, S., 2006. The effect of calcium
on the anaerobic digestion treating swine waste water. Biochem.
Eng. J. 30, 33e38.
Ali, N., Kurchania, A.K., Babel, S., 2010. Bio-methanisation of Jatropha
curcas defatted waste. J. of Eng. Tech. Res. 2 (3), 038e043.
Almoustapha, O., Kenfack, S., Millogo-Rasolodimby, J., 2008. Biogas
production using water hyacinth to meet collective energy needs in
a Sahelian country. Fact Rep. 1, 72e79.
Amon, Th., Kryvoruchko, V., Amon, B., Zollitsch, W., Mayer, K., Buga, S.,
Amid, A., November 2003. Biogaserzeugung aus mais e einfluss der
inhaltstoffe uaf das specifische methanbildungsvermoegen von
fruhe- bis spaetreifen maisorten. In: Rueckenbauer (Ed.),
Arbeitstagung 2003 der Vereinigung Oestereichischer Planzenzuechter und Saatgutkaufleute zum thema Hybridmais, Zuechtung
und Verwertung. HBLFA, Raumberg-Gumpenstein, Austria,
pp. 25e27. (November).
Angelidaki, A., Ellegaard, L., 2005. Biogas reactors in series may yield
up to 15% more biogas. Bioenergy Res. 9, 2e3.
Antunuassi, U.R., Velini, E.D., Martins, D., 2002. Remocao mechanica
de plantas aquaticas: analise economica e operacional. Planta
Daninha 20, 35e42.
Balsari, P., Gioelli, F., Menardo, S., Paschetta, E., 2010. The (re)use of
mechanical separated solid fraction of digested or not digested
slurry in anaerobic digestion plants. In: Marques dos
Santos, C.S.C., Ferreira, C.L. (Eds.), Proceedings Fourteenth Ramiran Conference Lisboa, Portugal 13e15 September 2010. Universidade Technical de Lisboa, Portugal, pp. 1e4.
Bardiya, N., Gaur, A.C., 1999. Iron supplementation enhances biogas
generation. Bio Energy News 1, 6e19.
Barsega, U., Egger, K., Wellinger, A., 1994. Biogas aus festmist,
entwicklung einer kontinuierlich betriebene biogasanlage zur
vergaerung von strohreichen mist. FAT Berichte 451, 1e12.
Boese, S., 2010. Kosten sind nicht alles. Praxisnahe 4, 8e9.
Bonilla Garcia, S., Georg, R., Hoffmann, R., Ulrich, G., 1985. Fermentation of poultry excrement/rice chaff mixtures. Gate 4, 1e3.
Bossuwe, M., 2011. Voorbehandeling van Mais bij Inkuiling voor Toename van de Biogasproductie. HoWest Kortrijk, Belgium. pp. 1e121.
Buisonje, F., 2009. Stromest van vleeseenden. V-Focus 4, 36e37.
Busch, G., Sieber, M., 2006. Zweistufiges fest-fluessig-biogasverfahren
mit offener hydrolyse eein neues technologisches konzept fuer die
biogasgewinnung
aus
nachwachsender
rohstoffe
und
bioerfuegbare abfaellen. Forum der Forschung 19, 63e68.
Chandler, J.A., Jewell, J.M., Van Soest, P.J., Robertson, J.B., 1980. Predicting Methane Fermentation Biodegradability. Solar Energy
Research Institute, Golden CO, USA. pp. 1e234.
215
Chollet, J.D., 2011. Application de biocatþ dans le Digesteur de
Kompogas de Germanier Encore Cyclage S.A. Lavigny Morges.
Citadel Biocat, Moreg, Switserland. pp. 1e4.
Chynoweth, D.P., Isaacson, R., 1987. Anaerobic Digestion of Biomass.
Elsevier Applied Science, London, United Kingdom. pp. 1e279.
Cremers, M.F.G., 2009. Technical Status of Biomass Co-Firing. Kema,
Arnhem, Netherlands. pp. 1e43.
Demirel, B., Scherer, P., 2011. Trace element requirement of agricultural biogas digesters during biological conversion of renewable
biomass to methane. Biomass Bioenergy 35, 992e998.
Drift van der, A., 2008. Status of Biomass Gasification. ECN Petten,
Netherlands, 1e24.
Dvorak, S.W., Chen, S., Frear, C., VanLoo, B.J., Zhao, Q., 2011. Nutrient
recovery systems and method. Patent application WO/2011/156767.
Edelstein, M., Pitchforth, E., Asres, G., Silverman, M., Kulkarni, N.,
2008. Awareness of health effects of cooking smoke among women
in the Gondar Region of Ethiopia: a pilot survey. BMC Int. Health
Hum. Rights 8 (10), 1e4.
Field, J., 2012. Gasifying Cookstoves Database. Colorado State
University Fort Collins, USA. pp. 1e24.
Frederiks, B., 2012. Biogas Tests with Euphorbia Trirucali, Sugar Filter
Mud, Coffee Husk, Banana Skin, Gras Manure and Maize Mixture.
Fact Foundation, Wageningen, Netherlands. pp. 1e3.
Godin, B., Ghysel, F., Agneesens, R., Schmitt, T., Gofflot, S.,
Lamaudiere, S., Sinnaeve, G., Goffart, J.-P., Gerin, P.A., Stilmant, D.,
Delcarte, J., 2010. Determination de la cellulose, des hemicelluluse,
de la lignine et des cendres dan diverses cultures lignocelluloses
dediees a la production de bioethanol de deuxieme generation.
Biotechnol. Agron. Soc. Environ. 14, 561e566.
Guengoer-Demirci, G., Demirer, G.N., 2004. Effect of initial COD
concentration, nutrient addition, temperature and microbial acclimation on anaerobic treatability of broiler and cattle manure.
Bioresour Technol. 93 (2), 109e117.
Gunnerson, C.G., Stuckey, D.C., 1987. Integrated resource recovery:
Anaerobic digestion; Principles and practices for biogas systems.
UNDP, New York, USA., 1e154.
Halim, S.A., 2008. A Technical Manual for Improved Cooking Stoves.
Village education resource center, Dhaka, Bangladesh. pp. 1e68.
Halwachs, M., 2010. Rotary Kiln Pyrolysis e First Results of a 3 MW
Plant. Technical University Wien, Austria. pp. 1e21.
Hansen, L.A., Nielsen, H.P., Frandsen, F.J., Dam-Johansen, K.,
Horlyck, S., Karlsson, A., 2000. Influence of deposit formation on
corrosion at a straw fired boiler. Fuel Process. Technol. 63 (1e3),
189e209.
Haque, M.S., Haque, M.N., 2006. Studies on the effect of urine on
biogas production. Bangladesh J. Sci. Ind. Res. 41 (1), 23e32.
Helbig, S., 2009. Biogas Production from Common Reed. Mariental
Desert Foundation of Namibia, Windhoek, Namibia. pp. 1e46.
Herbes, C., 2010. Biomethane Production on Industrial Scale. Nawaro
bioenergie, Leipzig, Germany. pp. 1e19.
Hills, D.J., Roberts, D.W., 1981. Anaerobic digestion of dairy manure
and field crop residues. Agric. Wastes. 3, 179e189.
Hoogwijk, M., Faaij, A., Van den Broek, R., Berndes, G.,
Gielen, D., Turkenburg, W., 2003. Exploration of the ranges of
the global potential of biomass for energy. Biomass Bioenergy
25, 119e133.
Hossain, M.Z., 2001. Farmers view on soil organic matter depletion
and its management in Bangladesh. Nutrient Cycling in Agroecosystems 61, 197e204.
Jandl, O.M., 2010. Barriers for the Employment of Floating Invasive
Weeds for Biogas Production in Local Communities in West African Developing Countries. Thesis Technical University Eindhoven,
Netherlands. pp. 1e66.
Jensen, P.A., Dam-Johansen, K., 1999. Release of potassium and
chlorine during straw pyrolysis. In: Overend, R.P., Chornet, E.
216
13. USE OF VOLATILE SOLIDS FROM BIOMASS FOR ENERGY PRODUCTION
(Eds.), Fourth Biomass Conference of the Americas, Oakland, USA.
Elsevier Science, Oxford, UK, pp. 1169e1176.
Jerger, D.E., Conrad, J.R., Fannin, K.F., Cynoweth, D.P., 1982. Biogasification of woody biomass. In: White, J.W., McGrew, W.,
Sutton, M.R. (Eds.), Energy from Biomass and Wastes VI. Institute
of Gas Technology, Chicago Il, USA, pp. 341e372.
Jerger, D.E., Dolenc, D.A., Chynoweth, D.P., 1983. Biogasification
of woody biomass following physical and chemical pretreatment.
In: Klass, D.L., Eliott (Eds.), Seventh Symposium on Energy from
Biomass and Wastes Orlando. Institute of Gas technology, Chicago,
Ill. USA, pp. 233e234.
Joseph, O., Rouez, M., Meltivier-Pignon, H., Bayard, R., Emmanuel, E.,
Gourdon, R., 2009. Adsorption of heavy metals on sugarcane
bagasse: improvement of adsorption capacities due to anaerobic
degradation of the biosorbent. Environ. Technol. 30, 1371e1379.
Karakashev, D., Batstone, D.J., Angelidaki, I., 2005. Influence of environmental conditions on methanogenic compositions in anearobic
biogas reactors. Appl. Environ. Microbiol. 71 (1), 331e338.
Kelly, W.D., Martens, D.C., Reneau, R.B., Simpson, T.W., 1984. Agricultural Use of Sewage Sludge: A Literature Review. Bulletin 143.
Virginia Water Resources Research Centre, Gloucester Point Va,
USA, pp 1e47.
Kern, M., Siepenkothen, J., 2008. Potenziale fuer die erzeugung von
biogas in der deutschen abfalwirtschaft. In: ThomeKozmiensky, K.J., Beckmann, M., Versteyl, A. (Eds.), Berliner
Abfallwirtschafts- und Energiekonferenz Ersatzbrennstoffe und
Biogas, pp. 495e505. Berlin.
Klopfenstein, T., 1978. Chemical treatment of crop residues. J. Anim.
Sci. 46, 841e848.
Komatsu, T., Kudo, K., Himeno, S., 2007. Anaerobic digestion of
sewage sludge and rice straw. In: LeBlanc, R.J., Laughton, P.J.,
Rajesh, T. (Eds.), Moving Forward Waste Water Biosolids Sustainability Technical, Managerial, and Public Synergy June 24e27,
2007. Moncton, Greater Moncton Sewerage Commission Moncton,
NB Canada, pp. 495e501.
Lar, J.S., Li, R., Li, X., 2010. The influence of calcium and iron supplementation on the methane yield of biogas treating dairy
manure. Energy Sources Part A 32, 1651e1658.
Lebuhn, M., Andrade, D., Bauer, C., Gronauer, A., 2010. Intensivierung des anaeroben Biomassenabbaus zur Methanproduktion aus
Nachwachsende Rohstoffen. LfL Tier und Technik Freising,
Germany. pp. 1e132.
Lehtomaeki, A., 2006. Biogas Production from Energy Crops and Crop
Residues. Dissertation. University of Jyvaeskylae Finland. pp. 1e85.
Lindsey, K., Hirt, H.-M., 2000. Use Water Hyacinth: a Practical
Handbook of Uses for Water Hyacinth from across the World.
Aname, Winnenden Germany. pp. 1e114.
Loeffen, P., Geraats, B., 2005. Toekomstige Kwantiteit en Kwaliteit van
ZuiveringsslibStowa Rapport 2005e2006. Stowa, Amersfoort,
Netherlands. pp. 1e96.
Melse, R.W., Verdoes, N., 2005. Evaluation of four farm-scale systems
for the treatment of liquid pig manure. Biosyst. Eng. 92 (1), 47e57.
Mo, Z., Polarski, K., 2011. Preliminary comparison of biogas productivity from maize silage and maize straw silage. J. Res. Appl. Agric.
Eng. 56 (2), 108e110.
Moeller, H.B., Nielsen, A.M., 2006. Straw and energy crops in biogas
plants. BioEnergy Res. 14, 4e5.
Moorhead, K.K., Norstedt, R.A., 1993. Batch anaerobic digestion of
water hyacinth: effect of particle size, plant nitrogen content and
inoculum volume. Bioresour. Technol. 44, 71e76.
Mussoline, W.A., Esposito, G., Giordano, A., 2012. Optimization of
Anaerobic Digestion of Rice Straw Inoculated with Piggery Waste.
University of Paris-Est, France. pp. 1e2.
Oechsner, H., 2012. Forschungsinitiative und eprojecte zur biogasforschung. In: Kranert, M. (Ed.), 8e Biogastag Baden
Wuertenberg 13 Maerz 2012 Hohenheim. Oldenbourg Industrie
Verlag, Essen Germany, pp. 1e144.
Ofoefule, A.U., Uzodinma, E.O., Onukwuli, O.D., 2009. Comparative
study of the effect of different pre-treatment methods on biogas
yield from water hyacinth (Eichhornia crassipies). Int. J. Phys. Sci.
4 (8), 535e539.
Ong, H.K., Greenfield, P.F., Pullammanappallil, P.C., 2000. An operational strategy for improved biomethanation of cattle manure
slurry in an unmixed single stage digester. Bioresour. Technol. 73,
87e89.
Oosterkamp, W.J., 2003. Werkwijze voor het vergisten van biomassa.
Patent Nl 1023558. Bureau voor Industrieel Eigendom, Rijswijk,
Netherlands, pp 1e7.
Op den Camp, H.J.M., Gijzen, H.J., 1991. The Rudad process for
enhanced degradation of solid waste materials. In: Martin, A.M.
(Ed.), Biological Degradation of Waste. Elsevier Science Publishers,
Barking, England, pp. 281e306.
Owen, W.F., Stucley, D.C., Healy jr, J.B., Young, L.Y., McCarty, P.L.,
1979. Bioassay for monitoring biochemical methane potential and
anaerobic toxicity. Water Res. 13 (6), 485e492.
Padua Ferreira, R.V. de, Sakata, S.K., Bellini, M.H., Marumo, J.T., 2011.
Biosorption of Am-241 and CS-137 by Radioactieve Liquid Waste
by Coffee Husk. IPEN, Sao Paulo, SP, Brazil, 1e5.
Preeti Rao, P., Seenayya, G., 1993. Improvement of methanogenesis
from cow dung and poultry litter waste digesters by addition of
iron. World J. Microbiol. Biotechnol. 10, 211e214.
Preez, J., du, Norddahl, B., Christensen, K., 2005. The biorek concept: a
hybrid membrane reactor concept for very strong wastewater.
Desalination 183, 407e415.
Raju, C.S., Ward, A.J., Moeller, H.B., 2010. The effect of thermochemical pre-treatment on the ultimate biogas potential of straw.
In: Marques dos Santos, C.S.C., Ferreira, C.L. (Eds.), Proceedings
Fourteenth Ramiran Conference Lisboa, Portugal 13e15 September
201. Universidade Technical de Lisboa, Portugal, pp. 1e4.
Rashed, E.M., Fouad, H.N., Awadalla, M.S., 2008. Utilization of agricultural residues in improving sludge digester efficiency. J. Appl.
Sci. Res. 4 (12), 2127e2133.
Ravindranath, N.H., Ramakrishna, J., 1997. Energy options for cooking in India. Energy Policy 25 (1), 63e75.
Rossow, N., 2011. Verfahren und vorrichtung zur erhoehung der
besiedlungsdichte von methanbildende bakterienstaemme in biogasfermentern. European patent EP2314666 A1.
Sauter, 2012. Personal communication.
Scherer, P., 2011. Wirkungsweise von spurenelementen in der biovergasungskette. In: Binder, W. (Ed.), Fachtagung Spurenelementen
in Biogasanlagen. Energie Agentur, Goetingen, Germany, pp. 1e47.
Scherer, P., Lippert, H., Wollf, G., 1983. Composition of the minor
elements and trace elements of 10 methanogenic bacteria determined by inductively coupled plasma emission spectrometry. Biol.
Trace Elem. Res. 5 (3), 149e163.
Schmaltschinski, T., 2008. Optimierung des Strohtransportes zu einem
Saege- und Spanplattenwerk Abslussbericht zum Forschungsproject (11D/07Keil). Institut Fuer Forstbenutzung und Forstliche
Arbeitswissenschaft, Freiburg, Germany. pp. 1e21.
Schober, G., Wellinger, A., Widmer, C. 2006. Bau und Betrieb einer
Perkolationsanlage im Pilotmassstab zur Aufbereitung von Bioabfalle Projekt Nr. 38714. Nova Energie, Aadorf, Switserland,
pp. 1e34.
Schoenberg, M., Linke, B., 2012. Prozessoptimierung in der zweiphasigen/zweistufigen vergaerung festerbiomassen. Bornimer Agrartechnischen Ber. 79, 34e44.
Shyam, M., 2001. A biogas plant for the digestion of fresh undiluted
cattle dung. Boiling Point 47, 33e35.
Somayaji, D., Khanna, S., 1994. Biomethanation of rice and wheat
straw. World J. Microbiol. Biotechnol. 10, 521e523.
REFERENCES
Speece, R.E., 1987. Nutrient requirements. In: Chynoweth, D.P.,
Isaacson, R. (Eds.), Anaerobic Digestion of Biomass. Elsevier
Applied Science London, United Kingdom, pp. 109e127.
Speece, R.E., 1988. A survey of municipal anaerobic sludge digesters
and diagnostic activity assays. Water Res. 22 (3), 365e372.
Strecker, M., 2012. In: Willingmann, A. (Ed.), Das Ruminotec verfahren
zur Gewinnung von Biogas aus Primaer Cellulosehaltigen Reststoffen, pp. 1e55. Jahrestag des BWK 24 October, 2012. Regiona,
Wernigrode, Germany.
Tait, S., Tarnis, J., Edgeton, B., Batstone, D.J., 2009. Anaerobic digestion
of spent bedding from deep litter piggery housing. Bioresour.
Technol. 100 (7), 2210e2218.
Telschow, D. 2006. Untersuchung des Einflusses von Enzymen auf die
Biogasbildung thesis. Bauhaus Universitaet, Weimar, Germany.
Terefe, A., Edström, G., 1999. Ecosan-ecological sanitation. In:
Pickford, J. (Ed.), Twenty fifth WEDC Conference Integrated
Development for Water Supply and Sanitation, pp. 16e17. Addis
Ababa, Ethiopia; 1999 WEDC Loughborough University Leicestershire LE11 3TU, UK.
Torres-Castillo, R., Llabres-Luengo, P., Mata-Alvarez, J., 1995. Temperature effect on anaerobic digestion of bedding straw in a one-phase
system at different inoculum concentration. Agric., Ecosyst.
Environ. 54, 55e66.
Tsao, G.T., 1987. Pre-/posttreatment. In anaerobic digestion of
biomass. In: Chynoweth, D.P., Isaacson, R. (Eds.), Anaerobic
Digestion of Biomass. Elsevier Applied Science, London, United
Kingdom, pp. 91e107.
Tuerker, M., Celen, I., 2007. Removal of ammonia as struvite from
anaerobic digester effluents and recycling of magnesium and
phosphate. Bioresour. Technol. 98, 1529e1534.
Vaidyanathan, S., Kavadia, K.M., Schroff, K.C., Mahajan, S.P., 1985.
Biogas production in batch and semicontinuous digesters using
water hyacinth. Biotechnol. Bioeng. 28, 905e908.
Vandevivere, P., Baere, L., Verstraete, W., 2003. Types of anaerobic
digesters for solid wastes. In: Mata-Alvarez, J. (Ed.),
217
Biomethanization of the Organic Fraction of Municipal Solid
Wastes. IWA publishing, London, pp. 336e367.
Veitch, V.D., Burrows, Hudson, 2007. Trialling different low cost
methods of water hyacinth removal in tropical coastal wetlands. In:
Wilson, A.L., Dehaan, R.I., Watts, R.J., Page, K.J., Bowmar, K.H.,
Curtis, A. (Eds.), Proceedings of the Fifth Australian Stream Management Conference: Australian Rivers Making a Difference.
Charles Stuart University, Thurgoona, NSW, Australia, pp. 407e412.
Vevekanandan, S., Kamaraj, G., 2011. Investigation on cow dung as
co-substrate with pretreated sodium hydroxide on rice chaff for
efficient biogas production. Int. J. Sci. Adv. Technol. 1 (4),
76e80.
Weiss, S., Tauber, M., Somitscht, M., Meincke, R., Mueller, H., Berg, G.,
Guebitz, G.M., 2009. Enhancement of biogas production by addition of hemicellulolytic bacteria immobilised o activated zeolite.
Water Research 44 (6), 1970e1980.
Willms, M., Deumlich, D., Hufnagel, J., Reinicke, F., Wagner, B.,
Buttlar, C., 2009. Anbauverfahren fuer energiepflanzen e auswirkungen auf boden und umwelt. In: Schuette, A. (Ed.), Tagungsband zum KTBL/FNR biogas-Kongress von 15 bis 16 September
2009, Weimar. Fachagentur fuer nachwachsend Rohstoffe, Guelzow-Pruesen Germany, pp. 148e162.
Wolf, P de, Heeres, E., Postma, J., 2005. Compost voor de Biologische
Kringloop. Praktijkonderzoek plant en omgeving, Wageningen,
Netherland, 1e22.
Wolverton, B.C., McDonald, R.C., 1979. Energy from Aquatic Waste
Water Treatment Systems. NASA, United States.
Yang, Y.B., Shariff, V., Swithenbank, J., 2006. Simulation of a Large-Scale
Straw-fired Furnace. University of Sheffield England. pp. 1e18.
Zhang, Y., Walker, M., Banks, C.J., 2010. Optimising Processes for
the Stable Operation of Food Waste Digestion Defra WR1208.
University of Southampton, England. pp. 1e111.
Zoca, S., Rosolem, C., 2012. Potential Benefits of Coffee Cherry
Applications to Agricultural Soil. Okalahoma State University
Stilwater, O.K., USA, pp 1e3.
C H A P T E R
14
Biorefinery Systems: An Overview
Maria Gavrilescu
Department of Environmental Engineering and Management, Gheorghe Asachi Technical University of Iasi,
Iasi, Romania; Academy of Romanian Scientists, Bucharest, Romania
email: mgav@tuiasi.ro
O U T L I N E
IntroductiondBiorefinery, Concepts and Emerging
Opportunities for Sustainable Economy
219
Short History of Biorefineries and Bio-Based
Products
221
Biomass Feedstock
221
Structure of Biorefinery Concept
224
INTRODUCTIONdBIOREFINERY,
CONCEPTS AND EMERGING
OPPORTUNITIES FOR SUSTAINABLE
ECONOMY
In a continuous developing world, the industrial system needs to sustain an increasing Earth population,
which poses a high pressure on planet biocapacity. It is
generally recognized that the current industrial system
which generates products and services required by the
society is not sustainable: Earth continues to be depleted
of its resources at such rates that need to be diminished.
In addition, the current production efficiency is <10%,
while 90% of the material resources used in the production process end up as waste, with high impacts in the
environment. Furthermore, climate changes require significant minimization in the current greenhouse gases
emissions, by using new technologies able to bridge
the gap between the economic growth and environmental sustainability as well as alternative sources of energy (WEF, 2010). The rapid increase in energy
requirement run in parallel to the technological development, so as R&D activities are encouraged to study new
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00014-0
Biorefinery Platforms
227
Biorefinery Eco-Efficiency
231
Concluding Remarks and Perspectives
236
References
239
and biorenewable energy resources (Demirbas, 2010).
Recent data show that 25% of the population from
industrialized countries consumes about 75% of the
world natural resources, and controls about 88% of the
total production, 80% of trade and 94% of industrial
products of the entire world (Hens and Quynh, 2008).
These pressures on the planet biocapacity associated
to the unprecedented change in the climate and trends
in fossil fuel depletion generate premises to study and
evaluate the ways of producing and process the natural
resources in an efficient and sustainable way to ensure
an environmentally friendly and eco-efficient development (EEA, 1999; PP, 2012). Designing for sustainability
of smart production systems should be based on raw
materials renewability, correlated with the rate of use.
Therefore the design of production processes, products
and services needs to be developed on a broader
perspective having economic, technical, social and environmental efficiency, effectiveness, and performance as
red lines in minimizing the impact on humans and environment (Centi and Perathoner, 2009; Yuan et al., 2013).
The biological feedstock is an essential part of the
smart economy, since the preservation and management
219
Copyright Ó 2014 Elsevier B.V. All rights reserved.
220
14. BIOREFINERY SYSTEMS: AN OVERVIEW
of various resources are fundamental strategic lines to
foster sustainability tasks in the twentieth century
(Kamm et al., 2006). In this context, biotechnology becomes more and more a sustainable alternative to
various industrial sectors, in particular chemical industry since some shifts from production of goods and services from fossil to bio-based materials are essential
(Gavrilescu and Chisti, 2005). This would also constitute
the seeds of a new synergy of biological physical, chemical and technical sciences, as was pointed by Kamm
et al. (2006). So, biomass has been and continues to be
seen as the most promising carbon neutral source of energy able to mitigate greenhouse emissions because the
quantity of CO2 released during combustion is the
same as that absorbed by plants during photosynthesis
(Demirbas, 2010; Khan et al., 2009). Biomass is also
considered environmentally sustainable since it is
derived prevalent from industrial waste or resulted
from agriculture and forestry management.
An integrated production of food, feed, chemicals, materials, fuels, energy, and goods is possible by the development of technologies for processing biogenic raw
materials biomass, with those for the production of intermediate and final products (Kamm, 2013). These final
products, termed as “bio-based products” include three
categories: biofuels (biodiesel and bioethanol); bioenergy
(heat and power); and bio-based chemicals (Figure 14.1).
All are produced by “a biorefinery that integrates the
biomass conversion processes” (WEF, 2010). Therefore,
the biorefinery economy means a perspective in which
the fossil fuels are replaced with biorenewables. This
integration under the coverage of biorefineries registered notable accomplishments in research and development over the world, in several specific directions. First,
Plant raw
material
Crop
residues
Sugar
crops
Woody &
herbaceous
crops
FIGURE 14.1
Preprocessing
the development of knowledge in the area of biomass resources in parallel with the improvement of systems
sustainability which develop, harvest and process
biomass resources, contributes in a high degree to biorefinery establishment as a new and efficient way in
saving resources and improving the global ecological
and carbon foot prints (Kamm, 2013). This would shift
the actual promising state for the industrial biorefinery
(with most second generation plants), toward largescale commercial production (WEF, 2010). Furthermore,
the development of a multitude of products from biobased materials may be possible due to the increased efficiency and performance of process and technologies
conversion and distribution of these materials and products (Kamm, 2013).
In this context, biorefineries can be seen as “facilities
that convert biomass-biological materials from living or
recently living organisms-into fuels, energy, chemicals and
materials” (WEF, 2010).
The International Energy Agency (IEA) defines biorefineries in the IEA Bioenergy Task 42 on Biorefineries,
as “the sustainable processing of biomass into a spectrum of
marketable products and energy” (de Jong et al., 2009). In
this context, the similarity to the spectrum of light in
the image provided by the mentioned work is significant
in terms of biorefineries tasks (Figure 14.2).
In accordance with the concept of sustainability, a
new and more general definition was proposed by the
“Biorefinery Euroview” project. It defines biorefineries as
“. integrated bio-based industries, using a variety of
different technologies to produce chemicals, biofuels, food
and feed ingredients, biomaterials (including fibers) and
power from biomass raw materials” (Biorefinery
Euroview, 2008).
Final
processing
Feeds & foods
Functional
unit
Products to
replace
petroleum
based or
petroleum
dependent
products
Recycle or
disposal
Recycled
within
product
system
or
to other
product
system
Compost pile
or
landfill
Integrated production of bio-based products from biogenic raw materials with specific technologies for biomass processing
(Kim and Dale, 2004). Source: Reproduced with the permission of the author of PowerPoint presentation, Prof. Bruce E. Dale. (For color version of this
figure, the reader is referred to the online version of this book.)
221
BIOMASS FEEDSTOCK
Heat
power
fuels
chemicals
Biomass
Biorefinery
Food
feed
materials
FIGURE 14.2 The spectrum of sustainable products and energy
resulted through biomass processing (de Jong et al., 2009). Source:
Reproduced with the permission of IEA Bioenergy Task Leader, Dr. Ed de
Jong. (For color version of this figure, the reader is referred to the
online version of this book.)
Consequently, the biorefinery concept includes the
management of all sustainability issues, concerning economic, environmental and societal components entailing a valuable strategy focused on the green economy
era, being expected to play a significant role in supporting economical and social development (PP, 2012;
Gavrilescu and Chisti, 2005). It should be noted that all
these themes are in a tight connection with the development of regulations in the frame of bio-based products,
and of market environment as well, and will contribute
to overcome the differences in the status quo of industrial
commercialization.
These fundamental trends generated a very large interest in bio-based products, so as these products
become parts of strategic plans of numerous stakeholders in various industries (WEF, 2010). Since biorefineries can generate multiple products, they offer
the advantages of maximization the values derived
from biomass feedstock (Xin et al., 2011; WEF, 2010).
SHORT HISTORY OF BIOREFINERIES
AND BIO-BASED PRODUCTS
The industrial conversion of renewable resources has
a quite long history, lasting since 6000 BC, in particular
on the utilization of sugar cane (Demirbas, 2010;
Kamm et al., 2006). However, proofs on the production
of ethanol by distillation were found in China, in the
form of dried residues of 9000 years old. Also, the
ancient Egyptians used to produce alcohol by fermentations from vegetal materials (Demirbas, 2010).
An analysis of biorefineries history should entail
various aspects of wood saccharification, sugar production, synthesis of various bio-based products (furfural,
lipids, lactic acid and many others), energy sources,
and integrated processes (Kamm et al., 2006; Demirbas,
2010; de Jong and Marcotullio, 2010; Martin and
Grossmann, 2012). Therefore, the topic branches of biorefineries which process renewable materials became
well known and applied worldwide. These developments were more evident since the nineteenth and the
beginning of the twentieth century, distinctively in the
pulp and paper industry, where wood is the main raw
material and the derived wastes gave rise to various solutions for the exploitation of valuable components they
include (Rodsrud et al., 2012). Also, the food industry
was a sector with high potential of waste valorization
and recovery. Moreover, the increase in environmental
concerns, especially related to the use of fossil fuels,
has asked for sustainable solutions to limit the greenhouse gas effects and resources depletion. Table 14.1
provides a short outline of biorefinery evolution, based
on data existent in various sources (Demirbas, 2010;
Kamm et al., 2006; Rodsrud et al., 2012).
However, some voices claim that the concept of “biorefinery” appeared in the 1990s as reaction to some
trends of industry such as the need to use biomass resources in a more balanced way from both economic
and environmental perspectives; an emergent concern
in the promotion of low-quality lignocellulosic biomass
to valuable products; an increased attention to the production of starch for energy applications; a need to
develop extra high-value products and expand product
combinations to face global competition; and to exploit
an excess of biomass (especially in the pulp and paper
industry) (Alakangas and Mäkinen, 2008; Berntsson
et al., 2012).
BIOMASS FEEDSTOCK
Biorefineries process a bio-based feedstock input,
analogous to the petroleum refineries, where a variety
of different products may result, such as fuel, power,
or chemicals (WEF, 2013). Although biorefineries use a
large variety of different raw materials and conversion
technologies, a clear alternative to fossil-based products
does not exist still today (WEF, 2013). However, four
classes of feedstocks are established (Demirbas, 2010):
• First generation which entails edible biomass (starchrich, oily plants) to produce bioalcohols, vegetable oil,
biodiesel, biosyngas, and biogas.
• Second generation which uses biomass in the form of
nonfood sources and crops (residual nonfood parts
of crops, solid waste, wheat straw, etc.) to produce
bioalcohols, biooil, biohydrogen, bio-Fischere
Tropsch diesel.
• Third generation which includes algae to produce
vegetable oil and biodiesel.
• Fourth generation which uses vegetable oil and
biodiesel to produce biogasoline.
A more detailed presentation is done in Table 14.2.
The option to choose one or more of the four different
222
TABLE 14.1
14. BIOREFINERY SYSTEMS: AN OVERVIEW
Short History of Biorefineries and Bio-Based Products
Key Moment
Place and Actors
Innovations and Activities
References
9000 BC
China
e Discovery of the art of distillation,
which increases the concentration of
alcohol in fermented solutions
Demirbas, 2009
6000 BC
Asia
e Utilization of sugar cane
Demirbas, 2010
Fifteenth Century
American plantations
e Export of sugar cane
James et al., 1989
1748
Andreas Sigismund Margraff,
German scientist
e Key initiator of the modern sugar
industry
e Research on the isolation of crystalline
sugar from different roots and beet
Kamm et al., 2006,
Burton and Cox, 1998
1780
Carl Wilhem Scheele
e Discovery of lactic acid
Benninga, 1990
1801
Cunern/Schlesien Poland
e The first sugar refinery based on
sugar bet F.C. Achard
Paulik, 2011
Pennington and Baker, 1990
Early Nineteenth
Century
Samule Morey
e First tested ethanol in internal
combustion engine
Lee and Lavore, 2013
1806
Napoleon Bonaparte
e Economic continental blockade to
limit overseas trade in cane sugar
starch hydrolysis became of interest
for the economy
Brown, 2009
Harris, 1919
Paulik, 2011
1811
G.S.C. Kirchoff German
pharmacist
e Conversion of potato starch into
“grape sugar“
e The starting point of starch industry
Kamm et al., 2006,
Paulik, 2011
van der Maarel et al., 2002
1812
Weimar, Germany J.W.
Döbereiner
e The first starch sugar plant was
established
Jentoft, 2003
Kamm et al., 2006
1819
H. Braconnot, French plant
chemist
e Treatment of wood with concentrated
H2SO4 results in sugar (glucose)
Binder and Raines, 2010
Jeffries and Lindblad, 2009;
Paulik, 2011
1831
Döbereiner
e First report on the production and
separation of furfural by bran
distillation with diluted acid
de Jong and Marcotullio, 2010
Yang et al., 2011
1835
J.J. Berzelius, Swedish
Professor
e Development of enzymatic hydrolysis
of starch to sugar (“catalysis”)
Buchholz et al., 2005
Cheeptham and Lal, 2012
1839
A. Payen
e Cellulose was obtained by wood
treatment with nitric acid and
subsequent treatment with a sodium
hydroxide solution (“les cellules”)
Kamm et al., 2006
Paulik, 2011
1840
G.J. Mulder
e Synthesis of levulinic acid by
heating fructose with hydrochloride
Kamm et al., 2006
Paulik, 2011
1845
G. Fowners
e Proposed the name of “furfurol”
changed in “furfural” due to
aldehyde function
Kamm et al., 2006
1854
M.A.C Mellier
e Disintegration of cellulose pulp from
straw with caustic soda and steam
Hofmann, 1873
Jeffries and Lindblad, 2009
Kamm et al., 2006
1855
G.F. Melsens
e Wood conversion to sugar with dilute
acid
e Development of two approach on
wood hydrolysis
e Hydrolysis with concentrated acid at
low temperature; hydrolysis with
diluted acid at high temperature
Kamm et al., 2006
Kupiainen, 2012
1863
B.C. Tilghman
e The first patent for cellulose
production by use of calcium bisulphite
Gao et al., 2013
223
BIOMASS FEEDSTOCK
TABLE 14.1
Short History of Biorefineries and Bio-Based Productsdcont’d
Key Moment
Place and Actors
Innovations and Activities
References
1866
B.C. Tilghman and brother
(paper mill Harding and Sons)
e Start of the first industrial experiment
for the production of pulp from wood
and hydrogen sulphite
Antonsson, 2008; de Sa, 2004
1872
C.D. Ekman
e Production of cellulose sulfate using
magnesium sulfate as cracking agent
Kamm et al., 2006
1874
W. Haarman
F. Tiemann
e Vanillin synthesis from cambial juice
of coniferous wood
Kamm et al., 2006
Paulik, 2011
1875
Company Haarman and Reimer
e Coniferindthe first precursor for the
production of vanillin was isolated,
oxidized to glucovanillin and cleaved
into glucose and vanillin
e Industrial vanillin production
e The first industrial utilization of lignin
Kamm et al., 2006
Paulik, 2011
Wolfrom, 1970
1878
A. Mitscherlich
e Improved the sulfite pulp process by
fermentation of sugar from waste
liquor to ethanol
e Applied procedure to obtain paper
glue from the waste liquor
Kamm et al., 2006
Sindall, 1906
Watt, 1890
1895
A. Boehringer
e Industrial lactic acid fermentation
Benninga, 1990
The End of the
Nineteenth Century
e Ethanol was used in farm machinery
and introduced in the automobile
market
Lee and Lavore, 2013
1900
e Development of pulp and paper
mils (5200 worldwide)
Kamm et al., 2006
Paulik, 2011
1901
A. Classen
e The first commercial process of wood
saccharification (German Patent 130980)
with sulfuric acid
Kamm et al., 2006
Hajny, 1981
1902
W. Normann
e Liquid plant oils are converting into
tempered fat by augmentation of
hydrogen
e Hydration of liquid catalytic (Ni),
resulting tempered stearic acid
Kamm et al., 2006
WEF, 2010
1909
M. Ewen
G. Tomlinson
e The first commercial process of wood
working with dilute sulfuric acid (US
Patent 938208)
Kamm et al., 2006
Lloyd and Harris, 1955
Otulugbu, 2012
1893e1912
Company Boehringer-Ingelheim
e The pioneer of industrial biotechnology
Bio Deutschland, 2012
Interbelic Period
Friedrich Bergius
e Development technologically viable
processes for wood saccharification
e Ethanol production from the
fermentation of wood sugar
Kamm et al., 2006
Schobert, 2013
1920
Quaker Oats company
e Development of furfural production
from pentoses
Marcus, 2005
RIRDC, 2006
1925
W.J. Hale, H. Dow, C.H. Herty
e Chemurgy was founded in USA, having
as an objective the utilization of
agricultural resources in industry
Kamm et al., 2006
1927
American Maraton Corporation
e Development of commercial products
from the organic solids in the spent
sulfite liquor from pulp and paper
manufacture as leather tanning agents
and dispensing agents
Kamm et al., 2006
WEF, 2010
1932
W.H. Carothers Van Natta
e Discovery and developing a polyester
made from lactic acid
Huijser, 2009
Kobayashi, 2010
(Continued )
224
TABLE 14.1
14. BIOREFINERY SYSTEMS: AN OVERVIEW
Short History of Biorefineries and Bio-Based Productsdcont’d
Key Moment
Place and Actors
Innovations and Activities
References
1934
Cedar rapids, Iowa
e Furfural production was established
as an industrial process
Kamm et al., 2006
Peters, 1937
1940
A.E. Staley
Dectur Illinois
e Commercial production of levulinic
acid in autoclaves
e Utilization of hexoses from low cost
cellulose production was experimented
for the production of levulinic acid
Kamm et al., 2006
Kitano et al., 1975
1941
Henry Ford
e A car 100% biosynthetic composite
material made from cellulose meal, soy
meal, formaldehyde resin, with
methanol as fuel produced from
cannabis
Kamm et al., 2006
1990s
Company nature works
e Commercialization of the poly(lactic)
acid made from lactic acid
Vink et al., 2003
alternatives to replace the fossil fuels-based products
with biomass-based products depends on, among
others, the costs involved (Sanders et al., 2005; van Ree
and Annavelink, 2007).
There are different paths for biomass utilization
(Table 14.3) (Wagemann, 2012):
• integral unmodified or modified biomass, without
component separation;
• various individual components of biomass;
• biomass components in a complete way/form at
various location;
• the whole biomass in its complete forms.
However, any classification is generic only based on a
too large generalization and provides little information
on the intimacy of involved processes as well as of the
possibility to apply various technological processes to
different feedstocks (Cherubini et al., 2009). No classification criterion allows the combination of different biorefinery systems by linking different technologies
involved in both energy-driven biorefinery systems and
material-driven biorefinery systems. Cherubini et al.
(2009) mentioned some examples in this regard: “if the
carbohydrate fraction of a lignocellulosic feedstock is used to
produce cellulose and xylose, the system is classified as a
lignocellulosic feedstock biorefinery; but can also be classified
as a forest-based biorefinery and, if the lignin fraction is pyrolyzed, the same biorefinery is also suitable for classification as
a two-platform concept biorefinery”.
STRUCTURE OF BIOREFINERY CONCEPT
The biorefinery is more than a fixed technology since
it includes a collection of unitary processes, by several
different routes from feedstocks to products (Xiu et al.,
2011). Figure 14.3 shows the structural scheme of biorefinery concepts, including process types with the unitary processes and the primary products and
intermediates, as well as secondary products (Hackl
and Harvey, 2010).
The economic viability of bio-based products preparation involves different processes and methods: physical, chemical, biological, and thermal. Table 14.3
describes shortly some of these processes and methods.
However, a clear set of criteria to classify the different
biorefinery concepts is still missing. van Ree and
Annevelink (2007) considered a classification based on
the following:
• Raw material input, resulting in some classes of
biorefineries, like Green, Whole Crop, Lignocellulosic,
Feedstock, and Marine Biorefineries.
• Technologies applied for biomass processing: Two
Platform Concept, Thermo, Chemical Biorefineries.
• Products resulted (main, intermediate): Syngas, Sugar,
Lignin Platforms.
Due to the complexity of this structure, process
integration is the most sustainable approach to ensure
the system efficiency and products quality. In an integrated configuration, biorefinery systems are structured in various ways by considering the use of raw
materials, the environmentally sound character, and
the degree of integration as follows (van Ree and
Annevelink, 2007, Martin and Grossmann, 2012,
Wagemann, 2012):
• Lignocellulosic feedstock biorefinery is based on the
processing of lignocellulosic-rich biomass sources in
three steps (Figure 14.4): cellulose (sugar raw
material); hemicelluloses (polyses); and lignin. These
TABLE 14.2
Feedstock
Generation
First Generation
Feedstock Type and
Characteristics
Bio-Based
Products
Actual
Situation
Crops
(sugar/starch rich)
e Sugar cane: the most preferred, due to
case of production but it is restricted to
certain location (15e18% sugar)
e Sugar beet (15e18% sugar)
e Sweet sorghum, green grass (30% fibers,
20% proteins, aminoacids, 15%
polysaccharides, 9% mono/disaccharides,
3% oligosaccharides)
e corn, wheat, cassava: can be hydrolyzed
enzymatically and resulted sugars are
then fermented and processed in fuels
and chemicals
e Ethanol
e Bio-based
chemicals
e Proteins for
animals
e Over 400 operational first
generation refineries in the
world
e Risk of an excessive
consumption of food crop
competition between food
and biorefinery
e Risk of deforestation on
long term
e Excessive use of fertilizers
and pesticides to improve
production levels
Demirbas, 2009; Kamm et al.,
2006; McKillip et al., 2001;
WEF, 2010; van Ree and
Annevelink, 2007; Lee and
Lavore, 2013; Sanders et al.,
2005
Vegetable oil from oily
plants
e Palm (PPO) (40e50% oil)
e Soybean (PPO) (11e22% oil)
e Sunflower seed (PPO) (30e50% oil)
(All are limited by the agricultural
capacity of a country, and land-use
change limitations
e Jatropha (30e37% oil)
e Waste vegetable oil
e Biodiesel
(glycerol
as by-product)
e The process itself relies
on extracting the oils and
converting them into
biodiesel by breaking the
bonds linking the long
chain fatty acids to glycerol,
replacing it with methanol
(transesterification)
e Pure plant oil (PPO)
e Waste vegetable oil (WVO)
e 40e50%yield
WEF, 2010; Demirbas, 2009;
Kamm et al., 2006; Lee and
Lavore, 2013; Sanders et al.,
2005
Lignocellulosic biomass
(cellulose, hemicellulose,
and lignin)
e Inedible plants
e Homogeneous biomass: wood chips and
waste (16e24% lignin; 25e35%
hemicellulose, 43e47% cellulose)
e Quasihomogeneous biomass: straw
(8e14% lignin, 24e30% hemicellulose,
31e41% cellulose)
e Whole plants (25e31% lignin, 25e29%
hemicellulose, 40e44% cellulose)
e Switchgrass, miscanthus, short rotation
poplar (exclude the direct land-use
change)
e Nonhomogeneous biomass, including
low-value feedstock as municipal solid
wastes
e Biofuels
(bioethanol)
e Bio-based
chemicals
e Biomass to
liquid (BTL)
e Such biomass is generally
more complex to convert
and its production is
dependent on new technologies
significant breakthroughs in
enzymatic conversion process
e Significant and more abundant
than first generation
e Reduced dependence on
food crops
e Improvement of energy
and environmental cycles
e Improvement and costs of
production
e The conversion process is done
according to two different
approaches referred to as
“thermo” or “bio” pathways
Demirbas, 2009; Lee and
Lavore, 2013; Sanders et al.,
2005; Zhang et al., 2007;
Speight, 2011
Category
References
STRUCTURE OF BIOREFINERY CONCEPT
Second
Generation
Categories of Feedstock Utilized in Biorefineries for Various Bio-Based Products
(Continued)
225
226
TABLE 14.2
Feedstock
Generation
Categories of Feedstock Utilized in Biorefineries for Various Bio-Based Productsdcont’d
Feedstock Type and
Characteristics
Bio-Based
Products
Microalgae
e Unicellular photo and heterotrophic
organisms
e Contain storage lipids (as triacylglycerols,
transformed in biodiesel through
transesterification)
e Oil contents of some microalgae can
exceed 80% of dry weight (20 times than
traditional feedstock)
e Safe biodegradable, not competing with
arable land
e Are highly productive, quick to cultivate,
require CO2, sunlight and water for
growing
e Needs improvements in algal biology to
achieve high growth rates, high liquid
content, and ease extraction
e Developments in photobioreactor
engineering
e Biofuels
(ecologically
friendly)
Jatropha curcas tree
e Contains 27e40% inedible oil, ehich
can be converted to biodiesel
e Positive effects on the environment and
GHG emissions
e Possible to be cultivated on wasteland
or degraded ground.
Lestari et al., 2010; Speight,
2011; WEF, 2010
Third
Generation
Agricultural, forestry,
petrochemical and urban
waste and residues
e Agricultural waste and residues
e Forest waste and residues
e Municipal waste (paper cardboard,
town cleaning)
e Sludges (wet or dry biosolids)
e Residual biomass from process
industries
e Could be gasification or combustion
resulting in energy, heat, clan
synthesis gas
de Jong et al., 2009; Kamm
et al., 2006; Star-COLIBRI,
2011; WEF, 2010
Fourth
Generation
Vegetable oil
e Hydrolytic conversion
e Deoxygenation
Category
References
Lee and Lavore, 2013; Chisti,
2007; Chisti, 2008; Ribeiro
et al., 2007; Speight, 2011
Al-Zuhair, 2007; Demirbas,
2009; Cherubini, 2010
14. BIOREFINERY SYSTEMS: AN OVERVIEW
e Biogasoline
e Bio jet fuel
e Biodiesel
Actual
Situation
227
BIOREFINERY PLATFORMS
TABLE 14.3
Biomass Utilization Paths (Wagemann, 2012)
Biomass Utilization
Examples
Use of unmodified biomass
without component separation
for chemicals/materials or
bioenergy
Wood for wood-based raw
materials or sawing products
Wood used as fuel
Insulating materials made of
natural fibers
Linseed oil as solvent
Use of individual biomass
components for chemicals/
materials and/or bioenergy
Vegetable oil from rape or as
component of lacquers/dyes
Starch from cereal crops for the
production of bioethanol or for the
production of paper starch
Sugar from sugar beet used as a
fermentation raw material
Complete utilization of the
biomass components for
chemicals/materials and/or
bioenergy at various location
Biogas from corn for local
generation of electricity and heat
respectively for biomethane as
feed-in into grid for use in different
locations
Palm oil generation aboard, its
transportation to Europe, and its
domestic processing
Complete, integrated utilization
of the biomass components for
chemicals/materials and/or
bioenergy in one (networked)
location for chemicals/materials
and bioenergy
Biorefinery concepts using a
platform for the integrated
production of a spectrum of
products
Source: Adapted with the permission of the coordinator of “Biorefineries Roadmap as part
of the German Federal Government action plans for the material and energetic utilization
of renewable raw materials” brochure on behalf of The Federal Government, Professor
Kurt Wagemann.
processing steps result in feeds, chemicals,
biopolymers and other biomaterials. All residues are
incinerated for the cogeneration of heat and power
(van Ree and Annevelink, 2007).
• Whole crop biorefinery uses raw materials (cereals,
maize, and wheat) in the form of grain, flour (meal),
and straw (combination of ears, leaves, chaff and
nodes), based on dry or wet milling biomass. Their
processing results in feeds, chemicals and
biomaterials (Figure 14.5).
• Green biorefineries use “nature wet” (fresh) biomass
(green grass, clover, alfalfa, and immature cereals),
resulting in a fiber-rich press cake and a nutrient-rich
press juice (Figure 14.6).
• Thermochemical biorefinery (TCBR) entails the biomass
refining into a large portfolio of value-added
products, by applying several technologies such as
pyrolysis, gasification, torrefaction, and
hydrothermal upgrading. The resulting products
could be introduced into the existing infrastructures
and substituting fossil fuels (de Wild, 2011; Martin
and Grossmann, 2012). A particular concept derived
from TCBR and developed by de Wild (2011) relies
to Staged Catalytic Biorefinery Concept, which offers
the possibility to process biomass in different
sequential technological steps, with reducing the
severity of the processing conditions using suitable
catalysts, and to separate diverse products at
different stages.
• Marine biorefinery (MBR) is based on marine crops, i.e.
microalgae (diatoms; green, golden, and blue/green
algae) and macroalgae (brown, red and green
seaweeds), and their derived products (Bowles, 2007;
van Ree and Annevelink, 2007; Martin and
Grossmann, 2012).
Depending on the materials resulted after primary
refinery steps, the leading procedures applied for
further transformation and the integration degree of
these above mentioned biorefinery systems could
be included in various biorefinery platforms: biochemical, thermochemical, and microorganism platforms
(Cherubini et al., 2009; Kammm et al., 2006; WEF,
2010) (Table 14.4.)
In this context, the biorefinery is “an explicitly integrative, multifunctional overall concept that biomass as a
diverse source of raw materials for the sustainable generation of a spectrum of different intermediates and products (chemicals, materials, bioenergy/biofuels), allowing
the fullest possible use of all raw material components.
The coproducts can also be food and/or feed. These objectives necessitate the integration of a range of different
methods and technologies” (Wagemann, 2012).
The integration and multifunctionality in biorefineries can be performed at four levels raw material,
process, product, and industry (Martin and Grossmann,
2012; Wagemann, 2012) (Figure 14.7).
BIOREFINERY PLATFORMS
Biorefineries can produce chemicals and feels from
biomass on several integrated platforms (Figure 14.8)
(WEF, 2010).
1. The biochemical (sugar) platform, based on the
biochemical conversion of biomass, focusing on sugar
fermentation, and including steps dedicated to
products separation and purification.
2. The thermochemical platform, based on the
thermochemical conversion of biomass focusing on
the gasification of carbonaceous materials and
lignocellulosic biomass.
3. The microorganism platform, focusing on algae
biomass cultivated in raceway type ponds or in
photobioreactors.
228
14. BIOREFINERY SYSTEMS: AN OVERVIEW
Primary
process
Primary
products/
intermediates
Secondary
products
Gasification
Product gas
Syngas,
SNG,FT-fuels,
MeOH, olefins
etc.
Pyrolysis
Pyrolysis oil
Fuel, biochemicals
Torrefaction
Torrefied
biomass
Biofuel with
improved
properties
Combustion
Steam/heat
Heat and
electricity
Anaerobic
digestion
Biogas
Biomethane,
methanol,
olefins
Fermentation
Ethanol
Fuel, ethylene,
ETBE,
ethylamines
Enzymatic
hydrolysis
Fermentable
sugars
Ethanol,fuel,
ethylene,
ethylamines
Acid hydrolysis
Cellulose,
hemicelulose,
lignin
Fermentable
sugars, biofuel,
ethanol
Supercritical
conversion of
biomass
Cellulose,
hemicelulose,
lignin
Fermentable
sugars, biofuel,
ethanol
Solvent
extraction
Cellulose,
hemicelulose,
lignin,
polysaccharides
Ethanol,
extractives,
waxes
Separation
E.g. lignin
Biofuel, heat,
electricity,
materials
Drying and
pelletising
Biofuel
Heat and
electricity
Extraction
Vegetable oils,
organic acids,
extracts
Fuels,
oleochemicals
Process type
Thermochemical
conversion
Biological
conversion
Biomass
Chemical
conversion
Mechanical
conversion
FIGURE 14.3
Schematic structure of biomass conversion processes and potential products (Hackl and Harvey, 2010). Source: Reproduced with
the permission of authors, Dr. Roman Hackl and Prof. Simon Harvey, Department of Energy and Environment, Division of Heat and Power Technology
Chalmers University of Technology. (For color version of this figure, the reader is referred to the online version of this book.)
The biorefinery concept considered by the National
Renewable Energy Laboratory is based on two different
primary platforms integrating various routes included
in the biorefinery structure (NREL, 2009):
• The biochemical (sugar) platform performs the biomass
breakdown into sugars based on chemical and
biological processes:
• If lignin is the result of pretreatment and
enzymatic hydrolysis, two steps can be involved in
its further transformation:
e lignin upgrading, to etherified gasoline;
e lignin pulping to high quality paper.
• If aqueous sugars result after pretreatment and
enzymatic hydrolysis, they are involved in
fermentation processes, resulting in ethanol,
butanol, and hydrogen.
• The thermochemical platform is based on the
biomass conversion onto synthesis gas through
gasification, pyrolysis or hydrothermal
conversion.
• Gasification results in syngas, which can be further
transformed in alkanes, methanol or hydrogen by
FischereTropsch, catalysis, wateregas shift
processes.
BIOREFINERY PLATFORMS
Lignocellulosic feedstock biorefinery (LCFBR)
Cellulose
Hemicellulose
Lignin
Glucose polymer
Pentoses, hexoses
Phenol polymer
Residues
Cogeneration
heat and power,
extractions
Fuels
chemicals
biomaterials
FIGURE 14.4 Scheme of integrated biorefinery process of lignocellulosic feedstock biorefinery (LCFBR) type. Source: Adapted from Kamm
et al. (2006) and Wagemann (2012). (For color version of this figure, the
reader is referred to the online version of this book.)
FIGURE 14.5 Scheme of integrated biorefinery process of whole
crop biorefinery (WCBR) type. Source: Adapted from Kamm et al. (2006)
and Wagemann (2012). (For color version of this figure, the reader is
referred to the online version of this book.)
FIGURE 14.6 Scheme of integrated biorefinery process of green
biorefineries type. Source: Adapted from Kamm et al. (2006) and Wagemann
(2012). (For color version of this figure, the reader is referred to the
online version of this book.)
229
• Pyrolysis and hydrothermal conversion result in
biooil, which is further transformed during the
following processes:
e upgrading, when liquid fuel results;
e catalytic reforming, resulting in hydrogen;
e extraction, when various chemicals are
obtained;
e cross-linking resulting in various (bio) materials.
The third platformdmicroorganism platformdhas
been included in the biorefineries structure by the
National Renewable Energy Laboratory (WEF, 2010).
This structure demonstrates that various processes can
occur in a complex biorefinery, similar to a conventional
oil refinery. This similarity was also graphically demonstrated by Kamm et al. (2006) (Figure 1.3).
There are also some unclassified biorefineries, which
include (de Jong and van Ree, 2006) side and waste
streams, MBR, most generation III biorefineries, and
consortia of different industries. They are expected to
play a significant role in the future, since the classic
concept of biorefinery is tightly linked with the progress
of agriculture, the efficiency and availability of food and
feed production, with major consequences for the prime
arable land (PP, 2012). Considering these problems, it is
essential to promote integrated biorefinery models,
which would be able to surpass the challenges addressing retaining and recycling of phosphorous, finding new
sources of soil organic carbon, maintaining biodiversity
by adequate measures (PP, 2012; Star-COLIBRI, 2011).
Besides, a new and challenging development began to
be focused on the integrated valorization of organic
waste streams, such as agrofood by-products, effluents,
resulting in new value-added chemicals, biofuels,
biomaterial, and water (PP, 2012; Liu et al., 2010;
Visvanathan, 2010; Laufenberg et al., 2003).
This way, the integration of biorefinery platforms
would be able to generate the synergism, as the
underlying concept of industrial ecology. By closing
material cycles and cascade utilization and recycling,
it would be ensured a multilevel, explicitly integrative, multifunctional incorporation of raw materials,
processes, and products, belonging to various industrial
systems, simultaneously with preventing resource
loss by source reduction and waste minimization along
the entire biorefinery value chain. A full overview of
the platforms, products, feedstocks and conversion processes is given in Figure 14.9 (de Jong and Marcotullio,
2010).
Moreover, the eco-efficiency would become the leading concept governing the full system, since processes
for biomass treatment and conversion should be
resource efficient in terms of materials and energy use
and long lifetime of goods and products, along with consumption of auxiliaries, and should avoid adverse
230
14. BIOREFINERY SYSTEMS: AN OVERVIEW
TABLE 14.4 Conversion Processes, Methods and Techniques Employed by Biorefineries to Transform the Raw Biomass into Commercial
Products
Conversion Process
Description
Products
References
Fermentation of Sugar/Starch
Crops
• Raw materials
Bioethanol
e Starch crops
Butyric acid
e Lignocellulosic materials
Butanol
• Pretreatment
e Starch hydrolyzed
enzymatically to deliver
sugar solutions (b-glucosidase,
endocellulase, and exocellulase)
• Treatment
e Microbial fermentation to
produce bioethanol (S.
cerevisiae, P. skittles, Z. mobiles,
E. coli) or butanol (C.
tyrobutyricum, C. acetobutylicum)
Chen, 2011; Hodge et al., 2008;
Ramey, 1998; Sharara et al., 2012;
WEF, 2010
Fermentation of Lignocellulosic
Biomass
• Raw materials
e Lignocellulosic biomass
• Pretreatment
e Mechanical
e Chemical or thermal treatment
to separate the cellulosic and
hemicellulosic material from
the nonfermentable lignin
• Treatment
e Enzymatic hydrolysis of
cellulosic and hemicellulosic
components
e Fermentation of sugars
Cherubini, 2010; FitzPatrick et al.,
2010; Knauf and Moniruzzaman,
2004; WEF, 2010
Transesterification of
Triglycerides
• Raw materials
e Plant or algal oil
• Treatment
e Triglycerides are treated with
methanol in the presence of a
dedicated catalyst to deliver
fatty acids and methyl esters
Biodiesel
Asakuma et al., 2009; Chisti, 2007;
Meher et al., 2006
Gasification to Syngas
e Breakdown of carbonaceous
materials into H2 and CO
(syngas) through thermal
decomposition in the presence
of a limited quantity of oxygen
e H2 þ CO mixture can be further
converted by partial oxidation at
elevated temperature as
FischereTropsch reactions
e Physical and chemical properties
of feedstocks affects the quality
of syngas and process efficiency
(moisture, ash minerals)
e The use of catalysts can improve
the thermochemical conversion
by facilitating a preferred
reaction mechanism
e Syngas (H2 þ CO) can be
used to
e Direct combustion in boilers,
turbines or internal
combustion engines
e Produce hydrogen by
separation
e Produce chemical products
(ammoma) by chemical
synthesis
e Produce liquid fuels through
the FischereTropsch process
Devi et al., 2003; Göransson et al.,
2011; Huang and Ramaswamy,
2013; Hughes and Larson, 1998;
Pryadarsan et al., 2004; Van der
Drift et al., 2001; WEF, 2010
Fast Pyrolysis
e Thermal decomposition of
biomass in a biooil (in the
absence of oxygen), which
can be further converted through
hydrogenation or gasification
into certain hydrocarbon
e Reduced costs, compared to
gasification of solid biomass
About 100 chemicals species
biooil (biocrude) with 44e47%
carbon, 6e7% hydrogen, and
46e48% oxygen)
WEF, 2010; Sharara et al.; Evans
and Milne, 1987; Bridgwater et al.,
1999
231
BIOREFINERY ECO-EFFICIENCY
TABLE 14.4 Conversion Processes, Methods and Techniques Employed by Biorefineries to Transform the Raw Biomass into Commercial
Productsdcont’d
Conversion Process
Description
Products
References
FischereTropsch Synthesis
e Catalytic conversion of sugars
into liquid hydrocarbons
(C1eC50)
e The process in selective
depending on temperature,
pressure and catalysts
Synthetic fuel
Demirbas, 2010; Lappas and
Heracleous, 2011; NREL, 2009
Hydrogenation
e Hydrotreatment of biooils,
resulting hydrotreated
renewable jet fuels (HRJ)
e Removes oxygen and others
impurities from organic oils
(extracted directly from
feedstocks with high oil
content or produced by
pyrolysis)
HRJdhydrotreated renewable
jet fuels, with similar
properties as kerosene
Conversion of Syngas
to Methane (SNG)
e Thermal gasification and
particular FischereTropsch
reaction
SNGdsynthetic natural gas
(a good substitute of the
natural gas)
Martin and Grossmann, 2012
Aerobic Digestion
e Conversion of biodegradable
waste or energy crops into
a gaseous fuel biogas
e Conversion efficiency is
about 70%
Biogas (50% methane)
Martin and Grossmann, 2012
Catalytic Thermochemical
Conversion
e Increases the yield and
optimize the composition of
output products of
thermochemical conversion
e Helps in overcoming the
problematic qualities of
biooil (thermal and
temporal instability)
e Catalyst can be incorporated
during or after the production
process, or in both stages
(activated alumina, silicate,
Y-zeolite, ZSM-5)
Pyrolysis oil (biooil) which is
a chemical intermediate or
directly as liquid fuel
Carlson et al., 2009; de Wild, 2011;
Sharara et al., 2012; Zhang et al.,
2009
environmental impacts and risks (Gavrilescu, 2011;
Wagemann, 2012). Therefore, we can discuss about a
multilevel integration of the biorefinery concept along
the supplying chain: raw materialseprocesseseproductse
industrial platforms (Figure 14.10).
BIOREFINERY ECO-EFFICIENCY
Biorefineries offer numerous business opportunities
(WEF, 2010). It is expected that the global market of biofuels will increase from almost $80,000 million in 2011 to
$185,000 million in 2021. Moreover, it is estimated that
almost 136,000 million L of biofuels will be consumed
in 2020 in USA, which would require over 500
commercial-scale cellulosic ethanol refineries, with
capital requirements of $168,000 million (Solecki et al.,
2012). Other estimations showed that the actual revenue
potentials due to biomass conversion are of $80,000
million for biofuels, $10,000e15,000 million for biobased chemicals, including bioplastics (WEF, 2010).
There are important revenue potentials along the
entire biomass value chain, associated with the most relevant steps, as follows (WEF, 2010): agricultural inputs,
$15,000 million; biomass production, $89,000 million;
biomass trading, $30,000 million; biorefinering inputs,
$10,000 million; biorefinering chemicals and downstream chemistry, $6,000 million; biorefinering fuels,
$80,000 million; biomass power and heat, $65,000 million.
Today a major challenge is associated to the production of biofuels and bio-based chemicals in an ecoefficient manner. Since the production, the products
232
14. BIOREFINERY SYSTEMS: AN OVERVIEW
FIGURE 14.7
Levels of integration and multifunctionality already realized in biorefineries. Source: Adapted upon Wagemann (2012). Adapted
with the permission of the coordinator of “Biorefineries Roadmap as part of the German Federal Government action plans for the material and energetic
utilization of renewable raw materials” brochure on behalf of The Federal Government, Professor Kurt Wagemann. (For color version of this figure, the
reader is referred to the online version of this book.)
Biorefinery
platforms
Biochemical
platform
Crops (sugar) starch rich
Biobased products
Biomaterials
Vegetable oily plants
Thermochemical
platform
Feedstock
Lignocellulosic biomass
Biomass
Fuels
and energy
Microorganism
platform
Microalgae
Oily trees
Combined
heat and power
Biochemicals
• Composite materials
• Dyes and pigments
• Detergents and cleaners
• Adhesives
• Oils and inks
• Etc.
• Lignin, biogas, cake
• Ethanol, methanol,
fuel oil
• Syngas, methane,
• Hydrogen
• Agricultural chemicals
• Activated carbon
• Specialty chemicals
• Industrial surfactants
• Fatty acids
• Acetic acid
• Etc
FIGURE 14.8 Integration of three biorefinery-integrated platforms. Source: Adapted from WEF (2010) and Kamm et al. (2006). (For color version
of this figure, the reader is referred to the online version of this book.)
and by-products are quite numerous and diverse; a simple approach for the estimation of production economics
would be always opportune, so as to offer valuable information about the relative feasibility of various production alternatives and routes. For example, Melin
and Hurne (2011) developed an algorithm to find “the
production route with the minimum production costs
for a biofuel or a chemical, for each raw material,
when the process and the economic parameters occur
in a known range”. Other several studies have estimated
biofuel production costs from corn stove through gasification and FischereTropsch routes (Demirbas, 2010;
233
BIOREFINERY ECO-EFFICIENCY
Organic residues
and others
Starch
crops
Grasses
Fractionation
and/or
pressing
Separation
Grain
Sugar
crops
Lignocellulosic
crops
Lignocellulosic
residues
Oil based
residues
Oil crops
Straw
Straw
Pretreatment
Pressing
Lignin
Fiber
Separation
Gasification
Organic
solution
Oil
Pyrolysis, HTU
Hydrolysis
Syngas
Extraction
Anaerobic
digestion
Pyrolytic
liquid
C5 sugars
C6 sugars
Separation
Water
gas
shift
Hydrogenation
Biogas
Fermentation
Methanisation
Chemical
reaction
Chemical
reaction
Upgrading
Combustion
Steam
reforming
Water
electrolysis
H2
Chemical
reaction
Legend
Feedstock
Platform
Material
products
Chemical
process
Mechanical/
Physical process
Estherification
Thermochemical
process
Biochemical
processes
Bio-methane
Energy products
Link among biorefinery pathways
Bio-H2
Fertilizer
Organic acids &
extracts
Biomaterials
Synthetic liquid
biofuels (FT)
Bioethanol
Chemicals &
polymers
Glycerine
Food
Electricity
and heat
Animal
feed
Biodiesel
FIGURE 14.9 Full network of the platforms, products, feedstocks and conversion processes (de Jong et al., 2009). Source: Reproduced with the
permission of IEA Bioenergy Task Leader, Dr. Ed de Jong. (For color version of this figure, the reader is referred to the online version of this book.)
Batsi et al., 2012; Swanson et al., 2010). The objective was
to compare capital investment costs and production
costs for various biorefinery scenarios.
Building a bio-based economy must be able not only
to solve the current economic difficulties but also to
generate an economic system with minimal impact to
the environment.
Even though regarded as similar to petroleum refinery, a comparison of the biorefinery and petrochemical
value chains show some similarities but also a large
number of differences. Both result in complex product
trees, but one of the most relevant differences consists
in compositions of fossil raw materials and biogenic
raw materials (Kamm et al., 2006; Wagemann, 2012).
Table 14.5 illustrates some similarities and differences
between two value chains.
Consequently, for decision-making process, it is
necessary to develop a methodology to drive decisions
on biorefinery, with a focus on product design and process. Transition to a biorefinery economy could involve
significant investments in infrastructure to produce,
store and sell biorefinery products to customers
(Demirbas, 2010). A number of questions related to biorefinery diagnosis can be addressed using SWOT
analysis. Such an investigation of the opportunities
and strengths, weaknesses and threats of biorefineries
as developed by IEA within the Task 42 is illustrated
in Table 14.6 (de Jong et al., 2009).
The concept of eco-efficiencyddefined as “creating
more value with less impact”dhas been developed by
The World Business Council to weigh and compare
products and technologies in both aspects: environmental pressure and economic significance (WBCSD,
2000). The Organization for Economic Co-operation
and Development (OECD) has defined eco-efficiency
as the effectiveness with which ecological resources are
used to meet human needs.
Integrating the issues concerning the environmental
impacts and economic value resulting from biorefinery
processes allows decision makers in the business world
to evaluate and compare products and technologies
simultaneously, from both points of view. Organizations could be supported to establish measurable objectives of eco-efficiency and to facilitate comparisons
between companies and business sectors by the standardization of definitions and decision system for
calculating and reporting eco-efficiency indicators.
The environmental impact ratio, defined in Figure 14.11,
234
14. BIOREFINERY SYSTEMS: AN OVERVIEW
reflects how much environmental impact per environmental credit occurs in the product system (Hong
Chua and Replace with Steinmüller, 2010; Kim and
Dale, 2004). A scenario with a greater eco-efficiency
would be more sustainable, which means that it would
offer more economic value per unit of environmental
impact (Fig. 14.11).
Some eco-efficiency indicators were developed for
different levels of biorefinery integration, following the
physical flows of materials and energy (Hong Chua
and Steinmüller, 2010):
Level 1 addresses process integration and involves
the key processes (receiving and preparation of
feedstock, retreatment, conversion to bioproduct,
and wastewater treatment system).
Level 2 refers to agriculture integration, which means
that feedstocks, including agricultural waste, are
supplied in the biorefinery system at the business
level that is involving low costs, while biofuels,
bioelectricity and biochemicals from biorefinery are
sent to the agricultural sector.
Level 3 involves livestock farming integration at the
business level, meaning that the organic waste from
farms are supplied to the biorefinery system, while
animal feed products are sent to the farm.
FIGURE 14.10
The biorefinery process chain (Wagemann, 2012).
Source: Reproduced with the permission of the coordinator of “Biorefineries
Roadmap as part of the German Federal Government action plans for the material and energetic utilization of renewable raw materials” brochure on behalf
of The Federal Government, Professor Kurt Wagemann. (For color version of
this figure, the reader is referred to the online version of this book.)
TABLE 14.5
Estimated costs of production in biorefinery systems may be hampered by a number of driving forces
who can change their direction of action and/or
importance in time (agricultural development, raw
material costs, production scale, competing markets
evolution, their demands and access, waste recovery
Comparison of Biorefinery and Petrochemical Value Chains (Wagemann, 2012)
Value Chain
Biorefinery
Petrochemical
Raw Materials
Biomasses: very complex mixture of
organic compounds
Mineral oil, natural gas: mixture of
hydrocarbons
Carbon and heteroatoms (poor in
hydrogen, rich in oxygen)
Carbon and hydrogen (almost no hetero atoms,
poor in oxygen)
Contains inorganic compounds
Contains virtually no inorganic compounds
Hydrous
Waterless
Primary Refinery
Thermal and thermocatalytic (syngas) as
well as biochemical (biogas) cleavage
into simple molecules
Distillation and thermal and thermocatalytic
cleavage into simple molecules
Secondary Refinery
Build-up complex molecules from simple precursors (bottom-up principal)
Processes
Thermochemical, thermocatalytic and chemocatalytic processes
Product Classes
Chemicals and materials Combustibles and fuels
Source: Adapted with the permission of the coordinator of “Biorefineries Roadmap as part of the German Federal Government action plans for the
material and energetic utilization of renewable raw materials” brochure on behalf of The Federal Government, Professor Kurt Wagemann.
BIOREFINERY ECO-EFFICIENCY
TABLE 14.6
235
SWOT* Analysis of Biorefineries Processes (de Jong et al., 2009)
Strengths
Weaknesses
• Adds value to the sustainable use of biomass
• Maximizes biomass conversion efficiencydminimizing raw
material requirements
• Produces a spectrum of bio-based products (food, feed, materials,
and chemicals) and bioenergy (fuels, power and/or heat) feeding
the full bio-based economy
• Strong knowledge of infrastructure available to tackle any
nontechnical and technical issues potentially hindering the
deployment trajectory
• Is not new, and in some market sectors (food, paper, etc.), it is
common practice
• Broad undefined and unclassified area
• Needs involvement of stakeholders from different market sectors
(agro, energy, chemical,.) over the full biomass value chain
• Most promising biorefinery processes/concepts not clear
• Most promising biomass value chains, including current/future
market volumes/prices, not clear
• Still at a stage of studying and concept development instead of
real market implementation
• Variability of quality and energy density of biomass
Opportunities
Threats
• Make a significant contribution to sustainable development
• Challenging national, European and global policy
goalsdinternational focus on sustainable use of biomass
for the production of bioenergy
• Biomass availability is limited so the raw material should
be used as efficiently as possibledi.e. development of
multipurpose biorefineries in a framework of scarce raw
materials and energy
• International development of a portfolio of biorefinery
concepts, including designing technical processes
• Strengthening of the economic position of various market
sectors (e.g. agriculture, forestry, chemical and energy)
• Biorefinery is seen as hype that still has to prove its benefits in the
real market
• Economic change and drop in fossil fuel prices
• Fast implementation of other renewable energy technologies
filling market needs
• No level playing field concerning bio-based products and
bioenergy (assessed to a higher standard)
• Global, national and regional availability and contractibility of
raw materials (e.g. climate change, policies, and logistics)
• High-investment capital for pilot and demonstration initiatives
difficult to find, and existing industrial infrastructure is not
depreciated yet
• Fluctuating (long-term) governmental policies
• Questioning of food/feed/fuels (land use competition) and
sustainability of biomass production
• Goals of end users often focused upon single product
* Strengths, Weaknesses, Opportunities, and Threats
Source: Reproduced with the permission of IEA Bioenergy Task Leader, Dr. Ed de Jong.
and recycling alternatives, storage and production
costs, distribution costs, etc.), which could be associated with the components of a complex system with
various boundaries (Figure 14.12; Demirbas, 2010;
Kim and Dale, 2004).
Life cycle assessment (LCA) is an especially useful
tool to investigate the environmental performance of
l
nta
me
n
viro act
En Imp
Eco-efficiency =
AD
PO P, GW
CP
, H P, O
AP TP, E DP,
,E
P TP,
Economic value added
Capital
investment
Environmental impact ratio
NPV, IRR
Economic value added =
ic
om
on is
Ec alys
An
Market value of products
Cost of raw material & fuel
Environmental impact ratio =
product and/or technologies. The problem to be solved
in the case of biorefineries is not a simple one because
these systems are characterized by some particularities
that need to be considered in evaluating the processes
on an LCA basis and to ensure correct results in terms
of eco-efficiency (for example, sometimes it is not
obvious which product should be the main output;
Environmental impact
Environmental credit
FIGURE 14.11 Integration of economic analysis
and environmental impact for eco-efficiency (ADP,
abiotic resources depletion potential; GWP, global
warming potential; ODP, ozone layer depletion potential; POCP, photochemical oxidation potential;
HTP, human toxicity potential; ETP, ecological toxicity
potential; AP, acidification potential; EP, eutrophication potential; NPV, net present value; IRR, internal
rate of return). (For color version of this figure, the
reader is referred to the online version of this book.)
236
14. BIOREFINERY SYSTEMS: AN OVERVIEW
Surplus
Local
power grid
•Electricity
•Chemicals, enzyme
•Electricity
•Steam
System boundary
Pre-processing
Cropping
systems
Animal waste
treatment
Animal
operation
Final processing
Intermediates
Final product
•Sugars
•Ethanol
•Lipids
•Biodiesel
•Lignin
•Nutrient
Energy inputs
Biorefinery
•Steam
•Electricity
•Steam
Inputs
•Fertilizers
•Fuel
•Agrochemicals
Inputs
•Biopolymers
•Ash
•Protein
Final product
•Food
•Other products
•Chemicals
Cogeneration
•Ash
FIGURE 14.12
Boundaries of an integrated biorefinery system (Kim and Dale, 2004). Source: Reproduced with the permission of the author of
PowerPoint presentation, Prof. Bruce E. Dale. (For color version of this figure, the reader is referred to the online version of this book.)
Hong Chua and Steinmüller, 2010; Laser et al., 2009).
Further, the system boundaries could be different if
the biorefineries are nonintegrated or integrated and
this can determine the selection of system boundaries,
which could also affect the eco-efficiency results, while
allocation issues in particular are both important and
somewhat controversial (Figure 14.12). A very common
approach considers that all biomass is local since this
could improve the selection of crops and cropping systems
for local biorefineries, reduce opportunities for agendadriven manipulation of data and opportunities for system
integration and waste utilization could be better exploited
(Kim and Dale, 2004). The functional unit could be chosen
as unit area of land allocated for crop biomass for a certain
time period since cropping systems play an important
role in the environmental performance of bio-based
products, while impacts assessment could address global
warming potential, nonrenewable energy, crude oil consumption, water use, acidification, eutrophication, biodegradability, less toxicity, etc. (Laser et al., 2009; Demirbas,
2010). In their study, Hong Chua and Steinmüller (2010)
have identified the following main environmental
influences for a biorefinery: energy consumption, material
consumption, GHG emissions, acidification, and eutrophication. The eco-efficiency indicators used to account
for these environmental influences are as shown in
Table 14.7.
Ensuring biorefinery eco-efficiency is one of the most
relevant objectives of Task 42 of IEA in parallel with the
projection of new perspectives in terms of competitiveness, sustainability, and safety of processing routes for
biogenic raw materials to guarantee the concurrent
fabrication of biofuels, commodity chemicals, new materials, heat and power.
CONCLUDING REMARKS AND
PERSPECTIVES
Bioresource use in the forms of new and waste biomass
is a great opportunity and a challenge for the future since it
offers the chance of replacing fossil fuels for the production of energy carriers, materials and specialty chemicals
and diminishing the market pressure in an almost
carbon-neutral way. Industrial biorefineries are seen as
one of the most promising directions toward a sustainable
bio-based economy. Fully developed biorefineries
combine biological and physicochemical processes.
A weakness of biorefineries as an alternative to conventional oil refineries consists in the fact that the former
is based on biofeedstock, which can require an intensive
cultivation and land use.
Moreover, biorefineries could compete with food requirements and needs, which would limit the land allocated to biomass for biorefineries. As a result, the future
of biorefineries should consider the use of nonedible
biomass and the advanced processing of biomass waste,
as well as land which could not normally be used for
agriculture. This type of land could be used for microalgae cultures or renewable plants. Other sources of raw
material for biorefineries could be found on waste
from the food industry and urban organic waste. The
processing of this raw matter can be successfully and
eco-efficiently carried out through the development of
enzymatic systems and engineered microorganisms
capable of separating useful compounds from waste.
The development of these technologies should also
consider the important issue of costs, since, currently,
oil-based refineries offer more cost-effective solutions at
the expense of environmental degradation and pollution.
TABLE 14.7
Main Eco-Efficiency Indicators for Biorefineries (Hong Chua and Steinmüller, 2010)
Eco-Efficiency
Indicators (EEI)
Equation
Terms
Overall
P
EETEC;i ¼ PRi =
Biorefinery Material
Consumption (EETMC,i)
EETMC;i ¼ PRi =
Biorefinery GHG Emissions
(EEGHG,i)
EEGHG;i ¼ PRi =
Biorefinery Acidification
Emissions (EEAP,i)
EEAP;i ¼ PRi =
Biorefinery Eutrophication
Emissions (EEEP,i)
EEEP;i ¼ PRi =
TECi ¼ PRi =ðRECI þ RECII þ RECIII þ NRECI þ NRECII þ NRECIII Þ
P
P
P
P
TMCi ¼ PRi =ðRMCI þ RMCII þ RMCIII þ NRMCI þ NRMCII þ NRMCIII Þ
GHGi ¼ PRi =ðGHGI þ GHGII þ GHGIII Þ
APi ¼ PRi =ðAPI þ APII þ APIII Þ
EPi ¼ PRi =ðEPI þ EPII þ EPIII Þ
PRi, total profit from all productions sold
(country currency)
NRECi, total nonrenewable energy
consumption of biorefinery
RECi, total renewable energy consumption of
biorefinery (Megajoules)
RMCi, total renewable material consumption of
biorefinery (kg)
NRMCi , total nonrenewable material
consumption of biorefinery (kg)
GHGi, total greenhouse gas emissions of
biorefinery (kg)
APi, total acidification emissions of
biorefinery (kg)
EPi, total eutrophication emissions of
biorefinery (kg)
Energy Consumption
Total Energy Consumption
(EETEC;ij)
EETEC;ij ¼ PRij =
Nonrenewable Energy
Consumption (EENRE;ij)
EENRE;ij ¼ PRij =
P
TECij ¼ PRij =ðRECI þ RECII þ RECIII þ NRECI þ NRECII þ NRECIII Þj
P
NRECij ¼ PRij =ðNRECI þ NRECII þ NRECIII Þj
Renewable Energy Consumption EEREC;ij ¼ EETEC ð1=EETEC 1=EENRE Þ 100%
Rate (EEREC;ij)
PRij, allocated profit from productions sold
(country currency)
NRECij, allocated nonrenewable energy
consumption associated with the production of
bioproduct from feedstock and biorefinery
integration levels (Megajoules)
RECij, allocated renewable energy consumption
associated with the production of bioproduct
and biorefinery integration levels (Megajoules)
CONCLUDING REMARKS AND PERSPECTIVES
Biorefinery Energy
Consumption (EETEC,i)
Material Consumption
Total Material Consumption
(EETMC;ij)
EETMC;ij ¼ PRij =
EENRM;ij ¼ PRij =
P
P
TMCij ¼ PRij =ðRMCI þ RMCII þ RMCIII þ NRMCI þ NRMCII þ NRMCIII Þj
NRMCij ¼ PRij =ðNRMCI þ NRMCII þ NRMCIII Þj
PRij, allocated profit from productions sold
(country currency)
RMCij, allocated renewable materials
consumption associated with the production
(Continued)
237
238
TABLE 14.7
Main Eco-Efficiency Indicators for Biorefineries (Hong Chua and Steinmüller, 2010)dcont’d
Eco-Efficiency
Indicators (EEI)
Equation
Terms
EERMC;ij ¼ EETMC ð1=EETMC 1=EENRM Þ 100%
of bioproduct and biorefinery integration
levels (kg)
NRMCij, allocated nonrenewable materials
consumption associated with the production
of bioproduct and biorefinery integration
levels (kg)
Nonrenewable Material
Consumption (EENRM;ij)
Renewable Material
Consumption Rate (EERMC;ij)
Greenhouse Gases (GHG)
EEGHG;ij ¼ PRij =
P
GHGij ¼ PRij =ðGHGI þ GHGII þ GHGIII Þj
PRij, allocated profit from productions sold
(country currency)
GHGij, allocated greenhouse gas emissions
associated with the production of bioproduct
and biorefinery integration levels (kg)
Acidification Potential (AP)
Acidification Emissions (EEAP)
EEAP;ij ¼ PRij =
P
APij ¼ PRij =ðAPI þ APII þ APIII Þj
PRij, allocated profit from productions sold
(country currency)
APij, allocated acidification emissions
associated with the production of bioproduct
and biorefinery integration levels (kg SO2
equivalent)
EPij ¼ PRij =ðEPI þ EPII þ EPIII Þj
PRij, allocated profit from productions sold
(country currency)
EPij, allocated eutrophication emissions
associated with the production of bioproduct
and biorefinery integration levels (kg PO4
equivalent)
Eutrophication Potential (EP)
Eutrophication Emissions (EEEP) EEEP;ij ¼ PRij =
P
Notations: i refers to the level of integrations of the biorefinery; j refers to the product from the refinery.
Source: Development Of Eco-Efficiency Indicators for a Biorefinery, Authors: Celia Bee Hong Chua, Horst Steinmüller (http://www.energyefficiency.at/web/artikel/eco-efficiency_indicators.html).
14. BIOREFINERY SYSTEMS: AN OVERVIEW
GHG Emissions (EEGHG)
REFERENCES
Acknowledgments
This work was partially supported by the grant of the Romanian
National Authority for Scientific Research, CNCSdUEFISCDI, project
number PN-II-ID-PCE-2011-3-0559, Contract 265/2011.
References
Al-Zuhair, S., 2007. Production of Biodiesel: possibilities and challenges. Biofuels, Bioprod. Biorefin. 1, 57e66.
Alakangas, E., Mäkinen, T., 2008. BioRefine Programme 2007e2012.
Yearbook 2008, Tekes Review 293/2008. VTT Technical Centre of
Finland, Tekes, Finnish Funding Agency for Technology and
Innovation, Helsinki.
Antonsson, S., 2008. Strategies for Improving Kraftliner Pulp Properties (Ph.D. thesis). Royal Institute of Technology, Stockholm.
Asakuma, Y., Maeda, K., Kuramochi, H., Fukui, K., 2009. Theoretical
study of the transesterification of triglycerides to biodiesel fuel.
Fuel 88, 786e791.
Batsi, D.R., Solvason, C.C., Sammons, N.E., Chambost, V.,
Bilhartz, D.L., Eden, M.R., El-Halwagi, M.M., Stuart, P.R., 2012.
Product Portofolio Selection and Process Design for the Forest
Biorefinery. In: Stuart, P.R., El-Halwagi, M.M. (Eds.), Integrated
Biorefineries: Design, Analysis and Optimization. CRC Press, Boca
Raton, pp. 4e36.
Benninga, H., 1990. A History of Lactic Acid Making: A Chapter in the
History of Biotechnology. Springer.
Berntsson, T., Sanden, B., Olsson, L., Asblad, A., 2012. What is a biorefinery? In: Sanden, B. (Ed.), Systems Perspectives on Biorefineries. Chalmens University of Technology, Gäteborg,
pp. 16e25.
Binder, B., Raines, R.T., 2010. Fermentable sugars by chemical hydrolysis of biomass. Proc. Natl. Acad. Sci. USA 107, 4516e4521.
Bio Deutschland, 2012. White biotechnology a pillar of the bioeconomy.
Available from: http://www.biodeutschland.org/tl_files/content_
eng/Documents/2013/Flyer_White_Biotechnology.pdf.
Biorefinery Euroview, 2008. Current situation and potential of biorefinery concept in the EEC, strategic framework and guideline for
its development. FP6EEC Project. Available from: www.
biorefinery.euroview.eu.
Bowles, D. (Ed.), 2007. Micro and macro algae: Utility for industrial
applications. CPL Press, Newbury, University of York, UK.
Bridgwater, A., Meier, D., Radlein, D., 1999. An overview of fast
pyrolysis of biomass. Org. Geochem. 30, 1479e1493.
Brown, H., 2009. Starch Reality. Food Manufacture, UK. Available
from:
http://www.foodmanufacture.co.uk/Ingredients/Starchreality2.
Buchholz, K., Kasche, V., Bornscheuer, U.T., 2005. Biocatalysts and
Enzyme Technology. Wiley-VCH Verlag GmbH & Co. KGaA,
Weinheim.
Burton, R.A., Cox, P.A., 1998. Sugarbeet culture and mormon economic development in the Intermountain West. Econ. Bot. 52,
201e206.
Carlson, T., Tompsett, G., Conner, W., Huber, G., 2009. Aromatic
production from catalytic fast pyrolysis of biomass derived feedstocks. Top. Catal. 52, 241e252.
Centi, G., Perathoner, S., 2009. From green to sustainable industrial
chemistry. In: Cavani, F., Centi, S., Perathoner, S., Trifiro, F. (Eds.),
Sustainable Industrial Chemistry. John Wiley and Sons, New York,
pp. 11e20.
Cheeptham, N., Lal, A., 2012. Starch Agar Protocol. American Society
for Microbiology. Available from: http://www.microbelibrary.
org/library/laboratory-test/3780-starch-agar-protocol.
239
Chen, Y., 2011. Development and application of co-culture for ethanol
production by co-fermentation of glucose and xylose: a systematic
review. J. Ind. Microbiol. Biotechnol. 38, 581e597.
Cherubini, F., 2010. The biorefinery concept using biomass instead of
oil fro producing chemicals. Energy Convers. Manage. 51,
1412e1421.
Cherubini, F., Jungmeier, G., Wellisch, M., Willke, T., Skiadas, I., Van
Ree, R., de Jong, E., 2009. Toward a common classification approach
for biorefinery systems. Biofuels, Bioprod. Biorefin. 3, 534e546.
Chisti, Y., 2007. Biodiesel from microalgae. Biotechnol. Adv. 25,
294e306.
Chisti, Y., 2008. Biodiesel from microalgae beats bioethanol. Trends
Biotechnol. 26, 126e131.
de Jong, W., Marcotullio, G.L., 2010. Overview of biorefineries based
on coproduction of furfural, existing concepts and novel developments. Int. J. Chem. React. Eng. 8. Article A69.
de Jong, E., van Ree, R., 2006. Biorefineries- International Status
Quo and Future Direction. Wagenigen University. Available from:
www.biorefinery.nl/fileadmin/biorefinery/docs/publications/
presentations-kickoff/-2-current-status-on-biorefineries-IEA42150307.pdf.
de Jong, E., van Ree, R., Kwant, I.K., 2009. Biorefineries: Adding
Value to the Sustainable Utilization of Biomass. IEA Bioenergy.
Available from: http://www.ieabioenergy.com/Libitem.aspx?
id¼6420.
de Sa, D.O.J., 2004. Applied Technology and Instrumentation for
Process Control. Taylor & Francis, New York, London.
de Wild, R.J., 2011. Biomass Pyrolysis for Chemicals (Ph.D. thesis).
Rijksu University, Groningen, The Netherlands.
Demirbas, M.F., 2009. Biorefineries for biofuel upgrading: a critical
review. Appl. Energy 86, S151eS161.
Demirbas, A., 2010. Biorefineries: for Biomass Upgrading Facilities.
Springer Dordrecht, Heidelberg-London-New York.
Devi, L., Ptasinski, K.J., Janssen, F., 2003. A review of the primary
measures for tar elimination in biomass gasification processes.
Biomass Bioenergy 24, 125e140.
EEA, 1999. Making sustainability accountable: eco-efficiency resource
productivity and innovation topic report NO11/1999. In: Proceedings of a workshop on the occasion of the Fifth Anniversary of
the European Environment Agency. EEA, 28e30 October 1998,
Copenhagen, Denmark.
Evans, R., Milne, T., 1987. Molecular characterization of the pyrolysis
of biomass. Energy Fuels 1, 123e137.
FitzPatrick, M., Champagne, P., Cunningham, M.F., Whitney, R.A.,
2010. A biorefinery processing perspective: treatment of lignocellulosic materials for the production of value added products.
Bioresour. Technol. 101, 8915e8922.
Gao, J., Andreson, D., Levie, B., 2013. Saccharification of recalcitrant
biomass and integration options for lignocellulosic sugars from
Catchlight Energy’s sugar process (CLE Sugar). Biotechnol. Biofuels 6, 10. http://dx.doi.org/10.1186/1754-6834-6-10.
Gavrilescu, M., 2011. Sustainable Industrial Production. Ecozone
Press, Iasi, Romania.
Gavrilescu, M., Chisti, Y., 2005. Biotechnology e sustainable alternative for chemical industry. Biotechnol. Adv. 23, 471e499.
Goransson, K., Soderlind, U., He, J., Zhang, W., 2011. Review of syngas
production via biomass DFGs. Renewable Sustainable Energy Rev.
15, 482e492.
Hackl, R., Harvey, S., 2010. Opportunities for Process Integrated Biorefinery Concepts in the Chemical Cluster in Stenungsund.
Research Project Report, Department of Energy and Environment,
Division of Heat and Power Technology. Chalmers University of
Technology. Available from: http://publications.lib.chalmers.se/
records/fulltext/local_131485.pdf.
240
14. BIOREFINERY SYSTEMS: AN OVERVIEW
Hajny, G.J., 1981. Biological Utilization of Wood for Production of
Chemicals and Foodstuffs. United States Department of Agriculture. Available from: http://www.fpl.fs.fed.us/documnts/fplrp/
fplrp385.pdf.
Harris, F.S., 1919. The Sugar-Beet in America. The MacMillan Company,
New York. Available from: http://booksnow2.scholarsportal.info/
ebooks/oca4/33/sugarbeetinameri00harruoft/sugarbeetin
ameri00harruoft.pdf.
Hens, L., Quynh, L.X., 2008. Environmental space. In: Encyclopedia of
Ecology. Academic Press, pp. 1356e1363.
Hodge, D., Karim, N., Schell, D., MacMillan, J., 2008. Soluble and
insoluble solid contributions to high- solid enzymatic hydrolysis of
lignocellulose. Bioresour. Technol. 99, 8940e8948.
Hofmann, C., 1873. A Practical Treatise on the Manufacture of Paper
in All Its Branches. University Oxford.
Hong Chua, C.B., Steinmüller, H., 2010. Development of Eco-efficiency
Indicators for a Biorefinery. Energy Institute and Johannes Keppler
University Linz. Available from: http://www.energyefficiency.at/
web/artikel/eco-efficiency_indicators.html.
Huang, H.J., Ramaswamy, S., 2013. Overview of biomass conversion
processes and separation and purification technologies in biorefineries. In: Ramaswamy, S., Hunag, H.J., Ramarao, B.V. (Eds.),
Separation and Purification Technologies in Biorefineries. John
Wiley and Sons, Ltd, pp. 1e36.
Hughes, W., Larson, E., 1998. Effect of fuel moisture content on
biomass-IGCC performance. J. Eng. Gas Turbines Power 120,
455e459.
Huijser, S., 2009. Synthesis and Characterization of Biodegradable
Polyesters (Ph.D. thesis). Eindhoven University of Technology.
James, C.E., Hough, L., Khan, R., 1989. Sucrose and its derivatives. In:
Herz, W., Grisebach, H., Kirby, G.W., Tamm, Ch (Eds.), Progress in
the Chemistry of Organic Natural Products. Springer, Vienna,
pp. 117e184.
Jeffries, T., Lindblad, P., 2009. We march backward into the future.
Curr. Opin. Biotechnol. 20, 255e256.
Jentoft, F.C., 2003. Introduction: a historical approach to catalysis.
Mod. Methods Heterog. Catal. Available from: http://www.fhiberlin.mpg.de/acnew/department/pages/teaching/pages/
teaching__winterseme ster__2003_2004/jentoft_intro_241003.pdf.
Kamm, B., 2013. Introduction to biomass and biorefineries. In: Xie, H.,
Gathergood, N. (Eds.), The Role of Green Chemistry in Biomass
Processing and Conversion. Wiley, Hoboken, New Jersey,
pp. 1e26.
Kamm, B., Kamm, M., Gruber, P.R., 2006. Biorefinery systems e an
overview. In: Kamm, B., Gruber, R., Kamm, M. (Eds.), 2006. Biorefineries e Industrial Processes and Products. Status Quo and
Future Directions, vol. 1. Wiley WCH Verlag GmbH and Co.
KGaA, Weinheim, Germany, pp. 3e40.
Khan, A.A., de Jong, W., Jausens, P.J., Spliethoff, H., 2009. Biomass
contribution in fluidized bed boilers: potential problems and
remedies. Fuel Process. Technol. 90, 21e50.
Kim, S., Dale, B.E., 2004. Life cycle assessment of integrated biorefinery cropping systems: all biomass is local. In: Presentation
within Agriculture as a Producer and Consumer of energy, Michigan State University.
Kitano, M., Tanimoto, F., Okabayashi, M., 1975. Levulinic acid, a new
chemical raw material; its chemistry and use. Chem. Econ. Eng.
Rev. 7, 25e29.
Knauf, M., Moniruzzaman, M., 2004. Lignocellulosic biomass processing a perspective. Int. Sugar J. 106, 147e150.
Kobayashi, Y., 2010. Lipase-catalyzed polyester synthesis e a green
polymer chemistry. Proc. Jpn. Acad. Ser. B 86, 338e365.
Kupiainen, L., 2012. Dilute acid catalysed hydrolysis of cellulose e
extension to formic acid. Acta Universitatis Ouluensis, C TecHNICA 438 (Ph.D. thesis). University of Oulu.
Lapas, A., Herackeous, E., 2011. Production of Biofuels via Fischer e
Tropsch Synthesis: Biomass to Liquids in Handbook of Biofuels and
Technologies. Woodhead Publishing ltd, Cambridge UK, 493e529.
Laser, M., Larson, E., Bale, B., Wang, M., Greene, N., Lynd, L.R., 2009.
Comparative analysis of efficiency, environmental impact and
process economics for mature biomass refining scenario. Biofuels,
Bioprod. Biorefin. 3, 247e270.
Laufenberg, G., Kunz, B., Nystroem, M., 2003. Transformation of
vegetable waste into value e added products: (A) the upgrading
concept, (B) practical implementations. Bioresour. Technol. 87,
167e198.
Lee, R.A., Lavore, J.M., 2013. From first to third generation biofuels:
challenges of producing a from a biomass of microarating
complexity. Anim. Front. 3, 6e11.
Lestare, D., Mudler, W., Sanders, J., 2010. Improving Jatropha curcas
seed protein recovery by using counter current multistage extraction. Biochem. Eng. J. 50, 16e23.
Liu, X.Y., Ding, H.B., Wang, J.Y., 2010. Food waste to bioenergy. In:
Khanal, S.K., Surampalli, R.Y., Zhang, T.C., Lamsal, B.P.,
Tyagi, R.D., Kao, C.M. (Eds.), Bioenergy and Biofuel from Biowastes and Biomass. ASCE, Reston, Virginia, pp. 43e70.
Lloyd, R.A., Harris, J.F., 1955. Wood Hydrolysis for Sugar Production.
United States Department of Agriculture, Forest Products Laboratory. Available from: http://ir.library.oregonstate.edu/xmlui/
bitstream/handle/1957/2671/FPL_2029_ocr.pdf?sequence¼1.
Marcus, A.I., 2005. Engineering in a Land-Grant Context: The Past,
Present, and Future of an Idea. Purdue University Press.
Martin, M., Grossmann, I.E., 2012. Optimal synthesis of sustainable
biorefineries. In: Stuart, P.R., El-Halwagi, M.M. (Eds.), Integrated
Biorefineries: Design, Analysis and Optimization. CRC Press, Boca
Raton, pp. 325e347.
McKillip, W.J., Collin, G., Hoke, H., Zeitsch, K.J., 2001. Furan and
derivatives. In: Ulmann’s Encyclopedia of Industrial Chemistry.
Wiley-VCH, Weihneim.
Meher, L.C., Vidya Sagar, D., Naik, S.N., 2006. Technical aspects of
biodiesel production by trans esterification a review. Renewable
Sustainable Energy Rev. 10, 248e268.
Melin, K., Hurne, M., 2011. Lignocellulosic biorefinery economic
evaluation. Cellul. Chem. Technol. 45, 443e454.
NREL, 2009. Biomass research e what is a Biorefinery? Available
from: www.nrel.gov/biomass/biorefinery.html.
Otulugbu, K., 2012. Production of ethanol from cellulose (sawdust).
Available from: http://publications.theseus.fi/bitstream/handle/
10024/42578/Otulugbu.pdf?sequence¼1.
Paulik, C., 2011. Chemical Technology of Organic Materials. Institute of
Organic Technology and Organic Materials, Johannes Keppler University of Linz. Available from: http://www.jku.at/cto/content/
e34502/e116152/e118705/OT2Bio-Refinery_1handout_ger.pdf.
Peiyadarsan, S., Annamalai, K., Sweeten, J., Munkhtar, S.,
Holzapple, M.T., 2004. Fixed bed gasification of feedlot manure
and poultry litter biomass. Trans. ASAE. 47, 1689e1696.
Pennington, N.L., Baker, C.W., 1990. Sugar: User’s Guide to Sucrose.
Springer, Berlin, Heidelberg, New York.
Peters, F.N., 1937. Furfural as an outlet for cellulosic waste materials.
Chem.Eng. News 15, 269e270.
PP, 2012. Biowaste Biorefinery in Europe: Opportunities and R&D
Needs, Position Paper of the Section of Environmental Biotechnology. European Federation of Biotechnology. Available from:
www.efb-central.org/images/uploads/PP_biowastebiorefinery_
26.02.2012.pdf.
Ramey D., 1998. Continuous, two stage, dual path anaerobic
fermentation of butanol and other organic solvents using two
different strains of bacteria, US Patent 5753474.
Ribeiro, N., Pinto, A., Quintella, C., da Rocha, G., Teixeira, L.,
Guariero, L.L.N., da Corno Rangel, M., Veloso, M.C.C.,
REFERENCES
Rozende, M.J.C., da Cruz, R.S., de Oliveira, A.M., Torres, E.A., de
Andrade, J.B., 2007. The role of additives for diesel and diesel blended
(ethanol or biodiesel) fuels: a review. Energy Fuels 21, 2433e2445.
RIRDC, 2006. Furfural chemicals and biofuels from Agriculture.
Wondu Business and Technology Services, RIRDC Project No
WBT-2A. Available from: https://rirdc.infoservices.com.au/
downloads/06-127.pdf.
Rodsrud, G., Lersch, M., Sjode, A., 2012. History and future of world’s
most advanced biorefinery in operation. Biomass Bioenergy 46, 46e59.
Sanders, J., Scott, E., Mooibrock, H., 2005. Biorefinery the bridge between agricultural and chemistry In: Proceeding of the Fourteenth
European Biomass Conference and Exhibition: Biomass for Energy
Industry and Climate Protection, Paris, France, 17e21 October 2005.
Schobert, H., 2013. Chemistry of Fossil Fuels and Biofuels. Cambridge
University Press, Cambridge, UK.
Sharara, M.A., Clausen, E.C., Carrier, D.J., 2012. An overview of biorefinery technology. In: Bergeron, C., Carrier, D.J., Ramswamy (Eds.),
Biorefinery Co-products: Phytochemicals, Primary Metabolites and
Value Added Biomass Processing. John Wiley and Sons, pp. 1e18.
Sindall, R.W., 1906. Paper Technology. Charles Griffin and Company
Ltd, London.
Solecki, M.,Dougherty, A., Epstein, B., 2012. Advanced Biofuel Market.
In: Report 2012 Meeting US Fuel Standards, San Francisco, California USA.
Speight, J.G., 2011. The Biofuel Handbook. Royal Scociety of Chemistry, London, UK.
Star-COLIBRI, 2011. European Biorefinery Joint Strategic Research
Roadmap, Star-COLIBRI, Strategic targets for 2020-Collaboration
Initiatives on Biorefineries. Available from: www.star-colibri.eu/
files/files/road-map-web.pdf.
Swanson, R.M., Satrio, J.A., Brown, R.C., Platon, A., Hsu, D.D., 2010.
Technico- Economic Analysis of Biofuels Production Based on
Gasification. Technical Report NREL/TP-6A20e46587. National
Renewable Energy Laboratory, Golden Colorado USA.
van der Drift, van Doorn, J., van Verneulen, J., 2001. Ten residual
biomass fuels for circulating fluidized bed gasification. Biomass
Bioenergy 20, 45e56.
van der Maarel, M.J.E.C., Van der Veen, B., Uitdehaag, J.C.M.,
Leemhuis, H., Dijkhuizen, L., 2002. Properties and applications of
starch-converting enzymes of the a -amylase family. J. Biotechnol.
94, 137e155.
van Ree, R., Annevelink, B., 2007. Status Report Biorefinery 2007
Report 847, Agrotechnology and Food Sciences Group, Wageningen, Netherlands Available from: http://edepot.wur.nl/42141.
241
Vink, E.T.H., Rabago, K.R., Glasner, D.A., Gruber, P.R., 2003. Applications of life cycle assessment to natureworks polylactide (PLA)
production. Polym. Degrad. Stabil. 80, 403e419.
Visvanathan, C., 2010. Bioenergy production from organic fraction of
municipal solid waste (OFMSW) through dry anaerobic digestion.
In: Khanal, S.K. (Ed.), Bioenergy and Biofuel from Biowastes and
Biomass. ASCE Publications, pp. 71e86.
Wagemann, K. (Ed.), 2012. Biorefineries Roadmap, as Part of the
German Federal Government Action Plans for the Material and
Energetic Utilisation of Renewable Raw Materials. Federal Ministry of Food, Agriculture and Consumer Protection (BMELV),
Berlin. Available from: http://www.bmbf.de/pub/roadmap_
biorefineries.pdf.
Watt, A., 1890. The Art of Paper-making. Crosby Lockwood and Son,
London.
WBCSD, 2000. Eco-efficiency: creating more value with less impact,
World Business Council for Sustainable Development. Available
from: http://www.wbcsd.org/web/publications/eco_efficiency_
creating_more_value.pdf.
WEF, 2010. The Future of Industrial Biorefineries. World Economic
Forum, Cologny, Geneva, Switzerland. Available from: http://
www3.weforum.org/docs/WEF_FutureIndustrialBiorefineries_
Report_2010.pdf.
Wolfrom, M.L. (Ed.), 1970. Methods in Carbohydrate Chemistry.
Academic Press.
Xiu, S., Zhang, B., Shahbazi, A., 2011. Biorefinery processes for
biomass conversion to liquid fuel. In: dos Santos, M.A. (Ed.),
Biofuels Engineering Process Technology. Intech Open Science,
pp. 167e190.
Yang, X., li, T., Weston, D., Karve, A., Labbe, J.L., Gunter, L.E.,
Sukumar, P., Borland, A., Chen, J.-G., Wullschleger, S.D.,
Tschaplinski, T.J., Tuskan, G.A., 2011. Innovative biological solutions to challenges in sustainable biofuels production. In: dos
Santos Bernardes, M.A. (Ed.), Biofuel Production e Recent Developments and Prospects. InTech, Croatia, pp. 375e414.
Yuan, Z., Chen, B., Gani, R., 2013. Applications of process synthesis:
moving from conventional processes towards biorefinery processes. Comput. Chem. Eng. 49, 217e229.
Zhang, Q., Chang, J., Wang, T., Xu, Y., 2007. Review of biomass pyrolysis oil properties and upgrading research. Energy Convers.
Manage. 48, 87e92.
Zhang, H., Xiao, R., Huang, H., Xiao, G., 2009. Comparison of noncatalytic and catalytic fast pyrolysis of corncob in a fluidized bed
reactor. Bioresour. Technol. 100, 1428e1434.
C H A P T E R
15
Catalytic Thermochemical Processes
for Biomass Conversion to Biofuels
and Chemicals
Lin Mei Wu 1, Chun Hui Zhou 1,2,*, Dong Shen Tong 1, Wei Hua Yu 1
1
Research Group for Advanced Materials & Sustainable Catalysis (AMSC), Breeding Base of State Key Laboratory
of Green Chemistry Synthesis Technology, College of Chemical Engineering and Materials Science,
Zhejiang University of Technology, Hangzhou, Zhejiang, China,
2
The Institute for Agriculture and the Environment, University of Southern Queensland, Queensland, Australia
*Corresponding author email: clay@zjut.edu.cn, Chun.Zhou@usq.edu.au
O U T L I N E
Introduction
243
Pyrolysis of Biomass
Fast Pyrolysis
Catalytic Pyrolysis
Reactors
Entrained-Fow Reactors
Ablative Reactors
Bubbling Fluid Bed Reactor and Circulating
Fluidized Beds
Rotating Cone Reactor
New Systems
244
244
244
245
245
245
Gasification of Biomass
247
245
246
247
INTRODUCTION
Thermochemical processing usually refers to the one
in which solid reactants are heated at high temperatures for a certain period to yield the desired products.
In modern times, the thermochemical processing has
often been used in industry for the production of fuels,
chemicals and materials. Today, the production of fuels,
chemicals and materials from biomass become attractive because it has renewability, one of the advantages
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00015-2
Gasification
Catalytic Gasification
247
247
Hydrothermal Liquefaction of
Biomass
Hydrothermal Liquefaction
Feedstock
Reaction Conditions
Solvent
Catalyst
248
248
249
249
250
250
Conclusion
251
References
251
over the fossil oil sources (Zhou et al., 2011; Wu et al.,
2013). In a sense, thermochemical processing of
biomass is not a new technique. Wood combustion for
heating and cooking, a method that humanity have
been using since prehistoric time can be regarded as a
thermochemical processing of biomass. However, today’s need for thermal processing of biomass is far
beyond combustion. The combination of thermal processing and catalysis is bringing about new opportunities for using biomass to produce renewable fuels,
243
Copyright Ó 2014 Elsevier B.V. All rights reserved.
244
15. CATALYTIC THERMOCHEMICAL PROCESSES FOR BIOMASS CONVERSION TO BIOFUELS AND CHEMICALS
chemicals and materials (Brown, 2011). The past three
decades have witnessed rapid progress in catalytically
thermochemical technologies (Zhou et al., 2008; Huber
et al., 2006; Fan et al., 2009). Pyrolysis, gasification and
hydrothermal liquefaction are major methods
frequently tested for the catalytically thermochemical
conversion of biomass (Zhou et al., 2011). Other thermochemical technologies could be regarded as modification, in more or less ways, of these three methods.
Relevant studies and progress have shown that these
technologies are promising alternatives to process
diverse biomass feedstocks to yield fine chemicals
and biofuels.
reaction time of only several seconds or even less (Demirbas and Arin, 2002). Among these, fast pyrolysis is of
most commercial interest for production of chemicals
and liquid fuels (Zhou et al., 2011).
Fast pyrolysis is mainly intended to maximize the biooil yield as well as to increase the contents of the target
compounds in it. To this end, there are needs to use a
finely ground particle biomass feed of typically less
than 3 mm, selective catalysts, a well-controlled pyrolysis
temperature of around 773K, short hot vapor residence
time of typically less than 2 s and rapid removal and cooling of the products (Bridgwater, 2012).
Catalytic Pyrolysis
PYROLYSIS OF BIOMASS
Fast Pyrolysis
Pyrolysis of biomass is thermal depolymerization and
decomposition of biomass (TDP) in the absence of air/
oxygen. The temperature generally used is in the range
of 623e973K (Goyal et al., 2008). The products, charcoal
or biochar, gaseous and liquid chemicals, depend on the
biomass composition, the heating rate and the temperature. According to the heating rate, pyrolysis is classified
as slow pyrolysis, fast pyrolysis and flash pyrolysis.
Slow pyrolysis of biomass is conducted at slow heating
rates (0.1e1 C/s). In the relatively low temperatures of
573e673K, charcoal is the main product; when the temperature is are increased to >673K, the oil yield is
increased (Putun et al., 2001; Ӧzbay et al., 2001; Onay
and Kockar, 2004). By contrast, fast pyrolysis is conducted
at higher heating rates (about 10e200 C/s) and intended
to produce liquid bio-oil (Bridgwater, 2003). Flash pyrolysis is conducted at heating rates >1000 C/s within
TABLE 15.1
Table 15.1 shows some results from recent studies on
the pyrolysis of lignocellulosic biomass in the presence
or absence of catalysts. Earlier, catalysts such as carbonates and hydroxides were mainly tested for the catalytic
pyrolysis of lignocellulosic biomass. The use of alkaline
compounds like NaOH, Na2CO3 and Na2SiO3 resulted
in bio-oils rich in acetol and to some extent favored H2
formation. The use of Fe2(SO4)3 as a catalyst favored
the formation of furfural and 4-methyl-2-methoxyphenol (Chen et al., 2008). Lu et al. revealed that
SO2
4 /SnO2 were an effective catalyst to yield 5-methyl
furfural (Lu et al., 2009). The selectivity varied significantly once the catalyst support was altered. For
example, SO2
4 /TiO2 catalyst favored the formation of
furfural; SO2
4 /ZrO2 catalyst favored the formation of
furan (Lu et al., 2009).
Liquid acids such as H2SO4, hydrochloric acid, phosphoric acid and solid acids such as ZSM-5, Al-MCM-41
are also used as catalysts (Table 15.1), in addition to their
uses in the pretreatment of lignocellulosic biomass (Lu
et al., 2011). The typical products of liquid acid catalytic
Typical Pyrolysis of Lignocellulosic Biomass in the Presence or Absence of Catalysts
Feedstock
Catalyst
Reactor
Fast Pyrolysis
Typical Products
References
Cellulose
No
Entrained-flow reactor
1173 K, 3 h
CO, H2, CH4,
hydrocarbons
Lanza et al., 2009
Pine Sawdust
H-ZSM-5
Conical spouted-bed
reactor
673e773 K*, 50 ms,
under N2 flow
C4-hydrocarbons
Olazar et al., 2000
Wood
Ni-ZSM-5
Tubular quartz
microreactor
873 K, under He flow
Hydrocarbons toluene
French and Czernik,
2010
Cellulose
0.1 wt% sulfuric or
polyphosphoric acid
Quartz boat
773 K, under He flow
Levoglucosane
Levoglucosenone
Dobele et al., 2005
Pine Wood
Sawdust
NaOH
Conical spouted-bed
reactor
773 K, microwave
heating
Acetol
Chen et al., 2008
Pine Wood
Sawdust
Fe2(SO4)3
Conical spouted-bed
reactor
773 K, microwave
heating
Furfural
Chen et al., 2008
Corncob
ZnCl2
Quartz reactor
613 K
Furfural
Qiang et al., 2011
* Flash pyrolysis. All others are fast pyrolysis.
PYROLYSIS OF BIOMASS
pyrolysis of biomass are levoglucosenone, furfural and
levoglucosane (0.1 wt% sulfuric or polyphosphoric
acid as catalyst) (Dobele et al., 2005; Kawamoto et al.,
2007). Taking the separation between catalysts and
liquid products and corrosive into consideration, the
catalytic pyrolysis of lignocellulosic biomass over
zeolites and many other solid catalysts have recently
received much attention. Microporous zeolite and
mesoporous materials such as ZSM-5, Al-MCM-41
have been used catalysts in catalytic pyrolysis of lignocellulosic biomass. In particular, hydrocarbons can be
produced in considerable quantities by fast pyrolysis
of biomass over these catalysts (Pattiya et al., 2008). Olazar et al. conducted the fast pyrolysis of pine sawdust
catalyzed by ZSM-5 in a spouted-bed reactor using
nitrogen as the carrier gas. A 12% yield of aromatic compounds was obtained (carbon) (Olazar et al., 2000).
French et al. revealed that over nickel, cobalt, iron, or
gallium-substituted ZSM-5 at 673e873 K and with a
catalyst-to-biomass ratio of 5e10 by weight, lignocellulosic biomass was pyrolyzed to give an approximately
16 wt% yield of hydrocarbons (French and Czernik,
2010). The solid catalysts have advantages over the
liquid acid catalysts. However, for solid catalysts at
high temperatures, the cracking and deoxygenating
activity decreased with time because of the
coke formed on them (Carlson et al., 2008).
Reactors
Several types of reactors have been designed for fast
pyrolysis of biomass, and the reactor is very crucial to
fast pyrolysis of biomass. There are entrained-flow reactors (EFRs), fluid bed reactors, rotating cone reactors,
and ablative reactors.
Entrained-Fow Reactors
In EFRs, schematically shown in Figure 15.1, biomass
particles are usually fed into the reactor in a stream of
hot, inert gas. Reaction is typically completed at
973e1073K within a residence time of a few seconds.
Dupont et al. used the mixture of two softwoods
(sylvester pine and spruce) as a model of biomass to
pyrolyze in an EFR (Dupont et al., 2008). The influence
of the particle size (0.4 and 1.1 mm), temperature
(1073e1273 K), the presence of steam in the gas atmosphere (0 and 20 vol%) and the residence time (between
0.7 and 3.5 s for gas) on conversion and selectivity were
studied. Results showed that the particle size was the
most crucial parameter that influenced decomposition
and more than 70 wt% of gas was produced.
Ablative Reactors
An ablative pyrolysis reactor is considered as a
possible alternative to an EFR. The surface is heated by
245
FIGURE 15.1 Pressurized high temperature entrained flow
reactor (PiTER). Source: Tremel et al., 2012; Elsevier. (For color
version of this figure, the reader is referred to the online version of this
book.)
hot flue gas produced by combustion of pyrolysis gases
or char and rotates while biomass is pressed onto the hot
surface (873K).
However, in general, an ablative pyrolysis reactor
has difficulty in getting sufficient heat transfer from
hot gases to the ablative surface and in contacting feedstock of diverse morphologies (particle shape, structure, and density) with the ablative surface. In
practice, relatively few feedstocks would be suitable
for ablative pyrolysis.
Bubbling Fluid Bed Reactor and Circulating
Fluidized Beds
A fluid bed reactor is very suitable for fast pyrolysis, as the biomass is rapidly heated and there are
high heat and mass transfer rates between gas, particles and catalysts and any other objects in the reactor.
Vapor and solid residence time are controlled by the
fluidizing gas flow rate. Bubbling fluidized beds
(Figure 15.2(a)) are usually referred to simply as fluidized beds, which provide good temperature control
and very efficient heat transfer to biomass particles
due to high density of solids in the bed. Jung et al.
pyrolyzed rice straw and bamboo sawdust in a
bubbling fluidized bed equipped with a char separation system (Jung et al., 2008). They found that the
maximum bio-oil yield was above 70 wt% and a higher
feed rate and a smaller feed size were more favorable
to the production of bio-oil.
246
FIGURE 15.2
15. CATALYTIC THERMOCHEMICAL PROCESSES FOR BIOMASS CONVERSION TO BIOFUELS AND CHEMICALS
(a) Bubbling fluid bed reactor and (b) circulating fluid bed reactor. Source: Bridgwater, 2012; Elsevier.
While circulating fluid bed reactors have similar
features to bubbling fluid bed reactors. A main difference between them is the amount of gas used to fluidize the bed. In the circulating fluid bed reactor
(Figure 15.2(b)), the gas flow is intentionally set high
enough to transport particles out of the bed, which are
recovered by gas cyclones and then returned to the
fluidized bed. All char is burned in the secondary
reactor to reheat the circulating sand or is separated as
a fine powder.
The circulating fluidized bed can be divided into
two zones: pyrolysis zone; and reduction and cracking
zone (Wu et al., 1992). In the pyrolysis zone, biomass is
loaded into the bed and pyrolyzed very quickly to
form char, tar, H2O, and gas (CO2, CO, CH4, CnHm
and H2). In the reduction and cracking zone, pyrolysis
char of contributes to secondary cracking in the vapor
phase. For example, with the circulating fluidized bed
as reactor, Dai et al. pyrolyzed wood powder at 773K
(Dai et al., 2000). The main effects were: (1) the higher
temperature and longer residence time contributed to
the secondary reactions and then lead to less liquids;
(2) the lower heating rate favored the carbonization
and reduced the liquid production; and (3) most compounds in bio-oil were nonhydrocarbons and alkanes,
aromatics, and asphalt were relatively less.
Rotating Cone Reactor
Rotating cone reactor is designed to achieve the
intense mixing and heat transfer between biomass
and heat carrier without the use of a large amount of
fluidizing gas (Figure 15.3). Gas is needed for char
burn-off in a secondary bubbling fluid bed combustor
and sand transport recirculated to the pyrolyzer. Flash
FIGURE 15.3 Rotating cone pyrolysis
reactor and integrated process. Source:
Bridgwater, 2012; Elsevier.
247
GASIFICATION OF BIOMASS
pyrolysis of wood dust was processed in a rotating cone
reactor by Wagenaar et al. the cone geometry was specified by a top angle of p/2 radians and a maximum
diameter of 650 mm (Wagenaar et al., 1994). The
rotating cone reactor model included the description
of the particle flow behavior, the particle conversion
and the gas-phase cracking of tar vapors. It appeared
that the product distribution was affected by the gasphase reaction kinetics and residence time and the
gas-phase residence time was determined by the available reactor volume and the feed rate of the wood
particles.
New Systems
Recently, emerging technology is to couple a pyrolysis reactor with other catalytic reactors such as steam
reformer and hydrogenation. For example, the technology of hydroprocessing is intended to convert bio-oil
to petroleum-refinery compatible feedstock (Elliott
et al., 2012). The combination can also be used to build
a microscale pyrolysis reactor coupled to the
molecular-beam mass-spectrometer (Bahng et al.,
2009). It can be used as a very efficient tool for studying
mechanisms of thermal and catalytic processes and to
optimize process conditions for different products
from a variety of feedstocks.
GASIFICATION OF BIOMASS
Gasification
Gasification of biomass is to convert it into useful
gases such as carbon monoxide, hydrogen and light
hydrocarbons (Brown, 2003). Since the mid-1980s, interest has grown on the subject of catalysis for biomass
gasification. The advances in this area have been driven
by the need for producing tar-free gases from biomass.
The avoidance of tars and the yield of hydorgen are
deciding factors the economic viability of the biomass
TABLE 15.2
gasification process. Major reactions in gasification are
as follows (Brown, 2003).
1
carboneoxygen reactionk C þ O2 4 CO
2
Boudouard reaction
C þ CO2 4 2CO
carbonewater reaction
C þ H2 O 4 H2 þ CO
hydrogenation reaction
C þ 2H2 4 CH4
wateregas shift reaction
CO þ H2 O 4 H2 þ CO2
methanation
CO þ 3H2 4 CH4 þ H2 O
The desired product from gasification of biomass is
hydrogen or syngas. Syngas can be burned directly in
gas engines, be used to produce methanol, or be converted into synthetic fuels via the FischereTropsch
process. Though gases are target products, gasification
of biomass leaves behind solid residuals such carbon
and inorganic compounds (ash).
Gasification of biomass is normally performed in the
presence of steam and the process depends on the occurrence of the steam-reforming reactions. Water, in the form
of steam, is often added to promote additional production of hydrogen via the wateregas shift reaction. As
the biomass is heated, moisture contained in the biomass
is converted to steam, which can react with biomass.
However, in practice, proper drying of biomass before
feeding it into gasification equipment is still needed in
view of energy-input.
Small amounts of oxygen can also be added to the gas
feed. The heat from exothermic oxidation reactions can
then be used by the endothermic steam-reforming reaction. In addition, oxygen has a function to delay the catalyst deactivation by helping burn off some of the coke
formed.
Catalytic Gasification
Table 15.2 lists some typical results from the gasification of lignocellulosic biomass in the presence of catalysts.
Typical Gasification of Lignocellulosic Biomass in the Presence of Catalysts
Feedstock
Catalyst
Gasification
Typical Products
References
Cellulose
Pt/Al2O3
623 K, 10 min, 30 MPa (N2)
H2 and CH4
Usui et al., 2000
Sawdust
Ru/C
773 K, 20 min, 27 MPa (N2)
H2
Hao et al., 2005
Cellulose
SnO2, ZnO
573 K, 1 h, 8 Mpa
CO, CO2 and H2
Sinag et al., 2011
CH4 and CO2
Nieolivine
1073 K, 80 h
Rich H2 gas
Courson et al., 2002
Cellulose
Rh/CeO2/SiO2
823e923 K, 0.1 MPa
Syngas
Tomishige et al., 2004
Glucose
NaOH
723 K, 34 MPa
H2
Onwudili and Williams, 2009
Sawdust
Fe/CaO
933 K
H2
Huang et al., 2012
248
15. CATALYTIC THERMOCHEMICAL PROCESSES FOR BIOMASS CONVERSION TO BIOFUELS AND CHEMICALS
Biomass gasification is inevitably accompanied with tar
formation. Nevertheless, tar can be effectively minimized
by catalytic cracking. Naturally occurring dolomite
(CaMg(CO3)2), for example, has been used as a catalyst
for gasification of biomass in a fluid bed reactor to reduce
the tar content by transforming it to gases (Delgado and
Aznar, 1997). The mineral-based catalyst generally contains CaO, MgO, CO2 and trace minerals such as SiO2,
Fe2O3 and Al2O3. The tar cracking efficiency over the
dolomites depends on their chemical composition. In
general, dolomites with the lowest content of CaO and
MgO show the lowest tar cracking efficiency. Yu et al.
gasified birch on the four types of dolomites (deposites
in Zhenjiang, Nanjing, Shanxi, and Anhui, China) and
a Swedish dolomite (Sala) (Yu et al., 2009). The result
was that Anhui dolomite showed a low catalytic capacity
to crack tar at 973 and 1073K due to its lowest content of
CaO and MgO among the tested dolomites. An alternative can be naturally occurring particles of olivine, which
are a mineral containing magnesium oxide, iron oxide
and silica. Regarding their attrition resistance, Olivine
is advantageous over dolomite (Devi et al., 2005).
Alkali salts are often added to biomass by dry mixing
or wet impregnation and used as catalysts for the elimination of tar and upgrading of the product gas (Li et al.,
1996; Encinar et al., 1998). But it has considerable difficulty
in catalyst recovery and disposal of ash. Carbonates,
oxides and hydroxides of alkali metals can effectively catalyze the decomposition of tar during catalytic gasification
(McKee, 1983). Earlier, for example, Mudge et al. investigated the catalytic steam gasification of wood using alkali
carbonates and naturally occurring minerals (trona,
borax), which were either impregnated or mixed with
the biomass (Mudge and Baker, 1985). The order of
activity reported was potassium > carbonate > sodium
carbonate > trona > borax.
The Ni-based catalysts for biomass gasification in a
fluid bed reactor are typically Ni-Al based one (Garcı́a
et al., 2002; Arauzo et al., 1997) and Ni/olivine one
(Courson et al., 2002, 2000). Ni catalysts help to remove
tars and methane and to adjust the composition of synthesis gas. Sinag et al. studied the effect of nano-sized
and bulky ZnO and SnO2 at 573 K on the wateregas
shift reaction in gasification of cellulose. The results
showed that the wateregas shift reaction proceeded
faster over ZnO catalysts than that over SnO2 catalysts.
Therefore, a higher yield of hydrogen was obtained in
the presence of ZnO (Sinag et al., 2011).
However, catalysts often suffer from deactivation by
sintering and/or coke deposition. The use of supercritical water can prevent catalyst from deactivation by
means of extracting the coke precursor from the catalyst
surface (Baiker, 1999). In addition, it can improve solubility of cellulosic materials and thus reduce mass-transfer
limitation. It is also worth noting that, in addition to the
active component in a catalyst, usually the acidity and
basicity of a support is also an influential factor on
product distribution and coke formation. Tasaka and
coworkers disclosed that steam reforming of tar derived
from cellulose gasification was efficiently catalyzed by
12 wt% Co/MgO catalyst at 873 K in a fluidized bed
reactor (Tasaka et al., 2007).
Supported Ru, Pt or Pd catalysts also appear promising in the catalytic gasification of lignocellulosic
biomass. They were able to overcome the shortcomings
of Ni-based catalysts and dolomite catalysts, although
they are relatively costly. Usui et al. gasified cellulose
in hot-compressed water at 623 K in the presence of
a series of supported catalysts such as Zr(OH)4,
(CH3COCH]C(Oe)CH3)3Fe, ferrocene, Ru3(CO)12,
(CH3COCH]C(Oe)CH3)2Co, NiC2O4, NiO, Ni(OH)2,
PdI2 and Cu(OH)2. After reaction for 3 h, 5 wt% Pd supported on Al2O3 showed the highest catalytic activity,
leading to a 42.3 vol% yield of H2 and a 7.7 vol% yield
of CH4 (Usui et al., 2000). Tomishige et al. found that
the order of M/CeO2/SiO2 catalyst activity in the cedar
wood gasification at 823 K was the following:
Rh > Pd > Pt > Ni]Ru (Tomishige et al., 2004). For Rh/
CeO2/M-type (M]SiO2, Al2O3, and ZrO2) catalysts for
cellulose gasification in a continuous-feeding fluidizedbed reactor, Asadullah et al. found that Rh/CeO2/SiO2
exhibited the best performance in terms of generating
syngas or hydrogen (Asadullah et al., 2001, 2003).
HYDROTHERMAL LIQUEFACTION
OF BIOMASS
Hydrothermal Liquefaction
Hydrothermal liquefaction of biomass makes biomass react at high-temperature aqueous solutions under
high vapor pressures. In the field of geochemistry and
mineralogy, this method also was used for getting insights into the solubility of minerals in hot water
under high pressure (Zhang et al., 2010; Wu et al.,
2012; Tong et al., 2013). Hydrothermal liquefaction involves thermal depolymerization in an aqueous or
organic medium. In this context, it might be called a
depolymerization process using hydrous pyrolysis
for decomsition of complex organic materials (for
example, here biomass) into light crude oil. In this
way, it is expected that under pressure and upon
heat, long-chain lignocellulosic polymers decompose
into short-chain petroleum-like hydrocarbons and
chemicals. In this aspect, pyrolysis technologies are
best suited for the conversion of dry feedstocks
(<5% moisture), while hydrothermal liquefaction
of biomass is ideal for processing high-moisture
(i.e. wet) biomass.
HYDROTHERMAL LIQUEFACTION OF BIOMASS
Direct hydrothermal liquefaction involves converting biomass to an oily liquid under elevated pressures
(50e200 atm) and at low temperatures (473e673K) to
keep water in either liquid or supercritical state. In a
hot pressurized water for sufficient time, hydrothermal treatment of biomass breaks down the solid
biopolymeric structure to liquid components and
even gases. Usually, the liquid product from the
hydrothermal liquefaction of lignocellulosic biomass
is a complicated mixture with a wide range of compositions. It typically consists of glycoaldehyde dimers,
1,3-dihydroxyacetone dimers, anhydroglucose, soluble
polyols, 5-hydroxy-methylfurfural (HMF), furfural,
organic acids, phenolic compounds and even
hydrocarbons.
Feedstock
Hydrothermal liquefaction has been applied to a
wide range of biomass. One of the advantages of
hydrothermal processing is the use of high-moisture
biomass without the need for preliminary drying of
the biomass. The feedstocks can be cellulose, hemicellulose, lignin, aquatic biomass such as duckweed,
microalgae, microalgae, wastes animal manure and
human sewage. In general, the presence of high cellulose and hemicelluloses content in biomass yields
more bio-oil (Akhtar and Amin, 2011). For example,
hardwood samples (cherry) produced more oils than
softwood (cypress) due to the high lignin contents in
the latter biomass (Bhaskar et al., 2008). Besides the
oil yield, the oil composition is also different when
different feedstocks are used. Karagöz et al. made
analysis of oil compositions obtained from hydrothermal treatment of sawdust, rice husk, lignin and cellulose at 553K for 15 min (Karagöz et al., 2005b). The
conclusion was that the oil from the cellulose mainly
consisted of furan derivatives, whereas ligninderived oil mainly contained phenolic compounds.
The compositions of oils from sawdust and rice husk
contained both phenolic compounds and furans; however, phenolic compounds were dominant. But rice
husk-derived oil consists of more benzenediols than
sawdust-derived oil.
In addition, hydrothermal liquefaction of algae
biomass has also received much attention. The advantage of microalgae compared to terrestrial biomass is
its much higher photosynthetic efficiency, which results
in higher growth rates and improved CO2 mitigation
(Brennan and Owende, 2009). Studies on the hydrothermal processing of microalgae indicated that 30e60% of
the algal biomass can be converted to bio-oils (Tsukahara and Sawayama, 2005; Patil et al., 2008). With
different biochemical content of pristine microalgae,
the oil from the hydrothermal liquefaction of microalgae
249
is different. Biller and Ross liquefied microalgae and
cyanobacteria with different biochemical contents
(lipids, proteins and carbohydrates) under hydrothermal conditions at 623K, w200 bar in water (Biller and
Ross, 2011). The results indicated that bio-oil formation
followed the trend: lipids > proteins > carbohydrates,
and proteins produced large amounts of nitrogen heterocycles, pyrroles and indoles; carbohydrates produced cyclic ketones as well as phenols while lipids
were converted to fatty acids.
Reaction Conditions
Hydrothermal liquefaction of biomass need be
accomplished with careful choices of time, temperature,
pressure, catalyst and the use of reducing gases.
Increasing temperature in a certain range is favorable.
Temperature control is important because after reaching a maximum of the oil yield, further increase in temperature actually inhibits biomass liquefaction due to
the secondary decomposition, Bourdard gas reactions
and char formation (Mok and Antal, 1992; El-Rub
et al., 2004; Zhong and Wei, 2004). The choice of temperature also depends on the biomass types. Rogalinski
et al. carried out a kinetic study on hydrolysis of
different biopolymers (Rogalinski et al., 2008). It was
observed that cellulose hydrolysis rate in water at
25 MPa increased 10-fold between 513 and 583K and
at 553K, a 100% of cellulose conversion was achieved
within 2 min. Lignin showed a higher hydrothermal liquefaction temperature than hemicellulose and
cellulose. Zhang and Wei found that the optimal temperature of wood hydrothermal liquefaction shifted to
a higher value as the lignin content increased (Zhong
and Wei, 2004).
Pressure increases the density of solvent to facilitate
solvent penetration into molecules of biomass components, which results in enhanced decomposition and
extraction (Deshande et al., 1987). According to Le
Chatelier’s principle, one would expect that the higher
the pressure during liquefaction, the less liquid components are gasified. By maintaining pressure above
the critical pressure of medium, the rate of hydrolysis
and biomass dissolution can be controlled. This can be
used to enhance favorable reaction pathways thermodynamically for the production of liquid fuels. However, once supercritical conditions for liquefaction are
used, pressure has little or negligible influence on
the yield of liquid oil or gas yield because in the supercritical region influence of pressure on the properties
of water or solvent medium becomes very weak small
(Kersten et al., 2006; Sangon et al., 2006).
Reaction atmosphere also need to be considered. The
use of reductive gases (e.g. CO and H2) generally improves oil yields with higher H/C ratios (He et al.,
250
15. CATALYTIC THERMOCHEMICAL PROCESSES FOR BIOMASS CONVERSION TO BIOFUELS AND CHEMICALS
2001). The reducing gases stabilize the products of liquefaction by inhibiting the condensation, cyclization, or
repolymerization of free radicals. Hence, they help
reduce char formation (Xu and Etchevery, 2008). By
using H2 instead of Ar atmosphere for liquefaction,
Wang et al. found that both the conversion of sawdust
and the oil yield were able to be increased (Wang
et al., 2007a). Besides the oil yield, the quality of gaseous
product is also improved by using H2; For example, CO
and C1eC4 products increased and CO2 decreased.
Solvent
Water is the most common medium used for hydrothermal liquefaction of biomass. The bio-oil obtained
from hydrothermal liquefaction of lignocellulose in
water is usually a viscous tarry lump with a high oxygen content and low heat. To make bio-oils with low
viscosity and high yield, the use of organic solvents
is an alternative. The tested ones include ethyl acetate
(Demirbas, 2000), acetone (Liu and Zhang, 2008),
methanol, ethanol, propanol, butanol, propylene glycol, ethylene glycol, diethylene glycol and so forth
(Mun and Hassan, 2004; KrZan
et al., 2005).
Liquefaction of biomass with proper solvents is a process that can be integrated with optimized conditions to
produce fuel and valuable chemicals. Liu et al. liquefied
pinewood in the presence of various solvents (water,
acetone and ethanol) in the conditions of temperature
range 523e723 K, starting pressure 1 MPa, reaction
time 20 min (Demirbas, 2000). The results showed that
the highest oil yield reached 26.5% at 473 K in ethanol
and the product distribution was strongly affected by
the solvent type. The major compound was 2-methoxyphenol (17.20%) for liquefaction in water, while it was
2-methoxy-4-methyl-phenol (8.23%) for liquefaction
in ethanol and 4-methyl-1,2-benzenediol (9.49%) for
liquefaction in acetone. Recently, it was found that
co-solvents are a much more effective than the constituent monosolvents alone due to the synergistic effects of
different solvents. For example, biomass conversion in
TABLE 15.3
100% ethanol and 100% methanol at 573K is 43% and
42%, respectively, producing a bio-oil yield at approximately 26 and 23 wt%, while the liquefaction in the
mixed 50 wt% methanol-water solution or the 50 wt%
ethanol-water solution led to a conversion of biomass
>95 wt% and a bio-oil yield of as high as 65 wt% at
573K (Cheng et al., 2010).
The use of donor hydrogen solvents is a new option
to hydrogenate the biomass fragments. These solvents
not only donate hydrogen but also act as hydrogen
transport vehicle and it was found that the use of tetralin solvent enhanced liquid oil yield by suppressing the
formation of asphaltenes, preasphaltenes and gases
compared to toluene solvent (nonhydrogen donor)
(Wang et al., 2007b). For example, Wang et al. observed
that in the presence of solvent the yield of oil increased
to 33.1% in toluene (nonhydrogen donor) and 48.4% in
tetralin (Wang et al., 2007c). Besides, tetrahydrophenanthrene, octahydrophenanthrene, hexahydropyrene,
hexahydrofluorene, and tetrahydroacenaphthene are
also useful solvents for hydrogenation (Akhtar and
Amin, 2011).
Catalyst
Table 15.3 summarizes some results of catalytically
hydrothermal liquefaction of lignocellulosic biomass.
Hydrothermal liquefaction of biomass was significantly affected by catalyst. Lignocellulosic biomass
mainly contains cellulosic polymer and lignin polymer. The former readily interacts with acid; the latter
readily interacts with alkali. In the presence of alkaline
catalysts, liquefaction of lignocellulosic biomass
mainly leads to oil-like products (Meszaros et al.,
2004; Knill and Kennedy, 2003). The conversion and
yield of liquid products decreases in the following
order: K2CO3 > KOH > Na2CO3 > NaOH (Karagöz
et al., 2006; Akhtar et al., 2010).
Typically, the equipment corrosion by caustic hydroxides is severely enhanced under subcritical and
Catalytically Hydrothermal Liquefaction of Lignocellulosic Biomass
Catalyst
Feedstock
Reaction Conditions
Main Products
References
Ba(OH)2 or Rb2CO3
Lignin
1.5e8.6 MPa, 573 K, 1 h
Phenolic compounds
Tymchyshyn and Xu, 2010
Na2CO3
Woody biomass
653 K, 16e20 min, 8 MPa (H2)
Heavy oil
Qian et al., 2007
Ca(OH)2
Sawdust
553 K, 15 min
Oil
Karagöz et al., 2004a
CoSO4
Cellulose
573 K, 120 s
Lactic acid
Kong et al., 2008
K2CO3 þ ZrO2
Waste biomass
673 K, 10 min, 22.1 MPa
Oil
Hammerschmidt et al., 2011
FeSO4
Jack pine powder
623 K, 40 min, 5 MPa (H2)
Bio-oil
Xu and Etchevery, 2008
CrCl3
Cellulose
473 K, 3 h
Levulinic acid
Peng et al., 2010
REFERENCES
supercritical water conditions. Therefore, in this aspect,
alkali and alkaline earth carbonate salts are thought to
be optional catalysts. Karagöz et al. found that the alkali and alkaline salts enhanced bio-oil formation
from wood hydrothermal processing and the catalytic
activity of these catalysts shown a sequence of
K2CO3 > KOH > Na2CO3 > NaOH > RbOH > CsCO3
> RbCO3 > CsOH based on heavy oil yield (Karagöz
et al., 2004b, 2005a, 2005c). Jena et al. investigated the
thermochemical liquefaction of the microalga Spirulina
platensis over an alkali metal salt catalyst (Na2CO3), an
alkaline earth metal salt (Ca3(PO4)2), and a transition
metal oxide (NiO) and without a catalyst (Jena et al.,
2012). Results showed that Na2CO3 was found to increase biocrude oil yield, resulting in 51.6% biocrude
oil, which was w29.2% higher than that under noncatalytic conditions and w71% and w50% higher than
those when NiO and Ca3(PO4)2 were used as catalysts,
respectively.
Hydrothermal processing of biomass can also be carried out over halide catalysts. Lewis acid catalysts could
exhibit good catalytic properties in hydrothermal liquefaction of lignocellulosic biomass while catalytic hydrolysis is frequently conducted in the presence of Brønsted
acid catalysts. Transition metal chlorides such as CrCl3,
FeCl3, CuCl2 and AlCl3 (Zhang and Zhao, 2010; Li
et al., 2009), including a pair of these metal chlorides
(for example CuCl2 and CrCl2) (Su et al., 2009), exhibited
high catalytic activity.
In addition sulfates can also be used as catalysts for
the catalytic liquefaction of lignocellulosic biomass.
Kong et al. revealed, for example, that lactic acid can
be produced from the catalytic hydrothermal liquefaction of lignocellulosic biomass in the presence of
different transition metal ions like ZnSO4, NiSO4,
CoSO4 or Cr2(SO4)3 (Kong et al., 2008).
Recently, natural minerals are used as catalysts in
the hydrothermal liquefaction of biomass. Tekin et al.
reported the effects of a natural calcium borate mineral, colemanite, on the hydrothermal liquefaction of
beech wood biomass (Tekin et al., 2012). The highest
light bio-oil yield (11.1 wt%) and the highest heavy
bio-oil yield (29.8 wt%) were obtained at 573K over
colemanite catalysts. The total bio-oil yields were
about 22 and 41 wt% at 573K without and with colemanite, respectively.
CONCLUSION
Catalytically thermochemical technologies allowed
the possibilities to convert biomass into fuels and chemicals. The parameters such as temperature, pressure,
feedstock, catalysts, and medium have been extensively
studied. In the process of catalytic hydrothermal
251
gasification, catalysts can be naturally occurring minerals (dolomite and olivine); alkali metal catalysts; Ni,
Fe, Co, and Cu-based catalysts and supported noble
metal catalysts (Rh, Pd, Pt and Ru). Biomass gasification
has been profiled as being CO2-neutral, having a potential to produce hydrogen and syngas.
For pyrolysis, charcoal, gas and liquid are always produced simultaneously. However, by adjusting process
parameters (high heating rates and very high heat transfer rates, controlled pyrolysis reaction temperature at
around 773K, short hot vapor residence time, rapid
removal of product char and cooling of the pyrolysis vapors), maximizing bio-oil yield could be achieved. Fast
pyrolysis has now achieved a nearly commercial success
and is being actively developed for producing liquid
fuels. Catalytic pyrolysis of biomass could increase the
content of the target compounds in the mixture products. Besides, the catalytic pyrolysis of lignocellulosic
biomass over zeolites, along with integrated hydroprocesses, offer a new potential way to produce hydrocrabon fuels from biomass.
Catalytically hydrothermal liquefaction of lignocellulosic biomass produces a very complex mixture of
liquid products (typically consists of glycoaldehyde
dimers, 1,3-dihydroxyacetone dimers, anhydroglucose,
soluble polyols, 5-HMF, furfural, organic acids, phenolic compounds and even hydrocarbons). Therefore,
the novel technology for separation and extraction of
downstream products from hydrothermal liquefaction
of lignocellulosic biomass need to be developed (Miller
et al., 1999).
Acknowledgments
The authors wish to acknowledge the financial support from the
National Natural Scientific Foundation of China (21373185), the
Distinguished Young Scholar Grants from the Natural Scientific
Foundation of Zhejiang Province (ZJNSF, R4100436), ZJNSF
(LQ12B03004), Zhejiang “151 Talents Project”, and the projects
(2010C14013 and 2009R50020-12) from Science and Technology
Department of Zhejiang Provincial Government and the financial
support by the open fund from breeding base of state key laboratory
of green chemistry and synthesis technology.
References
Akhtar, J., Amin, N.A.S., 2011. A review on process conditions for
optimum bio-oil yield in hydrothermal liquefaction of biomass.
Renewable Sustainable Energy Rev. 15, 471e481.
Akhtar, J., Kuang, S.K., Amin, N.S., 2010. Liquefaction of empty palm
fruit bunch (EPFB) in alkaline hot compressed water. Renewable
Energy 35, 1220e1227.
Arauzo, J., Radlein, D., Piskorz, J., Scott, D.S., 1997. Catalytic pyrogasification of biomass. Evaluation of modified nickel catalysts.
Ind. Eng. Chem. Res. 36, 67e75.
Asadullah, M., Tomishige, K., Fujimoto, K., 2001. A novel catalytic process
for cellulose gasification to synthesis gas. Catal. Commun. 2, 63e68.
252
15. CATALYTIC THERMOCHEMICAL PROCESSES FOR BIOMASS CONVERSION TO BIOFUELS AND CHEMICALS
Asadullah, M., Tomishige, K., Fujimoto, K., 2003. Catalyst performance of Rh/CeO2/SiO2 in the pyrogasification of biomass.
Energy Fuels 17, 842e849.
Bahng, M.K., Mukarakate, C., Robichaud, D.J., Nimlos, M.R., 2009.
Current technologies for analysis of biomass thermochemical
processing: a review. Anal. Chim. Acta 651, 117e138.
Baiker, A., 1999. Supercritical fluids in heterogeneous catalysis. Chem.
Rev. 99, 453e473.
Bhaskar, T., Sera, A., Muto, A., Sakata, Y., 2008. Hydrothermal
upgrading of wood biomass: influence of the addition of K2CO3
and cellulose/lignin ratio. Fuel 87, 2236e2242.
Biller, P., Ross, A.B., 2011. Potential yields and properties of oil from
the hydrothermal liquefaction of microalgae with different
biochemical content. Bioresour. Technol. 102, 215e225.
Brennan, L., Owende, P., 2009. Biofuels from microalgae e a review of
technologies for production, processing, and extractions of biofuels
and co-products. Renewable Sustainable Energy Rev. 14, 557e577.
Bridgwater, A.V., 2003. Renewable fuels and chemicals by thermal
processing of biomass. Chem. Eng. J. 91, 87e102.
Bridgwater, A.V., 2012. Review of fast pyrolysis of biomass and
product upgrading. Biomass Bioenergy 38, 68e94.
Brown, R.C., 2003. Biorenewable Resources: Engineering New Products from Agriculture. Blackwell Publishing, Ames, IA.
Brown, R.C., 2011. Introduction to thermochemical processing of
biomass into fuels, chemicals, and power. In: Brown, R.C. (Ed.),
Thermochemical Processing of Biomass: Conversion into Fuels,
Chemicals and Power. John Wiley & Sons, Ltd., England, pp. 1e10.
Carlson, T.R., Vispute, T.P., Huber, G.W., 2008. Green gasoline by
catalytic fast pyrolysis of solid biomass derived compounds.
Chem. Sus. Chem. 1, 397e400.
Chen, M.Q., Wang, J., Zhang, M.X., Chen, M.G., Zhu, X.F., Min, F.F.,
Tan, Z.C., 2008. Catalytic effects of eight inorganic additives on
pyrolysis of pine wood sawdust by microwave heating. J. Anal.
Appl. Pyrolysis 82, 145e150.
Cheng, S., D’cruz, I., Wang, M., Leitch, M., Xu, C., 2010. Highly efficient liquefaction of woody biomass in hot-compressed alcoholwater co-solvents. Energy Fuels 24, 4659e4667.
Courson, C., Makaga, E., Petit, C., Kiennemann, A., 2000. Development of Ni catalysts for gas production from biomass gasification.
Reactivity in steam- and dry-reforming. Catal. Today 63, 427e437.
Courson, C., Udron, L., Swierczy
nski, D., Petit, C., Kiennemann, A.,
2002. Hydrogen production from biomass gasification on nickel
catalysts: tests for dry reforming of methane. Catal. Today 76,
75e86.
Dai, X.W., Wu, C.Z., Li, H.B., Chen, Y., 2000. The fast pyrolysis of
biomass in CFB reactor. Energy Fuels 14, 552e557.
Delgado, J., Aznar, M.P., 1997. Biomass gasification with steam in
fluidized bed: effectiveness of CaO, MgO, and CaOMgO for hot
raw gas cleaning. Ind. Eng. Chem. Res. 36, 1535e1543.
Demirbas, A., Arin, G., 2002. An overview of biomass pyrolysis.
Energy Sources 24, 471e482.
Demirbas, A., 2000. Effect of lignin content on aqueous liquefaction
products of biomass. Energy Convers. Manage. 41, 1601e1607.
Deshande, G.V., Holder, G.D., Shah, Y.T., 1987. Effect of solvent density on coal liquefaction under supercritical conditions. Available
from: <http://web.anl.gov/PCS/acsfuel/preprint%20archive/Files/
30_3_CHICAGO_09-85_0112.pdf> (accessed 14.01.13.).
Devi, L., Ptasinski, K.J., Janssen, F.J.J.G., van Paasen, S.V.B.,
Bergman, P.C.A., Kiel, J.H.A., 2005. Catalytic decomposition of
biomass tars: use of dolomite and untreated olivine. Renewable
Energy 30, 565e587.
Dobele, G., Rossinskaja, G., Diazbite, T., Telysheva, G., Meier, D.,
Faix, O., 2005. Application of catalysts for obtaining
1,6-anhydrosaccharides from cellulose and wood by fast pyrolysis.
J. Anal. Appl. Pyrolysis 74, 401e405.
Dupont, C., Commandre, J.M., Gauthier, P., Boissonnet, G.,
Salvador, S., Schweich, D., 2008. Biomass pyrolysis experiments in
an analytical entrained flow reactor between 1073 K and 1273 K.
Fuel 87, 1155e1164.
Elliott, D.C., Hart, T.R., Neuenschwander, G.G., Rotness, L.J.,
Olarte, M.V., Zacher, A.H., Solantausta, Y., 2012. Catalytic hydroprocessing of fast pyrolysis bio-oil from pine sawdust. Energy Fuel
26, 3891e3896.
El-Rub, A.Z., Bramer, E.A., Brem, G., 2004. Review of catalysts for tar
elimination in biomass gasification processes. Ind. Eng. Chem. Res.
43, 6911e6919.
Encinar, J.M., Beltran, F.J., Ramiro, A., González, J.F., 1998. Pyrolysis/
gasification of agricultural residues by carbon dioxide in the
presence of different additives: influence of variables. Fuel Process.
Technol. 55, 219e233.
Fan, Y.X., Zhou, C.H., Zhu, X.H., 2009. Selective catalysis of lactic acid
to produce commodity chemicals. Catal. Rev. 51, 293e324.
French, R., Czernik, S., 2010. Catalytic pyrolysis of biomass for biofuels production. Fuel Process. Technol. 91, 25e32.
Garcı́a, L., Benedicto, A., Romeo, E., Salvador, M.L., Arauzo, J.,
Bilbao, R., 2002. Hydrogen production by steam gasification of
biomass using NieAl coprecipitated catalysts promoted with
magnesium. Energy Fuel 16, 1222e1230.
Goyal, H.B., Seal, D., Saxena, R.C., 2008. Bio-fuels from thermochemical conversion of renewable resources: a review. Renewable
Sustainable Energy Rev. 12, 504e517.
Hammerschmidt, A., Boukis, N., Hauer, E., Galla, U., Dinjus, E.,
Hitzmann, B., Larsen, T., Nygaard, S.D., 2011. Catalytic conversion
of waste biomass by hydrothermal treatment. Fuel 9, 555e562.
Hao, X.H., Guo, L.J., Zhang, X.M., Guan, Y., 2005. Hydrogen production from catalytic gasification of cellulose in supercritical
water. Chem. Eng. J. 110, 57e65.
He, J.B., Zhang, Y., Yin, Y., Funk, T.L., Riskowski, G.L., 2001. Effects of
feedstock pH, initial CO addition, and total solids content on the
thermochemical conversion process of swine manure. Trans. ASAE
44, 697e701.
Huang, B.S., Chen, H.Y., Chuang, K.H., Yang, R.X., Yang, R.X.,
Wey, M.Y., 2012. Hydrogen production by biomass gasification in a
fluidized-bed reactor promoted by an Fe/CaO catalyst. Int. J.
Hydrogen Energy 37, 6511e6518.
Huber, G.W., Iborra, S., Corma, A., 2006. Synthesis of transportation
fuels from biomass: chemistry, catalysts, and engineering. Chem.
Rev. 106, 4044e4098.
Jena, U., Das, K.C., Kastner, J.R., 2012. Comparison of the effects of
Na2CO3, Ca3(PO4)2, and NiO catalysts on the thermochemical
liquefaction of microalga Spirulina platensis. Appl. Energy 98,
368e375.
Jung, S.H., Kang, B.S., Kim, J.S., 2008. Production of bio-oil from
rice straw and bamboo sawdust under various reaction conditions in a fast pyrolysis plant equipped with a fluidized bed
and a char separation system. J. Anal. Appl. Pyrolysis 82,
240e247.
Karagöz, S., Bhaskar, T., Muto, A., Sakata, Y., Uddin, M.A., 2004a.
Low-temperature hydrothermal treatment of biomass: effect of
reaction parameters on products and boiling point distributions.
Energy Fuels 18, 234e241.
Karagöz, S., Bhaskar, T., Muto, A., Sakata, Y., 2004b. Effect of Rb and
Cs carbonates for production of phenols from liquefaction of wood
biomass. Fuel 83, 2293e2299.
Karagöz, S., Bhaskar, T., Muto, A., Sakata, Y., 2005a. Catalytic hydrothermal treatment of pine wood biomass: effect of RbOH and CsOH
on product distribution. J. Chem. Technol. Biotechnol. 80, 1097e1102.
Karagöz, S., Bhaskar, T., Muto, A., Sakata, Y., 2005b. Comparative
studies of oil compositions produced from sawdust, rice husk,
lignin and cellulose by hydrothermal treatment. Fuel 84, 875e884.
REFERENCES
Karagöz, S., Bhaskar, T., Muto, A., Sakata, Y., Oshiki, T.,
Kishimoto, T., 2005c. Low temperature catalytic hydrothermal
treatment of wood biomass: analysis of liquid products. Chem.
Eng. J. 108, 127e137.
Karagöz, S., Bhaskar, T., Muto, A., Sakata, Y., 2006. Hydrothermal
upgrading of biomass: effect of K2CO3 concentration and biomass/
water ratio on products distribution. Bioresour. Technol. 97, 90e98.
Kawamoto, H., Saito, S., Hatanaka, W., Saka, S., 2007. Catalytic pyrolysis of cellulose in sulfolane with some acidic catalysts. J. Wood
Sci. 53, 127e133.
Kersten, S.R.A., Potic, B., Prins, W., Swaaij, W.P.M.V., 2006. Gasification of model compounds and wood in hot compressed water. Ind.
Eng. Chem. Res. 45, 4169e4177.
Knill, C.J., Kennedy, J.F., 2003. Degradation of cellulose under alkaline
conditions. Carbohydr. Polym. 51, 281e300.
Kong, L.Z., Li, G.M., Wang, H., He, W.Z., Ling, F., 2008. Hydrothermal
catalytic conversion of biomass for lactic acid production. J. Chem.
Technol. Biotechnol. 83, 383e388.
KrZan,
A., Kunaver, M., Tisler, V., 2005. Wood liquefaction using
dibasic organic acids and glycols. Acta Chim. Slov. 52, 253e258.
Lanza, R., Nogare, D.D., Canu, P., 2009. Gas phase chemistry in cellulose fast pyrolysis. Ind. Eng. Chem. Res. 48, 1391e1399.
Li, T.C., Yan, Y.J., Ren, Z., 1996. Study on gasification of peat and its
kinetic behavior. Fuel Sci. Technol. 14, 879.
Li, C.Z., Zhang, Z.H., Zhao, Z.B., 2009. Direct conversion of glucose
and cellulose to 5-hydroxymethylfurfural in ionic liquid under
microwave irradiation. Tetrahedron Lett. 50, 5403e5405.
Liu, Z.G., Zhang, F.S., 2008. Effect of various solvents on the liquefaction of biomass to produce fuels and chemical feedstocks.
Energy Convers. Manage. 49, 3498e3504.
Lu, Q., Xiong, W.M., Li, W.Z., Guo, Q.X., Zhu, X.F., 2009. Catalytic
pyrolysis of cellulose with sulfated metal oxides: a promising
method for obtaining high yield of light furan compounds. Bioresour. Technol. 100, 4871e4876.
Lu, Q.A., Dong, C.Q., Zhang, X.M., Tian, H.Y., Yang, Y.P., Zhu, X.F.,
2011. Selective fast pyrolysis of biomass impregnated with ZnCl2 to
produce furfural: analytical Py-GC/MS study. J. Anal. Appl.
Pyrolysis 90, 204e212.
McKee, D.W., 1983. Mechanisms of the alkali metal catalysed gasification of carbon. Fuel 62, 170e175.
Meszaros, E., Jakab, E., Varhegyi, G., Szepesvary, P., Marosvolgyi, B.,
2004. Comparative study of the thermal behavior of wood and
bark of young shoots obtained from an energy plantation. J. Anal.
Appl. Pyrolysis 72, 317e328.
Miller, J.E., Evans, L., Littlewolf, A., Trudell, D.E., 1999. Batch microreactor studies of lignin and lignin model compound depolymerization by bases in alcohol solvents. Fuel 78, 1363e1366.
Mok, W.S.L., Antal, M.J., 1992. Uncatalyzed solvolysis of whole
biomass hemicellulose by hot compressed liquid water. Ind. Eng.
Chem. Res. 31, 1157e1161.
Mudge, L.K., Baker, E.G., Mitchell, D.H., Brown, M.D., 1985. Catalytic
steam gasification of biomass for methanol and methane production. J. Sol. Energy Eng. 107, 88e92.
Mun, S.P., Hassan, E.B.M., 2004. Liquefaction of lignocellulosic
biomass with mixtures of ethanol and small amounts of phenol in
the presence of methanesulfonic acid catalyst. J. Ind. Eng. Chem.
10, 722e727.
Olazar, M., Aguado, R., Bilbao, J., Barona, A., 2000. Pyrolysis of
sawdust in a conical spouted-bed reactor with a HZSM-5 catalyst.
AIChE J. 46, 1025e1033.
Onay, O., Kockar, O.M., 2004. Fixed bed pyrolysis of rapeseed (Brassica
napus L.). Biomass Bioenergy 26, 289e299.
Onwudili, J.A., Williams, P.T., 2009. Role of sodium hydroxide in the
production of hydrogen gas from the hydrothermal gasification of
biomass. Int. J. Hydrogen Energy 34, 5645e5656.
253
Patil, V., Tran, K.Q., Giselrød, H.R., 2008. Towards sustainable production of biofuels from microalgae. Int. J. Mol. Sci. 9, 1188e1195.
Pattiya, A., Titiloye, J.O., Bridgwater, A.V., 2008. Fast pyrolysis of cassava
rhizome in the presence of catalysts. J. Anal. Appl. Pyrolysis 81, 72e79.
Peng, L., Lin, L., Zhang, J., Zhuang, J., Zhang, B., Gong, Y., 2010.
Catalytic conversion of cellulose to levulinic acid by metal chlorides. Molecules 15, 5258e5272.
Putun, A.E., Ozcan, A., Gercel, H.F., Putun, E., 2001. Production of
biocrudes from biomass in a fixed bed tubular reactor. Fuel 80,
1371e1378.
Qian, Y.J., Zuo, C.J., Tan, H., He, J.H., 2007. Structural analysis of biooils from sub-and supercritical water liquefaction of woody
biomass. Energy 32, 196e202.
Qiang, L., Wang, Z., Dong, C.Q., Zhang, Z.F., zhang, Y., Yang, Y.P.,
Zhu, X.F., 2011. Selective fast pyrolysis of biomass impregnated with
ZnCl2: furfural production together with acetic acid and activated
carbon as by-products. J. Anal. Appl. Pyrolysis 91, 273e279.
Rogalinski, T., Liu, K., Albrecht, T., Brunner, G., 2008. Hydrolysis kinetics of biopolymers in subcritical water. J. Supercrit. Fluids 46,
335e341.
Sangon, S., Ratanavaraha, S., Ngamprasertsith, S., Prasassarakich, P.,
2006. Coal liquefaction using supercritical tolueneetetralinmixture
in a semi-continuous reactor. Fuel Process. Technol. 87, 201e207.
Sinag, A., Yumak, T., Balci, V., Kruse, A., 2011. Catalytic hydrothermal
conversion of cellulose over SnO2 and ZnO nanoparticle catalysts.
J. Supercrit. Fluids 56, 179e185.
Su, Y., Brown, H.M., Huang, X.W., Zhou, X.D., Amonette, J.E.,
Zhang, Z.C., 2009. Single-step conversion of cellulose to
5-hydroxy-methylfurfural (HMF), a versatile platform chemical.
Appl. Catal. A 361, 117e122.
Tasaka, K., Furusawa, T., Tsutsumi, A., 2007. Biomass gasification in
fluidized bed reactor with Co catalyst. Chem. Eng. Sci. 62, 5558e5563.
Tekin, K., Karagöz, S., Bektas, S., 2012. Hydrothermal liquefaction of
beech wood using a natural calcium borate mineral. J. Supercrit.
Fluids 72, 134e139.
Tomishige, K., Asadullah, M., Kunimori, K., 2004. Syngas production
by biomass gasification using Rh/CeO2/SiO2 catalysts and fluidized bed reactor. Catal. Today 89, 389e403.
Tong, D.S., Xi, X., Luo, X.P., Wu, L.M., Lin, C.X., Yu, W.H., Zhou, C.H.,
Zhong, Z.K., 2013. Catalytic hydrolysis of cellulose to reducing
sugar over acid-activated montmorillonite catalysts. Appl. Clay Sci
74, 147e153.
Tremel, A., Haselsteiner, T., Spliethoff, H., Kunze, C., 2012. Experimental investigation of high temperature and high pressure coal
gasification. Appl. Energy 92, 279e285.
Tsukahara, K., Sawayama, S., 2005. Liquid fuel production using
microalgae. J. Jpn. Pet. Inst. 48, 251e259.
Tymchyshyn, M., Xu, C.B., 2010. Liquefaction of bio-mass in hot
compressed water for the production of phenolic compounds.
Bioresour. Technol. 101, 2483e2490.
Usui, Y., Minowa, T., Inoue, S., Ogi, T., 2000. Selective hydrogen
production from cellulose at low temperature catalyzed by supported group 10 metal. Chem. Lett. 10, 1166e1167.
Wagenaar, B.M., Prins, W., van Swaaiij, W.P.M., 1994. Pyrolysis of
biomass in the rotating cone reactor: modelling and experimental
justification. Chem. Eng. Sci. 49, 5109e5126.
Wang, G., Li, W., Chen, H., Li, B., 2007a. The Direct Liquefaction of
Sawdust in Tetralin. Energy Sources, Part A 29, 1221e1231.
Wang, G., Li, W., Li, B.Q., Chen, H.K., 2007b. Direct liquefaction of
sawdust under syngas. Fuel 86, 1587e1593.
Wang, G., Li, W., Li, B.Q., Chen, H.K., Bai, J., 2007c. Direct liquefaction
of sawdust under syngas with and without catalyst. Chem. Eng.
Process. 46, 187e192.
Wang, G., Li, W., Chen, H., Li, B., 2007b. The direct liquefaction of
sawdust in tetralin. Energy Sources, Part A 29, 1221e1231.
254
15. CATALYTIC THERMOCHEMICAL PROCESSES FOR BIOMASS CONVERSION TO BIOFUELS AND CHEMICALS
Wu, J.Z., Xu, B.Y., Lou, Z.F., Zhou, X.G., 1992. Performance analysis of
a biomass circulating fluidized bed gasifier. Biomass Bioenergy 3,
105e110.
Wu, L.M., Zhou, C.H., Keeling, J., Tong, D.S., Yu, W.H., 2012. Towards an understanding of the role of clay minerals in crude oil
formation, migration and accumulation. Earth-sci. Rev. 115,
373e386.
Wu, L.M., Zhou, C.H., Zhang, P.P., Yu, W.H., Tong, D.S., 2013. Oil-forming
potential from hydrothermal treatment of cellulose in the presence of
montmorillonite clay minerals. In: Proceedings of Second International Congress on Catalysis for Biorefineries. Dalian, China.
Xu, C., Etchevery, T., 2008. Hydro-liquefaction of woody biomass in
sub and supercritical ethanol with iron-based catalysts. Fuel 87,
335e345.
Yu, Q.Z., Brage, C., Nordgreen, T., Sjöström, K., 2009. Effects of
Chinese dolomites on tar cracking in gasification of birch. Fuel 88,
1922e1926.
Ӧzbay, N., Pütün, A.E., Uzun, B.B., Pütün, E., 2001. Biocrude from
biomass: pyrolysis of cottonseed cake. Renewable Energy 24,
615e625.
Zhang, Z.H., Zhao, Z.K., 2010. Microwave-assisted conversion of
lignocellulosic biomass into furans in ionic liquid. Bioresour.
Technol. 101, 1111e1114.
Zhang, D., Zhou, C.H., Lin, C.X., Tong, D.S., Yu, W.H., 2010. Synthesis
of clay minerals. Appl. Clay Sci. 50, 1e11.
Zhong, C., Wei, X., 2004. A comparative experimental study on the
liquefaction of wood. Energy 29, 1731e1741.
Zhou, C.H., Beltramini, J.N., Fan, Y.X., (Max) Lu, G.Q., 2008. Chemoselective catalytic conversion of glycerol as a biorenewable
source to valuable commodity chemicals. Chem. Soc. Rev. 37,
527e549.
Zhou, C.H., Xia, X., Lin, C.X., Tong, D.S., Beltramini, J., 2011. Catalytic
conversion of lignocellulosic biomass to fine chemicals and fuels.
Chem. Soc. Rev. 40, 5588e5617.
C H A P T E R
16
Applications of Heterogeneous Catalysts
in the Production of Biodiesel by Esterification
and Transesterification
Luiz P. Ramos*, Claudiney S. Cordeiro, Maria Aparecida F. Cesar-Oliveira,
Fernando Wypych, Shirley Nakagaki
Research Center in Applied Chemistry, Department of Chemistry, Federal University of Paraná, Curitiba, Paraná, Brazil
*Corresponding author email: luiz.ramos@ufpr.br
O U T L I N E
Introduction
255
Heteropolyacids
257
Zeolites
258
Clay Minerals
Clay Minerals Improving Acidity
Acid-Activated Clay Minerals in Biodiesel
Production
Case 1
Case 2
Case 3
260
262
263
263
263
264
INTRODUCTION
It is well known that most of the products derived
from the chemical industry involve a catalyst in at least
one step of synthesis (Figueiredo and Ribeiro, 1987).
However, traditional processes for chemical conversion
have numerous inconveniences such as the generation
of undesirable by-products and environmental pollution. For this reason, civil groups as well as governmental agencies are pressing the industrial sector to
overcome these problems by developing alternative
processes in which waste generation is minimized or
even eliminated. This concept is also part of the atom
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00016-4
Layered Materials
Layered Double Hydroxides
Layered Hydroxide Salts
Layered Carboxylates
Layered Materials as Heterogeneous Catalysts in
(Trans)Esterification Reactions
265
265
265
266
Polymeric Catalysts
269
Concluding Remarks
272
References
272
266
economy theory proposed by Trost (1991), in which the
majority of the atoms present in chemical reagents
must be incorporated into useful products.
Many traditional processes based on homogeneous
catalysis have been reviewed in order to minimize waste
generation. In addition, many researchers have shown
that heterogeneous catalysts are excellent alternatives
to generate lower amounts of waste streams and also
to improve the quality of coproducts, which may
contribute with additional revenue for the overall production process.
The biodiesel industry is another important sector
that is following the same strategic pathway (Cordeiro
255
Copyright Ó 2014 Elsevier B.V. All rights reserved.
256
16. APPLICATIONS OF HETEROGENEOUS CATALYSTS IN THE PRODUCTION OF BIODIESEL
et al., 2012; Di Serio et al., 2008). Biodiesel is a biodegradable fuel derived from renewable sources that can be
obtained by different routes such transesterification
and esterification. The traditional transesterification
process of oils and fats is based on the use of a homogeneous system, with methanol as the transesterification
agent and a base catalyst, usually an alkoxide or a
hydroxide (NaOH or KOH) that generates the corresponding alkoxide in situ (Van Gerpen and Knothe,
2009). Then, the synthetic mono alkyl esters can be
used as biodiesel after suitable purification. The main
problem of these processes is related to the required purification steps of the mono alkyl esters as well as glycerin, which must be recovered in good condition due to
their high commercial value.
Ideally, the biodiesel fuel must be free of residues
formed in the chemical process like free and bonded
glycerin, soaps and water, which are normally used in
washing stages for purification. The presence of glycerin
in the resulting biofuel is problematic because this polyol may undergo dehydration during combustion, producing a toxic unsaturated aldehyde named acrolein
that is not only a dangerous atmospheric pollutant but
also a reactive chemical that can be easily involved in
condensation reactions, producing an accumulation of
carbon deposits that may block filters and compromise
the engine performance (Mittelbach and Tritthart,
1988). Soaps and free fatty acids (FFAs) cause degradation of engine components and free water can interfere
with the biodiesel acid number and induce hydrolysis
and biological contamination under nonadequate storage conditions (Ramos et al., 2003).
The traditional fatty acid esterification processes in
homogeneous media uses Brønsted acids such as sulfuric or hydrochloric as reaction catalysts (Ilgen et al.,
2007). However, the extensive use of these catalysts
may induce corrosion in reactor components and pipelines. Also, the purification of the monoesters produced
in this way is also expensive and may require additional
washing steps and distillation (Altiparmak et al., 2007).
A traditional sequence for biodiesel production involves (1) the recovery of vegetable oil by pressing
and/or solvent extraction, (2) the oil pretreatment to
adjust its properties for transesterification, (3) the transesterification process, (4) the purification by several
stages of water washing and (5) the recovery of reaction
coproducts, especially glycerin. Each of these steps adds
costs to the overall process. Thus, the introduction of
operation units that are able to reduce the contamination
degree of mono alkyl esters and glycerin may be an
important measure to make biodiesel more competitive
from an economic and environmental point of view.
For these reasons, many researches had focused their
efforts to substitute homogeneous catalysts for heterogeneous ones. The biodiesel produced in a heterogeneous
system is easily purified and glycerin is of high purity,
diminishing the investment to achieve a suitable market
specification (Ramos et al., 2003). Many classes of chemical compounds have been tested as solids for heterogeneous catalysis to produce biodiesel by either
esterification or transesterification processes. Among
these, zeolites, ionic exchange resins, inorganic oxides,
layered compounds, guanidines and metal complexes
have been already used (Cordeiro et al., 2011).
In order to have a truly heterogeneous catalytic process, the solid catalyst must not leach into the reaction
medium and it also needs to be stable under the reaction
conditions and reusable. While using solid catalysts for
biodiesel preparation, whether by esterification or transesterification, the most common catalyst classifications
are solid Brønsted acids, Brønsted bases or Lewis acids.
The same solid catalyst, however, may present more
than one of these sites and depending on the acidity or
basicity of the solid, the catalytic performance can vary
considerably (Sharma et al., 2011).
Recently, in addition to this primary classification, a
number of other factors have been considered while
developing solid catalysts for esterification or transesterification reactions. The solids hydrophobicity, for once,
is used to unveil the water tolerance. The knowledge
of the pore and channel system is used to improve the
mass transfer of the catalytic substrate, which for this
kind of reaction presents a relatively high viscosity
(Wilson and Lee, 2012).
Metal oxides, mainly calcium (CaO), magnesium
(MgO) and strontium (SrO) oxides, are among the most
extensively studied solid bases for heterogeneous catalytic processes (Sharma et al., 2011). Among all alkaline
and alkaline earth metal oxides, CaO is the most widely
studied. Many are the reasons to explain this fact,
including its low cost, low toxicity and low solubility in
methanol, which is the most commonly used primary
alcohol for the catalytic transesterification of oils and
fats (Sharma et al., 2011; Kusdiana and Saka, 2001).
CaO also has a long catalytic life, with high activity in
many recycling processes under moderate reaction conditions (Lopez et al., 2007). Besides these advantages, CaO
can be obtained from various and sometimes unusual
natural sources. Naturally occurring minerals such as calcite (CaCO3) and several calcium salts (Lopez-Granados
et al., 2010) as well as mollusk shells and egg shells
(Cho and Seo, 2010) can be used as a source of CaO by
calcination.
The impregnation of different alkaline salts in zeolites
followed by appropriate thermal treatment can produce
basic zeolites and the resulting solids have shown good
activity as heterogeneous catalysts for transesterification. Studies have shown that the basicity of the resulted
zeolite can be related to the electropositive nature of the
exchanged alkaline cation (Philippou et al., 2000).
HETEROPOLYACIDS
The infrequent use of acid catalysis in transesterification reactions, in comparison to the base catalysis, is in
part justified by the lower catalytic activities of the
acid compounds. On the other hand, acid catalysts are
less sensitive to several contaminants such as water
and FFAs, which in many cases can deactivate the base
catalyst or drive the catalytic reaction to other products
(Van Gerpen and Knothe, 2009).
Notwithstanding this apparent disadvantage of the
acid catalyst, solid catalysts with Brønsted or Lewis
acid properties have been recently investigated in heterogeneous processes. These solids are promising solid
catalysts for the replacement of strong inorganic acids
that, although effective in both esterification and transesterification homogeneous catalytic systems, have
serious adverse factors such as corrosion of the reaction
vessels. Furthermore, the use of strong inorganic acids
leads to medium- and long-term environmental problems (Helwani et al., 2009a). Thus, the possibility of
using solids with acid properties, rather than highly
corrosive liquids, therefore replacing homogeneous processes by heterogeneous ones, may be advantageous
since higher catalytic efficiencies may be obtained in
more sustainable conversion processes. These are likely
to outweigh the higher costs that are often associated
with the rational synthesis and use of suitable solid acids.
Furthermore, the research of acid catalysts has also
been driven by the possible use of waste cooking oil
and other cheap and widely available raw materials
for biodiesel production. For such materials, the catalyst
must be suitable for acting in the presence of high water
and acid content, properties that are often found in low
cost feedstocks. In general, solids with high acid properties usually meet these prerequisites (Oliveira et al.,
2010; Zhao et al., 2012).
The present work presents a discussion about the
most important classes of inorganic solids and polymeric materials that have been applied in the synthesis
of (m)ethyl monoesters through esterification or transesterification. However, biological systems such as immobilized lipases are not treated in this book chapter.
Luckily, highly qualified reviews have been published
recently on this specific subject (Di Serio et al., 2008; Fjerbaek et al., 2009; Tana et al., 2010).
HETEROPOLYACIDS
The heteropolyacid (HPA) solids, related to the class
of polyoxometalates, are often remembered when there
is a need for catalysts that are tolerant to the large
amounts of water. As already discussed above, such
conditions are usually found in the catalytic conversion
of low-cost raw materials to liquid biofuels such as
biodiesel.
257
Besides their inherent superacidity (pKHþ > 12)
(Mizuno and Misono, 1998), which already ensures the
achievement of relatively high yields, these compounds
can be devised in such a way to produce a pore architecture and a chemical composition that meets the structure
and size of the molecules that are involved in both esterification and transesterification reactions.
Polyoxometalates, frequently named as POMs, are
anionic metal-oxo clusters whose chemical properties
can be modulated by the presence of one or more
different transition metal ions and the cation used to
generate the salt form.
The presence of two different metal atoms per polyoxometalate molecule generates compounds with mixed
metals, vanadium and molybdenum being the most
commonly used. Furthermore, the presence of other
atoms in the structure, besides the metal and the oxygen
atoms, leads to heteropolyoxometalate compounds with
the general formula (XnþMo12O40)(8n), where X can
often be as W (V), Si (IV), Ge (IV) and Ti (IV). These anions can be arranged in typical structures such as
Keggin and Dawson structures.
The protonated form of heteropolyoxometalate anions is referred to as heteropolyacids, which may be
defined as a condensed structure of different types of
oxyacids. In water, it is expected that all HPA protons
are dissociated. The strength of these acids in acetonitrile was estimated. For instance, the acidity of
H3PW12O40 in acetonitrile is greater than that observed
for the p-toluenesulfonic acid and H2SO4, two acids usually used as catalysts for (trans)esterification in homogeneous catalytic systems (Drago et al., 1997).
HPAs are generally soluble in water and other polar
media. Therefore, they are usually unsuitable for biodiesel production by heterogeneous catalysis. However,
these anionic compounds are water insoluble when presented as salts with large cations such as Csþ. Because of
this characteristic, there is a great interest in the application of this family of solid compounds in heterogeneous
process, acting as acid, redox and bifunctional catalysts
(Li et al., 2007).
The (C16TA)H4TiPW11O40 solid, resulting from the
combination of a surfactant (C16TA, cetyltrimethylammonium) with an HPA, was recently reported as a
water-tolerant solid for the heterogeneous catalytic
esterification of palmitic acid (Zhao et al., 2012). The
observed high conversion (94.7 wt%) and high efficiency
(91.8 wt% yield) were attributed to the presence of
Brønsted and Lewis acid sites, its amphiphilic property
and high water tolerance. The authors claimed that substrate molecules concentrate around the catalyst through
hydrophobic interactions with its lipophilic tail while
methanol molecules are absorbed by HPA through
hydrogen bonding. The hydrophobic surroundings
also promote the separation and/or repulsion of water
258
16. APPLICATIONS OF HETEROGENEOUS CATALYSTS IN THE PRODUCTION OF BIODIESEL
molecules from the surface of the catalyst. Also, the solid
catalyst showed a good recyclability and its heterogeneous character was proved through several cycles of recovery and reuse.
In order to heterogenize the HPAs and improve their
recovery and reuse, their impregnation on zirconia was
also investigated (Oliveira et al., 2010). The H3PW12O40
was immobilized on zirconia at different ratios and
calcined at 200 C for 4 h. No decomposition of the Keggin anion structure was observed under these conditions.
The resulting solids were used in the esterification of oleic
acid with ethanol at a 20 wt% loading, 100 C and 4 h
with an ethanol:acid molar ratio of 6:1, conditions under
which an 88 wt% conversion of oleic acid to ethyl oleate
was obtained. A small leaching (8 wt%) of the catalyst
was observed at the end of the reaction, therefore
affecting the reaction kinetics. The recyclability study
indicated that, after being recovered, washed and thermally treated, the solid presented conversion values as
high as 70 wt%, that is, 80% of the original value of
88 wt%.
These examples and many others have shown that
HPA and POWs represent promising catalysts for both
esterification and transesterification (Giri et al., 2005;
Caetano et al., 2008; Wee et al., 2010; Leng et al., 2009)
but their heterogenization in different supports still
needs to be improved in order to keep the process totally
heterogeneous.
ZEOLITES
Zeolites are natural or synthetic materials, classically
defined as crystalline aluminosilicate compounds (Cundy
and Cox, 2003). Zeolites can be prepared by different
synthetic routes with different Si/Al molar ratios, crystal
structures, and proton exchange levels. These modifications favor the rationalization of catalytic properties
such as pore size, hydrophobicity, strength and distribution of acid sites. When designed in a positive way, all
these properties can be interesting and useful for applications in heterogeneous catalysis (Corma et al., 1989).
The catalytic activity of zeolytes can derive from the
properties of the cation that is present in its chemical
composition. Moreover, the exchange of these cations
by protons may generate different degrees of zeolite
acidity, which is also an interesting property for various
catalytic processes (Csicsery, 1984). In fact, acid zeolites
are used in many industrial catalytic applications,
mainly in the petrochemical industry.
Another interesting property is the organized and
uniform pore distribution and the existence of a cavity system of regular molecular dimensions ranging
from <1 nm to over 10 nm, depending on the solid
material. This feature may bestow rather important
catalytic properties to the resulting material such as
selectivity.
In general, zeolites and other porous materials of
similar composition and textural properties, named
zeolite-like materials and zeotypes, have been prepared
and used as catalysts in various chemical processes.
These solid catalysts present a strong scientific appeal
for green chemistry applications since they are considered environmentally benign when one takes into
account their chemical composition.
There are several reports regarding the use of zeolitebased catalysts for various chemical reactions. Such uses
have been recently reviewed (Martinez and Corma,
2011; Rinaldi and Schuth, 2009). This versatility of uses
is not only justified for their great variety in chemical
composition but also because of the uniformity of their
pore structure.
Due to the presence of pores and channels, catalysts
based on zeolites can present size selectivity that is
rarely seen in other solids. This selectivity can be
observed for reactants, products and transition state intermediates that are expected to control a given catalytic
reaction (Csicsery, 1984). However, for the same reason,
these solids not always perform well in catalytic
processes that are dependent on one of their main chemical properties (e.g. acidity). This occurs mainly for processes in which the reactants have dimensions exceeding
the catalytic channels and pores provided by zeolitic
solid catalyst. Therefore, the structure of a zeolite catalyst must be idealized in order to have not only the
appropriate chemical property but also the textural
properties that would offer an array of pores and channels that are adequate for the diffusion of the chemical
reactants. The strategy to meet these two goals is a challenge for the catalytic application of these solids.
The preparation of zeotype materials with mesopores
(2e50 nm) appears to be the solution to avoid the mass
transfer limitations of zeolites in many catalytic processes. In this sense, many efforts have been made in
the scientific community to prepare zeolite-like mesoporous materials that are able to address this goal (Tao
et al., 2006).
For applications in the esterification of fatty acids or
in transesterification of oils and fats, in which large molecules are directly involved in the production of biofuels, it is expected that, apart from their high acidity,
the surface of the zeolitic and/or zeotype solids should
be hydrophobic enough to promote the adsorption of
the substrate on the catalyst surface. In this regard, the
adsorption of polar molecules may cause deactivation
of catalytic sites, such as in the case of water in esterification reactions (Helwani et al., 2009a). For example,
faujasite is a highly hydrophilic zeolite that presents
high levels of water adsorption. Hence, this material is
barely adequate for esterification because water may
ZEOLITES
not only inactivate the catalytic sites on the solid surface
but also compromise the reaction yields by interfering
with the reaction mechanism (Nijhuis et al., 2002).
The MCM-41 molecular sieves have been used as an
alternative to zeolite microporous materials. Since its
discovery in the early 1990s, these molecular sieves
have been used as catalyst in different chemical reactions (Beck et al., 1992; Climent et al., 1999), including
in the preparation of biofuels (Twaiq et al., 2003).
Similar to the zeolites, these mesoporous compounds
also have a regular and ordered distribution of pores
(mesopores) across the solid catalyst, allowing their
use for the conversion of larger molecules (Carmo
et al., 2009). Moreover, the incorporation of metals in
the structure of mesoporous solids may lead to acidic
materials with special characteristics such as a higher
hydrothermal stability. For example, the incorporation
of aluminum ions in zeolitic materials can lead to a
decrease in the Si/Al ratio and a subsequent increase
in the amount of the solid acid sites, since it is well
known that the lower the framework Si/Al ratio of the
zeolite, the lower the strength of its acid sites, regardless
of its higher density (Ma et al., 1996; Corma et al., 1989).
Furthermore, the catalyst hydrophobicity can also be
changed by modifying the Si/Al ratio. This leads to an
alteration in the ability of the solid to adsorb nonpolar
molecules in the catalytic reactions such as esterification
and transesterification, as well as in desorption of polar
molecules such as water (Luque et al., 2007). In general,
high Si/Al ratios (or low aluminum contents) leads to
high solid hydrophobicity. Thus, since the Si/Al ratio
modifies the acidity and hydrophobicity of the catalyst,
its influence on the catalytic properties is subtle, mainly
in esterification reactions.
The presence of water is an important factor in the
conversion outcome of esterification reactions. The equilibrium constant for ester formation is very low (3.38 for
the reaction of acetic acid with ethanol in nonpolar solvents) (Corma et al., 1989). So, to obtain high ester yields,
the reaction must be displaced toward the products, for
example, by the continuous removal of water from the
system. Furthermore, the reaction can be shifted toward
the products when working with a large excess of
reagents.
In order to segregate the water from the reaction environment, it is necessary to work with high reaction rates
and this can be achieved with homogeneous acid catalysts such as sulfuric acid. However, for solid catalysts
such as the zeolitic materials and zeotypes, the proper
balance between strength and density of the acid sites,
suitable for a good catalytic performance and water
segregation, is often difficult to achieve. The rate of reactions catalyzed by zeolite catalysts and other solid materials is usually very low compared to that of sulfuric acid
(Ma et al., 1996).
259
Ajaikumar and Pandurangan (2007) prepared AlMCM-41 materials with different Si/Al ratios (29, 52,
74 and 110) and used these solids in the esterification
of acetic and propionic acids with various alcohols
(1-hexanol, 2-ethyl-1-isoamyl alcohol and cyclohexanol).
With a small addition of aluminum, which translates
into a high Si/Al ratio of 110, these authors observed a
higher hydrophobicity and a higher catalyst hydrothermal stability of the material concerning the amount of
water formed during esterification. Furthermore, the
use of more hydrophobic solid materials prevented the
subsequent hydrolysis of the ester formed. On the other
hand, solid catalysts with lower Si/Al ratio promoted
lower levels of alcohol dehydration, which can also be
favored at high temperature. As a result, the selectivity
of the catalytic reaction is improved toward the ester production as the accumulation of possible by-products
(etherified and dehydrated compounds) is decreased.
Hence, the hydrophobicity achieved at higher Si/Al
ratios was an important factor for the best catalytic
performance (catalytic efficiency), whereas the use of
low aluminum contents led to more selective catalytic
systems.
Corma et al. (1989) reported that strong BrønstedeLowry acid sites are required for the catalytic
esterification of acetic acid since they are able to protonate the acetic acid carbonyl group. Working with protonated faujazite zeolite after dealuminization, these
authors observed that some dealuminized HY zeolites
with Si/Al ratio less than or equal to 15 had better catalytic performance. The strong acid sites present in that
solid, which correspond to those aluminum vacant sites
or sites occupied by one aluminum atom and the respective nearest neighbors, are more active for the catalytic
esterification of fatty acids. Zeolites with high Si/Al
ratio presented a more hydrophobic surface and this
hydrophobicity became the predominant factor for the
equilibrium shifting toward the production of alkyl
esters. Also, the higher the aluminum content of the
zeolite, the higher the observed adsorption effect.
Carmo et al. (2009) also prepared solids based on
Al-MCM-41 to investigate the relationship between the
high availability of acid sites, introduced by increasing
the aluminum content in the mesoporous solid, and
the degree of esterification of palmitic acid with methanol, ethanol and isopropanol. However, these authors
restricted their work to solids with low Si/Al ratios
(8, 16 and 32) whose hydrophobicity was much smaller
than the solids described in the previous work. This and
most of the data already available in the literature propose the utilization of solid catalysts for esterification
reactions. In general, these studies have been motivated
by the technological challenge of developing a suitable
catalytic system to convert vegetable oils or animal fats
of high acid number in biodiesel. Hence, by the catalytic
260
16. APPLICATIONS OF HETEROGENEOUS CATALYSTS IN THE PRODUCTION OF BIODIESEL
esterification of their fatty acid content, these materials
would be neutralized and become suitable for transesterification in alkaline media.
The high aluminum content solid catalysts (Si/Al
molar ratio of 8) prepared by Carmo et al. (2009) showed
relatively modest palmitic acid conversion values. The
highest value achieved in this study was 79 wt% of
methyl ester. The authors did not report the effect of
hydrophobicity on reaction conversion but only the
increased effect of aluminum incorporation in the catalytic activity of the resulting solids.
Ma et al. (1996) used different zeolitic solids (zeolite
ZSM-5 and three HY zeolites with Si/Al molar ratios
of 30, 5.1 and 9.3) to evaluate the relationship between
the solid hydrophobicity and its aluminum content
with the observed catalytic efficiency in the preparation
of ethyl, n-butyl, isopentyl and benzyl acetates; ethyl
and n-butyl benzoates and dioctyl phthalates. For all
the solid catalysts used in this study, a high selectivity
for the expected ester was observed without any formation of ether derivatives. The increase in selectivity
with decreasing aluminum content was also reported
by Corma et al. (1989).
Insoluble inorganic salts and other inorganic solids
based on transition metals can also be used as acid
catalysts for transesterification. The application of sodium molybdate (Na2MoO4) and sodium tungstate
(Na2WO4) has been recently reported as efficient catalysts for biodiesel production under relatively mild
experimental conditions (Nakagaki et al., 2008; Santos
et al., 2011). In these studies, soybean oil (0.7 mg/g
KOH of acid number), degummed soybean oil containing 180 ppm of phosphatides (1.0 mg/g of KOH), and
waste cooking oil (1.5 mg/g KOH) were transesterified
with methanol (methanolysis). At 65e80 C using a
54:1 methanol:oil molar ratio and 5 wt% catalyst for
3e5 h, both catalysts reached conversions higher than
92 wt% regardless of the feedstock used for methanolysis. The catalytic activity of these compounds was attributed to the presence of molybdenum(VI) or tungsten(VI)
strong Lewis acid sites that are probably able to polarize
the methanol OeH bond leading to intermediate species
that possibly have high nucleophilic character.
The heterogeneous nature of the above-mentioned
catalysts was investigated through their reuse in several
consecutive reaction cycles. Both compounds could be
reused for at least three catalytic cycles. However, part
of the solid catalysts was lost during the recycling processes due to their reduced particle size and noticeable
adherence to the walls of the reaction vessel. To circumvent these problems, both molybdenum (Bail et al., 2013)
and tungsten (Santos et al., 2011) compounds were heterogenized in silica obtained by the solegel process
and used in esterification of stearic and oleic acids. Improvements were observed in the catalysts’ recovery
and reuse and a good catalytic activity was obtained in
the first and subsequent recycling stages. Similarly, zirconia impregnated with tungsten oxide (ZrO2/WO2)
was also investigated as an acid catalyst for both esterification and transesterification reactions with methanol
(Lopez et al., 2007).
CLAY MINERALS
Clay minerals are composed of hydrous layered silicates that are part of the phyllosilicates family. The phyllosilicates family is broad and is roughly separated by
layer types, groups, subgroups and species (Brindley
and Brown, 1980). Two basic units are important to build
the clay minerals, the first is silicon atoms coordinated
tetrahedrically to oxygen atoms (SiO4) and divalent or
trivalent metals coordinated octahedrically to hydroxyls
(Mþ2/Mþ3(OH)6). The silicon tetrahedral face can share
the three corners with other silicon tetrahedral to build a
hexagonal two-dimensional pattern, the tetrahedral
sheet. A similar procedure can be adopted by the octahedral, where basically two differences can be obtained
when Mþ2 or Mþ3 atoms occupy the center of the
octahedral.
When Mþ2 is used and the octahedral share the edges,
all octahedral sites are occupied and a two-dimensional
unit is formed, the so-called octahedral sheet. This unit
resembles the structure of brucite (Mg(OH)2) and the
sheet is called trioctahedral. When Mþ3 is used and the
octahedral share the edges, only 2/3 of the octahedral
sites are occupied and the resulting two-dimensional
unit resembles the structure of gibbsite Al(OH)3; in
this case, the sheet is named dioctahedral. In the ideal
condition, the apical oxygen of the tetrahedral sheets
can be linked to one octahedral, building the clay minerals of the 1:1 layer type. The unshared hydroxyls of
the octahedra lie at the center of the tetrahedra at the
same “z” level of the shared apical oxygen.
Under ideal conditions, two clay minerals of the 1:1
layer type can be obtained. When Mþ2 occupies the center
of the octahedral, the structure of chrysotile (Mg3(OH)4
Si2O5) is obtained and the replacement of Mþ2 by Mþ3
yields the structure of kaolinite (Al2(OH)4Si2O5).
As both sides of the octahedral have hydroxyls to
share, one octahedral sheet can also be combined with
two tetrahedral sheets, originating the 2:1 layer-type
clay minerals. Again, when Mþ2 occupies the center
of the octahedral, the structure of talc is obtained
(Mg3(OH)2Si4O10) while its replacement by Mþ3 results
in the structure of pyrophyllite (Al2(OH)4Si4O10).
Figure 16.1 shows the lateral (A) and top (B) views of
the above-cited compounds.
In nature, the phyllosilicates are obtained through
weathering, which is the phenomenon related to the
CLAY MINERALS
261
FIGURE 16.1 Lateral view (A) and top view (B) of the layered structures of chrysotile (a), kaolinite (b), talc (c) and pyrophyllite (d). (For color
version of this figure, the reader is referred to the online version of this book.)
disintegration and chemical alteration of rocks and minerals at the Earth’s surface in direct contact with the atmosphere, water and organism. Through this process,
many different isomorphic substitutions occur either in
the tetrahedral (Si by Al or Feþ3) or in the octahedral
sheets (Al or Mg by Feþ2/þ3, Li, Ti, V, Cr, Mn, Co, Ni,
Cu and Zn), mainly in clay minerals of the 2:1 type.
The isomorphic substitution generates an excess of
negative charge into the layers, which needs to be
compensated with the intercalation of hydrated cations
between the layers. Hence, this substitution generates
the cationic exchange capacity and the plastic properties
of these clay minerals, particularly when they are
dispersed in water.
Using the example of talc and pyrophyllite, these
minerals can give origin to trioctahedral saponite
ððMþ
xy $nH2 OÞðM g3y ðA l; F eÞy ÞðS i4x A lx O1 0 ðO HÞ2 ÞÞ,
where Mgþ2 is substituted by Al and Fe and Si, by Al.
After this substitution, the excess of negative charges
in the clay layers are compensated by the intercalation
of hydrated cations ðMþ
xy $nH2 OÞ. Another example of
262
16. APPLICATIONS OF HETEROGENEOUS CATALYSTS IN THE PRODUCTION OF BIODIESEL
trioctahedral mineral derived from pyrophyllite is the
clay mineral hectorite ððMþ
y $nH2 OÞðMg3y Liy ÞðSi4 O10
ðOHÞ2 ÞÞ. In the case of dioctahedral talc, the derived clay
minerals are montmorillonite ððMþ
y $nH2 OÞðAl2y Mgy Þ
ðSi4 O10 ðOHÞ2 ÞÞ,
beidellite ððMþ
y $nH2 OÞAl2 ðSi4x Alx Þ
þ3
O10 ðOHÞ2 Þ and nontronite ððMþ
x $nH2 OÞFe ðSi4x Alx Þ
O10 ðOHÞ2 Þ.
Clay Minerals Improving Acidity
Most of the clay minerals have low Brønsted and
Lewis acidity. The Brønsted acidity and surface area
can be slightly improved during drying when interstitial
and intercalated cation hydration water molecules are
removed. Another way to improve the acidity is to
exchange the intercalated species with highly polarizing
species such M3þ cations, where the hydrolysis of the
solvated water molecules release protons.
The Lewis acidity is normally associated with
exposed Al3þ or Fe3þ at the broken crystalline edges,
which can be increased by heating the clay material to
temperatures above 300 C or by acid treatment
(Moronta, 2004). As the thermal treatment can lead to
the collapse of the clay mineral structure, the acid treatment is the most effective way to improve both Lewis
and Brønsted acidity. This procedure was already
described in the 1960s (Ryland et al., 1960), when
acid-modified smectites provided high gasoline yields
when used as a petroleum-cracking catalyst.
Acid activation of clay minerals using mineral acids is
not new and this procedure causes disaggregation of
clay particles, elimination of impurities and improvement of their surface area, porosity and catalytic properties. Acid-activated clays are broadly spread in different
industrial processes, being especially used as bleaching
agent and catalysts. Although this process was found
to be dependent on several factors such as the type of
mineral, its crystallinity, morphology and particle size,
the effects of the selective acid leaching of a 2:1 clay mineral, which is a first-order process, can be schematically
seen as shown in Figure 16.2.
The treatment of clay minerals with mineral acids at
room temperature tends to replace the intercalated cations by hydrated protons and/or by the leached cations,
improving Brønsted acidity while the structure is mostly
preserved (Figure 16.2(b)). By contrast, the thermal acid
activation has several effects on the structure of the clay
minerals, which depend on the temperature, time of
treatment and concentration and strength of the acid
used. Under mild temperatures, times and acid concentration, the first effect is usually the removal of
acid-soluble impurities and partial leaching of the octahedral coordinated metals from the octahedral sheet
FIGURE 16.2 Steps of the acid activation. (a) Raw 2:1 clay mineral; (b) original intercalated cations are replaced by hydrated protons;
(c) octahedral structural metals are leached out of the structure; (d) hydrated silica obtained by the collapse of the clay structure. (For color
version of this figure, the reader is referred to the online version of this book.)
CLAY MINERALS
263
Konwar et al., 2008; Nascimento et al., 2011; Neji et al.,
2011; Zatta et al., 2012, 2013; Rezende et al., 2012; Olowokere et al., 2012), as well as in the transesterification of
oils and fats (Bokade and Yadav, 2009). Some specific
cases will be evaluated in sequence.
FIGURE 16.3 Possible mechanism for the formation of Brønsted
and Lewis acid sites after treatment of a 2:1 clay mineral constituted
basically of Al in the octahedral sites and Si in the tetrahedral sites.
(For color version of this figure, the reader is referred to the online
version of this book.)
(Figure 16.2(c)). During this process, not only the Lewis
acidity is generated but also the anions of the acid can be
incorporated in the structure.
As clays having Mg or Fe in their structure are more
easily leached than octahedra occupied by Al, the acid
activation must be optimized to extract the best characteristic of each clay mineral. Under extreme conditions,
regardless of the type of clay mineral, all the octahedral
metals are removed to produce inactive hydrated
fibrous silica as reported previously (Figure 16.2(d))
(Wypych et al., 2005). The effect of an effective acid activation is the broadening of the basal X-ray diffraction
peak due to the damage of the layers and, finally, to
the total collapse of the structure. The physical effects
of this activation process are the improvement of the
surface area and pore volumes up to a specific point
from which these properties are decreased. The pore
radii are also constantly reduced during the treatment.
For each acid and activation conditions, a new optimization needs to be reported since each clay mineral has a
characteristic that depends not only on the mineralogical classification but also on the mine from which it
was extracted.
As an example for montmorillonites (Wilson and
Clark, 2001; Zatta et al., 2012), Figure 16.3 shows a
possible mechanism for the formation of Brønsted and
Lewis acid sites after mineral acid treatment of a 2:1
clay mineral constituted basically of Al in the octahedral
sites and Si in the tetrahedral sites.
Acid-Activated Clay Minerals in Biodiesel
Production
Just a small number of papers have been devoted so
far to the use of acid-activated clay minerals in the catalytic esterification of fatty acids (Vijayakumar et al., 2005;
Case 1 (Zatta et al., 2012)
Standard Texas Montmorillonite STx-1 with the
chemical formula ((Ca0.27Na0.04K0.01)[Al2.41Fe(III)0.09
Mg0.71Ti0.03]Si8.00O20(OH)4), supplied by the Clay Mineral Society repository, was activated using phosphoric,
nitric and sulfuric acids under different conditions of
temperature, time and acid concentrations and the
resulting materials were characterized by X-ray diffraction (XRD), nitrogen adsorption isotherms and Fourier
transform infrared spectroscopy. Also, the presence of
Lewis and Brønsted acid sites in the structure of the catalyst was characterized by pyridine adsorption. Afterward, the materials were evaluated as catalysts in the
methyl esterification of lauric acid. Blank reactions carried out in the absence of any added catalyst presented
conversions of 32.64, 69.79 and 79.23% for alcohol:lauric
acid molar ratios of 60:1, 12:1 and 6:1, respectively. In the
presence of the untreated clay and using molar ratios of
12:1 and 6:1 with 12 wt% of catalyst, conversions of 70.92
and 82.30% were obtained, respectively. For some key
samples obtained by the acid activation, conversions
up to 93.08% of lauric acid to methyl laurate were obtained, much higher than those observed for the thermal
conversion (TC) or using raw montmorillonite. Relative
good correlations were observed between the catalytic
activity and the development of acid sites and structural
and textural properties of the acid-leached materials.
Case 2 (Zatta et al., 2013)
The same sample of montmorillonite STx-1 described
above was submitted to acid activation using aqueous
solutions of phosphoric acid. The acid treatment was
carried out under vigorous stirring at 100 C in a flatbottomed flask connected to a reflux condenser and a
heating mantle. The mineral clay and the acid solution
were mixed in a 1:4 ratio (mass per volume) using acid
concentrations of 0.5, 1, 2 and 4 mol/l.
After the acid activation process, the samples were
repeatedly washed with distilled water until pH close
to 7, dried at 110 C for 24 h and then heated in an
oven at 250 C for 2 h. To check the influence of other
acids in the activation of montmorillonite STx-1, this
clay mineral was subjected to activation with hydrochloric (37% proof), nitric (65% proof) and sulfuric (98%
proof) acids. The resulting acid-activated clay materials
were characterized and subsequently used in the catalytic conversion of lauric, oleic and stearic acids, as
well as of a complex mixture of fatty acids (tall oil) to
their corresponding fatty acid methyl esters (FAMEs).
264
16. APPLICATIONS OF HETEROGENEOUS CATALYSTS IN THE PRODUCTION OF BIODIESEL
100
K10
95
K10
PA
PA
K10
100
PA
K10
PA
95
85
80
75
70
STX
STX
TC
TC
TC
STX
TC
STX
65
Conversion (%)
Conversion (%)
90
85
80
60
5
0
90
10
0
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16
1
Experiment
The results obtained for the best phosphoric acidactivated sample (PA) were compared to those of the
TC and from a standard commercial Lewis acid catalyst
(K10) and raw montmorillonite (STX). In all experiments, conversion of all samples were carried out for
2 h at a methanol:fatty acid molar ratio of 12:1 and
160 C with 12 wt% of the catalyst in relation to the oil
mass.
In general, the PAs and the standard Lewis acid catalyst (K10, SigmaeAldrich), which is produced by HCl
activation of mineral clays at boiling temperatures, had
similar catalytic activities. However, in some cases, the
catalytic performance of PA was even better than that
of K10 (Figure 16.4). These data were a strong indication
that PA and K10 have similar chemical and physical
characteristics, even though part of the layered structure
is still retained in PA after acid activation.
Tests of reuse of the best PA were performed
(Figure 16.5) and no significant losses of activity were
observed during the first four consecutive reaction cycles (see dotted horizontal line in Figure 16.5). This is a
very important observation since, from any practical
use in industrial processes, the catalysts must last for
long time before deactivation.
4
FIGURE 16.5 Reuse experiments of the best phosphoric acid
activated sample. The reactions were carried out at 160 C for 2 h with
a methanol:fatty acid molar ratio of 12:1 and a catalyst loading of
12 wt% in relation to the oil mass.
an oven at 110 C for 24 h and finally ground until passage through a 60 mesh sieve. The surface area of the
acid-activated clays was improved from 71 m2/g to
238 m2/g for the VLn sample and from 123 m2/g to
170 m2/g for the BBn sample. The catalytic activity of
these compounds was evaluated in the esterification of
different organic acids, using different acylating agents
and reflux conditions. In the case of methyl and hexyl
esterification of lauric acid (Figure 16.6), the sulfuric
acid activation of both clays greatly improved their catalytic activity and this was also valid for other acids and
acylating agents. As reported in Case 2, acid activation
led to catalytic activities higher than the standard Lewis
acid catalyst (K10).
100
80
BBa
Vla
BBa
Vla
K10
60
K10
40
Vln
BBn
20
Case 3 (Rezende et al., 2012)
Two different clay minerals (namely BBn and VLn),
mined in Boa Vista District of Paraı́ba (Brazil), were
acid-activated using a 10 wt% suspension of clay in
4 mol/l sulfuric acid at 90 C for 2 h to produce the
BBa and VLa solids, respectively. The solids were
filtered under reduced pressure and washed with
distilled water until the washing water had the same
pH of the original material. The material was dried in
3
Use cycle
Ester (%)
FIGURE 16.4 Comparison among the catalytic activities of the
best sample obtained after phosphoric acid (PA) activation with those
of the thermal conversion (TC), raw montmorillonite (STX) and a
standard commercial Lewis acid catalyst (K10). Experiments 1e4 were
carried out with stearic acid, 5e8 with lauric acid, 9e12 with oleic acid
and 13e16 with tall oil fatty acids.
2
BBn
0
1
Vln
2
3
4
5
6
7
8
9
10
Experiment
FIGURE 16.6 Comparison between the catalytic activity of two
Brazilian clay minerals (BBn and VLn) and their respective acidactivated counterparts (BBA and VLa) in the esterification of lauric
acid. A standard commercial Lewis acid catalyst (K10) was also used
for comparison. Experiments 1e5 correspond to the use of methanol,
whereas hexanol was used in experiments 6e10.
LAYERED MATERIALS
In conclusion, clay minerals are cheap inorganic materials that are readily available worldwide, environmentally friendly and suitable for the development of
reusable acid catalysts for the esterification of fatty acids
and the transesterification of oils and fats. The natural
acidity can be improved by thermal treatment and selective acid activation. Depending on the clay minerals’
origin and genesis, different chemical compositions are
possible and different acid treatments are needed to
optimize the acidic properties. Normally, the catalysts
can be used in several consecutive reaction cycles and,
after deactivation, the residual solids can be easily
disposed of or even incorporated in native clays for
the production of ceramic materials, bricks and roofs,
as well as in the production of porcelains.
LAYERED MATERIALS
Apart from clay materials, different types of layered
compounds have been tested as heterogeneous catalysts
in processes traditionally mediated by homogeneous
catalysts, which are in some cases expensive and highly
toxic. Reactions such as Michael addition, cyanoethylations of alcohols, aldolic condensations and condensation of nitro compounds with aldehydes and ketones,
and ring openings can be used as examples (Centi and
Perathoner, 2008).
Layered materials have also been used as solid catalysts for biodiesel production through esterification
and transesterification. Most applications involving
this class of compounds refer to the use of layered double oxides (LDOs), which are derived from layered double hydroxides (LDHs) by controlled calcination. LDHs,
layered hydroxide salts (LHSs) and layered carboxylates
are less commonly used for this purpose. This section
presents a brief review of the structure of these layered
materials in addition to the description of their use
and performance as catalysts.
Layered Double Hydroxides
LDHs are compounds whose individual layers are of
brucite-like (Mg(OH)2) structure. In brucite, the layers
are electrically neutral with magnesium cations located
in the center of an octahedron with six hydroxyl groups
in the vertices. The isomorphic substitution of magnesium
by trivalent cations forms positively charged layers, which
are stabilized by the presence of anions in the interlayer
space (Bravo-Suárez et al., 2004). LDHs are represented
m
3þ
by the formula ½M2þ
1x Mx ðOHÞ2 Ax=m $nH2 O, where
2þ
3þ
M and M are divalent and trivalent cations and Am
represents an anion with an m-charge and x usually has
a value between 0.25 and 0.33 (Crepaldi and Valim, 1998).
265
In this work, the chemical composition of a specific
LDH will be condensed as M2þ/M3þ-A. Thus, an LDH
whose layers contain Mg and Al and the counterion
between the layers is nitrate will be written as Mg/
Al-NO3.
One peculiar characteristic of LDHs is the memory effect. Calcination of Mg/Al or Zn/Al LDHs forms mixed
and nanostructured mixed metal oxides described as
LDO, which are able to reassemble the LDH structure
if added back to an aqueous solution containing a salt
whose anions will be intercalated between the layers
in order to stabilize the LDH structure (Carlino, 1997).
These anions can be different from the anions found in
the original LDH. This kind of materials can substitute
basic homogeneous catalysts like ammonia, ammonium
salts or amines and offer an option as nonpollutant solid
catalysts that can be easily separated from the reaction
system and recovered. Their catalytic activity is related
to the large surface area of LDHs, its solid base character
and layered morphology. For instance, Zn/Al LDH containing nitrate, sulfate or orthophosphate anions have
catalytic activity in esterification reactions (Hu and Li,
2004), while Mg/Al LDH intercalated with t-butoxide
is active in transesterification (Choudary et al., 2000).
Layered Hydroxide Salts
Some layered hydroxides can also undergo isomorphic
substitution of hydroxyl groups by other oxo-ions or by
water molecules. In the last case, additional anions will
be required to neutralize the excess of positive charge in
the layers, keeping the cations unaltered, i.e. only divalent
cations are present in the layers. The resulting compounds
are called layered hydroxide salts. According to this
description, LHSs can be classified based on the structure
of copper hydroxide nitratedCu2(OH)3NO3dand zinc
hydroxide nitratedZn5(OH)8(NO3)2$2H2O. The general
formula for an LHS is M2þ ðOHÞ2x ðAn Þx=n $mH2 O,
where M ¼ Mg, Ni, Zn, Cu, Co and A ¼ NO3 ; SO2
4
e Cl (Arizaga et al., 2007).
The layers in the copper hydroxide nitrate structure
are formed by octahedrons whose center is occupied
by Cu2þ cations and these are coordinated to hydroxyl
groups and nitrate ions that have substituted ¼ of the
hydroxyl sites. This example is the easiest description
of an LHS.
The structure of zinc hydroxide nitrate has two main
characteristics. The first is that ¼ of the Zn2þ cations in
octahedral coordination with hydroxyl groups migrate
out of the layers, leaving empty octahedrons and forming tetrahedrons up and down the empty octahedral
sites. Then, each layer is formed by zinc cations in octahedral coordination with hydroxyl groups and in tetrahedral coordination, whose base is formed by three
266
16. APPLICATIONS OF HETEROGENEOUS CATALYSTS IN THE PRODUCTION OF BIODIESEL
hydroxyl groups shared with the main octahedral layer
and its apex is occupied by water molecules. The resulting layers have residual positive charge with com2þ
tetr
position ½Znoct
3 Zn2 ðOHÞ8 ðH2 OÞ2 , where oct and
tetr indicate octahedral and tetrahedral sites (Stählin
and Oswald, 1971).
The residual positive charge in the layer is neutralized with nitrate ions in the interlayer space in a perpendicular position to the layers plane. Normally, nitrate
ions do not coordinate directly to the cations in the
layers; however, part of the nitrate ions can be grafted
to the layers by controlling the pH of the synthesis
(Arizaga et al., 2008). The stacking of layers is stable
because of the numerous hydrogen bonds that are
formed between OH groups in the layer, nitrate ions
and the interlayer water molecules. Two adjacent layers
are shifted by a factor of b/2 along the (001) plane and
are stacked along the basal axis.
Zinc laurate melts at approximately 137 C, when nearly
30% of the all-trans conformation is lost. As a result, the
structure is partially disassembled but the metals still
remain coordinated to oxygen atoms, either bridging
two different carboxylate groups or in a monodentade
form (Taylor et al., 2006).
The application of metal carboxylates in the production of biodiesel became more usual since Cordeiro
et al. (2008) demonstrated that the LHS zinc hydroxynitrate (ZHN, Zn5(OH)8(NO3)2$2H2O) is rapidly transformed into zinc carboxylates when used in the
catalytic conversion of lauric acid to methyl laurate.
According to these authors, zinc carboxylates, which
are produced in situ during a reaction course, is the
actual catalytic species in the reaction system. Recently,
the transformation of LDHs into metal carboxylates
was also demonstrated during the catalytic esterification
of fatty acids (Cordeiro et al., 2012).
Layered Carboxylates
Layered Materials as Heterogeneous Catalysts
in (Trans)Esterification Reactions
Layered carboxylates are also known as metal fatty
acid soaps. These carboxylates are used in several applications such as fuel additives, cosmetic products, components of lubricants greases, catalysts, among many
others. Carboxylates can be obtained by melting a
selected fatty acid or fatty acid mixture with the oxide
that contains the metal of interest. Temperatures above
200 C and vigorous agitation are usually required for
synthesis. However, the precipitation method is more
commonly used. In this method, fatty acids are neutralized with sodium hydroxide in ethanol under stoichiometric conditions. Afterward, an ion exchange is
promoted by adding a salt solution of the metal of interest to the sodium carboxylate solution obtained previously, also under stoichiometric conditions. As a result,
the desired metal carboxylate is precipitated and subsequently recovered by filtration (Barman and Vasudevan,
2006).
Zinc is among the metals most commonly used for the
synthesis of carboxylates. However, other cations,
mainly di- and trivalent, have also been used such as
manganese, nickel, copper and lantanum (Lisboa et al.,
2012). In the layered zinc(II) carboxylates, the central
zinc metal is tetrahedrally coordinated to bridging
bidentate carboxylate groups forming a bilayer of the
hydrocarbonic chain bridges in a syn-anti arrangement
(Lacouture et al. 2000; Barman and Vasudevan, 2006).
Layered carboxylates are isostructural materials and
this can be observed through the repeating basal planes
of their X-ray diffraction pattern below 20 of 2q (Taylor
and Ellis, 2007; Lisboa et al., 2012). In long-chain metal
carboxylates such as zinc laurate, methylenic groups of
the hydrocarbon chains are organized in a zigzag
conformation, which is also referred to as all-trans.
The use of LDH as catalysts for transesterification
reactions is less common if compared to the use of
LDOs derived from LDH by calcination. However, the
t-butoxide intercalated Mg/Al LDH (LDH/t-BU) was
shown to be catalytically active for production of
b-ketoesters by transesterification with primary, secondary and tertiary alcohols (Choudary et al., 2000).
Serio et al. (2006) synthesized Mg/Al LDHs by coprecipitation at pH 10. After washing and drying, the LDHs
were calcined at 500 C for 14 h to produce the corresponding oxides. Besides, two samples of oxides identified as MgO1 and MgO2 were obtained by calcination
of Mg(OH)2 and (MgCO3)4 Mg(OH)2 at 400 C. All these
oxides were tested as catalysts for soybean oil methanolysis. Reactions carried out with 10 wt% catalyst at 100 C
yielded about 80% of products using the LDO solids and
less than 20% with both MgO1 and MgO2. The higher
activity of LDO, with respect to other catalysts, was justified by the presence of a higher concentration of very
strong base sites and large pores that favored the reaction
by rendering the active sites more accessible to the bulky
triglyceride molecules. In another study (Serio et al.,
2007), an LDO obtained in the same way was used in
the methanolysis of soybean oil with and without the
addition of 10 wt% of its weight in oleic acid. The reaction
was carried out at 180 C for 1 h with 5 wt% catalyst using
commercial MgO as a reference material. The methanolysis of neutral soybean oil was catalyzed with LDO and
MgO and the yields were 92% and 75%, whereas the corresponding values for the acidified soybean oil were
80.3% and 76.6%, respectively.
Unlike the direct use of LDOs, Xi and Davis (2008)
rehydrated the LDO and tested the resulting material
LAYERED MATERIALS
as catalyst for transesterification. The experiments
started with the coprecipitation of an Mg/Al LDH
with an Mg:Al molar ratio of four. The material was
calcined at 500 C under nitrogen atmosphere to form
the LDO and then rehydrated with vapor under nitrogen atmosphere. The crystallinity of the resulting rehydrated LDH was lower than that of the initial LDH.
The absence of CO2 in the rehydration process avoided
formation of carbonate ions. Hence, the counterion in
the LDH structure was the hydroxyl ion. For this reason,
the hydrated LDH had more Brønsted sites than a
typical LDH. This material was subsequently used in
the methanolysis of tributyrine and the yield of monoesters was around 80% when the reaction conditions
involved 136.5 g of methanol, 43.0 g of tributyrine and
0.25 g of catalyst at 60 C for 400 min.
Zeng et al. (2008) synthesized various LDHs with
different Mg:Al molar ratios by coprecipitation and
ripened them at 65 C. The solid LDHs were washed
and dried at 90 C to be subsequently calcined in a
muffle at 673e1073 C for 7 h, with the resulting oxides
being tested in the transesterification of refined colza oil.
The catalytic activity was correlated with the temperature and time of calcination as well as with the Mg:Al
molar ratio. The best yield (90.5%) was obtained from
the oxide with Mg:Al molar ratio of three that was
calcined at 500 C for 12 h. In this case, the transesterification was carried out with 1.5% of catalyst in relation to
the oil mass, a methanol:oil molar ratio of 6:1 and stirring at 300 rpm for 4 h at 65 C. In addition, the reuse
assays showed that the catalytic activity was kept for
six cycles with a slight decrease in ester yield after
each cycle.
Mg/Al LDOs were also tested by Xie et al. (2006) in
the transesterification of soybean oil with methanol.
The precursor was synthesized by coprecipitation at
pH 7. The material was calcined for 8 h at different temperatures and the obtained LDO was tested in the transesterification of soybean oil with a methanol:oil molar
ratio of 15:1, 7.5% of catalyst and heating under reflux.
The Mg:Al molar ratio of three yielded 67% of ester,
which was the best result if compared to other molar
ratios of 2.0, 2.5, 3.5 and 4.0. The calcination temperature
also influenced the catalytic activity. Actually, the calcination temperature affected the basic strength of the
oxides as determined by the Hammett method. When
the calcination temperature was increased from 300 C
to 500 C, the methyl ester yield reached a maximum
of 66%. The highest yield was attributed to the achievement of the highest basicity after calcination. According
to XRD, this oxide corresponded to the MgO periclase
phase. Temperatures above 500 C transformed the
crystalline phase into spinel with less basicity and also
less catalytic activity. Calcination below 500 C led
Al3þ to replace Mg2þ sites and the basicity Al bonded
267
to O2 is lower than that of Mg bonded to O2. For the
LDH with an Mg:Al molar ratio of three, calcination at
500 C led to the optimal basicity for catalytic applications in the methanolysis of soybean oil.
Cantrell et al. (2005) reported the use of layered
materials for the catalytic transesterification of glycerin tributyrate. For this purpose, a series of ½Mgð1xÞ
Alx ðOHÞ2 xþ ðCO3 Þ2
x=2 compounds with the x value ranging from 0.25 to 0.55 were calcined at 500 C for 3 h
under wet N2 flux (95% humidity). Also, pure Al2O3
and samples of magnesium-impregnated calcined
hydrotalcite were used as reference materials and no
catalytic activity was detected in any of these compounds. On the other hand, the LDOs improved their
catalytic efficiency with an increase in their magnesium
content, achieving a maximum ester yield of 74.8% with
25% of magnesium in the LDO structure. The reactions
were always performed at the same experimental conditions (60 C for 3 h), in which pure MgO yielded only
11% of esters.
In another study, heterogeneous catalytic processes
were developed for the alcoholysis of triglycerides using
LDOs that were impregnated with alkaline metals
(Trakarnpruk and Porntangjitlikit, 2008). Mg/Al-NO3
LDHs were synthesized by coprecipitation and calcined
at 450 C for 35 h. The resulting oxide was added to a
potassium acetate solution in order to impregnate the
oxide with potassium ions. The material was recovered
from the solution, dried at 100 C for 12 h and calcined
again at 500 C for 2 h. The potassium content of the
resulting powder was 1.5%. FAMEs with a 96.9% ester
content and methyl ester yields of 86.6% were obtained
with these solids in reactions carried out at 100 C for
6 h, using 7% of catalyst and a methanol:oil molar ratio
of 30:1.
Liu et al. (2007) carried out the catalytic conversion
of chicken fat to methyl esters using oxides that were
derived from the Mg6Al2(CO3)(OH)16$4H2O hydrotalcite
by calcination at different temperatures (400e800 C) for
8 h. As a result, the effect of the calcination temperature
on the catalytic performance of the oxide was confirmed,
as already described by Xie et al. (2006). High yields of
94 wt% were obtained when the LDH was calcined at
550 C and the reaction was carried out at 120 C for 6 h
with a catalyst loading of 0.04 mg/l. The catalyst activity
decreased slightly in the first recycling stage but dropped
to only 60% of the original value after the fourth consecutive reaction cycle. However, the original activity could
be totally recovered by calcination of the spent catalyst
in air.
Antunes et al. (2008) catalyzed the methanolysis of
soybean oil with Mg/Al and Zn/Al oxides that were
obtained by calcination of the corresponding LDH at
450 C for 12 h. Transesterification was performed for
268
16. APPLICATIONS OF HETEROGENEOUS CATALYSTS IN THE PRODUCTION OF BIODIESEL
7 h at 70, 100 and 130 C with a methanol:oil molar ratio
of 55:1. The highest activity was detected at 130 C and
the yield at this temperature was 80% with MgO, 70%
with Mg/Al LDO, 63% with Zn/Al LDH, 30% with
ZnO, and 11% with Al2O3.
Ilgen et al. (2007) used LDOs derived from Mg6Al2(OH)16CO3$4H2O for the catalytic conversion of
canola oil into methyl esters. The LDH was prepared
by coprecipitation of magnesium and aluminum carbonate salts at pH 10 and ripened for 18 h. After separation,
the solid compound was dried at 80 C and then
calcined at 500 C for 16 h. The LDO with particle diameter of 150e177 mm gave a 63% ester yield when the reaction was carried out at 60 C with methanol:canola oil
molar ratio of 6:1. Higher molar ratios of 9:1, 12:1 and
16:1 decreased the ester yields to values lower than
60%, and when LDOs with other particle sizes (125,
125e150 and 150e177 mm) were used, the best performance was obtained in the range of 125e150 mm. In
the same report, the use of n-hexane as a cosolvent
was shown to be detrimental to methanolysis. Also,
methanol resulted in better ester yields than ethanol.
Barakos et al. (2008) calcined Mg/Al-CO3 at 350 C
for 6 h and tested it for methanolysis of cotton oil. Samples with 95% esters were obtained for reactions carried
out at 180 C, using methanol:oil molar ratios of 6:1 wt%
and 1 wt% of catalyst at 2200 kPa.
Albuquerque et al. (2008) prepared calcium and magnesium mixed layered hydroxides by coprecipitation
from which LDO catalysts were generated. The LDH
was calcined at 800 C and the resulting oxides were
tested in the catalytic methanolysis of sunflower oil at
60 C. Higher yields of 92.4% were obtained in methanolysis after 3 h using a methanol:oil molar ratio of 12:1 and
a 2.5 wt.% of the solid catalyst with a 3.8 Mg:Ca ratio.
Macedo et al. (2006) prepared (Al2O3)4(SnO) and
(Al2O3)4(ZnO) LDOs from the corresponding Sn/Al
and Zn/Al LDHs and both present catalytic activity in
the alcoholysis of soybean oil, even when branched alcohols were used. Yields higher than 80% were obtained
with methanol after 4 h at 60 C and the recycling tests
indicated that these materials did not lose their catalytic
activity.
Shumaker et al. (2008) used LDO catalysts to convert
soybean oil in methyl esters. The LDH precursors
(Mg/Al, Fe/Al and Li/Al) were obtained by coprecipitation and subsequently calcined at 450 C for 2 h. The
best catalytic performance was obtained with the oxide
derived from [LiAl2(OH)6](CO3)0,5.nH2O, reaching a
conversion of 83.1% in 2 h with a methanol:oil molar
ratio of 15:1. Under the same conditions, the LDO
derived from the Mg/Al precursor yielded only 13.6%
of products. The same catalysts were also tested in the
methanolysis of glyceryl tributyrate. The reactions
were carried out at 65 C under reflux for 1 h with
20 mmol of glyceryl tributyrate, 600 mmol of methanol
and 0.1 g of catalyst. The Li/Al LDO gave yields higher
than 98% while the LDOs with Mg/Al and Mg/Fe
yielded only 32% and 23.9%, respectively. These results
were close to the 37.1% yield that was achieved with
MgO under the same conditions. These authors also
observed the influence of the calcination temperature
on the catalytic performance and concluded that
the optimal temperature to obtain the best synthetic
LDOs is between 450 C and 500 C.
Ngamcharussrivichai et al. (2007) used CaO.ZnO
mixed oxides as heterogeneous catalysts for the methanolysis of palm kernel oil. A layered hydroxide formed
by a mixture of the divalent cations (Ca2þ and Zn2þ)
was coprecipitated in alkaline media. The mixed hydroxide was then subjected to calcination between 600 C
and 900 C for 2e6 h. Ester yields higher than 94%
were obtained with this catalyst after 1 h at 60 C using
a methanol:oil molar ratio of 30:1 and a catalyst loading
of 10 wt%. Also, the mixed oxide was shown to have a
Ca:Zn molar ratio of 0.25.
LDHs containing Zn/Al and Mg/Al with different
counterions (nitrate, chlorite and carbonate) and
M2þ/M3þ ratios were synthesized by Cordeiro et al.
(2012) and used as catalysts in the esterification of fatty
acids with methanol. The best conversion of 97 wt%
was obtained with Zn5AlCl for a reaction that was
carried at 140 C with a methanol:lauric acid molar ratio
of 6:1 and 2 wt% of the solid catalyst. However, all the
LDHs tested were converted in situ to layered carboxylates, which preserved their catalytic activity even after
several consecutive cycles of reuse.
LDH compounds containing Mg2þ, Ni2þ and Al3þ
were synthesized by Wang and Jehng (2011) and
calcined at 500 C for 10 h to produce heterogeneous
LDO catalysts for biodiesel production. The best condition for synthesis involved the use of a methanol:soybean oil molar ratio of 21, 0.3 wt% of catalyst, 105 C
and 1200 rpm for 4 h, when an 87% conversion of soybean oil to methyl esters was obtained. The observed
catalytic efficiency was related to the basicity and Mg
content of the Mg/Al/Ni catalysts.
Corma et al. (2005) also applied LDOs in the transesterification of monoesters with glycerol. LDHs were
initially calcined at 450 C under nitrogen flux for 8 h
to produce LDOs that were immediately rehydrated in
N2 atmosphere to avoid the presence of CO2. MgO
was also synthesized from magnesium oxalate by calcination at 500 C for 6 h and used as a control. The LDOs
containing Li/Al had a performance better than those
containing Mg/Al or MgO due to formation of stronger
Lewis basic sites, since Liþ ions, which are more electropositive than magnesium, increase the charge density of
the oxygen. Based on this, alumina was impregnated
with KF and the resulting material revealed basicity
269
POLYMERIC CATALYSTS
even higher than that of the Li/Al LDO. The catalytic
conversion of glycerol to glycerin oleate with KF/
Al2O3 was 98% with monoester selectivity of 69%.
Cordeiro et al. (2008) showed that the LHS zinc hydroxide nitrate [ZHN, Zn5(OH)8(NO3)2$2H2O] can be
used as a heterogeneous catalyst for the esterification
of fatty acids and for the transesterification of vegetable
oils. For the transesterification reaction carried out at
150 C for 2 h with 5 wt% of ZHN and a methanol:palm
oil molar ratio of 48:1, the resulting ester layer contained
95.7 wt% of methyl esters and the purity of the glycerin
layer was as high as 93 wt%. Also, when esterification
was carried at 140 C for 2 h with a methanol:lauric
acid molar ratio of 4:1, the final ester layer contained
97.4 wt% of methyl laurate. In addition, these authors
were able to demonstrate that ZHN turned into zinc
laurated(C12H23O2)2Zndduring the reaction course
and this new layered material was held responsible for
the observed catalytic activity.
LDHs containing Zn/Al and Mg/Al with different
counterions and M2þ/M3þ ratios were used as catalysts in the esterification of fatty acids with methanol.
All LDHs were synthesized by coprecipitation and
high conversion rates were obtained depending on the
reaction condition. For instance, a 97 wt% conversion
of lauric acid to methyl laurate was obtained using a
methanol:fatty acid molar ratio of 6:1 and 2 wt% of catalyst at 140 C for 2 h. However, all LDHs were also
converted in situ into layered carboxylates and this
new material was responsible for the observed catalytic
activity, which was preserved even after several consecutive cycles of reuse (Cordeiro et al., 2012).
Reinoso et al. (2012) used zinc carboxylates (acetate,
laurate, palmitate, stearate and oleate) as catalysts for
the methanolysis of soybean oil. Methyl ester conversions as high as 98 wt% were obtained for yields in the
range of 84 wt% when the reaction was carried out for
2 h with a 30:1 methanol:oil molar ratio and a catalyst
loading of 3 wt% in relation to the oil mass.
Jacobson et al. (2008) developed a solid catalyst by
immobilizing zinc stearate in silica using the solegel
method. The resulting solids contained 6 wt% of zinc
and a total available surface area of 35 m2/g. These
solids were shown to be catalytically active in the methanolysis of used frying oil with high acid number
(w15%). High yields of 98 wt% were obtained at
200 C for 10 h using a methanol:oil molar ratio of 18:1
and 3 wt% of catalyst in relation to the mass of the starting material.
Lisboa et al. (2012) described the synthesis and characterization of layered copper(II), manganese(II), lanthanum(III) and nickel(II) laurates as well as their catalytic
activity in the methyl and ethyl esterification of lauric
acid. Conversions between 80 wt% and 90 wt% were
observed for all catalysts when methanol was used for
esterification, whereas only manganese laurate gave a
reasonable catalytic activity of about 75 wt% with the
use of ethanol. In general, the best results were obtained
at temperatures around 140 C. Also, the structure of
copper(II) and lanthanum(III) laurates was shown to
be preserved after three consecutive reaction cycles.
POLYMERIC CATALYSTS
This section describes the application of functionalized polymers as catalysts for esterification and transesterification reactions to produce biodiesel. Polymeric
catalysts consist of functionalized polymeric matrixes
or polymeric matrixes that can be used as solid supports
for a variety of catalysts, constituting catalytic systems
(Coutinho et al., 2004a,b; Guerreiro et al., 2010; Lee
and Saka, 2010; Zieba et al., 2010). These materials
have long been studied as heterogeneous catalysts in
systems that traditionally employ acid or basic homogeneous catalysts.
Biodiesel production can be carried out in the presence of different types of catalysts. In the specific case
of polymeric catalysts belonging to the class of functionalized polymers, their use in biodiesel synthesis has
focused on acid catalysts, such as in the case of ion
exchange resins (Ma and Hanna, 1999; Guerreiro et al.,
2006; Knothe et al., 2006; Soldi et al., 2009; Rezende
et al., 2008; Helwani et al., 2009b; Lee and Saka, 2010).
Acid-catalyzed triacylglycerol transesterification is
not commercially applied as often as catalysis in basic
medium because acid catalysis in homogeneous medium is around 4000 times slower than the basecatalyzed reaction. However, acid catalysts can perform
esterification and transesterification simultaneously,
producing biodiesel directly from oils with high acid
number. These oils are not suitable for biodiesel production via alkaline catalysis, since the FFAs promptly react
with the base, generating soaps that make the separation
between the ester and glycerin difficult during the
washing step (Bondioli et al., 1995; Schuchardt et al.,
1998; Ma and Hanna, 1999; Vicente et al., 2004; Lotero
et al., 2005; Meher et al., 2006; Rezende et al., 2008; Lee
and Saka, 2010).
Several acid catalysts can be used in alcoholysis, especially sulfonic and sulfuric acids (Hayyan et al., 2011).
Although these catalysts afford high yields of mono
alkyl esters, they require high temperatures and long
reaction times to achieve a satisfactory conversion
rate. Another disadvantage is that residual acid catalysts can contaminate the fuel and attack the metal
parts of the engine, corroding it. To avoid this situation,
acid catalysts must be completely eliminated from the
final product, which demands many purification steps
(Canakci and Gerpen, 1999).
270
16. APPLICATIONS OF HETEROGENEOUS CATALYSTS IN THE PRODUCTION OF BIODIESEL
Some types of organic polymers and ion exchange
resins can be used as polymeric catalysts, behaving as
heterogeneous catalysts for esterification and transesterification reactions. Heterogeneous catalysts such as
these reduce the number of biodiesel purification steps,
facilitates catalyst reuse, and decreases production costs
(Schuchardt et al., 1998; Choudary et al., 2000; Fukuda
et al., 2001; Harmer and Sun, 2001; Ramos et al., 2003;
Abreu et al., 2004; Suppes et al., 2004; Chouhan and
Sarma, 2011).
The pioneering studies of Merrifield on the solidphase synthesis of polypeptides and subsequent works
have shown that polystyrene (PS) is a suitable polymeric
support for catalysts and reagents (Merrifield, 1963;
Fréchet, 1981). Styrene and divinylbenzene (DVB) are
among the monomers that are most often employed
to prepare solid polymeric matrixes. Polystyreneco-divinylbenzene (PS-DVB), an insoluble copolymer,
results from styrene polymerization in the presence of
varied amounts of DVB. The characteristics of this
copolymer depend on the quantity of DVB present in
the material (Kapura and Gates, 1973; Xia et al., 2012).
In many cases, the success of a heterogeneous
catalyst relies on the features of the polymeric material.
Bergbreiter (2002) proposed that some physicochemical
properties should be considered when choosing the
catalyst support, including the catalytic activity, surface
area, porosity, and thermal and mechanical stability of
the material in the conditions of the catalyzed reaction
(Bergbreiter, 2002; Chouhan and Sarma, 2011).
In the field of polymer chemistry, the term resin is
indistinctly employed to describe polymers with and
without cross-links (Akelah and Sherrington, 1981;
Sharma, 1995). Alternatively, in heterogeneous catalysis,
the term resin refers to species consisting of long
polymeric chains interconnected via cross-links, the
so-called polymeric matrix. Polymeric matrixes are
tridimensional, insoluble, and porous; their ability to exchange ions arises from the introduction of adequate
functional groups into the polymeric support (Kunin
et al., 1962; Akelah and Sherrington, 1981; Fréchet,
1981; Harmer and Sun, 2001; Hart et al., 2002).
Polymeric matrix functionalization can be achieved in
two ways: (1) monomers containing the desired functional groups (or precursors of this functional group)
can be directly polymerized; (2) the polymeric support
can be prepared first, and the functional group is introduced by chemical modification of the polymeric support (Molinari et al., 1979; Kucera and Jancar, 1998;
Harmer and Sun, 2001).
Coutinho and Rezende (2001) and Coutinho et al.
(2004a) showed that supported species can be prepared
by chemically modifying the copolymer base (polymeric
support). These authors reported the sulfonation of a support consisting of PS reticulated with DVB (Figure 16.7).
The aromatic rings on the insoluble PS-DVB copolymer
react with concentrated sulfuric acid in the presence of
1,2-dichloroethane; the latter compound expands the
polymeric matrix and allows sulfonation of the internal
surface as well. Most of the functional groups introduced
into polymeric matrices concentrate inside the resin beads
(Coutinho and Rezende, 2001).
Cationic resins can be used as an option for catalytic
reactions involving mineral or sulfonic acids. In the
presence of water, the cationic groups on the polymer
display different acidity constants, as in the case of compounds with low molecular mass.
The catalytic performance of an ion exchange resin is
associated with the concentration of functional groups
and the physicochemical properties of the support.
Therefore, compared with homogeneous catalysts,
different factors affect the activity of resins.
The use of ion exchange resins as catalysts has
many advantages: (1) despite being equivalent to strong
SO3H
CH2 – CH CH2 – CH CH2
CH2 – CH CH2 – CH CH2
+ H 2SO4
C H2
C H CH2 – CH CH 2
SO3H
ClCH2CH2Cl
94ºC/3,5 h
C H2
C H CH2 – CH CH2
SO3H
FIGURE 16.7
Sulfonation of a polymeric matrix consisting of polystyrene and divinylbenzene.
271
POLYMERIC CATALYSTS
FIGURE 16.8
mineral acids, resins are less oxidizing and corrosive,
since most of the catalytic sites are located inside the
beadsdtherefore, they do not pose any hazards to the
operator and are easy to store; (2) resins behave as selective catalysts and enable reaction control; (3) catalysts
with high purity are recovered at the end of the reaction
by simple filtration; (4) resins do not require neutralization before being separated from the reaction medium, a
step that usually reduces product yield; (5) resins eliminate the steps and equipment necessary to separate the
catalyst and purify the product, simplifying continuous
or batch procedures based on ion exchange resins; and
(6) if the resins undergo deactivation due to contamination or prolonged use, they can be reactivated via a simple procedure that does not release hazardous gases into
the atmosphere (Saha and Sharma, 1996; Coutinho and
Rezende, 2001; Harmer and Sun, 2001; Marquardt
and Eifler-Lima, 2001; Mitsutani, 2002; Coutinho et al.,
2003, 2004a,b; Kiss et al., 2006).
The main drawback of ion exchange resins is that
their maximum operation temperature is relatively
low. Literature suggests that they should be used below
125 C to ensure long catalyst lifetime (John and Israelstam, 1960; Akelah and Sherrington, 1981; Giménez
et al., 1987; Coutinho and Rezende, 2001; Rezende
et al., 2008).
Aromatic compounds are easy to functionalize, especially if they contain acid groups like sulfonic acids. The
sulfonation of organic compounds containing benzene
rings, including polymers, has been extensively reported (Ma and Hanna, 1999; Coutinho and Rezende,
2001; Coutinho et al., 2003, 2004a,b, 2006; Rezende
et al., 2008; Soldi et al., 2009). Figure 16.8 represents
the sulfonation PS (Soldi et al., 2009).
Sulfonation significantly modifies the physical properties of linear PS, especially the polarity. Hence, sulfonated PS should remain insoluble during biodiesel
production. Soldi et al. (2009) studied methods to sulfonate linear PS and applied the resulting sulfonated
material as heterogeneous polymeric catalyst to produce
soybean methyl esters. Raw materials with different
moisture degrees and the effect of different variables
on the conversion rate have been investigated; biodiesel
production from soybean oil and beef tallow led to
significantly improved yields.
Recently, much interest has been taken in utilizing
low-cost plant oil and fat containing a large amount of
PS sulfonation with acetyl sulfate.
FFAs. However, oils with high FFA content are difficult
to transesterify using the commercially available alkaline catalyst (Zhang et al., 2003; Tesser et al., 2005;
Marchetti and Errazu, 2008; Sharma et al., 2008; Liu
et al., 2009; Tesser et al., 2010; Chouhan and Sarma,
2011; Shahid and Jamal, 2011; Borges and Dı́az, 2012).
Canakci and Van Gerpen (1999, 2001) found the transesterification would not occur if the FFA content in the oil
was beyond 3%. According to the research paper by
Kouzu et al. (2011), the promising approach is to esterify
FFA into FAMEs with the help of the solid acid catalyst,
and there were some research papers studying utilization of several types of heterogeneous catalysts
including sulfonated cation exchange resin (Russnueldt
and Hoelderich, 2009; Tesser et al., 2010; Kouzu et al.,
2011). With respect to utilization of the sulfonated
resin for the preesterification of FFA, some researchers
focused their attention on the macroreticular type but
the use of two types of resins (macroreticular and gelular
types) were also studied by other authors (Ramadhas
et al., 2005; Soldi et al., 2009; Lam et al., 2010; Melero
et al., 2010; Kouzu et al., 2011; Semwal et al., 2011;
Li et al., 2012; Xia et al., 2012; Zhang et al., 2012a,b).
Feng et al. (2011) investigated the continuous esterification of FFAs from acidified oil with methanol by cation
exchange resin in a fixed bed reactor to prepare biodiesel
and the operational stability of continuous esterification
by resin in the fixed bed reactor was also conducted
(McNeff et al., 2008; Shibasaki-Kitagawa et al., 2010;
Feng et al., 2011; Cheng et al., 2012).
According to Feng et al. (2010), from the viewpoint of
cost savings, the use of cation exchange resins in heterogeneous catalytic processes may be advantageous over
enzymes and supercritical methanol (Feng et al., 2010).
These resins are composed of copolymers of DVB and
styrene containing sulfonic acid groups attached to benzene rings and these are the active sites for esterification
and transesterification (Marchetti and Errazu, 2008;
Rezende et al., 2008; Russnueldt and Hoelderich, 2009;
Feng et al., 2010; Kouzu et al., 2011). However, other sulfonated polymeric backbones such as AmberlystÒ , DowexÒ and NafionÒ , a perfluorinated ion exchange resin,
all of them having a very strong Brønsted acid character,
have also been used in these type of reactions (Özbay
et al., 2008; Talukder et al., 2009; Feng et al., 2010;
Park et al., 2010; Galia et al., 2011; Yin et al., 2012; Zhang
et al., 2012a). In general, cation exchange resins are
272
16. APPLICATIONS OF HETEROGENEOUS CATALYSTS IN THE PRODUCTION OF BIODIESEL
preferable for esterification (Giménez et al., 1987; Chen
et al., 1999; Coutinho et al., 2004b; Coutinho et al.,
2006; Grob and Hasse, 2006), while anionic resins may
be applied for transesterification of oils and fats (Shibasaki-Kitakawa et al., 2007; Ren et al., 2012).
CONCLUDING REMARKS
Truly heterogeneous catalytic processes are attractive
for many practical applications due to their recyclability,
structural stability, high selectivity and good catalytic
performance. However, all these properties are hardly
achievable in a single catalytic system. In most cases,
leachable catalytic species migrate to the reaction environment, causing a partial contamination of the final
product as well as a loss in catalytic activity when the
solids are applied in several consecutive reaction cycles.
Moreover, in many situations found in the literature, the
heterogeneity of catalytic systems is not approached
with proper analytical methods, resulting in wrong
conclusions and/or classification of the proposed solid
catalyst. These usually arise from poor data on catalyst
recovery and reuse, poor characterization of the catalyst
structure and high leaching levels of catalytic species.
Also, in many cases, no attempt is made to fully
characterize these properties and solids are classified
as heterogeneous catalysts just because they are partially
filterable after reaction completion. One of such flaws
was nicely demonstrated by Silva et al. (2013) using
bismuth-containing mixed oxides. Apart from these observations, the lack of suitable reaction controls such
as in the case of TC in esterification reactions reveal
unrealistic catalytic performance in reactions that are
known to be autocatalytic under appropriate experimental conditions. Nevertheless, a number of rather
attractive heterogeneous catalytic systems have been
discovered so far for biodiesel applications, probably
due to the wide scope of catalytic properties that are
influential in both esterification and transesterification.
However, many of these will never be able to reach industrial applications because their benchmarking was
never strong enough to support further investments at
large scale.
References
Abreu, F.R., Lima, D.G., Hamú, E.H., Wolf, C., Suarez, P.A.Z., 2004.
Utilization of metal complexes as catalysts in the transesterification
of Brazilian vegetable oils with different alcohols. J. Mol. Catal. A:
Chem. 209, 29e33.
Ajaikumar, S., Pandurangan, A., 2007. Esterification of alkyl acids
with alkanols over MCM-41 molecular sieves: influence of
hydrophobic surface on condensation reaction. J. Mol. Catal. A:
Chem. 266, 1e10.
Akelah, A., Sherrington, D., 1981. Application of functionalized
polymers in organic synthesis. Chem. Rev. 81, 557e587.
Albuquerque, M.C.G., Santamarı́a-González, J., Mérida-Robles, J.M.,
Moreno-Tost, R., Rodrı́guez-Castellón, E., Jiménez-López, A.,
Azevedo, D.C.S., Cavalcante Jr., C.L., Maireles-Torres, P., 2008.
MgM (M ¼ Al and Ca) oxides as basic catalysts in transesterification processes. Appl. Catal. A: Gen. 347, 162e168.
Altiparmak, D., Keskin, A., Koca, A., Gürü, M., 2007. Alternative fuel
properties of tall oil fatty acid methyl esterediesel fuel blends.
Bioresour. Technol. 98, 241e246.
Antunes, W.M., Veloso, C.O., Henriques, C.A., 2008. Transesterification of soybean oil with methanol catalyzed by basic
solids. Catal. Today 133e135, 548e554.
Arizaga, G.G.C., Satyanarayana, K.G., Wypych, F., 2007. Layered
hydroxide salts: synthesis, properties and potential applications.
Solid State Ionics 178, 1143e1162.
Arizaga, G.G.C., Mangrich, A.S., Gardolinski, J.E.F.C., Wypych, F.,
2008. Chemical modification of zinc hydroxide nitrate and Zn/
Al-layered double hydroxide with dicarboxylic acids. J. Colloid
Interface Sci. 320, 68e176.
Bail, A., Santos, V.C., Freitas, M.R., Ramos, L.P., Schreiner, W.H.,
Ricci, G.P., Ciuffi, K.J., Nakagaki, S., 2013. Investigation of a molybdenum-containing silica catalyst synthesized by the sol-gel
process in heterogeneous catalytic esterification reactions using
methanol and ethanol. Appl. Catal. B: Environ. 130e131, 314e324.
Barakos, N., Pasias, S., Papayannakos, N., 2008. Transesterification of
triglycerides in high and low quality oil feeds over an HT2
hydrotalcite catalyst. Bioresour. Technol. 99, 5037e5042.
Barman, S., Vasudevan, S., 2006. Melting of saturated fatty acid soaps.
J. Phys. Chem. B 110, 22407e22414.
Beck, J.S., Vartuli, J.C., Roth, W.J., Leonowicz, M.E., Kresge, C.T.,
Schmitt, K.D., Chu, C.T.W., Olson, D.H., Sheppard, E.W., 1992. A
new family of mesoporous molecular sieves prepared with liquid
crystal templates. J. Am. Chem. Soc. 114, 10834e10843.
Bergbreiter, D.E., 2002. Using soluble polymers to recover catalysts
and ligands. Chem. Rev. 102, 3345e3384.
Bokade, V.V., Yadav, G.D., 2009. Transesterification of edible and
nonedible vegetable oils with alcohols over heteropolyacids
supported on acid-treated clay. Ind. Eng. Chem. Res. 48,
9408e9415.
Bondioli, P., Gasparolia, A., Fedeli, E., Veronese, S., Sala, M., 1995.
Storage stability of biodiesel. J. Am. Oil Chem. Soc. 72, 669e702.
Borges, M.E., Dı́az, L., 2012. Recent developments on heterogeneous
catalysts for biodiesel production by oil esterification and transesterification reactions: a review. Renewable Sustainable Energy
Rev. 16, 2839e2849.
Bravo-Suárez, J.J., Páez-Mozo, E.A., Oyama, S.T., 2004. Review of the
synthesis of layered double hydroxides: a thermodynamic
approach. Quim. Nova 27, 601e614.
Brindley, G.W., Brown, G., 1980. Crystal structures of clay minerals
and their X-ray identification. Min. Soc. Lond. 2e115.
Caetano, C.S., Fonseca, I.M., Ramos, A.M., Vital, J., Castanheiro, J.E.,
2008. Esterification of free fatty acids with methanol using heteropolyacids immobilized on silica. Catal. Commun. 9, 1996e1999.
Canakci, M., Gerpen, J.V., 1999. Biodiesel production via acid catalysis.
Trans. Am. Soc. Agric. Eng. 42, 1203e1210.
Canakci, M., Gerpen, J.V., 2001. Biodiesel production from oils and
fats with high free fatty acids. Trans. Am. Soc. Agric. Eng. 44,
1429e1436.
Cantrell, D.G., Gillie, L.J., Lee, A.F., Wilson, K., 2005. Structurereactivity correlations in MgAl hydrotalcite catalysts for biodiesel
synthesis. Appl. Catal. A: Chem. 287, 183e190.
Carlino, S., 1997. The intercalation of carboxylic acids into layered
double hydroxides: a critical evaluation and review of the different
methods. Solid State Ionics 98, 73e84.
REFERENCES
Carmo Jr., A.C., Souza, L.K.C., Costa, C.E.F., Longo, E., Zamian, J.R.,
Rocha Filho, G.N., 2009. Production of biodiesel by esterification of
palmitic acid over mesoporous aluminosilicate Al-MCM-41. Fuel
88, 461e468.
Centi, G., Perathoner, S., 2008. Catalysis by layered materials: a review.
Microporous Mesoporous Mater. 107, 3e15.
Chen, X., Xu, Z., Okuhara, T., 1999. Liquid-phase esterification of
acrylic acid with 1-butanol catalyzed by solid acid catalysis. Appl.
Catal. A: Gen. 180, 261e269.
Cheng, Y., Feng, Y., Ren, Y., Liu, X., Gao, A., He, B., Yan, F., Li, J., 2012.
Comprehensive kinetic studies of acidic oil continuous esterification by cation-exchange resin in fixed bed reactors. Bioresour.
Technol. 113, 65e72.
Cho, Y.B., Seo, G., 2010. High activity of acid-treated quail eggshell
catalyst in the transesterification of palm oil with methanol.
Bioresour. Technol. 101, 8515e8519.
Choudary, B.M., Kantam, M.L., Reddy, Ch.V., Aranganathan, S.,
Santhi, P.L., Figueras, F., 2000. Mg-Al-O-t-Bu hydrotalcite: a new
and efficient heterogeneous catalyst for transesterification. J. Mol.
Catal. A: Chem. 159, 411e416.
Chouhan, A.P.S., Sarma, A.K., 2011. Modern heterogeneous catalysts
for biodiesel production: a comprehensive review. Renewable
Sustainable Energy Rev. 15, 4378e4399.
Climent, M.J., Corma, A., Iborra, S., Miguel, S., Primo, J., Rey, F.,
1999. Mesoporous materials as catalysts for the production of
chemicals: synthesis of alkyl glucosides on MCM-41. J. Catal 183,
76e82.
Cordeiro, C.S., Arizaga, G.G.C., Ramos, L.P., Wypych, F., 2008. A new
zinc hydroxide nitrate heterogeneous catalyst for the esterification
of free fatty acids and the transesterification of vegetable oils.
Catal. Commun. 9, 2140e2143.
Cordeiro, C.S., Silva, F.R., Wypych, F., Ramos, L.P., 2011. Catalisadores
Heterogêneos para a Produção de Monoésteres Graxos (biodiesel).
Quim. Nova 34, 477e486.
Cordeiro, C.S., Silva, F.R., Marangoni, R., Wypych, F., Ramos, L.P.,
2012. LDHs instability in esterification reactions and their conversion to catalytically active layered carboxylates. Catal. Lett. 142,
763e770.
Corma, A., Garcia, H., Iborra, S., Primo, J., 1989. Modified faujasite
zeolites as catalysts in organic reactions: esterification of carboxylic
acids in the presence of HY zeolites. J. Catal. 120, 78e87.
Corma, A., Hamid, S.B.A., Iborra, S., Velty, A., 2005. Lewis and
Brönsted basic active sites on solid catalysts and their role in the
synthesis of monoglycerides. J. Catal. 234, 340e347.
Coutinho, F.M.B., Rezende, S.M., 2001. Catalisadores sulfônicos
imobilizados em polı́meros: Sı́ntese, caracterização e avaliação.
Polı́meros 11, 222e233.
Coutinho, F.M.B., Aponte, M.L., Barbosa, C.C.R., Costa, V.G.,
Lachter, E.R., Tabak, D., 2003. Resinas sulfônicas: Sı́ntese, caracterização e avaliação em reações de alquilação. Polı́meros 13,
141e146.
Coutinho, F.M.B., Cunha, L., Gomes, A.S., 2004a. Suportes poliméricos
para catalisadores sulfônicos: Sı́ntese e caracterização. Polı́meros
14, 31e37.
Coutinho, F.M.B., Souza, R.R., Gomes, A.S., 2004b. Synthesis, characterization and evaluation of sulfonic resins as catalysts. Eur. Polym.
J. 40, 1525e1532.
Coutinho, F.M.B., Rezende, S.M., Soares, B.G., 2006. Characterization of
sulfonated poly(styrene-divinylbenzene) and poly(divinylbenzene)
and its application as catalysts in esterification reaction. J. Appl.
Polym. Sci. 102, 3616e3627.
Crepaldi, E.L., Valim, J.B., 1998. Hidróxidos duplos lamelares: sı́ntese,
estrutura, propriedades e aplicações. Quim. Nova 21, 300e311.
Csicsery, S.M., 1984. Shape-selective catalysis in zeolites. Zeolites 4,
202e213.
273
Cundy, C.S., Cox, P.A., 2003. The hydrothermal synthesis of zeolites:
history and development from the earliest days to the present time.
Chem. Rev. 103, 663e701.
Di Serio, M., Tesser, R., Pengmei, L., Santacesaria, E., 2008. Heterogeneous catalysts for biodiesel production. Energy Fuels 22,
207e217.
Drago, R.S., Dias, J.A., Maier, T.O., 1997. An acidity scale for Brønsted
acids including H3PW12O40. J. Am. Chem. Soc. 119, 7702e7710.
Feng, Y., He, B., Cao, Y., Li, J., Liu, M., Yan, F., Liang, X., 2010. Biodiesel
production using cation-exchange resin as heterogeneous catalyst.
Bioresour. Technol. 101, 1518e1521.
Feng, Y., Zhang, A., Li, J., He, B., 2011. A continuous process for
biodiesel production in a fixed bed reactor packed with cationexchange resin as heterogeneous catalyst. Bioresour. Technol. 102,
3607e3609.
Figueiredo, J.L., Ribeiro, F.R., 1987. Catálise Heterogénea, first ed.
Fundação Calouste Gulbenkian, Portugal. p. 1.
Fjerbaek, L., Christensen, K.V., Norddahl, B., 2009. A review of the
current state of biodiesel production using enzymatic transesterification. Biotechnol. Bioeng. 102, 1298e1315.
Fréchet, J.M.J., 1981. Synthesis and applications of organic polymers as
supports and protecting groups. Tetrahedron 37, 663e683.
Fukuda, H., Kondo, A., Noda, A., 2001. Biodiesel fuel production by
transesterification of oil. J. Biosci. Bioeng. 92, 405e416.
Galia, A., Scialdone, O., Tortorici, E., 2011. Transesterification of
rapeseed oil over acid resins promoted by supercritical carbon
dioxide. J. Supercrit. Fluids 56, 186e193.
Giménez, J., Costa, J., Cervera, S., 1987. Vapor-phase esterification of aceticacid with ethanol catalyzed by a macroporous sulfonated styrenedivinilbenzene (20%) resin. Ind. Eng. Chem. Res. 26, 198e2002.
Giri, B.Y., Rao, K.N., Prabhavathi Devi, B.L.A., Lingaiah, N.,
Suryanarayana, I., Prasad, R.B.N., Sai Prasad, P.S., 2005. Esterification of palmitic acid on the ammonium salt of 12-tungstophosphoric
acid: the influence of partial proton exchange on the activity of the
catalyst. Catal. Commun. 6, 788e792.
Grob, S., Hasse, H., 2006. Reaction kinetics of the homogeneously
catalyzed esterification of 1-butanol with acetic acid in a wide
range of initial compositions. Ind. Eng. Chem. Res. 45, 1869e1874.
Guerreiro, L., Castanheiro, J.E., Fonseca, I.M., Martin-Aranda, R.M.,
Vital, J., 2006. Transesterification of soybean oil over sulfonic acid
functionalized polymer membranes. Catal. Today 118, 166e171.
Guerreiro, L., Pereira, P.M., Fonseca, I.M., Martin-Aranda, R.M.,
Ramos, A.M., Dias, J.M.L., Oliveira, R., Vital, J., 2010. PVA
embedded hydrotalcite membranes as basic catalysts for biodiesel
synthesis by soybean oil methanolysis. Catal. Today 156, 191e197.
Harmer, M.A., Sun, Q., 2001. Solid acid catalysis using ion-exchange
resins. Appl. Catal. A: Gen. 221, 45e62.
Hart, M., Fuller, G., Brown, D.R., Dale, J.A., Plant, S., 2002. Sulfonated
poly(styrene-co-divinilbenzene) ion-exchange resins: acidities and
catalystic activities in aqueous reactions. J. Mol. Catal. A: Chem.
182e183, 439e445.
Hayyan, A., Mjalli, F.S., Hashim, M.A., Hayyan, M., AlNashef, I.M.,
Al-Zahrani, S.M., Al-Saadi, M.A., 2011. Ethanesulfonic acid-based
esterification of industrial acidic crude palm oil for biodiesel production. Bioresour. Technol. 102, 9564e9570.
Helwani, Z., Othman, M.R., Aziz, N., Kim, J., Fernando, W.J.N., 2009a.
Solid heterogeneous catalysts for transesterification of triglycerides
with methanol: a review. Appl. Catal. A: Gen. 363, 1e10.
Helwani, Z., Othman, M.R., Aziz, N., Fernando, W.J.N., Kim, J., 2009b.
Technologies for production of biodiesel focusing on green catalytic techniques: a review. Fuel Process. Technol. 90, 1502e1514.
Hu, C., Li, D., 2004. Polyoxometalate complexes of layered double
hydroxides. In: Wypych, F., Satyanarayana, K.G. (Eds.), Clay
Surfaces: Fundamentals and Applications, Interface Science and
Technology, first ed., vol. 1. Elsevier Academic Press, pp. 389e390.
274
16. APPLICATIONS OF HETEROGENEOUS CATALYSTS IN THE PRODUCTION OF BIODIESEL
Ilgen, O., Dinçer, I., Yildiz, M., Alptekin, E., Boz, N., Canakci, M.,
Akin, A.N., 2007. Investigation of biodiesel production from canola
oil using Mg/Al hydrotalcite catalysts. Turk. J. Chem. 31, 509e514.
Jacobson, K., Gopinath, R., Meher, L.C., Dalai, A.K., 2008. Solid acid
catalyzed biodiesel production from waste cooking oil. Appl.
Catal. B: Environ. 85, 86e91.
John, E.V.O., Israelstam, S.S., 1960. Use of cation exchange resin in
organic reaction. J. Org. Chem. 26, 240.
Kapura, J.M., Gates, B.C., 1973. Sulfonated polymers as alkylation
catalysts. Ind. Eng. Chem. Prod. Res. Dev. 12, 62e67.
Kusdiana, D., Saka, S., 2001. Fuel 80, 693e698.
Kiss, A.A., Dimin, A.C., Rothenberg, G., 2006. Solid acid catalysts for
biodiesel productiondtowards sustainable energy. Adv. Synth.
Catal. 348, 75e81.
Knothe, G., Krahl, J., Gerpen, J.V., Ramos, L.P. (Eds.), 2006. Manual de
biodiesel. Edgard Blücher, São Paulo, pp. 1e352.
Konwar, D., Gogoi, P.K., Gogoi, P., Borah, G., Baruah, R., Hazarika, N.,
Borgohain, R., 2008. Esterification of carboxylic acids by acid
activated kaolinite clay. Indian J. Chem. Technol. 15, 75e78.
Kouzu, M., Nakagaito, A., Hidaka, J., 2011. Pre-esterification of FFA in
plant oil transesterified into biodiesel with the help of solid acid
catalysis of sulfonated cation-exchange resin. Appl. Catal. A: Gen.
405, 36e44.
Kucera, F., Jancar, J., 1998. Homogenous and heterogeneous sulfonation of polymers: a review. Polym. Eng. Sci. 38, 1e12.
Kunin, R., Meitzner, E., Bortinick, N., 1962. Macroreticular ion exchange resins. J. Am. Chem. Soc. 84, 305e306.
Lacouture, F., Peultier, J., Francois, M., Steinmetz, J., 2000. Anhydrous
polymeric zinc(II) octanoate. Cryst. Struct. Commun. 56, 556e557.
Lam, M.K., Lee, K.T., Mohamed, A.R., 2010. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free
fatty acid oil (waste cooking oil) to biodiesel: a review. Biotechnol.
Adv. 28, 500e518.
Lee, J.S., Saka, S., 2010. Biodiesel production by heterogeneous catalysts
and supercritical technologies. Bioresour. Technol. 101, 7191e7200.
Leng, Y., Wang, J., Zhu, D.R., Wu, Y.J., Zhao, P.P., 2009. Sulfonated
organic heteropolyacid salts: recyclable green solid catalysts for
esterifications. J. Mol. Catal. A: Chem. 313, 1e6.
Li, G., Ding, Y., Wang, J., Wang, X., Suoa, Jishuan, 2007. New progress
of Keggin and WellseDawson type polyoxometalates catalyze acid
and oxidative reactions. J. Mol. Cat. A: Chem. 262, 67e76.
Li, J., Fu, Y.-J., Qu, X.-J., Wang, W., Luo, M., Zhao, C.-J., Zu, Y.-G., 2012.
Biodiesel production from yellow horn (Xanthoceras sorbifolia
Bunge) seed oil using ion exchange resin as heterogeneous catalyst.
Bioresour. Technol. 108, 112e118.
Lisboa, F.S., Gardolinski, J.E.F.C., Cordeiro, C.S., Wypych, F., 2012.
Layered metal laurates as active catalysts in the Methyl/Ethyl
esterification reactions of lauric acid. J. Braz. Chem. Soc. 23,
46e56.
Liu, Y., Lotero, E., Goodwin Jr., J.G., MO, X., 2007. Transesterification
of poultry fat with methanol using MgeAl hydrotalcite derived
catalysts. Appl. Catal. A: Gen. 331, 138e148.
Liu, Y., Wang, L., Yan, Y., 2009. Biodiesel synthesis combining preesterification with alkali catalyzed process from rapeseed oil
deodorizer distillate. Fuel Process. Technol. 90, 857e862.
Lopez, D.E., Suwannakarn, K., Bruce, D.A., Goodwin Jr, J.G., 2007.
Esterification and transesterification on tungstated zirconia: effect
of calcination temperature. J. Catal. 247, 43e50.
Lopez-Granados, M., Alba-Rubio, A.C., Vila, F., Martı́n, A.D.,
Marisacl, R., 2010. Surface chemical promotion of Ca oxide catalyst
in biodiesel production reaction by the addition of monoglycerides, diglycerides and glycerol. J. Catal. 276, 229e236.
Lotero, E., Liu, Y., Lopez, D.E., Suwannakarn, K., Bruce, D.A.,
Goodwin, J.G., 2005. Synthesis of biodiesel via acid catalysis. Ind.
Eng. Chem. Res. 44, 5353e5363.
Luque, R., Campelo, J.M., Luna, D., Marinas, J.M., Romero, A.A., 2007.
Catalytic performance of Al-MCM-41 materials in the N-alkylation
of aniline. J. Mol. Catal. A: Chem. 269, 190e196.
Ma, F., Hanna, M.A., 1999. Biodiesel production: a review. Bioresour.
Technol. 70, 1e15.
Ma, Y., Wang, Q.L., Yan, H., Ji, X., Qiu, Q., 1996. Zeolite-catalyzed
esterification I. Synthesis of acetates, benzoates and phthalates.
Appl. Catal. A: Gen. 139, 51e57.
Macedo, C.C.S., Abreu, F.R., Tavares, A.P., Alves, M.B., Zara, L.F.,
Rubim, J.C., Suarez, P.A.Z., 2006. New heterogeneous metal-oxides
based catalyst for vegetable oil trans-esterification. J. Braz. Chem.
Soc. 17, 1291e1296.
Marchetti, J.M., Errazu, A.F., 2008. Comparison of different heterogeneous catalysts and different alcohols for the esterification reaction
of oleic acid. Fuel 87, 3477e3480.
Marquardt, M., Eifler-Lima, V.L., 2001. A sı́ntese em fase sólida e
seus suportes poliméricos mais empregados. Quim. Nova 24,
846e855.
Martinez, C., Corma, A., 2011. Inorganic molecular sieves: preparation, modification and industrial application in catalytic processes.
Coord. Chem. Rev. 255, 1558e1580.
McNeff, V.C., McNeff, C.L., Yan, B., Nowlan, D.T., Rasmussen, M.,
Gyberg, A.E., Krohn, B.J., Fedie, R.L., Hoye, T.R., 2008. A continuous catalytic system for biodiesel production. Appl. Catal. A:
Gen. 343, 39e48.
Meher, L.C., Vidya, D., Naik, S.N., 2006. Technical aspects of biodiesel
production by transesterificationda review. Renewable Sustainable Energy Rev. 10, 248e268.
Melero, J.A., Bautista, L.F., Morales, G., Iglesias, J., SánchezVázquez, R., 2010. Biodiesel production from crude palm oil using
sulfonic acid-modified mesostructured catalysts. Chem. Eng. J.
161, 323e331.
Merrifield, R.B., 1963. Solid phase synthesis. I. The synthesis of a
tetrapeptide. J. Am. Chem. Soc. 85, 2149e2154.
Mitsutani, A., 2002. Future possibilities of recently commercialized
acid/base-catalyzed chemical processes. Catal. Today 73, 57e63.
Mittelbach, Tritthart, M.P., 1988. Diesel fuel derived from vegetable
oils, III. Emission tests using methyl ester of used frying oil. J. Am.
Oil Chem. Soc. 65, 1185e1187.
Mizuno, N., Misono, M., 1998. Heterogeneous catalysis. Chem. Rev.
98, 199e217.
Molinari, H., Montanari, F., Quici, S., Tundo, P., 1979. Polymersupported phase-transfer catalysts. High catalytic activity of
ammonium and phosphonium quaternary salts bonded to a
polystyrene matrix. J. Am. Chem. Soc. 1001, 3920e3927.
Moronta, A., 2004. In: Wypych, F., Satyanarayana, K.G. (Eds.), Clay
SurfacesdFundamentals and Applications. Elsevier, pp. 321e344.
Nakagaki, S., Bail, A., Santos, V.C., Souza, V.H.R., Vrubel, H.,
Nunes, F.S., Ramos, L.P., 2008. Use of anhydrous sodium molybdate as an efficient heterogeneous catalyst for soybean oil methanolysis. Appl. Catal. A: Gen. 351, 267e274.
Nascimento, L.A.S., Tito, L.M.Z., Angelica, R.S., Costa, C.E.F.,
Zamian, J.R., Rocha, G.N., 2011. Esterification of oleic acid over
solid acid catalysts prepared from Amazon flint kaolin. Appl.
Catal. B: Environ. 101, 495e503.
Neji, S.B., Trabelsi, M., Frikha, M.H., 2011. Esterification of fatty acid
over Tunisian acid activated clay: kinetic study. J. Oleo Sci. 60,
293e299.
Ngamcharussrivichai, C., Wiwatnimit, W., Wangnoi, S., 2007. Modified dolomites as catalysts for palm kernel oil transesterification.
J. Mol. Cat. A: Chem. 276, 24e33.
Nijhuis, T.A., Beers, A.E.W., Kapteijn, F., Moulijn, J.A., 2002.
Water removal by reactive stripping for a solid-acid catalyzed
esterification in a monolithic reactor. Chem. Eng. Sci. 57,
1627e1632.
REFERENCES
Oliveira, C.F., Dezaneti, L.M., Garcia, F.A.C., de Macedo, J.L.,
Dias, J.A., Dias, S.C.L., Alvim, K.S.P., 2010. Esterification of oleic
acid with ethanol by 12-tungstophosphoric acid supported on
zirconia. Appl. Catal. A: Gen. 372, 153e161.
Olowokere, J.A., Okafor, J.O., Mbah, G.O., 2012. Comparative studies
on the catalytic esterification of butanol with ethanoic acid by some
nigerian montmorillonite clays. Int. J. Environ. Sci. Manage. Eng.
Res. 1, 147e159.
Özbay, N., Oktar, N., Tapan, A.N., 2008. Esterification of free fatty
acids in waste cooking oils (WCO): role of ion-exchange resins.
Fuel 87, 1789e1798.
Park, J.Y., Kim, D.K., Lee, J.S., 2010. Esterification of free fatty acids
using water-tolerable Amberlyst as a heterogeneous catalyst. Bioresour. Technol. 101, S62eS65.
Philippou, A., Anderson, M.W., 2000. Aldol-Type Reactions over Basic
Microporous Titanosilicate ETS-10 Type Catalysts. J. Catal 189,
395e400.
Ramadhas, A.S., Jayaraj, S., Muraleedharan, C., 2005. Biodiesel production from high FFA rubber seed oil. Fuel 84, 335e340.
Ramos, L.P., Kucek, K.T., Domingos, A.K., Wilhelm, H.M., 2003. Biodiesel: Um projeto de sustentabilidade econômica e sócio-ambiental
para o Brasil. Biotecnologia Cienc. Desenvolv. 31, 28e37.
Reinoso, D.M., Damiani, D.E., Tonetto, G.M., 2012. Zinc carboxylic
salts used as catalyst in the biodiesel synthesis by esterification and
transesterification: study of the stability in the reaction medium.
Appl. Catal. A: Gen. 449, 88e95.
Ren, Y., He, B., Yan, F., Wang, H., Cheng, Y., Lin, L., Feng, Y., Li, J.,
2012. Continuous biodiesel production in a fixed bed reactor
packed with anion-exchange resin as heterogeneous catalyst. Bioresour. Technol. 113, 19e22.
Rezende, S.M., Reis, M.C., Reid, M.G., Silva Jr., P.L., Coutinho, F.M.B.,
Gil, R.A.S.S., Lachter, E.R., 2008. Transesterification of vegetable
oils promoted by poly(styrene-divinylbenzene) and poly(divinylbenzene). Appl. Catal. A: Gen. 349, 198e203.
Rezende, M.J.C., Pereira, M.S.C., Santos, G.F.N., Aroeira, G.O.P.,
Albuquerque, T.C., Suarez, P.A.Z., Pinto, A.C., 2012. Preparation,
characterisation and evaluation of Brazilian clay-based catalysts for
use in esterification reactions. J. Braz. Chem. Soc. 23, 1209e1215.
Rinaldi, R., Schuth, F., 2009. Design of solid catalysts for the conversion of biomass. Energy Environ. Sci. 2, 610e626.
Russnueldt, B.M.E., Hoelderich, W.F., 2009. New sulfonic acid ionexchange resins for the preesterification of different oils and fats
with high content of free fatty acids. Appl. Catal. A: Gen. 362,
47e57.
Ryland, L.B., Tamale, M.W., Wilson, J.N., 1960. In: Emmett, P.H. (Ed.),
1960. Catalysis, vol. 7, Reinhold, New York, pp. 1e91.
Saha, B., Sharma, M.M., 1996. Esterification of formic acid, acrylic acid
and methacrylic acid with cyclohexene in bath and distillation
column reactors: ion-exchange resins as catalysts. React. Funct.
Polym. 28, 263e278.
Santos, V.C., Bail, A., Okada, H.O., Ramos, L.P., Ciuffi, K.J., Lima, O.J.,
Nakagaki, S., 2011. Methanolysis of soybean oil using tungstencontaining, heterogeneous catalysts. Energy Fuels 25, 2794e2802.
Schuchardt, U., Sercheli, R., Vargas, R.M., 1998. Transesterification of
vegetable oils: a review. J. Braz. Chem. Soc. 9, 199e210.
Semwal, S., Arora, A.K., Badoni, R.P., Tuli, D.K., 2011. Biodiesel production using heterogeneous catalysts. Bioresour. Technol. 102,
2151e2161.
Serio, M., Ledda, M., Cozzolino, M., Minutillo, G., Tesser, R.,
Santacesaria, E., 2006. Transesterification of soybean oil to biodiesel
by using heterogeneous basic catalysts. Ind. Eng. Chem. Res. 45,
3009e3014.
Serio, M., Cozzolino, M., Giordano, M., Tesser, R., Patrono, P.,
Santacesaria, E., 2007. From homogeneous to heterogeneous catalysts in biodiesel production. Ind. Eng. Chem. Res. 46, 6379e6384.
275
Shahid, E.M., Jamal, Y., 2011. Production of biodiesel: a technical review. Renewable Sustainable Energy Rev. 15, 4732e4745.
Sharma, M.M., 1995. Some novel aspects of cationic ion-exchange
resins as catalysts. React. Funct. Polym. 26, 3e23.
Sharma, Y.C., Singh, B., Upadhyay, S.N., 2008. Advancements in
development and characterization of biodiesel: a review. Fuel 87,
2355e2373.
Sharma, Y.C., Singh, B., Korstad, J., 2011. Latest developments on
application of heterogeneous basic catalysts for an efficient and
ecofriendly synthesis of biodiesel: a review. Fuel 90, 1309e1324.
Shibasaki-Kitagawa, N., Tsuji, T., Chida, K., Kubo, M., Yonemoto, T.,
2010. Simple continuous production process of biodiesel fuel from
oil with high content of free fatty acid using ion-exchange resin
catalysts. Energy Fuels 24, 3634e3638.
Shibasaki-Kitakawa, N., Honda, H., Kuribayashi, H., Toda, T.,
Fukumura, T., Yonemoto, T., 2007. Biodiesel production using
anionic ion-exchange resin as heterogeneous catalyst. Bioresour.
Technol. 98, 416e421.
Shumaker, J.L., Crofcheck, C., Tackett, S.A., Santillan-jimenez, E.,
Morgan, T., Ji, Y., Crocker, M., Toops, T., 2008. Biodiesel synthesis
using calcined layered double hydroxide catalysts. Appl. Catal. A:
Gen. 82, 120e130.
Silva, F.R., Silveira, M.H.L., Cordeiro, C.S., Nakagaki, S., Wypych, F.,
Ramos, L.P., 2013. Esterification of Fatty Acids Using a BismuthContaining Solid Acid Catalyst. Energy Fuels 27, 2218e2225.
Soldi, R.A., Oliveira, A.R.S., Ramos, L.P., César-Oliveira, M.A.F., 2009.
Soybean oil and beef tallow alcoholysis by acid heterogeneous
catalysis. Appl. Catal. A: Gen. 361, 42e48.
Stählin, W., Oswald, H.R., 1971. The infrared spectrum and thermal
analysis of zinc hydroxide nitrate. J. Solid State Chem. 3, 252e255.
Suppes, G.J., Dasarik, M.A., Doskocil, E.J., Mankidy, P.J., Goff, M.,
2004. Transesterification of soybean oil with zeolite and metal
catalysts. Appl. Catal. A: Gen. 257, 213e223.
Talukder, M.M.R., Wu, J.C., Lau, S.K., Cui, L.C., Shimin, G., Lim, A.,
2009. Comparison of Novozym 435 and Amberlyst 15 as heterogeneous catalyst for production of biodiesel from palm fatty acid
distillate. Energy Fuels 23, 1e4.
Tana, T., Lua, J., Niea, K., Denga, L., Wanga, F., 2010. Biodiesel production with immobilized lipase: a review. Biotechnol. Adv. 28,
628e634.
Tao, Y., Kanoh, H., Abrams, L., Kaneko, K., 2006. Mesopore-modified
zeolites: preparation, characterization, and applications. Chem.
Rev. 106, 896e910.
Taylor, R.A., Ellis, H.A., 2007. Room temperature molecular and lattice
structures of a homologous series of anhydrous zinc(II) n-alkanoate. Spectrochim. Acta A. 68, 99e107.
Taylor, R.A., Ellis, H.A., Maragh, P.T., White, N.A.S., 2006. The room
temperature structures of anhydrous zinc(II) hexanoate and pentadecanoate. J. Mol. Struct. 787, 113e120.
Tesser, R., DiSerio, M., Guida, M., Nastai, M., Santacesaria, E., 2005.
Kinetics of oleic acid esterification with methanol in the presence
of triglycerides. Ind. Eng. Chem. Res. 44, 7978e7982.
Tesser, R., Casale, L., Verde, D., Di Serio, M., Santacesaria, E., 2010.
Kinetics and modeling of fatty acids esterification on acid exchange resins. Chem. Eng. J. 157, 539e550.
Trakarnpruk, W., Porntangjitlikit, S., 2008. Palm oil biodiesel synthesized with potassium loaded calcined hydrotalcite and effect of
biodiesel blend on elastomer properties. Renewable Energy 33,
1558e1563.
Trost, B.M., 1991. The atom economy: a search for synthetic efficiency.
Science 254, 1471e1477.
Twaiq, F., Zabidi, N.A.M., Mohamed, A.R., Bhatia, S., 2003. Catalytic
conversion of palm oil over mesoporous aluminosilicate MCM-41
for the production of liquid hydrocarbon fuels. Fuel Process.
Technol. 84, 105e120.
276
16. APPLICATIONS OF HETEROGENEOUS CATALYSTS IN THE PRODUCTION OF BIODIESEL
Van Gerpen, J., Knothe, G., 2009. Basis of the transesterification reaction. In: Knothe, G., Krahl, J., Van Gerpen, J. (Eds.), The Biodiesel
Handbook, second ed. AOCS Press, Urbana, pp. 31e46.
Vicente, G., Martinez, M., Aracil, J., 2004. Integrated biodiesel production: a comparison of different homogeneous catalysts systems.
Bioresour. Technol. 92, 297e305.
Vijayakumar, B., Reddy, C.R., Iyengar, P., Nagendrappa, G.,
Prakasha, B.S.J., 2005. Synthesis of p-tolyl stearate catalyzed by acid
activated Indian bentonite. Indian J. Chem. Technol. 12, 316e321.
Wang, Y., Jehng, J., 2011. Hydrotalcite-like compounds containing
transition metals as solid base catalysts for transesterification.
Chem. Eng. J. 175, 548e554.
Wee, L.H., Bajpe, S.R., Janssens, N., Hermans, I., Houthoofd, K.,
Kirschhock, C.E.A., Martens, J.A., 2010. Convenient synthesis of
Cu3(BTC)2 encapsulated Keggin heteropolyacid nanomaterial for
application in catalysis. Chem. Commun. 46, 8186e8188.
Wilson, K., Clark, J.H., 2001. Solid acids and their use as environmentally friendly catalysts in organic synthesis. Pure Appl. Chem.
72, 1313e1319.
Wilson, K., Lee, A.F., 2012. Rational design of heterogeneous catalysts
for biodiesel synthesis. Catal. Sci. Technol. 2, 884e897.
Wypych, F., Adad, L.B., Mattoso, N., Marangon, A.A.,
Schreiner, W.H., 2005. Synthesis and characterization of disordered layered silica obtained by selective leaching of octahedral
sheets from chrysotile and phlogopite structures. J. Colloid Interf.
Sci. 283, 107e112.
Xi, Y., Davis, R.J., 2008. Influence of water on the activity and stability
of activated Mg/Al hydrotalcites for the transesterification of
tributyrin with methanol. J. Catal. 254, 190e197.
Xia, P., Liu, F., Wang, C., Zuo, S., Qi, C., 2012. Efficient mesoporous
polymer based solid acid with superior catalytic activities towards
transesterification to biodiesel. Catal. Commun. 26, 140e143.
Xie, W., Peng, H., Chen, L., 2006. Calcined MgeAl hydrotalcites as solid
base catalysts for methanolysis of soybean oil. J. Mol. Catal. A:
Chem. 246, 24e32.
Yin, P., Chen, L., Wang, Z., Qu, R., Liu, X., Xu, Q., Ren, S., 2012. Biodiesel production from esterification of oleic acid over aminophosphonic acid resin D418. Fuel 102, 499e505.
Zatta, L., Ramos, L.P., Wypych, F., 2012. Acid activated montmorillonite as catalysts in methyl esterification reactions of lauric acid.
J. Oleo Sci. 61, 497e504.
Zatta, L., Ramos, L.P., Wypych, F., 2013. Acid-activated montmorillonites as heterogeneous catalysts for the esterification of lauric
acid with methanol. Appl. Clay Sci 80e81, 236e244.
Zeng, H., Feng, Z., Deng, X., Li, Y., 2008. Activation of MgeAl hydrotalcite catalysts for transesterification of rape oil. Fuel 87, 3071e3076.
Zhang, Y., Dube, M.A., Mclean, D.D., Kates, M., 2003. Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour. Technol. 89, 1e16.
Zhang, H., Ding, J., Qiu, Y., Zhao, Z., 2012a. Kinetics of esterification of
acidified oil with different alcohols by a cation ion-exchange resin/
polyethersulfone hybrid catalytic membrane. Bioresour. Technol.
112, 28e33.
Zhang, H., Ding, J., Zhao, Z., 2012b. Microwave assisted esterification of
acidified oil from waste cooking oil by CERP/PES catalytic membrane for biodiesel production. Bioresour. Technol. 123, 72e77.
Zhao, J., Guan, H., Shi, W., Cheng, M., Wang, X., Li, S., 2012. A
BrønstedeLewis-surfactant-combined heteropolyacid as an environmental benign catalyst for esterification reaction. Catal. Commun. 20, 103e106.
Zieba, A., Drelinkiewicz, A., Konyushenko, E.N., Stejskal, J., 2010. Activity and stability of polyaniline-sulfate-based solid acid catalysts
for the transesterification of triglycerides and esterification of fatty
acids with methanol. Appl. Catal. A: Gen. 383, 169e181.
C H A P T E R
17
Lignocellulose-Based Chemical Products
Ed de Jong 1,*, Richard J.A. Gosselink 2
1
Avantium Chemicals, Amsterdam, The Netherlands, 2Food and Biobased Research,
Wageningen UR, Wageningen, The Netherlands
*Corresponding author email: ed.dejong@avantium.com
O U T L I N E
Introduction
278
Occurrence and Composition of Lignocellulosic
Biomass
Storage Carbohydrates
Structural Carbohydrates
278
280
280
Cellulose
280
Hemicelluloses
Glucuronoxylans
Glucomannan
Xyloglucans
Galactoglucomannans
Arabinoglucuronoxylans
Arabinogalactan
Arabinoxylan
b-(1/3, 1/4)-Glucans
Complex Heteroxylans
Conclusions on Carbohydrate Feedstocks
280
280
282
282
282
282
282
283
283
283
283
Lignin
283
Pretreatment Technologies
Steam Explosion
Liquid Hot Water
Wet Oxidation
Dilute and Concentrated Acid Pretreatment
Alkaline (Lime) Pretreatment Process
286
286
288
288
289
289
Pretreatment Technologies Still at a Laboratory/
Conceptual Stage
Ammonia Fiber Explosion/Ammonia
Recycle Percolation)
Ionic Liquids
Sub/Supercritical Treatments
Summary of Lignocellulosic Biomass Pretreatments
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00017-6
290
290
291
291
291
Lignocellulosic BiorefineriesdClassification
292
C6 and C6/C5 Sugar Platform
Fermentation Products
Chemical Transformation Products
295
295
296
Lignin Platform
296
Importance of Furans and Aromatics as Building
Blocks for Chemicals and Fuels
297
Carbohydrate Dehydration
298
Introduction
298
Furfural Production and Applications
298
5-Hydroxymethylfurfural Formation from Hexose
Feedstock
301
Relevance of 5-Hydroxymethylfurfural as a Platform
304
Chemical
Conversion of Technical Lignins into
Monoaromatic Chemicals
Base-catalyzed Depolymerization
Acid-catalyzed Depolymerization
Pyrolysis
Oxidative Depolymerization
Reductive Hydrodeoxygenation
Solvolysis
Sub- and Supercritical Water
Supercritical Solvents
Ionic Liquids
Future Perspectives of Lignin Aromatics
305
305
305
305
306
306
307
307
308
308
308
Conclusions and Further Perspectives
309
References
309
277
Copyright Ó 2014 Elsevier B.V. All rights reserved.
278
17. LIGNOCELLULOSE-BASED CHEMICAL PRODUCTS
INTRODUCTION
Around the world significant steps are being taken to
move from today’s fossil-based economy to a more sustainable economy based on biomass. A key factor in the
realization of a successful biobased economy will be the
development of biorefinery systems allowing highly
efficient and cost-effective processing of biological feedstocks to a range of biobased products, and
successful integration into existing infrastructure. The
recent climb in oil prices and consumer demand for
environmentally friendly products have now opened
new windows of opportunity for biobased chemicals
and polymers. Industry is increasingly viewing chemical and polymer production from renewable resources
as an attractive area for investment. Within the biobased
economy and the operation of a biorefinery there are significant opportunities for the development of biobased
building blocks (chemicals and polymers) and materials
(fiber products, starch derivatives, coatings, resins, etc.).
In many cases this happens in conjunction with the production of bioenergy or biofuels. The production of biobased products could generate US$ 10e15 billion of
revenue for the global chemical industry. The economic
production of biofuels is often a challenge. The coproduction of chemicals, materials, food and feed can
generate the necessary added value.
The world is more and more confronted with the
reduction of fossil oil reserves, strong fluctuations of
fossil fuel prices and the increase in CO2 emissions
and the ensuing problem of the greenhouse gas effect.
Recent development on the production of shale gas at
various places in the world might change this picture
on the short term, but the disadvantages associated
with fossil resources stay in place. These environmental, social and economic challenges have created
the need for sustainable alternatives to fossil fuels
and chemicals (Brown, 2003; Kamm et al., 2006). The
use of plant biomass as starting material is one of the
alternatives to reduce the dependency on fossil oil for
transportation fuels and is the main alternative to
replace petrochemicals. The biomass can be transformed into energy, transportation fuels, various chemical compounds and materials such as natural fibers by
biochemical, chemical, physical and thermal processes
(Brown, 2003; Huber et al., 2006; Gallezot, 2012; Climent
et al., 2011a,b; Lichtenthaler and Peters, 2004). The
fermentation and the chemical conversion of carbohydrates into value-added compounds has received
increasing interest in the last decade, and in a biorefinery different advantages may be taken from both
processes (Kamm et al., 2006; Gallezot, 2012;
Climent et al., 2011a; Lichtenthaler and Peters, 2004;
Spiridon and Popa, 2008; Lin and Huber, 2009; Stöcker,
2008; Dhepe and Fukuoka, 2008). However, the potential competition with food and feed applications and
the consequent rise in feedstock prices is an important
aspect to take into consideration. Therefore the use of
lignocellulosic feedstocks (often referred to as secondgeneration feedstocks) is strongly advocated. In addition to carbohydrates also substantial amounts of lignin
is produced when using lignocellulosic feedstocks. In
this chapter the composition of lignocellulosic biomass
is discussed followed by an overview of the most
important pretreatment and fractionation technologies.
Especially the effect of the different technologies on
the subsequent fermentative/chemocatalytic conversions is addressed. The importance is illustrated by
an overview of the most important commercial as
well as anticipated chemical building blocks from carbohydrates and lignin with a special emphasis on the
production of furan-based building blocks from carbohydrates and aromatic building blocks originating
from lignin.
OCCURRENCE AND COMPOSITION
OF LIGNOCELLULOSIC BIOMASS
Lignocellulosic biomass is the most abundant
organic compound on Earth and represents the major
portion of the world’s annual production of renewable
biomass. The global biomass production is about
150 billion tons annually (Balat and Ayar, 2005).
Carbohydrates are by far the most omnipresent component of lignocellulosic biomass and are therefore often
the preferred feedstock for the biobased economy. In
fermentative processes there is sometimes more room
for feedstock flexibility (proteins, triglycerides/fatty
acids) but in the case of catalytic conversions such as
the transformation of biomass into furan molecules
you are restricted to carbohydrates. Sources of carbohydrates include conventional forestry, wood processing
by-products (e.g. wood chips, sawdust, bark, pulp
and paper industrial residue as black liquor), agricultural crops and surpluses (e.g. corn stover, wheat and
rice straw), and so-called energy crops (e.g. switchgrass, Miscanthus, willow) grown on degraded soils
and aquatic biomass (algae, seaweeds). In this chapter
we will focus on lignocellulosic biomass. Typical
carbohydrate compositions are shown in Table 17.1.
The majority of lignocellulosic biomass consists of
carbohydrates (60e80%); the other main component is lignin (20e25%); proteins are mainly found
in fresh (green) plant material. Amounts of
triglycerides, extractives and inorganic materials are
very much species as well as harvest time dependent.
The bulk of the carbohydrates present in biomass
are composed of poly/oligosaccharides, such as
TABLE 17.1
Carbohydrate Composition of the Main Biomass Types
C6-Sugars*
Origin
Species
Hardwoods
(Average)
Mixed (stem)
Softwoods
(Average)
Mixed (stem)
Grasses
Sugarcane
bagasse
Agricultural
Residues
Corn cobs
75
39
Wheat straw
57
Rice Husks
49
C5-Sugars*
Glu
Man
Gal
Rha
Fuc
Uro
Xyl
Ara
38e50% cellulose
67e75%
carbohydrate
43
0.4
0.9
0.5
0.1
0.2
16
40e50% cellulose
67e75%
carbohydrate
44
4.9
7.8
0.4
0.3
32e34
0.5
1.6
Lignin
Reference
1.3
18e25
Fengel and
Wegener 1984,
Ebringerova et al.,
2005
8.9
5.9
27e33
Fengel and Wegener
1984, Ebringerova et al.,
2005
20e23
2
19e24
Girio et al., 2010,
Han, J.S. 1998,
Martin et al., 2008
N.D.{
30
3.3
N.D.
Nabarlatz et al., 2007
32
N.D.{
20
2.8
2.6x
16e21
Nabarlatz et al., 2007,
Han, J.S. 1998
30
N.D.{
17
2
1.1x
21
Nabarlatz et al., 2007,
Han, J.S. 1998
* Glu, Glucose; Man, Mannose; Gal, Galactose; Rha, Rhamnose; Fuc, Fucose; UrA, Uronic acids; Xyl, Xylose; Ara, Arabinose; Others, e.g. ash.
x
Ace, Acetyl groups.
{
N.D., Not determined; d.m., dry mass.
Others*
1.4
4x
OCCURRENCE AND COMPOSITION OF LIGNOCELLULOSIC BIOMASS
Carbohydrate
Content (% d.m.){
279
280
17. LIGNOCELLULOSE-BASED CHEMICAL PRODUCTS
hemicelluloses, cellulose, starch, and inulin. Sucrose is
an omnipresent disaccharide consisting of a glucose
and fructose moiety, whereas monosaccharides such
as glucose and fructose are present in far lesser
amounts. In particular, lignocellulosic plant matter is
available in large quantities and is relatively cheap
while aquatic biomass is given great potential for the
future.
Storage Carbohydrates
Often the biological energy storage systems are also
based on carbohydrates like sucrose (saccharose), starch
and inulin, which will not be discussed here. In this
overview we will only focus on the structural carbohydrates of terrestrial biomass.
Structural Carbohydrates
Cellulose and hemicellulose can be found in the cell
wall of all terrestrial plant cells. In terrestrial biomass
the combined cellulose and hemicellulose fraction
represents almost always more than 50% of the total
biomass based on dry weight. Cellulose is a linear polymer composed of b-D-glucopyranose (glucose) units
forming microfibrils that give strength and resistance
to the cell wall. The hemicellulose consists of a wide
variety of polysaccharides (composed of pentoses,
hexoses, hexuronic acids), which are interspersed with
the microfibrils of cellulose, conferring consistency
and flexibility to the structure of the cell wall (Spiridon
and Popa, 2008).
CELLULOSE
Cellulose is the basic structural component of plant
cell walls and comprises about a third of all vegetable
materials. Cellulose is a complex polysaccharide, consisting of 3000 or more b-(1 / 4) linked D-glucose units
(Table 17.2). It is present in wood in quantities between
40% and 50% on dry matter basis (Table 17.1). It is the
most abundant of all naturally occurring organic
compounds, comprising over 50% of all the carbon in
vegetation. Cellulose is a straight-chain polymer where
no coiling or branching occurs, and the molecule adopts
an extended and rather stiff rodlike conformation.
Cellulose consists of crystalline parts together with
some amorphous regions. The chains can stack together
to form larger microfibrils, which make cellulose highly
insoluble in water. Cellulose microfibrils may also
associate with water and matrix polysaccharides,
such as the (1 / 3, 1 / 4)-b-D-glucans, heteroxylans
(arabinoxylans (AXs)) and glucomannans (GMs) (Sinha
et al., 2011; Fengel and Wegener, 1984).
HEMICELLULOSES
Hemicelluloses are the world’s second most abundant renewable polymers after cellulose in lignocellulosic materials. Hemicelluloses are a heterogeneous
class of polymers representing, in general, 15e35%
of plant biomass and which may contain pentoses
(b-D-xylose and a-L-arabinose), hexoses (b-D-mannose,
b-D-glucose, and a-D-galactose) and/or uronic acids
a-D-4-O-methylgalacturonic
and
(a-D-glucuronic,
a-D-galacturonic acids). Other sugars such as a-L-rhamnose and a-L-fucose may also be present in small
amounts and the hydroxyl groups of sugars can be
partially substituted with acetyl groups (Ebringerova
et al., 2005; Peng et al., 2012; Girio et al., 2010). Composition and amounts strongly depend on plant source,
plant tissue and geographical location. Hemicelluloses
are usually bonded to other cell wall components such
as cellulose, cell wall proteins, lignin, and phenolic compounds by covalent and hydrogen bonds, and by ionic
and hydrophobic interactions. The most relevant hemicelluloses are the xylans and the GMs, with xylans being
the most abundant. Xylans are the main hemicellulose
components of secondary cell walls constituting about
20e30% of the biomass of hardwoods (angiosperms)
and herbaceous plants. In some tissues of grasses and
cereals xylans can account up to 50% (Ebringerova
et al., 2005). Xylans are usually available in large
amounts as by-products of forest, agriculture, agroindustries, wood and pulp and paper industries.
Mannan-type hemicelluloses such as GMs and galactoglucomannans (GGMs) are the major hemicellulosic
components of the secondary wall of softwoods
(gymnosperms), whereas in hardwoods they occur in
minor mounts. Depending on their biological origin,
different hemicellulose structures can be found (Table
17.2). Upon hydrolysis, the hemicelluloses are converted
into the corresponding monosaccharides (Table 17.1).
The major hemicelluloses are discussed below.
Glucuronoxylans
Hemicelluloses in various hardwood species differ
from each other both quantitatively and qualitatively.
The main hemicelluloses of hardwood are glucuronoxylans
(O-acetyl-4-O-methylglucurono-b-(1,4)-D-xylan;
GXs), which can also contain small amounts of GMs.
In hardwoods, GXs represent 15e30% of their dry
mass and consist of a linear backbone of b-(1,4)-D-xylopyranosyl units. Some xylose units are acetylated at C2
and C3 and 1 in 10 molecules has an uronic acid group
(4-O-methylglucuronic acid) attached by a-(1,2) linkages
(Table 17.2). The percentage of acetyl groups ranges
between 8% and 17% of total xylan (about 3.5e7 seven
acetyl residues per 10 xylose units). The xylosidic bonds
TABLE 17.2 Main Types of Di-, Oligo- and Polysaccharides Present in Biomass based on Ebringerova et al., 2005, Girio et al., 2010 and Peng et al., 2012
Units
Linkage
DPx
b-(1 / 4)
100e>10,000
b-D-Galp
a-L-Araf
b-L-Arap
b-(1 / 6)
a-(1 / 3)
b-(1 / 3)
100e600
b-D-Glcp
b-D-Xylp
b-D-Xylp
b-D-Galp
a-L-Araf
a-L-Fucp
Acetyl
b-(1
a-(1
b-(1
a-(1
a-(1
10e25
b-D-Manp
b-D-Glcp
b-D-Galp
Acetyl
a-(1 / 6)
40e100
GM
2e5
b-D-Manp
b-D-Glcp
b-(1 / 4)
40e70
Hardwoods
GX
15e30
b-D-Xylp
4-O-Me-a-D-GlcpA
Acetyl
a-(1 / 2)
100e200
Arabinoglucuronoxylan
Grasses and cereals,
softwoods
AGX
5e10
b-D-Xylp
4-O-Me-a-D-GlcpAb-L-Araf
a-(1 / 2)
a-(1 / 3)
50e185
Arabinoxylans
Cereals
AX
0.15e30
b-D-Xylp
a-L-Araf-Feruloy
a-(1 / 2)
a-(1 / 3)
Glucuronoarabinoxylans
Grasses and cereals
GAX
15e30
b-D-Xylp
a-L-Araf
4-O-Me-a-D-GlcpA
Acetyl
a-(1 / 2)
a-(1 / 3)
Saccharide Type
Biological Origin
Cellulose
All terrestrial plants
Arabinogalactan
Softwoods
Xyloglucan
Abbreviation
Backbone
40e50%
b-D-Glcp
AG
1e3; 35**
b-D-Galp
Hardwoods, softwood,
grasses
XG
2e25
Galactoglucomannan
Softwoods
GGM
Glucomannan
Hardwoods and
softwoods
Glucuronoxylan
Side Chains$$
/
/
/
/
/
4)
3)
2)
2)
2)
HEMICELLULOSES
Amount*
$$
* %, Dry biomass.
x
Degree of polymerization.
** (Up to) in the heartwood of larches.
$$
p ¼ sugar in pyranose configuration, f ¼ sugar in furanose configuration.
281
282
17. LIGNOCELLULOSE-BASED CHEMICAL PRODUCTS
between the xylose units are easily hydrolyzed by acids,
but the linkages between the uronic acid groups and
xylose are very resistant. Acetyl groups are easily
cleaved by alkali, and the acetate formed during kraft
(alkaline) pulping of wood mainly originates from these
groups. Besides these main structural units, GXs may
also contain small amounts of L-rhamnose and galacturonic acid. The latter increases the polymer resistance to
alkaline agents. The average degree of polymerization
(DP) of GXs is in the range of 100e200 (Peng et al.,
2012; Girio et al., 2010).
Glucomannan
In addition to xylan, hardwoods contain 2e5% of a
GM, which is composed of b-glucopyranose and
b-mannopyranose units linked by b-(1 / 4) bonds
(Table 17.2). However, the mannose/glucose monomer
ratio may vary depending on the original source of
GM. The ratio of glucose to mannose varies between
1:2 and 1:1. Galactose is not present in hardwood
mannan. The mannosic bonds between the mannose
units are more rapidly hydrolyzed by acid than the corresponding glycosidic bonds, and GM is easily depolymerized under acidic conditions. There may be certain
short side branches at the C3 position of the mannoses
and acetyl groups randomly present at the C6 position
of a sugar unit. The acetyl groups frequently range
from 1 per 9 to 1 per 20 sugar units (Peng et al., 2012).
by a-D-galactopyranosyl units attached to glucose and
mannose by a-(1,6) bonds. Acetyl groups content of
GGM is around 6%, corresponding, on average, to one
acetyl group per three to four hexose units (Girio et al.,
2010) (Table 17.2). Some GGMs are water soluble,
presenting in that case higher galactose content than
the insoluble GGMs. There are two main types of
acetylgalactoglucomannans in softwoods, one being
galactose-poor (5e8% of dry wood) and the other
galactose-rich (10e15% of dry wood). The ratio of
galactose:glucose:mannose is approximately 0.1:1:3 and
1:1:3, for the two woods, respectively (Peng et al.,
2012). GGMs have an approximate DP between 100
and 150, which is equivalent to a molecular weight
around 16,000e24,000 Da. GGMs are easily depolymerized by acids, especially the bonds between galactose
and the main chain. The acetyl groups are much more
easily cleaved by alkali and acid (Peng et al., 2012).
GMs occur in minor amounts in the secondary wall of
hardwoods (<5% of the dry wood mass) (Girio et al.,
2010). As GGMs, they have a linear backbone of
b-D-glucopyranosyl (Glcp) and b-D-mannopyranosyl
(Manp) units but the ratio Glcp:Manp is lower. In
GGMs and GMs the extent of galactosylation governs
their association tendency to the cellulose microfibrils
and hence their extractability from the cell wall matrix
(Ebringerova et al., 2005).
Arabinoglucuronoxylans
Besides xylan and GM, xyloglucans (XGs) are also
present in the primary cell walls of some higher plants
(mainly in hardwoods, and less in softwoods) (Peng
et al., 2012). They can also appear in small amounts
(2e5%) in grasses. XGs consist of b-1,4-linked D-glucose
(cellulosic) backbone with 75% of these residues
substituted at O-6 with D-xylose. L-Arabinose and
D-galactose residues can be attached to the xylose residues forming di- or triglycosyl side chains. Also L-fucose
has been detected attached to galactose residues. In
addition, XGs can contain O-linked acetyl groups. XGs
interact with cellulose microfibrils by the formation of
hydrogen bonds, thus contributing to the structural
integrity of the cellulose network (Girio et al., 2010).
Arabinoglucuronoxylans
(AGXs)
(arabino-4-Ometylglucuronoxylans) are the major components of
nonwoody materials (e.g. agricultural crops) and a
minor component of softwoods (5e10% of dry mass).
They consist of a linear b-(1,4)-D-xylopyranose backbone
containing 4-O-methyl-D-glucuronic acid and a-L-arabinofuranosyl linked by a-(1,2) and a-(1,3) glycosidic
bonds (Table 17.2) (Girio et al., 2010). The xylopyranose
backbone might be slightly acetylated (Peng). The
typical ratio arabinose:glucuronic acid:xylose is 1:2:8.
Conversely to hardwoods xylan, AGXs might be less
acetylated, but may contain low amounts of galacturonic
acid and rhamnose. The average DP of AGXs ranges between 50 and 185 (26). In addition, because of their furanosidic structure, the arabinose side chains are easily
hydrolyzed by acids (Peng et al., 2012).
Galactoglucomannans
Arabinogalactan
The major hemicelluloses in softwoods (gymnosperms) are acetylated GGMs, accounting up to
20e25% of their dry mass (Girio et al., 2010). GGMs
consist of a linear backbone of b-D-glucopyranosyl and
b-D-mannopyranosyl units, linked by b-(1,4) glycosidic
bonds, partially acetylated at C2 or C3 and substituted
The heartwood of larches contains exceptionally large
amounts of water-soluble arabinogalactan (AG), which
is only a minor constituent in other softwood species
(Peng et al., 2012). Its concentration and quality are not
affected by seasonal variability. AGs are highly
branched polysaccharides with molecular weights
Xyloglucans
283
LIGNIN
ranging from 10,000 to 120,000 Da. All larch AGs isolated from the Larix sp. are of the b-(3,6)-D-galactan
type and consist of galactose and arabinose in a 6 to 1
ratio. Larch AG has a galactan backbone that features
b-(1 / 3) linkages and galactose b-(1 / 6) and arabinose b-(1 / 6 and 1 / 3) side chains (Peng et al.,
2012) (Table 17.2). The highly branched structure is
responsible for the low viscosity and high solubility in
water of this polysaccharide (Peng et al., 2012). It has
the ability to bind fat, retain liquid, and dispersing properties and AG also possesses a high biological activity.
Larch AG is currently used in a variety of food,
beverage, nutraceutical, and medicine applications
(Peng et al., 2012).
b-(1 / 4)-linked segments also occur. Cellulose is
also b-D-glucan, which is linked by (1 / 4)-glycosidic
bonds, and thus cellulose has high stiffness (crystallinity) and is insoluble in most solvents. Contrary to
cellulose the b-(1 / 3) linkages existing in 1314Gs
make glucans flexible and soluble (Peng et al., 2012).
Complex Heteroxylans
Complex heteroxylans are present in cereals, seeds,
gum exudates and mucilages and they are structurally
more complex. In this case the b-(1,4)-D-xylopyranose
backbone is decorated with single uronic acid and arabinosyl residues and also various mono- and oligoglycosyl
side chains (Girio et al., 2010).
Arabinoxylan
AXs are the main hemicelluloses of the grasses (Gramineae). AXs have been generally present in a variety of
tissues of the main cereals: wheat, rye, barley, oat, rice,
corn, and sorghum, as well as other plants (Peng et al.,
2012). AXs are generally present in the starchy endosperm (flour) and outer layers (bran) of cereal grain.
They are similar to hardwood xylan, but the amount of
L-arabinose is higher. In AX, the linear b-(1 / 4)-DXylp backbone is substituted by a-L-Araf units in the
positions 2-O and/or 3-O (Table 17.2). In addition, the
AXs are also substituted by a-D-glucopyranosyl uronic
unit or its 4-O-methyl derivative in the position 2-O, as
can be found in wheat straw, bagasse and bamboo.
O-acetyl substituents may also occur (Peng et al.,
2012). According to the amount of glucuronic acid
and arabinose, the types of AXs are classified as
AGX and glucuronoarabinoxylan (GAX), respectively
(Ebringerova et al., 2005). AGXs are the dominant hemicelluloses in the cell walls of grasses and cereals, such as
sisal, corncobs and straw. Compared to AGXs, the GAXs
have an AX backbone, which contains about 10 times
fewer uronic acid side chains than arabinose, and also
contains xylan that is double substituted by uronic
acid and arabinose units. Ferulic acid and p-coumaric
acid can occur esterified to the C-5 of arabinosyl units
of GAXs (Peng et al., 2012). The physical and/or
covalent interaction with other cell wall constituents
restricts xylan extractability (Girio et al., 2010).
b-(1 / 3, 1 / 4)-Glucans
b-(1 / 3, 1 / 4)-glucans (1314Gs) consist of a
linear chain of b-D-glucopyranosyl units linked by
(1 / 3) and (1 / 4) bonds (Table 17.1). 1314Gs are
present in Poaceae (grasses and cereals) as well as in equisetum, liveworts and Charophytes. The mixed-linkage
glucans are dominated by cellotriosyl and cellotetrasyl
units linked by b-(1 / 3) linkages, but longer
Conclusions on Carbohydrate Feedstocks
Storage carbohydrates are uniform in composition
and relatively easily to isolate and purify. Therefore
many fermentative and catalytic processes have identified these feedstocks as their initial feedstock of choice.
Because of costs and societal debates (food versus fuel
and indirect land use debates) many researchers both
from industry and academia are investigating the use
of lignocellulose as feedstock. Pure cellulose has the
same advantages as starch in that it is only build up
from glucose and relatively easy to hydrolyze (although
much more difficult than starch) when pure (and amorphous). However, to make use of lignocellulose economically also the hemicellulose needs to be used. This
overview clearly shows that due to the heterogeneity
of the monosaccharides incorporated and large diversity
in linkages and side groups, both enzymatic hydrolysis
system as well as a catalytic/fermentative conversion
system needs to be quite robust to make optimal use
of the cellulose and hemicellulose fractions.
LIGNIN
In addition to carbohydrates the major component of
lignocelullulosic biomass is lignin. Lignins are major
structural components of higher plants, and confer to
woody biomass its mechanical structure and resistance
to environmental stress and microbial decay. Lignin,
the name of which is derived from the Latin word for
“wood”, accounts for 15e30 wt% of woody biomass
and it is also available from agricultural residues such
as straw, grass and bagasse.
Lignins are built in plants starting from three basic
monolignols via oxidative phenolic coupling reactions
to generate the three-dimensional lignin polymer (Ralph
et al., 2007). The heterogeneity of lignin polymers exists in
molecular composition and linkage types between
284
17. LIGNOCELLULOSE-BASED CHEMICAL PRODUCTS
the phenylpropane monomers, p-hydroxyphenyl- (H),
guaiacyl- (G), and syringyl- (S) units. These are derived
from the monolignols sinapyl-, coniferyl-, and coumaryl
alcohol, respectively (Table 17.1). Lignin composition
will be different not only between species, but also
between different tissues of an individual plant. In softwood lignin coniferyl alcohol is the predominant building unit (over 95% guaiacyl structural elements), while
in hardwoods (and dicotyl fiber crops) the ratio
coniferyl/synapyl shows considerable variation. In
lignins of cereal straws and grasses the presence of coumaryl alcohol leading to p-hydroxyphenylpropane structures is typical. The lignin content ranges (Table 17.1) and
chemical structures of the three primary building blocks
in lignocellulosic biomass are given in Table 17.3 and
the occurrence and type of different interunit linkages
in Table 17.4.
For the production of aromatic chemicals from biorefinery lignin selection of suitable resources can be
made on the occurrence of building blocks and interunit
linkages next to the choice of pretreatment and isolation
procedure. The presence of one or two methoxyl groups
at the ring may for the production of some chemicals
(e.g. guaiacol, syringol) be a requirement. For the conversion of lignin into benzene, toluene, xylene (BTX) or
phenol the presence of coumaryl units (H-units) may
be an advantage due to the lack of side groups next to
the aromatic phenolic group (Table 17.3).
The complex structure of (isolated) lignins needs suitable characterization methods and an ongoing effort for
improvement of these methods have been performed
during the last decades. However, results of these
analytical procedures are not always consistent.
TABLE 17.3
Methods for lignin characterization can be found in
the literature (Gosselink et al., 2004; Baumberger et al.,
2007; Tejado et al., 2007; Monteil-Rivera et al.,
2013) and via the International Lignin Institute (www.
ili-lignin.com). Two-dimensional nuclear magnetic
resonance and pyrolysis gas chromatography mass
spectrometry are now established analytical techniques
for detailed structural lignin analysis (Table 17.4).
From a chemical perspective, lignins are highly complex polyphenolic biopolymers with aromatic units in
different configurations. Lignins are traditionally
produced in the pulp and paper mills by extracting
lignin upon liberation of cellulosic fibers used for paper
making. Most kraft lignins are burnt within paper mills
to generate heat and power, thus providing energy
autonomy and lowered operating costs. The majority
of lignosulfonates are used as additives in the building
sector, where they provide plasticity and flowability to
concrete. Lignosulfonates are also used as binders in
animal feed, in road building, oil well drilling and as
dispersants and coatings in pesticides used for agriculture applications. Sulfur-free lignins derived from soda
pulping of annual plants such as grass and wheat straw
are produced commercially and used among others in
wood adhesives and in animal feed. More recently,
biorefinery lignins are produced in so-called biorefinery
or fractionation processes, for example for the
manufacturing of cellulosic bioethanol. This side stream
is for the short term used primarily as energy source, but
for the medium to longer term utilization of these lignins
for the production of biofuels, aromatic chemicals and
materials are expected. So far limited industrial use of
technical lignins is seen mainly due to the easy use as
Lignin Content and Chemical Structures of Lignocellulosic Biomass
Lignin (wt%)
Phenylpropane Units (%)
Structure
Coumaryl (H)
Coniferyl (G)
Sinapyl (S)
Softwood
27e33
e
90e95
5e10
Hardwood
18e25
e
50
50
Grasses
17e24
5
75
25
Source: Azadi et al., 2013.
285
LIGNIN
TABLE 17.4 Frequencies of Different Interunit Linkage Types in Native Softwood and Hardwood Lignin per 100 C9 units
Name
Structure
Softwood
Hardwood
a-O-4
40e50
50e60
b-5
10e12
3
5-5
13
3
4-O-5
3
3
b-b
3
3
Bonds to
1-position
1e3
3
Source: Henriksson et al., 2010.
286
17. LIGNOCELLULOSE-BASED CHEMICAL PRODUCTS
energy source, the impurities in technical lignin sources,
tendency to form condensed structures, inferior performance compared to synthetic compounds, unique reactivity, lack of availability of high-purity lignins and a
large variety of different types of lignins (Vishtal and
Kraslawski, 2011). Additionally, traditional (heterogeneous) catalysts work inferior to biorefinery lignin and
need to be redesigned (Zakzeski et al., 2010).
Upon depletion of fossil resources, the production of
aromatic chemicals from these resources will come
under stress. As lignin is by far the most abundant aromatic renewable resource on earth, lignin is the only
resource that could fulfill the quantities needed for the
substitution of the main aromatic compounds used in industry (Holladay et al., 2007). These are phenol, BTX,
and terephthalic acid (Van Haveren et al., 2008). The
annual global production of these largest aromatic
chemicals is estimated at 103 million metric tons total,
with benzene at 44, toluene at 22, and xylenes at
37 million metric tons (Nexant Chem. Systems, 2012).
With an estimated global biomass production of about
150 billion tons annually about 30 billion tons of lignin is
generated each year globally (Balat and Ayar, 2005). This
amount of lignin is far exceeding the need for the conversion to aromatic chemicals even at low conversion degrees of about 10%. Currently there is a strong desire
from major brand owners (e.g. Coca Cola, Pepsi, Heinz)
to “green” their product portfolio by using biobased polymer building blocks. Lignin could play in the future an
important role as a biobased feedstock. However, there
are quite some challenges to overcome for the development of an economically viable process for the production
of aromatic chemicals from lignin (Gosselink, 2011).
PRETREATMENT TECHNOLOGIES
There are a number of key features for the effective
pretreatment of lignocellulosic biomass. The pretreatment process should have a low capital and operational
cost. It should be effective on a wide range and loading of
lignocellulosic feedstocks and should result in the recovery of most of the lignocellulosic components in a usable
form in separate fractions. The need for preparation/
handling or preconditioning steps prior to pretreatment
such as size reduction should be minimized. It should
produce no or limited amounts of sugar and lignin
degradation products that inhibit the growth of fermentative microorganisms or the action of hydrolytic enzymes, and it should have a low energy demand or be
performed in a manner that energy invested could be
used for other purposes such as secondary heating
(Agbor et al., 2011). The ideal pretreatment process
produces a disrupted, hydrated substrate that is easily
hydrolyzed and optimized to accommodate the
requirements of subsequent conversion steps, e.g. the
formation of sugar degradation products and fermentation inhibitors is avoided, inorganic materials is minimized and/or optimized separation of the main
constituents lignin, cellulose and hemicellulose is
achieved. Pretreatment technologies are always a combination of physical/physicochemical and chemical steps.
Physical pretreatment involves the size reduction by cutting, milling or grinding. Smaller particle sizes result in
improved hydrolysis/solvation because of increased surface/volume ratio of the substrate resulting in improved
mass transfer rates. Barakat and coworkers reviewed the
dry fractionation of lignocellulosic biomass. They
concluded that particle sizes must be reduced to
0.5e2 mm in order to decrease heat and mass transfer
limitations and to reach a well-accepted level of digestibility. However, currently mechanical size reduction
steps are not cost-effective because of too high energy
demands of dry grinding operations; therefore, innovative grinding and milling processes or combinations of
mechanical size reduction with others pretreatments
are still required (Barakat et al., 2013). Table 17.5
highlights the advantages and disadvantages of the
different pretreatment technologies that will be discussed in more detail below. The importance of highsolids loadings in biomass pretreatment has recently
been reviewed (Modenbach and Nokes, 2012).
Steam Explosion
Steam explosion pretreatment is one of the most
commonly used pretreatment technologies, as it uses a
combination of physical and chemical techniques in
order to open up and partly break down the structure
of lignocellulosic biomass. The steam-explosion pretreatment is a (autocatalytic) hydrothermal process,
which subjects the biomass to high pressures and temperatures for a short duration of time after which the
system is rapidly depressurized, causing a disrupting
of the three-dimensional structure of the biomass. The
disruption causes a partial solubilization of the hemicellulose and lignin fraction of the biomass subsequently
increasing the accessibility of the cellulose to the hydrolytic enzymes. Particle size is a major contributing factor
on the effectiveness of the process, and it has been seen
that relatively large particle sizes have been able to yield
maximum sugar concentrations. This is a promising
finding, as decreasing the particle sizes of the
material requires further mechanical processing of the
raw material driving up the production costs (Brodeur
et al., 2011). Temperatures ranging from 190 to 270 C
have been used with residence times of 1e10 min,
respectively. The addition of acidic catalysts has been
explored in minor amounts in order to improve hemicellulose hydrolysis during the pretreatment and cellulose
287
PRETREATMENT TECHNOLOGIES
TABLE 17.5
Advantages and Disadvantages of Different Pretreatment Methods of Lignocellulosic Biomass
Pretreatment Method
Advantages
Disadvantages
Processes Pursued at Commercial or Demonstration Scale
Mechanical
1. Reduce cellulose crystallinity
1. High power consumption
Steam Explosion
1. Cost-effective
2. Lignin transformation and hemicellulose
solubilization
3. High yield of glucose and hemicellulose
in two-step process
1. Partial hemicellulose degradation
2. Acid catalyst needed to make process efficient
with high lignin content material
3. Toxic compound generation
4. Incomplete destruction of lignin-carbohydrate
complexes
Liquid Hot Water
1. Separation of nearly pure hemicellulose
from rest of feedstock
2. No need for catalyst
3. Hydrolysis of hemicellulose
1. High energy/water input
2. Solid mass left over will need to be dealt with
(cellulose/lignin)
3. Long residence times
Wet Oxidation
1.
2.
3.
4.
Concentrated Acid
1. High glucose yield
2. Solubilizes hemicellulose
3. Ambient temperatures
1. High costs of acids and need for recovery and
recyclability
2. High costs of corrosion-resistant equipment
3. Formation of inhibitors
4. Hazardous and toxic process
Dilute Acid
1. Solubilizes hemicellulose
2. Tends to solubilize some lignin
1. Formation of inhibitors/degradation products
2. Risk of corrosion
Alkali
1.
2.
3.
4.
Organosolv
1. Efficient separation of lignin, cellulose and
hemicellulose fractions
2. Low molecular mass, reactive lignins
Removal of lignin
Solubilizes hemicellulose
Cellulose decrystallization
Exothermic process
Efficient removal of lignin
Low inhibitor formation
Increase accessible surface area
Proven technology
1. Cost of oxygen
2. Equipment requirements (temperature,
pressure)
1. High cost of alkaline catalyst
2. Alteration of lignin structure
3. Long residence times
1. Need for very efficient solvent recycling
2. Higher capex costs because of higher pressures
and safety concerns
Processes Pursued at a Laboratory or Conceptual Scale
AFEX
1. High effectiveness for herbaceous material
and low lignin content biomass
2. Cellulose becomes more accessible
3. Causes inactivity between lignin and enzymes
4. Low formation of inhibitors
5. Removes majority of lignin (ARP)
6. High cellulose content after pretreatment (ARP)
7. Herbaceous materials are most affected (ARP)
1. High energy costs and liquid loading (ARP)
2. Not efficient with lignin-rich feedstocks
Ionic Liquids
1. Lignin and hemicellulose hydrolysis
2. Ability to dissolve high loadings of different
biomass types
3. Mild processing conditions (low temperatures)
1. High solvent costs, therefore need for almost
complete solvent recovery and recycle
2. Difficult separation of solvent and products
3. Buildup of inorganics in the ionic liquids
4. Chemical modifications of the ionic liquid
Supercritical Fluids
1. Low degradation of sugars
2. Cost-effective
3. Increases cellulose accessible area
1. High pressure requirements
2. Lignin and hemicelluloses unaffected
Microbial
1. Low energy requirements
2. Degrades lignin and hemicellulose
1. Long time required
2. Some of the glucose is digested
3. No commercial value from impure lignin and
hemicellulose fractions
AFEX, ammonia fiber explosion; ARP, ammonia recycle percolation.
Source: Based on Agbor et al., 2011; Brodeur et al., 2011; Menon and Rao, 2012; Kumar et al., 2009; da Costa Sousa et al., 2009, Pedersen and Meyer, 2010; Limayem and Ricke, 2012;
Garlock et al., 2011.
288
17. LIGNOCELLULOSE-BASED CHEMICAL PRODUCTS
digestibility further on in the process. In addition, a
reduction of inhibitory compounds formed is seen.
The addition of acidic catalysts causes the hydrolysis
of acetyl groups into acetic acid. The physical pretreatment is realized during the rapid decompression of the
system. This causes a rapid expansion by vaporization
of the saturated water within the lignocellulosic
biomass; this results in the breakage of the molecular
linkages, and leads to a lignocellulosic matrix very susceptible to enzymatic hydrolysis. The steam-explosion
pretreatment process has been a proven technique for
the pretreatment of different biomass feedstocks. It is
able to generate complete sugar recovery while utilizing
a low capital investment and low environmental impacts concerning the chemicals and conditions being
implemented and has a higher potential for optimization and efficiency (Brodeur et al., 2011). The steamexplosion pretreatment has been demonstrated using a
wide range of biomass sources including poplar chips,
olive tree residues, wheat straw and corn stover. However, some disadvantages can be seen when using this
process. Dilute acids are needed when using softwoods
or even when increased yields are warranted for lower
acetylated feedstocks. However, the addition of acids
comes at a cost because it results in elevated equipment
requirements and the higher formation of degradation
products such as furfural and 5-hydroxymethyl furfural
(HMF), which is sometimes detrimental for subsequent
fermentations. In addition salts are formed because the
liquors need to be neutralized. These salts need subsequently to be separated from the system and disposed.
In the past the focus of many pretreatment technologies
was the optimization of the cellulose recovery and
subsequent conversion. However, cellulose content of
biomass is seldom above 45% making the economics
challenging especially because of rising feedstock and
energy costs. Therefore, research and developments
are now moving into the direction of complete utilization of the entire lignocellulosic biomass.
Liquid Hot Water
Liquid hot water (LHW) pretreatment uses water
at elevated temperatures (160 Ce240 C) and high
pressures to keep water in its liquid form in order to
promote disintegration and separation of the lignocellulosic matrix (Ruiz et al., 2013). Time ranges from a
few minutes up to an hour with temperatures between
160e240 C. The LHW process uses many of the same
features as steam explosion, primarily autohydrolysis,
although without the rapid decompression of the matrix. The LHW process utilizes flow through reactors
of varying configurations or batch techniques with the
latter being the primary emphasis at the laboratory
scale (Brodeur et al., 2011). A primary goal of this
process is to completely solubilize hemicellulose and
separate it from the rest of the solid material while
reducing the formation of inhibitors. The generation
of reactive cellulose fibers for the production of glucose
as well as disruption of the entire lignocellulosic matrix
is achieved through the cell penetration of the biomass
by the water. Both hemicellulose and part of the lignin
are solubilized by the LHW acting as an acid as well as
a base. On average between 40% and 60% of the total
biomass dissolved in the process (Ruiz et al., 2013).
There are two main product streams that are formed
at the outlet of this process: the solubilized
hemicellulose-rich slurry and the cellulose-rich solid
fraction. The solubilized product consists primarily of
(oligo)saccharides derived from nearly completely solubilized hemicellulose and lignin (35e60% of total starting material) and a minor amount of cellulose (4e15%).
Temperature plays an important role in this pretreatment as the quantity of inhibitor (e.g. furfural, acetic
acid, HMF, and formic acid) formation is mainly correlated with an increase in temperature. Johnson et al.
evaluated inhibitors and concluded that furfural and
HMF are not that toxic and that most likely other unidentified compounds are the main reason for the
observed toxicity of some of the pretreatment methods
(Johnson et al., 2013). The solid fraction consists mainly
of more accessible cellulose because of swelling and
disruption of the matrix but still needs further treatment (enzymatically/chemocatalytic) to convert it to
solubilized products. The primary objective of this pretreatment is, therefore, to reduce the solubilization of
cellulose as much as possible while simultaneously
maximizing hemicellulose and lignin solubilization.
Advantageous aspects of this pretreatment process are
the relative low costs because no solvents or additives
such as acid catalysts are required; furthermore, expensive reactor systems are not necessary due to the low
corrosive nature of this pretreatment technique and
the chemicals that are involved. As is the case with
many of the pretreatment methods, the severity of the
LHW process largely depends on the type of lignocellulosic material that is being used.
Wet Oxidation
Wet oxidation is a pretreatment technology using
water and air or oxygen to fractionate biomass at temperatures above 120 C. A clear advantage of wet oxidation,
in particular in combination with alkali, is the relatively
mild temperature and the limited formation of fermentation inhibitors (e.g. furan aldehydes and phenolaldehydes) (Klinke et al., 2002). Wet oxidation facilitates the
separation of cellulose after the majority of hemicelluloses and lignin has been solubilized. The amount of
lignin removed after pretreatment ranges from 50%
PRETREATMENT TECHNOLOGIES
to 70% depending on the type of biomass pretreated and
the conditions used. The solid material after wet oxidation displayed a higher enzymatic convertability than
the remaining solid material after steam explosion (Martin et al., 2008). Wet oxidation is effective in pretreating a
variety of biomass such as wheat straw, corn stover, sugarcane bagasse, cassava, peanuts, rye, canola, faba beans,
and reed (Brodeur et al., 2011; Martin et al., 2008). Wet
oxidation can be combined with other pretreatment
methods to further increase the yield of sugars after
enzymatic hydrolysis. Combining wet oxidation with
alkaline pretreatment has been shown to reduce the formation of by-products, thereby decreasing inhibition. In
combination with steam explosion, in a process called
wet explosion, the biomass undergoes not only the chemical reaction described above but also physical rupture.
The advantages to combining wet oxidation with steam
explosion includes the ability to process larger particle
sizes and to operate at higher substrate loadings, up to
50% substrate (Brodeur et al., 2011).
Dilute and Concentrated Acid Pretreatment
Acid pretreatment involves the use of concentrated
and diluted acids to break the rigid structure of the
lignocellulosic material. The most commonly used acids
are sulfuric (H2SO4) and hydrochloric (HCl). Dilute sulfuric acid has traditionally been used to manufacture
furfural (van Putten et al., 2013a,b) by hydrolyzing the
hemicellulose of mainly corncobs and bagasse into simple sugars. The pentose part (e.g. xylose) is subsequently
converted into furfural. Dilute sulfuric acid has also
been used commercially to pretreat a wide variety of
biomass types to subsequently convert both the C6
and C5 part. Feedstocks evaluated include switchgrass,
corn stover, spruce, and poplar (Brodeur et al., 2011
and references therein). Other acids have also been studied, such as phosphoric acid (H3PO4), nitric acid (HNO3)
and organic acids (Brodeur et al., 2011; Kootstra et al.,
2009). Due to its ability to remove hemicellulose, acid
pretreatments have also been integrated in other processes in fractionating the components of lignocellulosic
biomass such as the production of dissolving cellulose.
Acid pretreatment (removal of hemicellulose) followed
by alkali pretreatment (removal of lignin) results in relatively pure cellulose. This chemical pretreatment usually
consists of the addition of concentrated or diluted acids
(usually between 0.2% and 2.5% w/w) to the biomass,
followed by constant mixing at temperatures between
130 C and 210 C. Depending on the conditions of the
pretreatment, the hydrolysis of the sugars could take
from a few minutes to hours (Brodeur et al., 2011). A
key advantage of acid pretreatment is that a subsequent
enzymatic hydrolysis step is sometimes not required, as
the acid itself hydrolyzes the biomass to yield
289
fermentable sugars. This is especially true with concentrated acid treatments. Hemicellulose and lignin are
partly solubilized with relatively minor degradation
[75], and the hemicellulose is converted to monomeric
and oligomeric sugars with acid pretreatment. A potential drawback is the production of fermentation inhibitors like furfural and HMF, which reduce the
effectiveness of the further processes. Therefore, extensive washing and/or a detoxification step is sometimes
required to remove these inhibitors before a fermentation step. Most acids have a strong corrosive nature
asking for special reactor requirements (material for
the reactor) in order to withstand the required experimental conditions and corrosiveness of the acids.
The optimum conditions for the acid pretreatment
depend highly on the targeted sugars and the purpose
of the pretreatment. Up to now most times subsequent
conversions were based on fermentative processes.
These processes require low amounts of inhibitors
(furfural, HMF, organic acids) but are relatively tolerant
to inorganic components. But also for fermentative processes this is not clear cut. It was found that the optimal
conditions for obtaining the maximum sugar yield
depends on whether the goal is to maximize the yield
after the pretreatment or after the enzymatic hydrolysis
of the pretreated solids or if the goal is to obtain
maximum yield after both steps (Lloyd and Wyman,
2005). Delmas (2008) studied the use of formic acid/
acetic acid pretreatment at 105 C and atmospheric pressure to fractionate wheat straw into high purity fractions
of organosolv cellulose, lignin and a sugar syrup. The
raw straw pulp was separated from the dissolved lignin
and hemicelluloses. The pulp can be bleached with
hydrogen peroxide and the commercial value of the
raw pulp is close to that of eucalyptus chemical pulp.
A high-purity lignin, with linear structures, was
recovered from the organic medium.
Alkaline (Lime) Pretreatment Process
The kraft and soda processes used in chemical pulping processes are the predominant processes to produce
low lignin fibers suitable for papermaking. Alkaline
pretreatments have also widely been researched as a
pretreatment step for biorefineries, although in general
at more benign conditions compared to traditional
pulping processes. Alkaline pretreatment with alkali
such as NaOH, KOH, Ca(OH)2, hydrazine and anhydrous ammonia cause swelling of biomass, which
increases the internal surface area of the biomass, and
decreases both the DP and cellulose crystallinity. Alkaline pretreatment disrupts the lignin structure and
breaks the linkage between lignin and the other carbohydrate fractions in lignocellulosic biomass, thus making the carbohydrates in the heteromatrix more
290
17. LIGNOCELLULOSE-BASED CHEMICAL PRODUCTS
accessible. The reactivity of remaining polysaccharides
increases as the lignin is removed. Acetyl and other
uronic acid substitutions on hemicellulose that lessen
the accessibility of enzymes to cellulose surface are
also removed by alkali pretreatments (Mosier et al.,
2005). However, most of the alkali is consumed. Alkali
pretreatment is most effective with low lignin content
biomass like agricultural residues but becomes less
effective as lignin content of the biomass increases
(Agbor et al., 2011). Sodium hydroxide has been extensively studied for many years; another alkali that has
been used for the pretreatment of biomass is lime. Especially leftover lignocellulosic feedstocks have been
shown to benefit from this method of pretreatment
and include corn stover, switchgrass, bagasse, wheat,
and rice straw. The conditions for alkaline pretreatment
are usually less severe than other pretreatments. It can
be performed at ambient conditions, but longer pretreatment times are required than at higher temperatures. The alkaline process involves soaking the
biomass in alkaline solutions and mixing it at a target
temperature for a certain amount of time. A neutralizing step to remove lignin and inhibitors (salts,
phenolic acids, furfural, and aldehydes) is required
before enzymatic hydrolysis. The advantage of lime
pretreatment is that the cost of lime required to pretreat
a given quantity of biomass is lowest among alkaline
treatments (Brodeur et al., 2011). A number of studies
have combined alkaline pretreatment with other
pretreatment methods, such as wet oxidation, peroxide
treatment, steam explosion, ammonia fiber explosion
(AFEX), and ammonia recycled percolation (Brodeur
et al., 2011; Yamashita et al., 2010). In summary, the
mechanisms of alkaline pretreatment to increase cellulose accessibility is attributed to the separation of structural linkages between lignin and carbohydrates,
disruption of the lignin structure and removal of acetyl
group and uronic acid substitutions, with associated
swelling of cellulose, decrease in DP and crystallinity,
thus increasing the accessible surface area of cellulose
to enzymes (Zhao et al., 2012).
PRETREATMENT TECHNOLOGIES
STILL AT A LABORATORY/
CONCEPTUAL STAGE
Ammonia Fiber Explosion/Ammonia
Recycle Percolation)
The ammonia fiber/freeze explosion (AFEX) process
is a physicochemical process in which the biomass is
subjected to liquid anhydrous ammonia under high
pressures and moderate temperatures and then is
rapidly depressurized. The AFEX resembles very
much the steam-explosion pretreatment technology.
However, compared to the steam-explosion process
the temperatures (60e100 C) are much more moderate,
meaning less energy input and overall energy costs
associated with the AFEX process. Major variables in
the process are the operation temperature, ammonia
concentration and reaction time. The temperature will
influence the degree of disruption to the biomass structure, as it will affect the rapidness of the ammonia
vaporization within the reactor during depressurization. Typical ammonia loading for many feedstocks
are around 1 kg ammonia per kilogram dry biomass.
The residence time can be altered from minutes to
half an hour duration depending on the degree of saturation needed for the selected biomass (Chundawat
et al., 2007). The biomass is saturated for a period of
time with the ammonia in a pressurized reactor before
being released to atmospheric temperature resulting in
a rapid expansion of the ammonia gas causing swelling
of the biomass feedstock. This creates hydrolysis of the
hemicellulose fraction, a disruption in the lignincarbohydrate linkages, ammonolysis of glucuronic
cross-linked bonds, and partial decrystallization of the
cellulose structure, all leading to a higher accessible
surface area for enzymatic attack (Chundawat et al.,
2007). An important prerequisite to make the process
economic is a very efficient recovery of the ammonia
gas. Under typical AFEX conditions this pretreatment
does not remove lignin or any other substances from
the biomass; however, the lignin-carbohydrate complexes are cleaved, and the lignin is deposited on the
surfaces of the material possibly causing blockage of
cellulases to cellulose (Kumar et al., 2009; da Costa
Sousa et al., 2009). An overview of the advantages
and disadvantages is listed in Table 17.5. Ammonia
recycle percolation (ARP) has often been paired with
the AFEX pretreatment process, but it can have some
different characteristics. In the ARP process, aqueous
ammonia of concentration between 5 and 15% (wt%)
is sent through a packed bed reactor containing the
biomass feedstock at moderately high temperatures
(140e210 C) and longer reaction times compared to
the AFEX process, increasing the energy costs (Brodeur). The advantage of the ARP process over AFEX
is its ability to remove the majority of the lignin
(75e85%) as well as solubilize more than half of the
hemicellulose (50e60%) while keeping the cellulose in
its polymeric form. This results in short-chained cellulosic material containing a high amount of glucan
with a high degree (>86%) of enzymatic digestibility
and a limited amount of inhibitors. Up to now mostly
herbaceous biomass has been treated with this process.
Many of the primary concerns with the AFEX process
(high energy costs and liquid loadings, along with
many disadvantages associated with the AFEX process)
PRETREATMENT TECHNOLOGIES STILL AT A LABORATORY/CONCEPTUAL STAGE
need to be addressed before an economical process can
be envisioned (Brodeur et al., 2011).
Ionic Liquids
Room temperature ionic liquids (RTILs) were used
for the development of new technologies in chemical
and biological transformations, separations, and more
recently biomass pretreatment. RTILs consist of an
organic cation and an organic or inorganic anion. This
tremendous variation allows solvent properties to be
tailored to specific applications such as biocatalysis,
particularly as nonaqueous alternatives to organic
solvents. More recently, RTILs have been used as alternatives for lignocellulosic pretreatment (Mora-Pale
et al., 2011). Birch wood was pretreated with
N-methylmorpholine-N-oxide (NMMO or NMO) followed by enzymatic hydrolysis and fermentation to
ethanol or digestion to biogas. The pretreatments
were carried out with NMMO at 130 C for 3 h, and
the effects of drying after the pretreatment were investigated (Goshadrou et al., 2013). Another interesting
process is the use of concentrated phosphoric acid
(CPA) in the pretreatment of lignocellulosic biomass
(Zhao et al., 2012). After reprecipitation from CPA cellulose becomes completely amorphous and contains little
lignin and hemicellulose. Further research is needed to
evaluate and improve the economics of usage of ionic
liquids (ILs), NMMO and CPA for pretreatment of
lignocellulosic biomass. Also the integration with
subsequent chemocatalytic and enzymatic/fermentative processes such as simultaneous saccharification
and fermentation needs further research. Especially,
the ability of microorganisms to ferment sugars in the
presence of these solvents also needs to be tested to
carry out a continuous process. ILs are still very expensive and need to be synthesized at a much lower cost
and on a much larger scale. Other points of concern
are the buildup of inorganics in the ILs introduced
with the lignocellulosic biomass (especially a concern
with nonwoody lignocellulosic biomass such as straw
and bagasse) and chemical modifications of the ILs.
So it is rather questionable if the great potential
assigned to ILs can be fulfilled for bulk applications
such as biomass pretreatment taking into account the
aforementioned limitations.
Lignocellulosic biomass pretreatment in RTIL’s is an
alternative showing promise, with comparable or superior yields of fermentable sugars, than conventional pretreatments. The high number of RTILs that can be
synthesized allows the design of solvents with specific
physicochemical properties that play a critical role interacting with lignocellulosic biomass subcomponents.
Today, these interaction mechanisms are better understood. However, future challenges rely on the ability to
291
make this process economically feasible. This might be
achieved by optimizing large-scale pretreatment conditions, performing post-pretreatment steps in RTILs,
reusing RTILs, recycling the RTILs with reduced energy
consumption and enhancing process efficiency, and producing high-value biobased products and chemicals in
addition to ethanol. Moreover, the potential high value
of lignin suggests that it might instead be used in the
large-scale diversified manufacture of high-value chemicals, traditionally obtained from petroleum (Mora-Pale
et al., 2011).
Sub/Supercritical Treatments
Supercritical fluids (SCFs; conditions where the solvent is both above the critical temperature and critical
pressure of the chemical) show unique properties that
are different from those of either gases or liquids under
standard conditions. SCFs have liquidlike densities and
gaslike transport properties of diffusivity and viscosity.
So, SCFs have the ability to penetrate the crystalline
structure of lignocellulosic biomass overcoming the
mass transfer limitations encountered with other pretreatments. Another important advantage is the fact
that SCFs have tunable properties such as partition coefficients and solubility. Small changes in temperature or
pressure close to critical point can result in up to
100-fold changes in solubility, which can simplify separation. Supercritical carbon dioxide (CO2) with a critical
temperature (Tc) of 31 C and a critical pressure (Pc) of
7.4 MPa, as well as supercritical water has been used
for biomass pretreatment. REAC fuels and Renmatix
are examples of companies employing this kind of technology (Table 17.6).
Other technologies such as gamma rays, ozonolysis,
biological pretreatment (mainly with fungi) are still in
an earlier phase and currently face challenges in
scaling up and commercialization (Agbor et al., 2011;
Alvira et al., 2010).
Summary of Lignocellulosic Biomass
Pretreatments
Recently technoeconomic comparisons of some of
the different pretreatment technologies have been done
using identical feedstocks, and analytical methods to
generate comparable data (Wyman et al., 2005, 2011;
Eggeman and Elander, 2005). The results indicated that
no clear winning pretreatment technology could be
identified and that further optimization potential is
available in the pretreatment methods. It is also clear
that the optimal pretreatment technology is very much
substrate dependent further hampering the surfacing
of a predominant technology (Table 17.7). The effect of
pH on solubilization of the different lignocellulosic
292
17. LIGNOCELLULOSE-BASED CHEMICAL PRODUCTS
TABLE 17.6
Effect of Various Pretreatment Methods on the Chemical Composition and Chemical/Physical Structure of
Lignocellulosic Biomass
Increases
Accessible
Surface Area
Pretreatment
Sugar
Yieldc
Mechanical
L
Steam Explosion
H
H
Liquid Hot Water
H
H
Wet Oxidation
H or L
Dilute Acid
Concentrated Acid
H
H
Removes
Hemicellulose
Inhibitor
Formation
Removes
Lignin
Alters Lignin
Structure
Nil
ND
Reuse of
Chemicals
No
H
H
L
-
H
H
L
No
Nil
H
Lime (Alkaline)
Organosolv*
Decrystallizes
Cellulose
No
H
H
H
H
H
H
H
L
Yes
H
ND
L
L
H
H
Yes
H
H
H
L
Yes
H
AFEX/ARP
H
H
L
L
H
H
Yes
Ionic liquids*
(NMMO and
Ionic Liquids)
H
H
L
L
H or L*
L
Yes
Supercritical Fluid
H
H
H
L
* Depends on the chemical nature of the solvent.
H, high effect; L, low effect; ND, not determined.
Source: Adapted from Mosier et al., 2005; Brodeur et al., 2011; Menon and Rao, 2012.
components was nicely illustrated by Garlock et al.
(2011) as depicted in Figure 17.1. Table 17.6 summarizes
the effect of various pretreatment methods on the chemical composition and chemical/physical structure of
lignocellulosic biomass. It can be concluded that at the
moment there is no clearly winning technology also
because each subsequent conversion process (e.g.
fermentative, chemocatalytic) has its own set of requirements. Therefore, a wide range of technologies
are currently in the progress of being scaled-up. In
Table 17.7 an overview of currently worldwide developed demonstration and pilot plant facilities is presented for production of bioethanol and other chemicals.
LIGNOCELLULOSIC
BIOREFINERIESdCLASSIFICATION
Biorefineries can be classified on the basis of a number of their key characteristics. Major feedstocks
include perennial grasses, starch crops (e.g. wheat
and maize), sugar crops (e.g. beet and cane), lignocellulosic crops (e.g. managed forest, short rotation
coppice, and switchgrass), lignocellulosic residues
(e.g. stover and straw), oil crops (e.g. palm and oilseed
rape), aquatic biomass (e.g. algae and seaweeds), and
organic residues (e.g. industrial, commercial and postconsumer waste).
4 KEY BIOREFINERY
CHARACT E RISTICS
•
•
•
•
Feedstock utilized
Biorefinery platform
Process
Products
These feedstocks can be processed to a range of biorefinery streams termed platforms. These platforms
include single carbon molecules such as biogas and
syngas, five- and six-carbon carbohydrates from starch,
sucrose or cellulose; a mixed five- and six-carbon
carbo-hydrates stream derived from hemicelluloses,
lignin, oils (plant-based or algal); organic solutions
from grasses; and pyrolytic liquids. These primary platforms can be converted to a wide range of marketable
products using combinations of thermal, biological
and chemical processes (Table 17.8).
Knowledge of a biorefinery’s feedstock, platform and
product allows it to be classified in a systematic manner
(Cherubini et al., 2009). The classification of biorefineries
enables the comparisons of biorefinery systems, improves the understanding of global biorefinery development and allows the identification of technology gaps.
TABLE 17.7 Demonstration and Pilot Plant Facilities Developed Worldwide for Production of Bioethanol and Other Chemicals
Location
Products
Status
Raw Material
Pretreatment/Technology
Fate of Lignin
Abengoa
Bioenergia
Spain,
Kansas, USA
75,000 tons/a EtOH
Commercial facility, start-up
2013, 320,000 tons/year
Corn stover, wheat straw,
switchgrass
Acid-catalyzed steam explosion,
enzymatic hydrolysis
As coproduct, recovered
after distillation
Beta Renewables
Italy, Brazil
Variable, cellulose,
C5 sugars
Commercial facility, start-up
2013, 270,000 tons/year
Arundo donax, straw
Steam explosion/enzymatic
hydrolysis (PROESAÒ )
Solid biofuel
Borregard
Norway
Cellulose, glucose,
C5 sugars, lignin
Pilot plant 50 kg/h, 2011
Sugarcane bagasse, corn
stover, bamboo, eucalyptus,
switchgrass, straw, spruce
Modified neutral/acidic sulfite
cook (Bali process)
Performance chemicals
CIMV
France
Cellulose, lignin,
C5 sugar stream
Pilot plant, in operation
since 2006
Wheat straw
Concentrated organic acid
solvolysis
High value product, linear
structure
Chempolis
Finland
Cellulose, glucose,
C-5 sugars, lignin
Demo scale plant, Finland,
2009, 25,000 tons/year
Rice and wheat straw,
corn stover, Empty
Fruit Bunches, Oil
Palm Fronts,
bagasse, bamboo
Organosolv, (Formicobio/
Formicofib process)
Clariant
(Süd Chemie)
Germany
1000 tons/year ethanol
Pilot plant, 2012,
4500 tons/year
Wheat straw, corn stover or
other lignocellulosic material
Thermal pretreatment/enzymatic
hydrolysis (Sunliquid process)
Dupont
USA
750 tons/year
Pilot plant, 2010
Lignocellulosic, corn stover,
switchgrass
AFEX/enzymatic hydrolysis
Inbicon
(Dong Energy)
Denmark
4000 tons/a EtOH,
C5-molasses solid biofuel
Demo facility, start-up 2009
Wheat straw
Liquid hot water(hydrothermal,
autocatalyzed)
Solid biofuel for power-plant,
recovered after distillation
Iogen
Canada
70,000 tons/a EtOH
Commercial facility,
start-up 2011
Straw (wheat, barley, oat)
Modified steam explosion,
enzymatic hydrolysis
For steam and electricity
generation recovered after
enzymatic hydrolysis
Blue Sugars
Corporation
(KL Energy)
USA
4500 tons/a EtOH
Demo facility, operational
since 2007, 1e2 MT/h
Sugarcane bagasse, wood
waste, cardboard and paper
Thermomechanical
For steam or electricity
generation, or as wood pellet
Lignol
Canada
Lignin, cellulose, monomeric
hemicellulose stream
Pilot plant facility,
1 tons/day
Wood, agricultural waste
Organosolv (ethanol)
High value lignin
POET/DSM JC
USA
75,000 tons/a EtOH
Commercial facility,
start 2013
Corn cobs
Pretreatment/enzymatic
hydrolysis
Biogas production
Pure Lignin
Environmental
Technology (PLET)
Canada
Cellulose, proteins, lignin
Pilot plant since 2008,
demo plant planned (2012)
Softwood (pine)
Weak acid pretreatment (nitric
acid/ammonium hydroxide)
Water-soluble lignin
for products
Renmatix
USA
C6/C5 sugar syrups
Demo scale plant
(100 kg/day dry biomass)
Lignocellulose
Supercritical fluids
(Plantrose process)
Sweetwater
Energy/Biogasol
USA
Verenium
Process
USA
4200 tons/a EtOH
Demo facility,
operational since 2009
Sugarcane bagasse,
energy crops, wood
products and switchgrass
Mild acid hydrolysis and steam
explosion
Lignin-rich residue burned
for steam generation
recovered after distillation
Virdia (HCl
Cleantech)
USA
Sugars, lignin
Demo
Lignocellulose
Concentrated HCl,
(modified Bergius)
Solid fuel
Weyland AS
Norway
Sugars, lignin
Pilot plant, 2010, 75 kg/h
Lignocellulosedvarious
feedstocks, mostly
spruce & pine
Concentrated acids
Lignin as value-added
product
Demo facility
Wet oxidation/steam explosion
293
Source: Partly based on Menon and Rao, 2012; Bacovsky et al., 2013.
Solid biofuel for energy
generation
LIGNOCELLULOSIC BIOREFINERIESdCLASSIFICATION
Company
294
17. LIGNOCELLULOSE-BASED CHEMICAL PRODUCTS
FIGURE
17.1 Cell wall model
showing the general effect of pH on solubilization of hemicellulose and lignin.
(A) Untreated cell wall and (B) cell wall
during pretreatment. Cellulose can also be
degraded under extremely acidic conditions; however, that is not portrayed in
this diagram. Source: Designed by Garlock
et al., 2011 based on figures from Mosier et al.,
2005 and Pedersen and Meyer, 2010. (For
color version of this figure, the reader is
referred to the online version of this book.)
TABLE 17.8
Biomass-Derived Chemical Building Blocks
Cn
Chemical
Company
Potential
1
Formic acid
Maine BioProducts
Pipeline
Methane
Many companies
Growth
Ethylene
Braskem, DOW/Mitsui,
Songyuan Ji’an
Biochemical
Growth
Ethyl acetate
Zeachem
Pipeline
Ethanol
Many companies
Growth
Glycolic acid
Metabolic Explorer
(Metex)
Pipeline
Ethylene glycol
India Glycols Ltd,
Greencol Taiwan
Growth
Acetic acid
Wacker
Growth
Lactic acid
Purac, NatureWorks,
Galactic, Henan Jindan,
BBCA
Growth
Acrylic acid
Cargill, Perstorp,
OPXBio, DOW, Arkema
3-Hydroxy
propionic acid
Propylene
2
3
TABLE 17.8 Biomass-Derived Chemical Building
Blocksdcont’d
Cn
Chemical
Company
Potential
n-Propanol
Braskem
Pipeline
Ethyl lactate
Vertec BioSolvents
Growth
Isopropanol
Genomatica, Mitsui
chemicals
Pipeline
Propylene glycol
(1,2-propanediol)
ADM
Growth
n-Butanol
Cathay Industrial
Biotech, Butamax,
Butalco, Cobalt/Rhodia
Growth
1,4-Butanediol
Genomatica/M&G,
Genomatica/Mitsubishi
Chemical, Genomatica/
Tate & Lyle
Pipeline
Iso-butanol
Butamax, Gevo
Growth
Iso-butene
Gevo/Lanxess
Pipeline
Pipeline
Methyl
methacrylate
Lucite/Mitsubishi
Rayon, Evonik/Arkema
Pipeline
Cargill
Pipeline
Succinic acid
Growth
Braskem/Toyota Tsusho,
Mitsubishi Chemical,
Mitsui Chemicals
Pipeline
BioAmber, Myriant,
BASF/Purac, Reverdia
(DSM/Roquette), PTT
Chem/Mitsubishi CC
Iso-butene
Gevo/Lanxess
Pipeline
Epichlorohydrin
Solvay, DOW
Growth
Furfural
Many companies
Growth
1,3-Propanediol
DuPont/Tate & Lyle
Growth
Furfuryl alcohol
a.o. Transfurans Chemicals
Growth
4
5
295
C6 AND C6/C5 SUGAR PLATFORM
TABLE 17.8
Cn
6
Biomass-Derived Chemical Building
Blocksdcont’d
Chemical
Company
Potential
Itaconic acid
a.o. Qingdao Kehai
Biochemistry Co, Itaconix
Pipeline
Xylitol
a.o. Danisco/Lenzing,
Xylitol Canada
Growth
Isoprene/
Farnesene
Goodyear/Genencor,
GlycosBio, Amyris
Pipeline
Glutamic acid
a.o. Global Biotech,
Meihua, Fufeng, Juhua
Growth
Levulinic acid
Maine BioProducts,
Avantium, Segetis,
Circa Group
Pipeline
Sorbitol
a.o. Roquette, ADM
Growth
Adipic acid
Verdezyne, Rennovia,
BioAmber, Genomatica
Pipeline
Lysine
a.o. Global Biotech,
Evonik/RusBiotech,
BBCA, Draths, Ajinomoto
Growth
FDCA
Avantium
Pipeline
Isosorbide
Roquette
Growth
Fermentation Products
Benzene
Phenol(s)
7
fermentation processes providing access to a variety of
important chemical building blocks. Glucose can also
be converted by chemical processing to useful chemical
building blocks.
Mixed six- and five-carbon platforms are produced
from the hydrolysis of hemicelluloses. The fermentation
of these carbohydrate streams can in theory produce the
same products as six-carbon sugar streams; however,
technical, biological and economic barriers need to be
overcome before these opportunities can be exploited.
Chemical manipulation of these streams can provide a
range of useful molecules.
Growth
Glucaric acid
Rivertop Renewables
Pipeline
Citric acid
a.o. Cargill, DSM, BBCA,
Ensign, TTCA, RZBC
Growth
Caprolactam
DSM
Pipeline
Vanillin
o.a. Borregaard
Steady
Toluene
8
Para-xylene
Gevo, Draths*, UOP,
Annellotech, Virent
Pipeline
N**
PHA
Metabolix, Meridian
plastics (103),
Tianjin Green
Biosience Co.
Growth
Alkyl benzenes
* Draths is recently acquired by Amyris.
** N means unspecified number bigger than 8.
Source: Based on De Jong et al., 2012b.
The number of chemical building blocks accessible
through fermentation is considerable. Fermentation
has been used extensively by the chemical industry to
produce a number of products with chemical production through fermentation starting around the turn of
the twentieth century. Around 8 million tons of fermentation products are currently produced annually
(Bakker et al., 2010).
• Fermentation-derived fine chemicals are largely
manufactured from starch and sugar (wheat, corn,
sugarcane, etc.)
• The global market for fermentation-derived fine
chemicals in 2009 was $16 billion and is forecast to
increase to $22 billion by 2013 (Frost and Sullivan,
2011).
• The market is broken down as follows:
Chemical
2009 ($ millions)
2013 ($ millions)
Amino Acids
5410
7821
Enzymes
3200
4900
Organic Acids
(Lactic Acid 20%)
2651
4036
Vitamins and Related
Compounds
2397
2286
Antibiotics
1800
2600
Xanthan
443
708
Total
15,901
22,351
An overview of current feedstocks, platforms and products is given in Figure 17.2.
C6 AND C6/C5 SUGAR PLATFORM
Six-carbon sugar platforms can be accessed from sucrose or through the hydrolysis of starch or cellulose to
give glucose. Glucose serves as feedstock for (biological)
Modern biotechnology is allowing industry to target
new and previously abandoned fermentation products
and improve the economics of products with commercial potential. Coupled with increasing fossil feedstock
costs, cost reductions in the production of traditional
fermentation products such as ethanol and lactic acid
will allow derivative products to capture new or
296
17. LIGNOCELLULOSE-BASED CHEMICAL PRODUCTS
FIGURE 17.2 Overview of the different feedstocks, platforms, conversion steps and products leading to a novel biorefinery classification
system. Source: According to Cherubini et al., 2009. (For color version of this figure, the reader is referred to the online version of this book.)
increased market shares. Improving cost structures will
also allow previously abandoned products such as
butanol to reenter the market. Many see the future
abundant availability of carbohydrates derived from
lignocellulosic biomass as the main driver. However,
carbohydrate costs are increasing strongly in recent
years and its use for nonfood products is under pressure even in China. Fermentation also gives the industry access to new chemical building blocks previously
inaccessible due to cost constraints. The development
of cost-effective fermentation processes to succinic, itaconic and glutamic acids promises the potential for
novel chemical development.
INNOVATIVE F ERMENTA TIO N
PRODUCTS
Succinic acid
Itaconic acid
Adipic acid
3-Hydroxypropionic acid/aldehyde
Isoprene/farnesene
Glutamic acid
Aspartic acid
Chemical Transformation Products
Six- and five-carbon carbohydrates can undergo selective dehydration, hydrogenation and oxidation reactions
to give useful products, such as sorbitol, furfural,
glucaric acid, HMF and levulinic acid. Over 1 million
tons of sorbitol are produced per year as a food ingredient, personal care ingredient (e.g. toothpaste) and for
industrial use (ERRMA, 2011; Vlachos et al., 2010).
PROMISING GLUCOSE
CHEMICAL DERIVATIVES
Sorbitol
Levulinic acid
Glucaric acid
Hydroxymethylfurfural
2,5-Furan dicarboxylic acid
p-Xylene
LIGNIN PLATFORM
Lignin offers a significant opportunity for enhancing
the operation of a lignocellulosic biorefinery. It is an
extremely abundant raw material contributing as
much as 30% of the weight and 40% of the energy content of lignocellulosic biomass (Holladay et al., 2007).
Lignin’s native structure suggests that it could play a
central role as a new chemical feedstock, particularly
in the formation of supramolecular materials and aromatic chemicals (Holladay et al., 2007; Hatakeyama
and Hatakeyama, 2010). Up to now the vast majority
of industrial applications have been developed for
IMPORTANCE OF FURANS AND AROMATICS AS BUILDING BLOCKS FOR CHEMICALS AND FUELS
297
Lignin
Syngas products
Hydrocarbons
Methanol
DME
Ethanol
Mixed Alcohols
Fischer Tropsch
Liquids
C1-C7 gasses
Benzene
Toluene
Xylene
Cyclohexane
Styrenes
Biphenyls
Phenols
Oxidised products
Phenol
Substituted phenols
Catechols
Cresols
Resorcinols
Eugenol
Syringols
Coniferols
Guaiacols
Vanilin
Vanilic acid
DMSO
Aromatic acids
Aliphatic acids
Syringaidyde
Aldehydes
Quinones
Cyclohexanol
β-keto adipate
Macromolecules
Carbon fibre fillers
Polymer extenders
Substituted lignins
Themoset resins
Composites
Adhesives
Binders
Preservatives
Pharmaceuticals
Polyols
FIGURE 17.3 Potential products from lignin. (For color version of this figure, the reader is referred to the online version of this book.)
lignosulfonates. These sulfonates are isolated from acid
sulfite pulping and are used in a wide range of lower
value applications where the form but not the quality
is important. The solubility of this type of lignin in water
is an important requirement for many of these applications. Around 67.5% of world consumption of lignosulfonates in 2008 was for dispersant applications followed
by binder and adhesive applications at 32.5%. Major
end-use markets include construction, mining, animal
feeds and agriculture uses. The use of lignin for chemical
production has so far been limited due to contamination
from salts, carbohydrates, particulates, volatiles and the
molecular weight distribution of lignosulfonates. The
only industrial exception is the limited production of
vanillin from lignosulfonates (Evju, 1979). Besides lignosulfonates, kraft lignin is produced as commercial product at about 60 kton/year. New extraction technologies,
developed in Sweden, will lead to an increase in kraft
lignin production at the mill side for use as external
energy source and for the production of value-added
applications (Öhman et al., 2009).
The production of bioethanol from lignocellulosic
feedstocks could result in new forms of higher quality
lignin becoming available for chemical applications.
The Canadian company Lignol Energy has announced
the production of cellulosic ethanol at its continuous pilot plant at Burnaby, British Columbia. The process is
based on a wood pulping process using Canadian
wood species but the pilot plant will test a range of feedstocks while optimizing equipment configurations,
enzyme formulations and other process conditions
(Lignol Energy. 2013). The Lignol Energy process proÔ
duces a lignin product (HP-L lignin) upon which the
company is developing new applications together with
industrial partners. Also other lignin types will result
from the different biomass pretreatment routes under
development and unfortunately there is not one lignin
macromolecule that will fit all applications. However,
if suitable cost-effective and sustainable conversion
technologies can be developed, a lignocellulosic biorefinery can largely benefit from the profit obtained
from this side stream lignin (Gosselink, 2011).
The production of more value-added chemicals
from lignin (e.g. resins, composites and polymers, aromatic
compounds, carbon fibers) is viewed as a medium- to longterm opportunity that depends on the quality and functionality of the lignin that can be obtained (Figure 17.3,
Table 17.8). The potential of catalytic conversions of lignin
(degradation products) has been recently reviewed
(Zakzeksi et al., 2010).
The main chemical building blocks can be organized
by their carbon number, i.e. C1eCn. In the following sections, examples of biobased chemicals are discussed with
respect to their current status and the companies that are
pursuing the development of these new chemicals.
IMPORTANCE OF FURANS AND
AROMATICS AS BUILDING BLOCKS
FOR CHEMICALS AND FUELS
Aromatic compounds are important building blocks
for many chemicals and polymers as well as components
of fuel compositions. Furans, with their dienic structure,
can replace aromatic compounds in several applications
including polymers (e.g. Poly Ethylene Terephthalate by
Poly Ethylene Furanoate), fuels (diesel) and pharmaceuticals (de Jong et al., 2012a; de Jong et al., 2013; Van Putten
298
17. LIGNOCELLULOSE-BASED CHEMICAL PRODUCTS
et al., 2013a). In this paragraph we will discuss the formation of furans from carbohydrates and the formation of
aromatic compounds from lignin as an example how
all major components of lignocellulosic biomass can be
valorized by chemocatalytic routes. Some of the most
important chemical transformations of carbohydrates
are arguably the hydrolysis and subsequent dehydration
of polysaccharides into the furan platform products,
furfural and HMF (Dias et al., 2010; Van Putten et al.,
2013a,b). Furfural has a wide industrial application profile and is considered as one of the top 30 building blocks
that can be produced from biomass (Dias et al., 2010; Van
Putten et al., 2013b; Lange et al., 2012; Bozell and
Petersen, 2010; Zeitsch, 2000a; Hoydonckx et al., 2007).
HMF is promising as a versatile, renewable furan chemical for the production of chemicals, polymers and biofuels, similar to furfural (Van Putten et al., 2013a; Bozell
and Petersen, 2010). While furfural has been produced
on an industrial scale for decades (Dias et al., 2010; Van
Putten et al., 2013b), the production of HMF has not yet
reached industrial scale (Van Putten et al., 2013a; Bozell
and Petersen, 2010).
CARBOHYDRATE DEHYDRATION
Introduction
The formation of furans from sugars has been known
since the early nineteenth century (Dias et al., 2010; Van
Putten et al., 2013a,b). Furfural was discovered in 1821
by Döbereiner, by the distillation of bran with dilute sulfuric acid (Kamm et al., 2006; Van Putten et al., 2013b).
The resulting compound was first named furfurol (the
name comes from the Latin word furfur that means
bran cereal, while finishing ol means oil). The furfural
molecule has an aldehyde group and a furan ring with
aromatic character, and a characteristic smell of almonds.
In the presence of oxygen, a colorless solution of furfural
tends to become initially yellow, then brown, and finally
black. This color is due to the formation of oligomers/
polymers with conjugated double bonds formed by
radical mechanisms and can be observed even at concentrations as low as 105 M (Zeitsch, 2000a). Despite the
fact that furfural has an LD50 between 50 and 2330 mg/
kg for mice, rats, guinea pigs and dogs, man tolerates
its presence in a wide variety of fruit juices, wine, coffee
and tea (Zeitsch, 2000a; Hoydonckx et al., 2007). The
highest concentrations of furfural are present in cocoa
and coffee (55e255 ppm), in alcoholic beverages
(1e33 ppm) and in brown bread (26 ppm) (Zeitsch,
2000a). There is no commercially attractive route for the
production of furfural from petrochemical resources
(Mamman et al., 2008). The synthesis of HMF from
biomass was already described in 1895 by Düll (1895)
and Kiermayer (1895). Due to its high potential as a platform chemical for a variety of applications, furfural and
HMF were mentioned by Bozell in the “top 10 þ 4” list
ofbiobasedchemicals (Bozell and Petersen, 2010), along
with 2,5-furandicarboxylic acid (FDCA), which is formed
by oxidation of HMF (Van Putten et al., 2013a).
The formation of furans from sugars takes place
through an acid-catalyzed dehydration of sugar molecules at elevated temperature. In general furfural is
formed from C-5 sugars and HMF is formed from
C-6 sugars. It is therefore not surprising that furans,
especially HMF, can be found in essentially all carbohydrate containing heat-treated food. Furfural is known to
have some toxic effects, whereas for HMF it is still unclear (Van Putten et al., 2013a). The hydrolysis of polysaccharides and subsequent dehydration into furfural
and HMF may be promoted by Brönsted or Lewis acid
catalysts (Dias et al., 2010; Van Putten et al., 2013a).
Furfural production through traditional processes is
accompanied by acidic waste stream production and
high energy consumption. Marcotullio and de Jong state
that modern furfural production process concepts will
have to consider environmental concerns and energy
requirements besides economics moreover will have to
be integrated within widened biorefinery concepts
(Marcotullio and de Jong, 2010). The industrial use of
aqueous mineral acids as the catalysts, such as sulfuric
acid for furfural production, poses serious operational
(corrosion), safety and environmental problems (large
amounts of toxic waste). Hence, it is seen desirable to
replace conventional aqueous mineral acids by “green”
nontoxic catalysts for converting sugars into furfural
and HMF. The use of solid acids as catalysts may have
several advantages over liquid acids, such as easier
separation and reuse of the solid catalyst, longer catalyst
lifetimes, toleration of a wide range of temperatures and
pressures, and easier/safer catalyst handling, storage
and disposal. A road map to furfural, HMF and levulinic
acid has recently been presented by the group of
Dumesic (Wettstein et al., 2012).
Furfural Production and Applications
The industrial production of furfural was driven by
the need of the United States to become self-sufficient
during the First World War. Between 1914 and 1918,
intensive exploration for converting agricultural wastes
into industrially more valuable products was initiated.
In 1921, the Quaker Oats company in Iowa initiated the
production of furfural from oat hulls using “left over” reactors (Zeitsch, 2000a). Over time, there was an increased
industrial production of furfural and the discovery of
new applications. Nowadays, the annual world production of furfural is about 300,000 tons and, although there
is industrial production in several countries, the main
299
CARBOHYDRATE DEHYDRATION
FIGURE 17.4
Some of the main outlets of furfural. Source: Dias et al., 2010.
production units are located in China, the Dominican
Republic and South Africa (Kamm et al., 2006; Zeitsch,
2000a; Hoydonckx et al., 2007; Mamman et al., 2008).
Figure 17.4 gives an overview of some of the main outlets of furfural. Most of the furfural produced worldwide
is converted through a hydrogenation process into furfuryl alcohol, which is primarily used as foundry resin
but also increasingly applied as resin to improve wood
durability and for the manufacturing of polymers and
plastics (Dias et al., 2010). The aldehyde group and furan
ring furnish the furfural molecule with outstanding properties for use as a selective solvent (Zeitsch, 2000a;
Hoydonckx et al., 2007; Sain et al., 1982). Furfural has
the ability to form a conjugated double bond complex
with molecules containing double bonds, and therefore
is used industrially for the extraction of aromatics from
lubricating oils and diesel fuels, or unsaturated compounds from vegetable oils. Furfural is used as a fungicide
and nematocide in relatively low concentrations (Zeitsch,
2000a). Additional advantages of furfural as an agrochemical are its low cost, safe and easy application, and relatively low toxicity to humans. Nakagawa and Tomishige
(Nakagawa and Tomishige, 2012) have recently reviewed
HO
OH
HO
the catalyst system used to produce 1,5-pentanediol from
tetrahydrofurfuryl alcohol. Other furan compounds obtained from furfural include levulinic acid (Gürbüz
et al., 2012) and tetrahydrofuran. Furfural and many of
its derivatives can be used for the synthesis of new polymers based on the chemistry of the furan ring (Hoydonckx
et al., 2007; Sain et al., 1982; Win, 2005; Gandini and Belgacem, 1997; Moreau et al., 2004). Furfural derivatives are
also excellent starting points for fuel applications (Lange
et al., 2012; Gruter and de Jong, 2009; de Jong et al.,
2012a,b). Commercially, the pentosans (mainly xylan) present in the hemicellulose fraction of agricultural streams
such as corn cobs and sugarcane bagasse are hydrolyzed,
using homogeneous acid catalysts in water, giving rise to
pentose (xylose), which, by dehydration and cyclization
reactions, leads to furfural with a theoretical mass yield
of approximately 73% (Scheme 17.1). Nowadays also
other feedstocks are considered. Huber and his group
developed a new process to produce furfural from waste
aqueous hemicellulose solutions from the pulp and paper
and cellulosic ethanol industries using a continuous twozone biphasic reactor (Xing et al., 2011). A two-stage
hybrid fractionation process was investigated to produce
HO
OH
OH
H+
+
H
HO
O
O
O
Pentosans
H2O
OH
O
CHO
-3 H2O
Pentose
SCHEME 17.1
Net conversion of pentosans into furfural.
O
Furfural
300
17. LIGNOCELLULOSE-BASED CHEMICAL PRODUCTS
cellulosic ethanol and furfural from corn stover. In the first
stage, zinc chloride (ZnCl2) was used to selectively solubilize hemicellulose. During the second stage, the remaining
solids were converted into ethanol using commercial
cellulase and fermentative microorganisms. Yoo et al.
found that the furfural yield from the hemicellulose hydrolysates could be up to 58% based on carbon (Yoo
et al., 2012). Yemis and Mazza researched the potential
of a microwave-assisted process that provided a highly
efficient conversion of wheat straw, triticale straw, and
flax shives: obtained furfural yields based on carbon
were 48%, 46%, and 72%, respectively (Yemis and Mazza,
2011, 2012). Sahu and Dhepe also presented a solid acidcatalyzed one-pot method for the selective conversion of
solid hemicellulose without its separation from other
lignocellulosic components, such as cellulose and lignin
resulting in 56% furfural yields in biphasic systems
(Sahu and Dhepe, 2012). An interesting approach was disclosed by vom Stein and coworkers (vom Stein et al., 2011)
by working with “real samples”. They prepared aqueous
solutions of FeCl3eNaCl (or seawater) to evaluate the
dehydration of xylose into furfural, which can be
extracted in situ into 2-methyltetrahydrofuran (2-MTHF)
as second phase. Furfural was also successfully obtained
when aqueous nonpurified xylose effluents directly
from lignocellulose fractionation are tested (vom Stein
et al., 2011). Also Marcotullio and De Jong observed
good results with FeCl3 (Marcotullio and De Jong, 2010).
The hydrolysis of pentosans into pentoses in the
presence of H2SO4 is faster than the dehydration of
the pentose monomers into furfural (Zeitsch, 2000a; Hoydonckx et al., 2007). Hence, kinetic studies are generally
focused on the rate-limiting process, i.e. the dehydration
of pentoses. Xylose and arabinose are monomers found in
pentosans, which can be converted into furfural, and
some studies have shown that the dehydration of arabinose is slower than that of xylose (Zeitsch, 2000a; Kootstra
et al., 2009). The concentration of xylose in the various
raw materials is almost always much higher than that
of arabinose. Considering these factors, it seems reasonable to investigate the kinetics of the dehydration process
using xylose as substrate (Zeitsch, 2000a; Sain et al., 1982;
Win, 2005; Gandini and Belgacem, 1997; Moreau et al.,
2004, 1998; Antal et al., 1991; Root et al., 1959). In the dehydration and cyclization of xylose into furfural, three molecules of water are released per molecule of furfural
produced. Huber and coworkers developed a kinetic
model for the dehydration of xylose to furfural in a
biphasic batch reactor with microwave heating (62).
There are four key steps in their kinetic model: (1) xylose
dehydration to form furfural, (2) furfural reaction to form
degradation products, (3) furfural reaction with xylose to
form degradation products, and (4) mass transfer of
furfural from the aqueous phase into the organic phase
(methyl isobutyl ketone (MIBK)). It was estimated that
furfural yields in a biphasic system can reach 85%,
whereas at these same conditions in a monophase system
furfural yields of only 30% are obtained (Weingarten
et al., 2010). Also a kinetic model for the homogeneous
conversion of D-xylose in high-temperature water was
developed (Kim et al., 2011). Experimental testing evaluated the effects of operating conditions on xylose conversion and furfural selectivity, with furfural yields of up to
60% observed. Also the kinetics of formic acid-catalyzed
xylose dehydration into furfural and furfural decomposition was investigated using batch experiments within a
temperature range of 130e200 C (Lamminpää et al.,
2012). The study showed that the modeling must account
for other reactions from xylose besides dehydration into
furfural. Moreover, the reactions between xylose intermediate and furfural play only a minor role and that furfural
decomposition reactions must take the uncatalyzed reaction in water as solvent into account (Lamminpää et al.,
2012). By-products formed in the xylose reaction may
also derive from the fragmentation of xylose, such as glyceraldehyde, glycolaldehyde, formic acid, lactic acid, acetol (Antal et al., 1991; Ahmad et al., 1995).
As furfural is formed it can be transformed into higher
molecular weight products by (1) condensation reactions
between furfural and intermediates of conversion of
xylose to furfural (and not directly with xylose) and
(2) furfural polymerization (Zeitsch, 2000a). Aldol
condensation between two molecules of furfural does
not occur due to the absence of a carbon atom in Ha
position in relation to the carbonyl group (Chheda and
Dumesic, 2007). The side reactions (1) and (2) lead to oligomers and polymers with (1) are considered to be more
relevant than (2), although published characterization
studies of the by-products formed are scarce (Zeitsch,
2000a). The extent of these side reactions can be minimized by reducing the residence time of furfural in the
reaction mixture and by increasing the reaction temperature (Zeitsch, 2000a,b; Root et al., 1959; Zeitsch, 2000b). If
furfural is kept in the gas phase during the aqueous phase
reaction it will not react with intermediates, which are
“nonvolatile”. Agirrezabal-Telleria et al. (AgirrezabalTelleria et al., 2011) developed new approaches for the production of furfural from xylose. They propose to combine
relatively cheap heterogeneous catalysts (Amberlyst 70)
with simultaneous furfural stripping using nitrogen under semibatch conditions. Nitrogen, compared to steam,
does not dilute the vapor phase stream when condensed.
This system allowed stripping 65% of the furfural converted from xylose and almost 100% of selectivity in the
condensate. Moreover, high initial xylose loadings led to
the formation of two waterefurfural phases, which could
further reduce purification costs. Constant liquidevapor
equilibrium during stripping could be maintained for
different xylose loadings. The modeling of the experimental data was carried out in order to obtain a liquidevapor mass transfer coefficient. This value could be used for
future studies under steady-state continuous conditions
CARBOHYDRATE DEHYDRATION
in similar reaction systems (Agirrezabal-Telleria, 2011).
Formic acid, a by-product of furfural process (Root et al.,
1959), can be an effective catalyst for dehydration of xylose
into furfural. There is a growing interest in the use of formic acid as catalyst because it has low corrosiveness and
can be easily separated and reused. Using response surface methodology the optimal process parameters (xylose
concentration 40 g/l, formic concentration 10 g/l, and a
reaction temperature 180 C) were determined to obtain
high furfural yield and selectivity. Under these conditions,
a maximum furfural yield of 74% and selectivity of 78%
were achieved (Yang et al., 2012). Extraction using supercritical CO2 (scCO2) also enhances furfural yields (Kim
et al., 2011; Sako et al., 1991, 1992). The above mechanistic
considerations for the homogeneous conversion of xylose
into furfural using H2SO4 as catalyst may also be considered for solid acid catalysts. Nevertheless, differences in
product selectivity between homogeneous and heterogeneous catalytic processes are expected due to effects
such as shape/size selectivity, competitive adsorption
(related to hydrophilic/hydrophobic properties), and
strength of the acid sites.
Industrially, furfural is directly produced from the
lignocellulosic biomass in the presence of mineral acids,
mainly sulfuric acid, under batch or continuous mode
operation (Table 17.9). Attempts to improve furfural
yields have been made by process innovation, although
the use of mineral acids remains a drawback (Zeitsch,
2000a, 69. 70). The cost and inefficiency of separating
these homogeneous catalysts from the products makes
their recovery impractical, resulting in large volumes
of acid waste, which must be neutralized and disposed
off. Other drawbacks include corrosion and safety
problems. The production of furfural is therefore one
of many industrial processes where the reduction or
replacement of the “toxic liquid” acid catalysts by alternative “green” catalysts is of high priority. Recently Marcotullio and De Jong (Marcotullio and de Jong, 2010,
2011) shed new light on some particular aspects of the
chemistry of D-xylose reaction to furfural. Their aim
was to clarify the reaction mechanism leading to furfural
TABLE 17.9
Industrial Processes of Furfural Production
Industrial
Process
Catalyst
Reaction
Type
Temperature
( C)
Quaker Oats
H2SO4
Batch
153
Chinese
H2SO4
Batch
160
Agrifurane
H2SO4
Batch
177e161
Quaker Oats
H2SO4
Continuous
184
Escher Wyss
H2SO4
Continuous
170
Rosenlew
Acids formed
from the raw
material
Continuous
180
301
and to define new green catalytic pathways for its production. Specifically, their objective was to reduce the
use of mineral acids by the introduction of alternative
catalysts, e.g. halides, in dilute acidic solutions at temperatures between 170 and 200 C (Schädel et al.,
2010). Results indicate that the Cl- ions promote the formation of the 1,2-enediol from the acyclic form of xylose,
and thus the subsequent acid-catalyzed dehydration to
furfural. For this reason the presence of Cl- ions led to
significant improvements for H2SO4 catalyzed reactions.
The addition of NaCl to a 50 mM HCl aqueous solution
gave 90% selectivity to furfural. Follow-up experimental
results by the same group show the halides to influence
at least two distinct steps in the reaction leading from
D-xylose to furfural under acidic conditions, via different
mechanisms. The nucleophilicity of the halides appears
to be critical for the dehydration, but not for the initial
enolization reaction. By combining different halides synergic effects become evident resulting in very high selectivities and furfural yields (Marcotullio and de Jong,
2011). Also Rong et al. (2012) found that the addition
of inorganic salts (e.g. NaCl, FeCl3) promoted the yield
of furfural from xylose. Another approach to reduce
the inorganic waste streams is to perform the reaction
at high temperatures. It was shown that the reaction
pathway for the xylose decomposition in hightemperature liquid water can be changed by manipulating the temperature and pressure without any
catalyst with a maximum furfural yield of 50% (Jing
and Lu, 2007). Many attempts have been made to
develop heterogeneous catalytic processes for furfural
production that offer environmental and economic
benefits, but to the best of our knowledge none has
been commercialized (Van Putten et al., 2013b).
5-Hydroxymethylfurfural Formation
from Hexose Feedstock
HMF stands out among the platform chemicals for a
number of reasons: It has retained all six-carbon atoms
that were present in the hexoses and high selectivities
have been reported for its preparation, in particular
from fructose, which compares favorably with other
platform chemicals, such as levulinic acid or bioethanol.
HMF is formed through the acid-catalyzed dehydration
of a hexose, as described in Scheme 17.2. Initially the
synthesis of HMF from hexoses was performed in
aqueous systems, catalyzed by homogeneous acids.
SCHEME 17.2 The acid-catalyzed dehydration of hexose into
HMF.
302
17. LIGNOCELLULOSE-BASED CHEMICAL PRODUCTS
SCHEME 17.3 The dehydration of glucose and fructose through acyclic intermediates.
A number of mechanistic pathways have been proposed for this reaction, which can generally be divided
into two groups. The first group is based on a pathway
through acyclic intermediates and the second group is
based on a pathway through cyclic intermediates.
Although there are differences between the various
acyclic pathways proposed for the aqueous dehydration
of hexoses, they generally propose the formation of
the 1,2-enediol intermediate in the Lobry De BruijnAlberda Van Ekenstein transformation (Speck Jr, 1958)
between fructose and glucose as the key intermediate
(Anet, 1964; Feather and Harris, 1973; Kuster, 1990;
Newt, 1951). This intermediate is proposed to dehydrate
to a 3-deoxyglucosone, followed by further dehydration
and ring closure to form HMF. A schematic representation is provided in Scheme 17.3.
The proposed aqueous hexose dehydration pathways
through cyclic intermediates generally assume dehydration to start at the C2 hydroxyl position of fructose
(Scheme 17.4), leading to the formation of a tertiary carbocation (Van Putten et al., 2013a; Feather and Harris,
1973; Newt, 1951). This is then followed by consecutive
dehydrations at C3 and C4 to form HMF. It is clear that
in this proposed mechanism, glucose dehydration
requires glucose to first isomerize to fructose before it
can dehydrate to HMF. Under the acidic reaction conditions, however, this is unfavorable as the isomerization
is base catalyzed.
The HMF yields and selectivities from the dehydration of fructose, a ketose, are generally much higher
than those obtained from the dehydration of glucose,
which is an aldose (Van Putten et al., 2013a). The HMF
SCHEME 17.4 The dehydration of fructose through cyclic intermediates.
CARBOHYDRATE DEHYDRATION
yields for homogeneous acid-catalyzed fructose dehydration in water are limited to around 60% at full conversion, whereas for glucose this is only around 10% at full
conversion. Fructose is known to be significantly less stable than glucose, which shows in the required
reaction conditions for dehydration. Fructose dehydrates
to HMF at temperatures around 100 C in the presence of
acid, whereas glucose requires much more severe conditions of at least 140 C in the presence of catalyst to form
only small amounts of HMF (less than 10% yield). Quite
large variations are seen in the reaction conditions
applied by different groups. In some cases relatively
high catalyst concentrations in the order of 0.1e1 M mineral acid are applied in fructose dehydration at relatively
low temperatures between 100 and 150 C with reaction
times in the order of minutes. Others applied lower
acid concentrations, but at either longer reaction times
or higher temperatures (Van Putten et al., 2013a). Also
a significant amount of work has been done with heterogeneous acid catalysts, like ion exchange resins and zeolites, showing comparable selectivities and yields to the
homogeneous catalysts (Van Putten et al., 2013a).
The HMF yield is limited by its inherent instability
under aqueous acidic conditions. In the presence of
acid HMF reacts with water (so-called HMF hydration
reaction) to form levulinic acid and formic acid, as
described in Scheme 17.5 (Kuster, 1990). Other undesirable side reactions are the formation of polymeric material, often referred to as humins (Kuster, 1990), and
retroaldol reactions of sugars (Aida et al., 2007).
In order to minimize side reactions and HMF hydration, biphasic systems have been researched in which
the HMF is extracted to the organic phase (RománLeshkov et al., 2006; Cope, 1959; Kuster and van der
Steen, 1977; Kuster and Laurens, 1977; Moreau et al.,
1996). The major extraction solvents used are methylisobutylketone, 1-butanol and 2-butanol. The in situ
extraction has improved HMF yields from fructose
dehydration in some cases to around 70% at full conversion. Due to the high solubility of HMF in water relatively large amounts of solvent are needed, generally
at least two equivalents, in order to extract sufficient
amounts of HMF (Van Putten et al., 2013a).
In the early 1980s a number of researchers started performing HMF synthesis in organic solvents (Nakamura
and Morikawa, 1980; Szmant and Chundury, 1981; Brown
et al., 1982). The biggest initial challenge here is that,
except from high-boiling coordinating solvents like
SCHEME 17.5 The acid-catalyzed hydration of HMF to levulinic
acid and formic acid.
303
dimethyl sulfoxide (DMSO), N,N-dimethylformamide
(DMF) and N-methylpyrrolidinone, most organic solvents do not dissolve sugars very well. The focus was
mainly on solvents like DMSO and DMF, showing
significant improvements in yield and selectivity
(Nakamura and Morikawa, 1980; Szmant and
Chundury, 1981; Brown et al., 1982; Musau and Munavu,
1987). In DMSO reaction temperatures of 100e120 C are
generally applied and the solvent shows catalytic
activity as yields over 90% have been reported in the
absence of catalyst (Brown et al., 1982; Musau and
Munavu, 1987). An important issue here is the known
decomposition of DMSO at temperatures over 100 C.
Since 2003 ILs have been extensively researched as
solvents for HMF synthesis by many research groups;
however, 20 years before that HMF synthesis in pyridinium salts was already performed by Fayet and Gelas,
resulting in 70% yield starting from fructose (Fayet
and Gelas, 1983). Certain ILs are known to dissolve
sugars in high concentrations. The vast majority of this
research has been done in imidazolium-based ILs. As
is the case for the coordinating organic solvents, the
HMF yields for fructose dehydration to HMF in ILs, in
which the IL is often also the catalyst, are generally
high (70e90%) and levulinic acid formation is in most
cases not mentioned (Van Putten et al., 2013a; Zakrzewska et al., 2010). In the work on ILs some conflicting results have been published with the same or comparable
ILs. As was already mentioned, HMF synthesis from
glucose is much more challenging than from fructose.
In 2007 Zhang and coworkers published a breakthrough
in glucose dehydration to HMF by using CrCl2 as a catalyst in an imidazolium type IL (Zhao et al., 2007). They
achieved an HMF yield of around 70%, which was
essentially equal to the yield obtained from fructose in
the same system. It is believed that CrCl2 behaves as
an isomerization catalyst that forms fructose, which
can be dehydrated readily to HMF.
Earlier research on HMF synthesis focused mainly on
fructose and polymers thereof as substrates. Recent
years have seen an enormous increase in interest in the
development of biobased platform chemicals as a
replacement for fossil-oil based feedstock. For this
reason it is preferable to use cheap feedstocks that do
not compete with food. Many parties have placed their
focus on cellulose, a for humans nondigestible polymer
of glucose, as a feedstock. Cellulose is present in large
amounts in plant waste material. Application in HMF
synthesis will require both hydrolysis and dehydration
of the cellulose, either in one reactor or in two separate
steps. Recent years have shown a dramatic increase in
research on HMF synthesis from cellulose. The main
focus has been in line with the work on glucose,
applying bifunctional catalyst systems, especially chromium salts in combination with a Brønsted acid.
304
17. LIGNOCELLULOSE-BASED CHEMICAL PRODUCTS
Especially in ILs the yields approach those obtained
with glucose. The substrate concentration is mostly
significantly lower, due to the much lower solubility of
cellulose. Also the reaction times are typically much
longer for cellulose compared to glucose, likely due to
the required hydrolysis prior to dehydration to HMF
(Van Putten et al., 2013a).
Although sugar dehydration to furans is a hot topic in
academia, a lot of research has yet to be done in upscaling these processes to pilot plant and ultimately industrial scale. Especially for hexose dehydration to HMF
this holds true. Only two pilot-scale processes are
known for the production of HMF: a process from Süddeutsche Zucker-Aktiengesellschaft and a process from
Roquette Frères. The first process concerns HMF production in around 5 kg scale from fructose and inulin,
a polymer of mainly fructose, catalyzed by oxalic acid
at around 140 C in water in which the purification of
HMF is done by chromatographic separation (Rapp,
1987). The second process concerns fructose dehydration in a water-MIBK (1:9 v/v) biphasic system in the
presence of cationic resins at temperatures between
70 and 95 C (Fleche et al., 1982). In both processes the
fructose concentration in water was 20e25 wt% and
the HMF yields are in the range of 40e50%. The workup
procedures for HMF mentioned in these patents appear
unfavorable for a large-scale plant as large-scale chromatographic separation is expensive and a very high
solvent to water ratio requires a lot of energy for evaporation of the solvent from the product.
In order to produce HMF or a derivative thereof in a
cost-effective way, some challenges must be overcome.
HMF is unstable under the reaction conditions in the
presence of water, leading to the formation of levulinic
acid, formic acid and polymeric materials. For this
reason contact with water should be minimized. This
can be achieved by performing the reaction using other
solvents or by continuously extracting the HMF from the
aqueous phase.
The distribution of HMF over water and extraction
solvents is generally not highly favorable toward the solvent, demanding large excess of extraction solvents and
therefore energy-consuming workup (Román-Leshkov
and Dumesic, 2009).
Performing HMF synthesis in other solvents than
water is an appealing option. Here the choice has to be
made between solvents that have lower boiling points,
but exhibit low sugar solubility, and solvents that
dissolve high concentrations of sugar, like DMSO and
ILs, although from which product separation is difficult
due to the high affinity of HMF for these solvents. Two
processes focus on the production of derivatives of
HMF in order to produce the furanic product more effectively. Mascal and coworkers have focused their efforts
on the production of 5-chloromethylfurfural in a
biphasic system of concentrated hydrochloric acid and
1,2-dichloroethane (Mascal and Nikitin, 2008). Avantium Chemicals opened their pilot plant in December
of 2011 on alcohol-based production of HMF ethers,
which will be used for the production of furan-based
polymers (Gruter and Dautzenberg, 2007).
Relevance of 5-Hydroxymethylfurfural
as a Platform Chemical
HMF is a very important building block for a wide
range of applications. In this paragraph applications in
the areas of polymers, fine chemicals, and fuels are summarized. When HMF is produced at high efficiency
follow-up products will become an attractive option to
replace petrochemical analogs. An interesting molecule
that can be derived from HMF is FDCA. It can be
obtained via the oxidation of HMF; several oxidation
methods have been described in literature (Van Putten
et al., 2013a). FDCA was identified by the US Department
of Energy (Bozell and Petersen, 2010) to be a key bioderived platform chemical, which in itself is the building
block for polyesters, polyamides and plasticizers but
FDCA can also serve as starting point for several other
interesting molecules, including succinic acid, FDCA
dichloride, and FDCA dimethyl ester. In addition to
FDCA, other platform chemicals can be produced as
well. 5-Hydroxymethylfuroic acid, 2,5-diformyl furan,
the 2,5-diamino-methylfuran, and 2,5-bishydroxymethylfuran are most versatile intermediate chemicals of
high industrial potential because they are six-carbon
monomers that could replace, for example, adipic acid,
alkyldiols, or hexamethylenediamine in the production
of polymers (Van Putten et al., 2013a). 2,5-Furandicarboxaldehyde and 2,5-hydroxymethylfuroic acid can
be considered intermediates to FDCA in the oxidation
of HMF. De Vries, Heeres and coworkers (Buntara
et al., 2011) have shown an interesting route to convert
HMF into caprolactam, the monomer for nylon-6. In
addition to applications in the polymer field HMF can
also be used in many fine chemicals applications. In
view of the rigid furan structure and the two substituents
that can be easily modified, HMF has been used in quite a
number of pharmaceutical studies (Van Putten et al.,
2013a). HMF-derived 5-amino-levulinic acid (Binder
et al., 2010) and its derivatives are herbicides. A synthesis
route was published by Descotes in collaboration with
Südzucker (Schinzer et al., 2004).
The Maillard reaction between reducing carbohydrates and amino acids is undoubtedly one of the most
important reactions in the flavor and fragrance world,
leading to the development of the unique aroma and
taste as well as the typical browning, which contribute
to the sensory quality of thermally processed foods,
such as cooked or roasted meat, roasted coffee or cocoa.
CONVERSION OF TECHNICAL LIGNINS INTO MONOAROMATIC CHEMICALS
Although numerous studies have addressed the structures and sensory attributes of the volatile odor-active
compounds, the information available on nonvolatile,
sensory-active components generated during thermal
food processing is scarce but HMF derivatives play an
essential role (Van Putten et al., 2013a). HMF has also
been linked to natural products, sugar derivatives (e.g.
glucosylated HMF) and spiroketals (Van Putten et al.,
2013a). HMF can also be a precursor of fuel components.
HMF is a solid at room temperature with very poor fuel
blend properties; therefore, HMF cannot be used and has
not been considered as a fuel or a fuel additive. The Small
Medium-sized Enterprise (SME) company Avantium is
developing chemical, catalytic routes to produce furan
derivatives “furanics” for a range of biofuel applications
(de Jong et al., 2012a,b). Avantium targets biofuels with
advantageous qualities, both over existing biofuels
such as bioethanol and biodiesel as well as over traditional transportation fuels. Another major goal is minimizing the H2 demand for their production. These
C5-derived furanic monoethers and C6-derived furanic
diethers have a relatively high energy density, and
good chemical and physical characteristics, no difference
in the engine operation was observed and strongly
decreased smoke and particulates emissions. The use of
furans, such as HMF and furfural, as precursors of liquid
hydrocarbon fuels is also an option for the production of
linear alkanes in the molecular weight range appropriate
for diesel or jet fuel. The group of Dumesic has
researched and evaluated the different strategies
possible for upgrading HMF to liquid fuels (531 Alonso
et al., 2010). HMF can be transformed by hydrogenolysis
to 2,5-dimethyl furan. To form larger hydrocarbons,
HMF and other furfural products can be upgraded by
aldol condensation with ketones, such as acetone, over
a basic catalyst (NaOH) already at room temperatures
(West et al., 2008). Also several levulinic acid derivatives
have been proposed for fuel applications, for instance
ethyl levulinate, g-valerolactone, and MTHF (Geilen
et al., 2010). The conversion of HMF to fuels has recently
been reviewed (Mäki-Arvela et al., 2012).
CONVERSION OF TECHNICAL LIGNINS
INTO MONOAROMATIC CHEMICALS
The conversion of technical lignin into these
monoaromatic chemicals is assumed to be a long-term
application (Holladay et al., 2007). Increased worldwide
research activities can be observed in this area where
predominantly thermochemical approaches are under
study to convert lignin model compounds and depolymerize technical lignins into the desired aromatic compounds. In general, lignin depolymerization can not
only be performed in aqueous and organic phases, but
305
also in dry form. Complex mixtures are the result in
which the individual mass yields barely exceeds few
percent. Mostly, CeOeC bonds are cleaved, while the
CeC linkages in the lignin structures are very resistant
to cleavage. The use of catalysts seems to be a necessity
and these activities have been recently reviewed
(Zakzeski et al., 2010; Gallezot, 2012; Azadi et al., 2013)
showing the following main routes for technical lignin
depolymerization in (mono)aromatic chemicals.
Base-catalyzed Depolymerization
Most work related to base-catalyzed depolymerization (BCD) originates from the pulp and paper industry
where these alkaline processes are used to depolymerize
and liberate lignin from the lignocellulosic matrix as
described in the previous sections. Besides extensive
cleavage of the b-O-4 linkages under BCD conditions
the methoxyl contents in lignin decrease with the
severity of alkaline conditions. However, repolymerization of liberated lignin fragments to condensation
products may occur. Alcell organosolv lignin depolymerization in alkali (0e4%) yielded 7e30% liquid
products. The maximum concentration of identified phenols was 4.4%, mostly syringol (2.4%) and a limited
amount of guaiacol when less severe conditions were
applied. Catechol was found at higher pH and temperatures (Thring, 1994). More recently, Yuan et al. (2010)
studied the base-catalyzed degradation of kraft lignin
in watereethanol at 220e300 C, with phenol as the
capping agent into oligomers with a negligible char
and gas production. Under the conditions applied lignin
could not be degraded completely into lignin monomers.
Base-catalyzed lignin depolymerization with the
addition of boric acid greatly facilitates the depolymerization of lignin in water, increase product selectivity
and boric acid acts as a capping agent to suppress addition and condensation reactions (Roberts et al., 2011).
Acid-catalyzed Depolymerization
Depolymerization of Alcell lignin using Lewis acid
catalysts NiCl2 or FeCl3 yielded gas, solid and liquid
products including the formation of ether-soluble monomers under different reaction conditions. Both catalysts
favor condensation reactions leading to insoluble residues. The low yields of organic monomers were dominated by phenolics over ketones and aldehydes
(Hepditch and Thring, 2000).
Pyrolysis
Pyrolysis of isolated lignins gives a different product
distribution than pyrolysis of wood of other lignocellulosic materials. Lignin pyrolysis occurs in a wider
306
17. LIGNOCELLULOSE-BASED CHEMICAL PRODUCTS
temperature range (e.g. 160e900 C) compared to polysaccharides (e.g. 220e400 C) (Yang et al., 2007). Furthermore, the amount of char from isolated lignins is
significantly higher compared to whole biomass
pyrolysis. Solid acid catalysts such as H-Zeolite Socony
Mobil-5 can effectively shift the products toward more
deoxygenated compounds. Different isolated lignins pyrolyzed at temperature ranges of 500e800 C yielded
bio-oil, gas and char of 16e70%, 3e39%, and 17e81%,
respectively (Azadi et al., 2013). Several researchers
showed that inorganic alkaline catalysts such as NaOH
can facilitate depolymerization of lignin by pyrolysis
and influence the product composition (Amen-Chen
et al., 2001).
Recently, an international study of fast pyrolysis of
lignin was undertaken with contribution from 14 laboratories. Based on the results it was concluded that an
impure lignin containing up to 50% carbohydrates
behaves like whole biomass, while a purified lignin
was difficult to process in the fast pyrolysis reactors
and produced a much lower amount of a more enriched
aromatic bio-oil. It was concluded that for highly pure
lignin feedstocks new reactor designs will be required
other than the typical fluidized bed fast pyrolysis systems (Nowakowski et al., 2010).
Upgrading of lignin pyrolysis oil by catalytic hydrodeoxygenation (HDO) is often used as described by de
Wild et al. (2009). More stable oil due to partial removal
of oxygen is an important upgrading property. Bu et al.
(2012) made a review on the catalytic HDO upgrading of
lignin-derived phenols from biomass pyrolysis. This
study shows that further investigation of HDO is
needed to improve catalysts and optimize operation
conditions, further understanding of kinetics of complex
bio-oils, and availability of sustainable and cost-effective
hydrogen sources. Further HDO treatments are discussed in the next session.
Anellotech (2010) has developed a technology platform using catalytic pyrolysis for the claimed inexpensive production of chemicals and transportation fuels
from nonfood biomass. Vispute et al. (2010) claim that
all chemical conversions can be performed in one
reactor, using an inexpensive catalyst. Target green
chemicals are BTX.
Oxidative Depolymerization
In general oxidative depolymerization of lignin is
carried out to produce aromatics with an increase in
oxygen-containing groups, mostly aldehydes. The production of vanillin (3-methoxy-4-hydroxybenzaldehyde)
by oxidative depolymerization of lignin, mainly from
black liquor of sulfite pulping is the most well-known process. This commercial process is typically performed at
160e175 C under alkaline conditions using a copper
catalyst by Borregard in Norway. Especially softwood
lignin is yielding relatively higher amounts of vanillin
as compared to hardwood lignin where syringaldehyde
may prevail (Evju, 1979).
Other researchers used hydrogen peroxide for oxidative depolymerization. Kraft lignin was treated at 90 C
by a biomimetic system, using hemin as a catalyst and
hydrogen peroxide as an oxidizing agent, which mimics
the catalytic mechanism of lignin peroxidase. Relatively high yields of vanillin 19%, vanillic acid 9%,
2-methoxyphenol 2% and 4-hydroxybenzaldehyde 2%
were obtained (Suparno et al., 2005). Xiang and Lee
(2000) found that alkaline peroxide treatment of lignin
at 80e160 C yields mainly low molecular weight
organic acids (up to 50%) with only traces of aromatics,
which are rapidly degraded by hydrogen peroxide.
Sales et al. (2004, 2007) studied the alkaline oxidation
of sugarcane soda lignin with a continuous fluid bed
with a palladium chloride PdCl3.3H2O/g-Al2O3 catalyst
at 100e250 C and 2e10 bar partial oxygen pressure.
Total aldehyde yield on lignin was 12%. Zakzeski et al.
(2010) reported other predominantly catalytic lignin
oxidation processes yielding aromatic aldehydes and
acids, which do not exceed 10% on lignin basis. However, lignin model compounds show in some catalytic processes good conversions, which are promising to further
develop catalytic strategies for lignin depolymerization
in a biorefinery concept.
Voitl and Rudolf von Rohr (2010) studied a process
for producing vanillin and methyl vanillate from kraft
lignin by acidic oxidation in aqueous methanol with
H3PMo12O40 as a homogeneous catalyst in the presence
of 10 bar oxygen. A stable yield of 3.5 wt% vanillin and
3.5 wt% methyl vanillate can be obtained together with
60 wt% of oligomeric products in the extract. The monomers can be effectively separated using organic solvent
nanofiltration (Werhan et al., 2012).
Reductive Hydrodeoxygenation
HDO is a promising upgrading technology to
remove the oxygen from biomass-derived streams, for
example obtained after pyrolysis. Strong emphasis is
put on finding selective catalysts to minimize the use
of hydrogen while maintaining the aromatic functionality of lignin. HDO of lignin model compounds can
be efficiently performed over a copper chromite catalyst
(Deutsch and Shanks, 2012). The hydroxymethyl group
of benzyl alcohol is highly reactive to HDO. Demethoxylation of anisole is the primary reaction pathway in
contrast to demethylation and transalkylation. The
latter are more prevalent for conventional hydrotreating
catalysts. The hydroxyl group of phenol strongly activated the aromatic ring toward cyclohexanol and
cyclohexane.
CONVERSION OF TECHNICAL LIGNINS INTO MONOAROMATIC CHEMICALS
When applied directly to isolated technical lignin a
wide range of chemical reactions occur at 380e430 C
including cleavage of interunit linkages, deoxygenation,
ring hydrogenation, and removal of alkyl and methoxyl
moieties. A complex bio-oil is the result, but the oxygen
content of this hydropyrolysis oil is lower compared to
pyrolysis oil and therefore this HDO bio-oil is chemically more stable. The hydrogen pressure, typically
50e150 bar, strongly influences the oil yield. Ideal catalysts should have high activity for hydrogenolysis
and/or cracking of CeOeC and CeC linkages; low activity for ring hydrogenation; meaningful selectivity toward a certain aromatic compound or class of
compounds to allow effective product isolation; high
resistance against coke formation and easy regeneration;
high sulfur resistance for processing sulfur-containing
lignins. Bifunctional catalysts comprise an active hydrogenation metal (e.g. NiMo-Cr2O3, Pd, Co-Mo) and an
acidic support such as zeolites to selectively open
some CeC bonds. By using catalysts the yield of HDO
bio-oil has been improved from 15% up to 81% (Azadi
et al., 2013). For development of viable catalytic
HDO bio-oil upgrading technologies to produce transportation fuel include (1) improved catalysts, (2) alternative hydrogen source, (3) detailed kinetics study and
(4) optimizing the HDO reactions conditions suitable
for existing refinery infrastructure (Bu et al., 2012).
Solvolysis
Alternatively, instead of the use of metal catalysts and
hydrogen for hydrogenation, solvolytic depolymerization reactions were performed in the presence of
hydrogen donors such as tetralin or anthracene derivatives (Dorrestijn et al., 1999). However, the high costs
of these solvents that are consumed during the process
prevent practical implementation. A solution to this
problem could be the use of formic acid or 2-propanol
as hydrogen donors (Kleinert and Barth, 2008; Kleinert
et al., 2009). In the presence of relatively large amounts
of formic acid and a low chain alcohol the resulting
phenolic oil contains substantial amounts of aliphatic
hydrocarbons, indicating that extensive hydrogenation
of the resulting depolymerization products occurs
(Gellerstedt et al., 2008). Another advantage of this process is the negligible formation of char. Xu et al. (2012)
used this approach to depolymerize lignin with a combination of formic acid and a Pt/C catalyst in ethanol to
further promote the production of lower molar mass
fractions. After 4 h all lignin has been completely solubilized. The highest H/C and lowest O/C molar ratios
were obtained with prolonged reaction times.
Lignin depolymerization in aqueous ethanol leads to
a reduced formation of char, which might be attributed
to the solubility power of ethanol and the hydrogen
307
donation capability of ethanol to stabilize generated
free lignin radicals (Ye et al., 2012).
Zakzeski et al. 2012 used ethanol/water mixtures that
greatly enhanced the solubility of different technical lignins (e.g. kraft, organosolv and sugarcane bagasse
lignin) and consequently led to higher yields of monoaromatics in one-pot lignin liquid phase reforming (LPR)
reactions. During solubilization extensive cleavage of
various ether linkages in the macromolecule occurred.
The Pt/Al2O3-catalyzed LPR reactions yielded up to
17% of monomeric guaiacol-type products for kraft
lignin in the presence of H2SO4. Depending on the lignin
source and the used cocatalyst, different product distributions and light gases such as hydrogen and methane
were formed. Char formation was not observed in any
of the reactions. HDO reduction of solubilized lignin using transition metal catalysts led to the formation of
alkyl-substituted guaiacol-type molecules with isolated
yields of up to 6% for Pt/Al2O3.
Toledano et al. 2012 used a microwave-assisted
bifunctional catalytic process using tetralin or formic
acid as in situ hydrogen donating solvents lead to over
30% bio-oil yield mostly enriched in monomeric and
dimeric phenolic compounds. However, the amount of
biochar and residual lignin still needs to be reduced.
Organosolv and kraft lignin were depolymerized
using a silica-alumina catalyst in a water/1-butanol
mixture to a yield of 85e88 C-mol%. In a second step
the lignin-derived slurry was cracked over a ZrO2e
Al2O3eFeOx catalyst in water/1-butanol Total recovered
phenols is 6.6e8.6% and the conversion of methoxy
phenol reached 92e94% to phenol and cresol (Yoshikawa et al., 2013).
Sub- and Supercritical Water
Depolymerization of lignin in sub- and supercritical
water (pc > 22.1 MPa; Tc > 374 C) lead to extensive
lower molar mass fragments, dealkylation and demethoxylation, but a part of these fragments tend to
cross-link in larger fragments. The economic viability
of this process is severely controlled by the extent to
which the heat is recovered from the effluents. The yield
of monomers is positively correlated with base concentration added with maximum yield of one-third of the
initial lignin. Low molecular weight fraction yields
increased with longer reaction times in supercritical
water without catalysts at 350e400 C and 25e40 MPa.
The water-soluble fraction consists of catechol (28%),
phenol (7.5%), and cresols (11%), suggesting the cleavage of both ether and carbonecarbon (Wahyudiono
et al., 2008). Addition of phenolics (e.g. phenol and
p-cresol Okuda et al., 2004a,b, 2008; Fang et al., 2008)
gives a complete depolymerization of lignin into dimers
without char formation. Phenol and p-cresol depressed
308
17. LIGNOCELLULOSE-BASED CHEMICAL PRODUCTS
cross-linking reactions due to entrapment of reactive
fragments, like formaldehyde, and capping of active
sites like Ca in the lignin structure.
Supercritical Solvents
Lignin depolymerization in supercritical solvents
next to water includes ethanol, methanol, CO2, and
CO2/acetone/water. The supercritical properties of
these fluids are displayed in Table 17.10.
The choice for using CO2 as solvent is obvious as CO2 is
cheap, environmentally friendly and generally recognized as safe by the US Food and Drug Administration.
scCO2 has other advantages because of its high diffusivity
combined with its easily tunable solvent strength. To use
CO2 under supercritical conditions, the temperature
needed is low (>31 C) and the pressure needed relatively
low (>7.4 MPa) in comparison to other supercritical solvents (Table 17.10). Additionally, CO2 is a gas at room
temperature and pressure, which leads to a solvent-free
product after pressure expansion. A drawback of scCO2
is its low polarity, which is comparable to hexane, but
this problem can be overcome by using cosolvents to
change the polarity of the SCF (Herrero et al., 2010).
Furthermore, SCF processing based on CO2 enables the
easy recycling of CO2, which is advantageous for the
development of a sustainable process. Research performed on supercritical processing of lignin to produce
aromatic compounds has been summarized hereafter.
Depolymerization of lignin model compounds and
organosolv lignin have been studied in supercritical alcohols like methanol and ethanol in a temperature range
of >239 C and a pressure of >8.1 MPa. By using bases
such as KOH and NaOH a high depolymerization conversion was obtained. The dominant depolymerization
route is the solvolysis of ether linkages in the lignin
structure while the carbonecarbon linkages are mostly
stable (Miller et al., 1999; Minami et al., 2003).
Yuan et al. (2010) used BCD at mild temperatures
(220e300 C) of kraft lignin in watereethanol into oligomers with a negligible char and gas production.
TABLE 17.10
Supercritical Fluid Parameters
Solvent
Carbon Dioxide
Critical
Temperature Tc ( C)
Critical Pressure
Pc (MPa)
31
7.4
Water
374
22.1
Acetone
235
4.7
Methanol
239
8.1
Ethanol
241
6.2
1-Butanol
287
4.9
Source: Reid et al., 1987.
However, under the conditions applied lignin could
not be completely degraded into monomers.
Oxidation of lignin and lignin model compounds
with peroxide was studied under scCO2 conditions in
the absence of alkali. The 5-5 biphenols were shown to
be degraded and in this process mostly the formation
of carboxylic acids from kraft lignin was observed
(Argyropoulos et al., 2006).
Gosselink et al. (2012) found that hardwood and
wheat straw organosolv lignins were depolymerized in
supercritical carbon dioxide/acetone/water fluid at
300 C and 100 bar into 10e12% monomeric aromatic
compounds. Small amounts of formic acid were introduced as in situ hydrogen donor. Furthermore, lignin
is converted into a phenolic oil consisting of both monomeric and oligomeric aromatic compounds. Interestingly, maximum individual yields of 3.6% for syringol
and 2.0% for syringic acid based on lignin were obtained. Depolymerized phenolic products and char
were separated during this process by pressure expansion. As during this process competition occurs between
lignin depolymerization and recondensation of fragments a substantial amount of char is formed.
Ionic Liquids
Recent work has demonstrated that ILs are excellent
solvents for processing woody biomass and technical
lignin. Seeking to exploit ILs as media for depolymerization of lignin, lignin model compounds were treated using
Brønsted acid catalysts in 1-ethyl-3-methylimidazolium
triflate at temperatures below 200 C. A 11.6% molar yield
of the dealkylation product 2-methoxyphenol from the
model compound 2-methoxy-4-(2-propenyl)phenol and
cleaved 2-phenylethyl phenyl ether, a model for lignin
ethers, was obtained. However, depolymerization of organosolv lignin to monomers failed (Binder et al., 2009).
The oxidative depolymerization of lignin in
1-ethyl-3-methylimidazolium
trifluoromethanesulfonate with Mn(NO3)2 catalyst yielded 11.5 wt% of pure
2,6-dimethoxy-1,4-benzoquinone (Stark et al., 2010).
Hossain and Aldous (2012) reviewed the achieved
results for depolymerization of lignin model compounds in ILs, but for technical lignin samples mixed
results have been obtained. It should be emphasized
that conversion of lignin in ILs is still at its infancy,
but there is certain potential to make use of these solvents in the valorization of lignin into aromatic
chemicals.
Future Perspectives of Lignin Aromatics
Although the research activities show that in 2013
there is a great interest in using lignin as a renewable
resource for the production of aromatic chemicals, it
REFERENCES
is also clear that commercial utilization will take
substantial time. So far the literature results show that
relatively low conversion yields to about 10 wt% based
on dry lignin and resulting complex mixtures hinder
the commercial utilization of these processes.
The Netherlands can play an important role in the
lignin aromatics valorization technologies as technology
provider with the strong presence and strategic location
of academia, chemical industries and other stakeholders
in the value chain. In the Port of Rotterdam in the
Netherlands about 5 million tons of aromatic building
blocks are currently produced and distributed to the
chemical industry in the Netherlands, Germany,
Belgium and other countries. These aromatic bulk chemicals used and produced consist of so-called aromatic
monomers like BTX, styrene and phenol.
In the Netherlands in 2010 the Wageningen UR
Lignin Platform was established, which plays an important role in this lignin valorization value chain development (http://www.wageningenur.nl/Lignin-Platform.
htm). This is a joint research program with academia
and industry dedicated to develop the entire lignin bioaromatics value chain. Besides this initiative other networks in Canada and Scandinavia work on lignin
valorization topics.
Considering these increased research activities on
lignin conversion and valorization technologies it can
be concluded that the race to produce bioaromatics
from renewable feedstocks is wide open. Next to lignin
as aromatic feedstock conversion of carbohydrates to
aromatic chemicals is also under investigation (Dodds
and Humpheys, 2013). It should also be emphasized
that many of the above-discussed technologies are at a
very early stage, which makes it at present unclear if
and which of those routes can become cost competitive
as well as sustainable.
CONCLUSIONS AND FURTHER
PERSPECTIVES
The use of lignocellulosic feedstocks as an important
source for chemicals and fuels is gaining momentum.
This chapter has indicated that there are many variables to take into consideration. We have learned that
lignocellulosic biomass consists of three major groups:
the softwoods, hardwoods and grasses and that there
is also great heterogeneity within each group. There
are multiple pretreatment routes developed that are
currently scaled up to pilot, demonstration and commercial scales. The optimal pretreatment technology
needs to be selected based on the available feedstock
and the desired product. At the moment there are no
indications that one pretreatment method will be the
optimal route for all feedstocks and products. Many
309
routes toward chemical building blocks based on
monomeric carbohydrates are ready for scaling up;
lignin conversion into monomeric building blocks
needs substantial additional R&D before economical
processes are within reach.
References
Agbor, V.B., Cicek, N., Sparling, R., Berlin, A., Levin, D.B., 2011.
Biomass pretreatment: fundamentals toward application.
Biotechnol. Adv. 29, 675e685.
Agirrezabal-Telleria, I., Larreategui, A., Requies, J., Güemez, M.B.,
Arias, P.L., 2011. Furfural production from xylose using sulfonic
ion-exchange resins (Amberlyst) and simultaneous stripping with
nitrogen. Bioresour. Technol. 102, 7478e7485.
Ahmad, T., Kenne, L., Olsson, K., Theander, O., 1995. The formation of
2-furaldehyde and formic acid from pentoses in slightly acidic
deuterium oxide studied by 1H-NMR spectroscopy. Carbohydr.
Res. 276, 309e320.
Aida, T.M., Tajima, K., Watanabe, M., Saito, Y., Kuroda, K., Nonaka, T.,
Hattori, H., Smith Jr, R.L., Arai, K., 2007. Dehydration of d-glucose
in high temperature water at pressures up to 80 MPa. J. Supercrit.
Fluids 42, 110e119.
Alonso, M.D., Bond, J.Q., Dumesic, J.A., 2010. Catalytic conversion of
biomass to biofuels. Green Chem. 12, 1493e1513.
Alvira, P., Tomás-Pejó, E., Ballesteros, M., Negro, M.J., 2010. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour. Technol.
101, 4851e4861.
Amen-Chen, C., Pakdel, H., Roy, C., 2001. Production of monomeric
phenols by thermochemical conversion of biomass: a review.
Bioresour. Technol. 79, 277e299.
Anellotech Inc, 2010. Low Cost Petrochemicals from Non-food
Biomass. accessed December 2012. www.anellotech.com.
Anet, E.F.L.J., 1964. 3-deoxyglycosuloses (3-deoxyglycosones) and the
degradation of carbohydrates. Adv. Carbohydr. Chem. 19, 181e218.
Antal, M.J., Leesomboon Jr, T., Mok, W.S., Richards, G.N., 1991.
Mechanism of formation of 2-furaldehyde from D-xylose. Carbohydr. Res. 217, 71e86.
Argyropoulos, D.S., Gaspar, A.R., Lucia, L.A., Rojas, O.J., 2006.
Supercritical CO2 oxidation of lignin. Production of high value
added Products. Chim. l’Ind. 1 (88), 74e79.
Azadi, P., Inderwildi, O.R., Farnood, R., King, D.A., 2013. Liquid fuels,
hydrogen and chemicals from lignin: a critical review. Renewable
Sustainable Energy Rev. 21, 506e523.
Bacovsky, D., Ludwiczek, N., Ognissanto, M., Wörgetter, M., 2013.
Status of Advanced Biofuels Demonstration Facilities in 2012. a
report to IEA Bioenergy task 39. http://demoplants.bioenergy
2020.eu/files/Demoplants_Report_Final.pdf.
Bakker, R.R., den Uil, H., van Ree, R., et al., 2010. Financieel-Economische Aspecten van Biobrandstofproductie e Desktopstudie
Naar de Invloed van Co-Productie van Bio-Based Producten op de
Financiele Haalbaarheid van Biobrandstoffen. rapport 1175. WUR
Food and Biobased Research, Wageningen, Nederland. Oktober
2010 (in Dutch). http://edepot.wur.nl/202366.
Balat, M., Ayar, G., 2005. Biomass energy in the world, use of biomass
and potential trends. Energy Sources 27, 931e940.
Barakat, A., de Vries, H., Rouau, X., 2013. Dry fractionation process as
an important step in current and future lignocellulose biorefineries: a review. Bioresour. Technol. 134, 362e373.
Baumberger, S., Abaecherli, A., Fasching, M., Gellerstedt, G.,
Gosselink, R., Hortling, B., Li, J., Saake, B., De Jong, E., 2007. Molar
mass determination of lignins by size-exclusion chromatography:
towards standardisation of the method. Holzforschung 61, 459e468.
310
17. LIGNOCELLULOSE-BASED CHEMICAL PRODUCTS
Binder, J.G., Gray, M.J., White, J.F., Zhang, Z.C., 2009. Reactions of
lignin model compounds in ionic liquids. Biomass Bioenergy 33,
1122e1130.
Binder, J.B., Blank, J.J., Cefali, A.V., Raines, R.T., 2010. Synthesis of
furfural from xylose and xylan. ChemSusChem 3, 1268e1272.
Bozell, J.J., Petersen, G.R., 2010. Technology development for the
production of biobased products from biorefinery carbohydratesd
the US Department of Energy’s “Top 10” revisited. Green Chem.
12, 539e554.
Brodeur, G., Yau, E., Badal, K., Collier, J., Ramachandran, K.B.,
Ramakrishnan, S., 2011. Chemical and physicochemical pretreatment of lignocellulosic biomass: a review. Enzym. Res. 2011. http://
dx.doi.org/10.4061/2011/787532. Article ID 787532, 17 pages.
Brown, R.C., 2003. Biorenewable Resources e Engineering New
Products from Agriculture, first ed. Blackwell Publishing, USA.
Brown, D.W., Floyd, A.J., Kinsman, R.G., Roshan-Ali, Y., 1982. Dehydration reactions of fructose in non-aqueous media. J. Chem.
Technol. Biotechnol. 32, 920e924.
Bu, Q., Lei, H., Zacher, A.H., Wanga, L., Ren, R., Liang, J., Wei, Y.,
Liu, Y., Tang, J., Zhang, Q., Ruan, R., 2012. A review of catalytic
hydrodeoxygenation of lignin-derived phenols from biomass
pyrolysis. Bioresour. Technol. 124, 470e477.
Buntara, T., Noel, S., Phua, P.H., Melian-Cabrera, I., de Vries, J.G.,
Heeres, H.J., 2011. Caprolactam from renewable resources: catalytic conversion of 5-hydroxymethylfurfural into caprolactone.
Angew. Chem. Int. Ed. 50, 7083e7087.
Cherubini, F., Jungmeier, G., Wellisch, M., Willke, T., Skiadas, I.,
van Ree, R., de Jong, E., 2009. Toward a common classification
approach for biorefinery systems. Biofuels Bioprod. Biorefin. 3,
534e546.
Chheda, J.N., Dumesic, J.A., 2007. An overview of dehydration, aldolcondensation and hydrogenation processes for production of
liquid alkanes from biomass-derived carbohydrates. Catal. Today
123, 59e70.
Chundawat, S.P.S., Venkatesh, B., Dale, B.E., 2007. Effect of particle
size based separation of milled corn stover on AFEX pretreatment
and enzymatic digestibility. Biotechnol. Bioeng. 96, 219e231.
Climent, M.J., Corma, A., Iborra, 2011a. Heterogeneous catalysts for
the one-pot synthesis of chemicals and fine chemicals. Chem. Rev.
111, 1072e1133.
Climent, M.J., Corma, A., Iborra, 2011b. Converting carbohydrates to
bulk chemicals and fine chemicals over heterogeneous catalysts.
Green Chem. 13, 520e540.
Cope, A.C., 1959. Production and recovery of furans. US2917520.
da Costa Sousa, L., Chundawat, S.P.S., Balan, V., Dale, B.E., 2009.
‘Cradle-to-grave’ assessment of existing lignocellulose pretreatment technologies. Curr. Opin. Biotechnol. 20, 339e347.
de Jong, E., Vijlbrief, T., Hijkoop, R., Gruter, G.-J.M., van der Waal, J.C.,
2012a. Promising results with YXY Diesel components in an ESC
testcycle using a PACCAR Diesel engine. Biomass Bioenergy 36,
151e159.
de Jong, E., Higson, A., Walsh, P., Wellisch, M., 2012b. Product
developments in the bio-based chemicals arena. Biofuels, Bioprod.
Biorefin. 6, 606e624.
de Jong, E., Dam, M.A., Sipos, L., Gruter, G.-J.M., 2013. Furandicarboxylic acid (FDCA), a versatile building block for a very
interesting class of polyesters. In: Smith, P.B., Gross, R. (Eds.), ACS
Symposium Series “Biobased Monomers, Polymers and Materials”,
pp. 1e13. http://dx.doi.org/10.1021/bk-2012-1105.ch001.
de Wild, P., Van der Laan, R., Kloekhorst, A., Heeres, E., 2009. Lignin
valorisation for chemicals and (transportation) fuels via (catalytic)
pyrolysis and hydrodeoxygenation. Environ. Prog. Sustainable
Energy 28 (3), 461e469.
Delmas, M., 2008. Vegetal refining and agrichemistry. Chem. Eng.
Technol. 31 (5), 792e797.
Deutsch, K.L., Shanks, B.H., 2012. Hydrodeoxygenation of lignin
model compounds over a copper chromite catalyst. Appl. Catal., A
447-448, 144e150.
Dhepe, P.L., Fukuoka, A., 2008. Cellulose conversion under heterogeneous catalysis. ChemSusChem 1, 969e975.
Dias, A.S., Lima, S., Pillinger, M., Valente, A.A., 2010. Furfural and
furfural-based industrial chemicals. In: Pignataro, B. (Ed.), Ideas in
Chemistry and Molecular Sciences, Advances in Synthetic Chemistry, vol. 8. Wiley-VCH, Weinheim, pp. 167e186.
Dodds, D., Humpheys, B., 2013. Production of aromatic chemicals
from biobased feedstock. In: Imhof, P., van der Waal, J.C. (Eds.),
Catalytic Process Development for Renewable Materials. WileyVCH Verlag GmbH & Co, pp. 183e238.
Dorrestijn, E., Kranenburg, M., Poinsot, D., Mulder, P., 1999.
Lignin depolymerization in hydrogen-donor solvents. Holzforschung 53 (6), 611e616.
Düll, G., 1895. Über die einwirkung van oxalsäure auf inulin. Chem.
Ztg. 19, 216e220.
Ebringerova, A., Hromadkova, Z., Heinze, T., 2005. Hemicellulose.
Adv. Polym. Sci. 186, 1e67.
Eggeman, T., Elander, R.T., 2005. Process and economic analysis of
pretreatment technologies. Bioresour. Technol. 96, 2019e2025.
European Renewable Resources and Materials Association (ERRMA),
2011. EU-Public/Private Innovation Partnership “Building the Bioeconomy by 2020”. www.errma.com.
Evju, H., 1979. Process for the preparation of 3-methoxy4-hydroxybenzaldehyde. US Patent 4151207.
Fang, Z., Sato, T., Smith Jr, R.L., Inomata, H., Arai, K., Kozinski, J.A., 2008.
Reaction chemistry and phase behavior of lignin in high-temperature
and supercritical water. Bioresour. Technol. 99, 3424e3430.
Fayet, C., Gelas, J., 1983. Nouvelle méthode de préparation du
5-hydroxyméthyl-2-furaldéhyde par action de sels d’ammonium
ou d’immonium sur les mono-, oligo- et poly-saccharides. Accès
direct aux 5-halogénométhyl-2-furaldéhydes. Carbohydr. Res. 122,
59e68.
Feather, M.S., Harris, J.F., 1973. Dehydration reactions of carbohydrates. Adv. Carbohydr. Chem. 28, 161e224.
Fengel, D., Wegener, G., 1984. Wood: Chemistry, Ultrastructure,
Reactions. Kessel Verlag, Munich, Germany, ISBN 3-935638-39-6.
pp. 613.
Fleche, G., Gaset, A., Gorrichon, J.-P., Truchot, E., Sicard, P., 1982.
Procedé de fabrication du 5-hydroxymethylfurfural. US4339387.
Frost, Sullivan, 2011. Advances in Fermentation Technologies - An
Industry Overview. http://www.technicalinsights.frost.com.
Gallezot, P., 2012. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 41, 1538e1558.
Gandini, A., Belgacem, M.N., 1997. Furans in polymer chemistry. Prog.
Polym. Sci. 22, 1203e1379.
Garlock, R.J., Balan, V., Dale, B.E., Pallapolu, V.R., et al., 2011.
Comparative material balances around pretreatment technologies
for the conversion of switchgrass to soluble sugars. Bioresour.
Technol. 102, 11063e11071.
Geilen, F.M.A., Engendahl, B., Harwardt, A., Marquardt, W.,
Klankermayer, J., Leitner, W., 2010. Selective and flexible transformation of biomass-derived platform chemicals by a multifunctional catalytic system. Angew. Chem. 122, 5642e5646.
Gellerstedt, G., Li, J., Eide, I., Kleinert, M., Barth, T., 2008. Chemical
structures present in biofuel obtained from lignin. Energy Fuels 22,
4240e4244.
Girio, F.M., Fonseca, C., Carvalheiro, F., Duarte, L.C., Marques, S.,
Bogel-Łukasik, R., 2010. Hemicelluloses for fuel ethanol: a review.
Bioresour. Technol. 101, 4775e4800.
Goshadrou, A., Karimi, K., Taherzadeh, M.J., 2013. Ethanol and biogas
production from birch by NMMO pretreatment. Biomass Bioenergy 49, 95e101.
REFERENCES
Gosselink, R.J.A., 2011. Lignin as a Renewable Aromatic Resource for
the Chemical Industry, Dissertation. Wageningen University, The
Netherlands, ISBN 978-94-6173-100-5.
Gosselink, R.J.A., Abacherli, A., Semke, H., Malherbe, R., Kauper, P.,
Nadif, A., van Dam, J.E.G., 2004. Analytical protocols for characterisation of sulphur-free lignin. Ind. Crops Prod. 19, 271e281.
Gosselink, R.J.A., Teunissen, W., van Dam, J.E.G., de Jong, E.,
Gellerstedt, G., Scott, E.L., Sanders, J.P.M., 2012. Lignin depolymerisation in supercritical carbon dioxide/acetone/water fluid for the
production of aromatic chemicals. Bioresour. Technol. 106, 173e177.
Gruter, G.-J.M., Dautzenberg, F., 2007. Method for the Synthesis of
5-Alkoxymethylfurfural Ethers and Their Use. Furanix Technologies B. V., The Netherlands. WO2007104514.
Gruter, G.-J., de Jong, E., 2009. Furanics: novel fuel options from carbohydrates. Biofuels Technol. 1, 11e17.
Gürbüz, E.I., Wettstein, S.G., Dumesic, J.A., 2012. Conversion of
hemicellulose to furfural and levulinic acid using biphasic reactors
with alkylphenol solvents. ChemSusChem 5, 383e387.
Han, J.S., 1998. Properties of nonwood fibers. In: Proceedings of the
Korean Society of Wood Science and Technology Annual Meeting.
The Korean Society of Wood Science and Technology, Seoul, Korea,
pp. 3e12.
Hatakeyama, H., Hatakeyama, T., 2010. Lignin structure, properties
and applications. Adv. Polym. Sci. 232, 1e63.
Henriksson, G., Li, G., Zhang, L., Lindström, M.E., 2010. Lignin utilization. In: Crocker, M. (Ed.), Thermochemical Conversion of
Biomass to Liquid Fuels and Chemicals. Royal Society of Chemistry, pp. 223e262.
Hepditch, M.M., Thring, R.W., 2000. Degradation of solvolysis lignin
using Lewis acid catalysts. Can. J. Chem. Eng. 78 (1), 226e231.
Herrero, M., Mendiola, J.A., Cifuentes, A, Ibañeza, E., 2010. Supercritical fluid extraction: Recent advances and applications.
J. Chrom. A 1217, 2495e2511.
Holladay, J.E., Bozell, J.J., White, J.F., Johnson, D., 2007. Top ValueAdded Chemicals from Biomass Volume II - Results of Screening
for Potential Candidates from Biorefinery Lignin. http://www.
ntis.gov/ordering.htm.
Hossain, M.M., Aldous, L., 2012. Ionic liquids for lignin processing:
dissolution, isolation, and conversion. Aust. J. Chem. 65, 1465e1477.
Hoydonckx, H.E., van Rhijn, W.M., van Rhijn, W., de Vos, D.E.,
Jacobs, P.A., 2007. Furfural and derivatives. Ullmann’s Encycl. Ind.
Chem., 1e29.
Huber, G.W., Iborra, S., Corma, A., 2006. Synthesis of transportation
fuels from biomass: chemistry, catalysts, and engineering. Chem.
Rev. 106, 4044e4098.
Jing, Q., Lu, X.-Y., 2007. Kinetics of non-catalyzed decomposition of
D-xylose in high temperature liquid water. Chin. J. Chem. Eng. 15,
666e669.
Jönsson, L.J., Alriksson, B., Nilvebrant, N.O., 2013. Bioconversion of
lignocellulose: inhibitors and detoxification. Biotechnol. Biofuels 6,
16e26.
Kamm, B., Gruber, P.R., Kamm, M. (Eds.), 2006. Biorefineries e
Industrial Processes and Products, Status Quo and Future Directions, vols 1e2. Whiley-VCH, Weinheim.
Kiermayer, J., 1895. Über ein furfurolderivat aus lävulose. Chem. Ztg.
19, 1003e1006.
Kim, S.B., Lee, M.R., Park, E.D., Lee, S.M., Lee, H.K., Park, K.H.,
Park, M.J., 2011. Kinetic study of the dehydration of D-xylose in
high temperature water. React. Kinet. Mech. Catal. 103, 2267e2277.
Kleinert, M., Barth, T., 2008. Phenols from lignin. Chem. Eng. Technol.
31, 736e745.
Kleinert, M., Gasson, J.R., Barth, T., 2009. Optimizing solvolysis
conditions for integrated depolymerisation and hydrodeoxygenation of lignin to produce liquid biofuel. J. Anal. Pyrolysis 85, 108e117.
311
Klinke, H.B., Ahring, B.K., Schmidt, A.S., Thomsen, A.B., 2002.
Characterization of degradation products from alkaline wet
oxidation of wheat straw. Bioresour. Technol. 82, 15e26.
Kootstra, A.M.J., Mosier, N.S., Scott, E.L., Beeftink, H.H.,
Sanders, J.P.M., 2009. Differential effects of mineral and organic
acids on the kinetics of arabinose degradation under lignocellulose
pretreatment conditions. Biochem. Eng. J. 43, 92e97.
Kumar, P., Barrett, D.M., Delwiche, M.J., Stroeve, P., 2009. Methods for
pretreatment of lignocellulosic biomass for efficient hydrolysis and
biofuel production. Ind. Eng. Chem. Res. 48, 3713e3729.
Kuster, B.F.M., 1990. 5-hydroxymethylfurfural. A review focusing on
its manufacture. Starch/Stärke 43, 314e321.
Kuster,
B.F.M.,
Laurens,
J.,
1977.
Preparation
of
5-hydroxymethylfurfural. Part II: dehydration of fructose in a tube
reactor using polyethyleneglycol as solvent. Starch/Stärke 29,
172e176.
Kuster, B.F.M., van der Steen, H.J.C., 1977. Preparation of
5-hydroxymethylfurfural. Part I: dehydration of fructose in a
continuous stirred tank reactor. Starch/Stärke 29, 99e103.
Lamminpää, K., Ahola, J., Tanskanen, J., 2012. Kinetics of xylose
dehydration into furfural in formic acid. Ind. Eng. Chem. Res. 51,
6297e6303.
Lange, J.-P., van der Heide, E., van Buijtenen, J., Price, R., 2012.
Furfuralda promising platform for lignocellulosic biofuels.
ChemSusChem 5, 150e166.
Lichtenthaler, F.W., Peters, S., 2004. Carbohydrates as green raw
materials for the chemical industry. C. R. Chim. 7, 65e90.
Lignol Energy, 2013. Lignol Energy Corporation Announces First
Commercial Supply Agreement, for HP-L Lignin in the Thermoplastics Sector. [Online] 1 February 2013. [Cited: 18 April 2013].
http://www.lignol.ca/news/News-2013/News%20Release%
2031Jan3013%20final.pdf.
Limayem, A., Ricke, S.C., 2012. Lignocellulosic biomass for bioethanol
production: current perspectives, potential issues and future
prospects. Prog. Energy Combust. Sci. 38, 449e467.
Lin, Y.-C., Huber, G.W., 2009. The critical role of heterogeneous
catalysis in lignocellulosic biomass conversion. Energy Environ.
Sci. 2, 68e80.
Lloyd, T.A., Wyman, C.E., 2005. Combined sugar yields for dilute
sulfuric acid pretreatment of corn stover followed by enzymatic
hydrolysis of the remaining solids. Bioresour. Technol. 96,
1967e1977.
Mäki-Arvela, P., Salminen, E., Riittonen, T., Virtanen, P., Kumar, N.,
Mikkola, J.-P., 2012. The challenge of efficient synthesis of biofuels
from lignocellulose for future renewable transportation fuels. Int. J.
Chem. Eng. 2012 (674761).
Mamman, A.S., Lee, J.-M., Kim, Y.-C., Hwang, I.T., Park, N.-J.,
Hwang, Y.K., Chang, J.-S., Hwang, J.-S., 2008. Furfural: hemicellulose/xylose derived biochemical. Biofuels, Bioprod. Biorefin. 2,
438e454.
Marcotullio, G., de Jong, W., 2010. Chloride ions enhance furfural
formation from D-xylose in dilute aqueous acidic solutions. Green
Chem. 12, 1739e1746.
Marcotullio, G., de Jong, W., 2011. Furfural formation from D-xylose: the
use of different halides in dilute aqueous acidic solutions allows for
exceptionally high yields. Carbohydr. Res. 346, 1291e1293.
Martin, C., Marcet, M., Thomsen, A.B., 2008. Comparison between
wet oxidation and steam explosion as pretreatment methods for
enzymatic hydrolysis of sugarcane bagasse. Bioresources 3 (3),
670e683.
Mascal, M., Nikitin, E.B., 2008. Direct, high-yield conversion of cellulose into biofuel. Angew. Chem. 120, 1e4.
Menon, V., Rao, M., 2012. Trends in bioconversion of lignocellulose:
biofuels, platform chemicals & biorefinery concept. Prog. Energy
Combust. Sci. 38, 522e550.
312
17. LIGNOCELLULOSE-BASED CHEMICAL PRODUCTS
Miller, J.E., Evans, L., Littlewolf, A., Trudell, D.E., 1999. Batch microreactor studies of lignin and lignin model compound depolymerization by bases in alcohol solvents. Fuel 78, 1363e1366.
Minami, E., Kawamoto, H., Saka, S., 2003. Reaction behavior of lignin
in supercritical methanol as studied with lignin model compounds. J. Wood Sci. 49, 158e165.
Modenbach, A.A., Nokes, S.E., 2012. The use of high-solids loadings in
biomass pretreatment - a review. Biotechnol. Bioeng. 109,
1430e1442.
Monteil-Rivera, F., Phuong, M., Ye, M., Halasz, A., Hawari, J., 2013.
Isolation and characterization of herbaceous lignins for applications in biomaterials. Ind. Crops Prod. 41, 356e364.
Mora-Pale, M., Meli, L., Doherty, T.V., Linhardt, R.J., Dordick, J.S.,
2011. Room temperature ionic liquids as emerging solvents for the
pretreatment of lignocellulosic biomass. Biotechnol. Bioeng. 108
(6), 1229e1245.
Moreau, C., Durand, R., Razigade, S., Duhamet, J., Faugeras, P.,
Rivalier, P., Ros, P., Avignon, G., 1996. Dehydration of fructose to
5-hydroxymethylfurfural over H-mordenites. Appl. Catal., A 145,
211e224.
Moreau, C., Durand, R., Peyron, D., Duhamet, J., Rivalier, P., 1998.
Selective preparation of furfural from xylose over microporous
solid acid catalysts. Ind. Crops Prod. 7, 95e99.
Moreau, C., Belgacem, M.N., Gandini, A., 2004. Recent catalytic advances in the chemistry of substituted furans from carbohydrates
and in the ensuing polymers. Top. Catal. 27, 11e30.
Mosier, N., Wyman, C.E., Dale, B.E., Elander, R., Lee, Y.Y.,
Holtzapple, M., Ladisch, M., 2005. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour.
Technol. 96, 673e686.
Musau, R.M., Munavu, R.M., 1987. The preparation of
5-hydroxymethyl-2-furaldehyde (HMF) from D-fructose in the
presence of DMSO. Biomass 13, 67e75.
Nabarlatz, D., Ebringerová, A., Montané, D., 2007. Autohydrolysis
of agricultural by-products for the production of xylooligosaccharides. Carbohydr. Polym. 69, 20e28.
Nakagawa, Y., Tomishige, K., 2012. Production of 1,5-pentanediol
from biomass via furfural and tetrahydrofurfuryl alcohol. Catal.
Today 195, 136e143.
Nakamura, Y., Morikawa, S., 1980. The dehydration of D-fructose to
5-hydroxymethyl-2-furaldehyde. Bull. Chem. Soc. Jpn. 53,
3705e3706.
Newt, F.H., 1951. The formation of furan compounds from hexoses.
Adv. Carbohydr. Chem. 6, 83e106.
Nexant ChemSystems, 2012. Global Chemicals Demand Estimate.
White Plains, New York.
Nowakowski, D.J., Bridgwater, A.V., Elliott, D.C., Meier, D.,
de Wild, P., 2010. Lignin fast pyrolysis: results from an international collaboration. J. Anal. Appl. Pyrolysis 88 (1), 53e72.
Öhman, F., Theliander, H., Tomani, P., Axegard, P., 2009. A method
for separating lignin from black liquor, a lignin product, and use
of a lignin product for the production of fuels or materials.
WO104995.
Okuda, K., Man, X., Umetsu, M., Takami, S., Adschiri, T., 2004a.
Efficient conversion of lignin into single chemical species by solvothermal reaction in water-p-cresol solvent. J. Phys.: Condens.
Matter 16, 1325e1330.
Okuda, K., Umetsu, M., Takami, S., Adschiri, T., 2004b. Disassembly of
lignin and chemical recovery - rapid depolymerization of lignin
without char formation in water - phenol mixtures. Fuel Process.
Technol. 85, 803e813.
Okuda, K., Ohara, S., Umetsu, M., Takami, S., Adschiri, T., 2008.
Disassembly of lignin and chemical recovery in supercritical water
and p-cresol mixture: studies on lignin model compounds. Bioresour. Technol. 99 (6), 1846e1852.
Pedersen, P., Meyer, A.S., 2010. Lignocellulose pretreatment severity e
relating pH to biomatrix opening. New Biotechnol 27, 739e750.
Peng, F., Peng, P., Xu, F., Sun, R.-C., 2012. Fractional purification and
bioconversion of hemicelluloses. Biotechnol. Adv. 30, 879e903.
Ralph, J., Brunow, G., Boerjan, W., 2007. Lignins. John Wiley & Sons,
Ltd.
Rapp, K.M., 1987. Process for preparing pure 5-hydroxymethylfurfuraldehyde. Süddeutsche Zucker-Aktiengesellschaft. US
4740605A1.
Reid, R.C., Prausnitz, J.M., Poling, B.E., 1987. The Properties of Gases
and Liquids. McGraw-Hill, New York.
Roberts, V., Stein, V., Reiner, T., Lemonidou, A., Li, X., Lercher, J., 2011.
Towards quantitative catalytic lignin depolymerization. Chem.
Eur. J. 17, 5939e5948.
Román-Leshkov, Y., Dumesic, J.A., 2009. Solvent effects on fructose
dehydration to 5-hydroxymethylfurfural in biphasic systems
saturated with inorganic salts. Top. Catal. 52, 297e303.
Román-Leshkov, Y., Chheda, J.N., Dumesic, J.A., 2006. Phase modifiers promote efficient production of hydroxymethylfurfural from
fructose. Science 312, 1933e1937.
Rong, C., Ding, X., Zhu, Y., Li, Y., Wang, L., Qu, Y., Ma, X.,
Wang, Z., 2012. Production of furfural from xylose at atmospheric pressure by dilute sulfuric acid and inorganic salts.
Carbohydr. Res. 350, 77e80.
Root, D.F., Saeman, J.F., Harris, J.F., Neill, W.K., 1959. Chemical conversion of wood residues, part II: kintetics of the acid-catalyzed
conversion of xylose to furfural. For. Prod. J. 9, 158e165.
Ruiz, H.A., Rodriguez-Jasso, R.M., Fernandes, B.D., Vicente, A.A.,
Teixeira, J.A., 2013. Hydrothermal processing, as an alternative for
upgrading agriculture residues and marine biomass according to
the biorefinery concept: a review. Renewable Sustainable Energy
Rev. 21, 35e51.
Sahu, R., Dhepe, P.L., 2012. A one-pot method for the selective conversion of hemicellulose from crop waste into C5 sugars and
furfural by using solid acid catalysts. ChemSusChem 5, 751e761.
Sain, B., Chaudhuri, A., Borgohain, J.N., Baruah, B.P., Ghose, J.L., 1982.
Furfural and furfural-based industrial chemicals. J. Sci. Ind. Res.
41, 431e438.
Sako, T., Sugeta, T., Nakazawa, N., Okubo, T., Sato, M., Taguchi, T.,
Hiaki, T., 1991. Phase equilibrium study of extraction and concentration of furfural produced in a reactor using supercritical
carbon dioxide extraction. J. Chem. Eng. Jpn. 24, 449e455.
Sako, T., Sugeta, T., Nakazawa, N., Okubo, T., Sato, M., Taguchi, T.,
Hiaki, T., 1992. Kinetic study of furfural formation accompanying
supercritical carbon dioxide extraction. J. Chem. Eng. Jpn. 25, 372e377.
Sales, F.G., Abreu, C.A.M., Pereira, J.A.F.R., 2004. Catalytic wet-air
oxidation of lignin in a three-phase reactor with aromatic aldehyde production. Braz. J. Chem. Eng. 21 (2), 211e218.
Sales, F.G., Maranhão, L.C.A., Filho, N.M.L., Abreu, C.A.M., 2007.
Experimental evaluation and continuous catalytic process for fine
aldehyde production from lignin. Chem. Eng. Sci. 62, 5386e5391.
Schädel, C., Blöchl, A., Richter, A., Hoch, G., 2010. Quantification and
monosaccharide composition of hemicelluloses from different
plant functional types. Plant Physiol. Biochem. 48, 1e8.
Schinzer, D., Bourguet, E., Ducki, S., 2004. Synthesis of furanoepothilone D. Chem. Eur. J. 10, 3217e3224.
Sinha, A.K., Kumar, V., Makkar, H.P.S., De Boeck, G., Becker, K., 2011.
Non-starch polysaccharides and their role in fish nutrition e a
review. Food Chem. 127, 1409e1426.
Speck Jr, J.C., 1958. The Lobry de Bruyn- Alberda van Ekenstein
transformation. Adv. Carbohydr. Chem. 13, 63e103.
Spiridon, I., Popa, V.I., 2008. Hemicelluloses: major sources, properties
and applications. In: Belgacem, N.M., Gandini, A. (Eds.), Monomers, Polymers and Composites from Renewable Resources.
Elsevier, Amsterdam, pp. 289e304.
REFERENCES
Stark, K., Taccardi, N., Bosmann, A., Wasserscheid, P., 2010. Oxidative
depolymerisation of lignin in ionic liquids. ChemSusChem. 3,
719e723.
Stöcker, M., 2008. Biofuels and biomass-to-liquid fuels in the biorefinery: catalytic conversion of lignocellulosic biomass using
porous materials. Angew. Chem. Int. Ed. 47, 9200e9211.
Suparno, O., Dovington, A.D., Evans, C.S., 2005. Kraft lignin degradation products for tanning and dyeing of leather. J. Chem. Technol. Biotechnol. 80, 44e49.
Szmant, H.H., Chundury, D.D., 1981. The preparation of
5-hydroxymethylfurfuraldehyde from high fructose corn syrup
and other carbohydrates. J. Chem. Tech. Biotechnol. 31, 135e145.
Tejado, A., Pena, C., Labidi, J., Echeverria, J.M., Mondragon, I., 2007.
Physico-chemical characterization of lignins from different sources
for use in phenol-formaldehyde resin synthesis. Bioresour. Technol. 98, 1655e1663.
Thring, R.W., 1994. Alkaline degradation of ALCELLÒ lignin. Biomass
Bioenergy 7, 125e130.
Toledano, A., Serrano, L., Labidi, J., 2012. Organosolv lignin depolymerization with different base catalysts. J. Chem. Technol. Biotechnol. 87, 1593e1599.
Van Haveren, J., Scott, E.L., Sanders, J.P.M., 2008. Review: bulk
chemicals from biomass. Biofuels Bioprod. Biorefin. 2, 41e57.
Van Putten, R.-J., van der Waal, J.C., de Jong, E., Rasrendra, C.B.,
Heeres, H.J., de Vries, J.G., 2013a. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem.
Rev. 113, 1499e1597.
Van Putten, R.-J., Dias, A.S., de Jong, E., 2013b. Furan based building
blocks from carbohydrates. In: Imhof, P., van der Waal, J.C. (Eds.),
Catalytic Process Development for Renewable Materials. WileyVCH Verlag GmbH & Co., pp. 81e118.
Vishtal, A., Kraslawski, A., 2011. Challenges in industrial applications
of technical lignins. Bioresources 6, 1e22.
Vispute, T.P., Zhang, H., Sanna, A., Xiao, R., Huber, G.W., 2010.
Renewable chemical commodity feedstocks from integrated catalytic processing of pyrolysis oils. Science 330, 1222e1227.
Vlachos, D.G., Chen, J.G., Gorte, R.J., Huber, G.W., Tsapatsis, M., 2010.
Catalysis center for energy innovation for biomass processing:
research strategies and goals. Catal. Lett. 140, 77e84.
Voitl, T., Rudolf von Rohr, P., 2010. Demonstration of a process for the
conversion of kraft lignin into vanillin and methyl vanillate by acidic
oxidation in aqueous methanol. Ind. Eng. Chem. Res. 49, 520e525.
vom Stein, T., Grande, P.M., Leitner, W., Domı́nguez de Marı́a, P., 2011.
Iron-catalyzed furfural production in biobased biphasic systems:
from pure sugars to direct use of crude xylose effluents as feedstock. ChemSusChem 4, 1592e1594.
Wahyudiono, Sasaki, M., Goto, M., 2008. Recovery of phenolic compounds through the decomposition of lignin in near and supercritical
water. Chem. Eng. Process.: Process Intensif. 47 (9e10), 1609e1619.
Weingarten, R., Cho, J., Conner Jr, W.C., Huber, G.W., 2010. Kinetics of
furfural production by dehydration of xylose in a biphasic reactor
with microwave heating. Green Chem. 12, 1423e1429.
Werhan, H., Farshori, A., Rudolf von Rohr, P., 2012. Separation of
lignin oxidation products by organic solvent nanofiltration.
J. Membr. Sci. 423e424, 404e412.
West, R.M., Liu, Z.Y., Peter, M., Gartner, C.A., Dumesic, J.A., 2008.
Carbonecarbon bond formation for biomass-derived furfurals and
ketones by aldol condensation in a biphasic system. J. Mol. Catal.
A: Chem. 296, 18e27.
Wettstein, S.G., Alonso, D.M., Gürbüz, E.I., Dumesic, J.A., 2012. A
roadmap for conversion of lignocellulosic biomass to chemicals
and fuels. Curr. Opin. Chem. Eng. 1, 218e224.
Win, D.T., 2005. Furfural e gold from garbage. Aust. J. Technol. 8,
185e190.
313
Wyman, C.E., Dale, B.E., Elander, R.T., Holtzapple, M., Ladisch, M.R.,
Lee, Y.Y., 2005. Coordinated development of leading biomass
pretreatment technologies. Bioresour. Technol. 96, 1959e1966.
Wyman, C.E., Balan, V., Dale, B.E., Elander, R.T., et al., 2011.
Comparative data on effects of leading pretreatments and enzyme
loadings and formulations on sugar yields from different switchgrass sources. Bioresour. Technol. 102, 11052e11062.
Xiang, Q., Lee, Y.Y., 2000. Oxidative cracking of precipitated hardwood lignin by hydrogen peroxide. Appl. Biochem. Biotechnol.
84e86, 153e162.
Xing, R., Qi, W., Huber, G.W., 2011. Production of furfural and carboxylic acids from waste aqueous hemicellulose solutions from the
pulp and paper and cellulosic ethanol industries. Energy Environ.
Sci. 4, 2193e2205.
Xu, W., Miller, S.J., Agrawal, P.K., Jones, C.W., 2012. Depolymerization
and hydrodeoxygenation of switchgrass lignin with formic acid.
ChemSusChem 5 (4), 667e675.
Yamashita, Y., Shono, M., Sasaki, C., Nakamura, Y., 2010. Alkaline
peroxide pretreatment for efficient enzymatic saccharification of
bamboo. Carbohydr. Pol 79, 914e920.
Yang, H., Yan, R., Chen, H., Lee, D., Zheng, C., 2007. Characteristics of
hemicellulose, cellulose and lignin pyrolysis. Fuel 86, 1781e1788.
Yang, W., Li, P., Bo, D., Chang, H., 2012. The optimization of formic
acid hydrolysis of xylose in furfural production. Carbohydr. Res.
357, 53e61.
Ye, Y., Zhang, Y., Fan, J., Chang, J., 2012. Novel method for the production
of phenolics by combining lignin extraction with lignin depolymerisation in aqueous ethanol. Ind. Eng. Chem. Res. 51, 103e110.
Yemis, O., Mazza, G., 2011. Acid-catalyzed conversion of xylose, xylan
and straw into furfural by microwave-assisted reaction. Bioresour.
Technol. 102, 7371e7378.
Yemis, O., Mazza, G., 2012. Optimization of furfural and
5-hydroxymethylfurfural production from wheat straw by a
microwave-assisted process. Bioresour. Technol. 109, 215e223.
Yoo, C.G., Kuo, M., Kim, T.H., 2012. Ethanol and furfural production
from corn stover using a hybrid fractionation process with zinc
chloride and simultaneous saccharification and fermentation (SSF).
Process Biochem. 47, 319e326.
Yoshikawa, T., Yagia, T., Shinoharaa, S., Fukunagab, T., Nakasakaa, Y.,
Tagoa, T., Masudaa, T., 2013. Production of phenols from lignin via
depolymerization and catalytic cracking. Fuel Proc. Technol. 108,
69e75.
Yuan, Z., Cheng, S., Leitch, M., Xu, C., 2010. Hydrolytic degradation of
alkaline lignin in hot-compressed water and ethanol. Bioresour.
Technol. 101, 9308e9313.
Zakrzewska, M.E., Bogel-Łukasik, E., Bogel-Łukasik, R., 2010. Ionic
liquid-mediated formation of 5-hydroxymethylfurfurals. A promising biomass-derived building block. Chem. Rev. 111, 397e417.
Zakzeski, J., Bruijnincx, P.C., Jongerius, A.L., Weckhuysen, B.M., 2010.
The catalytic valorization of lignin for the production of renewable
chemicals. Chem. Rev. 110 (6), 3552e3599.
Zakzeski, J., Jongerius, A.L., Bruijnincx, P.C.A., Weckhuysen, B.M.,
2012. Catalytic lignin valorization process for the production of
aromatic chemicals and hydrogen. ChemSusChem 5, 1602e1609.
Zeitsch, K.J., 2000a. The chemistry and technology of furfural and its
many by-products. Sugar Series 13. Elsevier, The Netherlands.
Zeitsch, K.J., 2000b. Furfural production needs chemical innovation.
Chem. Innovation 30, 29e32.
Zhao, H., Holladay, J.E., Brown, H., Zhang, Z.C., 2007. Metal chlorides
in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural.
Science 316, 1597e1600.
Zhao, X., Zhang, L., Liu, D., 2012. Biomass recalcitrance. Part II: fundamentals of different pre-treatments to increase the enzymatic
digestibility of lignocellulose. Biofuels, Bioprod. Biorefin. 6, 561e579.
C H A P T E R
18
Industrial Lignins: Analysis,
Properties, and Applications
Alex Berlin 1,*, Mikhail Balakshin 2
1
Novozymes, Protein Chemistry Department, Davis, CA, USA, 2Renmatix, R&D Department, King of Prussia, PA, USA
*Corresponding author email: axbl@novozymes.com
O U T L I N E
The Potential of Technical Lignins as a Renewable
315
Raw Material Feedstock
Technical Lignins: Production, Properties,
and Analysis
Comparison of Analytical Methods for
Characterization of Technical Lignins
Reproducibility of 31P NMR Analytical Techniques
13
C NMR Analysis of Technical Lignins
Advanced NMR Methods
Molecular Weight Distribution
318
323
323
327
330
330
THE POTENTIAL OF TECHNICAL
LIGNINS AS A RENEWABLE
RAW MATERIAL FEEDSTOCK
Lignins in their native form are the most abundant
renewable aromatic polymers on earth (Kirk and Farrell,
1987). Consequently, lignins present great potential as a
source of energy due to their high fuel content
(26e28 MJ/ton dry lignin) rivaling the fuel content of
some coals (Lora, 2006; Tomani, et al., 2011). Lignins
can be combusted to produce “green” electricity, power,
fuel, steam, or syngas; all these are forms of energy
which are being or will be used in the future to operate
industrial plants where lignins are generated as byproducts. The lignin by-products are called “technical
lignins” or “industrial lignins” and they differ dramatically in properties from the native lignins found in
plants. Examples of the use of technical lignins as a
source of energy to run industrial plants are the pulp
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00018-8
StructureeProperties Correlations in Lignin
331
Technical Lignins: Traditional and Emerging
Applications
Traditional Lignin Applications
Emerging Lignin Applications
332
332
332
Conclusions
333
References
333
mills deployed worldwide and the emerging lignocellulose biorefineries. Energy production is, compared to all
other technical lignins applications, the one with the
lowest market value, estimated at approximately
10 US$ cents/kg as coal replacement (Holladay et al.,
2007). However, energy generation is the lignin application with the highest demand by volume and currently
the one with the lowest technical risk. Almost every major pulp chemical mill today utilizes lignin as a source of
energy. The latter is today’s common industrial practice
which will likely be mirrored by future cellulosic
biomass biorefineries which will use lignin as the main
energy source in combination with other fuels such as
raw biomass.
Technical lignins are available in large volumes, primarily in kraft mill spent liquors (“black liquors”),
and, to a less extent, in the spent liquors of the few
remaining sulfite mills (“brown liquors”). According to
our conservative estimate, ca. 6e7% of the spent liquor
315
Copyright Ó 2014 Elsevier B.V. All rights reserved.
316
18. INDUSTRIAL LIGNINS: ANALYSIS, PROPERTIES, AND APPLICATIONS
produced at a kraft pulp mill could be used for lignin
extraction without significantly affecting the plant
energy balance. This represents a potential average
lignin production capacity per plant in the order of
30e75 tons of lignin per kraft pulp plant per day
(Domtar, 2013) assuming an average annual pulp production capacity of ca. 0.5 million tons odw pulp (Table
18.1). On the contrary, in sulfite pulp mills, the majority
of the produced spent liquor can be used for
TABLE 18.1
Chemical Pulp Production Capacity of Typical
Pulp and Paper Mills
Corporation*
Plant Location
Pulp Production
Capacity
(1000 odw tons
per year)
Domtar (USA/
Canada)
Ashdown (AR, USA)
747
Dryden (Ontario, Canada)
328
Espanola (Ontario, Canada)
325
Hawesville (KY, USA)
430
Kamloops (BC, Canada)
380
Marlboro (SC, USA)
278
Plymouth (NC, USA)
438
Windsor (QC, Canada)
447
Fray Bentos (Uruguay)
1100
Kymi (Finland)
570
Pietarsaari (Finland)
790
Kaukas (Finland)
740
Husum (Sweden)
700
Joutseno (Finland)
670
Kaskinen (Finland)
300
Kemi (Finland)
590
Rauma (Finland)
630
Äänekoski (Finland)
520
Licantén (Chile)
140
Constitución (Chile)
355
Ránquil (Chile)
1027
Arauco (Chile)
790
San José de la
Mariquina (Chile)
550
Misiones Province
(Argentina)
350
Average production capacity
(1000s odw tons pulp/year)
550
UPM (Finland)
Metsä-Botnia
(Finland)
Arauco (Chile)
*The information contained in this table was obtained from the respective corporate
websites.
lignosulfonate production given the fact that not many
sulfite pulping players burn lignosulfonates for energy
generation. In 2004, it was reported that 2% of the lignin
available in the pulp and paper industry was commercially used comprising about 1,000,000 tons/year lignosulfonates from sulfite pulping brown liquors and
<100,000 tons/year from kraft spent liquors (Gosselink
et al., 2004). Assuming the forecasted annual growth
rate of 2.5% (IHS-Chemical, 2012), the current lignosulfonates production should be ca. 1,200,000 tons/year.
The global annual production of chemical pulps is
estimated at 150 million tons/year (Vappula, 2011) with
an average odw chemical pulp production per plant of
ca. 0.5 million tons (Table 18.1) and a total volume of dissolved lignin in pulp making of ca. 70 million tons globally (Lora, 2010). Assuming a lignin annual production
capacity per chemical pulp mill of ca. 27,000 metric tons
kraft lignin per year (Domtar, 2013), it can then be
concluded that the estimated annual global potential
for kraft lignin production capacity from pulp mills is
ca. 6e9 million tons depending on the feedstock species
and the plant design which is in good agreement with the
estimates reported by other authors (Glasser, 2010; Lora,
2010). When considering the potential replacement of the
main petrochemicals, namely, ethylene, propylene,
butadiene and benzene, toluene, and xylene (BTX) isomers, which are produced at a rate of about 300 million
tons/year with a total value of over $400 billion (Lucintel, 2013), by lignin it is relevant to consider the current
global potential production capacity of technical lignins.
It becomes clear from the analysis performed above that
in the best case scenario purified technical lignins produced globally at chemical pulp mills could potentially
replace a maximum of ca. 2% of the global volume of
main petrochemicals. However, the emerging lignocellulose biorefinery industry for production of biofuels and
chemicals might completely change this picture. For
instance, the US Department of Energy estimates that
1.3 billion tons of biomass is available in the United
States alone for biorefining into transportation fuels
and chemicals (Perlack et al., 2005). This amount of
biomass could make available additionally 225 million
tons of lignin which could be utilized for power, transportation fuels, products and various combinations of
the above (Holladay et al., 2007). Assuming that 20% of
this biorefinery lignin (45 million tons) will be converted
into BTX and linear hydrocarbons, the result could be ca.
10% replacement of these petrochemicals by lignins
produced at American biomass biorefineries alone. In
other words, if we consider, in addition to the American
biorefineries, a scenario where these biomass biorefining
technologies will be deployed globally, we could witness,
in the future, a hypothetical situation where a large
fraction of petroleum-derived BTX, and perhaps of other
petrochemicals too, could be replaced by lignin.
THE POTENTIAL OF TECHNICAL LIGNINS AS A RENEWABLE RAW MATERIAL FEEDSTOCK
Another abundant source of technical lignins, which
is often ignored, is the acid-hydrolysis (AH) lignin (“hydrolysis lignin”) which has been produced at Eastern
European wood and agricultural wastes AH plants since
the mid-1930s with yields in the range of 350e400 kg
lignin/ton odw softwood. The annual production of
such hydrolysis lignin in the former Soviet Union
reached 1.5 million tons by the end of the 1980s. However, only 30e40% of the hydrolysis lignin was really utilized, whereas the rest was disposed in giant landfills
nearby the wood hydrolysis plants, creating as a result
serious environmental problems caused primarily by
autoignition of these deposits. For example, the current
lignin waste stocks in the Irkutsk region (Siberia), where
only four plants are located, exceed 20 million tons
(Rabinovich, 2010) equivalent to ca. 20 times today’s
global commercial technical lignin market. The current
annual production of AH lignin in Belarus alone is in
the order of 100,000 tons (Podterob et al., 2004). The
main application of the hydrolysis lignin is the production of pellets for energy generation. However, highly
specialized applications, such as pharmaceutical enterosorbents, have been successfully developed and
commercialized on the basis of purified hydrolysis
lignin. An example of these commercial sorbents is the
enterosorbent “Polyphepan” (Podterob et al., 2004).
In addition to the low-value energy lignin application, a wide diversity of high-value industrial applications have been envisioned or industrially realized or
demonstrated including uses as novel materials, polymeric, oligomeric, and monomeric feedstock. Some
of these opportunities, such as the use of lignin or its
derivatives in animal feed additives, agriculture, construction, textile, oil drilling, binders, dispersants, and
composites, are today commercial realities but many
others such as the production of carbon fiber precursors, the broad incorporation of lignin in synthetic
polymeric blends, or the production of BTX remain
longer term opportunities with great value and market
potential.
Both low- and high-value lignin applications are
often seen as efficient vehicles to increase the productivity, reduce fossil fuel consumption, and increase the
profitability of the industrial plants where lignin is produced as a by-product. For instance, the LignoboostÔ
process (Tomani, 2010), a recently commercialized process by Metso Corporation (Helsinki, Finland) for lignin
production from alkaline black liquors, significantly improves the profitability of the pulp and paper mill by
debottlenecking the wood pulp production as a result
of increasing the recovery capacity of pulping chemicals
and valorizing the lignin stream. Commercial-scale
lignin production based on the LignoboostÔ process
has begun in February 2013 by Domtar Corp. at the Plymouth Mill (NC, USA) with a targeted rate of 75 tons/
317
day (w27,000 tons/year), destined for a wide range of
industrial applications as a bio-based alternative to
the use of petroleum and other fossil fuels (Domtar,
2013).
As it was mentioned earlier, in the case of emerging
industries, such as the cellulosic ethanol industry, the
smart utilization of residual lignin could dramatically
boost the profitability of the cellulosic biofuel plants if
converted into value-added chemicals such as BTX,
other monomeric and oligomeric phenolic compounds,
and suitable for material applications macromolecules
such as carbon fiber precursors, polymeric blends, adhesives, dispersants, and others. While energy and
monomeric applications for technical lignins and their
derivatives often target direct replacements of fossil
fuels and petrochemicals, the development of novel
lignin-derived oligomeric and macromolecular entities
has the potential of generating better alternatives or
synergy with petrochemical feedstocks. Recent examples of the latter have been reported in the literature
which illustrates this concept. For instance, recently
Berlin (2011) showed that the replacement of methylene
diphenyl diisocyanate (MDI) in engineered wood diisocyanate adhesives by organosolv (OS) lignin derivatives can lead to substantial improvements of the
adhesive binding properties (increased modulus of
rupture and modulus of elasticity) when applying the
lignineMDI adhesives in engineered wood composite
construction materials such as Oriented Strand Boards
(OSB) while still meeting the industry standard requirements for these adhesives. A similar observation was
documented when phenol in phenoleformaldehyde
resins was replaced by OS lignin derivatives which
resulted in a significant increase of the resin normalized
bond strength (Berlin, 2012b). These two examples are
important because they illustrate a fact often overseen
which is the evidence that lignin derivatives can technically outperform petrochemicals when used in conjunction with the latter in certain chemical formulations.
This observation hints at the possibility of not needing
to completely depolymerize lignin, a longstanding unresolved challenging technical problem, into the equivalent petrochemical monomers in order to achieve
similar or better performance of the lignin-derived
chemicals in formulated products. On the contrary,
further research efforts could be directed toward valorization strategies of technical lignins with preserve
natural backbone structures to produce viable novel
polymeric precursors alternative to petrochemicals.
The recent resurgence of interest in lignin as a renewable raw material feedstock is evidenced by the growing
number of patent applications containing the word
“lignin” which have been filed between 2003 and 2012
via the World Intellectual Property Organization
(WIPO; Figure 18.1). It is interesting to note the fact
318
18. INDUSTRIAL LIGNINS: ANALYSIS, PROPERTIES, AND APPLICATIONS
FIGURE 18.1 Number of WIPO patent applications containing the word “lignin” found in May 2013
by using the WIPO search tool Patentscope for the
period 2003e2012.
that 25% of all these patent applications were filed by
major chemical, pharmaceutical, and energy companies
such as BASF, Bayer, Ciba-Geigy, Monsanto, Sumitomo,
and Shell with the German chemical giants Bayer (10%
lignin filings) and BASF (8% lignin filings) leading the
group. The patents found in May 2013 by using the
WIPO search tool Patentscope for the period 2003e
2012 (25,974 documents) represent ca. 50% of all the patents registered in the WIPO which contain the word
“lignin” on the front page (52,895 documents).
Today’s global lignin market is dominated by the
Norwegian company Borregaard Lignotech (Norway)
followed by Tembec (North AmericaeFrance) and
MeadWestvaco (USA). There are a number of smaller
players such as Domsjö (Finland-India), Granit SA
(Switzerland), and CIMV (France), among others.
TECHNICAL LIGNINS: PRODUCTION,
PROPERTIES, AND ANALYSIS
A detailed understanding of the structure of technical
lignins is critically important in order to direct the efforts
toward their valorization (Glasser, 2000). Not surprisingly, there is in the literature significant evidence
suggesting that the performance of purified technical
lignins can be linked to their chemical structure
(Gosselink et al., 2010; Berlin, 2011, 2012a,b; Chung
and Washburn, 2012). However, it is recognized that
there is a fundamental lack of knowledge in the understanding of technical lignins as a polymer and their conversion to materials, so targeted modifications via
refining, chemical modifications, or fractionation can
be pursued to maximize their performance in formulated products (Baker and Rials, 2013). Hence, the
importance of the lignin analytical methods employed
to study the structure of these lignins will be discussed
in detail below.
Native lignin is an irregular heterogeneous polymer.
The same applies to technical lignins with the particularity that the lignin heterogeneity is typically increased by
the biomass processing. It is widely believed that the
lignin structure is tridimensional; however, there is no
solid evidence supporting this hypothesis. Some scientists question the latter claim (Ralph et al., 2004). Lignin
is optically inactive. The repeated (monomeric) unit in
lignin is the phenylpropane unit (or so-called the
“C9-unit”) of the p-hydroxyphenyl (H), guaiacyl (G)
and syringyl (S) types (Figure 18.2). Coniferous lignins
are predominantly of G-type. Hardwood lignins contain
both G- and S-units. The H-unit content in woody lignin
is usually low; however, they can significantly
contribute to the structure of nonwoody lignins, for
instance, from annual fibers. In addition, annual fiber
lignins contain significant amounts of cinnamic and
ferrulic acid derivatives attached to lignin predominantly via ester linkages with the gamma hydroxyl of
C9-units (Adler, 1977; Sakakibara, 1991; Ralph et al.,
2004). The lignin C9-units contain different functional
groups. The most common ones are aromatic methoxyl
and phenolic hydroxyl, primary and secondary aliphatic
hydroxyl, small amounts of carbonyl groups (of the
aldehyde and ketone types) and carboxyl groups. The
monomeric C9 lignin units are linked to form a polymer
by CeOeC and CeC linkages. The most abundant
lignin interunit linkage is the b-O-4 type of linkage
(structures 1e4, and 7; Figure 18.2) comprising about
50% of the interunit linkages in lignin (ca. 45% in softwoods and up to 60e65% in hardwoods). Other common lignin interunit linkages are the resinol (b-b)
(structure 6; Figure 18.2), phenylcoumaran (b-5) (structure 5; Figure 18.2), 5-50 (structure 12; Figure 18.2), and
TECHNICAL LIGNINS: PRODUCTION, PROPERTIES, AND ANALYSIS
319
FIGURE 18.2 Structural moieties of native lignins (1e20), technical lignins and major ligninecarbohydrate bonds (AeC).
4-O-5 (structure 11; Figure 18.2) moieties. The number of
these structures varies in different lignins, but rarely exceeds 10% of the total lignin moieties. The number of
other lignin moieties is usually below 5% (Adler, 1977;
Sakakibara, 1991; Balakshin et al., 2008).
The degree of lignin condensation (DC) is an important lignin characteristic as it is often correlated (negatively) with lignin reactivity. The definition of
condensed lignin moieties found in the literature is not
always clear. Most commonly, condensed lignin structures are lignin moieties linked to other lignin units
via 2, 5 or 6 positions of the aromatic ring (in H-units
also C-3 position). The most common condensed structures are 5-50 , b-5, and 4-O-50 structures. Since the C-5
position of the syringyl aromatic ring is occupied by a
methoxyl group and therefore it cannot be involved in
condensation, hardwood lignins are less condensed
than softwood lignins.
According to recent findings, almost all lignin in softwood and softwood pulps is linked to polysaccharides,
mainly via hemicelluloses (Lawoko et al., 2005). The
main types of ligninecarbohydrate (LC) linkages in
320
18. INDUSTRIAL LIGNINS: ANALYSIS, PROPERTIES, AND APPLICATIONS
wood are phenyl glycoside bonds (A), esters (B) and
benzyl ethers (C) (Figure 18.2; Helm, 2000; Koshijima
and Watanabe, 2003; Balakshin et al., 2007; Balakshin
et al., 2011; Balakshin et al., 2014). The occurrence of stable LC bonds in native lignins is one of the main reasons
preventing selective separation of the wood components
in biorefining processes.
Technical lignins are obtained as a result of lignocellulosic biomass processing. Technical lignins differ
dramatically from the corresponding native ones as a
result of a combination of multiple reactions including
catalyzed biomass hydrolysis, condensation of lignin
fragments, elimination of native lignin functional
groups, formation of new functional groups, and others.
They are appreciably more heterogeneous (in terms
of chemical structure and molecular mass) than the
native lignins. Technical lignins have a high variety of
structural moieties present in rather small amounts
(Balakshin et al., 2003; Liitiä et al., 2003).
Technical lignins can be classified from different
points of view (Table 18.2). From a practical point of
view, there are technical lignins originated from pulp
and paper industrial processes which are considered
mostly waste products without controllable chemical
properties. These are kraft and soda lignins (kraft and
soda pulping, correspondingly) and lignosulfonates
(sulfite pulping). On the other hand, there is a big group
of technical lignins from various emerging biomass biorefining processes such as different variations of AH,
steam explosion (SE), and OS pretreatment, in particular.
In terms of the process chemistry, and, correspondingly, the lignin chemical structure, lignins can be
derived from acid- or alkali-based processes. The former
includes most of the emerging biomass biorefinery pretreatments, such as AH, SE (except AFEX) and most of
OS processes as well as lignosulfonates. Alkaline processes are kraft and soda pulping, AFEX pretreatment,
and some OS processes. In addition to the process nature, the feedstock source has naturally an important
impact on the structure of technical lignins.
TABLE 18.2
Another consideration which can be used to classify
technical lignins, especially in view of their application,
is the presence or absence of sulfur in their structure.
Therefore, kraft lignin, and, especially lignosulfonates,
are sulfur-containing lignins whereas soda, OS, AH
and SE lignin are sulfur-free or low-sulfur-containing
lignins.
In terms of the chemical structure, native lignins undergo significant degradation/modification during
biomass processing. Lignin degradation occurs predominantly via cleavage of b-O-4 linkages (although the
mechanisms are different for different processes), which
results in an increase of phenolic hydroxyls and a
decrease in lignin molecular mass. The lignin degradation also leads to a decrease in aliphatic hydroxyls,
oxygenated aliphatic moieties and the formation of
carboxyl groups and saturated aliphatic structures. In
contrast to lignin degradation, some reversed reactions,
such as lignin repolymerization/condensation, take
place to some degree resulting in increase of lignin molecular mass and decrease of its reactivity. These changes
are common for most of the technical lignins although
the degree of transformation varies significantly depending on the process conditions (temperature, time,
pH, and others) and feedstock origin.
Each process provides the lignin with specific chemical characteristics. First, the reaction mechanism is quite
different in acidic and basic media. The cleavage of b-O4 linkages under alkaline conditions occurs via a
quinone methide intermediate which results in the formation of coniferyl alcohol-type moieties as a primary
reaction product (Figure 18.3). They are not accumulated
in the lignin; however, they undergo further secondary
rearrangement reactions forming various (aryl-)
aliphatic acids. b-5 and b-1 type of linkages of the native
lignin cannot be cleaved during the process but are
transformed into stilbene-type structures (structure 30;
Figure 18.2). The latter are stable and are accumulated
in alkaline lignins. In addition, a significant amount of
vinyl ether structures (structure 29; Figure 18.2) forms
Classification of Technical Lignins
Lignin Type
Scale
Chemistry
Sulphur content
Purity
Kraft
Industrial
Alkaline
Moderate
Moderate
Soda
Industrial
Alkaline
Free
Moderate-low
Lignosulfonate
Industrial
Acid
High
Low
Organosolv
Pilot/demo
Acid
Free
High
Hydrolysis
Industrial/pilot
Acid
Low-free
Moderate-low
SE
Demo/pilot
Acid
Low-free
Moderate-low
AFEX
Pilot
Alkaline
Free
Moderate-low
SE, steam-exploded lignin; AFEX, ammonia fiber expansion lignin.
TECHNICAL LIGNINS: PRODUCTION, PROPERTIES, AND ANALYSIS
FIGURE 18.3
321
The main lignin reactions in alkaline pulping.
during soda pulping and accumulates in lignin, in
contrast to kraft lignin. Another relevant structural difference between soda and kraft lignins, resulting from
differences in the reaction mechanism, is the presence
of aryl-glycerol type structures (structure 20; Figure 18.2)
in the former. On the other hand, lignin undergoes
demethylation reactions which result in formation of
o-quinone structures during kraft pulping (but not in
the case of soda pulping). In addition, kraft lignins
contain small amounts of organically bound sulfur,
likely in the form of thiol compounds (Marton, 1971;
Gierer, 1980; Gellerstedt, 1996; Balakshin et al., 2003).
Kraft and soda lignins show significantly higher degree
of condensation than the corresponding native lignins.
However, this is the result of accumulation of condensed
moieties of original native lignin rather than the result of
extensive condensation reactions during pulping (Balakshin et al., 2003). Kraft and soda lignins contain small
amounts of carbohydrate and ash impurities. The
amounts of these contaminants are dependent on feedstock origin and are significantly higher in annual fiber
lignins than in woody lignins.
The lignin chemistry originated from the emerging
acid-based biomass biorefinery processes is very diverse
(Glasser et al., 1983). The acid-based biomass biorefining
can be catalyzed by addition of mineral or organic acids
(from catalytic amounts to the use of organic acids as the
reaction media) or without acid addition (autohydrolysis) when organic acids are generated due to cleavage
of acetyl groups of lignocellulosics as well as due to
the formation of acidic reaction products. Technical lignins derived from biomass biorefinery processes have
been much less investigated than kraft lignins. Moreover, a high diversity of lignins is expected in the future
given the large number of technical biomass pretreatment processes under either R&D or industrial deployment and the high variety of potential raw materials
(softwoods, hardwoods, annual fibers, agricultural residues, etc.) as compared to the relative uniformity of
pulping processes.
The main pathway of lignin degradation under acidic
conditions is the acidic hydrolysis of b-O-4 linkages
(Figure 18.4). The major products of this reaction are
the so-called Hibbert ketones (Wallis, 1971). The accumulation of Hibbert ketones in lignin results in relatively
high content, as it compares to alkaline lignins, of
carbonyl groups and the corresponding saturated
aliphatic structures (Berlin et al., 2006). Although degradation of lignin under acidic condition occurs via vinyl
ether intermediates, they do not accumulate in the lignin
since vinyl ether structures are very unstable in acid media. Significant amounts of olefinic moieties were
observed in lignin obtained under acidic conditions,
but their nature is different from the olefinic structures
of kraft and soda lignins, their exact structure is still
not well understood (Berlin et al., 2006). Moreover,
lignin condensation reactions under acidic conditions
are more significant than those occurring in alkaline processes. Acidic lignin condensation occurs predominantly via 2 and 6 positions of the aromatic ring, in
contrast to alkaline condensation which occurs predominantly at the C-5 position of the aromatic ring (Glasser
et al., 1983). The DC is dependent on the acidity of the
reaction media (pH and solvent used) and the process
322
18. INDUSTRIAL LIGNINS: ANALYSIS, PROPERTIES, AND APPLICATIONS
FIGURE 18.4 Major lignin degradation reactions under acidolysis conditions (in acidic aqueous organic solvent). The cleavage of b-O-4
structures results in the formation of free phenolic moieties and Hibbert ketones.
severity (temperature and time). An extreme example of
highly modified technical lignins is the industrial AH
lignin produced in Russia or Belarus which is obtained
at 170e190 C, 2e3 h with 1% H2SO4. AH lignin is insoluble in polar organic solvents and alkaline solutions due
to the strong condensation/polymerization occurred
during the AH process. Hydrolysis lignin has high content of phenolic hydroxyl groups and olefinic structures.
In addition, it contains 10e30% residual carbohydrates
and up to 20% lipophilic extractives (Chudakov, 1983).
In contrast, a significant fraction of AH lignin obtained
at very high reaction temperature (>220 C) but short reaction time (<1 min) was soluble in 1 N NaOH (70e90%
of AH lignin) and dioxane (50%); the amounts of carbohydrates in these soluble lignins were significantly
lower, 2e4% (Glasser et al., 1983; Lora and Glasser,
2002). SE lignin is also quite degraded in terms of cleavage of b-O-4 linkages, but apparently much less
condensed than AH lignins (Glasser et al., 1983; Robert
et al., 1988; Li et al., 2009).
OS methods of lignin production are very diverse,
where different organic solvents and reaction pH can
be varied during the process. Each of these processes
produces lignins that are very different in their physicochemical and biochemical properties. Furthermore, the
physical conditions (e.g. temperature, time, and pressure) and chemical conditions (e.g. pH and concentration of solvents) under which these processes are
conducted can drastically affect the molecular weight
(Mw), chemical structure, and functional groups distribution in the generated lignin derivatives (Abdelkafi
et al., 2011; Balakshin et al., 2013a, b). The most investigated OS delignification technology is the Alcell process
deployed at industrial scale in Eastern Canada during
the 1980s. The Alcell process can be carried out in
aqueous ethanol liquor at moderate acidity (no exogenous acid is added; the acidic pH results from formation
of organic acids during the process). Alcell lignin is
practically sulfur-free and has significantly lower
amounts of carbohydrate and ash impurities compared
to kraft lignins.
Lignosulfonates are a special class of technical lignins
and they constitute the bulk of the commercial lignins
for materials and chemical applications. Lignosulfonates
are primarily isolated from sulfite spent liquors. However, sulfonated or sulfomethylated kraft lignins are often
used in similar to lignosulfonates applications but they
have different chemical properties, in particular the sulfonic acid groups in lignosulfonates are located in the
side alkyl chains, whereas in sulfonated kraft lignins
they are found in the aromatic ring. Sulfur-containing
lignins, in form of lignosulfonates or sulfomethylated
kraft lignins, are commercialized by Borregaard
(Sarpsborg, Norway), MeadWestvaco (Richmond, VA,
USA), Tembec (Montreal, Quebec, Canada) and other
smaller players for a wide variety of applications
including dispersants for wettable powders, binders
for granules and seed coatings, additives, etc. Although
sulfur-containing lignins are generated during acid sulfite pulping or are produced by sulfonation of kraft
lignin, the chemistry of the process and correspondingly
the lignin structure is quite specific. The main reaction in
sulfite pulping is sulfonation of lignin side chain, predominantly at the a-position of the propane chain as
well as at the conjugated a-hydroxyl group. In addition,
carbonyl groups also undergo sulfonation although the
TECHNICAL LIGNINS: PRODUCTION, PROPERTIES, AND ANALYSIS
mechanism is different from that for hydroxyl groups.
Introduction of highly polar sulfonate groups into the
lignin structure strongly increases its solubility in
aqueous solutions. Most of the lignosulfonates contain
about 1 sulfonate group per 2 monomeric units.
Although strong degradation of lignin is not needed to
transfer sulfonated lignin from solid phase into solution,
it still takes place and the degree of degradation is
dependent on the reaction conditions. Therefore, the
molecular mass of lignosulfonates is very high and
varies strongly. The average Mw of lignosulfonates has
been reported in the range of 10,000e40,000 Da and a
fraction with Mw up to 100,000 Da has been isolated.
An increased number of phenolic hydroxyl groups can
be observed in lignosulfonates and it is strongly dependent on reaction conditions (Glennie, 1971).
Comparison of Analytical Methods for
Characterization of Technical Lignins
There is a rather good correlation between different
analytical methods used for the analysis of native lignin
preparations, both in intralab (Evtuguin et al., 2001) and
in interlab studies (Sakakibara, 1991; Capanema et al.,
2004; Zhang and Gellerstedt, 2007; Balakshin et al.,
2008). An exception is the 31P NMR analytical methodology of native lignins which yielded ca. 30% lower
numbers for aliphatic hydroxyls (Pu et al., 2011)
compared to other methods (Sakakibara, 1991; Balakshin
et al., 2008).
A rather good correlation between various methods
for technical lignins analysis has been also reported
(Faix et al., 1994; Cateto et al., 2008). However, a comprehensive review of published analytical data leads to a
much less optimistic conclusion. Table 18.3 shows that
significant variability in the structure of the same technical lignins can be observed when these lignins are
analyzed by independent methods, in contrast to what
is seen with the analysis of native lignin preparations.
This deviation might be caused by the interference of
specific lignin moieties generated during the technical
process on the results of each specific analytical method
which, as a rule, was developed and validated for the
analysis of native lignin preparations.
Various wet chemistry techniques for the analysis of
lignin functional groups have been comprehensively
reviewed previously (Lin and Dence, 1992; Zakis, 1994).
Therefore, we will focus our discussion on major NMR
spectroscopic techniques for the analysis of technical lignins, 31P and 13C NMR, as well as advanced NMR
methods, which have received less attention.
As 31P NMR spectroscopy of derivatized lignins becomes one of the most common techniques for lignin analysis, it is important to critically evaluate the method in a
comprehensive manner. There are two main
323
modifications of the 31P NMR lignin analysis. Originally,
2-chloro-1,3,2-dioxaphospholane (31P-I method) was suggested (Archipov et al., 1991) as the derivatizing agent
in this method. Later, 2-chloro-4,4,5,5-tetramethylwas
reported
1,3,2-dioxaphospholane
(31P-II)
(Kostukevich et al., 1993; Granata and Argyropoulos,
1995) to provide better signal separation and it is currently
used as the major 31P method for lignin analysis.
Although good agreement has been reported between
the results obtained with these two derivatization reagents (Granata and Argyropoulos, 1995), data reported
later did not confirm this observation (Tables 18.3 and
18.4). The results obtained with the 31P-II method tend
to underestimate results compared to the data generated
by all other analytical methods. The 31P-I method tends
to report significantly higher amount of aliphatic and total
hydroxyl groups if compared to the data obtained by the
31
P-II methodology (Table 18.3), in contrast to the conclusions drawn in the original validation work (Granata and
Argyropoulos, 1995).
The significantly lower numbers reported by the
31
P-II NMR analysis, as it is compared to other analytical
methods, in the structural analysis of lignins might be
explained by the incomplete lignin derivatization with
the phosphorylating reagent PR-II possibly due to steric
hindrance of the bulky reagent containing four t-butyl
groups. The use of PR-I yields apparently more quantitative results. However, the signal resolution in 31P-I is
not high enough as it can clearly be seen in the publication by Akim et al. (2001). In this publication, the signals
of primary hydroxyls and 5-substituted phenolic hydroxyls are heavily overlapped. The main conclusion
derived from this observation is that even when the resolution of a resonance signal is formally acceptable
(Argyropoulos, 1994), one cannot conclude that the
resolved signals are reporting the correct values.
Reproducibility of 31P NMR
Analytical Techniques
Although, a very high reproducibility has been reported in a specific intralaboratory study (Granata and
Argyropoulos, 1995), the reproducibility of quantitative
31
P NMR spectroscopy reported in independent interlaboratory studies is much lower (Table 18.4). Moreover,
even in studies conducted at the same laboratory, one
can observe significant divergences between the results
reported earlier (Granata and Argyropoulos, 1995) and
more recently (Xia et al., 2001) for the same lignin samples (see SE aspen and poplar lignins in Table 18.3).
As expected, the worst reproducibility of 31P NMR
analytical methods has been observed for different
types of 5-substituted phenolic hydroxyls (S-units and
5-condensed G units) in a hardwood technical lignin
(Table 18.4) due to a very poor signal resolution.
324
In mmol/g
per 100 C9 (or 100 Ar)
Lignin
Method
References
Aliphatic
Phenolic
COOH
Total
OH£
Alcell
31
Average (Gosselink et al., 2010;
Wörmeyer et al., 2011; Vanderlaan
and Thring, 1997; Cateto et al., 2010;
Granata and Argyropoulos, 1995;
Balakshin and Capanema,
unpublished data; Saad et al., 2012)
1.51
3.80
0.32
5.31
2.54
31
(Argyropoulos, 1994)
2.68
3.91
0.34
6.59
1.46
13
(Balakshin and Capanema,
unpublished data)
1.78
4.00
1.22*
5.78
13
(Cateto et al., 2010)
1.58U
3.88
5.46
P-II
P-I
C
C
Wet chem
(Milne et al., 1992)
3.00
3.39
U
3.18
Alcell lab aspen
Wet chem
(Glasser et al., 1983)
1.59
Indulin AT (MWV)
31
Average (Gosselink et al., 2010;
Cateto et al., 2010; Granata and
Argyropoulos, 1995; Balakshin
and Capanema, unpublished data;
Beauchet et al., 2012)
2.34
3.66
31
(Argyropoulos, 1994)
3.04
3.15
13
(Balakshin and Capanema,
unpublished data)
2.82
3.70
P-II
P-I
C
6.39
Phenolic/
Aliphatic
0.88
Total
OH£
Conversion
Factor
96
17.9
48
70
118
17.9
2.25
32
72
104
17.9
2.46
29U
71
100
18.3
61
115
17.9
59
109
1.13
27
Phenolic
68
4.77
0.42
Aliphatic
54
50
U
U
5.99
1.57
42
66
108
18.1
6.19
1.04
55
57
112
18.1
6.52
1.13
51
67
118
18.1
18. INDUSTRIAL LIGNINS: ANALYSIS, PROPERTIES, AND APPLICATIONS
TABLE 18.3 Content of Major Lignin Functional Groups Determined by Different Analytical Methods
13
C
(Cateto et al., 2010)
Wet chem
Wet chem
(Milne et al., 1992)
(Glasser et al., 1983)
3.01
3.06
U
3.73
U
3.88
6.89
3.72
6.78
3.25
1.27
1.22
56
U
55
U
71
127
18.4
67
122
18.1
6.98
0.87
73
59
132
SW kraft
Wet chem
(Mörck et al., 1986)
2.2
4.3
0.80
6.5
1.95
40
79
119
18.3
Soda Sarkanda (Granit)
31
(Gosselink et al., 2010)
1.59
2.41
0.99
4.00
1.52
31
47
78
19.5
31
(Cateto et al., 2010)
1.89
2.74
0.62
4.63
1.45
37
53
90
19.5
13
(Cateto et al., 2010)
3.11
2.23
61
44
104
19.5
31
(Granata and Argyropoulos, 1995)
3.47
P-II
P-II
C
SE aspen
P-II
31
P-II
P-I
13
x
C-IS
SE poplar
2.44
(Xia et al., 2001)
3.01
1.75
(Argyropoulos, 1994)
4.20
2.18
(Xia et al., 2001)
U
2.93
U
(Milne et al., 1992)
4.46
2.44
31
(Granata and Argyropoulos, 1995)
2.72
2.92
P-II
P-II
31
P-I
13
x
C-IS
Wet chem
Wet chem
U
(Xia et al., 2001)
2.25
2.29
(Argyropoulos, 1994)
2.97
2.46
(Xia et al., 2001)
(Milne et al., 1992)
(Glasser et al., 1983)
U
2.12
3.08
U
3.25
0.21
1.72
Wet chem
31
0.31
2.16
3.08
2.53
5.91
0.70
67
47
114
19.3
4.76
0.58
58
34
92
19.3
6.37
0.52
81
42
123
19.3
46
126
27.1
4.65
0.41
0.00
0.59
U
80
U
6.89
0.55
86
47
133
19.3
5.64
1.08
53
57
110
19.5
4.54
1.02
44
45
89
19.5
5.44
0.83
58
48
106
19.5
51
104
24.3
60
120
19.5
48
111
4.28
6.16
5.78
1.02
1.00
0.78
*COOH þ COOR.
x
Quantitative 13C NMR using internal standard.
U
Calculated by difference: Aliphatic OH = Total OH Phenolic OH
£
COOH groups are not included
Regular font, as reported; italic font, recalculated using conversion factor; “wet chem”, wet chemistry method; MWV, MeadWestvaco; SW, softwood.
U
53
U
60
U
63
TECHNICAL LIGNINS: PRODUCTION, PROPERTIES, AND ANALYSIS
31
U
5.34
325
326
TABLE 18.4
Reproducibility of
31
P NMR (PR-II) for Some Reference Technical Lignins
Phenolic
References
Aliphatic
G-Condensed
S
Total
5-Substituted
G
H
Total
Phenolic
COOH
Total
Hydroxyl**
Phenolic/
Aliphatic
Alcell
1.08
0.76
1.05
1.81
0.70
0.20
2.71
0.30
3.79
2.51
(Wörmeyer, Ingram et al., 2011)
1.28
0.24
1.45
1.69
0.64
0.11
2.44
0.26
3.72
1.91
(Vanderlaan and Thring, 1997)
1.5
5.4
2.60
(Cateto, Barreiro et al., 2010)
1.10
(Granata and Argyropoulos, 1995)*
1.84
(Saad, Radovic-Hrapovic et al., 2012)
2.2
(Balakshin, and Capanema,
unpublished data)
1.57
0.77
1.55
2.32
0.99
Average
1.51
0.74
1.68
2.27
0.91
RSD, %
26.9
52.2
37.1
22.5
(Gosselink, van Dam et al., 2010)
2.08
1.3
(Beauchet, Monteil-Rivera et al., 2012)
2.25
(Granata and Argyropoulos, 1995)*
2.54
(Cateto, Barreiro et al., 2010)
2.34
1.58
(Balakshin, and Capanema,
unpublished data)
2.45
Average
2.34
RSD, %
7.8
3.9
1.18
1.63
2.81
0.80
0.13
3.74
0.23
4.84
3.40
2.74
2.74
1.45
0.39
4.59
0.33
6.43
2.48
5.73
0.42
7.93
2.60
0.21
3.52
0.37
5.09
2.24
0.21
3.80
0.32
5.31
2.54
29.3
22.1
27.9
17.9
35.5
53.0
Indulin AT
1.3
1.62
0.23
3.15
0.44
5.23
1.51
1.38
1.94
0.23
3.55
0.36
5.80
1.58
3.59
0.43
6.13
1.41
1.93
1.91
1.96
0.26
4.13
0.39
6.47
1.76
1.22
1.65
1.95
0.25
3.85
0.48
6.30
1.57
1.37
1.56
1.88
0.24
3.66
0.42
5.99
1.57
17.8
7.8
6.2
10.0
11.0
8.2
8.2
13.8
0.33(?)
*Recalculated from the original report (Granata and Argyropoulos, 1995) using a conversion factor reported earlier (Argyropoulos, 1994), see Table 18.3.
** COOH groups are not included; Bold font corresponds to statistically calculated values Average and Relative standard Deviation (RSD,%)
18. INDUSTRIAL LIGNINS: ANALYSIS, PROPERTIES, AND APPLICATIONS
(Gosselink, van Dam et al., 2010)
TECHNICAL LIGNINS: PRODUCTION, PROPERTIES, AND ANALYSIS
Therefore, in these cases, we consider erroneous reporting separately S-units and 5-condensed G-units, it is
more reasonable to report them as “5-substituted phenolics”. The reproducibility of 31P NMR is overall better for
major signals such as aliphatic hydroxyls (AliphOH),
phenolic hydroxyls (PhOH), and total OH, especially
for Indulin AT lignin. Surprisingly, it is still not of very
high quality the results reported for Alcell lignin (Table
18.4). In part, but not completely, this observation might
be explained by inconsistencies in the lignin itself. In
addition, 31P NMR reports much lower carboxyl numbers
than wet chemistry methods and 13C NMR methods (Table 18.3). In fact, 13C NMR methods usually report the
sum of carboxyl and ester structures in general. For
instance, the significantly higher numbers reported by
13
C NMR for Alcell lignin might be explained by the significant contribution of ester structures (predominantly
ethyl esters) in this lignin. However, it is quite unreasonable to expect significant amounts of esters in kraft lignins isolated from high alkaline solutions. Therefore, it
might be concluded that 31P NMR also underestimates
the amounts of carboxyl groups in lignins.
In summary, it appears that 31P NMR-I does not provide sufficient resolution even between major signals,
PhOH and AliphOH, while 31P NMR-II yields significantly lower values. Moreover, separation of S-units
and G-condensed (at C-5) structures is very ambiguous
in 31P NMR-II analysis of technical lignins, consequently
so is the evaluation of S/G ratio and the degree of
condensation. The amount of 5-substituted (S þ G-condensed) and 5-unsubstitued phenolic hydroxyl
should be reported instead. Comparative data for the
analysis of various technical lignins by the 31P-II NMR
method are summarized in Table 18.5.
Due to the high degree of variability in the structure of
lignins discussed above, it is difficult to make any general conclusions on the existing structural differences
among the lignins originated from various technical processes (Tables 18.3e18.5). In addition to the variability
linked to the feedstock origin and process variables, the
numbers for different structural moieties reported can
vary due to the particularities of the used analytical
methodologies (Table 18.3). There is overall a lack of
comprehensive comparison of technical lignins. In addition, the existing comparative studies are limited to a few
lignin functionalities only, such as amounts of phenolic,
aliphatic hydroxyl groups, or methoxyl groups.
13
C NMR Analysis of Technical Lignins
The 13C NMR analysis of lignin has been considered as
a very informative, but not very affordable method due to
the very long experimental time originally required for
quantitative lignin analysis (Chen and Robert, 1988).
Recently, we have optimized the experimental time
327
required for 13C NMR analysis and reduced it from 70
to 15e20 h (Capanema et al., 2004, 2005a). Thus, a large
amount of valuable structural information (20e30 results
on structural moieties per analyzed lignin sample) can be
obtained in a reasonably short experimental time which
permits considering the 13C NMR method as the most
productive one in lignin analysis. Furthermore, we
demonstrated that the use of a CryoProbe NMR (Bruker
BioSpin MRI GmbH, Germany) allows for 1 h total quantitative 13C NMR experimental time (Balakshin, Berlin
et al., 2013). Therefore, 13C NMR cannot be considered
a time consuming lignin analytical technique anymore.
In addition, the CryoProbe yields much better signal resolution, both for 13C and Heteronuclear Single Quantum
Coherence (HSQC) NMR methods. However, currently
the use of CryoProbe does require tedious and professional optimization of acquisition and processing parameters to adjust the baseline, specifically for 13C NMR
spectra of lignin samples. Therefore, we cannot recommend yet the use of this method on a routine basis.
Further development in the use of CryoProbe technology
and its use for lignin analytical chemistry is expected to
mitigate this limitation.
A careful optimization of the acquisition parameters
for lignin analysis using a traditional probe yields 13C
NMR spectra with a good and reproducible baseline,
which can be easily and reliably corrected during
spectra processing. This careful adjustment of the acquisition and processing parameters has enabled the
recording of reproducible results even in different
NMR spectrometers with a relative error of ca. 2e3%
for the major lignin peaks. Unfortunately, it is not
feasible for now to evaluate interlab variability of the
13
C NMR method as it has been used much less often
than the 31P NMR method and data for the same lignin
preparations are very limited. It is also very important to
consider some issues when calculating the amount of
various lignin moieties using the original 13C NMR
spectra as it has been discussed earlier (Capanema
et al., 2005b). However, our team has been acquiring
over the past few years significant information on
different technical lignins, which is hereby summarized
in Table 18.6. Since the data were produced and interpreted by the same analytical methodology, their comparison is more accurate than the comparisons based
on data obtained from various literature reports. The
analysis of technical lignins (Table 18.6) clearly showed
dramatic changes in lignin structure resulting from the
delignification process. In addition to between-process
variations, certain within-process lignin structural
changes could be documented. One of the most important factors in these variations is the feedstock origin.
Significant differences in the structure of technical lignins from different tree species were obvious and they
were significantly larger than the differences in the
328
TABLE 18.5
Analysis of Different Technical Lignins by 31P-II NMR Method (mmol/g Lignin)
Phenolic
References
Aliphatic
Total
5-Substituted
G-Non-condensed
H
Total
Phenolic
COOH
Phenolic/
Aliphatic
Alcell
Average (Gosselink et al., 2010;
Wörmeyer et al., 2011; Vanderlaan
and Thring, 1997; Cateto et al., 2010;
Granata and Argyropoulos, 1995;
Balakshin and Capanema,
unpublished data; Saad et al., 2012)
1.51
2.27
0.91
0.21
3.80
0.32
2.54
Pine organosolv
(Pu et al., 2011; Sannigrahi
et al., 2010)
7.3
0.60
1.4
0.4
2.7
0.3
0.37
Straw organosolv
(Wörmeyer et al., 2011)
4.69
0.34
0.57
0.36
1.27
0.12
0.27
Miscanthus organosolv
(Pu et al., 2011; 15)
1.26e3.11
1.58e0.91
0.49e0.65
2.12e3.93
0.16e0.28
Miscanthus organosolv
(Pu et al., 2011; 16)
1.19
1.33
0.61
3.07
0.22
2.58
Indulin AT
Average (Gosselink et al., 2010;
Cateto et al., 2010; Granata and
Argyropoulos, 1995; Balakshin and
Capanema, unpublished data;
Beauchet et al., 2012)
2.34
1.56
1.88
0.24
3.66
0.42
1.57
Curan 100
(Gosselink et al., 2010)
1.78
1.55
1.84
0
3.39
0.43
1.90
Sarkanda soda granit
Average (Gosselink et al., 2010;
Cateto et al., 2010)
1.74
1.34
0.77
0.47
2.58
0.81
1.48
Straw soda
(Wörmeyer et al., 2011)
3.18
0.32
0.66
0.16
1.14
1.07
0.36
Hardwood soda
(Gosselink et al., 2010)
1.34
1.62
0.51
0.34
2.47
1.06
1.84
Aspen steam explosion
(Granata and Argyropoulos, 1995)*
3.47
1.76
0.67
2.44
0.31
0.70
(Xia et al., 2001)
3.01
(Granata and Argyropoulos, 1995)*
2.72
(Xia et al., 2001)
2.25
Pine acid hydrolysis
(Pu et al., 2011; Sannigrahi
et al., 2008)
3.42
0.34
1.82
Switchgrass acid hydrolysis
(Pu et al., 2011; 17)
2.83
0.35
0.57
Poplar steam explosion
1.75
2.00
0.92
*Recalculated from the original report (Granata and Argyropoulos, 1995) using a conversion factor reported earlier (Argyropoulos, 1994), see Table 18.3.
2.92
0.58
0.41
1.08
2.29
1.02
0.06
2.22
0.65
0.33
1.25
0.33
0.44
18. INDUSTRIAL LIGNINS: ANALYSIS, PROPERTIES, AND APPLICATIONS
Lignin
329
TECHNICAL LIGNINS: PRODUCTION, PROPERTIES, AND ANALYSIS
TABLE 18.6
13
C NMR Analysis of Different Native and Technical Lignins (per 100 Ar)
Moieties
Birch
MWL
Spruce
MWL
Birch
Kraft
E. glob.
kraft
E. grandis
kraft
Pine
kraft
Indulin
AT
Alcell
Douglas
fir OS
Total CO
12
21
9
14
12
11
12
35
20
Non-conjugated
CO
3
5
4
5
4
4
5
16
7
Conjugated CO
9
14
5
9
8
7
7
19
13
Total COOR
4
5
20
18
16
21
16
19
6
Aliphatic COOR
3
4
18
16
13
20
15
15
5
Conjugated
COOR
1
1
2
2
3
1
1
4
1
Total OH
150
138
107
128
119
108
118
104
118
Aliphatic
129
107
27
51
39
34
51
32
37
Primary
73
68
23
29
24
23
32
18
27
Secondary
56
39
3
22
15
11
19
14
10
Phenolic
20
31
80
77
80
74
67
72
81
S/G
3.02
n.a.
1.7
2.5
1.4
n.a.
n.a.
1.3
n.a.
ArH
209
172
191
186
218
235
209
DC
16
38
65
37
55
82
65
33
73
b-O-4
66
45
2
12
5
3
7
8
<5
b-b
11
4
3
2
3
5
4
3
1
b-5
2
9
2
1
2
3
4
3
3
OCH3
177
95
141
141
125
81
80
117
85
Oxygen aliphatic
260
86
110
79
72
94
94
86
Saturated
aliphatic
15
.
.
68
96
62
-OEt
n.a.
n.a.
n.a.
n.a.
13
10
Sugars
4
1
1
<1
Alk-ether
68
61
52
43
55
48
38
32
17
>35
Alk-O-Alk
n.a.
42
n.a.
n.a.
These numbers could be recalculated on a mmol/g basis using the approximate mass of a C9-unit (ca. 180) for Organoslv and kraft Lignins (see Table 18.3).
(Source: Capanema, Balakshin et al., 2005b; Berlin et al., 2006; Balakshin et al., 2008; Balakshin, Capanema unpublished data.)
structure observed for native lignins in these tree species. For instance, it was shown that various hardwood
lignins degraded differently during kraft pulping resulting in variations of hydroxyl and carboxyl groups, b-O4, b-b, and b-5 linkages as well as in S/G ratio and
degree of condensation (Capanema et al., 2005b; Balakshin et al., 2008). In fact, species-originated variations
are similar or even larger than the variations in major
lignin functionalities caused by different delignification
technologies, such as kraft and OS processes. The only
significant differences observed between kraft and
ethanol OS lignins (as analyzed by 1-D NMR) are the
incorporation of ethoxyl groups and the significantly
higher amounts of carbonyl groups in the latter.
Most of the wet chemistry and 31P NMR methods
originally yield results in mmol/g (or mass %) units.
The 13C NMR method reports results in number of
functional groups per aromatic ring (Ar). A conversion
factor based on the C9-formulae is typically used to
correlate these values (mmol/g and units/Ar), but the
ratio is not obvious. The C9-formulae might not be accurate (even for high-purity lignins; contaminations
would also contribute to this NMR signal) as the lignin
side chain is degraded, to a certain extent, during
biomass processing. The 13C NMR lignin analysis
with Internal Standard (IS) allows for both types of
data presentation. A very good correlation between
13
C NMR with IS and 31P NMR data for the hydroxyl
330
18. INDUSTRIAL LIGNINS: ANALYSIS, PROPERTIES, AND APPLICATIONS
group content has been reported (Xia et al., 2001). However, in that publication, the authors did not specify if a
correction for lignin acetylation was applied to the 13C
NMR data or not. The proportion between the values
expressed “per 100 Ar” and those in mmol/g (for the
same lignin) allow us to calculate the weight of an
average C9-unit (or Ar). The numbers obtained are
271 and 243 for Aspen SE and Poplar SE lignin, correspondingly (Table 18.3). These values are much higher
than those calculated based on the C9-formulae (195
and 193, correspondingly; Table 18.3) and indicate
that the amounts of OH groups in the 13C-IS NMR experiments have been calculated based on acetylated
lignin. Therefore, recalculation as per the original
(non-derivatized) lignin would give numbers of ca.
25e40% higher for the 13C NMR (with IS) vs. 31P
NMR-II. It should be mentioned that a very good correlation between 13C-NMR-IS data (for a non-acetylated
lignin) and the methoxyl group wet chemistry analysis,
one of the most reliable analytical methods in lignin
chemistry, has been reported (Xia et al., 2001). This indicates that 13C NMR data should be considered as
more realistic and that the 31P-II NMR method produced significantly underestimated numbers (probably
due to incomplete derivatization), in agreement with
the earlier discussed results.
In summary, 13C NMR with IS is probably the best
analytical approach to obtain the most comprehensive
and reliable lignin structural information expressed,
both in mol% (units/Ar) and in mmol/g. Unfortunately, very little has been reported so far on the methodology development and its validation of the 13C
NMR lignin analysis with IS vs other analytical
techniques.
Overall, the lignin scientific community believes,
based on a few publications, that there is a good correlation between the different methods used for the
analysis of the technical lignin chemical structures.
However, a comprehensive review of the existing
database (especially of independent publications)
clearly shows that this is not the case. In fact, the deviation between reported data using even the same
analytical method (such as 31P NMR) for the same
lignin preparation is often very significant. Moreover,
the deviation between different analytical methods is
in the range of the differences observed between
different lignin types. This conclusion indicates that
significant efforts still should be made to address
these deviations and to standardize the analytical
methodology for technical lignins analysis. Meanwhile, we should remember the general principle
that it is naturally more accurate to compare structural data obtained with the same analytical method
in the same lab. The use of “reference” lignin samples
(well-investigated lignins, such as Alcell and Indulin
AT) would be also very beneficial to ensure at least
a reliable relative comparison.
Advanced NMR Methods
Routine analytical methods, even comprehensive
C NMR analysis, have failed to reveal the key lignin
structures originated by different biomass processes, for
example, differences between Alcell OS and kraft Indulin
lignins (Tables 18.3 and 18.6). Therefore, this methodology
is not sufficient to distinguish typical structural features of
different lignins neither in qualitative nor in quantitative
analytical mode. This indicates the necessity of advanced
analytical methods to describe the key characteristics in
the structure of technical lignins.
Two-dimensional NMR methods, specifically the
HSQC technique, allow to distinguish specific structural
characteristics of various technical lignins (Capanema
et al., 2001; Balakshin et al., 2003; Liitiä et al., 2003). The
most advanced structural characterization has been
achieved so far for kraft lignins. The HSQC analysis of
OS lignins showed significant amounts of specific structures (Balakshin et al., 2000; Capanema et al., 2001; Berlin
et al., 2006), however, their exact signal assignment was
not possible due to limited NMR data for specific model
compounds. Further studies are required in order to
perform the proper assignments in this type of lignins.
The first attempts to quantify specific lignin functionalities in different technical lignins have already been undertaken (Capanema et al., 2008). The 2D NMR
approach pursued by the authors of the latter article
focused on the quantification of lignin moieties, which
were not possible to quantify with 13C NMR alone (and
other 1D NMR techniques), especially of those structures
formed during pulping. The study provided important
quantitative information on various structural lignin
units, such as condensed lignin moieties, products of bO-4 bond cleavage, vinyl, and alkyl-aryl structures,
saturated aliphatic moieties and others, as well as
lignin-carbohydrate linkages (Table 18.7).
Another useful advanced NMR analytical method is
DEPT 13C NMR that allows for quantification of specific
lignin functionalities overlapped in routine 13C NMR
spectra (Gellerstedt and Robert, 1987). These advanced
NMR methods showed the possibility of expanding our
understanding of the structure of technical lignins. However, more comprehensive studies and cross-validation
of the advanced methodologies with independent
methods are needed before these methods can be
routinely used.
13
Molecular Weight Distribution
The accurate determination of lignin Mw is an important aspect of lignin characterization. The lignin Mw is
TECHNICAL LIGNINS: PRODUCTION, PROPERTIES, AND ANALYSIS
TABLE 18.7
Amounts of Different Structural Units per 100 Ar
(per 100 Monomeric Units) Quantified by Combination of 13C and HSQC NMR Techniques
Units
PSDL
PKRL
EKRL
EKDL
1-G
0.7
5.2
5.8
1.7
1-S
na
na
21.8
2.2
C
1.1
2.8
2.3
0.8
5
1.3
1.8
0.8
0.7
6
2.7
4.0
7.5
4.6
1.5
6.4
1.2
18
17
2.9
4.2
1.3
10
1.4
2.4
28
6.9
3.8
20
3.5
0.6
30
4.1
25.2
3.4
13.1
29
2.0
nd
nd
tr
21e26
4.7
2.1
nd
2.4
13
e
3.1
nd
0.2
16
2.8
2.2
nd
0.3
S/G
na
na
3.0
2.1
7.2
1.5
tr
PSDL, pine soda dissolved lignin; PKRL, pine kraft residual lignin; EKRL,
Eucalyptus globulus kraft residual lignin; EKDL, Eucalyptus grandis kraft dissolved
lignin. See Figure 18.2 for lignin structural units numbering reference.
Source: Capanema et al., 2008.
typically analyzed by a combination of size-exclusion
HPLC and a detection method (refractive index, evaporative light scattering, or others) using calibrants which
mimic the typical Mws and chemistry of lignins. However, lignin Mw determination still remains a challenge. For
instance, a recent joined study by seven major European
lignin research groups reported a reasonably small
(<15%) intralab variability in the determination of lignin
Mw. However, the interlab differences were very large,
up to 49 times(!) difference even under standardized conditions (Baumberger et al., 2007).
StructureeProperties Correlations in Lignin
In spite of the large number of studies dedicated to
the utilization of technical lignins, the correlation between lignin chemical structure and its properties and
functions has not been established yet for most industrial applications. Lignin functional properties are most
often correlated with physical properties, such as glass
transition point (Tg), solubility and, sometimes, with
molecular mass distribution (Glasser, 2000) as well as
with such compositional features as the ash, carbohydrate, sulfur, and carbon content. The effect of the chemical structure of lignins on lignin performance in specific
331
applications is usually anticipated based on fundamental knowledge rather than on experimentally established correlations. For example, the behavior of lignin
in polyurethane production is correlated with the
amount of hydroxyl groups predominantly. The utilization of lignin in phenoleformaldehyde (PF) resins requires the unsubstituted 5-position of the aromatic
ring (o-position to the phenolic hydroxyl group) and
therefore higher proportion of G-units is desirable, in
contrast to S-units, which cannot participate in this reaction. The comparison of lignin reactivity is typically
limited to the comparison of lignins originated from
different technical processes and different feedstock origins (Tejado, 2007; Mansouri and Salvado, 2006; Evtuguin et al. 1998; Rials and Glasser, 1986). An attempt to
correlate lignin structure with its performance includes,
for instance, the observation that the presence of ethyl
groups in Alcell lignin act as an internal plasticizer
and improve the lignin performance in poly(ethylene
oxide) blends (Kubo and Kadla, 2004). Another attempt
was the correlation of the quantity of aliphatic, phenolic
hydroxyl groups and methoxyl groups as well as the
lignin Mw with antioxidant lignin properties (Pan
et al., 2006). However, the correlations between the
amount of phenolic hydroxyls, Mn and the Radical Scavenging Index observed in this study were rather poor or
inexistent (R2 ¼ 0.53 or lower) implying that the dependence is more complex and it requires comprehensive
lignin structural elucidation.
In summary, it would appear that the main reason for
the lack of clear structureefunctional performance correlations is the high heterogeneity and variability of technical lignins and the absence of widely accepted and
understood, quantitative, fast, and simple analytical techniques (Glasser, 2000). In the past few years, leveraging
all the recent advances made in the development of
new lignin analytical techniques, a very comprehensive
work on correlation between process parameters and
the structure and properties of the produced technical lignins was undertaken at the R&D Department of Lignol
Innovations, Ltd (Vancouver, Canada) (Balakshin, Berlin
et al., 2013). Three categories of feedstock (softwoods,
hardwoods, and annual fibers) including various
biomass species sourced from different continents were
processed under at least 30 different combinations of process conditions (time, temperature, ethanol concentration, pH, and L:S ratio). The extracted lignins were
analyzed using advanced rapid and comprehensive 13C
high-resolution NMR spectroscopy coupled with a CryoProbe technology for lignin structural characterization, as
described above, along with traditional methods for
lignin composition analysis, Mw, thermal properties
(Tg), antioxidant activity, and other properties generating
results for over 50 different characteristics for each lignin
sample. This effort generated a very comprehensive
332
18. INDUSTRIAL LIGNINS: ANALYSIS, PROPERTIES, AND APPLICATIONS
database including several thousands of data points
covering a wide diversity of OS lignins (Balakshin and
Berlin, 2010). This unique database has been playing a
very important role in developing Lignol’s lignin applications helping in the selection of best lignin candidates for
specific customer needs. Moreover, very accurate models
correlating process parameters and lignin characteristics
have been also developed on the basis of these studies
(Balakshin, Berlin et al., 2013).
TECHNICAL LIGNINS: TRADITIONAL
AND EMERGING APPLICATIONS
According to the Global Industry Classification
Standard (GICSÒ) developed by Morgan Stanley Capital
International and Standard & Poor’s, the global industries can be divided into 10 sectors: energy, materials, industrials, consumer discretionary, consumer staples,
health care, financials, information technology, telecommunication services, and utilities. After a review of each
of the 10 GICSÒ industrial sectors, it should be noted
that lignin finds applications in almost every sector of
the industry with the exception of financials, telecommunication services, and information technology. The
latter illustrates the versatility of lignin chemistry and
its potential. Overall, lignin applications can be divided
into traditional and emerging. The traditional applications of lignosulfonates and sulfonated kraft lignins
are primarily lower value such as dust control (ca. 11%
market), concrete admixtures (ca. 50% market), and oil
well drilling muds (ca. 4% market) while most of the
emerging applications target higher value applications,
in which the chemical versatility of lignin could be fully
leveraged, or very large volume applications such as the
production of BTX and other petrochemicals.
Traditional Lignin Applications
The traditional lignin applications include, primarily,
lignin uses where lignin plays a replacement role for a
relatively low-value chemical or material. As it was
mentioned earlier, the largest current use of industrial
lignin is fuel. However, there are a number of matured
industrial lignin applications, constituting the bulk of
the higher value lignin commercial chemical market,
and this includes, primarily, lignosulfonates or sulfonated kraft lignins in large-medium-size markets such
as additives for concrete admixtures, dust control, feed
and food additives, dispersants, resin and binder compositions, and oil well drilling. Examples of smaller markets for technical lignins include carbon black,
emulsifiers, water treatment, cleaning chemicals, leather
tanning, battery expanders, and rubber additives
(Higson, 2011). There are many comprehensive reviews
in the literature which cover traditional lignin applications (ACS, 1999; Holladay et al., 2007); therefore, we
will not discuss them here in detail.
Emerging Lignin Applications
The growth of the lignin business in this century
depends, for the most part, on the ability of chemists
and materials scientists to develop novel original applications and processes for lignin valorization. From 1990
to 2010, a number of novel lignin applications have been
proposed and described in the scientific and patent literature. Some of these novel applications have been
piloted or demonstrated at larger than laboratory scale.
For instance, the production of lignin-based carbon fiber
(LCF) (Baker and Rials, 2013) is one of the brightest examples of successful lignin upgrading technology which
have been scaled up to pilot scale. Another example of
nontraditional lignin applications which is being
commercialized with a market potential comparable to
carbon fiber is the use of upgraded technical lignins in
polymers, in particular, in thermoplastic, thermoset, and
composite applications. Not far in the future, we will witness the rise of processes aiming at depolymerization
of lignins to produce valuable oxygenated aromatic compounds, and possibly olefins too, in replacement of petrochemicals. This section briefly summarizes these three
relevant examples of emerging nontraditional lignin
applications which in our opinion present the largest
potential, in terms of volume and value, for commercialization of technical lignins in the future.
Among all the emerging lignin applications, the
manufacturing of LCFs is perhaps the one with the
largest market potential in terms of value. This dream
finds its beginning in the early 1960s, in Japan, when a
group led by Dr. Sugio Otani from the Department of
Chemistry, Faculty of Technology, Gunma University
developed a technology to turn lignin into carbonized fibers (Otani et al., 1969). The Kajima Corporation, a giant
Japanese construction company, working together with
Dr. Otani, had developed, on the basis of this early
invention, a ligninefiber-based reinforced cement with
superior strength properties. Since the early work of
Dr. Otani, the quest to develop commercially viable
LCF applications has been focused on achieving lignin
and lignin blends with properties enabling high rate of
fiber spinning (>2000 m/s), yielding scalable and fast
conversion technologies, as well as high yield. However,
regardless of all the efforts made so far by multiple
research groups worldwide, in particular, by the Carbon
Fiber Composites Consortium (Oak Ridge National Laboratory), the development of high-quality structural
LCF has proved to be very challenging. The main challenge remains achieving the required LCF engineering
properties imposed by existing carbon fiber spinning
333
REFERENCES
technologies. In particular, the best LCF prototypes have
shown a relative low fiber tensile strength (w1 GPa)
and low fiber elastic modulus (<100 GPa) while most
structural applications (automotive, sport goods,
wind turbines, and aerospace applications) require tensile strengths well above 1 GPa and elastic moduli over
100 GPa. Attempts to overcome these limitations have
been made where lignin is blended with synthetic polymers, such as acrylonitrile, and copolymerized to yield
hybrid LCF with acceptable tensile strengths (Maradura et al., 2012). In addition to the LCF mechanical
limitations, the relatively high cost can be pointed out
as another limiting factor deterring LCF commercialization. Owing to these hurdles, the focus of LCF R&D
efforts has been recently shifting from structural applications toward functional uses where LCF seem better
suited. Examples of functional uses are hightemperature insulating materials, CO2 and other gases
capture sorbents, controlled adsorption and release of
macromolecules, and capacitors. On the cost reduction
side, besides the improvement of technical lignins as a
raw material, so their upgrading costs can be minimized, there is the potential to realize cost reductions
by way of alternative fiber spinning methods that are
not bound by the stringent technical requirements
needed for the traditional melt-spinning of carbon fiber
precursors (Baker and Rials, 2013). In addition to cost
reduction and attempts to improve LCF mechanical
properties, the development of novel technologies to
further upgrade lignin will be required to meet the
demanding industrial standards.
Valorization of lignin can be achieved also via its
incorporation in polymeric materials, in particular, in
composite materials containing thermosets, such as,
phenoleformaldehyde, ureaeformaldehyde, and epoxy
resins (Zhao et al., 2001; Mankar et al., 2012; Wang et al.,
2012; Yin and Di, 2012), and, thermoplastics, such as
lignin blends for extrusion applications with polyesters
(Li and Sarkanen, 2002), polyamides (Nitz et al., 2001),
polyacrylonitrile (Seydibeyo
glu, 2012), and polyethylenes (Casenave et al., 2009). Technical lignins find also
applications in structural materials such as polyurethanes which are recognized as one of the most versatile
classes of polymeric materials and they show good
compatibility with lignin given the presence of both aromatic and aliphatic hydroxyl groups within the lignin
structure (Cateto et al., 2010; Faria et al., 2012). The
cornerstone of a viable wide incorporation of technical
lignins in synthetic polymer blends is their compatibility
with the chemical matrices. The compatibility between
lignin and synthetic polymers, often more hydrophobic
than lignin, can be achieved, for instance, via chemical
modification of lignin through esterification (Li and Sarkanen, 2002) or during lignin production by modifying
the reaction conditions.
Finally, the depolymerization of lignins to produce
valuable oxygenated aromatic compounds is an application which, if successful, will consume most of the technical lignin supply in the future. However, this goal
faces, perhaps, one of the most challenging lignin technological barriers (Holladay et al., 2007). Multiple
approaches have been attempted to achieve the evasive
lignin depolymerization target. The thermochemical
methods including pyrolysis (thermolysis), gasification,
hydrogenolysis, chemical oxidation, and hydrolysis under supercritical conditions are the major methods studied with regard to lignin depolymerization (Pandey and
Kim, 2011). The biochemical route, due to its relative low
capital required for deployment and low-energy operation, has been extensively researched as a way of selectively depolymerizing lignin but its high cost and
relatively long reaction time continues to be a barrier
for commercialization (Chen et al., 2012a,b).
CONCLUSIONS
The low value of technical lignins as a fuel has been
evidenced in this review. However, new technological
developments for smart utilization of technical lignins
can lead to much higher value market opportunities,
possibly with lignin-based chemicals superior to
petrochemicals as it was illustrated in the case of
lignin-based hybrid resins. Further technological advancements in the upgrading and optimization of technical lignins for materials applications is currently
constrained by our ability to better understand their
chemical structure and reactivity as well as by our capacity to efficiently purify and adapt them to the needs of the
demanding chemical industry. Advancements in developing comprehensive and validated lignin analytical
methods applied to purposeful lignin applications will
help with mitigating the existing technological hurdles.
Regrettably, the efforts made toward developing lignin
as a chemical feedstock remain very modest compared,
for instance, to those made toward the utilization of other
biomass components such as cellulose and hemicellulose. The refining of complex raw chemical streams into
building blocks is a matured technology best exemplified
by the petroleum and gas industries. Therefore, the
development of lignin-refining technologies into valuable chemicals should also be possible in the near future
if adequate resources are directed toward this goal.
References
Abdelkafi, F., Ammar, H., et al., 2011. Structural analysis of alfa grass
(Stipa tenacissima L.) lignin obtained by acetic acid/formic acid
delignification. Biomacromolecules 12 (11), 3895e3902.
ACS, 1999. Lignin: Historical, Biological, and Materials Perspectives.
American Chemical Society, Washington, DC.
334
18. INDUSTRIAL LIGNINS: ANALYSIS, PROPERTIES, AND APPLICATIONS
Adler, E., 1977. Lignin chemistry: past, present and future. Wood Sci.
Technol. 11, 169e218.
Akim, L., Argyropoulos, D., et al., 2001. Quantitative 31P NMR spectroscopy of lignins from transgenic poplars. Holzforschung 55 (4),
386e390.
Archipov, Y., Argyropoulos, D., et al., 1991. 31P NMR spectroscopy in
wood chemistry. I. Model compounds. J. Wood Chem. Technol. 11
(2), 137e157.
Argyropoulos, D., 1994. Quantitative phosphorus-31 NMR analysis of
six soluble lignins. J. Wood Chem. Technol. 14, 65e82.
Baker, D., Rials, T., 2013. Recent advances in low-cost carbon fiber
manufacture from lignin. J. Appl. Polym. Sci. http://dx.doi.org/
10.1002/APP.39273.
Balakshin, M., Berlin, A., 2010. Lignol’s Biorefinery Technology - Unlocking the Biomass Value. Bio-Based Chemicals East: Strategic Science
and the Business of Breakthrough Technologies, Boston, MA, USA.
Balakshin, M., Capanema E. unpublished data
Balakshin, M., Capanema, E., et al., 2000. The Use of 2D NMR Spectroscopy on Structural Analysis of Residual and Technical Lignin.
Proceedings of the Sixth European Workshop on Lignocellulosics
and Pulp, Bordeaux, France.
Balakshin, M., Capanema, E., et al., 2003. Elucidation of the structures
of residual and dissolved pine kraft lignins using an HMQC NMR
technique. J. Agric. Food Chem. 51 (21), 6116e6127.
Balakshin, M., Capanema, E., et al., 2007. A fraction of MWL with high
concentration of lignin-carbohydrate linkages: isolation and analysis
with 2D NMR spectroscopic techniques. Holzforschung 61, 1e7.
Balakshin, M., Capanema, E., et al., 2008. Recent advances in the
isolation and analysis of lignins and ligninecarbohydrate complexes. In: Hu, T. (Ed.), Characterization of Lignocellulosic Materials. Blackwell, Oxford, UK, pp. 148e170.
Balakshin, M., Capanema, E.A., et al., 2011. Quantification of lignincarbohydrate linkages with high-resolution NMR spectroscopy.
Planta 233, 1097e1110.
Balakshin, M., Berlin, A., et al., 2013. Derivatives of Native Lignin
from Softwood Feedstocks. USPTO. USA. Lignol Innovations, Ltd.
US8431635 B2.
Balakshin, M., Berlin, A., et al., 2013. Processes for Recovery of
Derivatives of Native Lignin. USPTO. USA. Lignol Innovations,
Ltd. US8378020 B1.
Balakshin, M., Yu, M., Capanema, E.A., et al., 2014. Isolation and
analysis of Lignin-Carbohydrate Complexes (LCC) preparations
with traditional and advanced methods. A review. In: Studies in
Natural Products Chemistry. Elsevier, Amsterdam, in press.
Baumberger, S., Abaecherli, A., et al., 2007. Molar mass determination
of lignins by size-exclusion chromatography: towards standardisation of the method. Holzforschung 61 (4), 459e468.
Beauchet, R., Monteil-Rivera, F., et al., 2012. Conversion of lignin to
aromatic-based chemical (L-chems) and biofuels (L-fuels).
Bioresour. Technol. 121, 328e334.
Berlin, A., Balakshin, M., et al., 2006. Inhibition of cellulase, xylanase
and beta-glucosidase activities by softwood lignin preparations.
J. Biotechnol. 125 (2), 198e209.
Berlin, A., 2011. Binder Compositions Comprising Lignin Derivatives.
W.I.P.O., WO2011097719.
Berlin, A., 2012a. Carbon Fibre Compositions Comprising Lignin
Derivatives. E. P. Office. Europe. Lignol Innovations, Ltd.
Berlin, A., 2012b. Resin Compositions Comprising Lignin Derivatives.
European Patent Office, EP2435457.
Capanema, E., Balakshin, M., et al., 2001. Structural analysis of residual and technical lignins by 1H-13C correlation 2D NMRspectroscopy. Holzforschung 55 (3), 302e308.
Capanema, E., Balakshin, M., et al., 2004. A comprehensive approach
for quantitative lignin characterization by NMR spectroscopy.
J. Agric. Food Chem. 52 (7), 1850e1860.
Capanema, E., Balakshin, M., et al., 2005a. Quantitative characterization of a hardwood milled wood lignin by nuclear magnetic
resonance spectroscopy. J. Agric. Food Chem. 53 (25), 9639e9649.
Capanema, E., Balakshin, M., et al., 2005b. Isolation and Characterization of Residual Lignins from Hardwood Pulps: Method Improvements. Proceedings of the Thirteenth International
Symposium on Wood, Fibre, and Pulping Chemistry (ISWFPC),
Auckland, New Zealand.
Capanema, E.A., Balakshin, M.Yu., et al., 2008. Quantitative analysis
of technical lignins by a combination of 1H-13C HMQC and 13C
NMR methods. In: Proceedings of International Conference on
Pulping, Papermaking and Biotechnology. Nov. 4-6. Nanjing,
China. 647e651.
Casenave, S., Aı̈t-Kadi, A., et al., 2009. Mechanical behaviour of highly
filled lignin-polyethylene compistes made by catalytic grafting.
Can. J. Chem. Eng. 74 (2), 308e315.
Cateto, C., Barreiro, M., et al., 2008. Lignins as macromonomers for
polyurethane synthesis: a comparative study on hydroxyl group
determination. J. Appl. Polym. Sci. 109 (5), 3008e3017.
Cateto, C., Barreiro, M., et al., 2010. Polyurethane as a Viable Route to
Valorise Lignin. XXI Encontro Nacional da TECNICELPA/VI
CIADICYP 2010, Lisbon, Portugal.
Chen, C.-L., Robert, D., 1988. Characterization of Lignin by 1H and 13C
NMR Spectroscopy, Vol. 61B. Academic Press Inc, New York
pp. 137e174.
Chen, Q., Marshall, M.N., et al., 2012. Effects of laccase on lignin
depolymerization and enzymatic hydrolysis of ensiled corn stover.
Bioresour. Technol. 117 (0), 186e192.
Chen, Y., Sarkanen, S., et al., 2012. Lignin-degrading enzyme activities.
Methods Mol. Biol. 908, 251e268.
Chudakov, M., 1983. Industrial Utilization of Lignin. Lesnaya Promishlennost, Moscow, Russia.
Chung, H., Washburn, N., 2012. Improved Lignin Polyurethane
Properties with Lewis Acid Treatment. ACS Applied Materials and
Interfaces.
Domtar, March 12, 2013. Domtar inaugurates commercial lignin
production. Biomass Mag. Retrieved June 19, 2013, from
biomassmagazine.com.
Evtuguin, D.V., Andreolety, J.P., et al., 1998. Polyurethanes based on
oxygen-organosolv lignin. Eur. Polym. J. 34, 1163e1169.
Evtuguin, D., Neto, C., et al., 2001. Comprehensive study on the
chemical structure of dioxane lignin from plantation Eucalyptus
globulus wood. J. Agric. Food Chem. 49, 4252e4261.
Faix, O., Argyropoulos, D., et al., 1994. Determination of hydroxyl
groups in lignins. Evaluation of 1H-, 13C-, 31P-, FTIR and wet
chemistry methods. Holzforschung 48, 387e394.
Faria, F., Evtuguin, D., et al., 2012. Lignin-based polyurethane doped
with carbon nanotubes for sensor applications. Polym. Int. 61 (5),
788e794.
Gellerstedt, G., Robert, D., 1987. Quantitative 13C NMR analysis of
kraft lignins. Acta Chem. Scand., Ser. B 41, 541e546.
Gellerstedt, G. (Ed.), 1996. Chemical Structure of Pulp Components.
Pulp Bleaching: Principles and Practice. Tappi Press, Peachtree
Corners, GA.
Gierer, J., 1980. Chemical aspects of kraft pulping. Wood Sci. Technol.
14, 241e266.
Glasser, W., Barnett, C., et al., 1983. The chemistry of several
novel bioconversion lignins. J. Agric. Food Chem. 31 (5),
921e930.
Glasser, W., 2000. Classification of lignin according to chemical and
molecular structure. In: Glasser, W., Northey, R., Schultz, T. (Eds.),
Lignin: Historical, Biological, and Material Perspectives,
pp. 216e238. Washington, DC.
Glasser, W., 2010. New Perspectives on Lignin Conversion (International Lignin Biochemicals Conference).
REFERENCES
Glennie, D., 1971. Reactions in Sulfite Pulping. Wiley e Interscience,
New York.
Gosselink, R., de Jong, E., et al., 2004. Co-ordination network for
lignin - standardisation, production, and applications adapted to
market requirements (EUROLIGNIN). Ind. Crops Prod. 20,
121e129.
Gosselink, R., van Dam, J., et al., 2010. Fractionation, analysis, and
PCA modeling of properties of four technical lignins for prediction
of their application potential in binders. Holzforschung 64 (2),
193e200.
Granata, A., Argyropoulos, D., 1995. 2-chloro-4,4,5,5-tetramethyl1,3,2-dioxaphospholane, a reagent for the accurate determination
of the uncondensed and condensed phenolic moieties in lignins.
J. Agric. Food Chem. 43 (6), 1538e1544.
Helm, R. (Ed.), 2000. Lignin-Polysaccharide Interaction in Woody
Plants. Lignin: Historical, Biological, and Material Perspectives.
ACS Symposium Series, Washington, DC.
Higson, A., 2011. Lignin Factsheet.
Holladay, J.E., White, J.F., et al., 2007. Top Value-added Chemicals from
Biomass. In: Results of Screening for Potential Candidates from
Biorefinery Lignin, Vol. 2. Pacific Northwest National Laboratory
(PNNL) and the National Renewable Energy Laboratory (NREL).
IHS-Chemical, 2012. Lignosulfonates.
Kirk, T.K., Farrell, R.L., 1987. Enzymatic "Combustion": the microbial
degradation of lignin. Annu. Rev. Microbiol. 41, 465e501.
Koshijima, T., Watanabe, T., 2003. Association between Lignin and
Carbohydrates in Wood and Other Plant Tissues. Springer-Verlag
Berlin Heidelberg GmbH, New York.
Kostukevich, N., Filipov, V., et al., 1993. Determination of the Hydroxyl
Containing Functional Groups of the Oxygen-acetic Lignins by 31P
NMR Spectroscopy. Proceedings of the Seventh International Symposium on Wood and Pulping Chemistry, Beijing, China.
Kubo, S., Kadla, J., 2004. Poly(ethylene oxide)/organosolv lignin
blends: relationship between thermal properties, chemical structure, and blend behavior. Macromolecules 37, 6904e6911.
Lawoko, M., Henriksson, G., et al., 2005. Structural differences between the lignincarbohydrate complexes present in wood and in
chemical pulps. Biomacromolecules 6 (6), 3467e3473.
Li, Y., Sarkanen, S., 2002. Alkylated kraft lignin-based thermoplastic
blends with aliphatic polyesters. Macromolecules 35 (26),
9707e9715.
Li, J., Gellerstedt, G., et al., 2009. Steam explosion lignins; their
extraction, structure and potential as feedstock for biodiesel and
chemicals. Bioresour. Technol. 100 (9), 2556e2561.
Liitiä, T., Maunu, S., et al., 2003. Analysis of technical lignins by twoand three-dimensional NMR spectroscopy. J. Agric. Food Chem. 51
(8), 2136e2143.
Lin, S., Dence, C., 1992. Methods of Lignin Chemistry. Springer-Verlag,
Heidelberg/Berlin/New York.
Lora, J., Glasser, W., 2002. Recent industrial applications of lignin:
a sustainable alternative to nonrenewable materials. J. Polym.
Environ. 10 (1e2), 39e48.
Lora, J., 2006. Biorefinery Non-wood Lignins: Potential Commercial
Impact, 3e6. PAPTAC Ninety-Second. Annual Meeting.
Lora, J., 2010. Utilization Opportunities for Biorefinery Lignins: an
Industrial Perspective (International Lignin Biochemicals Conference, Toronto, Canada).
Lucintel, 2013. Global Basic Petrochemicals Industry 2013e2018:
Trend, Profit, and Forecast Analysis.
Mankar, S., Chaudhari, A., et al., 2012. Lignin in phenol-formaldehyde
adhesives. Int. J. Knowl. Eng. 3 (1), 116e118.
Mansouri, N.-E., Salvado, J., 2006. Structural characterization of
technical lignins for the production of adhesive: Application of
lignosulfonates, kraft, soda-anthraquinone, organosolv and
ethanol process lignins. Industrial Crops and Products. 24, 8e16.
335
Maradura, S., Kimb, C., et al., 2012. Preparation of carbon fibers from a
lignin copolymer with polyacrylonitrile. Synth. Mater. 162,
453e459.
Marton, J. (Ed.), 1971. Reaction in Alkaline Pulping. Lignins. Occurrence, Formation, Structure and Reactions. Wiley e Interscience,
New York.
Milne, T., Chum, H., et al., 1992. Standardized analytical methods.
Biomass Bioenergy 2 (1e6), 341e366.
Mörck, R., Yoshida, H., et al., 1986. Fractionation of kraft lignin by
successive extraction with organic solvents. I. Functional groups,
13
C-NMR-spectra and molecular weight distribution. Holzforschung (Suppl. 51e60).
Nitz, H., Semke, H., et al., 2001. Influence of lignin type on the mechanical properties of lignin based compounds. Macromol. Mater.
Eng. 286 (12), 737e743.
Otani, S., Fukuoka, K., et al., 1969. Method for Producing Carbonized
Lignin Fiber. U. S. P. Office. USA. 3,461,082.
Pan, X., Kadla, J., et al., 2006. Organosolv ethanol lignin from hybrid
poplar as a radical scavenger: relationship between lignin structure, extraction conditions, and antioxidant activity. J. Agric. Food
Chem. 54 (16), 5806e5813.
Pandey, M., Kim, C., 2011. Lignin depolymerization and conversion: a
review of thermochemical methods. Chem. Eng. Technol. 34 (1),
29e41.
Perlack, R., Wright, L., et al., 2005. Biomass as Feedstock for a Bioenergy and Bioproducts Industry: The Technical Feasibility of a
Billion-Ton Annual Supply.
Podterob, A., Bogdanovich, Y., et al., 2004. Detoxicating properties of
polyphepan evaluated in model experiments. Pharm. Chem. J. 38
(8), 49e54.
Pu, Y., Cao, S., et al., 2011. Application of quantitative 31P NMR in
biomass lignin and biofuel precursors characterization. Energy
Environ. Sci. 4, 3154e3166.
Rabinovich, M., 2010. Wood hydrolysis industry in the Soviet Union and
Russia: a mini-review. Cellul. Chem. Technol. 44 (4e6), 173e186.
Ralph, J., Lundquist, K., et al., 2004. Lignins: natural polymers from
oxidative coupling of 4-hydroxyphenylpropanoids. Phytochem.
Rev. 3, 29e60.
Rials, T.G., Glasser, W.G., 1986. Engineering plastics from lignin. XIII
Effect of lignin structure on polyurethane network formation.
Holzforschung. 40, 353e360.
Robert, D., Bardet, M., et al., 1988. Structural changes in aspen lignin
during steam explosion treatment. Cellul. Chem. Technol. 22,
221e230.
Saad, R., Radovic-Hrapovic, Z., et al., 2012. Sorption of
2,4-dinitroanisole (DNAN) on lignin. J. Environ. Sci. 24 (5),
808e813.
Sakakibara, A. (Ed.), 1991. Chemistry of Lignin. Wood and Cellulose
Chemistry. Marcel Dekker Inc, New York.
Sannigrahi, P., Ragauskas, A., et al., 2008. Effects of two-stage dilute
acid pretreatment on the structure and composition of lignin and
cellulose in loblolly pine. BioEnergy Res. 1, 205e214.
Sannigrahi, P., Ragauskas, A., et al., 2010. Lignin structural modifications resulting from ethanol organosolv treatment of loblolly
pine. Energy Fuels 24 (1), 683e689.
Seydibeyo
glu, M., 2012. A novel partially biobased PAN-lignin blend
as a potential carbon fiber precursor. J. Biomed. Biotechnol.
Tejado, A., Pena, C., et al., 2007. Phisico-chemical characterization of
lignins from different sources for use in phenol-formaldehyde
resin synthesis. Bioresource Technology. 98, 1655e1663.
Tomani, P., Axegard, P., et al., 2011. LignoBoost Kraft Lignin - A New
Renewable Fuel and a Valuable Fuel Additive (International Bionergy & Bioproducts Conference. Atlanta, GA, USA).
Tomani, P., 2010. The Lignoboost process. Cellul. Chem. Technol. 44
(1e3), 53e58.
336
18. INDUSTRIAL LIGNINS: ANALYSIS, PROPERTIES, AND APPLICATIONS
Vanderlaan, M., Thring, R., 1997. Polyurethanes from Alcell lignin
fractions obtained by sequential solvent extraction. Biomass Bioenergy 14, 525e531.
Vappula, H., 2011. Pulp Market Review - Energy and Pulp Business
Group. U. T. B. Company, Helsinki.
Wallis, A., 1971. Solvolysis by acids and bases. In: Sarkanen, K.,
Ludvig, C. (Eds.), Lignins. Occurrence, Formation, Structure and
Reactions. Wiley e Interscience, New York.
Wang, Z., Xue, J., et al., 2012. Synthesis of wood lignin-ureaformadehyde resin adhesive. Adv. Mater. Res. 560e561,
242e246.
Wörmeyer, K., Ingram, B., et al., 2011. Comparison of different pretreatment methods for lignocellulisic materials. Part II: influence of
pretreatment on the properties of rye straw lignin. Bioresour.
Technol. 102, 4157e4164.
Xia, Z., Akim, L., et al., 2001. Quantitative 13C NMR of lignins with
internal standards. J. Agric. Food Chem. 49, 3573e3578.
Yin, Q., Di, M., 2012. Preparation and mechanical properties of lignin/epoxy resin composites. Adv. Mater. Res. 482-484, 1959e1962.
Zakis, G., 1994. Functional Analysis of Lignins and Their Derivatives.
Tappi Press, Atlanta, GA.
Zhang, L., Gellerstedt, G., 2007. Quantitative 2D HSQC NMR determination of polymer structures by selecting suitable internal
standard references. Magn. Reson. Chem. 45 (1), 37e45.
Zhao, B., Chen, G., et al., 2001. Synthesis of lignin-based expoxy resin
and its characterization. J. Mater. Sci. Lett. 20 (9), 859e862.
C H A P T E R
19
Amino-Based Products from Biomass and
Microbial Amino Acid Production
K. Madhavan Nampoothiri*, Vipin Gopinath a, M. Anusree a,
Nishant Gopalan, Kiran S. Dhar
Biotechnology Division, National Institute for Interdisciplinary Science and Technology (NIIST),
CSIR, Trivandrum, Kerala, India, aEqual contributors
*Corresponding author email: madhavan85@hotmail.com
O U T L I N E
Amino Acids
Glutamic Acid
Lysine
Methionine
Threonine
Arginine
Aromatic Amino Acids
337
338
339
340
340
340
341
Aspartame
341
Poly(Amino Acid)s
Cyanophycin/Cyanophycin Granule Polypeptide
Production of Cyanophycin
Biodegradability of Cyanophycin
Applications for Cyanophycin
341
342
343
343
343
AMINO ACIDS
The amino acid industry has shown an exponential
growth since its infancy in the 1950s. It has grown
from extracting flavor enhancers from seaweeds, to fermenting high-purity, optically active forms in hundreds
and thousands tons. The isolation of a bacterial strain
producing glutamic acid and an efficient screening
method to identify the highest producer by the Japanese
researchers of the Kyowa Hakko Kogyo Co. was the key
event in the amino acid fermentation industry. Until
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00019-X
Poly-g-Glutamic Acid
Production of PGA
Biodegradability of PGA
ε-Poly-L-Lysine
Production of ε-Poly-L-Lysine
Degradation of Polylysine
Applications of Polylysine
343
344
344
345
345
345
345
Polyamines
Putrescine
Cadaverine
345
346
348
Conclusion and Perspectives
349
References
349
then, there was no suitable commercial process for the
mass production of amino acids. Later on, it received
further boost when the workers of the same organization
reported a homoserine auxotrophic lysine producer.
This discovery led to the development of a commercially
viable fermentation process for lysine fermentation with
a conversion efficiency of 26% from glucose. Bioprocess
engineering and strain improvement methods have
contributed to the massive growth of the industry.
The essential amino acids hold a major place in the
global amino acid market, as these cannot be
337
Copyright Ó 2014 Elsevier B.V. All rights reserved.
338
19. AMINO-BASED PRODUCTS FROM BIOMASS AND MICROBIAL AMINO ACID PRODUCTION
synthesized in the organisms and have to be supplied
externally. The annual demand for feed-grade amino
acids globally is about 2.43 million tons with an estimated value of US $6 109. The global amino acid market is estimated to hit US $12.8 109 by the end of 2017
(Chapman, 2012). There has been a substantial increase
in the demand for amino acids in the last 30 years with
a steady growth rate in the market. It is estimated that
in that last 10 years the market demand for amino acids
has doubled with glutamic acid and lysine on the top of
the chart. Corynebacterium glutamicum is generally used
for amino acid production, with an estimated annual
production of 2,160,000 tons of L-glutamate and
1,480,000 tons of L-lysine (Zahoor et al., 2012).
The commonly used methods for amino acid production are extraction, chemical synthesis, enzymatic conversion and fermentation. Selection of the best method
depends on the cost of the raw materials used, overall
production cost, purification methods adopted, marketability and demand. Cost of production and environmental impact can further be reduced by using sugars
from agricultural, industrial or municipal wastes than
pure and refined sugars. Microbial amino acid process
for biorefining applications will lead to a cleaner environment and lesser production costs.
Glutamic Acid
Glutamic acid is a nonessential amino acid finding its
major application in the flavor industry. It was first isolated in its pure form from wheat gluten by Ritthausen
(1866) and Dr Kikunae Ikeda (1908) found that monosodium glutamate was responsible for the flavor of brown
kelp used in Japanese food preparations. The discovery
was soon patented, and Ajinomoto began the commercial production of monosodium glutamate from acid hydrolysate of wheat gluten and defatted soybean in 1909.
At the end of the 1950s, the team led by Kinoshita, of
Kyowa Hakko Kogyo, isolated the glutamate-excreting
soil bacterium, C. glutamicum. This led to the beginning
of the fermentative production of amino acids in large
quantities, which in turn made revolutionary changes
in the amino acid production and the flavor industry.
The bacterium was able to accumulate large amounts
of glutamate in the cell, and was excreted out of the
cell when triggered either by change in temperature,
addition of surfactants, antibiotics or biotin deficiency
(Shimizu and Hirasawa, 2006). These triggering mechanisms altered cell wall permeability to help excrete the
amino acid. Nampoothiri et al. (2002) showed that
expression of genes of lipid synthesis and altered lipid
composition modulates L-glutamate efflux of C. glutamicum. In the same year as Kinoshita, Donald J Kita and
Jackson Heights reported glutamic acid production
from Cephalosporium species (Kita and Jackson, 1957).
Generally, the preferred sources of glutamic acid production were refined sugars like glucose, fructose and sucrose. In a short period, diverse substrates were explored
in place of refined sugars for glutamic acid production,
including starch hydrolysates, molasses hydrocarbons
and methanol. Enzymatic hydrol, a waste product after
enzymatic hydrolysis of grains and starch in industries
was fermented by Brevibacterium divaricatum with a yield
of 0.19 g glutamic acid per gram of enzymatic hydrolysate
(McCutchan et al., 1962). Fermentation with wheat bran
and rice bran extracts gave maximum of 50% w/w glutamic acid by Bacillus ammoniagenes (Hong et al., 1974).
Glutamic acid production has already been demonstrated
with palm waste hydrolysate, cassava starch hydrolysate,
date waste hydrolysate and rice hydrolysate by different
bacterial strains (Das et al., 1995; Nampoothiri and
Pandey, 1999; Tavakkoli et al., 2009). Different fermentation methods also were developed so as to utilize agricultural wastes. Sugarcane bagasse was used as inert
substrate for glutamate production by solid state fermentation (Nampoothiri and Pandey, 1996) and similarly
sugarcane molasses-enriched medium was also used
with carrageenan-immobilized C. glutamicum cells.
Advances in the biorefinery system research basically
demands value addition in the overall process. Cell
surface expression of alpha amylase was reported in
C. glutamicum for glutamate production, which could
find use in whole crop biorefinery. The heterologous
expression of pentose sugar utilizing genes in
C. glutamicum helped to establish a way into the lignocellulosic feedstock biorefinery for amino acid production.
Here, the heterologous expression of araBAD operon
from Escherichia coli resulted in the utilization of arabinose for production of L-glutamate. Coutilization of
xylose and arabinose along with glucose has also been
demonstrated in rice straw and wheat bran hydrolysates
for the amino acid production (Gopinath et al., 2011).
Later this strain was further improved for accelerated
growth and production of lysine, glutamate, ornithine
and putrescine, by overexpressing xyl B of C. glutamicum
along with other genes involved in pentose sugar utilization (Meiswinkel et al., 2012). A detailed review on
pentose sugar utilization by C. glutamicum for production of various value-added commodities was made by
Gopinath et al. (2012). The biodiesel industry generates
waste glycerol streams, and the engineering of glycerol
utilization pathway in C. glutamicum points toward an
efficient utilization of glycerol for glutamate production
(Rittmann et al., 2008). Efforts were also made for the
direct utilization of cellulosic materials. The heterologous expression of Corynebacterium thermocellum endoglucanase in C. glutamicum resulted in the production
of 178 mg/l glutamate from 15 g/l barley b-glucan,
with the synergistic action of external b-glucosidase
from Aspergillus oryzae (Tsuchidate et al., 2011).
AMINO ACIDS
Lysine
Lysine was first isolated from casein by Drechsel
(1889). However, microbial conversion of a-amino
adipic acid and diaminopimelic acid to lysine was reported much later (Haulaham and Mitchell, 1948; Davis,
1952). Microbial fermentation was initiated, when the
Kyowa Hakko Kogyo group reported a homoserine
auxotroph of C. glutamicum that produced increased
amounts of lysine. Classical mutagenesis, strain improvements and metabolic pathway engineering led to
increased production of lysine (Wittmann and Becker,
2007). Genes coding for enzymes in the amino acid
biosynthetic pathway were overexpressed or disrupted,
either alone or in combinations for amino acid overproduction. For example, the identification and cloning of
339
lysine exporter gene lysE helped to increase lysine excretion (Vrljic et al., 1996). Point mutations in the pyc, lysC
and hom genes (Figure 19.1) were introduced into the
wild-type C. glutamicum chromosome to get the engineered lysine producer DM1729 (Georgi et al., 2005).
Pyruvate carboxylase and phosphoenol pyruvate
carboxykinase are two anaplerotic enzymes in Corynebacterium for growth on carbohydrates. The results
from various studies indicate that overexpression of
pyc gene encoding pyruvate carboxylase redirects the
carbon flux toward lysine production and overexpression of pck encoding phosphoenol pyruvate carboxykinase is counteractive to amino acid production (Peters
Wendisch et al., 1998, 2001; Riedel et al., 2001).
Substrate range for lysine production is very vast
including dextrose, sucrose and fructose as the refined
FIGURE 19.1 Genes involved in lysine biosynthetic pathways in Corynebacterium glutamicum. pyc, pyruvate carboxylase; lysC, aspartate kinase;
dapA, dihydrodipicolinate synthase; dapD, succinyl transferase; dapC, aminotransferase; dapE, desuccinylase; dapF, epimerase; ddh, diaminopimelate
dehydrogenase and hom, homoserine dehydrogenase. (For color version of this figure, the reader is referred to the online version of this book.)
340
19. AMINO-BASED PRODUCTS FROM BIOMASS AND MICROBIAL AMINO ACID PRODUCTION
sugars. Organic acids such as acetic acid, propionic acid,
benzoic acid, formic acid, malic acid, citric acid and
fumaric acid; alcohols such as ethanol, propanol, inositol
and glycerol and hydrocarbons, oils and fats such as soybean oil, sunflower oil, groundnut oil and coconut oil as
well as fatty acids such as palmitic acid, stearic acid
and linoleic acid were also used. These substances may
be used individually or as mixtures (Anastassiadis,
2007). An array of nondefined sugar substrates like
cane and beet molasses, blackstrap molasses and starch
hydrolysates were used for industrial fermentation
(Ikeda, 2003). Inability of lysine-producing microbes
was circumvented by engineering them for direct starch
utilization. The expression of amylase genes on the cell
surface of C. glutamicum enabled simultaneous saccharification and fermentation of raw corn starch, potato
starch and sweet potato starch, rather than using starch
hydrolysates and refined sugars (Tateno et al., 2007;
Berens et al., 2001). In the direction of enabling the
efficient utilization and conversion of hemicellulosic
biomass-derived sugars, C. glutamicum has been engineered to utilize arabinose and xylose. In the fermentation section of biorefinery, downstream processes with
least steps help in better energy efficiency, low product
cost and waste management exemplified by spray
dried lysine with 78% purity called “Biolys”. Under the
greenbiorefineries, the brown juice produced from the
green crop drying units is acidified with lactic acid
fermentation by Lactobacillus species and is stored and
transported for lysine fermentation (Thomsen et al.,
2004). Biodiesel industry generated glycerol can also be
utilized for lysine production.
Methionine
Methionine is a limiting amino acid in the monogastric’s feed. Its isolation was first reported from casein
(Mueller, 1923) and since then methionine was commercially produced either by chemical synthesis or enzymatic
methods. Unlike other amino acids, methionine has an
advantage that it can be supplied to animal feed as a racemate or a racemic mixture as the mammals are able to
convert it to utilizable form with a methionine racemase
enzyme. Even so, the microbial production has added advantages over the racemate that it helps optimal nutrient
utilization. With the discovery of fermentative production
of amino acids by Kinoshita, attempts were being made to
commercialize methionine production by submerged
fermentation. The initial attempts on microbial fermentation were done in the 1970s (Kase and Nakayama, 1974).
As amino acid synthesis is an energy expensive process
and is feedback inhibited, the wild-type bacteria were
not reported to overproduce methionine. Hence, overproduction was achieved either by classical mutagenesis or
deregulation of the biosynthetic pathway. Species of
Corynebacterium and Brevibacterium have much simpler
regulatory mechanisms than E. coli for methionine biosynthesis and are the preferred microbes for overproduction.
Corynebacterium is also able to switch between the
transsulfuration pathway and the direct sulfhydrylation
for methionine production (Hwang et al., 2002). Even
though attempts are still being made to tailor the methionine producers to utilize raw sugars from complex
sources, glucose and maltose are most extensively used.
Substrates for fermentation varied over coconut water,
banana, cassava, molasses, sugarcane juice, etc. (Pham
et al., 1992). Methanol and n-alkanes were also used
(Morinaga et al., 1982; Ghosh and Banerjee, 1986).
Threonine
Threonine is the third limiting amino acid after lysine
and methionine in the animal feeds and was discovered
by W.C. Rose (Rose, 1931). Presently, the major share of
commercially produced threonine employs fermentative
production. The microbial strains employed in the production process were genetically manipulated for threonine overproduction including Serratia marcescens
(Komatsubara et al., 1978), C. glutamicum and E. coli
(Dong et al., 2011). Escherichia coli dominates as the threonine producer due to their dynamic growth pattern
and better substrate utilization, but cannot be used in
the synthesis of pharmaceutical grade amino acid owing
to their endotoxin production. The generally recognized
as safe status, the highly defined genome database and
scope for further genetic manipulations make C. glutamicum dominate the pharmaceutical grade threonine production. Generally the strains employed in methionine
fermentation utilize directly available monosaccharide
sugars. The broadening of substrate utilization range to
polysaccharides and biomass-derived sugars including
pentoses is highly desired, as this helps in cost reduction
and flexibility of the overall industrial production process. Most importantly it will help utilize the renewable
sugar sources that will otherwise go underutilized. But
this will require manipulations in the sugar uptake systems of the microorganisms under concern, as both the
industrial strainsdE. coli and C. glutamicumdare unable
to directly utilize polysaccharides. Escherichia coli strains
are reported to have pentose utilization systems, but this
capability has to be incorporated in the strains with
amino acid production ability and resistance to biomass
pretreatment-derived inhibitors. Thus, the extended substrate utilization spectrum will be a step toward production of threonine from biorefinery.
Arginine
Isolation of L-arginine was first reported from lupin
seedlings. The analog-resistant mutants of E. coli,
341
POLY(AMINO ACID)S
Saccharomyces cerevisiae and Bacillus subtilis excreted
arginine in production medium, but were lower than
C. glutamicum (Utagawa, 2004). An arginine overproducer with an argR (arginine repressor) deletion and
expression of feedback-insensitive N-acetylglutamate
kinase was modified with E. coli araBAD. The recombinant strain was able to utilize 77% of arabinose from
the production medium to accumulate arginine, when
glucose and arabinose were used as the sole carbon
source. Arginine production can be further increased
by the overexpression of argininosuccinate synthase
gene.
An ornithine overproducer C. glutamicum argFR double mutant was manipulated to utilize arabinose as the
carbon source (Schneider et al., 2011). It was able to utilize
98% arabinose (244 mM) from the production medium.
The ability of arabinose utilization points to the possibilities of amino acid production from lignocellulosic hydrolysates. The ability of simultaneous utilization of
mixed sugars (Sasaki et al., 2008) and ability to withstand
lignocellulosic pretreatment-derived inhibitors were
demonstrated earlier in C. glutamicum. The integration
of amino acid production excellence with these characters will boost the chances of amino acid production
from the lignocellulosic feedstock biorefinery.
Aromatic Amino Acids
Aromatic amino acids include tryptophan, phenylalanine, tyrosine and histidine and all of them were isolated
in the 1880s. Tyrosine was isolated by Liebig in 1846 and
phenylalanine from lupins by Schulze in 1881. While
histidine was reported by Kossel and Hedin in 1896,
tryptophan was first isolated from casein by Frederick
Hopkins in 1901. Even though the aromatic amino acids
are produced by microbial fermentation, high production levels are not reached. Commercial production of
these amino acids also includes extraction and enzymatic
conversion. In 2006, Kyowa Hakko Kogyo claimed development of the world’s first fermentation-based method
for the commercial production of L-tyrosine. Biodiesel
industry-generated crude glycerol can be biorefined by
phenylalanine-producing E. coli cells (Khamduang
et al., 2009). The use of glycerol resulted in phenylalanine
yields up to 0.58 g/g, which is twice as compared to production with sucrose. Tryptophan and histidine were
produced from mixed sugars pentoses and hexoses by
genetically modified E. coli (Savrasova et al., 2004).
Microbial amino acid production process for biorefining application will be technically feasible, only if
the nutrient requirements are met in invariably same
quantity and quality. These raw material production
costs must be lower than starch hydrolysates or refined
sugars and the coproducts have to use the existing
fermentation machinery and infrastructure for economic
feasibility. Most of the microbial amino acid fermentations occurs at a temperature range of 30e40 C and at
near neutral pH. The fermentation medium has to be
neutralized and proper cooling systems for temperature
maintenance, agitation and aeration has to be in place for
the amino acid production to match with the expected
values. Alternatives are development or utilization of
strains that are pH and temperature tolerant and overproduce amino acids or choosing coproducts and organisms having the same substrate utilization spectrum and
physical requirements. The use of microbial amino acid
fermentation for biorefining resulted in improved
ground water quality, lower ammonia and nitrate excretion from poultry and livestock. This is due to the substitution of optimal quantities of the limiting amino acids in
place of soybean meal. The sugar solutions from the
lignocellulose feedstock biorefinery will have a fair concentration of inhibitors like furfural and hydroxymethyl
furfurals and syringaldehyde, which is toxic to the microorganisms. The inhibitor tolerance of amino acid producers will also be a deciding factor in the biorefining
concept. Fortunately, the amino acid producer C. glutamicum has shown tolerance toward inhibitors at growtharrested conditions and high cell densities.
ASPARTAME
Aspartame is a methyl ester of dipeptides consisting of
aspartic acid and phenylalanine. It was accidentally
discovered in 1965 by the chemist James M. Schlatter while
working on an antiulcer drug (Walters, 1991). Aspartame
is 160e220 times sweeter than sucrose and is used as artificial sweetener in foods and beverages. Aspartame is produced by coupling microbial fermentation and synthesis.
Phenylalanine and aspartic acid are produced by microbial fermentation and phenylalanine is reacted with methanol to form the methyl ester. Aspartic acid is also
treated in such a way as to protect active sites by benzyl
rings. Then the modified amino acids are mixed in a reaction tank at appropriate temperatures to get aspartame intermediates (Figure 19.2). It is further treated with acetic
acid, purified, crystallized and powdered to produce
aspartame. Methods for direct enzymatic synthesis and
chemical synthesis are also reported.
POLY(AMINO ACID)S
In a microbial (or higher) system, proteins are synthesized with the help of an information template in the
form of an mRNA molecule and the translatory machinery of ribosomes and amino acylated tRNA; however,
poly(amino acid)s are synthesized enzymatically
without the requirement of an information template.
342
19. AMINO-BASED PRODUCTS FROM BIOMASS AND MICROBIAL AMINO ACID PRODUCTION
FIGURE 19.2
Aspartame synthesis. (For
color version of this figure, the reader is
referred to the online version of this book.)
While proteins owe their functionality to the tertiary
structure that they attain, the poly(amino acid)s owe it
normally to their physical properties, usually the number of repeating units more dominant in a pool of poly
(amino acid)s. Another striking difference between proteins and poly(amino acid)s is the fact that one type of a
protein will contain an exact number of amino acids,
while poly(amino acid)s display wide polydispersity,
i.e. in a single organism, the size of the same poly
(amino acid) will vary. The fact that these polymers are
biocompatible with human physiology and for applications other than pharmacology, the polymer is biodegradable, makes it an attractive alternative to the
widely used polymers obtained through the petrochemical route, which are often nonbiodegradable, and end
up accumulating in the environment. These poly(amino
acid)s have been shown to be useful in multitude of
applications, like controlled drug release, preparation
of bioplastics, use as antimicrobial additive in food, as
well as superabsorbers (replacement of polyacrylate
gels) (Obst and Steinbüchel, 2004). They may also be
used as a viable source of dipeptide neutraceuticals
(Sallam and Steinbüchel, 2010).
There are three identified poly(amino acid)s so far
that have been reported to be produced from microbial
source; they are cyanophycin, poly-g-glutamate (PGA)
and ε-poly lysine (Figure 19.3). In poly(glutamic acid)
the amide linkages are formed on the a-amino group
to then g-carboxyl group in the polymer backbone,
whereas in poly(lysine), the a-carboxyl group is linked
to the ε-amino group of lysine. In the case of cyanophycin, almost equivocal amounts of arginine and aspartic
acid are arranged as a polyaspartate backbone, with
arginine moieties linked to the b-carboxyl group of
almost every aspartic acid residue.
Cyanophycin/Cyanophycin Granule
Polypeptide
Cyanophycin is the ideal nitrogen storing molecule,
because every repeating unit has about five atoms of nitrogen, and it is insoluble at physiological conditions inside the cell protoplasm. Due to its insolubility, it does
not cause detrimental shifts in the osmolarity of the
cell, hence helps in cell survival (Oppermann-Sanio
and Steinbüchel, 2002). Cyanobacteria normally produce cyanophycin when the organism senses a decrease
in sulfur, phosphate and significantly by the decrease in
nitrogen concentration in the surrounding milieu (Lin
et al., 2012). Apart from cyanobacteria, cyanophycin
granule polypeptide (CGP) has been found in some
strains of Synechococcus sp. (Hai et al., 1999).
FIGURE 19.3 Amino compounds from microbial
sources. (For color version of this figure, the reader is
referred to the online version of this book.)
POLY(AMINO ACID)S
Production of Cyanophycin
Cyanophycin is enzymatically produced by the action
of cyanophicin synthases on small primers of cyanophycin. Because of the slow growth of cyanobacteria in photobioreactors, large-scale production of CGP is hindered
by the lower cell densities and thus used to get only low
yield of CGP with respect to the cell dry mass (CDM). To
solve this problem cyanophycin synthase gene or CphA
gene has been identified and cloned into a wide range of
organisms, from E. coli to eukaryotic microbes like S. cerevisiae (Steinle et al., 2008), commercially important
strains like Ralstonia eutropha, C. glutamicum, Pseudomonas spp., etc. (Aboulmagd et al., 2001) and even
higher plants like potato and tobacco (Neumann et al.,
2005). Recombinant CGP produced were shorter in
length (21e35 repeating units), along with a small percentage of arginine replaced by incorporated lysine.
Till some time, the highest CGP production was attained
by using Acinetobacter calcoaceticus, with a value of 48%
CDM, with the addition of exogenous arginine, along
with the addition of other carbon and nitrogen sources
(Elbahoul et al., 2005). The economic viability of recombinant strains is always a problem for largeescale usage,
due to the sheer amount of antibiotics that have to be
added to the medium. However, the hunt for a commercially compatible strain for economical production of
CGP led to a method for obtaining high cell densities
and high yield of CGP with Ralstonia eutropha. The strain
is poly hydroxy butyrate (PHB) negative (the wild type
produces PHB) and was devoid of the 2-keto-3-deoxyphosphogluconate aldolase (eda) gene (Lin et al.,
2012). The plasmid carrying the cphA gene was constructed along with the eda gene; for the microbe to survive, the plasmid had to be retained, and in other words,
a selective pressure for plasmid retention was achieved,
without the use of antibiotic resistance genes, thus
making the process economically viable.
In general, the production was optimized with a basic
mineral medium, Mineral Salts Medium (MSM), with
sufficient supplements of fructose, NH3, K2SO4,
MgSO4.7H2O, Fe(III) NH4-citrate, CaCl2.2H2O, and trace
elements. A 30 l pilot study gave promising yields of
water-insoluble CGP and water-soluble CGP, contributing to 47.5% and 5.8% (w/w) of CDM, and a cell density as high as 57 g/l CDM was obtained (Elbahloul
et al., 2005). CGP is normally purified by acid extraction,
which involves the solubilization of CGP in acidic solutions of pH 1, followed by washes with distilled water,
which renders it insoluble again.
Alternative production strategies include the use of
molecular farming approach, and expression of the
cphA gene in certain specific tissues of selected plants.
Potato and tobacco have been successfully transformed
with the cphA gene; however, production of CGP within
343
the plant tissues would lead to slow growth and fleshy
leaves. Use of the stable cyanophycin synthase for direct
synthesis of cyanophycin has been suggested by certain
authors as an alternative to the intricately controlled
fermentative production of cyanophycin (Hai et al., 2002).
Biodegradability of Cyanophycin
Biodegradation of cyanophycin is observed in all the
organisms that naturally produce the polymer, as it
serves as a reserve carbon and nitrogen pool. Cyanophycin can be depolymerized by intracellular cyanophycinase, which also has been isolated from Synechocystis
sp. strain PCC6308 (Hai et al., 2002). The cyanophycinase gene or the cphE gene has been found to be located
downstream of the cphA gene. The cyanophycinase
enzyme does not cleave the polymer into arginine and
aspartate. Recent studies have shown that cyanophycin
is degraded by most of the gut bacteria through the
anaerobic route within a time period ranging from
1 day to 7 days (Sallam and Steinbüchel, 2009). The
above studies have opened the doors to the use of cyanophycin directly as nutrient supplement.
Applications for Cyanophycin
Cyanophycin can be hydrolyzed to its constituent
amino acids, aspartic acid, and arginine. These amino
acids may be utilized directly in food and pharmaceutical applications. Cyanophycin can be stripped off of
arginine through chemical modifications, so as to produce polyaspartate. Polyaspartate is a polyanionic polymer that can be utilized for production of biodegradable
surfactants, and can be utilized for applications pertaining to polyacrylate (Schwamborn M, 1998). It has been
discovered recently that cyanophycin can be degraded
by the gut bacteria obtained from a diverse group of organisms ranging from mammals, birds, and fishes. This
opens routes for the use of cyanophycin directly as a
nutritional substrate, instead of constituent dipeptides
or amino acids, which would require additional investments of time and money (Sallam A and Steinbüchel A
2009). Even though a considerable amount of research
has been carried out for the viable production of cyanophycin, the complete potential for the various bulk
chemicals that may be obtained from cyanophycin is
not attained yet.
Poly-g-Glutamic Acid
PGA was first observed by Ivanovics et al. produced
by Bacillus anthracis strain during 1937. The traditional
Japanese food natto (fermented product made from
soyabean) consists of PGA and a fructan, and is produced through fermentation using the strain Bacillus
natto. PGA produced can be essentially of three types,
344
19. AMINO-BASED PRODUCTS FROM BIOMASS AND MICROBIAL AMINO ACID PRODUCTION
a polymer purely made of either D-glutamic acid, L-glutamic acid, or D and L-glutamic acid. Besides Bacillus,
other microbial species from Archaea (Natrialba aegyptiaca, Natronococcus occultus), eubacteria as well as eukaryotes (hydra) were found to produce PGA
(Kocianova et al., 2005; Hezayen et al., 2001). PGA is
one of the most utilized poly(amino acid)s, with multitude of applications ranging from agriculture to cosmetics, food industry and even pharmaceuticals
(Buescher and Margaritis, 2007).
Production of PGA
PGA can be produced only through the microbial
route, unlike alpha poly glutamic acid, which can be
chemically synthesized. Among PGA producers, a broad
classification was established in the form of glutamic
acid-dependent PGA producers and glutamic acidindependent PGA producers, depending on the mandatory requirement or nonrequirement for glutamic acid as
a major component in the nutrient medium. Examples of
glutamic acid-dependent producers of PGA include B.
subtilis IFO 3335, B. subtilis NX-2, and Bacillus licheniformis
ATCC 9945A and examples of glutamic acid-independent
PGA producers include B. subtilis TAM-4 and B. licheniformis SAB-26. Initial studies for the production of PGA
was carried out using the strain B. licheniformis ATCC
9945A. The production medium formulated for PGA
production contains a relatively high concentration
(20e1230 mM) of Mnþ2 and the more it was, the amount
of D-glutamic acid present as well (Leonard et al.,
1958a,b). Bacillus subtilis IFO 3335 was originally isolated
from natto, a traditional fermented food in Japan, which
has mucilage containing PGA and a levan. PGA productivity of this strain was higher than that of B. licheniformis
ATCC 9945A. Bacillus subtilis IFO 3335 could produce
PGA at levels of 9.6 g/l, with an optimized medium
with major components including 30 g/l of L-glutamic
acid, 20 g/l of citrate, and 5 g/l ammonium sulfate. It
was found that L-glutamic acid merely stimulated the
production of PGA, which could initiate PGA productions at lower concentrations (0.1 g/l), without the addition of 30 g/l of L-glutamic acid (Kunioka and Goto, 1994;
Kunioka, 1995). Cheng et al. (1989) isolated B. licheniformis A35 while looking for amino acid producer under
denitrifying conditions. A strain with extremely high
production rates of PGA was isolated from fermented
bean curd (Shi et al., 2006). The strain was found to be
B. subtilis ZJU7. In an optimized culture medium containing 60 g/l sucrose, 60 g/l tryptone and 80 g/l L-glutamic
acid and after cultivated at 37 C for 24 h, the yield of
g-PGA reached 54.4 g/l. In an interesting study, a coculture of C. glutamicum S9114 along with B. subtilis ZJU7
was attempted to reduce the input costs of addition of
L-glutamic acid into the medium, and it yielded 32.8 g/l
of PGA after 24 h of fermentation (Shi et al., 2007).
Various studies regarding the fermentative production,
downstream processing and characterization of PGA
have been reported in the literature. The review by Bajaj
and Singal (2011) provides updated information on
fermentative production of PGA by various bacterial
strains and effect of fermentation conditions and media
component on production of PGA in submerged as well
as solid state fermentation.
PGA can be extracted from the fermentation medium
by two different methods. A crude method involves
centrifuging cells followed by precipitation of PGA by
methanol or ethanol (chilled) (Goto and Kunioka,
1992). Subsequent purification steps, involving gel
permeation chromatography followed by reprecipitation have to be used to get PGA in a pure form. Another
method exploiting the specific interaction of Cuþ2 ions
with PGA, gives relatively purer PGA (Troy, 1973).
Further, the purity of the polymer is checked through
peptide hydrolysis, followed by thin layer chromatography, to assess the constituent amino acids. Large
amounts of PGA are produced microbially by the
Japanese company Meiji Seika Kaisha Ltd employing
B. subtilis strain F-021.
Biodegradability of PGA
PGA like the other poly(amino acid)s is a degradable
polymer. The polymer can withstand temperatures up to
60 C, beyond which the amide bonds start getting hydrolyzed. PGA is resistant to proteases that cleave alpha
peptide bonds. Two types of enzymes are involved in
the degradation of PGA, endo-g-glutamyl peptidase
and exo-g-glutamyl peptidase. Exo-g-glutamyl peptidase consists of two subunits and is a key enzyme in
glutathione metabolism (Ogawa et al., 1991; Xu and
Strauch, 1996). This enzyme catalyzes the formation of
g-glutamic acid di- and tripeptides in vitro. Endo-gglutamyl peptidase is secreted into the medium by
g-PGA-producing B. subtilis and B. licheniformis. It
subsequently cleaves high molecular weight g-PGA
into fragments as small as 105 Da (Goto and Kunioka,
1992). Attempts to isolate microbes that can utilize
PGA as the sole source of carbon and nitrogen source
were also successful (Obst and Steinbüchel, 2004).
APPLICATIONS OF PGA
PGA has been a keen interest of research as far as applications are concerned and hence a plethora of applications for this biopolymer has been developed. PGA
has been used in the food industry as an additive to flour
to increase the moisture-retaining capacity of the dough,
as well as to improve the texture and shelf life of
bread. The calcium salt of g-PGA can be added to health
food in order to increase the Ca2þ concentration, thus
contributing to the prevention of osteoporosis (Ashiuchi
et al., 2004). Addition of PGA improved the solubility
345
POLYAMINES
and hence the availability of vitamins and also caused
sustained release of these vitamins, which led to
increased absorption of these vitamins. PGA salts are
known to be used as antifreeze agents in food. The antifreeze action of the salt increases with the decreasing
size of the salt of the polymer (Shih et al., 2003). PGA
has been suggested for water treatment, as PGA complexes with a lot of metal ions, like Caþ2, Feþ3, Alþ3,
etc. (Kunioka, 2004). Esters of PGA have been used to
test the ability of PGA to form bioplastics with required
properties (Kubota et al., 1995). Hydrogels that can be
used for applications such as controlled drug release,
biosensors, diagnostics, and bioseparators can be produced by using g-PGA and poly(ethylene glycol)methacrylate (Yang et al., 2002). PGA has been used as
adjuvants in vaccines, and also as a delivery agent for
hydrophobic drugs, increasing their bioavailability.
PGA has also been used as a medical adhesive for surgical wounds (sutureless wound closure). PGA hydrogels
can be used as three-dimensional scaffolds for tissue engineering (Matsusaki et al., 2005).
ε-Poly-L-Lysine
The polymer was discovered by (Shima and Sakai,
1977). The polymer has been found to be heat stable,
and can even withstand autoclaving for 20 min. These
properties led to the use of polylysine as a food preservative on a commercial scale in Japan (Yoshida and
Nagasawa, 2003). Production of the compound is influenced substantially by the pH of the medium. Polylysine
has not known to form any secondary or tertiary structure, and its microbicidal activity is attributed to its polycationic nature.
Production of ε-Poly-L-Lysine
The strain Streptomyces albulus 346 spp. lysinopolymerus was the first organism to be isolated as a polylysine
producer, following which many improvements were
carried out on the same for industrial production of
polylysine. Later more producers from the genus
Streptomyces, Kitasatospora, and an ergot fungus epichole
were found to produce polylysine. Polylysine is
now industrially produced by aerobic fermentation, using a mutant derived from S. albulus 346, isolated from
soil. A maximum amount of polylysine, 0.5 g/l was
reported under optimized conditions set at pH 6.0
(Shima and Sakai, 1981). A mutant strain resistant to
S-(2-aminoethyl)-L-cysteine, an analogue for lysine and
glycine were derived and gave higher productivity
values of up to 20 mg/l of polylysine, after 120 h, with
glucose as the carbon source and ammonium sulfate as
the nitrogen source (Hiraki et al., 1998).
Streptomyces albulus 410, the strain that has been
exploited for commercial production of polylysine
displayed the two-stage polylysine production (pH 6.0
and pH 3.0e5.0). Accurate control of the process and
pH led to a maximal production of about 48.3 g/l. It
was found that the best pH for increasing the cellular
mass was pH 6.0, while pH below 4.2 was favorable
for high levels of polylysine production (Shi et al.,
2007). Every poly(amino acid) produced is poly
dispersed (has variable molecular weight), which in
turn makes it difficult to obtain the product of the
required specification. This problem was recently
tackled to an extent, when new isolates belonging to
the genus Streptomyces was obtained, which could produce nearly monodispersed polylysine.
Degradation of Polylysine
There is not much report on the biodegradation of
polylysine. The polylysine-resistant strain Chryseobacterium sp. OJ7 also was postulated to have a polylysinedegrading enzyme, with an exopeptidase activity
(Obst and Steinbüchel, 2004). Polylysine was shown to
be susceptible and was degraded by commercially available enzymes proteases A, P and peptidase R from
A. oryzae, Aspergillus melleus and Rhizopus oryzae (Kito
et al., 2002).
Applications of Polylysine
The application of polylysine as a food preservative
has been established. Other uses of polylysine include
the production of an emulsifying agent through its
conjugation with dextran. Polylysine can be used to
coat biochips and surfaces of cell culture flasks, so as
to provide a biocompatible adherent surface. One of
the most industrially relevant applications of polylysine
is its use as a drug delivery agent and an aid in cell transformation due to its polycationic nature.
POLYAMINES
Alkaline organic compounds with an aliphatic, saturated carbon backbone having at least two primary
amino groups, and a varying number of secondary
amino groups are referred to as polyamines (Schneider
and Wendisch, 2011). The polyamines were first discovered by Antonie van Leeuwenhoek (1678) when he isolated some “three-sided” crystals (sperminephosphate
crystals) from human semen. The charge on the polyamines is distributed along the entire length of the carbon chain, making them unique and distinct from the
point charges of the cellular bivalent cations. Their positive charge enables polyamines to interact electrostatically withpolyanionicmacromolecules within the cell.
Due to this they can modulate diverse cellular processes
such as transcription and translation (Wallace et al.,
2003), biosynthesis of siderophores (Brickman and
346
19. AMINO-BASED PRODUCTS FROM BIOMASS AND MICROBIAL AMINO ACID PRODUCTION
Armstrong, 1996), take part in acid resistance (Foster,
2004), protect from oxygen toxicity (Jung et al., 2003),
etc. They have a role in signaling for cellular differentiation (Sturgill and Rather, 2004) and are essential for plaque biofilm formation (Patel et al., 2006). They are also
found as a part of gram-negative bacterial outer membranes (Takatsuka and Kamio, 2004). Transgenic activation of polyamine catabolism profoundly disturbs
polyamine homeostasis in most tissues, creates a complex phenotype affecting skin, female fertility, fat depots, pancreatic integrity and regenerative growth
(Janne et al., 2004). In the nucleosome, polyamine depletion results in partial unwinding of DNA and unmasking of sequences previously buried in the particle.
These sequences are potential binding sites for factors
regulating transcription (Morgan et al., 1987). This,
together with the fact that polyamines favor the formation of triplex DNA at neutral pH, may provide a mechanism whereby polyamines regulate the transcription of
growth regulatory genes such as c-myc (Hampel et al.,
1991; Celano et al., 1992). Since polyamines play a
wide range of activities in a living cell their relative
intracellular concentrations may vary from species to
species, and they can reach up to the millimolar range
(Miyamoto et al., 1993).
The most common polyamines in bacteria and
Archaea are putrescine (a diamine also named as
1,4-diaminobutane) and cadaverine (diamine also
named 1,5-diaminopentane) (Figure 19.3). In addition
to the above-mentioned polyamines, the pathways for
the biosynthesis of 1,3-diaminopropane, norspermidine,
homospermidine, and thermine are known in some bacteria and Archaea (Tabor and Tabor, 1985). The polyamine family also contains a number of uncommon
longer or branched-chain polyamines, which were
found in extremophiles and which seem to play an
essential role for growth under such extreme conditions
(Oshima, 2007). Polyamines are found in all living species, except two orders of Archaea, Methanobacteriales
and Halobacteriales (Hamana and Matsuzaki, 1992).
Polyamines are used in a wide variety of commercial
applications due to their unique combination of reactivity,
basicity, and surface activity. With a few exceptions, they
are used predominantly as intermediates in the production of functional products (e.g. polyamides/epoxy
curing, fungicide, anthelmintics/pharmaceuticals, petroleum production, oil and fuel additives, paper resins,
chelating agents, fabric softeners/surfactants, bleach activator, asphalt chemicals) (Kroschwitz and Seidel, 2004).
The main commercial interest in biogenic polyamines is
their use in the polymer industry. Today, the only example
of an industrial polyamide containing a biogenic diamine,
which can also be synthesized by bacteria, is nylon-4, 6.
This polyamide is produced from putrescine and adipic
acid (hexanedioic acid).
Putrescine
Putrescine apparently has a specific role in skin physiology and neuroprotection (Janne et al., 2005). Fermentative production of putrescine can be achieved by
manipulating arginine decarboxylase (ADC) pathway
or ornithine decarboxylase (ODC) pathway in E. coli
(Figure 19.4(a)) and C. glutamicum (Figure 19.4(b)) and
of which, the ODC pathway is preferable as it comprises
only a single reaction compared to two or three reactions
of the ADC pathway. To increase L-ornithine formation,
its conversion to L-arginine may be blocked; however,
this results in unfavorable auxotrophy for L-arginine.
Thus, the maintenance of prototrophy with concomitant
high L-ornithine supply is a focus in strain construction.
The pathway for biosynthesis of L-arginine and L-ornithine, the substrates of the initial decarboxylase reactions in the ADC and ODC pathway, respectively, are
similar in E. coli and C. glutamicum. There is some difference in ornithine synthesis between them; C. glutamicum
has a cyclic pathway while E. coli has a linear pathway
(Glansdorff and Xu, 2007). The cyclic pathway is
economical in terms of metabolic cost for ornithine synthesis when compared to linear pathway, because in
linear pathway there is a concomitant hydrolysis of
acetyl-CoA to acetic acid. The L-ornithine was then converted to citrulline by ornithine carbamoyl phosphate
transferase ArgF (EC 2.1.3.3). The synthesis of all enzymes in the pathway is subject to repression by L-arginine, which is mediated by the repressor ArgR in E. coli
and C. glutamicum (Glansdorff and Xu, 2007). In order to
use a microorganism in industrial fermentations or biotransformations the organism should possess high tolerance to the desired product. Concentrations of up to
66 g/l putrescine reduced the growth rate of C. glutamicum by 34% and that of E. coli by 78% (Schneider and
Wendisch, 2010).
In order to overproduce putrescine in E. coli, several
attempts have been done so far. The ADC pathway is
completed by agmatinase SpeB, which hydrolyzes
agmatine to putrescine and urea. While urea cannot be
reused by E. coli, putrescine can be utilized by E. coli
as a sole carbon source. The overexpression of ODC
genes speC (b2965) and of speF (b0693) in the wildtype genetic background led to comparable results as
0.72 or 0.87 g/l of putrescine accumulated in batch cultures (Eppelmann, 2006). The simultaneous overexpression of speF and speAB, the ADC encoding gene speA as
well as speB coding for the agmatinase of E. coli (b2938,
b2937) increased putrescine accumulation up to 1.03 g/l
(Eppelmann et al., 2006). In order to increase the putrescine production a base strain was constructed, by inactivating the putrescine degradation and utilization
pathways, and the engineered E. coli strain was able to
produce 1.68 g/l of putrescine with a yield of 0.168 g/
POLYAMINES
347
FIGURE 19.4 (a) Engineered putrescine and cadaverine production pathways used in E. coli. GdH, glutamic acid dehydrogenase (EC1.4.1.4);
ArgA, amino acid N-acetyltransferase (EC 2.3.1.1); ArgB, acetylglutamic acid kinase (EC 2.7.2.8); ArgC, N-acetylglutamylphosphate reductase (EC
1.2.1.38); ArgD, acetylornithine aminotransferase (EC 2.6.1.11); ArgE, acetylornithinase (EC 3.5.1.16); ArgF, ornithine carbamoyltransferase (EC 2.1.3.3);
ArgG, argininosuccinic acid synthetase (EC 6.3.4.5); ArgH, argininosuccinic acid lyase (EC 4.3.2.1); Pepck, phosphoenolpyruvic acid carboxykinase (EC
4.1.1.32); Ppc, phosphoenolpyruvic acid carboxylase (EC 4.1.1.31); Pyc, pyruvic acid carboxylase (EC 6.4.1.1); AspC, aspartic acid aminotransferase (EC
2.6.1.1); LysC, aspartokinase (EC 2.7.2.4); Asd, aspartic acid semialdehyde dehydrogenase (EC 1.2.1.11); MetL, ThrA bifunctional aspartokinase/
homoserine dehydrogenase (EC 2.7.2.4/1.1.1.3); DapA, dihydrodipicolinic acid synthase (EC 4.2.1.52); DapB, dihydrodipicolinic acid reductase (EC
1.3.1.26); DdH, meso-diaminopimelic acid dehydrogenase (EC 1.4.1.16); LysA, diaminopimelic acid decarboxylase (EC 4.1.1.20); ODC, ornithine
decarboxylase (EC 4.1.1.17); ADC, arginine decarboxylase (EC 3.5.3.1).
(b) Engineered putrescine and cadaverine production pathways used in C. glutamicum. GdH, glutamic acid dehydrogenase (EC1.4.1.4); ArgJ,
bifunctional ornithine acetyltransferase/N-acetylglutamic acid synthase (EC 2.3.1.35/2.3.1.1); ArgB, acetylglutamic acid kinase (EC 2.7.2.8); ArgC, Nacetylglutamylphosphate reductase (EC 1.2.1.38); ArgD, acetylornithine aminotransferase (EC 2.6.1.11); ArgE, acetylornithinase (EC 3.5.1.16); ArgF,
ornithine carbamoyltransferase (EC 2.1.3.3); ArgG, argininosuccinic acid synthetase (EC 6.3.4.5); ArgH, argininosuccinic acid lyase (EC 4.3.2.1); Pepck,
phosphoenolpyruvic acid carboxykinase (EC 4.1.1.32); Ppc, phosphoenolpyruvic acid carboxylase (EC 4.1.1.31); Pyc, pyruvic acid carboxylase
(EC 6.4.1.1); AspC, aspartic acid aminotransferase (EC 2.6.1.1); LysC, aspartokinase (EC 2.7.2.4); Asd, aspartic acid semialdehyde dehydrogenase
(EC 1.2.1.11); MetL, ThrA, bifunctional aspartokinase/homoserine dehydrogenase (EC 2.7.2.4/1.1.1.3); DapA, dihydrodipicolinic acid synthase
(EC 4.2.1.52); DapB, dihydrodipicolinic acid reductase (EC 1.3.1.26); DdH, meso-diaminopimelic acid dehydrogenase (EC 1.4.1.16); LysA, diaminopimelic acid decarboxylase (EC 4.1.1.20); ODC, ornithine decarboxylase (EC 4.1.1.17); ADC, arginine decarboxylase (EC 3.5.3.1).
348
19. AMINO-BASED PRODUCTS FROM BIOMASS AND MICROBIAL AMINO ACID PRODUCTION
g glucose. A further optimization by 25% was achieved
by promoter exchange of genes encoding the enzymes
converting L-glutamic acid into L-ornithine, as well as
the exchange of speFepotE promoter (potE encodes
the ornithineeputrescine antiporter) (Qian et al., 2009).
In contrast to E. coli, C. glutamicum is unable to
degrade and utilize putrescine as a carbon source. The
expression of genes of the ADC and ODC pathway
from E. coli in the wild-type background of C. glutamicum only led to minor amounts of putrescine. The deletion of argR and argF led to accumulation of L-ornithine
but rendered the resulting strain arginine auxotrophic.
When speC and speF from E. coli were expressed in
the argReargF deletion strain of C. glutamicum, production of 5 g/l putrescine resulted, which was about 50
times higher than strains endowed with the ADC
pathway. To avoid costly supplementation with
L-arginine and the strong feedback inhibition of the
key enzyme N-acetylglutamate kinase (ArgB) by L-arginine, a plasmid addiction system for low-level argF
expression was developed. This strain resulted in putrescine yields on glucose from less than 0.001 up to
0.26 g/g, the highest yield in bacteria reported to date
and was named as PUT21. In fed-batch cultivation
with C. glutamicum PUT21, a putrescine titer of 19 g/l
at a volumetric productivity of 0.55 g/l h and a yield
TABLE 19.1
of 0.16 g/g glucose was achieved (Schneider et al.,
2012). Moreover, while plasmid segregation of the initial
strain required antibiotic selection, plasmid segregation
in C. glutamicum PUT21 was fully stable for more than 60
generations without antibiotic selection even in the presence of L-arginine.
Cadaverine
Cadaverine can be overproduced by introduction of
an overproduced lysine decarboxylase. The corresponding substrate, L-lysine, is synthesized in E. coli and
C. glutamicum by similar pathways covering 10 enzymatic steps initiating from the tricarboxylic acid cycle intermediate oxaloacetate. The three initial steps in this
pathway lead to aspartic acid semialdehyde, which is
the branch point for biosynthesis of the amino acids,
L-methionine, L-threonine, L-isoleucine and L-lysine
(Figure 19.4). However, there were substantial differences in the enzyme systems possessed by E. coli and
C. glutamicum. When it is LysC from C. glutamicum that
is additionally feedback inhibited by L-threonine, it
was ThrA from E. coli that is subject to feedback inhibition by L-threonine (Park and Lee, 2010). The tolerance of
E. coli for cadaverine seems to be lower compared to putrescine. The biomass formed in the presence of 51 g/l
Characteristics of Microbial Putrescine and Cadaverine Production
Polyamine
Substrate
Organism
Cultivation Method
C [g/l]
Y(P/S) [g/g]
References
Putrescine
Glucose
E. coli
Fermentor (fed-batch)
5.1
nd
Eppelmann et al. (2006)
Putrescine
Glucose
E. coli
Fermentor (fed-batch)
24.2
nd
Qian et al. (2009)
Putrescine
Glucose
C. glutamicum
Shake flask
6
0.12
Schneider and
Wendisch (2010)
Putrescine
Glucose
C. glutamicum
Fermentor (fed-batch)
19
0.16
Schneider et al. (2012)
Cadaverine
Lysine
E. coli
Fermentor (fed-batch)
69
e
Nishi et al. (2007)
Cadaverine
Glucose
E. coli
Fermentor (fed-batch)
9.6
0.12
Qian et al. (2011)
Cadaverine
Glucose
C. glutamicum
Fermentor (fed-batch)
2.6
0.05
Mimitsuka et al. (2007)
Cadaverine
Glucose
C. glutamicum
Shake flask
3.4
nd
Verseck et al. (2008)
Cadaverine
Glucose
C. glutamicum
Fermentor (fed-batch)
5.0
0.09
Tateno et al. (2007)
Cadaverine
Starch
C. glutamicum
Fermentor (fed-batch)
2.4
0.05
Tateno et al. (2007)
Cadaverine
Glucose
C. glutamicum
Shake flask
1.7
0.17
Kind et al. (2010b)
Cadaverine
Glucose
C. glutamicum
Shake flask
1.1
0.11
Kind et al. (2010b)
Cadaverine
Glucose
C. glutamicum
Shake flask
1.3
0.13
Kind et al. (2010a)
Cadaverine
Glucose
C. glutamicum
Fermentor (fed-batch)
nd
Völkert et al. (2010)
Cadaverine
Xylose
C. glutamicum
Shake flask
1.4
0.11
Buschke et al. (2011)
Cadaverine
Hemicellulose
hydrolysate
C. glutamicum
Shake flask
2
nd
Buschke et al. (2011)
nd - Not determined
Source: Schneider, J. and Wendisch, V.F. (2011); with modification.
72
REFERENCES
cadaverine was reduced by 30% in comparison to the
same molar concentration of putrescine (Qian et al.,
2011, 2009). Corynebacterium glutamicum was tested for
growth on solid medium and grew even at concentrations of up to 31 g/l cadaverine (Mimitsuka et al., 2007).
Escherichia coli strains overexpressing the lysine
decarboxylase gene cadA (b4131) in the wild-type genetic background led to accumulation of 0.8 g/l cadaverine by growing cells. To avoid side reactions of
enzymes active with putrescine toward cadaverine, a
number of genes were deleted: the spermidine synthase
gene speE, the spermidine acetyltransferase gene speG,
the putrescine importer gene puuP, the putrescine
aminotransferase gene puuA and ygjG, which encodes
the initial enzyme of the second putrescine degradation
pathway and is known to be active in vitro with cadaverine. The resulting strain was able to accumulate 1.2 g/l
cadaverine. Production of cadaverine was increased by
10% as a consequence of enhancing the flux of L-aspartic
acid toward L-lysine by overexpression of dapA via promoter exchange. In fed-batch cultivation, this strain produced 9.6 g/l cadaverine (Qian et al., 2011).
Cadaverine production in C. glutamicum was also
achieved by insertional inactivation of homoserine dehydrogenase gene, hom (cg1337, Figure 19.4, B-1) with
cadA from E. coli. The resultant strain secretes 2.6 g/l
cadaverine in the supernatant. The expression of cadA
was driven by the strong kanamycin resistance gene
promoter. But the strain was auxotrophic for L-methionine, L-threonine, and L-isoleucine (Mimitsuka et al.,
2007). A different approach with biosynthetic lysine
decarboxylase (LdcC) from E. coli led to 30% more
cadaverine production than overexpression of cadA
(Kind et al., 2010b). Later the C. glutamicum DAP-3c
cadaverine-producing strain’s substrate spectrum was
broadened for hemicellulose utilization by introducing
xylA and xylB genes from E. coli (Buschke et al., 2011).
Through various studies reasonable titers and productivities were achieved for putrescine and cadaverine
(Table 19.1).
CONCLUSION AND PERSPECTIVES
This chapter outlined the microbial production of
amino acids, poly(amino acid)s and polyamines known
so far. Biotechnological production of amino acids today
serves a market with strong prospects of growth. In the
foreground are the fermentation processes, which are
now widely established in the production of proteinogenic amino acids; this can be extended to the production of other amino products like poly(amino acid)s
and polyamines. The potential that will be leveraged
in the future by modern methods and new findings in
system biology will further stimulate and strengthen
349
microbial production of amino products. Modern
methods such as directed evolution will allow development of customized, highly selective, and stable enzymes and whole cell biocatalysts, as well as efficient
and ecologically sustainable production of the required
products. It became a need to assess the feasibility of
implementing, in addition to the established chemical
processes, a biorefinery concept based on renewable
raw materials. The poly(amino acid)s production does
not have the luxury of background knowledge
regarding the metabolic process leading to their synthesis when compared to the amino acids and polyamines.
Even then, poly(amino acid)s was produced in recently
good titers by using newly isolated strains and their
genetically manipulated versions. However, the genetic
engineering strategies were yet to attain maximal potential in polyamine and poly(amino acid)s producing
strains.
Acknowledgments
The authors are thankful to various funding agencies such as DBT,
New Delhi, DST New Delhi, and BMBF, Germany for different grants
to work on microbial production of amino acids.
References
Aboulmagd, E., Voss, I., Oppermann-Sanio, F.B., Steinbüchel, A., 2001.
Heterologous expression of cyanophycin synthetase and cyanophycin synthesis in the industrial relevant bacteria Corynebacterium
glutamicum and Ralstonia eutropha and in Pseudomonas putida. Biomacromolecules 2, 1338e1342.
Anastassiadis, S., 2007. L-Lysine fermentation. Recent Pat Biotechnol.
1, 11e24.
Ashiuchi, M., Shimanouchi, K., Nakamura, H., Kamei, T., Soda, K.,
Park, C., Sung, M.H., Misono, H., 2004. Enzymatic synthesis of
highmolecular mass poly-gamma-glutamate and regulation of its
stereochemistry. Appl. Environ. Microbiol. 70, 4249e4255.
Bajaj, I., Singhal, R., 2011. Poly (glutamic acid)dan emerging
biopolymer of commercial interest. Bioresour. Technol. 102,
5551e5561.
Berens, S., Kalinowski, J., Puehler, A., 2001. Corynebacterium glutamicum expressing heterologous amylase. European Patent Application EP1156113.
Brickman, T.J., Armstrong, S.K., 1996. The ornithine decarboxylase
gene odc is required for alcaligin siderophore biosynthesis in
Bordetella spp.: putrescine is a precursor of alcaligin. J. Bacteriol.
178, 54e60.
Buescher, J.M., Margaritis, A., 2007. Microbial biosynthesis of polyglutamic acid biopolymer and applications in the biopharmaceutical, biomedical and food industries. Crit. Rev. Biotechnol. 27,
1e19.
Buschke, N., Schröder, H., Wittmann, C., 2011. Metabolic engineering
of
Corynebacterium
glutamicum
for
production
of
1,5-diaminopentane from hemicellulose. Biotechnol. J. 6, 306e317.
Celano, P., Berchold, C.M., Kizer, D.L., Weeraratna, A., Nelkin, B.D.,
Baylin, S.B., Casero Jr, R.A., 1992. Characterisation of an endogenous RNA transcript with homology to the antisense strand of the
human c-myc gene. J. Biol. Chem. 267, 15092e15096.
Chapman, P., 3 July, 2012. Amino Acids Market to Hit $12.8 Billion by
2017. Available from: http://www.companiesandmarkets.com/
350
19. AMINO-BASED PRODUCTS FROM BIOMASS AND MICROBIAL AMINO ACID PRODUCTION
News/Chemicals/Amino-acids-market-to-hit-12-8- billion-by-2017/
NI3838.
Cheng, C., Asada, Y., Aida, T., 1989. Production of g-polyglutamic by
Bacillus licheniformis A35 under denitrifying conditions. Agric. Biol.
Chem. 53, 2369e2375.
Das, K., Anis, M., Azemi, B.M., Ismail, N., 1995. Fermentation and
recovery of glutamic acid from palm waste hydrolysate by ionexchange resin column. J. Biotechnol. Bioeng. 48, 551e555.
Davis, B., 29 Mar, 1952. Biosynthetic interrelations of lysine, diaminopimelic acid, and threonine in mutants of E. coli. Nature 169
(4300), 534e536.
Dong, X.Y., Quinn, P.J., Wang, X.Y., 2011. Metabolic engineering of
Escherichia coli and Corynebacterium glutamicum for the production
of L-threonine. Biotechnol. Adv. 29, 11e23.
Drechsel, E., 1889. Zur Kenntniss der Spaltungsprodukte des Caseı̈ns.
J. Prakt. Chem. 39, 425e429.
Elbahloul., Y., Krehenbrink, M., Reichelt, R., Steinbüchel, A., 2005.
Physiological conditions conducive to high cyanophycin content in
biomass of Acinetobacter calcoaceticus strain ADP1. Appl. Environ.
Microbiol. 71, 858e866.
Eppelmann, K., Nossin, P.M.M., Raeven, L.J.R.M., Kremer, S.M.,
Wubbolts, M.G., 2006. Biochemical Synthesis of 1,4-butanediamine.
WO2006005603.
Foster, J.W., 2004. Escherichia coli acid resistance: tales of an amateur
acidophile. Nat. Rev. Microbiol. 2, 898e907.
Georgi, T., Rittmann, D., Wendisch, V.F., 2005. Lysine and glutamate
production by Corynebacterium glutamicum on glucose, fructose and
sucrose: roles of malic enzyme and fructose-1,6-bisphosphatase.
Metab. Eng. 7, 291e301.
Ghosh, B.B., Banerjee, A.K., 1986. Production of methionine and glutamic acid from n-alkanes by Serratia marcescens var. kiliensis. Folia
Microbiol. 31, 106e112.
Glansdorff, N., Xu, Y., 2007. Amino acid biosynthesisdpathways,
regulation and metabolic engineering. In: Wendisch, V.F. (Ed.),
Microbial Arginine Biosynthesis: Pathway, Regulation and Industrial Production, Microbiology Monographs, vol. 5. Springer Berlin
Heidelberg, Berlin, pp. 219e257.
Gopinath, V., Meiswinkel, T.M., Wendisch, V.F., Nampoothiri, K.M.,
2011. Amino acid production from rice straw and wheat bran hydrolysates by recombinant pentose-utilizing Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 92, 985e996.
Gopinath, V., Anusree, M., Dhar, K.S., Nampoothiri, K.M., 2012.
Corynebacterium glutamicum as a potent biocatalyst for the bioconversion of pentose sugars to value-added products. Appl. Microbiol. Biotechnol. 93, 95e106.
Goto, A., Kunioka, M., 1992. Biosynthesis and hydrolysis of poly(gglutamic acid) from Bacillus subtilis IFO3335. Biosci. Biotechnol.
Biochem. 56, 1031e1035.
Hai, T., Oppermann-Sanio, F.B., Steinbüchel, A., 1999. Purification and
characterization of cyanophycin and cyanophycin synthetase from
the thermophilic Synechococcus sp. MA19. FEMS Microbiol. Lett.
181, 229e236.
Hai, T., Oppermann-Sanio, F.B., Steinbüchel, A., 2002. Molecular
characterization of a thermostable cyanophycin synthetase from
the thermophilic cyanobacterium Synechococcus sp. strain MA19
and in vitro synthesis of cyanophycin and related polyamides.
Appl. Environ. Microbiol. 68, 93e101.
Hamana, K., Matsuzaki, S., 1992. Polyamines as a chemotaxonomic
marker in bacterial systematics. Crit. Rev. Microbiol. 18, 261e283.
Hampel, K.J., Crosson, P., Lee, J.S., 1991. Polyamines favour triplex
DNA formation at neutral pH. Biochemistry 30, 4455e4459.
Hezayen, F.F., Rehm, B.H., Tindall, B.J., Steinbüchel, A., 2001. Transfer
of Natrialba asiatica B1T to Natrialba taiwanensis sp. nov. and
description of Natrialba aegyptiaca sp. nov., a novel extremely
halophilic, aerobic, non-pigmented member of the Archaea from
Egypt that produces extracellular poly(glutamic acid). Int. J. Syst.
Evol. Microbiol. 51, 1133e1142.
Hiraki, J., Hatakeyama, M., Morita, H., Izumi, Y., 1998. Improved epolyL-lysine production of an S-(2-aminoethyl)-L-cysteine resistant
mutant of Streptomyces albulus. Seibutsu Kogaku Kaishi 76, 487e493.
Hong, W., Soon, H., Yung, Chil, Seung Hee, C., 1974. Kor. Jour.
Microbiol. 12, 115e130.
Houlahan, M.B., Mitchell, H.K., 1948. Evidence for an interrelation in
the metabolism of lysine, arginine and pyrimidines in Neurospora.
Proc. Natl. Acad. Sci. USA 34, 465e470.
Hwang, B.J., Yeom, H.J., Kim, Y., Lee, H.S., 2002. Corynebacterium
glutamicum utilizes both transsulfuration and direct sulfhydrylation pathways for methionine biosynthesis. J. Bacteriol. 184,
1277e1286.
Ikeda, K., 1908. Japanese patent 4805.
Ikeda, M., 2003. Amino acid production processes. Adv. Biochem. Eng.
Biotechnol. 79, 1e35.
Janne, J., Alhonen, L., Pietila, M., Keinanen, T.A., 2004. Genetic approaches to the cellular functions of polyamines in mammals. Eur.
J. Biochem. 271, 877e894.
Janne, J., Alhonen, L., Pietila, M., Keinanen, T.A., Pietila, M.,
Uimari, A., Pirinen, E., Hyvonen, M.T., Jarvinen, A., 2005. Animal
disease models generated by genetic engineering of polyamine
metabolism. J. Cell. Mol. Med. 9, 865e882.
Jung, I.L., Oh, T.J., Kim, G., 2003. Abnormal growth of polyamine
deficient Escherichia coli mutant is partially caused by oxidative
stress-induced damage. Arch. Biochem. Biophys. 418, 125e132.
Kase, H., Nakayama, K., 1974. Production of O-acetyl-L-homoserine by
methionine analog-resistant mutants and regulation of homoserine-O-transacetylase in Corynebacterium glutamicum. Agr. Biol.
Chem. 38, 2021e2030.
Khamduang, M., Packdibamrung, K., Chutmanop, J., Chisti, Y.,
Srinophakun, P., 2009. Production of L-phenylalanine from glycerol
by a recombinant Escherichia coli. J. Ind. Microbiol. Biotechnol. 36,
1267e1274.
Kind, S., Jeong, W.K., Schröder, H., Zelder, O., Wittmann, C., 2010a.
Identification and elimination of the competing N-acetyldiaminopentane pathway for improved production of diaminopentane by
Corynebacterium glutamicum. Appl. Environ. Microbiol. 76, 5175e5180.
Kind, S., Jeong, W.K., Schröder, H., Wittmann, C., 2010. Systemswide metabolic pathway engineering in Corynebacterium glutamicum for bio-based production of diaminopentane. Metab. Eng.
12, 341e351.
Kita, D.A., Jackson Heights, N.Y., 1957. Production of glutamic acid by
cephalasporum. United States Patent 2789939.
Kito, M., Onji, Y., Yoshida, T., Nagasawa, T., 2002. Occurrence of
epsilon-poly-L-lysine-degrading enzyme in epsilon-poly-L-lysinetolerant Sphingobacterium multivorum OJ10: purification and characterization. FEMS Microbiol. Lett. 207, 147e151.
Kocianova, S., Vuong, C., Yao, Y., Voyich, J.M., Fischer, E.R.,
DeLeo, F.R., Otto, M., 2005. Key role of poly-gamma-DL-glutamic
acid in immune evasion and virulence of Staphylococcus epidermidis.
J. Clin. Invest. 115, 688e694.
Komatsubara, S., Kisumi, M., Murata, K., Chibata, I., 1978. Threonine
production by regulatory mutants of Serratia marcescens. Appl.
Environ. Microbiol. 35, 834e840.
Kroschwitz, J.I., Seidel, A., 2004. KirkeOthmer Encyclopedia of
Chemical Technology, fifth ed. Wiley-Interscience, Hoboken.
Kubota, H., Nambu, Y., Endo, T., 1995. Convenient esterification of
poly(-glutamic acid) produced by microorganism with alkyl
halides and their thermal properties. J. Polym. Sci. A: Polym.
Chem. 33, 85e88.
Kunioka, M., 1995. Biosynthesis of poly(g-glutamic acid) from
L-glutamine, citric acid, and ammonium sulfate in Bacillus subtilis
IFO3335. Appl. Microbiol. Biotechnol. 44, 501e506.
REFERENCES
Kunioka, M., 2004. Biodegradable water absorbent synthesized from
bacterial poly(amino acid)s. Macromol. Biosci. 4, 324e329.
Kunioka, M., Goto, A., 1994. Biosynthesis of poly(g-glutamic acid)
from L-glutamic acid, citric acid, and ammonium sulfate in Bacillus
subtilis IFO3335. Appl. Microbiol. Biotechnol. 40, 867e872.
Leonard, C.G., Houseright, R.D., Thorne, C.B., 1958a. Effects of some
metallic ions on glutamyl polypeptides, by Bacillus subtilis.
J. Bacteriol. 76, 499e503.
Leonard, C.G., Houseright, R.D., Thorne, C.B., 1958b. Effects of some
metallic ions on the optical specificity of glutamyl synthase and
glutamyl transferase of Bacillus licheniformis. Biochem. Biophys.
Acta 62, 432e434.
Lin, K., Elbahloul, Y., Steinbüchel, A., 2012. Physiological conditions
conducive to high cell density and high cyanophycin content in
Ralstonia eutropha strain H16 possessing a KDPG aldolase genedependent addiction system. Appl. Microbiol. Biotechnol. 93,
1885e1894.
Matsusaki, M., Serizawa, T., Kishida, A., Akashi, M., 2005. Novel
functional biodegradable polymer. III. The construction of
poly(gamma-glutamic acid)-sulfonate hydrogel with fibroblast
growth factor-2 activity. J. Biomed. Mater. Res. A 73, 485e491.
McCutchan, et. al., 1962. Glutamic acid fermentation. US patent
3061521.
Meiswinkel, M.T., Gopinath, V., Lindner, S.N., Nampoothiri, K.M.,
Wendisch, V.F., 2012. Accelerated pentose utilization by Corynebacterium glutamicum for accelerated production of lysine, glutamate, ornithine and putrescine. Microbial. Biotechnol.. http://
dx.doi.org/10.1111/1751-7915.12001.
Mimitsuka, T., Sawai, H., Hatsu, M., Yamada, K., 2007. Metabolic
engineering of Corynebacterium glutamicum for cadaverine
fermentation. Biosci. Biotechnol. Biochem. 71, 2130e2135.
Miyamoto, S., Kashiwagi, K., Ito, S., Watanabe, S., Igarashi, K., 1993.
Estimation of polyamine distribution and polyamine stimulation of
protein synthesis in Escherichia coli. Arch. Biochem. Biophys. 300,
63e68.
Morgan, J.E., Blankenship, J.W., Matthews, H.R., 1987. Polyamines
and acetylpolyamines increase the stability and alter the
conformation of nucleosome core particles. Biochemistry 26,
3643e3649.
Morinaga, Y., Tani, Y., Yamada, H., 1982. L-Methionine production by
ethionine resistant mutant of facultative methylotroph Pseudomonas FM 518. Agric. Biol. Chem. 46, 473e480.
Mueller, J.H., 1923. A new sulfur-containing amino-acid isolated from
the hydrolytic products of protein. J. Biol. Chem. 56, 157e169.
Nampoothiri, K.M., Pandey, A., 1996. Solid state fermentation for Lglutamic acid production using Brevibacteriuı́m sp. Biotech. Lett. 18,
199e204.
Nampoothiri, K.M., Pandey, A., 1999. Fermentation and recovery of Lglutamic acid from cassava starch hydrolysate by ion-exchange
resin column. Rev. Microbiol. 30, 258e264 (online).
Nampoothiri, K.M., Hoischen, C., Bathe, B., Mockel, B., Pfefferle, W.,
Krumbach, K., Sahm, H., Eggeling, L., 2002. Expression of genes of
lipid synthesis and altered lipid composition modulate L-glutamate
efflux of C. glutamicum. Appl. Microbiol. Biotechnol. 58, 89e96.
Neumann, K., Stephan, D.P., Ziegler, K., Hühns, M., Broer, I.,
Lockau, W., Pistorius, E.K., 2005. Production of cyanophycin, a
suitable source for the biodegradable polymerpolyaspartate, in
transgenic plants. Plant. Biotechnol. J. 3, 249e258.
Nishi, K., Endo, S., Mori, Y., Totsuka, K., Hirao, Y., 2007. Method for
producing cadaverine dicarboxylate. US 7189543 B2.
Obst, M., Steinbüchel, A., 2004. Microbial degradation of poly(amino
acid)s. Biomacromolecules 5 (4), 1166e1176.
Ogawa, Y., Hosoyama, H., Hamano, M., Motai, H., 1991. Purification
and properties of gamma-glutamyltranspeptidase from Bacillus
subtilis (natto). Agric. Biol. Chem. 55, 2971e2977.
351
Oppermann-Sanio, F.B., Steinbüchel, A., 2002. Occurrence, functions
and biosynthesis of polyamides in microorganisms and biotechnological production. Naturwissenschaften 89, 11e22.
Oshima, T., 2007. Unique polyamines produced by an extreme thermophile, Thermus thermophilus. Amino Acids 33, 367e372.
Park, J.H., Lee, S.Y., 2010. Metabolic pathways and fermentative production of L-aspartate family amino acids. Biotechnol. J. 5,
560e577.
Patel, C.N., Wortham, B.W., Lines, J.L., Fetherston, J.D., Perry, R.D.,
Oliveira, M.A., 2006. Polyamines are essential for the formation of
plague biofilm. J. Bacteriol. 188, 2355e2363.
Peters-Wendisch, P.G., Kreutzer, C., Kalinowski, J., Pátek, M.,
Sahm, H., Eikmanns, B.J., 1998. Pyruvate carboxylase from Corynebacterium glutamicum: characterization, expression and inactivation of the pyc gene. Microbiology 144, 915e927.
Peters-Wendisch, P.G., Schiel, B., Wendisch, V.F., Katsoulidis, E.,
Möckel, B., Sahm, H., Eikmanns, B.J., 2001. Pyruvate carboxylase is
a major bottleneck for glutamate and lysine production by Corynebacterium glutamicum. J. Mol. Microbiol. Biotechnol. 3, 295e300.
Pham, C.B., Galvez, F.C.F., Padolina, W.G., 1992. Methionine production by batch fermentation from various carbohydrates. ASEAN
Food J. 7, 34e37.
Qian, Z., Xia, X., Lee, S.Y., 2009. Metabolic engineering of Escherichia
coli for the production of putrescine: a four carbon diamine. Biotechnol. Bioeng. 104, 651e662.
Qian, Z., Xia, X., Lee, S.Y., 2011. Metabolic engineering of Escherichia
coli for the production of cadaverine: a five carbon diamine. Biotechnol. Bioeng. 108, 93e103.
Riedel, C., Rittmann, D., Dangel, P., Möckel, B., Petersen, S., Sahm, H.,
Eikmanns, B.J., 2001. Characterization of the phosphoenolpyruvate
carboxykinase gene from Corynebacterium glutamicum and significance of the enzyme for growth and amino acid production. J. Mol.
Microbiol. Biotechnol. 3, 573e583.
Ritthausen, H., 1866. Über die Glutaminsaure [About glutamic acid].
J. Prakt. Chem. 99, 454e462.
Rittman, D., Lindner, S.N., Wendisch, V.F., 2008. Engineering of a
glycerol utilization pathway for amino acid production by
Corynebacterium glutamicum. Appl. Environ. Microbiol. 74,
6216e6222.
Rose, W.C., 1931. Feeding Experiments with Mixtures of Highly
Purified Amino Acids. I. The Inadequacy of Diets Containing
Nineteen Amino Acids. J. Biol. Chem. 94, 155e165.
Sallam, A., Steinbüchel, A., 2009. Cyanophycin-degrading bacteria in
digestive tracts of mammals, birds and fish and consequences for
possible applications of cyanophycin and its dipeptides in nutrition and therapy. J. Appl. Microbiol. 107, 474e484.
Sallam, A., Steinbüchel, A., 2010. Dipeptides in nutrition and therapy:
cyanophycin-derived dipeptides as natural alternatives and their
biotechnological production. Appl. Microbiol. Biotechnol. 87,
815e828.
Sasaki, M., Jojima, T., Inui, M., Yukawa, H., 2008. Simultaneous utilization of D-cellobiose, D-glucose, and D-xylose by recombinant
Corynebacterium glutamicum under oxygen-deprived conditions.
Appl. Microbiol. Biotechnol. 81, 691e699.
Savrasova, E.A., Sycheva, E.V., Michurina, T.A., Kozlov, Y.I., 2004.
Process for producing l-amino acids by fermentation of a mixture
of glucose and pentoses. US Patent 20040229321A1.
Schneider, J., Wendisch, V.F., 2010. Putrescine production by engineered Corynebacterium glutamicum. Appl. Microbiol. Biotechnol.
88, 859e868.
Schneider, J., Wendisch, V.F., 2011. Biotechnological production of
polyamines by bacteria: recent achievements and future perspectives. Appl. Microbiol. Biotechnol. 91, 17e30.
Schneider, J., Niermann, K., Volker, V.F., 2011. Production of the amino
acids L-glutamate, L-lysine, L-ornithine and L-arginine from
352
19. AMINO-BASED PRODUCTS FROM BIOMASS AND MICROBIAL AMINO ACID PRODUCTION
arabinose by recombinant Corynebacterium glutamicum. J. Biotechnol. 154, 191e198.
Schneider, J., Eberhardt, D., Wendisch, V.F., 2012. Improving putrescine production by Corynebacterium glutamicum by fine-tuning
ornithine transcarbamoylase activity using a plasmid addiction
system. Appl. Microbiol. Biotechnol. 95, 169e178.
Schwamborn, M., 1998. Chemical synthesis of polyaspartates: a
biodegradable alternative to currently used polycarboxylate homoand copolymers. Polym. Degrad. Stabil. 59, 39e45.
Shi, F., Xu, Z.N., Cen, P.L., 2006. Efficient production of poly-gglutamic acid by Bacillus subtilis ZJU-7. Appl. Biochem. Biotechnol.
133, 271e282.
Shi, F., Xu, Z.N., Cen, P.L., 2007. Microbial production of natural poly
amino acid. Sci. China Ser. B: Chem. 50, 291e303.
Shih, I.L., Van, Y.T., Sau, Y.Y., 2003. Antifreeze activities of
poly(gamma-glutamic acid) produced by Bacillus licheniformis.
Biotechnol. Lett. 25, 1709e1712.
Shima, S., Sakai, H., 1977. Polylysine produced by streptomyces.
Agric. Biol. Chem. 41, 1807e1809.
Shima, S., Sakai, H., 1981. Poly-L-lysine produced by Streptomyces. Part
II. Taxonomy and fermentation studies. Agric. Biol. Chem. 45,
2497e2502.
Shimizu, H., Hirasawa, T., 2006. Production of glutamate and
glutamate-related amino acids: molecular mechanism analysis and
metabolic engineering. In: Wendisch, V.F. (Ed.), Microbiology
Monographs, Amino Acid BiosynthesisdPathways, Regulation
and Metabolic Engineering, vol. 5. Springer Berlin Heidelberg,
Berlin, pp. 1e38.
Steinle, A., Oppermann-Sanio, F.B., Reichelt, R., Steinbüchel, A., 2008.
Synthesis and accumulation of cyanophycin in transgenic strains of
Saccharomyces cerevisiae. Appl. Environ. Microbiol. 74, 3410e3418.
Sturgill, G., Rather, P.N., 2004. Evidence that putrescine acts as an
extracellular signal required for swarming in Proteus mirabilis. Mol.
Microbiol. 51, 437e446.
Tabor, C.W., Tabor, H., 1985. Polyamines in microorganisms. Microbiol. Rev. 49, 81e99.
Takatsuka, Y., Kamio, Y., 2004. Molecular dissection of the Selenomonas ruminantium cell envelope and lysine decarboxylase
involved in the biosynthesis of a polyamine covalently linked to
the cell wall peptidoglycan layer. Biosci. Biotechnol. Biochem. 68,
1e19.
Tateno., T., Fukuda, H., Kondo, A., 2007. Production of L-lysine from
starch by Corynebacterium glutamicum displaying alpha-amylase on
its cell surface. Appl. Microbiol. Biotechnol. 74, 1213e1220.
Tavakkoli, M., Hamidi-Esfahani, Z., Azizi, M.H., 2009. Optimization of
Corynebacterium bacterium glutamic acid production by response
surface methodology. Food Bioprocess Technol. 5, 92e99.
Thomsen, M.H., Bech, D., Kiel, P., 2004. Manufacturing of stabilised
brown juice for L-lysine from university lab scale over pilot scale to
industrial production. Chem. Biochem. Eng. Q. 18, 37e46.
Troy, F.A., 1973. Chemistry and biosynthesis of the poly(g-D-glutamyl)
capsule in Bacillus licheniformis. I. Properties of the membranemediated biosynthetic reaction. J. Biol. Chem. 248, 305e315.
Tsuchidate, T., Tateno, T., Okai, N., Tsutomu, T., Ogino, C., Kondo, A.,
2011. Glutamate production from b-glucan using endoglucanasesecreting Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 90, 895e901.
Utagawa, T., 2004. Production of arginine by fermentation. J. Nutr.
134, 2854Se2857S.
van Leeuwenhoek, A., 1678. Observationes D. Anthonii Leeuwenhoek, de Natis e semine genitali Animalculis. Phil. Trans. R.
Soc. Lond. 12, 1040e1043.
Verseck, S., Häger, H., Karau, A., Eggeling, L., Sahm, H., 2008.
A process for the fermentative production of cadaverine.
DE 102007005072 A1.
Völkert, M., Zelder, O., Ernst, B., Jeong, W.K., 2010. Method for fermentatively producing 1,5-diaminopentane. US 20100292429 A1.
Vrljic, M., Sahm, H., Eggeling, L., 1996. A new type of transporter with
a new type of cellular function: L-lysine export from Corynebacterium glutamicum. Mol. Microbiol. 22, 815e826.
Wallace, H.M., Fraser, A.V., Hughes, A., 2003. A perspective of polyamine metabolism. Biochem. J. 376, 1e14.
Walters, E.D., 1991. The rational discovery of sweeteners. In:
Walters, D.E., Orthoefer, F.T., DuBois, G.E. (Eds.), Sweeteners.
Discovery, Molecular Design, and Chemoreception. American
Chemical Society, Washington DC, pp. 1e11.
Wittmann, C., Becker, J., 2007. The L-lysine story: from metabolic
pathways to industrial production. In: Wendisch, V.F. (Ed.),
Microbiology Monographs, Amino Acid BiosynthesisdPathways,
Regulation and Metabolic Engineering, vol. 5. Springer Berlin
Heidelberg, Berlin, pp. 39e70.
Xu, K., Strauch, M.A., 1996. Identification, sequence, and expression of
the gene encoding gamma-glutamyltranspeptidase in Bacillus
subtilis. J. Bacteriol. 178, 4319e4322.
Yang, G., Chen, J., Qu, Y.B., Lun, S.Y., 2002. Effects of metal ions on
gamma-poly (glutamic acid) synthesis by Bacillus licheniformis.
Sheng Wu Gong Cheng Xue Bao 17, 706e709.
Yoshida, T., Nagasawa, T., 2003. Epsilon-poly-L-lysine: microbial production, biodegradation and application potential. Appl. Microbiol. Biotechnol. 62, 21e26.
Zahoor, A., Linder, S.N., Wendisch, V.F., 2012. Metabolic engineering
of Corynebacterium glutamicum aimed at alternative carbon sources
and new products. Computational Struct. Biotechnol. J. 3 (4).
e201210004. http://dx.doi.org/10.5936/csbj.201210004.
C H A P T E R
20
Production of Phytochemicals, Dyes and
Pigments as Coproducts in Bioenergy Processes
Hanshu Ding*, Feng Xu
Department of Protein Chemistry, Novozymes Inc., Davis, California, USA
*Corresponding author email: hdin@novozymes.com; fxu@novozymes.com
O U T L I N E
Industrial Phytochemicals
Overview
Colorants (Pigment, Dye, and Ink)
Dietary, Nutraceutical, Food or Feed Additives
Bioactive or Pharmaceutical Phytochemicals
Phytochemicals for Personal Care or Other Uses
353
353
355
356
356
358
Production of Industrial Phytochemicals
Extraction and Isolation from Specific Plants
Coproduction from Processing (Biorefinery)
of Staple Crops
Production from Cultured Plant Cells
Production from Microbial Fermentation
Production from Algae via Aquaculture
358
358
Coproduction of Phytochemicals in Bioenergy
Processes
358
360
360
361
References
361
361
362
362
363
363
363
361
INDUSTRIAL PHYTOCHEMICALS
Overview
Phytochemicals may be defined as chemicals derived
or derivable from plants. Phytochemical sources may
include not only unprocessed trees, crops, grains, fruits,
nuts, vegetables and legumes, but also processed
plant-derived materials such as starch, sugars and oil.
Phytochemicals of commercial interest have demonstrated or suspected utilities for dietary, bioactive,
therapeutic and industrial technical uses. Based on
chemical structure, major groups of phytochemicals
include (Figure 20.1) the following:
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00020-6
Coproduction from Starch- or Sugar-Based Bioenergy
Processes
Coproduction from Plant Oil-Based Bioenergy
Processes
Coproduction from Lignocellulose (Biomass)-Based
Bioenergy Processes
Coproduction from Bio-Oil, Syn-Gas, or Algal
Bioenergy Processes
Colocation of Fermentative Phytochemicals
Production with Bioenergy Processes
Utilization of Phytochemical Production By-Products
for Bioenergy
1. Carotenoids (carotenes or xanthophylls, e.g. a- or
b-carotene, b-cryptoxanthin, lycopene, lutein, and
zeaxanthin) and homologs (e.g. crocin)
2. Flavonoids (anthocyanins, flavanols, flavanones,
flavonols, flavones, and isoflavones), and condensed
tannin and xanthones
3. Other phenolic/quinonics (e.g. tocopherols,
curcumin, resveratrol, carminic acids, alizarin,
purpurin, lignans (dimeric phenyl propanoid), tannic
acid, thymol, and capsaicin)
4. Alkaloids (e.g. caffeine, nicotine, quinine, vinblastine,
and opiates) and other N-contained compounds (e.g.
chlorophylls, flavins, betalain, indole-3-carbinol,
galanthamine, and indigo)
353
Copyright Ó 2014 Elsevier B.V. All rights reserved.
354
20. PRODUCTION OF PHYTOCHEMICALS, DYES AND PIGMENTS AS COPRODUCTS IN BIOENERGY PROCESSES
O
H3C
H3C
H 3C
N
+
CH3
N
O
N
Carotenoids:
β-Carotene
N
CH3
H3C
O
CH3
CH3
Flavonoids:
Anthocyanin
CH3
N
N
Alkaloids:
Caffeine
Alkaloids:
Nicotine
H3C
CH3
H3C
CH3
H 3C
H3C
H3C
CH3
O
CH3
H3C
H3C
CH3
H3C
H
H
OH
H3C
CH3
H
CH3 H
CH3
H3C
HO
Phenolic/quinonics: Tocopherols
Phytosterols: Sitosterol
O
CH3
H3C
SH
HO
O
HN
H
O
O
O
H 2N
HN
Protease inhibitors:
Papain-inhibitor E64
Lipids: Lecithin
O
OH
O
O
O
HO
H
O
HO
O
H
O
OH
O
O
OH
O
O
HO
O
H
HO
O
H
O
OH
O
O
HO
H
O
OH
O
O
HO
O
H
HO
O
H
O
OH
O
O
HO
H
O
OH
O
O
HO
HO
O
O
H
H
O
OH
O
O
OH
HO
O
H
HO
O
HO
O
O
O
H
HO
O
OH
CH3O
OCH3
O
OH
O
HO
O
OH
O
O
O
OH
HO
O
OCH3
HO
HO
OCH3
O
HO
O
OH
O
H
Carbohydrates: Cellulose
O
CH3O
OCH3
O
OH
O
OCH3
OH
O
HO
O
OCH3
O
O
H
HO
O
OH
OH
O
OH
OCH3
CH3O
OH
HO
HO
O
H
OCH3
OH
HO
OH
O
OH
OH
OH
O
OH
OH
HO
HO
H
O
N
H
H2N
H3C
S-compounds:
γ-glutamylcysteines
NH
O H3C
CH3 O
O
O
N+
H3C
O
N
H
O
P
O
CH3
O
HO
O
O
HO
CH3
OCH3
OH
OH
OCH3
Carbohydrates: Lignin
FIGURE 20.1
Major groups of phytochemicals. Source: Drawings from ChemSpider and Sigmaaldrich.com are used in this and Figures 20.2e20.4.
(For color version of this figure, the reader is referred to the online version of this book.)
5. S-contained compounds (e.g. g-glutamylcysteines,
(allyl)cysteine sulfoxides, and isothiocyanates)
6. Phytosterols (e.g. sitosterol, stigmasterol, campesterol
or 4-desmethyl sterols), saponin, digoxin, and other
terpenoids (e.g. artemisinin, paclitaxel, and camphor)
7. Polymeric carbohydrates (e.g. cellulose,
hemicellulose, b-glucan, pectin, gum, inulin, and
resistance starch), oligosaccharides (e.g.
oligofructose), and lignin
8. Lipids and volatiles such as lecithin, essential oils,
and menthol
9. Proteases such as bromelain and papain, as well as
protease inhibitors
It is reported that about 8000 phenolics (including
w4000 flavonoids), w20,000 terpenoids, w10,000 alkaloids, w700 carotenoids, and w250 phytosterols are
known, and many have been shown with various
355
INDUSTRIAL PHYTOCHEMICALS
Colorants (Pigment, Dye, and Ink)
functions (Watkins and Chaudhry, 2013). Comprehensive studies have been carried out on phytochemicals
from whole grains, fruits and vegetables (Liu, 2007;
Piironen et al., 2000).
Phytochemicals may have different physical, chemical
and biological properties, thus suitable for different
industrial usages as colorants (pigment, dye, and ink),
dietary food/feed additives or nutraceuticals, bioactive/
pharmaceutical ingredients, personal care (cosmetic,
perfume) agents, or other useful materials. For instance,
carotenoids, polyphenols, flavonoids and tocopherols
may be used as antioxidant or antiinflammatory agents;
alkaloids may be used as analgesic, antispasmodic or
mental disorder-relieving agents; and carotenoids may
be used as coloring agents.
Phytochemicals are of great interest for industrial,
technical, household, health care or other uses, due to
their renewability, performance, safety, environmentfriendliness, and diversity in structure and activity.
The use of phytochemicals started at the dawn of
humanity, has contributed to the civilization, and is
reemerging along with the advancement of bioenergy,
biobased chemicals, and biorefinery.
Many phytochemicals are chromophoric, reflecting
lights that cover the visible wavelength range. Ubiquitous phytocolorants include chlorophyll (green) and
carotenoids (yellow-red) from leaves and stems of
plants, while more specific colorants may exist in
flowers, fruits or other parts of plants (Figure 20.2).
Colorants could also be produced from algae, bacteria,
or fungi including the saprophytes (Gupta et al., 2011;
Rymbai et al., 2011; Matthews and Wurtzel, 2007;
Mortensen, 2006; Dufossé, 2006; Mapari et al., 2005;
Adrio and Demain, 2003; Sengupta, 2003).
Colorants are used mostly as dyestuff, food/feed additives or cosmetic agents. Traditional plant-extracted/
derived dyestuffs include saffron from saffron crocus
plant, madder (red) from madder plants (Rubia), and
indigo from Indigofera plants (at present chemical
synthesis from fossil feedstocks provides most indigo
dyes, although microbial route has been explored).
Commonly used food or feed colorants derived from
plants include extracts or isolates from specifically
grown plants, such as bixin and norbixin (annatto), betalains (including betanin), curcumin (turmeric), crocin
CH2
CH3
-
N
H3C
Chlorophyll
CH3
Mg2+ N
N
Betanin
O
O
H3C
CH
H3C
O CH3
H3C
O
O
O
OH
O
H 3C
H
N
O
HO
N
H3C
H3C
OH
N+
HO
OH
HO
O
OH
H3C
O
OH
O
Curcumin
HO
Purpurin
Indigo
O
OH
O
N
H
O
O
H 3C
OH
O
O
O
H
N
OH OH
OH
H 3C
H
CH3
CH3
H3C
CH3
O
CH3
CH3
H3C
HO
CH3
CH3
CH3
Canthaxanthin
H3C
CH3
H3C
CH3
OH
O
OH
H3C
H3C
CH3
H3C
Astaxanthin
HO
CH3
OH
HO
H3C
CH3
CH2
Crocin
OH
H3C
CH3
OH
O
HO
CH3
H3C
H 3C
H3C
H 3C
OH
O
OH
O
CH3
Lutein
OH
OH
O
O
O
O
O
H3C
H3C
CH3
O
CH3
HO
O
O
OH
OH
OH
FIGURE 20.2 Representative phytocolorants. (For color version of this figure, the reader is referred to the online version of this book.)
356
20. PRODUCTION OF PHYTOCHEMICALS, DYES AND PIGMENTS AS COPRODUCTS IN BIOENERGY PROCESSES
FIGURE 20.3 Plant-derived dietary, food or feed additives, and nutraceuticals. (For color version of this figure, the reader is referred to the
online version of this book.)
(saffron), and carotenoids (including b-carotene, lutein,
canthaxanthin and astaxanthin). The colorants also
include extracts or isolates from agricultural residues,
such as anthocyanins and carotenoids. The colorants
may be produced microbially, as exemplified by the
carotenoids such as astaxanthin, b-carotene, lutein, and
riboflavin (Chattopadhyay et al., 2008).
Some plant-derived colorants, such as lutein and
b-carotene, are used as cosmetic agents. In addition to
plants, algae also produce colorants of industrial interest. For instance, phycobiliproteins have uses as natural
dyes, cosmetic agents or food colorants (in addition to
health applications) (Spolaore et al., 2006). Phytocolorants may also be used for thermoplastic (van den Oever
et al., 2004).
Dietary, Nutraceutical, Food or Feed Additives
A wide range of phytochemicals have long been used
for dietary or neutraceutical purposes (Rao, 2012; Wang
and Weller, 2006). Several vitamin homologs or precursors, such as b-carotene (for vitamin A), tocopherol (for
vitamin E), and ascorbic acid (vitamin C), are widely produced from plants. Numerous nutraceutical phytochemicals, such as anthocyanin and flavonoids, are known for
antioxidant or other bioactivities. Commonly used food
or feed additives include lutein, canthaxanthin and
b-carotene as dietary or coloring agent, astaxanthin for
aquaculture such as salmon farming, essential oils,
menthol, camphor, caffeine, tannin, capsaicin, wood flavor or liquid smoke (water-diluted bio-oil; Venderbosch
and Prins, 2010; Di Blasi et al., 2010) for flavor or aroma,
anthocyanins as antimicrobial agents (Chattopadhyay
et al., 2008), papain and bromelain for meat processing,
lecithin for emulsification, and dietary fibers. Figure 20.3
shows representative dietary phytochemicals.
Bioactive or Pharmaceutical Phytochemicals
Phytochemicals with a wide diversity in structure
and bioactivity have long been sources for pharmaceutical agents (“phytomedicines”, Pandey et al., 2011).
One important bioactive group is carotenoids.Astaxanthinhas uses as anticarcinogens, antioxidant, antiinflammation (da Fonseca et al., 2011), cholesterol effector, pain
reliever (Skjanes et al., 2012) or immune system booster
(Abad and Turon, 2012). Canthaxanthin also suits for
antioxidant or antiinflammation uses (Skjanes et al.,
2012). b-Carotene may serve to prevent erythema or
arthritis (Skjanes et al., 2012).
Another major bioactive phytochemical group is isoflavones, especially those from soybean. For instance,
daidzein, genistein and glycitein are antioxidative or
estrogenic, potentially beneficial in cancer, heart disease
or obesity prevention.
Phytosterols or triterpenes are phytochemicals beneficial for cholesterol, cancer or immune system-related considerations. Saponins (saponenols glycosides) are also
thought to be beneficial for certain cancer, heart, liver
illness treatments (Wu and Kang, 2011; Guclu-Ustundag
and Mazza, 2007; Zhao and Moghadasian, 2010). Organic
sulfur compounds, such as allylsulfides, also have
INDUSTRIAL PHYTOCHEMICALS
anticancer potential (Cerella et al., 2011). Polyaminehydroxycinnamic amide conjugates are antioxidative
and antimelanogenic (Choi et al., 2007). Lignans are
also antioxidants; so are phytic and cinnamic acid ester
glycosides (Wu and Kang, 2011; Guclu-Ustundag and
Mazza, 2007). Menthol and essential oils are used as
topical analgesic or antiitching agents, decongestants or
oral hygiene ingredients. Capsaicin also has topical uses
for relieving pain, itch or inflammation.
Highly effective, pharmacologically well-studied medicines originated from plants include quinine, ephedrine,
artemisinin, paclitaxel (Taxol) and vinblastine, galanthamine and digoxin, as well as opiates like morphine
and cocaine. Plant-derived precursors for medicines
include 10-deacetylbaccatin (for paclitaxel), ()-shikimic
FIGURE 20.4
this book.)
357
acid (for oseltamivir phosphate or Tamiflu), diosgenin
(for various steroid hormones), salicylic acid or salicin
(for acetylsalicylic acid or aspirin) (Pandey et al., 2011).
There are also phytochemicals that can be used for
agriculture or forestry protection (‘agrochemicals’,
Huter, 2011; Dayan et al., 2009). For example,leptospermonefromCallistemoncitrinusplant is used as herbicide
(Salim et al., 2008); lemongrass oil as pesticide or herbicide; essential oils (e.g.D-limonene), pyrethrum, nicotine
and rotenone as insecticide; thymol and pyrolyzed tobacco bio-oil as biocide (O’Brien et al., 2009; Dayan
et al., 2009); and corn gluten meal and essential oils for
weed control.
The structures of many bioactive phytochemicals are
shown in Figure 20.4.
Bioactive or pharmaceutical phytochemicals. (For color version of this figure, the reader is referred to the online version of
358
20. PRODUCTION OF PHYTOCHEMICALS, DYES AND PIGMENTS AS COPRODUCTS IN BIOENERGY PROCESSES
Phytochemicals for Personal Care or
Other Uses
Various phytochemicals are used for personal care
because of their performance and renewability. Betaine
(trimethylglycine ammonium salt) has significant potential for hair care (Kripp, 2006), lutein and b-carotene as
colorant, and menthol and citronella oil for insect
repelling.
There are other industrial uses of phytochemicals.
Lecithin is useful for antifoaming, dispersion, stabilization, or wetting. Tannin is used for leather processing
(tannery), wood products (e.g. particle board) adhesion
(Frihart, 2010), or anticorrosion. Lignin may be used for
making resins.
PRODUCTION OF INDUSTRIAL
PHYTOCHEMICALS
Extraction and Isolation from Specific Plants
Conventional productions involve various mostly
physical but sometimes chemical methods to isolate
and enrich phytochemicals from selected wild or purposely farmed plants. Representative methods consist
of solid-liquor extraction (including steam distillation),
liquideliquid extraction, or membrane separation, whose
choices are based on effectiveness (low cost) and efficiency (recovery, especially for labile or low-abundance
phytochemicals). In principle, phytochemical productions may involve mechanical grinding of feedstocks, single or multiple steps of extraction, and enrichment or
purification of final products. The extraction (leaching)
parts may be simple binary systems or assisted by
enhanced energy inputs (ultrasound, microwave, high
pressure, sub- or supercritical condition) (Huang and
Ramaswamy, 2012). Proper selection of solvent, adsorbent, and other conditions is critical. Chemical transformation is also applied to convert phytochemical
precursors to final products, as exemplified by sulfuric
acid treatment of madder to yield alizarin or purpurin.
Plant-derived colorants are mostly produced by the
methods listed above: betalains (including betanin)
extracted from red beet (Beta vulgaris); bixin/norbixin
(annatto) from the tree Bixa orellana (Chattopadhyay
et al., 2008); gossypol from cotton seed; lutein from marigold (Tagetes erecta); capsanthin/capsorubin from
paprika; capsorubin from Capsicum annuum; crocin
from saffron (Crocus sativus) flower; anthocyanins from
grape skin, apple or cranberry; acylated anthocyanins
from black carrot; curcumin (turmeric) from Curcuma
longa; carminic acid from Dactylopius coccus; alizarin or
purpurin from madder (Rubia) plants; chlorophyll
from spinach; and indigo from Indigofera or Isatistinctoria
(woad) plants.
For plant-derived S-compounds, glutamylcysteine or
(allyl)cysteine sulfoxide are prepared from Allium species (including garlic); betaine from sugar beet (Kripp,
2006); tannin from tea, quebracho, chestnut or barks;
caffeine from coffee and tea plant; nicotine from tobacco;
camphor from camphor laurel; bromelain from pineapple; papain from papaya; essential oils from a variety
of fruits, seeds, leaves, woods, barks and roots; and
menthol from mint.
Plant-derived drugs or precursors have been prepared
by combinations of the methods listed above: quinine
from cinchona tree, artemisinin from sweet wormwood
(Artemisia annua), paclitaxel from Pacific yew (Taxus brevifolia)’s endophytic fungi, 10-deacetylbaccatin from a few
yews, ()-shikimic acid from shikimi tree, diosgenin
from Dioscorea plants, cytisine from Cytisus laburnum,
vinblastine from Madagascar periwinkle (Catharanthus
roseus), salicylic acid from willow bark, salicin from
meadow sweet (Filipendula ulmaria), galanthamine from
Caucasian snowdrop (Galanthus caucasicus), digoxin
from foxglove (Digitalis lanata), and ephedrine from
Ephedra sinica (Simard et al., 2012; Braz-Filho, 1999).
Plant-derived phytochemicals active as plant protection and other bioactive agents are also produced from
specific plants. For instance, pyrethrum is prepared
from Chrysanthemum cinerariifolium and Chrysanthemum
coccineum, rotenone from jicama vine, thymol from
thyme (O’Brien et al., 2009; Dayan et al., 2009), and flavonoid glycosides or polymethoxylated flavones from
citrus peels (juice-extracted residues) (Manthey, 2012).
Coproduction from Processing (Biorefinery)
of Staple Crops
In addition to specific plants, staple crops also provide phytochemicals at large scale, as coproducts of
well-developed, comprehensive food production processes, which may be considered as the first generation
of biorefineries.
Soybean processing involves multitier steps yielding
multiple streams. The major soybean products include
oil, feedstuff, and fermented soy food. Minor products
include full-fat soy flours, soy concentrate, soy protein isolates, and lecithin. Phytochemicals that can be produced
as coproducts from soybean processing include carotenoids, isoflavones and saponin; protease inhibitors from
protein fractions; as well as lecithin, phytosterols, and
tocopherols from oil. Soybean processing in general
consists of preparatory steps (cleaning, drying, mechanic
disruption or grinding, or conditioning) and oil-extraction
steps (mechanical pressing or solvent extraction, refining,
bleaching, and hydrogenation). Coproducts are prepared
by extractive distillation, adsorption, membrane filtration,
and super- or subcritical fluid extraction (Kannan et al.,
2012; Zijlstra et al., 2012) (Figure 20.5).
359
PRODUCTION OF INDUSTRIAL PHYTOCHEMICALS
Soybean
Crushing, grinding
Extraction
Wet meal
Miscella
Toaster Drying
Desolventizing
Soybean meal
Crude soybean oil
Carotenoids
Isoflavones
Saponin
Protease inhibitor
Refining
Soy lecithin
Lecithin
Phytosterols
Tocopherols
Refined soybean oil
FIGURE 20.5
Schematic soybean processing, with potential
phytochemical coproduction.
One of the two major corn processings is wet milling.
Wet milling yields major products ranging from starch,
starch-fermented ethanol (first-generation bioethanol),
Wet or Dried Distillers Grains (residues from ethanol
fermentation) or Dried Distillers Grains and Solubles
or Stillage (WDDG or DDG, DDGS, which are widely
used as feed), and steep liquor. Phytochemicals that
might be generated as coproducts include flavonoids,
phytosterols, carotenoids, polyamine-hydroxycinnamic
acid amide conjugates (Rausch, 2012; Moreau et al.,
2009) from steep liquor, steeped corn, oil-extracted residues, stillage, or unfermented residues, although their
production has not been widely integrated in current
corn wet milling factories. Corn wet milling process
include mechanic disruption, liquid extraction (steeping, acidic, basic or SO2 impregnation), screening, oil
pressing, evaporation, centrifugation, fermentation and
distillation. Corn dry milling is another major corn processing, mainly geared for bioethanol production. The
process does not have steeping and germ-processing
(oil extraction) as the wet milling does, resulting in separation and enrichment of most corn phytochemicals in
unfermented residues and stillage (Rausch, 2012;
Rausch and Belyea, 2006) (Figure 20.6).
Wet milling
Dry milling
Corn
Corn
Steeping
Corn steep liquor
First milling
germ separation
Germ for
oil and
to CGF
Second milling
fiber separation
Fiber to
corn gluten
feed (CGF)
Starch-protein
separation
Grinding
Water
Cooking
Saccharification
Fermentation
Corn gluten
meal
(CGM)
Enzymes
Yeast
CO2
Downstream
processing
Ethanol
DDGs
Unfermented
residues
Starch washing
Ethanol
Water Starch
Polyolefins
polyurethane
DDG, DDGS
Corn steep liquor
Flavonoids
Phytosterols
Carotenoids
Polyamine-hydroxycinnamic acid amide conjugates
FIGURE 20.6 Schematic corn wet milling and dry milling processes, with potential phytochemical coproduction.
360
20. PRODUCTION OF PHYTOCHEMICALS, DYES AND PIGMENTS AS COPRODUCTS IN BIOENERGY PROCESSES
and germ as primary products, but also gluten, fiber,
bran oil, or other phytochemicals as secondary products.
The processing mainly comprises different levels of milling and fractionation (air classification, sieving, etc.),
sometimes also with extraction (Kraus, 2006).
Soybean, rapeseed, sun flower seed,
peanut, olive, coconut, etc
Handling, conditioning
Hexane
Hexane extraction
Hexane recovery
(Steam distillation)
Water,
acid, base,
or enzyme
Methanol
Meal processing
Meal
Carotenoids
Isoflavones
Saponin
Protease inhibitor
Oil degumming
Lecithin
Oil deodorizing
Phytosterols
Tocopherols
Transesterification
Catalyst
Phase separation
Crude biodiesel
Acid
Free fatty acid
separation
Glycerin/water/methan
ol separation
Free fatty acid
Crude glycerin
FIGURE 20.7 Schematic vegetable oil and biodiesel production
processes, with potential phytochemical coproduction.
Vegetable oil production involves multitier, multiphase steps (Figure 20.7). Major plant sources for vegetable oils include palm, soybean, rapeseed, sunflower
seed, peanut, cotton seed, coconut, and olive, and (to
less extent) corn, hazelnut, grape seed, sesame, flax
seed, safflower, rice bran, etc. Besides oil and cake (or
meal, oil-extracted residues), other coproducts come
from mechanically or chemically separated substances
prior to oil extraction as well as refining by-products.
Degumming and deodorizing of crude vegetable oils
result in the production of lecithin and tocopherols
or phytosterols, respectively. General processes of
vegetable oil production include feedstock disruption
by mechanical, chemical or enzymatic means, mechanical pressing, phase separation, solvent extraction, and
refining (degumming, neutralizing, bleaching and deodorizing) (Panpipat et al., 2012; Febrianto and Yang,
2011; Muth et al., 1998; Dunford, 2012).
Postharvest processing of wheat, rice, oat, or other
cereals generates not only flour or milled rice, bran,
Production from Cultured Plant Cells
High-value phytochemicals may be produced from
cultured plant cells, which can be far more expensive
and demand far more complex, highly specialized
technologies (to promote and sustain the growth, propagation, vitality and productivity of the cells) in comparison with other methods. Such approaches are generally
developed for phytochemicals of pharmaceutical uses,
as exemplified by the production of paclitaxel or other
taxoids from cultured Taxus plants cells. Anthocyanin
production from Ajugareptans, Aralia, Euphorbia milli,
Fragaria, Oxalis, Perilla, Vitis, grapes or carrot have also
been explored (Chattopadhyay et al., 2008; Tripathi
and Tripathi, 2003).
Production from Microbial Fermentation
Microbial fermentation may be a viable
phytochemical-producing technology, alternative to
those directly targeting plants. A fermentative process
is independent of plant harvesting cycle, suits for process engineering and control, and could be more
economical than plant cell culturing. Specifically
selected wild-type or genetically engineered microbes
are fed (in addition to N sources, growth-stimulators,
and other medium ingredients) with inexpensive
fermentable sugars such as isolated glucose, corn steep
liquor, whey or other coproducts from various plants,
crops or dairy processings (Gupta et al., 2011; Dufosse,
2006; Mapari et al., 2005; Adrio and Demain, 2003). After
fermentation, various steps such as cell disruption and
solvent extraction may be applied to obtain, enrich or
purify phytochemical products.
Full or semicommercial processes for phytochemical
production by microbial fermentation have been developed, as exemplified by the production of riboflavin
from fungi (Eremothecium ashbyii, Ashbya gossypi), yeast
(Candida guilliermondii, Debaryomyces subglobosus), or
bacteria (Clostridium acetobutylicum) (Chattopadhyay
et al., 2008), as well as other vitamins (Shimizu, 2008).
Carotenoids may also be produced by microbial fermentation, as exemplified by the production of b-carotene
from B. trispora or Phycomyces blakesleeanus; lycopene
from Fusarium sporotrichioides or bacterium Erwinia
uredovora; zeaxanthin from a Flavobacterium sp.; astaxanthin from Xanthophyllomyces dendrorhous, Rhodotorula
glutinis, Rhodotorula gracilis, Rhodotorula rubra or
Rhodotorula graminis; canthaxanthin from bacterium
COPRODUCTION OF PHYTOCHEMICALS IN BIOENERGY PROCESSES
Bradyrhizobium sp.; and isorenieratene from bacterium
Brevibacterium aurartiacum, Streptomyces mediolani, or
Mycobacterium aurum. Production of certain therapeutic
phytochemicals in microbial fermentation has been
reported as well (Demain and Adrio, 2008).
Production from Algae via Aquaculture
As known producers of many compounds identical
or homologous to plant-derived phytochemicals of
industrial interest, algae have been explored for phytochemical production. Currently, the majority of
commercial b-carotene is produced from Dunaliella salina and Dunaliella bardawil. Astaxanthin may be produced from Haematococcus lacustris; canthaxanthin
from H. lacustris, Coelastrella striolata or Chlorella zofingiensis; and lutein from Muriellopsis sp., Scenedesmus
almeriensis or Chlamydomonas zofingiensis (Skjanes et al.,
2012; Chattopadhyay et al., 2008). Algae may also
produce vitamins and bioactive or dietary amino acids,
proteins (e.g. phycobiliproteins from Spirulina (Arthospira) platensis), lipids or fatty acids, or phycocolloids
(agar, carrageenan and alginate) (Brennan et al., 2012;
Becker, 2004). Those algae may be grown and harvested
either outdoor (aquaculture) or indoor inside factory
tanks, as selected wild types or genetically engineered
strains.
COPRODUCTION OF PHYTOCHEMICALS
IN BIOENERGY PROCESSES
Productions of biofuels (bioenergy processes) use
biobased (sustainable) feedstocks, and convert energylatent plant or algal molecules (formed by photosynthesis) to molecules more suited or amenable as fuels
(see chapters 10, 11, 15, 18e20 of this book). The key intermediate, platform molecules of bioenergy processes,
such as glucose for starchy or cellulosic bioethanol
production, may be used to produce nonfuel biochemicals, such as organic acids, polyols, polymers or plastics
(Clark et al., 2012; Dapsens et al., 2012; Koutinas et al.,
2007, 2006). As the economics and scale of biofuel and
biochemical productions continue to grow, it becomes
more important to enhance the values of various coand by-products, so that the biofuel or biochemical production can be upgraded or expanded to a more efficient
biorefinery capable of maximally utilizing biobased
feedstocks. Such expansion is desirable considering the
colocation and energy/material source sharing, as well
as the integrability of the existing phytochemical
production technologies (mentioned in Section (Production of Industrial Phytochemicals)), with the bioenergy
processes. Many approaches might be taken to recover
phytochemicals during bioenergy or biochemical
361
production processes. For instance, an upstream fractionation (e.g. dry milling or air classification) might
be added to allow further processing of crudely separated feedstock components.
Coproduction from Starch- or Sugar-Based
Bioenergy Processes
Starch- or sugar(cane)-based bioethanol processes are
fully commercialized, and are the major bioethanol providers at present. The main feedstocks are corn and
sugarcane, and to a less extent, potato, cassava and
sugar beet. Starch is converted by amylolytic enzymes
to fermentable sugars (mainly glucose), sucrose
is squeezed out from cane or beet, and sugars are fermented by yeast to ethanol. For corn ethanol processes,
the main coproducts are DDGS for feed, as well as steep
liquor, gluten meal, corn oil, and fiber from wet milling
(Zhang et al., 2012, Figure 20.6). Main by-products are
corn stover and cob for corn ethanol processes, and
bagasse for sugarcane ethanol process. These byproducts are currently being developed as feedstocks
for lignocellulosic ethanol. Many phytochemicals of
industrial interest might be obtained or derived from
the co- or by-products of starch- or sugar-based bioenergy processes, as exemplified in Section (Coproduction from Processing (Biorefinery) of Staple Crops).
Obtaining betaine from sugar beet has been shown
(Kripp, 2006). It has also been reported that polyolefins
(e.g. polypropylene and polyethylene), polymerized
polyurethane or other biomaterials may be made from
DDGS (Diebel et al., 2012, Tatara et al., 2007; Cheesbrough et al., 2008).
Coproduction from Plant Oil-Based Bioenergy
Processes
Plant oil-based biodiesel processes are commercialized (Figure 20.7). Biodiesel productions use mostly
extracted plant or vegetable oils, isolated animal fats
(from meat or dairy productions), or to less extent
recycled cooking oils, and transesterify the triglycerides
with short-chain primary alcohols (e.g. methanol or
(bio)ethanol) to make fatty acid esters (diesels). The
oil extraction from plants (solideliquid extraction)
may allow coextraction of numerous phytochemicals
as coproducts. The main coproduct from the transesterification is glycerol, which can be used directly as
solvent, or as feedstock for microbial fermentation
production of other biochemicals, such as propanediol, dihydroxyacetone, succinic acid, polyglycerols or
polyhydroxyalkanoate, or for hydrothermal-chemical
conversion to H2 (Khanna et al., 2012; Kosmider
et al., 2011; Kannan et al., 2012). Grown on glycerol,
362
20. PRODUCTION OF PHYTOCHEMICALS, DYES AND PIGMENTS AS COPRODUCTS IN BIOENERGY PROCESSES
astaxanthin production by bacterium Phaffia rhodozyma
(X. dendrorhous) or Sporobolomyces ruberrimus (Valduga
et al., 2009), b-carotene by B. trispora (Mantzouridou
et al., 2008), prodigiosin or other carotenoids by
R. glutinis (da Silva et al., 2009), various carotenoids
by Rhodosporidium paludigenum (Yimyoo et al., 2011),
as well as b-carotene by microalgae Clamidomonas acidophila (Langner et al., 2009), astaxanthin by Schizochytrium sp., and various carotenoids by Thraustochytrium
have been demonstrated.
Coproduction from Lignocellulose (Biomass)Based Bioenergy Processes
Lignocellulosic (or second-generation) bioenergy is
being intensively developed, due to its use of renewable
but nonfood or feed feedstocks; valorization of agricultural, forestry, first-generation bioenergy, or municipal
by-products or waste; and potential to significantly
replace fossil feedstocks for energy or chemical industries. From lignocellulosic biomass materials, cellulose
and hemicellulose are converted by (hemi)cellulolytic
enzymes or chemical means to fermentable sugars
(mainly glucose and xylose), which are then fermented
by yeast or bacteria to ethanol or other chemicals. The
processes could run alone, fed by selected biomass feedstocks, or along with the starch/sugar bioethanol or biodiesel processes, fed by the lignocellulosic by-products
from firstegeneration bioenergy processes.
The main by-product from lignocellulosic bioenergy
processes (Figure 20.8) is lignin or lignaceous residue,
whose valorizations are the focus of rigorous research
efforts and may include uses for the production of phenolics (e.g. vanillin, vinyl guaiacol, ferulic acid), lignans,
carbon fiber, or as additives for paper and pulp industry,
roadbed construction, or soil augmentation (Ceylan
et al., 2012; Chapters 22, 23 of this book). Other byproducts from lignocellulosic bioenergy processes
include stillage and pretreatment liquor, which may be
inhibitory to fermentation or enzymatic hydrolysis but
rich in phenolics and oligosaccharides (Klinke et al.,
2002; Persson et al., 2002). Mechanically separated
biomass components (upstream to pretreatment, hydrolysis, and fermentation) may also serve as sources for
Biomass feedstock
Enzymes
Biomass
pretreatment
(Hemi)cellulose
hydrolysis
Pretreatment liquor
Phenolics
Oligosaccharides
phytochemicals, such as tree barks for tannin
production.
Woods, especially those not suited for conventional
forestry products, are attractive feedstocks for lignocellulosic bioenergy. Prior to enzymatic or chemical conversion to fermentable sugars, woody materials might be
subjected to treatments (such as the leaching processes
widely used for dedicated phytochemical production,
as mentioned in Section (Extraction and Isolation from
Specific Plants) to yield extractives comprising phenolics (phenols, flavonoids, and anthocyanins), terpenoids
(essential oils), nitrogen-containing phytochemicals
(alkaloids) or organic acids (citric, oxalic, acetic, malic,
benzoic, etc.) (Huang and Ramaswamy, 2012; Turley
et al., 2006). In addition to agricultural and forestry
by-products (e.g. corn stover, wheat straw, and wood
residues), switchgrass and other dedicated “energy
crops” may serve as viable feedstocks for not only bioenergy but also phytochemical coproducts. For instance,
valued phytochemicals like antioxidants and flavonoids
might be extracted from switchgrass prior to the pretreatment of the bioenergy process (Huang and
Ramaswamy, 2012; Uppugundla et al., 2009; Wang and
Weller, 2006).
Coproduction from Bio-Oil, Syn-Gas, or Algal
Bioenergy Processes
Bio-oil and syn-gas are fuels chemically converted
from lignocellulosic feedstocks. Aggressively pursued
and developed as viable bioenergy, bio-oil and syn-gas
productions rely on thermal-chemical conversion (or
pyrolysis) of biomass or other lignocellulosic feedstocks
to combustible oily substances (comprising numerous
oxygenated hydrocarbons) or H2eCO gas mix, respectively. Depending on process conditions, up to hundreds
of compounds may be present in bio-oils, with
numerous chemicals of interest (other than combustibility) among them (Abou-Zaid and Scott, 2012; Venderbosch and Prins, 2010; Briens et al., 2008; Demirbas,
2009). These may include polyphenols, proanthocyanidins, tannins, flavonoids or organic acids. Some of the
(phenolic) compounds may possess biocidal activity,
making them useful as pesticide, bactericide, antitermite
Ethanol
Glucose, xylose
fermentation
Phenolics
Lignans
Carbon fiber
Distillation:
ethanol recovery
Lignin residue
FIGURE 20.8 A schematic lignocellulosic
bioenergy process, with potential phytochemical
coproduction.
REFERENCES
agent, or wood preservatives (Di Blasi et al., 2010). For
instance, bio-oil made from tobacco can have antimicrobes or insect activity (Hossain et al., 2013). Bio-oil
made from lignin may provide substances that replace
formaldehyde-phenol resins in particle board (Venderbosch and Prins, 2010).
Algae have attracted intensive research and development efforts for bioenergy production, because algal
processes might directly be driven by photosynthesis
(thus fixing CO2) or yield hydrocarbons (for drop-in
refining or use as fuel). The main by-product from algal
bioenergy production is the post-hydrocarbon-harvest
algal mass, which might be used as feed or fertilizer.
Some algae species can produce phenolics, terpenoids,
carotenoids, alkaloids or sterols at significant levels
(Huang and Ramaswamy, 2012; Brennan et al., 2012).
Phytochemical productions from algae (as mentioned
in Section (Production from Algae via Aquaculture))
might be combined with hydrocarbon production, to
further valorize algal bioenergy processes.
Colocation of Fermentative Phytochemicals
Production with Bioenergy Processes
Microbial fermentation production of valuable
phytochemicals may be colocated (onsite, integral use
of materials or energy streams) with starch- or
lignocellulose-based bioenergy processes, to benefit
from locally produced, inexpensive fermentable sugars
(intermediates or by-products of bioenergy processes)
(Thomsen et al., 2006). In principle, all microbial fermentations for biochemicals production may be run on
sugars converted from starch, sugarcane or lignocellulose in various bioenergy processes, to yield chemicals
like surfactants, polyols or organic acids (Choi et al.,
2007; Aalford and Morel, 2006; Mapari et al., 2005). For
instance, X. dendrorhous can be grown on cellulasesdigested pine and produce carotenoids (Chattopadhyay
et al., 2008), and Serratia marcescens can be grown
on processed cassava waste to produce prodigiosin
(Casullo de Araújo et al., 2010).
Utilization of Phytochemical Production
By-Products for Bioenergy
Production of valuable phytochemicals (such as those
for therapeutic, cosmetic, dietary or agricultural uses)
from either wild-type or transgenic plants generates
lignocellulosic by-products. Such materials might serve
as feedstocks for (onsite) bioenergy production. This
might add value, reduce waste, and enhance raw material or energy use efficiency. For instance, the woody
residues from vinblastine or vincristine production in
C. roseus (Braz-Filho, 1999) or other natural products
363
production (Simard et al., 2012), or the citrus peel residues from furanocoumarins, flavonoid glycosides, polymethoxylated flavones, triterpenoids, limonoids or peel
oil extraction (Manthey, 2012), have potential as bioenergy feedstocks.
Phytochemical production has focused mainly on
therapeutic, dietary or cosmetic agents from specific
fruits, flowers, nuts, vegetables, or other plant sources.
In comparison, less attention has been paid on phytochemicals coproduction in current or future bioenergy
processes. Integral coproductions of biofuels, biochemicals, phytochemicals and other valuable materials are
imperative for highly efficient and viable bioenergy
and biorefinery processes (Huang and Ramaswamy,
2012). In some cases, relatively simple combinations
or colocations of existing bioenergy and phytochemicals processes may suffice to coproduce biofuels,
biochemicals and phytochemicals. In other cases, new
production technology or process engineering may
need to be developed. To maximize the economy of
raw materials and energy utilization and minimize
the carbon footprint, bioenergy processes will evolve
into more comprehensive biorefineries in which the
coproduction of industrial phytochemicals plays an
important role.
References
Aalford, S.N., Morel du Boil, P.G., 2006. A survey of value addition in
the sugar industry. Proc. S. Afr. Sug. 80, 39e61.
Abad, S., Turon, X., 2012. Valorization of biodiesel derived glycerol as
a carbon source to obtain added-value metabolites: focus on
polyunsaturated fatty acids. Biotechnol. Adv. 30, 733e741.
Abou-Zaid, M., Scott, I.M., 2012. Pyrolysis bio-oils from temperate
forests: fuels, phytochemicals and bioproducts. In: Bergeron, C.,
Carrier, D.J., Ramaswamy, S. (Eds.), Biorefinery Co-products.
Phytochemicals, Primary Metabolites and Value-Added Biomass
Processing. John Wiley, Chichester, UK, pp. 311e325.
Adrio, J.L., Demain, A.L., 2003. Fungal biotechnology. Ind. Microbiol.
6, 191e199.
Becker, W., 2004. Microalgae in human and animal nutrition. In:
Richmond, A. (Ed.), Handbook of Microalgae Culture: Biotechnology and Applied Phycology. Wiley-Blackwell, Oxford,
pp. 312e351.
Braz-Filho, R., 1999. Brazilian phytochemical diversity: bioorganic
compounds produced by secondary metabolism as a source of new
scientific development, varied industrial applications and to
enhance human health and the quality of life. Pure Appl. Chem.
71, 1663e1672.
Brennan, L., Mostaert, A., Murphy, C., Owende, P., 2012. Phytochemicals from algae. In: Bergeron, C., Carrier, D.J.,
Ramaswamy, S. (Eds.), Biorefinery Co-products. Phytochemicals,
Primary Metabolites and Value-added Biomass Processing. John
Wiley, Chichester, UK, pp. 199e240.
Briens, C., Piskorz, J., Berruti, F., 2008. Biomass valorization for fuel and
chemicals production - a review. Int. J. Chem. React. Eng. 6, 1e49.
Casullo de Araújo, H.W., Fukishima, K., Campos Takaki, G.M., 2010.
Prodigiosin production by Serratia marcescens USC 1549 using
renewable-resources as a low cost substrate. Molecules 15,
6931e6940.
364
20. PRODUCTION OF PHYTOCHEMICALS, DYES AND PIGMENTS AS COPRODUCTS IN BIOENERGY PROCESSES
Cerella, C., Kelkel, M., Viry, E., Dicato, M., Jacob, C., Diederich, M.,
2011. Naturally occurring organic sulfur compounds: an example
of a multitasking class of phytochemicals. In: Rasooli, I. (Ed.),
Phytochemicals - Bioactivities and Impact on Health. InTech,
Rijeka, Croatia, pp. 3e42.
Ceylan, H., Kim, S., Gopalakrishnan, K., 2012. Sustainable utilization
of
bio-fuel co-product in
roadbed stabilization.
In:
Gopalakrishnan, K., van Leeuwen, J.H., Brown, R.C. (Eds.),
Sustainable Bioenergy and Bioproducts: Value Added Engineering
Applications (Green Energy and Technology). Springer-Verlag,
London, pp. 117e130.
Chattopadhyay, P., Chatterjee, S., Sen, S.K., 2008. Biotechnological
potential of natural food grade biocolorants. Afr. J. Biotechnol. 7,
2972e2985.
Cheesbrough, V., Rosentrater, K.A., Visser, J., 2008. Properties of distillers grains composites: a preliminary investigation. J. Polym.
Environ. 16, 40e50.
Choi, S.W., Lee, S.K., Kim, E.O., Oh, J.H., Yoon, K.S., Parris, N.,
Hicks, K.B., Moreau, R.A., 2007. Antioxidant and antimelanogenic
activities of polyamine conjugates from corn bran and related
hydroxycinnamic acids. J. Agric. Food Chem. 55, 3920e3925.
Clark, J.H., Luque, R., Matharu, A.S., 2012. Green chemistry, biofuels,
and biorefinery. Annu. Rev. Chem. Biomol. Eng. 3, 183e207.
da Fonseca, R.A.S., da Silva Rafael, R., Kalil, S.J., Burkert, C.A.V.,
Burkert, J.F.M., 2011. Different cells disruption methods for astaxanthin recovery by Phaffia rodozyma. Afr. J. Biotechnol. 10,
1165e1171.
da Silva, G.P., Mack, M., Contiero, J., 2009. A promising and abundant
carbon source for industrial microbiology. Biotechnol. Adv. 27,
30e39.
Dapsens, P.Y., Mondelli, C., Pérez-Ramı́rez, J., 2012. Biobased chemicals from conception toward industrial reality: lessons learned
and to be learned. ACS Catal. 2, 1487e1499.
Dayan, F.E., Cantrell, C.L., Duke, S.O., 2009. Natural products in crop
protection. Bioorg. Med. Chem. 17, 4022e4034.
Demain, A.L., Adrio, J.L., 2008. Strain improvement for production of
pharmaceuticals and other microbial metabolites by fermentation.
Prog. Drug Res. 65, 251e289.
Demirbas, A., 2009. Biorefineries: current activities and future
developments. Energ. Convers. Manage. 50, 2782e2801.
Di Blasi, C., Branca, C., Galgano, A., 2010. Biomass screening for the
production of furfural via thermal decomposition. Ind. Eng. Chem.
Res. 49, 2658e2671.
Diebel, W., Reddy, M.M., Misra, M., Mohanty, A.K., 2012. Material
property characterization of co-products from biofuel industries:
potential uses in value-added biocomposites. Biomass Bioenergy
37, 88e96.
Dufossé, L., 2006. Microbial production of food grade pigments. Food
Technol. Biotechnol. 44, 313e321.
Dunford, N.T., 2012. Co-products from cereal and oilseed biorefinery
systems. In: Bergeron, C., Carrier, D.J., Ramaswamy, S. (Eds.),
Biorefinery Co-Products. Phytochemicals, Primary Metabolites and
Value-Added Biomass Processing. John Wiley, Chichester, UK,
pp. 93e115.
Febrianto, N.A., Yang, T.A., 2011. Producing high quality edible oil by
using eco-friendly technology: a review. Adv. J. Food Sci. Technol.
3, 317e326.
Frihart, C., 2010. Biobased adhesives and non-conventional bonding.
In: Rowell, R.M., Caldeira, F., Rowell, J.K. (Eds.), Sustainable
Development in the Forest Products Industry. Universidade
Fernando Pessoa, Porto, Portugal, pp. 98e113.
Guclu-Ustundag, O., Mazza, G., 2007. Saponins: properties, applications and processing. Crit. Rev. Food Sci. Nutr. 47, 231e258.
Gupta, C., Garg, A.P., Prakash, D., Goyal, S., Gupta, S., 2011. Microbes
as potential source of biocolours. Pharmacol Online. 2, 1309e1318.
Hossain, M.M., Scott, I.M., McGarvey, B.D., Conn, K., Ferrante, L.,
Berruti, F., Briens, C., 2013. Toxicity of lignin, cellulose and
hemicellulose-pyrolyzed bio-oil combinations: estimating pesticide
resources. J. Anal. Appl. Pyrol. 99, 211e216.
Huang, H.J., Ramaswamy, S., 2012. Separation and purification of
phytochemicals as co-products in biorefineries. In: Bergeron, C.,
Carrier, D.J., Ramaswamy, S. (Eds.), Biorefinery Co-Products.
Phytochemicals, Primary Metabolites and Value-Added Biomass
Processing. John Wiley, Chichester, UK, pp. 37e53.
Huter, O.F., 2011. Use of natural products in the crop protection
industry. Phytochem. Rev. 10, 185e194.
Kannan, A., Rayaprolu, S., Hettiarachchy, N., 2012. Bioactive soy
co-products. In: Bergeron, C., Carrier, D.J., Ramaswamy, S. (Eds.),
Biorefinery Co-Products. Phytochemicals, Primary Metabolites and
Value-Added Biomass Processing. John Wiley, Chichester, UK,
pp. 117e131.
Khanna, S., Goyal, A., Moholkar, V.S., 2012. Microbial conversion of
glycerol: present status and future prospects. Crit. Rev. Biotechnol.
32, 235e262.
Klinke, H.B., Ahring, B.K., Schmidt, A.S., Thomsen, A.B., 2002.
Characterization of degradation products from alkaline wet
oxidation of wheat straw. Bioresour. Technol. 82, 15e26.
Kosmider, A., Leja, K., Czaczyk, K., 2011. Improved utilization of
crude glycerol by-product from biodiesel production. In:
Montero, G. (Ed.), Biodiesel - Quality, Emissions and By-products.
InTech, Rejika, Croatia, pp. 341e364.
Koutinas, A.A., Wang, R., Campbell, G.M., Webb, C., 2006. A whole
crop biorefinery system: a closed system for the manufacture of nonfood products from cereals. In: Kamm, B., Gruber, P.R., Kamm, M.
(Eds.), Biorefineries - Industrial Processes and Products: Status Quo
and Future Directions, vol. 1. Wiley-VCH, Weinheim, pp. 165e191.
Koutinas, A.A., Wang, R.H., Webb, C., 2007. The biochemurgist bioconversion of agricultural raw materials for chemical production. Biofuels., Bioprod. Biorefin. 1, 24e38.
Kraus, G.A., 2006. Phytochemicals, dyes, and pigments in the
biorefinery context. In: by Kamm, B., Gruber, P.R., Kamm, M.
(Eds.), Biorefineries - Industrial Processes and Products: Status
Quo and Future Directions, vol. 2. Wiley-VCH, Weinheim,
pp. 315e323.
Kripp, T.C., 2006. Biobased consumer products for cosmetics. In:
Kamm, B., Gruber, P.R., Kamm, M. (Eds.), Biorefineries - Industrial
Processes and Products: Status Quo and Future Directions, vol. 2.
Wiley-VCH, Weinheim, pp. 409e442.
Langner, U., Jakob, T., Stehfest, K., Wilhelm, C., 2009. An energy balance
from absorbed photons to new biomass for Chlamydomonas reinhardtii and Chlamydomonas acidophila under neutral and extremely
acidic growth conditions. Plant Cell Environ. 32, 250e258.
Liu, R.H., 2007. Whole grain phytochemicals and health. J. Cereal Sci.
46, 207e219.
Manthey, J.A., 2012. Potential value-added co-products from citrus
fruit processing. In: Bergeron, C., Carrier, D.J., Ramaswamy, S.
(Eds.), Biorefineries, Biorefinery Co-products. Phytochemicals,
Primary Metabolites and Value-added Biomass Processing. John
Wiley, Chichester, UK, pp. 153e178.
Mantzouridou, F., Naziri, E., Tsimidou, M.Z., 2008. Industrial glycerol
as a supplementary carbon source in the production of betacarotene by Blakeslea trispora. J. Agric. Food Chem. 56, 2668e2675.
Mapari, S.A.S., Nielsen, K.F., Larsen, T.O., Frisvad, J.C., Meyer, A.S.,
Thrane, U., 2005. Exploring fungal biodiversity for the production
of water-soluble pigments as potential natural food colorants.
Curr. Opin. Biotechnol. 16, 231e238.
Matthews, P.D., Wurtzel, E.T., 2007. Biotechnology of food colorant
production. In: Socaciu, C. (Ed.), Food Colorants: Chemical and
Functional Properties. CRC Press, Boca Raton, FL, USA,
pp. 347e398.
REFERENCES
Moreau, R.A., Lampi, A.M., Hicks, K.B., 2009. Fatty acid, phytosterol,
and polyamine conjugate profiles of edible oils extracted from corn
germ, corn fiber, and corn kernels. J. Am. Oil Chem. Soc. 86,
1209e1214.
Mortensen, A., 2006. Carotenoids and other pigments as natural colorants. Pure Appl. Chem. 78, 1477e1491.
Muth, M.K., Depro, B.M., Domanico, J.L., 1998. Vegetable Oil Production: Industry Profile. US Environmental Protection Agency
document A.98.41. http://www.epa.gov/ttnecas1/regdata/IPs/
Vegetable%20Oil_IP.pdf.
O’Brien, K.P., Franjevic, S., Jones, J., 2009. Green Chemistry and Sustainable Agriculture: The Role of Biopesticides. http://
advancinggreenchemistry.org/wp-content/uploads/Green-Chemand-Sus.-Ag.-the-Role-of-Biopesticides.pdf.
Pandey, M., Debnath, M., Gupta, S., Chikara, S.K., 2011. Phytomedicine: an ancient approach turning into future potential source of
therapeutics. J. Pharmacogn. Phytother. 3, 27e37.
Panpipat, W., Xu, X., Guo, Z., 2012. Towards a commercially
potential process: enzymatic recovery of phytosterols from
plant oil deodoriser distillates mixture. Process Biochem. 47,
1256e1262.
Persson, P., Andersson, J., Gorton, L., Larsson, S., Nilvebrant, N.O.,
Jönsson, L.J., 2002. Effect of different forms of alkali treatment on
specific fermentation inhibitors and on the fermentability of
lignocellulose hydrolysates for production of fuel ethanol. J. Agric.
Food Chem. 50, 5318e5325.
Piironen, V., Lindsay, D.G., Miettinen, T.A., Toivo, J., Lampi, A.M.,
2000. Plant sterols: biosynthesis, biological function and their
importance to human nutrition. J. Sci. Food Agric. 80, 939e966.
Rao, V., 2012. In: Phytochemicals as Nutraceuticals e Global
Approaches to Their Role in Nutrition and Health. (ed.). InTech,
Rijeka, Croatia.
Rausch, K., 2012. Phytochemicals from corn: a processing perspective.
In: Bergeron, C., Carrier, D.J., Ramaswamy, S. (Eds.), Biorefineries,
Biorefinery Co-products. Phytochemicals, Primary Metabolites and
Value-added Biomass Processing. John Wiley, Chichester, UK,
pp. 55e92.
Rausch, K.D., Belyea, R.L., 2006. The future of co-products from corn
processing. Appl. Biochem. Biotechnol., 47e86.
Rymbai, H., Sharma, R.R., Srivastav, M., 2011. Biocolorants and its
implications in health and food industry - a review. Int. J. Pharm.
Tech. Res. 3, 2228e2244.
Salim, A.A., Chin, Y.W., Kinghorn, A.D., 2008. Drug discovery from
plants. In: Ramawat, K.G., Mérillon, J.M. (Eds.), Bioactive Molecules and Medicinal Plants. Springer, Berlin, pp. 1e24.
Sengupta, S., 2003. Natural, “green” Dyes for the Textile Industry. The
Massachusetts Toxics Use Reduction Institute University of Massachusetts Lowell Technical Report No. 57. http://www.google.
com/url?sa¼t&rct¼j&q¼natural%20green%20dyes%20for%20the
%20textile%20industry&source¼web&cd¼1&ved¼0CC8QFjAA
&url¼http%3A%2F%2Fwww.turi.org%2Fcontent%2Fdownload%
2F3242%2F29575%2Ffile%2FReport%252057%2520-%2520
sengupta.pdf&;ei¼tuovUdOTGZCZhQfu74CYCg&usg¼AFQj
CNGU8AeXikvG83-fwL6jvTrs8t2qXw" (accessed 28.02.13.).
Shimizu, S., 2008. Vitamins and Related Compounds: Microbial Production. In Biotechnology Set Second edition volume 10eSpecial
Processes IVeSpecial Substances. Wiley-VCH Verlag, Weinheim.
pp. 318e340.
Simard, F., Pichette, A., Legault, J., 2012. New bioactive natural products
from Canadian boreal forest. In: Bergeron, C., Carrier, D.J.,
365
Ramaswamy (Eds.), Biorefineries, Biorefinery Co-products. Phytochemicals, Primary Metabolites and Value-added Biomass Processing. John Wiley, Chichester, UK, pp. 241e258.
Skjånes, K., Rebours, C., Lindblad, P., 2012. Potential for green
microalgae to produce hydrogen, pharmaceuticals and other high
value products in a combined process. Crit. Rev. Biotechnol.
http://dx.doi.org/10.3109/07388551.2012.681625.
Spolaore, P., Joannis-Cassan, C., Duran, E., Isambert, A., 2006. Commercial applications of microalgae. J. Biosci. Bioeng. 101, 87e96.
Tatara, R.A., Suraparaju, S., Rosentrater, K.A., 2007. Compression
molding of phenolic resin and corn-based DDGS blends. J. Polym.
Environ. 15, 89e95.
Thomsen, M.H., Andersen, M., Kiel, P., 2006. Plant juice in the biorefinery - use of plant juice as fermentation medium. In: Kamm, B.,
Gruber, P.R., Kamm, M. (Eds.), Biorefineries - Industrial Processes
and Products: Status Quo and Future Directions, vol. 1. WileyVCH, Weinheim, pp. 295e374.
Tripathi, L., Tripathi, J.N., 2003. Role of biotechnology in medicinal
plants. Trop. J. Pharm. Res. 2, 243e253.
Turley, D.B., Chaudhry, Q., Watkins, R.W., Clark, J.H., Deswarte, F.E.I.,
2006. Chemical products from temperate forest tree species developing strategies for exploitation. Ind. Crop Prod. 24, 238e243.
Uppugundla, N., Engelberth, A., Ravindranath, S.V., Clausen, E.C.,
Lay, J.O., Gidden, J., Carrier, D.J., 2009. Switchgrass water extracts:
extraction, separation and biological activity of rutin and quercitrin. J. Agric. Food Chem. 57, 7763e7770.
Valduga, E., Tatsch, P.O., Tiggemann, L., Zeni, J., Colet, R.,
Cansian, J.M., Treichel, H., Luccio, M., 2009. Evaluation of the
conditions of carotenoids production in a synthetic medium by
Sporidiobolus salmonicolor (CBS 2636) in a bioreactor. Int. J. Food Sci.
Technol. 44, 2445e2451.
van den Oever, M.J.A., Boeriu, C.G., Blaauw, R., van Haveren, J., 2004.
Colorants based on renewable resources and food-grade colorants
for application in thermoplastics. J. Appl. Polym. Sci. 92,
2961e2969.
Venderbosch, R.H., Prins, W., 2010. Fast pyrolysis technology development. Biofuel. Bioprod. Biorefin. 4, 178e208.
Wang, L., Weller, C.L., 2006. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 17, 300e312.
Watkins, R., Chaudhry, Q., 2013. Useful Chemicals from the Main
Commercial Tree Species in the UK. The Food and Environment
Research Agency of UK, online database (accessed 28.02.13.).
https://secure.fera.defra.gov.uk/treechemicals/.
Wu, X., Kang, J., 2011. Phytochemicals in soy and their health effects.
In: Rasooli, I. (Ed.), Phytochemicals - Bioactivities and Impact on
Health. InTech, Rijeka, Croatia, pp. 43e76.
Yimyoo, T., Yongmanitchai, W., Limtong, S., 2011. Carotenoid production by Rhodosporidium paludigenum DMKU3-LPK4 using
glycerol as the carbon source. Kasetsart J. Nat. Sci. 45, 90e100.
Zhang, X., Beltranena, E., Christensen, C., Yu, P., 2012. Use of a dry
fractionation process to manipulate the chemical profile and
nutrient supply of a coproduct from bioethanol processing.
J. Agric. Food Chem. 60, 6846e6854.
Zhao, Z., Moghadasian, M.H., 2010. Bioavailability of hydroxycinnamates: a brief review of in vitro and in vivo studies. Phytochem. Rev. 9, 133e145.
Zijlstra, R.T., van Kessel, A.G., Drew, M.D., 2012. Ingredient Fractionation: The Value of Value-added Processing for Animal
Nutrition (accessed 28.02.13.). http://www.prairieswine.com/
pdf/1713.pdf.
C H A P T E R
21
Recent Developments on Cyanobacteria and
Green Algae for Biohydrogen Photoproduction
and Its Importance in CO2 Reduction
Y. Allahverdiyeva*, E.M. Aro, S.N. Kosourov
Department of Biochemistry, University of Turku, Turku, Finland
*Corresponding author email: allahve@utu.fi
O U T L I N E
Introduction
367
Mechanisms of Hydrogen Photoproduction
Oxygenic Photosynthesis
Nitrogenases
Alternative Nitrogenases
Hydrogenases
Uptake Hydrogenases
Bidirectional Hydrogenases
Green Algal [FeeFe]-Hydrogenases
368
368
369
369
370
370
370
371
Hydrogen Photoproduction by Cyanobacteria
Strategies to Improve H2 Production in
Cyanobacteria
Genetic Modifications
Introducing Foreign Enzymes and Semiartificial
Systems
372
373
373
374
374
375
Hydrogen Photoproduction by Green Algae
Light-Dependent Hydrogen Production Pathways
Role of H2 Photoproduction in Green Algae
Long-Term H2 Production by Green Algae
Hydrogen Photoproduction by Nutrientdeprived Green Algae
Strategies to Improve H2 Photoproduction
in Green Algae
375
375
377
377
References
382
378
381
373
INTRODUCTION
It is well known that the fossil energy resources are
limited. Despite the fact that millions of years of photosynthesis were required to ensure the fossil fuel formation and accumulation, the current consumption of
fossil fuels occurs at a rapid rate. Such utilization of fossil fuels creates extreme damage to the environment,
increasing the CO2 level in atmosphere and leading to
global warming and pollution on the Earth. Future scenarios predict an increase in CO2 partial pressure in the
atmosphere from the current levels of approximately
380, to about 750 and up to 1000 matm until the end of
this century (Raupach et al., 2007).
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00021-8
Elimination of Competing Electron Transfer
Routes
Biodiversity
Immobilization
There is an urgent need to switch to alternative, environmentally friendly and renewable energy sources. An
efficient strategy for production of bioenergy would
employ photosynthetic microorganisms, which are
collectively, a significant player in the global carbon
cycle. Cyanobacteria and green algae have inherited
mechanisms for production of hydrogen, which possesses all properties of a clean and efficient energy
carrier. Although the natural production of hydrogen
by these microorganisms is negligible at the current
state, there is a huge potential for engineering and
synthetic biology advances of cyanobacteria and green
algae toward commercially profitable production of
hydrogen and other biofuels.
367
Copyright Ó 2014 Elsevier B.V. All rights reserved.
368
21. RECENT DEVELOPMENTS ON CYANOBACTERIA AND GREEN ALGAE FOR BIOHYDROGEN PHOTOPRODUCTION
MECHANISMS OF HYDROGEN
PHOTOPRODUCTION
The electrons extracted from water on the lumenal side
of PSII are transferred via the PSII reaction center, plastoquinone (PQ), the Cyt b6f complex and plastocyanin to
PSI, which after excitation directs electrons to ferredoxin
(Fd), ferredoxin-NADPþ reductase (FNR) and, finally, to
generate reduced nicotinamide adenine dinucleotide
phosphate (NADPH). This process is known as the linear
electron transport (LET). Concomitantly with electron
transfer reactions, protons are transferred inside of
the thylakoid lumen creating a proton gradient across
the thylakoid membrane, which in turn drives adenosine
triphosphate (ATP) production via the ATP synthase
complex. Sometimes, the electrons are recycled from
NADPH or Fd to PQ in the process known as the cyclic
electron transport, whereby DpH is generated without
production of NADPH. NADPH produced by LET is
further used by carbon metabolism, and many other
metabolic pathways. The excess of reduced carbon is
stored in cells as carbohydrates or lipids. An unique
feature of photosynthetic microorganisms is that under
specific conditions, most of them are able to redirect the
flow of electrons originated from water splitting to the
enzymes that mediate H2 production (Figure 21.1).
Biophotolysis of water by microalgae has been under
investigation for over 70 years. H2 production by the
anaerobically adapted and CO2-depleted suspension of
Scenedesmus obliquus in light was reported for the first
time by Gaffron and Rubin (1942). Three decades later,
it was revealed that filamentous cyanobacteria, Anabaena
cylindrica is also able to evolve H2 and O2 simultaneously under Ar atmosphere (Benemann and Weare,
1974). Despite intensive research on the structure and
function of photosynthetic protein complexes, we are
still lacking a fundamental understanding of the molecular factors regulating the entire electron transfer chain
from water to H2 in oxygenic photosynthetic organisms.
In this chapter, we mainly focus on H2 production
by oxygenic photosynthetic microorganisms via the
Oxygenic Photosynthesis
Cyanobacteria and green algae are photosynthetic
microorganisms widespread in nature that survive even
in extreme climatic conditions. They are able to harness
solar energy and convert it into chemical energy by simultaneous splitting of water to molecular oxygen and
protons with following fixation of CO2, according to the
general equation of photosynthesis:
6CO2 þ 6H2 O / 6ðCH2 OÞ þ 6O2
(21.1)
The photosynthetic electron transfer reactions are
usually divided into two stagesdthe “light reactions”,
where light energy is converted into the chemical energy
of strong reductants and the “dark reactions”, where
CO2 is reduced into organic compounds by using
chemical energy obtained from the light reactions. The
simplified scheme of photosynthetic light reactions is
presented in Figure 21.1. Photosynthetic light reactions
involve electron flow through three major protein complexes: photosystem II (PSII), Cytochrome b6f (Cyt b6 f),
and photosystem I (PSI) embedded into the thylakoid
membrane. The light reactions start with capture of photons by the pigment molecules in the antenna complexes
and subsequent transfer of light energy to PSI and PSII
reaction centers, where primary charge separation
occurs and photosynthetic electron transport reactions
are initiated.
The two reaction centers, PSII and PSI, function simultaneously, but in series. PSII is the only known biocatalyst
that can oxidize water, which is energetically a poor electron donor. The oxidationereduction midpoint potential
of water is þ0.82 Vat pH 7. In PSII the photolysis of water
is driven by the oxidized reaction center, P680þ (the
midpoint potential of P680/P680þ is þ1.2 V at pH 7).
FIGURE 21.1 Simplified schematic
CO2
H2
2H+
H2 metabolism
NAD(P)H
2H
Carbohydrates
2O2
2H2O
O2
RubisCO
?
Carbon metabolism
Flv1, Flv3
(Mehler)
NADPH NADP+
?
NAD(P)
ADP + P
ATP
FNR
Fd
QA
QB
? NDH-2
Membrane
Cyt b
PQH
Pheo
PQ
FB
FA
PQ
FX
A1
Cyt f
PQH
FeS
P680
A0
P700
PC
2H
O2 + 4H+
2H2O
PSII
PC
H+
NDH-1
Cyt b6 f
PSI
H+
ATP synt
model of the electron transport routes in
unicellular cyanobacteria. For details, see
the text. Black arrows show the routes
of photosynthetic electron transport.
Dashed arrow shows that under certain
conditions the electrons from the photosynthetic electron transport chain can be
redirected to H2 metabolism allowing H2
photoproduction to occur. Dashed lines
marked with ‘?’ show possible electron
transfer routes via the NDH-2 complex
and photorespiratory pathway. (For color
version of this figure, the reader is
referred to the online version of this
book.)
MECHANISMS OF HYDROGEN PHOTOPRODUCTION
light-dependent direct and indirect biophotolysis
pathways. During direct biophotolysis H2 is derived
from the electrons originated from water splitting at
PSII, whereas for indirect biophotolysis electrons are
mainly supplied by degradation of intracellular carbon
compounds produced in photosynthetic carbon reduction reactions.
Nitrogenases
Many cyanobacteria are able to fix atmospheric N2
into ammonia (NH3) and produce H2 as a by-product.
The reaction of nitrogen fixation is catalyzed by nitrogenase, a complex metalloenzyme and results in the
formation of 1 mol of H2 per 1 mol of fixed N2 (Phelps
and Wilson, 1942):
þ
N2 þ 8H þ 8e þ 16ATP / 2NH3
þ 16ðADP þ Pi Þ þ H2 ;
(21.2)
where Pi is inorganic phosphate.
Nitrogenases have relatively low turnover numbers.
N2 fixation is an energy-expensive process that requires
two ATP molecules per electron transfer. It has, however, an advantage of catalyzing an irreversible reaction
and not being inhibited by H2 accumulation.
The best studied type of nitrogenase is conventional
molybdenum (Mo)-nitrogenase, which is encoded by
the structural genes nifHDK1. Very little is known about
the regulation of nif genes. Nitrogenase consists of two
components: dinitrogenase, or the MoFe protein
composed of NifD and NifK subunits, and dinitrogenase
reductase, or the Fe protein, consisting of two subunits
of NifH. The substrate-binding and reducing active
site is located in the MoFe protein. The Fe protein containing a [4Fee4S] cluster and a Mg-ATP binding site
acts as electron donor to a MoFe protein. This cluster
accepts electrons from Fd or flavodoxin. The fixation
of N2 is always accompanied by H2 evolution (Hadfield
and Bulen, 1969). The reason for production of H2 as a
by-product is not yet clear. It could be a result of
unavoidable leakage of reducing potential, or formation
of H2 could be a prerequisite for binding of N2 to the
active site (Burgess and Lowe, 1996). Besides reducing
N2, nitrogenase can reduce a number of other substrates
with triple bonds. Importantly, in the absence of N2
as a substrate, nitrogenase exclusively catalyzes
ATP-dependent reduction of Hþ to H2 (Benemann and
Weare, 1974; Pickett, 1996).
8Hþ þ 8e þ 16ATP / 16ðADP þ Pi Þ þ 4H2
(21.3)
Indeed, in terms of H2 production by N2 fixation in heterocystous cyanobacteria the N2 is a much more potent
inhibitor than O2 (Yeager et al., 2011). This is logical,
369
due to the fact that heterocysts can protect
enzymes from external O2. Since the replacement of N2
with argon (Ar) gas is an expensive approach for optimization of H2 production, an alternative method to genetically modify the catalytic site of the nitrogenase enzyme
has been chosen as more appropriate. Recently, sitedirected mutations have been introduced to several
amino acid residues coordinating the MoeFe active site
of the nif1-enzyme in attempts to direct the electron flow
selectively to H2 production in atmospheric N2 condition
(Masukawa et al., 2010). Importantly, several mutant
strains demonstrated nearly similar rate of H2 production under N2 and Ar atmosphere. Moreover, these
strains accumulated significantly high levels of H2 under
atmospheric N2 as compared to the reference strains.
Alternative Nitrogenases
In addition to the conventional Mo-nitrogenase, nif1,
N2-fixing microorganisms possess also alternative nitrogenases: second type of Mo-nitrogenase, nif 2, vanadium
(V)-nitrogenase, vnf, and Fe-nitrogenase, anf (Bothe
et al., 2010). Presence of the Fe-nitrogenase in cyanobacteria has not yet been documented.
Mo-nitrogenase 2, encoded by nifHDK2, is expressed
in both vegetative cells and heterocysts of Anabaena
variabilis under N2-fixing and anaerobic conditions
(Schrautemeier et al., 1995; Thiel et al., 1995). Unicellular
Chroococcidiopsis, inhabiting in a gypsum rock, where the
shards provide a microaerobic, low light environment,
also possesses the alternative nif 2 system. Based on the
phylogenetic analysis of nif H sequences, it has been
suggested that nif 2 is characteristic of unicellular or filamentous nonheterocystous cyanobacteria fixing N2 only
under microaerobic conditions (Boison et al., 2004).
Weyman and coworkers reported the amino acid substitution in nifD2 as a first step toward the development of
nitrogenase mutants in A. variabilis, which produces
large amounts of H2 in N2 containing atmosphere
(Weyman et al., 2010).
V-nitrogenase has a V-Fe cofactor in the active site. It
is encoded by vnfHDGK genes and is expressed in heterocysts only under Mo-deficient conditions, in the presence of V (Kentemich et al., 1988; Thiel, 1993; Thiel et al.,
1995). Biochemical and spectroscopic investigations of
purified proteins isolated from Azotobacter vinelandii
have revealed a mechanistic difference between the
MoeFe and VeFe catalytic site and in H2 evolution
mechanisms (Lee et al., 2009).
N2 þ 12Hþ þ 12e þ 24ATP / 2NH3
þ 24ADP þ 24Pi þ 3H2
(21.4)
As can be seen from the Eqns (21.2) and (21.4), the distribution of electrons and protons are different for Mo- and
V-nitrogenases. V-nitrogenase can produce three
370
21. RECENT DEVELOPMENTS ON CYANOBACTERIA AND GREEN ALGAE FOR BIOHYDROGEN PHOTOPRODUCTION
times more H2 per mole of N2 reduced compared to
Mo-nitrogenase (Eady, 1996). For this reason, the production of H2 by vnf system is likely to be more efficient
and it is, therefore, worth searching for organisms possessing alternative nitrogenases. For a long time the
presence of alternative nitrogenases was confirmed only
for A. variabilis and Anabaena azollae (Ni et al., 1990; Thiel,
1993). Recent screening of 14 different cyanobacterial
strains has revealed 8 strains with nif2, and 4 strains
with vnf nitrogenases (Masukawa et al., 2009), suggesting that alternative hydrogenases are not unique.
Hydrogenases
There are two classes of hydrogenases that commonly
present in phototrophic organisms: the [FeeFe]hydrogenase and the [NieFe]-hydrogenase. The [FeeFe]hydrogenase is found in green algae and some bacteria,
and it is the most active H2-forming enzyme. It demonstrates about 100 times higher activity than the [NieFe]hydrogenase. However, it is irreversibly inactivated
when exposed to O2 (see Section Green Algal [FeeFe]-Hydrogenases in this chapter). Cyanobacteria possess two
types of [NieFe]-hydrogenases (Houchins, 1984), which
are more tolerant to O2 and only temporarily inactivated
upon exposure to O2.
Uptake Hydrogenases
In cyanobacteria, the uptake hydrogenases (encoded
by hupSL genes) catalyze the consumption of H2 produced by the nitrogenase. Thus, the net H2 evolution
by N2-fixing cyanobacteria is barely observed under natural conditions. Uptake hydrogenase has been found in
all N2-fixing cyanobacteria studied so far. Nevertheless,
a few N2-fixing Synechococcus strains lacking an uptake
hydrogenase have been reported (Ludwig et al., 2006;
Steunou et al., 2008). It is believed that the uptake
hydrogenase transfers electrons from H2 back to the
photosynthetic and respiratory electron transport
chains, and thus partially regains the energy used for
N2 fixation. The cellular/subcellular localization of the
uptake hydrogenase is controversial and seems to be
species specific. The data obtained from the N2-fixing
filamentous nonheterocystous cyanobaterium, Lyngbya
majuscule, revealed higher specific labeling associated
with the thylakoid membranes, suggesting that the cyanobacterial uptake hydrogenase is a membrane-bound
protein (Seabra et al., 2009). However, it lacks a
membrane-spanning region. Therefore, the presence of
the third subunit, which would anchor the uptake hydrogenase to the membrane and link electron transfer
from the enzyme to the respiratory or photosynthetic
chains, has been suggested (Tamagnini et al., 2007).
In some heterocystous cyanobacteria, such as
Anabaena PCC 7120, the uptake hydrogenase enzyme
was detected only in heterocysts, while in other cyanobacteria, such as Nostoc punctiforme, it is localized in
both vegetative and heterocyst cells, corresponding
most probably to inactive and active pools of the enzyme
(Camsund et al., 2011; Seabra et al., 2009).
Since the uptake hydrogenase is an obstacle for H2
production, mutations disrupting the structural hupSL
genes have been constructed to improve the H2 production in N2-fixing cyanobacteria (Happe et al., 2000;
Lindberg et al., 2002; Masukawa et al., 2002; Schutz
et al., 2004; Yoshino et al., 2007; Khetkorn et al., 2012).
These mutants produced about four- to sevenfold
more H2 than the control strain. In addition, inactivation
of uptake hydrogenase had no major effect on cell
growth and heterocyst differentiation. Quantitative
shotgun proteomics and physiological approaches on
the uptake hydrogenase mutant of N. punctiforme
demonstrated that the mutant strain undergoes metabolic and structural alterations to compensate for the
amount of electrons lost as a release of H2 (Ekman
et al., 2011). Construction of mutant strains combining
several improvements is likely to be a better approach
toward sustainable H2 production. To this end, the
single- and the double-mutant strains lacking the
homocitrate synthase genes, nifV1 and nifV2, were constructed using the DhupL strain of Anabaena PCC 7120 as
the parental strain (Masukawa et al., 2007). The catalytic
MoeFe center binds homocitrate, which is necessary for
N2 fixation, but in the absence of homocitrate gene
MoeFe center binds citrate: in a Klebsiella mutant this
was shown to demonstrate low N2 fixation but high
H2 production activity in a N2 atmosphere (Mayer
et al., 2002). In line with this result, the DhupLDnifV1
cells also demonstrated high H2 production rate and
heterocyst frequency compared to the parental DhupL
in N2 atmosphere (Masukawa et al., 2007).
Bidirectional Hydrogenases
The bidirectional hydrogenases can either produce or
consume H2 according to the cellular redox environment. The enzyme functions in dark fermentation and
under specific conditions in photoproduction of H2.
In cyanobacteria the bidirectional hydrogenase consists of two structural moieties: the hydrogenase
(encoded by hoxYH) and the diaphorase unit (encoded
by hoxUFE) capable of oxidation of NAD(P)H. Over the
last several years, significant progress has been achieved
in the identification of the transcription factors, such
as LexA and AbrB-like proteins, which are members of
the complex signal cascade that directs the expression
of the bidirectional hydrogenase genes (Oliveira and
Lindblad, 2009).
On the basis of high sequence similarity, it has been hypothesized that the diaphorase subunit of the bidirectional hydrogenase also serves as the three missing
MECHANISMS OF HYDROGEN PHOTOPRODUCTION
activity subunits of cyanobacterial respiratory NDH-1
complex (Appel and Schulz, 1996). However, more recent
results have not supported this hypothesis since the mutants lacking the diaphorase subunits do not show malfunction of the respiratory activity (Boison et al., 1999).
Bidirectional hydrogenase has been found in all
non-N2-fixing and some N2-fixing cyanobacteria. Thus,
many filamentous N2-fixing cyanobacteria contain both
the bidirectional and the uptake hydrogenase. However,
a few species have only the uptake hydrogenase. In
cyanobacteria, the bidirectional hydrogenase is constitutively expressed under both aerobic and anaerobic conditions but is active only in the dark, anoxic conditions or
during the transition from dark to light (Cournac et al.,
2004; Schutz et al., 2004). The biological function of bidirectional hydrogenase in filamentous cyanobacteria is not
well understood (Tamagnini et al., 2007). Mutational
studies with hox-defective mutants suggested that the
bidirectional hydrogenase in N2-fixing cyanobacteria
does not support N2 fixation (Masukawa et al., 2002). In
non-N2-fixing cyanobacteria the bidirectional hydrogenase is the main H2-producing enzyme and it is thought
to interact with photosynthetic pathways (Ludwig et al.,
2006). However, H2 production catalyzed by bidirectional
hydrogenases is only transient (less than 30 s in light)
since it is quickly inhibited by increasing photosynthetic
O2 evolution (Cournac et al., 2004). In line with this, no
transient H2 evolution was detected in different Hox
deletion mutants studied by Aubert-Jousset et al. (2011).
It is hypothesized that the bidirectional hydrogenase
functions as a safety electron sink thereby removing
excess reducing equivalents during the dark, anaerobic
to light transition in unicellular Synechocystis cells (Appel
et al., 2000; McIntosh et al., 2011). This hypothesis is interesting due to the natural environment of cyanobacteria
being highly dynamic, with rapid fluctuations in light intensity. Such fluctuations might strongly unbalance the
function of the photosynthetic complexes, resulting in
production of reactive oxygen species and destroying
photosynthetic apparatus. Cyanobacteria have unique
flavodiiron proteins, Flv1 and Flv3, functioning as a
strong electron sink at the end of light reactions by directing excess electrons to O2 without production of reactive
oxygen species, thus maintaining the redox balance of the
electron transport chain (Helman et al., 2003; Allahverdiyeva et al., 2011, 2012). A bidirectional hydrogenase
possibly takes over the role of a strong electron sink
upon dark to light transitions during anaerobiosis, the
condition created in cyanobacterial mats and blooms,
and where the Flv1 and Flv3 pathway is not functional
(Gutthann et al., 2007).
In Synechocystis, both NADPH and NADH can act as
electron donors for the bidirectional hydrogenase.
Recent studies showed that NADH is a preferential substrate of the diaphorase moiety, whereas NADPH is an
371
efficient activator of the bidirectional hydrogenase
(Aubert-Jousset et al., 2011). These results are in line
with the observed dynamics of H2 production during
darkelight transition. In the dark anaerobic conditions,
H2 is produced by oxidation of NADH, the major product of glycolysis assimilation. Sudden exposure to light
produces NADPH by photosynthetic electron transfer
chain, which functions as an activator of the hydrogenase and begins consumption of H2. Although there is
no strong evidence for direct electron donation from
reduced Fd (E0 ¼ 0.42 V), which is a stronger reductant
than NADH (E0 ¼ 0.315 V), to the bidirectional
hydrogenase, such an electron transfer cannot be
completely excluded (McNeely et al., 2011). Direct
linkage of Fd to the bidirectional enzyme in mutants
lacking the diaphorase domain could be employed to
improve cyanobacterial H2 production.
Accounting for the high affinity of the bidirectional
hydrogenase to H2, it has been suggested that the
enzyme can function in utilization of H2 under physiological conditions. However, it should be kept in mind
that the bidirectional hydrogenase reversibly evolves
H2 under dark, anaerobic conditions as a result of
fermentation of photosynthetically stored carbon intermediates in cyanobacteria. Recent electrochemical
investigations of the bidirectional enzyme from Synechocystis PCC 6803 have revealed unexpected properties.
The rate of H2 production at low pH and low H2 pressure was shown to be about 1.4 times faster than the
rate of H2 consumption at high pH and high H2 pressure
(McIntosh et al., 2011).
Green Algal [FeeFe]-Hydrogenases
Hydrogen photoproduction in green algae is catalyzed
by [FeeFe]-hydrogenases. Earlier reports have suggested
the existence of [NieFe]-hydrogenase in S. obliquus (Zinn
et al., 1994), but presently S. obliquus is considered as
having only the [FeeFe]-hydrogenase (Wunschiers et al.,
2001; Florin et al., 2001). Some green algal species do not
show hydrogenase activity at all (Brand et al., 1989;
Boichenko and Hoffmann, 1994). Currently, the presence
of genes encoding [FeeFe]-hydrogenases has been
proved in the following species: Shlamydomonas reinhardtii (Happe and Kaminski, 2002; Forestier et al., 2003),
Chlorella fusca (Winkler et al., 2002), Shlamydomonas noctigama (Skjanes et al., 2010), Volvox carteri (Prochnik et al.,
2010), Tetraselmis subcordiformis (Yan et al., 2011) and
Chlorella variabilis (Meuser et al., 2011). Algal [FeeFe]hydrogenases in vivo interact with Fd, a terminal acceptor
of photosynthetic electron transport chain (Chang et al.,
2007). In contrast to [NieFe]-hydrogenase enzymes,
[FeeFe]-hydrogenases have significantly higher turnover
rate (6000e9000/s) and usually catalyze H2 production
instead of H2 uptake (Frey, 2002). However, the possible
role of these enzymes in H2 uptake under high H2 partial
372
21. RECENT DEVELOPMENTS ON CYANOBACTERIA AND GREEN ALGAE FOR BIOHYDROGEN PHOTOPRODUCTION
pressure has also been suggested (Kosourov et al., 2012).
Unfortunately, the [FeeFe]-hydrogenases are extremely
sensitive to O2 that irreversibly inactivates purified enzymes within seconds (Ghirardi et al., 1997).
The most studied green alga, C. reinhardtii has two
monomeric [FeeFe]-hydrogenases: HydA1 and HydA2
with a molecular mass of around 48 kD (Happe and
Kaminski, 2002; Forestier et al., 2003). Both proteins
are nuclear encoded and contain putative transit sequences that target them to the chloroplast. The hydA1
gene shows 74% similarity to hydA2 and encodes protein
that is 68% identical to HydA2. Two homologous
hydrogenases are typically observed in almost all green
algae showing hydrogenase activity (Winkler et al.,
2004). Nevertheless, some species have three [FeeFe]hydrogenase enzymes (Skjanes et al., 2010). The physiological basis for the presence of two and more
hydrogenases in green algae has not been determined.
In C. reinhardtii cells, HydA1 most probably participates
in the light-dependent H2 production pathway (Happe
and Naber, 1993). Examination of relative enzyme activities by gene-silencing techniques indicate that HydA1
catalyzes the majority of the hydrogenase activity, but
the role of HydA2 in algal H2 production has not been
clearly resolved (Godman et al., 2010). Recently, Meuser
and et al. (2012) using the single hydA1, hydA2 and
double hydA1/hydA2 knockout mutants showed
that HydA2 also participates in H2 photoproduction.
However, according to the authors, its contribution in
the light-dependent process does not exceed 25%. The
next important step in this direction should be investigation of the role of these enzymes in the H2 uptake,
including the mechanisms of photoreduction (Kosourov
et al., 2012).
HYDROGEN PHOTOPRODUCTION
BY CYANOBACTERIA
Cyanobacteria have different life forms: some species
are unicellular, others form colonies and filaments, or
live in symbiosis with eukaryotic organisms. Accordingly,
the protection of O2 sensitive enzymes from photosynhetically evolved oxygen has evolved through several
different strategies.
In the absence of combined nitrogen, many filamentous N2-fixing cyanobacteria physically separate
oxygenic photosynthesis and N2-fixing enzymes by
differentiating specialized heterocyst cells, which are
regularly spaced among vegetative cells. Mature heterocysts are unique cells providing a microaerobic environment suitable for the enzymes involved in N2 fixation.
The microaerobic environment inside of heterocysts is
maintained by an elevated rate of respiration, lack of
active PSII complexes resulting in an absence of photosynthetic O2 evolution, and a thick cell wall (Wolk
et al., 1994). These two cell types, the vegetative cells
and heterocysts, depend on each other. During diazotrophic growth the vegetative cells perform photosynthetic
CO2 fixation and provide the heterocysts with organic
carbon intermediates, like sucrose (Lopez-Igual et al.,
2010), whereas heterocysts provide vegetative cells
with fixed nitrogen required for cell growth (Figure 21.2).
Since H2 production in heterocysts depends on carbohydrates produced in vegetative cells, this process of
H2 production has been classified as indirect water
biophotolysis.
Heterocyst differentiation is tightly regulated by
NtcA, a global transcription factor of carbon and nitrogen metabolism (Zhao et al., 2010). HetR is another
essential protein specifically involved in the initial steps
of heterocyst development. The patterned differentiation of heterocysts is controlled by the ratio of activator,
HetR, and suppressor molecules, peptides derived from
PetS and HetN (Muro-Pastor and Hess, 2012; Risser and
Callahan, 2009). For the heterocystous strain, Anabaena
PCC 7120 the frequency of heterocysts is approximately
10% under optimal laboratory growth conditions. Such
a low frequency of heterocysts in the filament might
result in modest yields of net H2 production. Thus, one
possible strategy to improve H2 production is to increase
the number of heterocysts in the filaments. However,
although the overexpression of HetR resulted in an overall enhancement of heterocyst frequency up to 29% in
FIGURE 21.2 Simplified schematic view of spatial separation of oxygenic photosynthesis in the vegetative cells and N2 fixation/H2 production in heterocysts. (For color version of this figure, the reader is referred to the online version of this book.)
HYDROGEN PHOTOPRODUCTION BY CYANOBACTERIA
Anabaena PCC 7120 mutant, no increase in the nitrogenase activity of the filaments took place (Buikema and
Haselkorn, 2001). It is possible that the relative decrease
in the number of vegetative cells makes them incapable
of producing enough reducing power to be transferred
to heterocysts for enhanced H2 production.
Unicellular and filamentous nonheterocystous
N2-fixing cyanobacteria apply mostly temporal separation mechanism, by performing photosynthesis during
the daytime and N2 fixation at night (Compaore and
Stal, 2010). The energy generated by photosynthesis is
stored in glycogen granules, which are later subjected
to oxidative breakdown.
Trichodesmium are unique cyanobacteria, because these
filamentous nonheterocystous cyanobacteria are able to
fix N2 simultaneously with oxygenic photosynthesis
during the photoperiod (Berman-Frank et al., 2001).
Nitrogenase is localized in subsets of cells in each
trichome, which also contain photosynthetic complexes.
During hours of high N2 fixation the cells can turn photosynthetic activity down within 10 min, which is observed
as unequally distributed inactive zones in whole filaments. Importantly, the PSII activity was shown to be
essential for N2 fixation in Trichodesmium (Berman-Frank
et al., 2001). According to the authors, Trichodesmium
utilizes photosynthetic electron transport to support N2
fixation and concomitantly enhances the Mehler reaction,
which efficiently eliminates the evolved O2. Recently
published complete genome sequence of Trichodesmium
erythraeum (http://www.ncbi.nlm.nih.gov) shows that
the strain indeed possesses the genes encoding the Flv1
and Flv3 proteins, which are involved in the “Mehlerlike” reaction in cyanobacteria. Thus, the nitrogenase
enzyme in this organism is protected from O2 by a combined and modulated temporal and spatial segregation of
N2 fixation and oxygenic photosynthesis within individual cells (Berman-Frank et al., 2001).
The N2-fixing, unicellular cyanobacteria Cyanothece
has recently attracted lots of research interest as a highly
efficient H2 producer under natural aerobic conditions.
Cyanothece sp. ATCC 51142 is the best hydrogen producer among the known wild-type cyanobacterial
strains (Bandyopadhyay et al., 2010). Also, the ability
to grow phototrophically, mixotrophically, and heterotrophically makes this strain an attractive organism for
biotechnology. Cyanothece demonstrates temporal separation of oxygenic photosynthesis and N2 fixation by
performing photosynthesis in daytime and N2 fixation
at night. Moreover, these alternating processes are regulated by an intrinsic circadian rhythm. The genome
sequence reveals the presence of the bidirectional
[NieFe]-hydrogenase, uptake hydrogenase, and the
conventional MoeFe nitrogenase. Diazotrophically
grown Cyanothece cells entrained in 12-h light/12-h
dark cycles exhibit a light-induced H2 production
373
(specific rate >150e300 mmol H2 mg/Chl h) under aerobic conditions during “subject dark” (Bandyopadhyay
et al., 2010). Interestingly, the robust circadian rhythm
of Cyanothece allows cells to fix N2 and produce H2 at
reasonably high rates even when grown under continuous light (Min and Sherman, 2010).
In the presence of combined nitrogen, Cyanothece produces H2 at very low rates, 2e10 mmol H2 mg/Chl h.
This H2 production is catalyzed by the bidirectional
hydrogenase and is dependent on PSII activity. In diazotrophically grown cultures, the production of H2 is
driven by the nitrogenase enzyme and the activity of
the enzyme is linked to PSI and respiratory electron
flow (Min and Sherman, 2010). Moreover, the rates of
H2 production in Cyanothece 51142 could be greatly
enhanced when cells were grown in the presence of
additional carbon sources, as observed in cultures supplemented with high concentrations of CO2 or glycerol
(Bandyopadhyay et al., 2010). Photoproduction of H2
can be significantly enhanced by increasing reductant
availability via dark anaerobic preincubation. This indicates the tight coupling of H2 photoproduction to the
dark, anaerobic metabolism (Skizim et al., 2012).
Recently, it was reported that Cyanothece can coproduce H2 and O2 over 100 h under continuous illumination and uninterrupted photosynthetic electron
transport (Melnicki et al., 2012). Of course, Cyanothece
has a very flexible metabolism and the existence of intracellular O2 gradient within the cells cannot be excluded.
Despite many interesting papers describing the H2
production in Cyanothece, the molecular mechanisms
behind the regulation of the nitrogenase and protection
against oxygenic photosynthesis are still under debate.
Strategies to Improve H2 Production in
Cyanobacteria
Genetic Modifications
Most important genetic modifications applied to cyanobacteria in order to improve their H2 production
capacity have already been discussed above. Therefore,
just a few recent and important findings, will be
mentioned below.
Introducing Foreign Enzymes and Semiartificial
Systems
Most attempts of heterologous expression of genetically modified hydrogenase in cyanobacteria have not
been successful due to the complexity of transcriptional
regulation and maturation of the hydrogenases.
A successful heterologous expression of Fd-dependent
hydrogenase from Clostridium in the Synechococcus
PCC7942 was demonstrated and resulted in about threefold higher H2 production activity compared to the wild
type (Asada et al., 2000). More recent studies related to
374
21. RECENT DEVELOPMENTS ON CYANOBACTERIA AND GREEN ALGAE FOR BIOHYDROGEN PHOTOPRODUCTION
the identification and characterization of the genes
involved in maturation and regulation of the [FeeFe]and [NieFe]-hydrogenases and nitrogenases (Rubio
and Ludden, 2005) as well as the development of heterologously expressed enzyme systems have opened new
opportunities. In an attempt to engineer an organism,
which can produce H2 even under aerobic conditions,
the [FeeFe]-hydrogenase operon from Shewanella oneidensis MR-1 containing all maturation genes was successfully expressed in the heterocysts of Anabaena PCC
7120 (Gartner et al., 2012). Active [FeeFe]-hydrogenase
was detected in aerobically grown Anabaena PCC 7120
under diazotropic growth. Despite significantly higher
turnover number of the [FeeFe]-hydrogenase, in situ
H2 production rate was only about 20% of that by nitrogenases (Masukawa et al., 2010). One of the reasons for
such low activity might be that Fd in Anabaena is not
an effective electron donor to the [FeeFe]-hydrogenase.
Some hydrogenases, like the [NieFe]-hydrogenase of
Ralstonia eutropha are able to perform H2 cycling in the
presence of ambient O2. Employing this interesting
property of the Ralstonia hydrogenase, a [NieFe]hydrogenase (Hox) from Ralstonia has been fused with
the extrinsic PsaE subunit, which is located at the
acceptor site of PS I, by genetic engineering. The resulted
Hox/PsaE fusion exhibited in vitro self-assembly with a
cyanobacterial PSI lacking the PsaE subunit and lightdriven H2 was evolved (0.58 mmol H2 mg/Chl h, Ihara
et al., 2006a). In another study, Cytochrome c3 (Cyt c3)
from Desulfovibrio vulgaris was chemically cross-linked
to PsaE protein and the Cyt c3/PsaE complex was
rebound to a PsaE-free PSI complex and introduced
to a solution containing the D. vulgaris [NieFe]hydrogenase enzyme (Ihara et al., 2006b). When illuminated, light-induced H2 generation was observed at a
maximum rate of 0.30 mmol H2 mg/Chl h in the
presence of Fd and FNR, physiological acceptors of
PSI. These results suggest that the Cyt c3/PSI complex
may produce H2 in vivo. However, the observed in vitro
H2 production rate was quite low, most probably due to
poor electron transfer coupling between PSI and the hydrogenase in solution.
A further coupling approach has been applied where
the FB cluster of PSI and catalytic nanoparticle surface
have been covalently bound via a “molecular wire”.
Upon illumination, this semiartificial system generated
up to 70 mol H2 PSI/mol min (Grimme et al., 2008,
2009). In this innovative system, covalent binding
between FB (an electron cofactor of PSI) and a “molecular wire” (catalytic nanoparticle surface) enables high
rates of H2 evolution.
Finally, the direct coupling of photosynthesis and H2
production has also been performed on a gold surface
(Krassen et al., 2009). For stable connection of the
[NieFe]-hydrogenase to PSI, the extrinsic PsaE subunit
was fused to the electron-transferring subunit of the
membrane-bound [NieFe]-hydrogenase by genetic
engineering. The resulting Hox/PsaE protein was purified and incubated with isolated PSI from Synechocystis
sp. PCC 6803 lacking the PsaE subunit (PSIDPsaE).
PSIDPsaE and HoxPsaE were assembled on a gold surface and electrons provided by the gold electrode were
transferred to PSI with the aid of the soluble electron carrier N-methylphenazonium methyl sulfate. Upon light
illumination the hydrogenase-PSI hybrid system
demonstrated H2 production a rate of 4500 mol H2/min
mol (Krassen et al., 2009).
Elimination of Competing Electron Transfer
Routes
For surviving under different environmental conditions, cyanobacteria have developed a number of
different alternative electron transfer pathways, which
at the same time decrease the photosynthetic and H2 production efficiency. In order to improve H2 photoproduction from cyanobacteria, the competing alternative
electron transport pathways should be diminished. This
will guide future genetic and metabolic engineering efforts to modulate the major energetic pathways to avoid
“wasteful” electron flow and to channel major electron
flux to H2 production. Recent studies have confirmed
that the alternative electron-transport routes in cyanobacteria are also strong competitors for H2 production.
Under Ci-deprivation conditions the Flv1/Flv3 proteins
in cooperation with photorespiratory pathway might
flux up to 60% of electrons to O2, functioning as a powerful electron sink for the electrons originated from watersplitting PSII (Allahverdiyeva et al., 2011). In line with
this, recent studies on deletion mutants of the respiratory
electron transport complexes, terminal oxidases and the
NdhB subunit of the NDH-1 complex have revealed
increased hydrogenase activity and the production of
H2 (Cournac et al., 2004; Gutthann et al., 2007).
Biodiversity
Cyanobacteria are very diverse organisms. For many
decades only a limited number of cyanobacterial strains
have been used as model laboratory organisms for H2
research and by the end of 2000 only a few attempts to
screen large cyanobacterial culture collections for H2
production were recorded (Berchtold and Bachofen,
1979; Lambert and Smith, 1977).
Recently, more emphasis has been given to the biodiversity of cyanobacteria in order to identify promising
H2 producers with flexible metabolic pathways (Yeager
et al., 2011; Yoshino et al., 2007; Allahverdiyeva et al.,
2010). Screening of 400 cyanobacteria strains from the
University of Helsinki Culture Collection revealed that
about 50% of these strains produced easily detectable
amounts of H2. Ten of them produced similar or up to
HYDROGEN PHOTOPRODUCTION BY GREEN ALGAE
four times as much of H2 as the uptake hydrogenase mutants of Anabaena PCC 7120 (Masukawa et al., 2002) and
N. punctiforme ATCC 29133 (Lindberg et al., 2002), specifically engineered to produce higher amounts of H2
(Allahverdiyeva et al., 2010). All 10 best H2 producers
were N2-fixing, heterocystous filamentous strains.
Notably, the changes in environmental parameters had
differential effects on H2 production, depending on the
strain. Therefore, it is necessary to test multiple environmental conditions when screening for superior H2producing strains (Yeager et al., 2011). Optimization of
culture conditions and genetic modification of new
strains would enhance further H2 production.
Immobilization
In general, cyanobacterial H2 production is difficult to
sustain for long time periods in liquid cultures in
photobioreactors. Utilization of suspension cultures in
a two-stage system for H2 production is an even more
complicated and energy-consuming process due to the
centrifugation or sedimentation steps required for cell
harvesting, media changes, and dilutions of cell density
during the switch between the different phases. Suspension cultures require intensive mixing, which in turn
causes damage to the fragile cyanobacteria filaments.
This system is hard to scale up. Use of immobilized
cyanobacterial cells in specially designed laboratoryscale photobioreactors would be a good solution to the
above-mentioned problems of liquid cultures.
Immobilization of biomolecules and whole cells on
various substrates and into different gels, such as solid
surfaces like porous glass, supported films, (nano)
porous materials, (nano)fibers, foams, inorganic and
organic hydrogels, latex, nanotubes, and nanoparticles,
has been studied extensively (see for review Meunier
et al., 2011). Application of immobilization usually improves the stability of the enzymes, and increases
light-utilization efficiency. Immobilization of algal cells
on a solid phase made of glass has been used for
extended H2 production (Laurinavichene et al., 2006).
A green alga, C. reinhardtii entrapped in thin alginate
films demonstrated extended H2 photoproduction due
to increased light-utilization efficiency and better tolerance against O2 (Kosourov and Seibert, 2009). Several
attempts also have been made to immobilize cyanobacterial cells in order to improve H2 production. These
include Plectonema boryanum within alginate beads (Sarkar et al., 1992), Oscillatoria in agar matrix (Phlips and
Matsui, 1986) Phormidium valderianum together with
Halobacterium halobium and E. coli within polyvinyl
alcohol-alginate beads (Bagai and Madamwar, 1998).
Recent immobilization of the Calothrix 336/3 strain in
thin alginate film resulted in extended production of
H2 even after 40 days of immobilization (Leino et al.,
2012). Immobilization has also been found to have a
375
positive effect also on viability of cells. Twelve weeks
after initial immobilization, entrapped cells from recovered films produced H2 nearly as efficiently as the fresh
cells in newly made films. Moreover, the immobilized
cells of Calothrix 336/3, Anabaena PCC 7120 and DhupL
mutant of Anabaena were viable for over 10 months in
the initial nutrient medium without addition of CO2.
The demonstrated long-term viability of entrapped cells
is a very important issue for economical use of cyanobacterial in H2 production systems.
HYDROGEN PHOTOPRODUCTION BY
GREEN ALGAE
Light-Dependent Hydrogen Production
Pathways
Like some cyanobacteria, many species of eukaryotic
green algae are capable of direct water biophotolysis
(Boichenko and Hoffmann, 1994). In green algae, water
biophotolysis typically proceeds in two steps:
H2 O þ 2Fdox / 2Hþ þ 1=2O2 þ 2Fdred
2Hþ þ 2Fdred / H2 þ 2Fdox
(21.5)
(21.6)
The first reaction is common to all oxygenic phototrophs,
and was explained above (Section Oxygenic Photosynthesis). The second step, however, occurs only under
anaerobic or microaerobic conditions, since it is
extremely oxygen sensitive (Ghirardi et al., 1997). The
reaction is catalyzed by the [FeeFe]-hydrogenase
enzyme that accepts electrons from photosynthetically
reduced Fd and reduces protons of water to molecular
hydrogen (Happe and Naber, 1993; Happe and Kaminski, 2002; Foriestier et al., 2003). Although six different
Fds are known in green algae, it is most likely that only
PetF serves as the physiological electron donor to
[FeeFe]-hydrogenase and, thus, links the photosynthetic
electron-transport chain to the hydrogenase-driven
reaction in vivo (Happe and Naber, 1993; Winkler et al.,
2009; Long et al., 2008).
In photosynthetically active algal cells, the direct
water biophotolysis process usually occurs when cultures are exposed to the light after a period of dark,
anaerobic adaptation (Gaffron and Rubin, 1942; Greenbaum, 1982; Appel and Schulz, 1998). It proceeds at
very high initial rates (up to 300 mmol H2 mg/Chl h)
that are comparable to the rates of O2 evolution in green
algae under optimal light conditions (Boichenko and
Hoffmann, 1994; Boichenko et al., 2004). It should be
noted, however, that such high rates of H2 photoproduction in healthy algal cells occur only for a very short
period of time and that the duration of the process
376
21. RECENT DEVELOPMENTS ON CYANOBACTERIA AND GREEN ALGAE FOR BIOHYDROGEN PHOTOPRODUCTION
depends directly on the light intensity. For example, in
dark-adapted Chlorella vulgaris cultures the H2 photoproduction rate reaches the maximum in 2.5 s after
exposing cells to about 0.03 W/m2 light and the kinetics
is linear for at least 1 min (Boichenko et al., 1983). Under
2 W/m2 illumination, cells show the maximum rate
after 0.6 s, which starts declining soon after 1 s. The process can also be extended in the presence of 3-(30 ,40 dichlorophenyl)-1,1-dimethylurea (DCMU), a specific
inhibitor of electron transport from PSII to the PQ-pool
(Happe and Naber, 1993; Florin et al., 2001). In this
case, however, H2 photoproduction is driven through
the photofermentation pathway (see below) and thus
depends on the level of stored carbohydrates and proteins. Nevertheless, the extension of the H2 photoproduction period in DCMU-treated cultures indicates
that inhibition of H2 evolution in dark-adapted and
DCMU-untreated algae in light is mainly due to a fast
accumulation of O2 inside algal chloroplasts. This
inhibits hydrogenase-driven reaction and switches the
physiological state of cells from anaerobic to aerobic.
Indeed, using the Clark-type O2 and H2 sensors,
Boichenko and et al. (1983) showed that the decrease
in the rate of H2 photoproduction in algae is always
followed by accumulation of O2 in the culture. The
sensitivity to O2 occurs at four levels: (1) gene transcription, (2) [FeeFe]-hydrogenase maturation, (3) activity of
the hydrogenase catalytic site, and (4) competition for
photosynthetic reductants (Ghirardi, 2006).
Although H2 photoproduction in green algae is a
short-term phenomenon, its theoretical sunlight to
hydrogen conversion efficiency (STHE) is higher than
in N2-fixing cyanobacteria. In addition, algae split water
and, contrasting many anoxygenic photosynthetic bacteria, they do not require any organic substrates for production of H2 gas. The maximum efficiency for the
direct water biophotolysis process has been estimated
at around 10% (Bolton, 1996; Akkerman et al., 2002).
Since this value is comparable to the power conversion
efficiency of present commercial silicon solar cell modules, which are rated at around 10e11% with watersplitting process considered (Blankenship et al., 2011),
the high efficiency of algal H2 photoproduction raises
the possibility of industrial application of the process
in the future. At the current state, however, the direct
water biophotolysis systems are not cost-effective and
will require significant biochemical and engineering
improvements to achieve commercial viability.
Taking into account a high sensitivity of algal hydrogenases to O2, much attention in the past was focused on
the development of the efficient methods of keeping cultures anaerobic throughout the H2 production period.
These methods included the addition of O2 scavengers
(Healey, 1970; Randt and Senger, 1985), the use of added
reductants (Randt and Senger, 1985), sparging cultures
with inert gases (e.g. argon or helium) (Greenbaum,
1982; Greenbaum et al., 2001) and the addition of PSII inhibitors (Gfeller and Gibbs, 1984; Fouchard et al., 2005).
Although some of these methods indeed prolong H2
production in algae, none of them resulted in bulk production of H2 gas. The expense of such approach also
limits their application on larger scales. Many early
research efforts also concentrated on screening for naturally better H2 producers. As a result, a few dozen algal
species were tested for their ability to photoproduce
H2 gas after the period of dark anaerobic adaptation.
Many of them, but not all, were capable of direct water
biophotolysis only for a very short period of time
(Ben-Amotz et al., 1975; Boichenko and Hoffmann, 1994).
According to Eqns (21.5) and (21.6), direct water
biophotolysis gives a maximum theoretical H2 to O2
(mol:mol) ratio of 2 to 1. Since both PSII and PSI are
involved in the process, the minimum number of quanta
required for generating one H2 molecule is equal to four.
In many short-term experiments performed with darkadapted algae, this value was above 4, but in some cases
only 2e2.5 quanta were required (Greenbaum, 1988;
Boichenko et al., 1989, 2004). The latter value indicates
that H2 photoproduction in green algae may occur
through a mechanism independent of water oxidation.
The existence of this pathway was also confirmed by
the experiments with inhibitors of the photosynthetic
electron transport chain. It was found that DCMU, a
PSII inhibitor (see above), does not completely block
H2 photoproduction in algal cells, while 2,5-dibromo3-methyl-6-iso-propyl-p-benzoquinone, which blocks
PQ oxidation by the Cyt b6 f complex, inhibits the
process almost completely (Ben-Amotz et al., 1975;
Kosourov et al., 2003; Antal et al., 2009). In contrast to
water biophotolysis, this pathway depends on the metabolic oxidation of organic substrates that are coupled to
PSI and the [FeeFe]-hydrogenases through the PQ-pool
(Stuart and Gaffron, 1972; Gfeller and Gibbs, 1984; Gibbs
et al., 1986). According to Gibbs and coworkers, starch,
acetate and proteins could be the main substrates for
photofermentation in algae, which results in production
of CO2 and H2 gases. For example, the full degradation
of 1 mol (in glucose equivalents) starch will give up to
6 mol CO2 and up to 12 mol H2:
C6 H12 O6 þ 6H2 O / 6CO2 þ 12H2
(21.7)
In C. reinhardtii and other green algae, degradation of
substrates through photofermentation is not complete.
The major by-products are formate, acetate, ethanol,
and, in some rare cases, lactate and glycerol (Gfeller and
Gibbs, 1984; Kreuzberg, 1984; Zhang et al., 2002;
Kosourov et al., 2003). According to a number of studies,
the final composition varies significantly on the strain
and the environmental condition. Since accumulation of
HYDROGEN PHOTOPRODUCTION BY GREEN ALGAE
organic substrates, mainly starch, also depends on PSII
activity, the photofermentation pathway in green algae
proceeds in two stages and, thus, can be considered as
an indirect water biophotolysis. Starch accumulated
through photosynthetic activity later undergoes degradation through glycolysis that yields pyruvate, ATP and
reductants. In green algae, oxidation of reductants may
occur via Nda2, a monomeric class-II type NAD(P) dehydrogenase, which feeds electrons into the photosynthetic electron transport chain at the point of the
PQ-pool (Jans et al., 2008; Desplats et al., 2008). From
the PQ-pool, electrons follow to [FeeFe]-hydrogenase
through PSI and Fd. Since both direct and indirect
water biophotolysis pathways are linked to [FeeFe]hydrogenase via PSI, it becomes clear that in algae they
operate simultaneously most of the time. Nevertheless,
their contribution into the overall H2 photoproduction
process varies depending on the organism (Meuser
et al., 2009) and the physiological state of algal cells
(Laurinavichene et al., 2004).
In the indirect water biophotolysis process, O2 evolution and H2 production stages can be separated from
each other either temporally or spatially, thus circumventing the apparently inherent O2 sensitivity of H2
photoproduction. Despite lower efficiency (as compared
to the direct water splitting), separation of the process
into two distinct stages gives a significant advantage to
the biotechnological applications (Benemann, 1994,
1996). According to the early Benemann’s concept,
during the first aerobic stage algal cultures accumulate
biomass enriched in carbohydrates as a result of photosynthetic CO2 fixation. During the second, anaerobic
stage carbohydrates and other materials stored in
biomass are processed to H2 gas. The two stages are separated either physically in two different photobioreactors
or temporally through additional dark adaptation and
fermentation periods. In this approach, O2-evolving
activity inside the cells is totally inactivated during the
H2 production stage without application of any inhibitors, and algae evolve H2 gas through the photofermentation pathway linked to PSI. The second stage can also be
driven in the dark by fermentative bacteria. Thus, the
discovery of the indirect water biophotolysis pathway
was the first step toward development of the protocol
for long-term H2 photoproduction in green algae.
Role of H2 Photoproduction in Green Algae
Despite extensive research, the role of H2 photoproduction in physiology of green algae still remains unclear.
Some genera of green algae do not show hydrogenase
activity and so do not produce H2 even under dark anaerobic conditions (Brand et al., 1989; Winkler et al., 2002;
Boichenko et al., 2004). Some strains demonstrate a very
low rate of H2 production in light, even if they have a
377
high hydrogenase activity (Boichenko and Hoffmann,
1994; Guan et al., 2004; Skjanes et al., 2008). In algae
showing active H2 photoproduction, hydrogenase has
been proposed as a regulatory valve preventing overreduction of photosynthetic apparatus during transition
from dark anaerobic to light aerobic conditions (Appel
and Schulz, 1998). Under anaerobic conditions, CO2 fixation in algal cells is repressed due to inactivation of
Rubisco, the key enzyme of the CalvineBenson cycle.
Reactivation of Rubisco upon illumination is a very
slow process, which takes up to 2 min (Campbell and
Ogren, 1990). During this time, hydrogenase may act as
an alternative sink of electrons for the electron transport
chain, preventing photodamage of the photosynthetic
apparatus. Although very attractive, this hypothesis has
never been proved experimentally. Moreover, experiments performed by Tsygankov and coauthors have
demonstrated results contradicting the hypothesis, as
cell viability between the mutant lacking the hydrogenase enzyme and the parental strain was almost the
same after the period favorable to H2 photoproduction
(Tsygankov, 2012).
Long-Term H2 Production by Green Algae
As mentioned above, all metabolic pathways leading
to H2 production in green algae are extremely sensitive
to O2 due to fast and irreversible inhibition of [FeeFe]hydrogenase enzyme(s) and competition from different
respiratory processes for the reductants. Significant
efforts to surmount the O2 sensitivity issue have been
made, but still the algal strain with the O2-tolerant H2
photoproduction has not been generated (Ghirardi,
2006; Ghirardi and Mohanty, 2010). Therefore, in
photosynthetically active algal cells under saturating
light conditions the most efficient H2 photoproduction
process (via direct biophotolysis) lasts for a few seconds.
For the industrial system, however, H2 photoproduction
should be extended from the scale of seconds to at least
days. When optimized, such process may yield H2 at a
cost of around $3/kg H2 for the upper bound
performance (w9% STHE) and slightly above $8/kg
H2 for the near term performance (w1.5e2% STHE)
(Blake et al., 2008; James et al., 2009). Unfortunately, at
the current state sustained H2 photoproduction in
photosynthetically active green algae is only possible
at the expense of efficiency. For example, long-term H2
production is usually observed in cultures under very
low light intensities or even in the dark when O2 evolution proceeds very slowly or does not proceed at all
(Kondratieva and Gogotov, 1983; Aparicio et al., 1985).
Interestingly, Batyrova et al. (2012) recently observed a
stable but negligible rate of H2 production in very dense
cultures placed under normal light conditions. The algal
cells demonstrated a high hydrogenase activity, but
378
21. RECENT DEVELOPMENTS ON CYANOBACTERIA AND GREEN ALGAE FOR BIOHYDROGEN PHOTOPRODUCTION
efficient H2 photoproduction was not observed. Most
probably, under these conditions H2 evolution was
driven by the cells in the inner part of the photobioreactor, while H2 photoproduction in the illuminated algae
was limited by the coevolved O2.
One of the possibilities of driving the process under
normal photosynthetic conditions is to sparge cultures
continuously with inert gas, such as argon or nitrogen,
that removes rapidly the PSII-evolved O2 gas
(Greenbaum, 1982; Greenbaum et al., 2001). Before the
sulfur-deprivation protocol was developed, this was
the only way for the long-term H2 generation in algal
cultures. Using a confined bioreactor, Greenbaum et al.
(2001) showed several cycles of simultaneous H2 and
O2 photoproduction during 1 h intervals after extensive
purging the cultures in the dark for 2 h by N2. The experiment lasted for over 1400 h (58 days) and required periodic additions of CO2 gas into the photobioreactor for
restoration of photosynthetic activity and replenishment
of carbohydrates in algal cells. The average stoichiometric ratio of H2 to O2 was 2.8, indicating that reducing
equivalents for H2 were derived from endogenous
reductants, most likely starch, as well as water. Therefore, H2 photoproduction in this case was partly driven
by the cells via indirect biophotolysis pathway. Nevertheless, even under extensive purging of the cultures
with N2 and good mixing conditions in the photobioreactor, the H2 evolution rate was limited due to O2
buildup in the liquid phase (Greenbaum et al., 2001).
These experiments were later repeated under sulfurdeprived conditions (but with some modifications) and
demonstrated a simultaneous improvement in the rate
of H2 photoproduction upon declining the O2-evolving
activity in algal cultures (Ghirardi et al., 2000).
The prolonged H2 evolution by green algae can also
be induced by a full or partial inhibition of the watersplitting activity of PSII in cells. The full inhibition can
be achieved by applying DCMU (Gfeller and Gibbs,
1984; Fouchard et al., 2005). In contrast to the
N2-sparging approach, H2 photoproduction in
DCMU-treated cultures depends totally on the amount
of carbohydrates or other substrates stored by the cells
during the growth period and, thus, the process is
limited to only one cycle. The partial inhibition of the
PSII activity occurs in sulfur-deprived algae (Melis
et al., 2000) and in certain mutants with manipulated
expression levels of the D1 reaction center protein
(Surzycki et al., 2007) or in the mutant cells affected in
the PSII water-splitting complex (Makarova et al., 2005,
2007). For the establishment of an anaerobic environment and H2 production in algal cultures, the partial
inhibition of the PSII activity in cells should achieve
the point when the O2 produced by the PSII centers is
consumed sufficiently by respiration. However, H2
photoproduction under these conditions usually
proceeds at lower rates as compared to the initial rates
in dark-adapted algae exposed to the light. In contrast
to the full inhibition approach, several cycles of H2 production are possible (Ghirardi et al., 2000), and thus the
process can be driven continuously (Laurinavichene
et al., 2006, 2008; Kim et al., 2010).
Hydrogen Photoproduction by
Nutrient-deprived Green Algae
One of the most remarkable events in investigating
H2 metabolism in green algae was the discovery of sustained H2 photoproduction in C. reinhardtii cultures under sulfur-deprived conditions (Melis et al., 2000;
Ghirardi et al., 2000). In this approach, the long-term
H2 photoproduction is possible due to a metabolic
switch occurring in sulfur-deprived algal cells, which
separate temporarily the O2-evolving, aerobic (Eqn
(21.5)) and H2-producing, anaerobic (Eqn (21.6)) stages
in the same culture.
Sulfur deprivation causes the partial and reversible
inhibition of PSII-dependent water-splitting activity in
algae. As demonstrated by Wykoff et al. (1998), C. reinhardtii cells lose gradually up to 75% of the initial PSII activity within the first 24 h of sulfur starvation. The
reduction of H2O-splitting activity was also shown
under deprivation of other nutrients, such as nitrogen,
phosphorus, Fe and Mn (Wykoff et al., 1998; Ghirardi
et al., 2000; Philipps et al., 2012) but usually with a
significant delay, as compared to sulfur starvation. The
repression of the linear electron flow from the PSII
centers under nutrient starvation is a common phenomenon not only for green algae but also for cyanobacteria
(Sauer et al., 2001) and high plants (Dietz and Heilos,
1990; Ferreira and Teixeira, 1992), and is a good example
of how photosynthetic organisms adjust the rate of
photosynthesis to the stress conditions. Continuous
nutrient starvation reduces the capacity for de novo protein biosynthesis and CO2 fixation, and, as a result,
decreases the demand of the cells in the photosynthetic
reductants (Grossman, 2000). Under these conditions,
the repression of the O2-evolving activity and linear
electron flow protects the photosynthetic apparatus
from overreduction, generation of reactive oxygen species and photoinhibition. Numerous experiments
showed that the inhibition of O2-evolving activity in
nutrient-deprived cells is mostly caused by the loss of
PSII centers (Kolber et al., 1988; Wykoff et al., 1998). In
the absence of basic nutrients such as nitrogen, phosphorus or sulfur, the cells cannot efficiently resynthesize
D1 protein, the key component of the PSII complex, and
the PSII repair cycle is blocked (Melis and Chen, 2005).
Nutrient deprivation, however, has little effect on
cellular respiration, especially in the first few days
(Melis et al., 2000). As a result, the rate of photosynthetic
HYDROGEN PHOTOPRODUCTION BY GREEN ALGAE
O2 evolution falls below the rate of respiratory O2 uptake and algal cultures, if sealed in photobioreactors
with a little headspace volume, become anaerobic in
the light (Melis et al., 2000). In sulfur-deprived cultures,
this usually happens within the first 24 h. The establishment of anaerobiosis in the sealed photobioreactor
induces the expression of [FeeFe]-hydrogenase
enzymes in algal cells (Happe and Kaminski, 2002;
Forestier et al., 2003). [Fe-Fe]-hydrogenase accepts electrons from the photosynthetic electron-transport chain
and algae start producing H2 in the light. If not optimized, H2 photoproduction lasts for several days (Melis
et al., 2000; Ghirardi et al., 2000). Under continuous flow
of the medium containing sulfur in a micromolar range,
algae produce H2 gas for several months, although at
substantially low rates (Fedorov et al., 2005; Laurinavichene et al., 2006). The most interesting results obtained
from sulfur-deprivation experiments are summarized in
Table 21.1. As shown in the table, the rates and the yields
of H2 photoproduction in algal cultures vary depending
on the experimental conditions. In C. reinhardtii wildtype strains the rate usually does not exceed 13 mmol
mg/Chl h, while some genetically modified strains are
able to produce H2 with rates up to 27 mmol mg/Chl h.
In green algae, sulfur deprivation demonstrates the
strongest inhibitory effect on PSII (Wykoff et al., 1998;
Ghirardi et al., 2000) most probably due to the lowest
intracellular sulfur reserves. The later studies showed
that the same principle works for phosphorusdeprived (Batyrova et al., 2012) and nitrogen-deprived
(Philipps et al., 2012) microalgae. Phosphorus-depleted
cultures start producing H2 gas only after the initial
growth period on the phosphorus-free medium.
Growing algae utilize an intracellular pool of reserved
phosphorus. When they reach the point of phosphorus
starvation, PSII in algal cells is inactivated in a manner
similar to sulfur-starved algae. Despite a considerable
delay in the establishment of anaerobic conditions,
phosphorus-deprived algae produce only slightly less
H2 gas than sulfur-deprived cultures under the same
experimental conditions, but they also accumulate less
starch reserves during the growth stage (Batyrova
et al., 2012). Nitrogen-deprived algae behave in a similar
way. They produce H2, but with a significant delay
(Philipps et al., 2012). In contrast to phosphorusdeprived cells, the delay in nitrogen-deprived cultures
seems to be caused by slower inactivation of PSII centers. These algae also accumulate significantly more
starch reserves than sulfur-deprived algae, but degrade
them slower. As a result, they produce considerably less
H2 overall. Inability to efficiently channel electrons from
carbohydrate oxidation toward the hydrogenase
enzyme likely causes the degradation of the Cyt b6 f
complex upon nitrogen starvation and lowers amounts
of PetF. Nevertheless, nitrogen-deprived cultures may
379
have a higher potential for the light-independent H2
production pathway (Philipps et al., 2012).
The vast majority of experiments completed on H2
production by nutrient-deprived microalgae have been
undertaken so far with C. reinhardtii cultures. However,
other species of green algae also produce H2 gas under
this condition (Winkler et al., 2002; Skjanes et al., 2008;
Meuser et al., 2009). Successful H2 production has been
demonstrated by sulfur-depriving C. noctigama and
Chlamydomonas euryale (Skjanes et al., 2008). Sulfurdeprived cultures of S. obliquus, Platymonas subcordiformis, Scenedesmus vacuolatus, Chlamydomonas vectensis,
Chlamydomonas pyrenoidosa, Desmodesmus subspicatus,
Pseudokirchneriella subcapitata, Chlamydomonas moewusii
and Lobochlamys culleus generate only minor amounts
of H2 gas (Winkler et al., 2002; Guan et al., 2004; Skjanes
et al., 2008; Meuser et al., 2009). Some other tested species, such as Dunaliella salina and C. vulgaris demonstrate
no detectible hydrogenase activities and do not produce
H2 under sulfur-deprived conditions (Cao et al., 2001;
Winkler et al., 2002).
H2 photoproduction in nutrient-deprived algae depends both on the residual PSII activity remaining in
cells after inactivation (Antal et al., 2003; Kosourov
et al., 2003) and on the catabolism of starch accumulated
during the first 18e24 h of sulfur deprivation (Fouchard
et al., 2005; Ghirardi et al., 2000; Kosourov et al., 2003;
Tsygankov et al., 2002; Zhang et al., 2002). The contribution of these two pathways in H2 photoproduction
varies depending on the stage of sulfur deprivation
(Laurinavichene et al., 2004) and, most probably, on
the strain used in the experiment (Chochois et al.,
2009). In the wild-type C. reinhardtii CC-124 strain, starch
degradation may donate up to 20% electrons to hydrogenase enzymes in the middle of the H2 production stage
(Kosourov et al., 2003). Besides contribution to H2
photoproduction, the degradation of starch and other
stored organic substrates fuels the respiratory consumption of O2 produced by the residual PSII activity and
therefore is responsible for maintaining culture anaerobiosis and for protecting hydrogenase enzymes from
O2 inactivation (Fouchard et al., 2005; Kosourov et al.,
2007). The importance of efficient respiration for H2
photoproduction was further proved by inhibitory
analysis (Antal et al., 2009) and in the respiratorydeficient mutants (Table 21.1).
Under photoheterotrophic conditions (when acetate
is the only substrate), accumulation of starch in algae
in the beginning of sulfur deprivation is tightly linked
to consumption of acetate from the medium. The respiration of acetate provides the cells with a substrate for
CO2 fixation. It also helps with the establishment of
anaerobiosis in the photobioreactor (Kosourov et al.,
2007). The use of acetate in the growth medium, however,
increases the expense associated with maintenance of
380
21. RECENT DEVELOPMENTS ON CYANOBACTERIA AND GREEN ALGAE FOR BIOHYDROGEN PHOTOPRODUCTION
TABLE 21.1
The Rates and Yields of H2 Photoproduction by the Sulfur-Deprived, Wild-Type C. reinhardtii Strains and Some Mutants
under Different Experimental Conditions
Maximum Specific
Rate of H2 Production,
mmol mg/Chl h
Total Yield of
H2 Gas, mmol/l
References
e
4.7
Melis et al., 2000
12.5
23.1
Kosourov et al., 2012
e
2.3
Tsygankov et al., 2006
e
1.6
Lecler et al., 2011
1. Photoheterotrophic, 150 mmol/
m2 s PAR from two sides, 28 C,
initial pH at 7.3, synchronized
culture
5.9
6.6
Kosourov et al., 2002
2. The same as above, but initial pH
at 7.7 and unsynchronized culture
9.4
7.7
Kosourov et al., 2003
3. The same as above, but 140 mmol/
m2 s PAR from two sides and
improved culture mixing
9.8
6.9
Giannelli et al., 2009
e
w22
Kruse et al., 2005
Hemschemeier et al.,
2008
Strain
Experimental Condition
WT,
137C mtþ
1. Photoheterotrophic, 25 C,
200 mmol/m2 s PAR from two
sides
2. The same as above, but 70 mmol/
m2 s PAR from one side and low
H2 partial pressure, initial pH 7.3
3. Photoautotrophic, 28 C,
110 mmol/m2 s PAR during the
photosynthetic stage and
20 mmol/m2 s PAR during the
hydrogen production stage (from
two sides), pH was stabilized at
7.4 during the first stage
4. Photoheterotrophic, 25 C,
500 mmol/m2 s PAR
WT,
CC-124 mt-
Stm6 (Affected in the
State Transition)
Photoheterotrophic, 100 mmol/m2 s
PAR, 25 C
CC-2803 (RubiscoDeficient Strain)
1. Photoheterotrophic, 100 mmol/
m2 s PAR
3.8
e
2. The same as above, but sulfurreplete
5.4
e
CC-4169 (Antennae
Mutant, Affected in
tla1)
Photoheterotrophic, 285 mmol/m2 s
PAR, 25 C immobilized in alginate
films
3.8
e
Kosourov et al., 2011
Respiratory-Deficient
Mutants
1. Photoheterotrophic, 500 mmol/
m2 s PAR, 25 C, mutant defective
in mitochondrial complex I
(NADH:ubiquinone
oxidoreductase)
e
1.3
Lecler et al., 2011
2. The same as above, mutant
defective in mitochondrial
complex III (ubiquinol cytochrome
c oxidoreductase)
e
0.3
3. The same as above, mutant
defective in both I and III
complexes
e
0.07
1. Photoheterotrophic, 28 C,
improved culture mixing,
70 mmol/m2 s PAR from two sides
19
21
Torsillo et al., 2009
2. The same as above, but 140 mmol/
m2 s PAR from two sides
27.5
23.6
Scoma et al., 2012
L159I-N230Y
(Substitution in the D1
PSII Protein)
WT, wild type; PAR, photosynthetic active radiation.
HYDROGEN PHOTOPRODUCTION BY GREEN ALGAE
the system and should therefore be avoided. Recently,
Tsygankov et al. (2006) showed that H2 photoproduction in green algae is also possible under autotrophic
conditions, when cultures are supplied with CO2 gas
instead of acetate. In this experiment, authors used
the microprocessor-controlled bioreactor system for a
controllable addition of CO2 gas. The unique aspect of
this system is that cells are provided with appropriate
amounts of CO2, in accordance with the demands of the
culture. Under these conditions, algae accumulate
enough starch that can later be used for the establishment of anaerobiosis in the culture and for the removal
of O2 during the H2 production stage (Kosourov et al.,
2007). Using the special light regime, the authors generated almost the same amounts of H2 gas as in photoheterotrophic cultures (Tsygankov et al., 2006; Tolstygina et al.,
2009).
Strategies to Improve H2 Photoproduction
in Green Algae
Among major barriers to optimal H2-production yields
in algal cultures are high sensitivity of algal [FeeFe]hydrogenases to O2 inactivation, low light saturation
levels of photosynthesis, competition for reductant
from alternative metabolic pathways, state transition
and establishment of cyclic electron flow around
PSI, and the reversible nature of the hydrogenasee
driven reaction. All these barriers have been extensively studied by different research groups in the last
few years.
Clearly, the O2 sensitivity of [FeeFe]-hydrogenases is
the major barrier preventing the application of green
algal H2 photoproduction in commercial systems.
Several approaches for solving the O2-sensitivity issue
have been suggested: (1) identifying and implementing
mutations, which narrow the channel(s) of the
[FeeFe]-hydrogenase enzyme for blocking access of O2
molecules to the catalytic center (Cohen et al., 2005;
Posewitz et al., 2009); (2) selecting for O2-tolerant enzymes through random mutagenesis (Nagy et al., 2007;
Stapleton and Swartz, 2010); (3) introducing enzymes
from other organisms, which are more stable to O2 inactivation, into algal cells. None of these approaches have
yet resulted in a mutant with improved O2 tolerance.
Nevertheless, Stapleton and Swartz (2010) applying
the directed evolution approach identified a version of
C. reinhardtii HydA1 with a fourfold increase in catalytic
activity as compared to the wild-type enzyme.
Low light-utilization efficiency in mass cultures
is another important factor precluding the use of H2producing green algae in practical applications (Torzillo
et al., 2003). In algal suspensions, light intensity
decreases with the depth of the culture. The light attenuation is more pronounced in dense cultures, where
381
shading limits the productivity of inner parts of the culture. On the contrary, algae in the upper layers suffer
from photoinhibition, which is more pronounced under
high light intensities. The latter significantly limits
application of high light intensities for improving the
overall algae productivity. The problem can be
addressed in part by immobilizing algae in thin layers
or films. Immobilization fixes algal cells within a
controllable volume and allows uniform light distribution to the cells that makes light utilization per volume
basis more efficient. Indeed, immobilization of sulfurdeprived C. reinhardtii cultures on glass fiber matrices
demonstrated significant improvements both in the
volumetric rate of H2 photoproduction and in the duration of the process (Laurinavichene et al., 2006).
This technique used the property of microalgae to
form biofilm on the glass surface. The attachment of
cells occurred through natural colonization that, if
required, can be accelerated by activating glass fibers
with 3-(2-aminoethyl-aminopropyl)-trimethoxysilane
(Tsygankov et al., 1994). Later studies of immobilized
algae with either a constant flow of medium containing
micromolar sulfate concentrations or cycling of immobilized cells between minus and plus sulfate conditions
improved the duration of H2 production up to at least
3 months (Laurinavichene at al., 2008). However, due
to irregular colonization of glass fibers by the algal cells,
the system showed significant physical and physiological heterogeneities in different parts of the matrix,
resulting in irregular light and nutrient distributions,
and decreasing the overall performance of H2 photoproduction. In order to improve the light absorption properties of immobilized microalgae, Kosourov and Seibert
(2009) entrapped cells within thin alginate films. This
technique produced films with uniform distribution of
algal cells within the matrix that had very high cell
densities (up to 2000 mg total Chl per ml of the matrix).
As a result, the light conversion efficiency in alginate
films at w60 mE/m2 s PAR (photosynthetic active radiation) achieved 1.5% for the period of the maximum H2production rate and was close to 1% for the whole
period of nutrient deprivation.
Another approach for improving light utilization efficiency in mass algal cultures is to find or generate algal
mutants with a small chlorophyll antenna size. Strains
with the truncated antennae allow greater transmittance
of irradiance through the ultrahigh cell density culture
without significant dissipation of light energy and, as a
result, have a higher photosynthetic productivity in outdoor conditions. Recently, C. reinhardtii mutants with
truncated chlorophyll antennae were generated and
characterized (Polle et al., 2000, 2003). These mutants
have shown promise in increasing the light utilization
efficiency and the overall productivity in mass cultures
(Polle et al., 2002, 2003), but suspensions have not
382
21. RECENT DEVELOPMENTS ON CYANOBACTERIA AND GREEN ALGAE FOR BIOHYDROGEN PHOTOPRODUCTION
established anaerobiosis and so have failed to produce
H2 gas under sulfur-deprived conditions. Despite this,
nutrient-deprived mutants with truncated chlorophyll
antennae produced H2 after immobilization within
thin alginate films (Table 21.1). These mutants showed
higher efficiency of H2 photoproduction than the
parental CC-425 strain under saturating light conditions
(Kosourov et al., 2011).
H2 photoproduction in green algae competes with a
number of different metabolic pathways for the reductant originated in photosynthesis (Hemschemeier and
Happe, 2011). Here, CO2 fixation is one of the most
important. The affinity of Fd to FNR is very high and
on the order of 0.6 mM (Kurisu et al., 2005), while the affinity of Fd to hydrogenase enzyme is only about 10 mM
(Roessler and Lien, 1984). It is clear that electrons in
healthy algal cells will be preferably directed toward
reduction of NADPþ and, hence, toward CO2 fixation.
Sulfur-deprived algae, however, inactivate Rubisco, the
key enzyme of CO2 fixation, by the time of the establishment of anaerobiosis in the photobioreactor (Zhang
et al., 2002). Zhang and coauthors showed that only
about 3% of this protein is present in cells during the
H2 production stage. This finding suggests that the
photosynthetically generated reductants in sulfurdeprived algae are preferably used for generation of
H2, but not for CO2 fixation. It is important to note
here, that according to Hemschemeier et al. (2008) the
Rubisco-deficient C. reinhardtii, CC-2803 strain produces
H2 gas even under sulfur-replete conditions (Table 21.1).
H2 evolution in this strain is almost completely dependent on electron flow from PSII. This finding shows
that flow of electrons in engineered green algae can be
successfully redirected toward H2 photoproduction.
The competition for the photosynthetically generated
reductant from other metabolic pathways is less studied.
There has been some evidence for the competition from
nitrate reductase (Aparicio et al., 1985). However, the
wild-type CC-124 and 137C strains of C. reinhardtii that
are commonly used in sulfur-deprivation experiments
(Table 21.1) carry the nit1 and nit2 mutations and cannot
grow on nitrate. Therefore, the question about possible
competition for the reductant between hydrogenase
and nitrate reductase should be studied in detail
using the Sager’s line of C. reinhardtii wild-type strains
(Pröschold et al., 2005).
A novel approach for preventing competition for the
reductant from other metabolic pathways is formation of
a fused complex of Fd and hydrogenase. In vitro analysis
of such a complex showed that replacing the hydrogenase with the Fd/hydrogenase fusion switches the bias
of electron transfer from FNR to hydrogenase and results in an increased rate of H2 photoproduction (Yacoby
et al., 2011). This experiment indicates that the idea of
the formation of a fused Fd/hydrogenase complex is
promising, but should be checked in the C. reinhardtii
mutant in vivo.
Another barrier for the industrial H2 photoproduction system involves the redirection of photosynthetic
electron flow from linear to cyclic and production of
ATP, which results in a nonproductive pathway and
decreased H2 production under anaerobic conditions.
In green algae, this process also involves phosphorylation and dissociation of PSII-light-harvesting antenna
and results in the so-called state 1 to state 2 transitions
that lead to higher excitation of PSI over PSII. A promising approach for prolongation of H2 production in
algae has recently been proposed by Kruse et al.
(2005). They generated the mutants affected in state transition. These mutants are blocked in state 1 that inhibits
cyclic electron flow around PSI. One of these mutants,
stm6, accumulated larger starch reserves under sulfur
deprivation and produced almost five times more H2
gas than the wild type (Table 21.1).
H2 photoproduction in green algae is driven by the
bidirectional [FeeFe]-hydrogenase enzyme that catalyzes not only the forward (H2 photoproduction) but
also the reverse (H2 uptake) reaction. Under high H2
partial pressure in the photobioreactor, the rate of
reverse reaction is significant (Kosourov et al., 2012).
The authors showed that the decrease in H2 partial pressure improves significantly the yields and rates of H2
photoproduction in algal cultures. They also suggested
the existence in sulfur-deprived algae of H2-uptaking
pathways, either photoreduction or oxy-hydrogen reaction. The possibility of photoreduction in nutrientdeprived algae is questionable because of the significant
degradation of the Rubisco enzyme by the time H2
photoproduction begins (Zhang et al., 2002). Therefore,
it is most likely that nutrient-deprived algae utilize H2
gas through the indirect oxy-hydrogen reaction
involving the chlororespiration pathway from [FeeFe]hydrogenase(s) to O2 through Fd, NADPþ/NADPH
and the PQ-pool. If H2-uptaking pathway(s) does exist,
H2 photoproduction in green algae can be further
improved by downregulating this pathway(s).
Acknowledgments
This work was financially supported by the Academy of Finland
Center of Excellence project (118637) and by the Kone foundation (YA,
SNK).
References
Akkerman, I., Janssen, M., Rocha, J., Wijffels, R.H., 2002. Photobiological hydrogen production: photochemical efficiency and bioreactor design. Int. J. Hydrogen Energy 27, 1195e1208.
Allahverdiyeva, Y., Leino, H., Saari, L., Fewer, D., Shunmugam, S.,
Sivonen, K., Aro, E.M., 2010. Screening for biohydrogen production by cyanobacteria isolated from the Baltic Sea and Finnish
lakes. Int. J. Hydrogen Energy 35, 1117e1127.
REFERENCES
Allahverdiyeva, Y., Ermakova, M., Eisenhut, M., Zhang, P.,
Richaud, P., Hagemann, M., Cournac, L., Aro, E.M., 2011. Interplay
between flavodiiron proteins and photorespiration in Synechocystis
sp. PCC 6803. J. Biol. Chem. 286, 24007e24014.
Allahverdiyeva, Y., Mustila, H., Ermakova, M., Bersanini, L.,
Richaud, P., Ajlani, G., Battchikova, N., Cournac, L., Aro, E.M.,
2012. Flavodiiron proteins Flv1 and Flv3 enable cyanobacterial
growth and photosynthesis under fluctuating light. Proc. Natl.
Acad. Sci. USA 110, 4111e4116.
Antal, T.K., Krendeleva, T.E., Laurinavichene, T.V., Makarova, V.V.,
Ghirardi, M.L., Rubin, A.B., Tsygankov, A.A., Seibert, M., 2003. The
dependence of algal H2 production on photosystem II and O2
consumption activities in sulfur-deprived Chlamydomonas
reinhardtii cells. Biochim. Biophys. Acta 1607, 153e160.
Antal, T.K., Volgusheva, A.A., Kukarskih, G.P., Krendeleva, T.E.,
Rubin, A.B., 2009. Relationships between H2 photoproduction
and different electron transport pathways in sulfur-deprived
Chlamydomonas reinhardtii. Int. J. Hydrogen Energy 34, 9087e9094.
Aparicio, P.J., Azuara, M.P., Ballesteros, A., Fernandez, V.M., 1985. Effects of light intensity and oxidized nitrogen sources on hydrogen
production by Chlamydomonas reinhardii. Plant Physiol. 78, 803e806.
Appel, J., Schulz, R., 1996. Sequence analysis of an operon of a
NAD(P)-reducing nickel hydrogenase from the cyanobacterium
Synechocystis sp. PCC 6803 gives additional evidence for direct
coupling of the enzyme to NAD(P)H-dehydrogenase (complex I).
Biochim. Biophys. Acta 1298, 141e147.
Appel, J., Schulz, R., 1998. Hydrogen metabolism in organisms with
oxygenic photosynthesis: hydrogenases as important regulatory
devices for a proper redox poising? J. Photochem. Photobiol. Biol.
47, 1e11.
Appel, J., Phunpruch, S., Steinmuller, K., Schulz, R., 2000. The bidirectional hydrogenase of Synechocystis sp. PCC 6803 works as an electron valve during photosynthesis. Arch. Microbiol. 173, 333e338.
Asada, Y., Koike, Y., Schnackenberg, J., Miyake, M., Uemura, I.,
Miyake, J., 2000. Heterologous expression of clostridial hydrogenase in the Cyanobacterium synechococcus PCC7942. Biochim.
Biophys. Acta 1490, 269e278.
Aubert-Jousset, E., Cano, M., Guedeney, G., Richaud, P., Cournac, L.,
2011. Role of HoxE subunit in Synechocystis PCC 6803 hydrogenase.
FEBS J. 278, 4035e4043.
Bagai, R., Madamwar, D., 1998. Prolonged evolution of photohydrogen by intermittent supply of nitrogen using a combined
system of Phormidium valderianum, Halobacterium halobium, and
Escherichia coli. Int. J. Hydrogen Energy 23, 545e550.
Bandyopadhyay, A., Stöckel, J., Min, H., Sherman, L.A., Pakrasi, H.B.,
2010. High rates of photobiological H2 production by a cyanobacterium under aerobic conditions. Nat. Commun. 1, 139.
Batyrova, K.A., Tsygankov, A.A., Kosourov, S.N., 2012. Sustained
hydrogen photoproduction by phosphorus-deprived Chlamydomonas reinhardtii cultures. Int. J. Hydrogen Energy 37, 8834e8839.
Ben-Amotz, A., Erbes, D.L., Riederer-Henderson, M.A., Peavey, D.G.,
Gibbs, M., 1975. H2 metabolism in photosynthetic organisms: I.
dark H2 evolution and uptake by algae and mosses. Plant Physiol.
56, 72e77.
Benemann, J.R., 1994. Feasibility analysis of photobiological hydrogen
production. In: Block, D.L., Versiroglu, T.N. (Eds.), Hydrogen
Energy Progress X, Proceedings of Tenth World Hydrogen Energy
Conference, Cocoa Beach, Florida, pp. 931e940.
Benemann, J.R., 1996. Hydrogen biotechnology: progress and prospects. Nat. Biotech. 14, 1101e1103.
Benemann, J.R., Weare, N.M., 1974. Hydrogen evolution by nitrogenfixing Anabaena cylindrica cultures. Science 184, 174e175.
Berchtold, M., Bachofen, R., 1979. Hydrogen formation by cyanobacteria cultures selected for nitrogen fixation. Arch. Microbiol. 123,
227e232.
383
Berman-Frank, I., Lundgren, P., Chen, Y.B., Küpper, H., Kolber, Z.,
Bergman, B., Falkowski, P., 2001. Segregation of nitrogen fixation
and oxygenic photosynthesis in the marine cyanobacterium Trichodesmium. Science 294, 1534e1537.
Blake, D.M., Amos, W.A., Ghirardi, M.L., Seibert, M., 2008. Chapter 5:
materials requirements for photobiological hydrogen production.
In: Jones, R.H., Thomas, G.J. (Eds.), Materials for the Hydrogen
Economy. CRC Press, Boca Raton, FL, pp. 123e145.
Blankenship, R.E., Tiede, D.M., Barber, J., Brudvig, G.W., Fleming, G.,
Ghirardi, M., Gunner, M.R., Junge, W., Kramer, D.M., Melis, A.,
Moore, T.A., Moser, C.C., Nocera, D.G., Nozik, A.J., Ort, D.R.,
Parson, W.W., Prince, R.C., Sayre, R.T., 2011. Comparing
photosynthetic and photovoltaic efficiencies and recognizing the
potential for improvement. Science 332, 805e809.
Boichenko, V.A., Hoffmann, P., 1994. Photosynthetic hydrogen
production in prokaryotes and eukaryotes e occurrence, mechanisms and functions. Photosynthetica. 30, 527e552.
Boichenko, V.A., Arkhipov, V.N., Litvin, F.F., 1983. Simultaneous measurements of fluorescence induction and hydrogen evolution of
Chlorella under anaerobic conditions. Biophys. (Russ). 28, 976e979.
Boichenko, V.A., Satina, L.Y., Litvin, F.F., 1989. Efficiency of hydrogen
photoproduction in algae and cyanobacteria. Fiziol. Rast. 36,
239e247.
Boichenko, V.A., Greenbaum, E., Seibert, M., 2004. Hydrogen
production by photosynthetic microorganisms. In: Archer, M.D.,
Barber, J. (Eds.), Photoconversion of Solar Energy: Molecular to
Global Photosynthesis. Imperial College Press, London,
pp. 397e452.
Boison, G., Bothe, H., Hansel, A., Lindblad, P., 1999. Evidence against
a common use of the diaphorase subunits by the bidirectional
hydrogenase and by the respiratory complex I in cyanobacteria.
FEMS Microbiol. Lett. 174, 159e165.
Boison, G., Mergel, A., Jolkver, H., Bothe, H., 2004. Bacterial life and
dinitrogen fixation at a gypsum rock. Appl. Environ. Microbiol. 70,
7070e7077.
Bolton, J.R., 1996. Solar photoproduction of hydrogen. In: Report to
the International Energy Agency, under agreement on the production and utilization of hydrogen. IEA/H2/TR-96. pp. 1e48.
Bothe, H., Schmitz, O., Yates, M.G., Newton, W.E., 2010. Nitrogen
fixation and hydrogen metabolism in cyanobacteria. Microbiol.
Mol. Biol. Rev. 74, 529e554.
Brand, J.J., Wright, J., Lien, S., 1989. Hydrogen production by
eukaryotic algae. Biotechnol. Bioengy 33, 1482e1488.
Buikema, W.J., Haselkorn, R., 2001. Expression of the Anabaena hetR
gene from a copper-regulated promoter leads to heterocyst
differentiation under repressing conditions. Proc. Natl. Acad. Sci.
USA 98, 2729e2734.
Burgess, B.K., Lowe, D.J., 1996. Mechanism of molybdenum nitrogenase. Chem. Rev. 96, 2983e3011.
Campbell, W.J., Ogren, W.L., 1990. Electron transport through photosystem I stimulates light activation of ribulose bisphosphate
carboxylase/oxygenase (rubisco) by rubisco activase. Plant Physiol. 94, 479e484.
Camsund, D., Devine, E., Holmqvist, M., Yohanoun, P., Lindblad, P.,
Stensjö, K., 2011. A HupS-GFP fusion protein demonstrates a
heterocyst-specific localization of the uptake hydrogenase in
Nostoc punctiforme. FEMS Microbiol. Lett. 316, 152e159.
Cao, H., Zhang, L., Melis, A., 2001. Bioenergetic and metabolic
processes for the survival of sulfur-deprived Dunaliella salina
(Chlorophyta). J. Appl. Phycol. 13, 25e34.
Chang, C.H., King, P.W., Ghirardi, M.L., Kim, K., 2007. Atomic resolution modeling of the ferredoxin:[FeFe] hydrogenase complex
from Chlamydomonas reinhardtii. Biophys. J. 93, 3034e3045.
Chochois, V., Dauvillee, D., Beyly, A., Tolleter, D., Cuine, S.,
Timpano, H., Ball, S., Cournac, L., Peltier, G., 2009. Hydrogen
384
21. RECENT DEVELOPMENTS ON CYANOBACTERIA AND GREEN ALGAE FOR BIOHYDROGEN PHOTOPRODUCTION
production in Chlamydomonas: PSII-dependent and independent
pathways differ in their requirement on starch metabolism. Plant
Physiol. 151, 613e640.
Cohen, J., Kim, K., Posewitz, M., Ghirardi, M.L., Schulten, K.,
Seibert, M., King, P., 2005. Molecular dynamics and experimental
investigation of H(2) and O(2) diffusion in [Fe]- hydrogenase.
Biochem. Soc. Trans. 33, 80e82.
Compaore, J., Stal, L.J., 2010. Oxygen and the light-dark cycle of
nitrogenase activity in two unicellular cyanobacteria. Environ.
Microbiol. 12, 54e62.
Cournac, L., Guedeney, G., Peltier, G., Vignais, P.M., 2004. Sustained
photoevolution of molecular hydrogen in a mutant of Synechocystis
sp strain PCC 6803 deficient in the type I NADPH-dehydrogenase
complex. J. Bacteriol. 186, 1737e1746.
Desplats, C., Mus, F., Cuine, S., Billon, E., Cournac, L., Peltier, G., 2008.
Characterization of Nda2, a plastoquinone-reducing type II
NAD(P)H dehydrogenase in Chlamydomonas chloroplasts. J. Biol.
Chem. 284, 4148e4157.
Dietz, K.J., Heilos, L., 1990. Carbon metabolism in spinach leaves as
affected by leaf age and phosphorus and sulfur nutrition. Plant
Physiol. 93, 1219e1225.
Eady, R.R., 1996. Structurefunction relationships of alternative nitrogenases. Chem. Rev. 96, 3013e3030.
Ekman, M., Ow, S.Y., Holmqvist, M., Zhang, X., van Wagenen, J.,
Wright, P.C., Stensjo, K., 2011. Metabolic adaptations in a H2 producing heterocyst-forming cyanobacterium: potentials and implications for biological engineering. J. Proteome Res. 10, 1772e1784.
Fedorov, A.S., Kosourov, S., Ghirardi, M.L., Seibert, M., 2005.
Continuous hydrogen photoproduction by Chlamydomonas
reinhardtii: using a novel two-stage, sulfate-limited chemostat
system. Appl. Biochem. Biotechnol. 121-124, 403e412.
Ferreira, R.M.B., Teixeira, A.R.N., 1992. Sulfur starvation in Lemna
leads to degradation of ribulose-bisphosphate carboxylase without
plant death. J. Biol. Chem. 267, 7253e7257.
Florin, L., Tsokoglou, A., Happe, T., 2001. A novel type of iron
hydrogenase in the green alga Scenedesmus obliquus is linked to the
photosynthetic electron transport chain. J. Biol. Chem. 276,
6125e6132.
Forestier, M., King, P., Zhang, L., Posewitz, M., Schwarzer, S.,
Happe, T., Ghirardi, M.L., Seibert, M., 2003. Expression of two [Fe]hydrogenases in Chlamydomonas reinhardtii under anaerobic conditions. Eur. J. Biochem. 270, 2750e2758.
Fouchard, S., Hemschemeier, A., Caruana, A., Pruvost, J., Legrand, J.,
Happe, T., Peltier, G., Cournac, L., 2005. Autotrophic and
mixotrophic hydrogen photoproduction in sulfur-deprived
Chlamydomonas cells. Appl. Environ. Microbiol. 71, 6199e6205.
Frey, M., 2002. Hydrogenases: hydrogen-activating enzymes. Chembiochem 3, 153e160.
Gaffron, H., Rubin, J., 1942. Fermentative and photochemical production of hydrogen in algae. J. Gen. Physiol. 26, 219e240.
Gfeller, R.P., Gibbs, M., 1984. Fermentative metabolism of Chlamydomonas reinhardtii, I: analysis of fermentative products from starch
in dark and light. Plant Physiol. 75, 212e218.
Ghirardi, M.L., 2006. Hydrogen production by photosynthetic green
algae. Ind. J. Biochem. Biophys. 43, 201e210.
Ghirardi, M., Mohanty, P., 2010. Oxygenic hydrogen photoproductionecurrent status of the technology. Curr. Sci. 98,
499e507.
Ghirardi, M.L., Togasaki, R.K., Seibert, M., 1997. Oxygen sensitivity of
algal hydrogen production. Appl. Biochem. Biotechnol. 63, 141e151.
Ghirardi, M.L., Zhang, L., Lee, J.W., Flynn, T., Seibert, M.,
Greenbaum, E., Melis, A., 2000. Microalgae: a green source of
renewable H2. Trends Biotechnol. 18, 506e511.
Giannelli, L., Scoma, A., Torzillo, G., 2009. Interplay between light
intensity, chlorophyll concentration and culture mixing on the
hydrogen production in sulfur-deprived Chlamydomonas reinhardtii
cultures grown in laboratory photobioreactors. Biotechnol.
Bioengy 104, 76e90.
Gibbs, M., Gfeller, R.P., Chen, C., 1986. Fermentative metabolism of
Chlamydomonas reinhardtii. III: photoassimilation of acetate. Plant
Physiol. 82, 160e166.
Godman, J.E., Molnar, A., Baulcombe, D.C., Balk, J., 2010. RNA
silencing of hydrogenase(-like) genes and investigation of their
physiological roles in the green alga Chlamydomonas reinhardtii.
Biochem. J. 431, 345e351.
Greenbaum, E., 1982. Photosynthetic hydrogen and oxygen production: kinetic studies. Science 196, 879e880.
Greenbaum, E., 1988. Energetic efficiency of hydrogen photoevolution
by algal water splitting. Biophys. J. 54, 365e368.
Greenbaum, E., Blankinship, S.L., Lee, J.W., Ford, R.M., 2001. Solar photobiochemistry: simultaneous photoproduction of hydrogen and
oxygen in a confined bioreactor. J. Phys. Chem. B. 105, 3605e3609.
Grimme, R.A., Lubner, C.E., Bryant, D.A., Golbeck, J.H., 2008.
Photosystem I/molecular wire/metal nanoparticle bioconjugates
for the photocatalytic production of H2. J. Am. Chem. Soc. 130,
6308e6309.
Grimme, R.A., Lubner, C.E., Golbeck, J.H., 2009. Maximizing H2
production in photosystem I/dithiol molecular wire/platinum
nanoparticle bioconjugates. Dalton Trans. 10106e10113.
Grossman, A., 2000. Acclimation of Chlamydomonas reinhardtii to its
nutrient environment. Protist 151, 201e224.
Guan, Y.F., Deng, M.C., Yu, X.J., Zhang, W., 2004. Two-stage photobiological production of hydrogen by marine green alga Platymonas subcordiformis. Biochem. Eng. J. 19, 69e73.
Gutthann, F., Egert, M., Marques, A., Appel, J., 2007. Inhibition of
respiration and nitrate assimilation enhances photohydrogen
evolution under low oxygen concentrations in Synechocystis sp.
PCC 6803. Biochim. Biophys. Acta 1767, 161e169.
Gärtner, K., Lechno-Yossef, S., Cornish, A.J., Wolk, P.C., Hegg, E.L.,
2012. Expression of Shewanella oneidensis MR-1 [FeFe]-Hydrogenase
genes in Anabaena sp. Strain PCC 7120. Appl. Environ. Microbiol.
78, 8579e8586.
Hadfield, K.L., Bulen, W.A., 1969. Adenosine triphosphate requirement of nitrogenase from Azotobacter vinelandii. Biochem. 8,
5103e5108.
Happe, T., Kaminski, A., 2002. Differential regulation of the
Fe-hydrogenase during anaerobic adaptation in the green alga
Chlamydomonas reinhardtii. Eur. J. Biochem. 269, 1022e1032.
Happe, T., Naber, J.D., 1993. Isolation, characterization and N-terminal
amino acid sequence of hydrogenase from the green alga
Chlamydomonas reinhardtii. Eur. J. Biochem. 214, 475e481.
Happe, T., Schutz, K., Bohme, H., 2000. Transcriptional and mutational
analysis of the uptake hydrogenase of the filamentous cyanobacterium Anabaena variabilis ATCC 29413. J. Bacteriol. 182, 1624e1631.
Healey, F.P., 1970. The mechanism of hydrogen evolution by
Chlamydomonas moewusii. Plant Physiol. 45, 153e159.
Helman, Y., Tchernov, D., Reinhold, L., Shibata, M., Ogawa, T.,
Schwarz, R., Ohad, I., Kaplan, A., 2003. Genes encoding a-type
flavoproteins are essential for photoreduction of O2 in cyanobacteria. Curr. Biol. 13, 230e235.
Hemschemeier, A., Fouchard, S., Cournac, L., Peltier, G., Happe, T.,
2008. Hydrogen production by Chlamydomonas reinhardtii: an elaborate interplay of electron sources and sinks. Planta 227, 397e407.
Hemschemeier, A., Happe, T., 2011. Alternative photosynthetic
electron transport pathways during anaerobiosis in the green
alga Chlamydomonas reinhardtii. Biochim. Biophys. Acta 1807,
919e926.
Houchins, J.P., 1984. The physiology and biochemistry of hydrogen
metabolism in cyanobacteria. Biochim. Biophys. Acta 768,
227e255.
REFERENCES
Ihara, M., Nishihara, H., Yoon, K., Lenz, O., Friedrich, B.,
Nakamoto, H., Kojima, K., Honma, D., Kamachi, T., Okura, I.,
2006a. Light-driven hydrogen production by a hybrid complex of a
[NiFe]-hydrogenase and the cyanobacterial photosystem I. Photochem. Photobiol. 82, 676e682.
Ihara, M., Nakamoto, H., Kamachi, T., Okura, I., Maedal, M., 2006b.
Photoinduced hydrogen production by direct electron transfer
from photosystem I cross-linked with cytochrome c3 to [NiFe]hydrogenase. Photochem. Photobiol. 82, 1677e1685.
James, B.D., Baum, G.N., Perez, J., Baum, K.N., 2009. Technoeconomic
Boundary Analysis of Biological Pathways to Hydrogen Production. Report No. SR-560-46674. NREL. p. 207.
Jans, F., Mignolet, E., Houyoux, P.A., Cardol, P., Ghysels, B., Cuine, S.,
Cournac, L., Peltier, G., Remacle, C., Franck, F., 2008. A type II
NAD(P)H dehydrogenase mediates light-independent plastoquinone reduction in the chloroplast of Chlamydomonas. Proc. Natl.
Acad. Sci. USA 105, 20546e20551.
Kentemich, T., Danneberg, G., Hundeshagen, B., Bothe, H., 1988. Evidence for the occurrence of the alternative, vanadium-containing
nitrogenase in the cyanobacterium Anabaena variabilis. FEMS
Microbiol. Lett. 51, 19e24.
Khetkorn, W., Lindblad, P., Incharoensakdi, A., 2012. Inactivation of
uptake hydrogenase leads to enhanced and sustained hydrogen
production with high nitrogenase activity under high light exposure in the cyanobacterium Anabaena siamensis TISTR 8012. J. Biol.
Eng. 6, 19.
Kim, J.P., Kim, K.-R., Choi, S.P., Han, S.J., Kim, M.S., Sim, S.J., 2010.
Repeated production of hydrogen by sulfate re-addition in sulfur
deprived culture of Chlamydomonas reinhardtii. Int. J. Hydrogen
Energy 35, 13387e13391.
Kolber, Z., Zehr, Z., Falkowski, P., 1988. Effects of growth irradiance
and nitrogen limitation on photosynthetic energy conversion in
photosystem II. Plant Physiol. 88, 923e929.
Kondratieva, E., Gogotov, I., 1983. Production of molecular hydrogen
in microorganisms. In: Advances in Biochemical Engineering/
Biotechnology. Microbial Activities, vol. 23. Springer-Verlag,
Berlin, Heidelberg, New York, Tokyo, pp. 139e191.
Kosourov, S.N., Seibert, M., 2009. Hydrogen photoproduction by
nutrient-deprived Chlamydomonas reinhardtii cells immobilized
within thin alginate films under aerobic and anaerobic conditions.
Biotechnol. Bioengy 102, 50e58.
Kosourov, S., Tsygankov, A., Seibert, M., Ghirardi, M.L., 2002. Sustained hydrogen photoproduction by Chlamydomonas reinhardtii:
effects of culture parameters. Biotechnol. Bioeng. 78, 731e740.
Kosourov, S., Seibert, M., Ghirardi, M.L., 2003. Effects of extracelular
pH on the metabolic pathways of sulfur-deprived, H2-producing
Chlamydomonas reinhardtii cultures. Plant Cell Physiol. 44,
146e155.
Kosourov, S., Patrusheva, E., Ghirardi, M.L., Seibert, M.,
Tsygankov, A., 2007. A comparison of hydrogen photoproduction
by sulfur-deprived Chlamydomonas reinhardtii under different
growth conditions. J. Biotechnol. 128, 776e787.
Kosourov, S.N., Ghirardi, M.L., Seibert, M., 2011. A truncated antenna mutant of Chlamydomonas reinhardtii can produce more
hydrogen than the parental strain. Int. J. Hydrogen Energy 36,
2044e2048.
Kosourov, S.N., Batyrova, K.A., Petushkova, E.P., Tsygankov, A.A.,
Ghirardi, M.L., Seibert, M., 2012. Maximizing the hydrogen
photoproduction yields in Chlamydomonas reinhardtii cultures: the
effect of the H2 partial pressure. Int. J. Hydrogen Energy 37,
8850e8858.
Krassen, H., Stripp, S., von Abendroth, G., Ataka, K., Happe, T.,
Heberle, J., 2009. Immobilization of the [FeFe]-hydrogenase
CrHydA1 on a gold electrode: design of a catalytic surface for
the production of molecular hydrogen. J. Biotechnol. 142, 3e9.
385
Kreuzberg, K., 1984. Starch fermentation via a formate-producing
pathway in Chlamydomonas reinhardtii, Chlorogonium elongatum
and Chlorella fusca. Physiol. Plant 61, 87e94.
Kruse, O., Rupprecht, J., Bader, K.P., Thomas-Hall, S., Schenk, P.M.,
Finazzi, G., Hankamer, B., 2005. Improved photobiological H2
production in engineered green algal cells. J. Biol. Chem. 280,
34170e34177.
Lambert, G.R., Smith, G.D., 1977. Hydrogen formation by marine
blue-green algae. FEBS Lett. 83, 159e162.
Laurinavichene, T., Tolstygina, I., Tsygankov, A., 2004. The effect of
light intensity on hydrogen production by sulfur-deprived Chlamydomonas reinhardtii. J. Biotechnol. 114, 143e151.
Laurinavichene, T.V., Fedorov, A.S., Ghirardi, M.L., Seibert, M.,
Tsygankov, A.A., 2006. Demonstration of sustained hydrogen
photoproduction by immobilized, sulfur-deprived Chlamydomonas
reinhardtii cells. Int. J. Hydrogen Energy 31, 659e667.
Laurinavichene, T.V., Kosourov, S.N., Ghirardi, M.L., Seibert, M.,
Tsygankov, A.A., 2008. Prolongation of H2 photoproduction by
immobilized, sulfur limited Chlamydomonas reinhardtii cultures.
J. Biotechnol. 134, 275e277.
Lecler, R., Godaux, D., Vigeolas, H., Hiligsmann, S., Thonart, P.,
Franck, F., Cardol, P., Remacle, C., 2011. Functional analysis of
hydrogen photoproduction in respiratory-deficient mutants of
Chlamydomonas reinhardtii. Int. J. Hydrogen Energy 36,
9562e9570.
Lee, C.C., Hu, Y., Ribbe, M.W., 2009. Unique features of the nitrogenase VFe protein from Azotobacter vinelandii. Proc. Natl. Acad. Sci.
USA 106, 9209e9214.
Leino, H., Kosourov, S.N., Saari, L., Sivonen, K., Tsygankov, A.A.,
Aro, E.M., Allahverdiyeva, Y., 2012. Extended H2 photoproduction
by N2-fixing cyanobacteria immobilized in thin alginate films. Int.
J. Hydrogen Energy 37, 151e161.
Lindberg, P., Schutz, K., Happe, T., Lindblad, P., 2002. A hydrogenproducing, hydrogenase-free mutant strain of Nostoc punctiforme
ATCC 29133. Int. J. Hydrogen Energy 27, 1291e1296.
Long, H., Chang, C.H., King, P.W., Ghirardi, M.L., Kim, K., 2008.
Brownian dynamics and molecular dynamics study of the association between hydrogenase and ferredoxin from Chlamydomonas
reinhardtii. Biophys. J. 95, 3753e3766.
Lopez-Igual, R., Flores, E., Herrero, A., 2010. Inactivation of a heterocystspecific invertase indicates a principal role of sucrose catabolism in
heterocysts of Anabaena sp. J. Bacteriol. 192, 5526e5533.
Ludwig, M., Schulz-Friedrich, R., Appel, J., 2006. Occurrence of hydrogenases in cyanobacteria and anoxygenic photosynthetic bacteria: implications for the phylogenetic origin of cyanobacterial
and algal hydrogenases. J. Mol. Evol. 63, 758e768.
Makarova, V.V., Kosourov, S.N., Krendeleva, T.E., Kukarskikh, G.P.,
Ghirardi, M.L., Seibert, M., Rubin, A.B., 2005. Photochemical
activity of photosystem II and hydrogen photoproduction in
sulfur-deprived Chlamydomonas reinhardtii mutants D1-R323D and
D1-R323L. Biofizika 50, 1070e1078.
Makarova, V.V., Kosourov, S., Krendeleva, T.E., Semin, B.K.,
Kukarskikh, G.P., Rubin, A.B., Sayre, R.T., Ghirardi, M.L.,
Seibert, M., 2007. Photoproduction of hydrogen by sulfur-deprived
Chlamydomonas reinhardtii mutants with impaired photosystem II
photochemical activity. Photosynth. Research. 94, 79e89.
Masukawa, H., Mochimaru, M., Sakurai, H., 2002. Disruption of the
uptake hydrogenase gene, but not of the bi-directional hydrogenase gene, leads to enhanced photobiological hydrogen production
by the nitrogen-fixing cyanobacterium Anabaena sp. PCC 7120.
Appl. Microbiol. Biotechnol. 58, 618e624.
Masukawa, H., Inoue, K., Sakurai, H., 2007. Effects of disruption of
homocitrate synthase genes on Nostoc sp. strain PCC 7120 photobiological hydrogen production and nitrogenase. Appl. Environ.
Microbiol. 73, 7562e7570.
386
21. RECENT DEVELOPMENTS ON CYANOBACTERIA AND GREEN ALGAE FOR BIOHYDROGEN PHOTOPRODUCTION
Masukawa, H., Zhang, X., Yamazaki, E., Iwata, S., Nakamura, K.,
Mochimaru, M., Inoue, K., Sakurai, H., 2009. Survey of the
distribution of different types of nitrogenases and hydrogenases
in heterocyst-forming cyanobacteria. Mar. Biotechnol. 11,
397e409.
Masukawa, H., Inoue, K., Sakurai, H., Wolk, C.P., Hausinger, R.P.,
2010. Site-directed mutagenesis of the Anabaena sp. strain PCC 7120
nitrogenase active site to increase photobiological hydrogen production. Appl. Environ. Microbiol. 76, 6741e6750.
Mayer, S.M., Gormal, C.A., Smith, B.E., Lawson, D.M., 2002. Crystallographic analysis of the MoFe protein of nitrogenase from a nifV
mutant of Klebsiella pneumoniae identifies citrate as a ligand to the
molybdenum of iron molybdenum cofactor (FeMoco). J. Biol.
Chem. 277, 35263e35266.
McIntosh, C.L., Germer, F., Schulz, R., Appel, J., Jones, A.K., 2011. The
[NiFe]-hydrogenase of the cyanobacterium Synechocystis sp. PCC
6803 works bidirectionally with a bias to H2 production. J. Am.
Chem. Soc. 133, 11308e11319.
McNeely, K., Xu, Y., Ananyev, G., Bennette, N., Bryant, D.A.,
Dismukes, G.C., 2011. Synechococcus sp. strain PCC 7002 nifJ
mutant lacking pyruvate: ferredoxin oxidoreductase. Appl.
Environ. Microbiol. 77, 2435e2444.
Melis, A., Chen, H.C., 2005. Chloroplast sulfate transport in green
algae: genes, proteins and effects. Photosynth. Res. 86, 299e307.
Melis, A., Zhang, L., Forestier, M., Ghirardi, M.L., Seibert, M., 2000.
Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiol. 122, 127e136.
Melnicki, M.R., Pinchuk, G.E., Hill, E.A., Kucek, L.A.,
Fredrickson, J.K., Konopka, A., Beliaev, A.S., 2012. Sustained H-2
production driven by photosynthetic water splitting in a unicellular cyanobacterium. mBio 3, e00197.
Meunier, C.F., Yang, X.-Y., Rooke, J.C., Su, B.-L., 2011. Biofuel cells
based on the immobilization of photosynthetically active bioentities. Chem. Cat. Chem. 3, 476e488.
Meuser, J.E., Ananyev, G., Wittig, L.E., Kosourov, S., Ghirardi, M.L.,
Seibert, M., Dismukes, G.C., Posewitz, M.C., 2009. Phenotypic diversity of hydrogen production in chlorophycean algae reflects
distinct anaerobic metabolisms. J. Biotechnol. 142, 21e30.
Meuser, J.E., Boyd, E.S., Ananyev, G., Karns, D., Radakovits, R.,
Murthy, U.M.N., Ghirardi, M.L., Dismukes, G.C., Peters, J.W.,
Posewitz, M.C., 2011. Evolutionary significance of an algal gene
encoding an [FeFe]-hydrogenase with F-domain homology and
hydrogenase activity in Chlorella variabilis NC64A. Planta. 234,
829e843.
Meuser, J.E., D’Adamo, S., Jinkerson, R.E., Mus, F., Yang, W.,
Ghirardi, M.L., Seibert, M., Grossman, A.R., Posewitz, M.C., 2012.
Genetic disruption of both Chlamydomonas reinhardtii [FeFe]hydrogenases: Insight into the role of HYDA2 in H2 production.
Biochem. Biophys. Res. Commun. 417, 704e709.
Min, H.T., Sherman, L.A., 2010. Genetic transformation and mutagenesis via single-stranded DNA in the unicellular, diazotrophic
cyanobacteria of the genus Cyanothece. Appl. Environ. Microbiol.
76, 7641e7645.
Muro-Pastor, A.M., Hess, W., 2012. Heterocyst differentiation: from
single mutants to global approaches. Trends Microbiol. 20,
548e557.
Nagy, L., Meuser, J., Plummer, S., Seibert, M., Ghirardi, M., King, P.,
Ahmann, D., Posewitz, M., 2007. Application of gene-shuffling
for the rapid generation of novel [FeFe]-hydrogenase libraries.
Biotechnol. Lett. 29, 421e430.
Ni, C.V., Yakuninin, A.F., Gogotov, I.N., 1990. Influence of molybdenum, vanadium, and tungsten on growth and nitrogenase
synthesis of the free-living cyanobacterium Anabaena azollae.
Microbiology 59, 395e398.
Oliveira, P., Lindblad, P., 2009. Transcriptional regulation of the cyanobacterial bidirectional Hox-hydrogenase. Dalton Trans. 45,
9990e9996.
Phelps, A.S., Wilson, P.W., 1942. Occurrence of hydrogenase in
nitrogen-fixing organisms. Proc. Soc. Exp. Biol. Med. 47, 473e476.
Philipps, G., Happe, T., Hemschemeier, A., 2012. Nitrogen deprivation
results in photosynthetic hydrogen production in Chlamydomonas
reinhardtii. Planta 235, 729e745.
Phlips, E.J., Mitsui, A., 1986. Characterization and optimization of
hydrogen production by a salt water blue-green algae Oscillatoria
sp. strain Miami BG 7. II Use of immobilization for enhancement of
hydrogen production. Int. J. Hydrogen Energy 11, 83e89.
Pickett, C.J., 1996. The Chatt cycle and the mechanism of enzymic
reduction of molecular nitrogen. J. Biol. Inorg. Chem. 1, 601e606.
Polle, J.E.W., Benemann, J.R., Tanaka, A., Melis, A., 2000. Photosynthetic apparatus organization and function in wild type and a Chl
b-less mutant of Chlamydomonas reinhardtii. Dependence on carbon
source. Planta 211, 335e344.
Polle, J.E.W., Kanakagiri, S., Jin, E., Masuda, T., Melis, A., 2002. Truncated chlorophyll antenna size of the photosystems e a practical
method to improve microalgal productivity and hydrogen
production in mass culture. Int. J. Hydrogen Energy 27, 1257e1264.
Polle, J.E.W., Kanakagiri, S., Melis, A., 2003. tla1, a DNA insertional
transformant of the green alga Chlamydomonas reinhardtii with a
truncated light-harvesting chlorophyll antenna size. Planta 217,
49e59.
Posewitz, M.C., Dubini, A., Meuser, J.E., Seibert, M., Ghirardi, M.L.,
2009. Hydrogenases, hydrogen production, and anoxia. In:
Stern, D.B. (Ed.), The Chlamydomonas Sourcebook, Second ed.,
vol. 2. Academic Press, Oxford, UK, pp. 217e255.
Prochnik, S.E., Umen, J., Nedelcu, A.M., Hallmann, A., Miller, S.M.,
Nishii, I., Ferris, P., Kuo, A., Mitros, T., Fritz-Laylin, L.K.,
Hellsten, U., Chapman, J., Simakov, O., Rensing, S.A., Terry, A.,
Pangilinan, J., Kapitonov, V., Jurka, J., Salamov, A., Shapiro, H.,
Schmutz, J., Grimwood, J., Lindquist, E., Lucas, S., Grigoriev, I.V.,
Schmitt, R., Kirk, D., Rokhsar, D.S., 2010. Genomic analysis of
organismal complexity in the multicellular green alga Volvox carteri. Science 329, 223e226.
Pröschold, T., Harris, E.H., Coleman, A.W., 2005. Portrait of a species:
Chlamydomonas reinhardtii. Genetics 170, 1601e1610.
Randt, C., Senger, H., 1985. Participation of the two photosystems in
light dependent hydrogen evolution in Scenedesmus obliquus. Photochem. Photobiol. 42, 553e557.
Raupach, M.R., Marland, G., Ciais, P., Le Quere, C., Canadell, J.G.,
Klepper, G., Field, C.B., 2007. Global and regional drivers of accelerating CO2 emissions. Proc. Natl. Acad. Sci. USA. 104, 10288e10293.
Risser, D.D., Callahan, S.M., 2009. Genetic and cytological evidence
that heterocyst patterning is regulated by inhibitor gradients
that promote activator decay. Proc. Natl. Acad. Sci. USA. 106,
19884e19888.
Roessler, P.G., Lien, S., 1984. Purification of hydrogenase from Chlamydomonas reinhardtii. Plant. Physiol 75, 705e709.
Rubio, L., Ludden, P., 2005. Maturation of nitrogenase: a biochemical
pazzle. J. Bacteriol. 187, 405e414.
Sarkar, S., Pandey, K.D., Kashyap, A.K., 1992. Hydrogen photoproduction by filamentous nonheterocystous cyanobacterium Plectonema boryanum and simultaneous release of ammonia. Int.
J. Hydrogen Energy 17, 689e694.
Sauer, J., Schreiber, U., Schmid, R., Volker, U., Forchhammer, K., 2001.
Nitrogen starvation-induced chlorosis in Synechococcus PCC 7942.
Low-level photosynthesis as a mechanism of long-term survival.
Plant Physiol. 126, 233e243.
Schrautemeier, B., Neveling, U., Schmitz, S., 1995. Distinct and
differentially regulated Mo-dependent nitrogen-fixing systems
evolved for heterocysts and vegetative cells of Anabaena variabilis
REFERENCES
ATCC 29413: characterization of the fdX1/2 gene regions as part of
the nif1/2 gene clusters. Mol. Microbiol. 18, 357e359.
Schutz, K., Happe, T., Troshina, O., Lindblad, P., Leitao, E., Oliveira, P.,
Tamagnini, P., 2004. Cyanobacterial H2 production e a comparative analysis. Planta 218, 350e359.
Scoma, A., Krawietz, D., Faraloni, C., Giannelli, L., Happe, T.,
Torzillo, G., 2012. Sustained H2 production in a Chlamydomonas
reinhardtii D1 protein mutant. J. Biotechnol. 157, 613e619.
Seabra, R., Santos, A., Pereira, S., Moradas-Ferreira, P., Tamagnini, P.,
2009. Immunolocalization of the uptake hydrogenase in the marine
cyanobacterium Lyngbya majuscula CCAP 1446/4 and two Nostoc
strains. FEMS Microbiol. Lett. 292, 57e62.
Skizim, N.J., Ananyev, G.M., Krishnan, A., Dismukes, G.C., 2012.
Metabolic pathways for photobiological hydrogen production by
nitrogenase-and hydrogenase-containing unicellular cyanobacteria
Cyanothece. J. Biol. Chem. 287, 2777e2786.
Skjånes, K., Knutsen, G., Kallqvist, T., Lindblad, P., 2008. H2 production from marine and freshwater species of green algae during
sulfur deprivation and considerations for bioreactor design. Int. J.
Hydrogen Energy 33, 511e521.
Skjånes, K., Pinto, F.L., Lindblad, P., 2010. Evidence for transcription
of three genes with characteristics of hydrogenases in the green
alga Chlamydomonas noctigama. Int. J. Hydrogen Energy 35,
1074e1088.
Stapleton, J.A., Swartz, J.R., 2010. A cell-free microtiter plate screen for
improved [FeFe] hydrogenases. PLoS ONE 5, e10554.
Steunou, A.S., Jensen, S.I., Brecht, E., Becraft, E.D., Bateson, M.M.,
Kilian, Q., Bhaya, D., Ward, D.M., Peters, J.W., Grossman, A.R.,
Kuhl, M., 2008. Regulation of nif gene expression and the energetics of N2 fixation over diel cycle in a hot spring microbial mat.
ISME J. 2, 364e378.
Stuart, T.S., Gaffron, H., 1972. The mechanism of hydrogen photoproduction by several algae. Planta. 106, 101e112.
Surzycki, R., Cournac, L., Peltier, G., Rochaix, J., 2007. Potential
for hydrogen production with inducible chloroplast gene
expression in Chlamydomonas. Proc. Natl. Acad. Sci. USA. 104,
17548e17553.
Tamagnini, P., Leita, E., Oliveira, P., Ferreira, D., Pinto, F., Harris, D.J.,
Heidorn, T., Lindblad, P., 2007. Cyanobacterial hydrogenases: diversity, regulation and applications. FEMS Microbiol. Rev. 31,
692e720.
Thiel, T., 1993. Characterization of genes for an alternative nitrogenase
in the cyanobacterium Anabaena variabilis. J. Bacteriol. 175,
6276e6286.
Thiel, T., Lyons, E.M., Erker, J., Ernst, A., 1995. A second nitrogenase in
vegetative cells of a heterocyst-forming cyanobacterium. Proc.
Natl. Acad. Sci. USA 92, 9358e9362.
Tolstygina, I.V., Antal, T.K., Kosourov, S.N., Krendeleva, T.E.,
Rubin, A.B., Tsygankov, A.A., 2009. Hydrogen production by
photoautotrophic sulfur-deprived Chlamydomonas reinhardtii pregrown and incubated under high light. Biotechnol. Bioengy 102,
1055e1061.
Torzillo, G., Pushparaj, B., Masojidek, J., Vonshak, A., 2003. Biological
constraints in algal biotechnology. Biotechnol. Bioprocess. Eng. 8,
338e348.
Torzillo, G., Scoma, A., Faraloni, C., Ena, A., Johanningmeier, U., 2009.
Increased hydrogen photoproduction by means of a sulfurdeprived Chlamydomonas reinhardtii D1 protein mutant. Int. J.
Hydrogen Energy 34, 4529e4536.
Tsygankov, A.A., 2012. Hydrogen production: light-driven
processes e green algae. In: Hallenbeck, P.C. (Ed.), Microbial
Technologies, Advanced Biofuels Production, pp. 29e51.
387
Tsygankov, A.A., Hirata, Y., Miyake, M., Asada, Y., Miyake, J., 1994.
Photobioreactor with photosynthetic bacteria immobilized on
porous-glass for hydrogen photoproduction. J. Ferment. Bioeng.
77, 575e578.
Tsygankov, A., Kosourov, S., Seibert, M., Ghirardi, M.L., 2002.
Hydrogen photoproduction under continuous illumination by
sulfur-deprived, synchronous Chlamydomonas reinhardtii cultures.
Int. J. Hydrogen Energy 27, 1239e1244.
Tsygankov, A.A., Kosourov, S.N., Tolstygina, I.V., Ghirardi, M.L.,
Seibert, M., 2006. Hydrogen production by sulfur-deprived Chlamydomonas reinhardtii under photoautotrophic conditions. Int. J.
Hydrogen Energy 31, 1574e1584.
Weyman, P.D., Pratte, B., Thiel, T., 2010. Hydrogen production in
nitrogenase mutants in Anabaena variabilis. FEMS Microbiol. Lett.
304, 55e61.
Winkler, M., Hemschemeier, A., Gotor, C., Melis, A., Happe, T., 2002.
[Fe]-hydrogenases in green algae: photo-fermentation and
hydrogen evolution under sulfur deprivation. Int. J. Hydrogen
Energy 27, 1431e1439.
Winkler, M., Maeurer, C., Hemschemeier, A., Happe, T., 2004. The
isolation of green algal strains with outstanding H2eproductivity.
In: Miyake, J., Igarashi, Y., Roegner, M. (Eds.), Biohydrogen III.
Elsevier Science, Oxford, pp. 103e115.
Winkler, M., Kuhlgert, S., Hippler, M., Happe, T., 2009. Characterization of the key step for light-driven hydrogen evolution in green
algae. J. Biol. Chem. 284, 36620e36627.
Wolk, C.P., Ernst, A., Elhai, J., 1994. In: Bryant, D.A. (Ed.), Heterocyst
Metabolism and Development. The Molecular Biology of Cyanobacteria. Kluwer, Dordrecht, pp. 769e823.
Wünschiers, R., Stangier, K., Senger, H., Schulz, R., 2001. Molecular
evidence for a Fe-hydrogenase in the green alga Scenedesmus obliquus. Curr. Microbiol. 42, 353e360.
Wykoff, D.D., Davies, J.P., Melis, A., Grossman, A.R., 1998. The regulation of photosynthetic electron transport during nutrient deprivation in Chlamydomonas reinhardtii. Plant Physiol. 117, 129e139.
Yacoby, I., Pochekailov, S., Toporik, H., Ghirardi, M.L., King, P.W.,
Zhang, S., 2011. Photosynthetic electron partitioning between
[FeFe]-hydrogenase
and
ferredoxin:NADPþ-oxidoreductase
(FNR) enzymes in vitro. Proc. Natl. Acad. Sci. USA 108,
9396e9401.
Yan, F., Chen, Z., Li, W., Cao, X., Xue, S., Zhang, W., 2011. Purification
and characterization of a hydrogenase from the marine green alga
Tetraselmis subcordiformis. Process Biochem. 46, 1212e1215.
Yeager, C.M., Milliken, C.E., Bagwell, C.E., Staples, L., Berseth, P.A.,
Sessions, H.T., 2011. Evaluation of experimental conditions that
influence hydrogen production among heterocystous cyanobacteria. Int. J. Hydrogen Energy 36, 7487e7499.
Yoshino, F., Ikeda, H., Masukawa, H., Sakurai, H., 2007. High photobiological hydrogen production activity of a Nostoc sp. PCC 7422
uptake hydrogenase-deficient mutant with high nitrogenase
activity. Mar. Biotechnol. 9, 101e112.
Zhang, L., Happe, T., Melis, A., 2002. Biochemical and morphological
characterization of sulfur-deprived and H2-producing Chlamydomonas reinhardtii (green alga). Planta. 214, 552e561.
Zhao, M.X., Jiang, Y.-L., He, Y.X., Chen, Y.F., Teng, Y.B., Chen, Y.,
Zhang, C.C., Zhou, C.Z., 2010. Structural basis for the allosteric
control of the global transcription factor NtcA by the nitrogen
starvation signal 2-oxoglutarate. Proc. Natl. Acad. Sci. USA 107,
12487e12492.
Zinn, T., Schnackenberg, J., Haak, D., Romer, S., Schulz, R., Senger, H.,
1994. Evidence for nickel in the soluble hydrogenase from the
unicellular green alga Scenedesmus obliquus. J. Biosci. 49, 33e38.
C H A P T E R
22
Engineered Cyanobacteria: Research
and Application in Bioenergy
Gustavo B. Leite, Patrick C. Hallenbeck*
Département de Microbiologie et Immunologie, Université de Montréal, Montréal, Québec, Canada
*Corresponding author email: patrick.hallenbeck@umontreal.ca
O U T L I N E
Introduction
389
Engineering Cyanobacteria
Strains, Tools and Methods
392
392
Cyanobacteria as a Production System for Biofuels:
393
Current Status
Hydrogen
393
Hydrogen Bioproduction
394
Hydrogen-Evolving Enzymes
394
Hydrogen Bioproduction
395
Ethanol
398
INTRODUCTION
Paleontological and geochemical data as well as
molecular analysis of the plastid genome point to a single
prokaryote as the origin of several groups of organisms
scattered throughout the tree of life, including the entire
kingdom of Plantae (Knoll, 2008; Yoon, 2004). A cyanobacterial ancestor is believed to be the only organism
ever to couple together two photosystems, harvesting
electrons from water to produce energy-rich molecules
such as adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide phosphate (NADPH)
(Knoll, 2008) (Figure 22.1). These molecules provide the
necessary chemical energy, protons and electrons for
cellular reactions and the synthesis of other molecules,
most importantly powering CO2 fixation through the
Calvin-Benson-Bassham cycle. This event is thought to
have happened between the mid-Archean and early Proterozoic eras (2000e3000 millions of years ago). The
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00022-X
Ethylene
Microbial Production of Ethylene
Bioproduction of Ethylene Using efe
Isoprene
Butyraldehyde and Butanol
Photosynthetic Production of Aliphatic Alcohols and
Alkanes
398
399
399
400
401
402
Conclusion and Outlook
403
References
403
atmosphere was poor in oxygen and rich in CO2, and
the oceans were rich in salts and minerals; perfect
conditions for the first algal blooms. The invention of
oxygenic photosynthesis conferred a great advantage to
this ancient cyanobacterium, starting widespread
speciation and changing the composition of the atmosphere through the oxidation of water into protons and
molecular oxygen (Figure 22.1). This was probably the
first universally relevant instance of primary production
and established a food chain by transforming inorganic
nutrients into organic molecules that could be used by
heterotrophic organisms (Knoll, 2008). The role of
primary producers, so important in fully establishing
life on earth, is still equally important today, when cyanobacteria are thought to be responsible for 25% of all
carbon dioxide fixation and together with eukaryotic
microalgae sustain most of oceanic life, fixing CO2 and
carrying out important steps in various biogeochemical
nutrient cycles (Field et al., 1998).
389
Copyright Ó 2014 Elsevier B.V. All rights reserved.
390
22. ENGINEERED CYANOBACTERIA: RESEARCH AND APPLICATION IN BIOENERGY
FIGURE 22.1 Scheme of light reactions in oxygenic photosynthesis. Photosystem II oxidizes a water molecule, harvesting the electron that
will be used to synthesize NADPH, and producing an electrochemical gradient through the release of protons (Hþ) that will be used by ATP
synthase to drive phosphorylation of ADP. The ATP and NADPH that are produced are used by the Calvin cycle for CO2 fixation (dark
reactions). (For color version of this figure, the reader is referred to the online version of this book.)
Humanity is totally dependent on photosynthesis for
food and fuel. As well as a source of organic carbon,
mankind relies on photosynthesis as energy source,
through the use of fossil fuels, ancient photosynthetic
products stored and cooked under pressure for millions
of years, the burning of readily available biomass, or
more recently through the use of biofuel crops as a
new source of liquid fuels. Sugarcane or corn ethanol
and biodiesel have been produced from crops for more
than 40 years, with a greatly increased role the last two
decades. These first-generation biofuels are presently
being produced at large scale, with worldwide production of ethanol and biodiesel of 50 billion and 9 billion
liters, respectively, in 2007. Even though these seem
like significant quantities, biofuels still represent a
miniscule fraction of the world’s primary energy use;
in 2011, 161 tons per day of renewable liquid biofuels
were produced, whereas 12 million tons per day of
crude oil were consumed (BP, 2012).
Humans have been constantly perfecting agricultural
technology since the dawn of civilization, and with the
green revolution, food crop yields have shown considerable increases decade after decade, although this
progress is now stagnating in many food producing
areas (Ray et al., 2012). At any rate, given the enormous
demand for energy and the predicted increase in the
world’s population to 9 billion by 2050, it is evident
that there is not enough arable land to satisfy both
nutritional and energy demands through food and fuel
crops. Of course, in addition to renewable energy
derived through photosynthesis, other sources of
sustainable energy exist: solar, wind, geothermal, hydroelectric, etc., but together these energy sources cannot
supply the quantity and types of energy demanded
worldwide since electricity is not suitable for all applications. Modern society is built around liquid and gaseous
fuels, which are very efficient energy carriers suitable for
a variety of applications, in particular mobile power.
Liquid biofuels are essentially photosynthetically
derived compounds, at present sustainably produced
through the cultivation of energy crops, but as discussed
above, this directly competes with the production of
food crops.
A possible and promising alternative for sustainable
energy production system is intimately related to crude
oil formation over the previous millions of years. Before
the appearance of vascular land plants on earth,
ancestral cyanobacteria were already occupying a large
variety of environments and now, after a long period
of evolution, cyanobacteria and the microalgae formed
through endosymbiosis of cyanobacteria, can be isolated
from virtually any natural water sample, from extremely
fresh water to hypersaline lakes, from snow in the Arctic
Circle to hot or relatively dry environments. The
richness of this speciation over billions of years can be
appreciated through the variety of morphological forms
that are found. These organisms show themselves to be a
promising system for the production of hydrocarbons
and other desirable products. Cultivation can be carried
out using nonarable land; seawater and wastewater
have been shown to support growth, bioremediating
effluents while fixing atmospheric carbon dioxide into
INTRODUCTION
possible commercial products. The rather simple nutrition requirements of these organisms highlight the capability of their metabolism to produce all the molecules
needed for cellular growth. Their pathways frequently
contain metabolites with commercial interest that can
be readily used or easily processed into a final product
(Figure 22.2).
391
Although cyanobacteria and eukaryotic algae share
these attributes, cyanobacteria have the additional
advantage of being relatively easily manipulated genetically. Thus, using cyanobacteria, if a desired product is
not naturally produced, genetic engineering techniques
allow the insertion of genes or even entire pathways to
make novel products, either high-value compounds or
FIGURE 22.2 Scheme of the TCA cycle with the alternatives proposed for cyanobacteria, blue pathways on the bottom (Zhang et al., 2011),
and for production of ethylene through the ethylene-forming enzyme isolated from P. syringae (orange pathway in the center). The lack of
homologous genes for 2-oxoglutarate dehydrogenase in cyanobacteria led to the idea that they have an incomplete TCA cycle, working as two
branched chains of reactions (oxidative and reductive) generating succinate from fumarate. However, a new 2-oxoglutarate decarboxylase
recently described in Synechococcus sp. was the missing piece that closes the TCA cycle in cyanobacteria. Homologs to this gene were found in all
cyanobacteria already sequenced with the exception of Prochlorococcus and marine Synechococcus sp. Source: Zhang et al., 2011. (For interpretation
of the references to color in this figure legend, the reader is referred to the online version of this book.)
392
22. ENGINEERED CYANOBACTERIA: RESEARCH AND APPLICATION IN BIOENERGY
commodity chemicals such as biofuels. Of course any
molecule that is produced using cyanobacteria could
be produced in other microorganisms, especially
fermentation workhorses such as Saccharomyces cerevisiae or Escherichia coli, but these are heterotrophs
requiring carbon compounds previously fixed through
photosynthesis, i.e. agriculturally produced.
Thus, the cyanobacteria are uniquely positioned to
carry out CO2 fixation driven by solar energy capture
while at the same time being amenable of genetic engineering to produce a wide variety of liquid and gaseous
biofuels. In this chapter the current achievements on
research toward the production of biofuels and crude
oil substitutes using cyanobacteria as a model organism
are reviewed. As will be seen, although much has
already been achieved in terms of engineering toward
the production of biofuels, in most cases productivity
is the greatest bottleneck, although some steps in downstream processing also present many challenges. Thus,
at present, use of a cyanobacterial system for commercial production of biofuels at cost-effective levels still
faces significant hurdles.
ENGINEERING CYANOBACTERIA
The major argument for using cyanobacteria or
eukaryote microalgae for biofuel production is the possibility to directly couple photosynthesis with product
formation. This strategy could have sustainable and
economic advantages. The financial appeal is related to
the production chain, with CO2 fixation directly producing the desired fuel in a single organism. Thus, the biofuel is recovered at the production site, avoiding as a
consequence the processing of photosynthetically produced sugars in a second-stage microbial fermentation.
This process also has great ecological and sustainability
appeal since atmospheric CO2 is being recycled into
fuels without using the conventional agriculture system,
leaving arable land available for food crops. Nevertheless, the inherently low value and high demand characteristics of fuels present a challenge for the development
of biofuel production. The volume of fuel required to
fulfill the needs of the transportation sector is massive,
in contrast to their low market value, which must be at
least as cheap as bottled water. The achievement of
this goal requires the solution of major challenges in civil
and mechanical engineering, chemistry, and biology.
In the biological arena, the main challenge is strain
development. The ideal cyanobacterium for biofuel production would have a high quantum efficiency of photosynthesis and well-defined carbon partitioning, where
the CO2 fixed would be primarily directed to “housekeeping” metabolism and the targeted product. To
achieve this goal, two main venues are being followed:
high-throughput bioprospecting, which seeks naturally
occurring species, enzymes and pathways adaptable
for cultivation and economic exploitation, or the use of
genetic engineering, where a model organism is genetically modified to introduce and/or to enhance the production of a desired molecule. In this section the
available tools are discussed as well as some paths
toward the improvement of photosynthetic quantum
efficiency.
Strains, Tools and Methods
Originating in an environment without available
fixed carbon, cyanobacteria have evolved as versatile
organisms, capable of producing a large variety of
organic compounds from simple inorganic sources that
can be directly used or transformed into a commercial
product. When the desired molecule is not naturally
produced, genes or entire pathways can be introduced
through a variety of methods and product yields
can be increased by driving cell metabolism toward
the desired product. There are more than 3350 species
of cyanobacteria already described, with hundreds available in culture collections (Guiry and Guiry). To date, 87
cyanobacterial genomes have been sequenced and deposited in public databases but only a few strains have been
used in genetic manipulation studies (Heidorn et al.,
2011). Many molecular tools are currently available and
genetic manipulation can be pursued through conjugation, electroporation or natural transformation. These
techniques are constantly being revised or optimized
for each host species and sample protocols are available
elsewhere (Heidorn et al., 2011). So far, no cyanophage
able to perform transduction has been described, nevertheless this technique is still the object of great interest
(Koksharova and Wolk, 2002).
Natural transformation is an appealing feature found
in some cyanobacterial strains, with two standing out as
being frequently used in genetic manipulation studies,
Synechocystis sp. PCC 6803 (Pasteur Culture Collection)
and Synechococcus sp. PCC 7002 (Grigorieva and Shestakov, 1982). These two strains are of significant interest
due to the high yield of mutants achieved through this
technique, making it widely used for both pure and
applied science, from plant physiology studies to metabolic engineering aiming for the commercial production
of biomolecules.
The high frequency of transformants with natural
transformation is intimately linked with the nature of
the transferred genetic material, with chromosomal
DNA reaching up to 100-fold more viable transformants
than when replicative plasmids are used as the source of
DNA (Golden and Sherman, 1983; Shestako and Khyen,
1970). In fact, this is true specifically for replicative plasmids since most of the transformation efficiency is
CYANOBACTERIA AS A PRODUCTION SYSTEM FOR BIOFUELS: CURRENT STATUS
recovered when a suicide plasmid is used (Tsinoremas
et al., 1994). Thus, it would seem that the final localization of the inserted DNA plays a key role in the transformation efficiency. This is argued to be related to the
postreplicative processing of chromosomal DNA
together with a putative robust recombination mechanism in these species (Flores et al., 2008). Natural transformation has being reported to be associated with
pilus-related genes (Yoshihara et al., 2001; Yura, 1999),
a natural machinery putatively adapted to take up exogenous DNA with such high efficiency that different
artificial procedures intended to increase the transformation yield fail to improve the frequency of viable
mutants (Zang et al., 2007). Unfortunately, natural transformation is not widespread in the cyanobacterial
phylum and many species require other techniques for
the efficient introduction of exogenous DNA.
Electroporation was first demonstrated in Anabaena sp.
(Thiel and Poo, 1989) and today has been optimized for
many strains. It has been shown to be effective despite
the low yield in many cases (Koksharova and Wolk,
2002). Unlike what is observed for green algae (Kilian
et al., 2011), the procedures and electric pulse settings
are not very different from those used with other bacterial phyla (Heidorn et al., 2011). However, even though it
can be an effective method, the ease of natural transformation and the higher yield of conjugation have left
electroporation behind as a choice for mutagenesis.
Conjugation is the most commonly used technique
for genetic engineering in terms of the diverse species
with which it can be used, and, with the filamentous
N2 fixing (heterocyst forming) cyanobacteria, it is the
only effective technique thus far described. With the
advent of molecular biology, plasmids of cyanobacterial
origin were actively sought with the intention of producing shuttle vectors allowing their transfer from E. coli to
Synechococcus (Golden and Sherman, 1983). Since then,
E. coli has been widely used for conjugation with
many filamentous strains, such as Nostoc sp. and
Anabaena sp., and single cell strains, like Synechococcus
sp. and Synechocystis sp. Although incorporation of
DNA into the chromosome of many strains has proved
to be relatively easily achieved when using linear
DNA or suicide plasmids, it has proved challenging to
make cyanobacteria harbor replicative plasmids. During
conjugation, the plasmid is relaxed and single-stranded
DNA is driven to the recipient cell through the type four
secretion system by the enzyme relaxase. Once in the
recipient cell, the transferred DNA will have its antisense strand resynthesized and this newly reformed
plasmid can integrate itself into the genome or autoreplicate. The vectors used in cyanobacteria must contain
the replicons for both organisms, donor and recipient, a
mobilization site (origin of transfer, e.g. bom, nic and
oriT), a selective marker effective for both organisms,
393
and a codon optimization to avoid the broad range of
restriction enzymes harbored by cyanobacteria, which
has been found to be an important hurdle to successful
conjugation (Elhai et al., 1997; Flores et al., 2008; Wolk
et al., 1984). Extra enzymes might be needed to ensure
a successful transfer, which could be encoded on secondary (aka helper) plasmids. Among these special
enzymes are some endonucleases, intended to cut the
cargo plasmid at the bom site and promote transfer,
and methylases to protect the transferred DNA against
the restriction enzymes in the recipient. Detailed procedures, strategies and strains used are amply reviewed
elsewhere (Heidorn et al., 2011).
CYANOBACTERIA AS A PRODUCTION
SYSTEM FOR BIOFUELS: CURRENT
STATUS
Hydrogen
Frequently cited as the fuel of the future, hydrogen
production, storage and utilization are being widely
investigated. As a transportation fuel it presents a series
of challenges in every link of the chain, from production
to storage and distribution. Although having a low volumetric energy density, hydrogen has the highest energy
density per mass and the simple fact that its combustion
generates almost only water and heat has seduced entire
generations. “Yes, my friends, I believe that water will
one day be employed as fuel, that hydrogen and oxygen
which constitute it, used singly or together, will furnish
an inexhaustible source of heat and light, of an intensity
of which coal is not capable” (Verne). Cars that could run
on water with minimal energy consumption have
captured the imagination of many people and, not surprisingly, have inspired frauds like the almost magical
conversion of saltwater into fuel using radiofrequency
radiation, claimed by John Kanzius and broadcast live
countrywide from Philadelphia, or the notorious “StanleyMeyer’s water fuel cell” to be used in an internal
combustion engine, where a special device could split
water giving an energy output sufficient to generate mechanical energy for the vehicle with enough leftover to
power a fuel cell that would provide more hydrogen
and oxygen through water splitting. Considering that
the combustion of hydrogen and oxygen regenerates
water, both systems obviously defy the first and second
laws of thermodynamics (Ball, 2007).
Despite the motivation behind these schemes, they
touched upon the most limiting step in the development
of the hydrogen fuel technology: production. In current
industrial practice, hydrogen can be produced by pyrolysis, electrolysis or by steam reforming of hydrocarbons.
The last is the dominant method, applied to fossil fuels,
394
22. ENGINEERED CYANOBACTERIA: RESEARCH AND APPLICATION IN BIOENERGY
usually natural gas (methane). This makes hydrogen
both expensive and unsustainable.
Hydrogen Bioproduction
Molecular hydrogen (H2) is the lightest gas possible.
When released into the atmosphere it diffuses quickly
toward the troposphere, thus, at the sea level it can
only be found in trace amounts. For this reason, very little naturally occurring H2 is available and therefore a
sustainable production system must be found if this
molecule is to be used as a fuel. Efficient biological
production of hydrogen could represent a breakthrough
in the development of this energy carrier and many
different approaches are being followed toward this
goal. Undoubtedly, among all the possible fuels
that could be produced by cyanobacteria, it is
hydrogen that has received the most attention. Here
we discuss the biological mechanisms for hydrogen
production and advances toward yield improvements
in cyanobacteria.
In the light reactions of photosynthesis, light is
captured by photosystems I and II, acting together to
transform solar energy into chemical energy, splitting
water into molecular oxygen and protons (Hþ) and the
reducing agent NADPH. The transmembrane proton
gradient that is formed is used by ATP synthase to
combine adenosine diphosphate (ADP) þ Pi into ATP
(Figure 22.1). This set of reactions is rather interesting
because it effectively conserves ubiquitous solar energy
in energy-dense molecules using an abundant substrate,
water. Ironically, cyanobacteria (and all plants) had been
all along for millions of years the very sought after solution for breaking the strong bond between oxygen and
hydrogen in the water molecule without using the special radiofrequency of John Kanzius or the mysterious
fuel cell of Stanley Meyer.
During the water-splitting process, oxygen is released
in its molecular form (O2), while hydrogen, in the form
of protons, is further used to produce two molecules of
high-energy content: ATP and NADPH. Together, they
feed energy into the Calvin-Benson-Bassham cycle,
where CO2 is fixed into organic molecules, as well as
into many other reactions related to cellular homeostasis
or secondary metabolism. Alternatively, before it is
used to generate NADPH, the high-energy electron
generated by photosynthesis can be directly used for
the evolution of hydrogen, a process called direct biophotolysis (Benemann and Weare, 1974). Therefore,
hydrogen evolution through this route does not require
CO2 fixation, and solar energy and water, together with
the required enzymes, are sufficient for H2 formation
(Hallenbeck and Benemann, 2002). The major problem
with this process is that hydrogenases, the hydrogenevolving enzymes, are extremely sensitive to oxygen
(O2) and are irreversibly inactivated by even small
concentrations of this gas. Thus, hydrogen evolution is
usually a short-lived process, with a burst of hydrogen
evolution when transitioning from a dark cycle into light
as increasing oxygenic photosynthesis quickly inactivates
the hydrogenase. Some species, especially filamentous
ones (e.g. Anabaena sp. and Nostoc sp.), capable of forming
specialized cells called heterocysts, can be shown to
produce hydrogen over prolonged periods in light, as
the heterocysts provide an oxygen-free environment
that protects the hydrogenase against inactivation. In
indirect biophotolysis, the captured light energy is
used to fix CO2 and the organic molecules that are produced are stored as reserve material. Under normal
conditions, part of these carbon reserves will be
oxidized over the dark period to maintain cellular homeostasis. However, under proper conditions such a
culture can be induced to produce hydrogen, thus
separating hydrogen evolution temporally and
spatially from the oxygen evolved by oxygenic photosynthesis (Hallenbeck, 2011). Thus, hydrogenase activity is maintained and the simultaneous production of
hydrogen and oxygen, an explosive mixture when
concentrated in the headspace of a bioreactor, is
avoided.
Hydrogen-Evolving Enzymes
Hydrogenases in cyanobacteria have been studied for
over 35 years (Benemann and Weare, 1974; Hallenbeck
and Benemann, 1978) and many variations of hydrogenases have been described in different bacterial phyla
(Vignais and Billoud, 2007). These enzymes are
frequently classified into three different groups: nitrogenase, the reversible hydrogenase (Hox), and the uptake
hydrogenase (Hup) (Ghirardi et al., 2007).
HUPdHYDROGEN UPTAKE ENZYME
Hup is a [NiFe] hydrogenase that occurs associated
with the thylakoid membrane (Seabra et al., 2009). This
enzyme shows the least sensitivity to oxygen among
the three classes. Its function is in the oxidation of H2,
returning the captured electrons to cellular electron
transfer reactions. To date it has been found only in
N2-fixing strains and appears to have an intimate relationship with nitrogenase (Marreiros et al., 2013). Under
natural conditions, nitrogenase functions to reduce atmospheric N2 to NH3, producing H2 in an unavoidable side
reaction. It is thought that Hup functions to recycle the
recently formed H2, which is oxidized back into protons
or reacted with O2 in a respiratory oxyhydrogen reaction,
protecting the nitrogenase from O2 inactivation, avoiding
an excessive build up of H2 in the cell and recovering
part of the ATP used in its formation (Bothe et al., 2010;
Tamagnini et al., 2007). In the nitrogen-fixing cyanobacteria, transcription of the Hup-encoding genes hupSL is
associated with the nitrogen depletion response and
CYANOBACTERIA AS A PRODUCTION SYSTEM FOR BIOFUELS: CURRENT STATUS
395
is under the regulation of the NtcA, the global nitrogen
regulator (Weyman et al., 2008). Hup inactivation
increases the production of H2 two- to threefold in
most cyanobacteria (Ludwig et al., 2006; Tamagnini
et al., 2007).
heterocyst can maintain an internal anoxic environment
since the expression of PSII is repressed. Hydrogen
production therefore is supported through the use of
carbon compounds delivered by the neighboring
vegetative cells.
NITROGENASEdA GRATUITOUS HYDROGENASE
REVERSIBLE HYDROGENASE (HOX)
In nature this complex enzyme carries out a critical
function, breaking the three covalent bonds of molecular nitrogen (N2) providing ammonia to the cell and
closing the nitrogen cycle. This process consumes a
large amount of energy in the form of ATP and highenergy electrons (Eqn (22.1)), producing NH3 with the
coproduction of hydrogen in an unavoidable side
reaction.
In addition to nitrogenase, N2-fixing cyanobacteria
can have a second hydrogen-evolving enzyme, the
so-called reversible hydrogenase (Hox). This enzyme is
a heteropentameric complex that is formed by a hydrogenase module (HoxHY) and a diaphorase module (HoxEFU), which transfers electrons from NAD(P)H to the
hydrogenase module (Bothe et al., 2010). Like Hup,
Hox is a [NiFe] hydrogenase, but in this case it shows
a high sensitivity to O2. Its expression is totally independent from that of nitrogenase and varies among species.
In some cases it is under the control of the circadian
clock, where it is shown to promote hydrogen production in the dark (Hallenbeck and Benemann, 1978;
Schmitz et al., 2001). The bidirectional hydrogenase is
not taxon specific, being found in many different groups
of cyanobacteria, and its location and organization in the
chromosome are also heterogeneous. Recent studies
regarding Hox transcription factors have elucidated
many aspects of its regulatory mechanisms, which are
reviewed elsewhere (Oliveira and Lindblad, 2009).
N2 þ 10Hþ þ 8e þ 16ATP/2NH3 þ H2 þ 16ADP
(22.1)
The most common nitrogenase is the Mo-Fe nitrogenase,
which is characterized by a complex iron-sulfur cluster
containing molybdenum. While performing nitrogen
fixation, up to one-fourth of the electron flux goes toward the reduction of hydrogen. Variations of this
enzyme includes the substitution of the molybdenum by
vanadium or iron (V-Fe and FeeFe nitrogenases,
respectively), which, although a greater proportion of
electrons are allocated to hydrogen production, in fact
show a lower net flux of electrons to hydrogen since
their overall reaction rates are much lower than that of
the Mo-Fe enzyme, limiting the application of these
variants in bioproduction systems. One option that is an
interesting strategy for H2 production, to increase the
electron flux into H2, is cultivation in the absence of N2,
since nitrogenase turnover continues, but now the electron flux goes totally toward hydrogen evolution. In
addition, the growth arrest caused by the nutrient limitation is of interest as this decouples hydrogen evolution from biomass production, therefore potentially
leaving more energy available for H2 production
(Benemann and Weare, 1974). Even so, the expression of
an oxygen-sensitive enzyme in an O2 rich milieu is
counter productive. To overcome this problem, temporal
separation between N2 fixation and photosynthesis can
be used, where during the day the photosynthetic
machinery works toward the carbon fixation, which
then can be consumed to power nitrogenase and
consequently proton reduction. Interestingly, the peak of
hydrogen production in indirect biophotolysis occurs
when the cell is reilluminated, possibly due to a burst in
ATP synthesis before the oxygen formed by PSII
(Figure 22.1) reaches a toxic level for the nitrogenase.
Heterocyst forming species on the other hand can
perform direct biophotolysis by carrying out nitrogen
fixation in the differentiated cell during the day. The
Hydrogen Bioproduction
As discussed above, nonbiological production of
hydrogen is energy intensive and often associated
with the production of greenhouse gas. Biologically,
hydrogen can be produced by a variety of microorganisms possessing one of several different hydrogenases.
In the cyanobacteria, enzymes involved in hydrogen
metabolism belong to one of the three families discussed
above: Hox, Hup or nitrogenase. The uptake hydrogenase (Hup) is not useful for hydrogen evolution since
it is poised to work unidirectionally, toward the recycling of H2 into Hþ. When hydrogen is produced by a
heterotrophic organism, an organic carbon source (ultimately derived from photosynthesis) is used to provide
protons and chemical energy to fuel hydrogen evolution. Ironically, this is also true for cyanobacteria
carrying out direct or indirect biophotolysis, at least on
the molecular level. As discussed above, a complete
photosynthetic apparatus uses water as proton donor,
releasing molecular oxygen (Figure 22.1). Thus, the
high sensitivity of hydrogenase to this gas dictates that
both reactions cannot occur in the same place at the
same time. The solution found by Nature was the
most obvious one: changing the timing (indirect
biophotolysis) or the space (direct biophotolysis).
In indirect biophotolysis the cell uses the chemical
energy stored through the capture of sunlight, as
396
22. ENGINEERED CYANOBACTERIA: RESEARCH AND APPLICATION IN BIOENERGY
NADPH and ATP (Figure 22.1), to fix CO2 into organic
compounds. These energy reserve molecules are then
consumed in the dark to drive cellular metabolism,
including nitrogen fixation by nitrogenase. The separation of these reactions occurs naturally in several cyanobacterial species by circadian control and in these strains
dark hydrogen production by either nitrogenase or the
bidirectional hydrogenase is frequently reported (Prabaharan et al., 2010; Troshina et al., 2002). An interesting
characteristic found in many of these strains is a burst
of hydrogen production when cells are reilluminated.
This phenotype was characterized as a function of the
bidirectional hydrogenase and hydrogen production
ceases quickly as the O2 produced by photosystem II
(Figure 22.1) accumulates in the cell, inactivating
hydrogenase The production of H2 is thought to serve
as an electron sink, helping the cell return to the proper
redox state for carrying out the light reactions. In practice, indirect biophotolysis could possibly be done as a
large-scale production using a two-stage cultivation
system. In a first stage, the cells are cultivated in the light
and biomass is formed through photosynthesis. When
the desired cell concentration is achieved and the cells
have stored enough fixed carbon, a dark anaerobic cultivation could follow, favoring proton reduction to
hydrogen by hydrogenase. Thus, the water-splitting
reaction is separated from H2 production in time and
space. This system has being already demonstrated,
where nitrogen limitation was also used to induce
glycogen accumulation and increase hydrogen production yield in the second stage through the nitrogenase
enzyme (Huesemann et al., 2009). In a similar approach
with Synechococcus sp., the carbon accumulated in the
first stage was converted into hydrogen in a second
stage by a [NiFe] hydrogenase (McNeely et al., 2010).
Even so, some continual synthesis of nitrogenase is
necessary to replace oxygen-damaged nitrogenase
(Murry et al., 1983).
As discussed above, since heterocysts lack a complete
photosynthetic apparatus, the necessary reductant is
derived from fixed carbon imported from the neighboring vegetative cells through specialized interconnecting pore structures (Mariscal and Flores, 2010). The
imported sugar is sucrose (Lopez-Igual et al., 2010)
and it is metabolized though the oxidative pentose
pathway (Summers et al., 1995) Thus, hydrogen production by heterocysts is essentially indirect biophotolysis
on a microscopic scale, and since the energy captured
by photosynthesis is first stored as fixed carbon, the
maximal possible theoretical conversion efficiencies are
reduced.
However, this system has been attractive due to its
inherent robustness and has been studied for almost
four decades (Benemann and Weare, 1974). Very reasonable conversion efficiencies, sustained for days to weeks,
were achieved in early studies using nitrogen-limited
cultures. Under laboratory conditions where higher efficiencies can be expected, conversion efficiencies (total
incident light energy to free energy of hydrogen produced) were shown to be 0.4% (Weissman and Benemann, 1977). Cultures incubated under natural
sunlight (Figure 22.3) were able to attain an average conversion efficiency of 0.1% (Miyamoto et al., 1979a).
Remarkably, even though there have been a large number of studies since, very little improvement in yields
H2 PRODUCTION BY HETEROCYSTOUS
CYANOBACTERIA
Solar energy capture and hydrogen evolution by
some filamentous cyanobacterial strains proceeds naturally in the presence of oxygen by confining the
oxygen-sensitive processes to the heterocyst, a cell type
that emerged shortly after the oxygenation of the earth’s
atmosphere in what has been called the Oxygen Catastrophe or Great Oxidation Event 2.6 billion years ago
(Kumar et al., 2010; Mariscal and Flores, 2010). In this
case the evolved hydrogen is produced by nitrogenase
whose expression is restricted to the heterocyst under
normal aerobic conditions (Murry et al., 1984). A number of mechanisms are employed to protect nitrogenase
from oxygen damage; heterocysts lack photosystem II so
do not produce oxygen, gas diffusion into the heterocyst
is restricted by a unique cell wall structure, and heterocysts possess a very active membrane-bound respiratory
system that consumes trace amounts of entering oxygen.
FIGURE 22.3 Tubular photobioreactors operating under “airlift”conditions were used to demonstrate prolonged (over 30 days)
simultaneous oxygen and hydrogen evolution by nitrogen-limited
cultures of the heterocystous cyanobacterium, Anabaena cylindrica.
Source: Miyamoto et al 1979d. (For color version of this figure, the reader
is referred to the online version of this book.)
CYANOBACTERIA AS A PRODUCTION SYSTEM FOR BIOFUELS: CURRENT STATUS
has been obtained. Thus, recent reports of conversion
efficiencies found z0.7% under laboratory conditions
(Berberoglu, 2008; Sakurai and Masukawa, 2007; Yoon
et al., 2006) and 0.03e0.1% with natural sunlight
(Sakurai and Masukawa, 2007; Tsygankov et al., 2002).
Similar low efficiencies have been found with thermophilic strains, which at least have the possible advantage
of requiring less cooling (Miyamoto et al., 1979b,c).
There should be room for improvement as theoretical
efficiencies with this nitrogenase-based system have
been calculated to be around 4.6% (Hallenbeck, 2011).
Since observed conversion efficiencies are lower than
predicted, different strategies might be employed in
order to improve overall performance, which is critically
important since light conversion efficiencies directly
impact on the photobioreactor footprint (doubling efficiency should halve the required surface area for the
same amount of fuel production). For one thing, genetic
engineering could be applied to optimizing the size of
the photosynthetic antenna, since part of the reduction
in efficiency is thought to be due to inefficient use of
light energy at high intensities where more photons
are captured than can be used and the excess energy is
wasted. Another point that could be addressed is the
hydrogen producing catalyst. Since half of the photon
requirement is needed to provide ATP to nitrogenase action, replacing it with a hydrogenase, which does not
require ATP for proton reduction, should in principle
have an energy sparing effect. In a recent attempt to
verify this, the [FeFe] hydrogenase from Shewanella oneidensis was expressed in Anabaena sp. under the control of
a heterocyst-specific promoter with the required maturation genes (Gartner et al., 2012). Although it could be
shown that active hydrogenase was made under the
proper conditions, the increase in hydrogen production
above the levels due to the coexisting nitrogenase was
disappointingly small. Of course, under these conditions the two enzymes compete for the reductant; the
true test would be to do this in a strain lacking nitrogenase activity. Finally, it might in principle be a possible
way to increase hydrogen production by increasing heterocyst frequency. However, heterocyst frequency might
already be close to optimal since even in long-term
studies the H2/O2 ratio is close to the desired stoichiometry of two, what one would expect for optimal coupling
between oxygen-generating photosynthesis in the vegetative cells and hydrogen production by heterocysts.
H2 PRODUCTION BY NONHETEROCYSTOUS
CYANOBACTERIA
Although the heterocyst/nitrogenase-based system
has been the most studied, some other known cyanobacterial hydrogen-producing reactions could potentially be used for biological hydrogen production.
These include the unicellular and nonheterocystous
397
filamentous cyanobacteria, which possess nitrogenase
and are able to fix nitrogen in nature. Two strategies
are employed to avoid oxygen inhibition. In some unicellular species, oxygen evolution and nitrogen fixation
(or hydrogen production) are separated in time since
photosynthesis and nitrogen fixation are under circadian control with photosynthesis taking place during
the day and nitrogen fixation being maximal during
the night period. The filamentous cyanobacterium Trichodesmium uses a strategy of spatial segregation where
nitrogen fixation occurs in cells located in the middle of
the bundle carrying out the oxygen-sensitive nitrogenase reactions and the others carrying out the normal
photosynthetic reactions (Berman-Frank, 2001).
The unicellular cyanobacterium Cyanothece has been
the subject of a number of recent studies demonstrating
prolonged hydrogen production in the light mediated
by nitrogenase. In one study, considerable hydrogen
production (up to 465 mmol per milligram of chlorophyll
per hour) was shown, the growth conditions were very
stringent and hydrogen production was only observed
when the culture was submitted to nitrogen starvation,
sparged with argon to remove any oxygen formed
through photosynthesis, supplemented with glycerol
and cultivated under low light (Bandyopadhyay et al.,
2010; Min and Sherman, 2010). Glycerol, in addition to
serving as a possible additional energy source to support nitrogenase activity, appears to release nitrogenase
from diurnal control (Aryal et al., 2013). Another recent
study found appreciable hydrogen and oxygen production with nitrogen-depleted cultures that were incubated under continuous illumination (Melnicki et al.,
2012). Light saturation curves and photosynthesis inhibition studies indicate that the hydrogen is evolved indirectly from the fixed carbon produced through
photosynthesis. Here again, the requirements for continuous illumination (it can hardly be energetically positive
to produce hydrogen using artificial illumination) and
for argon sparging raise serious hurdles to practicality.
Thus, although a nice proof of principle, such a system
would hardly be economically viable.
Many cyanobacteria also possess Hox, a soluble
reduced nicotinamide adenine dinucleotide (NADH)linked [NiFe] hydrogenase. This reversible hydrogenase
is capable of hydrogen evolution, in particular when
dark-adapted cells are reilluminated (Schwarz et al.,
2010). As discussed above, this forms an electron valve,
readjusting the poise of the photosynthetic apparatus,
but activity is quickly inhibited with renewed oxygen
evolution. A recent survey showed that a diversity of cyanobacteria contains this enzyme and that there is great
variability in both the amounts of hydrogen made by
this enzyme and the pattern of hydrogen evolution
(Kothari et al., 2012). This enzyme is also responsible
for evolution during dark fermentation of endogenous
398
22. ENGINEERED CYANOBACTERIA: RESEARCH AND APPLICATION IN BIOENERGY
reserves, principally glycogen, and hydrogen production
by this pathway can be enhanced through lowering of the
hydrogen partial pressure (Ananyev et al., 2012). At least
in Synechocystis, hydrogen production by Hox can be
increased by eliminating the master regulator AbrB2,
which normally represses synthesis of Hox (Dutheil
et al., 2012; Leplat et al., 2013). In a recent attempt to
increase hydrogen production, heterologous expression
of the [FeFe] hydrogenase from Clostridium acetobutylicum
was carried out in the non-nitrogen-fixing cyanobacterium Synechococcus (Ducat et al., 2011). Active hydrogenase was formed under proper conditions, but in vivo
light-driven hydrogen production from this system was
significant only when the cultures were incubated under
an inert atmosphere and oxygenic photosynthesis was
completely inhibited.
carbon, to ethanol, pyruvate decarboxylase (pdc) and
alcohol dehydrogenase (adh). This alone is sufficient to
produce low millimolar levels of ethanol in the medium
upon prolonged (5e10 days) incubation and growth.
Further increases, obviously necessary for practical production, have been achieved through a variety of means,
including better transcriptional control and further
metabolic engineering. Most of this development work
is being done at private enterprise laboratories, but a
recent published report (Gao et al., 2012) shows that
impressive increases in yields can be achieved by integrating a foreign pdc and a native adh into the genome
of Synechocystis and abolishing carbon flux into polyhydroxybutyrate synthesis.
Ethanol
The chemical industry relies on simpler molecules to
build complex compounds, which are used in a variety
of applications. Among these organic compounds,
ethylene is the building block with the highest demand,
being used to manufacture “everyday” products such as
polyethylene terephthalate (PET bottles), polyester, antifreeze, and others (Ungerer et al., 2012). Ethylene (or
ethene) is the second simplest unsaturated hydrocarbon,
it consists of two carbons, with a double bond, and four
hydrogens (H2C]CH2). It is one of the products of
pyrolysis, and has been used as a fuel since the early
nineteenth century as one of constituents of the gaseous
fuel in gas lamps. Ethylene is the most important compound in the chemical industry in terms of market
volume, it has a heat of combustion higher than that of
gasoline or diesel and can be used as a transportation
fuel or to produce electric energy in stationary plants.
Currently, ethylene is a petroleum derivative produced
through steam cracking. It reached a production of 100
million metric tones in 2005, accounting for 30% of all
petrochemical commodities (McCoy et al., 2006; Saini
and Sigman, 2012). The fluctuation in crude oil prices
over the last few years (EIADOE, 2012), the imminent
threat of peak oil (Nashawi et al., 2010), and the existence of biological pathways for its production coupled
with the ease of harvesting a gas like ethylene, make
this chemical a good target for the development of a sustainable biological production system.
The most common occurrence of ethylene in nature is
as a hormone found in vascular plants, where it is associated with many effects such as defoliation, responses
to temperature stress, mechanical injury, and for
promoting fruit ripening (Abeles, 1972). In addition to
vascular plants, many other plants and algae have
been shown to be able to produce ethylene and even if
it not found in animals, this gaseous hormone has
been shown to induce regulatory responses in invertebrate and mammalian cells (Perovic et al., 2001).
While hydrogen production, or at least direct biophotolysis, can be driven directly by photosynthesis, all
other biofuels must use the capacity of cyanobacteria to
drive carbon dioxide fixation with photosynthetically
derived energy, ATP and reductant. However, once fixed
by the Calvin-Benson-Bassham cycle, the newly recycled
carbon can be converted to useful biofuels through
the introduction of novel (to cyanobacteria) metabolic
pathways (Angermayr et al., 2009). The first such
cyanobacterial-derived biofuel that was demonstrated
was ethanol (Deng and Coleman, 1999; Dexter and Fu,
2009), and its production is the only cyanobacterialproduced biofuel under active investigation and commercial development (Algenol Biofuels: http://www.
algenolbiofuels.com/). Algenol Biofuels is presently
claiming production at “around $1.00 per gallon using
sunlight, carbon dioxide and saltwater at production
levels above 9000 gallons of ethanol per acre per year”.
At an average solar insolation for Florida of 19.8 MJ/day
and since ethanol has a higher heating value of 29.7 MJ/
kg, this translates to a claim of a very impressive 2.8%
conversion efficiency. Now another company, Joule
Unlimited (http://www.jouleunlimited.com/), has stepÒ
ped into the picture, offering to sell SunFlow-E through
its fuel company, Joule Fuels. Their process uses genetically modified thermophilic cyanobacterium containing
Moorella alcohol dehydrogenase, and their Web site
claims are even more spectacular with targets of up to
25,000 gallons per acre (7.8% conversion efficiency) and
$0.60 per gallon at full-scale commercial production.
Cyanobacteria can naturally produce relatively minute amounts of ethanol so at the simplest level, creating
a cyanobacterium that produces higher levels of ethanol
involves boosting flux through the ethanol pathway
through the introduction of the key enzymes for conversion of pyruvate, generated by glycolysis of the fixed
Ethylene
CYANOBACTERIA AS A PRODUCTION SYSTEM FOR BIOFUELS: CURRENT STATUS
The most common biosynthetic pathway for
ethylene production is the Yang cycle that occurs in
plants, where it is produced from methionine in a
three-step reaction, having S-adenosylmethionine
(AdoMet) and 1-aminocyclopropane-1-carboxylic acid
(ACC) as precursors. However, the cellular response
to this hormone occurs at very low concentrations, a
characteristic that, together with the fast and easy
diffusion of this gas into plant tissues, makes the conversion of AdoMet to ACC, catalyzed by ACC synthase, and from ACC to ethylene (ACC oxidase) a
tightly regulated process. Both enzymes are multigenic
with differential regulation through distinct promoters
and operators for groups of genes of the same enzyme
(Nakatsuka et al., 1998). The methionine used in this
pathway is recycled through the Yang cycle (Taiz and
Zeiger, 2002; Wang et al., 2002).
Microbial Production of Ethylene
The great influence of this gaseous hormone on
different plant organs made it an interesting target for
pathogens. Indeed, the mold Penicillium digitatum has
been known to produce ethylene since the mid-1950s
(Wang et al., 1962) and its production was shown for
prokaryotic plant pathogens in the early 1960s (Freebairn
and Buddenhagen, 1964). Not surprisingly, the pathways
found in these microorganisms are not analogous to
the one in plants. So far, two distinct routes for ethylene
production have been described in microbes: a
2-oxoglutarate-dependent pathway and the 2-keto4-methyl-thiobutyric acid (KMBA) pathway (Nagahama
et al., 1991, 1992). The latter is the most common among
microorganisms, composed of a series of chemical and
enzymatic reactions, by which only trace amounts of
ethylene are usually produced (Ogawa et al., 1990). The
former pathway has been found to be more efficient,
with 2-oxoglutarate being used as substrate in a singlestep reaction by the ethylene-forming enzyme (EFE).
This pathway has been found in several different microorganisms, including P. digitatum, Chaetomium globosum,
Phycomyces nitens, Fusarium oxysporum, and in different
pathovars of Pseudomonas syringae, where a comparison
study found the pv. phaseolicola to be the most efficient
ethylene-producing strain (Weingart et al., 1999). This
enzyme catalyzes simultaneously two reactions (Fukuda
et al., 1992b):
2 oxoglutarate4ethylene þ 3CO2 þ H2 O
(22.2)
2 oxoglutarate þ L arginine þ O2 4succinate
þCO2 þ guanidine þ ðSÞ 1 pyrroline 5
carboxylate þ H2 O
(22.3)
399
These reactions are rather interesting as they keep the
tricarboxylic acid (TCA) cycle closed through a shortcut,
converting 2-oxoglutarate directly into succinate with
the formation of ethylene “as a by-product” (Figure 22.2),
and therefore substituting for the steps catalyzed by
2-oxoglutarate dehydrogenase and succinyl-CoA synthetase (Figure 22.2). The original two-step reaction between
2-oxoglutarate and succinate generates one NADH and
one guanosine triphosphate, which are not produced by
EFE. Thus, competition of the two pathways for substrate
2-oxoglutarate would lower the formation of NADH.
Since NADH also has a role as an inhibitor for four
enzymes associated with the TCA cycle, pyruvate dehydrogenase, isocitrate dehydrogenase, 2-oxoglutarate dehydrogenase, and citrate synthase (Figure 22.2), this
could potentially upregulate the reactions performed by
these enzymes, from the decarboxylation of pyruvate to
2-oxoglutarate. The last is a direct and indirect substrate
to the EFE, directly to generate ethylene (Eqn (22.2)) and
indirectly, as it is also a substrate for the synthesis of arginine, required for the simultaneous reaction of this
enzyme (Eqn (22.3) and Figure 22.2).
Bioproduction of Ethylene Using efe
When producing any molecule of commercial interest
with a microorganism, prospecting for the key gene is as
important as the choice of the host to be used. The evolutionary convergence of ethylene production is highlighted by the three pathways delineated above: Yang
cycle, KMBA and 2-oxoglutarate, with the last having
been found to be the most efficient when overproduction is desired. The evolutionary radiation of the mobile
plasmid encoding the efe gene among the different
species and strains might have produced a naturally
optimized gene that could be used in commercial production. A comparison between 20 P. syringae strains
revealed a high amino acid sequence similarity between
five pathovars, with P. syringae pv. phaseolicola PK2 being
the most efficient, giving a twofold higher production of
ethylene (Weingart et al., 1999). However, these variations are likely to be due to differences in regulation as
the sequence of amino acids of efe of these five strains
differs by only one codon.
Using the efe gene encoded by an indigenous plasmid
from P. syringae pv. phaseolicola PK2, ethylene production
was reported in E. coli with a tenfold increase when
compared to the original strain, P. syringae (Fukuda
et al., 1992a; Ishihara et al., 1995), showing that the efe
gene alone was sufficient for ethylene production.
When a high-copy-number plasmid containing efe was
transconjugated into Pseudomonas putida and P. syringae,
ethylene production was increased, but surprisingly,
production was 27- and 8-fold higher, respectively,
than the wild type, whereas the amount of protein produced in the cloned P. syringae was 20-fold higher
400
22. ENGINEERED CYANOBACTERIA: RESEARCH AND APPLICATION IN BIOENERGY
(Ishihara et al., 1996), suggesting the presence of a posttranscription regulatory system. Using cellulose as substrate, Tao et al. showed the production of ethylene in
Trichoderma viride through the heterologous expression
of efe from P. syringae pv. glycinea. Thus, the use of agriculture wastes as substrate for ethylene production was
proved to be feasible, but the recombinant filamentous
fungus produced only very small amounts of ethylene
(Tao et al., 2008).
So far, cyanobacteria have been shown to be the best
model for the bioproduction of ethylene. Of course,
many barriers still have to be crossed and commercial
production is far from reality at present, but the last
few years have seen encouraging reports where the productivity was increased several fold without compromising cell fitness, suggesting that the true production
limit might be much higher. The efe (EFE) from P. syringae was originally cloned into Synechococcus elongatus
PCC 7942 (Fukuda et al., 1994; Sakai et al., 1997; Takahama et al., 2003). The first problem area, the production
of only trace amounts of ethylene by the transformants
(Fukuda et al., 1994), was later shown to be due to the
nature of the promoter used. A systematic evaluation
of different promoters showed the psbA1 promoter is
more efficient for efe expression than those (lac and efe)
previously used in other reports, achieving production
rates up to 240 nl/ml h or 451 nl/ml h OD730 (Takahama et al., 2003). However, these recombinants showed
high genetic instability. Sequencing of the heterologous
gene from mutants that had ceased to produce ethylene
showed punctual mutations at a defined sequence of
five nucleotides, suggested to be a possible hot-spot
site for spontaneous mutagenesis (Takahama et al.,
2003). Nevertheless, active ethylene-producing strains
showed signs of metabolic stress, evidenced by their
yellow-green color. When these strains had ceased
ethylene production due to spontaneous mutation of
efe (genetic instability), they recovered the normal
blue-green phenotype.
In another strategy (Ungerer et al., 2012), Synechocystis sp. PCC 6803 was used as model organism. Toxicity to
ethylene was tested, efe was codon optimized and artificially synthesized, eliminating the bases at the putative
mutational hot spot by conservative substitution. As
well, efe was placed under the control of the psbA1 promoter. A semicontinuous culture using a clone containing two copies of efe was sustained over a three-week
period, reaching a constant production of 3100 nl/ml h,
compared to the previous result of 240 nl/ml h (Takahama et al., 2003). The peak of the specific productivities
was 380 nl/ml h OD730 for one efe copy and 580 nl/
mL h OD730 for two copies, respectively, and when in
semicontinuous culture, the average rate was 200 nl/
ml h OD730. The additional copy of the efe gene presented some production improvement when compared
with the previous work from Takahama et al., (451 nl/
ml h OD730 compared to 580 nl/ml h OD730) but the
real advance for the field can be seen from the healthy
state of the culture. The growth rate, the color of the culture and the growth curve were the same for wild type
and the mutants containing one or two copies of efe.
This shows that there is no toxicity either by the product
or by the metabolic route used to produce ethylene. In
addition to the zero toxicity, the release of five carbons
per ethylene formed does not seem to present a burden
to the cell, as shown by the growth pattern of the single
and double mutant when compared with the wild type.
Nevertheless, the metabolic consequences to the cell of a
higher rate of ethylene production are unknown and a
physiological approach would help to understand how
far ethylene production can be pushed and what to
target to improve the final yield.
Isoprene
As with ethylene, isoprene is a medium-value
biochemical that is produced through steam cracking
of oil. It is actually an important by-product of ethylene
production and is almost entirely used for production of
a synthetic substitute for natural rubber. It is also naturally produced by many plants as a heat stress response,
where it was shown to increase the stability of photosynthetic membranes at high temperatures (Sharkey et al.,
2001). It can represent as much as 2% of all carbon fixed
by oak leaves at a temperature of 30 C (Sharkey, 1996),
showing the physiological importance of this compound. The enzyme isoprene synthase (ispS) was shown
to produce isoprene in plants, converting one of the
products of the methylerythritol phosphate (MEP)
pathway, dimethylallyl-diphosphate (DMADP), into
isoprene (Silver and Fall, 1991; Silver and Fall, 1995).
Prokaryotes were suggested to be able to produce
isoprene after reports of the detection of this compound
in the headspace of culture broth on many species
(Kuzma et al., 1995), with emphasis on Bacillus subtilis.
Not surprisingly, sequence analysis of bacterial genome
could not identify any gene homologous to the ispS. found
in plants (Withers et al., 2007). So far, functional genomics
has also failed to identify the pathway for isoprene production in prokaryotes. Sequence-independent methods
showed that 19,000 E. coli clones transformed with
DNA fragments from B. subtilis in an environment where
DMADP and IPP (isopentenyl pyrophosphate) levels
were selectively toxic, showed that no single enzyme
was sufficient to convert DMADP to isoprene, where the
few clones that managed to survive, preferably converted
it to a prenyl alcohol (Withers et al., 2007). As all isoprenoids are thought to be solely produced from DMADP
and IPP (Xue and Ahring, 2011), the conversion of the
metabolites involved in MEP or mevalonate pathway
CYANOBACTERIA AS A PRODUCTION SYSTEM FOR BIOFUELS: CURRENT STATUS
401
FIGURE 22.4 Pathway alternatives for n-butanol bioproduction. The alcohol n-butanol is naturally produced in different microorganisms in
small quantities, where it can be synthesized either through the CoA-dependent pathway or the keto acids pathway. (For color version of this
figure, the reader is referred to the online version of this book.)
to isoprene in bacteria could be a phenotype derived
from convergent evolution using a multistep reaction
diverged from those pathways (Izumikawa et al., 2010;
Withers et al., 2007; Xue and Ahring, 2011).
Bioproduction of isoprene is feasible and has already
been demonstrated in E. coli expressing heterologous
ispS (Miller et al., 2001; Zhao et al., 2011). Of course productivity is an issue and different strategies were tried
to increase isoprene production. Simultaneous expression of heterologous enzymes involved in MEP or mevalonate pathways was shown to be effective in both cases
(Yang et al., 2012; Zhao et al., 2011). Julsing et al. also
showed that the individual expression of the genes
encoding enzymes involved in the MEP pathway did
not affect isoprene production with the exception of the
dxs gene, encoding the enzyme that catalyzes the first reaction of the MEP pathway, which significantly
improved isoprene production (Julsing et al., 2007). Cyanobacteria produce DMAPP through the MEP pathway
for secondary metabolites and, albeit with no natural production of isoprene being reported yet, transformation
and expression of heterologous ispS were shown to be
sufficient for production of isoprene. Lindberg et al. reported isoprene production using Synechocystis sp. PCC
6803 as a model organism harboring ispS from Pueraria
montana (Kudzu) (Lindberg et al., 2010). The transgene
was inserted at the psbA2 locus and mutants did not
show any disturbance in growth when compared to the
wild type. This was a well-achieved proof of concept,
and the low productivity reported, 50 mg per gram of
dry cell weight per day, can be much improved through
metabolic engineering. However, the use of cyanobacteria to produce isoprene has issues different from metabolic yield: to develop a production system of a
molecule with a half-life of only a couple hours in the
presence of light is particularly challenging in a photosynthetic organism. To overcome this issue, the development of special photobioreactors is made in parallel to the
molecular research, where the properties of isoprene as a
volatile hydrophobic compound, easily separated from a
culture broth and concentrating in the headspace, are
exploited (Lindblad et al., 2012). The production of a
gas in microorganisms is an interesting strategy because
one does not need to harvest the cells, the product is
concentrated in the gaseous phase of the culture. However, the cultivation techniques and the purification of this
gas from a complex mixture represents an important
step in the production chain and, as shown in this case,
should develop together.
Butyraldehyde and Butanol
Butanol has many desirable properties as a fuel
and thus is a suitable target for modification of
402
22. ENGINEERED CYANOBACTERIA: RESEARCH AND APPLICATION IN BIOENERGY
cyanobacteria. In fact, as a fuel it is superior to ethanol,
being less corrosive and less volatile. Thus, it can easily
be mixed with hydrocarbon-based fuels and used in the
same infrastructure. A number of recent studies have
shown that engineered cyanobacteria can in fact make
surprisingly high levels of this compound, at rates that
in fact surpass published rates for ethanol production
by engineered cyanobacteria. Since cyanobacteria produce this fuel directly through photosynthetically
driven CO2 fixation, it is appropriate to compare the productivity per area of this process, as presently described,
with that required for other biofuels, be it growing corn
to produce the necessary sugars, or growing algae to
produce biodiesel. Such a comparison shows that
butanol production by cyanobacteria could be much better than making fuels from corn and very comparable to
making biodiesel from microalgae (Sheehan, 2009).
Many cyanobacteria are capable of producing volatile
compounds, including higher alcohols, but the natural
production levels are miniscule (Hasegawa et al.,
2012). In order to make fuel molecules at significant
quantities, new pathways must be introduced as well
as changes made to the native metabolic pathways.
Studies on creating heterotrophic bacterial strains
capable of producing butanol demonstrated that two
possible routes were useful: the 2-ketoacid pathway,
normally involved in amino acid biosynthesis, and the
acetyl-CoA pathway, found in organisms such as Clostridium that naturally produce butanol during fermentation (Figure 22.4).
The first successful attempt in this direction was to
engineer S. elongates to produce isobutyraldehyde
through the 2-ketoacid pathway (Atsumi et al., 2009).
Isobutyraldehyde is a precursor for isobutanol and other
chemicals of interest and has the advantage of being
highly volatile, easing its recovery from the culture
broth thus removing product inhibition. The strategy
applied consisted of boosting carbon flux through the
pathway from pyruvate to 2-ketoisovalerate by integration into the genome of three foreign genes, alsS, ilvC
and ilvD, catalyzing these steps, as well as kivd from
Lactococcus lactis, the gene encoding the ketoacid decarboxylase enzyme that converts 2-ketoisovalerate to isobutyraldehyde. Overall carbon flux was then increased
by integrating an additional copy of Rubisco (rbcLS)
and the resulting strain produced 6230 mg isobutyraldehyde per liter per hour , a production rate that is
higher than any other fuel molecule made by cyanobacteria to date. Additionally, it was demonstrated that
isobutanol could be formed if a foreign alcohol dehydrogenase (YqhD from E. coli) was introduced, but titers
were lower, presumably due to product inhibition.
However, the isomer that is made by the
2-ketoisovalerate pathway is isobutanol, a fuel additive,
but not nearly as desirable in itself as a fuel as
n-butanol, the product of the acetyl-CoA pathway or
the 2-ketobutyrate pathway (Figure 22.4). Metabolic
engineering was used to create an n-butanol-producing
strain of S. elongatus by introducing the hbd, crt, and
adhE2 genes from C. acetobutylicum, the ter gene from
Treponema denticola, and the atoB gene (instead of thl)
from E. coli (Lan and Liao, 2011). However, n-butanol
was only produced by this strain under anaerobic conditions, either in the light when photosystem II was
inhibited by DCMU, or in the dark, which gave the
highest production, a meager 20.8 mg/L/h. It was suggested that anaerobic conditions were necessary since
some of the enzymes introduced are oxygen sensitive,
severely limiting its usefulness. On the other hand,
metabolic fluxes are obviously different during dark
metabolism than during photosynthesis and the difference could be in the supply of a key metabolite. In line
with this, in a more recent attempt to create an
n-butanol-producing strain, flux through acetyl-CoA
was increased by substituting an irreversible ATP
hydrolysis step leading to the formation of acetoacetyl
CoA (Lan and Liao, 2011). Other improvements consisted of substituting NADPH-requiring enzymes for
NADH enzymes. With these changes, it was possible
to demonstrate light-dependent n-butanol production,
but at 62.5 mg/L/h this is well below (by a factor of
100) the initial promising results with butyraldehyde.
This system would need very significant improvement
before it could be considered for practical biofuel
production.
Photosynthetic Production of Aliphatic
Alcohols and Alkanes
For many fuel purposes, alkanes are more desirable
than the other biofuels already discussed. For example,
jet fuel standards (Jet-A or JP-8) demand a fuel with
high energy density, low viscosity, low freezing point
and good physical-chemical compatibility. These criteria
cannot be met with fuels such as ethanol or fatty acid
methyl esters, biodiesel. Being able to directly make alkanes would have a great payoff as these biofuels are
“drop-in” fuels, able to directly substitute for presently
used petroleum-based fuels as they could be used with
existing infrastructure and would require no engine
modification, etc.
Cyanobacteria, like some other bacteria, have long
been recognized as being able to synthesize at least
very small quantities of alkanes, which in fact can serve
as a biogeochemical marker for their presence in the past
(Han et al., 1968; Winters et al., 1969). This was taken
advantage of in a recent demonstration of the heterotrophic production of alkanes using a modified E. coli that
expressed the alkane biosynthetic pathway from a
REFERENCES
cyanobacterium, consisting of an acyl-carrier protein
reductase, which produces a fatty aldehyde, and an
aldehyde decarbonylase (Schirmer et al., 2010). This
allowed the production and secretion of a variety of
C13eC17 alkanes and alkenes. Of course it would be
desirable to actually do this in a cyanobacterium, and
one study examined this through the heterologous
expression of fatty acyl-CoA reductase in Synechocystis
(Tan et al., 2011), which allowed the production of small
quantities of aliphatic alcohols. The acc genes, encoding
acetyl-CoA carboxylase (ACCase), which catalyses what
is believed to be the rate-limiting step of fatty acid
biosynthesis, were introduced into the genome in hopes
of boosting alkane production, but only insignificant
quantities were made. Further work is required to
demonstrate significant alkane synthesis by a cyanobacterium. However, it may prove difficult to greatly boost
alkane synthesis in this oxygen-evolving organism as
the critical enzyme, aldehyde decarbonylase, has
recently been shown to be a di-iron enzyme with an
unusual mechanism that requires anaerobic conditions
for full activity (Das et al., 2011).
CONCLUSION AND OUTLOOK
As discussed in this chapter, recent studies have
shown the great promise for biofuels production by
cyanobacteria. Unique among possible biofuel
producers, cyanobacteria combine the attributes of
being able to carry out photosynthesis-driven carbon
dioxide fixation and to be easily manipulated genetically. The next few years should see advances in
increasing the production rates and titers of the
different demonstrated biofuels as well as perhaps
the widening of the spectrum of possible biofuels.
Nevertheless, for cyanobacterial systems to live up to
their potential, a number of serious hurdles must be
overcome. These include the development of reliable
methods of stable cyanobacterial mass culture at high
levels of productivity and the demonstration of costeffective harvesting strategies. Harvesting presents a
real dilemma no matter what the biofuel. If the biofuel
is contained within the cell, then the biomass has to be
removed from the culture medium, of which it is less
than 1% by weight. If the biofuel is an excreted liquid,
then this will necessarily be quite dilute and require
substantial concentration. If the biofuel is a gaseous
product, the culture will have to be enclosed in airtight transparent material at a substantial cost given
the large surface areas that would be required. Of
course, the payoff to solving these problems would be
enormous and this is likely to inspire future research
and development in this area.
403
References
Abeles, A.L., 1972. Biochemical pathway of stress-induced ethylene.
Plant Physiol. 50, 496e498.
Ananyev, G.M., Skizim, N.J., Dismukes, G.C., 2012. Enhancing biological hydrogen production from cyanobacteria by removal of
excreted products. J. Biotechnol. 162, 97e104.
Angermayr, S.A., Hellingwerf, K.J., Lindblad, P., Teixeira de
Mattos, M.J., 2009. Energy biotechnology with cyanobacteria. Curr.
Opin. Biotechnol. 20, 257e263. NCBI: http://www.ncbi.nlm.nih.gov.
Aryal, U.K., Callister, S.J., Mishra, S., Zhang, X., Shutthanandan, J.I.,
Angel, T.E., Shukla, A.K., Monroe, M.E., Moore, R.J.,
Koppenaal, D.W., Smith, R.D., Sherman, L., 2013. Proteome analyses
of strains ATCC 51142 and PCC 7822 of the diazotrophic cyanobacterium Cyanothece sp. under culture conditions resulting in
enhanced H2 production. Appl. Environ. Microbiol. 79, 1070e1077.
Atsumi, S., Higashide, W., Liao, J.C., 2009. Direct photosynthetic
recycling of carbon dioxide to isobutyraldehyde. Nat. Biotechnol.
27, 1177e1180.
Ball, P., 2007. Burning Water and Other Myths. news@nature.
Bandyopadhyay, A., Stöckel, J.S.O., Min, H., Sherman, L.A.,
Pakrasi, H.B., 2010. High rates of photobiological H2 production by
a cyanobacterium under aerobic conditions. Nat. Commun. 1,
139e147.
Benemann, J.R., Weare, N.M., 1974. Hydrogen evolution by nitrogenfixing Anabaena cylindrical cultures. Science 184, 174e175.
Berberoglu, H., 2008. Effect of nutrient media on photobiological
hydrogen production by Anabaena variabilis ATCC 29413. Int. J.
Hydrogen Energy 33, 1172e1184.
Berman-Frank, I., 2001. Segregation of nitrogen fixation and oxygenic
photosynthesis in the marine cyanobacterium Trichodesmium.
Science 294, 1534e1537.
Bothe, H., Schmitz, O., Yates, M.G., Newton, W.E., 2010. Nitrogen
fixation and hydrogen metabolism in cyanobacteria. Microbiol.
Mol. Biol. Rev. 74, 529e551.
BP, 2012. BP Statistical Review of World Energy June 2012. BP. p. 48.
http://bp.com/statisticalreview.
Das, D., Eser, B.E., Han, J., Sciore, A., Marsh, E.N.G., 2011. OxygenIndependent Decarbonylation of Aldehydes by Cyanobacterial
Aldehyde Decarbonylase: A New Reaction of Diiron Enzymes.
Angewandte Chemie International Edition 50 (31), 7148e7152.
Deng, M.D., Coleman, J.R., 1999. Ethanol synthesis by genetic engineering in cyanobacteria. Appl. Environ. Microbiol. 65, 523e528.
Dexter, J., Fu, P., 2009. Metabolic engineering of cyanobacteria for
ethanol production. Energy Environ. Sci. 2, 857.
Ducat, D.C., Way, J.C., Silver, P.A., 2011. Engineering cyanobacteria to
generate high-value products. Trends. Biotechnol. 29, 95e103.
Dutheil, J., Saenkham, P., Sakr, S., Leplat, C., Ortega-Ramos, M.,
Bottin, H., Cournac, L., Cassier-Chauvat, C., Chauvat, F., 2012. The
AbrB2 autorepressor, expressed from an atypical promoter,
represses the hydrogenase operon to regulate hydrogen production in Synechocystis strain PCC6803. J. Bacteriol. 194, 5423e5433.
EIADOE, 2012. Primary energy consumption by source and sector,
2011 384(null). In: EIA - US Energy Information Administration,
vol. 1. http://www.eia.gov/aer.
Elhai, J., Vepritskiy, A., MuroPastor, A.M., Flores, E., Wolk, C.P., 1997.
Reduction of conjugal transfer efficiency by three restriction
activities of Anabaena sp. strain PCC 7120. J. Bacteriol. 179, 1998e2005.
Field, C.B., Behrenfeld, M.J., Randerson, J.T., Falkowski, P., 1998.
Primary production of the biosphere: integrating terrestrial and
oceanic components. Science 281, 237e240.
Flores, E., Muro-Pastor, A.M., Meeks, J.C., 2008. Gene transfer to
cyanobacteria in the laboratory and in nature. In: Herrero, A.,
Flores, E. (Eds.), The Cyanobacteria: Molecular Biology, Genomics
and Evolution. Caister Academic Press.
404
22. ENGINEERED CYANOBACTERIA: RESEARCH AND APPLICATION IN BIOENERGY
Freebairn, H.T., Buddenhagen, I.W., 1964. Ethylene production by
Pseudomonas solanacearum. Nature 202, 313e314.
Fukuda, H., Ogawa, T., Ishihara, K., Fujii, T., Nagahama, K., Omata, T.,
Inoue, Y., Tanase, S., Morino, Y., 1992a. Molecular-cloning in
Escherichia coli, expression, and Nucleotide-sequence of the gene
for the ethylene-forming enzyme of Pseudomonas syringae pv phaseolicola-Pk2. Biochem. Biophys. Res. Commun. 188, 826e832.
Fukuda, H., Ogawa, T., Tazaki, M., Nagahama, K., Fujii, T., Tanase, S.,
Morino, Y., 1992b. Two reactions are simultaneously catalyzed by a
single enzyme: the arginine-dependent simultaneous formation of
two products, ethylene and succinate, from 2-oxoglutarate by an
enzyme from Pseudomonas syringae. Biochem. Biophys. Res. Commun. 188, 483e489.
Fukuda, H., Sakai, M., Nagahama, K., Fujii, T., Matsuoka, M.,
Inoue, Y., Ogawa, T., 1994. Heterologous expression of the gene for
the ethylene-forming enzyme from Pseudomonas syringae in the
cyanobacterium Synechococcus. Biotechnol. Lett. 16, 1e6.
Gao, Z., Zhao, H., Li, Z., Tan, X., Lu, X., 2012. Photosynthetic production of ethanol from carbon dioxide in genetically engineered
cyanobacteria. Energy Environ. Sci. 5, 9857.
Gartner, K., Lechno-Yossef, S., Cornish, A.J., Wolk, C.P., Hegg, E.L.,
2012. Expression of Shewanella oneidensis MR-1 [FeFe]-Hydrogenase
genes in Anabaena sp. Strain PCC 7120. Appl. Environ. Microbiol.
78, 8579e8586.
Ghirardi, M.L., Posewitz, M.C., Maness, P.-C., Dubini, A., Yu, J.,
Seibert, M., 2007. Hydrogenases and hydrogen photoproduction in
oxygenic photosynthetic organisms. Annu. Rev. Plant Biol. 58,
71e91.
Golden, S.S., Sherman, L.A., 1983. A hybrid plasmid is a stable cloning
vector for the cyanobacterium Anacystis nidulans R2. J. Bacteriol.
155, 966e972.
Grigorieva, G., Shestakov, S., 1982. Transformation in the cyanobacterium Synechocystis sp. 6803. FEMS. Microbiol. Lett. 13, 367e370.
Guiry, M.D., Guiry, G.M. Algaebase. National University of Ireland,
Galway. http://www.algaebase.org.
Hallenbeck, P.C., Benemann, J.R., 1978. Characterization and partial
purification of reversible hydrogenase of Anabaena cylindrica. FEBS
Lett. 94, 261e264.
Hallenbeck, P.C., Benemann, J.R., 2002. Biological hydrogen production; fundamentals and limiting processes. Int. J. Hydrogen.
Energy. 27, 1185e1193.
Hallenbeck, P.C., 2011. Hydrogen production by cyanobacteria. In:
Hallenbeck, P.C. (Ed.), Microbial Technologies in Advanced
Biofuels Production. Springer US, Boston, MA, pp. 15e28.
Han, J., McCarthy, E.D., Hoeven, W.V., Calvin, M., Bradley, W.H.,
1968. Organic geochemical studies, II. a preliminary report on
the distribution of aliphatic hydrocarbons in algae, in bacteria,
and in a recent lake sediment. Proc. Natl. Acad. Sci. USA. 59,
29e33.
Hasegawa, M., Nishizawa, A., Tsuji, K., Kimura, S., Harada, K.-I.,
2012. Volatile organic compounds derived from 2-Keto-Acid
decarboxylase in Microcystis aeruginosa. Microb. Environ. 27,
525e528.
Heidorn, T., Camsund, D., Huang, H.-H., Lindberg, P., Oliveira, P.,
Stensjö, K., Lindblad, P., 2011. Synthetic biology in cyanobacteria:
engineering and analyzing novel functions. In: Voigt, C. (Ed.),
Methods in Enzymology, Synthetic Biology, Methods for Part/
Device Characterization and Chassis Engineering, Part A, vol. 497.
Elsevier academic press inc, 525 B Street, suite 1900, San Diego, CA
92101-4495, USA, pp. 539e579.
Huesemann, M.H., Hausmann, T.S., Carter, B.M., Gerschler, J.J.,
Benemann, J.R., 2009. Hydrogen generation through indirect biophotolysis in batch cultures of the nonheterocystous nitrogenfixing cyanobacterium Plectonema boryanum. Appl. Biochem. Biotechnol. 162, 208e220.
Ishihara, K., Matsuoka, M., Inoue, Y., Tanase, S., Ogawa, T.,
Fukuda, H., 1995. Overexpression and in-vitro reconstitution of the
ethylene-forming enzyme from Pseudomonas syringae. J. Ferment.
Bioeng. 79, 205e211.
Ishihara, K., Matsuoka, M., Ogawa, T., Fukuda, H., 1996. Ethylene
production using a broad-host-range plasmid in Pseudomonas
syringae and Pseudomonas putida. J. Ferment. Bioeng. 82, 509e511.
Izumikawa, M., Khan, S.T., Takagi, M., Shin-ya, K., 2010. Spongederived Streptomyces producing isoprenoids via the mevalonate
pathway. J. Nat. Prod. 73, 208e212.
Julsing, M.K., Rijpkema, M., Woerdenbag, H.J., Quax, W.J., Kayser, O.,
2007. Functional analysis of genes involved in the biosynthesis of
isoprene in Bacillus subtilis. Appl. Microbiol. Biotechnol. 75,
1377e1384.
Kilian, O., Benemann, C.S.E., Niyogi, K.K., Vick, B., 2011. Highefficiency homologous recombination in the oil-producing alga
Nannochloropsis sp. Proc. Natl. Acad. Sci. USA. 108, 21265e21269.
Knoll, A., 2008. Cyanobacteria and earth history. In: Herrero, A.,
Flores, E. (Eds.), The Cyanobacteria: Molecular Biology, Genomics
and Evolution. Caister Academic Press.
Koksharova, O.A., Wolk, C.P., 2002. Genetic tools for cyanobacteria.
Appl. Microbiol. Biotechnol. 58, 123e137.
Kothari, A., Potrafka, R., Garcia-Pichel, F., 2012. Diversity in hydrogen
evolution from bidirectional hydrogenases in cyanobacteria from
terrestrial, freshwater and marine intertidal environments. J. Biotechnol. 162, 105e114.
Kumar, K., Mella-Herrera, R.A., Golden, J.W., 2010. Cyanobacterial
heterocysts. Cold Spring Harbor Perspect. Biol. 2, a000315.
Kuzma, J., Nemecek-Marshall, M., Pollock, W.H., Fall, R., 1995. Bacteria produce the volatile hydrocarbon isoprene. Curr. Microbiol.
30, 97e103.
Lan, E.I., Liao, J.C., 2011. Metabolic engineering of cyanobacteria for
1-butanol production from carbon dioxide. Metab. Eng. 13,
353e363.
Leplat, C., Champeimont, R., Saenkham, P., Cassier-Chauvat, C., JeanChristophe, A., Chauvat, F., 2013. Genome-wide transcriptome
analysis of hydrogen production in the cyanobacterium Synechocystis: towards the identification of new players. Int. J. Hydrogen
Energy 38, 1866e1872.
Lindberg, P., Park, S., Melis, A., 2010. Engineering a platform for
photosynthetic isoprene production in cyanobacteria, using Synechocystis as the model organism. Metab. Eng. 12, 70e79.
Lindblad, P., Lindberg, P., Oliveira, P., Stensjö, K., Heidorn, T., 2012.
Design, engineering, and construction of photosynthetic microbial
cell factories for renewable solar fuel production. Ambio 41,
163e168.
Lopez-Igual, R., Flores, E., Herrero, A., 2010. Inactivation of a
heterocyst-specific invertase indicates a principal role of sucrose
catabolism in heterocysts of Anabaena sp. J. Bacteriol. 192,
5526e5533.
Ludwig, M., Schulz-Friedrich, R., Appel, J., 2006. Occurrence of
hydrogenases in cyanobacteria and anoxygenic photosynthetic
bacteria: Implications for the phylogenetic origin of cyanobacterial
and algal hydrogenases. J. Mol. Evol. 63, 758e768.
Mariscal, V., Flores, E., 2010. In: Hallenbeck, P.C. (Ed.), 2010.
Multicellularity in a Heterocyst-forming Cyanobacterium: Pathways for Intercellular Communication, vol. 675. Springer-Verlag
Berlin, Heidelberger Platz 3, D-14197, Berlin, Germany,
pp. 123e135.
Marreiros, B.C., Batista, A.P., Duarte, A.M.S., Pereira, M.M., 2013.
A missing link between complex I and group 4 membrane-bound
[NiFe] hydrogenases. Biochim. Biophys. 1827, 198e209.
McCoy, M., Reisch, M., Tullo, A.H., Short, P.L., Tremblay J-, F., 2006.
Production: growth is the norm. Chem. Eng. News Arch. 84,
59e68.
REFERENCES
McNeely, K., Xu, Y., Bennette, N., Bryant, D.A., Dismukes, G.C., 2010.
Redirecting reductant flux into hydrogen production via metabolic
engineering of fermentative carbon metabolism in a cyanobacterium. Appl. Environ. Microbiol. 76, 5032e5038.
Melnicki, M.R., Pinchuk, G.E., Hill, E.A., Kucek, L.A.,
Fredrickson, J.K., Konopka, A., Beliaev, A.S., 2012. Sustained H2
production driven by photosynthetic water splitting in a unicellular cyanobacterium. mBio 3, e00197ee00212.
Miller, B., Oschinski, C., Zimmer, W., 2001. First isolation of an
isoprene synthase gene from poplar and successful expression of
the gene in Escherichia coli. Planta 213, 483e487.
Min, H., Sherman, L.A., 2010. Hydrogen production by the unicellular,
diazotrophic cyanobacterium Cyanothece sp. strain ATCC 51142
under conditions of continuous light. Appl. Environ. Microbiol. 76,
4293e4301.
Miyamoto, K., Hallenbeck, P.C., Benemann, J.R., 1979a. Effects of nitrogen supply on hydrogen production by cultures of Anabaena
cylindrica. Biotechnol. Bioeng. 21, 1855e1860.
Miyamoto, K., Hallenbeck, P.C., Benemann, J.R., 1979b. Hydrogen
production by the thermophilic alga Mastigocladus laminosus effects of nitrogen, temperature, and inhibition of photosynthesis.
Appl. Environ. Microbiol. 38, 440e446.
Miyamoto, K., Hallenbeck, P.C., Benemann, J.R., 1979c. Nitrogenfixation by thermophilic blue-green algae (cyanobacteria) temperature characteristics and potential use in biophotolysis.
Appl. Environ. Microbiol. 37, 454e458.
Miyamoto, K., Hallenbeck, P.C., Benemann, J.R., 1979d. Solar energy
conversion by nitrogen limited cultures of Anabaena cylindrical.
J. Ferment. Technol. 57, 287e293.
Murry, M.A., Hallenbeck, P.C., Benemann, J.R., 1984. Immunochemical
evidence that nitrogenase is restricted to the heterocysts in Anabaena cylindrica. Arch. Microbiol. 137, 194e199.
Murry, M.A., Hallenbeck, P.C., Esteva, D., Benemann, J.R., 1983.
Nitrogenase inactivation by oxygen and enzyme turnover in
Anabaena cylindrica. Can. J. Microbiol. 29, 1286e1294.
Nagahama, K., Ogawa, T., Fujii, T., Fukuda, H., 1992. Classification of
ethylene-producing bacteria in terms of biosynthetic pathways to
ethylene. J. Ferment. Bioeng. 73, 1e5.
Nagahama, K., Ogawa, T., Fujii, T., Tazaki, M., Tanase, S., Morino, Y.,
Fukuda, H., 1991. Purification and properties of an ethyleneforming enzyme from Pseudomonas syringae pv. phaseolicola PK2.
J. Gen. Microbiol. 137, 2281e2286.
Nakatsuka, A., Murachi, S., Okunishi, H., Shiomi, S., Nakano, R.,
Kubo, Y., Inaba, A., 1998. Differential expression and internal
feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxylate oxidase, and ethylene
receptor genes in tomato fruit during development and ripening.
Plant. Physiol. 118, 1295e1305.
Nashawi, I.S., Malallah, A., Al-Bisharah, M., 2010. Forecasting world
crude oil production using multicyclic Hubbert model. Energy
Fuels 24, 1788e1800.
Ogawa, T., Takahashi, M., Fujii, T., Tazaki, M., Fukuda, H., 1990.
The role of NADH: Fe(III)EDTA oxidoreductase in ethylene
formation from 2-Keto-4-methylthiobutyrate. J. Ferment. Bioeng.
69, 287e291.
Oliveira, P., Lindblad, P., 2009. Transcriptional regulation of the cyanobacterial bidirectional Hox-hydrogenase. Dalton Trans., 9990.
Perovic, S., Seack, J., Gamulin, V., Muller, W., Schroder, H.C., 2001.
Modulation of intracellular calcium and proliferative activity of
invertebrate and vertebrate cells by ethylene. BMC Cell Biol. 2. art.
no.e7.
Prabaharan, D., Kumar, D.A., Uma, L., Subramanian, G., 2010. Dark
hydrogen production in nitrogen atmosphere - an approach for
sustainability by marine cyanobacterium Leptolyngbya valderiana
BDU 20041. Int. J. Hydrogen Energy 35, 10725e10730.
405
Ray, D.K., Ramankutty, N., Mueller, N.D., West, P.C., Foley, J.A., 2012.
Recent patterns of crop yield growth and stagnation. Nat.
Commun. 3, 1293.
Saini, V., Sigman, M.S., 2012. Palladium-catalyzed 1,1-difunctionalization
of ethylene. J. Am. Chem. Soc. 134, 11372e11375.
Sakai, M., Ogawa, T., Matsuoka, M., Fukuda, H., 1997. Photosynthetic
conversion of carbon dioxide to ethylene by the recombinant
cyanobacterium, Synechococcus sp. PCC 7942, which harbors a gene
for the ethylene-forming enzyme of Pseudomonas syringae.
J. Ferment. Bioeng. 84, 434e443.
Sakurai, H., Masukawa, H., 2007. Promoting R & D in photobiological
hydrogen production utilizing mariculture-raised cyanobacteria.
Mar. Biotechnol. 9, 128e145.
Schirmer, A., Rude, M.A., Li, X., Popova, E., del Cardayre, S.B., 2010.
Microbial biosynthesis of alkanes. Science 329, 559e562.
Schmitz, O., Boison, G., Bothe, H., 2001. Quantitative analysis of
expression of two circadian clock-controlled gene clusters coding
for the bidirectional hydrogenase in the cyanobacterium Synechococcus sp. PCC7942. Mol. Microbiol. 41, 1409e1417.
Schwarz, C., Poss, Z., Hoffmann, D., Appel, J., 2010. In: Hallenbeck, P.C.
(Ed.), 2010. Hydrogenases and Hydrogen Metabolism in Photosynthetic Prokaryotes, vol. 675. SPRINGER-VERLAG BERLIN,
Heidelberger Platz 3, D-14197, BERLIN, GERMANY, pp. 305e348.
Seabra, R., Santos, A., Pereira, S., Moradas-Ferreira, P., Tamagnini, P.,
2009. Immunolocalization of the uptake hydrogenase in the marine
cyanobacterium Lyngbya majuscula CCAP 1446/4 and two Nostoc
strains. FEMS Microbiol. Lett. 292, 57e62.
Sharkey, T.D., 1996. Emission of low molecular mass hydrocarbons
from plants. Trends Plant Sci. 1, 78e82.
Sharkey, T.D., Chen, X.Y., Yeh, S., 2001. Isoprene increases thermotolerance of fosmidomycin-fed leaves. Plant Physiol. 125,
2001e2006.
Sheehan, J., 2009. Engineering direct conversion of CO2 to biofuel.
Nat. Biotechnol. 27, 1128e1129.
Shestako, S., Khyen, N.T., 1970. Evidence for genetic transformation in
blue-green alga Anacystis nidulans. Mol. Gen. Genet. 107, 372.
Silver, G.M., Fall, R., 1991. Enzymatic synthesis of isoprene from
dimethylallyl diphosphate in aspen leaf extracts. Plant Physiol. 97,
1588e1591.
Silver, G.M., Fall, R., 1995. Characterization of aspen isoprene synthase, an enzyme responsible for leaf isoprene emission to the
atmosphere. J. Biol. Chem. 270, 13010e13016.
Summers, M.L., Wallis, J.G., Campbell, E.L., Meeks, J.C., 1995. Genetic
evidence of a major role for glucose-6-phosphate-dehydrogenase
in nitrogen-fixation and dark growth of the cyanobacterium Nostoc
sp strain Atcc-29133. J. Bacteriol. 177, 6184e6194.
Taiz, L., Zeiger, E., 2002. In: Taiz, Lincoln (Ed.), Plant Physiology, third
ed. Sinauer Associates, Sunderland, MA, p. 672.
Takahama, K., Matsuoka, M., Nagahama, K., Ogawa, T., 2003.
Construction and analysis of a recombinant cyanobacterium
expressing a chromosomally inserted gene for an ethylene-forming
enzyme at the psbAI locus. J. Biosci. Bioeng. 95, 302e305.
Tamagnini, P., Leitao, E., Oliveira, P., Ferreira, D., Pinto, F., Harris, D.J.,
Heidorn, T., Lindblad, P., 2007. Cyanobacterial hydrogenases:
diversity, regulation and applications. FEMS Microbiol. Rev. 31,
692e720.
Tan, X., Yao, L., Gao, Q., Wang, W., Qi, F., Lu, X., 2011. Photosynthesis
driven conversion of carbon dioxide to fatty alcohols and hydrocarbons in cyanobacteria. Metab. Eng. 13, 169e176.
Tao, L., Dong, H.-J., Chen, X., Chen, S.-F., Wang, T.-H., 2008. Expression of ethylene-forming enzyme (EFE) of Pseudomonas syringae pv.
glycinea in Trichoderma viride. Appl. Microbiol. Biotechnol. 80,
573e578.
Thiel, T., Poo, H., 1989. Transformation of a filamentous cyanobacterium by electroporation. J. Bacteriol. 171, 5743e5746.
406
22. ENGINEERED CYANOBACTERIA: RESEARCH AND APPLICATION IN BIOENERGY
Troshina, O., Serebryakova, L., Sheremetieva, M., Lindblad, P., 2002.
Production of H-2 by the unicellular cyanobacterium Gloeocapsa
alpicola CALU 743 during fermentation. International Journal of
Hydrogen Energy 27, 1283e1289.
Tsinoremas, N.F., Kutach, A.K., Strayer, C.A., Golden, S.S., 1994.
Efficient gene-transfer in Synechococcus sp strains Pcc-7942 and Pcc6301 by Interspecies conjugation and chromosomal recombination.
J. Bacteriol. 176, 6764e6768.
Tsygankov, A.A., Fedorov, A.S., Kosourov, S.N., Rao, K.K., 2002.
Hydrogen production by cyanobacteria in an automated outdoor
photobioreactor under aerobic conditions. Biotechnol. Bioeng. 80,
777e783.
Ungerer, J., Tao, L., Davis, M., Ghirardi, M., Maness, P.-C., Yu, J., 2012.
Sustained photosynthetic conversion of CO2 to ethylene in
recombinant cyanobacterium Synechocystis 6803. Energy Environ.
Sci. 5, 8998e9006.
Verne, J. The Mysterious Island. Plain Label Books.
Vignais, P.M., Billoud, B., 2007. Occurrence, classification, and biological function of hydrogenases: an overview. Chem. Rev. 107,
4206e4272.
Wang, C.H., Persyn, A., Krackov, J., 1962. Role of the Krebs cycle in
ethylene biosynthesis. Nature 195, 1306e1308.
Wang, K., Li, H., Ecker, J.R., 2002. Ethylene biosynthesis and signaling
networks. Plant Cell 14, S131eS151.
Weingart, H., Völksch, B., Ullrich, M.S., 1999. Comparison of ethylene
production by Pseudomonas syringae and Ralstonia solanacearum.
Phytopathology 89, 360e365.
Weissman, J.C., Benemann, J.R., 1977. Hydrogen production by
nitrogen-starved cultures of Anabaena cylindrica. Appl. Environ.
Microbiol. 33, 123e131.
Weyman, P.D., Pratte, B., Thiel, T., 2008. Transcription of hupSL in
Anabaena variabilis ATCC 29413 is regulated by NtcA and Not by
Hydrogen. Appl. Environ. Microbiol. 74, 2103e2110.
Winters, K., Parker, P.L., Vanbaale, C., 1969. Hydrocarbons of bluegreen algae - geochemical significance. Science 163, 467.
Withers, S.T., Gottlieb, S.S., Lieu, B., Newman, J.D., Keasling, J.D.,
2007. Identification of isopentenol biosynthetic genes from Bacillus
subtilis by a screening method based on isoprenoid precursor
toxicity. App. Environ. Microbiol. 73, 6277e6283.
Wolk, C.P., Vonshak, A., Kehoe, P., Elhai, J., 1984. Construction of
shuttle vectors capable of conjugative transfer from Escherichia coli
to nitrogen-fixing filamentous cyanobacteria. Proc. Natl. Acad. Sci.
USA. 81, 1561e1565.
Xue, J., Ahring, B.K., 2011. Enhancing isoprene production by
genetic modification of the 1-Deoxy-D-Xylulose-5-Phosphate
pathway in Bacillus subtilis. Appl. Environ. Microbiol. 77, 2399e2405.
Yang, J., Zhao, G., Sun, Y., Zheng, Y., Jiang, X., Liu, W., Xian, M., 2012.
Bio-isoprene production using exogenous MVA pathway and
isoprene synthase in Escherichia coli. Bioresour. Technol. 104,
642e647.
Yoon, H.S., 2004. A molecular timeline for the origin of photosynthetic
eukaryotes. Mol. Biol. Evol. 21, 809e818.
Yoon, H.S., Haeshin, J., Kim, M., Junsim, S., Park, T., 2006. Evaluation
of conversion efficiency of light to hydrogen energy by Anabaena
variabilis. Int. J. Hydrogen Energy 31, 721e727.
Yoshihara, S., Geng, X., Okamoto, S., Yura, K., Murata, T., Go, M.,
Ohmori, M., Ikeuchi, M., 2001. Mutational analysis of genes
involved in pilus structure, motility and transformation competency in the unicellular motile cyanobacterium Synechocystis sp.
PCC 6803. Plant Cell. Physiol. 42, 63e73.
Yura, K., 1999. Putative mechanism of natural transformation as
deduced from genome data. DNA Res. 6, 75e82.
Zang, X., Liu, B., Liu, S., Arunakumara, K.K.I.U., Zhang, X., 2007.
Optimum conditions for transformation of Synechocystis sp. PCC
6803. J. Microbiol. 45, 241e245.
Zhang, S., Bryant, D.A., 2011. The Tricarboxylic Acid Cycle in Cyanobacteria. Science 334 (6062), 1551e1553.
Zhao, Y., Yang, J., Qin, B., Li, Y., Sun, Y., Su, S., Xian, M., 2011. Biosynthesis
of isoprene in Escherichia coli via methylerythritol phosphate (MEP)
pathway. Appl. Microbiol. Biotechnol. 90, 1915e1922.
C H A P T E R
23
Sustainable Farming of Bioenergy Crops
Adrian Muller
Research Institute of Organic Agriculture FiBL, Zurich, Switzerland; Institute for Environmental Decisions,
Swiss Federal Institutes of Technology (ETH), Zurich, Switzerland
email: adrian.mueller@fibl.org
O U T L I N E
Introduction
407
Criteria for Sustainable Farming and Sustainable
Food Systems
Conventional Agricultural
Production
Sustainable Agricultural Production
Sustainable Food Systems
What is Sustainable Bioenergy
Production?
Ways of Comparisons
409
409
409
410
410
410
INTRODUCTION
Is bioenergy a sustainable energy source? A positive
answer to this question is a key if bioenergy shall
become a significant sustainable energy source for
future societies. The answer to this question depends
on various aspects of the production and use of bioenergy. The most prominent topic there is the greenhouse gas (GHG) balance, as this is the key
motivation to investigate bioenergy at all. For most
cases, the GHG balance is positive, albeit not at a
tremendously high rate and negative values are due
to large emissions from direct and indirect land use
change (ILUC), e.g. if palm oil plantations are established on peatland rainforest (Faist Emmenegger
et al., 2012; PBL, 2010; Fargione et al., 2008). Clearly,
in the use phase, GHG emissions are counted as zero
due to the overall assumption of renewable biomass
provision for bioenergy. However, over the whole life
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00023-1
Sustainability Criteria for Biofuel Production
Biomass Use
410
412
How Much Bioenergy may be Produced
Sustainably?
Global Bioenergy Potential
Bioenergy Potential on Farm Level
412
413
414
Conclusions
415
References
415
cycle of bioenergy, GHG emissions arise at various
steps, in particular, in the agricultural production
phase (Faist Emmenegger et al., 2012). The GHG balance usually plays the role of a fundamental decision
criterion in favor or against bioenergy types. With a
zero or negative balance, bioenergy will not contribute
to and may even adversely affect climate change mitigation. However, even in this case, one could promote
one argument for bioenergy, namely that it replaces
nonrenewable energy sources with renewable ones.
This is particularly attractive for liquid fuels as there
are currently no other alternatives available than liquid
fuels from biological sources. In the following, we often
focus on liquid biofuels as the discussion of sustainability of bioenergy is developed furthest for those and
most data are available for those bioenergy types. The
findings on the sustainability of agricultural production of crops for liquid biofuel, however, apply to agricultural production of any bioenergy type and we will
407
Copyright Ó 2014 Elsevier B.V. All rights reserved.
408
23. SUSTAINABLE FARMING OF BIOENERGY CROPS
also report findings for biogas production, for example,
on which there is also a considerable amount of
research.
Besides the GHG balance, many other criteria are
needed to assess the sustainability of bioenergy. They
range from environmental impacts of the agricultural production process over emissions in the use phase
(e.g. nitrous oxide emissions from biomass-fueled power
plants) to socioeconomic aspects, such as production costs
or effects on labor (Elbehri et al., 2013; Faist Emmenegger
et al., 2012; Delucchi, 2010). The assessments of environmental impacts show that most bioenergy crops perform
worse than the fossil fuel baseline regarding many criteria
of sustainability in agricultural production. The best performance is realized for some residue or forestry-based
bioenergy sources such as fuels from wood products
(Faist Emmenegger et al., 2012). The negative performance of bioenergy crops is due to the fact that current
conventional agricultural production has many adverse
environmental effects (Matson et al., 1997; IAASTD,
2009; see e.g. Tomei and Upham, 2009 on soy-based
biodiesel in Argentina for an exemplification).
As important as these findings on environmental performance is, the key point in the current discussion
besides the GHG balance is land competition between
bioenergy crops and food production (Rathman et al.,
2010; Delzeit et al., 2010; HLPE, 2013). Bioenergy crops
compete for fertile land with food crops and bioenergy
use of food crops such as maize directly competes
with food use. This dynamics has been behind the volatile and high food prices in recent years as, e.g. for maize
and grain in 2007e2008 (NYT, 2007; HLPE, 2013).
There are several options available to address all these
challenges. First, there are alternative ways of agricultural production that reduce the adverse impacts from
farming, a key example being organic agriculture (Rossi,
2012). These alternatives usually focus on systemic aspects of the whole production systems, emphasize closed
nutrient cycles, sustaining soil fertility and plant health
and the role of ecosystem services, e.g. for pest and disease control. Second, how strong land competition may
become depends on the concrete situation, i.e. on total
bioenergy demand, relative prices for food and energy,
the policy environment with incentives and regulations,
etc. Very circumspect planning of any larger scale bioenergy strategy seems crucial to have a chance to avoid
such adverse effects (e.g. Damen, 2010 for Thailand, wa
Gathui and Ngugi, 2010 for vulnerable regions in Kenya,
or Palola and Walker, 2010 on oil palms). Standards and
certification are also suggested as a means to address
the potential land use competition, e.g. by explicitly
excluding bioenergy from fertile croplands (e.g. GEF
et al., 2013). Third, new forms of bioenergy emerge
(IEA, 2010). Instead of the so-called “first-generation”
bioenergy that is based on use of oil, starch or sugar
contents of crops to manufacture biodiesel, “second-generation” bioenergy relies on the lignocellulosic contents
of the crops. This allows a much wider range of crops
to be utilized for biofuel production, in particular
nonfood woody crops such as switchgrass or Eucalyptus
(IEA, 2010). It also allows utilization of basically the
whole plant and of crop residues for biofuel production.
This reduces land demand per unit energy for bioenergy.
However, it is not expected that the second-generation
biofuels will play a significant role in the near future as
much research is still needed (IEA, 2010).
An aspect that is largely missing in this discussion on
environmental impacts of bioenergy production and land
competition is the role of biomass quantities. Large quantities of biomass are needed for bioenergy strategies that
significantly contribute to the global energy supply. On
the other hand, biomass plays a key role as a fertilizer
in sustainable agricultural production systems. There is
thus a direct competition for biomass between exporting
it from the agricultural production system for bioenergy
use and recycling it as a fertilizer (Muller, 2009). Data
and studies on how much biomass may be exported for
bioenergy use from sustainable agricultural farming systems are scarce, but indications that it will not be much
dominate, as we will discuss further down.
The key topic in this discussion is thus less whether
agricultural production of bioenergy crops can be done
in a sustainable way, as sustainable agricultural production is well established and can be implemented for any
crop production and as options to reduce land competition are around in principle, albeit challenging to implement. The question is thus rather how much bioenergy
can be sustainably produced in such a context where
biomass is not a waste output from agricultural production but an essential fertilizer input in sustainable agricultural production systems and where fertile land is
needed for food production.
In this chapter, we focus on the sustainable production
of bioenergy crops with a focus on the farm level. This
thus covers the farm operations, but it does not cover
processing, transport, storage and use of bioenergy.
Possible disposal of waste after bioenergy use is shortly
addressed in the context of fertilizer use (cf. Section
Bioenergy Potential on Farm Level). This chapter also
covers more aggregate aspects of agricultural production
such as land and water resource use and more systemic
aspects related to the whole food system, such as the
competition for land, water and biomass between food
and bioenergy production. In this context, we also
address general aspects of food security and in the conclusions also relate to the role of meat and milk production as another sector that competes for scarce land
resources with crop-based food production.
We restrict this analysis to agricultural bioenergy. We
thus address forestry only occasionally and do not
CRITERIA FOR SUSTAINABLE FARMING AND SUSTAINABLE FOOD SYSTEMS
address aquaculture such as for algae-based biomass production for energy use or biomass production in industrial contexts, such as in bioreactors (Chen et al., 2011).
As biological processes are involved in all these cases,
the key topics we discuss in the following are relevant
also there: resource use and resource competition, environmental impacts of the production (e.g. water and air
pollution, intoxication due to pesticide use, etc.), and effects on the sustainability of the food production system
as a whole. On the other hand, harvesting the energy capture potential of photosynthesis in purely industrial production of biomass in bioreactors could be an option, as
the production system can work well separated from
the natural environment. For such production, no
ecosystem inputs such as fertile soils and water bodies
are used as inputs or sinks (e.g. for nutrient runoff) and
thus potentially depleted. Environmental impacts can
thus be kept to a minimum. Such operations do however
not refer to agricultural but rather to industrial production of biomass for bioenergy use and we do not further
pursue this here. Furthermore, they are still in the
research and development phase and commercial use is
not expected in the near future (Pragya et al., 2013).
This chapter is organized as follows. In Section
Criteria for Sustainable Farming and Sustainable Food
Systems, we discuss the criteria of sustainable agricultural production and sustainable food systems. In Section What is Sustainable Bioenergy Production? we
assess how bioenergy production may be implemented
if it needs to meet these criteria. We also address sustainability criteria for bioenergy production as formulated
by a range of institutions and relate them to the criteria
for sustainable agricultural production from Section
Criteria for Sustainable Farming and Sustainable Food
Systems. Section How Much Bioenergy may be Produced Sustainably? provides some discussion on how
much agricultural bioenergy may be produced in a sustainable way and Section Conclusions concludes.
CRITERIA FOR SUSTAINABLE FARMING
AND SUSTAINABLE FOOD SYSTEMS
Conventional Agricultural Production
Over the decades from 1960 to 2010, agricultural production has increased more than threefold and per capita provision of calories has increased by one-third
(FAOSTAT, 2013). This has greatly contributed to
feeding an ever-increasing population (from 3109 to
6.7109 billion over this period). This development is
usually subsumed under the label “Green revolution”
(IFAD, 2001; Evenson and Gollin, 2003). Starting in the
1960s, this development was based on a strict focus on
monocropping with high yielding species and varieties,
409
irrigation and mechanization where available and
increased use of mineral fertilizers, pesticides and herbicides. The successes of the green revolution are evident,
but so are the downturns related to it (Matson et al.,
1997; DFID, 2004). The focus on monocropping, chemical fertilizers, pesticides, irrigation and mechanization
has left an increasingly negative legacy regarding
adverse effects on soil fertility, i.e. increased soil degradation, salinization and depletion of water bodies, on
intoxication of the environment, biodiversity loss, loss
of ecosystem services, on eutrophication of water bodies
and animal health (Matson et al., 1997). Current agriculture is well able to feed the world and will be able in
2050 to feed more than 9 billion people, given projected
yield increases realize (Alexandratos and Bruinsma,
2012). The challenge is not the average supply of calories
per capita but their distribution globally and the fact that
a third is lost or wasted globally (Godfray et al., 2010).
However, in the light of the adverse effects of the green
revolution and climate change, such yield increases may
be compromised and sustained agricultural production
calls for alternative cropping practices and a fundamental shift in the agricultural production system
(IAASTD, 2009; Müller et al., 2010).
Sustainable Agricultural Production
A new revolution in agricultural production is thus
needed. On the production level, a sustainable future
agricultural system needs to focus on mitigating and
avoiding the adverse effects of current agricultural practices. It needs to focus on crop diversity, ecosystem services, soil protection and fertility, nutrient and water
use cycling, biocontrol of pests, diseases and weeds
and reduced pesticide use. A range of alternative production approaches are available (Eyhorn et al., 2003;
Pretty et al., 2006; Rossi, 2012), such as agroecologybased approaches, focusing on utilization of ecological
concepts (Altieri, 1995), or integrated pest management,
focusing on reducing pesticide use via managing pest
populations in such a way that damages remain low
(Bajwa and Kogan, 2002). The role model for these alternative approaches is organic agriculture with its ban on
most pesticides, focus on soil fertility, plant health and
closed nutrient cycles, utilization of optimized crop rotations and crop diversity, organic fertilizers and
ecosystem functions for pest and weed control (FAO,
2002; Eyhorn et al., 2003; IFOAM, 2006). Organic agriculture is the role model as it addresses all adverse effects of
conventional production, adopts a systemic approach
and is well established and tested for decades and
embedded in a context of governance, information provision, training and extension institutions that make it
the best-developed alternative production system.
Organic agriculture performs better than conventional
410
23. SUSTAINABLE FARMING OF BIOENERGY CROPS
agriculture with respect to most environmental indicators on a per-hectare basis (Schader et al., 2012). The
biggest drawback is its generally lower yields (Seufert
et al., 2012; De Ponti et al., 2012; Badgley et al., 2007).
Lower yields predominantly manifest in comparison to
high-yielding intensive conventional agriculture. In
developing countries, in a context of currently nooptimal conventional production systems, organic
yields are on par or even higher for well-managed
organic farms. The lower yields can result in a less favorable per kilogram produce assessment of environmental
impacts for some products in organic agriculture
(Schader et al., 2012). We emphasize that we do not
address socioeconomic aspects of organic production
here, such as the need for information and extension services to train farmers and potential challenges of the
conversion from organic to conventional agriculture.
Interestingly, the key principles and practices of
organic agriculture become increasingly important in
conventional agriculture, mainly due to the increasing
need to contribute to climate change mitigation and
adaptation but also due to the increasingly important
discussion on global biodiversity losses. Optimized
crop rotations with deep-rooting forage legumes and
use of organic fertilizers, for example, are promoted in
the context of climate change mitigation and adaptation
to improve soil fertility and increase soil organic carbon
levels (Smith et al., 2008) and reducing nitrogen loads
are key to protect biodiversity.
consumption of animal products that are mainly based
on grassland feed (and some by-products of food production) thus comprise an optimal option for a sustainable food system (e.g. preliminary results from the
FAO-SOL-model, Schader et al., 2012).
WHAT IS SUSTAINABLE BIOENERGY
PRODUCTION?
Ways of Comparisons
When assessing the sustainability of energy crop production, the first criterion is usually its GHG performance with regard to a fossil baseline. For this
comparison, the baseline fuel mix plays a crucial role,
as the increasing importance of unconventional fossil
fuel sources such as oil sands will increase GHG emissions from the baseline and its general environmental
impacts, thus relatively improving the performance of
bioenergy production (Faist Emmenegger et al., 2012).
This bears the danger that biofuel options with
increasing environmental impacts and less favorable
GHG balances become relatively more sustainable.
Here, we adopt a different focus as we are primarily
interested in the sustainability of bioenergy production
with reference to sustainability in agricultural production systems and in food systems in general. This takes
all sustainability criteria into account and does not focus
on the GHG balance.
Sustainable Food Systems
Agricultural production is only one aspect of food
systems. Sustainability in agriculture also needs to
address more encompassing concepts such as food security and land availability on a regional level. If agriculture would switch to organic, 10e20% more land
would be needed due to lower yields, given diets do
not change and the same amount of wastage is produced as today. However, dietary change is a key topic
for sustainable food systems, as a large part of agriculture’s environmental charge stems from animal husbandry. Reducing meat, egg and dairy product
consumption levels would greatly help to reduce environmental pressure from agricultural production.
Focusing on feeding animals on grasslands and not on
food crops such as soy and maize would reduce the
need for land, as calorie production from crops is
much more efficient than from animals. Furthermore,
about 30% of agricultural production are lost or wasted
globally (Godfray et al., 2010). Reducing this would also
contribute to reduced agricultural land use. Such
reduced land use would on the other hand reduce
pressure to further increase yields. Organic production
in combination with reduced waste and lower
Sustainability Criteria for Biofuel Production
There exist several approaches proposing sustainability criteria for bioenergy (e.g. Cramer et al., 2007;
EU, 2009; IEA, 2010; RSB, 2011; GEF et al., 2013, where
the last two are very detailed). They usually cover
some goal for GHG emission reductions, e.g. a 35%
reduction of aggregate emissions over some time period
with respect to the baseline as suggested in EU regulations (50% from 2017 to 60% from 2018 onwards, EU,
2009). While this seems a clear criterion, its assessment
is complex. The choice of different default values, soil
carbon stock data and land use change definitions, for
example, is behind the huge differences in GHG balances between two GHG calculation tools as assessed
in Hennecke et al. (2013), one of them being the tool
used by the Roundtable of Sustainable Biomaterials
(RSB) whose sustainability criteria are discussed below.
Other aspects are decisive as well. The choice of the time
horizon over which aggregate reductions have to be
achieved and the choice of the social discount rate,
which influences the relative importance of current
and future emissions, also greatly influence the
outcome (De Gorter and Tsur, 2009).
WHAT IS SUSTAINABLE BIOENERGY PRODUCTION?
The sustainability criteria proposed for biofuel production that relate to agricultural production and the
food system, address land competition, biodiversity,
environmental impacts on soil, water and air, and social
aspects. Land competition, resp. absence thereof and the
related food security are by far the most prominent
criteria in the discussion (HLPE, 2013). Potential drivers
for land competition are many. First, there is the fact that
bioenergy crops need fertile land to achieve economically interesting yields. Biofuels on marginal lands
are some option in smallholder and communityself-sufficiency contexts, but for commercial supply of
biofuels in significant shares of total global energy demand, production on fertile land that potentially is
used for food production is necessary (cf. Section Bioenergy Potential on Farm Level). Similarly, bioenergy
crop production depends on water availability and
nutrient inputs as any other agricultural production system. Thus, a second potential competition is not only on
fertile lands, but also on land with sufficient water availability (Lysen and van Egmond, 2008), in particular in
the context of climate change, where water scarcity
will become a prevalent problem in many regions
(Meehl et al., 2007). Third, relative price differences between bioenergy and food production will be and have
been a key driver behind land competition as without
further regulation, land will be allocated to the most
profitable production. Stated differently, an increasing
demand for biofuels leads to higher prices, which triggers an increasing supply for it, with corresponding
land use (HLPE, 2013). It has to be emphasized that
land competition between food and other uses is not
new and relative profitability has always been a key
driver behind this. As Nhantumbo and Salomão (2010)
state, “Competition for higher-value resources existed
well before the biofuels campaign was initiated. In this
sense, biofuels production per se cannot be blamed for
land use conflicts, as the same types of conflicts have
occurred in other economic activities. But, in conjunction with other activities like mining, forestry and
tourism, biofuels projects further exacerbate competition for land, water and other resources” (p. 4). The
key point is thus that biofuel expansion increases the
pressure on the already scarce resource of fertile land.
In principle, policy measures can be used to mitigate
these adverse effects. However, their implementation
is often riddled with difficulties and land-use rights protection, enforcement of laws and regulations, etc. have to
be carefully considered when establishing potentially
promising institutions for sustainable land use. Nhantumbo and Salomão (2010) illustrate these challenges
for the case of Mozambique and draw a rather pessimistic picture.
The land use debate is further complicated by ILUCs
(Wicke et al., 2012). Those arise, for example, if biofuel
411
expansion in one region (e.g. sugar cane in southern
Brazil) leads to land use change in another region (in
this case, deforestation for livestock production in northern Brazil). The rationale behind this example is the fact
that expanding sugarcane in the South is at the expense
of already existing pastures in this region, that then
themselves relocate at the expense of other uses such
as natural forests (Andrade De Sa et al., 2013). Such effects are very difficult to clearly identify and assess
(Wicke et al., 2012). This is also the case in the detailed
analysis in Andrade De Sa et al. (2013) who find only
very weak significant statistical effects. Nevertheless,
there is evidence from many descriptive studies that
the potential presence of such mechanisms must not
be neglected (PBL, 2010). ILUC is not only relevant for
the competition between different land uses but also
for the GHG balances of biofuels, as it can have considerable negative effects on those (PBL, 2010; Faist
Emmenegger et al., 2012).
Biodiversity criteria mainly refer to the ban of using
forests or protected areas for bioenergy production
(e.g. Cramer et al., 2007; EU, 2009) or to being attentive
not to use invasive species as bioenergy crops (UNEP,
2010). The use of protected areas can also be seen as a
particular aspect of land competition from biofuel production. As mentioned above, biofuel production competes not only with food for land but also with other
uses, such as biodiversity protection and also with fiber
and biomaterial production that all depend on land
availability. Invasive species are seen as a potential
danger, due to already existing cases but also due to general characteristics of biofuel crops that also correlate
with invasiveness (e.g. fast growth or tolerance to
wide range of soil and climate conditions, UNEP,
2010). Much less prominent in this discussion are the
adverse effects of current agricultural production on
biodiversity (mainly due to overfertilization with nitrogen and pesticide use), albeit those are a key driver
behind biodiversity losses (Galloway et al. 2008). This
is mentioned in Bindraban et al. (2009) and adoption
of agricultural practices with low negative effects on
biodiversity is a criterion in GEF (2013), but not in EU
(2009) or RSB (2011).
Other environmental impacts largely remain rather
unspecific in the criteria suggested, although the range
of adverse environmental effects of current agricultural
production as described above will also realize in bioenergy cropping systems. EU (2009) for example only
posits that the production has to meet the Community
environmental requirements and in GEF et al. (2013),
water contamination is assumed to be no issue if legal
requirements are met. The size of the adverse environmental effects depends on the types of crops. Grassland
or wood products usually perform better than annual
crops, for example, WBGU (2009). Somewhat more
412
23. SUSTAINABLE FARMING OF BIOENERGY CROPS
detailed criteria are usually given in reference to soilrelated aspects such as soil fertility and soil organic carbon contents (see e.g. Cramer et al., 2007; EU, 2009; RSB,
2011; GEF et al., 2013). Regarding soil organic carbon,
some sustainability criteria explicitly exclude bioenergy
cropping on peatlands and other carbon-rich soils (EU,
2009). On the other hand, some bioenergy crops are
judged to be advantageous for soil carbon levels, mainly
grassland and forest-based bioenergy. The effect on soil
carbon is not that clear for some perennial crops and
rather negative for annual crops (WBGU, 2009).
Social sustainability criteria, finally, sometimes tend
to be formulated on a very general level. EU (2009), for
example, only requires that source countries for bioenergy have “ratified and implemented” (p. 97) a range
of conventions referring to labor rights, gender aspects,
etc. RSB (2011) and GEF et al. (2013), on the other hand,
are quite detailed on the social aspects that cover a range
of important criteria for social sustainability in agricultural production. RSB (2011) and GEF et al. (2013) also
make long-term economic viability of bioenergy projects
a criterion for their sustainability assessment. It is not
mentioned as a criterion, but bioenergy crops can have
some risk-spreading characteristics as they can increase
production diversity of a farm and as their demand and
price dynamics likely follows different patterns than
food or fiber crop demand and prices. Some types of bioenergy crops can also be used for direct on-farm energy
provision without much investment needs, such as
Jatropha, for example. These energy crops thus have
the potential to increase energy access and reduce workload of women considerable in case they have to collect
fuel-wood from far away, which is a common situation
in many poor regions in developing countries. Energy
crops can also provide specific income sources for
women, as many case-studies show (Karlsson and
Banda, 2009). However, there is similar evidence of
problematic situations from case-studies, and whether
a bioenergy project is advantageous for single farmers,
the community and women in particular strongly depends on the concrete design and institutional context.
Further example of positive cases are given in Practical
Action Consulting (2009), and some negative cases for
example in Ribeiro and Matavel (2009), focusing on
Jatropha in Mozambique.
The choice of sustainability criteria for bioenergy thus
reflects the classical topics of sustainability criteria, with
a focus on environmental aspects and climate change in
particular. An additional aspect is land competition,
which is covered extensively in the discussion. The
focus on environmental criteria is understandable as
bioenergy is a climate change mitigation strategy and
prime impacts of agricultural production are in the environment. However, besides GHG emissions and, partly,
biodiversity, the assessment of environmental criteria
remains rather weak. For a comprehensive sustainability
assessment, the topical breadth and depth in analysis
must be improved. Generally, bioenergy production
has the same impacts as any agricultural production
and sustainability in bioenergy production largely links
to sustainable agricultural production.
Biomass Use
The assessment of proposed sustainability criteria for
bioenergy production shows that the competition for
biomass between bioenergy use and for fertilizing sustainable agricultural production systems is no topic. It
is covered marginally in some other publications on sustainable bioenergy, e.g. in Bindraban et al. (2009) or Blonz
et al. (2008), although it is of key relevance for sustainable
agricultural production. Some publications address this
topic as a caveat of agricultural or forestry residues use,
as exporting too much of them causes soil carbon losses
and soil degradation (e.g. WBGU, 2009). Only Muller
(2009) takes up this topic in depth. The export of biomass
from the fields for bioenergy use also exports nutrients
that have to be replaced by other fertilizers, i.e. mineral
fertilizers. The overuse of mineral fertilizers is however
a key driver behind many environmental problems of
current agricultural production. Sustainable agricultural
production systems are based on closed nutrient cycles
and organic fertilizers (compost from crop residues, roots
and residues that remain on and in the fields, and manure
from livestock operations). Those are keys for soil fertility
and increased soil organic carbon levels (Lal, 2008; Gattinger et al., 2012). The nutrient export becomes particularly relevant for second-generation biofuels, where
basically the whole plant can be used and no unused residues remain, resp. where cellulosic residues from any
crops can be utilized (IEA, 2010). This is even suggested
as a strategy to mitigate land use competition, as residues
come without additional land requirements and feedstock for second-generation biofuel is claimed to often
grow on marginal lands (IEA, 2010, 2011). On marginal
lands in particular, high organic matter inputs are key
to improve soil fertility, though. Also for bioenergy crops,
yields tend to be lower and erratic on marginal lands and
economic viability of bioenergy projects is often given on
fertile land only (Bindraban et al., 2009). Thus, regarding
the biomass competition, the most promising options to
avoid land use competition seem particularly
problematic.
HOW MUCH BIOENERGY MAY BE
PRODUCED SUSTAINABLY?
As illustrated above, sustainable production of
biomass for energy use is in principle possible. Thus,
HOW MUCH BIOENERGY MAY BE PRODUCED SUSTAINABLY?
the key question is how much biomass may be produced
sustainably on a global level. We basically discern two
types of approaches to this question. First, there are
various assessments of the global biomass potential for
energy use. They are based on assessments of the suitability of global land areas for biomass production and
corresponding yields, usually imposing the condition
that food, feed and fiber demand need to be met. The
second approach focuses on single farms or farming systems and estimates how much bioenergy may be produced in such, given the specific agronomic
characteristics. These latter studies can also involve
experimental data from case studies.
Global Bioenergy Potential
A range of literature assesses the global bioenergy potential employing various models and assumptions.
WBGU (2009), Chum et al. (2011) and HLPE (2013)
contain some recent reviews of this literature. Some
very gross global comparisons are illustrative. If all harvested biomass today (including crops, forage, wood,
and residues) would be used for energy use, this would
cover about one-third of today’s energy supply (HLPE,
2013). This total harvested biomass corresponds to about
230 Exajoule (EJ) of primary energy per year. Chum et al.
(2011) give a gross estimate for the technical bioenergy
potential of 100e300 EJ/a in 2050, showing a wide range
of uncertainty, though. This amount of bioenergy would
very roughly cover between 10% and 60% of total primary energy supply in 2050, which ranges between
500 and 1000 EJ/a, based on 164 scenarios (Chum
et al., 2011, Figure 10.3). Nevertheless, this number is
illustrative as it roughly corresponds to a situation,
where a biomass quantity equaling the total current
biomass production was used for bioenergy in 2050.
Current biomass energy use is about 50 EJ/a. Chum
et al. (2011) base these estimates on a literature review
that assess the physical biomass production potential
based on land suitability and crop yields. These assessments also rely on considerably gains from yield increases and agricultural technology progress. While
Chum et al. (2011) evaluate these numbers rather positively, HLPE (2013) considers them very problematic.
WBGU (2009) in detail presents their model for the
assessment of bioenergy potentials, relating to clearly
stated general sustainability boundaries (biodiversity
conservation, food security, climate change and acidification mitigation, and soil protection) and arrive at an
estimate of 80e170 EJ/a.
A key drawback in these estimates is the lack of economic and agronomic considerations in a systematic
way. Both Chum et al. (2011) and HLPE (2013) emphasize the role of economic aspects, i.e. costs of such bioenergy development. WBGU (2009) also emphasize
413
that their estimate is purely technical given some sustainability boundaries and that the economic potential
likely is considerably lower. But only few studies
address market interactions between food and energy
crops in economic equilibrium models and none of those
was used to assess the bioenergy potential presented.
Supply costs curves for various bioenergy crops resp.
food crops for energy use are provided for illustration
in Chum et al. (2011), for example, but no consequences
on crop prices in interaction with demand are derived
from this. Infrastructure and access to the suitable land
are neither addressed explicitly. However, to realistically
assess any competition for land between food and energy crop production, biomass and land markets need
to be included in the model, as the relative profitability
of food or bioenergy production on a certain area of
land will drive production decisions and food and bioenergy supplydunless strong governmental regulations
are imposed on the bioenergy and land markets. Thus,
even if the biomass potential for energy uses is very
large, its effect on food market prices needs to be
assessed in detail to derive robust statements on land
competition.
The second aspect that is missing is agronomic characteristics of biomass production. The estimates
reviewed in Chum et al. (2011) consider some sustainability criteria when trying to assess how much biomass
may be produced without additional conversion of forests and grassland to cropland, which may be the effects
on biodiversity, or via investigating whether bioenergy
crops may even serve to improve degraded soils. However, agronomic aspects of the crop production system
are largely neglected. Water requirements and potential
problems related to that are mentioned explicitly, but are
not captured explicitly in the models referred. Furthermore, any crop production needs to be fertilized if yield
decreases after some years and soil degradation should
be avoided. Yield assessments for biomass production
do however not differentiate for nitrogen inputs. In
addition, fertilizing with mineral fertilizers only is not
enough, as organic fertilizers are crucial to halt soil
degradation (e.g. Lal, 2008; Blanco-Canqui and Lal,
2009). This problem is mentioned in Chum et al.
(2011), but it does not become effective for determining
the biomass potential although it directly conflicts with
the basic mechanism of bioenergy cropping, which is
exporting a high amount of biomass, resp. using
biomass residues formerly left on the field. Utilizing
the biomass potential referred to above may thus result
in considerably increased total global fertilizer use with
respect to today (and in addition, crop production has to
increase by 70% as well, Alexandratos and Bruinsma,
2012). This would put additional pressure on the nitrogen cycle and lead to corresponding adverse environmental effects (Erisman et al., 2010). WBGU (2009)
414
23. SUSTAINABLE FARMING OF BIOENERGY CROPS
contains a review of several bioenergy crops that also
contains some agronomic aspects. They conclude that
only grasslands and some forestry have a sustainable
potential for bioenergy provision. This is also reflected
in their model, which assesses the bioenergy potential
on additional grassland and forestry use, as only those
meet their sustainability boundaries, besides some use
of residues.
Bioenergy Potential on Farm Level
As we have seen in the previous section, assessments
of the global bioenergy potential are based on land use
and land availability consideration subject to several
sustainability criteria. These assessments thus tend to
disregard agronomic boundary conditions. WBGU
(2009) is one exception and also explicitly includes
such aspects on a very aggregate level in their model,
by assuming that only 60% of residues can be used for
energy production technically (and only 30% economically), given that part of the residue biomass needs to
be left on the fields in order to avoid soil degradation.
In contrast to such global or regional assessments,
farm or farming system-based assessments are in principle able to account for such agronomic boundaries.
Rossi (2012) reviews a range of sustainable farming systems as options for sustainable biomass production. He
points out the role of biomass as a fertilizer and for soil
fertility, but does not provide quantitative assessments
of how much biomass may be exported from these systems for bioenergy use. Even more, the case studies presented in Rossi (2012) often do not address bioenergy
production at all but only illustrate the advantageous
performance of the respective farming system along a
range of sustainability criteria.
There is however other research that provides
detailed quantitative analysis. Meyer and Priefer (2012)
for example discuss the potential of biogas production
in organic agriculture, based on case-study farms in Germany. Biogas fits neatly into organic production systems,
as in organic farms, much biomass that can be used as
feedstock for biogas plants is around (from grassclover leys in the crop rotations, for example) and the
biogas slurry can be used as a fertilizer. Meyer and Priefer (2012) provides also some forecast on the potential for
such bioenergy production in Germany, assuming that
the biogas is used for electricity production and also utilizing the heat generated in the power plants. Assuming
20% of agricultural production being organic (political
goals for 2020 are 20% in Germany) and equipped with
biogas facilities, 7 TWh/a electricity could be provided
plus 50% of this energy in heat. Assuming a total electricity demand of 535 TWh/a in Germany in 2030
BMU 2011), similar biogas production on all farms
would provide 6e7% of this (35 TWh/a). Also, Anspach
(2009) finds that biogas production fits well into organic
production systems. Using biogas slurry as fertilizer has
also some additional advantages regarding yields, environmental impacts and weed control (as seeds of weeds
e.g. in manure are killed in the biogas digester). The potential of biogas production is also recognized by authors of more aggregate studies, e.g. Bindraban et al.
(2009). This biogas production is assumed to work
largely without bioenergy cropping and only uses residues and manure. Thus, it does not lead to competition
with food production. Currently, the reality in Germany
is different, though, as co-substrates are imported to a
significant part in biogas digesters and part of
those are specifically grown for biogas production (e.g.
maize).
Another body of literature focuses on energy selfsufficiency of organic farms, motivated by the unsustainable use of fossil fuels also in organic production
systems (Carter et al., 2012; Christen and Dalgaard,
2013; Halberg et al., 2008; Oleskowicz-Popiel et al.,
2012; Pugesgaard et al., 2013). Those studies are from
Denmark and serve as further illustration for the
bioenergy production in sustainable agricultural production systems. They generally find that energy
self-sufficiency of organic farms is possible and that
sometimes even some small energy surplus can be
generated. Carter et al. (2012) are somewhat different,
as they focus on a GHG life-cycle analysis and do not
address nutrient recycling aspects at all. Pugesgaard
et al. (2013) find that energy self-sufficiency is also
possible with nitrogen self-sufficiency. The energy selfsufficiency described in these studies comes at the
expense of increased land demand or lower yields,
though a fact that is not emphasized in these studies
but that is crucial for our more encompassing assessment of sustainable bioenergy production. Fredriksson
et al. (2006) find 4e10% increased land demand for energy self-sufficiency of the farm. We emphasize that
self-sufficiency means that such a farm does not produce
any energy for the wider society. In Fredriksson et al.
(2006), this is achieved with utilization of firstgeneration bioenergy, thus the agronomy is similar to ordinary food production and biomass exports are also
similar. Halberg et al. (2008) achieve energy selfsufficiency and improved nutrient availability by using
land that has been set-aside in the baseline (8.5% of total
farmland) for energy production. It is not discussed
which environmental effects this has. Pugesgaard et al.
(2013) use 10e20% of the farm area for biogas feedstock
production and report lower food yields. Either are milk
yields reduced by more than 50% due to lower cattle
numbers (while cash crop yields are increased by
60e120% due to improved N fertilization of cash crops),
or cash crop yields are reduced by 10e30%. The scenario
with 120% increased cash crop utilizes additional 20%
REFERENCES
farmland of meadows and is thus not fully comparable
to the baseline. Also in this case, energy production
thus comes at the expense of lower yields or higher
land use. A clear assessment of what this means
regarding food security is however not possible, as the
differences should be translated in total calorie and protein provision for human nutrition. Interesting though is
the fact that part of this energy provision is possible in
scenarios that go along with some dietary change only,
as animal products are reduced.
CONCLUSIONS
Our analysis shows that bioenergy without land
competition is difficult. While general land use models
exhibit quite some potential for bioenergy production
also under several sustainability constraints, they lack
a due assessment of nutrient use, supply and demand
in the agricultural production phase. On-farm studies
reveal that increased land use or reduced yields cannot
be avoided even for moderate bioenergy generation
(e.g. to make a farm energy self-sufficient) unless only
biogas is produced.
We draw several conclusions from this assessment of
sustainable farming of bioenergy crops. First, for a thorough assessment of the sustainability of bioenergy, systemic views have to be adopted. It is not enough to
assess the GHG balance on a life-cycle basis. Bioenergy
as a climate change mitigation strategy needs to be
analyzed in the context of the whole food system
including agricultural production. Much work has
been done in this direction. Land use modeling and
also sustainability criteria for bioenergy account for a
wide range of aspects, such as the competition for
land. However, as a second point, we want to emphasize
that fertilization and nutrient cycles play a minor role in
the assessment of bioenergy and its sustainable production only. This is a significant lack in analysis, as biomass
plays a key role as fertilizer in sustainable agricultural
production systems and as feedstock for bioenergy
production. Agronomic aspects of crop fertilization
and nitrogen use need to play a significant role in
sustainability assessments of bioenergy.
Third, we may point out biogas production as one
viable option, where biomass can in principle be used
for both ends at the same timedas feedstock for biogas
plants and as fertilizer in the form of biogas slurry, after
having passed through the biogas digester. Biogas production can be designed in such a way that it fits into
agricultural production systems without additional
land demand. However, as promising as it is for local
energy generation, the aggregate potential remains
small. In addition, it is no option for producing liquid
biofuels.
415
Fourth, land competition is a key challenge, in particular for liquid biofuel production. Many models to
assess the bioenergy potential globally or regionally
exist, but they should be improved by adding much
more detailed interaction with the energy markets.
Such models need to be able to capture land use allocation based on the relative profitability of energy or food
production. Most models focus on assessing physical
potentials which is a key basis for this, and they mention
economic constraints for developing the technical bioenergy potential, but how strong a land competition
will emerge hinges on such relative profitability, resp.
prices and on demand and supply elasticities, i.e. how
much demand and supply changes with prices. In addition, these land use models need to incorporate agronomic aspects. Nitrogen demand of energy crops,
corresponding fertilizer demand, its environmental effects and linkages between yields and nutrient inputs
need to be captured in much more detail to arrive at reliable conclusions. If it comes to assessing bioenergy potentials in the context of sustainable agricultural
production systems, the need to capture fertilizer and
nutrient dynamics in more detail is directly linked to
biomass flows that must be captured adequately between energy and fertilizer use.
Fifth, some improved standard for sustainable bioenergy could help in this. We thus suggest to combine
the RSB (2011) and GEF et al. (2013) standards and to
enhance them with agronomic aspects related to
nutrient and biomass use and recycling.
References
Alexandratos, N., Bruinsma, J., 2012. World Agriculture Towards
2030/2050. The 2012 Revision, ESA Working Paper No. 12-03. FAO,
Rome.
Altieri, M., 1995. Agroecology: The Science of Sustainable Agriculture,
second ed. Westview Press, Boulder, CO.
Andrade de Sá, S., Di Falco, S., Palmer, C., 2013. Dynamics of indirect
land-use change: empirical evidence from Brazil. J. Environ. Econ.
Manage.
Anspach, V., 2009. Status quo, Prespektiven und wirtschafltiche
Potenziale der Biogaserzeugung auf landwirtschaftlichen Betrieben im ökologischen Landbau (Ph.D. thesis). University of Kassel.
Badgley, C., Moghtader, J., Quintero, E., Zakem, E., Chappell, M.,
Aviles-Vazquez, K., Samulon, A., Perfecto, I., 2007. Organic agriculture and the global food supply. Renewable Agric. Food Syst.
22, 86e108.
Bajwa, W.I., Kogan, M., 2002. Compendium of IPM Definitions
(CID)dWhat Is IPM and How Is It Defined in the Worldwide
Literature?. IPPC Publication no. 998. Integrated Plant Protection
Center (IPPC), Oregon State University, Corvallis, OR.
Bindraban, P., Bulte, E., Conjin, S., Eickhout, B., Hoogwijk, M., Londo,
M., 2009. Can biofuels be sustainable by 2020? An assessment for
an obligatory blending target of 10% in the Netherlands, Scientific
Assessment and Policy Analysis Report WAB 500102 024, Research
Programme on Scientific Assessment and Policy Analysis for
Climate Change (WAB).
416
23. SUSTAINABLE FARMING OF BIOENERGY CROPS
Blanco-Canqui, H., Lal, R., 2009. Corn stover removal for expanded
uses reduces soil fertility and structural stability. Soil Sci. Soc. Am.
J. 73 (2), 418e426.
Blonz, J., Vajjhala, S., Safirova, E., 2008. Growing Complexities: A
Cross-sector Review of U.S. Biofuels Policies and Their Interactions. RFF Discussion Paper 08e47. Resources for the Future
RFF, Washington DC.
BMU, 2011. Leitstudie 2010-Langfristszenarien und Strategien für den
Ausbau der erneuerbaren Energien in Deutschlandbei Berücksichtigung der Entwicklung in Europa und global. Bundesministerium für Umwelt, Naturschutz und Reaktorsicherheit.
Carter, S., Hauggaard-Nielsen, H., Heiske, S., Jensen, M., Thomsen, S.,
Schmidt, J., Johansen, A., Ambus, P., 2012. Consequences of field
N2O emissions for the environmental sustainability of plant-based
biofuels produced within an organic farming system. Global
Change Biol. Bioenergy 4 (4), S. 435e452.
Chen, C., Yeh, K., Aisyah, R., Lee, D., Chang, J., 2011. Cultivation,
photobioreactor design and harvesting of microalgae for biodiesel
production: a critical review. Bioresour. Technol. 102, 71e81.
Christen, B., Dalgaard, T., 2013. Buffers for biomass production in
temperate European agriculture: a review and synthesis on function, ecosystem services and implementation. Biomass Bioenergy
55, S. 53e67.
Chum, H., Faaij, A., Moreira, J., Berndes, G., Dhamija, P., Dong, H.,
Gabrielle, B., Goss Eng, A., Lucht, W., Mapako, M., Masera
Cerutti, O., McIntyre, T., Minowa, T., Pingoud, K., 2011. Bioenergy.
In: Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Seyboth, K.,
Matschoss, P., Kadner, S., Zwickel, T., Eickemeier, P., Hansen, G.,
Schlömer, S., von Stechow, C. (Eds.), IPCC Special Report on
Renewable Energy Sources and Climate Change Mitigation.
Cambridge University Press, Cambridge, United Kingdom and
New York, NY, USA.
Cramer, J., Wissema, E., de Bruijne, M., Lammers, E., Dijk, D.,
Jager, H., van Bennekom, S., Breunesse, E., Horster, R., van
Leenders, C., Wonink, S., Wolters, W., Kip, H., Stam, H., Faaij, A.,
Kwant, K., 2007. Testing Framework for Sustainable Biomass,
EnergieTransitie, the Netherlands. http://www.senternovem.nl/
energietransitiegg/documentatie/downloads_rapporten_en_achter
grondinformatie.asp.
Damen, B., 2010. BEFS Thailand e Key Results and Policy Recommendations for Future Bioenergy Development. FAO Environment
and Natural Resources Working Paper 43.
De Gorter, H., Tsur, Y., 2009. Towards a Genuine Sustainability Standard for Biofuel Production. Working Paper of the Department of
Applied Economics and Management. Cornell University, NY.
Delucchi, M., 2010. Impacts of biofuels on climate change, water use,
and land use. Ann. N. Y. Acad. Sci. 1195, 28e45.
Delzeit, R., Gömann, H., Holm-Müller, K., Kreins, P., Kretschmer, B.,
Münch, J., Peterson, S., 2010. Analysing Bioenergy and Land Use
Competition in a Coupled Modelling System: the Role of Bioenergy in Renewable Energy Policy in Germany. Kiel Working
Papers No 1653.
De Ponti, T., Rijk, B., van Ittersum, M., 2012. The crop yield gap
between organic and conventional agriculture. Agric. Syst. 108,
1e9.
DFID, 2004. Agricultural Sustainability. Working Paper Nr. 12. UK
Department of International Development (DFID), London. http://
dfid-agriculture-consultation.nri.org/summaries/wp12.pdf.
Elbehri, A., Segerstedt, A., Liu, P., 2013. Biofuels and the Sustainability
Challenge. Food and Agriculture Organization of the United
Nations FAO.
Erisman, J., van grinsven, H., Leip, A., Mosier, A., Blecker, A., 2010.
Nitrogen and biofuels; an overview of the current state of knowledge. Nutr. Cycling Agroecosyst. 86, 211e223.
EU, 2009. Directive 2009/30/EC of the European parliament and of
the council of 23 April 2009. Off. J. Eur. Union. L. 140/88, 5.6. 2009.
Evenson, R.E., Gollin, D., 2003. Assessing the impact of the green
revolution, 1960e2000. Science 300, 758e762.
Eyhorn, F., Heeb, M., Weidmann, G., 2003. IFOAM Training Manual
for Organic Agriculture in the Tropics. International Federation of
Organic Agriculture Movements (IFOAM). http://www.fibl.org/
english/publications/training-manual/.
Faist Emmenegger, M., Gmünder, S., Reinhard, J., Zah, R.,
Nemecek, T., Schnetzer, J., Bauer, C., Simons, A., Doka, G., 2012.
Harmonisation and Extension of the Bioenergy Inventories and
Assessment. End Report to the Swiss Federal Office of energy, 31th
August 2012.
FAO, 2002. In: El-Hage Scialabba, N., Hattam, C. (Eds.), Organic
Agriculture, Environment and Food Security. Food and agriculture
organisation of the United Nations FAO. http://www.fao.org/
docrep/005/y4137e/y4137e00.htm.
FAOSTAT, 2013. Food Balance Sheets, Food and Agriculture Organization of the United Nations FAO, Rome.
Fargione, J., Hill, J., Tilman, D., Polasky, S., Hawthorne, P., 7 February
2008. Land clearing and the biofuel carbon Debt. Sci. Express.
http://dx.doi.org/10.1126/science.1152747.
Fredriksson, H., Baky, A., Bernesson, S., Nordberg, A., Noren, O.,
Hansson, P.-A., 2006. Use of on-farm produced biofuels on organic
farms e evaluation of energy balances and environmental loads
for three possible fuels. Agric. Syst. 89, 184e203.
Galloway, J., Townsend, A., Erisman, J., Bekunda, M., Cai, Z.,
Freney, J., Martinelli, L., Seitzinger, S., Sutton, M., 2008. Transformation of the nitrogen cycle: resent trends, questions, and potential Solutions. Science 320 (5878), 889e892.
Gattinger, A., Muller, A., Häni, M., Skinner, C., Fliessbach, A.,
Buchmann, N., Mäder, P., Stolze, M., Smith, P., El-Hage
Scialabba, N., Niggli, U., 2012. Enhanced top soil carbon stocks
under organic farming. Proc. Natl. Acad. Sci. USA. 109 (44),
18226e18231. http://dx.doi.org/10.1073/pnas.1221886110.
GEF/UNEP/FAO/UNIDO, 2013. Global Assessments and Guidelines
for Sustainable Liquid Biofuel Production in Developing Countries. GEF, UNEP, FAO, UNIDO. http://energy-l.iisd.org/news/
experts-assess-biofuels-screening-toolkit-at-unido-biofuelsconference/.
Godfray, H., Beddington, J., Crute, I., Haddad, L., Lawrence, D.,
Muir, J., Pretty, J., Robinson, S., Thomas, S., Toulmin, C., 2010.
Food security: the challenge of feeding 9 billion people. Science
327 (6).
Halberg, N., Dalgaard, R., Olesen, J.E., Dalgaard, T., 2008. Energy selfreliance, net-energy production and GHG emissions in Danish
organic cash crop farms. Renewable Agric. Food Syst. 23 (1),
30e37.
Hennecke, A., Faist, M., Reinhardt, J., Junquera, V., Neeft, J.,
Fehrenbach, H., 2013. Biofuel greenhouse gas calculations under
the European renewable energy directive e a comparison of the
bioGrace tool vs the tool of the roundtable on sustainable biofuels.
Appl. Energy 102, 55e62. http://www.sciencedirect.com/science/
article/pii/S0306261912003066.
HLPE, 2013. Biofuels and Food Security. High Level Panel of Experts
on Food Security and Nutrition HLPE, Committee on World Food
Security.
IAASTD, 2009. Agriculture at the Crossroads, International Assessment of Agricultural Scinece and Technology for Development
(IAASTD), Island Press, Washington DC.
IEA, 2010. Sustainable Production of Second-generation Biofuels. International Energy Association (Information Paper).
IEA Bioenergy, 2011. Bioenergy, Land Use Change and Climate
Change Mitigation (Background report).
REFERENCES
IFAD, 2001. Rural Poverty Report 2001: The Challenge of Ending
Rural Poverty. International Fund for Agricultural Development
(IFAD). http://www.ifad.org/poverty/.
IFOAM, 2006. The Principles of Organic Agriculture. International
Federation of Organic Agriculture Movements (IFOAM). http://
www.ifoam.org/about_ifoam/principles/index.html.
Karlsson, G., Banda, K. (Eds.), 2009. Biofuels for Sustainable Rural
Development and Empowerment of Women e Case Studies from
Africa and Asia. Energia.
Lal, R., 2008. Crop residues as soil amendments and feedstock for
bioethanol production. Waste Manage. 28 (4), 747e758.
Lysen, E., van Egmond, S. (Eds.), 2008. Biomass AssessmentdAssessment of Global Biomass Potentials and Their Links
to Food, Water, Biodiversity, Energy Demand and Economy. Report
500102 012, Netherlands Research Programme on Scientific
Assessment and Policy Analysis for Climate Change (WAB)
http://www.rivm.nl/bibliotheek/rapporten/500102012.pdf.
Matson, P.A., Parton, W.J., Power, A.G., Swift, M.J., 1997. Agricultural
intensification and ecosystem properties. Science 277, 504e509.
Meehl, G.A., Stocker, T.F., Collins, W.D., Friedlingstein, P., Gaye, A.T.,
Gregory, J.M., Kitoh, A., Knutti, R., Murphy, J.M., Noda, A.,
Raper, S.C.B., Watterson, I.G., Weaver, A.J., Zhao, Z.-C., 2007.
Global climate projections. In: Solomon, S., Qin, D., Manning, M.,
Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L. (Eds.),
Climate Change 2007: The Physical Science Basis. Cambridge
University Press, Cambridge, United Kingdom and New York, NY,
USA. Contribution of Working Group I to the Fourth Assessment
Report of the Intergovernmental Panel on Climate Change.
Meyer, R., Priefer, C., 2012. Ökologischer Landbau und Bioenergieerzeugung e Zielkonflikte und Lösungsansätze. Büro für
Technikfolgenabschätzung beim deutschen Bundestag. Arbeitsbericht Nr. 151.
Müller, C., Bondeau, A., Popp, A., Waha, K., Fader, M., 2010. Climate
Change Impacts on Agricultural Yields. Background Note to the
World Development Report 2010.
Muller, A., 2009. Sustainable agriculture and the production of
biomass for energy use. Clim. Change 94, 319e331.
Nhantumbo, I., Salomão, A., 2010. Biofuels, Land Access and Rural
Livelihoods in Mozambique. International Institute for Environment and Development IIED, London.
NYT, February 1, 2007. Thousands in Mexico City protest rising food
prices. N. Y. Times.
Oleskowicz-Popiel, P., Kádár, Z., Heiske, S., Klein-Marcuschamer, D.,
Simmons, B., Blanch, H., Schmidt, J., 2012. Co-production of
ethanol, biogas, protein fodder and natural fertilizer in organic
farming e evaluation of a concept for a farm-scale biorefinery.
Bioresour. Technol. 104, 440e446.
Palola, E., Walker, N., 2010. Food, Fuel, or Forests? Charting a
Responsible U.S. Role in Global Palm Oil Expansion. National
Wildlife Federation.
PBL, 2010. How to Deal with Indirect Landuse Change in the EU
Renewable Energy Directive? Netherlands Environmental
Assessment Agency PBL.
Practical Action Consulting, January 2009. Small-Scale Bioenergy Initiatives: Brief Description and Preliminary Lessons on Livelihood
Impacts from Case Studies in Asia, Latin America and Africa.
Prepared for PISCES and FAO by Practical Action Consulting.
417
Pragya, N., Pandey, K., Sahoo, P., 2013. A review on harvesting, oil
extraction and biofuels production technologies from microalgae.
Renewable Sustainable Energy Rev. 24, 159e171.
Pretty, J.N., Noble, A.D., Bossio, D., Dixon, J., Hine, R.E., Penning de
Vries, F., Morison, J., 2006. Resource conserving agriculture increases yields in developing countries. Environ. Sci. Technol. 40 (4),
1114e1119.
Pugesgaard, S., Olesen, J., Dalgaard, T., Jørgensen, U, 2013. Biogas in
organic agriculture - effects on productivity, energy selfsufficiency and greenhouse gas emissions. Renewable Agric.
Food Syst.
Rathman, R., Szklo, A., Schaeffer, R., 2010. Land use competition
for production of food and liquid biofuels: an analysis of
the arguments in the current debate. Renewable Energy 35,
14e22.
Ribeiro, D,Matavel, N., 2009. Jatropha! A socio-economic pitfall for
Mozambique, Justiça Ambiental & União Nacional de Caponeses,
Alliance Sud, Arbeitsgruppe Schweiz Kolumbien, Basler Appell
gegen Gentechnologie, Bio Suisse, Brot für Alle, Caritas, erklärung
von Bern, Fastenopfer, HEKS, Kleinbauern-Vereinigung, l Pro
Natura, Reformierte Kirchen, SWISSAID, Terre des Hommes,
Uniterre.
Rossi, A., 2012. Good Environmental Practices in Bioenergy Feedstock
Production e Making Bioenergy Work for Climate and Food Security. FAO Environment and Natural Resources Management.
Working Paper Nr. 49.
RSB, 2011. Consolidated RSB EU RED Principles & Criteria for
Sustainable Biofuel Production. Roundtable of Sustainable Biofuels
RSB. http://rsb.org/pdfs/standards/RSB-EU-RED-Standards/1105-10-RSB-STD-11-001-01-001-vers-2-0-Consolidated-RSB-EU-REDPCs.pdf.
Schader, C., Stolze, M., Gattinger, A., 2012. Environmental performance of organic agriculture. In: Boye, J., Arcand, Y. (Eds.), Green
Technologies in Food Production and Processing. Springer, New
York.
Seufert, V., Ramankutty, N., Foley, J., 2012. Comparing the yields of
organic and conventional agriculture. Nature.
Smith, P., Martino, D., Cai, Z., Gwary, D., Janzen, H.H., Kumar, P.,
McCarl, B., Ogle, S., O’Mara, F., Rice, C., Scholes, R.J.,
Sirotenko, O., Howden, M., McAllister, T., Pan, G.,
Romanenkov, V., Schneider, U., Towprayoon, S., Wattenbach, M.,
Smith, J.U., 2008. Greenhouse gas mitigation in agriculture. Philos.
Trans. R. Soc.
Tomei, J., Upham, P., 2009. Argentinean soy-based biodiesel: an introduction to production and impacts. Energy Policy 37, 3890e3898.
UNEP, 2010. Gain or Pain e Biofuels and Invasive Species. Bioenergy
Issue Paper Series Nr. 3. United Nations Environmental Programme UNEP.
wa Gathui, T., Ngugi, W., 2010. Bioenergy and Poverty in Kenya:
Attitude, Actors and Activities (Policy Innovation Systems for
Clean Energy Security PISCES Working Paper).
WBGU, 2009. Welt im Wandel. Zukunftsfähige Bioenergie und nachhaltige Landnutzung. Wissenschaftlicher Beirat Globale Umweltveränderung der Bundesregierung WBGU, Berlin.
Wicke, B., Verweij, P., van Meij, H., van Vuuren, D., Faaij, A., 2012.
Indirect land use change: review of existing models and strategies
for mitigation. Biofuels 3 (1), 87e100.
C H A P T E R
24
Bioenergy Technology and Food Industry
Waste Valorization for Integrated Production
of Polyhydroxyalkanoates
Vasiliki Kachrimanidou 1, Nikolaos Kopsahelis 1, Colin Webb 2,
Apostolis A. Koutinas 1,*
1
2
Department of Food Science and Human Nutrition, Agricultural University of Athens, Athens, Greece,
School of Chemical Engineering and Analytical Science, University of Manchester, Manchester, England,
United Kingdom
*Corresponding author email: akoutinas@aua.gr
O U T L I N E
Introduction
419
PHA Structure and Properties
420
PHA Production Integrated in Biorefinery Concepts 421
Valorization of Biodiesel Industry By-Products
424
Valorization of Second-Generation Bioethanol
427
Industry By-Products
Valorization of By-Product Streams from
427
Food Industries
INTRODUCTION
The imminent depletion of fossilized raw materials
and increasing environmental concerns has paved
the way toward the development of a sustainable
bio-based economy. Biorefinery concepts constitute a
significant aspect of the future bioeconomy era where
renewable raw materials, such as widely available lignocellulosic biomass in conjunction with industrial
by-products and waste streams, will be utilized for the
production of value-added commercial products,
including biofuels, chemicals, biodegradable polymers
and antioxidants among others. However, the establishment of a new industrial sector is a difficult task not only
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00024-3
PHA Production from Winery By-Products
PHB Production from Confectionery and Bakery
Industry Waste Streams
PHB Production from Whey
428
429
430
Conclusions and Future Perspectives
430
References
430
because of the viability of new technological advances
but also because of the transition from the nonrenewable
to the sustainable era should occur smoothly in order to
avoid job losses and economic turmoil. A smooth transition can be achieved through the integration of sustainable processing schemes in those conventional
industrial plants that generate waste and by-product
streams suitable for bioconversion or green chemical
conversion into value-added products.
Petroleum-derived plastics constitute an everyday
commodity used mainly for packaging and disposable
materials. According to the U.S. Environmental Protection Agency, 31 million t of waste was generated in
2010, while only 8% of the total plastic waste was
419
Copyright Ó 2014 Elsevier B.V. All rights reserved.
420
24. BIOENERGY TECHNOLOGY AND FOOD INDUSTRY WASTE VALORIZATION FOR INTEGRATED PRODUCTION
recovered for recycling (Anonymous, 2012a). Other conventional waste management approaches, such as incineration and discarding to landfills that are currently
applied for plastic disposal, are not considered as sustainable options. Bearing in mind that plastic consumption rates are constantly increasing, it is nowadays
common knowledge that the only way to deal with
petroleum-derived plastics is the development of biodegradable counterparts that serve the same, or even more,
market outlets and societal needs.
The production of fully biodegradable polymers can
be achieved either via microbial production of small
molecules (e.g. organic acids and alcohols) that could
be subsequently used as monomers for polymer synthesis or through direct fermentative production of tailormade biodegradable polymers. Polyhydroxyalkanoates
(PHAs) are biodegradable polyesters accumulated intracellularly during fermentation that belong to the second
category. The wide versatility of PHA properties
coupled with their potential to substitute major
petroleum-derived plastics has driven research studying
their synthesis and exploring industrial implementation
for more than 30 years. Despite the numerous attempts
to create industrial processes for PHA production by
many manufacturers in different countries, their industrial production is still limited due mainly to high production costs that should coincide with the production
of tailor-made PHAs possessing the required thermophysical and mechanical properties. The high production cost is mainly dependent on the use of
commercial sources of carbon and nutrients and the significant cost required for downstream separation and
purification of PHAs from microbial mass. Moreover,
fermentation parameters, such as carbon source to
PHA conversion yield, productivity, final PHA concentration and PHA content, are critical for the development of highly efficient production processes.
Consequently, the use of low- or even no-cost waste
and by-products streams could provide alternative
renewable feedstocks to reduce process costs. In recent
years, research focuses on the evaluation of various
low-cost renewable resources that may lead to reduction
of PHA production costs. However, it is nowadays clear
that traditional fermentation processes should be
restructured and integrated in advanced biomass
refining schemes in order to reduce process economics,
improve environmental impact, produce many products
for different markets and create synergies between
different industrial sectors.
This study reports recent efforts targeting PHA production from low-cost renewable resources and proposes potential biomass refining schemes that could be
developed for simultaneous production of fuels, chemicals, value-added products and PHAs. Such biorefinery
schemes could be either developed in new industrial
plants or integrated in existing industrial plants through
the valorization of waste or by-product streams.
PHA STRUCTURE AND PROPERTIES
PHAs are a group of polyesters produced intracellularly as carbon and energy reserve granules. Intracellular accumulation of PHAs is usually observed when
one nutrient (e.g. nitrogen, phosphorus, and oxygen) is
present in the fermentation broth in limiting concentration, while, in the same time, there is an available excess
source of carbon. Most bacterial strains, such as Cupriavidus necator (formerly classified as Ralstonia eutropha
and Alcaligenes eutrophus), accumulate PHAs as secondary products under nutrient-limiting conditions. However, there are some bacterial strains, such as
recombinant Escherichia coli and Alcaligenes latus, that
synthesize PHAs as a primary metabolite during microbial growth.
The generic chemical formula of PHAs is presented in
Figure 24.1, where R represents the alkyl side groups
of varying chain lengths. The most common monomers
are 3-hydroxybutyrate (3HB, m ¼ 1 and R]CH3), 3hydroxyvalerate (3HV, m ¼ 1 and R]CH2CH3) and
3-hydroxyhexanoate (3HHx, m ¼ 1 and R]CH2CH2CH3).
The simplest and most widely studied member of the
PHA family is called poly-(3-hydroxybutyrate) (PHB).
Three of the most widely studied copolymers are poly-(3hydroxybutyrate-co-3-hydroxyvalerate) [P(3HB-co-3HV),
y ¼ 1], poly-(3-hydroxybutyrate-co-3-hydroxyhexanoate)
[P(3HB-co-3HHx), y ¼ 2] and poly-(3-hydroxybutyrate-co4-hydroxybutyrate) [P(3HB-co-4HB]. Recent research
has also focused on the production of terpolymers, such
as P(3HB-co-4HB-co-3HV) and P(3HB-co-3HV-co-3HHx)
O
R
CH
(CH2)m C
O
CH3
ΟH
Ο
CH2
CH
C
ΟH
Generic formula of hydroxy alkanoates (HA)
n
PHB
CH3
Ο
CH
(CH2)y
O
CH3
CH2
C
Ο
CH
O
CH2
n
C
x
P(3HB-co-3HA)
O
CH3
Ο
CH
CH2
O
C
Ο
n
CH2
CH2
CH2
C
x
P(3HB-co-4HB)
FIGURE 24.1 Generic chemical formula of HA monomers and
common PHA copolymers.
PHA PRODUCTION INTEGRATED IN BIOREFINERY CONCEPTS
(Bhubalana et al., 2010; Cavalheiro et al., 2012). PHAs can be
categorized according to the number of carbon atoms into
short-chain-length PHAs (monomers containing three to
five carbon atoms) and medium-chain-length PHAs
(monomers containing more than six carbon atoms) (Asbhy
et al., 2011; Du et al., 2012). A historical overview of PHA
research and industrial applications is presented in previous publications (Solaiman et al., 2006; Verlinden et al.,
2007; Du et al., 2012).
As previously mentioned, one of the most important
advantages of PHAs is their biodegradability and
biocompatibility. Under aerobic conditions, PHAs are
degraded to carbon dioxide and water, while under
anaerobic conditions, methane and water are the final
products. Hence, these compounds can be utilized
from various microorganisms living in soil and water
as carbon source for their growth, without toxic effects
to the environment.
PHB was the first member of the PHA family that
was identified after isolation from Bacillus megaterium
(Lemoigne, 1926). It can be produced by many bacterial
strains (especially various strains of C. necator) in high
concentrations (more than 150 g/l) and intracellular
content (more than 80% on a dry weight basis) from
commercial carbon sources (mainly glucose as well as
fructose and sucrose) and starch hydrolysates (Ryu
et al., 1997; Yu et al., 2003). In addition, the physical
properties of PHB are similar to polypropylene. However, the brittle and thermally unstable nature of PHB
limits its commercial applications and constitutes one
of the major reasons that have prevented its production
in large-scale operations. The high crystallinity of PHB
(55e80%), associated with the formation of large spherulites, is the main reason that causes the brittle nature
of PHB. It should be stressed though that the application of appropriate processing methodologies could
reduce the undesirable mechanical properties of PHB,
which could be used for the production of ductile films
(Barham et al., 1992). Furthermore, the molecular
weight of the PHB homopolymer produced by many
bacterial strains, under varying fermentation condition
and utilization of different feedstocks may also result in
a biopolymer with improved characteristics (Kusaka
et al., 1999).
P(3HB-co-3HV) was the first copolymer of the PHA
family that was identified and subsequently produced
on industrial scale by Imperial Chemical Industries
using a R. eutropha strain. The incorporation of 3HV
units in different proportions in the copolymer by
this R. eutropha strain was only possible after the
addition of propionic acid as a carbon source precursor that induced the metabolic synthesis of 3HV units.
The production of P(3HB-co-3HV) also demonstrated
that it is feasible to alter the properties of PHAs
by controlling fermentation conditions. For instance,
421
the addition of increasing propionic acid concentrations during PHA accumulation results in increasing
proportions of 3HV units (expressed as mol%) in
the P(3HB-co-3HV) copolymer. In this way, it was
demonstrated that the incorporation of 3HV units in
the P(3HB-co-3HV) copolymer results in improved
mechanical properties (Byrom, 1987; Choi and Lee,
1997).
Since the identification and commercial production
of P(3HB-co-3HV) copolymers, research has focused
on the identification or modification of microbial
strains capable of producing PHA copolymers without
addition of carbon source precursors or the production
of different PHA copolymers, with addition of carbon
source precursors, containing two, three or four monomers that demonstrate desired properties (Madden
et al., 2000; Loo et al., 2005; Koller et al., 2007a). For
instance, the archeon Haloferax mediterranei accumulates 72.8% (w/w) of P-(3HB-co-3HV) that contains
6 mol% 3HV units directly from whey sugars, while it
produces the terpolymer P(3HB-co-3HV-co-4HB) when
it is supplemented with 3HV and 4HB precursors
(Koller et al., 2007a). Table 24.1 presents the specific
properties of various PHAs compared with major
petroleum-derived plastics. Nowadays, it is widely
accepted that PHA physical properties can vary from
brittle PHB homopolymers with high crystallinity to
flexible PHA copolymers with lower crystallinity,
such as P(3HB-co-3HV) and P(3HB-co-3HHx), to elastic
PHA copolymers, such as P(3HB-co-4HB) and P(3hydroxyoctanoate-co-3-hydroxydecanoate) (Wolf et al.,
2005; Whitehouse et al., 2006). In the last 30 years,
PHAs have been identified as potential biopolymers
for a wide spectrum of end-uses including food packaging, flushable hygiene products, tissue engineering
applications, adhesives, agriculture and biocomposites
(Wolf et al., 2005).
PHA PRODUCTION INTEGRATED
IN BIOREFINERY CONCEPTS
It is nowadays widely recognized that successful
implementation of industrial PHA production will
only be achieved through satisfaction of sustainability
aspects coupled with production of biodegradable polymers with desirable properties. Sustainability aspects
include cost-competitiveness, environmental benignness and production of biodegradable polymers that
serve certain market and societal needs. Additional advantages will be provided through the ability to produce
PHAs with adjustable properties that could be used in
different end-uses by simple modification of fermentation conditions. For instance, the production of different
types of PHAs that could be used for both commodity
422
TABLE 24.1
24. BIOENERGY TECHNOLOGY AND FOOD INDUSTRY WASTE VALORIZATION FOR INTEGRATED PRODUCTION
Thermophysical Properties of Various PHAs
Polymer
Co-Monomer
(mol%)
Tg*
( C)
Tmx
( C)
DHm{
(J/g)
Xc**
%
Td(50%)xx
( C)
References
PHB
e
4
177
84
60
5
e
Shimamura et al. (1994)
PHB
e
4
180
e
e
e
Akaraonye et al. (2010)
2.3
163
e
e
252
Han et al. (2010)
1.2
169
49.00
33
e
Lee et al. (2008)
P(3HB-co-3HV)
P(3HB-co-3HV)
{{
4.1
{{
8
{{
P(3HB-co-3HV)
12
e
153
66.40
45
e
Gunaratne and Shanks
(2005)
P(3HB-co-3HV)
12{{
1.9
155
72.61
49
271
Garcia et al. (2013)
{{
P(3HB-co-3HV)
30
2.1
111
e
e
352
He et al. (2001)
P(3HB-co3HHx)
3.5***
1
140 and 151
44
e
e
Tsuge et al. (2004)
P(3HB-co3HHx)
5***
1 to 3
125e138 and
142e155
38e47
e
e
Loo et al. (2005)
P(3HB-co3HHx)
11***
1
136
60
40
5
e
Shimamura et al. (1994)
P(3HB-co3HHx)
17***
2
130
39
29
5
e
Shimamura et al. (1994)
P(3HB-co-4HB)
12xxx
4.3
124
56
38.2
270{{{
Luo et al. (2009)
Polypropylene
e
10
176
e
70
e
Akaraonye et al. (2010)
Polystyrene
e
100
240
e
e
e
Akaraonye et al. (2010)
* Glass transition temperature.
x
Melting temperature.
{
Enthalpy of fusion.
** Percentage of crystallinity.
xx
Degradation temperature.
{{
3-hydroxyvalerate.
***
3-hydroxyhexanoate.
xxx
4-hydroxybutyrate.
{{{
Td(5%).
(e.g. food packaging) and specialty (e.g. scaffolds for tissue engineering applications) end-uses by simple modification of fermentation parameters could provide
process flexibility.
An important innovation on future PHA-based processes will be the creation of cascade processing schemes
in order to increase resource efficiency (Anonymous,
2012b). Cascade processing is based on the reutilization
of packaging material after its use (also called postconsumer plastics) for other commercial purposes. For
instance, hydrolysis into monomers could create valueadded platform molecules for the chemical industry. In
addition, bioplastics could be used as replacements for
coal and heating fuel due to their high calorific value
(Anonymous, 2012b). Reutilization of PHA-based packaging materials is strongly dependent on the development of suitable recycling technologies.
Despite their significant advantages, industrial production of PHAs is hindered by high production cost.
Previous attempts to produce PHAs in large scale had
to rely on conventional fermentation technologies that
cannot compete with low-cost petroleum-derived plastics. As mentioned earlier, raw material supply is one
of the most important factors that should be optimized
in order to reduce processing costs. For this reason,
recent research focuses on the utilization of low-cost
feedstock for PHA production (e.g. molasses, crude
glycerol, whey, animal fats, and waste cooking oils
among others) aiming to substitute for conventional
and expensive carbon sources. Table 24.2 presents the results regarding PHA production from various waste and
by-product streams. However, even if waste or byproduct streams are used as fermentation feedstocks,
aerobic cultivation for PHA production in industrial
scale operations is still an expensive unit operation.
For this reason, integration of PHA production into
existing industrial plants or the development of new industrial plants for PHA production should be combined
TABLE 24.2 PHA Production from Various Crude Renewable Resources, Waste and By-Product Streams
By-Product or
Waste Stream
Type of
PHA
Strain
Maximum
Cell Weight
Max PHA
Concentration
(g/l)
PHA
Content
(%)
Productivity
(g/l h)
References
38
0.84
Cavalheiro et al. (2009)
Cupriavidus necator
DSM 545
PHB
68.8
26.1
Bagasse hydrolysates
Ralstonia eutropha
PHA
11.1
6.3
56.5
e
Yu and Stahl (2008)
Crude glycerol and
rapeseed hydrolysates
Cupriavidus necator
DSM 545
P(3HB-co-3HV)
19.6
10.9
55.6
0.12
Garcia et al. (2013)
Wheat-derived media
(shake flask cultures)
Cupriavidus necator
NCIMB
11599
PHB
73.2
51.1
70
0.3
Koutinas et al. (2007b)
Wheat-derived media
(bioreactor cultures)
Wautersia eutropha NCIMB
11599
PHB
175.2
162.8
93
0.89
Xu et al. (2010)
Soybean oil
Ralstonia eutropha H16
PHB
126
95.8
76
0.99
Kahar et al. (2004)
Ralstonia eutropha PHB 4
(DSM 541)
P(3HB-co-3HHx)
138
102.1
74
1.06
Oleic acid
Pseudomonas putida PGA1
PHAs-mcl
30.2
44.8
0.19
Marsudi et al. (2007)
Hydrolyzed whey
Haloferax mediterranei
DSM 1411
PHA
11
5.5
50
0.05
Koller et al. (2007b)
Pseudomonas hydrogenovora
DSM 1749
10.83
1.3
12
0.03
Hydrogenophaga pseudoflava
DSM 1034
6.75
2.7
40
0.05
-
13.52
Pseudomonas hydrogenovora
DSM 1749
PHB
10.58
1.27
12
0.03
P(3HB-co-3HV)
12
1.44
12
0.05
Cheese whey
Methylobacterium sp. ZP24
PHB
3.54
64
0.09
Nath et al. (2008)
Saccharified waste
potato starch
Ralstonia eutropha NCIMB
11599
PHB
179
94
55
1.47
Haas et al. (2008)
Extruded rice bran and
extruded corn starch
Haloferax mediterranei
ATCC 33500
PHB
140
77.8
55.6
0.65
Huang et al. (2006)
Sugarcane molasses and
corn steep liquor
Bacillus megaterium
PHB
3.6
2.2
59.4
e
Gouda et al. 2001
Sugarcane molasses and urea
Bacillus megaterium BA-019
PHB
72.6
30.5
42
1.27
Kulpreecha et al. 2009
Hydrolyzed whey permeate
Hydrolyzed whey permeate
and valerate
5.53
Koller et al. (2008)
PHA PRODUCTION INTEGRATED IN BIOREFINERY CONCEPTS
Waste glycerol
423
424
24. BIOENERGY TECHNOLOGY AND FOOD INDUSTRY WASTE VALORIZATION FOR INTEGRATED PRODUCTION
with the production of value-added co-products. This
can be achieved through fractionation of agricultural resources or by-product and waste streams from existing
industrial processes.
PHA production cost increases further due to downstream separation and purification of PHAs from residual microbial mass. Several methods have been reported
for the recovery of PHAs based on the utilization of
organic solvents such as acetone, chloroform or dichloroethane. However, these methods are unfavorable for
large-scale production since solvents increase operational cost and additional equipment for solvent recovery is often needed. Alternative extraction methods
have been also proposed including enzymatic lysis of residual microbial mass (Kapritchkoff et al., 2006; Verlinden et al., 2007), supercritical fluid extraction (Hejazi
et al., 2003), mechanical disruption of bacterial cells
coupled with chemical treatment, autolysis of bacterial
cells, and chemical treatment under acidic or alkaline
conditions (Yu and Chen, 2006; Verlinden et al., 2007).
Several studies have also focused on the estimation of
PHA production costs from different feedstocks (Choi
and Lee, 1997; van Wegen et al., 1998; Posada et al.,
2011). However, there are limited studies on the evaluation of integrated biorefineries focusing on the fractionation of the initial raw material combining the
production of PHAs with the extraction or production
of value-added co-products. In addition, future costing
studies should also focus on the evaluation of the potential to integrate PHA production in existing industries.
In recent years, several studies focused on the production of PHAs from low-cost renewable resources
(Akaraonye et al., 2010; Koller et al., 2010; Du et al.,
2012). This study focuses on the presentation of representative biorefinery concepts targeting the production
of PHAs and other value-added products. In particular,
PHA production could be combined with biofuel and
bioenergy production.
Valorization of Biodiesel Industry By-Products
Both edible (e.g. rapeseed, soybean or palm) and
nonedible (e.g. Jatropha) oilseeds can be used for biodiesel production. Biodiesel production is mainly
achieved from soybean in the USA, rapeseed (or sunflower in lower quantities) in Europe and palm oil in
South-East Asian countries. Biodiesel production could
be also achieved using nonconventional resources
including microbial oil produced by yeast, fungi and
algae (Meng et al., 2009). The continuous growth of biodiesel production coincides with proportional production of by-products streams. The main by-product is
glycerol that is generated during transesterification of
triglycerides with predominantly methanol leading to
the production of fatty acid methyl esters and glycerol
(10%, w/w). It has been estimated that by 2021, the share
of biodiesel production from vegetable oils will increase
and the worldwide biodiesel production from such
oils is projected to reach approximately 30 106 t
(Anonymous, 2012c). This means that approximately
3 106 t of glycerol will be available for chemical
and biopolymer production. Crude glycerol streams
produced from biodiesel plants have purities in the
range of 77e90% (w/w) (Mothes et al., 2007). The
main impurities in crude glycerol are water, methanol,
residual fatty acids and corresponding esters, and salts
(NaCl or K2SO4) in varying proportions depending on
the extent of glycerol purification (Mothes et al., 2007).
Purification methods for glycerol have been proposed
(Chatzifragkou and Papanikolaou, 2012) for the removal
of impurities and salts after biodiesel production but
they seem to be rather unprofitable, especially for small
industries. Novel uses of glycerol involve both green
chemical conversions and microbial bioconversions.
Glycerol represents an easily assimilated carbon source
for many microorganisms. Crude glycerol has been evaluated as carbon source for various microbial bioconversions, such as 1,3-propanediol, citric acid, ethanol,
succinic acid, propionic acid, microbial oil and PHAs
(Koutinas et al., 2007a; da Silva et al., 2009; Koutinas
and Papanikolaou, 2011; Sarris et al., 2011).
Biodiesel production from oilseeds leads to the production of oilseed meals as a valuable by-product
stream. Oilseed meal is the protein- and carbohydraterich fraction that remains after the extraction of oil.
The main conventional commercial outlet for oilseed
meals is as animal feed. In the period 2012e2021, biodiesel production from edible vegetable oils will still
rely mainly on rapeseed and sunflower. However, biodiesel production from palm oil is projected to increase
twofold (Anonymous, 2012c). Based on recent estimates,
approximately 315 106 t of oilseed meals are expected
to be produced by 2021, corresponding to an increase up
to 23% based on current production capacities (Anonymous, 2012c). Future biodiesel industries could be converted into novel biorefineries through valorization of
crude glycerol and oilseed meal streams leading to the
production of biodiesel, chemicals, food and feed ingredients and biopolymers such as PHAs.
Ashby et al. (2004, 2011) evaluated the production
and properties of PHAs accumulated by the bacterial
strains Pseudomonas oleovorans NRRL B-14682 and Pseudomonas corrugata 388 cultivated on crude glycerol.
Ashby et al. (2011) reported that the molecular weight
of PHB was decreased with increasing methanol concentration in crude glycerol. Mothes et al. (2007) and Garcia
et al. (2013) evaluated the effect of NaCl and K2SO4 on
PHA production during fermentation with the bacterial
strains Paracoccus denitrificans, C. necator JMP 134 and C.
necator DSMZ 545. These salts are present in crude
PHA PRODUCTION INTEGRATED IN BIOREFINERY CONCEPTS
glycerol depending on the catalyst (NaOH or KOH)
employed during transesterification of triglycerides.
The inhibition caused by NaCl on PHA production is
more pronounced at significantly lower concentrations
than K2SO4. Mothes et al. (2007) reported that bioreactor
fermentations with C. necator JMP 134 cultivated on
crude glycerol and inorganic chemicals as additional nutrients could lead to the production of PHB contents up
to 70% (w/w). Crude glycerol has also been employed in
bioreactor fermentations for the production of PHB using the bacterial strain C. necator DSM 545 leading to
50% (w/w) PHB content and 1.1 g/l h PHB productivity
(Cavalheiro et al., 2009). Tanadchangsaeng and Yu (2012)
stressed that the productivity (around 0.92 g/l ) of glycerol fermentation to PHB synthesis is lower than the one
achieved from glucose. Crude glycerol could be also
combined with other carbon sources that could be
used as precursors for the production of PHA copolymers (Cavalheiro et al., 2012). The production of
P(3HB-co-4HB-co-3HV) was reported when C. necator
DSM 545 was cultivated on crude glycerol, propionic
acid (stimulator of 4HB accumulation and 3HV precursor) and g-butyrolactone (4HB precursor). In all studies
presented above, inorganic chemicals were used as
nutrient supplements.
Apart from fermentation efficiency of PHA production, it is also crucial to assess the properties of the polymer produced and the associated production cost.
Tanadchangsaeng and Yu (2012) reported that although
the thermal and physical properties of the PHB produced from glycerol is similar to the one produced
from glucose, the molecular weight of the glycerolderived homopolymer is lower than the molecular
weight of the PHB produced from glucose. Posada
et al. (2011) presented a comparative technoeconomic
evaluation of PHB production from crude glycerol using
two different bacterial strains, C. necator and B. megaterium, and three different downstream separation strategies. Fermentation of C. necator resulted in the
production of 81.6 g/l of which 57.1 g/l was PHB,
significantly higher than B. megaterium. The most costcompetitive process involved PHB production in fedbatch fermentations with C. necator followed by PHB
separation and purification with heat pretreatment,
enzymatic alkaline digestion, centrifugation, washing,
evaporation, and spray drying. Posada et al. (2011) reported also that glycerol purification to 98% (w/w) contributes approximately 6% of the overall PHB production
cost, thereby slightly affecting the total cost. In this
study, it was concluded that the PHB production
cost from crude glycerol could be as low as US$2/kg
depending on the downstream process utilized.
PHA production from crude glycerol could be combined with the valorization of oilseed meals or residues
remaining after extraction of microbial oil. For instance,
425
rapeseed meal could be utilized for the production of
various value-added fractions including protein isolates, carbohydrates, hulls, phenolic compounds and
glucosinolates with various applications such as animal
feed, pesticidal agent, bioactive proteins, glues and adhesives, paper coatings and ingredients for cosmetics
among others (Anonymous, 2011; Egues et al., 2010).
Another alternative application of oilseed meals is based
on the production of complex nutrient supplements for
fermentation processes including PHA production. In
this way, commercial inorganic chemicals will be
replaced improving the sustainability of the whole biorefinery concept. Oilseed meals contain significant
quantities of protein, minerals and other necessary nutrients for microbial growth. Enzymatic hydrolysis of
protein to amino acids and peptides, and phytic acid
to phosphorus could provide a hydrolysate suitable for
PHA production. Crude enzymes could be produced
via solid-state fermentation employing appropriate
fungal strains and oilseed meals as substrates (Wang
et al., 2010; Kachrimanidou et al. 2013). Wang et al.
(2010) reported the production of a nutrient-rich hydrolysate from rapeseed meal with a free amino nitrogen
content of 2016.2 mg/l and inorganic phosphorus (IP)
of 304 mg/l that was subsequently used successfully
as nutrient supplement combined with glucose as carbon source for the cultivation of Saccharomyces cerevisiae.
Garcia et al. (2013) investigated the generation of a microbial feedstock through hydrolysis of rapeseed meal,
which was combined with crude glycerol as the sole
fermentation medium for PHA production. Fed-batch
fermentations resulted in a production of 10.9 g/l
P(3HB-co-3HV) without addition of any precursor. The
properties of the biopolymer produced were also examined, leading to the conclusion that this bioprocess could
be incorporated in rapeseed-based biodiesel plants
contributing to the sustainability of biodiesel biorefineries. Kachrimanidou et al. (2013) reported the utilization of sunflower meal for the production of
nutrient-rich hydrolysates that could be subsequently
supplemented with crude glycerol for the production
of 9.9 g/l P(3HB-co-3HV) with a PHA content of 50%
(w/w) in shake-flask fermentations using the microbial
strain C. necator DSM 545 without addition of any
precursor.
Figure 24.2 presents a biorefinery concept in which
sunflower (or other oilseed) meal is utilized only for
the production of fermentation feedstock involving production of crude enzymes via solid-state fermentation
followed by hydrolysate production via enzymatic hydrolysis. Preliminary bioreactor fermentations carried
out in fed-batch mode at the Agricultural University of
Athens with the microbial strain C. necator DSM 7237
cultivated on sunflower meal hydrolysate and crude
glycerol lead to the production of more than 20 g/l
426
24. BIOENERGY TECHNOLOGY AND FOOD INDUSTRY WASTE VALORIZATION FOR INTEGRATED PRODUCTION
FIGURE 24.2 Utilization of sunflower-derived
Sunflower seed
biodiesel industry by-products for PHA production.
Mechanical pressing and
hexane extraction
Vegetable Oil
Transesterification
Sunflower meal
Solid state
fermentation
Aspergillus
oryzae
Crude enzymes
Biodiesel
Cupriavidus
necator
Crude glycerol
Enzymatic hydrolysis
Microbial
bioconversion
Carbon source
Nitrogen-rich
source
Polyhydroxyalkanoates
PHB with a PHB content of approximately 70% (w/w).
However, this processing scheme does not take advantage of the full potential of sunflower meal that contains
value-added ingredients that could be isolated contributing in the development of a more sustainable biorefinery approach.
Figure 24.3 presents a sunflower-based biorefinery
where besides fermentation feedstock, sunflower meal
is also used for the production of an antioxidant-rich
stream and a protein isolate product. The sunflower
seed is covered by the hull that could be removed before
oil separation by mechanical pressing and solvent
extraction in biodiesel production processes. The protein
content in sunflower meals can be increased via dehulling and complete oil extraction. The composition of
sunflower meal is variable and is highly dependent on
cultivation conditions, sunflower variety and the industrial process used for biodiesel production. Dehulling or
partial dehulling of sunflower seeds could provide a byproduct that could be used for the production of energy,
hemicelluloses, organic amendment for the soils, and
biomaterial (Anonymous, 2011). The sunflower meal
that remains as a by-product after (partial) dehulling
and complete oil extraction could be fractionated in
three different fractions (i.e. a protein-rich fraction, a
lignocellulose-rich fraction and a liquid fraction) by a
simple sedimentation/flotation process based on the
formation of an aqueous suspension (Bautista et al.,
1990; Parrado et al., 1991). This separation is based on
the different densities of major components in sunflower
Sunflower
seeds
Energy generation
biomaterial
hemicellulose production
Biodiesel
Transesterification
Oil
Crude
glycerol
Partial
dehulling
Mechanical pressing and
hexane extraction
Protein
isolate
Carbon source
Natural adhesive
food/feed additive
biopolymers
Protein-rich
fraction
Antioxidants
Microbial
fermentation
Polyhydroxyalkanoates
FIGURE 24.3
Partly dehulled
sunflower meal
Aqueous extraction
Lignocellulosic
fraction
Solid state
fermentation
Nutrient
supplement
Enzymatic
hydrolysis
Advanced sunflower-based biorefinery concept.
Liquid
fraction
PHA PRODUCTION INTEGRATED IN BIOREFINERY CONCEPTS
meal. Subsequently, antioxidants can be removed from
the protein-rich fraction, as well as from the lignocellulosic fraction. The most important of the phenolic compounds found in sunflower is chlorogenic acid.
The protein isolate extracted from the protein-rich
fraction, after treatment with acid and alkaline solutions,
can be utilized for the production of biopolymers and
edible films. Yust et al. (2003) improved protein extraction from sunflower meal through treatment with alkalase. Remaining fractions can be used as substrate in
solid-state fermentation with a fungal strain of Aspergillus oryzae, an efficient producer mostly of proteolytic
enzymes. The solids at the end of solid-state fermentation can be used as enzyme-rich medium for hydrolysis
of macromolecules contained in remaining sunflower.
The liquid fraction from sunflower meal fractionation
can be used as suspension liquid in enzymatic hydrolysis, aiming at the generation of a nutrient-rich supplement. At the end of hydrolysis, remaining solids are
separated from the hydrolysate by centrifugation, and
can be possibly used for combustion to generate heat
or as a carbohydrate-rich resource for the production
of hydrolysates for other fermentations. The nutrientrich supplement has been used successfully for
enhanced production of PHB. The advanced biorefinery
concept results in the production of three products (antioxidants, protein isolate and PHB) from the same raw
material presenting a high potential of improved process economics.
Valorization of Second-Generation Bioethanol
Industry By-Products
Cellulose, hemicellulose and lignin are the main components found in lignocellulosic raw materials and the
corresponding composition is dependent on the biomass
resource. Production of sugar-rich hydrolysates from
lignocellulosic biomass requires treatment with combined thermochemical treatment and enzymatic hydrolysis. Previous studies on utilization of lignocellulosic
resources have focused on hydrolysis of cellulose and
hemicellulose fractions to simple sugars for microbial
fermentation mainly aiming to bioethanol production.
Nonetheless, given the interest arising in biopolymers
production, bioethanol production could be combined
with PHA production. Cellulose could be utilized for
the production of bioethanol, while sugars from hemicellulose could be utilized for the production of PHAs.
In this way, conventional processes employed for the
production of bioethanol from lignocellulosic biomass
could be upgraded into advanced biorefinery concepts.
Silva et al. (2004) screened 55 strains as potential PHB
producers from xylose and identified Burkholderia sacchari IPT 101 and Burkholderia cepacia IPT 048 that were
subsequently evaluated via cultivations on xylose and
427
bagasse hydrolysates. Intracellular PHB content reached
62% and 53% for the two strains, respectively, when
grown on bagasse hydrolysates. Keenan et al. (2006)
utilized detoxified hemicellulose hydrolysates from
lignocellulosic resources for the production of P(3HBco-3HV) with B. cepacia through supplementation with
levulinic acid (0.25e0.5%) to achieve a P(3HB-co-3HV)
concentration of 2 g/l, a P(3HB-co-3HV) content of 40%
(w/w) and 3HV composition of 16e52 mol%. When
xylose and levulinic acid were used in microbial bioconversions with B. cepacia, the P(3HB-co-3HV) concentration and 3HV composition achieved were up to 4.2 g/l
and 61 mol%, respectively. Sugarcane bagasse hydrolysates were evaluated for PHA synthesis via fermentation
of R. eutropha (Yu and Stahl, 2008). The effect of inoculum concentration, dilution of hydrolysate and implementation of an adapted strain was studied regarding
PHA accumulation, which reached up to 57% (w/w)
polymer content. PHB was the major polymer accumulated, whereas copolymers could be also produced that
presented high ductility.
PHA production could be incorporated in existing
bioethanol production facilities from both sugar cane
in Brazil and cereals, such as wheat and corn, in other
countries worldwide. Sugar cane utilization for bioethanol production generates significant quantities of
bagasse, a lignocellulosic raw material that could be
used for combined production of ethanol from cellulose
and PHAs from hemicellulose sugars (mainly xylose).
Integration of PHA production in existing cereal-based
facilities used for bioethanol production could be
achieved by incorporating straw utilization as raw material for combined production of bioethanol and PHAs.
Such integrated biorefinery concepts could improve
the sustainability of first-generation bioethanol production plants.
Valorization of By-Product Streams from
Food Industries
The term “food waste” covers the wastes (and byproduct streams) that are generated during the whole
food supply chain starting from production of the raw
material followed by the processing into edible products
by the food industry and the final disposal by consumers, restaurants or catering services. Valorizing the
waste derived from the food industry sector would
result in the creation of novel biorefineries leading to
restructured and advanced industrial plants that will
not only satisfy the traditional market of food production but also other markets that are nowadays dependent on petroleum to provide the necessary feedstocks.
Food processing waste streams constitute renewable resources enriched in carbohydrates, protein, oils and fats,
phenolic compounds and various micronutrients.
428
24. BIOENERGY TECHNOLOGY AND FOOD INDUSTRY WASTE VALORIZATION FOR INTEGRATED PRODUCTION
PHA Production from Winery By-Products
utilization of this waste stream, since it contains lignocellulosic fractions that can be hydrolyzed and further
used in microbial bioconversions. Solid-state fermentation for production of hydrolytic enzymes has also
been reported using grape marc as solid support (Botella
et al., 2005).
Wine lees is the remaining residue after the end of the
fermentation stage. It is a rich source of ethanol, tartaric
acid, phenolic compounds and yeast cells. Wine lees can
be used for the production of potable alcohol (wine lees
mainly produced by large wineries), as nutrient supplement for fermentation (Bustos et al., 2004; Salgado et al.,
2010), for the production of tartaric acid (Versari et al.,
2001; Rivas et al., 2006) and as raw material for composting (Diaz et al., 2002; Nogales et al., 2005). A novel process has been developed at the Agricultural University
of Athens targeting the creation of a novel biorefinery
concept based on wine lees valorization (Figure 24.4).
The process starts with centrifugation or filtration of
wine lees in order to separate the liquid stream that
can be used for ethanol production via distillation. The
ethanol produced can be used as potable or fuel ethanol
depending on the purity. Current processes produce
potable ethanol. Ethanol could be also used as a platform chemical to supply the future sustainable chemical
industry. Alternatively, ethanol could be also utilized as
carbon source for microbial fermentation aiming to PHB
production by the bacterial strain C. necator NCIMB
12080 (Senior et al., 1986). This, however, may not be a
cost-competitive alternative when compared to the
traditional potable ethanol market. The remaining liquid
after ethanol extraction can be used in subsequent
hydrolysis stages to increase the presence of nutrients.
Wine production constitutes an important industrial
sector in many countries around the world, such as the
South European countries, United States, Chile and
Australia. Wine making generates both solid and liquid
by-products. Residues from wine production involve
mainly trimming wastes, grape stalk, grape pomace or
marc, wine lees and winery wastewater. These byproducts are currently supplied to ethanol distilleries
(e.g. in the case of wine lees), used (if possible) as fertilizers or processed as wastes in order to reduce the environmental impact caused by their disposal to the
environment. However, given the fact that environmental
policies are changing, new practices should be applied
aiming at valorization of winery by-product streams.
Ongoing research focuses on valorization of residues
from wine making. Trimming wastes are rich in cellulose, hemicellulose and lignin. Combined thermochemical treatment with enzymatic hydrolysis can be
applied to convert cellulose and hemicelluloses into C5
and C6 sugars that can be assimilated by microorganisms. Delignification steps are usually required since
the complex structure of lignin prevents hydrolysis of
polysaccharide. Bustos et al. (2005) evaluated the use
of trimming wastes and wine lees aiming at the production of lactic acid through simultaneous saccharification
and fermentation carried out by Lactobacillus rhamnosus.
Trimming wastes could be also used as solid support in
solid-state fermentations for the production of various
enzymes (Sanchez et al., 2002).
Grape pomace or marc is the solid fraction remaining
after the extraction and it consists of skins, pulp, seeds
and stems of grapes. Research has focused on efficient
Wine lees
Liquid
Distillation
Ethanol
Solids
Residual solids 1
Alcohol-free
nutrient rich
liquid
Potable alcohol
Biofuel
Platform chemical
Treatment with HCl
Residual solids2
(rich in yeast cells)
Enzymatic
hydrolysis of
yeast cells
Polyhydroxyalkanoates
FIGURE 24.4
Antioxidants
Nutrient-rich
supplement for
microbial
fermentations
Liquid rich in
tartaric acid
Tartrate salts
Tartaric acid
Crude enzymes
produced via solid
state fermentation
Addition of carbon
sources (e.g. crude
glycerol, lignocellulosic
hydrolysates)
Valorization of wine lees.
PHA PRODUCTION INTEGRATED IN BIOREFINERY CONCEPTS
The solid fraction that remains after centrifugation of
wine lees contains phenolic compounds with antioxidant properties, tartrate salts and yeast cells.
A phenolic-rich fraction can be easily isolated via solvent extraction. Tartrate salts can be subsequently separated from yeast cells via treatment with hydrochloric
acid. Versari et al. (2001) extracted tartaric acid with purity up to 99% from three different winery by-product
streams, including wine lees. Moreover, Nurgel and
Canbas (1998) investigated the production of tartaric
acid from grape pomace. Use of tartaric acid is well
established in wine making in order to adjust the pH
of the must prior to fermentation. Tartaric acid could
be also used as food additive.
After the extraction of phenolic compounds and
tartrate salts, residual wine lees solids are subjected to
enzymatic hydrolysis with the addition of crude enzymes produced via solid-state fermentation of a fungal
strain of A. oryzae on wheat bran. The ethanol-free medium that remains after the distillation step is used as
liquid in the hydrolysis stage. In this stage, yeast cells
are lysed and converted into a nutrient-rich supplement
similar to yeast extract. This supplement is rich in
various sources of nitrogen (e.g. amino acids and peptides), phosphorus and various trace elements. This
nutrient supplement can be combined with a carbon
source (e.g. crude glycerol from biodiesel industries) as
fermentation media for the production of PHB with
C. necator. Preliminary experiments with C. necator
DSM 7237 and crude glycerol as carbon source showed
that PHB production is feasible using wine lees hydrolysates. However, supplementation with a low quantity of
minerals is necessary showing that this nutrient supplement is deficient in some minerals. The wine lees hydrolysate could be combined with a sugar-rich hydrolysate
derived from treatment of lignocellulosic streams
derived during wine production.
PHB Production from Confectionery and Bakery
Industry Waste Streams
Significant quantities of waste streams are generated
annually from confectionery industries and bakeries.
The waste streams from the industrial sectors mentioned
above produce flour-, starch- or sugar-rich waste
streams generated either during processing or as endof-date products returned from the market. Confectionery waste streams are currently used as animal feed, for
composting or are discarded to landfills. However, these
low-cost materials constitute renewable feedstocks that
could be used for the development of novel biorefinery
schemes. Anaerobic digestion from various food waste
streams and biodiesel production from cooking oils are
predominant alternatives that have been proposed for
the utilization of various food waste streams. Current
research on confectionery waste streams and waste
bread is rather limited, but in recent years research has
started to focus on the valorization of such waste
streams. Dorado et al. (2009) utilized hydrolysates
derived from wheat milling by-products as fermentation
media for the production of succinic acid (50.6 g/l).
Leung et al. (2012) developed a two-stage bioprocess
involving solid-state fermentation and enzymatic hydrolysis of waste bread to produce a fermentation feedstock for the production of succinic acid (47.3 g/l at a
conversion yield of 0.55 g SA/g bread) using the bacterial strain Actinobacillus succinogenes.
A potential biorefining concept for the production of
PHAs and biodiesel from confectionery industry waste
streams is presented in Figure 24.5. In the case of confectionery wastes that contain high oil content, this could
be removed via solvent extraction. The oil obtained
from this step can be used for biodiesel production.
Remaining fractions will be rich in directly assimilable
sugars such as glucose, fructose, sucrose and lactose as
well as starch and protein. Utilizing starch- and
Biodiesel
production
Oil extraction
(if required)
Flour-and starchbased waste
streams
Enzymatic
hydrolysis
Crude enzyme
production
Wheat milling
by-products
Solid state
fermentation
429
Fermentation
medium
Microbial
fermentation
Polyhydroxyalkanoates
Additional
wastes
Aspergillus
awamori
FIGURE 24.5 Valorization of confectionery industry waste streams.
430
24. BIOENERGY TECHNOLOGY AND FOOD INDUSTRY WASTE VALORIZATION FOR INTEGRATED PRODUCTION
protein-rich waste streams as sources of carbon and nitrogen in fermentation processes demands the conversion of starch into glucose and protein into amino
acids and peptides. The amylolytic and proteolytic enzymes required for the hydrolysis of these macromolecules could be produced via solid-state fermentation
using the fungal strain Aspergillus awamori cultivated
on wheat milling by-products. The fermented solids,
rich in amylolytic and proteolytic enzymes, are subsequently combined with confectionery waste to produce
hydrolysates that can be used in fermentation processes
for the production of platform chemicals, microbial oil
or PHB. The production of PHB or PHAs from confectionery industry wastes could be employed for the production of biodegradable packaging materials for the
same industry.
The proposed process is based on the results that
were achieved for the production of PHB using wheat
as the whole raw materials (Koutinas et al., 2007a,
2007b; Xu et al., 2010). In this biorefinery concept,
wheat is fractionated into bran and gluten as valueadded co-products, while remaining fractions are used
for the production of fermentation media suitable for
the production of PHB via fed-batch cultures using the
microbial strain Wautersia eutropha NCIMB 11599. Xu
et al. (2010) developed a fermentation process for the
production of PHB from wheat-derived fermentation
media during fed-batch cultures in a bioreactor. The
highest PHB concentration achieved was 162.8 g/l.
However, wheat is regarded a food resource and should
not be used for chemical production. Starch- or flourrich food wastes could be used, instead of wheat, as a
renewable resource for the production of PHB.
PHB Production from Whey
Whey is the main by-product occurring from cheese
manufacture and lactose is one of the primary components. Current whey valorization processes mainly
focus on the production of whey powder, whey protein
concentrate or whey protein isolate. Utilization of whey
in fermentation processes has been widely investigated,
given the fact that it is produced in many countries in
significant quantities. Furthermore, whey valorization
will also contribute to the improvement of the environmental impact of the cheese industry because whey
disposal is a notorious environmental burden.
Future cheese industries could incorporate integrated
processing schemes for the production of whey protein
and PHAs. Koller et al. (2010) reviewed various bioconversions that employed whey permeate as carbon source
aiming at the production of PHAs. Different strategies
were proposed concerning uses of whey permeate;
direct conversion as substrate or hydrolysis of lactose
to glucose and galactose were examined. Moreover,
Wong and Lee (1998) presented PHB production from
whey powder with recombinant E. coli in pH-stat cultures. In fed-batch cultures with additions of concentrated whey solution, the corresponding dry cell
weight and PHB concentrations were 87 and 69 g/l,
respectively. The PHB content reached up to 80% (w/
w). These results established that PHB fermentation process from whey could be industrially employed,
increasing the sustainability and market alternatives of
traditional cheese producing plants.
Whey protein concentrate and isolate that could be
extracted from whey by ultrafiltration and evaporation
steps can be applied as food additives. Moreover, they
are considered to possess therapeutic properties and
for this reason, whey protein concentrate was applied
for treatment of various clinical disorders. Furthermore,
whey protein ingredients are added to food targeting to
improve their functional or technological properties.
Hence, keeping that in mind, biorefinery schemes based
on whey utilization could be easily proposed.
CONCLUSIONS AND FUTURE
PERSPECTIVES
The necessity to eliminate our dependence on fossil
resources will lead to an inevitable reconstruction of
the current industry in order to introduce the utilization
of renewable resources and produce chemicals, fuels
and materials in a sustainable manner. Implementation
of biorefinery concepts into existing industrial facilities
provide an alternative processing option, taking into
consideration that industrial by-products and waste
streams are generated in significant quantities and
currently, they are inadequately utilized. Consequently,
production of value-added products from waste and
by-product streams will enhance sustainability and
diversify market opportunities. Furthermore, production of biofuels should coincide with chemical and
biodegradable polymer production to enhance their sustainability. This study showed potential industries
where biofuel and food production could coincide
with PHA production. This research area is currently
at the inception phase and significant effort is required
in order to develop the technologies that will be implemented on industrial scale.
References
Akaraonye, E., Keshavarz, T., Roy, I., 2010. Production of polyhydroxyalkanoates: the future green materials of choice. J. Chem.
Technol. Biotechnol. 85, 732e743.
Anonymous, 2011. WP2: Optimisation of Primary Processing. Report
of Deliverables. Project Title: Developing advanced Biorefinery
schemes for integration into existing oil production/transesterification plants. Project Number: 213637 http://www.york.ac.
uk/res/sustoil/Pages/Deliverable%202-5.pdf.
REFERENCES
Anonymous, 2012c. OECD-FAO Agricultural Outlook 2012e2021
(Chapter 5): Oilseeds and Oilseed products http://www.fao.org/
fileadmin/templates/est/COMM_MARKETS_MONITORING/
Oilcrops/Documents/OECD_Reports/Ch5StatAnnex.pdf.
Anonymous, 2012a. Wastes-Resource Conservation- Common Wastes
& Materials. U.S. Environmental Protection Agency. http://www.
epa.gov/osw/conserve/materials/plastics.htm.
Anonymous, 2012b. Renewable Resources for the Production of Bioplastics: Impact on Agriculture - Status and Outlook. Fact sheet.
European Bioplastics http://en.european-bioplastics.org/wpcontent/uploads/2011/04/fs/Renewable_resources_eng.pdf.
Ashby, R.D., Solaiman, D.K.Y., Strahan, G.D., 2011. Efficient utilization
of crude glycerol as fermentation substrate in the synthesis of
poly(3-hydroxybutyrate) biopolymers. J. Am. Oil Chem. Soc. 88,
949e959.
Ashby, R.D., Solaiman, D.K.Y., Foglia, T.A., 2004. Bacterial
poly(hydroxyalkanoate) polymer production from the biodiesel
co-product stream. J. Polym. Environ. 12, 105e112.
Barham, P.J., Barker, P., Organ, S.J., 1992. Physical properties of
poly(hydroxybutyrate) and copolymers of hydroxybutyrate and
hydroxyvalerate. FEMS Microbiol. Lett. 103, 289e298.
Bautista, J., Parrado, J., Machado, A., 1990. Composition and fractionation of sunflower meal: use of the lignocellulosic fraction as
substrate in solid state fermentation. Biol. Wastes 32, 225e233.
Bhubalana, K., Rathia, D.N., Abeb, H., Iwatac, T., Sudesh, K., 2010.
Improved synthesis of P(3HB-co-3HV-co-3HHx) terpolymers by
mutant Cupriavidus necator using the PHA synthase gene of Chromobacterium sp. USM2 with high affinity towards 3HV. Polym.
Degrad. Stab. 95, 1436e1442.
Botella, C., de Ory, I., Webb, C., Cantero, D., Blandino, A., 2005. Hydrolytic enzyme production by Aspergillus awamori on grape
pomace. Biochem. Eng. J. 26, 100e106.
Bustos, G., Moldes, A.B., Cruz, J.M., Domı́nguez, J.M., 2004. Evaluation of vinification lees as a general medium for Lactobacillus
strains. J. Agric. Food Chem. 52, 5233e5239.
Bustos, G., Moldes, A.B., Cruz, J.M., Domı́nguez, J.M., 2005. Production of lactic acid from vine-trimming wastes and viticulture lees
using a simultaneous saccharification fermentation method. J. Sci.
Food Agric. 85, 466e472.
Byrom, D., 1987. Polymer synthesis by microorganisms: technology
and economics. Trends Biotechnol. 5, 246e250.
Cavalheiro, J.M.B.T., de Almeida, M.C.M.D., Grandfils, C., da
Fonseca, M.M.R., 2009. Poly(3-hydroxybutyrate) production by
Cupriavidus necator using waste glycerol. Process. Biochem. 44,
509e515.
Cavalheiro, J.M.B.T., Raposo, R.S., de Almeida, M.C.M.D.,
Cesário, M.T., Sevrin, C., Grandfils, C., da Fonseca, M.M.R.,
2012. Effect of cultivation parameters on the production
of
poly(3-hydroxybutyrate-co-4-hydroxybutyrate)
and
poly(3-hydroxybutyrate-4-hydroxybutyrate-3-hydroxyvalerate) by
Cupriavidus necator using waste glycerol. Bioresour. Technol. 111,
391e397.
Chatzifragkou, A., Papanikolaou, S., 2012. Effect of impurities in
biodiesel-derived waste glycerol on the performance and feasibility of biotechnological processes. Appl. Microbiol. Biotechnol.
95, 13e27.
Choi, J., Lee, S.Y., 1997. Process analysis and economic evaluation for
poly(3-hydroxyutyrate) production by fermentation. Bioprocess
Eng. 17, 335e342.
da Silva, G.P., Mack, M., Contiero, J., 2009. Glycerol: a promising and
abundant carbon source for industrial microbiology. Biotechnol.
Adv. 27, 30e39.
Diaz, M.J., Madejón, E., López, F., López, R., Cabrera, F., 2002. Optimization of the rate vinasse/grape marc for co-composting process. Process. Biochem. 37, 1143e1145.
431
Dorado, M.P., Lin, S.K.C., Koutinas, A., Du, C., Wang, R., Webb, C.,
2009. Cereal-based biorefinery development: utilisation of wheat
milling by-products for the production of succinic acid. J. Biotechnol. 143, 51e59.
Du, C., Sabirova, J., Soetaert, W., Lin, C., 2012. Polyhydroxyalkanoates
production from low-cost sustainable raw materials. Curr. Chem.
Biol. 6, 14e25.
Egues, I., Gonzalez Alriols, M., Herseczki, Z., Marton, G., Labidi, J.,
2010. Hemicelluloses obtaining from rapeseed cake residue
generated in the biodiesel production process. J. Ind. Eng. Chem.
(Seoul, Repub. Korea) 16, 293e298.
Garcia, I.L., Dorado, M.P., Kopsahelis, N., Alexandri, M.,
Papanikolaou, S., Villar, M.A., Koutinas, A.A., 2013. Evaluation of
by-products from the biodiesel industry as fermentation feedstock
for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production by
Cupriavidus necator. Bioresour. Technol. 130, 16e22.
Gouda, M.K., Swellam, A.E., Omar, S.H., 2001. Production of PHB by a
Bacillus megaterium strain using sugarcane molasses and corn steep liquor as sole carbon and nitrogen sources. Microbiol. Res. 156, 201e207.
Gunaratne, L.M.W.K., Shanks, R.A., 2005. Multiple melting behaviour
of poly(3-hydroxybutyrate-co-hydroxyvalerate) using step-scan
DSC. Eur. Polym. J. 41, 2980e2988.
Haas, R., Jin, B., Zepf, F.T., 2008. Production of poly(3-hydroxybutyrate)
from waste potato starch. Biosci. Biotechnol. Biochem. 72,
253e256.
Han, J., Li, M., Hou, J., Wu, L., Zhou, J., Xiang, H., 2010. Comparison
of four phaC genes from Haloferax mediterranei and their function in
different PHBV copolymer biosyntheses in Haloarcula hispanica.
Saline Syst. 6, 1e10.
He, J.-D., Cheung, M.K., Yu, P.H., Chen, G.-Q., 2001. Thermal
analyses of poly(3-hydroxybutyrate), poly(3-hydroxybutyrate-co3-hydroxyvalerate),
and
poly(3-hydroxybutyrate-co3-hydroxyhexanoate). J. Appl. Polym. Sci. 82, 90e98.
Hejazi, P., Vasheghani-Farahani, E., Yamini, Y., 2003. Supercritical fluid
disruption of Ralstonia eutropha for poly(beta-hydroxybutyrate)
recovery. Biotechnol. Prog. 19, 1519e1523.
Huang, T.Y., Duan, K.J., Huang, S.Y., Chen, C.W., 2006. Production of
polyhydroxyalkanoates from inexpensive extruded rice bran and
starch by Haloferax mediterranei. J. Ind. Microbiol. Biotechnol. 33,
701e706.
Kachrimanidou,
V.,
Kopsahelis,
N.,
Chatzifragkou,
A.,
Papanikolaou, S., Yanniotis, S., Kookos, I., Koutinas, A.A., 2013.
Utilisation of by-products from sunflower-based biodiesel production processes for the production of fermentation feedstock.
Waste Biomass Valor 4, 529e537.
Kahar, P., Tsuge, T., Taguchi, K., Doi, Y., 2004. High yield production of
polyhydroxyalkanoates from soybean oil by Ralstonia eutropha and
its recombinant strain. Polym. Degrad. Stab. 83, 79e86.
Kapritchkoff, F.M., Viotti, A.D., Alli, R.C.P., Zuccolo, M.,
Pradella, J.G.C., Maiorano, A.E., Miranda, E.A., Bonomi, A., 2006.
Enzymatic recovery of polyhydroxybutyrate produced by Ralstonia
eutropha. J. Biotechnol. 122, 453e462.
Keenan, T.M., Nakas, J.P., Tanenbaum, S.W., 2006. Polyhydroxyalkanoate copolymers from forest biomass. J. Ind. Microbiol. Biotechnol. 33, 616e626.
Koller, M., Atlic, A., Dias, M., Reiterer, A., Braunegg, G., 2010. Microbial PHA production from waste raw materials. In: Chen, G.Q.
(Ed.), Plastics from Bacteria: Natural Functions and Applications,
vol. 14. Springer-Verlag, Berlin, pp. 85e119.
Koller, M., Hesse, P., Bona, R., Kutschera, C., Atlic, A., Braunegg, G.,
2007b. Potential of various Archae- and Eubacterial strains as industrial polyhydroxyalkanoate producers from whey. Macromol.
Biosci. 7, 218e226.
Koller, M., Hesse, P., Bona, R., Kutschera, C., Atlic, A., Braunegg, G.,
2007a. Biosynthesis of high quality polyhydroxyalkanoate co- and
432
24. BIOENERGY TECHNOLOGY AND FOOD INDUSTRY WASTE VALORIZATION FOR INTEGRATED PRODUCTION
terpolyesters for potential medical application by the archaeon
Haloferax mediterranei. Macromol. Symp. 253, 33e39.
Koller, M., Bona, R., Chiellini, E., Grillo Fernandes, E., Horvat, P.,
Kutschera, C., Hesse, P., Braunegg, G., 2008. Polyhydroxyalkanoate
production from whey by Pseudomonas hydrogenovora. Bioresour.
Technol. 99, 4854e4863.
Koutinas, A.A., Papanikolaou, S., 2011. Biodiesel production from
microbial oil. In: Luque, R., Campelo, J., Clark, J.H. (Eds.), Handbook of Biofuels Production - Processes and Technologies. Woodhead Publishing Limited, pp. 177e198.
Koutinas, A.A., Wang, R.H., Webb, C., 2007a. The biochemurgist bioconversion of agricultural raw materials for chemical production. Biofuels, Bioprod. Biorefin. 1, 24e38.
Koutinas, A.A., Xu, Y., Wang, R., Webb, C., 2007b. Polyhydroxybutyrate
production from a novel feedstock derived from a wheat-based
biorefinery. Enzyme Microb. Technol. 40, 1035e1044.
Kulpreecha, S., Boonruangthavorn, A., Meksiriporn, B., Thongchu, N.,
2009. Inexpensive fed-batch cultivation for high poly(3hydroxybutyrate) production by a new isolate of Bacillus megaterium. J. Biosci. Bioeng. 107, 240e245.
Kusaka, S., Iwata, T., Doi, Y., 1999. Properties and biodegradability of
ultra-high-molecular-weight poly[(R)-3-hydroxybutyrate] produced by a recombinant Escherichia coli. Int. J. Biol. Macromol. 25,
87e94.
Lee, W.H., Loo, C.Y., Nomura, C.T., Sudesh, K., 2008. Biosynthesis of
polyhydroxyalkanoate copolymers from mixtures of plant oils and
3-hydroxyvalerate precursors. Bioresour. Technol. 99, 6844e6851.
Lemoigne, M., 1926. Produits de déshydration et de polymerization de
l’acide b-oxybutyrique. Bull. Soc. Chim. Biol. 8, 770e782.
Leung, C.C.J., Cheung, A.S.Y., Zhang, A.Y.Z., Lam, K.F., Lin, C.S.K.,
2012. Utilisation of waste bread for fermentative succinic acid
production. Biochem. Eng. J. 65, 10e15.
Loo, C.Y., Lee, W.H., Tsuge, T., Doi, Y., Sudesh, K., 2005. Biosynthesis and
characterization of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)
from palm oil products in a Wautersia eutropha mutant. Biotechnol.
Lett. 27, 1405e1410.
Luo, L., Wei, X., Chen, G.Q., 2009. Physical properties and biocompatibility of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) blended
with poly(3-hydroxybutyrate-co-4-hydroxybutyrate). J. Biomater. Sci.
20, 1537e1553.
Madden, L.A., Anderson, A.J., Asrar, J., Berger, P., Garrett, P., 2000.
Production and characterization of poly(3-hydroxybutyrate-co3-hydroxyvalerate-co-4-hydroxybutyrate) synthesized by Ralstonia
eutropha in fed-batch cultures. Polymer 41, 3499e3505.
Marsudi, S., Tan, I.K.P., Gan, S.N., Ramachandran, K.B., 2007. Production of medium chain length polyhydroxyalkanoates from oleic
acid using Pseudomonas putida PGA1 by fed batch culture. Makara,
Teknologi 11, 1e4.
Meng, X., Yang, J., Xu, X., Zhang, L., Nie, Q., Xian, M., 2009. Biodiesel
production from oleaginous microorganisms. Renewable Energy
34, 1e5.
Mothes, G., Schnorpfeil, C., Ackermann, J.-U., 2007. Production of
PHB from crude glycerol. Eng. Life Sci. 7, 475e479.
Nath, A., Dixit, M., Bandiya, A., Chavda, S., Desa, A.J., 2008.
Enhanced PHB production and scale up studies using cheese whey
in fed batch culture of Methylobacterium sp. ZP24. Bioresour.
Technol. 99, 5749e5755.
Nogales, R., Cifuentes, C., Benı́tez, E., 2005. Vermicomposting of
winery wastes: a laboratory study. J. Environ. Sci. Health Part B 40,
659e673.
Nurgel, C., Canbas, A., 1998. Production of tartaric acid from pomace of
some anatolian grape cultivars. Am. J. Enol. Vitic. 49, 95e99.
Parrado, J., Bautista, J., Machado, A., 1991. Production of soluble
enzymatic protein hydrolysate from industrially defatted nondehulled sunflower meal. J. Agric. Food Chem. 39, 447e450.
Posada, J.A., Naranjo, J.M., López, J.A., Higuita, J.C., Cardona, C.A., 2011.
Design and analysis of poly-3-hydroxybutyrate production processes
from crude glycerol. Process. Biochem. 46, 310e331.
Rivas, B., Torrado, A., Moldes, A.B., Domı́nguez, J.M., 2006. Tartaric
acid recovery from distilled lees and use of the residual solid as an
economic nutrient for Lactobacillus. J. Agric. Food Chem. 54,
7904e7911.
Ryu, H.W., Hahn, S.K., Chang, Y.K., Chang, H.N., 1997. Production of
poly(3-hydroxybutyrate) by high cell density fed-batch culture of
Alcaligenes eutrophus with phosphate limitation. Biotechnol. Bioeng.
55, 28e32.
Salgado, J.M., Rodriguez, N., Cortés, S., Domı́nguez, J.M., 2010.
Improving downstream processes to recover tartaric acid, tartrate
and nutrients from vinasses and formulation of inexpensive
fermentative broths for xylitol production. J. Sci. Food Agric. 90,
2168e2177.
Sanchez, A., Ysunza, F., Beltran-Garcia, M., Esqueda, M., 2002.
Biodegradation of viticulture wastes by Pleurotus: a source of microbial and human food and its potential use in animal feeding. J.
Agric. Food Chem. 50, 2537e2542.
Sarris, D., Galiotou-Panayotou, M., Koutinas, A., Komaitis, M.,
Papanikolaou, S., 2011. Citric acid, biomass and cellular lipid production by Yarrowia lipolytica strains cultivated on olive mill
wastewater-based media. J. Chem. Technol. Biotechnol. 86, 1439e1448.
Senior, P.J., Collins, S.H., Richardson, K.R., 1986. Copolymers of
Poly(b-Hydroxybutyric Acid) and Poly(b -hydroxyvaleric Acid)
Are Produced by Culturing Alcohol-utlising Strains of Alcaligenes
eutrophus on a Carbon Source Including Primary Alcohols Having
an Odd Number of Carbon Atoms Such as Propan-1-ol. European
Patent Office. Publication Number 0204442 A2.
Shimamura, E., Kasuya., K., Kobayashi, G., Shiotani, T., Shima, Y.,
Doi, Y., 1994. Physical properties and biodegradability of microbial
poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Macromolecules
27, 878e880.
Silva, L.F., Taciro, M.K., Ramos, M.E.M., Carter, J.M., Pradella, J.G.C.,
Gomez, J.G.C., 2004. Poly-3-hydroxybutyrate (P3HB) production
by bacteria from xylose, glucose and sugarcane bagasse hydrolysate. J. Ind. Microbiol. Biotechnol. 31, 245e254.
Solaiman, D.K.Y., Ashby, R.D., Foglia, T.A., Marmer, W.N., 2006.
Conversion of agricultural feedstock and coproducts into
poly(hydroxyalkanoates). Appl. Microbiol. Biotechnol. 71, 783e789.
Tanadchangsaeng, N., Yu, J., 2012. Microbial synthesis of polyhydroxybutyrate from glycerol: gluconeogenesis, molecular
weight and material properties of biopolyester. Biotechnol. Bioeng.
109, 2808e2818.
Tsuge, T., Saito, Y., Kikkawa, Y., Hiraishi, T., Doi, Y., 2004. Biosynthesis
and compositional regulation of poly[(3-hydroxybutyrate)-co(3-hydroxyhexanoate)] in recombinant Ralstonia eutropha expressing mutated polyhydroxyalkanoate synthase genes. Macromol.
Biosci. 4, 238e242.
Van Wegen, R.J., Ling, Y., Middelberg, A.P.J., 1998. Industrial production of polyhydroxyalkanoates using Escherichia coli: an economic analysis. Trans. IChemE 76, 417e426.
Verlinden, R.A.J., Hill, D.J., Kenward, M.A., Williams, C.D.,
Radecka, I., 2007. Bacterial synthesis of biodegradable polyhydroxyalkanoates. J. Appl. Microbiol. 102, 1437e1449.
Versari, A., Castellari, M., Spinabelli, U., Galassi, S., 2001. Recovery of
tartaric acid from industrial enological wastes. J. Chem. Technol.
Biotechnol. 76, 485e488.
Wang, R., Shaarani, S.M., Godoy, L.C., Melikoglu, M., Vergara, C.S.,
Koutinas, A., Webb, C., 2010. Bioconversion of rapeseed meal for
the production of a generic microbial feedstock. Enzyme Microb.
Technol. 47, 77e83.
Whitehouse, R.S., Zhong, L., Daughtry, S., 2006. Compositions
Comprising Low Molecular Weight Polyhydroxyalkanoates and
REFERENCES
Methods Employing Same. United States Patent No. US
7,094,840 B2.
Wolf, O., Crank, M., Patel, M., Marscheider-Weidemann, F.,
Schleich, J., Husing, B., Angerer, G., 2005. Techno-economic
Feasibility of Large Scale Production of Bio-based Polymers in
Europe. Technical Report EUR 22103 EN.
Wong, H.H., Lee, S.Y., 1998. Poly-(3-hydroxybutyrate) production
from whey by high-density cultivation of recombinant Escherichia
coli. Appl. Microbiol. Biotechnol. 50, 30e33.
Xu, Y., Wang, R.-H., Koutinas, A.A., Webb, C., 2010. Microbial
biodegradable plastic production from a wheat-based biorefining
strategy. Process. Biochem. 45, 153e163.
433
Yu, H., Shi, Y., Yin, J., Shen, Z., Yang, S., 2003. Genetic strategy for
solving chemical engineering problems in biochemical engineering. J. Chem. Technol. Biotechnol. 78, 283e286.
Yu, J., Stahl, H., 2008. Microbial utilization and biopolyester synthesis
of bagasse hydrolysates. Bioresour. Technol. 99, 8042e8048.
Yu, J., Chen, L.X.L., 2006. Cost-effective recovery and purification of
polyhydroxyalkanoates by selective dissolution of cell mass. Biotechnol. Prog. 22, 547e553.
Yust, M.M., Pedroche, J., Megı́as, C., Girón-Calle, J., Alaiz, M.,
Millán, F., Vioque, J., 2003. Improvement of protein extraction from
sunflower meal by hydrolysis with alcalase. Grasas Aceites 54,
419e423.
C H A P T E R
25
Advances and Innovations in Biochar
Production and Utilization for Improving
Environmental Quality
Charles Hyland*, Ajit K. Sarmah
Department of Civil & Environmental Engineering, The University of Auckland, Auckland, New Zealand
*Corresponding author email: chyl531@aucklanduni.ac.nz
O U T L I N E
Introduction
435
Properties of Biochar
Chemical and Physical
Microbiological Effects and Synergisms
436
436
437
Utilization of Biochar for Environmental Quality
Carbon Sequestration
Sorption of Plant Nutrients and Other Pollutants
Soil Greenhouse Gas Emissions
Soil-Specific Biochar Design
438
438
438
439
440
INTRODUCTION
In its simplest material context, biochar is incompletely combusted organic matter that is applied to
soil; however, this material has attracted considerable
attention in recent years due to goals that may be achievable through its production and end use. In this chapter,
advances and innovations in biochar production and intermediate uses are presented and discussed. A considerable proportion of the world’s natural soil organic
carbon content comprises black carbon, a pool that is
resistant to microbial degradation and was deposited
from historic fires (Krull et al., 2008). Augmenting this
nearly ubiquitous pool with additional recalcitrant carbon has recently been the subject of much scientific
research focused on soil improvement and carbon (C)
sequestration, which has garnered notable support
from some of the world’s most well-known climate
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00025-5
Postpyrolysis Indirect Application of Biochar
Water Filtration
Soil Nutrient Reclamation
Biochar As Container Growth Medium and
Container
440
440
441
441
Conclusions, Knowledge Gaps, and Research Needs 443
References
444
scientists and environmental advocates, such as Al
Gore (Gore, 2009), Tim Flannery (Flannery, 2009), James
Hansen (Hansen et al., 2008), and James Lovelock
(Lovelock, 2009). Biochar is a form of anthropogenic
recalcitrant carbon produced for the purpose of application to soil that draws inspiration from the practices of
precolonial Native Amazonians who transformed
some of the world’s poorest soils into extremely fertile
soils that remain productive into the present, centuries
after their production ceased (Lehmann, 2008). This
rediscovered concept represents both a stable form of
C-rich soil organic matter and a means to ameliorate
degraded soils into fertile soils (Lehmann et al., 2006).
C sequestration is achievable through a range of thermochemical conversion processes (Table 25.1), including
pyrolysis, a process that produces biochar as a byproduct. Recalcitrant carbon, including particulates
emitted by fossil fuel combustion, may represent up to
435
Copyright Ó 2014 Elsevier B.V. All rights reserved.
436
25. ADVANCES AND INNOVATIONS IN BIOCHAR PRODUCTION AND UTILIZATION FOR IMPROVING ENVIRONMENTAL QUALITY
TABLE 25.1
Biomass Pyrolytic Conversion Processes
Process
Temperature ( C)
Heating
Rate
End Products
Torrefaction
230e300
Very low
Biochar
Slow Pyrolysis
380e530
Low
Biochar,
bio-oils, gas
Fast Pyrolysis
380e530
High
Bio-oils, relatively
little biochar
Combustion
700e1400
Very high
Gas
Gasification
>750
Very high
Gas
30% of all soil C globally (Schmidt et al., 2001). Woolf
et al. (2010) reported that by increasing biochar production net anthropogenic greenhouse gas (GHG) emissions
could be reduced by as much as 12% through the substitution of pyrolysis oils and gases for fossil fuels.
Recent reviews and studies have highlighted the
potential environmental as well as agronomic benefits
of biochar (Kookana et al., 2011). While many of these
potential environmental benefits of biochar are largely
known through glasshouse and field trials, there have
been findings that also showed negative impacts of
biochar utilization as soil amendment (Clough et al.,
2010). Given that biochar can be produced from a variety
of feedstocks, and quality of biochar is dependent on the
type of feedstock and pyrolysis conditions, not all biochars are made equal.
The pyrolysis bioenergy industry is currently in the
early stages of commercialization (Downie and van
Zwieten, 2013) and is growing slowly due to the vast
heterogeneity of biochar feedstocks, production
methods, and end uses (Jahirul et al., 2012). The economic viability of commercial pyrolysis is likely to increase in tandem with the price of C credits assigned
to biochar (Laird et al., 2009). Due to the characteristic
persistence of biochar after incorporation into soil, prerequisite testing of biochars is crucial in order to avoid
production of biochars that either form toxic compounds, such as polycyclic aromatic hydrocarbons
formed as a result of pyrolysis (Verheijen et al., 2010;
Kloss et al., 2012), or retain toxins, such as heavy metals,
that were present in a feedstock, such as municipal solid
waste or sewage sludge (Sparkes and Stoutjesdijk, 2011;
Kookana et al., 2011). Minimal predictive capability
currently exists in relation to biochar performance in
the field (Sohi et al., 2010). Field trials, preceded by
initial laboratory and Glasshouse testing, are the
preferred biochar efficacy evaluation methods for conventional biochar, as well as novel biochar uses that
are currently being investigated. Thorough research
must be conducted in order to ensure that biochars
suited to address specific issues are selected from the
diverse array of biochars that are currently being produced, in order to avoid potential socioeconomic and
environmental damage (Barrow, 2012).
Rather than applying arbitrarily chosen biochars and
passively observing their effects on the environment, a
forefront of biochar research is the custom design of biochar for specific targeted end uses. Biochar is commonly
referred to as a soil conditioner (Lehmann et al., 2006) in
order to contextually differentiate the material from
direct fertilizers. Given this, it rationally follows that biochars and their deployment methods be engineered to
enhance the soil and the surrounding environment in a
contextually desirable manner. For example, if it is
known that biochar efficacy is increased once the
biochar surfaces are coated with clay minerals in a given
soil after a period of time, then producing biochars that
already possess this quality at the time of incorporation
into soil would be a significant advancement. Evaluating
intermediate uses for biochar, that is functions that biochar can perform after production and before soil incorporation, may simultaneously improve the efficacy of
the biochar and reduce preexistent downstream and
atmospheric pollution. An example of an intermediate
use discussed in this chapter is the employment of biochar contained within permeable mesh bags for the
filtration of runoff and leachate water.
PROPERTIES OF BIOCHAR
As mentioned earlier, two important factors in biochar production are the type of feedstock used and
pyrolysis conditions as they affect the physical and
chemical properties of the biochar that is produced.
Depending on the origin of the feedstock (e.g. cellulose,
lignin, lingocellulose, hemicellulose) the chemical and
structural composition of pyrolyzed biochar can change,
and thus when biochar is used as a soil amendment, its
behavior, function and fate in soils could be different.
For instance, Winsley (2007) showed that when woodbased feedstocks are pyrolyzed, coarse and resistant biochars were generated with nearly 80% carbon contents,
because the rigid ligninolytic nature of the source material is still retained in the biochar residue. The following
sections discuss the chemical, physical, and biological
properties of soil amended with biochar with particular
focus on how the various properties influence the soilbiochar interactions and consequently improve the soil’s
biological health and crop productivity.
Chemical and Physical
In an effort to offset anthropogenic C emissions to the
atmosphere, a number of geoengineering technologies
have been proposed, which can be divided into the
437
PROPERTIES OF BIOCHAR
Respiration
Respiration
CO2
CO2
Photosynthesis
Photosynthesis
5%
25%
50%
50%
50%
Pyrolysis
25%
Photosynthetic carbon cycle
Photosynthetic + biochar carbon cycle
FIGURE 25.1 Carbon sequestration through pyrolysis of plant biomass. Source: Adapted from Lehmann, 2007.
two broad categories of solar radiation management and
CO2 removal, with C sequestration through biochar
incorporation into soil in the latter (Vaughan and Lenton,
2011). What distinguishes biochar CO2 sequestration
(Figure 25.1) from competing CO2 removal technologies,
such as ocean iron fertilization and CO2 geological injection, are two factors. First, biochar C sequestration relies
on photosynthesizing plants to draw CO2 from the atmosphere and is stored in the form of charcoal, whereas
methods such as CO2 geological injection rely on relatively new and untested storage methods, including mechanically forcing supercritical CO2 into depleted
fossil fuel reservoirs or deep sea sediments (Vaughan
and Lenton, 2011). Second, charcoal storage in soils is
already ubiquitous and the clearest challenges in the
short term involve optimizing economic profitability,
rather than projected efficacy (Spokas et al., 2012a).
C sequestration through pyrolysis for biochar production is a result of the conversion of C forms present
in the feedstock into recalcitrant forms present in
biochar. Pyrolysis consists of a succession of changes
in chemical structural composition as temperature increases. Cellulose and lignin are degraded as volatile
compounds are driven off between 250 C and 350 C,
followed by lateral growth and coalescence of polyaromatic graphene sheets, and culminating with carbonization, constituted by the expulsion of the majority of
non-C atoms above 600 C (Verheijen et al., 2010). Additionally, as pyrolysis temperature and residence time
increase, H/C and O/C ratios of biochar decrease and
aromaticity, the extent to which aromatic rings are connected, increases, resulting in greater recalcitrance
against degradation (Kookana et al., 2011). Recent
research suggests that biochars intended for both soil
improvement and C sequestration should possess recalcitrant carbon of >15%, O/C ratios of <0.4, H/C ratios
of <0.6, polyaromatic hydrocarbon contents below background soil values, and a surface area of >100 m2 g
(Schimmelpfennig and Glaser, 2012). As pyrolysis temperatures increase, biochar specific surface area and
microporosity analogously increase. Biochar chemical
and physical properties are due to both the composition
of the feedstock and the extent of the alterations undergone during pyrolysis (Kookana et al., 2011). The diversity of biochar chemical and physical qualities
achievable through varied pyrolysis conditions and
feedstocks is reflected in the diversity of effects on soil
biota that may be achievable. Biochar is sterile when
produced, yet has been observed to have beneficial
effects on soil microbes that play essential roles in
nutrient cycling. These effects are complex; however,
they are linked to the chemical and physical properties
of the biochar employed, which can serve as both a
source of nutrients and as a habitat for soil microbes
(Lehmann and Rondon, 2006; Lehmann et al., 2011).
Microbiological Effects and Synergisms
Literature focused on the effects of biochar on soil
biota is currently sparse relative to biochar chemical
438
25. ADVANCES AND INNOVATIONS IN BIOCHAR PRODUCTION AND UTILIZATION FOR IMPROVING ENVIRONMENTAL QUALITY
and physical effects on soil (Lehmann et al., 2011). Published research fails to adequately address the diverse
spectrum of biochars within a single study, typically
evaluating the effects of a small number of biochars on
microbial and root abundance for a small number of
soil types. Depending on the feedstock from which biochar is produced and the pyrolysis conditions involved
in the feedstock conversion, biotoxic substances may
persist through or be generated during pyrolysis. Standardized methods, including germination rates of
various crops and degree of earthworm avoidance,
have recently been proposed in order to assess biochar
toxicity to avoid inadvertent detrimental effects on
crop production and the environment (Busch et al.,
2012; Rogovska et al., 2012).
The addition of biochar to compost systems and the
resultant effects on both the microbial community dynamics and final compost quality have been evaluated,
and the potential for synergistic effects and increased
soil C stability are known (Fischer and Glaser, 2012).
While the aromatic core of biochar has been observed
to remain unchanged during the composting process
with manure, C and plant-available nutrients are drawn
into its pores and adhere to its surfaces, elevating the
cation exchange capacity (CEC) and acid neutralizing
capacity, and enhancing the functionalization of biochar
surfaces, although the mechanisms responsible warrant
additional research (Prost et al., 2013). Biochar has been
observed to affect mycorrhizal symbioses, yet the mechanisms responsible are still being determined (Warnock
et al., 2007) and recently, it has been proposed that soil
nitrogen may act as a switch controlling the proliferation
of mycorrhizae and the subsequent oxidation of fresh
biochar surfaces (LeCroy et al., 2013). Compared to
compost, biochar is a much more stable soil amendment
and the addition of biochar to composts has been shown
to dramatically increase compost stability (Bolan et al.,
2012).
UTILIZATION OF BIOCHAR FOR
ENVIRONMENTAL QUALITY
Carbon Sequestration
The potential of biochar to sequester atmospheric C
for centuries is certainly one of its most attractive
qualities. As global anthropogenic C emissions continue
to increase, C sequestration using biochar employs
photosynthesis to draw C from the atmosphere, and
pyrolysis to convert that photosynthetically sequestered
C into forms that are mostly not biologically degradable.
Fossil fuel-derived energy and most biofuels are
regarded as carbon-positive, due to the positive net emissions of C from their production and use. Carbon-neutral
biofuels are those that result in no net emissions of C
resulting from their production and use. Pyrolysis energy
production combined with biochar incorporation into
soil has been described as what may be the only
carbon-negative energy production system known
(Mathews, 2008). Carbon-negative energy (Figure 25.2)
sequesters more C from the atmosphere than is released
through its production and use. If biochar is produced from waste feedstocks that would otherwise be
microbially degraded and contribute to anthropogenic
C emissions, then it is possible to sequester a significant
portion of anthropogenic C emissions through pyrolysis
(Lehmann et al., 2006). While estimates of biochar
C sequestration potential vary between studies depending on methodological details, recent studies reported
1.65 GtC/y or 19% of anthropogenic carbon dioxide emissions could be offset (Lee and Day, 2013; Lee et al., 2013).
When combined with projected adoptions of renewable
energy systems by the year 2100, it has been estimated
that through the pyrolysis of agricultural residues, silvicultural residues, organic waste from industry, and
urban waste, 5.5e9.5 GtC/y is achievable (Lehmann
et al., 2006). This would exceed current fossil fuel emissions and thereby represent a potential remediation, as
opposed to a conservation tool. Others have recently
projected the possibility of up to 15.6 GtC/y (Smith
et al., 2013). While the C sequestration potential of
biochar is currently appealing, it must be deployed
carefully in order to minimize the risk of damaging the
soil, due to the irreversibility of biochar application to
soil (Sohi et al., 2008).
Sorption of Plant Nutrients and Other
Pollutants
The potential benefits of biochar to improving soil
health through nutrient addition, and consequent improvements in fertilizer use efficiency have been well
recognized through glasshouse and field trials (Sohi
et al., 2010; Verheijen et al., 2010). These studies have
shown that biochar and biochar-amended soil help
retain plant nutrients and this is one of the means by
which biochar application to soils is known to improve
soil quality and increase crop yields in some cases. However, it is important to recognize that if the nutrients are
retained by biochar particles in soil to a degree that
plants are unable to take up the nutrients, this could
impact productivity (Kookana et al., 2011).
Studies have shown that retention of nitrogen (N)
(Hollister et al., 2013; Liu et al., 2013), as well as phosphorus (P) and potassium (K) (Schnell et al., 2012), is
achievable through incorporation of biochar, demonstrating potential agronomic and wider environmental
benefits. Within the context of an agroecosystem, plant
nutrients are essential and often the limiting factor
439
UTILIZATION OF BIOCHAR FOR ENVIRONMENTAL QUALITY
Energy use
Carbon
Carbon
Carbon
Carbon-neutral
Carbon
Energy use
Carbon
Carbon-positive
Carbon
Carbon
Carbon
Energy use
Carbon-negative
FIGURE 25.2 Carbon-positive, carbon-neutral, and carbon-negative bioenergy systems. Source: Adapted from Mathews, 2008.
determining the extent of crop growth. However, once N
or P leave an agroecosystem, either through overland
flow or leaching, they could potentially pose a threat to
surface waters, with P being the limiting nutrient governing eutrophication of fresh water and N being the limiting
nutrient governing the eutrophication of estuarine and
ocean systems (Brady and Weil, 2008). Similar to gaseous
C emissions, aqueous N and P losses from agricultural
soil have global effects. While the majority of biochar
research focuses on short-term impacts of its application,
more long-term field research focused on net C sequestration, net GHG emissions, microbial community dynamics, nutrient use efficiency, and water use efficiency
is needed (Ippolito et al., 2012). Furthermore, an increased
fundamental understanding of the mechanisms underlying the interactions between biochar and soil in order to
optimize agricultural systems and protect the environment should be a further focus (Spokas et al., 2012b).
While applied plant nutrients outside of an agricultural context represent a threat to the environment that
biochar has demonstrated potential to address, it is
possible to retain other pollutants (e.g. heavy metals
and pesticides) using biochar, as well. When biochar is
applied to cadmium (Cd)-, copper (Cu)-, and lead
(Pb)-contaminated soils, these metals have been
observed to become immobilized, decreasing phytotoxicity and bioavailability, and vastly improved crop production (Park et al., 2011). Other studies have shown
biochar to exhibit strong sorption and degradation
inhibition of pesticide residues, leading to potential concerns regarding long-term accumulation in biochar
amended soils treated with pesticides (Kookana, 2010).
Some biochars have also been shown to retain estrogenic
steroid hormones on dairy farm soils (Sarmah et al.,
2010). While the potential for soil organic and inorganic
contaminants (e.g. metal remediation, pesticide accumulation, and hormone retention) remediation are valid, it
is important to consider that the enormous heterogeneity of biochars with respect to their chemical qualities
and resultant effects on soil pollutants remain largely
uninvestigated (Kookana et al., 2011). Research on
potential agronomic and environmental applications of
biochar is currently in its infancy and it is through the
establishment and monitoring of additional long-term
field trials that its full potential could be realized
(Sarmah, 2009).
Soil Greenhouse Gas Emissions
In addition to the potential of biochar to partially
offset anthropogenic C emissions through C sequestration, biochar has been observed to inhibit the release of
GHGs from soil, thereby reducing net emissions of
GHGs as a side effect of C sequestration. Decreased carbon dioxide (CO2), methane (CH4), and nitrous oxide
(N2O) emissions from soils have been observed
following biochar applications (Spokas et al., 2010),
although increased emissions of N2O have been
440
25. ADVANCES AND INNOVATIONS IN BIOCHAR PRODUCTION AND UTILIZATION FOR IMPROVING ENVIRONMENTAL QUALITY
(a)
(b)
Feedstock drying
and shredding
Feedstock drying and shredding
Preparation
Pyrolysis of
feedstock
Pyrolysis of feedstock
Preparation
Mixing with clay, minerals
Biochar employed for surface
water filtration
Pyrolysis
Pyrolysis
Surface loading
Conversion
Application
to soil
Incorporation
Incorporation
Soil amelioration
Soil amelioration
Direct application
Indirect application
Torrefaction
End use
Biochar, bio oil,
and biogas use
Biochar
FIGURE 25.3
Biochar, bio oil, and biogas use
Biochar mineral complex
End use
(a) Biochar enhancement using clay. Adapted from Joseph et al., 2013. (b) Direct and indirect application of biochar to soil.
observed in some cases (Clough et al., 2010). N2O, a
GHG with a global warming potential hundreds of
times greater than CO2, emissions have been shown to
be reduced by a variety of biochars, yet the mechanisms
responsible vary depending on soil moisture (Saarnio
et al., 2013) and N content and forms (Kammann
et al., 2012; van Zwieten et al., 2010). Additionally, biochar has been demonstrated to specifically reduce
earthworm-derived CO2 and N2O emissions (Augustenborg et al., 2012). While there is an abundance of shortterm field and laboratory derived data to indicate the
N2O emissions reduction potential of biochar, there is
a dearth of information on the long-term field studies
on this topic (Ussiri and Lal, 2013).
Biochar is becoming increasingly broad in its contextual definition. At least one study has been conducted to
evaluate the potential of biochar to reduce landfillderived CH4 emissions. A recent study reported that
when landfill cover soil was mixed with 20% biochar,
nearly 200% more CH4 was adsorbed compared to control soil, and 100% biochar was found to adsorb over
10-fold more CH4 than control soil (Yaghoubi, 2011). Biochar has also been shown to effectively decrease cattle
enteric CH4 emissions while increasing animal growth,
by 22% and 25%, respectively, when biochar was mixed
with animal feed (Leng et al., 2012).
alleviate the organic matter constraints of a given soil,
using a procedure similar in effect to those used by
chemical fertilizer companies to determine the macroand micronutrient requirements of a given soil (Joseph
et al., 2013). While some researchers aim to produce
improved biochars by simply blending multiple biochars together (Novak and Busscher, 2013), truly novel
biochars are being produced by pyrolyzing an organic
feedstock mixed with clay and other minerals, in order
to produce a biochar mineral complex (BMC), which
may represent the current forefront of custom biochar
design. This BMC production process (Figure 25.3(a))
enables the production and customization of biochars
through blending of materials before pyrolysis, followed
by subsequent blending of materials with desirable
chemical qualities, which are then subjected to torrefaction treatments. This can facilitate the loading of the biochar surfaces with additional plant-available nutrients
and enhanced CEC, representing a clear progression
from biochar as a soil conditioner toward biochar as an
organic fertilizer (Lin et al., 2012a,b).
POSTPYROLYSIS INDIRECT
APPLICATION OF BIOCHAR
Water Filtration
Soil-Specific Biochar Design
Published data on biochar and its interactions with
soil are increasingly detailed in relation to feedstocks
and production conditions (Sohi et al., 2010). As this
body of literature grows, it will enable biochar producers to more predictably custom design biochars to
While considerable research has been conducted on
soil remediation through adsorption of pollutants
following biochar application, relatively little data
have been published on the sorption of pollutants by
biochars preceding biochar application to soil. Most of
the few studies that have been published on this subject
441
POSTPYROLYSIS INDIRECT APPLICATION OF BIOCHAR
TABLE 25.2
Selected Examples of Biochar Employed As an Adsorbent to Remove Contaminants from
Aqueous Solutions
Biochar
Temperature ( C)
Function
Source
Pine Needles
180
U adsorption
Zhang et al., 2013a
Mulch
200
Urban road runoff Cu, Zn,
and Cd adsorption
Xaio-Jun et al., 2012
Dairy Manure
200, 350
Cu, Zn, and Cd adsorption
Xu et al., 2013
Pine Wood
400e450
-
Mohan et al., 2012
-
F adsorption
Pine Bark
400e450
F adsorption
Mohan et al., 2012
Rice Husk
500e550
C6H5OH adsorption
Liu et al., 2011
Corncob
500e550
C6H5OH adsorption
Liu et al., 2011
Cottonwood and
Maghemite (g-Fe2O3)
600
As adsorption
Zhang et al., 2013b
use biochar to filter substances from surface waters that
are not suitable for application to agricultural soil, such
as arsenic (As), Cd, Cu, fluoride (F), phenol (C6H5OH),
uranium (U), and Zn (Table 25.2). Considering that
these substances are better suited to be contained in a
secure landfill than be applied on productive agricultural soil, further research should be conducted to determine if these biochars could be as effective at reducing
CH4 emissions from soil as demonstrated by Yaghoubi
(2011).
Soil Nutrient Reclamation
While some substances, such as heavy metals, pesticides, and hormones, are not desirable in soil amendments, other substances, such as N and P, are regarded
as pollutants in surface waters, yet are essential plant
nutrients within agroecosystems. If biochar is used to filter N and P from surface waters before in is incorporated
into agricultural soil (Figure 25.3(b)), the process could
reclaim a portion of the nutrients that are lost from agroecosystems to surface waters, and benefit both crop
yield and surface water quality. If effective designer biochars are created for the purpose of N and P retention, it
is feasible that those biochars, once saturated with N and
P, could supply a crop area of some size with an
adequate supply of runoff-derived nutrients to replace
N and P fertilizer inputs entirely, and becoming effectively nutrient-neutral. Furthermore, it is feasible that
if an analogous relocation of nutrients is performed
where the deposition site is an area that would otherwise not receive fertilizer, and not be a likely source of
runoff, such as a well-managed silvicultural system,
then a nutrient-negative system could be created
(Figure 25.4).
Runoff and water-induced erosion occur when precipitation exceeds the infiltration capacity of a soil, and
gravity carries it downhill over the soil surface into a
drainage ditch or natural waterway. Common practices
employed to reduce erosion caused by runoff and retain
eroded soil particles by slowing the velocity of water
flow within drainage ditches are well established. These
practices include lining the sides of channels with either
large angular rocks or rectangular wire mesh containers
filled with smaller rocks, lining the bottom of the channel with grass sod, various bioengineering techniques
involving the establishment of trees along the channel
edge, and fixing straw bales in order to intercept eroded
soil particles (Brady and Weil, 2008). Biochar contained
within either reusable synthetic or single use biodegradable mesh containers could be used to simultaneously
slow and filter overland flow (Figure 25.5). This may
be complementary or even preferable to the aforementioned runoff and erosion control methods due to the
added benefits of nutrient-saturated biochar application
to soil (Figure 25.6). In areas where runoff currently
flows directly into natural waterways, an enclosed biochar overland flow filter (Figure 25.7) fitted with mesh
containers of biochar may be an effective option for
reducing nutrient losses to surface water, allowing those
nutrients to be relocated to soil and ultimately into plant
biomass. Similarly, tile drain effluent may be filtered using mesh containers of biochar. Like the biochar overland flow filter discussed above, end caps on the tile
drain with drain holes only in the upper half will increase the residence time of nutrient-laden water with
the biochar and may increase filtration efficiency
(Figure 25.8).
Biochar As Container Growth Medium and
Container
Biochar has been reported to have the potential
to be used as an amendment in plant nurseries.
442
25. ADVANCES AND INNOVATIONS IN BIOCHAR PRODUCTION AND UTILIZATION FOR IMPROVING ENVIRONMENTAL QUALITY
Nutrients
Nutrients
Nutrients
Nutrients
Nutrients
Nutrient
use
Nutrient
use
Nutrients
Nutrients
Nutrients
Nutrients
Nutrient positive
Nutrient
use
Nutrient neutral
Nutrient negative
FIGURE 25.4 Nutrient-positive, nutrient-neutral, and nutrient-negative agricultural and silvicultural systems.
For example, when 25% biochar was mixed with 75%
peat, enhanced hydraulic conductivity and water
retention were observed (Dumroese et al., 2011). Additionally, this study showed that the expansion of
pelletized biochar (biochar that has been compressed
with a binding agent in order to increase particle
size), when wetted nearly offset the shrinkage typically exhibited by peat over time. A coconut fiber
and tuff growing medium was shown to induce
improved resistance of tomatoes to the necrotrophic
Run
off
and
ero
sion
Bio
ov char
flow erland
fil
dra
ina ter in
ge
ditc
h
Slope
fungus Botrytis cinerea when mixed with biochar at
rates as low as 0.5% w/w (Elad et al., 2011). In another
study, coconut fiber and tuff growing medium was
shown to increase leaf size, plant height, flower development, and crop yield in pepper plants across all
application rates from 1% to 5% w/w (Graber et al.,
2010). In addition to container growth media, research
is currently being conducted into the effects of plant
containers constructed from molded biochar (Pulver,
2013).
Run
off
and
ero
sion
Bio
ov char
flow erland
fil
dra
ina ter in
ge
ditc
h
Slope
Nutrient saturated biochar
FIGURE 25.5 Biochar overland flow filter stage 1. (For color
version of this figure, the reader is referred to the online version of
this book.)
FIGURE 25.6 Biochar overland flow filter stage 2. (For color
version of this figure, the reader is referred to the online version of
this book.)
443
CONCLUSIONS, KNOWLEDGE GAPS, AND RESEARCH NEEDS
Pipe
Runoff inlet
Hinge
Pipe section connector
End cap and drain
holes
Soil / slope
Water line
Runoff inlet
Hinge
FIGURE 25.7
Enclosed biochar overland flow filter concept with and without end cap to maximize liquid residence time for use in areas
where runoff currently flows directly into natural waterways. (For color version of this figure, the reader is referred to the online version of
this book.)
End cap without drain
holes
End cap with drain
holes
Pipe section connector
Water
line
FIGURE 25.8 Biochar tile drain flow filter concept with end cap for maximizing liquid residence time. (For color version of this figure, the
reader is referred to the online version of this book.)
CONCLUSIONS, KNOWLEDGE GAPS,
AND RESEARCH NEEDS
It is critical to understand that biochar is not a single
material, but rather an entire class of materials (Spokas
et al., 2012a) with a broad spectrum of chemical,
physical, and biological properties that are drawn from
both the diversity of feedstocks, production methods,
and postproduction intermediary uses. It is also equally
important to recognize the environmentally beneficial
functions that biochar can perform after production
and before application to soil and that there may be
444
25. ADVANCES AND INNOVATIONS IN BIOCHAR PRODUCTION AND UTILIZATION FOR IMPROVING ENVIRONMENTAL QUALITY
Feedstock production
and preparation
Pyrolysis
Ruminant feed
supplement
Landfill
cover
Water filtration, container
media, and containers
Soil
FIGURE 25.9
Biochar end use decision process.
desirable uses for biochars that are not suited to soil
amelioration (Figure 25.9). Long-term field trial data
related to biochar functions and properties as they
change over time are extremely limited (Verheijen
et al., 2010). Glasshouse projects that may display potential field scale benefits of biochar should be conducted,
and continually monitored in order to measure, rather
than project, what may be achievable for agriculture
and the environment using biochar. It will be essential
moving forward to be able to predict the sorption
longevity and saturation point of biochars for pesticides
and other pollutants. It is unknown if over time biochar
in soil will lose or retain its ability to deactivate
herbicides (Kookana et al., 2011). Similar temporal
uncertainties exist in relation to most other biochar characteristics, aside from C stability. The understanding of
both the short- and long-term effects of biochar on soil
microbial communities remains limited (Sohi et al.,
2008), yet is of critical importance due to the important
role of microbes in many nutrient cycles and pollutant
degradation pathways. Biochar uses that precede its
incorporation into soil remain largely uninvestigated.
Research related to the potential suitability of biochar
for intermediate uses before application to soil, such as
surface water filtration, enteric mitigation of methane
production in ruminants, container media, and landfill
cover is almost nonexistent. However, biochar itself
has only recently expanded to become the focus of
scientific research worldwide, so perhaps research into
indirect biochar uses will progress accordingly.
Acknowledgments
The authors would like to thank Christian Pulver at Cornell University
for his comments on the chapter.
References
Augustenborg, C.A., Hepp, S., Kammann, C., Hagan, D., Schmidt, O.,
Müller, C., 2012. Biochar and earthworm effects on soil nitrous
oxide and carbon dioxide emissions. J. Environ. Qual. 41,
1203e1209.
Barrow, C., 2012. Biochar: potential for countering land degradation
and for improving agriculture. Appl. Geogr. 34, 21e28.
Bolan, N.S., Kunhikrishnan, A., Choppala, G.K., Thangarajan, R.,
Chung, J.W., 2012. Stabilization of carbon in composts and biochars
in relation to carbon sequestration and soil fertility. Sci. Total Environ. 424, 264e270.
Brady, N.C., Weil, R.R., 2008. The Nature and Properties of Soils.
Prentice Hall, Upper Saddle River pp. 1e980.
Busch, D., Kammann, C., Grünhage, L., Müller, C., 2012. Simple biotoxicity tests for evaluation of carbonaceous soil additives: establishment and reproducibility of four test procedures. J. Environ.
Qual. 41, 1023e1032.
Clough, T.J., Bertram, J.E., Ray, J.L., Condron, L.M., O’Callaghan, M.,
Sherlock, R.R., Wells, N.S., 2010. Unweathered wood biochar
impact on nitrous oxide emissions from a bovine-urine-amended
pasture soil. Soil Sci. Soc. Am. J. 74, 852e860.
Downie, A., van Zwieten, L., 2013. Biochar: a coproduct to bioenergy
from slow-pyrolysis technology. In: Lee, J.W. (Ed.), Advanced
Biofuels and Bioproducts. Springer ScienceþBusiness Media, New
York, pp. 97e117.
Dumroese, R.K., Heiskanen, J., Englund, K., Tervahauta, A., 2011.
Pelleted biochar: chemical and physical properties show potential
use as a substrate in container nurseries. Biomass Bioenergy 35,
2018e2027.
Elad, Y., Cytryn, E., Meller Harel, Y., Lew, B., Graber, E.R., 2011. The
biochar effect: plant resistance to biotic stresses. Phytopathol.
Mediterr. 50, 335e349.
Fischer, D., Glaser, B., 2012. Synergisms between compost and biochar
for sustainable soil amelioration. In: Kumar, S. (Ed.), Management
of Organic Waste. InTech, New York, pp. 167e198.
Flannery, T., 2009. Foreword. In: Lehmann, J., Joseph, S. (Eds.), Biochar
for Environmental Management: Science and Technology. Earthscan Publications, Oxford, pp. xxviiexxviii.
Gore, A., 2009. Our Choice: A Plan to Solve the Climate Crisis. Rodale
Books, Emmaus, 1e416.
Graber, E.R., Harel, Y.A., Kolton, M., Cytryn, E., Silber, A., David, D.R.,
Tsechansky, L., Borenshtein, M., Elad, Y., 2010. Biochar impact on
development and productivity of pepper and tomato grown in
fertigated soilless media. Plant Soil 337, 481e496.
Hansen, J., Sato, M., Kharecha, P., Beerling, D., Berner, R., MassonDelmotte, V., Pagani, M., Raymo, M., Royer, D.L., Zachos, J.C.,
2008. Target atmospheric CO2: where should we aim? Open
Atmos. Sci. J. 2, 217e231.
Hollister, C.C., Bisogni, J.J., Lehmann, J., 2013. Ammonium, nitrate,
and phosphate sorption to and solute leaching from biochars
prepared from corn stover (Zea mays L.) and oak wood (Quercus
spp.). J. Environ. Qual. 42, 137e144.
Ippolito, J.A., Laird, D.A., Busscher, W.J., 2012. Environmental benefits
of biochar. J. Environ. Qual. 41, 967e972.
Jahirul, M.I., Rasul, M.G., Chowdhury, A.A., Ashwath, N., 2012. Biofuels production through biomass pyrolysis-a technological
review. Energies 5, 4952e5001.
Joseph, S., van Zwieten, L., Chia, C., Kimber, S., Munroe, P., Lin, Y.,
Marjo, C., Hook, J., Thomas, T., Nielsen, S., Donne, S., Taylor, P.,
2013. Designing specific biochars to address soil constraints: a
developing industry. In: Ladygina, N., Rineau, F. (Eds.), Biochar
and Soil Biota. CRC Press, Boca Raton, pp. 165e201.
Kammann, C., Ratering, S., Eckhard, C., Müller, C., 2012. Biochar and
hydrochar effects on greenhouse gas (carbon dioxide, nitrous oxide, and methane) fluxes from soils. J. Environ. Qual. 41,
1052e1066.
Kloss, S., Zehetner, F., Dellantonio, A., Hamid, R., Ottner, F.,
Liedtke, V., Schwanninger, M., Gerzabek, M.H., Soja, G., 2012.
Characterization of slow pyrolysis biochars: effects of feedstocks
REFERENCES
and pyrolysis temperature on biochar properties. J. Environ. Qual.
41, 990e1000.
Kookana, R.S., 2010. The role of biochar in modifying the environmental fate, bioavailability, and efficacy of pesticides in soils: a
review. Aust. J. Soil Res. 48, 627e637.
Kookana, R.S., Sarmah, A.K., van Zwieten, L., Krull, E., Singh, B.P., 2011.
Biochar application to soil: agronomic and environmental benefits
and unintended consequences. In: Sparks, D.L. (Ed.). Advances in
Agronomy, vol. 112. Elsevier, Amsterdam, pp. 103e143.
Krull, E., Lehmann, J., Skjemstad, J., Baldock, J., Spouncer, L., 2008.
The global extent of black carbon in soils: is it everywhere? In:
Schröder, H.G. (Ed.), Grasslands: Ecology, Management and
Restoration. Nova Science Publishers, Inc, New York, pp. 13e17.
Laird, D.A., Brown, R.C., Amonette, J.E., Lehmann, J., 2009. Review of
the pyrolysis platform for co-producing bio-oil and biochar. Biofuels, Bioprod. Biorefin. 3, 547e562.
LeCroy, C., Masiello, C.A., Rudgers, J.A., Hockaday, W.C., Silberg, J.J.,
2013. Nitrogen, biochar, and mycorrhizae: alteration of the symbiosis and oxidation of the char surface. Soil Biol. Biochem. 58,
248e254.
Lee, J.W., Day, D.M., 2013. Smokeless biomass pyrolysis for producing
biofuels and biochar as a possible arsenal to control climate
change. In: Lee, J.W. (Ed.), Advanced Biofuels and Bioproducts.
Springer ScienceþBusiness Media, New York, pp. 23e34.
Lee, J.W., Hawkins, B., Li, X., Day, D.M., 2013. Biochar fertilizer for soil
amendment and carbon sequestration. In: Lee, J.W. (Ed.),
Advanced Biofuels and Bioproducts. Springer ScienceþBusiness
Media, New York, pp. 57e68.
Lehmann, J., 2007. Bio-energy in the black. Front. Ecol. Environ. 5,
381e387.
Lehmann, J., 2008. Terra preta nova: where to from here? In:
Woods, W.I., Teixeira, W.G., Lehmann, J., Steiner, C.,
WinklerPrins, A.M.G.A., Rebellato, L. (Eds.), Amazonian Dark
Earths: Wim Sombroek’s Vision. Springer ScienceþBusiness
Media, New York, pp. 473e486.
Lehmann, J., Gaunt, J., Rondon, M., 2006. Bio-char sequestration in
terrestrial ecosystems-a review. Mitig. Adapt. Strat. Gl. 11,
403e427.
Lehmann, J., Rillig, M.C., Thies, J., Masiello, C.A., Hockaday, W.C.,
Crowley, D., 2011. Biochar effects on soil biota-a review. Soil Biol.
Biochem. 43, 1812e1836.
Lehmann, J., Rondon, M., 2006. Bio-char soil management on highly
weathered soils in the humid tropics. In: Uphoff, N., Ball, A.S.,
Fernandes, E., Herron, H., Husson, O., Liang, M., Palm, C.,
Pretty, J., Sanchez, P., Sanginga, N., Thies, J. (Eds.), Biological
Approaches to Sustainable Soil Systems. CRC Press, Boca Raton,
pp. 517e530.
Leng, R.A., Preston, T.R., Inthapanya, S., 2012. Biochar reduces enteric
methane and improves growth and feed conversion in local “Yellow” cattle fed cassava root chips and fresh cassava foliage. Livest.
Res. Rural Dev. 24. Article #212. Retrieved March 14, 2013, from:
http://www.lrrd.org/lrrd24/12/sang24212.htm.
Lin, Y., Munroe, P., Joseph, S., Henderson, R., 2012a. Migration of
dissolved organic carbon in biochars and biochar-mineral complexes. Pesqui. Agropecu. Bras. 47, 677e686.
Lin, Y., Munroe, P., Joesph, S., Ziolokowski, A., van Zwieten, L.,
Kimber, S., Rust, J., 2012b. Chemical and structural analysis of
enhanced biochars: thermally treated mixtures of biochar, chicken
litter, clay and minerals. Chemosphere 91, 35e40.
Liu, N., Sun, Z., Wu, Z., Zhan, X., Zhang, K., Zhao, E., Han, X., 2013.
Adsorption characteristics of ammonium nitrogen by biochar from
diverse origins in water. Adv. Mater. Res. 664, 305e312.
Liu, W., Zeng, F., Jiang, H., Zhang, X., 2011. Preparation of high
adsorption capacity bio-chars from waste biomass. Bioresour.
Technol. 102, 8247e8252.
445
Lovelock, J., 2009. The Vanishing Face of Gaia: A Final Warning. Basic
Books, New York, 1e304.
Mathews, J.A., 2008. Carbon-negative biofuels. Energy Policy 36,
940e945.
Mohan, D., Sharma, R., Singh, V.K., Steele, P., Pittman, C.U., 2012.
Fluoride removal from water using bio-char, a green waste, lowcost adsorbent: equilibrium uptake and sorption dynamics
modeling. Ind. Eng. Chem. Res. 51, 900e914.
Novak, J.M., Busscher, W.J., 2013. Selection and use of designer biochars to improve characteristics of southeastern USA coastal plain
degraded soils. In: Lee, J.W. (Ed.), Advanced Biofuels and Bioproducts. Springer ScienceþBusiness Media, New York, pp. 69e96.
Park, J.H., Choppala, G.K., Bolan, N.S., Chung, J.W., Chuasavathi, T.,
2011. Biochar reduces the bioavailability and phytotoxicity of
heavy metals. Plant Soil 348, 439e451.
Prost, K., Borchard, N., Siemens, J., Kautz, T., Séquaris, J., Möller, A.,
Amelung, W., 2013. Biochar affected by composting with farmyard
manure. J. Environ. Qual. 42, 164e172.
Pulver, C., 2013. Personal communication.
Rogovska, N., Laird, D., Cruse, R.M., Trabue, S., Heaton, E., 2012.
Germination tests for assessing biochar quality. J. Environ. Qual.
41, 1014e1022.
Saarnio, S., Heimonen, K., Kettunen, R., 2013. Biochar addition indirectly affects N2O emissions via soil moisture and plant N uptake.
Soil Biol. Biochem. 58, 99e106.
Sarmah, A.K., 2009. Potential risk and environmental benefits of waste
derived from animal agriculture. In: Ashworth, G.S., Azevedo, P.
(Eds.), Agricultural Wastes. Nova Science Publishers, Inc, New
York, pp. 1e18.
Sarmah, A.K., Srinivasan, P., Smernik, R.J., Manley-Harris, M.,
Antal Jr., M.J., Downie, A., van Zwieten, L., 2010. Retention capacity of biochar-amended New Zealand dairy farm soil for an
estrogenic steroid hormone and its primary metabolite. Aust. J. Soil
Res. 48, 648e658.
Schmidt, M.W.I., Skjemstad, J.O., Czimczik, C.I., Glaser, B.,
Prentice, K.M., Gelinas, Y., and Kuhlbusch, T.A.J., 2001. Comparative analysis of black carbon in soils. Global Biogeochem. Cy. 15,
163e167.
Schimmelpfennig, S., Glaser, B., 2012. One step forward toward
characterization: some important material properties to distinguish biochars. J. Environ. Qual. 41, 1001e1013.
Schnell, R.W., Veitor, D.M., Provin, T.L., Munster, C.L., Capareda, S.,
2012. Capacity of biochar application to maintain energy crop
productivity: soil chemistry, sorghum growth, and runoff water
quality effects. J. Environ. Qual. 41, 1044e1051.
Smith, P., Haberl, H., Popp, A., Erb, K., Lauk, C., Harper, R., Tubiello,
F., de Siqueira Pinto, A., Jafari, M., Sohi, S., Masera, O., Böttcher,
H., Berndes, G., Bustamante, M., Ahammad, H., Clark, H., Dong,
H., Elsiddig, E. A., Mbow, C., Ravindranath, N. H., Rice, C. W.,
Robledo-Abad, C., Romanovskaya, A., Sperling, F., Herrero, M.,
House, J. I., Rose, S, in press. How much land based greenhouse
gas mitigation can be achieved without compromising food security and environmental goals? Glob. Change Biol. 19, 2285-2302
Sohi, S.P., Krull, E., Lopez-Capel, E., Bol, R., 2010. A review of biochar
and its use and function in soil. In: Sparks, D.L. (Ed.). Advances in
Agronomy, vol. 112. Elsevier, Amsterdam, pp. 47e82.
Sohi, S., Lopez-Capel, E., Krull, E., Bol, R., 2008. In: Krull, E. (Ed.),
Biochar, Climate Change and Soil: a Review to Guide Future
Research. CSIRO, Collingwood, pp. 1e56.
Sparkes, J., Stoutjesdijk, P., 2011. Biochar: Implications for Agricultural
Productivity. ABARES, Canberra, 1e48.
Spokas, K.A., Baker, J.M., Reicosky, D.C., 2010. Ethylene: potential key
for biochar amendment impacts. Plant Soil 333, 443e452.
Spokas, K.A., Cantrell, K.B., Novak, J.M., Archer, D.W., Ippolito, J.A.,
Collings, H.P., Boateng, A.A., Lima, I.M., Lamb, M.C.,
446
25. ADVANCES AND INNOVATIONS IN BIOCHAR PRODUCTION AND UTILIZATION FOR IMPROVING ENVIRONMENTAL QUALITY
McAloon, A.J., Lentz, R.D., Nichols, K.A., 2012a. Biochar: a synthesis of its agronomic impact beyond carbon sequestration.
J. Environ. Qual. 41, 973e989.
Spokas, K.A., Novak, J.M., Venterea, R.T., 2012b. Biochar’s role as an
alternative N-fertilizer: ammonia capture. Plant Soil 350, 35e42.
Ussiri, D., Lal, R., 2013. Nitrous oxide sources and mitigation strategies. In: Ussiri, D., Lal, R. (Eds.), Soil Emission of Nitrous Oxide
and Its Mitigation. Springer ScienceþBusiness Media, New York,
pp. 243e275.
van Zwieten, L., Kimber, S., Morris, S., Downie, A., Berger, E., Rust, J.,
Scheer, C., 2010. Influence of biochars on flux of N2O and CO2 from
a ferrosol. Aust. J. Soil Res. 48, 555e568.
Vaughan, N.E., Lenton, T.M., 2011. A review of climate geoengineering
proposals. Clim. Change 109, 745e790.
Verheijen, F., Jeffery, S., Bastos, A.C., van der Velde, M., Diafas, I., 2010.
Biochar Application to Soils - A Critical Scientific Review of Effects
on Soil Properties, Processes and Functions. EUR 24099 EN. Office
for the Official Publications of the European Communities,
Luxembourg. pp. 1e149.
Warnock, D.D., Lehmann, J., Kuyper, T.W., Rillig, M.C., 2007. Mycorrhizal responses to biochar in soildconcepts and mechanisms.
Plant Soil 300, 9e20.
Winsley, P., 2007. Biochar and bioenergy production for climate
change mitigation. N. Z. Sci. Rev. 64, 5e10.
Woolf, D., Amonette, J.E., Perrott, F.A., Lehmann, J., Joseph, S., 2010.
Sustainable biochar to mitigate global climate change. Nat. Commun. 1, 1e9.
Xiao-Jun, Z., Da-Fang, F., He, L., 2012. Adsorption removal of
copper, zinc and cadmium in aqueous solutions and road
runoff by carbonized mulch: heavy metal removal by
carbonized mulch. In: Hang, P.S. (Ed.), Proceedings of the
2012 International Conference on Biomedical Engineering
and Biotechnology. IEEE Computer Society, Washington,
pp. 1180e1185.
Xu, X., Cao, X., Zhao, L., Wang, H., Yu, H., Gao, B., 2013. Removal of
Cu, Zn, and Cd from aqueous solutions by the dairy manurederived biochar. Environ. Sci. Pollut. Res. 20, 358e368.
Yaghoubi, P., 2011. Development of Biochar-Amended Landfill Cover
for Landfill Gas Mitigation (Thesis). University of Illinois at
Chicago, USA.
Zhang, M., Gao, B., Varnoosfaderani, S., Hebard, A., Yao, Y.,
Inyang, M., 2013a. Preparation and characterization of a novel
magnetic biochar for arsenic removal. Bioresour. Technol. 130,
457e462.
Zhang, Z., Cao, X., Liang, P., Liu, Y., 2013b. Adsorption of uranium from aqueous solution using biochar produced by hydrothermal carbonization. J. Radioanal. Nucl. Chem. 295,
1201e1208.
C H A P T E R
26
Biochar Processing for Sustainable
Development in Current and Future
Bioenergy Research
Mark P. McHenry
School of Engineering and Information Technology, Murdoch University, Perth, Western Australia, Australia
email: mpmchenry@gmail.com
O U T L I N E
Can Biochars Increase Livestock Growth Rates,
or Provide a New Market for Semiarid Forestry?
453
A Comparison of Biochar Carbon Value for Different
454
Potential Income Streams
Introduction
447
Theoretical Income Streams
Renewable Energy and Fuel Generation
Carbon Sequestration of Biochars and
Carbon Markets
448
448
449
Conclusion
454
Agricultural Benefits
450
Disclaimers
455
Economic Analysis
451
Can Biochar Be a Cost-effective Fertilizer Substitute? 451
Can Biochar Be a Cost-Effective Approach to
452
Increase Grain Crop Primary Productivity?
References
455
INTRODUCTION
Rural biomass energy and carbon options seem to
offer increased financial resilience to agricultural enterprises relative to fluctuating seasonal growing conditions and uncertain market prices of inputs, products,
and exchange rates. The projected increases in farming
costs from any future inclusion of the agricultural sector
from carbon pricing may be offset by additional net income from such rural biomass-based sequestration and
renewable energy activities. Cellulose, hemicelluloses,
and lignin are the main components of wood and crop
residues of known potential for bioenergy and stable
carbon forms, and the management of which requires
detailed agronomic, technical, and market information.
Thus, there is a synergistic match between growing
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00026-7
food and growing biomass for energy and carbon in
the same rural enterprise.
Modern concepts of biochar-agricultural systems and
their respective projected financial viabilities have been
outlined in the existing literature (Lehmann and Joseph,
2009). These systems commonly incorporate complex
semi-industrial operations with rural and forestry
biomass as well as small-scale low-technology concepts
with farm waste and domestic heating. To narrow research
specificity, this work focused on the West Midlands of the
Northern Agricultural Region of Western Australia (WA),
and uses Australian dollars. (At the time of writing the
Australian and US currencies were roughly parity.) To
date, this region is one of the few regions of Australia
that has exhibited economically encouraging agricultural
responses from biochar addition, and has an established
447
Copyright Ó 2014 Elsevier B.V. All rights reserved.
448
26. BIOCHAR PROCESSING FOR SUSTAINABLE DEVELOPMENT IN CURRENT AND FUTURE BIOENERGY RESEARCH
practice of profitable grazing leguminous fodder shrubs,
which is a potentially large and sustainable biomass
supply.
THEORETICAL INCOME STREAMS
The potential income streams in the West Midlands
from above ground rural biomass include renewable
energy and fuel generation, carbon sequestration of biochars, and agricultural benefits from the use of biochar
and ash from energy and fuel generation or charring
alone. It is likely that tradable sequestered carbon will
be reliant on the supplies from bioenergy generation
plants that are able to comply with both emission and
biochar quality standards. However, a price on carbon
may help offset the additional costs of the coproduction
of electricity and biochar from biomass. (Table 26.1
outlines key benefits, costs and barriers to biochar
compliance to carbon markets.) The Australian Farm
Institute (2011) estimates an income reduction of between 1.4% and 1.6% from a carbon price based on electricity consumption for a WA mixed farming enterprise
of 4900 ha (2400 ha cropped and about 2000 head of livestock, mainly sheep), assuming agriculture and transport fuels are excluded from any carbon liability
(Australian Farm Institute, 2011). In contrast to concerns
of a carbon price reducing agricultural profitability, this
work presents the case that integrating new sequestration options into conventional production systems
from low-cost biochars produced from agricultural
wastes (with sufficient operational safety considerations) may offset costs in the West Midlands. The profitability of income streams (presented in Table 26.1) are
highly sensitive to (and often dependent upon) government subsidies for renewable energy, a carbon price, and
TABLE 26.1
also the location-specific demand for biochar and
energy. Similarly, agricultural effects of biochar addition
will vary more with soil type, seasonal conditions, and
animal nutrition characteristics. In complex and uncertain circumstances, predictive modeling can become
particularly challenging. However, the agricultural
effects (where they occur) will likely provide a more
solid basis for emerging industry development than
the highly sensitive and evolving carbon and electricity
markets. If agricultural benefits, initially at least, exhibit
less risky investments to individual farms than bioenergy cooperatives or carbon sequestration pooling
activities, then agricultural benefits may be a more suitable foundation for the establishment of biomass-based
industrial developments than energy or carbon sequestration policies in the initial phases.
Renewable Energy and Fuel Generation
In simple terms, wood-fired stoves, barbeques, or water heaters are a biomass-based renewable energy system.
Yet, the growing range of new medium- to large-scale
bioenergy technologies include gasifier and pyrolysis
power stations coproducing electricity, heat, and a range
of biofuels. Nonetheless, all traditional and new technologies convert the complex hydrocarbon molecules in
biomass to hydrogen, methane, carbon monoxide, carbon
dioxide, and numerous other gasses, including polyaromatic hydrocarbons and dioxins. Some technologies
also produce liquid and soil fuels (such as biochar)
from the same biomass. In general, while small-scale
and simple technology designs have less control and
efficiency, they exhibit lower capital and operating costs,
although they are usually more labor intensive per unit
production of output (McHenry, 2012b). At the regional
scale biomass power plant technology choices often
Outline of Key Potential Income Streams from Rural Biomass in the West Midlands
Income stream
Carbon Form
Benefits
Costs
Barriers
Renewable Energy
from Biomass
Crop residues, woody
harvest/coppice,
manures
Electricity, fuel
Capital and running
costs
Competition from other
renewable energy
technologies
Carbon Markets
Trees > 2 m, soil
carbon, biochar
Carbon sequestration
Establishment, practice
change, manufacture/
purchase and
application, monitoring
Accreditation and
acceptable
methodologies
Agricultural Benefits
Biochar from crop
waste or woody
biomass or manures
Increased grain yield at
maintenance P, reduced
fertilizer requirement,
detannification of
livestock feed,
composting accelerant
Manufacture/
purchase, application
High soil P levels, crop
and pasture benefits
restricted to sand and
gravelly soil types
(these are more
common in the West
Midlands than some
other regions)
449
THEORETICAL INCOME STREAMS
TABLE 26.2
Performance of a Selected Range of Available Biomass Conversion Technologies that May be Suitable
to Some West Midland Applications
Technology
Cost
Electrical Output
Application
Challenges
Gasifier Power Station
(Waste to Energy)
w$50 million
w30 GWh/yr
Regional landfill
Biochar contamination,
transport costs, gas cleaning
Rainbow Bee-Eater
(Crucible Carbon Slow
Pyrolysis Design)
e
w1 MWh/t
dry straw and
350 kg char
Regional center
near substation
New technology but clean gas
Slow Pyrolysis (BEST)
w$15 million
e
Regional center near
substation or customer
Gas cleaning
Updraft Gasifier (Big Char)
w$0.25 million
Nil
Mobile plant for biomass
conversion to biochar
(w25% efficiency)
Conversion rate and
biochar quality?
Woodgas Genset
(Powerpallet)
w$25,000
20 kW
On farm
Current price of diesel,
biochar production rate,
emissions?
Simple Drum Kilns
Low
Nil
On farm
Biochar production rate,
emissions, biochar quality
include gasifiers (which optimize gas production), and
slow pyrolysers (which optimize biochar production).
A general outline of the variations in biomass renewable
energy technologies are shown in Table 26.2. In terms
of developing a regional energy/biochar industry,
medium-sized biochar production units may address
concerns of soil nutrient loss from harvested biomass.
Despite the generally high costs of transporting timber
trees, transporting returned biochar is relatively efficient
on a weight basis, as the biochar mass is 70e80% less than
the original dry biomass (Lehmann, 2007). Nonetheless,
industrial biochar production and use will require a
number of safeguards. Handling risks include flammability concerns, and the dusts can spontaneously combust
in enclosed spaces and is comparable to the risk of
handling some metals, foods (flour, etc.), coal, plastics,
and woods (Joseph, 2007).
TABLE 26.3
Carbon Sequestration of Biochars and
Carbon Markets
Biochars contain very stable forms of carbon (fixed
carbon) (Blackwell et al., 2010). The proportion of the
original biomass carbon as total solid carbon in biochar
ranges from around 5% from gasification technologies
to about 35% in slow pyrolysis technologies. Wellmanaged large-scale biomass power plants can produce
biochar of a consistent quality, whereas often smallscale “low-tech” technologies tend to produce very
variable quality biochars, and are highly dependent on
the homogeneity of the heating regime and the duration
of heating. (See Tables 26.3 and 26.4.) A low-cost highvolume supply of sustainable biochar with a high
carbon fraction will be needed to generate meaningful
climate change mitigation benefits. However, currently
Conversion Efficiencies and Outputs of a Range of Technologies Producing Biochars
Technology
Power Output
Biochar Yield
Fixed C
Limitations
Gasification
Maximum
w5%
e
Some waste contamination. Biochar
credit may be owned by the power
plant operators
Slow Pyrolysis
Some
w35%
w65%
Biochar credit may be owned
by the power plant operators
Crucible Carbon (Slow Pyrolysis)
Some
35%
70%
Project developing
Kiln Pyrolysers (Big Char)
Nil
25%
e
Simple Drum Kiln (Ogawa)
Nil
e
Output rate
Drum Kiln (TLUD)
Nil
e
Output rate
Pit Kiln
Nil
12.5e30% (Brown, 2009)
Output rate
450
26. BIOCHAR PROCESSING FOR SUSTAINABLE DEVELOPMENT IN CURRENT AND FUTURE BIOENERGY RESEARCH
TABLE 26.4
Carbon Analysis of Biochars Used in Research in the West Midlands
Biochar Source (Temp of Slow
Pyrolysis)
Total Carbon, %
Fixed Carbon, %
K, %
P, %
Wood Jarrah (600 C Simcoa)*
65
69
e
e
45.7
76
e
e
58.6
e
34
4
40.1
e
14
12
Wood Jarrah (600 C Wundowie)*
y
Wheat Chaff (550 C)
y
Chicken Manure (550 C)
* Blackwell et al., 2010.
y
Krull, personal communication, Department of Agriculture, Fisheries and Forestry (a national initiative for biochar research), and Grains Research and
Development Corporation (biochar for agricultural productivity), 2010.
low carbon prices will not provide sufficient commercial
incentives to simply apply biochar in soils for mitigation
alone. The unacceptably high uncertainties of the direct
and indirect influences and residence times of biochars
and other organic carbon species in soils, their suitability
for carbon markets (Intergovernmental Panel on Climate
Change, 2000), and even the commercial incentives of
high-volume production are fundamental barriers to
widespread biochar use. Therefore, there is a growing
need for researchers to quantify the net effect of specific
biochars and application methods within niche agroecological systems (particularly grains and livestock) and to
verify any stable sequestration of carbon fractions
(McHenry, 2011). Furthermore, in terms of farm application risks, some biochars can contain toxic materials that
are controlled by “permissible exposure limit” standards. The levels of these toxic materials in the biochar
is dependent on both the biomass feedstock and the biochar manufacture process, thus no simple permissible
exposure limit is available for biochar to date (Blackwell
et al., 2009). Thus, the development of a secure and
responsible biochar industry will require awareness of
safe methods of handling agricultural inputs and
will need to be justified economically, and be integrated with existing agricultural production systems
(McHenry, 2011).
AGRICULTURAL BENEFITS
Available research to date has shown that biochar alters various soil properties in a number of ways. (See
Table 26.5.) In the context of the siliceous sandy soils
of the West Midlands, the most sought effects are
improved microbial habitats and improved nutrient
supplies from relatively low (w1 t/ha) rates of biochar
use. (See Table 26.6 for crop and pasture research
responses in the West Midlands.) It is clear that more
research is needed on how various biochars influence
the flows of nutrients through the soil profile (Lehmann
et al., 2006; Laird et al., 2008), particularly under Australian conditions (McHenry, 2011). To date, the major
claims have been related to biological immobilization
of inorganic N, adsorption of dissolved ammonium, nitrates, P, and hydrophobic organic pollutants (Beaton
et al., 1960; Gustafsson et al., 1997; Accardi-Dey and
Gschwend, 2002; Lehmann et al., 2003; Bridle, 2004;
Mizuta et al., 2004). However, the available research
scope does not include an assessment of whether this
adsorption could reduce some transport of agricultural
fertilizers or other pollutants into ground and surface
waters in agricultural catchments (Lehmann et al.,
2006; Lehmann, 2007). Early work by Bridle (2004) suggested that biochar applications reduce nitrate leaching,
as his research found levels of nitrate and ammonium
did not change in soils for 56 days after application.
The soil incubation study further revealed that in
contrast, soil bicarbonate availability and plant available
P levels would increase slowly (Bridle, 2004). The laboratory results suggested that biochar would provide a
source of P for plant growth and could have applications
on soils as a slow release form of P, yet some research
suggest a reduced uptake of N. This may be more useful
in deep sandy soils where P leaches from the surface into
groundwater. Biochars are also hypothesized to slow the
N cycle by increasing the carbon to N soil ratio, possibly
due to increased soil aeration reducing anaerobic
conditions (Lehmann et al., 2006). Rondon et al. (2005)
found a significant reduction of nitrous oxide emissions,
and a near-complete suppression of methane emissions
in glasshouse environments at biochar additions of
30 g/kg of soil for some crops (Rondon et al., 2005;
Lehmann et al., 2006). However, in some circumstances
a high carbon to N ratio and abiotic buffering of mineral
N may lead to low N availability (Lehmann and
Rondon, 2006). Therefore, medium-scale crop biochar
trials are required with regionally common soil biota
and mineralogy, and also crop, pasture, and animals
for greater understanding of commercial agricultural
applicability in a particular region.
451
ECONOMIC ANALYSIS
TABLE 26.5
Positive and Negative Effects on Plant Growth of Biochar Additions to Soil
Effect (D) or (L)
Process
Rate of Application
Appropriate Soils
Neutralization (þ)
Most biochars are alkaline and
can adsorb Al3þ ions
Often 10þ t/ha mixed in topsoil.
Has worked on Krasnozems.
Possibly very acid sands (Wodjils).
Increase Water
Holding (þ)
Increased microporosity
Effect proportional to rate and
biochar character
More for lower clay contents
Increase Nutrient
Holding (þ)
Increased cation exchange capacity
and anion exchange capacity?
Effect proportional to rate and
biochar character
More for lower clay contents
Increased Nutrient
Supply (þ)
Direct supply from biochar
inorganic fraction
Effect proportional to rate and
biochar character
All soils
Reduced Mechanical
Strength (þ)
Lowers soil cohesion
Higher rates
Non sands
Reduced N2O
Emission (þ)
Unclear
Higher rates? 10 t/haþ?
Especially poorly drained soils at
risk of denitrification
Microbial Habitat
Improvement (þ)
Micropores in the biochar help
mycorrhizal fungi and bacteria
survival, which use symbiosis
to improve nutrient and water
supply to plants which host them
w1 t/ha banded
Mainly low available P soils
(Colwell <20 ppm)
Phytotoxins ()
Carbon compounds such as phenols
are retained on low temperature
biochars and ones cooled with their
own emissions
Quenching, soaking and resting in
the soil after incorporation may
lower the concentration of these
substances
Depends on biomass and charring
process
Herbicide Adsorption
(þ) and ()
Can increase application
requirement and/or reduce
leaching
All rates
All soils
TABLE 26.6
Summary of Benefits to Crop and Pasture Production from Applied Biochars
in the West Midlands (2007e2010)
Crop Trial (Place)
Yield/DM Increase
Increase, %
Fertilizer
Soil
Wheat (Mingenew)
0.23 t/ha
40
96 kg/ha super
Colwell P 5 ppm
Wheat (Mingenew)
0.45 t/ha
25
25 kg/ha DAP
Colwell P 5.5 ppm
Pasture (Irwin)
40 kg/ha DM
20
Nil
Colwell P 10 ppm
Clover (Tubes)
0.15 kg/ha at the
rate of flowering
75
Nil
Colwell P 5.5 ppm
Clover (Badgi)
Very visible
Leached biosolids
Gravelly sand
DM, Dry matter.
DAP, Di-ammonium phosphate.
ECONOMIC ANALYSIS
A recent analysis by Blackwell et al. (2010) on biochar
effects on profitability of dryland wheat production in
WA provided a perspective of the breakeven investment
costs per hectare of different responses over the medium
term. Table 26.7 shows that for the West Midlands area
(high rainfall north) a 10% yield increase from 1 t/ha
application of banded biochar with a declining response
over 12 years would break even at $130/ha, based on the
previous 12-year data. This breakeven cost included
estimated biochar application costs of between $20 and
$50/ha; thus a production/purchase and transport cost
would need to be no higher than about $50e$100/t to
enable some income from the biochar use, which
encourages further work toward low-cost biochar production technology development (Blackwell et al., 2010).
Can Biochar Be a Cost-effective Fertilizer
Substitute?
An analysis by McHenry (2012a,b) quantified the potential of using biochar as a soil amendment to displace
annual applications of single superphosphate (SSP)
452
26. BIOCHAR PROCESSING FOR SUSTAINABLE DEVELOPMENT IN CURRENT AND FUTURE BIOENERGY RESEARCH
TABLE 26.7
Breakeven Cost of Applied Biochar in Six Rainfall Regions in WA
Trial
Low Rainfall
North (const.)
Low Rainfall
North (decl.)
Medium Rainfall
North (decl.)
High Rainfall
North (decl.)
Low Rainfall
South (decl.)
Medium Rainfall
South (decl.)
High Rainfall
South (decl.)
10% Yield Increase
170
100
140
130
100
120
140
70
40
50
50
40
40
40
240
140
190
190
150
160
180
50% P Fertilizer
Reduction
10% Yield
Increase and 50%,
P Fertilizer
Reduction
With and without initial yield increase and/or P fertilizer reduction responses, which decline linearly to nit after 12 years (decl.) or are constant for 12 years (const.).
(0% N, 8.8% P, 0% K, 11% S) in wheat cropping systems
in WA. The analysis assumed two biochar applications
over a 15-year period, applied in year zero, and year
eight. The analysis ignored all production inputs and
outputs, and only calculated the difference between using an average “full rate” of SSP (90 kg/ha), and a “half
rate” of SSP with deep banded biochar equivalent
to 1 t/ha. The 45 kg/ha year half-rate SSP application
is approximately equivalent to an annual application
of 4 kg of P/ha. The simplified analysis assumed that
the use of either method would achieve an identical
wheat yield, negating the requirement to model wheat
prices. The application cost of deep banding the biochar
(tons per hectare per application) was assumed to be
$110. The annual application costs of both the rates of
SSP were assumed to be $20/ha, goods and services
tax (GST)1 inclusive. A range of biochar prices (delivered to farm, per ton) was analyzed: $0, $50, $100,
$150, $200, $250, $300, $350, $400, and $450/t. Similarly,
a range of SSP costs (delivered to the farm, per ton) were
calculated: $250, $300, $350, $1250. A carbon price was
included in the analysis, and was analyzed at intervals
of $5 tCO2-e, between $0 and $100 tCO2-e. The analysis
assumed a 0.8 carbon fraction recalcitrance. A real discount rate of 8% p.a. was used, and all capital and maintenance costs were based on average current prices and
were GST inclusive. In summary, the results showed that
without a carbon value the “half rate” of SSP (45 kg/
ha year) and biochar (1 t/ha application) were only
cost competitive with the full rate of SSP (90 kg/ha year)
when the biochar purchase price was unreasonably low
(<w$20). At 2012 prices of SSP (generally between $200
and $450/t), the choice of using half SSP application
rates with biochar additions at the above application
rate assumptions were not an attractive option unless
the biochar purchase price was practically zero. The
net cost was also calculated assuming the carbon in
the biochar was eligible in soil carbon markets, and
the various potential prices of carbon were subtracted
from the gross biochar purchase price. While the introduction of a carbon price would effectively subsidize
biochar costs, very high carbon prices (>$100/t)
were required for the sequestration value of biochar
to simply equal the purchase price and cover costs of
soil application (McHenry, 2012a). The low SSP price,
the high market prices for biochar, and the high biochar soil application cost of deep banding relative to
conventional broadcasting, all resulted in the option
of halving SSP applications by using biochar an unattractive practice.
Can Biochar Be a Cost-Effective Approach to
Increase Grain Crop Primary Productivity?
For comparison, a further analysis by McHenry
(2012a,b) was undertaken of the value of applying biochar at 1 t/ha with the full rate of SSP as described
above. This analysis was undertaken to explore the relative impact of using biochar to increase yield, as
opposed to increasing fertilizer use efficiency. The analysis assumed that using the full rate of SSP (90 kg/ha
year) with a 1 t/ha year application of biochar increased
wheat yields by 15% on average over the 15 years relative to full SSP applications only, in the southwest of
WA2. The baseline yield used for the scenario was
1.75 t/ha, an approximate average wheat yield for WA.
The assumptions of the model, including a total area
wheat return increase of $71.75/ha, were based on an
increased production of an additional 15% wheat yield
from the 1.75 t/ha at a constant value of $350/t over
1
The Australian GST (goods and services tax) is a value-added tax of 10%, paid only by the final consumer of a good or service.
2
This assumption requires basic research to verify, although some agronomic studies indicate that this may be possible for certain crops
and soil types (Lehmann, J. and S. Joseph. 2008). Many individual studies are detailed in the book Biochar for Environmental Management:
Science and Technology (2008) published by Earthscan.
ECONOMIC ANALYSIS
the 15 years using the 8% real discount rate. The scenario
did not include additional harvesting or transport costs
for the additional wheat yield. The results indicated that
the required carbon prices to recoup biochar purchase
price costs were lower when biochar is used to increase
yield, rather than reduce fertilizer use. When biochar
purchase prices were below $250/t, the application of
biochar was attractive without any carbon price,
assuming the 15% yield is achieved (McHenry, 2012a).
Therefore, these relatively simple analyses suggest that
the most cost-effective on-farm use for biochar is to
simply increase the wheat yield. The results confirm previous assertions that agricultural biomass production
for the sole purpose of producing biochar for soil carbon
sequestration may not be economically feasible
(Lehmann et al., 2006).
Can Biochars Increase Livestock Growth Rates,
or Provide a New Market for Semiarid Forestry?
It is now clear that forestry carbon offsets are resilient
features of Australian climate change policies. To participate in such markets, farmers must be able to
adequately measure, and verify the mitigation achieved
(The CRC for Greenhouse Accounting & Tony Beck
Consulting Services Pty Ltd, 2003). Forestry plantations
that include some rotational harvesting for biochar or
bioenergy will require more sophisticated carbon
accounting than a simple revegetation project (Independent Pricing and Regulatory Tribunal, 2008). The establishment of tree fodder plantations has long offered a
significant productivity option for some farmers
(Sanford et al., 2003). Deferring the early grazing of
annual pastures and reduce dry season hand-feeding
has long generated interest (Patabendige et al., 1992;
Cleugh et al., 2002), and perennial fodder tree plantations offer another source to supplement stock feed in
the summer/autumn period (Sanford et al., 2003).
Deep-rooted perennials are well known to use available
water when annual pastures are dead, recover nutrients
from deeper soils, reduce soil acidification, minimize
erosion, and some leguminous species also fix nitrogen
(Patabendige et al., 1992; Cransberg and McFarlane,
1994; Hatton and Nulsen, 1999; Wise and Cacho, 1999;
Valzano et al., 2005). Adding value to these conventional
applications in such regions is the use of tree woody
wastes to produce biochar as a feed additive which
may improve ruminant growth when fed on the trees
(which may be of lower grade and/or be a “high tannin”
content), and in the process sequester carbon in the soil
(McHenry, 2010). The mechanism for this improvement
is generally known as “detannification”, and may enable
the use of potentially large resources of high-tannin fodder species (such as Acacia sp.) by increasing the availability of leaf protein (Van et al., 2006; Blackwell et al.,
453
2009). Acacia sp. fodder plantations require annual pruning of the higher branches to provide fodder for grazing
animals. Animals eat the leaves from the branches on the
ground, leaving the inedible woody waste components
in the paddock to dry and be collected as a potential
source of biomass for biochar manufacture. The
improved digestibility of some high-tannin fodder trees
with biochar feed additives may expand their utility
within agricultural production systems (McHenry,
2010). In particular, if an Acacia sp. biochar feed additive
is effective in Australian semiarid production systems
(such as the West Midlands), this might provide a
further incentive to revegetate semiarid sandy soils suitable to many native Acacia sp. to attain a combination of
positive benefits (Graetz and Skjemstad, 2003; Antle
et al., 2007). These options are currently based on a
12-week experiment by Van et al. (2006) comparing
goat growth rates fed on tannin-rich Acacia sp. fodder.
The goats were either fed biochar (produced from
bamboo) at a feed rate of <1 g per day per kilo of live
weight, or no biochar for the control group. The experimental group exhibited notably higher growth rates
(w20%) than the control goats that received no biochar
feed additive on the same feed regime. Over the 12
weeks the experimental goats fed biochar weighed
5.2% heavier than their controls (Van et al., 2006). This
may be a sufficient commercial incentive to drive demand and subsequent biomass conversion technology
investment without a carbon price (McHenry, 2010).
The work by Van et al. (2006) also presents a mechanism
(via animal excreta) that may be assessed for efficacy
when avoiding relatively expensive biochar soil application options such as deep banding, broadcasting,
seeding application, topdressing, aerial delivery, or precision application to ailing plants (Blackwell et al., 2009).
In addition to researching the efficacy of small
biochar additions to the diet of grazing animals, the
opportunity arises to simultaneously investigate the
reported capacity and magnitude of numerous other
biochar benefits (McHenry, 2010), including the ecologically delivered biochar to biosequester C; biologically
immobilize inorganic N; retain soil N; increase soil pH;
adsorb dissolved ammonium, nitrates, phosphate, as
well as hydrophobic organic soil pollutants such as
polycyclic aromatic hydrocarbons (Beaton et al., 1960;
Gustafsson et al., 1997; Accardi-Dey and Gschwend,
2002; Lehmann et al., 2003). The remaining levels of biochar in the animal excreta would also determine the carbon fractions that survive the digestive system to
determine the maximum available long-lived carbon
species fractions to be sequestered in soils via the ecological delivery method (McHenry, 2010). Long-term soil
testing may also be able to detect the stable fraction of
the ecologically delivered biochar after being exposed
to the soil environment. However, there is clearly
454
26. BIOCHAR PROCESSING FOR SUSTAINABLE DEVELOPMENT IN CURRENT AND FUTURE BIOENERGY RESEARCH
much research required to verify a number of assertions
and assumptions to provide a level of certainty acceptable to farmers and investors who collectively command
much of Australia’s productive capacity (Intergovernmental Panel on Climate Change, 2000; Barker et al.,
2007).
A Comparison of Biochar Carbon Value for
Different Potential Income Streams
A simple analysis of potential value per unit of dry
biomass associated with various potential production
systems may help identify suitable uses of agricultural
biomass (Table 26.8.) These theoretical comparative
financial values are exclusive of costs, which are
extremely variable according to the application and
scale of operation. These basic scenarios seem to indicate
that the highest values of agricultural residues are animal husbandry or cropping applicationsdonly if the
biochar can increase conventional yields. This demonstrates that a key focus for the development of a sustainable biochar industry is the value of the product to an
industry, rather than the cost of production per se.
This also illuminates the aspects of supplying biochar
with appropriate characteristics for the specific application, as it is likely that biochar applications will mature,
TABLE 26.8
and standards for biochars will be sought by users
assessing cost-effective product suppliers. In any case,
it seems reasonable that small-scale waste-to-energy
suppliers will be established at some point near rural
settlements with the assistance of government subsidies
in Australia. It also seems reasonable that various agricultural wastes will be co-fired, as well as potential
adjustments installed to increase clean biochar production options. These projects can be a sound foundation
to understand biomass-to-biochar technology by
supplying sufficient volumes of relatively cheap and
consistent biochars suitable for numerous medium- to
large-scale research trials. It is further likely that
bioenergy and biochar cogeneration at a regional level
may be more cost-effective when agricultural wastes
are leveraged by municipal solid waste resources, if
quality control of municipal wastes is maintained.
However, this will also require much evaluation and
research for processing technology and downstream
application suitability.
CONCLUSION
Taken in isolation, the cost and benefits of using
biochar for only farm soil carbon sequestration may
Comparative Values per Unit Wood Biomass for Different Mitigation and Sequestration Applications
Product
Product Value
Value per Ton
Dry Biomass
Biomass Energy
(Combustion)
Mitigation
$23 tCO2-e1
$32.20/t*
Carbon Credit
(Uncut Forestry)
Sequestration
$23 tCO2-e1
$42.16/ty
Income
Biochar Effect
Biochar Used
Grain Production
20% yield
increase
0.3 t of biochar
used on 0.3 ha
at a rate of 1 t/ha
Wheat yield at the
rate of 2 t/ha
$300/t (wheat),
$23 tCO2-e1
$36 þ $17.71/tyy
Detannification
20% increase in
liveweight gain
over 12 weeks
relative to controls
0.75 g/kg
liveweight
Live goat growth
at the rate of
10 kg/goat
(liveweight)
$1000/t liveweight,
or $1/kg), $23 tCO2-e1
$359 þ $17.71/tx
* 1 ton of wood with a 0.5 carbon dry fraction and a dry energy content of 21 MJ/kg can generate 1.75 MWh with a 30% conversion technology. If this technology was able to directly
displace electricity from the South West Interconnected System, using the latest estimate for the scope 2 emission factor (0.80 kgCO2-e/kWh) this would mitigate up to 1.4 tCO2-e, or at
$23 tCO2-e1 a gross mitigation value of up to $32.20.
y
If this same ton of wood remained in the paddock unharvested and was part of a carbon sequestration plantation, the theoretical sequestration of the carbon fraction would be
1.833 tCO2-e (500 kg C 3.666 tCO2-e t/C). At a price of $23 tCO2-e1, the gross value of the wood is now $42.16, or 25% more than the bioenergy option.
yy
In this scenario the ton of dry wood is assumed to be converted to biochar at an efficiency of 30%, with a stable carbon fraction of 0.7. Therefore, the 1 ton yields 300 kg of biochar
containing 210 kg C or 0.770 tCO2-e (210 kg C 3.666 tCO2-e t/C). If the paddock yield for the wheat crop increases 20% above the 2 t/ha, in the 0.3 ha the biochar from the 1 ton of
wood was applied to, the additional yield is 120 kg (2 t/ha 0.3 ha ¼ 0.600 t, 0.600 t 0.2 ¼ 0.120 t). Therefore, the additional 120 kg of wheat at $300/t is worth $36. The additional
stable fraction of 0.770 tCO2-e at a carbon price of $23 tCO2-e1 is also theoretically worth $17.71.
x
In this scenario the ton of dry wood is also converted to biochar at an identical efficiency (30%), with a stable carbon fraction of 0.7. The 300 kg of biochar is fed to 10 kg (liveweight)
goats as a detannification feed using the Van et al. (2006) methodology. The 300 kg would be sufficient for 40,000 daily doses for a goat weighing exactly 10 kg per head when given
0.75 g for each kg of liveweight each day. The 300 kg is the theoretical equivalent dose for 476 goats over the 12-week, or 84-day period (40,000/84). At a 20% growth increase relative to
controls on the same diet of high tannin fodder of an assumed 9 g per day, this is an additional liveweight of 0.756 kg per animal over the interval, or 359 kg for the total gain of the 476
goats. Therefore, the 1 ton of wood (or the 300 kg of char) has a value of $359 when the value of goats is assumed to be $1 per kg of liveweight. In terms of carbon sequestration, the
carbon fraction of 0.7 (assuming the digestion process does not influence the char), the additional $17.71.
455
REFERENCES
not be a profitable activity. Yet, the net sum over the agricultural system in terms of biochars increasing
conventional productivity may prove to be a more
cost-effective option than existing operations in some
areas (Antle et al., 2007). Notwithstanding economic
issues, the greater scientific challenge is determining
the efficacy of biochar carbon species in a range of
specific agricultural production systems over both the
long and the short term (McHenry, 2009, 2011). Integrated agricultural production systems require suitably
high-resolution data to determine the agricultural systems and regions that may be able to implement options
cost-effectively and sustainably (McHenry, 2010). Thus,
a coordinated and cross-disciplinary research approach
will likely be the most effective means of utilizing existing biomass/bioenergy activities for new agricultural
applications (Nabuurs et al., 2007). Providing greater
scientific rigor and certainty to farmers, environmentalists, governments and the broader community require
undertaking biochar research alongside their impacts
on upstream and downstream activities (McHenry,
2011). Once this research becomes available, it may provide a form of indemnity to farmers before prematurely
applying new systems and technologies that may be
only cost-effective in highly specific situations.
Conversely, if biochar feed additives prove effective,
even in localized regions, a major source of biochar
will be required, and as Acacia sp. are native to Australia,
and also most major continents, this may have extensive
global implications in arid, semiarid, and even some
temperate regions (McHenry, 2010). Nonetheless,
complex biological and agroecological production systems require high-resolution information to determine
where the best opportunities are to integrate these new
diversification options into their existing production
systems. In conclusion, the author offers a selection
of key biochar-related knowledge deficiencies for
Australian agriculture in bullet points,3 and also
numbered suggestions for groups in the West Midlands
of WA:
• The sensitivity of biochar industry to policy change
and administrative changes;
• Development of biochar research that aims to create
major benefits to agricultural productivity.
1. Proceed with caution
2. Understand carbon credit ownership in biomass
provided to regional power stations
3. Test cropping benefits with affordable biochar
4. Using appropriate safety precautions, experiment
with on-farm production and application of biochar
on a small scale
5. Encourage research into effects of biochar on crops,
animal nutrition, and animal health
6. Monitor technical developments of small scale
(2e20 MW) gasifier power units
7. Consider relationships with local waste-to-energy
projects using landfill
• Key sensitivities of biochars in major West
Australian agricultural operations (grains and
livestock);
• Key sensitivities of biochar carbon sequestration in
major agricultural operations;
• Energy, material, and cost flows of various biochar/
bioenergy conversion systems;
• Major feedstock availability in different regions, costs,
and transportation logistics;
• Efficacy and cost of various biochar application
technologies for West Australian conditions;
References
3
DISCLAIMERS
This material has been written for Western Australian
conditions, and many conclusions do not imply suitability to other areas. The inclusion of biochar/bioenergy products or trade names do not imply
recommendation, the comparisons are simply for a general audience, and are not sufficiently detailed for commercial comparisons or technical appropriateness for
any one or range of applications. The omission of any
locally available technology is unintentional.
Acknowledgments
This chapter would not have been written without the considerable
experience and expertise of Dr Paul Blackwell, Department of Agriculture and Food, Western Australia’s (DAFWA) Geraldton Regional
Office. Dr Blackwell’s long-time contribution to this field of research in
WA under particularly limiting funding and time allocations is an
example to those of us following in his notable footsteps.
Accardi-Dey, A., Gschwend, P.M., 2002. Assessing the combined roles
of natural organic matter and black carbon as sorbents in sediments. Environ. Sci. Technol. 36, 21e29.
Antle, J.M., Stoorvogel, J.J., Valdivia, R.O., 2007. Assessing the economic
impacts of agricultural carbon sequestration: terraces and agroforestry in the Peruvian Andes. Agric. Ecosyst. Environ. 122 (4), 435e445.
Australian Farm Institute, 2011. The Impact of a Carbon Price on
Australian Farm Businesses: Grain Production. Australian Farm
Institute, Sydney, New South Wales, Australia.
Barker, T., Bashmakov, I., Alharthi, A., Amann, M., Cifuentes, L.,
Drexhage, J., Duan, M., Edenhofer, O., Flannery, B., Grubb, M.,
Hoogwijk, M., Ibitoye, F.I., Jepma, C.J., Pizer, W.A., Yamaji, K.,
These requirements are in addition to the major research projects underway regarding the influence of various biochar feedstocks and
conversion technologies on the characteristics of the final biochar product.
456
26. BIOCHAR PROCESSING FOR SUSTAINABLE DEVELOPMENT IN CURRENT AND FUTURE BIOENERGY RESEARCH
2007. mitigation from a cross-sectoral perspective. Contribution of
Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. In: Climate Change 2007:
Mitigation. Cambridge University Press, Cambridge, United
Kingdom and New York, USA.
Beaton, J.D., Peterson, G.A., Bauer, N., 1960. Some aspects of phosphate adsorption by charcoal. Soil Sci. Soc. Am. J. 24, 340e346.
Blackwell, P., Krull, E.S., Butler, G., Herbert, A., Solaiman, Z., 2010.
Effect of banded biochar on dryland wheat production and fertiliser use in south-western Australia: an agronomic and economic
perspective. Aust. J. Soil Res. 48, 531e545.
Blackwell, P., Reithmuller, G., Collins, M., 2009. Biochar application to
soil. In: Lehmann, J., Joseph, S. (Eds.), Biochar for Environmental
Management: Science and Technology. Earthscan, London, United
Kingdom, pp. 207e226.
Bridle, T.R., 2004. Use of Pyrolysis to Recover Energy and Nutrients
from Biosolids. Cited 25.07.08. Available from: http://www.wef.
org/NR/rdonlyres/7DA581D9-C0D3-4E5C-B127AC68B7ABA6DD/0/Bridle_Paper.pdf.
Brown, R.C., 2009. Biochar production technology. In: Lehmann, J.,
Joseph, S. (Eds.), Biochar for Environmental Management: Science
and Technology. Earthscan, London, United Kingdom, pp. 127e145.
Cleugh, H.A., Prinsley, R., Bird, R.P., Brooks, S.J., Carberry, P.S.,
Crawford, M.C., Jackson, T.T., Meinke, H., Mylius, S.J.,
Nuberg, I.K., Sudmeyer, R.A., Wright, A.J., 2002. The Australian
national windbreaks program: overview and summary of results.
Aust. J. Exp. Agric. 42, 649e664.
Cransberg, L., McFarlane, D.J., 1994. Can perennial pastures provide
the basis for a sustainable farming system in southern Australia?
N. Z. J. Agric. Res. 37, 287e294.
Graetz, R.D., Skjemstad, J.O., 2003. The Charcoal Sink of Biomass
Burning on the Australian Continent. CSIRO Atmospheric Research
Technical Paper No. 64. CSIRO, Aspendale, Victoria, Australia.
Gustafsson, O., Haghseta, F., Chan, C., Macfarlane, J., Gschwend, P.M.,
1997. Quantification of the dilute sedimentary soot phase: implications for PAH speciation and bioavailability. Environ. Sci. Technol. 31, 203e209.
Hatton, T.J., Nulsen, R.A., 1999. Towards achieving functional
ecosystem mimicry with respect to water recycling in southern
Australian agriculture. Agroforestry Syst. 45, 203e214.
Independent Pricing and Regulatory Tribunal, 2008. Compliance and
Operation of the NSW Greenhouse Gas Reduction Scheme 2007.
The New South Wales Government & The Independent Pricing
Regulatory Tribunal of New South Wales, Sydney, Australia.
Intergovernmental Panel on Climate Change, 2000. Land Use, Land
Use Change, and Forestry. Cambridge University Press, Cambridge, United Kingdom. pp. 25e45.
Joseph, G., 2007. Combustible dusts: a serious industrial hazard.
J. Hazard. Mater. 142, 589e591.
Laird, D., Fleming, P., Wang, B., Horton, R., Karlen, D.L., 2008. Impact
of soil biochar applications on nutrient leaching. In: The 2008 Joint
Annual Meeting of Black Carbon in Soils and Sediments: III.
Environmental Function Symposium, Houston, Texas, USA.
Lehmann, J., 2007. Bio-energy in the black. Front. Ecol. Environ. 5 (7),
381e387.
Lehmann, J., Gaunt, J., Rondon, M., 2006. Bio-char sequestration in
terrestrial ecosystems. Mitigation Adapt. Strategies. Global Change
11, 315e419.
Lehmann, J., Joseph, S., 2008. Biochar for Environmental Management:
Science and Technology. Earthscan, London, United Kingdom.
Lehmann, J., Joseph, S., 2009. Biochar systems. In: Lehmann, J.,
Joseph, S. (Eds.), Biochar for Environmental Management: Science
and Technology. Earthscan, London, United Kingdom, pp. 147e168.
Lehmann, J., Pereira da Silva Jr, J., Steiner, C., Nehls, T., Zech, W.,
Glaser, B., 2003. Nutrient availability and leaching in an archaeological
Anthrosol and a Ferralsol of the Central Amazon basin: fertiliser,
manure and charcoal amendments. Plant Soil 249, 343e357.
Lehmann, J., Rondon, M., 2006. Bio-char Soil Management on Highly
Weathered Soils in the Humid Tropics. CRC Press, Boca Raton,
Florida, USA.
McHenry, M.P., 2009. Agricultural bio-char production, renewable
energy generation and farm carbon sequestration in WA: certainty,
uncertainty & risk. Agric. Ecosyst. Environ. 129, 1e7.
McHenry, M.P., 2010. Carbon-based stock feed additives: a research
methodology that explores ecologically delivered C biosequestration, alongside live-weights, feed-use efficiency, soil
nutrient retention, and perennial fodder plantations. J. Sci. Food
Agr. 90, 183e187.
McHenry, M.P., 2011. Soil organic carbon, biochar, and applicable
research results for increasing farm productivity under Australian
agricultural conditions. Commun. Soil Sci. Plan 42, 1187e1199.
McHenry, M.P., 2012a. Sensitive variables for applying biochar as a
fertiliser substitute and a method to sequester carbon in soils: a
wheat crop scenario. In: Ryan, B.J., Anderson, D.E. (Eds.), Carbon
Sequestration: Technology, Measurement Technologies and Environmental Effects. Nova Science Publishers, Hauppauge, New
York, USA.
McHenry, M.P., 2012b. Small-scale (6 kWe) stand-alone and gridconnected photovoltaic, wind, hydroelectric, biodiesel, and wood
gasification system’s simulated technical, economic, and mitigation analyses for rural regions in Western Australia. Renewable
Energy 38, 195e205.
Mizuta, K., Matsumoto, T., Hatate, Y., Nishihara, K., Nakanishi, T.,
2004. Removal of nitrate-nitrogen from drinking water using
bamboo powder charcoal. Bioresour. Technol. 95, 255e257.
Nabuurs, G.J., Masera, O., Andrasko, K., Benitez-Ponce, P., Boer, R.,
Dutschke, M., Elsiddig, E., Ford-Robertson, J., Frumhoff, P.,
Karjalainen, T., Krankina, O., Kurz, W.A., Matsumoto, M.,
Oyhantcabal, W., Ravindranath, N.H., Sanz Sanchez, M.J., Zhang, X.,
2007. Forestry, Climate Change 2007: Mitigation. Contribution of
Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University
Press, Cambridge, United Kingdom and New York, USA.
Patabendige, D.M., Scott, P.R., Lefroy, E.C., 1992. Fodder Trees and
Shrubs for High Rainfall Areas of South Western Australia.
Department of Agriculture Western Australia, Perth, Western
Australia.
Rondon, M., Ramirez, J.A., Lehmann, J., 2005. Greenhouse gas emissions decrease with charcoal additions to soils. The Third USDA
Symposium on Carbon Sequestration. Baltimore, USA.
Sanford, P., Wang, X., Greathead, K.D., Gladman, J.H., Speijers, J.,
2003. Impact of Tasmanian blue gum belts and kikuyu-based
pasture on sheep production and groundwater recharge in
south-western Western Australia. Aust. J. Exp. Agric. 43 (8),
755e767.
The CRC for Greenhouse Accounting & Tony Beck Consulting Services Pty Ltd, 2003. Opportunities for the Western Australian Land
Management Sector Arising from Greenhouse Gas Abatement.
Western Australian State Government, Perth, Western Australia.
Valzano, F., Murphy, B., Koen, T., 2005. The Impact of Tillage on
Changes in Soil Carbon Density with Special Emphasis on
Australian Conditions. Report No. 43. National Carbon Accounting System, Australian Greenhouse Office, Canberra, Australia.
Van, D.T.T., Mui, N.T., Ledin, I., 2006. Effect of method of processing
foliage of Acacia mangium and inclusion of bamboo charcoal in the
diet on performance of growing goats. Anim. Feed Sci. Tech. 130,
242e256.
Wise, R., Cacho, O., 1999. A Bioeconomic Analysis of Soil Carbon
Sequestration in Agroforests. Cited 16.07.12. Available from:
http://www.une.edu.au/carbon/CC02.PDF.
C H A P T E R
27
Development of Thermochemical and
Biochemical Technologies for Biorefineries
Michael P. Garver, Shijie Liu*
Department of Paper and Bioprocess Engineering, College of Environmental Science and Forestry,
State University of New York, Syracuse, NY, USA
*Corresponding author email: sliu@esf.edu
O U T L I N E
Introduction
457
Characteristics of Lignocellulosic Biomass
458
An Overview on Biomass Conversion
461
PretreatmentdBiomass Size Reduction by Physical
or Mechanical Methods
Mechanical PretreatmentdChipping, Grinding,
Milling, Refining
Irradiation Pretreatment by Electron Beam, Gamma
Ray, or Microwave
Ammonia Recycle Percolation Pretreatment
Ozonolysis Pretreatment
Organosolv Pretreatment
Oxidation Pretreatment
Ionic Liquid Pretreatment
Sulfite Pretreatment to Overcome Recalcitrance
of Lignocelluloses
Hot Water
462
470
473
473
474
474
475
Hydrolysis
476
BioconversiondConverting Sugars to Products
477
465
465
465
466
466
467
Thermochemical Conversion
Combustion
Gasification
Pyrolysis
Direct Liquefaction
478
478
478
481
481
Conclusion
482
468
469
References
482
463
INTRODUCTION
A biorefinery is a complex industrial system to
convert raw biologically derived materials into usable
and valuable products. The actual design of a biorefinery depends on the desired product, the raw materials available, and the method of conversion desired.
For the purposes of this chapter, the raw material
considered is woody biomass or more generally,
Bioenergy Research: Advances and Applications
http://dx.doi.org/10.1016/B978-0-444-59561-4.00027-9
Steam Explosion
Ammonia Fiber Explosion
Supercritical Carbon Dioxide Explosion
Biological Pretreatment
Acid Hydrolysis
Alkaline Hydrolysis
lignocellulosic biomass (LB). LB may originate from forest, herbaceous plants or organic waste streams such as
sewage, food processing waste, or animal manure.
LB is a source of energy that can reduce the consumption of fossil fuels. Energy independence is an important
economic and political goal. Renewable sources of
energy are also critical for a balanced ecological policy.
Biorefineries may be designed to output a specific set
of products and by-products. These products include
457
Copyright Ó 2014 Elsevier B.V. All rights reserved.
458
27. DEVELOPMENT OF THERMOCHEMICAL AND BIOCHEMICAL TECHNOLOGIES FOR BIOREFINERIES
biofuels, adhesives, surfactants, biochemicals, biopolymers, food and medicine. This chapter will focus on
some common products such as acetic acid, ethanol,
butanol, acetone, hydrogen, and polyhydroxyalkanoates. These products stem from the fermentation of
sugars derived from LB or they may be derived from
thermochemical conversion processes.
The first objective in any conversion is to reduce the
size and increase the surface area of the raw material.
This enables subsequent treatment methods to attack
and exploit specific properties of LB more effectively
to obtain sugars for bioconversion or obtain products
from thermochemical conversion.
Secondary treatment or conversion methodologies
include some form of hydrolysis, fermentation or any
of a variety of thermochemical conversion treatments.
The objective of these methods is to break lignin and
complex carbohydrates into either simple sugars or
intermediate products or even down to CO and H2 (syngas) for further fermentation (bioconversion) or thermochemical conversion. Fermentation is usually followed
by separations or filtrations as final steps in the acquisition of a desired product in a bioconversion.
CHARACTERISTICS OF
LIGNOCELLULOSIC BIOMASS
Understanding the characteristics of LB is necessary
for the effective application of a conversion technology
or a pretreatment method. LB is the most abundant
organic and renewable resource on the planet (Klass,
1998). Man has been producing chemicals, materials,
and energy from LB since his origin. These activities
continue to be the most promising activities to pursue
in order to address our contemporary challenges. For
(a)
example, we currently look to use LB to reduce dependence on fossil fuels.
There are three families of LBs: grassy plants, shrubs,
and trees, each possess four primary components: cellulose, hemicellulose, lignin and extractives. Each species
possesses these components in different proportions.
Hardwood trees (angiosperms), shrubs and grassy
plants (graminoids) usually possess less lignin than softwood trees (gymnosperms) (Liu, 2012).
Figure 27.1 illustrates the variation in cellular structure between hardwood and softwood. Notice that in
both images the structure is porous, where the pores
are empty spaces and run longitudinally. Figure 27.2 is
a compilation of sketches of wood cells. The cellular
structure of each plant species is of different sizes and
shapes and varies in the size and number of pores.
From Figure 27.1, one can note that there is no space
between the cells. Regardless of size and shape, each cell
is glued tightly to its neighbors. The intercellular spaces
are called middle lamellae. The great majority of the
middle lamellae, over 80%, contain lignin (Liu, 2012).
Lignin is the glue that binds the cells together and provides the rigid structure of wood. The remaining volume
of the middle lamellae consists of hemicellulose and
extractives. Conversely, the cell wall is mostly made of
cellulose and to a much lesser degree contains lignin and
hemicellulose. The great majority of biomass dry weight
is derived from the cell wall. A much smaller portion of
the biomass dry weight comes from the middle lamellae.
Most of the total lignin content of LB comes from the cell
wall. Despite the high concentration of lignin in the
middle lamellae, over 60% of the lignin from LB comes
from the cell wall portion of the material (Liu, 2012).
Table 27.1 shows that cellulose is the largest portion
of LB. Cellulose, which represents between 40% and
50% of the dry weight of wood, is a homopolymer of
(b)
Softwood
FIGURE 27.1
Hardwood
Typical structures of wood (a) Softwood (b) Hardwood. Source: Liu, 2012.
459
CHARACTERISTICS OF LIGNOCELLULOSIC BIOMASS
Southern
yellow pine
Western
hemlock
Ray tracheid
Softwood
Redgum
Parenchyma
cells
Hardwood
200 µm
Birch
Oak early wood
Redgum
Aspen
Alder
Eucalyptus Gmelina
Oak
tracheid
Softwood fibers
Tracheid
Fiber
Hardwood fibers
Birch
Aspen
Oak
late wood
Hardwood vessel elements
FIGURE 27.2 Diagrams of major cell types in softwood and hardwood. All the diagrams are shown at the same magnification to illustrate
the relative sizes of these elements. Source: Parham, 1983.
b-D-glucopyranose where dehydration of the b-Dglucose units forms a linear chain with a degree of polymerization (DP) between a few hundred and several
thousand b-D-glycosidic bonds. The dehydration occurs
between the one and four carbons of b-D-glucopyranose
units and leaves an oxygen atom to join the two units,
which is written as, b-1-O-4 glycosidic bonds. The formula for cellulose is He(C6H10O5)neOH, where “n” represents the DP. This highly ordered, tightly bound
pattern is made of bonds that are quite strong and are
difficult to break.
Cellulose grows into microfibrils with crystalline and
amorphous regions. The crystalline portions of the
molecule line up side by side. Hydrogen bonds, between
the hydroxyl groups, provide strong, sturdy and stable
links between and within these crystalline units. When
these microfibrils form macrofibrils and interact with
noncellulosic material in the cell walls of plants, the
result is strength and rigidity.
While the crystalline regions are stable and strong,
the amorphous regions provide an opportunity to break
down the large structure into smaller saccharides. Solvents, reagents and enzymes may be used to penetrate
and hydrolyze the structure. Hydration requires the
addition of energy or a strong acid. Alternatively, enzymes, such as cellulase, may facilitate the conversion.
Enzymatic hydrolysis tends to be much slower than
acid hydrolysis. Reducing the chip size or increasing
the exposed surface area of LB increases the effectiveness of these solvents, reagents and enzymes.
Hemicelluloses compose another large portion of LB,
between 20% and 30% of the dry weight of wood, see
Table 27.1. These are heteropolymers, or heterosaccharides of five- and six-carbon sugars. They are found
mostly in the cell walls of LB. Common hemicellulose
sugars are D-glucose, D-mannose, D-galactose, D-xylose,
L-arabinose, and to a lesser degree, L-rhamnose. Hemicellulose has a low DP, around 100e200, and thus is
more easily hydrolyzed into their monomeric sugar
components (Glaudemans and Timell, 1958; Goring
and Timell, 1960; Koshijima et al., 1965; Timell, 1960).
The structure of a hemicellulose tends to possess a
primary backbone, off of which might hang a variety
of residual units. These residual units are nonpolymeric
acids and sugars. The degree of branching or number of
residual units depends on the origin or species of the
biomass. For hardwood, the backbone is xylan, containing b-linked bonds at carbons one and four, like cellulose. Unlike cellulose, residual units can hang off
from the other carbon positions. These residues may
include those of acetic acid, glucuronic acid, mannose,
arabinose and galactose. Softwood is even more variable in that the backbone may be made of more diverse
materials. The backbone is typically made of galactoglucomannan units or arabinoglucuronoxylan units.
Galactoglucomannan is a polymer that is a primarily
460
TABLE 27.1
27. DEVELOPMENT OF THERMOCHEMICAL AND BIOCHEMICAL TECHNOLOGIES FOR BIOREFINERIES
Major Components of Wood
Distribution, wt%
Type
Cellulose
OH
O
O
HO
OH
OH
HO
O
O
O
OH
OH
HO
O
OH
HO
O
Softwoods
Hardwoods
40e50
40e50
20e30
20e25
OH
O
OH
OH
Lignin
Phenolic eOH
Per C6C3 unit
0.2e0.3
0.1e0.2
Aliphatic eOH
Per C6C3 unit
1.15e1.2
1.1e1.15
Methoxyl eOCH3
Per C6C3 unit
0.9e0.95
1.4e1.6
Carbonyl >CaO
Per C6C3 unit
0.2
0.15
Hemicellulose
25e30
25e35
Galactoglucomannan
(1:1:3)
5e8
0
(Galacto)glucomannan
(0.1:1:4)
10e15
0
0
2e5
7e10
Trace
Trace
15e30
5e8
2e4
The OH groups in the xylose units were partially substituted by OAc
on the C-2 or C-3 positions, i.e. RaCH3CO (Ac) or H
Glucomannan
(1:2 to 1:1)
OH
OH
HO
O
O
O
O
HO
OH
OH
O
OH
HO
O
OH
O
O
HO
OH
O
OH
Arabinoglucuronoxylan
Glucuronoxylan
OH in the xylo-units were partially substituted by OAc on the C-2 or
C-3 positions (about 7 in 10 xylo-units), i.e. RaCH3CO (Ac) or
Extractives
Aliphatic and alicyclic
Terpenes, terpenoids, esters, fatty acids, alcohols, etc.
Phenolics
Phenols: p-cresol, p-ethylphenol, guaiacol, salicyl alcohol, eugenol, vanillin, coniferyl aldehyde, acetovanillone,
propioguaiacone, salicylic acid, ferulic acid, syringaldehyde, sinapaldehyde, and syringic acid; stilbenes: pinosylvin,
pinosylvin monomethyl and dimethyl ethers, 4-hydroxystilbene, 4-hydroxystilbene monomethyl ether; lignans;
hydrolyzable and condensed tannins; flavonoids; isoflavones or isoflavonoids
Carbohydrates
Arabinose, galactose, glucose, xylose, raffinose, starch, pectic material
Inorganics
Ca, K, Mg, Na, Fe, SO 2
4 , Cl , etc.
Others
Cyclitols; tropolones; amino acids, protein, alkaloids, etc.
Ash
Source: Fengel, 1989.
0.2e0.5
0.2e0.8
AN OVERVIEW ON BIOMASS CONVERSION
linear and perhaps mildly branched chain. In hemicellulose, the residual units take the place of the strong
hydrogen bonding that occurred with cellulose
components.
Recall that cellulose is highly ordered and tightly
bound and thus resistant to hydrolysis. Hemicellulose
is not. Hemicellulose tends to be more randomly organized with a more variable and loosely bound structure
(amorphous). Therefore, it can be hydrolyzed by weaker
or more dilute acids and bases, or at milder conditions.
Lignin is the third largest component of LB at 25e35%
of the LB dry weight (Boerjan et al., 2003). Lignin is a heteropolymer with methoxylated phenylpropylene alcohol
units. Its structure tends to be amorphous and variable.
These units are interconnected by stable ether and ester
linkages. It is hydrophobic and aromatic. It covalently
links to hemicelluloses and cross-links different plant
polysaccharides giving mechanical strength to the cell
wall (Mielenz, 2001). Additionally, lignin is highly resistant to biological degradation and thus it protects cellulose
and hemicellulose from decay.
Lignin from different plant families vary in their
alcohol content and composition. These lignins are
thus defined by these components into different types.
The lignin precursor in gymnosperms is coniferyl
alcohol. The precursor in angiosperms is p-coumaryl
alcohol and sinapyl alcohol. The corresponding lignins
are guaiacyl (G), p-hydroxyphenyl (H) and syringal
(S), respectively. Grasses tend to contain G while palm
trees contain mostly S (Sjöstrom, 1993).
The next largest component of LB is the extractives.
These make up between 2% and 8% of the total dry
weight (Table 27.1). Extractives are compounds found
in LB that are soluble in neutral organic solvents or
water at standard temperatures and atmospheric conditions. Extractives vary in solubility. Some are lipophilic
and others are hydrophilic. Lipophilic extractives that
are soluble in nonpolar organic solvents are called
resins. There is a large diversity in the number of extractives. Additionally, the concentrations of extractives are
highly variable throughout the plant depending on the
tissue type, i.e. root, stem, bark, branch, needle or leaf.
It is important to note that over 70 metal, earth elements,
and inorganic compounds may be found in LB. The
extractives are the first components that can be extracted
from wood. This is advantageous for using LB as a bioremediation for toxic soil and wastewater in addition to
being a source for biofuel and other products.
AN OVERVIEW ON BIOMASS
CONVERSION
Conversion refers to the collection of processes
employed to modify a feedstock into desired product(s).
461
Given that LB is composed of a number of distinct
components, there are a variety of treatment options
available that one can use to change these components
into fuel, chemicals and other products.
With a harsh condition (high temperature, strong
acid/base, strong solvents, or a combination of these
agents), LB can be turned into small molecular units
(such as C, CO2, CO, H2 and H2O) and then further converted to a desired product. Thermochemical conversion technologies usually employ this strategy to break
down LB unselectively to accommodate further conversions either catalytically or biologically. Therefore, thermochemical conversion technologies can be versatile.
The structure of wood (or LB in general) is sufficiently
strong and complex that it is not feasible to attack the
whole complex at mild conditions in a single step, nor
is it feasible to isolate the components and attack them
individually. When mild conditions are desired, one
must attack at least one portion of the whole structure
and weaken it. Follow up with another treatment to
break down the first component or attack a second
component. Continue to treat the biomass until the
desired composition is obtained. These treatment options are classified into mechanical, thermal, chemical,
or biological processes. These are not discrete classifications. In other words, a process can be considered to
belong to more than one category. For example, if one
were to saturate LB with water, then heat it under high
pressure and rapidly release the pressure, the hot water
could vaporize into steam and thus explode apart
the woody cells it had penetrated. This is called steam
explosion and it uses a thermal process to accomplish
a mechanical breakdown of the woody material. Steam
explosion will be discussed later in this chapter.
While there are several LB conversion technologies
available, this chapter will focus on biochemical conversion technologies with some discussions in thermochemical conversions.
These treatments may be applied at various points
across the process. The typical process to acquire fuel
products using a bioconversion methodology is generally described in four parts: pretreatment, hydrolysis,
fermentation and distillation, separation and filtration.
As discussed previously, cellulose, hemicellulose and
lignin are strong, stable structures. These structures are
challenging for one to convert into fermentable components (Mielenz, 2001). Of these three components, hemicellulose is the most vulnerable and easiest to degrade.
Recall that compared to cellulose, hemicellulose is a
lower molecular weight and is less uniform as it is
composed of a variety of sugar polymers and residual
units.
In bioconversions, the objective of pretreatment is to,
as efficiently as possible, prepare LB for fermentation
into products. The amount of energy required to break
462
27. DEVELOPMENT OF THERMOCHEMICAL AND BIOCHEMICAL TECHNOLOGIES FOR BIOREFINERIES
FIGURE 27.3 Schemes of biochemical conversion to materials, chemicals and fuels: (1) sequential incremental deconstruction; (2) two-step
saccharification and fermentation; (3) simultaneous saccharification and fermentation; (4) gasification and fermentation. Source: Wang et al., 2012.
(For color version of this figure, the reader is referred to the online version of this book.)
down LB is fixed. No matter what suite of treatment
options used to convert LB to product, the thermodynamic barrier is the same. It requires the same amount
of energy to completely biologically degrade wood as
it does to chemically treat it or gasify it. One trades off
time for allowing organisms to invest energy on one’s
behalf versus applying heat or concentrated chemicals
to accomplish the same task more quickly. Additionally,
biological methods allow for greater selection of the
portion of biomass to convert it into product, and usually by selecting a descending route of molecular chemical energy or intermediates that are not down to
simplistic building blocks if possible (green chemistry).
By selecting only a portion of the LB to convert, one
lowers the amount of investment energy required.
Biochemical processes operate at moderate or low temperatures. These milder conditions may be slightly
more efficient than their thermochemical counterparts.
However, a burden of biological or biochemical processes could arrive for the need of detoxification. One
must often remove toxic components resulting from
the pretreatment methods employed.
Figure 27.3 illustrates a set of four treatment pathways to convert LB into various products. These are
not the only methods available but merely an example
of commonly used methods. This pathway represents
one of the most popular biorefinery designs used to biochemically convert LB into biofuels and bioproducts.
Pathway 4 shows a gasification process to produce syngas. This is a thermochemical process. The sugars in syngas are subsequently fermented into liquid fuels similar
to those produced by the more biochemical methods.
The four pathways shown vary in the number of steps,
or time, required to acquire the product.
Figure 27.4 provides a slightly more detailed look at
pathway 1 from Figure 27.3. Different pretreatment
methods have different desired characteristics (Limayem
and Ricke, 2012). A summary of pretreatment methods to
be discussed in this chapter and their characteristics is
shown in Table 27.2.
PRETREATMENTdBIOMASS SIZE
REDUCTION BY PHYSICAL OR
MECHANICAL METHODS
The first and most important step in any conversion
process is to reduce the physical size of LB. In order to
obtain the high yields required for commercial success
in bioconversion operations, it is vital to pretreat and
reduce the biomass into an effective size (Mosier et al.,
2005). Reducing the LB size from a log to wood chips
to even fine powders improves mass and heat transfer
as well as increases the surface area of the particle.
Increasing the surface area exposes a higher percentage
of the glycosidic or ester bonds to the agents in solution
463
PRETREATMENTdBIOMASS SIZE REDUCTION BY PHYSICAL OR MECHANICAL METHODS
Lignocellulosic
biomass
Size reduction
Hemicelluloses
extraction
(Pretreatment)
Hemicelluloses
extracts
Hydrolysis
Detoxification
&
neutralization
Residual
biomass
Enzymatic
hydrolysis
Fermentation of
hemicellulosic
sugars
Product
recovery
Fermentation of
cellulosic
sugars
Ethanol
butanol
biopolymer
...
Residue
processing
Co-products
FIGURE 27.4 Schematic flow sheet for biomass conversion to bioproducts. Source: US DOE, 2006. (For color version of this figure, the reader
is referred to the online version of this book.)
(Mosier et al., 2005). Catalysts, such as a proton or an
enzyme, can only access active chemical bonds when
exposed at the solideliquid interface (Liu, 2003; Yang
and Liu, 2005). Smaller particles translate into faster,
more uniform reactions and a more complete
conversion.
The energy required to reduce the biomass into a
treatable size depends on the density of the biomass
source. Herbaceous materials do not require as much
processing to achieve the needed particle size as it
does to reduce wood (Cadoche and Lopez, 1989). Since
LB reduction is much more energy intensive, it is imperative to adequately define the reduction process. This requires an understanding of the quality and condition of
the source materials. Qualities such as moisture content,
soil particles, foreign matter, and initial cut length will
impact efficiency, energy requirements and downstream
treatment conditions and requirements. Pretreatment is
costly and greatly influences the cost and effectiveness
of downstream operations. It affects fermentation
toxicity, the rate of enzymatic hydrolysis, enzyme load,
powder mix, product concentration, product purity,
waste treatment requirements, energy requirements
and a host of other process variables (Zhu and Pan,
2010). Thus, it is important to begin the design process
with the end in mind. Much effort should be invested
to design the whole process up front with specific source
materials and conditions defined.
Mechanical PretreatmentdChipping, Grinding,
Milling, Refining
At the time of harvest, an operation is performed in
the field to presize the LB. Herbaceous biomass is prepared by shredding or forage cutting. Chipping is the
preferred method for reducing the size of wood. Chipping reduces wood to 10e50 mm in two dimensions
and 5e15 mm in the third (Zhu and Pan, 2010). This is
the minimum treatment necessary to begin conversion.
However, additional reduction is often performed. For
example, wood chips may subsequently be refined to fibers such as that in fiber production, pulverized into
wood fibers or wood flour (Zhu and Pan, 2010). Pulverization requires much more energy than chipping (Zhu
and Pan, 2010).
In addition to chipping and shredding, hammer milling, knife milling, disk or attrition milling, and ball milling are viable alternatives to reduce biomass sizes.
Large-scale reduction operations have favored hammer
and disk milling (Tienvieri et al., 1999). Chip refining
is also an alternative as it can have a large throughput.
Hammer milling is primarily used for making wood
flours for composites and pellets. Disk milling is used
for wood fiber production at a commercial scale, around
1000 tons per day. Disk milling operations are dependent on environmental conditions and the quality of
source materials. The energy requirement and the
wood particle size and shape depend on these operational parameters (Tienvieri et al., 1999).
Milling operations have a significant impact on
downstream energy requirements and the efficiency of
enzymatic cellulose saccharification. Since the goal of a
biorefinery is to optimize the conversion process, to
reduce energy requirement and maximize the enzymatic
cellulose saccharification, it is important to attend to the
biomass size reduction portion of the process. Failure at
this stage amplifies the cost of energy requirements and
reduces the effectiveness of subsequent treatments.
Since these mechanical processes can produce a range
of particle sizes it is often necessary to control the
464
TABLE 27.2
27. DEVELOPMENT OF THERMOCHEMICAL AND BIOCHEMICAL TECHNOLOGIES FOR BIOREFINERIES
Summary of Pretreatment Methods and Key Characteristics
Pretreatments
Key Characteristics
References
Dilute Acid (H2SO4,
HCl (0.5e5%)
• Practical and simple technique. Does not require
thermal energy
• Effective hydrolysis of hemicelluloses with high
sugar yield
• Generates toxic inhibitors
• Requires recovery steps
(Chandel et al., 2007; Chaudhary et al., 2012;
Gamez et al., 2004; Li et al., 2012; Lloyd and
Wyman, 2005; Schell et al., 2003; Um and van
Walsum, 2012; Wyman et al., 2005; Yasuda and
Murase, 1995)
Hot Water
•
•
•
•
(Banerjee et al., 2009; Hu et al., 2008;
Kemppainen et al., 2012; Ladisch et al., 1998;
Laser et al., 2002; Lynd et al., 2002; Mosier et al.,
2005; Shupe and Liu, 2009; Weil et al., 1994)
Lime
• High total sugar yield including pentose and
hexose sugars
• Effective against hardwood and agricultural
residues
• High pressure and temperature hinder chemical
operation
• Commercial scalability problem
(Kim and Holtzapple, 2006; Weil et al., 1994;
Zhu and Pan, 2010)
Ammonia Fiber
Explosion (AFEX)
• Effective against agricultural residues mainly
corn stover without formation of toxic end
products
• Not suitable for high-lignin materials
• Ammonia recovery
• No wastewaters
(Bisaria and Ghose, 1981; Dale et al., 1984;
Hendriks and Zeeman, 2009; Jin et al., 2012; Lau
and Dale, 2008; Speers and Reguera, 2012; Sun
and Cheng, 2002)
Ammonia Recycle
Percolation (ARP)
• High redistribution of lignin (85%)
• Recycling ammonia
• Theoretical yield is attained
(Drapcho et al., 2008; Gupta and Lee, 2009; Kim
and Dale, 2005)
Steam Explosion
with Catalyst
• Effective against agricultural residues and
hardwood
• High hemicelluloses fraction removal
• Not really effective with softwood
(Bisaria and Ghose, 1981; Bura et al., 2009; Galbe
and Zacchi, 2002; Kemppainen et al., 2012;
Lloyd and Wyman, 2005; Monavari et al., 2009;
Park et al., 2012)
Organosolv
•
•
•
•
•
•
(Cybulska et al., 2012; Koo et al., 2012; Monavari
et al., 2009; Pan et al., 2005)
Sulfite Pretreatment to
Overcome Recalcitrance
(SPORL)
• Effective against high-lignin materials, both
softwood and hardwood
• Highest pretreatment energy efficiency
• Minimum of inhibitors formation
• Accommodate feedstocks versatility
• Steam explosion combined to SPORL in
presence of catalyst becomes effective against
softwood materials
• Cost-effective
(Li et al., 2012; Shuai et al., 2010; Tian et al., 2011;
Wang et al., 2009; Zhu et al., 2010a,c, 2009; Zhu
and Pan, 2010)
Ozone
• Effectively remove lignin from a wide range of
cellulosic material without generating inhibitors
• Expensive
(Garcı́a-Cubero et al., 2009; Mvula et al., 2009;
Sun and Cheng, 2002)
Alkaline Wet Oxidation
• The combination of oxygen, water, high
temperature and alkali reduce toxic inhibitors
• High delignification and solubilization of
cellulosic material
• Low hydrolysis of oligomers
(Chaudhary et al., 2012; Klinke et al., 2004;
Monavari et al., 2009)
Fungal Bioconversion
• Environmentally friendly
• Low use of energy and chemical
• Slow bioconversion
(Dashtban et al., 2010; Nguyen et al., 2000)
The majority of hemicelluloses can be dissolved
No chemicals and toxic inhibitors
Average solid load
Not successful with softwood
High yield is enhanced by acid combination
Effective against both hardwood and softwood
Low hemicellulosic sugar concentration
Formation of toxic inhibitors
Organic solvent requires recycling
High capital investment
PRETREATMENTdBIOMASS SIZE REDUCTION BY PHYSICAL OR MECHANICAL METHODS
particle size used in the biorefinery. Size characterization
is accomplished using sieves, screens and imaging
analysis. The particle surface area is the most relevant
determination of effectiveness, and thus, it is the quality
to be controlled. Specific surface area correlates to
energy consumption and the efficiencies of a variety
of size reduction processes have been compared
(Holtzapple et al., 1989).
There is a limit to the effectiveness of size reduction.
At this point, additional surface area increases, or particle size reductions will not improve substrate enzymatic
digestibility. This critical size is proportional to the pore
size in and along the wood cells. Refer to Figures 27.1
and 27.2. A common target size is one that maintains
the cell structure while allowing for lignin removal
from the middle lamellae.
Size reduction below the cell size will provide a more
efficient conversion. To reduce particle size to this
smaller level is done by comminution (Vidal et al.,
2011). Comminution of biomass, especially at the final
sizing stage, is energy intensive and the product is of
low value. Thus, there is much interest in finding the
most efficient milling processes.
To that end, ball milling has been extensively studied.
It has been shown to deliver excellent results in terms of
the hydrolysis rate and sugar yield. Additionally, this
pretreatment method is clean and easy to do. Vibratory
ball milling has been shown to be more effective at
breaking down the crystallinity of cellulose and
improving the digestibility of the biomass over ball milling alone (Millet et al., 1976). Mechanical milling requires
long operation times and a large amount of energy (Lynd
et al., 1996). The smaller the desired particle size the
greater the comminution requirements will be in terms
of time and energy (Cadoche and Lopez, 1989).
Irradiation Pretreatment by Electron Beam,
Gamma Ray, or Microwave
Irradiation is an option for biomass size reduction.
Following a gross procedure to reduce field supplies
into at least chip size, one can employ high-energy
radiation such as gamma radiation and/or microwave
radiation to accomplish fine particle reduction
(Wasikiewicz et al., 2005). Not only does a high-energy
radiation treatment produce fine particles, but it can
also favorably alter the physical and chemical properties
of the biomass, depending on dosage (Bouchard et al.,
2006). Irradiation has been shown to decrease the DP
(Bouchard et al., 2006) and make microstructural
changes to the irradiated cellulose pulp (Dubey et al.,
2004). These changes include an increase in the carbonyl
contents and an overall improvement in the vulnerability of the cellulose crystalline regions to reagents
(Stepanik et al., 1998). This in turn leads to a higher
465
rate of enzymatic hydrolysis. Furthermore, irradiation
leads to a significant increase in sugar yield (Yang
et al., 2008).
An electron beam cuts biopolymers such as cellulose,
hemicellulose and lignin into smaller chains. Analysis
by powder X-ray diffractometer and Fourier transform
infrared spectroscopy confirm the electron beam treatments reduce the degree of crystallinity and improve
the sugar yields from enzymatic hydrolysis from treated
samples (Karthika et al., 2012).
Electron beam irradiation is preferred over irradiation using a radioisotope. First of all, electron beam is
safer. Turn off the power and the electron beam stops.
A radioisotope is continuous and thus requires significant safety precautions to handle and dispose of.
Furthermore, dosages delivered by high-energy electron
beam can be controlled and they can provide more power per dose. This is a feature that would be useful in
the continuous treatment of LB (Auslender et al., 2002).
Compared with microwave and gamma ray treatments, treatment by electron beam is more energy
effective. The larger particle sizes, that it can treat, significantly offset the negative effect of higher dosage. That
said, there still remains the challenge of the limitation
of electron beam source and the potential limitation on
the scale of operations. Even though all these irradiation
options reduce the particle size and reduce the DP, they
are too expensive to use in full-scale operations.
Currently, the prospects of engaging an irradiation treatment, even if in conjunction with other environmentally
friendly treatment options, does not look promising due
to the excessive energy requirements. Table 27.3 shows a
comparison of these irradiation treatment methods on a
variety of wood species.
Ammonia Recycle Percolation Pretreatment
When aqueous ammonia, 10e15 wt%, is percolated
through biomass at temperatures between 150 and
170 C with a fluid velocity of 1 cm/min and a residence
time of 14 min, lignin depolymerizes and the lignincarbohydrate linkages break. This process is known as
ammonia recycle percolation (ARP) (Iyer et al., 1996).
This process is advantageous in that it does not inhibit
downstream biological processes. A water wash is therefore not necessary (Kumar et al., 2009a). Additionally, it
is possible to recover and recycle the ammonia. On the
downside, ARP is inefficient when used to pretreat softwood pulp (Mosier et al., 2005) where lignin had already
been removed.
Ozonolysis Pretreatment
A pretreatment option that is appropriate for
grassy biomass and some softwood is ozonolysis.
466
TABLE 27.3
27. DEVELOPMENT OF THERMOCHEMICAL AND BIOCHEMICAL TECHNOLOGIES FOR BIOREFINERIES
The Effects of Cellulose Conversion by Different Irradiation Pretreatments
Wood Species
Maximum
Dose
Electron Beam Irradiation
(Khan et al., 1986)
Spruce
2 MGy
1e2 mm in thickness
and 10e20 cm2
Gamma Irradiation
(Betiku et al., 2009)
Softwood
(Triplochiton scleroxylon)
40 kGy
32e42 mesh
8
0.673 0.10
Gamma Irradiation
(Betiku et al., 2009)
Hardwood
(Khaya senegalensis)
90 kGy
32e42 mesh
8
0.803 0.10
Microwave
(Verma et al., 2011)
Beech
400 W
30e42 mesh
48
Particle Size
Enzymatic Hydrolysis
Duration (h)
Cellulose
Conversion
72
0.89
0.561
Source: Wang and Liu, 2012.
In this case, ozone is used to degrade the lignin and
hemicellulose in bagasse, green hay, peanut, pine,
cotton straw, wheat straw and poplar sawdust (BenGhedalia and Miron, 1981; Vidal and Molinier, 1988).
The process primarily acts on the lignin component
and only mildly affects the hemicellulose component,
while cellulose is negligibly affected. Ozonolysis is
notable in that it removes lignin effectively and the
reactions take place at room temperature and standard pressure (Ben-Ghedalia and Miron, 1981). The
most significant advantage is that following an ozone
pretreatment where 60% of the lignin is removed
from wheat straw, the rate of enzymatic hydrolysis
increases by 500% (Ben-Ghedalia and Miron, 1981).
The most notable drawback is that the process is
expensive due to the large volume of ozone required
(Sun and Cheng, 2002).
Raising the operational temperature above 185 C
eliminates the need for a catalyst, either for an inorganic
acid or for an organic acid. At this condition, the amount
of delignification is quite satisfactory. Adding acid
yields a high quantity of xylose.
Since the organic solvents inhibit downstream biological processes, such as organism growth and enzymatic
hydrolysis, it is necessary to remove these solvents
from the system. This is quite difficult as some quantity
of solvent is likely to reside in the system even after efforts to remove them. Organic solvents tend to evaporate into the atmosphere and are hazardous to the
environment and one’s health. Containing the solvent
is another challenge. Given these challenges, an organosolv pretreatment is not necessarily ideal for large-scale
or commercial operations.
Oxidation Pretreatment
Organosolv Pretreatment
Take an organic or aqueous organic solvent such as
formic acid, acetic acid, methanol, ethanol, acetone,
ethylene glycol, oxalic acid, triethylene glycol or tetrahydrofurfuryl alcohol and combine it with an inorganic
acid catalyst such as hydrochloric acid or sulfuric acid
and one can eliminate the internal lignin and hemicellulose bonds. This is known as an organosolv process (Pan
et al., 2006; Sarkanen, 1980; Thring et al., 1990). Alternatively, an organic acid, such as oxalic acid, acetylsalicylic
acid and salicylic acid may be substituted for the inorganic catalyst.
It has been observed that approximately 72% of
xylose in untreated wood, in both its monomeric and
oligomeric forms, could be recovered using an organosolv pretreatment process (Pan et al., 2006). Pan et al.
(2006) also investigated a bioconversion of hybrid poplar to ethanol at 180 C, for 60 min, with 1.25% H2SO4,
and 60% ethanol. They observed that nearly 74% of the
lignin was removed as a precipitate in the ethanol
extraction.
Oxidation is a pretreatment option whereby an oxidizing agent, such as hydrogen peroxide or peracetic
acid, is applied to LB. The result is the removal of hemicellulose and lignin and thus, an increased accessibility to
cellulose to enzymatic hydrolysis. This result is the
culmination of several reactions: electrophilic substitution, displacement of side chains, cleavage of alkyl aryl
ether linkages, or the oxidative cleavage of aromatic
nuclei (Hon and Shiraishi, 2001). Often the oxidative
agent is not selective and a significant loss of hemicellulose and cellulose may occur. Additionally, there is a high
risk of forming downstream inhibitors as soluble aromatic compounds are formed while the lignin oxidizes.
When using hydrogen peroxide as the oxidative
agent on sugarcane bagasse, the rate of enzymatic hydrolysis improves. In one study, hemicelluloses and
approximately 50% lignin were solubilized by 2%
hydrogen peroxide at 30 C over 8 h. This was followed
by enzymatic hydrolysis, or saccharification, using
cellulase at 45 C within 24 h. The result was 95% efficiency in glucose production (Azzam, 1989).
PRETREATMENTdBIOMASS SIZE REDUCTION BY PHYSICAL OR MECHANICAL METHODS
In another study, peracetic acid was applied at
ambient temperatures to a hybrid poplar and sugarcane
bagasse mixture (Teixeira et al., 2000). It was determined
that peracetic acid was very selective for lignin and in
some cases, no significant carbohydrate was lost.
When peracetic acid was applied at 21%, the enzymatic
hydrolysis of cellulose increased from 6.8% for untreated biomass to 98% in the peracetic acid-pretreated
biomass.
Ionic Liquid Pretreatment
Ionic liquids are strong solvents. They are able to
dissolve the components of LB at ambient to moderate
temperatures. Furthermore, ionic liquids are highly
tunable through the selection of anion and cation.
Beyond toxicity and corrosivity, other considerations
affecting the selection of an ionic liquid include price,
availability, water tolerance, biodegradability, and physical properties such as viscosity, melting point, dipolarity and hydrogen bond basicity. An effective wood
dissolution is possible when both the ionic liquid and
conditions are properly identified and employed.
The most significant consideration for practical
large-scale operations is the toxicity of the ionic liquid
to be used. For example, 1-butyl-3-methylimidazolium
chloride ([BMIM][Cl]) is a good solvent to use on
cellulose as it is only moderately toxic compared to
that of 1-ethyl-3-methylimidazolium chloride ([EMIM]
[Cl]) (Swatloski et al., 2004; Wu et al., 2004).
Corrosivity of the selected ionic liquid is also important. It plays a large role in the economics of a commercial
operation. One can minimize corrosivity by selecting an
ionic liquid that is halogen free. Good choices include
1-ethyl-3-methylimidazolium acetate ([EMIM][OAc])
(Liebert, 2010) and 1,3-dimethylimidazolium-dimethylphosphate ([MMIM][(MeO)2PO2]) (Zavrel et al., 2009).
Balancing the physical properties and operational
conditions is important to obtaining the most ideal dissolution of LB. For example, if the viscosity of an ionic
liquid is high, it may be necessary to operate the pretreatment at a high temperature to obtain a practical dissolution. As a result, the reactions may become unstable and
may give rise to undesirable reactions and by-products.
A solution to this problem is to reduce the viscosity of
the ionic liquid by combining it with a cosolvent. A
good viscosity-reducing cosolvent is polyethylene glycol
(Willauer et al., 2000).
The dissolution rate is inversely proportional to wood
chip sizes. For example, ball-milled wood powder
produces a higher dissolution rate than does sawdust.
The dissolution rate for TMP fibers is higher than that
for sawdust, which is much greater than that of wood
chips (Kilpeläinen et al., 2007; Sun et al., 2009; Zavrel
et al., 2009).
467
In addition to particle size, dissolution efficiency is
also highly sensitive to the water content. Water attenuates the dissolution effectiveness of an ionic liquid.
Studies have shown that storing wood chips at warm
temperatures, e.g. 50 C or 90 C, reduces the water content of the wood and thus improves the pretreatment
effectiveness (Kilpeläinen et al., 2007; Sun et al., 2009).
Reducing the water content improves the dissolution
power of an ionic liquid regardless of the type of
wood being treated. However, if the wood becomes
too dry, the wood composition may change unfavorably.
Determining the precise water content of LB is quite
difficult and is complicated due to the diversity of environmental conditions of the regions from which the
wood studied grew. Variables such as humidity and variances in species present a challenge when comparing
literature on the subject (Wang et al., 2012).
The type of LB, dissolution time, temperature and
ionic liquid to wood ratio, are all factors that contribute
to the dissolution power of an ionic liquid. That said,
those ionic liquids that were effective at dissolving
both lignin and cellulose were also excellent at overall
LB dissolution. One of the best solvents for wood chips
is the combination of 1-allyl-3-methylimidazolium chloride ([AMIM][Cl]) and [EMIM][OAc]. Ionic liquids
derived from polycyclic amidine bases have been shown
to dissolve aspen wood chips completely (D’Andola
et al., 2008). The ionic liquids used in this study
were 1,8-diazabicyclo[5,4,0] undec-7-enium salt, and
1,8-diazabicyclo[5,4,0] undec-7-enium chloride [HDBU]
[Cl] (D’Andola et al., 2008). It has been observed that
[AMIM][Cl] can effectively dissolve both hardwood
and softwood wood chips. However, the same solvent
only partially dissolved Norway spruce (Kilpeläinen
et al., 2007). The efficiency of [AMIM][Cl] in dissolution
of wood is due to the presence of p-electrons both in the
alkenyl chain as well as in the imidazolium ring.
Possible pep interactions may occur between the aromatic part of lignin and the ionic liquid (Hunter and
Sanders, 1990; Kilpeläinen et al., 2007). The highest solubility of maple wood powder was achieved using
[AMIM][Cl] and [BMIM][Cl] (Lee et al., 2009).
[EMIM][OAc] can completely treat three types of
wood chips. It is used to treat spruce, beech and chestnut.
However, it only partially dissolves silver fir (Abies alba)
wood chips (Zavrel et al., 2009). A plausible explanation
for this difference is that silver fir contains more cellulose
(50.3%) and lignin (27.7%) than the other wood species
(Kuznetsov et al., 2002). When comparing the dissolution
effectiveness of [EMIM][OAc] to [BMIM][Cl] and controlling for species, wood chip size, and temperature one can
obtain a 3.6-fold increase in dissolution effectiveness using [EMIM][OAc] vs [BMIM][Cl]. In this case, southern
yellow pine wood chips were treated at 110 C. This
3.6-fold increase in dissolution effectiveness is
468
27. DEVELOPMENT OF THERMOCHEMICAL AND BIOCHEMICAL TECHNOLOGIES FOR BIOREFINERIES
FIGURE 27.5 A process for the
dissolution of wood and regeneration of
ionic liquid.Source: Sun et al., 2009. (For
color version of this figure, the reader is
referred to the online version of this
book.)
Wood chips
Ground wood
IL
Cooking
IL
Recycle
Wood/IL solution
Cellulose-rich
materials
Filtration
Regeneration
Acetone/H2O
Wash, dry
Lignin in solution
Evaporation of
acetone
Lignin
attributable to the basicity of the acetate anion, higher
than that of the chloride anion. Thus, [EMIM][OAc] is
stronger at breaking the intramolecular hydrogen bonds
(Fort et al., 2007; Sun et al., 2009).
An opportunity for improvement in using ionic liquids is better recovery of solubilized cellulosic materials
and lignin. A significant drawback is that much of the
hemicellulose is washed away during the recovery process. Figure 27.5 illustrates the process.
Following pretreatment with an ionic liquid, an enzymatic hydrolysis pretreatment is applied to produce the
sugars for downstream fermentation. This pretreatment
can recover as much as 90% of the cellulose for enzymatic hydrolysis. While cellulose is recovered at a high
rate, the hemicelluloses are not as they are washed
away. As Figure 27.5 illustrates, the ionic liquids are
recovered and recycled for reuse. Even so, the problems
of price, toxicity, and the lost hemicellulose persist,
which inhibit wide adoption in industrial scale
operations.
Sulfite Pretreatment to Overcome Recalcitrance
of Lignocelluloses
Sulfites are found to be efficient agents for pretreating
LB, in both hardwoods and softwoods (Zhu et al., 2009).
In sulfite pretreatment to overcome recalcitrance of lignocelluloses (SPORL), the sulfite refers to any sulfite,
bisulfate or combination. A combination may contain
any two of the following three reagents: sulfite (SO2
3 ),
bisulfite (HSO
3 ), and sulfur dioxide (SO2, or H2SO3).
The specific combination to use depends on the pH of
the pretreatment liquor and the temperature (Zhu
et al., 2009).
The first step in the process is to treat wood chips or
another LB feedstock with a sulfite salt solution where
the salt may be sodium, magnesium or calcium. This
first step usually operates at a temperature between
160 C and 190 C and at a pH between two and four
for 10e30 min. The second step is to fiberize the resultant biomass using a disk mill. This yields a fine fibrous
substrate suitable for robust saccharification and
fermentation (Shuai et al., 2010).
The typical acid charge on oven-dried wood is
0.5e1% for hardwood and 1e2% for softwood. The
typical bisulfite charge is 1e3% for hardwood and
4e8% for softwood (Zhu et al., 2010b). More than 90%
of the cellulose was converted from SPORL-treated
spruce chips. In this case the oven-dried wood chips
were treated with an 8e10% bisulfate and 1.8e3.7% sulfuric acid combination at 180 C for 30 min. The resultant material was treated with enzyme hydrolysis for
48 h using 14.6 cellulase and 22.5 b-glucosidase per
gram of substrate. Shuai et al., and Zhu et al., have performed comparative SPORL studies using dilute-acid
pretreatments for both softwoods and hardwoods
(Shuai et al., 2010; Zhu et al., 2010b). In these studies,
it was observed that SPORL is better at saccharification
of hexoses and pentoses than was a dilute acid (DA)
treatment. In one case, where oven-dried spruce was
treated at 180 C for 30 min with 1% H2SO4 at a 5:1
liquor-to-wood ratio, 87.9% of the hexoses and pentoses
were recovered using SPORL versus a similar DA treatment where 56.7% of the saccharides were recovered.
469
PRETREATMENTdBIOMASS SIZE REDUCTION BY PHYSICAL OR MECHANICAL METHODS
TABLE 27.4
Pretreatment
(180 C, 30 min)
Comparison of Chemical Pretreatment Method on Lodgepole Pine Wood Chips
Initial
Liquid pH
Untreated
Disk-Milling Energy
(kWh/ton wood)
Size Reduction
Energy Savings (%)
699
(%)
1.7
Hot Water
5.0
680
2.7
16.0
Acid
1.1
412
41.0
41.6
SPORL
4.2
594
15.0
75.1
SPORL
1.9
153
78.1
91.6
About 92.5% of the cellulose was recovered in an SPORL
process utilizing a 9% sodium sulfite (w/w of wood)
and 77.7% for the DA (Shuai et al., 2010). In another
study of aspen, or Populus tremuloides, a comparison
was made between an SPORL pretreatment using a
combination of sulfuric acid and sodium bisulfite and
a dilute sulfuric acid (DA) pretreatment. It was observed
that nearly 60% more ethanol was produced from the
SPORL-treated wood than from the DA-treated wood.
In both cases enzymatic hydrolysis was conducted using
10 FPU cellulase per gram glucan for 120 h (Zhu et al.,
2010b).
Table 27.4 highlights a handful of comparisons
between treatment methods and their effectiveness
where the pretreatment conditions were L/W ¼ 3,
disk-milling solids loading ¼ 30% (the solid contents of
pretreated wood chips), and disk plate gap ¼ 0.76 mm.
Sodium bisulfite charge was 8% on oven-dried wood
for the two SPORL runs; sulfuric acid charge was 2.21
(w/w) on oven-dried wood for the DA and low pH
SPORL runs, and 0 for the hot water and high pH
SPORL runs.
SPORL is an attractive pretreatment method due to
several features. SPORL generates much less furfural
and hydroxymethylfurfural (HMF) than does a simple
DA pretreatment. SPORL significantly enhances
fermentation yields by weakening the hydrophobic relationship between lignin and enzymes and enhancing
saccharification of cellulose. One of the products of
SPORL is a sulfonified lignin, which has potential economic value as a directly marketable coproduct within
existing markets and for opening new markets. The
energy consumption in the size reduction process is
reduced by an order of magnitude. Lastly, SPORL has
demonstrated commercial scalability with low technological and environmental risks (Zhu et al., 2010b).
Hot Water
Utilizing liquid water by itself, as the only pretreatment reagent, is an option of interest as it is environmentally friendly and inexpensive compared to other
pretreatment methods (Amidon et al., 2008; Liu, 2010;
Mosier et al., 2005). High pressure is applied to keep
the water in a liquid state while it is at elevated temperatures (Hendriks and Zeeman, 2009). This enables the
water to penetrate the cell structure of the biomass and
thus hydrate the cellulose and remove the hemicelluloses. Another feature of water is that it has a high
dielectric constant. This facilitates ionic substances to
disassociate and allows for the dissolution of hemicelluloses and a portion of the lignin.
When the water temperature exceeds 150 C, the
hemicellulose begins to solubilize. The degree to which
this occurs is determined by thermal, acid and alkali
stability of the hemicellulose, which is dependent on
the composition of the hemicellulose backbone and the
branching groups. Temperature of the water can selectively solubilize hemicelluloses. A 75% maximum xylan
solubilization in the hot water extract of sugar maple
was obtained at 175 C after 2 h, whereas only 30% of
the initial xylan was removed from a 2 h treatment at
152 C (Mittal et al., 2009). When the water temperature
exceeds 180 C an exothermal reaction begins. It is most
likely related to the solubilization of the hemicelluloses
(Brasch and Free, 1965).
Another result of the thermal process is that the pH of
the extract decreases to 3e4 (Gregg and Saddler, 1996a).
Portions of the hemicelluloses are hydrolyzed, which
form acids such as acetic acid. These are released from
acetylated polysaccharides in the wood. These acids
lower the pH and catalyze the additional hydrolysis of
hemicellulose (Liu and Wyman, 2003; Liu, 2008; Tunc
and van Heiningen, 2008; Zhu et al., 2005).
Depending on the intensity of the hot water extraction,
sugars may dehydrate. When hexose sugar dehydrates
HMF, also known as HFM or 5-hydroxymethyl2-furaldehyde, is formed. When pentose sugar dehydrates, furfural is formed. In addition to solubilizing
hemicellulose, hot water treatment can lead to solubilization of portions of lignin (Ramos, 2003). Regardless, the
produced compounds are usually phenolic heterocyclic
compounds such as vanillin, vanillin alcohol, furfural
and HMF. This is especially true in strong acidic
470
27. DEVELOPMENT OF THERMOCHEMICAL AND BIOCHEMICAL TECHNOLOGIES FOR BIOREFINERIES
conditions. Additionally, these compounds tend to
inhibit or toxify bacteria, yeast, methanogens and archae.
This is a significant disadvantage in using hot water to
extract cellulose and hemicellulose (Brownell et al., 1986).
Hot water extracts can be converted to desired
products as well, i.e. via separation and fermentation
(Liu et al., 2009; Shupe and Liu, 2009). Fermentation is
also strongly inhibited when a hydrolysate is produced
from a treatment containing 3% or more of solids or the
treatment temperature exceeded 220 C for 2 min. These
conditions likely yield furfural or soluble lignin compounds. At temperatures in excess of 250 C pyrolysis
begins to take place (Laser et al., 2002). Therefore, one
should avoid these high temperatures. Another undesirable effect of thermal pretreatment is that it may increase
the crystallinity index (CrI) of cellulose (Weimer et al.,
1995). It is important to remove the soluble lignin compounds quickly. Since lignin is highly reactive, the
disengaged lignin will recondense and precipitate onto
the biomass (Liu and Wyman, 2003). This seems to be
more prevalent in cases where severe pretreatment conditions are used. In these cases, more condensation and
precipitation of lignin compounds takes place and
sometimes, soluble hemicellulosic compounds such as
furfural and HMF are also produced (Mittal et al.,
2009) and polymerized (condensed) and deposited
onto the extracted biomass.
Despite the undesirable effects above, when compared
to other pretreatment methods, liquid hot water wood
extraction still has a major advantage. Since a large volume of water is used the solubilized hemicelluloses and
lignin compounds appear in lower concentrations. As a
result, the risk of undesirable degradation products is
reduced. The substances in the extract can be separated
and converted to desired products.
Figure 27.6 illustrates the three methods of liquid hot
water reactors. They are differentiated by their configurations. One is cocurrent, another is countercurrent and
the third is a flow-through reactor.
Briefly, in cocurrent pretreatment, the biomass and
water are heated and held at the desired conditions
for a specific residence time prior to allowing it to
cool. In the countercurrent design, water and lignocellulosic material flow in opposite directions through the
reactor. The flow-through reactor is designed such that
hot water is passed over a stationary bed of LB and
carries the hydrolysate and dissolved lignocellulosic
components out of the reactor (Hendriks and Zeeman,
2009).
Steam Explosion
LB is bulky and much of the volume is “empty”
or saturated by air. Air in LB can be replaced by
liquid water at high temperature and pressure. When
water-saturated LB is suddenly exposed to low pressures, liquid water suddenly expands when vaporized
forcing LB to disintegrate into fine particles. The
Masonite process was invented in 1926 (Mason, 1926)
employing this water to steam explosion process. Since
then, the steam explosion pretreatment (SEP) has been
a common technique (Mason, 1928). SEP is used to break
the crystalline and lignocellulosic structure of biomass
into its three major components, cellulose, hemicellulose
and lignin. SEP enhances the resultant cellulose’s susceptibility to enzymatic hydrolysis. High-pressure, saturated steam is applied to biomass for a brief period and
then allowed to rapidly decompress to atmospheric
pressure, hence the term explosion.
The explosion breaks up solid particles and is used as
a standard practice in chemical pulping operations. The
steam is vented and the biomass is discharged to a larger
vessel for rapid flash cooling (Mosier et al., 2005). SEP is
as much a mechanical process as it is a thermal process
(Holtzapple et al., 1989). Regardless, the explosion per
se, whether it causes particle disintegration or not,
does not play a significant role in producing a product
that is easily digested by enzymes (Brownell et al.,
1986). A more likely mechanism at play is the treatment’s effect in removing hemicellulose (Mosier et al.,
2005). Applying acid catalysts, usually SO2 (or sulfite),
enhances this effect by reacting, in conjunction with water, within the interstitial spaces to form sulfuric acid
and thus catalyze hemicellulose degradation (Gregg
and Saddler, 1996b).
SEP effectiveness and the chemical changes that take
place depend on residence time, temperature, chip size,
and moisture content. Effectiveness is determined by the
amount of hemicellulose solubilized and the rate of subsequent enzymatic hydrolysis. Optimal outcomes are
obtained when pretreatment occurs at either high temperature or short residence time, such as 270 C for
1 min, or at lower temperature and longer residence
time, such as 190 C for 10 min. Generally, initial treatment pressures range from 0.69 to 4.83 MPa and treatment temperature ranges from 160 C to 260 C.
At high temperatures water acts as an acid. Thus, during the treatment time, the hemicellulose hydrolyzes into
soluble sugars. The hemicellulose is considered to autohydrolyze as a result of exposure to the acetyl groups
in the organic acids formed at these high temperatures.
Acetic acid is formed from the acetylated hemicelluloses.
The pH during SEP is kept quite low, near pH 3e4. SEP
degrades a significant portion of the hemicellulose (Sun
et al., 2005). However, degradation of hemicellulose
may not stop at this point. If the treatment conditions
are severe, the solubilized hemicellulose may undergo a
series of secondary reactions that yield furfural and
HMF. These severe conditions may be high temperature
or a long incubation time. Furfural and HMF are
PRETREATMENTdBIOMASS SIZE REDUCTION BY PHYSICAL OR MECHANICAL METHODS
(a)
471
Co-current reactor
Check valve
Pretreated
biomass
Water
Biomass
Steam
Insulated coil
(b)
Counter-current reactor
Water with
dissolved extracts
Biomass
Jacketed
reactor
Pretreated
biomass
Water
(c) Flow-through reactor
Water
Jacketed
reactor
Biomass
Water with
dissolved extracts
FIGURE 27.6 Schematic diagram illustrating three types of liquid hot water reactors: (a) cocurrent, (b) countercurrent, (c) flow through.
(For color version of this figure, the reader is referred to the online version of this book.)
undesirable products that inhibit enzymatic hydrolysis
and limit the effectiveness of fermentation.
Meanwhile, lignin is partially depolymerized, some
lignin is redistributed within the material and some
may be removed completely from the fibers, each of
which contribute to an improved exposure of the cellulose domains (Chen and Qiu, 2010). The reduction in
hemicellulose and partial removal of lignin exposes
the cellulose surface and thus improves the ability of
the enzyme to attack the cellulose microfibrils (Alvira
et al., 2010). If the treatment conditions are severe,
some degradation of cellulose to glucose can occur.
One study reported an enzymatic hydrolysis efficiency
of 90% over 24 h using poplar chips using SEP. This
was significantly better than the control where the enzymatic hydrolysis efficiency was 15% from untreated
472
27. DEVELOPMENT OF THERMOCHEMICAL AND BIOCHEMICAL TECHNOLOGIES FOR BIOREFINERIES
poplar chips (Grous et al., 1986). That said, SEP is more
effective on agricultural residues than in wood as a
result of the lower acetic acid content in the hemicellulose portion of the biomass.
Adding a supplemental acid to the SEP reduces both
residence time and temperature. Adding an acid such as
H2SO4 (or SO2) or CO2, typically 0.3e3% (w/w), improves hydrolysis, decreases the production of inhibitory compounds and leads to a more complete
removal of hemicellulose (Kumar et al., 2009a). For the
effective treatment of softwoods, adding an acid catalyst
is essential to make the substrate susceptible to enzymatic hydrolysis. Adding a supplemental acid also improves the enzymatic hydrolysis of the residual solids
and decreases the production of inhibitory compounds
(Morjanoff and Gray, 1987).
These three parameters, the level of H2SO4 (or SO2) or
CO2, the residence time and the temperature, are the
most influential parameters on total sugar yield. For
SEP treatment of sugarcane bagasse, the optimal conditions are 1% H2SO4, 220 C; 30 s residence time, and a
water-to-solids ratio of 2:1 (Holtzapple et al., 1989). After
SEP treatment under these conditions sugar production
was determined to be 65.1 g sugar/100 g.
A two-step SEP is a good pretreatment for softwood
(Söderström et al., 2003). In this case, the first step is to
optimize the amount of hydrolyzed hemicellulose by
employing low severity conditions where the biomass
is treated at 180 C for 10 min with 0.5% H2SO4. In the
second step, the solid material from the first step is
washed and impregnated again with H2SO4. SEP is
applied again using more severe conditions. This time
the biomass is treated at 180 Ce220 C for a longer
time, between 2 and 10 min, and with a higher concentration of acid catalyst, 1e2% H2SO4. These treatments
appear to hydrolyze a portion of the cellulose and
make it more accessible to enzymatic attack (Sassner
et al., 2008). The most favorable conditions for
Salix wood is to impregnate it with 0.5% H2SO4 at
200 C for between 4 and 8 min. The yield is thus
55.6 g glucose and xylose per 100 g dry biomass (Sassner
et al., 2008).
If one uses SO2 as the impregnating agent in spruce
chips, the sugar yield is almost independent of impregnation time and slightly increases with decreasing chip
size (Monavari et al., 2009). Shorter impregnation times
result in slightly lower mannose yields in larger chips.
The optimum pretreatment conditions when using
SO2-catalyzed SEP for lodgepole and Douglas fir pine
is 200 C for 5 min with 4% SO2 (w/w) (Ewanick et al.,
2007; Kumar et al., 2010).
Another option for an impregnating agent is to use a
weak organic acid, in particular, lactic acid (Monavari
et al., 2011). It was observed that it was not efficient
and resulted in lower sugar yields in spruce, with or
without the addition of SO2. However, using a weak
organic acid is more environmentally friendly than using an inorganic acid as it would biodegrade in a waste
stream or be used for production of a biogas such as
methane.
Particle size, by itself, is not a significant contributor
to SEP effectiveness. Some studies report that larger particle size may improve the outcomes from SEP (Cullis
et al., 2004). In these studies, pretreated Douglas fir, a
softwood, was milled to three particle sizes:
<0.422 mm screenings, 1.5 1.5 cm and 5 5 cm. They
were then steam exploded using SO2. It was observed
that the largest particle size suffered less from pretreatment severity and had the highest cellulose recovery. It
had larger quantities of solubilized carbohydrate and
contained fewer furan degradation products. The
smaller particle sizes produced outcomes containing
more solubilized hemicellulose and lignin. If the resultant biomass is further refined to particles of a finer
size by plate milling, with a 0.178 mm gap, the initially
larger particle size showed a higher lignin removal
with peroxide washing and a greater rate of enzymatic
hydrolysis. This is likely due to the reduction in lignin
redeposition as a result of treatment severity.
These findings were substantiated in studies of
steam-exploded pine, another softwood (Ballesteros
et al., 2000). The largest of the sizes exhibited a higher
cellulose recovery and also a higher content of solubilized hemicellulose. Conversion of cellulose to glucose
was only slightly higher from the larger particle sizes.
However, the total recovered glucose, including the solubilized glucose from the steam explosion, was much
higher when starting with the largest particle size
(Ballesteros et al., 2000).
Starting with a herbaceous feedstock, such as Brassica
carinata residues, produced different results. Although
the cellulose recovery was still higher for the 8 and
12 mm fractions, the smaller particle sizes performed
better during enzyme hydrolysis. The 5e8 mm and
2e5 mm fractions yielded 100% while the 8e12 mm
fraction produced 85% (Ballesteros et al., 2002). This
suggests that lignin condensation is not as influential
in herbaceous feedstock. It is a critical factor when
pretreating softwood.
In softwood, the larger particle size produces a higher
maximum glucose yield, over 80%, compared to smaller
particle sizes with yields under 70% (Ballesteros et al.,
2002). SEP-treated hardwood exhibited no difference in
either enzyme digestibility or ethanol yield between
disparate particle sizes, in particular between 2e5 mm
and 12e15 mm (Negro et al., 2003). This indicates that
the severity of the treatment plays a larger role than particle size when using softwood. In these cases, smaller
particles increase the lignin condensation and recalcitrance to enzyme hydrolysis.
PRETREATMENTdBIOMASS SIZE REDUCTION BY PHYSICAL OR MECHANICAL METHODS
Overall, SEP is an attractive pretreatment method
considering the low amount of energy required to
reduce the biomass size compared to mechanical
comminution. Conventional mechanical size reduction
methods require 70% more energy than SEP to achieve
the same particle size reduction (Mittal et al., 2009).
Additionally, SEP is attractive because there are no recycling or environmental concerns. This too lowers cost.
SEP is thus recognized as one of the most cost-effective
pretreatment methods for hardwoods and agricultural
wastes. It has been extensively tested on a wide
array of lignocellulosic feedstock. It has been observed
that SEP is less effective for the pretreatment of
softwoods.
The most significant limitations include the partial
destruction of xylan, incomplete disruption of the
lignin-carbohydrate matrix, low lignin removal, and
lignin redistributes over the surface of cellulose (Chen
et al., 2010). Additionally, there is a risk of producing undesirable compounds such as furfural, HMF and other
soluble phenolic compounds. These undesirables inhibit
microbial growth and enzymatic hydrolysis. Thus, prior
to fermentation, SEP-treated LB must be washed with
water to remove these undesirable materials along
with water-soluble hemicellulose. Unfortunately, this
wash lowers the overall effectiveness as it washes
away around 20e25% of the initial dry matter and a
portion of the soluble sugars (Sun and Cheng, 2002).
Ammonia Fiber Explosion
Another explosion pretreatment is the ammonia fiber
explosion (AFEX) process. Instead of using liquid water
under high pressure, liquid ammonia is used. AFEX is
an effective and somewhat economically attractive
method to increase the yields of fermentable sugars
from LB (Holtzapple et al., 1991; Holtzapple et al.,
1992). In this method LB is exposed to liquid ammonia,
not ammonium hydroxide (i.e. no water/moisture), at
moderate temperatures and elevated pressures for a
longer period of time. After the appropriate residence
time, the system is rapidly vented allowing the liquid
to vaporize and literally explode the fibrous material.
Typically, 1e2 kg of liquid ammonia is used for each
kg of dry biomass. The system operates at temperatures
below 100 C, pressures above 3 MPa, and is quite
tolerant of pH. Any pH under 12 appears suitable. The
residence time is between 10 and 60 min. Under these
conditions, the system forms few degraded sugar products yet gives a high yield of desirable sugar products
(Mosier et al., 2005).
AFEX is an attractive treatment method for a variety
of herbaceous crops and grasses as it significantly improves the saccharification rates. It has been tested on
a variety of LB including aspen chips, softwood and
473
kenaf newspaper, alfalfa, wheat chaff, wheat straw,
barley straw, rice straw, bagasse, coastal Bermuda grass,
switchgrass, corn stover, and municipal solid waste. One
of the benefits is that AFEX only solubilizes a trivial
amount of solid material. Also, compared to acid pretreatment and acid-catalyzed steam explosion, very little
hemicellulose or lignin is removed. Lastly, the structure
of the material changes such that the result is an increase
in water-holding capacity and improved digestibility.
Although physically modified, the chemical composition of the material following AFEX pretreatment is
essentially unchanged from its original condition. The
benefit is illustrated as follows: over 90% hydrolysis of
the cellulose and hemicellulose may be obtained after
AFEX pretreatment of Bermuda grass where 5% of that
is lignin. The result is similar for bagasse except 15%
of the hydrolysate is lignin (Holtzapple et al., 1992).
These low-lignin containing biomasses readily hydrolyze at near theoretical yields of sugars. The resulting
sugars ferment rapidly with a high yield into a variety
of desired products. Since the AFEX treatment produces
very few inhibitors to the downstream biological processes, a water wash is not necessary (Dale et al., 1984;
Mes-Hartree et al., 1988).
Materials with a high lignin content, around 25%,
have proved to be recalcitrant to AFEX. Therefore,
AFEX is a less effective pretreatment method for hardwood chips, some newspaper material, and nut shells
(Teymouri et al., 2005). AFEX does not require a small
particle size for it to be an effective treatment option
(Larson and King, 1986) like steam explosion and hot
water treatments.
The most significant cost is that associated with recycling the ammonia following pretreatment (Kumar et al.,
2009a). Since pure ammonia is used in the process, more
stringent environmental and recovery procedures are
required. Thus, recycling is necessary to reduce the environmental impact and the cost of the procedure. To
recover the ammonia, a superheated ammonia vapor,
at temperatures upward of 200 C, is used to vaporize
and strip the residual ammonia from the pretreated
biomass. The evaporated ammonia is then drawn off
the system by a pressure controller for final recovery
(Holtzapple et al., 1990). Using this recovery method
has demonstrated that over 99% of the ammonia can
be recycled successfully. Even so, the overall capital
and operating costs are higher than other comparable
methods.
Supercritical Carbon Dioxide Explosion
To address the expense of the AFEX method, the supercritical carbon dioxide explosion method was developed. Compared to steam explosion, the supercritical
CO2 explosion method produces fewer inhibitory
474
27. DEVELOPMENT OF THERMOCHEMICAL AND BIOCHEMICAL TECHNOLOGIES FOR BIOREFINERIES
compounds. Additionally, CO2 is much more environmental friendly than organic solvents used in the organosolv method and the ammonia used in the AFEX
method. Because carbon dioxide is nontoxic, physiologically safe and inexpensive it is used in a variety of industries, for example, in food and pharmaceutical
production. The critical temperature of CO2 is 31.1 C
and its critical pressure is 73 atm. The term supercritical
refers to a fluid that at standard temperature and pressure would exist in its gaseous state. However, when
compressed using high pressures and at temperatures
above the critical point, the gas condenses into a liquidlike density. In this state it retains the characteristics of
mass transfer that are “gaslike” but with the solvating
power that is “liquidlike” (Kim and Hong, 2001). Carbon
dioxide molecules are small, like water and ammonia,
and thus it penetrates the small pores of LB. It is believed
that CO2 forms carbonic acid and thus it should increase
the hydrolysis rate. Furthermore, at low temperatures it
is thought to prevent significant decomposition of the
monosaccharides by the weak acid. However, the primary effect of supercritical carbon dioxide explosion is
from the explosion whereby it disrupts the biomass
structure and increases the surface area and improves
its vulnerability to enzymatic attack (Conner and
Lorenz, 1986; Zheng et al., 1998).
Despite these advantages, the operating and capital
costs of the supercritical carbon dioxide explosion pretreatment option remain prohibitive.
Biological Pretreatment
Perhaps the most natural pretreatment of biomass is a
purely biological method. Nature commonly employs
lignin-degrading microorganisms such as white, brown
or soft-rot fungi (Lee, 1997; McMillan et al., 1999). A
study that investigated the effect of high-yield concentrated recombinant MnP (rMnP), produced from the
yeast Pichia pastoris on the biobleaching of kraft pulps
found that rMnP applied at 30 U/g pulp for 24 h followed by alkali extraction removed a significant quantity of lignin from both hardwood and softwood
unbleached kraft pulps (Xu et al., 2010). The rMnPtreated pulp was more susceptible to subsequent
peroxide bleaching compared to the control pulp.
More than 60% of the kappa number was reduced by
sequential rMnP treatments and alkaline extractions.
When using white-rot fungi, such as Ceriporiopsis subyermispora, to treat sugar maple chips, the amount of
extracted hemicellulose can be increased (Barber,
2007). The biotreatment alters the physical and chemical
structures of the LB and removes a portion of the noncarbohydrate mass.
Because biological pretreatment is safe, environmentally friendly and energy saving it is gaining more
attention (Okano et al., 2005). The downside is that biological pretreatment is too slow for some industrial applications and some material is lost to the
microorganism as it is a consumer of hemicellulose, cellulose and lignin (Bohlmann, 2006). The microorganisms
are also susceptible to poisoning by lignin derivatives
(Hamelinck et al., 2003). Biological pretreatment by itself
may not be the best solution but it could provide value
when employed in conjunction with other pretreatment
options.
Acid Hydrolysis
Water is a weak acid by itself; however, adding a salt
to water will enhance the activity of the acid. Aqueous
acids, especially those with a salt, autoseparate into
hydrogen cations and hydroxyl anions, where one side
of the cleaved sugar polymer receives the hydrogen
cation and the other receives the hydroxyl group. One
can apply acid hydrolysis either as a pretreatment or
as a main hydrolysis step. A variety of acids act well at
ambient temperatures to pretreat LB and prepare the
material for anaerobic digestion. LB is eventually hydrolyzed into monosaccharides, furfural, HMF and other
volatile products. The lignin, however, condenses and
precipitates out as a result of the pretreatment (Esteghlalian et al., 1997; Liu and Wyman, 2003; Shevchenko et al.,
1999).
Concentrated acids are quite powerful, act at mild
temperatures and result in rapid reactions. However,
H2SO4, H3PO4 and HCl are highly toxic, corrosive, and
hazardous. Reactors for acid hydrolysis need to resist
corrosion. Furthermore, recovering the concentrated
acid from the hydrolysis effluent is important to reduce
the negative environmental consequences and to reduce
costs.
Hydrolysis using a DA is an effective pretreatment
for LB (Hinman et al., 1992). It produces high sugar
yields from some hardwoods, like poplar and aspen.
In one study, poplar wood was pretreated with a 2%
sulfuric acid at 190 C for 1.1 min and this was followed
by an enzymatic hydrolysis (Wyman et al., 2009). In this
particular study, the xylose yield was 18.5% and the
glucose yield was 64.3% where the raw material
contained 25.8% xylose and 74.2% glucose (Wyman
et al., 2009). In another study of aspen wood, the wood
was pretreated with a 1.1% sulfuric acid at 170 C for
30 min and followed by enzymatic hydrolysis (Tian
et al., 2011). The xylose and mannose yield was 13 wt%
(18 wt% theoretical contents) and the glucose yield
was 85% following treatment (Tian et al., 2011). See
Table 27.5 for a comparison of concentrated and DA
pretreatments.
There are essentially two classes of DA pretreatment
processes: high-temperature continuous-flow and low
PRETREATMENTdBIOMASS SIZE REDUCTION BY PHYSICAL OR MECHANICAL METHODS
TABLE 27.5
Concentrated
Acid
Dilute Acid
475
Comparison of Concentrated and Dilute Acid Pretreatments
Wood Species
Particle Size
Acid
Reaction Condition
Effects and Results
Hybrid poplar
(Zhang et al., 2007)
40e60 mesh
H3PO4 83e85.9%
50 C, 30e60 min
Enzymatic cellulose
digestibility 97% for 24 h
Douglas fir
(Zhang et al., 2007)
40e60 mesh
H3PO4 83e85.9%
50 C, 30e60 min
Enzymatic cellulose
digestibility 75% for 24 h
Spruce
1e5 mm
H2SO4 70 wt%
and 30 wt%
70 wt% H2SO4
(50 C, 2 h); 30 wt%
H2SO4 (80 C, 6 h)
74% pentoses, 69% hexoses
Birch
1e5 mm
H2SO4 70 wt%
and 30 wt%
70 wt% H2SO4
(50 C, 2 h); 30 wt%
H2SO4 (80 C, 6 h)
73% pentoses, 68% hexoses
Poplar
(Wyman et al., 2009)
6.35 mm
H2SO4 2%
190 C, 1.1 min
25.8% xylose 74.2% glucose
Aspen (Tian et al., 2011)
6e38 mm
H2SO4 1.1%
170 C, 30 min
72.2% pentoses 84% glucoses
Loblolly pine
(Marzialetti et al., 2008)
35e60 mesh
Trifluoroacetic
acid pH 1.65
150 C, 60 min
70.3% pentoses, 22.9% hexoses
Pine (Orozco et al., 2011)
1 mm
H3PO4 2.5 wt%
175 C, 10 min
100% xylose 13% glucose
Source: Wang and Liu, 2012.
temperature batch processes. High-temperature systems
operate at temperatures over 160 C and are appropriate
for solutions with a low concentration of solids, between
5% and 10%. Low-temperature systems operate under
160 C and are appropriate for solutions with a high
concentration of solids, between 10% and 40%.
Even though a simple acid pretreatment significantly
improves the rate of a hydrolysis process, it costs higher
than other physicochemical pretreatment processes. One
such process is steam explosion and was discussed
previously in this chapter. Another consideration for
an acid hydrolysis pretreatment is that one must
neutralize the hydrolysate prior to subsequent enzymatic hydrolysis or fermentation (Sun and Cheng, 2002).
Alkaline Hydrolysis
Alkaline pretreatment is viewed as a viable treatment
method because of its low energy requirement and low
capital equipment and operational costs (Zhao et al.,
2008). This process operates at lower temperatures and
pressures than other pretreatment methods. However,
at these conditions, the process is measured in hours
or days vs. minutes or seconds for high-temperature,
high-pressure methods (Karr and Holtzapple, 2000).
Additionally, one may recover or regenerate many of
the caustic salts.
Alkaline pretreatment may follow an SEP and may be
followed by an enzymatic hydrolysis pretreatment
(Montane et al., 1994; Pan et al., 2006). The initial reactions of alkaline pretreatment involve solvation and
saponification. Solvation, similarly associated with
dissolution or diffusion, is where the solvent surrounds
an ion, typically sodium dissolved in water. Traditional
NaOH treatment requires high temperatures to be effective (Zhao et al., 2008). It may be supplemented with
urea to lower operational temperatures and improve
dissolution (Zhao et al., 2008).
The alkaline solvent then saponifies the intermolecular ester bonds that cross-link xylan hemicelluloses
and other components including lignin and other hemicelluloses. Removing these cross-links increases the
porosity of the lignocellulosic materials. This improves
the penetrability of the material to the solvent and
swelling of the biomass follows. The swollen biomass is
thus more vulnerable to enzymatic and bacterial activity.
Compared with acid hydrolysis, alkaline hydrolysis
generally causes less sugar degradation. That said,
dissolution or solubilization of LB increases with alkali
concentrations. At strong alkali concentrations, peeling
of end-groups may occur. This leads to alkaline hydrolysis and degradation of the dissolved polysaccharides.
Furthermore, this may also produce unwanted byproducts. However, there may be a downstream advantage in
subsequent conversion treatments. It increases the internal surface area, decreases the DP, decreases crystallinity
and separates linkages between lignin and carbohydrates causing an overall disruption of the lignin structure (Fengel, 1984). This provides opportunity for
increased enzymatic and bacterial activity in downstream processes.
An alternative process to improve sugar content is to
use aqueous potassium hydroxide, which selectively
removes xylan. Keeping the temperature low, at or
476
27. DEVELOPMENT OF THERMOCHEMICAL AND BIOCHEMICAL TECHNOLOGIES FOR BIOREFINERIES
below room temperature, prevents peeling (Hon and
Shiraishi, 2001).
It appears that monomeric forms of hemicelluloses
are easily degradable to other volatile compounds. Glucomannans and xylans are particularly vulnerable to
peeling. However, by pretreating with a 3% NaOH
and 12% urea at 15 C one can achieve a 60% glucose
conversion (Zhao et al., 2008).
Calcium hydroxide, or slake lime, is yet another
effective alkaline pretreatment agent. It is one of the
least expensive and it is highly recyclable (Karr and
Holtzapple, 2000). Using common lime kiln technology,
one can recover calcium hydroxide by regenerating it
from insoluble calcium carbonate. Lime pretreatment
removes lignin and hemicellulose and increases the CrI.
Pretreatment with dilute NaOH decreases the lignin
content within a range of 24e55% to 20% and increases
the digestibility of NaOH-treated hardwood from 14%
to 55%. No effect was observed for softwoods with
lignin content greater than 26% (Bjerre et al., 1996).
Dilute NaOH pretreatment causes swelling, which, as
stated previously, has downstream benefits.
The overall effectiveness of alkaline pretreatment
depends on the lignin content of the biomass. Furthermore, it changes the cellulose structure such that it is
less dense and more thermodynamically stable than
native cellulose (Hendriks and Zeeman, 2009; Liu and
Wyman, 2003).
HYDROLYSIS
Hydrolysis is generally defined as the depolymerization of a substance via hydration. An aqueous acid’s ions
act to cleave long polymers like cellulose, hemicellulose
and lignin into smaller chains. Pretreating LB to undergo
hydrolysis or converting polysaccharides into monosaccharides will enhance later fermentation by improving
TABLE 27.6
the ability of anaerobic organisms to digest the resultant,
simpler sugars. Hydrolysis requires extended residence
time. Unfortunately, monosaccharides degrade into
other nonsugar molecules when subjected for extended
times to relatively high temperatures and acid conditions (Hsu, 1996; Wyman et al., 2005). The hydrolysis
reaction rate accelerates when either a chemical or an
enzymatic catalyst is used and when the material to by
hydrolyzed is concentrated.
Enzyme hydrolysis is highly specific and relatively
fast. Using an enzyme to act on its target polysaccharide
will convert it rapidly into its component monomers.
Additionally, this will convert the insoluble polysaccharide into a soluble monomer. Enzymatic hydrolysis is
best applied after other pretreatment methods that leave
cellulose as a major component. The most common
method of saccharification is enzymatic hydrolysis
following acid hydrolysis (Harun, 2010).
The enzyme cellulase converts cellulose into glucose.
Cellulases are so specific that they only affect cellulose
and do not treat hemicelluloses in the LB (Wang et al.,
2012). There are five general types of cellulases. They
are classified by the reactions they catalyze. These five
cellulases are endocellulases, exocellulases, cellobiases,
oxidative cellulases and cellulose phosphorylases (Bayer
et al., 1998). Table 27.6 summarizes the effectiveness
on the hydrolysis of wheat straw of Cellulase, alphaGlucosidase and Xylananse from T. reesei, A. niger,
and T. longibrochiatum after various pre-treatments.
The high yields and mild conditions are attractive for
commercial applications.
These enzyme structures are complex and can be
found in various bacteria as organized supramolecular
complexes called cellulosomes (Bayer et al., 1998). These
enzymes are commonly found in fungi such as Trichoderma reesei and Aspergillus niger and in bacteria such
as Clostridium cellulovorans (Arai et al., 2006). These
source organisms are either aerobic or anaerobic and
Sugar Yield in the Enzymatic Hydrolysis of Wheat Straw after Various Pretreatments
Pretreatment
Enzymes Mixture
Source of Enzyme
Hydrolysis
Condition
Sugar Yield,
g/g-DM
% Max.
Theoretical
Dilute H2SO4
Impregnation þ
Cellulase
a-Glucosidase
T. reesei
A. niger
40 C; pH 5.0
96 h
0.612
99.6
0.75% (v/v)
H2SO4,
121 C
Cellulase
a-Glucosidase
Xylananse
T. reesei
A. niger
T. longibrochiatum
45 C; pH 5.0
72 h
0.565
74
2.15% (v/v)
H2O2
35 C
Cellulase
a-Glucosidase
Xylananse
T. reesei
A. niger
T. longibrochiatum
45 C; pH 5.0
120 h
0.672
96.7
Fine Grinding þ Wet
Oxidation
Cellulase
a-Glucosidase
T. reesei
A. niger
50 C; pH 5.0
24 h
0.638
92
Source: Talebnia et al., 2010.
BIOCONVERSIONdCONVERTING SUGARS TO PRODUCTS
are either mesophilic or thermophilic. Commercial production of cellulase is focused on fungal sources because
bacterial sources tend to be anaerobic and thus are slow
to grow (Duff and Murray, 1996).
It appears that at least three classes of enzymes act
together, synergistically, to hydrolyze cellulose: endocellulase, exocellulase, and cellobiase. Endocellulase (EC
3.2.1.4) randomly breaks internal (b-D-1,4) bonds at
amorphous sites that create new chain ends. Exocellulase (EC 3.2.1.91) cleaves two to four units from the
ends of the exposed chains produced by the endocellulase and results in tetrasaccharides or disaccharides.
Lastly, the cellobiase (EC 3.2.1.21), otherwise known as
b-glucosidase, hydrolyzes the exocellulase products
into individual monosaccharides (Coughlan and Ljungdahl, 1988; Galbe and Zacchi, 2002; Rabinovich et al.,
2002; Zhang et al., 2006).
The cellulase action occurs in three steps. The first is
adsorption of cellulase onto the surface of the cellulose.
The second is biodegradation of cellulose into fermentable sugars. Lastly, desorption of cellulase occurs
completing the catalytic cycle.
Enzyme activity is affected by a variety of environmental and substantive conditions. Temperature and
pH are known to affect enzyme activity. Most cellulose
enzymes show an optimum activity at temperatures in
the range of 45e55 C and at pH values between 4 and
5 (Galbe and Zacchi, 2002). For LB applications, the
optimum pH is shifted upward to between 5 and 6.5
due to the presence of lignin in the system (Lucas
et al., 2012). These are mild operational conditions.
These mild conditions lower the overall operational
costs compared to purely chemical hydrolysis methods.
Additionally, substrate concentration, product concentration, activators, inhibitors and cellulose structure
are also significant determiners of enzyme effectiveness
(Detroy and Julian, 1982).
Cellobiase is itself an inhibitor to endo- and exocellulases. Thus, the b-glucosidase activity is crucial for the
efficiency of the hydrolysis process (Coughlan and
Ljungdahl, 1988; Galbe and Zacchi, 2002; Rabinovich
et al., 2002; Zhang et al., 2006).
The structure of cellulose affects the rate of hydrolysis. The cellulose features known to affect the rate of hydrolysis include (1) molecular structure of cellulose, (2)
crystallinity of cellulose, (3) surface area of
cellulose fiber, (4) degree of swelling of cellulose fiber,
(5) DP, and (6) associated lignin or other materials (Detroy and Julian, 1982). The purer and more refined the
cellulose is, the more ideal the cellulase activity will
be. Higher enzyme activity lowers the enzyme load
and cost for the enzymatic hydrolysis process.
Lastly, even under ideal conditions, the activity of the
cellulase enzyme is affected by the age of the enzyme itself. The overall activity of the enzyme decreases rapidly
477
and slows the rate of enzymatic hydrolysis. There is
currently much research devoted to improving the overall yield and maintaining a high rate of hydrolysis (Sun
and Cheng, 2002).
Supplementing cellulase enzymes with other enzymes is another area of current focus. Conjugating
the action of cellulases and hemicellulases is known to
increase the rate of enzymatic hydrolysis and result in
an overall higher sugar yield. Cellulose is a homopolysaccharide, hemicelluloses are heteropolysaccharides.
To obtain a more complete hydrolysis of LB one must
consider a multiple-enzyme system and reap the yield
of the combined activities.
BIOCONVERSIONdCONVERTING
SUGARS TO PRODUCTS
Following hydrolysis, converting the resultant
sugars to products is the next step. Fermentation is a biological option and is the focus of this section. Both
chemical/catalytic and biochemical conversions are
common.
At this point, the pretreatment and hydrolysis activities were designed and executed all with the intent of
optimizing and preparing for fermentation, the capstone
process of the bioconversion (Gamage et al., 2010).
Fermentation is referred to as anaerobic digestion.
Fermentation is the chemical breakdown of a substance
by bacteria, yeasts, or other microorganisms to produce
ethanol or other alcohols, lactic acid, lactose, and
hydrogen (Chandel et al., 2007; Wheals et al., 1999).
One of the most significant factors in fermentation is
the choice of organism or modification to an organism to
acquire a desired product. Some organisms only metabolize hexoses while others may metabolize both hexoses
and pentoses. Saccharomyces cerevisiae is an old and very
popular strain of yeast used throughout the food and
fuel industries. When added to a batch of material,
it will metabolize the glucose component, almost exclusively, into ethanol and carbon dioxide. It will generally
follow the Embden-Myerhof pathway under anaerobic
conditions when the temperature is controlled around
30 C (Limayem and Ricke, 2012). S. cerevisiae grows
optimally at this temperature and it also resists high osmotic pressure and it is tolerant of pH as low as 4.0 and it
is tolerant of many inhibitory products (Hahn-Hagerdal
et al., 2007). S. cerevisiae remains popular because of its
high ethanol yield from hexose sugars; it generates
12.0e17.0% w/v, which is 90% of the theoretical
maximum (Bayrock and Ingledew, 2001; Claassen
et al., 1999).
Despite all its great characteristics, S. cerevisiae
cannot metabolize both hexoses and pentoses and
thus it is not a great organism for converting LB. In
478
27. DEVELOPMENT OF THERMOCHEMICAL AND BIOCHEMICAL TECHNOLOGIES FOR BIOREFINERIES
LB, there is a significant portion of the hydrolysate
containing hemicelluloses, pentose sugars such as
D-xylose, which may potentially enhance yields
(Martin et al., 2002). Identifying and employing an
optimal organism is a great opportunity in fermentation. The optimal organism ought to be high yielding,
able to metabolize both hexose and pentose sugars,
tolerant to high ethanol concentration and tolerant to
chemical inhibitors left over from pretreatment and
hydrolysis. There are numerous naturally occurring
organisms that possess a subset of these characteristics,
but none are ideal. To develop a more advantageous
organism one might have to genetically modify an
organism to achieve one’s goals. Table 27.7 lists several
naturally occurring organisms and their features and
liabilities (Limayem and Ricke, 2012).
Reducing operating costs and product inhibition is
another important goal. There are strategies that
combine hydrolysis and fermentation together. Simultaneous saccharification and fermentation (SSF) is one
strategy that has just that in mind. The needed enzyme(s) and the corresponding organisms are added
together so that enzymatic saccharification of cellulose
and subsequent fermentation of the resultant sugars
takes place at the same time in the same reactor
(Dowe and McMillan, 2008). However, SSF requires
an overall compromise between saccharification and
fermentation, usually resulting in a less optimum operation. Another strategy is to employ an organism that
is capable of making its own enzymes for hydrolysis
and of fermenting the resultant sugars. Consolidated
bioprocessing lowers the cost of bioconversion by
reducing enzymatic saccharification and fermentation
into a single step and eliminates the need for cellulase
enzymes (Ladisch et al., 2010; Lynd et al., 2005).
Despite the number of prokaryotic and eukaryotic
microorganisms that convert sugars to ethanol, most
remain limited in terms of cofermentation, ethanol
yields, and tolerance to chemical inhibitors, high
temperatures and ethanol.
THERMOCHEMICAL CONVERSION
The process of converting LB to products using primarily heat as the engine of conversion is thermochemical conversion. Thermochemical processing appears
more promising than bioconversion of the lignin fraction of the LB in that it serves as a source of process
energy and the coproducts have benefits in a life-cycle
context; however, it has a detrimental effect on enzymatic hydrolysis (Lynd et al., 1999, 2005; Lynd and
Wang, 2004). This method differentiates on how much
air is supplied to the conversion, as shown in Figure 27.7.
If LB is heated in the presence of excessive amounts of
air, specifically oxygen, then the biomass will combust.
If the amount of air or elements of air is limited then
gasification will occur. Lastly, if no air is allowed then
pyrolysis or hydrothermal liquefaction is the outcome.
Combustion
Combustion is a result of a complicated network of
exothermic chemical reactions. The reaction generates
copious amounts of heat and radiation. The reaction
tends to be self-perpetuating and continues spontaneously due to the large amount of heat generated by the
reaction. Specifically, combustion is when carbon,
hydrogen, combustible sulfur, and nitrogen in LB react
with oxygen. The process includes fusion, evaporation,
pyrolysis, a gas phase, and surface reactions.
Combustion of solids can take place in many forms
including evaporation combustion, decomposition combustion, surface combustion, and smoldering combustion. Evaporation combustion is when fuel, with a
relatively low fusing point, fuses and evaporates by
heating, and reacts with gaseous oxygen and burns.
When gasses such as H2, CO, CmHn, H2O, and CO2 are
produced by thermal decomposition and react with
O2, it is called decomposition combustion. A common
by-product of evaporation and decomposition combustion is char. Char burns by surface combustion. Surface
combustion occurs when the material only contains carbon and small amounts of volatile compounds such as
charcoal, oxygen, carbon dioxide, or steam. When these
compounds diffuse into pores inside or on the surface of
the solid they burn in a reaction of the surface of the material. Lastly, smoldering combustion is a slower and
lower temperature reaction. It is a form of thermal
decomposition that takes place at a temperature below
the ignition temperature of the volatile components of
the LB. If the temperature is forced up, smoke might
be produced or the reaction may ignite. If it ignites the
reaction is referred to as flammable combustion. Industrial LB conversion processes commonly employ decomposition or surface combustion.
Gasification
Gasification is the conversion of LB from its solid
form to fuel gas or syngas. Syngas is simply a chemical
feedstock in its gas phase. These might be CO, H2, CH4,
CO2 and N2 as well as char (Balat, 2008b; Demirbas,
2004; Li and Suzuki, 2009).
Gasification is a broad treatment method and produces a variety of different results depending on how
it is controlled. Manipulating pressure, temperature,
heating method, and conversion agent produces specific
results. Pressure is usually controlled for either normal
pressure (0.1e0.12 MPa) or high-pressure conditions
TABLE 27.7
Advantages and Drawbacks of Potential Organisms in Lignocellulosic-Based Bioethanol Fermentation
Characteristics
Advantages
Drawbacks
References
Saccharomyces cerevisiae
Facultative anaerobic yeast
• Naturally adapted to ethanol
fermentation
• High alcohol yield (90%)
• High tolerance to ethanol (up
to 10% v/v) and chemical
inhibitors
• Amenability to genetic
modifications
• Not able to ferment xylose
and arabinose sugars
• Not able to survive high
temperature of enzyme
hydrolysis
(Gamage et al., 2010; HahnHagerdal et al., 2007; Jorgensen,
2009; McMillan, 1994; Rogers
et al., 2007; Talebnia et al., 2010)
Candida shehatae
Microaerophilic yeast
• Ferment xylose
• Low tolerance to ethanol
• Low yield of ethanol
• Require microaerophilic
conditions
• Does not ferment xylose
at low pH
(Banerjee et al., 2009; Ligthelm
et al., 1988; McMillan, 1994; Sun
et al., 2011; Zaldivar et al., 2001)
Zymomonas mobilis
Ethanologenic gram-negative
bacteria
• Ethanol yield surpasses
S. cerevisiae (97% of the
theoretical)
• High ethanol tolerance
(up to 14% v/v)
• High ethanol productivity
(fivefold more than S. cerevisiae
volumetric productivity)
• Amenability to genetic
modification
• Does not require additional
oxygen
• Not able to ferment xylose
sugars
• Low tolerance to inhibitors
• Neutral pH range
(Balat and Balat, 2008; Herrero,
1983; Liu et al., 2010; McMillan,
1994)
Pichia stipites
Facultative anaerobic yeast
• Best performance xylose
fermentation
• Ethanol yield (82%)
• Intolerant to a high
concentration of ethanol
above 40 g/L
(Jeffries et al., 2007; McMillan,
1994; Nigam, 2001; Shupe and
Liu, 2012; Zaldivar et al., 2001)
479
(Continued)
THERMOCHEMICAL CONVERSION
Species
Advantages and Drawbacks of Potential Organisms in Lignocellulosic-Based Bioethanol Fermentationdcont’d
Species
Characteristics
Drawbacks
• Able to ferment most of
cellulosic-material sugars
including glucose, galactose,
and cellobiose
• Possess cellulase enzymes
favorable to SSF process
• Does not ferment xylose
at low pH
• Sensitive to chemical inhibitors
• Requires microaerophilic
conditions to reach peak
performance
• Reassimilates formed ethanol
• Low yield of ethanol
• Require microaerophilic
conditions
• Does not ferment xylose at low
pH
References
Pachysolen tannophilus
Aerobic fungus
• Ferment xylose
Escherichia coli
Mesophilic gram-negative
bacteria
• Ability to use both pentose and
hexose sugars
• Amenability for genetic
modifications
• Repression catabolism
interfere to cofermentation
• Limited ethanol tolerance
• Narrow pH and temperature
growth range
• Production of organic acids
• Genetic stability not proved
yet
• Low tolerance to inhibitors
and ethanol
(Gamage et al., 2010; Liu et al.,
2010; Weber and Boles, 2010;
Zayed and Meyer, 1996)
Kluveromyces marxianus
Thermophilic yeast
• Able to grow at a high
temperature above 52 C
• Suitable for SSF/CBP process
• Reduces cooling cost
• Reduces contamination
• Ferments a broad spectrum of
sugars
• Amenability to genetic
modifications
• Excess of sugars affect its
alcohol yield
• Low ethanol tolerance
• Fermentation of xylose is poor
and leads mainly to the
formation of xylitol
(Banat et al., 1992; Kumar et al.,
2009b; Weber et al., 2010)
Thermophilic bacteria
• Thermoanaerobacterium
saccharolyticum
• Thermoanaerobacter ethanolicus
• Clostridium thermocellum
Extreme anaerobic bacteria
• Resistance to an extremely
high temperature of 70 C
• Suitable for SSCombF/CBP
processing
• Ferment a variety of sugars
• Display cellulolytic activity
• Amenability to genetic
modification
• Low tolerance to ethanol
(Georgieva et al., 2008; Kumar
et al., 2009b; Lynd et al., 2002;
Shaw et al., 2008; Zeikus et al.,
1981)
CBP, consolidated bioprocessing.
(Zaldivar et al., 2001; Zayed and
Meyer, 1996)
27. DEVELOPMENT OF THERMOCHEMICAL AND BIOCHEMICAL TECHNOLOGIES FOR BIOREFINERIES
Advantages
480
TABLE 27.7
481
THERMOCHEMICAL CONVERSION
FIGURE 27.7
Overview of thermochemical conversion technologies. Source:
Bain, 2004.
Thermochemical
conversion
Excess air
combustion
Partial air
gasification
No air
pyrolysis
hydrothermal liquefaction
(0.5e2.5 MPa). Temperatures are usually either operated
under low-temperature (<700 C) or high-temperature
(>700 C) conditions. High-temperature decomposition
may occur at the ash fusion point or above. Indirect
gasification occurs when heating the raw material
and gasification agent using an external heat source.
Direct gasification occurs when heat generated from
the reaction of partial gasification of raw material and
oxygen is used as the heating source. The gasification
agent is another variable with significant influence on
the product. An agent is any combination of air, oxygen,
or steam. Additionally, carbon dioxide may be used for a
set period of time to affect the product.
There are a variety of gasifiers in use today. Fixed bed,
flow bed, circulating flow bed, entrained bed, mixing
bed, rotary kiln, twin tower, and molten furnace are
examples of industrial gasifiers (Yokoyama, 2008).
Another method, supercritical water gasification
(SCWG), is interesting because water under supercritical
conditions has properties that facilitate the transport
processes of compounds while remaining a benign media for processing. It even acts as a catalyst for acid/
base reactions under these conditions (Calzavara et al.,
2005; Nolen et al., 2003; Savage et al., 1995). Of note in
SCWG is that it takes place in either high- or lowtemperature conditions (Matsumura et al., 2005). However, if one adds an alkali catalyst to the processing at
low temperatures, it increases the conversion into oil
and gas. Additionally, the catalyst lowers the temperature at which the cellulose decomposes, or the onset of
the gasification process (Minowa et al., 1997, 1995,
1998a,b, Murakami et al., 2012).
Pyrolysis
The conversion of LB by heating is pyrolysis (Balat,
2008a; Bridgwater, 2003; Mohan et al., 2006). Depending
on the desired product, one chooses the operational conditions for pyrolysis. Factors such as heating rate,
reactor temperature, residence time and composition
of the feedstock determine the product. The goal of pyrolysis is to execute the process in the absence of oxygen
and thus avoid the burning of the feedstock and instead
break down the lignocellulosic bonds and crystalline
structure. By doing so under these conditions, new compounds are formed. Compounds such as char, bio-oil,
and gasses are produced (Thuijl et al., 2003). The biooil formed by pyrolysis is not easily stored because it
is unstable and requires additional processing prior to
long-term storage (Adam, 2005).
There are three categories of pyrolysis: conventional,
fast and flash. Conventional pyrolysis produces solids,
liquids and gasses. Fast and flash pyrolysis produces
primarily liquid and gaseous products (Demirbas, 2005).
Direct Liquefaction
Direct conversion of LB to biofuels is liquefaction.
Typical products include biodiesel and heavy oils that
are typically very viscous. Adding an alkali to the conversion will enhance the liquefaction process (Itoh
et al., 1994; Demirbas, 2005). Hydrothermal liquefaction
is an application where thermal depolymerization, or
hydrous pyrolysis, is accomplished using superheated
water under pressure.
Drying feedstock in unnecessary when using hydrothermal liquefaction. Consequently, it is suitable for converting any biomass regardless of its moisture content.
Aquatic biomass, garbage, organic sludge as well as
LB are all good feedstock candidates for hydrothermal
liquefaction.
At the boiling point of water, 100 C, extraction of
aqueous soluble components is possible. At temperatures above 150 C hydrolysis begins and biomass
polymers, such as cellulose, hemicellulose, proteins,
and so on, degrade into monomers. Then at 200 C
and 1 MPa solidlike biomass changes into a slurry, a
process called liquidization. At higher temperatures
below the critical point of water, around 300 C and
10 MPa, liquefaction takes place and oily product is
obtained. If one changes the reaction conditions such
as reaction time or the catalyst, the main product can
be changed to char, a process referred to as hydrothermal carbonization. Finally, at a temperature around the
critical point and in the presence of a catalyst, the
biomass will gasify.
482
27. DEVELOPMENT OF THERMOCHEMICAL AND BIOCHEMICAL TECHNOLOGIES FOR BIOREFINERIES
CONCLUSION
Utilizing LB for energy and fuel production is as old
as mankind. However, modern sources of fuel are
more cost-effective and convenient to drive and meet
our contemporary energy demands. Liberating carbon
that has accumulated over millennia in such an unnaturally condensed period of time threatens to alter current
climate conditions. Our economic engine has created dependencies on fossil fuels and has encouraged unhealthy
relationships between highly industrialized societies like
the United States and China and some energy suppliers
around the world. The cycle of growing and using LB for
fuel and energy is a closed and therefore, sustainable system that consumes as much carbon dioxide as is liberated. Finding technologies that can return modernized
societies around the globe to more sustainable and
self-reliant sources of energy is critical to reduce the environmental impact and improve national security and
sovereignty. To that end, this chapter has presented
and reviewed a wide array of biochemical and thermochemical LB conversion options. These methods have
developed into capable methods of converting LB into
fermentable intermediates such as sugars or products.
Much development has already taken place and there
is still much more needed until utilizing LB for energy
and fuel can compete with and replace more convenient
and less expensive sources of fuel.
Industrializing the process of converting LB into
valuable materials is the function of a biorefinery and
it employs a complex and diverse set of conversion technologies to accomplish its tasks. There are several pretreatment options available that either exploit thermal,
mechanical and chemical mechanisms or use biological
and chemical mechanisms. Ultimately the treatments
chosen will depend on the desired product and desired
specifications. These products may include liquid biofuels, biochemicals as well as steam, heat and electricity.
Regardless, the objectives of the pretreatment are the
same: to break down the strong crystalline lignin and
cellulosic structures such that the biomass becomes
vulnerable to conversion processes, such as hydrolysis
treatment that yields fermentable sugars that are free
from microbial growth inhibitors, or a thermochemical
conversion such as gasification or liquefaction. No treatment option is ideal. There are often trade-offs between
competing features and liabilities such as high yield and
long retention time, low concentration of inhibitors and
high cost, significant environmental and safety concerns
and high efficacy.
The biochemical and thermochemical pretreatments
and conversions have developed and improved over
time independently and in conjunction with a series of
pretreatment methods. The goal of the development of
these pretreatments is to improve the quantity and quality of materials available to conversion, whether it is
bioconversion or thermochemical conversion. In order
to compete with contemporary and convenient sources
of energy it is important to maximize the pretreatment
and conversion processes as well as to eliminate and
recycle waste and energy.
The biorefinery holds great promise to enable the usage of biomass as a sustainable and reliable source of energy and fuel. The biochemical and thermochemical
conversion options described here are by no means an
exhaustive representation of all the studied methods.
Many more exist in the literature and maybe the best
methods are yet to come.
References
Adam, J., 2005. Catalytic Conversion of Biomass to Produce Higher
Quality Liquid Bio-fuels. Norwegian University of Science and
Technology, Trondheim, Norway, pp. 142.
Alvira, P., Tomas-Pejo, E., Ballesteros, M., Negro, M.J., 2010. Pretreatment technologies for an efficient bioethanol production process
based on enzymatic hydrolysis. Bioresour. Technol. 101, 4851e4861.
Amidon, T.E., Wood, C.D., Shupe, A.M., Wang, Y., Graves, M., Liu, S.,
2008. Biorefinery: conversion of woody biomass to chemicals,
energy and materials. J. Biobased Mater. Bioenergy 2, 100e120.
Arai, T., Kosugi, A., Chan, H., Koukiekolo, R., Yukawa, H., Inui, M.,
Doi, R.H., 2006. Properties of cellulosomal family 9 cellulases from
Clostridium cellulovorans. Appl. Microbiol. Biotechnol. 71, 654e660.
Auslender, V.L., Ryazantsev, A.A., Spiridonov, G.A., 2002. The use of
electron beam for solution of some ecological problems in pulp
and paper industry. Radiat. Phys. Chem. 63, 641e645.
Azzam, A.M., 1989. Pretreatment of cane bagasse with alkaline
hydrogen peroxide for enzymatic hydrolysis of cellulose and
ethanol fermentation. J. Environ. Sci. Health, Part B 24, 421e433.
Bain, R.L., 2004. An introduction to biomass thermochemical conversion. In: Laboratory, N.R.E. (Ed.), DOE/NASLUGC Biomass and
Solar Energy Workshops. US Department of Energy.
Balat, M., 2008a. Mechanisms of thermochemical biomass conversion process. Part 1: reactions of pyrolysis. Energy Sources, Part
A 30, 620.
Balat, M., 2008b. Mechanisms of thermochemical biomass conversion
processes. Part 2: reactions of gasification. Energy Sources, Part A
30, 636.
Balat, M., Balat, H., 2008. Progress in bioethanol processing. Prog.
Energy Combust. Sci. 34, 551e573.
Ballesteros, I., Oliva, J.M., Navarro, A.A., Gonzalez, A., Carrasco, J.,
Ballesteros, M., 2000. Effect of chip size on steam explosion pretreatment of softwood. Appl. Biochem. Biotechnol. 84e86, 97e110.
Ballesteros, I., Oliva, J.M., Negro, M.J., Manzanares, P., Ballesteros, M.,
2002. Enzymic hydrolysis of steam exploded herbaceous agricultural waste (Brassica carinata) at different particle sizes. Process
Biochem. 38, 187e192.
Banat, I., Nigam, P., Marchant, R., 1992. Isolation of thermotolerant,
fermentatitve yeasts growing at 52 C and producing ethanol at
45 C and 52 C. World J. Microbiol. Biotechnol. 8, 259e263.
Banerjee, S., Mudliar, S., Sen, R., Giri, B., Satpute, D., Chakrabarti, T.,
Pandey, R.A., 2009. Commercializing lignocellulosic bioethanol:
technology bottlenecks and possible remedies. Biofuels, Bioprod.
Biorefin. 4, 77e93.
Barber, V.A., 2007. State University of New York, College of Environmenatl Science and Forestry.
REFERENCES
Bayer, E.A., Chanzy, H., Lamed, R., Shoham, Y., 1998. Cellulose,
cellulases and cellulosomes. Curr. Opin. Struct. Biol. 8, 548e555.
Bayrock, D., Ingledew, W.M., 2001. Changes in steady state on
introduction of a Lactobacillus contaminant to a continuous culture ethanol fermentation. J. Ind. Microbiol. Biotechnol. 27,
39e45.
Ben-Ghedalia, D., Miron, J., 1981. The effect of combined chemical and
enzyme treatments on the saccharification and in vitro digestion
rate of wheat straw. Biotechnol. Bioeng. 23, 823e831.
Betiku, E., Adetunji, O.A., Ojumu, T.V., Solomon, B.O., 2009. A
comparative study of the hydrolysis of gamma irradiated lignocelluloses. Braz. J. Chem. Eng. 26, 251.
Bisaria, V., Ghose, T., 1981. Biodegradation of cellulosic materials:
substrate, microorganisms, enzymes, and products. Enzyme
Microb. Technol. 3, 90e104.
Bjerre, A.B., Olesen, A.B., Fernqvist, T., Ploger, A., Schmidt, A.S., 1996.
Pre-treatment of wheat straw using combined wet oxidation and
alkaline hydrolysis resulting in convertible cellulose and hemicellulose. Biotechnol. Bioeng. 49, 568e577.
Boerjan, W., Ralph, J., Baucher, M., 2003. Lignin biosynthesis. Annu.
Rev. Plant Biol. 54, 519e546.
Bohlmann, G.M., 2006. Process economic considerations for production of ethanol from biomass feedstocks. Ind. Biotechnol. 2, 14e20.
Bouchard, J., Methot, M., Jordan, B., 2006. The effect of ionizing
radiation on the cellulose of wood-free paper. Cellulose 13,
601e610.
Brasch, D.J., Free, K.W., 1965. Prehydrolysis-kraft pulping of Pinus
radiata grown in New Zealand. Tappi 48, 245e248.
Bridgwater, A., 2003. Renewable fuels and chemicals by thermal
processing of biomass. Chem. Eng. J. 91, 87e102.
Brownell, H.H., Yu, E.K.C., Saddler, J.N., 1986. Steam-explosion pretreatment of wood: effect of chip size, acid, moisture content and
pressure drop. Biotechnol. Bioeng. 28, 792e801.
Bura, R., Chandra, R., Saddler, J., 2009. Influence of xylan on the
enzymatic hydrolysis of steam-pretreated corn stover and hybrid
poplar. Biotechnol. Prog. 25 (2), 315e322.
Cadoche, L., Lopez, G.D., 1989. Assessment of size reduction as a
preliminary step in the production of ethanol from lignocellulosic
wastes. Biol. Wastes 30 (2), 153e157.
Calzavara, Y., Joussot-Dubien, C., Boissonnet, G., Sarrade, S., 2005.
Evaluation of biomass gasification in supercritical water process
for hydrogen production. Energy Convers. Manage. 46, 615e631.
Chandel, A.K., Chan, E., Rudravaram, R., Narasu, M.L., Rao, L.V.,
Ravindra, P., 2007. Economics and environmental impact of bioethanol production technologies: an appraisal. Biotechnol. Mol.
Biol. Rev. 2, 14e32.
Chaudhary, G., Singh, L.K., Ghosh, S., 2012. Alkaline pretreatment
methods followed by acid hydrolysis of Saccharum spontaneum for
bioethanol production. Bioresour. Technol. 124, 111e118.
Chen, H.Z., Qiu, W.H., 2010. Key technologies for bioethanol production from lignocellulose. J. Biotechnol. Adv. 28, 556e562.
Chen, Y., Dong, B., Qin, W., Xiao, D., 2010. Xylose and cellulose
fractionation from corncob with three different strategies and
separate fermentation of them to bioethanol. Bioresour. Technol.
101 (18), 6994e6999.
Claassen, P.A.M., Lopez-Contreras, A.M., Sijtsma, L., Weusthuis, R.A.,
van Lier, J.B., van Niel, E.W.J., Stams, A.J.M., De Vries, S.S., 1999.
Utilization of biomass for the supply of energy carriers. Appl.
Microbiol. Biotechnol. 52, 741e755.
Conner, A.H., Lorenz, L.F., 1986. Kinetic modeling of hardwood prehydrolysis. 3. Water and dilute acetic-acid prehydrolysis of
southern red oak. Wood Fiber Sci. 18 (5), 248e263.
Coughlan, M.P., Ljungdahl, L.G., 1988. Comparative biochemistry of
fungal and bacterial cellulolytic enzyme system. FEMS Symp. 43,
11e30.
483
Cullis, I.F., Saddler, J.N., Mansfield, S.D., 2004. Effect of initial moisture content and chip size on the bioconversion efficiency of softwood lignocellulosics. Biotechnol. Bioeng. 85, 413e421.
Cybulska, I., Brudecki, G., Rosentrater, K., Julson, J.L., Lei, H., 2012.
Comparative study of organosolv lignin extracted from prairie
cordgrass, switchgrass and corn stover. Bioresour. Technol. 118,
30e36.
D’Andola, G., Szarvas, L., Massonne, K., Stegmann, V., 2008. Ionic
liquids for solubilizing polymers. WO Pat. 2008/043837.
Dale, B.E., Henk, L.L., Shiang, M., 1984. Fermentation of lignocellulosic materials treated by ammonia freeze-explosion. Dev. Ind.
Microbiol. 26, 223e233.
Dashtban, M., Schraft, H., Qin, W., 2010. Fungal bioconversion of
lignocellulosic residues: opportunities & perspectives. Int. J. Biol.
Sci. 5, 578e595.
Demirbas, A., 2004. Current technologies for thermo-conversion of
biomass into fuels and chemicals. Energy Sources 26, 715e730.
Demirbas, A., 2005. Bioethanol from cellulosic materials: a renewable
motor fuel from biomass. Energy Sources 21, 327e337.
Detroy, R.W., Julian, G.S., 1982. Biomass conversion: fermentation
chemicals and fuels. Crit. Rev. Microbiol. 10, 203e228.
Dowe, N., McMillan, J., 2008. SSF experimental protocols - lignocellulosic biomass hydrolysis and fermentation. In: Office of Energy
Efficiency and Renewable Energy NREL. Midwest Research Insitute, Battelle, Golden, CO.
Drapcho, C., Nhuan, N., Wlaker, T., 2008. Biofuel Engineering Process Technology. McGraw Hill Companies, Inc, New York, NY,
pp. 371.
Dubey, K.A., Pujari, P.K., Rammani, S.P., Kadam, R.M., Sabharwal, S.,
2004. Microstructural studies of electron beam-irradiated cellulose
pulp. Radiat. Phys. Chem. 69, 395.
Duff, S.J.B., Murray, W.D., 1996. Bioconversion of forest products industry waste cellulosics to fuel ethanol: a review. Bioresour.
Technol. 55, 1e33.
Esteghlalian, A., Hashimoto, A.G., Fenske, J.J., Penner, M.H., 1997.
Modeling and optimization of the dilute-sulfuric-acid pretreatment of corn stover, poplar and switchgrass. Bioresour. Technol.
59, 129e136.
Ewanick, S.M., Bura, R., Saddler, J.N., 2007. Acid-catalyzed steam pretreatment of lodgepole pine and subsequent enzymatic hydrolysis
and fermentation to ethanol. Biotechnol. Bioeng. 98, 737e746.
Fengel, D.W.G., 1984. Wood: Chemistry, Ultrastructure, Reactions.
Walter de Gruyter, Berlin and New York.
Fengel, D.W.G., 1989. Wood: Chemistry, Ultrastructure, Reactions.
Walter de Gruyter, New York.
Fort, D.A., Remsing, R.C., Swatloski, R.P., Moyna, P., Moyna, G.,
Rogers, R.D., 2007. Can ionic liquids dissolve wood? Processing
and analysis of lignocellulosic materials with 1-n-butyl3-methylimidazolium chloride. Green Chem. 9, 63e69.
Galbe, M., Zacchi, G., 2002. A review of the production of ethanol
from softwood. Appl. Microbiol. Biotechnol. 59, 618e628.
Gamage, J., Lam, H., Zhang, Z., 2010. Bioethanol production from
lignocellulosic biomass. J. Biobased Mater. Bioenergy 4, 3e11.
Gamez, S., Ramirez, J., Garrote, G., Vazquez, M., 2004. Manufacture of
fermentable sugar solutions from sugarcane bagasse hydrolyzed
with phosphoric acid at atmospheric pressure. J. Agric. Food
Chem. 52, 4172e4177.
Garcı́a-Cubero, M.A.T.M.A., González-Benito, G.G., Indacoechea, I.I.,
Coca, M.M., Bolado, S.S., 2009. Effect of ozonolysis pretreatment on
enzymatic digestibility of wheat and rye straw. Bioresour. Technol.
100 (4), 1608e1613.
Georgieva, T., Mikkelsen, M., Ahring, B., 2008. Ethanol production
from wet exploded wheat straw hydrolysate by thermophilic
anaerobic bacterium Thermoanaerobacter BG1L1 in a continuous
immobilized reactor. Appl. Biochem. Biotechnol. 145, 99e110.
484
27. DEVELOPMENT OF THERMOCHEMICAL AND BIOCHEMICAL TECHNOLOGIES FOR BIOREFINERIES
Glaudemans, C., Timell, T.E., 1958. The polysaccharides of white Brich
(Betula papyrifera) IV, the constitution of the hemicellulose. J. Am.
Chem. Soc. 80, 1209e1213.
Goring, D., Timell, T., 1960. Molecular properties of six 4-O-methylglucuronoxylans. J. Phys. Chem. 64, 1426e1430.
Gregg, D., Saddler, J.N., 1996a. A technoeconomic assessment of the
pretreatment and fractionation steps of a biomass-to-ethanol process. Appl. Biochem. Biotechnol. 57, 711e727.
Gregg, D.J., Saddler, J.N., 1996b. Factors affecting cellulose hydrolysis
and the potential of enzyme recycle to enhance the efficiency of an
integrated wood to ethanol process. Biotechnol. Bioeng. 51,
375e383.
Grous, W.R., Converse, A.O., Grethlein, H.E., 1986. Effect of steam
explosion pre-treatment on pore size and enzymatic hydrolysis of
poplar. Enzyme Microb. Technol. 8, 274e280.
Gupta, R., Lee, Y., 2009. Pretreatment of hybrid poplar by aqueous
ammonia. Biotechnol. Prog. 25, 357e364.
Hahn-Hagerdal, B., Karhumaa, K., Fonseca, C., Spencer-Martins, I.,
Gorwa-Grauslund, M.F., 2007. Towards industrial pentosefermenting yeast strains. Appl. Microbiol. Biotechnol. 74,
937e953.
Hamelinck, C.N., van Hooijdonk, G., Faaij, A.P.C., 2003. Prospects for
Ethanol from Lignocellulosic Biomass: Techno-economic Performance as Development Progresses. Utrecht University. Report nr
NWS-E-2003-55, p. 35.
Harun, R., Jason, W.S.Y., Cherrington, T., Danquah, M.K., 2010.
Biomass as a cellulosic fermentation feedstock for bioethanol production. Renewable Sustainable Energy Rev. http://dx.doi.org/
10.1016/j.rser.2010.07.071.
Hendriks, A.T., Zeeman, G., 2009. Pretreatments to enhance the
digestibility of lignocellulosic biomass. Bioresour. Technol. 100,
10e18.
Herrero, A., 1983. End product inhibition in anaerobic fermentation.
Trends Biotechnol. 1, 49e53.
Hinman, N.D., Schell, D.J., Riley, C.J., Bergeron, P.W., Walter, P.J., 1992.
Preliminary estimate of the cost of ethanol production for SSF
technology. Appl. Biochem. Biotechnol. 34, 639e649.
Holtzapple, M.T., Humphrey, A.E., Taylor, J.D., 1989. Energy requirements for the size reduction of poplar and aspen wood.
Biotechnol. Bioeng. 33, 207e210.
Holtzapple, M.T., Jun, J. H., Ashok, G., Patibandla, S.L., Dale, B.E.,
1990. Ammonia fiber explosion (AFEX) pretreatment of lignocellulosic wastes. In: Proceedings of the American Institute of
Chemical Engineers National Meeting. Chicago, IL, USA.
Holtzapple, M.T., Jun, J.H., Ashok, G., Patibandla, S.L., Dale, B.E.,
1991. The ammonia freeze explosion (AFEX) process: a practical
lignocellulosic pretreatment. Appl. Biochem. Biotechnol. 28,
59e74.
Holtzapple, M.T., Davison, R.R., Stuart, E.D., 1992. Biomass refining
process. USA Patent 5, 171, 592.
Hon, D.N.S., Shiraishi, N., 2001. In: Wood and Cellulose Chemistry,
second ed. Marcel Dekker Inc, New York.
Hsu, T.A., 1996. Pretreatment of biomass. In: Wyman, C.E. (Ed.),
Handbook on Bioethanol, Production, and Utilization. Taylor &
Francis, Washington, D. C.
Hu, G., Heitmann, J.A., Rojas, O.J., 2008. Feedstock pretreatment
strategies for producing ethanol from wood, bark and forest residues. Bioresources 3 (1), 270e294.
Hunter, C.A., Sanders, K.M., 1990. The nature of pep interaction.
J. Am. Chem. Soc. 112, 5525e5534.
Itoh, S., Suzuki, A., Nakamura, T., Yokoyama, S., 1994. Production
of heavy oil from sewage sludge by direct thermochemical
liquefaction. Proceedings of the IDA and WRPC World Conference on Desalination and Water Treatment. 98 (1e3),
127e133.
Iyer, P.V., Wu, Z.W., Kim, S.B., Lee, Y.Y., 1996. Ammonia recycled
percolation process for pretreatment of herbaceous biomass. Appl.
Biochem. Biotechnol. 57-58, 121e132.
Jeffries, T.W., Grigoriev, I.V., Grimwood, J., Laplaza, J.M., Aerts, A.,
Salamov, A., 2007. Genome sequence of the lignocellulose bioconverting and xylose-fermenting yeast Pichia stipitis. Nat. Biotechnol. 25 (3), 319e326.
Jin, M., Gunawan, C., Balan, V., Lau, M.W., Dale, B.E., 2012. Simultaneous saccharification and co-fermentation (SSCF) of AFEX(TM)
pretreated corn stover for ethanol production using commercial
enzymes and Saccharomyces cerevisiae 424A(LNH-ST). Bioresour.
Technol. 110, 587e594.
Jorgensen, H., 2009. Effect of nutrients on fermentation of pretreated
wheat straw at very high dry matter content by Saccharomyces
cerevisiae. Appl. Biochem. Biotechnol. 153, 44e57.
Karr, W.E., Holtzapple, T., 2000. Using lime pretreatment to facilitate the
enzymatic hydrolysis of corn stover. Biomass Bioenergy 18, 189e199.
Karthika, K., Arun, A.B., Rekha, P.D., 2012. Enzymatic hydrolysis and
characterization of lignocellulosic biomass exposed to electron
beam irradiation. Carbohydr. Polym. 90 (2), 1038e1045.
Kemppainen, K., Inkinen, J., Uusitalo, J., Nakari-Setälä, T., Siikaaho, M., 2012. Hot water extraction and steam explosion as pretreatments for ethanol production from spruce bark. Bioresour.
Technol. 117, 131e139.
Khan, A.W., Labrie, J.P., McKeown, J., 1986. Effect of electron-beam
irradiation pretreatment on the enzymatic hydrolysis of softwood. Biotechnol. Bioeng. 28, 1449e1453.
Kilpeläinen, I., Xie, H., King, A., Granstrom, M., Heikkinen, S.,
Argyropoulos, D.S., 2007. Dissolution of wood in ionic liquids.
J. Agric. Food Chem. 55, 9142.
Kim, S., Dale, B., 2005. Global potential bioethanol production from
wasted crops and crop residues. Biomass Bioenergy 29, 361e375.
Kim, S., Holtzapple, M., 2006. Delignification kinetics of corn stover in
lime pretreatment. Bioresour. Technol. 97, 778e785.
Kim, K.H., Hong, J., 2001. Supercritical CO2 pretreatment of lignocellulose enhances enzymatic cellulose hydrolysis. Bioresour.
Technol. 77, 139e144.
Klass, D., 1998. Biomass for Renewable Energy, Fuels, and Chemicals.
Academic Press, New York.
Klinke, H., Thomsen, A., Ahring, B., 2004. Inhibition of ethanolproducing yeast and bacteria by degradation products produced
during pretreatment of biomass. Appl. Microbiol. Biotechnol. 66,
10e26.
Koo, B.-W., Min, B.-C., Gwak, K.-S., Lee, S.-M., Choi, J.-W., Yeo, H.,
Choi, I.-G., 2012. Structural changes in lignin during organosolv
pretreatment of Liriodendron tulipifera and the effect on enzymatic
hydrolysis. Biomass Bioenergy 42, 24e32.
Koshijima, T., Timell, T.E., Zinbo, M., 1965. The number-average molecular weight of native hardwood xylans. J. Polym. Sci. Part C:
Polym. Symp. 11, 265e279.
Kumar, A., Singh, L.K., Ghosh, S., 2009a. Bioconversion of lignocellulosic fraction of water-hyacinth (Eichhornia crassipes) hemicellulose acid hydrolysate to ethanol by Pichia stipitis. Bioresour.
Technol. 100 (13), 3293e3297.
Kumar, S., Singh, S., Mishra, I., Adhikari, D., 2009b. Recent advances
in production of bioethanol from lignocellulosic biomass. Chem.
Eng. Technol. 32, 517e526.
Kumar, L., Chandra, R., Chung, P.A., Saddler, J.N., 2010. Can the same
steam pretreatment conditions be used for most softwoods to
achieve good, enzymatic hydrolysis and sugar yields? Bioresour.
Technol. 101, 7827e7833.
Kuznetsov, B.N., Kuznetsova, S.A., Danilov, V.G., Kozlov, I.A.,
Taraban’ko, V.E., Ivanchenko, N.M., Alexandrova, N.B., 2002. New
catalytic processes for a sustainable chemistry of cellulose production from wood biomass. Catal. Today 75, 211e217.
REFERENCES
Ladisch, M., Kohlmann, K., Westgate, P., Weil, J. Yang, Y., 1998. Processes for treating cellulosic material. USA Patent 5, 846, 787.
Ladisch, M., Mosier, N., Kim, Y., Ximenes, E., Hogsett, D., 2010.
Converting cellulose to biofuels. SBE special supplement biofuels.
CEP 106 (3), 56e63.
Larson, K.A., King, M.L., 1986. Evaluation of supercritical fluid extraction
in the pharmaceutical industry. Biotechnol. Prog. 2, 73e82.
Laser, M., Schulman, D., Allen, S.G., Lichwa, J., Antal, M.J., Lynd, L.R.,
2002. A comparison of liquid hot water and steam pretreatments of
sugarcane bagasse for bioconversion to ethanol. Bioresour. Technol. 81, 33e44.
Lau, M., Dale, B., 2008. Cellulosic ethanol production from AFEX-treated
corn stover using Saccharomyces cerevisiae 424(LNH-ST). Proc. Natl.
Acad. Sci. USA. 106 (5), 1368e1373.
Lee, J., 1997. Biological conversion of lignocellulosic biomass to
ethanol. J. Biotechnol. 56 (1), 1e24.
Lee, S.H., Doherty, T.V., Linhardt, J.S., 2009. Ionic liquid-mediated
selective extraction of lignin from wood leading to enhanced
enzymatic cellulose hydrolysis. Biotechnol. Bioeng. 102,
1368e1376.
Li, C., Suzuki, K., 2009. Tar property, analysis, reforming mechanism
and model for biomass gasification - an overview. Renewable
Sustainable Energy Rev. 13, 594.
Li, X., Luo, X., Li, K., Zhu, J.Y., Fougere, J.D., Clarke, K., 2012. Effects of
SPORL and dilute acid pretreatment on substrate morphology, cell
physical and chemical wall structures, and subsequent enzymatic
hydrolysis of lodgepole pine. Appl. Biochem. Biotechnol. 168 (6),
1556e1567.
Liebert, T., 2010. Cellulose solvents-remarkable history, bright future,
cellulose solvents: for analysis, shaping and chemical modification.
In: Liebert, T., Heinze, T., Edgar, K. (Eds.). ACS Symposium Series,
vol. 1033. American Chemical Society, Washington, DC, USA,
pp. 3e54.
Ligthelm, M.E., Prior, B.A., Preez, J.C., 1988. The oxygen requirements
of yeasts for the fermentation of D-xylose and D-glucose to ethanol.
Appl. Microbiol. Biotechnol. 28 (1), 63e68.
Limayem, A., Ricke, S.C., 2012. Lignocellulosic biomass for bioethanol
production: current perspectives, potential issues and future
prospects. Prog. Energy Combust. Sci. 38 (4), 449e467.
Liu, S., 2003. Chemical kinetics of alkaline peroxide brightening of
mechanical pulps. Chem. Eng. Sci. 58, 2229e2244.
Liu, S., 2008. A Kinetic model on autocatalytic reactions in woody
biomass hydrolysis. J. Biobased Mater. Bioenergy 2 (5), 135e147.
Liu, S., 2010. Woody biomass: niche position as a source of sustainable
renewable chemicals and energy and kinetics of hot-water
extraction/hydrolysis. J. Biotech. Adv. 28, 563e582.
Liu, S., 2012. Woody biomass conversion: sustainability and waterbased processes. J. Bioprocess Eng. Biorefin. 1 (1), 6e32.
Liu, C., Wyman, C.E., 2003. The effect of flow rate of compressed hot
water on xylan, lignin and total mass removal from corn stover.
Ind. Eng. Chem. Res. 42, 5409e5416.
Liu, S., Mishra, G., Amidon, T.E., Gratien, K., 2009. Effect of hot-water
extraction of woodchips on the kraft pulping of Eucalyptus
woodchips. J. Biobased Mater. Bioenergy 3 (6), 363e372.
Liu, T., Lin, L., Sun, Z., Hu, R., Liu, S., 2010. Bioethanol fermentation
by recombinant E. coli FBR5 and its robust mutant FBHW using
hot-water wood extract hydrolyzate as substrate. Biotechnol. Adv.
28 (5), 602e608.
Lloyd, T., Wyman, C., 2005. Combined sugar yields for dilute sulfuric
acid pretreatment of corn stover followed by enzymatic hydrolysis
of the remaining solids. Bioresour. Technol. 96, 1967e1977.
Lucas, M., Hanson, S.K., Wagner, G.L., Kimball, D.B., Rector, K.D.,
2012. Evidence for room temperature delignification of wood using
hydrogen peroxide and manganese acetate as a catalyst. Bioresour.
Technol. 119, 174e180.
485
Lynd, L.R., 1996. Overview and evaluation of fuel ethanol from
cellulosic biomass: technology, economics, the environment, and
policy. Annu. Rev. Energy Environ. 21, 403e465.
Lynd, L.R., Wang, M., 2004. A product-nonspecific framework for
evaluating the potential of biomass-based products to displace
fossil fuels. J. Ind. Ecol. 7, 17e32.
Lynd, L.R., Elander, R.T., Wyman, C.E., 1996. Likely features and costs
of mature biomass ethanol technology. Appl. Biochem. Biotechnol.
57, 741e761.
Lynd, L.R., Gerngross, T., Wyman, C., 1999. Biocommodity engineering. Biotechnol. Prog. 15, 777e793.
Lynd, L.R., Weimer, P.J., van Zyl, W.H., Pretorius, I.S., 2002. Microbial
cellulose utilization: fundamentals and biotechnology. Microbiol.
Mol. Biol. Rev. 66, 506e577.
Lynd, L.R., van Zyl, W.H., McBride, J.E., Laser, M., 2005. Consolidated
bioprocessing of cellulosic biomass: an update. Curr. Opin. Biotechnol. 16, 577e583.
Martin, C., Galbe, M., Wahlbom, C.F., Hahn-Hägerdal, B.,
Jönsson, L.J., 2002. Ethanol production from enzymatic hydrolysates of sugarcane bagasses using recombinant xylose - utilizing Saccharomyces cerevisiae. Enzyme Microb. Technol. 31 (2),
274e282.
Marzialetti, T., Olarte, M.B.V., Sievers, C., Hoskins, T.J.C., Agrawal, P.K.,
Jones, C.W., 2008. Dilute acid hydrolysis of loblolly pine: a
comprehensive approach. Ind. Eng. Chem. Res. 47, 7131e7140.
Mason, W.H., 1926. Process and apparatus for disintegration of wood
and the like. USA Patent 1, 578, 609.
Mason, W.H., 1928. Apparatus for the process of explosion fibration of
lignocellulose material. USA Patent 1, 655, 618.
Matsumura, Y., Minowa, T., Potic, B., Kersten, S., Prins, W., van
Swaaij, W.P., van de Beld, B., Elliot, D.C., Neuenschwander, G.G.,
Kruse, A., Antal Jr, M.J., 2005. Biomass gasification in near- and
super-critical water: status and prospects. Biomass Bioenergy 29,
269e292.
McMillan, J.D., 1994. Pretreatment of lignocellulosic biomass. In:
Himmel, M.E., Baker, J.O., Overend, R.P. (Eds.), Enzymatic Conversion of Biomass for Fuels Production. American Chemical
Society, Washington, D. C., pp. 292e324.
McMillan, J.D., Newman, M., Templeton, D., Mohagheghi, A., 1999.
Simultaneous saccharification and cofermentation of dilute-acid
pretreated yellow poplar hardwood to ethanol using xylosefermenting Zymomonas mobilis. Appl. Biochem. Biotechnol. 79,
649e665.
Mes-Hartree, M., Dale, B.E., Craig, W.K., 1988. Comparison of steam
and ammonia pretreatment for enzymatic hydrolysis of cellulose.
Appl. Microbiol. Biotechnol. 29, 462e468.
Mielenz, J.R., 2001. Ethanol production from biomass: technology
and commercialization status. Curr. Opin. Microbiol. 4,
324e329.
Millet, M.A., Baker, A.J., Scatter, L.D., 1976. Physical and chemical
pretreatment for enhancing cellulose saccharification. Biotech.
Bioeng. 6, 125e153.
Minowa, T., Ogi, T., Yokoyama, S.-Y., 1995. Hydrogen production from
wet cellulose by low temperature gasification using a reduced
nickel catalyst. Chem. Lett. 10, 937e938.
Minowa, T., Fang, Z., Ogi, T., Varhegyi, G., 1997. Liquefaction of
cellulose in hot compressed water using sodium carbonate: products distribution at different reaction temperatures. J. Chem. Eng.
Jpn. 30, 186e190.
Minowa, T., Fang, Z., Ogi, T., Varhegyi, G., 1998a. Decomposition of
cellulose and glucose in hot compressed water under catalyst-free
conditions. J. Chem. Eng. Jpn. 31, 131e134.
Minowa, T., Zhen, F., Ogi, T., 1998b. Cellulose decomposition in hot
compressed water with alkali or nickel catalyst. J. Supercrit. Fluids
13, 253e259.
486
27. DEVELOPMENT OF THERMOCHEMICAL AND BIOCHEMICAL TECHNOLOGIES FOR BIOREFINERIES
Mittal, A., Chatterjee, S.G., Scott, G.M., Amidon, T.E., 2009. Modeling
xylan solubilization during autohydrolysis of sugar maple and
aspen wood chips: reaction kinetics and mass transfer. Chem. Eng.
Sci. 64, 3031e3041.
Mohan, D., Pittman, C., Steele, P., 2006. Pyrolysis of wood/biomass for
bio-oil: a critical review. Energy Fuels 20, 848e889.
Monavari, S., Galbe, M., Zacchi, G., 2009. Impact of impregnation time
and chip size on sugar yield in pretreatment of softwood for
ethanol production. Bioresour. Technol. 100, 6312e6316.
Monavari, S., Galbe, M., Zacchi, G., 2011. Influence of impregnation
with lactic acid on sugar yields from steam pretreatment of sugarcane bagasse and spruce, for bioethanol production. Biomass
Bioenergy 35, 3115e3122.
Montane, D., Salvado, J., Farriol, X., Jollez, P., Chornet, E., 1994.
Phenomenological kinetics of wood delignification - application of
a time-dependent rate-constant and a generalized severity
parameter to pulping and correlation of pulp properties. Wood Sci.
Technol. 28, 387e402.
Morjanoff, P.J., Gray, P.P., 1987. Optimization of steam explosion as a
method for increasing susceptibility of sugarcane bagasse to
enzymatic saccharification. Biotechnol. Bioeng. 29, 733e741.
Mosier, N., Wyman, C.E., Dale, B., Elander, R., Lee, Y.Y.,
Holtzapple, M., Ladisch, M., 2005. Features of promising technologies for pre-treatment of lignocellulosic biomass. Bioresour.
Technol. 96, 673e686.
Murakami, K., Kasai, K., Kato, T., Sugawara, K., 2012. Conversion of
rice straw into valuable products by hydrothermal treatment and
steam gasification. Fuel 93, 37e43.
Mvula, E.E., Naumov, S.S., von Sonntag, C.C., 2009. Ozonolysis of
lignin models in aqueous solution: anisole, 1,2-dimethoxybenzene,
1,4-dimethoxybenzene, and 1,3,5-trimethoxybenzene. Environ. Sci.
Technol. 43 (16), 6275e6282.
Negro, M.J., Manzanares, P., Ballesteros, I., Oliva, J.M., Cabañas, A.,
Ballesteros, M., 2003. Hydrothermal pretreatment conditions to
enhance ethanol production from poplar biomass. Appl. Biochem.
Biotechnol. 105e108, 87e100.
Nguyen, D., Naotsugu, N., Tamikazi, K., 2000. Effect of radiation and
fungal treatment on lignocelluloses and their biological activity.
Radiat. Phys. Chem. 59, 393e398.
Nigam, J.N., 2001. Ethanol production from wheat straw hemicellulose hydrolysate by Pichia stipitis. J. Biotechnol. 87 (4), 17e27.
Nolen, S., Liotta, C., Eckert, C., Glaser, R., 2003. The catalytic opportunities of near-critical water: a benign medium for conventionally
acid and based catalyzed condensations for organic synthesis.
Green Chem. 5, 663e669.
Okano, K., Kitagaw, M., Sasaki, Y., Watanabe, T., 2005. Conversion of
Japanese red cedar (Cryptomeria japonica) into a feed for ruminants
by white-rot basidiomycetes. Anim. Feed Sci. Technol. 120,
235e243.
Orozco, A.M., Al-Muhtaseb, A.H., Albadarin, A.B., Rooney, D.,
Walker, G.M., Ahmad, M.N.M., 2011. Dilute phosphoric acidcatalysed hydrolysis of municipal bio-waste wood shavings using autoclave parr reactor system. Bioresour. Technol. 102,
9076e9082.
Pan, X., Xie, D., Gilkes, N., Gregg, D., Saddler, J., 2005. Strategies to
enhance the enzymatic hydrolysis of pretreated softwood with high
residual lignin content. Appl. Biochem. Biotechnol. 121, 1069e1079.
Pan, X., Gilkes, N., Kadla, J., Pye, K., Saka, S., Gregg, D., Ehara, K., Xie, D.,
Lam, D., Saddler, J., 2006. Bioconversion of hybrid poplar to ethanol
and co-products using an organosolv fractionation process: optimization of process yields. Biotechnol. Bioeng. 94 (5), 851e861.
Parham, R.A., 1983. Wood structure-hardwood, in pulp and paper
manufacture. In: Kocurek, M.J., Stevens, F. (Eds.), Properties of
Fibrous Raw Materials and Their Preparation for Pulping. CPPA,
Montreal, Canada.
Park, J.-Y., Kang, M., Kim, J.S., Lee, J.-P., Choi, W.-I., Lee, J.-S., 2012.
Enhancement of enzymatic digestibility of Eucalyptus grandis pretreated by NaOH catalyzed steam explosion. Bioresour. Technol.
123, 707e712.
Rabinovich, M.L., Melnik, M.S., Boloboba, A.V., 2002. Microbial cellulases (review). Appl. Biochem. Microbiol. 38, 305e321.
Ramos, L.P., 2003. The chemistry involved in the steam treatment of
lignocellulosic materials. Quim. Nova 26, 863e871.
Rogers, P., Jeon, Y., Lee, K., Lawford, H., 2007. Zymomonas mobilis for
Fuel Ethanol and Higher Value Products. Springer-Verlag, Berlin.
Adv Biochem. Eng. Biotechnol 108, 263e288.
Sarkanen, K.V., 1980. Acid-catalyzed delignification of lignocellulosics
in organic solvents. Prog. Biomass Convers. 2, 127e144.
Sassner, P., Mårtensson, C.G., Galbe, M., Zacchi, G., 2008. Steam pretreatment of H2SO4-impregnated Salix for the production of bioethanol. Bioresour. Technol. 99, 137e145.
Savage, P., Goplan, S., Mizan, T., Martino, C., Brock, E., 1995. Reactions
at supercritical conditions: applications and fundamentals. AIChE
J. 41, 1723e1778.
Schell, D., Farmer, J., Newman, M., McMillan, J., 2003. Dilute-sulfuric
acid pretreatment of corn stover in pilot-scale reactor: investigation
of yields, kinetics and enzymatic digestibilities of solids. Appl.
Biochem. Biotechnol. 105, 69e85.
Shaw, A., Jennery, F., Adams, M., Lynd, L., 2008. End-product pathways in xylose fermenting bacterium Thermoanaerobacterium saccharolyticum. Enzyme Microb. Technol. 42, 453e458.
Shevchenko, S.M., Beatson, R.P., Saddler, J.N., 1999. The nature of
lignin from steam explosion/enzymatic hydrolysis of softwoods:
structural and possible uses. Appl. Biochem. Biotechnol. 77,
867e876.
Shuai, L., Yang, Q., Zhu, J.Y., Lu, F.C., Weimer, P.J., Ralph, J., Pan, X.J.,
2010. Comparative study of SPORL and dilute-acid pretreatments
of spruce cellulosic ethanol production. Bioresour. Technol. 101,
3106e3114.
Shupe, A., Liu, S., 2009. Ethanol fermentation from hot-water wood
extract hydrolysate with a modified Pichia stipitis strain HWR.
AIChE Annu. Meet. (AIChE 2009).
Shupe, A.M., Liu, S., 2012. Ethanol fermentation from hydrolysed hotwater wood extracts by pentose fermenting yeasts. Biomass Bioenergy 39 (0), 31e38.
Sjöstrom, E., 1993. Wood Chemistry: Fundamentals and Applications.
Academic Press, New York.
Söderström, J., Pilcher, L., Galbe, M., Zacchi, G., 2003. Two-step steam
pretreatment of softwood by dilute H2SO4 impregnation for
ethanol production. Biomass Bioenergy 24, 475e486.
Speers, A.M., Reguera, G., 2012. Consolidated bioprocessing of
AFEX-pretreated corn stover to ethanol and hydrogen in a
microbial electrolysis cell. Environ. Sci. Technol. 46 (14),
7875e7881.
Stepanik, T.M., Rajagopal, S., Ewing, D., Whitehouse, R., 1998. Electron-processing technology: a promising application for the viscose
industry. Radiat. Phys. Chem. 52, 505e509.
Sun, Y., Cheng, J.Y., 2002. Hydrolysis of lignocellulosic materials for
ethanol production: a review. Bioresour. Technol. 83, 1e11.
Sun, X.F., Xu, F., Sun, R.C., Geng, Z.C., Fowler, P., Baird, M.S., 2005.
Characteristics of degraded hemicellulosic polymers obtained
from steam exploded wheat straw. Carbohydr. Polym. 60, 15e26.
Sun, N., Rahman, M., Qin, Y., Maxim, M.L., Rodriguez, H.,
Roger, R.D., 2009. Complete dissolution and partial delignification
of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate.
Green Chem. 11, 646e655.
Sun, Z., Shupe, A., Liu, T., Hu, R., Amidon, T.E., Liu, S., 2011. Particle
properties of sugar maple hemicellulose hydrolysate and its influence on growth and metabolic behavior of Pichia stipitis. Bioresour. Technol. 102 (2), 2133e2136.
REFERENCES
Swatloski, R.P., Holbrey, J.D., Memon, S.B., Caldwell, G.A.,
Caldwell, K.A., Rogers, R.D., 2004. Using Caenorhabditis elegans to
probe toxicity of 1-alkyl-3-methylimidazolium chloride based ionic
liquids. Chem. Commun. 6, 668e669.
Talebnia, F., Karakashev, D., Angelidaki, I., 2010. Production of bioethanol from wheat straw: an overview on pretreatment, hydrolysis and fermentation. Bioresour. Technol. 101 (13), 4744e4753.
Teixeira, L.C., Linden, J.C., Schroeder, H.A., 2000. Simultaneous
saccharification and cofermentation of peracetic acid-pretreated
biomass. Appl. Biochem. Biotechnol. 84e86 (1e9), 111e127.
Teymouri, F., Laureano-Perez, L., Alizadeh, H., Dale, B.E., 2005.
Optimization of the ammonia fiber explosion (AFEX) treatment
parameters for enzymatic hydrolysis of corn stover. Bioresour.
Technol. 96 (18), 2014e2201.
Thring, R.W., Chorent, E., Overend, R., 1990. Recovery of a solvolytic
lignin: effects of spent liquor/acid volume ratio, acid concentration, and temperature. Biomass 23, 289e305.
Thuijl, E., Roos, C., Beurskens, L., 2003. An Overview of Biofuel
Technologies, Markets, and Policies in Europe. Energy Research
Centre of the Netherlands, Amsterdam, Netherlands.
Tian, S., Zhu, W., Gleisner, R., Pan, X.J., Zhu, J.Y., 2011. Comparisons of
SPORL and dilute acid pretreatments for sugar and ethanol productions from aspen. Biotechnol. Prog. 27 (2), 419e427.
Tienvieri, T., Huusari Jr., E.S., Vuorio, P., Kortelainen, J., Ystedt, H.,
Artamo, A., 1999. Thermomechanical pulping. In: Sundholm, J.,
Gullichsen, J., Paulapuro, H. (Eds.). Mechanical Pulping, vol. 5.
Papermaking Science and Technology. Fapet, OyJyvaskyla,
Finland, pp. 157e221.
Timell, T.E., 1960. Determination of the degree of polymerization of
reducing pentose and hexose oligosaccharides. Sven. Papperstid.
63, 668e671.
Tunc, M.S., van Heiningen, A.R.P., 2008. Hemicellulose extraction of
mixed southern hardwood with water at 150 C: effect of time. Ind.
Eng. Chem. Res. 47 (21), 7031e7037.
Um, B.-H., van Walsum, G.P., 2012. Effect of pretreatment severity on
accumulation of major degradation products from dilute acid pretreated corn stover and subsequent inhibition of enzymatic hydrolysis of cellulose. Appl. Biochem. Biotechnol. 168 (2), 406e420.
US DOE, 2006. Breaking the biological barriers to cellulosic ethanol: a
joint research agenda. Report from the December 2005 Workshop.
DOE/SC-0095.
Verma, P., Watanabe, T., Honda, Y., Watanabe, T., 2011. Microwaveassisted pretreatment of woody biomass with ammonium molybdate activated by H2O2. Bioresour. Technol. 102, 3941e3945.
Vidal, P.F., Molinier, J., 1988. Ozonolysis of LignindImprovement of in
vitro digestibility of poplar sawdust. Biomass 16, 1e17.
Vidal, B.C., Dien, B.S., Ting, K.C., Singh, V., 2011. Influence of feedstock particle size on lignocellulose conversionda review. Appl.
Biochem. Biotechnol. 164, 1405e1421.
Wang, Y., Liu, S., 2012. Pretreatment technologies for biological and
chemical conversion of woody biomass. Tappi 11 (1), 9e16.
Wang, G., Pan, X., Zhu, J., Gleisner, R., 2009. Sulfite pretreatment to
overcome recalcitrance of lignocellulose (SPORL) for robust
enzymatic saccharification of hardwoods. Biotechnol. Prog. 25,
1086e1093.
Wang, Y., Liu, S., Liang, L., Buyondo, J.P., Wang, Y., Garver, M., 2012.
Biochemical conversion. In: Stuart, P.R., El-Halwagi, M.M. (Eds.),
Integrated Biorefineries: Design, Analysis and Optimization. CRC
Press, New York, pp. 591e650.
Wasikiewicz, J.M., Yoshii, F., Nagasawa, N., Wach, R.A, Mitomo, H.,
2005. Degradation of chitosan and sodium alginate by gamma
radiation, sonochemical and ultraviolet methods. Radiat. Phys.
Chem. 73, 287e295.
Weber, C., Boles, E., 2010. Sugar-hungry yeast to boost biofuel production. Sci. News Sci. Dly. 92, 881e882.
487
Weber, C., Farwick, A., Benisch, F., Brat, D., Dietz, H., Subtil, T.,
Boles, E., 2010. Trends and challenges in the microbial production
of lignocellulosic bioalcohol fuels. Appl. Microbiol. Biotechnol. 87,
1303e1315.
Weil, J., Westgate, P., Kohlmann, K., Ladisch, M., 1994. Cellulose
pretreatments of lignocellulosic substrates. Enzyme Microb. Technol. 16 (11), 1002e1004.
Weimer, P.J., Hackney, J.M., French, A.D., 1995. Effects of chemical
treatments and heating on the crystallinity of celluloses and their
implications for evaluating the effect of crystallinity on cellulose
biodegradation. Biotechnol. Bioeng. 48, 169e178.
Wheals, A., Basso, L., Alves, D., Amorim, H., 1999. Fuel ethanol after
25 years. Trends Biotechnol. 17, 482e487.
Willauer, H.D., Huddleston, J.G., Li, M., Rogers, R.D., 2000. Investigation of aqueous biphasic systems for the separation of lignins
from cellulose in the paper pulping process. J. Chromatogr. B
Biomed. Sci. Appl. 743, 127e135.
Wu, J., Zhang, J., Zhang, H., He, J., Ren, Q., Guo, M., 2004. Homogeneous acetylation of cellulose in a new ionic liquid. Biomacromolecules 5, 266e268.
Wyman, C.E., Dale, B.E., Elandert, R., Holtzapple, M., Ladisch, M.R.,
Lee, Y.Y., 2005. Coordinated development of leading biomass
pretreatment technologies. Bioresour. Technol. 96 (18),
1959e1966.
Wyman, C.E., Dale, B.E., Elander, R.T., Holtzapple, M.,
Ladisch, M.R., Lee, Y.Y., Mitchinson, C., Saddler, J.N., 2009.
Comparative sugar recovery and fermentation data following
pretreatment of poplar wood by leading technologies. Biotechnol.
Prog. 25, 333e339.
Xu, H.W., Scott, G.M., Jiang, F., Kelly, C., 2010. Recombinant manganese peroxidase (rMnP) from Pichia pastoris. Part 1: kraft pulp
delignification. Holzforschung 64, 137e143.
Yang, L., Liu, S., 2005. Kinetic model for kraft pulping process. Ind.
Eng. Chem. Res. 44, 7078e7085.
Yang, C.P., Shen, Z.Q., Yu, G.C., Wang, J.L., 2008. Effect and aftereffect
of g radiation pre-treatment on enzymatic hydrolysis of wheat
straw. Bioresour. Technol. 99, 6240e6245.
Yasuda, S., Murase, N., 1995. Chemical structures of sulfuric-acid
lignin. 12. Reaction of lignin models with carbohydrates in
72-percent H2SO4. Holzforschung 49, 418e422.
Yokoyama, S., 2008. The Asian biomass handbook. In: Yokoyama, S.,
Matsumura, Y. (Eds.), A Guide for Biomass Production and Utilization. The Japan Institute of Energy, Japan.
Zaldivar, J., Nielsen, J., Olsson, L., 2001. Fuel ethanol production from
lignocellulose: a challenge for metabolic engineering and process
integration. Appl. Microbiol. Biotechnol. 56, 17e34.
Zavrel, M., Bross, D., Funke, M., Buchs, J., Spiess, A.C., 2009. Highthroughput screening for ionic liquids dissolving (ligno-)cellulose.
Bioresour. Technol. 100, 2580e2587.
Zayed, G., Meyer, O., 1996. The single-batch bioconversion of wheat
straw to ethanol employing the fungus Trichoderma viride and the
yeast Pachysolen tannophytus. Appl. Microbiol. Biotechnol. 45,
551e555.
Zeikus, J., Ben-Bassat, A., Ng, T., Lamed, R., 1981. Thermophilic
ethanol fermentation. Basic Life Sci. 18, 441e461.
Zhang, Y.H.P., Himmel, M.E., Mielenz, J.R., 2006. Outlook for cellulase
improvement: screening and selection strategies. Biotechnol. Adv.
24, 452e481.
Zhang, Y.H.P., Ding, S.Y., Mielenz, J.R., Elander, R., Laser, M.,
Himmel, M., McMillan, J.D., Lynd, L.R., 2007. Fractionating
recalcitrant lignocellulose at modest reaction conditions. Biotechnol. Bioeng. 97, 214e223.
Zhao, Y., Wang, Y., Zhu, J.Y., Ragauskas, A., Deng, Y., 2008. Enhanced
enzymatic hydrolysis of spruce by alkaline pretreatment at low
temperature. Biotechnol. Bioeng. 99 (6), 1320e1328.
488
27. DEVELOPMENT OF THERMOCHEMICAL AND BIOCHEMICAL TECHNOLOGIES FOR BIOREFINERIES
Zheng, Y.Z., Lin, H.M., Tsao, G.T., 1998. Pretreatment of cellulose hydrolysis by carbon dioxide explosion. Biotechnol. Prog. 14, 890e896.
Zhu, J.Y., Pan, X.J., 2010. Woody biomass pretreatment for cellulosic
ethanol production: technology and energy consumption evaluation. Bioresour. Technol. 101 (13), 4992e5002.
Zhu, Y., Lee, Y.Y., Elander, R.T., 2005. Optimization of dilute-acid
pretreatment of corn stover using a high-solids percolation
reactor. Appl. Biochem. Biotechnol. 121, 1045e1054.
Zhu, J.Y., Pan, X.J., Wang, G.S., Gleisner, R., 2009. Sulfite pretreatment
(SPORL) for robust enzymatic saccharification of spruce and red
pine. Bioresour. Technol. 100 (8), 2411e2418.
Zhu, J., Pan, X., Zalesny Jr, R., 2010a. Pretreatment of woody biomass
for biofuel production: energy efficiency, technologies, and recalcitrance. Microbiol. Biotechnol. 87, 847e857.
Zhu, J., Pan, X., Zalesny Jr, R., 2010b. On the energy consumption for
size-reduction and yield from subsequent enzymatic saccharification of pretreated lodgepole pine. Bioresour. Technol. 101 (8),
2782e2792.
Zhu, J.Y., Zhu, W.Y., O’Bryan, P., Dien, B.S., Tian, S., Gleisner, R.,
Pan, X.J., 2010c. Ethanol production from SPORL-pretreated lodgepole pine: preliminary evaluation of mass balance and process energy efficiency. Appl. Microbiol. Biotechnol. 86 (5), 1355e1365.
Index
Note: Page numbers with “f” denote figures; “t” tables; and “b” boxes.
A
Ablative reactors, 245
Accelerated electron transfer, mediators for
flavins, 142
phenazines, 143
Acetone butanol ethanol (ABE), 116
Acid-catalyzed transesterification reaction,
121
Acid-driven hydrolysis, 161e163, 162f
Acidic lignin condensation, 321e322
Advanced reactor system, 9
AFEX. See Ammonia fiber explosion (AFEX)
Agricultural residues, 50
Agroindustrial residues, for bioethanol
production
raw material, 50e52
cellulose-containing residues, 52
starch-containing residues, 51e52, 52t
sugar-containing residues, 51, 51t
sugar production and fermentation
SHF, 52e55
SSF, 55
Algae biomass, 249
Alkali-catalyzed transesterification, 13
Alkaline neutralization, 195
Alkaline pulping, lignin reactions in, 321f
Alkaline salts, in zeolites, 256
Alkaloids, 353
American Society for Testing and Materials
(ASTM), 177
Amino acids, 110e112, 110f, 337e341
arginine, 340e341
aromatic amino acids, 341
glutamic acid, 338
lysine, 339e340
methionine, 340
threonine, 340
Amino-based products
amino acids, 337e341
arginine, 340e341
aromatic amino acids, 341
glutamic acid, 338
lysine, 339e340
methionine, 340
threonine, 340
aspartame, 341
polyamines
cadaverine, 348e349
putrescine, 346e348
poly amino acids, 341e345
cyanophycin/cyanophycin granule
polypeptide, 342e343
poly-g-glutamic acid, 343e345
ε-poly-L-Lysine, 345
Ammonia fiber explosion (AFEX), 290e291,
473
Ammonia recycled percolation (ARP),
61e62, 290e291, 465
Anabaena cylindrica, 368
Anaerobic biodegradation, 204
Anaerobic digestion (AD), 14e15, 15f
of microalgae, 164, 164f
Animal-derived biolipids, 188e189
Animal fat wastes
algae as biodiesel source, 12e13
biodiesel from, 11e12
beef tallow and chicken fat, 12
pork lard, 12
waste cooking oils, 12
Astaxanthin, 361
B
Bacillus coagulans, 84
Bacteria, 77
Bacterial metabolism
cytochromes (cell-bound), 140e142
shewanella oneidensis
MR-1 bioelectrochemical machinery, 142,
144f
riboflavin (vitamin B2) biosynthetic
genes of, 142, 145f
TFP, 139e140
Bidirectional hydrogenase, 371
Bioactive phytochemicals, 357, 357f
Biobased fats, 197, 198f
Biocathode chamber, microbial species in,
137e139, 138t
Biocathodes, 136e137
Biochar processing
agricultural benefits, 450
economic analysis, 451e454
cost-effective fertilizer substitute,
451e452
different potential income streams,
biochar carbon value for, 454
grain crop primary productivity, costeffective approach to, 452e453
livestock growth rates, 453e454
postpyrolysis indirect application of,
440e442
489
container growth medium and container,
441e442
soil nutrient reclamation, 441
water filtration, 440e441
properties of, 436e438
chemical and physical, 436e437
microbiological effects and synergisms,
437e438
theoretical income streams, 448e450
biochars and carbon markets, carbon
sequestration of, 449e450
renewable energy and fuel generation,
448e449
utilization of
carbon sequestration, 438
plant nutrients and other pollutants,
sorption of, 438e439
soil greenhouse gas emissions, 439e440
soil-specific biochar design, 440
Biochar overland flow filter, 441, 442f
Biochemical (sugar) platform, 227e228
Biocrude, 166
Biodegradability, 204, 205t
Biodiesel, 25
bioreactors for, 13e14
feedstocks for, 10e13
algae as source, 12e13
from animal fat wastes, 11e12
from pure vegetable oil, 10e11
waste cooking oils, 12
fuel properties of, 160t
and petrodiesel, 160e161, 160t
properties of, 196e197
Biodiversity, 374e375
Bioenergy production, technological routes
for
advanced biomass-to-biofuels
development platform, 30e33
biomass pretreatment, 28e29
combined pretreatment/hydrolysis and
fermentation strategies, 29
fermentation, 29
hydrolysis, 29
Bioenergy-related enzymes
databases and web servers, 98e103
CAT and dbCAN, 101e102
CAZy database, 98e101
FOLy database, 102
plant coexpression network databases,
102e103
490
Bioenergy-related enzymes (Continued)
purdue cell wall genomics and
UC-riverside cell wall navigator
databases, 102
plant biomass, 95e96
and regulation, 96e98
Bioenergy resources, 34
Bioethanol
in Brazil, feedstock for, 25
ethanol production
bioreactors in, 8e9
immobilization of cells for, 9
feedstock for, 3e4
fermentation, 7e8
lignocelluloses, pretreatment of, 4e7, 5f
biological pretreatment, 5e6
chemical pretreatment, 6e7
physical pretreatment, 6
molecular biology trends in, 8
Bioethanol production process, 4, 4f,
161e164, 162f
Biofuel production, representative
microorganisms features for, 83t
Biofuels development program, bioenergy
resources and, 33e34
Biogas
feedstock, 15
household digesters for, 15e17
fixed dome digesters, 15e16, 16f
floating drum digesters, 16e17, 17f
social and environmental aspects of, 17
Biohydrogen, 165e166, 165f
Biohydrogen photoproduction
by cyanobacteria, 372e375
H2 production strategies in, 373e375
by green algae
hydrogen photoproduction, nutrientdeprived green algae by, 378e381
light-dependent hydrogen production
pathways, 375e377
long-term H2 production by, 377e378
H2 photoproduction in, 377, 381e382
mechanisms of, 368e372
alternative nitrogenases, 369e370
hydrogenases, 370e372
nitrogenases, 369e370
oxygenic photosynthesis, 368e369
Biolipids
biodiesel, properties of, 196e197
energy balance, 192e193
liquid fuels, biomass to, 197e198
cleaning process, 197
gasification, 197
synthesis, 197e198
processing of
countercurrent extraction, 194
enzymatic hydrolytic maceration,
193e194
expression, 194
extraction, 193
hot continuous extraction, 194
maceration, 193
steam distillation, 193
supercritical fluid extraction, 194e195
ultrasound extraction, 194
INDEX
pure plant oil, properties of, 195e196
alkaline neutralization, 195
bleaching, 196
degumming, 195
transesterification, 196
winterization, 195e196
sources of
animal-derived biolipids, 188e189
edible lipids, 186e187
microalgae and other oleaginous
microorganisms, 189e190
nonedible lipids, 187
plant-derived biolipids, 186
waste edible oil, 187e188
supply and projected/purrent volume,
190e192
Biological pretreatment, 5e6
microorganisms for, 73e79
bioconversion, practical applications in,
74e77
genetically modified microorganisms for,
77e79
Biomass
addition of enzymes, 206
addition of microbes, 205e206
aquatic weeds, 209e211
biodegradability, 204, 205t
digesters, loading and unloading of, 212
digestion systems, 211e212
family-size biogas plant, 211
scum layer digester, 211e212
solid biomass digester, 212
wet digesters, 211
energy crops, 207
food processing residues, 207e209
bagasse, 207e208
coffee husks and mucilage, 208e209
rice husks, 207
gasification of, 247e248
catalytic gasification, 247e248
hydrothermal liquefaction of, 248e251
catalyst, 250e251
feedstock, 249
reaction conditions, 249e250
solvent, 250
kitchen and garden waste, 209
longer retention times, 207
macro and micronutrients, 204e205, 206t
pretreatments, 207
chemical pretreatment, 207
enzymes, biological pretreatment with,
207
hot water treatment, 207
mechanical pretreatment, 207
spent bedding, 209
use of methane, 213
utilization paths, 224, 227t
volatile solids
chemical conversion of, 213e214
thermal conversion of, 214
wet digesters
digestate treatment in, 212e213
solids content in, 212
pyrolysis of
catalytic pyrolysis, 244e245
fast pyrolysis, 244
reactors, 245e247
Biomass-based renewable energy system,
448e449
Biomass conversion, 461e462, 462f
to bioproducts, 462, 463f
Biomass-derived chemical building blocks,
297
Biomass pyrolytic conversion processes,
435e436, 436t
Biomass sources, 33, 33f
Biomethane, 25, 164e165
Biorefinery system, 32e33
and bio-based products, 221
bioconversion, converting sugars to
products, 477e478
biomass conversion, overview on,
461e462
eco-efficiency, 231e236
and emerging opportunities for sustainable
economy, 219e221
feedstock, 221e224
hydrolysis, 476e477
integration and multifunctionality in, 227,
232f
lignocellulosic biomass, characteristics of,
458e461
mechanical pretreatment
acid hydrolysis, 474e475
alkaline hydrolysis, 475e476
ammonia fiber explosion (AFEX), 473
ammonia recycle percolation
pretreatment, 465
biological pretreatment, 474
chipping/grinding/milling and refining,
463e465
electron beam/gamma ray and
microwave, irradiation pretreatment
by, 465
hot water, 469e470
ionic liquid pretreatment, 467e468
organosolv pretreatment, 466
overcome recalcitrance of
lignocelluloses, sulfite pretreatment to,
468e469
oxidation pretreatment, 466e467
ozonolysis pretreatment, 465e466
steam explosion, 470e473
supercritical carbon dioxide explosion,
473e474
physical/mechanical methods,
pretreatment-biomass size reduction
by, 462e476
platforms, 227e231, 232f
structure of, 224e227
thermochemical conversion, 478e481
combustion, 478
direct liquefaction, 481
gasification, 478e481
pyrolysis, 481
Biopellets, 3
Bleaching, 196
Brown-rot fungi, 75e76
Bubbling fluid bed reactor,
245e246, 246f
491
INDEX
C
D
H
Calcium oxide (CaO), 256
Carbohydrate composition, 278e280, 279t
Carbohydrate esterases (CEs), 98e100
Carbon dioxide pretreatment, 61
Carbon-negative bioenergy systems, 439f
Carbon sequestration, of biochars and
carbon markets, 449e450
Carboxymethyl cellulose (CMC), 78
Carotenoids, 353
Catalyst hydrophobicity, 259
Catalytic gasification, 247e248
Catalytic thermochemical processes, for
biomass conversion
gasification, 247e248
hydrothermal liquefaction, 248e251
pyrolysis, 244e247
CAZy database, 98e101
CAZyme Analysis Toolkit (CAT), 101
Cellobiose hydrolyzation, 161e163
Cellulose, into monomeric units of glucose,
161e163, 163f
Cellulose raw material, 52
Cell wall model, pH on solubilization,
291e292, 294f
Cell wall related (CWR), 96, 97t
C9-formulae, 329e330
Chemical pretreatment
acid hydrolysis, 6e7
alkaline hydrolysis, 7
ammonia hydrolysis, 7
ozonolysis, 7
Chemical pulp, productions of, 316, 316t
Chlamydomonas, 166
Circulating fluidized beds, 245e246, 246f
Clay minerals, 260e265
acid activation of, 262, 262f
biodiesel production, acid-activated clay
minerals in, 263e265
improving acidity, 262e263
treatment of, 262e263
Concept of eco-efficiency, 233
Confectionery industry waste streams,
429e430, 429f
Conjugation, 393
Conserved domain database (CDD), 101
Contamination, 156
Corrosion, 121
Corn dry milling, 359
Countercurrent extraction, 194
CMC. See Carboxymethyl cellulose (CMC)
Crude glycerol streams, 424
Crystalline cellulose, 115
Cyanobacteria, 165
biofuels, production system for, 393e403
aliphatic alcohols and alkanes,
photosynthetic production of, 402e403
butyraldehyde and butanol, 401e402
ethanol, 398
ethylene, 398e400
hydrogen, 393e398
isoprene, 400e401
engineering, 392e393
strains/tools and methods, 392e393
Cyanothece, 373, 397
Dedicated bioenergy crops, 38e39
Dedicated energy crops (DECs), 15
Degree of lignin condensation (DC), 319
Degree of polymerization (DP), 458e459
Degumming, 195
DET. See Direct electron transfer (DET)
Diacylglycerol acyltransferases (DGATs),
175e176
Digesters, 214
Digestion systems, 211e212
family-size biogas plant, 211
scum layer digester, 211e212
solid biomass digester, 212
wet digesters, 211
Dimethylallyl-diphosphate (DMADP),
400e401
Direct electron transfer (DET), 134, 134f, 137,
143f
Direct hydrothermal liquefaction, 249
Hardwood
cellular structure of, 458, 459f
lignins, 318e319
Hemicellulose, 76
Heterocyst differentiation, 372e373
Heterocyst forming species, 395
Heterogeneity, in feedstock, 30e32
Heterogeneous catalysts
clay minerals, 260e265
biodiesel production, acid-activated clay
minerals in, 263e265
improving acidity, 262e263
heteropolyacids, 257e258
layered materials, 265e269
layered carboxylates, 266
layered double hydroxides, 265
layered hydroxide salts, 265e266
transesterification reactions,
heterogeneous catalysts in, 266e269
polymeric catalysts, 269e272
zeolites, 258e260
Heteropolyacids, 257e258
Heteropolyoxometalate anions, 257
Heterotrophic microalgae, 155e156
High-efficiency algal-derived biocrude,
second-generation biofuel from, 153,
154f
biocrude, 166
biodiesel, 158e159
biodiesel and petrodiesel, 160e161, 160t
bioethanol production process, 161e164,
162f
biohydrogen, 165e166, 165f
biomethane, 164e165
contamination, 156
culture techniques, 156
heterotrophic microalgae, 155e156
HTL, 167
hydrothermal catalytic liquefaction, 167
microalgae
biomass/biofuel production-cultivation,
155
production of biodiesel from, 159e160
microalgal biofuels, 154e155
microalgal biomass to, 158
mixing, 156
nutrients, 156
open-pond culture, 157, 157f
photobioreactors, 157e158, 157f
phototrophic microalgae, 155
processing microalgal biomass for, 158, 158f
subcritical water, properties of, 166e167,
167f
Hot continuous extraction, 194
HOX. See Reversible hydrogenase (HOX)
Hydrogen
hydrogen bioproduction, 394
H2 production, heterocystous
cyanobacteria by, 396e397
H2 production, nonheterocystous
cyanobacteria by, 397e398
hydrogen-evolving enzymes, 394e395
hup-hydrogen uptake enzyme, 394e395
nitrogenase, gratuitous hydrogenase, 395
E
Edible lipids, 186e187
Escherichia coli, 8, 8f
Ethanol, 50
Ethylene, 398e400
bioproduction of, 399e400
microbial production of, 399
Energy balance, 192e193
Entrained-fow reactors, 245
Environmental impact ratio, 233e234,
235f
Enzymatic hydrolytic maceration,
193e194
Enzyme-mediated transesterification,
119e120
Extracellular/free lipases, 121e122
F
FAMEs. See Fatty acid methyl esters
(FAMEs)
Fatty acids, in animal fats, 188t
Fatty acid methyl esters (FAMEs), 119e120,
159e160, 263e264
First-generation bioenergy, 408
Fish pond discharge (FPD), 180e181
Flavins, 142
Flavonoids, 353
FOLy database, 102
Fomitopsis pinicola, 76
Fossil fuel-derived petrodiesel, 160e161
Fossil fuels, 24e25
FPD. See Fish pond discharge (FPD)
Fuel ethanol production, 25
Furfural, main outlets of, 299e300, 299f
G
Gasification, 197
Global energy production chart, 1e2, 2f
Gloeophyllum trabeum, 76
Glycoside hydrolases (GHs), 96
Glycosyltransferases (GTs), 96
Green biorefineries, 227, 229f
Greenhouse gases (GHGs), 1e2, 23e24, 65,
407e408
492
Hydrogen (Continued)
reversible hydrogenase (HOX), 395
Hydrogen-evolving enzymes, 394e395
hup-hydrogen uptake enzyme, 394e395
nitrogenase, gratuitous hydrogenase, 395
reversible hydrogenase (HOX), 395
Hydrothermal liquefaction (HTL), 154e155,
167, 248e251
Hydrothermal pretreatment methods, 65
Hyper-isobutanol-producing strains,
113e114
I
ILUC. See Indirect land use change (ILUC)
Immobilization, 375
Indirect land use change (ILUC), 407e408
Insoluble inorganic salts, 260
Integrated biorefinery system, 236f
Intracellular/immobilized lipases, 122, 122t
Ionic liquid pretreatment, 467e468
Irradiation pretreatment, 465
Isobutanol production
chemistry of fermentation, 112e113, 116
higher alcohol production, Keto acid
pathways for, 110e112
microorganisms, metabolic engineering of,
113e114
overview, 109e110
sustainable feedstocks, bioenergy crops as,
114e115
technologies, 115e116
Isoprene, 400e401
J
Jatropha curcas (Jatropha), 10, 172
K
2-ketoisovalerate (KIV), 111e112
biosynthetic genes, 113e114
L
Layered carboxylates, 266
Layered double hydroxides, 265
Layered hydroxide salts, 265e266
Layered materials, 265e269
carboxylates, 266
double hydroxides, 265
hydroxide salts, 265e266
transesterification reactions, heterogeneous
catalysts in, 266e269
Lewis acidity, 262
Light-dependent hydrogen production
pathways, 375e377
Lignin
biological degradation of, 73
classification of, 320, 320t
degradation, 320
under acidolysis conditions, 321e322,
322f
potential products from, 297, 297f
production/properties and analysis,
318e332
advanced NMR methods, 330
analytical methods for characterization
of, 323
INDEX
molecular weight distribution, 330e331
C NMR analysis of, 327e330, 329t
31
P NMR reproducibility analytical
techniques, 323e327, 326t
structure-properties correlations in,
331e332
renewable raw material feedstock,
315e318
traditional and emerging applications
emerging lignin applications, 332e333
traditional lignin applications, 332
valorization, 333
Lignocellulose-based chemical products
C6 and C6/C5 sugar platform, 295e296
chemical transformation products, 296
fermentation products, 295e296
carbohydrate dehydration, 298e305
furfural production and applications,
298e301
hexose feedstock, 5hydroxymethylfurfural formation
from, 301e305
cellulose, 280
chemicals and fuels, furans/aromatics as
building blocks for, 297e298
hemicelluloses
arabinogalactan, 282e283
arabinoglucuronoxylans, 282
arabinoxylan, 283
carbohydrate feedstocks, 283
complex heteroxylans, 283
galactoglucomannans, 282
glucomannan, 282
glucuronoxylans, 280e282
xyloglucans, 282
b-(1 / 3, 1 / 4)-glucans, 283
lignin, 283e286
lignin platform, 296e297
lignocellulosic biorefineries, 292e295
monoaromatic chemicals, technical lignins
into, 305e309
acid-catalyzed depolymerization, 305
base-catalyzed depolymerization, 305
ionic liquids, 308
lignin aromatics, future perspectives of,
308e309
oxidative depolymerization, 306
pyrolysis, 305e306
reductive hydrodeoxygenation, 306e307
solvolysis, 307
sub-and supercritical water, 307e308
supercritical solvents, 308
occurrence and composition of, 278e280
storage carbohydrates, 280
structural carbohydrates, 280
pretreatment technologies, 286e290
alkaline (lime) pretreatment process,
289e290
dilute and concentrated acid
pretreatment, 289
at laboratory/conceptual stages,
290e292
liquid hot water (LHW), 288
steam explosion, 286e288
wet oxidation, 288e289
13
Lignocellulose pretreatment, 64e65
Lignocellulosic bioenergy processes, 362,
362f
Lignocellulosic biomass
catalytically hydrothermal liquefaction of,
250, 250t
lignin content and chemical structures of,
284, 284t
pretreatment methods of, 286, 287t
pyrolysis of, 244, 244t
chemical composition of, 4e7, 5f
ethanol production from, 25e26
pretreatment of, 58
microorganisms, biological pretreatment
with, 73
nonbiological pretreatment, 72e73
pyrolysis of, 244, 244t
utilization of, 50
Lignocellulosic energy crops, 50
Lignocellulosic feedstock biorefinery
(LCFBR), 37e38, 224e227, 229f
Lignocellulosic raw materials, 32e33
Lignocellulosic sources, cell wall
compositions of, 30
Lignocellulosic waste materials, 50
Lignosulfonates, 322e323
Linear electron transport (LET), 368
Lipase-catalyzed biodiesel production
acyl acceptors, 126
catalyzed transesterification done in,
121e122
chemical transesterification, disadvantages
of, 120e121
chemistry of biodiesel, 120, 120f
choice of enzyme, 124
feedstock, 123e124
algae oils, 124
animal oils/fats, 123
vegetable oils, 123
waste oils/fats, 123e124
historical background of, 121
immobilized, advantages of,
122e123
molar ratio, 124
reactor system, 126e127
solvents, 126
technical challenges, 123
temperature, 124e126
transesterification, 120, 120f
using lipases, advantages of, 121
water content, 126
Lipid content, and biomass productivity,
190, 191t
Liquid fuels, 25
biomass to, 197e198
cleaning process, 197
gasification, 197
synthesis, 197e198
Liquid hot water (LHW), 73
Liquefaction of biomass, 250
Long-term soil testing, 453e454
Low light-utilization efficiency, 381
Lowry acid sites, 259
Lysophosphatidate acid acyl-transferase
(LPAT), 176
INDEX
M
Maceration, 193
Madhuca indica (Mahua), 11
Main technology platforms
for bioenergy production, 26, 26f
Marine biorefinery (MBR), 227
MCM-41 molecular sieves, 259
Mediated electron transfer (MET), 134e135,
137e139, 143f
through exogenous redox mediators,
134e135, 135f
through secondary metabolites, 135, 135f
Membrane bioreactor, 9, 9f
Metabolic engineering poses, 78
Metabolic flux analysis (MFA), 78e79
Methane emissions, 450
31
P-II methodology, 323
Methylene diphenyl diisocyanate (MDI),
317
Methyl esters, purification of, 121
MET. See Mediated electron transfer (MET)
MFA. See Metabolic flux analysis (MFA)
Microalgae
biofuel, 154e155
biomass, 158
biofuel production-cultivation, 155
cultivation of, 40
and oleaginous microorganisms, 189e190
production of biodiesel from, 159e160
uses of, 41
Microbial conversion, of biomass
biodiesel, 172
production, 177, 178t
viable feedstocks for, 173
waste utilization for, 179e181
biological pretreatment, microorganisms
for, 73e79
bioconversion, practical applications in,
74e77
genetically modified microorganisms for,
77e79
fatty acid methyl esters and fuel properties,
179
genetic engineering approach, 175e177
lignocellulosic biomass and pretreatment,
72e73
microorganisms, biological pretreatment
with, 73
nonbiological pretreatment, 72e73
microbial pretreatment
for biogas production, 80e81
for biomass conversion, 81e84
strategies of using, 79e84
petroleum fuel scenario, India, 172
potent strains, selection of, 173e175
RAS, 180f
renewable energy, 171
Microbial electrolysis cells (MECs), 143e144
Microbial fuel cells (MFC)
anode reaction in, 132e133, 132f
biocathodes, electron transfer for, 136e139
DET for, 137
MET for, 137e139
bioelectrochemistry of, 132e139
biofilm electrochemistry for, 139e143
accelerated electron transfer in,
142e143
bacterial metabolism, 139
mediator-less factors affecting,
139e142
biofilms, electrogens in, 135e136
cathode reaction in, 132f, 133
concomitant electricity, wastewater
treatment with,
143e147
reactor designs, 143e144
substrates used in, 145e147
electrode reactions in, 132e133
anode reaction, 132e133
cathode reaction, 133
electron transfer methods, 133e135
anodic biofilms, DET for, 134
anodic biofilms, MET for, 134e135
Microbial pretreatment
for biogas production, 80e81
for biomass conversion, 81e84
strategies of using, 79e84
Microbial putrescine, and cadaverine
production, 348t, 349
Microorganism platform, 227
Microwave-based heating, 63
Miscanthus species, 114e115
Miscanthus x giganteus, 114e115
Molecular hydrogen, 394
Multiple sequence alignment (MSA), 101
N
Napier grass biomass, 114e115
Native lignins, structural moieties of,
318e319, 319f
Negative performance, of bioenergy crops,
408
Neisseria gonorrhoeae, 139, 140f
Nicotiana tabacum (Tobacco), 11
Nicotinamide adenine dinucleotide
(NADH), 397e398
Nonbiological pretreatment, potential
advantages over,
73
Nonedible lipids, 187
Nutrient recycling, 214
O
Octahedral structural metals, 262e263, 262f
Oilseeds, biodiesel production from, 424
Oil transesterification, 13, 13f
Open-pond culture, 157, 157f
Organic agriculture, 409e410
Organosolv pretreatment, 60e61
advantages of, 61
Oriented Strand Boards (OSB), 317
Oxidation pretreatment, 466e467
Oxygenic photosynthesis, 368e369
P
Pelletization process, 3, 3f
Penicillium digitatum, 399
Perennial grasses, 114e115
Perennial herbaceous energy crops, 24
Permissible exposure limit, 449e450
493
Petrodiesel, fuel properties of, 160t
Phanerochaete chrysosporium, 5
Phenazines, 143
Phenoleformaldehyde (PF), 331
Photobioreactors, 157e158, 157f
Photosynthetic electron transfer reactions,
368
Photosynthetic light reactions, 368
Phototrophic microalgae, 155
Physical pretreatment
extrusion, 6
mechanical comminution, 6
steam explosion, 6
ultrasonic pretreatment, 6
Phytochemicals
bioactive/pharmaceutical phytochemicals,
356e357
colorants, 355e356
coproduction of, 361e363
bio-oil/syn-gas and algal bioenergy
processes, 362e363
fermentative phytochemicals
production, colocation of, 363
phytochemical production by-products,
utilization of, 363
plant oil-based bioenergy processes,
361e362
starch/sugar-based bioenergy processes,
361
dietary/nutraceutical/food or feed
additives, 356
major groups of, 353e354, 354f
for personal care, 358
production of, 358e361
algae via aquaculture, 361
cultured plant cells, 360
extraction and isolation from specific
plants, 358
microbial fermentation, 360e361
staple crops, biorefinery processing of,
358e360
Phytosterols, 354
Plant biomass, 95e96
Plant coexpression network databases,
102e103
Plant-derived biolipids, 186
Plant-derived colorants, 358
Plant oil-based biodiesel processes, 360f,
361e362
Plant oil, properties of, 195e196
alkaline neutralization, 195
bleaching, 196
degumming, 195
transesterification, 196
winterization, 195e196
Polyamines
cadaverine, 348e349
putrescine, 346e348
Poly amino acids, 341e345
cyanophycin/cyanophycin granule
polypeptide, 342e343
applications for, 343
biodegradability of, 343
production of, 343
poly-g-glutamic acid
494
Poly amino acids (Continued)
applications of, 344e345
biodegradability of, 344e345
production of, 344
poly-g-glutamic acid, 343e345
ε-poly-L-Lysine, 345
applications of, 345
degradation of, 345
production of, 345
Poly (3-hydroxybutyrate) (PHB)
confectionery and bakery industry waste
streams, production from, 429e430
production from whey, 430
Polyhydroxyalkanoates
biodiesel industry by-products,
valorization of, 424e427
biorefinery concepts, production integrated
in, 421e430
food industries, by-product streams from,
427e430
second-generation bioethanol industry byproducts, 427
structure and properties of, 420e421
winery by-products, production from,
428e429
Polymeric carbohydrates, 354
Polymeric catalysts, 269e272
Polyoxometalates, 257
Polyvinyl chloride (PVC), 14
Pongamia pinnata (Karanja), 11
Postharvest processing, 360
Postpyrolysis indirect application,
440e442
container growth medium and container,
441e442
soil nutrient reclamation, 441
water filtration, 440e441
Preliminary bioreactor fermentations,
425e426
Pressurized high temperature entrained
flow reactor (PiTER), 245, 245f
Pretreatments, 207
enzymes, biological pretreatment with, 207
modeling, 65
trends in, 62e65
technologies, laboratory/conceptual stages
AFEX, 290e291
ionic liquids, 291
lignocellulosic biomass pretreatments,
291e292
sub/supercritical treatments, 291
types of, 58e62
biological pretreatments, 58e59
chemical pretreatments, 60e61, 207
hot water treatment, 207
mechanical pretreatment, 207
physical pretreatments, 59e60
physicochemical pretreatments, 61e62
Proton exchange membrane (PEM), 132
Pseudomonas aeruginosa, 139, 140f, 143
Pseudomonas cepacia, 124
Purdue cell wall genomics, and UCriverside cell wall navigator
databases, 102
Pyrolysis bioenergy industry, 436
INDEX
R
RAS. See Recirculatory aquaculture system
(RAS)
Recirculatory aquaculture system (RAS),
180, 180f
Reed canary grass, 39
Renewable energy (REN), 24
Reversible hydrogenase (HOX), 395
Rotating cone reactor, 246e247, 246f
Roundtable of sustainable biomaterials
(RSB), 410, 412
S
Saccharomyces cerevisiae, 29, 161
Saturated fatty acids, 179
Scenedesmus obliquus, 368
S-contained compounds, 354
Second-generation bioenergy, 408
Separate hydrolysis and fermentation
(SHF), 52e55
SHF. See Separate hydrolysis and
fermentation (SHF)
Simultaneous saccharification and
fermentation (SSF), 29, 52, 55
Single superphosphate (SSP), 451e452
Soaking aqueous ammonia (SAA),
61e62
Soft-rot fungi, 76e77
Softwood, cellular structure of, 458, 459f
Soil fertility, 214
Soil greenhouse gas emissions, 439e440
Soil-specific biochar design, 440
Solid state cultivation (SSC), 73
Solid state fermentation approach, 9
Soybean processing, 358, 359f
SSF. See Simultaneous saccharification and
fermentation (SSF)
Starch raw material, 51e52, 52t
Steam explosion, 470e473
Steam distillation, 193
Steam pretreatment, objective of, 61
Stoichiometric models, 78e79
Sugarcane bagasse, 63
Sugar raw material, 51, 51t
Sunflower-based biorefinery concept,
426e427, 426f
Supercritical fluid
extraction, 194e195
fluid parameters, 308, 308t
Sustainability, 37f
biodiesel, feedstocks for, 39e41
bioenergy feedstocks and dedicated biofuel
crops, 37e39
dedicated bioenergy crops, 38e39
lignocellulosic feedstocks, 37e38
Sustainable farming
bioenergy production
biofuel production, sustainability criteria
for, 410e412
biomass uses, 412
farm level, potential on, 414e415
global bioenergy potential, 413e414
ways of comparisons, 410
criteria for
agricultural production, 409e410
conventional agricultural production,
409
food systems, 410
SWOT analysis, 233, 235t
Synechococcus elongatus, 113
T
Technoeconomic analysis, 66
TFP. See Type IV pili (TFP)
Thermal depolymerization, 244
Thermochemical biorefinery (TCBR), 227
Thermochemical platform, 227e229
Thioesterase (TE), 176e177
Torrefaction, 64
Traditional fatty acid esterification
processes, 256
Transesterification, 159e160, 160f, 196
heterogeneous catalysts in, 266e269
use of acid catalysis, 257
Transgenic organisms, enhance lipid
biosynthesis in, 175e176, 176t
Triacylglycerol (TAG), 175
chemical structure of, 120, 120f
Trichodesmium, 373
Tricarboxylic acid (TCA), 399
Tubular photobioreactor (TPBR), 14, 14f,
396f
Type IV pili (TFP), 139e140
U
Ubiquitous phytocolorants, 355, 355f
UC-riverside cell wall navigator databases,
purdue cell wall genomics and, 102
Ultrasound extraction, 194
Unclassified biorefineries, 229
Unicellular cyanobacterium, 397
V
Vegetable oil, production of, 360, 360f
VFAs. See Volatile fatty acids (VFAs)
Vegetative cells, 372, 372f
Vibrio cholerae, 139, 140f
Volatile fatty acids (VFAs), 145
Volatile solids
chemical conversion of
combustion, 213
gasification, 213e214
thermal conversion of, 214
V-nitrogenase, 369
W
Waste edible oil, 187e188
Wet digesters
digestate treatment in, 212e213
solids content in, 212
Wet milling yields, 359
Wet oxidation, 61
White-rot fungus application
for animal feed, 75
biofiber, biomass for, 75
in biological pulping, 75
corn stover, 74
cotton stalks, 74
paddy straw, 75
rice straw, 74e75
495
INDEX
Whole crop biorefinery (WCBR), 227, 229f
Wine lees valorization, 428, 428f
Winterization, 195e196
WIPO. See World Intellectual Property
Organization (WIPO)
Wood
major components of, 459, 460t, 461
typical structures of, 458, 458f
World Intellectual Property Organization
(WIPO), 317e318, 318f
X
X-ray diffraction (XRD), 263
Z
Zeolites, 258e260
Zeotype materials, 258